the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Physiological responses to ultra-high CO2 levels in an evergreen tree species
Ben-El Levy
Yedidya Ben-Eliyahu
Yaniv-Brian Grunstein
Itay Halevy
Although numerous experiments have been dedicated to studying plant response to elevated CO2, almost none crossed the level of 1000 ppm. Plant responses to high CO2 levels importantly inform our understanding of plant physiology in ultra-high CO2 environments, e.g. in Earth's history, in the case of unmitigated anthropogenic emissions, and for future colonisation of Mars.
Here, we challenged 2-year-old seedlings of fruit trees grown in soil in a mesocosm, with CO2 levels of 400, 1600, and 6000 ppm, the highest of which is approximately equivalent to that of Mars' atmosphere. Plant growth and leaf–gas exchange (transpiration, stomatal conductance, and CO2 assimilation) were measured on a weekly basis for 23–25 consecutive days. We hypothesised that elevated CO2 levels will induce a decrease in transpiration, primarily attributed to reduced stomatal conductance. Indeed, leaf transpiration was decreased at 1600 ppm CO2 and remained low at 6000 ppm, concurrent with a 50 % decrease in stomatal conductance. The CO2-induced stomatal closure appears to have saturated between 850 and 1600 ppm CO2. Due to this effect, net assimilation was only mildly changed at 1600 ppm CO2 but significantly increased at 6000 ppm. As a result, water-use efficiency (WUE) quadrupled at 6000 ppm CO2. Stem height increment did not change significantly across the CO2 treatments.
Taken together, our measurements demonstrated both the potential and the limit of CO2-induced stomatal closure, with positive implications for fruit tree growth in ultra-high CO2 environments, as on Earth in the case of unmitigated anthropogenic CO2 emissions and on Mars.
- Article
(2253 KB) - Full-text XML
-
Supplement
(705 KB) - BibTeX
- EndNote
Understanding the implications of elevated atmospheric CO2 levels on plant physiology is paramount, as it has profound consequences for global ecosystems, water economy, and terrestrial carbon cycling (Bazzaz, 1990). At the microscopic level, the sensitivity of plants to altered CO2 levels directly impacts carbon assimilation through photosynthesis. When the level of CO2 within the plant diminishes, a regulatory response is triggered within the stomatal guard cells, and the stomata open, allowing the diffusive uptake of atmospheric CO2 (Lawson and Morison, 2004). This gas exchange comes at a cost. When the stomata are open, the plant loses water to the air in transpiration (Brodribb et al., 2009). This loss of water, while essential for photosynthesis and temperature regulation, can become a significant challenge for the plant in water-limited environments (Wagner et al., 2021, 2022).
The concentration of CO2 in the leaf and the surrounding atmosphere is a critical factor for photosynthetic carbon fixation. Changes in CO2 levels can trigger adjustments in stomatal conductance to optimise photosynthesis and water-use efficiency (WUE; Klein et al., 2013). However, stomata are sensitive to many additional factors. Stomata generally open in the presence of light, as photosynthesis, the process that converts CO2 and light energy into sugars, is active during daylight hours. Moreover, the relative humidity inside and outside the leaf affects stomatal conductance. Lower external humidity levels can downregulate stomatal conductance, leading to a decrease in water loss through transpiration (Wagner et al., 2022). Leaf temperature can also directly impact stomatal conductance. Warmer conditions often lead to increased transpiration rates, causing stomata to open wider to cool the leaf (Uni et al., 2023). Fluctuations in nutrient availability, particularly potassium, can influence stomatal conductance by affecting the turgor pressure of guard cells, which control stomatal opening and closing (Lebaudy et al., 2008). Often, these responses act together in shaping stomatal conductance at a given atmospheric CO2 concentration (Bartlett et al., 2016).
On a macroscopic scale, elevated CO2 exerts significant influence over planetary climate, presenting both challenges and opportunities for life on Earth and beyond (Forget et al., 2013; Ozak et al., 2016). For example, on Mars, where the partial pressure of CO2 is over 1 order of magnitude higher than that of Earth's atmosphere, understanding the impact of high CO2 on plant physiology and growth may aid efforts of human colonisation, for which suitable conditions are thought to exist (Slobodian, 2015). Mars' atmosphere is about 95 % CO2, and, at an average surface pressure of ≈6.5 mbar, this is equivalent to ≈6200 ppm CO2 on Earth (Franz et al., 2017). At the same time, Mars' atmosphere contains only trace amounts of O2 (0.16 %) and H2O (0.03 % on average but highly variable). Water is not only rare in the Martian atmosphere, but also on its surface, present mainly as ice or in hydrated salts, inaccessible to plants (Diez, 2018; Nazari-Sharabian et al. 2020). Despite the growing interest in colonising Mars, plant growth under such conditions has been explored in only a few studies (e.g. Richards et al., 2006; Wamelink et al., 2014).
Ultra-high atmospheric CO2 levels are also relevant on Earth, in the past and future. Looking ahead, if industrial development continues as usual (IPCC emission scenario RCP8.5), the CO2 concentration may rise to 1000 ppm by the end of this century and double that by the end of the year 2200. The effects of these changes on plant development are unknown and so are the implications for the carbon and water cycles on Earth. This said, such high CO2 levels existed in Earth's deep past. Recent reconstructions indicate that, between ≈65 and ≈40 million years ago, atmospheric CO2 levels were close to ≈1000 ppm, peaking at ≈1600 ppm around 51 million years ago (CenCO2PIP et al., 2023). Over this time interval, all extant plant families already existed (Li et al., 2019). Therefore, we can assume that extant plant species have evolved through large changes in atmospheric CO2 levels, up to 4-fold the current level or higher. At a CO2 level of 1000 ppm, net assimilation is close to saturation, with values typically at 79 %–91 % of the maximum rate, depending on temperature (Kirschbaum, 1994). However, at 400 ppm and 25 °C, net assimilation is at 64 % of the maximum rate.
Interestingly, the high abundance of CO2 in the Martian atmosphere (comparable to ≈6200 ppm on Earth) may compensate for water shortage on the planet's surface. Plants open their stomata to capture CO2 from the atmosphere, inevitably losing water vapour (Brodribb et al., 2009). Under rising CO2 levels, stomatal opening can be minimised, thus saving water for the plant and providing an advantage in dry conditions (Elliott-Kingston et al., 2016; Paudel et al., 2018). Preliminary research hints at the potential outcomes of our exploration: short-term (minutes) responses to an increase in atmospheric CO2 levels often involve a decrease in stomatal conductance (Medlyn et al., 2001; Paudel et al., 2018). This initial reduction is a reversible response and is linked to an increase in carbon fixation during photosynthesis. Essentially, when more CO2 is available, the plant can achieve the same level of carbon uptake at lower stomatal opening, thereby conserving water in the process. In addition, leaf surface pH might decrease due to increased concentrations of dissolved inorganic carbon in leaf surface solutions (Zeebe and Wolf-Gladrow, 2001). However, leaves can regulate their leaf surface pH through mechanisms that are still under study (Gilbert and Renner, 2021). On longer timescales, from weeks through the lifetime of an individual plant (Hasanuzzaman et al., 2023) to generations over evolutionary timescales (Steinthorsdottir et al., 2019), plants can undergo morphological and developmental changes in response to altered CO2 concentrations. Fossilised leaves from times in Earth's history when CO2 levels were higher display a lower areal density of stomata (Beerling et al., 1993; Haworth et al., 2012). As atmospheric CO2 concentrations decreased over time, many plant species evolved to increase stomatal density. It is believed that this long-term adaptation to slowly evolving CO2 levels enabled plants to thrive under changing environmental circumstances. Stomatal density has hence been used extensively as a palaeo-CO2 proxy (Steinthorsdottir et al., 2019; Konrad et al., 2021). However, this use has rarely been validated experimentally but rather calibrated against other palaeo-CO2 proxies (Konrad et al., 2021; but see Brownlee, 2001).
Despite the decrease in leaf–gas exchange, on a timescale of weeks, carbon assimilation still increases under elevated CO2 due to passive diffusion, as shown in both fruit trees (Paudel et al., 2018) and forest trees (Dror and Klein, 2022) grown under controlled conditions. Among plant species, trees demonstrate the highest CO2-induced photosynthetic increase, about a 45 % increase from 370–570 ppm CO2, as shown in a meta-analysis of free-air CO2 enrichment experiments (Ainsworth and Rogers, 2007). The higher assimilation can lead to increased carbon storage (Kinsman et al., 1997; Paudel et al., 2018) and increased growth. For example, elevated CO2 levels resulted in an elongation of branches and stems by 33 % in Garcinia mangostana (Downton et al., 1990) and by 15 % in Glycine max (Rogers et al., 1992). However, in contrast to growth in controlled environments, it has been established that, under field conditions, where competition over limited resources prevails, tree growth is mostly unaffected by elevated CO2 (Korner et al., 2005; Klein et al., 2016; Jiang et al., 2020; Norby et al., 2022). However, increased tree growth is still observed in some cases (Kim et al., 2020; Norby et al., 2024). Common to all these experiments is the moderate level of elevated CO2, up to 1000 ppm, motivated by the gradual increase in atmospheric CO2 concentrations on Earth (Klein and Ramon, 2019), with the exception of a few studies that were conducted at ultra-high CO2 levels of up to 50 000 ppm. Experimenting with seedlings in small chambers, grown on artificial media, it was shown that the fresh weight of lettuce, mint, and thyme increased up to 10 000 ppm CO2, decreasing somewhat at 30 000 ppm but still 5-fold higher than at ambient CO2 (Tisserat and Silman, 2000, Tisserat and Vaughn, 2001). A similar trend was observed in young seedlings of loblolly pine grown on artificial media (Tisserat and Vaughn, 2003). The effect was smaller in plants grown in soil, with a 2-fold increase in fresh weight at 3000 ppm CO2 (Tisserat and Vaughn, 2001). These experiments importantly constrained aspects of plant growth at ultra-high CO2 levels, but they did not address the matter of the plant's water budget.
High concentrations of CO2, which increase CO2 assimilation and decrease the stomatal conductance, lead to a notable reduction in water loss and an increase in the plant's water-use efficiency (WUE), the ratio of net CO2 assimilation to transpiration, representing the plant's ability to maximise carbon gain while minimising water loss (Mathias and Thomas, 2021; Dror and Klein, 2022). Experiments have demonstrated that tree seedlings exposed to double the ambient CO2 levels exhibit a decrease in stomatal conductance ranging from 12 %–36 % (Klein and Ramon, 2019). This reduction in stomatal conductance signifies that, at elevated CO2 levels, fewer signals are sent to keep stomata open, resulting in reduced plant water loss. However, it is crucial to recognise that the response to elevated CO2 is not uniform across all plant species. Factors such as the duration of exposure to high CO2, the availability of other essential resources like water and nutrients, and inherent genetic differences between species can modulate the plant's response. Some plant species may even exhibit reduced growth or alterations in their architecture, including shorter stems or smaller leaves, in response to elevated CO2 levels.
Among tree species, evergreen broadleaf species of tropical biomes have shown higher sensitivity to CO2 levels in terms of reduced stomatal conductance (Klein and Ramon, 2019). Taking advantage of this sensitivity, in this study, an experiment was designed to investigate the response of dwarf guava (Psidium cattleyanum), an evergreen broadleaf of the Amazon rainforest, to ultra-high CO2 levels. This short-stature tree species (growing up to 6 m in height) is also well adapted to medium light levels. Dwarf guava is an economically important species in some areas where it is cultivated (Patel, 2012) and an invasive species in others, like Hawaii (Huenneke and Vitousek, 1990). We used an isolated mesocosm chamber that was uniquely constructed to maintain CO2 carbon dioxide as the only variable, while the other variables (temperature, relative humidity, illumination intensity, nutrient supply) remained constant. In this mesocosm chamber, we placed 10 2-year-old guava seedlings and increased the CO2 levels to about 4 times the ambient atmospheric concentration, 1600 ppm, and to a Mars-like atmospheric concentration, 6000 ppm (although not attempting to simulate Mars' conditions per se). We hypothesised that elevated CO2 concentrations would induce a decrease in leaf transpiration, primarily by a reduction in stomatal conductance. This research may advance our understanding of plant growth and water-use efficiency under conditions expected on Earth by the year ≈2100 if anthropogenic CO2 emissions are unmitigated, and, beyond the terrestrial realm, it serves as a steppingstone towards ecological solutions in planetary environments.
2.1 Experimental design
A total of 10 2-year-old dwarf guava plants (Psidium cattleyanum) of similar size were transferred to 1 L pots. Plants were placed in a sealed mesocosm chamber (; COY, MI, USA; Rog et al., 2021). This chamber is designed to control humidity and temperature conditions. The humidity was maintained at 70 %, and the temperature was maintained at 24–26 °C, producing a vapour pressure deficit of 1.3–1.4 kPa. Tubes from CO2 and N2 cylinders were connected to the chamber to regulate gas concentrations, allowing three experimental treatments with varying CO2 levels: ambient, high, and ultra-high (400, 1600, and 6000 ppm CO2, respectively). Experimenting with such high CO2 levels required special safety precautions, and a CO2 detector alarming of leaks was installed outside the chamber. This was complemented by periodic CO2 measurements around the chamber, constantly showing levels of 400–480 ppm, typical of an indoors environment and assuring no leak from the chamber. To eliminate potential effects of differences among plants and chambers, the entire experiment was conducted on the same plants within the same chamber. Therefore, treatment periods were limited to just over 3 weeks (23–25 d) so that all treatments were applied on plants at the same developmental stage. This period is sufficient to allow plant acclimation to the CO2 treatment (Poorter et al., 2022). The experiment was conducted between 4 June and 27 August 2023. Plants were firstly exposed to 400 ppm CO2, followed by 1600 ppm, and then 6000 ppm, i.e. from ambient to high and ultra-high CO2. Prior to each treatment, plants underwent an additional 1-week acclimation period within the mesocosm chamber. Chamber CO2 was regulated using an SCD30 sensor with a measurement accuracy of ±30 ppm at a range of 400–10 000 ppm (Sensirion, Chicago, IL, USA) and an ESP32 controller (Espressif Systems, Shanghai, China). Notably, at 400, 1600, and 6000 ppm CO2, fluctuations of ±30 ppm are equal to ±7.5 %, 1.9 %, and 0.5 %, which are small. For this reason, we consider the CO2 levels to have been steady. We utilised an HLG-320H-36 A solar lamp (Agro Light, Beit Yehoshua, Israel) as the primary light source, positioned on top of the chamber to provide a photosynthetically active radiation (PAR) range of 550–700 at the canopy height. However, most leaves grew below the canopy leaves, under a lower PAR of 50–400 , which was still adequate for this tree species (see next paragraph). The day/night cycle was set to 12 h12 h. A light distribution map within the mesocosm chamber showed that plants were exposed to a homogeneous light regime. Individual plant positions were rotated every other day to mitigate any positional effects within the chamber.
2.2 Plant response to light intensity and instantaneous changes in CO2 level
To validate that Psidium cattleyanum is suitable for growth in the mesocosm chamber conditions, a photosynthetic light response curve was constructed in a pilot experiment. Six plants were exposed to increasing light levels, and their net assimilation rate () was measured. Determination of the assimilation rate was achieved with an LI-6800 infrared gas analyser (IRGA; LI-COR Biosciences, Lincoln, NE, USA). Temperature, relative humidity, and CO2 level within the leaf cuvette were adjusted to ambient during the measurement. PAR was adjusted to 1000, 800, 600, 400, 300, 200, 100, 50, and 0 , in this order. The value of the assimilation rate increased linearly from −1.89 at darkness (PAR=0) to 5.93 at a PAR of 200 (Fig. S1 in the Supplement). The compensation point was at 45 . At , increases in the assimilation rate were minor, and the assimilation rate saturated. A second pilot experiment tested the plant response to instantaneous changes in CO2 level. Here, three plants grown at ambient CO2 level were exposed to increasing CO2 levels, and their net assimilation rate () and stomatal conductance () were measured with the LI-6800 infrared gas analyser. The temperature, relative humidity, and PAR level within the leaf cuvette were set to 26 °C, 75 %, and 600 , respectively. The CO2 level was adjusted every 3–4 min to 400, 100, 50, 0, 150, 250, 300, 400, 500, 600, 700, 800, 900, 1000, 1100, 1200, 1300, 1400, 1500, 1600, 1700, 1800, 1900, 2000, and 400 , in this order.
2.3 Plant growth
Plants were actively growing throughout the experiment. We measured four growth parameters on a weekly basis: plant height (cm), leaf surface area (cm2), branch number, and shoot number. Stem diameter growth was too small for a manual measurement, as expected in saplings of this size. The height of each guava plant was measured from the base of the stem to the tip of the plant using a tape measure. The number of new branches was counted manually, with the appearance of clear protrusions consisting of four or more leaves considered to be new branches. The number of shoots extending from the ground was recorded separately. The leaf surface area was determined by counting the number of leaves on each plant and multiplying by an average leaf size. The average leaf size was obtained using the Easy Leaf Area application developed by Hsien Ming Easlon (https://github.com/heaslon/Easy-Leaf-Area, last access: 10 June 2025). In this process, leaf tops were photographed close to a 2×2 cm2 area painted red over a white background. The leaf area was then derived from the ratio of green to red pixels present in the image.
2.4 Leaf–gas exchange
Stomatal conductance and transpiration were measured using an LI-600 infrared gas analyser (LI-COR Biosciences, Lincoln, NE, USA). The LI-600 was applied to determine the gas exchange during photosynthesis in fresh leaves, with a known leaf surface area of 1 cm2. The rates of transpiration (E in ) and stomatal conductance (gs in ) were measured between 09:00 and 11:00 LT. The rate of CO2 assimilation was measured with a leaf cuvette at 400 ppm µmol CO2 using the LI-6800 infrared gas analyser (see under Plant response to light intensity) but could not be measured using the leaf cuvette at the higher CO2 levels due to the instrument limitations. Instead, we measured the assimilation rate across the three CO2 levels by analysing the rate of decrease in the CO2 concentration (ppm) within the controlled mesocosm chamber over a known duration (measured in s), which was then divided by the corresponding leaf surface area of all plants (m2). The amount of assimilated CO2 was transformed from the change in ppm CO2 into µmol CO2 by multiplying the ppm value by 14.558, which is the number of moles of air in the chamber, according to the ideal gas law, considering atmospheric pressure and mesocosm volume and temperature (above). The SCD30 sensor data, responsible for monitoring the CO2 levels within the chamber, were collected at regular intervals of 1.5–2.3 min. Notably, a decrease in the CO2 level was considered when the sensor data showed a negative difference persisting for three or more consecutive intervals, and the net change in the CO2 level was computed and subsequently divided by the total duration of the decrease. Data collection for this process were conducted between 08:00 and 16:00 LT exclusively, during daylight hours and on days with no disturbances within the mesocosm chamber. For each CO2 level, the assimilation rate was calculated for 20 periods, from which the mean and standard error were calculated. These measurements facilitated a comprehensive evaluation of the plants' CO2 assimilation. Water-use efficiency (WUE) was calculated as the ratio of the rate of carbon assimilation to the rate of transpiration (E) in the guava plants on 4 different days for each CO2 concentration.
2.5 Leaf parameters
Leaf stomatal density was determined on newly matured leaves formed in the new CO2 environment (3 d old leaves from the top of the canopy, grown at PAR of 550 ; n=10 plants) by calculating the number of stomata per unit area (mm2). The lower epidermal layer was carefully affixed to a slide using a transparent contact adhesive. Subsequently, the slide was captured under a light microscope (Leica DM500, Wetzlar, Germany) at a 20× magnification. Utilising the ImageJ software, images were analysed, and the number of stomata was divided by the defined area obtained from the slide, with a 0.005 mm line reference. The pH level on leaf surfaces was measured non-invasively using a Hanna device (Hanna Instruments Inc., Woonsocket, USA). The leaves were carefully positioned on a stable cardboard surface, and the device was affixed directly to them.
2.6 Statistical analysis
We tested for significant differences among CO2 treatments for specific parameters. Prior to statistical tests, the distribution of data was presented as a histogram for each parameter. Then, a normal distribution was fit to each histogram, and the goodness of fit was tested by a Shapiro–Wilk test. P values of the Shapiro–Wilk tests were >0.05 (often >0.2), indicating that all parameters were normally distributed. Differences in transpiration and stomatal conductance were analysed using ANOVA at a significance level α=0.05. For the analysis, CO2 treatments were transformed into a nominal parameter (ambient, elevated, and ultra-high). All analyses were performed in the JMP software (SAS, Cary, NC, USA). Differences in stomatal density, assimilation, WUE, height increment, and leaf surface pH were analysed by t tests on pairwise comparisons. P values are reported in the figures.
Examining leaf transpiration (Fig. 1a), a 20 % decrease was observed upon the transition from 400–1600 ppm CO2 (from ∼4.4 to ; F=44.9, P<0.0001). The subsequent increase in CO2 concentration to 6000 ppm resulted in no statistically significant changes in leaf transpiration compared to the 1600 ppm treatment, suggesting a potential threshold effect in the modulation of transpiration rates by elevated CO2. In terms of stomatal conductance (gs), the transition from 400–1600 ppm CO2 resulted in a 50 % reduction in gs, which persisted at the 6000 ppm treatment (from ∼0.85 to ; F=22.6, P<0.0001; Fig. 1b). Overall, both transpiration and gs fluctuated across measurement days; however, only under 400 ppm CO2 was there an increasing trend. Considering that this treatment was applied immediately after plants were brought in from the greenhouse, values during the first 10 d under 400 ppm CO2 might still reflect acclimation to the chamber conditions. In contrast to the decrease in gs with CO2 increase, stomatal density increased mildly with CO2, with a significant 20 % increase between the 400 and 6000 ppm CO2 treatments (Fig. 2).
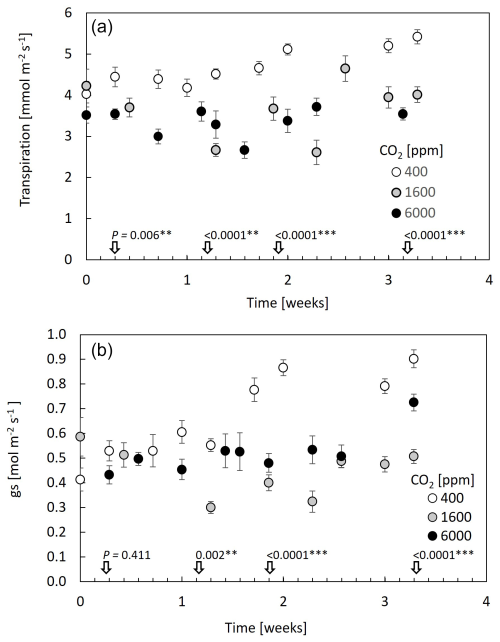
Figure 1Leaf transpiration and stomatal conductance are decreased at 1600 ppm CO2 and remain low at 6000 ppm. Data points are means of 10 guava saplings subjected to different CO2 concentrations. Measurements were made with a leaf cuvette. Error bars represent standard errors. P values are from ANOVA on transpiration and stomatal conductance levels at specific dates (±1 d), and *, **, and *** indicate differences among CO2 levels at 0.01, 0.001, and 0.0001 significance levels.
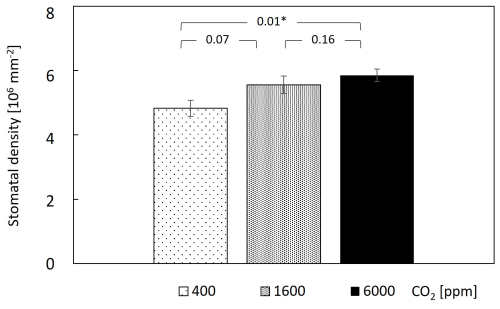
Figure 2Stomatal density mildly increased under elevated CO2. Data points and bar heights are means of 10 guava saplings subjected to different CO2 concentrations. Error bars represent standard errors. P values are from paired t tests, and * indicates significant differences among CO2 levels. Stomatal density was measured under a light microscope.
The assimilation rate of CO2 (A) was calculated for the entire mesocosm and showed a value of 1.5 at 400–1600 ppm CO2. While this value is relatively low, it was relatively close to the photosynthetic rate of Psidium cattleyanum leaves under PAR of , which is close to the light intensity experienced by most leaves in the chamber (Fig. S1 and Methods). The assimilation rate exhibited a mild, yet significant, decrease over the transition from 400–1600 ppm CO2, demonstrating a major increase only at 6000 ppm (Fig. 3a). Water-use efficiency was unchanged upon the shift from 400–1600 ppm CO2 and displayed a 4-fold increase upon the subsequent shift to 6000 ppm (Fig. 3b). Measurements of the weekly growth height did not reveal a significant change (Fig. 4), nor did the weekly increment of leaf surface area (Fig. S2 in the Supplement). The height increment was , which is equal to 4 %–5 % growth, regardless of CO2 level. Stalled branch growth or even branch degradation appeared to have occurred upon the change to 6000 ppm CO2 (data not shown).
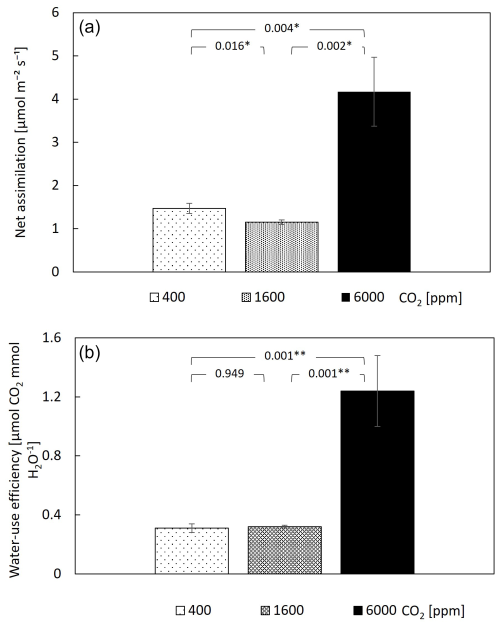
Figure 3Net assimilation is mildly changed at 1600 ppm CO2 and increases at 6000 ppm, driving a major increase in water-use efficiency. Bar heights are means of 10 guava saplings subjected to different CO2 concentrations. Error bars represent standard errors. P values are from paired t tests, and * and ** indicate differences among CO2 levels at 0.01 and 0.001 significance levels. (a) Net assimilation was calculated from chamber CO2 dynamics. (b) Water-use efficiency was calculated as net assimilation divided by transpiration.
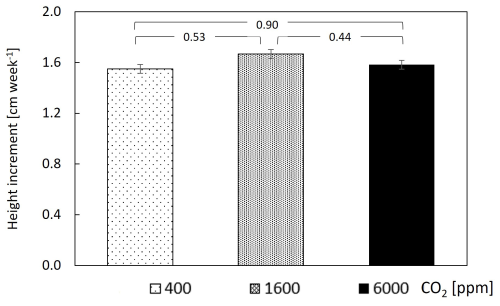
Figure 4Plant growth is similar under different CO2 concentrations. Data points are means of 10 guava saplings subjected to different CO2 concentrations. Error bars represent standard errors. P values are from paired t tests.
Plants that were grown at ambient CO2 level were also tested for their A and gs responses to instantaneous changes in CO2 level (Fig. S3 in the Supplement). Assimilation increased linearly from at 0 ppm CO2 to at 800 ppm CO2 (with a compensation point around 100 ppm CO2). At higher CO2 levels, A nearly saturated but continued to increase up to at 2000 ppm CO2, the highest level permitted by the IRGA instrument. In parallel, gs decreased in two measured plants from 0.14–0.11 and from 0.09–0.05 .
Decades of research on physiological tree responses to elevated CO2 have shaped our understanding of vegetation feedbacks to the increasing level of atmospheric CO2 on Earth (Medlyn et al., 2001; Korner et al., 2005; Klein et al., 2016). However, since most experiments were limited to CO2<1000 ppm, the limit of stomatal sensitivity to elevated CO2 has remained unclear, and, specifically, the limit of the effect of CO2-induced decrease in stomatal conductance on tree transpiration is unknown. Previous experiments with ultra-high CO2 levels indicated consistent benefits in small plants grown in small chambers, mostly on artificial media (Tisserat and Silman, 2000; Tisserat and Vaughn, 2001, 2003). However, these former experiments focused mostly on plant growth, leaving the physiological mechanisms and plant water use unresolved. Here, we challenged seedlings of fruit trees grown in a mesocosm, with ultra-high CO2 levels of 1600 and 6000 ppm. We hypothesised that elevated CO2 levels will induce a decrease in plant water use, primarily by a reduction in stomatal conductance. Indeed, transpiration was decreased at 1600 ppm CO2 and remained low at 6000 ppm (Fig. 1a), due to reduced stomatal conductance (Fig. 1b). The effects on assimilation, WUE, and growth were also measured (Figs. 3 and 4). Taken together, our measurements demonstrate both the potential and limit of CO2-induced stomatal closure, with positive implications for fruit tree growth in ultra-high CO2 environments, as on Earth in the case of unmitigated anthropogenic CO2 emissions (e.g. IPCC emission scenario RCP8.5) and on Mars (Franz et al., 2017; although not attempting to simulate Mars' conditions per se).
Experimenting with lemon tree saplings, we previously showed that CO2-induced stomatal closure can decrease transpiration, while still increasing assimilation, at CO2 levels as high as 850 ppm (Paudel et al., 2018). Here we showed that this effect was active upon an increase from 400–1600 ppm CO2 but that no further change occurred upon a further increase to 6000 ppm. On the contrary, stomatal conductance was sometimes higher at 6000 than at 1600 ppm CO2 (but still lower than at 400 ppm; Fig. 1b). Across the measurements, stomatal conductance at 1600 ppm CO2 was mostly ∼50 % of its value at 400 ppm. The calculated change in stomatal conductance within this range (400–1600 ppm) was per 100 ppm CO2 increase. For comparison, this CO2-induced stomatal closure is higher than that calculated for lemon ( per 100 ppm CO2 increase; Paudel et al., 2018), very similar to that measured in mango ( per 100 ppm CO2 increase), and identical to the mean calculated for broadleaf evergreen tree species across 39 different experiments (−33; Klein and Ramon 2019). However, due to the absence of a significant change in stomatal conductance from 1600–6000 ppm CO2, the calculated change in stomatal conductance over the range 400–6000 ppm was only per 100 ppm CO2 increase. Integrating our current and previous experiments, we can conclude that, for an evergreen fruit tree such as guava, CO2-induced stomatal closure probably saturates between 850 and 1600 ppm CO2. Do these responses reflect an adaptation to elevated CO2 or an instantaneous response? Experimenting with trees with no previous exposure to elevated CO2, we observed a similar trend in stomatal closure (a 20 %–50 % reduction in gs between 400–1600 ppm CO2; Fig. S3) which seemed to level off at higher CO2 levels. Despite this reduction, net carbon assimilation increased, mostly below 800 ppm CO2, while measurements were limited to 2000 ppm CO2 due to the IRGA constraints.
The measured stomatal response to the ultra-high CO2 levels can explain the observed changes in carbon assimilation. Since assimilation of CO2 into the leaf occurs by passive diffusion, most tree species increase assimilation despite CO2-induced stomatal closure (Dror and Klein, 2022). However, in our case, the strong decrease in stomatal conductance at 1600 ppm was not compensated by the higher CO2 availability, and the assimilation rate mildly decreased (Fig. 3a). Only at 6000 ppm did the surplus CO2, which was not accompanied by additional stomatal closure, result in a significant increase in assimilation. This indicates that the maximum rate of carboxylation increased under ultra-high CO2 level, as previously shown in lemon under 650 and 850 ppm CO2 (Paudel et al., 2018). The CO2-induced photosynthetic increase observed here agrees with earlier studies at lower levels of elevated CO2, which showed that photosynthesis is far from saturation at 400 ppm (Kirschbaum, 1994) and that trees, more than shrubs, grasses, and crop plants, increase their photosynthesis under elevated CO2 (Ainsworth and Rogers, 2007). In turn, these physiological mechanisms yielded a major increase in WUE (Fig. 3b). It is noteworthy that the stable WUE (400–1600 ppm CO2) was driven by proportional reductions in assimilation and transpiration, whereas the major WUE increase (at the 1600–6000 ppm CO2 transition) was driven by increased assimilation. The stable WUE (400–1600 ppm CO2) contrasts an overall increase in WUE in the terrestrial biosphere at this CO2 range (Walker et al., 2021) but can be expected due to the reduced stomatal conductance in a broadleaf tropical tree species (Paudel et al., 2018), which is not a ubiquitous response across tree species (Klein and Ramon, 2019). Finally, seedling growth, measured here as the height increment, was consistent across the large CO2 range (Fig. 4), meaning that the surplus carbon may have been allocated to sinks other than stem growth, such as storage (Paudel et al., 2018), respiration, or root growth (Dror and Klein, 2022). In addition, there was an increase in new shoot production at 1600 ppm (data not shown). Our results are in apparent disagreement with those of the early ultra-high CO2 experiments, in which the fresh weight of seedlings of several herb species and pine increased at ultra-high CO2 and saturated only at levels of 10 000 ppm (Tisserat and Silman, 2000, Tisserat and Vaughn, 2001, 2003). Several differences between the early studies and our experiments may account for the different results. Unlike our experiments, in the earlier studies, plants were grown on artificial media, sometimes supplemented with sucrose. The different growth conditions and substrate availability are expected to lead to differences in carbon assimilation of the observed sense (i.e. a greater biomass increase under the near-optimal conditions of the past studies). Indeed, the fresh weight increase measured in past experiments was smaller when plants were grown in soil, with only a 2-fold increase in fresh weight at 3000 ppm (Tisserat and Vaughn, 2001). Lastly, at least for pine, stomata are almost insensitive to increases in CO2 levels (Klein and Ramon, 2019) and especially in loblolly pine (Will and Teskey, 1997). Therefore, in pine variants, little or no CO2-induced stomatal closure is expected, and increases in carbon fixations are expected to be larger.
In contrast to the expected decrease in stomatal density in response to higher CO2 (Beerling et al., 1993; Ainsworth and Rogers, 2007), stomatal density increased modestly, but significantly, at the higher CO2 levels in our experiment (Fig. 2b). For example, stomatal density increased by 15 % between 400 and 1600 ppm CO2, while stomatal size did not change. This was unexpected, since, overall, stomatal conductance decreased (e.g. by 50 % between 400 and 1600 ppm CO2). Therefore, it can be deduced that stomatal aperture was in fact reduced by 65 %. Previous studies showed that stomatal conductance of mature leaves has a regulatory effect on the stomatal development of expanding leaves (Brownlee, 2001, Miyazawa et al. 2006). We do not fully understand why stomatal density increased and whether this change represents a long-term response. However, previous research shows that, even for plants that were exposed for generations to elevated CO2, responses can vary by species. Among 17 plant species growing in a naturally enriched CO2 spring in Italy, stomatal density was higher under the higher CO2 levels in 7 species, including two tree species (Buxus sempervirens and Ruscus aculeatus; Bettarini et al., 1998). Similarly, stomatal density increased in 10 out of 27 free-air CO2 enrichment experiments (Ainsworth and Rogers, 2007). More importantly, the assumption that plants decrease stomatal density in response to higher CO2 has not been rigorously tested in biological experiments. Rather, stomatal density was tested against other palaeo-CO2 proxies, such as contemporaneous CO2 measurements from glacial ice cores, and showed limited utility, especially under warm and moist conditions, as in our experiment (Konrad et al., 2021).
Other leaf traits that were examined did not show any sensitivity to CO2 levels, including leaf surface pH, which was expected to decrease due to a higher dissolved inorganic carbon content in leaf surface solutions (Zeebe and Wolf-Gladrow, 2001) but remained at 5.0 (Fig. 4). Variations among plant species in leaf surface pH range between 1.0 and 11.0, yet the pH of 5.0 measured here is lower than the pH values of other rosids, typically between 6.0 and 10.0 (Gilbert and Renner, 2021). In a preliminary experiment, we observed black stains on some higher-canopy leaves at 6000 ppm CO2 (not shown), but these were probably related to high light conditions at the top of the mesocosm chamber. Finally, we did not detect any signs related to oxygen deficiency caused by the ultra-high CO2 levels, as reported in humans at CO2>1000 ppm (Azuma et al., 2018).
Our experiment is not free of limitations. For example, a relatively low number of seedlings (n=10) with natural interplant heterogeneity yielded high variation in some parameters (e.g. net assimilation; Fig. 3a). Our calculation method for net assimilation was also unique, since a cuvette gas analyser could not be used at the high CO2 levels studied here. A comparison between the methods, permitted at 400 ppm CO2, indicated an underestimation by the mesocosm method (1.5 compared with 2.4 ) under PAR of (Fig. S1). It is possible that, due to self-shading of the leaves, PAR levels were for most leaves. Regardless, our calculation method was identical across the three CO2 levels; hence the relative changes persist. In addition, our choice of running the experiment over three consecutive periods (one for each of the three CO2 concentrations) meant that plants were slightly different at each period. Such a difference was minimised by limiting the periods to just slightly over 3 weeks each so that all treatments were applied on plants at the same developmental stage. As a result, the study results cannot be generalised to longer timescales of months and years. We reiterate that this choice of continuous experimentation with the same plants eliminated potential effects of differences among plants and chambers and that, prior to each treatment, plants underwent a 1-week acclimatisation period within the chamber.
Studying the physiological responses to ultra-high CO2 levels in evergreen tree species can inform our basic understanding of plant physiology and the cultivation of fruit trees under high anthropogenic CO2 emission scenarios and in extreme environments, as on Mars (although not attempting to fully simulate Mars' conditions). Except for a few pioneering studies in small plants grown on artificial media in small chambers, it has been unknown whether plants can thrive or even survive under such high-CO2 conditions, as has the effect of ultra-high CO2 levels on plant water use. In this respect, our results bring encouraging prospects of three sorts: (1) guava seedlings were actively growing at 6000 ppm CO2, at a rate similar to their growth rate under ambient CO2 levels. We note that other tree species may even show growth benefits, as observed at CO2 levels between 400 and 1000 ppm (Paudel et al., 2018; Dror and Klein, 2022; Norby et al., 2024). (2) Net assimilation increased significantly at 6000 ppm CO2. This means that cultivation at elevated CO2 can serve as a carbon sink, with a possible role in the mitigation of anthropogenic CO2 emissions. (3) Transpiration at 6000 ppm was 20 % lower than at 400 ppm CO2. Together with the increase in assimilation, we measured an almost 4-fold increase in WUE across this CO2 range. Our results are valuable both for the future of the Earth (e.g. emission scenario RCP8.5) and for Mars colonisation (Richards et al., 2006, Wamelink et al., 2014; Slobodian et al., 2015). On Earth, indoor environments are already experiencing excessive CO2 levels, and plants in green walls help to decrease indoor CO2 levels (Agra et al., 2021). On Mars, low temperature and low atmospheric O2 levels mean that plant cultivation will require an engineered environment. In this sense, our mesocosm can be regarded as a simulation of an artificial greenhouse (though we made no attempt to simulate Mars conditions). Considering the scarcity of water on Mars (Diez, 2018; Nazari-Sharabian, 2020) and in some regions on Earth, especially under climate change scenarios (Gosling and Arnell, 2016; Rosa et al., 2020), the increase in WUE at high CO2 levels observed in this study is a major advantage stemming from plant physiology. Overall, the high abundance of CO2 in Earth's future atmosphere and in the present Martian atmosphere might compensate for water shortage.
Data are available in an open repository: https://doi.org/10.6084/m9.figshare.29626349 (Klein et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-5069-2025-supplement.
IH and TK initiated the study. YBG and YBE established the experimental system and the first measurements. BEL performed the experiment and the analyses. BEL, TK, and IH wrote the article.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors thank the Weizmann Center for Planetary Science for supporting the project.
This research has been supported by the Helen Kimmel Center for Planetary Sciences, Weizmann Institute of Science.
This paper was edited by Andrew Feldman and reviewed by Anju Manandhar and two anonymous referees.
Agra, H. E., Uni, D., Horwitz, R., Klein, T., and Blaustein, L.: Leaf color segmentation and pot volume influence on the CO2 absorption efficiency in two common green-wall plants, J. Green Build., 16, 3–12, https://doi.org/10.3992/jgb.16.3.3, 2021.
Ainsworth, E. A. and Rogers, A.: The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions, Plant Cell Environ., 30, 258–270, https://doi.org/10.1111/j.1365-3040.2007.01641.x, 2007.
Azuma, K., Kagi, N., Yanagi, U., and Osawa, H.: Effects of low-level inhalation exposure to carbon dioxide in indoor environments: A short review on human health and psychomotor performance, Environ. Int., 121, 51–56, https://doi.org/10.1016/j.envint.2018.08.059, 2018.
Bartlett, M. K., Klein, T., Jansen, S., Choat, B., and Sack, L.: The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought, P. Natl. Acad. Sci. USA, 113, 13098–13103, https://doi.org/10.1073/pnas.1604088113, 2016.
Bazzaz, F. A.: The response of natural ecosystems to the rising global CO2 levels, Annu. Rev. Ecol. Evol. S., 21, 167–196, https://doi.org/10.1146/annurev.es.21.110190.001123, 1990.
Beerling, D. J., Chaloner, W. G., Huntley, B., Pearson, J. A., and Tooley, M. J.: Stomatal density responds to the glacial cycle of environmental change, P. Roy. Soc. Lond. B Bio., 251, 133–138, https://doi.org/10.1098/rspb.1993.0019, 1993.
Bettarini, I., Vaccari, F. P., and Miglietta, F.: Elevated CO2 concentrations and stomatal density: observations from 17 plant species growing in a CO2 spring in central Italy, Glob. Change Biol., 4, 17–22, https://doi.org/10.1046/j.1365-2486.1998.00098.x, 1998.
Brodribb, T. J., McAdam, S. A. M., Jordan, G. J., and Feild, T. S.: Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants, New Phytol., 183, 839–847, https://doi.org/10.1111/j.1469-8137.2009.02844.x, 2009.
Brownlee, C.: The long and the short of stomatal density signals, Trends Plant Sci., 6, 441–442, https://doi.org/10.1016/S1360-1385(01)02095-7, 2001.
CenCO2PIP (Cenozoic CO2 Proxy Integration Project Consortium), Hönisch, B., Royer, D. L., Breecker, D. O., Polissar, P. J., Bowen, G. J., and Zhang, L.: Toward a Cenozoic history of atmospheric CO2, Science, 382, eadi5177, https://doi.org/10.1126/science.adi5177, 2023.
Diez, A.: Liquid water on Mars, Science, 361, 448–449, https://doi.org/10.1126/science.aar7268, 2018.
Downton, W. J. S., Grant, W. J. R., and Chacko, E. K.: Effect of elevated carbon dioxide on the photosynthesis and early growth of mangosteen (Garcinia mangostana L.), Sci. Hortic., 44, 215–225, https://doi.org/10.1016/0304-4238(90)90121-T, 1990.
Dror, A. and Klein, T.: The effect of elevated CO2 on aboveground and belowground carbon allocation and eco-physiology of four species of angiosperm and gymnosperm forest trees, Tree Physiol., 42, 831–847, https://doi.org/10.1093/treephys/tpab136, 2022.
Elliott-Kingston, C., Haworth, M., Yearsley, J. M., Batke, S. P., Lawson, T., and McElwain, J. C.: Does size matter? Atmospheric CO2 may be a stronger driver of stomatal closing rate than stomatal size in taxa that diversified under low CO2, Front. Plant Sci., 7, 1253, https://doi.org/10.3389/fpls.2016.01253, 2016.
Forget, F., Wordsworth, R., Millour, E., Madeleine, J.-B., Kerber, L., Leconte, J., Marcq, E., and Haberle, R. M.: 3D modelling of the early martian climate under a denser CO2 atmosphere: Temperatures and CO2 ice clouds, Icarus, 222, 81–99, https://doi.org/10.1016/j.icarus.2012.10.019, 2013.
Franz, H. B., Trainer, M. G., Malespin, C. A., Mahaffy, P. R., Atreya, S. K., Becker, R. H., and Wong, M. H.: Initial SAM calibration gas experiments on Mars: Quadrupole mass spectrometer results and implications, Planet. Space Sci., 138, 44–54, https://doi.org/10.1016/j.pss.2017.01.014, 2017.
Gilbert, K. J. and Renner, T.: Acid or base? How do plants regulate the ecology of their phylloplane?, AoB Plants, 13, plab032, https://doi.org/10.1093/aobpla/plab032, 2021.
Gosling, S. N. and Arnell, N. W.: A global assessment of the impact of climate change on water scarcity, Climatic Change, 134, 371–385, https://doi.org/10.1007/s10584-013-0853-x, 2016.
Hasanuzzaman, M., Zhou, M., and Shabala, S.: How does stomatal density and residual transpiration contribute to osmotic stress tolerance?, Plants, 12, 494, https://doi.org/10.3390/plants12030494, 2023.
Haworth, M., Elliott-Kingston, C., Gallagher, A., Fitzgerald, A., and McElwain, J. C.: Sulphur dioxide fumigation effects on stomatal density and index of non-resistant plants: Implications for the stomatal palaeo-CO2 proxy method, Rev. Palaeobot. Palynol., 182, 44–54, https://doi.org/10.1016/j.revpalbo.2012.06.006, 2012.
Huenneke, L. F. and Vitousek, P. M.: Seedling and clonal recruitment of the invasive tree Psidium cattleianum: Implications for management of native Hawaiian forests, Biol. Conserv., 53, 199–211, https://doi.org/10.1016/0006-3207(90)90086-5, 1990.
Jiang, M., Medlyn, B. E., Drake, J. E., Duursma, R. A., Anderson, I. C., Barton, C. V. M., Boer, M. M., Carrillo, Y., Castañeda-Gómez, L., Collins, L., Crous, K. Y., De Kauwe, M. G., dos Santos, B. M., Emmerson, K. M., Facey, S. L., Gherlenda, A. N., Gimeno, T. E., Hasegawa, S., Johnson, S. N., Kännaste, A., Macdonald, C. A., Mahmud, K., Moore, B. D., Nazaries, L., and Ellsworth, D. S.: The fate of carbon in a mature forest under carbon dioxide enrichment, Nature, 580, 227–231, https://doi.org/10.1038/s41586-020-2128-9, 2020.
Kim, D., Medvigy, D., Maier, C. A., Johnsen, K., and Palmroth, S.: Biomass increases attributed to both faster tree growth and altered allometric relationships under long-term carbon dioxide enrichment at a temperate forest, Glob. Change Biol., 26, 2519–2533, https://doi.org/10.1111/gcb.14971, 2020.
Kinsman, E. A., Lewis, C., Davies, M. S., Young, J. E., Francis, D., Vilhar, B., and Ougham, H. J.: Elevated CO2 stimulates cells to divide in grass meristems: A differential effect in two natural populations of Dactylis glomerata, Plant Cell Environ., 20, 1309–1316, https://doi.org/10.1046/j.1365-3040.1997.d01-21.x, 1997.
Kirschbaum, M. U. F.: The sensitivity of C3 photosynthesis to increasing CO2 concentration: a theoretical analysis of its dependence on temperature and background CO2 concentration, Plant Cell Environ., 17, 747–754, https://doi.org/10.1111/j.1365-3040.1994.tb00167.x, 1994.
Klein, T., Bader, M. K. F., Leuzinger, S., Mildner, M., Schleppi, P., Siegwolf, R. T. W., and Körner, C.: Growth and carbon relations of mature Picea abies trees under 5 years of free-air CO2 enrichment, J. Ecol., 104, 1720–1733, https://doi.org/10.1111/1365-2745.12621, 2016.
Klein, T. and Ramon, U.: Stomatal sensitivity to CO2 diverges between angiosperm and gymnosperm tree species, Funct. Ecol., 33, 1411–1424, https://doi.org/10.1111/1365-2435.13379, 2019.
Klein, T., Shpringer, I., Fikler, B., Elbaz, G., Cohen, S., and Yakir, D.: Relationships between stomatal regulation, water use, and water-use efficiency of two coexisting key Mediterranean tree species, Forest Ecol. Manag., 302, 34–42, https://doi.org/10.1016/j.foreco.2013.03.044, 2013.
Klein, T. Levy, B.-E., and Halevy, I.: Data for the paper: Physiological responses to ultra-high CO2 levels in an evergreen tree species, figshare [data set], https://doi.org/10.6084/m9.figshare.29626349.v1, 2025.
Konrad, W., Royer, D. L., Franks, P. J., and Roth-Nebelsick, A.: Quantitative critique of leaf-based paleo-CO2 proxies: Consequences for their reliability and applicability, Geol. J., 56, 886–902, https://doi.org/10.1002/gj.3807, 2021.
Körner, C., Asshoff, R., Bignucolo, O., Hättenschwiler, S., Keel, S. G., Peláez-Riedl, S., and Zotz, G.: Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2, Science, 309, 1360–1362, https://doi.org/10.1126/science.1113977, 2005.
Lawson, T. and Morison, J. I. L.: Stomatal function and physiology, in The Evolution of Plant Physiology: From whole plants to ecosystems, edited by: Hemsley, A. R. and Poole, I., Academic Press, 217–242, https://doi.org/10.1016/B978-012339552-8/50013-5, 2004.
Lebaudy, A., Vavasseur, A., Hosy, E., Dreyer, I., Leonhardt, N., Thibaud, J. B., Véry, A.-A., Simonneau, T., and Sentenac, H.: Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels, P. Natl. Acad. Sci. USA, 105, 5271–5276, https://doi.org/10.1073/pnas.0709732105, 2008.
Li, H. T., Yi, T. S., Gao, L. M., Ma, P. F., Zhang, T., Yang, J. B., Gitzendanner, M. A., Fritsch, P. W., Cai, J., Luo, Y., Wang, H., van der Bank, M., Zhang, S. D., Wang, Q. F., Wang, J., Zhang, Z. R., Fu, C. N., Yang, J., Hollingsworth, P. M., Chase, M. W., Soltis, D. E., Soltis, P. S., and Li, D. Z.: Origin of angiosperms and the puzzle of the Jurassic gap, Nat. Plants, 5, 461–470, https://doi.org/10.1038/s41477-019-0421-0, 2019.
Mathias, J. M. and Thomas, R. B.: Global tree intrinsic water use efficiency is enhanced by increased atmospheric CO2 and modulated by climate and plant functional types, P. Natl. Acad. Sci. USA, 118, e2014286118, https://doi.org/10.1073/pnas.2014286118, 2021.
Medlyn, B. E., Barton, C. V. M., Broadmeadow, M. S. J., Ceulemans, R., De Angelis, P., Forstreuter, M., Freeman, M., Jackson, S. B., Kellomäki, S., Laitat, E., Rey, A., Roberntz, P., Sigurdsson, B. D., Strassemeyer, J., Wang, K., Curtis, P. S., and Jarvis, P. G.: Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis, New Phytol., 149, 247–264, https://doi.org/10.1046/j.1469-8137.2001.00028.x, 2001.
Miyazawa, S.-I., Livingston, N. J., and Turpin, D. H.: Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa × P. deltoides), J. Exp. Bot., 57, 373–380, https://doi.org/10.1093/jxb/eri278, 2006.
Nazari-Sharabian, M., Aghababaei, M., Karakouzian, M., and Karami, M.: Water on Mars – a literature review, Galaxies, 8, 40, https://doi.org/10.3390/galaxies8020040, 2020.
Norby, R. J., Warren, J. M., Iversen, C. M., Childs, J., Jawdy, S. S., Walker, A. P.: Forest stand and canopy development unaltered by 12 years of CO2 enrichment, Tree Physiol., 42, 428–440, https://doi.org/10.1093/treephys/tpab107, 2022.
Norby, R. J., Loader, N. J., Mayoral, C., Ullah, S., Curioni, G., Smith, A. R., Reay, M. K., van Wijngaarden, K., Amjad, M. S., Brettle, D., Crockatt, M. E., Denny, G., Grzesik, R. T., Hamilton, R. L., Hart, K. M., Hartley, I. P., Jones, A. G., Kourmouli, A., Larsen, J. R., Shi, Z., Thomas, R. M., and MacKenzie, A. R.: Enhanced woody biomass production in a mature temperate forest under elevated CO2, Nat. Clim. Change, 14, 983–988, https://doi.org/10.1038/s41558-024-02090-3, 2024.
Ozak, N., Aharonson, O., and Halevy, I.: Radiative transfer in CO2-rich atmospheres: 1. Collisional line mixing implies a colder early Mars, J. Geophys. Res.-Planet., 121, 965–985, https://doi.org/10.1002/2015JE004871, 2016.
Patel, S.: Exotic tropical plant Psidium cattleianum: a review on prospects and threats, Rev. Environ. Sci. Bio., 11, 243–248, https://doi.org/10.1007/s11157-012-9269-8, 2012.
Paudel, I., Halpern, M., Wagner, Y., Raveh, E., Yermiyahu, U., Hoch, G., and Klein, T.: Elevated CO2 compensates for drought effects in lemon saplings via stomatal downregulation, increased soil moisture, and increased wood carbon storage, Environ. Exp. Bot., 148, 117–127, https://doi.org/10.1016/j.envexpbot.2018.01.004, 2018.
Poorter, H., Knopf, O., Wright, I. J., Temme, A. A., Hogewoning, S. W., Graf, A., Cernusak, L. A., and Pons, T. L.: A meta-analysis of responses of C3 plants to atmospheric CO2: dose–response curves for 85 traits ranging from the molecular to the whole-plant level, New Phytol., 233, 1560–1596, https://doi.org/10.1111/nph.17802, 2022.
Richards, J. T., Edney, S. L., Yorio, N. C., Stutte, G. W., and Wheeler, R. M.: Yields of salad crops grown under potential lunar or Mars habitat environments: effect of temperature and lighting intensities, SAE Techn. Paper, 2006-01-2029, https://doi.org/10.4271/2006-01-2029, 2006.
Rog, I., Jakoby, G., & Klein, T.: Carbon allocation dynamics in conifers and broadleaved tree species revealed by pulse labeling and mass balance, Forest ecology and management, 493, 119258, 2021.
Rogers, H. H., Peterson, C. M., McCrimmon, J. N., and Cure, J. D.: Response of plant roots to elevated atmospheric carbon dioxide, Plant Cell Environ., 15, 749–752, https://doi.org/10.1111/j.1365-3040.1992.tb01009.x, 1992.
Rosa, L., Chiarelli, D. D., Rulli, M. C., Dell'Angelo, J., and D'Odorico, P.: Global agricultural economic water scarcity, Sci. Adv., 6, eaaz6031, https://doi.org/10.1126/sciadv.aaz6031, 2020.
Slobodian, R. E.: Selling space colonization and immortality: A psychosocial, anthropological critique of the rush to colonize Mars, Acta Astronaut., 113, 89–104, https://doi.org/10.1016/j.actaastro.2015.03.027, 2015.
Steinthorsdottir, M., Vajda, V., Pole, M., and Holdgate, G.: Moderate levels of Eocene pCO2 indicated by Southern Hemisphere fossil plant stomata, Geology, 47, 914–918, https://doi.org/10.1130/G46274.1, 2019.
Tisserat, B. and Silman, R.: Ultra-high carbon dioxide levels enhances in vitro shoot growth and morphogenesis in Labiatae, J. Herbs Spices Med. Plants, 7, 43–55, https://doi.org/10.1300/J044v07n02_06, 2000.
Tisserat, B. and Vaughn, S. F.: Ultra-high CO2 levels enhance loblolly pine seedling growth, morphogenesis, and secondary metabolism, HortScience, 38, 1083–1085, https://doi.org/10.4271/2006-01-2029, 2003.
Tisserat, B. R. E. N. T. and Vaughn, S. F.: Essential oils enhanced by ultra-high carbon dioxide levels from Lamiaceae species grown in vitro and in vivo, Plant Cell Rep., 20, 361–368, https://doi.org/10.1007/s00299-001-0428-6, 2001.
Uni, D., Sheffer, E., Winters, G., Lima, A. C., Fox, H., and Klein, T.: Peak photosynthesis at summer midday in Acacia trees growing in a hyper-arid habitat, Trees, 37, 255–267, https://doi.org/10.1007/s00468-022-02344-7, 2023.
Wagner, Y., Pozner, E., Bar-On, P., Ramon, U., Raveh, E., Neuhaus, E., Cohen, S., Grünzweig, J., and Klein, T.: Rapid stomatal response in lemon saves trees and their fruit yields under summer desiccation, but fails under recurring droughts, Agr. Forest Meteorol., 307, 108487, https://doi.org/10.1016/j.agrformet.2021.108487, 2021.
Wagner, Y., Feng, F., Yakir, D., Klein, T., and Hochberg, U.: In situ, direct observation of seasonal embolism dynamics in Aleppo pine trees growing on the dry edge of their distribution, New Phytol., 235, 1344–1350, https://doi.org/10.1111/nph.18208, 2022.
Walker, A. P., De Kauwe, M. G., Bastos, A., Belmecheri, S., Georgiou, K., Keeling, R. F., McMahon, S. M., Medlyn, B. E., Moore, D. J. P., Norby, R. J., Zaehle, S., Anderson-Teixeira, K. J., Battipaglia, G., Brienen, R. J. W., Cabugao, K. G., Cailleret, M., Campbell, E., Canadell, J. G., Ciais, P., Craig, M. E., Ellsworth, D. S., Farquhar, G. D., Fatichi, S., Fisher, J. B., Frank, D. C., Graven, H., Gu, L., Haverd, V., Heilman, K., Heimann, M., Hungate, B. A., Iversen, C. M., Joos, F., Jiang, M., Keenan, T. F., Knauer, J., Körner, C., Leshyk, V. O., Leuzinger, S., Liu, Y., MacBean, N., Malhi, Y., McVicar, T. R., Penuelas, J., Pongratz, J., Powell, A. S., Riutta, T., Sabot, M. E. B., Schleucher, J., Sitch, S., Smith, W. K., Sulman, B., Taylor, B., Terrer, C., Torn, M. S., Treseder, K. K., Trugman, A. T., Trumbore, S. E., van Mantgem, P. J., Voelker, S. L., Whelan, M. E., and Zuidema, P. A.: Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2, New Phytol., 229, 2413–2445, https://doi.org/10.1111/nph.16866, 2021.
Wamelink, G. W. W., Frissel, J. Y., Krijnen, W. H. J., Verwoert, M. R., and Goedhart, P. W.: Can plants grow on Mars and the Moon: a growth experiment on Mars and Moon soil simulants, PLoS One, 9, e103138, https://doi.org/10.1371/journal.pone.0103138, 2014.
Will, R. E. and Teskey, R. O.: Effect of irradiance and vapour pressure deficit on stomatal response to CO2 enrichment of four tree species, J. Exp. Bot., 48, 2095–2102, https://doi.org/10.1093/jxb/48.12.2095, 1997.
Zeebe, R. E. and Wolf-Gladrow, D. A.: CO2 in seawater: equilibrium, kinetics, isotopes, Gulf Prof. Publ., Oceanogr. Ser., Elsevier Oceanography book series, 65, 346 pp, https://doi.org/10.1016/S0422-9894(01)80001-5, 2001.




