the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Temperature fluctuation alleviates the negative effects of warming on marine diatoms: comparison between Thalassiosira sp. and Nitzschia closterium f. minutissima
Yangjie Sheng
Yanan Wang
Ting Cai
Yuntao Wang
Afef Fathalli
Sana Ben Ismail
Yuanyuan Feng
Marine phytoplankton are subjected to a wide range of environmental heterogeneity, from mean climate change to natural fluctuations under the climate change scenario. These changes include changes in the frequency of temperature fluctuations in the sea surface. Here we conducted semi-continuous incubation experiments on two ecologically significant marine diatom species, Thalassiosira sp. and Nitzschia closterium f. minutissima, to examine their physiological responses to ocean warming and temperature fluctuation (±4 °C) under low (20 °C) and high (25 °C) average temperatures. Our results demonstrate that temperature fluctuation alleviated the negative effects of elevated temperatures on the growth of both species. For Thalassiosira sp., warming under constant temperature significantly reduced the growth rate but significantly increased the cellular elemental contents and sinking rate. However, warming significantly reduced cellular particulate organic carbon (POC) content, biogenic silica (BSi) content, and the sinking rate, while increasing the protein content to cope with thermal stress under temperature fluctuation. The effects of temperature fluctuation were dependent on the average temperature: at low average temperatures, temperature fluctuation increased cellular POC, BSi, POC productivity, and sinking rates, whereas at high average temperatures, these parameters were decreased significantly. For Nitzschia closterium f. minutissima, warming under both constant and fluctuated temperatures significantly increased the POC, particulate organic nitrogen (PON), and POP quotas. The interaction between warming and temperature fluctuation had antagonistic effects on most parameters examined for Thalassiosira sp., whereas it had synergistic effects on the physiological parameters of Nitzschia closterium f. minutissima. Overall, Nitzschia closterium f. minutissima exhibited stronger tolerance to warming and temperature fluctuation, suggesting species-specific responses of diatoms to warming and temperature fluctuations. These findings highlight the important yet often underestimated influence of temperature fluctuation on the physiology of marine diatoms in the context of global warming, thus having implications for a further understanding of biogeochemical feedbacks.
- Article
(751 KB) - Full-text XML
- BibTeX
- EndNote
The continuous anthropogenic emissions of carbon dioxide (CO2) have led to a steady increase in atmospheric CO2 concentrations (IPCC, 2023). This will in turn induce global warming, with an average increase in global temperature by approximately 1 °C above 1850–1900 in 2011–2020 (IPCC, 2023). Under current high-emission scenarios, it is projected that the global sea surface temperature will increase by at least 2–4 °C by the end of the 21st century (Bindoff et al., 2019). Temperature is a key factor affecting enzyme activity and plays a crucial role in regulating the metabolic rates of phytoplankton (Eisenthal et al., 2006; Eppley, 1972; Falkowski and Oliver, 2007). According to model projections, the net primary productivity of marine phytoplankton may decline by around 20 % during the 21st century as a result of ocean warming (Steinacher et al., 2010). Within a certain temperature range, the growth rate, photosynthesis, respiration, and other key physiological processes of phytoplankton increase with rising temperature (Barton et al., 2020), which in turn affects the utilization of nutrients within cells (Raven and Geider, 2006). Moreover, warming favors smaller-sized phytoplankton, as these cells can more effectively acquire nutrients and utilize CO2 (Zaoli et al., 2019; Morán et al., 2010). Therefore, small-sized phytoplankton typically demonstrate stronger responses to warming in terms of both growth rates and photosynthetic activity (Wang et al., 2024), affecting the size fractionation of marine primary producers and thus carbon sequestration (Anderson et al., 2021).
In addition, marine phytoplankton are also subjected to environmental heterogeneity in the ocean (Bernhardt et al., 2020). As climate change accelerates, the frequency and amplitude of marine environmental fluctuations are also expected to change (Perkins-Kirkpatrick and Lewis, 2020). Ocean warming not only increases the average ocean temperature but also enhances the frequency and intensity of temperature fluctuations, which may have more complex effects on marine organisms than warming alone (Ketola and Saarinen, 2015). It has been reported that high-frequency temperature fluctuation (2 d) reduced the mortality rate of Emiliania huxleyi, a dominant species of the calcifying phytoplankton group that plays a key role in calcium carbonate production and marine carbon cycling (Wang et al., 2019). For the marine diazotroph Trichodesmium, temperature fluctuation was found to reduce the growth rate under phosphorus-replete conditions (Qu et al., 2019). Additionally, a study on green algae has shown that temperature fluctuation slowed the growth rate of Chlorella and Micromonas but did not affect the growth of Microcystis aeruginosa (Zhang et al., 2015). Furthermore, temperature fluctuation may enhance the ability of the diatom Thalassiosira pseudonana to better adapt to high temperature (Schaum, 2018; Schaum et al., 2018). Therefore, different phytoplankton groups exhibit species-specific responses to temperature fluctuation, and temperature fluctuation may alter the taxonomic composition of the phytoplankton community. A mesoscale enclosure experiment conducted in the coastal water of southern China found that temperature fluctuation reduced the abundance of diatoms, while increasing the proportion of Synechococcus and Prasinophytes, which may consequently influence the upper trophic levels in the marine food web (Zhang et al., 2025).
Marine diatoms, a critical phytoplankton functional group, contribute approximately 40 % of the ocean's primary productivity and play a key role in biogeochemical cycles as a central element of the marine biological carbon pump (Qiao et al., 2021; Sanders et al., 2014; Field et al., 1998). The growth responses to temperature variations are delineated by thermal tolerance curves (Thomas et al., 2012). An increase in temperature promotes growth until the optimal temperature is reached, beyond which further elevation in temperature results in a decrease in growth rate and even cessation of growth (Edullantes et al., 2023).
Different diatom species have distinct optimal growth temperatures. For instance, Paralia suicata demonstrates optimal growth at 6.25 °C (He et al., 2024), while Thalassiosira weissflogii thrives at 19.1 °C (Rossi et al., 2023), and Thalassiosira pseudonana and Nitzschia frustulum exhibit optimal growth at 20.9 °C (Kuefner et al., 2020) and 28.5 °C (Rossi et al., 2023), respectively. As such, the response of diatoms to warming may be species specific. For instance, 5 °C of warming significantly reduced the biomass of Phaeodactylum tricornutum, while having no significant effect on Thalassiosira weissflogii (Zeng et al., 2020). Warming of 4 °C significantly reduced the particulate organic carbon content of Thalassiosira sp., whereas the rate of particulate organic carbon production increased with temperature for Nitzschia closterium f. minutissima (Cai et al., 2022). The effects of warming also vary with the duration of exposure; short-term warming has little effect on the growth rate of P. tricornutum, while long-term exposure significantly inhibits its growth (Hong et al., 2023).
Although the impacts of warming on marine diatoms have been extensively investigated, our understanding of how temperature fluctuations affect the physiological responses of different diatom species remains limited. This knowledge gap represents a critical constraint in developing a comprehensive understanding of marine diatoms' adaptive responses to environmental changes under more ecologically realistic scenarios. In this study, we investigated the individual and interactive effects of warming and temperature fluctuations on the physiology of marine diatoms by conducting semi-continuous incubation experiments. Two representative diatom species were selected: the centric diatom Thalassiosira sp. and the pennate diatom Nitzschia closterium f. minutissima. Both species are model diatoms, representing the two major taxonomic classes of diatoms (centric vs. pennate), allowing for comparison of responses to environmental changes between two distinct diatom groups. To assess their physiological responses and potential implications for the marine carbon cycle considering both warming and temperature fluctuation, we systematically measured key parameters, including growth rate, macromolecular composition, elemental stoichiometry, and sinking rate.
2.1 Algae culture
The marine diatoms Thalassiosira sp. and Nitzschia closterium f. minutissima were originally isolated from surface seawater (depth 3–12 m; salinity 34.78) at 118°58.055′ E, 38°39.111′ N (China) in August 2019. At the time of sampling, the sea surface temperature was approximately 15 °C. Stock cultures of the two diatoms were maintained in sterilized medium natural seawater in Nalgene polycarbonate bottles in a constant-temperature and illumination incubator (GXZ-280D, NingBo, China) at 20 °C.
2.2 Experimental setup
Four temperature treatments were established (Fig. 1, Table 1): (i) LTCT – constant 20 °C; (ii) HTCT – constant 25 °C; (iii) LTFT – fluctuated between 16 and 24 °C d−1 (20 ± 4 °C); and (iv) HTFT – fluctuated based on warming, which cycled between 21 and 29 °C d−1 (25 ± 4 °C). The selection of 20 and 25 °C was based on both ecological relevance and experimental design considerations. We have previously obtained the thermal response curves of the two species; the optimal growth temperature for Thalassiosira sp. was ∼19 °C and ∼22 °C for Nitzschia closterium f. minutissima (Cai et al., 2022). The temperature condition in each treatment was maintained in a water tank, connected to a seawater temperature controller (HC150A, Hailea, China). During the course of incubation, temperature in the water tanks was monitored continuously using a HOBO underwater temperature logger (MX2201, USA). The measured temperature was within a range of ±0.2 °C from the preset temperature.
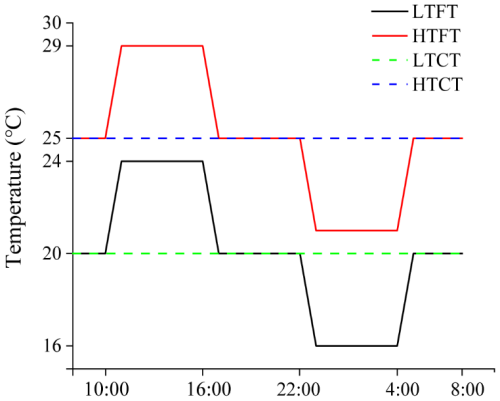
Figure 1Temperature schematic diagram. LTCT, 20 °C; LTFT, 20 ± 4 °C; HTCT, 25 °C; and HTFT, 25 ± 4 °C.
For each temperature treatment, stock cultures in the logarithmic growth phase were inoculated into triplicate polycarbonate culture flasks (1 L, Nalgene, USA), with an initial cell abundance of 1×104 cells mL−1. The light intensity was maintained at 170–200 with a light : dark cycle of 12 h : 12 h. The culture medium was prepared with seawater collected from the Yellow Sea, China, filtered using a 0.2 µm membrane (Whatman, USA) with nutrients added according to the recipe. A total of 2 mL of sample from the incubation bottle was taken from the cultures every 24 h to measure in vivo chlorophyll a (chl a) fluorescence using a Turner fluorometer (Trilogy, Turner Designs, USA). The cultures were diluted with a freshly prepared medium to supply enough nutrients. Final sampling was carried out once the cell growth rate had stabilized, with variations of less than 10 % for over 5 consecutive days.
2.3 Physiological and biogeochemical analyses
2.3.1 Chlorophyll a content and growth rate
For chlorophyll a (chl a) measurement, 20 mL of sample was filtered onto GF/F filters (Whatman, USA). After extraction with 5 mL of 90 % acetone for 24 h (stored at −20 °C in the dark), the chl a content was measured using a fluorometer (Trilogy, Turner Designs, USA).
The 2 mL sample was kept in the dark for 20 min, followed by measurement of the in vivo chl a fluorescence using a fluorometer (Trilogy, Turner Designs, USA). For the in vivo fluorescence measurements, the culture medium was always used as the blank; therefore the potential interference from the culture medium was eliminated. In addition, we conducted a semi-continuous incubation with cells growing at the exponential phase, and the cultures in the incubation bottles were well mixed two to three times a day; as such, there was no cell aggregation observed in the culture system. The growth rate (μ, d−1) was then calculated using the following equation:
where N2 and N1 represent the chl a fluorescence at times T2 and T1, respectively.
2.3.2 Cellular macromolecule content
The algal cultures were filtered through 0.4 µm polycarbonate filters (PALL, USA) and stored at −80 °C until the determination of protein and carbohydrate contents.
Protein content was measured using a Coomassie Brilliant Blue G-250 method (Bradford, 1976). The algal cells on the filter were eluted with 1 mL of Milli-Q water, followed by the addition of 4 mL of Coomassie Brilliant Blue solution. After thorough mixing, the mixture was left for 5 min to develop color. Absorbance was then measured at 595 nm using a UV–visible spectrophotometer (UV-2550, Shimadzu, Japan).
Carbohydrate content was determined using the sulfuric acid–phenol method (Dubois et al., 1956). The filter was removed and placed in 5 mL of 0.05 mol L−1 H2SO4, then heated in a water bath at 60 °C for 10 min and cooled to room temperature. A 2 mL sample was taken, to which 0.05 mL of 80 % phenol solution and 5 mL of concentrated sulfuric acid reagent was added. After standing for 30 min for color development, absorbance was measured at 485 nm using a UV–visible spectrophotometer (UV-2550, Shimadzu, Japan).
2.3.3 Elemental composition
Biogenic silica (BSi) was analyzed using a spectrophotometer (Nelson et al., 1995). Samples were filtered onto 0.4 µm polycarbonate filters (Millipore, USA), dried in an oven at 60 °C (DPG-9420A, Shanghai), and stored in a desiccator until analysis.
Samples for particulate organic phosphorus (POP) measurement were filtered onto pre-combusted GF/F filters (450 °C for 4 h). The filters were then rinsed with 2 mL of 0.17 M Na2SO4 solution and transferred to 20 mL pre-combusted (8 h at 450 °C) glass vials, with addition of 2 mL of 0.017 mol L−1 MgSO4 to each vial.
Particulate organic carbon (POC) and particulate organic nitrogen (PON) were analyzed using a CHN elemental analyzer (Costech, Italy). A 100 mL sample of algal culture was filtered onto a pre-combusted GF/F filter. The filter was then fumed with concentrated HCI for 3 h to remove inorganic carbon and subsequently dried in a 60 °C oven. The organic carbon fixation rate (CR, ) was calculated as
in which POCcell is the cellular content of POC (pg C cell−1) obtained from the measurement of elemental content, and μ is the growth rate on the last day of the culture.
2.3.4 Cell size and sinking rate
Cell size was measured using a laser particle size analyzer (LS 13320, Beckman, USA). Measurements were taken after the laser particle size analyzer had completed its preheating process. Each sample was measured three times.
The sinking rate was determined using the SETCOL method (Bienfang, 1981). The samples were filled into the column and stood in the dark for 3 h. Subsequently, samples were collected from the upper, middle, and lower sections of the column. Each sample was filtered through GF/F filters, and the chl a content was measured. The sinking rates were calculated using the following equation:
where Bt is the total biomass in the column (µg L−1), Bs is the biomass in the bottom layer of the column (µg L−1), l is the height of the column (cm), and t is the sinking time (h).
Significance analysis and interaction effects were assessed by two-way ANOVA using Origin 2021 software (OriginLab Corporation, USA). Differences between treatments were considered significant at the level of p<0.05. The pairwise tests between treatments were conducted using Tukey's multiple-comparison post hoc analysis. Three independent replicates (n=3) were performed for each experiment, and differences between treatments were considered significant at the level of p<0.05. The interaction effects for warming and temperature fluctuations were calculated as follows:
where ME1+2 is the interaction effect of warming and temperature fluctuation, and OE1 and OE2 are the apparent effects of warming and temperature fluctuation on phytoplankton physiological parameters, respectively. When , the interaction between the two environmental factors is synergistic, whereas when , the interaction becomes antagonistic (Folt et al., 1999).
4.1 Growth rate
Warming significantly decreased the growth rate of both species at constant temperature. At HTCT, the growth rates of Thalassiosira sp. and N. closterium f. minutissima were 1.67 ± 0.09 and 1.06 ± 0.14 and significantly decreased by 31.33 % and 12.46 % compared to LTCT, respectively (p<0.05, Fig. 2). In contrast, temperature fluctuation alleviated the negative effects of warming. At HTFT, the growth rates of Thalassiosira sp. and N. closterium f. minutissima exhibited a significant increase to 1.42 ± 0.07 and 1.18 ± 0.04 (p<0.05, Fig. 2).
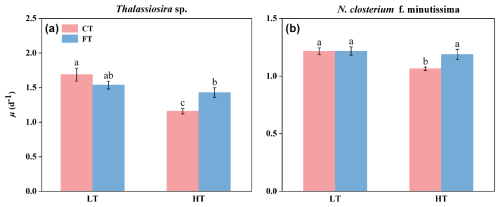
Figure 2Growth rate of Thalassiosira sp. (a) and N. closterium f. minutissima (b) under LTCT (20 °C), LTFT (20 ± 4 °C), HTCT (25 °C), and HTFT (25 ± 4 °C). The error bars represent the standard deviations (n=3), and the letters above the bars denote the statistically significant differences (p<0.05).
4.2 Chlorophyll a, protein, and carbohydrate contents
The chl a contents of Thalassiosira sp. and N. closterium f. minutissima were significantly increased by 124.12 % and 66.44 % in HTCT compared to LTCT, respectively (p<0.05, Fig. 3a and b). Compared to LTFT, the chl a content of N. closterium f. minutissima was significantly increased to 0.16 ± 0.00 under HTFT (p<0.05, Fig. 3b), while no significant effect was observed on the chl a content of Thalassiosira sp. (p>0.05, Fig. 3a).
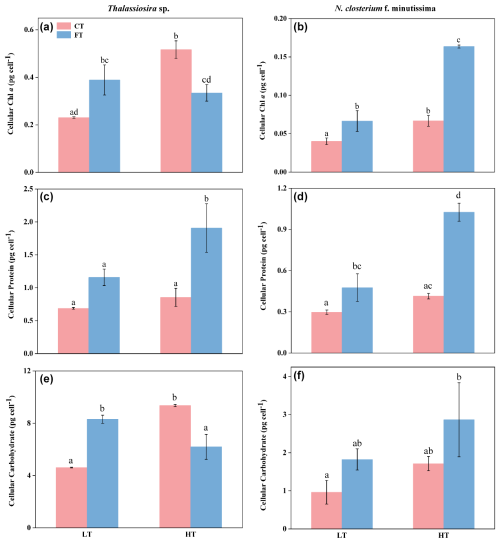
Figure 3The cellular contents of chl a (a, b), protein (c, d), and carbohydrate (e, f) of Thalassiosira sp. (a, c, e) and N. closterium f. minutissima (b, d, f) under LTCT (20 °C), LTFT (20 ± 4 °C), HTCT (25 °C), and HTFT (25 ± 4 °C). The error bars represent the standard deviations (n=3), and the letters above the bars denote the statistically significant differences (p<0.05).
At LTFT, the chl a contents of Thalassiosira sp. and N. closterium f. minutissima were significantly increased by 68.71 % and 65.78 % compared to LTCT, respectively (p<0.05, Fig. 3a and b). At HTFT, the chl a content of Thalassiosira sp. was 0.33 ± 0.03, significantly decreased by 35.23 % compared to HTCT (p<0.05, Fig. 3a), while that of N. closterium f. minutissima was significantly increased by 144.87 % (p<0.05, Fig. 3b).
At HTFT, the cellular protein contents of Thalassiosira sp. and N. closterium f. minutissima were 1.90 ± 0.37 and 1.03 ± 0.07, significantly increased by 64.53 % and 115.40 % compared to LTFT, respectively (p<0.05, Fig. 3c and d). The cellular protein content of Thalassiosira sp. was significantly increased by 123.48 % at HTFT compared to HTCT (p<0.05, Fig. 3c), but no significant effect was observed at LTFT compared to LTCT (p>0.05, Fig. 3c). For N. closterium f. minutissima, temperature fluctuation significantly increased the cellular protein content by 65.78 % and 144.87 % compared to constant temperature at low and high temperatures, respectively (p<0.05, Fig. 3d).
HTCT significantly increased the cellular carbohydrate content of Thalassiosira sp. by 103.09 % compared to LTCT, but HTFT markedly reduced it by 25.36 % compared to LTFT (p<0.05, Fig. 3e). Warming had no significant effect on the cellular carbohydrate content of N. closterium f. minutissima under either constant temperature or temperature fluctuation (p>0.05, Fig. 3f). At LTFT, the cellular carbohydrate content of Thalassiosira sp. was 8.31 ± 0.32, significantly increased by 80.25 % compared to LTCT, but the carbohydrate content of Thalassiosira sp. was 1.82 ± 0.28, decreased by 33.76 % at HTFT compared to HTCT (p<0.05, Fig. 3e).
4.3 Elemental composition
Compared to LTCT, the POC, POP, and BSi contents of Thalassiosira sp. increased by 79.08 %, 119.27 %, and 76.68 % at HTCT, respectively (p<0.05, Fig. 4a, e, and g). The PON content increased by 238.34 % at HTFT compared to LTFT (p<0.05, Fig. 4c). However, the POC and BSi contents decreased by 52.62 % and 43.69 % at HTFT compared to LTFT, respectively (p<0.05, Fig. 4a, e, and g). For N. closterium f. minutissima, HTCT significantly increased PON and POP contents with 68.99 % and 53.70 %, respectively, compared to LTCT (Fig. 4d and f). At HTFT, the PON and POP contents significantly increased by 544.91 % and 110.45 % compared to LTFT, respectively (p<0.05, Fig. 4d and f).
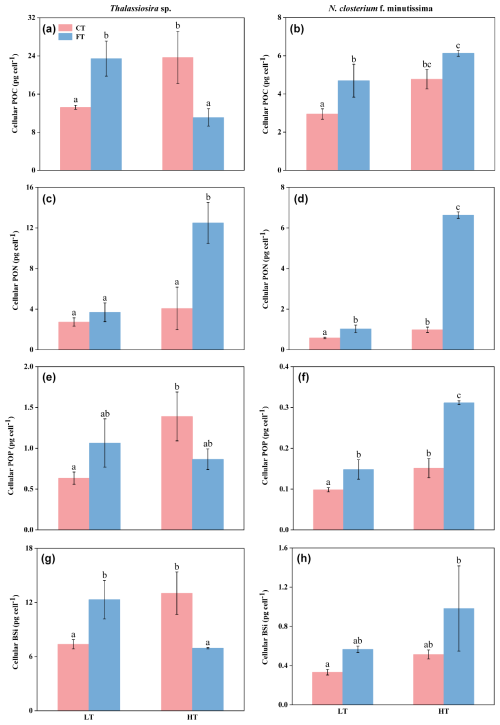
Figure 4Cellular contents (pg cell−1) of particulate organic carbon (POC; a, b), particulate organic nitrogen (PON; c, d), particulate organic phosphorus (POP; e, f), and biogenic silica (BSi; g, h) for Thalassiosira sp. (a, c, e, g) and N. closterium f. minutissima (b, d, f, h) grown under LTCT (20 °C), LTFT (20 ± 4 °C), HTCT (25 °C), and HTFT (25 ± 4 °C). The error bars show the standard deviations (n=3), and the letters above the bars denote the statistically significant differences (p<0.05).
The POC and BSi contents of Thalassiosira sp. were both significantly higher at LTFT compared to LTCT, with an increase by 77.2 % and 67.1 %, respectively (p<0.05, Fig. 4a and g). However, HTFT significantly decreased the POC and BSi contents of Thalassiosira sp. with 53.12 % and 46.74 % compared to HTCT, respectively (p<0.05, Fig. 4a and g). For N. closterium f. minutissima, the POC, PON, and POP contents significantly increased by 59.09 %, 76.85 %, and 50.3 % at LTFT compared to LTCT, respectively (p<0.05, Fig. 4b, d, and f). At HTFT, the PON and POP contents of N. closterium f. minutissima increased by 574.88 % and 105.81 % compared to HTCT, respectively (p<0.05, Fig. 4d and f).
The elemental ratios of Thalassiosira sp. and N. closterium f. minutissima responded similarly to warming and temperature fluctuation. Compared to LTCT, under HTCT, there were no significant changes in the C:N, N:P, C:P, C:BSi, and C:chl a ratios of Thalassiosira sp. and N. closterium f. minutissima. In contrast, compared to LTFT, HTFT significantly reduced the C:N, C:P, C:BSi, and C:chl a ratios of Thalassiosira sp. and N. closterium f. minutissima, while it significantly increased the N:P ratio. No significant changes in the C:N, N:P, C:P, C:BSi, and C:chl a ratios of Thalassiosira sp. and N. closterium f. minutissima were observed under LTFT compared to LTCT. However, HTFT significantly reduced the C:N, C:P, C:BSi, and C:chl a ratios of Thalassiosira sp. and N. closterium f. minutissima and significantly raised the N:P ratio relative to HTCT.
4.4 Particulate organic carbon productivity and sinking rate
No significant changes in the POC productivities of Thalassiosira sp. and N. closterium f. minutissima were observed under HTCT compared to LTCT (Fig. 5a and b). In contrast, the POC productivity of Thalassiosira sp. decreased significantly by 42.44 % from 27.41 ± 6.09 to 15.78 ± 2.02 under HTFT compared to HTCT, while the POC productivity of N. closterium f. minutissima exhibited a non-significant increase (Fig. 5a and b).
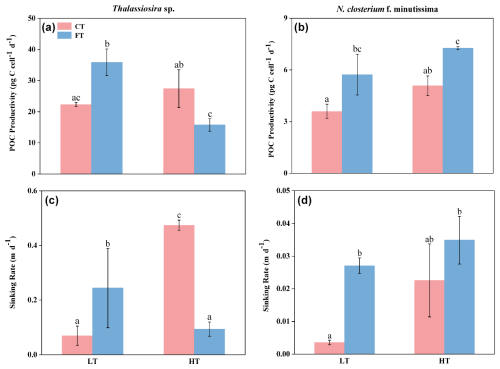
Figure 5The POC productivity (a, b) and sinking rate (c, d) of Thalassiosira sp. (a, c) and N. closterium f. minutissima (b, d) grown under LTCT (20 °C), LTFT (20 ± 4 °C), HTCT (25 °C), and HTFT (25 ± 4 °C). The error bars show the standard deviations (n=3), and the letters above the bars denote the statistically significant differences.
The POC productivities of Thalassiosira sp. and N. closterium f. minutissima responded to temperature fluctuation under low temperature (20 °C) but showed opposite responses to temperature fluctuation under elevated temperature of 25 °C (Fig. 5a). At LTFT, the POC productivities of Thalassiosira sp. and N. closterium f. minutissima were 35.88 ± 4.27 and 5.73 ± 0.57, increased by 60.98 % and 59.49 % compared to LTCT, respectively (p<0.05, Fig. 5a and b). However, at HTFT, the POC productivity of Thalassiosira sp. significantly decreased by 42.44 %, and the POC productivity of N. closterium f. minutissima significantly increased by 43.08 % (p<0.05, Fig. 5a and b).
Compared to LTCT, HTCT resulted in a significant increase of 582.44 % from 0.07 ± 0.04 to 0.47 ± 0.02 in the sinking rate of Thalassiosira sp., whereas the sinking rate of N. closterium f. minutissima showed a non-significant rise (p<0.05, Fig. 5c and d). At HTFT, the sinking rate of Thalassiosira sp. showed a significant decline of 70.63 % relative to LTFT, whereas the sinking rate of N. closterium f. minutissima remained significantly unchanged (p<0.05, Fig. 5c and d). The sinking rates of Thalassiosira sp. and N. closterium f. minutissima increased significantly by 359.77 % and 655.22 %, respectively, at LTFT relative to LTCT (p<0.05, Fig. 5c and d). However, at HTFT, the sinking rate of Thalassiosira sp. was 0.09 ± 0.03, decreased significantly by 80.20 %, while the sinking rate of N. closterium f. minutissima exhibited no significant change from 0.02 ± 0.01 to 0.03 ± 0.01 compared to HTCT (p<0.05, Fig. 5c and d).
4.5 Interactive effects of warming and temperature fluctuations
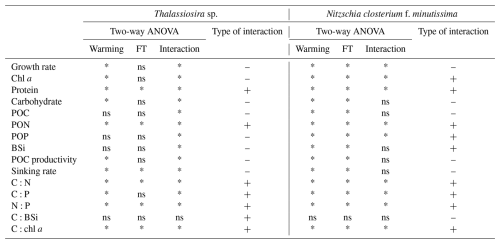
Two-way ANOVA revealed that warming had significant effects on most physiological parameters of Thalassiosira sp., including growth rate; chl a content; protein; carbohydrate content; PON; POC productivity; sinking rate; and C:N, C:P, N:P, and C:chl a ratios (Table 3). Temperature fluctuation had significant effects on the content of protein and PON; the sinking rate; and C:N, N:P, and C:chl a ratios (Table 3). Significant interactive effects between warming and temperature fluctuation were observed for all the examined physiological parameters of Thalassiosira sp., except for cellular BSi content (Table 3). For N. closterium f. minutissima, both warming and temperature fluctuations had significant effects on all physiological parameters, except for the C:BSi ratio (Table 3). Warming and temperature fluctuations produced significant interactive effects on the growth rate; cellular chl a, protein, PON, and POP contents; and C:N, C:P, N:P, and C:chl a ratios of N. closterium f. minutissima (Table 3).
Warming and temperature fluctuations had significant antagonistic interactive effects on the growth rates of Thalassiosira sp. and N. closterium f. minutissima but showed significant synergistic interactions on cellular protein and PON content, as well as C:N, C:P, N:P, and C:chl a ratios (Table 3). For Thalassiosira sp., warming and temperature fluctuation had significant antagonistic interactions on carbohydrate, BSi content, POC productivity, and sinking rate, whereas no significant interactive effects were found on these parameters in N. closterium f. minutissima (Table 3). For elemental ratios, significant synergistic interactions between warming and temperature fluctuation were found in both Thalassiosira sp. and N. closterium f. minutissima, with no significant interactive effects on the C:BSi ratio in either species (Table 3). In addition, warming and temperature fluctuation produced significant antagonistic interactive effects on the POP content of Thalassiosira sp., while in N. closterium f. minutissima, significant synergistic interactions were observed on POP content (Table 3).
Our experimental results demonstrate that both Thalassiosira sp. and N. closterium f. minutissima exhibit enhanced thermal tolerance under fluctuating-temperature regimes compared to constant-temperature conditions (Fig. 6). Our findings also reveal significant species-specific variability in physiological responses to warming and temperature fluctuations, with the pennate diatom N. closterium f. minutissima demonstrating greater resilience to elevated temperatures than Thalassiosira sp (Fig. 6). Particularly noteworthy are the differential responses observed in POC productivity and sinking rates between the two species under varying thermal regimes, which may alter their respective contributions to carbon export (Fig. 6). These results not only emphasize the critical importance of incorporating natural temperature variability but also highlight the necessity of considering species-specific physiological responses when modeling and predicting the ecological impacts of climate change on marine ecosystems.
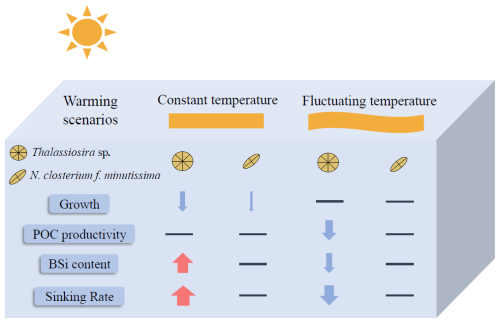
Figure 6Schematic diagram of the responses of Thalassiosira sp. and N. closterium f. minutissima to warming under constant temperature and fluctuating temperature. Arrow thickness represents the magnitude of change, with red arrows indicating significant increases, blue arrows indicating significant decreases, and horizontal lines denoting no significant changes.
5.1 Temperature fluctuation alleviated the negative effects of warming on growth
When the temperature was raised from 20 to 25 °C in our investigation, the growth rates of Thalassiosira sp. and N. closterium f. minutissima both dropped dramatically (Fig. 1, constant-temperature treatments). Similarly, a previous study on the marine diatom Thalassiosira weissflogii suggests an optimal temperature of 20 °C for the growth of T. weissflogii (Taucher et al., 2015). The optimal temperature of the marine diatom N. closterium f. minutissima is 22 °C. Therefore, here we assume that the 25 °C of our high-temperature treatments has exceeded the optimal temperature of both species. In general, below the optimal temperature, rising temperature enhances enzyme activity, which in turn stimulates cellular metabolism and promotes growth (Arrhenius, 1889). At temperatures higher than the optimum, the inhibition of the growth occurs due to cellular stress (Cai et al., 2022; Edullantes et al., 2023).
However, in our investigation, temperature variation had distinct effects on growth at low and high temperatures. Temperature variation had no discernible effect on either species' development rate at 20 °C (Fig. 2, Table 2). This tolerance may reflect their coastal habitat, where phytoplankton cells are subjected to frequent temperature fluctuations (annual temperature range between 15 and 30 °C, where daily temperature increases can reach up to 5 °C). Phytoplankton in these dynamic environments may have developed mechanisms to cope with environmental heterogeneity, maintaining stable growth even under fluctuating conditions. Our results are consistent with models that predict enhanced growth under non-optimal temperature fluctuations, especially in species adapted to variable coastal environments (Bernhardt et al., 2018).
Table 2The elemental molar ratios of C:N, N:P, C:P, and C:BSi (mol : mol) and the quality ratio of C:chl a (g : g) in the four experimental treatments of Thalassiosira sp. and N. closterium f. minutissima. Superscript letters represent significant differences (p<0.05).
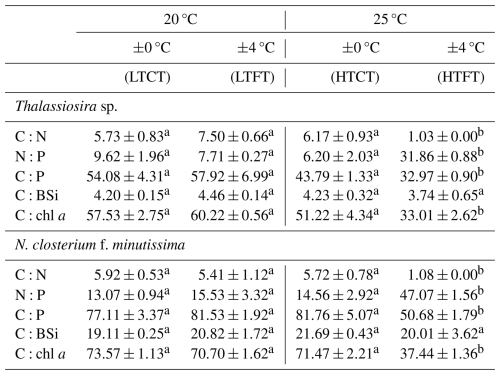
Table 3Interactive effects of warming and temperature fluctuations on the physiological parameters of Thalassiosira sp. and N. closterium f. minutissima. “–” represents antagonistic effects, and “+” represents synergistic effects. “*” represents significance, and “ns” represents non-significance (two-way ANOVA, p=0.05).
Interestingly, our study observed that temperature fluctuation increased the resilience of the growth of both diatom species to warming. At high temperature (25 °C), temperature fluctuation mitigated the negative effects on growth by warming. Especially for N. closterium f. minutissima, the growth rate under HTFT treatment showed no significant difference from that at low temperature (Fig. 2). This indicates that temperature fluctuations may intermittently expose cells to more favorable conditions during the cooling phase (Wolf et al., 2024; Schaum et al., 2018), facilitating recovery from stress. Similarly, a previous study on Thalassiosira pseudonana demonstrated that temperature fluctuation (cycling between 22 and 32 °C) facilitated rapid cellular adaptation by modulating transcription and oxidative stress responses (Schaum et al., 2018). Therefore, temperature fluctuation in natural environments plays a critical role in driving adaptation processes. The significant antagonistic interactions of warming and temperature fluctuation on growth rates of both diatom species (Table 2) have suggested that temperature fluctuations might alleviate the stress caused by extreme temperatures, enhancing the resilience of marine diatoms under warming scenarios.
5.2 Elemental composition and resource allocation
Temperature significantly affects phytoplankton metabolism and the utilization of nutrients within cells, which in turn affects the elemental composition of phytoplankton (Toseland et al., 2013; Spilling et al., 2015). In our study, warming (from 20 to 25 °C) at constant temperature led to a significant increase in the cellular contents of POC, POP, and BSi in Thalassiosira sp. and significantly elevated POC, PON, and POP quotas in N. closterium f. minutissima (Fig. 4). While the cellular POC, POP, and BSi contents of a temperate diatom species Thalassiosira sp. decreased with warming (from 15 to 20 °C) in a previous study (Cai et al., 2022), this difference may be due to the fact that the temperatures of the experimental setups were on different sides of the thermal response curve of Thalassiosira sp.
Both species showed elevated cellular protein and PON contents at HTFT compared to LTFT, indicating a reallocation of nitrogen to protein synthesis. This adaptive mechanism presumably facilitated the upregulation of stress-responsive proteins, thereby enhancing the capacity of the cells to cope with environmental stressors, such as extreme temperatures and thermal fluctuation (O'Donnell et al., 2018). For the larger-celled Thalassiosira sp., the higher metabolic demand for protein synthesis led to a reduction in the cellular quotas of macromolecules, including carbohydrates, POC, and BSi at HTFT compared to LTFT (Figs. 3 and 4). In comparison, N. closterium f. minutissima exhibited similar responses to temperature fluctuation at low and high temperatures. The increased protein, PON, carbohydrate, and POP content at HTFT compared to LTFT likely facilitated recovery of growth rates, highlighting the higher tolerance of N. closterium to temperature fluctuations and thermal stress (Figs. 3 and 4).
Phytoplankton exhibit nonlinear responses to temperature, and temperature fluctuation thus influences their elemental composition in distinct ways (Wang et al., 2019). These variations drive changes in cellular resource allocation, which in turn impact their stoichiometry and ultimately shape the competitive dynamics among different species (O'Donnell et al., 2018; Baker et al., 2016). Despite these changes in elemental content, we observed that warming had no significant effects on elemental ratios at constant temperature (Table 1). Similarly, no significant effects of warming on C:N and N:P were observed by a previous study on the Antarctic diatoms Pseudo-nitzschia subcurvata and Chaetoceros sp. (Zhu et al., 2016). In our study, HTCT did not significantly affect the elemental ratios of either species compared to LTCT. However, at HTFT, the C:N, C:P, and C:chl a ratios were significantly reduced in both species, while the N:P ratio increased compared to LTFT (Table 1).
5.3 Organic carbon fixation and sinking rate – oceanic implications
Marine diatoms are key primary producers in the ocean (Field et al., 1998). Due to the ballast effect of their silica frustules and their ability to produce POC, studying their POC production and sinking rate in response to warming and temperature fluctuations is crucial for understanding their role in the ocean's carbon cycle.
For Thalassiosira sp., HTCT had no significant effect on POC productivity compared to LTCT (Fig. 5a), likely because warming under constant temperature reduced growth rate but increased cellular POC content, offsetting the decline in POC productivity caused by slower growth. In contrast, the sinking rate of Thalassiosira sp. significantly increased under HTCT compared to LTCT (Fig. 5c), potentially due to elevated cellular POC and BSi content (Fig. 4). However, warming under temperature fluctuation elicited different responses. The POC productivity of Thalassiosira sp. significantly decreased under HTFT compared to LTFT (Fig. 5a), attributed to unchanged growth rates but reduced cellular POC content (Figs. 2 and 4). Similarly, HTFT significantly lowered the sinking rate of Thalassiosira sp. compared to LTFT (Fig. 5c), likely driven by declines in cellular POC and BSi content (Fig. 4). Thus, warming under constant temperature and temperature fluctuation had distinct impacts on Thalassiosira sp. At low temperature, temperature fluctuation enhanced POC productivity (Fig. 5a), supported by stable growth rates and increased POC content (Figs. 2a and 4a). At high temperature, despite accelerated growth rates under temperature fluctuation (Fig. 2a), POC productivity significantly declined due to reduced POC content (Fig. 4a).
For N. closterium f. minutissima, warming under both constant and fluctuating temperature had no significant effect on POC productivity or sinking rate (Fig. 5b and d). This further indicates that the smaller-celled N. closterium f. minutissima exhibits a superior ability to balance resource acquisition and utilization at elevated temperatures, conferring greater resilience to warming (Fan et al., 2023). A comparative study on diatoms of varying sizes confirmed that cell size decreases with rising temperatures, with larger species like Thalassiosira punctigera showing greater vulnerability to thermal stress, while smaller species exhibit enhanced tolerance to warming (Fan et al., 2023). However, temperature fluctuation increased POC productivity at both low and high temperatures (Fig. 5). At low temperature, this was due to increased POC content and stable growth rate, while at high temperature, it resulted from stable POC content and accelerated growth rate (Figs. 2 and 4). For N. closterium f. minutissima, temperature fluctuation at low temperature elevated POC content and sinking rates, although BSi content remained unaffected (Fig. 4). Previous studies have indicated that the sinking rate of Thalassiosira sp. is approximately 5 times higher than that of N. closterium f. minutissima, consistent with the general observation that larger diatoms exhibit higher sinking rates and contribute more significantly to deep-sea carbon export (Cai et al., 2022; Kiørboe, 1993). Thus, Thalassiosira sp. is expected to play a more prominent role in carbon export flux under warming conditions.
In summary, temperature fluctuation exerted contrasting effects on the carbon export flux of Thalassiosira sp. under different temperature regimes. At low temperature, it increased POC, BSi content, and sinking rates (Figs. 4 and 5), thereby enhancing carbon export. Conversely, at high temperature, it reduced POC, BSi content, and sinking rates (Figs. 4 and 5), leading to diminished carbon export to the deep ocean. These findings underscore the importance of incorporating temperature fluctuation into studies of phytoplankton and marine carbon dynamics.
5.4 Differential thermal responses driven by species traits
The differential physiological responses of Thalassiosira sp. and Nitzschia closterium f. minutissima to warming and temperature fluctuations are likely attributable to inherent differences in their morphology and ecological niches. Generally, algal cellular utilization of both light energy and nutrients, as well as metabolic efficiency, is intrinsically associated with cell size (Marañón, 2015; Marañón et al., 2012). As a representative centric diatom, Thalassiosira sp. typically has a larger cell size (∼30 µm), leading to fast sinking into depth but also imposing higher metabolic costs under thermal stress. Conversely, the smaller pennate diatom N. closterium f. minutissima (∼15 µm) exhibits a higher surface-area-to-volume ratio, promoting more efficient nutrient uptake and gas exchange, especially in variable environmental conditions. Additionally, pennate diatoms are commonly found in benthic or nearshore habitats that experience greater environmental heterogeneity (Burden et al., 2020), thus having increased adaptability and thermal resilience to temperature fluctuations, as observed in the present study. These morphological and geographical differences likely underpin species-specific strategies to thermal tolerance and consequent resource allocation and carbon export, highlighting the necessity of incorporating taxonomic and functional diversity when evaluating phytoplankton responses to climate change.
Our findings demonstrated that temperature fluctuations enhance the thermal tolerance of diatoms, though the degree of adaptation varies between species. The large-celled Thalassiosira sp. redirected resources toward protein synthesis to cope with thermal stress, while the small-celled N. closterium f. minutissima exhibited higher sensitivity to temperature fluctuations and a greater capacity for cellular repair. The divergent responses in carbon fixation and sinking rates further underscore species-specific contributions to oceanic carbon fluxes. These findings further provide insight into our understanding of how future climate conditions may alter phytoplankton productivity and biogeochemical cycling in marine ecosystems. Future studies should also investigate the combined effects of multiple stressors, such as nutrient availability and light conditions, with temperature fluctuations, as well as long-term adaptive responses of marine phytoplankton to environmental heterogeneity.
The research data are available at https://doi.org/10.5281/zenodo.15274949 (Sheng et al., 2025).
Conceptualization: YaW, YF; methodology: YW, YF; investigation: YW, YF; supervision: YF, YW, AF, SBI; writing – original draft: YS; writing – review and editing: YS, YF, AF, SBI.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
The authors would like to thank the laboratory of Xinwei Wang and Haibo Jiang at Ningbo University for helping with the analysis of the elemental compositions; Ruoyu Guo at the Second Institute of Oceanography, Ministry of Natural Resources, for providing phytoplankton stock cultures; Guisheng Song at Tianjin University for helping with chl a analysis; and the Shanghai Frontiers Science Center of Polar Science, Shanghai Jiao Tong University, 1954 Huashan Road, Shanghai 200030, China.
This work was financially supported by the National Natural Science Foundation of China (grant no. 42276093), the Impact and Response of Antarctic Seas to Climate Change funded by the Chinese Arctic and Antarctic Administration under contract no. IRASCC 1-02-01B, and the Oceanic Interdisciplinary Program of Shanghai Jiao Tong University (grant no. SL2022PT203).
This paper was edited by Andrew Thurber and reviewed by two anonymous referees.
Anderson, S. I., Barton, A. D., Clayton, S., Dutkiewicz, S., and Rynearson, T. A.: Marine phytoplankton functional types exhibit diverse responses to thermal change, Nat. Commun., 12, 6413, https://doi.org/10.1038/s41467-021-26651-8, 2021.
Arrhenius, S.: Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte, Zeitschrift für Physikalische Chemie, 4, 96–116, https://doi.org/10.1515/zpch-1889-0408, 1889.
Baker, K. G., Robinson, C. M., Radford, D. T., McInnes, A. S., Evenhuis, C., and Doblin, M. A.: Thermal Performance Curves of Functional Traits Aid Understanding of Thermally Induced Changes in Diatom-Mediated Biogeochemical Fluxes, Front. Mar. Sci., 3, 44, https://doi.org/10.3389/fmars.2016.00044, 2016.
Barton, S., Jenkins, J., Buckling, A., Schaum, C. E., Smirnoff, N., Raven, J. A., Yvon-Durocher, G., and Ezenwa, V.: Evolutionary temperature compensation of carbon fixation in marine phytoplankton, Ecol. Lett., 23, 722–733, https://doi.org/10.1111/ele.13469, 2020.
Bernhardt, J. R., Sunday, J. M., Thompson, P. L., and O'Connor, M. I.: Nonlinear averaging of thermal experience predicts population growth rates in a thermally variable environment, P. Roy. Soc. B, 285, 20181076, https://doi.org/10.1098/rspb.2018.1076, 2018.
Bernhardt, J. R., O'Connor, M. I., Sunday, J. M., and Gonzalez, A.: Life in fluctuating environments, Philos. T. R. Soc. B, 375, 1814, https://doi.org/10.1098/rstb.2019.0454, 2020.
Bienfang, P. K.: SETCOL – A Technologically Simple and Reliable Method for Measuring Phytoplankton Sinking Rates, Can. J. Fish. Aquat. Sci., 38, 1289–1294, https://doi.org/10.1139/f81-173, 1981.
Bindoff, N. L., Cheung, W. W. L., Kairo, J. G., Arístegui, J., Guinder, V. A., Hallberg, R., Hilmi, N., Jiao, N., Karim, S., Levin, L., O'Dongue, S., Purca, S., Rinkevich, B., Suga, T., Tagliabue, A., and Williamson, P.: Changing ocean, marine ecosystems, and dependent communities, Cambridge University Press, 447–587, https://doi.org/10.1017/9781009157964.007, 2019.
Bradford, M. M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 72, 248–254, https://doi.org/10.1016/0003-2697(76)90527-3, 1976.
Burden, A., Smeaton, C., Angus, S., Garbutt, A., Jones, L., Lewis, H., and Rees, S.: Impacts of climate change on coastal habitats, relevant to the coastal and marine environment around the UK, MCCIP Sci. Rev., 228–255, https://doi.org/10.14465/2020.arc11.chb, 2020.
Cai, T., Feng, Y., Wang, Y., Li, T., Wang, J., Li, W., and Zhou, W.: The Differential Responses of Coastal Diatoms to Ocean Acidification and Warming: A Comparison Between Thalassiosira sp. and Nitzschia closterium f. minutissima, Front. Microbiol., 13, 851149, https://doi.org/10.3389/fmicb.2022.851149, 2022.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F.: Colorimetric method for determination of sugars and related substances, Anal. Chem., 28, 350–356, https://doi.org/10.1021/ac60111a017, 1956.
Edullantes, B., Low-Decarie, E., Steinke, M., and Cameron, T.: Comparison of thermal traits between non-toxic and potentially toxic marine phytoplankton: Implications to their responses to ocean warming, J. Exp. Mar. Biol. Ecol., 562, 151883, https://doi.org/10.1016/j.jembe.2023.151883, 2023.
Eisenthal, R., Peterson, M. E., Daniel, R. M., and Danson, M. J.: The thermal behaviour of enzyme activity: implications for biotechnology, Trends Biotechnol., 24, 289–292, https://doi.org/10.1016/j.tibtech.2006.05.004, 2006.
Eppley, R. W. J. F. B.: Temperature and Phytoplankton Growth in the Sea, Environ. Sci., 70, 1063–1085, 1972.
Falkowski, P. G. and Oliver, M. J.: Mix and match: how climate selects phytoplankton, Nat. Rev. Microbiol., 5, 813–819, https://doi.org/10.1038/nrmicro1751, 2007.
Fan, J., Li, F., Hu, S., Gao, K., and Xu, J.: Larger diatoms are more sensitive to temperature changes and prone to succumb to warming stress, Limnol. Oceanogr., 68, 2512–2528, https://doi.org/10.1002/lno.12438, 2023.
Field, C. B., Behrenfeld, M. J., Randerson, J. T., and Falkowski, P.: Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components, Science, 281, 237–240, https://doi.org/10.1126/science.281.5374.237, 1998.
Folt, C. L., Chen, C. Y., Moore, M. V., and Burnaford, J.: Synergism and antagonism among multiple stressors, Limnol. Oceanogr., 44, 864–877, https://doi.org/10.4319/lo.1999.44.3_part_2.0864, 1999.
He, M., Zhou, X., Lv, T., Wang, Y., Liu, D., and Wang, Y.: The optimal realized niches of the diatom Paralia sulcata in the Yellow Sea and its environmental implication, Ecol. Indic., 158, 111362, https://doi.org/10.1016/j.ecolind.2023.111362, 2024.
Hong, T., Huang, N., Mo, J., Chen, Y., Li, T., and Du, H.: Transcriptomic and physiological responses of a model diatom (Phaeodactylum tricornutum) to heat shock and heat selection, Ecological Indicators, 153, 110420, https://doi.org/10.1016/j.ecolind.2023.110420, 2023.
IPCC: 2023: Summary for Policymakers, in: Climate Change 2023: Synthesis Report.Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Core Writing Team, Lee, H., and Romero, J., IPCC, Geneva, Switzerland, 1–34, https://doi.org/10.59327/IPCC/AR6-9789291691647.001, 2023.
Ketola, T. and Saarinen, K.: Experimental evolution in fluctuating environments: tolerance measurements at constant temperatures incorrectly predict the ability to tolerate fluctuating temperatures, J. Evolution. Biol., 28, 800–806, https://doi.org/10.1111/jeb.12606, 2015.
Kiørboe, T.: Turbulence, Phytoplankton Cell Size, and the Structure of Pelagic Food Webs, Adv. Mar. Biol., 29, 1–72, https://doi.org/10.1016/s0065-2881(08)60129-7, 1993.
Kuefner, W., Ossyssek, S., Geist, J., and Raeder, U.: The silicification value: a novel diatom-based indicator to assess climate change in freshwater habitats, Diatom Res., 35, 1–16, https://doi.org/10.1080/0269249x.2020.1722246, 2020.
Marañón, E.: Cell Size as a Key Determinant of Phytoplankton Metabolism and Community Structure, Annu. Rev. Mar. Sci., 7, 241–264, https://doi.org/10.1146/annurev-marine-010814-015955, 2015.
Marañón, E., Cermeño, P., López-Sandoval, D. C., Rodríguez-Ramos, T., Sobrino, C., Huete-Ortega, M., Blanco, J. M., Rodríguez, J., Fussmann, G.: Unimodal size scaling of phytoplankton growth and the size dependence of nutrient uptake and use, Ecol. Lett., 16, 371–379, https://doi.org/10.1111/ele.12052, 2012.
Morán, X. A. G., López-Urrutia, Á., Calvo-Díaz, A., and Li, W. K. W.: Increasing importance of small phytoplankton in a warmer ocean, Global Change Biol., 16, 1137–1144, https://doi.org/10.1111/j.1365-2486.2009.01960.x, 2010.
Nelson, D. M., Tréguer, P., Brzezinski, M. A., Leynaert, A., and Quéguiner, B.: Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation, Global Biogeochem. Cy., 9, 359–372, https://doi.org/10.1029/95GB01070, 1995.
O'Donnell, D. R., Hamman, C. R., Johnson, E. C., Kremer, C. T., Klausmeier, C. A., and Litchman, E.: Rapid thermal adaptation in a marine diatom reveals constraints and trade-offs, Global Change Biol., 24, 4554–4565, https://doi.org/10.1111/gcb.14360, 2018.
Perkins-Kirkpatrick, S. E. and Lewis, S. C.: Increasing trends in regional heatwaves, Nat. Commun., 11, 3357, https://doi.org/10.1038/s41467-020-16970-7, 2020.
Qiao, H., Zang, S., Yan, F., Xu, Z., Wang, L., and Wu, H.: Physiological responses of the diatoms Thalassiosira weissflogii and Thalassiosira pseudonana to nitrogen starvation and high light, Mar. Environ. Res., 166, 105276, https://doi.org/10.1016/j.marenvres.2021.105276, 2021.
Qu, P., Fu, F.-X., Kling, J. D., Huh, M., Wang, X., and Hutchins, D. A.: Distinct Responses of the Nitrogen-Fixing Marine Cyanobacterium Trichodesmium to a Thermally Variable Environment as a Function of Phosphorus Availability, Front. Microbiol., 10, 1282, https://doi.org/10.3389/fmicb.2019.01282, 2019.
Raven, J. A. and Geider, R. J.: Temperature and algal growth, New Phytol., 110, 441–461, https://doi.org/10.1111/j.1469-8137.1988.tb00282.x, 2006.
Rossi, S., Carecci, D., and Ficara, E.: Thermal response analysis and compilation of cardinal temperatures for 424 strains of microalgae, cyanobacteria, diatoms and other species, Sci. Total Environ., 873, 162275, https://doi.org/10.1016/j.scitotenv.2023.162275, 2023.
Sanders, R., Henson, S. A., Koski, M., De La Rocha, C. L., Painter, S. C., Poulton, A. J., Riley, J., Salihoglu, B., Visser, A., Yool, A., Bellerby, R., and Martin, A. P.: The Biological Carbon Pump in the North Atlantic, Prog. Oceanogr., 129, 200–218, https://doi.org/10.1016/j.pocean.2014.05.005, 2014.
Schaum, C. E.: Enhanced biofilm formation aids adaptation to extreme warming and environmental instability in the diatom Thalassiosira pseudonana and its associated bacteria, Limnol. Oceanogr., 64, 441–460, https://doi.org/10.1002/lno.11050, 2018.
Schaum, C. E., Buckling, A., Smirnoff, N., Studholme, D. J., and Yvon-Durocher, G.: Environmental fluctuations accelerate molecular evolution of thermal tolerance in a marine diatom, Nat. Commun., 9, 1719, https://doi.org/10.1038/s41467-018-03906-5, 2018.
Sheng, Y., Wang, Y., Cai, T., Fathalli, A., Ben Ismail, S., and Feng, Y.: Temperature fluctuation alleviates the negative effects of warming on marine diatoms: comparison between Thalassiosira sp. and Nitzschia closterium f. minutissima, Zenodo, https://doi.org/10.5281/zenodo.15274950, 2025.
Spilling, K., Ylostalo, P., Simis, S., and Seppala, J.: Interaction Effects of Light, Temperature and Nutrient Limitations (N, P and Si) on Growth, Stoichiometry and Photosynthetic Parameters of the Cold-Water Diatom Chaetoceros wighamii, PLoS One, 10, e0126308, https://doi.org/10.1371/journal.pone.0126308, 2015.
Steinacher, M., Joos, F., Frölicher, T. L., Bopp, L., Cadule, P., Cocco, V., Doney, S. C., Gehlen, M., Lindsay, K., Moore, J. K., Schneider, B., and Segschneider, J.: Projected 21st century decrease in marine productivity: a multi-model analysis, Biogeosciences, 7, 979–1005, https://doi.org/10.5194/bg-7-979-2010, 2010.
Taucher, J., Jones, J., James, A., Brzezinski, M. A., Carlson, C. A., Riebesell, U., and Passow, U.: Combined effects of CO2 and temperature on carbon uptake and partitioning by the marine diatoms Thalassiosira weissflogii and Dactyliosolen fragilissimus, Limnol. Oceanogr., 60, 901–919, https://doi.org/10.1002/lno.10063, 2015.
Thomas, M. K., Kremer, C. T., Klausmeier, C. A., and Litchman, E.: A Global Pattern of Thermal Adaptation in Marine Phytoplankton, Science, 338, 1085–1088, https://doi.org/10.1126/science.1224836, 2012.
Toseland, A., Daines, S. J., Clark, J. R., Kirkham, A., Strauss, J., Uhlig, C., Lenton, T. M., Valentin, K., Pearson, G. A., Moulton, V., and Mock, T.: The impact of temperature on marine phytoplankton resource allocation and metabolism, Nat. Clim. Change, 3, 979–984, https://doi.org/10.1038/nclimate1989, 2013.
Wang, J., Zeng, C., and Feng, Y.: Meta-analysis reveals responses of coccolithophores and diatoms to warming, Mar. Environ. Res., 193, 106275, https://doi.org/10.1016/j.marenvres.2023.106275, 2024.
Wang, X., Fu, F., Qu, P., Kling, J. D., Jiang, H., Gao, Y., and Hutchins, D. A.: How will the key marine calcifier Emiliania huxleyi respond to a warmer and more thermally variable ocean?, Biogeosciences, 16, 4393–4409, https://doi.org/10.5194/bg-16-4393-2019, 2019.
Wolf, K. K. E., Hoppe, C. J. M., Rehder, L., Schaum, E., John, U., and Rost, B.: Heatwave responses of Arctic phytoplankton communities are driven by combined impacts of warming and cooling, Sci. Adv., 10, eadl5904, https://doi.org/10.1126/sciadv.adl5904, 2024.
Zaoli, S., Giometto, A., Marañón, E., Escrig, S., Meibom, A., Ahluwalia, A., Stocker, R., Maritan, A., and Rinaldo, A.: Generalized size scaling of metabolic rates based on single-cell measurements with freshwater phytoplankton, P. Nat. A. Sci., 116, 17323–17329, https://doi.org/10.1073/pnas.1906762116, 2019.
Zeng, X., Jin, P., Jiang, Y., Yang, H., Zhong, J., Liang, Z., Guo, Y., Li, P., Huang, Q., Pan, J., Lu, H., Wei, Y., Zou, D., and Xia, J.: Light alters the responses of two marine diatoms to increased warming, Mar. Environ. Res., 154, 104871, https://doi.org/10.1016/j.marenvres.2019.104871, 2020.
Zhang, M., Qin, B., Yu, Y., Yang, Z., Shi, X., and Kong, F.: Effects of temperature fluctuation on the development of cyanobacterial dominance in spring: implication of future climate change, Hydrobiologia, 763, 135–146, https://doi.org/10.1007/s10750-015-2368-0, 2015.
Zhang, Y., Gao, G., Xue, H., and Gao, K.: Reduced primary productivity and notable resilience of phytoplankton community in the coastal water of southern China under a marine heatwave, Environ. Res., 264, 120286, https://doi.org/10.1016/j.envres.2024.120286, 2025.
Zhu, Z., Xu, K., Fu, F., Spackeen, J. L., Bronk, D. A., and Hutchins, D. A.: A comparative study of iron and temperature interactive effects on diatoms and Phaeocystis antarctica from the Ross Sea, Antarctica, Mar. Ecol. Prog. Ser., 550, 39–51, https://doi.org/10.3354/meps11732, 2016.