the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Vertical transport of sediment-associated metals and cyanobacteria by ebullition in a stratified lake
Kyle Delwiche
Junyao Gu
Harold Hemond
Sarah P. Preheim
Bubbles adsorb and transport particulate matter in a variety of natural and engineered settings, including industrial, freshwater, and marine systems. While methane-containing bubbles emitted from anoxic sediments are found widely in freshwater ecosystems, relatively little attention has been paid to the possibility that these bubbles transport particle-associated chemical or biological material from sediments to surface waters of freshwater lakes. We triggered ebullition and quantified transport of particulate material from sediments to the surface by bubbles in Upper Mystic Lake, MA, and in a 15 m tall experimental column. Particle transport was positively correlated with the volume of gas bubbles released from the sediment, and particles transported by bubbles appear to originate almost entirely in the sediment, rather than being scavenged from the water column. Concentrations of arsenic, chromium, lead, and cyanobacterial cells in bubble-transported particulate material were similar to those of bulk sediment, and particles were transported from depths exceeding 15 m, implying the potential for daily average fluxes as large as 0.18 µg arsenic m−2 and 2×104 cyanobacteria cells m−2 in the strongly stratified Upper Mystic Lake. Bubble-facilitated arsenic transport currently appears to be a modest component of total arsenic cycling in this lake. Although more work is needed to reduce uncertainty in budget estimates, bubble-facilitated cyanobacterial transport has the potential to contribute substantially to the cyanobacteria cell recruitment to the surface of this lake and may thus be of particular importance in large, deep, stratified lakes.
- Article
(723 KB) - Full-text XML
-
Supplement
(2593 KB) - BibTeX
- EndNote
Deterioration of water quality is widespread and expected to become more acute with increased urbanization and climate change (Zhang, 2016; Paerl et al., 2011). In a 2012 national assessment, 15.2 % of surveyed lakes in the US were categorized as “most disturbed” due to the concentration of cyanobacteria, a significant increase in lakes with this categorization (8.3 %, 95 % confidence intervals 4.0 %–12.5 %) over the 2007 assessment (U.S. Environmental Protection Agency, 2016). A concentration of 105 cyanobacteria cells mL−1 is considered to present a risk of both acute and chronic health effects (Backer, 2002), and many states, including Massachusetts, issue public health warnings for recreational water bodies when the cyanobacteria cell concentration exceeds this value. Metals are also important contaminants in freshwater systems because of their persistence and toxicity (Bronmark and Hansson, 2002). In 2004, 1.5 million lake acres in the US were impaired by metals such as lead, chromium, and arsenic (Environmental Protection Agency, 2004). Identifying the sources and mechanisms of transport of these substances within lake ecosystems can help predict the fate of contaminants and aid remediation efforts.
Because sediments are typically major repositories of contaminants (Nriagu et al., 1996; Pan and Wang, 2012; Taylor and Owens, 2009), it is important to understand the processes leading to contaminant mobilization. Metals can be mobilized from sediments via solubilization by oxidation-reduction reactions, as well as by sediment resuspension, acidification, or bioturbation (Calmano et al., 1993; Eggleton and Thomas, 2004; Schaller, 2014; Schindler et al., 1980). Likewise, overwintering cyanobacteria and algae concentrated in the sediments are mobilized through germination, wind-induced resuspension, or bioturbation (Ramm et al., 2017; Verspagen et al., 2004; Stahl-Delbanco and Hansson, 2002). In some cases, the number of resting cells in sediment can be predictive of the severity of subsequent bloom events (Anderson et al., 2005). Previous research showed that recruitment from sediments of the potentially toxic cyanobacterium Microcystis was a major driver of the summer bloom (Verspagen et al., 2005). Cyanobacterial recruitment to surface waters from deep sediments is inhibited by stratification, low oxygen concentration, and low light levels (Ramm et al., 2017). Metals mobilized from sediment under stratified water columns will also be inhibited from reaching surface waters due to stratification (Wetzel, 2001).
An alternative mechanism for vertical transport of metals and cells from sediment to surface water could be bubble-facilitated transport. Bubbling from anoxic sediments, driven by methanogenesis, is widespread in freshwater systems (Bastviken et al., 2011; Deemer et al., 2016), and bubbles are known to be effective particle transporters. Bubble particle flotation, a process by which amphiphilic particles attach to a bubble's gas–water interface and are transported upwards during bubble rise, is used extensively in industry for applications such as separating valuable minerals from gangue (Min et al., 2008; Rodrigues and Rubio, 2007), removing ink during paper recycling (Vashisth et al., 2011), recovering desirable proteins and microorganisms from industrial bioreactors (Schugerl, 2000), and treating wastewaters (Aldrich and Feng, 2000; Lin and Lo, 1996; Rubio et al., 2002). Bubble-mediated particle transport also occurs in the open ocean where bubbles are injected into the water by breaking waves, scavenge surface-active particles as they rise, and then deposit these particles on the ocean surface (Aller et al., 2005; Blanchard, 1975; Wallace et al., 1972; Liss, 1975).
Despite this previous work, little is known about the importance of particle transport by bubbles in freshwater systems. Bubbles produced by methanogenesis in anoxic sediments are prevalent in freshwater systems, and bubbles are released to the surface during drops in hydrostatic pressure, sediment disturbance, or upon sufficient gas accumulation (Chanton et al., 1989; Joyce and Jewell, 2003; Scandella et al., 2011; Liu et al., 2016; Maeck et al., 2014; Varadharajan and Hemond, 2012). Bubble flotation could thus potentially provide a chemical and biological link from deep water to surface waters that would otherwise not occur through advective or eddy-diffusive transport alone. Additionally, the relatively rapid rise time of bubbles limits the time available for oxidation reactions and suggests that particulate matter from the hypolimnion could reach the lake surface in a reduced state, with possible consequences for both toxicity and reactivity. Some evidence does suggest that bubbles can transport polycyclic aromatic hydrocarbons (Viana et al., 2012) and manufactured gas plant tar from sediments (McLinn and Stolzenburg, 2009). Additional work has shown that bubble-mediated transport of microorganisms including methane-oxidizing bacteria (MOB) is an important mechanism connecting benthic and pelagic populations at 10 m water depth (Schmale et al., 2015). However, researchers in the previous study were unable to quantify the importance of bubble-mediated transport to overall recruitment of pelagic MOB populations, and the extent of bubble particle flotation in aquatic systems remains unknown.
The present study is motivated by authors' observations of particle accumulations associated with bubbling events at Upper Mystic Lake (UML), where bursting bubbles often left black particles distributed on the water surface in a ring pattern (Fig. S1 in the Supplement). Particles were also observed at the air–water interface in bubble traps during long-term deployments (data not shown). The significant volume of gas observed to bubble from UML during previous studies (Delwiche and Hemond, 2017; Varadharajan and Hemond, 2012), together with strong thermal stratification suppressing other mechanisms of sediment transport to the surface, led to the hypothesis that bubbles could serve as a relatively important mode of particle transport from the sediment to the water surface. This potential transport pathway could be relatively more important for metal and cyanobacteria transport in eutrophic, deep, stratified lakes, such as UML.
In the present study, we quantified particle transport by bubbles in UML, an urban lake with a history of sediment contamination. We also used a 15 m tall bubble column to study bubble-mediated particle transport under controlled lab conditions. Given the expected importance of bubble size on key characteristics (e.g., surface area, buoyancy, diffusion of gas), we used a bubble size sensor (Delwiche et al., 2015; Delwiche and Hemond, 2017) to measure bubble diameter distribution both in the lake and in the laboratory. We address the following questions:
-
How much sediment is transported to the surface through ebullition?
-
How does bubble-mediated sediment transport contribute to metal cycling?
-
How does bubble-mediated sediment transport contribute to cyanobacteria recruitment to the upper water column?
2.1 Upper Mystic Lake field site history
UML in Arlington, MA, is an urban, dimictic kettle lake with an average depth of 15 m, a maximum depth of 24 m, and a surface area of 0.58 km2. The lake is used extensively for recreational and scientific purposes, and previous studies have characterized several aspects of methane ebullition (Delwiche and Hemond, 2017; Scandella et al., 2016; Varadharajan and Hemond, 2012) and microbial community structure and function (Preheim et al., 2016; Arora-Williams et al., 2018). Chemical manufacturing and leather tanning industries during the late 1800s and 1900s released toxic metals such as arsenic, chromium, and lead that flowed into the lake and were deposited in the lake sediments. Sediment cores reveal a distinct layered pattern with peak metal/metalloid concentrations traceable to years of peak manufacturing or subsequent earth moving (Spliethoff and Hemond, 1996). Additionally, high nutrient loading promotes the growth of algal and cyanobacterial blooms. A public health advisory was issued for UML as recently as July 2017 for cyanobacteria cell concentrations >70 000 cells mL−1 (https://www.arlingtonma.gov/Home/Components/News/News/4965/16, last access: 6 May 2019).
Years of field observations at UML have provided a thorough picture of the typical hydrological conditions in the lake. Significant volumes of gas are produced from the sediments, which escape to the surface via ebullition, resulting in an average release rate of 22 mL bubble volume m−2 d−1 (Varadharajan, 2009). From June–October, the oxycline and thermocline are typically found between 6–12 and 3–9 m, respectively (Varadharajan, 2009; Delwiche and Hemond, 2017). The Secchi depth in the lake is typically 2–3 m during the same period (Varadharajan, 2009). Light was sufficient for germination down to 12 m in lake Scharmützelsee with a similar average Secchi depth (Ramm et al., 2017); thus we assume light does not limit cyanobacteria germination down to a depth of at least 12 m when estimating the impact of bubbling on cyanobacteria recruitment.
2.2 Field sampling
Bubble-transported particles were collected from both the laboratory column and the lake in 350 mL plastic sampling cups affixed either to the top of a custom bubble size sensor (sensor described previously; Delwiche et al., 2015; Delwiche and Hemond, 2017) or to the top of a collection funnel (the bubble sensor was used in 2017 sampling; the funnel alone was used for sampling in 2018). The plastic sampling cup lid contained a barbed bulkhead fitting connected via flexible plastic tubing to an on–off valve and a quick-release adapter (Fig. S2). The sampling cup, valve, and adapter were connected to the custom bubble size sensor or collection funnel with flexible tubing. All bubbles rising through the bubble size sensor or collection funnel entered the flexible tubing and rose into the sample cup. The interaction of bubbles with the flexible tubing resulted in visible particle attachment to the tubing, making our estimates of particle mass transport a lower bound. The sample cup lid contained a secondary valve to release water upon bubble entry. All sample cups were soaked in 5 %–10 % reagent grade HNO3 for 24 h and rinsed and filled with Milli-Q water prior to use. Gas and associated particles accumulated in the collection cup during sampling and were then transported back to the lab for analysis.
On 17 October 2017 we sampled for bubble-mediated sediment mass fluxes and associated particulate metal fluxes in an area of the lake previously found to have relatively high ebullition rates (42.432 latitude, −71.151 longitude; 16 m deep; Delwiche and Hemond, 2017). This previous work showed that sediment ebullition rates from this location remain high from July to November, yet the water column remains stratified, preventing mixing of sediment to the surface. Previous work at this particular location within the lake indicated that natural bubble fluxes were around 45 mL m−2 d−1 with high spatial and temporal variability (Delwiche and Hemond, 2017). Given the need to collect samples as soon after bubbling as possible to minimize potential changes in cyanobacteria population, and the difficulty with predicting flux from natural bubble events, we chose to trigger ebullition manually by dropping a cinder-block anchor into the sediment. This procedure enabled us to collect multiple samples during a single field trip, with minimal time for samples to change after collection in the sampling cup. Since anchor triggering was expected to release a plume of sediment, we used laboratory experiments to explore whether bubbles rising through suspended sediment would scavenge particles (more details below).
After bubble triggering, the bubble size sensor was positioned above the bubble plume and 1 m below the water surface. Bubbles exiting the sensor, together with any particles adhered to the bubble–water interface, were collected in the sample cup described previously. Several anchor drops within an area of approximately 10 m by 10 m were required to intercept a sufficient number of bubbles for mass quantification per sample, and we intentionally collected samples with different total gas volumes. We collected blank water samples to correct for background contributions of particulate matter, arsenic, lead, and chromium. Bubbling resulted in the visual accumulation of particles at the surface (Fig. S1) and in the sampling cup. During a separate field visit in November 2017 we used an Ekman dredge to collect sediment and stored the sediment in a 5 gal (18.9 L) bucket below 4 ∘C until use in February 2018 for bubble column experiments.
On 26 June 2018 we sampled for cyanobacteria bubble transport using similar procedures, except we used a simple inverted funnel instead of a custom bubble size sensor to intercept rising bubbles. The sampling funnel was placed 10 m below the water surface, where cyanobacteria concentrations were expected to be lower than the surface based on previous observations (Preheim et al., 2016) to reduce sample contamination with cyanobacteria from the surrounding water column. Water temperature measurements taken using a Hydrolab sonde (Hach Co.) confirmed that the thermocline depth was above 10 m in this location during sampling (Fig. S3). We collected 30–40 mL of water samples at 15, 11, 10, and 1 m depths for background cell concentration counts and gathered sediment grab samples with an Ekman dredge. All sample cups were sterilized prior to use by rinsing with 10 % bleach followed by 70 % ethanol and deionized, sterile water, and cups were filled with sterile water prior to sample collection. Samples were stored in a dark cooler on ice and were refrigerated upon return to the lab. On 26 June 2018 we also used an Ekman dredge to collect a bulk sediment sample, which was kept in a dark refrigerator at 4 ∘C until use in February 2019 for cyanobacteria transport in the experimental bubble column.
2.3 Large laboratory column design and sampling
To study bubble particle shedding and scavenging, we built a 15 m tall bubble column in the laboratory stairwell. The column is composed of four sections of 6 in. (15.3 cm) nominal diameter transparent polyvinyl chloride (PVC) pipe joined by threaded unions with O-ring seals. The base of the column is a reducing tee fitting with a removable spigot for drainage, and the column was filled from the top with tap water. We built a sediment container connected to 1∕8 in. (3.1 mm) outer diameter copper tubing that could be lowered into the column and secured at any depth. The container was filled with sediment originally collected with an Ekman dredge from the same place in UML used for field sampling. We used a syringe pump to push air into the sediment through the tubing at a controlled rate, resulting in bubble release from the sediment. The bubbling rate was calibrated to achieve a relatively steady release of bubbles without substantial wait time in between. While we expect that much of the gas naturally existing within the sediment was released during sediment collection and as it was transferred to the sample bed (indeed we did not observe natural bubble release from the sediment bed prior to experimental trials), remaining gas could have been incorporated into rising bubbles.
We conducted one set of column experiments in February 2018 to quantify shedding, scavenging, and metal transport and another set of column experiments in February 2019 to quantify cyanobacterial transport. For the shedding and metal transport experimental runs, we filled the sediment bed with sediment collected from the same site as ebullition experiments during our November 2017 field visit, and we injected 50 mL of air at 0.7 mL min−1 into the sediment bed. Prior to the start of each run we collected water samples to correct for background contributions to particulate matter and arsenic concentrations in bubble-transported particle data. Three experimental runs were each conducted at each of three depths: 5, 10, and 15 m, with the mobile sediment bed being repositioned between runs. To quantify particle scavenging rates, we also conducted trials in which we injected air into the water column several centimeters above the sediment surface. Scavenging tests were conducted after particle transport tests, so the water column above the sediment bed was turbid and contained a plume of sediment particles. For the cyanobacterial transport experiments, we used sediment from the June 2018 field visit and injected variable volumes of air into the sediment bed. We ran four experiments each at 6 and 13 m depth, with the sediment being replenished between the 6 and 13 m runs. Six surface water grab samples were collected at multiple times throughout the experiment to quantify background cell concentrations, and at each depth one trial was run where air was bubbled into the water directly below sensor. For both sets of experiments, bubbles passed through the same customized bubble size sensor (Delwiche et al., 2015; Delwiche and Hemond, 2017) and sample cup apparatus used in the field setting.
2.4 Sample processing for particle mass and heavy metals analysis
We filtered the field samples collected from UML for metals analysis within 24 h of sampling with preweighed Whatman grade 41 quantitative cotton filters (nominal pore size 20 µm, 25 mm diameter). Due to filter clogging, we typically used multiple filters for each sample, and total particulate transport per sample was calculated by summing the particle mass on each filter and dividing by the total gas volume associated with the sample. After filtering we air-dried the filters, weighed them, transferred each to microwave digestion vessels, and added 10 mL of nitric acid from Fisher Scientific (Optima grade for ultratrace elemental analysis). Samples were digested in a MARS6 microwave oven, diluted with 30 mL of Milli-Q water, and then filtered with a 0.2 µm polyethersulfone membrane syringe filter. For analysis, we diluted samples to 2 % nitric acid, added a rhodium internal standard, and analyzed the samples using an Agilent 7900 inductively coupled plasma mass spectrometer (ICP-MS) with a five-point calibration curve from 0.05 to 10 ppb. Blank analysis to determine background arsenic concentrations in the Whatman cotton filter paper found levels of less than a nanogram of arsenic. For metal analysis in bulk sediment sample we added 100 mg of dried sediment to 10 mL of nitric acid and digested as described above. The relative standard deviation (RSD) values for counts per second from the ICP data were on average 5.2 %±2.8 % for the bubble-transported sediment particles and were 1.1 %±0.6 % for the bottom sediment digests (which contained more particle mass per digest). These relatively low RSD values indicate that analytical uncertainty is low, especially compared experimental uncertainty.
We filtered bubble column samples using preweighed 5.0 and 0.2 µm Whatman Nuclepore membrane filters (47 mm diameter). Filters were dried, weighed, digested, diluted, and analyzed as described above. Duplicate analysis of clean Nuclepore membranes (blank) was used to determine arsenic contamination of the filters and was below the detection limit for the 5 µm filters and 0.003±0.002 µg per filter for the 0.2 µm filter (less than 1 % of the arsenic found in the least-concentrated sample).
2.5 Sample processing for cyanobacteria analysis
For both the field and bubble column cyanobacterial transport experiments, we filtered a subset of the samples within 24 h with 0.2 µm pore size filters held in autoclaved Swinnex filter holders (25 mm diameter). Filters were then removed from the filter holders and transferred to PowerWater bead beating tubes (Qiagen, Inc.). Approximately 8–9 mL of remaining liquid for each sample was preserved with 1–2 mL of formamide (10 % final concentration volume ∕ volume) for microscopic cell counts. Lastly, the remaining sample volume was filtered on preweighed Whatman Nuclepore membrane filters (0.2 µm pore size, 47 mm diameter), air-dried, and reweighed to estimate bulk mass transport.
For quantitative polymerase chain reaction (qPCR) analysis on the June 2018 bulk sediment samples, 0.13 g of wet sediment was suspended in 15 mL of sterile water and then filtered as described above. For microscopy cell counts, 0.14 g wet sediment was preserved in 2 % by volume paraformaldehyde. Water column samples from the June 2018 field campaign were also preserved in 2 % by volume paraformaldehyde for cell counts. For qPCR analysis of the June 2018 sediment samples before use in the bubble column, we filtered 0.7 g of wet sediment (0.007 g dry sediment). For microscopy cell counts of the June 2018 sediment samples before use in the experimental bubble columns, we placed 0.8 and 2.0 mg of wet sediment (0.08 and 0.18 mg dry weight, respectively) into 10 mL of 10 % formalin.
2.6 Cyanobacteria cell quantification
Cyanobacteria cell counts were assessed through quantitative polymerase chain reaction and microscopy. These two methods estimate cyanobacteria cell numbers by targeting different features of cyanobacterial cells. qPCR targets the unique genetic signatures in the 16S ribosomal RNA (rRNA) gene of cyanobacteria (Nubel et al., 1997) to estimate cell number from gene copy numbers. Microscopy takes advantage of the unique fluorescence spectra of cyanobacterial photosynthetic pigments to identify cells (Salonen et al., 1999). Positive control Microcystis aeruginosa UTEX LB 2386 and negative control Pseudomonas aeruginosa samples were used to optimize amplification conditions to ensure specificity for cyanobacteria qPCR. Microcystis and Pseudomonas cultures were grown overnight (12 h) under fluorescent lights at 25 ∘C in BG11 and Luria broth media, respectively. Microcystis stock culture was serially diluted in phosphate-buffered saline to make a standard curve, filtered onto 0.22 µm polyethersulfone membrane filters (Millipore Sigma, Inc.) and frozen at −80 ∘C until DNA extraction. Additionally, serial dilutions of Microcystis cultures were fixed with 1 % formalin (final concentration, volume ∕ volume) for microscopy. While Microcystis cells were used as a positive control to test the method, qPCR primers targeted all cyanobacteria cells (not limited to Microcystis).
To estimate the total number of cells in the Microcystis stock culture and samples with microscopy, between 4.6 and 10.4 mL of fixed water samples or 1000 µL fixed Microcystis stock culture were filtered onto 0.22 µm polyethersulfone membrane filters (Millipore Sigma, Inc.). Cells were visualized under a Zeiss Axio Observer Epifluorescence SIM microscope (excitation: 545 nm; emission: 572 nm; Salonen et al., 1999). The total number of autofluorescent cells per filter was estimated from 20 to 40 random fields of view spanning the entire area of each filter. Cells were identified from images with ImageJ (Schneider et al., 2012). First, background noise was reduced by excluding low-intensity pixels, with threshold values ranging between 14 and 162 (pixel intensities ranged from 0 to 255 for 8 bit gray-scale images). Next, only particles within the size range of 0.1–29.4 µm2 were counted as cells. A dilution series of Microcystis fixed culture was created by diluting cultures 2-fold in 1 % formalin to test the variance and accuracy of this counting method (Fig. S4). We did not test the quantification below 20 cells per field of view, and all the experimental samples (not controls) had an average of less than 20 cells per field of view, so microscopy measurements were only used for detection and not quantification.
For cyanobacteria cell quantification with (qPCR), DNA was extracted using PowerWater kits (Qiagen, Inc) following the manufacturer's protocol, with the addition of 20 µL proteinase K and incubation at 65 ∘C for 10 min before bead beating as an alternative lysis step. Primers were used to amplify Cyanobacteria 16S rRNA genes as previously described (Nubel et al., 1997), with CYA359F (5′-GGG GAA TYT TCC GCA ATG GG) and an equal mixture of CYA781R(a) (5′-GAC TAC TGG GGT ATC TAA TCC CAT T) and CYA781R(b) (5′-GAC TAC AGG GGT ATC TAA TCC CTT T). qPCR reactions contained 10 µL of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc.), 1.6 µL DNA template, 2 µL forward primer (10 mM), 2 µL reverse primer (10 mM), and 4.4 µL deionized, reagent grade sterile water. The following cycling conditions were used: denaturation at 98 ∘C for 30 s, annealing at 68 ∘C for 30 s, and elongation at 72 ∘C for 30 s followed by visualization step for 40 cycles. A dilution series of Microcystis was created by diluting cells 10-fold in phosphate-buffered saline before filtration and DNA extraction. Cell numbers for environmental samples were determined from a linear regression of threshold cycle number (Cq) values of Microcystis and the number of cells calculated for each dilution (e.g., Fig. S5), and different batches were calibrated with internal standards of Microcystis culture. Inhibition was determined for a subset of samples by spiking known concentrations of Microcystis DNA into environmental DNA extracts and measuring the resulting threshold cycle number (Fig. S6). In all cases tested, inhibition was negligible. The limit of quantification is five cells per filter, based on a signal-to-noise ratio (SNR) 2–3 times the average cell concentration of the blanks (2.76 SNR).
2.7 Cyanobacterial recruitment estimates for cells from Upper Mystic Lake
The contribution (%) of ebullition to cyanobacteria recruitment (Pe) was calculated as
where Ce is the average cell flux from ebullition to the lake surface, Cg is the recruitment rate due to germination, SA is lake surface area, and Fg is the fraction of the lake surface area that could support recruitment through germination. We estimated Ce using our measured range of potential particle transport and the concentration of cells in the lake sediment. Values for recruitment estimates were calculated assuming ebullition occurs at an average rate of 22 mL m−2 d−1 for the entire summer from all areas of the lake equally based on previous lake-wide ebullition surveys, (Varadharajan, 2009). We used the average cyanobacteria cell concentration from this study of 880 cells per milliliter of gas volume to calculate the average flux of cells to the surface via ebullition (Ce) of 2×104 cells m−2 d−1. We estimated the recruitment rate due to resuspension and germination (Cg) as the maximum observed rate from a previous experiment in Lake Limmaren of 2.3×105 cells m−2 d−1 (Brunberg and Blomqvist, 2003) and applied this recruitment rate to areas of the lake suitable for germination, although this is likely an overestimation. We conservatively assumed that germination could occur to a depth of 12 m based on typical light, temperature, and oxygen levels observed in UML (Varadharajan, 2009). The fraction (Fg) of the surface area (SA=580 000 m2) of lake above 12 m that could support cyanobacterial recruitment through germination is approximately 0.50 (Varadharajan, 2009).
3.1 Rate of bubble particle transport
Both field and bubble column experiments demonstrate that bubbles can transport particles from the sediment to the lake surface. A positive correlation (p<0.05 level for October 2017 (r2=0.76), p=0.15 (r2=0.38) for June 2018) was found between total particle mass and gas volume in bubble traps for both field sampling campaigns (Fig. 1). The general magnitudes of particle loadings on bubbles in column experiments and on bubbles observed in triggered experiments in the field were of similar magnitude: 0.01±0.006 mg mL−1 in the column vs. 0.09±0.07 mg mL−1 on October 2017 and 0.01±0.01 mg mL−1 on June 2018 in the field.
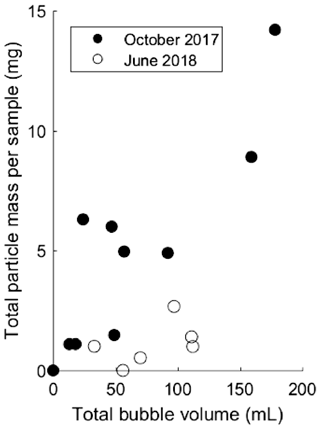
Figure 1Total particle mass in milligrams (mg) associated with the bubbles captured during each field campaign with bubble-triggering events in October 2017 (filled circles) and June 2018 (open circles). Triggering events yielded different bubble volumes (given in mL).
These particle loadings on bubbles, and any ecosystem-wide flux estimates derived from them, must be qualified by the fact that neither triggered bubbles nor bubbles in the bubble column fully replicate natural bubbling. In particular, the triggering of bubbles with an anchor may have raised plumes of suspended sediment through which some fraction of produced bubbles had to rise, and within which the possibility of scavenging should be considered. Likewise, bubbles could shed particles partway up the water column during rise. To estimate the significance of particle shedding, we used the bubble column to compare transport rates from bubbles released at 5, 10, and 15 m depths (Fig. 2). We found no significant difference in transport rates from any depths, suggesting that net particle shedding was not a major process. We did however note that the first bubble column test conducted after repositioning the sediment source yielded a higher particle transport rate than those found in subsequent tests (Fig. 2), consistent with the intuitively reasonable possibility that mechanical sediment disturbance can affect particle loading on bubbles. We also note that while bubbles do dissolve as they rise, bubbles in the size range seen during this study remain relatively constant in volume during their rise through 15 m of water column because dissolution is partially compensated for by bubble expansion during rise (Delwiche and Hemond, 2017), and we therefore do not expect bubble dissolution to substantively impact particle shedding.
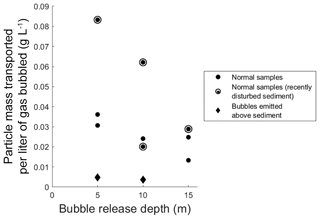
Figure 2Transported particle mass per liter of gas bubbled in the large bubble column, as a function of bubble release depth. Solid circles represent samples where bubbles were emitted from the sediment bed; diamonds represent samples where gas was bubbled directly above the sediment bed. Hollow circles around solid circles denote samples with recently disturbed sediments.
To observe the possible extent of bubble scavenging of particles from the water column, we compared data from 5 and 10 m column experiments to samples gathered when gas was bubbled from several centimeters above sediment, thus allowing maximum opportunity for scavenging to occur. We conducted the scavenging tests when the water column was visibly turbid and contained a plume of suspended particles from previous tests. Particle mass scavenging represented only around 10 % of the mean particle loading for bubbles in the 5 and 10 m experiments (gray diamonds in Fig. 2), indicating that while scavenging rates were nonzero, the large majority of the particulate matter transported to the top of the water column originated in the sediment. Taken together, the minimal particle shedding and particle scavenging in column experiments suggests that particles observed on bubbles in the field, even when bubble release was triggered, mainly originated in the sediment.
While bubbles transported sediment directly from the bottom of the laboratory column to the water surface, a vertical distance of 15 m, there appears to be no reason that transport of particles from significantly larger depths cannot occur. Such transport provides a direct chemical and biological link between sediment and surface waters, and this could be the dominant link between deep sediments and the surface water during months of stratification. However, many questions remain regarding bubble-mediated transport in natural systems, including how the change in water density at the thermocline affects bubble rise and associated chemical and biological material.
3.2 Bubble size distribution similar between field triggered and natural bubbles
Bubble volume has been found to significantly affect particle flotation rates in industrial processes (Yoon and Luttrell, 1989), and therefore it is important to compare the anchor-triggered bubble sizes to naturally occurring bubble sizes to understand how our measured transport rates may reflect naturally occurring transport rates. Anchor-triggered bubbles were significantly smaller (average diameter 5.6 mm) than those measured for natural bubbling events (average diameter 6.4 mm) during a 2016 field campaign (Fig. S7; Delwiche and Hemond, 2017), even though relatively high bubble flux events (such as those triggered by anchor dropping) can lead to some bubble coalescence within the funnel constriction in the bubble size sensor (as described previously; Delwiche and Hemond, 2017).
However, both natural and triggered bubbles were still very large compared to bubbles used in traditional flotation chambers (Yoon and Luttrell, 1989; Rubio et al., 2002). While research on particle flotation for large bubbles is limited, several previous studies have found that differences in particle transport rates decrease for bubbles above 1 mm diameter (Dai et al., 1998; Koh and Schwarz, 2008), indicating that particle transport rates should be similar between natural and triggered bubbles. Bubble sizes measured in the cyanobacteria transport experiment displayed a bimodal distribution (Fig. S8) that was not observed in other bubble experiments. This bimodal distribution could be a result of artificially pumping gas into the sediment, but the impact of this on particle transport is unknown.
3.3 Bubble-transported particles have chemical and biological characteristics similar to sediment
The data on bubble particle mass transport clearly shows that bubbles are capable of transporting particles from relatively deep depths, and minimal rates of particle shedding and scavenging in the water column suggest that these particles originate primarily in the sediment. Concentrations of arsenic, chromium, and lead in the bubble-transported particulate matter collected during field experiments were similar to concentrations in the sediment (Fig. 3). Bubble-transported particles contain arsenic, chromium, and lead at average ratios of 100, 120, and 240 µg kg−1 (respectively, excluding outlier in chromium data; see Fig. 3), compared to 136, 160, and 330 µg kg−1 (respectively) in bulk sediment samples. In the bubble column, arsenic and chromium levels are similar to the bulk sediment in the column experiments (Fig. S9), although lead levels appear to be higher. Overall, this similarity supports our conclusion that bubbles are primarily transporting sediment matter to the lake surface with only modest amounts of scavenging or particle shedding, despite the relatively deep water column.
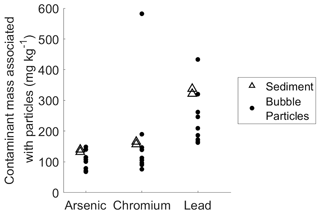
Figure 3Comparison between mass of arsenic, chromium, and lead per kilogram of sediment (open triangles) and bubble-transported particulate matter (solid circles). The standard deviation scale is similar to point size and therefore omitted for figure clarity.
In addition to the heavy metal results indicating that the transported particles are from the sediment, biological evidence also suggests a sedimentary origin. All particle samples transported by bubbles contained an abundance of biological structures (Fig. S10), such as the apparent head shields and carapaces of Bosmina spp., which have also been found extensively in other freshwater lake sediments (Kerfoot, 1995). These particle samples also contained structures that appear to be ephippia (Fig. S10b), the protective cases enclosing diapausing eggs produced by zooplankton such as Daphnia. Ephippia can overwinter in lake sediments or survive periods of desiccation, providing a seed bank to recolonize the water column when favorable conditions return (Caceres and Tessier, 2003; Hairston, 1996). These biological findings further support the finding that bubbles are transporting sediment particles through the profundal water column. There remains the possibility that our measured bubble particle transport rates differ significantly from those from naturally emitted bubbles, and this remains an important area for future research. However, despite this uncertainty, broad-scale estimates of arsenic and cyanobacteria cycling can provide important context as to whether these processes may be significant in UML.
3.4 Implications for arsenic and heavy metal cycling
The presence of arsenic and other heavy metals in the bubble-transported particles could have significant implications for chemical cycling in aquatic ecosystems. Measured rates of arsenic flotation in field samples were about 8±4 µg arsenic L−1 of gas bubbled (Fig. 4a). Typical natural bubble flux for UML was estimated as 0.02±0.02 L m−2 d−1 during previous ebullition studies (Varadharajan, 2009), which corresponds to a potential arsenic flux of 0.2±0.2 µg m−2 d−1 from the sediment to the lake surface. This flux would be highly episodic given the spatial and temporal heterogeneity of methane bubbling in UML (Varadharajan, 2009; Scandella et al., 2016).
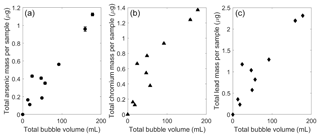
Figure 4Chemical amounts observed in bubble traps associated with bubble-mediated transport of sediment particles. (a) Arsenic mass, (b) chromium mass, and (c) lead mass (in µg) transported versus the bubble volume of each sample (in mL, as measured at the lake surface a–c). The standard deviation is added to each measurement but the scale is similar to point size for most measurements.
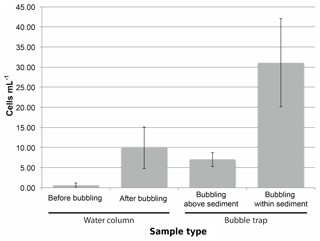
Figure 5The concentration of cyanobacteria cells (as measured by quantitative PCR) increases in the experimental water column and bubble traps after initiating bubbling within sediments. The background concentration of cyanobacteria cells in the water column was initially low (“Before bubbling”) but increased after bubbling air through the sediment. The concentration of cells in the bubble trap increased even if bubbles did not pass directly through sediment but instead originated above the sediment bed (“Bubbling above sediment”) from cells contaminating the surrounding water column. However, the highest concentration of cyanobacteria in the bubble trap was observed when initiating bubbling from within the sediment (“Bubbling within sediment”) from direct transport of cells from the sediment into the bubble trap. The increase in cell concentration in both the water column and the bubble trap after bubbling within sediment is evidence for cyanobacteria transport via bubble floatation. Error bars show standard deviation across measurements.
This estimate of daily arsenic flux can be compared with historical measurements showing significant arsenic accumulation within the epilimnion at rates exceeding 30 µg m−2 d−1 (Knauer et al., 2000). This flux is 2 orders of magnitude larger than our estimate for bubble-transported arsenic of 0.2 µg m−2 d−1, indicating that bubble-arsenic transport may be of relatively low importance in UML, where a significant fraction of the arsenic input to epilimnetic waters can be attributed to inflow from the Aberjona River (Hemond, 1995). However, bubble-mediated fluxes of arsenic or other sediment-borne metals may represent a larger fraction of epilimnetic input in other lakes having lower influx rates from surface water inflow or other external sources, such as atmospheric deposition (Csavina et al., 2012).
Although bubble-facilitated transport does not appear to dominate arsenic transport in UML, much higher ebullition rates have been reported elsewhere in the world (Deemer et al., 2016). For example, a midlatitude reservoir in Switzerland was reported to have an order of magnitude higher ebullition flux (0.225 L m−2 d−1; Delsontro et al., 2010). Co-occurrence of high ebullition rates and contaminated sediment could lead to significant bubble-facilitated contaminant cycling.
3.5 Implications for cyanobacteria transport and possible bloom initiation
Since cyanobacteria are known to overwinter in lake sediments, bubble-mediated transport could be one mechanism by which resting cells inoculate the upper water column. Bubble column experiments showed that bubble-transported particulate matter contained cells at approximately 30 cells mL−1 gas (Fig. 5), indicating that bubbles are capable of transporting cyanobacteria through deep water columns. We also measured cyanobacteria transport in the field with bubble traps, but our measurements were contaminated by cyanobacteria in the surrounding water column (see Supplement for results and discussion). While we could not directly measure bubble cyanobacteria transport in the field, we can estimate it using a combination of measured bubble particle flotation rates and the average cyanobacteria cell concentration in lake sediment. Estimated cell transport using this method is 880±1140 cyanobacteria cells mL−1 of bubble volume. Although this is significantly higher than the measurements made in the bubble column, the conditions in the column are substantially different from the conditions in the field, and the sediments used in column had been stored for 8 months, so the cyanobacteria cell concentration was 10 times less than fresh sediments. While this variability in cell transport between column measurements and estimates of potential field transport highlights the need for continued research, it is useful to estimate the potential range of cyanobacterial transport.
To assess the likelihood that bubble-mediated cell transport could significantly inoculate surface waters, we use the upper transport estimate of 880±1140 cyanobacteria cells mL−1 of bubble volume and the bubble flux estimate mentioned previously of 22±20 mL m−2 d−1 to estimate a daily transport of cells m−2 d−1. If cyanobacteria cells concentrate within the upper 1 m of the lake, outcompeting other phytoplankton species for sunlight (Xiao et al., 2018), this results in an increase in concentration of 20±30 cells L−1 d−1. While this concentration is not a human health concern, such a concentration from the average rate of bubbling could represent a significant inoculum. At a maximum growth rate of approximately 1 d−1 (Robarts and Zohary, 1987) and absent significant losses, this cell concentration alone would result in a cell density greater than the Massachusetts health limit of 7×104 cells mL−1 in about 3 weeks. Larger bubbling events (e.g., Deemer et al., 2016) could result in the same cyanobacteria cell concentration within approximately 15 d. The estimated growth of bubble-transported cyanobacterial cells is dependent on the cells being viable. Incubating cells to assess viability will be an important step for future studies. These calculations demonstrate that bubble-transported cyanobacteria could negatively impact water quality, though more research is warranted to improve these estimates.
Given the potential impact on bloom formation, we compared this source of cells to other pathways of cell recruitment to the lake surface, especially in deep, stratified lakes like UML. Cyanobacteria are thought to largely be recruited to surface waters from shallow areas due to a combination of higher light, temperature, and oxygen levels that promote germination, as well as increased wind-driven sediment resuspension (Ramm et al., 2017). While sediment cyanobacteria concentrations are higher in deeper areas of the lake, cells are not able to germinate because of the dark, anoxic conditions in deep, eutrophic lakes (Ramm et al., 2017). Bubble-mediated transport is a mechanism by which this large reservoir of “lost” cells in deep sediments could contribute to overall recruitment to the surface waters. To determine the potential contribution of bubble-mediated transport to cyanobacteria recruitment to the surface, we assume that germination does not occur significantly past the oxycline (12 m) in UML between June and October, as low oxygen concentrations and low light levels prevent germination, and wind-driven mixing cannot resuspend sediments across the shallow thermocline (Varadharajan, 2009). We also assume that cells resuspended in the spring overturn in March would have germinated, settled, lysed or have been consumed by grazers by June (e.g., Tijdens et al., 2008; Verspagen et al., 2005). Furthermore, we do not include external inputs of cyanobacteria to the lake, such as from the river (e.g., Bouma-Gregson et al., 2019) or air (Seifried et al., 2015; Lewandowska et al., 2017; Evans et al., 2019). Since literature estimates of recruitment rates for these sources are lacking, we assume these inputs are small compared to shallow sediment and bubble-mediated recruitment. Using the maximum observed recruitment rate of 2.3×105 cells m−2 d−1 (Brunberg and Blomqvist, 2003) from sediments for the area of the lake above 12 m, we estimate that bubbling could contribute 14 % of cyanobacterial recruitment in the lake, but 95 % confidence intervals range from less than 0 % to 46 % of overall recruitment. While we cannot rule out the possibility that this is an insignificant source of cells given the large uncertainty in these measurements, the potential for bubble-mediated transport to contribute substantially to the source of cyanobacteria cells at the lake surface warrants further investigation.
Bubble particle transport between the sediment and surface of UML is a novel transport pathway capable of moving particulate matter upwards through a stratified water column, over depths of 15 m or greater, without shedding a major fraction of their particle burden or accumulating large amounts of additional particles as they rise. Bubble-facilitated metal transport in present-day UML appears minor compared to surface inflows, but lakes with higher ebullition flux or more contaminated surficial sediments may experience more significant chemical transport from contaminated sediments. Bubble-mediated transport of cyanobacteria cells may contribute substantially to cellular recruitment from the sediment, but the uncertainties in our measurements make these estimates speculative. Bubble transport is expected to be particularly important in deep, eutrophic lakes in which alternative mechanisms of sediment regeneration to surface waters are limited. Further work is warranted to more thoroughly quantify this ebullitive transport pathway and its implications for chemical and biological cycling. In addition, future work should include alternative methods of bubble triggering as well as the quantification of particle transport rates on naturally occurring bubbles.
All data necessary to validate the research findings are available on JHU Dataverse, https://doi.org/10.7281/T1/7WXPIN (Delwiche et al., 2019).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-17-3135-2020-supplement.
KD, HH and SPP designed the experiments and KD and JG carried out experimental work. KD, JG and SPP analyzed the data. KD prepared the manuscript with contributions from all coauthors.
The authors declare that they have no conflict of interest.
Resources for this work were provided by the Singapore-MIT Alliance for Research and Technology, the MIT Center for Environmental Health Science, MIT Superfund Research Program, the MIT Martin Family Fellowship to Kyle Delwiche, and the W. E. Leonhard 1941 professorship to Harold Hemond.
This research has been supported by the National Science Foundation, Division of Earth Sciences (grant no. EAR-1045193), and the National Institutes of Health, National Institute of Environmental Health Sciences (grant no. P42 ES027707 and P30-ES002109).
This paper was edited by Manmohan Sarin and reviewed by Dileep Kumar and three anonymous referees.
Aldrich, C. and Feng, D.: Removal of heavy metals from wastewater effluents by biosorptive flotation, Miner. Eng., 13, 1129–1138, https://doi.org/10.1016/S0892-6875(00)00096-0, 2000.
Aller, J. Y., Kuznetsova, M. R., Jahns, C. J., and Kemp, P. F.: The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols, J. Aerosol Sci., 36, 801–812, https://doi.org/10.1016/j.jaerosci.2004.10.012, 2005.
Anderson, D. M., Stock, C. A., Keafer, B. A., Nelson, A. B., Thompson, B., McGillicuddy, D. J., Keller, M., Matrai, P. A., and Martin, J.: Alexandrium fundyense cyst dynamics in the Gulf of Maine, Deep-Sea Res. Pt. II, 52, 2522–2542, https://doi.org/10.1016/j.dsr2.2005.06.014, 2005.
Arora-Williams, K., Olesen, S. W., Scandella, B. P., Delwiche, K., Spencer, S. J., Myers, E. M., Abraham, S., Sooklal, A., and Preheim, S. P.: Dynamics of microbial populations mediating biogeochemical cycling in a freshwater lake, Microbiome, 6, 165, https://doi.org/10.1186/s40168-018-0556-7, 2018.
Backer, L. C.: Cyanobacterial harmful algal blooms (CyanoHABs): Developing a public health response, Lake Reserv. Manage., 18, 20–31, https://doi.org/10.1080/07438140209353926, 2002.
Bastviken, D., Tranvik, L. J., Downing, J. A., Crill, P. M., and Enrich-Prast, A.: Freshwater Methane Emissions Offset the Continental Carbon Sink, Science, 331, 50–50, https://doi.org/10.1126/science.1196808, 2011.
Blanchard, D. C.: Bubble Scavenging and the Water-to-Air Transfer of Organic Material in the Sea, in: Applied Chemistry at Protein Interfaces, Advances in Chemistry, 145, AMERICAN CHEMICAL SOCIETY, 360–387, 1975.
Bouma-Gregson, K., Olm, M. R., Probst, A. J., Anantharaman, K., Power, M. E., and Banfield, J. F.: Impacts of microbial assemblage and environmental conditions on the distribution of anatoxin-a producing cyanobacteria within a river network, Isme J., 13, 1618–1634, https://doi.org/10.1038/s41396-019-0374-3, 2019.
Bronmark, C. and Hansson, L. A.: Environmental issues in lakes and ponds: current state and perspectives, Environ. Conserv., 29, 290–307, https://doi.org/10.1017/S0376892902000218, 2002.
Brunberg, A. K. and Blomqvist, P.: Recruitment of Microcystis (Cyanophyceae) from lake sediments: The importance of littoral inocula, J. Phycol., 39, 58–63, https://doi.org/10.1046/j.1529-8817.2003.02059.x, 2003.
Caceres, C. E. and Tessier, A. J.: How long to rest: The ecology of optimal dormancy and environmental constraint, Ecology, 84, 1189–1198, https://doi.org/10.1890/0012-9658(2003)084[1189:HLTRTE]2.0.CO;2, 2003.
Calmano, W., Hong, J., and Forstner, U.: Binding and Mobilization of Heavy-Metals in Contaminated Sediments Affected by Ph and Redox Potential, Water Sci. Technol., 28, 223–235, 1993.
Chanton, J. P., Martens, C. S., and Kelley, C. A.: Gas-Transport from Methane-Saturated, Tidal Fresh-Water and Wetland Sediments, Limnol. Oceanogr., 34, 807–819, https://doi.org/10.4319/lo.1989.34.5.0807, 1989.
Csavina, J., Field, J., Taylor, M. P., Gao, S., Landazuri, A., Betterton, E. A., and Saez, A. E.: A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations, Sci. Total Environ., 433, 58–73, https://doi.org/10.1016/j.scitotenv.2012.06.013, 2012.
Dai, Z. F., Dukhin, S., Fornasiero, D., and Ralston, J.: The inertial hydrodynamic interaction of particles and rising bubbles with mobile surfaces, J. Colloid Interf. Sci., 197, 275–292, https://doi.org/10.1006/jcis.1997.5280, 1998.
Deemer, B. R., Harrison, J. A., Li, S. Y., Beaulieu, J. J., Delsontro, T., Barros, N., Bezerra-Neto, J. F., Powers, S. M., dos Santos, M. A., and Vonk, J. A.: Greenhouse Gas Emissions from Reservoir Water Surfaces: A New Global Synthesis, Bioscience, 66, 949–964, https://doi.org/10.1093/biosci/biw117, 2016.
Delsontro, T., McGinnis, D. F., Sobek, S., Ostrovsky, I., and Wehrli, B.: Extreme Methane Emissions from a Swiss Hydropower Reservoir: Contribution from Bubbling Sediments, Environ. Sci. Technol., 44, 2419–2425, https://doi.org/10.1021/es9031369, 2010.
Delwiche, K., Senft-Grupp, S., and Hemond, H.: A novel optical sensor designed to measure methane bubble sizes in situ, Limnol. Oceanogr. Methods, 13, 712–721, https://doi.org/10.1002/lom3.10060, 2015.
Delwiche, K., Gu, J., Hemond, H., and Preheim, S.: Data Associated with publication “Vertical transport of sediment-associated metals and cyanobacteria by ebullition in a stratified lake”, https://doi.org/10.7281/T1/7WXPIN, Johns Hopkins University Data Archive, V1, 2019.
Delwiche, K. B. and Hemond, H. F.: Methane Bubble Size Distributions, Flux, and Dissolution in a Freshwater Lake, Environ. Sci. Technol., 51, 13733–13739, https://doi.org/10.1021/acs.est.7b04243, 2017.
Eggleton, J. and Thomas, K. V.: A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events, Environ. Int., 30, 973–980, https://doi.org/10.1016/j.envint.2004.03.001, 2004.
Environmental Protection Agency: National Water Quality Inventory: Report to Congress, Office of Water (Ed.), Washington, D.C., 2004.
Evans, S. E., Dueker, M. E., Logan, J. R., and Weathers, K. C.: The biology of fog: results from coastal Maine and Namib Desert reveal common drivers of fog microbial composition, Sci. Total Environ., 647, 1547–1556, https://doi.org/10.1016/j.scitotenv.2018.08.045, 2019.
Hairston, N. G.: Zooplankton egg banks as biotic reservoirs in changing environments, Limnol. Oceanogr., 41, 1087–1092, https://doi.org/10.4319/lo.1996.41.5.1087, 1996.
Hemond, H. F.: Movement and Distribution of Arsenic in the Aberjona Watershed, Environ. Health Persp., 103, 35–40, 1995.
Joyce, J. and Jewell, P. W.: Physical controls on methane ebullition from reservoirs and lakes, Environ. Eng. Geosci., 9, 167–178, https://doi.org/10.2113/9.2.167, 2003.
Kerfoot, W. C.: Bosmina Remains in Lake Washington Sediments - Qualitative Heterogeneity of Bay Environments and Quantitative Correspondence to Production, Limnol. Oceanogr., 40, 211–225, 1995.
Knauer, K., Nepf, H. M., and Hemond, H. F.: The production of chemical heterogeneity in Upper Mystic Lake, Limnol. Oceanogr., 45, 1647–1654, https://doi.org/10.4319/lo.2000.45.7.1647, 2000.
Koh, P. T. L. and Schwarz, M. P.: Modelling attachment rates of multi-sized bubbles with particles in a flotation cell, Miner. Eng., 21, 989–993, https://doi.org/10.1016/j.mineng.2008.02.021, 2008.
Lewandowska, A. U., Sliwinska-Wilczewska, S., and Wozniczka, D.: Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea, Mar. Pollut. Bull., 125, 30–38, https://doi.org/10.1016/j.marpolbul.2017.07.064, 2017.
Lin, S. H. and Lo, C. C.: Treatment of textile wastewater by foam flotation, Environ. Technol., 17, 841–849, https://doi.org/10.1080/09593331708616452, 1996.
Liss, P. S.: Chemistry of the sea surface microlayer, in: Chemical oceanography, 2d ed., edited by: Riley, J. P., Skirrow, G., and Chester, R., Academic Press, London, New York, San Francisco, 193, 1975.
Liu, L., Wilkinson, J., Koca, K., Buchmann, C., and Lorke, A.: The role of sediment structure in gas bubble storage and release, J. Geophys. Res.-Biogeo., 121, 1992–2005, https://doi.org/10.1002/2016jg003456, 2016.
Maeck, A., Hofmann, H., and Lorke, A.: Pumping methane out of aquatic sediments – ebullition forcing mechanisms in an impounded river, Biogeosciences, 11, 2925–2938, https://doi.org/10.5194/bg-11-2925-2014, 2014.
McLinn, E. L. and Stolzenburg, T. R.: Ebullition-Facilitated Transport of Manufactured Gas Plant Tar from Contaminated Sediment, Environ. Toxicol. Chem., 28, 2298–2306, https://doi.org/10.1897/08-603.1, 2009.
Min, Q., Duan, Y. Y., Peng, X. F., Mujumdar, A. S., Hsu, C., and Lee, D. J.: Froth flotation of mineral particles: Mechanism, Dry Technol., 26, 985–995, https://doi.org/10.1080/07373930802115628, 2008.
Nriagu, J. O., Lawson, G., Wong, H. K. T., and Cheam, V.: Dissolved trace metals in Lakes Superior, Erie, and Ontario, Environ. Sci. Technol., 30, 178–187, https://doi.org/10.1021/es950221i, 1996.
Nubel, U., GarciaPichel, F., and Muyzer, G.: PCR primers to amplify 16S rRNA genes from cyanobacteria, Appl. Environ. Microbiol., 63, 3327–3332, 1997.
Paerl, H. W., Hall, N. S., and Calandrino, E. S.: Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change, Sci. Total Environ., 409, 1739–1745, https://doi.org/10.1016/j.scitotenv.2011.02.001, 2011.
Pan, K. and Wang, W. X.: Trace metal contamination in estuarine and coastal environments in China, Sci. Total Environ., 421, 3–16, https://doi.org/10.1016/j.scitotenv.2011.03.013, 2012.
Preheim, S. P., Olesen, S. W., Spencer, S. J., Materna, A., Varadharajan, C., Blackburn, M., Friedman, J., Rodríguez, J., Hemond, H., and Alm, E. J.: Surveys, simulation and single-cell assays relate function and phylogeny in a lake ecosystem, Nature Microbiol., 1, 16130, https://doi.org/10.1038/nmicrobiol.2016.130, 2016.
Ramm, J., Rucker, J., Knie, M., and Nixdorf, B.: Lost in the dark: estimation of the akinete pool for the recruitment of Nostocales populations (cyanobacteria) in a temperate deep lake, J. Plankton Res., 39, 392–403, https://doi.org/10.1093/plankt/fbx010, 2017.
Robarts, R. D. and Zohary, T.: Temperature Effects on Photosynthetic Capacity, Respiration, and Growth-Rates of Bloom-Forming Cyanobacteria, New Zeal. J. Mar. Fresh, 21, 391–399, https://doi.org/10.1080/00288330.1987.9516235, 1987.
Rodrigues, R. T. and Rubio, J.: DAF-dissolved air flotation: Potential applications in the mining and mineral processing industry, Int. J. Miner. Process., 82, 1–13, https://doi.org/10.1016/j.minpro.2006.07.019, 2007.
Rubio, J., Souza, M. L., and Smith, R. W.: Overview of flotation as a wastewater treatment technique, Miner. Eng., 15, 139–155, https://doi.org/10.1016/S0892-6875(01)00216-3, 2002.
Salonen, K., Sarvala, J., Jarvinen, M., Langenberg, V., Nuottajarvi, M., Vuorio, K., and Chitamwebwa, D. B. R.: Phytoplankton in Lake Tanganyika – vertical and horizontal distribution of in vivo fluorescence, Hydrobiologia, 407, 89–103, https://doi.org/10.1023/A:1003764825808, 1999.
Scandella, B. P., Varadharajan, C., Hemond, H. F., Ruppel, C., and Juanes, R.: A conduit dilation model of methane venting from lake sediments, Geophys. Res. Lett., 38, L06408, https://doi.org/10.1029/2011gl046768, 2011.
Scandella, B. P., Pillsbury, L., Weber, T., Ruppel, C., Hemond, H. F., and Juanes, R.: Ephemerality of discrete methane vents in lake sediments, Geophys. Res. Lett., 43, 4374–4381, https://doi.org/10.1002/2016gl068668, 2016.
Schaller, J.: Bioturbation/bioirrigation by Chironomus plumosus as main factor controlling elemental remobilization from aquatic sediments?, Chemosphere, 107, 336–343, https://doi.org/10.1016/j.chemosphere.2013.12.086, 2014.
Schindler, D. W., Hesslein, R. H., and Wagemann, R.: Effects of Acidification on Mobilization of Heavy-Metals and Radionuclides from the Sediments of a Fresh-Water Lake, Can. J. Fish. Aquat. Sci., 37, 373–377, https://doi.org/10.1139/f80-051, 1980.
Schmale, O., Leifer, I., Deimling, J. S. V., Stolle, C., Krause, S., Kiesslich, K., Frahm, A., and Treude, T.: Bubble Transport Mechanism: Indications for a gas bubble-mediated inoculation of benthic methanotrophs into the water column, Cont. Shelf Res., 103, 70–78, https://doi.org/10.1016/j.csr.2015.04.022, 2015.
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W.: NIH Image to ImageJ: 25 years of image analysis, Nat. Methods, 9, 671–675, https://doi.org/10.1038/nmeth.2089, 2012.
Schugerl, K.: Recovery of proteins and microorganisms from cultivation media by foam flotation, Advances in Biochemical Engineering/Biotechnology, 68, 191–233, 2000.
Seifried, J. S., Wichels, A., and Gerdts, G.: Spatial distribution of marine airborne bacterial communities, Microbiologyopen, 4, 475–490, https://doi.org/10.1002/mbo3.253, 2015.
Spliethoff, H. M. and Hemond, H. F.: History of toxic metal discharge to surface waters of the Aberjona Watershed, Environ. Sci. Technol., 30, 121–128, https://doi.org/10.1021/es950169q, 1996.
Stahl-Delbanco, A. and Hansson, L. A.: Effects of bioturbation on recruitment of algal cells from the “seed bank” of lake sediments, Limnol. Oceanogr., 47, 1836–1843, https://doi.org/10.4319/lo.2002.47.6.1836, 2002.
Taylor, K. G. and Owens, P. N.: Sediments in urban river basins: a review of sediment-contaminant dynamics in an environmental system conditioned by human activities, J. Soil Sediment, 9, 281–303, https://doi.org/10.1007/s11368-009-0103-z, 2009.
Tijdens, M., van de Waal, D. B., Slovackova, H., Hoogveld, H. L., and Gons, H. J.: Estimates of bacterial and phytoplankton mortality caused by viral lysis and microzooplankton grazing in a shallow eutrophic lake, Freshwater Biol., 53, 1126–1141, https://doi.org/10.1111/j.1365-2427.2008.01958.x, 2008.
U.S. Environmental Protection Agency: National Lakes Assessment 2012: A Collaborative Survey of Lakes in the United States. Interactive NLA Dashboard, available at: https://nationallakesassessment.epa.gov (last access: 15 June 2020), 2016.
Varadharajan, C.: Magnitude and spatio-temporal variability of methane emissions from a eutrophic freshwater lake/by Charuleka Varadharajan, PhD thesis, Civil and Environmental Engineering, Massachusetts Institute of Technology, 2009.
Varadharajan, C. and Hemond, H. F.: Time-series analysis of high-resolution ebullition fluxes from a stratified, freshwater lake, J. Geophys. Res.-Biogeo., 117, G02004, https://doi.org/10.1029/2011jg001866, 2012.
Vashisth, S., Bennington, C. P. J., Grace, J. R., and Kerekes, R. J.: Column Flotation Deinking: State-of-the-art and opportunities, Resour. Conserv. Recy., 55, 1154–1177, https://doi.org/10.1016/j.resconrec.2011.06.013, 2011.
Verspagen, J. M. H., Snelder, E. O. F. M., Visser, P. M., Huisman, J., Mur, L. R., and Ibelings, B. W.: Recruitment of benthic Microcystis (Cyanophyceae) to the water column: Internal buoyancy changes or resuspension?, J. Phycol., 40, 260–270, https://doi.org/10.1111/j.1529-8817.2004.03174.x, 2004.
Verspagen, J. M. H., Snelder, E. O. F. M., Visser, P. M., Johnk, K. D., Ibelings, B. W., Mur, L. R., and Huisman, J.: Benthic-pelagic coupling in the population dynamics of the harmful cyanobacterium Microcystis, Freshwater Biol., 50, 854–867, https://doi.org/10.1111/j.1365-2427.2005.01368.x, 2005.
Viana, P. Z., Yin, K., and Rockne, K. J.: Field Measurements and Modeling of Ebullition-Facilitated Flux of Heavy Metals and Polycyclic Aromatic Hydrocarbons from Sediments to the Water Column, Environ. Sci. Technol., 46, 12046–12054, https://doi.org/10.1021/es302579e, 2012.
Wallace, G. T., Loeb, G. I., and Wilson, D. F.: On the flotation of particulates in sea water by rising bubbles, J. Geophys. Res., 77, 5293–5301, https://doi.org/10.1029/JC077i027p05293, 1972.
Wetzel, R. G.: Limnology: lake and river ecosystems, Academic Press, San Diego, Calif., 2001.
Xiao, M., Li, M., and Reynolds, C. S.: Colony formation in the cyanobacterium Microcystis, Biol. Rev., 93, 1399–1420, https://doi.org/10.1111/brv.12401, 2018.
Yoon, R. H. and Luttrell, G. H.: The Effect of Bubble Size on Fine Particle Flotation, Mineral Processing and Extractive Metallurgy Review, 5, 101–122, https://doi.org/10.1080/08827508908952646, 1989.
Zhang, X. Q.: The trends, promises and challenges of urbanisation in the world, Habitat Int., 54, 241–252, https://doi.org/10.1016/j.habitatint.2015.11.018, 2016.





