the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Deep-sea sponge grounds as nutrient sinks: denitrification is common in boreo-Arctic sponges
Christine Rooks
James Kar-Hei Fang
Pål Tore Mørkved
Rui Zhao
Hans Tore Rapp
Joana R. Xavier
Friederike Hoffmann
Sponges are commonly known as general nutrient providers for the marine ecosystem, recycling organic matter into various forms of bioavailable nutrients such as ammonium and nitrate. In this study we challenge this view. We show that nutrient removal through microbial denitrification is a common feature in six cold-water sponge species from boreal and Arctic sponge grounds. Denitrification rates were quantified by incubating sponge tissue sections with -amended oxygen-saturated seawater, mimicking conditions in pumping sponges, and de-oxygenated seawater, mimicking non-pumping sponges. It was not possible to detect any rates of anaerobic ammonium oxidation (anammox) using incubations with . Denitrification rates of the different sponge species ranged from below detection to 97 nmol N cm−3 sponge d−1 under oxic conditions, and from 24 to 279 nmol N cm−3 sponge d−1 under anoxic conditions.
A positive relationship between the highest potential rates of denitrification (in the absence of oxygen) and the species-specific abundances of nirS and nirK genes encoding nitrite reductase, a key enzyme for denitrification, suggests that the denitrifying community in these sponge species is active and prepared for denitrification. The lack of a lag phase in the linear accumulation of the 15N-labelled N2 gas in any of our tissue incubations is another indicator for an active community of denitrifiers in the investigated sponge species.
Low rates for coupled nitrification–denitrification indicate that also under oxic conditions, the nitrate used to fuel denitrification rates was derived rather from the ambient seawater than from sponge nitrification. The lack of nifH genes encoding nitrogenase, the key enzyme for nitrogen fixation, shows that the nitrogen cycle is not closed in the sponge grounds. The denitrified nitrogen, no matter its origin, is then no longer available as a nutrient for the marine ecosystem.
These results suggest a high potential denitrification capacity of deep-sea sponge grounds based on typical sponge biomass on boreal and Arctic sponge grounds, with areal denitrification rates of 0.6 mmol N m−2 d−1 assuming non-pumping sponges and still 0.3 mmol N m−2 d−1 assuming pumping sponges. This is well within the range of denitrification rates of continental shelf sediments. Anthropogenic impact and global change processes affecting the sponge redox state may thus lead to deep-sea sponge grounds changing their role in marine ecosystem from being mainly nutrient sources to becoming mainly nutrient sinks.
- Article
(1000 KB) - Full-text XML
-
Supplement
(173 KB) - BibTeX
- EndNote
Sponges are sessile filter feeders with an immense capacity to process large volumes of seawater (Kahn et al., 2015; Reiswig, 1974). As such, they play a critical role in benthic–pelagic coupling, recycling particulate or dissolved organic matter from the water column into various forms of bioavailable nutrients (Reiswig, 1974; Yahel et al., 2003; Hoffmann et al., 2009; Schläppy et al., 2010a; Maldonado et al., 2012; de Goeij et al., 2013; Rix et al., 2016). Sponges show active nitrogen metabolism, as recently reviewed by Feng and Li (2019), Pawlik and McMurray (2019), Zhang et al. (2019), and Folkers and Rombouts (2020). Actively pumping sponges have been associated with the release of dissolved inorganic nitrogen (DIN), enriching ex-current waters with excess ammonium () and/or nitrite and nitrate ( and/or , summarized as ; Southwell et al., 2008; Fiore et al., 2013; Keesing et al., 2013; Leys et al., 2018; Hoer et al., 2018). Whilst is excreted by sponge cells as a metabolic waste product (Yahel et al., 2003), is derived from the microbial oxidation of , through , to in aerobic nitrification (Painter, 1970; Corredor et al., 1988; Diaz and Ward, 1997; Jiménez and Ribes, 2007; Southwell et al., 2008; Fiore et al., 2010; Schläppy et al., 2010a; Radax et al., 2012;). Nitrogen fixation has also been reported in shallow-water sponges (Wilkinson and Fay, 1979; Wilkinson et al., 1999; Mohamed et al., 2008; Ribes et al., 2015), reducing biologically inaccessible N2 gas to , which represents yet another source of DIN from sponges. DIN release has been affiliated with a number of deep-sea and shallow-water sponges and varies according to species (Schläppy et al., 2010a; Radax et al., 2012; Keesing et al., 2013), as well as on temporal (Bayer et al., 2008; Radax et al., 2012) and spatial scales (Fiore et al., 2013; Archer et al., 2017). Such variations have been linked to abiotic conditions, as well as the availability of organic matter or nutrients in the water column (Bayer et al., 2008; Fiore et al., 2013; Archer et al., 2017).
In any case, since nitrification is dependent on oxygen, release is dependent on active filtration, delivering an excess of O2 to sponge tissues (Reiswig, 1974; Hoffmann et al., 2008; Southwell et al., 2008; Pfannkuchen et al., 2009; Fiore et al., 2013; Keesing et al., 2013; Leys et al., 2018). Fluctuations in pumping activity, however, disrupt the delivery of O2 to sponge tissues, resulting in either heterogeneous oxygenation within the sponge matrix or complete anoxia (Hoffmann et al., 2005, 2008; Schläppy et al., 2007, 2010b). Under such conditions, a paucity of oxygen would inevitably promote anaerobic microbial processes.
Anaerobic N transformations have been quantified using 15N tracer experiments in deep-sea (Hoffmann et al., 2009) and shallow-water sponges (Schläppy et al., 2010a; Fiore et al., 2013). In the deep-sea sponge Geodia barretti, the removal of fixed nitrogen via heterotrophic denitrification (the sequential and anaerobic reduction of , via , to N2) was shown to exceed sedimentary denitrification rates at equivalent depths by a factor of 2 to 10 (Hoffmann et al., 2009). Also, anaerobic ammonium oxidation (anammox, transforming and to N2) was quantified in that study, as well as nitrification performed simultaneously with denitrification (coupled nitrification–denitrification). Given that marine sediments are considered the major sites of marine N transformations (Seitzinger, 1988; Middelburg et al., 1996), sponges may thus represent a significant, yet largely overlooked sink for bioavailable nitrogen (Hoffmann et al., 2009).
The redox state of the sponge tissue as well as the rates of the different N transforming processes could thus determine whether the sponge may act as a nutrient source or a nutrient sink. It has been observed in field studies that sponges can act as both net sources or sinks for and (Fiore et al., 2013; Archer et al., 2017); however the balance of the underlying processes and their controlling factors have not as yet been quantified.
The understanding of such processes and their dynamics is particularly relevant for areas where sponges occur in high densities, forming highly structured habitats as is the case of the sponge grounds found widely distributed across the deeper areas of the oceans. In such areas, sponges can represent up to 95 % of the total invertebrate biomass (Murillo et al., 2012) and attain densities of up to 20 individuals m−2 (Hughes and Gage, 2004). In the North Atlantic boreo-Arctic region the widely distributed sponge grounds have traditionally been divided into two main types. The cold-water (Arctic) type, generally found along continental slopes and mid-ocean ridges at negative temperatures, or at least below 3–4 ∘C, and comprising a multi-specific assemblage of demosponges (the astrophorids Geodia parva, G. hentscheli, and Stelletta rhaphidiophora) and glass sponges (the hexactinellids Schaudinnia rosea, Trichasterina borealis, Scyphidium septentrionale, and Asconema foliata; Klitgaard and Tendal, 2004; Cardenas et al., 2013; Roberts et al., 2018) . The boreal type is mainly found along continental shelves and upper slopes and at temperatures above 4 ∘C. These grounds are dominated by the astrophorids Geodia barretti, G. atlantica, Stryphnus fortis, and Stelletta normani (Klitgaard and Tendal, 2004; Murillo et al., 2012; Cardenas et al., 2013). To make reliable estimates on the potential nitrogen sink function of these deep-sea sponge grounds, denitrification rates from more sponge ground species are needed.
In this study we quantify the potential nutrient sink function of six sponge species which characterize the two main types of boreo-Arctic tetractinellid sponge grounds. We aim to test our hypothesis that nutrient removal through microbial denitrification is a common feature in cold-water sponges, and that rates are dependent on oxygen availability in the sponge tissue. Based on these results we aim to estimate the potential nutrient sink function of boreo-Arctic sponge grounds for the marine ecosystem.
2.1 Site description
Arctic sponge species were collected at the Schulz Bank (73∘50′ N, 7∘34′ E). This is a large seamount located at the transition between the Mohn and the Knipovich ridges, two of the main sections of the Arctic Mid-Ocean Ridge (AMOR). The seamount rises from more than 2500 m depth and its summit and shallower areas (550–700 m depth) host a dense and diverse sponge ground composed of a multispecific assemblage of species dominated by tetractinellid demosponges (Geodia parva, G. hentscheli, and Stelletta rhaphidiophora) and hexactinellid sponges (Schaudinnia rosea, Trichasterina borealis, Scyphidium septentrionale, and Asconema foliata). The exact hydrodynamic settings at the summit are not known, but conditions measured using a benthic lander at 670 m (i.e. 70–80 m below the summit) revealed a water temperature just below 0 ∘C, salinity of 34.9, and dissolved oxygen between 12.4 and 12.6 mg L−1. Near-bed suspended particulate matter concentrations were determined to be 3.2 mg L−1, considerably larger than those observed both in surface and deeper waters (where values range from below 1 to 2 mg L−1; Roberts et al., 2018).
Boreal sponge species were collected on the hard-bottom slope of the Korsfjord (60∘09′12′′ N, 05∘08′52′′ E) near the city of Bergen, on the west coast of Norway. Hard-bottom slopes of these fjords, which can be several hundred metres in deep, host dense assemblies of typical boreal sponges, dominated by tetractinellid demosponges such as different species of the Geodiidae. Site characteristics are described elsewhere (Hoffmann et al., 2003); see Supplement figure for locations of sampling sites.
Average sponge biomass (kg m−2) in both Arctic and boreal grounds was estimated from trawl catches and underwater imagery collected in the course of various sampling campaigns.
2.2 Sample collection and preparation
Intact individuals from each of the key Arctic species, Geodia hentscheli (n=3), Geodia parva (n=3), and Stelletta rhaphidiophora (n=3), were retrieved from a depth of 700 m at the top of Schulz Bank. Sponges were collected with a remotely operated vehicle (ROV) on board the R/V GO Sars in June 2016
Intact individuals from each of the key boreal species, Geodia barretti (n=3), Geodia atlantica (n=3), and Stryphnus fortis (n=3), were collected from a depth of 200 m at the slope of Korsfjord, Norway. Sponge individuals were retrieved using a triangular dredge deployed from the R/V Hans Brattström in November 2016.
Upon retrieval, samples were immediately transferred into containers holding low-nutrient seawater, directly recovered from the sampling site. Following species identification, intact individuals were either transported to the aquaria at the University of Bergen (ca. 1 h; boreal species), or immediately to the lab on board the R/V GO Sars (Arctic species). Sponge tissue, from three intact individuals, was then dissected for use in either 15N-labelled tissue incubations or preserved for subsequent DNA extraction for each species.
Whilst completely immersed in site water, the massive sponge individuals were cut into three sections of approximately equal size to aid dissection. Using an autoclaved stainless steel core (internal diameter = 0.74 cm; length = 7 cm), the choanosomal portion of the sponge was sliced from each section to produce cylindrically shaped tissue samples. Three whole sponges (n=3) were collected for each species. The dissected tissue from a single sponge individual represented one replicate. Avoiding exposure to air, tissue samples were then transferred to 1 L containers holding site water. Using a sterile scalpel, the tissue cylinders were further sectioned (under water) into pieces of equal size (volume = 0.45 cm3). The samples were then either distributed into 12 mL gas-tight vials (Exetainer, Labco, High Wycombe, UK) for incubation with 15N isotopes, or into 1.5 mL microcentrifuge tubes, and then snap frozen and stored at −80 ∘C for subsequent DNA extraction.
Sediment was collected from the Arctic sponge grounds using a box corer. The upper few centimetres were sampled, homogenized and packed into 10 mL sterile cut-off syringes. One millilitre of sediment was then either distributed into 3 mL gas-tight vials (Exetainer, Labco, High Wycombe, UK) for 15N isotope incubations, or into 1.5 mL microcentrifuge tubes (Eppendorf), and then snap-frozen and stored at −80 ∘C for subsequent DNA extraction. At the boreal sponge ground, sponges were collected from the rocky slope of the fjord. It was therefore not possible to collect sediment from this site.
2.3 Quantifying rates of N-removal processes in sponge tissues and deep-sea sediments
2.3.1 Sponge tissue incubations
For simulating conditions in pumping and non-pumping sponges, sponge tissue sections were incubated with oxygen-saturated (standard temperature and pressure) and degassed site water (oxygen-free seawater, degassed with ultra-high-purity He). Site water was retrieved using 10 L Niskin flasks mounted on a CTD rosette water sampler aboard the R/V GO Sars. This water was collected at a depth of approximately 650 m, just above the summit of the seamount. It was then filtered to remove water column bacteria and or phytoplankton (0.2 µm polycarbonate filters, Whatman Nucleopore) and added to all incubations with Arctic specimens. Boreal specimens were incubated with sand-filtered seawater, pumped into the aquaria at the University of Bergen from a local fjord. This water was sourced from a depth of 130 m.
To ensure that all labelled N2 gas was retained, it was necessary to maintain gas-tight conditions in each of the incubations. Consequently, no oxygen could be added during the experiment. Estimating from typical respiration rates of 0.32 µmol O2 mL sponge−1 h−1 in G. barretti (Leys et al., 2018), this would suggest the complete removal of oxygen (by sponge cells and associated microbes) following 26 h of incubation (12 mL Exetainer vials, 0.45 cm3 sponge pieces, 313 µmol L−1 oxygen concentration at experiment start). This means that oxygen concentrations in the aerobic incubation continuously decreased from oxygen saturation to zero throughout the course of the experiment, thus mimicking conditions where a sponge has recently ceased pumping, or where pumping occurs at a low rate (Hoffmann et al., 2008; Schläppy et al., 2010b; Fang et al., 2018). Nevertheless, we can assume that oxygen was available during the first 26 h of incubation in the oxic experiment, in contrast to the anoxic experiment where oxygen was absent from the beginning of the incubation, thus mimicking non-pumping conditions (Hoffmann et al., 2008; Schläppy et al., 2010b).
For the oxic incubations, 12 mL of air-saturated (standard temperature and pressure) seawater was transferred into 12 mL gas-tight vials. Using autoclaved forceps, one piece of freshly dissected tissue was then placed into each gas-tight vial until a sufficient number of samples were prepared for the incubations. The caps were then replaced and the vial was carefully sealed to exclude any air bubbles.
For the anoxic incubations, 2 L of site water was degassed with ultra-high-purity He for 2 h. To verify the absence of oxygen in the degassed water, an anaerobic strip test (colour change from pink to white under anaerobic conditions; Sigma Aldrich) was performed prior to transfer into 12 mL Exetainer vials. The caps were then replaced and the gas-tight vials were carefully sealed to exclude any air bubbles. An anaerobic strip was added to control Exetainer vials (seawater only) in order to verify the absence of oxygen during anaerobic incubations.
Incubations were prepared in four sets of one unamended reference (no isotope added) and five amended (15N-labelled) samples per intact sponge (three intact sponge individuals/species). Each set was then either injected (gas-tight luer lock syringes, VICI, USA) with air-saturated (at standard temperature and pressure, for oxic incubations) or oxygen-free (degassed; for anoxic incubations) concentrated stock solutions of (i) Na (99.2 15N atm %), screening for denitrification; or (ii) Cl− (≥98 15N atm %) and , screening for anammox. The solutions were shaken vigorously. The final concentrations of (i) ; or (ii) ; were 100 µM and 10 µM respectively. These values were essentially 10 times greater than ambient (10 µM ) and concentrations (< 1 µM ) present in the seawater. Prior to the incubations, however, background nutrient concentrations were unknown. In this regard, to ensure that the availability of 15N was sufficient for the measurement of denitrification and or anammox (e.g. at least 50 % above the ambient pool of 14N), we selected high concentrations of stock solutions (Holtappels et al., 2011). To enable continuous homogenization of the isotopic label with sponge tissue, Exetainer vials were placed on rollers (Spiromix, Denley) and incubated at (6 ∘C) in the dark. At zero hours, and at subsequent 3–6 h intervals, a selection of samples were injected with 2 mL of ultra-high-purity helium to create an oxygen-free headspace using a gas-tight syringe. The vials were then injected with 200 µL of formaldehyde, and shaken vigorously to inhibit further microbial activity. This was repeated over a period of 48 h.
2.3.2 Sediment slurry incubations
One millilitre of the homogenized sediment was distributed into 3 mL gas-tight vials (Exetainer, Labco, High Wycombe, UK) with 1 mL of degassed site water (as above). The cap was replaced, the headspace (1 mL) flushed with ultra-high purity helium, and each vial was shaken vigorously to produce an anaerobic sediment slurry. Anaerobic slurries were prepared as two sets of un-amended references (no isotopic mixture added) and five amended samples in incubations, screening for either anammox and or denitrification. Amended samples were injected with oxygen-free isotopic mixtures (as above) and placed on rotating rollers (Spiromix, Denley) in a constant-temperature room (6 ∘C) in the dark. At zero hours, and every subsequent 3–6 h, 3 parallel samples were injected with 200 µL of formaldehyde, and shaken vigorously to inhibit further microbial activity. This was repeated over a period of 48 h. Concentrations of 28N2, 29N2, and 30N2 were measured as above and calculations for denitrification and or anammox were performed as per Thamdrup and Dalsgaard (2002) and Risgaard-Petersen et al. (2003).
2.3.3 Calculation of denitrification and anammox rates
Concentrations of 28N2, 29N2, and 30N2 were measured by directly sub-sampling 70 µL from the gas headspace on a gas chromatograph (Trace GC, Thermo Fisher Scientific, Bremen) connected to a continuous-flow isotope ratio mass spectrometer (Delta V plus, Thermo Fisher Scientific, Bremen) calibrated with in-house reference gas and air. Though we never observed visual signs of tissue degradation (see for example Osinga et al., 1999, 2001; and Hoffmann et al., 2003 for a description of how to spot signs of sponge tissue degradation), some samples showed an abrupt increase in N2 production, indicating the onset of tissue degradation. These were not included in the analyses and rate calculations. Calculations for rates of both anammox and denitrification were based on established methods for measuring these processes in sediments (Thamdrup and Dalsgaard, 2002; Risgaard-Petersen et al., 2003). Rates were calculated from the linear increase in excess N2 accumulation over time as measured from the isotope ratio mass spectrometer.
The accumulation of excess 29N2 and 30N2, from incubations with, was linear over a 24 h period (p<0.05) and precluded an initial lag phase (Fig. 1a and b). This was the case for all species. In the oxic incubations, after 24 h a sharp non-linear increase in labelled N2 was detected. This is in good agreement with our calculations for oxygen depletion (26 h, see above). Since we observed no signs of tissue degradation during the 48 h of incubation, this increase was taken to indicate a switch of metabolic processes within the sponge towards predominantly anaerobic pathways, and thus, a different denitrification rate. For the anoxic incubations, N2 production was also linear during the first 24 h of incubations, although the data were more scattered when compared with oxic incubations. The scatter increased after 24 h, though most incubations still followed a similar linear trend.
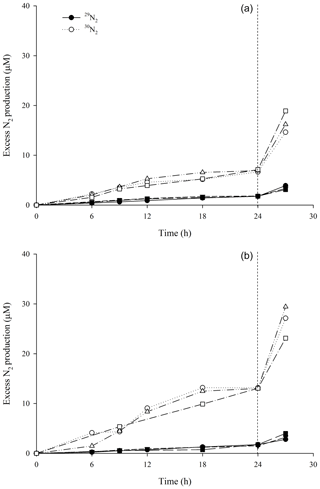
Figure 1Production of 29N2 (filled symbols) and 30N2 (open symbols) as a function of time after the addition of in incubations with (a) air-saturated (simulating pumping conditions) and (b) degassed site water (simulating non-pumping conditions) with tissue from Geodia barretti (n=3 individuals). Data associated with an individual sponge is represented by a set of symbols. Linear regressions of N2 production within the first 24 h of the experiments were used to calculate denitrification rates.
For best comparability of denitrification rates from oxic and anoxic incubations, only the first 24 h, where N2 production was linear in all experiments, and where oxygen was assumed to be present in the Exetainer vials of the oxic incubation, were used to calculate denitrification rates.
No 29N2 production was detected following labelling with and , suggesting an absence of anammox activity. Therefore, no anammox rates could be calculated. The N2 produced during the experiments is assumed to originate entirely from denitrification.
2.3.4 Calculation of coupled nitrification–denitrification and the denitrification of derived from ambient seawater
To determine the predominant source of which fuels denitrification, rates of coupled nitrification–denitrification and the denitrification of supplied by the ambient seawater were calculated according to the methods of Nielsen (1992). Production of can occur endogenously via the aerobic oxidation of to within the sponge tissues. In turn, this represents a source of for denitrification which “couples” nitrification to denitrification. Alternatively, denitrification can simply be fuelled by diffusing from the ambient seawater. By taking into consideration the frequency of 14 and availability, in addition to random isotope pairing, it is possible to calculate the source of denitrified from the abundance of 28,29 and 30N2 in all oxic incubations.
Denitrification rates were calculated from the production of 15N isotopes (see below) according to the method described by Nielsen (1992).
The rate of denitrification was measured from 15N isotope production (Eqs. 1 and 2). D15 and D14 represent denitrification of labelled 15 and 14 respectively. p(14N15N) and p(15N15N) are the production rates of the two labelled N2 species 14N15N and 15N15N (Rysgaard et al., 1995). Essentially, D15 is indicative of denitrification of labelled and D14 represents in situ denitrification of 14.
To estimate denitrification of from the ambient water (Dw), in terms of D14, the following calculation was applied (Eq. 3):
where []w and []w represent the concentration of unlabelled and labelled in the ambient water. Dw thus represents an estimate of denitrification of at ambient concentrations (approximately 10 µM), and in the rest of the publication we refer to this as denitrification rates unless otherwise stated.
In situ coupled denitrification (Dn), in terms of D14, was calculated using Eq. (4) (see below).
2.4 Screening and quantifying the abundance of nirS, nirK, and nifH genes
Total DNA was extracted from dissected sponge pieces (0.45 cm3 of sponge tissue) using a FastDNA Spin Kit for Soil (mpbio, Santa Ana, CA, USA) following the manufacturer's instructions. In total, DNA was extracted from three tissue samples retrieved from each of the intact sponges (three intact individuals sampled/key species) as well sediment samples (1 mL, ∼2 g sediment slurry) and sample blanks (RNAse-free water). DNA extracts were eluted into 100 µL of PCR-grade double-distilled H2O and stored at −20 ∘C until further analysis.
The functional genes diagnostic of nitrogen fixation (nifH encoding nitrogenase) and denitrification (nirS/K encoding nitrite reductase) in sponges were screened using conventional PCR of 40 cycles. nifH gene was amplified using the primer pair nifHfw/nifHrv (Mehta et al., 2003) with the following thermal conditions: 94 ∘C for 15 min, and 40 cycles of 94 ∘C for 30 s, 55 ∘C for 30 s, and 72 ∘C for 60 s. nirS/K genes were amplified using the primers and thermal conditions as described below. Each reaction mixture (25 µL total volume) contained the following: one HotStar Taq[U+F8E8] Master Mix (Qiagen, Hilden, Germany), 1.2 µM of each primer, and 1 µL template DNA. PCR products were evaluated by visual inspection of 1 % agarose gels.
The abundance of nirS or nirK genes of denitrifying bacteria were quantified using quantitative PCR (qPCR) on a StepOne Real-Time PCR system (Applied Biosystems). nirS genes were amplified using the primer pair nirS_cd3aF/nirS_R3cd (Throback et al., 2004), with thermal conditions as follows: 95 ∘C for 15 min, 45 cycles of denaturing at 95 ∘C for 15 s, annealing at 51 ∘C for 30 s, and elongation at 72 ∘C for 45 s. The nirK gene was amplified using the primer pair nirK_F1aCu/nirK_R3Cu, with the following thermal conditions: 95 ∘C for 15 min, 45 cycles of denaturing at 95 ∘C for 30 s, annealing at 51 ∘C for 45 s, and elongation at 72 ∘C for 45 s. All qPCR reactions were run in triplicate and each reaction mixture contained one QuantiTech SybrGreen PCR master mixture (QIAgen, Germany), 0.5 µM forward and reverse primer, and 1 µL of DNA template in a final volume of 20 µL. The qPCR standard of each gene was linear DNA, containing the respective genes from an uncultured denitrifying bacterium in an Arctic permafrost soil. For each gene, the DNA concentration of the standard was measured using Bioanalyzer (DNA 1000 chips, Agilent Technologies) and a DNA abundance gradient of 10–105 copies µL−1 were prepared via serial dilution 10 times.
2.5 Statistical analyses
Statistical analyses were performed to test for significant differences in (i) species-specific rates of denitrification or (ii) variations in the rates of denitrification according to oxygen availability. The data set failed to meet the assumptions of normality or equal variance. As a result, the data set was transformed by rank prior to two-way ANOVA. All pairwise multiple comparisons were performed using the Holm–Sidak method at species level. In all cases, the level of significance was set to at least p<0.05. Statistical analyses were performed using the software SigmaPlot 13.0 (Systat Software, CA, USA).
3.1 Denitrification activity in sponge tissues
The lack of 29N2 production following labelling with as observed in our study suggests an absence of anammox, since N2 production via anammox requires 1 N from (which is not labelled) and 1 N from (which is 15N labelled). Therefore, no anammox rates could be calculated and the labelled N2 produced during the 15 incubations is assumed to originate entirely from denitrification. Denitrification rates at ambient concentrations as calculated from this linear N2 release (Eqs. 1–4) were quantified in all six sponge species and are shown in Fig. 2. Mean rates of denitrification varied significantly between species (two-way ANOVA, , p<0.01) and in the presence or absence of dissolved oxygen (two-way ANOVA, , p<0.01). A significant interaction between species and the availability of dissolved oxygen was also identified using two-way ANOVA (, p=0.037). Mean rates of denitrification were always greater in incubations with degassed seawater relative to incubations with fully air-saturated seawater (Fig. 2). Under oxic conditions, mean rates varied from below detection in Stryphnus fortis to a maximum of 96 nmol N cm−3 sponge d−1 in Geodia barretti. However, under anoxic conditions, rates of denitrification ranged from 24 nmol N cm−3 sponge d−1 in Geodia atlantica to 280 nmol N cm−3 sponge d−1 in Geodia parva (Fig. 2). Differences in the rates of denitrification under either aerobic or anaerobic conditions were significant in Stryphnus fortis (t=6.591, p<0.05), Geodia barretti (t=2.197, p<0.05), Geodia hentscheli (t=4.577, p<0.05) Geodia parva (t=8.788, p<0.05), and Stelletta rhaphidiophora (t=6.408, p<0.05). Notably, the Arctic sponge ground species G. hentscheli and G. parva showed the highest anaerobic denitrification rates, with the boreal species G. barretti only slightly below.
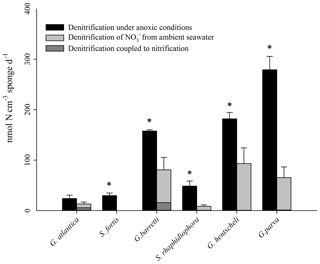
Figure 2Sponge species-specific rates of denitrification in incubations with degassed site water (anoxic conditions, black bars) and air-saturated site water (oxic conditions, grey bars) for six key species from boreal and arctic sponge grounds. Statistically significant differences between denitrification rates in the presence and absence of dissolved oxygen are indicated by an asterisk for each species. Error bars indicate SE (n=3 individuals). Coupled nitrification–denitrification under oxic conditions is visualized with dark grey colour in the grey bars. Compare with Table 1.
No labelled N2 production was detected in the surface sediment slurry screening for denitrification or anammox.
3.2 Coupled nitrification–denitrification and the absence of nitrogen fixation
In incubations with air-saturated seawater, denitrifying activity was detected in all sponges with the exception of Stryphnus fortis (Fig. 2). The rates for coupled nitrification–denitrification (Dn, Eq. 4) were generally low, with 16 % for G. barretti and 30 % for G. atlantica as the highest values (Table 1). This shows that seawater nitrate was the predominant source of nitrate for denitrification also under oxic conditions.
Table 1Nitrate sources for denitrification in the presence of dissolved oxygen. Most nitrate removed by sponge denitrification in oxic incubations originates from seawater, while some originates from sponge nitrification (coupled nitrification–denitrification). Denitrification rates in anoxic incubations (no coupled nitrification–denitrification) are also shown. Data are also presented in Fig. 2.
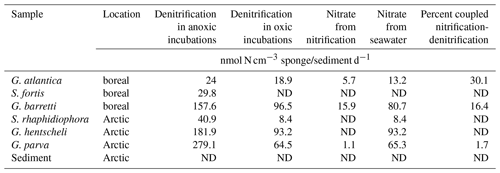
ND: not detectable.
Functional genes for nitrogen fixation were not detected in any of the six sponge species, pointing towards the absence of nitrogen-fixing microorganisms in these species.
3.3 Correlation between denitrification rates and the abundance of nitrite reductase
Copies of the nitrite reductase genes, nirS and nirK, were detected in all six sponges, though in different quantities (Table 2). The total nitrite reductase copy number (the sum of mean nirS and nirK gene copies cm−3 sponge tissue) ranged from 2.19 × 103 copies cm−3 sponge in Stryphnus fortis to 1.03 × 109 copies cm−3 sponge in Geodia parva (Table 2). Although no denitrification activity was detected in the sediment slurry incubations, nitrite reductase was present at an abundance of 2.77 × 104 copies cm−3 sediment.
Table 2Abundance of the nitrite reductase genes nirS and nirK in sponge and sediment samples. The nitrite reductase copy number is the sum of the mean number of nirS and nirK copies cm−3 of sponge tissue (n=3).
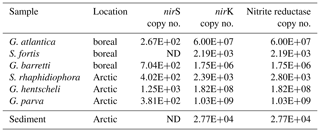
ND: not detectable.
We observed a positive relationship between denitrification rates under anoxic conditions and total nir copy number, for all species except G. atlantica, the species with the lowest denitrification rate. No correlation to nir copy number was detected for denitrification rates under oxic conditions (Fig. 3).
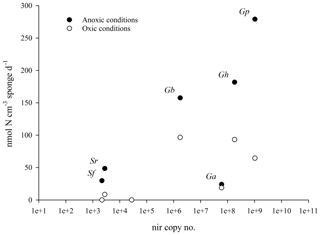
Figure 3Mean species-specific denitrification rates in incubations with air-saturated site water (with O2, open circles) and degassed site water (without O2, closed circles) as a function of nitrite reductase copy number. The nitrite reductase gene copy number is the sum of the mean number of nirS and nirK copies cm−3 of sponge tissue (n=3). There is a positive relationship between denitrification rates (in the absence of oxygen) and the species-specific abundance of nirS and nirK for five of the six sponge species.
4.1 Denitrification as a common feature of cold-water sponges
The purpose of this study was to quantify the potential nutrient sink function of six key sponge species from boreal and Arctic sponge grounds. We aimed to test our hypotheses that (1) nutrient removal through microbial denitrification is a common feature in cold-water sponge species, and that (2) rates are dependent on oxygen availability in the sponge tissue.
All six species investigated in this study showed denitrification rates under anoxic conditions, five of them even under oxic conditions. Rates were always higher in the absence of oxygen compared to in the presence of oxygen. All our denitrification rates are within the same range as rates previously reported for cold- and warm-water sponges: Hoffmann et al. (2009) reported 92 nmol N cm−3 sponge d−1 for explants of G. barretti incubated under oxic conditions, which is very close to our average rate of 97 nmol N cm−3 sponge d−1 for G. barretti sections incubated under oxic conditions. Rates reported by Schläppy et al. (2010a) for two Mediterranean shallow water sponges Chondrosia reniformis and Dysidea avara, also measured from tissue sections incubated under oxic conditions, were 240 and 357 nmol N cm−3 sponge d−1 respectively – well above our maximum rates measured under oxic conditions, but close to our maximum rates measured under anoxic conditions. Higher metabolic rates in warm- and shallow-water sponges compared to cold- and deep-water sponges is not surprising. In addition to these rather few direct quantifications of denitrification rates in sponges, the presence of denitrification activity has been indicated by isotopic tracer experiments in a tropical sponge (Fiore et al., 2013), as well as by numerous reports on the presence of functional genes for denitrification in sponge microbes or by demonstrating the ability to undertake denitrification in sponge-derived microbial isolates from a variety of marine habitats (Fiore et al., 2010, 2015; Liu et al., 2012, 2016; Webster and Taylor, 2012; Han et al., 2013; Zhang et al., 2013; Bayer et al., 2014; Li et al., 2014; Cleary et al., 2015; Zhuang et al., 2018).
We could not detect any anammox rates in any of the sponges investigated in this study. The only literature report for anammox rates quantified in a sponge was a very low rate of 3 nmol cm−3 sponge d−1 in explants of G. barretti (Hoffmann et al., 2009). In the present study, we could not reproduce these rates in the tissue sections of G. barretti nor detect the functional genes associated with this process. There are no other quantifications of anammox rates in sponges and only a few studies on the presence of anammox bacteria and genes in some sponge species (Mohamed et al., 2010; Han et al., 2012; Webster and Taylor, 2012).
Our study further clearly shows that denitrification rates are generally higher under anoxic conditions. As denitrification is an anaerobic process, this is not surprising. More surprising is our detection of considerable denitrification rates (up to 96 nmol N cm−3 sponge d−1) when sponge tissue sections were incubated in oxygenated seawater. Furthermore, evidence for coupled nitrification–denitrification proves that both aerobic and anaerobic processes can happen in the sponge sections at the same time. Oxygen was assumed to be present in the experimental vial at least during the first 26 h of the experiment, though continuously decreasing due to sponge respiration (see calculation in method section), because we did not have control over oxygen concentration in the sponge tissue pieces during the experiment. From marine sediments, there are numerous studies reporting denitrification in bulk oxic conditions, either in anoxic microniches or under complete oxygenated conditions (e.g. Wilson, 1978; Robertson et al., 1995; Chen and Strous, 2013; Marchant et al., 2017). For the present study, we do not know if denitrification actually happened in the presence of oxygen in anoxic microniches, which were already present in the sponge tissue at the experiment's start, or in tissue sections which rapidly become anoxic when not continuously flushed with oxygen. Nevertheless since all these scenarios reflect the situation in a sponge which is pumping at a low rate or occasionally stops pumping (Schläppy et al., 2007, 210b; Hoffmann et al., 2008), typical features in sponges, we assume that our results are representative for sponges under normal conditions.
Our study further indicates significant differences in anaerobic denitrification rates between most sponge species, indicating species-specific differences in maximum potential denitrification rates. Two of the Arctic sponges (G. hentscheli and G. parva) showed the highest denitrification rates. It is worth noting that for technical reasons the Arctic incubations had to be performed at a higher temperature (6 ∘C) compared to actual in situ conditions (0 ∘C), which may have led to an overestimation of the potential rates for the Arctic species.
Our systematic screening of six cold-water sponge species, together with reports of denitrification activity from other sponge species from around the world and from different habitats (see above) strengthens the view that denitrification is a common feature in many sponge species – both under oxygenated (pumping) and deoxygenated (non-pumping) tissue conditions, with rates being highest when oxygen is absent. Anammox in contrast seems to be a rarer and occasional feature in sponges, which might not have quantitative importance for sponge-mediated nitrogen cycling.
4.2 The fate of nitrogen in sponges
With the exception of Stryphnus fortis, denitrification was verified in the presence of dissolved oxygen across all species. For most species, denitrification was partly coupled to nitrification. For G. barretti, 16 % of the nitrate used for denitrification under oxic conditions was derived from nitrification, which is very close to previously reported values of 26 % as reported for the same species (Hoffmann et al., 2009). Evidence for coupled nitrification–denitrification in most sponge species of this study indicates that nitrification was present in these species. Nitrification rates have been quantified in the cold-water species Phakellia ventilabrum, Antho dichotoma, Geodia barretti, and Stryphnus fortis (120–1880 nmol N cm−3 sponge d−1; Hoffmann et al., 2009; Radax et al., 2012; Fang et al., 2018), and we may assume similar rates for the species in this study. Since the ammonium concentration in bottom seawater at our sampling sites is far too low (under the detection limit of 1 µM ) to fuel these nitrification rates, ammonium needs to originate from organic nitrogen remineralized from organic matter by the sponge cells or by heterotrophic sponge microbes. Under anoxic conditions, there is no nitrification, and the nitrate used to fuel the much higher denitrification rates has to be retrieved directly from the seawater. We did not detect any genes for nitrogen fixation; the N cycle is not closed in the cold-water sponges. The denitrified nitrogen, no matter its origin, is no longer available as a nutrient and thus is inevitably lost as a good and service for the marine ecosystem.
4.3 The sponge microbial community is ready for denitrification
NirS and nirK are functionally equivalent genes that code for the reduction of nitrite to nitric oxide, the first step towards the production of a gas in denitrification (Shapleigh, 2013). Copies of nirS and nirK were quantified in all six sponge species, and also in the sediment (Table 2). Scattering denitrification rates against nitrite reductase copy numbers revealed a clear positive relationship between denitrification rates (in the absence of oxygen) and the species-specific abundance of nirS and nirK (Fig. 3) for five of the six sponge species. This relationship suggests that there is an active denitrifying community present in these species.
This is further corroborated by our observation of a linear accumulation of 15N-labelled N2 gas at the start of our 15N incubation experiments, as shown in Fig. 1. The lack of a lag phase is frequently associated with “active” denitrification (Ward et al., 2009; Bulow et al., 2010). Conversely, denitrifiers in pure culture require a 24–48 h reactivation period to recover from dormancy (Baumann et al., 1996, 1997). There was no lag phase in any of our sponge tissue incubations, which strengthens our conclusion that the denitrifying community is active and prepared for the denitrification rates observed in our experiments. This again means that the measured maximum denitrification rates are likely to occur in situ in situations where the sponge tissue becomes completely anoxic. This also suggests that the heterotrophic microflora in these sponges regularly find themselves in an anoxic or microoxic environment where it is beneficial to have the denitrification genes readily expressed.
In the slurries of surface sediments from the Schulz Massive, nirK and nirS copy numbers were comparable to those in the sponges (Table 2); however, in these samples we did not detect any labelled N2 production within 48h of incubation. This would suggest that although a microbial community capable of denitrification is present in the surface sediments of the Schulz Bank, its activity was under the detection limit. Low availability of reactive carbon in these Arctic sediments (Baumberger et al., 2016) may be the reason for this lack of detectable denitrification activity, in contrast to a high availability of reactive carbon within a living sponge. Our results indicate that in the Arctic deep sea, sponge grounds play a much more important role for nitrogen cycling and benthic–pelagic coupling than the surrounding sediment.
4.4 Sponge grounds as nutrient sinks
Denitrification rates in this study were quantified in lab experiments, and therefore show potential rates of these species under certain conditions, not real rates under current in situ conditions. Keeping this in mind, and also considering that denitrification rates are calculated to represent that of the ambient , no carbon source was added, and the incubation temperature was realistic for natural conditions, our results allow estimates of the potential denitrification capacity of sponge grounds. Our results reveal average nitrogen removal rates for boreal sponge grounds of 70 nmol N cm−3 sponge d−1 assuming all sponges are not pumping (results from the anoxic experiment), and 38 when all sponges are pumping (results from the oxic experiment). For Arctic sponge grounds the rates will be 167 and 55 for non-pumping and pumping sponges respectively. Based on our own observations from trawl catches and underwater imagery from several cruises, we estimate that masses of 10 kg m−2 are common in boreal sponge grounds, while smaller areas both in shelves and fjords may even reach densities of 30 kg m−2. In other areas masses can be considerably lower and more patchy, e.g. 3.5 kg in the Traena area, as reported by Kutti et al. (2013). In the Arctic sponge grounds investigated in this study we estimate the sponge biomass to be approximately 4 kg m−2.
These estimates reveal a potential areal denitrification rate for the boreal sponge grounds of up to 0.587 mmol N m−2 assuming non-pumping and still show 0.321 mmol N m−2 assuming pumping sponges. For Arctic sponge grounds the numbers are quite similar (sponge biomass is lower but sponge denitrification rates are higher): 0.608 for non-pumping and 0.201 for pumping sponges. These rates are well within the range – or, for the non-pumping situation with anoxic tissue, on the upper end – of denitrification rates from continental shelf sediments, which are 0.1–1 mmol N m−2 d−1 (Middelburg et al., 1996; Seitzinger and Giblin, 1996). For the densest boreal sponge grounds, with sponge densities up to 30 kg m−2, rates are up to 1.7 mmol N m−2 d−1, well above typical rates for continental shelf sediments.
While our denitrification rates in sponges incubated under oxic conditions may reflect normal in situ conditions for pumping sponges, our numbers on denitrification rates in sponges incubated under anoxic conditions are theoretical extremes, since we know little about the in situ pumping patterns of deep-sea sponges or the environmental factors influencing them. Seawater nitrate, which fuels most of the denitrification under anoxic conditions, enters the sponge through pumping. The maximum denitrification rates in non-pumping sponges can therefore only be maintained until the nitrate in the sponge pore water is used up. The length and frequency of these anoxic spells will thus determine the variability of in situ sponge denitrification rates. Observations by Schläppy et al. (2010b) showed non-pumping periods of sponges in situ of up to 2 h, leading to complete tissue anoxia, followed by several hours of high pumping activity. Sponges with dense tissue and high loads of associated microbes (high-microbial abundance, HMA, sponges, such as most sponges in our study) generally show slower volume pumping rates than sponges with low microbial numbers and loose tissue structure (Weisz et al., 2008). Slow pumping rates lead to reduced and heterogeneous oxygen concentrations in sponges (e.g. Schläppy et al., 2007, 2010b), while they still may supply sufficient nitrate from ambient seawater to fuel denitrification. Even though our calculated areal denitrification rates of sponge grounds so far only point out potential capacity, our study clearly shows that both boreal and Arctic sponge grounds can function as efficient nutrient sinks, especially when they reduce or stop pumping and the tissue becomes anoxic. Environmental and anthropogenic stressors such as increased sediment loads (Bell et al., 2015) reduce pumping activity and increase anoxic conditions in sponges (Kutti et al., 2015; Tjensvoll et al., 2013; Fang et al., 2018), and thus stimulate nutrient removal through denitrification. Elevated ambient nitrate concentrations have been linked to increased nitrate removal by sponges (Archer et al., 2017). Global change processes affecting sponge redox state will impact the sponge holobiont (Pita et al., 2018), and may thus lead to deep-sea sponge grounds changing their role in marine ecosystems from functioning mainly as nutrient sources to functioning mainly as nutrient sinks.
In this study we have shown that several sponge species actively remove the bioavailable nutrients ammonium and nitrate from the marine ecosystem by denitrification and coupled nitrification–denitrification, which challenges the common view of sponges as DIN providers through mineralization of organic matter and nitrification. While variations in sponge remineralization activity only postpone the delivery of nutrients, denitrification inevitably removes these nutrients from the marine ecosystem. The nitrogen cycle is not closed in the sponge grounds; denitrified nitrogen, no matter its origin, is no longer available as a nutrient and is efficiently removed from the marine ecosystem. We further showed that the investigated sponges host an active community of denitrifiers which show the highest denitrification rates under anoxic conditions. Anthropogenic impacts and global change processes affecting the sponge redox state may thus lead to deep-sea sponge grounds changing their role in marine ecosystems from functioning mainly as nutrient sources to functioning mainly as nutrient sinks.
The data are available in the data publisher PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.899821 (Rooks et al., 2019).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-17-1231-2020-supplement.
FH and CR designed the study. CR and JKHF performed the sponge experiments. CR and PTM performed the stable isotope analyses. CR and RZ quantified the functional genes. CR analysed all the data. HTR organized the cruises, quantified sponge biomass at key sites, and determined the sponge species. CR wrote the first draft of the manuscript, and all authors contributed substantially to writing and revision. FH supervised and coordinated the writing process, and finalized the manuscript.
The authors declare that they have no conflict of interest.
This study was performed in the scope of the SponGES project, which received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 679849. This document reflects only the authors' views and the Executive Agency for Small and Medium-sized Enterprises (EASME) is not responsible for any use that may be made of the information it contains.
This research has been supported by the European Union Horizon 2020 programme (grant no. 679849), the Norwegian Research Council (grant no. 225283), and the Norwegian National Research Infrastructure (grant no. 245907). Joana R. Xavier's research is further supported by the strategic funding (grant no. UID/Multi/04423/2019) provided by the Portuguese Foundation for Science and Technology (FCT) to CIIMAR.
This paper was edited by Jack Middelburg and reviewed by three anonymous referees.
Archer, S. K., Stevens, J. L., Rossi, R. E., Matterson, K. O., and Layman, C. A.: Abiotic conditions drive significant variability in nutrient processing by a common Caribbean sponge, Ircinia felix, Limnol. Oceanogr., 62, 1783–1793, https://doi.org/10.1002/lno.10533, 2017.
Baumann, B., Snozzi, M., Zehnder, A. J., and Van Der Meer, J. R.: Dynamics of denitrification activity of Paracoccus denitrificans in continuous culture during aerobic-anaerobic changes, J. Bacteriol., 178, 4367–4374, https://doi.org/10.1128/jb.178.15.4367-4374.1996, 1996.
Baumann, B., Snozzi, M., Van Der Meer, J. R., and Zehnder, A. J. B.: Development of stable denitrifying cultures during repeated aerobic-anaerobic transient periods, Water Res., 31, 1947–1954, https://doi.org/10.1016/S0043-1354(97)00053-5, 1997.
Baumberger, T., Früh-Green, G. L., Thorseth, I. H., Lilley, M. D., Hamelin, C., Bernasconi, S. M., Okland, I. E., and Pedersen, R. B.: Fluid composition of the sediment-influenced Loki's Castle vent field at the ultra-slow spreading Arctic Mid-Ocean Ridge, Geochim. Cosmochim. Ac., 187, 156–178, https://doi.org/10.1016/j.gca.2016.05.017, 2016.
Bayer, K., Schmitt, S., and Hentschel, U.: Physiology, phylogeny and in situ evidence for bacterial and archaeal nitrifiers in the marine sponge Aplysina aerophoba, Environ. Microbiol., 10, 2942–2955, https://doi.org/10.1111/j.1462-2920.2008.01582.x, 2008.
Bayer, K., Moitinho-Silva, L., Brummer, F., Cannistraci, C. V., Ravasi, T., and Hentschel, U.: GeoChip-based insights into the microbial functional gene repertoire of marine sponges (high microbial abundance, low microbial abundance) and seawater, Fems Microbiol. Ecol., 90, 832–843, https://doi.org/10.1111/1574-6941.12441, 2014.
Bell, J. J., McGrath, E., Biggerstaff, A., Bates, T., Bennett, H., Marlow, J., and Shaffer, M.: Sediment impacts on marine sponges, Mar. Pollut. Bull., 94, 5–13, https://doi.org/10.1016/j.marpolbul.2015.03.030, 2015.
Bulow, S. E., Rich, J. J., Naik, H. S., Pratihary, A. K., and Ward, B. B.: Denitrification exceeds anammox as a nitrogen loss pathway in the Arabian Sea oxygen minimum zone, Deep-Sea Res. Pt. I, 57, 384–393, https://doi.org/10.1016/j.dsr.2009.10.014, 2010.
Cardénas, P., Rapp, H. T., Klitgaard, A. B., Best, M., Thollesson, M., and Tendal, O. S.: Taxonomy, biogeography and DNA barcodes of Geodia species (Porifera, Demospongiae, Tetractinellida) in the Atlantic boreo-arctic region, Zool. J. Linn. Soc.-Lond., 169, 251–311, https://doi.org/10.1111/zoj.12056, 2013.
Chen, J. W. and Strous, M.: Denitrification and aerobic respiration, hybrid electron transport chains and co-evolution, Biochim. Biophys. Ac., 1827, 136–144, https://doi.org/10.1016/j.bbabio.2012.10.002, 2013.
Cleary, D. F. R., de Voogd, N. J., Polonia, A. R. M., Freitas, R., and Gomes, N. C. M.: Composition and Predictive Functional Analysis of Bacterial Communities in Seawater, Sediment and Sponges in the Spermonde Archipelago, Indonesia, Microb. Ecol., 70, 889–903, https://doi.org/10.1007/s00248-015-0632-5, 2015.
Corredor, J., Wilkinson, C., P. Vicente, V., Morell, J., and Otero Morales, E.: Nitrate release by Caribbean reef sponges, 114–120, 1988.
de Goeij, J. M., van Oevelen, D., Vermeij, M. J. A., Osinga, R., Middelburg, J. J., and de Goeij, A. F. P. M.: Surviving in a marine desert: the sponge loop retains resources within coral reefs, Science, 342, 108–110, https://doi.org/10.1126/science.1241981, 2013.
Diaz, M. C. and Ward, B. B.: Sponge-mediated nitrification in tropical benthic communities, Mar. Ecol. Prog. Ser., 156, 97–107, https://doi.org/10.3354/meps156097, 1997.
Fang, J. K. H., Rooks, C. A., Krogness, C. M., Kutti, T., Hoffmann, F., and Bannister, R. J.: Impact of particulate sediment, bentonite and barite (oil-drilling waste) on net fluxes of oxygen and nitrogen in Arctic-boreal sponges, Environ. Pollut., 238, 948–958, https://doi.org/10.1016/j.envpol.2017.11.092, 2018.
Feng, G. and Li, Z.: Carbon and Nitrogen Metabolism of Sponge Microbiome, in: Symbiotic Microbiomes of Coral Reefs Sponges and Corals, edited by: Li, Z., Springer, Dordrecht, 145–169, 2019.
Fiore, C. L., Jarett, J. K., Olson, N. D., and Lesser, M. P.: Nitrogen fixation and nitrogen transformations in marine symbioses, Trend. Microbiol., 18, 455–463, https://doi.org/10.1016/j.tim.2010.07.001, 2010.
Fiore, C. L., Baker, D. M., and Lesser, M. P.: Nitrogen Biogeochemistry in the Caribbean Sponge, Xestospongia muta: A Source or Sink of Dissolved Inorganic Nitrogen?, PLOS ONE, 8, e72961, https://doi.org/10.1371/journal.pone.0072961, 2013.
Fiore, C. L., Labrie, M., Jarettt, J. K., and Lesser, M. P.: Transcriptional activity of the giant barrel sponge, Xestospongia muta Holobiont: molecular evidence for metabolic interchange, Front. Microbiol., 6, 1–18, https://doi.org/10.3389/fmicb.2015.00364, 2015.
Folkers, M. and Rombouts, T.: Sponges Revealed: A Synthesis of Their Overlooked Ecological Functions Within Aquatic Ecosystems, in: YOUMARES 9 – The Oceans: Our Research, Our Future, edited by: Jungblut, S., Liebich, V., and Bode-Dalby, M., Springer, Cham, 181–193, 2020.
Han, M. Q., Liu, F., Zhang, F. L., Li, Z. Y., and Lin, H. W.: Bacterial and Archaeal Symbionts in the South China Sea Sponge Phakellia fusca: Community Structure, Relative Abundance, and Ammonia-Oxidizing Populations, Mar. Biotechnol., 14, 701–713, doi10.1007/s10126-012-9436-5, 2012.
Han, M. Q., Li, Z. Y., and Zhang, F. L.: The Ammonia Oxidizing and Denitrifying Prokaryotes Associated with Sponges from Different Sea Areas, Microb. Ecol., 66, 427–436, https://doi.org/10.1007/s00248-013-0197-0, 2013.
Hoer, D. R., Tommerdahl, J. P., Lindquist, N. L., and Martens, C. S.: Dissolved inorganic nitrogen fluxes from common Florida Bay (USA) sponges, Limnol. Oceanogr., 63, 2563–2578, https://doi.org/10.1002/lno.10960, 2018.
Hoffmann, F., Rapp, H., Zöller, T., and Reitner, J.: Growth and regeneration in cultivated fragments of the boreal deep water sponge Geodia barretti Bowerbank, 1858 (Geodiidae, Tetractinellida, Demospongiae), 109–118, 2003.
Hoffmann, F., Larsen, O., Thiel, V., Rapp, H. T., Pape, T., Michaelis, W., and Reitner, J.: An Anaerobic World in Sponges, Geomicrobiol. J., 22, 1–10, https://doi.org/10.1080/01490450590922505, 2005.
Hoffmann, F., Røy, H., Bayer, K., Hentschel, U., Pfannkuchen, M., Brümmer, F., and de Beer, D.: Oxygen dynamics and transport in the Mediterranean sponge Aplysina aerophoba, Mar. Biol., 153, 1257–1264, https://doi.org/10.1007/s00227-008-0905-3, 2008.
Hoffmann, F., Radax, R., Woebken, D., Holtappels, M., Lavik, G., Rapp, H. T., Schläppy, M.-L., Schleper, C., and Kuypers, M. M. M.: Complex nitrogen cycling in the sponge Geodia barretti, Environ. Microbiol., 11, 2228–2243, https://doi.org/10.1111/j.1462-2920.2009.01944.x, 2009.
Holtappels, M., Lavik, G., Jensen, M. M., and Kuypers, M. M. M.: Chapter ten – 15N-Labeling Experiments to Dissect the Contributions of Heterotrophic Denitrification and Anammox to Nitrogen Removal in the OMZ Waters of the Ocean, in: Methods in Enzymology, edited by: Klotz, M. G., Academic Press, 223–251, 2011.
Hughes, D. J. and Gage, J. D.: Benthic metazoan biomass, community structure and bioturbation at three contrasting deep-water sites on the northwest European continental margin, Prog. Oceanogr., 63, 29–55, https://doi.org/10.1016/j.pocean.2004.09.002, 2004.
Jiménez, E. and Ribes, M.: Sponges as a source of dissolved inorganic nitrogen: Nitrification mediated by temperate sponges, Limnol. Oceanogr., 52, 948–958, https://doi.org/10.4319/lo.2007.52.3.0948, 2007.
Kahn, A. S., Yahel, G., Chu, J. W. F., Tunnicliffe, V., and Leys, S. P.: Benthic grazing and carbon sequestration by deep-water glass sponge reefs, Limnol. Oceanogr., 60, 78–88, https://doi.org/10.1002/lno.10002, 2015.
Keesing, J. K., Strzelecki, J., Fromont, J., and Thomson, D.: Sponges as important sources of nitrate on an oligotrophic continental shelf, Limnol. Oceanogr., 58, 1947–1958, https://doi.org/10.4319/lo.2013.58.6.1947, 2013.
Klitgaard, A. B. and Tendal, O. S.: Distribution and species composition of mass occurrences of large-sized sponges in the northeast Atlantic, Prog. Oceanogr., 61, 57–98, https://doi.org/10.1016/j.pocean.2004.06.002, 2004.
Kutti, T., Bannister, R. J., and Fossa, J. H.: Community structure and ecological function of deep-water sponge grounds in the Traenadypet MPA-Northern Norwegian continental shelf, Cont. Shelf Res., 69, 21–30, https://doi.org/10.1016/j.csr.2013.09.011, 2013.
Kutti, T., Bannister, R. J., Fossa, J. H., Krogness, C. M., Tjensvoll, I., and Sovik, G.: Metabolic responses of the deep-water sponge Geodia barretti to suspended bottom sediment, simulated mine tailings and drill cuttings, J. Exp. Mar. Biol. Ecol., 473, 64–72, https://doi.org/10.1016/j.jembe.2015.07.017, 2015.
Leys, S. P., Kahn, A. S., Fang, J. K. H., Kutti, T., and Bannister, R. J.: Phagocytosis of microbial symbionts balances the carbon and nitrogen budget for the deep-water boreal sponge Geodia barretti, Limnol. Oceanogr., 63, 187–202, https://doi.org/10.1002/lno.10623, 2018.
Li, Z. Y., Wang, Y. Z., He, L. M., and Zheng, H. J.: Metabolic profiles of prokaryotic and eukaryotic communities in deep-sea sponge Lamellomorpha sp indicated by metagenomics, Sci. Rep., 4, 1–11, https://doi.org/10.1038/srep03895, 2014.
Liu, F., Li, J. L., Feng, G. F., and Li, Z. Y.: New Genomic Insights into “Entotheonella” Symbionts in Theonella swinhoei: Mixotrophy, Anaerobic Adaptation, Resilience, and Interaction, Front. Microbiol., 7, 1–11, https://doi.org/10.3389/fmicb.2016.01333, 2016.
Liu, M., Fan, L., Zhong, L., Kjelleberg, S., and Thomas, T.: Metaproteogenomic analysis of a community of sponge symbionts, Isme J., 6, 1515–1525, https://doi.org/10.1038/ismej.2012.1, 2012.
Maldonado, M., Ribes, M., and van Duyl, F. C.: Chapter three – Nutrient Fluxes Through Sponges: Biology, Budgets, and Ecological Implications, in: Advances in Marine Biology, edited by: Becerro, M. A., Uriz, M. J., Maldonado, M., and Turon, X., Academic Press, 113–182, 2012.
Marchant, H. K., Ahmerkamp, S., Lavik, G., Tegetmeyer, H. E., Graf, J., Klatt, J. M., Holtappels, M., Walpersdorf, E., and Kuypers, M. M.: Denitrifying community in coastal sediments performs aerobic and anaerobic respiration simultaneously, ISME J., 11, 1799–1812, 2017.
Mehta, M. P., Butterfield, D. A., and Baross, J. A.: Phylogenetic diversity of nitrogenase (nifH) genes in deep-sea and hydrothermal vent environments of the Juan de Fuca ridge, Appl. Environ. Microbiol., 69, 960–970, https://doi.org/10.1128/aem.69.2.960-970.2003, 2003.
Middelburg, J. J., Soetaert, K., Herman, P. M. J., and Heip, C. H. R.: Denitrification in marine sediments: A model study, Global Biogeochem. Cy., 10, 661–673, https://doi.org/10.1029/96GB02562, 1996.
Mohamed, N., S Colman, A., Tal, Y., and Hill, R.: Diversity and expression of nitrogen fixation genes in bacterial symbionts of marine sponges, Environ. Microbiol., 10, 2910–2921, https://doi.org/10.1111/j.1462-2920.2008.01704.x, 2008.
Mohamed, N. M., Saito, K., Tal, Y., and Hill, R. T.: Diversity of aerobic and anaerobic ammonia-oxidizing bacteria in marine sponges, Isme J., 4, 38–48, https://doi.org/10.1038/ismej.2009.84, 2010.
Murillo, F. J., Muñoz, P. D., Cristobo, J., Ríos, P., González, C., Kenchington, E., and Serrano, A.: Deep-sea sponge grounds of the Flemish Cap, Flemish Pass and the Grand Banks of Newfoundland (Northwest Atlantic Ocean): Distribution and species composition, Mar. Biol. Res., 8, 842–854, https://doi.org/10.1080/17451000.2012.682583, 2012.
Nielsen, L. P.: Denitrification in sediment determined from nitrogen isotope pairing, FEMS Microbiol. Ecol., 9, 357–361, https://doi.org/10.1111/j.1574-6941.1992.tb01771.x, 1992.
Osinga, R., Tramper, J., and Wijffels, R. H.: Cultivation of marine sponges, Mar. Biotechnol., 1, 509–532, https://doi.org/10.1007/Pl00011807, 1999.
Osinga, R., Armstrong, E., Burgess, J. G., Hoffmann, F., Reitner, J., and Schumann-Kindel, G.: Sponge-microbe associations and their importance for sponge bioprocess engineering, Hydrobiologia, 461, 55–62, https://doi.org/10.1023/A:1012717200362, 2001.
Painter, H.: A review of literature on inorganic nitrogen metabolism in microorganisms, Water Res., 4, 393–450, https://doi.org/10.1016/0043-1354(70)90051-5, 1970.
Pawlik, J. R. and McMurray, S. E.: The Emerging Ecological and Biogeochemical Importance of Sponges on Coral Reefs, Ann. Rev. Mar. Sci., 12, 315–337, https://doi.org/10.1146/annurev-marine-010419-010807, 2019.
Pfannkuchen, M., Fritz, G. B., Schlesinger, S., Bayer, K., and Brümmer, F.: In situ pumping activity of the sponge Aplysina aerophoba, Nardo 1886, J. Exp. Mar. Biol. Ecol., 369, 65–71, https://doi.org/10.1016/j.jembe.2008.10.027, 2009.
Pita, L., Rix, L., Slaby, B. M., Franke, A., and Hentschel, U.: The sponge holobiont in a changing ocean: from microbes to ecosystems, Microbiome, 6, 1–18, https://doi.org/10.1186/s40168-018-0428-1, 2018.
Radax, R., Hoffmann, F., Rapp, H. T., Leininger, S., and Schleper, C.: Ammonia-oxidizing archaea as main drivers of nitrification in cold-water sponges, Environ. Microbiol., 14, 909–923, https://doi.org/10.1111/j.1462-2920.2011.02661.x, 2012.
Reiswig, H. M.: Water transport, respiration and energetics of three tropical marine sponges, J. Exp. Mar. Biol. Ecol., 14, 231–249, https://doi.org/10.1016/0022-0981(74)90005-7, 1974.
Ribes, M., Dziallas, C., Coma, R., and Riemann, L.: Microbial Diversity and Putative Diazotrophy in High- and Low-Microbial-Abundance Mediterranean Sponges, Appl. Environ. Microbiol., 81, 5683–5693, https://doi.org/10.1128/aem.01320-15, 2015.
Risgaard-Petersen, N., Nielsen, L. P., Rysgaard, S., Dalsgaard, T., and Meyer, R. L.: Application of the isotope pairing technique in sediments where anammox and denitrification coexist, Limnol. Oceanogr.-Method., 1, 63–73, https://doi.org/10.4319/lom.2003.1.63, 2003.
Rix, L., de Goeij, J. M., Mueller, C. E., Struck, U., Middelburg, J. J., van Duyl, F. C., Al-Horani, F. A., Wild, C., Naumann, M. S., and van Oevelen, D.: Coral mucus fuels the sponge loop in warm- and cold-water coral reef ecosystems, Sci. Rep., 6, 18715, https://doi.org/10.1038/srep18715, 2016.
Roberts, E. M., Mienis, F., Rapp, H. T., Hanz, U., Meyer, H. K., and Davies, A. J.: Oceanographic setting and short-timescale environmental variability at an Arctic seamount sponge ground, Deep-Sea Res. Pt. I, 138, 98–113, https://doi.org/10.1016/j.dsr.2018.06.007, 2018.
Robertson, L. A., Dalsgaard, T., Revsbech, N. P., and Kuenen, J. G.: Confirmation of “aerobic denitrification” in batch cultures, using gas-chromatography and 15 mass spectrometry, FEMS Microbiol. Ecol., 18, 113–119, https://doi.org/10.1111/j.1574-6941.1995.tb00168.x, 1995.
Rooks, C., Fang, J. K.-H., Mørkved, P. T., Zhao, R., Rapp, H. T., Xavier, J. R., and Hoffmann, F.: Denitrification rates in boreo-arctic sponges – data of sponge species from Korsfjord (Norway) and the Schulz Bank (Arctic Ocean), PANGAEA, https://doi.org/10.1594/PANGAEA.899821, 2019.
Rysgaard, S., Christensen, P. B., and Nielsen, L. P.: Seasonal-Variation in Nitrification and Denitrification in Estuarine Sediment Colonized by Benthic Microalgae and Bioturbating Infauna, Mar. Ecol. Prog. Ser., 126, 111–121, https://doi.org/10.3354/meps126111, 1995.
Schläppy, M.-L., Hoffmann, F., Røy, H., Wijffels, R. H., Mendola, D., Sidri, M., and de Beer, D.: Oxygen dynamics and flow patterns of Dysidea avara (Porifera: Demospongiae), J. Mar. Biol. Assoc. UK, 87, 1677–1682, https://doi.org/10.1017/S0025315407058146, 2007.
Schläppy, M.-L., Schöttner, S. I., Lavik, G., Kuypers, M. M. M., de Beer, D., and Hoffmann, F.: Evidence of nitrification and denitrification in high and low microbial abundance sponges, Mar. Biol., 157, 593–602, https://doi.org/10.1007/s00227-009-1344-5, 2010a.
Schläppy, M. L., Weber, M., Mendola, D., Hoffmann, F., and de Beer, D.: Heterogeneous oxygenation resulting from active and passive flow in two Mediterranean sponges, Dysida avara and Chondrosia reniformis, Limnol. Oceanogr., 55, 1289–1300, https://doi.org/10.4319/lo.2010.55.3.1289, 2010b.
Seitzinger, S. P.: Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance, Limnol. Oceanogr., 33, 702–724, https://doi.org/10.4319/lo.1988.33.4part2.0702, 1988.
Seitzinger, S. P. and Giblin, A. E.: Estimating denitrification in North Atlantic continental shelf sediments, Biogeochemistry, 35, 235–260, https://doi.org/10.1007/Bf02179829, 1996.
Shapleigh, J. P.: Denitrifying Prokaryotes, in: The Prokaryotes: Prokaryotic Physiology and Biochemistry, edited by: Rosenberg, E., DeLong, E. F., Lory, S., Stackebrandt, E., and Thompson, F., Springer Berlin Heidelberg, Berlin, Heidelberg, 405–425, 2013.
Southwell, M. W., Popp, B. N., and Martens, C. S.: Nitrification controls on fluxes and isotopic composition of nitrate from Florida Keys sponges, Mar. Chem., 108, 96–108, https://doi.org/10.1016/j.marchem.2007.10.005, 2008.
Thamdrup, B. and Dalsgaard, T.: Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments, Appl. Environ. Microbiol., 68, 1312–1318, https://doi.org/10.1128/AEM.68.3.1312-1318.2002, 2002.
Throback, I. N., Enwall, K., Jarvis, A., and Hallin, S.: Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE, FEMS Microbiol. Ecol., 49, 401–417, https://doi.org/10.1016/j.femsec.2004.04.011, 2004.
Tjensvoll, I., Kutti, T., Fossa, J. H., and Bannister, R. J.: Rapid respiratory responses of the deep-water sponge Geodia barretti exposed to suspended sediments, Aquat. Biol., 19, 65–73, https://doi.org/10.3354/ab00522, 2013.
Ward, B. B., Devol, A. H., Rich, J. J., Chang, B. X., Bulow, S. E., Naik, H., Pratihary, A., and Jayakumar, A.: Denitrification as the dominant nitrogen loss process in the Arabian Sea, Nature, 461, 78–81, https://doi.org/10.1038/nature08276, 2009.
Webster, N. S. and Taylor, M. W.: Marine sponges and their microbial symbionts: love and other relationships, Environ. Microbiol., 14, 335–346, https://doi.org/10.1111/j.1462-2920.2011.02460.x, 2012.
Weisz, J. B., Lindquist, N., and Martens, C. S.: Do associated microbial abundances impact marine demosponge pumping rates and tissue densities?, Oecologia, 155, 367–376, https://doi.org/10.1007/s00442-007-0910-0, 2008.
Wilkinson, C. R. and Fay, P.: Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria, Nature, 279, 527–529, https://doi.org/10.1038/279527a0, 1979.
Wilkinson, C. R., Summons, R. E., and Evans, E.: Nitrogen fixation in symbiotic marine sponges: Ecological significance and difficulties in detection, 667–673, 1999.
Wilson, T. R. S.: Evidence for denitrification in aerobic pelagic sediments, Nature, 274, 354–356, https://doi.org/10.1038/274354a0, 1978.
Yahel, G., Sharp, J. H., Marie, D., Häse, C., and Genin, A.: In situ feeding and element removal in the symbiont-bearing sponge Theonella swinhoei: Bulk DOC is the major source for carbon, Limnol. Oceanogr., 48, 141–149, https://doi.org/10.4319/lo.2003.48.1.0141, 2003.
Zhang, F., Jonas, L., Lin, H., and Hill, R. T.: Microbially mediated nutrient cycles in marine sponges, FEMS Microbiol. Ecol., 95, 1–14, https://doi.org/10.1093/femsec/fiz155, 2019.
Zhang, X., He, L. M., Zhang, F. L., Sun, W., and Li, Z. Y.: The Different Potential of Sponge Bacterial Symbionts in N2 Release Indicated by the Phylogenetic Diversity and Abundance Analyses of Denitrification Genes, nirK and nosZ, Plos One, 8, e65142, https://doi.org/10.1371/journal.pone.0065142, 2013.
Zhuang, L. P., Lin, B. B., Qin, F., and Luo, L. Z.: Zhouia spongiae sp nov., isolated from a marine sponge, Int. J. System. Evolut. Microbiol., 68, 2194–2198, https://doi.org/10.1099/ijsem.0.002808, 2018.





