the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Is litter biomass a driver of soil volatile organic compound fluxes in Mediterranean forest?
Julien Kammer
Mathieu Santonja
Brice Temime-Roussel
Cassandra Saignol
Caroline Lecareux
Etienne Quivet
Henri Wortham
Elena Ormeño
Soil biogenic volatile organic compound (BVOC) emissions have been studied in different biomes, showing that their emissions are considerable. However, so far, previous studies have neglected the role of litter accumulation (considered here to be the amount of litter) in soil BVOC fluxes, and most of them refer to coniferous and evergreen forests, while litter emissions from Mediterranean deciduous forests remain poorly explored. To fill these gaps, the present work aimed to study BVOC fluxes in a Mediterranean deciduous forest, with particular attention being paid to the relationships between litter biomass accumulation in soil, microbial abundance, and soil BVOC fluxes. Measurements were performed in southern France in the downy oak (Quercus pubescens Willd.) forest of the Observatoire de Haute Provence (O3HP) during the late spring of 2023 using dynamic chambers coupled to an online proton transfer reaction time-of-flight mass spectrometer (PTR-ToF-MS). We investigated in situ daily BVOC fluxes from bare soil and different litter biomasses mimicking current, lower, or higher litter production as both decreases and increases in litter accumulation are expected in the Mediterranean region under the current context of climate change and greening management policies. The results showed a high BVOC diversity, with more than 135 emitted compounds. For a large majority of the measured compounds, fluxes were negative, suggesting that soil (bare soil covered by litter) takes up compounds through biochemical and/or physical processes. Some compounds, such as acetone, methanol, or sesquiterpenes, increased with increasing litter biomass, suggesting the importance of considering litter accumulation when assessing soil BVOC emissions from Mediterranean deciduous forests. Microbial abundance was highlighted as a potential driver of this relation between litter biomass and VOC fluxes.
- Article
(6173 KB) - Full-text XML
-
Supplement
(821 KB) - BibTeX
- EndNote
Biogenic volatile organic compounds (BVOCs) are key components of the atmosphere's oxidative capacity through their influence on the OH radical, O3, and NOx budgets, among others. They also play a critical role in the formation of the secondary organic aerosol (SOA) (Hallquist et al., 2009; Kulmala et al., 2004; Mahilang et al., 2021; Seinfeld and Pandis, 2016), contributing to health and climate impacts (Seinfeld and Pandis, 2016; Thornhill et al., 2021). Numerous studies have documented BVOC emissions from the aerial parts of terrestrial plants, specifically at the canopy, branch, and leaf scales (Artaxo et al., 2022; Gros et al., 2022; Mu et al., 2022; Rinnan, 2024), and they are estimated to release between 300 and 1000 Tg C yr−1 on a global scale (Guenther et al., 2012; Sindelarova et al., 2014; Wang et al., 2024). However, the contributions of soil (bare soil covered by litter) VOC exchange (emission and immission) processes are still unclear due to the scarcity of studies. Recent studies on soil BVOCs have shown bi-directional VOC fluxes (i.e., positive and negative) with net emissions from soils covered by litter (Bourtsoukidis et al., 2018; Legros et al., 2025; Peñuelas et al., 2014; Viros et al., 2020; Yang et al., 2024b). Soil could thus be a significant source of BVOCs affecting atmospheric chemistry and related climate impacts (Kramshøj et al., 2019; Yang et al., 2024a).
Soil–atmosphere exchanges have been measured in different regions of the world, such as in tropical (Artaxo et al., 2022; Bourtsoukidis et al., 2018; Jardine et al., 2015, 2017), boreal (Aaltonen et al., 2013; Artaxo et al., 2022; Mäki, 2019), temperate (Isidorov et al., 2024; Isidorov and Zaitsev, 2022; Leff and Fierer, 2008; Mäki, 2019; Svendsen et al., 2018), and Mediterranean forests (Asensio et al., 2008; Rezaie et al., 2023; Viros et al., 2020; Yang et al., 2024b). Recent studies have reviewed most VOCs emitted from soil compartments (roots, bare soil, soil covered by litter, or litter alone) (Tang et al., 2019; Yang et al., 2024a). The most frequently reported VOCs are isoprene, monoterpenes, sesquiterpenes, and oxygenated VOCs such as methanol (Gray et al., 2010). Methanol is both emitted and consumed by soil bacteria and rhizosphere microorganisms (Asensio et al., 2007a). Other studies have reported important VOC deposition fluxes of hexanal or acetone (Peñuelas et al., 2014, and references therein).
Abiotic (soil moisture and temperature) and biotic (e.g., microbial community composition and biomass, soil and litter nutrient availability) parameters can drive VOC degradation (Cleveland and Yavitt, 1998; Rinnan and Albers, 2020; Trowbridge et al., 2020). Processes and sources related to soil VOC emissions are reviewed in Isidorov and Zaitsev (2022) and Tang et al. (2019) and highlight the importance of both biotic and abiotic parameters, as evaluated in several studies (Abis et al., 2020; Asensio et al., 2007a; Jiao et al., 2023; Leff and Fierer, 2008; Mackie and Wheatley, 1999; Wilkins, 1996). BVOC emissions are known to increase with microbial activity through microbial decomposition of litter or soil organic carbon and evaporation of litter-stored VOCs (Aaltonen et al., 2013; Insam and Seewald, 2010; Leff and Fierer, 2008; Stahl and Parkin, 1996). Plant roots are also known to be a source of VOCs, acting as mediators of belowground interactions between plants and soil microorganisms (Asensio et al., 2007a; Wenke et al., 2010; Yang et al., 2024a).
Soil also acts as a sink for BVOCs as they can be assimilated through microbial metabolism as a source of carbon and energy for certain heterotrophic microbes (Jiao et al., 2023; Kramshøj et al., 2018; McGenity et al., 2018; Pugliese et al., 2023; Shennan, 2006; Zhang et al., 2020). BVOCs can also diffuse through soil pores, where they can be adsorbed into soil particles or dissolved in soil water until equilibrium is reached (Ahn et al., 2020; Ruiz et al., 1998). Although these uptake processes are less well understood, VOC uptake is considered to be a widespread process in soil (Jiao et al., 2023; Rinnan and Albers, 2020), as well as in the canopy (Niinemets et al., 2014). Among the different drivers of soil VOC fluxes, temperature and drought are important abiotic factors (Asensio et al., 2007a; Legros et al., 2025; Rezaie et al., 2023; Trowbridge et al., 2020), suggesting that these fluxes will be affected by climate change.
Indeed, Mediterranean ecosystems are strongly affected by increasing warming and aridity (Peñuelas, 2008), which can alter soil microbial communities (i.e., diversity, biomass, and activity; Aupic-Samain et al. 2021; Santonja et al. 2017; Shihan et al. 2017), limit soil functioning (e.g., litter decomposition, soil organic carbon sequestration, nutrient release; Quer et al., 2022; Santonja et al., 2017, 2022), and thus affect BVOC exchanges between the soil and atmosphere (Peñuelas et al., 2017; Yang et al., 2024b). While bare soil appears to be a sink for VOCs in Mediterranean ecosystems and other biomes (Asensio et al., 2007b), the soil surfaces covered by litter and litter alone have been highlighted as a source of BVOCs, with negligible to moderate fluxes compared to leaf emissions (Legros et al., 2025; Peñuelas et al., 2014; Viros et al., 2020). However, the influence of the amount of litter biomass on soil VOC fluxes in Mediterranean forests has never been investigated. On the one hand, increasing litter accumulation is expected in this region as intensive drought reduces litter mixture interactions and decomposition, leading to litter accumulation on the soil (Santonja et al., 2015, 2017). Moreover, greening policies and gradual abandonment (in the years around 1855–1870) of wood in shipbuilding have contributed to an increase in forest area, which has doubled since the middle 19th century. On the other hand, chronic limited precipitation expected in the coming decades in the Mediterranean region will lead to lower leaf production and, thus, a lower litterfall in these ecosystems.
In this context, the present study aimed to investigate in situ BVOC emissions from soils in a Mediterranean deciduous forest dominated by downy oak (Quercus pubescens Willd.), constituting the predominant deciduous forest in the southern Mediterranean part of France. In particular, we sought to verify the hypothesis that litter BVOC fluxes are affected by litter biomass accumulation on the soil surface and the associated microbial communities.
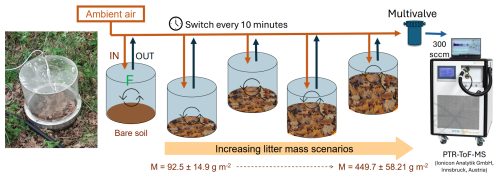
Figure 1Experimental setup for bare soil and soil covered by different litter accumulations. Photo of the PTR-ToF-MS reproduced with permission from Ionicon Analytik GmbH (source: https://www.ionicon.com/, last access: 24 July 2025).
2.1 Experimental site and sampling strategy
Measurements of litter BVOC emissions were performed at the O3HP experimental site, an AnaEE (Analysis and Experimentation on Ecosystems, https://www.anaee.eu, last access: 24 July 2025) in situ platform located at the research center Observatoire de Haute Provence (43°55′ N, 5°42′ E; 650 ; Saint-Michel l'Observatoire, France). AnaEE is a European network providing ecosystem data, research tools, and experimental facilities to study real ecosystems under environmental stress. The climate is typical of the Mediterranean region, characterized by a dry and hot summer (mean air temperature between 5.4 °C in January to 20.2 °C in July and precipitation rates of ∼ 500 mm yr−1; Rameau et al., 2008). The O3HP site was created in 2009 in order to study the Q. pubescens forest ecosystem (≈ 90 % of the biomass and ≈ 75 % of the trees) in different compartments (soil, leaf, and canopy scales). A rainfall exclusion setup (an automated, monitored roof that is deployed during rain events) is installed over part of the O3HP canopy to study both natural and intensified water stress conditions in this forest. Litter production of Q. pubescens within the site ranges between 1.4 and 1.6 , that is 166 (Genard-Zielinski et al., 2015; Viros et al., 2020). A dense network of sensors in the soil and under and above the canopy continuously recorded the climatic and edaphic parameters (air and soil temperatures and relative humidity, photosynthetically active radiation or PAR). More details can be found in Garnier et al. (2021).
The experiment was conducted in spring, from 23 to 26 May 2023. Five dynamic soil chambers (14 L volume) consisting of polycarbonate cylinders with a stainless-steel basement were used (for a full description, see Legros et al., 2025). Each chamber covered 0.067 m2 of soil surface. Fresh litters were removed from the soil and then were weighted and installed for the experiment. Aliquots were made to calculate equivalent dry masses of the 12 litter biomass pools used in this experiment (Table S1 in the Supplement). Chamber 1 covered bare soil (i.e., 0 g m−2 of litter), while chambers 2 to 5 circled bare soil fully covered by litter dry mass of Q. pubescens ranging from 92.5 to 449.7 g m−2 over the 3 d experiment period (Table S1, Fig. 1). All chambers were flushed using ambient air at 0.3 L min−1, and an inlet to measure ambient air was placed at 50 cm above ground level at a central position in relation to the five chambers. Ambient concentrations varied from 0.001 to 3.660 ppbv. Diurnal variations were observed for most of the compounds and are related to transported air masses and VOC emissions from the canopy. The most abundant ambient VOC concentrations are reported in Table S2 in the Supplement. Emissions from each chamber were monitored over a 24 h period. A new litter pool was added each day in chambers 2 to 5, resulting in a total of 15 samples over the 3 d experiment period, i.e., 3 bare soils and 12 different litter biomasses representing the gradient of increasing litter biomass accumulation.
2.2 PTR-ToF-MS (proton transfer reaction time-of-flight mass spectrometer) parameters, data treatment, and flux calculation
BVOCs were monitored by a PTR-ToF-MS (proton transfer reaction time-of-flight mass spectrometer 6000X2, Ionicon Analytik GmbH, Innsbruck, Austria). Each chamber and the ambient inlet were connected to a flow through a multi-valve (Valco Instruments Co. Inc, VICI VALCO, Houston, USA) placed inside the PTR-ToF-MS through ∼ 20 m long Teflon lines, with a flow of around 0.3 L min−1 in each line. Parameters of the PTR-ToF-MS were set to a reaction chamber pressure of 2.6 ± 0.001 mbar and to a drift tube voltage and temperature of 450 V and 120 °C, respectively, corresponding to an ratio (electric field strength over buffer gas number density) of ≈ 125 Td (1 Td = 10−17 V cm−2). Each of the 15 samples (bare soil or soil covered by litter) and the ambient air were sequentially monitored every 10 min over 24 h, leading to a 1 h cycle. From these 10 min, the first 3 min after the switch were removed to avoid accounting for the initial peak. Measurements were performed over 3 d using a new pool of litter each day within each chamber.
A large range of BVOCs was identified, with a mass-to-charge ratio () of up to 500. PTR-ToF-MS data were post-processed with IDA software (Ionicon Data Analyzer, Ionicon Analytik GmbH, Innsbruck, Austria). First, mass calibration was performed based on the H3O+ isotope (, 21.022), the isotope (, 39.033), and the diiodobenzene (an internal calibrant) parent ion (, 330.848) and its main fragment (, 203.943). Then peak fitting and high-resolution integration were performed. Peak identification was done based on peak position and the most probable combination of C, H, N, and O atoms. When possible, molecular formulas were assigned to organic compounds based on previous references for BVOC emissions (Inomata et al., 2014; Meischner et al., 2022; Yáñez-Serrano et al., 2021). BVOC mixing ratios were finally calculated using proton transfer theory, where the rate constant k (in cm3 s−1) was determined for each molecular formula based on the method proposed by Cappellin et al. (2012). The relative ion transmission efficiency was calculated using a standard gas calibration mixture containing 14 different VOCs (summarized in Table S3 in the Supplement) at 100 ± 10 ppb in nitrogen (TO-14A Aromatic Mix, Restek Corporation, Bellefonte, USA).
BVOC fluxes (F) from soil were calculated as described in Yang et al. (2024a):
where Cchamber and Cambient are BVOC concentrations in ng L−1 at the chamber outlet and in ambient air, respectively; Q is the flow rate inside the chamber (= 0.3 L min−1); and A is the soil surface (0.0616 m2).
2.3 Phospholipid fatty acid (PLFA) analysis measurements
After the end of BVOC measurements, the 15 soil and 12 litter samples were collected, frozen, lyophilized over 72 h, and then ground into powder prior to microbial analyses. The phospholipid fatty acids (PLFAs) are essential components of all living cells and are used as biomarkers of soil microbial communities (Frostegård and Bååth, 1996). The PLFA extraction, identification, and quantification followed the protocol described by Aupic-Samain et al. (2021) and Biryol et al. (2024). Litter lipid contents were extracted with Bligh–Dyer solution containing a quantification standard (C19:0; MIDI Inc., Newark, DE, USA). The lipids were then separated using a 96-well solid-phase extraction (SPE) plate (SilactSPE Silica Affinisep®). To identify and quantify the lipids present, a gas chromatography mass spectrometer (a GC-MS Agilent 7890 system with a MSD5977A Network mass detector, an ALS7693 automatic injector, and an HP5-MS apolar column) was employed with MassHunter and Sherlock software (MIDI Inc., Newark, DE, USA). Among the PLFAs identified in the samples, 10 were analyzed because they are markers for gram-positive bacteria, gram-negative bacteria, Actinobacteria, saprotrophic fungi, and arbuscular mycorrhizal (AM) fungi (Biryol et al., 2024; Frostegård and Bååth, 1996). Microbial biomasses were obtained by converting the peak areas into µg g−1 of litter or soil.
2.4 Measurements of environmental variables
Ambient meteorological parameters (temperature, relative humidity and precipitation, wind speed and direction, rain, solar radiation) were recorded using a Sentinel weather station. Temperature and precipitation within the plot over 1 year prior to this study are shown in the ombrothermic diagram in Fig. 2 (for more details on climate at O3HP, refer to Garnier et al., 2021). It is illustrated that 2022 was very dry, with 5 months of drought (January, March, June, July, and October 2022), while May 2023 (the measurement period for this study) was rainy, with an accumulated precipitation of 120 mm.
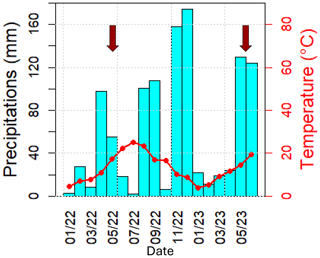
Figure 2Ombrothermic diagram from January 2022 to June 2023 (months per year). Red arrows refer to the field campaign in June 2022 (Legros et al., 2025), performed within the natural and accentuated water stress condition plots, and in May 2023 (this study).
Temperature and humidity inside the chambers were continuously monitored by sensors (i-buttonsLink, Whitewater, WI 53190, USA). Litter humidity was estimated from measurements of fresh and dry litter using three aliquots of 8 g each day (Table S1). Litter aliquots were dried using oven-drying at 65 °C for 3 d. Litter humidity calculated as ranged from 14 % to 41 % (Table S1).
2.5 Statistical analysis
Data were analyzed, statistically computed, and plotted using RStudio (version 2024.12.0). A holistic approach was used to investigate emissions, meaning that all VOCs detected by the PTR-ToF-MS (710 compounds) were considered in the data analysis steps described as follows. First, a Welch t test was used to only select ions showing a significantly different average concentration when comparing ambient and chamber measurements. The dataset was filtered to keep ions presenting a significant flux difference between ambient and chamber measurements. The BVOCs of interest are summarized in Table S4 in the Supplement. VOC data were filtered to keep the most abundant VOC fluxes in each chamber by applying a filter and removing all fluxes > 0.002 (in terms of absolute value).
To investigate the link between environmental parameters and the main measured VOC fluxes, a correlation matrix has been built based on Pearson correlations by merging all chamber data. A heatmap correlation plot was classified into five clusters of VOC fluxes and environmental and biological parameters. The optimal number of clusters was estimated using a k-means clustering method technique (Steinley, 2006).
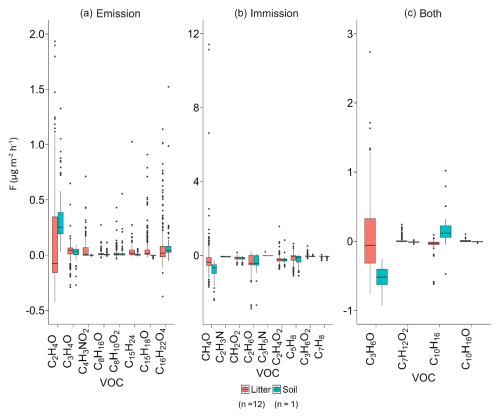
Figure 3Measured VOC fluxes (in ) during the field campaign for (a) major emitted compounds, (b) major immitted compounds, and (c) both emitted and immitted compounds for litter chambers and bare-soil chambers. The line that divides the box represents the median of measured fluxes, the ends of the box indicate the upper (Q3) and lower (Q1) quartiles, the upward and downward whiskers represent the maximum and minimum values, and outliers are represented by dots. VOC fluxes for this figure were obtained from the 24 h measurements of 12 samples of soil covered by litter (276 VOC flux measurements) and 3 bare-soil samples (69 flux measurements).
3.1 Diversity of VOC fluxes
Considering all treatments, we identified more than 135 compounds that may originate from different sources as ambient air was flushed into the chambers. However, these numerous measured VOCs reflect the high diversity of VOCs exchanged (emission or deposition) by Q. pubescens litter, which is much more than in previous studies focusing on VOC fluxes from litter soil (Asensio et al., 2007b; Legros et al., 2025; Viros et al., 2020; Yang et al., 2024b). This finding might be the consequence of contrasting conditions compared to other studies. Furthermore, the holistic approach used in this study tends to include a higher diversity of ions. Altogether, these compounds represent a total negative flux (or immission) of −0.85 . Considering the most abundant VOC fluxes (compounds with an absolute value of average fluxes above 0.01 , a total of 46 compounds) in each chamber, the average total flux of VOC reached −0.72 , representing 90 % of the total flux (Fig. S1, Table S4).
In comparison, Legros et al. (2025) reported emissions of 9.11 ± 0.64 when considering total VOC fluxes. Yang et al. (2024b) showed that fluxes are dependent on seasonality. Indeed, they found negative fluxes in summer and winter (−25.9 ± 9.36 and −2.77 ± 3.84 , respectively) and positive fluxes in autumn and spring (4.54 ± 7.18 and 3.86 ± 2.59 , respectively).
Regarding individual compounds, positive and negative fluxes occurred from bare soil and soil covered by litter, as often reported in earlier studies (Legros et al., 2025; Yang et al., 2024b). Figure 3 presents the positive and negative net fluxes for major compounds, while results for all VOCs are reported in the Data S1 in the Supplement. Almost 48 % of the compounds showed negative fluxes, meaning they were immitted by the soil and soil covered by litter through degradation or adsorption processes; 37 % were both emitted and immitted; and 15 % were only emitted (Data in S2 in the Supplement).
Soil covered by litter and bare soil mainly differed in terms of methanol (CH4O), acetone (C3H6O), and isoprene (C5H8) fluxes, which were bidirectional (emitted and immitted) in the presence of litter, whereas they were only emitted or immitted by bare soil (Fig. 3). For monoterpene (C10H16) and acetaldehyde (C2H4O) fluxes, higher net positive emissions were observed for bare soil than for soil covered by litter. This suggests that soil can be a source of monoterpene and acetaldehyde emissions to the atmosphere. The presence of litter on the soil physically reduces the transfer to the atmosphere or favors the presence of microorganisms that consume these two VOCs (Marmulla and Harder, 2014; McBride et al., 2023).
VOC emissions
In this section, VOCs are only considered to be emitted when they have positive average VOC fluxes for each modality (bare soil and soil covered by litter); the opposite is the case for immission (next section). The compounds with the highest emissions were acetaldehyde (C2H4O), acrolein (C3H4O), 1H-pyrrole-2,5-dione (C4H3NO2), lindestrene (C15H18O), creosol (C8H10O2), sesquiterpenes (C15H24), octanal (C8H16O), and dibutyl phthalate (C16H22O4).
On average, we measured positive acetaldehyde fluxes of 0.056 ± 0.192 and 0.186 ± 0.226 from soil covered by litter and from bare soil, respectively. In both cases, the fluxes were highly variable, resulting in positive and negative fluxes during the diurnal cycle (Sects. 3.3 and 3.4). Emissions of acetaldehyde have been measured during previous litter emission experiments (Asensio et al., 2007a; Gray et al., 2010, 2014). The origin of acetaldehyde can be attributed to root and leaf litter emissions (Schade and Goldstein, 2001; Warneke et al., 1999) formed by the oxidation of VOCs, but this second source is expected to be minor considering the residence time in dynamic chambers.
Although acrolein is mostly considered to be an anthropogenic compound originating from combustion sources (Koss et al., 2018; Schieweck et al., 2021), positive acrolein fluxes were observed in all chambers, with an average flux of 0.009 ± 0.033 . Acrolein has previously been associated with the decomposition of litter biomass in a mixed broadleaf and coniferous forest (Ehrlich and Cahill, 2018). Indeed, higher concentrations were measured in fresh leaves compared to in senescent or decomposing leaves. Acrolein was also previously detected in the litter of Pinus halepensis Mill. by Viros et al. (2021) and in the litter of Q. pubescens in a previous study performed at the O3HP experimental site (Legros et al., 2025), which showed an average flux 100 times higher than that in the present study (0.79 ± 0.04 ). Drier conditions during the study of Legros et al. (2025) compared to the conditions of our experiment (Fig. 2) could explain the differences observed between the two studies.
C4H3NO2 was tentatively assigned as 1H-pyrrole-2,5-dione is observed for the first time in litter VOC fluxes. This compound is a monopyrrolic derivative of the maleimide class/family corresponding to a transformation product of chlorophyll (Naeher et al., 2016). Maleimides are commonly studied in paleoecological and human-based studies and possess various biological characteristics, such as antibacterial and antifungal properties (Ma et al., 2022).
Two other compounds not previously measured were identified in this study as C15H18O, proposed to be lindestrene (average flux of 0.006 ± 0.014 ) and creosol (C8H10O2, 0.003 ± 0.006 ). To our knowledge, no previous study documented lindestrene emissions (or immissions) from soil, but it has been reported in emissions from tree resins (Taiti et al., 2018). Regarding creosol, it is a natural organic compound degradation in creosote, which can be further degraded by several fungal species (Atagana et al., 2006; Lee et al., 2005). This degradation process highlights the role of fungi in the decomposition of complex organic compounds, potentially contributing to soil and ecosystem health by removing phenols from contaminated soils (Atagana, 2004; Atagana et al., 2006).
Sesquiterpenes were also detected, with average fluxes of 0.003 ± 0.006 , and are frequently reported in the litter of terpene-storing species (Asensio et al., 2008; Viros et al., 2021; Yang et al., 2024b). Viros et al. (2020) reported VOC fluxes from Q. pubescens litter, but they did not observe sesquiterpenes, and this was only reported in the form of minor emissions in a previous field study at the O3HP experimental site (Legros et al., 2025). Since no storage organs are present in Q. pubescens leaves, the observed emission can be related to fungi, known to release high amounts of sesquiterpenes (Mäki et al., 2017; Weikl et al., 2016). However, no studies in the Mediterranean region report a relationship between fungi and sesquiterpene fluxes.
Octanal (C8H16O, detected at 129.132) fluxes reached, on average, 0.002 ± 0.004 for litter. Octanal has been previously found in Mediterranean litters for Q. pubescens, Quercus suber L., and Ulex parviflorus Pourr. (Viros et al., 2020; Legros et al., 2025) and, more specifically, in tundra soils, where it accounts for about 75 % of the total VOC emissions (Kramshøj et al., 2016). The processes responsible for octanal emissions remain unexplored.
Dibutyl phthalate (C16H20O4) fluxes were similar for both bare soil and soil with litter (on average: 0.007 ± 0.019 ). The occurrence of this compound in a rural environment such as that of O3HP seems surprising as it is known as a plasticizer compound, but emission can be related to microbial taxa, as shown by Sillo et al. (2024), who reported a significant correlation between dibutyl phthalate fluxes and the abundance of the Paenarthrobacter genus (Actinomycetota class such as Micrococcaceae).
VOC immission
Immission was frequently observed for oxygenated compounds, such as alcohols (methanol, CH4O, ethanol, C2H6O) and carboxylic acids (acetic, C2H4O2, propanoic, C3H6O2, and formic CH2O2 acids), and also for alkenes (isoprene, C5H8 and toluene, C7H8) and two nitrogenous compounds (acetonitrile, C2H3N and propanenitrile, C3H5N).
Average fluxes for both bare soil and soil covered by litter were negative for methanol (−0.033 ± 0.038 and −0.044 ± 0.047 , respectively) and ethanol (−0.383 ± 0.468 and −0.346 ± 0.447 , respectively). In contrast, most previous studies suggest that soil is a source of alcohols (Asensio et al., 2008; Gray et al., 2010; Yang et al., 2024a), except for Asensio et al. (2007a), who reported both methanol and ethanol deposition in a Mediterranean Quercus ilex L. forest in northern Spain (from −95.04 to −71.28 ). Thus, whether these compounds are primarily emitted from soils or deposited is strongly dependent on both biotic (microbial activity) and abiotic (meteorological parameters) factors. For example, Asensio et al. (2007a) showed that VOC immission of alcohol is related to soil moisture. More generally, lignocellulose, which is the most widespread and abundant source of carbon in nature for microorganisms (Abdeshahian et al., 2020), reached concentrations of about 187.6 ± 1.5 in Q. pubescens litter (Santonja et al., 2015), which is considered to be the preferred biomass consumed by bacteria for the production of ethanol (Du et al., 2015).
Carboxylic acids showed negative fluxes, with acetic acid having the highest deposition rate (−0.088 ± 0.115 on average for litters and −0.096 ± 0.117 for bare soil), followed by formic acid (−0.082 ± 0.099 on average for litters and −0.089 ± 0.105 for bare soil) and propanoic acid (−0.011 ± 0.022 on average for litter and −0.017 ± 0.22 for bare soil). Previous studies showed contrasting features: while in agreement with our results, some studies observed deposition in Mediterranean forests (Asensio et al., 2007b, a). Legros et al. (2025) observed emissions of acetic and formic acids. These compounds are sensitive to soil moisture, which favors microbial uptake and deposition of VOC on wet surfaces (Asensio et al., 2007a). Propanoic acid is known to be released during microbial fermentation (Leff and Fierer, 2008; Wheatley et al., 1996), while acetic or formic acids are reported to be produced by bacteria and fungi (Mielnik et al., 2018; Wheatley et al., 1996). Given the environmental conditions (i.e., humid) inside the chambers, we hypothesize that carboxylic acid deposition processes linked to the soil moisture dominate, resulting in net deposition (further discussed in Sect. 3.4).
Isoprene showed an average immission rate of −0.048 ± 0.106 for litters and −0.082 ± 0.128 for bare soil. Accordingly, most studies have shown negative fluxes for isoprene (Cleveland and Yavitt, 1998; Gray et al., 2014, 2015; Legros et al., 2025; McGenity et al., 2018). Higher negative fluxes were estimated by Legros et al. (2025) at −0.92 ± 0.20 and by Gray et al. (2014), with values up to −138.04 ± 89.76 . The isoprene fluxes are driven by the microbial activity as this compound is consumed by bacterial and fungal taxa (Gray et al., 2015) and can also be released by root exudates (Asensio et al., 2007a). In addition, isoprene is well known as being emitted by the canopy of Q. pubescens (Genard-Zielinski et al., 2015), and it is possible that specific bacteria or fungi that can use isoprene as a carbon source developed in the soil and litter at the O3HP experimental site.
Toluene (C7H8) immission occurred in both bare soil and soil covered by litter (−0.013 ± 0.014 on average), indicating that litter and soil are a sink for this compound. This VOC is well known as being of anthropogenic origin, associated with fossil fuel and biomass burning (Hanif et al., 2021), and, to a lesser extent, of plant stress origin (Heiden et al., 1999; Misztal et al., 2015). This compound may thus have been transported in the air masses affected by anthropogenic emissions and deposited on this rural ecosystem.
Toluene, acetonitrile (C2H3N), and propanenitrile (C3H5N) are mainly known to be emitted from anthropogenic sources and, more particularly, from biomass burning (Holzinger et al., 1999; Sarkar et al., 2016; Yang et al., 2016). However, acetonitrile emission has been reported in some plant species such as Gynandropsis gynandra L. (Briq.), where it is a repellent against spider mites (Nyalala et al., 2011, 2013). In our study, the averaged fluxes of acetonitrile and propanenitrile were negative, with −0.038 ± 0.040 and −0.005 ± 0.006 , respectively. Acetonitrile deposition has already been measured in Mediterranean forest soils, ranging from −75.6 to −15 (Asensio et al., 2008). In the present study, the origin of the acetonitrile and propanenitrile is probably regional rather than local since these compounds are well known as having a long lifetime (more than a year, Andersen et al., 2018; de Gouw et al., 2003), and deposition of this compound occurred in the forest soil of this study.
VOCs both emitted and immitted
Many oxygenated compounds showed bidirectional fluxes or fluxes of opposite magnitude when comparing bare-soil average fluxes to the fluxes soil covered by litter, including acetone (C3H6O), cyclohexanecarboxylic acid (CHC acid, C7H12O2), monoterpenes (C10H16), and oxygenated monoterpenes (C10H16O).
Among these compounds, acetone is one of the most commonly detected volatiles released from soils (Asensio et al., 2008; Gray et al., 2010), with an average immission of −0.217 ± 0.220 from bare soil and with, by contrast, a positive flux of 0.019 ± 0.195 from soil covered by litter. The positive flux is lower than that of Legros et al. (2025), who reported an average acetone flux of 1.15 ± 0.30 . This can be attributed to the different environmental conditions encountered during measurements.
Cyclohexanecarboxylic acid (CHC acid, C7H12O2) was found to have an average positive flux in litter chambers (0.003 ± 0.006 ) and an average negative flux for bare soil (−0.002 ± 0.003 ). Like lindestrene and 1H-pyrrole-2,5-dione, CHC acid has not yet been reported on in other studies about bare soil, litter alone, or bare soil covered by litter. CHC acid is produced naturally as part of the shikimate pathway, a major metabolic pathway for the biosynthesis of aromatic compounds in bacteria (Alicyclobacillus acidocaldarius and Streptomyces collinus), plants, and fungi (Shende et al., 2024). This pathway is key to the formation of phenolic compounds (Laoué et al., 2022). Thus, this novel finding highlights the potential for further exploration of its sources and ecological role.
Monoterpene fluxes were negative when the soil was covered by litter (−0.007 ± 0.015 on average). By contrast, bare soil showed positive fluxes of monoterpenes (0.026 ± 0.043 on average), as a previous study already observed (Leff and Fierer, 2008). The negative fluxes when the soil was covered by litter suggest that microorganisms consume monoterpenes as these compounds have been reported as a carbon and energy source for microorganisms (Marmulla and Harder, 2014; White, 1994). Bacteria also consume monoterpenes in order to protect themselves from the monoterpene-related toxic effects (Marmulla and Harder, 2014), and fungi are known to transform monoterpenes during growth (Farooq et al., 2004).
Fluxes of oxygenated monoterpenes were measured to be 0.001 ± 0.002 for litter emissions and at −0.001 ± 0.002 for bare soil on average. While this has not been reported for Q. pubescens forests, a previous study has shown concentrations of camphor from Rosmarinus officinalis in the Mediterranean region (3.18 ± 0.42 by Ormeño et al. (2008) and 9.9 ± 3.4 by Staudt et al. (2017)). Oxygenated monoterpenes (C10H16O, such as camphor, fenchone, or carveol) may originate from litter decomposed by fungi, as proposed by Isidorov and Jdanova (2002).
3.2 VOC variation with different litter masses
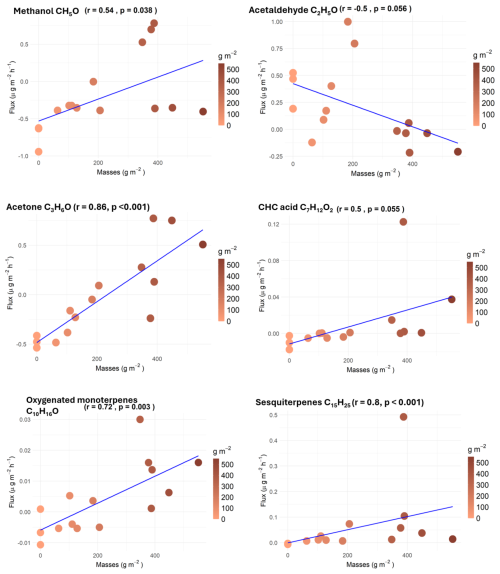
Correlations of VOC fluxes with the litter masses inside the chambers were performed (Fig. 4). Surprisingly, only a few compounds showed significant relationships between increasing litter masses and fluxes. Correlations were positive only for methanol, acetone, oxygenated monoterpenes, CHC acid, and sesquiterpenes. For other compounds, the lack of correlation may be due to a high variability or other environmental factors such as temperature and humidity inside the chambers, which are more important drivers of emission and/or deposition.
Methanol, acetone, oxygenated monoterpenes, and CHC acid fluxes showed a positive correlation with litter masses (Fig. 4), but average bare-soil fluxes were negative. For methanol and CHC acid, the correlation with litter mass is weaker (r = 0.54 and pvalue = 0.038 for methanol; r = 0.50 and pvalue = 0.055 for CHC acid) than for acetone and oxygenated monoterpenes (r = 0.63 and pvalue < 0.001 for acetone; r = 0.86 and pvalue < 0.001 for oxygenated monoterpenes). For these four compounds, the net flux increases with litter mass from a negative flux in bare soil to a positive flux at high litter mass. As a result, it is suggested that the soil alone acts as a sink for these four compounds, while litter mostly emits them, with the resulting net fluxes depending on the litter mass amount and maybe environmental conditions. However, a deeper study dedicated to separating the respective contributions of soil and litter by increasing the number of replicates in different locations will be necessary to fully elucidate this point.
A negative response has only been observed between acetaldehyde flux and litter mass (r = −0.50 and pvalue = 0.056). In opposition to the previous four compounds, emissions are observed for bare soil, while the net fluxes turned negative with increasing litter mass, suggesting that the soil is a source of acetaldehyde, further deposited on litter. This may be due to the presence of microorganisms on leaf litter that can take up acetaldehyde.
Finally, sesquiterpenes were the most significant VOC linked to the litter mass (r = 0.80 and pvalue < 0.001), with positive fluxes for all modalities increasing together with litter mass. If no storage organs are present in Q. pubescens leaves, the abundance of potential fungi associated with sesquiterpene emissions, as suggested by Mäki et al. (2017) and Weikl et al. (2016), should depend on litter mass.
Only a few compounds have shown a significant relationship with the variation of litter mass. Further tests with longer time series are needed to complete our observations.
3.3 VOC diurnal variations
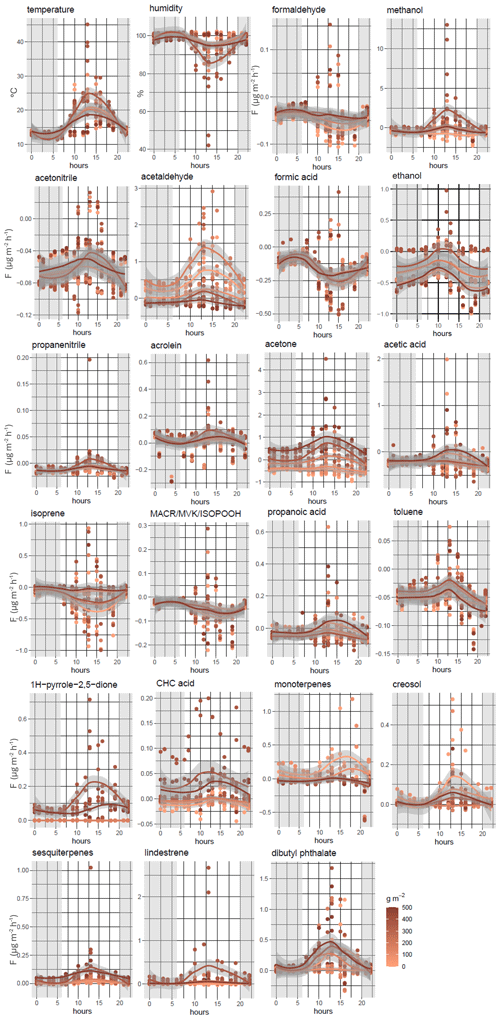
The diurnal variations of the main VOC fluxes (F > 0.005 ; 13 % of the total compounds, equating to 21 compounds; ∼ 75 % of the total fluxes) and temperature and relative humidity are shown in Figs. 5 and S2. The temperature was highest between 08:00 and 15:00 LT (local time), ranging from 15 to 20 °C and, exceptionally, reaching up to 30 °C in some litter samples. By contrast, the humidity decreased by up to 75 % during the same hours and reached 100 % during the nighttime.
Most of the compounds showed an increase in their fluxes between 10:00 and 15:00 LT. Strong diurnal variations were measured, with high fluxes during the daytime for methanol (0.797 ± 2.355 ), 1H-pyrrole-2,5-dione (0.050 ± 0.040 ), CHC acid (0.009 ± 0.014 ), and acetone (0.165 ± 0.402 ).
Patterns can vary between compounds within the litter mass gradient. Indeed, similar patterns can be highlighted for 1H-pyrrole, CHC acid, lindestrene, and sesquiterpenes, with higher emissions being associated with high litter masses (> 400 g m−2) during the day and very low levels during the nighttime. The 1H-pyrrole-2,5-dione, lindestrene, and CHC acid fluxes were only significant in the two highest litter mass scenarios (information in Data S2 in the Supplement, averaged fluxes). These fluxes are dependent on the litter mass but not linearly and include a threshold effect, explaining why they were not discussed in the previous section.
Monoterpenes were released from bare soil and low litter masses between 12:00 and 17:00 LT and were then taken up from 18:00 LT until midnight (Fig. 5). A specific pattern was shown for isoprene, formaldehyde (HCHO), and formic acid. Indeed, these fluxes decreased between 05:00 and 10:00 LT and increased around 12:00 LT, when isoprene emissions from trees were maximal, being linked to temperature and solar radiation (Kesselmeier et al., 1998; Monson et al., 1992; Owen et al., 2002). Around midnight, isoprene fluxes were stable in all litter masses, and no exchange occurred between the soil and the atmosphere (i.e., concentrations inside the chamber were identical to ambient concentrations). As expected, isoprene oxidation products (methacrolein, MACR; methyl vinyl ketone, MVK; and isoprene hydroxy hydroperoxide, ISOPOOH) showed the same pattern as isoprene, with an uptake of this compound in bare soil and all litter masses. Their fluxes increased between 10:00 and 12:00 LT, with averaged fluxes of up to −0.021 ± 0.022 during the daytime.
The uptake of formic acid, acetone, and formaldehyde by the soil was particularly high at night when the humidity increased and reached 100 % (−0.052 ± 0.023, −0.059 ± 0.073, and −0.027 ± 0.008 , respectively). Propanoic and acetic acids also showed negative fluxes (−0.008 ± 0.027 and −0.077 ± 0.114 ) during the daytime. These differences can be attributed to the fact that fluxes of these oxygenated compounds are influenced by abiotic factors, particularly soil moisture and temperature. Jacob et al. (2002) have demonstrated that both acetone fluxes and concentrations increase with rising temperatures. Furthermore, the physicochemical degradation of VOCs can act as a sink for VOCs at the soil level (Peñuelas et al., 2014). Our results are in line with those of Legros et al. (2025) for daytime acetone and isoprene fluxes, with the aforementioned authors having measured positive and negative fluxes for acetone and isoprene (3.52 ± 0.40 and −1.45 ± 0.41 , respectively).
Methanol fluxes were recorded for all chambers and were essentially negative during the nighttime (Fig. 5) and slightly positive during the daytime, except for litter masses between 120 g m−2 and 300 g m−2, where the flux is higher than on the other days (up to 0.797 ± 2.354 ). For litter masses ∼ 350 g m−2, the environmental conditions showed few differences with higher temperature and then lower humidity during the measurements (temperature and humidity inside the chambers in Fig. 5), which can explain the differences in the observed fluxes. The role of environmental parameters in the processes of consumption or emission of methanol by microorganisms has been already studied (Asensio et al., 2007b; Cleveland and Yavitt, 1998; Gray et al., 2015; Jiao et al., 2023; Schade and Goldstein, 2001).
Positive fluxes were observed during the day for acetaldehyde (0.184 ± 0.245 ), except for litter masses > 400 g m−2, and negative acetaldehyde fluxes were observed during the nighttime for litter masses in the medium–high scenario (300 g m−2 < M < 400 g m−2; −0.076 ± 0.029 and −0.066 ± 0.033 , respectively). This result is in line with previous studies that showed that acetaldehyde can be both emitted and taken up by soil (Asensio et al., 2008; Jiao et al., 2023; Peñuelas et al., 2014). Ethanol fluxes showed the same pattern as acetaldehyde, with higher fluxes between 10:00 and 12 00 LT (local time) (−0.017 ± 0.022 on average during the daytime).
In Legros et al. (2025), acrolein was shown to have high flux variations during the day and night (diurnal cycle). Our measurements were made almost 1 year after the Legros et al. (2025) study at the same site. However, temperature and humidity were strongly different, and the soil was not submitted to the same constraints, explaining the differences observed.
Toluene, creosol, propanenitrile, and acetonitrile are compounds with long lifetimes (> 1 year). Increases in the magnitude of fluxes during the daytime have been reported. However, the fluxes remained negative for toluene (−0.012 ± 0.009 ), propanenitrile (−0.004 ± 0.007 ), and acetonitrile (−0.034 ± 0.018 ) and turned positive for creosol (0.006 ± 0.011 ). Dibutyl phthalate fluxes were positive and higher during the daytime (0.016 ± 0.025 ) than during the nighttime (0.003 ± 0.003 ).
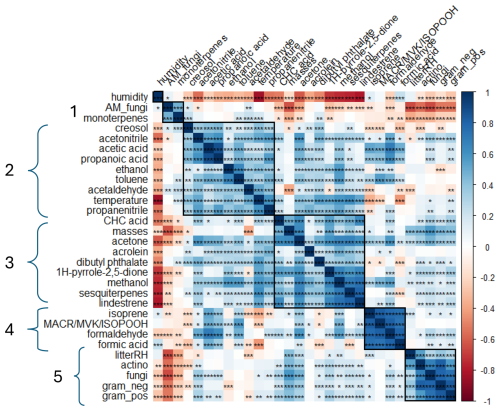
Figure 6Correlation matrix between BVOC fluxes and abiotic (air temperature and humidity, litter humidity) and biotic (litter mass and microbial biomass) factors, merging the data of the 15 chambers (n = 345). Positive correlations (r > 0) are highlighted in blue, and negative correlations (r < 0) are highlighted in red. Stars represent pvalues and their confidence: p ≥ 0.05 (∗), p < 0.01 (), p < 0.001 (). The cluster number is on the left of the correlation plot. AM_fungi denotes arbuscular mycorrhiza fungi, CHC acid denotes cyclohexanecarboxylic acid, and litterRH denotes litter humidity.
3.4 VOC fluxes linked to environmental and biological parameters
As the previous section illustrates, in relation to the strong variability during the hours of the day but also between the different scenarios, we investigated the relation between BVOC fluxes and biotic and abiotic factors to better understand the main drivers of these fluxes. For this purpose, a correlation analysis was performed based on hourly fluxes, merging the data of the 15 chambers (n = 345), as presented in Fig. 6.
First, a strong positive correlation can be observed between monoterpenes and arbuscular mycorrhizal (AM) fungi, forming the first cluster (r = 0.48, pvalue < 0.001; Fig. 6). We suggested earlier that soil fungi are the main source of monoterpenes as negative monoterpene fluxes were observed when litter mass increased. The concentration of AM fungi is observed to be higher in the bare soil compared to in the soil covered by litter masses, which confirms the fact that AM fungi can be the source of monoterpenes from the soil. This confirms the results of previous studies, showing that fungi are producers of monoterpenes (Farooq et al., 2004; Marmulla and Harder, 2014).
Compounds observed in cluster 2 were positively related to the temperature inside the chambers and, logically, were negatively related to relative humidity ( = −0.85). Most of the compounds are deposited (except acetaldehyde and creosol), and their correlation with temperature indicates that deposition is higher when temperature is lower or when humidity is higher. Acetonitrile and propanenitrile were among the compounds with the strongest response to temperature ( = 0.50, = 0.59). As mentioned previously, acetonitrile and propanenitrile's main atmospheric sources are considered to be combustion processes, even if a few studies showed biogenic origins (Heiden et al., 1999; Misztal et al., 2015; Nyalala et al., 2011, 2013). This result may indicate that deposition is favored by lower temperatures or higher humidity, with lower temperatures reducing the volatility of these species and humidity favoring adsorption and dissolution in water (especially for hydrophilic compounds). Acetonitrile fluxes were linked to propanenitrile, propanoic and acetic acids, and acetone ( = 0.55, = 0.60, = 0.55, and = 0.58, respectively). As a few positive fluxes were observed for these nitrogen-containing VOCs, it is suggested that their potential emissions can be biogenic as acetone and acids have a microbial fermentation origin. Acetaldehyde and creosol are also included in this second cluster but are mainly emitted compounds (see Sect. 3.1). Acetaldehyde has been described as an oxidation product of ethanol. However, only moderate correlations were measured between these two compounds (r = 0.36, pvalue < 0.001), confirming that there is no strong photochemistry inside the chambers. However, strong relationships were observed between toluene and ethanol and between methanol and ethanol ( = 0.64, = 0.56), indicating that these three compounds are likely to share a common origin.
Cluster 3 gathers compounds positively correlated with the litter masses highlighted in Sect. 3.2, as well as some other compounds (CHC acid, acrolein, dibutyl phthalates, and 1H-pyrrole-2,5-dione; e.g., = 0.63, = 0.58). In addition, we showed previously (Fig. 4) that these compounds present the same pattern, with higher emissions during the daytime for medium–high and high amounts of litter (> 300 g m−2). This means that the emissions of these compounds are either directly related to the litter (emission from tissues) or indirectly related to the litter through the presence of microorganisms. However, our study did not show a link between dibutyl phthalate fluxes and actinomycetes, as suggested by Sillo et al. (2024). This difference may be explained by the type of soil since the aforementioned study focused on urban soils that can be more polluted. Studies have demonstrated that both wet and dry conditions may influence microbial biomass by creating environments unfavorable for aerobic gram-positive (g+) and gram-negative (g−) bacteria, as well as for mycorrhizal fungi (Borowik and Wyszkowska, 2016). Most particularly, actinomycete abundance has been shown to increase with decomposing organic matter. However, actinomycetes are highly pH-sensitive organisms, and their abundance decreases significantly at pH levels below 5 (Kovacs et al., 2023). This parameter was not measured in our experiments, and so we cannot establish a link with actinomycete abundance.
Temperature was negatively related to compounds in cluster 4. Surprisingly, isoprene fluxes were negatively correlated with temperature (r = −0.35, pvalue < 0.001) and so were their oxidation products (MACR, MVK, and ISOPOOH) (r = −0.24, pvalue < 0.001) and formic acid (r = −0.43, pvalue < 0.001). In other words, as these compounds are mostly deposited, the magnitude of deposition increases with temperature. Indeed, Q. pubescens is well documented as a strong isoprene emitter, with emissions being well known to be positively influenced by temperatures (Genard-Zielinski et al., 2015). The temperature dependance of the deposition can thus be explained by the increasing production of isoprene by the green parts of Q. pubescens, which makes it more available for deposition. All compounds in this cluster are strongly correlated. MACR, MVK, and ISOPOOH; formaldehyde; and formic acid are mostly associated with being secondary products formed by the oxidation of isoprene. Isoprene fluxes have been found to be significantly correlated with MACR, MVK, and ISOPOOH; formaldehyde; and formic acid ( = 0.83, = 0.78, and = 0.75, with pvalue < 0.001). These compounds can be attributed to secondary origins from the degradation of isoprene. In addition, regarding the daily fluxes, isoprene; MACR, MVK, and ISOPOOH; formaldehyde; and formic acid fluxes presented the same pattern, supporting the stronger relationship between these compounds. We can hypothesize here that the production of these compounds follows isoprene emissions and that the deposition rate simply increases accordingly. This hypothesis is further supported by the absence of a relationship between these clusters' compound fluxes and PLFAs (g+, g−, actinomycetes, and fungi).
Finally, soil moisture (litterRH in Fig. 6) showed positive correlations with gram-positive and gram-negative bacteria (g+ and g−), actinomycetes, and fungi ( = 0.29, = 0.37, = 0.55, and = 0.29, with pvalue < 0.001; values are reported in Data S3 in the Supplement), all forming cluster 5. Negatively significant but moderate relationships of monoterpene fluxes with fungi have been observed ( = −0.33, pvalue < 0.001). Monoterpene fluxes were likely to be driven by the abundance of actinomycetes and gram-negative bacteria and were consumed by these microorganisms. This explains why we observed a predominance of negative fluxes for these compounds when litter accumulation increased inside the chambers.
A wide range of VOCs were measured from bare soil and litter, with over 135 compounds being identified. Positive and negative fluxes were observed, with some fluxes appearing to be independent of litter mass, such as toluene or formaldehyde. About 48 % of the compounds showed negative fluxes, indicating uptake by the soil and soil covered by litter; 37 % showed both emissions and uptake; and 15 % were emitted. The compounds with the highest uptake rates were ethanol, formaldehyde, isoprene, acetonitrile, toluene, and formic and acetic acids. In the holistic analysis of detected compounds, some VOCs such as lindestrene, cyclohexanecarboxylic acid (CHC acid), and 1H-pyrrole-2,5-dione were identified from the litter for the first time. The origin of these compounds can be attributed to biological sources, particularly microbial activity.
The diurnal variation of the main VOC fluxes and environmental parameters showed an increase in their fluxes between 10:00 and 15:00 LT (local time). Following the studied VOCs, we observed positive or negative fluxes representing, respectively, emission or immission over bare soil and soil covered by litter. Indeed, temperature and humidity conditions were different in scenarios of medium and high litter amounts (masses comprising between 120 and 300 g m−2), which we showed to be correlated with higher fluxes for some compounds, such as those tentatively assigned as lindestrene, CHC acid, or 1H-pyrrole-2,5-dione. We showed correlations between VOC fluxes, environmental parameters (temperature and humidity), and microbial biomasses. Monoterpene fluxes were negatively correlated with actinomycete and gram-negative bacteria biomasses and are therefore likely to be consumed by these organisms. However, monoterpene fluxes were strongly positively correlated with AM fungi that were more observed in bare soil, where fluxes of monoterpenes were positive. We also observed higher fluxes of acetonitrile, propanenitrile, and acetaldehyde with increasing temperature, showing that these compounds can be biogenically emitted, but we cannot rigorously explain their origins at this time.
Based on the results of this study, we can conclude that, in the short term, no clear relationship between litter accumulation and VOC fluxes could be established, except for a few compounds such as acetone, sesquiterpenes, CHC acid, or methanol. This study represents only a first step toward understanding the relationship between litter and VOC fluxes. It is not sufficient to conclude that litter mass has no effect on VOC emissions. We recommend that further experiments be conducted under varying environmental conditions and that time series analysis be conducted to fully evaluate the evolution and seasonality of VOC emissions. Finally, experiments in different locations are required to gain a deeper understanding of the role and effect of the amount of litter accumulated in relation to VOC fluxes.
All of the raw data can be provided by the corresponding authors upon request, and most of the data are provided in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-3661-2025-supplement.
MR and JK analyzed the data. MR and JK wrote the paper draft. EO, BTR, and MS reviewed and edited the paper. EO, MS, BTR, and EO planned the campaign and performed the measurements. CS and CL analyzed the PLFAs and provided PLFA raw data. EQ and HW reviewed the paper and served as the principal investigators responsible for securing funding for this project.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors gratefully acknowledge the MASSALYA instrumental platform (Aix Marseille Université, https://lce.univ-amu.fr, last access: 24 July 2025) for the provision of the analysis and measurements. We are grateful to IMBE, who manages the O3HP site, and ECCOREV, as well as to OSU Pytheas and, especially, to OHP for the accommodation, facilities, and technical support through the engineering work. We thank the AnaEE-ERIC and AnaEE-France networks and, specifically, the PIA (“Plan d'Investissements d'Avenir”) from ANR (grant no. ANR-11-INBS-0001 AnaEE France) for providing the long-term funding for the site functioning and the SEE-life network from CNRS for funding the long-term studies in Ecology and Evolution at the O3HP site. We thank Ilja Reiter for providing the climatic data. This work received support from the French government under the France 2030 investment plan as part of the Initiative d'Excellence d'Aix Marseille Université – A∗MIDEX (grant no. AMX-21-RID-009, LITOSMED).
This research has been supported by the Agence Nationale de la Recherche (grant no. ANR-11-INBS-0001 AnaEE France) and the SEE-life network from CNRS.
This paper was edited by Kerneels Jaars and reviewed by two anonymous referees.
Aaltonen, H., Aalto, J., Kolari, P., Pihlatie, M., Pumpanen, J., Kulmala, M., Nikinmaa, E., Vesala, T., and Bäck, J.: Continuous VOC flux measurements on boreal forest floor, Plant Soil, 369, 241–256, https://doi.org/10.1007/s11104-012-1553-4, 2013.
Abdeshahian, P., Kadier, A., Rai, P. K., and da Silva, S. S.: Lignocellulose as a Renewable Carbon Source for Microbial Synthesis of Different Enzymes, in: Lignocellulosic Biorefining Technologies, edited by: Avinash, P. I., Chandel, A. K., and da Silva, S. S., John Wiley & Sons, Ltd, https://doi.org/10.1002/9781119568858.ch9, 185–202, 2020.
Abis, L., Loubet, B., Ciuraru, R., Lafouge, F., Houot, S., Nowak, V., Tripied, J., Dequiedt, S., Maron, P. A., and Sadet-Bourgeteau, S.: Reduced microbial diversity induces larger volatile organic compound emissions from soils, Sci. Rep.-UK, 10, 6104, https://doi.org/10.1038/s41598-020-63091-8, 2020.
Ahn, J., Rao, G., Mamun, M., and Vejerano, E. P.: Soil–air partitioning of volatile organic compounds into soils with high water content, Environ. Chem., 17, 545–557, https://doi.org/10.1071/EN20032, 2020.
Andersen, S. T., Kyte, M., Andersen, L. L., Nielsen, O. J., and Sulbaek Andersen, M. P.: Atmospheric chemistry of n-CH3(CH2)xCN (x = 0–3): Kinetics and mechanisms, Int. J. Chem. Kinet., 50, 813–826, https://doi.org/10.1002/kin.21215, 2018.
Artaxo, P., Hansson, H. C., Machado, L. A. T., and Rizzo, L. V.: Tropical forests are crucial in regulating the climate on Earth, PLOS Clim., 1, e0000054, https://doi.org/10.1371/journal.pclm.0000054, 2022.
Asensio, D., Peñuelas, J., Filella, I., and Llusià, J.: On-line screening of soil VOCs exchange responses to moisture, temperature and root presence, Plant Soil, 291, 249–261, https://doi.org/10.1007/s11104-006-9190-4, 2007a.
Asensio, D., Peñuelas, J., Ogaya, R., and Llusià, J.: Seasonal soil VOC exchange rates in a Mediterranean holm oak forest and their responses to drought conditions, Atmos. Environ., 41, 2456–2466, https://doi.org/10.1016/j.atmosenv.2006.05.007, 2007b.
Asensio, D., Peñuelas, J., Prieto, P., Estiarte, M., Filella, I., and Llusià, J.: Interannual and seasonal changes in the soil exchange rates of monoterpenes and other VOCs in a Mediterranean shrubland, Eur. J. Soil Sci., 59, 878–891, https://doi.org/10.1111/j.1365-2389.2008.01057.x, 2008.
Atagana, H. I.: Biodegradation of phenol, o-cresol, m-cresol and p-cresol by indigenous soil fungi in soil contaminated with creosote, World J. Microb. Biot., 20, 851–858, https://doi.org/10.1007/s11274-004-9010-z, 2004.
Atagana, H. I., Haynes, R. J., and Wallis, F. M.: Fungal Bioremediation of Creosote-Contaminated Soil: A Laboratory Scale Bioremediation Study Using Indigenous Soil Fungi, Water Air Soil Poll., 172, 201–219, https://doi.org/10.1007/s11270-005-9074-x, 2006.
Aupic-Samain, A., Santonja, M., Chomel, M., Pereira, S., Quer, E., Lecareux, C., Limousin, J.-M., Ourcival, J.-M., Simioni, G., Gauquelin, T., Fernandez, C., and Baldy, V.: Soil biota response to experimental rainfall reduction depends on the dominant tree species in mature northern Mediterranean forests, Soil Biol. Biochem., 154, 108122, https://doi.org/10.1016/j.soilbio.2020.108122, 2021.
Biryol, C., Aupic-Samain, A., Lecareux, C., Gauquelin, T., Baldy, V., and Santonja, M.: Interactive effects of soil moisture, air temperature and litter nutrient diversity on soil microbial communities and Folsomia candida population, Oikos, 2024, e10345, https://doi.org/10.1111/oik.10345, 2024.
Borowik, A. and Wyszkowska, J.: Soil moisture as a factor affecting the microbiological and biochemical activity of soil, Plant Soil Environ., 62, 250–255, https://doi.org/10.17221/158/2016-PSE, 2016.
Bourtsoukidis, E., Behrendt, T., Yañez-Serrano, A. M., Hellén, H., Diamantopoulos, E., Catão, E., Ashworth, K., Pozzer, A., Quesada, C. A., Martins, D. L., Sá, M., Araujo, A., Brito, J., Artaxo, P., Kesselmeier, J., Lelieveld, J., and Williams, J.: Strong sesquiterpene emissions from Amazonian soils, Nat. Commun., 9, 2226, https://doi.org/10.1038/s41467-018-04658-y, 2018.
Cappellin, L., Karl, T., Probst, M., Ismailova, O., Winkler, P. M., Soukoulis, C., Aprea, E., Märk, T. D., Gasperi, F., and Biasioli, F.: On Quantitative Determination of Volatile Organic Compound Concentrations Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry, Environ. Sci. Technol., 46, 2283–2290, https://doi.org/10.1021/es203985t, 2012.
Cleveland, C. C. and Yavitt, J. B.: Microbial Consumption of Atmospheric Isoprene in a Temperate Forest Soil, Appl. Environ. Microb., 64, 172–177, https://doi.org/10.1128/AEM.64.1.172-177.1998, 1998.
de Gouw, J. A., Warneke, C., Parrish, D. D., Holloway, J. S., Trainer, M., and Fehsenfeld, F. C.: Emission sources and ocean uptake of acetonitrile (CH3CN) in the atmosphere, J. Geophys. Res.-Atmos., 108, 1–8, https://doi.org/10.1029/2002JD002897, 2003.
Du, R., Yan, J., Li, S., Zhang, L., Zhang, S., Li, J., Zhao, G., and Qi, P.: Cellulosic ethanol production by natural bacterial consortia is enhanced by Pseudoxanthomonas taiwanensis, Biotechnol. Biofuels, 8, 10, https://doi.org/10.1186/s13068-014-0186-7, 2015.
Ehrlich, J. and Cahill, T. M.: Identification of broadleaf and coniferous trees as a primary source of acrolein, Atmos. Environ., 191, 414–419, https://doi.org/10.1016/j.atmosenv.2018.08.033, 2018.
Farooq, A., Atta-ur-Rahman, and Choudhary, M. I.: Fungal Transformation of Monoterpenes, Curr. Org. Chem., 8, 353–366, https://doi.org/10.2174/1385272043485945, 2004.
Frostegård, A. and Bååth, E.: The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil, Biol. Fert. Soils, 22, 59–65, https://doi.org/10.1007/BF00384433, 1996.
Garnier, S., Giordanengo, E., Saatkamp, A., Santonja, M., Reiter, I. M., Orts, J.-P., Gauquelin, T., and Meineri, E.: Amplified drought induced by climate change reduces seedling emergence and increases seedling mortality for two Mediterranean perennial herbs, Ecol. Evol., 11, 16143–16152, https://doi.org/10.1002/ece3.8295, 2021.
Genard-Zielinski, A.-C., Boissard, C., Fernandez, C., Kalogridis, C., Lathière, J., Gros, V., Bonnaire, N., and Ormeño, E.: Variability of BVOC emissions from a Mediterranean mixed forest in southern France with a focus on Quercus pubescens, Atmos. Chem. Phys., 15, 431–446, https://doi.org/10.5194/acp-15-431-2015, 2015.
Gray, C. M., Monson, R. K., and Fierer, N.: Emissions of volatile organic compounds during the decomposition of plant litter, J. Geophys. Res.-Biogeo., 115, G03015, https://doi.org/10.1029/2010JG001291, 2010.
Gray, C. M., Monson, R. K., and Fierer, N.: Biotic and abiotic controls on biogenic volatile organic compound fluxes from a subalpine forest floor: Controls on BVOC fluxes from forest soil, J. Geophys. Res.-Biogeo., 119, 547–556, https://doi.org/10.1002/2013jg002575, 2014.
Gray, C. M., Helmig, D., and Fierer, N.: Bacteria and fungi associated with isoprene consumption in soil, Elem. Sci. Anthr., 3, 000053, https://doi.org/10.12952/journal.elementa.000053, 2015.
Gros, V., Lathière, J., Boissard, C., Jambert, C., Delon, C., Staudt, M., Fernandez, C., Ormeño, E., Baisnée, D., and Sarda-Estève, R.: Emissions from the Mediterranean Vegetation, in: Atmospheric Chemistry in the Mediterranean Region: Volume 2 – From Air Pollutant Sources to Impacts, edited by: Dulac, F., Sauvage, S., and Hamonou, E., Springer International Publishing, Cham, https://doi.org/10.1007/978-3-030-82385-6_325–49, 25–49, 2022.
Guenther, A. B., Jiang, X., Heald, C. L., Sakulyanontvittaya, T., Duhl, T., Emmons, L. K., and Wang, X.: The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions, Geosci. Model Dev., 5, 1471–1492, https://doi.org/10.5194/gmd-5-1471-2012, 2012.
Hallquist, M., Wenger, J. C., Baltensperger, U., Rudich, Y., Simpson, D., Claeys, M., Dommen, J., Donahue, N. M., George, C., Goldstein, A. H., Hamilton, J. F., Herrmann, H., Hoffmann, T., Iinuma, Y., Jang, M., Jenkin, M. E., Jimenez, J. L., Kiendler-Scharr, A., Maenhaut, W., McFiggans, G., Mentel, Th. F., Monod, A., Prévôt, A. S. H., Seinfeld, J. H., Surratt, J. D., Szmigielski, R., and Wildt, J.: The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 9, 5155–5236, https://doi.org/10.5194/acp-9-5155-2009, 2009.
Hanif, N. M., Hawari, N. S. S. L., Othman, M., Hamid, H. H. A., Ahamad, F., Uning, R., Ooi, M. C. G., Wahab, M. I. A., Sahani, M., and Latif, M. T.: Ambient volatile organic compounds in tropical environments: Potential sources, composition and impacts – A review, Chemosphere, 285, 131355, https://doi.org/10.1016/j.chemosphere.2021.131355, 2021.
Heiden, A. C., Kobel, K., Komenda, M., Koppmann, R., Shao, M., and Wildt, J.: Toluene emissions from plants, Geophys. Res. Lett., 26, 1283–1286, https://doi.org/10.1029/1999GL900220, 1999.
Holzinger, R., Warneke, C., Hansel, A., Jordan, A., Lindinger, W., Scharffe, D. H., Schade, G., and Crutzen, P. J.: Biomass burning as a source of formaldehyde, acetaldehyde, methanol, acetone, acetonitrile, and hydrogen cyanide, Geophys. Res. Lett., 26, 1161–1164, https://doi.org/10.1029/1999GL900156, 1999.
Inomata, S., Fujitani, Y., Fushimi, A., Tanimoto, H., Sekimoto, K., and Yamada, H.: Field measurement of nitromethane from automotive emissions at a busy intersection using proton-transfer-reaction mass spectrometry, Atmos. Environ., 96, 301–309, https://doi.org/10.1016/j.atmosenv.2014.07.058, 2014.
Insam, H. and Seewald, M. S. A.: Volatile organic compounds (VOCs) in soils, Biol. Fert. Soils, 46, 199–213, https://doi.org/10.1007/s00374-010-0442-3, 2010.
Isidorov, V. and Jdanova, M.: Volatile organic compounds from leaves litter, Chemosphere, 48, 975–979, https://doi.org/10.1016/S0045-6535(02)00074-7, 2002.
Isidorov, V., Maslowiecka, J., and Sarapultseva, P.: Bidirectional emission of organic compounds by decaying leaf litter of a number of forest-forming tree species in the northern hemisphere, Geoderma, 443, 116812, https://doi.org/10.1016/j.geoderma.2024.116812, 2024.
Isidorov, V. A. and Zaitsev, A. A.: Reviews and syntheses: VOC emissions from soil cover in boreal and temperate natural ecosystems of the Northern Hemisphere, Biogeosciences, 19, 4715–4746, https://doi.org/10.5194/bg-19-4715-2022, 2022.
Jacob, D. J., B. D. Field, E. M. Jin, I. Bey, Q. Li, J. A. Logan, R. M. Yantosca, and H. B. Singh, Atmospheric budget of acetone, J. Geophys. Res., 107, ACH 5-1–ACH 5-17, https://doi.org/10.1029/2001JD000694, 2002.
Jardine, K., Yañez-Serrano, A. M., Williams, J., Kunert, N., Jardine, A., Taylor, T., Abrell, L., Artaxo, P., Guenther, A., Hewitt, C. N., House, E., Florentino, A. P., Manzi, A., Higuchi, N., Kesselmeier, J., Behrendt, T., Veres, P. R., Derstroff, B., Fuentes, J. D., Martin, S. T., and Andreae, M. O.: Dimethyl sulfide in the Amazon rain forest, Glob. Biogeochem. Cy., 29, 19–32, https://doi.org/10.1002/2014GB004969, 2015.
Jardine, K. J., Jardine, A. B., Holm, J. A., Lombardozzi, D. L., Negron-Juarez, R. I., Martin, S. T., Beller, H. R., Gimenez, B. O., Higuchi, N., and Chambers, J. Q.: Monoterpene `thermometer' of tropical forest-atmosphere response to climate warming, Plant Cell Environ., 40, 441–452, https://doi.org/10.1111/pce.12879, 2017.
Jiao, Y., Kramshøj, M., Davie-Martin, C. L., Albers, C. N., and Rinnan, R.: Soil uptake of VOCs exceeds production when VOCs are readily available, Soil Biol. Biochem., 185, 109153, https://doi.org/10.1016/j.soilbio.2023.109153, 2023.
Kesselmeier, J., Bode, K., Schäfer, L., Schebeske, G., Wolf, A., Brancaleoni, E., Cecinato, A., Ciccioli, P., Frattoni, M., Dutaur, L., Fugit, J. L., Simon, V., and Torres, L.: Simultaneous field measurements of terpene and isoprene emissions from two dominant mediterranean oak species in relation to a North American species, Atmos. Environ., 32, 1947–1953, https://doi.org/10.1016/S1352-2310(97)00500-1, 1998.
Koss, A. R., Sekimoto, K., Gilman, J. B., Selimovic, V., Coggon, M. M., Zarzana, K. J., Yuan, B., Lerner, B. M., Brown, S. S., Jimenez, J. L., Krechmer, J., Roberts, J. M., Warneke, C., Yokelson, R. J., and de Gouw, J.: Non-methane organic gas emissions from biomass burning: identification, quantification, and emission factors from PTR-ToF during the FIREX 2016 laboratory experiment, Atmos. Chem. Phys., 18, 3299–3319, https://doi.org/10.5194/acp-18-3299-2018, 2018.
Kovacs, E. D., Kovacs, M. H., Kovacs, E. D., and Kovacs, M. H.: Global Change Drivers Impact on Soil Microbiota: Challenges for Maintaining Soil Ecosystem Services, in: Vegetation Dynamics, Changing Ecosystems and Human Responsibility, edited by: Hufnagel, L. and El-Esawi, M. A., IntechOpen, https://doi.org/10.5772/intechopen.111585, 2023.
Kramshøj, M., Vedel-Petersen, I., Schollert, M., Rinnan, Å., Nymand, J., Ro-Poulsen, H., and Rinnan, R.: Large increases in Arctic biogenic volatile emissions are a direct effect of warming, Nat. Geosci., 9, 349–352, https://doi.org/10.1038/ngeo2692, 2016.
Kramshøj, M., Albers, C. N., Holst, T., Holzinger, R., Elberling, B., and Rinnan, R.: Biogenic volatile release from permafrost thaw is determined by the soil microbial sink, Nat. Commun., 9, 1–9, https://doi.org/10.1038/s41467-018-05824-y, 2018.
Kramshøj, M., Albers, C. N., Svendsen, S. H., Björkman, M. P., Lindwall, F., Björk, R. G., and Rinnan, R.: Volatile emissions from thawing permafrost soils are influenced by meltwater drainage conditions, Glob. Change Biol., 25, 1704–1716, https://doi.org/10.1111/gcb.14582, 2019.
Kulmala, M., Vehkamäki, H., Petäjä, T., Maso, M. D., Lauri, A., Kerminen, V. M., Birmili, W., and McMurry, P. H.: Formation and growth rates of ultrafine atmospheric particles: a review of observations, J. Aerosol Sci., 35, 143–176, https://doi.org/10.1016/J.JAEROSCI.2003.10.003, 2004.
Laoué, J., Fernandez, C., and Ormeño, E.: Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress, Plants, 11, 172, https://doi.org/10.3390/plants11020172, 2022.
Lee, K., Lee, S., Takeoka, G. R., Kim, J., and Park, B.: Antioxidant activity and characterization of volatile constituents of beechwood creosote, J. Sci. Food Agr., 85, 1580–1586, https://doi.org/10.1002/jsfa.2156, 2005.
Leff, J. W. and Fierer, N.: Volatile organic compound (VOC) emissions from soil and litter samples, Soil Biol. Biochem., 40, 1629–1636, https://doi.org/10.1016/j.soilbio.2008.01.018, 2008.
Legros, T., Temime-Roussel, B., Kammer, J., Quivet, E., Wortham, H., Reiter, I. M., Santonja, M., Fernandez, C., and Ormeño, E.: Decline of soil volatile organic compounds from a Mediterranean deciduous forest under a future drier climate, Atmos. Environ., 340, 120909, https://doi.org/10.1016/j.atmosenv.2024.120909, 2025.
Ma, Z., Qiu, S., Chen, H.-C., Zhang, D., Lu, Y.-L., and Chen, X.-L.: Maleimide structure: a promising scaffold for the development of antimicrobial agents, J. Asian Nat. Prod. Res., 24, 1–14, https://doi.org/10.1080/10286020.2021.1877675, 2022.
Mackie, A. E. and Wheatley, R. E.: Effects and incidence of volatile organic compound interactions between soil bacterial and fungal isolates, Soil Biol. Biochem., 31, 375–385, https://doi.org/10.1016/S0038-0717(98)00140-0, 1999.
Mahilang, M., Deb, M. K., and Pervez, S.: Biogenic secondary organic aerosols: A review on formation mechanism, analytical challenges and environmental impacts, Chemosphere, 262, 127771, https://doi.org/10.1016/j.chemosphere.2020.127771, 2021.
Mäki, M.: Volatile organic compound fluxes from northern forest soils, Diss. For., 2019, https://doi.org/10.14214/df.275, 2019.
Mäki, M., Heinonsalo, J., Hellén, H., and Bäck, J.: Contribution of understorey vegetation and soil processes to boreal forest isoprenoid exchange, Biogeosciences, 14, 1055–1073, https://doi.org/10.5194/bg-14-1055-2017, 2017.
Marmulla, R. and Harder, J.: Microbial monoterpene transformations – a review, Front. Microbiol., 5, 346, https://doi.org/10.3389/fmicb.2014.00346, 2014.
McBride, S. G., Osburn, E. D., Lucas, J. M., Simpson, J. S., Brown, T., Barrett, J. E., and Strickland, M. S.: Volatile and Dissolved Organic Carbon Sources Have Distinct Effects on Microbial Activity, Nitrogen Content, and Bacterial Communities in Soil, Microb. Ecol., 85, 659–668, https://doi.org/10.1007/s00248-022-01967-0, 2023.
McGenity, T. J., Crombie, A. T., and Murrell, J. C.: Microbial cycling of isoprene, the most abundantly produced biological volatile organic compound on Earth, ISME J., 12, 931–941, https://doi.org/10.1038/s41396-018-0072-6, 2018.
Meischner, M., Haberstroh, S., Daber, L. E., Kreuzwieser, J., Caldeira, M. C., Schnitzler, J.-P., and Werner, C.: Soil VOC emissions of a Mediterranean woodland are sensitive to shrub invasion, Plant Biol., 24, 967–978, https://doi.org/10.1111/plb.13445, 2022.
Mielnik, A., Link, M., Mattila, J., Fulgham, S. R., and Farmer, D. K.: Emission of formic and acetic acids from two Colorado soils, Environ. Sci.-Proc. Imp., 20, 1537–1545, https://doi.org/10.1039/C8EM00356D, 2018.
Misztal, P. K., Hewitt, C. N., Wildt, J., Blande, J. D., Eller, A. S. D., Fares, S., Gentner, D. R., Gilman, J. B., Graus, M., Greenberg, J., Guenther, A. B., Hansel, A., Harley, P., Huang, M., Jardine, K., Karl, T., Kaser, L., Keutsch, F. N., Kiendler-Scharr, A., Kleist, E., Lerner, B. M., Li, T., Mak, J., Nölscher, A. C., Schnitzhofer, R., Sinha, V., Thornton, B., Warneke, C., Wegener, F., Werner, C., Williams, J., Worton, D. R., Yassaa, N., and Goldstein, A. H.: Atmospheric benzenoid emissions from plants rival those from fossil fuels, Sci. Rep.-UK, 5, 1–10, https://doi.org/10.1038/srep12064, 2015.
Monson, R. K., Jaeger, C. H., Adams, W. W., Driggers, E. M., Silver, G. M., and Fall, R.: Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature, Plant Physiol., 98, 1175–1180, https://doi.org/10.1104/pp.98.3.1175, 1992.
Mu, Z., Llusià, J., Zeng, J., Zhang, Y., Asensio, D., Yang, K., Yi, Z., Wang, X., and Peñuelas, J.: An Overview of the Isoprenoid Emissions From Tropical Plant Species, Front. Plant Sci., 13, 833030, https://doi.org/10.3389/fpls.2022.833030, 2022.
Naeher, S., Lengger, S. K., and Grice, K.: A new method for the rapid analysis of 1H-Pyrrole-2,5-diones (maleimides) in environmental samples by two-dimensional gas chromatography time-of-flight mass spectrometry, J. Chromatogr. A, 1435, 125–135, https://doi.org/10.1016/j.chroma.2016.01.026, 2016.
Niinemets, Ü., Fares, S., Harley, P., and Jardine, K. J.: Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition, Plant Cell Environ., 37, 1790–1809, https://doi.org/10.1111/pce.12322, 2014.
Nyalala, S. O., Petersen, M. A., and Grout, B. W. W.: Acetonitrile (methyl cyanide) emitted by the African spider plant (Gynandropsis gynandra L. (Briq)): Bioactivity against spider mite (Tetranychus urticae Koch) on roses, Sci. Hortic.-Amsterdam, 128, 352–356, https://doi.org/10.1016/j.scienta.2011.01.036, 2011.
Nyalala, S. o., Petersen, M. a., and Grout, B. w. w.: Volatile compounds from leaves of the African spider plant (Gynandropsis gynandra) with bioactivity against spider mite (Tetranychus urticae), Ann. Appl. Biol., 162, 290–298, https://doi.org/10.1111/aab.12021, 2013.
Ormeño, E., Baldy, V., Ballini, C., and Fernandez, C.: Production and Diversity of Volatile Terpenes from Plants on Calcareous and Siliceous Soils: Effect of Soil Nutrients, J. Chem. Ecol., 34, 1219–1229, https://doi.org/10.1007/s10886-008-9515-2, 2008.
Owen, S. M., Harley, P., Guenther, A., and Hewitt, C. N.: Light dependency of VOC emissions from selected Mediterranean plant species, Atmos. Environ., 36, 3147–3159, https://doi.org/10.1016/S1352-2310(02)00235-2, 2002.
Peñuelas, J.: An increasingly scented world, New Phytol., 180, 735–738, https://doi.org/10.1111/j.1469-8137.2008.02658.x, 2008.
Peñuelas, J., Asensio, D., Tholl, D., Wenke, K., Rosenkranz, M., Piechulla, B., and Schnitzler, J. P.: Biogenic volatile emissions from the soil, Plant Cell Environ., 37, 1866–1891, https://doi.org/10.1111/pce.12340, 2014.
Peñuelas, J., Sardans, J., Filella, I., Estiarte, M., Llusià, J., Ogaya, R., Carnicer, J., Bartrons, M., Rivas-Ubach, A., Grau, O., Peguero, G., Margalef, O., Pla-Rabés, S., Stefanescu, C., Asensio, D., Preece, C., Liu, L., Verger, A., Barbeta, A., Achotegui-Castells, A., Gargallo-Garriga, A., Sperlich, D., Farré-Armengol, G., Fernández-Martínez, M., Liu, D., Zhang, C., Urbina, I., Camino-Serrano, M., Vives-Ingla, M., Stocker, B. D., Balzarolo, M., Guerrieri, R., Peaucelle, M., Marañón-Jiménez, S., Bórnez-Mejías, K., Mu, Z., Descals, A., Castellanos, A., and Terradas, J.: Impacts of Global Change on Mediterranean Forests and Their Services, Forests, 8, 463, https://doi.org/10.3390/f8120463, 2017.
Pugliese, G., Ingrisch, J., Meredith, L. K., Pfannerstill, E. Y., Klüpfel, T., Meeran, K., Byron, J., Purser, G., Gil-Loaiza, J., van Haren, J., Dontsova, K., Kreuzwieser, J., Ladd, S. N., Werner, C., and Williams, J.: Effects of drought and recovery on soil volatile organic compound fluxes in an experimental rainforest, Nat. Commun., 14, 5064, https://doi.org/10.1038/s41467-023-40661-8, 2023.
Quer, E., Pereira, S., Michel, T., Santonja, M., Gauquelin, T., Simioni, G., Ourcival, J.-M., Joffre, R., Limousin, J.-M., Aupic-Samain, A., Lecareux, C., Dupouyet, S., Orts, J.-P., Bousquet-Mélou, A., Gros, R., Sagova-Mareckova, M., Kopecky, J., Fernandez, C., and Baldy, V.: Amplified Drought Alters Leaf Litter Metabolome, Slows Down Litter Decomposition, and Modifies Home Field (Dis)Advantage in Three Mediterranean Forests, Plants, 11, 2582, https://doi.org/10.3390/plants11192582, 2022.
Rameau, J.-C., Mansion, D., Dumé, G., and Gauberville, C.: Flore forestière française tome 3, région méditerranéenne: Guide écologique illustré, CNPF-IDF, Paris, 2438 pp., 2008.
Rezaie, N., Pallozzi, E., Ciccioli, P., Calfapietra, C., and Fares, S.: Temperature dependence of emission of volatile organic compounds (VOC) from litters collected in two Mediterranean ecosystems determined before the flaming phase of biomass burning, Environ. Pollut., 338, 122703, https://doi.org/10.1016/j.envpol.2023.122703, 2023.
Rinnan, R.: Volatile organic compound emissions in the changing Arctic, Ann. Rev. Ecol. Evol. Syst., 55, 227–249, https://doi.org/10.1146/annurev-ecolsys-102722-125156, 2024.
Rinnan, R. and Albers, C. N.: Soil Uptake of Volatile Organic Compounds: Ubiquitous and Underestimated?, J. Geophys. Res.-Biogeo., 125, e2020JG005773, https://doi.org/10.1029/2020JG005773, 2020.
Ruiz, J., Bilbao, R., and Murillo, M. B.: Adsorption of Different VOC onto Soil Minerals from Gas Phase: Influence of Mineral, Type of VOC, and Air Humidity, Environ. Sci. Technol., 32, 1079–1084, https://doi.org/10.1021/es9704996, 1998.
Santonja, M., Fernandez, C., Gauquelin, T., and Baldy, V.: Climate change effects on litter decomposition: intensive drought leads to a strong decrease of litter mixture interactions, Plant Soil, 393, 69–82, https://doi.org/10.1007/s11104-015-2471-z, 2015.
Santonja, M., Fernandez, C., Proffit, M., Gers, C., Gauquelin, T., Reiter, I. M., Cramer, W., and Baldy, V.: Plant litter mixture partly mitigates the negative effects of extended drought on soil biota and litter decomposition in a Mediterranean oak forest, J. Ecol., 105, 801–815, https://doi.org/10.1111/1365-2745.12711, 2017.
Santonja, M., Pereira, S., Gauquelin, T., Quer, E., Simioni, G., Limousin, J.-M., Ourcival, J.-M., Reiter, I. M., Fernandez, C., and Baldy, V.: Experimental Precipitation Reduction Slows Down Litter Decomposition but Exhibits Weak to No Effect on Soil Organic Carbon and Nitrogen Stocks in Three Mediterranean Forests of Southern France, Forests, 13, 1485, https://doi.org/10.3390/f13091485, 2022.
Sarkar, C., Sinha, V., Kumar, V., Rupakheti, M., Panday, A., Mahata, K. S., Rupakheti, D., Kathayat, B., and Lawrence, M. G.: Overview of VOC emissions and chemistry from PTR-TOF-MS measurements during the SusKat-ABC campaign: high acetaldehyde, isoprene and isocyanic acid in wintertime air of the Kathmandu Valley, Atmos. Chem. Phys., 16, 3979–4003, https://doi.org/10.5194/acp-16-3979-2016, 2016.
Schade, G. W. and Goldstein, A. H.: Fluxes of oxygenated volatile organic compounds from a ponderosa pine plantation, J. Geophys. Res.-Atmos., 106, 3111–3123, https://doi.org/10.1029/2000JD900592, 2001.
Schieweck, A., Uhde, E., and Salthammer, T.: Determination of acrolein in ambient air and in the atmosphere of environmental test chambers, Environ. Sci.-Proc. Imp., 23, 1729–1746, https://doi.org/10.1039/D1EM00221J, 2021.
Seinfeld, J. H. and Pandis, S. N.: Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, John Wiley & Sons, 1152 pp., ISBN: 978-1-118-94740-1, 2016.
Shende, V. V., Bauman, K. D., and Moore, B. S.: The shikimate pathway: gateway to metabolic diversity, Nat. Prod. Rep., 41, 604–648, https://doi.org/10.1039/D3NP00037K, 2024.
Shennan, J. L.: Utilisation of C2–C4 gaseous hydrocarbons and isoprene by microorganisms, J. Chem. Technol. Biot., 81, 237–256, https://doi.org/10.1002/jctb.1388, 2006.
Shihan, A., Hättenschwiler, S., Milcu, A., Joly, F.-X., Santonja, M., and Fromin, N.: Changes in soil microbial substrate utilization in response to altered litter diversity and precipitation in a Mediterranean shrubland, Biol. Fert. Soils, 53, 171–185, https://doi.org/10.1007/s00374-016-1166-9, 2017.
Sillo, F., Neri, L., Calvo, A., Zampieri, E., Petruzzelli, G., Ferraris, I., Delledonne, M., Zaldei, A., Gioli, B., Baraldi, R., and Balestrini, R.: Correlation between microbial communities and volatile organic compounds in an urban soil provides clues on soil quality towards sustainability of city flowerbeds, Heliyon, 10, e23594, https://doi.org/10.1016/j.heliyon.2023.e23594, 2024.
Sindelarova, K., Granier, C., Bouarar, I., Guenther, A., Tilmes, S., Stavrakou, T., Muller, J.-F., Kuhn, U., Stefani, P., Knorr, W., Sindelarova, K., Granier, C., Bouarar, I., Guenther, A., and Müller, J.-F.: Atmospheric Chemistry and Physics, Eur. Geosci. Union, 14, 9317–9341, https://doi.org/10.5194/acp-14-9317, 2014.
Stahl, P. D. and Parkin, T. B.: Microbial Production of Volatile Organic Compounds in Soil Microcosms, Soil Sci. Soc. Am. J., 60, 821–828, https://doi.org/10.2136/sssaj1996.03615995006000030020x, 1996.
Staudt, M., Bourgeois, I., Al Halabi, R., Song, W., and Williams, J.: New insights into the parametrization of temperature and light responses of mono - and sesquiterpene emissions from Aleppo pine and rosemary, Atmos. Environ., 152, 212–221, https://doi.org/10.1016/j.atmosenv.2016.12.033, 2017.
Steinley, D.: K-means clustering: A half-century synthesis, Brit. J. Math. Stat. Psy., 59, 1–34, https://doi.org/10.1348/000711005X48266, 2006.
Svendsen, S. H., Priemé, A., Voriskova, J., Kramshøj, M., Schostag, M., Jacobsen, C. S., and Rinnan, R.: Emissions of biogenic volatile organic compounds from arctic shrub litter are coupled with changes in the bacterial community composition, Soil Biol. Biochem., 120, 80–90, https://doi.org/10.1016/j.soilbio.2018.02.001, 2018.
Taiti, C., Costa, C., Figorilli, S., Billi, M., Caparrotta, S., Comparini, D., and Mancuso, S.: Volatome analysis approach for the taxonomic classification of tree exudate collection using Proton Transfer Reaction Time of Flight Mass Spectrometry, Flavour Frag. J., 33, 245–262, https://doi.org/10.1002/ffj.3439, 2018.
Tang, J., Schurgers, G., and Rinnan, R.: Process Understanding of Soil BVOC Fluxes in Natural Ecosystems: A Review, Rev. Geophys., 57, 966–986, https://doi.org/10.1029/2018RG000634, 2019.
Thornhill, G., Collins, W., Olivié, D., Skeie, R. B., Archibald, A., Bauer, S., Checa-Garcia, R., Fiedler, S., Folberth, G., Gjermundsen, A., Horowitz, L., Lamarque, J.-F., Michou, M., Mulcahy, J., Nabat, P., Naik, V., O'Connor, F. M., Paulot, F., Schulz, M., Scott, C. E., Séférian, R., Smith, C., Takemura, T., Tilmes, S., Tsigaridis, K., and Weber, J.: Climate-driven chemistry and aerosol feedbacks in CMIP6 Earth system models, Atmos. Chem. Phys., 21, 1105–1126, https://doi.org/10.5194/acp-21-1105-2021, 2021.
Trowbridge, A. M., Stoy, P. C., and Phillips, R. P.: Soil Biogenic Volatile Organic Compound Flux in a Mixed Hardwood Forest: Net Uptake at Warmer Temperatures and the Importance of Mycorrhizal Associations, J. Geophys. Res.-Biogeo., 125, e2019JG005479, https://doi.org/10.1029/2019JG005479, 2020.
Viros, J., Fernandez, C., Wortham, H., Gavinet, J., Lecareux, C., and Ormeño, E.: Litter of mediterranean species as a source of volatile organic compounds, Atmos. Environ., 242, 117815, https://doi.org/10.1016/j.atmosenv.2020.117815, 2020.
Viros, J., Santonja, M., Temime-Roussel, B., Wortham, H., Fernandez, C., and Ormeño, E.: Volatilome of Aleppo Pine litter over decomposition process, Ecol. Evol., 11, 6862–6880, https://doi.org/10.1002/ece3.7533, 2021.
Wang, H., Liu, X., Wu, C., and Lin, G.: Regional to global distributions, trends, and drivers of biogenic volatile organic compound emission from 2001 to 2020, Atmos. Chem. Phys., 24, 3309–3328, https://doi.org/10.5194/acp-24-3309-2024, 2024.
Warneke, C., Karl, T., Judmaier, H., Hansel, A., Jordan, A., Lindinger, W., and Crutzen, P. J.: Acetone, methanol, and other partially oxidized volatile organic emissions from dead plant matter by abiological processes: Significance for atmospheric HOx chemistry, Glob. Biogeochem. Cy., 13, 9–17, https://doi.org/10.1029/98GB02428, 1999.
Weikl, F., Ghirardo, A., Schnitzler, J.-P., and Pritsch, K.: Sesquiterpene emissions from Alternaria alternata and Fusarium oxysporum: Effects of age, nutrient availability and co-cultivation, Sci. Rep.-UK, 6, 22152, https://doi.org/10.1038/srep22152, 2016.
Wenke, K., Kai, M., and Piechulla, B.: Belowground volatiles facilitate interactions between plant roots and soil organisms, Planta, 231, 499–506, https://doi.org/10.1007/s00425-009-1076-2, 2010.
Wheatley, R. E., Millar, S. E., and Griffiths, D. W.: The production of volatile organic compounds during nitrogen transformations in soils, Plant Soil, 181, 163–167, https://doi.org/10.1007/BF00011303, 1996.
White, C. S.: Monoterpenes: Their effects on ecosystem nutrient cycling, J. Chem. Ecol., 20, 1381–1406, https://doi.org/10.1007/BF02059813, 1994.
Wilkins, K.: Volatile metabolites from actinomycetes, Chemosphere, 32, 1427–1434, https://doi.org/10.1016/0045-6535(96)00051-3, 1996.
Yáñez-Serrano, A. M., Filella, I., LLusià, J., Gargallo-Garriga, A., Granda, V., Bourtsoukidis, E., Williams, J., Seco, R., Cappellin, L., Werner, C., de Gouw, J., and Peñuelas, J.: GLOVOCS - Master compound assignment guide for proton transfer reaction mass spectrometry users, Atmos. Environ., 244, 117929, https://doi.org/10.1016/j.atmosenv.2020.117929, 2021.
Yang, B., Zhang, H., Wang, Y., Zhang, P., Shu, J., Sun, W., and Ma, P.: Experimental and theoretical studies on gas-phase reactions of NO3 radicals with three methoxyphenols: Guaiacol, creosol, and syringol, Atmos. Environ., 125, 243–251, https://doi.org/10.1016/j.atmosenv.2015.11.028, 2016.
Yang, K., Llusià, J., Preece, C., Tan, Y., and Peñuelas, J.: Exchange of volatile organic compounds between the atmosphere and the soil, Plant Soil, 501, 509–535, https://doi.org/10.1007/s11104-024-06524-x, 2024a.
Yang, K., Llusià, J., Preece, C., Ogaya, R., Márquez Tur, L., Mu, Z., You, C., Xu, Z., Tan, Y., and Peñuelas, J.: Impacts of seasonality, drought, nitrogen fertilization, and litter on soil fluxes of biogenic volatile organic compounds in a Mediterranean forest, Sci. Total Environ., 906, 167354, https://doi.org/10.1016/j.scitotenv.2023.167354, 2024b.
Zhang, Y., Zou, J., Meng, D., Dang, S., Zhou, J., Osborne, B., Ren, Y., Liang, T., and Yu, K.: Effect of soil microorganisms and labile C availability on soil respiration in response to litter inputs in forest ecosystems: A meta-analysis, Ecol. Evol., 10, 13602–13612, https://doi.org/10.1002/ece3.6965, 2020.