the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Elephant megacarcasses increase local nutrient pools in African savanna soils and plants
Michelle L. Budny
Johan T. du Toit
Ryan Helcoski
Joshua P. Schimel
Izak P. J. Smit
Tercia Strydom
Aimee Tallian
Dave I. Thompson
Helga van Coller
Nathan P. Lemoine
African elephants (Loxodonta africana) are the largest extant terrestrial mammals, with bodies containing enormous quantities of nutrients. Yet, we know little about how these nutrients move through the ecosystem after an elephant dies. Here, we investigated the initial effects (1–26 months postmortem) of elephant megacarcasses on savanna soil and plant nutrient pools in the Kruger National Park, South Africa. We hypothesized that (H1) elephant megacarcass decomposition would release nutrients into soil, resulting in higher concentrations of soil nitrogen (N), phosphorus (P), and micronutrients near the center of carcass sites; (H2) carbon (C) inputs into the soil would stimulate microbial activity, resulting in increased soil respiration potential near the center of carcass sites; and (H3) carcass-derived nutrients would be absorbed by plants, resulting in higher foliar nutrient concentrations near the center of carcass sites. To test our hypotheses, we identified 10 elephant carcass sites split evenly between nutrient-poor granitic and nutrient-rich basaltic soils. At each site, we ran transects in the four cardinal directions from the center of the carcass site, collecting soil and grass (Urochloa trichopus, formerly U. mosambicensis) samples at 0, 2.5, 5, 10, and 15 m. We then analyzed samples for C, N, P, and micronutrient concentrations and quantified soil microbial respiration potential. We found that concentrations of soil nitrate, ammonium, δ15N, phosphate, and sodium were elevated closer to the center of carcass sites (H1). Microbial respiration potentials were positively correlated with soil organic C, and both respiration and organic C decreased with distance from the carcass (H2). Finally, we found evidence that plants were readily absorbing carcass-derived nutrients from the soil, with foliar %N, δ15N, iron, potassium, magnesium, and sodium significantly elevated closer to the center of carcass sites (H3). Together, these results indicate that elephant megacarcasses release ecologically consequential pulses of nutrients into the soil which stimulate soil microbial activity and are absorbed by plants into the above-ground nutrient pools. These localized nutrient pulses may drive spatiotemporal heterogeneity in plant diversity, herbivore behavior, and ecosystem processes.
- Article
(3846 KB) - Full-text XML
-
Supplement
(2329 KB) - BibTeX
- EndNote
Living animals affect nutrient flows through ecosystems (Schmitz et al., 2018), but we have only recently acknowledged that the nutrients from animal carcasses could also influence ecosystem processes (Barton et al., 2013; Monk et al., 2024). In marine ecosystems, whale carcasses function as unique hotspots of nutrient cycling, biodiversity, and ecosystem processes (Roman et al., 2014). In terrestrial systems, mass mortality events (e.g., wildebeest, cicadas) create nutrient hotspots (Yang, 2004; Subalusky et al., 2020), while individual small- and medium-sized carcasses release pulses of nutrients into the soil (Towne, 2000; Barton et al., 2016; Olea et al., 2019). Yet we lack knowledge about the role of terrestrial megacarcasses (carcasses of animals such as elephants and rhinoceroses that are >1000 kg at death) as potential drivers of spatiotemporal heterogeneity in nutrient cycling and ecosystem processes. Importantly, these megacarcasses may be functionally different from smaller carcasses due to the extraordinarily high concentration of nutrients and the residence time of the decomposing animal (see reviews by Barton et al., 2013; Barton, 2016; Barton and Bump, 2019). This question around the role of megacarcasses is particularly relevant given the megaherbivore losses that occurred during the Pleistocene extinctions and that are still occurring today (Ripple et al., 2015). We are only beginning to understand how the “extinction aftershock” of losing the largest species impacts ecosystems (Owen-Smith, 1989; Flannery, 1990), and no study has yet investigated how the loss of megacarcasses might influence the dynamics of terrestrial ecosystems (Doughty et al., 2013; Doughty et al., 2016).
We can only evaluate the importance of terrestrial megacarcasses for nutrient cycling in ecosystems where megaherbivores still exist, such as African savannas. The African savanna elephant (Loxodonta africana) is the largest extant land animal and is known for its key ecological effects on savannas while alive (e.g., dispersing seeds, creating plant refuges, preventing woody encroachment) (Skarpe et al., 2004; Asner et al., 2009; Campos-Arceiz and Blake, 2011; Coverdale et al., 2016; Guy et al., 2021). Yet, the elephant's large body mass may mean that it also has an outsized impact on these ecosystems even after death. A 4000 kg elephant megacarcass likely represents ∼ 2000 kg carbon (C), ∼ 300 kg nitrogen (N), and ∼ 125 kg phosphorus (P) deposited in the savanna landscape (estimated from stoichiometry of elephants and other mammals in Sterner and Elser, 2002). The N deposition from one elephant megacarcass (in a 700 m2 impact zone, assuming a 15 m disturbance radius) is roughly equivalent to the N delivered to 10 000 m2 of savanna from ∼ 100 years of atmospheric deposition (Mphepya et al., 2006).
If megacarcasses provide large nutrient pulses then they likely create hotspots of important below- and above-ground processes. Below-ground, soil respiration and organic matter decomposition might increase with nutrient inputs from carcasses (Risch et al., 2020). Concentrations of C, N, P, and potassium (K) are often elevated near carcasses of medium-sized animals (e.g., bison, moose, kangaroo, vicuña) (Towne, 2000; Bump et al., 2009a; Macdonald et al., 2014; Risch et al., 2020; Monk et al., 2024), and nutrients such as P and calcium (Ca) continue leaching from bones even after soft tissues have been consumed or degraded (Coe, 1978; Keenan and Beeler, 2023). Above-ground, plant growth in African savannas is limited by nutrient availability, most commonly of N and P (Ries and Shugart, 2008; Pellegrini, 2016), and micronutrients such as sodium (Na) and K may co-limit plant productivity as well (Epron et al., 2012; Chen et al., 2024). Thus, the large influx of nutrients released from megacarcasses might increase the mobilization of nutrients by plants, potentially increasing nutrient accessibility for vertebrate and invertebrate herbivores (Yang, 2008; Grant and Scholes, 2006; Anderson et al., 2010; Joern et al., 2012). Indeed, carcasses of smaller vertebrates (e.g., salmon, deer) can increase the proportions of nitrogen and δ15N (an indicator of animal-driven N) in plants within just a few months postmortem (Hocking and Reynolds, 2012; van Klink et al., 2020).
To assess the effects of megacarcasses on local nutrient pools (Fig. 1), we measured the initial contributions of elephant carcasses (1–26 months postmortem) to soil and plant nutrients in the Kruger National Park (KNP), South Africa. Further, we examined the effects of elephant carcasses on the two main soil types in the KNP: sandy, relatively nutrient-poor granitic soils and clayey, relatively nutrient-rich basaltic soils (Venter et al., 2003). At each site, we ran transects in each cardinal direction from the center of the site where an elephant died, collecting samples of soil and a palatable grass species (Urochloa trichopus) at 0, 2.5, 5, 10, and 15 m. We then analyzed soil samples for C, N, P, and micronutrient content; quantified soil microbial respiration potential; and measured %N, δ15N, and macronutrient and micronutrient contents in grass tissue. We hypothesized that (H1) elephant megacarcass decomposition would release nutrients into soil, resulting in higher concentrations of soil N, P, and micronutrients near the center of carcass sites; (H2) C inputs into the soil would stimulate microbial activity, resulting in increased soil respiration potential near the center of carcass sites; and (H3) carcass-derived nutrients would move from soil into plants, resulting in higher foliar nutrient concentrations near the center of carcass sites. We predicted that enrichment effects from megacarcasses would be greater at sites with fresher carcasses relative to older carcasses and at nutrient-poor granitic sites compared to nutrient-rich basaltic sites.
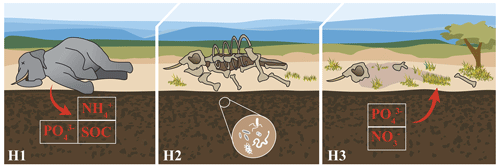
Figure 1Hypothesized impacts of elephant megacarcasses on soil and plant nutrients. First (H1), we hypothesized that elephant carcasses would release pulses of nutrients into the soil, resulting in higher concentrations of soil ions such as nitrogen (ammonium, [NH4]+), phosphorus (phosphate, [PO4]3−), and soil organic C. Second (H2), we hypothesized that C inputs from the carcass would result in increased soil microbial respiration potential. Third (H3), we hypothesized that plants would take up nutrients from the carcass soil, resulting in plants with distinct nutrient profiles and increased concentrations of key limiting nutrients such as N and P. Image credit: Kirsten Boeh.
2.1 Study system and sample collection
We performed this research in the southern part of the Kruger National Park (KNP), South Africa (24.996° S, 31.592° E; ∼ 275 m elevation). The two dominant soil types in the KNP are granitic soils (Inceptisols) and basaltic soils (Vertisols or Andisols) (Khomo et al., 2017). The clay-rich basaltic soils have a relatively large surface area, enabling them to retain larger quantities of water than granitic soils, which drain water more quickly and therefore are lower in water-soluble nutrients (Buitenwerf et al., 2014; Rughöft et al., 2016). The landscape of the KNP is a mix of savanna grasslands and broadleaf woodlands, with an overstorey dominated by trees from the genus Combretum (red bushwillow, C. apiculatum; russet bushwillow, C. hereroense; leadwood, C. imberbe) and trees formerly known as acacias (knobthorn, Senegalensis nigrescens; umbrella thorn, Vachellia tortilis). The park hosts a full suite of African savanna animals, including ∼ 30 000 elephants (Loxodonta africana) (Ferreira et al., 2024), with a mortality rate of ∼ 2 % (∼ 600 elephants per year). The targeted region of the KNP has a high density of scavengers and predators, including white-backed vultures (Gyps africanus), spotted hyenas (Crocuta crocuta), and lions (Panthera leo) (Owen-Smith and Mills, 2008).
During the wet season, in March 2023, we identified 10 elephant carcass sites (1–26 months postmortem), with 5 on relatively nutrient-rich basaltic soil and 5 on nutrient-poor granitic soil (Fig. S1). The KNP section rangers provided precise GPS locations of where elephant carcasses had been found. Most elephants died of old age; illness; injury; or, in the case of one young bull, fighting over territory. Carcass sites were recognizable in situ by a persistent bone field, undigested gut contents, and an absence of herbaceous vegetation. At each site, we hammered a rebar post into the center of the megacarcass disturbance and ran 15 m transects out from the post in each of the four cardinal directions. We collected green-leaf material from U. trichopus, a common and abundant palatable grass species, and used an auger to collect soil samples to a depth of 10 cm at five points along each transect (0.5, 2.5, 5, 10, and 15 m) (Bump et al., 2009b; Holdo and Mack, 2014; Gray and Bond, 2015; Monk et al., 2024). We treated the 10–15 m distances as representative of background concentrations of nutrients based on pilot data showing that the effect of elephant carcasses on soil nutrient concentrations was undetectable at this distance away from the carcass site, similarly to studies on the carcasses of other large vertebrates (e.g., Towne, 2000; Bump et al., 2009a). We pooled and homogenized the samples to yield one composite leaf and one composite soil sample per sampling distance from each carcass site. Soil samples were sieved in a 5 mm metal sieve, which was cleaned in between samples with 70 % ethanol. Soil samples were stored in a cooler during fieldwork. On the day they were collected, we used 5 g of each soil sample for soil respiration measurements (described below). The rest of each sample was placed in a plastic bag on the day of collection and stored in a −20 °C freezer for up to 1 month; samples were stored in coolers with ice blocks during the transition from the freezer at the field site to the lab for analysis. We chose to freeze samples rather than to store them at room temperature based on literature demonstrating that the impacts of freezing on soil nitrate and ammonium concentrations are fairly minimal, except in specific cases of high soil acidity or peaty soils, which were not present at our field site (Esala, 1995; Turner and Romero, 2009; Sollen-Norrlin and Rintoul-Hynes, 2024). Leaf samples were stored in paper bags at room temperature until they were dried for analysis (see below).
2.2 Hypothesis testing
We tested our first hypothesis that elephant carcass decomposition would release nutrients into the soil by performing soil nutrient analyses. We sent 250 g of each soil sample to the Eco-Analytica Laboratory at Northwest University in Potchefstroom, South Africa, for measurements of soil concentrations of ammonium [NH4]+, nitrate [NO3]−, phosphate [PO4]3−, and plant-available P. Samples were air-dried and sieved through <2 mm mesh prior to chemical analysis. Plant-available P was extracted from 4 g of soil and 30 mL extraction fluid (1:7.5 ratio) using an acid–fluoride solution (P Bray-1) (FAO, 2021), measured colorimetrically using a Systea EasyChem 200 analyzer, and expressed in mg kg−1. The detection limit was 0.5 mg kg−1, and plant-available P measurements <0.5 mg kg−1 were replaced with half the detection limit (0.25 mg kg−1) (Croghan and Egeghy, 2003). Water-soluble nitrate and phosphate anions were extracted from volume-on-volume 100 mg soil and 200 mL deionized water (Sonneveld and van den Ende, 1971), analyzed by means of ion chromatography using a Metrohm 930 Compact Flex System, and measured in mg L−1. Ammonium (also 1:2 water extract) was analyzed colorimetrically using a Systea EasyChem 200 analyzer and measured in mg L−1. Detection limits for soil ions were 0.01 mg L−1, and soil ion concentrations measured as <0.01 mg L−1 were replaced with half the detection limit (0.005 mg L−1). To convert the nitrate, ammonium, and phosphate units from mg L−1 to mg kg−1, we multiplied by 2 based on the 1:2 soil-to-water extraction ratio.
To determine whether soil micronutrients were distinct and elevated at the center of carcass sites relative to soil further from the center, we measured concentrations of sodium (Na), magnesium (Mg), iron (Fe), calcium (Ca), potassium (K), and phosphorus (P). Air-dried and sieved (>2 mm) soil samples, weighed to 0.2 g, were microwaved in 9 mL 65 % nitric acid (HNO3) and 3 mL 32 % hydrochloric acid (HCl) according to EPA 3051b in a Milestone ETHOS microwave digester with a UP MAXI44 rotor. A period of 20 min allowed the system to reach 1800 MW at a temperature of 200 °C, which was maintained for 15 min. After cooling, the samples were brought up to a final volume of 50 mL and analyzed on an Agilent 7500 CE ICP-MS fitted with CRC (collision reaction cell) technology for interference removal. The instrument was optimized using a solution containing Li, Y, Ce, and Tl (1 ppb) for standard low oxide and/or low interference levels (≤1.5 %) while maintaining high sensitivity across the mass range. The instrument was calibrated using ULTRASPEC®-certified custom mixed multi-element stock standard solutions containing all the elements of interest (De Bruyn Spectroscopic Solutions, South Africa). Calibrations spanned the range of 0–30 ppm for the mineral elements Ca, Mg, Na, and K and 0–0.3 ppm for the rest of the trace elements. Elemental concentrations were expressed in mg kg−1.
Finally, to determine whether elevated N levels in soils were derived from the carcass, we sent 10 g of each sample to the BIOGRIP laboratory within the Central Analytical Facility at Stellenbosch University for measurements of soil %N and δ15N, obtained using a Vario Isotope Select elemental analyzer connected to a thermal conductivity detector and an Isoprime precisION isotope ratio mass spectrometer (IRMS). Samples were oven-dried at 60 °C for 48 h and milled to a fine powder using a Retsch MM 400 mill (Germany). The powdered samples were weighed (2–60 mg) prior to combustion at 950 °C. The gases were reduced to N2 (undiluted) in the reduction column, which was held at 600 °C. A high-organic-carbon (HOC) soil standard (0.52 ± 0.02 %N), along with two international reference standards (USGS40 (δ15N − 4.52 % AIR) and USGS41 (δ15N + 47.57 % AIR)), was used for calibration. The N elemental content was expressed relative to atmospheric N as N2 δ15NAIR (‰). The quantification limit for δ15N on the IRMS was 1 nA (nanoamps), and the quantification limit for %N was 0.06 %. The precision for %N was 0.02 %, and that for δ15N was ±0.11 %, determined using the HOC standard, which was run multiple times throughout the analysis.
To test our second hypothesis that nutrient inputs into the soil would stimulate microbial activity, we measured soil organic C, water content, and microbial respiration potential. We sent 10 g of each sample to the BIOGRIP laboratory for measurements of soil organic C using a Vario TOC Cube (Elementar, Germany). Samples (dried and milled as above) were weighed (10–60 mg), acidified using 10 % HCl to remove the total inorganic C (carbonates), and dried overnight at 60 °C. All samples were analyzed through combustion at 950 °C. The released CO2 was measured by a non-dispersive infrared (NDIR) sensor. A high-organic-C (7.45 ± 0.14 %C) soil standard from Elemental Microanalysis Ltd (UK) was included during the analysis. The quantification limit for %C is 0.14 %. The precision for the %C was 0.09 %, and this was determined using the low-organic-C (LOC) standard (1.86 ± 0.14 %C), which was run multiple times throughout the analysis.
To quantify soil respiration and water content, we used an incubation method (Lemoine et al., 2023) in which 5 g (±0.2 g) of each sample was placed into a 100 mL clear glass bottle, sealed, and flushed with CO2-free air. Following flushing, we incubated the bottles for 1 h at 25 °C. We then recorded CO2 concentrations using an LI-850 infrared gas analyzer. After soil respiration measurements, we determined sample dry weight by drying each sample at 60 °C for 24–48 h until stable mass was achieved. We subtracted the dry weight from the starting weight to obtain soil water content. Finally, we used the dry weights and the ideal gas law to standardize all respiration measurements to CO2 µg h−1 g−1 dry soil.
To test our third hypothesis that carcass-derived nutrients would be incorporated by plants, we measured foliar nutrient concentrations in U. trichopus; 2 g of each dried leaf sample was sent to the BIOGRIP laboratory for preparation and measurements of %N and δ15N via stable isotope analysis, as described above. A sorghum flour standard (1.47 ± 0.25 %N) from Elemental Microanalysis Ltd (UK) was used for calibration, along with two international reference standards (USGS40 and USGS41). The quantification limit for δ15N on the IRMS was 1 nA, and the quantification limit for %N was 1.3 %. The precision for the %N was 0.02 %, and that for δ15N was ±0.08 ‰. Limits were determined using the sorghum flour standard, which was run multiple times throughout the analysis. Additionally, we sent 5 g per sample to Cedara Analytical Services Laboratory to quantify micronutrients in grass tissue (P, Na, Mg, K, Ca, and Fe) using inductively coupled plasma optical emission spectroscopy (ICP-OES 5800, Agilent, USA). Samples were dried (110 °C overnight) and milled to a fine powder. Subsamples (0.5 g) were ashed at 450 °C for 4 h, and the ash was re-wet using 2 mL HCl (32 %). Samples were evaporated to dryness then re-suspended in 25 mL 1 M HCl before filtering. Lastly, the filtrate was diluted with deionized water at a ratio of 5:20 filtrate to water. To calibrate the ICP-OES, solutions containing known amounts of each element were measured (10–20 ppm for Na and C, 200–1500 ppm for Fe, 0.5 %–3.75 % for K, and 0.125 %–0.5% for P), prepared from 1000 ppm primary single standards. At 3 of the 10 sites, we did not find sufficient plant material at the central point for analysis, resulting in a sample size of N=7 for the center (distance = 0.5 m) measurement for leaf nutrient analyses.
To test whether each response variable for the three hypotheses was significantly associated with soil type and/or distance from the carcass center, we performed a model selection procedure. For each response variable, we ran five generalized linear mixed models using the gamma family (link = log) in the package lme4 (Bates et al., 2015): (i) soil type, distance, and soil type–distance interaction; (ii) soil type and distance; (iii) soil type; (iv) distance; and (v) a null model indicating no significant difference in slope or intercept after accounting for the carcass site. All models included the carcass site as a random effect to account for individual variations. Each model included 50 observations (10 sites with five distances per site). For samples in which the nutrient level was listed as 0 or undetectable, we accounted for the uncertainty by using half the detection level as described above. The narrow distribution of ages (1–26 months since death) with the sample size of N=10 sites made testing for the effect of age challenging, and so we did not include carcass age in the models. We compared the models for each response variable using the Akaike information criterion (AIC). Models with a ΔAIC ≤ 2 were considered to be roughly equivalent in fit (Burnham and Anderson, 2002).
In addition to these models, for our second hypothesis, we regressed soil respiration potential against soil organic C, expecting that the two would be positively correlated. We ran a generalized linear mixed model with soil respiration potential as the response variable. The model included soil organic C, distance, and soil type, with carcass site as a random effect. We did not include an interaction with soil type in this model due to sample size restrictions. Respiration potential and organic C were both log-transformed to achieve normality.
To determine whether leaf and soil micronutrient composition differed with distance and soil type, we ran permutational analysis of variance (PERMANOVA) in the vegan R package (Oksanen et al., 2022). We ran the same model separately for soil and leaf micronutrient composition (soil type and distance). To determine which micronutrients contributed most to compositional differences across distances and soil types, we calculated sample-wise Bray–Curtis dissimilarity and performed principal component analysis. We also tested for differences in the variance in micronutrient composition across distances and soil types using “betadisper” in vegan (Oksanen et al., 2022). We ran linear models to test for correlations between the leaf and soil concentrations of each micronutrient. Each model included distance as a covariate and site as a random effect.
Finally, to test the impact of carcass age on key soil metrics, we ran exponential-decay functions for soil ammonium, nitrate, phosphate, and respiration versus carcass age for samples from the center of the carcass site (0.5 m sampling location). We also performed a t test to verify that there was no difference in mean carcass age across soil types.
All statistical analyses were performed in R version 4.2.1 (R Core Team, 2022).
3.1 Hypothesis 1: effects of megacarcasses on soil nutrient pools
We found partial support for our first hypothesis that soil N and P concentrations would be higher closer to the center of carcass sites (Table S1 in the Supplement). Overall, soil %N (Fig. 2a) was greater in basaltic soils, and it decreased with distance from the carcass site in granitic soils. Soil nitrate (Fig. 2b) decreased with distance from the carcass site but did not differ between soil types. Ammonium (Fig. 2c) also decreased with distance but only in granitic soils. δ15N (Fig. 2d) was greater in granitic soils and decreased with distance in both soil types, indicating that the proportion of animal-sourced N in the soil was greater near the center of the carcass site. Soil phosphate, plant-available P, and mineral P (Fig. 2e–g) all exhibited significant soil–distance interactions. Phosphate (Fig. 2e) was highly elevated at the center of carcass sites and decreased steeply with distance but only in granitic soils. Plant-available P (Fig. 2f) decreased with distance in both soil types, but the effect was strongest in granitic soils. Finally, mineral P (Fig. 2g) was greater in basaltic soils, and there was a small decrease with distance in granitic soils but not in basaltic soils.
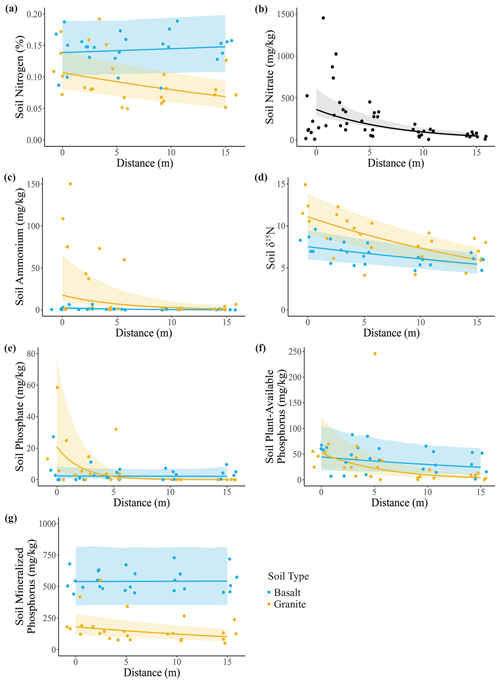
Figure 2Effects of elephant carcasses on soil N and P concentrations in granitic and basaltic soils. (a) Soil N (%) was greater in basaltic soils, and in granitic soils it decreased with distance from the carcass site. (b) Soil nitrate decreased with distance but did not differ between soil types. Soil (c) ammonium and (d) δ15N were both greater in granitic soils and decreased with distance from the carcass. (e) Soil phosphate, (f) plant-available P, and (g) mineralized P decreased with distance in granitic soils but not in basaltic soils. Points represent individual measurements from soil samples taken at 0, 2.5, 5, 10, and 15 m and are offset to be visible when they would otherwise overlap. Lines show predictions calculated from the top generalized linear mixed model, which may include soil type, distance, and soil type–distance interactions as covariates (Table S1). Only significant relationships are shown on plots. Shading indicates the 95 % confidence interval.
Contrary to our first hypothesis, soil micronutrient composition did not differ significantly with distance from the carcass center, nor did most individual micronutrients (Table S1). The PERMANOVA results showed that soil micronutrient composition did not differ significantly with distance (R2=0.00, , P=1.000) (Fig. S2a), but it did differ significantly with soil type (R2=0.71, , P=0.001) (Fig. S2b). There was no significant difference in variance with distance (, P=0.996) or soil type (, P=0.115). Principal component analysis showed that dimension 1 explained 53.6 % of the variation between soil types and was driven primarily by differences in Mg, Ca, and Fe. Dimension 2 explained 25.9 % of the variation and was driven primarily by differences in K. Soil Na (Fig. S3a) was marginally greater in granitic soils and decreased with distance from the carcass, with the effect greater in granitic soils. Soil K (Fig. S3b) was greater in basaltic soils and decreased marginally with distance. Soil Fe, Mg, and Ca (Fig. S3c–e) were greater in basaltic soils, with minimal effects of distance.
3.2 Hypothesis 2: effects of megacarcasses on soil carbon and respiration
Consistent with our second hypothesis, soil respiration potential was marginally positively correlated with soil organic carbon concentration and decreased significantly with distance but did not differ with soil type (Fig. 3). We found no evidence for differences in soil water content (Fig. S4a) or soil pH (Fig. S4b) with distance or soil type. In both cases, the null model ranked among the set of top models (Table S1).
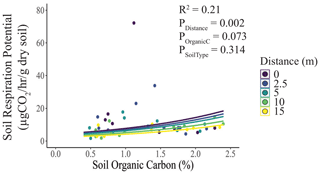
Figure 3Effects of elephant carcasses on soil respiration potential. We regressed soil respiration against soil organic carbon, with distance and soil type as covariates. Soil respiration potential was marginally positively correlated with soil organic C (%) and decreased significantly with distance from the carcass. Points represent individual measurements taken from soil samples at 0, 2.5, 5, 10, and 15 m and are offset to be visible when they would otherwise overlap. Lines represent model predictions. Only significant relationships are shown.
3.3 Hypothesis 3: effects of megacarcasses on plant nutrient pools
Consistent with our third hypothesis, we found elevated foliar nutrient concentrations in U. trichopus at elephant carcass sites. Leaf %N (Fig. 4a) and δ15N (Fig. 4b) both decreased with distance from the carcass center. δ15N exhibited a significant soil–distance interaction, which was greater overall in basaltic soils, but the difference between the two soil types was greater closer to the carcass site. Foliar P was greater in basaltic soils and decreased only marginally with distance in the granite soils. Finally, the foliar N:P ratio was greater in granitic soils and decreased with distance in the basaltic soils.
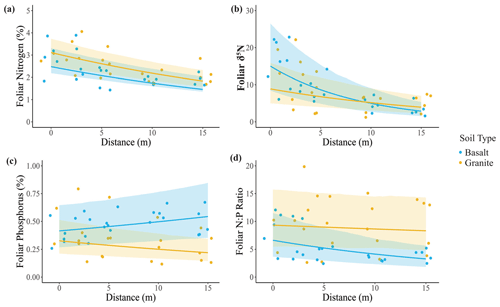
Figure 4Effects of elephant carcasses on foliar N and P concentrations in granitic and basaltic soils. (a) Foliar %N was higher in granitic soils and decreased with distance from the carcass center. (b) Foliar δ15N decreased with distance from the carcass center and exhibited a significant interaction in which δ15N decreased more rapidly with distance in basaltic soils. (c) Foliar P was greater in basaltic soils and decreased with distance in granitic soils. (d) Foliar N:P ratio was greater in granitic soils and decreased with distance from the carcass center for both soil types. Points represent individual measurements from soil samples taken at 0, 2.5, 5, 10, and 15 m and are offset to be visible when they would otherwise overlap. Lines show predictions calculated from the top generalized linear mixed model, which may include soil type, distance, and soil type–distance interactions as covariates (Table S2). Only significant relationships are shown on the plots. Shading indicates the 95 % confidence interval. A total of 3 of the 10 sites had bare ground at 0 m distance, resulting in a sample size of 7 sites for that distance and 10 for the other distances.
Leaf micronutrient composition did not differ significantly with distance (R2=0.13, , P=0.062; Fig. S5a) but did differ with soil type (R2=0.17, , P=0.001; Fig. S5b). There was no significant difference in variance with distance (, P=0.713) or soil type (, P=0.173). Dimension 1 explained 42.8 % of the variance across soil types and was primarily driven by Mg, Na, and P. Dimension 2 explained 26.6 % of the variance and was driven mainly by K, Ca, and Fe. Foliar Na (Fig. S6a) and Mg (Fig. S6b) were both greater in basaltic soils and decreased with distance from the carcass center. Foliar K (Fig. S6c) and Fe (Fig. S6d) both decreased with distance as well but did not differ with soil type. The null model was in the top set for foliar Ca, indicating no significant relationship between foliar Ca concentrations and soil type or distance from the carcass center. Individual micronutrients (K, Ca, Mg, Fe) were not correlated between leaf and soil samples, with the exception of Na (Table S3).
3.4 Effects of carcass age on soil ions and respiration potential
Soil ammonium (α=0.018, P<0.001), phosphate (α = 0.023, P<0.001), and respiration potential (α = 0.058, P<0.001) all decreased significantly with carcass age (Fig. 5a–c). The exponential-decay model for nitrate failed to converge due to an outlier with extremely high soil nitrate (1454 mg kg−1) at 258 d postmortem (Fig. 5d). We ran a t test to test for a difference in mean carcass age between soil types and found no significant difference between the two groups (P=0.294).
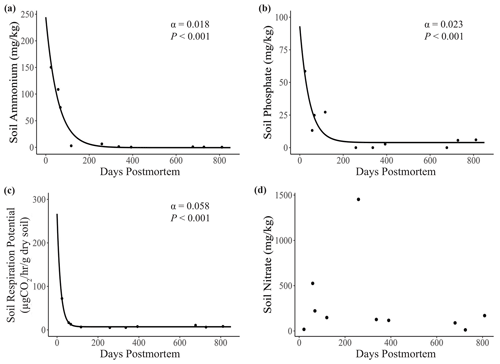
Figure 5Relationship between carcass age and key soil metrics (soil ion concentrations and respiration potential). Lines represent predictions from exponential-decay models, with α equal to the rate of decay. (a) Soil ammonium, (b) phosphate, and (c) respiration potential all decreased significantly with carcass age. The model for (d) soil nitrate failed to converge. Points represent individual measurements taken at the center of the carcass site (distance = 0.5 m). Only significant relationships are shown on plots.
Here, we show that elephant megacarcasses influence soil and foliar nutrients during at least the first 2 years following mortality. Consistent with our hypotheses, soil nitrate (Fig. 2b), ammonium (Fig. 2c), δ15N (Fig. 2d), and P (Fig. 2e–f) concentrations were all elevated at the center of carcass sites and decreased with distance from the center. Soil %N, nitrate, ammonium, and plant-available P concentrations at the 15 m point were consistent with those found in other studies of soil nutrient content in KNP (Aranibar et al., 2003; Rughöft et al., 2016), confirming that the 15 m point serves as an effective baseline control in this experiment. Microbial respiration potential was also elevated towards the center of carcass sites and was correlated with the abundance of organic C (Fig. 3). Finally, %N (Fig. 4a) and δ15N in a common grass (Fig. 4b) were both elevated closer to the centers of carcass sites compared to in grass farther from carcasses. Together, these results indicate that carcass-derived nutrients move into soil and are subsequently absorbed by plants over relatively short timescales, cycling essential nutrients such as N from carrion into the soil and then back into above-ground nutrient pools.
The initial influx of ammonium from elephant carcasses may have time-dependent impacts on plant abundance at elephant carcass sites. The mean ammonium level at the center of carcass sites (34.8 mg kg−1) was higher than the level generally considered to be toxic to plants (Britto and Kronzucker, 2002). Yet, we found living grass – typically U. trichopus – at the center of the carcass site at 7 out of our 10 sites (ammonium range 10–172 mg kg−1) and at the 2.5 m distance for all sites (ammonium range 0–72 mg kg−1). The three sites without vegetation in the center had the highest ammonium levels (70–144 mg kg−1), suggesting that U. trichopus has a higher degree of ammonium tolerance than some sympatric grass species but may still be limited by the high ammonium levels at the centers of these three relatively fresh carcass sites. However, the recentness of the disturbance from the carcass likely also plays a role in determining plant abundance near the center of the carcass. Because of the elephant carcass site age distribution (mean: 350 d postmortem; range: 24–811 d), this study may not have captured the full impact of ammonium release from carcasses during the early stages of decomposition. Soil ammonium spiked early and decreased rapidly (Fig. 5a), and future research on carcasses within the first few weeks postmortem would enhance our understanding of these early nutrient dynamics.
Soil nitrate (Fig. 2b) and soil respiration potential (Fig. 3) were also elevated near the center of carcass sites, indicating higher activity rates for nitrifying bacteria and heterotrophic microbes (Prosser, 2011). These results are consistent with other work on carrion, where microbial activity tends to be greater in soils near carcasses compared to in surrounding soil (Bump et al., 2009b). However, carcass effects on soil microbial respiration exhibit a high degree of intra-system variation (Risch et al., 2020), and the potentially short window during which increased respiration occurs may make capturing these variations challenging. For example, soil respiration potential at the center of the three youngest carcass sites was, on average, 2 times higher than at the seven older sites (18.43 and 9.62 µg CO2 h−1 g−1 of dry soil, respectively; Fig. 5d). Thus, the impact of increased organic C on soil microbial processes may be relatively short-lived and only last a matter of months (Keenan et al., 2018, 2019). These trends are consistent with soil ammonium and phosphate, both of which were highest at the youngest carcass sites (<200 d postmortem; Fig. 5a–b). Soil microbial respiration rate was also highly elevated early on, but it decreased at a faster rate over time than soil ions (Fig. 5c). Thus, soil dynamics during the first several months after death may play a crucial role in determining the long-term impacts of megacarcasses on savannas and therefore provide a promising avenue for future research.
Elevated soil phosphate (Fig. 2e) and plant-available P (Fig. 2f) at the center of carcass sites were also consistent with expectations from the literature (Bump et al., 2009a; Parmenter and MacMahon, 2009). However, elevated P levels in soil did not translate to elevated P in grass leaves (Fig. 4c), which could suggest a lag between trends in soil and plants that is longer for P than for N. This lag could occur because phosphate easily forms chemical bonds with other soil ions (e.g., iron and aluminum in acidic soils and calcium in basic soils). Nitrate does not form these bonds and therefore has greater water solubility and mobility in soils and may be more readily taken up by plants (Wiersum, 1962; Arai and Sparks, 2007). However, it is also possible that P limitation in KNP is not as strong as it is in some other African savanna systems (Pellegrini, 2016). The foliar N:P ratios measured in this experiment were higher closer to the center of the carcass site (median 9.38 at 0 m and 4.83 at 15 m), indicating that N limitation may be relatively stronger further from the carcass site, and P limitation may be relatively stronger closer to the center (Fig. 4d, Table S2). These relatively high foliar N:P ratios at the center of carcass sites are similar to those found in N fertilization studies in KNP (Craine et al., 2008), further supporting the idea that the influx of N from megacarcasses may shift the soil from relatively more N limited to more P limited.
The elevated plant-available P at the center of carcass sites likely came primarily from phosphate released from decomposing tissue (Yong et al., 2019). Bone decomposition, which is also likely to be a major source of P from animal carcasses (Subalusky et al., 2020), occurs over long timescales (Coe, 1978; Subalusky et al., 2020) and therefore should result in the slow release of P and a gradual decrease in the N:P ratio (Parmenter and MacMahon, 2009; Quaggiotto et al., 2019). Indeed, initial inorganic N influxes to the Mara River in Kenya from mass wildebeest die-offs are 10 times greater than concurrent increases in P, which is instead released slowly over about 7 years of bone decomposition (Subalusky et al., 2017). Research following megacarcasses over longer time frames postmortem is needed to clarify when P from enriched soil is absorbed by plants and at what stage megacarcass bones begin contributing to soil P dynamics. It is also possible that bone dispersal by scavengers may result in less P leaching from bones close to where the elephant died and more P being distributed across the landscape at distances far from the carcass site.
The contributions of megacarcasses to soil nutrient pools were strongly associated with soil type. Our results confirmed the previously established trend that basaltic soils are, overall, more micronutrient rich than granitic soils, with greater concentrations of P, K, Fe, Mg, and Ca (Figs. 2g, S3b–e; Gertenbach, 1983; Craine et al., 2008; Wigley et al., 2014). However, soil ammonium, δ15N, and phosphate were all higher in the granitic soils towards the center of carcass sites, decreasing steeply to be similar to basaltic soils about 10 m from the carcass center (Fig. 2c–e). These results indicate that the impact of organic matter from megacarcasses may be stronger in relatively nutrient-poor and sandy granitic soil compared with nutrient-rich and clayey basaltic soil. We were surprised that grass on basaltic soil did not consistently exhibit greater nutrient concentrations. One potential explanation is that grass may primarily be limited by macronutrients like N and P in both soil types (Craine et al., 2008; Holdo, 2013) rather than by micronutrients. Thus, even with increased micronutrient availability, their actual uptake may not differ substantially. Studies on ungulate carcasses (e.g., muskoxen, moose, zebra) have shown increased foliar N at carcass sites (Danell et al., 2002; Bump et al., 2009b; Turner et al., 2014), but, to date, there is little research on the flow of micronutrients from carrion to plants and none on the pipeline from megacarcasses to plants. Moreover, it remains to be seen whether increases in foliar N and other nutrients affect herbivory rates at carcass sites and how long such effects may last.
The magnitude of nitrogen inputs from megacarcasses, as well as the substantial size and duration of their impact zones, means their impacts on ecosystem processes may be functionally distinct from those of smaller carrion. Soil nitrate concentrations at elephant carcass sites are orders of magnitude higher than those at carcass sites of smaller carrion (e.g., rabbits, white-tailed deer, kangaroo) (Quaggiotto et al., 2019; Bump et al., 2009b; Barton et al., 2016). Even for large ungulates such as moose, total soil inorganic nitrogen (ammonium and nitrate) at carcass sites shows a mean of 300 mg kg−1 (Bump et al., 2009a), which is substantially lower than the mean total soil inorganic nitrogen at elephant carcass sites (2.5 m distance; 473 mg kg−1). Termite mounds, another long-lasting source of savanna nutrient heterogeneity, have mean soil nitrate concentrations (197 mg kg−1) that are lower than those of elephant carcass sites but maximum nitrate concentrations that are on par with them (974 mg kg−1) (Seymour et al., 2014), again indicating that elephant carcasses are one of the strongest known individual contributors of soil nitrogen in African savanna ecosystems, which may have important implications for savanna ecology. Indeed, there is evidence that carcass size strongly impacts scavenger food web structures (Moleón et al., 2015; Morris et al., 2023). Moreover, the attraction of animals to carcasses via scavenging, predation, or mourning (Goldenberg and Wittemyer, 2020) could have positive feedbacks on nutrient cycling (Bump et al., 2009a; Monk et al., 2024), which may be magnified by carcass size. Thus, the impacts of megacarcasses on savanna ecosystem processes may be dissimilar to the effects of small carrion and more similar to other more persistent contributors to savanna ecosystem processes, such as termite mounds (Davies et al., 2016), cattle bomas (Augustine, 2003), and even mass animal mortality events (Subalusky et al., 2017, 2020).
This study is an important first step in understanding the ecological legacies of megacarcasses on savanna ecosystem processes. During the first 2 years postmortem, elephant carcasses released pulses of nitrogen and phosphate, which influenced savanna primary productivity. These nutrients stimulated soil microbial activity and enriched foliar N, and the effects were strongest in nutrient-poor soil, with potential long-term impacts on savanna nutrient heterogeneity. These carcass-derived nutrient hotspots represent a previously unstudied function of megaherbivores in relation to savannas – one that we need to better understand in order to comprehend the full impacts of megaherbivore population declines on modern ecosystems.
Data and computer code are archived on the Dryad digital repository (https://doi.org/10.5061/dryad.wpzgmsbwm, Reed et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-1583-2025-supplement.
DEB, NPL, IPJS, TS, AT, JTdT, DIT, and JPS conceived the study. MLB, JTdT, NPL, JPS, IPJS, TS, AT, DIT, HvC, and DEB collected samples. CGR, NPL, DIT, and DEB analyzed the data. CGR drafted the paper, and all the authors contributed to editing.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
All research was completed under permits from South African National Parks (SANParks) (permit no. SS554). We thank the field assistants of SANParks for the guidance and protection in the field, as well as the section rangers and Sandra Snelling for the GPS locations and ages of carcasses. We thank Lucky Sithole (Cedara Analytical Services Laboratory), Terina Vermeulen (Eco-Analytica Laboratory), and Janine Colling (BIOGRIP laboratory) for the extensive laboratory support. Select data used in this research paper were generated using equipment in the DSI-funded BIOGRIP soil and water node at Stellenbosch University.
This research has been supported by the National Science Foundation Directorate for Biological Sciences (grant nos. 2128092, 2128093, and 2128094) and the University of California Santa Barbara Academic Senate.
This paper was edited by Erika Buscardo and reviewed by Shawn J. Leroux, Sarah Keenan, and one anonymous referee.
Anderson, T. M., Hopcraft, J. G. C., Eby, S., Ritchie, M., Grace, J. B., and Olff, H.: Landscape-scale analyses suggest both nutrient and antipredator advantages to Serengeti herbivore hotspots, Ecol., 91, 1519–1529, https://doi.org/10.1890/09-0739.1, 2010.
Aranibar, J. N., Macko, S. A., Anderson, I. C., Potgieter, A. L. F., Sowry, R., and Shugart, H. H.: Nutrient cycling responses to fire frequency in the Kruger National Park (South Africa) as indicated by stable isotope analysis, Isotopes Environ. Health Stud., 39, 141–158, https://doi.org/10.1080/1025601031000096736, 2003.
Arai, Y. and Sparks, D. L.: Phosphate reaction dynamics in soils and soil components: a multiscale approach, Adv. Agron., 94, 135–179, https://doi.org/10.1016/S0065-2113(06)94003-6, 2007.
Asner, G. P., Levick, S. R., Kennedy-Bowdoin, T., Knapp, D. E., Emerson, R., Jacobson, J., Colgan, M. S., and Martin, R. E.: Large-scale impacts of herbivores on the structural diversity of African savannas, P. Natl. Acad. Sci. USA, 106, 4947–4952, https://doi.org/10.1073/pnas.0810637106, 2009.
Augustine, D. J.: Long-term, livestock-mediated redistribution of nitrogen and phosphorus in an East African savanna, J. Appl. Ecol., 40, 137–149, https://doi.org/10.1046/j.1365-2664.2003.00778.x, 2003.
Barton, P. S.: Carrion Ecology, Evolution, and Their Applications, edited by: Benbow, M. E., Tomberlin, J. K., and Tarone, A. M., CRC Press, Boca Raton, FL, USA, ISBN 9781138893849, 2016.
Barton, P. S. and Bump, J. K.: Carrion decomposition, in: Carrion Ecology and Management, edited by: Olea, P. P., Mateo-Tomas, P., and Sanchez-Zapata, J. A., Springer, NY, USA, 101–124, 2019.
Barton, P. S., Cunningham, S. A., Lindenmayer, D. B., and Manning, A. D.: The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems, Oecologia, 171, 761–772, https://doi.org/10.1007/s00442-012-2460-3, 2013.
Barton, P. S., McIntyre, S., Evans, M. J., Bump, J. K., Cunningham, S. A., and Manning, A. D.: Substantial long-term effects of carcass addition on soil and plants in a grassy eucalypt woodland, Ecosphere, 7, e01537, https://doi.org/10.1002/ecs2.1537, 2016.
Bates, D., Mächler, M., Bolker, B., and Walker, S.: Fitting linear mixed-effects models using lme4, J. Stat. Softw., 67, 1–48, https://doi.org/10.18637/jss.v067.i01, 2015.
Britto, D. T. and Kronzucker, H. J.: NH toxicity in higher plants: a critical review, J. Plant Physiol., 159, 567–584, https://doi.org/10.1078/0176-1617-0774, 2002.
Buitenwerf, R., Kulmatiski, A., and Higgins, S. I.: Soil water retention curves for the major soil types of the Kruger National Park, Koedoe, 56, a1228, https://doi.org/10.4102/koedoe.v56i1.1228, 2014.
Bump, J. K., Peterson, R. O., and Vucetich, J. A.: Wolves modulate soil nutrient heterogeneity and foliar nitrogen by configuring the distribution of ungulate carcasses, Ecol., 90, 3159–3167, https://doi.org/10.1890/09-0292.1, 2009a.
Bump, J. K., Webster, C. R., Vucetich, J. A., Peterson, R. O., Shields, J. M., and Powers, M. D.: Ungulate carcasses perforate ecological filters and create biogeochemical hotspots in forest herbaceous layers allowing trees a competitive advantage, Ecosyst., 12, 996–1007, https://doi.org/10.1007/s10021-009-9274-0, 2009b.
Burnham, K. P. and Anderson, D. R.: Model Selection and Multimodel Inference, Springer New York, NY, https://doi.org/10.1007/b97636, 2002.
Campos-Arceiz, A. and Blake, S.: Megagardeners of the forest – the role of elephants in seed dispersal Acta Oecol., 37, 542–553, https://doi.org/10.1016/j.actao.2011.01.014, 2011.
Chen, B., Fang, J., Piao, S., Ciais, P., Black, T. A., Wang, F., Niu, S., Zeng, Z., and Luo, Y.: A meta-analysis highlights globally widespread potassium limitation in terrestrial ecosystems, New Phytol., 241, 154–165, https://doi.org/10.1111/nph.19294, 2024.
Coe, M.: The decomposition of elephant carcasses in the Tsavo (East) National Park, Kenya, J. Arid Environ., 1, 71–86, https://doi.org/10.1016/S0140-1963(18)31756-7, 1978.
Coverdale, T. C., Kartzinel, T. R., Grabowski, K. L., Shriver, R. K., Hassan, A. A., Goheen, J. R., Palmer, T. M., and Pringle, R. M.: Elephants in the understory: opposing direct and indirect effects of consumption and ecosystem engineering by megaherbivores, Ecol., 97, 3219–3230, https://doi.org/10.1002/ecy.1557, 2016.
Craine, J. M., Morrow, C., and Stock, W. D.: Nutrient concentration ratios and co-limitation in South African grasslands, New Phytol., 179, 829–836, https://doi.org/10.1111/j.1469-8137.2008.02513.x, 2008.
Croghan, C. and Egeghy, P. P.: Methods of Dealing with Values Below the Limit of Detection Using SAS, Presented at Southeastern SAS User Group, St. Petersburg, FL, September 22–24, EPA/600/A-03/176, https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=64046 (last access: 17 January 2025), 2003.
Danell, K., Berteaux, D., and Bråthen, K. A.: Effect of muskox carcasses on nitrogen concentration in tundra vegetation, Arctic, 55, 389–392, http://www.jstor.org/stable/40512497 (last access: 17 January 2025), 2002.
Davies, A. B., Levick, S. R., Robertson, M. P., van Rensburg, B. J., Asner, G. P., and Parr, C. L.: Termite mounds differ in their importance for herbivores across savanna types, seasons and spatial scales, Oikos, 125, 726–734, https://doi.org/10.1111/oik.02742, 2016.
Doughty, C. E., Wolf, A., and Malhi, Y.: The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia, Nat. Geosci., 6, 761–764, https://doi.org/10.1038/ngeo1895, 2013.
Doughty, C. E., Roman, J., Faurby, S., Wolf, A., Haque, A., Bakker, E. S., Malhi, Y., Dunning Jr., J. B., and Svenning, J.-C.: Global nutrient transport in a world of giants, P. Natl. Acad. Sci. USA, 113, 868–873, https://doi.org/10.1073/pnas.1502549112, 2016.
Epron, D., Laclau, J.-P., Almeida, J. C. R., Gonçalves, J. L. M., Ponton, S., Sette Jr., C. R., Delgado-Rojas, J. S., Bouillet, J.-P., and Nouvellon, Y.: Do changes in carbon allocation account for the growth response to potassium and sodium applications in tropical Eucalyptus plantations?, Tree Physiol., 32, 667–679, https://doi.org/10.1093/treephys/tpr107, 2012.
Esala, M. J.: Changes in the extractable ammonium- and nitrate-nitrogen contents of soil samples during freezing and thawing, Commun. Soil Sci. Plant Anal., 26, 61–68, https://doi.org/10.1080/00103629509369280, 1995.
FAO: Standard operating procedure for soil available phosphorus – Bray I and Bray II method, http://www.fao.org/3/cb3460en/cb3460en.pdf (last access: 17 January 2025), 2021.
Ferreira, S., Crowhurst, E., Greaver, C., and Simms, C.: Sample-Based Estimates of Elephants in Kruger National Park, South Africa, Afr. J. Wildl. Res., 54, https://doi.org/10.3957/056.054.0122, 2024.
Flannery, T. F.: Pleistocene faunal loss: implications of the aftershock for Australia's past and future, Archaeol. Ocean., 25, 45–55, https://doi.org/10.1002/j.1834-4453.1990.tb00232.x, 1990.
Gertenbach, W.: Landscapes of the Kruger National Park, Koedoe, 26, 9–121, https://doi.org/10.4102/koedoe.v26i1.591, 1983.
Goldenberg, S. Z. and Wittemyer, G.: Elephant behavior toward the dead: A review and insights from field observations, Primates, 61, 119–128, https://doi.org/10.1007/s10329-019-00766-5, 2020.
Grant, C. C. and Scholes, M. C.: The importance of nutrient hot-spots in the conservation and management of large wild mammalian herbivores in semi-arid savannas, Biol. Conserv., 130, 426–437, https://doi.org/10.1016/j.biocon.2006.01.004, 2006.
Gray, E. F. and Bond, W.: Soil nutrients in an African forest/savanna mosaic: Drivers or driven?, S. Afr. J. Bot., 101, 66–72, https://doi.org/10.1016/j.sajb.2015.06.003, 2015.
Guy, T. J., Hutchinson, M. C., Baldock, K. C. R., Kayser, E., Baiser, B., Staniczenko, P. P. A., Goheen, J. R., Pringle, R. M., and Palmer, T. M.: Large herbivores transform plant-pollinator networks in an African savanna, Curr. Biol., 31, 2964–2971, https://doi.org/10.1016/j.cub.2021.04.051, 2021.
Hocking, M. D. and Reynolds, J. D.: Nitrogen uptake by plants subsidized by Pacific salmon carcasses: a hierarchical experiment, Can. J. For. Res., 42, 908–917, https://doi.org/10.1139/x2012-045, 2012.
Holdo, R. M.: Effects of fire history and N and P fertilization on seedling biomass, specific leaf area, and root:shoot ratios in a South African savannah, S. Afr. J. Bot., 86, 5–8, https://doi.org/10.1016/j.sajb.2013.01.005, 2013.
Holdo, R. M. and Mack, M. C.: Functional attributes of savanna soils: contrasting effects of tree canopies and herbivores on bulk density, nutrients and moisture dynamics, J. Ecol., 102, 1171–1182, https://doi.org/10.1111/1365-2745.12290, 2014.
Joern, A., Provin, T., and Behmer, S. T.: Not just the usual suspects: insect herbivore populations and communities are associated with multiple plant nutrients, Ecol., 93, 1002–1015, https://doi.org/10.1890/11-1142.1, 2012.
Keenan, S. W. and Beeler, S. R.: Long-term effects of buried vertebrate carcasses on soil biogeochemistry in the Northern Great Plains, PloS One, 18, e0292994, https://doi.org/10.1371/journal.pone.0292994, 2023.
Keenan, S. W., Schaeffer, S. M., Jin, V. L., and DeBruyn, J. M.: Mortality hotspots: nitrogen cycling in forest soils during vertebrate decomposition, Soil Biol. Biochem., 121, 165–176, https://doi.org/10.1016/j.soilbio.2018.03.005, 2018.
Keenan, S. W., Schaeffer, S. M., and DeBruyn, J. M.: Spatial changes in soil stable isotopic composition in response to carrion decomposition, Biogeosciences, 16, 3929–3939, https://doi.org/10.5194/bg-16-3929-2019, 2019.
Khomo, L., Trumbore, S., Bern, C. R., and Chadwick, O. A.: Timescales of carbon turnover in soils with mixed crystalline mineralogies, SOIL, 3, 17–30, https://doi.org/10.5194/soil-3-17-2017, 2017.
Lemoine, N. P., Budny, M. L., Rose, E., Lucas, J., and Marshall, C. W.: Seasonal soil moisture thresholds inhibit bacterial activity and decomposition during drought in a tallgrass prairie, Oikos, 2024, e10210, https://doi.org/10.1111/oik.10201, 2023.
Macdonald, B. C. T., Farrell, M., Tuomi, S., Barton, P. S., Cunningham, S. A., and Manning, A. D.: Carrion decomposition causes large and lasting effects on soil amino acid and peptide flux, Soil Biol. Biochem., 69, 132–140, https://doi.org/10.1016/j.soilbio.2013.10.042, 2014.
Moleón, M., Sánchez-Sapata, J. A., Sebastián-González, E., and Owen-Smith, N.: Carcass size shapes the structure and functioning of an African scavenging assemblage, Oikos, 124, 1391–1403, https://doi.org/10.1111/oik.02222, 2015.
Monk, J. D., Donadio, E., Smith, J. A., Perrig, P. L., Middleton, A. D., and Schmitz, O. J.: Predation and biophysical context control long-term carcass nutrient inputs in an Andean ecosystem, Ecosyst., 27, 346–359, https://doi.org/10.1007/s10021-023-00893-7, 2024.
Morris, A. W., Smith, I., Chakrabarti, S., Lala, F., Nyaga, S., and Bump, J. K.: Eating an elephant, one bite at a time: predator interactions at carrion bonanzas, Food Webs, 37, e00304, https://doi.org/10.1016/j.fooweb.2023.e00304, 2023.
Mphepya, J. N., Galy-Lacaux, C., Lacaux, J. P., Held, G., and Pienaar, J. J.: Precipitation chemistry and wet deposition in Kruger National Park, South Africa, J. Atmos. Chem., 53, 169–183, https://doi.org/10.1007/s10874-005-9005-7, 2006.
Oksanen, J., Simpson, G., Blanchet, F., Kindt, R., Legendre, P., Minchin, P., O'Hara, R., Solymos, P., Stevens, M., Szoecs, E., Wagner, H., Barbour, M., Bedward, M., Bolker, B., Borcard, D., Carvalho, G., Chirico, M., De Caceres, M., Durand, S., Evangelista, H., FitzJohn, R., Friendly, M., Furneaux, B., Hannigan, G., Hill, M., Lahti, L., McGlinn, D., Ouellette, M., Ribeiro Cunha, E., Smith, T., Stier, A., Ter Braak, C., and Weedon, J.: Community Ecology Package, R package version 2.6-4 [code], https://CRAN.R-project.org/package=vegan (last access: 17 January 2025), 2022.
Olea, P. P., Mateo-Tomas, P., and Sanchez-Zapata, J. A. (Eds.): Carrion Ecology and Management, Springer, NY, USA, https://doi.org/10.1007/978-3-030-16501-7, 2019.
Owen-Smith, N.: Megafaunal extinctions: the conservation message from 11,000 years B.P., Conserv. Biol., 3, 405–412, https://www.jstor.org/stable/2386221 (last access: 17 January 2025), 1989.
Owen-Smith, N. and Mills, M. G. L.: Predator-prey size relationships in an African large-mammal food web, J. Anim. Ecol., 77, 173–183, https://doi.org/10.1111/j.1365-2656.2007.01314.x, 2008.
Parmenter, R. R. and MacMahon, J. A.: Carrion decomposition and nutrient cycling in a semiarid shrub–steppe ecosystem, Ecol. Monogr., 79, 637–661, https://doi.org/10.1890/08-0972.1, 2009.
Pellegrini, A. F. A.: Nutrient limitation in tropical savannas across multiple scales and mechanisms, Ecol., 97, 313–324, https://doi.org/10.1890/15-0869.1, 2016.
Prosser, J. I.: Soil Nitrifiers and Nitrification, in: Nitrification, ASM Press, 347–383, https://doi.org/10.2166/9781789064742_ch14, 2011.
Quaggiotto, M.-M., Evans, M. J., Higgins, A., Strong, C., and Barton, P. S.: Dynamic soil nutrient and moisture changes under decomposing vertebrate carcasses, Biogeochemistry, 146, 71–82, https://doi.org/10.1007/s10533-019-00611-3, 2019.
R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [code], https://www.R-project.org/ (last access: 17 January 2025), 2022.
Reed, C. G., Budny, M. L., du Toit, J. T., Helcoski, R., Schimel, J. P., Smit, I. P. J., Strydom, T., Tallian, A., Thompson, D. I., van Coller, H., Lemoine, N. P., and Burkepile, D. E.: Elephant megacarcasses increase local nutrient pools in African savanna soils and plants, Dryad [code and data set], https://doi.org/10.5061/dryad.wpzgmsbwm, 2025.
Ries, L. P. and Shugart, H. H.: Nutrient limitations on understory grass productivity and carbon assimilation in an African woodland savanna, J. Arid Environ., 72, 1423–1430, https://doi.org/10.1016/j.jaridenv.2008.02.013, 2008.
Ripple, W. J., Newsome, T. M., Wolf, C., Dirzo, R., Everatt, K. T., Galetti, M., Hayward, M. W., Kerley, G. I. H., Levi, T., Lindsey, P. A., Macdonald, D. W., Malhi, Y., Painter, L. E., Sandom, C. J., Terborgh, J., and Van Valkenburgh, B.: Collapse of the world's largest herbivores, Sci. Adv., 1, e1400103, https://doi.org/10.1126/sciadv.1400103, 2015.
Risch, A. C., Frossard, A., Schütz, M., Frey, B., Morris, A. W., and Bump, J. K.: Effects of elk and bison carcasses on soil microbial communities and ecosystem functions in Yellowstone, USA, Funct. Ecol., 34, 1933–1944, https://doi.org/10.1111/1365-2435.13611, 2020.
Roman, J., Estes, J. A., Morissette, L., Smith, C., Costa, D., McCarthy, J., Nation, J. B., Nicol, S., Pershing, A., and Smetacek, V.: Whales as marine ecosystem engineers, Front. Ecol. Environ., 12, 377–385, https://doi.org/10.1890/130220, 2014.
Rughöft, S., Hermann, M., Lazar, C. S., Cesarz, S., Levick, S. R., Trumbore, S. E., and Küsel, K.: Community composition and abundance of bacterial, archaeal and nitrifying populations in savanna soils on contrasting bedrock material in Kruger National Park, South Africa, Front. Microbiol., 7, fmicb.2016.01638, https://doi.org/10.3389/fmicb.2016.01638, 2016.
Schmitz, O. J., Wilmers, C. C., Leroux, S. J., Doughty, C. E., Atwood, T. B., Galetti, M., Davies, A. B., and Goetz, S. J.: Animals and the zoogeochemistry of the carbon cycle, Science, 362, eaar3213, https://doi.org/10.1126/science.aar3213, 2018.
Seymour, C. L., Milewski, A. V., Mills, A. J., Joseph, G. S., Cumming, G. S., Cumming, D. H. M., and Mahlangu, Z.: Do the large termite mounds of Macrotermes concentrate micronutrients in addition to macronutrients in nutrient-poor African savannas?, Soil Biology and Biogeochemistry, 68, 95–105, https://doi.org/10.1016/j.soilbio.2013.09.022, 2014.
Skarpe, C., Aarrestad, P. A., Andreassen, H. P., Dhillion, S. S., Dimakatso, T., du Toit, J. T., Duncan, Halley, J., Hytteborn, H., Makhabu, S., Mari, M., Marokane, W., Masunga, G., Ditshoswane, M., Moe, S. R., Mojaphoko, R., Mosugelo, D., Motsumi, S., Neo-Mahupeleng, G., Ramotadima, M., Rutina, L., Sechele, L., Sejoe, T. B., Stokke, S., Swenson, J. E., Taolo, C., Vandewalle, M., and Wegge, P.: The return of the giants: ecological effects of an increasing elephant population, Ambio, 33, 276–282, http://www.jstor.org/stable/4315497 (last access: 17 January 2025), 2004.
Sollen-Norrlin, M. and Rintoul-Hynes, N. L. J.: Soil sample storage conditions affect measurements of pH, potassium, and nitrogen, Soil Sci. Soc. Am. J., 88, 930–941, https://doi.org/10.1002/saj2.20653, 2024.
Sonneveld, C. and van den Ende, J.: Soil analysis by means of a 1:2 volume extract, Plant Soil, 63, 523–526, https://www.jstor.org/stable/42933277 (last access: 17 January 2025), 1971.
Sterner, R. W. and Elser, J. J.: Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere, Princeton University Press, http://www.jstor.org/stable/j.ctt1jktrp3 (last access: 17 January 2025), 2002.
Subalusky, A. L., Dutton, C. L., Rosi, E. J., and Post, D. M.: Annual mass drownings of the Serengeti wildebeest migration influence nutrient cycling and storage in the Mara River, P. Natl. Acad. Sci. USA, 114, 7647–7652, https://doi.org/10.1073/pnas.1614778114, 2017.
Subalusky, A. L., Dutton, C. L., Rosi, E. J, Puth, L. M., and Post, D. M.: River of bones: wildebeest skeletons leave a legacy of mass mortality in Mara River, Kenya. Front. Ecol. Evol., 8, fevo-08-00031, https://doi.org/10.3389/fevo.2020.00031, 2020.
Towne, E. G.: Prairie vegetation and soil nutrient responses to ungulate carcasses, Oecologia, 122, 232–239, https://www.jstor.org/stable/4222536 (last access: 17 January 2025), 2000.
Turner, B. L. and Romero, T. E.: Short-term changes in extractable inorganic nutrients during storage of tropical rainforest soils, Soil Sci. Soc. Am. J., 73, 1972–1979, https://doi.org/10.2136/sssaj2008.0407, 2009.
Turner, W. C., Kausrud, K. L., Krishnappa, Y. S., Cromsigt, J. P. G. M., Ganz, H. H., Mapaure, I., Cloete, C. C., Havarua, Z., Küsters, M., Getz, W. M., and Stenseth, N. C.: Fatal attraction: vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites, Proc. R. Soc. B, 281, 20141785, https://doi.org/10.1098/rspb.2014.1785, 2014.
van Klink, R., van Laar-Wiersma, J., Vorst, O., and Smit, C.: Rewilding with large herbivores: Positive direct and delayed effects of carrion on plant and arthropod communities, PloS One, 15, e0226946, https://doi.org/10.1371/journal.pone.0226946, 2020.
Venter, F. J., Scholes, R. J., and Eckhardt, H. C.: Abiotic template and its associated vegetation pattern, in: The Kruger experience: ecology and management of savanna heterogeneity, edited by: Du Toit, J. T., Rogers, K. H., and Biggs, H. C., Island Press, Washington, DC, USA, 83–129, ISBN 9781559639828, 2003.
Wiersum, L. K.: Uptake of nitrogen and phosphorus in relation to soil structure and nutrient mobility, Plant Soil, 16, 62–70, https://doi.org/10.1007/BF01378158, 1962.
Wigley, B. J., Coetsee, C., Fritz, C., and Bond, W. J.: Herbivores shape woody plant communities in the Kruger National Park: lessons from three long-term exclosures: original research, Koedoe, 56, a1165, https://doi.org/10.4102/koedoe.v56i1.1165, 2014.
Yang, L. H.: Periodical cicadas as resource pulses in North American forests, Science, 306, 1565–1567, https://doi.org/10.1126/science.1103114, 2004.
Yang, L. H.: Pulses of dead periodical cicadas increase herbivory of American bellflowers, Ecol., 89, 1497–1502, https://doi.org/10.1890/07-1853.1, 2008.
Yong, S. K., Jalaludin, N. H., Brau, E., Shamsudin, N. N., and Heo, C. C.: Changes in soil nutrients (ammonia, phosphate and nitrate) associated with rat carcass decomposition under tropical climatic conditions, Soil Res., 57, 482–488, https://doi.org/10.1071/SR18279, 2019.




