the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
External and internal drivers behind the formation, vegetation succession, and carbon balance of a subarctic fen margin
Teemu Juselius-Rajamäki
Sanna Piilo
Susanna Salminen-Paatero
Emilia Tuomaala
Tarmo Virtanen
Atte Korhola
Anna Autio
Hannu Marttila
Pertti Ala-Aho
Annalea Lohila
Minna Väliranta
Peatlands are the most carbon-dense terrestrial ecosystem, and recent studies have shown that the northern peatlands have been (and still are) expanding into new areas. However, depending on the vegetation and hydrological regime in the newly initiated areas, the climate forcing may vary. If these new areas developed as wet fen-type peatlands with high methane emissions, they would initially have a warming effect on the climate. On the other hand, if development began as dry bog-type peatlands, these new peatland areas would likely act as a strong carbon sink from early on. However, although some research has concentrated on the expansion of the new northern peatland areas, there remains a significant lack of studies on the successional development of the newly initiated peatland frontiers. In this research, we combine paleoecological, remote-sensing, and hydrological modeling methods to study the expansion and successional pathway dynamics in a subarctic fen margin in Finnish Lapland and discuss possible implications for the carbon balance of these marginal peatland areas. Our results show that (1) the studied peatland margins started to develop ca. 2000 years ago and have continued to expand thereafter and (2) this expansion has occurred in nonlinear fashion. In addition, wet fen-type vegetation persisted in the studied margin for the majority of the developmental history, and only dryer conditions after the Little Ice Age instigated the fen-to-bog transition. However, a notable part of the fen margins in the Lompolonvuoma and Lompolojänkkä basins has remained wet fen-type vegetation, and the persistence of this vegetation type was likely caused by the hydrological conditions in the peatland and surrounding catchment. Our findings show a large variation in the peatland expansion and succession dynamics, even within a single peatland basin. Although changes in climate conditions initiated the fen-to-bog process in some margins, some vegetation remained in the wet fen stage, showing resilience to allogenic forcings. Thus, when estimating the peatland carbon stocks and predicting the future trajectories for peatland development, this heterogeneity should be taken into account to avoid errors caused by the oversimplification of peatland lateral expansion dynamics.
- Article
(10135 KB) - Full-text XML
-
Supplement
(2086 KB) - BibTeX
- EndNote
After peatland initiation via primary peat formation, infilling (terrestrialization), or paludification, peatland area is increased by lateral expansion – the most important process for forming new peatland areas in the modern climate (Ruppel et al., 2013). In raised mires, these new peatland margin areas have been generally described as moist minerotrophic fens and spruce swamps (Howie and Meerveld, 2011; Rydin and Jeglum, 2013), whereas in aapa mires (patterned fens), the margins vary from dry ombrotrophic bogs to wetter lush swamps (Laitinen et al., 2005, 2007). However, although the current vegetation in aapa mire margins has been described in standard peatland literature, there is an obvious lack of studies on the long-term successional development of these transitional ecotones between peatlands and the surrounding mineral land. A recent study with a main focus on the aapa mire region of Finland showed that the northern peatlands are still expanding (Juselius-Rajamäki et al., 2023), and whether these newly forming peatland areas initiate and develop as moist fens or dryer bogs can markedly affect the climate forcing of this recent lateral expansion.
The lateral expansion process is driven by both allogenic and autogenic factors. For instance, forest fire or other disturbances in areas adjacent to a peatland decrease evapotranspiration and cause an increase in the water table that enables peatland expansion (Kuhry and Turunen, 2006). Similarly, waterlogging may be caused by the autogenic development of adjacent peatlands. As the peat accumulates vertically, the surface and groundwater flow pathways are directed towards the margins of peat mounds (Autio et al., 2023), creating suitable conditions for new peat formation (Korhola, 1996; Rydin and Jeglum, 2013). On the other hand, drainage ditches located in the mire margins can prevent natural discharge to peatlands and, thus, block lateral expansion (Sallinen et al., 2019), while high-intensity fires can destroy peat layers, thereby setting back the advance of peatland margins (Kuhry, 1994; Simard et al., 2007). Furthermore, climate affects the lateral expansion of peatlands; for example, during the warm and dry climate phase between 8000 and 5000 BP (before present), the expansion of peatlands slowed down, whereas the wet and humid climate from 5000 to 3000 BP promoted lateral peatland expansion (Korhola, 1994, 1995; Ruppel et al., 2013).
The development of vegetation communities in the newly initiated peatland margins varies according to the nonlinear successional trajectory and is driven by seasonal water availability and, consequently, the transportation of essential ions (Goud et al., 2018). Depending on the topography, surface flow may control the first appearance of vegetation communities. Later groundwater seepage, at the point scale or as a wider seepage front, transports moisture and dissolved elements to established plants. Compared to raised mires that have grown vertically above the surrounding marginal areas, and often the entire landscape (Howie and Meerveld, 2011; Rydin and Jeglum, 2013), the secondary peatland development pattern over the margins is more complex for aapa mires, as the shape of the peatland varies from flat to concave (Seppä, 2002) and the formation of new peatland habitats is dependent on the water supplied by snowmelt (Sallinen et al., 2023) and the dilution of ion concentrations (pH levels). Newly established habitat types may range from ombrotrophic bog types to minerotrophic swamps and fens (Foster and King, 1984; Laitinen et al., 2005, 2007; Mäkilä and Moisanen, 2007; Ruuhijärvi, 1983). However, mechanisms, such as surface water hydraulic forcing, that create different types of margins are currently poorly understood.
Differences in local hydrology mirrored in the current vegetation communities suggest opposite climatic feedback mechanisms for the peatland centers and marginal areas. The overall climatic effect of peatlands is (and has been) strongly controlled by the balance between the sequestration of carbon dioxide (CO2) and the release of methane (CH4) (Frolking and Roulet, 2007). Methane is produced under anoxic conditions and released into the atmosphere via vegetation, ebullition, or diffusion (Lai, 2009; Rydin and Jeglum, 2013). However, in areas where the acrotelm (i.e., the oxic and biologically active layer of the peat) is thick, most of the methane is oxidated to carbon dioxide (Lai, 2009). Thus, in the peatland margins where dry bog-type vegetation communities dominate, the climate forcing is most likely negative (i.e., a cooling impact on climate) due to the continuous uptake of CO2 and the low CH4 emissions. However, if the water table depth becomes too deep, accelerated decomposition can turn these locations to carbon sources due to increased CO2 emissions that negate the decrease in CH4 emissions (Evans et al., 2021). On the other hand, in wet fen-type margins, high methane emissions have the opposite effect at short timescales, further amplified by graminoid vegetation communities (Bubier et al., 1993; Juutinen et al., 2013; Kou et al., 2022; Ward et al., 2013).
Often, the interest of (paleo)peatland researchers has been focused on the deepest and oldest part of a peatland, while the development of peatland margins (i.e., younger areas) has attracted less consideration (Korhola et al., 2010; Ruppel et al., 2013). Only recently has the focus turned to peatland margins, and peat profile sampling has been extended to these regions (Juselius-Rajamäki et al., 2023; Lacourse et al., 2019; Le Stum-Boivin et al., 2019; Mathijssen et al., 2014, 2016, 2017; Peregon et al., 2009; Schaffhauser et al., 2017). Nevertheless, even these studies have focused more on the expansion dynamics of the peatlands, while the vegetation succession of the marginal areas has received less consideration. As past vegetation communities can be used to ascertain climate feedback, knowledge of vegetation succession in peatland margins can be used to better understand how lateral expansion has affected the past climates and, thus, helps us to predict the effects of lateral expansion in the context of future climate change.
Here, we studied the expansion and successional pathways of peatland margins in a subarctic fen, Lompolonvuoma, located in Finnish Lapland, using a novel approach combining paleoecological, remote-sensing, and hydrological modeling methods. To obtain a wider understanding of the development and diversity of plant communities in aapa mire margins, we used additional comparable peat profile data from three other peatlands from northern Finland as well as detailed remote-sensing-based vegetation and land cover classification information (Räsänen et al., 2021) from Lompolonvuoma fen margins. The aim of the research was to study when the development of the mire margins occurred and to establish the vegetation composition of the mire margins during the different stages of this development. In addition, we studied how the local and external factors contributed to the mire margin development. The results of our study provide insight into aapa mire margin succession patterns, an overview of their relation to hydrology, a basic understanding of the climate feedback of the peatland margin development, and a better grasp on the carbon balance related to peatland lateral expansion in subarctic areas.
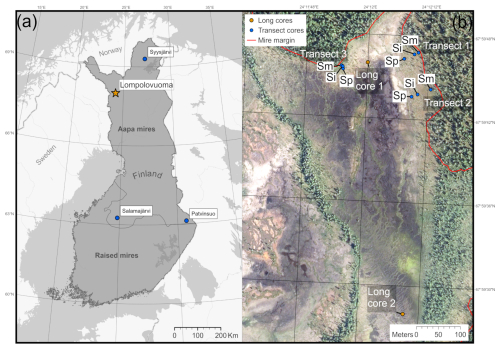
Figure 1(a) Location of the main Lompolonvuoma study site (marked with an orange star) and of the comparison sites (marked with blue circles). The border separating the aapa and raised mire complex areas in Finland is presented. (b) The study location within the Lompolonvuoma basin shows the study transect samples (blue circles) and the long cores (orange circles). For the transect samples, the sample code indicates the sample location within the transect: Sm for the sample closest to the mire margin, Si for the sample in the middle of the transect, and Sp for the sample closest to the peatland center. Mire margins are shown using a red line. The aerial image is from the National Land Survey of Finland and was taken in 2023. This figure contains data from the National Land Survey of Finland NLS Aerial photographs database.
2.1 Study sites
The Lompolonvuoma study site is a subarctic fen located in the municipality of Muonio in Finnish Lapland (67°59′42′′ N, 24°12′ E; Fig. 1a). The site belongs to the northern aapa mire zone, which has a more continental climate, a shorter growing season, and more profound frost effects than the aapa mires located further south (Ruuhijärvi, 1983). The mean annual temperature at the study site is 0.4 °C (2003–2019) and the mean annual precipitation is 647 mm (2008–2019) (Marttila et al., 2021).
We studied the margins of a sub-basin in a larger fen complex that was comprised of several elongated, north–south-aligned fen areas. The vegetation in the central areas of the study site is dominated by typical wet fen taxa, such as various Carex species and flark Sphagnum species. Strings mainly occur in the southern parts of the basin. A stream runs across the peatland basin from south to north towards Lake Pallasjärvi.
Vegetation communities in the studied peatland margins resemble raised pine bog habitats in the south, with low hummocks and narrow lawn areas (Laine et al., 2012). The ground layer consists of Sphagnum fuscum and Sphagnum angustifolium, and Cladonia sp. lichens also occur. In the field layer, Eriophorum vaginatum, Rubus chamaemorus, and various dwarf shrubs (such as Empetrum sp., Andromeda polifolia, and Vaccinium vitis-idaea) are found. In addition, stunted Pinus sylvestris individuals grow on the hummocks.
To expand our understanding of vegetation succession in aapa mire margins, we used three additional short profiles collected from aapa mires elsewhere in Finland: Syysjärvi, Salamajärvi, and Patvinsuo (Fig. 1a). These profiles enabled comparison between different local and geographic settings across Finland. For a full description of the study sites, field sampling, and laboratory analysis for supplementary sites, the reader is referred to Juselius-Rajamäki et al. (2023).
2.2 Field sampling
The field sampling for the study was conducted during the summer and the autumn of 2022. To study the lateral expansion dynamics, we sampled a total of three transects. The sample transects were coded as “T” followed by the transect number. Each transect comprised three peat core samples, which were coded as “S”. We then added information on the core location indicator, which was coded as “p” for the sample located closest to the peatland center, “i” for the intermediate sample, and “m” for the sample located closest to the margin. Each transect ran from the edge of the peatland towards the center. The transects were placed on the “bog-type” margin with variable width, which led to variable transect lengths. All profiles were collected from lawn microform with similar types of vegetation. The first sampling location for each transect was at the extreme end of the bog-type margin next to the central fen area. The other samples were collected along the transect from locations fulfilling the above criteria; samples were collected in such manner that entire length of bog-type margin at the transect location was covered. We established two transects from the east edge and one transect from the north edge of the fen sub-basin (Fig. 1b). The peat cores were taken with a box corer ( cm) down to mineral subsoil. To reconstruct Holocene peatland initiation, in addition to the peat cores sampled from the mire margin, four long cores were collected from two different locations in the central part of the study basin: two of the long cores, a and b, were located close to each other and represent replicates (Fig. 1b). These samples were collected using a Russian peat corer (3×50 cm). The profiles were described and classified in the field, and the length of the profile was measured. The location of each sampling point was recorded using a Trimble R8 GPS device with ±0.05 m accuracy, and the distance between each transect sampling point was measured using a tape measure. After sampling, the peat cores were carefully wrapped in plastic to avoid any contamination and transported to University of Helsinki premises. The samples were stored in a cold room prior to further analysis.
2.3 Laboratory analysis
The short profiles were cut into 1 cm subsamples. From these subsamples, dry bulk density (BD, g cm−3) and sediment organic matter (OM) based on the loss on ignition method (LOI) were determined (Heiri et al., 2001). We used LOI values to differentiate between the mineral subsoil and the peat. We defined the peat initiation depth based on the first layer where LOI ≥70 % (Korhola, 1994). In addition, we analyzed the C and N content at a 4 cm (transect 1 and 3) or 5 cm (transect 2) interval using a LECO TruSpec micro elemental determinator. For the long profiles, the contact layer between limnotelmatic Equisetum peat and fen peat, without visible Equisetum remains, was first determined in the field and then confirmed using a stereomicroscope.
To reconstruct past changes in vegetation, plant sub-macrofossil analysis for each short peat profile was conducted at 4 cm intervals; however, when prominent changes occurred, the interval was increased to every second centimeter. The percentage proportion of each peat-forming vegetation type of a total sample volume (100 %) was analyzed from 5 cm3 peat samples that were gently rinsed under running water in a 100 µm sieve. The residue was analyzed under a stereomicroscope following Väliranta et al. (2007) and Mauquoy et al. (2010). For example, seeds and leaves were counted as exact numbers, while the percentage of unidentified organic material (UOM) was estimated for highly decomposed organic remains that had lost their microscopical characteristics. A compound light microscope was used for higher-taxonomic-level identification. The Tilia (Grimm, 2011) and C2 (Juggins, 2007) software packages were used to create diagrams.
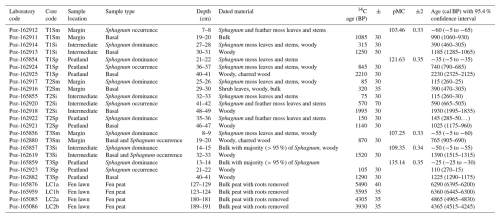
To study the lateral expansion and succession dynamics of the fen margins, we applied accelerator mass spectrometry (AMS) radiocarbon (14C) determinations to date the basal peat of each short profile and the depths corresponding to the major regime shifts in vegetation (e.g., the first occurrence of the Sphagnum mosses overlying sedge-dominated peat and the shift to Sphagnum dominance). For the long profiles, we dated the shift from limnotelmatic Equisetum peat to fen peat to gain an understanding of the long-term development of the Lompolonvuoma fen. Terrestrial plant remains and charcoal were prioritized over bulk peat samples for 14C analyses (Quik et al., 2022). However, in three cases regarding the short cores, the peat was highly decomposed and bulk peat had to be used (Table 1). In addition, bulk peat was used as material for the AMS dating of the long cores. Rootlets were carefully removed from the bulk peat samples. Samples were dated in the Poznań Radiocarbon Laboratory (Poznań, Poland). We calibrated 14C BP ages against the IntCal20 Northern Hemisphere calibration curve (Reimer et al., 2020) and modern dates (pMC % modern carbon) using the Bomb21 NH1 calibration curve (Hua et al., 2022). Finally, calibrated ages were rounded to the nearest 5 years.
Table 1Peat profile description. The core sample location describes the location of the sampling across the transects, with “Margin” being located closest to the mire–forest boundary and “Peatland” being located closest to the mire center. Sample type describes the location within the profile, with “Basal” representing the contact layer between peat and mineral subsoil, “Sphagnum occurrence” indicating the first occurrence of Sphagnum mosses, and “Sphagnum dominance” indicating the first layer with clear Sphagnum dominance. Sample description indicates the material used in 14C analyses. Age (cal BP) with the 95 % confidence interval shows the calibrated median age with the 95.4 % confidence intervals.
For the comparison profiles, radiocarbon dating results were acquired from Juselius-Rajamäki et al. (2023). In addition, radiolead (210Pb) dating was performed for the comparison profiles at the Department of Chemistry, University of Helsinki. The separation method used for 210Po was a combination of several previously published methods (Ali et al., 2008; Flynn, 1968; Kauranen and Miettinen, 1966; Sanderson, 2016). Dried peat samples were digested with concentrated HNO3 and HCl acid. The 209Po tracer spike was added to the samples at the beginning of the analysis to monitor the yield loss. After digestion, the samples were evaporated to dryness, dissolved into a dilute HCl solution, filtered, and transferred into deposition vessels made from PTFE. Ascorbic acid was added to reduce interfering impurities (e.g., Fe) in the samples. The 210Po was deposited spontaneously onto a silver disk in the deposition vessel using a heated water bath (65–75 °C) with constant stirring for 2.5–3 h. The activity concentration of 210Po was measured from the silver disk with a PIPS (passivated implanted planar silicon) detector. The activity concentration of 210Pb in the samples was obtained via the equilibrium of 210Po and 210Pb in the samples.
2.4 Age–depth models
Age–depth models with 14C ages were done using the bacon package ver. 3.2.0 (Blaauw and Christen, 2011) in R software ver. 4.3.1 (R Core Team, 2023). We assumed different peat accumulation rates for different vegetation community stages; these were acquired from the literature and represented similar vegetation communities and geographic locations (Granlund et al., 2022; Mäkilä et al., 2001; Mathijssen et al., 2014; Rydin and Jeglum, 2013; Zhang et al., 2020). We used these accumulation rates as a prior value for the age–depth models for corresponding vegetation community stages. After the initial model run, if the model fit was not satisfied (Blaauw and Christen, 2011), the prior values were altered to ensure the model fit. Boundaries were set for the profiles based on vegetation community shifts, and different accumulation rates were calculated for different plant communities. For the profiles with both 14C and 210Pb ages (e.g., SyJ T1Sm, SJ T3Sm, and PS T1Sm), we used the Plum package ver. 0.4.0 (Aquino-López et al., 2018) in R software ver. 4.3.1 (R Core Team, 2023). For the comparison peat profiles, the same prior accumulation rates were used as for the Lompolonvuoma study site. Again, to accommodate the individual peat profile characteristics, the rates were modified to ensure age–depth model fit. The individual age–depth models containing the accumulation rates and the boundaries used are presented in the Supplement (Figs. S1–S12).
2.5 Lateral expansion rates
Lateral expansion rates (cm yr−1) were calculated between adjacent peat sections in each transect. The rates were calculated by dividing the horizontal distance between adjacent dated profiles (cm) by the respective difference in the basal ages (years). Mean calibrated ages from the age–depth model were used.
2.6 Current vegetation community analysis
We used field- and remote-sensing-based land cover type data from Räsänen et al. (2021), who describe the methodology in detail, to estimate the proportion of vegetation communities in the peatland margins. Land cover classification was based on field verification data collected in summer 2019 and on multisource remotely sensed data. Classification was conducted in two steps. First a four-channel aerial image with a 0.5 m pixel size from summer 2018 was segmented. Second, values for these segments (mean size of 50 m2) were then calculated from several lidar, PlanetScope, and Sentinel images from the years 2018 and 2019, and these were classified using random forest classification. The final land cover product had 16 classes, and the overall classification accuracy was 76 %. Here, we used a simplified classification based on an ombrotrophic–minerotrophic gradient to describe habitat conditions and related vegetation community. In addition, tree-covered fens were separated from open fens. The applied vegetation communities are as follows: bog-type vegetation (referring to dry conditions), fen-type vegetation (referring to wet conditions), and tree-covered fens (referring to forested peatland). These community types enable comparison with the remote-sensing data and were combined from the land cover type classes with similar ecological characteristics: dwarf shrub pine bogs and dwarf shrub bogs as bogs; tall sedge fens and flarks as fens; and paludified spruce, birch, and mixed forests as tree-covered fens. We delineated our study basin as the Lompolonvuoma basin and the adjacent Lompolojänkkä basin based on the land cover dataset in ArcGis Pro ver. 3.1.0 (Esri, 2023) and calculated the proportion of each land cover type for the whole peatland area and for the peatland margins. For the peatland margins, we chose a 25 m distance from the peatland–forest border to represent the marginal peatland area. This distance prevented any overlap of the marginal areas, even in the narrowest parts of the peatland, and allowed non-biased analysis of the marginal peatland types irrespective of the topography or vegetation at the site.
2.7 Hydrological analyses
To study the hydrological drivers behind the development of divergent peatland types at the fen margins detected in our vegetation coverage analysis, we used the fully integrated physically based hydrogeological model HydroGeoSphere (Aquanty, 2015). The model allows explicit simulation of water exchange between groundwater and surface water and can be parameterized using physical properties of peat and mineral soils. The high spatial resolution of the model makes it suitable to estimate water fluxes at the scale of vegetation inventories and remote-sensing data. This model has previously been implemented for the Pallaslompolo catchment, and the full methodology for this hydrogeological model is described in Autio et al. (2023). Due to the original study boundaries, this model only covers the Lompolojänkkä sub-basin. In this study, we (1) investigated the resulting hydrological conditions in terms of groundwater–surface water exchange flux and (2) compared the impact of the current climate (baseline) and a drier climate in terms of the water table elevation (Helama et al., 2017).
In step (1), we investigated the prevailing groundwater–surface water exchange fluxes of the transient model run averaged over the summer of 2017 within each peatland type. For step (2), we studied the effect of drier climate conditions by comparing the outputs of the steady-state simulations for the current climate with an effective rainfall Peff equal to 385 mm (average for 2016–2018) and a drier climate with a Peff equal to 250 mm. The value of 250 mm is within the measured range that varied between 170 and 574 mm for the 2008–2018 period, but it represents a significantly lower value than the measured long-term mean of 358 mm for the 2008–2018 period. Due to the variable density of the model computing mesh, the model output was first plotted in the postprocessing visualization software Tecplot 360 EX 2022 R2, which accommodates value interpolation over element size. The variables were divided into separate bins according to magnitude, hereafter referred to as contour groups, showing spatial variation in model output. The resulting raster image was imported to GIS mapping software (Esri, 2023), georeferenced, and clipped according to the defined peatland margins for each peatland type. The areas of each contour group were then calculated for each respective peatland type.
3.1 Peat initiation and spatial development of the peatland margins
In transect 1, the oldest basal date of ca. 2230 cal BP was dated from the peat profile closest to the mire center (T1Sp) (Table 1). For the intermediate profile (T1Si), the basal age was ca. 1185 cal BP, whereas for the profile next to the forest (T1Sm), the basal age was ca. 990 cal BP. In transect 2, the oldest basal age found in the intermediate profile (T2Si) was 1930 cal BP, whereas younger basal ages of 1025 and 390 cal BP were found for the T3Sp and T3Sm profiles, respectively (Table 1) The oldest basal age in transect 3 was 1390 cal BP in the intermediate sample T2Si (Table 1), whereas the basal age in the sample closest to the mire center (T3Sp) was 1225 cal BP and that in the peatland margin (T3Sm) was 765 cal BP.
Long-core (LC) dating results suggest that a shift from limnotelmatic peat to fen peat occurred at ca. 6300 cal BP at the earliest and at around 4000 cal BP at the latest (Table 1). This change occurred earlier in the northern part of the sub-basin (LC1a at ca. 6290 cal BP and LC1b at ca. 6360 cal BP). In the southern part, this shift occurred at ca. 4865 cal BP for LC2a and at ca. 4365 cal BP for LC2b.
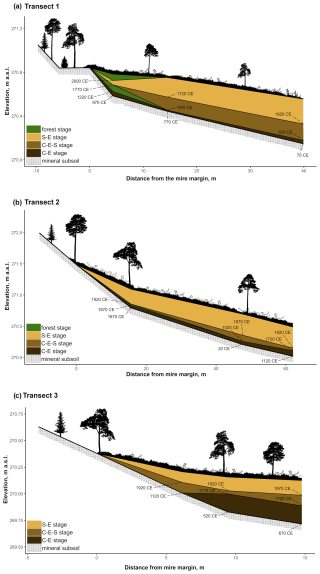
Figure 2Transect profiles. Panels show the vegetation community stages: C–E (Cyperaceae–Ericaceae), C–E–S (Cyperaceae–Ericaceae–Sphagnum), and S–E (Sphagnum–Ericaceae) as well as the forest community stages in the margins of (a) T1 and (b) T2. In addition, the onset of each stage at the peat profile location is shown, with ages (CE: common era) derived from the age–depth model. The ratios between the x and y axes vary between the illustrations. The vegetation is presented to give a rough impression of real-life conditions at the study transect locations but is not true to scale.
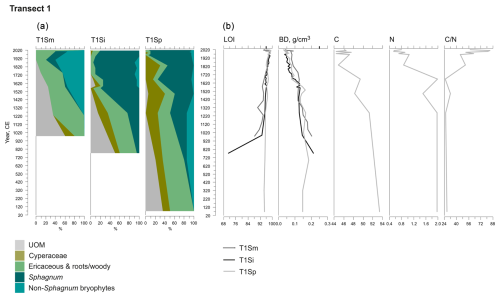
Figure 3Fossil plant records for transect 1: (a) undetected organic matter (UOM) and (b) loss on ignition (LOI), bulk density (BD), carbon and nitrogen contents, and the ratio. The proportion of vegetation type and the LOI are both given as a percentage (%), whereas bulk density is given in grams per cubic centimeter (g cm−3). Carbon content (%), nitrogen content (%), and the ratio are available for the T1Sp profile only.
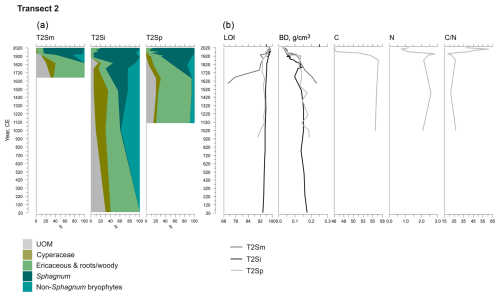
Figure 4Fossil plant records for transect 2: (a) undetected organic matter (UOM) and (b) loss on ignition (LOI), bulk density (BD), carbon and nitrogen contents, and the ratio. The proportion of vegetation type and the LOI are both given as a percentage (%), whereas bulk density is given in grams per cubic centimeter (g cm−3). Carbon content (%), nitrogen content (%), and the ratio are available for the T2Sp profile only.
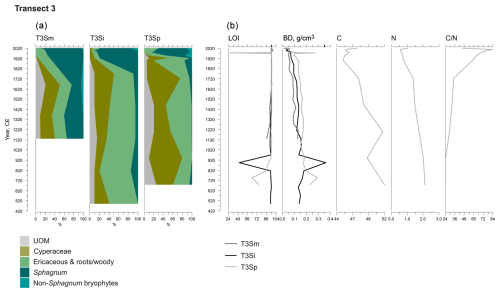
Figure 5Fossil plant records for transect 3: (a) undetected organic matter (UOM) and (b) loss on ignition (LOI), bulk density (BD), carbon and nitrogen contents, and the ratio. The proportion of vegetation type and LOI are both given as a percentage (%), whereas bulk density is given in grams per cubic centimeter (g cm−3). Carbon content (%), nitrogen content (%), and the ratio are available for the T3Sp profile only.
3.2 Peat properties
A shift from the mineral layer to the organic layer was sharp in all profiles, and the loss on ignition (LOI, %) values varied only slightly in the upper parts of the profiles (Figs. 3, 4, 5). In transect 3, the mineral material intruded into the peat at depths of 14 cm (T3Sp) and 23 cm (T3Si). Compared to LOI (%), more fluctuations were visible in bulk density (BD, g cm−3) values (Figs. 3, 4, 5). Above the sharp mineral subsoil–peat contact, the BD decreased towards the surface, with the lowest values found at the top of peat profiles. A stepwise decrease in BD occurred in peat profiles T1Sp, T2Si, T2Sp, and T3Sm, while a gradual decrease in BD values was observed in other profiles. Carbon content (%) above the mineral subsoil contact varied only slightly along the peat profiles (Figs. 3, 4, 5), and the highest nitrogen contents along the peat profiles were found in the layers closest to the mineral subsoil and the surface (Figs. 3, 4, 5).
3.3 Fossil plant communities and succession of the peatland margins
Three main vegetation stages were identified in the Lompolonvuoma peat margin profiles (Figs. 2a–c, 3, 4, 5). The first and oldest stage consisted of the remnants of cyperaceous and ericaceous vegetation (C–E), but it lacked the brown mosses usually associated with calcareous fens. This phase was characterized by a high proportion of unidentified organic matter (UOM), indicating a high level of humidification. The second stage contained the remains of mixed Cyperaceae–Ericaceae–Sphagnum (C–E–S) vegetation. The transition from stage 1 to 2 occurred gradually in some peat profiles, although the shift was sometimes abrupt in other profiles. In this transition Sphagnum sect. Acutifolia started to replace cyperaceous vegetation. In transects 1 and 3, the high level of decomposition prevented species-level identification of Sphagnum mosses in the early C–E–S stage. However, in transect 2, the C–E stage was directly overlain by Sphagnum fuscum. In the final Sphagnum–Ericaceae stage (S–E), the plant community is dominated by Sphagnum mosses, and the cyperaceous vegetation is nearly or completely missing. Sphagnum species consist of Sphagnum fuscum, S. capillifolium, S. russowii, and S. angustifolium. A varying amount (%) of ericaceous vegetation is usually mixed with the sphagna. Varying amounts of forest bryophytes, such as Pleurozium schreberi, are also detected through the peat layers. In addition, in the marginal profiles of transect 1, the mire vegetation was replaced twice by forest vegetation, and similar replacement occurred once in the margin of transect 2. Macrofossil data are presented in Figs. A2, A3, and A4 in the Appendix.
At the onset of peat development in the mire margins, the C–E vegetation community dominated (Figs. 2a–c, 3, 4, 5). In transects 1 and 2, this layer was thin (only up to 4 cm in transect 1 and from 5 to 9 cm in transect 2). In transect 3, the C–E layer was markedly thicker (16 cm in T3Si and 18 cm in T3Sp). The duration of the C–E stage was highly variable: in transect 1, the C–E stage lasted between ca. 250 (T1Sm) and 600 years (T1Sp); in transect 2, the C–E stage lasted between ca. 200 (T2Sm) and 1000 (T2Si) years; and in transect 3, the C–E stage was missing from the profile closest to the mire margin (T3Sm) and Sphagnum mosses established directly on top of the mineral subsoil, and the duration of the C–E stage was ca. 1250 years in T3Si and ca. 1050 years in T3Sp.
The C–E stage ended asynchronously across the Lompolonvuoma mire margin, and the C–E stage was followed by the mixed C–E–S stage in most of the cases, during which sphagna started to colonize the margins. The establishment of sphagna, marking the start of the C–E–S, occurred between ca. 670 and 970 CE in transect 1, between ca. 1020 and 1720 CE in transect 2, and between ca. 1720 and 1770 CE in transect 3. No C–E–S stage was detected in samples T1Sm and T2Sm. Instead, the vegetation shifted towards a mix of ericaceous vegetation, Pleurozium schreberi and Dicranum sp., suggesting a turn toward dryer conditions. In T3Sm, the C–E–S stage occurred directly over the mineral subsoil.
Contrary to the asynchronous shift from the C–E stage to the C–E–S stage, the change to an ombrotrophic vegetation community (S–E) with a high proportion of sphagna appeared nearly simultaneously across all studied margins. This stage started between ca. 1870 and 1970 in all peat sections in transects 2 and 3 and similarly also in T1Sp. Only in T1Sm (1770 CE) and T1Si (1720 CE) did the shift to an S–E vegetation community stage occur earlier. Currently, the S–E vegetation type is predominant across the transects.
A comparable successional pathway to that in Lompolonvuoma was detected at a Syysjärvi study site in eastern Lapland (Fig. A1). A 1 cm thick ericaceous vegetation layer overlaid mineral soil, and this community was shortly replaced by a 2 cm thick C–E layer, which is similar to the results found in Lompolonvuoma. These respective stages lasted only ca. 15 years, after which the C–E–S stage with some sphagna took over in ca. 1970 CE. Above the 3 cm thick C–E–S stage, the S–E stage mostly comprised Sphagnum capillifolium that took over in ca. 1980 and has persisted ever since.
Different successional pathways were found at the Salamajärvi and Patvinsuo peatland sites (Fig. A1). At the Salamajärvi site, there was no evidence of cyperaceous vegetation; rather, peat layers comprising ericaceous vegetation with a small amount of Sphagnum moss initiated directly on mineral subsoil in ca. 1830 CE in the margin of the Salamajärvi peatland. Afterwards, the proportion of sphagna gradually started to increase and Sphagnum mosses became dominant in ca. 1950 CE. Currently, Sphagnum capillifolium is the dominant moss species.
When peat formation started in the Patvinsuo margin (Fig. A1) in ca. 1850 CE, the initial community consisted of C–E–S vegetation. At first, the proportion of Sphagnum mosses started to increase, and these species were the dominant taxa by ca. 1915 CE. However, between ca. 1915 and 1950 CE, Sphagnum mosses and the remains of Cyperaceae nearly disappeared, and mostly ericaceous vegetation remained and was supplemented by the presence of Cenococcum sclerotia, which suggest dry-mire-margin conditions. However, towards the present, the amount of Sphagnum moss again increased, and Sphagnum species currently form most of the coring site vegetation, with Sphagnum russowii being the most common.
3.4 Lateral expansion rates and the vertical peat increment
The average rate of lateral expansion between dated peat profiles varied from 0.53 cm yr−1 (T3Si to T3Sm) to 5.23 cm yr−1 (T1Si to T1Sm). The median lateral expansion rate for all transects was 2.25 cm yr−1, with an interquartile range of 1.72–2.90 cm yr−1.
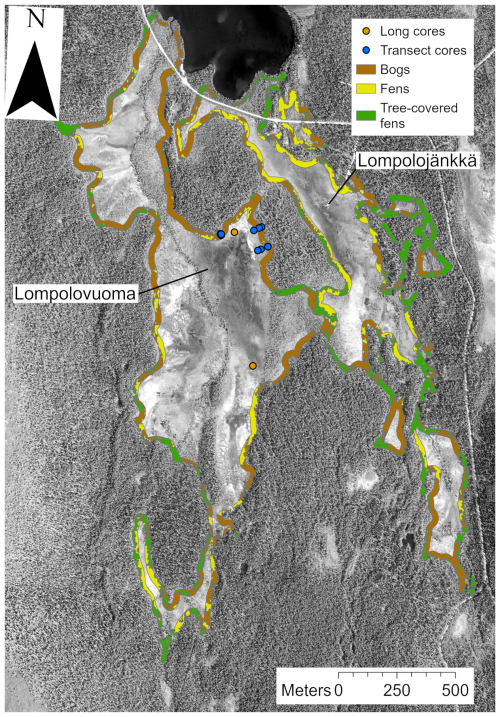
Figure 6Peatland margin vegetation communities. The area 25 m from the peatland margin is divided into bog-type vegetation (brown), fen-type vegetation (yellow), and tree-covered fens (green) in the Lompolonvuoma study basin and the adjacent Lompolojänkkä basin. In addition, the locations of the study transect peat cores (blue circles) and long cores (orange circles) are shown. This figure contains data from National Land Survey of Finland NLS Aerial photographs database.
Table 2The vegetation class coverage and peatland area. The table shows the total area of the Lompolonvuoma and Lompolojänkkä peatland basins and the proportion of three vegetation community classes in the peatland basins: bog, fen, and tree-covered fens. In addition, the total area of the 25 m margin and the proportions of the vegetation community classes are shown. In the final two columns, the proportions of the vegetation community classes are shown individually for the Lompolonvuoma and Lompolojänkkä basins.
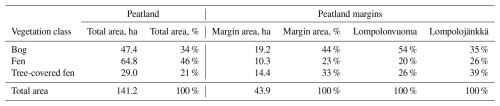
3.5 Vegetation community cover analysis
The total area of the Lompolonvuoma and Lompolojänkkä peatland basins is 141.2 ha, of which 34 % is classified as bog-type vegetation, 46 % is classified as fen-type vegetation, and 21 % is classified as tree-covered fens (Table 2). The area 25 m from the peatland border comprises, in total, 43.9 ha and covers 31 % of the total peatland area. In these marginal areas, bog-type vegetation constituted 44 %, fen-type vegetation constituted 23 %, and tree-covered fens constituted 33 % of the mire margin area (Fig. 6, Table 2). In Lompolonvuoma basin, where our study transects were located, the coverage of bog-type vegetation in the peatland margin is 54 %, whereas bog-type vegetation covers a smaller area (35 %) of the adjacent Lompolojänkkä peatland basin. On the contrary, a higher coverage of fen-type vegetation is found in the margins of the Lompolojänkkä basin (26 %) compared with the Lompolonvuoma basin (20 %). Similarly, larger areas were inhabited by tree-covered fens in the Lompolojänkkä basin (39 %) compared with the Lompolonvuoma basin (26 %).
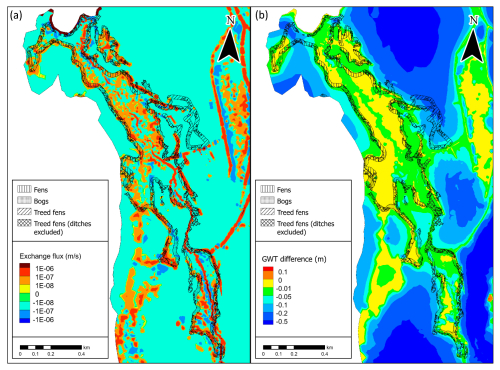
Figure 7(a) The GW–SW exchange flux patterns from the Lompolojänkkä sub-basin averaged for summer 2017, representing the current climate conditions. Positive flux values indicate the locations of groundwater exfiltration and infiltration towards groundwater. (b) The groundwater table elevation changes result from the drier climate conditions. Negative values indicate that the groundwater level decreases, whereas and positive values indicate that the groundwater level increases.
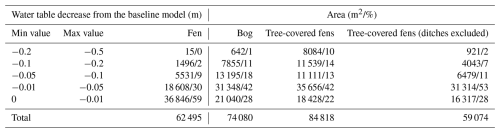
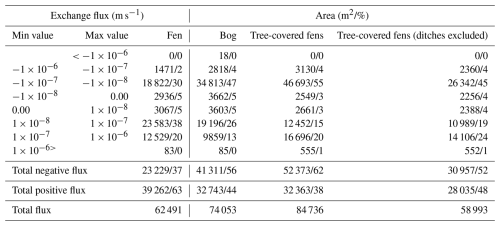
Table 3Exchange flux contour areas by vegetation type. Tree-covered fens (ditches excluded) exclude the open drainage areas.
3.6 Hydrological analyses
The simulated groundwater–surface water (GW–SW) exchange patterns for the current climatic and groundwater table (GWT) elevation change are shown in Fig. 7a and b, respectively. The calculated areas by contour group and peatland vegetation group are presented in Table 3 for the GW–SW exchange fluxes and in Table 4 for the changes in terms of GWT elevation.
In terms of exchange flux, the areas classified as fens indicate the dominance of the GW exfiltration over infiltration processes in the simulations. In contrast, the bog areas indicate more balance between infiltration and exfiltration processes, with a slight prevalence of the infiltration area. The areas classified as tree-covered fens show the dominance of infiltration. However, ∼30 % of the total tree-covered fen area is in the vicinity of the ditch network (the rightmost part of the peatland system), which impacted the peatland vegetation, as indicated by aerial photos (National Land Survey of Finland, 2023). After excluding the drained areas from tree-covered fens, the GW–SW exfiltration pattern is more balanced, with a slight prevalence of exfiltration.
In terms of GWT elevation changes, the simulated drier climatic conditions have a mild impact on the areas classified as fens, with 59 % of the water table decreasing by less than 1 cm and 89 % by less than 5 cm. In contrast, the areas classified as bog are more susceptible to GWT changes. They are characterized by significantly less extent with respect to the areas with a mild (less than 1 and 5 cm) water table decrease (only 28 % and 70 %, respectively) and a significant portion of areas (30 %) with a substantial decline (more than 5 cm). The tree-covered fen areas, excluding ditches, suggest that the water table decrease would be variable, with more GWT reduction than in the case of open fens but a lower reduction compared with bogs.
We studied lateral expansion and vegetation succession of peatland margins in a subarctic Lompolonvuoma fen in Finnish Lapland. Our results show that the studied margins in Lompolonvuoma started to develop ca. 2000 years ago, and the youngest basal age of 390 cal BP was still located a few meters from the current forest edge. Peat initiation in the margins occurred in several independent loci that only later coalesced into a continuous peatland. The initial wet Cyperaceae–Ericaceae marginal communities inhabited the fen margins over the time periods reaching from a few centuries to a millennium, and the following establishment of Sphagnum-moss-dominated communities was asynchronous. Starting from the end of the 18th century, these margins shifted to a climax bog plant community characterized by hummock sphagna and ericaceous vegetation. This change mostly occurred over a remarkably short time period (a few decades). However, our data also showed that forest vegetation had, on several occasions, intruded to the already established peatland, suggesting an ongoing “power struggle” between peatland and forest ecosystems. The marginal bog-type vegetation currently covers roughly 50 % of the margins in the Lompolonvuoma sub-basin, whereas only 35 % of margins have reached the ombrotrophic stage in the adjacent sub-basin of Lompolojänkkä. Our hydrological GW–SW model indicates that high water tables in the fen-type margins are sustained even under dry climatic conditions, showing a resistance potential to fen-to-bog transition.
4.1 Nonlinear development of peatland margins in Lompolonvuoma fen
The formation of Lompolonvuoma peat margins investigated here began ca. 2200 years ago. This differs from the central part of the mire, where limnotelmatic Equisetum peat found at the bottom the long peat profiles suggests that peat formation initially occurred over waterbodies. Similar to the results of Juselius-Rajamäki et al. (2023), these data contradict the traditional perception that peatland expansion has ceased or markedly slowed down in Fennoscandia over the last 2000 years (Ruuhijärvi, 1983; Sjörs, 1983). Instead, the current findings suggest that this presumption is due to an underrepresentation of studies and sample collection from the mire marginal areas, rather than an actual cessation of lateral expansion (Kuhry and Turunen, 2006; Ruppel et al., 2013). In transects 2 and 3, the expansion of new peat surfaces occurred from individual miniature loci; this is evidenced by that fact that the oldest basal ages were found in the middle of the profiles, whereas the oldest basal age in transect 1 was acquired for the profile closest to the main mire (Fig. 2a–c). However, the basal age and the basal elevation of T1Sp matches closely to the age and elevation of the oldest bottom age of transect 2, suggesting a relatively simultaneous initiation process.
The basal ages from the studied transects show that, after the initial peat formation, the individually formed peat patches spread both downhill towards the main mire area and uphill towards the adjacent forest. Only later were separate peat patches connected to main mire basin. Such convergence of the multiple smaller loci to a single peatland mass has been reported both during the early Holocene (Almquist-Jacobson and Foster, 1995; Korhola, 1992, 1994; Mathijssen et al., 2014, 2017) and for more recently developed mire margins (Juselius-Rajamäki et al., 2023). However, the mechanisms behind the development of individual peat patches and the later convergence have received only little attention and remain unresolved (Noble et al., 1984).
In Lompolonvuoma, peat initiation occurred on steep slopes (on average exceeding 0.5°, which is a threshold known to restrict peat formation in more continental regions where the availability of water is not excessive) (Almquist-Jacobson and Foster, 1995; Loisel et al., 2013; Zhao et al., 2014). Thus, in the past, suitable conditions promoting the initiation of individual peat patches must have existed. The peat patches may have started to form in small topographical depressions that, although initially well drained, may have become impervious due to the deposition of organic or fine inorganic matter, the formation of hardpans in the Spodosol layer, or the deposition of ash due to forest fires, thereby creating favorable conditions for peat formation (Klinger, 1996; Le Stum-Boivin et al., 2019; Mallik et al., 1984; Noble et al., 1984; Rydin and Jeglum, 2013). No full-scale subsoil topography measurements were conducted, but field survey data did not reveal any clear depressions underlying any of the oldest peat profiles. Another scenario is that, under sufficiently humid conditions, the peat formation began directly on the steep slopes, as suggested for southern Finland peatlands (Korhola, 1996). Climate reconstructions suggest that a wet climate phase prevailed in Lapland between 2500 and 2000 BP (Eronen et al., 1999; Luoto and Nevalainen, 2015), which may have promoted peat formation even on a relative steep slope, such as that presented here.
The vertical growth of peat as a driving mechanism for lateral expansion has been traditionally linked to raised mires (Foster and Wright, 1990). However, although the shape of the Lompolonvuoma surface has remained concave, the low hydraulic conductivity of saturated peat (Ingram, 1978; Rydin and Jeglum, 2013) combined with the large amounts of water flowing from surrounding uphill areas, especially during the snowmelt period (Autio et al., 2023), could still cause flooding at suitable locations, even if these locations were separated from the main mire body. Similarly, previous studies have shown that, although no elevated mire center exists, significant lateral expansion of peatland has occurred (Almquist-Jacobson and Foster, 1995; Korhola, 1994, 1996; Korhola et al., 2010; Mathijssen et al., 2017), suggesting that, even on flat or concave peatland basins, peat accumulation can lead to the redistribution of water towards mire margins. Low-severity fires in adjacent forests are also known to promote peatland lateral expansion, as the reduced tree cover decreases evapotranspiration and promotes colonization by Sphagnum due to increased light availability (Le Stum-Boivin et al., 2019; Novenko et al., 2021). However, in our basal layers, only a single charred wood piece used for dating was found, and the microscopic analysis of the basal layers did not reveal any charcoal (Figs. A2, A3, A4). Thus, forest fires did not likely play an important role in the peat initiation at the location in question.
4.2 Autogenic and allogenic drivers behind plant community succession
The initial Cyperaceae–Ericaceae-dominated stage found at our study site is commonly present in the basal layers of the peatland margins in Finland (Juselius-Rajamäki et al., 2023; Mathijssen et al., 2017). On the other hand, many studies have shown that sphagna are frequently found in the first stages of the paludification process (Le Stum-Boivin et al., 2019; Noble et al., 1984; Rydin and Jeglum, 2013). This variation can also be seen in our comparison profiles, as the margin of Syysjärvi site shows similar development to that in Lompolonvuoma, whereas Sphagnum mosses were already present during the initial paludification at the more southern Salamajärvi and Patvinsuo sites (Fig. A1). The lack of Sphagnum mosses in Lompolonvuoma margin during peatland initiation is likely explained by the hydrological conditions. At the onset of peatland expansion, the water table was likely fluctuating, as shown by the presence of both forest mosses and mycorrhizal fungi Cenococcum geophilum (van Geel, 1978), linked to dry conditions, and cyperaceous vegetation, for example, Carex limosa (Figs. A3, A4), usually associated with a relatively wet hydrological regime (Visser et al., 2000). Sphagnum mosses require constantly humid conditions for colonization (Fenton et al., 2007; Sundberg and Rydin, 2002), and even though they can tolerate limited periods of desiccation (Hájek and Vicherová, 2014), the prolonged occurrence of fluctuating water sources in marginal areas likely prevented early colonization by these species. Only after the gradual development of proper mire conditions in the margins was the spread of the peat mosses possible.
After the initial C–E stage, colonization of sphagna occurred asynchronously between 670 and 1770 CE. This gradual transition towards mixed Cyperaceae–Ericaceae–Sphagnum vegetation was likely molded by autogenic development, as changes driven by allogenic forcing would occur over large areas within a relatively short time span rather than over a millennium, as discussed in Väliranta et al. (2017). This conclusion is supported by the fact that no evidence of forest fires was found in the peat profiles. Similarly, no contemporary climate event has been detected that could promote such large-scale changes in vegetation and the simultaneous spatial colonization of sphagna (Hanhijärvi et al., 2013; Linderholm et al., 2018; Luoto and Nevalainen, 2015). The comparison profiles from Patvinsuo and Salamajärvi also show a gradual increase in the Sphagnum mosses, albeit at a much shorter timescale than witnessed in Lompolonvuoma, whereas the shift to Sphagnum moss dominance at Syysjärvi was extremely rapid (Fig. A1).
Although the decomposition of the bottommost layers of peat prevented complete species-level identification of cyperaceous vegetation, an increasing number of Eriophorum vaginatum remains were found in layers preceding the Sphagnum colonization (Figs. A2, A3, A4). Like Sphagnum mosses, tussock-forming cyperaceous species may act as “ecosystem engineers” (Palozzi and Lindo, 2017; Väliranta et al., 2017), and the importance of Eriophorum vaginatum with respect to facilitating the fen-to-bog transition has been recognized in various studies (Hughes, 2000; Hughes and Dumayne-Peaty, 2002; Väliranta et al., 2017). These species can alter local conditions, such as hydrology and acidity (Hughes, 2000; Hughes and Dumayne-Peaty, 2002), and produce litter highly resistant to decay, thus promoting peat accumulation (Wein, 1973). This accumulation process can be further amplified by the presence of ericaceous vegetation (Hughes, 2000). Although the accumulation of the peat during C–E stage was modest in the studied margins, the elevated surface combined with increased acidity seems to have been sufficient to create conditions suitable for the establishment of the Sphagnum species found in the studied margins, likely protecting them from alkaline waters and complete inundation, which are known to impede colonization by sphagna (Granath et al., 2010; Ruuhijärvi, 1983; Sallantaus, 2006).
After colonization, Sphagnum mosses accelerate the change in local conditions (Rydin and Jeglum, 2013), increasing their competitiveness against other mire vegetation and leading to ombrotrophication. In some cases, this change can occur rapidly (Tahvanainen, 2011) and synchronously over a wide area (Loisel and Bunsen, 2020), although more gradual changes have been observed (Väliranta et al., 2017). In Lompolonvuoma, the abundance of sphagna initially remained low after the first establishment, but a more dramatic change occurred towards the end of the 19th century, when Sphagnum mosses started dominating the marginal plant communities and most of the cyperaceous vegetation disappeared, leading to the current S–E vegetation stage. This change coincided with the end of the “Little Ice Age” (LIA), when humid and cool climate conditions were followed by increasingly warm temperatures (Hanhijärvi et al., 2013). Similar post-LIA fen-to-bog shifts have been reported in previous studies, in which data were captured from central parts of the peatland (Granlund et al., 2022; Kolari et al., 2022; Loisel and Yu, 2013; Magnan et al., 2018; Piilo et al., 2019; Primeau and Garneau, 2021; Robitaille et al., 2021), while our results show similar recent changes occurring in the margins. Current results are supported by a study from the adjacent Lompolojänkkä basin that showed a similar kind of recent vegetation shift in the margins (Kuuri-Riutta et al., 2024) as well as by those of our comparison profile from Syysjärvi (Fig. A1). Thus, although aapa mires are generally described as having wet central parts and dryer margins, our results show that dryer margins supporting sphagna may have formed rather recently.
Although these recent fen-to-bog transitions have occurred under dry post-LIA climatic conditions, a similar shift has also occurred during wet climate phases (Väliranta et al., 2017), as the only requirement for the process is the separation of the peat surface from the groundwater supply (Hughes, 2000; Hughes and Barber, 2003). During wet climatic conditions, the accumulation of peat is promoted, rather high water table levels are maintained, and the fen-to-bog transition leads to a bog pool and lawn communities (Hughes and Barber, 2003). On the other hand, dry climate conditions decrease the water table, thereby enabling species with tolerance towards drought or fluctuating water tables to outcompete other species (Hughes and Barber, 2004). In Lompolonvuoma margins, hummock-forming Sphagnum species, especially Sphagnum fuscum, increased markedly during the ultimate shift to ombrotrophic bog conditions. The final fen-to-bog transition in the studied mire margins appears to have been caused by the drier and warmer climate, as only the sporadic presence of non-hummock sphagna was detected in the peat profiles (Figs. A2, A3, A4). Moreover, the most marginal peat profiles in transect 1 and transect 2, as well as in the comparison profile from Patvinsuo, show that the peatland vegetation has been completely replaced by forest vegetation on several occasions. This suggests that peatland expansion may be reversed at least temporarily.
However, based on the remote-sensing data, similar ombrotrophication has not occurred across all margins in Lompolonvuoma and the adjacent Lompolojänkkä basin. The ombrotrophic S–E stage can currently be found roughly in 50 % of the margins of the Lompolonvuoma basin, whereas this stage has only been reached in ca. 35 % of the margins in the adjacent Lompolojänkkä basin. Similarly, the central part of the adjacent Lompolojänkkä basin has shown no evidence of fen-to-bog transition (Mathijssen et al., 2014), but transition is ongoing in the margins (Kuuri-Riutta et al., 2024). Thus, it appears that certain prerequisites and conditions must be met for the transition from fen to bog to occur. Our hydrological model, based on the Lompolojänkkä basin, showed that while marginal fens were generally groundwater recipients, the bog-type vegetation acted preferentially as surface water infiltration areas. By decreasing the effective precipitation in the hydrological model to mimic dryer conditions, the highest levels of water table drawdown were found in the current bog-type margins, making these locations more likely to suffer drying conditions. Although both the analysis of vegetation cover (Räsänen et al., 2021) and the hydrological model (Autio et al., 2023) contain some degree of uncertainty, the application of the hydrological model over the marginal peatland types supports our hypothesis of a drop in groundwater levels as a likely cause of the final shift towards the ombrotrophic climax stage.
4.3 Implication for carbon balance and future trajectories of vegetation succession in aapa mire margins
It has been shown that climate forcing from a peatland complex can remain positive (e.g., climate warming effect) for most of the development history under fen conditions, due to high methane emissions, and that forcing only becomes negative after continuous carbon uptake and the expansion of bog vegetation (Korhola et al., 1996; Mathijssen et al., 2017, 2022). For example, in the adjacent Lompolojänkkä basin, the modeled climate warming effect persisted for up to 2000 years (Mathijssen et al., 2014). Thus, it is likely that during the initial minerotrophic C–E stage, lasting between 150 and 1250 years, the mire margin had a climate warming effect. Afterwards, a shift to decay-resistant Sphagnum vegetation, a lower water table leading to reduced methane emissions, and continuous carbon uptake would likely have the same effect. A decrease in the cyperaceous vegetation, especially during the last ca. 100 years, would have reduced the methane emissions even further (Bubier et al., 1993; Ward et al., 2013). Although our study did not include carbon balance calculations, the shift towards a bog community in the studied margins suggests that the margins would likely proceed to have a climate cooling effect under current conditions. However, the drying trend detected in the European peatlands (Swindles et al., 2019) could also turn these locations into carbon sources if sufficient moisture conditions are not retained (Zhang et al., 2020).
As this study and studies by Juselius-Rajamäki et al. (2023) and Kuuri-Riutta et al. (2024) show, new peatland areas are currently widely being formed in the mire margins all over the subarctic and boreal zone under natural conditions. However, this development has been blocked in many places by the ditching of mire margins (Sallinen et al., 2019), while the widespread drying of peatland surfaces during the last ca. 300 years may suggest that detrimental climatic conditions for lateral expansion are forming (Swindles et al., 2019). In addition, as revealed by this study, the succession of mire margins can differ, even in the same peatland, with some margins retaining their initial wet minerotrophic characteristics, while others develop into ombrotrophic bogs. Due to the opposite climate forcing, the effect of this recent mire expansion on the climate depends on the scope of different peatland types across new mire margins and their later development. Knowledge on the developing peatland margins and their plant community succession still remains scarce. As the lateral expansion of peatlands has had a significant effect on atmospheric greenhouse gas concentrations in the past (Korhola et al., 2010; Peng et al., 2024), we suggest that more studies across the northern peatland margins are needed to reveal the wider effect of this recent lateral peatland expansion on the global carbon budgets.
Our research shows that the studied mire margin in Lompolonvuoma basin has continued to increase in area since ca. 2000 BP, but this development has not progressed linearly. Rather, the current mire margin has formed from several individual loci and via patches that have merged as the local hydrology has transformed and become suitable for peat formation. After the initial wet fen-type conditions, which persisted for a markedly long period, colonization by Sphagnum mosses and the change to current bog-type conditions represent a remarkable swift shift. This change was driven by dryer climatic conditions following the LIA, as shown by our hydrological model. However, not all margins in the Lompolonvuoma and Lompolojänkkä basins have shifted to bog-type communities, suggesting that wetter fen types are at least partially resistant to hydrologically driven regime shifts. This study shows that, even at the basin scale, peatland margins are highly heterogeneous systems, and this should be taken into account when assessing the effects of the past and future lateral expansion trend on the peatland area and peatland carbon dynamics.
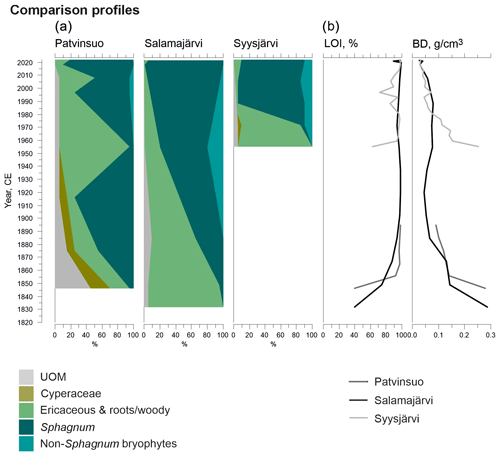
Figure A1Fossil plant records: (a) undetected organic material (UOM) and (b) loss on ignition (LOI) and bulk density (BD) for supplementary profiles. The proportion of vegetation type and LOI are both given as a percentage (%), whereas bulk density is given in grams per cubic centimeter (g cm−3).
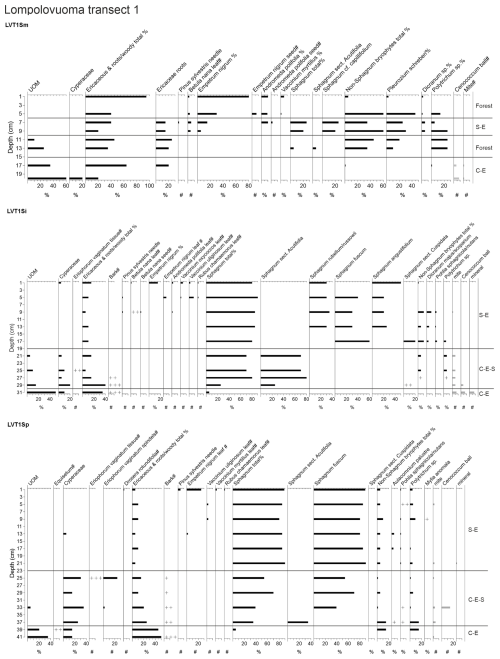
Figure A2Macrofossil data for peat profiles in transect 1: unrecognized organic matter (UOM); plant species or species group; and the remains of mites, Cenococcum, and mineral content are presented.
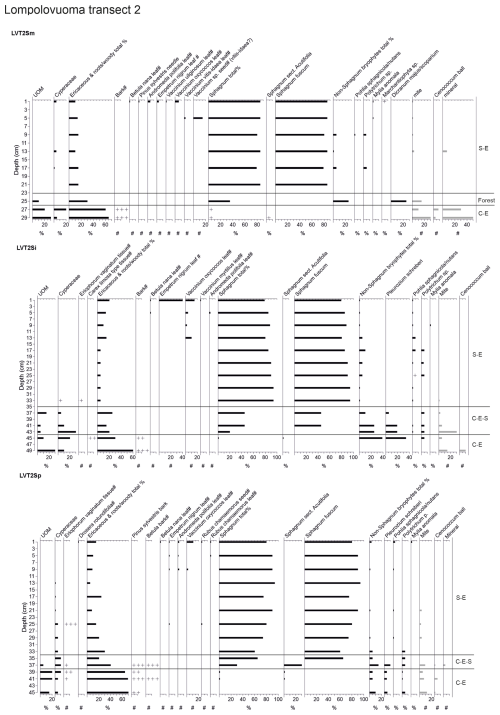
Figure A3Macrofossil data for peat profiles in transect 2: unrecognized organic matter (UOM); plant species or species group; and the remains of mites, Cenococcum, and mineral content are presented.
The data for peat properties, peat core locations, and age–depth models are available from figshare: https://doi.org/10.6084/m9.figshare.25941493.v1 (Juselius-Rajamäki, 2024).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-3047-2025-supplement.
TJR and MV conceived the idea for the article. TJR, ET, MV, AA, HM, and PAA collected the field data. TJR and SP performed the macrofossil analysis. TJR and SSP conducted the 210Pb analysis. TJR conducted the spatial analysis. AA, HM, and PAA conducted hydrological modeling. TJR created the initial draft of the manuscript. All authors contributed to the drafts and gave the final approval for publication.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Teemu Juselius-Rajamäki acknowledges funding from the Tellervo and Juuso Walden Foundation. The Groundwater modeling and the GPR data set were supported by the Freshwater Competence Centre (FWCC) and the Digital Waters Flagship (DIWA). The authors also acknowledge support from the Ministry of Transport and Communication, through ICOS Finland.
This research has been supported by the Research Council of Finland (grant nos. 338631, 349193, and 316349) and the WET HORIZONS project (Horizon Europe; grant no. GAP 101056848).
Open-access funding was provided by the Helsinki University Library.
This paper was edited by Petr Kuneš and reviewed by Paul J. Morris and one anonymous referee.
Ali, A. A., Ghaleb, B., Garneau, M., Asnong, H., and Loisel, J.: Recent peat accumulation rates in minerotrophic peatlands of the Bay James region, Eastern Canada, inferred by 210Pb and 137Cs radiometric techniques, Appl. Radiat. Isotopes, 66, 1350–1358, https://doi.org/10.1016/j.apradiso.2008.02.091, 2008.
Almquist-Jacobson, H. and Foster, D. R.: Toward an Integrated Model for Raised-Bog Development: Theory and Field Evidence, Ecology, 76, 2503–2516, 1995.
Aquanty: HydroGeoSphere user manual. Release 1, Aquanty Inc, Waterloo, Ontario, Canada, https://hydrogeosphere.blob.core.windows.net/hydrogeosphere/hgs/hydrosphere_ref.pdf (last access: 20 December 2023), 2015.
Aquino-López, M. A., Blaauw, M., Christen, J. A., and Sanderson, N. K.: Bayesian Analysis of 210Pb Dating, J. Agr. Biol. Envir. S., 23, 317–333, https://doi.org/10.1007/s13253-018-0328-7, 2018.
Autio, A., Ala-Aho, P., Rossi, P. M., Ronkanen, A. K., Aurela, M., Lohila, A., Korpelainen, P., Kumpula, T., Klöve, B., and Marttila, H.: Groundwater exfiltration pattern determination in the sub-arctic catchment using thermal imaging, stable water isotopes and fully-integrated groundwater-surface water modelling, J. Hydrol., 626, 130342, https://doi.org/10.1016/j.jhydrol.2023.130342, 2023.
Blaauw, M. and Christen, J. A.: Flexible paleoclimate age-depth models using an autoregressive gamma process, Bayesian Anal., 6, 457–474, https://doi.org/10.1214/11-ba618, 2011.
Bubier, J., Costello, A., Moore, T. R., Roulet, N. T., and Savage, K.: Microtopography and methane flux in boreal peatlands, northern Ontario, Canada, Can. J. Botany, 71, 1056–1063, https://doi.org/10.1139/b93-122, 1993.
Eronen, M., Lindholm, M., Saastamoinen, S., and Zetterberg, P.: Variable Holocene climate, treeline dynamics and changes in natural environments in northern Finnish Lapland, Chemosphere, 1, 377–387, https://doi.org/10.1016/S1465-9972(99)00042-2, 1999.
Environmental Systems Research Institute (Esri): ArcGIS Pro (Version 3.1), Redlands, CA, 2023.
Evans, C. D., Peacock, M., Baird, A. J., Artz, R. R. E., Burden, A., Callaghan, N., Chapman, P. J., Cooper, H. M., Coyle, M., Craig, E., Cumming, A., Dixon, S., Gauci, V., Grayson, R. P., Helfter, C., Heppell, C. M., Holden, J., Jones, D. L., Kaduk, J., Levy, P., Matthews, R., McNamara, N. P., Misselbrook, T., Oakley, S., Page, S, E., Rayment, M., Ridley, L. M., Stanley, K. M., Williamson, J. L., Worrall, F., and Morrison, R.: Overriding water table control on managed peatland greenhouse gas emissions, Nature, 593, 548–552, https://doi.org/10.1038/s41586-021-03523-1, 2021.
Fenton, N. J., Béland, C., De Blois, S., and Bergeron, Y.: Sphagnum establishment and expansion in black spruce (Picea mariana) boreal forests, Can. J. Bot., 85, 43–50, https://doi.org/10.1139/B06-148, 2007.
Flynn, W. W.: The determination of low levels of Polonium-210 in environmental materials, Anal. Chim. Acta, 43, 221–227, 1968.
Foster, D. R. and King, G. A.: Landscape Features, Vegetation and Developmental History of a Patterned Fen in South-Eastern Labrador, Canada, J. Ecol., 72, 115–143, https://doi.org/10.2307/2260009, 1984.
Foster, D. R. and Wright, H. E.: Role of ecosystem development and climate change in bog formation in central Sweden, Ecology, 71, 450–463, https://doi.org/10.2307/1940300, 1990.
Frolking, S. and Roulet, N. T.: Holocene radiative forcing impact of northern peatland carbon accumulation and methane emissions, Glob. Change Biol., 13, 1079–1088, https://doi.org/10.1111/j.1365-2486.2007.01339.x, 2007.
Goud, E. M., Watt, C., and Moore, T. R.: Plant community composition along a peatland margin follows alternate successional pathways after hydrologic disturbance, Acta Oecol., 91, 65–72, https://doi.org/10.1016/j.actao.2018.06.006, 2018.
Granath, G., Strengbom, J., and Rydin, H.: Rapid ecosystem shifts in peatlands: Linking plant physiology and succession, Ecology, 91, 3047–3056, https://doi.org/10.1890/09-2267.1, 2010.
Granlund, L., Vesakoski, V., Sallinen, A., Kolari, T. H. M., Wolff, F., and Tahvanainen, T.: Recent Lateral Expansion of Sphagnum Bogs Over Central Fen Areas of Boreal Aapa Mire Complexes, Ecosystems, 25, 1455–1475, https://doi.org/10.1007/s10021-021-00726-5, 2022.
Grimm, E. C.: Tilia 1.7.16 Software. Illinois State Museum, Research and Collection Center, 2011.
Hájek, T. and Vicherová, E.: Desiccation tolerance of Sphagnum revisited: A puzzle resolved, Plant Biol., 16, 765–773, https://doi.org/10.1111/plb.12126, 2014.
Hanhijärvi, S., Tingley, M. P., and Korhola, A.: Pairwise comparisons to reconstruct mean temperature in the Arctic Atlantic Region over the last 2,000 years, Clim. Dynam., 41, 2039–2060, https://doi.org/10.1007/s00382-013-1701-4, 2013.
Heiri, O., Lotter, A. F., and Lemcke, G.: Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results, J. Paleolimnol., 25, 101–110, https://doi.org/10.1023/A:1008119611481, 2001.
Helama, S., Jones, P. D., and Briffa, K. R.: Dark Ages Cold Period: A literature review and directions for future research, Holocene, 27, 1600–1606, https://doi.org/10.1177/0959683617693898, 2017.
Howie, S. A. and Meerveld, I. T. Van.: The essential role of the lagg in raised bog function and restoration: A review, Wetlands, 31, 613–622, https://doi.org/10.1007/s13157-011-0168-5, 2011.
Hua, Q., Turnbull, J. C., Santos, G. M., Rakowski, A. Z., Ancapichún, S., De Pol-Holz, R., Hammer, S., Lehman, S. J., Levin, I., Miller, J. B., Palmer, J. G., and Turney, C. S. M.: Atmospheric Radiocarbon for the Period 1950–2019, Radiocarbon, 64, 723–745, https://doi.org/10.1017/RDC.2021.95, 2022.
Hughes, P. D. M.: A reappraisal of the mechanisms leading to ombrotrophy in British raised mires, Ecol. Lett., 3, 7–9, https://doi.org/10.1046/j.1461-0248.2000.00118.x, 2000.
Hughes, P. D. M. and Barber, K. E.: Mire development across the fen-bog transition on the Teifi floodplain at Tregaron Bog, Ceredigion, Wales, and a comparison with 13 other raised bogs, J. Ecol., 91, 253–264, https://doi.org/10.1046/j.1365-2745.2003.00762.x, 2003.
Hughes, P. D. M. and Barber, K. E.: Contrasting pathways to ombrotrophy in three raised bogs from Ireland and Cumbria, England, Holocene, 14, 65–77, https://doi.org/10.1191/0959683604hl690rp, 2004.
Hughes, P. D. M. and Dumayne-Peaty, L.: Testing Theories of Mire Development Using Multiple Successions at Crymlyn Bog, West Glamorgan, South Wales, UK, J. Ecol., 90, 456–471, 2002.
Ingram, H. A. P.: Soil Layers in Mires: Function and Terminology, J. Soil Sci., 29, 224–227, https://doi.org/10.1111/j.1365-2389.1978.tb02053.x, 1978.
Juggins, S.: User Guide: C2 Software for ecological and palaeoecological data analysis and visualisation, User guide Version 1.5 (vols. 1-73), University of Newcastle, http://www.staff.ncl.ac.uk/stephen.juggins (last access: 24 April 2024), 2007.
Juselius-Rajamäki, T.: Mire edge is not a marginal thing - data for manuscript, figshare [data set], https://doi.org/10.6084/m9.figshare.25941493.v1, 2024.
Juselius-Rajamäki, T., Väliranta, M., and Korhola, A.: The ongoing lateral expansion of peatlands in Finland, Glob. Change Biol., 29, 7173–7191, https://doi.org/10.1111/gcb.16988, 2023.
Juutinen, S., Väliranta, M., Kuutti, V., Laine, A. M., Virtanen, T., Seppä, H., Weckström, J., and Tuittila, E. S.: Short-term and long-term carbon dynamics in a northern peatland-stream-lake continuum: A catchment approach, J. Geophys. Res.-Biogeo., 118, 171–183, https://doi.org/10.1002/jgrg.20028, 2013.
Kauranen, P. and Miettinen, J. K.: 210Po and 210Pb in environmental samples in Finland, in: Radioecological concentration processes, Proceedings of an International Symposium Held in Stockholm, 25–29 April, https://doi.org/10.1016/C2013-0-02040-3, ISBN 978-0-08-012122-2, 1966.
Klinger, L. F.: Coupling of Soils and Vegetation in Peatland Succession, Arct. Alp. Res., 28, 380–387, 1996.
Kolari, T. H. M., Sallinen, A., Wolff, F., Kumpula, T., Tolonen, K., and Tahvanainen, T.: Ongoing Fen–Bog Transition in a Boreal Aapa Mire Inferred from Repeated Field Sampling, Aerial Images, and Landsat Data, Ecosystems, 25, 1166–1188, https://doi.org/10.1007/s10021-021-00708-7, 2022.
Korhola, A.: Mire Induction, ecosystem dynamics and lateral expansion on raised bogs in the southern coastal area of Finland, Fennia, 170, 25–94, 1992.
Korhola, A.: Radiocarbon Evidence for Rates of Lateral Expansion in Raised Mires in Southern Finland, Quaternary Res., 42, 299–307, https://doi.org/10.1006/qres.1994.1080, 1994.
Korhola, A.: Holocene climatic variations in southern Finland reconstructed from peat-initiation data, Holocene, 5, 43–58, https://doi.org/10.1177/095968369500500106, 1995.
Korhola, A.: Initiation of a sloping mire complex in southwestern Finland: Autogenic versus allogenic controls, Ecoscience, 3, 216–222, https://doi.org/10.1080/11956860.1996.11682334, 1996.
Korhola, A., Alm, J., Tolonen, J., Turunen, J., and Jungner, H.: Three-dimensional reconstruction of carbon accumulation and CH4 emission during nine millenia in a raised mire, J. Quaternary Sci., 11, 161–165, 1996.
Korhola, A., Ruppel, M., Seppä, H., Väliranta, M., Virtanen, T., and Weckström, J.: The importance of northern peatland expansion to the late-Holocene rise of atmospheric methane, Quaternary Sci. Rev., 29, 611–617, https://doi.org/10.1016/j.quascirev.2009.12.010, 2010.
Kou, D., Virtanen, T., Treat, C. C., Tuovinen, J. P., Räsänen, A., Juutinen, S., Mikola, J., Aurela, M., Heiskanen, L., Heikkilä, M., Weckström, J., Juselius, T., Piilo, S. R., Deng, J., Zhang, Y., Chaudhary, N., Huang, C., Väliranta, M., Biasi, C., Liu, X., Guo, M., Zhuang, Q., Korhola, A. and Shurpali, N. J.: Peatland Heterogeneity Impacts on Regional Carbon Flux and Its Radiative Effect Within a Boreal Landscape, J. Geophys. Res.-Biogeo., 127, e2021JG006774, https://doi.org/10.1029/2021JG006774, 2022.
Kuhry, P.: The Role of Fire in the Development of Sphagnum-Dominated Peatlands in Western Boreal Canada, J. Ecol., 82, 899–910, 1994.
Kuhry, P. and Turunen, J.: The Postglacial Development of Boreal and Subarctic Peatlands, in: Boreal peatland ecosystems. Ecological studies, vol. 188, edited by: Wieder, R. K., Vitt, D., and Jackson, R. B., Springer, Berlin Heidelberg, Germany, 25–46, https://doi.org/10.1007/978-3-540-31913-9_3, 2006.
Kuuri-Riutta, O., Pilkama, E., Salminen-Paatero, S., Vögeli, C., Mitchell, E. A. D., Lohila, A., Tuittila, E. S., and Väliranta, M.: Recent hummock establishment in the margin of a subarctic fen, Finnish Lapland, Boreas, 53, 282–295, https://doi.org/10.1111/bor.12651, 2024.
Lacourse, T., Adeleye, M. A., and Stewart, J. R.: Peatland formation, succession and carbon accumulation at a mid-elevation poor fen in Pacific Canada, The Holocene, 29, 1694–1707, https://doi.org/10.1177/0959683619862041, 2019.
Lai, D. Y. F.: Methane Dynamics in Northern Peatlands: A Review, Pedosphere, 19, 409–421, https://doi.org/10.1016/S1002-0160(09)00003-4, 2009.
Laine, J., Vasander, H., Hotanen, J.-P., Nousiainen, H., Saarinen, M., and Penttilä, T.: Suotyypit ja turvekankaat – kasvupaikkapaikkaopas, Metsäkustannus Oy, Hämeenlinna, ISBN 978-952-5694-89-5, 2012.
Laitinen, J., Rehell, S., and Huttunen, A.: Vegetation-related hydrotopographic and hydrologic classification for aapa mires (Hirvisuo, Finland), Ann. Bot. Fenn., 42, 107–121, 2005.
Laitinen, J., Rehell, S., Huttunen, A., Tahvanainen, T., Heikkilä, R., and Lindholm, T.: Mire systems in Finland – Special view to aapa mires and their water-flow pattern, Suo, 58, 1–26, 2007.
Le Stum-Boivin, É., Magnan, G., Garneau, M., Fenton, N. J., Grondin, P., and Bergeron, Y.: Spatiotemporal evolution of paludification associated with autogenic and allogenic factors in the black spruce-moss boreal forest of Québec, Canada, Quaternary Res., 91, 520–532, https://doi.org/10.1017/qua.2018.101, 2019.
Linderholm, H. W., Nicolle, M., Francus, P., Gajewski, K., Helama, S., Korhola, A., Solomina, O., Yu, Z., Zhang, P., D'Andrea, W. J., Debret, M., Divine, D. V., Gunnarson, B. E., Loader, N. J., Massei, N., Seftigen, K., Thomas, E. K., Werner, J., Andersson, S., Berntsson, A., Luoto, T. P., Nevalainen, L., Saarni, S., and Väliranta, M.: Arctic hydroclimate variability during the last 2000 years: current understanding and research challenges, Clim. Past, 14, 473–514, https://doi.org/10.5194/cp-14-473-2018, 2018.
Loisel, J. and Bunsen, M.: Abrupt Fen-Bog Transition Across Southern Patagonia: Timing, Causes, and Impacts on Carbon Sequestration, Front. Ecol. Evol., 8, 1–19, https://doi.org/10.3389/fevo.2020.00273, 2020.
Loisel, J. and Yu, Z.: Recent acceleration of carbon accumulation in a boreal peatland, south central Alaska, J. Geophys. Res.-Biogeo., 118, 41–53, https://doi.org/10.1029/2012JG001978, 2013.
Loisel, J., Yu, Z., Parsekian, A., Nolan, J., and Slater, L.: Quantifying landscape morphology influence on peatland lateral expansion using ground-penetrating radar (GPR) and peat core analysis, J. Geophys. Res.-Biogeo., 118, 373–384, https://doi.org/10.1002/jgrg.20029, 2013.
Luoto, T. P. and Nevalainen, L.: Late Holocene precipitation and temperature changes in Northern Europe linked with North Atlantic forcing, Clim. Res., 66, 37–48, https://doi.org/10.3354/cr01331, 2015.
Magnan, G., van Bellen, S., Davies, L., Froese, D., Garneau, M., Mullan-Boudreua, G., Zaccone, C., and Shotyk, W.: Impact of the Little Ice Age cooling and 20th century climate change on peatland vegetation dynamics in central and northern Alberta using a multi-proxy approach and high-resolution peat chronologies, Quaternary Sci. Rev., 185, 230–243, 2018.
Mäkilä, M. and Moisanen, M.: Holocene lateral expansion and carbon accumulation of Luovuoma, a northern fen in Finnish Lapland, Boreas, 36, 198–210, https://doi.org/10.1080/03009480600994460, 2007.
Mäkilä, M., Saarnisto, M., and Kankainen, T.: Aapa mires as a carbon sink and source during the Holocene, J. Ecol., 89, 589–599, https://doi.org/10.1046/j.0022-0477.2001.00586.x, 2001.
Mallik, A. U., Gimingham, C. H., and Rahman, A. A.: Ecological Effects of Heather Burning: I. Water Infiltration, Moisture Retention and Porosity of Surface Soil, J. Ecol., 72, 767–776, https://doi.org/10.2307/2259530, 1984.
Marttila, H., Lohila, A., Ala-Aho, P., Noor, K., Welker, J. M., Croghan, D., Mustonen, K., Meriö, L. J., Autio, A., Muhic, F., Bailey, H., Aurela, M., Vuorenmaa, J., Penttilä, T., Hyöky, V., Klein, E., Kuzmin, A., Korpelainen, P., Kumpula, T., Rauhala, A., and Kløve, B.: Subarctic catchment water storage and carbon cycling – Leading the way for future studies using integrated datasets at Pallas, Finland, Hydrol. Process., 35, e14350, https://doi.org/10.1002/hyp.14350, 2021.
Mathijssen, P. J. H., Tuovinen, J. P., Lohila, A., Aurela, M., Juutinen, S., Laurila, T., Niemelä, E., Tuittila, E. S., and Väliranta, M.: Development, carbon accumulation, and radiative forcing of a subarctic fen over the Holocene, Holocene, 24, 1156–1166, https://doi.org/10.1177/0959683614538072, 2014.
Mathijssen, P. J. H., Väliranta, M., Korrensalo, A., Alekseychik, P., Vesala, T., Rinne, J., and Tuittila, E. S.: Reconstruction of Holocene carbon dynamics in a large boreal peatland complex, southern Finland, Quaternary Sci. Rev., 142, 1–15, https://doi.org/10.1016/j.quascirev.2016.04.013, 2016.
Mathijssen, P. J. H., Kähkölä, N., Tuovinen, J. P., Lohila, A., Minkkinen, K., Laurila, T., and Väliranta, M.: Lateral expansion and carbon exchange of a boreal peatland in Finland resulting in 7000 years of positive radiative forcing, J. Geophys. Res.-Biogeo., 122, 562–577, https://doi.org/10.1002/2016JG003749, 2017.
Mathijssen, P. J. H., Tuovinen, J. P., Lohila, A., Väliranta, M., and Tuittila, E. S.: Identifying main uncertainties in estimating past and present radiative forcing of peatlands, Glob. Change Biol., 28, 4069–4084, https://doi.org/10.1111/gcb.16189, 2022.
Mauquoy, D., Hughes, P. D. M., Mauquoy, D., Hughes, P. D. M., and Van Geel, B.: A protocol for plant macrofossil analysis of peat deposits, Mires Peat, 7, 1–5, 2010.
National Land Survey of Finland: Aerial photo V4134, https://asiointi.maanmittauslaitos.fi/karttapaikka/tiedostopalvelu/ortoilmakuva (last access: 1 April 2024), 2023.
Noble, M., Lawrence, D., and Streveler, G.: Sphagnum Invasion beneath an Evergreen Forest Canopy in Southeastern Alaska, The Bryologist, 87, 119–127, 1984.
Novenko, E. Y., Mazei, N. G., Kupriyanov, D. A., Kusilman, M. V., and Olchev, A. V.: Peatland initiation in Central European Russia during the Holocene: Effect of climate conditions and fires, Holocene, 31, 545–555, https://doi.org/10.1177/0959683620981709, 2021.
Palozzi, J. E. and Lindo, Z.: Boreal peat properties link to plant functional traits of ecosystem engineers, Plant Soil, 418, 277–291, https://doi.org/10.1007/s11104-017-3291-0, 2017.
Peng, H., Nijp, Jelmer, J., Ratcliffe, J. L., Li, C., Hong, B., Lidberg, W., Zeng, M., Mauquoy, D., Bishop, K., and Nilsson, M. B.: Climatic controls on the dynamic lateral expansion of northern peatlands and its potential implication for the “anomalous” atmospheric CH4 rise since the mid-Holocene, Sci. Total Environ., 908, 168450, https://doi.org/10.1016/j.scitotenv.2023.168450, 2024.
Peregon, A., Uchida, M., and Yamagata, Y.: Lateral extension in Sphagnum mires along the southern margin of the boreal region, Western Siberia, Environ. Res. Lett., 4, 045028, https://doi.org/10.1088/1748-9326/4/4/045028, 2009.
Piilo, S. R., Zhang, H., Garneau, M., Gallego-Sala, A., Amesbury, M. J., and Väliranta, M. M.: Recent peat and carbon accumulation following the Little Ice Age in northwestern Québec, Canada, Environ. Res. Lett., 14, 075002, https://doi.org/10.1088/1748-9326/ab11ec, 2019.
Primeau, G. and Garneau, M.: Carbon accumulation in peatlands along a boreal to subarctic transect in eastern Canada, Holocene, 31, 858–869, https://doi.org/10.1177/0959683620988031, 2021.
Quik, C., Palstra, S. W. L., van Beek, R., van der Velde, Y., Candel, J. H. J., van der Linden, M., Kubiak-Martens, L., Swindles, G. T., Makaske, B., and Wallinga, J.: Dating basal peat: The geochronology of peat initiation revisited, Quat. Geochronol., 72, 101278, https://doi.org/10.1016/j.quageo.2022.101278, 2022.
Räsänen, A., Manninen, T., Korkiakoski, M., Lohila, A., and Virtanen, T.: Predicting catchment-scale methane fluxes with multi-source remote sensing, Landscape Ecol., 36, 1177–1195, https://doi.org/10.1007/s10980-021-01194-x, 2021.
R Core Team: R: A language and environment for statistical computing (4.2.2), R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org (last access: 5 March 2024), 2023.
Reimer, P. J., Austin, W. E. N., Bard, E., Bayliss, A., Blackwell, P. G., Bronk Ramsey, C., Butzin, M., Cheng, H., Edwards, R. L., Friedrich, M., Grootes, P. M., Guilderson, T. P., Hajdas, I., Heaton, T. J., Hogg, A. G., Hughen, K. A., Kromer, B., Manning, S. W., Muscheler, R., Palmer, J. G., Pearson, C., van der Plicht, J., Weimer, R. W., Richards, D. A., Scott, M. E., Southon, J. R., Turney, C. S. M., Wacker, L., Adolphi, F., Büntgen, U., Capano, M., Fahrni, S. M., Fogtmann-Schulz, A., Friedrich, R., Köhler, P., Kudsk, S., Miyake, F., Olsen, J., Reinig, F., Sakamoto, M., Sookdeo, A., and Talamo, S.: The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP), Radiocarbon, 62, 725–757, https://doi.org/10.1017/RDC.2020.41, 2020.
Robitaille, M., Garneau, M., van Bellen, S., and Sanderson, N. K.: Long-term and recent ecohydrological dynamics of patterned peatlands in north-central Quebec (Canada), Holocene, 31, 844–857, https://doi.org/10.1177/0959683620988051, 2021.
Ruppel, M., Väliranta, M., Virtanen, T., and Korhola, A.: Postglacial spatiotemporal peatland initiation and lateral expansion dynamics in North America and northern Europe, Holocene, 23, 1596–1606, https://doi.org/10.1177/0959683613499053, 2013.
Ruuhijärvi, R.: Finnish mire types and their regional distribution, in: Ecosystems of the world (Vol. 4B), edited by: Gore, A. J. P., Elsevier, Amsterdam, The Netherlands, 47–67, 1983.
Rydin, H. and Jeglum, J. K. (Eds.): The Biology of Peatlands, Oxford University Press, New York, United States of America, https://doi.org/10.1093/acprof:osobl/9780199602995.001.0001, 2013.
Sallantaus, T.: Mire ecohydrology in Finland, in: Finland – Land of Mires, edited by: Lindholm, T. and Heikkilä, R., Finnish Environmental Institute, Vammalan kirjapaino Oy, Vammala, Finland, 105–108, 2006.
Sallinen, A., Tuominen, S., Kumpula, T., and Tahvanainen, T.: Undrained peatland areas disturbed by surrounding drainage: A large scale GIS analysis in Finland with a special focus on Aapa mires, Mires Peat, 24, 1–22, https://doi.org/10.19189/MaP.2018.AJB.391, 2019.
Sallinen, A., Akanegbu, J., Marttila, H., and Tahvanainen, T.: Recent and future hydrological trends of aapa mires across the boreal climate gradient, J. Hydrol., 617, 129022, https://doi.org/10.1016/j.jhydrol.2022.129022, 2023.
Sanderson, N. K.: Patterns and Drivers of Recent Peatland Carbon Accumulation in Northeastern Canada, University of Exeter, UK, http://hdl.handle.net/10871/24223 (last access: 2 February 2025), 2016.
Schaffhauser, A., Payette, S., Garneau, M., and Robert, É. C.: Soil paludification and Sphagnum bog initiation: the influence of indurated podzolic soil and fire, Boreas, 46, 428–441, https://doi.org/10.1111/bor.12200, 2017.
Seppä, H.: Mires of Finland: Regional and local controls of vegetation, landforms, and long-term dynamics, Fennia, 180, 43–60, 2002.
Simard, M., Lecomte, N., Bergeron, Y., Bernier, P. Y., and Paré, D.: Forest productivity decline caused by successional paludification of boreal soils, Ecol. Appl., 17, 1619–1637, https://doi.org/10.1890/06-1795.1, 2007.
Sjörs, H.: Mires of Sweden, in: Ecosystems of the world 4B, edited by: Gore, A. J. P., Elsevier, Amsterdam, The Netherlands, 69–94, 1983.
Sundberg, S. and Rydin, H.: Habitat requirements for establishment of Sphagnum from spores, J. Ecol., 90, 268–278, https://doi.org/10.1046/j.1365-2745.2001.00653.x, 2002.
Swindles, G. T., Morris, P. J., Mullan, D. J., Payne, R. J., Roland, T. P., Amesbury, M. J., Lamentowicz, M., Turner, T. E., Gallego-Sala, A., Sim, T., Barr, I. D., Blaauw, M., Blundell, A., Chambers, F. M., Charman, D. J., Feurdean, A., Galloway, J. M., Gałka, M., Green, S. M., Kajukało, K., Karofeld, E., Korhola, A., Lamentowicz, Ł., Langdon, P., Marcisz, K,. Mauquoy, D., Mazei, Y. A., McKeown, M. M., Mitchell, E. A. D., Novenko, E., Plunkett, G., Roe, H. M., Schoning, K., Sillasoo, Ü., Tsyganov, A. N., van der Linden, M., Väliranta, M., and Warner, B.: Widespread drying of European peatlands in recent centuries, Nat. Geosci., 12, 922–928, https://doi.org/10.1038/s41561-019-0462-z, 2019.
Tahvanainen, T.: Abrupt ombrotrophication of a boreal aapa mire triggered by hydrological disturbance in the catchment, J. Ecol., 99, 404–415, https://doi.org/10.1111/j.1365-2745.2010.01778.x, 2011.
Väliranta, M., Korhola, A., Seppä, H., Tuittila, E. S., Sarmaja-Korjonen, K., Laine, J., and Alm, J.: High-resolution reconstruction of wetness dynamics in a southern boreal raised bog, Finland, during the late Holocene: A quantitative approach, Holocene, 17, 1093–1107, https://doi.org/10.1177/0959683607082550, 2007.
Väliranta, M., Salojärvi, N., Vuorsalo, A., Juutinen, S., Korhola, A., Luoto, M., and Tuittila, E. S.: Holocene fen–bog transitions, current status in Finland and future perspectives, Holocene, 27, 752–764, https://doi.org/10.1177/0959683616670471, 2017.
van Geel, B.: A Palaeoecological study of Holocene peat bog sections in Germany and the Netherlands, Rev. Palaeobot. Palyno., 25, 1–120, https://doi.org/10.2307/1216527, 1978.
Visser, E. J. W., Bogemann, G. M., Van de Steeg, H. M., Pierik, R., and Blom, C. W. P. M.: Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation, New Phytol., 148, 93–103, https://doi.org/10.1046/j.1469-8137.2000.00742.x, 2000.
Ward, S. E., Ostle, N. J., Oakley, S., Quirk, H., Henrys, P. A., and Bardgett, R. D.: Warming effects on greenhouse gas fluxes in peatlands are modulated by vegetation composition, Ecol. Lett., 16, 1285–1293, https://doi.org/10.1111/ele.12167, 2013.
Wein, R. W.: Eriophorum Vaginatum L, J. Ecol., 61, 601–615, 1973.
Zhang, H., Väliranta, M., Piilo, S., Amesbury, M. J., Aquino-López, M. A., Roland, T. P., Salminen-Paatero, S., Paatero, J., Lohila, A., and Tuittila, E. S.: Decreased carbon accumulation feedback driven by climate-induced drying of two southern boreal bogs over recent centuries, Glob. Change Biol., 26, 2435–2448, https://doi.org/10.1111/gcb.15005, 2020.
Zhao, Y., Tang, Y., Yu, Z., Li, H., Yang, B., Zhao, W., Li, F., and Li, Q.: Holocene peatland initiation, lateral expansion, and carbon dynamics in the Zoige Basin of the eastern Tibetan Plateau, Holocene, 24, 1137–1145, https://doi.org/10.1177/0959683614538077, 2014.