the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Effects of pH/pCO2 fluctuations on photosynthesis and fatty acid composition of two marine diatoms, with reference to consequences of coastal acidification
Yu Shang
Jingmin Qiu
Yuxi Weng
Xin Wang
Di Zhang
Yuwei Zhou
Juntian Xu
Futian Li
Coastal waters are impacted by a range of natural and anthropogenic factors, which superimpose on effects of increasing atmospheric CO2, resulting in dynamically changing seawater carbonate chemistry. Research on the influences of dynamic pH/pCO2 on marine ecosystems is still in its infancy, although effects of ocean acidification have been extensively studied. In the present study, we manipulated the culturing pH to investigate physiological performance and fatty acid (FA) composition of two coastal diatoms, Skeletonema costatum and Thalassiosira weissflogii, in both steady and fluctuating pH regimes. Generally, seawater acidification and pH variability showed neutral or positive effects on the specific growth rate, chlorophyll a, and biogenic silica contents of the two species. Decreased pH inhibited the net photosynthetic rate by 27 % and enhanced the mitochondrial respiration rate of S. costatum by 36 % in the steady pH regime, while these rates were unaltered by decreased pH in the fluctuating regime. Acidification conditions led to lower saturated FA and higher polyunsaturated FA proportions in both species, regardless of steady or fluctuating regimes. Our results indicate that coastal acidification could affect primary production in a different way from ocean acidification. Together with the altered nutritional quality of prey for higher trophic levels, coastal acidification might have far-reaching consequences for marine ecosystem functioning.
- Article
(2784 KB) - Full-text XML
- BibTeX
- EndNote
Carbonate chemistry of coastal waters is impacted by biological metabolism, tidal cycles, upwelling, wind, and terrestrial nutrient inputs, in addition to the dissolution of atmospheric CO2 (Carstensen and Duarte, 2019; Duarte et al., 2013; Kapsenberg and Cyronak, 2019). This results in dynamic changes in carbonate chemistry parameters such as pH and pCO2 (García-Ibáñez et al., 2024). The amplitude of pH changes in coastal regions could be greater than 1 unit within 24 h (Duarte et al., 2013), which is larger than the average expected change of 0.3 units by the end of this century (Gattuso et al., 2015). Thus, short-term pH fluctuations will superimpose on the downward trend of pH in coastal waters in the context of ocean acidification. These fluctuations may have potential impacts on marine organisms at different trophic levels, as suggested by limited research (Li et al., 2021; Raven et al., 2020; Schaum et al., 2016; Wahl et al., 2018).
Diatoms are usually one of the dominant phytoplankton taxa in coastal waters, where they contribute to a large proportion of primary production (Tréguer et al., 2018). Coastal diatoms are characterized by a high tolerance to dynamic changes in abiotic factors (Key et al., 2010; Li et al., 2016; Strzepek and Harrison, 2004). This tolerance is supported by their special cell structure or fast acclimation rate and broad ecological niche (Armbrust, 2009). The pH tolerance of diatoms varies among species, with some capable of adapting to a wide range of pH levels and exhibiting positive growth (Hansen, 2002; Hinga, 2002). Our previous study found that coastal diatom species may benefit from or be tolerant to diurnal pH fluctuation (Li et al., 2016; Shang et al., 2024a). This phenomenon is suggested to be related to the larger pH differences experienced by larger coastal phytoplankton cells between the diffusion boundary layer (DBL) surrounding the cells and the bulk seawater (Flynn et al., 2012). In addition, coastal waters are characterized by large-amplitude and high-frequency fluctuations in seawater carbonate chemistry parameters, particularly pH and pCO2 (Duarte et al., 2013). Thus, larger phytoplankton dwelling in coastal waters with turbulent conditions should show a high tolerance to changes in carbonate chemistry.
Effects of ocean acidification and underlying mechanisms have been extensively studied at different trophic levels on both short- and long-term timescales (Doney et al., 2020; Hancock et al., 2020), yet research on the effects of dynamic pH is still in its infancy. Ocean acidification might have various effects on diatoms based on simulated laboratory and field studies, and other environmental drivers could mediate the effects (Gao and Campbell, 2014). These studies are important for revealing the comprehensive consequences of ocean acidification. However, the impacts of fluctuating carbonate chemistry might differ from those of decreased pH and increased CO2 in steady regimes.
Limited studies have focused on how marine phytoplankton perform under fluctuating pH/pCO2 conditions, leaving the effects of coastal acidification poorly understood. This knowledge gap impedes accurate predictions of acidification impacts in coastal regions. To capture more details in the fluctuating pH regime, we manipulated the culturing pH/pCO2 in a stepwise way by adjusting pCO2 aerated into cultures in the present study, and each step lasted for 24 h. This enabled us to investigate cell performance at each pH level in the fluctuating regime, besides the overall responses. We hypothesize that coastal diatoms could tolerate environmental pH fluctuation, given the dynamic carbonate chemistry in coastal waters and the unignorable pH difference between the DBL of cells and bulk seawater.
2.1 Culture conditions and experiment setup
Two typical diatoms, Skeletonema costatum (originally isolated from coastal waters of Gaogong Island, Jiangsu Province, China) and Thalassiosira weissflogii (CCMA 102; originally isolated from Daya Bay, Guangdong Province, China), were cultured in polycarbonate bottles with 500 mL sterile artificial seawater (Sunda et al., 2005). Nutrients were added according to the F/2 recipe (Guillard and Ryther, 1962) to ensure cells were not limited by nutrients. Triplicate cultures were set for each treatment, and they were cultured in one incubator with a light intensity of 150 µmol photons m−2 s−1 and a 12 : 12 h light and dark cycle. The culturing temperature was set at 20 °C, which is in the optimal temperature range for the growth of the two species. Cultures were diluted every 3 or 4 d to make sure cells were in the exponential phase, with maximum cell densities below 160 000 and 10 000 cells mL−1 for S. costatum and T. weissflogii, respectively.
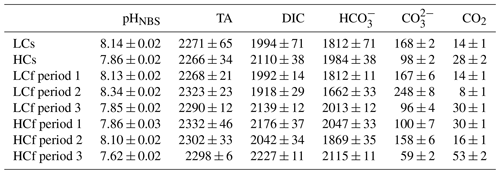
To compare the effects of pH level and variability in two diatoms, four pH/pCO2 treatments were set: (1) steady ambient pH/pCO2 levels (LCs), (2) steady future pH/pCO2 levels (HCs), (3) fluctuating ambient pH/pCO2 levels with similar mean values of pH/pCO2 to those in LCs treatment (LCf), and (4) fluctuating future pH/pCO2 levels with similar mean values of pH/pCO2 to those in HCs treatment (HCf) (see Fig. 1). The pHNBS values were measured using a pH meter (FE20, Mettler Toledo) with the NBS buffer system and three-point calibration. The CO2 partial pressure of the aerating air was measured with a CO2 detector (GM70, Vaisala Oyj). To verify the seawater carbonate chemistry parameters, total alkalinity (TA) was also determined. For TA measurement, samples were filtered through cellulose acetate membranes (0.45 µm, Xinya) and determined using the pH method after Anderson and Robinson (1946). The remaining carbonate chemistry parameters were calculated using the CO2SYS program, based on pH and TA (see Table 1), with the carbonic acid dissociation constants from Mehrbach et al. (1973), refitted by Dickson and Millero (1987), and those for sulfuric acid from Dickson (1990). LCs and HCs cultures were aerated with ambient air and CO2-enriched air, respectively. The CO2-enriched air was achieved by mixing air and CO2 with a CO2 enricher (CE100, Ruihua). The target pCO2 level (1000 µatm) for HCs cultures was set according to the projected range in the sixth assessment report of the Intergovernmental Panel on Climate Change (Lee et al., 2021). For fluctuating regimes, the pCO2 of aerating air was adjusted every 24 h in a stepwise way. The pCO2 was set as follows: 400–280–400–1000–400 µatm for LCf treatments and 1000–400–1000–1750–1000 µatm for HCf treatments, and each step lasted for 24 h. This resulted in a pH ranging from 7.85 to 8.35 and from 7.6 to 8.1 under LCf and HCf conditions, respectively (Fig. 1). The amplitude and frequency of fluctuations were set based on reported habitat conditions and experimental feasibility. The aerating rate was controlled at 100 mL min−1 by a gas flowmeter, and filter units (SLGPR33RB, Millipore) were used to sterilize the aerating air. Cultures were acclimated to four treatments for at least 10 d (i.e. two pH variation cycles for fluctuating regime) before the following parameters were measured.
Specific growth rates in fluctuating regimes were measured four times, covering each pH period at 24 h intervals. For comparison, the same sampling frequency was applied to cells in steady regimes. Net photosynthetic and mitochondrial respiration rates were determined during each pH period in fluctuating regimes, and photosynthetic rates in steady regimes were measured twice on different days to monitor potential variations over time. Other parameters were assessed when pH levels in fluctuating regimes matched those of the corresponding steady regimes. Fatty acid compositions were analyzed at the end of the experiment due to the high biomass requirement.
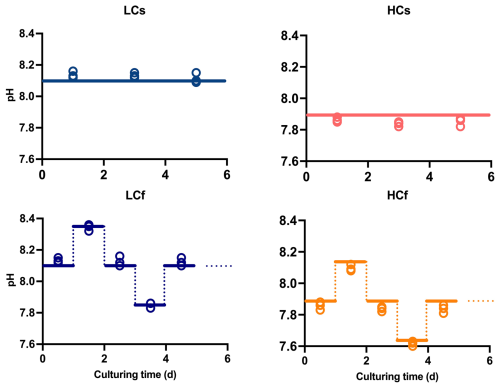
Figure 1Target (lines) and measured culturing pH (open circles) of steady ambient pH/pCO2 (LCs), fluctuating ambient pH/pCO2 (LCf), steady future pH/pCO2 (HCs), and fluctuating future pH/pCO2 (HCf) treatments.
2.2 Specific growth rate
Subsamples were collected and fixed with Lugol's solution for cell density measurement. Then samples were counted with a plankton counting chamber (DSJ-01, Xundeng) under an optical microscope (DM500, Leica). Specific growth rate was calculated according to the following equation: µ = ln (N2/N1)/(T2-T1), where N1 and N2 represent cell densities at T1 and T2, respectively.
2.3 Chlorophyll a and biogenic silica contents
Subsamples were filtered onto GF/F filters (Whatman) for subsequent chlorophyll a extraction in 100 % methanol at 4 °C. Then they were centrifuged at 5000×g for 10 min before the absorption of supernatant was determined at 632, 665, and 750 nm with a spectrophotometer (Ultrospect 3300 pro, Amersham Bioscience). Biogenic silica (BSi) samples were collected onto polycarbonate membranes (ATTP02500, Millipore). Membranes with cells were digested in NaOH at 95 °C for 45 min, and then HCl was added to terminate extraction. Then ammonium molybdate and mixture of metol-sulfite, oxalic acid, sulfuric acid, and Milli-Q water were added and let the color develop for 2 h. Then the absorption of the samples was determined at 810 nm to measure the BSi concentration (Brzezinski and Nelson, 1995). Cell concentration, filtration volume, and dilution factor during extraction and measurement were taken into account for calculating chlorophyll a and BSi contents.
2.4 Quantum yield of PSII
The AquaPen Chlorophyll Fluorometer (AP-C100, Photon Systems Instruments) was used to measure effective quantum yield of PSII (ΦPSII). Subsamples were maintained under the same light and temperature conditions as the culturing environment for at least 15 min prior to measurements. For fluorometer settings, blue light was chosen and the saturating pulse was set at 100 %. It was calculated as , where and Ft represent the maximum chlorophyll fluorescence of light-adapted samples and the steady-state chlorophyll fluorescence, respectively.
2.5 Net photosynthetic and mitochondrial respiration rates
Subsamples were gently filtered (<0.02 MPa) onto cellulose acetate membranes and then re-suspended into 20 mmol L−1 Tris-buffered medium. The pH values of the Tris-buffered media were pre-adjusted with HCl and NaOH to the corresponding culturing values. Then re-suspended samples were injected into the chamber of a Clark-type oxygen electrode (Oxygraph+, Hansatech), and the changes in the oxygen level were recorded for at least 10 min for each sample. Light intensity was set as 150 µmol photons m−2 s−1 for net photosynthetic rate measurement, and a halogen lamp (QVF135, Philips) was used as the light source. For mitochondrial respiration rate measurement, the chamber was covered by aluminum foil to achieve dark conditions. The water jacket temperature of the chamber was controlled at 20 °C with a thermostatic water bath (DHX-2005, Xianou). Net photosynthetic and respiration rates were measured at each culturing pH level in fluctuating regimes. Cell density after concentrating was counted as mentioned above to calculate the net photosynthetic and mitochondrial respiration rate per cell.
2.6 Fatty acid composition
Cells were collected by gentle filtration (<0.02 MPa) and centrifugation (3500×g, 5 min), then samples were dried (80 °C, 36 h) and pulverized to fine powders. Then fatty acids (FAs) in samples were converted into fatty acid methyl esters (FAMEs) by chloroform : methanol (), and their compositions were analyzed with a Shimadzu GC-2010 gas chromatography–flame ionization detector equipped with a fused silica column (100 m ×0.25 mm ×0.2 µm film thickness, Agilent CP-Sil 88). Standards were used to identify FAMEs by comparing retention times, and proportions of FAs were quantified by the percentage of each peak area to the total area.
2.7 Statistical analysis
All data are reported as the mean ± standard deviation (SD). Shapiro–Wilk and Levene tests were used to test the normality and equal variance of data, respectively. A one-way analysis of variance (ANOVA) and a post-hoc Tukey–Kramer test were used to analyze the differences among four treatments.
3.1 Specific growth rate, chlorophyll a, and BSi contents
There were no significant effects of pH treatments, including both mean levels and variability, on the specific growth rates of S. costatum and T. weissflogii (p=0.103 and 0.661, respectively), although growth rates varied across sampling periods (Fig. 2). Similarly, pH treatments did not significantly affect the chlorophyll a content of S. costatum, despite lower average values under HC conditions (Fig. 3a). This lack of significance was attributed to relatively high variance in the HC treatments. For T. weissflogii, no differences were observed between steady and fluctuating regimes under either LC or HC conditions. HCs cells had 44 % more chlorophyll a content compared to LCs cells (p=0.002), but LCf and HCf cells showed similar content (Fig. 3c; p=0.999). In terms of BSi content, both species had similar content regardless of treatments (Fig. 3b and d; p=0.312 and 0.600 for S. costatum and T. weissflogii, respectively).
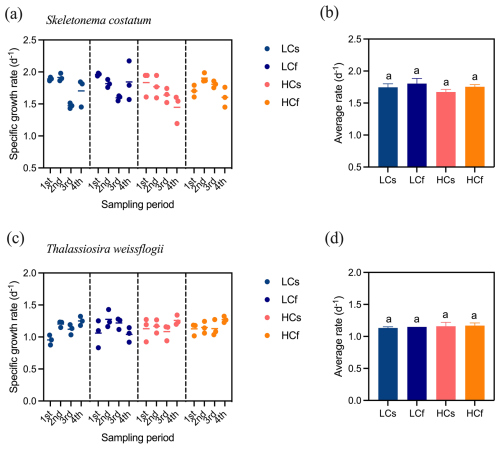
Figure 2Specific growth rate of S. costatum and T. weissflogii cells grown under steady ambient pH/pCO2 (LCs), fluctuating ambient pH/pCO2 (LCf), steady future pH/pCO2 (HCs), and fluctuating future pH/pCO2 (HCf). Scatter plots show the specific growth rate of all replicates in each sampling period, and the short line indicates the mean value of three replicates. Four sampling periods include all pH levels in the fluctuating regime, and average rates are calculated from the means of triplicate cultures across four sampling periods. The different letters indicate significant (p<0.05) differences among treatments.
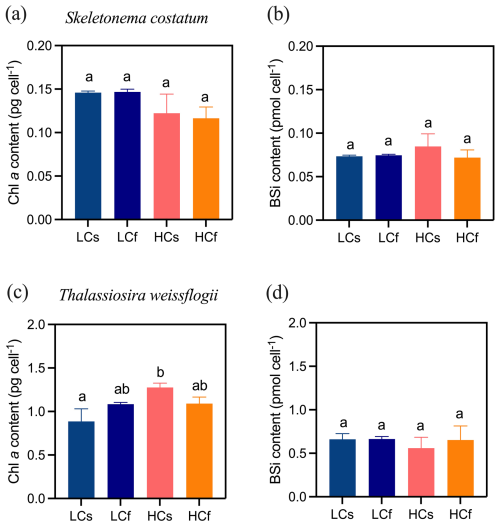
Figure 3Chlorophyll a and biogenic silica contents of S. costatum and T. weissflogii cells grown under steady ambient pH/pCO2 (LCs), fluctuating ambient pH/pCO2 (LCf), steady future pH/pCO2 (HCs), and fluctuating future pH/pCO2 (HCf). Values are the means ± SD of triplicate cultures. The different letters indicate significant (p<0.05) differences among treatments.
3.2 Quantum yield of PSII
Lower pH enhanced effective quantum yield of PSII of S. costatum by 9 % and 13 % compared to ambient pH conditions for steady and fluctuating regimes, respectively (Fig. 4a; p=0.001 for steady regimes and <0.001 for fluctuating regimes). No difference between steady and fluctuating regimes was found under LC or HC conditions. Similarly, no difference between steady and fluctuating regimes was found for T. weissflogii, and effects of decreased pH were only observed in the fluctuating regime, with 10 % higher effective quantum yield of PSII in HCf cells than LCf ones (Fig. 4b; p=0.032).
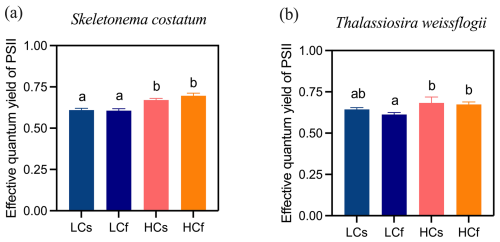
Figure 4Effective quantum yield of PSII of S. costatum and T. weissflogii cells grown under steady ambient pH/pCO2 (LCs), fluctuating ambient pH/pCO2 (LCf), steady future pH/pCO2 (HCs), and fluctuating future pH/pCO2 (HCf). Values are the means ± SD of triplicate cultures. The different letters indicate significant (p<0.05) differences among treatments.
3.3 Net photosynthetic and mitochondrial respiration rates
In the steady regime, seawater acidification inhibited the net photosynthetic rate of S. costatum by 27 % (p=0.005), while no effects of decreased pH on average net photosynthetic rates in the fluctuating regime were observed (Fig. 5a). Although photosynthetic rate changed with pH in the fluctuating regime (Fig. 5b), the average rates of cells cultured under LCf and HCf conditions were similar to the rates under corresponding steady conditions (Fig. 5a). For T. weissflogii, its photosynthetic rate was generally insensitive to changes in mean level of pH or pH variability (Fig. 5e; p=0.345), and no general relationship between net photosynthetic rate and pH was observed in fluctuating regimes (Fig. 5f).
Seawater acidification enhanced the mitochondrial respiration rate of S. costatum by 36 % in the steady regime (p=0.025), while there was no difference in the rate between LCf and HCf conditions (Fig. 5c). S. costatum cells cultured under LCf conditions showed an increased mitochondrial respiration rate with increasing pH levels in the regime, while the rates of HCf cells were similar among three pH levels (Fig. 5d). For T. weissflogii, its mitochondrial respiration rate varied at different pH levels in the fluctuating regime (Fig. 5h), but the average rates of LCf and HCf conditions were similar to the rates under corresponding steady conditions, and no effects of seawater acidification were found (Fig. 5g, p=0.982).
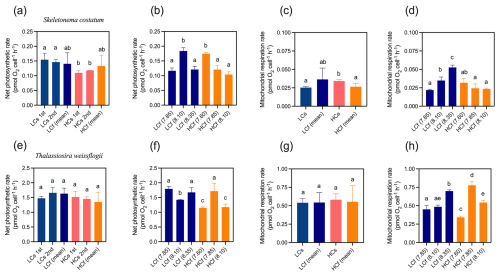
Figure 5Net photosynthetic rate and mitochondrial respiration rate of S. costatum and T. weissflogii cells grown under steady ambient pH/pCO2 (LCs), fluctuating ambient pH/pCO2 (LCf), steady future pH/pCO2 (HCs), and fluctuating future pH/pCO2 (HCf). Values are the means ± SD of triplicate cultures. The different letters indicate significant (p<0.05) differences among treatments. In the steady regime, the net photosynthetic rate was measured twice on different days to monitor potential variations over time; in the fluctuating regime, the net photosynthetic rate and mitochondrial respiration rate at each pH level were measured.
3.4 Fatty acid composition
The proportion of saturated FAs (SFAs) of S. costatum slightly decreased at lower pH, while the proportions of monounsaturated FAs (MUFAs) and polyunsaturated FAs (PUFAs) increased slightly (Fig. 6a; p=0.001, 0.015, <0.001 for SFAs, MUFAs, and PUFAs, respectively). Effects of pH fluctuation were only observed for PUFAs of S. costatum at ambient pH levels, with a 2 % lower proportion under LCf conditions (p=0.001). FA compositions of T. weissflogii were markedly altered by seawater acidification rather than pH fluctuation, with 13 % lower SFAs and 2-fold increase in PUFAs compared with ambient pH level (Fig. 6b; p=0.001 and <0.001 for SFAs and PUFAs, respectively). The decrease in SFA proportion of T. weissflogii was contributed by all main SFAs except C17:0, which showed a higher proportion under seawater acidification conditions (Fig. 7). Enhanced PUFAs mainly resulted from higher eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) under seawater acidification conditions regardless of steady or fluctuating regimes (p<0.001 for EPA and DHA).
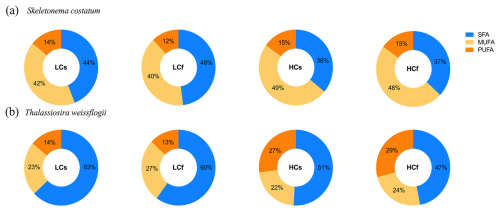
Figure 6Saturated FA (SFA), monounsaturated FA (MUFA), and polyunsaturated FA (PUFA) proportions of S. costatum and T. weissflogii cells grown under steady ambient pH/pCO2 (LCs), fluctuating ambient pH/pCO2 (LCf), steady future pH/pCO2 (HCs), and fluctuating future pH/pCO2 (HCf). Values are the means of triplicate cultures.
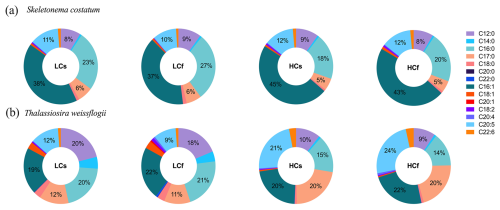
Figure 7FA composition of S. costatum and T. weissflogii cells grown under steady ambient pH/pCO2 (LCs), fluctuating ambient pH/pCO2 (LCf), steady future pH/pCO2 (HCs), and fluctuating future pH/pCO2 (HCf). Values are the means of triplicate cultures.
4.1 Effects of pH changes on the physiological performance of two diatoms
Previous studies have shown that T. weissflogii and S. costatum exhibit high tolerance to changes in pH levels. T. weissflogii has been found to tolerate decreased pH/increased pCO2, while seawater alkalization significantly inhibited growth when the medium pH exceeded 8.44 (Li et al., 2019). For S. costatum, growth remained nearly constant within a pH range of 6.5 to 8.5 (Taraldsvik and Myklestad, 2000), and the effective quantum yield of PSII in cells acclimated to the seawater acidification condition was unresponsive to pH changes between 7.6 and 8.2 (Zheng et al., 2015). In the present study, growth remained unchanged in both diatoms, although chlorophyll content, net photosynthetic rate, and the quantum yield of PSII were either enhanced or inhibited, depending on the species. Growth, as a proxy of the overall cellular response, is influenced by a range of metabolic processes and may remain insensitive to pH variations, despite changes in photosynthetic parameters.
The DBL exists at the interface of cells, in which the microenvironment is different from the bulk seawater. For example, pH in DBL could increase substantially when photosynthesis happens inside the cell, and larger cells could experience more significant changes in pH within DBL (Chrachri et al., 2018). Thus, just like the condition in coastal waters, stable seawater carbonate chemistry is not realistic in the DBL of large cells. For this reason, it seems reasonable to expect neutral or positive effects of pH fluctuation on coastal phytoplankton. Our previous comparative study showed that diurnal pH fluctuations depressed the growth and photosynthesis of the oceanic diatom Thalassiosira oceanica under either ambient or low pH conditions, whilst the coastal diatom T. weissflogii was insensitive to pH fluctuations, and the enhanced production rate of particulate organic carbon was even observed in fluctuating regimes (Li et al., 2016). In that study, seawater carbonate chemistry was manipulated to achieve high-frequency pH change, with a full pH fluctuation period of 24 h (pH increased during the light period and decreased during the dark period, with the CO2 partial pressure of the aerating gas adjusted every 12 h). This approach provided a holistic view of phytoplankton response to fluctuating pH but lacked specific analysis of the response to each pH period within the fluctuating regime. The high-pH/low-CO2 period might have negative effects if the treatment duration extends beyond several hours. In the present study, the fluctuation period was set to 5 d, and we found that the average net photosynthetic and respiration rates of T. weissflogii were unaltered by treatments (Fig. 5e), although these rates varied at different pH levels in fluctuating regimes (Fig. 5f). For S. costatum, no differences were observed in average photosynthetic rates between steady and fluctuating regimes under ambient and low pH levels. However, cells cultured under steady acidification conditions exhibited a 27 % reduction in net photosynthetic rate and a 36 % increase in respiration rate compared to cells grown under steady ambient pH.
The two species in this study exhibited different photosynthetic responses to seawater acidification, potentially due to their distinct inorganic carbon utilization strategies. The biochemical CO2-concentrating mechanism (i.e. unicellular C4 pathway) has been suggested to play a key role in the plasticity of inorganic carbon utilization in T. weissflogii (Reinfelder et al., 2000). This mechanism is crucial for maintaining relatively high photosynthetic rates in responses to changes in pH. Additionally, differences in membrane permeability and fluidity may contribute to these species' varied responses. FAs are incorporated into phospholipids, which are major structural components of cell membranes, and FA composition can influence membrane characteristics. Theoretically, the cell membranes of T. weissflogii are less fluid and less permeable to CO2 (Maulucci et al., 2016), due to higher proportions of SFAs (Fig. 6). This helps cells cope with decreased intracellular pH and maintain homeostasis under seawater acidification conditions (Rossoll et al., 2012).
Although there was a 10-fold difference in net photosynthetic rates between the two species, their chlorophyll a-normalized photosynthetic rates were comparable. This is because the larger T. weissflogii contains 10 times more chlorophyll a than S. costatum. Seawater acidification increased chlorophyll a content in T. weissflogii, while the content tended to decrease in S. costatum. The “pigment economy” hypothesis, proposed in previous studies, explains the reduction in pigment content under seawater acidification conditions by suggesting that cells eliminate chlorophyll molecules that are inefficient for light capture during photosynthesis (Gordillo et al., 1998, 2015). However, this hypothesis does not apply to T. weissflogii, indicating that different species employ distinct strategies for pigment synthesis in response to acidification. Both species in this study maintained stable BSi content regardless of pH changes. In contrast, the content and production rate of BSi in the oceanic diatom T. oceanica decreased under seawater acidification and fluctuating pH conditions (Li et al., 2016). Research on the effects of fluctuating pH on oceanic phytoplankton is limited, and further studies are needed to determine whether oceanic species are generally more susceptible to fluctuating pH than coastal species.
Based on the results of the present and previous studies, the effects of seawater acidification may differ depending on whether pH fluctuations are considered in experiments (Table 2). While the trends between steady and fluctuating seawater acidification regimes are similar, the variation amplitudes differ. If the steady regime was used to simulate coastal acidification, inhibited primary production could be observed in some species. However, no such effects were observed when coastal acidification was simulated under more realistic fluctuating pH conditions. In the complex and turbulent environment of coastal waters, dynamic pH fluctuations may have more diverse influences on phytoplankton than steady pH. Therefore, it is important to consider pH variation for more accurate predictions regarding the consequences of acidification in coastal waters.
4.2 Fatty acid composition was altered by mean pH level
Omega-3 long-chain essential fatty acids are integral to key functions in aquatic and terrestrial organisms. These FAs are directly or indirectly contributed by phytoplankton in the marine ecosystem (Hixson and Arts, 2016). Among them, DHA and EPA are well known due to their benefits in enhancing the nutritional quality of marine primary consumers (Kainz et al., 2004), especially herbivorous copepods and rotifers, which serve as prey for secondary consumers such as fishes and crustaceans. The only way of obtaining essential FAs for marine animals is through their diet, as they cannot synthesize them de novo (Brett and Müller-Navarra, 1997). EPA plays a critical role in the growth, development, and reproduction of marine consumers, and diatoms are the main producers of EPA in the marine ecosystem (Budge et al., 2014). EPA is the major PUFA in both species tested here, with its proportion accounting for more than 10 % of the total FAs under all conditions. DHA is the second most abundant PUFA in two diatoms, although its proportion is much lower than EPA. The proportions of EPA and DHA observed in this study fall within the ranges reported for marine microalgae (Boelen et al., 2013). However, it is important to note that these proportions can vary significantly both between species and within a single species under different conditions. For example, the growth phase of cells can have a substantial impact on fatty acid composition (Schwenk et al., 2013). Therefore, it is crucial to specify the growth phase of cells when comparing results with other studies. In the present study, cells were maintained in the exponential phase under conditions with sufficient nutrients. These conditions are not optimal for lipid accumulation, suggesting that there is potential for further enhancement of essential FAs.
Species- or even strain-specific responses of FAs to ocean acidification have been documented in previous studies. In terms of FA composition, different phytoplankton strains of one species appeared to respond to increased CO2 in a varied way. For instance, the EPA and DHA fractions of total FAs in a highly CO2-tolerant strain of T. weissflogii were lower when cells were cultured under 5 %, 10 %, and 20 % CO2, compared with control air conditions (Ishida et al., 2000), while EPA and DHA or total PUFA proportions of T. weissflogii (CCMP2599) were not altered when pCO2 levels increased from 320 to 690 and 2900 ppm (King et al., 2015). These studies indicate that changes in FAs in response to seawater acidification are species- or strain-dependent and are influenced by the culture conditions employed in studies. Although the mechanisms underlying these changes remain unclear, they are believed to be related to the regulation of lipid metabolites (Jin et al., 2021) and processes closely associated with lipid metabolisms, such as carbon concentration mechanisms (Abreu et al., 2020) and the regulation of intracellular pH homeostasis (Rossoll et al., 2012). It has been proposed that pH may act as a regulatory signal for the formation of cell membranes by controlling the production of the synthesizing enzymes of FAs (Young et al., 2010).
In the present study, PUFAs and MUFAs of S. costatum were slightly enhanced by decreased pH, whilst PUFAs of T. weissflogii increased substantially, without the changes observed in MUFAs. The increased proportion of PUFAs was mainly attributable to higher EPA and DHA proportions. Given the varied responses among species, the effects of ocean acidification on the FA composition of phytoplankton community would be mediated by community structure. Indeed, the increase in PUFAs in response to seawater acidification is likely linked to changes in taxonomic composition, as reported in a study of a natural plankton community in the Arctic (Leu et al., 2013). These changes could be transferred to higher trophic levels through the marine food web. This is evidenced by the study in a mixed phytoplankton assemblage including T. weissflogii, which found that seawater acidification was conducive to the accumulation of unsaturated FAs (Wang et al., 2017). However, for copepods fed a high-pCO2 Thalassiosira pseudonana diet, a decrease in both copepod somatic growth and egg production was found, attributed to the lower PUFA content in their diet (Rossoll et al., 2012). Thus, seawater acidification might have complex and far-reaching consequences for marine ecosystem functioning through altering intracellular macromolecules in primary producers.
Although temperature, light intensity, and nutrient limitation usually have prominent influences on photosynthetic performance and nutritional quality of primary producers, the regulating effects of seawater acidification should not be ignored, especially given the cascading effects throughout marine food webs. In the present study, the growth and BSi content of both species and the photosynthetic and respiration rates of T. weissflogii were impacted by neither decreased pH nor pH fluctuation, indicating their tolerance to pH changes. Nevertheless, fatty acid compositions of two species were altered by seawater acidification, with lower SFA and higher PUFA proportions compared to ambient pH conditions. Although the deterioration of nutritional quality (Rossoll et al., 2012) and the lower production of PUFAs (Hixson and Arts, 2016) were projected in the more warmed and acidified ocean, our results suggest that seawater acidification may enhance PUFA production without affecting growth in coastal diatoms, particularly T. weissflogii. Furthermore, taking dynamic carbonate chemistry into account would help investigate and predict the consequences of coastal acidification more thoroughly.
The data presented in this study have been deposited in the Zenodo repository: https://doi.org/10.5281/zenodo.13142180 (Shang et al., 2024b).
YS and JQ: methodology, formal analysis, investigation, and writing (original draft). YW, XW, and YZ: investigation. JX and DZ: conceptualization, writing (review and editing), and supervision. FL: conceptualization, methodology, validation, investigation, writing (review and editing), and funding acquisition.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This research has been supported by the National Natural Science Foundation of China (grant no. 42206138), the Natural Science Foundation of Jiangsu Province (grant no. BK20220684), the Qinglan Project of Jiangsu Province of China, the Innovation and Entrepreneurship Training Program of Jiangsu Province (grant no. SZ202411641631002), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
This paper was edited by Tyler Cyronak and reviewed by two anonymous referees.
Abreu, I.N., Aksmann, A., Bajhaiya, A.K., Benlloch, R., Giordano, M., Pokora, W., Selstam, E., and Moritz, T.: Changes in lipid and carotenoid metabolism in Chlamydomonas reinhardtii during induction of CO2-concentrating mechanism: Cellular response to low CO2 stress, Algal Res., 52, 102099, https://doi.org/10.1016/j.algal.2020.102099, 2020.
Anderson, D. H. and Robinson, R. J.: Rapid electrometric determination of alkalinity of sea water using glass electrode, Ind. Eng. Chem., 18, 767–769, https://doi.org/10.1021/i560160a011, 1946.
Armbrust, E. V.: The life of diatoms in the world's oceans, Nature, 459, 185–192, https://doi.org/10.1038/nature08057 2009.
Boelen, P., van Dijk, R., Sinninghe Damsté, J. S. Rijpstra, W. I. C., and Buma, A. G. J.: On the potential application of polar and temperate marine microalgae for EPA and DHA production, AMB Express, 3, 26, https://doi.org/10.1186/2191-0855-3-26, 2013
Brett, M. and Müller-Navarra, D.: The role of highly unsaturated fatty acids in aquatic foodweb processes, Freshwater Biol., 38, 483–499, https://doi.org/10.1046/j.1365-2427.1997.00220.x, 1997.
Brzezinski, M. A. and Nelson, D. M.: The annual silica cycle in the Sargasso Sea near Bermuda, Deep-Sea Res. Pt. I, 42, 1215–1237, https://doi.org/10.1016/0967-0637(95)93592-3, 1995.
Budge, S. M., Devred, E., Forget, M.-H., Stuart, V., Trzcinski, M. K., Sathyendranath, S., and Platt, T.: Estimating concentrations of essential omega-3 fatty acids in the ocean: Supply and demand, ICES J. Mar. Sci., 71, 1885–1893, https://doi.org/10.1093/icesjms/fsu003, 2014.
Carstensen, J. and Duarte, C. M.: Drivers of pH variability in coastal ecosystems, Environ. Sci. Technol., 53, 4020–4029, https://doi.org/10.1021/acs.est.8b03655, 2019.
Chrachri, A., Hopkinson, B. M., Flynn, K., Brownlee, C., and Wheeler, G. L.: Dynamic changes in carbonate chemistry in the microenvironment around single marine phytoplankton cells, Nat. Commun., 9, 74, https://doi.org/10.1038/s41467-017-02426-y, 2018.
Dickson, A. G.: Standard potential of the reaction: AgCl(s)+1/2H2(g)=Ag(s)+HCl(aq), and the standard acidity constant of the ion HSO in synthetic seawater from 273.15 to 318.15 K, J. Chem. Thermodyn., 22, 113–127, https://doi.org/10.1016/0021-9614(90)90074-Z, 1990.
Dickson, A. G. and Millero, F. J.: A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media, Deep-Sea Res., 34, 1733–1743, https://doi.org/10.1016/0198-0149(87)90021-5, 1987.
Doney, S. C., Busch, D. S., Cooley, S. R., and Kroeker, K. J.: The impacts of ocean acidification on marine ecosystems and reliant human communities, Annu. Rev. Env. Resour., 45, 83–112, https://doi.org/10.1146/annurev-environ-012320-083019, 2020.
Duarte, C. M., Hendriks, I. E., Moore, T. S., Olsen, Y. S., Steckbauer, A., Ramajo, L., Carstensen, J., Trotter, J. A., and McCulloch, M.: Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH, Estuar. Coast., 36, 221–236, https://doi.org/10.1007/s12237-013-9594-3, 2013.
Flynn, K. J., Blackford, J. C., Baird, M. E., Raven, J. A., Clark, D. R., Beardall, J., Brownlee, C., Fabian, H., and Wheeler, G. L.: Changes in pH at the exterior surface of plankton with ocean acidification, Nat. Clim. Change, 2, 510–513, https://doi.org/10.1038/nclimate1489, 2012.
Gao, K. and Campbell, D. A.: Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: A review, Funct. Plant Biol., 41, 449–459, https://doi.org/10.1071/fp13247, 2014.
García-Ibáñez, M. I., Guallart, E. F., Lucas, A., Pascual, J., Gasol, J. M., Marrasé, C., Calvo, E., and Pelejero, C.: Two new coastal time-series of seawater carbonate system variables in the NW Mediterranean Sea: Rates and mechanisms controlling pH changes, Front. Mar. Sci., 11, 1348133, https://doi.org/10.3389/fmars.2024.1348133, 2024.
Gattuso, J.-P., Magnan, A., Billé, R., Cheung, W. W. L., Howes, E. L., Joos, F., Allemand, D., Bopp, L., Cooley, S. R., Eakin, C. M., Hoegh-Guldberg, O., Kelly, R. P., Pörtner, H.-O., Rogers, A. D., Baxter, J. M., Laffoley, D., Osborn, D., Rankovic, A., Rochette, J., Sumaila, U. R., Treyer, S., and Turley, C.: Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios, Science, 349, aac4722, https://doi.org/10.1126/science.aac4722, 2015.
Gordillo, F. J. L., Jiménez, C., Figueroa, F. L., and Niell, X.: Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira), J. Appl. Phycol., 10, 461–469, https://doi.org/10.1023/A:1008090402847, 1998.
Gordillo, F. J. L., Aguilera, J., Wiencke, C., and Jiménez, C.: Ocean acidification modulates the response of two Arctic kelps to ultraviolet radiation, J. Plant Physiol., 173, 41–50, https://doi.org/10.1016/j.jplph.2014.09.008, 2015.
Guillard, R. R. L. and Ryther, J. H.: Studies of marine planktonic diatoms: I. Cyclotella nana hustedt, and Detonula confervacea (Cleve) Gran, Can. J. Microbiol., 8, 229–239, https://doi.org/10.1139/m62-029, 1962.
Hancock, A. M., King, C. K., Stark, J. S., McMinn, A., and Davidson, A. T.: Effects of ocean acidification on Antarctic marine organisms: A meta-analysis, Ecol. Evol., 10, 4495–4514, https://doi.org/10.1002/ece3.6205, 2020.
Hansen, P. J.: Effect of high pH on the growth and survival of marine phytoplankton: Implications for species succession, Aquat. Microb. Ecol., 28, 279–288, https://doi.org/10.3354/ame028279, 2002.
Hinga, K. R.: Effects of pH on coastal marine phytoplankton, Mar. Ecol. Prog. Ser., 238, 281–300, https://doi.org/10.3354/meps238281, 2002.
Hixson, S. M. and Arts, M. T.: Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton, Glob. Change Biol., 22, 2744–2755, https://doi.org/10.1111/gcb.13295, 2016.
Ishida, Y., Hiragushi, N., Kitaguchi, H., Mitsutani, A., Nagai, S., and Yoshimura, M.: A highly CO2-tolerant diatom, Thalassiosira weissflogii H1, enriched from coastal sea, and its fatty acid composition, Fisheries Sci., 66, 655–659, https://doi.org/10.1046/j.1444-2906.2000.00105.x, 2000.
Jin, P., Liang, Z., Lu, H., Pan, J., Li, P., Huang, Q., Guo, Y., Zhong, J., Li, F., Wan, J., Overmans, S., and Xia, J.: Lipid remodeling revels the adaptations of a marine diatom to ocean acidification, Front. Microbiol., 12, 748445, https://doi.org/10.3389/fmicb.2021.748445, 2021.
Kainz, M., Arts, M. T., and Mazumder, A.: Essential fatty acids in the planktonic food web and their ecological role for higher trophic levels, Limnol. Oceanogr., 49, 1784–1793, https://doi.org/10.4319/lo.2004.49.5.1784, 2004.
Kapsenberg, L. and Cyronak, T.: Ocean acidification refugia in variable environments, Glob. Change Biol., 25, 3201–3214, https://doi.org/10.1111/gcb.14730, 2019.
Key, T., McCarthy, A., Campbell, D. A., Six, C., Roy, S., and Finkel, Z. V.: Cell size trade-offs govern light exploitation strategies in marine phytoplankton, Environ. Microbiol., 12, 95–104, https://doi.org/10.1111/j.1462-2920.2009.02046.x, 2010.
King, A. L., Jenkins, B. D., Wallace, J. R., Liu, Y., Wikfors, G. H., Milke, L. M., and Meseck, S. L.: Effects of CO2 on growth rate, C: N: P, and fatty acid composition of seven marine phytoplankton species, Mar. Ecol. Prog. Ser., 537, 59–69, https://doi.org/10.3354/meps11458, 2015.
Lee, J.-Y., Marotzke, J., Bala, G., Cao., L., Corti, S., Dunne, J. P., Engelbrecht, F., Fischer, E., Fyfe, J. C., Jones, C., Maycock, A., Mutemi, J., Ndiaye, O., Panickal, S., and Zhou, T.: Future global climate: Scenario-based projections and near-term information, in: Climate Change 2021: The physical Science Basis. Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change, edited by: Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S. L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M. I., Huang, M., Leizell, K., Lonnoy, E., Matthews, J. B. R., Maycock, T. K., Waterfield, T., Yelekci, O., Yu, R., and Zhou, B., Cambridge University Press, Cambridge and New York, 553–672, https://doi.org/10.1017/9781009157896.006, 2021.
Leu, E., Daase, M., Schulz, K. G., Stuhr, A., and Riebesell, U.: Effect of ocean acidification on the fatty acid composition of a natural plankton community, Biogeosciences, 10, 1143–1153, https://doi.org/10.5194/bg-10-1143-2013, 2013.
Li, F., Wu, Y., Hutchins, D. A., Fu, F., and Gao, K.: Physiological responses of coastal and oceanic diatoms to diurnal fluctuations in seawater carbonate chemistry under two CO2 concentrations, Biogeosciences, 13, 6247–6259, https://doi.org/10.5194/bg-13-6247-2016, 2016.
Li, F., Fan, J., Hu, L., Beardall, J., and Xu, J.: Physiological and biochemical responses of Thalassiosira weissflogii (diatom) to seawater acidification and alkalization, ICES J. Mar. Sci., 76, 1850–1859, https://doi.org/10.1093/icesjms/fsz028, 2019.
Li, F., Xu, J., Beardall, J., and Gao, K.: Diurnally fluctuating pCO2 enhances growth of a coastal strain of Emiliania huxleyi under future-projected ocean acidification conditions, ICES J. Mar. Sci., 78, 1301–1310, https://doi.org/10.1093/icesjms/fsab036, 2021.
Maulucci, G., Cohen, O., Daniel, B., Sansone, A., Petropoulou, P.I., Filou, S., Spyridonidis, A., Pani, G., Spirito, M. De, Chatgilialoglu, C., Ferreri, C., Kypreos, K. E., and Sasson, S.: Fatty acid-related modulations of membrane fluidity in cells: detection and implications, Free Radical Res., 50, 40–50, https://doi.org/10.1080/10715762.2016.1231403, 2016.
Mehrbach, C., Culberson, C. H., Hawley, J. E., and Pytkowicz, R. M.: Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure, Limnol. Oceanogr., 18, 897–907, https://doi.org/10.4319/lo.1973.18.6.0897, 1973.
Raven, J. R., Gobler, C. J., and Hansen, P. J.: Dynamic CO2 and pH levels in coastal, estuarine, and inland waters: Theoretical and observed effects on harmful algal blooms, Harmful Algae, 91, 101594, https://doi.org/10.1016/j.hal.2019.03.012, 2020.
Reinfelder, J. R., Kraepiel, A. M. L., and Morel, F. M. M.: Unicellular C4 photosynthesis in a marine diatom, Nature, 407, 996–999, https://doi.org/10.1038/35039612, 2000.
Rossoll, D., Bermúdez, R., Hauss, H., Schulz, K. G., Riebesell, U., Sommer, U., and Winder, M.: Ocean acidification-induced food quality deterioration constrains trophic transfer, PLoS ONE, 7, e34737, https://doi.org/10.1371/journal.pone.0034737, 2012.
Schaum, C. E. and Collins, S.: Plasticity predictes evolution in a marine alga, P. R. Soc. B., 281, 20141486, https://doi.org/10.1098/rspb.2014.1486, 2014.
Schaum, C. E., Rost, B., and Collins, S.: Environmental stability affects phenotypic evolution in a globally distributed marine picoplankton, ISME J., 10, 75–84, https://doi.org/10.1038/ismej.2015.102, 2016.
Schwenk, D., Seppala, J., Spilling, K., Virkki, A., Tamminen, T., Oksman-Caldentey, K. M., and Rischer, H.: Lipid content in 19 brackish and marine microalgae: influence of growth phase, salinity and temperature, Aquat. Ecol., 47, 415–424, https://doi.org/10.1007/s10452-013-9454-z, 2013.
Shang, Y., He, J., Qiu, J., Hu, S., Wang, X., Zhang, T., Wang W., Yuan, X., Xu, J., and Li, F.: The tolerance of two marine diatoms to diurnal pH fluctuation under dynamic light condition and ocean acidification scenario, Mar. Environ. Res., 196, 106425, https://doi.org/10.1016/j.marenvres.2024.106425, 2024a.
Shang, Y., Qiu, J., Weng, Y., Wang, X., Zhang, D., Zhou, Y., Xu, J., and Li, F.: Effects of pH/pCO2 fluctuation on photosynthesis and fatty acid composition of two marine diatoms, Zenodo [data set], https://doi.org/10.5281/zenodo.13142180, 2024b.
Strzepek, R. F. and Harrison, P. J.: Photosynthetic architecture differs in coastal and oceanic diatoms, Nature, 431, 689–692, https://doi.org/10.1038/nature02954, 2004.
Sunda, W. G., Price, N. M., and Morel, F. M. M.: Trace metal ion buffers and their use in culture studies, in : Algal Culturing Techniques, edited by: Aderson, R. A., Elsevier Academic Press, Burlington, 35–63, ISBN 0120884267, 2005.
Taraldsvik, M. and Myklestad, S.: The effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum, Eur. J. Phycol., 35, 189–194, https://doi.org/10.1080/09670260010001735781, 2000.
Tréguer, P., Bowler, C., Moriceau, B., Dutkiewicz, S., Gehlen, M., Aumont, O., Bittner, L., Dugdale, R., Finkel, Z., Iudicone, D., Jahn, O., Guidi, L., Lasbleiz, M., Leblanc, K., Levy, M., and Pondaven, P.: Influence of diatom diversity on the ocean biological carbon pump, Nat. Geosci., 11, 27–37, https://doi.org/10.1038/s41561-017-0028-x, 2018.
Wahl, M., Covachã, S.S., Saderne, V., Hiebenthal, C., Müller, J.D., Pansch, C., and Sawll, Y.: Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations, Limnol. Oceanogr., 63, 3–21, https://doi.org/10.1002/lno.10608, 2018.
Wang, T., Tong, S., Liu, N., Li, F., Wells, M. L., and Gao, K.: The fatty acid content of plankton is changing in subtropical coastal waters as a result of OA: Results from a mesocosm study, Mar. Environ. Res., 132, 51–62, https://doi.org/10.1016/j.marenvres.2017.10.010, 2017.
Young, B. P., Shin, J. J. H., Orij, R., Chao, J. T., Li, S. C., Guan, X. L., Khong, A., Jan, E., Wenk, M. R., Prinz, W. A., and Smits, G. J.: Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism, Science, 329, 1085–1088, https://doi.org/10.1126/science.1191026, 2010.
Zheng, Y., Giordano, M., and Gao, K.: Photochemical responses of the diatom Skeletonema costatum grown under elevated CO2 concentrations to short-term changes in pH, Aquat. Biol., 23, 109–118, https://doi.org/10.3354/ab00619, 2015.