the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Methane oxidation in the waters of a humic-rich boreal lake stimulated by photosynthesis, nitrite, Fe(III) and humics
Sigrid van Grinsven
Kirsten Oswald
Bernhard Wehrli
Corinne Jegge
Jakob Zopfi
Moritz F. Lehmann
Carsten J. Schubert
Small boreal lakes are known to contribute significantly to global CH4 emissions. Lake Lovojärvi is a eutrophic lake in southern Finland with bottom water CH4 concentrations up to 2 mM. However, the surface water concentration, and thus the diffusive emission potential, was low (< 0.5 µM). We studied the biogeochemical processes involved in CH4 removal by chemical profiling and through incubation experiments. δ13C-CH4 profiling of the water column revealed a methane-oxidation hotspot just below the oxycline and zones of CH4 oxidation within the anoxic water column. In incubation experiments involving the addition of light and/or oxygen, CH4 oxidation rates in the anoxic hypolimnion were enhanced 3-fold, suggesting a major role for photosynthetically fueled aerobic CH4 oxidation. We observed a distinct peak in CH4 concentration at the chlorophyll-a maximum, caused by either in situ CH4 production or other CH4 inputs such as lateral transport from the littoral zone. In the dark anoxic water column at 7 m depth, nitrite seemed to be the key electron acceptor involved in CH4 oxidation, yet additions of Fe(III), anthraquinone-2,6-disulfonate and humic substances also stimulated anoxic CH4 oxidation. Surprisingly, nitrite seemed to inhibit CH4 oxidation at all other depths. Overall, this study shows that photosynthetically fueled CH4 oxidation can be a key process in CH4 removal in the water column of humic, turbid lakes, thereby limiting diffusive CH4 emissions from boreal lakes. Yet, it also highlights the potential importance of a whole suite of alternative electron acceptors, including humics, in these freshwater environments in the absence of light and oxygen.
- Article
(440 KB) - Full-text XML
-
Supplement
(344 KB) - BibTeX
- EndNote
Lacustrine water bodies represent a substantial natural source of atmospheric methane (CH4), a major contributor to global warming. They may release up to ∼ 72 Tg CH4 a−1 (12 % of total global emissions) (Bastviken et al., 2011), despite covering a relatively small proportion of the land surface area (> 3 %; Downing et al., 2006). In temperate and northern boreal regions, small lakes generally emit more CH4 per unit area than larger systems (Juutinen et al., 2009; Kortelainen et al., 2000, 2004; Michmerhuizen et al., 1996). Northern lakes alone are estimated to contribute 24.2 ± 10.5 Tg CH4 a−1 to global CH4 emissions (Walter et al., 2007).
The majority of lacustrine CH4 is produced by anaerobic methanogenic archaea as the end product of remineralization of organic matter in anoxic sediments (Bartlett and Harriss, 1993; Rudd, 1980). From the sediments, CH4 can diffuse into the water column and may be emitted to the atmosphere at the water–air interface. Large fractions of this CH4 may, however, be consumed by microbial CH4 oxidation, decreasing the CH4 concentration and emissions. Research has shown that microbial CH4 oxidation may be the single most important control on CH4 emissions from lakes and other ecosystems (Chistoserdova, 2015).
The vast majority of CH4 consumption in limnic systems has been assigned to bacterial CH4 oxidation (Hanson and Hanson, 1996; King, 1992). This process is performed by methane-oxidizing bacteria (MOB), affiliated with either gamma- or alphaproteobacteria. Typically, oxygen is used as the terminal electron acceptor (TEA) in the respiratory chain. However, some aerobic gamma-MOB like Methylomonas denitrificans (Kits et al., 2015a) and Methylomicrobium album (Kits et al., 2015b) can switch to the use of nitrate () or nitrite () as their TEA. The hybrid metabolism of Methylomirabilis oxyfera combines partial denitrification ( to NO) and classical aerobic CH4 oxidation, fueled by internal O2 generation (splitting NO to N2 and O2) (Ettwig et al., 2010). While M. oxyfera has similar metabolic traits as proteobacterial methanotrophs, it is associated with the novel phylum NC10 (Holmes et al., 2001; Rappé and Giovannoni, 2003). Recently, methanotrophs of the genera Methylomonas and Methylosinus have been shown to couple CH4 oxidation to Fe(III) reduction (Zheng et al., 2020). Bacterial methanotrophs require trace amounts of O2 for the activation of their enzymatic CH4 oxidation pathway. Completely O2-independent CH4 consumption is assigned to three distinct groups of anaerobic methanotrophic archaea (ANME-1, ANME-2 and ANME-3), which, at least in marine settings, are often found in a syntrophic relationship with sulfate-reducing bacteria (Boetius et al., 2000; Michaelis et al., 2002; Orphan et al., 2001) and have been estimated to remove 90 % of all produced CH4 in marine systems (Hinrichs and Boetius, 2002; Reeburgh, 2007). Although rare, ANME can be present in lake waters (Durisch-Kaiser et al., 2011; Eller et al., 2005; Oswald et al., 2016a) and sediments (Schubert et al., 2011; Su et al., 2020). Interestingly, studies reporting CH4 oxidation in anoxic zones of lakes, in the absence of ANME and in the presence of MOB, are increasing (Biderre-Petit et al., 2011; Blees et al., 2014; van Grinsven et al., 2020b; Oswald et al., 2016b; Schubert et al., 2010). While oxygen supplied by episodic down-welling of cold O2-laden water (Blees et al., 2014) or low-light photosynthesis (Milucka et al., 2015; Oswald et al., 2015) may explain this phenomenon to some degree, CH4 oxidation may also be coupled to the reduction of electron acceptors other than O2, such as nitrite or nitrate (Deutzmann et al., 2014; Graf et al., 2018; Oswald et al., 2016b), Fe(III) (Norði et al., 2013; Sivan et al., 2011), Mn(IV) (Crowe et al., 2011; Oswald et al., 2016a) and humic substances (Valenzuela et al., 2019).
The role of boreal lakes in worldwide greenhouse gas emissions is receiving increasing attention. Earlier studies mainly highlighted the large role of aerobic CH4 oxidation in the lake carbon cycle (Kankaala et al., 2006). More recent studies have shown that boreal lakes can exhibit highly active CH4-oxidizing communities both in the oxic and anoxic parts of the water column (Taipale et al., 2011). A recent study by Kallistova et al. (2019) showed a peak in CH4 oxidation rates at the oxycline, but also in the hypolimnion of boreal Lake Svetloe. No terminal electron acceptor (TEA) could, however, be identified in the ferruginous hypolimnion. Rissanen et al. (2018) demonstrated enhanced CH4 oxidation in the anoxic zone by light and nitrate but at the same time an inhibitory effect of sulfate and Fe(III). The environmental controls on the modes of anaerobic oxidation of methane (AOM) in boreal lakes, and the TEAs involved, are therefore still poorly understood. Here, we studied the microbial CH4 turnover, in particular the oxidative side, in a small lake rich in humic substances in southern Finland (Lake Lovojärvi). Sedimentation regime, stratigraphy and phytoplankton community have been studied intensively in this lake (Keskitalo, 1977; Saarnisto et al., 1977; Simola et al., 1990). A recent study by Rissanen et al. (2021) provided insight into the genomic potential of methanotrophic species living in the Lake Lovojärvi water column, revealing microbial community variation along the oxygen gradient that suggests adaptation and specialization of specific MOB types. To further reveal the methanotrophic potential in the water column of Lake Lovojärvi, and to gain an increased understanding of the biogeochemical controls on its biological CH4 consumption, we combined physical and chemical water column profiling with incubation experiments with different electron acceptors and light–dark conditions. Furthermore, we performed 16S rRNA gene sequencing to characterize the key microbial players involved.
2.1 Study site
Lake Lovojärvi is a small (5.4 ha) eutrophic lake near the town of Lammi in southern Finland. It is part of a glaciofluvial esker deposit (Simola, 1979), which gives the lake its elongated shape (600 m long, 130 m wide) and shields it from strong winds (Hakala, 2004). Lake Lovojärvi is shallow, with an average depth of 7.7 m (Ilmavirta et al., 1974) and a maximum depth of 17.5 m in the southeastern part (Simola, 1979). Due to the sheltered location and basin morphology, the lake undergoes strong thermal stratification and has a permanently anoxic hypolimnion (Saarnisto et al., 1977). The catchment of Lake Lovojärvi is 7.2 km2 and drains water from predominantly agricultural and swampy areas (Simola, 1979). It has been suggested that anthropogenic pollution of Lake Lovojärvi started as early as the Iron Age, by the soaking of hemp and flax (Tolonen et al., 1976). Hydrologically connected to marsh/wetlands (Limminjärvi), the lake receives high inputs of humic substances and dissolved ions (Hakala, 2004). To our knowledge, no information on groundwater inflow is available.
2.2 In situ profiling and sample collection
Profiling and sample collection were carried out in September 2015, at the deepest part of the lake (61∘04.584′ N, 25∘02.116′ E). A custom-made profiling device equipped with various probes and sensors was used to measure the following parameters in situ: conductivity, turbidity, temperature, depth (pressure) and pH (XRX 620, RBR); photosynthetically active radiation (PAR; LI-193 Spherical Underwater Quantum Sensor, LI-COR); chlorophyll a (ECO-FL, Wetlands, = ); and dissolved O2 (micro-optodes PSt1 and TOS7, PreSens). The detection limits of the two O2 optodes were 125 and 20 nM, respectively.
Samples for the analysis of all other parameters were pumped to the surface with a peristatic pump (Zimmermann AG Elektromaschinen, Horw, Switzerland) connected to gas-tight tubing (PVC Solaflex, Maagtechnic) attached to the profiler. To guarantee that water was taken from the correct depth, a custom-built inlet system was used (designed after Miracle et al., 1992), and water was pumped for 2 min (time necessary to replace the entire tube volume) prior to filling 60 mL syringes directly from the tube outlet avoiding air contact. Water from the syringes was then sub-sampled into different vials for further processing: for total sulfide analysis (HS− + H2S) zinc acetate was added (1.3 % final concentration). To quantify dissolved (< 0.45 µm) and total fractions of metals, and organic carbon, samples were acidified immediately to a final concentration of 0.1 M (Suprapur HNO3, Merck), 0.5 M (HCl) and 0.02 M (HCl), respectively. Aliquots were sterile filtered (< 0.22 µm) to analyze concentrations of dissolved nitrogen species (, and ), sulfate (), phosphate () and dissolved inorganic carbon (DIC). DIC samples were filled into gas-tight 12 mL Exetainers (Labco Ltd.) without a headspace and stored upside down. Water samples intended for hybridization techniques were fixed immediately with formaldehyde (2 % [] final concentration) and stored in the dark at 4 ∘C. All other samples requiring larger water volumes were taken directly from the tube outlet anoxically (without headspace or bubbles and by letting water overflow two to three volumes). For CH4 concentration and isotopic measurements, 120 mL serum bottles were filled prior to adding Cu(I)Cl (∼ 0.15 % [] final concentration) and sealing the bottles with butyl stoppers (Geo-Microbial Technologies, Inc.) and aluminum crimp caps. Similarly, sterile 160 mL serum bottles or 1 L Schott bottles served to store water for incubation experiments and DNA analysis. These were sealed with butyl stoppers and crimp or screw caps and were kept in the dark at 4 ∘C.
2.3 Carbon and isotopic parameters
A headspace was created by exchanging 20 mL lake water with 20 mL N2 gas. The bottles were then left for at least 24 h to equilibrate the gas content between the gas and water phase. Afterwards, headspace gas samples were used to measure the CH4 concentration by gas chromatography (GC; Agilent 6890N, Agilent Technologies) using a Carboxen 1010 column (30 m × 0.53 mm, Supelco), a flame ionization detector and an auto-sampler (Valco Instruments Co. Inc.). Resulting headspace concentrations were converted to dissolved water-phase CH4 by applying calculated Bunsen solubility coefficients (Wiesenburg and Guinasso, 1979). Stable carbon isotopes of CH4 were analyzed in the same headspace by isotope ratio mass spectrometry (IRMS; GV Instruments, Isoprime). For this, injected gas samples first passed through a trace gas unit (T/GAS PRECON, Micromass UK Ltd) for purification, concentration and combustion to CO2 (for details see Oswald et al., 2016a, b). Isotopic ratios of are presented in the standard δ13C notation (relative to the Vienna Pee Dee Belemnite (VPDB) reference) with a precision of ∼ 1.2 ‰.
Total organic carbon (TOC), dissolved organic carbon (DOC) and DIC were quantified with a total carbon analyzer (TOC-L, Shimadzu) equipped with a nondispersive infrared detector (NDIR). TOC was measured as CO2 after combustion (680 ∘C) of the untreated sample. For DOC determination, the samples were acidified before combustion. For DIC analysis, unacidified samples were injected and DIC was volatilized to CO2 (internal addition of HCl, pH < 3, in a CO2-free closed reaction chamber) and quantified subsequently. For carbon isotope analysis, 1 mL of the remaining liquid was then transferred to a He-flushed 3.7 mL exetainer and acidified (100 µL 85 % H3PO4). The δ13C of the released CO2 (overnight equilibration) was measured with a gas-bench system (MultiFlow, Isoprime) connected to an IRMS (Micromass, Isoprime). Isotopic ratios of the DIC are also expressed in the δ13C notation (VPDB reference) with a precision of ∼ 0.15 ‰.
2.4 Nutrients and metals
Nitrite, ammonium, sulfide and concentrations were measured on the same day as sampled using photometric protocols according to Griess (1879), Krom (1980), Cline (1969) and Stookey (1970), respectively. High background concentrations of organic carbon in the deep water column (9–17 m) may have affected the nitrite concentration measurements, along with possible oxidation of small amounts of ammonium during sample processing. Fe(III) concentrations were determined as the difference between total iron, after reduction with hydroxylamine hydrochloride, and Fe(II), which was measured directly (Viollier et al., 2000). Concentrations of nitrate and phosphate were quantified by flow injection analysis (SAN, Skalar), and sulfate concentrations were determined by ion chromatography (882 Compact IC plus, Metrohm). Total and dissolved Mn concentrations were analyzed by inductively coupled plasma–mass spectrometry (ICP-MS; Element2, Thermo-Fisher).
2.5 Catalyzed reporter deposition – fluorescence in situ hybridization (CARD-FISH)
Formaldehyde-fixed lake water samples (15 mL, incubated for ∼ 12 h at 4 ∘C) were filtered onto 0.2 µM polycarbonate filters (GTTP, Millipore) and rinsed 2× with 1× phosphate-buffered saline. Filters were stored at −20 ∘C until standard CARD-FISH (Pernthaler et al., 2002) was carried out using specific oligonucleotide probes with horseradish peroxidase labels (purchased from Biomers). An overview of the probes and percentage formamide used is supplied in Table S1 in the Supplement. Probes EUB338 I-III and Mgamma84+705 were applied as a mix of equal proportions. Background signals were assessed with probe NON338. Permabilization of cell walls, inactivation of endogenous peroxidase activity, hybridization, amplification (Oregon Green 488, Thermo-Fischer Scientific), counter staining (4′,6-diamidino-2-phenylindole, DAPI) and embedding of the filter pieces were carried out as described in detail previously (Oswald et al., 2016b). Total cell numbers (DAPI-stained cells) and cells belonging to the different targeted groups (CARD-FISH signals) were enumerated in 20 randomly selected fields of view using the grid ocular of the Axioskop 2 (Zeiss) epifluorescence microscope. Proportions of the microbial groups are based on total DAPI cell counts (260–550 cells counted per sample, distributed over 20 randomly chosen fields of view).
2.6 DNA extraction and 16S rRNA gene amplicon sequencing
Microbial biomass from different depths of the water column was collected on 0.2 µm polycarbonate membrane filters (Cyclopore, Whatman) and kept frozen (−20 ∘C) until DNA extraction using the FastDNA SPIN Kit for Soil (MP Biomedicals). A two-step PCR approach (Monchamp et al., 2016) was applied in order to prepare the library for Illumina sequencing at the Genomics Facility Basel. Briefly, 10 ng of extracted DNA was used, and a first PCR of 25 cycles was performed using universal primers 515F-Y (5′-GTGYCAGCMGCCGCGGTAA) and 926R (5′-CCGYCAATTYMTTTRAGTTT-3′) targeting the V4 and V5 regions of the 16S rRNA gene (Parada et al., 2016). The primers of this first PCR were composed of the target region and an Illumina Nextera XT specific adapter sequence. Four sets of forward and reverse primers, which contained zero to three additional and ambiguous bases after the adapter sequence, were used in order to introduce frame shifts to increase complexity (details described in Su et al., 2020). Sample indices and Illumina adaptors were added in a second PCR of eight cycles. Purified, indexed amplicons were finally pooled at equimolar concentration, denatured, spiked with 10 % PhiX and sequenced on an Illumina MiSeq platform using the 2 × 300 bp paired-end protocol (V3-Kit), resulting in 24 000–123 000 reads per sample. The initial sequence treatment was done at the Genetic Diversity Center (ETH Zurich) where FastQC (v 1.2.11; Babraham Bioinformatics) was used to check the quality of the raw reads, and FLASH (Magoč and Salzberg, 2011) was used to merge forward and reverse reads into amplicons of about 374 bp length. The procedure allowed a minimum overlap of 15 nucleotides and a mismatch density of 0.25. Full-length primer regions were trimmed using USEARCH (v10.0.240), allowing a maximum of one mismatch. Merged and primer-trimmed amplicons were quality-filtered (size range: 250–550, no ambiguous nucleotides, minimum average quality score of 20) using PRINSEQ (Schmieder and Edwards, 2011). OTU (operational taxonomic unit) clustering with a 97 % identity threshold was performed using the UPARSE-OTU algorithm in USEARCH v10.0.240 (Edgar, 2010, 2013). Taxonomic assignment of OTUs was done using SINTAX (Edgar, 2016) and the SILVA 16S rRNA reference database v128 (Quast et al., 2013). Downstream sequence analyses were done in R v3.5.1 using Phyloseq v1.25.2 (McMurdie and Holmes, 2013). Raw sequences have been deposited at NCBI under the BioProject number PRJNA717665 with the accession numbers SAMN18500068 to SAMN18500079.
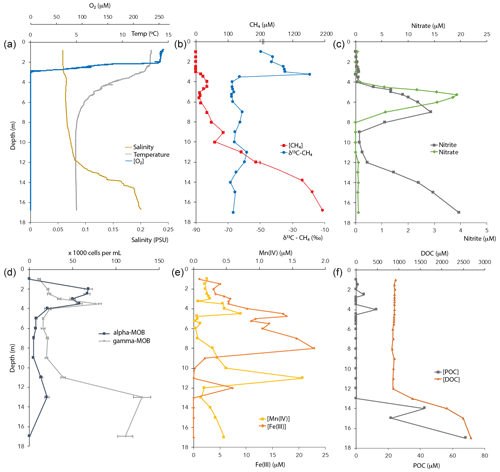
Figure 1Physicochemical characteristics and CH4-oxidizing bacterial (MOB) abundance in the Lake Lovojärvi water column in September 2015. POC – particulate organic carbon. DOC – dissolved organic carbon. Note the break at the [CH4] axis in (b). The oxygen profile combines data obtained by two different oxygen sensors, for low and high concentrations (see Methods).
2.7 CH4 oxidation incubation experiments
To determine the CH4 oxidation potential and possible stimulation by potential electron acceptors, incubation experiments were set up with water from 3, 4, 5, 7 and 9 m depth no later than 2 h after sampling. These depths were selected based on their expected relevance for CH4 turnover: previous research has repeatedly shown the highest CH4 oxidation rates to occur around the oxycline (Blees et al., 2014; Mayr et al., 2020; Milucka et al., 2015; Oswald et al., 2015; Panganiban et al., 1979; Sundh et al., 2005). The followed approach is described in detail by Oswald et al. (2016b) and is based on adapted protocols for 15N incubations (Holtappels et al., 2011). Briefly, water collected in 160 mL serum bottles was first degassed (10–15 min with He) and then individually amended with the different electron acceptors tested, except for the dark and light setups (Table S2 in the Supplement). After this, 5 mL of a saturated 13CH4 (99 atom%, Campro Scientific) solution was injected under anoxic and sterile conditions into each bottle to a final concentration of ∼ 50 µM CH4. Finally, water was dispensed into 12 mL Exetainers without headspace and incubated at ∼ 8 ∘C (average lake temperature between 3–9 m) under dark or light (∼5 ) conditions. At selected time points (∼ 0, 6, 12, 24 and 48 h), ZnCl2 (200 µL, 50 % [] solution) was used to stop microbial activity in one exetainer per setup to analyze δ13C-DIC by GC-IRMS (see above). CH4 oxidation rates were estimated by linear regression of the change of 13C-DIC over the experimental interval, under consideration of the in situ DIC concentration at the different incubation depths (1–1.2 mM) (for details see Oswald et al., 2015, 2016a). For comparison between all setups and depths, the CH4 oxidation potential was always determined over the initial 24 h time interval, as the production of 13C-DIC remained linear during this time period in all setups.
3.1 Physicochemical conditions in the water column
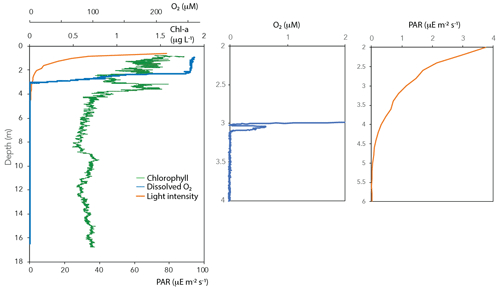
Oxygen concentrations were around 250 µM in the top 2 m of the Lake Lovojärvi water column (Fig. 1a). Below, the O2 profile displayed a sharp gradient between 2–3 m depth, and complete oxygen depletion was observed already below 3.1 m. A small peak in the O2 concentration was observed between 3 and 3.1 m depth (Fig. 2). The thermo- and pycnoclines were evidenced by gradients in temperature between 3–5 m (surface temperature 13 ∘C, bottom 5 ∘C) and in salinity between 12–14 m, respectively (Fig. 1a). Compared to the total radiation at the surface, PAR decreased from 27 % (80 ) at 0.6 m to 1 % (3 ) at 2.2 m (Fig. 2). Light diminished between 5 and 6.6 m (0.05–0.01 ; Fig. 2). Nitrate concentrations peaked between 4–7 m, with the highest concentrations of 19 µM at 5.25 m (Fig. 1c). Above and below the nitrate peak, concentrations averaged at 0.3 µM. A nitrite peak was visible at similar depths, but with the maximum concentration found at 7 m (3 µM, Fig. 1c). Below 12 m, nitrite increased to 4 µM (Fig. 1c). Sulfate concentrations in the top were relatively invariant around 150 µM and declined sharply to ∼ 12 µM at 12 m depth, whereas total sulfide was < 1 µM down to 9 m, from where it increased steadily to ∼ 14 µM at 14 m (Fig. S1 in the Supplement). Fe(III) showed a peak at 4–9 m depth, with a maximum of 23 µM at 8 m (Fig. 1e). Dissolved Fe(II) increased from 8 m downwards to reach a concentration of 830 µM at 17 m (Fig. S1). Manganese concentrations were much lower than those of iron, with particulate Mn(IV) ranging around 0.3 µM showing subtle peaks at 4.5 m (0.7 µM) and 11 m (1.7 µM; Fig. 1e). Dissolved Mn(II) was nearly undetectable in the top 3 m of the water column (100 nM average), yet reached rather constant values of ∼ 2 µM below (3–11 m), before increasing towards the sediment (16 µM at 17 m, Fig. S1).
3.2 CH4 and other carbon compounds
CH4 was present throughout the water column of Lake Lovojärvi, yet increased by more than 4 orders of magnitude from the surface (0.3 µM) to the sediment (∼ 2 mM; Fig. 1b). The profile exposed four “zones”: (i) low (≤0.3 µM) concentrations in the epilimnion; (ii) a distinct peak in [CH4] below the oxycline, from 3–5 m (max concentration 33 µM); (iii) a zone of gradual increase, from 11 µM at 5.5 m to 140 µM at 11 m; and (iv) a zone of rapid increase, from 190 µM at 12 m to 1990 µM at 17 m (Fig. 1b). The δ13C-CH4 profile showed values of −50 ‰ to −35 ‰ in the epilimnion and of −58 ‰ to −69 ‰ in the hypolimnion, with a trend towards heavier values directly at the oxycline: the δ13C-CH4 increased from −63 ‰ (3.5 m) to −19 ‰ (3.25 m) to decline to −35 ‰ at 3 m (Fig. 1b).
The majority of organic carbon was present in its dissolved form, with DOC concentrations being 100× higher than POC concentrations (Fig. 1f). Both DOC and POC profiles showed a constant concentration from the surface to the chemocline at 12 m depth, where both DOC and POC concentration profiles indicated a strong increase towards the sediment surface.
The DIC concentration profile followed that of CH4 closely. Concentrations of DIC also increased by an order of magnitude from the surface (700 µM) to the sediment (5.6 mM), with a peak just below the oxycline (Fig. S2 in the Supplement). δ13C-DIC values decreased from the surface waters (−11.5 ‰) to the oxycline (−18 ‰), remained relatively constant until 12 m depth and then increased strongly towards the sediment (−4 ‰ at 17 m; Fig. S2), a trend that could not be linked to that of δ13C-CH4 (Fig. 1b).
3.3 Microbial community and chlorophyll-a distribution
Cell counts showed that both gamma- (probes Mgamma84+705) and alpha-MOB (probe Ma450) abundances showed a distinct peak near the oxycline (Fig. 1d). Gamma-MOB were present at all sampled depths, with peaks at 3.5 m (8.0 × 104 cells mL−1; 1.8 % of DAPI counts), and in the hypolimnion at 13 m (1.3 × 105 cells mL−1; 3.5 % of DAPI counts). Alpha-MOB were most numerous near the oxycline at 2–3.5 m, where they comprised a relatively large proportion of the total community (6.8 × 104 cells mL−1; 3.6 % of DAPI counts). A second, smaller peak was observed at 13 m (2.0 × 104 cells mL−1, 0.5 % of DAPI counts). Both types of MOB were least abundant between 4–9 m depth. Known representatives of ANME-1 (probe ANME-1-350) and ANME-2 (probe ANME-2-538) did not exceed 0.4 % of total DAPI counts at any depth of the water column (data not shown).
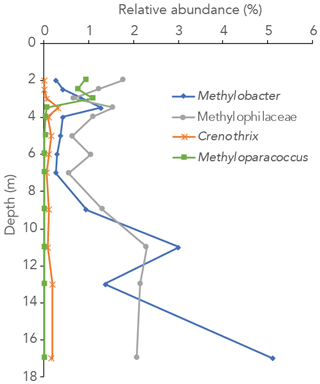
Figure 3Relative abundance of 16S rRNA gene sequences annotated to the methanotrophic genera Methylobacter, Methyloparacoccus and Crenothrix and the methylotrophic family Methylophilaceae in the water column of Lake Lovojärvi.
The 16S rRNA gene-sequencing data showed that the archaeal relative abundance was below 0.5 % throughout the upper and middle water column. Only between 11 and 17 m depth was the archaeal abundance higher than 0.5 % (0.7 %, 1.0 % and 4.0 % of all reads at 11, 13 and 17 m, respectively). The only known archaeal methanogens present belonged to the genus Methanoregula and were detected at 9, 11 and 17 m depth (0.1 %, 0.1 % and 0.3 %; at all other depths < 0.05 % and thus considered insignificant). Gammaproteobacterial methane-oxidizing bacteria reads were detected throughout the water column and were dominantly assigned to the genus Methylobacter (0.3 %–5 % of total 16S rRNA reads) and to a lesser extent to the genus Crenothrix (0 %–0.3 %; Fig. 3). Methyloparacoccus dominated the oxic epilimnion (0.9 %–1.1 %; Fig. 3) but was undetectable below 3.5 m depth. At 3.5, 13 and 17 m, respectively 0.3 %, 0.1 % and 0.3 % of “other Methylococcaceae”, specified as 16S rRNA sequence assigned to the family Methylococcaceae but not to the above-mentioned genera, were found. Alphaproteobacteria were highly abundant in the oxic water column (14 %–15 %), but only 0.1 %–0.3 % of these reads were assigned to the genus Methylocystaceae. A total of 30 %–35 % of the Alphaproteobacterial reads at 2–3 m depth were, however, assigned to unknown bacteria of the Rhizobiales order, the order to which the alpha-MOB belong (Fig. S3 in the Supplement). Possibly, part of these unknown Rhizobiales-assigned sequences belong to methane-oxidizing bacteria. Bacteria of the family Methylophilaceae were present throughout the water column (0.6 %–2.3 %, Fig. 3). Sequence reads of Canditatus Methylomirabilis sp., belonging to the NC10 phylum, were detected only at one single depth (13 m) but at a comparatively high relative abundance (2.3 %).
Chlorophyll a was present throughout the water column (Fig. 2). Yet, concentrations were highest in the surface waters (1.8 µg L−1), from where they decreased towards 2 m depth. A second peak in chlorophyll a was visible at 3–4 m depth (1.6 µg L−1; Fig. 2).
3.4 Potential CH4 oxidation rates
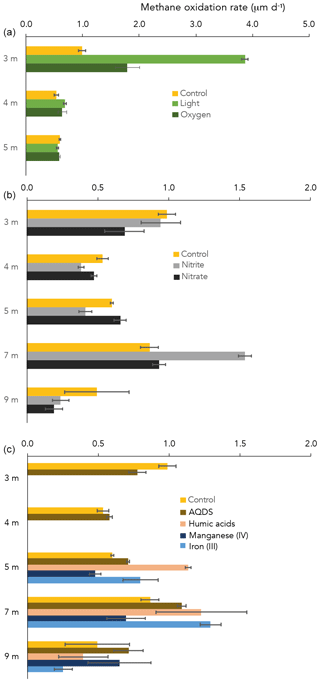
CH4 oxidation under “control” conditions (dark, starting concentration ∼ 50 µM CH4 after 13CH4 addition) peaked at the oxycline (3 m) and at 7 m depth (1.0 and 0.9 µM d−1, respectively; Fig. 4). At 3 and 4 m depth, of all dark incubations with substrate additions (overview in Table S2), only the addition of oxygen enhanced the CH4 oxidation rate (from 1.0 in the control to 1.8 µM d−1 with oxygen at 3 m; Fig. 4). Even more pronounced was the effect of light on the potential CH4 oxidate rate at 3 m depth, which accelerated the CH4 oxidation rate to 3.9 µM d−1 (Fig. 2). At 4 m, the effects of light and oxygen addition were minor (0.5, 0.7 and 0.6 µM d−1 in the control, light and O2 incubations, respectively; Fig. 2). At 5 m depth, neither light nor oxygen increased CH4 oxidation rates (Fig. 2). Additions of anthraquinone-2,6-disulfonate (AQDS), humic substances and Fe(III) increased the CH4 oxidation rate at 5 and 7 m depth (Fig. 4). Mn(IV) and nitrite increased the CH4 oxidation rate only at one specific depth (9 and 7 m, respectively; Fig. 1). Nitrate did not enhance CH4 oxidation at any of the depths (Fig. 4).
Despite extremely high CH4 concentrations in the bottom waters of Lake Lovojärvi (up to 2000 µM), the surface water CH4 concentration, and thus the diffusive emission potential, remained relatively low (< 0.5 µM). The pycnocline and thermocline seem to act as a physical barrier, hindering diffusive transport and containing dissolved CH4 in certain water layers, where the process of CH4 oxidation can consume CH4 and diminish the CH4 concentration. Lake Lovojärvi incubation experiments and the natural abundance δ13C-CH4 signal in the water column suggest that natural CH4 oxidation rates are highest at 3 and 7 m depth (Figs. 1 and 4).
4.1 Aerobic and photosynthesis-fueled CH4 oxidation
Oxygen was detected down to a depth of 3.1 m (oxycline) within Lake Lovojärvi (Figs. 1a and 2). Immediately below this depth, δ13C-CH4 showed a pronounced shift to high values from −63 ‰ at 3.5 m to −19 ‰ at 3.25 m (Fig. 1b). As methanotrophs fractionate carbon isotopes (just like many other biological reactions breaking carbon bonds), and preferentially oxidize the light carbon 12C isotopes, the residual pool of CH4 becomes enriched in the heavier 13C isotopes with fractional CH4 turnover. Hence, the distinct change in δ13C at 3–3.5 m pinpoints a hotspot of CH4 oxidation (Barker and Fritz, 1981). The relatively high abundance of both types of aerobic methanotrophs (i.e., gamma- and alpha-MOB; Fig. 1d) supports the existence of a CH4 oxidation hotspot at the oxycline depth. Furthermore, CH4 oxidation rates were highest directly at the oxycline (∼ 1 µM d−1 at 3 m; Fig. 4), confirming that aerobic methanotrophs are most active at the oxic–anoxic transition, where both substrates (CH4 and O2) overlap and conditions are most favorable for aerobic CH4 oxidation (Rudd et al., 1976, Blumenberg et al., 2007; Fenchel and Blackburn, 1979). These findings correspond well with previous studies in stratified lakes, where the highest CH4 turnover was also shown to occur in the vicinity of the oxycline (Blees et al., 2014; Mayr et al., 2020; Milucka et al., 2015; Oswald et al., 2015; Panganiban et al., 1979; Sundh et al., 2005).
The oxygen availability at 3 m depth is likely rate-limiting for CH4 oxidation, given the in situ concentration of 0.5 µM (Fig. 2) and the enhanced CH4 oxidation rate upon the addition of oxygen (Fig. 2). Oxygen availability below the oxycline of stratified lakes is often limited due to the low speed of diffusive oxygen transport across the oxycline (Kreling et al., 2014). In shallow Lake Lovojärvi, another source of oxygen besides diffusive supply is likely enhancing oxygen availability to methanotrophs, stimulating CH4 removal rates. A strong peak in chlorophyll-a concentration was observed at 3–4 m depth, where the light intensity was 0.3–1.14 (Fig. 2), still exceeding the threshold for photosynthesis (0.09 ; Gibson, 1985). At that same depth, a small peak in the O2 concentration is observed (Fig. 2), indicating in situ oxygen production. Milucka et al. (2015) and Oswald et al. (2015, 2016b) showed that photosynthetic oxygen production can fuel aerobic CH4 oxidation deep within the anoxic water column, where CH4 is often replete. Produced oxygen is immediately consumed by the oxygen-limited aerobic methanotrophs, keeping the dissolved oxygen concentrations in the water column low. Our experimental results indicate that photosynthetically fueled CH4 oxidation is also a key process in CH4 removal in the water column of this humic, turbid lake. The photosynthesis effect on methanotrophy is most pronounced at 3 m depth, where the CH4 oxidation rates increased significantly from 0.99 ± 0.06 µM d−1 under dark conditions to 3.9 ± 0.06 µM d−1 under light conditions. Why light stimulates the CH4 oxidation rate at 3 m much stronger than the addition of O2 directly (1.8 ± 0.2 µM d−1) remains unclear. Perhaps the oxygen availability and consumption are better balanced in the case of light stimulation, with a direct linkage between the production by phytoplankton and the consumption by methanotrophs, possibly even via a physical interaction, allowing the produced O2 to be more efficiently, and exclusively, used for CH4 oxidation. In the case of an O2 pulse, as in the oxygen addition experiment, part of the O2 may be used for non-CH4-oxidation-related processes (including, e.g., dark respiration by phototrophs). It is also possible that the methanotrophs were partly inhibited by the higher O2 concentrations, as methanotrophs have been suggested to be microaerophiles (Van Bodegom et al., 2001; Rudd and Hamilton, 1975; Thottathil et al., 2019).
In incubations with water from 4 m depth, there was only a minor observable effect of O2 addition and light on the CH4 oxidation rate (0.5, 0.7 and 0.6 µM d−1 for control, light and O2, respectively; Fig. 2). Oxygen availability may not be the rate-limiting factor here. The dark incubation experiments indicate that natural CH4 oxidation rates are lower at 4 m than at 3 m (Fig. 4). The addition of nitrate, nitrite and AQDS did not enhance CH4 oxidation at 4 m either (Fig. 4). Hence, what the dominant terminal electron acceptor(s) involved in CH4 oxidation at 4 m depth is/are, and why oxidation rates and methanotroph abundance were lower at 4 m than at 3 m, despite the elevated CH4 concentrations, remains uncertain.
4.2 Water column CH4 production
The major part of CH4 production in Lake Lovojärvi takes place in the sediment, where high amounts of the CH4 diffuse up into the water column (∼ 2 mM at 17 m; Fig. 1b). The carbon isotopic signature (δ13C of −66 ‰, Fig. 1b) is indicative of a biogenic origin, the production by methanogens (Whiticar, 1999). The concentration declines rapidly by an order of magnitude (∼ 200 µM at 12 m) upwards through the pycnocline (Fig. 1b), further decreases from 12 to 6 m depth, but then shows another maximum at 3–5 m depth. The observed peak in the CH4 concentration at this depth, just below the oxycline, suggests in situ CH4 production (Fig. 1b). CH4 is generally produced by methanogens, anaerobic archaea that do not tolerate oxygen (Kiener and Leisinger, 1983). It would therefore be remarkable that a zone of CH4 production is observed just below the oxycline, where traces of oxygen are still present, and where oxygen is likely produced by the highly abundant phototrophs (Fig. 2). These phototrophs may, however, play a role not only in enabling aerobic methanotrophy but also in CH4 production. Recent research has suggested that cyanobacteria are capable of forming CH4 as a by-product of photosynthesis (Bižić et al., 2020) and that this might contribute to CH4 emissions from oxic waters (Günthel et al., 2020). As the zone of CH4 production in Lake Lovojärvi coincides with the chlorophyll peak (Figs. 1 and 2), phytoplankton-mediated CH4 production may be responsible for the observed CH4 production near the oxycline. CH4 production under oxic conditions is, however, still highly debated. Another reasonable explanation for the observed CH4 peak could be lateral transport of CH4 produced in sediments in the littoral zone (Peeters et al., 2019). Archaeal methanogens of the genus Methanoregula were detected in the water column, but only at 9, 11 and 17 m depth (0.1 %, 0.1 % and 0.3 %).
4.3 CH4 oxidation in the anoxic water column
Besides the peak in CH4 oxidation at 3 m depth, high CH4 oxidation rates were also detected at 7 m, within the anoxic part of the water column (Fig. 4). Both the incubation experiments and the δ13C-CH4 profile, which showed a slight increase in the δ13C-CH4 values, suggest active CH4 oxidation within the anoxic hypolimnion (4–9 m). The δ13C-CH4 and methanotroph abundance profiles also suggest a zone of active CH4 oxidation between 11 and 13 m depth (Figs. 1 and 3). Earlier studies have demonstrated high CH4 oxidation rates in the anoxic water column of lakes, which exceeded oxic CH4 oxidation rates in some cases (Blees et al., 2014; van Grinsven et al., 2020b). In the anoxic water column of Lake Lovojärvi, nitrate, nitrite, sulfate, Fe(III) and organic matter are all present, in varying concentrations with water column depth (Figs. 1 and S1). These compounds have all been recognized as electron acceptors potentially involved in lacustrine CH4 oxidation (Ettwig et al., 2010; Kits et al., 2015a; Saxton et al., 2016; Schubert et al., 2011). Lake Lovojärvi incubation experiments showed that nitrite, AQDS, humic substances and Fe(III) all enhanced CH4 oxidation at 7 m (Fig. 4). This stands in contrast to a study by Rissanen et al. (2018) in a nearby lake, where nitrate stimulated CH4 oxidation, but Fe(III) inhibited CH4 oxidation instead. Although each of the aforementioned substances may have stimulated CH4 oxidation directly, as a terminal electron acceptor for CH4 oxidation, they may also have stimulated the internal cycling of other redox components instead, fostering CH4 oxidation indirectly. For example, Su et al. (2020) showed Mn and Fe oxides can support sulfate-dependent AOM. The stimulating effect of nitrite on the CH4 oxidation rate was the strongest among all substrates tested (1.5 ± 0.1 µM d−1 with nitrate, 0.9 ± 0.1 µM d−1 in the control experiment; Fig. 4). As CH4 oxidation coupled to the reduction of nitrite yields the largest Gibbs free energy (ΔG∘ = −1007 kJ mol−1 CH4), this form of CH4 oxidation may outcompete CH4 oxidation coupled to the reduction of Fe(III) (ΔG∘ = −571 kJ mol−1 CH4) or AQDS (ΔG∘ = −41 kJ mol−1 CH4; Reed et al. 2017). Nitrite was present in the water column of Lake Lovojärvi at relatively high concentrations (3 µM) at 7 m and below 12 m (Fig. 1c), supporting the hypothesis that nitrite could serve as an electron acceptor involved in natural CH4 oxidation in the Lake Lovojärvi water column. Nitrite has been found to support CH4 oxidation by Candidatus Methylomirabilis oxyfera and Methylomicrobium album (Ettwig et al., 2010; Kits et al., 2015b) but is also known to inhibit CH4 oxidation at higher concentrations (Dunfield and Knowles, 1995; Hütsch, 1998). Surprisingly, nitrite stimulated CH4 oxidation at 7 m but seemed to inhibit CH4 oxidation at all other depths (Fig. 4). As the same amounts of nitrite were added at all depths, it is unclear why an inhibitory effect would occur at all depths but 7 m. It may be reasonable to assume that the overall microbial community is involved in the (de)toxification of compounds inhibitory for methanotrophs, or that the differential response is caused by the presence of diverse methanotrophic communities, with different tolerance levels. The methanotrophic community composition is, however, similar at 7 m compared to the other depths (Fig. 3).
Organic material is present throughout the water column of Lake Lovojärvi (Fig. 1f). Potential involvement of organic molecules in CH4 oxidation is generally tested with the humic acid analogue AQDS (Saxton et al., 2016; Scheller et al., 2016) or a standard mixture of humic substances provided by commercial companies or the International Humic Substances Society (van Grinsven et al., 2020a; Valenzuela et al., 2019). In this study, both AQDS and leonardite humic acids were used as potential electron acceptors in the incubation experiments (Fig. 1f). A difference in the effect of these two humic substrates was observed, with the humic substances providing a stronger stimulating effect on the CH4 oxidation rates than the AQDS at both 5 and 7 m (Fig. 4). As organic matter in natural systems is highly diverse and complex in composition, it is difficult to assess how similar the added material is to the natural organic material present in the water column and what causes the observed difference between the two organic materials used in this study. Independent of the exact mechanisms/controls with regards to the role of humics in CH4 oxidation, our results show, however, that a whole spectrum of organic substrates maybe able to support AOM.
4.4 CH4-oxidizing community
Both alpha- and gammaproteobacterial CH4-oxidizing bacteria are present throughout the water column according to our cell-count data (Fig. 1d). Although concentrations of CH4 were very low above the oxycline (∼ 300 nM), alpha-MOB still make up several percent of microbial community here (3.5 % of DAPI counts at 2 m). Possibly, these methanotrophs are supported by CH4 that reaches the upper water column via ebullition, in contrast to the continuous CH4 supply by diffusion to MOB in the lower water layers. CH4 is a gas with a low solubility and can therefore form bubbles at high sedimentary concentrations, which are then released into the water column at instability events (Joyce and Jewell, 2003). These bubbles exchange gas with the water during their travel upwards through the water column (Delsontro et al., 2010). Possibly, pulses of CH4 are regularly delivered to the surface water via ebullition, feeding the epilimnetic methanotrophic community. Another possibility is the influx of CH4 from the littoral zone, via lateral transport. Alpha-MOB are known to predominantly occur at higher O2 levels, whereas gamma-MOB tend to prefer high CH4 levels (Amaral and Knowles, 1995; Crevecoeur et al., 2017). This zonation is visible in the Lake Lovojärvi water column, with alpha-MOB abundance peaking at 2 m (6.8 × 104 cells mL−1, Fig. 1d). The gamma-MOB abundance peaks just below the oxycline (8.0 × 104 cells mL−1, Fig. 1d), at the same depth where the peaks in δ13C-CH4 and CH4 oxidation rate were observed. A second peak in gamma-MOB abundance was observed in the deep water column, at 13 m (13 × 104 cells mL−1, Fig. 1d). These patterns are in line with a recent 16S rRNA gene and metagenomic sequencing study in Lake Lovojärvi (Rissanen et al., 2021), which also showed the presence of nitrite-reduction genes in Methylococcales metagenome assemblies of the water column, as well as genes related to extracellular electron transfer. Our 16S rRNA gene-sequencing data suggest that Methylobacter sp. represent the dominant methanotrophs in the water column (Fig. 3), both at the oxycline and in the deep water column. This is in line with previous findings, suggesting that Methylobacter sp. is a versatile methanotroph that can use both oxygen and other substrates, such as nitrate and nitrite, for CH4 oxidation (van Grinsven et al., 2020b; Martinez-Cruz et al., 2017; Smith et al., 2018). Methanotrophs belonging to the genus Methyloparacoccus dominate the oxic epilimnion, but they are absent in the zone with the highest chlorophyll-a concentrations (3–4 m; Fig. 3). Bacteria of the family Methylophilaceae were also found throughout the water column, with the highest abundances at depths were CH4 oxidation occurred (Figs. 1, 3 and 4). Methylophilaceae are methylotrophs that do not possess genes encoding for CH4 monooxygenases (pMMO nor sMMO) and are therefore incapable of methanotrophy. They are known to oxidize methanol and methylamine (Jenkins et al., 1987), which can be released by methanotrophs (Oshkin et al., 2014; Tavormina et al., 2017; Wei et al., 2016). These may be consumed by methylotrophs belonging to the Methylophilaceae (van Grinsven et al., 2020c), explaining the spatial co-occurrence of the two groups in the lake water column. Candidatus Methylomirabilis sp. was only detected at 13 m depth, but at a relatively large abundance (2.3 % of 16S rRNA reads).
Similar CH4 oxidation rates were measured at 3 and 7 m depth (1.0 ± 0.1 and 0.9 ± 0.1 µM d−1, respectively; Fig. 4), despite a large difference in methanotroph abundance (8.5 and 2.6 × 104 cells mL−1, respectively; Fig. 1d). Water column CH4 oxidation rates therefore seem not necessarily coupled to methanotroph cell numbers, but rather to cell activity rates instead.
Lake Lovojärvi is a productive humic lake. Despite the extremely high CH4 concentrations in its bottom waters, it is likely not a major source of CH4 to the atmosphere due to effective CH4 consumption in the water column, combined with limited gas diffusion from the deep water layers. Nitrite seems to serve as the main TEA for CH4 oxidation at the most active anoxic CH4 oxidation hotspot, yet a number of other potential organic and inorganic electron acceptors for CH4 oxidation are present in the water column and were demonstrated to stimulate AOM, demonstrating the high versatility of aerobic and anaerobic methanotrophic communities in freshwater environments. Near the oxycline, aerobic methanotrophy is supported by oxygen, via diffusion from above and by local production by phototrophs, and by a local input of CH4, either provided by in situ production of CH4 by the phototrophic community or by lateral transport. Overall, our study in Lake Lovojärvi shows that even in shallow lakes, CH4 oxidation in the water column can form an efficient two-step (anaerobic/aerobic) biological CH4 removal process, limiting CH4 emissions from highly productive systems.
Raw sequences have been deposited at NCBI under the BioProject number PRJNA717665 with the accession numbers SAMN18500068 to SAMN18500079.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-3087-2021-supplement.
KO, CJ and CJS were involved in designing the study, sampling campaign and experimental setups while CJS and BW developed the overall project. KO and CJ conducted the field sampling and experiments as well as the subsequent laboratory analyses. Amplicon sequence analyses were done by SvG and JZ. SvG and KO wrote the original draft. SvG adapted successive versions of the manuscript that led to the final version. CJS, BW, MFL and JZ reviewed and commented on the manuscript.
The authors declare that they have no conflict of interest.
The authors thank Christian Dinkel for his help in conducting the sampling campaign and operating measuring equipment in the field. We kindly thank the staff at the Lammi Biological Station in Finland for helping us arrange our stay there, as well as organizing a boat for the sampling campaign and the use of the laboratory. We appreciate the support of Andreas Brand in analyzing the oxygen measurements. We thank Patrick Kathriner, Serge Robert, David Kistler and Irene Brunner for their assistance in the laboratory. The Swiss National Science Foundation (SNF grant 153091) and Eawag funded this work.
This research has been supported by the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant no. 153091).
This paper was edited by Ji-Hyung Park and reviewed by two anonymous referees.
Amaral, J. A. and Knowles, R.: Growth of methanotrophs in methane and oxygen counter gradients, FEMS Microbiol. Lett., 126, 215–220, https://doi.org/10.1111/j.1574-6968.1995.tb07421.x, 1995.
Barker, J. F. and Fritz, P.: Carbon isotope fractionation during microbial methane oxidation, Nature, 293, 289–291, https://doi.org/10.1038/293289a0, 1981.
Bartlett, K. B. and Harriss, R. C.: Review and assessment of methane emissions from wetlands, Chemosphere, 26, 261–320, https://doi.org/10.1016/0045-6535(93)90427-7, 1993.
Bastviken, D., Tranvik, L. J., Downing, J. A., Crill, P. M., and Enrich-Prast, A.: Freshwater Methane Emissions Offset the Continental Carbon Sink, Science, 331, 50, https://doi.org/10.1126/science.1196808, 2011.
Biderre-Petit, C., Jézéquel, D., Dugat-Bony, E., Lopes, F., Kuever, J., Borrel, G., Viollier, E., Fonty, G., and Peyret, P.: Identification of microbial communities involved in the methane cycle of a freshwater meromictic lake, FEMS Microbiol. Ecol., 77, 533–545, https://doi.org/10.1111/j.1574-6941.2011.01134.x, 2011.
Bižić, M., Klintzsch, T., Ionescu, D., Hindiyeh, M. Y., Günthel, M., Muro-Pastor, A. M., Eckert, W., Urich, T., Keppler, F., and Grossart, H. P.: Aquatic and terrestrial cyanobacteria produce methane, Science Advances, 6, 1–10, https://doi.org/10.1126/sciadv.aax5343, 2020.
Blees, J., Niemann, H., Wenk, C. B., Zopfi, J., Schubert, C. J., Kirf, M. K., Veronesi, M. L., Hitz, C., and Lehmann, M. F.: Micro-aerobic bacterial methane oxidation in the chemocline and anoxic water column of deep south-Alpine Lake Lugano (Switzerland), Limnol. Oceanogr., 59, 311–324, https://doi.org/10.4319/lo.2014.59.2.0311, 2014.
Blumenberg, M., Seifert, R., and Michaelis, W.: Aerobic methanotrophy in the oxic–anoxic transition zone of the Black Sea water column, Org. Geochem., 38, 84–91, https://doi.org/10.1016/J.ORGGEOCHEM.2006.08.011, 2007.
Boetius, A., Ravenschlag, K., Schubert, C. J., Rickert, D., Widdel, F., Gieseke, A., Amann, R., Jørgensen, B. B., Witte, U., and Pfannkuche, O.: A marine microbial consortium apparently mediating anaerobic oxidation of methane, Nature, 407, 623–626, https://doi.org/10.1038/35036572, 2000.
Chistoserdova, L.: Methylotrophs in natural habitats: current insights through metagenomics, Appl. Microbiol. Biot., 99, 5763–5779, https://doi.org/10.1007/s00253-015-6713-z, 2015.
Cline, J. D.: Spectrophotometric determination of hydrogen sulfide in natural waters, Limnol. Oceanogr., 14, 454–458, https://doi.org/10.4319/lo.1969.14.3.0454, 1969.
Crevecoeur, S., Vincent, W. F., Comte, J., Matveev, A., and Lovejoy, C.: Diversity and potential activity of methanotrophs in high methane-emitting permafrost thaw ponds, PLoS One, 12, e0188223, https://doi.org/10.1371/journal.pone.0188223, 2017.
Crowe, S. A., Katsev, S., Leslie, K., Sturm, A., Magen, C., Nomosatryo, S., Pack, M. A., Kessler, J. D., Reeburgh, W. S., Roberts, J. A., González, L., Douglas Haffner, G., Mucci, A., Sundby, B., and Fowle, D. A.: The methane cycle in ferruginous Lake Matano, Geobiology, 9, 61–78, https://doi.org/10.1111/j.1472-4669.2010.00257.x, 2011.
Delsontro, T., Mcginnis, D. F., Sobek, S., Ostrovsky, I., and Wehrli, B.: Extreme methane emissions from a swiss hydropower Reservoir: Contribution from bubbling sediments, Environ. Sci. Technol., 44, 2419–2425, https://doi.org/10.1021/es9031369, 2010.
Deutzmann, J. S., Stief, P., Brandes, J., and Schink, B.: Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake, P. Natl. Acad. Sci. USA, 111, 18273–18278, https://doi.org/10.1073/pnas.1411617111, 2014.
Downing, J. A., Prairie, Y. T., Cole, J. J., Duarte, C. M., Tranvik, L. J., Striegl, R. G., McDowell, W. H., Kortelainen, P., Caraco, N. F., Melack, J. M., and Middelburg, J. J.: The global abundance and size distribution of lakes, ponds, and impoundments, Limnol. Oceanogr., 51, 2388–2397, https://doi.org/10.4319/lo.2006.51.5.2388, 2006.
Dunfield, P. and Knowles, R.: Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol, Appl. Environ. Microb., 61, 3129–3135, 1995.
Durisch-Kaiser, E., Schmid, M., Peeters, F., Kipfer, R., Dinkel, C., Diem, T., Schubert, C. J., and Wehrli, B.: What prevents outgassing of methane to the atmosphere in Lake Tanganyika?, J. Geophys. Res., 116, G02022, https://doi.org/10.1029/2010JG001323, 2011.
Edgar, R.: SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences, bioRxiv, https://doi.org/10.1101/074161, 2016.
Edgar, R. C.: Search and clustering orders of magnitude faster than BLAST, Bioinformatics, 26, 2460–2461, https://doi.org/10.1093/bioinformatics/btq461, 2010.
Edgar, R. C.: UPARSE: Highly accurate OTU sequences from microbial amplicon reads, Nat. Methods, 10, 996–998, https://doi.org/10.1038/nmeth.2604, 2013.
Eller, G., Känel, L., Krüger, M., Ka, L., and Kru, M.: Cooccurrence of Aerobic and Anaerobic Methane Oxidation in the Water Column of Lake Plußsee, Appl. Environ. Microb., 71, 8925–8928, https://doi.org/10.1128/AEM.71.12.8925, 2005.
Ettwig, K. F., Butler, M. K., Le Paslier, D., Pelletier, E., Mangenot, S., Kuypers, M. M. M., Schreiber, F., Dutilh, B. E., Zedelius, J., de Beer, D., Gloerich, J., Wessels, H. J. C. T., van Alen, T., Luesken, F., Wu, M. L., van de Pas-Schoonen, K. T., Op den Camp, H. J. M., Janssen-Megens, E. M., Francoijs, K.-J., Stunnenberg, H., Weissenbach, J., Jetten, M. S. M., and Strous, M.: Nitrite-driven anaerobic methane oxidation by oxygenic bacteria., Nature, 464, 543–548, https://doi.org/10.1038/nature08883, 2010.
Fenchel, T. and Blackburn, T. H.: Bacteria and mineral cycling, Academic Press, London, 1979.
Gibson, C. E.: Growth rate, maintenance energy and pigmentation of planktonic Cyanophyta during one-hour light: Dark cycles, Brit. Phycol. J., 20, 155–161, https://doi.org/10.1080/00071618500650161, 1985.
Graf, J. S., Mayr, M. J., Marchant, H. K., Tienken, D., Hach, P. F., Brand, A., Schubert, C. J., Kuypers, M. M. M., and Milucka, J.: Bloom of a denitrifying methanotroph, 'Candidatus Methylomirabilis limnetica', in a deep stratified lake, Environ. Microbiol., 20, 2598–2614, https://doi.org/10.1111/1462-2920.14285, 2018.
Griess, P.: “Über einige Azoverbindungen”, Ber. Dtsch. Chem. Ges., 12, 426–428, https://doi.org/10.1002/cber.187901201117, 1879.
Günthel, M., Klawonn, I., Woodhouse, J., Bižić, M., Ionescu, D., Ganzert, L., Kümmel, S., Nijenhuis, I., Zoccarato, L., Grossart, H. P., and Tang, K. W.: Photosynthesis-driven methane production in oxic lake water as an important contributor to methane emission, Limnol. Oceanogr., 65, 2853–2865, https://doi.org/10.1002/lno.11557, 2020.
Hakala, A.: Meromixis as a part of lake evolution; observations and a revised classification of true meromictic lakes in Finland, Boreal Environ. Res., 9, 37–53, 2004.
Hanson, R. S. and Hanson, T. E.: Methanotrophic bacteria, Microbiol. Rev., 60, 439–471, 1996.
Hinrichs, K.-U. and Boetius, A.: The Anaerobic Oxidation of Methane: New Insights in Microbial Ecology and Biogeochemistry, Ocean Margin Systems, Springer, Berlin, Heidelberg, 457–477, 2002.
Holmes, A. J., Tujula, N. A., Holley, M., Contos, A., James, J. M., Rogers, P., and Gillings, M. R.: Phylogenetic structure of unusual aquatic microbial formations in Nullarbor caves, Australia, Environ. Microbiol., 3, 256–264, https://doi.org/10.1046/j.1462-2920.2001.00187.x, 2001.
Holtappels, M., Lavik, G., Jensen, M. M., and Kuypers, M. M. M.: 15N-Labeling Experiments to Dissect the Contributions of Heterotrophic Denitrification and Anammox to Nitrogen Removal in the OMZ Waters of the Ocean, Methods Enzymol., 486, 223–251, https://doi.org/10.1016/B978-0-12-381294-0.00010-9, 2011.
Hütsch, B. W.: Methane oxidation in arable soil as inhibited by ammonium, nitrite, and organic manure with respect to soil pH, Biol. Fert. Soils, 28, 27–35, https://doi.org/10.1007/s003740050459, 1998.
Ilmavirta, V., Ilmavirta, K., and Kotimaa, A.-L.: Phytoplanktonic primary production during the summer stagnation in the eutrophicated lakes Lovojärvi and Ormajärvi, southern Finland, Ann. Bot. Fenn., 11, 121–132, https://doi.org/10.2307/23725044, 1974.
Jenkins, O., Byrom, D., and Jones, D.: Methylophilus: A New Genus of Methanol-Utilizing Bacteria, Int. J. Syst. Bacteriol., 37, 446–448, https://doi.org/10.1099/00207713-37-4-446, 1987.
Joyce, J. and Jewell, P. W.: Physical controls on methane ebullition from reservoirs and lakes, Environ. Eng. Geosci., 9, 167–178, https://doi.org/10.2113/9.2.167, 2003.
Juutinen, S., Rantakari, M., Kortelainen, P., Huttunen, J. T., Larmola, T., Alm, J., Silvola, J., and Martikainen, P. J.: Methane dynamics in different boreal lake types, Biogeosciences, 6, 209–223, https://doi.org/10.5194/bg-6-209-2009, 2009.
Kallistova, A., Kadnikov, V., Rusanov, I., Kokryatskaya, N., Beletsky, A., Mardanov, A., Savvichev, A., Ravin, N., and Pimenov, N.: Microbial communities involved in aerobic and anaerobic methane cycling in a meromictic ferruginous subarctic lake, Aquat. Microb. Ecol., 82, 1–18, https://doi.org/10.3354/ame01878, 2019.
Kankaala, P., Huotari, J., Peltomaa, E., Saloranta, T., and Ojala, A.: Methanotrophic activity in relation to methane efflux and total heterotrophic bacterial production in a stratified, humic, boreal lake, Limnol. Oceanogr., 51, 1195–1204, https://doi.org/10.4319/lo.2006.51.2.1195, 2006.
Keskitalo, J.: The species composition and biomass of phytoplankton in the eutrophic Lake Lovojärvi, southern Finland, Ann. Bot. Fenn., 14, 71–81, https://www.jstor.org/stable/43922123 (available at: 19 May 2021), 1977.
Kiener, A. and Leisinger, T.: Oxygen Sensitivity of Methanogenic Bacteria, Syst. Appl. Microbiol., 4, 305–312, https://doi.org/10.1016/S0723-2020(83)80017-4, 1983.
King, G.: Ecological aspects of methane oxidation, a key determinant of global methane dynamics, Adv. Microb. Ecol., 431–468, 1992.
Kits, D. K., Campbell, D. J., Rosana, A. R., and Stein, L. Y.: Diverse electron sources support denitrification under hypoxia in the obligate methanotroph Methylomicrobium album strain BG8, Front. Microbiol., 6, 1–11, https://doi.org/10.3389/fmicb.2015.01072, 2015a.
Kits, D. K., Klotz, M. G., and Stein, L. Y.: Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1, Environ. Microbiol., 17, 3219–3232, https://doi.org/10.1111/1462-2920.12772, 2015b.
Kortelainen, P., Huttunen, J. T., Väisänen, T., Mattsson, T., Karjalainen, P., and Martikainen, P. J.: CH4, CO2 and N2O supersaturation in 12 Finnish lakes before and after ice-melt, SIL Proceedings, 1922–2010, 27, 1410–1414, https://doi.org/10.1080/03680770.1998.11901468, 2000.
Kortelainen, P., Pajunen, H., Rantakari, M., and Saarnisto, M.: A large carbon pool and small sink in boreal Holocene lake sediments, Glob. Change Biol., 10, 1648–1653, https://doi.org/10.1111/j.1365-2486.2004.00848.x, 2004.
Kreling, J., Bravidor, J., McGinnis, D. F., Koschorreck, M., and Lorke, A.: Physical controls of oxygen fluxes at pelagic and benthic oxyclines in a lake, Limnol. Oceanogr., 59, 1637–1650, https://doi.org/10.4319/lo.2014.59.5.1637, 2014.
Krom, M. D.: Spectrophotometric determination of ammonia: a study of a modified Berthelot reaction using salicylate and dichloroisocyanurate, Analyst, 105, 305, https://doi.org/10.1039/an9800500305, 1980.
Magoč, T. and Salzberg, S. L.: FLASH: Fast length adjustment of short reads to improve genome assemblies, Bioinformatics, 27, 2957–2963, https://doi.org/10.1093/bioinformatics/btr507, 2011.
Martinez-Cruz, K., Leewis, M. C., Herriott, I. C., Sepulveda-Jauregui, A., Anthony, K. W., Thalasso, F., and Leigh, M. B.: Anaerobic oxidation of methane by aerobic methanotrophs in sub-Arctic lake sediments, Sci. Total Environ., 607–608, 23–31, https://doi.org/10.1016/j.scitotenv.2017.06.187, 2017.
Mayr, M. J., Zimmermann, M., Dey, J., Brand, A., Wehrli, B., and Bürgmann, H.: Growth and rapid succession of methanotrophs effectively limit methane release during lake overturn, Communications Biology, 3, 1–9, https://doi.org/10.1038/s42003-020-0838-z, 2020.
McMurdie, P. J. and Holmes, S.: Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data, PLoS One, 8, 1–11, https://doi.org/10.1371/journal.pone.0061217, 2013.
Michaelis, W., Seifert, R., Nauhaus, K., Treude, T., and Thiel, V.: Microbial Reefs in the Black Sea fueled by anaerobic Oxidation of Methane, Science, 297, 1013–1015, https://doi.org/10.1126/science.1072502, 2002.
Michmerhuizen, C. M., Striegl, R. G., and McDonald, M. E.: Potential methane emission from north-temperate lakes following ice melt, Limnol. Oceanogr., 41, 985–991, https://doi.org/10.4319/lo.1996.41.5.0985, 1996.
Milucka, J., Kirf, M., Lu, L., Krupke, A., Lam, P., Littmann, S., Kuypers, M. M. M., and Schubert, C. J.: Methane oxidation coupled to oxygenic photosynthesis in anoxic waters, ISME J., 9, 1991–2002, https://doi.org/10.1038/ismej.2015.12, 2015.
Miracle, M., Vicente, E., and Pedrós-Alió, C.: Biological studies of Spanish meromictic and stratified karstic lakes, Limnetica, 8, 59–77, 1992.
Monchamp, M. E., Walser, J. C., Pomati, F., and Spaak, P.: Sedimentary DNA reveals cyanobacterial community diversity over 200 years in two perialpine lakes, Appl. Environ. Microb., 82, 6472–6482, https://doi.org/10.1128/AEM.02174-16, 2016.
Norði, K. à., Thamdrup, B., and Schubert, C. J.: Anaerobic oxidation of methane in an iron-rich Danish freshwater lake sediment, Limnol. Oceanogr., 58, 546–554, https://doi.org/10.4319/lo.2013.58.2.0546, 2013.
Orphan, V. J., House, C. H., and Hinrichs, K.: Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis, Science, 293, 484–488, https://doi.org/10.1126/science.1061338, 2001.
Oshkin, I. Y., Beck, D. A., Lamb, A. E., Tchesnokova, V., Benuska, G., McTaggart, T. L., Kalyuzhnaya, M. G., Dedysh, S. N., Lidstrom, M. E., and Chistoserdova, L.: Methane-fed microbial microcosms show differential community dynamics and pinpoint taxa involved in communal response, ISME J., 9, 1–11, https://doi.org/10.1038/ismej.2014.203, 2014.
Oswald, K., Milucka, J., Brand, A., Littmann, S., Wehrli, B., Kuypers, M. M. M., and Schubert, C. J.: Light-dependent aerobic methane oxidation reduces methane emissions from seasonally stratified lakes, PLoS One, 10, e0132574, https://doi.org/10.1371/journal.pone.0132574, 2015.
Oswald, K., Milucka, J., Brand, A., Hach, P., Littmann, S., Wehrli, B., Kuypers, M. M. M., and Schubert, C. J.: Aerobic gammaproteobacterial methanotrophs mitigate methane emissions from oxic and anoxic lake waters, Limnol. Oceanogr., 61, S101–S118, https://doi.org/10.1002/lno.10312, 2016a.
Oswald, K., Jegge, C., Tischer, J., Berg, J., Brand, A., Miracle, M. R., Soria, X., Vicente, E., Lehmann, M. F., Zopfi, J., and Schubert, C. J.: Methanotrophy under versatile conditions in the water column of the ferruginous meromictic Lake La Cruz (Spain), Front. Microbiol., 7, 1–16, https://doi.org/10.3389/fmicb.2016.01762, 2016b.
Panganiban, A. T., Patt, T. E., Hart, W., and Hanson, R. S.: Oxidation of methane in the absence of oxygen in lake water samples, Appl. Environ. Microb., 37, 303–309, https://doi.org/10.1128/aem.37.2.303-309.1979, 1979.
Parada, A. E., Needham, D. M., and Fuhrman, J. A.: Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples, Environ. Microbiol., 18, 1403–1414, https://doi.org/10.1111/1462-2920.13023, 2016.
Peeters, F., Encinas Fernandez, J., and Hofmann, H.: Sediment fluxes rather than oxic methanogenesis explain diffusive CH4 emissions from lakes and reservoirs, Sci. Rep.-UK, 9, 1–10, https://doi.org/10.1038/s41598-018-36530-w, 2019.
Pernthaler, A., Pernthaler, J., and Amann, R.: Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria, Appl. Environ. Microb., 68, 3094–3101, https://doi.org/10.1128/AEM.68.6.3094-3101.2002, 2002.
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J., and Glöckner, F. O.: The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 41 (Database issue), D590-6, https://doi.org/10.1093/nar/gks1219, 2013.
Rappé, M. S. and Giovannoni, S. J.: The Uncultured Microbial Majority, Annu. Rev. Microbiol., 57, 369–394, https://doi.org/10.1146/annurev.micro.57.030502.090759, 2003.
Reeburgh, W. S.: Oceanic Methane Biogeochemistry, Chem. Rev., 107, 486–513, https://doi.org/10.1021/cr050362v, 2007.
Reed, D. C., Deemer, B. R., van Grinsven, S., and Harrison, J. A.: Are elusive anaerobic pathways key methane sinks in eutrophic lakes and reservoirs?, Biogeochemistry, 134, 29–39, https://doi.org/10.1007/s10533-017-0356-3, 2017.
Rissanen, A. J., Saarenheimo, J., Tiirola, M., Peura, S., Aalto, S. L., Karvinen, A., and Nykänen, H.: Gammaproteobacterial methanotrophs dominate methanotrophy in aerobic and anaerobic layers of boreal lake waters, Aquat. Microb. Ecol., 81, 257–276, https://doi.org/10.3354/ame01874, 2018.
Rissanen, A. J., Saarela, T., Jäntti, H., Buck, M., Peura, S., Aalto, S. L., Ojala, A., Pumpanen, J., Tiirola, M., Elvert, M., and Nykänen, H.: Vertical stratification patterns of methanotrophs and their genetic controllers in water columns of oxygen-stratified boreal lakes, FEMS Microbiol. Ecol., 97, 1–16, https://doi.org/10.1093/femsec/fiaa252, 2021.
Rudd, J. W. M.: Methane cycling in aquatic environments, Advances in Aquatic Microbiology, 1, 77–150, 1980.
Rudd, J. W. M. and Hamilton, R. D.: Factors controlling rates of methane oxidation and the distribution of the methane oxidizers in a small stratified lake, Arch. Hydrobiol., 75, 522–538, https://doi.org/10.1126/science.aad7154, 1975.
Rudd, J. W. M., Furutani, A., Flett, R. J., and Hamilton, R. D.: Factors controlling methane oxidation in shield lakes: The role of nitrogen fixation and oxygen concentration, Limnol. Oceanogr., 21, 357–364, https://doi.org/10.4319/lo.1976.21.3.0357, 1976.
Saarnisto, M., Huttunen, P., and Tolonen, K.: Annual lamination of sediments in Lake Lovojärvi, southern Finland, during the past 600 years, Ann. Bot. Fenn., 14, 35–45, https://www.jstor.org/stable/23726048 (last access: 19 May 2021), 1977.
Saxton, M. A., Samarkin, V. A., Schutte, C. A., Bowles, M. W., Madigan, M. T., Cadieux, S. B., Pratt, L. M., and Joye, S. B.: Biogeochemical and 16S rRNA gene sequence evidence supports a novel mode of anaerobic methanotrophy in permanently ice-covered Lake Fryxell, Antarctica, Limnol. Oceanogr., 61, S119–S130, https://doi.org/10.1002/lno.10320, 2016.
Scheller, S., Yu, H., Chadwick, G. L., and Mcglynn, S. E.: Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction, Science, 351, 703–707, 2016.
Schmieder, R. and Edwards, R.: Quality control and preprocessing of metagenomic datasets, Bioinformatics, 27, 863–864, https://doi.org/10.1093/bioinformatics/btr026, 2011.
Schubert, C. J., Lucas, F. S., Durisch-Kaiser, E., Stierli, R., Diem, T., Scheidegger, O., Vazquez, F., and Muller, B.: Oxidation and emission of methane in a monomictic lake (Rotsee, Switzerland), Aquat. Sci., 72, 455–466, https://doi.org/10.1007/s00027-010-0148-5, 2010.
Schubert, C. J., Vazquez, F., Loesekann-Behrens, T., Knittel, K., Tonolla, M., and Boetius, A.: Evidence for anaerobic oxidation of methane in sediments of a freshwater system (Lago di Cadagno), FEMS Microbiol. Ecol., 76, 26–38, https://doi.org/10.1111/j.1574-6941.2010.01036.x, 2011.
Simola, H.: Micro-stratigraphy of sediment laminations deposited in a chemically stratifying eutrophic lake during the years 1913–1976, Ecography, 2, 160–168, https://doi.org/10.1111/j.1600-0587.1979.tb00696.x, 1979.
Simola, H., Hanski, I., and Liukkonen, M.: Stratigraphy, species richness and seasonal dynamics of plankton diatoms during 418 years in Lake Lovojärvi, South Finland, Ann. Bot. Fenn., 27, 241–259, https://www.jstor.org/stable/23725364 (last access: 19 May 2021), 1990.
Sivan, O., Adler, M., Pearson, A., Gelman, F., Bar-Or, I., John, S. G., and Eckert, W.: Geochemical evidence for iron-mediated anaerobic oxidation of methane, Limnol. Oceanogr., 56, 1536–1544, https://doi.org/10.4319/lo.2011.56.4.1536, 2011.
Smith, G. J., Angle, J. C., Solden, L. M., Daly, R. A., Johnston, M. D., Borton, M. A., Wolfe, R., Stefanik, K. C., Morin, T. H., Gil, B., and Wrighton, K. C.: Members of the Genus Methylobacter Are Inferred To Account for the Majority of Aerobic Methane Oxidation in Oxic Soils from a Freshwater Wetland, mBio, 9, 1–17, https://doi.org/10.1128/mbio.00815-18, 2018.
Stookey, L. L.: Ferrozine – a new spectrophotometric reagent for iron, Anal. Chem., 42, 779–781, https://doi.org/10.1021/ac60289a016, 1970.
Su, G., Zopfi, J., Yao, H., Steinle, L., Niemann, H., and Lehmann, M. F.: Manganese/iron-supported sulfate-dependent anaerobic oxidation of methane by archaea in lake sediments, Limnol. Oceanogr., 65, 863–875, https://doi.org/10.1002/lno.11354, 2020.
Sundh, I., Bastviken, D., and Tranvik, L. J.: Abundance, activity, and community structure of pelagic methane-oxidizing bacteria in temperate lakes, Appl. Environ. Microb., 71, 6746–6752, https://doi.org/10.1128/AEM.71.11.6746-6752.2005, 2005.
Taipale, S., Kankaala, P., Hahn, M., Jones, R., and Tiirola, M.: Methane-oxidizing and photoautotrophic bacteria are major producers in a humic lake with a large anoxic hypolimnion, Aquat. Microb. Ecol., 64, 81–95, https://doi.org/10.3354/ame01512, 2011.
Tavormina, P. L., Kellermann, M. Y., Antony, C. P., Tocheva, E. I., Dalleska, N. F., Jensen, A. J., Valentine, D. L., Hinrichs, K. U., Jensen, G. J., Dubilier, N., and Orphan, V. J.: Starvation and recovery in the deep-sea methanotroph Methyloprofundus sedimenti, Mol. Microbiol., 103, 242–252, https://doi.org/10.1111/mmi.13553, 2017.
Thottathil, S. D., Reis, P. C. J., and Prairie, Y. T.: Methane oxidation kinetics in northern freshwater lakes, Biogeochemistry, 143, 105–116, https://doi.org/10.1007/s10533-019-00552-x, 2019.
Tolonen, K., Tolonen, M., Honkasalo, L., Lehtovaara, A., Sorsa, K., and Sundberg, K.: The influence of of prehistoric and historic land use on Lake Lampellonjärvi, South Finland, Luonnon Tutkija, 80, 1–15, 1976.
Valenzuela, E. I., Avendaño, K. A., Balagurusamy, N., Arriaga, S., Nieto-Delgado, C., Thalasso, F., and Cervantes, F. J.: Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments, Sci. Total Environ., 650, 2674–2684, https://doi.org/10.1016/J.SCITOTENV.2018.09.388, 2019.
Van Bodegom, P., Stams, F., Mollema, L., Boeke, S., and Leffelaar, P.: Methane Oxidation and the Competition for Oxygen in the Rice Rhizosphere, Appl. Environ. Microb., 67, 3586–3597, https://doi.org/10.1128/AEM.67.8.3586-3597.2001, 2001.
van Grinsven, S., Sinninghe Damsté, J. S., and Villanueva, L.: Assessing the effect of humic substances and Fe(III) as potential electron acceptors for anaerobic methane oxidation in a marine anoxic system, Microorganisms, 8, 1–15, https://doi.org/10.3390/microorganisms8091288, 2020a.
van Grinsven, S., Sinninghe Damsté, J. S., Abdala Asbun, A., Engelmann, J. C., Harrison, J., and Villanueva, L.: Methane oxidation in anoxic lake water stimulated by nitrate and sulfate addition, Environ. Microbiol., 22, 766–782, https://doi.org/10.1111/1462-2920.14886, 2020b.
van Grinsven, S., Sinninghe Damsté, J. S., Harrison, J., Polerecky, L., and Villanueva, L.: Nitrate promotes the transfer of methane-derived carbon from the methanotroph Methylobacter sp. to the methylotroph Methylotenera sp. in eutrophic lake water, Limnol. Oceanogr., 2, 1–14, https://doi.org/10.1002/lno.11648, 2020c.
Viollier, E., Inglett, P. W., Hunter, K., Roychoudhury, A. N., and Van Cappellen, P.: The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters, Appl. Geochem., 15, 785–790, https://doi.org/10.1016/S0883-2927(99)00097-9, 2000.
Walter, K. M., Smith, L. C., and Chapin, S. F.: Methane bubbling from northern lakes: Present and future contributions to the global methane budget, Philos. T. R. Soc. A, 365, 1657–1676, https://doi.org/10.1098/rsta.2007.2036, 2007.
Wei, X. M., He, R., Chen, M., Su, Y., and Ma, R. C.: Conversion of methane-derived carbon and microbial community in enrichment cultures in response to O2-availability, Environ. Sci. Pollut. R., 23, 7517–7528, https://doi.org/10.1007/s11356-015-6017-y, 2016.
Whiticar, M. J.: Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane, Chem. Geol., 161, 291–314, https://doi.org/10.1016/S0009-2541(99)00092-3, 1999.
Wiesenburg, D. A. and Guinasso, N. L.: Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water, J. Chem. Eng. Data, 24, 356–360, https://doi.org/10.1021/je60083a006, 1979.
Zheng, Y., Wang, H., Liu, Y., Zhu, B., Li, J., Yang, Y., Qin, W., Chen, L., Wu, X., Chistoserdova, L., and Zhao, F.: Methane-Dependent Mineral Reduction by Aerobic Methanotrophs under Hypoxia, Environ. Sci. Tech. Let., 7, 606–612, https://doi.org/10.1021/acs.estlett.0c00436, 2020.