the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The application of dendrometers to alpine dwarf shrubs – a case study to investigate stem growth responses to environmental conditions
Svenja Dobbert
Roland Pape
Considering the recent widespread greening and browning trends associated with shrubs in arctic–alpine ecosystems, further understanding of how these shrubs respond in a rapidly changing environment is of crucial importance.
We here monitor shrub growth, using high-precision dendrometers to produce fine-scale intra-annual growth patterns from hourly stem diameter variability in a widespread evergreen species (Empetrum nigrum ssp. hermaphroditum). Measurements were taken at a micrometer scale for the period 2015 till 2018 on exposed and mostly snow-free ridge positions. With the same temporal resolution, we collected near-ground environmental data and identified on-site controls of growth behavior.
We found high inter-plant variability in radial stem growth but strong similarities in response patterns to the local environment. Our results suggest that the evergreen species is highly adapted to the specific local conditions, remaining partly photosynthetically active during the snow-free winter, which facilitates carbohydrate accumulation for early-season physiological activities. Additionally, we discovered a phase of radial stem shrinkage during the winter months, which can be attributed to an active cell water reduction to protect the plant from frost damage.
We conclude that soil moisture availability and winter snow conditions are the main drivers of radial stem growth of E. hermaphroditum in arctic and alpine regions and could negatively affect the species' distribution in a warming climate.
- Article
(11761 KB) - Full-text XML
- BibTeX
- EndNote
Arctic and alpine ecosystems are especially sensitive to recent climate variability, with temperatures increasing thrice as much as the global average in the past decades, caused by a rising concentration of atmospheric CO2 and accompanied by a substantial lengthening of the growing period (e.g., IPCC, 2014; Post et al., 2019; AMAP, 2021). This trend has favored growth, abundance, and biomass production of numerous shrub species, resulting in a widespread, yet spatially heterogenic, greening of the affected areas – with potentially global effects (Myers-Smith et al., 2011; Gough et al., 2015; Brodie et al., 2019; Myers-Smith et al., 2020). The observed greening has been verified using remote sensing techniques (e.g., Carlson et al., 2017) and is caused by both evergreen and broadleaved species, although in different ways (Vowles and Björk, 2019; Weijers and Löffler, 2020). Relations between carbon assimilation through photosynthesis, environmentally controlled wood formation, and associated plant growth were shown to be complex, complicating the understanding of underlying processes, as well as predictions of future trends (Fatichi et al., 2019; Peters et al., 2021). In general, shrubs are considered one of the most responsive plant functional groups to climate variability (Elmendorf et al., 2012). Their expanding trend, in turn, has been associated with climatic feedbacks, such as influence on surface albedo and frozen-ground processes (Sturm et al., 2001; Chapin et al., 2005; Blok et al., 2011; Aartsma et al., 2021). Therefore, an understanding of shrub growth physiology and its environmental controls is of crucial importance.
Over the past decade, dendroecological studies have identified temperatures and soil moisture as the most important drivers in controlling cambial activity in shrubs, potentially independent of carbon assimilation (Van der Wal and Stien, 2014; Cabon et al., 2020). Here, conditions during the main growing season have proven especially important (Elmendorf et al., 2012; Hollesen et al., 2015; Ackerman et al., 2017; Weijers et al., 2017). Additionally, most recent studies have suggested that snow cover and winter warming may play an important role in promoting shrub growth (Hollesen et al., 2015; Weijers et al., 2018a; Francon et al., 2020), as well as spring warming (Weijers et al., 2018a). Yet, an increased frequency of spring freezing events might counteract these positive effects (Choler, 2018). Collectively, these studies agree on the fine-scale complexity of growth behavior, niche shifts, and local adaptation of shrubs in arctic and alpine regions, with a multitude of still little understood, site-related environmental drivers (Graae et al., 2018; Pape and Löffler, 2017; Löffler and Pape, 2020). Such studies, however, mostly rely on measurements of stem or shoot growth, obtained from shrub-ring series (Macias-Fauria et al., 2012; Shetti, 2018; Le Moullec et al., 2019), extraction of micro-cores, or wood anatomical analyses (Rossi et al., 2006; Weijers et al., 2010; Liang et al., 2012; Francon et al., 2020) – all of which are conducted at an inter-annual timescale, which might not be sufficient in explaining the observed complexity due to a too coarse temporal resolution.
High-resolution data, as provided by dendrometer measurements, have the potential to bridge this knowledge gap. They are likely to provide valuable insights into fine-scale response mechanisms to a changing environment, including information on stem water dynamics and carbon fluxes, with higher quality and resolutions than previously attainable (Fritts, 1976; De Schepper and Steppe, 2010; Steppe et al., 2015; Zweifel, 2016; González-Rodríguez et al., 2017). In general, stem diameter increase can be described as a result of cambial division and cell enlargement, which are in turn closely linked to temperature and water potential and therefore strongly affected by environmental stressors. Thus, stem diameter variability is closely related to cambial activity and underlying fine-scale, eco-physiological mechanisms, including water-driven turgor pressure changes in the xylem (Steppe et al., 2015; Drew and Downes, 2009; Cuny et al., 2015; Chan et al., 2016; Zweifel, 2016; Peters et al., 2021; Körner, 2021). Because cambial activity occurs at timescales ranging from hours to days (Deslauriers et al., 2007; Köcher et al., 2012; Liu et al., 2018), the fine temporal resolution that is gained by dendrometers provides valuable additional insights compared to traditional methods. These include intra-annual and seasonal growth behavior of shrubs in alpine environments, thereby bridging existing knowledge gaps regarding plant productivity in remote ecosystems (Le Moullec et al., 2019).
In tree physiology and forest sciences, dendrometers have already proven useful for monitoring tree responses to environmental fluctuations (Breitsprecher and Bethel, 1990; Duchesne et al., 2012; Ježík et al., 2016; Van der Maaten et al., 2018; Smiljanić and Wilmking, 2018), as recent dendrometers can detect radial stem dimensions at hourly or even shorter intervals (Drew and Downes, 2009; Liu et al., 2018). Starting with the early designs, first described in the 1930s and the 1940s (Reineke, 1932; Daubenmire, 1945), dendrometers have been widely used, focusing on long-term monitoring of growth responses to environmental variables (e.g., Duchesne et al., 2012; Liu et al., 2018; Van der Maaten et al., 2018). Recently, a first study using band dendrometers to monitor radial stem growth of tree-like shrubs was presented (González-Rodríguez et al., 2017). Because current dendrometers are designed to measure at a micrometer scale, they have the potential to be used on shrubs to provide fine-scale, intra-annual, continuous, and highly comparable information (Dobbert et al., 2021a, b).
In this context, we monitored intra- and inter-annual stem diameter variability at alpine ridge positions, testing this novel approach using high-precision dendrometer data derived from individual specimens of the shrub species Empetrum nigrum ssp. hermaphroditum (hereafter E. hermaphroditum), an evergreen shrub that is almost circumpolar in distribution (Bell and Tallis, 1973) and abundant in the Scandes mountain chain. E. hermaphroditum has been identified as a niche constructor species with strong direct effects on tundra communities, including a potential slowing of process rates and lowering of biodiversity with E. hermaphroditum encroachment (Bråthen et al., 2018). Because of its complex response to variation in snow cover, it is most common at positions with either shallow or relatively deep snow cover (Bienau et al., 2014, 2016). Additionally, E. hermaphroditum has been described as comparatively resistant to low winter temperatures (Stushnoff and Junttila, 1986; Ögren, 2001) and is usually not affected by grazing (Weijers and Löffler, 2020). The species' stem anatomy was described by Carlquist (1989) and is characterized by a narrow vessel diameter, which can be interpreted as a form of adaptation to drought or physiological drought due to cold as it impedes embolism formation (Fig. A1 in Appendix A). In general, the family of heath-like shrubs is known to match extreme environments by adapting stem anatomy (Carlquist, 1989) and generally occurs in a wide phytogeographic range at various sites along the alpine elevational gradient. We therefore expect to find the monitored specimens highly adapted to their local environment at the exposed ridge positions, possibly independent from larger-scale environmental variability associated with the elevational gradient. Thus, we aim to (1) explain major growth patterns and their variation between years and specimens, (2) identify the most important environmental drivers controlling these patterns across sites, and (3) gain insights into potential response to environmental change. The main objective of our work is thus to gain detailed understanding of the growth patterns of one common arctic–alpine dwarf shrub (Empetrum nigrum ssp. hermaphroditum) and its relation to its immediate environmental surroundings. With this, we hope to bridge the gap between observed large-scale vegetational shifts and the fine-scale physiological mechanisms driving these complex changes within the highly relevant arctic–alpine ecosystems.
2.1 Study sites
We conducted our study in two alpine mountain regions of central Norway. To the west, the Geiranger, Møre og Romsdal, region (62∘03′ N, 7∘15′ E) is located within the slightly to markedly oceanic climatic section (O1–O2; Moen, 1999) of the inner fjords. It is characterized by humid conditions, with total annual precipitation of 1500–2000 mm in the valleys (Aune, 1993) and a mean annual ambient air temperature of 1.9 ∘C (range −23.2 to 17.2 ∘C) (Löffler, 2003). To the east, the Vågåmo, Innlandet, region (61∘53′ N, 9∘15′ E) is located within the continental climatic section (C1; Moen, 1999). The total annual precipitation is low, approximately 300–500 mm in the valleys (Kleiven, 1959), and the mean annual ambient air temperature is −1.2 ∘C (range −29.2 to 16.7 ∘C) (Löffler, 2003). Our own measurements in the alpine parts of the studied regions indicated that the annual liquid precipitation was 900 mm in the west and 375 mm in the east. The additional amount of snow and its water equivalent remains unknown, but snowdrift leads to an uneven distribution of the snowpack within the complex alpine topography (Löffler, 2007).
Across both regions, we chose micro-topographical positions at exposed, wind-blown ridges as study sites. These positions likely represent the most extreme thermal regimes, with discontinuous snow cover and deeply frozen ground during winter. Within the framework of our long-term alpine ecosystem research project (LTAER; e.g., Löffler and Finch, 2005; Hein et al., 2014; Frindte et al., 2019; Löffler et al., 2021), sites were stratified randomly chosen along the elevational gradient to represent the full elevational range of the focal species within our sampled sites. The elevational gradient was stratified into six elevational bands from the treeline upwards, shifted by 100 m between regions to account for slightly different conditions and a diverging position of the treeline. In the oceanic region, we used 900, 1000, 1100, 1200, 1300, and 1400 m a.s.l. (above sea level), in accordance with the treeline in this region, which is located at about 750 to 800 m a.s.l. In the continental region, we used 1000, 1100, 1200, 1300, 1400, and 1500 m a.s.l. Here, the treeline is situated slightly higher, at about 1000 m a.s.l. (Rößler et al., 2008; Rößler and Löffler, 2007). Thus, all of our studied sites were located above the treeline. Our study design resulted in a total of 12 sites, i.e., 2 regions × 6 elevational bands, with one specimen monitored per site (N=12), resulting in 48 annual dendrometer curves, i.e., 12×4 years. A summary of the total stem diameter variation and environmental conditions measured at each site is presented in Fig. A2.
2.2 Dendrometric data and monitoring setup
Here, we applied a technological approach, commonly used for trees, to our multi-stemmed specimens of E. hermaphroditum, taking radial stem measurements using dendrometers. The general idea was to apply well-established methods from dendroecology and tree growth analysis within a novel setting to assess intra- and inter-annual variation in growth patterns and environmental factors controlling this variation. We mounted our dendrometers on one major aboveground stem of a randomly chosen specimen per site, horizontally to the ground surface and as close to the assumed root collar as possible. During this process, we removed the dead outer bark (periderm) to place the sensor as close to the living tissue as possible, following a common practice for dendrometer measurements of trees (Oberhuber et al., 2020; Wang et al., 2020; Grams et al., 2021). This ensures that hygroscopic shrinkage and swelling of dead tissues from the outer bark do not influence the diameter measurements. Such processes have been previously addressed in trees (Zweifel and Häsler, 2000; Gall et al., 2002; Ilek et al., 2016), and comparative studies revealed a complex interplay of xylem as well as phloem growth and pressure-induced size changes, which simultaneously affect radial stem change and are thus captured by the dendrometers (Turcotte et al., 2011; Zweifel et al., 2014b; Oberhuber et al., 2020; Knüsel et al., 2021) (Fig. A1). Additionally, we avoided specific micro-positions near stones and depressions, inside the radius of other larger shrub species, and near patches of wind erosion (Fig. A3). Stem diameter data were measured at 1 min intervals using dendrometers (type DRO; Ecomatik, Dachau, Germany). The sensor has a temperature coefficient of <0.2 µm K−1. To ensure that the dendrometers produce meaningful data, unaffected by the mounting process and bark removal, we tested the study design for several years before presenting the final study period here. In order to facilitate the following analysis, we aggregated the measured hourly values to obtain daily stem diameter variability, defined as the maximum stem diameter measured each day, following the “daily maximum approach” (Deslauriers et al., 2007). This approach assumes suppressed radial growth during times of stem shrinking, in accordance with Zweifel (2016). All calculations were made using R statistical software (R Development Core Team, 2020).
2.3 Analysis of seasonal growth patterns
To assess major growth patterns and growth variation between years, we first defined specific parameters and dates of annual stem growth for each year, resulting in a set of growth-defining parameters for each individual dendrometer curve. This included (a) total growth, defined as growth-induced stem expansion. To separate this expansion from reversible shrinking and swelling associated with stem water fluctuations, we chose the approach proposed by Zweifel (2016), which assumes radial stem growth to be suppressed during times of stem shrinking due to the transpiration-induced lowering of the turgor pressure, preventing cell expansion and cell division. Growth is thus defined as an increase in stem radius when the measured radius is larger than it was at any point in the past. Consequently, total growth realized throughout the year can be derived from the original measured data by calculating the sum of the cumulative maxima (Zweifel et al., 2014a; Zweifel, 2016; Zweifel et al., 2021). Following this approach, we were able to extract the growth-induced stem increment during the main growing season, as well as patterns of radial stem shrinkage (expressed as stem water deficit and derived by subtracting daily stem diameter values from the previously obtained cumulative growth curves; Zweifel, 2016). From these patterns we derived (b) peak shrinking, which we defined as the maximum stem water deficit and which usually occurred during the winter months. Subsequently, we aimed to further define the timing of the main growing season, calculating accurate dates for the parameter (c) peak growth (maximum daily growth rate), as well as (d) growth initiation (start of the growing season) and (e) growth cessation (end of the growing season). Here, we found sigmoid Gompertz models to be the best fit for our growth curves. Although multiple models have been used to describe growth, the Gompertz equation is the most widely used in dendrochronological studies and has been proven to explain the variations in dendrometer measurements (for trees) well (e.g., Rossi et al., 2003, 2006; Duchesne et al., 2012; Van der Maaten et al., 2018; Liu et al., 2019). The equation used for the model was Eq. (1):
where α is the upper asymptote, β is the x-axis placement parameter, and k is the growth rate. We calculated these input parameters from our original data using the equations defined by Fekedulegn et al. (1999). To assess how well the models fit our data, we calculated a goodness-of-fit (GoF) measure using the least-squares method with the formula Eq. (2):
where f is the original and f2 is the modeled stem diameter.
We determined growth initiation (onset) and cessation (offset) from this modeled curve. Since raw dendrometer data are known to deliver rough estimates of cambial activity and less reliable critical dates (Deslauriers et al., 2007; van der Maaten et al., 2018; Cruz-García et al., 2019), this modeling approach allowed us to clearly define the main growth phase and ensure high comparability between years and specimens. Growth initiation and cessation were defined as the time when 10 % and 90 %, respectively, of the total annual modeled growth occurred. We chose these thresholds in accordance with our data, and they were slightly higher than the thresholds used in similar studies for trees (e.g., Van der Maaten-Theunissen et al., 2013; Van der Maaten et al., 2018; Drew and Downes, 2009). This is a result of the unique growth patterns of the sampled shrubs.
Some of our specimens did not experience any growth in specific years. Years with no or little growth have been detected in other shrubs, for example, in Salix arctica by Polunin (1955). Buchwal et al. (2013) assumed such mechanisms to be related to carbon allocation and to occur irregularly along the stem because growth is not homogeneously allocated within the different plant segments. In accordance with their findings for Salix polaris, the specimens might preferentially allocate resources to less exposed parts (e.g., roots) in these years. In general, such partial dormancy (Preece et al., 2012) might reflect insufficient resources for homogenous growth across the entire plant. We did not calculate a growing season for these years, and the analyses proceeded separately, excluding them from most of our calculations.
2.4 Environmental data collection
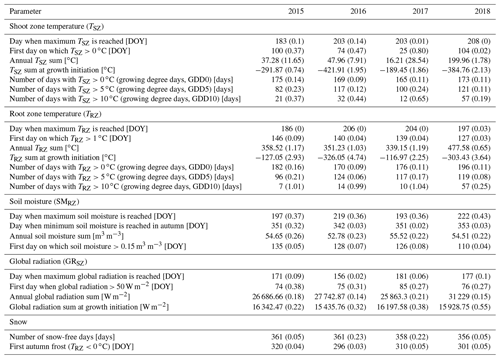
To identify the thermal constraints of our species at both the critical location and the timescale of action (cf. Körner and Hiltbrunner, 2018), we measured soil temperatures (∘C) at a depth of 15 cm below the ground surface (i.e., within the root zone, hereafter TRZ) and air temperatures 15 cm above the ground surface (i.e., within the shoot zone, hereafter TSZ), at all sites. Temperatures were measured at 1 min intervals and recorded as hourly means using Onset's HOBO loggers (type H21-002) and type S-TMB-M002 temperature sensors (±0.2 ∘C accuracy). For the TSZ measurements, the sensors were equipped with passively ventilated radiation shields. Moreover, to identify the soil moisture constraints in the root zone of our specimens, we measured the volumetric soil water content (m3 m−3) 15 cm below the soil surface (hereafter SMRZ) at all sites. The uncalibrated SMRZ was measured at 1 min intervals and recorded as hourly means using Onset's HOBO type S-SMD-M005 soil moisture sensors (±3 % accuracy). Additionally, we measured the shoot zone global radiation (W m−2) at 1 cm above the ground surface in close proximity to the plant (hereafter GRSZ) using Onset's HOBO type S-LIB-M003 silicon pyranometers (±10 W m−2 accuracy). We made sure that those measurements were not affected by the canopy. Our data covered a period of 4 full calendar years from 1 January 2015 to 31 December 2018. Missing data did not occur at the chosen sites.
We did not explicitly measure data regarding snow cover but calculated snow cover from the daily shoot zone temperature amplitude and validated those calculations using radiation sensor measurements. We assumed that a daily amplitude of less than 5 % of the maximum amplitude reached throughout the year indicated that a layer of snow restricted daily air temperature fluctuations at the measured height of 15 cm. The respective periods were therefore defined as snow-covered. However, because of the chosen positions on wind-blown ridges, most of our monitored sites did not experience long periods of snow cover. Nonetheless, snow and its presumed effects, such as mitigating extreme negative temperatures by acting as an isolating barrier and, hence, reducing the effects of frost (Körner, 2021; Bienau et al., 2014), might play a role in influencing the growth response and were therefore included in our analysis.
For further analysis and to assess inter-annual variability, we calculated a set of 25 annual parameters from the collected raw data (Table 1), defining the near-ground environmental conditions experienced by each specimen for each year, based on the expected effects on different growth mechanisms.
2.5 Statistical analysis of climate–growth relations and potential drivers of radial stem change
To analyze the effect of the on-site environmental conditions on the observed growth patterns and to identify the most important environmental drivers controlling these patterns across sites, we utilized linear mixed-effect models, alongside correlation analyses. All statistical analyses were carried out in R (R Development Core Team, 2020), and site-specific values of the previously defined growth-defining and environmental parameters for each sampled specimen and year were entered into each analysis to overcome common misconceptions regarding the averaging of environmental data (cf. Körner and Hiltbrunner, 2018; Löffler and Pape, 2020).
First, we were interested in how the timing and duration of the growing season are linked to total realized growth and the observed shrinking phase during the winter months. Therefore, we tested relations among our previously defined parameters (cf. Sect. 2.3) by modeling total annual growth and peak shrinking (dependent variables) from growth initiation, cessation, and peak growth (independent variables), using linear mixed-effect models with the study sites included as a random effect to account for inter-site variability (Table 2).
Table 2Results of linear mixed-effect modeling of total annual growth (measured cumulative stem diameter increment in comparison to the previous year's maximum stem diameter) and annual peak shrinking (maximum stem water deficit), modeled from growth parameters (growth initiation, growth cessation, and peak growth). Sites (including study region and elevation) entered into the models as random effects. R2 for the models was calculated using the r.squaredGLMM function from the MuMIn package (Barton, 2020).
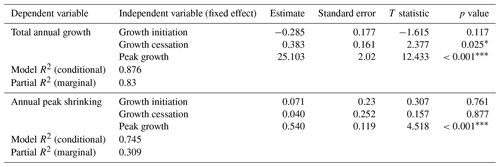
Asterisks indicate the significance based on the p value: * is significant (p value < 0.1); is strongly significant (p value < 0.01).
Having thus identified the role of these parameters in determining overall growth, we then analyzed the environment–growth relationship by modeling the parameters (total annual growth, peak shrinking, growth initiation, peak growth, and growth cessation) from the potential micro-environmental drivers presented in Table 1, similarly using linear mixed-effect models with the study sites included as a random effect. Since we observed a prominent period of radial stem shrinking during the winter months, we additionally included two parameters defining the timing of this period here (start of the shrinking period and day of year (DOY) on which peak shrinking occurred).
In addition to these inter-annual assessments of growth patterns and environmental controls, we subsequently analyzed the fine-scale, intra-annual seasonality in stem diameter variability to gain insight into seasonal response patterns of stem shrinking and swelling to local environmental surroundings. We performed a moving-window correlation, correlating daily rates of stem diameter variability (compared to the previous day) with measured daily environmental values for each of our four environmental parameters (i.e., TRZ, TSZ, GRSZ, and SMRZ). Coefficients are calculated over lagged time windows of 3 and 30 d, after testing window widths of up to 6 months. Overall, window width showed surprisingly little effect on the results.
3.1 Intra-annual stem diameter variability and growth patterns
In general, the seasonal variability in stem diameter was well explained by non-linear, sigmoid regressions (Gompertz curves) with a GoF between 0.90 and 0.94 (Fig. 1), and all specimens experienced distinct growing seasons starting in May or June, with little variation between the two study regions (Fig. A4). Moreover, our data revealed a distinct phase of radial stem shrinking following the growing phase towards the end of the year, starting in October, with remarkably little variation in timing between years (on average starting between the 287th and the 311th Julian day). In most cases, the stem radius remained below the previously achieved maximum for the entire winter and started to increase again with the following year's growing season (Fig. 1). The start of this shrinking period was significantly linked to the day when peak growth occurred (R=0.50; p=0.004), as well as to growth initiation (R=0.40; p=0.023) and cessation (R=0.52; p=0.0023).
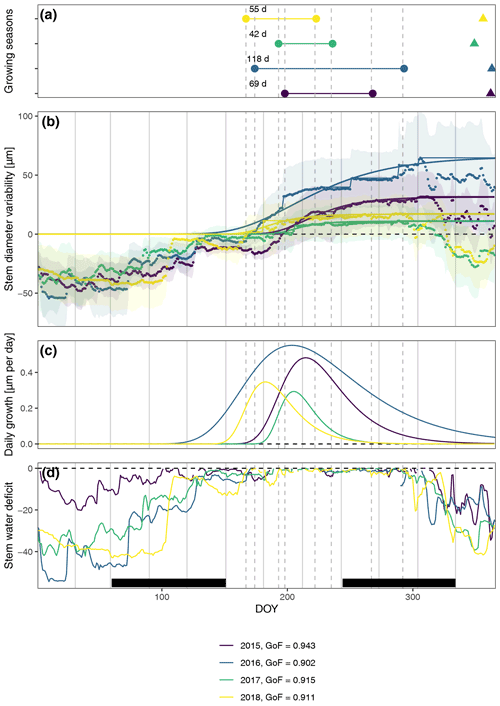
Figure 1Observed and modeled variability in stem diameter. (a) Growing seasons derived from fitted Gompertz models and timing of peak shrinking defined as the maximum stem water deficit (triangles). (b) Averaged measured daily stem diameter variability and fitted Gompertz models (a goodness-of-fit (GoF) measure was calculated using the least-squares method). Models were fitted to zero growth curves derived from the original measurements as cumulative maxima (thin lines), assuming zero growth during phases of prolonged stem shrinkage. In this way, annual growth and, consequently, growth start are directly linked to growth during the previous year, and additional rehydrating processes before the start of the main growing season are excluded. Shaded areas indicate standard deviation, showing the variability among the specimens. (c) Daily growth rates derived from Gompertz models. (d) Stem water deficit defined as reversible radial stem variability at times when no growth-induced irreversible stem expansion was measured. Black bars indicate meteorological seasons, while dashed lines show timing of the respective growing seasons to aid visual interpretation.
Table 3Parameters of stem change (means). Numbers in parentheses represent variability between specimens (coefficient of variation, CV).
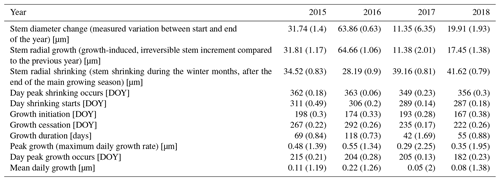
The observed total annual growth ranged from 11 µm in 2017 to 65 µm in 2016, on average (Table 3), with high variation between specimens and sites. Some specimens (31 % of the dendrometer curves) experienced zero growth in one or more years, and these dormant years were linked to comparatively long periods of snow cover during the previous winter, with a highly negative correlation between stem diameter change and the number of snow-free days (; p=0.024; Fig. A5) during these years. Interestingly, patterns of stem diameter variability and timing of the growing season were similar in the two studied regions (Fig. A4). Furthermore, while our data showed slight differences in total annual growth between the two regions (Fig. A6), there were no clear overall patterns in our data related to the elevational gradient or study regions, beyond the high inter-specimen variability observed in the whole dataset (Fig. A2c). Our chosen growth parameters – growth initiation, peak growth, and growth cessation – together explained 88 % of the variance in total annual growth, with peak growth (the maximum daily growth rate) having by far the greatest influence, indicating that the overall duration of the growth phase was less important for overall growth than the daily growth rate. For stem contraction (shrinking), the same parameters explained 75 % of the variance (Table 2 and Fig. A7), suggesting that the observed winter shrinking in E. hermaphroditum might be linked to growth during the growing season.
3.2 On-site environmental conditions
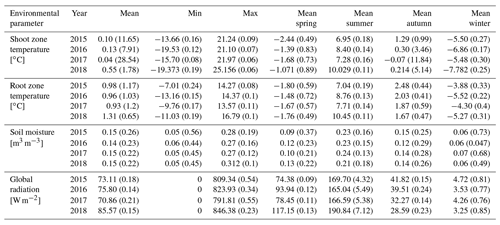
The different near-surface regimes of TRZ, TSZ, SMRZ, and GRSZ at our monitored sites are illustrated in Fig. 2. Averaged values for all sites (N=12) are summarized in Table 4. In all four monitored years, our sampled specimens experienced the highest temperatures during summer and, due to the exposed, wind-blown nature of the studied sites, only short periods of shallow snow cover during winter (Fig. 3). The period in which our specimens were snow-covered varied considerably between the monitored winters and between sites. The winter of 2015/16 had comparatively little snow, whereas 2017/18 was snow-covered for the longest period. In 2018, the highest mean temperatures were expressed because of exceptionally high summer temperatures (number of days with TSZ > 10 ∘C – i.e., GDD10 – is 57), whereas 2015 and 2017 both experienced shorter periods of high temperatures. Temperatures varied slightly among sites (Table 4 and Fig. A2), with a noticeable but not linear temperature decline with elevation. As expected, the shoot and root zone temperature curves were well coupled. Additionally, we detected slight variability between the two studied regions but overall similar seasonal temperature patterns on the measured micro-scale (Figs. 3 and A1), which differs from the regional climate signal. Global radiation showed similar patterns at all sites as well, following the course of the astronomic sun angle, with a mid-summer maximum; however, there were large variations according to cloud coverage. As such, 2018 experienced a short period of temperature and radiation decrease during summer (Fig. 2).
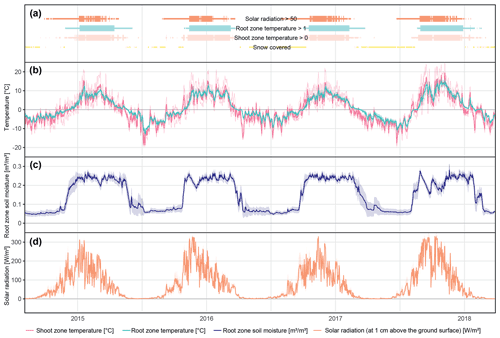
Figure 2Micro-environmental data. Daily mean values of shoot and root zone temperatures (b), soil moisture (c), and global radiation (d). Measurements were taken at each site individually but were averaged here over all sites for visualization. Shaded areas indicate standard deviations. Bars (a) show time spans for certain environmental conditions, with narrow bars marking the time at which the given condition was present at one or more (but not all) of the monitored sites, whereas broad bars mark the time for which the given condition was detected at all sites.
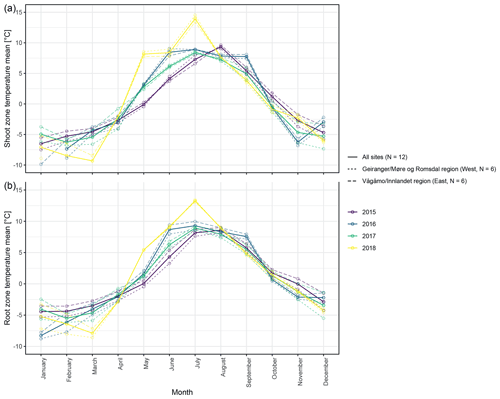
Figure 3Mean monthly shoot (a) and root (b) zone temperatures from 2015 to 2018, for visualization purposes averaged (i) over the measured sites per study region and (ii) over all measured sites.
3.3 Environmental controls of stem diameter variability
We investigated how the specific on-site environmental conditions were linked to the parameters described above. Moving-window correlations revealed a high seasonal variation in the relation of stem diameter variability and environmental conditions, with additional high variation across years. Overall, on-site environmental conditions had comparatively strong explanatory power during spring, when many of the specimens started stem increment. This spring stem increment was positively linked to radiation during cold spring conditions in 2015 and 2017 and showed no clear relation to spring temperatures (Figs. 2 and 4).
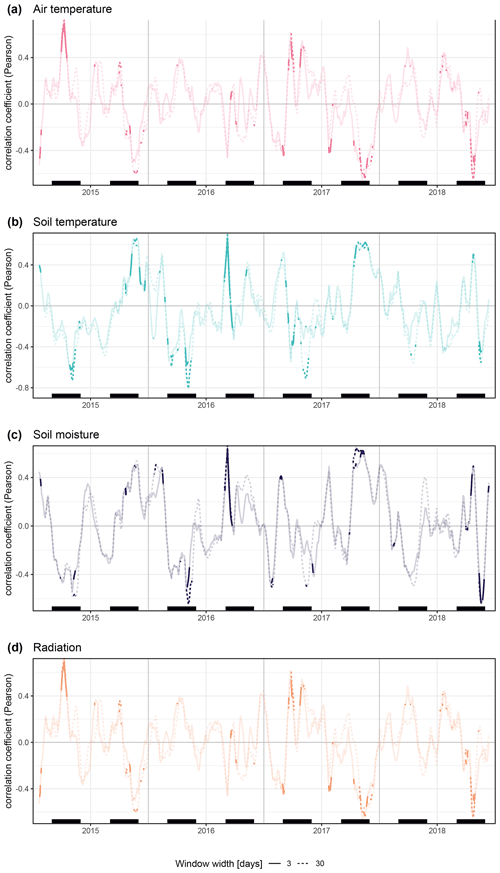
Figure 4Moving Pearson's correlation coefficients for daily rates of radial stem diameter variability against measured daily values of on-site environmental parameters (TSZ, TRZ, SMRZ, GRSZ). Results are shown for window widths of 3 (solid lines) and 30 (dashed lines) days. Non-significance (p>0.05) is indicated by transparency, and black bars show meteorological seasons. All individual specimens are entered into the analysis separately.
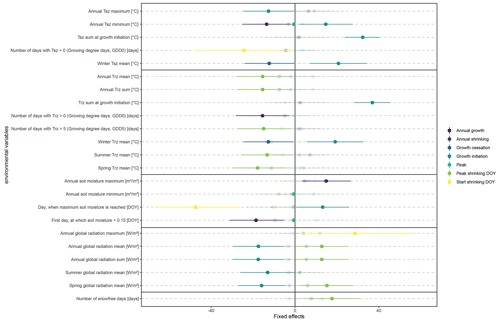
Figure 5Fixed effects and associated confidence intervals from linear mixed-effect models, modeling key parameters of stem change (dependent variables) from key environmental parameters characterizing on-site environmental conditions (for years with a total annual growth > 0). The environmental parameters are presented in Table 1, while the dependent variables included (i) total annual growth, defined as irreversible stem increment in comparison to the previous year; (ii) total annual shrinking, defined as stem shrinking during the winter months, after the end of the growing season; (iii) timing of the main growing season (start and end); (iv) peak growth, defined as the maximum daily growth rate; and (v) timing of the winter shrinking phase, including the start of stem shrinking (usually co-occurring with the maximum annual stem diameter) and peak shrinking, or the day when the minimum winter stem diameter is reached. All dates were given as day of the year (DOY). Only those environmental parameters which were significantly linked to at least one growth parameter are included in the plot. Nontransparent colors indicate significance.
The linear mixed models (Fig. 5) confirmed the lack of uniformity, indicating that a multitude of environmental drivers influenced growth, albeit to a very low degree and with high inter-annual variation. During 2016, the year with the highest radial stem growth, we also measured the highest number of days with soil temperatures above 5 ∘C, with temperatures rising quickly and steadily to 5 ∘C without reaching continuously higher values during the summer (Fig. 2). This indicates that optimum growth conditions lie within a soil temperature span of 5 to 10 ∘C. At the same time, maximum soil moisture had a strong positive influence on total annual growth, and growth was also linked to the timing of soil moisture rise in spring, resulting from thawing soils. Overall, our results indicate a strong influence of soil moisture on growth processes, with especially winter stem diameter variability closely linked to soil moisture availability and singular thawing events (Fig. A8). Growth initiation, on the other hand, was closely linked to radiation and winter temperatures. Here, our results show no clear influence of rising spring temperatures, and, counterintuitively, high winter temperatures led to a delayed growth start (Fig. 5).
In this study, we demonstrated that our focal species Empetrum hermaphroditum displayed distinct annual patterns of stem diameter variability in response to near-ground environmental drivers and in close accordance with distinct conditions caused by the local topographical characteristics of the studied wind-blown ridge positions. Previous dendroecological studies of shrub growth have commonly used macroclimatic data, such as free atmospheric air temperature, in combination with ring width measurements (e.g., Bär et al., 2007, 2008; Hollesen et al., 2015; Weijers et al., 2018a). While a direct comparison of annual growth derived from our dendrometer measurements and such ring width measurements at the studied sites revealed high synchrony (Fig. A9), dendrometer measurements have the potential to reveal much deeper insights into complex functional aspects of stem diameter variability and, in combination with on-site environmental data, might help in rethinking climate–growth relations. For example, while a phase of winter stem shrinking has been described in trees (Winget and Kozlowski, 1964; Zweifel and Häsler, 2000), the distinct and strongly pronounced phase found in our sampled specimens might be described as a unique feature of shrub growth, which we documented for the first time in this study. The reason why this phase has not been described earlier might be attributable to the methods used in the past to measure stem change in shrubs, which were insufficient to document intra-annual variability at the appropriate timescale. High-precision dendrometers can reveal these patterns in stem diameter variability, demonstrating the large amount of additional information gained from this method compared to traditional measuring methods (Knüsel et al., 2021). For our focal species Empetrum hermaphroditum, dendrometer measurements yielded several novel insights into phenology and growth physiology.
At the same time, common challenges of dendrometer use, including the separation of reversible (water-related) and irreversible (growth-related) stem increment, which have been widely discussed for trees (Zweifel, 2016; Cruz-García et al., 2019; Knüsel et al., 2021) proved similarly complex for our data. Here, the observed strong contraction and expansion patterns of the stems, most likely controlled by stem water variability within the plant, linked to the extreme thawing and freezing processes prominent at our study sites, provided an additional challenge. To fully explore these processes and possible links to transpiration dynamics and carbon fluxes, on-site measurements of the vapor pressure deficit, as a measure of atmospheric water demand, might yield additional insights (Novick et al., 2016; Peters et al., 2021; Zweifel et al., 2021). Furthermore, our study showed high inter-plant and inter-site variation in total annual growth, which has been previously described in E. hermaphroditum (Bär et al., 2008) and could be a result of internal growth variation within the multi-stemmed plant itself, which has not yet been fully explored (Bär et al., 2007). Overall, further studies are necessary to fully explore how dendrometer measurements compare to traditional measurement methods and which methodical adjustments are necessary for the extraction of physiologically meaningful data. Still, the comparative data presented in Fig. A9 clearly show that both types of measurement have the potential to reveal important physiological aspects of stem diameter variability and growth.
4.1 Radial stem growth variation
In general, we found total annual growth to be largely determined by peak growth. Thus, we cannot confirm a clear relation of the growing-season length and overall growth (Rammig et al., 2010; Blok et al., 2011; Prislan et al., 2019). The predominant role of peak growth in controlling total growth suggested that the shrubs were usually able to invest in new cells following the rise in water availability caused by thawing conditions in spring, most likely affected by prior carbohydrate storage. At the same time, an early growth start after favorable winter conditions may not be efficient in terms of total growth if conditions in early summer prevent the survival of the formed cells. For most snow-free ridges, budburst and flowering are not influenced by snowmelt and can therefore occur early on, causing high vulnerability to late frost events (Weijers et al., 2018b). This is in accordance with the findings of Choler (2018) and Weijers et al. (2018a), who suggested a strong influence of subzero temperatures in spring, counteracting improved conditions during summer, which would lead to the observed decoupling of the growing-season length from total realized growth. In our data, we found strong evidence of this relation in inter-annual comparison, with low growth rates in 2018 despite favorable summer conditions after an early growth start followed by a short cold snap and ground frost. Additionally, for some specimens we found total annual growth to be limited by long periods of snow cover during the previous winter, which might prevent E. hermaphroditum from photosynthetic activity and resource accumulation and thus may limit a crucial precondition of growth success. In accordance with Buchwal et al. (2013), we assumed that during years of no apparent radial growth, dwarf shrubs might prioritize growth in the more protected and long-living belowground segments, instead of investing in the more vulnerable shoots. This ability to reduce cambial activity to a minimum and cease aboveground wood formation is a trait common among woody plants (Wilmking et al., 2012).
4.2 Environmental controls of shrub growth
E. hermaphroditum at the monitored ridge positions is faced with unique near-ground environmental conditions, which are a feature of the heterogeneous topography that characterizes alpine terrain (Scherrer and Körner, 2011). These conditions are caused by the exposed, wind-blown nature of the ridges and thus are distinctly different from the immediate surroundings and to some extent decoupled from the regional climate signal. They include high exposure to global radiation and very little, temporary snow cover during the winter months, associated with very low temperatures (Wundram et al., 2010). In general, the effects of winter snow cover on shrub growth are a critical topic in arctic and alpine ecology, with findings ranging from positive (Blok et al., 2015; Addis and Bret-Harte, 2019) to negative (Schmidt et al., 2010) growth responses, depending on the snow depth and vegetation type.
Our results highlight the importance of the unique winter conditions for early growth, indicating that for our sampled evergreen species, the degree to which photosynthetic activity was effective in synthesizing carbohydrates during the winter months was especially important. During this time, extreme temperatures prevent cell production and differentiation, resulting in a carbon overflow (Körner, 2015; Saccone et al., 2017), which gives E. hermaphroditum the ability to start growth activity as soon as liquid water is available in the root zone (Starr and Oberbauer, 2003). In general, carbon assimilation may be greater or lower than the demand for plant functions, resulting in periods of carbon surplus and deficit, respectively (e.g., Martínez-Vilalta et al., 2016). Though there is never a critical depletion of carbohydrates in alpine plants, resources at or shortly after snowmelt and thus at the onset of the growing season are still diminished – due to respiratory consumption during winter (Körner, 2021). Any surplus of carbohydrates gained by photosynthetic activity in late winter and spring is, therefore, likely to be beneficial for growth throughout the growing period. Such continued photosynthetic activity was found in E. hermaphroditum, as well as several other evergreen shrub species before (e.g., Gimeno et al., 2012; Bienau et al., 2014; Wyka and Oleksyn, 2014; Blok et al., 2015), and photosynthetic activity of high-latitude vascular plants and shrubs has previously been observed under extreme thermal conditions (Semikhatova et al., 1992; Lundell et al., 2008). Accordingly, we concluded that growth initiation might be driven by the constantly increasing radiation with the astronomic rise of the angle of the sun. At that time of the year, energy transfer from global radiation into thermal heat was low, but radiation was high enough for photosynthetic activity, which might explain the decoupling of thermal and radiation drivers of growth initiation. Under snow-free conditions, it is likely that, in our study design, global radiation is directly linked to soil thawing close to the soil surface, which was previously linked to growth onset (Descals et al., 2020). Furthermore, we found growth initiation in E. hermaphroditum to be positively linked to winter temperatures, indicating that low winter temperatures were correlated with an early start of the growing season, in contrast to common assumptions relating high temperatures during late winter to an early growth start (Dolezal et al., 2020). This highlights the influence of high radiation on energy storage during periods of absent snow cover, which are usually accompanied by low temperatures, whereas mild winters are often associated with cloudy, humid weather and snow cover on the ridges.
Photosynthetic activity during winter, however, forced by the exposure of the plant to high incoming solar radiation (Saccone et al., 2017), causes continued water transport under extreme temperatures, increasing the risk of cavitation (Tyree and Sperry, 1989; Venn and Green, 2018) while long and severe ground frosts might limit access to soil moisture and frost-triggered droughts might thus result in tissue damage caused by an internal water deficit (Mayr et al., 2006). Yet, E. hermaphroditum at our studied sites proved mostly frost-hardy, drought-tolerant, and highly adapted to these conditions (Carlquist, 1989; Hacke et al., 2001). In this context, we assume that E. hermaphroditum uses cell dehydration to actively protect living cells from the consequences of freezing (e.g., ice nucleation), causing the radial stem contraction during phases of extreme subzero temperatures evident in our data. In trees, radial stem shrinkage has been related to sap flow and tree water content (Winget and Kozlowski, 1964; Zweifel and Häsler, 2000; Zweifel et al., 2006; Tian et al., 2019). When temperatures sink below approximately −5 ∘C, extra-cellular water begins to freeze, inducing the osmotic withdrawal of intra-cellular water and thus cell and ultimately stem shrinkage (Zweifel et al., 2000; King et al., 2013). As such, stem shrinkage could be interpreted as a result of freezing processes causing living cell shrinkage because of water losses, as well as a strategy to avoid frost damage, especially where the protective effects of snow cover are missing (Kuprian et al., 2014; Neuner, 2014; Charra-Vaskou et al., 2016).
Furthermore, our findings confirmed commonly assumed positive effects of temperatures on radial stem growth in E. hermaphroditum to some extent (Shaver and Chapin, 1986; Shevtsova et al., 1997; Hollesen et al., 2015; Ackerman et al., 2017; Bråthen et al., 2018), with optimum growth conditions within a soil temperature span of 5 to 10 ∘C, which is in accordance with previously reported temperature thresholds for alpine plant distribution (Körner and Paulsen, 2004; Rossi et al., 2008; Steppe et al., 2015). On the other hand, our analysis indicated no direct relationship between total growth and near-surface temperatures, and a clear link between near-surface thermal conditions and stem diameter variability was lacking, suggesting more complex relations, probably influenced by the evident temperature extremes at our chosen sites, as well as complex interconnections with soil water availability (Körner and Hiltbrunner, 2018). A critical temperature threshold, as present in many trees (Rossi et al., 2007, 2008; Deslauriers et al., 2008) and found for xylem growth of alpine rhododendron shrubs (Li et al., 2016), could not be determined for E. hermaphroditum. In trees, the timing of the maximum growth and growth cessation in cold environments has been linked to day length (Heide, 1985; Rossi et al., 2006; Duchesne et al., 2012). This cannot be confirmed for our monitored shrubs because of the high variability observed between specimens. Therefore, timing of these critical parameters is most likely controlled by different factors in shrubs than those assumed for trees. Here, our results highlight soil moisture availability as the main driver for the end of the growing season. This accounts for the species dependency on a damp climate and high rainfall (Bell and Tallis, 1973).
Overall, we conclude that surviving during extreme winters was the main principle governing E. hermaphroditum growth when existing at alpine ridge positions, causing unique adaptations to local micro-site conditions. Additionally, our results highlight the overall importance of the root zone soil moisture as the key driver of growth in E. hermaphroditum.
4.3 Potential response to environmental change
The fine-scale data provided by dendrometer measurements proved highly important, since they allowed for a detailed growth analysis, revealing a growth mechanism that is highly adapted to the local micro-environmental conditions at our studied exposed ridge positions. The ability of continuing photosynthetic activity throughout the year and thus of aggregating resources for use in early cell formation, as observed in other evergreen plants (Wyka and Oleksyn, 2014), might provide a competitive advantage of the evergreen species over deciduous species in the same habitat, limiting the risk of losing resources through competition. However, it is most likely a unique feature of the snow-free ridge positions and thus not present at other micro-topographic positions, where the energy budget and water balance are strongly altered (Dahl, 1956; Fritts, 1976; Löffler et al., 2006; Pape et al., 2009).
To sustain the continued metabolism throughout the year, we found that the overall growth mechanism of E. hermaphroditum was mainly defined by moisture, as well as solar radiation. Hence, temperatures mainly played a role in freezing and thawing processes, on an intra-annual scale. We can thus confirm that while there is a link between shrub growth and warming conditions, it is most likely not uniform and highly variable over spatial and temporal scales (Elmendorf et al., 2012). The high local adaptation explains the wide distribution and competitive ability of the species at these sites (Bienau et al., 2014, 2016; Löffler and Pape, 2020) and highlights the prominent role of on-site near-ground environmental conditions in controlling growth processes (Zellweger et al., 2020). In a changing climatic regime, this dependency on specific winter radiation conditions and soil moisture availability might become a disadvantage, complicating the adaptation to warming winters and longer snow-covered periods, coupled with prolonged dry periods during summer (Hollesen et al., 2015; Weijers et al., 2018a), which might cause early growth cessation. Thus, we conclude that changing winter conditions and altered snow regimes represent one of the most serious threats to evergreen shrub growth in tundra ecosystems and E. hermaphroditum will not be able to persist at exposed positions in a changing climate or respond with longer periods of dormancy to warming conditions. This will potentially promote the spread of competing deciduous species and thus contribute to the arctic–alpine greening trend.
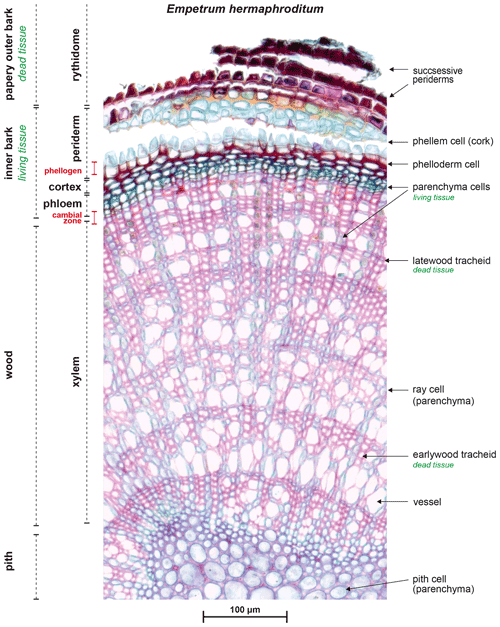
Figure A1Radial micro-slide of a stem from Empetrum nigrum ssp. hermaphroditum (1 : 1000 magnification). The outermost layers of the papery outer bark, which repeat the successive pattern of the shown periderm, were lost while cutting. In our dendrometer approach, we removed the outer layers of the bark, most likely down to the phellogen. As shown here, the loose bark structure allows removal without severe damage of the inner tissue. We aimed at mounting our dendrometer sensor as close to the still protected cambial zone to obtain data on physiologically active stem diameter variability such as growth, excluding swelling and shrinking of the passive outer bark tissue.
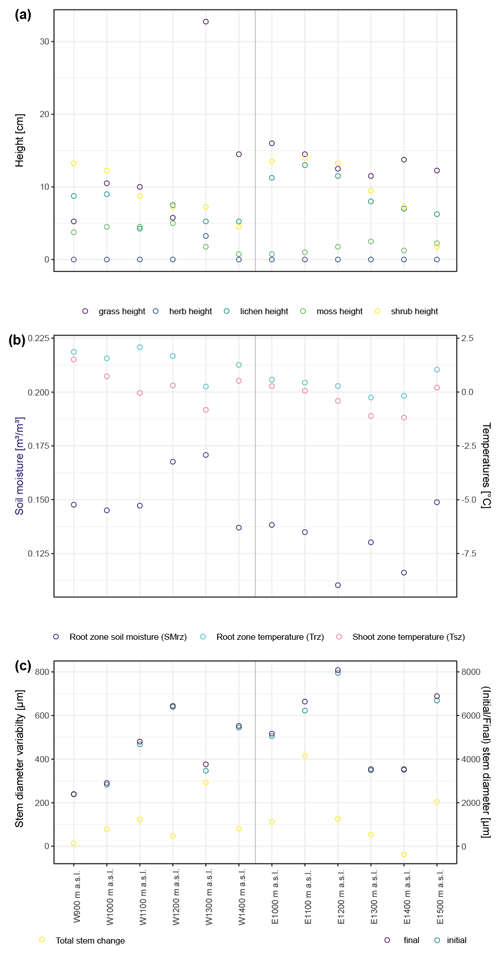
Figure A2Summary of canopy height at the 12 studied sites by major vegetation type (a). Trees are not included because there were no trees present. Panel (b) shows micro-environmental conditions averaged over the studied period, as well as minima and maxima for temperatures measured within the shoot and root zones and for soil moisture in the root zone. Panel (c) summarizes the total stem diameter change as well as stem diameter at the start and end of the studied period (2015–2018).
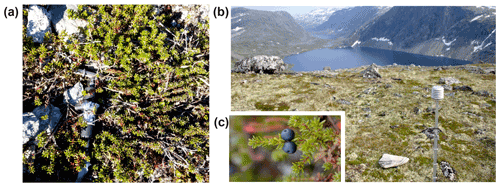
Figure A3Dendrometer setup (a), the studied species E. hermaphroditum in the studied region in central Norway (b), and the species fruits and leaves (c).
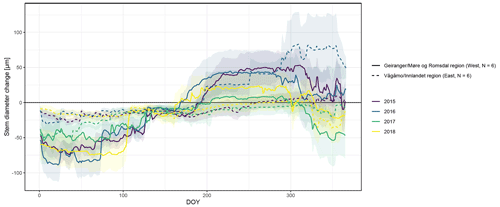
Figure A4Observed stem diameter change averaged over the monitored specimens within each of the two study regions (Vågåmo, Innlandet, region (east, N=6) and Geiranger, Møre og Romsdal, region (west, N=6)). Transparency indicates standard deviation.
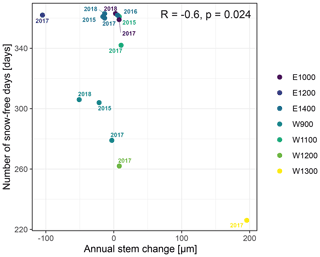
Figure A5Number of snow-free days and annual stem diameter change in years in which no irreversible growth occurred (total annual growth = 0). Colors indicate the monitored specimens at the individual sites (E denotes east; W denotes west; numbers indicate elevation, m a.s.l.).
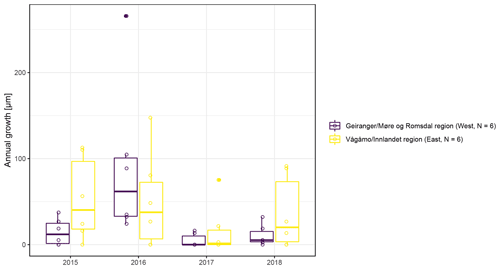
Figure A6Boxplots of observed annual growth within each of the two study regions (Vågåmo, Innlandet, region (east, N=6) and Geiranger, Møre og Romsdal, region (west, N=6)).
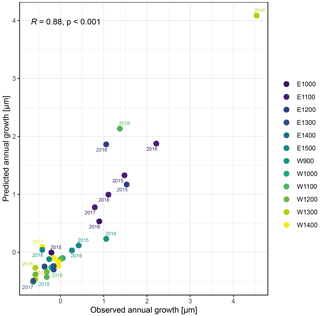
Figure A7Observed annual growth and annual growth predicted by a linear regression model including timing (growth initiation and growth cessation), as well as peak growth as independent variables. Colors indicate the monitored specimens at the individual sites (E denotes east; W denotes west; numbers indicate elevation, m a.s.l.).
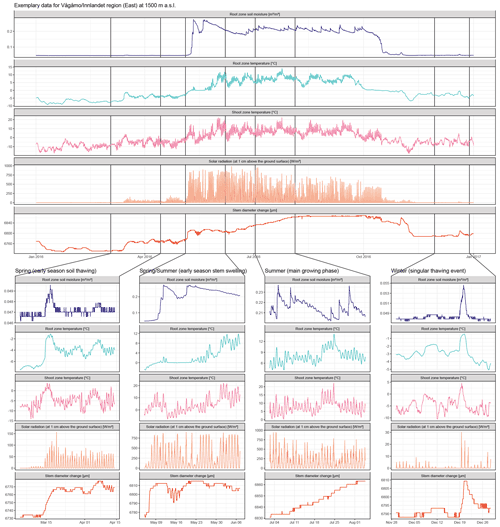
Figure A8Raw data for one exemplary specimen from the Vågåmo, Innlandet, region at 1500 m a.s.l. Here, we present hourly data of stem diameter variability and the respective micro-environmental conditions. The four sections show important phases of the annual stem diameter variability and their relation to the micro-environment in detail. Coupling of soil moisture and stem diameter during the winter and spring months, when water-induced stem swelling and shrinking occur and decoupling during the main growing phase is clearly evident. Additionally, the direct response of the stem diameter to singular soil thawing events in winter is clearly visible in the curves.
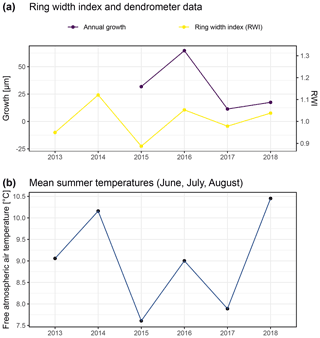
Figure A9Comparison of annual growth measured using dendrometers (see “Material and methods”) and ring width derived by measuring annual growth rings of 12 specimens from similar positions on exposed ridges, presented here as the ring width index (RWI). Ring width was measured from multiple micro-slices per specimen (following Bär et al., 2006; a). Panel (b) shows free atmospheric air temperatures measured at 2 m above ground in both study regions. Such temperature data are commonly used for comparison of climate–growth relationships in dendroecological studies. With this figure we aimed to reproduce previous studies (Bär et al., 2006, 2007) for comparison with our dendrometer measurements.
All underlying data pertinent to the results presented in this publication are publicly available in a data publication in ERDKUNDE – Archive for Scientific Geography (https://www.erdkunde.uni-bonn.de, last access: 8 March 2022; https://doi.org/10.3112/erdkunde.2021.dp.01, Löffler et al., 2021).
JL had the idea, designed the research platform, conducted the fieldwork, and together with RP ran the long-term project. SD analyzed the data, led the writing of the manuscript, and arranged the figures, with contributions from RP and JL.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors thank Eike Albrecht, Niklas Beckers, Elise Dierking, Nils Hein, Stef Weijers, and Dirk Wundram for collaboration within our LTAER project; Ole Øvsteng and Anders Svare for hospitality; and both the landowners and Norwegian authorities (Vågå and Stranda municipalities) for overall support. This work was supported by the Open Access Publication Fund of the University of Bonn.
This research has been supported by the Deutsche Forschungsgemeinschaft (grant nos. LO 830/16-1 and LO 830/32-1).
This open-access publication was funded by the University of Bonn.
This paper was edited by Michael Bahn and reviewed by five anonymous referees.
Aartsma, P., Asplund, J., Odland, A., Reinhardt, S., and Renssen, H.: Microclimatic comparison of lichen heaths and shrubs: shrubification generates atmospheric heating but subsurface cooling during the growing season, Biogeosciences, 18, 1577–1599, https://doi.org/10.5194/bg-18-1577-2021, 2021.
Ackerman, D., Griffin, D., Hobbie, S. E., and Finlay, J. C.: Arctic shrub growth trajectories differ across soil moisture levels, Glob. Change Biol., 23, 4294–4302, https://doi.org/10.1111/gcb.13677, 2017.
Addis, C. E. and Bret-Harte, M. S.: The importance of secondary growth to plant responses to snow in the arctic, Funct. Ecol., 33, 1050–1066, https://doi.org/10.1111/1365-2435.13323, 2019.
AMAP: Arctic Climate Change Update 2021: Key Trends and Impacts, Summary for Policy-makers, Arctic Monitoring and Assessment Programme (AMAP), Tromsø, Norway, 1–16, https://oaarchive.arctic-council.org/handle/11374/2621 (last access: 8 March 2022), 2021.
Aune, B. (Ed.): National atlas of Norway: climate, Norwegian Mapping Authority, Hønefoss, Norway, ISBN 829-0408-145, 978-8-2904-081-40, 1993.
Bär, A., Bräuning, A., and Löffler, J.: Dendroecology of dwarf shrubs in the high mountains of Norway – A methodological approach, Dendrochronologia, 24, 17–27, https://doi.org/10.1016/j.dendro.2006.05.001, 2006.
Bär, A., Bräuning, A., and Löffler, J.: Ring-width chronologies of the alpine dwarf shrub Empetrum hermaphroditum from the Norwegian mountains, IAWA J., 28, 325–338, https://doi.org/10.1163/22941932-90001644, 2007.
Bär, A., Pape, R., Bräuning, A., and Löffler, J.: Growth-ring variations of dwarf shrubs reflect regional climate signals in alpine environments rather than topoclimatic differences, J. Biogeogr., 35, 625–636, https://doi.org/10.1111/j.1365-2699.2007.01804.x, 2008.
Barton, K.: MuMIn: Multi-Model Inference, R package version 1.43.17, https://CRAN.R-project.org/package=MuMIn (last access: 8 March 2022), 2020.
Bell, J. N. B. and Tallis, J. H.: Empetrum nigrum L, J. Ecol., 61, 289–305, https://doi.org/10.2307/2258934, 1973.
Bienau, M. J., Hattermann, D., Kröncke, M., Kretz, L., Otte, A., Eiserhardt, W. L., Milbau, A., Graae, B. J., Durka, W., and Eckstein, R. L.: Snow cover consistently affects growth and reproduction of Empetrum hermaphroditum across latitudinal and local climatic gradients, Alp. Bot., 124, 115–129, https://doi.org/10.1007/s00035-014-0137-8, 2014.
Bienau, M. J., Eckstein, R. L., Otte, A., and Durka, W.: Clonality increases with snow depth in the arctic dwarf shrub Empetrum hermaphroditum, Am. J. Bot., 103, 2105–2114, https://doi.org/10.3732/ajb.1600229, 2016.
Blok, D., Sass-Klaassen, U., Schaepman-Strub, G., Heijmans, M. M. P. D., Sauren, P., and Berendse, F.: What are the main climate drivers for shrub growth in Northeastern Siberian tundra?, Biogeosciences, 8, 1169–1179, https://doi.org/10.5194/bg-8-1169-2011, 2011.
Blok, D., Weijers, S., Welker, J. M., Cooper, E. J., Michelsen, A., Löffler, J., and Elberling, B.: Deepened winter snow increases stem growth and alters stem δ13C and δ15N in evergreen dwarf shrub Cassiope tetragona in high-arctic Svalbard tundra, Environ. Res. Lett., 10, 44008, https://doi.org/10.1088/1748-9326/10/4/044008, 2015.
Bråthen, K. A., Gonzalez, V. T., and Yoccoz, N. G.: Gatekeepers to the effects of climate warming? Niche construction restricts plant community changes along a temperature gradient, Perspect. Plant Ecol. Evol. Syst., 30, 71–81, https://doi.org/10.1016/j.ppees.2017.06.005, 2018.
Breitsprecher, A. and Bethel, J. S.: Stem-growth periodicity of trees in a tropical wet forest of Costa Rica, Ecology, 71, 1156–1164, https://doi.org/10.2307/1937383, 1990.
Brodie, J. F., Roland, C. A., Stehn, S. E., and Smirnova, E.: Variability in the expansion of trees and shrubs in boreal Alaska, Ecology, 100, e02660, https://doi.org/10.1002/ecy.2660, 2019.
Buchwal, A., Rachlewicz, G., Fonti, P., Cherubini, P., and Gärtner, H.: Temperature modulates intra-plant growth of Salix polaris from a high Arctic site (Svalbard), Polar Biol., 36, 1305–1318, https://doi.org/10.1007/s00300-013-1349-x, 2013.
Cabon, A., Peters, R. L., Fonti, P., Martínez-Vilalta, J., and Cáceres, M.: Temperature and water potential co-limit stem cambial activity along a steep elevational gradient, New Phytol., 226, 1325–1340, https://doi.org/10.1111/nph.16456, 2020.
Carlquist, S.: Wood and bark anatomy of empetraceae; comments on paedomorphosis in woods of certain small shrubs, Aliso, 12, 497–515, 1989.
Carlson, B. Z., Corona, M. C., Dentant, C., Bonet, R., Thuiller, W., and Choler, P.: Observed long-term greening of alpine vegetation – a case study in the French Alps, Environ. Res. Lett., 12, 114006, https://doi.org/10.1088/1748-9326/aa84bd, 2017.
Chan, T., Hölttä, T., Berninger, F., Mäkinen, H., Nöjd, P., Mencuccini, M., and Nikinmaa, E.: Separating water-potential induced swelling and shrinking from measured radial stem variations reveals a cambial growth and osmotic concentration signal, Plant Cell Environ., 39, 233–244, https://doi.org/10.1111/pce.12541, 2016.
Chapin, F. S., Sturm, M., Serreze, M. C., McFadden, J. P., Key, J. R., Lloyd, A. H., McGuire, A. D., Rupp, T. S., Lynch, A. H., Schimel, J. P., Beringer, J., Chapman, W. L., Epstein, H. E., Euskirchen, E. S., Hinzman, L. D., Jia, G., Ping, C.-L., Tape, K. D., Thompson, C. D. C., Walker, D. A., and Welker, J. M.: Role of land-surface changes in arctic summer warming, Science, 310, 657–660, https://doi.org/10.1126/science.1117368, 2005.
Charra-Vaskou, K., Badel, E., Charrier, G., Ponomarenko, A., Bonhomme, M., Foucat, L., Mayr, S., and Améglio, T.: Cavitation and water fluxes driven by ice water potential in Juglans regia during freeze-thaw cycles, J. Exp. Bot., 67, 739–750, https://doi.org/10.1093/jxb/erv486, 2016.
Choler, P.: Winter soil temperature dependence of alpine plant distribution: Implications for anticipating vegetation changes under a warming climate, Perspect. Plant Ecol. Evol. Syst., 30, 6–15, https://doi.org/10.1016/j.ppees.2017.11.002, 2018.
Cruz-García, R., Balzano, A., Čufar, K., Scharnweber, T., Smiljanić, M., and Wilmking, M.: Combining Dendrometer Series and Xylogenesis Imagery – DevX, a Simple Visualization Tool to Explore Plant Secondary Growth Phenology, Front. For. Glob. Change, 2, 60, https://doi.org/10.3389/fpls.2021.674438, 2019.
Cuny, H. E., Rathgeber, C. B. K., Frank, D., Fonti, P., Mäkinen, H., Prislan, P., Rossi, S., Del Castillo, E. M., Campelo, F., Vavrčík, H., Camarero, J. J., Bryukhanova, M. V., Jyske, T., Gričar, J., Gryc, V., Luis, M. d., Vieira, J., Čufar, K., Kirdyanov, A. V., Oberhuber, W., Treml, V., Huang, J.-G., Li, X., Swidrak, I., Deslauriers, A., Liang, E., Nöjd, P., Gruber, A., Nabais, C., Morin, H., Krause, C., King, G., and Fournier, M.: Woody biomass production lags stem-girth increase by over one month in coniferous forests, Nat. Plants, 1, 15160, https://doi.org/10.1038/nplants.2015.160, 2015.
Dahl, E.: Rondane, mountain vegetation in south Norway and its relation to the environment, Skrifter utgitt av det Norske Videnskaps-Akademi i Oslo, Mathematisk-Naturvidenskapelig Klasse, 3, 1–374, 1956.
Daubenmire, R. F.: An improved type of precision dendrometer, Ecology, 26, 97–98, 1945.
De Schepper, V. and Steppe, K.: Development and verification of a water and sugar transport model using measured stem diameter variations, J. Exp. Bot., 61, 2083–2099, https://doi.org/10.1093/jxb/erq018, 2010.
Descals, A., Verger, A., Filella, I., Baldocchi, D., Janssens, I. A., Fu, Y. H., Piao, S., Peaucelle, M., Ciais, P., and Peñuelas, J.: Soil thawing regulates the spring growth onset in tundra and alpine biomes, Sci. Total Environ., 742, 140637, https://doi.org/10.1016/j.scitotenv.2020.140637, 2020.
Deslauriers, A., Rossi, S., and Anfodillo, T.: Dendrometer and intra-annual tree growth: What kind of information can be inferred?, Dendrochronologia, 25, 113–124, https://doi.org/10.1016/j.dendro.2007.05.003, 2007.
Deslauriers, A., Rossi, S., Anfodillo, T., and Saracino, A.: Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy, Tree Physiol., 28, 863–871, https://doi.org/10.1093/treephys/28.6.863, 2008.
Dobbert, S., Pape, R., and Löffler, J.: Contrasting growth response of evergreen and deciduous arctic-alpine shrub species to climate variability, Ecosphere, 12, e03688, https://doi.org/10.1002/ecs2.3688, 2021a.
Dobbert, S., Pape, R., and Löffler, J.: How does spatial heterogeneity affect inter- and intraspecific growth patterns in tundra shrubs?, J. Ecol., 7, 4115–4131, https://doi.org/10.1111/1365-2745.13784, 2021b.
Dolezal, J., Kurnotova, M., Stastna, P., and Klimesova, J.: Alpine plant growth and reproduction dynamics in a warmer world, New Phytol., 228, 1295–1305, https://doi.org/10.1111/nph.16790, 2020.
Drew, D. M. and Downes, G. M.: The use of precision dendrometers in research on daily stem size and wood property variation: A review, Dendrochronologia, 27, 159–172, https://doi.org/10.1016/j.dendro.2009.06.008, 2009.
Duchesne, L., Houle, D., and D'Orangeville, L.: Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada, Agr. Forest Meteorol., 162–163, 108–114, https://doi.org/10.1016/j.agrformet.2012.04.016, 2012.
Elmendorf, S. C., Henry, G. H. R., Hollister, R. D., Björk, R. G., Boulanger-Lapointe, N., Cooper, E. J., Cornelissen, J. H. C., Day, T. A., Dorrepaal, E., Elumeeva, T. G., Gill, M., Gould, W. A., Harte, J., Hik, D. S., Hofgaard, A., Johnson, D. R., Johnstone, J. F., Jónsdóttir, I. S., Jorgenson, J. C., Klanderud, K., Klein, J. A., Koh, S., Kudo, G., Lara, M., Lévesque, E., Magnússon, B., May, J. L., Mercado-Díaz, J. A., Michelsen, A., Molau, U., Myers-Smith, I. H., Oberbauer, S. F., Onipchenko, V. G., Rixen, C., Martin Schmidt, N., Shaver, G. R., Spasojevic, M. J., Şórhallsdóttir, Ş. E., Tolvanen, A., Troxler, T., Tweedie, C. E., Villareal, S., Wahren, C.-H., Walker, X., Webber, P. J., Welker, J. M., and Wipf, S.: Plot-scale evidence of tundra vegetation change and links to recent summer warming, Nat. Clim. Change, 2, 453–457, https://doi.org/10.1038/nclimate1465, 2012.
Fatichi, S., Pappas, C., Zscheischler, J., and Leuzinger, S.: Modelling carbon sources and sinks in terrestrial vegetation, New Phytol., 221, 652–668, https://doi.org/10.1111/nph.15451, 2019.
Fekedulegn, D., Mac Siurtain, M., and Colbert, J.: Parameter estimation of nonlinear growth models in forestry, Silva Fenn., 33, 653, https://doi.org/10.14214/sf.653, 1999.
Francon, L., Corona, C., Till-Bottraud, I., Choler, P., Carlson, B. Z., Charrier, G., Améglio, T., Morin, S., Eckert, N., Roussel, E., Lopez-Saez, J., and Stoffel, M.: Assessing the effects of earlier snow melt-out on alpine shrub growth: The sooner the better?, Ecol. Indic., 115, 106455, https://doi.org/10.1016/j.ecolind.2020.106455, 2020.
Frindte, K., Pape, R., Werner, K., Löffler, J., and Knief, C.: Temperature and soil moisture control microbial community composition in an arctic-alpine ecosystem along elevational and micro-topographic gradients, ISME J., 13, 2031–2043, https://doi.org/10.1038/s41396-019-0409-9, 2019.
Fritts, H. (Ed.): Tree Rings and Climate, Elsevier Science, Oxford, 583 pp., ISBN 978-0-12268-450-0, 1976.
Gall, R., Landolt, W., Schleppi, P., Michellod, V., and Bucher, J. B.: Water content and bark thickness of Norway spruce ( Picea abies) stems: phloem water capacitance and xylem sap flow, Tree Physiol., 22, 613–623, https://doi.org/10.1093/treephys/22.9.613, 2002.
Gimeno, T. E., Camarero, J. J., Granda, E., Pías, B., and Valladares, F.: Enhanced growth of Juniperus thurifera under a warmer climate is explained by a positive carbon gain under cold and drought, Tree Physiol., 32, 326–336, https://doi.org/10.1093/treephys/tps011, 2012.
González-Rodríguez, Á. M., Brito, P., Lorenzo, J. R., Gruber, A., Oberhuber, W., and Wieser, G.: Seasonal cycles of sap flow and stem radius variation of Spartocytisus supranubius in the alpine zone of Tenerife, Canary Islands, Alpine Bot., 127, 97–108, https://doi.org/10.1007/s00035-017-0189-7, 2017.
Gough, L., Bass, H., and McLaren, J. R.: Effects of Increased Soil Nutrients on Seed Rain: A Role for Seed Dispersal in the Greening of the Arctic? Arct. Antarct Alp. Res., 47, 27–34, https://doi.org/10.1657/AAAR0014-055, 2015.
Graae, B. J., Vandvik, V., Armbruster, W. S., Eiserhardt, W. L., Svenning, J.-C., Hylander, K., Ehrlén, J., Speed, J. D.M., Klanderud, K., Bråthen, K. A., Milbau, A., Opedal, Ø. H., Alsos, I. G., Ejrnæs, R., Bruun, H. H., Birks, H. J. B., Westergaard, K. B., Birks, H. H., and Lenoir, J.: Stay or go – how topographic complexity influences alpine plant population and community responses to climate change, Perspect. Plant Ecol. Evol. Syst., 30, 41–50, https://doi.org/10.1016/j.ppees.2017.09.008, 2018.
Grams, T. E. E., Hesse, B. D., Gebhardt, T., Weikl, F., Rötzer, T., Kovacs, B., Hikino, K., Hafner, B. D., Brunn, M., Bauerle, T., Häberle K.-H., Pretzsch, H., and Pritsch, K.: The Kroof experiment: realization and efficacy of a recurrent drought experiment plus recovery in a beech/spruce forest, Ecosphere, 12, e03399, https://doi.org/10.1002/ecs2.3399, 2021.
Hacke, U. G., Sperry, J. S., Pockman, W. T., Davis, S. D., and McCulloh, K. A.: Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure, Oecologia, 126, 457–461, https://doi.org/10.1007/s004420100628, 2001.
Heide, O. M.: Physiological aspects of climatic adaptation in plants with special reference to high-latitude environments, in: Plant production in the North: Proceedings from Plant Adaptation Workshop, edited by: Kaurin, A., Junttila, O., and Nilsen J., Norwegian University Press, Tromsö, 1–22, ISBN 978-82-0007-385-7, 1985.
Hein, N., Pape, R., Finch, O.-D., and Löffler, J.: Alpine activity patterns of Mitopus morio (Fabricius, 1779) are induced by variations in temperature and humidity at different scales in central Norway, J. Mt. Sci., 11, 644–655, https://doi.org/10.1007/s11629-013-2913-0, 2014.
Hollesen, J., Buchwal, A., Rachlewicz, G., Hansen, B. U., Hansen, M. O., Stecher, O., and Elberling, B.: Winter warming as an important co-driver for Betula nana growth in western Greenland during the past century, Glob. Change Biol., 21, 2410–2423, https://doi.org/10.1111/gcb.12913, 2015.
Ilek, A., Kucza, J., and Morkisz, K.: Hygroscopicity of the bark of selected forest tree species, IForest, 10, 220–226, https://doi.org/10.3832/ifor1979-009, 2016.
IPCC: Climate change 2013: The physical science basis Working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change, Cambridge University Press, New York, 2014.
Ježík, M., Blaženec, M., Kučera, J., Strelcová, K., and Ditmarová, L.: The response of intra-annual stem circumference increase of young European beech provenances to 2012–2014 weather variability, IForest, 9, 960–969, https://doi.org/10.3832/ifor1829-009, 2016.
King, G., Fonti, P., Nievergelt, D., Büntgen, U., and Frank, D.: Climatic drivers of hourly to yearly tree radius variations along a 6 ∘C natural warming gradient, Agr. Forest Meteorol., 168, 36–46, https://doi.org/10.1016/j.agrformet.2012.08.002, 2013.
Kleiven, M.: Studies on the xerophile vegetation in northern Gudbrandsdalen, Norway, Nytt. Mag. Bot., 7, 1–60, 1959.
Knüsel, S., Peters, R. L., Haeni, M., Wilhelm, M., and Zweifel, R.: Processing and Extraction of Seasonal Tree Physiological Parameters from Stem Radius Time Series, Forests, 12, 765, https://doi.org/10.3390/f12060765, 2021.
Köcher, P., Horna, V., and Leuschner, C.: Environmental control of daily stem growth patterns in five temperate broad-leaved tree species, Tree Physiol., 32, 1021–1032, https://doi.org/10.1093/treephys/tps049, 2012.
Körner, C.: Paradigm shift in plant growth control, Curr. Opin. Plant Biol., 25, 107–114, https://doi.org/10.1016/j.pbi.2015.05.003, 2015.
Körner, C. (Ed.): Alpine plant life: Functional plant ecology of high mountain ecosystems, 3rd Edn., Springer, Berlin, Heidelberg, 500 pp., https://doi.org/10.1007/978-3-030-59538-8, 2021.
Körner, C. and Hiltbrunner, E.: The 90 ways to describe plant temperature, Perspect. Plant Ecol. Evol. Syst., 30, 16–21, https://doi.org/10.1016/j.ppees.2017.04.004, 2018.
Körner, C. and Paulsen, J.: A world-wide study of high altitude treeline temperatures, J. Biogeogr., 31, 713–732, https://doi.org/10.1111/j.1365-2699.2003.01043.x, 2004.
Kuprian, E., Briceño, V. F., Wagner, J., and Neuner, G.: Ice barriers promote supercooling and prevent frost injury in reproductive buds, flowers and fruits of alpine dwarf shrubs throughout the summer, Environ. Exp. Bot., 106, 4–12, https://doi.org/10.1016/j.envexpbot.2014.01.011, 2014.
Le Moullec, M., Buchwal, A., Wal, R., Sandal, L., and Hansen, B. B.: Annual ring growth of a widespread high arctic shrub reflects past fluctuations in community-level plant biomass, J. Ecol., 107, 436–451, https://doi.org/10.1111/1365-2745.13036, 2019.
Li, X., Rossi, S., Liang, E., and Julio Camarero, J.: Temperature thresholds for the onset of xylogenesis in alpine shrubs on the Tibetan Plateau, Trees, 30, 2091–2099, https://doi.org/10.1007/s00468-016-1436-z, 2016.
Liang, E., Lu, X., Ren, P., Li, X., Zhu, L., and Eckstein, D.: Annual increments of juniper dwarf shrubs above the tree line on the central Tibetan Plateau: a useful climatic proxy, Ann. Bot., 109, 721–728, https://doi.org/10.1093/aob/mcr315, 2012.
Liu, X., Nie, Y., and Wen, F.: Seasonal dynamics of stem radial increment of Pinus taiwanensis Hayata and its response to environmental factors in the Lushan Mountains, Southeastern China, Forests, 9, 387, https://doi.org/10.3390/f9070387, 2018.
Löffler, J.: Micro-climatic determination of vegetation patterns along topographical, altitudinal, and oceanic-continental gradients in the high mountains of Norway, Erdkunde, 57, 232–249, https://doi.org/10.3112/erdkunde.2003.03.05, 2003.
Löffler, J.: Snow cover dynamics, soil moisture variability and vegetation ecology in high mountain catchments of central Norway, Hydrol. Process., 19, 2385–2405, https://doi.org/10.1002/hyp.5891, 2005.
Löffler, J.: The influence of micro-climate, snow cover, and soil moisture on ecosystem functioning in high mountains, J. Geogr. Sci., 17, 3–19, https://doi.org/10.1007/s11442-007-0003-3, 2007.
Löffler, J. and Finch, O.-D.: Spatio-temporal Gradients between high mountain ecosystems of central Norway, Arct. Antarct. Alp. Res., 37, 499–513, https://doi.org/10.1657/1523-0430(2005)037[0499:SGBHME]2.0.CO;2, 2005.
Löffler, J. and Pape, R.: Thermal niche predictors of alpine plant species, Ecology, 101, e02891, https://doi.org/10.1002/ecy.2891, 2020.
Löffler, J., Pape, R., and Wundram, D.: The climatologic significance of topography, altitude and region in high mountains – A survey of oceanic-continental differentiations of the Scandes, Erdkunde, 60, 15–24, https://doi.org/10.3112/erdkunde.2006.01.02, 2006.
Löffler, J., Dobbert, S., Pape, R., and Wundram, D.: Dendrometer measurements of arctic-alpine dwarf shrubs and micro-environmental drivers of plant growth – Dataset from long-term alpine ecosystem research in central Norway (LTAER-NO), Erdkunde, DP311201, https://doi.org/10.3112/erdkunde.2021.dp.01, 2021.
Lundell, R., Saarinen, T., Åström, H., and Hänninen, H.: The boreal dwarf shrub Vaccinium vitis-idaea retains its capacity for photosynthesis through the winter, Botany, 86, 491–500, https://doi.org/10.1139/B08-022, 2008.
Macias-Fauria, M., Forbes, B. C., Zetterberg, P., and Kumpula, T.: Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems, Nat. Clim. Change, 2, 613–618, https://doi.org/10.1038/NCLIMATE1558, 2012.
Martínez-Vilalta, J., Sala, A., Asensio, D., Galiano, L., Hoch, G., Palacio, S., Piper, F. I., and Lloret, F.: Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis, Ecol. Monogr., 86, 495–516, https://doi.org/10.1002/ecm.1231, 2016.
Mayr, S., Hacke, U., Schmid, P., Schwienbacher, F., and Gruber, A.: Frost drought in conifers at the alpine timberline: xylem dysfunction and adaptations, Ecology, 87, 3175–3185, https://doi.org/10.1890/0012-9658(2006)87[3175:FDICAT]2.0.CO;2, 2006.
Moen, A.: National atlas of Norway: vegetation, edited by: Lillethun, A., Norwegian Mapping Authority, ISBN 82-7945-0000-9, 1999.
Myers-Smith, I. H., Forbes, B. C., Wilmking, M., Hallinger, M., Lantz, T., Blok, D., Tape, K. D., Macias-Fauria, M., Sass-Klaassen, U., Lévesque, E., Boudreau, S., Ropars, P., Hermanutz, L., Trant, A., Collier, L. S., Weijers, S., Rozema, J., Rayback, S. A., Schmidt, N. M., Schaepman-Strub, G., Wipf, S., Rixen, C., Ménard, C. B., Venn, S., Goetz, S., Andreu-Hayles, L., Elmendorf, S., Ravolainen, V., Welker, J., Grogan, P., Epstein, H. E., and Hik, D. S.: Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities, Environ. Res. Lett., 6, 45509, https://doi.org/10.1088/1748-9326/6/4/045509, 2011.
Myers-Smith, I. H., Kerby, J. T., Phoenix, G. K., Bjerke, J. W., Epstein, H. E., Assmann, J. J., John, C., Andreu-Hayles, L., Angers-Blondin, S., Beck, P. S. A., Berner, L. T., Bhatt, U. S., Bjorkman, A. D., Blok, D., Bryn, A., Christiansen, C. T., Cornelissen, J. H. C., Cunliffe, A. M., Elmendorf, S. C., Forbes, B. C., Goetz, S. J., Hollister, R. D., Jong, R. de, Loranty, M. M., Macias-Fauria, M., Maseyk, K., Normand, S., Olofsson, J., Parker, T. C., Parmentier, F.-J. W., Post, E., Schaepman-Strub, G., Stordal, F., Sullivan, P. F., Thomas, H. J. D., Tømmervik, H., Treharne, R., Tweedie, C. E., Walker, D. A., Wilmking, M., and Wipf, S.: Complexity revealed in the greening of the Arctic, Nat. Clim. Change, 10, 106–117, https://doi.org/10.1038/s41558-019-0688-1, 2020.
Neuner, G.: Frost resistance in alpine woody plants, Front. Plant. Sci., 5, 654, https://doi.org/10.3389/fpls.2014.00654, 2014.
Novick, K. A., Ficklin, D. L., Stoy, P. C., Williams, C. A., Bohrer, G., Oishi, A. C., Papuga, S. A., Blanken, P. D., Noormets, A., Sulman, B. N., Scott, R. L., Wang, L., and Phillips, R. P.: The increasing importance of atmospheric demand for ecosystem water and carbon fluxes, Nat. Clim. Change, 6, 1023–1027, https://doi.org/10.1038/nclimate3114, 2016.
Oberhuber, W., Sehrt, M., and Kitz, F.: Hygroscopic properties of thin dead outer bark layers strongly influence stem diameter variations on short and long time scales in Scots pine (Pinus sylvestris L.), Agr. Forest Meteorol., 290, 108026, https://doi.org/10.1016/j.agrformet.2020.108026, 2020.
Ögren, E.: Effects of climatic warming on cold hardiness of some northern woody plants assessed from simulation experiments, Physiol. Plant, 112, 71–77, https://doi.org/10.1034/j.1399-3054.2001.1120110.x, 2001.
Pape, R. and Löffler, J.: Determinants of arctic-alpine pasture resources: the need for a spatially and functionally fine-scaled perspective, Geogr. Ann. A., 99, 353–370, https://doi.org/10.1080/04353676.2017.1368833, 2017.
Pape, R., Wundram, D., and Löffler, J.: Modelling near-surface temperature conditions in high mountain environments: an appraisal, Clim. Res., 39, 99–109, https://doi.org/10.3354/cr00795, 2009.
Peters, R. L., Steppe, K., Cuny, H. E., Pauw, D. J. W. de, Frank, D. C., Schaub, M., Rathgeber, C., Cabon, A., and Fonti, P.: Turgor – a limiting factor for radial growth in mature conifers along an elevational gradient, New Phytol., 229, 213–229, https://doi.org/10.1111/nph.16872, 2021.
Polunin, N.: Attempted dendrochronological dating of ice Island T-3, Science, 122, 1184–1186, https://doi.org/10.1126/science.122.3181.1184, 1955.
Post, E., Alley, R. B., Christensen, T. R., Macias-Fauria, M., Forbes, B. C., Gooseff, M. N., Iler, A., Kerby, J. T., Laidre, K. L., Mann, M. E., Virginia, R. A., Oloffson, J., Stroeve, J., Ulmer, F., and Wang, M.: The polar regions in a 2 ∘C warmer world, Sci. Adv., 5, eaaw9883, https://doi.org/10.1126/sciadv.aaw9883, 2019.
Preece, C., Callaghan, T. V., and Phoenix, G. K.: Impacts of winter icing events on the growth, phenology and physiology of sub-arctic dwarf shrubs, Physiol. Plant, 146, 460–472, https://doi.org/10.1111/j.1399-3054.2012.01640.x, 2012.
Prislan, P., Gričar, J., Čufar, K., Luis, M. D., Merela, M., and Rossi, S.: Growing season and radial growth predicted for Fagus sylvatica under climate change, Climatic Change, 153, 181–197, https://doi.org/10.1007/s10584-019-02374-0, 2019.
Rammig, A., Jonas, T., Zimmermann, N. E., and Rixen, C.: Changes in alpine plant growth under future climate conditions, Biogeosciences, 7, 2013–2024, https://doi.org/10.5194/bg-7-2013-2010, 2010.
R Development Core Team: R: A language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing, https://www.R-project.org (last access: 8 March 2022), 2020.
Reineke, L. H.: A precision dendrometer, J. Forest., 30, 692–697, 1932.
Rößler, O. and Löffler, J.: Uncertainties of treeline alterations due to climatic change during the past century in the central Norwegian Scandes, Geoöko, 28, 104–114, 2007.
Rößler, O., Bräuning, A., and Löffler, J.: Dynamics and driving forces of treeline fluctuation and regeneration in Central Norway during the past decades, Erdkunde, 62, 117–128, https://doi.org/10.3112/erdkunde.2008.02.02, 2008.
Rossi, S., Deslauriers, A., and Morin, H.: Application of the Gompertz equation for the study of xylem cell development, Dendrochronologia, 21, 33–39, https://doi.org/10.1078/1125-7865-00034, 2003.
Rossi, S., Deslauriers, A., Anfodillo, T., Morin, H., Saracino, A., Motta, R., and Borghetti, M.: Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length, New Phytol., 170, 301–310, https://doi.org/10.1111/j.1469-8137.2006.01660.x, 2006.
Rossi, S., Deslauriers, A., Anfodillo, T., and Carraro, V.: Evidence of threshold temperatures for xylogenesis in conifers at high altitudes, Oecologia, 152, 1–12, https://doi.org/10.1007/s00442-006-0625-7, 2007.
Rossi, S., Deslauriers, A., Griçar, J., Seo, J.-W., Rathgeber, C. B. K., Anfodillo, T., Morin, H., Levanic, T., Oven, P., and Jalkanen, R.: Critical temperatures for xylogenesis in conifers of cold climates, Global Ecol. Biogeogr., 17, 696–707, https://doi.org/10.1111/j.1466-8238.2008.00417.x, 2008.
Saccone, P., Hoikka, K., and Virtanen, R.: What if plant functional types conceal species-specific responses to environment? Study on arctic shrub communities, Ecology, 98, 1600–1612, https://doi.org/10.1002/ecy.1817, 2017.
Scherrer, D. and Körner, C.: Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming, J. Biogeogr., 38, 406–416, https://doi.org/10.1111/j.1365-2699.2010.02407.x, 2011.
Schmidt, N. M., Baittinger, C., Kollmann, J., and Forchhammer, M. C.: Consistent dendrochronological response of the dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types, Arct. Antarct. Alp. Res., 42, 471–475, https://doi.org/10.1657/1938-4246-42.4.471, 2010.
Semikhatova, O. A., Gerasimenko, T. V., and Ivanova T. I.: Photosynthesis, respiration, and growth of plants in the Soviet Arctic, in: Arctic ecosystems in a changing climate: an ecophysiological perspective, edited by: Chapin, F. S., Jefferies, R. L., Reynolds, J. F., Shaver, G. R., and Svoboda, J., Academic Press, San Diego, California, USA, 169–192, https://doi.org/10.1016/B978-0-12-168250-7.50014-6, 1992.
Shaver, G. R. and Chapin III, F. S.: Effect of fertilizer on production and biomass of tussock tundra, Alaska, U.S.A, Arct. Alp. Res., 18, 261–268, https://doi.org/10.2307/1550883, 1986.
Shetti, R.: Methods in shrub dendro-ecology: Understanding the processes influencing shrub growth in the Arctic and Alpine ecosystems, PhD thesis, University of Greifswald, Mathematisch-Naturwissenschaftlichen Fakultät, Greifswald, 209 pp., http://nbn-resolving.org/urn:nbn:de:gbv:9-opus-24118 (last access: 8 March 2022), 2018.
Shevtsova, A., Haukioja, E., and Ojala, A.: Growth Response of Subarctic Dwarf Shrubs, Empetrum nigrum and Vaccinium vitis-idaea, to Manipulated Environmental Conditions and Species Removal, Oikos, 78, 440–458, https://doi.org/10.2307/3545606, 1997.
Smiljanić, M. and Wilmking, M.: Drivers of stem radial variation and its pattern in peatland Scots pines: A pilot study, Dendrochronologia, 47, 30–37, https://doi.org/10.1016/j.dendro.2017.12.001, 2018.
Starr, G. and Oberbauer, S. F.: Photosynthesis of arctic evergreens under snow: Implications for tundra ecosystem carbon balance, Ecology, 84, 1415–1420, https://doi.org/10.1890/02-3154, 2003.
Steppe, K., Sterck, F., and Deslauriers, A.: Diel growth dynamics in tree stems: linking anatomy and ecophysiology, Trends Plant. Sci., 20, 335–343, https://doi.org/10.1016/j.tplants.2015.03.015, 2015.
Sturm, M., Racine, C., and Tape, K.: Climate change. Increasing shrub abundance in the Arctic, Nature, 411, 546–547, https://doi.org/10.1038/35079180, 2001.
Stushnoff, C. and Junttila, O.: Seasonal development of cold stress resistance in several plant species at a coastal and a continental location in North Norway, Polar Biol., 5, 129–133, https://doi.org/10.1007/BF00441691, 1986.
Tian, Q., He, Z., Xiao, S., Du, J., Peng, X., Lin, P., and Ding, A.: Effects of artificial warming on stem radial changes in Qinghai spruce saplings in the Qilian Mountains of China, Dendrochronologia, 55, 110–118, https://doi.org/10.1016/j.dendro.2019.04.009, 2019.
Turcotte, A., Rossi, S., Deslauriers, A., Krause, C., and Morin, H.: Dynamics of depletion and replenishment of water storage in stem and roots of black spruce measured by dendrometers, Front. Plant Sci., 2, 21, https://doi.org/10.3389/fpls.2011.00021, 2011.
Tyree, M. T. and Sperry, J. S.: Vulnerability of xylem to cavitation and embolism, Annu. Rev. Plant Phys., 40, 19–36, https://doi.org/10.1146/annurev.pp.40.060189.000315, 1989.
Van der Maaten, E., Pape, J., van der Maaten-Theunissen, M., Scharnweber, T., Smiljanic, M., Cruz-García, R., and Wilmking, M.: Distinct growth phenology but similar daily stem dynamics in three co-occurring broadleaved tree species, Tree Physiol., 38, 1820–1828, https://doi.org/10.1093/treephys/tpy042, 2018.
Van der Maaten-Theunissen, M., Kahle, H.-P., and van der Maaten, E.: Drought sensitivity of Norway spruce is higher than that of silver fir along an altitudinal gradient in southwestern Germany, Ann. Forest Sci., 70, 185–193, https://doi.org/10.1007/s13595-012-0241-0, 2013.
Van der Wal, R. and Stien, A.: High-arctic plants like it hot: a long-term investigation of between-year variability in plant biomass, Ecology, 95, 3414–3427, https://doi.org/10.1890/14-0533.1, 2014.
Venn, S. E. and Green, K.: Evergreen alpine shrubs have high freezing resistance in spring, irrespective of snowmelt timing and exposure to frost: an investigation from the Snowy Mountains, Australia, Plant. Ecol., 219, 209–216, https://doi.org/10.1007/s11258-017-0789-8, 2018.
Vowles, T. and Björk, R. G.: Implications of evergreen shrub expansion in the Arctic, J. Ecol., 107, 650–655, https://doi.org/10.1111/1365-2745.13081, 2019.
Wang, Y., Wang, Y., Li, Z., Yu, P., and Han, X.: Interannual variation of transpiration and its modeling of a larch plantation in semiarid Northwest China, Forests, 11, 1303, https://doi.org/10.3390/f11121303, 2020.
Weijers, S. and Löffler, J.: Auswirkungen des Klimawandels auf das Wachstum von Zwergsträuchern in Hochgebirgen, in: Warnsignal Klima: Hochgebirge im Wandel, edited by: Lozán, J. L., Breckle, S.-W., Grassl, H., Kasang, D., Paul, F., and Schickhoff, U., Wissenschaftliche Auswertungen, Hamburg, Germany, 23–27, https://doi.org/10.25592/uhhfdm.9253, 2020.
Weijers, S., Broekman, R., and Rozema, J.: Dendrochronology in the High Arctic: July air temperatures reconstructed from annual shoot length growth of the circumarctic dwarf shrub Cassiope tetragona, Quaternary Sci. Rev., 29, 3831–3842, https://doi.org/10.1016/j.quascirev.2010.09.003, 2010.
Weijers, S., Buchwal, A., Blok, D., Löffler, J., and Elberling, B.: High Arctic summer warming tracked by increased Cassiope tetragona growth in the world's northernmost polar desert, Glob. Change Biol., 23, 5006–5020, https://doi.org/10.1111/gcb.13747, 2017.
Weijers, S., Pape, R., Löffler, J., and Myers-Smith, I. H.: Contrasting shrub species respond to early summer temperatures leading to correspondence of shrub growth patterns, Environ. Res. Lett., 13, 34005, https://doi.org/10.1088/1748-9326/aaa5b8, 2018a.
Weijers, S., Beckers, N., and Löffler, J.: Recent spring warming limits near-treeline deciduous and evergreen alpine dwarf shrub growth, Ecosphere, 9, e02328, https://doi.org/10.1002/ecs2.2328, 2018b.
Wilmking, M., Hallinger, M., van Bogaert, R., Kyncl, T., Babst, F., Hahne, W., Juday, G. P., Luis, M. D., Novak, K., and Völlm, C.: Continuously missing outer rings in woody plants at their distributional margins, Dendrochronologia, 30, 213–222, https://doi.org/10.1016/j.dendro.2011.10.001, 2012.
Winget, C. H. and Kozlowski, T. T.: Winter shrinkage in stems of forest trees, J. Forest., 62, 335–337, 1964.
Wundram, D., Pape, R., and Löffler, J.: Alpine soil temperature variability at multiple scales, Arct. Antarct. Alp. Res., 42, 117–128, https://doi.org/10.1657/1938-4246-42.1.117, 2010.
Wyka, T. P. and Oleksyn, J.: Photosynthetic ecophysiology of evergreen leaves in the woody angiosperms – a review, Dendrobiology, 72, 3–27, https://doi.org/10.12657/denbio.072.001, 2014.
Zellweger, F., Frenne, P. de, Lenoir, J., Vangansbeke, P., Verheyen, K., Bernhardt-Römermann, M., Baeten, L., Hédl, R., Berki, I., Brunet, J., van Calster, H., Chudomelová, M., Decocq, G., Dirnböck, T., Durak, T., Heinken, T., Jaroszewicz, B., Kopecký, M., Máliš, F., Macek, M., Malicki, M., Naaf, T., Nagel, T. A., Ortmann-Ajkai, A., Petřík, P., Pielech, R., Reczyńska, K., Schmidt, W., Standovár, T., Świerkosz, K., Teleki, B., Vild, O., Wulf, M., and Coomes, D.: Forest microclimate dynamics drive plant responses to warming, Science, 368, 772–775, https://doi.org/10.1126/science.aba6880, 2020.
Zweifel, R.: Radial stem variations – a source of tree physiological information not fully exploited yet, Plant Cell. Environ., 39, 231–232, https://doi.org/10.1111/pce.12613, 2016.
Zweifel, R. and Häsler, R.: Frost-induced reversible shrinkage of bark of mature subalpine conifers, Agr. Forest Meteorol., 102, 213–222, https://doi.org/10.1016/S0168-1923(00)00135-0, 2000.
Zweifel, R., Item, H., and Hasler, R.: Stem radius changes and their relation to stored water in stems of young Norway spruce trees, Trees Struct. Funct., 15, 50–57, https://doi.org/10.1007/s004680000072, 2000.
Zweifel, R., Zimmermann, L., Zeugin, F., and Newbery, D. M.: Intra-annual radial growth and water relations of trees: implications towards a growth mechanism, J. Exp. Bot., 57, 1445–1459, https://doi.org/10.1093/jxb/erj125, 2006.
Zweifel, R., Drew, D. M., Schweingruber, F., and Downes, G. M.: Xylem as the main origin of stem radius changes in Eucalyptus, Funct. Plant. Biol., 41, 520–534, https://doi.org/10.1071/FP13240, 2014a.
Zweifel, R., Drew, D. M., Schweingruber F., and Downes, G. M.: Xylem as the main origin of stem radius changes in Eucalyptus, Funct. Plant. Biol., 41, 520–53, https://doi.org/10.1071/FP13240, 2014b.
Zweifel, R., Sterck, F., Braun, S., Buchmann, N., Eugster, W., Gessler, A., Häni, M., Peters, R. L., Walthert, L., Wilhelm, M., Ziemińska, K., and Etzold, S.: Why trees grow at night, New Phytol., 231, 2174–2185, https://doi.org/10.1111/nph.17552, 2021.