the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Nitrogen concentrations in boreal and temperate tree tissues vary with tree age/size, growth rate, and climate
Kailiang Yu
Stefano Manzoni
Anatoly Prokushkin
Melanie A. Thurner
Zhiqiang Wang
Thomas Hickler
Photosynthesis, growth, and plant maintenance respiration are closely related to tree tissue nitrogen (N) concentrations. While earlier studies of the variation in tissue N concentrations and underlying controls have mostly focused on leaves, here we identify the large-scale controls of N concentration in other tree compartments for the first time. This is achieved by constructing and analysing a novel database of N concentrations in stems, roots, and branches covering all common Northern Hemisphere boreal and temperate tree genera, combined with data for leaves mostly from existing databases. This database allows us to explore the large-scale abiotic (climate, soil N concentration) and biotic controls (tree age/size, leaf type, growth rate) of tree tissue N concentration. We find that N concentrations decrease with increasing tree age (or size) and are significantly higher in deciduous compared to evergreen trees in all tissues. Low growth rates or unfavourable climate conditions (very cold or dry climate) significantly decrease leaf (the latter only for needleleaf deciduous and needleleaf evergreen trees) but not stem N concentration, indicating their effects on N allocation. Plant traits and environmental conditions together explain very large parts of the variation in tissue N concentrations. These results suggest that changes in the distribution of tree age/size, species, and extreme climate, induced by climate change, forest management, or disturbances, will have substantial consequences for the carbon (C) sequestration potential of boreal and temperate forests by altering tissue N concentrations. We expect that the expansion of tree species better adapted to dry conditions in European temperate forests will result in a higher N concentration in all tree tissues and elevated N allocation fractions to stems, which might lead to higher productivity but also higher maintenance respiration. The identified relationships need to be represented in dynamic global vegetation models (DGVMs) to estimate future effects of N limitation on the C cycle.
- Article
(4438 KB) - Full-text XML
-
Supplement
(2198 KB) - BibTeX
- EndNote
Nitrogen (N) acquired by plants is incorporated into amino acids and thus proteins and enzymes, nucleic acids, and chlorophyll, and, as such, it is critical for photosynthesis and plant growth. Since leaf N concentration is strongly related to carboxylation capacity (Dong et al., 2022), increases in leaf N concentration are associated to higher photosynthetic rates, especially in N-limited ecosystems (Wright et al., 2004). Most terrestrial ecosystems are affected by N limitation (LeBauer and Treseder, 2008), resulting in a reduced response of photosynthesis and growth to global warming and increasing atmospheric carbon dioxide (CO2; Luo et al., 2004; Reich et al., 2006a; Terrer et al., 2019; Kou-Giesbrecht et al., 2023). N limitation is particularly relevant in northern boreal and temperate ecosystems (Du et al., 2020). At the same time, increased N concentrations in leaves, but also in other tissues (branches, stems, roots), directly translate into higher maintenance respiration (Rm) rates (Ryan, 1991; Reich et al., 2006b). Accordingly, not only plant growth, but also respiration, is directly related to the vegetation N content (Reich et al., 2006b), since Rm (respiratory costs that plants have to invest to maintain a healthy state) supports protein repair and replacement and most plant organic N is in proteins (Ryan, 1991). Moreover, litter decomposition is also driven by the plant tissue N content (Parton et al., 2007).
These relationships are represented in dynamic global vegetation models (DGVMs), but how tissue N content is prescribed or modelled differs between models, which indicates high uncertainty (Kou-Giesbrecht et al., 2023). Tissue-specific N concentrations are either prescribed and more or less specific for certain plant functional types (PFTs), or they change in relation to environmental factors (Meyerholt and Zaehle, 2015). A common approach is to optimise leaf N concentration for maximum net carbon gain, e.g. in Lund–Potsdam–Jena (LPJ) type of models (Haxeltine and Prentice, 1996; Sitch et al., 2003), and wood and fine-root N concentrations are usually simply assumed to vary proportionally with leaf N concentration (Meyerholt and Zaehle, 2015).
Despite the potential role of N concentration across plant tissues, previous studies have largely focused on global biogeographic understanding of leaf N concentration (Butler et al., 2017; Moreno-Martínez et al., 2018). These studies are facilitated by extensive leaf N concentration data from databases like TRY (Kattge et al., 2020). However, extrapolation to whole plants has been hampered by relatively sparse data on tissue N concentration in other tree compartments (i.e. branches, stems, and roots). While numerous N concentration measurements are available for fine roots (Iversen et al., 2017; Wang et al., 2019, 2021), N concentration data representative for the entire root system, including coarse roots, are comparatively sparse due to the complexity of such measurements. To address this knowledge gap, and since our study aims to facilitate large-scale estimates of tissue N contents and Rm in boreal and temperate forests in future studies, here we focus on total root N concentrations. Such estimates of tissue N contents and Rm are dependent on remote sensing biomass data and measurements of biomass allometry, which (in contrast to measurements of N concentrations) more frequently include total root biomass but rarely fine-root biomass separately (Thurner et al., 2014, 2019; Schepaschenko et al., 2017). Estimates of root N concentrations, root N contents, and root respiration are important, for instance, for improving estimates of the land C sink in C budgets (Friedlingstein et al., 2023).
N concentrations are highly variable among tissues and are 1 order of magnitude lower in structural compartments (i.e. branches, stems, and coarse roots) compared to leaves. Hence, information on distinct N concentrations for all living tree compartments (leaves, branches, stem sapwood, roots) and underlying environmental controls is required to better constrain the influence of N limitation on the response of the vegetation C cycle to environmental changes. Although the influence of many environmental and biological factors on tree tissue N concentration has been identified in certain experiments or stands, it has not been determined at global scale. The combined effects of tree species identity and their growth rates, climatic conditions, soil N availability, and tree size/age on N concentrations in leaves, but especially stems, roots, and branches, remain largely unexplored across boreal and temperate forest ecosystems. Here we compile an extensive database of N concentration measurements in boreal and temperate tree stems, roots, and branches (Thurner et al., 2025) from the literature and our own measurements in regions where other data are sparse (Siberia), in addition to measurements for leaves that are, to a large extent, available from TRY. Especially with regard to stem, root, and branch N concentrations, our database is novel, since it integrates numerous studies that focused on selected species and forest stands. Moreover, we collect information on simultaneously measured environmental controls (tree species, climate, tree size/age, soil N concentration). These data allow us to investigate the controls of N concentration in tree compartments other than leaves for the first time across the entire Northern Hemisphere boreal and temperate forests.
We use our compiled N concentration database to test the following hypotheses:
-
Tissue N concentration decreases with tree age/size.
N concentration has been reported to decrease in stem and branch segments (Bosc et al., 2003; Feng et al., 2008) and also in roots (Ceccon et al., 2016) of increasing age or increasing diameter (Ceschia et al., 2002) but only for single trees or stands and selected tree species. Other studies of certain needleleaf evergreen species at the stand scale, however, found N concentrations in stems and bark, but not branches and foliage, to decrease with stand age (Sprugel, 1984; Ranger et al., 1995; Ponette et al., 2001). Accordingly, the generality of this relationship has not yet been confirmed for all common boreal and temperate tree genera at global scale. Possible underlying mechanisms are (a) a decline in photosynthetic capacity with increasing tree age/size and associated decline in required N to support photosynthesis (Yoder et al., 1994; Steppe et al., 2011); (b) a decreasing share of tissues with high N concentrations in older trees due to the conversion of living cells in the sapwood to heartwood and due to N retranslocation (Augusto et al., 2008; Thurner et al., 2019); and (c) a depletion of soil N during early growth stages or a stabilisation of N in organic matter (especially in boreal forests), which limits growth in mature forests (Norby et al., 2010).
-
Deciduous trees have higher tissue N concentrations than evergreen trees.
Both leaf and woody tissue N concentrations differ strongly between tree species (e.g. Martin et al., 2015). Leaf N concentration is much higher in deciduous than in evergreen broadleaf and needleleaf trees, since trees with thin, short-living leaves have higher N concentrations and in general also higher growth rates to support photosynthesis of foliage with shorter lifespan (Chapin et al., 1993; Reich et al., 1992; Reich, 2014; Schulze et al., 1994). Similar relationships have been observed between fine-root N concentration and fine-root longevity (Withington et al., 2006). Fast-growing, deciduous species also have a greater capacity to acquire nutrients or usually live in nutrient-rich areas (Lambers and Poorter, 1992). For these reasons, deciduous trees are supposed to exhibit higher N concentrations compared to evergreen trees not only in their living tissue, but also in their structural woody components. However, the significance of the difference in branch, stem, and coarse-root N concentration between deciduous and evergreen boreal and temperate trees still has to be demonstrated based on an extensive database. An earlier study by Meerts (2002), for instance, relied solely on nine samples of sapwood and heartwood N concentration in gymnosperms. In addition, little is known about tissue N concentrations in needleleaf deciduous trees, i.e. larch (Larix).
-
Trees that are slow-growing or grow under unfavourable climatic conditions (very cold or dry climate) allocate a lower share of N to their leaves and a higher share of N to their stems compared to trees that are fast-growing or grow under favourable conditions.
Fast-growing species have been found to allocate relatively more N to their leaves and less N to their stems compared to slow-growing species (Poorter et al., 1990), due to their different defence and allocation strategies. However, these observations were based on a greenhouse experiment considering only non-woody herbaceous species and thus still need to be verified for boreal and temperate tree species at global scale. Plants face a trade-off when investing resources into growth or defence (Bazzaz et al., 1987; Herms and Mattson, 1992), and, because N is critically involved in defence mechanisms (Ullmann-Zeunert et al., 2013), their N economy is central in this trade-off. Specifically, N is required for chemical defence against herbivores and pathogens through N-based secondary metabolites, for instance, alkaloids (Herms and Mattson, 1992). However, how defence mechanisms are controlled by N is yet not fully understood (Sun et al., 2020) because of research having mostly focused on herbivory and pathogens but less on defence against environmental stresses (Loehle, 1988).
While, in fast-growing species, higher rates of photosynthesis and thus growth require more N to be allocated to their leaves, it has been suggested that slow-growing species tend to allocate relatively more N to their stems to support defence mechanisms (Loehle, 1988). In addition to being the result of a growth–defence trade-off, relatively more N might as well be stored in reserves in stems of slow-growing compared to fast-growing trees due to a relative oversupply of N as they grow in ecosystems limited by other resources (Chapin et al., 1990), including low temperatures, water, or light.
Climatic conditions affect tissue N via species sorting but also via acclimation mechanisms. Unfavourable climatic conditions (very cold or dry climate) favour tree species with slow growth and high investment into defence against cold stress and drought, respectively (Chapin, 1991), leading to relatively lower N concentrations in leaves and higher N concentrations in stems.
Up to now, the effects of temperature and water availability on N allocation have rarely been analysed at global scale. Although leaf N concentration is not strongly related to mean annual temperature (MAT; Laughlin et al., 2011), it tends to decrease with decreasing MAT in the high latitudes (Reich and Oleksyn, 2004). This relationship might be due to different interacting effects of acclimation and adaptation of plant physiology to temperature on the one hand but also to gradients in soil nutrient availability on the other hand (Reich and Oleksyn, 2004). In contrast, according to Tang et al. (2018), the N concentration not only in leaves, but also in stems and roots, decreases with increasing MAT and mean annual precipitation (MAP) across all ecosystems in China. In general, they found that the N concentration in stems and roots is more strongly related to abiotic factors than leaf N concentration. These contrasting results motivate a more complete analysis at the global scale.
-
Tissue N concentration increases with soil N concentration.
In addition, tissue N concentrations vary with soil N because higher N availability in the soil supports higher levels of N uptake. However, the relationship between soil N and the N concentration in structural tree compartments (i.e. branches, stems, and coarse roots) remains rarely investigated, and available studies have been limited to single or a few field sites or forest stands and a selection of tree species. For instance, higher soil N has been observed to result in elevated N concentrations in all tree tissues in Populus trees grown in a field experiment (Pregitzer et al., 1995).
This relationship has been studied more extensively for leaves and fine roots. Fine-root N concentration has been found to be correlated with soil nitrate availability in US temperate forests (Hendricks et al., 2000) and negatively correlated with the soil C:N ratio in boreal and temperate forests in Europe (Ostonen et al., 2017). In contrast, Tateno and Takeda (2010) reported decreasing leaf, but surprisingly not fine-root, N concentrations with decreasing soil N availability in a temperate deciduous forest in Japan. In permafrost regions, foliar N concentration has been reported to decrease with decreasing active layer thickness and consequently fewer available nutrients (Prokushkin et al., 2018). These partly contradictory results and the scarcity of studies on structural tree compartments show that further investigation of the relationship between tree tissue and soil N concentration considering all common boreal and temperate tree genera at global scale is required.
-
Both plant traits and environmental conditions are important controls of tissue N concentrations and together explain large parts of the variation therein.
As discussed above, tree tissue N concentrations have been shown to be related to different plant traits and environmental conditions. However, previous studies have usually focused on single factors but have not comprehensively studied effects and interactions of multiple controls for tissues other than leaves (e.g. Reich and Oleksyn, 2004) and fine roots (e.g. Yuan et al., 2011; Wang et al., 2020). For the first time, we investigate the relationships between N concentrations in branches, stems and (coarse) roots, and plant traits (tree age/size, leaf type, growth rate), as well as environmental conditions (temperature, water availability, soil nutrient availability) across the entire Northern Hemisphere boreal and temperate forests.
2.1 A novel database of N concentration measurements in tree tissues
We collect a novel database of N concentration measurements in stems (i.e. trunks), roots, and branches of Northern Hemisphere boreal and temperate trees (Thurner et al., 2025) by consulting extensive research literature. For this task, we search Web of Science for stem, root, and branch nitrogen concentrations for all common boreal and temperate tree genera (for search criteria, see Sect. S1 in the Supplement). To a lesser extent, we also collect leaf N concentration measurements from the literature because numerous measurements of leaf N concentration are already available from the TRY database (Kattge et al., 2020). Since measurements are rare in Russian boreal forests, we include our own measurements for Larix gmelinii in the central part of the Nizhnyaya Tunguska River basin in central Siberia (ca. 64° N, 100° E; Larjavaara et al., 2017; Prokushkin et al., 2018). Moreover, data sources from the Russian and Chinese literature, the TRY database (Kattge et al., 2020) and the Biomass And Allometry Database (BAAD; Falster et al., 2015), are considered.
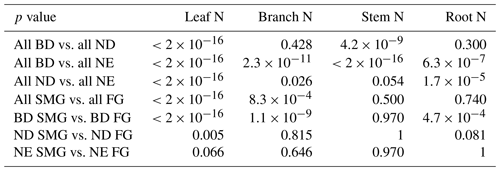
Only measurements of N concentration under natural conditions (no greenhouses, no trees grown in pots, no fertiliser, and no other experiments) are included in the database. In addition, we only include studies with explicit information on the measurement location and the investigated tree species. We only analyse measurements of total root N concentration but do not include measurements of N concentration specifically for fine roots. In cases where separate measurements are available for (stem) sapwood and heartwood, we include only N concentrations of sapwood. Replicate measurements, if available from the studies, are retained. All tissue N concentrations are expressed in gram nitrogen per gram of dry weight. In total, the compiled database investigated here comprises 1048 stem, 267 root, 599 branch, and 5944 leaf N concentration measurements. A list of the data sources is found in Sect. S2. While almost all of the stem (911 collected from literature, 1 own, 52 from TRY, 84 from BAAD), root (266 collected from literature, 1 own), and branch (all collected from literature) N concentration measurements have been collected from 192 studies in the literature, leaf N concentration measurements are to a large extent available from existing databases (188 collected from literature, 5 own, 5522 from TRY, 229 from BAAD). The spatial distribution of N concentration measurements applied in this study is shown in Fig. 1.
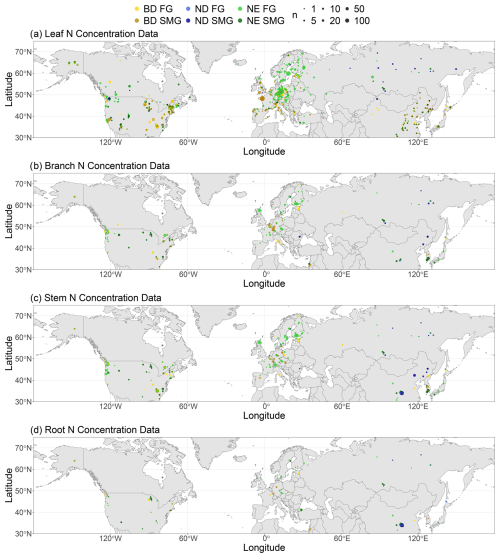
Figure 1Spatial distribution of N concentration measurements applied in this study in (a) leaves, (b) branches, (c) stems, and (d) roots of boreal and temperate tree species, grouped according to their leaf type (BD: broadleaf deciduous; ND: needleleaf deciduous; NE: needleleaf evergreen) and growth rate. n denotes the number of measurements.
2.2 Explanatory variables
To explain the variation in tree tissue N concentrations, we consider the following explanatory variables: tree species grouped according to growth/leaf type classes, mean annual temperature (MAT; °C), mean annual precipitation (MAP; mm), tree height (m), and soil total N concentration (g N g−1 dry weight). Additional analyses also include tree age (years) and compartment biomass per area (kg dry weight m−2 ground). The choice of this selection of variables is motivated by their hypothesised control on tissue N concentration (see Sect. 1) and the availability of corresponding measurements from studies contained in the compiled database. In addition, spatially extensive information is available for most of these variables, which will allow spatial products of tissue N concentration to be derived in subsequent studies. The relatively low sample numbers of many species, especially in the case of root N concentration, but also in the case of branch and stem N concentration, prevent an analysis of the large-scale controls of tissue N concentrations at species level. Therefore, we aggregate species by leaf types and analyse these relationships for different leaf types separately. Furthermore, we investigate the influence of variations in tissue N concentrations with season and needle age on our results.
Information on MAT, MAP, soil N concentration, tree height, age, and biomass is extracted from the respective studies, when available. Growth/leaf type classes categorise tree species according to their growth rate (fast-growing, slow-/medium-growing) and leaf type (BD: broadleaf deciduous; ND: needleleaf deciduous; NE: needleleaf evergreen). By combining these two characteristics, we classify species into six growth/leaf type classes. We exclude data without information on tree species and broadleaf evergreen trees from the analysis, since available measurements for this leaf type are scarce. Due to missing information on actual growth rates of the species at the specific measurement sites, we assign their typical growth rate (slow/medium: ≤60.96 cm yr−1; fast: >60.96 cm yr−1; threshold corresponds to 2 feet yr−1) to each investigated tree species based on our expert judgement and online research (see Sect. S3). In addition, we classify MAT (MAT <0 °C vs. MAT ≥0 °C) and MAP (MAP <500 mm vs. MAP ≥500 mm) into climatic classes to separate very cold and dry conditions from more favourable climatic conditions for plant growth. As an alternative measure of dryness, we calculate the aridity index (AI = MAP potential evapotranspiration) from CHELSA Version 2.1 long-term climate data at the study locations (1981–2010; 30 arcsec resolution; Brun et al., 2022), as information on potential evapotranspiration is usually not available in the compiled studies. Similarly, we separate dry (AI <0.65) from humid (AI ≥0.65) conditions following the UNEP classification (UNEP, 1992).
2.3 Regression analysis and generalised additive models
We apply linear regression and also partial regression (because of its ability to account for interaction effects between explanatory variables) to explore how the variation in tree tissue N concentration can be explained by the abovementioned explanatory variables. The low susceptibility of partial regression analysis to overfitting allows high confidence in the detected relationships. Measurements of tree age and soil N concentration are relatively sparse, thus reducing the available data for partial regression analyses with each included explanatory variable. Thus, we perform the partial regressions by controlling for only one explanatory variable at a time. Model accuracy is quantified in terms of modelling efficiency (MEF; Nash and Sutcliffe, 1970), pairwise partial correlations, and the p values of the partial regressions. Significance of differences in N concentration between tree tissues, growth/leaf type classes, and climatic classes is quantified by the p values of pairwise t tests. Although the distributions of tissue N concentrations are positively skewed and thus deviate from a normal distribution (as evident in Q–Q plots in Fig. S5 and S6 in Sect. S11), t tests are applied here, since they are relatively robust to deviations from normality, especially for large sample sizes (e.g. Fagerland, 2012).
In addition, we apply generalised additive models (GAMs) to investigate how much of the variation in tree tissue N concentration can be explained by the selected explanatory variables and to gain additional insights into the relative importance of different individual controls and their interactions. GAMs are employed because of their ability to account for non-linear relationships and interaction effects between explanatory variables and to include both numerical and factorial variables (Hastie and Tibshirani, 1990; Wood, 2006). A total of 17 model setups are implemented for each tree tissue N concentration, using different combinations of explanatory variables and considering either plant trait variables (leaf type, growth rate, tree age/height/biomass), environmental condition variables (MAT, MAP, soil N concentration), or both (see Sect. S4). For each of the implemented GAM setups with two or more variables, we compare models with and without interaction terms and select as the best model either the model with the lowest Akaike information criterion (AICmin) or a simpler model if AIC values differ by at most two units following Burnham and Anderson (2004). Due to the relative sparseness of measurements of tree age (and tree height and biomass) and soil N concentration, we can include only one of these variables in a GAM at a time. For their application in the GAMs, MAT and MAP are derived from CHELSA Version 2.1 long-term climate data at the study locations (1981–2010; 30 arcsec resolution; Brun et al., 2022) when not available from the compiled studies in order to increase the sample size of GAMs considering these variables. Model predictive power is quantified in terms of MEF (Nash and Sutcliffe, 1970).
Tree tissue N concentration is highest in leaves (median = 0.0167 g N g−1; see Table S5 in Sect. S5), followed by roots (median = 0.0060 g N g−1; Table S8) and branches (median = 0.0035 g N g−1; Table S6), and much lower in stems (median = 0.0010 g N g−1; Table S7). The differences in N concentration between these compartments are highly significant (see p values of pairwise t tests in Sect. S6).
There are strong differences in N concentrations between different tree species in all tissues (see Fig. S1 and Tables S10–S13 in Sect. S7). Especially in leaves, BD species (e.g. different species of Acer, Betula, Fagus, Fraxinus, Populus, and Quercus) have higher levels of N concentrations than NE species (e.g. different species of Abies, Picea, and Pinus). In some cases, even different species of the same genus exhibit strongly different tissue N concentrations. However, these differences between species are also influenced by other controls and might be due to differences in specific growing conditions and sometimes low sample numbers.
3.1 Relationship between tissue N concentrations and tree age, height, and tissue biomass
We find that tree tissue N concentration decreases with both tree age and tree height and compartment biomass in leaves, branches, stems, and roots (Fig. 2). This negative correlation (with MEFs up to 0.302) is evident in most cases (and in many cases significant at the 5 % level) when looking at leaf types (BD, ND, NE) separately. Note that we do not correct for heteroscedasticity occurring in some of the linear relationships identified in Fig. 2 (see Fig. S7 in Sect. S12), since one major reason for heteroscedasticity in these linear models is their non-consideration of other important explanatory variables (see below). When accounting for the influence of other explanatory variables (MAT, MAP, soil N concentration), the partial correlation analysis reveals that N concentration is in most cases negatively correlated to tree age for all investigated tree tissues (leaves, branches, stems, roots) and leaf types (Table S14 in Sect. S8). These negative correlations are sometimes, but not always, significant due to few available measurements in some cases. Note that the partial correlation can be analysed only for a subset of the data, since measurements of the included explanatory variables are not available for all measurements of tissue N concentration. In particular, measurements of tree age and soil N concentration are relatively sparse.
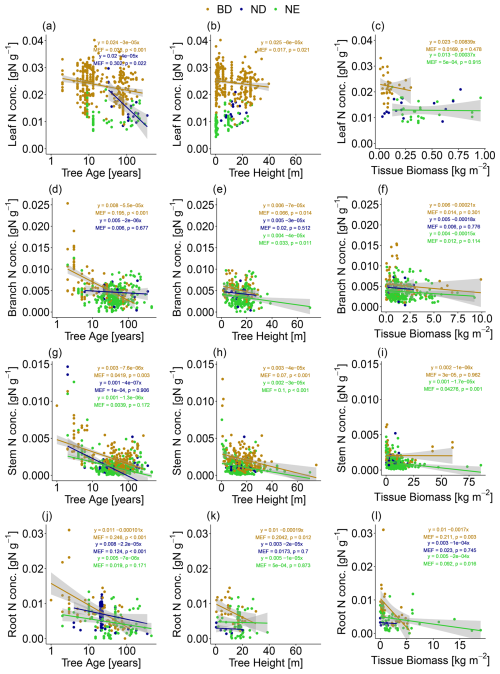
Figure 2The relationship between (a–c) leaf, (d–f) branch, (g–i) stem, and (j–l) root N concentration and tree age, tree height, and compartment biomass. Linear models have been fitted for leaf types (broadleaf deciduous, BD; needleleaf deciduous, ND; needleleaf evergreen, NE) separately and only in the case of negative correlation. The strength of the linear relationships is quantified by their modelling efficiency (MEF), and their significance is quantified by the p value. Confidence intervals of 95 % are shown in grey.
3.2 Relationships between tissue N concentrations and leaf type, season, and needle age
In addition to tree age/size, we find that tree tissue N concentration is also related to leaf type (BD, ND, NE; Fig. 3). Compared to NE trees, BD trees have significantly higher N concentrations in leaves (median = 0.0222 g N g−1 vs. 0.0124 g N g−1; Table S5), branches (median = 0.0042 g N g−1 vs. 0.0030 g N g−1; Table S6), stems (median = 0.0017 g N g−1 vs. 0.0008 g N g−1; Table S7), and roots (median = 0.0064 g N g−1 vs. 0.0038 g N g−1; Table S8; p values and pairwise t tests for these comparisons are reported in Table 1). ND trees on average show intermediate levels of N concentration in their leaves (median = 0.0185 g N g−1) and stems (median = 0.0010 g N g−1), but high levels in their branches (median = 0.0049 g N g−1) and roots (median = 0.0071 g N g−1).
Among other things, variations in tissue N concentrations with season and needle age could potentially affect our results. However, the vast majority of measurements included in the compiled database were taken during the summer season (June–September). In addition, we do not find significantly (at the 5 % level) lower leaf N concentrations outside the summer season or with increasing needle age in additional analyses, which are, however, based on limited data for which information on measurement time and needle age is available (see Figs. S2 and S3 in Sect. S9).
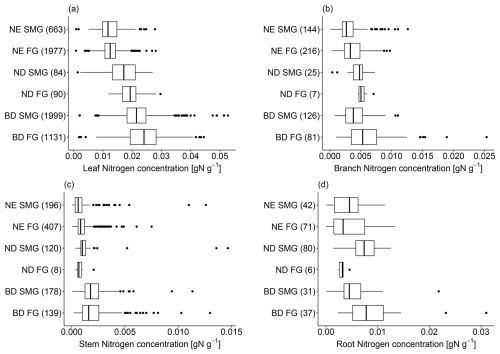
Figure 3N concentration in (a) leaves, (b) branches, (c) stems, and (d) roots of boreal and temperate tree species, grouped according to their leaf type (BD: broadleaf deciduous; ND: needleleaf deciduous; NE: needleleaf evergreen) and growth rate (SMG: slow-/medium-growing; FG: fast-growing). The number of observations in each growth/leaf type class is stated in parentheses. The box and whisker plots show the median and the interquartile range of values. The whiskers extend up to the most extreme data point, which is no more than 1.5 times the interquartile range away from the box. Outliers are drawn as points.
3.3 Relationship between tissue N concentrations and tree growth rate and climate
Tissue N concentration varies systematically with tree growth rate (fast-growing, slow-/medium-growing; Fig. 3). However, the identified relationships are sometimes different among tree compartments. Leaf and branch N concentration tends to be higher in fast-growing than in slow-/medium-growing tree species across all leaf types (Tables S5 and S6). In contrast, only NE stem N concentration shows this behaviour, while fast-growing trees exhibit a lower stem N concentration than slow-/medium-growing trees in BD and ND trees (Table S7). In roots, in turn, fast-growing trees show a higher N concentration compared to slow-/medium-growing trees in the case of BD trees but a lower N concentration in NE and ND trees (Table S8). However, these findings are not always significant (Table 1), and the results for branch, stem, and root N concentration of fast-growing ND trees are to be interpreted with care due to the very few values available.
The leaf N concentration of ND and NE trees is significantly lower under very cold climate conditions (MAT <0 °C) compared to more favourable conditions (MAT ≥0 °C). Similar differences are observed for root N concentration of ND trees (Fig. 4; Table 2). In contrast, branch and stem N concentration of NE trees is significantly higher under a very cold compared to a more favourable climate. Similarly, for these leaf types, leaf and root N concentrations are significantly lower, but branch and stem N concentrations are significantly higher under dry climate conditions (MAP <500 mm) compared to more favourable conditions (MAP ≥500 mm; Fig. 4; Table 2). When considering an alternative dryness indicator (AI; see Sect. 2), we also observe a significantly lower leaf N concentration of ND and NE trees under dry (AI <0.65) compared to more favourable (AI ≥0.65) conditions but opposite patterns for BD trees (see Fig. S4 in Sect. S10; Table 2). Root N concentration is significantly lower not only for ND but also NE trees, whereas branch N concentration is significantly higher for BD trees when AI <0.65. Note that, in some cases, few available measurements of tissue N concentrations of specific leaf types under extreme climate hamper the detection of significant differences.
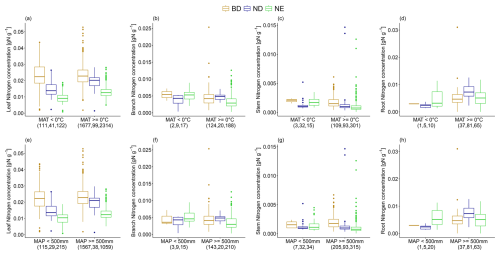
Figure 4The variation in leaf, branch, stem, and root N concentration for (a–d) mean annual temperature (MAT) classes (MAT <0 °C vs. MAT ≥0 °C) and (e–h) mean annual precipitation (MAP) classes (MAP <500 mm vs. MAP ≥500 mm) and for leaf types (BD: broadleaf deciduous; ND: needleleaf deciduous; NE: needleleaf evergreen) separately. The number of observations in each climatic class and for each leaf type is stated in brackets. The box and whisker plots show the median and the interquartile range of values. The whiskers extend up to the most extreme data point, which is no more than 1.5 times the interquartile range away from the box. Outliers are drawn as points.
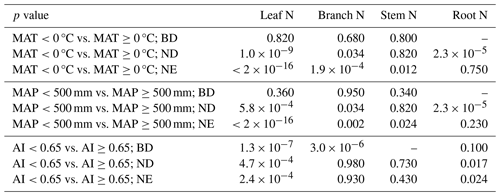
Table 2Significance of differences in leaf, branch, stem, and root N between climatic classes and for leaf types (BD: broadleaf deciduous, ND: needleleaf deciduous, NE: needleleaf evergreen) separately, quantified by the respective p values of pairwise t tests (MAT: mean annual temperature, MAP: mean annual precipitation sum, AI: aridity index). In some cases, not enough measurements are available (–).
Accordingly, the partial correlation analysis (Table S14 in Sect. S8) shows that leaf N concentration is significantly positively correlated with MAT when controlled for tree age and MAP for NE trees, whereas root N concentration is significantly positively correlated with MAT when controlled for MAP and soil N concentration for BD trees and when controlled for tree age for ND trees. Branch and stem N concentrations are significantly negatively correlated with MAT when controlled for tree age and MAP for NE trees. In addition, stem N concentration of ND trees is significantly negatively correlated with MAT when controlled for soil N concentration. However, for BD trees, there are opposite patterns for certain control variables (consistent significant negative correlation between leaf N concentration of BD trees and MAT; significant positive correlation for BD trees between their branch N concentration and MAT when controlled for MAP and soil N concentration and their stem N concentration and MAT when controlled for tree age and MAP).
With regard to MAP, we find significant positive correlations with leaf N concentration of ND trees when controlled for tree age and MAT and of NE trees when controlled for MAT. Similarly, root N concentration of ND trees is consistently significantly positively correlated with MAP. Negative correlations between branch N concentration and MAP are significant for BD trees when controlled for tree age and MAT and for NE trees across all control variables. Stem N concentration and MAP are most often negatively correlated but only in few cases significantly. Again, there are, in some cases, also opposite patterns for certain control variables and leaf types (significant negative correlation between leaf N concentration of BD trees and MAP when controlled for MAT and soil N concentration; significant positive correlation between branch N concentration of ND trees and MAP when controlled for MAT; significant negative correlation between root N concentration of BD trees and MAP when controlled for MAT and soil N concentration).
3.4 Relationship between tissue and soil N concentrations
Tissue N concentrations increase with increasing soil N concentration (MEF up to 0.084) in some cases when looking at leaf types separately (Fig. 5). The strongest relationships (in terms of MEF) are detected for root N concentrations of BD and NE trees (not significant at the 5 % level) and stem N concentration of NE trees (significant at the 5 % level). Note again that we do not correct for heteroscedasticity occurring in some of the linear models in Fig. 5 (see Fig. S8 in Sect. S12) because it can be explained by their non-consideration of other important explanatory variables. When accounting for the influence of other explanatory variables in the partial correlation analyses (Table S14 in Sect. S8), we detect a significant positive correlation between root and soil N concentration when controlled for tree age and MAP for BD trees and between stem and soil N concentration when controlled for tree age for NE trees. In most cases, there is no significant correlation, but, for ND trees, the partial correlation analysis shows even significant negative correlations between stem and soil N concentration when controlled for MAT and between root and soil N concentration when controlled for tree age and MAT.
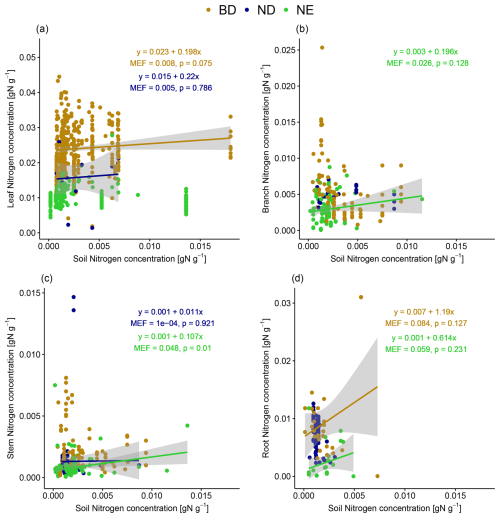
Figure 5The relationship between (a) leaf, (b) branch, (c) stem, and (d) root N concentration and soil N concentration. Linear models have been fitted for leaf types (BD: broadleaf deciduous; ND: needleleaf deciduous; NE: needleleaf evergreen) separately and only in the case of positive correlation. The strength of the linear relationships is quantified by their modelling efficiency (MEF), and their significance is quantified by the p value. Confidence intervals of 95 % are shown in grey.
3.5 Generalised additive model (GAM) results
The GAMs that considered combinations of multiple explanatory variables investigated here and their interactions can explain very large parts of the variation in tree tissue N concentrations. MEFs reach values up to 0.779 for leaves (considering leaf type, compartment biomass, MAT, and MAP), 0.702 for branches (considering leaf type, compartment biomass, MAT, and MAP), 0.922 for stems (considering leaf type, MAT, MAP, and soil N concentration), and 0.928 for roots (considering leaf type, compartment biomass, MAT, and MAP) (Fig. 6 and Tables S1–S4 in Sect. S4). While GAMs considering only plant trait variables (GAMs 1–9) show a better performance compared to GAMs considering only environmental condition variables (GAMs 10–12) for leaf (MEF =0.772 vs. MEF =0.516; number of available measurements n=73 vs. n=624) and stem (MEF =0.605 vs. MEF =0.488; n=823 vs. n=323) N concentrations, the opposite is the case for branch (MEF =0.402 vs. MEF =0.692; n=437 vs. n=201) and root (MEF =0.568 vs. MEF =0.862; n=98 vs. n=136) N concentrations when comparing the best models of these different setups (GAMs 1–9 vs. GAMs 10–12) in terms of their MEF. Single variables in general explain relatively small fractions of the variation in tree tissue N concentrations (MEF <0.3), with the exception of leaf type (MEF =0.51), tree height (MEF =0.336), and compartment biomass (MEF =0.368) for leaves; climate variables (MAT and MAP; MEF =0.428) for branches; tree age (MEF =0.366) and height (MEF =0.315) for stems; and climate variables (MEF =0.352) and soil N concentration (MEF =0.552) for roots. Note that comparisons of the individual GAMs have to be interpreted with care due to differences in the available number of measurements for each explanatory variable. Accordingly, the different GAMs rely on different sample sizes.

Figure 6Modelling efficiencies (MEFs) of a selection of 11 of the total 17 applied generalised additive models (GAMs) for modelling (a) leaf, (b) branch, (c) stem, and (d) root N concentration using different combinations of explanatory variables. GAMs (1)–(4), (7), and (10)–(15) as described in the Sect. S4 are shown. Numbers in each bar indicate the number of available measurements for each GAM. For values of the MEFs for all the GAMs implemented for each tissue, refer to Tables S1–S4.
At global scale, when incorporating measurements from the entire Northern Hemisphere boreal and temperate forests, we find that tissue N concentration decreases consistently (and in many cases significantly at the 5 % level) with tree age/size in leaves, branches, stems, and roots (in agreement with Hypothesis 1). This relationship is especially evident for relatively young and small trees before it levels out for more mature trees. This finding is in accordance with N concentrations in stem, branch (Bosc et al., 2003), and root (Ceccon et al., 2016) segments of different age observed in individual trees. In contrast to earlier studies at stand scale (Sprugel, 1984; Ranger et al., 1995; Ponette et al., 2001), N concentration decreases with tree age/size in all compartments and not only in stems. Thus, at global scale, reductions in tree age (by forest management or disturbances) would in general result in higher N concentrations in all tissues of boreal and temperate trees. This finding is in line with different mechanisms that can explain the decline in tissue N concentration with tree age/size, including a decline in photosynthetic capacity (Yoder et al., 1994; Steppe et al., 2011), a decreasing share of tissues with high N concentrations (Augusto et al., 2008; Thurner et al., 2019), and a depletion of soil N (Norby et al., 2010).
While BD trees exhibit significantly higher N concentrations than NE trees in all tissues (in agreement with Hypothesis 2), the N concentrations in leaves, branches, and roots (but not stems) of ND trees are significantly higher compared to NE trees. The observed relation between leaf lifespan and tissue N concentration based on our global database confirms earlier results from smaller datasets for leaves (Chapin et al., 1993; Reich et al., 1992; Reich, 2014; Schulze et al., 1994) and structural woody components (Meerts, 2002). Note, however, that Meerts (2002) discussed that his sample size was too low for drawing definite conclusions. The higher tissue N concentrations of BD trees can be explained by a higher proportion of living parenchyma cells in angiosperms compared to gymnosperms (Merrill and Cowling, 1966), but they are also influenced by environmental effects, as evergreen trees often grow in harsher environments with low N availability. Accordingly, the lower N concentration of needle-leaved trees is generally thought to be part of a more nutrient-conserving strategy.
Other studies have rarely covered ND trees, i.e. larch (Larix) species prevalent in boreal forests mainly in Siberia but also in North America and in high alpine regions. In these regions generally characterised by N limitation (Schulze et al., 1995; Beer et al., 2007; Du et al., 2020), Larix species allocate little N to stems but relatively more N to their needles compared to NE trees in order to support photosynthesis of their short-lived foliage. This is likely due to their high N resorption efficiency allowing them to use N resorbed from senescing leaves at the beginning of the next growing season when the soil is still frozen (Prokushkin et al., 2018). In boreal forests in eastern Siberia, climate change may lead to a replacement of Larix by pine (Pinus; Shuman et al., 2011), which may result in decreased levels of N concentration in tree tissues (except in stems) according to our findings. In contrast, in temperate forests in central Europe, spruce (Picea) and Pinus (amongst others) are expected to be replaced by oak (Quercus; Hanewinkel et al., 2013), leading to increased N concentrations in tree tissues. It should be noted that changes in tissue-level N concentrations do not necessarily match trends in the total N stock in vegetation, as the proportion and turnover times of various tissues will also vary as species change.
Moreover, we find that low growth rates or unfavourable climatic conditions (very cold or dry climate) significantly decrease leaf (the latter only in case of ND and NE trees) but not stem N concentration, indicating that growth conditions affect N allocation (in agreement with Hypothesis 3). This finding can be explained by the higher investment of trees into defence mechanisms (Loehle, 1988; Chapin, 1991) or, alternatively, accumulation of N in reserves (Chapin et al., 1990) in the stem under unfavourable growth conditions, whereas trees allocate more N to leaves in order to support higher growth rates under favourable conditions (growth–defence trade-off; Bazzaz et al., 1987; Herms and Mattson, 1992). This result is also in line with observations by Poorter et al. (1990), who demonstrated that fast-growing species allocate relatively more N to their leaves and less N to their stems compared to slow-growing species. However, while their results were based on a greenhouse experiment considering only non-woody herbaceous species, we show here that this relationship is also applicable to boreal and temperate tree species at large spatial scales. Our observation of lower leaf N concentrations of ND and NE trees under very cold temperatures is also in accordance with a decrease in leaf N with decreasing MAT in the high latitudes detected by Reich and Oleksyn (2004). However, while Tang et al. (2018) found that N concentration in leaves, stems, and roots decreases with increasing temperature and precipitation across all ecosystems in China, we find a consistently significant negative correlation only between leaf N concentration of BD trees and MAT and between branch N concentration of NE trees and MAP based on our database integrating over the entire northern boreal and temperate forests. The decrease in leaf N concentration of BD trees with increasing temperature has also been observed by Yin (1993) and discussed by Haxeltine and Prentice (1996) but, according to our results, does not apply to ND and NE trees. As noted above, these trends in N concentrations do not necessarily translate into trends in whole-plant N requirements. In fact, unfavourable conditions decrease overall plant growth, so higher N concentrations do not imply that slow-growing species have higher N requirements than fast-growing ones.
Extrapolating from the relation between unfavourable growth conditions and tree tissue N concentrations that we observe, an increase in MAT caused by climate change may on the one hand reduce the requirement of adaptation to cold stress and also the limitation of growth by low temperatures in boreal regions. This would result in relatively higher allocation of N to leaves than to stems. On the other hand, drier conditions in certain temperate regions will both require intensified defence against drought stress and increase the water limitation of growth, which could lead to opposite effects on N allocation. In turn, water limitation might increase leaf N concentrations to improve photosynthetic capacity when stomatal closure limits CO2 uptake (Wright et al., 2001); however, this mechanism is not reflected in our finding of lower leaf N concentration of ND and NE trees in dry conditions. In addition, changes in the distribution of tree species with diverging growth rates may have important consequences on N allocation to leaves and stems in boreal and temperate forests.
Regarding branch (significant decrease for low growth rates; for NE trees, significant increase under very cold and dry climate) and root (no consistent effect of growth rates; for ND trees, significant decrease under very cold and dry climate) N concentration, growth rates and unfavourable climate show opposite effects or no consistent effects. Interpretability of results for these compartments is hampered, despite our efforts, by the relatively low number of available measurements of branch and (total) root N concentrations, especially under extreme climatic conditions. Disentangling the controls of N allocation to branches and roots under unfavourable growth conditions will require further measurement campaigns.
In addition, we observe an increase in root N concentrations of BD and NE trees (not significant at the 5 % level) and in stem N concentration of NE trees (significant at the 5 % level) with soil N concentration. Although there is also a positive correlation for some other tissues and leaf types, we do not find a consistent significant increase in tissue N with soil N concentration across the boreal and temperate forest regions (contrary to Hypothesis 4). Thus, at such spatial scales and integrating over all common boreal and temperate tree species, we cannot confirm observations from field experiments of increases in N concentrations of all tissues of Populus trees with higher soil N availability (Pregitzer et al., 1995). The N limitation in boreal forests estimated by, for instance, Du et al. (2020) may not be strong enough to be reflected in tissue N concentrations of boreal (and also temperate) trees, except maybe in root N concentrations of BD and NE trees and stem N concentration of NE trees, which indeed seem to be limited by soil N availability. Consequently, increased N deposition (Schwede et al., 2018) may lead to elevated N concentrations in roots and stems of these leaf types but not necessarily other tissues and leaf types in boreal and temperate forests. However, we note that our findings are based on relationships between tree tissue N concentrations and total soil N concentration instead of plant-available soil N. For instance, in permafrost regions, plant-available soil N might be low despite sufficient total soil N concentration levels (Prokushkin et al., 2018). Although plant-available soil N could thus be an important explanatory variable of tree tissue N concentrations, we had to rely on total soil N concentration measurements, since they are more widely available from the studies contained in our database.
The GAMs that considered multiple explanatory variables and their interactions can explain very large fractions of the variation in tree tissue N concentration, strongly improving predictions compared to univariate models. Both plant traits and environmental conditions are important controls of tissue N concentrations (in agreement with Hypothesis 5), with plant traits (leaf type, growth rate, tree age/height/biomass) explaining larger fractions of the variation in leaf and stem but not branch and root N concentrations compared to environmental conditions (MAT, MAP, soil N concentration). These findings support the hypothesis that leaf and stem N concentrations are considerably influenced by plant strategies related to ecological trade-offs (growth–defence trade-off). In contrast, the spatial distributions of branch and (coarse-) root N concentrations at biome scale in boreal and temperate forests seem to be more strongly determined by gradients in climate and soil conditions. Until now, it has not been possible to investigate these relationships for branch, stem, and root N concentrations at biome scale. The current theory on the global relationships between plant traits and environmental conditions (e.g. Bruelheide et al., 2018; Joswig et al., 2022; Maynard et al., 2022) is based on plant traits which have been more extensively available.
Based on limited data on measurement time and needle age, we do not detect significantly lower leaf N concentrations outside the summer season or with increasing needle age. To further improve the robustness of the results of this study, additional efforts in future field measurement campaigns are required, including
-
additional measurements of N concentration in currently underrepresented regions (high latitudes except Scandinavia and Mediterranean regions) and PFTs (broadleaf evergreen trees);
-
more simultaneous measurements of N concentration in different tree tissues and in general more measurements of underrepresented tissues (branches, roots);
-
more simultaneous measurements of explanatory variables, especially of tree age, height, and biomass and soil N concentration but also simultaneous measurements of actual tree growth rates at the specific sites; of plant-available soil N; of other nutrients; or of different plant nutrient-acquisition strategies, for instance, by different types of mycorrhizal fungi (e.g. Thurner et al., 2024);
-
improved coverage of other potential confounding factors (e.g. season; Vose and Ryan, 2002; Damesin, 2003), including differences between green and senesced plant material, for instance, due to N resorption and translocation from senescing leaves (e.g. Vergutz et al., 2012); variation within tree stems (e.g. Pruyn et al., 2005; Merrill and Cowling, 1966; Schowalter and Morrell, 2002), between branch and root orders (e.g. Mei et al., 2015; Liu et al., 2016), across canopy height (Meir et al., 2002), with leaf age (e.g. Oren et al., 1988), and across soil horizons (e.g. Oren et al., 1988); and N deposition (e.g. Magill et al., 1997)); and
-
more standardised measurement procedures (e.g. concerning sampling of tree tissues).
Our findings have important implications for the coupling of the C and N cycles in vegetation. For instance, changes in climate are expected to lead to the expansion of tree species better adapted to dry conditions in large parts of European temperate forests (e.g. Quercus species; Hanewinkel et al., 2013), which replace (amongst others) NE with BD trees, exhibit relatively low growth rates, are initially of younger age, and meet soil conditions affected by increased N deposition (Schwede et al., 2018). In this example, as a result of these species shifts, we would expect a higher N concentration in all tree tissues and elevated N allocation fractions to stems. An increased leaf N concentration will, in turn, support higher photosynthesis (especially in N-limited ecosystems), but higher tissue N concentrations would also result in higher Rm, and the elevated N allocation fraction to stems might lead to a reduced C use efficiency (CUE; Manzoni et al., 2018) due to elevated stem sapwood Rm (Thurner et al., 2019). However, depending on the interplay of changes in the controls of tree tissue N concentration and other processes, the resulting net effects on N and C cycles remain largely unknown and require further investigation. In particular, our analyses do not cover effects of increasing atmospheric CO2.
The relationships found (except for differences in tissue N concentration between leaf types) are not represented in current DGVMs, which usually assume fixed ratios between leaf, wood, and fine-root N concentrations (Meyerholt and Zaehle, 2015). Unrealistic representations of tissue N concentrations in DGVMs and other carbon cycle models could be quite crucial because future predictions of climate impacts and carbon cycle changes by these models heavily depend on CO2 fertilisation effects and the extent to which they are constrained by N limitation (Hickler et al., 2015; Arora et al., 2020; Kou-Giesbrecht et al., 2023). Not considering the decrease in tissue N concentration with tree age, for example, implies that the effects of forest management and disturbances on the coupling of the C and N cycles cannot be realistically reproduced by DGVMs. Differences in tissue N concentrations between pioneer and late-successional trees could be incorporated by DGVMs that distinguish these growth types, such as LPJ-GUESS (Hickler et al., 2012). Moreover, the difference in the relationship between leaf N concentration and temperature that we observe here between different leaf types reveals a potential shortcoming in current DGVM parameterisations (see Haxeltine and Prentice, 1996). In addition to their critical importance for the improvement of N allocation in DGVMs, the identified relationships, together with available data on tree tissue biomass (Thurner et al., 2014; 2019), will also be the basis for spatially extensive mapping of tissue N concentration and content and highly novel spatial estimates of plant respiration in boreal and temperate forests in future studies.
Here, for the first time, we identified the large-scale abiotic and biotic controls of tree tissue N concentrations based on a novel database of N concentrations in stems, roots, and branches of all common Northern Hemisphere boreal and temperate tree genera that we compiled. In conclusion, our findings emphasise that N concentrations in boreal and temperate trees at large spatial scales consistently decrease with tree age/size and are significantly higher in deciduous compared to evergreen trees in all tissues (leaves, branches, stems, roots) but increase with soil N concentration only in roots of BD and NE trees. Low growth rates or unfavourable climatic conditions are found to decrease leaf (the latter only in the case of ND and NE trees) but not stem N concentration, indicating that growth conditions affect N allocation. Both plant traits and environmental conditions are important controls of tissue N concentrations and together explain very large parts of the variation therein. These relationships have considerable implications for the coupling of the C and N cycles in vegetation, since photosynthesis, growth, and plant respiration, as well as litter decomposition, are closely related to tissue N concentrations. Thus, changes in the distribution of tree age/size, tree species, and extreme climate, induced by climate change, forest management, or disturbances, may have substantial consequences for the C sequestration potential of boreal and temperate forests by their effects on tree tissue N concentrations. The identified relationships are only poorly represented in current DGVMs and need to be incorporated in order to realistically estimate future effects of N limitation on the C cycle.
The “Nitrogen concentrations in boreal and temperate tree tissues” dataset is available from the Zenodo repository (https://doi.org/10.5281/zenodo.14742947; Thurner et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-1475-2025-supplement.
MT designed the study with input from KY, SM, MAT, and TH. MT collated measurements from the literature and compiled the tree tissue N concentration database with contributions from KY, AP, and ZW. AP contributed own measurements. MT analysed the data and wrote most of the article. All authors contributed to the interpretation of results and writing of the article.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Martin Thurner has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Actions (grant no. 891402). Stefano Manzoni acknowledges support from the Swedish Research Council (grant no. 2020-03910) and Formas (grant no. 2021-02121). We sincerely thank the TRY initiative on plant traits (http://www.try-db.org, last access: 24 July 2018) for contributing to leaf N and the Biomass And Allometry Database (BAAD; https://github.com/dfalster/baad, last access: 23 April 2021) for contributing to leaf and stem N concentration data used in this study. The TRY initiative and database is hosted, developed, and maintained by Jens Kattge and Gerhard Boenisch (Max Planck Institute for Biogeochemistry, Jena, Germany). The BAAD is hosted, developed, and maintained by Daniel Falster (University of New South Wales, Sydney, Australia).
This research has been supported by the H2020 Marie Skłodowska-Curie Actions (grant no. 891402), the Vetenskapsrådet (grant no. 2020-03910), and the Svenska Forskningsrådet Formas (grant no. 2021-02121).
This paper was edited by Andreas Ibrom and reviewed by two anonymous referees.
Arora, V. K., Katavouta, A., Williams, R. G., Jones, C. D., Brovkin, V., Friedlingstein, P., Schwinger, J., Bopp, L., Boucher, O., Cadule, P., Chamberlain, M. A., Christian, J. R., Delire, C., Fisher, R. A., Hajima, T., Ilyina, T., Joetzjer, E., Kawamiya, M., Koven, C. D., Krasting, J. P., Law, R. M., Lawrence, D. M., Lenton, A., Lindsay, K., Pongratz, J., Raddatz, T., Séférian, R., Tachiiri, K., Tjiputra, J. F., Wiltshire, A., Wu, T., and Ziehn, T.: Carbon–concentration and carbon–climate feedbacks in CMIP6 models and their comparison to CMIP5 models, Biogeosciences, 17, 4173–4222, https://doi.org/10.5194/bg-17-4173-2020, 2020.
Augusto, L., Meredieu, C., Bert, D., Trichet, P., Porté, A., Bosc, A., Lagane, F., Loustau, D., Pellerin, S., Danjon, F., Ranger, J., and Gelpe, J.: Improving models of forest nutrient export with equations that predict the nutrient concentration of tree compartments, Ann. For. Sci., 65, 808–808, https://doi.org/10.1051/forest:2008059, 2008.
Bazzaz, F. A., Chiariello, N. R., Coley, P. D., and Pitelka, L. F.: Allocating Resources to Reproduction and Defense: New assessments of the costs and benefits of allocation patterns in plants are relating ecological roles to resource use, BioScience, 37, 58–67, https://doi.org/10.2307/1310178, 1987.
Beer, C., Lucht, W., Gerten, D., Thonicke, K., and Schmullius, C.: Effects of soil freezing and thawing on vegetation carbon density in Siberia: A modeling analysis with the Lund-Potsdam-Jena Dynamic Global Vegetation Model (LPJ-DGVM), Global Biogeochem. Cycles, 21, GB1012, https://doi.org/10.1029/2006gb002760, 2007.
Bosc, A., De Grandcourt, A., and Loustau, D.: Variability of stem and branch maintenance respiration in a Pinus pinaster tree, Tree Physiol., 23, 227–236, https://doi.org/10.1093/treephys/23.4.227, 2003.
Bruelheide, H., Dengler, J., Purschke, O., et al.: Global trait–environment relationships of plant communities, Nature Ecology & Evolution, 2, 1906–1917, https://doi.org/10.1038/s41559-018-0699-8, 2018.
Brun, P., Zimmermann, N. E., Hari, C., Pellissier, L., and Karger, D. N.: Global climate-related predictors at kilometer resolution for the past and future, Earth Syst. Sci. Data, 14, 5573–5603, https://doi.org/10.5194/essd-14-5573-2022, 2022.
Burnham, K. P. and Anderson, D. R.: Multimodel Inference: Understanding AIC and BIC in Model Selection, Sociol. Methods Res., 33, 261–304, https://doi.org/10.1177/0049124104268644, 2004.
Butler, E. E., Datta, A., Flores-Moreno, H., Chen, M., Wythers, K. R., Fazayeli, F., Banerjee, A., Atkin, O. K., Kattge, J., Amiaud, B., Blonder, B., Boenisch, G., Bond-Lamberty, B., Brown, K. A., Byun, C., Campetella, G., Cerabolini, B. E. L., Cornelissen, J. H. C., Craine, J. M., Craven, D., de Vries, F. T., Diaz, S., Domingues, T. F., Forey, E., Gonzalez-Melo, A., Gross, N., Han, W., Hattingh, W. N., Hickler, T., Jansen, S., Kramer, K., Kraft, N. J. B., Kurokawa, H., Laughlin, D. C., Meir, P., Minden, V., Niinemets, U., Onoda, Y., Penuelas, J., Read, Q., Sack, L., Schamp, B., Soudzilovskaia, N. A., Spasojevic, M. J., Sosinski, E., Thornton, P. E., Valladares, F., van Bodegom, P. M., Williams, M., Wirth, C., and Reich, P. B.: Mapping local and global variability in plant trait distributions, P. Natl. Acad. Sci. USA, 114, E10937–E10946, https://doi.org/10.1073/pnas.1708984114, 2017.
Ceccon, C., Tagliavini, M., Schmitt, A. O., and Eissenstat, D. M.: Untangling the effects of root age and tissue nitrogen on root respiration in Populus tremuloides at different nitrogen supply, Tree Physiol., 36, 618–627, https://doi.org/10.1093/treephys/tpw022, 2016.
Ceschia, E., Damesin, C., Lebaube, S., Pontailler, J.-Y., and Dufrene, E.: Spatial and seasonal variations in stem respiration of beech trees (Fagus sylvatica), Ann. For. Sci., 59, 801–812, https://doi.org/10.1051/forest:2002078, 2002.
Chapin, F. S.: Integrated Responses of Plants to Stress: A centralized system of physiological responses, BioScience, 41, 29–36, https://doi.org/10.2307/1311538, 1991.
Chapin, F. S., Schulze, E.-D., and Mooney, H. A.: The Ecology and Economics of Storage in Plants, Annu. Rev. Ecol. Syst., 21, 423–447, 1990.
Chapin, F. S., Autumn, K., and Pugnaire, F.: Evolution of Suites of Traits in Response to Environmental Stress, Am. Nat., 142, S78–S92, https://doi.org/10.1086/285524, 1993.
Damesin, C.: Respiration and photosynthesis characteristics of current-year stems of Fagus sylvatica: from the seasonal pattern to an annual balance, New Phytol., 158, 465–475, https://doi.org/10.1046/j.1469-8137.2003.00756.x, 2003.
Dong, N., Prentice, I. C., Wright, I. J., Wang, H., Atkin, O. K., Bloomfield, K. J., Domingues, T. F., Gleason, S. M., Maire, V., Onoda, Y., Poorter, H., and Smith, N. G.: Leaf nitrogen from the perspective of optimal plant function, J. Ecol., 110, 2585–2602, https://doi.org/10.1111/1365-2745.13967, 2022.
Du, E., Terrer, C., Pellegrini, A. F. A., Ahlström, A., van Lissa, C. J., Zhao, X., Xia, N., Wu, X., and Jackson, R. B.: Global patterns of terrestrial nitrogen and phosphorus limitation, Nat. Geosci., 13, 221–226, https://doi.org/10.1038/s41561-019-0530-4, 2020.
Fagerland, M. W.: t-tests, non-parametric tests, and large studies – a paradox of statistical practice?, BMC Medical Research Methodology, 12, 78, https://doi.org/10.1186/1471-2288-12-78, 2012.
Falster, D. S., Duursma, R. A., Ishihara, M. I., et al.: BAAD: a biomass and allometry database for woody plants, Ecology, 96, 1445, https://doi.org/10.1890/14-1889.1, 2015.
Feng, Z., Brumme, R., Xu, Y. J., and Lamersdorf, N.: Tracing the fate of mineral N compounds under high ambient N deposition in a Norway spruce forest at Solling/Germany, Forest Ecol. Manag., 255, 2061–2073, https://doi.org/10.1016/j.foreco.2007.12.049, 2008.
Friedlingstein, P., O'Sullivan, M., Jones, M. W., et al.: Global Carbon Budget 2023, Earth Syst. Sci. Data, 15, 5301–5369, https://doi.org/10.5194/essd-15-5301-2023, 2023.
Hanewinkel, M., Cullmann, D. A., Schelhaas, M.-J., Nabuurs, G.-J., and Zimmermann, N. E.: Climate change may cause severe loss in the economic value of European forest land, Nat. Clim. Change, 3, 203–207, https://doi.org/10.1038/nclimate1687, 2013.
Hastie, T. J. and Tibshirani, R. J.: Generalized additive models, Chapman & Hall/CRC, Boca Raton, FL, 1990.
Haxeltine, A. and Prentice, I. C.: A General Model for the Light-Use Efficiency of Primary Production, Funct. Ecol., 10, 551–561, https://doi.org/10.2307/2390165, 1996.
Hendricks, J. J., Aber, J. D., Nadelhoffer, K. J., and Hallett, R. D.: Nitrogen Controls on Fine Root Substrate Quality in Temperate Forest Ecosystems, Ecosystems, 3, 57–69, https://doi.org/10.1007/s100210000010, 2000.
Herms, D. A. and Mattson, W. J.: The Dilemma of Plants: To Grow or Defend, The Quarterly Review of Biology, 67, 283–335, 1992.
Hickler, T., Rammig, A., and Werner, C.: Modelling CO2 Impacts on Forest Productivity, Current Forestry Reports, 1, 69–80, https://doi.org/10.1007/s40725-015-0014-8, 2015.
Hickler, T., Vohland, K., Feehan, J., Miller, P. A., Smith, B., Costa, L., Giesecke, T., Fronzek, S., Carter, T. R., Cramer, W., Kühn, I., and Sykes, M. T.: Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model, Global Ecol. Biogeogr., 21, 50-63, https://doi.org/10.1111/j.1466-8238.2010.00613.x, 2012.
Iversen, C. M., McCormack, M. L., Powell, A. S., Blackwood, C. B., Freschet, G. T., Kattge, J., Roumet, C., Stover, D. B., Soudzilovskaia, N. A., Valverde-Barrantes, O. J., van Bodegom, P. M., and Violle, C.: A global Fine-Root Ecology Database to address below-ground challenges in plant ecology, New Phytol., 215, 15–26, https://doi.org/10.1111/nph.14486, 2017.
Joswig, J. S., Wirth, C., Schuman, M. C., Kattge, J., Reu, B., Wright, I. J., Sippel, S. D., Rüger, N., Richter, R., Schaepman, M. E., van Bodegom, P. M., Cornelissen, J. H. C., Díaz, S., Hattingh, W. N., Kramer, K., Lens, F., Niinemets, Ü., Reich, P. B., Reichstein, M., Römermann, C., Schrodt, F., Anand, M., Bahn, M., Byun, C., Campetella, G., Cerabolini, B. E. L., Craine, J. M., Gonzalez-Melo, A., Gutiérrez, A. G., He, T., Higuchi, P., Jactel, H., Kraft, N. J. B., Minden, V., Onipchenko, V., Peñuelas, J., Pillar, V. D., Sosinski, Ê., Soudzilovskaia, N. A., Weiher, E., and Mahecha, M. D.: Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation, Nat. Ecol. Evol., 6, 36–50, https://doi.org/10.1038/s41559-021-01616-8, 2022.
Kattge, J., Bönisch, G., Diaz, S., et al.: TRY plant trait database - enhanced coverage, open access, Glob. Chang. Biol., 26, 119–188, https://doi.org/10.1111/gcb.14904, 2020.
Kou-Giesbrecht, S., Arora, V. K., Seiler, C., Arneth, A., Falk, S., Jain, A. K., Joos, F., Kennedy, D., Knauer, J., Sitch, S., O'Sullivan, M., Pan, N., Sun, Q., Tian, H., Vuichard, N., and Zaehle, S.: Evaluating nitrogen cycling in terrestrial biosphere models: a disconnect between the carbon and nitrogen cycles, Earth Syst. Dynam., 14, 767–795, https://doi.org/10.5194/esd-14-767-2023, 2023.
Lambers, H. and Poorter, H.: Inherent Variation in Growth Rate Between Higher Plants: A Search for Physiological Causes and Ecological Consequences, Adv. Ecol. Res., 23, 187–261, https://doi.org/10.1016/s0065-2504(08)60148-8, 1992.
Larjavaara, M., Berninger, F., Palviainen, M., Prokushkin, A., and Wallenius, T.: Post-fire carbon and nitrogen accumulation and succession in Central Siberia, Sci. Rep., 7, 12776, https://doi.org/10.1038/s41598-017-13039-2, 2017.
Laughlin, D. C., Fulé, P. Z., Huffman, D. W., Crouse, J., and Laliberté, E.: Climatic constraints on trait-based forest assembly, J. Ecol., 99, 1489–1499, https://doi.org/10.1111/j.1365-2745.2011.01885.x, 2011.
LeBauer, D. S. and Treseder, K. K.: Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed, Ecology, 89, 371–379, https://doi.org/10.1890/06-2057.1, 2008.
Liu, B., He, J., Zeng, F., Lei, J., and Arndt, S. K.: Life span and structure of ephemeral root modules of different functional groups from a desert system, New Phytol., 211, 103–112, https://doi.org/10.1111/nph.13880, 2016.
Loehle, C.: Tree life history strategies: the role of defenses, Can. J. Forest Res., 18, 209–222, https://doi.org/10.1139/x88-032, 1988.
Luo, Y., Su, B., Currie, W. S., Dukes, J. S., Finzi, A., Hartwig, U., Hungate, B., McMurtrie, R. E., Oren, R., Parton, W. J., Pataki, D. E., Shaw, R. M., Zak, D. R., and Field, C. B.: Progressive Nitrogen Limitation of Ecosystem Responses to Rising Atmospheric Carbon Dioxide, BioScience, 54, 731–739, https://doi.org/10.1641/0006-3568(2004)054[0731:Pnloer]2.0.Co;2, 2004.
Magill, A. H., Aber, J. D., Hendricks, J. J., Bowden, R. D., Melillo, J. M., and Steudler, P. A.: Biogeochemical Response of Forest Ecosystems to Simulated Chronic Nitrogen Deposition, Ecol. Appl., 7, 402–415, https://doi.org/10.1890/1051-0761(1997)007[0402:BROFET]2.0.CO;2, 1997.
Manzoni, S., Čapek, P., Porada, P., Thurner, M., Winterdahl, M., Beer, C., Brüchert, V., Frouz, J., Herrmann, A. M., Lindahl, B. D., Lyon, S. W., Šantrůčková, H., Vico, G., and Way, D.: Reviews and syntheses: Carbon use efficiency from organisms to ecosystems – definitions, theories, and empirical evidence, Biogeosciences, 15, 5929–5949, https://doi.org/10.5194/bg-15-5929-2018, 2018.
Martin, A. R., Gezahegn, S., and Thomas, S. C.: Variation in carbon and nitrogen concentration among major woody tissue types in temperate trees, Can. J. Forest Res., 45, 744–757, https://doi.org/10.1139/cjfr-2015-0024, 2015.
Maynard, D. S., Bialic-Murphy, L., Zohner, C. M., Averill, C., van den Hoogen, J., Ma, H., Mo, L., Smith, G. R., Acosta, A. T. R., Aubin, I., Berenguer, E., Boonman, C. C. F., Catford, J. A., Cerabolini, B. E. L., Dias, A. S., González-Melo, A., Hietz, P., Lusk, C. H., Mori, A. S., Niinemets, Ü., Pillar, V. D., Pinho, B. X., Rosell, J. A., Schurr, F. M., Sheremetev, S. N., da Silva, A. C., Sosinski, Ê., van Bodegom, P. M., Weiher, E., Bönisch, G., Kattge, J., and Crowther, T. W.: Global relationships in tree functional traits, Nat. Commun., 13, 3185, https://doi.org/10.1038/s41467-022-30888-2, 2022.
Meerts, P.: Mineral nutrient concentrations in sapwood and heartwood: a literature review, Ann. For. Sci., 59, 713–722, https://doi.org/10.1051/forest:2002059, 2002.
Mei, L., Xiong, Y., Gu, J., Wang, Z., and Guo, D.: Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees, Oecologia, 177, 333–344, https://doi.org/10.1007/s00442-014-3186-1, 2015.
Meir, P., Kruijt, B., Broadmeadow, M., Barbosa, E., Kull, O., Carswell, F., Nobre, A., and Jarvis, P. G.: Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area, Plant Cell Environ., 25, 343–357, https://doi.org/10.1046/j.0016-8025.2001.00811.x, 2002.
Merrill, W. and Cowling, E. B.: Role of nitrogen in wood deterioration: amounts and distribution of nitrogen in tree stems, Can. J. Bot., 44, 1555–1580, https://doi.org/10.1139/b66-168, 1966.
Meyerholt, J. and Zaehle, S.: The role of stoichiometric flexibility in modelling forest ecosystem responses to nitrogen fertilization, New Phytol., 208, 1042–1055, https://doi.org/10.1111/nph.13547, 2015.
Moreno-Martínez, Á., Camps-Valls, G., Kattge, J., Robinson, N., Reichstein, M., van Bodegom, P., Kramer, K., Cornelissen, J. H. C., Reich, P., Bahn, M., Niinemets, Ü., Peñuelas, J., Craine, J. M., Cerabolini, B. E. L., Minden, V., Laughlin, D. C., Sack, L., Allred, B., Baraloto, C., Byun, C., Soudzilovskaia, N. A., and Running, S. W.: A methodology to derive global maps of leaf traits using remote sensing and climate data, Remote Sens. Environ., 218, 69–88, https://doi.org/10.1016/j.rse.2018.09.006, 2018.
Nash, J. E. and Sutcliffe, J. V.: River flow forecasting through conceptual models part I – a discussion of principles, J. Hydrol., 10, 282–290, 1970.
Norby, R. J., Warren, J. M., Iversen, C. M., Medlyn, B. E., and McMurtrie, R. E.: CO2 enhancement of forest productivity constrained by limited nitrogen availability, P. Natl. Acad. Sci. USA, 107, 19368–19373, https://doi.org/10.1073/pnas.1006463107, 2010.
Oren, R., Werk, K. S., Schulze, E. D., Meyer, J., Schneider, B. U., and Schramel, P.: Performance of Two Picea abies (L.) Karst. Stands at Different Stages of Decline. VI. Nutrient Concentration, Oecologia, 77, 151–162, 1988.
Ostonen, I., Truu, M., Helmisaari, H. S., Lukac, M., Borken, W., Vanguelova, E., Godbold, D. L., Lohmus, K., Zang, U., Tedersoo, L., Preem, J. K., Rosenvald, K., Aosaar, J., Armolaitis, K., Frey, J., Kabral, N., Kukumagi, M., Leppalammi-Kujansuu, J., Lindroos, A. J., Merila, P., Napa, U., Nojd, P., Parts, K., Uri, V., Varik, M., and Truu, J.: Adaptive root foraging strategies along a boreal-temperate forest gradient, New Phytol., 215, 977–991, https://doi.org/10.1111/nph.14643, 2017.
Parton, W., Silver, W. L., Burke, I. C., Grassens, L., Harmon, M. E., Currie, W. S., King, J. Y., Adair, E. C., Brandt, L. A., Hart, S. C., and Fasth, B.: Global-scale similarities in nitrogen release patterns during long-term decomposition, Science, 315, 361–364, https://doi.org/10.1126/science.1134853, 2007.
Ponette, Q., Ranger, J., Ottorini, J.-M., and Ulrich, E.: Aboveground biomass and nutrient content of five Douglas-fir stands in France, Forest Ecol. Manag., 142, 109–127, https://doi.org/10.1016/S0378-1127(00)00345-5, 2001.
Poorter, H., Remkes, C., and Lambers, H.: Carbon and Nitrogen Economy of 24 Wild Species Differing in Relative Growth Rate, Plant Physiol., 94, 621–627, https://doi.org/10.1104/pp.94.2.621, 1990.
Pregitzer, K. S., Zak, D. R., Curtis, P. S., Kubiske, M. E., Teeri, J. A., and Vogel, C. S.: Atmospheric CO2, soil nitrogen and turnover of fine roots, New Phytol., 129, 579–585, https://doi.org/10.1111/j.1469-8137.1995.tb03025.x, 1995.
Prokushkin, A., Hagedorn, F., Pokrovsky, O., Viers, J., Kirdyanov, A., Masyagina, O., Prokushkina, M., and McDowell, W.: Permafrost Regime Affects the Nutritional Status and Productivity of Larches in Central Siberia, Forests, 9, 314, https://doi.org/10.3390/f9060314, 2018.
Pruyn, M. L., Gartner, B. L., and Harmon, M. E.: Storage versus substrate limitation to bole respiratory potential in two coniferous tree species of contrasting sapwood width, J. Exp. Bot., 56, 2637–2649, https://doi.org/10.1093/jxb/eri257, 2005.
Ranger, J., Marques, R., Colin-Belgrand, M., Flammang, N., and Gelhaye, D.: The dynamics of biomass and nutrient accumulation in a Douglas-fir (Pseudotsuga menziesii Franco) stand studied using a chronosequence approach, Forest Ecol. Manag., 72, 167–183, https://doi.org/10.1016/0378-1127(94)03469-D, 1995.
Reich, P. B.: The world-wide “fast-slow” plant economics spectrum: a traits manifesto, J. Ecol., 102, 275–301, https://doi.org/10.1111/1365-2745.12211, 2014.
Reich, P. B. and Oleksyn, J.: Global patterns of plant leaf N and P in relation to temperature and latitude, P. Natl. Acad. Sci. USA, 101, 11001–11006, https://doi.org/10.1073/pnas.0403588101, 2004.
Reich, P. B., Walters, M. B., and Ellsworth, D. S.: Leaf Life-Span in Relation to Leaf, Plant, and Stand Characteristics among Diverse Ecosystems, Ecol. Monogr., 62, 365–392, https://doi.org/10.2307/2937116, 1992.
Reich, P. B., Tjoelker, M. G., Machado, J. L., and Oleksyn, J.: Universal scaling of respiratory metabolism, size and nitrogen in plants, Nature, 439, 457–461, https://doi.org/10.1038/nature04282, 2006b.
Reich, P. B., Hobbie, S. E., Lee, T., Ellsworth, D. S., West, J. B., Tilman, D., Knops, J. M., Naeem, S., and Trost, J.: Nitrogen limitation constrains sustainability of ecosystem response to CO2, Nature, 440, 922–925, https://doi.org/10.1038/nature04486, 2006a.
Ryan, M. G.: Effects of Climate Change on Plant Respiration, Ecol. Appl., 1, 157–167, https://doi.org/10.2307/1941808, 1991.
Schepaschenko, D., Shvidenko, A., Usoltsev, V., Lakyda, P., Luo, Y., Vasylyshyn, R., Lakyda, I., Myklush, Y., See, L., McCallum, I., Fritz, S., Kraxner, F., and Obersteiner, M.: A dataset of forest biomass structure for Eurasia, Sci. Data, 4, 170070, https://doi.org/10.1038/sdata.2017.70, 2017.
Schowalter, T. D. and Morrell, J. J.: Nutritional Quality of Douglas-Fir Wood: Effect of Vertical and Horizontal Position on Nutrient Levels, Wood and Fiber Science, 34, 158–164, 2002.
Schulze, E.-D., Kelliher, F. M., Körner, C., Lloyd, J., and Leuning, R.: Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: a global ecology scaling exercise, Annu. Rev. Ecol. Syst., 25, 629–662, https://doi.org/10.1146/annurev.es.25.110194.003213, 1994.
Schulze, E. D., Schulze, W., Koch, H., Arneth, A., Bauer, G., Kelliher, F. M., Hollinger, D. Y., Vygodskaya, N. N., Kusnetsova, W. A., Sogatchev, A., Ziegler, W., Kobak, K. I., and Issajev, A.: Aboveground biomass and nitrogen nutrition in a chronosequence of pristine Dahurian Larix stands in eastern Siberia, Can. J. Forest Res., 25, 943–960, https://doi.org/10.1139/x95-103, 1995.
Schwede, D. B., Simpson, D., Tan, J., Fu, J. S., Dentener, F., Du, E., and deVries, W.: Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale, Environ. Pollut., 243, 1287–1301, https://doi.org/10.1016/j.envpol.2018.09.084, 2018.
Shuman, J. K., Shugart, H. H., and O'Halloran, T. L.: Sensitivity of Siberian larch forests to climate change, Global Change Biol., 17, 2370–2384, https://doi.org/10.1111/j.1365-2486.2011.02417.x, 2011.
Sitch, S., Smith, B., Prentice, I. C., Arneth, A., Bondeau, A., Cramer, W., Kaplan, J. O., Levis, S., Lucht, W., Sykes, M. T., Thonicke, K., and Venevsky, S.: Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model, Global Change Biol., 9, 161–185, 2003.
Sprugel, D. G.: Density, Biomass, Productivity, and Nutrient-Cycling Changes During Stand Development in Wave-Regenerated Balsam Fir Forests, Ecol. Monogr., 54, 165–186, https://doi.org/10.2307/1942660, 1984.
Steppe, K., Niinemets, Ü., and Teskey, R. O.: Tree Size- and Age-Related Changes in Leaf Physiology and Their Influence on Carbon Gain, in: Size- and Age-Related Changes in Tree Structure and Function, Tree Physiol., 235–253, https://doi.org/10.1007/978-94-007-1242-3_9, 2011.
Sun, Y., Wang, M., Mur, L. A. J., Shen, Q., and Guo, S.: Unravelling the Roles of Nitrogen Nutrition in Plant Disease Defences, Int. J. Mol. Sci., 21, 572, https://doi.org/10.3390/ijms21020572, 2020.
Tang, Z., Xu, W., Zhou, G., Bai, Y., Li, J., Tang, X., Chen, D., Liu, Q., Ma, W., Xiong, G., He, H., He, N., Guo, Y., Guo, Q., Zhu, J., Han, W., Hu, H., Fang, J., and Xie, Z.: Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China's terrestrial ecosystems, P. Natl. Acad. Sci. USA, 115, 4033–4038, https://doi.org/10.1073/pnas.1700295114, 2018.
Tateno, R. and Takeda, H.: Nitrogen uptake and nitrogen use efficiency above and below ground along a topographic gradient of soil nitrogen availability, Oecologia, 163, 793–804, https://doi.org/10.1007/s00442-009-1561-0, 2010.
Terrer, C., Jackson, R. B., Prentice, I. C., Keenan, T. F., Kaiser, C., Vicca, S., Fisher, J. B., Reich, P. B., Stocker, B. D., Hungate, B. A., Peñuelas, J., McCallum, I., Soudzilovskaia, N. A., Cernusak, L. A., Talhelm, A. F., Van Sundert, K., Piao, S., Newton, P. C. D., Hovenden, M. J., Blumenthal, D. M., Liu, Y. Y., Müller, C., Winter, K., Field, C. B., Viechtbauer, W., Van Lissa, C. J., Hoosbeek, M. R., Watanabe, M., Koike, T., Leshyk, V. O., Polley, H. W., and Franklin, O.: Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass, Nat. Clim. Change, 9, 684–689, https://doi.org/10.1038/s41558-019-0545-2, 2019.
Thurner, M., Beer, C., Santoro, M., Carvalhais, N., Wutzler, T., Schepaschenko, D., Shvidenko, A., Kompter, E., Ahrens, B., Levick, S. R., and Schmullius, C.: Carbon stock and density of northern boreal and temperate forests, Global Ecol. Biogeogr., 23, 297–310, https://doi.org/10.1111/geb.12125, 2014.
Thurner, M., Beer, C., Crowther, T., Falster, D., Manzoni, S., Prokushkin, A., Schulze, E. D., and Gillespie, T.: Sapwood biomass carbon in northern boreal and temperate forests, Global Ecol. Biogeogr., 28, 640–660, https://doi.org/10.1111/geb.12883, 2019.
Thurner, M., Yu, K., Manzoni, S., Prokushkin, A., Thurner, M. A., Wang, Z., and Hickler, T.: Nitrogen concentrations in boreal and temperate tree tissues (1.0.0), Zenodo [data set], https://doi.org/10.5281/zenodo.14742947, 2025.
Thurner, M. A., Caldararu, S., Engel, J., Rammig, A., and Zaehle, S.: Modelled forest ecosystem carbon–nitrogen dynamics with integrated mycorrhizal processes under elevated CO2, Biogeosciences, 21, 1391–1410, https://doi.org/10.5194/bg-21-1391-2024, 2024.
Ullmann-Zeunert, L., Stanton, M. A., Wielsch, N., Bartram, S., Hummert, C., Svatos, A., Baldwin, I. T., and Groten, K.: Quantification of growth-defense trade-offs in a common currency: nitrogen required for phenolamide biosynthesis is not derived from ribulose-1,5-bisphosphate carboxylase/oxygenase turnover, Plant J., 75, 417–429, https://doi.org/10.1111/tpj.12210, 2013.
UNEP: World Atlas of Desertification. United Nations Environment Programme, edited by: Middleton, N. and Thomas D. S. G., Edward Arnold, London, 1992.
Vergutz, L., Manzoni, S., Porporato, A., Novais, R. F., and Jackson, R. B.: Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants, Ecol. Monogr., 82, 205–220, https://doi.org/10.1890/11-0416.1, 2012.
Vose, J. M. and Ryan, M. G.: Seasonal respiration of foliage, fine roots, and woody tissues in relation to growth, tissue N, and photosynthesis, Global Change Biol., 8, 182–193, https://doi.org/10.1046/j.1365-2486.2002.00464.x, 2002.
Wang, Z., Yu, K., Lv, S., Niklas, K. J., Mipam, T. D., Crowther, T. W., Umaña, M. N., Zhao, Q., Huang, H., Reich, P. B., and Niu, S.: The scaling of fine root nitrogen versus phosphorus in terrestrial plants: A global synthesis, Funct. Ecol., 33, 2081–2094, https://doi.org/10.1111/1365-2435.13434, 2019.
Wang, Z., Lv, S., Song, H., Wang, M., Zhao, Q., Huang, H., and Niklas, K. J.: Plant type dominates fine-root stoichiometry across China: A meta-analysis, J. Biogeogr., 47, 1019–1029, https://doi.org/10.1111/jbi.13791, 2020.
Wang, Z., Huang, H., Yao, B., Deng, J., Ma, Z., and Niklas, K. J.: Divergent scaling of fine-root nitrogen and phosphorus in different root diameters, orders and functional categories: A meta-analysis, Forest Ecol. Manag., 495, 119384, https://doi.org/10.1016/j.foreco.2021.119384, 2021.
Withington, J. M., Reich, P. B., Oleksyn, J., and Eissenstat, D. M.: Comparisons of Structure and Life Span in Roots and Leaves among Temperate Trees, Ecol. Monogr., 76, 381–397, 2006.
Wood, S. N.: Generalized additive models: an introduction with R, Chapman & Hall/CRC Press, Boca Raton, FL, 2006.
Wright, I. J., Reich, P. B., and Westoby, M.: Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats, Funct. Ecol., 15, 423–434, https://doi.org/10.1046/j.0269-8463.2001.00542.x, 2001.
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., Cavender-Bares, J., Chapin, T., Cornelissen, J. H. C., Diemer, M., Flexas, J., Garnier, E., Groom, P. K., Gulias, J., Hikosaka, K., Lamont, B. B., Lee, T., Lee, W., Lusk, C., Midgley, J. J., Navas, M.-L., Niinemets, Ü., Oleksyn, J., Osada, N., Poorter, H., Poot, P., Prior, L., Pyankov, V. I., Roumet, C., Thomas, S. C., Tjoelker, M. G., Veneklaas, E. J., and Villar, R.: The worldwide leaf economics spectrum, Nature, 428, 821–827, https://doi.org/10.1038/nature02403, 2004.
Yin, X.: Variation in foliar nitrogen concentration by forest type and climatic gradients in North America, Can. J. Forest Res., 23, 1587–1602, https://doi.org/10.1139/x93-199, 1993.
Yoder, B. J., Ryan, M. G., Waring, R. H., Schoettle, A. W., and Kaufmann, M. R.: Evidence of Reduced Photosynthetic Rates in Old Trees, Forest Sci., 40, 513–527, https://doi.org/10.1093/forestscience/40.3.513, 1994.
Yuan, Z. Y., Chen, H. Y. H., and Reich, P. B.: Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus, Nat. Commun., 2, 344, https://doi.org/10.1038/ncomms1346, 2011.