the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Benthic ostracod diversity and biogeography in an urban semi-enclosed eutrophic riverine bay
Jialu Huang
Moriaki Yasuhara
He Wang
Pedro Julião Jimenez
Jiying Li
Minhan Dai
The benthic ecosystem has been greatly altered by environmental pressure over the past several decades. Compared to some well-studied large bays, the situation in populated small bay areas is still under-investigated. In this study, we investigated the abundance, diversity, composition, and distribution of ostracods (a meiobenthic group) and their interactions with eutrophication and pollution through high-resolution sampling of surface sediment in Deep Bay, a small semi-enclosed riverine bay adjacent to two of the world's most populated cities. We found that ostracod abundance and diversity exhibited an inner–outer gradient in Deep Bay, shaped by eutrophication and pollution from human activities. Faunal composition was also characterized by inner–outer difference, as well as difference between Hong Kong and Shenzhen areas, whereby Hong Kong was more influenced by eutrophication, and Shenzhen was more affected by metal pollution. Ostracod distribution and their environmental preference in Deep Bay were consistent with broader studies in Hong Kong, strongly supporting the idea that ostracods are a useful bioindicator of coastal benthic ecosystems. Our study emphasizes the importance of studying an uncomplicated system with fine-scale sampling, as this approach offers a clear and direct understanding of how benthic ecosystems are shaped by distinct coastal environmental problems.
- Article
(8767 KB) - Full-text XML
- BibTeX
- EndNote
Coastal bays that are highly influenced by adjacent populated cities are facing incremental environmental problems and ecosystem degradation due to poor water exchange and intensive human activities (Dai et al., 2023; Nichols et al., 2019; Breitburg et al., 2018). River discharge, aquaculture, and maritime activities cause eutrophication and pollution of coastal areas, ultimately leading to altered marine biological diversity (Glibert, 2017). Deep Bay is a semi-enclosed bay influenced by several small rivers inside the bay and connected with the Pearl River Estuary outside the bay. The bay is surrounded by Hong Kong and Shenzhen, two of the world's largest urbanized cities with 17.8 million and 7.5 million people, respectively. It is also a well-known region for both oyster culture and hinterland mangrove ecosystems (Mohyuddin et al., 2023; Feng et al., 2020). Under the influence of nutrient and pollution inputs from rivers, the aquaculture industry, and large-scale reclamation projects, combined with limited water exchange, Deep Bay is currently experiencing increasing environmental degradation (Xu et al., 2019).
Benthic ecosystem alteration has been recognized as a main consequence of environmental degradation (Dorgham, 2014). Ostracoda (Crustacea), which are abundantly fossilized in marginal marine sediments, have been used as a useful metazoan group bioindicator (Yasuhara et al., 2007). Previous studies on the Hong Kong coast have suggested that ostracods can be used as a bioindicator for the diversity and biogeography of broader soft-sediment benthos (Hong et al., 2022; Mamo et al., 2023). Statistical relationships between ostracod species and environmental factors have been indicated (Hong et al, 2019). On this topic, some large, populated bays, including Chesapeake Bay (Cronin and Vann, 2003) and Osaka Bay (Yasuhara et al., 2007), as well as the Baltic Sea (Frenzel et al., 2010), were well studied; small bays with a lower population were also investigated before (e.g., Irizuki et al., 2014). However, semi-enclosed, intensively human-influenced small bay areas remain under-studied, especially with high-resolution sampling.
Previous studies in Deep Bay have primarily focused on sediment chemistry (Lee, 2000), pollutants (Zhang et al., 2021; Li et al., 2024), land reclamation (Ren et al., 2011), and mangrove restoration (Feng et al., 2020). Some ecological studies were conducted solely in mangrove reserves (Zan et al., 2003). And due to the boundary control of the sea area, sampling has typically been restricted to one side (either Hong Kong area or Shenzhen area only). As a result, detailed biodiversity and biogeographic distributions for marine organisms in the broader area of Deep Bay are largely unknown.
In our study, we provided a comprehensive understanding on benthic community distribution and related environmental factors in Deep Bay, a small semi-enclosed bay adjacent to two of the world's largest cities. We investigated the geographical structure of ostracod abundance, diversity, and composition and how they interacted with eutrophication and pollution through doing high-resolution sampling of surface sediments in both Hong Kong and Shenzhen areas of the bay.
2.1 Site description
Deep Bay (22.41–22.53° N, 113.88–114.00° E) is located between the Shenzhen Special Economic Zone of China and Hong Kong Special Administrative Region in the eastern Pearl River Estuary (Fig. 1). At 17 km long and 4–10 km wide, it is a semi-enclosed bay with limited water exchange. The main rivers flowing into the bay include the Shenzhen River and Shan Pui River in Hong Kong (Fig. 1). The hydrography of the bay is also strongly influenced by the discharge from the Pearl River. Deep Bay features a semidiurnal tidal system, with an annual tidal range of 1.4 m (Feng et al., 2020). At the northeastern end of the bay are the Futian and Mai Po mangrove reserves (Fig. 1). Along the Hong Kong coast and in the central bay area, oyster farming rafts have expanded over the last several decades (Bowler et al., 1984; Fig. 1). Deep Bay is experiencing rapid urbanization and population growth, accompanied by intensive human activities such as agricultural, municipal, and industrial water discharge into the bay (Yang et al., 2021).
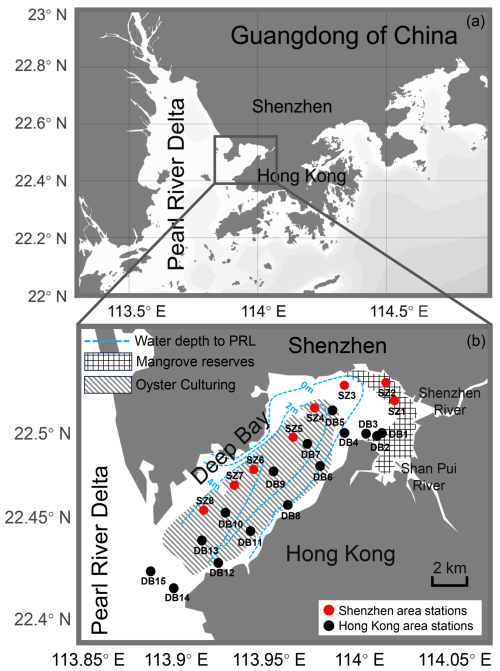
Figure 1Locality map of Deep Bay and sampling sites. Red dots represent stations in the Shenzhen area sampled in July 2024. Black dots represent stations in the Hong Kong area sampled in September 2023. Grid filling represents Futian (north) and Mai Po (south) mangrove reserves (adapted from Feng et al., 2020). Dashed filling represents areas of oyster culturing in Deep Bay (adapted from © Google Maps). Blue dashed lines represent relative water depth to Pearl River level (PRL), which reflect local bathymetry (adapted from Ye et al., 2018).
2.2 Materials
2.2.1 Ostracod samples and processing
Surface sediment samples of 100 mL were collected by using an Ekman grab from 23 locations that cover the entire Deep Bay area (Fig. 1), including 15 sites collected in September 2023 from the Hong Kong area and 8 sites collected in July 2024 from the Shenzhen area. Several sites, including DB3, DB5, DB9, DB10, and DB15, overlapped with the Hong Kong Environmental Protection Department (EPD) monitoring stations DM1 (DS1), DM2, DM3 (DS2), DM4 (DS3), and DM5 (DS4), respectively (DM represents seawater quality monitoring stations, and DS represents sediment quality monitoring stations; Environmental Protection Department of Hong Kong, 2024).
Sediment samples were washed through a 63 µm sieve and dried at 40°C in an oven. The dried samples were then sieved using a 150 µm sieve, and ostracod specimens larger than 150 µm were picked out for analysis. All individuals were picked out from the samples if the total number of specimens was less than 200. For samples containing substantially more than 200 specimens, we divided them into fractions using a sample splitter. The sample materials primarily consisted of death assemblages, with some articulated carapaces and a few containing soft parts inside. Each single valve was considered one individual; each carapace was considered two individuals (Yasuhara et al., 2017).
2.2.2 Environmental variables and data compilation
Additional surface sediment samples were collected at the same stations from the same cruise above to examine total organic carbon (TOC), total carbon (TC), total nitrogen (TN), total sulfur (TS), and their isotopes. Sediment chemical oxygen demand and the concentrations of heavy metals, including copper, chromium, barium, cadmium, and mercury, and the pollutant total cyanide were obtained from EPD sediment monitoring stations DS1–4 (Environmental Protection Department of Hong Kong, 2024). Data on bottom water hypoxia frequency (with thresholds of 2, 3, and 4 mg L−1, respectively), surface nutrient levels of dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP), and physical parameters including bottom temperature and salinity were obtained from EPD seawater monitoring stations DM1–5 (Environmental Protection Department of Hong Kong, 2024). As our grab sampler penetrated about 10 cm into the sediments, and the sedimentation rate was approximately 1 cm yr−1 in Deep Bay (Yan et al., 2019), we used a recent 10-year average (2012–2022) of the sediment/seawater monitoring result for analysis. We also compiled perfluoroalkyl and polyfluoroalkyl substances (PFAs) and heavy-metal data from recent studies (Zhang et al., 2021; Li et al., 2024), selecting sites in proximity to those in our study.
2.3 Methods
2.3.1 Diversity analysis
We used Hill numbers, or the effective numbers of equally abundant species, to estimate ostracod diversity at each station or group of stations. Hill numbers offer several major advantages and have been increasingly adopted by ecologists (Chao et al., 2020). The order q of Hill numbers represents the sensitivity of the diversity metric to relative species abundance. Specifically, Hill number 0D with order q=0 measures species richness; 1D measures the diversity of the abundant species, and 2D measures the diversity of dominant species (Chao et al., 2014). The Hill numbers 1D and 2D are equivalent to the exponential of Shannon entropy and Simpson diversity index, respectively (Chao et al., 2014).
2.3.2 Multivariate analysis
We applied hierarchical cluster analysis to measure the dissimilarity among ostracod fauna from different sites, using diversity decomposition to obtain dissimilarity measures. We also performed non-metric multidimensional scaling (nMDS) to show faunal similarities among ostracod assemblages in two-dimensional space. We used dissimilarity indices of Sørensen (Sørensen, 1948), Horn (Horn, 1966), and Morisita–Horn (Morisita, 1959), to estimate the effective proportion of unshared species in the ostracod assemblages. While classical Sørensen dissimilarity is presence–absence-based, Horn and Morisita–Horn are designed to quantify the compositional dissimilarities of abundant and dominant species. The environmental variables that we used for multivariate analysis included TOC, TC, TN, and TS in sediment; heavy metals (As, Cd, Cu, Pb, Zn) adapted from Zhang et al. (2021); PFAs adapted from Li et al. (2024); and 10-year average hypoxia frequency, pH, DIN, and chlorophyll a (Chl a) calculated from EPD seawater monitoring data (Environmental Protection Department of Hong Kong, 2024).
3.1 Environmental settings
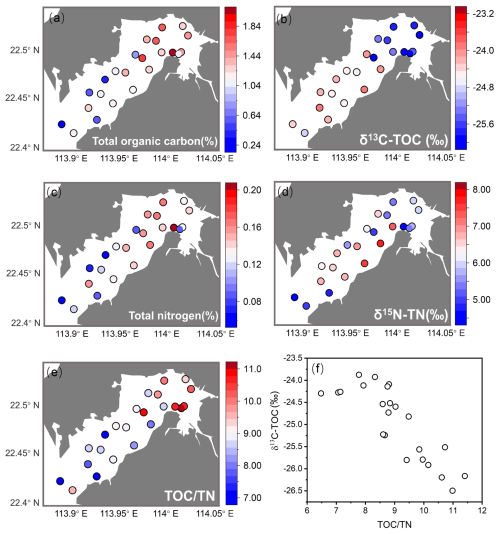
Deep Bay exhibited a riverine–oceanic salinity gradient from riverine freshwater input in the inner bay to more saline water influenced by the Pearl River Estuary in the outer bay (Fig. 2c). This gradient is also reflected in other related parameters, such as nutrients (Fig. 2b), heavy metals, and other pollutants (Fig. 2e, f), and TOC and TN levels (Fig. 3a, c). Hypoxia frequency was much higher in the inner bay than the outer bay, while surface DIN and DIP were higher in the inner bay (Fig. 2a, b). Sediment chemical oxygen demand, heavy metals, and other pollutants were relatively high in the inner bay and gradually decreased towards the outer bay (Fig. 2d–f). In the inner bay, beside the Shenzhen River and Shan Pui River, mangrove forests expand along the coastline, forming the Futian and Mai Po mangrove reserves (Fig. 1). Oyster shelves expand throughout most of the remaining bay area, particularly in the central area and Hong Kong area of the bay (Fig. 1). The bathymetry of Deep Bay is shallower in the inner part and deeper in the outer part (Ye et al., 2018; Fig. 1).
Eutrophication and pollution were predominant in the inner bay (Figs. 2, 3). Hypoxia frequency and nutrient concentration were elevated at EPD water monitoring stations DM1 and DM2 (Fig. 2a, b). Chemical oxygen demand, heavy metals, and other pollutants in sediment were also relatively high at EPD sediment monitoring stations DS1 and DS2 in the inner bay (Fig. 2d–f). Our TOC and TN results showed the same trend that inner bay stations had higher values compared to the outer stations (Fig. 3a, c). DB3 had the highest TOC and TN values, substantially exceeding those of the other sites (Fig. 3a, c). The lower δ13C–TOC and δ15N–TN values in the inner bay indicated a predominant organic matter source from terrestrial detritus, while the higher values in the outer bay suggest a predominant organic matter source of marine origin (Fig. 3a–d). The negative linear relationship between δ13C–TOC and TOC TN of the entire bay indicated a mixing between marine algae (with higher δ13C–TOC and lower TOC TN value) and C3 terrestrial plants like mangroves (with lower δ13C–TOC and higher TOC TN values) (Fig. 3e, f).
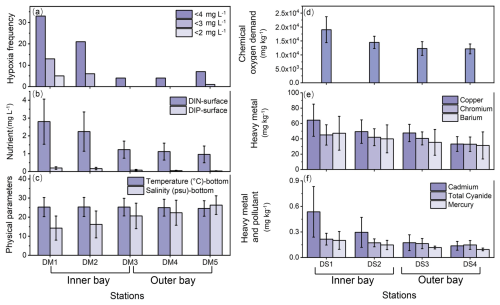
Figure 2Sediment and seawater properties vary from the inner bay to the outer bay based on EPD monitoring data. Left panels (a–c) show bottom-water hypoxia frequency (with thresholds of 2 mg L−1, 3 mg L−1, 4 mg L−1, respectively), surface nutrient DIN and DIP, and physical parameters bottom temperature and salinity from DM1 to DM5. Right panels (d–f) show sediment chemical oxygen demand and concentrations of heavy metals including copper, chromium, barium, cadmium, and mercury and pollutant total cyanide from DS1 to DS4. The data are annual averages from 2012 to 2022.
3.2 Abundance and diversity
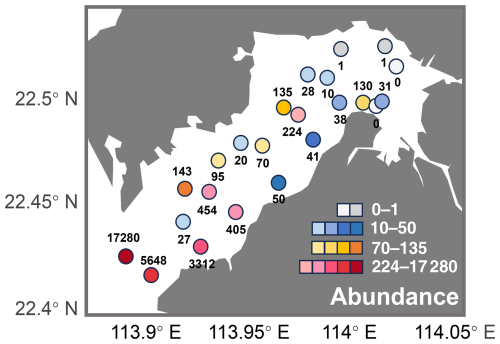
Abundance of ostracods in Deep Bay generally followed a gradient corresponding to the environmental conditions from the inner to outer bay. There tended to be lower abundance in the inner bay sites, with abundance increasing exponentially toward the bay mouth (Fig. 4). The lowest abundance was found inside the mangrove conservation area with almost zero specimens (Fig. 4). This may be due to the acidic conditions in mangrove sediments, which negatively affect the preservation of calcium carbonate ostracod shells (Plazziat et al., 1983). The inner bay, characterized by eutrophication and riverine conditions, generally had ostracod abundance below 100, while the outer bay exhibited a much higher abundance, especially at sites DB12, DB14, and DB15 (Fig. 4).
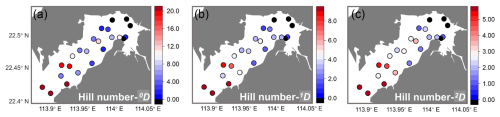
The diversity of ostracods in Deep Bay similarly followed the same inner–outer bay gradient (Fig. 5). The number of species, indicated by Hill number 0D, was relatively high at the bay mouth sites SZ8, DB10, DB12, DB14, and DB15, ranging from 20 to 33. The other stations mostly had values below 10 (Fig. 5a). Similarly, the diversity of abundant species and dominant species (1D and 2D) were also higher among the stations at the bay mouth (Fig. 5b, c). Notably, DB7 and DB11 exhibited relatively high diversity in both abundant and dominant species (Fig. 5b, c).
3.3 Multivariate analysis
Cluster analysis based on Sørensen, Horn, and Morisita–Horn dissimilarities delineated biofacies considering faunal composition in terms of species occurrence, relative abundance of abundant species, and relative abundance of dominant species, respectively. These three dissimilarities all showed a relatively clear separation both in geographical distribution and nMDS results (Fig. 6). The nMDS results showed a separation of five biofacies based on Sørensen and Morisita–Horn and six biofacies based on Horn dissimilarity (Fig. 6a, c, e).
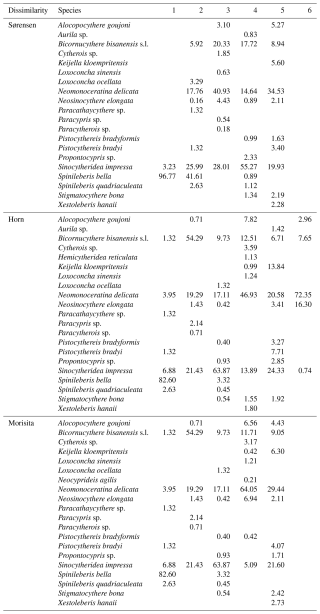
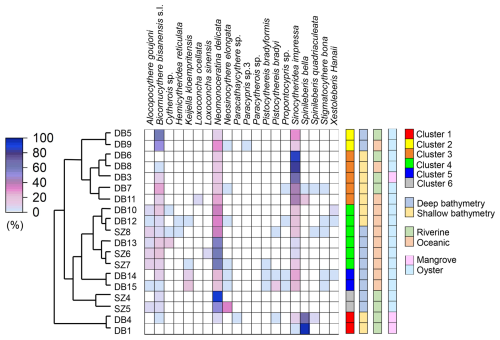
Sørensen dissimilarity showed a separation between inner bay and outer bay of Deep Bay into Biofacies 3 and Biofacies 5, respectively (Fig. 6a). The inner bay (Biofacies 3) was related to almost all the environmental parameters we used (Fig. 6b), which showed that the inner bay was strongly influenced by eutrophication, hypoxia, and pollutants. Biofacies 3 was characterized by the presence of Cytherois sp., Loxoconcha sinensis, Paracypris sp., and Paracytherois sp., as well as the absence of species that characterized other biofacies such as Spinileberis bella and Spinileberis quadriaculeata (Table 1). Biofacies 5 was characterized by the presence of marine species Alocopocythere goujoni, Keijella kloempritensis, and Xestoleberis hanaii (Table 1). Except the two main groups above, inner-bay Biofacies 1 was characterized by the presence of Spinileberis bella and Sinocytheridea impressa and the absence of other species (Table 1). Biofacies 2 was characterized by the occurrence of brackish-water species Spinileberis bella, Spinileberis quadriaculeata, Loxoconcha ocellata, and Paracathaycythere sp. (Table 1). Biofacies 4 was similar to Biofacies 2 but distinguished by the occurrence of Stigmatocythere bona and Propontocypris sp. (Table 1).
Horn and Morisita–Horn dissimilarities revealed a distinction between Hong Kong area stations and Shenzhen area stations, i.e., the Hong Kong area group (Biofacies 3) and the Shenzhen area group (Biofacies 4) (Fig. 6c, e). The Hong Kong area group was more eutrophication-influenced and characterized by the dominance of Sinocytheridea impressa (Table 1), while the Shenzhen area group was more related to heavy metals and other pollutants (Fig. 6d, f) and characterized by the dominance of Neomonoceratina delicata (Table 1). Other biofacies were distributed in the bay mouth (Biofacies 5) and inner bay (Biofacies 1, 2, and 6) (Fig. 6c, e). The bay mouth Biofacies 5 was unrelated to all eutrophication/pollution environmental parameters (Fig. 6) and characterized by moderately high abundance of both Sinocytheridea impressa and Neomonoceratina delicata without any distinctively dominant species (Table 1). The inner-most bay Biofacies 1 was distinguished by the dominance of Spinileberis bella (Table 1). Biofacies 2 was distinguished by the dominance of Bicornucythere bisanensis s.l. (Table 1). Biofacies 6 in Horn dissimilarity was less diverse and characterized by the dominance of Neomonoceratina delicata and Neosinocythere elongata (Table 1).
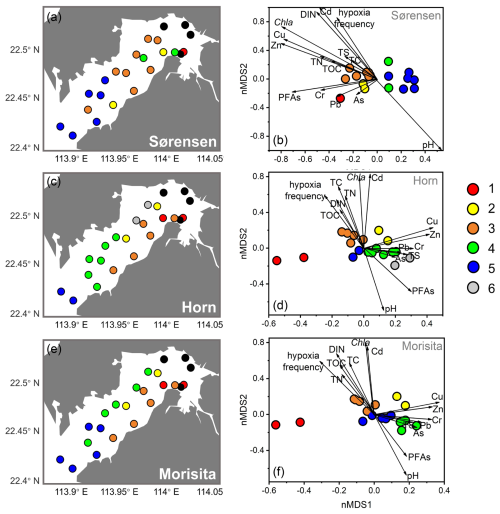
Figure 6Distribution map in left panels (a, c, e) and nMDS ordination in right panels (b, d, f) showing biofacies based on Sørensen, Horn, and Morisita–Horn dissimilarities. Vectors on the nMDS ordination show the strength and direction of relation between environmental variables and the nMDS axes. Black dots represent stations with low abundance, which are not included in calculation.
3.4 Biogeography
Geographical distributions of individual species are shown in Figs. 7 and 8. Neomonoceratina delicata was mostly distributed in the Shenzhen area (Fig. 7a). Sinocytheridea impressa had higher abundance in the Hong Kong area (Fig. 7b). Spinileberis bella was concentrated in the river mouth (Fig. 7c). Bicornucythere bisanensis s.l. was distributed with high abundance in the inner bay eutrophic/polluted area (Fig. 7d). Alocopocythere goujoni was distributed mostly in the outer bay, Shenzhen area (Fig. 7e). Neosinocythere elongata was abundant at the inner bay station SZ5 (Fig. 7f). Keijiella kloempritensis was abundant only at stations DB14 and DB15 located near the bay mouth (Fig. 7g). Pistocythereis bradyi was abundant at the bay mouth station DB15 (Fig. 7h). Cytherois sp. occurred mostly at the outer bay station DB13 (Fig. 7i). Stigmatocythere bona was abundant at outer bay stations SZ7 and SZ8 (Fig. 8a). Xestoleberis hanaii was abundant at outer–central bay stations, Shenzhen area, including SZ7, SZ8, and DB9 (Fig. 8b). Propontocypris sp. was relatively broadly distributed from the inner bay to the outer bay, with a higher abundance observed in the outer region (Fig. 8c). Pistocythereis bradyformis tended to show a higher abundance in the outer bay and at the bay mouth, while it was absent at most stations of the inner bay and at the Hong Kong area stations (Fig. 8d). Spinileberis quadriaculeata was abundant at central–outer bay stations SZ7 and SZ8 (Fig. 8e). Hemicytheridea reticulata was abundant at central bay stations SZ6, SZ7, and DB11 (Fig. 8f). Loxoconcha sinensis was distributed in the middle of the bay, Shenzhen area, with a high abundance observed at the relatively inner station SZ4 and the outer station SZ8 (Fig. 8g). Loxoconcha ocellata was abundant at outer bay stations SZ7 and SZ8 (Fig. 8h). Aurila cf. disparata was mostly found at station DB11 (Fig. 8i). Shenzhen area stations SZ7 and SZ8 had distinct characteristics with a relatively high abundance of Stigmatocythere bona, Xestoleberis hanaii, Spinileberis quadriaculeata, and Loxoconcha ocellata. The bay mouth, particularly the oceanic-influenced stations DB14 and DB15, exhibited a distinctly high abundance of Keijella kloempritensis and Pistocythereis bradyi. These stations also recorded the highest total ostracod abundance.
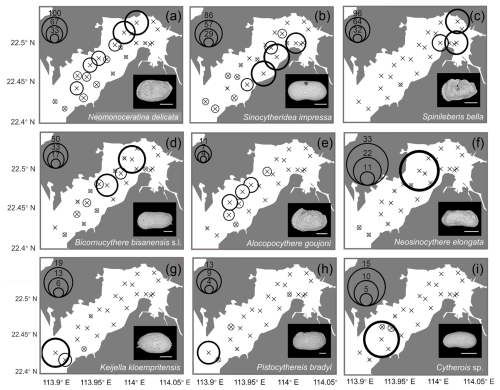
Figure 7Spatial distribution of the relative (%) abundance for the top nine abundant species (relative abundance more than 1 % among all species) in Deep Bay: (a) Neomonoceratina delicata, (b) Sinocytheridea impressa, (c) Spinileberis bella, (d) Bicornucythere bisanensis s.l., (e) Alocopocythere goujoni, (f) Neosinocythere elongata, (g) Keijella kloempritensis, (h) Pistocythereis bradyi, and (i) Cytherois sp. The scale bar represents 200 µm for scanning electron microscope images at the lower-right corner of each subplot.
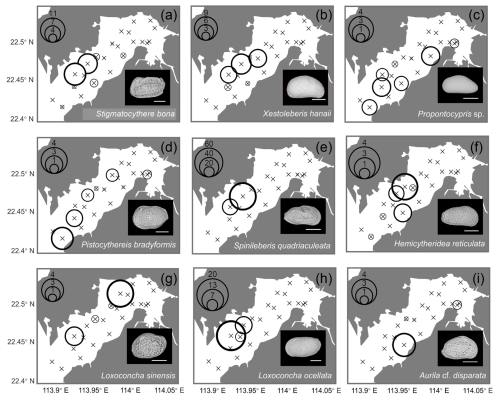
Figure 8Spatial distribution of the relative (%) abundance for top 10–18 abundant species (relative abundance more than 0.3 % among all species) in Deep Bay: (a) Stigmatocythere bona, (b) Xestoleberis hanaii, (c) Propontocypris sp., (d) Pistocythereis bradyformis, (e) Spinileberis quadriaculeata, (f) Hemicytheridea reticulata, (g) Loxoconcha sinensis, (h) Loxoconcha ocellata, and (i) Aurila cf. disparata. The scale bar represents 200 µm for scanning electron microscope images at the lower-right corner of each subplot.
4.1 Abundance and diversity
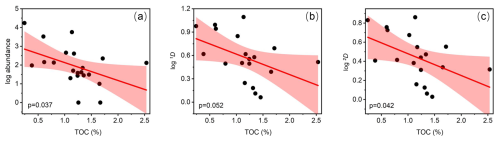
Both ostracod abundance and diversity distribution followed a riverine–oceanic gradient in Deep Bay, driven by riverine input of excessive human waste leading to eutrophication and pollution (note that the bay mouth area of Deep Bay is a part of Pearl River Estuary but is still much more oceanic compared to the inner Deep Bay) (Figs. 4, 5). Recent studies showed that large amounts of nitrogen and phosphorus are transported to the inner-most Deep Bay by rainwater runoff mainly via Shenzhen River from urban catchments, inducing eutrophication (Xu et al., 2019). Hypoxia frequency and nutrient concentration were much higher in the inner-bay, river-influenced sites as shown in the 10-year-averaged EPD data (Fig. 2a, b), indicating that eutrophication and hypoxia had already become long-term environmental problems in Deep Bay. In Asian urban marginal marine areas, eutrophication has been identified as one of the main drivers altering benthic ostracod community, both contemporarily and historically (Yasuhara et al., 2007; Cheung et al., 2019; Hong et al., 2022). Ostracod abundance and diversity (Hill number 1D and 2D) of the entire bay had a significantly negative correlation with TOC content in sediments, which also supported our perspective (Fig. 10). Metal pollution was investigated to be the dominant environmental factor structuring meiobenthic diversity in Hong Kong coastal waters (Hong et al., 2021; Hong et al., 2022). Relevant investigations in Deep Bay also showed a riverine–oceanic gradient on heavy-metal pollutants and PFAs (Zhang et al., 2021; Li et al., 2024), suggesting that the riverine–oceanic distribution of ostracod abundance and diversity may result from the combined effect of both eutrophication and pollution. Comparing Hill number values between riverine and oceanic sites (Figs. 9b, 11), we found that the diversity in oceanic sites was substantially higher when Hill number order was low. The diversity between riverine and oceanic sites was almost the same in higher-order Hill numbers, indicating that eutrophication did not influence much of the structure of the dominant species among stations. This difference in diversity between riverine and oceanic sites was mostly because of rare and abundant species. Low salinity and very shallow intertidal water depth could also be a reason for the lower diversity in riverine sites (Fig. 2c), as a brackish-water environment is commonly characterized by much lower diversity compared to a full-marine environment (Frenzel and Boomer, 2005; Whitfield et al., 2012), and aerial exposure at low tides could be stressful for marine organisms (Tomanek and Helmuth, 2002).
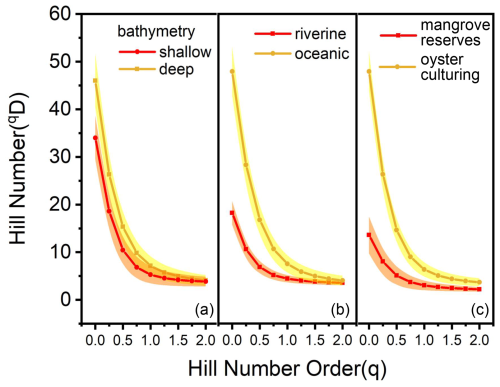
Figure 9Hill number profile of deep-shallow bathymetry, riverine–oceanic area, and mangrove reserve–oyster culturing zone. The shaded areas show the 95 % confidence interval of the profile. (a) Deep bathymetry indicates topography lower than PRL, while shallow bathymetry indicates higher than PRL. (b) Riverine stations included stations located inner than DB9 (DM3), while oceanic included outer stations and DB9 itself. (c) Stations in mangrove reserves included DB1–4 and SZ1–3, while other stations were regarded as oyster culturing zones.
4.2 Faunal distribution
In our study, the three eutrophication/hypoxia-tolerant species Neomonoceratina delicata, Sinocytheridea impressa, and Bicornucythere bisanensis s.l. (Irizuki et al., 2003; Yasuhara et al., 2007, 2012) were abundantly distributed in most of the inner bay stations where TOC was high (Figs. 3a, 7). The Sørensen dissimilarity result showed a distinction between riverine and oceanic (inner bay and middle–outer bay) stations (Fig. 6a). The outer-bay Biofacies 5 in Sørensen dissimilarity was characterized by the lowest TOC and TN values, along with highest abundance and highest diversity. This biofacies exhibited oceanic characteristics and was less related to riverine eutrophication and pollution (Figs. 3a, c, 4, 5, 6a). In contrast, the inner-bay Biofacies 3 in Sørensen dissimilarity was composed of brackish-water species and related to almost all the eutrophication and pollution parameters that we used in this study, accompanied by high TOC and TN values, low abundance, and low diversity (Figs. 3a, c, 4, 5, 6a, b). These results indicated that eutrophication and pollution were the drivers that differentiated the inner-bay fauna from the outer-bay ones. The inner-most bay Biofacies 1 (of all dissimilarities) was probably a river-mouth, brackish-water fauna, because it was located near the Shenzhen River mouth and characterized by low salinity (Figs. 2, 6a, c, e). The dominant species in this biofacies was Spinileberis bella, which is most likely a brackish-water species (Hou and Gou, 2007), as most Spinileberis species are known as brackish-water inhabitants (e.g., Zhao and Wang, 1988).
In addition to this major faunal trend of the inner–outer bay gradient, another major faunal trend was the separation of Hong Kong area and Shenzhen area faunas, clearly represented as Biofacies 3 and 4, respectively, in the Horn and Morisita–Horn dissimilarity results (Fig. 6c, e). Correspondingly, Hong Kong area stations were more eutrophication-influenced, while Shenzhen area stations were more affected by heavy-metal pollution (Fig. 6d, f). Biofacies 3 showed the highest abundance in eutrophication-tolerant Sinocytheridea impressa (Hong et al., 2019) in both Horn-based and Morisita–Horn-based cluster analysis (Table 1), indicating that the Hong Kong area benthic ecosystem was influenced more by eutrophication. In contrast, Biofacies 4 was characterized by a very high abundance in metal-pollution-tolerant Neomonoceratina delicata (Hong et al., 2019) and a much lower abundance of Sinocytheridea impressa (Table 1), indicating that the Shenzhen area benthic ecosystem was influenced more by metal pollution. Most eutrophication parameters were related to Biofacies 3 in the Hong Kong area, including hypoxia frequency, Chl a, DIN, and related sediment eutrophication parameters including TC, TN, and TOC (Fig. 6d, f). Pollutant PFAs and heavy metals Cu, Zn, Pb, Cr, and As were related to Biofacies 4 in the Shenzhen area (Fig. 6d, f), and TS was also related to Biofacies 4, potentially because heavy-metal availability affects the sediment sulfur cycle (Jørgensen et al., 2019).
The reason for the distinction between the Hong Kong area and Shenzhen area remained unclear due to limited evidence. However, there are several possibilities. Stations in Biofacies 3 (Hong Kong area) were shallower than stations in Biofacies 4 (Shenzhen area) (Zhang and Mao, 2015) (also see Fig. 1). With shallower water depth in the Hong Kong area, organic matter could be less decomposed in the water column with its short distance from the surface water to the bottom sediment, resulting in higher sedimentation of organic materials in the sediments compared to the deeper Shenzhen area. This is also shown in sedimentary TOC and TN results, with shallower Hong Kong area stations having relatively high TOC and TN concentrations compared to the Shenzhen area stations (Fig. 3a, c), indicating more eutrophic conditions in the Hong Kong area. Notably, α diversity was lower at shallower water depth (Figs. 9a, 11), potentially because of the stress from eutrophication. Another difference is that the Shenzhen area of Deep Bay is surrounded by Futian and Nanshan districts, which are two of the most populated districts of Shenzhen City (Zhou et al., 2020). Comparably, the Hong Kong area of Deep Bay is close to the Hong Kong rural area. Moreover, secondary industry was transferred from Hong Kong to Shenzhen after 1978 (Sit, 2020). Now secondary industry accounts for 37.6 % GDP in Shenzhen, which is almost 5 times higher than 6.5 % in Hong Kong (data from the website of the Greater Bay Area https://www.bayarea.gov.hk/sc/home/index.html (last access: 3 September 2025). More industrial waste could be the reason for pollutant-driven ostracod fauna distribution in the Shenzhen area. Possible roles of oyster aquaculture were uncertain, because oyster aquaculture rafts are distributed almost across the entire Deep Bay except at the inner-most bay, mangrove area (Fig. 1). Although diversity was much higher in the oyster aquaculture sites compared to the inner-most bay mangrove sites (Figs. 9c, 11), it might be complicated by the fact that mangrove sites were characterized by low salinity, acidic conditions, and intertidal water depth as discussed earlier.
4.3 Comparison with broader Hong Kong study
Species distribution of ostracod species has been investigated in the broader Hong Kong region (Hong et al., 2019). While Alocopocythere goujoni was common in Hong Kong's western water, albeit with low abundance (Hong et al., 2019), we found that this species was most abundant in the middle–outer Deep Bay (Fig. 7e) among Hong Kong's waters (Hong et al., 2019). Hong et al. (2019) suggested that this species is a high-salinity indicator. Our study partially supports this finding, as Alocopocythere goujoni was abundant in the middle–outer Deep Bay. However, other factors may also be at play, considering that Deep Bay is not the area with highest salinity in Hong Kong (Fig. 2c; Hong et al., 2019). Neosinocythere elongata was favorable to high turbidity conditions in Hong Kong broadly (Hong et al., 2019) and was indeed distinctly abundant in inner-bay Biofacies 6 in Horn dissimilarity in Deep Bay (Table 1). Neomonoceratina delicata was broadly distributed in all Hong Kong waters (Hong et al., 2019), and it was also broadly distributed with a relatively high abundance in Deep Bay (Fig. 7a). Sinocytheridea impressa and Bicornucythere bisanensis s.l. were more abundant in semi-enclosed bay areas, such as Tolo Harbour and inner Port Shelter in Hong Kong (Hong et al., 2019). Consistently, we found that they were also abundant mostly in the inner Deep Bay (Fig. 7b, d). In contrast, Keijella kloempritensis and Pistocythereis bradyi were distributed more in open waters, such as the southern and eastern part of Hong Kong, as they preferred deep waters and high-salinity environments, respectively (Hong et al., 2019). Consistently, they are distributed mostly at the bay mouth of Deep Bay (Fig. 7g, h). Xestoleberis hanaii had a preference for more transparent water conditions in Hong Kong (Hong et al., 2019) and, in our Deep Bay study, was located more in the deeper and less eutrophic Shenzhen area (Fig. 8b). Propontocypris sp., which preferred lower productivity in Hong Kong (Hong et al., 2019), was distributed more in the outer Deep Bay (Fig. 8c). In summary, we found consistent spatial trends of species distribution between our Deep Bay study and the previous broader Hong Kong study of Hong et al. (2019). Phytal species such as Cytherois sp., Xestoleberis hanaii, and Aurila cf. disparata were mostly distributed in oyster aquaculture areas (Figs. 1, 7i, 8b, i), which may be related to phytal habitats associated with oyster rafts (cf. Irizuki et al., 2008). However, this is speculative, as oyster aquaculture covers almost the entire area of Deep Bay and not just the inner bay and mangrove areas (Fig. 1).
The wide distribution of ostracod biofacies in Hong Kong is complex and cannot be explained straightforwardly by one or two environmental factors (Hong et al., 2022). This complexity likely arises from the region's multiple embayments, complicated coastline, numerous small and large islands, and varied environmental regimes influenced by multiple sources of pollutants, nutrients, freshwater, etc. However, our high-resolution study in a much simpler, smaller system as an important part of Hong Kong, i.e., Deep Bay, clearly confirmed that the river-influenced eutrophication is a critical factor controlling marginal marine benthic communities. While an intermediate level of eutrophication may be beneficial to soft-sediment benthos, the excess food supply caused by eutrophication, along with the consequent bottom deoxygenation, can lead to the dominance of a few opportunistic species, finally resulting in low diversity, as observed in East Asia and globally (Yasuhara et al., 2007, 2012). Riverine metal pollution may amplify this benthic community change and contribute to form the gradient of opportunistic species under stress in the inner bay to healthier fauna in the outer bay in Deep Bay.
Ostracod abundance, diversity, composition, and distribution, as well as their interactions with eutrophication and pollution, were investigated through high-resolution sampling of surface sediments in Deep Bay, a small semi-enclosed bay adjacent to two of the most populated cities in the world. We found the following:
-
Eutrophication and pollution were the most dominant drivers of ostracod abundance and diversity, creating a pronounced inner–outer bay gradient in Deep Bay.
-
Faunal composition in Deep Bay was characterized by differences between the inner and outer bays, as well as between the Hong Kong and Shenzhen areas. The inner–outer difference was controlled by eutrophication along with pollution from rivers. The Hong Kong–Shenzhen area difference was associated with eutrophication in the Hong Kong area and metal pollution in the Shenzhen area. However, the mechanism and dominant factor driving the Hong Kong–Shenzhen gradient remain unclear.
-
Our results in Deep Bay were consistent with broader studies on ostracod diversity and distribution in Hong Kong, strongly supporting the idea that ostracods are a useful bioindicator of coastal benthic ecosystems. Moreover, in our smaller urban bay case, our investigation provided strong evidence of how eutrophication shapes benthic ostracod communities – an effect that may not be as apparent at larger spatial scales, especially in the regions with complicated geographic settings like Hong Kong, which features multiple embayments, complex coast lines, and numerous islands. This underscores the importance of fine-scale sampling for regional studies. Eutrophication and pollution in small urban bay area have already had profound consequences.
The data are available in a Zenodo open-data repository (https://doi.org/10.5281/zenodo.15775088, Huang et al., 2025).
JH, MY, and HW designed the research; JH, HW, PJ, and JL performed the research; JH analyzed data; JH and MY wrote the paper with intellectual contributions from all authors.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Any opinions, findings, conclusions, or recommendations expressed in this publication do not reflect the views of the Hong Kong SAR Government or the ITC.
We thank Natalia Albarran-Melzer for help with sampling; Yuxuan Lin and Yafei Sun for help on board; Rachel P. P. Wong, Lifang Wang, Zhe Wang, and Qing Li for technical support; Mark Lever for editing; and the two anonymous reviewers for helpful comments.
The work described in this paper was partially supported by the Research Grants Council of the Hong Kong Special Administrative Region, China (project codes: RFS2223-7S02 to Moriaki Yasuhara and AoE/P-601/23-N to Minhan Dai); Seed Funding for Basic Research of the University of Hong Kong (project codes: 2302101483, 2202100581, 202111159167 to Moriaki Yasuhara); and the SKLMP Seed Collaborative Research Fund (project codes: SKLMP/SCRF/0073 and SKLMP/SCRF/0055 to Moriaki Yasuhara). The State Key Laboratory of Marine Pollution (now the State Key Laboratory of Marine Environmental Health) receives regular research funding from the Innovation and Technology Commission (ITC) of the Hong Kong SAR Government.
This paper was edited by Mark Lever and reviewed by two anonymous referees.
Bowler, R. A., Yang, D. S. C., and Smith, A. J. E.: The pearl river estuary oyster industry in and around deep bay, Journal of the Hong Kong Branch of the Royal Asiatic Society, 24, 162–181, http://www.jstor.org/stable/23902772 (last access: 3 September 2025), 1984.
Breitburg, D., Levin, L., Oschlies, A., Grégoire, M., Chavez, F., Conley, D., Garcon, V., Gilbert, D., Gutiérrez, D., Isensee, K., Jacinto, G., Limburg, K., Montes, I., Naqvi, S. W. A., Pitcher, G., Rabalais, N., Roman, M., Rose, K., Seibel, B., and Zhang, J.: Declining oxygen in the global ocean and coastal waters, Science, 359, eaam7240, https://doi.org/10.1126/science.aam7240, 2018.
Chao, A., Gotelli, N., Hsieh, T. C., Sander, E., Ma, K., Colwell, R., and Ellison, A.: Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies, Ecol. Monogr., 84, 45–67, https://doi.org/10.1890/13-0133.1, 2014.
Chao, A., Kubota, Y., Zelený, D., Chiu, C. H., Li, C. F., Kusumoto, B., Yasuhara, M., Thorn, S., Wei, C.–L., Costello, M., and Colwell, R.: Quantifying sample completeness and comparing diversities among assemblages, Ecol. Res., 35, 292–314, https://doi.org/10.1111/1440-1703.12102, 2020.
Cheung, R., Yasuhara, M., Iwatani, H., Wei, C.-L., and Dong, Y.: Benthic community history in the Changjiang (Yangtze River) mega-delta: Damming, urbanization, and environmental control, Paleobiology, 45, 1–15, https://doi.org/10.1017/pab.2019.21, 2019.
Cronin, T. and Vann, C.: The Sedimentary Record of Climatic and Anthropogenic Influence on the Patuxent Estuary and Chesapeake Bay Ecosystems, Estuar. Coasts, 26, 196–209, https://doi.org/10.1007/BF02695962, 2003.
Dai, M., Zhao, Y., Chai, F., Chen, M., Chen, N., Chen, Y., Cheng, D., Gan, J., Guan, D., Hong, Y., Huang, J., Lee, Y., Leung, K., Lim, P. E., Lin, S., Lin, X., Liu, X., Liu, Z., Luo, Y.-W., and Zhang, Z.: Persistent eutrophication and hypoxia in the coastal ocean, Cambridge Prisms: Coastal Futures, 1, 1–71, https://doi.org/10.1017/cft.2023.7, 2023.
Dorgham, M.: Effects of Eutrophication, in: Eutrophication: Causes, Consequences and Control, edited by: Ansari, A. A. and Gill, S., Springer Science & Business Media, USA, 29–44, https://doi.org/10.1007/978-94-007-7814-6, 2014.
Environmental Protection Department of Hong Kong: Marine Water Quality in Hong Kong 2024, https://www.epd.gov.hk/epd/english/environmentinhk/water/hkwqrc/waterquality/marine.html (last access: 3 September 2025), 2024.
Feng, Z., Tan, G., Xia, J., Shu, C., Chen, P., Wu, M., and Wu, X.: Dynamics of mangrove forests in Shenzhen Bay in response to natural and anthropogenic factors from 1988 to 2017, J. Hydrol., 591, 125271, https://doi.org/10.1016/j.jhydrol.2020.125271, 2020.
Frenzel, P. and Boomer, I.: The use of ostracods from marginal marine, brackish waters as bioindicators of modern and Quaternary environmental change, Palaeogeogr. Palaeocl., 225, 68–92, https://doi.org/10.1016/j.palaeo.2004.02.051, 2005.
Frenzel, P., Keyser, D., and Viehberg, F.: An illustrated key and (palaeo)ecological primer for Postglacial to Recent Ostracoda (Crustacea) of the Baltic Sea, Boreas, 39, 567–575, https://doi.org/10.1111/j.1502-3885.2009.00135.x, 2010.
Glibert, P.: Eutrophication, harmful algae and biodiversity – Challenging paradigms in a world of complex nutrient changes, Mar. Pollut. Bull., 124, 591–606, https://doi.org/10.1016/j.marpolbul.2017.04.027, 2017.
Hong, Y., Yasuhara, M., Iwatani, H., and Mamo, B.: Baseline for ostracod-based northwestern Pacific and Indo-Pacific shallow-marine paleoenvironmental reconstructions: ecological modeling of species distributions, Biogeosciences, 16, 585–604, https://doi.org/10.5194/bg-16-585-2019, 2019.
Hong, Y., Yasuhara, M., Iwatani, H., Chao, A., Harnik, P., and Wei, C.-L.: Ecosystem turnover in an urbanized subtropical seascape driven by climate and pollution, Anthropocene, 36, 100304, https://doi.org/10.1016/j.ancene.2021.100304, 2021.
Hong, Y., Yasuhara, M., Iwatani, H., Harnik, P., Chao, A., Cybulski, J., Liu, Y., Ruan, Y., Li, X., and Wei, C.-L.: Benthic ostracod diversity and biogeography in an urbanized seascape, Mar. Micropaleontol., 174, 102067, https://doi.org/10.1016/j.marmicro.2021.102067, 2022.
Horn, H.: Measurement of “Overlap” in Comparative Ecological Studies, Am. Nat., 100, 419–424, https://doi.org/10.1086/282436, 1966.
Hou, Y. and Gou, Y.: Fossil Ostracoda of China. Volume 2: Cytheracea and Cytherellidae, Science Publishing House, Beijing, 1034 pp., ISBN 9787030191830, 2007.
Huang, J., Yasuhara, M., Wang, H., Jimenez, P. J., Li, J., and Dai, M.: Benthic ostracod diversity and biogeography in an urban semi-enclosed eutrophic riverine bay, Zenodo [data set], https://doi.org/10.5281/zenodo.15775088, 2025.
Irizuki, T., Nakamura, Y., Takayasu, K., and Sakai, S.: Faunal changes in Ostracoda (Crustacea) in Lake Nakaumi, southwest Japan, over the last 40 years, Geoscience Report of Shimane University, 22, 149–160, 2003.
Irizuki, T., Seto, K., and Nomura, R.: The impact of fish farming and bank construction on Ostracoda in Uranouchi Bay on the Pacific coast of southwest Japan-Faunal changes between 1954 and 2002/2005, Paleontol. Res., 12, 283–302, https://doi.org/10.2517/1342-8144-12.3.283, 2008.
Irizuki, T., Ito, H., Sako, M., Yoshioka, K., Kawano, S., Nomura, R., and Tanaka, Y.: Anthropogenic impacts on meiobenthic Ostracoda (Crustacea) in the moderately polluted Kasado Bay, Seto Inland Sea, Japan, over the past 70 years, Mar. Pollut. Bull., 91, 149–159, https://doi.org/10.1016/j.marpolbul.2014.12.013, 2014.
Jørgensen, B., Findlay, A., and Pellerin, A.: The Biogeochemical Sulfur Cycle of Marine Sediments, Front. Microbiol., 10, 849, https://doi.org/10.3389/fmicb.2019.00849, 2019.
Lee, S.: Carbon dynamics of Deep Bay, eastern Pearl River estuary, China. II: Trophic relationship based on carbon – and nitrogen-stable isotopes, Mar. Ecol. Prog. Ser., 205, 1–10, https://doi.org/10.3354/meps205001, 2000.
Li, H., Wang, Z., Zhou, Y., Shi, C., Gan, H., Chen, F., Xing, L., Guo, D., Zhu, L., Wang, N., Fang, S., and Bao, R.: Spatial distribution characteristics of perfluoroalkyl substances in bulk and grain size fractionated sediments in Shenzhen Bay, Mar. Pollut. Bull., 199, 115931, https://doi.org/10.1016/j.marpolbul.2023.115931, 2024.
Mamo, B., Cybulski, J., Hong, Y., Harnik, P., Chao, A., Tsujimoto, A., Wei, C.-L., Baker, D., and Yasuhara, M.: Modern biogeography of benthic foraminifera in an urbanized tropical marine ecosystem, Geol. Soc. Lond. Spec. Publ., 529, 79–98, https://doi.org/10.1144/SP529-2022-175, 2023.
Mohyuddin, S., Mangi, A., Fahim, U., Khan, F., Rose, J., and Mao, Y.: Oyster bio–deposition enhanced the functional potential of labile and recalcitrant carbon in Shenzhen bay's sediments, Pakistan Journal of Science, 75, 729–734, https://doi.org/10.57041/pjs.v75i04.1031, 2023.
Morisita, M.: Measuring of dispersion of individuals and analysis of the distributional patterns, Memories of the Faculty of Science, Kyushu University. Series E (Biology), 2, 215–235, https://reference.morisita.or.jp/paper_pdf/55.pdf (last access 3 September 2025)1959.
Nichols, C., Zinnert, J., and Young, D.: Degradation of Coastal Ecosystems: Causes, Impacts and Mitigation Efforts, Tomorrow's Coasts: Complex and Impermanent, edited by: Wright, L., Nichols, C., Coastal Research Library, Springer, Cham, 27, 119–136, https://doi.org/10.1007/978-3-319-75453-6_8, 2019.
Plazziat, J., Koeniguer, J., and Baltzer, F.: Des mangroves actuelles aux mangroves anciennes, B. Soc. Geol. Fr., S7-XXV, 499–504, https://doi.org/10.2113/gssgfbull.S7-XXV.4.499, 1983.
Ren, H., Wu, X., Ning, T., Huang, G., Wang, J., Jian, S., and Lu, H.: Wetland changes and mangrove restoration planning in Shenzhen Bay, Southern China, Landsc. Ecol. Eng., 7, 241–250, https://doi.org/10.1007/s11355-010-0126-z, 2011.
Sit, V. F. S.: Industrial Transformation of Hong Kong, The Hong Kong–Guangdong Link, 163–186, 288 pp., ISBN 9789622094017, 2020.
Sørensen, T. A.: A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons, Biol. Skrif., 5, 1–34, 1948.
Tomanek, L. and Helmuth, B.: Physiological Ecology of Rocky Intertidal Organisms: A Synergy of Concepts, Integr. Comp. Biol., 42, 771–775, https://doi.org/10.1093/icb/42.4.771, 2002.
Whitfield, A., Elliott, M., Basset, A., Blaber, S., and West, R. J.: Paradigms in estuarine ecology – A review of the Remane diagram with a suggested revised model for estuaries, Estuar. Coast. Shelf S., 97, 78–90, https://doi.org/10.1016/j.ecss.2011.11.026, 2012.
Xu, H., Zhang, Y., Zhu, X., and Zheng, M.: Effects of rainfall–runoff pollution on eutrophication in coastal zone: a case study in Shenzhen Bay, southern China, Hydrol. Res., 50, 1062–1075, https://doi.org/10.2166/nh.2019.012, 2019.
Yan, H., He, X., Lei, Y., Wang, Y., Su, H., and Jiang, S.: Land use–induced change in trophic state of Shenzhen Bay (South China) over the past half–century, Mar. Pollut. Bull., 145, 208–213, https://doi.org/10.1016/j.marpolbul.2019.05.046, 2019.
Yang, Y., Gao, S., and Tong, X.: The Study on Hydrodynamic Characteristics of Qianhai Bay in Shenzhen, IOP C. Ser. Earth Env., 768, 012011, https://doi.org/10.1088/1755--1315/768/1/012011, 2021.
Yasuhara, M., Hideo, Y., Tsujimoto, A., and Hirose, K.: The effect of long–term spatiotemporal variations in urbanization–induced eutrophication on a benthic ecosystem, Osaka Bay, Japan, Limnol. Oceanogr., 52, 1633–1644, https://doi.org/10.4319/lo.2007.52.4.1633, 2007.
Yasuhara, M., Tittensor, D., Hillebrand, H., and Worm, B.: Combining marine macroecology and palaeoecology in understanding biodiversity: Microfossils as a model, Biol. Rev., 92, 199–215, https://doi.org/10.1111/brv.12223, 2017.
Yasuhara, M., Hunt, G., Breitburg, D., Tsujimoto, A., and Katsuki, K.: Human–induced marine ecological degradation: Micropaleontological perspectives, Ecol. Evol., 2, 3242–3268, https://doi.org/10.1002/ece3.425, 2012.
Ye, R., Zhang, C., Kong, J., Jin, G., Zhao, H., Song, Z.–y., and Li, L.: A non–negative and high–resolution finite volume method for the depth–integrated solute transport equation using an unstructured triangular mesh, Environ. Fluid Mech., 18, 1379–1411, https://doi.org/10.1007/s10652-018-9598-4, 2018.
Zan, Q. J., Wang, B. S., Wang, Y. J., and Li, M. G.: Ecological assessment on the introduced Sonneratia caseolaris and S. apetala at the Mangrove Forest of Shenzhen Bay, China, Acta Bot. Sin., 45, 544–551, 2003.
Zhang, Q., Ren, F., Xiong, X., Gao, H., Wang, Y., Sun, W., Peifang, L., Li, Z., and Bai, Y.: Spatial distribution and contamination assessment of heavy metal pollution of sediments in coastal reclamation areas: a case study in Shenzhen Bay, China, Environmental Sciences Europe, 33, 90, https://doi.org/10.1186/s12302-021-00532-9, 2021.
Zhang, S. and Mao, X.-Z.: Hydrology, sediment circulation and long–term morphological changes in highly urbanized Shenzhen River estuary, China: A combined field experimental and modeling approach, J. Hydrol., 529, 1562–1577, https://doi.org/10.1016/j.jhydrol.2015.08.027, 2015.
Zhao, Q. and Wang, P.: Distribution of Modern Ostracoda in the Shelf Seas off China, Developments in Palaeontology and Stratigraphy, 11, 805–821, https://doi.org/10.1016/S0920-5446(08)70223-1, 1988.
Zhou, Q., Xu, Y., Zheng, Y., Shao, J., Lin, Y., and Wang, H.: An Improved Method of Determining Human Population Distribution Based on Luojia 1–01 Nighttime Light Imagery and Road Network Data – A Case Study of the City of Shenzhen, Sensors, 20, 5032, https://doi.org/10.3390/s20185032, 2020.