the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Technical note: A low-cost, automatic soil–plant–atmosphere enclosure system to investigate CO2 and evapotranspiration flux dynamics
Maren Dubbert
Jörg Schaller
Matthias Lück
Marten Schmidt
Mathias Hoffmann
Investigating greenhouse gases (GHGs) and water flux dynamics within the soil–plant–atmosphere interphase is key for understanding ecosystem functioning, as they reflect the ecosystem's responses to environmental changes. Understanding these responses is essential for developing sustainable agricultural systems that can help to adapt to global challenges such as increased drought. Typically, an initial understanding of GHGs and water flux dynamics is gained through laboratory or greenhouse pot experiments, where gas exchange is often measured using commercially available manual closed-chamber (leaf) systems. However, these systems are rather expensive and often labor-intensive, thus limiting the number of different treatments and their repetitions that can be studied. Here, we present a fully automatic, low-cost (EUR <1000 per unit) multi-chamber system based on Arduino, termed “greenhouse coffins”. It is designed to continuously measure canopy CO2 and evapotranspiration (ET) fluxes. It can operate in two modes: an independent and a dependent measurement mode. The independent measurement mode utilizes low-cost NDIR (non-dispersive infrared) CO2 (K30 FR) and relative humidity (SHT31) sensors, thus making each greenhouse coffin a fully independent measurement device. The dependent measurement mode connects multiple greenhouse coffins via a low-cost multiplexer (EUR <250) to a single infrared gas analyzer (LI-850, LI-COR Inc., Lincoln, USA), allowing for measurements in series, achieving cost efficiency while also gaining more flexibility in terms of target GHG fluxes (potential extension to N2O, CH4 and stable isotopes). In both modes, CO2 and ET fluxes are determined through the respective concentration increase during closure time. We tested both modes and demonstrated that the presented system is able to deliver precise and accurate CO2 and ET flux measurements using low-cost sensors, with an emphasis on calibrating the sensors to improve measurement precision. By connecting multiple greenhouse coffins via our low-cost multiplexer to a single infrared gas analyzer in the dependent mode, we could additionally show that the system can efficiently measure CO2 and ET fluxes in a high temporal resolution across various treatments with both labor and cost efficiency. Therefore, the developed system is expected to be a valuable tool for conducting greenhouse experiments, enabling comprehensive testing of plant–soil dynamic responses to various treatments and conditions.
- Article
(5674 KB) - Full-text XML
- BibTeX
- EndNote
Agricultural systems are particularly vulnerable to more frequent, less predictable extreme weather events (e.g. droughts, heat waves) wrought by climate change (Altieri et al., 2015; Ummenhofer and Meehl, 2017). Moreover, agricultural systems have the potential to both contribute to (Tubiello et al., 2013; Chataut et al., 2023) and mitigate (Lal, 2004; Powlson et al., 2016) greenhouse gas (GHG) emissions, influenced by the practices implemented and the specific environmental contexts in which they operate. Therefore, to best mitigate the challenges of extreme weather (especially drought and heat waves) and to characterize the potential of agricultural fields to decrease or even reverse GHG emissions, it is essential to better monitor (and thus understand) gas and water fluxes between those systems and the atmosphere (Zhang et al., 2002; Fisher et al., 2017).
Chamber-based systems (automatic or manual) in conjunction with high-temporal-resolution gas analyzers are one of the most common techniques for directly measuring CO2 and evapotranspiration (ET), providing precise data on a leaf-to-plot scale and allowing us to assess small-scale heterogeneity (Smith et al., 2010; Dubbert et al., 2014; Riederer et al., 2014). However, it is challenging to study the effects of climate change on agricultural GHG dynamics given the difficulties inherent to both field- and laboratory-based research on soil–plant–atmosphere systems. Field-based research comes at the expense of high variability, environmental noise, and the labor and cost associated with large-scale, high-resolution data collection and equipment, whereas lab-based research is limited by a lack of environmental context and replicability besides the high cost of equipment (Savage and Davidson, 2003; Sun and May, 2013; Martin et al., 2017; Blackstock et al., 2019). Mesocosm-scale experiments performed in greenhouses or climate-controlled chambers allow researchers to mimic the in situ environmental conditions of many different settings and provide the opportunity to variably manipulate those conditions. In this way, researchers can explore the impacts of precisely isolated environmental treatments, bridging the gap between lab-based studies of single plants and field-based studies, thus facilitating a more nuanced understanding of ecological dynamics (Riebesell et al., 2013; Stewart et al., 2013).
In recent years, researchers have been increasingly developing low-cost devices for chamber-based gas-exchange systems using a do-it-yourself (DIY) approach. These DIY systems reduce the generally high cost per device (Fisher and Gould, 2012; D'Ausilio, 2012), allowing for higher replicability than has previously been possible using commercial systems. They leverage affordable microcontrollers and sensors to build custom measurement tools designed for specific research needs. By integrating sensors for CO2 and/or ET with microcontrollers, researchers were able to develop portable, precise, and cost-effective devices for monitoring CO2 and ET fluxes, such as in Macagga et al. (2024) and Bonilla-Cordova et al. (2024). Others went a step further and developed fully automated measurement systems to determine CO2 efflux, such as “fluxbots” (Forbes et al., 2023).
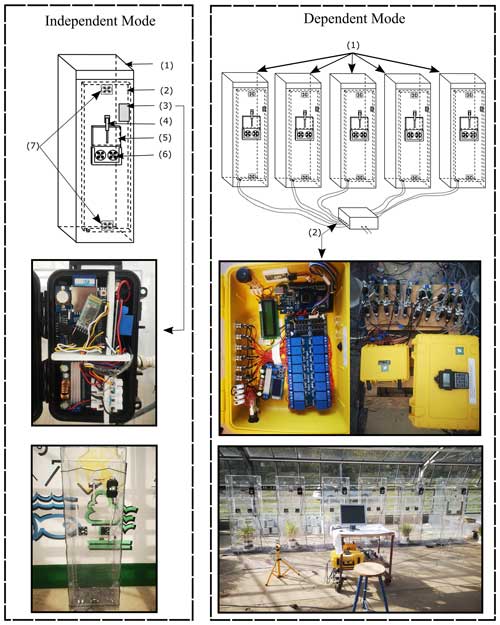
Figure 1Sketch illustrating the greenhouse coffin system. Left: the independent mode with a single unit comprising (1) the chamber, (2) the front door, (3) the control unit, (4) the linear actuator, (5) the sliding door, (6) ventilation fans, and (7) air mixing fans. Right: the dependent mode consists of (1) multiple greenhouse coffins and (2) a low-cost multiplexer connected to a single gas analyzer.
To expand the application space of such DIY devices to the mesocosm scale, we developed and validated greenhouse coffins, a novel low-cost, automatic soil–plant enclosure system, designed to monitor CO2 and ET fluxes within greenhouse experiments in a fully automatic manner. We hypothesize that (1) a single greenhouse coffin employing low-cost sensors can measure CO2 and ET fluxes accurately and reliably, comparable to a high-cost gas analyzer. (2) By combining several greenhouse coffins and adding a low-cost self-constructed multiplexer, we are able to monitor gas fluxes via one infrared gas analyzer for different treatments cost-efficiently. To test these hypotheses, we performed a number of experiments validating the different components of the greenhouse coffins. Additionally, we evaluated the accuracy and precision of the low-cost NDIR (non-dispersive infrared) CO2 and relative humidity (RH) sensors used (independent mode) by comparing measured CO2 and ET fluxes with those obtained using a commercial infrared gas analyzer (LI-850, LI-COR Inc., Lincoln, USA). Furthermore, we tested the DIY, low-cost multiplexer's ability to link multiple greenhouse coffins to one commercial gas analyzer (dependent mode).
2.1 Hardware and software implementation
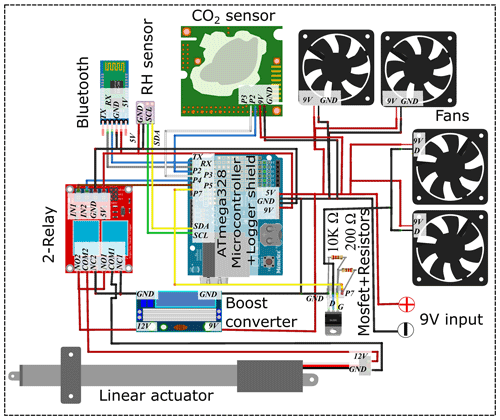
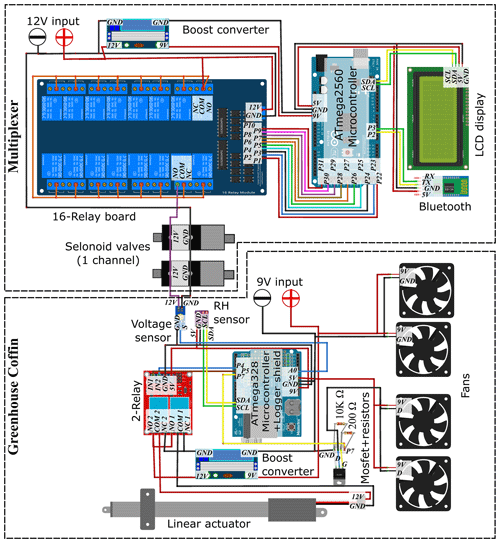
The greenhouse coffin system consists of one to multiple enclosed transparent chambers (PVCs; cm) that can house an entire soil–plant–atmosphere system (Fig. 1). Each chamber can be accessed through a front door sealed using a rubber rope. The front door is equipped with a sliding-window mechanism, which is opened and closed by a linear actuator moving it along guiding rails. The sliding window covers two openings, behind which two opposing directed 9 V axial fans are installed with a volumetric flow rate of 76.4 m3 h−1, allowing for a complete air exchange within 20 s during the opening period. Ventilation within each chamber is enabled through two additional axial fans at the bottom and top of the door. Each chamber is operated individually by a control unit consisting of a microcontroller (ATmega328-Board) with an attached logger shield module. This module is equipped with an SD card reader and a 2 GB SD card for data storage, along with a real-time clock (RTC), ensuring accurate timekeeping while off power. A Bluetooth module is connected to the microcontroller for direct operation and data monitoring of the microcontroller using, e.g., a smartphone via the Serial Bluetooth Terminal application. To steer the opening and closure of the sliding window, the microcontroller switches a double relay, which is connected to the linear actuator (Fig. 2). During the closure of the sliding door, the two axial fans behind it are switched off via a Mosfet (IRLZ44N) connected to two resistors (200 and 10 000 Ω). The power supply for each greenhouse coffin system is provided by a 9 V charger, connected to the microcontroller and axial fans, as well as a linear actuator (requiring 12 V) through a DC–DC buck boost power converter. The control unit is fitted in a waterproof, sealed outdoor box ( cm) in the top-right corner of the door. When operated independently (independent mode), each greenhouse coffin utilizes a low-cost NDIR-based CO2 sensor (0–5000 ppm, ± 30 ppm, ± 3 % accuracy; K30 FR, Senseair AB, Sweden) and an air humidity and temperature sensor (SHT31, ± 2 % accuracy, Sensirion AG, Switzerland) placed on the inner side of the door (Macagga et al., 2024).
The individual coffins (independent mode) can be operated together by connecting multiple greenhouse coffins with a low-cost multiplexer unit (dependent mode). This multiplexer unit switches a series of normally closed solenoid valves acting as air inlets and outlets, thus enabling researchers to chain each greenhouse coffin together and connect them to a single gas analyzer. The multiplexer is controlled by a microcontroller (ATmega2560), which steers a 16-fold relay model. Each of the 16 relays is linked to two solenoid valves, which open and close the air inlet and outlet of a greenhouse coffin. Relays are operated in series. When a relay is powered up, the normally closed solenoid valves connected to it open, connecting the greenhouse coffin to the gas analyzer in a closed loop. A voltage sensor connecting the two solenoid valves and the control unit of the greenhouse coffin signalizes when the solenoid valves are open so that the sliding door is closed to conduct the measurement, thus enabling indirect communication and synchronization between the multiplexer and each attached greenhouse coffin. The specific greenhouse coffin being measured at any given moment is indicated by a liquid-crystal display (LCD) connected to the microcontroller of the multiplexer unit. A Bluetooth module (HC-05) allows for easy data access. To enable remote access to the system during 24/7 measurements, a second Bluetooth module connected to a microcontroller (ATmega328-Board) with a logger shield acts as an uplink station. When connected to a stationary PC with internet access, incoming data transferred between both Bluetooth modules can be accessed on time using remote access software (e.g., AnyDesk). The power supply for the 16-fold relay is provided by a 12 V charger, connected to a boost converter step-up/step-down (HW-140 DC–DC), adjusting the energy to 9 V for the microcontroller. Figure 3 shows the assembled connection of the different components. Detailed information on component prices and distributors for both modes can be found in Table 1. The software was developed using Arduino IDE 2.0.0.
Table 1Components needed to construct one greenhouse coffin and a multiplexer. The prices are based on orders placed on 30 July 2023.
2.2 Sealing test
We performed three sealing tests to check for any leakage from different system components. Sealing tests included evaluation of (1) the sealing of the entire coffin with the door (check for serious leakages in the construction), (2) the suitability of the sliding window to sufficiently seal the greenhouse coffin when closed and exchange air when open, and (3) the proper sealing of the solenoid valves of the multiplexer.
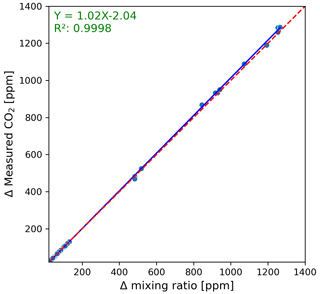
To assess for serious leakages from the greenhouse coffin construction itself (more importantly, where precisely on the construction it occurs), we used a smoke bomb as suggested by Hoffmann et al. (2018). The same method was also implemented by Olfs et al. (2018) for the leakage test on their chamber design used to measure nitrous oxide emissions. We placed the smoke bomb inside the greenhouse coffin and lit it. Subsequently, the greenhouse coffin was closed for a 15 min observation period. During this time, any escaping smoke would indicate potential leakage. To check the suitability of the sliding window to sufficiently seal the greenhouse coffin airtight when closed and exchange air when open in its final setup (complete hardware implementation), we repeatedly injected distinct amounts of technical gas containing 1 000 000 ppm CO2 ranging from 15 to 450 mL into its sealed headspace using a syringe. Prior, during, and after each injection, chamber headspace CO2 concentrations were continuously recorded in a 5 s interval using an infrared CO2 gas analyzer (LI-850, LI-COR Inc., Lincoln, USA) connected to the inlet and outlet of the coffin. In more detail, the following procedure was followed: (1) after the sliding door was closed and stable CO2 concentrations were obtained (ca. 1 min), (2) technical gas was injected into the chamber headspace, and CO2 concentration development was recorded over the next 5 min before (3) the sliding door was opened again, and CO2 concentration depletion was monitored until stabilization (ca 1 min). The average CO2 concentrations of the initial 1 min (12 records; after closure and before injection) and last 4 min (48 records; after injection/stabilization and before opening) of CO2 concentration records were then used to calculate the change in CO2 concentration from before to after injection (ΔCO2 in ppm). In the case of proper sealing of the coffin, the thus determined ΔCO2 should match the calculated mixing ratio.
To assess the absence of cross-contamination within the dependent mode, out of the six greenhouse coffins connected to the CO2 gas analyzer (LI-820, LI-COR Inc., Lincoln, USA) through the solenoid valves for the inlets and outlets, five were equipped with plants changing the headspace CO2 concentration during measurements, while one remained empty. We then measured this empty system and checked for potential CO2 concentration changes. We repeated these measurements for each of the six connected greenhouse coffins, altering coffins filled with plants.
2.3 Validation experiment
For the independent mode, we conducted a greenhouse experiment to test the accuracy of the low-cost sensors (K30 FR and SHT31) and the capability of the greenhouse coffin system. Therefore, we placed two pots planted with sorghum inside a greenhouse coffin. For 5 continuous days with a 30 min chamber closure frequency and 5 min chamber closure time, we measured the CO2 and ET fluxes using both low-cost sensors (K30 FR and SHT31) and an infrared gas analyzer (LI-850, LI-COR Inc., Lincoln, USA), resulting in ∼ 48 CO2 and ∼ 48 ET fluxes per day.
For the dependent mode, to test the ability of the system to continuously monitor CO2 and ET fluxes across various treatments in a fully automated manner using a single gas analyzer, we connected six greenhouse coffins (two empty, two with sorghum plants, and two with maize plants) to a single infrared gas analyzer (LI-850, LI-COR Inc., Lincoln, USA) via the low-cost multiplexer. Subsequently, we measured the CO2 and ET fluxes for each of the six chambers. Similarly to the independent mode, we continuously measured 5 d with a 30 min chamber closure frequency and 5 min chamber closure time, resulting in ∼ 48 CO2 and ∼ 48 ET fluxes per day and a greenhouse coffin. We obtained the environmental variables inside the greenhouse (air temperature, relative humidity, and photosynthetically active radiation (PAR)) from the greenhouse's climate station.
2.4 Data processing
2.4.1 CO2 and ET calculations
The first and last 10 % of each CO2 and ET measurement were removed to exclude any potential noises from turbulence and pressure fluctuations during the closing and opening of the sliding window (Hoffmann et al., 2015). Additionally, the CO2 concentrations measured with the LI-850 were corrected for changes in water vapor concentration during each chamber measurement (correction for dilution by foreign gas; Webb et al., 1980; Hupp et al., 2011) in Eq. (1):
where is the mole fraction of CO2 in the sample (µmol mol−1) corrected to the water vapor content of the reference measurement wr (mmol mol−1), is the mole fraction of CO2 measured in the sample (µmol mol−1), and ws is the water vapor content in the sample (µmol mol−1). To calculate ET fluxes using the low-cost RH sensor (SHT31), measured RH needed to be converted to mass concentration following Hamel et al. (2015) (Eq. 2):
where RH is relative humidity, es is saturated vapor pressure calculated according to Allen et al. (1998), and P is gas pressure (Pa). Modular R scripts, as described by Hoffmann et al. (2015) for CO2 and Dahlmann et al. (2023) for ET, were used to calculate CO2 and ET fluxes measured during the validation experiment. CO2 and ET fluxes were calculated using the ideal gas law and using a linear regression approach (Eq. 3):
where M is the molar mass of the gas (g mol−1), p is the ambient air pressure (Pa), V is the chamber volume (m3), R is the gas constant (8.314 m3 Pa K−1 mol−1), T is the temperature inside the chamber (K), A is the basal area (m2), and represents the linear concentration changes in CO2 (e.g., Leiber-Sauheitl et al., 2014) and H2O over time (e.g., Dahlmann et al., 2023). A variable moving window (window size 0.5 to 5 min) was applied to each chamber measurement to obtain the variables T and . Accordingly, the multiple resulting ET and CO2 fluxes per measurement (using the generated variable moving window data subset) were evaluated based on specific criteria, including fulfilled prerequisites for applying (1) linear regression (normality (Lilliefors adaption of the Kolmogorov–Smirnov test), homoscedasticity (Breusch–Pagan test), and linearity), (2) a regression slope (p≤0.1, t test), and (3) a range of within-chamber air temperature not larger than ± 1.5 K and a PAR deviation (only for day measurements) not larger than ± 20 % of the average to ensure stable environmental conditions within the chamber throughout the respective measurement window and having (4) no outliers present (± 6 times the interquartile range (IQR)). Calculated CO2 and ET fluxes meeting all criteria were retained. In cases where multiple fluxes per measurement met all criteria, the CO2 and ET fluxes with the steepest slope and closest timing to chamber closure were selected.
2.4.2 Statistical analysis
The statistical analysis was done using the SciPy and Sklearn packages in Python (version 3.9.12). To determine a suitable statistic test for the collected data during the laboratory validation and greenhouse trial, a Kolmogorov–Smirnov test (p<0.05) to assess the normal distribution was carried out. A pairwise Wilcoxon signed rank was employed to determine the significance of the CO2 and ET fluxes measured by the low-cost NDIR sensor and LI-850 sensor and to determine the significance of the CO2 concentration measured during the cross-contamination test. A concordance correlation coefficient was employed to determine the accuracy of the low-cost sensors, while the precision was determined by root mean square error (RMSE) and Pearson correlation. The error calculation for CO2 fluxes was quantified using a comprehensive error prediction algorithm described in detail by Hoffmann et al. (2015) using R software (version 3.6.1). The approaches utilize bootstrapping alongside k-fold subsampling to estimate uncertainties for each flux measurement and subsequent Reco (ecosystem respiration) and gross primary production (GPP) parameterization. This approach was adapted to calculate the error for ET fluxes by Dahlmann et al. (2023).
3.1 Sealing test
During our smoke bomb test, no visible leakage was detected, indicating the absence of serious leaks from both the greenhouse coffin and the sliding window in the coffin's door. However, a properly sealed coffin does not mean that its ventilation system is also sufficient. For repeated measurements, it is essential to replace the chamber headspace air after each measurement, thus recreating the atmospheric starting concentrations of CO2 and H2O. To test this, we compared the CO2 starting concentrations (n=38) obtained during the gas injection test. With an average of 413 ± 12 ppm, the CO2 starting concentrations not only were close to the atmospheric CO2 concentration (419.3 ppm in 2023; NOAA, 2024) but also showed a minor variation, with a minimum and maximum CO2 starting concentration of 378 and 425 ppm, respectively. Additionally, no significant difference (pairwise Wilcoxon signed rank, p>0.01) between ΔCO2 measured by the LI-850 and the calculated mixing ratio was observed (Fig. 4). Hence, the gas injection test evidenced the coffin ventilation system's effectiveness and overall airtightness when used in the independent mode. While the coffins are properly sealed, insufficient solenoid valve closure could lead to cross-contamination, with concentration increases in one coffin affecting another. Therefore, the cross-contamination test was performed to test for proper sealing of the coffins and the connected multiplexer when used in dependent mode. In the case of no cross-contamination, an empty coffin should show no concentration change during its measurement, irrespective of plants being present in the other coffins connected to the multiplexer. When comparing the measured ΔCO2 concentration of the performed cross-contamination, no significant difference from 0 was found (pairwise Wilcoxon signed-rank test, p>0.05). These results show that no cross-contamination due to the multiplexer occurred within the independent mode. However, since solenoid valves have moving parts that can show wear and tear with long-term use, it is recommended to repeat the cross-contamination test periodically and change the non-tight valve.
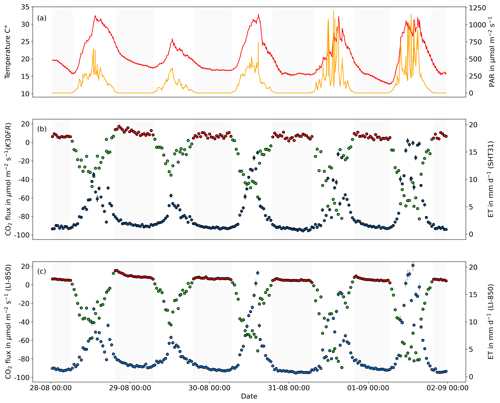
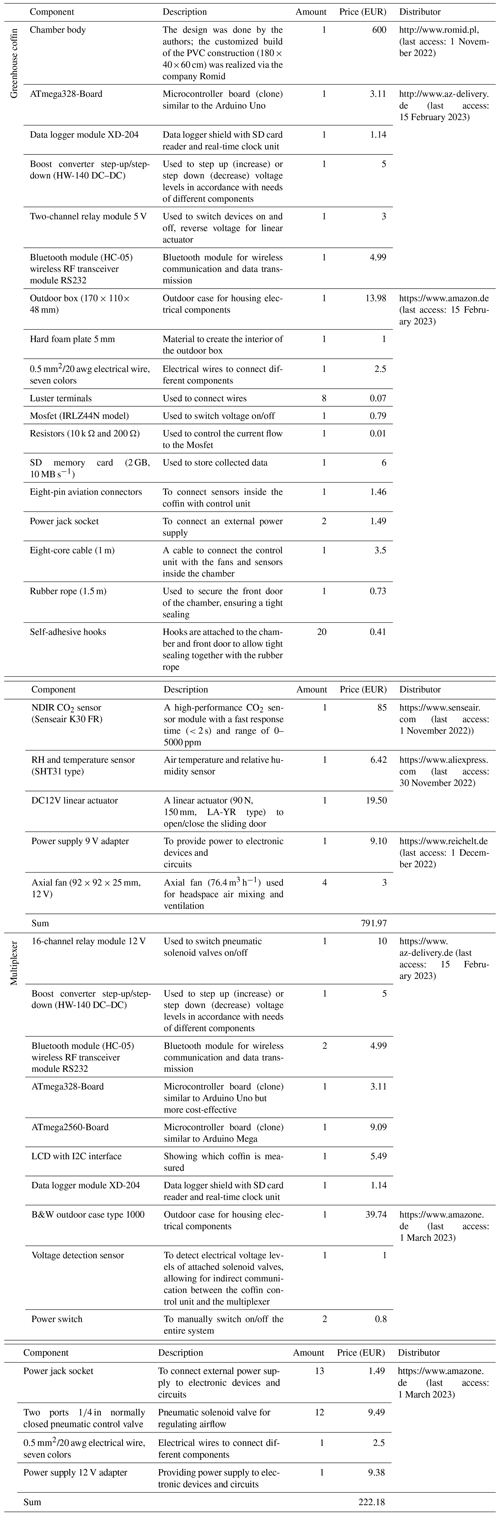
Figure 5The figure shows the 5 d trial conducted for the dependent mode. Panel (a) shows the air temperature (red line) and PAR (orange line), and (b) shows the diurnal cycle of CO2 (Reco: red points, net ecosystem exchange (NEE): green points) and ET fluxes (blue points) measured with low-cost sensors (CO2: K30 FR, ET: SHT31). Panel (c) shows the diurnal cycle of CO2 (Reco: red points, NEE: green points) and ET fluxes (blue points) measured with an infrared gas analyzer (LI-820, LI-COR Inc., Lincoln, USA). The gray-shaded areas represent nighttime. Error bars indicate calculated flux error.
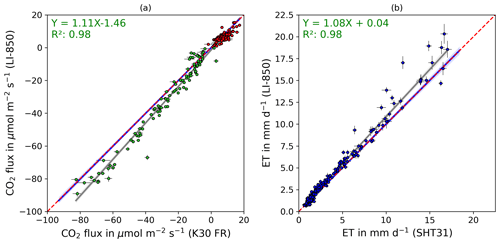
Figure 6The 1:1 agreement between (a) CO2 fluxes (Reco: red points, NEE: green points) measured with an infrared gas analyzer (LI-850, LI-COR, USA) and low-cost NDIR sensor (K30 FR) and (b) ET fluxes (blue points) measured with an infrared gas analyzer (LI-820, LI-COR Inc., Lincoln, USA) and low-cost RH sensor (SH31). The dashed red line indicates the 1:1 agreement. The gray line shows the linear regression of the measured CO2 and ET fluxes, while the gray-shaded area represents the respective confidence band of the regression line. The blue line shows the linear regression of the corrected measured CO2 and ET fluxes, while the blue-shaded area represents the respective confidence band of the regression line. Error bars indicate calculated flux errors.
3.2 Validation experiment
3.2.1 Independent mode
The validation experiment, performed continuously over 5 d using a single greenhouse coffin in independent mode, demonstrated that CO2 and ET fluxes can be measured reliably and accurately in a fully automated chamber using low-cost sensors. Out of 223 conducted automatic measurements, more than 99 % passed the flux calculation algorithm for CO2 and ET. The rate is only slightly below a 100 % pass rate of CO2 and ET flux calculation when using the LI-850 for CO2 and H2O concentration measurements. The low-cost- and LI-850-derived CO2 and ET fluxes showed mainly an identical diurnal pattern, with low fluxes during nighttime, higher ET fluxes, and a strong CO2 uptake during daytime (Fig. 5). The observed diurnal pattern clearly followed monitored environmental parameters, with higher ET fluxes and CO2 uptake with higher PAR and a higher Reco (nighttime measurements) with higher air temperatures. Figure 6 shows the 1:1 agreement and correlation between (a) calculated CO2 and (b) ET fluxes based on low-cost and LI-850 measurements of CO2 and H2O concentrations, as well as RH. The overall accuracy of both CO2 and ET fluxes derived by low-cost sensors is indicated by the high concordance correlation coefficient of 0.98 and 0.98 for CO2 and ET, respectively. These results align well with the findings of Macagga et al. (2024), who tested the accuracy and suitability of the same sensors for in situ manual closed-chamber measurements and suggested a high degree of precision (scatter of measurement) and trueness (proximity to the true value) of the low-cost sensors used. Moreover, other studies have highlighted the precision and trueness of the K30 FR and SHT31 sensors (Ali et al., 2016; Martin et al., 2017; Cannon et al., 2022). This is important, as a low trueness level can result in significant deviations from the actual value, while low precision can introduce noise/scatter into flux measurements (Werle, 2011) and might hamper the repeatability of measurement results. However, while an RMSE of 5.05 µmol m−2 s−1 and 2.84 mm d−1 and Pearson correlation coefficient of 0.99 and 0.98 proved the high precision for CO2 and ET measured within this study, respectively, this was not equally the case for the trueness. On the one hand, calculated fluxes derived from CO2 concentration and RH measurements using the low-cost sensors correlated nearly perfectly (R2: 0.98) with CO2 and ET fluxes calculated based on LI-850 measurements of CO2 and H2O concentrations (Fig. 6). On the other hand, a clear underestimation in the case of higher CO2 uptake by plants and ET flux rates for the low-cost sensors is evident in Fig. 6. This is confirmed by conducted pairwise Wilcoxon signed-rank tests, which resulted in significant differences between fluxes calculated based on measurements with the K30 FR, SHT31, and LI-850 sensors (p<0.05).
This underestimation, while potentially relevant for calculating fluxes, was not detected in other studies such as Macagga et al. (2024), which is likely due to the considerably larger observation range in this study when compared to previous studies (Ali et al., 2016; Cannon et al., 2022; Macagga et al., 2024). For example, Macagga et al. (2024) report a flux range of −17.05 to 13.74 µmol m−2 s−1 for CO2 and 1.2 to 3.0 mm d−1 for H2O, while in our study, the CO2 and ET flux amplitude was up to 6 times higher with CO2 and ET fluxes ranging from −89.06 to 15.37 µmol m−2 s−1 and 0.7 to 18.66 mm d−1, respectively. Since the underestimation was much more pronounced at higher CO2 uptake and ET, the trueness for the flux range of 20 to −30 µmol m−2 s−1 for CO2 and range of 0 to 5 mm d−1 was hence comparable with findings of Macagga et al. (2024). This highlights the importance of assessing sensor performance for a wide range of concentrations during validation experiments when aiming to use low-cost sensors and the independent mode to obtain accurate results. In the case of CO2 in particular, these experiments should consider conditions not only above ambient but also below ambient. However, the precision of the low-cost sensors used throughout the entire flux measurement range for CO2 and ET enabled us to derive and apply a correction function. After applying the correction function (CO2: , ET: ) on low-cost sensor-based CO2 and ET fluxes, cumulated CO2 and ET fluxes for the 5 d validation experiment period (e.g., 5 d flux balance), derived using low-cost and LI-850 measurements, differed by <1.5 %.
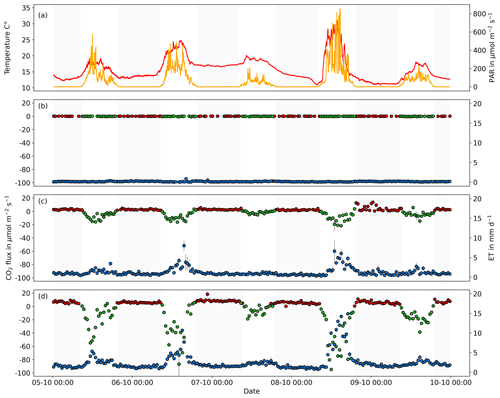
Figure 7The 5 d trial conducted for the dependent mode. Panel (a) shows the air temperature (red line) and PAR (orange line); panels (b), (c), and (d) show the diurnal cycle of CO2 (Reco: red points, NEE: green points) and ET fluxes (blue points) measured with an infrared gas analyzer (LI-850, LI-COR, USA) for three different chambers (a without plant, b maize plant, d sorghum plant). The gray-shaded areas represent nighttime. Error bars indicate calculated flux errors.
3.2.2 Dependent mode
The performed validation experiment, testing multiple greenhouse coffins with different treatments in the dependent mode, proved that by connecting multiple greenhouse coffins via a low-cost multiplexer to a single infrared gas analyzer, CO2 and ET fluxes can be fully automatically measured in a reliable and accurate manner. During the non-stop 5 d validation experiment for the dependent mode, the tested greenhouse coffins and the low-cost multiplexer used functioned reliably, with no system errors occurring. Thus, out of 237 conducted automatic measurements, more than 75 % and 99 % passed the flux calculation algorithm for CO2 and ET for the treatments with no plants (empty chamber), respectively. At the same time, 99 % passed the flux calculation algorithm for CO2 and ET for the other two treatments involving sorghum and maize, respectively. The limited number of valid CO2 fluxes for the treatments with no plants can be attributed to the absence of significant changes in CO2 fluxes during the measurement period. Consequently, many fluxes did not meet the IQR criteria set by the R module used for analysis. Moreover, the CO2 fluxes showed no significant difference from zero (pairwise Wilcoxon signed-rank test, p>0.05), which indicates the absence of cross-contamination due to the multiplexer (Fig. 7). The CO2 and ET fluxes from the greenhouse coffins containing sorghum and maize exhibited distinct diurnal patterns. Both treatments showed low fluxes during nighttime and higher ET fluxes and CO2 uptake during daytime, clearly following monitored environmental parameters with higher ET fluxes and CO2 uptake with higher PAR and a higher Reco (nighttime measurements) with higher air temperatures (Fig. 7). Notably, sorghum treatment showed higher ET fluxes and CO2 uptake compared to maize treatment. This disparity can be explained by variations in transpiration and physiological responses to environmental conditions between these two plants (Farré and Faci, 2006). The results highlight the system's ability to detect the diurnal cycles of CO2 and ET for different treatments. This feature is highly advantageous for greenhouse studies as it allows researchers to focus on specific conditions or treatments while keeping the complexity of uncontrolled conditions, such as mesocosm experiments. Zaman et al. (2021) and Bréchet et al. (2021) demonstrated the benefits of high-frequency measurements for monitoring gas fluxes from different treatments; however, their studies were conducted under field conditions using commercial multiplexers. Furthermore, the system's capacity to link multiple greenhouse coffins to one gas analyzer and carry out measurements automatically serves to cut down on the money and time that would otherwise be spent in such experiments. Finally, the choice between a standalone, fully low-cost-based mode and a cost-efficient multiplexer-connected system allows for a large degree of flexibility when planning experiments in terms of the target fluxes to be analyzed (e.g. only CO2 and H2O compared to other trace gases or stable isotope analysis, where low-cost sensors are not available).
The presented novel, low-cost, automatic soil–plant enclosure system allows for accurate and precise continuous monitoring of gaseous exchange fluxes during pot or mesocosm experiments. This was exemplarily shown during a greenhouse pot experiment for CO2 and ET fluxes of maize and sorghum. Performed system validation proved that, after calibration, CO2 and ET fluxes can be determined accurately and precisely using low-cost NDIR and RH sensors (independent mode). However, more importantly, by adding a low-cost multiplexer to the enclosure system, other GHGs can be measured, as well as by adding a gas analyzer and measuring the greenhouse coffins in series (dependent mode). Both modes allow for cost-effective, high-temporal-resolution measurements of soil–plant gas exchange across various treatments. In addition, the low-cost modular character of the system allows for multiple further enhancements:
- i.
Parallel, high-resolution measurements of various gases, such as CO2, CH4, N2O, and H2O, and stable isotopes can be conducted by combining high- and low-cost sensors, thus allowing us to determine water use efficiency, net ecosystem carbon exchange, and full GHG balances. However, to ensure proper sealing, thorough sealing tests are crucial, particularly since gases like N2O and CH4 have low mixing ratios. Additionally, careful consideration must be given to the materials used in the construction, as they may emit, e.g., volatile organic compounds that could affect the accuracy of their measurements.
- ii.
Proximal sensing of crop health and development can be integrated using available low-cost measurement systems to detect spectral crop indices such as the normalized difference vegetation index (NDVI) or ratio vegetation index (RVI).
In summary, the developed and presented system can be a valuable tool for conducting greenhouse experiments, particularly those with a high level of complexity (e.g., mesocosm experiment), allowing for holistic assessment of the dynamic responses of plants to various treatments and conditions while significantly reducing the required cost and labor.
The data and code referred to in this study are publicly accessible at https://doi.org/10.4228/ZALF-JG04-HV79 (Al Hamwi et al., 2024).
MH, WA, and MD conceptualized and developed the system and codes. WA carried out the sealing and validation experiments. WA, MH, MD, and JS wrote and prepared the paper with contributions from all co-authors. All authors reviewed and agreed to the final version of the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Special thanks go to Andrea Hoppe for her help in the greenhouse during the system's construction.
This research has been supported by the Leibniz Society Germany for their funding through the Leibniz Cooperative Excellence program (K378/2021) awarded to Joerg Schaller and Maren Dubbert.
The publication of this article was funded by the Open Access Fund of the Leibniz Association.
This paper was edited by Tyler Cyronak and reviewed by two anonymous referees.
Al Hamwi, W., Dubbert, M., Schaller, J., Lueck, M., Schmidt, M., and Hoffmann, M.: Data set and Arduino scripts for “Technical note: A low-cost, automatic soil-plant-atmosphere enclosure system to investigate CO2 and evapotranspiration flux dynamics”, Bonares [code, data set], https://doi.org/10.4228/ZALF-JG04-HV79, 2024.
Ali, A. S., Zanzinger, Z., Debose, D., and Stephens, B.: Open Source Building Science Sensors (OSBSS): A low-cost Arduino-based platform for long-term indoor environmental data collection, Build. Environ., 100, 114–126, https://doi.org/10.1016/j.buildenv.2016.02.010, 2016.
Allen, R. G., Pereira, L. S., Raes, D., and Smith, M.: Crop evapotranspiration-Guidelines for computing crop water requirements, FAO Irrigation and Drainage Paper 56, ISBN 92-5-104219-5, https://www.avwatermaster.org/filingdocs/195/70653/172618e_5xAGWAx8.pdf (last access: 25 September 2023), 1998.
Altieri, M. A., Nicholls, C. I., Henao, A., and Lana, M. A.: Agroecology and the design of climate change-resilient farming systems, Agron. Sustain. Dev., 35, 869–890, https://doi.org/10.1007/s13593-015-0285-2, 2015.
Blackstock, J. M., Covington, M. D., Perne, M., and Myre, J. M.: Monitoring Atmospheric, Soil, and Dissolved CO2 Using a Low-Cost, Arduino Monitoring Platform (CO2-LAMP): Theory, Fabrication, and Operation, Front. Earth Sci., 7, 461703, https://doi.org/10.3389/feart.2019.00313, 2019.
Bonilla-Cordova, M., Cruz-Villacorta, L., Echegaray-Cabrera, I., Ramos-Fernández, L., and Del Flores Pino, L.: Design of a Portable Analyzer to Determine the Net Exchange of CO2 in Rice Field Ecosystems, Sensors, 24, 402, https://doi.org/10.3390/s24020402, 2024.
Bréchet, L. M., Daniel, W., Stahl, C., Burban, B., Goret, J.-Y., Salomn, R. L., and Janssens, I. A.: Simultaneous tree stem and soil greenhouse gas (CO2, CH4, N2O) flux measurements: a novel design for continuous monitoring towards improving flux estimates and temporal resolution, New Phytol., 230, 2487–2500, https://doi.org/10.1111/nph.17352, 2021.
Cannon, J. B., Warren, L. T., Ohlson, G. C., Hiers, J. K., Shrestha, M., and Mitra, C.: Applications of low-cost environmental monitoring systems for fine-scale abiotic measurements in forest ecology, Agr. Forest Meteorol., 321, 108973, https://doi.org/10.1016/j.agrformet.2022.108973, 2022.
Chataut, G., Bhatta, B., Joshi, D., Subedi, K., and Kafle, K.: Greenhouse gases emission from agricultural soil: A review, J. Agr. Food Res., 11, 100533, https://doi.org/10.1016/j.jafr.2023.100533, 2023.
Dahlmann, A., Hoffmann, M., Verch, G., Schmidt, M., Sommer, M., Augustin, J., and Dubbert, M.: Benefits of a robotic chamber system for determining evapotranspiration in an erosion-affected, heterogeneous cropland, Hydrol. Earth Syst. Sci., 27, 3851–3873, https://doi.org/10.5194/hess-27-3851-2023, 2023.
D'Ausilio, A.: Arduino: a low-cost multipurpose lab equipment, Behav. Res., 44, 305–313, https://doi.org/10.3758/s13428-011-0163-z, 2012.
Dubbert, M., Cuntz, M., Piayda, A., and Werner, C.: Oxygen isotope signatures of transpired water vapor: the role of isotopic non-steady-state transpiration under natural conditions, New Phytol., 203, 1242–1252, https://doi.org/10.1111/nph.12878, 2014.
Farré, I. and Faci, J. M.: Comparative response of maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) to deficit irrigation in a Mediterranean environment, Agr. Water Manage., 83, 135–143, https://doi.org/10.1016/j.agwat.2005.11.001, 2006.
Fisher, D. K. and Gould, P. J.: Open-Source Hardware Is a Low-Cost Alternative for Scientific Instrumentation and Research, Modern Instrumentation, 1, 8–20, https://doi.org/10.4236/mi.2012.12002, 2012.
Forbes, E., Benenati, V., Frey, S., Hirsch, M., Koech, G., Lewin, G., Mantas, J. N., and Caylor, K.: Fluxbots: A method for building, deploying, collecting and analyzing data from an array of inexpensive, autonomous soil carbon flux chambers, J. Geophys. Res.-Biogeo., 128, e2023JG007451, https://doi.org/10.1029/2023JG007451, 2023.
Hamel, P., Mchugh, I., Coutts, A., Daly, E., Beringer, J., and Fletcher, T. D.: Automated chamber system to measure field evapotranspiration rates, J. Hydrol. Eng., 20, 04014037, https://doi.org/10.1061/(ASCE)HE.1943-5584.0001006, 2015.
Hoffmann, M., Jurisch, N., Borraz, E. A., Hagemann, U., Drösler, M., Sommer, M., and Augustin, J.: Automated modeling of ecosystem CO2 fluxes based on periodic closed chamber measurements: A standardized conceptual and practical approach, Agr. Forest Meteorol., 200, 30–45, https://doi.org/10.1016/j.agrformet.2014.09.005, 2015.
Hoffmann, M., Pehle, N., Huth, V., Jurisch, N., Sommer, M., and Augustin, J.: A simple method to assess the impact of sealing, headspace mixing and pressure vent on airtightness of manually closed chambers, J. Plant Nutr. Soil Sc., 181, 36–40, https://doi.org/10.1016/j.agrformet.2014.09.005, 2018.
Hupp, J.: The importance of water vapor measurements and corrections. LI-COR Biosciences Inc. Application Note, 129, 8, https://www.licor.com/env/support/TechTips/irg4110-h2o-measurement.html (last access: 1 May 2024), 2011.
Fisher, J. B., Melton, F., Middleton, E., Hain, C., Anderson, M., Allen, R., McCabe, M. F., Hook, S., Baldocchi, D., Townsend, P. A., Kilic, A., Tu, K., Miralles, D. D., Perret, J., Lagouarde, J.-P., Waliser, D., Purdy, A. J., French, A., Schimel, D., Famiglietti, J. S., Stephens, G., and Wood, E. F.: The future of evapotranspiration: Global requirements for ecosystem functioning, carbon and climate feedbacks, agricultural management, and water resources, Water Resour. Res., 53, 2618–2626, https://doi.org/10.1002/2016WR020175, 2017.
Lal, R.: Soil carbon sequestration to mitigate climate change, Geoderma, 123, 1–22, https://doi.org/10.1016/j.geoderma.2004.01.032, 2004.
Leiber-Sauheitl, K., Fuß, R., Voigt, C., and Freibauer, A.: High CO2 fluxes from grassland on histic Gleysol along soil carbon and drainage gradients, Biogeosciences, 11, 749–761, https://doi.org/10.5194/bg-11-749-2014, 2014.
Macagga, R., Asante, M., Sossa, G., Antonijević, D., Dubbert, M., and Hoffmann, M.: Validation and field application of a low-cost device to measure CO2 and evapotranspiration (ET) fluxes, Atmos. Meas. Tech., 17, 1317–1332, https://doi.org/10.5194/amt-17-1317-2024, 2024.
Martin, C. R., Zeng, N., Karion, A., Dickerson, R. R., Ren, X., Turpie, B. N., and Weber, K. J.: Evaluation and environmental correction of ambient CO2 measurements from a low-cost NDIR sensor, Atmos. Meas. Tech., 10, 2383–2395, https://doi.org/10.5194/amt-10-2383-2017, 2017.
NOAA: Trends in Atmospheric Carbon Dioxide (CO2), https://www.noaa.gov/, last access: 1 May 2024.
Olfs, H.-W., Westerschulte, M., Ruoss, N., Federolf, C.-P., Zurheide, T., Vergara Hernandez, M. E., Neddermann, N., Trautz, D., Pralle, H., Fuß, R., and Well, R.: A new chamber design for measuring nitrous oxide emissions in maize crops, J. Plant Nutr. Soil Sc., 181, 69–77, https://doi.org/10.1002/jpln.201700008, 2018.
Powlson, D. S., Stirling, C. M., Thierfelder, C., White, R. P., and Jat, M. L.: Does conservation agriculture deliver climate change mitigation through soil carbon sequestration in tropical agro-ecosystems, Agr. Ecosyst. Environ., 220, 164–174, https://doi.org/10.1016/j.agee.2016.01.005, 2016.
Riebesell, U., Czerny, J., von Bröckel, K., Boxhammer, T., Büdenbender, J., Deckelnick, M., Fischer, M., Hoffmann, D., Krug, S. A., Lentz, U., Ludwig, A., Muche, R., and Schulz, K. G.: Technical Note: A mobile sea-going mesocosm system – new opportunities for ocean change research, Biogeosciences, 10, 1835–1847, https://doi.org/10.5194/bg-10-1835-2013, 2013.
Riederer, M., Serafimovich, A., and Foken, T.: Net ecosystem CO2 exchange measurements by the closed chamber method and the eddy covariance technique and their dependence on atmospheric conditions, Atmos. Meas. Tech., 7, 1057–1064, https://doi.org/10.5194/amt-7-1057-2014, 2014.
Savage, K. E. and Davidson, E. A.: A comparison of manual and automated systems for soil CO2 flux measurements: Trade-offs between spatial and temporal resolution, J. Exp. Bot., 54, 891–899, https://doi.org/10.1093/jxb/erg121, 2003.
Smith, P., Lanigan, G., Kutsch, W. L., Buchmann, N., Eugster, W., Aubinet, M., Ceschia, E., Béziat, P., Yeluripati, J. B., Osborne, B., Moors, E. J., Brut, A., Wattenbach, M., Saunders, M., and Jones, M.: Measurements necessary for assessing the net ecosystem carbon budget of croplands, Agr. Ecosyst. Environ., 139, 302–315, https://doi.org/10.1016/j.agee.2010.04.004, 2010.
Stewart, R. I. A., Dossena, M., Bohan, D. A., Jeppesen, E., Kordas, R. L., Ledger, M. E., Meerhoff, M., Moss, B., Mulder, C., Shurin, J. B., Suttle, B., Thompson, R., Trimmer, M., and Woodward, G.: Mesocosm experiments as a tool for ecological climate-change research, Adv. Ecol. Res., 48, 71–181, https://doi.org/10.1016/B978-0-12-417199-2.00002-1, 2013.
Sun, X. and May, A.: A comparison of field-based and lab-based experiments to evaluate user experience of personalised mobile devices, Advances in Human-Computer Interaction, 2013, 619767, https://doi.org/10.1155/2013/619767, 2013.
Tubiello, F. N., Salvatore, M., Rossi, S., Ferrara, A., Fitton, N., and Smith, P.: The FAOSTAT database of greenhouse gas emissions from agriculture, Environ. Res. Lett., 8, 015009, https://doi.org/10.1088/1748-9326/8/1/015009, 2013.
Ummenhofer, C. C. and Meehl, G. A.: Extreme weather and climate events with ecological relevance: a review, Philos. T. Roy. Soc. B, 372, 20160135, https://doi.org/10.1098/rstb.2016.0135, 2017.
Webb, E. K., Pearman, G. I., and Leuning, R.: Correction of flux measurements for density effects due to heat and water vapour transfer, Q. J. Roy. Meteor. Soc., 106, 85–100, https://doi.org/10.1002/qj.49710644707, 1980.
Werle, P.: Accuracy and precision of laser spectrometers for trace gas sensing in the presence of optical fringes and atmospheric turbulence, Appl. Phys. B, 102, 313–329, https://doi.org/10.1007/s00340-010-4165-9, 2011.
Zaman, M., Heng, L., and Müller, C.: Measuring emission of agricultural greenhouse gases and developing mitigation options using nuclear and related techniques: Applications of nuclear techniques for GHGs, p. 337, Springer Nature, https://doi.org/10.1007/978-3-030-55396-8, 2021.
Zhang, Y., Li, C., Zhou, X., and Moore III, B.: A simulation model linking crop growth and soil biogeochemistry for sustainable agriculture, Ecol. Model., 151, 75–108, https://doi.org/10.1016/S0304-3800(01)00527-0, 2002.