the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The distinct roles of two intertidal foraminiferal species in phytodetrital carbon and nitrogen fluxes – results from laboratory feeding experiments
Julia Wukovits
Max Oberrauch
Annekatrin J. Enge
Petra Heinz
Benthic foraminifera play a major role as primary consumers and detrivores redistributing organic carbon and nitrogen in intertidal environments. Here we compared the differences of phytodetrital carbon and nitrogen intake and turnover of two dominant intertidal foraminifera, Ammonia tepida and Haynesina germanica. Their lifestyles in relation to feeding behavior (feeding preferences, intake and turnover of phytodetrital carbon and nitrogen) and temperature adaptations were compared to obtain a closer definition of their specific roles in intertidal organic matter processing. For this comparison, we carried out a series of short-term laboratory incubations with stable-isotope-labeled (13C and 15N) detritus as the food source. We compared the response of the two species to diatom detritus at three different temperatures (15, 20, 25 ∘C). Ammonia tepida showed a very high, temperature-influenced intake and turnover rates with more excessive carbon turnover, compared to nitrogen. The fairly low metabolic nitrogen turnover in H. germanica was not affected by temperature and was higher than the carbon turnover. This might be related with the chloroplast husbandry in H. germanica and its lower demands for food-derived nitrogen sources. Ammonia tepida prefers a soft chlorophyte food source over diatom detritus, which is harder to break down. In conclusion, A. tepida shows a generalist behavior that links with high fluxes of organic matter (OM). Due to its high rates of OM processing and abundances, we conclude that A. tepida is an important key player in intertidal carbon and nitrogen turnover, specifically in the short-term processing of OM and the mediation of dissolved nutrients to associated microbes and primary producers. In contrast, H. germanica is a highly specialized species with low rates of carbon and nitrogen budgeting.
- Article
(1181 KB) - Full-text XML
-
Supplement
(6447 KB) - BibTeX
- EndNote
Benthic foraminifera are ubiquitous marine protists and highly abundant in coastal sediments (Lei et al., 2014; Mojtahid et al., 2016; Murray and Alve, 2000). Coastal sediments represent the largest pool of marine particulate organic matter (OM), despite their rather small area (less than 10 % of the ocean floor), and play an essential role in global carbon and nitrogen cycles (Jahnke, 2004). Oceanic and terrestrial systems are connected by the carbon cycling in coastal waters, which contribute to a major part of the global carbon cycles and budgets (Bauer et al., 2013; Cai, 2011; Cole et al., 2007; Regnier et al., 2013). Estuaries are an important source of organic matter in coastal systems and were estimated to account for ∼40 % of oceanic phytoplankton primary productivity (Smith and Hollibaugh, 1993). Most estuarine areas are considered to be net heterotrophic or act as carbon sinks (Caffrey, 2003, 2004; Cai, 2011; Herrmann et al., 2015). In general, 30 % of overall coastal carbon is lost by metabolic oxidation (Smith and Hollibaugh, 1993). Foraminifera are highly abundant in estuarine sediments and contribute strongly to these processes (Alve and Murray, 1994; Cesbron et al., 2016; Moodley et al., 2000; Murray and Alve, 2000). They feed on various sources of labile particulate OM, including microalgae and detritus, and provide a pivotal link in marine carbon cycles and food webs (Bradshaw, 1961; Goldstein and Corliss, 1994; Heinz, 2001; Lee et al., 1966; Lee and Muller, 1973; Nomaki et al., 2005a, b, 2006, 2009, 2011). The nitrogen compounds of OM particles are usually remineralized to ammonium (). In this way, nitrogen becomes available again as a nutrient for primary productivity. A major part of this process is attributed to prokaryotic degraders, but protists are also involved in the process of regeneration of organic nitrogen compounds (Ferrier-Pages and Rassoulzadegan, 1994; Ota and Taniguchi, 2003; Verity et al., 1992). Due to their high abundances, we consider that foraminifera contribute a large part to this OM reworking and the regeneration of carbon and nitrogen compounds from particulate OM sources, e.g., phytodetritus. In this study, we quantify the bulk OM-derived carbon and nitrogen release, which originates rather via excretion of organic carbon and nitrogen compounds (vesicular transport of metabolic waste products), respiration, or diffusion of inorganic carbon and nitrogen by these single celled microorganisms.
Environmental conditions of temperate tidal flats are physiologically challenging (high fluctuations of physical and chemical parameters, e.g., temperature and/or OM quality) and therefore host very few, highly adapted foraminifera species. Monospecific or near monospecific foraminiferal communities are characteristic of temperate, estuarine regions (Alve and Murray, 1994, 2001; Hayward, 2014; Martins et al., 2015; Saad and Wade, 2017). Ammonia tepida and Haynesina germanica are typical representatives of these communities and their standing crop can reach more than 150 individuals per cm3 (Alve and Murray, 2001; Mojtahid et al., 2016; Wukovits et al., 2018). Typically, tidal flats offer a high availability of food sources for phytodetrivores or herbivores feeding on microalgae. But dense populations of A. tepida communities can deplete sediments from OM sources and consequently control benthic meiofaunal community structures (Chandler, 1989). Therefore, resource partitioning or different metabolic strategies can be beneficial for foraminifera which share the same spatial and temporal habitats.
Early experimental investigations and monitoring studies suggest feeding preferences or selective feeding in littoral foraminifera. However, these studies rely on indirect observations from environmental monitoring (Hohenegger et al., 1989; Papaspyrou et al., 2013) or from a laboratory study focusing on the more diverse salt marsh communities (Lee and Muller, 1973). The latter study revealed that foraminiferal salt marsh communities are characterized by highly specialized feeding strategies. Analogically, the close spatial coexistence of A. tepida and H. germanica might also be based on different feeding strategies and different preferences of other environmental variables. A major, important difference between the two species subject to this study is the fact that H. germanica hosts functional plastids derived from ingested microalgae (Jauffrais et al., 2016; Lopez, 1979), a phenomenon known as kleptoplasty, which was first described for a sacoglossan opisthobranch (Trench, 1969). It was shown that diatom-derived chloroplasts in the cytoplasm of H. germanica retain their function (as photosynthetically active kleptoplasts) for up to 2 weeks (Jauffrais et al., 2016). Further, there is recent proof that H. germanica takes up inorganic carbon and nitrogen sources (HCO3 and ) from the surrounding seawater, most likely to generate metabolites in autotrophic–heterotrophic interactions with its kleptoplasts (LeKieffre et al., 2018). Consequently, the mixotrophic lifestyle of H. germanica might lead to a lower demand of carbon and nitrogen sources and thus to a lower ingestion of various particulate OM sources as food sources. In contrary, food-derived chloroplasts in A. tepida lose their photosynthetic activity after a maximum of 24 h (Jauffrais et al., 2016). Species of the genus Ammonia are described to take up significant amounts of microalgae and phytodetritus of different origin. Laboratory feeding experiments have shown that A. tepida responds to several food sources, including different live microalgae (chlorophytes and diatoms) and chlorophyte and diatom detritus (Bradshaw, 1961; LeKieffre et al., 2017; Linshy et al., 2014; Pascal et al., 2008; Wukovits et al., 2017, 2018), whereas H. germanica shows a low affinity to chloroplast detritus food sources (Wukovits et al., 2017), but feeds actively on diatoms (Ward et al., 2003) and takes up inorganic, dissolved carbon and nitrogen compounds (LeKieffre et al., 2018). Both species are found in muddy coastal sediments containing high loads of nutrients or OM (Armynot du Châtelet et al., 2009, 2004). But considering their different feeding strategies, both species might play distinct roles in the reworking of OM. Recent literature still lacks direct, quantitative comparisons of foraminiferal species-specific OM-derived C and N ingestion and release. Therefore, this study aims to compare and quantify variations in their respective uptake of OM (phytodetritus).
Temperature has a strong impact on metabolic rates and can therefore play another major role in niche separation or in species-specific adaptations in the consumer community. Benthic foraminifera show strong metabolic responses to temperature fluctuations (Bradshaw, 1961; Cesbron et al., 2016; Heinz et al., 2012). Therefore, seasonal temperature fluctuations and human-induced global warming can have a strong impact on foraminiferal community compositions and foraminiferal carbon and nitrogen fluxes. In estuaries, e.g., temperature acts in many cases as the most controlling factor on metabolic rates and on net ecosystem metabolism (Caffrey, 2003). To examine the effect of temperature on foraminiferal OM processing, temperature variations were included in our studies. In summary, the aim of this study was to obtain a closer definition of the ecological feeding niches of A. tepida and H. germanica in relation to intertidal fluxes of OM and OM processing at different temperatures. Additionally, this study offers the first estimates for the release of OM-derived carbon and nitrogen in foraminifera. To reach our aim, we carried out laboratory feeding experiments with stable-isotope-labeled (13C and 15N) food sources (chlorophyte detritus: Dunaliella tertiolecta, diatom detritus: Phaeodactylum tricornutum). We compared diatom detritus intake and retention of phytodetrital carbon (pC) and nitrogen (pN) of A. tepida and H. germanica at three different temperatures (15, 20, 25 ∘C). The evaluation of the metabolic costs of pC and pN during a 24 h starvation period can further help to explain species-specific OM processing due to metabolic nutrient budgets. Further, both food sources were offered simultaneously to A. tepida to identify feeding preferences of this species. Finally, we collected quantitative data of the abundances of both species in the sampling area to estimate species-specific contributions to intertidal fluxes of OM-derived carbon and nitrogen.
2.1 Sampling area and sample preparation
The sampling area is located at the Elbe river estuary in the German Wadden Sea (Fig. 1). Samples were collected at low tide in April 2016, close to the shoreline. Three sediment cores (4.5 cm diameter) were taken in random spacing within an area of ∼4 m2. The uppermost centimeter of the cores was fixed with a mixture of ethanol and Rose Bengal to stain the cytoplasm of live foraminifera. At the University of Vienna, the sediment core material was sieved to obtain size fractions of 125–250, 250–355, and <355 µm. Brightly stained (living) foraminifera were identified and counted to calculate abundances (individuals per m2) to estimate the relevance of A. tepida and H. germanica in intertidal OM fluxes.
For the laboratory experiments, sediment was collected at low tide from the uppermost sediment layer and sieved in the field over 125 and 500 µm to remove larger meiofauna and organic components. Sampling trips to collect material for laboratory experiments were done in April 2015 and 2016. The sediment was filled into plastic containers with seawater and transported back to the University of Vienna. The sediment samples were kept within aquaria, containing filtered water collected at the sampling site. Foraminifera were picked from the sediment in sufficient number and collected in crystallizing dishes, containing a layer of North Sea sediment (<63 µm) and filtered North Sea water (NSW). They were fed with a mixture of live D. tertiolecta and P. tricornutum once to twice a week until the beginning of the experiments. Live individuals were identified by showing bright and intensive cytoplasm color, cyst formation (in case of A. tepida), material gathered around the aperture, and movement tracks in the sediment. The experiments started after accumulation of sufficient foraminiferal material 3 weeks after the field sampling.
2.2 Production of artificial phytodetritus
Labeled food was produced by growing D. tertiolecta and P. tricornutum (SAG 1090-1a) in a stable isotope-enriched growth medium. Algae were cultured in sterile 5 L Erlenmeyer bottles, containing an F1/2 growth medium (Guillard, 1975; Guillard and Ryther, 1962) enriched with aliquots of 98 at. % NaH13CO3 and 98 at. % Na15NO3 (SigmaAldrich). The algae culture medium for Experiment 1 (P. tricornutum) was produced with filtered NSW and enriched with 0.6 mM NaH13CO3 and 0.9 mM NaNO3 (), along with the stock solutions for the F/2 standard protocol. The culture medium for D. tertiolecta (13C single-labeled) in Experiment 2 was produced with filtered NSW, the stock solutions according to the F/2 standard protocol, and additionally enriched with 1.5 mM NaH13CO3 and for P. tricornutum (15N single-labeled) with 1.5 mM NaHCO3 (natural abundance) and with 0.9 mM NaNO3 (), along with the stock solutions for the F/2 standard protocol. The algae cultures were incubated at 20 ∘C (type ST 2 POL-ECO Aparatura incubation chambers) at a 18 h:6 h light:dark cycle and bubbled with ambient air. Cultures were harvested at stationary growth (after 14–16 days) by centrifugation, washed three times in sterile, carbon, and nitrogen-free artificial seawater, shock frozen with liquid nitrogen, and lyophilized to get 13C- and 15N-labeled phytodetritus (cf. Wukovits et al., 2017). Three batches of algae were produced. Final isotopic concentrations were P. tricornutum 7 at. %13C and 15 at. %15N (Experiment 1), D. tertiolecta 22 at. %13C (Experiment 2), and P. tricornutum 14 at. %15N (Experiment 2).
2.3 Experiment 1: Nutrient demand and temperature response of A. tepida and H. germanica
A total of 50 to 55 specimens of A. tepida and or H. germanica, respectively, of the size fraction 250–355 µm were distributed into separate wells on a 6-well plate, containing NSW (12 mL per well, salinity: 28 PSU, practical salinity units, which lies in the range of our measurements from seawater at the sampling site: 24–30 PSU). In total, triplicate samples were prepared. The food source, P. tricornutum (1.5 g dry weight m−2), was added into each well. Wells were then covered with a headspace to prevent evaporation and were incubated at 15, 20, or 25 ∘C (Table 1).The specimens were incubated at a 12 h:12 h light:dark cycle, starting the incubation with the light cycle. Two equal setups were prepared for incubation. The first setup was terminated after a 24 h incubation period to determine the intake of P. tricornutum detritus per species and temperature (“24 h fed”). The experimental period of 24 h was chosen to avoid potential bacterial activity and to maintain system stability. The specimens were removed from the wells, transferred to Eppendorf© tubes, and frozen at −20 ∘C. The specimens of the second setup were washed three times in carbon- and nitrogen-free artificial seawater after the 24 h incubation period and transferred to crystallizing dishes (9 cm diameter), containing 150 mL filtered NSW and covered with parafilm. Subsequently, the dishes were incubated for another 24 h (15, 20, 25 ∘C; 12 h light, 12 h dark, starting with the light cycle) without food. These samples were analyzed to determine the remaining phytodetrital carbon and nitrogen after a 24 h starvation period (“24 h starved”).
2.4 Experiment 2: Feeding preferences of A. tepida
This experiment was carried out at 20 ∘C, since A. tepida specimens collected in this area showed a good feeding response at this temperature (Wukovits et al., 2017). Ammonia tepida individuals were incubated at 20 ∘C within 6 well plates (55 individuals per triplicate/well, size fraction 250–355 µm). Each well was filled with 12 mL NSW. After acclimation of the individuals within the plates, three different dietary setups were established (Table 1). The first diet consisted of chlorophyte-derived detritus, uniformly 13C-labeled (D. tertiolecta, 1.5 g dry weight cm−2), the second was diatom detritus (P. tricornutum, 1.5 g dry weight cm−2), uniformly 15N-labeled, and the third consisted of a homogenized mixture of both food sources (0.73 g cm−2 each). The differential labeling approach allows calculation of nutrient uptake for the distinct phytodetritus source after determination of respective algal carbon and nitrogen composition. Triplicate samples were taken after 1, 3, 6, 12, and 24 h, and specimens were frozen at −20 ∘C for subsequent isotope (13C∕12C and 15N∕14N) and elemental analysis (total organic carbon, TOC, and total nitrogen, TN). Similarly as in Experiment 1, plates were incubated at a 12 h:12 h light:dark cycle, starting the incubation with the light cycle. The algal C:N ratio was used to calculate the pN aliquot for pC of the 13C-labeled chlorophyte and pC for the 15N-labeled diatom food source, for a better visual comparison of the food intake (this serves as a rough estimate of equivalent pC or pN intake in the two diets). This experiment was solely carried out with A. tepida, since the sediment did not contain sufficient individuals of H. germanica to set up a parallel run with this species.
2.5 Sediment core data and foraminiferal abundances
Sediment core samples (uppermost cm) were sieved to fractionate size classes (125–250, 250–355, <355 µm). Rose Bengal-stained individuals were counted for each size fraction to obtain abundance data for the live foraminiferal community at the sampling date. Nutrient budget data from the laboratory experiments (individual TOC, TN, pC, pN), together with the foraminiferal abundances counted from the sediment cores, were used to estimate the range of foraminiferal contributions to sedimentary carbon and nitrogen pools and fluxes. In the case of H. germanica, these contributions were only estimated for the 250–355 µm fraction (as used in laboratory experiments). For A. tepida, the 125–250 µm fraction was included in the estimation, using size fraction and feeding relationships from Wukovits et al. (2018). Further, the abundances of A. tepida, as derived by the latter study, were compared with the recent study.
2.6 Sample preparation and isotope analysis
Prior to cytoplasm isotope analysis, foraminifera were carefully cleaned from adhering particles in carbon and nitrogen-free artificial seawater, rinsed with ultrapure water in a last cleaning step to remove salts, transferred to tin capsules, and dried at 50 ∘C for several hours. Subsequently, the foraminifera were decalcified with 10–15 µL 4 % HCl, and kept at 50 ∘C for 3 days in a final drying step (Enge et al., 2014, 2016; Wukovits et al., 2017, 2018). The optimum range for isotope and elemental analysis was 0.7–1.0 mg cytoplasmic dry weight. In the 250 µm size fraction, 30–40 individuals met this criterion. Tools for preparation (hairbrush, needles, tin capsules, tweezers) were rinsed with dichloromethane (CH2Cl2) and methanol (CH4O) (1:1, v:v). Glassware for microscopy was combusted at 500 ∘C for 5 h. The samples were analyzed at the Large-Instrument Facility for Advanced Isotope Research at the University of Vienna (SILVER). Ratios of 13C ∕ 12C and 15N ∕ 14N and the content of organic carbon and nitrogen were analyzed with an Isotope Ratio Mass Spectrometer (IRMS; DeltaPLUS, Thermo Finnigan) coupled with an interface (ConFlo III, Thermo Finnigan) to an elemental analyzer (EA 1110, CE Instruments). Isotope ratio data, the Vienna Pee Dee Belemnite standard for C (RVPDB = 0.0112372) and the standard for atmospheric nitrogen for N (RatmN=0.0036765), were used to calculate at. % of the samples, where X is 13C or 15N:
Intake of pC and pN into foraminiferal cytoplasm was calculated by determining the excess (E) of isotope content within the samples using natural abundance data and data of enriched samples (Middelburg et al., 2000):
where X is 13C or 15N. Excess and content of total organic carbon and nitrogen (TOC and TN per individual) were used to calculate incorporated isotopes (Iiso) derived from the food source:
The amount of pC (µg ind−1) and pN (µg ind−1) within foraminiferal cytoplasm was calculated as follows (Hunter et al., 2012):
2.7 Statistical analysis
Experiment 1: The temperature effect on pC and pN within the foraminiferal cytoplasm, and pC:pN was tested using permutation tests and pairwise permutation tests for post hoc testing (R package rcompanion). Homogeneity of variances was tested using the Fligner–Killeen test. Relationships of pC and pN after feeding and starvation were explored using linear regression for both species, to observe if pC and pN processing are coupled processes in the two species. Finally, the relative amount of food-source-derived carbon and nitrogen after 24 h starvation was evaluated, to compare the metabolic carbon and nitrogen loss from the two species during the period without food.
Experiment 2: To describe and compare uptake dynamics for the different diets, Michaelis–Menten curves were applied on pC and pN data. The models were tested by applying the lack-of-fit method (R package drc). To compare pC and pN values for both diets, pN was calculated from pC for D. tertiolecta, and pC from pN for P. tricornutum. Estimates for pC and pN acquired in this way might be underestimated or overestimated, respectively, due to possible differences in the ratios of carbon:nitrogen excretion or remineralization, respectively.
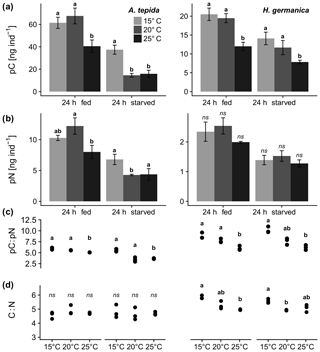
Figure 2Comparison of pC and pN from diatom feeding in A. tepida and H. germanica after a 24 h feeding period (24 h fed) and 24 h without food (24 h starved) at 15, 20, and 25 ∘C. Letters show significant differences of (a) cytoplasmic pC, (b) pN between incubation temperatures within the 24 h feeding period/24 h fed and the 24 h incubation without food/24 h starved, (c) pC:pN ratio (n=3 in all cases), and (d) ratios of foraminiferal cytoplasmic C:N; p<0.05, pairwise permutation tests; ns denotes values that are not significant.
3.1 Experiment 1: Nutrient demand and temperature response of A. tepida and H. germanica
Phytodetrital pC and pN levels derived from P. tricornutum detritus were 2–5 times higher in A. tepida compared to H. germanica (Fig. 2a, b). Different incubation temperatures resulted in significant effects on pC levels after 24 h feeding and 24 h starvation in both species. Ammonia tepida showed a significantly lowered pC content when feeding at 25 ∘C (Fig. 2a; A. tepida, 24 h fed, p<0.05). The 24 h incubation period with no food resulted in significantly lowered pC levels at 20 and 25 ∘C (Fig. 2a; A. tepida, 24 h starved, p<0.05). In H. germanica, the 24 h feeding period had a similar effect like on A. tepida, resulting in significantly lowered pC levels at 25 ∘C (Fig. 2a; H. germanica, 24 h fed, p<0.05). A strong effect of increased temperature after the starvation period was present at 25 ∘C (Fig. 2a; H. germanica, 24 h starved, p<0.05).
The pN levels in A. tepida were considerably affected by temperature after feeding and starvation, whereas there was no apparent effect on H. germanica pN levels, neither after feeding, nor after incubation without food (Fig. 2b). Ammonia tepida reacted with simultaneously lowered pN and pC levels at 25 ∘C after feeding and starvation (Fig. 2b; A. tepida, p<0.05).
The ratios of pC:pN were affected by temperature in both species during feeding and starvation (Fig. 2c; p<0.05). Increased temperatures promoted a drop of pC:pN ratios in A. tepida during the starvation period (Fig. 2c; A. tepida, p<0.05). In contrast, temperature-specific pC:pN ratios in H. germanica showed no change between the incubations with food (24 h fed) and the starvation period (24 h starved; Fig. 2c; H. germanica). Ratios of C:N show significant temperature-related changes in H. germanica (p<0.05), but not in A. tepida (Fig. 2d). The relatively high pN content in A. tepida also shows a steeper relationship of cytoplasmic pN and pC, compared to H. germanica (Fig. 3a). Further, there is a far higher metabolic turnover of pC and pN in A. tepida than in H. germanica, specifically at 20 ∘C (Fig. 3b).
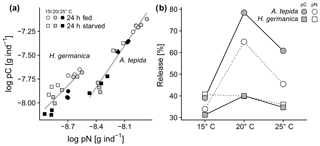
Figure 3(a) Relationship of pC and pN in A. tepida and H. germanica (A. tepida: R2=0.96, , p<0.01; H. germanica: R2=0.64, , p=0.011), and (b) phytodetrital carbon and nitrogen turnover as percent release (of total intake of pC or pN per day, respectively).
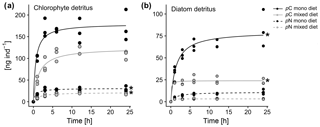
Figure 4Comparison of chlorophyte and diatom phytodetritus feeding in A. tepida for 24 h, presenting feeding dynamics for (a) chlorophyte detritus and (b) diatom detritus. Curves show Michaelis–Menten fits through triplicates for each approach (stars indicate calculated values for pC or pN).
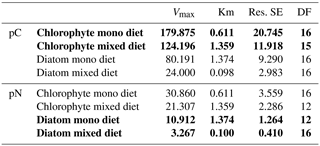
Table 2Michaelis–Menten parameters of curves for pC and pN intake in Fig. 4 (bold font shows data from measured values, and regular font shows data from calculated values; Vmax is the maximum pC/pN, Km is the half saturation for pC/pN, Res. SE is the residual standard error, and DF is the degrees of freedom).
3.2 Experiment 2: Feeding preferences of A. tepida
Michaelis–Menten curves fitted with no significant deviation of variance within the sample replicates. Enrichment of algal nutrients in foraminiferal cytoplasm was highest when a single diet of D. tertiolecta was available (Fig. 4a). Here, saturation levels (max. 180 ng C ind−1) were already reached within 3 h of detritus introduction, and half saturation with pC in A. tepida was reached after 0.6 h (Table 2). In contrast, a single P. tricornutum diet resulted in a slower food intake (Fig. 4b), with a half saturation of pN levels after 1.4 h (Table 2). Further, diatom phytodetritus intake resulted in lower levels of pC (max. ∼80 ng C ind−1). In the mixed feeding approach, half saturation of chlorophyte pC was reached after 1.4 h and diatom pN half saturation was already reached after 0.1 h. Further, the maximum pC levels of the chlorophyte diet still reached ∼70 % of those in the single chlorophyte diet, whereas the pN levels of the diatom diet only reached about 30 % of those in the single diatom diet (Fig. 4, Table 2). Chlorophyte intake was faster and higher, both in the single and mixed diet, and diatom pN stagnated already after less than 1 h in the mixed diet, but after this time period, chlorophyte detritus intake in the mixed diet had continued with increasing pC levels, saturating between 6 and 10 h (Fig. 4a, b).
Table 3Mean abundances (±SD) of live A. tepida and H. germanica (0–1 cm sediment depth), TOC, TN, and carbon and nitrogen flux calculated from sediment cores (early May 2015 n=1, late April 2016 n=3). Data for 15 ∘C of Experiment 1 were used to estimate carbon and nitrogen fluxes (n.d. denotes values that are not determined).

a Data from Wukovits et al. (2018)
3.3 Relevance of A. tepida and H. germanica in intertidal OM fluxes
Data for the live foraminiferal community in 2016 from the three stained sediment cores showed a typical, low-biodiversity mudflat community consisting of A. tepida, H. germanica, and very low abundances of Elphidium williamsonii (<1258 ind m−2, all size fractions). Abundances of A. tepida and H. germanica were equal and decreased with increasing size fraction. The calculated total biomass of live foraminifera in units of TOC is max. ∼120 mg C m−2 (both species, all size fractions; Table 3). From combining in situ abundances and pC values from Experiment 1 (15 ∘C), this foraminiferal community has the potential to take up at least 4 , when taking only diatom detritus into account. The contribution of H. germanica to this OM processing is only at about 15 %.
Different ecologic lifestyles or adaptations to environmental parameters are important organismic attributes to avoid inter- and intra-specific competition. Further, different metabolic adaptations result in species-specific rates of organic matter turnover. Our results clearly demonstrate that food resource partitioning and different temperature adaptations contribute to the fluctuating, temporal distribution and abundance of A. tepida and H. germanica. Due to these specific adaptations, both species play different roles in intertidal organic matter fluxes. There are, however, limitations for the interpretation of results derived from laboratory incubations. A laboratory setup cannot reproduce natural conditions completely. Therefore, the foraminiferal responses might deviate slightly from their natural behavior. However, laboratory experiments enable the analysis of the direct response of specimens to a single factor, while maintaining other factors at a stable level. To enable a compatible comparison, we incubated freshly sampled individuals at stable, near-natural conditions. Both tested food sources are considered good food sources for intertidal foraminifera (Lee et al., 1966). Dunaliella tertiolecta is commonly used in feeding experiments with foraminifera due to its easy culturing. Phaeodactylum tricornutum, which represents a more stable (due to the silicate frustule) source of OM, is a common food source of intertidal foraminifera (Murray, 1963). Additional tested food sources would give a more comprehensive picture, but there were limitations in time and material. In the following sections, our results are discussed with respect to these restrictions.
4.1 Experiment 1: Nutrient demand and temperature response of A. tepida and H. germanica
Experiment 1 shows clear differences in the amount of phytodetritus intake and different carbon and nitrogen budgeting between the two species (Figs. 2, 3). Ammonia tepida has a higher affinity to the diatom detritus food source with an intake of diatoms at the two lower temperatures 3 times higher than H. germanica. This lower food intake by H. germanica could be explained by the mixotrophic lifestyle of this species. Haynesina germanica is known to host kleptoplasts, exploiting the photosynthetic activity of ingested chloroplasts as an additional energy source (Lopez, 1979; Pillet et al., 2011). This species might therefore utilize nutrients (carbohydrates) derived from the photosynthetic activity of incorporated chloroplasts (Cesbron et al., 2017). This lifestyle could cause a lower demand for and lower turnover of OM as food source (Cesbron et al., 2017). In our study, the pC intake in H. germanica was ∼67 % lower than that of A. tepida (Fig. 2). Highly specialized sea slugs use plastids as energy reservoirs at times of low food availability (Cartaxana et al., 2017; Hinde and Smith, 1972; Marín and Ros, 1993), where carbon supply from chloroplasts can cover 60 % of total carbon input (Raven et al., 2001). In kleptoplast-hosting sea slugs, free from the seawater is a primary source of the generation of amino acids via kleptoplast metabolism within the slug (Teugels et al., 2008). A similar mechanism in H. germanica might explain the high relative turnover of pN (Fig. 3b). Phytodetrital nitrogen might therefore be disposed at a higher rate in a relatively temperature independent process, probably in the form of dissolved organic nitrogen, further causing a higher pC:pN ratio in the cytoplasm of H. germanica (Fig. 2).
In addition to the higher rates of phytodetritus intake, A. tepida shows a considerably higher metabolic turnover of pC and pN than H. germanica (Fig. 3b). According to Cesbron et al. (2016), respiration rates (normalized to ) are about 2–12 times higher in A. tepida specimens than in H. germanica specimens from the same location. In this study, a 4–7 times higher release of phytodetritus-derived pC per individual and day (size fraction 250–355 µm) was observed in A. tepida. Interestingly, this study shows similar reactions of both species in carbon loss due to increased temperature. An earlier study on the temperature effect on D. tertiolecta detritus intake of the two species showed a higher sensitivity to increased temperatures in H. germanica, and far lower rates of chlorophyte detritus intake compared to this study (Wukovits et al., 2017). In contrast, A. tepida seems to be more tolerant to higher temperatures when feeding on chlorophyte detritus. The results of Experiment 1 suggest a niche separation of the two species with respect to phytodetritus or OM availability and temperature.
4.2 Experiment 2: Feeding preferences of A. tepida
The findings of Experiment 2 suggest that A. tepida might prefer OM food sources, which are easy to exploit and to break down. The high intake values in the D. tertiolecta mono diet 1 h after incubation and the saturation of cytoplasmic pC levels after 3 h indicate a high affinity to chlorophyte detritus (Fig. 4, Table 2). Earlier studies also observed quick and high ingestion rates of chlorophyte detritus (Chlorella sp.) by the genus Ammonia (Linshy et al., 2014; Wukovits et al., 2017, 2018). The fast saturation with diatom detritus after 1 h in the mixed diet and the advanced and high intake of D. tertiolecta could even indicate an avoidance of P. tricornutum and selective feeding on D. tertiolecta. Probably, the soft cells of chlorophytes enable a faster and easier metabolic processing of this food source compared to the harder diatom frustules. The recognition of such food sources could be achieved by chemosensory behavior of the foraminifera (cf. Langer and Gehring, 1993) and the attraction to specific substances attached to, or leaking from the food particles, similar to some other protists, which react to food-specific amino acids (Almagor et al., 1981; Levandowsky et al., 1984). Microalgal communities in tidal sediments typically consist of microphytobenthic diatoms, which are considered to be the main food source for intertidal foraminifera. An isotope labeling study has shown that diatoms (Navicula salinicola) are taken up by A. tepida at high rates, but the complete release of the content of the diatom frustules can take several days (LeKieffre et al., 2017). This might not fit the nutrient demands of A. tepida at times of high metabolic activity. Therefore, a shift from microphytobenthos to particulate OM from riverine or tidal transport might be a feeding strategy in A. tepida, specifically at higher temperatures, when more energy is needed to maintain metabolic activities.
In general, food sources of A. tepida include microalgae, phytodetritus, bacteria, and sometimes metazoans (Bradshaw, 1961; Dupuy et al., 2010; Moodley et al., 2000; Pascal et al., 2008). Bacteria are considered to play a minor role in the diet of A. tepida (Pascal et al., 2008), and reports on metazoan feeding in A. tepida are restricted to a single observation (Dupuy et al., 2010). In contrast to A. tepida, H. germanica does actively ingest bacteria and they can occasionally be preferred over diatoms (Brouwer et al., 2016). Diatoms are reportedly taken up by H. germanica, and conical test structures serve as tools to crack diatom frustules open (Austin et al., 2005; Ward et al., 2003). These chloroplasts derived from diatoms remain as functional kleptoplasts, as mentioned above, within the cytoplasm of H. germanica.
4.3 Relevance of A. tepida and H. germanica for intertidal OM fluxes
Data of foraminiferal abundances or foraminiferal biomass are important variables to estimate foraminiferal nutrient fluxes. In this section, we discuss the relevance of A. tepida or H. germanica in intertidal fluxes of phytodetrital carbon and nitrogen as estimated from sediment core data in combination with results from the laboratory feeding experiments of this study. The total biomass of the two species in the sampling area ranges between ∼116 and >380 mg TOC m−2 (size fraction 125–355 µm) at the sampling dates in late April/early May in two consecutive years (Table 3). This lies within the range of estimations for hard-shelled foraminifera in other areas of the Wadden Sea (van Oevelen et al., 2006a, b; TOC max. ∼160–750 mg C m−2). Our phytodetritus uptake estimates propose that the foraminiferal biomass consists of ∼6 %–8 % diatom-derived pC/TOC, with the major amount contained within A. tepida (compare Table 3). An in situ feeding experiment with deep-sea foraminifera resulted in values of ∼1 %–12 % pC/TOC (Nomaki et al., 2005b). Similar in situ incubations in the core of the oxygen minimum zone of the Arabian Sea report ∼15 % pC/TOC in epifaunal and shallow infaunal foraminiferal carbon uptake (Enge et al., 2014). In situ incubations offer results closest to the natural responses of organisms in their natural habitat and enable precise estimates of foraminiferal nutrient fluxes. Although specific microhabitat conditions can have a strong influence on organismic behavior, the artificial conditions in laboratory experiments also have an influence on physiological analysis; therefore the obtained results should be treated with caution. However, our estimates lie in the same order of magnitude as the above-mentioned in situ studies and offer a basis for estimations of foraminiferal carbon and nitrogen fluxes. General variations in foraminiferal carbon and nitrogen budgets can be caused by different adaptations to variable food availability in different habitats. This can be achieved by different controls of energy metabolism (e.g., Linke, 1992) or different trophic strategies (e.g., Lopez, 1979; Nomaki et al., 2011; Pascal et al., 2008). Our results suggest A. tepida has a higher relevance for intertidal OM processing than H. germanica. This can be mainly attributed to the sequestered chloroplasts within the cytoplasm of H. germanica. Kleptoplasty is a widespread phenomenon in foraminifera, specifically in species inhabiting dysoxic sediments, where kleptoplasts could promote survival in anoxic porewaters (Bernhard and Bowser, 1999). They might be involved in biochemical pathways within the foraminiferal cytoplasm, e.g., the transport of inorganic carbon and nitrogen (LeKieffre et al., 2018). Further, transmission electron microscopic investigations on H. germanica report a very limited abundance of food vesicles (Goldstein and Richardson, 2018). Kleptoplast-bearing species might occupy a distinct niche concerning their energetic demands. Additionally, they might play an importance in the fluxes of inorganic or dissolved carbon and nitrogen compounds that has not yet been discovered. However, secondary producers with high uptake rates and a quick response to particulate OM sources like A. tepida play a strong role in the biogeochemical carbon and nitrogen recycling.
The high rates of OM carbon and nitrogen turnover are mainly caused by A. tepida populations (Table 3). The process of carbon and nitrogen regeneration by OM remineralization might play an important role in marine biogeochemical cycling. Carbon loss, e.g., due to organismic respiration or OM remineralization to CO2, reduces the availability of organic carbon sources in the heterotrophic food web. As mentioned above, in the heterotrophic coastal zone, 30 % of the carbon pool is lost via respiration, whereas dissolved organic carbon sources from organismic excretion can serve as an important nutrient source for bacteria (Kahler et al., 1997; Snyder and Hoch, 1996; Zweifel et al., 1993). Therefore, the fast processing of OM in A. tepida might be an important sink for inorganic carbon (CO2 respiration) and at the same time a link for dissolved organic carbon sources in intertidal carbon and nitrogen fluxes. According to this study, the maximum pC flux through A. tepida can reach values of ∼36 when feeding on chlorophytes at 20 ∘C (estimated from Experiment 2, Fig. 3 relative release, and max. abundances). Therefore, A. tepida could contribute up to 10 % of the turnover of OM derived from gross particulate phytoplankton production on the sampling date in April/May 2016, with a gross particulate primary production between ∼230 and 1500 (Tillmann et al., 2000). This is comparable with the study of Moodley et al. (2000), in which Ammonia sp. incorporated ∼7 % within 53 h in sediment core incubations' feeding experiments in sediment incubations with added labeled chlorophyte detritus.
Planktonic protozoa are the primary regenerators of marine nitrogen, transforming OM-derived nitrogen to their primary N excretion product, (Glibert, 1997). The excretion of by marine protists can contribute a large part to the nutritional demands of marine primary productivity (Ferrier-Pages and Rassoulzadegan, 1994; Ota and Taniguchi, 2003; Verity, 1985). Nitrogen regeneration by protozoa was supposed to play a far higher role than bacterial nitrogen regeneration in the marine microbial food chain (Goldman and Caron, 1985). Indeed, excreted nitrogen can serve as an important nutrient sources for microbes (Wheeler and Kirchman, 1986). The release of dissolved organic nitrogen and by, e.g., copepods, can be a major driver for marine microbial production (Valdés et al., 2018). Here, foraminiferal nitrogen excretion values are in the range of estimations for weight-specific excretion in marine protozoa according to Dolan (1997) (for data for foraminiferal weight, cf. Supplement Fig. S2). Due to their high abundances, nitrogen release by A. tepida as observed in this study could reach 2.5 or ∼73 , respectively, at 15 ∘C and high diatom availability (cf. Table 3). As a rough estimate for A. tepida feeding at high abundances and high availability of chlorophyte detritus at 20 ∘C, these values could increase to ∼22 or ∼0.6 (Fig. 1, Table 3). Therefore, foraminiferal nitrogen release as or amino acids could cover a considerable amount of the nutritional nitrogen demand in marine bacteria (cf. Wheeler and Kirchman, 1986), which assimilate (and amino-acid-derived ) to sustain their glutamate–glutamine cycle. Vice versa, the labile dissolved organic matter derived from the bacterial decomposition of refractory organic matter provides a valuable food source for some benthic foraminifera, and is indispensable for the reproduction of some foraminiferal species (Jorissen et al., 1998; Muller and Lee, 1969; Nomaki et al., 2011). In many marine diatoms, which are the main drivers of marine primary productivity, is the preferred source of nitrogen uptake over (Sivasubramanian and Rao, 1988). Foraminifera could act as important nutrient providers for closely associated diatoms, which are also considered as one of their main food sources (Lee et al., 1966). Consequently, the kleptoplast-hosting metabolism in H. germanica could benefit from regenerated nitrogen sources by the high OM mineralization rates in A. tepida. In summary, foraminiferal carbon and nitrogen fluxes constitute an important link in the food web complex of primary consumers and decomposers.
This study compares differences in the feeding behavior, nutrient demand, and OM flux of two intertidal foraminiferal species. Our results clearly show that A. tepida has a higher impact on the fluxes of phytodetrital carbon and nitrogen in intertidal sediments than H. germanica. This can partly be explained by their different lifestyles. Differences in temperature acclimatization or preferences for different food sources can serve as strategies to avoid spatial and temporal interspecific competition, resulting in a niche separation of the two species with respect to phytodetritus or OM availability and temperature. Accordingly, H. germanica could be associated with environmental conditions of moderate availability of microphytobenthos and lower temperatures, as given prior to the diatom spring bloom, whereas A. tepida could take advantage of seasons characterized by higher input of allochthonous OM. Further, temperature fluctuations in combination with allochthonous OM availability have less effect on the carbon and nitrogen processing in A. tepida. These differentiations in their metabolic OM processing and lifestyles suggest a far higher relevance of A. tepida in the mediation of the fluxes of intertidal carbon and nitrogen.
All data used in this study are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-6185-2018-supplement.
JW designed the study, carried out field sampling, laboratory experiments, and data analysis, and wrote the paper. MO processed the Rose Bengal samples and counted foraminiferal abundances. AJE made major contributions to the design of the study, carried out field sampling, was involved in data collection and interpretation, and carried out critical revisions of manuscript drafts. PH was involved in data interpretation and carried out critical revisions of manuscript drafts.
The authors declare that they have no conflict of interest.
We thank Patrick Bukenberger and Murtaza Kulaksiz for help
with sampling and culture maintenance and Margarete Watzka for EA-IRMS
analysis. We also thank Chiara Borrelli and one anonymous referee for their contribution to
the improvement of the earlier version of the manuscript. Open access funding
is provided by University of Vienna.
Edited by: Tina Treude
Reviewed by: Chiara Borrelli and one anonymous referee
Almagor, M., Ron, A., and Bar-Tana, J.: Chemotaxis in Tetrahymena thermophila, Cell Motil., 1, 261–268, https://doi.org/10.1002/cm.970010208, 1981.
Alve, E. and Murray, J. W.: Ecology and taphonomy of benthic foraminifera in a temperate mesotidal inlet, J. Foraminifer. Res., 24, 18–27, 1994.
Alve, E. and Murray, J. W.: Temporal variability in vertical distributions of live (stained) intertidal foraminifera, southern England, J. Foraminifer. Res., 31, 12–24, https://doi.org/10.2113/0310012, 2001.
Armynot du Châtelet, E., Debenay, J.-P., and Soulard, R.: Foraminiferal proxies for pollution monitoring in moderately polluted harbors, Environ. Pollut. Barking Essex 1987, 127, 27–40, 2004.
Armynot du Châtelet, E., Degre, D., Sauriau, P.-G., and Debenay, J.-P.: Distribution of living benthic foraminifera in relation with environmental variables within the Aiguillon cove (Atlantic coast, France): improving knowledge for paleoecological interpretation, Bull. Soc. Geol. Fr., 180, 131–144, 2009.
Austin, H. A., Austin, W. E. N., and Paterson, D. M.: Extracellular cracking and content removal of the benthic diatom Pleurosigma angulatum (Quekett) by the benthic foraminifera Haynesina germanica (Ehrenberg), Mar. Micropaleontol., 57, 68–73, https://doi.org/10.1016/j.marmicro.2005.07.002, 2005.
Bauer, J. E., Cai, W.-J., Raymond, P. A., Bianchi, T. S., Hopkinson, C. S., and Regnier, P. A. G.: The changing carbon cycle of the coastal ocean, Nature, 504, 61–70, https://doi.org/10.1038/nature12857, 2013.
Bernhard, J. M. and Bowser, S. S.: Benthic foraminifera of dysoxic sediments: chloroplast sequestration and functional morphology, Earth-Sci. Rev., 46, 149–165, https://doi.org/10.1016/S0012-8252(99)00017-3, 1999.
Bradshaw, J. S.: Laboratory experiments on the ecology of foraminifera, Contrib. Cushman Found. Foraminifer. Res., 7, 87–106, 1961.
Brouwer, G. M., Duijnstee, I. A. P., Hazeleger, J. H., Rossi, F., Lourens, L. J., Middelburg, J. J., and Wolthers, M.: Diet shifts and population dynamics of estuarine foraminifera during ecosystem recovery after experimentally induced hypoxia crises, Estuar. Coast. Shelf Sci., 170(Supplement C), 20–33, https://doi.org/10.1016/j.ecss.2015.12.015, 2016.
Caffrey, J. M.: Production, Respiration and Net Ecosystem Metabolism in U.S. Estuaries, Environ. Monit. Assess., 81, 207–219, https://doi.org/10.1023/A:1021385226315, 2003.
Caffrey, J. M.: Factors controlling net ecosystem metabolism in U.S. estuaries, Estuaries, 27, 90–101, https://doi.org/10.1007/BF02803563, 2004.
Cai, W.-J.: Estuarine and Coastal Ocean Carbon Paradox: CO2 Sinks or Sites of Terrestrial Carbon Incineration?, Annu. Rev. Mar. Sci., 3, 123–145, https://doi.org/10.1146/annurev-marine-120709-142723, 2011.
Cartaxana, P., Trampe, E., Kühl, M., and Cruz, S.: Kleptoplast photosynthesis is nutritionally relevant in the sea slug Elysia viridis, Sci. Rep.-UK, 7, 7714, https://doi.org/10.1038/s41598-017-08002-0, 2017.
Cesbron, F., Geslin, E., Jorissen, F. J., Delgard, M. L., Charrieau, L., Deflandre, B., Jézéquel, D., Anschutz, P., and Metzger, E.: Vertical distribution and respiration rates of benthic foraminifera: Contribution to aerobic remineralization in intertidal mudflats covered by Zostera noltei meadows, Estuar. Coast. Shelf Sci., 179, 23–38, https://doi.org/10.1016/j.ecss.2015.12.005, 2016.
Cesbron, F., Geslin, E., Kieffre, C. L., Jauffrais, T., Nardelli, M. P., Langlet, D., Mabilleau, G., Jorissen, F. J., Jézéquel, D., and Metzger, E.: Sequestered Chloroplasts in the Benthic Foraminifer Haynesina germanica: Cellular Organization, Oxygen Fluxes and Potential Ecological Implications, J. Foraminifer. Res., 47, 268–278, https://doi.org/10.2113/gsjfr.47.3.268, 2017.
Chandler, G. T.: Foraminifera may structure meiobenthic communities, Oecologia, 81, 354–360, https://doi.org/10.1007/BF00377083, 1989.
Cole, J. J., Prairie, Y. T., Caraco, N. F., McDowell, W. H., Tranvik, L. J., Striegl, R. G., Duarte, C. M., Kortelainen, P., Downing, J. A., Middelburg, J. J., and Melack, J.: Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget, Ecosystems, 10, 172–185, https://doi.org/10.1007/s10021-006-9013-8, 2007.
Dolan, J. R.: Phosphorus and ammonia excretion by planktonic protists, Mar. Geol., 139, 109–122, https://doi.org/10.1016/S0025-3227(96)00106-5, 1997.
Dupuy, C., Rossignol, L., Geslin, E., and Pascal, P.-Y.: Predation of Mudflat Meio-Macrofaunal Metazoans by a Calcareous Foraminifer, Ammonia tepida (cushman, 1926), J. Foraminifer. Res., 40, 305–312, https://doi.org/10.2113/gsjfr.40.4.305, 2010.
Enge, A. J., Witte, U., Kucera, M., and Heinz, P.: Uptake of phytodetritus by benthic foraminifera under oxygen depletion at the Indian margin (Arabian Sea), Biogeosciences, 11, 2017–2026, https://doi.org/10.5194/bg-11-2017-2014, 2014.
Enge, A. J., Wukovits, J., Wanek, W., Watzka, M., Witte, U. F. M., Hunter, W. R., and Heinz, P.: Carbon and Nitrogen Uptake of Calcareous Benthic Foraminifera along a Depth-Related Oxygen Gradient in the OMZ of the Arabian Sea, Aquat. Microbiol., 71, 1–12, https://doi.org/10.3389/fmicb.2016.00071, 2016.
Ferrier-Pages, C. and Rassoulzadegan, F.: N remineralization in planktonic protozoa, Limnol. Oceanogr., 39, 411–419, https://doi.org/10.4319/lo.1994.39.2.0411, 1994.
Glibert, P. M.: Interactions of top-down and bottom-up control in planktonic nitrogen cycling, Hydrobiologia, 363, 1–12, https://doi.org/10.1023/A:1003125805822, 1997.
Goldman, J. C. and Caron, D. A.: Experimental studies on an omnivorous microflagellate: implications for grazing and nutrient regeneration in the marine microbial food chain, Deep-Sea Res. Pt. I, 32, 899–915, https://doi.org/10.1016/0198-0149(85)90035-4, 1985.
Goldstein, S. T. and Corliss, B. H.: Deposit feeding in selected deep-sea and shallow-water benthic foraminifera, Deep-Sea Res. Pt. I, 41, 229–241, https://doi.org/10.1016/0967-0637(94)90001-9, 1994.
Goldstein, S. T. and Richardson, E. A.: Fine structure of the foraminifer Haynesina germanica (Ehrenberg) and its sequestered chloroplasts, Mar. Micropaleontol., 138, 63–71, https://doi.org/10.1016/j.marmicro.2017.10.010, 2018.
Guillard, R. R. L.: Culture of phytoplankton for feeding marine invertebrates, Culture of marine invertebrate animals, Springer, Boston, MA, 29–60, 1975.
Guillard, R. R. L. and Ryther, J. H.: Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran, Can. J. Microbiol., 8, 229–239, 1962.
Hayward, B. W.: “Monospecific” and near-monospecific benthic foraminiferal faunas, New Zealand, J. Foraminifer. Res., 44, 300–315, 2014.
Heinz, P.: Laboratory feeding experiments: response of deep-sea benthic foraminifer to simulated phytoplankton pulses, Rev. Paleobiol Geneve, 20, 643–646, 2001.
Heinz, P., Marten, R. A., Linshy, V. N., Haap, T., Geslin, E., and Kohler, H. R.: 70 kD stress protein (Hsp70) analysis in living shallow-water benthic foraminifera, Mar. Biol. Res., 8, 677–681, https://doi.org/10.1080/17451000.2011.650177, 2012.
Herrmann, M., Najjar, R. G., Kemp, W. M., Alexander, R. B., Boyer, E. W., Cai, W.-J., Griffith, P. C., Kroeger, K. D., McCallister, S. L., and Smith, R. A.: Net ecosystem production and organic carbon balance of U.S. East Coast estuaries: A synthesis approach, Global Biogeochem. Cy., 29, 96–111, https://doi.org/10.1002/2013GB004736, 2015.
Hinde, R. and Smith, D. C.: Persistence of Functional Chloroplasts in Elysia viridis (Opisthobranchia, Sacoglossa), Nature. New Biol., 239, 30–31, https://doi.org/10.1038/newbio239030a0, 1972.
Hohenegger, J., Piller, W., and Baal, C.: Reasons for spatial microdistributions of foraminifers in an intertidal pool (Northern Adriatic Sea), Mar. Ecol., 10, 43–78, https://doi.org/10.1111/j.1439-0485.1989.tb00065.x, 1989.
Hunter, W. R., Veuger, B., and Witte, U.: Macrofauna regulate heterotrophic bacterial carbon and nitrogen incorporation in low-oxygen sediments, The ISME Journal, 6, 2140–2151, 2012.
Jahnke, R. A.: Transport processes and organic matter cycling in coastal sediments, in: The Global Coastal Ocean, edited by: Robinson, A. R. and Brink, K. H., Harvard University Press, USA, 163–192, 2004.
Jauffrais, T., Jesus, B., Metzger, E., Mouget, J.-L., Jorissen, F., and Geslin, E.: Effect of light on photosynthetic efficiency of sequestered chloroplasts in intertidal benthic foraminifera (Haynesina germanica and Ammonia tepida), Biogeosciences, 13, 2715–2726, https://doi.org/10.5194/bg-13-2715-2016, 2016.
Jorissen, F. J., Wittling, I., Peypouquet, J. P., Rabouille, C., and Relexans, J. C.: Live benthic foraminiferal faunas off Cape Blanc, NW-Africa: Community structure and microhabitats, Deep-Sea Res. Pt. I, 45, 2157–2188, https://doi.org/10.1016/S0967-0637(98)00056-9, 1998.
Kahler, P., Bjornsen, P. K., Lochte, K., and Antia, A.: Dissolved organic matter and its utilization by bacteria during spring in the Southern Ocean, Deep-Sea Res. Pt. II, 44, 341–353, https://doi.org/10.1016/S0967-0645(96)00071-9, 1997.
Langer, M. and Gehring, C.: Bacteria Farming – a Possible Feeding Strategy of Some Smaller, Motile Foraminifera, J. Foraminifer. Res., 23, 40–46, https://doi.org/10.2113/gsjfr.23.1.40, 1993.
Lee, J. J. and Muller, W. A.: Trophic dynamics and niches of salt marsh foraminifera, Am. Zool., 13, 215–223, https://doi.org/10.1093/icb/13.1.215, 1973.
Lee, J. J., McEnery, M., Pierce, S., Freudent, H., and Muller, W. A.: Tracer experiments in feeding littoral foraminifera, J. Protozool., 13, 659–670, https://doi.org/10.1111/j.1550-7408.1966.tb01978.x, 1966.
Lei, Y.-L., Stumm, K., Wickham, S. A., and Berninger, U.-G.: Distributions and biomass of benthic ciliates, foraminifera and amoeboid protists in marine, brackish, and freshwater sediments, J. Eukaryot. Microbiol., 61, 493–508, https://doi.org/10.1111/jeu.12129, 2014.
LeKieffre, C., Spangenberg, J. E., Mabilleau, G., Escrig, S., Meibom, A., and Geslin, E.: Surviving anoxia in marine sediments: The metabolic response of ubiquitous benthic foraminifera (Ammonia tepida), PLoS ONE, 12, e0177604, https://doi.org/10.1371/journal.pone.0177604, 2017.
LeKieffre, C., Jauffrais, T., Geslin, E., Jesus, B., Bernhard, J. M., Giovani, M.-E., and Meibom, A.: Inorganic carbon and nitrogen assimilation in cellular compartments of a benthic kleptoplastic foraminifer, Sci. Rep.-UK, 8, 10140, https://doi.org/10.1038/s41598-018-28455-1, 2018.
Levandowsky, M., Cheng, T., Kehr, A., Kim, J., Gardner, L., Silvern, L., Tsang, L., Lai, G., Chung, C., and Prakash, E.: Chemosensory Responses to Amino-Acids and Certain Amines by the Ciliate Tetrahymena – a Flat Capillary Assay, Biol. Bull., 167, 322–330, https://doi.org/10.2307/1541279, 1984.
Linke, P.: Metabolic Adaptations of Deep-Sea Benthic Foraminifera to Seasonally Varying Food Input, Mar. Ecol. Prog. Ser., 81, 51–63, https://doi.org/10.3354/meps081051, 1992.
Linshy, V. N., Nigam, R., and Heinz, P.: Response of shallow water benthic foraminifera to a 13C-labeled food pulse in the laboratory, in: Approaches to Study Living Foraminifera. Collection, Maintenance and Experimentation, edited by: Kitazato, H., Bernhard, J. M., Förstner, U., and Salomons, W., Springer, Japan, 2014.
Lopez, E.: Algal chloroplasts in the protoplasm of 3 species of benthic foraminifera – taxonomic affinity, viability and persistence, Mar. Biol., 53, 201–211, https://doi.org/10.1007/bf00952427, 1979.
Marín, A. and Ros, J.: Ultrastructural and ecological aspects of the development of chloroplast retention in the sacoglossan gastropod Elysia timida, J. Molluscan Stud., 59, 95–104, https://doi.org/10.1093/mollus/59.1.95, 1993.
Martins, M. V. A., Silva, F., Laut, L. L. M., Frontalini, F., Clemente, I. M. M. M., Miranda, P., Figueira, R., Sousa, S. H. M., and Dias, J. M. A.: Response of Benthic Foraminifera to Organic Matter Quantity and Quality and Bioavailable Concentrations of Metals in Aveiro Lagoon (Portugal), PLoS ONE, 10, https://doi.org/10.1371/journal.pone.0118077, 2015.
Middelburg, J. J., Barranguet, C., Boschker, H. T. S., Herman, P. M. J., Moens, T., and Heip, C. H. R.: The fate of intertidal microphytobenthos carbon: An in situ 13C-labeling study, Limnol. Oceanogr., 45, 1224–1234, 2000.
Mojtahid, M., Geslin, E., Coynel, A., Gorse, L., Vella, C., Davranche, A., Zozzolo, L., Blanchet, L., Bénéteau, E., and Maillet, G.: Spatial distribution of living (Rose Bengal stained) benthic foraminifera in the Loire estuary (western France), J. Sea Res., 118, 1–16, https://doi.org/10.1016/j.seares.2016.02.003, 2016.
Moodley, L., Boschker, H. T. S., Middelburg, J. J., Pel, R., Herman, P. M. J., de Deckere, E., and Heip, C. H. R.: Ecological significance of benthic foraminifera: 13C labelling experiments, Mar. Ecol. Prog. Ser., 202, 289–295, https://doi.org/10.3354/meps202289, 2000.
Muller, W. A. and Lee, J. J.: Apparent indispensability of bacteria in foraminiferan nutrition, J. Protozool., 16, 471–478, https://doi.org/10.1111/j.1550-7408.1969.tb02303.x, 1969.
Murray, J. W.: Ecological experiments on Foraminifera, J. Mar. Biol. Assoc. UK, 43, 621–642, https://doi.org/10.1017/S0025315400025571, 1963.
Murray, J. W. and Alve, E.: Major aspects of foraminiferal variability (standing crop and biomass) on a monthly scale in an intertidal zone, J. Foraminifer. Res., 30, 177–191, https://doi.org/10.2113/0300177, 2000.
Nomaki, H., Heinz, P., Hemleben, C., and Kitazato, H.: Behavior and response of deep-sea benthic foraminifera to freshly supplied organic matter: A laboratory feeding experiment in microcosm environments, J. Foraminifer. Res., 35, 103–113, https://doi.org/10.2113/35.2.103, 2005a.
Nomaki, H., Heinz, P., Nakatsuka, T., Shimanaga, M., and Kitazato, H.: Species-specific ingestion of organic carbon by deep-sea benthic foraminifera and meiobenthos: In situ tracer experiments, Limnol. Oceanogr., 50, 134–146, 2005b.
Nomaki, H., Heinz, P., Nakatsuka, T., Shimanaga, M., Ohkouchi, N., Ogawa, N. O., Kogure, K., Ikemoto, E., and Kitazato, H.: Different ingestion patterns of 13C-labeled bacteria and algae by deep-sea benthic foraminifera, Mar. Ecol. Prog. Ser., 310, 95–108, https://doi.org/10.3354/meps310095, 2006.
Nomaki, H., Ohkouchi, N., Heinz, P., Suga, H., Chikaraishi, Y., Ogawa, N. O., Matsumoto, K., and Kitazato, H.: Degradation of algal lipids by deep-sea benthic foraminifera: An in situ tracer experiment, Deep-Sea Res. Pt. I, 56, 1488–1503, https://doi.org/10.1016/j.dsr.2009.04.013, 2009.
Nomaki, H., Ogawa, N. O., Takano, Y., Suga, H., Ohkouchi, N., and Kitazato, H.: Differing utilization of glucose and algal particulate organic matter by deep-sea benthic organisms of Sagami Bay, Japan, Mar. Ecol. Prog. Ser., 431, 11–24, https://doi.org/10.3354/meps09144, 2011.
Ota, T. and Taniguchi, A.: Standing crop of planktonic ciliates in the East China Sea and their potential grazing impact and contribution to nutrient regeneration, Deep-Sea Res. Pt. II, 50, 423–442, https://doi.org/10.1016/S0967-0645(02)00461-7, 2003.
Papaspyrou, S., Diz, P., Garcia-Robledo, E., Corzo, A., and Jimenez-Arias, J.-L.: Benthic foraminiferal community changes and their relationship to environmental dynamics in intertidal muddy sediments (Bay of Cadiz, SW Spain), Mar. Ecol. Prog. Ser., 490, 121–135, https://doi.org/10.3354/meps10447, 2013.
Pascal, P.-Y., Dupuy, C., Richard, P., and Niquil, N.: Bacterivory in the common foraminifer Ammonia tepida: Isotope tracer experiment and the controlling factors, J. Exp. Mar. Biol. Ecol., 359, 55–61, https://doi.org/10.1016/j.jembe.2008.02.018, 2008.
Pillet, L., de Vargas, C., and Pawlowski, J.: Molecular Identification of Sequestered Diatom Chloroplasts and Kleptoplastidy in Foraminifera, Protist, 162, 394–404, https://doi.org/10.1016/j.protis.2010.10.001, 2011.
Raven, J. A., Walker, D. I., Jensen, K. R., Handley, L. L., Scrimgeour, C. M., and McInroy, S. G.: What fraction of the organic carbon in sacoglossans is obtained from photosynthesis by kleptoplastids? An investigation using the natural abundance of stable carbon isotopes, Mar. Biol., 138, 537–545, https://doi.org/10.1007/s002270000488, 2001.
Regnier, P., Friedlingstein, P., Ciais, P., Mackenzie, F. T., Gruber, N., Janssens, I. A., Laruelle, G. G., Lauerwald, R., Luyssaert, S., Andersson, A. J., Arndt, S., Arnosti, C., Borges, A. V., Dale, A. W., Gallego-Sala, A., Goddéris, Y., Goossens, N., Hartmann, J., Heinze, C., Ilyina, T., Joos, F., LaRowe, D. E., Leifeld, J., Meysman, F. J. R., Munhoven, G., Raymond, P. A., Spahni, R., Suntharalingam, P., and Thullner, M.: Anthropogenic perturbation of the carbon fluxes from land to ocean, Nat. Geosci., 6, 597–607, https://doi.org/10.1038/ngeo1830, 2013.
Saad, S. A. and Wade, C. M.: Seasonal and Spatial Variations of Saltmarsh Benthic Foraminiferal Communities from North Norfolk, England, Microb. Ecol., 73, 539–555, https://doi.org/10.1007/s00248-016-0895-5, 2017.
Sivasubramanian, V. and Rao, V. N. R.: Uptake and assimilation of nitrogen by marine diatoms – I. Kinetics of nitrogen uptake, Proc. Plant Sci., 98, 71–88, https://doi.org/10.1007/BF03053393, 1988.
Smith, S. V. and Hollibaugh, J. T.: Coastal metabolism and the oceanic organic carbon balance, Rev. Geophys., 31, 75–89, https://doi.org/10.1029/92RG02584, 1993.
Snyder, R. A. and Hoch, M. P.: Consequences of protist-stimulated bacterial production for estimating protist growth efficiencies, Hydrobiologia, 341, 113–123, https://doi.org/10.1007/BF00018115, 1996.
Teugels, B., Bouillon, S., Veuger, B., Middelburg, J. J., and Koedam, N.: Kleptoplasts mediate nitrogen acquisition in the sea slug Elysia viridis, Aquat. Biol., 4, 15–21, https://doi.org/10.3354/ab00092, 2008.
Tillmann, U., Hesse, K.-J., and Colijn, F.: Planktonic primary production in the German Wadden Sea, J. Plankton Res., 22, 1253–1276, https://doi.org/10.1093/plankt/22.7.1253, 2000.
Trench, R. K.: Chloroplasts as Functional Endosymbionts in the Mollusc Tridachia crispata (Bërgh), (Opisthobranchia, Sacoglossa), Nature, 222, 1071–1072, https://doi.org/10.1038/2221071a0, 1969.
Valdés, V., Fernandez, C., Molina, V., and Escribano, R.: Nitrogen excretion by copepods and its effect on ammonia-oxidizing communities from a coastal upwelling zone, Limnol. Oceanogr., 63, 278–294, https://doi.org/10.1002/lno.10629, 2018.
van Oevelen, D., Middelburg, J. J., Soetaert, K., and Moodley, L.: The fate of bacterial carbon in an intertidal sediment: Modeling an in situ isotope tracer experiment, Limnol. Oceanogr., 51, 1302–1314, https://doi.org/10.4319/lo.2006.51.3.1302, 2006a.
van Oevelen, D., Moodley, L., Soetaert, K., and Middelburg, J. J.: The trophic significance of bacterial carbon in a marine intertidal sediment: Results of an in situ stable isotope labeling study, Limnol. Oceanogr., 51, 2349–2359, 2006b.
Verity, P. G.: Grazing, respiration, excretion, and growth rates of tintinnids, Limnol. Oceanogr., 30, 1268–1282, 1985.
Verity, P. G., Robertson, C. Y., Tronzo, C. R., Andrews, M. G., Nelson, J. R., and Sieracki, M. E.: Relationships between cell volume and the carbon and nitrogen content of marine photosynthetic nanoplankton, Limnol. Oceanogr., 37, 1434–1446, https://doi.org/10.4319/lo.1992.37.7.1434, 1992.
Ward, J. N., Pond, D. W., and Murray, J. W.: Feeding of benthic foraminifera on diatoms and sewage-derived organic matter: an experimental application of lipid biomarker techniques, Mar. Environ. Res., 56, 515–530, https://doi.org/10.1016/S0141-1136(03)00040-0, 2003.
Wheeler, P. A. and Kirchman, D. L.: Utilization of inorganic and organic nitrogen by bacteria in marine systems1, Limnol. Oceanogr., 31, 998–1009, https://doi.org/10.4319/lo.1986.31.5.0998, 1986.
Wukovits, J., Enge, A. J., Wanek, W., Watzka, M., and Heinz, P.: Increased temperature causes different carbon and nitrogen processing patterns in two common intertidal foraminifera (Ammonia tepida and Haynesina germanica), Biogeosciences, 14, 2815–2829, https://doi.org/10.5194/bg-14-2815-2017, 2017.
Wukovits, J., Bukenberger, P., Enge, A. J., Gerg, M., Wanek, W., Watzka, M., and Heinz, P.: Food supply and size class depending variations in phytodetritus intake in the benthic foraminifer Ammonia tepida, Biol. Open, 7, bio.030056, https://doi.org/10.1242/bio.030056, 2018.
Zweifel, U., Norrman, B., and Hagstrom, A.: Consumption of Dissolved Organic-Carbon by Marine-Bacteria and Demand for Inorganic Nutrients, Mar. Ecol. Prog. Ser., 101, 23–32, https://doi.org/10.3354/meps101023, 1993.