the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
An elucidatory model of oxygen's partial pressure inside substomatal cavities
A parsimonious model based on Dalton's law reveals substomatal cavities to be dilute in oxygen (O2), despite photosynthetic O2 production. Transpiration elevates the partial pressure of water vapour but counteractively depresses the partial pressures of dry air's components – proportionally including O2 – preserving cavity pressurization that is negligible as regards air composition. Suppression of O2 by humidification overwhelms photosynthetic enrichment, reducing the O2 molar fraction inside cool or warm leaves by hundreds or thousands of parts per million. This elucidates the mechanisms that realize O2 transport: diffusion cannot account for up-gradient conveyance of O2 from dilute cavities through stomata to the more aerobic atmosphere. Rather, leaf O2 emissions depend on non-diffusive transport via mass flow forced by cavity pressurization, which is not negligible in the context of dynamics. Non-diffusive O2 expulsion overcomes massive inward O2 diffusion to force net O2 emission. At very high leaf temperatures, mass flow also influences transport of water vapour and carbon dioxide, physically decoupling their exchanges and reducing water-use efficiency, independently of stomatal regulation.
- Article
(336 KB) - Full-text XML
- BibTeX
- EndNote
The partial pressure of water vapour (e) inside substomatal cavities is well known to be greatly elevated by transpiration, as reflected by the ambient vapour pressure deficit (VPD). However, both the total pressure (p) and partial pressures of dry-air components such as oxygen () have been fixed as parameters that are independent of plant functioning (e.g. Farquhar and Wong, 1984). This oversight neglects the implications of Dalton's law of partial pressures. Here, a very simple model is presented that accurately estimates , offering insights into the mechanisms of stomatal gas transport, which, contrary to long-standing assumptions (Moss and Rawlins, 1963), are not exclusively diffusive.
Dalton's law of partial pressures,
defines p as the sum of e with the partial pressure of dry air, within the parentheses, which in turn is the sum of the partial pressures of nitrogen (N2), O2, and argon (Ar), neglecting gases with mere trace contributions. Equation (1) can be expressed for both the substomatal-cavity interior (i),
and the ambient atmosphere (a) outside the leaf,
If Δ denotes a cavity surplus versus the ambient atmosphere, subtracting Eq. (3) from Eq. (2) yields
where Δe quantifies cavity humidification and reflects the ambient VPD. In the context of Eq. (4) for substomatal cavities, water vapour's substantial surplus (Δe>0) implies either cavity pressurization (Δp>0) or depressed partial pressures of dry air's components ( or a combination of both. Since cavity pressurization would drive mass flow out of the aperture, theoretical considerations from micro-scale fluid dynamics can establish an upper limit for Δp.
Despite the fact that stomata are not cylindrical, the Poiseuille equation derivation (Giancoli, 1984) can be used to show that Δp negligibly counterbalances Δe in Eq. (4). This is done below by exaggerating the parameters of cylindrical geometry to put a bound on the Δp required to force viscous flow. The axial velocity v of a laminar flow through a cylinder of length L and radius R is given as
where η is air's dynamic viscosity (18 µPa s). Solving for Δp yields
Here, parameters are chosen so as to maximize the Δp required to drive viscous flow.
-
Stomatal dimensions are exaggerated based on Lawson et al. (1998):
- –
Pore depth is overestimated as L= 10 µm.
- –
Stomatal aperture is underestimated using R= 2 µm (area ∼13 µm2).
- –
-
An air velocity of v= 6 mm s−1 escaping the stomatal aperture (Kowalski, 2017) represents an upper bound in the sense that traditional plant physiological models assume all transport to be diffusive, with no relevant role played by mass flow, effectively assuming a null value of v.
Plugging these values into Eq. (6) results in Δp= 0.0011 kPa, indicating that a very slight pressure difference is required to drive viscous flow. Given this, in the context of Eq. (4) regarding air composition and with resolution sufficient to characterize the VPD (to ±0.01 kPa), we can neglect substomatal pressurization in Eq. (4), taking Δp=0. This means that any increase in the cavity's Δe forced by transpiration must be counterbalanced by a reduction in the partial pressure of dry air (.
With transpired water vapour supplanting substomatal dry air, the simplest model is proportional depression of the partial pressures of dry air's components. In light of the ideal gas law, this implies that, for every 1000 dry-air molecules displaced by water vapour, we can expect N2 : O2 : Ar proportions of 781 : 210 : 9. Therefore O2's partial pressure inside substomatal cavities is modelled succinctly by
indicating O2 depression (versus ambient air) that is 21 % of the vapour pressure surplus of the substomatal cavity or about 21 % of the environmental VPD.
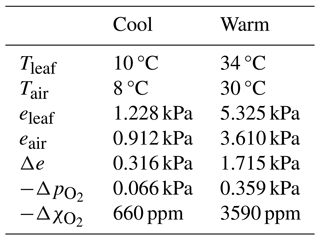
Oxygen deficits prevail within substomatal cavities because photosynthetic enrichment (µmol m−2 s−1) of O2 is vastly overwhelmed by O2 dilution and displacement due to transpiration (mmol m−2 s−1). Oxygen represents a sizeable fraction (about one-fifth) of ambient air but a far smaller fraction of leaf gas emissions, which are nearly pure water vapour and so dilute O2 to force hypoxic conditions inside substomatal cavities. The degree of O2 depression depends strongly on the VPD and therefore leaf temperature (T), as illustrated by representative examples of cool and warm leaves (Table 1). Notably, even the cool leaf has a significant O2 pressure deficit of 0.066 kPa. Near sea level (defined hereinafter as p= 100 kPa), this corresponds to an O2 molar fraction (referencing moist air) that is 660 ppm below ambient air. In warm leaves O2 depression reaches several thousand parts per million, and in torrid environments it can be far greater.
Table 1Consequences of negligible stomatal-cavity pressurization regarding air composition. Representative temperatures, water vapour pressures, stomatal-cavity vapour pressure surplus (Δe), oxygen pressure deficits (, and oxygen concentration deficits () for cool and warm leaves and their ambient atmospheres. Leaves are taken as saturated and ambient air at 85 % relative humidity; is calculated using Eq. (7); is calculated for conditions “near sea level” (p= 100 kPa).
The most noteworthy inference from this Daltonian model relates to the mechanisms of gas transport through stomata, since O2 produced by photosynthesis cannot diffuse out of stomata as has been traditionally assumed (Parkhurst, 1994). Equation (7) implies that substomatal cavities are generally much more dilute in O2 than their environments, whatever the leaf T. Although traditional thinking would explain O2 transport in terms of diffusive flows within a ternary system (Jarman, 1974; von Caemmerer and Farquhar, 1981), diffusive transport from dilute towards enriched regions is impossible – it would violate the second law of thermodynamics. Rather, non-diffusive transport by the viscous flow – driven by pressurization that is negligible in the context of Eq. (4) but nonzero nonetheless – is required to overcome inward O2 diffusion and drive O2 out of substomatal cavities. Diffusion of O2 into substomatal cavities is massive due to concentration differences of hundreds or thousands of parts per million across the leaf's pore depth. Gradients and levels of diffusion of O2 exceed those of CO2 by orders of magnitude.
However simplistic, the model improves upon the accuracy of previous assumptions regarding substomatal that neglected Dalton's law. These include the assumption that is a fixed parameter that does not depend substantially on plant functioning (Farquhar and Wong, 1984), as well as the notion that substomatal cavities are enriched in O2 (Parkhurst, 1994), purporting outward O2 diffusion while overlooking the dominant effects of transpiration on O2 abundance. The greatest inaccuracies of the Daltonian model presented here can be bounded by considering the chief processes that it does not take into account.
Adhering to the principle of parsimony, the model neglects the effects of two lesser and partially offsetting influences on , neither of which can alter the above conclusion regarding O2 transport mechanisms. Firstly, photosynthetic O2 production must reduce the O2 pressure deficit, increasing substomatal O2 somewhat but certainly not by the many hundreds of parts per million (or thousands for warm leaves) that would be required to make positive. This seems clear when recalling the stoichiometric relation between O2 and CO2 and the trace amounts of the latter gas that limit the possible magnitude of photosynthetic . Secondly, molecular diffusion's discrimination among dry-air species must increase the O2 deficit since N2 (28 g mol−1), representing 78.1 % of atmospheric dry-air molecules, diffuses upstream into substomatal cavities more rapidly than does O2 (32 g mol−1) according to Graham's law. Unaffected by these inaccuracies, the deduction that substomatal cavities generally are very dilute in O2 is ineluctable, as is the conclusion that stomatal O2 transport is predominantly non-diffusive. Specifically, it is due to a mass flow that indiscriminately pushes all gases outwards (Kowalski, 2017). Although previously couched in terms of “stomatal jets”, this is a low-velocity viscous flow (low Reynolds number) whose conveyance neither discriminates among gas species nor depends on concentration gradients, unlike diffusion. Its relevance to the transport of other gases depends on air's state conditions within stomata.
At very high leaf T, these implications from gas physics become relevant to the behaviour of CO2 and water vapour.
Regarding CO2, non-diffusive transport cannot be neglected universally. The model presented here is not valid for estimating , whose fluctuations are principally determined by photosynthesis. However, independently of photosynthetic drawdown (well, physically independently), the assumption of proportional depression of the partial pressures of dry air's components when supplanted by water vapour seems valid. Accordingly, just as Eq. (7) apportions 21 % of supplanted dry air to O2 depression, for a CO2 concentration of 420 ppm we can expect 0.042 % of the dry-air depression described by Eq. (4) to correspond to . This influence is negligible for temperate leaves with modest VPDs. For example, for the cool leaf in Table 1, it implies CO2 depression of ∼ 0.0001 kPa; near sea level, this is about 1 ppm and pales in comparison to photosynthetic drawdown. By contrast, for the warm leaf also near sea level, it means substomatal CO2 depression by over 7 ppm, which is no longer negligible and drives inward CO2 diffusion that is not due to photosynthesis. During heatwaves, with extreme values of VPD, substomatal CO2 depression due to humidification can be much larger. Thus, at very high leaf T, non-diffusive transport can appreciably suppress photosynthesis via CO2 limitation, but it has the opposite effect on transpiration.
Water vapour is also forced out of stomata by non-discriminating mass flow, with relevance that depends on water vapour abundance. Applying Newtonian physics to the momentum of air within stomata, Kowalski (2017) showed that the water vapour mass fraction, or specific humidity (q), defines the fraction of water vapour transport that is non-diffusive. Within substomatal cavities that are essentially saturated, the state variable q is largely determined by T. For the cool leaf in Table 1 (q<1 %), non-diffusive transport can reasonably be neglected. But this is not so for the warm leaf (q>3 %), and furthermore q increases rapidly as leaf T rises. If these increases in water vapour transport rates seem modest versus what can be achieved by diffusion alone, they grow in importance when considered in combination with reduced photosynthesis via suppression of substomatal by Δe as described above.
The consequences of gas physics at high leaf T are disparate for water vapour and CO2 exchanges. Ejecting all gases, mass flow enhances water vapour loss and opposes CO2 ingress, boosting transpiration and suppressing photosynthesis versus the capabilities of diffusive transport alone. It thereby reduces water-use efficiency via effects on each gas. Therefore, dry-air depression and non-diffusive transport likely explain the decoupling of transpiration and photosynthesis that has been observed widely at very high leaf T (Aparecido et al., 2020; De Kauwe et al., 2019; Diao et al., 2024; Krich et al., 2022; Marchin et al., 2023; Pankasem et al., 2024; Sun et al., 2024). In very hot substomatal cavities where water vapour is not a mere trace gas, transport due to mass flow casts doubt on the very meaning of stomatal conductance. Additionally, non-diffusive transport is gaining in relevance regarding leaf gas exchanges as the Earth warms and heatwaves increase in frequency and intensity (IPCC, 2021).
Evaporation within a moist cavity and vapour egress through a small aperture aptly describe not only leaf gas exchanges but also a whistling tea kettle. At the boiling point, a steam jet drives out dry air (including O2 and CO2) and the water vapour pressure approaches the total pressure (e∼p). This marks the humid extreme (q∼100 %) of a spectrum regarding fractional transport by different mechanisms, with the diffusion-only modelling framework valid at the other extreme (dry; q∼0 %). In state conditions that categorize stomatal air, q is limited to below 10 % and non-diffusive transport plays a role that is secondary, although sometimes not negligible. Insight into the consequences of such mass flow might be gained by investigating gas exchanges at intermediate values of q.
Artificial experiments may be helpful in this regard, and there are several strategies that can elevate q and that can be pursued individually or in combination. Gas exchange measurements can be made at very high temperatures (exceeding 50 °C) using artificial leaves (Schymanski and Or, 2017) since they suffer no heat stress or loss of functionality under conditions that would endanger life. Evaporation from such leaves with rising q but at constant VPD is predicted to be practically constant according to stomatal conductance models but to increase when taking non-diffusive transport into account. Similar experiments might be conducted on living leaves with hot but tolerable temperatures in conditions nearer to boiling due to reduced p, as within a hypobaric chamber. Finally, for leaves functioning in a “helox” environment (Mott and Parkhurst, 1991) – a mixture of helium and O2 whose density (hence inertia) is just 29 % that of dry air – non-diffusive transport would be elevated more than 3-fold (Kowalski, 2017). Assessments of leaf functioning in such conditions should help to shed light on the implications of non-diffusive transport for stomatal gas exchanges.
Water vapour's elevated partial pressure inside substomatal cavities implies depressed partial pressures of dry-air components including oxygen (O2), according to Dalton's law with negligible cavity pressurization. Substomatal cavities, not photosynthetically enriched in O2, are dilute because of transpiration. Only non-diffusive conveyance can account for transport of O2 from these O2-poor cavities into the more aerobic, ambient atmosphere. Slight substomatal pressurization, however negligible in the context of Dalton's law, is sufficient to drive mass flow of air out of stomatal apertures. The relevance of mass flow to gas transport cannot be neglected universally in plant physiology, becomes important for water vapour and CO2 in leaves at very high T, and therefore is increasing with global warming.
No data sets were used in this article.
The author has declared that there are no competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Constructive suggestions from the editor and referees improved the clarity and contribution of this paper.
This research has been supported by the Ministerio de Ciencia e Innovación (grant nos. PID2021-128463OB-I00, 2822/2021, PID2020-117825GB-C21, and TED2021-129499A-I00), the Organismo Autónomo Parques Nacionales (grant no. PN2021-2820s), and the Universidad de Granada (grant nos. PPJIB2022-08 and C‐EXP‐366‐UGR23).
This paper was edited by Paul Stoy and reviewed by three anonymous referees.
Aparecido, L. M. T., Woo, S., Suazo, C., Hultine, K. R., and Blonder, B.: High water use in desert plants exposed to extreme heat, Ecol. Lett., 23, 1189–1200, https://doi.org/10.1111/ele.13516, 2020.
De Kauwe, M. G., Medlyn, B. E., Pitman, A. J., Drake, J. E., Ukkola, A., Griebel, A., Pendall, E., Prober, S., and Roderick, M.: Examining the evidence for decoupling between photosynthesis and transpiration during heat extremes, Biogeosciences, 16, 903–916, https://doi.org/10.5194/bg-16-903-2019, 2019.
Diao, H., Cernusak, L. A., Saurer, M., Gessler, A., Siegwolf, R. T. W., and Lehmann, M. M.: Uncoupling of stomatal conductance and photosynthesis at high temperatures: mechanistic insights from online stable isotope techniques, New Phytol., 241, 2366–2378, https://doi.org/10.1111/nph.19558, 2024.
Farquhar, G. D. and Wong, S. C., An empirical model of stomatal conductance, Aust. J. Plant Physiol., 11, 191–210, 1984.
Giancoli D. C.: General Physics, Prentice-Hall, Englewood Cliffs, 892 pp., ISBN 0-13-350884-6, 1984.
IPCC: Climate change 2021 – the physical science basis, Cambridge University Press, 43, 22–23, https://doi.org/10.1017/9781009157896, 2021.
Jarman, P. D.: The diffusion of carbon dioxide and water vapour through stomata, J. Exp. Bot., 25, 927–936, 1974.
Kowalski, A. S.: The boundary condition for vertical velocity and its interdependence with surface gas exchange, Atmos. Chem. Phys., 17, 8177–8187, https://doi.org/10.5194/acp-17-8177-2017, 2017.
Krich, C., Mahecha, M. D., Migliavacca, M., De Kauwe, M. G., Griebel, A., Runge, J., and Miralles, D. G., Decoupling between ecosystem photosynthesis and transpiration: A last resort against overheating, Environ. Res. Lett., 17, 044013, https://doi.org/10.1088/1748-9326/ac583e, 2022.
Lawson, T., James, W., and Weyers, J., A surrogate measure of stomatal aperture, J. Exp. Bot., 49, 1397–1403, 1998.
Marchin, R. M., Medlyn, B. E., Tjoelker, M. G., and Ellsworth, D. S.: Decoupling between stomatal conductance and photosynthesis occurs under extreme heat in broadleaf tree species regardless of water access, Glob. Change Biol., 29, 6319–6335, https://doi.org/10.1111/gcb.16929, 2023.
Moss, D. N. and Rawlins, S. L., Concentration of carbon dioxide inside leaves, Nature, 197, 1320–1321, 1963.
Mott, K. A. and Parkhurst, D. F.: Stomatal responses to humidity in air and helox, Plant Cell Environ., 14, 509–515, 1991.
Pankasem, N., Hsu, P.-K., Lopez, B. N. K., Franks, P. J., and Schroeder, J. I.: Warming triggers stomatal opening by enhancement of photosynthesis and ensuing guard cell CO2 sensing, whereas higher temperatures induce a photosynthesis-uncoupled response, New Phytol., 244, 1847–1863, https://doi.org/10.1111/nph.20121, 2024.
Parkhurst, D. F.: Diffusion of CO2 and other gases inside leaves, New Phytol., 126, 449-479, 1994.
Schymanski, S. J. and Or, D.: Leaf-scale experiments reveal an important omission in the Penman–Monteith equation, Hydrol. Earth Syst. Sci., 21, 685–706, https://doi.org/10.5194/hess-21-685-2017, 2017.
Sun, W., Maseyk, C., Lett, C., and Seibt, U., Restricted internal diffusion weakens transpiration–photosynthesis coupling during heatwaves: Evidence from leaf carbonyl sulphide exchange, Plant Cell Environ., 47, 1813–1833, 2024.
von Caemmerer, S. and Farquhar, G. D.: Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves, Planta, 153, 376–387, 1981.




