the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The incubation history of soil samples strongly affects the occlusion of particulate organic matter
Frederick Büks
Sabine Dumke
Julia König
Soil structure is a key proxy for carbon and nutrient storage, stable pore space, and rootability. It is often quantified based on the degree of aggregation or the mechanical stability of soil aggregates. This work compares two methods representing basic principles of aggregate measurement. Undisturbed soil samples of loamy sand, clayey silt and silty loam were analyzed by ultrasonication/density fractionation (USD) to quantify different soil organic carbon (SOC) pools and by wet sieving to measure the amount of water-stable aggregates (%WSA). The measurements were carried out on field-fresh soils at field capacity (pF 1.8) as well as samples that were air-dried; reset to pF 1.8 by capillary action; and incubated for 0, 1 and 4 weeks. Our results show that the strength of particulate organic matter (POM) occlusion sharply decreases after rewetting, indicated by the reduction in the more strongly bound occluded carbon fraction. The respective amounts decreased by −4.5 wt % for loamy sand, −6.8 wt % for clayey silt and −16.3 wt % for silty loam, and the field fresh values are not fully recovered within the following 4 weeks. In contrast, the amount of water-stable aggregates (%WSA) remains largely stable except in clayey silt, which shows an increase by +5.9 wt % directly after rewetting. In consequence, field-fresh measurements are highly recommended to avoid overestimation of free and weakly bound soil organic matter (SOM) fractions or the degree of aggregation.
- Article
(1052 KB) - Full-text XML
-
Supplement
(415 KB) - BibTeX
- EndNote
It has been over 90 years since soil structure began to be a focus of agricultural research (Russell, 1928; Christensen, 1930). First seen under the aspect of soil plowability, the 1960s brought attention to well-aggregated soils regarding support for root growth and against soil compaction by heavy machinery (Rosenberg, 1964). Today, good soil structure is seen as an eminent proxy for soil quality, as it not only provides rootability and stable pore space but also is related to water holding capacity, the drainage of excess rain or flood water, enhanced aeration, and carbon and nutrient storage within aggregates (Bronick and Lal, 2005).
The quantification of soil aggregates was initially performed by the use of dry and wet sieving (Yoder, 1936; Chepil and Bisal, 1943). From here, the methods branched out into approaches still used to describe the amount and size distribution of soil aggregation. One branch comprised the weight fraction of water-stable aggregates (%WSA) and, based on the same wet-sieving principle, the mean weight diameter (Bryant et al., 1948; Bavel, 1950; Angulo et al., 2024; Meidl et al., 2024). The other branch was aimed to measure soil structure for its mechanical stability. Wet-sieving methods had been applied to quantify the mechanical integrity of soils (aggregate stability) after a certain amount of stress provided by sieve movements (Russell and Feng, 1947). In the second half of the 20th century, ultrasonic dispersion introduced by Edwards and Bremner (1967a) increasingly replaced sieving methods for the quantification of aggregate stability, since now the energy input to the soil could be estimated from the power output of the sonotrode (North, 1976). Nowadays, ultrasonication is a common tool for achieving a semi-quantitative view on soil structural stability by comparing the mass of aggregate size fractions or mean weight diameter of water-stable aggregates (WSA) after the application of defined quantities of ultrasonic power (J mL−1 s−1) (e.g., Lehtinen et al., 2014; Jouquet et al., 2016; Cavael et al., 2020). Beyond that, ultrasonication is combined with density fractionation (USD) of particulate organic matter (POM), which is successively released with increasing energy input and used for the quantification of soil carbon pools (Edwards and Bremner, 1967b; Golchin et al., 1994; Kaiser and Berhe, 2014; Graf-Rosenfellner et al., 2016).
However, carbon pool measurements that apply USD extraction are prone to a number of artifacts. A density cut-off at 1.6 g cm−3 is mandatory because lower concentrated solutions might not completely separate the respective POM fraction, while higher densities cause co-extraction of the mineral matrix (Cerli et al., 2012). Ultrasound treatments with energy levels > 50 J mL−1 can cause comminution of POM and sorption to mineral surfaces and lead to a reduced recovery rate, as demonstrated by Büks et al. (2021). This causes a carry-over of POM from fractions with a lower to those with a higher binding strength and a false estimation of both. Furthermore, adding the dense solution to the soil sample results in a low recovery rate of the free POM (fPOM) fraction due to burying within the matrix (Büks, 2023). This problem is addressed by rinsing the sample into the solution or gentle rotation, which significantly increases the recovery of fPOM and reduces overestimation of occluded POM (oPOM) mass. If the samples are air-dried, the abrupt addition of water or dense solutions to the soil causes rupture of WSA and release of oPOM by a process called slaking (Emerson, 1967; Bossuyt et al., 2001), which could in turn result in an overestimation of the fPOM fraction. In consequence, comparability of fractionation results is only given under standardized test conditions that represent natural conditions of the soil sample as well as possible.
The present work focuses on another parameter that can potentially cause carbon pool artifacts: the wetting history of the sample. When normally measured under field-fresh conditions, the extent of a sampling campaign or samples from soil archives can make it necessary to quantify structural characteristics of already air-dried soil. This may have an influence on the measured soil structural parameters. Aggregate stability increases from low to high soil moisture as, e.g., shown with sieving experiments (Liu et al., 2025) and rainfall simulators (Martínez-Mena et al., 1998). Air drying, however, can increase the mechanical stability of soil aggregates by precipitation of various inorganic (and organic) cementation agents (Amézketa, 1999) and, potentially, the transfer of dissolved organic matter (DOM) from outer- to inner-spherical binding patterns (Kaiser et al., 2015).
Another factor of aggregate stability, which is influenced by not only the actual water content but also its recent history, is the soil microbiome and its biofilm matrix. The composition and activity of soil bacterial and fungal communities adapt to the soil water content, and bacterial rather than fungal abundance is significantly reduced under dry conditions (Drenovsky et al., 2004; Chowdhury et al., 2011). Since biofilms consist of 90 wt %–99 wt % water, they reshape during the drying process, suggesting altered mechanical strength. The air drying and long-term storage of soil samples therefore may alter the biofilm matrix and fungal hyphae networks that have been shown to raise the quantity and stability of soil aggregates (e.g., Büks and Kaupenjohann, 2016; Bossuyt et al., 2001; Tang et al., 2011).
This, however, implies a decline in soil structural stability due to air drying and long-term storage, when samples are rewet, compared to field-fresh measurements. The destabilization of soil aggregates should have a similar effect on results of both USD and WSA measurements, since acting as a nucleus in aggregate formation POM is widely bound to the mineral matrix (e.g., Witzgall et al., 2021) and POM and mineral-associated organic matter (MAOM) carbon fractions are correlated with WSA (Bouajila and Gallali, 2010; Veum et al., 2012). Past studies on soil structural stability, POM occlusion and the amount of WSA have been conducted with either field-fresh, air-dried or re-moistened soil samples (e.g., Oztas and Fayetorbay, 2003; Annabi et al., 2007; Büks and Kaupenjohann, 2016). The aim of this work is to elucidate effects of these different practices on the comparability of the resulting data. We hypothesize that the air drying of soil samples causes the weakening of soil structure expressed by the decline in parameters such as the percentage by weight of WSA and the POM occlusive strength and that this effect can be attenuated by re-incubation that is long enough under conditions of field capacity.
2.1 Sites and sample preparation
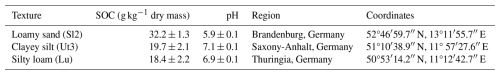
Undisturbed moist soil samples were taken in late March 2024 at three different organic farming sites in eastern Germany within a homogeneous area of 1 m2 each (Table 1). The organic litter and top 10 cm were removed, and sampling was carried out in 10–20 cm depth of the mineral topsoil by the use of metal rings (∅i= 5.6 cm, h= 4.0 cm, V= 100 mL, n= 25 per site). The rings containing the soil core were capped and tightly packed in closed plastic bags to preserve soil humidity for transpiration. Additionally, soil organic carbon (SOC) concentrations of the sites were determined by five mixed samples (each from three instances of drilling) of the upper 30 cm of topsoil along the bed.
2.2 Setting to field capacity
Back in the laboratory, 10 of the cores of each soil type were set on a porous plate, saturated via capillary action for 24 h and then drained by a hydrostatic head of pF 1.8 until constant weight was achieved. Half of these cores were weighed, dried for 24 h at 105 °C and weighed again, and the field capacity was determined from the difference minus the ring weight. The other five were directly measured (called the “field-fresh” treatment) for soil organic carbon (SOC) fractions and the percentage by weight of water-stable aggregates (%WSA). The remaining 3×5 cores were air-dried at 35 °C with strong air circulation until a constant weight was reached and then set to pF 1.8 as described above. Five of these were directly measured for the occlusion of POM carbon and %WSA (W0); the others were incubated at a constant water content and 20 °C in the dark for 1 and 4 weeks and then treated similarly.
2.3 fPOM, oPOM50 and residual SOC
For analysis of SOC fractions (Fig. 1), small metal rings (∅= 2.4 cm, h= 4.0 cm, V= 18.1 mL) were used to subsample approx. 20 g dry-soil equivalent from each of the field-fresh and re-incubated soil cores (n= 5). The samples were gently removed from the ring by the use of a spatula, weighted and rinsed into 200 mL polyethylene (PE) flasks with 100 mL of sodium polytungstate solution (SPT) following Büks (2023). As the soils differ in their field capacity, the density of the SPT solution was adapted to match 1.6 g cm−3 after addition to the sample by following Eq. (1):
with m as the dry mass of the soil sample (g), V as the volume of the soil solution per g soil (mL g−1) and ρSPT as the required density of the added SPT solution. Subsequently, the sample was stored at room temperature for 30 min to allow for infiltration of the SPT solution into the pore space.
The samples were then centrifuged for 26 min at 3500 g. The floating fPOM was separated by the use of a water-jet pump, filtered through a 0.45 µm cellulose acetate filter and cleaned with deionized water until the electrical conductivity of the filtrate dropped below 50 µS cm−1. The samples were flushed with deionized water though a 2 mm sieve to remove coarse material and into aluminum bottles, freezed at −20 °C, lyophilized, dried for 24 h at 105 °C and stored in an exsiccator with a desiccant battery.
After separation of the fPOM, the PE flasks were refilled to their initial weight with a 1.6 g cm−1 dense SPT solution. Cavitational stress was applied to the samples by the use of a sonotrode (Branson© Sonifier 250, sonotrode diameter of 13 mm, frequency of 40 kHz, immersion depth of 15 mm, power output of 50.02 ± 1.29 J s−1) as described by Büks and Kaupenjohann (2016). Sonication time corresponding to the applied energy density of q= 50 J mL−1 was determined based on the energy output of the sonotrode calculated following North (1976). The subsequent extraction and preparation of the respective fraction (oPOM50) was conducted in a similar fashion to the fPOM.
The residuum (R), containing the oPOM releasable with q>50 J mL−1 and the mineral-associated organic matter (MAOM), was washed approx. 6 times with 100 mL deionized water until the solution dropped below 50 µS cm−1 or showed no further decrease in conductivity by washing. Then, these samples were treated in a similar fashion to the fPOM and oPOM50 fractions.
All fPOM, oPOM50 and R samples were weighted and ground, and the SOC concentrations were determined by the use of an Elementar UNICUBE® CNS analyzer.
2.4 Water-stable aggregates
The %WSA was quantified for each soil type and treatment by the use of a wet-sieving apparatus (Eijkelkamp) following DIN 19683-16 (2015) and the manual instructions (Fig. 1, Eq. 2) (Kemper and Rosenau, 1986; Vrána et al., 2024). Similar to the above preparation, undisturbed subsamples of about 4 g dry-weight equivalent were taken from the five cores of each treatment by the use of a smaller metal ring (∅= 1.6 cm, h= 1.7 cm, V= 3.4 mL). The soil was carefully transferred from the ring to a 250 µm sieve, which was placed into a beaker with 90 mL of deionized water.
The samples were sieved for 3 min with a frequency of 34 min−1. The sieving sample remained within the first beaker, while the sieve was placed into a new one filled with 70 mL of deionized water. The submerged sample was treated ultrasonically by the use of a sonotrode (50.02 ± 1.29 J s−1) for 2 min (∼60 J mL−1) to fully disaggregate all macroaggregates. Then, 20 mL of deionized water was added, and the samples were sieved again as described above. The matter remaining within the sieve (R) was wet-sieved by the use of a 2 mm mesh to remove coarse material. The sieving sample of the first (m1) and second step (m2) as well as R were dried for 24 h at 105 °C and left at room temperature in an exsiccator with a desiccant battery.
The mass fraction m1 represents primary particles and microaggregates with diameters < 250 µm that were not part of water-stable macroaggregates, whereas m2 represents all objects < 250 µm that were released through the ultrasonication of water-stable macroaggregates. The fraction R is the sum of objects > 250 µm that did not pass through the mesh during both steps of separation. The fraction
with m2 as the matter < 250 µm of water-stable aggregates, m1 as the matter < 250 µm of water-labile aggregates and free primary particles, and R as the residuum of matter between 250 and 2000 µm, allows for semi-quantitative measurement of water-stable macroaggregates. Particles > 2 mm are excluded from the assessment, as samples can differ greatly in their coarse fraction due to small stones and litter.
2.5 Statistics
A pre-search with a 1.5× interquartile range method (1.5xIQR) was applied to identify potential outliers within the SOC and %WSA data sets, and the regular data of each treatment were tested for the normal distribution by the use of the Shapiro–Wilk test (α= 0.05). Since all WSA and SOC data were normally distributed, Grubbs' test for outliers was applied (α= 0.05) despite the low number of replications (n= 5). A few outliers were identified across all of the data that, however, do not affect the significance of the data (see Supplement). Data of %WSA and SOC fractions were also positively tested for regarding the homogeneity of variance (Levene's test, p>0.05). For each soil type separately, the data were compared by Student's t test between the field-fresh and each of the incubation treatments. In addition, linear regression analyses of the %WSA with the SOC fraction data were carried out.
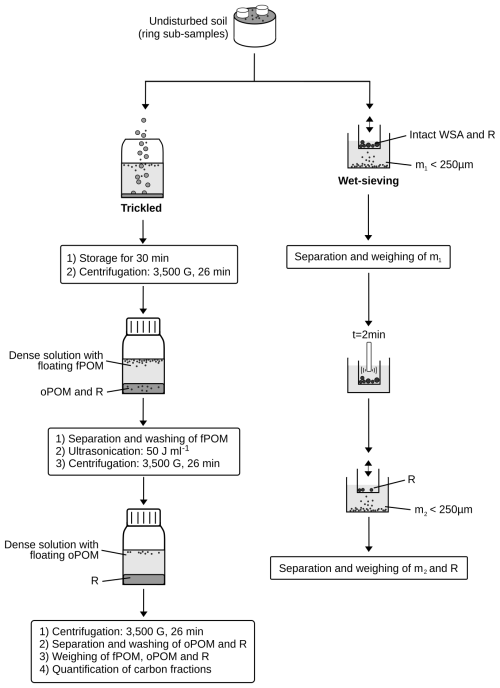
Figure 1Process chart of the SOC pool (left) and water-stable macroaggregate measurement (right). Abbreviations refer to free (fPOM) and occluded particulate organic matter (oPOM), non-aggregated particles < 250 µm (m1), particles < 250 µm of water-stable macroaggregates (m2), and the residua of both methods (R).
3.1 Shifts in SOC fractions
Directly after being rewet (0 weeks), the soils consistently show increased amounts of the fPOM-C and oPOM50-C fractions as well as corresponding decreases in the residual fractions, compared with the field-fresh treatments (Fig. 2). This shift is significant (p<0.05) for all samples except the fPOM of clayey silt (p= 0.22) and the oPOM50 of loamy sand (p= 0.06). The loamy sand thereby shows the largest increase in fPOM (+3.3 wt %) directly after being rewet, which mainly corresponds to the decrease in the residual fraction. The clayey silt and the silty loam, in contrast, have a rather small release of additional fPOM, and the decrease in the residual fraction corresponds with the oPOM50 fraction (+5.8 wt % and +14.8 wt %, respectively).
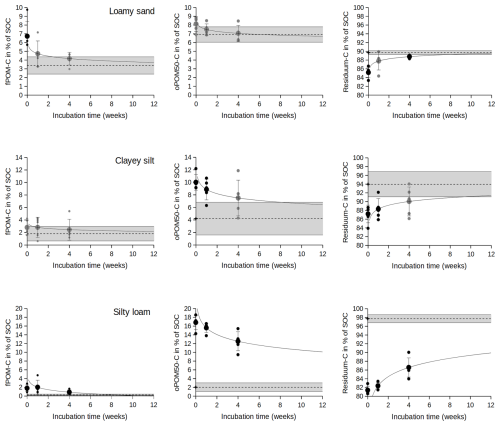
Figure 2Development of the fPOM-C, oPOM50-C and residual C fraction of a loamy sand; a clayey silt; and a silty loam after drying and re-incubation for 0, 1 and 4 weeks. The gray bar refers to the respective values of the field-fresh samples (dotted = mean value, outer lines = standard deviation). Large dots are mean values, and small dots are single measurements. Black dots refer to treatments with values significantly different from the field-fresh samples, and gray marks similarity (α= 0.05). The logarithmic fits illustrate the trend towards the field-fresh values with full lines within and dotted lines beyond the measured span.
All samples, furthermore, show a consistent trend towards the initial values of the field-fresh samples. The loamy sand reaches non-significant differences with field-fresh values in the fPOM and oPOM50 fraction within 1 week (t test, p≥0.05). The clayey silt takes 4 weeks in all SOC fractions, while the silty loam is not restored in any fraction.
3.2 Alteration of water stability and correlation with SOC fractions
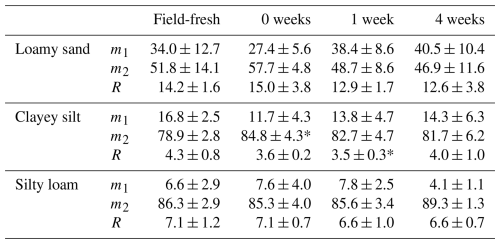
Of the three soil types, only the clayey silt shows significant (p<0.05) increases in %WSA directly after re-incubation and returns to non-significant differences compared to the field-fresh values within 1 week (Table 2). The loamy sand has a tendency for increased %WSA directly after being rewet. All soils thereby show higher variation compared to the above SOC measurements (1.4 wt %), with mean standard deviations of 4.2 wt % for the heavy soils and 9.1 wt % for the sand. The residual fractions of the respective soils have nearly constant mean values across all treatments differing by < 1.6 wt % (see Supplement).
Table 2Development of the amount of water-stable macroaggregates (m2, <250 µm), unbound particles (m1, <250 µm) and residual particles (R, 250–2000 µm) of loamy sand, clayey silt and silty loam in field-fresh state as well as after drying and re-incubation for 0, 1 and 4 weeks. The asterisk (*) refers to significant differences compared to the field-fresh samples (Student's t test, p<0.05).
The regression of %WSA (m2) with oPOM50-C plus residual C (the amount of SOC that should be occluded within water-stable aggregates) shows no correlation, neither for loamy sand (R2= 0.16) nor for clayey silt (R2= 0.10) and silty loam (R2= 0.11). Regression of m1 with the fPOM-C and %WSA with only the oPOM50-C or only the residual C lead to similar results (R2<0.21, R2<0.04 and R2<0.16, respectively).
Our results show that POM occlusion in sandy, silty and loamy soils is substantially decreased by air drying and subsequent rewetting. The effect diminishes over time with re-incubation but did not restore field-fresh values of all SOC fractions within a span of 4 weeks. Longer periods of incubation were avoided in this experiment, since they increase the risk that the isolated soil ecosystem within the sampling ring will be overgrown by fungal hyphae, most probably affecting aggregate characteristics (Tisdall et al., 1997; Fan et al., 2022).
The decreased strength of POM occlusion is indicated by an increased mass of the fPOM-C and weakly bound oPOM50-C fraction as well as the corresponding decrease in the residual C fraction, which contains the more strongly bound oPOM and the MAOM. This implies an initial section-by-section shift from the residual C to the oPOM50-C and further to the fPOM-C fraction as a result of air drying and rewetting. The sandy loam, which generally has lower aggregate stability due to its low clay content, thereby shows a stronger increase in the fPOM-C fraction, compared to the heavier soils. This can be interpreted as partial destruction of soil aggregates causing the release of POM. In contrast, clayey silt and silty loam have strong shifts from the residual to the oPOM50-C fraction, which accounts for the majority of destabilized POM and indicates a weakening of soil aggregate structure without the release of POM. Having decreasing mechanical stability of soil structure in common, all three soils show a different pattern of destabilization with mainly destruction (loamy sand), mainly weakening (clayey silt), and both destruction and weakening. In field studies, the observed weakening of soil structure after re-incubation will lead to an overestimation of fPOM and the loosely occluded POM fraction, while the respective residuum is underestimated. This causes overestimation of the labile C pool and underestimation of the binding forces within aggregates. This underpins the need for USD measurements with field-fresh samples.
In contrast to POM occlusion, the percentage by weight of water-stable aggregates is less affected by air drying and gentle rewetting. Only the clayey silt (and the loamy sand has a similar tendency) shows a significant increase in water-stable aggregates directly after re-incubation with a subsequent restoration of field-fresh values, while the other treatments have a largely constant state. Deviations within the residuum and a significant increase in the clayey silt residual fraction after 1 week can be better explained by a different composition of the 250–2000 µm mineral and organic matter fraction than by incomplete dispersion of macroaggregates. The mainly constant amount of water-stable aggregates matches results of Mikha et al. (2005), who observed constant aggregate size classes after dry–wet cycling. This is in contrast to different works that show a loss of macroaggregates after mainly fast rewetting events (e.g., Denef et al., 2001; Bossuyt et al., 2001). Haynes and Swift (1990) compared field-fresh and air-dried soil samples after wet sieving and found the mean weight diameter of macroaggregates in grassland soils to be increased, while arable soils showed decreased values compared to field-fresh samples. Due to the low number of studies on this topic, there can only be speculation as whether and to which extent these opposite observations are caused by soil texture, SOM quantity and quality, and methodology.
The observed lack of correlation with the SOC measurements seems contradictory at first, as it could be assumed that an unchanged amount of WSA implies unchanged oPOM fractions and, thus, decreasing the amount of water-stable macroaggregates causes an increase in fPOM-C at the expense of the oPOM fraction. However, both methods use incongruent parameters, as shown by Almajmaie et al. (2017), who stated there is a poor correlation between different methods of soil structure measurement. The WSA approach represents the ratio of soil particles <250 µm of water-stable macroaggregates to the total sample mass. If measurements are conducted with samples set to pF 1.8, shear forces of wet sieving are the prominent agent of dispersion. In contrast, the USD measurement provides the amount of fPOM that is fractionated by trickling through the SPT solution containing approx. 1.2 M Na+ as the chemical dispersion agent and oPOM fraction that is released from aggregates under certain levels of cavitational stress in the same Na+-rich environment. With both methods addressing different binding mechanisms and using different forces of disaggregation, results may, arguably, diverge. As an example, drying could weaken but not fully destroy the structural integrity of existing soil aggregates by dehydration of biofilms and fungal hyphae, which work as important aggregation agents in soils, and cause an increased release of weakly bound oPOM after the USD treatment, while the amount of non-aggregated material passing through the mesh in the WSA measurement remains constant as shown for silty loam. Air drying was also shown to cause extensive death of the soil microbiome, and rewetting can induce rapid mineralization of unprotected SOC such as biofilm components, known as the “Birch effect” (Birch, 1958; Kaiser et al., 2015; Schroeder et al., 2021). This may alter the binding pattern of POM within soil aggregates. As a result of a dehydration treatment, the increase in %WSA may occur through the formation of slightly soluble precipitates, which influences soil structural stability directly after rewetting. This points out the fact that WSA measurements should also be carried out with field-fresh samples to avoid, e.g., overestimation of the amount of soil aggregates of soils. If changes in structural integrity are associated with altering the size of water-stable macroaggregates, they can be addressed by measuring the mean weight diameter (MWD) as an extension of the %WSA method (Kemper and Rosenau, 1986).
From a mechanistic perspective, drying and rewetting are not clearly attributed to a positive or negative influence on aggregate stability, since different effects such as the loss of OM as a binding agent, partial damage of biofilms, cementation and the evolution of SOM–mineral interaction from outer- to inner-spherical binding pattern might work in opposite directions. Also the magnitude and duration of the respective effects after rewetting are still not estimated and require further studies, which should also include the shrinkage and swelling of clay-rich soils or the span of storage under air-dried conditions.
In consequence, studies on soil structure in mostly humid regions should generally use field-fresh samples. If there is no option except storing the soil samples, defined re-incubation should be carefully applied for ≥ 1 week. Furthermore, Kühnel et al. (2019) showed that similar masses of SOM fractions were measured in soil samples that were both air-dried and frozen for long-term storage, which makes freezing an additional storage option if microbial analyses are also on the schedule. In regions with dry seasons, however, severe droughts during the summer months and punctual raining events are regular phenomena. Most of the time, the topsoil is close to air dry, and rainfall sharply increases the soil water content, likely leading to aggregate breakdown. If analyzing WSA, POM occlusion or aggregate geometry in such cases, fast rewetting without subsequent incubation, even with the acceptance of slaking, can be suitable for simulating natural conditions properly. On the other hand, the slow introduction of moisture by capillary action is indicated if soil structure needs to be preserved. From our point of view, rinsing dry-soil aggregates into the SPT solution should be avoided to prevent enhanced slaking due to high ionic strength of dissolved Na+, similar to the solution of sodic soils (Rengasamy and Olsson, 1991; Liu et al., 2021). If comparing samples from different sites that are taken in different seasons with different cropping or weather history, the influence of seasonally changing aggregate stability (e.g., Tian et al., 2023) as well as underlying factors such as the adapting microbiome (McDaniel and Grandy, 2016; Kim et al., 2020) should be taken into account with regard to the respective research question.
The present study shows that measuring structural characteristics of soils with very different soil textures is strongly affected by drying and re-incubation treatments. The strength of POM occlusion decreases after rewetting, even if slaking is avoided, and is not recovered within the following 4 weeks. In contrast, the amount of water-stable macroaggregates remains stable and only increased significantly in one soil directly after rewetting. This work shows the importance of soil structure measurements with field-fresh samples to avoid overestimation of free and weakly occluded POM fractions and water-stable aggregates. The structure of measurement campaigns should be adapted in line with this issue. It further underpins that both investigated methods differ in their measured characteristics and should be used together and not substitute each other.
All of the data published within this paper are available in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-4679-2025-supplement.
FB developed the experimental concept, organized and conducted the soil sampling and the laboratory work, analyzed the data, and prepared the manuscript. SD planned the extraction process, adapted the USD method, and conducted and managed the experiment. JK conducted the laboratory work and data collection.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Many thanks go to Sandra Reimann, Carlotta Kollmann and Christine Beusch for their laboratory support. We are also very grateful to the farmers for the opportunity to sample their soils.
The research and publication of this article was funded by the Federal Ministry of Research, Technology and Space (BMFTR) (funding no. 01UU2202).
This paper was edited by Bertrand Guenet and reviewed by two anonymous referees.
Almajmaie, A., Hardie, M., Acuna, T., and Birch, C.: Evaluation of methods for determining soil aggregate stability, Soil Till. Res., 167, 39–45, https://doi.org/10.1016/j.still.2016.11.003, 2017.
Amézketa, E.: Soil Aggregate Stability: A Review, J. Sustain. Agr., 14, 83–151, https://doi.org/10.1300/j064v14n02_08, 1999.
Angulo, V., Bleichrodt, R. J., Dijksterhuis, J., Erktan, A., Hefting, M. M., Kraak, B., and Kowalchuk, G. A.: Enhancement of soil aggregation and physical properties through fungal amendments under varying moisture conditions, Environ. Microbiol., 26, e16627, https://doi.org/10.1111/1462-2920.16627, 2024.
Annabi, M., Houot, S., Francou, C., Poitrenaud, M., and Bissonnais, Y. L.: Soil Aggregate Stability Improvement with Urban Composts of Different Maturities, Soil Sci. Soc. Am. J., 71, 413, https://doi.org/10.2136/sssaj2006.0161, 2007.
Bavel, C. V.: Mean weight-diameter of soil aggregates as a statistical index of aggregation, Proceedings, Soil Sci. Soc. Am., 14, 20–23, https://doi.org/10.2136/sssaj1950.036159950014000C0005x, 1950.
Birch, H. F.: The effect of soil drying on humus decomposition and nitrogen availability, Plant Soil, 10, 9–31, https://doi.org/10.1007/BF01343734, 1958.
Bossuyt, H., Denef, K., Six, J., Frey, S., Merckx, R., and Paustian, K.: Influence of microbial populations and residue quality on aggregate stability, Appl. Soil Ecol., 16, 195–208, https://doi.org/10.1016/s0929-1393(00)00116-5, 2001.
Bouajila, A. and Gallali, T.: Land use effect on soil and particulate organic carbon, and aggregate stability in some soils in Tunisia, Afr. J. Agr. Res., 5, 764–774, 2010.
Bronick, C. J. and Lal, R.: Soil structure and management: a review, Geoderma, 124, 3–22, https://doi.org/10.1016/j.geoderma.2004.03.005, 2005.
Bryant, J. C., Bendixen, T. W., and Slater, C. S.: Measurement of the water-stability of soils, Soil Sci., 65, 341–346, 1948.
Büks, F. and Kaupenjohann, M.: Enzymatic biofilm digestion in soil aggregates facilitates the release of particulate organic matter by sonication, SOIL, 2, 499–509, https://doi.org/10.5194/soil-2-499-2016, 2016.
Büks, F., Kayser, G., Zieger, A., Lang, F., and Kaupenjohann, M.: Particles under stress: ultrasonication causes size and recovery rate artifacts with soil-derived POM but not with microplastics, Biogeosciences, 18, 159–167, https://doi.org/10.5194/bg-18-159-2021, 2021.
Büks, F.: Technical note: The recovery rate of free particulate organic matter from soil samples is strongly affected by the method of density fractionation, Biogeosciences, 20, 1529–1535, https://doi.org/10.5194/bg-20-1529-2023, 2023.
Cavael, U., Tost., P., Diehl, K., Büks, F., and Lentzsch, P.: Correlations of Soil Fungi, Soil Structure and Tree Vigour on an Apple Orchard with Replant Soil, Soil Syst., 4, 70, https://doi.org/10.3390/soilsystems4040070, 2020.
Cerli, C., Celi, L., Kalbitz, K., Guggenberger, G., and Kaiser, K.: Separation of light and heavy organic matter fractions in soil – Testing for proper density cut-off and dispersion level, Geoderma, 170, 403–416, https://doi.org/10.1016/j.geoderma.2011.10.009, 2012.
Chepil, W. and Bisal, F.: A Rotary Sieve Method for Determining the Size Distribution of Soil Clods, Soil Sci., 56, 95–100, https://doi.org/10.1097/00010694-194308000-00002, 1943.
Chowdhury, N., Marschner, P., and Burns, R.: Response of microbial activity and community structure to decreasing soil osmotic and matric potential, Plant Soil, 344, 241–254, https://doi.org/10.1007/s11104-011-0743-9, 2011.
Christensen, O.: An Index of Friability of Soils, Soil Sci., 29, 119–136, 1930.
Denef, K., Six, J., Paustian, K., and Merckx, R.: Importance of macroaggregate dynamics in controlling soil carbon stabilization: short-term effects of physical disturbance induced by dry–wet cycles, Soil Biol. Biochem., 33, 2145–2153, https://doi.org/10.1016/S0038-0717(01)00153-5, 2001.
DIN 19683-16:2015-12: Soil quality - Physical laboratory tests - Part 16: Determination of aggregate stability using the method of wet sieving, https://doi.org/10.31030/2360384, 2015.
Drenovsky, R. E., Vo, D., Graham, K. J., and Scow, K. M.: Soil water content and organic carbon availability are major determinants of soil microbial community composition, Microb. Ecol., 48, 424–430, https://doi.org/10.1007/s00248-003-1063-2, 2004.
Edwards, A. and Bremner, J.: Dispersion of Soil Particles by Sonic Vibration, J. Soil Sci., 18, 47–63, https://doi.org/10.1111/j.1365-2389.1967.tb01487.x, 1967a.
Edwards, A. P. and Bremner, J.: Microaggregates in Soils, J. Soil Sci., 18, 64–73, https://doi.org/10.1111/j.1365-2389.1967.tb01488.x, 1967b.
Emerson, W. W.: A classification of soil aggregates based on their coherence in water, Soil Res., 5, 47–57, https://doi.org/10.1071/SR9670047, 1967.
Fan, X., Pan, H., Ping, Y., Jin, G., and Song, F.: The underlying mechanism of soil aggregate stability by fungi and related multiple factor: a review, Eurasian Soil Sci., 55, 242–250, https://doi.org/10.1134/S1064229322020065, 2022.
Golchin, A., Oades, J. M., Skjemstad, J. O., and Clarke, P.: Study of free and occluded particulate organic matter in soils by solid state 13C CP/MAS NMR spectroscopy and scanning electron microscopy, Soil Res., 32, 285–309, https://doi.org/10.1071/SR9940285, 1994.
Graf-Rosenfellner, M., Cierjacks, A., Kleinschmit, B., and Lang, F.: Soil formation and its implications for stabilization of soil organic matter in the riparian zone, Catena, 139, 9–18, https://doi.org/10.1016/j.catena.2015.11.010, 2016.
Haynes, R. J. and Swift, R. S.: Stability of soil aggregates in relation to organic constituents and soil water content, J. Soil Sci., 41, 73–83, https://doi.org/10.1111/j.1365-2389.1990.tb00046.x, 1990.
Jouquet, P., Chintakunta, S., Bottinelli, N., Subramanian, S., and Caner, L.: The influence of fungus-growing termites on soil macro and micro-aggregates stability varies with soil type, Appl. Soil Ecol., 101, 117–123, https://doi.org/10.1016/j.apsoil.2016.02.001, 2016.
Kaiser, M. and Berhe, A. A.: How does sonication affect the mineral and organic constituents of soil aggregates? – A review, J. Plant. Nutr. Soil Sc., 177, 479–495, https://doi.org/10.1002/jpln.201300339, 2014.
Kaiser, M., Kleber, M., and Berhe, A. A.: How air-drying and rewetting modify soil organic matter characteristics: an assessment to improve data interpretation and inference, Soil Biol. Biochem., 80, 324–340, https://doi.org/10.1016/j.soilbio.2014.10.018, 2015.
Kemper, W. D. and Rosenau, R. C.: Aggregate stability and size distribution, in: Methods of soil analysis: Part 1 Physical and mineralogical methods, 5, 425–442, https://doi.org/10.2136/sssabookser5.1.2ed.c17, 1986.
Kim, N., Zabaloy, M. C., Guan, K., and Villamil, M. B.: Do cover crops benefit soil microbiome? A meta-analysis of current research, Soil Biol. Biochem., 142, 107701, https://doi.org/10.1016/j.soilbio.2019.107701, 2020.
Kühnel, A., Wiesmeier, M., Spörlein, P., Schilling, B., and Kögel-Knabner, I.: Influence of drying vs. freezing of archived soil samples on soil organic matter fractions, J. Plant Nutr. Soil Sci., 182, 772–781, https://doi.org/10.1002/jpln.201800529, 2019.
Lehtinen, T., Lair, G. J., Mentler, A., Gísladóttir, G., Ragnarsdóttir, K. V., and Blum, W. E.: Soil aggregate stability in different soil orders quantified by low dispersive ultrasonic energy levels, Soil Sci. Soc. Am. J., 78, 713–723, https://doi.org/10.2136/sssaj2013.02.0073, 2014.
Liu, J., Hu, F., Xu, C., Wang, Z., Ma, R., Zhao, S., and Liu, G.: Comparison of different methods for assessing effects of soil interparticle forces on aggregate stability, Geoderma, 385, 114834, https://doi.org/10.1016/j.geoderma.2020.114834, 2021.
Liu, J., Yang, Y., Zhou, J., Feng, X., Li, Y., Li, Y., Zi, J., Wang, C., Wang, E., and Jia, Y.: Evaluation of Soil Aggregate Sieving: The Impact of Field Moisture Content on Size Distribution and Stability, Agronomy, 15, 558, https://doi.org/10.3390/agronomy15030558, 2025.
Martínez-Mena, M., Williams, A. G., Ternan, J. L., and Fitzjohn, C.: Role of antecedent soil water content on aggregates stability in a semi-arid environment, Soil Till. Res., 48, 71–80, https://doi.org/10.1016/S0167-1987(98)00131-7, 1998.
McDaniel, M. D. and Grandy, A. S.: Soil microbial biomass and function are altered by 12 years of crop rotation, SOIL, 2, 583–599, https://doi.org/10.5194/soil-2-583-2016, 2016.
Meidl, P., Lehmann, A., Bi, M., Breitenreiter, C., Benkrama, J., Li, E., Riedo, J., and Rillig, M. C.: Combined application of up to ten pesticides decreases key soil processes, Environ. Sci. Pollut. Res., 31, 11995–12004, https://doi.org/10.1007/s11356-024-31836-x, 2024.
Mikha, M. M., Rice, C. W., and Milliken, G. A.: Carbon and nitrogen mineralization as affected by drying and wetting cycles, Soil Biol. Biochem., 37, 339–347, https://doi.org/10.1016/j.soilbio.2004.08.003, 2005.
North, P.: Towards an absolute measurement of soil structural stability using ultrasound, J. Soil Sci., 27, 451–459, https://doi.org/10.1111/j.1365-2389.1976.tb02014.x, 1976.
Oztas, T. and Fayetorbay, F.: Effect of freezing and thawing processes on soil aggregate stability, CATENA, 52, 1–8, https://doi.org/10.1016/s0341-8162(02)00177-7, 2003.
Rengasamy, P. and Olsson, K. A.: Sodicity and soil structure, Soil Res., 29, 935–952, https://doi.org/10.1071/SR9910935, 1991.
Rosenberg, N. J.: Response of plants to the physical effects of soil compaction, Adv. Agron., 16, 181–196, https://doi.org/10.1016/S0065-2113(08)60024-3, 1964.
Russell, J.: Report of the subcommittee on soil structure and consistency, Soil Sci. Soc. Am. J., 9, 10–22, https://doi.org/10.2136/sssaj1928.0361599500B920010002x, 1928.
Russell, M. B. and Feng, C. L.: Characterization of the stability of soil aggregates, Soil Sci., 63, 299–304, 1947.
Schroeder, J., Kammann, L., Helfrich, M., Tebbe, C. C., and Poeplau, C.: Impact of common sample pre-treatments on key soil microbial properties, Soil Biol. Biochem., 160, 108321, https://doi.org/10.1016/j.soilbio.2021.108321, 2021.
Tang, J., Mo, Y., Zhang, J., and Zhang, R.: Influence of biological aggregating agents associated with microbial population on soil aggregate stability, Appl. Soil Ecol., 47, 153–159, https://doi.org/10.1016/j.apsoil.2011.01.001, 2011.
Tian, J., Wu, X., Li, J., Guo, M., Zhang, X., and Chen, Q.: Seasonal and Long-Term Variability in Soil Structure and Erodibility under Different Land-Use Patterns in the Mollisols Region of Northeast China, Agronomy, 13, 449, https://doi.org/10.3390/agronomy13020449, 2023.
Tisdall, J. M., Smith, S. E., and Rengasamy, P.: Aggregation of soil by fungal hyphae, Soil Res., 35, 55–60, https://doi.org/10.1071/S96065, 1997.
Veum, K. S., Goyne, K. W., Kremer, R., and Motavalli, P. P.: Relationships among water stable aggregates and organic matter fractions under conservation management, Soil. Sci. Soc. Am. J., 76, 2143–2153, https://doi.org/10.2136/sssaj2012.0089, 2012.
Vrána, M., Kubát, J. F., Kavka, P., and Zumr, D.: A laser diffractometry technique for determining the soil water stable aggregates index, Geoderma, 441, 116756, https://doi.org/10.1016/j.geoderma.2023.116756, 2024.
Witzgall, K., Vidal, A., Schubert, D. I., Höschen, C., Schweizer, S. A., Buegger, F., Pouteau, V., Chenu, C., and Mueller, C. W.: Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun., 12, 4115, https://doi.org/10.1038/s41467-021-24192-8, 2021.
Yoder, R. E.: A direct method of aggregate analysis of soils and a study of the physical nature of erosion losses, Agron. J., 28, 337–351, https://doi.org/10.2134/agronj1936.00021962002800050001x, 1936.