the Creative Commons Attribution 3.0 License.
the Creative Commons Attribution 3.0 License.
Effect of light on N2 fixation and net nitrogen release of Trichodesmium in a field study
Yangyang Lu
Zuozhu Wen
Dalin Shi
Mingming Chen
Yao Zhang
Sophie Bonnet
Yuhang Li
Jiwei Tian
Shuh-Ji Kao
Dinitrogen fixation (NF) by marine cyanobacteria is an important pathway to replenish the oceanic bioavailable nitrogen inventory. Light is the key to modulating NF; however, field studies investigating the light response curve (NF-I curve) of NF rate and the effect of light on diazotroph-derived nitrogen (DDN) net release are relatively sparse in the literature, hampering prediction using models. A dissolution method was applied using uncontaminated 15N2 gas to examine how the light changes may influence the NF intensity and DDN net release in the oligotrophic ocean. Experiments were conducted at stations with diazotrophs dominated by filamentous cyanobacterium Trichodesmium spp. in the western Pacific and the South China Sea. The effect of light on carbon fixation (CF) was measured in parallel using the 13C tracer method specifically for a station characterized by Trichodesmium bloom. Both NF-I and CF-I curves showed a Ik (light saturation coefficient) range of 193 to 315 µE m−2 s−1, with light saturation at around 400 µE m−2 s−1. The proportion of DDN net release ranged from ∼ 6 to ∼ 50 %, suggesting an increasing trend as the light intensity decreased. At the Trichodesmium bloom station, we found that the CF ∕ NF ratio was light-dependent and the ratio started to increase as light was lower than the carbon compensation point of 200 µE m−2 s−1. Under low-light stress, Trichodesmium physiologically preferred to allocate more energy for CF to alleviate the intensive carbon consumption by respiration; thus, there is a metabolism tradeoff between CF and NF pathways. Results showed that short-term (< 24 h) light change modulates the physiological state, which subsequently determined the C ∕ N metabolism and DDN net release by Trichodesmium. Reallocation of energy associated with the variation in light intensity would be helpful for prediction of the global biogeochemical cycle of N by models involving Trichodesmium blooms.
- Article
(993 KB) - Full-text XML
-
Supplement
(374 KB) - BibTeX
- EndNote
The bioavailable nitrogen introduced via nitrogen fixation (NF) by cyanobacteria is important to fertilize the tropical and subtropical oligotrophic surface ocean (Karl et al., 1997). In such environments, nitrate supplied from the subsurface is generally limited by thermostructure-induced stratification and NF can directly input bioavailable nitrogen to the euphotic zone (Capone et al., 2005). Among the variety of diazotrophs, the filamentous non-heterocystous cyanobacterium Trichodesmium is recognized as a major player, contributing up to 80–110 Tg N annually, i.e. ∼ 50 % of global marine NF (Capone et al., 1997). It often forms colonies or aggregates and under appropriate circumstances it forms large surface blooms (Zehr, 2011).
Light is the primary energy source for the photoautotrophic diazotrophs, and the energy-exhausting NF process is tightly linked with photosynthesis (LaRoche and Breitbarth, 2005, and reference therein). Regarding the light response of Trichodesmium, several previous field studies put efforts into carbon fixation (CF) and oxygen production in response to irradiance (PI curve) and showed that photosynthetic rates of Trichodesmium were proportional to light intensities, and a relatively high irradiance requirement and a high respiration rate were needed to protect the nitrogenase enzyme from O2 deactivation (Lewis et al., 1988; Carpenter and Roenneberg, 1995). By using the C2H2 reduction method, Carpenter et al. (1993) investigated the light response of nitrogenase activity for the field-towed Trichodesmium, which showed a response pattern as a function of irradiance and resembling the PI curve. Similarly, by using 13C ∕ 15N isotope labeling techniques, Holl et al. (2007) found that NF and CF rates of field-towed Trichodesmium were attenuated as light intensity decreased. In controlled laboratory experiments, Breitbarth et al. (2008) suggested that both nitrogenase activity and growth rates of Trichodesmium (IMS-101) are light-dependent (15 to 1100 µE m−2 s−1), and Bell and Fu (2005) observed increasing NF rates with the increase in light intensity (PAR 10–160 µE m−2 s−1), and the cellular concentrations of Chl a and phycobiliproteins (PBPs) increased under low-light conditions.
Meanwhile, statistical analysis performed on the global dataset of field NF suggests that light is an important environmental factor explaining most of the spatial variance in NF on the global scale (Luo et al., 2014). However, it has to be noted that some of the NF rate measurements available in this global database might be questionable due to previously unrealized technical problems, e.g., incomplete 15N2 dissolution in the 15N2 bubble tracer method (Mohr et al., 2010), bioavailable 15N forms contamination in some commercial 15N2 gas (Dabundo et al., 2014) and inconsideration of diazotroph-derived N (DDN) release in the filtrate fraction (Konno et al., 2010). Nevertheless, the abovementioned experiments and global analysis support the idea of a light control on NF activity, CF and oxygen evolution of Trichodesmium; however, limited field experiments have been conducted on studying the effect of light on CF and NF of bulk seawater, particularly during naturally occurring Trichodesmium blooms. Moreover, to our knowledge, no study using the improved 15N2 dissolution tracer method (Mohr et al., 2010) has been implemented yet.
During the NF process, Trichodesmium release 10 to 50 % of the DD15N in the dissolved pool (Glibert and Bronk, 1994; Konno et al., 2010), primarily as dissolved organic N (DON, such as dissolved free amino acid) and NH (Capone et al., 1994; Mulholland et al., 2004). High DON and NH concentrations are often measured within Trichodesmium blooms (Karl et al., 1992; Lenes et al., 2001), being supportive of DDN release. As most NF rate measurements were via incorporation of 15N2 into particulate organic N (PON), the 15N enrichment in the dissolved pool had not been taken into account, resulting in the aforementioned potential underestimation of NF rates. Conversely, diatom and dinoflagellate blooms have often been observed following Trichodesmium blooms, suggesting that DDN potentially supported non-diazotrophic phytoplankton growth (Devassy et al., 1978; Lenes et al., 2001). By using nanometer-scale secondary ion mass spectrometry, Bonnet et al. (2016a) recently showed that the DDN is quickly (1–3 days) transferred to surrounding plankton, predominantly diatoms and bacteria, during Trichodesmium blooms. A mesocosm experiment performed in the western tropical South Pacific (VAHINE) revealed an incommensurately high contribution of NF to export production (> 50 %; Knapp et al., 2016) during a bloom of UCYN-C bloom. The contribution of NF to export can be up to 92 % in some studies (Kumar et al., 2017). However, the effect of NF on export was largely indirect, i.e., attributable to quick recycling processes of DDN transfer to non-diazotrophs that were subsequently exported (Bonnet et al., 2016b, c; Knapp et al., 2016). In spite of the importance of DDN release in C and N cycles, the factors controlling Trichodesmium DDN release remained unclear. In particular, the effect of light on DDN release has been poorly studied. To date, only one Trichodesmium culture study has reported a significant release of DDN and DOC after a rapid shift from low-light to high-light regimes to protect the photosynthetic apparatus (Wannicke et al., 2009).
Here we investigated the effect of light on DDN release and C ∕ N fixation stoichiometry of Trichodesmium in the field under contrasting situations, i.e. during a Trichodesmium bloom in the western equatorial Pacific and in a non-bloom area in the South China Sea.
This study was performed onboard the R/V Dong Fang Hong 2 during two cruises to the western equatorial Pacific Ocean (6 December 2015 to 12 January 2016) and the South China Sea (15 May to 7 June 2016). Experiments were conducted at three stations (Supplement Fig. S1), among which one of them was characterized by the presence of a Trichodesmium bloom (western equatorial Pacific Ocean station S0320); the other two were located in the South China Sea (A3, D5).
2.1 Seawater sampling and experimental procedures
Water samples were collected from 3 to 5 m depth using 10 L Go-Flo bottles that were attached to a CTD rosette (Sea-Bird 911 CTD). In our experiments, the same 4.5 L surface water samples were collected in the polycarbonate (PC) bottles and then put in six on-deck incubators with different light intensities for NF rate incubations. The light source was natural solar irradiance. Light intensity gradients (92, 54, 28, 14, 8 and 1 % of surface irradiance) were created by using neutral density and blue (061 Mist Blue; 172 Lagoon Blue) filters to adjust the light level (Fernandez et al., 2013; Rijkenberg et al., 2011; Mourino-Carballido et al., 2011). During the incubation period, the light intensity was monitored on deck with a flat 2π photosynthetically available radiation (PAR) sensor (PQS 1 PAR Quantum sensor, Kipp & Zonen) at 1 min intervals. We took the average light intensity of the incubation light period (> 1 µE m−2 s−1) as the surface irradiance to calculate light intensities of the six light gradients.
2.2 Nutrients, Chl a and abundance of Trichodesmium
Nutrient samples were collected in 100 mL high-density polyethylene (HDPE) bottles and kept frozen at −20 ∘C until analysis. Nanomolar levels of soluble reactive phosphorous (SRP) were determined according to Ma et al. (2008) with a detection limit of 1.4 nM and relative precision of ±2.5 %. Nanomolar levels of nitrate were analyzed using the chemiluminescent method (Garside, 1982) with a detection limit of 2 nM.
For Chl a concentration determination, 1 L of seawater was filtered on GF/F filters, wrapped in aluminum foil and stored at −20 ∘C until analysis onshore. Chl a was extracted in 90 % acetone, refrozen for 24 h and analyzed fluorometrically according to the method described by Welschmeyer (1994).
For Trichodesmium abundance determination, 1 L of seawater was sampled in HDPE bottles and immediately fixed with 10 mL Lugol's solution. Onshore, subsamples were settled for 48 h, the supernatant was removed and Trichodesmium filaments (trichomes) were counted on a Nikon Eclipse 50i optical microscope.
2.3 Molecular assessment of diazotrophs
For DNA analysis, 4 L of seawater was filtered through 0.2 µm pore-sized membrane filters (Supor 200, Pall Gelman, NY, USA), which were stored in liquid nitrogen until analysis. DNA was extracted according to Massana et al. (1997) with some modifications. Briefly, each filter was cut into pieces and placed into a 2 mL sterile screw cap micro-tube containing 0.2 g autoclaved glass beads and 0.8 mL GTE buffer (100 mM EDTA, 50 mM tris, 0.75 M sucrose). The tubes were agitated three times for 40 s in a homogenizer (FastPrep-24, MP Bio, USA) at 4.5 m s−1, and they were then frozen and thawed three times in liquid nitrogen. The next steps followed the protocol of Massana et al. (1997).
Four published quantitative polymerase chain reaction (qPCR) probe–primer sets (Church et al., 2005a, b) were used for qPCR analysis. Relevantly, the nifH genes of four photoautotrophic diazotroph groups were targeted: Trichodesmium spp., Richelia spp. associated with Rhizosolenia spp. (het-1), and the unicellular groups A (UCYN-A) and B (UCYN-B). We used the thermal cycling conditions and reaction mixtures as described previously by Zhang et al. (2011) with slight modifications. Triplicate 20 µL qPCR mixtures were used for each sample and standard, reaction mixes contained 10 µL Premix Ex Taq (probe qPCR) (RR390A, Takara Bio Inc., Dalian, China), 400 nM each of forward and reverse primer, 400 nM of fluorogenic probe, and 1 µL of environmental DNA or plasmid standards. We used dilution series of four linearized plasmids as standards, which contained inserts matching four primer–probe sets. The real-time quantitative PCR was performed on a CFX96 real-time system (Bio-Rad Laboratories, USA) with the following thermal cycling conditions: 50 ∘C for 2 min, 95 ∘C for 2 min and 45 cycles of 95 ∘C for 15 s, followed by 60 ∘C for 1 min. The quantification limit was determined empirically to be one copy per reaction. The amplification efficiency varied between 90 and 100 %. The negative controls contained complete reaction ingredients except environmental DNA or standards; no amplification was found in the negative controls.
2.4 N2 and carbon fixation rate measurements
NF rates were determined according to the dissolution method: the 15N2-enriched seawater was prepared following the same device and procedure as described in Shiozaki et al. (2015) and 200 mL 15N2-enriched seawater was added into each 4.5 L PC incubation bottle (at bloom station S0320, 1.2 L PC bottles were used) triplicated. The 15N2 gas (98.9 %) from Cambridge Isotope Laboratories was used. We conducted a blank check for 15N2 gas (contamination of bioavailable non-N215N) as mentioned in Dabundo et al. (2014). Briefly, triplicate 2 mL 15N2 gas and 10 mL natural seawater were injected into 20 mL headspace vials, sealed with a septum stopper and then shaken overnight. The δ15N of total dissolved nitrogen (TDN) was measured and compared with the δ15N of natural seawater samples. Values of δ15N TDN of the blank seawater and test seawater groups were 4.7 and 5.0 ‰, respectively, suggesting no contamination of the 15N2 gas.
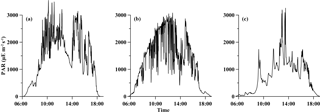
Figure 1Temporal variations in photosynthetically active radiation (PAR µE m−2 s−1) obtained on deck during the experiment periods, (a) for station S0320, (b) for station A3 and (c) for station D5.
At station S0320, the Trichodesmium bloom station, 13C-labeled sodium bicarbonate (99 atom% 13C; Cambridge Isotope Laboratories) was added in parallel with 15N2 to each bottle at a final tracer concentration of 70 µmol L−1 to simultaneously measure the CF and NF rates. At each irradiance level, triplicate water samples (4.5 L or 1.2 L PC bottle) were incubated on deck in incubators with surface seawater flow through. The surface-cooling seawater was connected with incubators in parallel to keep the temperature variations synchronous in six incubators. Thus, temperature was not a variable parameter that could influence the variability of final rates.
After 24 h incubation, water samples were gently filtered (< 200 mm Hg) onto precombusted (450 ∘C, 4 h) 25 mm Whatman GF/F (0.7 µm) filters, preserved at −20 ∘C and then dried in an oven overnight (50 ∘C). The particulate organic C (POC) ∕ PON concentrations and isotopic values were analyzed on a Flash EA (Thermo Fisher Flash HT 2000) IRMS (Thermo Fisher Delta V plus). International reference material (USGS40) with a different amount of C ∕ N and certified δ15N and δ13C values of −4.5 and −26.2 ‰, respectively, was inserted every eight samples to check the drift and ensure the accuracy of the measurements. The reproducibility for δ15N and δ13C measurements were both better than 0.3 ‰. The NF and CF rates were calculated by using similar equations proposed by Montoya et al. (1996) and Hama et al. (1983), respectively.
2.5 Light response curves for N2 fixation and carbon fixation
We follow the photosynthetic model by Webb et al. (1974):
where Nm is the maximum rate of NF at light-saturating irradiance, Nd is the rate measured in darkness, I is the natural irradiance and α is the light affinity coefficient for NF rate, to construct the irradiance curves for NF. Similarly, the light response curve of CF was obtained. The light saturation coefficient Ik was defined as Nm∕α.
2.6 DDN net release to the dissolved pool
To determine the TDN concentration and δ15N TDN according to Knapp et al. (2005), 40 mL of the filtrate (passed through precombusted GF/F filters) of each NF incubation bottle was collected and preserved at −20 ∘C. Briefly, TDN was oxidized to nitrate by a persulphate oxidation reagent (purified by recrystallization three to four times) and the concentration was measured using the chemiluminescent method (Garside, 1982). The δ15N TDN-derived nitrate was analyzed by using the denitrifier method (Sigman et al., 2001). The reproducibility for δ15N TDN measurements was better than 0.5 ‰. The DDN released to the dissolved pool was calculated following the equation proposed by Bonnet et al. (2016a).
2.7 Transfer of DDN into non-diazotrophic plankton
To evaluate the short time (24 h) DDN transfer to non-diazotrophic plankton, we followed the method by Adam et al. (2015). Briefly, for the control group, a 10 µm sieve was used to remove most Trichodesmium colonies and the remaining community was incubated for 24 h with 15N2-enriched seawater. In another group, the whole community was incubated for 24 h and Trichodesmium colonies were removed after incubation was terminated. Each experiment was performed in triplicate. The δ15N difference between the two treatments was considered to be a proxy of the DDN transfer to non-diazotrophic plankton.
3.1 Environmental conditions
The temporal patterns of PAR were shown in Fig. 1. The sun rose at ∼ 06:00 and set at ∼ 18:00 (all measurements at the S0320 station were made in local time and all measurements at the other two stations were made in Beijing time). The value of PAR (sampling at 10 s intervals) varied rapidly in a wide range from 0 to 3000 µE m−2 s−1, which is the typical range of values observed at low latitudes, yet much higher than those generally used in laboratory culture experiments (Bell and Fu, 2005; Wannicke et al., 2009). Although incubations were conducted for 24 h, average PAR during the incubation period (light intensity > 1 µE m−2 s−1) was applied for discussion. The average PAR values were 1464 ± 888 (61 %), 1293 ± 903 (70 %) and 743 ± 619, (83 %) µE m−2 s−1 for stations S0320, A3 and D5, respectively.
Table 2Synthesis of PON, POC, and DON concentrations, C ∕ N, carbon consumption and the corresponding NF and CF rates, and NF ∕ CF at station S0320. The “< 10 µm-a” represents a NF rate of < 10 µm community incubated with > 10 µm Trichodesmium colonies, “< 10 µm-b” represents the background NF rate of < 10 µm community, and carbon consumption was calculated using POC concentration variation from each irradiance point final concentration to initial POC concentration minus the carbon fixation rate at the corresponding irradiance point.

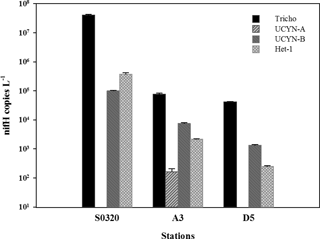
Figure 2Cyanobacteria diazotroph nifH phylotype abundances (nifH gene copies L−1). Tricho: Trichodesmium spp.; UCYN: unicellular N2-fixing cyanobacteria from Group A, B; Het-1: heterocystous cyanobacteria from Group 1. Error bars represent the standard deviation for triplicate natural samples.
The hydrographic and biogeochemical parameters are shown in Table 1. All three stations were characterized by low nutrient concentrations (NO 6 to 11 nM, PO 13 to 100 nM), relatively high salinity (34.5–34.6) and high sea surface temperature (27.6–29.7 ∘C). At the Trichodesmium bloom station (station S0320), Chl a concentrations were 1.2 mg m−3, much higher than those measured at the other stations (0.25 and 0.39 mg m−3). Results from the nifH phylotype abundances showed that Trichodesmium accounted for > 98.8, 88.6 and 96.4 % of the diazotrophic community at stations S0320, A3 and D5, respectively (Fig. 2). The dominant Trichodesmium species was Trichodesmium thiebautii for stations S0320 and D5, with an abundance of 4227 ± 679 (n=6) and 190 ± 50 (n=6) trichomes L−1, respectively. POC ∕ N concentration of the < 10 µm fraction represented < 25 % of the bulk POC ∕ N (see Table 2 and below), supporting that Trichodesmium was the dominant phytoplankton community at the blooming station.
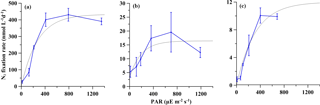
Figure 3Net (particulate) NF versus irradiance. The gray curves represent the fitted NF-I curves. The error bar represents the standard deviation of triplicate incubations. (a) Station S0320; (b) station A3; (c) station D5.
The net NF rates in the surface light intensity were 390.6 ± 20.4, 12.2 ± 1.8 and 9.9 ± 0.4 nM N d−1 at stations S0320, A3 and D5, respectively. The NF rate at the blooming station was 30–40 times higher than that of the two non-bloom stations. Detailed experimental data, including concentrations and isotopic values, for initial and final time points are listed in Tables S1–S3 in the Supplement. However, trichome-normalized rates were 92 and 52 pM N trichomes−1 d−1, respectively, for stations S0320 and D5, revealing a more consistent rate per trichome.
3.2 Light response of net (particulate) N2 fixation
As shown in Fig. 3, these NF-I curves showed a general pattern indicating that net NF rates increased significantly with light intensity from 10 to 400 µE m−2 s−1. The R2 values of fitted NF-I curves were 0.92, 0.71 and 0.95 at stations S0320, A3 and D5, respectively (all p values < 0.0001) and then saturated at around 400 µE m−2 s−1. The simulated Ik values for NF were 271, 193 and 315 µE m−2 s−1, respectively, for stations S0320, A3 and D5, with an average value of 260 ± 51 µE m−2 s−1.
Results of CF for stations S0320 showed a traditional PI curve pattern without apparent light inhibition (solid curve in Fig. 4a). The fitted curve of CF showed a pattern consistent with those of NF (dashed curve in Fig. 4a), giving an Ik value of 292 µE m−2 s−1 falling within the Ik range of the three NF-I curves, and the R2 of the fitted CF-I curve was 0.90 (p value < 0.0001).
3.3 Particulate C ∕ N metabolism of Trichodesmium bloom
The ratio of CF to NF was variable as light varied (Fig. 4b). The values of CF ∕ NF ranged from 7.4 ± 0.6 to 9.3 ± 1.0 when light intensities were saturated, while the ratios increased significantly from 7.4 ± 0.6 to 16.8 ± 3.2 as light intensities decreased from 410 to 15 µE m−2 s−1.
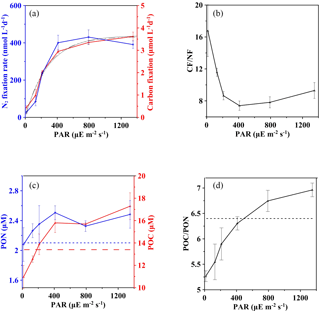
Figure 4Effect of light on the carbon and nitrogen budget at station S0320 with a Trichodesmium thiebautii bloom. (a) Carbon (red solid line) and nitrogen (blue solid line) fixation at different light intensities with fitted light response curves (black solid line for CF and black dotted line for NF). (b) The CF ∕ NF ratios at different light intensities. (c) The final concentrations of POC (red solid line) and PON (blue solid line) after incubations under different light intensities and initial values of POC (red dashed line) and PON (blue dashed line) concentration. (d) C ∕ N ratios after incubation in different light conditions. Error bars represent the standard deviation of triplicates.
The initial concentrations (n=3) of POC and PON were 13.4 ± 0.1 and 2.1 ± 0.0 µM, respectively, with a mean C : N molar ratio of 6.4 (horizontal lines in Fig. 4c and d), which is almost identical to the Redfield C ∕ N ratio of 6.6. After incubations under various light intensities, the final POC concentrations showed a decreasing trend (p value < 0.0001) ranging from 17.3 ± 1.2 to 10.9 ± 1.0 µM as the irradiance decreased. Below ∼ 200 µE m−2 s−1, the final POC concentration was even lower than the initial POC concentration (red dashed line in Fig. 4c), suggesting that the light compensation point (Ic) is around 200 µE m−2 s−1. A similar light-dependent pattern was found for PON. However, final PON concentrations, varying from 2.1 ± 0.2 to 2.5 ± 0.2 µM, were always higher than the initial concentration (blue dashed line in Fig. 4c) without a compensation point.
The observed C : N ratio of bulk particulate matter (5.3–7.0; Fig. 4d) is consistent with previously reported ranges for Trichodesmium (LaRoche and Breitbarth, 2005; Mulholland, 2007). However, a strong light dependency was also observed for the final C ∕ N after incubation. The saturated irradiance of ∼ 400 µE m−2 s−1 was likely a threshold; below the saturation light the final C ∕ N tended to be lower than the initial C ∕ N of 6.4 (dashed horizontal line in Fig. 4d).
3.4 DDN net release to the dissolved pool
The rate of DD15N net release in the TDN pool ranged from 7.7 ± 0.4 to 54.1 ± 7.8 nM N d−1 for station S0320, from 0.7 ± 0.2 to 1.0 ± 0.1 nM N d−1 for D5 and from 1.9 ± 1.5 to 5.0 ± 1.6 nM N d−1 for A3. The contribution of DDN net release to gross NF ranged from 8 ± 0 to 25 ± 6, 6 ± 6 to 45 ± 14 and 14 ± 11 to 50 ± 5 % for stations S0320, D5 and A3, respectively (Fig. 5). The overall range agrees well with previous field studies (Glibert and Bronk, 1994; Mulholland et al., 2006; Bonnet et al., 2016a; Konno et al., 2010; Benavides et al., 2013; Berthelot et al., 2015). Our data revealed that the fraction of DDN release to gross NF increased as light decreased (all p values < 0.05).
3.5 DDN transfer to non-diazotroph biomass
After 24 h of incubation, the DDN transfer rates (transferred to the non-diazotrophic plankton) were 18.6 ± 3.6, 0.5 ± 0.3 and 0.7 ± 0.5 nM N d−1 corresponding to 5 % ± 1 %, 4 % ± 3 % and 5 % ± 4 % of total NF (net plus dissolved), respectively, for stations S0320, D5 and A3 (Fig. 6). Our fractions are consistent with previous reports by Bonnet et al. (2016a), in which 6 % ± 1 % of DD15N was transferred to non-diazotrophic plankton in naturally occurring Trichodesmium blooms. Our fractions are slightly lower than the DD15N transfer (∼ 12 %) by Berthelot et al. (2016), who inoculated Trichodesmium erythraeum into natural surface oligotrophic seawater. Our results confirm that Trichodesmium could actively transfer newly fixed nitrogen to non-diazotrophs.
4.1 High light demand for Trichodesmium N2 fixation
The simulated Ik values in this field study for Trichodesmium fell within the high end of the reported Ik values for photosynthesis (LaRoche and Breitbarth, 2005). These values suggest a high light demand for Trichodesmium NF. The high energy requirement of Trichodesmium is not only for breaking the strong triple bond of the N2 molecule but also for numerous strategies, such as high respiration rates and the Mehler reaction, to protect the sensitive nitrogenase against the oxygen evolved by photosynthesis during daytime (Kana, 1993). Thus, Trichodesmium generally dwells in the upper euphotic zone of the tropical and subtropical ocean to meet the high light demands (Capone et al., 1997; Gandhi et al., 2011).
Generally, in the tropical and subtropical regions, average surface light intensities are around 1000 µE m−2 s−1 on sunny days. By taking into account the light extinction coefficient of seawater, the maximum depth for Trichodesmium to perform NF would be shallower than 15–40 m. This result matches well with many field observations that most NF occurs in the well-lit (0–45 m) region of the euphotic zone (Capone et al., 1997; Böttjer et al., 2016). This also agrees well with the observation that maximum Trichodesmium densities often appear at around 15 m depth and typically form blooms at the surface (Carpenter and Price 1977; Capone et al., 1997; Gandhi et al., 2011).
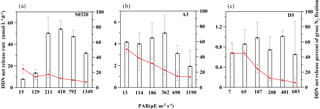
Figure 5DDN net release rate (bar charters) and percentage of total NF (red lines) under different light intensities for stations S0320, A3 and D5. Error bars represent the standard deviation of triplicates.
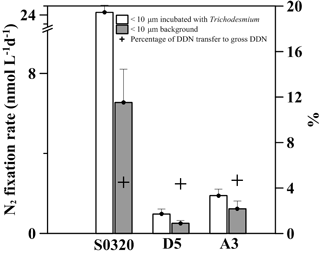
Figure 6The NF and DDN transfer measured in two treatment groups for stations S0320, D5 and A3. The black bars represent a background NF rate of < 10 µm community. White bars represent a NF rate of < 10 µm community incubated with > 10 µm Trichodesmium colonies. The error bar represents the standard deviation of the triplicate. Crosses stand for the percentage of DDN transferred to the < 10 µm community to total N2 fixation.
Our results also suggest that NF of Trichodesmium could respond to variable light intensity in the field within a short time period (24 h). Such results mean that light conditions during on-deck incubations should also be presented along with rate data if we want to compare field NF results among different studies. Unfortunately, the field NF rates had rarely been reported with consideration of in situ light conditions, although the light control on NF is well known to researchers.
Compared with laboratory strains acclimated to low light, field-observed NF-I curves are more representative of the real ocean with greater applicability. The parameter consistency among our three stations in the NF-I curves regardless of the wide range of trichome biomass and maximum NF rates, offers critical information on light-associated parameters in model predictions of global nitrogen fixation (Fennel et al., 2001; Hood et al., 2001).
4.2 Metabolism tradeoff between carbon and nitrogen fixation under light stress
In our field incubations, bulk C ∕ N molar ratios were always lower than the corresponding net CF : NF ratios at all light intensities (Fig. 4b, d). As reported in both culture and field studies, Trichodesmium usually exhibits a higher CF : NF ratio than the expected stoichiometric value of 6.6 (Mulholland, 2007). Several hypotheses have been proposed: (1) the underestimation of gross NF rates by overlooking the 15N signal in the dissolved pool (Glibert and Bronk, 1994; Mulholland et al., 2004); (2) the underestimation of N assimilation rates if there is uptake of other N sources such as nitrate or ammonium (Mulholland et al., 1999); (3) high carbon requirements to synthesize carbohydrate as a ballast for vertical migration (Villareal and Carpenter, 1990); (4) the support of the high energy cost, high respiration and Mehler reaction pathways (Carpenter and Roenneberg, 1995); and (5) the CF by non-diazotrophic phytoplankton.
Here, the low DDN net release rate is not supportive of the first hypothesis. As the incubation experiments were used the same bulk water and only light intensity was manipulated, the initial bioavailable nitrogen concentration between different treatments was almost the same. Thus, no apparent evidence supports the second hypothesis. Meanwhile, the third and fourth hypotheses could not explain the increased CF : NF ratio trend with the decrease in light intensity over the low-light condition (p value was 0.0005). In fact, the contribution from non-diazotrophic phytoplankton to CF cannot be excluded from bulk water incubation; however, the contribution is limited even at low light after assessment (see Supplement). As aforementioned, Trichodesmium was the dominant phytoplankton species; thus, the variation pattern of CF rates and POC concentrations against different light intensities mainly reflects the carbon metabolism of Trichodesmium.
In fact, in unialgal culture experiments (Berthelot et al., 2015), CF : NF ratios (1.8–5.6) were quite close to the POC : PON ratio (3.8–5.5) of a variety of diazotrophs including Trichodesmium. In our field study, the abundance of Trichodesmium was up to 4227 trichomes L−1, and the measured CF : NF ratios (9.3) at in situ light were close to the initial POC : PON ratio (6.4). Similarly, in a surface bloom of Trichodesmium in the Arabian Sea, Gandhi et al. (2011) also observed a low CF : NF ratio of ∼ 4 (NF rate of 1125 nM N h−1 and CF rate of 4594 nM C h−1), even lower than the Redfield ratio. Consistency among aforementioned laboratory and field studies suggested that CF : NF ratios of Trichodesmium should not be particularly high.
Under light limitation where Trichodesmium faced severe carbon consumption and energy shortage, energy was likely reallocated between CF and NF. We hypothesized that under low-light stress, Trichodesmium physiologically preferred to allocate more energy for CF to alleviate the intensive carbon consumption by respiration. This is analogous to the Trichodesmium iron limitation metabolism, of which photosynthesis takes priority over NF to get iron (Shi et al., 2007). Since the short-term (< 24 h) light manipulation in our experiments resembles the natural variation in irradiance, such a metabolism tradeoff between CF and NF under low light for Trichodesmium may happen frequently and widespread in the field, for example, on cloudy days and rainy days.
The proper allocation and utilization of energy (ATP) and reductant (NADPH) among various cellular processes determines the growth rate of Trichodesmium. Light-dependent reactions of photosynthesis are the major pathways to produce these molecules. In cyanobacteria, both respiratory and photosynthetic electron transport occur in the thylakoid membrane and compete for the electron transport chain (Oliver et al., 2012). When light intensity decreases, the light-dependent reactions of photosynthetic activity decrease concurrently, resulting in reduced production of ATP and NADPH and increased respiration activity. The negative feedback of POC consumption lead to more ATP and NADPH being reallocated to CF process, and in turn, the NF process was down-regulated.
4.3 Light modulation of DDN net release fraction
A previous study found that Trichodesmium trichomes contain only 15–20 % of diazocyte cells capable of NF (Kranz et al., 2011, and references therein). The remaining non-diazocyte cells rely on the release of bioavailable N, mainly the form of ammonium or amino acid, from diazocytes (Mulholland et al., 2004; Kranz et al., 2011). This process is directly proved by 15N labeling and the NanoSIMS method in which the 15N signal is rapidly distributed into the majority cells of Trichodesmium trichomes and even the 15N label signal is relatively lower in the center cells, which is probably a zone of diazocytes (Finzi-Hart et al., 2009; Bergman et al., 2013). Our results demonstrated that light does not directly regulate the absolute amount of DDN release. However, to discuss the physiological status for DDN distribution in the dissolved pool and particulate pool (mainly Trichodesmium), the proportion of DDN released into the dissolved pool is a proper indicator. In this study, the proportion of DDN in the dissolved pool increases with the light intensity decrease. This suggests that the physiology status of diazotrophs was modulated by light and could then take control of the DDN release process. At station A0320 high light intensities (> 400 µE m−2 s−1), the final POC and PON concentrations increased significantly, also implying an active physiology status of Trichodesmium, and the fraction of DD15N release in the dissolved pool ranged from 6 % ± 6 % to 23 % ± 5 %. Actually, several unialgal culture studies, including Trichodesmium and UCYN-B and UCYN-C, showed less than 2 % DD15N release in the dissolved pool (Berthelot et al., 2015; Benavides et al., 2013). These low values were attributable to the exponential growth phase and optimal growth conditions and a lack of exogenous factor influence such as viral lysis (Hewson et al., 2004) and sloppy feeding (O'Neil et al., 1996). Nevertheless, our values at high light are congruent with the field study (7–17 %) by Berthelot et al. (2016). Similar to their findings, we suggested that the active cell status and exposure to the exogenous factor may only lead to a slightly higher proportion of DDN net release. Under the light limitation stress, the inactive physiology state condition of Trichodesmium was reflected by the decrease in POC concentrations and activity of the CF and NF; thus, the DDN fixed by diazocytes was likely not efficiently transferred to other cells along the trichomes, therefore accumulating in the dissolved pool. Furthermore, a number of cells could breakdown and directly release intracellular bioavailable nitrogen. The fraction of DD15N release in the dissolved pool ranged from 17 % ± 4 % to 50 % ± 5 % at low-light conditions (400 µE m−2 s−1). This conclusion is also consistent with Bonnet et al. (2016a) for two natural Trichodesmium bloom studies that in the decaying bloom case, high ammonium concentration accumulation (3.4 µmol L−1) and a high proportion of DDN release (20 ± 5 to 48 ± 5 %) were observed, while in the exponentially growing bloom case, the proportion of DDN release only ranged from 13 ± 2 to 28 ± 6 % and without apparent accumulation of ammonium.
As summarized in Berthelot et al. (2015), most of the higher end of reported DDN net release values were estimated using the difference between gross NF rates measured with acetylene reduction assays (ARAs) and the net NF measured using the 15N2 bubble labeling technique (Montoya et al., 1996). The known uncertainty of the conversion factor for acetylene to N2 for the ARA method (Montoya et al., 1996; Shiozaki et al., 2010) may bias the DDN release estimate, while potential underestimation of net NF from the 15N2 bubble method may result in a higher DDN net release. In this study the direct measurement of the DD15N in the dissolved pool using the improved dissolution 15N2-enriched seawater method (Mohr et al., 2010) was applied to assess the DDN net release; thus, our data were quite reliable.
In this study, we provide quantitative information on the effect of light on NF and DDN net release of field Trichodesmium and found that the NF was a function of light intensity and biomass. The light requirement of Trichodesmium NF was higher relative to its photosynthesis light demand. The empirical Ik value suggests that the Trichodesmium population maxima should appear at < 15 m depth to obtain sufficient light energy. Furthermore, light intensity is a crucial factor to drive the physiological state of Trichodesmium, which subsequently determined the C ∕ N metabolism and DDN net release. Thus, we suggest the necessity to provide field light data along with nitrogen fixation data for on-deck incubation for the future studies.
Recent studies suggested that unicellular cyanobacteria diazotrophs, inhabiting different niches, especially UCYN-A, are distributed more widely in the global ocean and may contribute equally to the NF flux with Trichodesmium (Zehr et al., 2016; Martínez-Pérez et al., 2016). More field studies are needed in future to explore the light response of those UCYN diazotrophs to further understand their light behavior and to optimize the role of diazotrophs in global NF models.
The data associated with the paper are available from the corresponding author upon request.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-1-2018-supplement.
The authors declare that they have no conflict of interest.
We sincerely thank Guanghe Shao, Wenfang Lin, and Jian Pang at the State Key Laboratory of Marine Environmental
Science (Xiamen University, China) for their valuable help with
the water sampling and pretreatment during cruises. Ting-Chang Hsu from the Department
of Geology at the National Taiwan University in Taiwan is thanked for his valuable
discussion on and suggestions for the paper. This research was funded by
the National Natural Science Foundation of China (NSFC 2014CB953702, 91328207, 2015CB954003).
This is MEL contribution number #melpublication2017225.
Edited by: Manmohan Sarin
Reviewed by: three
anonymous referees
Adam, B., Klawonn, I., Svedén, J. B., Bergkvist, J., Nahar, N., Walve, J., Littmann, S., Whitehouse, M. J., Lavik, G., and Kuypers, M. M.: N2-fixation, ammonium release and N-transfer to the microbial and classical food web within a plankton community, ISME J., 10, 450–459, https://doi.org/10.1038/ismej.2015.126, 2015.
Bell, P. R. and Fu, F.-X.: Effect of light on growth, pigmentation and N2 fixation of cultured Trichodesmium sp. from the Great Barrier Reef lagoon, Hydrobiologia, 543, 25–35, https://doi.org/10.1007/s10750-004-5713-2, 2005.
Benavides, M., Bronk, D. A., Agawin, N. S., Pérez-Hernández, M. D., Hernández-Guerra, A., and Arístegui, J.: Longitudinal variability of size-fractionated N2 fixation and DON release rates along 24.5∘ N in the subtropical North Atlantic, J. Geophys. Res., 118, 3406–3415, https://doi.org/10.1002/jgrc.20253, 2013.
Bergman, B., Sandh, G., Lin, S., Larsson, J., and Carpenter, E. J.: Trichodesmium – a widespread marine cyanobacterium with unusual nitrogen fixation properties, FEMS Microbiol. Rev., 37, 286–302, https://doi.org/10.1111/j.1574-6976.2012.00352.x, 2013.
Berthelot, H., Bonnet, S., Camps, M., Grosso, O., and Moutin, T.: Assessment of the dinitrogen released as ammonium and dissolved organic nitrogen by unicellular and filamentous marine diazotrophic cyanobacteria grown in culture, Front. Mar. Sci., 2, 80, https://doi.org/10.3389/fmars.2015.00080, 2015.
Berthelot, H., Bonnet, S., Grosso, O., Cornet, V., and Barani, A.: Transfer of diazotroph-derived nitrogen towards non-diazotrophic planktonic communities: a comparative study between Trichodesmium erythraeum, Crocosphaera watsonii and Cyanothece sp., Biogeosciences, 13, 4005–4021, https://doi.org/10.5194/bg-13-4005-2016, 2016.
Bonnet, S., Berthelot, H., Turk-Kubo, K., Cornet-Barthaux, V., Fawcett, S., Berman-Frank, I., Barani, A., Grégori, G., Dekaezemacker, J., and Benavides, M.: Diazotroph derived nitrogen supports diatom growth in the South West Pacific: a quantitative study using nanoSIMS, Limnol. Oceanogr., 61, 1549–1562, https://doi.org/10.1002/lno.10300, 2016a.
Bonnet, S., Baklouti, M., Gimenez, A., Berthelot, H., and Berman-Frank, I.: Biogeochemical and biological impacts of diazotroph blooms in a low-nutrient, low-chlorophyll ecosystem: synthesis from the VAHINE mesocosm experiment (New Caledonia), Biogeosciences, 13, 4461–4479, https://doi.org/10.5194/bg-13-4461-2016, 2016b.
Bonnet, S., Berthelot, H., Turk-Kubo, K., Fawcett, S., Rahav, E., L'Helguen, S., and Berman-Frank, I.: Dynamics of N2 fixation and fate of diazotroph-derived nitrogen in a low-nutrient, low-chlorophyll ecosystem: results from the VAHINE mesocosm experiment (New Caledonia), Biogeosciences, 13, 2653–2673, https://doi.org/10.5194/bg-13-2653-2016, 2016c.
Böttjer, D., Dore, J. E., Karl, D. M., Letelier, R. M., Mahaffey, C., Wilson, S. T., Zehr, J., and Church, M. J.: Temporal variability of nitrogen fixation and particulate nitrogen export at Station ALOHA, Limnol. Oceanogr., 62, 200–216, https://doi.org/10.1002/lno.10386, 2016.
Breitbarth, E., Wohlers, J., Kläs, J., LaRoche, J., and Peeken, I.: Nitrogen fixation and growth rates of Trichodesmium IMS-101 as a function of light intensity, Mar. Ecol.-Prog. Ser, 359, 25–36, https://doi.org/10.3354/meps07241, 2008.
Capone, D. G., Ferrier, M. D., and Carpenter, E. J.: Amino acid cycling in colonies of the planktonic marine cyanobacterium Trichodesmium thiebautii, Appl. Environ. Microb., 60, 3989–3995, 1994.
Capone, D. G., Zehr, J. P., Paerl, H. W., Bergman, B., and Carpenter, E. J.: Trichodesmium, a globally significant marine cyanobacterium, Science, 276, 1221–1229, https://doi.org/10.1126/science.276.5316.1221, 1997.
Capone, D. G., Burns, J. A., Montoya, J. P., Subramaniam, A., Mahaffey, C., Gunderson, T., Michaels, A. F., and Carpenter, E. J.: Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean, Global BIogeochem. Cy., 19, GB2024, https://doi.org/10.1029/2004GB002331, 2005.
Carpenter, E. J. and Price, C. C.: Nitrogen Fixation, Distribution, and Production of Oscillatoria (Trichodesmium) Spp. in the Western Sargasso and Caribbean Seas, Limnol. Oceanogr., 22, 60–72, https://doi.org/10.4319/lo.1977.22.1.0060, 1977.
Carpenter, E. J. and Roenneberg, T.: The marine planktonic cyanobacteria Trichodesmium spp.: photosynthetic rate measurements in the SW Atlantic Ocean, Mar. Ecol.-Prog. Ser., 118, 267–273, https://doi.org/10.3354/meps118267, 1995.
Carpenter, E. J., ONeil, J. M., Dawson, R., Capone, D. G., Siddiqui, P. J. A., Roenneberg, T., and Bergman, B.: The tropical diazotrophic phytoplankter Trichodesmium: biological characteristics of two common species, Mar. Ecol.-Prog. Ser, 95, 295–304, https://doi.org/10.3354/meps095295, 1993.
Church, M. J., Jenkins, B. D., Karl, D. M., and Zehr, J. P.: Vertical distributions of nitrogen-fixing phylotypes at Stn ALOHA in the oligotrophic North Pacific Ocean, Aquat. Microb. Ecol., 38, 3–14, https://doi.org/10.3354/ame038003, 2005a.
Church, M. J., Short, C. M., Jenkins, B. D., Karl, D. M., and Zehr, J. P.: Temporal patterns of nitrogenase gene (nifH) expression in the oligotrophic North Pacific Ocean, Appl. Environ. Microb., 71, 5362–5370, https://doi.org/10.1128/AEM.71.9.5362-5370.2005, 2005b.
Dabundo, R., Lehmann, M. F., Treibergs, L., Tobias, C. R., Altabet, M. A., Moisander, P. H., and Granger, J.: The Contamination of Commercial 15N2 Gas Stocks with 15N–Labeled Nitrate and Ammonium and Consequences for Nitrogen Fixation Measurements, PloS one, 9, e110335, https://doi.org/10.1371/journal.pone.0110335, 2014.
Devassy, V. P., Bhattathiri, P. M. A., and Qasim, S. Z.: Trichodesmium phenomenon, Indian J. Mar. Sci., 7, 168–186, 1978.
Fennel, K., Spitz, Y. H., Letelier, R. M., Abbott, M. R., and Karl, D. M.: A deterministic model for N2 fixation at stn. ALOHA in the subtropical North Pacific Ocean, Deep-Sea Res. Pt. II, 49, 149–174, https://doi.org/10.1016/S0967-0645(01)00098-4, 2001.
Fernández, A., Graña, R., Mourino-Carballido, B., Bode, A., Varela, M., Domínguez-Yanes, J. F., Escánez, J., de Armas, D., and Marañón, E.: Community N2 fixation and Trichodesmium spp. abundance along longitudinal gradients in the eastern subtropical North Atlantic, ICES J. Mar. Sci., 70, 223–231, https://doi.org/10.1093/icesjms/fss142, 2013.
Finzi-Hart, J. A., Pett-Ridge, J., Weber, P. K., Popa, R., Fallon, S. J., Gunderson, T., Hutcheon, I. D., Nealson, K. H., and Capone, D. G.: Fixation and fate of C and N in the cyanobacterium Trichodesmium using nanometer-scale secondary ion mass spectrometry, P. Natl. Acad. Sci. USA, 106, 6345–6350, https://doi.org/10.1073/pnas.0810547106, 2009.
Gandhi, N., Singh, A., Prakash, S., Ramesh, R., Raman, M., Sheshshayee, M., and Shetye, S.: First direct measurements of N2 fixation during a Trichodesmium bloom in the eastern Arabian Sea, Global Biogeochem. Cy., 25, GB4014, https://doi.org/10.1029/2010GB003970, 2011.
Garside, C.: A chemiluminescent technique for the determination of nanomolar concentrations of nitrate and nitrite in seawater, Mar. Chem., 11, 159–167, https://doi.org/10.1016/0304-4203(82)90039-1, 1982.
Glibert, P. M. and Bronk, D. A.: Release of dissolved organic nitrogen by marine diazotrophic cyanobacteria, Trichodesmium spp., Appl. Environ. Microb., 60, 3996–4000, https://doi.org/10.3354/ame01621, 1994.
Hama, T., Miyazaki, T., Ogawa, Y., Iwakuma, T., Takahashi, M., Otsuki, A., and Ichimura, S.: Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope, Mar. Biol., 73, 31–36, https://doi.org/10.1007/BF00396282, 1983.
Hewson, I., Govil, S. R., Capone, D. G., Carpenter, E. J., and Fuhrman, J. A.: Evidence of Trichodesmium viral lysis and potential significance for biogeochemical cycling in the oligotrophic ocean, Aquat. Microb. Ecol., 36, 1–8, https://doi.org/10.3354/ame036001, 2004.
Holl, C. M., Villareal, T. A., Payne, C. D., Clayton, T. D., Hart, C., and Montoya, J. P.: Trichodesmium in the western Gulf of Mexico: 15N2-fixation and natural abundance stable isotopic evidence, Limnol. Oceanogr., 52, 2249–2259, https://doi.org/10.4319/lo.2007.52.5.2249, 2007.
Hood, R. R., Bates, N. R., Capone, D. G., and Olson, D. B.: Modeling the effect of nitrogen fixation on carbon and nitrogen fluxes at BATS, Deep-Sea Res. Pt. II, 48, 1609–1648, https://doi.org/10.1016/S0967-0645(00)00160-0, 2001.
Kana, T. M.: Rapid oxygen cycling in Trichodesmium thiebautii, Limnol. Oceanogr., 38, 18–24, https://doi.org/10.4319/lo.1993.38.1.0018, 1993.
Karl, D., Letelier, R., Tupas, L., Dore, J., Christian, J., and Hebel, D. V.: The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean, Nature, 388, 533–538, 1997.
Karl, D. M., Letelier, R., Hebel, D. V., Bird, D. F., and Winn, C. D.: Trichodesmium blooms and new nitrogen in the North Pacific Gyre, Kluwer Academic Publishers, Dordrecht, 1992.
Knapp, A. N., Sigman, D. M., and Lipschultz, F.: N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic Time-series Study site, Global Biogeochem. Cy., 19, GB1018, https://doi.org/10.1029/2004GB002320, 2005.
Knapp, A. N., Fawcett, S. E., Martínez-Garcia, A., Leblond, N., Moutin, T., and Bonnet, S.: Nitrogen isotopic evidence for a shift from nitrate- to diazotroph-fueled export production in the VAHINE mesocosm experiments, Biogeosciences, 13, 4645–4657, https://doi.org/10.5194/bg-13-4645-2016, 2016.
Konno, U., Tsunogai, U., Komatsu, D. D., Daita, S., Nakagawa, F., Tsuda, A., Matsui, T., Eum, Y.-J., and Suzuki, K.: Determination of total N2 fixation rates in the ocean taking into account both the particulate and filtrate fractions, Biogeosciences, 7, 2369–2377, https://doi.org/10.5194/bg-7-2369-2010, 2010.
Kranz, S. A., Eichner, M., and Rost, B.: Interactions between CCM and N2 fixation in Trichodesmium, Photosynth. Res., 109, 73–84, 2011.
Kumar, P. K., Singh, A., Ramesh, R., and Nallathambi, T.: N2 Fixation in the Eastern Arabian Sea: Probable Role of Heterotrophic Diazotrophs, Front. Mar. Sci., 4, 80, https://doi.org/10.3389/fmars.2017.00080, 2017.
LaRoche, J. and Breitbarth, E.: Importance of the diazotrophs as a source of new nitrogen in the ocean, J. Sea Res., 53, 67–91, https://doi.org/10.1016/j.seares.2004.05.005, 2005.
Lenes, J. M., Darrow, B. P., Cattrall, C., Heil, C. A., Callahan, M., Vargo, G. A., Byrne, R. H., Prospero, J. M., Bates, D. E., and Fanning, K. A.: Iron fertilization and the Trichodesmium response on the West Florida shelf, Limnol. Oceanogr., 46, 1261–1277, https://doi.org/10.4319/lo.2001.46.6.1261, 2001.
Lewis, M. R., Ulloa, O., and Platt, T.: Photosynthetic action, absorption, and quantum yield spectra for a natural population of Oscillatoria in the North Atlantic, Limnol. Oceanogr., 33, 92–98, https://doi.org/10.4319/lo.1988.33.1.0092, 1988.
Luo, Y.-W., Lima, I. D., Karl, D. M., Deutsch, C. A., and Doney, S. C.: Data-based assessment of environmental controls on global marine nitrogen fixation, Biogeosciences, 11, 691–708, https://doi.org/10.5194/bg-11-691-2014, 2014.
Ma, J., Yuan, D., and Liang, Y.: Sequential injection analysis of nanomolar soluble reactive phosphorus in seawater with HLB solid phase extraction, Mar. Chem., 111, 151–159, https://doi.org/10.1016/j.marchem.2008.04.011, 2008.
Martínez-Pérez, C., Mohr, W., Löscher, C. R., Dekaezemacker, J., Littmann, S., Yilmaz, P., Lehnen, N., Fuchs, B. M., Lavik, G., and Schmitz, R. A.: The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle, Nat. Microbiol., 1, 16163, https://doi.org/10.1038/NMICROBIOL.2016.163, 2016.
Massana, R., Murray, A. E., Preston, C. M., and DeLong, E. F.: Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel, Appl. Environ. Microb., 63, 50–56, 1997.
Mohr, W., Grosskopf, T., Wallace, D. W. R., and LaRoche, J.: Methodological underestimation of oceanic nitrogen fixation rates, PloS one, 5, e12583, https://doi.org/10.1371/journal.pone.0012583, 2010.
Montoya, J. P., Voss, M., Kahler, P., and Capone, D. G.: A Simple, High-Precision, High-Sensitivity Tracer Assay for N2 Fixation, Appl. Environ. Microb., 62, 986–993, 1996.
Mourino-Carballido, B., Graña, R., Fernández, A., Bode, A., Varela, M., Domínguez, J., Escánez, J., de Armas, D., and Marañón, E.: Importance of N2 fixation vs. nitrate eddy diffusion along a latitudinal transect in the Atlantic Ocean, Limnol. Oceanogr., 56, 999–1007, https://doi.org/10.4319/lo.2011.56.3.0999, 2011.
Mulholland, M. R.: The fate of nitrogen fixed by diazotrophs in the ocean, Biogeosciences, 4, 37–51, https://doi.org/10.5194/bg-4-37-2007, 2007.
Mulholland, M. R., Ohki, K., and Capone, D. G.: Nitrogen utilization and metabolism relative to patterns of N2 fixation in cultures of Trichodesmium NIBB1067, J. Phycol, 35, 977–988, https://doi.org/10.1046/j.1529-8817.1999.3550977.x, 1999.
Mulholland, M. R., Bronk, D. A., and Capone, D. G.: Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101, Aquat. Microb. Ecol., 37, 85–94, https://doi.org/10.3354/ame037085, 2004.
Mulholland, M. R., Bernhardt, P. W., Heil, C. A., Bronk, D. A., and O'Neil, J. M.: Nitrogen fixation and release of fixed nitrogen by Trichodesmium spp. in the Gulf of Mexico, Limnol. Oceanogr., 51, 1762–1776, https://doi.org/10.4319/lo.2006.51.4.1762, 2006.
O'Neil, J. M., Metzler, P. M., and Glibert, P. M.: Ingestion of 15N2-labelled Trichodesmium spp. and ammonium regeneration by the harpacticoid copepod Macrosetella gracilis, Mar. Biol., 125, 89–96, https://doi.org/10.1007/BF00350763, 1996.
Oliver, R. L., Hamilton, D. P., Brookes, J. D., and Ganf, G. G.: Physiology, blooms and prediction of planktonic cyanobacteria, in: Ecology of cyanobacteria II, Springer, the Netherlands, 155–194, 2012.
Rijkenberg, M. J., Langlois, R. J., Mills, M. M., Patey, M. D., Hill, P. G., Nielsdóttir, M. C., Compton, T. J., LaRoche, J., and Achterberg, E. P.: Environmental forcing of nitrogen fixation in the eastern tropical and sub-tropical North Atlantic Ocean, PLoS One, 6, e28989, https://doi.org/10.1371/journal.pone.0028989, 2011.
Shi, T., Sun, Y., and Falkowski, P. G.: Effects of iron limitation on the expression of metabolic genes in the marine cyanobacterium Trichodesmium erythraeum IMS101, Environ. Microbiol., 9, 2945–2956, https://doi.org/10.1111/j.1462-2920.2007.01406.x, 2007.
Shiozaki, T., Furuya, K., Kodama, T., Kitajima, S., Takeda, S., Takemura, T., and Kanda, J.: New estimation of N2 fixation in the western and central Pacific Ocean and its marginal seas, Global Biogeochem. Cy., 24, GB1015, https://doi.org/10.1029/2009GB003620, 2010.
Shiozaki, T., Nagata, T., Ijichi, M., and Furuya, K.: Nitrogen fixation and the diazotroph community in the temperate coastal region of the northwestern North Pacific, Biogeosciences, 12, 4751–4764, https://doi.org/10.5194/bg-12-4751-2015, 2015.
Sigman, D., Casciotti, K., Andreani, M., Barford, C., Galanter, M., and Böhlke, J.: A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater, Anal. Chem., 73, 4145–4153, https://doi.org/10.1021/ac010088e, 2001.
Villareal, T. A. and Carpenter, E. J.: Diel buoyancy regulation in the marine diazotrophic cyanobacterium Trichodesmium thiebautii, Limnol. Oceanogr., 35, 1832–1837, https://doi.org/10.4319/lo.1990.35.8.1832, 1990.
Wannicke, N., Koch, B. P., and Voss, M.: Release of fixed N2 and C as dissolved compounds by Trichodesmium erythreum and Nodularia spumigena under the influence of high light and high nutrient (P), Aquat. Microb. Ecol., 57, 175–189, https://doi.org/10.3354/ame01343, 2009.
Webb, W. L., Newton, M., and Starr, D.: Carbon dioxide exchange of Alnus rubra: a mathmatical model, Oecologia, 17, 281–291, https://doi.org/10.1007/BF00345747, 1974.
Welschmeyer, N. A.: Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments, Limnol. Oceanogr., 39, 1985–1992, https://doi.org/10.1007/BF02026767, 1994.
Zehr, J. P.: Nitrogen fixation by marine cyanobacteria, Trends Microbiol., 19, 162–173, https://doi.org/10.1016/j.tim.2010.12.004, 2011.
Zehr, J. P., Shilova, I. N., Farnelid, H. M., del Carmen Muñoz-MarínCarmen, M., and Turk-Kubo, K. A.: Unusual marine unicellular symbiosis with the nitrogen-fixing cyanobacterium UCYN-A, Nat. Microbiol., 2, 16214, https://doi.org/10.1038/nmicrobiol.2016.214, 2016.
Zhang, Y., Zhao, Z., Sun, J., and Jiao, N.: Diversity and distribution of diazotrophic communities in the South China Sea deep basin with mesoscale cyclonic eddy perturbations, FEMS Microbiol. Ecol., 78, 417–427, https://doi.org/10.1111/j.1574-6941.2011.01174.x, 2011.






