the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Evaluating the potential for Haloarchaea to serve as ice nucleating particles
Jessie M. Creamean
Julio E. Ceniceros
Lilyanna Newman
Allyson D. Pace
Thomas C. J. Hill
Paul J. DeMott
Matthew E. Rhodes
Aerosols play a crucial role in cloud formation. Biologically derived materials from bacteria, fungi, pollen, lichen, viruses, algae, and diatoms can serve as ice nucleating particles (INPs), some of which initiate glaciation in clouds at relatively warm freezing temperatures. However, determining the magnitude of the interactions between clouds and biologically derived INPs remains a significant challenge due to the diversity and complexity of bioaerosols and limited observations of such aerosols facilitating cloud ice formation. Additionally, microorganisms from the domain Archaea have, to date, not been evaluated as INPs. Here, we present the first results reporting the ice nucleation activity of four species in the class Haloarchaea. Intact cells of Halococcus morrhuae and Haloferax sulfurifontis demonstrated the ability to induce immersion freezing at temperatures up to −18 ∘C, while lysed cells of Haloquadratum walsbyi and Natronomonas pharaonis were unable to serve as immersion INPs. Exposure to heat and peroxide digestion indicated that the INPs of intact cells were driven by organic (H. morrhuae and H. sulfurifontis) and possibly also heat labile materials (H. sulfurifontis only). While halophiles are prominent in hypersaline environments such as the Great Salt Lake and the Dead Sea, other members of the Archaea, such as methanogens and thermophiles, are prevalent in anoxic systems in seawater, sea ice, marine sediments, glacial ice, permafrost, and other cold niches. Archaeal extremophiles are both diverse and highly abundant. Thus, it is important to assess their ability to serve as INPs as it may lead to an improved understanding of biological impacts on clouds.
- Article
(2328 KB) - Full-text XML
-
Supplement
(538 KB) - BibTeX
- EndNote
Through their impact on the atmospheric energy budget and hydrological cycle, clouds play a prominent role in shaping Earth's climate at both global and regional scales (Baker and Peter, 2008; Boucher et al., 2013). However, due to the complexity of the microphysical processes associated with cloud formation and dynamics, clouds remain some of the most poorly constrained atmospheric features in climate models. In turn, while atmospheric aerosols strongly impact cloud formation, albedo, lifetime, and precipitation formation processes, disentangling the relationships and feedbacks among aerosols, clouds, and precipitation remains a significant challenge (Sato and Suzuki, 2019; Stevens and Feingold, 2009). In particular, the role of aerosols called ice nucleating particles (INPs), which induce glaciation in the mixed phase and ice clouds, is highly uncertain relative to other aerosol–cloud processes (Kanji et al., 2017). Improving our understanding of such ice formation processes is crucial given that most precipitation, globally, is initiated via the ice phase (Lohmann and Feichter, 2005).
In the Earth's troposphere, pure water remains in a supercooled liquid state until below −38 ∘C. At temperatures greater than −38 ∘C, the assistance of INPs, such as mineral dust, volcanic ash, and select biologically produced macromolecules (e.g., from pollen, fungi, bacteria, and other sources), is required to initiate the heterogeneous formation of primary ice embryos that continue to grow into larger ice crystals (Hoose and Möhler, 2012; Kanji et al., 2017). Depending on their surface properties and structural makeup, mineral dust and volcanic ash can raise the freezing point of water from −38 ∘C to as high as −12 to −15 ∘C (Murray et al., 2012). At temperatures above −15 ∘C, and specifically in the immersion freezing regime (i.e., heterogeneous glaciation of a supercooled droplet), the majority of naturally occurring INPs are biological in origin (Fröhlich-Nowoisky et al., 2016; Hoose and Möhler, 2012; Kanji et al., 2017; Morris et al., 2004). Intact and lysed cell wall fragments (Anderson and Ashworth, 1986; Du et al., 2017; O'Sullivan et al., 2015; Šantl-Temkiv et al., 2015), viable and nonviable cells (Anderson and Ashworth, 1986), and cellular byproduct materials such as exopolymeric substances, saccharides, and biosurfactants (Albers et al., 2017; Decho and Gutierrez, 2017; Demott et al., 2018; Dreischmeier et al., 2017; Perkins et al., 2020b; Zeppenfeld et al., 2019) have all been demonstrated to serve as biologically derived INPs (Després et al., 2012; Fröhlich-Nowoisky et al., 2016). The most thoroughly studied biologically derived INPs of measurable abundance are strains of the bacterium Pseudomonas syringae, which is capable of forming ice at temperatures as high as −1 ∘C (Maki et al., 1974; Morris et al., 2008). The ability of P. syringae to effectively facilitate ice formation is due to a specific ice nucleating protein (i.e., a protein that is ice nucleation active; designated as Ina) that coordinates the crystallization of ice (Cochet and Widehem, 2000; Davies, 2014; Failor et al., 2017; Gurian-Sherman and Lindow, 1993; Hartmann et al., 2013; Šantl-Temkiv et al., 2015). The Ina protein is found in select lineages of bacteria all within the class Gammaproteobacteria (Warren, 1995). There is some evidence that slightly less effective but highly abundant proteinaceous ice nucleating proteins can be found within fungi, but they remain to be fully categorized (Fröhlich-Nowoisky et al., 2015; Kunert et al., 2019). Furthermore, a recent study identifying the first Gram positive ice nucleating bacterium from cloud precipitation suggests that ice nucleating proteins are far more widespread within the bacterial domain than previously thought (Failor et al., 2017).
Even though many laboratory and field-based investigations have alluded to the importance of biologically derived INPs, the relatively limited available observational data have caused models to produce equivocal results regarding the global significance of biological ice nucleation in cloud and precipitation formation (Burrows et al., 2013; Hoose et al., 2010a; Hummel et al., 2018; Phillips et al., 2009; Sesartic et al., 2012; Twohy et al., 2016; Vergara-Temprado et al., 2017). This modeling issue is further complicated by a very limited understanding and representation of secondary ice formation processes and their links to biologically derived INPs in clouds. Climate models of all scales require information on INP sources to accurately represent ice nucleation and, thus, cloud microphysics, especially considering that (1) biologically derived INPs form ice at cloud temperatures as high as −1 ∘C while certain mineral dusts glaciate modestly starting at −12 ∘C, and (2) biologically derived and mineral INP concentrations can vary by several orders of magnitude at any given temperature (Burrows et al., 2013; Demott et al., 2016; Hoose et al., 2010b; Kanji et al., 2017; Mccluskey et al., 2019; Petters and Wright, 2015; Vergara-Temprado et al., 2017).
While there have been ongoing efforts to identify and characterize INPs in the eukaryotic and bacterial domains (Dreischmeier et al., 2017; Failor et al., 2017; Fröhlich-Nowoisky et al., 2016; Hill et al., 2014; Kanji et al., 2017; Kunert et al., 2019; Ling et al., 2018; Morris et al., 2008; Pummer et al., 2012; Qiu et al., 2019), to date, there has been no study that has attempted to identify INPs within the domain Archaea. This is in no small part due the fact that, until recently, the Archaea were believed to be largely relegated to marginal existences in extreme environments on our planet. Though recent studies have begun to reveal the true ubiquity and abundance of Archaea in Earth's ecosystems – including seawater, ocean sediment, plankton, soil, marine and terrestrial biofilms, and sea ice, where they can comprise up to 40 % of the microbial taxa in an ecosystem (Cavicchioli, 2011; Flemming and Wuertz, 2019; Hoshino and Inagaki, 2019; Junge et al., 2004; Mondav et al., 2014; Munson et al., 1997; Ochsenreiter et al., 2002; Santoro et al., 2019) – many Archaea remain uncultured and unculturable in laboratory settings, further complicating investigations into their possible propensities to serve as INPs. At the same time, however, the archaeal domain contains both unique cell wall compositions and cell surface structures not present in the other domains (Albers and Meyer, 2011). While appearing highly similar to the bacterial domain in both size and appearance, the archaeal cell envelope is distinct in several ways. In contrast to the bacteria, most cultured Archaea maintain a proteinaceous surface layer, or S layer, that provides the cell with structural stability. In many Archaea, the S layers provide the entirety of the cell envelope. Comparatively few Archaea contain additional cell envelope polymers, and the ones that do, do not produce the near-ubiquitous bacterial polymer peptidoglycan. Some Archaea do produce a structurally similar polymer, pseudomurein. The archaeal S layers are often composed of a single protein or glycoprotein arranged in symmetrical patterns, with hexagonal symmetry being the most common (Albers and Meyer, 2011). As with the bacteria, many surface-exposed proteins are modified in a variety of ways. In addition to the S layer itself, the archaeal domain contains its own regimen of surface proteins and structures that interact with the external environment. Thus, an entire domain worth of cell surface structures remains to be assessed for potential INP activity.
Here we present a first attempt to assess the potential for members of the domain Archaea to serve as INPs. We initiated our investigation with the following four members of the obligate halophilic lineage, Haloarchaea: Halococcus morrhuae, Haloferax sulfurifontis, Haloquadratum walsbyi, and Natronomonas pharaonis. Together, these four Haloarchaea represent a variety of cell surface designs. Halococcus is one of a limited number of genera belonging to the Archaea that does not possess an S layer. Instead they possess a cell envelope that includes highly sulfated heteropolysaccharides (Schleifer et al., 1982). They can be several microns in size, exhibit a cocci (i.e., spheroidal) morphology, and do not lyse in fresh water (Legat et al., 2010; Leuko et al., 2004). Haloferax possesses an exclusively sulfur-rich S layer and exists as rod- or irregular-shaped cells several microns in size (Elshahed et al., 2004). Haloquadratum can produce halomucin in addition to its S layer and grows in its trademark square morphology, a couple of microns in size (Burns et al., 2007). Natronomonas exist as rods several microns in length and prefer alkaline hypersaline environments (Falb et al., 2005). Specifically, H. walsbyi and N. pharaonis are particularly sensitive to lysis under hyposaline conditions (Boring et al., 1963) – they readily lyse in salinities of roughly 5 % salt by weight and below. Assessing a variety of cells that lyse or remain intact is relevant for ice nucleation because cell fragments of other microorganisms have been shown to serve as INPs (Anderson and Ashworth, 1986; Du et al., 2017; O'Sullivan et al., 2015; Šantl-Temkiv et al., 2015), and Archaea might lyse naturally once exposed to atmospheric water vapor in the aerosol phase.
We opted to initiate our investigation with members of the Haloarchaea for a variety of reasons, including the following: (1) the diversity of cell surface compositions, (2) that they are relatively easy to culture compared to other archaeal lineages, and (3) that the regional dominance of the Haloarchaea in relatively large hypersaline environments. While few large-scale geographic areas are dominated by members of the domain Archaea, one notable exception is hypersaline bodies of water such as the north basin of the Great Salt Lake and the Dead Sea. These waterbodies extend over hundreds of square kilometers, often contain upwards of 90 % Archaea, and can impact the local climate and weather patterns (Carpenter, 1993). Thus, when investigating the impact of potential archaeal INPs in an environment, halophiles offer attractive starting points.
To fully understand the interaction of Earth's climate with the microbial world, it is imperative to include the impact of the archaeal domain since, even when less prevalent, the possession of ice nucleation activity will enable a species to exert an outsized effect on its environs. And to fully understand the potential impact of the archaeal domain on Earth's climate, it is important to assess the potential for members of the Archaea to serve as INPs and contribute to cloud formation.
2.1 Cell cultures
Cell cultures for all four haloarchaeal species tested were purchased from the DSMZ (German Collection of Microorganisms and Cell Cultures; https://www.dsmz.de/, last access: 1 April 2020). The associated saline medias were prepared for H. morrhuae (medium 97), H. sulfurifontis (medium 1018), H. walsbyi (medium 1091), and N. pharaonis (medium 371) with the following alterations: (1) for medium 97, only 150 g of NaCl was added instead of 250 g NaCl for the growth of H. morrhuae, and (2) 1 g L−1 of glycerol was supplemented to medium 1091 for the growth of H. walsbyi. The salinities of the media were confirmed using a handheld refractometer. All cultures were grown at 37 ∘C and 100 rpm (revolutions per minute) until mid-log phase. The purity and cell density were monitored optically using a Leica DM750 microscope (https://us.leica-camera.com/, last access: 3 July 2019) at 1000× magnification with a 100× oil immersion objective lens on a Petroff–Hausser 3900 counting chamber (http://hausserscientific.com/, last access: 3 July 2019). Cells were counted and monitored for growth until the mid-log phase, at which point they were shipped overnight on ice to Colorado and stored for up to 48 h at 4 ∘C. Cultures were checked a final time for cell density immediately prior to ice nucleation assays to ensure that no appreciable growth had occurred during transport and storage. Table 1 provides the cell concentrations and salinities of all four prepared cultures immediately prior to ice nucleation assays.
Table 1Samples produced from all four haloarchaeal species for ice nucleation testing. Initial sample cell concentrations and salinities are provided after culturing. Diluted cell concentrations and salinities were calculated based on the volume of culture mixed with media and dilution factor. The cell state is also provided after dilution.
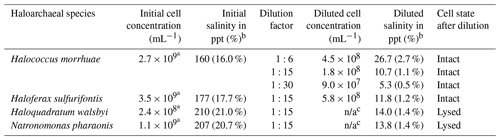
a Initial cell concentrations were too many to count optically and were estimated based on diluted samples, which were 1:15 in deionized water dilution for H. morrhuae and 1:6 for H. sulfurifontis and N. pharaonis.
b For initial and diluted salinity, “ppt” represents parts per thousand.
c For the diluted cell concentrations, “n/a” is not applicable due to lysing.
2.2 Preparation of samples for ice nucleation measurements
Due to the high salinities of the media which would have caused significant freezing point depression – for reference, seawater is typically 30–35 ppt (Bodnar, 1993) and can depress the freezing point of water by ≥2 ∘C (Irish et al., 2019; Schnell and Vali, 1975) – samples were diluted as shown in Table 1. Reductions in salinity can inherently cause the cells of certain haloarchaeal species to lyse (Boring et al., 1963; Legat et al., 2010; Leuko et al., 2004). Testing ice nucleation responses from cell lysis is relevant given that (1) cell fragments from fungi and bacteria have been previously observed to serve as INPs (Anderson and Ashworth, 1986; Du et al., 2017; O'Sullivan et al., 2015; Šantl-Temkiv et al., 2015), and (2) that a less saline environment is more atmospherically relevant for how Archaea might behave once exposed to atmospheric water vapor in the aerosol phase. Cell lysis was determined by diluting cultures with deionized water (as shown in Table 10) and microscopically assessing cellular survival. Samples were diluted 1:15 with deionized water to result in 1.1 %–1.4 % salinity. This dilution was chosen based on tests using saline solution controls (made with Instant Ocean®Sea Salt) and solutions with H. walsbyi and N. pharaonis whereby, for example, freezing would not be observed until ∘C and then only reach the ∼0.2 fraction frozen curve at the instrument's lower limit for a 10 % salt solution. Thus, a 1:15 dilution was found to be a good compromise for preventing significant freezing point depression while still obtaining full spectra (i.e., reaching a fraction frozen curve of 1). Additional tests were conducted on H. morrhuae cultures diluted to 1:6 and 1:30, with cells remaining intact at all dilutions tested. Cultures were checked microscopically after dilution for cell density and to ascertain if cell lysis had occurred.
2.3 Immersion freezing ice nucleation experiments
INP concentrations were measured using the Colorado State University (CSU) Ice Spectrometer (IS; Hiranuma et al., 2015; Mccluskey et al., 2018; Suski et al., 2018), which is an immersion freezing measurement device suited to testing aliquots of liquid culture. The IS is constructed using two 96-well aluminum incubation blocks, designed for incubating polymerase chain reaction (PCR) plates placed end-to-end and encased on their sides and base by cold plates. Immersion freezing temperature spectra were obtained by dispensing 50 µL aliquots of cell suspensions into sterile 96-well PCR trays in a laminar flow cabinet. Each sample consisted of 24 aliquots. PCR plates were then placed into the blocks of the IS, after which the device was cooled using SYLTHERM XLT in a recirculating low-temperature bath. Frozen aliquots were detected with a charged-coupled device (CCD) camera system controlled with LabVIEW (LabVIEW; Nation Instruments Corp.) as the temperature was lowered at approximately 0.33 to down to −29 ∘C. The temperature uncertainty of the IS is less than ±0.2 ∘C, which is a combination of the uncertainty in the thermocouples and the temperature variation across the blocks due to gradients in cooling. From the number of wells frozen at each temperature step, the fraction frozen curve is calculated. Confidence intervals (95 %) were calculated based on the methodology of Agresti and Coull (1998). Deionized water and uninoculated diluted media were run as controls for all species.
Heat and peroxide treatments were conducted to isolate heat labile (e.g., proteinaceous) and organic INPs in the diluted samples (Barry et al., 2021; Creamean et al., 2020; Hill et al., 2016; Mccluskey et al., 2018; Perkins et al., 2020a; Suski et al., 2018; Tobo et al., 2014). Each sample (i.e., all dilutions listed in Table 1) was subjected to heat and peroxide treatments to obtain the heat labile (proteinaceous) and organic frozen fractions in addition to the total (unamended) frozen fractions. The stability (or lack thereof) of INPs to these treatments provides an indication of the composition. To assess the contribution of heat labile entities, a 1.5 mL aliquot of suspension was tested after heating to 95 ∘C for 20 min. To remove all organic INPs, 0.75 mL of 30 % H2O2 was added to another 1.5 mL aliquot of suspension and the mixture heated to 95 ∘C for 20 min while illuminated with UVA/UVB fluorescent bulbs (Exo Terra Reptile UVB; 2×26 W providing ∼2000 µW cm−2 UVA and ∼300 µW cm−2 UVB at the distance used) to generate hydroxyl radicals (residual H2O2 was removed using catalase) prior to testing. Both heat- and peroxide-treated samples were tested simultaneous to the unamended samples in the IS for each species, since the CSU IS can house up to four 96-well plates at one time. Remaining INPs are possibly aggregates of cellular material that are not fully digested, inorganic INPs in the media, or other biological materials resistant to heat and peroxide digestion (Conen et al., 2011; Perkins et al., 2020a).
3.1 Interspecies comparison of haloarchaeal ice nucleation abilities
Figure 1 shows the results of the 1:15 dilutions of each of the haloarchaeal species. The media for all four species contributed modestly to background freezing as compared to deionized water controls. H. morrhuae performed best as an INP, initiating the freezing of water at temperatures as high as −17.6 ∘C. H. sulfurifontis also demonstrated enhanced capability to serve as an INP, freezing water at temperatures above that of deionized water and sterile media, with a freezing onset of −19.2 ∘C. These Haloarchaea are not as proficient at being ice nucleators as other more commonly studied biologically derived entities such as certain bacteria (up to −1.3 ∘C; Kim et al., 1987; Lindow et al., 1989; Maki et al., 1974; Vali et al., 1976), fungi (up to −1 ∘C; Kunert et al., 2019; Richard et al., 1996), lichen (up to −2 ∘C; Kieft, 1988; Kieft and Ruscetti, 1990), and pollen (up to −8 to −12 ∘C; Hader et al., 2014; Pummer et al., 2012). The haloarchaeal species fall closer in line with less effective biologically derived INPs such as fungal spores (onset freezing temperatures are reported as being up to −10 ∘C but typically initiate freezing ∘C; Iannone et al., 2011; Jayaweera and Flanagan, 1982) and diatoms (observed up to −24 ∘C; Knopf et al., 2011). H. morrhuae cells were also more effective at nucleating ice even though total cell concentrations were 3 times higher for H. sulfurifontis (Table 1). One possible explanation is that H. morrhuae is unusual among the Archaea in that it has a cell envelope composed of polysaccharides, which have been shown to serve as tracers for ice nucleating activity (Zeppenfeld et al., 2019). In general, although not warm temperature INPs (i.e., that glaciate ∘C), these Haloarchaea are comparable in ice nucleation activity to fungal spores and diatoms and can, thus, contribute to INP populations at very relevant atmospheric freezing temperatures.
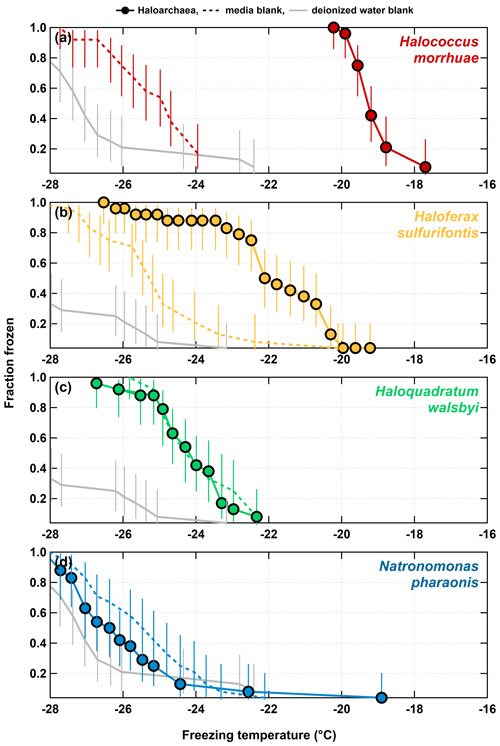
Figure 1Freezing spectra for each of the haloarchaeal species diluted 1:15 in deionized water. (a) H. morrhuae, (b) H. sulfurifontis, (c) H. walsbyi, and (d) N. pharaonis. H. morrhuae and H. sulfurifontis did not lyse, H. walsbyi lysed, and N. pharaonis partially lysed. Note that H. walsbyi and N. pharaonis do not reach a frozen fraction of 1 because not all drops were frozen at the lower limit of the IS tests. Error bars indicate 95 % confidence intervals.
As expected, ice nucleation activity did not occur in all tested haloarchaeal species, just as with bacteria (Karimi et al., 2020; Szyrmer and Zawadzki, 1997). Lysed cells of both H. walsbyi and N. pharaonis did not exhibit ice nucleation activity as the fractions frozen were not higher than the media blanks. Interestingly, the dilution of the log-phase cultures to 1:15 with deionized water resulted in the complete lysis of H. walsbyi and N. pharaonis as opposed to H. morrhuae and H. sulfurifontis, which both remained intact. Testing ice nucleation responses from cell lysis is relevant given that (1) cell fragments from fungi and bacteria have been previously observed to serve as INPs (Anderson and Ashworth, 1986; Du et al., 2017; O'Sullivan et al., 2015; Šantl-Temkiv et al., 2015) and (2) that a less saline environment is more atmospherically relevant for how Haloarchaea might behave once aerosolized and exposed to atmospheric water. However, the results presented here indicate that of the species studied, lysed haloarchaeal cells (i.e., cell fragments) do not enhance ice nucleation abilities and possibly even suppress it. This is analogous to previous work on bacteria, whereby it is well known that the lysing of ice nucleating active bacterial cells decreases the efficiency at which they are INPs (e.g., Lindow et al., 1989).
3.2 Response of haloarchaeal species to heat and peroxide treatments
Both H. morrhuae and H. sulfurifontis demonstrated responses to both the heat and peroxide treatments. Figure 2 shows the spectra for the 1:6, 1:15, and 1:30 samples (i.e., increasing dilutions or decreasing cell densities) of H. morrhuae. The ice nucleating activity of all three samples was reduced by heat by 0.3–0.9 ∘C on average (i.e., when calculating the average freezing temperature per spectrum and subtracting from average freezing temperature of the unamended INP spectrum per sample) but was especially reduced by peroxide (i.e., by 1.5–4.2 ∘C on average). These differences are statistically significant for decreases in the fraction frozen curve of 0.25 or more at similar temperatures when applying Fisher's exact test (p<0.0479), which was the case for all data except for the first three and the last data points for the 1:15 H. morrhuae unamended and heat spectra (Fig. 2b; Table S1 in the Supplement). These results indicate that the samples contained some heat labile, likely proteinaceous, INPs but contained a relatively larger contribution from other biogenic organic INPs (Conen et al., 2011; Hill et al., 2016; Mccluskey et al., 2018). Interestingly, increasing the dilution, such that the sample contained lower cell densities, led to a larger decrease in the fraction frozen curve from the peroxide treatments. For the 1:6 sample (i.e., highest cell density), frozen fractions dropped 0.9 and 1.5 ∘C on average for the heat and peroxide treatments, respectively. For the largest decrease, the 1:30 sample (i.e., lowest cell density) dropped by 0.7 and 4.2 ∘C on average for heat and peroxide treatments, respectively. One conceivable explanation for the increased efficacy with decreasing cell density is that the peroxide – the same volume was used in each sample, based on successful peroxide sample degradation reported in previous studies (e.g., Barry et al., 2021; Creamean et al., 2020; Suski et al., 2018) – remained in a higher concentration to digest a lower concentration of cells; thus, less residual organic material was available to serve as INPs. It is possible that higher volumes of peroxide would be needed for higher cell concentrations to eliminate all organic material, depending on the location of the organic material (i.e., if it is extra- or intercellular). A recent study demonstrated that excess peroxide was required to effectively reduce INP concentrations to background levels for the lignin biopolymer (Bogler and Borduas-Dedekind, 2020). We recommend that future work evaluating archaeal INPs should involve a more rigorous peroxide treatment regimen to test this hypothesis.
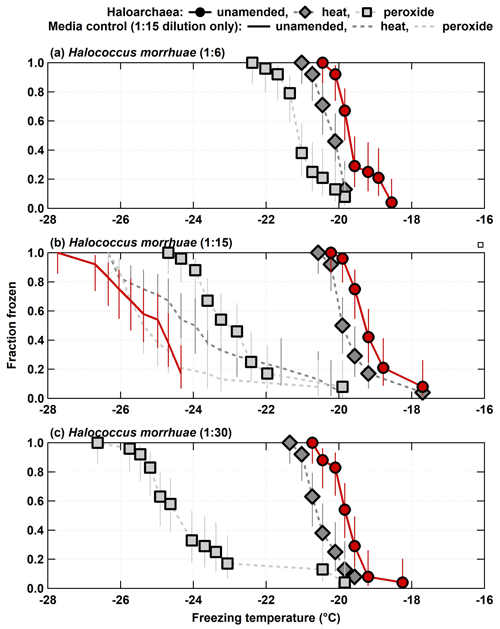
Figure 2Unamended, heat labile (heat), and organic (peroxide) frozen fractions for each of the three H. morrhuae dilutions from the processing treatments. Note that media controls were only conducted for the 1:15 dilution. Error bars indicate 95 % confidence intervals.
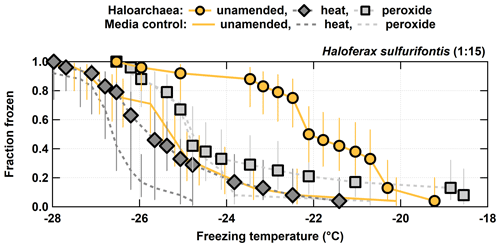
Figure 3Unamended, heat labile (heat), and organic (peroxide) frozen fractions for each of the 1:15 diluted H. sulfurifontis from the processing treatments. H. walsbyi and N. pharaonis are not shown since they exhibited no INP activity in their unamended spectra. Error bars indicate 95 % confidence intervals.
For the other three haloarchaeal species, only H. sulfurifontis exhibited a decrease in frozen fractions – the fraction frozen curve dropped by 2.7 and 1.8 ∘C on average for heat and peroxide, respectively (Fig. 3). H. walsbyi and N. pharaonis showed no response to the treatments because there were essentially no INPs to begin with; thus, these species are not discussed herein. For comparison between the H. sulfurifontis unamended and heat spectra, data and ∘C are the only data not statistically significant. For comparison between the unamended and peroxide spectra, only data ∘C are not statistically significant. These decreases were mostly observed and statistically significant when the fraction frozen curve was approximately 0.2 to 0.8 (average decrease in freezing temperatures within this range was 3.8 and 2.3 ∘C, respectively), as opposed to H. morrhuae, where the difference in temperature from the unamended to the treated frozen fractions was roughly equivalent throughout the spectra. Interestingly, H. sulfurifontis spectra were more responsive to the heat treatment than to peroxide (i.e., exhibited a larger decrease in average freezing temperatures for heat than for peroxide), indicating this species contained more heat labile, probably proteinaceous, INPs versus organic INPs – the opposite of H. morrhuae. Collectively, these results indicate that H. morrhuae contained more organic relative to heat labile INPs, while H. sulfurifontis contained more heat labile as opposed to organic INPs. These Haloarchaea have very different cellular envelope compositions. H. sulfurifontis contains a proteinaceous S layer, while H. morrhuae is devoid of such an S layer but instead possesses a cell envelope that is composed of highly sulfated heteropolysaccharides. Thus, it would make sense that H. sulfurifontis is more sensitive to heat than peroxide given its proteinaceous cell envelope (assuming that those proteins are ice nucleation active), and H. morrhuae is more sensitive to peroxide than heat given its polysaccharide-rich cell envelope.
Here, we present the first reported results on the ice nucleation activity of the domain Archaea. Specifically, we focus on four species within the Haloarchaea due to their diversity of cell surface compositions, ease of culturability, and regional presence within relatively large hypersaline environments. The freezing temperature ranges measured for the Haloarchaea species involved in this study are put into a broader context by comparing them to reported ranges for other known biologically derived INPs (Fig. 4). While not the most proficient of biologically derived INPs such as lichen, bacteria, fungi, viruses, algae, pollen, or pollen wash water, which nucleate ice at warmer temperatures, Haloarchaea fall within moderate freezing temperature ranges above phytoplankton exudates, diatoms, and fungal spores. However, this work is based on a limited subset of species, and future work should focus on other members of the Archaea.
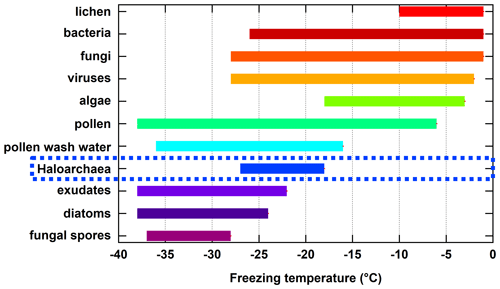
Figure 4Summary of approximate freezing temperature ranges reported for known biologically derived INPs. Haloarchaea data are those from H. morrhuae and H. sulfurifontis from the current work. Data for lichen, bacteria, fungi, algae, pollen, pollen wash water, phytoplankton exudates, diatoms, and fungal spores were obtained and compiled from reviews by Després et al. (2012), Huang et al. (2021), Kanji et al. (2017), and Murray et al. (2012) and references therein. Data for viruses are more limited and were obtained from Adams et al. (2021) and Orser et al. (1985). Note that freezing temperature ranges for each result from one to multiple studies and may be dependent on the technique used (e.g., instrumental freezing limits and drop size).
All Haloarchaea species were introduced to hyposaline conditions to reduce freezing point depression. While intact cells of H. morrhuae and H. sulfurifontis demonstrated their ability as INPs, inducing freezing up to −18 ∘C, lysed cells of H. walsbyi and N. pharaonis did not exhibit ice nucleation activity over the temperature range tested. The intact cells that demonstrated ice nucleation activity contained both heat stable organic ice nucleating entities (H. morrhuae) and heat labile material (H. sulfurifontis). This may be negated at cloud-level salinities where H. sulfurifontis would be expected to lyse as well. H. morrhuae, on the other hand, remained intact at all salinities (Legat et al., 2010; Leuko et al., 2004). It is also rare among both the Haloarchaea, in particular, and the Archaea, as a whole, that the surface is completely devoid of a proteinaceous S layer. Ice-binding ability is a characteristic of both ice nucleating and antifreeze proteins and is influenced primarily by their size (Eickhoff et al., 2019; Qiu et al., 2019). Therefore, further work is needed to directly evaluate the surface properties to both disentangle which constituents are responsible for ice formation in the Halococcus and assess the ice nucleating potential within other members of the Haloarchaea and Archaea, in general.
While halophilic Archaea are prominent in hypersaline environments throughout the globe, such as the Great Salt Lake and the Dead Sea, other members of the domain Archaea, such as methanogens and thermophiles, are prevalent in anoxic systems in seawater, sea ice, marine sediments, glacial ice, permafrost, hot springs, submarine hydrothermal vents, and hot, dry deserts (Amend and Shock, 2001; Collins et al., 2010; Oremland and Taylor, 1978; Price, 2007; Thauer et al., 2008; Thummes et al., 2007; Van Der Maarel et al., 1999). However, some studies allude to the fact that these extremophiles are not confined to extreme living conditions, which qualifies them as one of the most abundant prokaryotes on Earth (Delong, 1998). Thus, these microorganisms are more ubiquitous than one might think, are present in the atmosphere (Fröhlich-Nowoisky et al., 2014), and may affect cloud formation (Amato et al., 2017). Indeed, the order Halobacteriales, which contains H. morrhuae, has been found to be present in continental air and was relatively abundant among the Archaea found in marine air (Fröhlich-Nowoisky et al., 2014). Furthermore, Archaea accounted for several percent of all sequences in boundary layer air sampled from 45–50∘ S over the Southern Ocean (Uetake et al., 2020). Future work should focus on characterizing a wide range of environmentally relevant Archaea, such as methanogens (see Creamean et al., 2020) and ammonia oxidizers (see Fröhlich-Nowoisky et al., 2014), for their ice nucleating properties and address how they may be important for regional cloud formation and, hence, weather and climate, as the impacts of the Archaea on climate processes are currently unknown.
Data used in this paper can be accessed in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-3751-2021-supplement.
JMC and MER were responsible for overseeing these experiments. JMC lead the writing of this paper. JEC, LN, and ADP prepared the cultures and ran the immersion freezing tests. TCJH and PJD helped with interpretation of the results. All authors contributed to the writing of this paper.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors would like to acknowledge Stephen Schmidt and Pacifica Sommers of the University of Colorado, Boulder, for assisting with the preservation and culturing of the Haloarchaea after its arrival in Colorado.
Julio E. Ceniceros was supported by the US Department of Commerce, National Oceanic and Atmospheric Administration, Educational Partnership Program (grant no. NA16SEC4810006). This work was funded by a NASA EPSCoR Research Infrastructure Development for the state of South Carolina.
This paper was edited by Denise Akob and reviewed by Brent Christner and three anonymous referees.
Adams, M. P., Atanasova, N. S., Sofieva, S., Ravantti, J., Heikkinen, A., Brasseur, Z., Duplissy, J., Bamford, D. H., and Murray, B. J.: Ice nucleation by viruses and their potential for cloud glaciation, Biogeosciences Discuss. [preprint], https://doi.org/10.5194/bg-2020-474, in review, 2021.
Agresti, A. and Coull, B. A.: Approximate is better than “exact” for interval estimation of binomial proportions, Am. Stat., 52, 119–126, https://doi.org/10.2307/2685469, 1998.
Albers, S. V. and Meyer, B. H.: The archaeal cell envelope, Nat. Rev. Microbiol., 9, 414–426, https://doi.org/10.1038/nrmicro2576, 2011.
Albers, S. V., Eichler, J., and Aebi, M.: Chapter 22 Archaea, in: Essentials of Glycobiology (3rd edition), edited by: Varki, A., Cummings, R. D., and Esko, J. D., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2017.
Amato, P., Joly, M., Besaury, L., Oudart, A., Taib, N., Moné, A. I., Deguillaume, L., Delort, A.-M., and Debroas, D.: Active microorganisms thrive among extremely diverse communities in cloud water, PLOS ONE, 12, e0182869, https://doi.org/10.1371/journal.pone.0182869, 2017.
Amend, J. P. and Shock, E. L.: Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria, FEMS Microbiol. Rev., 25, 175–243, https://doi.org/10.1111/j.1574-6976.2001.tb00576.x, 2001.
Anderson, J. A. and Ashworth, E. N.: The Effects of Streptomycin, Desiccation, and UV Radiation on Ice Nucleation by Pseudomonas viridiflava, Plant Physiol., 80, 956–960, https://doi.org/10.1104/pp.80.4.956, 1986.
Baker, M. B. and Peter, T.: Small-scale cloud processes and climate, Nature, 451, 299–300, https://doi.org/10.1038/nature06594, 2008.
Barry, K. R., Hill, T. C. J., Levin, E. J. T., Twohy, C. H., Moore, K. A., Weller, Z. D., Toohey, D. W., Reeves, M., Campos, T., Geiss, R., Schill, G. P., Fischer, E. V., Kreidenweis, S. M., and DeMott, P. J.: Observations of Ice Nucleating Particles in the Free Troposphere From Western US Wildfires, J. Geophys. Res.-Atmos., 126, e2020JD033752, https://doi.org/10.1029/2020JD033752, 2021.
Bodnar, R. J.: Revised equation and table for determining the freezing point depression of H2O-NaCl solutions, Geochim. Cosmochim. Ac., 57, 683–684, https://doi.org/10.1016/0016-7037(93)90378-A, 1993.
Bogler, S. and Borduas-Dedekind, N.: Lignin's ability to nucleate ice via immersion freezing and its stability towards physicochemical treatments and atmospheric processing, Atmos. Chem. Phys., 20, 14509–14522, https://doi.org/10.5194/acp-20-14509-2020, 2020.
Boring, J., Kushner, D. J., and Gibbons, N. E.: Specificity of the Salt Requirement of Halobacterium Cutirubrum, Can. J. Microbiol., 9, 143–154, https://doi.org/10.1139/m63-020, 1963.
Boucher, O., Randall, D., Artaxo, P., Bretherton, C., Feingold, G., Forster, P., Kerminen, V.-M., Kondo, Y., Liao, H., Lohmann, U., Rasch, P., Satheesh, S. K., Sherwood, S., Stevens, B., and Zhang, X. Y.: Clouds and Aerosols, in: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia, Y., Bex, V., and Midgley, P. M., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 571–658, https://doi.org/10.1017/CBO9781107415324.016, 2013.
Burns, D. G., Janssen, P. H., Itoh, T., Kamekura, M., Li, Z., Jensen, G., Rodríguez-Valera, F., Bolhuis, H., and Dyall-Smith, M. L.: Haloquadratum walsbyi gen. nov., sp. nov., the square haloarchaeon of Walsby, isolated from saltern crystallizers in Australia and Spain, Int. J. Syst. Evol. Micr., 57, 387–392, https://doi.org/10.1099/ijs.0.64690-0, 2007.
Burrows, S. M., Hoose, C., Pöschl, U., and Lawrence, M. G.: Ice nuclei in marine air: biogenic particles or dust?, Atmos. Chem. Phys., 13, 245–267, https://doi.org/10.5194/acp-13-245-2013, 2013.
Carpenter, D. M.: The Lake Effect of the Great Salt Lake: Overview and Forecast Problems, Weather Forecast., 8, 181–193, https://doi.org/10.1175/1520-0434(1993)008<0181:TLEOTG>2.0.CO;2, 1993.
Cavicchioli, R.: Archaea – Timeline of the third domain, Nat. Rev. Microbiol., 9, 51–61, https://doi.org/10.1038/nrmicro2482, 2011.
Cochet, N. and Widehem, P.: Ice crystallization by Pseudomonas syringae, Appl. Microbiol. Biot., 54, 153–161, https://doi.org/10.1007/s002530000377, 2000.
Collins, R. E., Rocap, G., and Deming, J. W.: Persistence of bacterial and archaeal communities in sea ice through an Arctic winter, Environ. Microbiol., 12, 1828–1841, https://doi.org/10.1111/j.1462-2920.2010.02179.x, 2010.
Conen, F., Morris, C. E., Leifeld, J., Yakutin, M. V., and Alewell, C.: Biological residues define the ice nucleation properties of soil dust, Atmos. Chem. Phys., 11, 9643–9648, https://doi.org/10.5194/acp-11-9643-2011, 2011.
Creamean, J. M., Hill, T. C. J., DeMott, P. J., Uetake, J., Kreidenweis, S., and Douglas, T. A.: Thawing permafrost: an overlooked source of seeds for Arctic cloud formation, Environ. Res. Lett., 15, 084022, https://doi.org/10.1088/1748-9326/ab87d3, 2020.
Davies, P. L.: Ice-binding proteins: a remarkable diversity of structures for stopping and starting ice growth, Trends Biochem. Sci., 39, 548–555, https://doi.org/10.1016/j.tibs.2014.09.005, 2014.
Decho, A. W. and Gutierrez, T.: Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems, Front. Microbiol., 8, 922, 2017.
DeLong, E.: Archaeal Means and Extremes, Science, 280, 542–543, https://doi.org/10.1126/science.280.5363.542, 1998.
DeMott, P. J., Hill, T. C. J., McCluskey, C. S., Prather, K. A., Collins, D. B., Sullivan, R. C., Ruppel, M. J., Mason, R. H., Irish, V. E., Lee, T., Hwang, C. Y., Rhee, T. S., Snider, J. R., McMeeking, G. R., Dhaniyala, S., Lewis, E. R., Wentzell, J. J. B., Abbatt, J., Lee, C., Sultana, C. M., Ault, A. P., Axson, J. L., Diaz Martinez, M., Venero, I., Santos-Figueroa, G., Stokes, M. D., Deane, G. B., Mayol-Bracero, O. L., Grassian, V. H., Bertram, T. H., Bertram, A. K., Moffett, B. F., and Franc, G. D.: Sea spray aerosol as a unique source of ice nucleating particles, 113, 5797–5803, https://doi.org/10.1073/pnas.1514034112, Proc. Natl. Acad. Sci. USA, 2016.
DeMott, P. J., Mason, R. H., McCluskey, C. S., Hill, T. C. J., Perkins, R. J., Desyaterik, Y., Bertram, A. K., Trueblood, Jonathan V., Grassian, V. H., Qiu, Y., Molinero, V., Tobo, Y., Sultana, C. M., Lee, C., and Prather, K. A.: Ice nucleation by particles containing long-chain fatty acids of relevance to freezing by sea spray aerosols, Environ. Sci.-Proc. Imp., 20, 1559–1569, https://doi.org/10.1039/C8EM00386F, 2018.
Després, V., Huffman, J. A., Burrows, S. M., Hoose, C., Safatov, A., Buryak, G., Fröhlich-Nowoisky, J., Elbert, W., Andreae, M., Pöschl, U., and Jaenicke, R.: Primary biological aerosol particles in the atmosphere: a review, Tellus B, 64, 15598–15598, https://doi.org/10.3402/tellusb.v64i0.15598, 2012.
Dreischmeier, K., Budke, C., Wiehemeier, L., Kottke, T., and Koop, T.: Boreal pollen contain ice-nucleating as well as ice-binding “antifreeze” polysaccharides, Sci. Rep., 7, 41890, https://doi.org/10.1038/srep41890, 2017.
Du, R., Du, P., Lu, Z., Ren, W., Liang, Z., Qin, S., Li, Z., Wang, Y., and Fu, P.: Evidence for a missing source of efficient ice nuclei, Sci. Rep., 7, 39673, https://doi.org/10.1038/srep39673, 2017.
Eickhoff, L., Dreischmeier, K., Zipori, A., Sirotinskaya, V., Adar, C., Reicher, N., Braslavsky, I., Rudich, Y., and Koop, T.: Contrasting Behavior of Antifreeze Proteins: Ice Growth Inhibitors and Ice Nucleation Promoters, J. Phys. Chem. Lett., 10, 966–972, https://doi.org/10.1021/acs.jpclett.8b03719, 2019.
Elshahed, M. S., Savage, K. N., Oren, A., Gutierrez, M. C., Ventosa, A., and Krumholz, L. R.: Haloferax sulfurifontis sp. nov., a halophilic archaeon isolated from a sulfide- and sulfur-rich spring, Int. J. Syst. Evol. Microbiol., 54, 2275–2279, https://doi.org/10.1099/ijs.0.63211-0, 2004.
Failor, K. C., Schmale, D. G., Vinatzer, B. A., and Monteil, C. L.: Ice nucleation active bacteria in precipitation are genetically diverse and nucleate ice by employing different mechanisms, ISME J., 11, 2740–2753, https://doi.org/10.1038/ismej.2017.124, 2017.
Falb, M., Pfeiffer, F., Palm, P., Rodewald, K., Hickmann, V., Tittor, J., and Oesterhelt, D.: Living with two extremes: conclusions from the genome sequence of Natronomonas pharaonis, Biotechfor., 15, 1336–1343, https://doi.org/10.1101/gr.3952905, 2005.
Flemming, H.-C. and Wuertz, S.: Bacteria and archaea on Earth and their abundance in biofilms, Nat. Rev. Microbiol., 17, 247–260, https://doi.org/10.1038/s41579-019-0158-9, 2019.
Fröhlich-Nowoisky, J., Ruzene Nespoli, C., Pickersgill, D. A., Galand, P. E., Müller-Germann, I., Nunes, T., Gomes Cardoso, J., Almeida, S. M., Pio, C., Andreae, M. O., Conrad, R., Pöschl, U., and Després, V. R.: Diversity and seasonal dynamics of airborne archaea, Biogeosciences, 11, 6067–6079, https://doi.org/10.5194/bg-11-6067-2014, 2014.
Fröhlich-Nowoisky, J., Hill, T. C. J., Pummer, B. G., Yordanova, P., Franc, G. D., and Pöschl, U.: Ice nucleation activity in the widespread soil fungus Mortierella alpina, Biogeosciences, 12, 1057–1071, https://doi.org/10.5194/bg-12-1057-2015, 2015.
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., Lang-Yona, N., Burrows, S. M., Gunthe, S. S., Elbert, W., Su, H., Hoor, P., Thines, E., Hoffmann, T., Després, V. R., and Pöschl, U.: Bioaerosols in the Earth system: Climate, health, and ecosystem interactions, Atmos. Res., 182, 346–376, https://doi.org/10.1016/j.atmosres.2016.07.018, 2016.
Gurian-Sherman, D. and Lindow, S. E.: Bacterial ice nucleation: Significance and molecular basis, FASEB J., 7, 1338–1343, https://doi.org/10.1096/fasebj.7.14.8224607, 1993.
Hader, J. D., Wright, T. P., and Petters, M. D.: Contribution of pollen to atmospheric ice nuclei concentrations, Atmos. Chem. Phys., 14, 5433–5449, https://doi.org/10.5194/acp-14-5433-2014, 2014.
Hartmann, S., Augustin, S., Clauss, T., Wex, H., Šantl-Temkiv, T., Voigtländer, J., Niedermeier, D., and Stratmann, F.: Immersion freezing of ice nucleation active protein complexes, Atmos. Chem. Phys., 13, 5751–5766, https://doi.org/10.5194/acp-13-5751-2013, 2013.
Hill, T. C. J., Moffett, B. F., Demott, P. J., Georgakopoulos, D. G., Stump, W. L., and Franc, G. D.: Measurement of ice nucleation-active bacteria on plants and in precipitation by quantitative PCR, Appl. Environ. Microb., 80, 1256–1267, https://doi.org/10.1128/AEM.02967-13, 2014.
Hill, T. C. J., DeMott, P. J., Tobo, Y., Fröhlich-Nowoisky, J., Moffett, B. F., Franc, G. D., and Kreidenweis, S. M.: Sources of organic ice nucleating particles in soils, Atmos. Chem. Phys., 16, 7195–7211, https://doi.org/10.5194/acp-16-7195-2016, 2016.
Hiranuma, N., Augustin-Bauditz, S., Bingemer, H., Budke, C., Curtius, J., Danielczok, A., Diehl, K., Dreischmeier, K., Ebert, M., Frank, F., Hoffmann, N., Kandler, K., Kiselev, A., Koop, T., Leisner, T., Möhler, O., Nillius, B., Peckhaus, A., Rose, D., Weinbruch, S., Wex, H., Boose, Y., DeMott, P. J., Hader, J. D., Hill, T. C. J., Kanji, Z. A., Kulkarni, G., Levin, E. J. T., McCluskey, C. S., Murakami, M., Murray, B. J., Niedermeier, D., Petters, M. D., O'Sullivan, D., Saito, A., Schill, G. P., Tajiri, T., Tolbert, M. A., Welti, A., Whale, T. F., Wright, T. P., and Yamashita, K.: A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: a comparison of 17 ice nucleation measurement techniques, Atmos. Chem. Phys., 15, 2489–2518, https://doi.org/10.5194/acp-15-2489-2015, 2015.
Hoose, C. and Möhler, O.: Heterogeneous ice nucleation on atmospheric aerosols: a review of results from laboratory experiments, Atmos. Chem. Phys., 12, 9817–9854, https://doi.org/10.5194/acp-12-9817-2012, 2012.
Hoose, C., Kristjánsson, J. E., and Burrows, S. M.: How important is biological ice nucleation in clouds on a global scale?, Environ. Res. Lett., 5, 024009, https://doi.org/10.1088/1748-9326/5/2/024009, 2010a.
Hoose, C., Kristjansson, J. E., Chen, J. P., and Hazra, A.: A Classical-Theory-Based Parameterization of Heterogeneous Ice Nucleation by Mineral Dust, Soot, and Biological Particles in a Global Climate Model, J. Atmos. Sci., 67, 2483–2503, https://doi.org/10.1175/2010jas3425.1, 2010b.
Hoshino, T. and Inagaki, F.: Abundance and distribution of Archaea in the subseafloor sedimentary biosphere, ISME J., 13, 227–231, https://doi.org/10.1038/s41396-018-0253-3, 2019.
Huang, S., Hu, W., Chen, J., Wu, Z., Zhang, D., and Fu, P.: Overview of biological ice nucleating particles in the atmosphere, Environ. Int., 146, 106197, https://doi.org/10.1016/j.envint.2020.106197, 2021.
Hummel, M., Hoose, C., Pummer, B., Schaupp, C., Fröhlich-Nowoisky, J., and Möhler, O.: Simulating the influence of primary biological aerosol particles on clouds by heterogeneous ice nucleation, Atmos. Chem. Phys., 18, 15437–15450, https://doi.org/10.5194/acp-18-15437-2018, 2018.
Iannone, R., Chernoff, D. I., Pringle, A., Martin, S. T., and Bertram, A. K.: The ice nucleation ability of one of the most abundant types of fungal spores found in the atmosphere, Atmos. Chem. Phys., 11, 1191–1201, https://doi.org/10.5194/acp-11-1191-2011, 2011.
Irish, V. E., Hanna, S. J., Xi, Y., Boyer, M., Polishchuk, E., Ahmed, M., Chen, J., Abbatt, J. P. D., Gosselin, M., Chang, R., Miller, L. A., and Bertram, A. K.: Revisiting properties and concentrations of ice-nucleating particles in the sea surface microlayer and bulk seawater in the Canadian Arctic during summer, Atmos. Chem. Phys., 19, 7775–7787, https://doi.org/10.5194/acp-19-7775-2019, 2019.
Jayaweera, K. and Flanagan, P.: Investigations on biogenic ice nuclei in the Arctic atmosphere, Geophys. Res. Lett., 9, 94–97, https://doi.org/10.1029/GL009i001p00094, 1982.
Junge, K., Eicken, H., and Deming, J. W.: Bacterial Activity at −2 to −20 ∘C in Arctic Wintertime Sea Ice, Appl. Environ. Microb., 70, 550–557, https://doi.org/10.1128/AEM.70.1.550-557.2004, 2004.
Kanji, Z. A., Ladino, L. A., Wex, H., Boose, Y., Burkert-Kohn, M., Cziczo, D. J., and Krämer, M.: Overview of ice nucleating particles, Meteor. Mon., 58, 1.1–1.33, https://doi.org/10.1175/AMSMONOGRAPHS-D-16-0006.1, 2017.
Karimi, B., Nosrati, R., Fazly Bazzaz, B. S., Mirpour, M., Malboobi, M., and Owlia, P.: A comparative evaluation of freezing criteria and molecular characterization of epiphytic ice-nucleating (Ice+) and non-ice-nucleating (Ice−) Pseudomonas syringae and Pseudomonas fluorescens, J. Plant Pathol., 102, 169–178, https://doi.org/10.1007/s42161-019-00402-7, 2020.
Kieft, T. L.: Ice Nucleation Activity in Lichens, Appl. Environ. Microbiol., 54, 1678–1681, 1988.
Kieft, T. L. and Ruscetti, T.: Characterization of biological ice nuclei from a lichen, J. Bacteriol., 172, 3519–3523, https://doi.org/10.1128/jb.172.6.3519-3523.1990, 1990.
Kim, H. K., Orser, C., Lindow, S. E., and Sands, D. C.: Xanthomonas campestris pv. translucens strains active in ice nucleation, Plant Dis, 71, 994–997, https://doi.org/10.1094/pd-71-0994, 1987.
Knopf, D. A., Alpert, P. A., Wang, B., and Aller, J. Y.: Stimulation of ice nucleation by marine diatoms, Nat. Geosci., 4, 88–90, https://doi.org/10.1038/ngeo1037, 2011.
Kunert, A. T., Pöhlker, M. L., Tang, K., Krevert, C. S., Wieder, C., Speth, K. R., Hanson, L. E., Morris, C. E., Schmale III, D. G., Pöschl, U., and Fröhlich-Nowoisky, J.: Macromolecular fungal ice nuclei in Fusarium: effects of physical and chemical processing, Biogeosciences, 16, 4647–4659, https://doi.org/10.5194/bg-16-4647-2019, 2019.
Legat, A., Gruber, C., Zangger, K., Wanner, G., and Stan-Lotter, H.: Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species, Appl. Microbiol. Biot., 87, 1119–1127, https://doi.org/10.1007/s00253-010-2611-6, 2010.
Leuko, S., Legat, A., Fendrihan, S., and Stan-Lotter, H.: Evaluation of the LIVE/DEAD BacLight Kit for Detection of Extremophilic Archaea and Visualization of Microorganisms in Environmental Hypersaline Samples, Appl. Environ. Microb., 70, 6884, https://doi.org/10.1128/AEM.70.11.6884-6886.2004, 2004.
Lindow, S. E., Lahue, E., Govindarajan, A. G., Panopoulos, N. J., and Gies, D.: Localization of ice nucleation activity and the iceC gene product in Pseudomonas syringae and Escherichia coli, Mol. Plant Microbe In., 2, 262–272, https://doi.org/10.1094/mpmi-2-262, 1989.
Ling, M. L., Wex, H., Grawe, S., Jakobsson, J., Löndahl, J., Hartmann, S., Finster, K., Boesen, T., and Šantl-Temkiv, T.: Effects of Ice Nucleation Protein Repeat Number and Oligomerization Level on Ice Nucleation Activity, J. Geophys. Res.-Atmos., 123, 1802–1810, https://doi.org/10.1002/2017JD027307, 2018.
Lohmann, U. and Feichter, J.: Global indirect aerosol effects: a review, Atmos. Chem. Phys., 5, 715–737, https://doi.org/10.5194/acp-5-715-2005, 2005.
Maki, L. R., Galyan, E. L., Chang-Chien, M. M., and Caldwell, D. R.: Ice nucleation induced by pseudomonas syringae, Applied Microbiol., 28, 456–459, 1974.
McCluskey, C. S., Hill, T. C. J., Sultana, C. M., Laskina, O., Trueblood, J., Santander, M. V., Beall, C. M., Michaud, J. M., Kreidenweis, S. M., Prather, K. A., Grassian, V., and DeMott, P. J.: A Mesocosm Double Feature: Insights into the Chemical Makeup of Marine Ice Nucleating Particles, J. Atmos. Sci., 75, 2405–2423, https://doi.org/10.1175/Jas-D-17-0155.1, 2018.
McCluskey, C. S., DeMott, P. J., Ma, P. L., and Burrows, S. M.: Numerical Representations of Marine Ice-Nucleating Particles in Remote Marine Environments Evaluated Against Observations, Geophys. Res. Lett., 46, 7838–7847, https://doi.org/10.1029/2018gl081861, 2019.
Mondav, R., Woodcroft, B. J., Kim, E.-H., McCalley, C. K., Hodgkins, S. B., Crill, P. M., Chanton, J., Hurst, G. B., VerBerkmoes, N. C., Saleska, S. R., Hugenholtz, P., Rich, V. I., and Tyson, G. W.: Discovery of a novel methanogen prevalent in thawing permafrost, Nat. Commun., 5, 3212, https://doi.org/10.1038/ncomms4212, 2014.
Morris, C. E., Georgakopoulos, D. G., and Sands, D. C.: Ice nucleation active bacteria and their potential role in precipitation, J. Phys. IV, 121, 87–103, https://doi.org/10.1051/jp4:2004121004, 2004.
Morris, C. E., Sands, D. C., Vinatzer, B. A., Glaux, C., Guilbaud, C., Buffière, A., Yan, S., Dominguez, H., and Thompson, B. M.: The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle, ISME J., 2, 321–334, https://doi.org/10.1038/ismej.2007.113, 2008.
Munson, M. A., Nedwell, D. B., and Embley, T. M.: Phylogenetic diversity of Archaea in sediment samples from a coastal salt marsh, Appl. Environ. Microbiol., 63, 4729–4733, 1997.
Murray, B. J., O'Sullivan, D., Atkinson, J. D., and Webb, M. E.: Ice nucleation by particles immersed in supercooled cloud droplets, Chem. Soc. Rev., 41, 6519–6554, https://doi.org/10.1039/c2cs35200a, 2012.
O'Sullivan, D., Murray, B. J., Ross, J. F., Whale, T. F., Price, H. C., Atkinson, J. D., Umo, N. S., and Webb, M. E.: The relevance of nanoscale biological fragments for ice nucleation in clouds, Sci. Rep., 5, 8082, https://doi.org/10.1038/srep08082, 2015.
Ochsenreiter, T., Pfeifer, F., and Schleper, C.: Diversity of Archaea in hypersaline environments characterized by molecular-phylogenetic and cultivation studies, Extremophiles, 6, 267–274, https://doi.org/10.1007/s00792-001-0253-4, 2002.
Oremland, R. S. and Taylor, B. F.: Sulfate reduction and methanogenesis in marine sediments, Geochim. Cosmochim. Ac., 42, 209–214, https://doi.org/10.1016/0016-7037(78)90133-3, 1978.
Orser, C., Staskawicz, B. J., Panopoulos, N. J., Dahlbeck, D., and Lindow, S. E.: Cloning and expression of bacterial ice nucleation genes in Escherichia coli, J. Bacteriol., 164, 359–366, https://doi.org/10.1128/JB.164.1.359-366.1985, 1985.
Perkins, R. J., Gillette, S. M., Hill, T. C. J., and DeMott, P. J.: The Labile Nature of Ice Nucleation by Arizona Test Dust, ACS Earth and Space Chemistry, 4, 133–141, https://doi.org/10.1021/acsearthspacechem.9b00304, 2020a.
Perkins, R. J., Vazquez de Vasquez, M. G., Beasley, E. E., Hill, T. C. J., Stone, E. A., Allen, H. C., and DeMott, P. J.: Relating Structure and Ice Nucleation of Mixed Surfactant Systems Relevant to Sea Spray Aerosol, J. Phys. Chem. A, 124, 8806–8821, https://doi.org/10.1021/acs.jpca.0c05849, 2020b.
Petters, M. D. and Wright, T. P.: Revisiting ice nucleation from precipitation samples, Geophys. Res. Lett., 42, 8758–8766, https://doi.org/10.1002/2015gl065733, 2015.
Phillips, V. T. J., Andronache, C., Christner, B., Morris, C. E., Sands, D. C., Bansemer, A., Lauer, A., McNaughton, C., and Seman, C.: Potential impacts from biological aerosols on ensembles of continental clouds simulated numerically, Biogeosciences, 6, 987–1014, https://doi.org/10.5194/bg-6-987-2009, 2009.
Price, P. B.: Microbial life in glacial ice and implications for a cold origin of life, FEMS Microbiol. Ecol., 59, 217–231, https://doi.org/10.1111/j.1574-6941.2006.00234.x, 2007.
Pummer, B. G., Bauer, H., Bernardi, J., Bleicher, S., and Grothe, H.: Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen, Atmos. Chem. Phys., 12, 2541–2550, https://doi.org/10.5194/acp-12-2541-2012, 2012.
Qiu, Y., Hudait, A., and Molinero, V.: How Size and Aggregation of Ice-Binding Proteins Control Their Ice Nucleation Efficiency, J. Am. Chem. Soc., 141, 7439–7452, https://doi.org/10.1021/jacs.9b01854, 2019.
Richard, C., Martin, J. G., and Pouleur, S.: Ice nucleation activity identified in some phytopathogenic Fusarium species, Phytoprotection, 77, 83–92, https://doi.org/10.7202/706104ar, 1996.
Šantl-Temkiv, T., Sahyoun, M., Finster, K., Hartmann, S., Augustin-Bauditz, S., Stratmann, F., Wex, H., Clauss, T., Nielsen, N. W., Sørensen, J. H., Korsholm, U. S., Wick, L. Y., and Karlson, U. G.: Characterization of airborne ice-nucleation-active bacteria and bacterial fragments, Atmos. Environ., 109, 105–117, https://doi.org/10.1016/j.atmosenv.2015.02.060, 2015.
Santoro, A. E., Richter, R. A., and Dupont, C. L.: Planktonic Marine Archaea, Annu. Rev. Mar. Sci., 11, 131–158, https://doi.org/10.1146/annurev-marine-121916-063141, 2019.
Sato, Y. and Suzuki, K.: How do aerosols affect cloudiness?, Science, 363, 580, https://doi.org/10.1126/science.aaw3720, 2019.
Schleifer, K. H., Steber, J., and Mayer, H.: Chemical Composition and Structure of the Cell Wall of Halococcus morrhuae, Zbl. Bakt. Mik. Hyg. I C, 3, 171–178, https://doi.org/10.1016/S0721-9571(82)80030-6, 1982.
Schnell, R. C. and Vali, G.: Freezing nuclei in marine waters, Tellus, 27, 321–323, https://doi.org/10.3402/tellusa.v27i3.9911, 1975.
Sesartic, A., Lohmann, U., and Storelvmo, T.: Bacteria in the ECHAM5-HAM global climate model, Atmos. Chem. Phys., 12, 8645–8661, https://doi.org/10.5194/acp-12-8645-2012, 2012.
Stevens, B. and Feingold, G.: Untangling aerosol effects on clouds and precipitation in a buffered system, Nature, 461, 607–613, https://doi.org/10.1038/nature08281, 2009.
Suski, K. J., Hill, T. C. J., Levin, E. J. T., Miller, A., DeMott, P. J., and Kreidenweis, S. M.: Agricultural harvesting emissions of ice-nucleating particles, Atmos. Chem. Phys., 18, 13755–13771, https://doi.org/10.5194/acp-18-13755-2018, 2018.
Szyrmer, W. and Zawadzki, I.: Biogenic and Anthropogenic Sources of Ice-Forming Nuclei: A Review, B. Am. Meteorol. Soc., 78, 209–228, https://doi.org/10.1175/1520-0477(1997)078<0209:BAASOI>2.0.CO;2, 1997.
Thauer, R. K., Kaster, A.-K., Seedorf, H., Buckel, W., and Hedderich, R.: Methanogenic archaea: ecologically relevant differences in energy conservation, Nat. Rev. Microbiol., 6, 579–591, https://doi.org/10.1038/nrmicro1931, 2008.
Thummes, K., Schäfer, J., Kämpfer, P., and Jäckel, U.: Thermophilic methanogenic Archaea in compost material: Occurrence, persistence and possible mechanisms for their distribution to other environments, Syst. Appl. Microbiol., 30, 634–643, https://doi.org/10.1016/j.syapm.2007.08.001, 2007.
Tobo, Y., DeMott, P. J., Hill, T. C. J., Prenni, A. J., Swoboda-Colberg, N. G., Franc, G. D., and Kreidenweis, S. M.: Organic matter matters for ice nuclei of agricultural soil origin, Atmos. Chem. Phys., 14, 8521–8531, https://doi.org/10.5194/acp-14-8521-2014, 2014.
Twohy, C. H., McMeeking, G. R., DeMott, P. J., McCluskey, C. S., Hill, T. C. J., Burrows, S. M., Kulkarni, G. R., Tanarhte, M., Kafle, D. N., and Toohey, D. W.: Abundance of fluorescent biological aerosol particles at temperatures conducive to the formation of mixed-phase and cirrus clouds, Atmos. Chem. Phys., 16, 8205–8225, https://doi.org/10.5194/acp-16-8205-2016, 2016.
Uetake, J., Hill, T. C. J., Moore, K. A., DeMott, P. J., Protat, A., and Kreidenweis, S. M.: Airborne bacteria confirm the pristine nature of the Southern Ocean boundary layer, Proc. Natl. Acad. Sci. USA, 117, 13275, https://doi.org/10.1073/pnas.2000134117, 2020.
Vali, G., Christensen, M., Fresh, R. W., Galyan, E. L., Maki, L. R., and Schnell, R. C.: Biogenic Ice Nuclei. Part II: Bacterial Sources, J. Atmos. Sci., 33, 1565–1570, https://doi.org/10.1175/1520-0469(1976)033<1565:BINPIB>2.0.CO;2, 1976.
van der Maarel, M. J. E. C., Sprenger, W., Haanstra, R., and Forney, L. J.: Detection of methanogenic archaea in seawater particles and the digestive tract of a marine fish species, FEMS Microbiol. Lett., 173, 189–194, https://doi.org/10.1111/j.1574-6968.1999.tb13501.x, 1999.
Vergara-Temprado, J., Murray, B. J., Wilson, T. W., O'Sullivan, D., Browse, J., Pringle, K. J., Ardon-Dryer, K., Bertram, A. K., Burrows, S. M., Ceburnis, D., DeMott, P. J., Mason, R. H., O'Dowd, C. D., Rinaldi, M., and Carslaw, K. S.: Contribution of feldspar and marine organic aerosols to global ice nucleating particle concentrations, Atmos. Chem. Phys., 17, 3637–3658, https://doi.org/10.5194/acp-17-3637-2017, 2017.
Warren, G. J.: Biological Ice Nucleation and Its Applications, American Phyotpathological Society Press, St. Paul, MN, 1995.
Zeppenfeld, S., van Pinxteren, M., Hartmann, M., Bracher, A., Stratmann, F., and Herrmann, H.: Glucose as a Potential Chemical Marker for Ice Nucleating Activity in Arctic Seawater and Melt Pond Samples, Environ. Sci. Technol., 53, 8747–8756, https://doi.org/10.1021/acs.est.9b01469, 2019.





