the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Main drivers of plant diversity patterns of rubber plantations in the Greater Mekong Subregion
Chuan Yang
Zhixiang Wu
Xicai Zhang
The Greater Mekong Subregion (GMS) is one the global biodiversity hotspots. However, the diversity has been seriously threatened due to environmental degradation and deforestation, especially by expansion of rubber plantations. Yet, little is known about the impact of expansion of rubber plantations on regional plant diversity as well as the drivers for plant diversity of rubber plantations in this region. In this study, we analyzed plant diversity patterns of rubber plantations in the GMS based on a ground survey of a large number of samples. We found that diversity varied across countries due to varying agricultural intensities. Laos had the highest diversity, followed China, Myanmar, and Cambodia. Plant species richness of Laos was about 1.5 times that of Vietnam. We uncovered latitudinal gradients in plant diversity across these artificial forests of rubber plantations and these gradients caused by environmental variables such as temperature. Results of redundancy analysis (RDA), multiple regression, and random forest demonstrated that latitude and temperature were the two most important drivers for the composition and diversity of rubber plantations in the GMS. Meanwhile, we also found that higher dominance of some exotic species (such as Chromolaena odorata and Mimosa pudica) was associated with a loss of plant diversity within rubber plantations; however, not all exotic plants cause the loss of plant diversity in rubber plantations. In conclusion, not only environmental factors (temperature), but also exotic species were the main factors affecting plant diversity of these artificial stands. Much more effort should be made to balance agricultural production with conservation goals in this region, particularly to minimize the diversity loss in Vietnam and Cambodia.
- Article
(4853 KB) - Full-text XML
-
Supplement
(765 KB) - BibTeX
- EndNote
Many tropical regions contain hotspots of biodiversity (Myers et al., 2000), especially for the Great Mekong Subregion (GMS), which is threatened by agriculture (Delzeit et al., 2017; Egli et al., 2018; Shackelford et al., 2014; Kehoe et al., 2017). Much of the land has recently been converted from forest to agriculture (Li et al., 2007), and rubber plantations have quickly expanded throughout the region (Ziegler et al., 2009; Li et al., 2015; Ahrends et al., 2015) due to a surge in the global demand for natural rubber, driven largely by the growth of tire and automobile industries. For example, 23.5 % of Cambodia's forest cover was destroyed between 2001 and 2015 to make way for crops such as rubber (Fig. S1h in the Supplement) and palm oil (Grogan et al., 2019). In southwest China, nearly 10 % of the total area of nature reserves had been converted to rubber monoculture by 2010 (Chen et al., 2016). At present, the GMS is a globally important rubber-planting region (Xiao et al., 2021).
Agricultural land uses can exacerbate many infectious diseases in Southeast Asia (Shah et al., 2019) and reduce biodiversity (Xu, 2011; Warren-Thomas et al., 2018; Fitzherbert et al., 2018; Zabel et al., 2019; Singh et al., 2019). Previous studies have shown that rubber cultivation not only affects plant diversity (Hu et al., 2013), but also affects the soil fauna (Chaudhuri et al., 2013; Xiao et al., 2014), bird diversity (Aratrakorn et al., 2006; Li et al., 2013), and bat diversity (Phommexay et al., 2011). There is also a large body of literature on the effects of forest conversion from tropical forest to rubber plantations on soil microbial composition and diversity (Tripathi et al., 2012; Schneider et al., 2015; Kerfahi et al., 2016, Lan et al., 2017a, b, c, 2020a, b, 2021; Cai et al., 2018). However, the impact of expansion of rubber plantations on regional plant diversity as well as the drivers for plant diversity of rubber plantations in the GMS are still unclear.
Latitudinal gradients in species diversity are well known (Mccoy and Connor, 1980), which holds that there is a fairly regular increase in the number of species of some higher taxon from the poles to the Equator. It has been suggested that the latitudinal diversity gradient could be caused by environmental variables such as temperature and precipitation. Previous studies have also demonstrated that temperature (Nottingham et al., 2018) and soil nutrients (Soons et al., 2017) as well as water resource utilization efficiency (Han et al., 2020) were the dominant drivers of plant diversity. However, whether latitudinal gradients in species diversity exist in rubber plantations, which are greatly affected by management measures, is still unknown.
In addition, rubber plantations have lower biodiversity than natural forests (Chaudhary et al., 2016). Generally speaking, species-rich zones showed a higher proportion of alien plant species in their flora (Stadler et al., 2000); thus, exotic plants are ubiquitous in rubber plantations which indicate that. Although the fact that exotic invasive species significantly decrease plant diversity (Xu et al., 2022) is universally known, we still do not know whether exotic species are the main driver for the sharp decline in plant diversity in rubber plantations. Thus, we hypothesize that (1) latitudinal gradients in plant diversity would not exist in rubber plantations due to the strong intensity of management and (2) exotic plants will result in a sharp decline in the plant diversity of rubber plantations because areas of low plant species richness may be invaded more easily than areas of high plant species richness (Stohlgren et al., 1999) and exotic species may results in loss of plant diversity (Xu et al., 2022). To test these hypotheses, we surveyed a large number of plots on rubber plantations in the GMS to investigate plant diversity and analyzed the associated drivers. Our study provides an empirical case for understanding the effect of rubber plantations on plant diversity in the Greater Mekong region and the restoration and protection of biodiversity in this region.
2.1 Study area
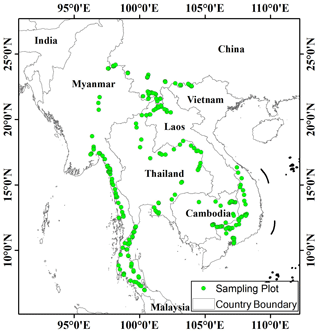
The Mekong River basin has a total length of 4880 km and a drainage area of 795 000 km2, with 326 million people living in the basin. The GMS encompasses a variety of climate types and geographical characteristics and is rich in water and biological resources (Wu et al., 2020). Rubber plantations are one of the most widespread vegetation types in the region and are distributed throughout the south of Yunnan province, almost all states of Thailand and Laos, the southern half of Vietnam and Myanmar, and the eastern half of Cambodia.
2.2 Sampling methods
Before the field investigation, we first determined the investigation route according to the distribution of rubber plantations in this regions. Then, plots were randomly selected approximately equidistant from each other (every 10–20 km according to the actual situation) along the investigation route (Yaseen, 2013). We did not deliberately select plots according types of rubber plantations, and thus these plots were independent from each other. Consequently, a total of 240 plots, each with an area of 100 m2 (10 m × 10 m), were selected in the GMS, with 32 plots in Vietnam, 24 in Cambodia, 15 in Laos, 73 in Thailand, 47 in Myanmar, and 49 in China (Fig. 1).
We started the investigation only after the guide (local people) asked the farmer's consent. Plot measurements, such as longitude, latitude, elevation, slope degree, slope aspect, rubber tree height, and canopy density, were recorded in detail (Table S1 in the Supplement). Annual and perennial plant species, shrubs, trees, and lianas as well as theirs seedlings were recorded. We do not investigate bryophytes, but ferns were investigated. Species information, such as species name, height and coverage, and life form (non-woody, shrub, liana, or tree) (Lan et al., 2014), from each plot in the rubber plantations was also recorded. We visually assigned a cover value to each species in each quadrant of the plot, using an ordinal cover class scale with class limits 0.5 %, 1 %, 2 %, 5 %, 10 %, 15 %, 20 %, and thereafter every 10 % up to 100 %. The cover values for each species in the plot were then averaged across the four quadrants (Sabatini et al., 2016). Climate data, including annual average temperature and annual average precipitation, were obtained from WorldClim 2 (Fick and Hijmans, 2017) (http://worldclim.org, last access: 8 March 2021) based on the geographic coordinates of each sample site.
2.3 Data analysis
Relative height (RH), relative dominance (RD, using coverage), and relative frequency (RF) were calculated for each species to estimate the importance value (IV). Importance value, as defined here, differs from previous studies (e.g., Curtis and Mcintosh, 1950, 1951; Greig-Smith, 1983; Linares-Palomino and Alvarez, 2005) because most understory species are herbs, which make the precise measure of abundance difficult. We define the importance value as
the relative frequency as
the relative height as
and the relative dominance as
where Fj is the number of plots containing species j, Dj is the coverage of species j, and Hj is the height of species j. For the local community, there were no frequency data; therefore, the importance value is defined as
Species richness and the Shannon index were used to measure the α diversity of each plot. It should be noted that the importance values of each species were used to calculate the Shannon diversity (i.e., replace “abundance” or “number of individuals” with “important value”). Principal coordinate analysis (PCoA) based on Bray–Curtis distance of species IVs (importance values) was performed to compare plant species composition across countries using the R package “amplicon”. Analysis of similarity (ANOSIM) was used to test for differences in diversity indices among countries. Multiple linear regression was used to find whether there were positive or negative correlations between diversity (richness) and environmental variables including latitude, longitude, elevation, rainfall, temperature, slope degree, tree age, tree height, and canopy density. A machine learning algorithm, random forest (Breiman, 2001), was used to model α diversity (richness) and rank the feature importance of environmental factors with 999 iterations. In order to understand how plant compositions are structured by environmental factors, a redundancy analysis (RDA) for the importance value of species was carried out using the vegan package (version 2.5–7) (Oksanen et al., 2020) in the R (version 4.04) environment (R Core Team, 2021). Statistical significance was assessed using Monte Carlo tests with 999 permutations.
3.1 Plant composition of rubber plantations
A total of 949 plant species, representing 550 genera and 153 families, were recorded across rubber plantations of the six countries (Tables 1 and S2 in the Supplement). Our results also showed that 445 (46.89 %) were herbs, with a large number of Compositae (Table 1). Plant communities of rubber plantations tended to be dominated by Fabaceae, Euphorbiaceae, Poaceae, Rubiaceae, and Compositae (Table S3 in the Supplement). The five most common species observed were Cyrtococcum patens, Chromolaena odorata, Asystasia chelonoides, Axonopus compressus, and M. pudica (Table S4 in the Supplement). From 237 plots containing exotic plant species, most of them were from tropical America. A total of 121 (12.75%) species were identified as exotic (belonging to 45 families and 91 genera). The five most common exotic species were C. odorata, M. pudica, Axonopus compressus, Ageratum conyzoides, and Borreria latifolia. C. odorata and M. pudica were recorded in almost every plot (Fig. 2).
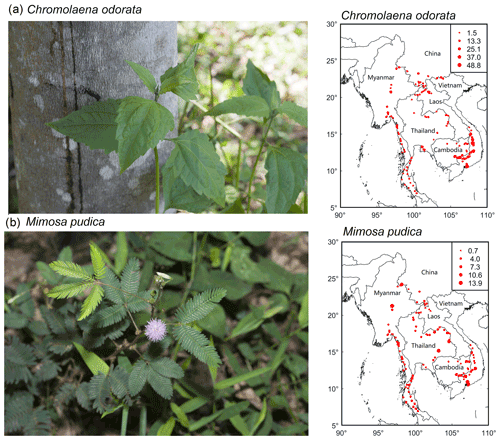
Figure 2Distribution maps of two common exotic species (a: Chromolaena odorata; b: Mimosa pudica) of rubber plantations in the GMS (circle size is proportional to importance value).
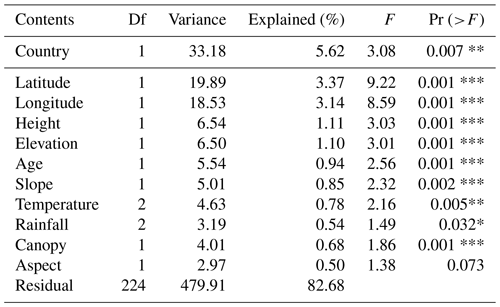
PCoA and ANOSIM were used to reveal the difference in plant compositions among these six countries. And the results showed significant differences (R=0.383, P=0.001) in species composition among these countries (Fig. 3a–b). Meanwhile, the first and second axes of RDA explained 5.95 % and 3.11 % of variation of species compositions, respectively (Fig. 4a). All environmental factors explained 18.65 % of the total variation (Fig. 4b). Countries, latitude, longitude, canopy height, and elevation all significantly impacted plant compositions of rubber plantations in the GMS and explained 5.62 %, 3.37 %, 3.14 %, 1.11 %, and 1.10 % of the total variations (Table 2).
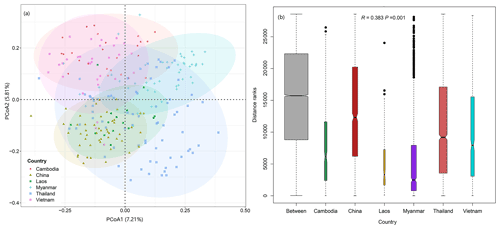
Figure 3Significant difference in plant community compositions of rubber plantations among countries in the GMS. (a) Principal coordinate analysis (PCoA) based on Bray–Curtis distance; (b) analysis of similarity among countries.
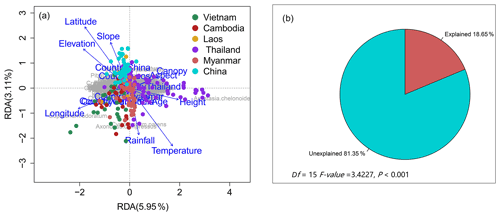
Figure 4Redundancy analysis of plant community compositions of rubber plantations in the GMS (a: RDA ordination; b: percentage of explained and unexplained by RDA results).
3.2 Plant diversity of rubber plantations
Species richness of rubber plantations in Laos was the highest among the six countries, followed by China and Myanmar, while the richness of Thailand, Cambodia, and Vietnam was relatively lower (Fig. 5a). The same was true for Shannon diversity (Fig. 5b).
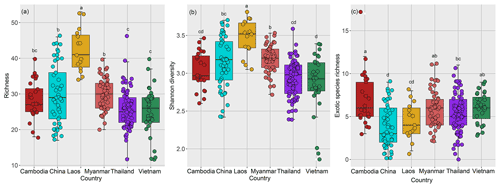
Figure 5Plant species diversity of rubber plantations across countries in the GMS (a: species richness; b: Shannon diversity; c: exotic species richness).
The results of multiple linear regression (R2=0.3227, P<0.001) showed that temperature (P<0.001), tree height (P<0.001), latitude (P<0.01), and slope degree (P<0.001) were positively correlated with the species richness (Fig. 6a). Among these factors, temperature (with the highest intercept 1.3) is the most important factor affecting plant diversity. Random forest results showed that high mean squared errors of latitude, temperature, and countries were the top three features affecting plant diversity of rubber plantations (Fig. 6b).
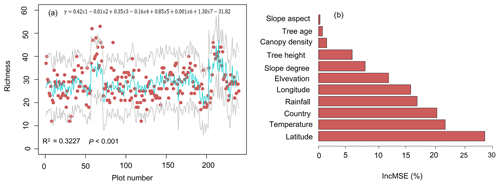
Figure 6Factors affecting plant diversity of rubber plantations. (a) Predicting species richness by using multiple linear regression (the red point was the observed richness, the green solid line was the estimated richness, and the grey solid line was the 95 % confidence interval; y: richness; x1: latitude; x2: elevation; x3: slope; x4: age; x5: height; x6: rainfall; x7: temperature). (b) Predictions of the importance of environmental variables based on random forest. IncMSE stands for increase in mean squared error.
3.3 Effects of exotic species on plant diversity of rubber plantations
The exotic species richness of rubber plantations was relatively higher in Cambodia, Vietnam, and Myanmar compared to China, Laos, and Thailand (Fig. 3c). In order to clarify whether exotic species can reduce plant diversity, we analyzed the relationship between the dominance of exotic species and the species richness in the plot. In view of the fact that C. odorata and M. pudica are the two most common exotic species in rubber plantations (Fig. 7a), the two species were selected for analysis. The importance values of exotic species C. odorata (Fig. S2a in the Supplement) and M. pudica (Fig. S2b) were negatively correlated with species richness, suggesting that exotic species with high dominance will reduce rubber plantation diversity. However, exotic species richness was positively correlated with species richness (Fig. 7c). Richness of communities where C. odorata (M. pudica) was present was not lower than those where it was absent (Fig. 7b). In sum, diversity of the community was reduced only when the dominance of exotic species was high.
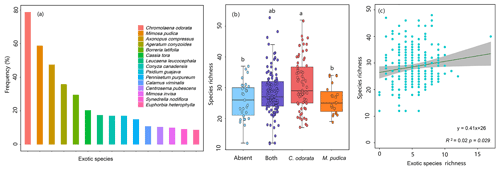
Figure 7Effects of exotic species on plant diversity of rubber plantations in the GMS (a: frequency of the most common exotic species; b: richness comparison of different communities (sky-blue box plot: plots without C. odorata and M. pudica; purple box plot: plots with both C. odorata and M. pudica; red box plot: plots only with C. odorata; orange box plot: plots only with M. pudica); c: relationship between exotic species richness of given plot and species richness of given plot).
4.1 Main drivers for plant composition and diversity of rubber plantations
Rubber plantations constitute one of the most important agro-ecosystems of tropical regions and play an important role in their carbon budgets (Chen et al., 2020). For plant composition, latitude ranks second (Table 2) in terms of its impact on plant composition, indicating that latitude is an important driver of plant composition of rubber plantations. For plant diversity, both multiple linear regression and random forest showed that temperature was the most important factor for plant diversity of rubber plantations. Our results are consistent with a previous study which revealed that temperature is the main driver for plant diversity (Nottingham et al., 2018).
We were surprised to find that understory plant diversity of artificial rubber plantations increased with latitude, similar to that of the global diversity patterns (Rohde, 1992; Perrigo et al., 2013) that latitudinal gradients are known for in which maximum diversity does not occur near the Equator (Mccoy and Connor, 1980). One study suggest that the diversity of plant communities was directly affected by latitude (Li et al., 2020). Our results showed that elevation was not as important as other factors, which is different from a previous study in which elevation significantly affects plant species diversity (Li et al., 2020).
Plant diversity of north Laos and south China was relatively higher than other countries. This observation may be due to the large variation in elevation in these areas, which translates into greater environmental heterogeneity. In addition, greater slope may increase environmental heterogeneity and expand niche space (Morrison-Whittle and Goddard, 2015). In any case, temperature could largely contribute to explaining the latitudinal diversity gradient patterns of rubber plantations.
4.2 Not all exotic plants cause the loss of plant diversity in rubber plantations
Rubber plantation expansion and intensification has occurred in many regions that are key for biodiversity conservation. Monoculture plantations have been promoted to restore the world's forested areas but have done little to slow the loss of biodiversity (Zhang et al., 2021). It has been hypothesized that exotic species might more easily invade areas of low species diversity than areas of high species diversity (Stohlgren et al., 1999). A recent study shows exotic plants account for ∼17 % of the total importance value for natural ecosystems and ∼35 % of the total importance value for human-modified ecosystems (Chandrasekaran et al., 2001). Here, in rubber plantations, exotic plants made up roughly 12% of the total recorded species and 22.80 % of the coverage. C. odorata is a noxious perennial weed in many parts of the world (Kushwaha et al., 1981), and it is unsurprising that it was recorded in almost all plantation plots in our study. These indicated that invasion by exotic species has either already occurred or is inevitable in many systems (Stohlgren et al., 1999). M. pudica, the “sensitive plant”, is a worldwide, pan-tropical invasive species (Melkonian et al., 2014). M. pudica, as many tropical grasses and herbs, is tolerant of low pH (Humphreys, 1997; Paudel, 2018), which explains its ubiquity in acidic rubber plantation soil.
More importantly, our study demonstrated that the diversity of the community was reduced only when the importance value of exotic species is large enough; not all exotic species cause the loss of plant diversity in rubber plantations, which follows the theory that many species can coexist in spatially heterogeneous areas as long as nutrients and light are not limiting (Huston and DeAngelis, 1994). Our results also were consistent with the idea that inhibition of plant diversity by exotic species invasion gradually weakened with increased precipitation (Xu et al., 2022) due to higher precipitation in the GMS. In addition, management of rubber plantations reduces the dominance of exotic species to a great extent, thus providing space for the survival of other plants.
4.3 Plant composition and diversity is largely affected by management
Forests that are intensively managed for production purposes generally have lower biodiversity than natural forests (Chaudhary et al., 2016), and this is especially true for rubber plantations (He and Martin, 2016). In artificial forests such as rubber plantations, there is no doubt that management measures and agricultural intensity are two of the most important factors affecting plant diversity. The application of herbicides and sprout control causes low diversity of understory plants; this is especially true of rubber plantations of Vietnam (Fig. S1f). Also, it is not easy for farmers to clear understory plants on the steep slopes of rubber plantations at high elevation; thus, high slope degree indirectly results in low agricultural intensity and high diversity. RDA analysis only explained 18.65 % of the variation of community compositions, and multiple linear regression only explained 32.27 % of the variation of plant diversity. Most of the unexplained variations are caused by management intensity and measures. In sum, plant compositions and diversity are largely affected by the measures and intensity of management.
In poor areas, we cannot just talk about ecological goals without first understanding local cultures and economies. Well-managed forests can alleviate poverty in rural areas, as outlined by the United Nations Sustainable Development Goals (Lewis et al., 2019). A previous study conducted in India demonstrated that a no-weeding practice in mature rubber plantations did not affect rubber yield (Abraham and Joseph, 2016). A similar study conducted in China also showed that natural management strategies can improve biodiversity without reducing latex production (Lan et al., 2017d). There is strong evidence that adopting more natural management strategies improves plant diversity without reducing latex production (Lan et al., 2017d). More innovative management measures, such as cease of weeding and herbicide application (He and Martin, 2015), must be implemented to improve the biodiversity of rubber plantations, so as to promote the biodiversity of the region.
We provide a large regional study on the plant diversity of rubber plantations in a global biodiversity hotspot. Plant diversity followed global trends with respect to latitude and temperature. Exotic species were very common in rubber plantations, especially where agricultural intensity was strong. However, not all exotic species directly drive the loss of biodiversity. Only higher dominance of some exotic species was associated with a loss of plant diversity within rubber plantations. We must make greater efforts to balance agricultural production with conservation goals in this region, particularly in Vietnam and Cambodia, to minimize the loss of biodiversity.
Data generated in this study are available from the corresponding authors upon reasonable request.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-19-1995-2022-supplement.
GL contributed to the conceptualization, methodology, writing, review, and editing. BC contributed to the methodology, review, and editing. CY, RS, BC, ZW, and XZ performed the investigation.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank the help of the Rubber Authority of Thailand, Perennial Crop Research Institute of Myanmar, the Rubber Research Institute of Cambodia, Vietnam National University of Agriculture, and the Yunnan Rubber Group. We would like to thank Fangliang He at the University of Alberta and Ian Gilman at Yale University for their assistance with English language and grammatical editing.
This study was supported by the National Natural Science Foundation of China (grant no. 42071418), the Lancang–Mekong international cooperation project of the Ministry of Foreign Affairs (grant no. 081720203994192003), and the Earmarked Fund for China Agriculture Research System (grant no. CARS-33-ZP3).
This paper was edited by Ben Bond-Lamberty and reviewed by Yuwu Li and one anonymous referee.
Abraham, J. and Joseph, P.: A new weed management approach to improve soil health in a tropical plantation crop, rubber (Hevea brasiliensis), Exp. Agr., 52, 36–50, 2016.
Ahrends, A., Hollingsworth, P. M., Ziegler, A. D., Fox, J. M., Chen, H., Su, Y., Xu, and J. X.: Current trends of rubber plantation expansion may threaten biodiversity and livelihoods, Global Environ. Chang., 34, 48–58, 2015.
Aratrakorn, S., Thunhikorn, S., and Donald, P. F.: Changes in bird communities following conversion of lowland forest to oil palm and rubber plantations in southern Thailand, Bird Conserv. Int., 16, 71–82, 2006.
Breiman, L.: Random Forests, Mach. Learn., 45, 5–32, 2001.
Cai, Z. Q., Zhang, Y. H., Yang, C., and Wang, S.: Land-use type strongly shapes community composition, but not always diversity of soil microbes in tropical China, Catena, 165, 369–380, 2018.
Chandrasekaran, S., Sundarapandian, S. M., Chandrasekar, P., and Swamy, P. S.: Exotic plant invasions in disturbed and man-modified forest ecosystems in the Western Ghats of Tamil Nadu. Tropical Forestry Research: Challenges in the New Millennium, in: Proceedings of the International Symposium, Peechi, India, 2–4 August 2000, 32–39, 2001.
Chaudhary, A., Burivalova, Z., Koh, L. P., and Hellweg, S.: Impact of forest management on species richness: Global meta-analysis and economic trade-offs, Sci. Rep., 6, 23954, https://doi.org/10.1038/srep23954, 2016.
Chaudhuri, P. S., Bhattacharjee, S., Dey, A., Chattopadhyay, S., and Bhattacharya, D.: Impact of age of rubber (Hevea brasiliensis) plantation on earthworm communities of West Tripura (India), J. Environ. Biol., 34, 59–65, 2013.
Chen, B. Q., Yun, T., Ma, J., Kou, W. L., Li, H. L., Yang, C., Xiao, X. M., Zhang, X., Sun, R., Xie, G. S., and Wu, Z. X.: High-Precision stand age data facilitate the estimation of rubber plantation biomass: A case study of Hainan Island, China, Remote Sens., 12, 3853, https://doi.org/10.3390/rs12233853, 2020.
Chen, H., Yi, Z. F., Schmidt-Vogt, D., Ahrends, A., Beckschäfer, P., Kleinn, C., Ranjitkar, S., and Xu, J. C.: Pushing the Limits: The Pattern and Dynamics of Rubber Monoculture Expansion in Xishuangbanna, SW China, PLoS ONE, 11, e0150062, https://doi.org/10.1371/journal.pone.0150062, 2016.
Curtis, J. T. and Mclntosh, R. P.: The interactions of certain analytic and synthetic phytosociological characters, Ecology, 31, 435–455, 1950.
Curtis, J. T. and Mclntosh, R. P.: An upland forest continuum in the prairie-forest border region of Wisconsin, Ecology, 32, 476–496, 1951.
Delzeit, R., Zabel, F., Meyer, C., and Václavík, T.: Addressing future trade-offs between biodiversity and cropland expansion to improve food security, Reg. Environ. Change, 17, 1429–1441, 2017.
Egli, L., Meyer, C., Scherber, C., Kreft, H., and Tscharntke, T.: Winners and losers of national and global efforts to reconcile agricultural intensification and biodiversity conservation, Global Change Biol., 24, 2212–2228, 2018.
Fick, S. E. and Hijmans, R. J.: WorldClim 2: new 1 m spatial resolution climate surfaces for global land areas, Int. J. Climatol., 37, 4302–4315, https://doi.org/10.1002/joc.5086, 2017.
Fitzherbert, E. B., Struebig, M. J., Morel, A., Danielsen, F., Carsten, A., Brühl, Donald, P. F., and Phalan, B.: How will oil palm expansion affect biodiversity?, Trends Ecol. Evol., 23, 538–545, 2008.
Greig-Smith, P. W.: Use of perches as vantage points during foraging by male and female Stonechats Saxicola torquata, Behaviour, 86, 215–236, 1983.
Grogan, K., Pflugmacher, D., Hostert, P., Mertz, O., and Fensholt, R.: Unravelling the link between global rubber price and tropical deforestation in Cambodia, Nat. Plants, 5, 47–53, 2019.
Han, Z. Q., Liu, T., Wang, T., Liu, H. F., and Li, B. L.: Quantification of water resource utilization efficiency as the main driver of plant diversity in the water-limited ecosystems, Ecol. Model., 429, 108974, https://doi.org/10.1016/j.ecolmodel.2020.108974, 2020.
He, P. and Martin, K.: Effects of rubber cultivation on biodiversity in the Mekong Region, CAB Reviews, 10, 44, https://doi.org/10.1079/PAVSNNR201510044, 2016.
Humphreys, L. R.: The Evolving Science of Grassland Improvemen, Cambridge University Press, Cambridge, UK, ISBN: 9780511525803, https://doi.org/10.1017/CBO9780511525803, 1997.
Huston, M. A. and DeAngelis. D. L.: Competition and coexistence: the effects of resource transport and supply rates, Am. Nat., 144, 954–977, https://doi.org/10.2307/2463137, 1994.
Hu, Y. H., Sheng D. Y., Xiang Y. Z., Yang Z. J., Xu D. P., Zhang N. N., and Shi L. L.: The Environment, Not Space, Dominantly Structures the Landscape Patterns of the Richness and Composition of the Tropical Understory Vegetation, PLoS ONE, 8, e81308, https://doi.org/10.1371/journal.pone.0081308, 2013.
Kehoe, L., Romero-Muoz, A., Polaina, E., Estes, L., and Kuemmerle, T.: Biodiversity at risk under future cropland expansion and intensification, Nat. Ecol. Evol., 1, 1129–1135, 2017.
Kerfahi, D. B. M. T., Dong, K., Go, R., and Adams, J. M.: Rainforest conversion to rubber plantation may not result in lower soil diversity of bacteria fungi and nematodes, Microb. Ecol., 72, 359–371, 2016.
Kushwaha, S. P. S. and Tripathi, P. S. R. S.: Population dynamics of Chromolaena odorata in successional environments following slash and burn agriculture, J. Appl. Ecol., 18, 529–535, 1981.
Lan, G. Y., Wu, Z. X., and Xie, G. X.: Characteristics of plant species diversity of rubber plantation in Hainan Island, Biodivers. Sci., 22, 658–666, 2014.
Lan, G. Y., Li, Y. W., Wu, Z. X., and Xie, G. S.: Impact of tropical forest conversion on soil bacterial diversity in tropical region of China, Eur. J. Soil Biol., 83, 91–97, 2017a.
Lan, G. Y., Li, Y. W., Wu, Z. X., and Xie, G. S.: Soil bacterial diversity impacted by conversion of secondary forest to rubber or eucalyptus plantations-A case study of Hainan Island, South China, Forest Sci., 63, 87–93, 2017b.
Lan, G. Y., Li, Y. W., Jatoi, M. T., Tan, Z. H., Wu, Z. X., and Xie, G. S.: Change in soil microbial community compositions and diversity following the conversion of tropical forest to rubber plantations in Xishuangbanan, Southwest China, Trop. Conserv. Sci., 10, 1–14, 2017c.
Lan, G. Y., Wu, Z. X., Chen, B. Q., and Xie, G. S.: Species diversity in a naturally managed rubber plantation in Hainan Island, South China, Trop. Conserv. Sci., 10, 1–7, 2017d.
Lan, G. Y., Wu, Z. X., Sun, R., Yang, C., Chen, B. Q., and Zhang, X.: Tropical rainforest conversion into rubber plantations results in changes in soil fungal composition, but underling mechanisms of community assembly remain unchanged, Geoderma, 375, 114505, https://doi.org/10.1016/j.geoderma.2020.114505, 2020a.
Lan, G. Y., Wu, Z. X., Li, Y. W., and Chen, B. Q.: The drivers of soil bacterial communities in rubber plantation at local and geographic scales, Arch. Agron. Soil Sci., 66, 358–369, 2020b.
Lan, G. Y., Wu, Z. X., Sun, R., Yang, C., Chen, B. Q., and Zhang, X. C.: Forest conversion changed the structure and functional process of tropical forest soil microbiome, Land Degrad. Dev., 32, 613–627, 2021.
Lewis, S. L., Wheeler, C. E., Mitchard, E. T. A., and Koch, A.: Regenerate natural forests to store carbon, Nature, 568, 25–28, 2019.
Li, H. M., Aide, T., Ma, Y. X., Liu, W. J., and Cao, M.: Demand for rubber is causing the loss of high diversity rain forest in SW China, Biodivers. Conserv., 16,1731–1745, 2007.
Linares-Palomino, R. and Alvarez, S. I. P.: Tree community patterns in seasonally dry tropical forests in the Cerros de Amotape Cordillera, Tumbes, Peru, Forest Ecol. Manag., 209, 261–272, 2005.
Li, S., Zou, F., Zhang, Q., and Sheldon, F. H.: Species richness and guild composition in rubber plantations compared to secondary forest on Hainan Island, China, Agroforestry Syst., 87, 1117–28, 2013.
Li, T., Xiong, Q., Luo, P., Zhang, Y., Gu, X., and Lin, B.: Direct and indirect effects of environmental factors, spatial constraints, and functional traits on shaping the plant diversity of montane forests, Ecol. Evol., 10, 557–568, https://doi.org/10.1002/ece3.5931, 2020.
Li, Y. W., Xia, Y. J., Lei, Y. B., Deng, Y., Chen, H., Sha, L. Q., Cao, M., and Deng, X. B.: Estimating changes in soil organic carbon storage due to land use changes using a modified calculation method, Iforest, 8, 45–52, 2015.
Mccoy, E. D. and Connor, E. F.: Latitudinal gradients in the species diversity of north American mammals, Evolution, 34, 193–203, 1980.
Melkonian, R., Moulin, L., Béna, G., Tisseyre, P., Chaintreuil, C., Heulin, K., Rezkallah, N., Klonowska, A., Gonzalez, S., Simon, M., Chen, W. M., James, E. K., and Laguerre, G.: The geographical patterns of symbiont diversity in the invasive legume mimosa pudicacan be explained by the competitiveness of its symbionts and by the host genotype, Environ. Microbiol., 16, 2099–2111, 2014.
Morrison-Whittle, P. and Goddard, M. R.: Quantifying the relative roles of selective and neutral processes in defining eukaryotic microbial communities, ISME J., 9, 2003–2011, 2015
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A. B. D., and Kent, J.: Biodiversity hotspots for conservation priorities, Nature, 403, 853–858, 2000.
Nottingham, A., Fierer, N., Turner, B. L., Whitaker, J., Ostle, N. J., Mcnamara, N. P., Bardgett, R. D., Leff, J. W., Salinas, N., Silman, M. R., Kruuk, L. E. B., and Meir, P.: Temperature drives plant and soil microbial diversity patterns across an elevation gradient from the Andes to the Amazon, Ecology, 99, 2455–2466, 2018.
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P. R., O'Hara, R. B., Simpson, G. L., Solymos, P., Stevens, M. H. H., Szoecs, E., and Wagner, H.: vegan: Community Ecology, R package, version 2.5–7, https://cran.r-project.org/web/packages/vegan/ (last access: 4 April 2022), 2020.
Paudel, N.: Seasonal variation in phenophases of Mimosa pudica (fabaceae) in grazed pasture of Barandabhar corridor forest Chitwan, Nepal, Curr. Trends Biomed. Eng. Biosci., 11, 101–102, https://juniperpublishers.com/ctbeb/pdf/CTBEB.MS.ID.555825.pdf (last access: 4 April 2022), 2018.
Perrigo, A. L., Baldauf, S. L., and Romeralo, M.: Diversity of dictyostelid social amoebae in high latitude habitats of Northern Sweden, Fungal Divers., 58, 185–198, 2013.
Phommexay, P., Satasook, C., Bates, P., Pearch, M., and Bumrungsri, S.: The impact of rubber plantations on the diversity and activity of understory insectivorous bats in southern Thailand, Biodivers. Conserv., 20, 1441–56, 2011.
R Core Team: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/, last access: 1 March 2021.
Rohde, K.: Latitudinal gradients in species diversity: The search for the primary cause, Oikos, 65, 514–527, 1992.
Sabatini, F. M., Burrascano, S., Azzella, M. M., Barbati, A., De Paulis, S., Di Santo, D. Facioni, L., Giuliarelli, D., Lombardi, F., Maggi, O., Mattioli, W., Parisi, F., Persiani, A., Ravera, S., and Blasi, C.: Herb-layer diversity and stand structural complexity are weak predictors of biodiversity in Fagus sylvatica forests, Ecol. Indic., 69, 126–137, 2016.
Schneider, D., Engelhaupt, M., Allen, K., Kurniawan, S., Krashevska, V., Heinemann, M., and Scheu, S.: Impact of lowland rainforest transformation on diversity and composition of soil prokaryotic communities in Sumatra Indonesia, Front. Microbiol., 6, 296, https://doi.org/10.3389/fmicb.2015.01339, 2015.
Shackelford, G. E., Steward, P. R., German, R. N., Sait, S. M., and Benton, T. G.: Conservation planning in agricultural landscapes: hotspots of conflict between agriculture and nature, Divers. Distrib., 21, 357–367, 2014.
Shah, H. A., Huxley, P., Elmes, J., and Murray, K. A.: Agricultural land-uses consistently exacerbate infectious disease risks in Southeast Asia, Nat. Commun., 10, 4299, https://doi.org/10.1038/s41467-019-12333-z, 2019.
Singh, D., Slik, J. W. F., Jeon, Y. S., Tomlinson, K. W., Yang, X. D., Wang, J., Kerfahi, D., Porazinska, D. L., and Adams, J. M.: Tropical forest conversion to rubber plantation affects soil micro and mesofaunal community and diversity, Sci. Rep., 9, 5893, https://doi.org/10.1038/s41598-019-42333-4, 2019.
Soons, M. B., Hefting, M. M., Dorland, E., Lamers, L. P. M., Versteeg, C., and Bobbink, R.: Nitrogen effects on plant species richness in herbaceous communities are more widespread and stronger than those of phosphorus, Biol. Conserv., 212, 390–397, 2017.
Stadler, J., Trefflich, A., Klotz, S., and Brandl, R.: Exotic plant species invade diversity hot spots: the alien flora of northwestern Kenya, Ecography, 23, 169–176, 2000.
Stohlgren, T. J. , Binkley, D., Chong, G. W., Kalkhan, M. A., Schell, L. D., Bull, K. A., Otsuki, Y., Newman, G., Bashkin, M., and Son, Y.: Exotic plant species invade hot spots of native plant diversity, Ecol. Monogr., 69, 25–46, 1999.
Tripathi, B. M., Kim, M., Singh, D., Lee-Cruz, L., Lai-Hoe, A., Ainuddin, A. N., and Adams, J. M.: Tropical soil bacterial communities in Malaysia: pH dominates in the equatorial tropics too, Microb. Ecol., 64, 474–484, 2012.
Warren-Thomas, E. M. , Edwards, D. P. , Bebber, D. P. , Chhang, P., Diment, A. N., Evans, T. D., Lambrick, F. H., Maxwell, J. F., Nut, M., O'Kelly, H. J., Theilade, I., and Dolman, P. M.: Protecting tropical forests from the rapid expansion of rubber using carbon payments, Nat. Commun., 9, 911, https://doi.org/10.1038/s41467-018-03287-9, 2018.
Wu, J., Wang, X., Zhong, B., Yang, A., and Liu, Q.: Ecological environment assessment for Greater Mekong Subregion based on pressure-state-response framework by remote sensing, Ecol. Indic., 117, 106521, https://doi.org/10.1016/j.ecolind.2020.106521, 2020.
Xiao, C. W., Li, P., Feng, Z. M., Yang, Y. Z., You, Z. L., Yu, M., and Zhang, X. Z.: Latest 30-m map of mature rubber plantations in Mainland Southeast Asia and Yunnan province of China: Spatial patterns and geographical characteristics, Prog. Phys. Geog., 45, 736–756, https://doi.org/10.1177/0309133320983746, 2021.
Xiao, H. F., Tian, Y. H., Zhou, H. P., Ai, X. S., Yang, X. D., and Schaefer, D. A.: Intensive rubber cultivation degrades soil nematode communities in Xishuangbanna, southwest China, Soil Biol. Biochem., 76, 161–169, 2014.
Xu, H. W., Liu, Q., Wang, S. Y., Yang, G. S., and Xue, S.: A global meta-analysis of the impacts of exotic plant species invasion on plant diversity and soil properties, Sci. Total Environ., 810, 152286, https://doi.org/10.1016/j.scitotenv.2021.152286, 2022.
Xu, J. C.: China's new forests aren't as green as they seem, Nature, 477, 371, https://doi.org/10.1038/477371a, 2011.
Yaseen, S. D.: Prevalence of economically important fungal diseases at different phenological stages of peanut (Arachis hypogaea L.), pearl millet (Pennisetum glaucum L.) and sorghum (Sorghum bicolor L.) in sub-zone Hamelmalo, J. Agr. Econ. Dev., 2, 237–245, 2013.
Zabel, F., Delzeit, R., Schneider, J. M., Seppelt, R., Mauser, W., and Václavík, T.: Global impacts of future cropland expansion and intensification on agricultural markets and biodiversity, Nat. Commun., 10, 2844, https://doi.org/10.1038/s41467-019-10775-z, 2019.
Zhang, J., Fu, B., Stafford-Smith, M., Wang, S., and Zhao, W.: Improve forest restoration initiatives to meet sustainable development goal, Nat. Ecol. Evol., 5, 10–13, 2021.
Ziegler, A. D., Fox, J. M., and Xu, J.: The rubber juggernaut, Science, 324, 1024–1025, https://doi.org/10.1126/science.1173833, 2009,