the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The patterns of elemental concentration (Ca, Na, Sr, Mg, Mn, Ba, Cu, Pb, V, Y, U and Cd) in shells of invertebrates representing different CaCO3 polymorphs: a case study from the brackish Gulf of Gdańsk (the Baltic Sea)
Anna Piwoni-Piórewicz
Stanislav Strekopytov
Emma Humphreys-Williams
Piotr Kukliński
The shells of calcitic arthropod Amphibalanus improvisus; aragonitic bivalves Cerastoderma glaucum, Limecola balthica, and Mya arenaria; and bimineralic bivalve Mytilus trossulus were collected in the brackish waters of the southern Baltic Sea in order to study patterns of bulk elemental concentration (Ca, Na, Sr, Mg, Ba, Mn, Cu, Pb, V, Y, U and Cd) in shells composed of different crystal lattices (calcite and aragonite). The factors controlling the elemental composition of shells are discussed in the context of crystal lattice properties, size classes of organisms and potential environmental differences between locations. Clams that precipitate fully aragonitic shells have a clear predominance of Sr over Mg in shells, contrary to predominant accumulation of Mg over Sr in calcitic shells of barnacles. However, the barnacle calcite shell contains higher Sr concentration than bivalve aragonite. The elemental variability between size-grouped shells is different for each studied species, and the elemental concentrations tend to be lower in the large size classes compared to the smaller size classes. Biological differences between and within species, such as growth rate, feeding strategy (including feeding rate and assimilation efficiency or composition) and contribution of organic material, seem to be important factors determining the elemental accumulation in shells. Because specimens used in this study were obtained from different sampling sites within the gulf, the impact of location-specific environmental factors, such as sediment type, cannot be excluded.
- Article
(3619 KB) - Full-text XML
-
Supplement
(99 KB) - BibTeX
- EndNote
Marine invertebrates such as molluscs, brachiopods, corals, echinoderms, bryozoans and some groups of protozoa (foraminifera) are able to form skeletons built of calcium carbonate. The combination of inorganic CaCO3 crystals and organic compounds with a characteristic and ordered structure, mainly polymers, creates a composite strengthening material. As a result of long-term adaptation, calcifiers are armed with hard protective internal and external body parts such as shells, tubes, walls, or plates of various colours, shapes, and functions. The biogenic CaCO3 is deposited mainly in the form of two polymorphs: calcite and aragonite, which in some taxa are found co-existing in the same specimen and appear to be precipitated in the same environment (Cusack and Freer, 2008; Morse et al., 2007; Taylor et al., 2008; Stanley, 2008). Furthermore, various trace elements, especially those forming divalent ions – mainly Mg and Sr, as well as Mn, Br, Cd, Cu, Pb, V, etc. – are present as impurities in skeletons. The major sources of trace elements for organisms during the calcification process are aquatic soluble phases and the food base that may consist of suspended and/or sedimented particles (Newman and Unger, 2003; Rainbow, 1995; Rainbow and Phillips, 1993). However, the bioavailability of trace elements depends on a combination of environmental (e.g. salinity, pH, sediment type, and oxygen conditions) and chemical factors (e.g. element–particle binding strength, the composition of particle, and the presence of other trace elements and compounds; Blackmore and Wang, 2002).
Biological carbonates determine a very important component of the hydrosphere's carbon reservoir, and the biogenic production of CaCO3 has a strong interdependence on the ocean composition, biogeochemistry and the carbon cycle (Cohen and McConnaughey, 2003). In the last few decades, increasing attention has been paid to the relationship between the composition of shells and external environmental factors. It has been repeatedly observed that the mineral type and accumulated trace element concentrations in CaCO3 shells of marine invertebrates are integrated records of environmental conditions (Marchitto et al., 2000; Rodland et al., 2006). The elemental composition of the carbonate skeleton can provide records of seawater chemistry and has a significant potential for the fields of palaeoceanography and palaeoclimatology (Freitas et al., 2006; Gillikin et al., 2006; Khim et al., 2003; Ponnurangam et al., 2016; Vander Putten et al., 2000). However, as recent studies have indicated (Dove, 2010), the influence of environmental parameters on shell precipitation could be very complex. The mineralogy and chemistry of shells are likely to be both linked to environmental conditions and controlled by the organism itself. Even a single population or closely related species within the same habitat may exhibit different accumulation strategies (Rainbow et al., 2000).
Due to its spatial structure, aragonite shows preferential substitution with larger cations, such as Sr, while smaller cations, such as Mg, are energetically favoured in calcite. In natural systems, calcite commonly incorporates Mg (Morse et al., 2007; Reeder, 1983; Wang and Xu, 2001), and the solubility of Mg-containing calcite is known to increase with increasing Mg substitution (Kuklinski and Taylor, 2009; Smith et al., 2006). At the temperatures and pressures of the Earth's surface, low-Mg calcite is the most stable form of CaCO3 (de Boer, 1977). Nevertheless, in many marine organisms, aragonite and high-Mg calcite are the dominant phases precipitated from seawater (Dickson, 2004). The solution chemistry (Cusack and Freer, 2008), temperature (Balthasar and Cusack, 2015), pressure (Allison et al., 2001), CaCO3 saturation state (Watson et al., 2012), pCO2 (Lee and Morse, 2010) and phylogenesis (Kuklinski and Taylor, 2009; Smith et al., 1998; Smith and Girvan, 2010) are known to influence shell mineralogy. The main driving force controlling the mineralogy of precipitated CaCO3 is the ratio of Mg to Ca ions in seawater (Cusack and Freer, 2008; Morse et al., 2007). A Mg∕Ca ratio >2 favours the precipitation of aragonite and high-Mg calcite. At high Mg∕Ca ratios, such as those in modern seawater (Mg∕Ca = 5.2), calcitic structures incorporate Mg, which is observed to inhibit calcite nucleation and growth, whereas aragonite nucleation is not affected by Mg in solution (De Choudens-Sanchez and Gonzalez, 2009; Morse et al., 2007). Most taxa producing low-Mg calcite are known to actively control the amount of incorporated Mg (Bentov and Erez, 2005; De Nooijer et al., 2014). Several studies of organisms that secrete calcareous skeletons have shown that lower seawater temperatures are correlated with the secretion of calcite skeletons with low Mg contents rather than more soluble high-Mg calcite or aragonite skeletons (Taylor and Reid, 1990). The mineralogy of many calcareous structures changes with latitude, likely as a result of the temperature gradient from the poles to the Equator (Kuklinski and Taylor, 2009; Loxton et al., 2014; Taylor et al., 2014). Thermodynamics predicts that aragonite is the stable phase at pressures higher than 5000 hPa (roughly 40 m depth), and calcite is the stable phase at lower pressures. However, aragonite is still the major constituent of shells or pearls, indicating its metastable formation in shallow waters (Sunagawa et al., 2007). The incorporation of Sr was suggested to play a significant role in the biomineralogical precipitation of aragonite (Allison et al., 2001). Many studies have demonstrated a clear correlation between the concentration of Sr in the hard parts and precipitation of the aragonite layer (Iglikowska et al., 2016; Reeder, 1983). The ionic radius of Sr is larger than that of Ca; thus, Sr is more likely to form ninefold coordination, which triggers metastable aragonite nucleation (Sunagawa et al., 2007).
Many studies have demonstrated that the biological control of shell composition is often more important than the environmental control (Carré et al., 2006; Freitas et al., 2005, 2006; Gillikin, 2005; Gillikin et al., 2005b). Elements incorporated into skeletons originate from the environment, yet as some of them are the components of enzymes or body fluids, the trace element pathway to the skeleton can be altered by the biological processes (Cubadda et al., 2001; Luoma and Rainbow, 2008), which can affect the relationship between the concentrations of a given trace element between skeleton and environment. In biologically controlled mineralization, the organism drives the process of nucleation and growth of the minerals in a way that is not entirely dependent on the environmental conditions. Endogenous factors manifest themselves through co-regulation of all the structures and functions of the organism, including its sex, growth rate, metabolism and feeding strategy (Lowenstam and Weiner, 1989). The main physiological processes involved in element accumulation are ingestion, assimilation, elimination and growth (Wang and Fisher, 1997). Throughout the lifespan, the biological system experiences ontogenetic trends and seasonal variations in physiology, determining metabolic expenses based on life's needs. Biological effects have been repeatedly used to explain shifts of elemental concentrations in shells from a theoretical equilibrium (Davis et al., 2000; Roger et al., 2017; Watson et al., 1995). Ontogenetic fluctuations of the growth rate and metabolic activity affect the intensity of the element uptake (Lee et al., 1998). Vander Putten et al. (2000) concluded that the seasonality of the accumulation of Mg, Sr and Pb in Mytilus edulis shells shows significant similarity across individuals, with a maximum during spring and early summer, and that the elemental profiles cannot be explained by seasonal variations in the seawater composition. Carré et al. (2006) developed a model of ion transport in bivalve shells that shows that Ca2+ channels are less ion selective when Ca2+ fluxes are higher. Other studies have found that the rate of trace element uptake increases as mussel filtration rate increases (Janssen and Scholtz, 1979).
The aim of this study is to assess the patterns of elemental variability (Ca, Na, Sr, Mg, Mn, Ba, Cu, Pb, V, Y, U and Cd) in shells of mussels and barnacles representing three possible mineralogical forms. The selected species are aragonitic clams Cerastoderma glaucum, Mya arenaria, and Limecola balthica; bimineralic mussel Mytilus trossulus; and barnacle Amphibalanus improvisus with a fully calcitic shell, which are all collected from the low-salinity environment of the southern Baltic Sea. A comparison of elemental concentration levels between size classes is also performed. Assuming the larger specimens are older than the smaller specimens of the same species, we assess how different ontogenetical stages influence elemental accumulation. The potential influence of biological control and local environmental conditions on the observed element concentrations in shells is briefly discussed. The study area offers a variety of fluctuating factors, giving the opportunity to increase our understanding of elemental variation patterns of skeletons formed in the area. Brackish waters affect the activity and speciation of elements, enhancing their bioavailability (Fritioff et al., 2005). The seasonal changes (e.g. surface temperature, primary production and freshwater inflow) determine the element sources and drive the physiological processes of living organisms (Urey et al., 1951).
2.1 Study area
The study area is located in the Gulf of Gdańsk in the southern Baltic Sea; more precisely, it is in the outer Puck Bay and the central Gulf of Gdańsk (Fig. 1). The north-western part of the gulf is separated by the Hel Peninsula and in the west and south by the coastline stretches (Kruk-Dowgiałło and Szaniawska, 2008; Rainbow et al., 2004). This location makes the seawater the most turbulent in January and the calmest in June, with weak bottom currents and minimal tidal amplitudes. The hydrophysical parameters of the gulf are mostly driven by the temperate climate and the following seasonal changes. Differences in air temperature and water mixing cause seasonal fluctuations of the surface water temperature, ranging from approximately 4 to 22 ∘C (Uścinowicz, 2011). The Gulf of Gdańsk is a low-salinity system under the influence of brackish water from the open southern Baltic Sea and fresh waters from rivers, mainly the Vistula river; the Vistula is the largest river in Poland and has an average annual inflow into the estuary of 1080 m3 s−1, which varies seasonally from 250 to 8000 m3 s−1 and has a maximum in spring (Cyberski et al., 2006). Thus, the average water salinity in the gulf is 7, varying from approximately 5.5 in summer to 8.4 in winter (Bulnheim and Gosling, 1988; Szefer, 2002).
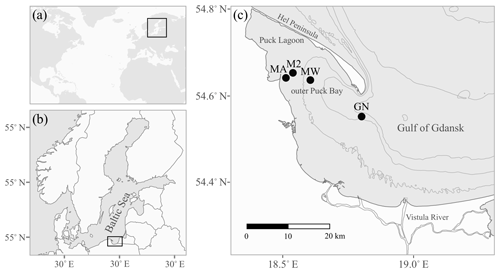
Figure 1The location of the study area: the Baltic Sea (a) and the Gulf of Gdańsk (b) are marked by black rectangles. Panel (c) shows the sampling stations as black circles (see Table 1 for station details). The grey lines indicate 20 m isobaths.
The Gulf of Gdańsk is an area highly influenced by human activities. This is due to intensive usage of its resources and to anthropogenic emissions originating from various coastal sources, river inflows and atmospheric deposition. The most significant input of industrial and municipal pollution into the gulf is derived from the Vistula river, which transports pollutants from a catchment area of 194 000 km2 (Pruszak et al., 2005). Both the water discharge and sediment load into the gulf are strongly seasonally dependent. Because of the local conditions, mainly the limited water exchange, river-borne contaminants remain in the ecosystem for decades, accumulating in the sediments and in living organisms (Glasby et al., 2004; Szumiło-Pilarska et al., 2016).
Most regions of the Baltic Sea have lower salinity and alkalinity (that is, lower Ca2+ and CO concentrations) than oceanic surface waters (Beldowski et al., 2010; Cai et al., 2010; Findlay et al., 2008). Due to the seasonality of temperature and biogeochemical cycle, the amplitude of CaCO3 saturation state (, where is a solubility product of calcite or aragonite under in situ conditions; e.g. Kawahata et al., 2019) in the Baltic Sea is high in comparison to saline waters. It alternates between approximately 1 to 5 for calcite and 0.5 to 2.5 for aragonite (Findlay et al., 2008).
The community of calcifiers from the Gulf of Gdańsk is characterized by the dominance of benthic filter feeders and deposit feeders (Kruk-Dowgiałło and Dubrawski, 1998). These organisms exploit their growth potential during the productive seasons of the year when the availability of suspended matter, mainly phytoplankton, is the highest (Pierscieniak et al., 2010; Staniszewska et al., 2016).
2.2 Species
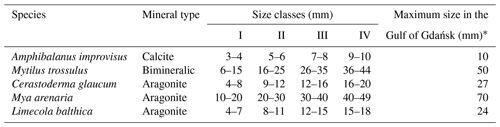
The clam Cerastoderma glaucum (Mollusca, Bivalvia; Fig. 2a), commonly known as the lagoon cockle, is a saltwater organism found along the coasts of Europe and northern Africa, including in the Mediterranean Sea, Black Sea, Caspian Sea and the low-salinity Baltic Sea. It is a euryhaline species living in salinities between 4 and 84. Clam C. glaucum can tolerate habitats with a wide range of temperatures, from periodically freezing to above 30 ∘C. It is a filter feeder that actively lives near the sediment surface, acting as a biodiffuser (Urban-Malinga et al., 2013). The clam is surrounded by a ribbed aragonite shell, which is externally yellowish to greenish brown (Jelnes et al., 1971). In the brackish environment of the Gulf of Gdańsk, C. glaucum spawns in May–July and typically lives up to 4 years, achieving a height of 27 mm (Żmudziński, 1990).

Figure 2The investigated species photographed in situ: clams (a) Cerastoderma glaucum, (b) Mya arenaria, and (c) Limecola balthica; mussels (d) Mytilus trossulus; and barnacle (e) Amphibalanus improvisus.
The soft-shell clam Mya arenaria (Mollusca, Bivalvia; Fig. 2b) is a marine invasive species introduced into European waters from the Atlantic coasts of North America (Behrends et al., 2005). It has a wide global distribution, mainly due to its adaptability to varying environments with salinities between 4 and 35 and temperatures between −2 and 28 ∘C (Gofas, 2004; Strasser et al., 1999). M. arenaria is a filter feeder, filtering organic particles and microinvertebrates using long fused siphons, and a deposit feeder. In the Gulf of Gdańsk, M. arenaria is a common inhabitant of shallow waters down to a depth of 30 m. It spawns once or twice a year in spring or summer, at temperatures of 10–15 ∘C. Individuals live 10–12 years. They have aragonitic shells and grow up to 70 mm (Żmudziński, 1990).
The clam Limecola balthica (Mollusca, Bivalvia; Fig. 2c) lives in the northern parts of the Atlantic and Pacific oceans, in sub-Arctic and European waters from southern France to the White Sea and Pechora Sea, including the Baltic Sea (Strelkov et al., 2007). It is a euryhaline clam capable of living in a wide range of water salinities from 3 to 40 and at temperatures from −2 to above 30 ∘C (Sartori and Gofas, 2016). L. balthica is a filter feeder and a deposit feeder and has a semi-sessile lifestyle, with the ability to undertake periodic migrations (Hiddink et al., 2002). In the Baltic Sea, L. balthica lives at depths down to 40 m and grows to 24 mm. Adults reproduce in spring when the water temperature reaches 10 ∘C and live 12 years (Żmudziński, 1990). They have aragonitic shells varying in colour between individuals and locations, mainly exhibiting white, pink, yellow and orange (Sartori and Gofas, 2016).
The mussel Mytilus trossulus (Mollusca, Bivalvia; Fig. 2d) is one of three closely related taxa in the Mytilus edulis complex of blue mussels, which, collectively, are widely distributed in the temperate and cold-water coasts of the Northern Hemisphere and are often dominant organisms on hard substrates of shallow nearshore habitats (Rainbow et al., 1999; Wenne et al., 2016). Generally, M. trossulus has a lifespan of approximately 12 years and grows to 100 mm, yet in the estuarine environment of the Gulf of Gdańsk, it reaches a maximum length of approximately 50 mm (Gofas, 2004; Żmudziński, 1990). Mussels are sessile filter feeders, mainly depending on phytoplankton. They reproduce from late spring to early autumn, depending on the temperature and food abundance (Larsson et al., 2017; Lauringson et al., 2014; Rainbow et al., 2004). The shell of M. trossulus is bimineralic and consists of two calcium carbonate layers: an outer calcite and inner aragonite layer in variable proportions between individuals (Dalbeck, 2008; Piwoni-Piórewicz et al., 2017).
The barnacle Amphibalanus improvisus (Arthopoda, Maxillopoda; Fig. 2e), commonly named the bay barnacle, is a small sessile crustacean that typically exists in shallow coastal zones that are less than 10 m deep. It is widespread around the Atlantic and has been dispersed by shipping to many parts of the world, now having a worldwide distribution. It is a euryhaline and eurythermal species that is absent only from the Arctic and Antarctic seas (Kerckhof, 2002). A. improvisus is a filter feeder that inhabits hard substrates. In the Baltic Sea, the reproduction of barnacles starts in spring with temperatures over 10 ∘C and ends in autumn. The species grows to approximately 10 mm in diameter with a maximum height of approximately 6 mm, and generally, it has a longevity of 1 year (Żmudziński, 1990). It has a conical shell composed of six fused calcite plates (Weidema, 2000).
2.3 Sample collection and preparation
Samples of shells were gathered by a Van Veen grab sampler from four stations: GN (May 2013), MA, M2 and MW (June 2014), located in the Gulf of Gdańsk (Table 1, Fig. 1). One species was found at stations MA (Mya arenaria), M2 (Cerastoderma glaucum) and MW (Limecola balthica), while two species were collected at station GN (Mytilus trossulus, Amphibalanus improvisus; Table 1). To ensure that the samples were not contaminated or modified by solutions of preservatives, the collected material was transported alive in tanks filled with seawater to the laboratory in which sample preparation was performed.
Table 1Details of the sampling locations. Temperature (T) and salinity were measured near the bottom during sample collection.
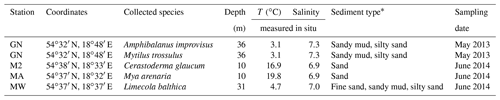
* Based on Uścinowicz (2011).
By measuring the shell heights of clams and shell diameters of barnacles using a calliper with an accuracy of ±1 mm, the shells were classified into four size classes. The division into size classes was performed based on the size reached by each species in the southern Baltic Sea environment (Table 2). Forty shells (10 in each class) were selected for A. improvisus, C. glaucum, M. arenaria and L. balthica. For M. trossulus, 20 shells were selected, with 5 in each class, while the results for the rest were obtained from Piwoni-Piórewicz et al. (2017). After the removal of soft tissues, each shell was viewed under a stereoscopic microscope to check for the presence of epibiotic flora and fauna, which could contaminate the sample and bias the chemical analysis. If any organisms were present on the shell, they were carefully removed. To remove the biofilm, the periostracum was scraped with a scalpel, and pre-cleaned shells were placed in an ultrasonic bath (InterSonic IS-7S) in ultra-pure water for 30 min and then dried at 70 ∘C for 24 h. The shells were then crushed and ground into a fine powder with an agate mortar and pestle. Aliquots (2.8–849 mg, mean of 132 mg) of the powdered samples were weighed using a five-digit analytical balance, placed into a 15 mL plastic tube (Sarstedt™) and dissolved in a mixture of 1.5 mL concentrated nitric acid (HNO3, Sigma-Aldrich®, TraceSELECT for trace analysis), 1.5 mL ultra-pure water and 0.3 mL 30 % hydrogen peroxide (H2O2, Merck® Suprapure grade). After 24 h at 70 ∘C, the liquid samples were diluted to ca. 15 mL by weight with ultra-pure water.
2.4 Elemental analysis
Concentrations of chemical elements in the digested samples were determined at the Natural History Museum, London, using a Thermo iCap 6500 Duo inductively coupled plasma optical emission spectrometer (ICP-OES) for Ca, Na, Sr and Mg and an Agilent 7700x inductively coupled plasma mass spectrometer (ICP-MS) for Mn, Ba, Cu, Pb, V, Y, U and Cd. Calibration of the ICP-OES analysis was performed using solutions containing 1–50 mg L−1 Ca, 0.01–5 mg L−1 Na, and 0.001–0.5 mg L−1 Sr and Mg in 0.8 M HNO3. Na, Mg and Sr calibration solutions all contained Ca at 100 times the Mg concentration, to account for the effects on other wavelengths by high Ca contents in the samples and standards. Multiple wavelengths for each element were recorded, and line selection was performed by accounting for the suitability of the wavelength to the concentrations in the samples and accounting for any potential spectral interferences. The accuracy and reproducibility of the analyses were checked using two calcium-rich certified reference materials (CRMs): JLs-1 Limestone and JDo-1 Dolomite (both from the Geological Survey of Japan). The reference materials were diluted to match the concentrations of Ca in the sample solutions. Ca, Mg and Sr concentrations were found to be within 1 standard deviation (SD) of the reported values (Imai et al., 1996).
The limit of quantification (LOQ) of the ICP-MS analysis was generally determined as the concentration corresponding to 10 times the standard deviation of the signal obtained by analysing 0.8 M HNO3 solution (six to seven times) in each individual run. ICP-MS was run in helium (He) mode (5 mL min−1 He, 99.9995 % purity) for lighter elements (V, Mn, Cu, Y and Cd) to minimize the molecular interference from plasma and solution components and Ca from the samples.
The accuracy and reproducibility were checked by analyses of JLs-1 and JDo-1 before and after every batch of samples. The results obtained for all elements were within ±2.5 SD of the recommended values (Imai et al., 1996). Accuracy of Pb determination could not be checked using these CRMs because of the large spread of reference values probably due to insufficient homogeneity of Pb distribution in these materials. Based on the analyses of CRMs and matrix-matched solutions, the maximum analytical error for the typical range of concentrations in the shells can be estimated (in relative percentage) as 1.5 % for Ca, Mg and Sr; 3 % for Ba; 20 % for Cu and U; and 10 % for all other elements. More details on method validation were reported previously (Piwoni-Piórewicz et al., 2017).
2.5 Statistical analyses
To evaluate the effect of the shell size (ontogenesis stage) on the concentrations of trace elements in calcareous parts of A. improvisus, C. glaucum, M. arenaria, L. balthica and M. trossulus, the concentrations of Ca, Na, Sr, Mg, Mn, Ba, Cu, Pb, V, Y, U and Cd were examined in the four size classes separately for each species. The data were not normally distributed (Shapiro–Wilk test); therefore, significant differences between the mean concentrations of the selected trace elements in the size classes were identified by one-way Kruskal–Wallis nonparametric ANOVA (p value = 0.05) and post hoc Dunn's tests for multiple independent groups. Statistical computing and graphical visualizations were performed in RStudio (R Core Team, 2019).
The concentrations of trace elements in studied individuals can be found in Table S1 in the Supplement. The mean concentrations in all studied organisms decreased, being the highest for Ca and the lowest for Cd, in the following order . However, the concentrations of given elements were different for Cerastoderma glaucum, Mya arenaria, Limecola balthica, Mytilus trossulus and Amphibalanus improvisus, showing high variability both between and within species (Table 3, Fig. 3).
Table 3Elemental concentrations in shells of studied organisms, presented in decreasing order of means for each species (SD represents standard deviation).
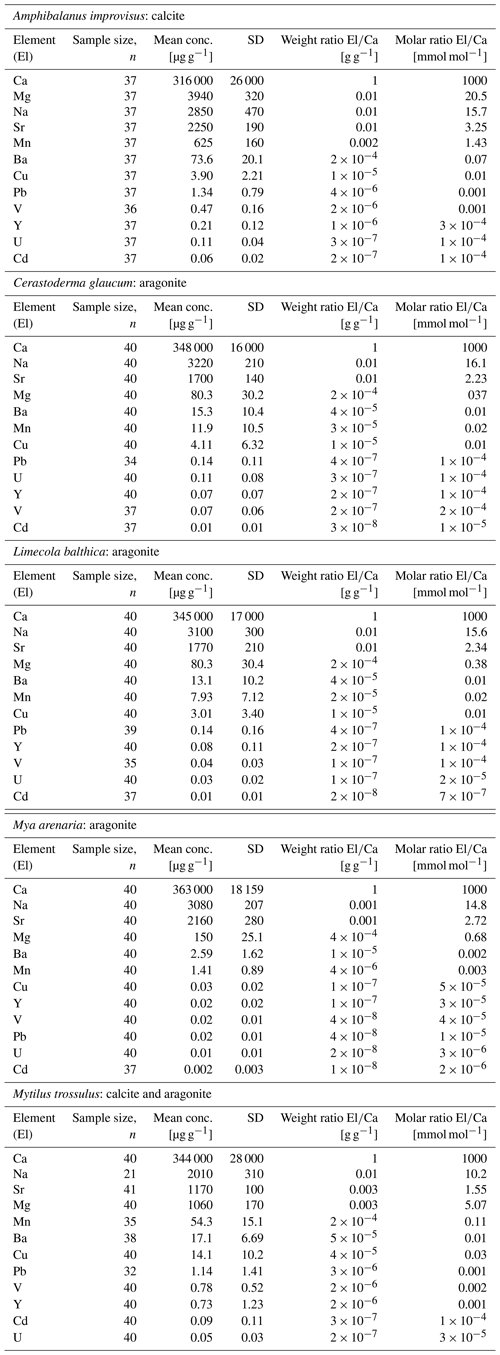
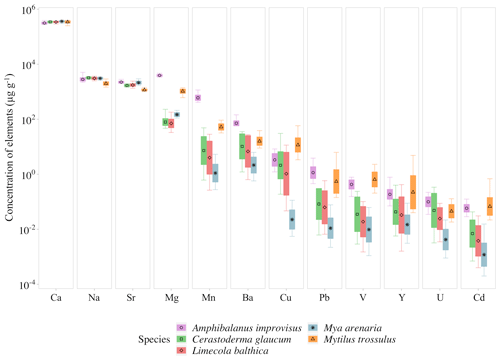
Figure 3Mean elemental concentrations in shells of studied species. The boxplots represent standard deviations (±1 SD) of values, and whiskers indicate the minimum and maximum concentrations for each studied species. Concentrations of elements are presented using a logarithmic scale.
There is a pattern of the highest concentration of Ca in all individuals, which ranges from 316±26 mg g−1 (mean ± 1 SD) in A. improvisus to 363±18 mg g−1 in M. arenaria. The rest of the elements had mean concentrations in shells below 4.0 mg g−1. In mussels, the most concentrated were in the order , while in barnacles the order was . The concentration of Na ranged, on average, from 2.01±0.31 mg g−1 in M. trossulus to 3.22±0.21 mg g−1 in C. glaucum. In A. improvisus, Mg (3.94±0.32 mg g−1) was dominant after Ca, while mean concentration of Sr (2.25±0.19 mg g−1) was lower than Na (2.85±0.47 mg g−1). Clams C. glaucum (Mg: 80.3±30.2 µg g−1, Sr: 1.70±0.14 mg g−1, Na: 3.22±0.21 mg g−1), M. arenaria (Mg: 150±25.1 µg g−1, Sr: 2.16±0.28 mg g−1, Na: 3.08±0.21 mg g−1) and L. balthica (Mg: 80.3±30.4 µg g−1, Sr: 1.77±0.21 mg g−1, Na: 3.10±0.30 mg g−1) had concentrations of Sr over 15 times higher than those of Mg. Mussels M. trossulus were characterized by concentrations of Mg and Sr reaching 1.06±0.17 and 1.17±0.10 mg g−1, respectively. This species also was characterized by a concentration of Na (2.01±0.31 mg g−1) higher than Mg and Sr (Table 3, Fig. 3).
The trace elements (Mn, Ba, Cu, Pb, V, Y, U and Cd) exhibit a general trend of highest concentrations in A. improvisus and M. trossulus, lower in C. glaucum and L. balthica, and the lowest in M. arenaria. Manganese was the most variable element and increased from 1.41±0.89 µg g−1 in M. arenaria to 620±160 µg g−1 in A. improvisus (Table 3, Fig. 3).
The results of the Kruskal–Wallis nonparametric ANOVA test, which was used to compare the element concentrations between the four size classes in each species, revealed the lack of variability within M. arenaria. The smallest variability was found in L. balthica. Only the concentration of Na (H=10.586, p=0.014) decreased with shell growth for this clam. The third clam species, C. glaucum, showed high variability in the four trace elements. Shells of C. glaucum had increased concentrations of Sr (H=14.584, p=0.002), contrary to the decreasing concentration of Na (H=10.529, p=0.015), Mn (H=10.658, p=0.014) and Cd (H=11.655, p=0.009). Similarly, shells of A. improvisus also showed variability in four trace elements between size classes, namely, Mg (H=11.996, p=0.007), V (H=11.206, p=0.011), Cu (H=9.146, p=0.027) and Pb (H=13.308, p=0.004). However, in this case, the sequences of the changes were not straightforward but, rather, had a tendency to fluctuate between the smallest and the largest individuals. The highest variability was found within the bimineralic shells of M. trossulus. The size classes differed in terms of five trace elements. The incorporation of V (H=22.595, p<0.001), Cu (H=26.18, p<0.001), Y (H=10.819, p=0.013), Cd (H=15.353, p=0.002) and U (H=18.202, p<0.001) into shells decreased in larger mussels (Fig. 4).
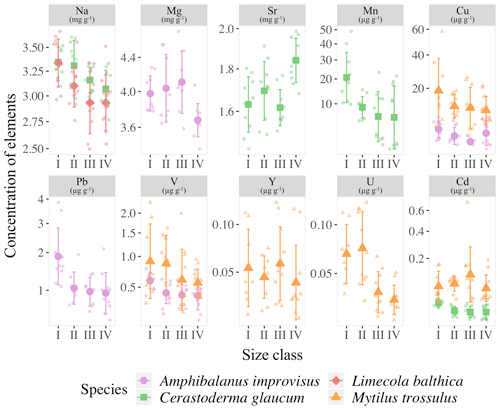
Figure 4Mean concentrations of elements with statistically significant differences between the four size classes (for size class details see Table 2) in the shells of studies species; error bars indicate standard deviations (±1 SD). Concentrations of Mn, Cu, Pb, Cd, V and Y are presented using a square root scale.
Detailed analyses of the differences in the studied elements between the size classes based on post hoc Dunn's tests for multiple independent groups indicated that the significant variations were not linear (Fig. 4). In L. balthica, Na concentration decreased in larger shells, showing differences between the size classes I and III and I and IV. In the shells of C. glaucum, Sr concentration increased gradually, reaching a peak in the size class IV, while statistically significant differences were observed between the size classes I and IV and between III and IV. An inverse pattern was observed for Na concentration, which, however, differed only between the smallest and largest clams. Shells of C. glaucum were also characterized by a common trend of the Mn and Cd, which decreased from size class I to size class II, reaching then a plateau. In shells of A. improvisus, the concentrations of Mg, V, Cu and Pb decreased in larger individuals. The levels of Mg in shells statically differed between the size classes III and IV; likewise, shells from the size class III had the highest concentrations. The elements V and Pb occurred at the highest concentrations in shells of the smallest individuals, and later, they showed no trend in classes II–IV. Copper decreased in growing shells of barnacles, reaching the minimum in the size class III. However, it is worth noting that Cu concentrations in many of the oldest shells are several times higher. Shells of M. trossulus were characterized by the highest variability of trace element concentrations between size classes. The trend of decreasing concentrations was clearly marked for V, Cu, Cd and U. The concentration of Y showed significant differences between the size classes I and III (Fig. 5).
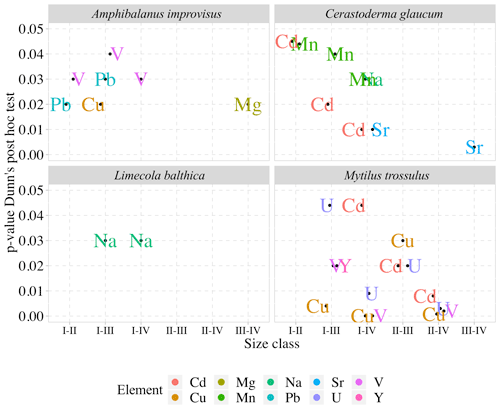
Figure 5Pairwise comparisons of elements in the shells of studied species with statistically significant differences (p<0.05) between size classes (for size class details see Table 2).
The comparison of elemental concentrations in shells of mussels and barnacles from different regions, based on literature data, is presented in Table 4.
Table 4Elemental concentrations in shells from different regions based on this study (in bold) and literature data.
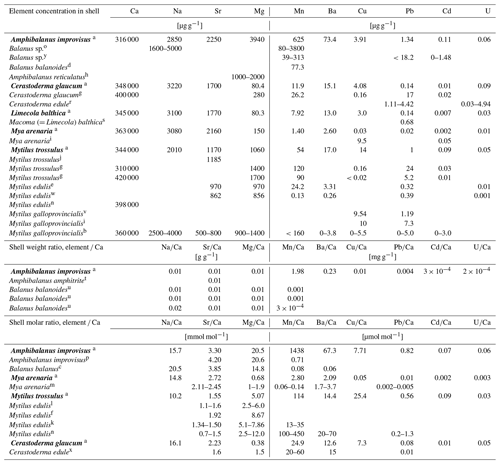
a Baltic Sea, Gulf of Gdańsk (this study); b Adriatic Sea, Croatia (Rončević et al., 2010); c Arctic (Iglikowska et al., 2018); d Atlantic, USA (Blanchard and Chasteen, 1976); e North Sea, Germany (Ponnurangam, 2018); f Baltic Sea, Germany (Heinemann et al., 2008); g Baltic Sea, Gulf of Gdańsk (Szefer and Szefer, 1985); h Bay of Bengal, India (Raman and Kumar, 2011); i Black Sea (Mititelu et al., 2014); j Canada (Klein et al., 1996); k cultured (Freitas et al., 2009); l cultured (Heinemann et al., 2011); m cultured (Strasser et al., 2008); n cultured (Vander Putten et al., 2000); o cultured (Gordon et al., 1970); p Denmark (Ullmann et al., 2018); r England (Price and Pearce, 1997); s Canada, estuary (Thomas and Bendell-Young, 1998); t Hong Kong (Zhang et al., 2015); u Irish Sea, UK (Bourget, 1974); v Spain (Puente et al., 1996); w Portugal (Ponnurangam, 2018); x Portugal (Ricardo et al., 2015); y south-west coast of India (Ashraf et al., 2007).
4.1 CaCO3 polymorph type and elemental concentrations
The average Ca concentration in the skeletons of all species collected in the Baltic Sea was found to be 343 mg g−1 (Table 3), which corresponds to ca. 86 % weight per weight (w∕w) of pure CaCO3. For comparison, same species or congeners from different regions contain between 310 and 420 mg g−1 of Ca in shell (Table 4), with the results above (400 mg g−1) probably being caused by analytical bias.
Among shell impurities, the most concentrated elements were Na, Sr and Mg in all studied CaCO3 polymorphs (Fig. 3). Such compositions are considered to be typical for calcareous skeletons of marine invertebrates, as those elements are energetically favoured in CaCO3 crystal lattice, substituting for Ca (Allison et al., 2001; Iglikowska et al., 2016; Reeder, 1983; Sugawara and Kato, 2000; Wang and Xu, 2001; see also Table 4). The main elemental constituents of the Baltic shells showed generally a uniform distribution in collected samples and were within the ranges typical for a given species. Clams that precipitate fully aragonitic shells and bimineralic mussels were characterized by the following order of accumulated concentrations: . In barnacles, however, this order was different: (Fig. 3). The most evident observed feature was that the aragonitic shells of C. glaucum, M. arenaria and L. balthica contained over 15 times more Sr than Mg. In the calcitic A. improvisus, Mg was 1.8 times more concentrated than Sr, while the bimineralic shells of M. trossulus containing layers of calcite and aragonite were distinguished by equalized concentrations of Mg and Sr in shells (Table 3, Fig. 3). Such a pattern of Mg and Sr is observed in a number of calcareous species including bivalves and barnacles (Table 4; Dalbeck, 2008; Iglikowska et al., 2016; Wang and Xu, 2001; Zhao et al., 2017). However, inter-species comparison showed that the calcite of barnacles A. improvisus deviated from this general trend: both Mg and Sr concentrations were higher in calcitic barnacles than in aragonitic clams (Table 3, Fig. 3). Kinetic and biological effects influence the partitioning of Sr between the shell and seawater (Urey et al., 1951), and Sr in shells is known to significantly exceed its concentration expected at the thermodynamic equilibrium (Schöne et al., 2010). Furthermore, previous studies revealed that barnacles have significantly higher Sr shell concentration (up to 2.3 mg g−1) than other marine invertebrates (Carpenter and Kyger, 1992; Ullmann et al., 2018). The concentration of Sr in shells of A. improvisus from this study (2.25±0.19 mg g−1) supports these findings, suggesting that the barnacle precipitation process differs from other marine organisms. Carpenter and Kyger (1992) concluded that Sr is a sensitive indicator of precipitation rate; this may explain the elevated Sr concentrations in barnacles. The shell increment in barnacles is rapid (15–30 mm per year; Milliman, 1974) due to their relatively short lifespan (approximately 1 year). Consequently, the observed Sr concentration of A. improvisus may be caused by a higher precipitation rate of barnacle calcite in comparison with the precipitation rate of aragonite in long-lived bivalves (Tables 3 and 4, Fig. 3). Crystal lattice distortions in barnacle calcite induced by rapid incorporation of impurities (Pokroy et al., 2006) may allow for even higher Sr incorporation and support the high Sr concentration in A. improvisus shell. This indicates that Mg and Sr concentrations in shell are controlled by CaCO3 lattice properties yet in a strongly species-specific way (Skinner and Elderfield, 2005).
The concentrations of Mn and Ba are several orders of magnitude higher in the calcitic shells of barnacle A. improvisus than in other species (Table 3, Fig. 3). Based on the ionic radii of Mn and Ba, it is expected that aragonite shells would incorporate Ba more intensively than would calcitic shells (Findlater et al., 2014; Gillikin et al., 2006), yet this trend is not observed in this study, indicating that Ba is not clearly related to the crystal lattice orientation. Furthermore, barnacles appear to have a stronger capacity for Mn incorporation into shells than other benthic calcifiers (Pilkey and Harris, 1966), including A. improvisus collected at the same location (GN, Fig. 1), possibly due to the species-specific biological factors (Bourget, 1974). Yet, as both species with highest concentration of Mn produced calcite (Table 3, Fig. 3), we should not rule out that the polymorph type of CaCO3 regulates, to some degree, the level of shell Mn in this low-salinity environment.
It was observed that an increased incorporation of Mg and Sr into shell can contribute to distortion of CaCO3 crystal lattice, which, in turn, results in increased incorporation of trace elements into the shells (Davis et al., 2000; Dalbeck, 2008). In our study, shell lattices of mussels and barnacles could be affected by the concentrations of Mg and Sr higher than those in clams. Therefore, the concentrations of Cu, V, Cd, Y and U seem to be driven to some degree by crystal lattice properties having the highest values in calcite-containing shells (Fig. 3). It is also important to note that the smaller ionic radii of V, Cd, Y, U and Cu are energetically favoured in calcite, while the larger Pb radius is favoured in aragonite structure (Morse et al., 1997; Reeder, 1983; Wang and Xu, 2001). However, trace elements Cu, V, Cd and Y were present at the highest levels in bimineralic M. trossulus, while only U, as well as Pb, was present in calcitic A. improvisus (Table 3), which suggests that additional factors, other than the crystal lattice effects, determine those concentrations. Furthermore, this study likewise revealed inconsistent variability of trace element concentrations between aragonitic clams. Out of the three species of clams, M. arenaria was characterized by the lowest concentration of all trace elements (Table 3). This also indicates that factors other than mineral properties co-regulate trace element accumulation in shells.
This study considers element concentrations in shells that were not subjected to chemical removal of organic matter prior to the dissolution of the carbonate matrix. Chemical cleaning of carbonate skeletons prior to chemical analysis and both improvement of the data quality and potential artefacts associated with this are widely discussed in the literature (Barker et al., 2003; Holcomb et al., 2015; Loxton et al., 2017), but a plausible pre-treatment method for the removal of organics still needs to be found (Inoue et al., 2004). Mannella et al. (2020) showed that the suitability of chemical pre-treatments for organic matter removal from carbonate matrices should be evaluated on a case-by-case basis and, in case of relatively low organic content, should be avoided. In addition to CaCO3 crystal lattice, shells of barnacles and bivalves usually contain up to 5 % of organic matter (Bourget, 1987; Wolowicz and Goulletquer, 1999; Marin and Luquet, 2004; Rueda and Smaal, 2004). However, the specific features and composition of a particular organic matrix might result from the inter-species and inter-individual variability of elemental concentration in shells (Fig. 3, Table 3; Takesue and van Geen, 2004). The organic fraction was found to be generally not associated with significant levels of trace elements (Lingard et al., 1992; Takesue et al., 2008), which are strongly incorporated into the crystal phase. Yet, many authors found correlations, especially for Mg and Mn, associated with the shell organic matrix (Bourget, 1974; Walls et al., 1977; Lorens et al., 1980; Rosenberg et al., 2001; Takesue and van Geen, 2004). These elements are biologically essential (Bellotto and Miekeley, 2007) and inter-species variability of Mg and Mn between barnacles, mussels and clams (Fig. 3) could be enhanced by specific properties of organic phases. Furthermore, trace elements in shells may be present in microscopic aqueous fluid inclusions that were trapped within crystals during their growth from solution (Gaffery, 1998). Lécuyer and O'Neil (1994) found that such inclusion waters constitute up to 2 % of the shell and probably represent the remnants of metabolic fluids produced by the mantle epithelium. Therefore, their composition most likely results from the specific biological features of an organism rather than from the structural properties of calcium carbonate.
4.2 Size classes and potential biological impact on elemental concentrations
The recorded concentrations of trace elements in all populations exhibited marked inter-individual variability (Table 3, Fig. 3), which is a feature previously recorded by several authors (Gillikin et al., 2005a; Vander Putten et al., 2000). In this study, individuals were collected over a wide range of sizes (Table 2), representing different ages and lifespans. Bivalves are long-living organisms, and those of the Gulf of Gdańsk have a life expectancy of 4–12 years (Gofas, 2004; Żmudziński, 1990), contrary to barnacles with the relatively short lifespan of approximately 1 year (Bornhold and Milliman, 1973). The southern Baltic Sea is driven by cyclical environmental dynamics, which evoke physiological stress, determine the food base and drive its biogeochemical cycles (Elder and Collins, 1991). Shells of A. improvisus experienced 1-year variability of environmental factors, while bivalves represent long-term variability. Thus, the lifespan may explain, to some extent, the lowest variability of trace elements in barnacles (Fig. 3). However, this relationship is not noticeable in mussels, for which the inter-individual variability of trace elements in the youngest individuals (size class I) was not lower than in the oldest ones (size class IV, Fig. 4). Thébault et al. (2009) revealed low inter-individual elemental variability in bivalves and on this basis indicated the environment as a factor controlling their incorporation within shells. Therefore, the variability in elemental concentrations between individuals from the brackish Gulf of Gdańsk may to some extent be caused by biological factors, which could lead to a deviation from what is expected with purely environmental control. The biological influence on the shell chemistry in the southern Baltic Sea could be reinforced by unfavourable conditions for calcification. The low salinity (∼7) and alkalinity, which is typical for the studied area of the Gulf of Gdańsk, cause a reduced CaCO3 saturation state (Beldowski et al., 2010; Cai et al., 2010; Findlay et al., 2008). Ions of Ca2+ and CO are essential components for the crystal formation and, when their concentrations in seawater are low, calcifying organisms exert selective Ca2+ channels to enable an active ion capture from solution (Sather and McCleskey, 2003). The required higher contribution of Ca2+ active pumping results in a greater degree of biological control over the calcification process (Sather and McCleskey, 2003; Waldbusser et al., 2016), and shells are not produced in equilibrium with environmental conditions when it comes to an elemental concentration.
Within species, organisms from juveniles to adults experience morphological and functional changes related to sex, metabolic rate or reproductive stage, which complicate the biomineralization process (Carré et al., 2006; Freitas et al., 2006; Gillikin et al., 2005b; Schöne et al., 2010, 2011; Warter et al., 2018). The size-related elemental patterns in shells of A. improvisus, C. glaucum, M. arenaria, L. balthica and M. trossulus from the Gulf of Gdańsk indicate that if a significant variability exists, it is specifically expressed in trace element concentrations. The studied mussel M. trossulus and barnacle A. improvisus showed the greatest variability between size classes, while the size class effects were less pronounced in clams (Fig. 4). However, the variability of trace elements was not uniform for M. trossulus and A. improvisus, even though the organisms came from the same location (Table 1). The size-related trend was observed for V, Cu, Y, Cd and U in molluscs and Mg, V, Cu and Pb in barnacles (Fig. 3). Among clams, we found a lack of size-dependent changes within M. arenaria. In L. balthica only the concentration of Na decreased with shell growth, while C. glaucum showed variability of Sr, Na, Mn and Cd (Fig. 4). Species-specific patterns of elemental accumulation within the same habitat were observed before (Rainbow, 2002, 1995). Rainbow et al. (2000) tested the potential of A. improvisus and M. trossulus from the Gulf of Gdańsk to be used as environmental biomonitors by measuring the concentrations of Co, Zn, Fe, Cd, Pb, Mn and Ni in soft tissues. They found that mussels and barnacles occurring at the same location did not show the same variation in elemental bioavailabilities, probably because barnacles were particularly strong accumulators of trace elements (Rainbow, 1998, 2002). This shows that biological differences between species, such as growth rate, feeding rate, assimilation efficiency (Luoma and Rainbow, 2005), and route and degrees of element uptake (Rainbow and Wang, 2001) are significant factors determining the elemental accumulation in shells.
In this study, it was generally observed that, when statistical differences between size classes were recorded, the concentrations of trace elements decreased with the shell size. The reverse was found only for Sr in the shells of C. glaucum, in which the concentration of Sr increased with size (Figs. 4 and 5). Large mussels pump less water per unit body weight, and their uptake of trace elements is lower than that in smaller individuals. When the concentrations of trace elements decrease with increasing shell size (Fig. 4), the incorporation might depend on the growth rate. The younger specimens could have a greater growth rate and shell precipitation rate, resulting in a greater uptake of trace elements (Dalbeck, 2008; Szefer et al., 2002). Rosenberg and Hughes (1991) suggested that areas of higher shell curvature, such as the umbo, require greater metabolic expenditure, resulting in an increase in element uptake. When the metabolic activity of an organism decreases, the ionic flux likewise decreases, increasing the tendency of Ca2+ to block other ion fluxes (Carré et al., 2006; Friel and Tsien, 1989). Therefore, in a low-salinity environment of the Gulf of Gdańsk, metabolic fluctuations of organisms can have an exceptionally strong effect on elemental variability, which was high among studied individuals (Table 3, Fig. 3). The surface-to-volume ratio decreases with size and affects the contribution of the adsorbed element content to the bulk concentration (Azizi et al., 2018). Therefore, the negative correlation between the bulk elemental concentration and the shell size (Fig. 4), noted in some previous studies (Martincic et al., 1992; Piwoni-Piórewicz et al., 2017; Ritz et al., 1982), could have been caused by a greater potential of surface adsorption in smaller individuals. This is most pronounced for some trace elements, the concentrations of which decreased across the four size classes in shells of A. improvisus (V, Cu and Pb), M. trossulus (V, Cu, Y, Cd and U) and C. glaucum (Mn and Cd) (Figs. 4 and 5). Catsiki et al. (1994) suggested that, apart from metabolic processes, an active detoxification mechanism in tissues is responsible for this trend, and its efficiency is higher in older and larger individuals.
Nevertheless, many of the trace elements studied herein showed a lack of statistically significant relationships with the shell size. Some trace elements in the shells of A. improvisus (Y, U and Cd), M. trossulus (Pb), and C. glaucum (V, Cu, Y, Pb and U) and all trace elements in the shells of L. balthica and M. arenaria (V, Cu, Y, Pb, U and Cd) showed no significant variability related to the size of organisms (Figs. 4 and 5). This is not an unusual pattern for marine invertebrates and has been shown by a number of studies. Saavedra et al. (2004) observed no differences between Cd, Pb, Cr, Ni, As, Cu or Zn concentrations for different shell lengths of the raft Mytilus galloprovincialis separated into four size classes. Protasowicki et al. (2008) similarly found that the concentrations of Hg, Pb, Cd, Cu, Zn, Cr, Ni, Fe, Mn, V, Li or Al in the shells of the mussel Mytilus edulis from the Polish coast of the Baltic Sea did not vary between shell sizes. This inconsistent relationship shows that trace element concentrations in shells of different sizes might be under the influence of a number of factors including species-specific biological mechanisms (e.g. metabolic rate).
4.3 Environmental factors and elemental concentrations
Organisms derive trace elements in dissolved and particulate forms primarily from surrounding water, sediments and their food base (Freitas et al., 2006; Gillikin et al., 2005a; Poulain et al., 2015). The concentration of major constituents Na, Mg and Sr in surrounding seawater is generally proportional to salinity (Beldowski et al., 2010; Cai et al., 2010; Findlater et al., 2014; Wit et al., 2013); therefore, the environmental level of these elements should be rather homogenous in the study area (salinity range 6.9–7.3, Table 1). Indeed, our analysis has shown low variability of the concentrations of Na (from 2.01±0.31 to 3.22±0.21 mg g−1) and Sr (from 1.17±0.10 to 2.25±0.19 mg g−1; Table 3) in the shells of studied species, which might reflect environmental stability. However, the concentration of Mg in shells was highly variable (from 80.3±30.2 µg g−1 to 3.94±0.32 mg g−1; Table 3, Fig. 3). Although salinity was indicated as the factor controlling the concentration of Na, Sr and Mg in the environment and skeletons built there (Elderfield and Ganssen, 2000; Rosenheim et al., 2004), the range of surface salinity in the study region was unlikely to explain the observed range of Mg concentrations. Magnesium is the most energetically preferred skeletal element (Allison et al., 2001; Wang and Xu, 2001), is associated with the shell organic matrix (Bourget, 1974) and its concentrations have been found to depend on seawater temperature (Freitas et al., 2005; Dalbeck, 2008; Schöne et al., 2011). Vander Putten at al. (2000) observed that the skeletal Mg∕Ca variations in M. edulis could not be interpreted based solely on variations in the seawater Mg, while Dodd and Crisp (1982) showed that the Mg∕Ca ratios of most estuarine waters only differ significantly from the open-ocean ratios at salinities below 10. Therefore, the variations in the shell Mg concentration must be the combination of many biological and environmental factors.
Among trace elements (Mn, Ba, Cu, Pb, V, Y, U and Cd), Mn and Ba were definitely the most concentrated in shells (Fig. 3), as previously reported for bivalves (Lazareth et al., 2003; Vander Putten et al., 2000). Such high concentrations of Mn and Ba are usually associated with freshwater inputs to estuarine systems, which likewise causes phytoplankton blooms (Gillikin et al., 2006; Vander Putten et al., 2000; Thébault et al., 2009). Consequently, Ba and Mn may be taken up by calcifiers through ingestion of phytoplankton (Stecher et al., 1996) or decaying algal flocs (Brannon and Rao, 1979; Stecher et al., 1996). The elemental incorporation into shell has been shown to correlate at species level with changes in primary production and phytoplankton blooms (Freitas et al., 2006; Lazareth et al., 2003; Vander Putten et al., 2000). Therefore, the food base and elemental transport in the trophic chain might cause the spatial and species-specific elemental variability in shells (Fig. 3). This pattern is generally confirmed in different regions by the contents of Mn and Ba relative to other trace elements (Fig. 4).
Seawater trace element concentrations in the study area depend on seasonality and human activity; however, elemental concentrations in sediments represent long-term chemical background. In coastal regions, trace elements discharged from various sources, such as the atmosphere, rivers and plankton blooms, can be rapidly transported from the water column to the bottom sediments (Bendell-Young et al., 2002; Szefer et al., 1995). In this study, samples were collected in two regions of the Gulf of Gdańsk: the outer part of Puck Bay (stations MA, M2 and MW) and central part of the Gulf of Gdańsk (station GN, Fig. 1). The shells of clams collected in the outer Puck Bay, with sandy sediment (Szefer et al., 1998; Szefer and Grembecka, 2009; Uścinowicz, 2011), were depleted in trace elements. On the other hand, mussels and barnacles from the central Gulf of Gdańsk, with sandy mud, were characterized by higher elemental concentrations than clams (Fig. 3). On this basis, we can assume that the concentrations of trace elements Mn, Ba, Cu, Pb, V, Y, U and Cd in shells might be related to sediment granulometry and increase from sandy to silty types (Góral et al., 2009; Uścinowicz, 2011). The maximum concentration of trace elements within a given region is commonly associated with the finest sediment fraction (<2 µm) as compared to the sandy fraction (Kim et al., 2004; Szefer et al., 1998). In addition, the formation of Mn-Fe oxyhydroxides in the surface sediments (Glasby and Szefer, 1998; Szefer et al., 2002) has a particular role in absorbing trace elements in the fine sediments (Pruysers et al., 1991; Szefer et al., 1995; Tessier et al., 1979). It has been previously shown that for mussel M. trossulus, clam L. balthica and barnacle A. improvisus from the central part of the Gulf of Gdańsk (near the GN station), elemental concentrations in shells (Szefer and Szefer, 1985) and tissues (Rainbow et al., 2000, 2004; Sokolowski et al., 2001) were similar to that in the Vistula river plume and higher than trace element concentrations in macrozoobenthos from the outer Puck Bay.
The studied trace elements exhibited variations in accumulated concentrations between species, both within Puck Bay (M2: C. glaucum, MA: M. arenaria, MW: L. balthica) and within the central Gulf of Gdańsk (GN station: M. trossulus. and A. improvisus, Fig. 3). The highest concentration of trace elements in C. glaucum (M2: 10 m) and L. balthica (MW: 31 m) within clams from the sandy Puck Bay could be driven by elevated amounts of trace elements in oxygenated zones where Fe-Mn oxyhydroxides accumulate. At the Mn(II)∕Mn(IV) redox interface manganese oxides may predominantly precipitate on the periostracum of molluscs comparing to inorganic surfaces (Strekopytov et al., 2005), which may, in turn, influence the incorporation of trace elements into shells. M. arenaria likewise was collected in a shallow zone (MA: 10 m), but had, nevertheless, the lowest concentrations of all studied trace elements (Fig. 3). This bivalve mainly spends life buried 20–30 cm in a sediment (Żmudziński, 1990). Szefer et al. (1998) reported that in the Gulf of Gdańsk the enrichment factors for Cu, Zn, Ag, Cd and Pb are highest in the <2 µm fraction and decrease with increasing both fraction size and depth of the sediment. This may by a reason why C. glaucum and L. balthica have similar patterns of elemental accumulation, while M. arenaria was characterized by the lowest values (Fig. 3). Such dependence indicates that the sediment properties is one of the factors controlling concentration of trace elements in the shells.
Mussels M. trossulus and barnacles A. improvisus from the same location (Fig. 1, Table 1) showed greater chemical differentiation than clams. This relationship is most evident for Mn and Ba. Much lower concentrations of Mn and Ba were found in the shells of molluscs (Mn: 54.3±15.1 µg g−1, Ba: 17.1±6.69 µg g−1) than in barnacles (Mn: 625±160 µg g−1, Ba: 73.6±20.1 µg g−1, Table 3). A similar relationship was found in the soft tissues of M. trossulus and A. improvisus collected from different locations in the Gulf of Gdańsk in May 1998 (Rainbow et al., 2000). The range of Mn in the soft tissues of M. trossulus varied from 19.0 to 41.0 µg g−1, while that in A. improvisus ranged from 187 to 307 µg g−1 and was interpreted as species-specific accumulation efficiency. The concentration of Mn has been repeatedly reported to be influenced by phytoplankton blooms (Vander Putten et al., 2000; Gillikin et al., 2006; Zhao et al., 2017); thus, diet seems to affect the shell Mn concentration. Due to the size of the organism, the diet of A. improvisus more strongly depends on Mn-rich phytoplankton than that of larger M. trossulus that easily picks zooplankton. The lower proportion of zooplankton in the barnacle diet could hereby add to a higher Mn concentration in their shells (Fig. 3). This suggests that the Mn concentration in shell is highly species dependent.
Some studies have investigated trace element concentrations in shells (but many more have dealt with the soft tissues only) of marine invertebrates as a tool to assess trace elements contamination of the aquatic environment. The concentrations of trace elements Mn, Ba, Cu, Pb, Cd and U in the shells of a studied organism in Gulf of Gdańsk and in other regions were found to be highly variable, even within a single taxon (Table 4). Therefore, the environmental conditions prevailing during biomineralization are largely reflected in the trace element concentrations of the shells, although their interpretation requires consideration of species-specific biological factors.
The shells of calcitic Amphibalanus improvisus; aragonitic Cerastoderma glaucum, Limecola balthica, and Mya arenaria; and bimineralic Mytilus trossulus from the Gulf of Gdańsk are accumulators of a wide spectrum of trace elements from the surrounding environment. The elemental concentration levels in studied species are not determined solely by their bioavailability in the environment. Many biotic and abiotic factors are acting on the shell incorporation mechanism, and their effect is likely to be species specific.
By determining Ca, Na, Sr, Mg, Mn, Ba, Cu, Pb, V, Y, U and Cd in the shells of a given species, we found some patterns of elemental accumulation. At a local scale of the Gulf of Gdańsk, the main elements Na, Sr and Mg are mostly dependent on crystal lattice properties of calcite and aragonite. Clams that precipitate fully aragonitic shells have a clear preference for accumulating Sr over Mg in shells, contrary to dominant Mg content over Sr in barnacle shell calcite. It is energetically more favourable for larger cations such as Na and Sr to enter the aragonite lattice while smaller cations (e.g. Mg) favour calcite. However, this relationship breaks down when comparing shells of organisms belonging to different taxonomic groups. For example, the barnacle calcite contains higher Sr concentration than the bivalve aragonite. The level of main elements, especially Sr and Mg, seems to be determined by specific biological factors, such as growth rate.
In the case of trace elements Mn, Ba, Cu, Pb, V, Y, U and Cd, factors other than the given crystal lattice presence seem to determine their concentrations. The elemental variability between size-grouped shells indicates that trace elements were more variable than Na, Sr and Mg but this varies between species. Moreover, there is a trend for the elemental concentrations being lower in larger shells than in smaller shells. Biological differences between and within species, such as feeding (including its rate and assimilation efficiency) related to age of organisms (size of the shell), are potentially important factors determining the elemental accumulation in shells.
Given that the specimens were obtained from two different regions of the Gulf of Gdańsk (the outer part of Puck Bay and the central part of the Gulf of Gdańsk), an impact of location-specific environmental factors, such as the sediment type and the food base, on the concentration of trace elements in shells cannot be excluded.
The underlying research data can be accessed at the Institute of Oceanology, Polish Academy of Sciences, Powstańców Warszawy 55, 81-712, Sopot, Poland.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-707-2021-supplement.
APP and PK designed and led the study. StS and EHW conducted elemental analysis. APP prepared the original draft, which was reviewed and edited by all co-authors.
The authors declare that they have no conflict of interest.
We would like to thank Halina Kendzierska from the University of Gdańsk for cooperation during sampling and Piotr Bałazy, Radosław Brzana and Jerzy Abramowicz for photos of the studied species presented in Fig. 2.
The research leading to these results received funding from the Polish National Science Centre in the frame of project contracts LOGGER/2017/25/N/ST10/02305 and PANIC/2016/23/B/ST10/01936.
This paper was edited by Lennart de Nooijer and reviewed by two anonymous referees.
Allison, N., Finch, A. A., Sutton, S. R., and Newville, M.: Strontium heterogeneity and speciation in coral aragonite: implications for the strontium paleothermometer, Geochim. Cosmochim. Ac., 65, 2669–2676, https://doi.org/10.1016/S0016-7037(01)00628-7, 2001.
Ashraf, M. P., Meenakumari, B., and Thomas, S. N.: Seasonal variation of metal concentration in barnacles (Balanus spp.) of Cochin estuary, south-west coast of India, Fish. Technol., 44, 73–84, 2007.
Azizi, G., Akodad, M., Baghour, M., Layachi, M., and Moumen, A.: The use of Mytilus spp. mussels as bioindicators of heavy metal pollution in the coastal environment, a review, J. Mater. Environ. Sci., 9, 1170–1181, 2018.
Balthasar, U. and Cusack, M.: Aragonite-calcite seas – quantifying the gray area, Geology, 43, 99–102, https://doi.org/10.1130/G36293.1, 2015.
Barker, S., Greaves, M., and Elderfield, H.: A study of cleaning procedures used for foraminiferal Mg∕Ca paleothermometry, Geochem. Geophy. Geosys., 4, 8407, https://doi.org/10.1029/2003GC000559, 2003.
Blanchard, S. C. and Chasteen, N. D.: Determination of manganese (II) in powdered barnacle shells by electron paramagnetic resonance, Anal. Chim. Acta, 82, 113–119, https://doi.org/10.1016/S0003-2670(01)82209-1, 1976.
Behrends, B., Hertweck, G., Liebezeit, G., and Goodfriend, G.: Earliest Holocene occurrence of the soft-shell clam, Mya arenaria, in the Greifswalder Bodden, southern Baltic, Mar. Geol., 216, 79–82, https://doi.org/10.1016/j.margeo.2005.01.002, 2005.
Beldowski, J., Löffler, A., Schneider, B., and Joensuu, L.: Distribution and biogeochemical control of total CO2 and total alkalinity in the Baltic Sea, J. Mar. Syst., 81, 252–259, https://doi.org/10.1016/j.jmarsys.2009.12.020, 2010.
Bellotto, V. R. and Miekeley, N.: Trace metals in mussel shells and corresponding soft tissue samples: a validation experiment for the use of Perna perna shells in pollution monitoring, Anal. Bioanal. Chem., 389, 769–776, https://doi.org/10.1007/s00216-007-1420-y, 2007.
Bendell-Young, L. I., Thomas, C. A., and Stecko, J. P.: Contrasting the geochemistry of oxic sediments across ecosystems: a synthesis, Appl. Geochem., 17, 1563–1582, 2002.
Bentov, S. and Erez, J.: Novel observations on biomineralization processes in foraminifera and implications for Mg∕Ca ratio in the shells, Geology, 33, 841, https://doi.org/10.1130/G21800.1, 2005.
Blackmore, G. and Wang, W. X.: Uptake and efflux of Cd and Zn by the green mussel Perna viridis after metal preexposure, Environ. Sci. Technol., 36, 989–995, https://doi.org/10.1021/es0155534, 2002.
Bornhold, B. D. and Milliman, J. D.: Generic and environmental control of carbonate mineralogy in serpulid (Polychaete) tubes, J. Geol., 81, 363–373, https://doi.org/10.1086/627876, 1973.
Bourget, E.: Barnacle shells: composition, structure and growth, in: Barnacle Biology, edited by: Southward, A. J. and Balkema, A. A., Rotterdam, the Netherlands, https://doi.org/10.1201/9781315138053-14, 267–285, 1987.
Bourget, F.: Environmental and structural control of trace elements in barnacle shells, Mar. Biol., 28, 27–36, https://doi.org/10.1007/BF00389114, 1974.
Brannon, A. C. and Rao, K. R.: Barium, strontium and calcium levels in the exoskeleton, hepatopancreas and abdominal muscle of the grass shrimp, Palaemonetes pugio: relation to molting and exposure to barite, Comp. Biochem. Phys. A, 63, 261–274, https://doi.org/10.1016/0300-9629(79)90158-0, 1979.
Bulnheim, H. P. and Gosling, E.: Population genetic structure of mussels from the Baltic Sea, Helgoländer Meeresunters., 42, 113–129, https://doi.org/10.1007/BF02364207, 1988.
Cai, W. J., Hu, X., Huang, W. J., Jiang, L. Q., Wang, Y., Peng, T. H., and Zhang, X.: Alkalinity distribution in the western North Atlantic Ocean margins, J. Geophys. Res., 115, C08014, https://doi.org/10.1029/2009JC005482, 2010.
Carpenter, J. and Kyger, C.: Sr∕Mg ratios of modern marine calcite: empirical indicators of ocean chemistry and precipitation rate, Geochim. Cosmochim. Ac., 56, 1837–1849, https://doi.org/10.1016/0016-7037(92)90314-9, 1992.
Carré, M., Bentaleb, I., Bruguier, O., Ordinola, E., Barrett, N. T., and Fontugne, M.: Calcification rate influence on trace element concentrations in aragonitic bivalve shells: evidences and mechanisms, Geochim. Cosmochim. Acta, 70, 4906–4920, https://doi.org/10.1016/j.gca.2006.07.019, 2006.
Catsiki, V. A., Katsilieri, C., and Gialamas, V.: Chromium distribution in benthic species from a gulf receiving tannery wastes (Gulf of Geras – Lesbos island, Greece), Sci. Total Environ., 145, 173–185, https://doi.org/10.1016/0048-9697(94)90308-5, 1994.
Cohen, A. L. and McConnaughey, T. A.: Geochemical perspectives on coral mineralization, Rev. Mineral. Geochem., 354, 151–187, https://doi.org/10.2113/0540151, 2003.
Cubadda, F., Superiore, I., Conti, M. E., Cubadda, F., Enrique, M., and Campanella, L.: Size-dependent concentrations of trace metals in four Mediterranean gastropods, Chemosphere, 45, 561–569, https://doi.org/10.1016/S0045-6535(01)00013-3, 2001.
Cusack, M. and Freer, A.: Biomineralization: elemental and organic influence in carbonate systems, Chem. Rev., 108, 4433–4454, https://doi.org/10.1021/cr078270o, 2008.
Cyberski, J., Grześ, M., Gurty-Korycka, M., Nachlik, E., and Kundziewicz, W. W.: History of floods on the river Vistula, Hydrol. Sci. J., 51, 799–817, https://doi.org/10.1623/hysj.51.5.799, 2006.
Dalbeck, P.: Crystallography, Stable Isotope and Trace Element Analysis of Mytilus edulis Shells in the Context of Ontogeny, PhD thesis, University of Glasgow, UK, 235 pp., 2008.
Davis, K. J., Dove, P. M., and De Yoreo, J. J.: The role of Mg2+ as an impurity in calcite growth, Science, 290, 1134–1138, https://doi.org/10.1126/science.290.5494.1134, 2000.
de Boer, R. B.: Stability of Mg-Ca carbonates, Geochim. Cosmochim. Ac., 41, 265–270, https://doi.org/10.1016/0016-7037(77)90234-4, 1977.
De Choudens-Sanchez, V. and Gonzalez, L. A.: Calcite and aragonite precipitation under controlled instantaneous supersaturation: elucidating the role of CaCO3 saturation state and Mg∕Ca ratio on calcium carbonate polymorphism, J. Sediment. Res., 79, 363–376, https://doi.org/10.2110/jsr.2009.043, 2009.
De Nooijer, L. J., Spero, H. J., Erez, J., Bijma, J., and Reichart, G. J.: Biomineralization in perforate foraminifera, Earth-Sci. Rev., 135, 48–58, https://doi.org/10.1016/j.earscirev.2014.03.013, 2014.
Dickson, J. A. D.: Echinoderm skeletal preservation; calcite-aragonite seas and the Mg∕Ca ratio of Phanerozoic oceans, J. Sediment. Res., 74, 355–365, https://doi.org/10.1306/112203740355, 2004.
Dodd, J. R. and Crisp, E. L.: Non-linear variation with salinity of Sr∕Ca and Mg∕Ca ratios in water and aragonitic bivalve shells and implications for paleosalinity studies, Paleogeogr. Paleocl., 38, 45–56, 1982.
Dove, P. M.: The rise of skeletal biominerals, Elements, 6, 37–42, https://doi.org/10.2113/gselements.6.1.37, 2010.
Elder, J. F. and Collins, J.: Freshwater molluscs as indicators of bioavailability and toxicity of metals in surface-water systems, in: Reviews of Environmental Contamination and Toxicology, vol. 122, edited by: Ware, G. W., Springer, New York, USA, 37–69, https://doi.org/10.1007/978-1-4612-3198-1_2, 1991.
Elderfield, H. and Ganssen, G.: Past temperature and δ18O of surface ocean waters inferred from foraminiferal Mg∕Ca ratios, Nature, 405, 442–445, https://doi.org/10.1038/35013033, 2000.
Findlater, G., Shelton, A., Rolin, T., and Andrews, J.: Sodium and strontium in mollusc shells: preservation, palaeosalinity and palaeotemperature of the Middle Pleistocene of eastern England, P. Geologist. Assoc., 125, 14–19, https://doi.org/10.1016/j.pgeola.2013.10.005, 2014.
Findlay, H. S., Tyrrell, T., Bellerby, R. G. J., Merico, A., and Skjelvan, I.: Carbon and nutrient mixed layer dynamics in the Norwegian Sea, Biogeosciences, 5, 1395–1410, https://doi.org/10.5194/bg-5-1395-2008, 2008.
Freitas, P., Clarke, L. J., Kennedy, H., Richardson, C., and Abrantes, F.: Mg/Ca, Sr/Ca, and stable-isotope (δ18O and δ13C) ratio profiles from the fan mussel Pinna nobilis: seasonal records and temperature relationships, Geochem. Geophy. Geosy., 6, Q04D14, https://doi.org/10.1029/2004GC000872, 2005.
Freitas, P. S., Clarke, L. J., Kennedy, H., Richardson, C. A., and Abrantes, F.: Environmental and biological controls on elemental (Mg∕Ca, Sr∕Ca and Mn∕Ca) ratios in shells of the king scallop Pecten maximus, Geochim. Cosmochim. Ac., 70, 5119–5133, https://doi.org/10.1016/j.gca.2006.07.029, 2006.
Friel, D. D. and Tsien, R. W.: Voltage-gated calcium channels: direct observation of the anomalous mole fraction effect at the single-channel level, P. Natl. Acad. Sci. USA, 86, 5207–5211, https://doi.org/10.1073/pnas.86.13.5207, 1989.
Fritioff, A., Kautsky, L., and Greger, M.: Influence of temperature and salinity on heavy metal uptake by submersed plants, Environ. Pollut., 133, 265–274, https://doi.org/10.1016/j.envpol.2004.05.036, 2005.
Gaffey, S. J.: Water in skeletal carbonates, J. Sediment. Res., 58, 397–414, 1998.
Gillikin, D. P.: Geochemisry of Marine Bivalve Shells: the Potential for Paleoenvironmental Reconstruction, PhD thesis, Vrije Universiteit Brussel, Belgium, 258 pp., 2005.
Gillikin, D. P., Dehairs, F., Baeyens, W., Navez, J., Lorrain, A., and Andre, L.: Inter- and intra-annual variations of Pb∕Ca ratios in clam shells (Mercenaria mercenaria): a record of anthropogenic lead pollution?, Mar. Pollut. Bull., 50, 1530–1540, https://doi.org/10.1016/j.marpolbul.2005.06.020, 2005a.
Gillikin, D. P., Lorrain, A., Navez, J., Taylor, J. W., Andre, L., Keppens, E., Baeyens, W., and Dehairs, F.: Strong biological controls on Sr∕Ca ratios in aragonitic marine bivalve shells, Geochem. Geophy. Geosy., 6, Q05009, https://doi.org/10.1029/2004GC000874, 2005b.
Gillikin, D. P., Dehairs, F., Lorrain, A., Steenmans, D., Baeyens, W., and André, L.: Barium uptake into the shells of the common mussel (Mytilus edulis) and the potential for estuarine paleo-chemistry reconstruction, Geochim. Cosmochim. Ac., 70, 395–407, https://doi.org/10.1016/j.gca.2005.09.015, 2006.
Glasby, G. P. and Szefer, P.: Marine pollution in Gdansk Bay, Puck Bay and the Vistula Lagoon, Poland: an overview, Sci. Total Environ., 212, 49–57, https://doi.org/10.1016/S0048-9697(97)00333-1, 1998.
Glasby, G. P., Szefer, P., Geldon, J., and Warzocha, J.: Heavy-metal pollution of sediments from Szczecin Lagoon and the Gdansk Basin, Poland, Sci. Total Environ., 330, 249–269, https://doi.org/10.1016/j.scitotenv.2004.04.004, 2004.
Gofas, S.: Mytilus trossulus Gould, 1850, MolluscaBase, available at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=140482 (last access: 26 January 2021), 2004.
Góral, M., Szefer, P., Ciesielski, T., and Warzocha, J.: Distribution and relationships of trace metals in the isopod Saduria entomon and adjacent bottom sediments in the southern Baltic, J. Environ. Monit., 11, 1875–1882, https://doi.org/10.1039/b900366e, 2009.
Gordon, C., Carr, R., and Larson, R.: Influence of environmental factors on the sodium and manganese content of barnacle shells, Limnol. Oceanogr., 15, 461–466, 1970.
Heinemann, A., Fietzke, J., Eisenhauer, A., and Zumholz, K.: Modification of Ca isotope and trace metal composition of the major matrices involved in shell formation of Mytilus edulis, Geochem. Geophy. Geosy., 9, Q01006, https://doi.org/10.1029/2007GC001777, 2008.
Heinemann, A., Hiebenthal, C., Fietzke, J., Eisenhauer, A., and Wahl, M.: Disentangling the biological and environmental control of M. edulis shell chemistry, Geochem. Geophy. Geosy., 12, Q03009, https://doi.org/10.1029/2010GC003340, 2011.
Hiddink, J. G., Marijnissen, S. A., Troost, K., and Wolff, W. J.: Predation on 0-group and older year classes of the bivalve Macoma balthica: interaction of size selection and intertidal distribution of epibenthic predators, J. Exp. Mar. Bio. Ecol., 269, 223–248, https://doi.org/10.1016/S0022-0981(02)00002-3, 2002.
Holcomb, M., DeCarlo, T. M., Schoepf, V., Dissard, D., Tanaka, K., and McCulloch, M.: Cleaning and pre-treatment procedures for biogenic and synthetic calcium carbonate powders for determination of elemental and boron isotopic compositions, Chem. Geol., 398, 11–21, https://doi.org/10.1016/j.chemgeo.2015.01.019, 2015.
Iglikowska, A., Beldowski, J., Chelchowski, M., Chierici, M., Kedra, M., Przytarska, J., Sowa, A., and Kukliński, P.: Chemical composition of two mineralogically contrasting Arctic bivalves' shells and their relationships to environmental variables, Mar. Pollut. Bull., 114, 903–916, https://doi.org/10.1016/j.marpolbul.2016.10.071, 2016.
Iglikowska, A., Ronowicz, M., Humphreys-Williams, E., and Kukliński, P.: Trace element accumulation in the shell of the Arctic cirriped Balanus balanus, Hydrobiologia, 818, 43–56, https://doi.org/10.1007/s10750-018-3564-5, 2018.
Imai, N., Terashima, S., Itoh, S., and Ando, A.: Compilation of analytical data on nine GSJ geochemical reference samples, “Sedimentary rock series”, Geostand. Newsl., 20, 165–216, https://doi.org/10.1111/j.1751-908X.1996.tb00184.x, 1996.
Inoue, M., Nohara, M., Okai, T., Suzuki, A., and Kawahata, H.: Concentrations of trace elements in carbonate reference materials Coral JCp-1 and Giant Clam JCt-1 by inductively coupled plasma-mass spectrometry, Geostand. Geoanal. Res., 28, 411–416, https://doi.org/10.1111/j.1751-908X.2004.tb00759.x, 2004.
Janssen, H. H. and Scholtz, N.: Uptake and cellular distribution of cadmium in Mytilus edulis, Mar. Biol., 55, 133–141, https://doi.org/10.1007/BF00397309, 1979.
Jelnes, J. E., Petersen, G. H., and Russell, P. C.: Isoenzyme taxonomy applied on four species of Cardium from Danish and British waters with a short description of the distribution of the species (Bivalvia), Ophelia, 9, 15–19, https://doi.org/10.1080/00785326.1971.10430087, 1971.
Kawahata, H., Fujita, K., Iguchi, A., Inoue, M., Iwasaki, S., Kuroyanagi, A., Maeda, A., Manaka, T., Moriya, K., Takagi, H., Toyofuku, T., Yoshimura, T., and Suzuki, A.: Perspective on the response of marine calcifiers to global warming and ocean acidification – behavior of corals and foraminifera in a high CO2 world “hot house”, Prog. Earth Planet. Sci., 6, 5, https://doi.org/10.1186/s40645-018-0239-9, 2019.
Kerckhof, F.: Barnacles (Cirripedia, Balanomorpha) in Belgian waters, an overview of the species and recent evolutions, with emphasis on exotic species, Bull. Inst. Roy. Sci. Nat. Belg., Biol., 72, 93–104, 2002.
Khim, B., Krantz, D. E., Cooper, L. W., and Grebmeie, J. M.: Seasonal discharge of estuarine freshwater to the western Chukchi Sea shelf identified in stable isotope profiles of mollusk shells, J. Geophys. Res.-Ocean., 108, 3300, https://doi.org/10.1029/2003JC001816, 2003.
Kim, G., Alleman, L. Y., and Church, T. M.: Accumulation records of radionuclides and trace metals in two contrasting Delaware salt marshes, Mar. Chem., 87, 87–96, https://doi.org/10.1016/j.marchem.2004.02.002, 2004.
Klein, R. T., Lohmann, K. C., and Thayer, C. W.: Sr∕Ca and 13C ∕ 12C ratios in skeletal calcite of Mytilus trossulus: covariation with metabolic rate, salinity, and carbon isotopic composition of seawater, Geochim. Cosmochim. Ac., 60, 4207–4221, https://doi.org/10.1016/S0016-7037(96)00232-3, 1996.
Kruk-Dowgiałło, L. and Szaniawska, A.: Gulf of Gdańsk and Puck Bay, in: Ecology of Baltic Coastal Waters, edited by: Schiewer, U., Springer, Berlin, Heidelberg, Germany, 139–165, https://doi.org/10.1007/978-3-540-73524-3_7, 2008.
Kuklinski, P. and Taylor, P. D.: Mineralogy of Arctic bryozoan skeletons in a global context, Facies, 55, 489–500, https://doi.org/10.1007/s10347-009-0179-3, 2009.
Larsson, J., Lind, E. E., Corell, H., Grahn, M., Smolarz, K., and Lönn, M.: Regional genetic differentiation in the blue mussel from the Baltic Sea area, Estuar. Coast. Shelf Sci., 195, 98–109, https://doi.org/10.1016/j.ecss.2016.06.016, 2017.
Lauringson, V., Kotta, J., Orav-Kotta, H., and Kaljurand, K.: Diet of mussels Mytilus trossulus and Dreissena polymorpha in a brackish nontidal environment, Mar. Ecol., 35, 56–66, https://doi.org/10.1111/maec.12120, 2014.
Lazareth, C. E., Vander Putten, E., André, L., and Dehairs, F.: High-resolution trace element profiles in shells of the mangrove bivalve Isognomon ephippium: a record of environmental spatio-temporal variations?, Estuar. Coast. Shelf Sci., 57, 1103–1114, https://doi.org/10.1016/S0272-7714(03)00013-1, 2003.
Lécuyer, C. and O'Neil, J. R.: Stable isotope compositions of fluid inclusions in biogenic carbonates, Geochim. Cosmochim. Ac., 58, 353–363, https://doi.org/10.1016/0016-7037(94)90469-3, 1994.
Lee, B. G., Wallace, W. G., and Luoma, S. N.: Uptake and loss kinetics of Cd, Cr and Zn in the bivalves Potamocorbula amurensis and Macoma balthica: effects of size and salinity, Mar. Ecol. Prog. Ser., 175, 177–189, https://doi.org/10.3354/meps175177, 1998.
Lee, J. and Morse, J. W.: Influences of alkalinity and pCO2 on CaCO3 nucleation from estimated Cretaceous composition seawater representative of “calcite seas”, Geology, 38, 115–118, https://doi.org/10.1130/G30537.1, 2010.
Lingard, S. M., Evans, R. D., and Bourgoin, B. P.: Method for the estimation of organic-bound and crystal-bound metal concentrations in bivalve shells, Bull. Environ. Contam. Toxicol., 48, 179–184, https://doi.org/10.1007/BF00194369, 1992.
Lorens, R. B., Bender, M. L., and Island, R.: The impact of solution chemistry on Mytilus edulis calcite and aragonite, Geochim. Cosmochim. Ac., 44, 1265–1278, https://doi.org/10.1016/0016-7037(80)90087-3, 1980.
Lowenstam, H. A. and Weiner, S.: On Biomineralization, Oxford University Press, New York, 1989.
Loxton, J., Kuklinski, P., Barnes, D., Najorka, J., Spencer Jones, M., and Porter, J.: Variability of Mg-calcite in Antarctic bryozoan skeletons across spatial scales, Mar. Ecol.-Prog. Ser., 507, 169–180, https://doi.org/10.3354/meps10826, 2014.
Loxton, J., Najorka, J., Humphreys-Williams, E., Kuklinski, P., Smith, A. M., Porter, J. S., and Spencer Jones, M.: The forgotten variable: impact of cleaning on the skeletal composition of a marine invertebrate, Chem. Geol., 474, 45–57, https://doi.org/10.1016/j.chemgeo.2017.10.022, 2017.
Luoma, S. N. and Rainbow, P. S.: Why is metal bioaccumulation so variable? Biodynamics as a unifying concept, Environ. Sci. Technol., 39, 1921–1931, https://doi.org/10.1021/es048947e, 2005.
Luoma, S. N. and Rainbow, P. S.: Metal Contamination in Aquatic Environments: Science and Lateral Management, Cambridge University Press, Cambridge, 2008.
Mannella, G., Zanchetta, G., Regattieri, E., Perchiazzi, N., Drysdale, R. N., Giaccio, B., Leng, M. J., and Wagneret, B.: Effects of organic removal techniques prior to carbonate stable isotope analysis of lacustrine marls: a case study from palaeo-lake Fucino (central Italy), Rapid Commun. Mass Sp., 34, e8623, https://doi.org/10.1002/rcm.8623, 2020.
Marchitto, T. M., Jones, G. A., Goodfriend, G. A., and Christopher, R. W.: Precise temporal correlation of Holocene mollusk shells using sclerochronology, Quaternary Res., 53, 236–246, https://doi.org/10.1006/qres.1999.2107, 2000.
Marin, F. and Luquet, G.: Molluscan shell proteins, Compt. Rend. Palevol, 3, 469–492, https://doi.org/10.1016/j.crpv.2004.07.009, 2004.
Martincic, D., Kwokal, Z., Peharec, Z., Margus, D., and Branica, M.: Distribution of Zn, Pb, Cd and Cu between seawater and transplanted mussels (Mytilus galloprovincialis), Sci. Total Environ., 119, 211–230, https://doi.org/10.1016/0048-9697(92)90265-T, 1992.
Milliman, J. D.: Recent Sedimentary Carbonates. Part 1. Marine Carbonates, Springer, Berlin, Heidelberg, Germany, 1974.
Mititelu, M., Ioniṭă, C., Ioniṭă, E., Neacşu, S. M., Amzoiu, E., Averis, L. M. E., and Moroşan, E.: Detection of heavy metals from molluscs shells, Ann. Univ. Craiova – Agric., Montanology, Cadastre Ser., 44, 163–166, 2014.
Morse, J. W., Wang, Q., and Tsio, M. Y.: Influences of temperature and Mg : Ca ratio on CaCO3 precipitates from seawater, Geology, 25, 85–87, https://doi.org/10.1130/0091-7613(1997)025<0085:IOTAMC>2.3.CO;2, 1997.
Morse, J. W., Arvidson, R. S., and Lüttge, A.: Calcium carbonate formation and dissolution, Chem. Rev., 107, 342–381, https://doi.org/10.1016/0166-445X(82)90002-9, 2007.
Newman, M. C. and Unger, M. A.: Uptake, biotransformation, detoxification, elimination, and accumulation, in: Fundamentals of Ecotoxicology, 2nd Edn., CRC Press, Boca Raton, Florida, USA, 53–73, 2003.
Pierscieniak, K., Grzymala, J., Wolowicz, M., Pierścieniak, K., Grzymała, J., and Wołowicz, M.: Differences in reproduction and condition of Macoma balthica and Mytilus trossulus in the Gulf of Gdansk (southern Baltic Sea) under anthropogenic influences, Oceanol. Hydrobiol. Stud., 39, 17–32, https://doi.org/10.2478/v10009-010-0054-0, 2010.
Pilkey, O. H. and Harris, R. C.: The effect of intertidal environment on the composition of calcareous skeletal material, Limnol. Oceanogr., 11, 381–385, https://doi.org/10.4319/lo.1966.11.3.0381, 1966.
Piwoni-Piórewicz, A., Kukliński, P., Strekopytov, S., Humphreys-Williams, E., Najorka, J., and Iglikowska, A.: Size effect on the mineralogy and chemistry of Mytilus trossulus shells from the southern Baltic Sea: implications for environmental monitoring, Environ. Monit. Assess., 189, 197, https://doi.org/10.1007/s10661-017-5901-y, 2017.
Pokroy, B., Fitch, A. N., Marin, F., Kapon, M., Adir, N., and Zolotoyabko, E.: Anisotropic lattice distortions in biogenic calcite induced by intra-crystalline organic molecules, J. Struct. Biol., 155, 96–103, https://doi.org/10.1016/j.jsb.2006.03.008, 2006.
Ponnurangam, A., Bau, M., Brenner, M., and Koschinsky, A.: Mussel shells of Mytilus edulis as bioarchives of the distribution of rare earth elements and yttrium in seawater and the potential impact of pH and temperature on their partitioning behavior, Biogeosciences, 13, 751–760, https://doi.org/10.5194/bg-13-751-2016, 2016.
Ponnurangam, A. E.: Trace Elements in Mussel Shells as Potential Tools for Environmental Reconstructions, PhD thesis, Jacobs University, Bremen, Germany, 154 pp., 2018.
Poulain, C., Gillikin, D. P., Thébault, J., Munaron, J. M., Bohn, M., Robert, R., Paulet, Y. M., and Lorrain, A.: An evaluation of Mg∕Ca, Sr∕Ca, and Ba∕Ca ratios as environmental proxies in aragonite bivalve shells, Chem. Geol., 396, 42–50, https://doi.org/10.1016/j.chemgeo.2014.12.019, 2015.
Price, G. D. and Pearce, N. J. G.: Biomonitoring of pollution by Cerastoderma edule from the British Isles: a laser ablation ICP-MS study, Mar. Pollut. Bull., 34, 1025–1031, https://doi.org/10.1016/S0025-326X(97)00098-2, 1997.
Protasowicki, M., Dural, M., and Jaremek, J.: Trace metals in the shells of blue mussels (Mytilus edulis) from the Poland coast of Baltic Sea, Environ. Monit. Assess., 141, 329–337, https://doi.org/10.1007/s10661-007-9899-4, 2008.
Pruszak, Z., van Ninh, P., Szmytkiewicz, M., Hung, N. M., and Ostrowski, R.: Hydrology and morphology of two river mouth regions (temperate Vistula delta and subtropical Red River delta), Oceanologia, 47, 365–385, 2005.
Pruysers, P. A., de Lange, G. J., and Middelburg, J. J.: Geochemistry of eastern Mediterranean sediments: primary sediment composition and diagenetic alterations, Mar. Geol., 100, 137–154, https://doi.org/10.1016/0025-3227(91)90230-2, 1991.
Puente, X., Villares, R., Carral, E., and Carballeira, A.: Nacreous shell of Mytilus galloprovincialis as a biomonitor of heavy metal pollution in Galiza (NW Spain), Sci. Total Environ., 183, 205–211, https://doi.org/10.1016/0048-9697(96)05066-8, 1996.
Rainbow, P. S.: Physiology, physicochemistry and metal uptake – A crustacean perspective, Mar. Pollut. Bull., 31, 55–59, https://doi.org/10.1016/0025-326X(95)00005-8, 1995.
Rainbow, P. S.: Phylogeny of trace metal accumulation in crustaceans, in: Metal Metabolism in Aquatic Environments, edited by: Langston, W. J. and Bebianno, M. J., Springer, Dordrecht, The Netherlands, 285–319, https://doi.org/10.1007/978-1-4757-2761-6_9, 1998.
Rainbow, P. S.: Trace metal concentrations in aquatic invertebrates: why and so what?, Environ. Pollut., 120, 497–507, https://doi.org/10.1016/S0269-7491(02)00238-5, 2002.
Rainbow, P. S. and Phillips, D. J. H.: Cosmopolitan biomonitors of trace metals, Mar. Pollut. Bull., 26, 593–601, https://doi.org/10.1016/0025-326X(93)90497-8, 1993.
Rainbow, P. S. and Wang, W.: Comparative assimilation of Cd, Cr, Se, and Zn by the barnacle, Mar. Ecol.-Prog. Ser., 218, 239–248, https://doi.org/10.3354/meps218239, 2001.
Rainbow, P. S., Amiard-Triquet, C., Amiard, J. C., Smith, B. D., Best, S. L., Nassiri, Y., and Langston, W. J.: Trace metal uptake rates in crustaceans (amphipods and crabs) from coastal sites in NW Europe differentially enriched with trace metals, Mar. Ecol.-Prog. Ser., 183, 189–203, https://doi.org/10.3354/meps183189, 1999.
Rainbow, P. S., Wolowicz, M., Fialkowski, W., Smith, B. D., and Sokolowski, A.: Biomonitoring of trace metals in the Gulf of Gdansk, using mussels (Mytilus trossulus) and barnacles (Balanus improvisus), Water Res., 34, 1823–1829, https://doi.org/10.1016/S0043-1354(99)00345-0, 2000.
Rainbow, P. S., Fialkowski, W., Sokolowski, A., Smith, B. D., and Wolowicz, M.: Geographical and seasonal variation of trace metal bioavailabilities in the Gulf of Gdansk, Baltic Sea using mussels (Mytilus trossulus) and barnacles (Balanus improvisus) as biomonitors, Mar. Biol., 144, 271–286, https://doi.org/10.1007/s00227-003-1197-2, 2004.
Raman, S. and Kumar, R.: Construction and nanomechanical properties of the exoskeleton of the barnacle, Amphibalanus reticulatus, J. Struct. Biol., 176, 360–369, https://doi.org/10.1016/j.jsb.2011.08.015, 2011.
R Core Team: R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, available at: https://www.R-project.org/, last access: 10 August 2019.
Reeder, R. J.: Crystal chemistry of the rhombohedral carbonates, Rev. Mineral., 11, 1–47, 1983.
Ricardo, F., Génio, L., Costa Leal, M., Albuquerque, R., Queiroga, H., Rosa, R., and Calado, R.: Trace element fingerprinting of cockle (Cerastoderma edule) shells can reveal harvesting location in adjacent areas, Sci. Rep.-UK, 5, 11932, https://doi.org/10.1038/srep11932, 2015.
Ritz, D. A., Swain, R., and Elliott, N. G.: Use of the mussel Mytilus edulis planulatus (Lamarck) in monitoring heavy metal levels in seawater, Mar. Freshw. Res., 33, 491–506, https://doi.org/10.1071/MF9820491, 1982.
Rodland, D. L., Schöne, B. R., Helama, S., Nielsen, J. K., and Baier, S.: A clockwork mollusc: ultradian rhythms in bivalve activity revealed by digital photography, J. Exp. Mar. Bio. Ecol., 334, 316–323, https://doi.org/10.1016/j.jembe.2006.02.012, 2006.
Roger, L. M., George, A. D., Shaw, J., Hart, R. D., Roberts, M., Becker, T., McDonald, B. J., and Evans, N. J.: Geochemical and microstructural characterisation of two species of cool-water bivalves (Fulvia tenuicostata and Soletellina biradiata) from Western Australia, Biogeosciences, 14, 1721–1737, https://doi.org/10.5194/bg-14-1721-2017, 2017.
Rončević, S., Svedružić, L. P., Smetiško, J., and Medaković, D.: ICP-AES analysis of metal content in shell of mussel Mytilus galloprovincialis from Croatian coastal waters, Int. J. Environ. Anal. Chem., 90, 620–632, https://doi.org/10.1080/03067310903410956, 2010.
Rosenberg, G. D. and Hughes, W. W.: A metabolic model for the determination of shell composition in the bivalve mollusc, Mytilus edulis, Lethaia, 24, 83–96, https://doi.org/10.1111/j.1502-3931.1991.tb01182.x, 1991.
Rosenberg, G. D., Hughes, W. W., Parker, D. L., and Ray, B. D.: The geometry of bivalve shell chemistry and mantle metabolism, Am. Malacol. Bull., 16, 251–261, 2001.
Rosenheim, B. E., Swart, P. K., Thorrold, S. R., Willenz, P., Berry, L., and Latkoczy, C.: High-resolution Sr∕Ca records in sclerosponges calibrated to temperature in situ, Geology, 32, 145–148, https://doi.org/10.1130/G20117.1, 2004.
Rueda, J. L. and Smaal, A. C.: Variation of the physiological energetics of the bivalve Spisula subtruncata (da Costa, 1778) within an annual cycle, J. Exp. Mar. Bio. Ecol., 301, 141–157, https://doi.org/10.1016/j.jembe.2003.09.018, 2004.
Saavedra, Y., Gonzalez, A., Fernandez, P., and Blanco, J.: The effect of size on trace metal levels in raft cultivated mussels (Mytilus galloprovincialis), Sci. Total Environ., 318, 115–124, https://doi.org/10.1016/S0048-9697(03)00402-9, 2004.
Sartori, A. F. and Gofas, S.: Macoma balthica (Linnaeus, 1758), MolluscaBase, available at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=141579 (last access: 26 January 2021), 2016.
Sather, W. A. and McCleskey, E. W.: Permeation and selectivity in calcium channels, Annu. Rev. Physiol., 65, 133–159, https://doi.org/10.1146/annurev.physiol.65.092101.142345, 2003.
Schöne, B. R., Zhang, Z., Jacob, D., Gillikin, D. P., Tütken, T., Garbe-Schönberg, D., McConnaughey, T., and Soldati, A.: Effect of organic matrices on the determination of the trace element chemistry (Mg, Sr, Mg∕Ca, Sr∕Ca) of aragonitic bivalve shells (Arctica islandica) – comparison of ICP-OES and LA-ICP-MS data, Geochem. J., 44, 23–37, https://doi.org/10.2343/geochemj.1.0045, 2010.
Schöne, B. R., Zhang, Z., Radermacher, P., Thébault, J., Jacob, D. E., Nunn, E. V., and Maurer, A.: Sr∕Ca and Mg∕Ca ratios of ontogenetically old, long-lived bivalve shells (Arctica islandica) and their function as paleotemperature proxies, Palaeogeogr. Palaeocl., 302, 52–64, https://doi.org/10.1016/j.palaeo.2010.03.016, 2011.
Skinner, L. C. and Elderfield, H.: Constraining ecological and biological bias in planktonic foraminiferal Mg∕Ca and δ18Occ: a multispecies approach to proxy calibration testing, Paleoceanography, 20, PA1015, https://doi.org/10.1029/2004PA001058, 2005.
Smith, A. M. and Girvan, E.: Understanding a bimineralic bryozoan: skeletal structure and carbonate mineralogy of Odontionella cyclops (Foveolariidae: Cheilostomata: Bryozoa) in New Zealand, Palaeogeogr. Palaeocl., 289, 113–122, https://doi.org/10.1016/j.palaeo.2010.02.022, 2010.
Smith, A. M., Nelson, C. S., and Spencer, H. G.: Skeletal carbonate mineralogy of New Zealand bryozoans, Mar. Geol., 151, 27–46, https://doi.org/10.1016/S0025-3227(98)00055-3, 1998.
Smith, A. M., Key, M. M., and Gordon, D. P.: Skeletal mineralogy of bryozoans: taxonomic and temporal patterns, Earth Sci. Rev., 78, 287–306, https://doi.org/10.1016/j.earscirev.2006.06.001, 2006.
Sokolowski, A., Wolowicz, M., and Hummel, H.: Distribution of dissolved and labile particulate trace metals in the overlying bottom water in the Vistula River plume (southern Baltic Sea), Mar. Pollut. Bull., 42, 967–980, 2001.
Staniszewska, M., Nehring, I., and Mudrak-Cegiołka, S.: Changes of concentrations and possibility of accumulation of bisphenol A and alkylphenols, depending on biomass and composition, in zooplankton of the southern Baltic (Gulf of Gdansk), Environ. Pollut., 213, 489–501, https://doi.org/10.1016/j.envpol.2016.03.004, 2016.
Stanley, S. M.: Effects of global seawater chemistry on biomineralization: past, present, and future, Chem. Rev., 108, 4483–4498, https://doi.org/10.1021/cr800233u, 2008.
Stecher, H. A., Krantz, D. E., Lord, C. J., Luther, G. W., and Bock, K. W.: Profiles of strontium and barium in Mercenaria mercenaria and Spisula solidissima shells, Geochim. Cosmochim. Acta, 60, 3445–3456, https://doi.org/10.1016/0016-7037(96)00179-2, 1996.
Strasser, M., Walensky, M., and Reise, K.: Juvenile–adult distribution of the bivalve Mya arenaria on intertidal flats in the Wadden Sea: why are there so few year classes?, Helgol. Mar. Res., 33, 45–55, https://doi.org/10.1007/PL00012137, 1999.
Strasser, C. A., Mullineaux, L. S., and Walther, B. D.: Growth rate and age effects on Mya arenaria shell chemistry: implications for biogeochemical studies, J. Exp. Mar. Bio. Ecol., 355, 153–163, https://doi.org/10.1016/j.jembe.2007.12.022, 2008.
Strekopytov, S. V., Uspenskaya, T. Yu., Vinogradova, E. L., and Dubinin, A. V.: Geochemistry of early diagenesis of sediments of Kandalaksha Bay of the White Sea, Geochem. Int., 43, 117–130, 2005.
Strelkov, P., Nikula, R., and Väinölä, R.: Macoma balthica in the White and Barents seas: properties of a widespread marine hybrid swarm (Mollusca: Bivalvia), Mol. Ecol., 16, 4110–4127, https://doi.org/10.1111/j.1365-294X.2007.03463.x, 2007.
Sugawara, A. and Kato, T.: Aragonite CaCO3 thin-film formation by cooperation of Mg2+ and organic polymer matrices, Chem. Commun., 6, 487–488, https://doi.org/10.1039/A909566G, 2000.
Sunagawa, I., Takahashi, Y., and Imai, H.: Strontium and aragonite-calcite precipitation, J. Mineral. Petrol. Sci., 102, 174–181, https://doi.org/10.2465/jmps.060327a, 2007.
Szefer, P.: Metal pollutants and radionuclides in the Baltic Sea – an overview, Oceanologia, 44, 129–178, 2002.
Szefer, P. and Grembecka, M.: Chemometric assessment of chemical element distribution in bottom sediments of the southern Baltic Sea including Vistula and Szczecin lagoons – an overview, Polish J. Environ. Stud., 18, 25–34, 2009.
Szefer, P. and Szefer, K.: Occurrence of ten metals in Mytilus edulis L. and Cardium glaucum L. from the Gdańsk Bay, Mar. Pollut. Bull., 16, 446–450, https://doi.org/10.1016/0025-326X(85)90415-1, 1985.
Szefer, P., Kusak, A., Szefer, K., Jankowska, H., Wołowicz, M., and Ali, A. A.: Distribution of selected metals in sediment cores of Puck Bay, Baltic Sea, Mar. Pollut. Bull., 30, 615–618, https://doi.org/10.1016/0025-326X(95)00079-3, 1995.
Szefer, P., Glasby, G. P., Kusak, A., Szefer, K., Jankowska, H., Wolowicz, M., and Ali, A. A.: Evaluation of the anthropogenic influx of metallic pollutants into Puck Bay, southern Baltic, Appl. Geochemistry, 13, 293–304, https://doi.org/10.1016/S0883-2927(97)00098-X, 1998.
Szefer, P., Frelek, K., Szefer, K., Lee, C. B., Kim, B. S., Warzocha, J., Zdrojewska, I., and Ciesielski, T.: Distribution and relationships of trace metals in soft tissue, byssus and shells of Mytilus edulis trossulus from the southern Baltic, Environ. Pollut., 120, 423–444, https://doi.org/10.1016/S0269-7491(02)00111-2, 2002.
Szumiło-Pilarska, E., Grajewska, A., Falkowska, L., and Hajdrych, J.: Species differences in total mercury concentration in gulls from the Gulf of Gdansk (southern Baltic), J. Trace Elem. Med. Biol., 33, 100–109, https://doi.org/10.1016/j.jtemb.2015.09.005, 2016.
Takesue, R. K. and van Geen, A.: Mg∕Ca, Sr∕Ca, and stable isotopes in modern and Holocene Protothaca staminea shells from a northern California coastal upwelling region, Geochim. Cosmochim. Acta, 68, 3845–3861, https://doi.org/10.1016/j.gca.2004.03.021, 2004.
Takesue, R. K., Bacon, C. R., and Thompson, J. K.: Influences of organic matter and calcification rate on trace elements in aragonitic estuarine bivalve shells, Geochim. Cosmochim. Acta, 72, 5431–5445, https://doi.org/10.1016/j.gca.2008.09.003, 2008.
Taylor, J. D. and Reid, D. G.: Shell microstructure and mineralogy of the Littorinidae: ecological and evolutionary significance, in: Progress in Littorinid and Muricid Biology: Proceedings of the Second European Meeting on Littorinid Biology, Tjärnö Marine Biological Laboratory, Sweden, 4–8 July 1988 (Developments in Hydrobiology, vol. 56), edited by: Johannesson, K., Raffaelli, D. G., and Hannaford Ellis, C. J., Kluwer, Dordrecht, the Netherlands, 199–215, https://doi.org/10.1007/978-94-009-0563-4_16, 1990.
Taylor, P. D., James, N. P., Bone, Y., Kuklinski, P., and Kyser, T. K.: Evolving mineralogy of cheilostome bryozoans, Palaios, 24, 440–452, https://doi.org/10.2110/palo.2008.p08-124r, 2008.
Taylor, P. D., Kudryavtsev, U. A. B., and Schopf, J. W.: Calcite and aragonite distributions in the skeletons of bimineralic bryozoans as revealed by Raman spectroscopy, Invertebr. Biol., 127, 87–97, https://doi.org/10.1111/j.1744-7410.2007.00106.x, 2014.
Tessier, A., Campbell, P. G. C., and Bisson, M.: Sequential extraction procedure for the speciation of particulate trace metals, Anal. Chem., 51, 844–851, https://doi.org/10.1021/ac50043a017, 1979.
Thébault, J., Chauvaud, L., Helguen, L., Clavier, J., and Pe, C.: Barium and molybdenum records in bivalve shells: geochemical proxies for phytoplankton dynamics in coastal environments?, Limnol. Oceanogr., 54, 1002–1014, https://doi.org/10.4319/lo.2009.54.3.1002, 2009.
Thomas, C. A. and Bendell-Young, L. I.: Linking the sediment geochemistry of an intertidal region to metal bioavailability in the deposit feeder Macoma balthica, Mar. Ecol. Prog. Ser., 173, 197–213, https://doi.org/10.3354/meps173197, 1998.
Ullmann, C. V., Gale, A. S., Huggett, J., Wray, D., Frei, R., Korte, C., Broom-Fendley, S., Littler, K., and Hesselbo, S. P.: The geochemistry of modern calcareous barnacle shells and applications for palaeoenvironmental studies, Geochim. Cosmochim. Ac., 243, 149–168, https://doi.org/10.1016/j.gca.2018.09.010, 2018.
Urban-Malinga, B., Warzocha, J., and Zalewski, M.: Effects of the invasive polychaete Marenzelleria spp. on benthic processes and meiobenthos of a species-poor brackish system, J. Sea Res., 80, 25–34, https://doi.org/10.1016/j.seares.2013.02.005, 2013.
Urey, H. C., Lowenstam, H. A., Epstein, S., and McKinney, C. R.: Measurement of paleotemperatures and temperatures of the Upper Cretaceous of England, Denmark, and the southeastern United States, Geol. Soc. Am. Bull., 62, 399–416, https://doi.org/10.1130/0016-7606(1951)62[399:MOPATO]2.0.CO;2, 1951.
Uścinowicz, S.: Geochemia osadów powierzchniowych Morza Bałtyckiego (Geochemistry of the Baltic Sea surface sediments), Państwowy Instytut Geologiczny – Państwowy Instytut Badawczy (Polish Geological Institute – National Research Institute), Warsaw, Poland, 2011 (in Polish).
Vander Putten, E., Dehairs, F., Keppens, E., and Baeyens, W.: High resolution distribution of trace elements in the calcite shell layer of modern Mytilus edulis: environmental and biological controls, Geochim. Cosmochim. Ac., 64, 997–1011, https://doi.org/10.1016/S0016-7037(99)00380-4, 2000.
Waldbusser, G. G., Hales, B., and Haley, B. A.: Calcium carbonate saturation state: on myths and this or that stories, ICES J. Mar. Sci., 73, 563–568, https://doi.org/10.1093/icesjms/fsv174, 2016.
Walls, R. A., Ragland, P. C., and Crisp, E. L.: Experimental and natural early diagenetic mobility of Sr and Mg in biogenic carbonates, Geochim. Cosmochim. Acta, 41, 1731–1737, https://doi.org/10.1016/0016-7037(77)90204-6, 1977.
Wang, W. and Fisher, N. S.: Modeling the influence of body size on trace element accumulation in the mussel Mytilus edulis, Mar. Ecol.-Prog. Ser., 161, 103–115, https://doi.org/10.3354/meps161103, 1997.
Wang, Y. and Xu, H.: Prediction of trace metal partitioning between minerals and aqueous solutions: a linear free energy correlation approach, Geochim. Cosmochim. Acta, 65, 1529–1543, https://doi.org/10.1016/S0016-7037(01)00551-8, 2001.
Warter, V., Erez, J., and Müller, W.: Environmental and physiological controls on daily trace element incorporation in Tridacna crocea from combined laboratory culturing and ultra-high resolution LA-ICP-MS analysis, Palaeogeogr. Palaeocl., 496, 32–47, 2018.
Watson, D., Foster, P., and Walker, G.: Barnacle shells as biomonitoring material, Mar. Pollut. Bull., 31, 111–115, https://doi.org/10.1016/0025-326X(95)00021-E, 1995.
Watson, S. A., Peck, L. S., Tyler, P. A., Southgate, P. C., Tan, K. S., Day, R. W., and Morley, S. A.: Marine invertebrate skeleton size varies with latitude, temperature and carbonate saturation: implications for global change and ocean acidification, Glob. Change Biol., 18, 3026–3038, https://doi.org/10.1111/j.1365-2486.2012.02755.x, 2012.
Weidema, I. R.: Introduced Species in the Nordic Countries, Nordic Council of Ministers, Copenhagen, Denmark, 2000.
Wenne, R., Bach, L., Zbawicka, M., Strand, J., and McDonald, J. H.: A first report on coexistence and hybridization of Mytilus trossulus and M. edulis mussels in Greenland, Polar Biol., 39, 343–355, https://doi.org/10.1007/s00300-015-1785-x, 2016.
Wit, J. C., de Nooijer, L. J., Wolthers, M., and Reichart, G. J.: A novel salinity proxy based on Na incorporation into foraminiferal calcite, Biogeosciences, 10, 6375–6387, https://doi.org/10.5194/bg-10-6375-2013, 2013.
Wolowicz, M. and Goulletquer, P.: The shell organic content in the energy budget of Mytilus trossulus from the South Baltic, Haliotis, 28, 1–10, 1999.
Zhang, G., He, L. S., Wong, Y. H., Xu, Y., Zhang, Y., and Qian, P. Y.: Chemical component and proteomic study of the Amphibalanus (= Balanus) amphitrite shell, Plos One, 10, e0133866, https://doi.org/10.1371/journal.pone.0133866, 2015.
Zhao, L., Schöne, B. R., and Mertz-Kraus, R.: Delineating the role of calcium in shell formation and elemental composition of Corbicula fluminea (Bivalvia), Hydrobiologia, 790, 259–272, https://doi.org/10.1007/s10750-016-3037-7, 2017.
Żmudziński, L.: Świat zwierzȩcy Bałtyku – atlas makrofauny (Animal World of the Baltic Sea: Atlas of Macrofauna), WSiP, Warsaw, Poland, 1990 (in Polish).