the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Effect of organic carbon addition on paddy soil organic carbon decomposition under different irrigation regimes
Masuda Akter
Samuel Bodé
Orly Mendoza
Haichao Li
Pascal Boeckx
Steven Sleutel
Anaerobic decomposition of organic carbon (OC) in submerged rice paddies is coupled to the reduction of alternative soil electron acceptors, primarily Fe3+. During reductive dissolution of Fe3+ from pedogenic oxides, previously adsorbed native soil organic carbon (SOC) could be co-released into solution. Incorporation of crop residues could hence indirectly, i.e. through the stimulation of microbially mediated Fe3+ reduction, promote the loss of native SOC via enhanced dissolution and subsequent mineralisation to CO2 and CH4. Our aim was to estimate the relevance of such a positive feedback during the degradation of added OC, and to investigate the impact of irrigation management on this mechanism and on priming effects on native SOC decomposition in general. In a six-week pot experiment with rice plants, two Bangladeshi soils with contrasting SOC to oxalate-extractable Fe (SOC : Feox) ratios were kept under a regime of alternate wetting and drying (AWD) or continuous flooding (CF), and were either amended with maize shoots or not. The δ13C signatures of dissolved organic C and emitted CH4 and CO2 were used to infer the decomposition of added maize shoots (δ13C = −13.0 ‰) versus native SOC (δ13C = −25.4 ‰ and −22.7 ‰). Addition of maize residues stimulated the reduction of Fe as well as the dissolution of native SOC, and the latter to a larger extent under CF, especially for the soil with the highest SOC : Feox ratio. Estimated Fe-bound SOC contents denote that stimulated SOC co-release during Fe reduction could explain this positive priming effect on SOC dissolution after the addition of maize. However, priming effects on SOC mineralisation to CO2 and CH4 were lower than for SOC dissolution, and were even negative under AWD for one soil. Enhanced reductive dissolution of Fe-bound SOC upon exogenous OC addition therefore does not necessarily lead to stimulated SOC mineralisation. In addition, AWD irrigation was found to decrease the above-mentioned priming effects.
- Article
(1165 KB) - Full-text XML
-
Supplement
(503 KB) - BibTeX
- EndNote
Anaerobiosis in flooded paddy fields thoroughly affects soil chemical processes, as in the absence of oxygen, the decomposition of organic carbon (OC) requires alternative terminal electron acceptors like manganese (Mn4+), iron (Fe3+), sulphate, acetate or carbon dioxide (CO2). Anaerobic reduction of the latter two electron acceptors, which also depends on the production of dissolved organic carbon (DOC) as an electron donor, leads to the production and emission of methane (CH4) (Kögel-Knabner et al., 2010; Ponnamperuma, 1972). Obviously, irrigation management strongly affects the prevalence of aerobic versus anaerobic OC decomposition. The impact of irrigation management on soil processes is an extensively studied topic because of the demand to grow rice more efficiently with less water (Carrijo et al., 2017). In particular, the adoption of alternate wetting and drying (AWD), a periodic drying and reflooding irrigation practice, is increasingly promoted (Lampayan et al., 2015). However, our understanding of how the alternation of redox conditions under AWD irrigation affects the decomposition of native soil organic carbon (SOC) and exogenous OC in comparison with more consistent anaerobic conditions is still rather limited. This limited insight mainly stems from difficulties in discerning native SOC and exogenous OC mineralisation based on soil gaseous emissions. In upland soils, assessments usually rely on δ13C isotopic signatures of emitted CO2 derived from C sources with distinct δ13C (Hayes, 1983; Werth and Kuzyakov, 2010). However, in submerged soils, emission of CH4 also needs to be accounted for. Since most CH4 and CO2 is emitted through rice plant aerenchyma transport, a realistic experiment necessitates the presence of growing rice plants, which further complicates the tracking of OC mineralisation in paddy soils. It is then important to account for C isotope fractionation during the OC decomposition process, mainly caused by the microbial community discriminating for or against 13C. In particular, during anaerobic CH4 production, the shift in δ13C is typically large and moreover, production pathway-dependent (Conrad, 2005; Sugimoto and Wada, 1993; Schweizer et al., 1999; Werth and Kuzyakov, 2010; Conrad et al., 2012). The δ13C of emitted CO2 is on the other hand influenced by CO2 consumption during hydrogenotrophic CH4 production (i.e. with as substrate) and its partial storage as dissolved bicarbonate and CO2. As a result, in the absence of oxygen, it is much less straightforward to link the δ13C-CO2 and δ13C-CH4 of emissions to the δ13C of the original C substrate (Conrad et al., 2012).
Furthermore, the incorporation of crop residues is a common practice in paddy soil (Ponnamperuma, 1984; Mandal et al., 2004). It is known that exogenous OC addition can modify the rate of SOC decomposition in upland soils via so-called priming effects. Positive priming could for example result from soil microbial growth upon the addition of fresh OC as an energy source, resulting in the co-metabolism of biologically more recalcitrant SOC (Kuzyakov et al., 2000; Blagodatskaya and Kuzyakov, 2008). Positive as well as negative priming effects of OC addition on the dissolution of native SOC and on its further mineralisation into CO2 and CH4 have also been reported for flooded paddy soils (Bertora et al., 2018; Conrad et al., 2012; Ye et al., 2015; Ye and Horwath, 2017; Yuan et al., 2014), but again these processes are much less well understood. Indeed, considering the unique interlinkage between anaerobic microbial activity and redox reactions with mineral soil oxidants, priming mechanisms could be quite different than in upland soils. For example, Bertora et al. (2018) and Ye and Horwath (2017) observed that rice straw addition enhances the reductive dissolution of pedogenic Fe, and found indications that this in turn led to a stimulated co-release of OC originating from the Fe-OC complex. Pedogenic Fe3+ oxides, hydroxides and oxyhydroxides (hereafter collectively referred to as “Fe oxides”), which are often abundant in tropical soils, are indeed relevant sorbents of SOC owing to their high reactive specific surface area or through complexation (Wagai et al., 2013; Chen et al., 2020; Kaiser and Guggenberger, 2000). Dissolution of OC from these minerals following the establishment of anaerobic conditions could thus be a considerable C-releasing process and may take place (i) by desorption due to a pH increase, caused by the consumption of protons during reduction reactions; or (ii) by co-release of sorbed OC along with reductive dissolution of short-range-ordered Fe3+ minerals such as ferrihydrite and goethite (Grybos et al., 2009; Said-Pullicino et al., 2016). However, it is still unknown whether such stimulated native SOC dissolution would in turn significantly promote further conversion into CO2 and CH4. This would seem plausible as DOC is assumed to be one of the most bioavailable SOC fractions, especially under submerged conditions (Marschner and Kalbitz, 2003; Said-Pullicino et al., 2016). The indirect stimulation of SOC mineralisation by OC amendment through enhanced reduction of Fe in paddy soil may furthermore be expected to depend on the water regime, as this overridingly determines the extent of pedogenic Fe reduction and increase in pH. Lastly, opposite to the co-dissolution of Fe and OC, DOC can likewise be adsorbed onto or can co-precipitate with the newly formed Fe3+ oxides as soon as the Eh again increases and Fe2+ is reoxidised (Sodano et al., 2017). Such C removal pathways might again strongly depend on irrigation management.
Our aim was to investigate how the addition of exogenous OC influences the dissolution and mineralisation of native SOC in paddy soils in function of water management, with particular attention to the role of the co-release of Fe-bound SOC. To this end, patterns of DOC and the emission of CO2 and CH4 were compared in a six-week pot experiment with living rice plants in a tropical greenhouse. Two common irrigation practices were compared, i.e. the water-saving AWD practice next to continuous flooding (CF). We hypothesised that maize shoot addition would result in positive priming of native SOC mineralisation. This priming should be stronger under CF than under AWD, owing to enhanced pedogenic Fe3+ reduction with stimulated net co-release of Fe-bound native SOC. Maize shoots were used as an external OC source, because of the contrast of their δ13C (C4 crop) with the δ13C of the native SOC (largely C3-derived due to long-term rice cultivation) in both soils. A secondary aim was to see if the proportion of SOC compared to pedogenic Fe, with the latter considered to be the main pool of readily reducible Fe, interacts with the effect of OC amendment on native SOC decomposition. Therefore, we compared two soils from Bangladeshi young floodplain paddy fields that were specifically chosen for their contrasting SOC to oxalate-extractable Fe (Feox, as a proxy for short-range-ordered Fe oxides that can be considered easily reducible; Postma, 1993; van Bodegom et al., 2003) ratio. We expected a stronger stimulation of native SOC release from the soil with the higher SOC : Feox ratio.
2.1 Soils
Two young floodplain paddy soils (from soil series Balina and Sonatala) from northern Bangladesh were selected from a larger set of soils previously used by Akter et al. (2018) and Kader et al. (2013). Per soil series, the puddle layer soil (0–15 cm) of 15 locations within one field was sampled and then bulked by means of a clean spade in May 2014, after which the soil was stored in air-dried, ground and sieved form. Both soils were selected based on their contrasting SOC : Feox ratios with other traits remaining largely similar (Table 1).
Table 1Initial properties of the selected two paddy soil series and maize shoots used in the soil incubation experiment.
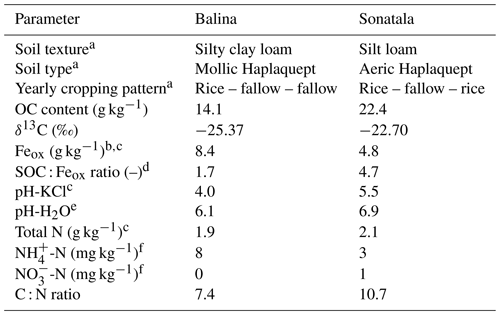
a Data taken from Kader et al. (2013). b Oxalate-extractable Fe. c Data taken from Akter et al. (2018). d Ratio of SOC to oxalate-extractable Fe. e pH-H2O by inserting a glass pH electrode in 1 : 5 soil–water extracts after 18 h equilibration. f Exchangeable mineral N determined in 1 M KCl extracts.
X-ray diffraction analyses confirmed that both soils contained mica, vermiculite, chlorite and kaolinite, and Sonatala also some crystalline goethite (Kader et al., 2013). The presence of Fe3+ in chlorite and vermiculite was confirmed by Mössbauer spectroscopy (Akter et al., 2018), but ferrihydrite and poorly crystalline goethite were the main Fe3+ pools in both soils.
2.2 Pot experiment
A six-week pot experiment with living rice plants was run in the tropical greenhouse of the Faculty of Bioscience Engineering (Ghent University) in Melle, Belgium, from 23 April to 4 June 2018. The mean ambient temperature was 28 ∘C and the relative humidity was 82.2 %. PVC tubes (diameter: 18.5 cm; height: 25.0 cm) were filled with soil from either Balina (4.8 kg dry soil) or Sonatala (4.0 kg dry soil) until a height of 17.5 cm, matching their respective bulk densities, and were subjected to two contrasting irrigation treatments, as described below. Ground maize shoots were mixed into the soil at a dose of 4 g dry mass kg−1 soil (i.e. 1.90 g C kg−1) to three replicate pots per soil type and per irrigation regime. Maize shoots had an OC content of 474.4 g kg−1, a δ13C of −13.04 ‰ and a total N content of 13.0 g kg−1. Two replicate pots were installed as controls without maize added, and two more replicates per irrigation and soil combination received maize (same dose of 4 g kg−1) but had no living rice plants. Macronutrients were added once as basal fertilisation to all pots as urea, KCl and Ca3(PO4)2, at doses of 60 kg N ha−1, 40 kg K ha−1 and 10 kg P ha−1, respectively. Five 15 d-old rice seedlings were transplanted to each pot in a single hill. These seedlings were obtained by germinating rice seeds of BINA dhan14 (Oryza sativa L.), a Bangladeshi short-duration (maturity 120–130 d) dry season rice variety, and sowing those in seeding beds that were kept saturated in the tropical greenhouse for 15 d.
A standing water table of 2.5 cm was initially maintained in all pots by adding demineralised water every one or two days to enable seedling establishment. Starting from 11 d after transplanting (DAT), the two different irrigation regimes were imposed. In pots under CF, the 2.5 cm water table was maintained until the end of the experiment. Pots under AWD, in contrast, were left to dry out until the water table dropped to between −8 and −14 cm, after which they were reflooded until a 2 cm standing water table was reached. Water table depths were monitored using a perforated tube (diameter: 3 cm) that was permanently installed in the pots. Soil drying was only due to evapotranspiration. Drying cycles took on average 6 (±3) d and shortened towards the end of the experiment.
2.3 Monitoring of biochemical soil parameters
The soil redox potential (Eh) was measured manually daily or every two days at 4 and 12 cm depth in eight pots with plants (i.e. 2 irrigation treatments × 2 soil series × with versus without maize). This was achieved by connecting a redox mV meter (Paleo Terra, the Netherlands) to permanently installed platinum (Pt) probes and one Ag|AgCl reference electrode (Paleo Terra, the Netherlands) per pot, and expressed relative to the standard hydrogen electrode. Soil pH was measured six times during the experiment in every pot at about 2 cm depth by inserting a glass pH electrode into the saturated soil.
To place CH4 and CO2 flux measurements in context, KCl-extractable ammonium (NH) and nitrate (NO) were determined at the start of the experiment (0 DAT), and at 11, 23, 30 and 45 DAT. For this, soil samples were taken by carefully inserting a tube (diameter: 1 cm) until 10 cm depth about four times at different locations near the side of each PVC pot. Soil samples were then stored at −20 ∘C until analysis. The contents of NH and NO were determined with a continuous flow auto-analyser (Skalar, the Netherlands) after extracting soil samples with 1 M KCl at a 1 : 5 ratio.
At the end of the experiment, the above- and below-ground biomass of the rice plants was determined after drying at 60 ∘C for three days. The δ13C of SOC was determined before the experiment for solid native SOC and maize, and after the experiment in SOC, with an EA-IRMS (automated nitrogen carbon analyser – solids and liquids – coupled to a SerCon 20-20 IRMS, Syscon Electronics, the Netherlands).
2.4 Soil solution sampling and analyses of Fe2+, Mn2+, DOC and δ13C-DOC
To track dissolved Fe, Mn, Ca, Mg and DOC, soil solution samples (2×9 mL per pot) were taken ten times throughout the experiment. Solution samples were collected by connecting a pre-evacuated 12 mL glass vial through a needle, 2-way stopcock and extension tube to a porous MacroRhizon soil moisture sampler (Rhizosphere Research Products, the Netherlands) that was permanently installed in each pot in a vertical position until about 9 cm depth. Solutions were analysed for their Fe, Mn, Ca and Mg concentrations by ICP-OES (Thermo Scientific, Unites States) without filtration, after acidifying the solutions with some drops of concentrated HNO3 to redissolve precipitates. The sum of the molar concentrations of dissolved Fe and the increment in dissolved Ca and Mg (compared to their initial level) was taken as a proxy for total reduced Fe since Fe2+ dissolves easily, but readily exchanges with Ca2+ and Mg2+ on colloid surfaces (Saeki, 2004). In the same samples, the DOC concentration and δ13C-DOC were measured by FIA-IRMS (flow injection analysis with isotope ratio mass spectrometry, DELTA V Plus Advantage, Thermo Scientific, United States) after dilution in 0.85 % H3PO4, evacuation and ultrasonication to remove carbonates and dissolved gases. To account for small differences in soil to soil solution ratios, soil solution concentrations of Fe equivalents and DOC were for each time point converted to mmol dissolved Fe equivalents kg−1 soil and mg C kg−1 soil, respectively.
2.5 Estimation of SOC bound to pedogenic Fe and its δ13C
We furthermore estimated the content and δ13C signature of SOC bound to weakly crystalline pedogenic Fe in the initial Balina and Sonatala soils based on the release of SOC after reduction with hydroxylamine (NH2OH • HCl), i.e. by assessing the change in OC content and δ13C of the solid soil samples with versus without NH2OH • HCl treatment. In all samples, particulate organic matter was firstly removed through ultrasonication at 60 J mL−1 and subsequent wet sieving at 53 µm (Sleutel et al., 2007). After drying the sieved suspensions, 0.8 g soil and 40 mL of a solution of 0.25 M NH2OH • HCl and 0.25 M HCl were mixed in a 85 mL Nalgene centrifuge tube, similarly to Chao and Zhou (1983). The tubes were then kept in a water bath at 50 ∘C for 30 min with regular stirring. After centrifuging at 1000 g for 15 min, the supernatant was decanted, and traces of NH2OH • HCl and HCl were removed by washing the residue soil three times with 40 mL demineralised water. Samples without the NH2OH • HCl treatment were treated identically, but the NH2OH • HCl solution was replaced by demineralised water. Finally, all samples were dried for four days at 40 ∘C, ground and analysed for their C content and δ13C by means of elemental analyser – isotope ratio mass spectrometry (EA-IRMS), i.e. an ANCA-SL (automated nitrogen carbon analyser – solids and liquids) interfaced with a 20-22 IRMS (Sercon Ltd., United Kingdom). The content of Fe-bound SOC was then estimated as the difference in SOC between treated samples and samples without NH2OH • HCl treatment. Analogous to Keeling (1958), the δ13C of Fe-bound SOC (δ13C-SOCFe) was calculated based on the following mass balance:
where δ13C-SOC and [C]-SOC refer respectively to the δ13C and C concentration of samples treated with NH2OH • HCl, and δ13C-SOCuntreated and [C]-SOCuntreated to those of samples without NH2OH • HCl treatment.
2.6 Gaseous C fluxes
In order to estimate the decomposition of OC to gaseous CH4 and CO2, we measured emission fluxes and δ13C isotopic signatures of CO2 and CH4 at ten different moments for pots under CF (most frequently after the onset of the experiment) and at twenty-six moments for pots under AWD (spread evenly over the course of the experiment). These extra measurements were required to sufficiently capture changing emissions during soil wetting and drying cycles. Gaseous efflux measurements were conducted by connecting a cavity ring-down spectrometer (G2201-i CRDS isotopic analyser, Picarro, United States) in a loop to an opaque PVC gas flux chamber (diameter: 18.5 cm; height: 25.0 cm) with an internal battery-operated fan. For each measurement, this flux chamber was secured onto the pots by means of a PVC connection ring (diameter: 18.5 cm; height: 16.0 cm), and the headspace accumulation of CO2 and CH4 as well as the evolution of their ratio (as opposed to the initial headspace air) was recorded every 4 s during 12 min. The CRDS was calibrated using standard gases with known δ13C signatures of −35.95 ‰ and −26.43 ‰. Starting from 28 DAT, an extra PVC extension ring (diameter: 18.5 cm; height: 25.0 cm) was added to the chambers because rice plants had outgrown the initial chamber.
CH4 and CO2 fluxes were determined as the slope of the accumulating headspace CH4 and CO2 concentrations as a function of time, and were converted into a mass-based unit (mg C kg−1 soil h−1) by means of the ideal gas law. Based on the conservation of mass, ratios of the emitted CH4 and CO2 were determined as the intercept of the linear regression from the isotopic signature of the headspace gas in function of the reciprocal of the headspace concentration, also known as the Keeling plot method (Keeling, 1958). All ratios were expressed relative to the international VPDB (Vienna Pee Dee Belemnite) standard as δ13C (‰). When rice seedlings were young and pots were flooded, a considerable part of the produced CO2 and CH4 appeared to be transported to the atmosphere through ebullition. In those cases, their accumulation in the headspace did not follow a linear course but was stepwise, with alternating time intervals of high and low accumulation rates, which were assumed to be dominated by ebullition versus by plant-mediated transport and molecular diffusion, respectively. When this was the case, additional C emission fluxes and δ13C signatures were derived for shorter time intervals during which emissions were dominated by ebullition or by plant-mediated transport and diffusion.
Average daily fluxes were estimated from the measured fluxes by accounting for diurnal fluctuation of the actual temperature using a temperature dependency model:
where Tavg and Tmeas (in ∘C) respectively represent the average daily soil temperature and the temperature during measurement. The Q10 temperature coefficient was set to 3.1 for CH4 (Wang et al., 2015; Wei et al., 2021; Hattori et al., 2001) and to 2.3 for CO2 (Huang et al., 2015; P. Zhou et al., 2014; Zhang et al., 2007; Wei et al., 2021). Overall, Tavg was 29.5±0.6 ∘C while Tmeas was 32.6 ± 1.0 ∘C, as gas efflux measurements were always carried out around noon.
2.7 Source partitioning of gaseous and dissolved C
The fractions of emitted CO2 and CH4 derived from added maize shoots (C4) ( and ) versus from native SOC (C3) ( and ) were inferred from δ13C signatures of emissions from (i) the “mixed pool” pots (to which maize was added); (ii) control pots without maize added (δ13C-CO2 | SOC and δ13C-CH4 | SOC as reference for the C3-derived emission endmember); and (iii) a shorter ancillary incubation with maize shoots as the only OC source (δ13C-CO2 | maize and δ13C-CH4 | maize as reference for the C4-derived emission endmember) (see Supplement). The latter two were used instead of the δ13C of respectively native SOC and maize-C in order to account for isotopic fractionation during microbial decomposition and emission of CO2 and CH4. Any effect of the presence of rice plants (plant respiration or decomposition of plant photosynthates to CO2 and CH4) is accounted for by the fact that the control pots without maize added (i.e. δ13C-CO2 | SOC and δ13C-CH4 | SOC) contained rice plants too. Based on the conservation of mass, the fraction of maize-derived CO2 is for example (Werth and Kuzyakov, 2010; Rochette and Flanagan, 1997; Hayes, 1983):
The CO2 or CH4 fluxes derived from native SOC (CO2 | SOC-derived or CH4 | SOC-derived) or maize (CO2| maize-derived or CH4 | maize-derived) in the mixed pots were then obtained by multiplying emissions with or versus or . Then, cumulative emissions of CH4, CO2, maize-derived C and native SOC-derived C were calculated.
We calculated a dimensionless priming effect (PE) coefficient of maize addition on SOC-derived CO2 or CH4 emission by comparing SOC-derived CO2 and CH4 fluxes with those from control pots without maize (CO2 | SOC or CH4| SOC) (Conrad et al., 2012). For example, relative priming of CO2 emission was defined as
In addition, the fraction of DOC derived from maize (fDOC | maize) was calculated as well based on δ13C signatures of DOC in amended pots (δ13C-DOC), of control pots without amendment (δ13C-DOCSOC), of solid maize-C (δ13Cmaize) and of solid SOC (δ13CSOC), as follows:
DOC contents derived from SOC (DOCSOC-derived) in amended soils were obtained by multiplying their total DOC contents with (1− fDOC | maize), and the relative priming effect of maize addition on SOC-derived C dissolution was then calculated in an analogous way to Eq. (4).
Because of the presence of rice plants, DOC contents as well as CH4 and CO2 emissions originate not only from native SOC versus maize shoots, but also from rice plant photosynthates. Having a similar δ13C as SOC, rice plant-derived DOC, CH4 and CO2 will contribute to what is partitioned as SOC-derived C, leading to overestimation of SOC decomposition. However, this contribution is believed to be very low in our experiment considering the young age of the rice plants. An estimate of the plant-derived DOC contents in our pots based on literature (Lu et al., 2004; Nguyen, 2003) was as low as 14 mg C kg−1 soil, with therefore indeed low potential contributions to soil C emissions, in agreement with observations of Yuan et al. (2012) at 41 d, and comparison of our emissions from maize-amended pots with and without rice plants. SOC-derived emission estimates based on isotopic mixing (Eq. 3) are henceforth assumed to be derived from native SOC decomposition.
2.8 Statistical analyses
Statistical tests were conducted with R 3.6.1 for Windows (R Core Team, 2019), with P=0.05 taken as the default significance level. Mostly, results for both soils (Balina and Sonatala) were analysed separately. The effect of irrigation treatment (starting from 11 DAT in the case of time series) on measured variables was assessed by two-way or three-way ANOVA or by Kruskal-Wallis tests with irrigation treatment and maize amendment as factors, and DAT as a third factor in the case of time series. In the case of interaction between the irrigation management and maize amendment factors, the tests were repeated for data from maize-amended versus control pots separately. In the case of interaction with DAT, differences between irrigation treatments were also assessed separately for individual points in time. Student's t-tests or Wilcoxon-Mann-Whitney tests were used to test the significance of priming effects for dissolution (i.e. comparing averages after integration over time from SOC-derived DOC in maize-amended and unamended soil) as well as emission (i.e. comparing SOC-derived emissions from maize-amended and unamended soil), to compare if dissolution and emission priming effects differed from one another, and to test if the δ13C of CH4 and CO2 differed when emissions were dominated versus not dominated by ebullition.
3.1 Redox potential and iron reduction
Within one to two weeks the soil redox potential (Eh) generally decreased to ca. −250 to −150 mV (Fig. 1). The effect of the irrigation treatment on Eh seemed to depend on both soil and addition of maize, but temporary increases in Eh were observed upon soil drying. The Eh was more responsive to wetting and drying at 4 cm than at 12 cm depth. Maize addition did not particularly induce a lower Eh, irrespective of soil type, though it did stimulate reductive dissolution of Fe (P<0.001 for both soils between 2 and 11 DAT) during the observed initial increase in dissolved Fe equivalents (Fig. 2). Similar trends were observed for Mn2+ (data not shown). When maize was added, Fe concentrations after 11 DAT dropped to lower levels in the case of AWD compared to CF (P<0.001 for Balina and P=0.04 for Sonatala). In control pots without maize shoots added, there was no significant effect of irrigation treatment on the decrease in dissolved Fe.
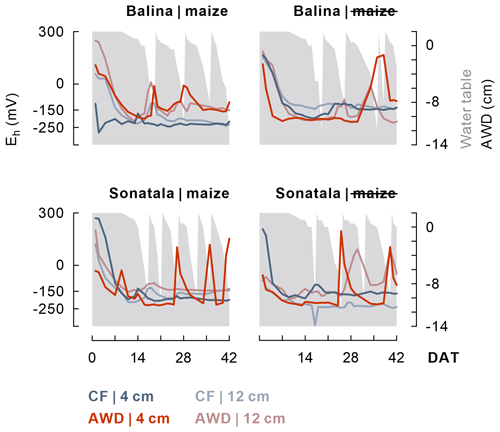
Figure 1After a general initial drop, the soil redox potential (Eh) (values per replicate at 4 and 12 cm depth) depended to some extent on the irrigation treatment in both paddy soils (Balina and Sonatala). Water table fluctuations in the case of AWD are indicated in grey.
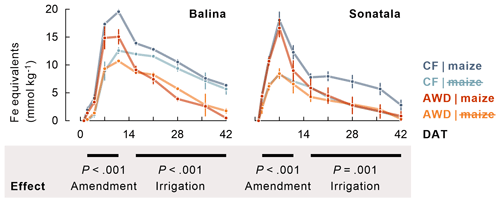
Figure 2Maize addition stimulated reductive dissolution of Fe (average ± standard error of three or two replicates, calculated as sum of dissolved Fe and increments in dissolved Ca and Mg), and Fe2+ disappeared to a larger extent from the soil solution under AWD than under CF in maize-amended pots. The P-values indicate the effect of maize addition on the time series between 2 and 11 DAT, and the effect of irrigation treatment in maize-amended pots on the time series between 16 and 42 DAT.
3.2 Exchangeable NH, pH and rice plant biomass
Concentrations of exchangeable NH increased until 11 DAT, partly owing to the initial urea additions of 39 and 47 mg N kg−1 to the Balina and Sonatala soils, respectively. Thereafter they remained more or less constant (Fig. S2 in the Supplement). Overall, exchangeable NH levels reached in the Balina soil were about double those for Sonatala. The effects of the presence of rice plants or irrigation treatment were at times significant but tended to be small and are not further discussed in detail. NH levels were mostly significantly higher with maize added than without, probably because of net N mineralisation. Concentrations of NO were always minor, i.e. below 1 mg N kg−1 (data not shown).
The soil solution pH of the Balina soil increased from an initial value of 6.1 to 6.8 (±0.1) after 17 DAT and then remained constant onwards (Fig. S3). In the Sonatala soil, pH remained nearly constant throughout (initially 6.9, then 6.8±0.1 starting from 17 DAT).
After 42 d, rice plants had reached a height of 76±5 cm, with an above-ground dry biomass of 2.7±0.7 g per pot and a below-ground dry biomass of 0.6 ± 0.3 g per pot. There was no effect from soil type or irrigation practice on the above-ground biomass. Below-ground biomass was not affected by soil type either but was significantly higher for plants grown under CF (0.8±0.3 g per pot) than under AWD (0.4±0.2 g per pot) (P<0.001).
3.3 Organic carbon dissolution
Extremely high total DOC peaks were reached 1 to 2 weeks after submergence in all treatments (maximally 1912 and 1329 mg C kg−1 soil for maize-amended Balina and Sonatala soils, respectively), which were also much higher with maize added (P<0.001 for time series until 11 DAT). Not only maize-derived DOC (data not shown), but also enhanced dissolution of native SOC caused these DOC peaks (Fig. 3). Over the course of the experiment, SOC-derived DOC was indeed higher with maize added than without in both soils, which was significant for Sonatala under CF (averaged concentrations over the course of the season: P=0.003). In other words, there was a positive priming effect on SOC dissolution (i.e. stimulation) during most of the incubation. This priming effect was irrespective of the irrigation regime in the Balina soils (P=0.97), while it was stronger under CF than under AWD in the Sonatala soils (P<0.001) starting from 16 DAT. Priming of SOC dissolution was also stronger for Sonatala than for Balina (P<0.001). Dissolution of maize-C was, in contrast, nearly identical in both soils, with an average peak concentration of 811 mg C kg−1 at 7 DAT, followed by a near-complete disappearance of maize-derived DOC around 16 DAT (<79 mg C kg−1) (data not shown).
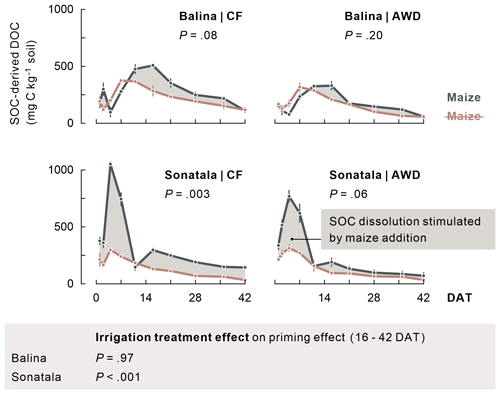
Figure 3Dissolution of native SOC (average ± standard error of three or two replicates) was stimulated upon addition of maize, as it appears when comparing with DOC in unamended pots (indicated in brown). P-values of the priming effect significance using average values per season (in the figure) and of the irrigation treatment effect on the priming effect time series starting from 16 DAT (below the figure) are indicated.
3.4 Gaseous carbon emission
Gaseous C emissions consisted on average of 31 % CH4 and 69 % CO2. Irrigation management did not affect the cumulative C emissions (CO2 + CH4), and neither CO2 nor CH4 emissions individually in the case of the Balina soil (Fig. 4). For the Sonatala soil, however, the effect of irrigation depended on whether or not the soils were amended with maize. With maize added, total gaseous C (P=0.04) and CH4 (P=0.03) emissions were higher under CF than AWD, but CO2 emissions were not significantly affected. On the other hand, in unamended Sonatala pots, total C (P=0.02) and CO2 (P=0.003) emissions were higher under AWD than under CF, while CH4 emission was not significantly different. CH4 and CO2 emissions were in general higher with maize added than without (P<0.001). Total gaseous C emission was furthermore lower from the Sonatala than from the Balina soil (P=0.01). In general, gaseous C emissions were similar with or without living rice plants in pots with maize added (P=0.23). Both the addition of maize (P<0.001) and the implementation of CF (P<0.001) led to more gaseous C emission via ebullition: 60±15 % of C was emitted through ebullition under CF compared to only 37±11 % under AWD. With maize added, 56±16 % of C was emitted via ebullition, compared to only 37±12 % for unamended control pots.
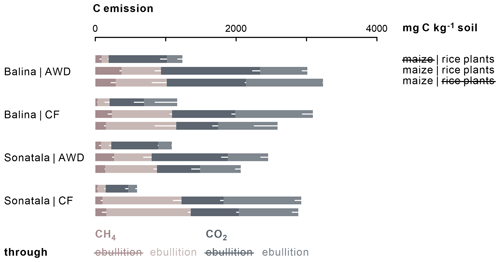
Figure 4Total cumulative C emissions (average − standard error of three or two replicates, represented by white bars) from maize-amended soils were two to fourfold of those from unamended pots (P<0.001). In addition, relatively more C was emitted through ebullition under CF (P<0.001) and upon addition of maize shoots (P<0.001).
In addition, the course of CH4 and CO2 fluxes seemed linked to the wetting and drying cycles under AWD (Fig. 5) as (i) more gaseous C was emitted when the soils dried; (ii) most ebullition transport occurred when the water table was at the soil surface, usually a few days before rewetting; and (iii) δ13C-CO2 signatures increased as water tables declined.
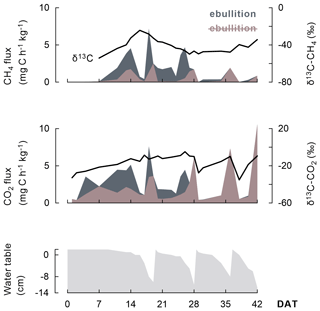
Figure 5As this representative example (one replicate of the Balina soil with maize shoots added and under AWD) shows, the progression of CH4 and CO2 emission fluxes (shaded) and even their δ13C signature (black line) appear linked to the change in water table depth under AWD. A distinction is made between emission fluxes dominated by ebullition (dark grey shaded) versus by diffusion or plant transport (brown shaded). Transport through ebullition was particularly considerable when rice plants were juvenile.
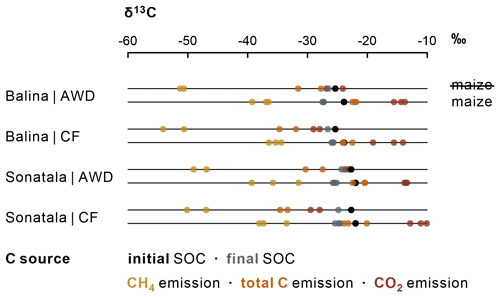
CH4 and CO2 emissions were clearly more enriched in 13C when maize was added (Figs. 6 and S1), evidently indicating the contribution of maize C decomposition. The δ13C of CH4 increased to a distinct maximum at 16 DAT (Balina) or 13 DAT (Sonatala) (Fig. S1). In control pots without maize, the δ13C of emitted CH4 was higher for Sonatala than for Balina, in line with the difference in δ13C of native SOC (Fig. 6). Implementation of AWD to control pots resulted in higher δ13C signatures of emitted C (CO2 + CH4) compared to CF (P=0.02). Isotopic fractionation took place during mineralisation of maize and native SOC into CH4 and CO2 (Figs. 6 and S1). Lastly, the δ13C of CH4 (P=0.02) and CO2 (P<0.001) was on average 2.62 ‰ and 3.45 ‰ higher when emissions were dominated by ebullition than when they were dominated by plant transport and diffusion (data not shown).
3.5 Source partitioning of emitted CO2 and CH4
The fractions of maize-derived and SOC-derived CO2 and CH4 were calculated based on isotopic mixing, considering the δ13C of SOC and maize, and accounting for isotopic fractionation during decomposition to CO2 and CH4 (Fig. S1). Surprisingly, the δ13C-CH4 of emissions from an ancillary C4-reference incubation with only maize and no native SOC as OC (δ13C-CH4 | maize) was usually lower (instead of higher) than from control pots with no maize added (δ13C-CH4| SOC) (Fig. S1). The difference in isotopic signature between maize and SOC was therefore not further reflected in a likewise difference in δ13C of the CH4 derived from both sources. In addition, the δ13C-CH4 was often higher than the δ13C of both endmembers in the case of the maize-amended Balina and Sonatala soils. For these reasons, the fraction of CH4 emission derived from maize () could unfortunately not be reliably deduced, in spite of the high measurement frequency across the experiment. As a best estimate, we therefore assumed that the contributions of emitted CH4 from maize decomposition would equal . After about 16 DAT, this derived fraction of maize-derived CO2 increased to about 100 % for most of the pots (data not shown). The calculated cumulative maize-derived CO2 and CH4 emissions ranged between 1737 and 2757 mg C kg−1, in most cases exceeding the theoretical maximum of 1898 mg C kg−1, i.e. the amount of initially added maize. Therefore, in those cases we instead consider complete degradation of all the initially added maize for further calculation of cumulative SOC-derived C emissions, which seems reasonable considering the complete disappearance of dissolved maize-C around 16 DAT (Fig. 3), and the fact that the δ13C of SOC was not higher after than before the experiment (Fig. 6).
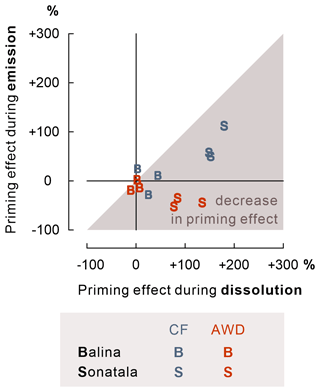
The effect of maize addition on SOC-derived C emission was negligible in the case of Balina, while for Sonatala, it depended on the irrigation treatment (Fig. 7). CF irrigation led to a positive priming (P=0.05) (i.e. stimulated SOC-derived gaseous C emissions upon maize addition), whereas negative priming on SOC-derived C emissions in maize-amended soil occurred under AWD (P=0.01). In addition, priming on SOC-derived C emission was higher under CF than AWD for Sonatala (P=0.004).
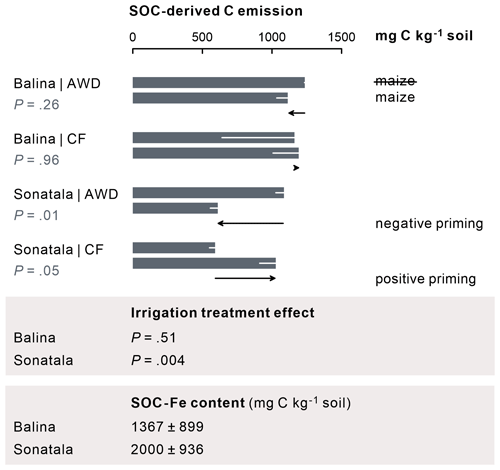
Figure 7For the Sonatala soil, maize addition led to positive priming of gaseous C (CO2 + CH4) emission under CF and negative priming under AWD. P-values of the priming effect significance (in the figure) and of the irrigation treatment effect on the priming effect (below the figure) are indicated. Standard errors (three or two replicates) are indicated. Fe-bound SOC levels (average ± standard error, estimated by means of NH2OH • HCl) exceed the positive priming effect of Sonatala under CF, so stimulated Fe-C dissolution and emission has the potential to explain this priming.
4.1 Are Fe reduction and SOC dissolution linked?
With the onset of anaerobic conditions, exemplified by a decreasing soil Eh (Fig. 1), there was a strong increase in dissolved Fe (Fig. 2), which shows that part of the soil Fe was quickly reduced and dissolved. At the same time, DOC concentrations increased rapidly until maxima at 11 DAT (Balina) and at 4–7 DAT (Sonatala). The synchrony of OC dissolution and Fe reduction patterns prompts the question of whether both processes are physically linked. Said-Pullicino et al. (2016) suggested that observed DOC increases could have been due to the co-release of OC during reductive dissolution of Fe oxides. With an adapted NH2OH • HCl extraction procedure, we estimated that the amount of C bound onto reducible Fe3+ minerals was 1367 mg kg−1 for Balina and 2000 mg kg−1 for Sonatala (Fig. 7), constituting approximately 9 % of SOC. Release of this substantial Fe-bound SOC pool might have been a crucial source of DOC, but other important C dissolution pathways need to be considered as well. Firstly, a rise in pH upon soil reduction under anaerobic conditions can lead to SOC desorption (Grybos et al., 2009) but this mechanism was most likely unimportant here because of the initially high pH that did not considerably change during the incubation, especially in the Sonatala soil (Fig. S3). Secondly, rhizodeposition from rice plants can also cause an increase in DOC (Said-Pullicino et al., 2016; Lu et al., 2004). However, based on values from Lu et al. (2004) and Nguyen (2003), maximum DOC contents derived from rice plant photosynthates in our soils are estimated to be around 14 mg C kg−1 soil, which is again limited since rice plants were juvenile during the experiment. Next to the direct contribution of rhizodeposition, some root photosynthates (e.g. oxalate and citric acid) can also indirectly increase DOC levels by promoting the release of Fe-bound SOC in the rhizosphere through their strong metal-complexing capacity (Keiluweit et al., 2015; Yu et al., 2017). This mechanism was, however, likewise restricted here considering its local impact and the juvenile age of the rice plants. Lastly, fermentation of SOC and added maize into water-soluble organic metabolites must obviously have largely contributed to the accumulation of DOC. As such, based on DOC patterns alone it is not possible to unequivocally state that reductive dissolution of Fe with co-release of Fe-bound OC drove DOC build-up.
The stable isotope approach then allowed to discriminate between SOC-derived and maize-derived DOC. Maize addition roughly doubled dissolution of native SOC (Fig. 3). Since also Fe reduction was stimulated upon the addition of maize (Fig. 2), it seems likely that, next to stimulated SOC fermentation, promoted SOC co-release upon enhanced Fe reduction was indeed a potentially important priming mechanism, in agreement with the observations of Ye and Horwath (2017) and Bertora et al. (2018). Dissolution of all Fe-bound SOC present might produce a DOC peak of approximately 584 mg C kg−1 (Balina) or 854 mg C kg−1 (Sonatala), when roughly comparing it to the DOC production and the DOC mineralisation kinetics of maize (where we know that 1898 mg added maize-C kg−1 yielded a peak of 811 mg C kg−1 maize-derived DOC). While just a tentative approximation, it becomes clear that the magnitude of SOC-derived DOC peaks suffices to explain the observed increment in SOC-derived DOC with maize added (Fig. 3).
Comparison of the δ13C of Fe-bound SOC and DOC could further contribute to the quantification of DOC derived from pedogenic Fe. Attempts to measure δ13C of NH2OH • HCl extracts directly by means of FIA-IRMS unfortunately failed owing to interference of the matrix, even after trying to remove NH2OH • HCl and Cl− by oxidation and precipitation. However, from the difference in soil δ13C before and after NH2OH • HCl extraction, the δ13C of Fe-bound SOC could be estimated indirectly with reasonable uncertainty, i.e. ‰ for Balina and ‰ for Sonatala. Apparently, Fe-C was a bit more depleted in 13C than bulk SOC (Table 1). In control pots, with only Fe-bound SOC and bulk SOC as C sources, δ13C signatures of DOC were also lower, supporting the assumption that DOC partly consists of released Fe-bound C. To robustly derive the contribution of potentially enhanced Fe-bound C release to stimulated SOC dissolution in maize-amended pots, three-source partitioning with an additional contrast in 14C next to the existing variation in would be required (when rice plants can be disregarded).
4.2 Extent of priming of SOC mineralisation as compared to SOC dissolution
Our objective was then to more specifically assess whether the stimulated SOC dissolution would be reflected in enhanced native SOC-derived mineralisation to CO2 and CH4. As it turns out, native SOC-derived gaseous C (i.e. CO2 + CH4) emissions were not significantly modified by the addition of maize in the Balina soil (Fig. 7). For Sonatala, there was however positive priming (i.e. more SOC-derived C emissions in soil with maize addition than in soil without) under CF irrigation (P=0.05) and a negative priming under AWD (P=0.01). For the CF treatment, the discussed stimulation of native SOC dissolution, probably partially by enhanced co-release of Fe-bound SOC, may have promoted SOC mineralisation upon maize addition. Yet, priming effect coefficients were overall larger for dissolution than for gaseous C emission in Sonatala (P<0.001) and not significantly for Balina (P=0.18) (Fig. 8), which suggests that the enhanced SOC dissolution would not have resulted in a proportionate SOC mineralisation. Hanke et al. (2013) likewise found that extra solubilised C after paddy soil submergence did not cause a proportional rise in gaseous C emissions. They assumed that the availability of alternative terminal electron acceptors was limiting mineralisation. However, as SOC mineralisation (indicated by SOC-derived CO2 and CH4 emission) was actually slower under AWD than under CF in the Sonatala soil, electron acceptor availability must not really have limited priming of SOC-derived C emission. It instead seems more likely that preferences of microorganisms that decompose DOC explain the observed smaller priming of soil C emission compared to dissolution. DOC-decomposing microorganisms must have preferred the abundantly available maize-derived DOC at the expense of SOC-derived DOC mineralisation in maize-amended soils. This hypothesis is supported by the faster removal of maize-derived compared to SOC-derived DOC (data not shown). Maize shoots and native SOC obviously differ in their biochemical quality, where SOC in paddy soils is generally quite aromatic with an accumulation of hardly degradable lignin components (Olk et al., 2002; Z. Zhou et al., 2014). In fact, phenol accumulation in paddy soil in part explains their larger topsoil OC content compared to upland counterparts (Chen et al., 2021). The energy yield of oxidation of such reduced SOC compounds (phenols and lipids) becomes low when coupled to Fe reduction (Keiluweit et al., 2017), and therefore it is logical that maize-derived DOC would have been preferred over SOC-derived DOC as a microbial substrate. Lastly, enhanced Fe reduction might also abiotically mediate positive priming of native SOC mineralisation through Fenton reactions that lead to the production of reactive hydroxyl radicals (Yu and Kuzyakov, 2021). However, as priming effect coefficients for gaseous C emissions were low compared to those for dissolution (Fig. 8), and since the relative contribution of CO2 to gaseous C emissions in maize-amended pots was lower than in control pots (Fig. 4), it seems unlikely that the potentially enhanced production of hydroxyl radicals would have played a considerable role in stimulating SOC decomposition in maize-amended pots.
4.3 Influence of irrigation management and soil type
Furthermore, we wanted to verify whether irrigation management plays a role in the priming effect on SOC-derived dissolution and mineralisation. It is of interest that the enhancement of SOC dissolution by maize addition was stronger under CF than AWD, especially for the Sonatala soil (Fig. 3). Under permanently flooded conditions, the addition of maize was probably more effective in promoting anaerobic microbial fermentation resulting in dissolution of SOC, as the biomass of anaerobes could grow under less disturbed conditions than in the case of a regime with repeated soil drying. In addition, DOC may have been removed from soil solution through net association with Fe oxides to a higher extent under AWD because of net higher reoxidation of soil solution Fe2+. This irrigation treatment effect on DOC priming was also manifested in higher priming on SOC emission under CF than AWD in the case of Sonatala (P=0.004) (Fig. 7). Furthermore, the decrease in priming effect was slightly more pronounced for AWD than CF for Sonatala (P=0.08) (Fig. 8). The negative priming of gaseous C emission under AWD in Sonatala was therefore possibly not only due to a lesser stimulation of SOC-derived dissolution under AWD than under CF. On top of that, the microbial community under AWD might have been less adapted to anaerobic mineralisation than under CF, resulting in an even larger preference for maize-derived than SOC-derived DOC.
Furthermore, the effect of irrigation management on overall gaseous emissions was rather limited, especially for Balina soil, and surprisingly partially determined by ebullition. With no maize added, CO2 emission (P=0.003) was lower under CF than under AWD for Sonatala (Fig. 4). Indeed, SOC mineralisation is often retarded under anaerobic conditions (Kögel-Knabner et al., 2010; Sahrawat, 2004). However, CH4 effluxes were also surprisingly lower under CF, while the more reducing conditions compared to under AWD were expected to stimulate CH4 production (Jiang et al., 2019; Peyron et al., 2016). One cause for this contrasting outcome may be the often considerable temporal storage of produced CH4 and CO2 in submerged CF pots. Indeed, transport of CH4 and CO2 in paddy soils usually proceeds predominantly via rice plant aerenchyma, whereas diffusion-based outgassing is retarded by permanently standing water. With rice plants still juvenile and relatively limited CO2 and CH4 production in the unamended pots, it is thus possible that a larger part of these gases was stored dissolved or as gas bubbles in the CF pots. With AWD on the other hand, the capacity of the standing water to store gases decreases as the water table goes down regularly (Green, 2013; Tokida et al., 2013). Transport of the produced CH4 and CO2 to the atmosphere was indeed clearly facilitated by episodic flushes, e.g. through ebullition, when the water table decreased (Fig. 5), in line with the observations of Yagi et al. (1996) and Han et al. (2005). With maize added, on the other hand, CH4 emission (P=0.03) was 53 % higher under CF than AWD in the case of the Sonatala soil. The much faster production of CH4 compared to in unamended pots likely led to oversaturation of the dissolved CH4 pool, resulting in less restricted CH4 emission. For Balina, on the contrary, the irrigation regime did not significantly affect CO2 and CH4 emission. This all in all low effect of water management on gaseous C emissions in the Balina soil was in line with its limited impact on the progression of Eh at 12 cm depth (Fig. 1), pH (Fig. S3) and the fact that exchangeable NH levels (Fig. S2) only differed during the final two weeks.
Lastly, the contrast in Fe-bound SOC estimates between Sonatala and Balina corresponded with the difference in their respective SOC : Feox ratios (4.7 versus 1.7), and moreover with the higher SOC-derived DOC levels and stronger priming of SOC dissolution in Sonatala compared to Balina. Ye et al. (2016) found that a soil with less electron acceptors gave rise to stronger priming on CH4 emission in a laboratory incubation under submerged conditions, which corresponds with our findings on C (CH4 + CO2) emission priming for the CF treatment. However, the higher SOC : Feox ratio cannot immediately explain the more pronounced decrease in priming effect on C emission compared to dissolution for Sonatala than for Balina (P<0.001), except if the DOC derived from pedogenic Fe would be more recalcitrant, leading to a bigger preference of DOC decomposers for maize-derived DOC. Soil type also influenced temporal evolutions of biochemical parameters, but only to some extent. For Sonatala, redox-related processes took place three to five days earlier than for Balina, such as: (i) the initial decline of Eh (Fig. 1); (ii) the peak in dissolved Fe (Fig. 2); (iii) the peak in DOC, in particular in SOC-derived DOC (Fig. 3); and (iv) the “peak” in emission δ13C-CH4 signatures (Fig. S1). This probably all relates to the higher electron surplus (i.e. higher amount of electron donors as compared to the main electron acceptor Fe3+) in combination with a lower Feox content for Sonatala, with its higher SOC : Feox ratio, resulting in a faster decrease in soil Eh. In Sonatala, it is likely that microbes quickly needed to couple OC oxidation with the reduction of alternative electron acceptors other than Fe3+, so that Eh dropped below the level enabling CH4 production (−150 mV) after 4 d on average for Sonatala while only after 9 d for Balina. In general, the impact of irrigation management and maize residue addition on DOC release and emissions seemed to depend on the soil type (in particular the SOC : Feox ratio), and soil type therefore seems to be an equally important factor controlling the progression of reductive processes.
4.4 Using an isotope mass balance to infer native SOC mineralisation in paddy soils
Because of different types of isotopic fractionation, most importantly caused by microbial discrimination for or against 13C as a substrate, source partitioning of gaseous emissions was not straightforward. Unlike the results of Conrad et al. (2012) but in agreement with the observations of Ye et al. (2016), isotopic fractionation was different for maize than for SOC. For example, we observed a very consistent initial enrichment in 13C-CH4 over time until strikingly high δ13C-CH4 signatures of ‰ (Balina; 16 DAT) and ‰ (Sonatala; 13 DAT), with thereafter 13C depletion (Figs. S1 and 5). As such trends were inexistent for δ13C-CO2, these δ13C evolutions are likely the result of gradually changing dominant CH4 production pathways. Indeed, CH4 can be produced with acetate, CO2 or other methylated compounds as precursors. Among any of these precursors, the lowest fractionation (i.e. resulting in CH4 with the least negative δ13C) occurs with the acetate-dependent pathway, resulting in a δ13C-CH4 as high as −27 ‰ (relative to VPDB) (Conrad, 2005). The even further enrichment of 13C-CH4 in the maize-amended plots can be explained by the higher δ13C of maize (Fig. 6). Another explanation for the constantly changing δ13C-CH4 could be that the contributions of various gas transport mechanisms and associated isotopic fractionation would have changed over time. The δ13C-CO2 increased as water tables declined during AWD cycles, particularly towards the end of the experiment (Fig. 5). In the unamended pots, the δ13C of CO2 (P=0.02) was also higher under AWD than under CF (Fig. 6). Both observations clearly suggest that change in transport fractionation affected the evolving δ13C-CO2 and δ13C-CH4 patterns. Interestingly, continuous CRDS-based recording of CO2, CH4 and their δ13C allowed to discern sudden ebullition events over the course of a measurement. Emissions dominated by ebullition indeed displayed higher δ13C-CH4 but also higher δ13C-CO2 signatures than emissions during which ebullition appeared absent. The evolving contribution of diffusion and plant-mediated versus ebullition transport throughout the experiment thus must have altered the δ13C of emitted CO2 and CH4. In the case of CH4, it is indeed well known that δ13C isotopic fractionation not only takes place during plant-mediated transport (Zhang et al., 2014, 2015) but also during gas bubble formation (Zhang et al., 2014). Our results suggest that the δ13C of emitted CO2 is also impacted by the contribution of ebullition transport. Using pots without maize added, we quantified the overall isotopic fractionation between SOC and SOC-derived CO2 and CH4. We then also attempted to quantify isotopic fractionation between maize-C and maize-derived CO2 and CH4 by means of an ancillary incubation experiment (Fig. S1), but this was only successful for CO2. In addition, maize addition lifted the contribution of ebullition towards 56 % of all emitted gaseous C compared to 37 % without maize. Because of that, the estimated δ13C-CO2 | SOC and δ13C-CH4 | SOC used in Eq. (3) were perhaps not representative for the maize-amended pots, since the proportion of ebullition transport was particularly large in our experiment. However, decreasing ebullition by retarding mineralisation, e.g. by lowering soil temperatures or by working with a less labile substrate than the finely ground maize, would after all have but a limited impact. To improve the source partitioning of emissions, the use of exogenous OC with a more strongly contrasting δ13C would help the most, as then the relative effect of isotopic fractionation declines.
Using a stable isotope approach, it was confirmed that the addition of high-quality OC like maize shoots stimulates Fe reduction and dissolution of native SOC. The synchrony of both processes suggests that priming of SOC dissolution after addition of maize could result from increased net co-release of OC during reductive dissolution of Fe oxides, next to enhanced fermentation of native SOC to DOC. In support of this hypothesis, the pool of Fe-bound C (1.3–2.0 g C kg−1), estimated by means of reduction with NH2OH • HCl, was large enough to explain this. Moreover, we found that maize addition stimulated SOC dissolution more strongly in the soil with the highest SOC : Feox ratio and Fe-bound SOC content. However, positive priming of SOC mineralisation into CO2 and CH4 only occurred under CF in one out of the two investigated soils. Priming of SOC dissolution therefore does not necessarily result in proportional priming of native SOC-derived emissions, most likely owing to an overall preference of microbes for maize-derived DOC in anaerobic conditions. However, it turned out that it is particularly difficult to assess priming effects on native SOC mineralisation in paddy soils, because source partitioning of C emissions based on a stable isotope mass balance is inherently complicated due to various isotopic fractionation effects. Nevertheless, our study indicated that particularly in soil with a high SOC : Feox ratio, the addition of labile OC can result in substantial extra dissolution and mineralisation of native SOC under CF irrigation, with a possible adverse impact on the SOC balance in the longer term. The adoption of water-saving irrigation can instead successfully decrease the stimulation of SOC dissolution caused by OC addition and inhibit the positive priming of SOC mineralisation.
The codes used in the preparation of this paper are available from the corresponding author upon request.
The raw data obtained in this study are available from the corresponding author upon request.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-5035-2021-supplement.
SS, HD and PB designed the experiment, with input of MA. MA and SS selected and provided paddy soils from Bangladesh. HD, OM and HL conducted the incubation experiment with the support of MA. HD did the lab analyses with the support of SS, MA, OM and HL. SB and HD performed the dissolved organic C content and δ13C analyses, and SS, SB and HD developed and performed the procedure to quantify Fe-bound soil organic C. HD and SS conducted the data analyses and wrote the manuscript, with input from all other authors.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We would like to genuinely thank the involved staff of the tropical greenhouse, the lab of the Soil Fertility and Nutrient Management (SoFer) group and the Isotope Bioscience Laboratory (ISOFYS) from Ghent University (Belgium).
This research has been supported by the European Research Area Network (ERA-Net) and the Research Foundation Flanders (FWO) in the framework of the project “Greenhouse gas emissions from paddy rice soils under alternative irrigation management” (GreenRice) (grant no. GA.010.14N).
This paper was edited by Luo Yu and reviewed by Guanghui Yu and one anonymous referee.
Akter, M., Deroo, H., De Grave, E., Van Alboom, T., Kader, M. A., Pierreux, S., Begum, M. A., Boeckx, P., and Sleutel, S.: Link between paddy soil mineral nitrogen release and iron and manganese reduction examined in a rice pot growth experiment, Geoderma, 326, 9–21, https://doi.org/10.1016/j.geoderma.2018.04.002, 2018.
Bertora, C., Cucu, M. A., Lerda, C., Peyron, M., Bardi, L., Gorra, R., Sacco, D., Celi, L., and Said-Pullicino, D.: Dissolved organic carbon cycling, methane emissions and related microbial populations in temperate rice paddies with contrasting straw and water management, Agr. Ecosyst. Environ., 265, 292–306, https://doi.org/10.1016/j.agee.2018.06.004, 2018.
Blagodatskaya, Å. and Kuzyakov, Y.: Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review, Biol. Fert. Soils, 45, 115–131, 2008.
Carrijo, D. R., Lundy, M. E., and Linquist, B. A.: Rice yields and water use under alternate wetting and drying irrigation: A meta-analysis, Field Crop Res, 203, 173–180, https://doi.org/10.1016/j.fcr.2016.12.002, 2017.
Chao, T. and Zhou, L.: Extraction techniques for selective dissolution of amorphous iron oxides from soils and sediments, Soil Sci. Soc. Am. J., 47, 225–232, 1983.
Chen, C., Hall, S. J., Coward, E., and Thompson, A.: Iron-mediated organic matter decomposition in humid soils can counteract protection, Nat. Commun., 11, 2255, https://doi.org/10.1038/s41467-020-16071-5, 2020.
Chen, X., Hu, Y., Xia, Y., Zheng, S., Ma, C., Rui, Y., He, H., Huang, D., Zhang, Z., and Ge, T.: Contrasting pathways of carbon sequestration in paddy and upland soils, Glob. Change Biol., 27, 2478–2490, https://doi.org/10.1111/gcb.15595, 2021.
Conrad, R.: Quantification of methanogenic pathways using stable carbon isotopic signatures: a review and a proposal, Org. Geochem., 36, 739–752, https://doi.org/10.1016/j.orggeochem.2004.09.006, 2005.
Conrad, R., Klose, M., Yuan, Q., Lu, Y., and Chidthaisong, A.: Stable carbon isotope fractionation, carbon flux partitioning and priming effects in anoxic soils during methanogenic degradation of straw and soil organic matter, Soil Biol. Biochem., 49, 193–199, https://doi.org/10.1016/j.soilbio.2012.02.030, 2012.
Green, S. M.: Ebullition of methane from rice paddies: the importance of furthering understanding, Plant Soil, 370, 31–34, 2013.
Grybos, M., Davranche, M., Gruau, G., Petitjean, P., and Pédrot, M.: Increasing pH drives organic matter solubilization from wetland soils under reducing conditions, Geoderma, 154, 13–19, https://doi.org/10.1016/j.geoderma.2009.09.001, 2009.
Han, G. H., Yoshikoshi, H., Nagai, H., Yamada, T., Saito, M., Miyata, A., and Harazono, Y.: Concentration and carbon isotope profiles of CH4 in paddy rice canopy: Isotopic evidence for changes in CH4 emission pathways upon drainage, Chem. Geol., 218, 25–40, https://doi.org/10.1016/j.chemgeo.2005.01.024, 2005.
Hanke, A., Cerli, C., Muhr, J., Borken, W., and Kalbitz, K.: Redox control on carbon mineralization and dissolved organic matter along a chronosequence of paddy soils, Eur. J. Soil Sci., 64, 476–487, 2013.
Hattori, C., Ueki, A., Seto, T., and Ueki, K.: Seasonal variations in temperature dependence of methane production in paddy soil, Microbes Environ., 16, 227–233, 2001.
Hayes, J. M.: Practice and principles of isotopic measurements in organic geochemistry, in: Organic Geochemistry of Contemporaneous and Ancient Sediments, edited by: Meinschein, W. G., Great Lakes Section, Society of Economic Paleontologists and Mineralogists, Bloomington, Indiana, 5–31, 1983.
Huang, S., Sun, Y., Yu, X., and Zhang, W.: Interactive effects of temperature and moisture on CO2 and CH4 production in a paddy soil under long-term different fertilization regimes, Biol. Fert. Soils, 52, 285–294, https://doi.org/10.1007/s00374-015-1075-3, 2015.
Jiang, Y., Carrijo, D., Huang, S., Chen, J., Balaine, N., Zhang, W., van Groenigen, K. J., and Linquist, B.: Water management to mitigate the global warming potential of rice systems: A global meta-analysis, Field Crop. Res., 234, 47–54, https://doi.org/10.1016/j.fcr.2019.02.010, 2019.
Kader, M., Sleutel, S., Begum, S. A., Moslehuddin, A. Z. M., and De Neve, S.: Nitrogen mineralization in sub-tropical paddy soils in relation to soil mineralogy, management, pH, carbon, nitrogen and iron contents, Eur. J. Soil Sci., 64, 47–57, 2013.
Kaiser, K. and Guggenberger, G.: The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils, Org. Geochem., 31, 711–725, https://doi.org/10.1016/S0146-6380(00)00046-2, 2000.
Keeling, C. D.: The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas, Geochim. Cosmochim. Ac., 13, 322–334, 1958.
Keiluweit, M., Bougoure, J. J., Nico, P. S., Pett-Ridge, J., Weber, P. K., and Kleber, M.: Mineral protection of soil carbon counteracted by root exudates, Nat. Clim. Change, 5, 588–595, https://doi.org/10.1038/nclimate2580, 2015.
Keiluweit, M., Wanzek, T., Kleber, M., Nico, P., and Fendorf, S.: Anaerobic microsites have an unaccounted role in soil carbon stabilization, Nat. Commun., 8, 1771, https://doi.org/10.1038/s41467-017-01406-6, 2017.
Kögel-Knabner, I., Amelung, W., Cao, Z., Fiedler, S., Frenzel, P., Jahn, R., Kalbitz, K., Kölbl, A., and Schloter, M.: Biogeochemistry of paddy soils, Geoderma, 157, 1–14, 2010.
Kuzyakov, Y., Friedel, J., and Stahr, K.: Review of mechanisms and quantification of priming effects, Soil Biol. Biochem., 32, 1485–1498, 2000.
Lampayan, R. M., Rejesus, R. M., Singleton, G. R., and Bouman, B. A. M.: Adoption and economics of alternate wetting and drying water management for irrigated lowland rice, Field Crop Res., 170, 95–108, https://doi.org/10.1016/j.fcr.2014.10.013, 2015.
Lu, Y., Watanabe, A., and Kimura, M.: Contribution of plant photosynthates to dissolved organic carbon in a flooded rice soil, Biogeochemistry, 71, 1–15, https://doi.org/10.1007/s10533-004-3258-0, 2004.
Mandal, K. G., Misra, A. K., Hati, K. M., Bandyopadhyay, K. K., Ghosh, P. K., and Mohanty, M.: Rice residue-management options and effects on soil properties and crop productivity, J. Food Agr. Environ., 2, 224–231, 2004.
Marschner, B. and Kalbitz, K.: Controls of bioavailability and biodegradability of dissolved organic matter in soils, Geoderma, 113, 211–235, https://doi.org/10.1016/S0016-7061(02)00362-2, 2003.
Nguyen, C.: Rhizodeposition of organic C by plants: mechanisms and controls, Agronomie, 23, 375–396, 2003.
Olk, D., Dancel, M., Moscoso, E., Jimenez, R., and Dayrit, F. M.: Accumulation of lignin residues in organic matter fractions of lowland rice soils: a pyrolysis-GC-MS study, Soil Sci., 167, 590–606, 2002.
Peyron, M., Bertora, C., Pelissetti, S., Said-Pullicino, D., Celi, L., Miniotti, E., Romani, M., and Sacco, D.: Greenhouse gas emissions as affected by different water management practices in temperate rice paddies, Agr. Ecosyst. Environ., 232, 17–28, https://doi.org/10.1016/j.agee.2016.07.021, 2016.
Ponnamperuma, F. N.: Straw as a source of nutrients for wetland rice, in: Organic Matter and Rice, edited by: Banta, S., International Rice Research Institute, Manila, Philippines, 117–136, 1984.
Ponnamperuma, F. N.: The chemistry of submerged soils, Adv. Agron., 24, 29–96, 1972.
Postma, D.: The reactivity of iron oxides in sediments: A kinetic approach, Geochim. Cosmochim. Ac., 57, 5027–5034, https://doi.org/10.1016/S0016-7037(05)80015-8, 1993.
R Core Team: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, available at: https://www.R-project.org/ (last access: 21 August 2021), 2019.
Rochette, P. and Flanagan, L. B.: Quantifying rhizosphere respiration in a corn crop under field conditions, Soil Sci. Soc. Am. J., 61, 466–474, https://doi.org/10.2136/sssaj1997.03615995006100020014x, 1997.
Saeki, K.: Adsorption of Fe2+ and Mn2+ on silica, gibbsite, and humic acids, Soil Sci., 169, 832–840, 2004.
Sahrawat, K. L.: Organic matter accumulation in submerged soils, Adv. Agron., 81, 170–203, 2004.
Said-Pullicino, D., Miniotti, E. F., Sodano, M., Bertora, C., Lerda, C., Chiaradia, E. A., Romani, M., De Maria, S. C., Sacco, D., and Celi, L.: Linking dissolved organic carbon cycling to organic carbon fluxes in rice paddies under different water management practices, Plant Soil, 401, 273–290, 2016.
Schweizer, M., Fear, J., and Cadisch, G.: Isotopic (13C) fractionation during plant residue decomposition and its implications for soil organic matter studies, Rapid Commun. Mass Sp., 13, 1284–1290, 1999.
Sleutel, S., Kader, M. A., Leinweber, P., D'Haene, K., and De Neve, S.: Tillage management alters surface soil organic matter composition: a pyrolysis mass spectroscopy study, Soil Sci. Soc. Am. J., 71, 1620–1628, 2007.
Sodano, M., Lerda, C., Nisticò, R., Martin, M., Magnacca, G., Celi, L., and Said-Pullicino, D.: Dissolved organic carbon retention by coprecipitation during the oxidation of ferrous iron, Geoderma, 307, 19–29, https://doi.org/10.1016/j.geoderma.2017.07.022, 2017.
Sugimoto, A. and Wada, E.: Carbon isotopic composition of bacterial methane in a soil incubation experiment: Contributions of acetate and CO2 H2, Geochim. Cosmochim. Ac., 57, 4015–4027, 1993.
Tokida, T., Cheng, W., Adachi, M., Matsunami, T., Nakamura, H., Okada, M., and Hasegawa, T.: The contribution of entrapped gas bubbles to the soil methane pool and their role in methane emission from rice paddy soil in free-air [CO2] enrichment and soil warming experiments, Plant Soil, 364, 131–143, 2013.
van Bodegom, P. M., van Reeven, J., and Denier van der Gon, H. A. C.: Prediction of reducible soil iron content from iron extraction data, Biogeochemistry, 64, 231–245, https://doi.org/10.1023/A:1024935107543, 2003.
Wagai, R., Mayer, L. M., Kitayama, K., and Shirato, Y.: Association of organic matter with iron and aluminum across a range of soils determined via selective dissolution techniques coupled with dissolved nitrogen analysis, Biogeochemistry, 112, 95–109, https://doi.org/10.1007/s10533-011-9652-5, 2013.
Wang, C., Lai, D. Y. F., Tong, C., Wang, W., Huang, J., and Zeng, C.: Variations in Temperature Sensitivity (Q10) of CH4 Emission from a Subtropical Estuarine Marsh in Southeast China, PloS one, 10, e0125227, https://doi.org/10.1371/journal.pone.0125227, 2015.
Wei, L., Zhu, Z., Liu, S., Xiao, M., Wang, J., Deng, Y., Wu, J., and Ge, T.: Temperature sensitivity (Q10) of stable, primed and easily available organic matter pools during decomposition in paddy soil, Appl. Soil Ecol., 157, 103752, https://doi.org/10.1016/j.apsoil.2020.103752, 2021.
Werth, M. and Kuzyakov, Y.: 13C fractionation at the root–microorganisms–soil interface: a review and outlook for partitioning studies, Soil Biol. Biochem., 42, 1372–1384, 2010.
Yagi, K., Tsuruta, H., Kanda, K. I., and Minami, K.: Effect of water management on methane emission from a Japanese rice paddy field: Automated methane monitoring, Global Biogeochem. Cy., 10, 255–267, 1996.
Ye, R. and Horwath, W. R.: Influence of rice straw on priming of soil C for dissolved organic C and CH4 production, Plant Soil, 417, 231–241, https://doi.org/10.1007/s11104-017-3254-5, 2017.
Ye, R., Doane, T. A., Morris, J., and Horwath, W. R.: The effect of rice straw on the priming of soil organic matter and methane production in peat soils, Soil Biol. Biochem., 81, 98–107, https://doi.org/10.1016/j.soilbio.2014.11.007, 2015.
Ye, R., Doane, T. A., and Horwath, W. R.: Comparison of isotope methods for partitioning methane production and soil C priming effects during anaerobic decomposition of rice residue in soil, Soil Biol. Biochem., 95, 51–59, https://doi.org/10.1016/j.soilbio.2015.12.010, 2016.
Yu, G. and Kuzyakov, Y.: Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling, Earth-Sci. Rev., 214, 103525, https://doi.org/10.1016/j.earscirev.2021.103525, 2021.
Yu, G., Xiao, J., Hu, S., Polizzotto, M. L., Zhao, F., McGrath, S. P., Li, H., Ran, W., and Shen, Q.: Mineral availability as a key regulator of soil carbon storage, Environ. Sci. Technol., 51, 4960–4969, 2017.
Yuan, Q., Pump, J., and Conrad, R.: Partitioning of CH4 and CO2 production originating from rice straw, soil and root organic carbon in rice microcosms, PloS one, 7, e49073, https://doi.org/10.1371/journal.pone.0049073, 2012.
Yuan, Q., Pump, J., and Conrad, R.: Straw application in paddy soil enhances methane production also from other carbon sources, Biogeosciences, 11, 237–246, https://doi.org/10.5194/bg-11-237-2014, 2014.
Zhang, G., Ji, Y., Liu, G., Ma, J., and Xu, H.: Carbon isotope fractionation during CH4 transport in paddy fields, Sci. China Earth Sci., 57, 1664–1670, https://doi.org/10.1007/s11430-014-4879-3, 2014.
Zhang, G., Zhang, W., Yu, H., Ma, J., Xu, H., and Yagi, K.: Fraction of CH4 oxidized in paddy field measured by stable carbon isotopes, Plant Soil, 389, 349–359, https://doi.org/10.1007/s11104-014-2365-5, 2015.
Zhang, X., Li, L., and Pan, G.: Topsoil organic carbon mineralization and CO2 evolution of three paddy soils from South China and the temperature dependence, J. Environ. Sci., 19, 319–326, https://doi.org/10.1016/S1001-0742(07)60052-7, 2007.
Zhou, P., Li, Y., Ren, X., Xiao, H. A., Tong, C., Ge, T., Brookes, P. C., Shen, J., and Wu, J.: Organic carbon mineralization responses to temperature increases in subtropical paddy soils, J. Soil. Sediment., 14, 1–9, https://doi.org/10.1007/s11368-013-0781-4, 2014.
Zhou, Z., Cao, X., Schmidt-Rohr, K., Olk, D. C., Zhuang, S., Zhou, J., Cao, Z., and Mao, J.: Similarities in chemical composition of soil organic matter across a millennia-old paddy soil chronosequence as revealed by advanced solid-state NMR spectroscopy, Biol. Fert. Soils, 50, 571–581, https://doi.org/10.1007/s00374-013-0875-6, 2014.