the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Light-dependent calcification in Red Sea giant clam Tridacna maxima
Susann Rossbach
Vincent Saderne
Andrea Anton
Carlos M. Duarte
Tropical giant clams of the subfamily Tridacninae, including the species Tridacna maxima, are unique among bivalves as they live in a symbiotic relationship with unicellular algae and generally function as net photoautotrophs. Light is therefore crucial for these species to thrive. Here we examine the light dependency of calcification rates of T. maxima in the central Red Sea as well as the patterns of its abundance with depth in the field. Red Sea T. maxima show the highest densities at a depth of 3 m with 0.82±0.21 and 0.11±0.03 individuals m−2 (mean ± SE) at sheltered and exposed sites, respectively. Experimental assessment of net calcification (µmol CaCO3 cm−2 h−1) and gross primary production (µmol O2 cm−2 h−1) under seven light levels (1061, 959, 561, 530, 358, 244, and 197 µmol quanta m−2 s−1) showed net calcification rates to be significantly enhanced under light intensities corresponding to a water depth of 4 m (0.65±0.03 µmol CaCO3 cm−2 h−1; mean ± SE), while gross primary production was 2.06±0.24 µmol O2 cm−2 h−1 (mean ± SE). We found a quadratic relationship between net calcification and tissue dry mass (DM in gram), with clams of an intermediate size (about 15 g DM) showing the highest calcification. Our results show that the Red Sea giant clam T. maxima stands out among bivalves as a remarkable calcifier, displaying calcification rates comparable to other tropical photosymbiotic reef organisms such as corals.
- Article
(1613 KB) - Full-text XML
-
Supplement
(102 KB) - BibTeX
- EndNote
Giant clams (family Cardiidae, subfamily Tridacninae) are among the largest and fastest growing bivalves on earth, reaching up to 1 m in size (Rosewater, 1965) and growth rates of up to 8–12 cm yr−1 in the largest species, Tridacna gigas (Beckvar, 1981). In the Indo-Pacific, giant clams are considered ecosystem-engineering species (Neo et al., 2015), playing multiple roles in the framework of coral reef communities, such as providing food for a number of predators and scavengers (Alcazar, 1986), shelter for commensal organisms (De Grave, 1999), and substrate for epibionts (Vicentuan-Cabaitan et al., 2014). By producing calcium carbonate shell material they can occasionally even form reef-like structures (Andréfouët et al., 2005). However, due to their specific habitat preference (Yonge, 1975; Hart et al., 1998) and their presumed longevity (Chambers, 2007), Tridacninae are exceedingly vulnerable to exploitation and environmental degradation (Ashworth et al., 2004; Van Wynsberge et al., 2016). In South-East Asia, giant clams have been harvested for human consumption (adductor muscle and mantle meat) and for their shells (Lucas, 1994) already since pre-historic times (Hviding, 1993). Giant clams are also reared in aquaculture farms for the fishkeeping market (Bell et al., 1997) and in an effort to restock the natural population (Gomez and Mingoa-Licuanan, 2006). Currently, all giant clam species are listed in the IUCN Red List of Threatened Species (IUCN, 2016) and protected under Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Most of them are considered to be lower risk/conservation-dependent status; however, the IUCN status of tridacnine species is in need of updating according to Neo et al. (2017). Besides the pressure of fishing on natural stocks, giant clams are also predicted to be vulnerable to the effects of climate change, including heat waves which have been associated with mass die-off events of Tridacninae in French Polynesia (Andréfouët et al., 2013).
Giant clams are one of the few molluscan groups living in symbiotic relationship with dinoflagellates of the genus Symbiodinium (Yonge, 1936; Taylor, 1969; LaJeunesse et al., 2018), likewise to corals and sea anemones. They are generally described as being mixotrophic (Klumpp et al., 1992), obtaining their energy from both filter feeding and photosynthesis; however, some species appear to be even functionally autotrophic (Beckvar, 1981; Jantzen et al., 2008). This dual capacity is assumed to support their fast calcification and growth rates, exceeding those of most other bivalves (Klumpp and Griffiths, 1994). Thus, the availability of light seems to be a critical factor affecting the growth and overall performance of giant clams (Lucas et al., 1989). To date, several studies have examined long-term growth rates of giant clams in response to different environmental factors, such as nutrient enrichment (Hastie et al., 1992; Hoegh-Guldberg, 1997; Belda-Baillie et al., 1998), water temperature (Hart et al., 1998; Schwartzmann et al., 2011), and wave exposure (Hart et al., 1998). Only a few studies assessed net calcification of Tridacninae as a short-term process and how environmental factors, especially light, are influencing the calcification, physiology, and general metabolic rates of Tridacninae.
A positive correlation between light and calcification has been observed in several photosynthetic calcifying organisms, symbiotic (e.g. scleractinian corals) or not (e.g. coccolithophorids and calcifying algae) (Allemand et al., 2011). For corals, the term light-enhanced calcification (LEC) has been coined (Yonge, 1931); however, the underlying mechanisms remain poorly understood and various hypotheses have been proposed. (1) The photosynthetic uptake of carbon dioxide by the symbionts lowers CO2 levels while increasing pH and the concentration of carbonate ions at the calcification site, which eventually could favour calcium carbonate precipitation (McConnaughey and Whelan, 1997); (2) the removal of inhibiting substances (such as phosphates) by the symbionts during photosynthesis (Simkiss, 1964) or (3) the light-induced production of signalling molecules by the symbionts could lead to an increase in enzymatic activity, essential for the calcification of the host (Ip et al., 2015). Only within the last years has it been possible to investigate LEC mechanisms at the molecular level (Moya et al., 2008; Bertucci et al., 2015), leading to an increasing number of publications reporting light-enhanced expression of enzymes, such as carbonic anhydrase, supporting shell formation in giant clams (Ip et al., 2006, 2015, 2017; Hiong et al., 2017a, b; Chan et al., 2018; Chew et al., 2019). There is also evidence of the light-enhanced expression of gene encoding for those transporters/enzymes needed for calcification within the inner mantle and ctenidium of Tridacna squamosa (Hiong et al., 2017a, b; Ip et al., 2017; Chew et al., 2019). As both tissues lack the presence of symbiotic algae, it has been supposed that light could also directly affect the giant clam host. Despite recent progress in understanding LEC processes in Tridacninae, much remains unknown to date. Previous studies mostly focussed on molecular processes or long-term (several months) effects of light on growth rates, assessed either as an increase in shell length (Lucas et al., 1989; Adams et al., 2013) or total weight (Adams et al., 2013), and did not differentiate between different light intensities. Only a small number of studies actually reported short-term (hours to few days) effects of light on calcification. They either focused on the development of proxies (strontium ∕ calcium ratio) for parameters of the daily light cycle (Sano et al., 2012) through tracer (strontium) incorporation or aimed to understand environmental and physiological parameters controlling daily trace element incorporation, using the total alkalinity (TA) anomaly technique (Warter et al., 2018). As growth and calcification rates in calcifying organisms are considered to be controlled by the corresponding light intensities (Barnes and Taylor, 1973) and as the penetration of light decreases with depth, so the calcification rate is expected to decrease (Goreau, 1963). Tridacna maxima, the most abundant giant clam species in the Red Sea, can be found on shallow reef flats and edges, usually shallower than 10 m, where light intensity is high due to these transparent waters of tropical, oligotrophic oceans (Van Wynsberge et al., 2016). Although tridacnid clams are one of the most dominant and charismatic molluscan taxa in the Red Sea (Zuschin et al., 2000), little is known about their ecology in this area. In addition, the majority of studies on Tridacninae in the region exclusively focused on the Gulf of Aqaba in the northern Red Sea (Roa-Quiaoit, 2005; Jantzen et al., 2008; Richter et al., 2008), which represents less than 2 % of the entire basin of the Red Sea (Berumen et al., 2013).
In the present study, we assessed the net calcification rates (as µmol calcium carbonate per hour) of T. maxima in two short incubation experiments under seven different incident light levels (corresponding to a water depth of 0–14 m) and in the dark, as well as photosynthetic rates at three experimental light levels corresponding to the high-light conditions in shallow waters (0–4 m). Further, we assessed in situ abundances of T. maxima in different depth zones (0.5–11 m) at a sheltered reef and an exposed reef in the central Red Sea. To our knowledge, this is the first study quantifying the light dependence of short-term net calcification rates of tridacnid clams of the Red Sea, interrelating these rates to their abundances in the field.
2.1 Clam abundance surveys
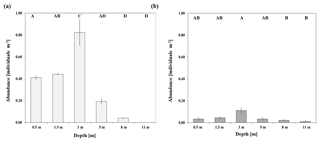
Abundance surveys on T. maxima were conducted either via snorkelling or SCUBA diving at two reefs in the eastern central Red Sea (Fig. 1). The first station was Abu Shosha (22.303833∘ N, 39.048278∘ E), a small inshore reef, where abundances were examined at the sheltered, leeward side (south-east) of the reef which are relatively protected from wave action and currents (Khalil et al., 2013). Additionally, abundances were assessed at a second station (20.753764∘ N, 39.442561∘ E), a fringing reef close to Almojermah, where we conducted transects at the exposed, windward side (north-west) of the reef. At both stations, belt transects were conducted at six different depths (0.5, 1.5, 3, 5, 8, and 11 m). At the sheltered reef, a total area of 1000 m2 was covered, and we conducted six transects at each depth. At the exposed reef 560 m2 were covered, with three transects at each depth. Transect lines of 25 m were deployed and all T. maxima specimens within 2 m of the transect were counted (e.g. 50 m2 area was covered on each transect). In addition, their length (maximum anterior to posterior distance) was recorded at the sheltered reef, using a measuring tape to the nearest centimetre.
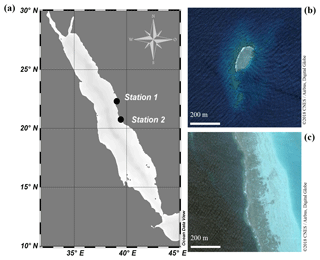
Figure 1(a) Map of the Red Sea. Abundance surveys and sampling of clams for incubation experiments were conducted at both a sheltered reef (Station 1; 22.303833∘ N, 39.048278∘ E) and an exposed reef (Station 2; 20.753764∘ N, 39.442561∘ E). (b) Satellite image of the sheltered reef (Station 1). (c) Satellite image of the exposed, fringing reef (Station 2).
2.2 Clam incubations to obtain net calcification rates
We determined net calcification (see Sect. 2.4 below) in T. maxima during two consecutive incubation experiments. During the first incubations, conducted in December 2016, we assessed net calcification of T. maxima under four different, moderate experimental light levels, mimicking light intensities at different water depths ranging from 4 to 14 m and during a dark incubation. In November 2016, 20 specimens of T. maxima (shell length of 17±2 cm; mean ± SD) were collected at a water depth of about 4 m at a sheltered reef site (Station 1) (Fig. 2). As T. maxima is often embedded in the substrate, specimens were removed by carefully cutting their byssus with a knife. The incubations took place in December 2016 at the Coastal and Marine Resources Core Lab (CMOR) of King Abdullah University of Science and Technology (KAUST) in Thuwal, Saudi Arabia. The experimental setup consisted of 10 flow-through independent LDPE (low-density polyethylene) outdoor aquaria (30 L). Each aquarium contained 2 clams (in total 20), cleaned with a brush from epibionts prior to the experiment. Aquaria were supplied with water by gravity through an intermediate PVC (polyvinylchloride) tank of 77 L, itself receiving water pumped from the adjacent Red Sea surface water at a flow of 0.22 m3 h−1, leading to a complete water exchange in each single aquarium every 80 min. To maintain ambient Red Sea surface water temperatures, all aquaria were immerged to the last top centimetre in a large flow-through pool of 12 m3, receiving the overflowing water from the intermediate tank and the 10 experimental tanks. An Exo1 probe (YSI Incorporated, Yellow Springs, USA) was used to log water temperature and salinity at 30 min frequency. Both remained constant during the experimental period, with an average temperature of 27.2±0.8 ∘C (mean ± SD, n=672) and salinity of 38.4±0.8 (mean ± SD, n=672). Experimental aquaria were shaded with nets to reproduce light levels that mimicked natural conditions at different depths on the reef. We conducted short-term incubations of 6 h (from approximately 09:30 to 15:30 mean solar time) under four different shadings and one dark incubation (at night) (n=10), allowing a 3 d acclimatization period to the clams prior to each incubation. During the incubations, the flow-through system was turned off in order to determine changes in seawater carbon chemistry over time as a measure for calcification processes. Photosynthetically active radiation (PAR) was recorded with a light logger (Odyssey Logger, Dataflow Systems Ltd., New Zealand) as µmol quanta m−2 s−1 and averaged over the incubation period, as natural light conditions fluctuated over the course of the day. Experimental light levels comprised 530, 358, 244, and 197 µmol quanta m−2 s−1. Using data on depth-dependent decrease in light levels (Dishon et al., 2012), we calculated the extinction of light with water depth. The experimental irradiation levels therefore correspond to incident light conditions at about 4, 8, 12, and 14 m water depths. No additional food was provided, as natural and unfiltered seawater was flowing into the tanks. During the subsequent incubation, conducted in April 2018, we examined net calcification and primary production of T. maxima under three additional experimental high-light levels, addressing light effects encountered in very shallow waters (between 0 and 4 m). We collected eight specimens of T. maxima (shell length of 17±1 cm; mean ± SD) at a water depth of about 4 m at an exposed, fringing reef close to Almojermah (Station 2) (Fig. 2). The incubation experiment was conducted onboard R/V Thuwal in a setup consisting of two big PVC flow-through tanks (350 L each) containing nine individual PVC tanks (10 L), eight of them containing one clam each (cleaned from epibionts) and one serving as a control tank. To maintain ambient Red Sea surface water temperatures, all aquaria were immerged into the flow-through pool and water was constantly pumped (0.36 m3 h−1), ensuring a constant water exchange and movement in the individual tanks. Temperature and salinity were checked four times a day using a handheld CTD (conductivity, temperature, depth) probe (CastAway-CTD, SonTek, USA). Both remained constant during the experimental period, with an average temperature of 31.5±0.3 ∘C (mean ± SD, n=16) and salinity of 38.2±0.1 (mean ± SD, n=16). During the incubations, the individual tanks were closed air-tight with see-through PVC lids and water movement was generated with battery-driven motors (Underwater motor, Playmobil, Germany). Nets were used for shading and therefore to reproduce light levels that mimicked natural conditions at different depths on the reef. We conducted closed short-term incubations of 3 h (from approx. 11:00 to 14:00 mean solar time) under three different shadings and one dark incubation (at night), allowing 1 d acclimatization to the clams prior to each incubation. Measurements of PAR intensities were identical to the first round of incubations. Experimental light levels comprised 561, 959, and 1061 µmol quanta m−2 s−1. The amount of light received by the highest experimental light level was identical to light received directly at the water surface in the reef of collection at the same time of the day. Experimental irradiation levels correspond to incident light conditions at 0, 0.5, and 4 m. No additional food was provided, as raw unfiltered seawater was used.
2.3 Carbonate chemistry
At the start, after 3 h and after 6 h of incubation, seawater was sampled from each experimental aquarium in gas-tight 100 mL borosilicate bottles (Schott Duran, Germany) and poisoned with mercury chloride, following Dickson et al. (2007). Each sample was analysed for TA by open-cell titration with an AS-ALK2 titrator (Apollo SciTech,USA) using certified seawater reference material (CRM) (Andrew Dickson, Scripps Institution of Oceanography). During the incubations at moderate light levels (530, 358, 244, and 197 µmol quanta m−2 s−1), additional samples for dissolved inorganic carbon (DIC) were analysed using an AS-C3 infrared DIC analyser (Apollo SciTech, USA). Further components of the carbonate system were calculated with R package Seacarb (Lavigne and Gattuso, 2013) using first and second carbonate system dissociation constants of Millero (2010) as well as the dissociations of HF and (Dickson, 1990; Dickson and Goyet, 1994), respectively. Carbonate chemistry at the beginning of each incubation and in all experimental aquaria were comparable with mean (± SD) TA of 2324±83 and ΩAra of 3.44±0.33 (n=50) during the moderate light incubations and a TA of 2489±38 (n=4) during the high-light incubations (Sect. S1 in the Supplement).
2.4 Net calcification
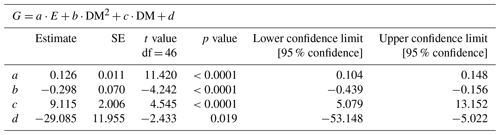
Net calcification (G in µmol CaCO3 h−1) was estimated from changes in total alkalinity (TA) using the alkalinity anomaly technique (Smith and Key, 1975) using the following equation (Eq. 1):
where ΔTA is the variation of TA during the time (t) of the incubations and the factor 2 accounts for a decrease in TA by two equivalents per CaCO3 precipitated (Zeebe and Wolf-Gladrow, 2001). Calcification rates were expressed relative to either mantle surface area (cm2) or tissue dry mass (g). For mantle surface area, the power relationship between standard length in centimetres (L) and mantle area (cm2) (Jantzen et al., 2008) was used to calculate the mantle surface in cm2. For tissue dry mass (DM in gram) of clams, all clams were dissected and their biomass was determined subsequently to the incubation experiment. Clams were opened by cutting the adductor muscle with a scalpel, and the mantle and other tissues were separated from the shells and dried at 60 ∘C for 24 to 48 h to determine tissue DM to the nearest 0.01 g. Experimentally determined net calcification rates (µmol CaCO3 h−1) of T. maxima for different DM under four moderate light levels (197, 244, 358, and 530 µmol quanta m−2 s−1) were used to create a multiple regression modelling net calcification for any given light level and DM.
2.5 Primary production
Primary production was assessed during the high-light incubations (561, 959, and 1061 µmol quanta m−2 s−1) only. Therefore, oxygen (µmol L−1) content in the incubation chambers was automatically logged (miniDOT, Precision Measurement Engineering, Inc., USA) in 15 min intervals over the 3 h incubation period. Net photosynthesis (NPP) was calculated from the variation of oxygen concentration over time and normalized for clam mantle surface area (µmol O2 cm−2 h−1). Dark respiration rates (R), also given in µmol O2 cm−2 h−1, were used to calculate gross primary production (GPP) as Eq. (2):
2.6 Statistical analyses
To assess the comparisons of clam abundance at the six survey depths, an analysis of variance (ANOVA) and pairwise post hoc Tukey analysis (Tukey HSC) were performed. A statistical model was built to explain calcification rates from the combination of PAR and clam tissue dry mass. The model chosen was a multiple non-linear relationship built as the combination of a linear dependency between PAR and calcification rates and a quadratic dependency of net calcification rates and clam tissue mass. This model was selected against other concurrent models by using the Akaike information criterion (AIC) (Anderson and Burnham, 2004). Statistical analyses were performed using R (Foundation for Statistical Computing, Vienna, Austria, Version 3.4.2) and Statistica (Dell Software).
3.1 Depth-dependent abundances
At the sheltered reef site, significantly highest abundances of T. maxima (0.82±0.21 individuals m−2; mean ± SE) were observed at a water depth of 3 m (ANOVA, p<0.001, F=35.6; post hoc Tukey test p<0.001; Sect. S2.1), being twice as high as in shallower waters (between 0.41±0.02 and 0.44±0.01 individuals m−2 mean ± SE; at 0.5 and 1.5 m, respectively) (Fig. 2). No clams were found at the deepest survey depth of 11 m and abundances at 8 m were low, at 0.04±0.01 individual m−2 (mean ± SE). Giant clams were significantly less abundant in deeper water when compared to shallow reef areas (p<0.001 for both 0.5 and 1.5 m when compared to 8 and 11 m). On average, the density of T. maxima at the sheltered reef (0.5–11 m depth) was 0.32±0.05 individuals m−2; mean ± SE). The average size of clams was 16.6±5.1 cm (mean ± SD, n=422) and their calculated mantle surface area was 140.4±90.4 cm2 (mean ± SD, n=422), respectively. At the exposed reef, abundances of T. maxima were overall lower, at 0.04±0.01 individuals m−2 (mean ± SE); however, we also found highest densities of clams at a water depth of 3 m (0.11±0.03 individuals m−2; mean ± SE) (ANOVA, p=0.027, F=3.813; Sect. S2.2). However, they were only significantly higher than those found at 8 and 11 m (with mean ± SE of 0.02±0.01 and 0.01±0.01, respectively) (post hoc Tukey test; Sect. S2.2) (Fig. 2).
3.2 Net calcification and primary production
We combined observed net calcification (as the balance between calcification and dissolution) at all seven experimental incident light levels and the dark incubation and identified a polynomial relationship (R2=0.77) between net calcification (NC, µmol CaCO3 cm−2 h−1) and incident light (I, µmol quanta m−2 s−1) (Eq. 3) (Fig. 3):
Among all light incubations, net calcification rates of T. maxima were highest (mean ± SE 0.65±0.03 µmol CaCO3 cm−2 h−1) at experimental incident light levels of 530 to 561 µmol quanta m−2 s−1 (Fig. 3). T. maxima still showed positive but low net calcification during the night (0.18±0.02 µmol CaCO3 cm−2 h−1; mean ± SE). The lowest NC rates (mean ± SE of 0.01±0.01 µmol CaCO3 cm−2 h−1) were observed at the highest incident irradiance of 1061 µmol quanta m−2 s−1. Overall, we observed a decline in net calcification with both decreasing and increasing light intensities (Table 1), with polynomial regression indicating the maximum calcification (NCmax) to be reached at an incident light level of 475 µmol quanta m−2 s−1. From an incident light level of 1033 µmol quanta m−2 s−1 on, we expect to see dissolution processes outweighing calcification (NCmin, −0.01 µmol CaCO3 cm−2 h−1). Gross primary production (GPP) under the high-light incubations (561, 959, and 1061 µmol quanta m−2 s−1) showed an identical decreasing trend with increase in incident light as observed for net calcification. At 561 µmol quanta m−2 s−1, GPP was highest (2.06±0.24 µmol O2 cm−2 h−1; mean ± SE) and production rates were significantly lower (ANOVA, p=0.039, F=4.982; Sect. S3) during the incubations at 959 and 1061 µmol quanta m−2 s−1 (Table 1, Fig. 3), with mean ± SE of 1.76±0.28 and 0.87±0.37 µmol O2 cm−2 h−1, respectively. Two specimens died after the second highest light treatment of 959 µmol quanta m−2 s−1.
Table 1Net calcification (µmol CaCO3 cm−2 h−1; ± SE) and gross primary production (µmol O2 cm−2 h−1; ± SE) under the seven experimental incident light levels (µmol quanta cm−2 h−1) and in the dark.
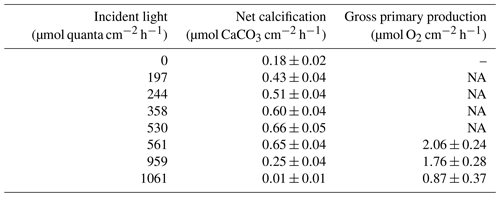
NA – not available
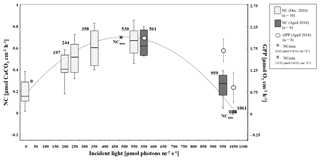
Figure 3Boxplots showing net calcification rates (µmol CaCO3 cm−2 h−1) of T. maxima under seven different light regimes (197, 244, 358, 530, 561, 959, and 1061 µmol quanta m−2 s−1) (n=10 in December 2016 and n=8 in April 2018) and in the dark, as well as gross primary production (µmol O2 cm−2 h−1) (n=8) as dots (± SE), under three high-light regimes (561, 959, and 1061 µmol quanta m−2 s−1). Calculated maximum net calcification (NCmax) at 475 µmol quanta m−2 s−1 and incident light level where dissolution outweighs calcification processes (NCmin) are symbolized by a cross (×). Net calcification rates obtained during incubations under moderate light conditions are symbolized by light grey boxplots and those from the high-light incubations by dark grey boxplots, the central line represents the median, the boxes encompass the central 50 % of the data, and the lines extend to the 95 % quartiles.
We identified a quadratic relationship between net calcification and tissue dry mass, with clams of an intermediate size (DM of about 15 g), showing the highest calcification rates at the four incubations at moderate light level (197, 244, 358, 530 µmol quanta m−2 s−1) (Fig. 4). Therefore, we combined the influence of light and dry mass into a statistical model, explaining 77 % of the variance in observed calcification rates (all parameters p<0.05, Table 2, Fig. 5). Based on this model, we identify maximum rates on clams of an intermediate size (DM of about 15 g), showing the highest calcification rates at the four light-level incubations.
Table 2Description of the statistical model parameters (Fig. 5) combining the influence of irradiance (E, µmol m−2 s−1) and clam dry mass (DM; g) on calcification (G; µmol CaCO3 ind−1 h−1), where d is the fitted intercept.
R2: 0.77
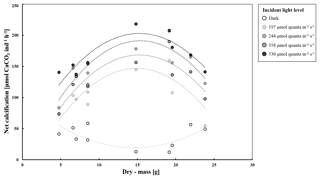
Figure 4Net calcification (µmol CaCO3 ind−1 h−1) (n=10) in T. maxima at four different incident light levels (197, 244, 358, and 531 µmol photons cm−2 h−1) and in the dark, plotted against tissue dry mass (g). Data are shown with polynomial trend lines.
4.1 Depth-dependent abundances
In the Red Sea, T. maxima shows a significant dependence of net calcification rates on incident light. This light dependency is consistent with significantly higher abundances of this species in shallow, sunlit reef flats. Globally, densities of T. maxima range between 0.1 and 0.0001 individuals m−2 (Van Wynsberge et al., 2016), with some exceptions such as at the Ningaloo Marine Park in Western Australia with 0.86 clams m−2 (Black et al., 2011), the Egyptian Sinai Peninsula with a peak value of 0.80 clams m−2 (Roa-Quiaoit, 2005), and 0.42 clams m−2 in Kiribati (Chambers, 2007).
At water depths between 0.5 and 11 m, we found averaged (± SD) abundances of T. maxima of 0.04±0.01 individuals m−2 and 0.32±0.05 individual per m−2 (mean ± SE) at an exposed reef and a sheltered reef, respectively. Abundances at the sheltered reef rank amongst the highest abundances reported worldwide, representing a 50 % higher abundance than previously reported for a local reef (Bodoy, 1984) with 0.22 clams m−2. This difference in average abundances between the two reefs observed in this study could be explained by the leeward and windward (sheltered or exposed, respectively) characters of the examined sites. As reviewed by Van Wynsberge et al. (2016), the “reef type” can influence Tridacna abundances, as it potentially affects the water exchange (and thus water temperature and nutrient availability) as well as the exposure to waves. Similarly to Roa-Quiaoit (2005), we found that T. maxima abundances in the Red Sea seem to display great differences between locations, as we found significant lower numbers of giant clams at the exposed reef, with an average of 0.04±0.01 individuals m−2; (mean ± SE) at water depths between 0.5 and 11 m. Explanations for the observed contrasts in numbers of clams per m2 at both reefs could lie in the probable differences in abiotic environmental conditions at the surveyed sites. For instance, giant clams at the exposed reef are potentially more at risk from high wave action than at the sheltered reef site, which could impact the initial settlement (Jameson, 1976) as well as the survival of juveniles (Foyle et al., 1997), as both have been shown to be influenced by geographical factors (Foyle et al., 1997). While a previous study (Militz et al., 2015), in which abundances of giant clam species in French Polynesia were examined, reports similar patterns for T. crocea, opposite patterns were observed for abundances of T. maxima in that region. In the reefs surveyed by Militz et al. (2015), T. maxima showed higher abundances at reef sites with a high exposure, in comparison to those with low exposure levels. However, additional factors such as temperature and local geomorphology might also have an influence on giant clam densities. Therefore, it is not possible to confidently identify the underlying causes of the observed differences by considering exposure alone. For example, T. maxima specimens from our study, which were located at the more southern reef, could possibly also be exposed to higher surface water temperatures due to the location of this reef at lower latitudes. Mean sea surface annual temperatures of the Red Sea have been shown to increase towards lower latitudes and can be as high as 33 ∘C in the central and southern Red Sea (Chaidez et al., 2017). Further, the local geomorphological features of each reef could influence the light availability of benthic habitats. Consequently, differences in the local topography could have led to different angles of incident light and shading conditions, which would then result in differences between reefs even though the examined depths are identical.
Contrasting findings to previously reported Tridacninae abundances in the central Red Sea could be further a result of differences in sampling depths in the respective studies, as e.g. Bodoy et al. (1984) only accounted for clams at water depths of a maximum of 2 m, while we assessed abundances of T. maxima at six different depths (0.5, 1.5, 3, 5, 8, and 11 m). Previous studies have shown that the depth of abundance surveys significantly impacts the estimates (Van Wynsberge et al., 2016), even though generally, the highest densities of T. maxima are always reported for shallow reefs (0–5 m) (Jantzen et al., 2008; Andréfouët et al., 2009). This is also reflected in the results of previous studies in the Red Sea (Roa-Quiaoit, 2005) showing the highest abundances of T. maxima in shallow water (<3 m). However, Roa-Quiaoit (2005) accumulated abundances at all depths less than 3 m, while we differentiated even between the 0.5, 1.5, and 3 m depth levels and thereby found that although T. maxima shows the highest density at 3 m, abundances in shallower depths are significantly reduced. Furthermore, we found only a few specimens of T. maxima at water depths between 5 and 11 m. This finding is similar to previous studies describing T. maxima as being mostly restricted to reefs shallower than 10 m, principally reef flats and edges (Van Wynsberge et al., 2016). This depth distribution is most likely a result from a trade-off between maximizing light-dependent photosynthesis while minimizing temperature stress, UV irradiation, wave exposure, and/or emersion stress. All these stressors have been previously reported to lead to massive bleaching and mass die-off events in T. maxima (Addessi, 2001) and prevent settlement and recruitment in the shallow waters of the reef flat (Watson et al., 2012). The average size of T. maxima specimens at the sheltered reef was 16.6±5.1 cm (± SD), similar to previous studies on this species in the Red Sea (Roa-Quiaoit, 2005), corresponding, according to the size classification by Manu and Sone (1995), to broodstock (i.e. sexually mature individuals) hermaphrodites. However, the number of small, juvenile specimens (<4 cm) is potentially underestimated, as they are extremely cryptic (Munro and Heslinga, 1983).
4.2 Light-dependent calcification and production in Red Sea giant clam T. maxima
Overall, we found significantly enhanced net calcification rates in Red Sea T. maxima during light incubations compared to the dark incubation. Net calcification rates also significantly increased with light intensity up to 475 µmol photons m−2 s−1 (incident light level corresponding to a water depth of approximately 5 m, at the same time of the day and season, when the incubations were conducted), and thenceforward decrease until an eventual dominance of dissolution over calcification at approximately 1033 µmol photons m−2 s−1 (corresponding to light conditions received directly at the air–water interface in the reef of the collection). Likewise to net calcification in T. maxima, we observed gross primary production (GPP) to be highest at intermediate light levels of around 560 µmol photons m−2 s−1 (corresponding to a water depth of about 4 m) and to decrease with increasing light intensities (at 959 and 1061 µmol quanta m−2 s−1, corresponding to 1.5 and 0.5 m water depth, respectively). We conclude that net calcification in the Red Sea giant clam T. maxima is not only enhanced by light, but is also likely coupled to the photosynthetic activity of their algal symbionts. Further, our results show that both net calcification and primary production in Red Sea T. maxima are highest at incident light levels received at water depths between 5 and 3 m at Red Sea reefs. This is especially noteworthy as these findings correlate with the observed depth-related abundances of T. maxima, displaying the highest densities at intermediate water depths around 3 m in the central Red Sea. The observed irradiance optima for both net calcification and primary production of T. maxima could therefore provide an explanation for the maximum in abundances in intermediate waters (3–5 m) and the decreasing numbers of observed clams at both shallower and deeper reef sites.
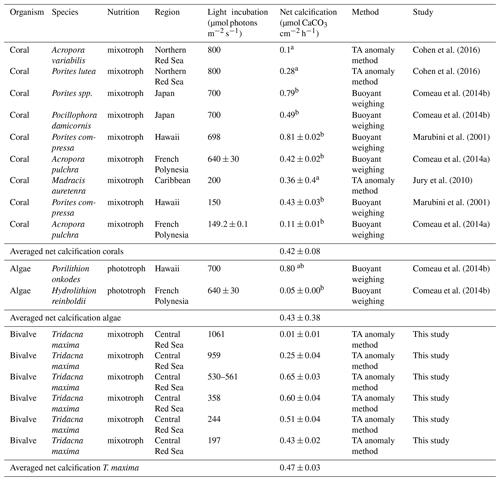
Table 3Comparison of net calcification rates in relation to light conditions in different marine phototrophic and mixotrophic calcifiers. Values are given as average value mean (± SE) or a (± SD). Experimental light incubation levels are given in µmol photons m−2 s−1. Net calcification values were converted to µmol CaCO3 cm−2 h−1 from bmg CaCO3 cm−2 d−1.
Overall, our findings of enhanced calcification rates under light are consistent with reports on the related species Tridacna gigas (Lucas et al., 1989), Tridacna derasa (Sano et al., 2012), and Tridacna squamosa (Adams et al., 2013). The mechanisms of LEC have been intensely studied in zooxanthellate scleractinian corals, leading to several hypotheses proposed to explain LEC (Tambutté et al., 2011). The majority of these refer to mechanisms that are influenced by the symbiotic relationship of host and Symbiodinium, with the most supported hypothesis relating photosynthetic CO2 uptake by the algal symbionts to increased pH and the concentration of carbonate ions, thereby favouring calcification through the corresponding elevated saturation state for carbonate minerals (McConnaughey and Whelan, 1997).
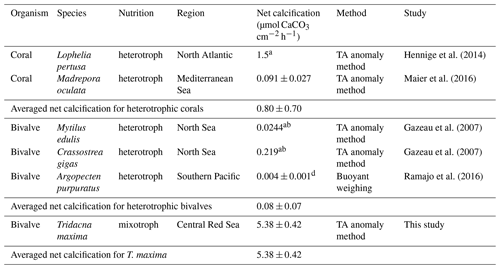
Table 4Comparison of net calcification rates of different marine heterotrophic calcifiers. Values are given as average value mean (± SE) or a (± SD). All net calcification values were normalized for gram dry mass (DM); rates given in fresh weight were converted to DM b following Ricciardi and Bourget (1998) or c Dame (1972). Net calcification values were converted to µmol CaCO3 g DM−1 h−1 from d µg CaCO3 g DM−1 d−1.
Giant clams, including T. maxima, can potentially harbour multiple genera of Symbiodiniaceae simultaneously (DeBoer et al., 2012; Ikeda et al., 2017), including Symbiodinium, Cladocopium, and Durusdinium (previously referred to as clades A, C, and D; LaJeunesse et al., 2018; DeBoer et al., 2012). The composition of these associated algal symbionts might therefore also impact the susceptibility to (high) light levels, as different genera of Symbiodiniaceae (in symbiosis) exhibit different physiological and ecological patterns, including sensitivity to light and temperature (Rowan et al., 1997; Berkelmans and Van Oppen, 2006). However, a previous study on Red Sea giant clams and their associated Symbiodiniaceae (Pappas et al., 2017) reports that T. maxima in the region exclusively associated with Symbiodinium spp. (previously clade A), which was thus assumed to represent an optimal group for the local environmental conditions. However, the reliance of calcification of host organisms (e.g. T. maxima) on their relationship with symbiotic algae could provide an explanation for the significant decrease in net calcification rates at the highest light treatment (1061 µmol photons m−2 s−1). These diminished rates could be the result of photoinhibition and even photodamage of the associated unicellular algae when exposed to these high incident light levels. This would also be supported by the pronounced decrease in gross primary production rates at this light treatment. High incident light levels, especially high levels of UV radiation in shallow waters, have been previously shown to be correlated with decreased calcification rates in other marine calcifiers such as stony corals, e.g. Porites compressa (Kuffner, 2001). However, recent findings for hermatypic corals also report that the contribution by the symbionts might not be the primary or sole driver for LEC, but the blue light spectrum could trigger the light sensors of the host itself, leading to higher calcification rates (Cohen et al., 2016). It is suggested that blue light photoreceptors in coral tissues of Porites lutea and Acropora variabilis could potentially sense the light which is ultimately activating a cascade of processes involved in blue light-enhanced calcification (Cohen et al., 2016). However, our experimental light level, produced by different layers of neutral screen shading, only differed in light intensities but not in the wavelength that T. maxima would receive in the respective water depth. In a previous study on Tridacna crocea, short-term calcification rates were also reported to be strongly light dependent (Warter et al., 2018). However, in this their experiment, Warter et al. (2018) exposed the clams not only to artificial light, but also light levels that were not comparable to actual conditions in the environment, as the average treatment comprised only 162±7 µmol quanta m−2 s−2 (corresponding to a water depth of approximately 16 m in an oligotrophic ocean such as the Red Sea).
4.2.1 Allometric relationship between calcification and biomass
We determined a non-linear relationship between net calcification and biomass (as tissue DM) in T. maxima. Clams of an intermediate DM of approximately 15 g showed the highest net calcification throughout the four incubations as moderate light levels (530, 358, 244, and 197 µmol quanta m−2 s−1). Specimens of a smaller or higher biomass calcified less during the incubations. A similar allometric relationship has been previously described for the photosynthetic metabolic performance of the zooxanthellae in T. maxima (Yau and Fan, 2012). This allometric pattern is most likely due to an optimal ratio of symbionts to clam body mass at intermediate sizes. As the clam grows, its mantle tissue increases in thickness and thus the three dimensional tubular systems bearing the utmost of symbionts (Fisher et al., 1985). However, as the mantle thickens, impinging light must penetrate through more tissue before reaching the stacked zooxanthellae (Trench et al., 1981), and there is evidence of increased shading of the symbionts in the mantles of bigger clams (Fisher et al., 1985). With further increasing size, the number of symbionts per unit clam biomass also decreases (Fisher et al., 1985; Fitt et al., 1993; Griffiths and Klumpp, 1996). In general, growth rates of giant clams seem to decrease with age once they reach the threshold for maturity and become broodstock hermaphrodites (Van Wynsberge et al., 2016). Past this age, a growing portion of their energy is invested in reproduction (Romanek and Grossman, 1989; Van Wynsberge et al., 2016), especially since there is an exponentially increase in produced egg numbers with increasing shell size (Jameson, 1976).
4.2.2 Comparison with other calcifiers
We compared net calcification rates of T. maxima with those of other benthic phototrophic and mixotrophic calcifiers (Table 3). In most calcifying organisms that live in symbiotic relationship with zooxanthellae (such as corals), metabolic rates and calcification are normalized by surface area. In contrast to corals, however, which host their symbiotic algae intracellularly in their endodermal cell layer, the symbionts of Tridacninae are located in delicately branching and specialized channels within the mantle, which extend from the stomach (Trench et al., 1981; Norton et al., 1992). Although this difference makes the comparison to other calcifiers conceptually difficult, normalization of calcification rates per mantle surface area would also be appropriate, as Symbiodinium cells in giant clams are mostly found in the upper 5 mm of the mantle (Ishikura et al., 1997). The Red Sea giant clam T. maxima shows averaged net calcification rates of 0.47±0.03 µmol CaCO3 cm−2 h−1 (mean ± SE), which are comparable to those reported for hermatypic corals (0.42±0.08 µmol CaCO3 cm−2 h−1; mean ± SE) and those for calcifying macroalgae (0.43±0.38 µmol CaCO3 cm−2 h−1; mean ± SE). However, in comparison with averaged rates of other heterotrophic, temperate bivalve species, such as Mytilus edulis, Argopecten purpuratus, and Crassostrea gigas with 0.08±0.07 µmol CaCO3 g DM−1 h−1 (mean ± SD), calcification in T. maxima is about 70 times higher (5.38±0.42 µmol CaCO3 g DM−1 h−1; mean ± SE) (Table 4). When compared to heterotrophic cold-water coral species, such as Lophelia pertusa and Madrepora oculata, net calcification rates of T. maxima are more than 7 times higher (0.80±0.70 µmol CaCO3 g DM−1 h−1; mean ± SD); Table 4). Our comparative assessment of the net calcification rates of giant clams with temperate/azooanthellate species show that rates in the Red Sea T. maxima tested here are comparable to other photosymbiotic organisms (such as corals) and calcifying algae.
The present study shows that net calcification and photosynthetic rates of Red Sea T. maxima are light dependent but show a maximum at intermediate irradiance, suggesting strong inhibition at the highest incident light levels received in very shallow (0–1.5 m) waters. This is consistent with the depth-related distribution of this species in the Red Sea, and elsewhere, which showed maximum abundances in shallow (3 m), sunlit coral reefs but a decrease in abundance from 3 m towards the surface and below. Although enhanced calcification is consequently beneficial for T. maxima, the light dependency of both calcification and production restricts them to shallow waters, which also makes them more vulnerable to potentially harmful environmental changes, such as predicted increasing water temperatures associated with global warming (Hughes et al., 2003) as well as high levels of incident light, including high levels of UV radiation (Shick et al., 1995). The present study provides an important baseline for future studies examining the impact of wavelength-specific responses of calcification and metabolic rates on giant clams as well as for a better overall understanding of light-enhanced calcification in Red Sea Tridacninae and their relationship with the symbiotic algae.
Data on abundance, net calcification, and primary production of Tridacna maxima of this study are available in the following data collection: Rossbach et al. (2019): Abundance, primary production rates and net calcification rates of Tridacna maxima giant clams at two reefs in the Central Red Sea, PANGAEA, https://doi.org/10.1594/PANGAEA.903560.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-16-2635-2019-supplement.
CMD, VS, and SR conceptualized the research; VS and SR collected the animals and performed the abundance surveys. Experimental execution was carried out by SR; AA and VS conducted the data curation and ran formal analyses. SR prepared the first draft of the manuscript and all the co-authors contributed substantially to subsequent versions, including the final draft.
The authors declare that they have no conflict of interest.
We thank Janna Leigh Randle and Felix Ivo Rossbach for assistance with field sampling and the KAUST Coastal and Marine Resources Core Lab for logistical support.
This research was funded by King Abdullah University of Science and Technology (KAUST), through baseline funding to Carlos M. Duarte, and a fellowship of the Visiting Student Research Program to Susann Rossbach.
This paper was edited by Hiroshi Kitazato and reviewed by two anonymous referees.
Adams, A. L., Needham, E. W., and Knauer, J.: The effect of shade on water quality parameters and survival and growth of juvenile fluted giant clams, Tridacna squamosa, cultured in a land-based growth trial, Aquacult. Int., 21, 1311–1324, https://doi.org/10.1007/s10499-013-9634-9, 2013.
Addessi, L.: Giant clam bleaching in the lagoon of Takapoto atoll (French Polynesia), Coral Reefs, 19, 220–220, https://doi.org/10.1007/PL00006957, 2001.
Alcazar, S. N.: Observations on predators of giant clams (Bivalvia: Family Tridacnidae), Silliman Journal, 33, 54–57, 1986.
Allemand, D., Tambutté, É., Zoccola, D., and Tambutté, S.: Coral Calcification, Cells to Reefs, in: Coral Reefs: An Ecosystem in Transition, edited by: Dubinsky, Z. and Stambler, N., Springer, Dordrecht, https://doi.org/10.1007/978-94-007-0114-4_9, 2011.
Anderson, D. R. and Burnham, K.: Model selection and multi-model inference, 2nd Edn., 63 pp., Springer-Verlag, NY, 2004.
Andréfouët, S., Gilbert, A., Yan, L., Remoissenet, G., Payri, C., and Chancerelle, Y.: The remarkable population size of the endangered clam Tridacna maxima assessed in Fangatau Atoll (Eastern Tuamotu, French Polynesia) using in situ and remote sensing data, ICES J. Mar. Sci., 62, 1037–1048, https://doi.org/10.1016/j.icesjms.2005.04.006, 2005.
Andréfouët, S., Friedman, K., Gilbert, A., and Remoissenet, G.: A comparison of two surveys of invertebrates at Pacific Ocean islands: the giant clam at Raivavae Island, Australes Archipelago, French Polynesia, ICES J. Mar. Sci., 66, 1825–1836, https://doi.org/10.1093/icesjms/fsp148, 2009.
Andréfouët, S., Van Wynsberge, S., Gaertner-Mazouni, N., Menkes, C., Gilbert, A., and Remoissenet, G.: Climate variability and massive mortalities challenge giant clam conservation and management efforts in French Polynesia atolls, Biol. Conserv., 160, 190–199, https://doi.org/10.1016/j.biocon.2013.01.017, 2013.
Ashworth, J. S., Ormond, R. F., and Sturrock, H. T.: Effects of reef-top gathering and fishing on invertebrate abundance across take and no-take zones, J. Exp. Mar. Biol. Ecol., 303, 221–242, https://doi.org/10.1016/j.jembe.2003.11.017, 2004.
Barnes, D. J. and Taylor, D. L.: In situ studies of calcification and photosynthetic carbon fixation in the coral Montastrea annularis, Helgoländer Wissenschaftliche Meeresuntersuchungen, 24, 284, 1973.
Beckvar, N.: Cultivation, spawning, and growth of the giant clams Tridacna gigas, T. derasa, and T. squamosa in Palau, Caroline Islands, Aquaculture, 24, 21–30, https://doi.org/10.1016/0044-8486(81)90040-5, 1981.
Belda-Baillie, C., Leggat, W., and Yellowlees, D.: Growth and metabolic responses of the giant clam-zooxanthellae symbiosis in a reef-fertilisation experiment, Mar. Ecol.-Prog. Ser., 170, 131–141, https://doi.org/10.3354/meps170131, 1998.
Bell, J., Lane, I., Gervis, M., Soule, S., and Tafea, H.: Village-based farming of the giant clam, Tridacna gigas (L.), for the aquarium market: initial trials in Solomon Islands, Aquacult. Res., 28, 121–128, https://doi.org/10.1046/j.1365-2109.1997.t01-1-00834.x, 1997.
Berkelmans, R. and Van Oppen, M. J.: The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change, P. Roy. Soc. Lond. B Bio., 273, 2305–2312, 2006.
Bertucci, A., Foret, S., Ball, E., and Miller, D. J.: Transcriptomic differences between day and night in Acropora millepora provide new insights into metabolite exchange and light-enhanced calcification in corals, Mol. Ecol., 24, 4489–4504, https://doi.org/10.1111/mec.13328, 2015.
Berumen, M. L., Hoey, A., Bass, W. H., Bouwmeester, J., Catania, D., Cochran, J. E., Khalil, M. T., Miyake, S., Mughal, M., and Spät, J. L.: The status of coral reef ecology research in the Red Sea, Coral Reefs, 32, 737–748, https://doi.org/10.1007/s00338-013-1055-8, 2013.
Black, R., Johnson, M. S., Prince, J., Brearley, A., and Bond, T.: Evidence of large, local variations in recruitment and mortality in the small giant clam, Tridacna maxima, at Ningaloo Marine Park, Western Australia, Mar. Freshwater Res., 62, 1318–1326, https://doi.org/10.1071/MF11093, 2011.
Bodoy, A. L. A. I. N.: Assessment of human impact on giant clams (Tridacna maxima) near Jeddah, Saudi Arabia, Proc. Symp. Coral Reef Environ, Red Sea, Jeddah, Saudi Arabia, 472–490, 1984.
Chaidez, V., Dreano, D., Agusti, S., Duarte, C. M., and Hoteit, I.: Decadal trends in Red Sea maximum surface temperature, Sci. Rep.-UK, 7, 8144, https://doi.org/10.1038/s41598-017-08146-z, 2017.
Chambers, C. N.: Pasua (Tridacna maxima) size and abundance in Tongareva Lagoon, Cook Islands, SPC Trochus Information Bulletin, 13, 7–12, 2007.
Chan, C. Y., Hiong, K. C., Boo, M. V., Choo, C. Y., Wong, W. P., Chew, S. F., and Ip, Y. K.: Light exposure enhances urea absorption in the fluted giant clam, Tridacna squamosa, and up-regulates the protein abundance of a light-dependent urea active transporter, DUR3-like, in its ctenidium, J. Exp. Biol., 221, 176313, https://doi.org/10.1242/jeb.176313, 2018.
Chew, S. F., Koh, C. Z., Hiong, K. C., Choo, C. Y., Wong, W. P., Neo, M. L., and Ip, Y. K.: Light-enhanced expression of Carbonic Anhydrase 4-like supports shell formation in the fluted giant clam Tridacna squamosa, Gene, 683, 101–112, https://doi.org/10.1016/j.gene.2018.10.023, 2019.
Cohen, I., Dubinsky, Z., and Erez, J.: Light Enhanced Calcification in Hermatypic Corals: New Insights from Light Spectral Responses, Front. Mar. Sci., 2, 122, https://doi.org/10.3389/fmars.2015.00122, 2016.
Comeau, S., Carpenter, R. C., and Edmunds, P. J.: Effects of irradiance on the response of the coral Acropora pulchra and the calcifying alga Hydrolithon reinboldii to temperature elevation and ocean acidification, J. Exp. Mar. Biol. Ecol., 453, 28–35, https://doi.org/10.1016/j.jembe.2013.12.013, 2014a.
Comeau, S., Carpenter, R. C., Nojiri, Y., Putnam, H. M., Sakai, K., and Edmunds, P. J.: Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification, P. Roy. Soc. B, 281, 20141339, https://doi.org/10.1098/rspb.2014.1339, 2014b.
Dame, R. F.: The ecological energies of growth, respiration and assimilation in the intertidal American oyster Crassostrea virginica, Mar. Biol., 17, 243–250, https://doi.org/10.1007/BF00366299, 1972.
DeBoer, T. S., Baker, A. C., Erdmann, M. V., Jones, P. R., and Barber, P. H.: Patterns of Symbiodinium distribution in three giant clam species across the biodiverse Bird's Head region of Indonesia, Mar. Ecol.-Prog. Ser., 444, 117–132, 2012.
De Grave, S.: Pontoniinae (Crustacea: Decapoda: Palaemonidea) associated with bivalve molluscs from Hansa Bay, Papua New Guinea, Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Biologie = Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie, 1999.
Dickson, A.: The oceanic carbon dioxide system: planning for quality data, US JGOFS News, 2, 2, 1990.
Dickson, A. G. and Goyet, C.: Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water, Version 2 (No. ORNL/CDIAC-74), Oak Ridge National Lab., TN, USA, https://doi.org/10.2172/10107773, 1994.
Dickson, A. G., Sabine, C. L., and Christian, J. R.: Guide to best practices for ocean CO2 measurements, North Pacific Marine Science Organization, Sidney, British Columbia, 2007.
Dishon, G., Dubinsky, Z., Fine, M., and Iluz, D.: Underwater light field patterns in subtropical coastal waters: A case study from the Gulf of Eilat (Aqaba), Isr. J. Plant Sci., 60, 265–275, https://doi.org/10.1560/ijps.60.1-2.265, 2012.
Fisher, C. R., Fitt, W. K., and Trench, R. K.: Photosynthesis and respiration in Tridacna gigas as a function of irradiance and size, The Biol. Bull., 169, 230–245, https://doi.org/10.2307/1541400, 1985.
Fitt, W., Rees, T., Braley, R., Lucas, J., and Yellowlees, D.: Nitrogen flux in giant clams: size-dependency and relationship to zooxanthellae density and clam biomass in the uptake of dissolved inorganic nitrogen, Mar. Biol., 117, 381–386, 1993.
Foyle, T. P., Bell, J. D., Gervis, M., and Lane, I.: Survival and growth of juvenile fluted giant clams, Tridacna squamosa, in large-scale grow-out trials in the Solomon Islands, Aquaculture, 148, 85–104, 1997.
Gazeau, F., Quiblier, C., Jansen, J. M., Gattuso, J. P., Middelburg, J. J., and Heip, C. H.: Impact of elevated CO2 on shellfish calcification, Geophys. Res. Lett., 34, L07603, https://doi.org/10.1029/2006GL028554, 2007.
Gomez, E. D. and Mingoa-Licuanan, S. S.: Achievements and lessons learned in restocking giant clams in the Philippines, Fish. Res., 80, 46–52, https://doi.org/10.1016/j.fishres.2006.03.017, 2006.
Goreau, T. F.: Calcium carbonate deposition by coralline algae and corals in relation to their roles as reef-builders, Ann. NY Acad. Sci., 109, 127–167, https://doi.org/10.1111/j.1749-6632.1963.tb13465.x, 1963.
Griffiths, C. and Klumpp, D.: Relationships between size, mantle area and zooxanthellae numbers in five species of giant clam (Tridacnidae), Mar. Ecol.-Prog. Ser., 139–147, https://doi.org/10.3354/meps137139, 1996.
Hart, A. M., Bell, J. D., and Foyle, T. P.: Growth and survival of the giant clams, Tridacna derasa, T. maxima and T. crocea, at village farms in the Solomon Islands, Aquaculture, 165, 203–220, https://doi.org/10.1016/S0044-8486(98)00255-5, 1998.
Hastie, L., Watson, T., Isamu, T., and Heslinga, G.: Effect of nutrient enrichment on Tridacna derasa seed: dissolved inorganic nitrogen increases growth rate, Aquaculture, 106, 41–49, https://doi.org/10.1016/0044-8486(92)90248-J, 1992.
Hennige, S. J., Wicks, L. C., Kamenos, N. A., Bakker, D. C., Findlay, H. S., Dumousseaud, C., and Roberts, J. M.: Short-term metabolic and growth responses of the cold-water coral Lophelia pertusa to ocean acidification, Deep-Sea Res. Pt. II, 99, 27–35, https://doi.org/10.1016/j.dsr2.2013.07.005, 2014.
Hiong, K. C., Cao-Pham, A. H., Choo, C. Y., Boo, M. V., Wong, W. P., Chew, S. F., and Ip, Y. K.: Light-dependent expression of a exchanger 3-like transporter in the ctenidium of the giant clam, Tridacna squamosa, can be related to increased H+ excretion during light-enhanced calcification, Physiological Reports, 5, e13209, https://doi.org/10.14814/phy2.13209, 2017a.
Hiong, K. C., Choo, C. Y., Boo, M. V., Ching, B., Wong, W. P., Chew, S. F., and Ip, Y. K.: A light-dependent ammonia-assimilating mechanism in the ctenidia of a giant clam, Coral Reefs, 36, 311–323, https://doi.org/10.1007/s00338-016-1502-4, 2017b.
Hoegh-Guldberg, O.: Effect of nutrient enrichment in the field on the biomass, growth and calcification of the giant clam Tridacna maxima, Mar. Biol., 129, 635–642, https://doi.org/10.1007/s002270050206, 1997.
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., Grosberg, R., Hoegh-Guldberg, O., Jackson, J., and Kleypas, J.: Climate change, human impacts, and the resilience of coral reefs, Science, 301, 929–933, 2003.
Hviding, E.: The rural context of giant clam mariculture in Solomon Islands: an anthropological study, WorldFish, International Center for Living Aquatic Resources Management, Makati, Philippines, 1993.
Ikeda, S., Yamashita, H., Kondo, S. N., Inoue, K., Morishima, S. Y., and Koike, K.: Zooxanthellal genetic varieties in giant clams are partially determined by species-intrinsic and growth-related characteristics, PloS one, 12, e0172285, https://doi.org/10.1371/journal.pone.0172285, 2017.
Ip, Y. K., Loong, A. M., Hiong, K. C., Wong, W. P., Chew, S. F., Reddy, K., Sivaloganathan, B., and Ballantyne, J. S.: Light induces an increase in the pH of and a decrease in the ammonia concentration in the extrapallial fluid of the giant clam Tridacna squamosa, Physiol. Biochem. Zool., 79, 656–664, https://doi.org/10.1086/501061, 2006.
Ip, Y. K., Ching, B., Hiong, K. C., Choo, C. Y., Boo, M. V., Wong, W. P., and Chew, S. F.: Light induces changes in activities of -ATPase, -ATPase and glutamine synthetase in tissues involved directly or indirectly in light-enhanced calcification in the giant clam, Tridacna squamosa, Front. Physiol., 6, 68, https://doi.org/10.3389/fphys.2015.00068, 2015.
Ip, Y. K., Hiong, K. C., Goh, E. J. K., Boo, M. V., Choo, C. Y. L., Ching, B., Wong, W. P., and Chew, S. F.: The Whitish Inner Mantle of the Giant Clam, Tridacna squamosa, Expresses an Apical Plasma Membrane Ca2+-ATPase (PMCA) Which Displays Light-Dependent Gene and Protein Expressions, Front. Physiol., 8, 781, https://doi.org/10.3389/fphys.2017.00781, 2017.
Ishikura, M., Kato, C., and Maruyama, T.: UV-absorbing substances in zooxanthellate and azooxanthellate clams, Mar. Biol., 128, 649–655, https://doi.org/10.1007/s002270050131, 1997.
IUCN: Red List of Threatened Species, Version 2016-3, available at: https://www.iucnredlist.org (last access: December 2018), 2016.
Jameson, S. C.: Early life history of the giant clams Tridacna crocea, Lamarck, Tridacna maxima (Roding), and Hippopus hippopus (Linnaeus), Pac. Sci., 30, 219–233, 1976.
Jantzen, C., Wild, C., El-Zibdah, M., Roa-Quiaoit, H. A., Haacke, C., and Richter, C.: Photosynthetic performance of giant clams, Tridacna maxima and T. squamosa, Red Sea, Mar. Biol., 155, 211–221, https://doi.org/10.1007/s00227-008-1019-7, 2008.
Jury, C. P., Whitehead, R. F., and Szmant, A. M.: Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (= Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates, Glob. Change Biol., 16, 1632–1644, https://doi.org/10.1111/j.1365-2486.2009.02057.x, 2010.
Khalil, M. T., Cochran, J. E., and Berumen, M. L.: The abundance of herbivorous fish on an inshore Red Sea reef following a mass coral bleaching event, Environ. Biol. Fish., 96, 1065–1072, https://doi.org/10.1007/s10641-012-0103-5, 2013.
Klumpp, D. and Griffiths, C.: Contributions of phototrophic and heterotrophic nutrition to the metabolic and growth requirements of four species of giant clam (Tridacnidae), Mar. Ecol.-Prog. Ser., 115, 103–115, 1994.
Klumpp, D., Bayne, B., and Hawkins, A.: Nutrition of the giant clam Tridacna gigas (L.) I. Contribution of filter feeding and photosynthates to respiration and growth, J. Exp. Mar. Biol. Ecol., 155, 105–122, https://doi.org/10.1016/0022-0981(92)90030-E, 1992.
Kuffner, I.: Effects of ultraviolet radiation and water motion on the reef coral Porites compressa Dana: a flume experiment, Mar. Biol., 138, 467–476, 2001.
LaJeunesse, T. C., Parkinson, J. E., Gabrielson, P. W., Jeong, H. J., Reimer, J. D., Voolstra, C. R., and Santos, S. R.: Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts, Curr. Biol., 28, 2570–2580, https://doi.org/10.1016/j.cub.2018.07.008, 2018.
Lavigne, H. and Gattuso, J.: Package “seacarb”: seawater carbonate chemistry with R, v. 2.4. 8, edtited by: R Development Core Team, available at: https://cran.r-project.org/web/packages/ seacarb/index.html (last access: March 2018), 2013.
Lucas, J., Nash, W., Crawford, C., and Braley, R.: Environmental influences on growth and survival during the ocean-nursery rearing of giant clams, Tridacna gigas (L.), Aquaculture, 80, 45–61, https://doi.org/10.1016/0044-8486(89)90272-X, 1989.
Lucas, J. S.: The biology, exploitation, and mariculture of giant clams (Tridacnidae), Rev. Fish. Sci., 2, 181–223, https://doi.org/10.1080/10641269409388557, 1994.
Maier, C., Popp, P., Sollfrank, N., Weinbauer, M. G., Wild, C., and Gattuso, J. P.: Effects of elevated pCO2 and feeding on net calcification and energy budget of the Mediterranean cold-water coral Madrepora oculata, J. Exp. Biol., 219, 3208–3217, https://doi.org/10.1242/jeb.127159, 2016.
Manu, N. and Sone, S.: Breeding season of the Tongan shellfish. 3. Elongated giant clam, Tridacna maxima, Fish. Res. Bull. Tonga, 3, 25–33, 1995.
Marubini, F., Barnett, H., Langdon, C., and Atkinson, M. J.: Dependence of calcification on light and carbonate ion concentration for the hermatypic coral Porites compressa, Mar. Ecol.-Prog. Ser., 220, 153–162, https://doi.org/10.3354/meps220153, 2001.
McConnaughey, T. and Whelan, J.: Calcification generates protons for nutrient and bicarbonate uptake, Earth-Sci. Rev., 42, 95–117, https://doi.org/10.1016/S0012-8252(96)00036-0, 1997.
Militz, T. A., Kinch, J., and Southgate, P. C.: Population demographics of Tridacna noae (Röding, 1798) in New Ireland, Papua New Guinea, J. Shellfish Res., 34, 329–336, 2015.
Millero, F. J.: Carbonate constants for estuarine waters, Mar. Freshwater Res., 61, 139–142, https://doi.org/10.1071/MF09254, 2010.
Moya, A., Tambutté, S., Bertucci, A., Tambutté, E., Lotto, S., Vullo, D., Supuran, C. T., Allemand, D., and Zoccola, D.: Carbonic anhydrase in the scleractinian coral Stylophora pistillata characterization, localization, and role in biomineralization, J. Biol. Chem., 283, 25475–25484, https://doi.org/10.1074/jbc.M804726200, 2008.
Munro, J. and Heslinga, G.: Prospects for the commercial cultivation of Giant Clams, Micronesian Mariculture Demonstration Center, Palau, Caroline Islands, 1983.
Neo, M. L., Eckman, W., Vicentuan, K., Teo, S. L.-M., and Todd, P. A.: The ecological significance of giant clams in coral reef ecosystems, Biol. Conserv., 181, 111–123, https://doi.org/10.1016/j.biocon.2014.11.004, 2015.
Neo, M. L., Wabnitz, C. C., Braley, R. D., Heslinga, G. A., Fauvelot, C., Van Wynsberge, S., Andrefouet, S., Waters, C., Tan, A. S.-H., and Gomez, E. D.: Giant clams (Bivalvia: Cardiidae: Tridacninae): a comprehensive update of species and their distribution, current threats and conservation status, Oceanogr. Mar. Biol., 55, 87–388, 2017.
Norton, J. H., Shepherd, M. A., Long, H. M., and Fitt, W. K.: The zooxanthellal tubular system in the giant clam, Biol. Bull., 183, 503–506, https://doi.org/10.2307/1542028, 1992.
Pappas, M. K., He, S., Hardenstine, R. S., Kanee, H., and Berumen, M. L.: Genetic diversity of giant clams (Tridacna spp.) and their associated Symbiodinium in the central Red Sea, Mar. Biol., 47, 1209–1222, https://doi.org/10.1007/s12526-017-0715-2, 2017.
Ramajo, L., Marbà, N., Prado, L., Peron, S., Lardies, M. A., Rodriguez-Navarro, A. B., Vargas, C. A., Lagos, N. A., and Duarte, C. M.: Biomineralization changes with food supply confer juvenile scallops (Argopecten purpuratus) resistance to ocean acidification, Glob. Change Biol., 22, 2025–2037, https://doi.org/10.1111/gcb.13179, 2016.
Ricciardi, A. and Bourget, E.: Weight-to-weight conversion factors for marine benthic macroinvertebrates, Mar. Ecol.-Prog. Ser., 163, 245–251, https://doi.org/10.3354/meps163245, 1998.
Richter, C., Roa-Quiaoit, H., Jantzen, C., Al-Zibdah, M., and Kochzius, M.: Collapse of a new living species of giant clam in the Red Sea, Curr. Biol., 18, 1349–1354, https://doi.org/10.1016/j.cub.2008.07.060, 2008.
Roa-Quiaoit, H. A. F.: The ecology and culture of giant clams (Tridacnidae) in the Jordanian sector of the Gulf of Aqaba, Red Sea, PhD thesis, Universitat Bremen, 2005.
Romanek, C. S. and Grossman, E. L.: Stable isotope profiles of Tridacna maxima as environmental indicators, Palaios, 4, 402–413, https://doi.org/10.2307/3514585, 1989.
Rosewater, J.: The family Tridacnidae in the Indo-Pacific, Department of Mollusks, Academy of Natural Sciences of Philadelphia, 1965.
Rossbach, S., Saderne, V., Anton, A., and Duarte, C. M.: Abundance, primary production rates and net calcification rates of Tridacna maxima giant clams at two reefs in the Central Red Sea, PANGAEA, https://doi.org/10.1594/PANGAEA.903560, 2019.
Rowan, R., Knowlton, N., Baker, A., and Jara, J.: Landscape ecology of algal symbionts creates variation in episodes of coral bleaching, Nature, 388, 265, https://doi.org/10.1038/40843, 1997.
Sano, Y., Kobayashi, S., Shirai, K., Takahata, N., Matsumoto, K., Watanabe, T., Sowa, K., and Iwai, K.: Past daily light cycle recorded in the strontium/calcium ratios of giant clam shells, Nat. Commun., 3, 761, https://doi.org/10.1038/ncomms1763, 2012.
Schwartzmann, C., Durrieu, G., Sow, M., Ciret, P., Lazareth, C. E., and Massabuau, J.-C.: In situ giant clam growth rate behavior in relation to temperature: A one-year coupled study of high-frequency noninvasive valvometry and sclerochronology, Limnol. Oceanogr., 56, 1940–1951, https://doi.org/10.4319/lo.2011.56.5.1940, 2011.
Shick, J., Lesser, M., Dunlap, W., Stochaj, W., Chalker, B., and Won, J. W.: Depth-dependent responses to solar ultraviolet radiation and oxidative stress in the zooxanthellate coral Acropora microphthalma, Mar. Biol., 122, 41–51, 1995.
Simkiss, K.: Phosphates as crystal poisons of calcification, Biol. Rev., 39, 487–504, https://doi.org/10.1111/j.1469-185X.1964.tb01166.x, 1964.
Smith, S. and Key, G.: Carbon dioxide and metabolism in marine environments, Limnol. Oceanogr., 20, 493–495, https://doi.org/10.4319/lo.1975.20.3.0493, 1975.
Taylor, D. L.: Identity of zooxanthellae isolated from some Pacific Tridacnidae, J. Phycol., 5, 336–340, https://doi.org/10.1111/j.1529-8817.1969.tb02623.x, 1969.
Trench, R., Wethey, D., and Porter, J.: Observations on the symbiosis with zooxanthellae among the Tridacnidae (Mollusca, Bivalvia), Biol. Bull., 161, 180–198, https://doi.org/10.2307/1541117, 1981.
Van Wynsberge, S., Andréfouët, S., Gaertner-Mazouni, N., Wabnitz, C. C. C., Gilbert, A., Remoissenet, G., Payri, C., and Fauvelot, C.: Drivers of density for the exploited giant clam Tridacna maxima: a meta-analysis, Fish Fish., 17, 567–584, https://doi.org/10.1111/faf.12127, 2016.
Vicentuan-Cabaitan, K., Neo, M. L., Eckman, W., Teo, S. L., and Todd, P. A.: Giant clam shells host a multitude of epibionts, B. Mar. Sci., 90, 795–796, https://doi.org/10.5343/bms.2014.1010, 2014.
Warter, V., Erez, J., and Müller, W.: Environmental and physiological controls on daily trace element incorporation in Tridacna crocea from combined laboratory culturing and ultra-high resolution LA-ICP-MS analysis, Palaeogeogr. Palaeocl., 496, 32–47, https://doi.org/10.1016/j.palaeo.2017.12.038, 2018.
Watson, S.-A., Southgate, P. C., Miller, G. M., Moorhead, J. A., and Knauer, J.: Ocean acidification and warming reduce juvenile survival of the fluted giant clam, Tridacna squamosa, Molluscan Res., 32, 177–180, 2012.
Yau, A. J.-Y. and Fan, T.-Y.: Size-dependent photosynthetic performance in the giant clam Tridacna maxima, a mixotrophic marine bivalve, Mar. Biol., 159, 65–75, https://doi.org/10.1007/s00227-011-1790-8, 2012.
Yonge, C. M.: The significance of the relationship between corals and zooxanthellae, Nature, 128, 309–311, 1931.
Yonge, C.: Giant clams, Sci. Am., 232, 96–105, 1975.
Yonge, C. M.: Mode of life, feeding, digesting and symbiosis with zooxanthellae, Scientific Reports/Great Barrier Reef Expedition, 1, 283–321, 1936.
Zeebe, R. E. and Wolf-Gladrow, D. A.: CO2 in seawater: equilibrium, kinetics, isotopes, Elsevier Oceanography Series, 65, edited by: Halpern, D., Gulf Professional Publishing, Amsterdam, the Netherlands, 2001.
Zuschin, M., Hohenegger, J., and Steininger, F. F.: A comparison of living and dead molluscs on coral reef associated hard substrata in the northern Red Sea – implications for the fossil record, Palaeogeogr. Palaeocl., 159, 167–190, https://doi.org/10.1016/S0031-0182(00)00045-6, 2000.