the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Reviews and syntheses: A trait-based approach to constrain controls on planktic foraminiferal ecology – key trade-offs and current knowledge gaps
Maria Grigoratou
Fanny M. Monteiro
Ruby Barrett
Daniela N. Schmidt
Planktic foraminifera are a major contributor to global marine inorganic carbon production. They leave abundant calcium carbonate shells on the seafloor that serve as prime proxies for the physical and chemical attributes of past oceans. Despite their well-preserved fossil record and widespread use in palaeoceanography, our understanding of their ecology remains limited due to their low-standing stocks in the modern ocean and the challenges in culturing multiple generations under laboratory conditions, even after decades of data collection. This limitation affects our ability to interpret their fossil remains to describe past ecosystems and predict their responses to ongoing environmental changes. Trait-based ecology offers a powerful framework to characterise how and why foraminifera interact with their environment. Here, we review the current state of knowledge on key planktic foraminifera traits, including morphological, physiological, behavioural, and life history traits. Most spinose taxa are carnivorous, host to dinoflagellate photosymbionts, and are abundant and diverse in oligotrophic environments. In contrast, non-spinose taxa are typically herbivorous and most common in high-productivity regions. We highlight the potential of trait modelling to generate hypotheses testable in the field. Integration of trait-based modelling with metabarcoding, environmental DNA, and enhanced standardised data collection made openly available will help to fill critical gaps in our understanding of planktic foraminiferal ecology and allow us to use foraminifera as a key model organism for addressing fundamental ecological questions.
- Article
(3600 KB) - Full-text XML
- BibTeX
- EndNote
Planktic foraminifera (heterotrophic marine protists; Fig. 1), along with phytoplankton coccolithophores and planktic snail pteropods, are the dominant calcifying plankton in the modern ocean (Deuser et al., 1981). Among these, planktic foraminifera constitute 23 %–56 % of the global CaCO3 flux from the top 100 m of surface waters and between 32 %–80 % of the total deep-marine calcite budget, with their carbonate shells covering much of the seafloor (Neukermans et al., 2023). Thus, these organisms have pronounced impacts on biogeochemical cycling, in particular the inorganic carbon cycle, by transferring carbon from surface to deep waters, and, over longer timescales, modulating ocean alkalinity, carbonate chemistry, and ultimately climate (Ridgwell and Zeebe, 2005). Moreover, in part due to their calcareous shells that can be preserved in marine sediments for millions of years, planktic foraminifera have one of the best fossil records of all plankton groups (Aze et al., 2011; Fenton et al., 2021). This makes them a critical group for studying the influence of climate change in the past and improving our understanding of the possible impacts of future climate (Strack et al., 2024; Woodhouse et al., 2023; Fenton et al., 2023). Their relative abundances, biometry, and a wealth of geochemical proxies based on shell chemistry are extensively used to reconstruct past climates and oceans, including temperature and ocean chemistry (Kucera, 2007).
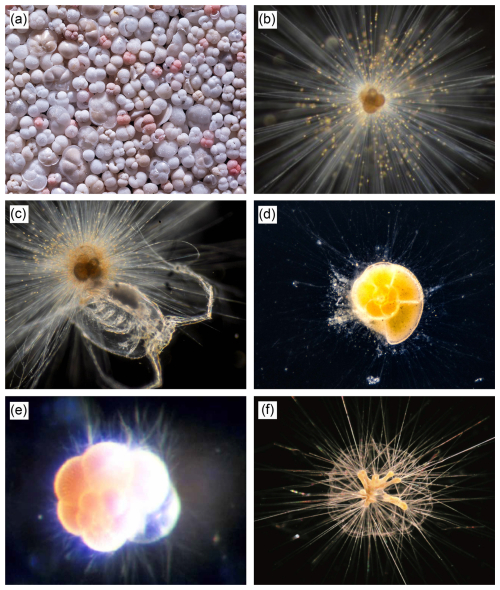
Figure 1Selection of living and recent planktic foraminifera. (a) Assorted shells of planktic foraminifera from sea floor sediment (image credit: Wilfried Rönnfeld). (b) Orbulina universa with symbionts attached along thin radial spines (Fig. 2 of Topa et al., 2017). (c) Orbulina universa eating a small copepod (image credit: Oscar Branson). (d) Globorotalia truncatulinoides in culture with pseudopodial network (a web-like structure formed of pseudopodia (see below) filaments, which interacts with the environment and provides physiological functions) extending from the shell (https://www.usgs.gov/media/images/live-foraminifera-globorotalia-truncatulinoides, last access: 13 July 2025). (e) Neogloboquadrina dutertrei suspended with pseudopodia (cytoplasmic projections that assist locomotion, feeding, and other physiological functions) extending from the shell (image credit: Kate Darling). (f) Hastigerinella digitata with triradiate spines and bubble capsule (Fig. 1a of Hull et al., 2011).
Despite their importance in understanding past oceans, planktic foraminifera ecology has received relatively little attention from modern ecologists due to their small contribution to the total plankton biomass (Michaels et al., 1995; Buitenhuis et al., 2013) and the challenges associated with culturing them (del Campo et al., 2024; Meilland et al., 2024). Developing a mechanistic understanding of the controls on planktic foraminifera diversity and distribution is essential for generating accurate predictions of how changing environmental conditions will impact their communities and, ultimately, biogeochemical cycles (Dutkiewicz et al., 2020).
An improved mechanistic understanding can be supported by trait-based approaches (Dutkiewicz et al., 2020), which describe how species interact with each other and their environment based on measurable organismal characteristics or traits (e.g. size, resource acquisition mode, and defence). Rather than focusing on individual species, these approaches group organisms with similar traits into functional groups, simplifying the characterisation of highly diverse ecosystems like plankton communities, where millions of specimens from thousands of species interact (De Vargas et al., 2015). This approach effectively links individual-level traits to larger ecosystem processes, helping to explain patterns of biodiversity, species distributions, and how communities respond to environmental changes. However, a key challenge is to identify the trade-offs between traits (i.e. costs and benefits) (e.g. Litchman et al., 2013; Barton et al., 2013; Violle et al., 2007; Westoby, 2024). Trade-offs arise when the optimisation of one trait occurs at the expense of another (Kiørboe et al., 2018). Overall, trait-based approaches offer a mechanistic yet computationally efficient means of explaining large-scale patterns of diversity and abundance across microbial, planktic, and nekton communities (e.g. Barton et al., 2013; Dutkiewicz et al., 2009; Follows et al., 2007; Grigoratou et al., 2019; Naidoo-Bagwell et al., 2024; Ying et al., 2024; Ward, 2013; Monteiro et al., 2016; Litchman et al., 2021).
Trait-based approaches are particularly promising for making predictions beyond the sampling domain and testing different (and often complex) hypotheses over longer timescales – both past and future (e.g. Barton et al., 2016; Grigoratou et al., 2022; Ying et al., 2024). This makes this approach particularly valuable in macroevolutionary studies spanning millions of years, where new traits evolve in response to changing environments and climates. By permitting functional groups with novel trait combinations, trait-based models can simulate emergent taxa that may not be present in the modern ocean but could have existed in the past or evolve in the future. For instance, the model of Knoll and Follows (2016) shows how the rise of the mixotrophy trait in the Mesozoic increases energy transfer efficiency to higher trophic levels in the food web. Similarly, Gibbs et al. (2020) used trait-based modelling to explore ecological selectivity in marine plankton following the Cretaceous–Paleogene mass extinction.
While trait-based models have significant potential, they have inherent limitations. Like any models, they are a highly simplified version of natural systems. They may simplify complex ecological interactions and environmental influences, and the expression and importance of traits may vary by environment, impacting generalisation. Additionally, trade-offs between traits are not always well understood or quantified as, for example, the absence or presence of symbionts in foraminifera. The eco-evolutionary model used by Gibbs et al. (2020), for instance, resulted in modelled trait evolution at rates orders of magnitude faster than observed in the fossil dataset, highlighting the need for caution when interpreting these model results. Despite these caveats, trait-based approaches present an exciting opportunity to leverage the exceptional fossil record of foraminifera to test our understanding outside of the modern range of environmental conditions, assess the universal applicability of traits and trade-offs through time, and ultimately improve our understanding of evolutionary processes.
Trait-based modelling has already provided novel insights into planktic foraminifera ecology and fitness. The first non-species-specific trait-based planktic foraminifera modelling study by Grigoratou et al. (2019) highlighted the cost and benefits of calcification and the influence of resource competition among planktic foraminifera and other zooplankton. Ying et al. (2023) further expanded this approach to characterise the main ecogroups of planktic foraminifera (Figs. 1 and 2). In this study, we aim to present a comprehensive review of our current knowledge of planktic foraminifera traits and trade-offs, identify critical knowledge gaps, and propose future research directions to advance the application of trait-based ecology in foraminifera research.
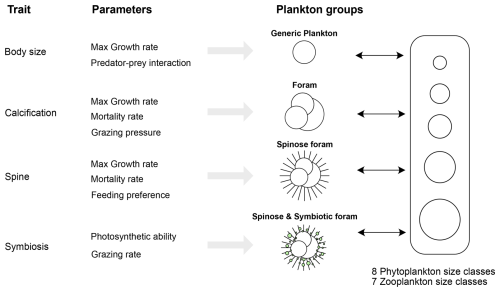
Figure 2Schematic representation of a trait-based ecosystem model incorporating planktic foraminifers. Key functional traits include size, calcification, spinosity, and symbiosis and are assumed to influence foraminiferal interactions within the food web by modifying maximum growth rate, mortality rate, feeding strategy, and other predator–prey interactions.
Planktic foraminifera have predominantly been studied by geologists for their palaeoecology and evolution and as proxy carriers for palaeoenvironmental studies, while most biological studies have, until recently, been from seminal papers by Allan Bé and co-workers, e.g. Bé et al. (1981, 1982), Bé and Anderson (1976), Anderson and Bé (1976), and Anderson et al. (1979), in the 1970s and 1980s. Classification of planktic foraminifera is primarily based on adult morphology, with ∼ 45 morphologically distinct species or “morphospecies” in the modern ocean (Brummer and Kučera, 2022; Schiebel and Hemleben, 2017), many but not all of which consist of cryptic species (i.e. organisms that look identical but represent distinct evolutionary lineages; Morard et al., 2024; Morard et al., 2016).
Planktic foraminifera spend their lives in the open ocean, predominantly the upper ∼ 200 m of the water column (Table 1), with very few individuals or species found below ∼ 1 km water depth (Rebotim et al., 2019; Vincent and Berger, 1981). Planktic foraminifera are absent in shallow marine seas and on coastal shelves (Schiebel and Hemleben, 2017). Some taxa have unique ecologies; for example, Neogloboquadrina pachyderma may spend parts of the year in sea ice (Dieckmann et al., 1991).
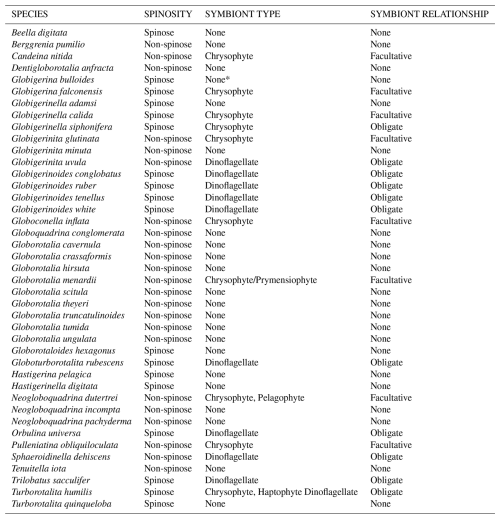
Table 1Modern planktic foraminiferal species list and associated key traits. Species are listed alphabetically and correspond to those listed in the ForCenS database of Siccha and Kucera (2017) and underpin the trait distribution maps shown in Fig. 3. Summary of ecological information from Schiebel and Hemleben (2017), Takagi et al. (2019), and Aze et al. (2011). Obligate symbiosis = essential for optimal host fitness and observed in the majority of individuals of a species. Facultative symbiosis = not essential for host success and only associated with some individuals of the species (Table 1; Takagi et al., 2019). Determining whether symbiosis is obligate or facultative is challenging as photosymbiosis is a spectrum as proposed by Stoecker et al. (2009) from non-symbiosis to robust symbiosis. bacterial endobionts Synechococcus reported by Bird et al. (2017) but unclear if symbionts.
Here we characterise planktic foraminiferal traits according to their type and function, following the approach of Litchman et al. (2013) (Table 2). We discuss all identified key traits, along with their individual impacts on foraminiferal fitness, as well as their main associated trade-offs. We recognise two levels of traits: (1) those that are common to all planktic foraminifera, referred to as “foraminiferal” traits, and (2) those that are specific to individual species or groups of species, referred to as “group-/species-specific” traits.
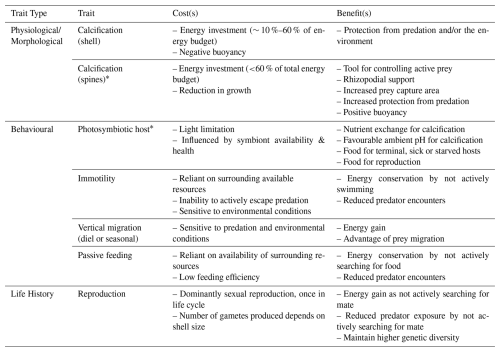
Table 2Summary of planktic foraminifera traits and associated trade-offs described in Sect. 2.1–2.3. Traits with “*” are group-/species-specific traits; all other traits are common to all planktic foraminifera. Rhizopodia refers to the network of cytoplasmic strands around the shell that help to capture, transport, and break up prey items and carry waste out of the cell.
2.1 Physiological/morphological traits
2.1.1 Body size (foraminiferal trait)
Size is often considered a master trait because it influences most relationships between organisms in an ecosystem and how they respond to and influence their environment (Brown et al., 2004; Peters, 1983). Body size directly impacts physiological and ecological aspects like metabolic rates (e.g. growth), diet, predator–prey relationships, abundance, biomass, and reproduction in organisms (e.g. Kiørboe et al., 2018; McKinney, 1990). For instance, the primary mode of resource acquisition for organisms shifts from osmotrophy in the smallest single-celled organisms to phototrophy, mixotrophy, and ultimately heterotrophy as body size increases (Andersen et al., 2016; Haldane, 1926). Body size also influences predator–prey relationships by impacting the range of prey sizes an organism can consume, with prey being typically smaller than the predator (Gaskell et al., 2019; Wirtz, 2012).
Planktic foraminifera grow their organic cell by adding multiple chambers in their calcite shell (Brummer et al., 1987; Caromel et al., 2016). Depending on the species, the shell size of adult individuals can vary from ∼ 100 to 1500 µm (Schmidt et al., 2004). At the individual level, organisms typically reach their largest shell size under specific optimal environmental growth conditions (Chernihovsky et al., 2023; Schmidt et al., 2004), although some show no relationships between size and environments (Rillo et al., 2020). Temperature and suitable prey availability have been identified as the primary environmental factors influencing shell growth, followed by pH, salinity, and light conditions – particularly for species that maintain symbiotic relationships with algae. As a group, planktic foraminifera reach large sizes in the tropics, while smaller individuals dominate at the higher latitudes and in equatorial upwelling regions (Schmidt et al., 2004). This size distribution contrasts with most zooplankton species, which exhibit their largest sizes in polar regions (Brandão et al., 2021; Horne et al., 2017). The size distribution of foraminifera may reflect a combination of factors including higher temperatures and thus metabolic rates promoting growth, higher carbonate saturation and light intensity promoting calcification, more energy subsidies from algal photosymbionts, increased volume for prey encounter, or enhanced niche diversity through stratification (Schmidt et al., 2004; Burke et al., 2025). Shell size not only responds to environmental conditions but also directly reflects individual fitness and reproduction (Hemleben, 1989). Larger foraminifera have a greater amount of cytoplasm available to generate gametes and hence potentially higher reproductive success (Bé and Anderson, 1976; Weinkauf et al., 2022). In the open ocean, a semi-lunar periodicity in shell fluxes (i.e. shells exported to the sea floor after death) of several species is interpreted as the presence of a circadian clock (Bijma et al., 1990; Jonkers and Kučera, 2015; Lončarić et al., 2005). This periodicity is modulated by seasonal changes in shell fluxes (Jonkers et al., 2015) with intervals characterised by smaller body sizes and extended life cycles during unfavourable conditions and food scarcity contrasting with times of high foraminiferal fluxes and larger shell sizes (Chernihovsky et al., 2023). For organisms with a circadian clock and therefore a fixed life duration, large sizes may reflect increased growth rates (rather than prolonged growth), versus rapid reproduction and smaller body size as seen in many phytoplankton (Schmidt et al., 2006).
2.1.2 Calcification (foraminiferal trait)
The primary function of producing a calcite shell in planktic foraminifera is still debated. However, one potential advantage is that the shell and, to a lesser extent, the spines in spinose species provide the cytoplasm with support and protection, increasing the individual's potential to survive to reproductive maturity. This is achieved by (1) reducing their palatability to predators, (2) increasing their body size to reduce predation pressure, and (3) forming a potential barrier from environmental conditions such as harmful UV rays or pathogens (Brasier and Armstrong, 2004).
The mineralised shell can comprise a significant proportion of an organism's total mass, making the construction and maintenance energetically expensive (Sanders et al., 2018). However, it is difficult to quantify the energetic costs of biomineralisation, and foraminifera (as many organisms) can modulate their energy use between different activities depending on the environment. An unclear understanding of how biomineralisation occurs for many groups further exacerbates the challenge (Gaylord et al., 2015). No laboratory assessments of the energy budget allotted to calcification in planktic foraminifera currently exist. However, theoretical trait-based models indicate a likely investment of ∼ 10 %–60 % of foraminifera's total energy budget to calcification (Grigoratou et al., 2021, 2022, 2019).
A calcite shell reduces the buoyancy of foraminifera; i.e. it increases their tendency to sink through the water column (Brasier and Armstrong, 2004; Caromel et al., 2014), although this is counterbalanced by low-density fibrillar bodies within the cytoplasm (Hemleben, 1989). Spinose foraminifera taxa have long calcite spines that may extend up to several centimetres from the shell surface (2–3× the shell size; Fig. 1b, c and f), which increases their drag and thus reduces their sinking rate (Takahashi and Bé, 1984). Other benefits of spines include an increased capture area for prey acquisition, reduced palatability to predators, a rigid skeleton to anchor pseudopodia to enable acquisition of higher-quality (active) prey, and a means of organising photosymbionts more effectively around the shell. Logically, the development of these compensatory features should increase the relative cost of calcification. However, trait-based modelling suggests similar calcification costs for both spinose and non-spinose foraminifera species (Grigoratou et al., 2021; Grigoratou et al., 2022; Grigoratou et al., 2019). This suggests that spine formation either does not significantly increase energy demand or is offset by nutritional benefits. Spinose species are predominantly carnivorous as adults, which provides more energy, and many also host algal photosymbionts, providing an additional carbon subsidy. While non-spinose species are omnivorous, they prefer a herbivorous diet (Hemleben et al., 1985) (see Sect. 2.2).
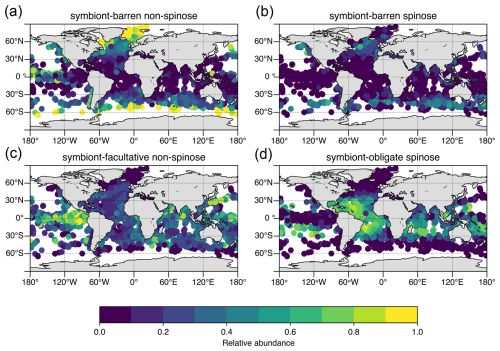
Figure 3Modern distribution and relative abundance of planktic foraminifera by ecological group in surface sediment samples. Underlying data from ForCenS database (Siccha and Kucera, 2017) and traits coded as presented in Table 1 (this study).
The range of estimated energetic calcification costs for foraminifera is broadly consistent with those of other calcifying groups (although estimates vary dramatically): coccolithophores at ∼ 30 % (Monteiro et al., 2016), marine benthic gastropods at <60 % (Palmer, 1992; Sanders et al., 2018), and <10 % across gastropods and bivalves globally (Watson et al., 2017). In the calcareous algae coccolithophores, the high energetic cost of calcification varies between species and environments, and protection from predation may be the primary benefit (Monteiro et al., 2016). However, the role of predation on foraminifera is unknown. Whilst adult foraminiferal shells have been found in pteropods, salps, shrimps and many other metazooplankton, we do not know of any specialised predators (Berger, 1971). Nevertheless, the role of predation on populations should not be underestimated, as many juveniles do not reach maturity (Schiebel et al., 1995) and are selectively found in faecal pellets of salp (Bé, 1977). Juvenile forms may have a higher palatability due to their higher ratio of cytoplasm to calcite (i.e. lack of numerous spines, thick calcite walls and gametogenic calcite) than adult forms (Meilland et al., 2016). In culture, damaged specimens or those undergoing gametogenesis were highly susceptible to digestion by other smaller protists (bacteria, sporozoans, and other parasitic organisms) that entered the foraminifera via the aperture (Hemleben, 1989).
Despite numerous data on the weight of foraminiferal shells (see Barrett et al., 2025, for a recent review), there are still large gaps in our understanding of the metabolic and energetic needs for calcification. However, it is clear that some specific traits within ecogroups, for example symbiosis (see Sect. 2.2.3), can positively impact calcification due to modification of the calcifying microenvironment and provision of an energy subsidy (e.g. Köhler-Rink and Kühl, 2005). Biomineralisation pathways in planktic foraminifera are poorly known but are inferred to be extracellular, with calcification occurring predominantly via endocytosis, based on analogy to benthic foraminifera, e.g. Amphistegina lobifera (Bentov et al., 2009; Schiebel and Hemleben, 2017). Briefly, seawater is engulfed in vacuoles by the foraminiferal plasma membrane, modified in composition to become “alkalinised” via assorted biological and chemical processes, and transported to the site of calcification. Enhanced respiration near the site of calcification locally increases the CO2 in the foraminiferal cytoplasm and ultimately the pool of total inorganic carbon available for calcification (Bentov et al., 2009). Alternative foraminiferal biomineralisation pathways are suggested (Erez, 2003; de Nooijer et al., 2014; Bentov and Erz, 2006; Nehrke et al., 2013). This includes the benthic foraminiferal group, the miliolids, which precipitate an imperforate porcelaneous shell intracellularly, but this is very different in structure to planktic foraminifera shells (Hemleben et al., 1985). Many studies identify species-specific calcification responses and sensitivities associated with a range of environmental drivers (Barker and Elderfield, 2002; Béjard et al., 2024; Weinkauf et al., 2016; Pallacks et al., 2023). Understanding those drivers is complicated by differential environmental preferences of cryptic species (De Vargas et al., 2001), regional plasticity, and a lack of understanding of the controls on the thickness of gametogenic calcite or the biomineralisation pathway (de Nooijer et al., 2023; Erez, 2003; LeKieffre et al., 2018; Nehrke et al., 2013). Furthermore, the variety of methods used to collect individuals for foraminifera weighing (i.e. the use of plankton tow, sediment trap, and core-top data, as well as differences in the weighing technique itself) (Beer et al., 2010) and inadequate metadata reporting lead to further difficulty in understanding calcification. Our understanding of what drives calcification could be enhanced by resolving the aforementioned biological factors (i.e. resolving cryptic species, understanding regional plasticity, identifying what controls the thickness of gametogenic calcite, and determining the biomineralisation pathway) and improving data collection and data management practices, e.g. by following FAIR principles (Wilkinson et al., 2016). In turn, this will result in better modelling of the potential changes in the pelagic carbonate production under future climate change (Barrett et al., 2025).
The cost of calcification increases under ocean acidification scenarios in a wide range of (though not all) marine calcifiers (Hoppit and Schmidt, 2022), e.g. corals, molluscs, and coccolithophores (Leung et al., 2022). Impacted groups typically show evidence of reduced growth, reduced calcification, muscle wastage, or weakened shells (e.g. Alma et al., 2020; Hill and Hoogenboom, 2022; Swezey et al., 2020), supporting the finding that the metabolic costs of calcification are at the expense of other life processes. While the allotment of the foraminifera energy budget to calcification is not well constrained, its cost is evidenced in the smaller terminal body sizes (e.g. maintaining calcification at the expense of growth) in field and laboratory studies (Schmidt et al., 2003; Russell et al., 2004; Lombard et al., 2010). This results in a reduced chance of individuals reaching sufficient size to obtain reproductive maturity (Bé et al., 1981) and potentially a smaller number of gametes based on the relationship between gamete number and body size, reducing reproductive success. This ultimately impacts the fitness of individuals by increasing their vulnerability to further environmental change or predation, etc., which may prove fatal.
2.2 Behavioural traits
2.2.1 Feeding (group-specific trait)
The biggest challenge faced by marine plankton is the acquisition of food from a very dilute suspension without being eaten, a challenge exacerbated in low-prey-density environments such as oligotrophic gyres. In these environments, organisms must maximise opportunities for finding food and capturing their prey. Marine plankton adopt a range of different feeding strategies to deal with the challenge, each with specific trade-offs that extend beyond an impact on feeding behaviour alone.
All planktic foraminifera are passive ambush feeders (Fenchel, 1986; Kiørboe, 2011). They do not actively detect or seek out their prey. Acquisition of food relies solely on the motility of their prey and the likelihood of direct interception, a function of prey density, and foraminifera's ability to “capture” prey (Kiørboe, 2011). The benefits of passive food capture are its low energy investment, as organisms do not need to move to acquire their prey, and reduced predation risk due to lower predator encounter rates. However, the cost of the passive ambush feeding strategy is a low feeding efficiency and potentially low mate encounter rate (Kiørboe, 2011). This trade-off is very well constrained in copepod populations, with passive ambush feeders having lower metabolic and mortality rates than their more active counterparts (Eiane and Ohman, 2004).
The importance of passive feeding as the main nutritional mode is supported by the correlation between growth rate and feeding frequency (Bé et al., 1981). Foraminifera are capable of digesting most organic materials, presumably a survival strategy for environments where one or more food sources may not be available and mitigating environmental change. They have an extensive network of thin, sticky strands of cytoplasm (rhizopodia) around their shell that are used to help capture, transport, and break up prey items, as well as carry waste products out of the cell (Hemleben, 1989). During the early life stages (prolocular-juvenile, ∼ 15–100 µm shell diameter; Spindler et al., 1984; Caron et al., 1987), foraminifera are omnivorous, although they primarily consume an herbivorous diet.
As some foraminiferal species transition from the juvenile to neanic stage (>100 µm shell diameter), they may become host to photosymbiotic algae and/or develop spines. Spines provide a rigid framework for rhizopodia to attach and increase foraminifera's capture area (Gaskell et al., 2019), allowing them to capture and hold active prey, facilitating a switch from a predominantly herbivorous to carnivorous diet (Fig. 1c; Anderson et al., 1979; Schiebel and Hemleben, 2017). For example, O. universa shifts from a herbivorous diet during its early and juvenile phase to a carnivorous diet in the spherical adult stage (Schiebel and Hemleben, 2017). A wide variety of animal prey can be consumed by spinose planktic foraminifera, including copepods, hyperiid amphipods, tunicates, ostracods, pteropods, gastropod larvae, ciliates, radiolarians, acanthurids, and polychaete larvae (Anderson et al., 1979). Observational evidence suggests that copepods account for >40 % of spinose taxa diets, with an additional ∼ 20 %–30 % from ciliates and the remainder made up of a variety of prey items (Schiebel and Hemleben, 2017). In a laboratory environment, spinose taxa exhibited higher acceptance rates of calanoid over cyclopoid copepods (Spindler et al., 1984), indicating some degree of prey selectivity. Spinose planktic foraminifera are capable of capturing and digesting prey items 2–3 times bigger than their body size (Schiebel and Hemleben, 2017). Carnivory is advantageous in a food-poor environment for organisms relying on chance encounters, because individual prey items have a higher calorific value relative to similarly sized phytoplankton (Boyd and Goodyear, 1971).
Non-spinose planktic foraminifera are omnivorous but prefer a herbivorous diet throughout their life cycle (Hemleben et al., 1985; Schiebel and Hemleben, 2017), preying on diatoms, dinoflagellates, and eukaryotic algae. Copepods (dead and alive) have been given in culture to several non-spinose species (Globorotalia truncatulinoides, G. hirsuta, G. inflata, Globigerinita glutinata, and Pulleniatina obliquiloculata), but it was found these taxa are unable to capture and hold live zooplankton and are only able to consume zooplankton if they are immobilised or dead (Spindler et al., 1984; Anderson et al., 1979). In the ocean, non-spinose species such as G. menardii can capture and control small ciliates using their rhizopodia, as evidenced by muscle and other animal tissues in food vacuoles (Anderson et al., 1979). Metabarcoding studies have shown that the Arctic non-spinose species Neogloboquadrina pachyderma include animal tissue in their cytoplasm (Greco et al., 2021); however, it remains unclear whether this tissue comes from live or dead prey. Hence, the predominantly herbivorous diet may largely be because of their difficulty in capturing living zooplankton. Under laboratory conditions, non-spinose species exhibit cannibalism, but whether they cannibalise in the natural habitat is unknown and considered unlikely due to the very low foraminiferal abundance (Schiebel and Hemleben, 2017; Westgård et al., 2023).
The temporal and spatial distribution of prey items is a major driver of the regional distribution of planktic foraminifera species, influencing their growth and fecundity in addition to sea surface temperature (Schiebel and Hemleben, 2017; Schmidt et al., 2004; Adebayo et al., 2023; Lombard et al., 2011). Spinose taxa are most abundant in (sub)tropical oligotrophic gyres (Fig. 3), where copepods are most abundant (Grice and Hart, 1962), whereas non-spinose species are most abundant in upwelling and coastal waters and at higher latitudes (>30°), which are rich in phytoplankton and small zooplankton (Grice and Hart, 1962).
Model simulations of trophic dynamics require data on prey preferences, e.g. prey acceptance rates, protein acquisition from zooplankton versus phytoplankton, and average digestion and capture times. Observational data can be collected by analysing feeding vacuoles and metabarcoding analyses of in situ samples. For planktic foraminifera, this information is scarce and limited to a few taxa (e.g. the spinose species Trilobatus sacculifer, Globigerinoides ruber, and Orbulina universa), which are most easily cultured in the laboratory. It is unclear, though, how representative laboratory environments are of natural feeding behaviours given the stress responses of some species, e.g. shortening or spine loss of G. ruber (Bijma et al., 1990). Furthermore, many critical parameters are known only from a single experiment. There is a clear need to capitalise on the recent success in culturing of a wider range of taxa, such as the eutrophic G. bulloides (Sykes et al., 2024) or the polar N. pachyderma (Meilland et al., 2023, 2024), to explore these questions across a wide range of foraminifera ecogroups. Multigenerational experiments would also allow closure of the important gap of quantitative data on the herbivory preferences or digestion rates of juveniles or the role of bacteria and organic matter in foraminiferal diet.
There are many further gaps in knowledge about the costs and benefits of being a passive ambush feeder, competition with other foraminifera or their close relatives, the siliceous radiolarians, all of which impact our ability to consider interactions with zooplankton in trait models. To advance trait models, data are needed on prey preferences, prey–predator optimum length ratio, and encounter rates (successful and unsuccessful) to cover the energetic needs for calcification and other metabolic costs.
2.2.2 Starvation tolerance/dormancy (group-specific trait)
Feeding experiments indicate that when food is available at the optimum frequency, foraminifera reproduce quickly, but the reverse is also true. In culture, planktic foraminifera can survive and grow for 16–46 d with little or no food after initial capture, i.e. in some cases beyond their “normal” lifespan in the ocean (Anderson et al., 1979; Bé et al., 1982). However, associated low growth leads ultimately to lower-standing stocks and/or smaller body sizes, threatening their ability to undergo gametogenesis or, as volume is linked to the number of gametes, fewer gametes (Bé et al., 1981). The taxa that have survived longest in culture (>230 d) are asymbiotic herbivorous taxa such as Neogloboquadrina pachyderma, with growth optimised for low temperatures (Lombard et al., 2010) with presumably lower metabolic rates (Spindler, 1996).
N. pachyderma is the dominant planktic foraminifera at high latitudes and thrives in upwelling and (sub)polar oceans, able to survive low temperatures (−2 to +15 °C) and a wider range of pH and salinities (Westgård et al., 2023). This is the only planktic foraminifera species for which dormancy is known. Specifically, Antarctic genotype IV of N. pachyderma of Darling and Wade (2008) is observed to overwinter in brine channels and pockets within Antarctic sea ice (Spindler and Dieckmann, 1986; Dieckmann et al., 1991; Berberich, 1996; Spindler, 1996). This species can survive and grow in sea ice but does not reproduce. Brine pockets and channels can have salinities up to 177 psu and temperatures as low as −15 °C. However, at >50 psu, individuals grew more slowly and reached smaller overall body sizes and were unable to undergo gametogenesis. At >73 psu, pseudopodal activity and movement ceased, but they were able to survive for up to several weeks at up to ∼ 82 psu without feeding (Spindler, 1996). This finding is supported by more recent culturing work suggesting tolerance to a wide range of salinities but also that specimens are less active and less likely to add new chambers at very high or low salinities or reproduce (Bertlich et al., 2021; Westgård et al., 2023). Indeed, in culture, specimens that were inactive (e.g. no growth, limited/no rhizopodal activity) or dormant (appeared to have empty shells/decaying cytoplasm and did not feed) were able to recover as conditions became more favourable (Westgård et al., 2023). The same brine pockets and channels also contain dense populations of phytoplankton, predominantly diatoms, a rich potential food source for N. pachyderma (Spindler and Dieckmann, 1986; Dieckmann et al., 1991). Overwintering via dormancy is thus advantageous because it allows N. pachyderma to suppress metabolic activity to reduce energy consumption (e.g. buoyancy compensation and growth) and survive unfavourable environmental circumstances, but it also provides a rich food source for when conditions are favourable and protection from predators (Dieckmann et al., 1991). Dormancy ultimately means that taxa inhabiting sea ice can significantly extend their “normal” lifespan, potentially surviving for up to 1 year from the formation of sea ice in the autumn until the following spring/summer (Spindler, 1996).
2.2.3 Photosymbioses (group-specific traits)
Symbiosis between different biological organisms is a common ecological strategy in the ocean from shallow benthic marine ecosystems (e.g. coral reefs) through to the nekton and plankton, a major source of evolutionary innovation and hence biodiversity (Decelle et al., 2015; Margulis, 1993). Planktic symbiotic relationships in the open ocean are relatively poorly constrained in contrast to the benthic shallow-water counterparts; however, symbiosis in planktic foraminifers appears to represent an adaptation to nutrient-poor, sunlit waters (Fig. 3d; Bé and Tolderlund, 1971).
Many of the largest benthic and planktic foraminifera in the modern ocean tend to host algal photosymbionts (Kucera, 2007), highlighting the important role of symbiosis in providing additional energy to support the energetic costs of building a larger skeleton. In foraminifera, species that occupy the photic zone are commonly host to algal photoendosymbionts, either chrysophytes or dinoflagellates (Fig. 1b and c). Symbionts are acquired by juvenile foraminifera from the water column following sexual reproduction (Hemleben, 1989) but potentially also via the parent by direct vertical transmission during the more poorly known asexual reproductive cycle (Takagi et al., 2020). Whilst many spinose taxa are host to dinoflagellate photosymbionts, there are exceptions. For example, G. bulloides IId is associated with bacterial endobionts, e.g. Synechococcus in the eastern Pacific Ocean (Bird et al., 2017), or none, e.g. Hastigerina pelagica (Takagi et al., 2019). Some non-spinose taxa such as G. glutinata or Globigerinella siphonifera may also host photosymbionts, mostly chrysophytes (Takagi et al., 2019). However, whether these species have a truly symbiotic relationship with foraminifera or just utilise waste products is currently unknown (Hemleben, 1989). Available data suggest that the type of symbiont dictates photophysiology rather than host size or spines (Hoadley et al., 2019; Takagi et al., 2019). This conclusion is supported by the likely evolution of dinoflagellate symbiosis in the Cretaceous before spines or spine-like structures evolved in the Palaeocene (Hoadley et al., 2019; Pearson et al., 2001; Bornemann and Norris, 2007), suggesting that whilst spines may help to optimise photosymbiont activities, they are not essential (and, therefore, this is not the primary function of spines).
Symbiosis can be described as obligate (essential for optimal host fitness and observed in the majority of individuals of a species, making them functionally mixotrophic) or facultative (not essential to the host success and thus only associated with some individuals of the species) (Table 1; Takagi et al., 2019). Determining whether symbiosis is obligate or facultative is challenging as photosymbiosis is a spectrum, as proposed by Stoecker et al. (2009), from non-symbiosis to robust symbiosis. We have direct observations of symbiotic presence or absence in more than 30 species (see Table 1), typically recognised via microscopic observations (Anderson and Bé, 1976) and/or molecular work (Gast and Caron, 1996; Shaked and de Vargas, 2006). However, it is difficult to clearly differentiate between digested and active symbiosis with these methods. Active chlorophyll fluorescence, a non-destructive and invasive approach that allows assessment of fluorescence through ontogeny, is a powerful technique helping to close this knowledge gap and advance modelling traits related to symbioses by enabling determination of the chlorophyll a content of specimens, health of symbionts, and their light-level adaptation (Takagi et al., 2019; Takagi et al., 2016).
Within planktic foraminifera, taxa with obligate (dinoflagellate) photosymbionts tend to dominate oligotrophic regions with expanded mixed layers and high light penetration (Fig. 3). Symbionts are rarer in cold, low-light high-latitude areas, in deep waters >200 m and in eutrophic regions of the ocean, presumably where they are either unable to survive or unnecessary (Hemleben, 1989). No symbiont-bearing taxa are found at very high latitudes (>∼ 50°; Fig. 3).
Photosymbionts provide an important energy supplement to their host in the form of photosynthetically fixed carbon, aiding growth, longevity, calcification, and reproductive potential (e.g. LeKieffre et al., 2018; Bé et al., 1982), but photosymbionts alone do not provide sufficient carbon subsidies to entirely support foraminiferal life processes. At the same time, symbionts preferentially use metabolites from the foraminifera for photosynthesis (Takagi et al., 2018). Algal photosymbionts also aid calcification by increasing the pH of the foraminifera's immediate microenvironment above ambient seawater by utilising CO2 during photosynthesis and therefore potentially enhancing calcification (Rink et al., 1998; Köhler-Rink and Kühl, 2005).
Photosymbionts are acquired as juveniles or, as new evidence suggests, provided to gametes during gametogenesis (Takagi et al., 2020) and are typically arranged in the external rhizopodial net during the day and brought into the shell at night (e.g. Anderson and Bé, 1976; LeKieffre et al., 2018). Symbiont biomass rises and falls during the host's life (Takagi et al., 2016), with the symbionts eventually being consumed immediately prior to gametogenesis (Bé et al., 1983). Digestion of photosymbionts may help to meet the energy demands of gametogenesis or provide energy during periods of prolonged darkness (e.g. Spero and Parker, 1985; Bé et al., 1983; Takagi et al., 2016). However, even under starvation, some symbionts are retained in culture until gametogenesis (Takagi et al., 2018).
Takagi et al. (2019) showed that photosynthetic activity in symbiont-facultative species tends to be weaker than in symbiont-obligate species, and thus nutritional benefit to the host may be smaller. However, the benefit of facultative symbiosis is still unclear. It may be opportunistic and may overall support a more flexible range of nutritional sources for the host. A lower reliance on symbiont activity in organisms might also allow these taxa to explore low-light eutrophic regions, including the deep-water layers and the turbulent upwelling regions. A recent study developing the ForamECOGEniE model to incorporate the different symbiosis types (Ying et al., 2023) replicated patterns of asymbiotic and obligate symbiotic global distributions but underestimated the abundance of the non-spinose symbiont-facultative group, particularly in the eastern equatorial Pacific. The challenge of modelling this group is the lack of information on what drives the symbiont-facultative group to acquire or lose their symbionts. Without a clear ecological/physiological understanding, trade-offs cannot be incorporated into the model. Intriguingly, if the abundances of the obligate and facultative symbiotic taxa are combined, there is a good fit between the model and observations (Ying et al., 2023). This may suggest that the facultative group overall exploits the same benefits from symbiosis. However, determining the mechanistic underpinning is critical to understand the data, particularly the environmental/biological conditions under which symbiosis is active or not, and the benefits of the relationship to the host. Thus, culture experiments and further observation of this phenomenon are still required.
The costs associated with hosting photosymbionts are that (1) they restrict foraminifera's depth habitat to the euphotic zone (generally <200 m water depth; Caron et al., 1987; Schiebel and Hemleben, 2017), and (2) the host is dependent on changes in the availability and health of the symbionts, which respond to environmental change. Studies suggest that foraminifera symbionts are not favoured in low pH waters (Henehan et al., 2017) or in eutrophic areas due to light limitation (Ortiz et al., 1995). Reduced photosynthetic activity results in smaller final body sizes and shorter survival times (Ortiz et al., 1995; Bé et al., 1981; Caron et al., 1982; Faber et al., 1989). During extreme temperatures in the geological record, planktic foraminiferal populations of “bleached” individuals may have persisted for thousands of years with smaller sizes and lower population abundances (Edgar et al., 2013; Wade et al., 2008), which may have impacted fitness and increased their susceptibility to extinction (e.g. Wade et al., 2008).
The knowledge gap relating to symbioses, beyond the fundamental knowledge of which symbiont each species hosts, includes what triggers changes in symbiont activity and mode of symbiosis, the degree of energy subsidy symbionts provide to their host, and further understanding of the use of symbionts as prey items. For G. bulloides, the nature of their association with bacterial endobionts also needs further exploration to understand how widespread the association is between genetic types and within the ocean. Without this understanding, and the ecological impact on the symbiont, the future fitness of planktic foraminifera cannot be explored fully, nor can the importance of the loss of symbiosis in the fossil record after extinctions (Birch et al., 2016).
2.3 Reproduction
2.3.1 Sexual reproduction (foraminiferal trait)
Planktic foraminifera were traditionally observed to only reproduce sexually (e.g. Bé and Anderson, 1976, Ketten and Edmond, 1979, and references in Schiebel and Hemleben, 2017) differing from their benthic counterparts that can alternate between sexual and asexual reproductive modes (Goldstein, 1999). Most shallow-dwelling planktic foraminifera reproduce on a semi-lunar or lunar synodic cycle (∼ 2–4 weeks), whereas intermediate- to deeper-dwelling species may live for up to 1 year (Spindler, 1979; Hemleben, 1989). Foraminifera migrate to reproduce at depth close to the deep chlorophyll maximum, where there are optimum feeding and grazing protection opportunities for offspring (Hemleben, 1989). Notably, most observational work was on spinose species. During gametogenesis, foraminifera undergo a suite of morphological, physiological, and ecological changes. They retract their rhizopodia, shorten and shed their spines by dissolution at the tips and resorption at the base, consume any photosymbionts, and precipitate an additional outer layer of calcite (gametogenic calcite) over the shell (Schiebel and Hemleben, 2017). Gametogenesis ends with the conversion of all cytoplasm into gametes via vacuolisation, which are released directly into the water column (broadcast spawning). Gametogenesis takes ∼ 1–3 d from the formation of the final chamber (Bé et al., 1983), resulting in an empty adult shell which sinks to the seafloor (Siebold and Berger, 1993).
Sexual reproduction allows populations to maintain higher genetic diversity and select for advantageous mutations or conversely eradicate unfavourable mutations (Otto and Lenormand, 2002). Therefore, sexual reproduction provides a definite (if though difficult to quantify) advantage for survival in the dynamic surface waters with constantly changing selective pressures (Lynch, 1991). In addition, the broadcast spawning strategy presumably confers energy savings compared to organisms actively searching for mates and reduced predator encounters. However, the typically low concentration of foraminifera in the water column (∼ 1 specimen per m3 in the open ocean) makes this a risky strategy. Further, any mismatches in the timing (or place) of reproduction between individuals would reduce the chance of fertilisation.
Planktic foraminifera have developed a number of strategies to maximise the successful fertilisation of gametes from different parents in the water column, including (1) the synchronisation of the timing and depth of reproduction between multiple individuals of the same species, (2) the release of a large number of gametes (200 000–400 000 individuals; dependent on both shell and gamete size) from each adult, thereby increasing encounter and survival rates, and (3) the development of motile gametes (see Weinkauf et al., 2022, and references therein).
2.3.2 Asexual reproduction (species-/group-specific traits)
Recent culture breakthroughs have shown that some non-spinose planktic foraminiferal species (N. pachyderma, G. glutinata, and G. uvula) can reproduce both asexually and sexually (Davis et al., 2020; Kimoto, 2006; Meilland et al., 2023; Meilland et al., 2024, Takagi et al., 2020). In N. pachyderma, precursors to asexual reproduction include feeding in large quantities, the development of bright red cytoplasm, and maintenance of the rhizopodial network, the latter in contrast to pre-gametogenic changes (Greco et al., 2023; Meilland et al., 2024). There also remains a positive relationship between the size of the foraminifera shell and the number of offspring under asexual reproduction, albeit with fewer offspring per individual (∼ 80–300) than via sexual reproduction (Meilland et al., 2024). However, there is generally considered to be higher survival of offspring compared to sexual reproduction; hence, this reproductive mode supports rapid growth of planktic foraminifera populations when conditions are optimal. Asexual reproduction also provides a mechanism to explain the survival and rapid population growth of N. pachyderma at high latitudes (Davis et al., 2020; Meilland et al., 2024).
The ability of at least some species of planktic foraminifera to switch between sexual and asexual reproduction helps to reconcile rapid population growth in response to temporally and spatially optimal conditions and represents a distinct advantage for survival of polar species in particular (Davis et al., 2020). It is highly likely that future targeted experimental work will expand the evidence base for the number of planktic foraminifera species that can reproduce asexually.
Modelling the development of foraminifera in a way that resembles their accretionary growth has not been achieved to date. The main challenge is the lack of information on the different traits and trade-offs in their development, such as changes in metabolic rates, and food uptake. The new culturing breakthrough, which enables us to explore the full life cycle of planktic foraminifera, opens the door to explore these questions and close our knowledge gaps.
Planktic foraminifera are an ideal target group for testing trait-based approaches, as a relatively small number of functional traits can define their ecological niches in the modern ocean. However, many of the traits identified here remain poorly qualified and quantitatively constrained, requiring further observational and experimental laboratory-based investigations.
A key trait requiring further study is calcification. Its trade-offs (Table 2), particularly its hypothesised benefit of protection against grazing, are fundamental to current trait-based models for calcifying plankton but still lack direct evidence (Barrett et al., 2025; Monteiro et al., 2016). We also require an understanding of the energetic costs of calcification and the mechanisms controlling biomineralisation. This requires moving away from analogies largely based on physiological calcification studies of benthic foraminifera (e.g. De Nooijer et al., 2009, 2014) to similar experiments on planktic foraminifera. At present, the poorly constrained relationship between calcification and seawater carbonate chemistry limits our ability to predict the impact of ocean acidification on foraminiferal growth and carbonate production. These knowledge gaps also hamper accurate modelling of ocean alkalinity distribution and carbonate production in response to a changing climate.
In other organisms, food availability can support metabolism to reduce the impacts of anthropogenic climate change on other physiological processes (Thomsen et al., 2013). Further understanding of foraminiferal predator–prey dynamics is therefore urgently needed. For example, cultures and in situ observations can improve our understanding of feeding by providing quantitative data on (1) the role of bacteria, (2) the role of organic matter in foraminiferal diet – specifically protein acquisition from zooplankton versus phytoplankton, (3) prey–predator optimum length ratio, (4) prey encounter rates (successful and unsuccessful), and (5) average digestion and capture time of prey. These data could (in part) be collected by analysing feeding vacuoles and metabarcoding analyses of samples.
Photosymbiosis, a major trait among symbiotic foraminifera, also requires further investigation. Culture and observational studies should focus on the impacts of environmental change on symbiosis. These include understanding what triggers change in symbiont hosting (e.g. bleaching and hosting for symbiont-facultative species), which symbiont each species hosts (facilitated by eDNA analyses), and the energy the symbiont provides to its host.
Experiments that exploit the new breakthrough in multigenerational planktic foraminifera culturing will improve our knowledge of their life cycle through understanding the traits and trade-offs associated with their development, e.g. their metabolic rates, food uptake, reproduction, and importantly, which species are capable of asexual reproduction and under what conditions this mode dominates. All these new insights will contribute to better data for improved modelling of planktic foraminifers.
Environmental DNA (eDNA) metabarcoding, which analyses the genetic material present in the environment, such as sediment or water, is a powerful new tool for identifying and monitoring biodiversity, biogeography and reconstructing ecosystems and ecologies (Ruppert et al., 2019). This technique can also provide insights into community composition over timescales spanning several hundreds of thousands of years or longer, improving our understanding of the relationships between biodiversity, environment, and climate (e.g. Armbrecht et al., 2019). However, the bulk of eDNA foraminiferal studies to date have focussed on benthic foraminifera. For instance, this technique has significantly increased the known diversity of organic walled and “naked” foraminifera that are rarely observed, have few morphological characters for traditional species delimitation, and do not preserve well in the fossil record (Pawlowski et al., 2014). Thus, eDNA holds great promise for investigating marine plankton, as it has the potential to overcome many of the data limitations that we currently face in this group with typically low-standing stocks (de Vargas et al., 2015). It is a potentially more effective means of detecting species presence in an environment than observations alone (Malviya et al., 2016; Ser-Giacomi et al., 2018; Barrenechea Angeles et al., 2020), but it can also contribute much more broadly, for instance, by providing insights into plankton population size (Andres et al., 2023), response to environmental change (Cao et al., 2022), or predator–prey dynamics (Ruppert et al., 2019). A combination of molecular and microscopic approaches can also yield new insights; for example, in benthic foraminifera it allowed rapid determination of multiple different feeding strategies driving diversity and abundance in several foraminiferal taxa (Schweizer et al., 2022), a question which is typically restricted to analysis of feeding vacuoles and laboratory experiments. However, further method development is still required as some groups are not as well represented by the eDNA technique as others. For example, specimens may visually be present in sediments but not found in the eDNA analysis, likely because of limitations of the primer to detect certain groups, e.g. Prymnesiophyceae (Barrenechea Angeles et al., 2020; Hoshino and Inagaki, 2024).
In general, there is a need to improve data reporting practices in the aforementioned studies by following guidance on data publishing (e.g. FAIR principles) and developing standardised, community-agreed protocols akin to other fields (e.g. Riebesell et al., 2010) for measuring traits to enhance our understanding of planktic foraminifera and how they are best modelled.
Ultimately, dynamic trait-based models are a useful tool to create theoretical frameworks for assessing ecosystem functions of foraminiferal traits and explore questions at scales and beyond those possible in laboratory or natural environments. For instance, ecological parameters measured in laboratory environments could be used in trait-based models to assess their impacts on the global biomass and calcite production, which are both poorly constrained in the modern carbon cycle (Ying et al., 2023). In addition, palaeoceanography models could help to elucidate the relationship between foraminiferal evolution (i.e. the emergence of new traits) and the background climate change, e.g. the evolution of deeper-water taxa as the oceans cooled over the past 15 Myr (Boscolo-Galazzo et al., 2021). For modern species with high mortality rates in laboratory culture or difficulties in maintaining multigenerational experiments (and high time and financial costs), trait-based models provide suitable setups to estimate potential physiological ecology in the absence of physical organisms (Grigoratou et al., 2019).
However, whilst a powerful tool, numerical models are ultimately just that, models. They are by necessity based on assumptions and simplifications of the natural world. Model reliability and performance is highly dependent on the specific research question asked and the observations available against which models can be validated. For planktic foraminifera, the most abundant data for model calibration are sediment core top data, and most foraminiferal models can reproduce the observed global biogeography of main species/ecogroups in sediment cores. However, less empirical data can be used for foraminiferal physiological model parameterisation. The growth rate and temperature dependency data from Lombard et al. (2009) are adopted by most plankton functional type models to parameterise foraminiferal physiology (e.g. PLAFOM, FORAMCLIM, and PLANKTOM). However, a lower constraint exists for food preference and grazing efficiency, hampering us from simulating the observed low foraminiferal biomass. Trait-based models can dynamically calculate grazing rate based on body size (allometric law) and temperature/food availability. However, a recent study shows that planktic foraminifera have lower allometric scaling of energetic needs compared to other plankton when considered in the context of organic density and catchment volume (Burke et al., 2025). Therefore, key parameters needed to improve foraminiferal model performance include more quantitative data on fundamental parameters such as growth rate, respiration rate, half-saturation constant, and grazing preferences.
Despite existing data gaps and model challenges, distinct groupings of planktic foraminifera traits identified in the modern ocean allow us to explore key drivers of past biogeography, changes in metabolic traits and trade-offs, and responses to extreme environments with moderate confidence (e.g. Deutsch et al., 2020). Closer collaboration between modellers and data scientists is essential to optimise the application of trait-based approaches to planktic foraminifera. Targeted data collection to fill specific critical knowledge gaps via models, laboratory culturing, field observations, molecular techniques, and more inclusive reporting will improve confidence in predictions from trait-based models and help to realise the full potential of this approach. Ultimately, understanding the associations of different traits and suites of traits with particular environments provides the basis for understanding how environmental factors structure planktic foraminifera communities in the past and in the future. This knowledge will be crucial for assessing the vulnerability of these communities to ongoing and future environmental changes.
All data referenced in the text are openly available and can be found in the cited sources. Figure 3 is plotted using the openly available database ForCenS of Siccha and Kucera (2017).
DNS, FMM, and KME conceived the idea for the manuscript. KME and MG prepared the original draft of the manuscript. KME, MG, and RY created the figures and tables. All of the authors contributed to reviewing and editing of the manuscript. DNS supervised the work.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Financial support was provided to Kirsty M. Edgar in the form of a Leverhulme Early Career Fellowship (ECF-2013-608) and NERC grant NE/X000567/1. Maria Grigoratou was supported by the European Research Council “PALEOGENiE” Project (ERC-2013-CoG617313). Ruby Barrett was supported by NE/S007504/1. Rui Ying was supported by a Chinese Scholarship Council grant (no. 202006380070). Fanny M. Monteiro was supported by NE/X001261/1, NE/V01823X/1, and DNS NERC grant NE/P019439/1.
This research has been supported by the Leverhulme Trust (grant no. ECF-2013-608), the Natural Environment Research Council (grant nos. NE/X000567/1, NE/S007504/1, NE/X001261/1, NE/V01823X/1, and NE/P019439/1), the European Research Council, EU H2020 European Research Council (grant no. ERC-2013-CoG617313), and the China Scholarship Council (grant no. 202006380070).
This paper was edited by Emilio Marañón and reviewed by two anonymous referees.
Adebayo, M. B., Bolton, C. T., Marchant, R., Bassinot, F., Conrod, S., and de Garidel-Thoron, T.: Environmental controls of size distribution of modern planktonic foraminifera in the tropical Indian Ocean, Geochem. Geophy. Geosy., 24, e2022GC010586, https://doi.org/10.1029/2022GC010586, 2023.
Alma, L., Kram, K. E., Holtgrieve, G. W., Barbarino, A., Fiamengo, C. J., and Padilla-Gamiño, J. L.: Ocean acidification and warming effects on the physiology, skeletal properties, and microbiome of the purple-hinge rock scallop, Comp. Biochem. Phys. A, 240, 110579, https://doi.org/10.1016/j.cbpa.2019.110579, 2020.
Andersen, K. H., Blanchard, J. L., Fulton, E. A., Gislason, H., Jacobsen, N. S., and van Kooten, T.: Assumptions behind size-based ecosystem models are realistic, ICES J. Mar. Sci., 73, 1651–1655, https://doi.org/10.1093/icesjms/fsv211, 2016.
Anderson, O. R. and Be, A. W. H.: The ultrastructure of a planktonic foraminifer, Globigerinoides sacculifer (Brady), and its symbiotic dinoflagellates, J. Foramin. Res., 6, 1–21, https://doi.org/10.2113/gsjfr.6.1.1, 1976.
Anderson, O. R., Spindler, M., Bé, A. W. H., and Hemleben, C.: Trophic activity of planktonic foraminifera, J. Mar. Biol. Assoc. UK, 59, 791–799, https://doi.org/10.1017/S002531540004577X, 1979.
Andres, K. J., Lodge, D. M., and Andrés, J.: Environmental DNA reveals the genetic diversity and population structure of an invasive species in the Laurentian Great Lakes, P. Natl. Acad. Sci. USA, 120, e2307345120, https://doi.org/10.1073/pnas.2307345120, 2023.
Armbrecht, L. H., Coolen, M. J. L., Lejzerowicz, F., George, S. C., Negandhi, K., Suzuki, Y., Young J., Foster N. R., Armand, L. K., Cooper, A., Ostrowski, M., Focardi, A., Stat, M., Moreau, J. W., and Weyrich, L. S.: Ancient DNA from marine sediments: precautions and considerations for seafloor coring, sample handling and data generation, Earth-Sci. Rev., 196, 102887, https://doi.org/10.1016/j.earscirev.2019.102887, 2019.
Aze, T., Ezard, T. H., Purvis, A., Coxall, H. K., Stewart, D. R., Wade, B. S., and Pearson, P. N.: A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data, Biol. Rev. Camb. Philos. Soc., 86, 900–927, https://doi.org/10.1111/j.1469-185X.2011.00178.x, 2011.
Barker, S. and Elderfield, H.: Foraminiferal calcification response to glacial-interglacial changes in atmospheric CO2, Science, 297, 833–836, https://doi.org/10.1126/science.1072815, 2002.
Barrenechea Angeles, I., Lejzerowicz, F., Cordier, T., Scheplitz, J., Kucera, M., Ariztegui, J., and Morard, R.: Planktonic foraminifera eDNA signature deposited on the seafloor remains preserved after burial in marine sediments, Sci. Rep., 10, 20351, https://doi.org/10.1038/s41598-020-77179-8, 2020.
Barrett, R., de Vries, J., and Schmidt, D. N.: What controls planktic foraminiferal calcification?, Biogeosciences, 22, 791–807, https://doi.org/10.5194/bg-22-791-2025, 2025.
Barton, A. D., Pershing, A. J., Litchman, E., Record, N. R., Edwards, K. F., Finkel, Z. V., Kiørboe, T., and Ward, B. A.: The biogeography of marine plankton traits, Ecol. Lett., 16, 522–534, https://doi.org/10.1111/ele.12063, 2013.
Barton, A. D., Irwin, A. J., Finkel, Z. V., and Stock, C. A.: Anthropogenic climate change drives shift and shuffle in North Atlantic phytoplankton communities, P. Natl. Acad. Sci. USA, 113, 2964–2969, https://doi.org/10.1073/pnas.1519080113, 2016.
Beer, C. J., Schiebel, R., and Wilson, P. A.: Technical Note: On methodologies for determining the size-normalised weight of planktic foraminifera, Biogeosciences, 7, 2193–2198, https://doi.org/10.5194/bg-7-2193-2010, 2010.
Berberich, D.: Die planktische Foraminifere Neogloboquadrina pachyderma (Ehrenberg) im Weddellmeer, Antarktis, Berichte zur Polarfroschung, 195, 1–193, https://doi.org/10.2312/BzP_0195_1996, 1996.
Berger, W. H.: Planktonic foraminifera; sediment production in an oceanic front, J. Foramin. Res., 1, 95–118, https://doi.org/10.2113/gsjfr.1.3.95, 1971.
Bijma, J., Erez, J., and Hemleben, C.: Lunar and semi-lunar reproductive cycles in some spinose planktonic foraminifers, J. Foramin. Res., 20, 117–127, https://doi.org/10.2113/gsjfr.20.2.117, 1990.
Birch, H. S., Coxall, H. K., Pearson, P. N., Kroon, D., and Schmidt, D. N.: Partial collapse of the marine carbon pump after the Cretaceous-Paleogene boundary, Geology, 44, 287–290, https://doi.org/10.1130/g37581.1, 2016.
Bird, C., Darling, K. F., Russell, A. D., Davis, C. V., Fehrenbacher, J., Free, A., Wyman, M., and Ngwenya, B. T.: Cyanobacterial endobionts within a major marine planktonic calcifier (Globigerina bulloides, Foraminifera) revealed by 16S rRNA metabarcoding, Biogeosciences, 14, 901–920, https://doi.org/10.5194/bg-14-901-2017, 2017.
Bornemann, A. and Norris, R. D.: Size-related stable isotope changes in Late Cretaceous planktic foraminifera: Implications for paleoecology and photosymbiosis, Mar. Micropaleontol., 65, 32–42, https://doi.org/10.1016/j.marmicro.2007.05.005, 2007.
Boscolo-Galazzo, F., Crichton, K. A., Ridgwell, A., Mawbey, E. M., Wade, B. S., and Paul N., and Pearson, P. N.: Temperature controls carbon cycling and biological evolution in the ocean twilight zone, Science, 371, 1148–1152, https://doi.org/10.1126/science.abb6643, 2021.
Boyd, C. E. and Goodyear, C. P.: Nutritive quality of food in ecological systems, Arch. Hydrobiol., 69, 256–270, 1971.
Brandão, M. C., Benedetti, F., Martini, S., Soviadan, Y. D., Irisson, J.-O., Romagnan, J.-B., Elineau, A., Desnos, C., Jalabert, L., Freire, A. S., Picheral, M., Guidi, L., Gorsky, G., Bowler, C., Karp-Boss, L., Henry, N., de Vargas, C., Sullivan, M. B., Acinas, S. G., Babin, M., Bork, P., Boss, E., Bowler, C., Cochrane, G., de Vargas, C., Sullivan, M. B., Tara Oceans Consortium, Coordinators, Stemman, L., and Lombard, F.: Macroscale patterns of oceanic zooplankton composition and size structure, Scientific Reports, 11, 15714, https://doi.org/10.1038/s41598-021-94615-5, 2021.
Brasier, M. D. and Armstrong, H.: Foraminifera, in: Microfossils, Blackwell Publishing, 142–187, ISBN 0-632-05279-1, https://doi.org/10.1002/9781118685440, 2004.
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B.: Toward a metabolic theory of ecology, Ecology, 85, 1771–1789, https://doi.org/10.1890/03-9000, 2004.
Brummer, G.-J. A., Hemleben, C., and Spindler, M.: Ontogeny of extant spinose planktonic foraminifera (Globigerinidae): A concept exemplified by Globigerinoides sacculifer (Brady) and G. ruber (d'Orbigny), Mar. Micropaleontol., 12, 357–381, https://doi.org/10.1016/0377-8398(87)90028-4, 1987.
Brummer, G.-J. A. and Kučera, M.: Taxonomic review of living planktonic foraminifera, J. Micropalaeontol., 41, 29–74, https://doi.org/10.5194/jm-41-29-2022, 2022.
Buitenhuis, E. T., Hashioka, T., and Quéré, C. L.: Combined constraints on global ocean primary production using observations and models, Global Biogeochem. Cy., 27, 847–858, https://doi.org/10.1002/gbc.20074, 2013.
Bé, A. W. H.: An ecological, zoogeographic and taxonomic review of Recent planktonic foraminifera, in: Oceanic Micropaleontology, edited by: Ramsay, A. T. S., Academic Press, London, 1–100, ISBN 0125773013, 1977.
Bé, A. W. H. and Anderson, O. R.: Gametogenesis in planktonic foraminifera, Science, 192, 890–892, https://doi.org/10.1126/science.946914, 1976.
Bé, A. W. H. and Tolderlund, D. S.: Distribution and ecology of living planktonic foraminifera in surface waters of the Atlantic and Indian oceans, in: The Micropaleontology of Oceans, edited by: Funnel, B. M. and Riedel, W. R., Cambridge University Press, Cambridge, 105–149, ISBN-10: 0521076420, 1971.
Bé, A. W. H., Caron, D. A., and Anderson, O. R.: Effects of feeding frequency on life processes of the planktonic foraminifer Globigerinoides sacculifer in laboratory culture, J. Mar. Biol. Assoc. UK, 61, 257–277, https://doi.org/10.1017/S002531540004604X, 1981.
Bé, A. W. H., Spero, H. J., and Anderson, O. R.: Effects of symbiont elimination and reinfection on the life processes of the planktonic foraminifer Globigerinoides sacculifer, Mar. Biol., 70, 73–86, https://doi.org/10.1007/BF00397298, 1982.
Bé, A. W. H., Anderson, O. R., Faber Jr., W. W., and Caron, D. A.: Sequence of morphological and cytoplasmic changes during gametogenesis in the planktonic foraminifer Globigerinoides sacculifer (Brady), Micropaleontology, 29, 310–325, 1983.
Béjard, T. M., Rigual-Hernández, A. S., Tarruella, J. P., Flores, J.-A., Sanchez-Vidal, A., Llamas-Cano, I., and Sierro, F. J.: Planktonic foraminifera assemblage composition and flux dynamics inferred from an annual sediment trap record in the central Mediterranean Sea, Biogeosciences, 21, 4051–4076, https://doi.org/10.5194/bg-21-4051-2024, 2024.
Bentov, S. and Erez, J.: Impact of biomineralization processes on the Mg content of foraminiferal shells: A biological perspective, Geochem. Geophy. Geosys., 7, Q01P08, https://doi.org/10.1029/2005GC001015, 2006.
Bentov, S., Brownlee, C., and Erez, J.: The role of seawater endocytosis in the biomineralization process in calcareous foraminifera, P. Natl. Acad. Sci. USA, 106, 21500–21504, https://doi.org/10.1073/pnas.0906636106, 2009.
Bertlich, J., Gussone, N., Berndt, J., Arlinghaus, H. F., and Dieckmann, G. S.: Salinity effects on cultured Neogloboquadrina pachyderma (sinistral) from high latitudes: new paleoenvironmental insights, Geo-Mar. Lett, 41, 2, https://doi.org/10.1007/s00367-020-00677-1, 2021.
Burke, J. E., Elder, L. E., Maas, A. E., Gaskell, D. E., Clark, E. G., Hsiang, A. Y., Foster, G. L., and Hull, P. M.: Physiological and morphological scaling enables gigantism in pelagic protists, Limnol. Oceanogr., 70, 461–476, https://doi.org/10.1002/lno.12770, 2025.
Cao, Y., Lei, Y., Fang, J. K. H., and Li, T.: Molecular diversity of foraminiferal eDNA in sediments and their correlations with environmental factors from the Yellow Sea, Ecol. Indic., 142, 109294, https://doi.org/10.1016/j.ecolind.2022.109294, 2022.
Caromel, A. G. M., Schmidt, D. N., Phillips, J. C., and Rayfield, E. J.: Hydrodynamic constraints on the evolution and ecology of planktic foraminifera, Mar. Micropaleontol., 106, 69–78, https://doi.org/10.1016/j.marmicro.2014.01.002, 2014.
Caromel, A. G. M., Schmidt, D. N., Fletcher, I., and Rayfield, E. J.: Morphological Change During The Ontogeny Of The Planktic Foraminifera, J. Micropalaeontol., 35, 2–19, https://doi.org/10.1144/jmpaleo2014-017, 2016.
Caron, D. A., Bé, A. W. H., and Anderson, O. R.: Effects of variations in light intensity on life processes of the planktonic foraminifer Globigerinoides sacculifer in laboratory culture, J. Mar. Biol. Assoc. UK, 62, 435–451, https://doi.org/10.1017/S0025315400057374, 1982.
Caron, D. A., Faber, W. W., and Bé, A. W. H.: Effects of temperature and salinity on the growth and survival of the planktonic foraminifer Globigerinoides sacculifer, J. Mar. Biol. Assoc. UK, 67, 323–341, https://doi.org/10.1017/S0025315400026643, 1987.
Chernihovsky, N., Torfstein, A., and Almogi-Labin, A.: Daily timescale dynamics of planktonic foraminifera shell-size distributions, Front. Mar. Sci., 10, 1126398, https://doi.org/10.3389/fmars.2023.1126398, 2023.
Darling, K. F. and Wade, C. M.: The genetic diversity of planktic foraminifera and the global distribution of ribosomal RNA genotypes, Mar. Micropaleontol., 67, 216–238, https://doi.org/10.1016/j.marmicro.2008.01.009, 2008.
Davis, C. V., Livsey, C. M., Palmer, H. M., Hull, P. M., Thomas, E., Hill, T. M., and Benitez-Nelson, C. R.: Extensive morphological variability in asexually produced planktic foraminifera, Science Advances, 6, eabb8930, https://doi.org/10.1126/sciadv.abb8930, 2020.
Decelle, J., Romac, S., Stern, R. F., Bendif, E. M., Zingone, A., Audic, S., Guiry, M. D., Guillou, L., Tessier, D., Le Gall, F., Gourvil, P., Dos Santos, A. L., Probert, I., Vaulot, D., de Vargas, C., and Christen, R.: PhytoREF: a reference database of the plastidial 16S rRNA gene of photosynthetic eukaryotes with curated taxonomy, Mol. Ecol. Resour., 15, 1435–1445, https://doi.org/10.1111/1755-0998.12401, 2015.
del Campo, J., Carlos-Oliveira, M., Čepička, I., Hehenberger, E., Horák, A., Karnkowska, A., Kolisko, M., Lara, E., Lukeš, J., Pánek, T., Piwosz, K., Richter, D. J., Škaloud, P., Sutak, R., Tachezy, J., and Hampl, V.: The protist cultural renaissance, Trends Microbiol., 32, 128–131, https://doi.org/10.1016/j.tim.2023.11.010, 2024.
de Nooijer, L. J., Langer, G., Nehrke, G., and Bijma, J.: Physiological controls on seawater uptake and calcification in the benthic foraminifer Ammonia tepida, Biogeosciences, 6, 2669–2675, https://doi.org/10.5194/bg-6-2669-2009, 2009.
de Nooijer, L. J., Spero, H. J., Erez, J., Bijma, J., and Reichart, G. J.: Biomineralization in perforate foraminifera, Earth-Sci. Rev., 135, 48–58, https://doi.org/10.1016/j.earscirev.2014.03.013, 2014.
de Nooijer, L. J., Pacho Sampedro, L., Jorissen, F. J., Pawlowski, J., Rosenthal, Y., Dissard, D., and Reichart, G. J.: 500 million years of foraminiferal calcification, Earth-Sci. Rev., 243, 104484, https://doi.org/10.1016/j.earscirev.2023.104484, 2023.
Deuser, W. G., Ross, E. H., Hemleben, C., and Spindler, M.: Seasonal changes in species composition, numbers, mass, size, and isotopic composition of planktonic foraminifera settling into the deep sargasso sea, Palaeogeogr. Palaeocl., 33, 103–127, https://doi.org/10.1016/0031-0182(81)90034-1, 1981.
Deutsch, C., Penn, J. L., and Seibel, B.: Metabolic trait diversity shapes marine biogeography, Nature, 585, 557–562, 2020.
de Vargas, C., Renaud, S., Hilbrecht, H., and Pawlowski, J.: Pleistocene adaptive radiation in Globorotalia truncatulinoides: genetic, morphologic, and environmental evidence, Paleobiology, 27, 104–125, https://doi.org/10.1666/0094-8373(2001)027<0104:PARIGT>2.0.CO;2, 2001.
de Vargas, C., Audic, S., Henry, N., Decelle, J., Mahé, F., Logares, R., Lara, E., Berney, C., Le Bescot, N., Probert, I., Carmichael, M., Poulain, J., Romac, S., Colin, S., Aury, J.-M,, Chaffron, S., Dunthorn, M., Engelen, S., Flegontova, O., Guidi, L., Horák, A., Jaillon, O., Lima-Mendez, G., Lukeš, J., Malviya, S., Morard, R., Mulot, M., calco, E., Siano, R., Vincent, F., Zingone, A., Dimier, C., Picheral, M,., Searson, S., Kandels-Lewis, S., Tara Oceans, Acinas, S.G., Bork, P., Bowler, C., Gorsky, G., Grimsley, N., Hingamp, P., Iudicone, D., Not, F., Ogata, H., Pesant, S., Raes, J., Sieracki, M.E., Speich, S., Stemmann, L., Sunagawa, S., Weissenbach, J., Wincker, P., and Karsenti, E.: Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean, Science, 348, 1261605, https://doi.org/10.1126/science.1261605, 2015.
Dieckmann, G. S., Spindler, M., Lange, M. A., Ackley, S. F., and Eicken, H.: Antarctic sea ice; a habitat for the foraminifer Neogloboquadrina pachyderma, J. Foramin. Res., 21, 182–189, https://doi.org/10.2113/gsjfr.21.2.182, 1991.
Dutkiewicz, S., Follows, M. J., and Bragg, J. G.: Modeling the coupling of ocean ecology and biogeochemistry, Global Biogeochem. Cy., 23, GB4017, https://doi.org/10.1029/2008GB003405, 2009.
Dutkiewicz, S., Cermeno, P., Jahn, O., Follows, M. J., Hickman, A. E., Taniguchi, D. A. A., and Ward, B. A.: Dimensions of marine phytoplankton diversity, Biogeosciences, 17, 609–634, https://doi.org/10.5194/bg-17-609-2020, 2020.
Edgar, K. M., Bohaty, S. M., Gibbs, S. J., Sexton, P. F., Norris, R. D., and Wilson, P. A.: Symbiont “bleaching” in planktic foraminifera during the Middle Eocene Climatic Optimum, Geology, 41, 15–18, https://doi.org/10.1130/g33388.1, 2013.
Eiane, K. and Ohman, M. D.: Stage-specific mortality of Calanus finmarchicus, Pseudocalanus elongatus and Oithona similis on Fladen Ground, North Sea, during a spring bloom, Mar. Ecol. Prog. Ser., 268, 183–193, 2004.
Erez, J.: The Source of Ions for Biomineralization in Foraminifera and Their Implications for Paleoceanographic Proxies, Rev. Mineral. Geochem., 54, 115–149, https://doi.org/10.2113/0540115, 2003.
Faber, W. W., Anderson, O. R., and Caron, D. A.: Algal-foraminiferal symbiosis in the planktonic foraminifer Globigerinella aequilateralis; II, Effects of two symbiont species on foraminiferal growth and longevity, J. Foramin. Res., 19, 185–193, https://doi.org/10.2113/gsjfr.19.3.185, 1989.
Fenchel, T.: The Ecology of Heterotrophic Microflagellates, in: Advances in Microbial Ecology, edited by: Marshall, K. C., Springer US, Boston, MA, 57–97, https://doi.org/10.1007/978-1-4757-0611-6_2, 1986.
Fenton, I. S., Woodhouse, A., Aze, T., Lazarus, D., Renaudie, J., Dunhill, A. M., Young, J. R., and Saupe, E. E.: Triton, a new species-level database of Cenozoic planktonic foraminiferal occurrences, Sci. Data, 8, 160, https://doi.org/10.1038/s41597-021-00942-7, 2021.
Fenton, I. S., Aze, T., Farnsworth, A., Valdes, P., and Saupe, E. E.: Origination of the modern-style diversity gradient 15 million years ago, Nature, 614, 708–712, https://doi.org/10.1038/s41586-023-05712-6, 2023.
Follows, M. J., Dutkiewicz, S., Grant, S., and Chisholm, S. W.: Emergent biogeography of microbial communities in a model ocean, Science, 315, 1843–1846, https://doi.org/10.1126/science.1138544, 2007.
Gaskell, D. E., Ohman, M. D., and Hull, P. M.: Zooglider-Based Measurements of Planktonic Foraminifera in the California Current System, J. Foramin. Res., 49, 390–404, https://doi.org/10.2113/gsjfr.49.4.390, 2019.
Gast, R. J. and Caron, D. A.: Molecular phylogeny of symbiotic dinoflagellates from planktonic foraminifera and radiolaria, Mol. Biol. Evol., 13, 1192–1197, https://doi.org/10.1093/oxfordjournals.molbev.a025684, 1996.
Gaylord, B., Kroeker, K. J., Sunday, J. M., Anderson, K. M., Barry, J. P., Brown, N. E., Connell, S. D., Dupont, S., Fabricius, K. E., Hall-Spencer, J. M., Klinger, T., Milazzo, M., Munday, P. L., Russell, B. D., Sanford, E., Schreiber, S. J., Thiyagarajan, V., Vaughan, M. L. H., Widdicombe, S., and Harley, C. D. G.: Ocean acidification through the lens of ecological theory, Ecology, 96, 3–15, https://doi.org/10.1890/14-0802.1, 2015.
Gibbs, S. J., Bown, P. R., Ward, B. A., Alvarez, S. A., Kim, H., Archontikis, O. A., Sauterey, B., Poulton, A. J., Wilson, J., and Ridgwell, A.: Algal plankton turn to hunting to survive and recover from end-Cretaceous impact darkness, Sci. Adv., 6, eabc9123, https://doi.org/10.1126/sciadv.abc9123, 2020.
Goldstein, S. T.: Foraminifera: A biological review in Modern Foraminifera, edited by: Sen Gupta, B. K., Kluwer Academic Publishers, Dordrecht, 37–55, https://doi.org/10.1007/0-306-48104-9_3, 1999.
Greco, M., Morard, R., and Kucera, M.: Single-cell metabarcoding reveals biotic interactions of the Arctic calcifier Neogloboquadrina pachyderma with the eukaryotic pelagic community, J. Plankton Res., 43, 113–125, https://doi.org/10.1093/plankt/fbab015, 2021.
Greco, M., Westgård, A., Sykes, F. E., Ezat, M. M., and Meilland, J.: Uncovering hidden structures: previously undescribed pseudopodia and ectoplasmic structures in planktonic foraminifera, J. Plankton Res., 45, 652–660, https://doi.org/10.1093/plankt/fbad031, 2023.
Grice, G. D. and Hart, A. D.: The Abundance, Seasonal Occurrence and Distribution of the Epizooplankton between New York and Bermuda, Ecol. Monogr., 32, 287–309, https://doi.org/10.2307/1942377, 1962.
Grigoratou, M., Monteiro, F. M., Schmidt, D. N., Wilson, J. D., Ward, B. A., and Ridgwell, A.: A trait-based modelling approach to planktonic foraminifera ecology, Biogeosciences, 16, 1469–1492, https://doi.org/10.5194/bg-16-1469-2019, 2019.
Grigoratou, M., Monteiro, F. M., Ridgwell, A., and Schmidt, D. N.: Investigating the benefits and costs of spines and diet on planktonic foraminifera distribution with a trait-based ecosystem model, Mar. Micropaleontol., 166, 102004, https://doi.org/10.1016/j.marmicro.2021.102004, 2021.
Grigoratou, M., Monteiro, F. M., Wilson, J. D., Ridgwell, A., and Schmidt, D. N.: Exploring the impact of climate change on the global distribution of non-spinose planktonic foraminifera using a trait-based ecosystem model, Glob. Change Biol., 28, 1063–1076, https://doi.org/10.1111/gcb.15964, 2022.
Haldane, J. B. S.: On Being the Right Size, Harper's Magazine, 152, 424–427, 1926.
Hemleben, C., Spindler, M., Breitinger, I., and Deuser, W.: Field and laboratory studies on the ontogeny and ecology of some globorotaliid species from the Sargasso Sea off Bermuda, J. Foramin. Res., 15, 254–272, https://doi.org/10.2113/gsjfr.15.4.254, 1985.
Hemleben, C., Spindler, M., Anderson, R. O.: Modern Planktonic Foraminifera, Springer-Verlag, Ney York, Berlin, Heidelberg, 363 pp., ISBN-13: 978-1-4612-8150-4, 1989.
Henehan, M. J., Evans, D., Shankle, M., Burke, J. E., Foster, G. L., Anagnostou, E., Chalk, T. B., Stewart, J. A., Alt, C. H. S., Durrant, J., and Hull, P. M.: Size-dependent response of foraminiferal calcification to seawater carbonate chemistry, Biogeosciences, 14, 3287–3308, https://doi.org/10.5194/bg-14-3287-2017, 2017.
Hill, T. S. and Hoogenboom, M. O.: The indirect effects of ocean acidification on corals and coral communities, Coral Reefs, 41, 1557–1583, https://doi.org/10.1007/s00338-022-02286-z, 2022.
Hoadley, K. D., Lewis, A. M., Wham, D. C., Pettay, D. T., Grasso, C., Smith, R., Kemp, D. W., LaJeunesse, T. C., and Warner, M. E.: Host–symbiont combinations dictate the photo-physiological response of reef-building corals to thermal stress, Scientific Reports, 9, 9985, https://doi.org/10.1038/s41598-019-46412-4, 2019.
Hoppit, G. and Schmidt, D. N.: A Regional View of the Response to Climate Change: A Meta-Analysis of European Benthic Organisms' Responses, Front. Mar. Sci., 9, 896157, https://doi.org/10.3389/fmars.2022.896157, 2022.
Horne, C. R., Hirst, A. G., and Atkinson, D.: Seasonal body size reductions with warming covary with major body size gradients in arthropod species, P. Roy. Soc. B-Biol. Sci., 284, 20170238, https://doi.org/10.1098/rspb.2017.0238, 2017.
Hoshino, T. and Inagaki, F.: Distribution of eukaryotic environmental DNA in global subseafloor sediments, Progress in Earth and Planetary Science, 11, 19, https://doi.org/10.1186/s40645-024-00621-2, 2024.
Hull, P. M., Osborn, K. J., Norris, R. D., and Robison, B. H.: Seasonality and depth distribution of a mesopelagic foraminifer, Hastigerinella digitata, in Monterey Bay, California, Limnol. Oceanogr., 56, 562–576, https://doi.org/10.4319/lo.2011.56.2.0562, 2011.
Jonkers, L. and Kučera, M.: Global analysis of seasonality in the shell flux of extant planktonic Foraminifera, Biogeosciences, 12, 2207–2226, https://doi.org/10.5194/bg-12-2207-2015, 2015.
Jonkers, L., Reynolds, C. E., Richey, J., and Hall, I. R.: Lunar periodicity in the shell flux of planktonic foraminifera in the Gulf of Mexico, Biogeosciences, 12, 3061–3070, https://doi.org/10.5194/bg-12-3061-2015, 2015.
Ketten, D. and Edmond, J.: Gametogenesis and calcification of planktonic Foraminifera, Nature 278, 546–548, https://doi.org/10.1038/278546a0, 1979.
Kimoto, K.: The “unusual” reproduction of planktonic foraminifera: an Asexual reproductive phase of Neogloboquadrina pachyderma (Ehrenberg), Anuario do Instituto de Geociencias, Universidade Federal do Rio de Janeiro, 29, 461, https://doi.org/10.11137/2006_1_461-461, 2006.
Kiørboe, T.: How zooplankton feed: mechanisms, traits and trade-offs, Biol. Rev., 86, 311–339, https://doi.org/10.1111/j.1469-185X.2010.00148.x, 2011.
Kiørboe, T., Visser, A., and Andersen, K. H.: A trait-based approach to ocean ecology, ICES J. Mar. Sci., 75, 1849–1863, https://doi.org/10.1093/icesjms/fsy090, 2018.
Knoll, A. H. and Follows, M. J.: A bottom-up perspective on ecosystem change in Mesozoic oceans, P. Roy. Soc. B-Biol. Sci., 283, 20161755, https://doi.org/10.1098/rspb.2016.1755, 2016.
Köhler-Rink, S. and Kühl, M.: The chemical microenvironment of the symbiotic planktonic foraminifer Orbulina universa, Mar. Biol. Res., 1, 68–78, https://doi.org/10.1080/17451000510019015, 2005.
Kucera, M.: Chapter Six Planktonic Foraminifera as Tracers of Past Oceanic Environments, Developments in Marine Geology, 1, 213–262, https://doi.org/10.1016/S1572-5480(07)01011-1, 2007.
LeKieffre, C., Spero, H. J., Russell, A. D., Fehrenbacher, J. S., Geslin, E., and Meibom, A.: Assimilation, translocation, and utilization of carbon between photosynthetic symbiotic dinoflagellates and their planktic foraminifera host, Mar. Biol., 165, 104, https://doi.org/10.1007/s00227-018-3362-7, 2018.
Leung, J. Y. S., Zhang, S., and Connell, S. D.: Is Ocean Acidification Really a Threat to Marine Calcifiers? A Systematic Review and Meta-Analysis of 980+ Studies Spanning Two Decades, Small, 18, 2107407, https://doi.org/10.1002/smll.202107407, 2022.
Litchman, E., Ohman, M. D., and Kiørboe, T.: Trait-based approaches to zooplankton communities, J. Plankton Res., 35, 473–484, https://doi.org/10.1093/plankt/fbt019, 2013.
Litchman, E., Edwards, K. F., and Boyd, P. W.: Toward trait-based food webs: Universal traits and trait matching in planktonic predator–prey and host–parasite relationships, Limnol. Oceanogr., 66, 3857–3872, https://doi.org/10.1002/lno.11924, 2021.
Lombard, F., Labeyrie, L., Michel, E., Spero, H. J., and Lea, D. W.: Modelling the temperature dependent growth rates of planktic foraminifera, Mar. Micropaleontol., 70, 1–7, https://doi.org/10.1016/j.marmicro.2008.09.004, 2009.
Lombard, F., da Rocha, R. E., Bijma, J., and Gattuso, J.-P.: Effect of carbonate ion concentration and irradiance on calcification in planktonic foraminifera, Biogeosciences, 7, 247–255, https://doi.org/10.5194/bg-7-247-2010, 2010.
Lombard, F., Labeyrie, L., Michel, E., Bopp, L., Cortijo, E., Retailleau, S., Howa, H., and Jorissen, F.: Modelling planktic foraminifer growth and distribution using an ecophysiological multi-species approach, Biogeosciences, 8, 853–873, https://doi.org/10.5194/bg-8-853-2011, 2011.
Lončarić, N., Brummer, G.-J. A., and Kroon, D.: Lunar cycles and seasonal variations in deposition fluxes of planktic foraminiferal shell carbonate to the deep South Atlantic (central Walvis Ridge), Deep-Sea Res. Pt. I, 52, 1178–1188, https://doi.org/10.1016/j.dsr.2005.02.003, 2005.
Lynch, M.: The Genetic interpretation of inbreeding depression and outbreeding depression, Evolution, 45, 622–629, https://doi.org/10.1111/j.1558-5646.1991.tb04333.x, 1991.
Malviya, S., Scalco, E., Audic, S., Vincent, F., Veluchamy, A., Poulain, J., Wincker, P., Iudicone, D., de Vargas, C., Bittner, L., Zingone, A., and Bowler, C.: Insights into global diatom distribution and diversity in the world's ocean, P. Natl. Acad. Sci. USA, 113, E1516–E1525, 2016.
Margulis, L.: Origins of species: acquired genomes and individuality, Biosystems, 31, 121–125, https://doi.org/10.1016/0303-2647(93)90039-F, 1993.
McKinney, M. L.: Trends in body-size evolution, in: Evolutionary Trends, edited by: McNamara, K. J., Belhaven Press, London, 75–118, ISBN-10: 0816512345 1990.
Meilland, J., Howa, H., Lo Monaco, C., and Schiebel, R.: Individual planktic foraminifer protein-biomass affected by trophic conditions in the Southwest Indian Ocean, 30° S–60° S, Mar. Micropaleontol., 124, 63–74, https://doi.org/10.1016/j.marmicro.2016.02.004, 2016.
Meilland, J., Ezat, M. M., Westgård, A., Manno, C., Morard, R., Siccha, M., and Kucera, M.: Rare but persistent asexual reproduction explains the success of planktonic foraminifera in polar oceans, J. Plankton Res., 45, 15–32, https://doi.org/10.1093/plankt/fbac069, 2023.
Meilland, J., Siccha, M., Morard, R., and Kucera, M.: Continuous reproduction of planktonic foraminifera in laboratory culture, J. Eukaryot. Microbiol., 71, e13022, https://doi.org/10.1111/jeu.13022, 2024.
Michaels, A. F., Caron, D. A., Swanberg, N. R., Howse, F., and Michaels, C. M.: Planktonic sarcodines (Acantharia, Radiolaria, Foraminifera) in surface waters near Bermuda: abundance, biomass and vertical flux, J. Plankton Res., 17, 131–163, 1995.
Monteiro, F. M., Bach, L. T., Brownlee, C., Bown, P., Rickaby, R. E. M., Poulton, A. J., Tyrrell, T., Beaufort, L., Dutkiewicz, S., Gibbs, S., Gutowska, M. A., Lee, R., Riebesell, U., Young, J., and Ridgwell, A.: Why marine phytoplankton calcify, Science Advances, 2, e1501822, https://doi.org/10.1126/sciadv.1501822, 2016.
Morard, R., Escarguel, G., Weiner, A. K., André, A., Douady, C. J., Wade, C. M., Darling, K. F., Ujiié, Y., Seears, H. A., Quillévéré, F., de Garidel-Thoron, T., de Vargas, C., and Kucera, M.: Nomenclature for the Nameless: A Proposal for an Integrative Molecular Taxonomy of Cryptic Diversity Exemplified by Planktonic Foraminifera, Syst. Biol., 65, 925–940, https://doi.org/10.1093/sysbio/syw031, 2016.
Morard, R., Darling, K. F., Weiner, A. K. M., Hassenrück, C., Vanni, C., Cordier, T., Henry, N., Greco, M., Vollmar, N. M., Milivojevic, T., Rahman, S. N., Siccha, M., Meilland, J., Jonkers, L., Quillévéré, F., Escarguel, G., Douady, C. J., de Garidel-Thoron, T., de Vargas, C., and Kucera, M.: The global genetic diversity of planktonic foraminifera reveals the structure of cryptic speciation in plankton, Biol. Rev., 99, 1218–1241, https://doi.org/10.1111/brv.13065, 2024.
Naidoo-Bagwell, A. A., Monteiro, F. M., Hendry, K. R., Burgan, S., Wilson, J. D., Ward, B. A., Ridgwell, A., and Conley, D. J.: A diatom extension to the cGEnIE Earth system model – EcoGEnIE 1.1, Geosci. Model Dev., 17, 1729–1748, https://doi.org/10.5194/gmd-17-1729-2024, 2024.
Nehrke, G., Keul, N., Langer, G., de Nooijer, L. J., Bijma, J., and Meibom, A.: A new model for biomineralization and trace-element signatures of Foraminifera tests, Biogeosciences, 10, 6759–6767, https://doi.org/10.5194/bg-10-6759-2013, 2013.
Neukermans, G., Bach, L. T., Butterley, A., Sun, Q., Claustre, H., and Fournier, G. R.: Quantitative and mechanistic understanding of the open ocean carbonate pump – perspectives for remote sensing and autonomous in situ observation, Earth-Sci. Rev., 239, 104359, https://doi.org/10.1016/j.earscirev.2023.104359, 2023.
Ortiz, J. D., Mix, A. C., and Collier, R. W.: Environmental control of living symbiotic and asymbiotic foraminifera of the California Current, Paleoceanography, 10, 987–1009, https://doi.org/10.1029/95PA02088, 1995.
Otto, S. P. and Lenormand, T.: Resolving the paradox of sex and recombination, Nat. Rev. Genet., 3, 252–261, https://doi.org/10.1038/nrg761, 2002.
Pallacks, S., Ziveri, P., Schiebel, R., Vonhof, H., Rae, J. W. B., Littley, E., Garcia-Orellana, J., Langer, G., Grelaud, M., and Martrat, B.: Anthropogenic acidification of surface waters drives decreased biogenic calcification in the Mediterranean Sea, Communications Earth & Environment, 4, 301, https://doi.org/10.1038/s43247-023-00947-7, 2023.
Palmer, A. R.: Calcification in marine molluscs: how costly is it?, P. Natl. Acad. Sci. USA, 89, 1379–1382, https://doi.org/10.1073/pnas.89.4.1379, 1992.
Pawlowski, J., Lejzerowicz, F., and Esling, P.: Next-Generation Environmental Diversity Surveys of Foraminifera: Preparing the Future, Biol. Bull., 227, 93–106, 2014.
Pearson, P. N., Ditchfield, P. W., Singano, J., Harcourt-Brown, K. G., Nicholas, C. J., Olsson, R. K., Shackleton, N. J., and Hall, M. A.: Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs, Nature, 413, 481–487, https://doi.org/10.1038/35097000, 2001.
Peters, R. H.: The Ecological Implications of Body Size, Cambridge Studies in Ecology, Cambridge University Press, Cambridge, https://doi.org/10.1017/CBO9780511608551, 1983.
Rebotim, A., Voelker, A. H. L., Jonkers, L., Waniek, J. J., Schulz, M., and Kucera, M.: Calcification depth of deep-dwelling planktonic foraminifera from the eastern North Atlantic constrained by stable oxygen isotope ratios of shells from stratified plankton tows, J. Micropalaeontol., 38, 113–131, https://doi.org/10.5194/jm-38-113-2019, 2019.
Ridgwell, A. and Zeebe, R. E.: The role of the global carbonate cycle in the regulation and evolution of the Earth system, Earth Planet. Sc. Lett., 234, 299–315, https://doi.org/10.1016/j.epsl.2005.03.006, 2005.
Riebesell, U., Fabry, V.J., Hansson, L., and Gattuso, J.-P. (Eds.): Guide to best practices for ocean acidification research and data reporting, Publications Office of the European Union, Luxembourg, 260 pp., https://doi.org/10.2777/66906, 2010.
Rillo, M. C., Miller, C. G., Kucera, M., and Ezard, T. H. G.: Intraspecific size variation in planktonic foraminifera cannot be consistently predicted by the environment, Ecol. Evol., 10, 11579–11590, https://doi.org/10.1002/ece3.6792, 2020.
Rink, S., Kühl, M., Bijma, J., and Spero, H. J.: Microsensor studies of photosynthesis and respiration in the symbiotic foraminifer Orbulina universa, Mar. Biol., 131, 583–595, https://doi.org/10.1007/s002270050350, 1998.
Ruppert, K. M., Kline, R. J., and Rahman, M. S.: Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA, Global Ecology and Conservation, 17, e00547, https://doi.org/10.1016/j.gecco.2019.e00547, 2019.
Russell, A. D., Hönisch, B., Spero, H. J., and Lea, D. W.: Effects of seawater carbonate ion concentration and temperature on shell U, Mg, and Sr in cultured planktonic foraminifera, Geochim. Cosmochim. Ac., 68, 4347–4361, https://doi.org/10.1016/j.gca.2004.03.013, 2004.
Sanders, T., Schmittmann, L., Nascimento-Schulze, J. C., and Melzner, F.: High Calcification Costs Limit Mussel Growth at Low Salinity, Front. Mar. Sci., 5, 352, https://doi.org/10.3389/fmars.2018.00352, 2018.
Schiebel, R. and Hemleben, C.: Planktic Foraminifers in the Modern Ocean, Springer, Heidelberg, ISBN 978-3-662-50295-2, 2017.
Schiebel, R., Hiller, B., and Hemleben, C.: Impacts of storms on Recent planktic foraminiferal test production and CaCO3 flux in the North Atlantic at 47° N, 20° W (JGOFS), Mar. Micropaleontol., 26, 115–129, https://doi.org/10.1016/0377-8398(95)00035-6, 1995.
Schmidt, D. N., Renaud, S., and Bollmann, J.: Response of planktic foraminiferal size to late Quaternary climate change, Paleoceanography, 18, 1039, https://doi.org/10.1029/2002PA000831, 2003.
Schmidt, D. N., Renaud, S., Bollmann, J., Schiebel, R., and Thierstein, H. R.: Size distribution of Holocene planktic foraminifer assemblages: biogeography, ecology and adaptation, Mar. Micropaleontol., 50, 319–338, https://doi.org/10.1016/S0377-8398(03)00098-7, 2004.
Schmidt, D. N., Lazarus, D., Young, J. R., and Kucera, M.: Biogeography and evolution of body size in marine plankton, Earth-Sci. Rev., 78, 239–266, https://doi.org/10.1016/j.earscirev.2006.05.004, 2006.
Schweizer, M., Jauffrais, T., Choquel, C., Méléder, V., Quinchard, S., and Geslin, E.: Trophic strategies of intertidal foraminifera explored with single-cell microbiome metabarcoding and morphological methods: What is on the menu?, Ecol. Evol., 12, e9437, https://doi.org/10.1002/ece3.9437, 2022.
Ser-Giacomi, E., Zinger, L., Malviya, S., De Vargas, C., Karsenti, E., Bowler, C., and De Monte, S., Ubiquitous abundance distribution of non-dominant plankton across the global ocean, Nature Ecology & Evolution, 2, 1243–1249, 2018.
Shaked, Y. and de Vargas, C.: Pelagic photosymbiosis: rDNA assessment of diversity and evolution of dinoflagellate symbionts and planktonic foraminiferal hosts, Mar. Ecol. Prog. Ser., 325, 59–71, 2006.
Siccha, M. and Kucera, M.: ForCenS, a curated database of planktonic foraminifera census counts in marine surface sediment samples, Scientific Data, 4, 170109, https://doi.org/10.1038/sdata.2017.109, 2017.
Siebold, E. and Berger, W.: The Sea Floor: An Introduction to Marine Geology, Springer Verlag, Berlin, Heidelberg, New York, 356 pp., ISSN 2510-1307, 1993.
Spero, H. J. and Parker, S. L.: Photosynthesis in the symbiotic planktonic foraminifer Orbulina universa, and its potential contribution to oceanic primary productivity, J. Foramin. Res., 15, 273–281, https://doi.org/10.2113/gsjfr.15.4.273, 1985.
Spindler, M.: On the salinity tolerance of the planktonic foraminifer Neogloboquadrina pachyderma from Antarctic sea ice, Polar Biol., 9, 85–91, 1996.
Spindler, M. and Dieckmann, G. S.: Distribution and abundance of the planktic foraminifer Neogloboquadrina pachyderma in sea ice of the Weddell Sea (Antarctica), Polar Biol., 5, 185–191, https://doi.org/10.1007/BF00441699, 1986.
Spindler, M., Hemleben, C., Bayer, U., Bé, A. W. H., Anderson, O.R.: Lunar Periodicity of Reproduction in the Planktonic Foraminifer Hastigerina pelagica, Mar. Ecol. Prog. Ser., 1, 61–64, 1979.
Spindler, M., Hemleben, C., Salomons, J. B., and Smit, L. P.: Feeding behavior of some planktonic foraminifers in laboratory cultures, J. Foramin. Res., 14, 237–249, https://doi.org/10.2113/gsjfr.14.4.237, 1984.
Stoecker, D. K., Johnson, M. D., deVargas, C., and Not, F.: Acquired phototrophy in aquatic protists, Aquat. Microb. Ecol., 57, 279–310, 2009.
Strack, T., Jonkers, L., C. Rillo, M., Baumann, K.-H., Hillebrand, H., and Kucera, M.: Coherent response of zoo- and phytoplankton assemblages to global warming since the Last Glacial Maximum, Global Ecol. Biogeogr., 33, e13841, https://doi.org/10.1111/geb.13841, 2024.
Swezey, D. S., Boles, S. E., Aquilino, K. M., Stott, H. K., Bush, D., Whitehead, A., Rogers-Bennett, L., Hill, T. M., and Sanford, E.: Evolved differences in energy metabolism and growth dictate the impacts of ocean acidification on abalone aquaculture, P. Natl. Acad. Sci. USA, 117, 26513–26519, https://doi.org/10.1073/pnas.2006910117, 2020.
Sykes, F. E., Meilland, J., Westgård, A., Chalk, T. B., Chierici, M., Foster, G. L., and Ezat, M. M.: Large-scale culturing of the subpolar foraminifera Globigerina bulloides reveals tolerance to a large range of environmental parameters associated to different life-strategies and an extended lifespan, J. Plankton Res., 46, 403–420, https://doi.org/10.1093/plankt/fbae029, 2024.
Takagi, H., Kimoto, K., Fujiki, T., Kurasawa, A., Moriya, K., and Hirano, H.: Ontogenetic dynamics of photosymbiosis in cultured planktic foraminifers revealed by fast repetition rate fluorometry, Mar. Micropaleontol., 122, 44–52, https://doi.org/10.1016/j.marmicro.2015.10.003, 2016.
Takagi, H., Kimoto, K., Fujiki, T., and Moriya, K.: Effect of nutritional condition on photosymbiotic consortium of cultured Globigerinoides sacculifer (Rhizaria, Foraminifera), Symbiosis, 76, 25–39, https://doi.org/10.1007/s13199-017-0530-3, 2018.
Takagi, H., Kimoto, K., Fujiki, T., Saito, H., Schmidt, C., Kucera, M., and Moriya, K.: Characterizing photosymbiosis in modern planktonic foraminifera, Biogeosciences, 16, 3377–3396, https://doi.org/10.5194/bg-16-3377-2019, 2019.
Takagi, H., Kurasawa, A., and Kimoto, K.: Observation of asexual reproduction with symbiont transmission in planktonic foraminifera, J. Plankton Res., 42, 403–410, https://doi.org/10.1093/plankt/fbaa033, 2020.
Takahashi, K. and Bé, A. W. H.: Planktonic foraminifera: factors controlling sinking speeds, Deep Sea Research Part A. Oceanographic Research Papers, 31, 1477–1500, https://doi.org/10.1016/0198-0149(84)90083-9, 1984.
Thomsen, J., Casties, I., Pansch, C., Körtzinger, A., and Melzner, F.: Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments, Glob. Change Biol., 19, 1017–1027, 2013.
Topa, P., Faber, Ł., Tyszka, J., and Komosinski, M.: Modelling ecology and evolution of Foraminifera in the agent-oriented distributed platform, Journal of Computational Science, 18, 69–84, https://doi.org/10.1016/j.jocs.2016.07.009, 2017.
Vincent, E. and Berger, W. H.: Planktonic foraminifera and their use in paleoceanography, in: The Sea, edited by: Emiliani, C., John Wiley & Sons, New York, 1035–1119, ISBN 9780674017368, 1981.
Violle, C., Navas, M.-L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., and Garnier, E.: Let the concept of trait be functional!, Oikos, 116, 882–892, https://doi.org/10.1111/j.0030-1299.2007.15559.x, 2007.
Wade, B. S., Al-Sabouni, N., Hemleben, C., and Kroon, D.: Symbiont bleaching in fossil planktonic foraminifera, Evol. Ecol., 22, 253–265, https://doi.org/10.1007/s10682-007-9176-6, 2008.
Ward, B. B.: Oceans. How nitrogen is lost, Science, 341, 352–353, https://doi.org/10.1126/science.1240314, 2013.
Watson, S.-A., Morley, S. A., and Peck, L. S.: Latitudinal trends in shell production cost from the tropics to the poles, Science Advances, 3, e1701362, https://doi.org/10.1126/sciadv.1701362, 2017.
Weinkauf, M. F. G., Kunze, J. G., Waniek, J. J., and Kučera, M.: Seasonal Variation in Shell Calcification of Planktonic Foraminifera in the NE Atlantic Reveals Species-Specific Response to Temperature, Productivity, and Optimum Growth Conditions, PLOS ONE, 11, e0148363, https://doi.org/10.1371/journal.pone.0148363, 2016.
Weinkauf, M. F. G., Siccha, M., and Weiner, A. K. M.: Reproduction dynamics of planktonic microbial eukaryotes in the open ocean, J. R. Soc. Interface, 19, 20210860, https://doi.org/10.1098/rsif.2021.0860, 2022.
Westgård, A., Ezat, M. E., Chalk, T. B., Chierici, M., Foster, G. L., and Meilland, J.: Large-scale culturing of Neogloboquadrina pachyderma, its growth in, and tolerance of, variable environmental conditions, J. Plankton Res., 45, 732–745, 2023.
Westoby, M.: Trait-based ecology, trait-free ecology, and in between, New Phytol., 245, 33–39, https://doi.org/10.1111/nph.20197, 2024.
Wilkinson, M. D., Dumontier, M., Aalbersberg, I. J., Appleton, G., Axton, M., Baak, A., Blomberg, N., Boiten, J.-W., da Silva Santos, L. B., Bourne, P. E., Bouwman, J., Brookes, A. J., Clark, T., Crosas, M., Dillo, I., Dumon, O., Edmunds, S., Evelo, C. T., Finkers, R., Gonzalez-Beltran, A., Gray, A. J. G., Groth, P., Goble, C., Grethe, J. S., Heringa, J., ’t Hoen, P. A. C., Hooft, R., Kuhn, T., Kok, R., Kok, J., Lusher, S. J., Martone, M. E., Mons, A., Packer, A. L., Persson, B., Rocca-Serra, P., Roos, M., van Schaik, R., Sansone, S.-A., Schultes, E., Sengstag, T., Slater, T., Strawn, G., Swertz, M. A., Thompson, M., van der Lei, J., van Mulligen, E., Velterop, J., Waagmeester, A., Wittenburg, P., Wolstencroft, K., Zhao, J., and Mons, B.: The FAIR Guiding Principles for scientific data management and stewardship, Scientific Data, 3, 160018, https://doi.org/10.1038/sdata.2016.18, 2016.
Wirtz, K. W.: Who is eating whom? Morphology and feeding type determine the size relation between planktonic predators and their ideal prey, Mar. Ecol. Prog. Ser., 445, 1–12, https://doi.org/10.3354/meps09502, 2012.
Woodhouse, A., Swain, A., Fagan, W. F., Fraass, A. J., and Lowery, C. M.: Late Cenozoic cooling restructured global marine plankton communities, Nature, 614, 713–718, https://doi.org/10.1038/s41586-023-05694-5, 2023.
Ying, R., Monteiro, F. M., Wilson, J. D., and Schmidt, D. N.: ForamEcoGEnIE 2.0: incorporating symbiosis and spine traits into a trait-based global planktic foraminiferal model, Geosci. Model Dev., 16, 813–832, https://doi.org/10.5194/gmd-16-813-2023, 2023.
Ying, R., Monteiro, F.M., Wilson, J.D., Ödalen, M., and Schmidt, D.N.: Past foraminiferal acclimation capacity is limited during future climate warming, Nature, 636, 385–289, https://doi.org/10.1038/s41586-024-08029-0, 2024.




