the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Past aridity's effect on carbon mineralization potentials in grassland soils
Zhenjiao Cao
Yufu Jia
Yue Cai
Xin Wang
Huifeng Hu
Jinbo Zhang
Juan Jia
Xiaojuan Feng
Mineralization potential is a key property for assessing carbon substrate's degradability and mineralization in biogeochemical models and studies. While mineralization potential is widely examined under controlled conditions, whether and how it is influenced by the past aridity of sample's origins remain poorly constrained, which is important for an accurate assessment and prediction of future CO2 emissions. Here we collect topsoils and subsoils from different aridity regimes along a 2100 km grassland transect of northern China and conduct a 91 d decomposition experiment with and without the addition of 13C-labeled leaf litter under controlled temperature and moisture. CO2 release from both soil organic carbon (SOC) and fresh litter is measured, along with microbial biomass, extracellular enzyme activities, and soil and mineral properties. We find that neither microbial carbon use efficiency nor biomass-normalized metabolic quotient (qCO2) is related to the aridity of sampling sites. However, both fresh litter and SOC display the highest mineralization potentials in soils originating from the driest site. Using pathway analysis, we demonstrate that past aridity's effect is mediated by differential mechanisms for substrates of varied complexity. While microbial biomass plays a more important role in the decomposition of fresh litter, enzyme-catalyzed extracellular reactions predominantly govern the mineralization of SOC. Our findings provide novel evidence on the mechanisms underlying past aridity's effect on the mineralization potentials of organic matter with different qualities, which has significant implications for assessing and modeling decomposition in different aridity regimes.
- Article
(953 KB) - Full-text XML
-
Supplement
(1222 KB) - BibTeX
- EndNote
Organic carbon mineralization is a critical process affecting global carbon and nutrient cycles as well as atmospheric CO2 levels (Wieder et al., 2015). Numerous experiments have demonstrated the primary control of contemporary or experimental climates (including temperature and moisture) on the decomposition of soil organic carbon (SOC) and litter (Davidson and Janssens, 2006; Conant et al., 2011). Recent studies have also underscored the effect of past climate or rainfall patterns on the contemporary processes of SOC mineralization (Strickland et al., 2015; Hawkes et al., 2017). By comparison, the influence of past aridity on the mineralization potentials of carbon substrates is less studied. Given that carbon mineralization potentials are commonly measured under controlled temperature and moisture conditions to assess substrate's degradability and potential decay rate (Shaver et al., 2006), it is vital to assess whether and how the past aridity of the soil's original site affects mineralization potentials, in order to fully understand environmental controls on carbon decomposition processes in different aridity regimes.
Past aridity of a sample's origins may influence carbon mineralization potentials via at least four pathways, i.e., through affecting (i) microbial biomass production, (ii) microbial carbon use efficiency (CUE) or metabolic quotient (qCO2), (iii) extracellular enzyme activity, and (iv) organic matter and edaphic properties associated with the original soil. The first two microbial responses have been invoked to explain the fast decomposition rate of SOC (after normalizing to SOC content) from arid regions under similar decomposition conditions (Li and Sarah, 2003; Li and Chen, 2004). Under optimal moisture conditions, microbial communities originating from drier soils show a higher growth rate than those from wetter soils, potentially implying a higher moisture sensitivity for microbes from arid soils (Li and Sarah, 2003). Moreover, microbes increase energy allocation for respiration under stress, including moisture constraint (Odum, 1985), and hence show a higher qCO2 or a lower microbial CUE from drier soils (Li and Sarah, 2003). Hence, microbes dwelling in soils of different aridity regimes may show varied activities under the same incubation conditions (Maestre et al., 2015).
Extracellular enzymes are direct regulators for ex vivo reactions that break organic matter macromolecules into smaller units for the subsequent microbial metabolism (Burns, 1978). The activity and turnover of extracellular enzymes, albeit linked to their microbial producers, are also regulated by abiotic factors, such as temperature, moisture and clay content, etc. (Sinsabaugh, 2010), and hence indicate different facets of microbial processes compared to microbial biomass and qCO2 (Sinsabaugh and Follstad Shah, 2012). Recently, enzyme activities are shown to be more sensitive to moisture changes in soils from historically drier than from wetter sites (Averill et al., 2016). Hence, it will be important to disentangle mechanisms mediated by extracellular enzymes versus the microbial community itself (i.e., biomass production and CUE) that contribute to past aridity's effect on carbon mineralization.
Moreover, past aridity may affect carbon mineralization by adjusting the physiochemical properties of organic matter from different aridity regimes (Silver and Miya, 2001). In terms of organic matter properties, soils from drier regions are shown to have higher organic carbon to nitrogen (N) ratios (Delgado-Baquerizo et al., 2013) and are postulated to show lower SOC mineralization rates under similar conditions compared with those from wetter climates (Marschner and Kalbitz, 2003). Alternatively, soil pH that directly mediates enzyme and microbial activities (Sinsabaugh, 2010) typically increases with increasing aridity (C. Wang et al., 2014). It is not clear how the above soil properties jointly affect past aridity's effect on carbon mineralization. Empirical evidence is greatly lacking for disentangling the different mechanisms.
Here we utilize soils collected from grassland sites with varied climatic aridity and conduct soil incubation experiments under controlled temperature and moisture conditions to examine the effect of past aridity (i.e., of soil sampling sites) on carbon mineralization. Given the positive correlation between mineralization rate and SOC concentrations (Harrison-Kirk et al., 2013), mineralization potential is measured as the percentage of respired CO2 in total organic carbon, as a commonly used parameter to assess the degradability of organic matter. Compared to previous studies that employed common litter (Strickland et al., 2015) or reciprocal transplant manipulations (Hawkes et al., 2017), carbon mineralization of soils from different aridity regimes may be further complicated by site-specific edaphic properties such as soil mineralogy and texture (Bronick and Lal, 2005). To control for such side effects, a detailed list of soil properties (including texture, reactive minerals and mineralogy) were examined and compared against mineralization rate. Moreover, we add 13C-labeled leaf litter to soils collected from different depths to examine litter mineralization without inducing site-specific edaphic properties and to compare the mineralization of organic matter with varied complexity (i.e., fresh litter, topsoils and subsoils). Finally, using pathway analysis coupled with measurements of microbial communities, extracellular enzyme activities and soil properties, we attempt to quantitatively assess mechanisms contributing to the effect of past aridity on mineralization potentials. Specifically, we hypothesize that past aridity mediates SOC mineralization via its effect on microbial, enzyme and soil organic matter (SOM) properties (hypothesis 1). Moreover, given the vital role of extracellular enzymes in macromolecule breakdown within complex soil matrix, extracellular enzymes have a stronger influence on regulating aridity's effect on the mineralization of SOC relative to fresh litter (hypothesis 2).
Table 1Location and basic information of the studied grassland sites.
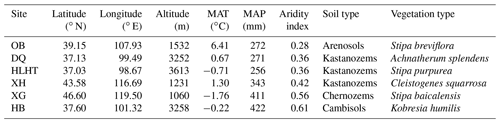
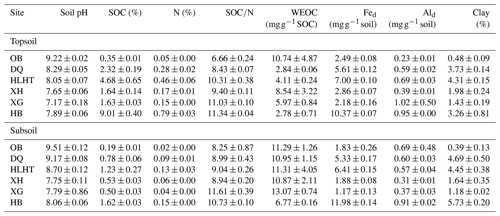
MAT: mean annual temperature; MAP: mean annual precipitation (aridity index is defined as the ratio of MAP to potential evapotranspiration and increases with increasing moisture); OB: Otog Banner; DQ: Daqiao; HLHT: Halihatu; XH: Xilinhot; XG: Xilingol League; and HB: Haibei.
2.1 Study area and soil sampling
Six grassland sites with varied climatic conditions are selected from a 2100 km transect from northern China (37.03–47.60∘ N, 98.67–119.50∘ E; 1060–3613 m above sea level; Fig. S1 in the Supplement). These sites represent typical grasslands with minimal human influence, including three alpine sites on the Qinghai–Tibetan Plateau (Halihatu, HLHT; Daqiao, DQ; and Haibei, HB) and three temperate sites in Inner Mongolia (Otog Banner, OB; Xilingol League, XG; and Xilinhot, XH). Mean annual temperature (MAT) ranges from −1.8 to 6.4 ∘C, and mean annual precipitation (MAP) varies from 256 to 422 mm (WorldClim database; http://www.worldclim.org, last access: 25 August 2019; Table 1). Aridity index, calculated as the ratio of precipitation to potential evapotranspiration, is used to indicate regional dryness of the sampling sites and ranges from 0.28 to 0.61 (http://www.cgiar-csi.org/, last access: 25 August 2019). Soil types include Arenosols, Kastanozems, Chernozems and Cambisols (IUSS Working Group WRB, 2006), and the dominant vegetation types are listed in Table 1. Parent materials are dominated by clastic and igneous rocks and the dominant minerals are similar across sites including quartz and feldspar besides small amounts of calcite (Table S1 in the Supplement).
Three random soil cores (up to 100 m in between) were taken from each site in July–August 2015. Soils from two horizons were collected with varying depths for the subsoil due to different development and depths of soil profiles at varied sites: topsoil (0–10 cm) from the A horizon and subsoil (50–70 cm for HLHT and DQ; 30–40 cm for HB; 30–50 cm for the other sites) from the B horizon. The samples were separated into two parts: one part was air-dried immediately for soil physiochemical analyses and the other part was stored in ziplock bags in the dark at 4 ∘C for the incubation experiment. Both parts were passed through a 2 mm sieve with visible roots removed and homogenized before further treatment. Soils from different cores were not mixed and hence represented authentic field replicates.
2.2 Analysis of soil properties
Total C and N contents were measured for the air-dried soils using an elemental analyzer (Vario EL III, Elementar, Hanau, Germany). SOC content was calculated by subtracting inorganic carbon from total carbon, with the former analyzed volumetrically by reaction with hydrochloric acid (HCl). Soil pH was analyzed by a pH meter in a soil:water suspension (1:2.5, w:v). Water-extractable organic carbon (WEOC) was extracted from air-dried original soils (∼5 g) by mixing with 12 mL Milli-Q water on a reciprocal shaker for 24 h. The supernatant was filtered through 0.45 µm PTFE filters after centrifugation and acidified to pH <2 with HCl for WEOC measurement on a Multi N/C 3100 total organic carbon analyzer (Analytik Jena, Germany). Soil texture was examined using Malvern Mastersizer 2000 particle analyzer after removing SOM and calcium carbonates (Ma et al., 2018). Reactive iron (Fed) and aluminum (Ald) were extracted by the citrate–bicarbonate–dithionite method (Lalonde et al., 2012), and their contents were determined on an inductively coupled plasma–atomic emission spectrometer (ICP-AES; ICAP 6300, Thermo Scientific, USA).
2.3 Incubation experiment
Within 1 month of sample collection, authentic field replicates of soils were incubated in the dark at 25 ∘C for 91 d to examine carbon mineralization. Varying amounts of soils were weighed into 165 mL brown glass flasks containing >60 mg of SOC. Soil water content was maintained at 55 %–60 % of the water holding capacity by regularly weighing and spraying MilliQ water over the soil. Before incubation, all samples were preincubated under the same condition for 2 weeks to activate soil microbes. On the first day of incubation, one half of the replicates were used as control without any amendment, while the other half were mixed with fine powders (∼2500 mesh) of 13C-labeled grass leaves (a mixture of Oplismenus undulatifolius folius and Miscanthus sinensis) to examine the mineralization of fresh litter relative to SOC. Due to logistic reasons, δ13C of the added leaves varied between HB (2067.75 ‰ in the first batch of incubation) and all other soils (1269.97 ‰ in the second batch). Both batches of grass leaves were continuously labeled with CO2 gas with 99.9 atom % 13C for 3 months in a growth chamber. Nonetheless, both δ13C values were substantially higher than those of SOC (−26.35 ‰ to −23.06 ‰) and did not influence the calculation. The added litter carbon corresponded to a higher proportion of SOC in the HB (0.7 %) than all other soils (0.29 %) due to an oversight in calculation. Nevertheless, the low amendment rates did not induce a priming effect on the mineralization of native SOC in any soil (details in Sect. 3).
Mineralization was monitored by quantifying CO2 accumulated in the headspace for 6 h on >12 selected days using gas chromatograph (GC; Agilent 7890A, USA), coupled with a flame ionization detector (FID). To differentiate SOC- and litter-derived CO2, δ13C of CO2 from litter-amended samples was measured periodically (five to six times in total) on an isotope ratio mass spectrometry (IRMS; Delta PLUS XP, Thermo Finnigan, Germany). The contribution of SOC- and litter-derived carbon to CO2 was calculated by the mass balance equations:
where r is cumulative CO2 (mg C g−1 soil) and the subscript t refers to total respired CO2 from the litter-amended sample. The mineralization potential for litter (Rlitter), as well as SOC in the control (Rcontrol) and litter-amended treatments (RSOC), was normalized to the corresponding organic carbon content. Microbial metabolic quotient (qCO2) was calculated as cumulative CO2 divided by microbial biomass (estimated using phospholipid fatty acids, PLFAs; Sect. 2.4) at the end of incubation (Martínez-García et al., 2018).
2.4 Analyses of PLFAs and extracellular enzyme activity
Microbial community structure and biomass were analyzed by PLFAs using a modified Bligh–Dyer extraction (Bligh and Dyer, 1959) at the end of the incubation (details in Supplement Sect. S1.1). PLFAs are categorized into nonspecific, fungi, gram-positive (G+) and gram-negative (G−) bacteria-derived (Harwood and Russell, 1984). The concentration of individual PLFAs was normalized to the SOC content. Microbial community composition is assessed by the ratio of fungal to bacterial PLFAs (F∕B) and the ratio of G+ to G− bacteria (). The δ13C values of individual PLFAs analyzed on gas chromatography coupled to a stable isotope ratio mass spectrometry via a combustion interface (GC-C-IRMS) and the proportion of litter-derived carbon in PLFAs was calculated using a mass balance approach (Supplement Sect. S1.1).
PLFA-based CUE (referred to as CUE' here), instead of microbial-biomass-carbon-based CUE, was calculated as follows (Kallenbach et al., 2016):
where PLFA−Clitter and CO2−Clitter are the amount of carbon in litter-derived PLFAs and CO2, respectively (Bradford et al., 2013).
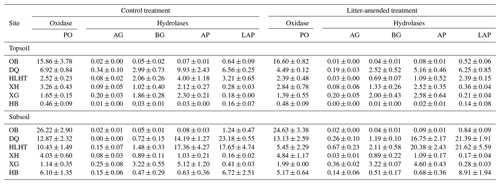
At the end of the incubation, the activity of one oxidase (phenol oxidase) and four hydrolases, including α-glucosidase, β-glucosidase, alkaline phosphatase and leucine-aminopeptidase, were measured according to Saiya-Cork et al. (2002; Supplement Sect. S1.2). Enzyme activity was expressed as specific activity normalized to the SOC content (Allison et al., 2014).
2.5 Data and statistical analysis
We assessed the homogeneity of variances and normal distribution of data using the Shapiro–Wilk test before applying parametric methods. Nonparametric tests (Kruskal-Wallis H or Wilcoxon) were conducted for non-normally distributed data. Differences of measured soil and microbial properties in soils from different depths or treatments were assessed by paired T or Wilcoxon test. Relationships between environmental factors and substrate mineralization potentials were assessed using Pearson correlations (for normally distributed data) or, otherwise, Spearman correlations by IBM SPSS 20.0 (IBM SPSS, Chicago, USA). Differences and correlations were considered to be significant at a level of p<0.05.
To delineate mechanisms regulating substrate mineralization potentials, structural equation modeling (SEM) was conducted to quantify the complex interactions between environmental variables and substrate mineralization potential using the “lavaan” package of R software (version 3.5.3; Rosseel, 2012). The selection of model parameters and optimization of the model were detailed in Supplement Sect. S1.3. To complement SEM in evaluating the main influencing variable(s) on mineralization potentials, multiple stepwise regression was conducted encompassing the same variables (aridity index, soil minerals, pH, SOM properties, PLFAs, phenol oxidase and hydrolases) using IBM SPSS 20.0 (IBM SPSS, Chicago, USA) at a level of p<0.05. Variables not contributing to the variation in substrate mineralization potential were excluded in the subsequent variable selection process based on the p values (i.e., only variables with p≤0.10 were retained in the model). After the establishment of regression models, normal distribution of model residues was checked, while collinearity among the selected variables was avoided based on the variance inflation factor (a cutoff value of 1 was chosen to define collinearity) to ensure robustness of the models. The explanatory power of the regression model is indicated by R2.
3.1 Soil bulk properties
Subsoils had higher pHs, SOC and N contents than their corresponding topsoil (p<0.05) but similar SOC∕N ratios (p>0.05; Table 2). Concentrations of WEOC were higher in the subsoil than the topsoil after normalization against SOC (p<0.05). The driest site (OB) showed the highest pH value, the lowest SOC and N contents, and SOC∕N ratios, while the wettest site HB showed the highest SOC, N contents, and SOC∕N ratios (p<0.05). Although the SOC-normalized concentration of WEOC was highest in the topsoil of OB, it was not related to the aridity index for either topsoils or subsoils. Contents of Fed and Ald did not show consistent patterns related to aridity or depth. Clay content was lowest for both topsoils and subsoils in OB (p<0.05).
3.2 Microbial PLFAs and δ13C
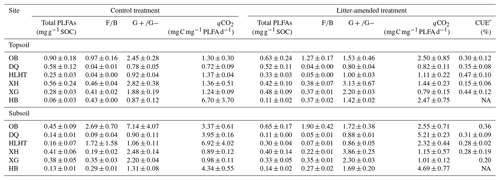
The topsoils and subsoils from the same site had similar concentrations of total PLFAs and F∕B and ratios (p>0.05; Table 3). The F∕B ratio was highest in OB at both depths (p<0.05). Litter amendment did not produce significant effects on either PLFA concentrations or ratios (p>0.05). PLFA concentrations were negatively correlated with aridity index in both treatments (p<0.05; Fig. S2). The δ13C of PLFAs was analyzed for all the sites except HB due to sample loss. There were no significant differences in δ13C of PLFAs for various microbial groups in either treatment (p>0.05; Fig. S3). The abundance-weighted average δ13C of PLFAs was higher in the topsoil of HLHT than the other sites (p<0.05) and was similar in all subsoils (p>0.05). The proportion of litter-derived C in PLFAs was around 1 %–3 % in the topsoil and 1 %–5 % in the subsoil (Table S2), showing no difference between depths (p>0.05).
Table 3Concentrations of microbial phospholipid fatty acids (PLFAs), ratios of fungal∕bacterial (F∕B) and gram-positive∕gram-negative bacterial PLFAs (), microbial metabolic quotient (qCO2), and PLFA-based microbial carbon use efficiency (CUE′) in the soil at the end of the incubation (mean±standard error; n=3).
NA: not available
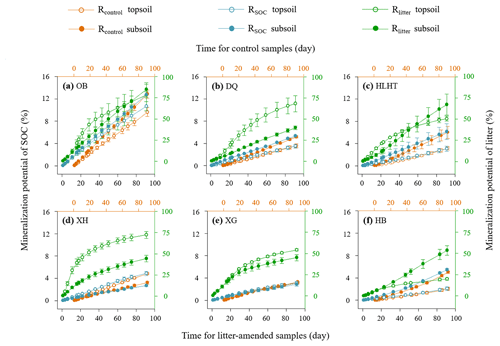
Figure 1Mineralization potential of soil organic carbon (SOC) and leaf litter during the 91 d incubation. Rcontrol and RSOC refer to the mineralization potential of SOC in the control and leaf-amended treatments, respectively, while Rlitter refers to the mineralization potential of leaf litter. Mean values are shown with standard error (n=3). The x axis (top) for Rcontrol is shifted to the right relative to the leaf-amended treatments (bottom axis) for better illustration.
3.3 Mineralization, qCO2 and CUE′
Rcontrol was 1.4 %–10.6 % and 3.0 %–14.2 % in the topsoils and subsoils (Fig. 1), similar to RSOC in the litter-amended soils (p>0.05). There was hence no priming effect in the litter-amended soils relative to the control (Fig. S4). Both Rcontrol and RSOC were highest in the driest OB soil with the lowest SOC contents. By comparison, Rlitter was higher (17.9 %–95.5 % and 35.6 %–92.3 % in the topsoils and subsoils, respectively; Fig. 1) and also highest in OB for both depths. There were no significant differences in Rcontrol or RSOC between depths (p>0.05) except HB having higher Rcontrol in the subsoil than topsoil (p<0.05). Rlitter was also similar between depths except that XH and HB showed higher values in topsoils and subsoils than their counterparts, respectively (p<0.05). All mineralization potentials were negatively correlated with aridity index (p<0.05; Fig. 2). Similar qCO2 values were found for the litter-amended and control treatments (p>0.05; Table 3). There were no significant differences between topsoils and subsoils except for OB and DQ. Microbial CUE′ did not significantly differ between depths (p>0.05; Table 3). Neither qCO2 nor CUE′ showed a relationship with aridity index (p>0.05; Fig. S2).
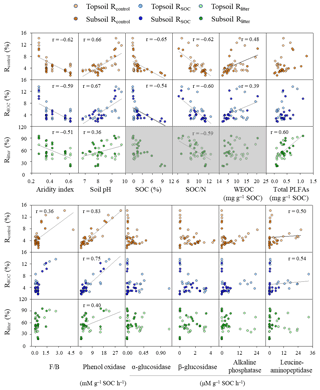
Figure 2Relationships between the mineralization potential of substrates and key environmental variables. Rcontrol and RSOC refer to the mineralization potential of soil organic carbon (SOC) in the control and leaf-amended treatments, respectively, while Rlitter refers to the mineralization potential of leaf litter. N: nitrogen; WEOC: water-extractable organic carbon; F∕B: ratios of fungal to bacterial PLFAs; PLFAs: phospholipid fatty acids. Field replicates are shown as individual data points. Black lines represent significant Spearman correlations for non-normally distributed data or Pearson correlations for normally distributed data (i.e., between pH and Rlitter; p<0.05). Three boxes of Rlitter are shaded in grey because the examined soil properties do not describe litter quality and hence should not be correlated.
3.4 Extracellular enzyme activity
The specific SOC-normalized activity of hydrolases was highly variable across sites while phenol oxidase activity was highest in OB at both depths (Table 4). Litter amendment did not produce any effect on enzyme activities at either depth (p>0.05). Enzyme activity did not show consistent variations between depths. Phenol oxidase and leucine-aminopeptidase activities were negatively correlated with aridity index in both treatments (p<0.05; Fig. S2).
3.5 Relationships between mineralization potentials and environmental variables
All mineralization potentials are negatively correlated with aridity index and positively correlated with soil pH and phenol oxidase activity (p<0.05; Fig. 2). Rcontrol and RSOC also show positive correlations with WEOC concentrations and leucine-aminopeptidase activities and negative correlations with SOC contents and SOC∕N ratios (p<0.05). Rcontrol is positively correlated with the F∕B ratio despite a low r value (r=0.36; p<0.05). By comparison, Rlitter is strongly and positively correlated with total PLFAs (r=0.60; p<0.05). This relationship is confirmed by positive correlations between Rlitter and various PLFA groups (i.e., fungal, G+ and G− bacterial PLFAs; p<0.05) while no significant correlations were observed for either Rcontrol (except with fungal PLFAs) or RSOC (Fig. S5). Soil minerals, CUE′ and ratios hardly show correlations with substrate mineralization potentials.
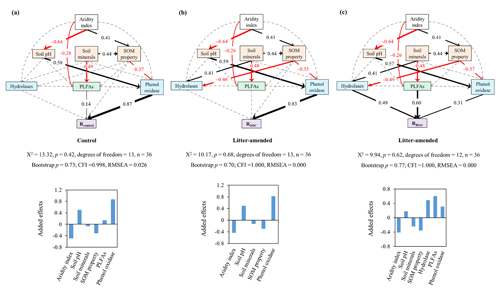
Figure 3The best-supported structural equation models (SEMs) for disentangling the cascading effects of environmental variables on substrate mineralization. Rcontrol (a) and RSOC (b) refer to the mineralization potential of soil organic carbon (SOC) in the control and leaf-amended treatments, respectively, while Rlitter (c) refers to the mineralization potential of leaf litter. Black and red arrows indicate positive and negative flows of causality (p<0.05), respectively. Dotted grey lines indicate insignificant pathways from a priori models (Fig. S7). Numbers on the arrows indicate significant standardized path coefficients, proportional to the arrow width. Environmental variables are categorized into climate (i.e., aridity index), extracellular enzymes (in blue) including hydrolases and phenol oxidase, microbial biomass (in green) represented by phospholipid fatty acids (PLFAs) and soil properties (in orange) including soil minerals, soil pH, and soil organic matter (SOM) properties. Soil minerals, SOM properties and hydrolases are defined by a principle component analysis (Table S5).
To disentangle the interactive mechanisms, we conducted an SEM analysis for the pooled data from both soil depths. Combining topsoils and subsoils allowed us to focus on the comparison of pathways affecting the mineralization of SOC versus litter and also improved the model performance by increasing the number of data. The constructed SEMs show a good model fit indicated by a nonsignificant χ2 test (p>0.05), a high comparative fit index (CFI>0.95), a low root-mean-square error of approximation (RMSEA<0.05) and a bootstrap p value (p>0.1; Schermelleh-Engel and Moosbrugger, 2003), and it explains 89 %, 79 % and 41 % of variations in Rcontrol, RSOC and Rlitter, respectively. Based on the SEMs, enzyme activities are the most important direct regulator for SOC mineralization in both control and litter-amended treatments (Fig. 3a–b). By contrast, microbial biomass (PLFAs) displays a stronger direct influence on Rlitter than either hydrolases or phenol oxidase (Fig. 3c). Phenol oxidase, rather than hydrolases, affects SOC mineralization potentials in both treatments, while hydrolases have a similar effect on Rlitter as phenol oxidase. Soil minerals and SOM properties have a relatively minor, negative effect on all mineralization potentials via influencing enzyme activities and PLFAs. In addition, soil pH has a positive effect on all mineralization potentials through positively influencing phenol oxidase activity. Aridity index has an overall negative effect on all mineralization potentials via exerting a positive effect on SOM properties and a negative effect on soil pH and PLFAs. It is also notable that hydrolases are influenced by soil minerals and SOM properties only in the litter-amended treatment. Generally, these pathways are consistent with correlations between the corresponding variables (Fig. S2) and mineralization potentials (Fig. 2).
To verify the differential impacts of PLFAs and enzyme activities on mineralization potentials, we further employed multiple stepwise regression encompassing the same environmental variables. The standardized partial regression coefficient is used to assess the relative importance of influencing factors; i.e., a higher value indicates a stronger influence. The regression analysis yields R2 of 0.85, 0.76 and 0.42, indicating a reasonable explanatory power of the model (Table 5). Consistent with the SEM results, phenol oxidase activity is the most important variable influencing SOC mineralization in both treatments. By comparison, PLFAs exert the strongest control on Rlitter. Furthermore, when the topsoils and subsoils are considered separately, phenol oxidase activities remain the most important regulator for SOC mineralization at both depths. PLFAs remain the only important regulator for Rlitter in the subsoil, while PLFAs, as well as aridity index, govern Rlitter in the topsoil.
4.1 Magnitude of mineralization potentials for litter versus SOC
Mineralization potential of SOC ranged from 1.4 % to 14.2 % in both control and litter-amended treatments in our studied grasslands, falling within the range reported for other grassland soils (Guenet et al., 2010; reference details in Table S3). By comparison, the mineralization potential of grass leaf litter (Rlitter) showed a wider range and higher values in this study (17.9 %–95.5 %) compared to the literature (Sievers and Cook, 2018; Table S4). As shown in Fig. 2 and discussed below, the mineralization potential of the same litter increases with increasing aridity of the original site. Sites in this study had an aridity index of 0.28–0.61, much dried than those in the above studies. Hence, the high values of Rlitter in our study may also reflect the high mineralization potential of litter in semiarid regions. Furthermore, the Rlitter values measured under optimal conditions in this study (25 ∘C; soil moisture: 55 %–60 % of water holding capacity) are comparable to the litter mineralization rates reported in “real-world” conditions such as in field litterbag experiments. For instance, G. Wang et al. (2014) reported that >70 % of E. speciosus litter degraded under warm (12–35 ∘C) and humid conditions within 90 d. Shaw and Harte (2001) found that nearly 73 % of forb litter was lost within 46 d in a subalpine meadow. Sievers and Cook (2018) also discovered that the litter of hairy vetch and cereal rye degraded by 90 % in cropland within 84 d. Hence, we consider the Rlitter measured in this study to reflect optimal decomposition rates of litter in semiarid regions. The mineralization potential is significantly higher for grass leaf litter than for SOC (p<0.05), reflecting the high degradability of fresh litter carbon due to microbial preference for litter enriched with labile carbon such as carbohydrates (Guenet et al., 2010) and the absence of mineral protection compared to SOC (Six et al., 2002).
Table 5Standardized partial regression coefficients of the multiple stepwise regression analysis for substrate mineralization potentials with environmental variables.
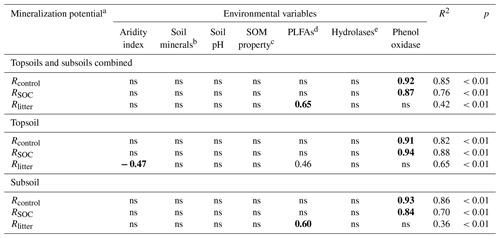
a Rcontrol and RSOC refer to the mineralization potential of soil organic carbon (SOC) in the control and leaf-amended treatments, respectively, while Rlitter refers to the mineralization potential of leaf litter. b Soil minerals are represented by the first principal component in the principal component analysis involving Fed, Ald and clay. c Soil organic matter (SOM) properties are represented by the first principal component in the principal component analysis involving SOC, SOC:N ratio and water-extractable organic carbon (WEOC) contents. d PLFAs: phospholipid fatty acids. ehydrolases are represented by the first principal component in the principal component analysis involving α-glucosidase, β-glucosidase, alkaline phosphatase and leucine-aminopeptidase. Bold fonts correspond to the highest coefficient values and hence the strongest influence by the corresponding environmental variable. ns: not significant.
4.2 Differential controls by extracellular enzymes and microbial biomass on the mineralization of SOC versus fresh litter
An important finding of this study is that while Rlitter is mainly affected by the SOC-normalized concentrations of PLFAs (i.e., microbial biomass) in the soil, extracellular enzyme (specifically, phenol oxidase) activities rather than PLFAs predominantly govern the mineralization of SOC. This result is supported by both SEM (Fig. 3) and multiple stepwise regression analyses (Table 5), in line with our second hypothesis. The production of extracellular enzymes is not only related to the size of microbial community (biomass) but also to its structure (Gallo et al., 2004). As >95 % of microbial biomass is considered to be dormant or inactive in the soil (Fierer, 2017), these microbes may not actively participate in enzyme production. Moreover, extracellular enzymes may experience inactivation or prolonged activity caused by sorption to minerals and/or complexation with SOM (Arnosti et al., 2014). Hence, microbial biomass and extracellular enzymes may act quite independently on carbon decomposition.
As SOM consists of a consortium of complex molecules, often in association with each other and/or minerals (Lehmann and Kleber, 2015), its decomposition is a multistep process initiated with the oxidation or hydrolysis by extracellular enzymes (Nannipieri et al., 2002). As such, macromolecular SOM structures are broken into molecules small enough to be transported through microbial cell membranes and utilized for respiration and biomass production (Sollins et al., 1996). Hence, enzymatic depolymerization is a crucial, rate limiting step for large, complex substrates (Conant et al., 2011). This explains the dominant role of extracellular enzyme activity in SOC mineralization in both treatments in our experiment. In contrast, for leaf litter that is relatively easy to degrade without complex mineral interactions (Bosatta and Ågren, 1999), microbial biomass predominantly controls its mineralization.
Interestingly, among the investigated enzymes, phenol oxidase, rather than hydrolases, plays a decisive role in SOC mineralization in our study, likely related to the much higher activity of phenol oxidase compared with hydrolases in the soil (Table 4). It also agrees with previous findings that oxidases (i.e., phenol oxidase and catalase) instead of hydrolases (i.e., urease and neutral phosphatase) are key players in SOM breakdown, controlling its decomposition rate (Hassan et al., 2013). Oxidative enzymes are shown to be more important in the soils of desert grasslands than temperate grasslands (Stursova and Sinsabaugh, 2008). Phenol oxidase activity is also documented to control SOC decomposition and CO2 emission in peatlands and some upland ecosystems (Freeman et al., 2001). By comparison, in contrast to SOC, hydrolases are more important than phenol oxidase in the mineralization of litter that contains more hydrolyzable carbon such as cellulose (Fig. 3).
4.3 Pathways regulating past aridity's effect on carbon mineralization potentials
Our study demonstrates that the aridity index of sampling sites has a strong negative effect on the mineralization potential of both SOC and litter added to the soil (Fig. 2), such that the driest site OB displays the highest mineralization potential for both substrates under controlled temperature and moisture conditions. This finding underscores past climate's effect on carbon mineralization potentials and agrees with other reports showing elevated soil respiration (after normalizing to SOC content) and/or microbial metabolic quotient in soils from arid regions over subhumid regions under similar incubation conditions (Li and Sarah, 2003). More importantly, employing SEM analysis (Fig. 3), we show that, consistent with our first hypothesis, the relationship between past aridity and substrate mineralization potential is jointly mediated through aridity's effect on microbial properties (i.e., phenol oxidase activity and PLFAs) and SOM properties (for mineralization of SOC only).
First, soils from drier regions (with a lower aridity index) exhibited higher phenol oxidase activity under incubation conditions (Fig. S2). This result agrees with the higher responsiveness of enzyme activities to water availability in drier soils (Averill et al., 2016), which is considered to reflect microbial strategies to cope with sporadic supply of water in arid environments. Aridity index also influences phenol oxidase activity via a negative effect on soil pH and a positive effect on SOM properties (affected by SOC contents and SOC∕N ratios; Fig. S2c). The former pathway is likely related to pH's positive effect on phenol oxidase activity in neutral-to-alkaline soils (Sinsabaugh, 2010). The latter pathway is attributed to increasing phenol oxidase activity with both decreasing SOC∕N ratios (Artigas et al., 2008) and increasing WEOC concentrations (Fang et al., 2015). With the above pathways combined, aridity index and pH indirectly exert a negative and positive effect on substrate mineralization potentials, respectively, with the latter relationship in accordance with previous reports (Whittinghill and Hobbie, 2011; Carrasco et al., 2017).
In contrast to phenol oxidase, hydrolases are unresponsive to the variation in aridity index or any other investigated variables in this study. With a lower activation energy (Ea), phenol oxidase (Ea of 32.5 kJ mol−1) is often more active than hydrolases (e.g., β-glucosidase: Ea of 61.8 kJ mol−1; Davidson et al., 2012) and less stable in the environment (Sinsabaugh, 2010). Additionally, cellulolytic activity shows small variations while phenol oxidase activity typically exhibits a large decline in decaying organic matter (Carreiro et al., 2000). Hence, phenol oxidase is more responsive to environmental variabilities than hydrolases and its activity is strongly linked to substrate mineralization potentials, especially for SOC (Fig. 3).
Second, in contrast to previous studies (Odum, 1985; Li and Sarah, 2003), neither qCO2 nor CUE′ in our experiment shows any consistent changes with shifting aridity of the original sites. Hence, aridity index mediates Rlitter mainly via negatively influencing PLFA concentrations (Fig. 3c). Similar to phenol oxidase activities, microbial growth in soils from drier regions may be strongly promoted during the incubation due to the release of moisture constraint (Li and Sarah, 2003), which in turn leads to a higher mineralization rate of the easy-to-degrade carbon (e.g., leaf litter).
Third, past aridity also affects SOM properties, which indirectly regulate mineralization potentials of both SOC and litter via affecting phenol oxidase and hydrolases (for Rlitter only). In this study, SOM property is an arbitrary term generated by principal component analysis (PCA) for SOC contents, SOC∕N ratios and WEOC concentrations (Table S5). Its negative effect on SOC mineralization potentials is related to SOC and SOC∕N's negative correlations and WEOC's positive correlation with Rcontrol and RSOC (Fig. 2), in agreement with the literature data (Fig. S6). In addition, high SOC∕N ratios and low WEOC concentrations may lower SOC mineralization potentials due to N and/or labile carbon constraints on microbial activities (Kalbitz et al., 2000; Schimel and Weintraub, 2003).
Last but not least, soil minerals that are strongly influenced by parent materials rather than aridity (Harradine and Jenny, 1958) may exert complicating effects on SOC mineralization potentials via interacting with SOC (von Lützow et al., 2006) and microbial activities (Bruun et al., 2010). In our studied soils, mineralization potentials are not directly correlated with Fed, Ald or clay contents (p>0.05). However, soil minerals have indirect effects on mineralization potentials via positively affecting SOM properties (mainly SOC and N contents), as well as hydrolases (in the litter-amended treatments), and negatively affecting PLFAs in the SEM. The former two relationships reflect minerals' protective effect on SOC (von Lützow et al., 2006) and extracellular enzymes (Wei et al., 2014), while the latter may be associated with the inhibitive effects of reactive Fe and Al on microbial respiration and growth (Bruun et al., 2010; Lemire et al., 2013). Nonetheless, soil minerals have a minimal added effect on Rcontrol and RSOC and a relatively minor effect on Rlitter compared to other aridity-influenced variables. We hence conclude that past aridity of the sampling sites has a strong control on carbon mineralization potentials of both SOC and litter mainly via mediating microbial biomass, enzyme activities and SOM property. It should be mentioned that our measured variables explained a relatively low proportion of Rlitter variance (R2=0.42). Hence, there are still other mechanisms regulating Rlitter (and similarly SOC decomposition rate) that are not depicted by our analysis, such as radical attack by reactive oxygen species (Georgiou et al., 2015) and protection by soil aggregation (Angst et al., 2017). These mechanisms also deserve attention in the future.
In summary, our study demonstrates that the aridity of sampling sites has a strong and consistent effect on the mineralization of both common litter and SOC from grasslands under controlled conditions. Such effects should be taken into account in the assessment of carbon release potentials, given the wide application of controlled incubation in studying carbon mineralization. Moreover, in comparison with the well-investigated microbial control on climate's legacy effect (Strickland et al., 2015; Hawkes et al., 2017), our study emphasizes the importance of extracellular processes catalyzed by enzymes (in particular, phenol oxidase) in the mineralization of more complex SOC relative to fresh litter. As extracellular enzyme and microbial activities may show varied responses to climatic variations, our findings suggest different vulnerabilities for organic matter of different qualities and originating from various aridity regimes. With aridity shifts in the future, soil carbon stocks in drylands may be more vulnerable to decomposition than those in humid regions.
All data are included in the paper and Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-16-3605-2019-supplement.
XF designed the study. ZC and YC carried out the experiment and analyses with help from JJ. HH, JZ, YC and XW collected soil samples. ZC analyzed the data with help from YJ and JJ. ZC, YJ and XF wrote the manuscript with input from all the other authors. ZC and YJ contributed equally to this work.
The authors declare that they have no conflict of interest.
We thank the sampling team for collecting the samples and the reviewers for their valuable comments.
This research has been supported by the Chinese National Key Development Program for Basic Research (grant nos. 2017YFC0503902 and 2015CB954201), the National Natural Science Foundation of China (grant nos. 41422304, 41773067 and 41807329) and the International Partnership Program of Chinese Academy of Sciences (grant no. 151111KYSB20160014).
This paper was edited by Jianming Xu and reviewed by Tida Ge and one anonymous referee.
Allison, S. D., Chacon, S. S., and German, D. P.: Substrate concentration constraints on microbial decomposition, Soil Biol. Biochem., 79, 43–49, 2014.
Angst, G., Mueller, K. E., Kögel-Knabner, I., Freeman, K. H., and Mueller, C. W.: Aggregation controls the stability of lignin and lipids in clay-sized particulate and mineral associated organic matter, Biogeochemistry, 132, 307–324, 2017.
Arnosti, C., Bell, C., Moorhead, D. L., Sinsabaugh, R. L., Steen, A. D., Stromberger, M., Wallenstein, M., and Weintraub, M. N.: Extracellular enzymes in terrestrial, freshwater, and marine environments: perspectives on system variability and common research needs, Biogeochemistry, 117, 5–21, 2014.
Artigas, J., Romaní, A. M., and Sabater, S.: Relating nutrient molar ratios of microbial attached communities to organic matter utilization in a forested stream, Fund. Appl. Limnol., 173, 255–264, 2008.
Averill, C., Waring, B. G., and Hawkes, C. V.: Historical precipitation predictably alters the shape and magnitude of microbial functional response to soil moisture, Glob. Change Biol., 22, 1957–1964, 2016.
Bligh, E. G. and Dyer, W. J.: A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol., 37, 911–917, 1959.
Bosatta, E. and Ågren, G. I.: Soil organic matter quality interpreted thermodynamically, Soil Biol. Biochem., 31, 1889–1891, 1999.
Bradford, M. A., Keiser, A. D., Davies, C. A., Mersmann, C. A., and Strickland, M. S.: Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth, Biogeochemistry, 113, 271–281, 2013.
Bronick, C. J. and Lal, R.: Soil structure and management: a review, Geoderma, 124, 3–22, 2005.
Bruun, T. B., Elberling, B., and Christensen, B. T.: Lability of soil organic carbon in tropical soils with different clay minerals, Soil Biol. Biochem., 42, 888–895, 2010.
Burns, R. G.: Soil enzymes, Academic Press, New York, 1978.
Carrasco, B., Cabaneiro, A., and Fernandez, I.: Exploring potential pine litter biodegradability as a natural tool for low-carbon forestry, Forest Ecol. Manag., 401, 166–176, 2017.
Carreiro, M. M., Sinsabaugh, R. L., Repert, D. A., and Parkhurst, D. F.: Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition, Ecology, 81, 2359–2365, 2000.
Conant, R. T., Ryan, M. G., Ågren, G. I., Birge, H. E., Davidson, E. A., Eliasson, P. E., Evans, S. E., Frey, S., Giardina, E. A., Hopkins, F. M., Hyvönen, R., Kirschbaum, M. U. F., Lavallee, J. M., Leifeld, J., Parton, W. J., Steinweg, J. M., Wallenstein, M. D., Wetterstedt, J. A. M., and Bradford, M. Å.: Temperature and soil organic matter decomposition rates – synthesis of current knowledge and a way forward, Glob. Change Biol., 17, 3392–3404, 2011.
Davidson, E. A. and Janssens, I. A.: Temperature sensitivity of soil carbon decomposition and feedbacks to climate change, Nature, 440, 165–173, 2006.
Davidson, E. A., Samanta, S., Caramori, S. S., and Savage, K.: The dual Arrhenius and Michaelis-Menten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales, Glob. Change Biol., 18, 371–384, 2012.
Delgado-Baquerizo, M., Maestre, F. T., Gallardo, A., Bowker, M. A., Wallenstein, M. D., Quero, J. L., Ochoa, V., Gozalo, B., García-Gómez, M., Soliveres, S., García-Palacios, P., Berdugo, M., Valencia, E., Escolar, C., Arredondo, T., Barraza-Zepeda, C., Bran, D., Carreira, J. A., Chaieb, M., Conceição, A. A., Derak, M., Eldridge, D. J., Escudero, A., Espinosa, C. I., Gaitán, J., Gatica, M. G., Gómez-González, S., Guzman, E., Gutiérrez, J. R., Florentino, A., Hepper, E., Hernández, R. M., Huber-Sannwald, E., Jankju, M., Liu, J., Mau, R. L., Miriti, M., Monerris, J., Naseri, K., Noumi, Z., Polo, V., Prina, A., Pucheta, E., Ramírez, E., Ramírez-Collantes, D. A., Romão, R., Tighe, M., Torres, D., Torres-Díaz, C., Ungar, E. D., Val, J., Wamiti, W., Wang, D., and Zaady, E.: Decoupling of soil nutrient cycles as a function of aridity in global drylands, Nature, 502, 672–676, 2013.
Fang, H., Cheng, S., Lin, E., Yu, G., Niu, S., Wang, Y., Xu, M., Dang, X., Li, L., and Wang, L.: Elevated atmospheric carbon dioxide concentration stimulates soil microbial activity and impacts water-extractable organic carbon in an agricultural soil, Biogeochemistry, 122, 253–267, 2015.
Fierer, N.: Embracing the unknown: disentangling the complexities of the soil microbiome, Nat. Rev. Microbiol., 15, 579–590, 2017.
Freeman, C., Ostle, N., and Kang, H.: An enzymic `latch' on a global carbon store – A shortage of oxygen locks up carbon in peatlands by restraining a single enzyme, Nature, 409, 149, 2001.
Gallo, M., Amonette, R., Lauber, C., Sinsabaugh, R. L., and Zak, D. R.: Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils, Microb. Ecol., 48, 218–229, 2004.
Georgiou, C. D., Sun, H. J., McKay, C. P., Grintzalis, K., Papapostolou, I., Zisimopoulos, D., Panagiotidis, K., Zhang, G., Koutsopoulou, E., Christidis, G. E., and Margiolaki, I.: Evidence for photochemical production of reactive oxygen species in desert soils, Nat. Commun., 6, 7100, https://doi.org/10.1038/ncomms8100, 2015.
Guenet, B., Neill, C., Bardoux, G., and Abbadie, L.: Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl. Soil Ecol., 46, 436–442, 2010.
Harradine, F. and Jenny, H.: Influence of parent material and climate on texture and nitrogen and carbon contents of virgin California soils: I. Texture and nitrogen contents of soils, Soil Sci., 85, 235–243, 1958.
Harrison-Kirk, T., Beare, M. H., Meenken, E. D., and Condron, L. M.: Soil organic matter and texture affect responses to dry/wet cycles: Effects on carbon dioxide and nitrous oxide emissions, Soil Biol. Biochem., 57, 43–55, 2013.
Harwood, J. L. and Russell, N. J.: Lipids in plants and microorganisms, George Allen and Unwin Ltd., London, 1984.
Hassan, W., Chen, W., Cai, P., and Huang, Q.: Oxidative enzymes, the ultimate regulator: implications for factors affecting their efficiency, J. Environ. Qual., 42, 1779–1790, 2013.
Hawkes, C. V., Waring, B. G., Rocca, J. D., and Kivlin, S. N.: Historical climate controls soil respiration responses to current soil moisture, P. Natl. Acad. Sci. USA, 114, 6322–6327, 2017.
IUSS Working Group WRB: World reference base for soil resources, World soil resources reports, No. 103, 2nd edn., FAO, Rome, 2006.
Kalbitz, K., Solinger, S., Park, J. H., Michalzik, B., and Matzner, E.: Controls on the dynamics of dissolved organic matter in soils: a review, Soil Sci., 165, 277–304, 2000.
Kallenbach, C. M., Frey, S. D., and Grandy, A. S.: Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls, Nat. Commun., 7, 13630, https://doi.org/10.1038/ncomms13630, 2016.
Lalonde, K., Mucci, A., Ouellet, A., and Gelinas, Y.: Preservation of organic matter in sediments promoted by iron, Nature, 483, 198–200, 2012.
Lehmann, J. and Kleber, M.: The contentious nature of soil organic matter, Nature, 528, 60–68, 2015.
Lemire, J. A., Harrison, J. J., and Turner, R. J.: Antimicrobial activity of metals: mechanisms, molecular targets and applications, Nat. Rev. Microbiol., 11, 371–384, 2013.
Li, X. and Chen, Z.: Soil microbial biomass C and N along a climatic transect in the Mongolian steppe, Biol. Fert. Soils, 39, 344–351, 2004.
Li, X. and Sarah, P.: Arylsulfatase activity of soil microbial biomass along a Mediterranean-arid transect, Soil Biol. Biochem., 35, 925–934, 2003.
Ma, T., Zhu, S., Wang, Z., Chen, D., Dai, G., Feng, B., Su, X., Hu, H., Li, K., Han, W., Liang, C., Bai, Y., and Feng, X.: Divergent accumulation of microbial necromass and plant lignin components in grassland soils, Nat. Commun., 9, 3480, https://doi.org/10.1038/s41467-018-05891-1, 2018.
Maestre, F. T., Delgado-Baquerizo, M., Jeffries, T. C., Eldridge, D. J., Ochoa, V., Gozalo, B., Quero, J. L., García-Gómez, M., Gallardo, A., Ulrich, W., Bowker, M. A., Arredondo, T., Barraza-Zepeda, C., Bran, D., Florentino, A., Gaitán, J., Gutiérrez, J. R., Huber-Sannwald, E., Jankju, M., Mau, R. L., Miriti, M., Naseri, K., Ospina, A., Stavi, I., Wang, D., Woods, N. N., Yuan, X., Zaady, E., and Singh, B. K.: Increasing aridity reduces soil microbial diversity and abundance in global drylands, P. Natl. Acad. Sci. USA, 112, 15684–15689, 2015.
Marschner, B. and Kalbitz, K.: Controls of bioavailability and biodegradability of dissolved organic matter in soils, Geoderma, 113, 211–235, 2003.
Martínez-García, L. B., Korthals, G., Brussaard, L., Jørgensen, H. B., and De Deyn, G. B.: Organic management and cover crop species steer soil microbial community structure and functionality along with soil organic matter properties, Agr. Ecosyst. Environ., 263, 7–17, 2018.
Nannipieri, P., Kandeler, E., and Ruggiero, P.: Enzyme activities and microbiological and biochemical processes in soil, in: Enzymes in the Environment, edited by: Burns, R. G. and Dick, R. P., Marcel Dekker Inc., New York, 1–34, 2002.
Odum, E. P.: Trends expected in stressed ecosystems, Bioscience, 35, 419–422, 1985.
Rosseel, Y.: Lavaan: an R package for structural equation modeling and more. Version 0.5–12 (BETA), J. Stat. Softw., 48, 1–36, 2012.
Saiya-Cork, K. R., Sinsabaugh, R. L., and Zak, D. R.: The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil, Soil Biol. Biochem., 34, 1309–1315, 2002.
Schermelleh-Engel, K. and Moosbrugger, H.: Evaluating the fit of structural equation models, tests of significance descriptive goodness-of-fit measures, Method. Psychol. Res., 8, 23–74, 2003.
Schimel, J. P. and Weintraub, M. N.: The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model, Soil Biol. Biochem., 35, 549–563, 2003.
Shaver, G. R., Giblin, A. E., Nadelhoffer, K. J., Thieler, K. K., Downs, M. R., Laundre, J. A., and Rastetter, E. B.: Carbon turnover in Alaskan tundra soils: effects of organic matter quality, temperature, moisture and fertilizer, J. Ecol., 94, 740–753, 2006.
Shaw, M. R., and Harte, J.: Control of litter decomposition in a subalpine meadow-sagebrush steppe ecotone under climate change, Ecol. Appl., 11, 1206–1223, 2001.
Sievers, T. and Cook, R. L.: Aboveground and root decomposition of cereal rye and hairy vetch cover crops, Soil Sci. Soc. Am. J., 82, 147–155, 2018.
Silver, W. L. and Miya, R. K.: Global patterns in root decomposition: comparisons of climate and litter quality effects, Oecologia, 129, 407–419, 2001.
Sinsabaugh, R. L.: Phenol oxidase, peroxidase and organic matter dynamics of soil, Soil Biol. Biochem., 42, 391–404, 2010.
Sinsabaugh, R. L. and Follstad Shah, J. J.: Ecoenzymatic stoichiometry and ecological theory, Annu. Rev. Ecol., Evol., S., 43, 313–343, 2012.
Six, J., Conant, R. T., Paul, E. A., and Paustian, K.: Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils, Plant Soil, 241, 155–176, 2002.
Sollins, P., Homann, P., and Caldwell, B. A.: Stabilization and destabilization of soil organic matter: mechanisms and controls, Geoderma, 74, 65–105, 1996.
Strickland, M. S., Keiser, A. D., and Bradford, M. A.: Climate history shapes contemporary leaf litter decomposition, Biogeochemistry, 122, 165–174, 2015.
Stursova, M. and Sinsabaugh, R. L.: Stabilization of oxidative enzymes in desert soil may limit organic matter accumulation, Soil Biol. Biochem., 40, 550–553, 2008.
von Lützow, M., Kögel-Knabner, I., Ekschmitt, K., Matzner, E., Guggenberger, G., Marschner, B., and Flessa, H.: Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions – a review, Eur. J. Soil Sci., 57, 426–445, 2006.
Wang, C., Wang, X., Liu, D., Wu, H., Lu, X., Fang, Y., Cheng, W., Luo, W., Jiang, P., Shi, J., Yin, H., Zhou, J., Han, X., and Bai, E.: Aridity threshold in controlling ecosystem nitrogen cycling in arid and semi-arid grasslands, Nat. Commun., 5, 4799, https://doi.org/10.1038/ncomms5799, 2014a.
Wang, G., Zhang, L., Zhang, X., Wang, Y., and Xu, Y.: Chemical and carbon isotopic dynamics of grass organic matter during litter decompositions: A litterbag experiment, Org. Geochem., 69, 106–113, 2014b.
Wei, H., Guenet, B., Vicca, S., Nunan, N., Asard, H., AbdElgawad, H., Shen, W., and Janssens, I. A.: High clay content accelerates the decomposition of fresh organic matter in artificial soils, Soil Biol. Biochem., 77, 100–108, 2014.
Whittinghill, K. A. and Hobbie, S. E.: Effects of pH and calcium on soil organic matter dynamics in Alaskan tundra, Biogeochemistry, 111, 569–581, 2011.
Wieder, W. R., Allison, S. D., Davidson, E. A., Georgiou, K., Hararuk, O., He, Y., Hopkins, F., Luo, Y., Smith, M. J., Sulman, B., Todd-Brown, K., Wang, Y. P., Xia, J., and Xu, X.: Explicitly representing soil microbial processes in Earth system models, Global Biogeochem. Cy., 29, 1782–1800, 2015.