the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Coccolithophore populations and their contribution to carbonate export during an annual cycle in the Australian sector of the Antarctic zone
Andrés S. Rigual Hernández
José A. Flores
Francisco J. Sierro
Miguel A. Fuertes
Lluïsa Cros
Thomas W. Trull
The Southern Ocean is experiencing rapid and relentless change in its physical and biogeochemical properties. The rate of warming of the Antarctic Circumpolar Current exceeds that of the global ocean, and the enhanced uptake of carbon dioxide is causing basin-wide ocean acidification. Observational data suggest that these changes are influencing the distribution and composition of pelagic plankton communities. Long-term and annual field observations on key environmental variables and organisms are a critical basis for predicting changes in Southern Ocean ecosystems. These observations are particularly needed, since high-latitude systems have been projected to experience the most severe impacts of ocean acidification and invasions of allochthonous species.
Coccolithophores are the most prolific calcium-carbonate-producing phytoplankton group playing an important role in Southern Ocean biogeochemical cycles. Satellite imagery has revealed elevated particulate inorganic carbon concentrations near the major circumpolar fronts of the Southern Ocean that can be attributed to the coccolithophore Emiliania huxleyi. Recent studies have suggested changes during the last decades in the distribution and abundance of Southern Ocean coccolithophores. However, due to limited field observations, the distribution, diversity and state of coccolithophore populations in the Southern Ocean remain poorly characterised.
We report here on seasonal variations in the abundance and composition of coccolithophore assemblages collected by two moored sediment traps deployed at the Antarctic zone south of Australia (2000 and 3700 m of depth) for 1 year in 2001–2002. Additionally, seasonal changes in coccolith weights of E. huxleyi populations were estimated using circularly polarised micrographs analysed with C-Calcita software. Our findings indicate that (1) coccolithophore sinking assemblages were nearly monospecific for E. huxleyi morphotype B/C in the Antarctic zone waters in 2001–2002; (2) coccoliths captured by the traps experienced weight and length reduction during summer (December–February); (3) the estimated annual coccolith weight of E. huxleyi at both sediment traps (2.11 ± 0.96 and 2.13 ± 0.91 pg at 2000 and 3700 m) was consistent with previous studies for morphotype B/C in other Southern Ocean settings (Scotia Sea and Patagonian shelf); and (4) coccolithophores accounted for approximately 2–5 % of the annual deep-ocean CaCO3 flux. Our results are the first annual record of coccolithophore abundance, composition and degree of calcification in the Antarctic zone. They provide a baseline against which to monitor coccolithophore responses to changes in the environmental conditions expected for this region in coming decades.
- Article
(2787 KB) - Full-text XML
-
Supplement
(244 KB) - BibTeX
- EndNote
1.1 Background and objectives
The rapid increase in atmospheric CO2 levels since the onset of the industrial revolution is modifying the environmental conditions of marine ecosystems in a variety of ways. The enhanced greenhouse effect, mainly driven by increased atmospheric CO2 levels, is causing ocean warming (Barnett et al., 2005), shallowing of mixed layer depths (Levitus et al., 2000) and changes in light penetration and nutrient supply (Bopp et al., 2001; Rost and Riebesell, 2004; Sarmiento et al., 2004b; Deppeler and Davidson, 2017). Moreover, the enhanced accumulation of CO2 in the ocean is giving rise to changes in the ocean carbonate system, including reduction of carbonate ion concentrations and lowering of seawater pH. Most evidence suggests that the ability of many marine calcifying organisms to form carbonate skeletons and shells may be reduced with increasing seawater acidification including some (but not all) species of coccolithophores, corals, pteropods and foraminifera (e.g. Orr et al., 2005; Moy et al., 2009; Lombard et al., 2010; Beaufort et al., 2011; Andersson and Gledhill, 2013). Since phytoplankton are extremely sensitive to global environmental change (Litchman et al., 2012) all predicted changes in marine environmental conditions are likely to modify the abundance, composition and distribution of phytoplankton communities.
Changes in the relative abundances of major phytoplankton functional groups are likely to influence ocean biogeochemistry and ocean carbon storage, with feedbacks to the rate of climate change (e.g. Boyd and Newton, 1995; Boyd et al., 1999; Falkowski et al., 2004; Cermeño et al., 2008). The precipitation and sinking of CaCO3 by coccolithophores has the potential for complex contributions to carbon cycling. Carbonate precipitation removes more alkalinity than dissolved inorganic carbon from surface waters, thereby acting to increase pCO2 in surface waters (the so-called carbonate counter pump; e.g. Zeebe, 2012). On the other hand, ballasting by carbonates appears to increase the transfer of organic carbon to the ocean interior (Armstrong et al., 2002; Klaas and Archer, 2002). On seasonal timescales the counter pump contribution dominates (Boyd and Trull, 2007), but more complex interactions can occur over longer timescales as a result of changing extents of carbonate dissolution in sediments, including the possibility that enhanced calcite dissolution in the Southern Ocean contributed to lower atmospheric CO2 levels during glacial maxima (Archer and Maier-Reimer, 1994; Sigman and Boyle, 2000; Ridgwell and Zeebe, 2005).
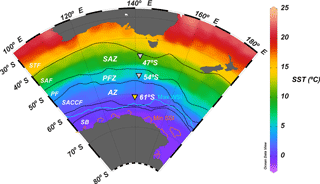
Figure 1Annual mean sea surface temperature map (World Ocean Atlas; Locarnini et al., 2013) of the Australian sector of the Southern Ocean, showing the position of the main frontal and zonal systems (adapted from Orsi et al., 1995) and the location of the 61, 54 and 47∘ S sediment trap stations (inverted triangles). Abbreviations: STF – subtropical front, SAZ – Subantarctic zone, SAF – Subantarctic Front, PFZ – Polar Frontal Zone, PF – Polar Front, AZ – Antarctic zone, SACCF – Southern Antarctic Circumpolar Current Front, SB – southern boundary, Max WSI – maximum winter sea ice extent (August 2001) and Min SSI – minimum summer sea ice extent (February 2002; Fetterer et al., 2017).
The Southern Ocean is a critical component of the Earth's ocean–climate system and plays a pivotal role in the global biogeochemical cycles of carbon and nutrients (Sarmiento et al., 2004a; Anderson et al., 2009). Despite the fact that the Southern Ocean accounts for about 25 % of the global ocean, it contains ∼ 40 % of the global ocean inventory of anthropogenic CO2 (Khatiwala et al., 2009; Takahashi et al., 2009; Frölicher et al., 2015), and it exports nutrients to more northern latitudes, ultimately supporting ∼ 75 % of the ocean primary production north of 30∘ S (Sarmiento et al., 2004a). Model projections suggest that the reduction in the saturation state of CaCO3 will reach critical thresholds sooner in cold, high-latitude ecosystems such as the Southern Ocean (Orr et al., 2005; McNeil and Matear, 2008; Feely et al., 2009). Therefore, calcifying organisms living in these regions will be the first to face the most severe impacts of ocean acidification.
In view of the rapid changes in climate and other environmental stressors presently occurring in the Southern Ocean, a major challenge facing the scientific community is to predict how phytoplankton communities will reorganise in response to global change. In this regard, two main aspects of the distributions of coccolithophores are emerging. Firstly, coccolithophores exhibit high concentrations in the Subantarctic Southern Ocean, a feature termed by Balch et al. (2011) as the “Great Calcite Belt” based on satellite reflectance estimates of PIC abundances. However, the PIC accumulations are significantly less than those that arise in the North Atlantic, and the satellite algorithm is not reliable in Antarctic waters where it badly overestimates PIC abundances (Balch et al., 2016; Trull et al., 2018). Secondly, recent studies suggest that the magnitude and geographical distribution of E. huxleyi blooms may be experiencing significant and rapid changes. Cubillos et al. (2007) and Winter et al. (2014) postulated that E. huxleyi has expanded its ecological niche south of the Polar Front in recent decades. Contrastingly, Freeman and Lovenduski (2015) suggested an overall decline in Southern Ocean PIC concentrations using satellite records between 1998 and 2014. The explanation of these contrasting results may lie in the methodologies applied. While shipboard surface water observations provide a highly detailed picture of a given ecosystem, they are very sparse, only represent a snapshot in time and can easily miss blooms of any given species. The satellite PIC signal has the great advantage of large-scale and repeated coverage, but can miss subsurface populations (e.g. Winter et al., 2014) and be mimicked by the spectral characteristics of other scattering sources. The most important among them are probably microbubbles (Zhang et al., 2002), glacial flour (Balch et al., 2011) and non-calcifying organisms such as Phaeocystis antarctica (Winter et al., 2014), a colonial prymnesiophyte algae very abundant in high-latitude systems of the Southern Ocean (e.g. Arrigo et al., 1999, 2000). Notably, the PIC algorithm performs particularly poorly in Antarctic waters (Balch et al., 2016; Trull et al., 2018).
For these reasons, year-round field observations of areas representative of key Southern Ocean regions are essential to determine the current state of coccolithophore communities and to develop baselines against which long-term trends can be detected. Moreover, a better understanding of coccolithophore distribution, ecology and seasonal dynamics is required to improve our interpretations of the sedimentary record and our models of biogeochemistry. Sediment traps are a direct method to collect data about calcareous and siliceous microplankton and nanoplankton. Traps allow us to monitor the seasonal and annual variability of plankton export, document species successions and determine the specific role of microplankton species in the biological and carbonate pumps. The autonomous collection capacity of sediment traps is particularly useful in the remote Southern Ocean, where inaccessibility and harsh working conditions prevent year-round ship-based sampling.
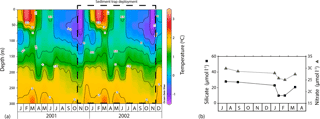
Figure 2(a) Seasonal variation in the vertical structure of temperature (∘C) between January 2001 and December 2002 for the 61∘ S site from the World Ocean Atlas 2009 (Locarnini et al., 2010). (b) Summary of seasonal evolution of macronutrient concentrations (silicate and nitrate) in the mixed layer at the 61∘ S site taken from the WOCE SR3 transects between 1993 and 1996 (modified from Trull et al., 2001b).
We present here the first record of the composition, abundance and seasonality of coccolithophore assemblages in the Antarctic zone of the Southern Ocean collected by two deep-ocean sediment traps deployed on a single mooring during 10 months south of Australia at the site of the Southern Ocean Iron Release Experiment (SOIREE) near 61∘ S, 140∘ E (Boyd et al., 2000a). Moreover, we report weight and length measurements on E. huxleyi coccoliths, thereby assessing the impact of seasonally varying environmental parameters on E. huxleyi coccoliths. This provides a baseline of coccolith dimensions for the populations living in this region. All the above information is needed for monitoring coccolithophore responses, if any, to changing environmental conditions in the Antarctic zone south of Australia during the coming decades.
1.2 Regional setting and oceanography
The southern Antarctic zone (AZ-S; Parslow et al., 2001) is delimited in the north by the southern branch of the Polar Front (PF) and in the south by the southern front of the Antarctic Circumpolar Current (SACCF; Fig. 1). Trull et al. (2001b) summarised the seasonal evolution of water column properties in the study region. The intense heat loss of surface waters during winter decreases sea surface temperature (SST) to values < 1 ∘C, resulting in strong vertical convection. Winter mixing extends to depths of about 120 m, replenishing the upper water column with nutrients. Chlorophyll a levels during winter are negligible throughout the region due to the reduced solar radiation and the deep, continuous vertical mixing. During summer, increasing solar radiation warms the surface ocean and a seasonal thermocline forms (Fig. 2). By late summer–early autumn (March) SST ranges between 2 and 3 ∘C. Considerable nutrient depletion associated with a moderate increase in algal biomass occurs within the mixed layer (Trull et al., 2001b). Nonetheless, due to the limited sampling of the study region, the timing of the summer nutrient minimum is not well constrained by the available data (Trull et al., 2001b). Silicate exhibits the strongest summer drawdown of all the macronutrients, reaching ∼ 30 % of its winter values (Fig. 2; Trull et al., 2001b) mainly due to diatom growth and subsequent biogenic silica export to the deep sea (Rigual-Hernández et al., 2015a). The low algal biomass accumulation in the region is attributed to the very low iron levels (0.1–0.2 nM; Boyd et al., 2000a; Sohrin et al., 2000). Mesozooplankton analysis during the SOIREE experiment by Zeldis (2001) indicates that the zooplankton community in the study region is dominated by copepods. Grazing pressure was low (< 1 % of the phytoplankton standing stock removed per day) and therefore is thought not to play an important role in the control of the microphytoplankton (primarily diatom) stocks, but nanoflagellate grazer abundances were significant and were likely to have regulated smaller phytoplankton abundances (Hall and Safi, 2001).
1.3 Water carbonate chemistry in the study region
Calcite solubility increases at higher pressures and lower temperatures so that dissolution increases with depth in the water column. Based on downward changes in the calcite dissolution rate, two critical depth horizons can be distinguished: the calcite saturation horizon (CSH) that can be defined as the depth at which the water becomes undersaturated with respect to calcite (i.e. where Ωcalcite=1) and the CaCO3 compensation depth (CCD), the depth at which the rate of calcite rain from the upper water column equals the dissolution rate. Figure 3 shows carbonate concentrations [CO] and calcite saturation (Ωcalcite) for the WOCE SR03 2001 transect between Antarctica and Tasmania along the 140∘ E meridian as estimated by Bostock et al. (2011). In the AZ-S waters south of Tasmania, the CSH and CCD occur at 3000 and 3700 m, respectively (Fig. 3). Therefore, the location of sediment traps at the 61∘ S site allows for the assessment of dissolution changes, if any, of coccolithophore assemblages between the two critical dissolution depth horizons: the CSH and CCD. Notably, both the progressive uptake of anthropogenic CO2 and increased upwelling of naturally CO2-rich deep waters over the past 20 years is leading to the shallowing of these features (Pardo et al., 2017).
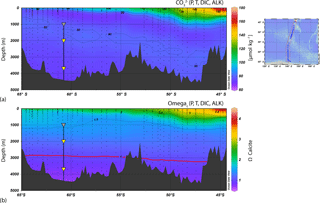
Figure 3Cross section of the mooring location in comparison to regional sea-floor bathymetry, carbonate concentrations [CO] and calcite saturation (Ωcalcite) for WOCE transect SR03 2001 from Bostock et al. (2011), who calculated them from the dissolved inorganic carbon (DIC) and alkalinity in the CARINA database (CARINA group, 2011). The location of the transects is shown on the map on the right top. The Ωcalcite=1 contour is highlighted with a red line to show the approximate depth of the CSH across the transect.
2.1 Sediment trap experiment
As part of the SAZ collaborative research programme (Trull et al., 2001c), a sediment trap experiment was carried out at the 61∘ S site (60∘44.43′ S; 139∘53.97′ E) in the Australian sector of the southern Antarctic zone within the region where the Southern Ocean Iron Release Experiment (SOIREE) was conducted (Boyd et al., 2000a). The 61∘ S site is characterised by weak currents with a mean eastward geostrophic surface velocity of approximately 0.03 ± 0.02 m s−1 (Trull et al., 2001b). The site is north of the seasonal sea ice zone (Massom et al., 2013; Rigual-Hernández et al., 2015a) and remote from any known iceberg pathway (Gladstone et al., 2001).
The mooring was equipped with three McLane Parflux time series sediment traps (Honjo and Doherty, 1988) for approximately 1 year (30 November 2001 to 29 September 2002, 317 days). The traps were located at 1000, 2000 and 3700 m below the surface in a water column of 4393 m (Fig. 3a and b). Each trap was provided with 21 cups. Sampling intervals were synchronised between traps in order to resolve the seasonal flux cycle and ranged from 8 days (in austral summer) to 55 days in austral winter. No samples were recovered from the shallowest trap owing to equipment malfunction, and therefore only results for the 2000 and 3700 m traps are presented here. Each trap was paired with an Aanderaa current meter and temperature sensors. The 250 mL collection cups were filled with a buffered solution of sodium tetraborate (1 g L−1), sodium chloride (5 g L−1), strontium chloride (0.22 g L−1) and mercury chloride (3 g L−1) in unfiltered deep (> 1000 m) seawater from the region. Risk of sample contamination by the unfiltered seawater is considered negligible due to the fact that the deep water exhibits low particle abundance and also because particle concentration in seawater is of the order of µg L−1, while concentration in the trap cups after recovery was of the order of mg L−1.
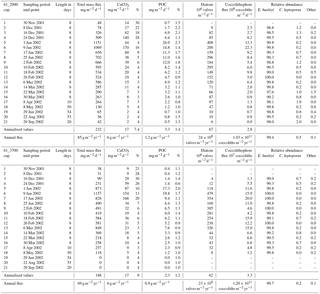
The two deeper traps completed their collection sequence as programmed, providing continuous time series for 1 year. Due to the low particle fluxes during the winter, insufficient material remained for phytoplankton analysis of cup 1 from the 2000 m trap and cups 1, 2, 19, 20 and 21 from the 3700 m trap (Table 1).
2.2 Sample processing and coccolithophore counting procedure
The sediment trap cup contents were washed through a 1 mm sieve after recovery and then divided into 10 aliquots using a rotary splitter (McLane, Inc.). A description of the analytical procedures for the estimation of geochemical fluxes is provided in Trull et al. (2001a) and Rigual-Hernández et al. (2015a). One aliquot was used for siliceous and calcareous microplankton and nanoplankton analyses. Each fraction for plankton analysis was refilled with distilled water to 40 mL, from which 10 mL was subsampled and buffered with a solution of sodium carbonate and sodium hydrogen carbonate (pH 8) and kept refrigerated for calcareous nanoplankton analysis. Samples for coccolithophore analysis were prepared following the methodology of Flores and Sierro (1997). In short, 300 µL were extracted with a micropipette and dropped onto a glass Petri dish previously filled with a buffered solution and with a coverslip on its bottom. After settling for 12 h, the buffer solution was removed using short strips of filter paper placed at the edge of the dish. Then, the coverslip was left to dry completely and mounted on a glass slide using Canada balsam. Coccoliths were identified and counted using a Nikon Eclipse 80i polarised light microscope at 1000 × magnification. A minimum of 400 coccoliths were counted in each sample. Coccospheres occurred in much lower numbers than loose coccoliths in these preparations. The coccolith counts were transformed into daily fluxes of specimens m−2 d−1 following the formula
where “F” is the daily coccolith flux, “N” the number of coccoliths, “A” the total area of a Petri dish, “n” the number of fields of view analysed, “a” the area of a single field of view, “V” the dilution volume, “S” the split of the cup, “d” the number of days of collection and “T” the aperture area of the sediment trap.
Since the sediment trap collection period was shorter than a full calendar year, an estimate of the annual coccolith flux of the 2000 m trap was calculated. This estimate takes into account the fact that the unsampled days occurred in winter when particle fluxes were low and were obtained using the flux for the last winter cup (no. 21 in 2002) to represent mean daily fluxes during the unobserved interval. Due to the lack of samples corresponding to winter 2002 for the 3700 m sediment trap record, the annualisation of the coccolith fluxes for this trap was made based only on the samples with available data. Therefore, the annualised and annual flux data for the 3700 m trap presented in Table 1 should be used with caution.
2.3 SEM analysis
As the resolution of the light microscope is insufficient to differentiate E. huxleyi morphotypes, the samples of the 2000 m trap record were analysed using a scanning electron microscope (SEM). Glass coverslips were prepared following the method outlined by Flores and Sierro (1997). The dried coverslips were mounted on aluminium stubs and coated with gold. An EVO HD25 SEM (Carl Zeiss) was used to determine the morphotype of E. huxleyi coccoliths found in the samples (magnification 5000–20 000 ×). Due to the large abundance of diatom valves and the scarcity of coccoliths in the samples, a compromise between the number of identified coccoliths and the time spent had to be reached. Therefore, a target minimum of 30 E. huxleyi coccoliths per sample were identified. The taxonomic concepts of Young and Westbroek (1991), Young et al. (2003), Cubillos et al. (2007) and Hagino et al. (2011) were followed to classify the E. huxleyi coccoliths into different morphotypes.
2.4 C-Calcita analyses
The glass slides used for coccolith counts were also analysed for coccolith mass and size measurements using a Nikon Eclipse LV100 POL polarised light microscope equipped with circular polarisation and a Nikon DS-Fi1 8-bit colour digital camera. Calibration images were performed on an apical rhabdolith of the genus Acanthoica collected by a sediment trap at the 47∘ S site (46∘48′ S, 142∘6′ E), which is located in the Australian sector of the Subantarctic zone. Camera parameters and microscope light settings were maintained as constant throughout the imaging session. Depending on coccolith concentration, between 13 and 28 random fields of view per sample were photographed. The images were then analysed by the image processing software C-Calcita (Fuertes et al., 2014). The output files for single E. huxleyi coccoliths were visually selected. Length and weight measurements were automatically performed by C-Calcita software. A total of 2328 coccoliths were analysed with a minimum of 50 coccoliths per sample. For more methodological details, see Fuertes et al. (2014).
An estimated range of annual contributions of coccoliths to total CaCO3 export was calculated for the 2000 m trap record by multiplying the coccolith flux of each sampling interval by the maximum and minimum standard deviations of coccolith weight values measured on each sample. Then, the minimum and maximum estimates of coccolith CaCO3 fluxes for each sampling interval (i.e. cup) were used to estimate the minimum and maximum annual contribution of coccoliths to total carbonate following the same procedure as for the annual coccolith fluxes.
2.5 Satellite imagery, meteorological and oceanographic data
Weekly mean SSTs for the 2001–2002 interval were obtained from the NOAA Optimum Interpolation SST Analysis database (Reynolds et al., 2002). The seasonal SST variation range was low, with maximum SSTs of 2.94 ∘C observed during March 2002 and minimum of 0.12 ∘C, in early October 2002. SST variations mirrored changes in the vertical structure of the water column temperature profile (Fig. 4) that displayed the vertical homogeneity of the water column in autumn and winter and a seasonal thermocline during the austral summer (Fig. 2a).
Sea surface salinity (SSS) climatology for the study site was obtained from the NOAA World Ocean Atlas 2005 (Antonov et al., 2006). SSS exhibited very low seasonal variability with values ranging between 33.7 and 33.9 psu.
Photosynthetically active radiation (PAR), monthly chlorophyll a concentration and particulate inorganic carbon (PIC) concentration estimates were obtained from NASA's Giovanni programme (Acker and Leptoukh, 2007; Fig. 4) for the region 130∘ E, 62.5∘ S, 150∘ E, 59.5∘ S. The chlorophyll a concentration was low throughout the year (ranging from 0.07 to 0.30 mg m−3) and in line with previous observations in the study region (Trull et al., 2001b). Algal biomass responded rapidly to the solar radiation increase in September 2001 and reached its highest levels in November 2001 (Fig. 4). The chlorophyll a concentration declined throughout the summer, reaching negligible values in autumn and winter (i.e. from March to August 2002). The satellite-derived PIC concentration exhibited a clear seasonal pattern similar to that of chlorophyll a with peak concentrations in November (up to 0.003 mol m−3) and values below the detection limit in winter (Fig. 4).
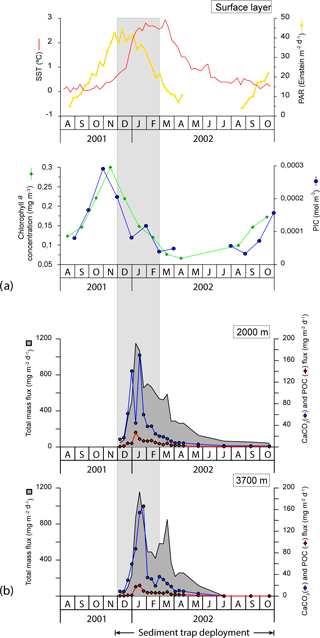
Figure 4(a) Satellite-derived SST (∘C), PAR (Einstein m−2 d−1), chlorophyll a concentration (mg m−3) and PIC concentration (mol m−3) for the period November 2001 to September 2002. It is important to note that satellite PIC concentration estimates have been reported to be biased for high-latitude systems of the Southern Ocean where the satellite algorithm is thought to produce overestimates (Balch et al., 2016; Trull et al., 2018). Therefore, the PIC data presented here should be viewed with caution. (b) Temporal variability of the total mass, CaCO3 and POC in the < 1 mm fraction at 2000 and 3700 m of water depth from November 2001 through November 2002 at the 61∘ S site (Rigual-Hernández et al., 2015a). Grey strips represent summer.
3.1 Seasonal dynamics of coccolith export fluxes
Coccolith fluxes showed a pronounced seasonal pattern at both sediment trap depths, roughly following the chlorophyll a dynamics in the surface layer with maximum fluxes during the austral summer and minima during winter (Figs. 4 and 5). The summer coccolith flux exhibited a bimodal distribution with a major peak registered in early January (2.2 × 109 coccoliths m−2 d−1 at 2000 m) and a secondary maximum recorded in mid-February (9.8 × 108 coccoliths m−2 d−1). The coccolith flux was low in autumn and winter (down to ∼ 7 × 107 coccoliths m−2 d−1). Coccolith fluxes in the deeper trap (3700 m) followed a similar pattern to that in the 2000 m trap with a delay of about one sampling interval.
The fluxes of all biogeochemical components were closely correlated (Table 2 in Rigual-Hernández et al., 2015a). Coccolith fluxes at both traps were broadly in line with biogenic particle fluxes estimated by Rigual-Hernández et al. (2015a), showing the strongest correlations with biogenic silica (R2=0.74 at 2000 m and R2=0.71 at 3700 m), followed by PIC (R2=0.62 at 2000 m and R2=0.47 at 3700 m) and POC (R2=0.56 at 2000 m and R2=0.41 at 3700 m).
Coccolithophore sinking assemblages captured by the traps were nearly monospecific, with an overwhelming dominance of E. huxleyi that represented > 99 % of the annual coccolith sinking assemblage at both trap depths. Background concentrations of Calcidiscus leptoporus (sensu lato), Gephyrocapsa spp. and Helicosphaera spp. were also registered, together representing 0.6 and 0.3 % of the coccolith assemblage at 2000 and 3700 m, respectively, of the total annual coccolith fluxes (Table 1). The seasonal changes in the coccolithophore species flux and relative abundance can be found in Fig. S1 in the Supplement. The seasonal pattern of C. leptoporus and Gephyrocapsa spp. followed that of E. huxleyi, with peak values during the summer and minima during winter. The numbers of coccospheres found in the samples were negligible in both sediment trap records.
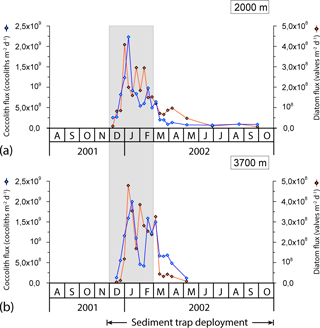
Figure 5Seasonal variation in total coccolith and diatom valve flux at the 2000 and 3700 m sediment traps at the 61∘ S site. Grey strips represent summer.
3.2 SEM analyses
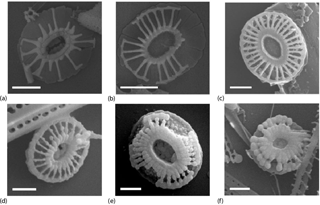
Emiliania huxleyi coccoliths correspond to morphotype B/C, having proximal shields slightly wider than the distal ones and with a central area usually filled by several (usually 5 to 11) flat, wide and thin tile-like elements (see Plate 1a). Distal shields of several are partially missing, most likely due to the slender and delicate structure of the laths. Distal shield measures ranged between 2 and 4.35 µm in the samples recovered from the 2000 m sediment trap. The coccoliths captured by the traps were clearly different than those of morphotype A, which is the other morphotype that has been reported in the Australian sector of the Southern Ocean (Cubillos et al., 2007). Morphotype A has a central area composed of curved elements (Young et al., 2003) and its distal shield elements are more robust than those of B/C (Young et al., 2003). Since the size of the coccoliths has been reported to vary significantly on the same coccosphere, coccolith size was not used as a discriminatory feature to differentiate between morphotypes following Cubillos et al. (2007).
It is conspicuous that most of the coccoliths display a morphology which is compatible with a secondary recrystallisation. Small spherule-like recrystallisations are present on these coccoliths, especially on the laths (Plate 1c–f). However, some coccoliths, mostly from cup 10 (February), have no spherules covering them (Plate 1a and b). Aside from this sample, no relationship between the morphology of the coccoliths and collection time was found. These coccoliths present very thin slender laths (usually from 20 to 26) and wider central areas than the coccoliths having spherules.
3.3 Coccolith weight and length changes
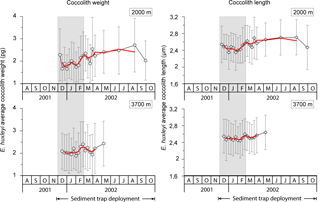
Average coccolith weight at both sediment trap depths exhibited a clear seasonal pattern with high values (2.28 ± 1.16 and 2.09 ± 0.80 pg coccolith−1 at 2000 and 3700 m, respectively) at the onset of the coccolithophore productive period in early spring, followed by a pronounced decrease (down to 1.65 ± 0.63 and 1.88 ± 0.63 pg at 2000 and 3700 m, respectively) in approximately January–early February. Average coccolith weight followed a gradual increasing trend from approximately mid-February into winter, reaching values up to 2.71 ± 1.20 pg in August 2002 at 2000 m and up to 2.43 ± 1.00 in May at 3700 m, respectively. Average annual coccolith weight was 2.11 ± 0.96 and 2.13 ± 0.91 pg at 2000 and 3700 m, respectively. The annual amplitude of the mean coccolith weight was approximately 1 pg at 2000 m and 0.5 pg at 3700 m. The lower annual amplitude exhibited by the coccolith assemblages captured at the 3700 m trap is attributed to the lower sampling duration at that depth over the winter season.
Mean coccolith length was greatest in early spring 2001 (2.54 ± 0.44 and 2.55 ± 0.40 µm at 2000 and 3700 m, respectively), followed by a decrease in early summer (down to 2.35 ± 0.43 and 2.44 ± 0.41 µm at 2000 and 3700 m, respectively; Fig. 6). From late February coccolith length increased again, reaching the highest values of the record in winter 2002 (up to 2.71 ± 0.42 and 2.64 ± 0.41 µm at 2000 and 3700 m, respectively).
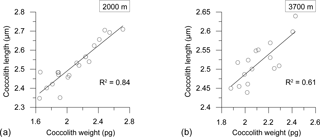
Seasonal variations in coccolith length and weight exhibited a strong correlation at both depths (R2=0.84, n=20 at 2000 m; R2=0.61, n=16 at 3700 m), indicating a clear, dependable relationship between the two variables (Fig. 7).
4.1 Origin, magnitude and composition of the coccolithophores
Since there is a current debate about the potential expansion of E. huxleyi populations south of the PF during recent decades (Cubillos et al., 2007; Saavedra-Pellitero et al., 2014; Winter et al., 2014; Malinverno et al., 2015; Patil et al., 2017), it is important to evaluate the likely origins of the sinking coccolith assemblages collected at station 61∘ S. This assessment is particularly needed in the case of deep-moored sediment trap experiments because the source area of the particles collected by the traps can be as wide as hundreds of square kilometres (Buesseler et al., 2007).
Several lines of evidence strongly suggest that the coccolithophore fluxes registered by the traps were produced in the waters of the Antarctic zone. Firstly, the mooring was deployed in a quiescent area of the AZ-S (Trull et al., 2001b) between the stronger flows associated with the southern branch of the PF and the SACCF (Fig. 1). The relatively weak currents around the sediment trap location greatly reduce the area of likely origins of the particles intercepted by the traps, i.e. the statistical funnel (Siegel and Deuser, 1997; Siegel et al., 2008). Moreover, the large magnitude of the coccolith export fluxes at both depths, plus the long duration of the period of enhanced coccolith flux (about 3 months), rules out the likelihood of a transient lateral transport event (e.g. transport by mesoscale eddies) of a coccolithophore bloom produced in more northerly latitudes. Lastly, the composition of the biogeochemical fluxes and diatom assemblages collected by the traps are characteristic of AZ waters (Rigual-Hernández et al., 2015a), further supporting the idea that the coccolithophores captured by the traps were produced close to the site. All this clearly indicates that in 2001 E. huxleyi was an established member of the phytoplankton communities of the Antarctic zone to the C-calcite south of Australia.
The annual coccolith export to the deep ocean at the 61∘ S site (1.03 × 1011 coccoliths m−2 yr−1) is one-sixth of that registered by Wilks et al. (2017; 6.5 × 1011 coccolith m−2 yr−1) in the SAZ waters (station 47∘ S; Fig. 1) north of the study site. The lower abundance of coccolithophores at the sampling site is most likely due to the negative effects of low temperature and low light levels on coccolithophore growth (Paasche, 2002; Boyd et al., 2010), but the competitive advantage of diatoms over coccolithophores in the silicate-rich waters of the AZ-S is also important. The lower coccolithophore production in the AZ-S is also reflected in the lower carbonate export at this site, i.e. 6 g m−2 yr−1 versus 10–13 g m−2 yr−1 at the 47∘ S site (Rigual-Hernández et al., 2015b; Wilks et al., 2017). The non-proportional latitudinal change in coccolith and carbonate fluxes (i.e. sixfold versus twofold changes, respectively) is most likely due to variations in the contribution of heterotrophic calcifiers (i.e. foraminifers and pteropods) to total carbonate export. There are also differences in the carbonate content per coccolith of the coccolithophore species and the morphotypes of E. huxleyi dwelling in each zonal system. Indeed, mean coccolith weight can vary up to 2 orders of magnitude between small species such as E. huxleyi (2–3.5 pg) and large and heavily calcified taxa such as Coccolithus pelagicus (∼ 150 pg; Giraudeau and Beaufort, 2007). Intraspecific size variability is also common in most coccolithophore species, mainly due to growth variations driven by different environmental factors and by genotypic variability (e.g. Knappertsbusch et al., 1997; Poulton et al., 2011).
Based on the significant genetic variability found between Southern Ocean populations of morphotypes A and B/C, Cook et al. (2011) classified these morphotypes as E. huxleyi var. huxleyi and E. huxleyi var. aurorae, respectively. Since only morphotype B/C had been reported at and south of the Antarctic Polar Front, Cook et al. (2013) concluded that the rapid drop in water temperature occurring at the Antarctic Polar Front may act as an open-ocean barrier to gene flow between these the two Southern Ocean E. huxleyi morphotypes or varieties. The nearly monospecific coccolith assemblages of E. huxleyi morphotype B/C collected by the 61∘ S site traps (Plate 1) are consistent with those studies and support the idea that the physiological differences in light-harvesting pigments of morphotype B/C compared to other E. huxleyi varieties (Cook et al., 2011) may represent a critical ecological advantage in the cold and low-light waters of the AZ-S south of Australia.
4.2 Seasonal dynamics of the calcareous and siliceous phytoplankton fluxes
The 8-day sampling resolution during spring and summer enabled us to monitor the detailed temporal dynamics of phytoplankton fluxes at the 61∘ S site. Comparison of satellite-derived PIC and chlorophyll a concentrations for the study region with coccolith fluxes registered by the sediment trap suggests a time lag of about 2 months between their surface maxima and peak coccolith fluxes registered by the shallower trap (Fig. 5). Therefore, the growth phase of the E. huxleyi bloom probably took place between October and December 2001, a period characterised by very low SSTs (0.1–0.9 ∘C). It was before the development of any significant stratification in the upper water column (Figs. 2a and 4a). These observations indicate that the very cold temperatures (near 0 ∘C) and strong mixing of the water column in the Antarctic waters during spring are not an impediment to the development of an E. huxleyi bloom. The very low C. leptoporus and Gephyrocapsa spp. fluxes throughout the annual cycle suggest that the environmental conditions of the AZ-S must represent an ecological limit for these species. Peak fluxes of C. leptoporus and Gephyrocapsa spp. at both sediment traps coincide with those of E. huxleyi, indicating that the summer solar irradiance increase is the main factor stimulating coccolithophore growth regardless of the species.
The onset of a seasonal increase in coccolithophore arrivals in the traps occurred at the same time as that of diatoms, suggesting a rapid response of both phytoplankton groups to enhanced light levels. Although both coccolith and diatom fluxes exhibited a pronounced and nearly parallel increase throughout December (Fig. 5), coccolith fluxes peaked 1 week later than those of diatoms. A similar succession was observed in late summer when coccoliths displayed a secondary flux maximum one sampling interval later (8 days) than that of diatoms (Fig. 5). These observations agree with the bloom dynamics scheme proposed by Barber and Hiscock (2006; the so-called coexistence theory) in that neither phytoplankton group seems to outcompete the other during the development of the bloom. Interestingly, diatoms seem to decline earlier than coccolithophores, a feature often (but not always) observed in other parts of the world oceans (e.g. Margalef, 1978; Holligan et al., 1983; Lochte et al., 1993; Sieracki et al., 1993; Thunell et al., 1996; Balch, 2004). Indeed, a recent study of the phenological characteristics of coccolithophore blooms by Hopkins et al. (2015) concluded that they often follow those of diatoms in many regions, with the sequencing driven by increasing stabilisation and/or nutrient depletion (mainly silicate and/or iron and possibly also favoured by an associated increase in carbonate saturation; Merico et al., 2004) of the surface layer. The slightly different seasonal pattern observed at both sampling depths (Fig. 5) is mainly attributed to the fact that the area of the ocean from which the particles have been produced increases with depth (Siegel and Deuser, 1997).
Lack of nutrient and mixed layer depth measurements during the sediment trap deployment precludes us from establishing robust links between changes in physical and chemical parameters in the upper water column and the observed phytoplankton succession. Nonetheless, shipboard observations of mixed layer properties from years prior to the sediment trap deployment (Fig. 2; Trull et al., 2001b) can provide some insight about the mechanisms driving the phytoplankton succession. Macronutrient measurements indicate that although considerable nutrient drawdown often occurs by midsummer, the AZ-S waters never reach potentially limiting concentrations (i.e. below 10 µM) of silicate, nitrate or phosphate (Fig. 2b; Trull et al., 2001b). Thus, macronutrient limitation was not a likely driver of the observed phytoplankton succession at the 61∘ S site traps. Iron levels in the AZ-S, on the other hand, are low year-round (0.1–0.2 nM; Boyd et al., 2000b; Sohrin et al., 2000) and exhibit clear seasonality in the AZ (Tagliabue et al., 2014). So, iron availability does represent a potential driver for the observed phytoplankton succession. Indeed, laboratory experiments have shown that E. huxleyi has lower minimum iron requirements for growth than oceanic diatoms (Brand et al., 1983; Muggli and Harrison, 1997). This physiology likely provides an ecological advantage over diatoms in the later stages of the spring–summer bloom when most iron has been stripped from the mixed layer.
In regard to the mechanism underlying the second diatom–coccolith succession observed at both depths in February (Fig. 5), it is possible that a vertical mixing event – as frequently reported in the AZ (e.g. Brzezinski et al., 2001) – supplied waters rich in iron and macronutrients to the euphotic zone, resetting the phytoplankton succession. Alternatively, the part of the E. huxleyi populations accumulated at or just above the nutricline may have increased using the iron moved by diapycnal diffusion through the pycnocline (Tagliabue et al., 2014). Their deposition in February could have been triggered by a drop in the light levels (Fig. 4). This second hypothesis is also consistent with the following observations: (1) the presence of a subsurface chlorophyll a maximum in the study region during spring and summer (Parslow et al., 2001; Trull et al., 2001b); (2) reports of high E. huxleyi cell accumulations associated with the nutricline in other settings of the world oceans (Beaufort et al., 2008; Henderiks et al., 2012) and (3) peak annual sedimentation in late February of the diatom Thalassiothrix antarctica (Rigual-Hernández et al., 2015a), a typical component of the “shade flora” (Kemp et al., 2000; Quéguiner, 2013). Further sampling and taxonomic analysis of the vertical distributions of phytoplankton in the AZ-S south of Australia are required to assess these hypotheses.
4.3 Seasonal variability in coccolith calcification
Two main factors have been proposed as driving seasonal changes in coccolith weights of E. huxleyi: a seasonal shift in the dominant morphotypes and/or ecotypes – each with a different degree of calcification (Poulton et al., 2011) – and the physiological response of a given morphotype to the seasonal variation in environmental parameters (e.g. Smith et al., 2012; Meier et al., 2014). SEM analysis of the sediment trap samples revealed that only morphotype B/C, as described by Young et al. (2003), thrives in the AZ-S waters south of Tasmania. This is consistent with a report by Cubillos et al. (2007) of the dominance of B/C south of 50∘ S. Therefore, a seasonal shift in the dominant morphotype can be ruled out with respect to changing coccolith weight. The observed decrease in coccolith weight could have been caused by a change in coccolith calcification or a reduction in coccolith dimensions. Young and Ziveri (2000) showed that coccolith weight is approximately linearly correlated with the cube of coccolith length. Applying that, the decrease in length by 7.5 % (a reduction to 92.5 %) observed from the pre-bloom to the summer bloom in the 2000 m traps (i.e. difference in minimum coccolith lengths in cups 5 and 8) corresponds to a coccolith weight loss of 21 % (0.9253 ≈ 0.79). This is similar to the observed weight reduction in the 2000 m trap between the pre-bloom and summer bloom coccolith assemblages (16.2–27.6 %; Fig. 6). When the linear correlation between coccolith length and weight proposed by Young and Ziveri (2000) is also applied to the 3700 m trap coccoliths, the predicted reduction of coccolith weight between the pre-bloom and bloom assemblages is 12 %. This is again very similar to the reduction in coccolith weight observed in the E. huxleyi coccoliths intercepted by the 3700 trap (10 %). It is strongly suggested that the seasonal changes in coccolith weight at the 61∘ S site were mainly driven by changes in coccolith length and were not due to significant changes in their degrees of calcification.
Laboratory, mesocosm and field studies have shown that multiple environmental factors including light, temperature, salinity, seawater carbonate chemistry, macronutrient concentrations and iron availability affect coccolith formation by E. huxleyi cells (e.g. Paasche, 2002; Zondervan, 2007; Langer and Benner, 2009; Feng et al., 2017). We examine each of these factors in turn, but note that all exhibit correlated seasonal cycles and thus the identification of a single driver is particularly difficult.
Since calcification in E. huxleyi is a light-dependent process (Paasche, 1999, 2002), the observed decrease in coccolith weight during summer in both traps is not an obvious response to increasing light in summer. However, Paasche and Brubak (1994) observed that calcification is less strongly curtailed than photosynthesis under low light conditions, so perhaps high calcification relative to growth in winter could lead to heavier coccolith weights in that part of the seasonal cycle. Interestingly, this would contrast with a recent synthesis of results for another coccolithophore, Gephyrocapsa oceanica, in which optimal light for calcification was found to be slightly higher than for photosynthesis or growth (Gafar et al., 2018), emphasising that the sensitivities of these processes may be organism and possibly even strain specific. Smith et al. (2012) previously documented a reduction in coccolith calcification of E. huxleyi coccospheres during the summer months in the Bay of Biscay, but advised caution in associating this with light intensity because calcification rates may not necessarily covary with the amount of calcite content per coccolith. Therefore, the possible effect of light intensity on coccolith weight in our traps is plausible but not demonstrable with our current data.
In terms of temperature effects, Saruwatari et al. (2016) described a decrease in coccolith size with increasing temperature by cultivating E. huxleyi strains (morphotype B/C, strains MR57N and MR70N) from the Bering and Chukchi seas. However, comparison of our results with those of Saruwatari et al. (2016) should be done with great caution for two reasons. Firstly, the E. huxleyi coccolithophores living in the Arctic seas most likely correspond to a different ecotype than those dwelling in the AZ waters, and therefore they may potentially exhibit different physiological responses to water temperature changes. Secondly, the SST range in our study site was remarkably lower (0–3 ∘C) than that used by Saruwatari et al. (2016) in their cultures (5–20 ∘C). These limitations make drawing inferences from Saruwatari et al. (2016) difficult. Feng et al. (2017), on the other hand, showed that the optimal temperature for calcification of E. huxleyi cells retrieved in the Southern Ocean (morphotype A, strain NIWA1108) was ∼ 20 ∘C, while temperatures below 10 ∘C resulted in a dramatic reduction of calcification rates and severe malformations of coccoliths, such as incomplete distal shield elements. Although E. huxleyi morphotype B/C found at the 61∘ S site likely represents an ecotype more tolerant to low temperatures than morphotype A (Cubillos et al., 2007; Cook et al., 2013), the frequent variations in the structure of the coccoliths (e.g. incomplete distal shield elements; Plate 1) captured by the traps suggest some degree of low-temperature stress. Despite the important role of temperature in coccolithophore growth (Paasche, 2002), enhanced summer SSTs may lead to an increase in coccolith weight, a response opposite to that observed at both traps. Therefore, it is unlikely that seasonal SST variations at the 61∘ S site are behind the observed variability in coccolithophore weight.
For salinity, Bollmann and Herrle (2007) identified a close relationship between changes in SSS (gradient from 33 to 38) and the length of E. huxleyi coccoliths using a global compilation of core top and plankton samples. However, based on the almost negligible annual variability in SSS (values ranging between 33.7 and 33.9 psu) in the study region, salinity most likely did not play a significant role in the seasonal variability in coccolith morphology observed in our traps.
In regard to the possible impact of macronutrient concentrations on coccolith weight, both nitrate and phosphate are known to have a pronounced effect on coccolith calcite content and morphology (Zondervan, 2007). However, as mentioned previously, none of these macronutrients reach limiting concentrations throughout the annual cycle in the AZ-S (Fig. 2; Trull et al., 2001b), and therefore their influence in the calcification of coccolithophores is likely to be low or negligible.
Seawater carbonate chemistry is a known driver of calcification in coccolithophores, with decreased growth and calcification attributable to both lower pH and carbonate saturation state, e.g. as summarised in a recent model capable of reproducing a wide range of experimental observations (Bach et al., 2015). But the seasonal cycle in carbonate saturation and pH in Antarctic waters is driven by net community production so that both are higher in summer (Shadwick et al., 2013, 2015a, b) and thus would be more likely to favour higher shell weights in contrast to the observations.
On the other hand, low iron levels have been reported to have a pronounced negative effect on CaCO3 production by E. huxleyi cells (Schulz et al., 2004), so it represents a candidate driver of seasonal changes in coccolith weight. During winter, deep water mixing restocks the mixed layer with iron (Tagliabue et al., 2014). As soon as light levels become sufficient for photosynthesis in early spring, phytoplankton rapidly develops under non-limiting concentrations of macronutrients and micronutrients. These favourable conditions for coccolithophore growth could explain the heavier and larger coccoliths registered in early December (Fig. 6). As the phytoplankton bloom develops, the dissolved iron stock is rapidly depleted in the photic zone, possibly resulting in a size and weight reduction of coccoliths of the already substantial E. huxleyi populations. From late summer throughout autumn, some recycling of iron in the upper water column by increasing summer populations of zooplankton feeding on the bloom (Tagliabue et al., 2014), coupled with increasing light levels and the continued shallowing of the mixed layer, would allow coccolithophores to again produce longer and heavier coccoliths (Fig. 6).
Changes in light intensity in the mixed layer and/or iron limitation therefore represent the most likely environmental driving factors for the seasonal variability in coccolith weight and length of E. huxleyi assemblages at the 61∘ S site. However, we note again that the lack of fitness response experiments on Southern Ocean strains of E. huxleyi morphotype B/C to varying environmental conditions and lack of in situ measurements of chemical and physical parameters of the water column mean that control of coccolith weight by light and/or iron availability in the AZ-S remains as a hypothesis needing validation by future studies.
4.4 Effects of calcite dissolution on the sinking coccolith assemblages
The similar average annual coccolith weight registered at both traps indicates that negligible coccolith dissolution occurs at mesopelagic and bathypelagic depths in the AZ-S south of Australia. That is despite the fact that coccolith sinking assemblages captured by the deeper trap were exposed to potentially intense dissolution after crossing the CSH (located at 3000 m in the study region; Fig. 3). The similar coccolith values observed at both depths can be attributed to the formation of algal and faecal aggregates in the mixed layer that include fine mineral particles (Passow and De La Rocha, 2006) and provide protection against dissolution. They also facilitate rapid transport of the coccoliths down through the water column. The aggregate formation hypothesis is supported by the findings of Closset et al. (2015), who estimated that sinking rates at the 61∘ S site were at least 213 m d−1 during the productive period, a value consistent with the sinking rates of algal and/or faecal aggregates (Turner, 2002, 2015).
Despite not finding increased dissolution with water depth between 2000 and 3700 m, it is possible that coccoliths experienced some carbonate dissolution before reaching the traps. Milliman et al. (1999) suggested that the same biological processes that facilitate aggregate formation and flocculation, such as ingestion, digestion and egestion by grazers, may be responsible for significant carbonate dissolution at epipelagic depths (i.e. depths shallower than 800–1000 m). Indeed, the negligible amounts of coccospheres found in both traps, together with the high sinking velocities, suggest that grazing could have been an important influence on export. This is supported by the findings of Ebersbach et al. (2011) in the PFZ north of our study location. They documented that an important fraction of the particles sinks from the mixed layer as faecal aggregates. On the other hand, the small spherules often observed on the coccoliths captured by the traps suggest some degree of coccolith dissolution followed by remineralisation. We speculate that some of the coccoliths captured by the traps could have experienced partial dissolution in the upper water column, leading to the exposure of their organic coccolith scaffold (Gal et al., 2016; Lee et al., 2016) to the environment. It is possible that salts dissolved in the water column subsequently precipitated over this scaffold structure, resulting in the formation of the recrystallised structures observed in some coccoliths (Plate 1e–g). However, the available data are insufficient to evaluate the impact of carbonate dissolution in the upper water column and processes leading to secondary recrystallisation in the coccoliths.
4.5 Calcium carbonate content of Emiliania huxleyi coccoliths
A broad range of calcite contents for E. huxleyi coccoliths (1.4–7.0 pg) has been proposed in the literature (e.g. Young and Ziveri, 2000; Beaufort, 2005; Holligan et al., 2010; Poulton et al., 2011). The differences in these estimates are most likely due to variability in the amount of coccolith calcite between morphotypes and to the varied methodological biases associated with the three main approaches for estimating coccolith mass: morphometrics, regression and birefringence. Since E. huxleyi morphotype B/C is considered to be geographically restricted to the Southern Ocean (Cubillos et al., 2007; Cook et al., 2013) we limit the comparison of our results to studies conducted only in the Southern Ocean reporting this morphotype.
Average annual coccolith quotas at both trap depths at the 61∘ S site (2.11 ± 0.96 and 2.13 ± 0.91 pg coccolith−1 at 2000 and 3700 m, respectively) are almost identical to that estimated by Holligan et al. (2010; 2.20 ± 0.60 pg; morphotype B/C) in the Scotia Sea using a regression line between the number of coccoliths against PIC. Moreover, our estimates are slightly higher, but with a considerable overlap in the ranges of coccolith weight, than those estimated by Poulton et al. (2011) for the E. huxleyi morphotype B/C populations found in Patagonian shelf waters (1.40 ± 0.6 pg). The greater standard deviation of our data is most likely due to the time periods compared. While the average coccolith weight estimated for our traps reflects an integration of the annual variability in coccolith weight, the shipboard observations by Poulton et al. (2011) provide a snapshot of the summer coccolithophore populations that likely exhibit lower coccolith size and thus variability.
Because our coccolith weight estimates are similar to those of Poulton et al. (2011) and Holligan et al. (2010), we can estimate the fractional contribution of coccolithophores to total carbonate production in the AZ-S south of Australia. Coccolithophores account for approximately 2–5 % of the annual deep-ocean CaCO3 fluxes at mesopelagic depths at the 61∘ S site. The contribution of coccolithophores to the annual CaCO3 budget in the AZ-S south of Australia is similar to the estimate by Salter et al. (2014) for the macronutrient-rich but iron-deficient M6 site in the Indian sector of the AZ (12 %) and remarkably lower than an estimate for the iron-fertilised station A3 over the central Kerguelen Plateau (85 %; Rembauville et al., 2016). Due to the different methodologies for estimating coccolithophore contributions to carbonate production, comparison of our results with these other studies should be treated with caution. While only whole coccoliths were counted for our calculation, therefore providing a conservative estimate, Salter et al. (2014) and Rembauville et al. (2016) estimated the weight of the < 20 µm fraction using inductively coupled plasma–atomic emission spectrometry. That approach often results in overestimates of the coccolith contribution to bulk carbonate content. There can be non-negligible contributions of non-coccolith fragments to the fine fraction (Giraudeau and Beaufort, 2007). Despite the biases associated with both methodologies, the general trend appears clear: the fractional contributions of coccolithophores to bulk carbonate export are lower in the iron-limited waters of the AZ compared to those in naturally iron-fertilised settings of the Southern Ocean. These findings underscore the secondary role of this phytoplankton group in the biological carbon pumps (both the in organic carbon and carbonate counter pumps) south of the PF where non-calcifying phytoplankton – mainly diatoms and Phaeocystis – largely control the biologically mediated CO2 exchange between the ocean and the atmosphere.
Analysis of the materials captured by two sediment traps deployed at the 61∘ S site allowed for the characterisation and quantification of coccolith assemblages in the Australian sector of the Antarctic zone. The data presented here provide a baseline of the state of coccolithophore populations in this region against which future changes can be assessed. More specifically, our study has shown the following.
Coccolithophores were a consistent member of the phytoplankton communities of the Antarctic zone south of Australia in year 2001. Coccolithophore assemblages in this region are monospecific, being composed almost entirely of Emiliania huxleyi morphotype B/C. This observation supports the hypothesis that the physiological differences in light-harvesting pigments of morphotype B/C (or E. huxleyi var. aurorae), compared to other Southern Ocean E. huxleyi varieties (Cook et al., 2011), may represent an ecological advantage in the cold, low-light and iron-limited environment of the Antarctic zone.
The onset of the coccolithophore productive period took place at the same time as that of diatoms, indicating that neither phytoplankton group outcompetes the other during the development of the bloom. We speculate that the diatom–coccolithophore succession observed during the peak phase of the productive period could result from the lower minimum iron requirements for the growth of E. huxleyi, a feature that may confer a competitive advantage over diatoms.
A decrease in coccolith weight and size during the summer months was observed at both sediment trap depths. After assessing the potential influence of several environmental parameters, changes in light intensity in the mixed layer and increasing iron limitation seem to be the most likely candidates to drive this change. These hypotheses, however, will need to be validated in future field and laboratory culture experiments with morphotype B/C.
The similar weight of E. huxleyi coccolith assemblages captured by the 2000 and 3700 m sediment traps indicates that negligible coccolith dissolution occurs during transit through mesopelagic and bathypelagic depths in the study region. This is most likely due to a rapid transport of the coccoliths in algal and/or faecal aggregates.
Coccolith weight values calculated for both sediment trap records using a birefringence-based approach were similar to previous estimates of E. huxleyi morphotype B/C in other Southern Ocean settings using regression and morphometric methods (Holligan et al., 2010; Poulton et al., 2011, respectively).
Coccolithophore fluxes at the 61∘ S site account for only 2–5 % of the annual deep-ocean CaCO3 fluxes, suggesting that heterotrophic calcifiers must represent the main biogenic carbonate producer in the AZ-S south of Australia.
All the data produced in this publication can be accessed here: https://data.aad.gov.au/metadata/records/ Coccolithophore_Fluxes_SAZ, https://doi.org/10.4225/15/5ab86f35e277e (Rigual-Hernandez and Trull, 2018).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-1843-2018-supplement.
The authors declare that they have no conflict of interest.
Sediment trap deployments and sample processing were carried out by ACE CRC
staff (including Stephen Bray and Diana Davies) with support from the
Australian Commonwealth Cooperative Research Centres Program and Australian
Antarctic Division (via AAS awards 1156 and 2256 to Thomas W. Trull).
Subsampling for coccolith analysis was made possible by the Australian
government's Australian Antarctic Science Grant Program (project number 4078)
and Macquarie University (Leanne K. Armand,
Andrés S. Rigual-Hernández and Thomas W. Trull). Leanne K. Armand is
acknowledged for her support in this study. We would like to express our
sincere thanks to two anonymous reviewers and Emilio Marañón for
valuable comments and remarks that greatly helped to improve the paper.
This project has received funding from the European Union's Horizon 2020
research and innovation programme under the Marie Skłodowska-Curie grant
agreement number 748690 – SONAR-CO2 (Andrés S. Rigual-Hernández
and José A. Flores). We thank Charlie B. Miller for English corrections
and comments on an earlier version of the paper.
Edited by:
Emilio Marañón
Reviewed by: two anonymous referees
Acker, J. G. and Leptoukh, G.: Online Analysis Enhances Use of NASA Earth Science Data, EOS T. Am. Geophys. Un., 88, 14–17, 2007.
Anderson, R. F., Ali, S., Bradtmiller, L. I., Nielsen, S. H. H., Fleisher, M. Q., Anderson, B. E., and Burckle, L. H.: Wind-Driven Upwelling in the Southern Ocean and the Deglacial Rise in Atmospheric CO2, Science, 323, 1443–1448, https://doi.org/10.1126/science.1167441, 2009.
Andersson, A. J. and Gledhill, D.: Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification, Annu. Rev. Mar. Sci., 5, 321–348, 2013.
Antonov, J. I., Locarnini, R. A., Boyer, T. P., Mishonov, A. V., and Garcia, H. E.: World Ocean Atlas 2005, Volume 2: Salinity. S. Levitus, Ed. NOAA Atlas NESDIS 62, U.S. Government Printing Office, Washington, D.C., 182 pp., 2006.
Archer, D. and Maier-Reimer, E.: Effect of deep-sea sedimentary calcite preservation on atmospheric CO2 concentration, Nature, 367, 260–263, 1994.
Armstrong, R. A., Lee, C., Hedges, J. I., Honjo, S., and Wakeham, S. G.: A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals, Deep-Sea Res. Pt. II, 49, 219–236, https://doi.org/10.1016/S0967-0645(01)00101-1, 2002.
Arrigo, K. R., Robinson, D. H., Worthen, D. L., Dunbar, R. B., DiTullio, G. R., VanWoert, M., and Lizotte, M. P.: Phytoplankton Community Structure and the Drawdown of Nutrients and CO2 in the Southern Ocean, Science, 283, 365–367, https://doi.org/10.1126/science.283.5400.365, 1999.
Arrigo, K. R., DiTullio, G. R., Dunbar, R. B., Robinson, D. H., VanWoert, M., Worthen, D. L., and Lizotte, M. P.: Phytoplankton taxonomic variability in nutrient utilization and primary production in the Ross Sea, J. Geophys. Res.-Oceans, 105, 8827–8846, https://doi.org/10.1029/1998JC000289, 2000.
Bach, L. T., Riebesell, U., Gutowska, M. A., Federwisch, L., and Schulz, K. G.: A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework, Prog. Oceanogr., 135, 125–138, https://doi.org/10.1016/j.pocean.2015.04.012, 2015.
Balch, W. M.: Re-evaluation of the physiological ecology of coccolithophores, in: Coccolithophores. From Molecular Processes to Global Impact, edited by: Thierstein, H. R. and Young, J. R., Springer-Verlag, Berlin, 165–190, 2004.
Balch, W. M., Drapeau, D. T., Bowler, B. C., Lyczskowski, E., Booth, E. S., and Alley, D.: The contribution of coccolithophores to the optical and inorganic carbon budgets during the Southern Ocean Gas Exchange Experiment: New evidence in support of the “Great Calcite Belt” hypothesis, J. Geophys. Res.-Oceans, 116, C00F06, https://doi.org/10.1029/2011JC006941, 2011.
Balch, W. M., Bates, N. R., Lam, P. J., Twining, B. S., Rosengard, S. Z., Bowler, B. C., Drapeau, D. T., Garley, R., Lubelczyk, L. C., Mitchell, C., and Rauschenberg, S.: Factors regulating the Great Calcite Belt in the Southern Ocean and its biogeochemical significance, Global Biogeochem. Cy., 30, 1124–1144, https://doi.org/10.1002/2016GB005414, 2016.
Barber, R. T. and Hiscock, M. R.: A rising tide lifts all phytoplankton: Growth response of other phytoplankton taxa in diatom-dominated blooms, Global Biogeochem. Cy., 20, GB4S03, https://doi.org/10.1029/2006GB002726, 2006.
Barnett, T. P., Pierce, D. W., AchutaRao, K. M., Gleckler, P. J., Santer, B. D., Gregory, J. M., and Washington, W. M.: Penetration of Human-Induced Warming into the World's Oceans, Science, 309, 284–287, https://doi.org/10.1126/science.1112418, 2005.
Beaufort, L.: Weight estimates of coccoliths using the optical properties (birefringence) of calcite, Micropaleontology, 51, 289–297, https://doi.org/10.2113/gsmicropal.51.4.289, 2005.
Beaufort, L., Couapel, M., Buchet, N., Claustre, H., and Goyet, C.: Calcite production by coccolithophores in the south east Pacific Ocean, Biogeosciences, 5, 1101–1117, https://doi.org/10.5194/bg-5-1101-2008, 2008.
Beaufort, L., Probert, I., de Garidel-Thoron, T., Bendif, E. M., Ruiz-Pino, D., Metzl, N., Goyet, C., Buchet, N., Coupel, P., Grelaud, M., Rost, B., Rickaby, R. E. M., and de Vargas, C.: Sensitivity of coccolithophores to carbonate chemistry and ocean acidification, Nature, 476, 80–83, 2011.
Bollmann, J. and Herrle, J. O.: Morphological variation of Emiliania huxleyi and sea surface salinity, Earth Planet. Sc. Lett., 255, 273–288, https://doi.org/10.1016/j.epsl.2006.12.029, 2007.
Bopp, L., Monfray, P., Aumont, O., Dufresne, J.-L., Le Treut, H., Madec, G., Terray, L., and Orr, J. C.: Potential impact of climate change on marine export production, Global Biogeochem. Cy., 15, 81–99, https://doi.org/10.1029/1999GB001256, 2001.
Bostock, H. C., Hayward, B. W., Neil, H. L., Currie, K. I., and Dunbar, G. B.: Deep-water carbonate concentrations in the southwest Pacific, Deep-Sea Res. Pt. I, 58, 72–85, https://doi.org/10.1016/j.dsr.2010.11.010, 2011.
Boyd, P. and Newton, P.: Evidence of the potential influence of planktonic community structure on the interannual variability of particulate organic carbon flux, Deep-Sea Res. Pt. I, 42, 619–639, https://doi.org/10.1016/0967-0637(95)00017-Z, 1995.
Boyd, P. W. and Trull, T. W.: Understanding the export of biogenic particles in oceanic waters: Is there consensus?, Prog. Oceanogr., 72, 276–312, https://doi.org/10.1016/j.pocean.2006.10.007, 2007.
Boyd, P. W., LaRoche, J., Gall, M. P., Frew, R., and McKay, R. M. L.: Role of iron, light, and silicate in controlling algal biomass in subantarctic waters SE of New Zealand, J. Geophys. Res.-Oceans, 104, 13395–13408, https://doi.org/10.1029/1999JC900009, 1999.
Boyd, P., Watson, A., Law, C., Abraham, E., Trull, T., Murdoch, R., Bakker, D., Bowie, A., Buesseler, K., and Chang, H.: Phytoplankton bloom upon mesoscale iron fertilisation of polar Southern Ocean waters, Nature, 407, 695–702, 2000a.
Boyd, P. W., Watson, A. J., Law, C. S., Abraham, E. R., Trull, T., Murdoch, R., Bakker, D. C. E., Bowie, A. R., Buesseler, K. O., Chang, H., Charette, M., Croot, P., Downing, K., Frew, R., Gall, M., Hadfield, M., Hall, J., Harvey, M., Jameson, G., LaRoche, J., Liddicoat, M., Ling, R., Maldonado, M. T., McKay, R. M., Nodder, S., Pickmere, S., Pridmore, R., Rintoul, S., Safi, K., Sutton, P., Strzepek, R., Tanneberger, K., Turner, S., Waite, A., and Zeldis, J.: A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization, Nature, 407, 695–702, 2000b.
Boyd, P. W., Strzepek, R., Fu, F., and Hutchins, D. A.: Environmental control of open-ocean phytoplankton groups: Now and in the future, Limnol. Oceanogr., 55, 1353–1376, https://doi.org/10.4319/lo.2010.55.3.1353, 2010.
Brand, L. E., Sunda, W. G., and Guillard, R. R. L.: Limitation of marine phytoplankton reproductive rates by zinc, manganese, and iron 1, Limnol. Oceanogr., 28, 1182–1198, https://doi.org/10.4319/lo.1983.28.6.1182, 1983.
Brzezinski, M. A., Nelson, D. M., Franck, V. M., and Sigmon, D. E.: Silicon dynamics within an intense open-ocean diatom bloom in the Pacific sector of the Southern Ocean, Deep-Sea Res. Pt. II, 48, 3997–4018, https://doi.org/10.1016/S0967-0645(01)00078-9, 2001.
Buesseler, K. O., Antia, A. N., Chen, M., Fowler, S. W., Gardner, W. D., Gustafsson, O., Harada, K., Michaels, A. F., der Loeff, M. R. v., and Sarin, M.: An assessment of the use of sediment traps for estimating upper ocean particle fuxes, J. Marine Res., 65, 345–416, 2007.
CARINA group: Temperature, salinity, nutrients, carbon, and other profile data collected worldwide as part of the CARINA project (NODC Accession 0057766), Version 2.2., https://doi.org/10.3334/CDIAC/OTG.NDP091, 2011.
Cermeño, P., Dutkiewicz, S., Harris, R. P., Follows, M., Schofield, O., and Falkowski, P. G.: The role of nutricline depth in regulating the ocean carbon cycle, P. Natl. Acad. Sci. USA, 105, 20344–20349, https://doi.org/10.1073/pnas.0811302106, 2008.
Closset, I., Cardinal, D., Bray, S. G., Thil, F., Djouraev, I., Rigual-Hernández, A. S., and Trull, T. W.: Seasonal variations, origin, and fate of settling diatoms in the Southern Ocean tracked by silicon isotope records in deep sediment traps, Global Biogeochem. Cy., 29, 1495–1510, https://doi.org/10.1002/2015GB005180, 2015.
Cook, S. S., Whittock, L., Wright, S. W., and Hallegraeff, G. M.: Photosynthetic pigment and genetic differences between two southern ocean morphotypes of Emiliania huxleyi (haptophyta), J. Phycol., 47, 615–626, 2011.
Cook, S. S., Jones, R. C., Vaillancourt, R. E., and Hallegraeff, G. M.: Genetic differentiation among Australian and Southern Ocean populations of the ubiquitous coccolithophore Emiliania huxleyi (Haptophyta), Phycologia, 52, 368–374, https://doi.org/10.2216/12-111.1, 2013.
Cubillos, J., Wright, S., Nash, G., De Salas, M., Griffiths, B., Tilbrook, B., Poisson, A., and Hallegraeff, G.: Calcification morphotypes of the coccolithophorid Emiliania huxleyi in the Southern Ocean: changes in 2001 to 2006 compared to historical data, Mar. Ecol.-Prog. Ser., 348, 47–54, 2007.
Deppeler, S. L. and Davidson, A. T.: Southern Ocean Phytoplankton in a Changing Climate, Front. Mar. Sci., 4, https://doi.org/10.3389/fmars.2017.00040, 2017.
Ebersbach, F., Trull, T. W., Davies, D. M., and Bray, S. G.: Controls on mesopelagic particle fluxes in the Sub-Antarctic and Polar Frontal Zones in the Southern Ocean south of Australia in summer – Perspectives from free-drifting sediment traps, Deep-Sea Res. Pt. II, 58, 2260–2276, https://doi.org/10.1016/j.dsr2.2011.05.025, 2011.
Falkowski, P. G., Katz, M. E., Knoll, A. H., Quigg, A., Raven, J. A., Schofield, O., and Taylor, F. J. R.: The Evolution of Modern Eukaryotic Phytoplankton, Science, 305, 354–360, https://doi.org/10.1126/science.1095964, 2004.
Feely, R. A., Doney, S. C., and Cooley, S. R.: Ocean acidification: present conditions and future changes in a high-CO2 world, Oceanography, 22, 36–47, 2009.
Feng, Y., Roleda, M. Y., Armstrong, E., Boyd, P. W., and Hurd, C. L.: Environmental controls on the growth, photosynthetic and calcification rates of a Southern Hemisphere strain of the coccolithophore Emiliania huxleyi, Limnol. Oceanogr., 62, 519–540, https://doi.org/10.1002/lno.10442, 2017.
Fetterer, F., Knowles, K., Meier, W., Savoie, M., and Windnagel, A. K.: Sea Ice Index, Version 3 [Sea Ice Extent], https://doi.org/10.7265/N5K072F8, 2017.
Flores, J. A. and Sierro, F. J.: A revised technique for the calculation of calcareous nannofossil accumulation rates, Micropaleontology, 43, 321–324, 1997.
Freeman, N. M. and Lovenduski, N. S.: Decreased calcification in the Southern Ocean over the satellite record, Geophys. Res. Lett., 42, 1834–1840, https://doi.org/10.1002/2014GL062769, 2015.
Frölicher, T. L., Sarmiento, J. L., Paynter, D. J., Dunne, J. P., Krasting, J. P., and Winton, M.: Dominance of the Southern Ocean in Anthropogenic Carbon and Heat Uptake in CMIP5 Models, J. Climate, 28, 862–886, https://doi.org/10.1175/jcli-d-14-00117.1, 2015.
Fuertes, M.-Á., Flores, J.-A., and Sierro, F. J.: The use of circularly polarized light for biometry, identification and estimation of mass of coccoliths, Mar. Micropaleontol., 113, 44–55, https://doi.org/10.1016/j.marmicro.2014.08.007, 2014.
Gafar, N. A., Eyre, B. D., and Schulz, K. G.: A Conceptual Model for Projecting Coccolithophorid Growth, Calcification and Photosynthetic Carbon Fixation Rates in Response to Global Ocean Change, Front. Mar. Sci., 4, https://doi.org/10.3389/fmars.2017.00433, 2018.
Gal, A., Wirth, R., Kopka, J., Fratzl, P., Faivre, D., and Scheffel, A.: Macromolecular recognition directs calcium ions to coccolith mineralization sites, Science, 353, 590–593, https://doi.org/10.1126/science.aaf7889, 2016.
Giraudeau, J. and Beaufort, L.: Coccolithophores: from extant populations to fossil assemblages, Proxies in Late Cenozoic paleoceanography–Developments in Marine Geology, edited by: Hillaire-Marcel, C. and De Vernal, A., Elsevier, 409–439, 2007.
Gladstone, R. M., Bigg, G. R., and Nicholls, K. W.: Iceberg trajectory modeling and meltwater injection in the Southern Ocean, J. Geophys. Res.-Oceans, 106, 19903–19915, https://doi.org/10.1029/2000JC000347, 2001.
Hagino, K., Bendif, E. M., Young, J. R., Kogame, K., Probert, I., Takano, Y., Horiguchi, T., de Vargas, C., and Okada, H.: New evidence for morphological and genetic variation in the cosmopolitan coccolithophore Emiliania huxleyi (prymnesiophyceae) from the COX1b-ATP4 genes1, J. Phycol., 47, 1164–1176, https://doi.org/10.1111/j.1529-8817.2011.01053.x, 2011.
Hall, J. A. and Safi, K.: The impact of in situ Fe fertilisation on the microbial food web in the Southern Ocean, Deep-Sea Res. Pt. II, 48, 2591–2613, https://doi.org/10.1016/S0967-0645(01)00010-8, 2001.
Henderiks, J., Winter, A., Elbrächter, M., Feistel, R., van der Plas, A., Nausch, G., and Barlow, R.: Environmental controls on Emiliania huxleyi morphotypes in the Benguela coastal upwelling system (SE Atlantic), Mar. Ecol.-Prog. Ser., 448, 51–66, 2012.
Holligan, P. M., Viollier, M., Harbour, D. S., Camus, P., and Champagne-Philippe, M.: Satellite and ship studies of coccolithophore production along a continental shelf edge, Nature, 304, 339–342, 1983.
Holligan, P. M., Charalampopoulou, A., and Hutson, R.: Seasonal distributions of the coccolithophore, Emiliania huxleyi, and of particulate inorganic carbon in surface waters of the Scotia Sea, J. Marine Syst., 82, 195–205, https://doi.org/10.1016/j.jmarsys.2010.05.007, 2010.
Honjo, S. and Doherty, K. W.: Large aperture time-series sediment traps; design objectives, construction and application, Deep-Sea Res. Pt. A, 35, 133–149, 1988.
Hopkins, J., Henson, S. A., Painter, S. C., Tyrrell, T., and Poulton, A. J.: Phenological characteristics of global coccolithophore blooms, Global Biogeochem. Cy., 29, 239–253, https://doi.org/10.1002/2014GB004919, 2015.
Kemp, A. E. S., Pike, J., Pearce, R. B., and Lange, C. B.: The “Fall dump” – a new perspective on the role of a “shade flora” in the annual cycle of diatom production and export flux, Deep-Sea Res. Pt. II, 47, 2129–2154, 2000.
Khatiwala, S., Primeau, F., and Hall, T.: Reconstruction of the history of anthropogenic CO2 concentrations in the ocean, 462, 346–349, https://doi.org/10.1038/nature08526, 2009.
Klaas, C. and Archer, D. E.: Association of sinking organic matter with various types of mineral ballast in the deep sea: Implications for the rain ratio, Global Biogeochem. Cy., 16, 63-1–63-14, https://doi.org/10.1029/2001GB001765, 2002.
Knappertsbusch, M., Cortes, M. Y., and Thierstein, H. R.: Morphologic variability of the coccolithophorid Calcidiscus leptoporus in the plankton, surface sediments and from the Early Pleistocene, Marine Micropaleontology, 30, 293–317, https://doi.org/10.1016/S0377-8398(96)00053-9, 1997.
Langer, G. and Benner, I.: Effect of elevated nitrate concentration on calcification in Emiliania huxleyi, Journal of Nannoplankton Research, 30, 77–80, 2009.
Lee, R. B. Y., Mavridou, D. A. I., Papadakos, G., McClelland, H. L. O., and Rickaby, R. E. M.: The uronic acid content of coccolith-associated polysaccharides provides insight into coccolithogenesis and past climate, Nat. Commun., 7, 13144, https://doi.org/10.1038/ncomms13144, 2016.
Levitus, S., Antonov, J. I., Boyer, T. P., and Stephens, C.: Warming of the World Ocean, Science, 287, 2225–2229, https://doi.org/10.1126/science.287.5461.2225, 2000.
Litchman, E., Edwards, K. F., Klausmeier, C. A., and Thomas, M. K.: Phytoplankton niches, traits and eco-evolutionary responses to global environmental change, Mar. Ecol.-Prog. Ser., 470, 235–248, 2012.
Locarnini, R. A., Mishonov, A. V., Antonov, J. I., Boyer, T. P., Garcia, H. E., Baranova, O. K., Zweng, M. M., and Johnson, D. R.: World Ocean Atlas 2009, Volume 1: Temperature, NOAA Atlas NESDIS 68, edited by: Levitus, S., U.S. Government Printing Office, Washington, D.C., 2010.
Locarnini, R. A., Mishonov, A. V., Antonov, J. I., Boyer, T. P., Garcia, H. E., Baranova, O. K., Zweng, M. M., Paver, C. R., Reagan, J. R., Johnson, D. R., Hamilton, M., and Seidov, D.: World Ocean Atlas 2013, Volume 1: Temperature, S. Levitus, edited by: Mishonov, A., NOAA Atlas NESDIS 73, 40 pp., 2013.
Lochte, K., Ducklow, H. W., Fasham, M. J. R., and Stienen, C.: Plankton succession and carbon cycling at 47∘ N 20∘ W during the JGOFS North Atlantic Bloom Experiment, Deep-Sea Res. Pt. II, 40, 91–114, https://doi.org/10.1016/0967-0645(93)90008-B, 1993.
Lombard, F., da Rocha, R. E., Bijma, J., and Gattuso, J.-P.: Effect of carbonate ion concentration and irradiance on calcification in planktonic foraminifera, Biogeosciences, 7, 247–255, https://doi.org/10.5194/bg-7-247-2010, 2010.
Malinverno, E., Triantaphyllou, M. V., and Dimiza, M. D.: Coccolithophore assemblage distribution along a temperate to polar gradient in theWest Pacific sector of the Southern Ocean (January 2005), Micropaleontology, 61, 489–506, 2015.
Margalef, R.: Life-forms of phytoplankton as survival alternatives in an unstable environment, Oceanol. Acta, 1, 493–509, 1978.
Massom, R., Reid, P., Stammerjohn, S., Raymond, B., Fraser, A., and Ushio, S.: Change and Variability in East Antarctic Sea Ice Seasonality, 1979/80–2009/10, PLOS ONE, 8, e64756, https://doi.org/10.1371/journal.pone.0064756, 2013.
McNeil, B. I. and Matear, R. J.: Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2, P. Natl. Acad. Sci., 105, 18860–18864, 2008.
Meier, K. J. S., Beaufort, L., Heussner, S., and Ziveri, P.: The role of ocean acidification in Emiliania huxleyi coccolith thinning in the Mediterranean Sea, Biogeosciences, 11, 2857–2869, https://doi.org/10.5194/bg-11-2857-2014, 2014.
Merico, A., Tyrrell, T., Lessard, E. J., Oguz, T., Stabeno, P. J., Zeeman, S. I., and Whitledge, T. E.: Modelling phytoplankton succession on the Bering Sea shelf: role of climate influences and trophic interactions in generating Emiliania huxleyi blooms 1997–2000, Deep-Sea Res. Pt. I, 51, 1803–1826, https://doi.org/10.1016/j.dsr.2004.07.003, 2004.
Milliman, J. D., Troy, P. J., Balch, W. M., Adams, A. K., Li, Y. H., and Mackenzie, F. T.: Biologically mediated dissolution of calcium carbonate above the chemical lysocline?, Deep-Sea Res. Pt. I, 46, 1653–1669, https://doi.org/10.1016/S0967-0637(99)00034-5, 1999.
Moy, A. D., Howard, W. R., Bray, S. G., and Trull, T. W.: Reduced calcification in modern Southern Ocean planktonic foraminifera, Nat. Geosci., 2, 276–280, 2009.
Muggli, D. L. and Harrison, P. J.: Effects of iron on two oceanic phytoplankters grown in natural NE subarctic pacific seawater with no artificial chelators present, J. Exp. Mar. Biol. Ecol., 212, 225–237, https://doi.org/10.1016/S0022-0981(96)02752-9, 1997.
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., and Joos, F.: Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms, Nature, 437, 681–686, 2005.
Orsi, A. H., Whitworth Iii, T., and Nowlin Jr., W. D.: On the meridional extent and fronts of the Antarctic Circumpolar Current, Deep-Sea Res. Pt. I, 42, 641–673, https://doi.org/10.1016/0967-0637(95)00021-W, 1995.
Paasche, E.: Reduced coccolith calcite production under light-limited growth: a comparative study of three clones of Emiliania huxleyi (Prymnesiophyceae), Phycologia, 38, 508–516, https://doi.org/10.2216/i0031-8884-38-6-508.1, 1999.
Paasche, E.: A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions, Phycologia, 40, 503–529, https://doi.org/10.2216/i0031-8884-40-6-503.1, 2002.
Paasche, E. and Brubak, S.: Enhanced calcification in the coccolithophorid Emiliania huxleyi (Haptophyceae) under phosphorus limitation, Phycologia, 33, 324–330, https://doi.org/10.2216/i0031-8884-33-5-324.1, 1994.
Pardo, P. C., Tilbrook, B., Langlais, C., Trull, T. W., and Rintoul, S. R.: Carbon uptake and biogeochemical change in the Southern Ocean, south of Tasmania, Biogeosciences, 14, 5217–5237, https://doi.org/10.5194/bg-14-5217-2017, 2017.
Parslow, J. S., Boyd, P. W., Rintoul, S. R., and Griffiths, F. B.: A persistent subsurface chlorophyll maximum in the Interpolar Frontal Zone south of Australia: Seasonal progression and implications for phytoplankton-light-nutrient interactions, J. Geophys. Res.-Oceans, 106, 31543–31557, https://doi.org/10.1029/2000JC000322, 2001.
Passow, U. and De La Rocha, C. L.: Accumulation of mineral ballast on organic aggregates, Global Biogeochem. Cy., 20, GB1013, https://doi.org/10.1029/2005GB002579, 2006.
Patil, S. M., Mohan, R., Shetye, S. S., Gazi, S., Baumann, K.-H., and Jafar, S.: Biogeographic distribution of extant Coccolithophores in the Indian sector of the Southern Ocean, Mar. Micropaleontol., 137, 16–30, https://doi.org/10.1016/j.marmicro.2017.08.002, 2017.
Poulton, A. J., Young, J. R., Bates, N. R., and Balch, W. M.: Biometry of detached Emiliania huxleyi coccoliths along the Patagonian Shelf, Mar. Ecol.-Prog. Ser., 443, 1–17, 2011.
Quéguiner, B.: Iron fertilization and the structure of planktonic communities in high nutrient regions of the Southern Ocean, Deep-Sea Res. Pt. II, 90, 43–54, https://doi.org/10.1016/j.dsr2.2012.07.024, 2013.
Rembauville, M., Meilland, J., Ziveri, P., Schiebel, R., Blain, S., and Salter, I.: Planktic foraminifer and coccolith contribution to carbonate export fluxes over the central Kerguelen Plateau, Deep-Sea Res. Pt. I, 111, 91–101, https://doi.org/10.1016/j.dsr.2016.02.017, 2016.
Reynolds, R. W., Rayner, N. A., Smith, T. M., Stokes, D. C., and Wang, W.: An Improved In Situ and Satellite SST Analysis for Climate, J. Climate, 15, 1609–1625, https://doi.org/10.1175/1520-0442(2002)015<1609:aiisas>2.0.co;2, 2002.
Ridgwell, A. and Zeebe, R. E.: The role of the global carbonate cycle in the regulation and evolution of the Earth system, Earth Planet. Sc. Lett., 234, 299–315, https://doi.org/10.1016/j.epsl.2005.03.006, 2005.
Rigual-Hernandez, A. S. and Trull, T. W.: Coccolithophore species fluxes in the Australian sector of the southern Antarctic Zone, Australian Antarctic Data Centre, https://doi.org/10.4225/15/5ab86f35e277e, 2018.
Rigual-Hernández, A. S., Trull, T. W., Bray, S. G., Closset, I., and Armand, L. K.: Seasonal dynamics in diatom and particulate export fluxes to the deep sea in the Australian sector of the southern Antarctic Zone, J. Marine Syst., 142, 62–74, https://doi.org/10.1016/j.jmarsys.2014.10.002, 2015a.
Rigual-Hernández, A. S., Trull, T. W., Bray, S. G., Cortina, A., and Armand, L. K.: Latitudinal and temporal distributions of diatom populations in the pelagic waters of the Subantarctic and Polar Frontal zones of the Southern Ocean and their role in the biological pump, Biogeosciences, 12, 5309–5337, https://doi.org/10.5194/bg-12-5309-2015, 2015b.
Rost, B. and Riebesell, U.: Coccolithophores and the biological pump: responses to environmental changes, in: Coccolithophores: From Molecular Processes to Global Impact, edited by: Thierstein, H. R. and Young, J. R., Springer Berlin Heidelberg, Berlin, Heidelberg, 99–125, 2004.
Saavedra-Pellitero, M., Baumann, K.-H., Flores, J.-A., and Gersonde, R.: Biogeographic distribution of living coccolithophores in the Pacific sector of the Southern Ocean, Mar. Micropaleontol., 109, 1–20, 2014.
Salter, I., Schiebel, R., Ziveri, P., Movellan, A., Lampitt, R., and Wolff, G. A.: Carbonate counter pump stimulated by natural iron fertilization in the Polar Frontal Zone, Nat. Geosci., 7, 885–889, https://doi.org/10.1038/ngeo2285, 2014.
Sarmiento, J. L., Gruber, N., Brzezinski, M. A., and Dunne, J. P.: High-latitude controls of thermocline nutrients and low latitude biological productivity, Nature, 427, 56–60, 2004a.
Sarmiento, J. L., Slater, R., Barber, R., Bopp, L., Doney, S. C., Hirst, A. C., Kleypas, J., Matear, R., Mikolajewicz, U., Monfray, P., Soldatov, V., Spall, S. A., and Stouffer, R.: Response of ocean ecosystems to climate warming, Global Biogeochem. Cy., 18, GB3003, https://doi.org/10.1029/2003GB002134, 2004b.
Saruwatari, K., Satoh, M., Harada, N., Suzuki, I., and Shiraiwa, Y.: Change in coccolith size and morphology due to response to temperature and salinity in coccolithophore Emiliania huxleyi (Haptophyta) isolated from the Bering and Chukchi seas, Biogeosciences, 13, 2743–2755, https://doi.org/10.5194/bg-13-2743-2016, 2016.
Schulz, K., Zondervan, I., Gerringa, L., Timmermans, K., Veldhuis, M., and Riebesell, U.: Effect of trace metal availability on coccolithophorid calcification, Nature, 430, 673–676, 2004.
Shadwick, E. H., Trull, T., Thomas, H., and Gibson, J. A. E.: Vulnerability of Polar Oceans to Anthropogenic Acidification: Comparison of Arctic and Antarctic Seasonal Cycles, Scientific Reports, 3, 1–7, 2013.
Shadwick, E. H., Tilbook, B., Cassar, N., Trull, T. W., and Rintoul, S. R.: Summertime physical and biological controls on O2 and CO2 in the Australian Sector of the Southern Ocean, J. Marine Syst., 147, 21–28, 2015a.
Shadwick, E. H., Trull, T. W., Tilbrook, B., Sutton, A. J., Schulz, E., and Sabine, C. L.: Seasonality of biological and physical controls on surface ocean CO2 from hourly observations at the Southern Ocean Time Series site south of Australia, Global Biogeochem. Cy., 29, 223–238, https://doi.org/10.1002/2014GB004906, 2015b.
Siegel, D. A. and Deuser, W. G.: Trajectories of sinking particles in the Sargasso Sea: modeling of statistical funnels above deep-ocean sediment traps, Deep-Sea Res. Pt. I, 44, 1519–1541, https://doi.org/10.1016/S0967-0637(97)00028-9, 1997.
Siegel, D. A., Fields, E., and Buesseler, K. O.: A bottom-up view of the biological pump: Modeling source funnels above ocean sediment traps, Deep-Sea Res. Pt. I, 55, 108–127, https://doi.org/10.1016/j.dsr.2007.10.006, 2008.
Sieracki, M. E., Verity, P. G., and Stoecker, D. K.: Plankton community response to sequential silicate and nitrate depletion during the 1989 North Atlantic spring bloom, Deep-Sea Res. Pt. II, 40, 213–225, https://doi.org/10.1016/0967-0645(93)90014-E, 1993.
Sigman, D. M. and Boyle, E. A.: Glacial/interglacial variations in atmospheric carbon dioxide, Nature, 407, 859–869, 2000.
Smith, H. E. K., Tyrrell, T., Charalampopoulou, A., Dumousseaud, C., Legge, O. J., Birchenough, S., Pettit, L. R., Garley, R., Hartman, S. E., Hartman, M. C., Sagoo, N., Daniels, C. J., Achterberg, E. P., and Hydes, D. J.: Predominance of heavily calcified coccolithophores at low CaCO3 saturation during winter in the Bay of Biscay, P. Natl. Acad. Sci. USA, 109, 8845–8849, https://doi.org/10.1073/pnas.1117508109, 2012.
Sohrin, Y., Iwamoto, S., Matsui, M., Obata, H., Nakayama, E., Suzuki, K., Handa, N., and Ishii, M.: The distribution of Fe in the Australian sector of the Southern Ocean, Deep-Sea Res. Pt. I, 47, 55–84, https://doi.org/10.1016/S0967-0637(99)00049-7, 2000.
Tagliabue, A., Sallee, J.-B., Bowie, A. R., Levy, M., Swart, S., and Boyd, P. W.: Surface-water iron supplies in the Southern Ocean sustained by deep winter mixing, Nat. Geosci., 7, 314–320, https://doi.org/10.1038/ngeo2101, 2014.
Takahashi, T., Sutherland, S. C., Wanninkhof, R., Sweeney, C., Feely, R. A., Chipman, D. W., Hales, B., Friederich, G., Chavez, F., Sabine, C., Watson, A., Bakker, D. C. E., Schuster, U., Metzl, N., Yoshikawa-Inoue, H., Ishii, M., Midorikawa, T., Nojiri, Y., Körtzinger, A., Steinhoff, T., Hoppema, M., Olafsson, J., Arnarson, T. S., Tilbrook, B., Johannessen, T., Olsen, A., Bellerby, R., Wong, C. S., Delille, B., Bates, N. R., and de Baar, H. J. W.: Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans, Deep-Sea Res. Pt. II, 56, 554–577, https://doi.org/10.1016/j.dsr2.2008.12.009, 2009.
Thunell, R., Pride, C., Ziveri, P., Muller-Karger, F., Sancetta, C., and Murray, D.: Plankton response to physical forcing in the Gulf of California, J. Plankton Res., 18, 2017–2026, https://doi.org/10.1093/plankt/18.11.2017, 1996.
Trull, T. W., Bray, S. G., Manganini, S. J., Honjo, S., and François, R.: Moored sediment trap measurements of carbon export in the Subantarctic and Polar Frontal zones of the Southern Ocean, south of Australia, J. Geophys. Res.-Oceans, 106, 31489–31509, https://doi.org/10.1029/2000JC000308, 2001a.
Trull, T. W., Rintoul, S. R., Hadfield, M., and Abraham, E. R.: Circulation and seasonal evolution of polar waters south of Australia: implications for iron fertilization of the Southern Ocean, Deep-Sea Res. Pt. II, 48, 2439–2466, https://doi.org/10.1016/S0967-0645(01)00003-0, 2001b.
Trull, T. W., Sedwick, P. N., Griffiths, F. B., and Rintoul, S. R.: Introduction to special section: SAZ Project, J. Geophys. Res.-Oceans, 106, 31425–31429, https://doi.org/10.1029/2001JC001008, 2001c.
Trull, T. W., Passmore, A., Davies, D. M., Smit, T., Berry, K., and Tilbrook, B.: Distribution of planktonic biogenic carbonate organisms in the Southern Ocean south of Australia: a baseline for ocean acidification impact assessment, Biogeosciences, 15, 31–49, https://doi.org/10.5194/bg-15-31-2018, 2018.
Turner, J. T.: Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms, Aquat. Microb. Ecol., 27, 57–102, 2002.
Turner, J. T.: Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump, Prog. Oceanogr., 130, 205–248, https://doi.org/10.1016/j.pocean.2014.08.005, 2015.
Wilks, J. V., Rigual-Hernández, A. S., Trull, T. W., Bray, S. G., Flores, J.-A., and Armand, L. K.: Biogeochemical flux and phytoplankton succession: A year-long sediment trap record in the Australian sector of the Subantarctic Zone, Deep-Sea Res. Pt. I, 121, 143–159, https://doi.org/10.1016/j.dsr.2017.01.001, 2017.
Winter, A., Henderiks, J., Beaufort, L., Rickaby, R. E., and Brown, C. W.: Poleward expansion of the coccolithophore Emiliania huxleyi, J. Plankton Res., 36, 316–325, 2014.
Young, J., Geisen, M., Cross, L., Kleijne, A., Sprengel, C., Probert, I., and Østergaard, J.: A guide to extant coccolithophore taxonomy, Journal of Nanoplankton Research Special Issue 1, International Nannoplankton Association, 124 pp., 2003.
Young, J. R. and Westbroek, P.: Genotypic variation in the coccolithophorid species Emiliania huxleyi, Mar. Micropaleontol., 18, 5–23, https://doi.org/10.1016/0377-8398(91)90004-P, 1991.
Young, J. R. and Ziveri, P.: Calculation of coccolith volume and it use in calibration of carbonate flux estimates, Deep-Sea Res. Pt. II, 47, 1679–1700, https://doi.org/10.1016/S0967-0645(00)00003-5, 2000.
Zeebe, R. E.: History of Seawater Carbonate Chemistry, Atmospheric CO2, and Ocean Acidification, Annu. Rev. Earth Planet. Sc., 40, 141–165, https://doi.org/10.1146/annurev-earth-042711-105521, 2012.
Zeldis, J.: Mesozooplankton community composition, feeding, and export production during SOIREE, Deep-Sea Res. Pt. II, 48, 2615–2634, https://doi.org/10.1016/S0967-0645(01)00011-X, 2001.
Zhang, X., Lewis, M., Lee, M., Johnson, B., and Korotaev, G.: The volume scattering function of natural bubble populations, Limnol. Oceanogr., 47, 1273–1282, 2002.
Zondervan, I.: The effects of light, macronutrients, trace metals and CO2 on the production of calcium carbonate and organic carbon in coccolithophores – A review, Deep-Sea Res. Pt. II, 54, 521–537, https://doi.org/10.1016/j.dsr2.2006.12.004, 2007.