the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Dynamics of rare earth elements and associated major and trace elements during Douglas-fir (Pseudotsuga menziesii) and European beech (Fagus sylvatica L.) litter degradation
Alessandro Montemagno
Christophe Hissler
Victor Bense
Adriaan J. Teuling
Johanna Ziebel
Laurent Pfister
Given the diverse physico-chemical properties of elements, we hypothesize that their incoherent distribution across the leaf tissues, combined with the distinct resistance to degradation that each tissue exhibits, leads to different turnover rates among elements. Moreover, litter layers of varying ages produce diverse chemical signatures in solution during the wet degradation. To verify our hypothesis, Na, K, Mg, Mn, Ca, Pb, Al and Fe were analysed together with the rare earth elements (REE) in the solid fractions and in the respective leachates of fresh leaves and different litter layers of two forested soils developed under Pseudotsuga menziesii and Fagus sylvatica L. trees. The results from the leaching experiment were also compared with the in situ REE composition of the soil solutions to clarify the impact that the litter degradation processes may have on soil solution chemical composition.
Both tree species showed similar biogeochemical processes dominating the element dynamics during litter degradation. REE, Al, Fe and Pb were preferentially retained in the solid litter material, in comparison with the other cations, and their concentrations increased over time during the degradation. Accordingly, different litter fractions produced different yields of elements and REE patterns in the leachates, indicating that the tree species and the age of the litter play a role in the chemical release during degradation. In particular, the evolution of the REE patterns, relative to the age of the litter layers, allowed us to deliver new findings on REE fractionation and mobilization during litter decay. Specifically, the degradation of the litter was characterized by a decrease in the ratio and an increase in the ratio. The relationship between these ratios provided information on the litter species-specific resistance to degradation, with Douglas-fir litter material showing a lower resistance.
During the litter degradation of the two tree species, two main differences were highlighted with the help of the REE: (i) in Pseudotsuga menziesii the behaviour of Eu appeared to be linked to Ca during leaf senescence and (ii) species-specific release of organic acids during litter degradation leads to a more pronounced middle REE (MREE) enrichment in the Fagus sylvatica leachates.
Finally, we showed the primary control effect that white fungi may have in Ce enrichment of soil solutions, which appears to be associated with the dissolution and/or direct transport of Ce-enriched MnO2 accumulated on the surface of the old litter due to the metabolic functioning of these microorganisms. Similar MREE and heavy REE (HREE) enrichments were also found in the leachates and the soil solutions, probably due to the higher affinity of these elements for the organic acids, which represent the primary products of organic matter degradation.
- Article
(4715 KB) - Full-text XML
- BibTeX
- EndNote
Nutrient cycling is key to forest ecosystem sustainability and productivity, especially in sites characterized by low fertility or degraded soils. There are three types of nutrient cycles, which relate to geochemical, biochemical or biogeochemical processes (Morris, 2004). The geochemical cycle encompasses all processes inherent to the introduction or removal of nutrients – excluding any kind of biological activity (e.g. input from aerosols, leaching of nutrients from rocks and their removal from the system through runoff). The biochemical cycle refers to processes involved in the transport and retention of nutrients inside the trees (such as the withdrawal of specific nutrients from leaves before senescence). The biogeochemical cycle encompasses the processes that occur outside the trees and lead to the degradation of organic waste material (such as exudates of leaves and stems, dead leaves and branches or even a whole tree) into its primary components and therefore to the release of nutrients in a form that is reusable by trees (Morris, 2004). Organic matter degradation, which represents part of the biogeochemical cycle, is a major contributor to the nutrient stock available to trees in forest ecosystems (Staaf, 1980; Guo and Sims, 1999; Chadwick et al., 1999; Pacyna, 2008; Krishna and Mohan, 2017) and, in this context, litter degradation is known to play a key role in the replenishment of the nutritional pools of forests (Tagliavini et al., 2007). Nutrient release from litter is possibly regulated by various biotic and abiotic factors. Temperature, abundance of precipitation, species of decomposers (including the microfauna and microorganism communities), litter composition and the chemical structure of its components are all factors that regulate the degradation rate and therefore the recirculation of the elements in a forest (Krishna and Mohan, 2017).
Trees absorb many nutrients to meet the metabolic demands for growth, the immune system and reproduction but at the same time unnecessary elements, such as toxic metals, can also be absorbed and “trapped” in specific tree compartments (Gómez et al., 2019). Indeed, once absorbed, the elements are distributed within different tissues depending on their metabolic role or on their affinities with various compounds (Shan et al., 2003; Ding et al., 2005; Brioschi et al., 2013; Page and Feller, 2015; Yuan et al., 2018). This distribution could play a key role in processes involved in element turnovers in forest ecosystems especially during litter degradation, which potentially leads to a preferential release into the environment of certain elements rather than others, depending on the substances to which they are bound. Litter degradation would preferably promote the release of elements from more labile fractions making them available for tree uptake during the first stages of the degradation (Swift et al., 1979), while pools of elements that are trapped inside the most refractory tissues would remain unavailable for longer time spans.
The study of the biogeochemical processes involved in the distribution of the different classes of elements (toxic and nutrients) among the various leaf tissues during the growth period, and their fractionation during the degradation of the litter, is of crucial importance for a better understanding (and forecast) of the dynamics of the aforementioned classes of elements in forest ecosystems. To investigate such processes, rare earth elements (REE) are interesting candidates thanks to their recognized use as tracers of geochemical processes, to existing knowledge of their partitioning in plant tissues and to recent studies related to their ecotoxicology as emerging pollutants (Liang et al., 2008; Li et al., 2013; Rim et al., 2013). Indeed, knowledge of the processes involved in REE dynamics during litter degradation is of importance in environmental and social matters due to the increase in environmental concentrations of REE linked to their extraction in mining areas and exploitation in modern technologies. REE are a group of elements composed of lanthanides (from 57La to 71Lu) and 39Y. Apart from Y, the other REE are usually divided into light (LREE: La to Nd), middle (MREE: Sm to Tb) and heavy (HREE: Dy to Lu) according to their atomic weight. To highlight the geochemical behaviour characteristics of the REE, it is convenient to consider the normalized concentrations rather than their absolute concentrations as is usually the case for other chemical elements. From the trends of the normalized concentrations (or patterns), it is then possible to determine the REE serial behaviour. These patterns can exhibit so-called anomalies, which represent an enrichment (positive anomalies) or depletion (negative anomalies) in certain elements of the series or also a fractionation occurring among the three groups of elements that are intimately linked to the environmental conditions. The characteristics of the patterns allow us to determine the biogeochemical processes occurring in the system.
REE have already proven to be among the best-suited tracers for investigating critical zone processes such as the origin of solid and dissolved load transported by streams (Aubert et al., 2001; Hissler et al., 2015a); metal adsorption in organic matter (Schijf and Zoll, 2011) and in bacterial cell walls (Takahashi et al., 2005, 2010); characterization of water–rock interactions and regolith weathering processes (Bau, 1991, 1996; Aubert et al., 2001; Stille et al., 2006; Ma et al., 2011; Möller et al., 2011; Cidu et al., 2013; Hissler et al., 2015b; Jin et al., 2017; Laveuf and Cornu, 2009; Moragues-Quiroga et al., 2017; Vázquez-Ortega et al., 2015, 2016); as an indicator of atmospheric dust composition in leaves (Censi et al., 2017) or wastewater spillage in freshwaters (Merschel et al., 2015; Hissler et al., 2016); and metal mobilization and fractionation in the soil–plant continuum (Liang et al., 2005, 2008; Censi et al., 2014; Cheng et al., 2015; Semhi et al., 2009).
Despite the large number of studies of REE in plant tissues (Fu et al., 1998; Wyttenbach et al., 1998; Zhenggui et al., 2001; Han et al., 2005; Ding et al., 2005; Brioschi et al., 2013; Censi et al., 2014; Zaharescu et al., 2017), their dynamics during litter degradation remains scarcely known, since research has mainly focused on changes in the concentrations of elements according to the total mass loss of litter material during the duration of the experiment (Albers et al., 2004; Tyler, 2004; Brun et al., 2008; Gautam et al., 2019). To the best of our knowledge, no studies regarding the processes involved in the regulation of such dynamics have been carried out. Our aim is to elucidate which processes may control the release and retention of REE in relation to major cations and other trace elements during the litter degradation in wet conditions – with a secondary focus on the qualitative impact of litter degradation on the REE patterns of soil solutions. We believe that REE environmental behaviour and its capacity to accurately trace biogeochemical processes add value to our understanding of nutrient cycles and, in particular, could provide more precise information on the processes that control the litter degradation stages and the release of nutrients in forest ecosystems. Since the different leaf tissues (across which leaves distribute the elements taken up during the life span) exhibit distinct resistance to degradation, we hypothesize that during litter decay the combination of such a distribution process with the different levels of degradation resistance of the tissues will lead to distinct turnover rates among the elements. Consequently, this leads to a specific chemical release into the environment depending on the degradation stage of litter and the different tissues among which the elements are distributed. To test our hypothesis, we designed a field experiment in the forested Weierbach experimental catchment (Hissler et al., 2021), relying on a series of biogeochemical tracers – including concentrations of Na, Mg, K, Ca, Mn, Fe, Pb, Al and REE measured in fresh leaves and different litter fractions of Douglas-fir (Pseudotsuga menziesii) and European beech (Fagus Sylvatica L.) grown on the same soil. Moreover, we carried out leaching experiments on these samples with ultrapure water (MilliQ) in order to observe how the different fractions of litter can contribute to element release and sequestration and how this release may affect soil solution chemistry. Finally, we compared the results of the leaching experiment with the chemical composition of soil solutions collected from the two tree stands. If our hypothesis is confirmed, we expect the older litter fractions to show an enrichment in specific elements, which would be linked to their distribution in the most refractory tissues, limiting their release into the environment during the degradation. Subsequently, we expect that the solutions obtained through the leaching experiment (hereafter called “leachates”) will exhibit different chemical signatures according to the degree of degradation of the samples. On the contrary, if different litter fractions showed similar chemical compositions and similar chemical release during the leaching experiment, it would imply that the distribution of the elements among the tissues is coherent and, therefore, the degradation stage of the litter does not affect the chemical release during the litter decay.
2.1 Study site
We selected two experimental plots in the Weierbach experimental catchment located in the Luxembourg Ardennes Massif, which have been monitored for ecohydrological purposes since 2012 (Hissler et al., 2021). The “Be” profile (BeP) shows a deciduous cover of European beech (Fagus sylvatica L.), while the “Do” profile (DoP) is covered with Douglas-fir (Pseudotsuga menziesii). The altitude ranges from 450 to 500 m above sea level and the geological substratum consists of Devonian metamorphic slates covered by 70–100 cm of Pleistocene periglacial slope deposit (PPSD) composed of past loamy aeolian deposition (Moragues-Quiroga et al., 2017). The soil, which develops below a Hemimoder type of humus (Jabiol et al., 2013) in the first 50 cm of the PPSD, presents homogeneous properties all over the catchment. It is at an early formation stage and classified as dystric cambisol according to the World Reference Base for soil resources (Juilleret et al., 2016).
2.2 Sampling and preparation
The different plant materials (i.e. fresh leaves and needles, as well as new and old litter) were collected on the same sampling day in May 2019 at both beech and Douglas-fir stands.
Fresh leaves (beech) and needles (Douglas-fir) (hereafter referred to as “FL”) were collected from 10 adult trees randomly selected per plot. The leaves were taken from different branches, accessible from the ground, at various heights and radial directions. All leaves were aggregated together in one sample per plot and stored in clean polypropylene bags. The litter material was collected from five different locations within an area of 500 m2 of each experimental plot using a 25×25 cm metallic frame and avoiding contamination by soil particles. During the collection, the different fractions of litter were sorted according to their degradation degree (Fig. 1) and stored in polypropylene bags.
In BeP, three litter fractions were identified: the new litter (OLn – unprocessed, unfragmented, light-brownish coloured), the old litter (OLv – slightly altered, bleached and softened, discoloured or dark-brownish coloured) and the fragmented litter (OF – partially decomposed and fragmented, grey-black coloured). For DoP only two fractions stood out: the new litter (OLn – unprocessed, unfragmented, light-brownish coloured) and the old litter (OLv – slightly altered, bleached and softened, grey-black coloured), whereas the fragmented litter layer was not sufficiently developed and was not considered in this study.

Figure 1Fresh leaves and litter of European beech and Douglas-fir collected at the Weierbach experimental catchment and sorted by degradation degree.
The atmospheric dust was collected in the Weierbach experimental catchment using a modified polypropylene version of the passive SIGMA-2 collectors produced by the German Meteorological Service in Freiburg, Germany (VDI 3787, 2010) installed at 1.5 m above ground level. The SIGMA-2 passive sampler enables the sampling of atmospheric particles greater than 2.5 µm (Grobéty et al., 2010). The atmospheric dust represents an integrated sample exposed to the atmospheric deposition from September 2018 to May 2019. The particles were collected and stored in an acid-cleaned Teflon vessel during the whole exposure period and then stored in a clean desiccator in the laboratory until their preparation before the analysis. After precise weighing of the atmospheric dust collected (2.3 mg), the sample was totally digested using concentrated ultrapure HNO3, HF and HClO4 acid mixture. The acids were then evaporated, and the residue was dissolved in a solution of HNO3 (1 % in volume) and stored at 4 ∘C before the analysis.
Fresh leaves and litter materials were cleaned with a strong air flux and with a brush avoiding the use of water in order not to disturb the chemical signature and the biotic communities existing on the surfaces of the samples. The samples were then dried in an oven at 40 ∘C and homogenized. Two aliquots were taken for the total chemical composition and the leaching experiment. The leaves and litter aliquots for the total chemical composition were preliminarily reduced to a fine powder and incinerated in closed ceramic cups at 550 ∘C before mineralization in order to destroy organic matter and to pre-concentrate the trace elements. We opted for a more convenient digestion protocol for the organic-derived samples by using aqua regia in a microwave-assisted oven (Anton Paar Multiwave PRO), which makes it possible to use a higher amount of sample (250 mg) and to reach high temperature (212 ∘C) and pressure (25 bars) conditions. Although aqua regia is not a total digestion method, among the different methods tested it yielded the best results without any precipitates or suspended particles in the digestates, not only at the moment of the digestion but also long after. The samples were then stored at 4 ∘C before the analysis.
For the leaching experiment, 2 L high-density polyethylene bottles were filled with the aliquots of leaves and litter fractions. A total of 1 L ultrapure water was then added. There were two reasons for putting as much material as possible into the bottles: first, part of the samples had to be above the water level in order to enhance the aerobic degradation; and second, we wanted to ensure that the release of elements during the experiment period was abundant enough to be detectable with the instrumentation. The bottles were left with the cup partially open in order to enable gas exchanges to encourage bacterial and fungi activity. Samples were agitated for 1 h d−1 for 7 d in an automatic vertical agitator (GFL type 3040) set at minimum speed to enhance the leaching process from all samples while avoiding further fragmentation. After 1 week, the leachates were separated from the solid material with a nylon sieve, filtered at 0.2 µm and acidified using HNO3 (1 % in volume). Subsequently, a 50 mL aliquot from each leachate was evaporated in Savillex PFA vessels placed on a hot plate allowing for the precipitation of the content. The residue was then sequentially mineralized with HF, HCl and HNO3. After evaporation, the residue was dissolved in HNO3 (1 % in volume) and samples were stored at 4 ∘C before the analysis. All the chemicals used for the different digestion procedures were of ultrapure quality. Aliquots were also taken for dissolved organic carbon (DOC) and pH measurements.
Thanks to the bi-weekly monitoring in place at the Weierbach catchment since 2009 (Hissler et al., 2021), we can rely on the chemical composition of soil solutions collected between 2012 and 2014 at the two sampling locations at 20, 40 and 60 cm depths. Sampling was carried out using Teflon/quartz suction cups (SDEC, Reignac-sur-Indre, France) connected to acid-clean 2 L Nalgene flasks under a vacuum of 0.8 bar.
2.3 Sample analysis
The concentrations of major cations and trace elements in all samples were analysed via inductively coupled plasma mass spectrometry (Agilent 7900). The measured isotopes were chosen with no isobaric interferences, and the polyatomic interferences were minimized by using the collision cell in He mode. Calibration standards and quality controls (QCs) were prepared with certified solutions (Chem-lab, Belgium and Merck, Belgium). QCs at low, medium and high levels of concentration of the calibration range were each analysed after 10 samples to control the validity of the measurements. Internal standards were prepared with Chem-lab (Belgium) Re and Rh certified solutions. To ensure the quality of the mineralization procedure, mineralization blanks were prepared following the same steps as for the samples and were analysed. The limit of quantification (LoQ) for the different analysed elements are reported in Table S1 in Hissler and Montemagno (2021, https://doi.org/10.5281/zenodo.6539436).
DOC was measured via a Teledyne Tekmar® Torch Combustion Analyser and pH with SenTix® 940 WTW.
2.4 REE normalization and anomaly calculations
All data presented in the following sections were normalized to the local atmospheric deposition (Table S2 in Hissler and Montemagno, 2021). This allowed us to compare our samples with a local reference for REE. Since atmospheric dust is an important input of cations and nutrients (Reynolds et al., 2006; Lequy et al., 2012), we also expected it to be important in terms of REE input in forest ecosystems. Moreover, with this normalization, we could directly differentiate the vegetation contribution to the REE patterns observed for the litter samples.
Gd, Eu and Ce anomalies were calculated from Eqs. (1) to (3), respectively:
with LaN, CeN, PrN, SmN, EuN, GdN and TbN indicating the REE normalized concentrations.
3.1 Chemical composition of the fresh leaves and litter bulk samples
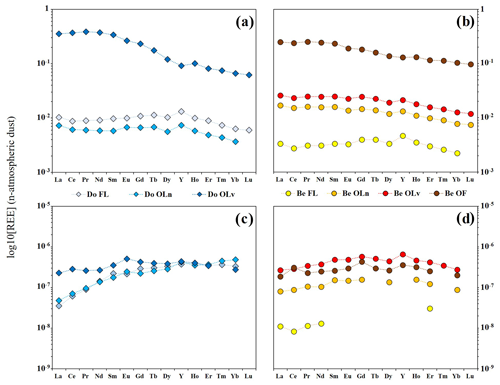
Concentrations of REE in fresh leaves and litter are reported in Table S3 in Hissler and Montemagno (2021). When normalized to the local atmospheric deposition (hereafter referred to as “dust”), the REE patterns of bulk fresh leaves and litter material show some similarities between the two tree species. The REE concentrations increase with the age of the litter, with the lowest concentration in fresh leaves and the highest in the most degraded litter layers (Fig. 2a, b and Table S3 in Hissler and Montemagno, 2021). Additionally, both tree species present a depletion in HREE if compared to LREE and MREE (Fig. 3a, b). Y appears to behave coherently for both tree species during the litter degradation, as illustrated by the evolution of the ratios. Indeed, the samples show a Y enrichment in comparison to the dust ( = 25.93) that is higher in the fresh leaves with ratios equal to 34.34 and 34.3 for Do FL and Be FL, respectively, which decreases progressively with the age of the litter showing the lowest values in the oldest litter fractions (23.58 and 25.43 in Do OLv and Be OF, respectively).
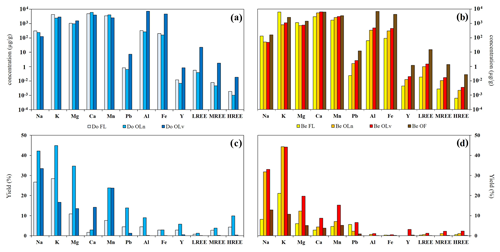
Figure 2Log10 of the concentrations of the study elements in fresh leaves and different litter fractions of (a) Douglas-fir and (b) European beech; element yields (% of the total mass) for the leaching experiment of (c) Douglas-fir and (d) European beech fresh leaves and litter samples.
In the bulk material, the REE concentration and the LREE enrichment also increase in line with the litter degradation stages but with differences between the Douglas-fir and the European beech. For the Douglas-fir samples, the REE total concentrations decrease from the fresh material to the Do OLn litter layer before drastically increasing in the Do OLv sample. The total REE concentrations are 0.78, 0.51 and 25.3 µg g−1 in Do FL, Do OLn and Do OLv, respectively. Here, the fractionations between the REE groups are not visible between the fresh leaves and the Do OLn litter layer. However, the fractionations between LREE, MREE and HREE in Do OLv are the highest for all the samples considered in this study, with and and having ratios of 5.35 and 1.53 and 3.5, respectively. For the European beech, the REE total concentration in the fresh leaves is the lowest for all leaves and litter samples (Fig. 3b) and increases progressively at each degradation stage to reach its maximum value of 17.7 µg g−1 for the Be OF litter layer. The LREE enrichment increases during the degradation as illustrated by the ratios, which progressively evolve from 1.49 in the fresh leaves to 2.40 in the Be OF. The degradation of the beech litter also leads to an MREE enrichment as shown by the ratio evolving from 0.84 in Be FL to 1.16 in Be OLn and finally 1.36 in the Be OF.
The major and trace elements can be sorted into three different groups according to the evolution of their concentrations in the bulk samples of the different litter layers (Fig. 2a, b). This classification remains coherent for the two tree species. Na, K and Mg have their highest concentrations in the fresh leaves and the older litter layers. Ca and Mn present a progressive increase in their concentration for the European beech, whereas they are less concentrated in the oldest litter layer of Douglas-fir in comparison with Do FL and Do OLn. The concentrations of the other trivalent metals (Fe, Al) and Pb evolve similarly to the REE with a progressive increase from the fresh leaves to the OL litters and an enrichment in the oldest litter layer for both species (Do OLV and Be OF). In contrast to the European beech, Douglas-fir fresh leaves have metal concentrations as high as in the Do OLn litter sample.
3.2 Chemical composition, pH and DOC content of leachates
The leaching experiment led to similar REE concentration ranges between the two tree species, except for the beech fresh leaves, which are 1 order of magnitude less concentrated (Table S4 in Hissler and Montemagno, 2021). The leachate patterns present strong differences to the REE characteristics of the bulk leaves and litter material and between the two tree species.
The total REE concentrations of the Douglas-fir leachates are similar in the Do FL and Do OLn samples and higher in the leachate of the Do OLv sample. Since the HREE show very similar concentrations in the three leachates, the difference is mainly related to the concentration of LREE, as shown by the dust-normalized REE patterns (Fig. 3c). Indeed, LREE are similarly depleted in the leachates of the two younger samples, showing ratios of 0.10, while Do OLv presents a ratio equal to 0.82. Noteworthy are the positive Eu anomalies () of 1.23 and 1.31 observed in the leachates of Do OLn and Do OLv, respectively. In Do OLv a slightly positive Ce anomaly () of 1.16 is also observed.
The REE concentrations in the leachates of beech samples increase from the fresh leaves to the highest stages of litter degradation but are higher for the Be OLv material. The dust-normalized REE patterns of beech leachates have an MREE enrichment in comparison with LREE and HREE where Gd shows the highest peak. The patterns of Be OLn and Be OLv leachates present very similar characteristics as indicated by their ratios (0.46 and 0.43, respectively), ratios (2.10 and 2.14, respectively) and the absence of any anomaly, whereas the Be OF leachate presents positive Ce and Gd anomalies ( = 1.49 and = 1.52). By contrast, the leachate of the fresh beech leaves presents a negative Ce anomaly ( = 0.74).
Similar trends in the percentage of elements leached from the material of both tree species were observed. The percentage of leaching of the study elements can be classified according to their valence, Na, K > Mg, Mn > Ca, Pb > Al, Fe, REE, with the trivalent elements being less leached. However, some differences can be highlighted between Douglas-fir and beech.
The Douglas-fir material released a higher quantity of Na, Mg, Mn, Pb, Al, Fe, MREE and HREE compared with the beech. Major elements and Mn are preferentially released compared with trivalent metals and Pb, as shown in Fig. 2c. The highest percentage of element release is observed for the Do OLn sample with Mn having similar values in Do OLn-L and Do OLv-L. An exception is noted for Ca, which presents similar release percentages as those of the trivalent metals during the first stages of degradation and which shows the highest release from the Do OLv fraction.
In beech leachates, the elements show similar release trends to Douglas-fir, but the highest percentages of release for all elements are in Be OLv (Fig. 2d), with Na and K having analogous values in Be OLn. In Be FL and Be OLn leachates, all the elements show lower release values than in the Douglas-fir samples. Trivalent metals and Pb, similarly to those of Douglas-fir, show a low release from the solid material during the experiment (in the case of Al, the release from fresh leaves is below the LoQ, as well as for many REE as shown in Tables S4 and S5 in Hissler and Montemagno, 2021).
The ratios of the leachates of Douglas-fir litter can explain the appearance of a small Y depletion in the oldest litter sample. The leachate of Do OLn, which represents the stage of degradation that brings about the formation of the OLv fraction, indeed shows a ratio equal to 31.29, indicating a preferential release of Y (when compared with the neighbour) that leads to a lower-than-atmospheric dust value in the Do OLv sample ( = 23.58 in Do OLv and = 25.93 in atmospheric dust).
In beech samples, we can observe a similar behaviour with values that are slightly higher. The ratio in the Be OLv leachate ( = 36.59) justifies the absence of Y enrichment in the Be OF fraction, which instead shows a dust-like ratio ( = 25.43).
The highest DOC concentrations (Table S4 in Hissler and Montemagno, 2021) were measured in the Douglas-fir leachates with values ranging from 10.39 mg L−1 in Do OLv to 29.37 mg L−1 in Do OLn, while beech leachates showed concentrations from 5.91 mg L−1 in Be OLn to 14.68 mg L−1 in Be OLv. Note that for both species, the highest DOC concentrations were measured in leachates of the second-to-last degradation stages with a decrease in the oldest fractions.
The pH appears to be inversely proportional to the DOC concentrations. Indeed, for the Douglas-fir leachates, the most acidic pH was found in Do OLn, i.e. pH = 4.26 (Table S4 in Hissler and Montemagno, 2021), while the pH of Do OLv was the highest with a value of 5.03. In beech leachates, the lowest pH was measured in Be OLv (pH = 4.07) and the highest in Be OLn (pH = 5.39).
3.3 Average REE in soil solutions
The average REE concentrations in soil solutions collected between 2012 and 2014 are reported in Table S6 in Hissler and Montemagno (2021). The REE total concentrations differed by 1 order of magnitude between the two experimental plots and were lower under the Douglas-fir stand at 20 and 40 cm depth (ΣREE = 0.88 and 0.92 µg L−1 in Do SS20 and Do SS40, respectively), whereas the highest concentration was observed in beech samples at 40 cm depth (ΣREE = 6.70 µg L−1 in Be SS40).
The dust-normalized REE patterns show an MREE enrichment for all soil solutions and positive Ce anomalies at 20 and 40 cm depth. Among the MREE, Eu shows a peak in the soil solutions under the Douglas-fir, whereas Gd is more enriched in beech soil solutions. The Do SS samples at 20 and 40 cm depth exhibit positive Ce anomalies ( = 1.14 and 1.21, respectively) that disappear at 60 cm. Moreover, Douglas-fir samples also show an LREE depletion as indicated by the ratios, which range from 0.50 to 0.66 in Do SS40 and Do SS60, respectively. Under the beech, the positive Ce anomaly is higher at 20 cm and decreases with depth until it becomes negative in soil solutions at 60 cm depth ( = 1.39, 1.20 and 0.82 in Be SS20, Be SS40 and Be SS60, respectively), whereas the LREE show a consistent depletion at 20 cm depth ( = 0.56) and a slight depletion at 60 cm depth ( = 0.85).
4.1 REE fractionation during litter degradation: similarities and differences with the other elements
The leaching yields clearly illustrate that during the leaching experiment, REE are preferentially leached following the HREE > MREE > LREE order (Fig. 2c, d). This justifies the decreasing trends (from La to Lu) observed in the REE patterns of litter fractions for both tree species and demonstrates the tendency of the litter to retain LREE rather than the other elements of the lanthanide series. This progressive LREE enrichment in the litter (Fig. 3a, b) proceeding towards degradation is more evident for the Douglas-fir samples as suggested by the ratios in the Do OLv and Be OF samples ( = 5.35 and = 2.40, respectively). The leaching experiment also showed that REE, together with the other trivalent metals (Fe and Al) and Pb, are preferentially held inside the solid material, while the other major elements and Mn are more easily released during litter degradation. This behaviour suggests a preferential fractionation of the nutrients studied (Na, K, Mg and Mn) into more labile tissues, probably due to their role in the metabolic functioning of the tissues of living leaves (Alejandro et al., 2020; Sardans and Peñuelas, 2021; Shaul, 2002; Maathuis, 2014). This partitioning results in a concentration decrease of these elements during litter degradation, while Fe, Al, Pb and REE tend to remain embedded inside the most refractory tissues – increasing their concentration over time in the remnant litter material. In our experiment, all elements behave coherently according to their oxidation number (Fig. 2c, d). Monovalent elements (Na, K) are more likely to be released than the divalent elements (Mg, Mn), while trivalent metals (Al, Fe, REE) tend to stay more tightly bound to the solid residual fraction. Exceptions can be seen for Ca in the Douglas-fir samples and for Pb in the oldest litter fractions of both tree species.
Given that REE are preferentially retained in the recalcitrant tissues together with the other trivalent metals and Pb, the higher yields for these elements obtained from Douglas-fir samples during the leaching experiment might suggest a faster degradation process for the Douglas-fir litter when compared with the beech litter.
The fact that LREE are preferentially retained in the litter of both tree species can be explained by referring to the existing literature and by normalizing the REE concentrations in the leachates to those of the respective litter material (Fig. 4).
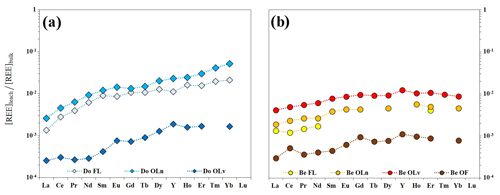
Figure 4Patterns of REE in leachates normalized to the REE concentrations in leaves and litter for Douglas-fir (a) and beech (b) samples.
The patterns of REE in Douglas-fir samples show an HREE enrichment when compared with the other elements of the series ( and ), indicating a preferential release of the HREE to the solution. In the European beech samples, the patterns are smoother between MREE and HREE (), while they conserve the depletion in LREE when compared with the other groups ( and ). In general, these patterns show a progressive increase from La to Lu that mirrors the trend of the stability constants of REE complexes with malate, EDTA, humic and fulvic acids (Suzuki et al., 1980; Pourret et al., 2007; Marsac et al., 2010; Sonke and Salters, 2006). Humic acids, fulvic acids and malic acid are indeed primary products of organic matter degradation (Adeleke et al., 2017 and reference therein) and have been reported to possess metal chelating properties. The increasing release of REE from La to Lu in our leachates is likely due to an increase in the specific affinity – proceeding along the lanthanide series – of these elements towards the degradation products present in the aqueous solution (Schijf and Zoll, 2011). This suggests that the nephelauxetic effect (Juranic, 1989; Tchougréeff and Dronskowski, 2009) plays a key role in the mechanism of REE complexation with the dissolved organic ligands during the wet degradation of litter and is in line with the results shown by Sonke and Salters (2006), who experimentally demonstrated that the lanthanide contraction is responsible for a gradual increase in the complexation strength with humic substances when decreasing the ionic radius. The bond strength, indeed, increases with increasing ionic potential .
Differences in the atmospheric dust-normalized REE patterns of leachates between the two tree species can be explained by the nature of the ligands present in the solutions. Tang and Johannesson (2010) showed that the REE complexation with organic ligands in natural waters would produce patterns enriched in HREE when the majority of the ligands in solutions are represented by low-molecular-weight dissolved organic compounds (for instance citric, oxalic, malic, succinic, malonic and maleic acids) while a preponderant presence of high-molecular-weight dissolved organic compounds (such as humic acid and fulvic acids) would produce an enrichment in MREE. Therefore, the results obtained indicate that the differences between the patterns of Douglas-fir and European beech leachates are likely due to the production of different classes of organic acids during the degradation of the samples.
The behaviours of Al, Fe, Pb and REE are coherent during litter degradation for both tree species, as their concentrations progressively increase towards the oldest litter fractions (Table S3 in Hissler and Montemagno, 2021, and Fig. 2a, b). We can explain such an accumulation in the oldest litter fractions by the binding with lignins. Lignins constitute the most degradation-resistant compounds in leaves. Their resilience lets these metals persist longer in the organic material than carbohydrates, lipids, proteins, hemicellulose and cellulose, which can degrade at a faster rate (Rahman et al., 2013). Lignins are a class of organic polymers that have many functions in vascular plants. They provide structural support, improve cellular adhesion, enhance water transport and defence towards pathogens and are mainly situated in the cell walls of vascular and support tissues (Weng and Chapple, 2010; Leisola et al., 2012; Labeeuw et al., 2015). Moreover, the chemical structure of these molecules exerts a strong control on litter decay rates (Talbot et al., 2012). Increased lignin concentrations inhibit biological activity and linearly increase photo-degradation due to its wide spectrum of absorbance (Austin and Ballaré, 2010; Cogulet et al., 2016).
While Fe, Al and Pb toxicity in plants is well known (Imadi et al., 2016; Bienfait, 1989; Woolhouse, 1983; Rout et al., 2001; Sharma and Dubey, 2005; Pourrut et al., 2011; Singh et al., 2017; Nikolic and Pavlovic, 2018), the toxicity of REE is not yet widely studied as their micropollutant nature has only recently emerged (Gwenzi et al., 2018). However, the toxicity of REE in plants is far beyond the scope of this work; we limit ourselves to mentioning that researchers observed REE displaying redox-related toxicity mechanisms (El-Ramady, 2010; Pagano et al., 2015; Tommasi et al., 2021) and we assume that plants can trap REE in lignified tissues as a defence to avoid the toxicity-related events with the same mechanism adopted for other potentially toxic metals, such as Pb and Al. Therefore, we propose that lignins constrain the REE in the oldest litter fractions during the degradation of the leaves. Given the high affinity of such metals for oxygen, the absorption operated by lignins through the binding with the oxygen-bearing functional groups (such as phenolic, hydroxyl) may be the mechanism involved and would explain the accumulation of these metals in the oldest litter fractions. Therefore, during the life cycle of leaves, lignins are able to sequestrate the elements that show higher affinity for the exposed functional groups. As lignins are the most resistant tree components in forests, they would prevent the release of the absorbed elements for longer during the litter degradation. The chemical elements that are more important for tree nutrition and metabolism would then be preferentially released to the soil solutions.
As shown by the evolution of the chemical composition of leaves and litter fractions along the different degradation stages, our hypothesis on the distribution of different elements among the different tissues is confirmed. This finding is further corroborated by the results of the leaching experiment, which clearly confirm our hypothesis that during litter decay, the release of elements is linked to the degradation stage of the litter itself. As conjectured, elements partitioned in the most labile tissues are more easily released during the degradation process than those bound to more refractory tissues, which are instead accumulated over time.
According to our findings, two main REE fractionation processes are specific to the life span of a leaf:
- i.
an inter-tissue fractionation occurring during the “living period” of the leaves, through which recalcitrant tissues would preferentially absorb REE as a result of binding with lignins, developing a particular signature;
- ii.
a degradation-driven fractionation, which has the different affinities of REE towards the products of the decay as the main factor for their partitioning between the remaining solid fraction of litter and the resulting solution.
4.2 Ce anomalies in leachates
Another interesting aspect of our results is the presence of positive Ce anomalies in the leachates of the oldest litter fractions ( and in Do OLv and Be OF leachates, respectively) and a slight W-type tetrad effect in the Be OF leachate. The tetrad effect can be defined as a graphical effect that divides the REE patterns into four segments, so-called tetrads (T1 from La to Nd; T2 from Pm to Gd; T3 from Gd to Ho; T4 from Er to Lu), resulting from the increased stability at a quarter, half, three-quarter and complete filling of the 4f orbital (McLennan, 1994). The tetrad effect is usually classified according to the shape of the patterns into the “W” type and “M” type.
Davranche et al. (2005) demonstrated that the REE complexation by organic acids inhibits the development of the tetrad effect and of Ce anomalies in the REE patterns of aqueous solutions. This is because the complexation operated by organic acids is not selective towards any specific lanthanide and therefore also Ce. The REE complexation with the organic acids can therefore explain the absence of Ce anomalies and of the tetrad effect in the REE patterns of the leachates of the younger litter fractions (Do FL, Do OLn, Be FL, Be OLn, Be OLv), but it does not justify the positive Ce anomalies found in the patterns of the leachates of the oldest fractions (Do OLv and Be OF) and the W-type tetrad effect observed in the pattern of the Be OF leachate. The presence of both positive Ce anomalies and the tetrad effect can be explained by a biologically driven accumulation of MnO2 on the surface of the components of the oldest litter layers and their subsequent transport into solution. Keiluweit et al. (2015) demonstrated how litter decomposition is controlled by the Mn redox cycle. The authors explained that, during the first 3 years of the litter degradation, specific microorganisms (in particular fungi) are able to transform the Mn2+ supplied by the decomposing organic material into the more reactive Mn3+ form. This latter would be subsequently used by other microorganisms for the degradation of the aromatic compounds (such as lignin and tannins) through redox reactions with the litter components, which would give the Mn back under its reduced Mn2+ form. After the first few years, the excess of Mn3+ produced by the biological activity precipitates under the form of oxides accumulating on the surface of the litter during more advanced stages of degradation (Keiluweit et al., 2015).
Unlike organic acids, MnO2 are capable of a selective adsorption of Ce, along the other REE, with a mechanism of oxidative scavenging through which Ce is preferentially trapped onto the surface of the aforementioned oxides (Bau, 1999; Bau and Koschinsky, 2009; Pourret and Davranche, 2013). The Ce enrichment linked to Mn oxides could be the reason for the formation of positive Ce anomalies in the waters that leached the litter material.
We conjecture that after a rainfall event, residual water that is deposited onto the surface of the oldest litter fraction has inherited a specific REE signature after passing through the younger litter layers above. We can assume that such a signature is similar to that of the leachates of the younger litter fractions recovered during our experiment (with the related MREE-HREE enrichment). Once the MnO2 deposited onto the surface of the old litter interacts with this solution, it would preferentially adsorb Ce with the scavenging mechanism previously mentioned. A question mark here is related to the form (complexed or free ions) of the REE when they come into contact with the MnO2. We assume that their main form during such an interaction occurs mainly as free ions since their complexation with organic acids could inhibit the preferential adsorption of Ce onto MnO2, as observed by Davranche et al. (2005, 2008). Moreover, these authors also highlighted a process of REE–organic acid complexes dissociating with time and with decreasing ratios. The reduced DOC concentrations (Table S4 in Hissler and Montemagno, 2021) and the presence of the MnO2 in Do OLv and Be OF would then lead to the dissociation of the OA–REE complexes and to the re-adsorption of the REE onto the MnO2 with a preferential intake of Ce. Note that when compared with the Do OLv leachate, the Be OF leachate shows a lower DOC, a higher Ce anomaly and the presence of tetrad effect, which may be a direct effect of the decrease of the ratios on the development of these specific REE features in the solutions during the litter degradation.
Interestingly, for both species the leachates of younger litter fractions (Do FL, Do OLn, Be FL, Be OLn and Be OLv) show higher DOC concentrations and lower pH than those of the oldest litter fractions (Do OLv and Be OF), as shown in Table S4 in Hissler and Montemagno (2021). This strengthens the assumption that the REE patterns in leachates of fresh leaves and young litter fractions are shaped by the presence of organic acids, which confers the typical increasing trend from La to Lu and the absence of positive and of tetrad effect (Fig. 4). On the contrary, leachates of the oldest litter fractions show higher pH, lower DOC, positive Ce anomalies and tetrad effect (in Be OF leachate), indicating that the shapes of the REE patterns in the leachates of the oldest litter fractions mainly result from the OA–REE dissociation accompanied by Ce-enriched MnO2. This would explain both the increasing trend from La to Lu and the development of positive Ce anomalies (with a tetrad effect in the Be OF sample) in the leachates of the oldest litter fractions. We propose that the process leading to Ce enrichment in waters that are in contact with the oldest litter fractions occurs in three steps, as reported below (and in more detail in Fig. 5):
- i.
biologically driven accumulation of MnO2 onto the surface of the old litter components;
- ii.
dissociation of OA–REE complexes and subsequent oxidative scavenging of Ce onto the surface of the MnO2 in the presence of stationary water in the litter surface during the degradation;
- iii.
dissolution of Ce-enriched MnO2 particles and/or their direct transport as MnO2 nanoparticles into solution operated by incident waters characterized by higher water volume and higher turbulence, which may then “wash” the surface of the oldest litter layers. One or the combination of these two processes would lead to Ce enrichment in the solutions, thus developing a positive anomaly.
One may argue that the yields of Mn during the leaching experiment are higher in the Do OLn and Be OLv leachates where the Ce anomalies do not appear. Here it is important to consider not the overall concentration of Mn in the leachates, but rather the chemical form in which this element is present in the litter layers. We recall, in fact, that the formation of Mn oxides (which lead to the development of Ce enrichment) only occurs during the last stages of the litter decay, in our case Do OLv and Be OF.
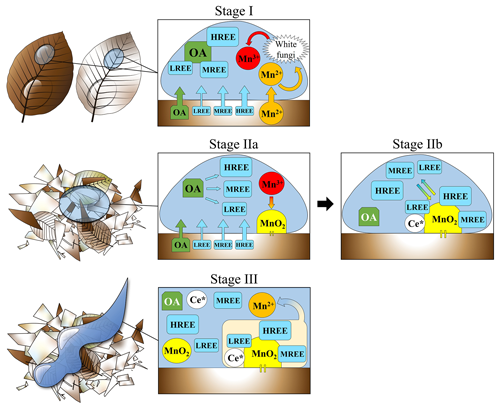
Figure 5Conceptual model representing the main processes treated in this study occurring during the litter degradation. Stage I – Do OLn, Be OLn and Be OLv degradation: Preferential release of HREE and MREE linked to their affinity for organic acids; REE complexation with organic acids and subsequent inhibition of Ce anomalies and tetrad effect; transformation of Mn2+ (coming from the litter) into Mn3+ operated by white fungi. Stage IIa – Do OLv and Be OF degradation: Preferential release of HREE and MREE linked to their affinity for organic acids; REE complexation with organic acids and subsequent inhibition of Ce anomalies and tetrad effect; accumulation and precipitation of Mn3+ under the form of Mn oxides; decrease in the ratios (due to increased concentration of MnO2 and decreased release of organic acids), leads to the REE–organic acid dissociation. Stage IIb – Do OLv and Be OF degradation: REE released from the organic acids in Stage IIa are re-adsorbed onto MnO2 positioned on the litter surface; scavenging of Ce and its subsequential enrichment on the Mn oxide surface. Stage III – Do OLv and Be OF degradation: Higher volume of water and higher turbulence lead to the dissolution and/or direct transportation of the Ce-enriched Mn oxides into solution, which inherits the enrichment in Ce and develops a tetrad effect (Be OF only).
For completeness of information, it is worth mentioning two other factors, which may play a role in the development of positive Ce anomalies in solution: the presence of siderophores and Fe oxides. Siderophores are a group of small molecules characterized by a strong affinity towards Fe3+ and are among the strongest Fe-chelating agents in nature. Plants (Poaceae), fungi and bacteria are able to synthesize and release these compounds to enhance the Fe assimilation in Fe-deficiency conditions (Chennappa et al., 2019). Accordingly, one would expect siderophores to be released by the aforementioned organisms to enhance the Fe assimilation especially during the first stages of the degradation where the Fe concentrations are lower when compared with the oldest fractions. Therefore, it is precisely in those early stages of decay where one would expect a greater participation of siderophores in the geochemical behaviour of Ce. Kraemer et al. (2017) demonstrated that siderophores are able to scavenge Ce by oxidizing it to Ce (IV), forming stable complexes in solution and leading to the development of positive Ce anomalies; anomalies that are not shown in the leachates of the younger litter fractions where one would expect them according to the Fe-deficiency. However, the Ce enrichment occurs only in the leachates of the oldest litter fractions, suggesting that the process acting in these circumstances is timeframe-specific (during specific stages of the degradation) rather than being condition-specific (Fe-deficiency). Other important aspects to consider are the much higher concentrations of Mn in the leachates when compared with Fe and the different leaching yields that Fe shows between the two tree species (Table S4 in Hissler and Montemagno, 2021), while Ce anomaly instead shows the same dynamics.
Fe oxides have been shown to potentially oxidize Ce(III) to Ce(IV) and scavenge this element from the water column leading to the development of positive Ce anomalies in the oxide fractions, as reported by Bau and Koschinsky (2009). Nevertheless, the ratios in the leachates of our litter samples () are much higher than those of the samples treated by the aforementioned authors (), suggesting that Mn rather than Fe might be a better candidate to act in the oxidative scavenging of Ce during the degradation of the litter.
4.3 Behaviour of Ca and Eu during litter degradation
Ca leaching yields are close to or even lower than those of trivalent metals during the first stages of Douglas-fir leaf degradation (Do FL and Do OLn), while its release increases from the oldest litter layer (Do OLv). In European beech samples, the leaching of Ca is not as low as for Douglas-fir samples but is lower than the other divalent elements (Mn and Mg). Ca is involved in many plant mechanisms and, among these, the stabilization of the cell wall structure is of vital importance. Indeed, it is an essential component of Ca pectate, an insoluble molecule that forms polymers between the cell walls linking them together (Bateman and Basham, 1976; Proseus and Boyer, 2012). The fact that Ca pectate is insoluble and that it is positioned between the cell walls makes this molecule less accessible to microorganisms during the initial stages of the degradation, leading to a reduced release of Ca. The fragmentation of the leaves and the decay of the weakest tissues during the first stages of degradation would therefore facilitate the accessibility of these insoluble components to biological degradation (Norman, 1929), thus contributing to a higher Ca release from the oldest litter fractions. However, the stabilization of cell walls cannot explain the increase in Ca concentrations observed in the litter fractions. This latter case, on the other hand, can be explained by the Ca-translocation mechanism occurring during leaf senescence described by Turpault et al. (2021). At high concentrations, Ca is a toxic element for trees and during the senescence is translocated from the other tree organs to the leaves, where it crystalizes under the form of insoluble Ca-bearing biominerals and is released to the litter material during the leaf fall as a form of an anti-toxicity mechanism.
Ca accumulation during leaf senescence can also explain the development of the slight Eu enrichment found in the Do OLn fraction in comparison with the fresh leaves ( and 0.93 in Do OLn and Do FL, respectively). The linkage between Ca and Eu in plants lies in the capability of Eu3+ to substitute Ca2+ in some physiological mechanisms due to their similar ionic radii. Stille et al. (2006) proposed that positive Eu anomalies in leaves can be related to the preferential uptake of Eu by plants. They argued that Eu3+ can follow the same Ca2+ fate in the cell cytoplasm, in which Ca concentrations are controlled via the precipitation of oxalate crystals leading to the accumulation of Eu in leaves and the consequential development of a positive anomaly. Gao et al. (2003) reported a preferential Eu accumulation in cell membranes and its capability to use Ca ion channels to enter cells and be absorbed by cytoplasmic organelles. Ding et al. (2005) linked Eu enrichments in roots to the precipitation of Eu-enriched phosphate particles, while Brioschi et al. (2013) proposed that the origin of such anomalies in roots should be attributed to Ca-depleted soils where plants may suffer Ca deficiency and where Eu substitution of Ca is responsible for the enrichments, especially in soils characterized by high ratios. In the case of the Weierbach soil, the ratios in the first 60 cm of soil under the Douglas-fir stand ranged from 0.0005 to 0.0044 (Moragues-Quiroga et al., 2017) which, according to Brioschi et al. (2013), indicates Ca depletion. Nonetheless, the slight enrichment in Eu occurs in the Do OLn fraction and not in the fresh leaves where instead the is below 1. Thus, it is our opinion that the Eu enrichment in the litter cannot be linked to the Ca-depleted soil as it does not occur during the entire life span of the leaf. In this study, the Eu enrichment in Do OLn indicates its involvement in Ca-dedicated biochemical pathways, which would lead to an anomalous accumulation of Eu with respect to the other REE in Douglas-fir litter. The fact that increases in Do OLn and not in Do FL suggests that Eu is involved in the same process of Ca translocation that occurs during leaf senescence.
Moreover, the positive Eu anomalies observed in the Do OLn and Do OLv leachates (Figs. 3c and 4a) suggest that at least part of the Eu is fractionated into slightly less degradation-resistant compounds – compared with the lignin where the other REE are supposed to stay bound – or into biogenic minerals, which are released from the cytoplasm after the cell wall breaks. The presence of these aforementioned compounds in the leachates and their subsequent digestion would deliver additional Eu content to the solution, leading to the development of positive anomalies in the leachates of Do OLv and Do OLn fractions. This would be in line with Gao et al. (2003), Turpault et al. (2021) and partially with Stille et al. (2006). In fact, if a preferential absorption of Eu by trees had played a role in the development of a positive anomaly in the leaves, we would have observed an enrichment already in the Do FL, which does not occur.
Although the reason for Ca behaving differently in the two tree species during the litter degradation cannot be explained with our experiment, it could be due to possible differences in the chemical and/or mechanical structures of the leaves or in the physiology of the tree species.
4.4 REE as a proxy for litter degradation resistance?
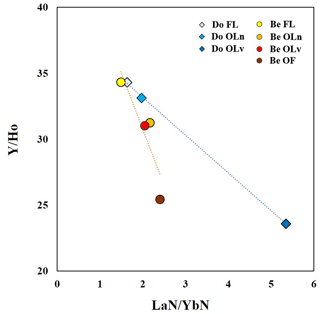
Both tree species show a progressive decrease in the ratios, indicating that during the degradation of the litter material, Y decouples from Ho, as it is preferentially leached. This trend in the ratios is also accompanied by the enrichment in LREE proceeding towards decay in both species, as shown by the ratios. These ratios can be thought of as proxies for classifying resistance to the litter degradation of the two tree species in the Weierbach forest. As illustrated in Fig. 6, the smaller the slope of the regression line, the lower the resistance. In accordance with this, Douglas-fir samples appear to be less resistant than the European beech samples. This is in line with the higher yields of trivalent metals we observed in the leachates of Douglas-fir samples since they are bound to the most resistant tissues. It is interesting to note that the fresh leaves from both species have a close position in the graph, indicating a similar stage of degradation (none) which changes over time.
4.5 REE in soil solutions
The differences in the average REE patterns of soil solutions observed between the two experimental sites in the Weierbach catchment seem to be linked to the different REE release occurring during the degradation of the litter at each plot. Indeed, from a depth close to the litter layers (soil solution at 20 cm depth) to the deepest soil layer (soil solution at 60 cm depth), the evolution of the HREE enrichment, Ce anomaly and specific MREE (Gd and/or Eu) enrichments in soil solutions (Fig. 7) could be discussed according to similarities with the litter leachates (Fig. 3c, d). It may be expected that if any degrading litter compound can contribute to the soil solution REE composition, it would be more easily observed close to the surface and would disappear progressively with depth, being diluted by the water–rock interaction processes and changing in redox conditions that control REE in soils (Braun et al., 1998; Laveuf and Cornu, 2009). Our results are in agreement with this expectation. For instance, particularities in the REE patterns of these litter leachates (especially for the last two stages of degradation for both species) seem to be mirrored by their respective soil solutions. Indeed, Eu and Gd enrichments, observed independently in both litter leachates (Do OLv and Be OF), were also found in the related soil solutions ( in Be SS and in Do SS). In both profiles, such anomalies are higher at 40 cm and decrease at 60 cm. This is in line with the leachate REE patterns normalized by the respective bulk litter concentrations reported in Fig. 4. Indeed, in the patterns of Be OLv and Be OF, Gd is the most enriched, while in the patterns of Do OLn and Do OLv, Eu leads the MREE enrichment. This may indicate a preferential release of these two elements to the soil solutions during the natural leaching operated by rainfall and throughfall of Do OLn and Do OLv fractions in the Douglas-fir stand and of Be OLv and Be OF fractions in the beech stand.
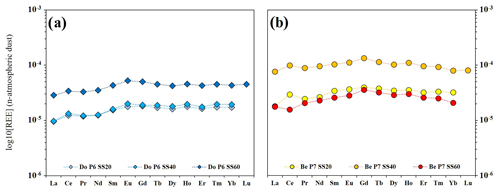
Figure 7Patterns of the average concentrations of rare earth elements in soil solutions of DoP (a) and BeP (b). REE concentrations have been normalized by the values in the local atmospheric dust.
Moreover, REE patterns of soil solutions from both profiles show positive Ce anomalies ( in Do SS20 and in Be SS20) that are close to those in the leachates of the oldest litter fractions. The amplitude of these anomalies decreases with the increasing depth until they disappear at 60 cm. Here again, the natural leaching of the oldest litter material could lead to the desorption of REE from the MnO2 or the direct transport into solution of Mn oxide nanoparticles enriched in Ce, finally leading to a positive Ce anomaly in soil solutions. However, due to its redox-sensitive nature, Ce dynamics are not easily understandable in soil solutions in which, due to the oxidative conditions, we would expect there to be a depletion (negative ) linked to the precipitation of Ce4+ as cerianite and adsorption onto Fe–Mn oxy-hydroxides.
Other important features of leachate patterns that are mirrored in the soil solutions are the HREE and MREE enrichments for the Douglas-fir and European beech stands, respectively, when compared with LREE. Indeed, average Douglas-fir soil solutions showed quite stable ratios at all depths with values between 0.50 (Do SS40) and 0.66 (Do SS60), which are in line with the ratios of Douglas-fir litter leachates (). Concerning the beech stand, the value of in average soil solutions is between 0.46 (Be SS20) and 0.57 (Be SS40), which is still in line with the values of beech litter leachates () obtained with our experiment. Similarities like these suggest a strong impact of the litter degradation on what is the REE signature of soil solutions, especially at shallower depths, and the fact that the anomalies tend to disappear in the deepest solutions strengthens this assumption.
It must be mentioned that the same environmental conditions to which the litter is generally exposed are not found in the laboratory. Conditions under which a greater degradation efficiency would be expected and that were avoided due to limitations present in the laboratory (such as the limited exchange of gases with the atmosphere, limited light, greater volume of water per litter surface area, lower concentration of microorganisms). Additional in situ studies regarding the REE dynamics in the Weierbach catchment soils are necessary to better understand and quantify the real contribution of litter degradation to the REE composition of soil solutions in a forest ecosystem. Moreover, chromatographic analysis of the leachates and SEM analysis of litter surfaces could help, respectively, to elucidate what kind of REE ligands are present in the different leachates and to observe the existence (or not) of MnO2 deposited on the surface of the oldest litter fractions.
We focused our attention on the role of forest vegetation in REE, major cations and trace elements sequestration and release into and from leaf tissues during litter degradation. As shown in our experiment, and similarly for both tree species, major cations and nutrients such as Na, Mg, K, and Mn are preferentially located in more labile tissues and are easily released during litter degradation, while Pb, Fe, Al and REE tend to be accumulated in the most recalcitrant tissues. We conjecture that such a sequestration in degradation-resistant tissues is imputable to the binding with lignins as the most resistant compounds in leaves.
Our results clearly show that litter degradation plays an important role in the REE dynamics in forest ecosystems. New findings related to REE dynamics during litter degradation and the potential of REE as complementary tracers for litter degradation processes were highlighted. The observation that the Ce anomaly and tetrad effect only occur in leachates of the oldest litter fractions can be linked to the accumulation of MnO2 on the surface of the litter after the first years of degradation. In comparison with the major cations, REE presented marked differences during the degradation of the litters of European beech and Douglas-fir. In the latter case, Eu seems to be involved in the same Ca translocation pathway that occurs during leaf senescence. Moreover, the evolution of the and ratios could be used as a proxy to analyse the resistance to the degradation of the leaves and litter between these two tree species.
Finally, the type of tree cover and the degradation stage of the litter are important parameters to consider when studying the chemistry of REE in forest soil waters. Similarities between the REE patterns of fresh leaves and litter leachates and REE patterns of soil solutions have been reported, possibly suggesting the importance of vegetation in determining the REE signatures in soil solutions. When compared with the other elements in the series, HREE are preferentially released from litter into solution due to the stronger affinity they have with the organic acids produced during the leaf degradation stages. This would also explain the unexpected positive Ce anomaly that can be observed in the shallower soil solutions of the Weierbach experimental catchment in Luxembourg.
The database used in this study is publicly available at Zenodo (https://doi.org/10.5281/zenodo.6539436, Hissler and Montemagno, 2021).
AM contributed to the field and laboratory experimental design and development; data interpretation; figure production; conceptual model idealization, development and drawing; and manuscript writing. CH contributed to the field and laboratory experimental design and development; data interpretation; figures production; conceptual model development; and manuscript writing. VB, AJT and LP contributed to the data interpretation and manuscript writing. JZ carried out the chemical analysis of the samples and the data treatment and contributed to the manuscript writing.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We would like to thank Olivier Pourret and an anonymous reviewer who, thanks to their suggestions, improved the quality of our manuscript.
We would like to thank Lindsey Auguin for the English proofreading of the manuscript.
This work is part of the HYDRO-CSI project and was supported by the Luxembourg National Research Fund (FNR) in the framework of the FNR/PRIDE research programme (contract no. PRIDE15/10623093/HYDRO-CSI/Pfister).
This paper was edited by Aninda Mazumdar and reviewed by Olivier Pourret and one anonymous referee.
Adeleke, R., Nwangburuka, C., and Oboirien, B.: Origins, roles and fate of organic acids in soils: A review, S. Afr. J. Bot., 108, 393–406, https://doi.org/10.1016/j.sajb.2016.09.002, 2017.
Albers, D., Migge, S., Schaefer, M., and Scheu, S.: Decomposition of beech leaves (Fagus sylvatica) and spruce needles (Picea abies) in pure and mixed stands of beech and spruce, Soil Biol. Biochem., 36, 155–164, https://doi.org/10.1016/j.soilbio.2003.09.002, 2004.
Alejandro, S., Höller, S., Meier, B., and Peiter, E.: Manganese in Plants: From Acquisition to Subcellular Allocation, Front. Plant Sci., 11, 1–23, https://doi.org/10.3389/fpls.2020.00300, 2020.
Aubert, D., Stille, P., and Probst, A.: REE fractionation during granite weathering and removal by waters and suspended loads: Sr and Nd isotopic evidence, Geochim. Cosmochim. Ac., 65, 387–406, https://doi.org/10.1016/S0016-7037(00)00546-9, 2001.
Austin, A. T. and Ballaré, C. L.: Dual role of lignin in plant litter decomposition in terrestrial ecosystems, P. Natl. Acad. Sci. USA, 107, 4618–4622, https://doi.org/10.1073/pnas.0909396107, 2010.
Bateman, D. F. and Basham, H. G.: Degradation of Plant Cell Walls and Membranes by Microbial Enzymes, Physiol. Plant Pathol., 316–355, https://doi.org/10.1007/978-3-642-66279-9_13, 1976.
Bau, M.: Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium, Chem. Geol., 93, 219–230, https://doi.org/10.1016/0009-2541(91)90115-8, 1991.
Bau, M.: Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from , , and lanthanide tetrad effect, Contrib. Mineral. Petr., 123, 323–333, https://doi.org/10.1007/s004100050159, 1996.
Bau, M.: Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect, Geochim. Cosmochim. Ac., 63, 67–77, https://doi.org/10.1016/S0016-7037(99)00014-9, 1999.
Bau, M. and Koschinsky, A.: Oxidative scavenging of cerium on hydrous Fe oxide: Evidence from the distribution of rare earth elements and yttrium between Fe oxides and Mn oxides in hydrogenetic ferromanganese crusts, Geochem. J., 43, 37–47, 2009.
Bienfait, H.: Prevention of stress in iron metabolism of plants, Acta Bot. Neerl., 38, 105–129, https://doi.org/10.1111/j.1438-8677.1989.tb02035.x, 1989.
Braun, J. J., Viers, J., Dupré, B., Polve, M., Ndam, J., and Muller, J. P.: Solid/liquid REE fractionation in the lateritic system of Goyoum, East Cameroon: The implication for the present dynamics of the soil covers of the humid tropical regions, Geochim. Cosmochim. Ac., 62, 273–299, https://doi.org/10.1016/S0016-7037(97)00344-X, 1998.
Brioschi, L., Steinmann, M., Lucot, E., Pierret, M. C., Stille, P., Prunier, J., and Badot, P. M.: Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE, Plant Soil, 366, 143–163, https://doi.org/10.1007/s11104-012-1407-0, 2013.
Brun, C. B., Åström, M. E., Peltola, P., and Johansson, M. B.: Trends in major and trace elements in decomposing needle litters during a long-term experiment in Swedish forests, Plant Soil, 306, 199–210, https://doi.org/10.1007/s11104-008-9572-x, 2008.
Censi, P., Saiano, F., Pisciotta, A., and Tuzzolino, N.: Geochemical behaviour of rare earths in Vitis vinifera grafted onto different rootstocks and growing on several soils, Sci. Total Environ., 473–474, 597–608, https://doi.org/10.1016/j.scitotenv.2013.12.073, 2014.
Censi, P., Cibella, F., Falcone, E. E., Cuttitta, G., Saiano, F., Inguaggiato, C., and Latteo, V.: Rare earths and trace elements contents in leaves: A new indicator of the composition of atmospheric dust, Chemosphere, 169, 342–350, https://doi.org/10.1016/j.chemosphere.2016.11.085, 2017.
Chadwick, O. A., Derry, L. A., Vitousek, P. M., Huebert, B. J., and Hedin, L. O.: Changing sources of nutrients during four million years of ecosystem development, Nature, 397, 491–497, https://doi.org/10.1038/17276, 1999.
Cheng, J., Ding, C., Li, X., Zhang, T., and Wang, X.: Rare Earth Element Transfer from Soil to Navel Orange Pulp (Citrus sinensis Osbeck cv. Newhall) and the Effects on Internal Fruit Quality, PLoS ONE, 10, e0120618, https://doi.org/10.1371/journal.pone.0120618, 2015.
Chennappa, G., Naik, M. K., Nidoni Udaykumar, Vidya, M., Sreenivasa, M. Y., Amaresh, Y. S., and Mathad, P. F.: Chapter 10 – Plant growth promoting microbes: a future trend for environmental sustainability, in: New and Future Developments in Microbial Biotechnology and Bioengineering, edited by: Shankar Singh, J., Elsevier, 163–178, https://doi.org/10.1016/B978-0-12-818258-1.00010-8, 2019.
Cidu, R., Buscaroli, A., Biddau, R., Da Pelo, S., Zannoni, D., Vianello, G., Dinelli, E., Vittori Antisari, L., and Carbone, S.: Dynamics of rare earth elements in water–soil systems: The case study of the Pineta San Vitale (Ravenna, Italy), Geoderma, 193–194, 52–67, https://doi.org/10.1016/j.geoderma.2012.10.009, 2013.
Cogulet, A., Blanchet, P., and Landry, V.: Wood degradation under UV irradiation: A lignin characterization, J. Photoch. Photobio. B, 158, 184–191, https://doi.org/10.1016/j.jphotobiol.2016.02.030, 2016.
Davranche, M., Pourret, O., Gruau, G., Dia, A., and Le Coz-Bouhnik, M.: Adsorption of REE(III)-humate complexes onto MnO2: Experimental evidence for cerium anomaly and lanthanide tetrad effect suppression, Geochim. Cosmochim. Ac., 69, 4825–4835, https://doi.org/10.1016/j.gca.2005.06.005, 2005.
Davranche, M., Pourret, O., Gruau, G., Dia, A., Jin, D., and Gaertner, D.: Competitive binding of REE to humic acid and manganese oxide: Impact of reaction kinetics on development of cerium anomaly and REE adsorption, Chem. Geol., 247, 154–170, https://doi.org/10.1016/j.chemgeo.2007.10.010, 2008.
Ding, S. M., Liang, T., Zhang, C. S., Yan, J. C., and Zhang, Z. L.: Accumulation and fractionation of rare earth elements (REEs) in wheat: Controlled by phosphate precipitation, cell wall absorption and solution complexation, J. Exp. Bot., 56, 2765–2775, https://doi.org/10.1093/jxb/eri270, 2005.
El-Ramady, H. R.: Ecotoxicology of rare earth elements: Ecotoxicology of rare earth elements within soil and plant environments, KG: VDM Verlag Dr. Muller Aktiengsellsellshaft & Co, 2010.
Fu, F., Akagi, T., and Shinotsuka, K.: Distribution pattern of rare earth elements in fern, Biol. Trace Elem. Res., 64, 13–26, 1998.
Gao, Y., Zeng, F., Yi, A., Ping, S., and Jing, L.: Research of the entry of rare earth elements Eu3+ and La3+ into plant cell, Biol. Trace Elem. Res., 91, 253–265, https://doi.org/10.1385/BTER:91:3:253, 2003.
Gautam, M. K., Lee, K. S., Berg, B., Song, B. Y., and Yeon, J. Y.: Trends of major, minor and rare earth elements in decomposing litter in a cool temperate ecosystem, South Korea, Chemosphere, 222, 214–226, https://doi.org/10.1016/j.chemosphere.2019.01.114, 2019.
Gómez, L., Contreras, A., Bolonio, D., Quintana, J., Oñate-Sánchez, L., and Merino, I.: Phytoremediation with trees, Adv. Bot. Res., 89, 281–321, https://doi.org/10.1016/bs.abr.2018.11.010, 2019.
Grobéty, B., Gieré, R., Dietze, V., and Stille, P.: Airborne particles in the urban environment, Elements, 6, 229–234, https://doi.org/10.2113/gselements.6.4.229, 2010.
Guo, L. B. and Sims, R. E. H.: Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests, Agr. Ecosyst. Environ., 75, 133–140, https://doi.org/10.1016/S0167-8809(99)00069-9, 1999.
Gwenzi, W., Mangori, L., Danha, C., Chaukura, N., Dunjana, N., and Sanganyado, E.: Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants, Sci. Total Environ., 636, 299–313, https://doi.org/10.1016/j.scitotenv.2018.04.235, 2018.
Han, F., Shan, X.-Q., Zhang, J., Xie, Y.-N., Pei, Z.-G., Zhang, S.-Z., Zhu, Y.-G., and Wen, B.: Organic acids promote the uptake of lanthanum by barley roots, New Phytol., 165, 481–492, https://doi.org/10.1111/j.1469-8137.2004.01256.x, 2005.
Hissler, C. and Montemagno, A.: Supplementary material for Dynamics of rare earth elements and associated major and trace elements during Douglas-fir (Pseudotsuga menziesii) and European beech (Fagus sylvatica L.) litter degradation (Version v2), Zenodo [data set], https://doi.org/10.5281/zenodo.6539436, 2021.
Hissler, C., Hostache, R., Iffly, J. F., Pfister, L., and Stille, P.: Anthropogenic rare earth element fluxes into floodplains: coupling between geochemical monitoring and hydrodynamic-sediment transport modelling, C.R. Geosci., 347, 294–303, https://doi.org/10.1016/j.crte.2015.01.003, 2015a.
Hissler, C., Stille, P., Juilleret, J., Iffly, J. F., Perrone, T., and Morvan, G.: Elucidating the formation of terra fuscas using Sr–Nd–Pb isotopes and rare earth elements, Appl. Geochem., 54, 85–99, https://doi.org/10.1016/j.apgeochem.2015.01.011, 2015b.
Hissler, C., Stille, P., Iffly, J. F., Guignard, C., Chabaux, F., and Pfister, L.: Origin and Dynamics of Rare Earth Elements during flood events in contaminated river basins: Sr-Nd-Pb evidence, Environ. Sci. Technol., 50, 4624–4631, https://doi.org/10.1021/acs.est.5b03660, 2016.
Hissler, C., Martínez-Carreras, N., Barnich, F., Gourdol, L., Iffly, J. F., Juilleret, J., Klaus, J., and Pfister, L.: The Weierbach experimental catchment in Luxembourg: A decade of critical zone monitoring in a temperate forest – from hydrological investigations to ecohydrological perspectives, Hydrol. Process., 35, 1–7, https://doi.org/10.1002/hyp.14140, 2021.
Imadi, S. R., Waseem, S., Kazi, A. G., Azooz, M. M., and Ahmad, P.: Chapter 1 – Aluminum Toxicity in Plants: An Overview, in: Plant Metal Interaction, edited by: Ahmad, P., Elsevier, 1–20, https://doi.org/10.1016/B978-0-12-803158-2.00001-1, 2016.
Jabiol, B., Zanella, A., Ponge, J. F., Sartori, G., Englisch, M., van Delft, B., de Waal, R., and Le Bayon, R. C.: A proposal for including humus forms in the World Reference Base for Soil Resources (WRB-FAO), Geoderma, 192, 286–294, https://doi.org/10.1016/j.geoderma.2012.08.002, 2013.
Jin, L., Ma, L., Dere, A., White, T., Mathur, R., and Brantley, S. L.: REE mobility and fractionation during shale weathering along a climate gradient, Chem. Geol., 466, 352–379, https://doi.org/10.1016/j.chemgeo.2017.06.024, 2017.
Juilleret, J., Dondeyne, S., Hissler, C., Vancampenhout, K., and Deckers, J.: Mind the gap: A classification system for integrating the subsolum into soil surveys, Geoderma, 264, 332–339, https://doi.org/10.1016/j.geoderma.2015.08.031, 2016.
Juranic, N.: Nephelauxetic Effect in Paramagnetic Shielding of Transition-Metal Nuclei in Octahedral d6 Complexes, J. Am. Chem. Soc., 111, 8326, https://doi.org/10.1021/ja00203a074, 1989.
Keiluweit, M., Nico, P., Harmon, M. E., Mao, J., Pett-Ridge, J., and Kleber, M.: Long-term litter decomposition controlled by manganese redox cycling, P. Natl. Acad. Sci. USA, 112, E5253–E5260, https://doi.org/10.1073/pnas.1508945112, 2015.
Kraemer, D., Tepe, N., Pourret, O., and Bau, M.: Negative cerium anomalies in manganese (hydr)oxide precipitates due to cerium oxidation in the presence of dissolved siderophores, Geochim. Cosmochim. Ac., 196, 197–208, https://doi.org/10.1016/j.gca.2016.09.018, 2017.
Krishna, M. P. and Mohan, M.: Litter decomposition in forest ecosystems: a review, Energy, Ecol. Environ., 2, 236–249, https://doi.org/10.1007/s40974-017-0064-9, 2017.
Labeeuw, L., Martone, P. T., Boucher, Y., and Case, R. J.: Ancient origin of the biosynthesis of lignin precursors, Biol. Direct, 10, 1–21, https://doi.org/10.1186/s13062-015-0052-y, 2015.
Laveuf, C. and Cornu, S.: A review on the potentiality of Rare Earth Elements to trace pedogenetic processes, Geoderma, 154, 1–12, https://doi.org/10.1016/j.geoderma.2009.10.002, 2009.
Leisola, M., Pastinen, O., and Axe, D. D.: Lignin–Designed Randomness, BIO-Complexity, 2012, 1–11, https://doi.org/10.5048/bio-c.2012.3, 2012.
Lequy, É., Conil, S., and Turpault, M. P.: Impacts of Aeolian dust deposition on European forest sustainability: A review, Forest Ecol. Manage., 267, 240–252, https://doi.org/10.1016/j.foreco.2011.12.005, 2012.
Li, X., Chen, Z., Chen, Z., and Zhang, Y.: A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China, Chemosphere, 93, 1240–1246, https://doi.org/10.1016/j.chemosphere.2013.06.085, 2013.
Liang, T., Zhang, S., Wang, L., Kung, H., Te, Wang, Y., Hu, A., and Ding, S.: Environmental biogeochemical behaviors of rare earth elements in soil-plant systems, Environ. Geochem. Hlth., 27, 301–311, https://doi.org/10.1007/s10653-004-5734-9, 2005.
Liang, T., Ding, S., Wenchong, S., Zhongyi, C., Zhang, C., and Li, H.: A review of fractionations of rare earth elements in plants, J. Rare Earths, 26, 7–15, https://doi.org/10.1016/S1002-0721(08)60027-7, 2008.
Ma, L., Jin, L., and Brantley, S. L.: How mineralogy and slope aspect affect REE release and fractionation during shale weathering in the Susquehanna/Shale Hills Critical Zone Observatory, Chem. Geol., 290, 31–49, https://doi.org/10.1016/j.chemgeo.2011.08.013, 2011.
Maathuis, F. J. M.: Sodium in plants: Perception, signalling, and regulation of sodium fluxes, J. Exp. Bot., 65, 849–858, https://doi.org/10.1093/jxb/ert326, 2014.
Marsac, R., Davranche, M., Gruau, G., and Dia, A.: Metal loading effect on rare earth element binding to humic acid: Experimental and modelling evidence, Geochim. Cosmochim. Ac., 74, 1749–1761, https://doi.org/10.1016/j.gca.2009.12.006, 2010.
McLennan, S.: Rare earth element geochemistry and the tetrad effect, Geochim. Cosmochim. Ac., 58, 2025–2033, https://doi.org/10.1016/0016-7037(94)90282-8, 1994.
Merschel, G., Bau, M., Baldewein, L., Dantas, E. L., Walde, D., and Bühn, B.: Tracing and tracking wastewater-derived substances in freshwater lakes and reservoirs: Anthropogenic gadolinium and geogenic REEs in Lake Paranoá, Brasilia, C.R. Geosci., 347, 284–293, https://doi.org/10.1016/j.crte.2015.01.004, 2015.
Möller, P.: The distribution of rare earth elements and yttrium in water-rock interactions: field observations and experiments, Water-Rock Interaction, Water Trans., 40, 97–123, https://doi.org/10.1007/978-94-010-0438-1_4, 2011.
Moragues-Quiroga, C., Juilleret, J., Gourdol, L., Pelt, E., Perrone, T., Aubert, A., Morvan, G., Chabaux, F., Legout, A., Stille, P., and Hissler, C.: Genesis and evolution of regoliths: Evidence from trace and major elements and Sr-Nd-Pb-U isotopes, Catena, 149, 185–198, https://doi.org/10.1016/j.catena.2016.09.015, 2017.
Morris, L. A.: Nutrient cycling, in: Encyclopedia of Forest Sciences, edited by: Burley, J., Evans, J., and Youngquist, J. A., Department of Plant Sciences, Oxford University, Oxford, UK, Academic Press, London, ISBN 0-12-145160-7, 2004.
Nikolic, M. and Pavlovic, J.: Chapter 3 – Plant Responses to Iron Deficiency and Toxicity and Iron Use Efficiency in Plants, in: Plant Micronutrient Use Efficiency, edited by: Hossain, M. A., Kamiya, T., Burritt, D. J., Tran, L.-S. P., and Fujiwara, T., Academic Press, 55–69, https://doi.org/10.1016/B978-0-12-812104-7.00004-6, 2018.
Norman, A. G.: The Biological Decomposition of Pectin, Ann. Bot., 43, 233–243, https://doi.org/10.1111/j.1469-8137.1941.tb07026.x, 1929.
Pacyna, J. M.: Atmospheric Deposition, in: Encyclopedia of Ecology, edited by: Jørgensen, S. E. and Fath, B. D., Academic Press, 275–285, https://doi.org/10.1016/B978-008045405-4.00258-5, 2008.
Pagano, G., Aliberti, F., Guida, M., Oral, R., Siciliano, A., Trifuoggi, M., and Tommasi, F.: Rare earth elements in human and animal health: State of art and research priorities, Environ. Res., 142, 215–220, https://doi.org/10.1016/j.envres.2015.06.039, 2015.
Page, V. and Feller, U.: Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level, Agronomy, 5, 447–463, https://doi.org/10.3390/agronomy5030447, 2015.
Pourret, O. and Davranche, M.: Rare earth element sorption onto hydrous manganese oxide: A modeling study, J. Colloid Interf. Sci., 395, 18–23, https://doi.org/10.1016/j.jcis.2012.11.054, 2013.
Pourret, O., Davranche, M., Gruau, G., and Dia, A.: Rare earth elements complexation with humic acid, Chem. Geol., 243, 128–141, https://doi.org/10.1016/j.chemgeo.2007.05.018, 2007.
Pourrut, B., Shahid, M., Dumat, C., Winterton, P., and Pinelli, E.: Lead Uptake, Toxicity, and Detoxification in Plants, Rev. Environ. Contam. Toxicol., 213, 113–136, https://doi.org/10.1007/978-1-4419-9860-6_4, 2011.
Proseus, T. E. and Boyer, J. S.: Pectate chemistry links cell expansion to wall deposition in Chara corallina, Plant Signal. Behav., 7, 1490–1492, https://doi.org/10.4161/psb.21777, 2012.
Rahman, M. M., Tsukamoto, J., Rahman, M. M., Yoneyama, A., and Mostafa, K. M.: Lignin and its effects on litter decomposition in forest ecosystems, Chem. Ecol., 29, 540–553, https://doi.org/10.1080/02757540.2013.790380, 2013.
Reynolds, R., Neff, J., Reheis, M., and Lamothe, P.: Atmospheric dust in modern soil on aeolian sandstone, Colorado Plateau (USA): Variation with landscape position and contribution to potential plant nutrients, Geoderma, 130, 108–123, https://doi.org/10.1016/j.geoderma.2005.01.012, 2006.
Rim, K. T., Koo, K. H., and Park, J. S.: Toxicological evaluations of rare earths and their health impacts to workers: A literature review, Saf. Health Work, 4, 12–26, https://doi.org/10.5491/SHAW.2013.4.1.12, 2013.
Rout, G., Samantaray, S., and Das, P.: Aluminium toxicity in plants: a review, Agronomie, 21, 3–21, https://doi.org/10.1051/agro:2001105, 2001.
Sardans, J. and Peñuelas, J.: Potassium Control of Plant Functions: Ecological and Agricultural Implications, Plants, 10, 419, https://doi.org/10.3390/plants10020419, 2021.
Schijf, J. and Zoll, A. M.: When dissolved is not truly dissolved-The importance of colloids in studies of metal sorption on organic matter, J. Colloid Interf. Sci., 361, 137–147, https://doi.org/10.1016/j.jcis.2011.05.029, 2011.
Semhi, K., Chaudhuri, S., and Clauer, N.: Fractionation of rare-earth elements in plants during experimental growth in varied clay substrates, Appl. Geochem., 24, 447–453, https://doi.org/10.1016/j.apgeochem.2008.12.029, 2009.
Shan, X., Wang, H., Zhang, S., Zhou, H., Zheng, Y., Yu, H., and Wen, B.: Accumulation and uptake of light rare earth elements in a hyperaccumulator Dicropteris dichotoma, Plant Sci., 165, 1343–1353, https://doi.org/10.1016/s0168-9452(03)00361-3, 2003.
Sharma, P. and Dubey, R. S.: Lead toxicity in plants, Braz. J. Plant Physiol., 17, 35–52, https://doi.org/10.1590/S1677-04202005000100004, 2005.
Shaul, O.: Magnesium transport and function in plants: The tip of the iceberg, BioMetals, 15, 309–323, https://doi.org/10.1023/A:1016091118585, 2002.
Singh, S., Tripathi, D. K., Singh, S., Sharma, S., Dubey, N. K., Chauhan, D. K., and Vaculík, M.: Toxicity of aluminium on various levels of plant cells and organism: A review, Environ. Exp. Bot., 137, 177–193, https://doi.org/10.1016/j.envexpbot.2017.01.005, 2017.
Sonke, J. E. and Salters, V. J. M.: Lanthanide-humic substances complexation. I. Experimental evidence for a lanthanide contraction effect, Geochim. Cosmochim. Ac., 70, 1495–1506, https://doi.org/10.1016/j.gca.2005.11.017, 2006.
Staaf, H.: Release of plant nutrients from decomposing leaf litter in a South Swedish beech forest, Ecography (Cop.), 3, 129–136, https://doi.org/10.1111/j.1600-0587.1980.tb00719.x, 1980.
Stille, P., Steinmann, M., Pierret, M. C., Gauthier-Lafaye, F., Chabaux, F., Viville, D., Pourcelot, L., Matera, V., Aouad, G., and Aubert, D.: The impact of vegetation on REE fractionation in stream waters of a small forested catchment (the Strengbach case), Geochim. Cosmochim. Ac., 70, 3217–3230, https://doi.org/10.1016/j.gca.2006.04.028, 2006.
Suzuki, Y., Yokoi, S., Katoh, M., Minato, M., and Takizawa, N.: Stability constants of Rare Earth complexes with some organic ligands, in: The Rare Earths in Modern Science and Technology, edited by: McCarthy, G. J., Rhyne, J. J., and Silber, H. B., Vol. 2, Springer, 121–126, https://doi.org/10.1007/978-1-4613-3054-7, 1980.
Swift, M. J., Heal, O. W., and Anderson, J. M.: Decomposition in Terrestrial Ecosystem, Studies in Ecology, Vol. 5, Oxford, Blackwell, 1979.
Tagliavini, M., Tonon, G., Scandellari, F., Quiñones, A., Palmieri, S., Menarbin, G., Gioacchini, P., and Masia, A.: Nutrient recycling during the decomposition of apple leaves (Malus domestica) and mowed grasses in an orchard, Agric. Ecosyst. Environ., 118, 191–200, https://doi.org/10.1016/j.agee.2006.05.018, 2007.
Takahashi, Y., Châtellier, X., Hattori, K. H., Kato, K., and Fortin, D.: Adsorption of rare earth elements onto bacterial cell walls and its implication for REE sorption onto natural microbial mats, Chem. Geol., 219, 53–67, https://doi.org/10.1016/j.chemgeo.2005.02.009, 2005.
Takahashi, Y., Yamamoto, M., Yamamoto, Y., and Tanaka, K.: EXAFS study on the cause of enrichment of heavy REEs on bacterial cell surfaces, Geochim. Cosmochim. Ac., 74, 5443–5462, https://doi.org/10.1016/j.gca.2010.07.001, 2010.
Talbot, J. M., Yelle, D. J., Nowick, J., and Treseder, K. K.: Litter decay rates are determined by lignin chemistry, Biogeochemistry, 108, 279–295, https://doi.org/10.1007/s10533-011-9599-6, 2012.
Tang, J. and Johannesson, K. H.: Ligand extraction of rare earth elements from aquifer sediments: Implications for rare earth element complexation with organic matter in natural waters, Geochim. Cosmochim. Ac., 74, 6690–6705, https://doi.org/10.1016/j.gca.2010.08.028, 2010.
Tchougréèff, A. and Drownkowski, R.: Nephelauxetic Effect Revisited, Int. J. Quantum Chem., 109, 2606–2621, https://doi.org/10.1002/qua.21989, 2009.
Tommasi, F., Thomas, P. J., Pagano, G., Perono, G. A., Oral, R., Lyons, D. M., Toscanesi, M., and Trifuoggi, M.: Review of Rare Earth Elements as Fertilizers and Feed Additives: A Knowledge Gap Analysis, Arch. Environ. Contam. Toxicol., 81, 531–540, https://doi.org/10.1007/s00244-020-00773-4, 2021.
Turpault, M. P., Kirchen, G., Calvaruso, C., Redon, P. O., and Dincher, M.: Exchanges of major elements in a deciduous forest canopy, Biogeochemistry, 152, 51–71, https://doi.org/10.1007/s10533-020-00732-0, 2021.
Tyler, G.: Rare earth elements in soil and plant systems – A review, Plant Soil, 267, 191–206, https://doi.org/10.1007/s11104-005-4888-2, 2004.
Vázquez-Ortega, A., Zapata-Ríos, X., Rasmussen, C., McIntosh, J., Brooks, P. D., Perdrial, J., Amistadi, M. K., Chorover, J., Schaap, M., Harpold, A., and Pelletier, J. D.: Rare earth elements as reactive tracers of biogeochemical weathering in forested rhyolitic terrain, Chem. Geol., 391, 19–32, https://doi.org/10.1016/j.chemgeo.2014.10.016, 2015.
Vázquez-Ortega, A., Huckle, D., Perdrial, J., Amistadi, M. K., Durcik, M., Rasmussen, C., McIntosh, J., and Chorover, J.: Solid-phase redistribution of rare earth elements in hillslope pedons subjected to different hydrologic fluxes, Chem. Geol., 426, 1–18, https://doi.org/10.1016/j.chemgeo.2016.01.001, 2016.
Weng, J. K. and Chapple, C.: The origin and evolution of lignin biosynthesis, New Phytol., 187, 273–285, https://doi.org/10.1111/j.1469-8137.2010.03327.x, 2010.
Woolhouse, H. W.: Toxicity and Tolerance in the Responses of Plants to Metals, in: Physiological Plant Ecology III, edited by: Lange, O. L., Nobel, P. S., Osmond, C. B., and Ziegler, H., Springer, Berlin, Heidelberg, 245–300, https://doi.org/10.1007/978-3-642-68153-0_8, 1983.
Wyttenbach, A., Furrer, V., Schleppi, P., and Tobler, L.: Rare earth elements in soil and in soil-grown plants, Plant Soil, 199, 267–273, https://doi.org/10.1023/A:1004331826160, 1998.
Yuan, M., Liu, C., Liu, W. S., Guo, M. N., Morel, J. L., Huot, H., Yu, H. J., Tang, Y. T., and Qiu, R. L.: Accumulation and fractionation of rare earth elements (REEs) in the naturally grown Phytolacca americana L. in southern China, Int. J. Phytoremediat., 20, 415–423, https://doi.org/10.1080/15226514.2017.1365336, 2018.
Zaharescu, D. G., Burghelea, C. I., Dontsova, K., Presler, J. K., Maier, R. M., Huxman, T., Domanik, K. J., Hunt, E. A., Amistadi, M. K., Gaddis, E. E., Palacios-Menendez, M. A., Vaquera-Ibarra, M. O., and Chorover, J.: Ecosystem Composition Controls the Fate of Rare Earth Elements during Incipient Soil Genesis, Sci. Rep.-UK, 7, 1–15, https://doi.org/10.1038/srep43208, 2017.
Zhenggui, W., Ming, Y., Xun, Z., Fashui, H., Bing, L., Ye, T., Guiwen, Z., and Chunhua, Y.: Rare earth elements in naturally grown fern Dicranopteris linearis in relation to their variation in soils in South-Jiangxi region (Southern China), Environ. Pollut., 114, 345–355, https://doi.org/10.1016/S0269-7491(00)00240-2, 2001.