the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Nine years of warming and nitrogen addition in the Tibetan grassland promoted loss of soil organic carbon but did not alter the bulk change in chemical structure
Huimin Sun
Michael W. I. Schmidt
Jintao Li
Jinquan Li
Xiang Liu
Nicholas O. E. Ofiti
Shurong Zhou
Ming Nie
Nitrogen (N) and warming effects on ecosystem carbon (C) budgets and stabilization are critical to understand as C sequestration is considered as a mechanism to offset anthropogenic CO2 emissions, which is important for accurately predicting ecosystem C sequestration and/or potential C loss, remaining controversial though. However, the relevant information, especially for the intervention of environmental controls on grassland soil, is limited in Tibetan Plateau (TP) regions. Here we used a 9-year two-way factorial experiment involving warming with open top chambers (+1.80 ∘C in the daytime and +0.77 ∘C in the nighttime at the soil surface) and multilevel nitrogen (N) enrichment treatments (0, 5, 10, and 15 ) in the TP to investigate the changes in soil organic carbon (SOC) pool size and chemical structure. Nine-year warming treatment significantly decreased SOC stock in the Tibetan grassland. We observed decreasing SOC concentrations which may be related to changes in the C-degrading enzymes. Surprisingly, the SOC molecular structure remained unchanged in all N-enrichment and warmed plots, suggesting that both treatments had affected all forms of SOC, from simple and complex polymeric in a similar way. Our results suggest that long-term warming stimulates soil C loss but no preference in SOC loss with different chemical structures.
- Article
(2095 KB) - Full-text XML
-
Supplement
(758 KB) - BibTeX
- EndNote
Soil organic matter is the largest organic carbon reservoir of near-surface terrestrial ecosystems (Dlamini et al., 2016). Even subtle acceleration in soil organic carbon (SOC) decomposition will result in large CO2 emissions (Davidson and Janssens, 2006). So, knowledge of the factors affecting SOC storage and decomposition is essential for understanding the dynamically changing global C cycle. The influence of global warming on the decomposition of soil carbon has been well documented (Poeplau et al., 2017; Guan et al., 2018; X. Ding et al., 2019), but there remains considerable uncertainty in the potential response of soil C dynamics to the rapid global increase in reactive nitrogen (N, coming largely from agricultural fertilizers and fossil fuel combustion) as well as the combined effects with warming (Liang and Balser, 2012; Devaraju et al., 2015; Li et al., 2017). For example, soil N availability would strongly affect microbial physiology and C-degrading enzymes (EnC), which can subsequently alter soil C feedbacks to warming (Mack et al., 2004; Contosta et al., 2015). EnC have been shown to play an important role in SOM nutrient cycling and catabolism (H. Chen et al., 2018), and information on such activity can be used to investigate substrate nutrient demand and responses to environmental changes (Allison et al., 2010; Wang et al., 2015). The knowledge gap demonstrated a need to focus research on biological and physicochemical controls of SOC stabilization and destabilization processes as a basis for understanding causal relationships and key processes that determine pool sizes and turnover rates of functional SOC pools (von Lützow and Kögel-Knabner, 2009).
Soil warming experiments in the field have shown that warming generates a considerable short-term soil C loss (Lu et al., 2013; Romero-Olivares et al., 2017). This loss declines over time (e.g., >2 years) (Romero-Olivares et al., 2017), although there is evidence that it can continue for longer (e.g., >20 years) (Melillo et al., 2017). Also, indirect effects of warming on nutrient cycling (Pendall et al., 2004) or plant inputs (Bradford et al., 2016) may have cascading effects on SOC quality and quantity (Lu et al., 2013) and consequently on microbial decomposition of SOC, including recent plant-derived material (Hicks Pries et al., 2017) or older SOC (Vaughn and Torn, 2019). Because ecosystems in alpine meadows are normally N-limited (Hobbie et al., 2002), increased N released from decomposing SOC could stimulate plant productivity, thereby increasing ecosystem C storage (Moscatelli et al., 2008). However, field evidence suggests that soil microbial activity and biomass may also be N-limited in some C-rich ecosystems (Mack et al., 2004; Rinnan et al., 2007). Therefore, increased N released from decomposition of SOC could further fuel microbial activity and decrease soil C storage. In addition, according to the priming effect hypothesis, the increase in N availability and labile C substrates promotes microbial C utilization, thereby increasing the degradation of less decomposable SOC and leading to a negative effect on soil C accumulation over the long term (Riggs and Hobbie, 2016). However, it has proven difficult to quantify bulk SOC stock changes and organic matter composition directly (Sistla et al., 2013; Van Gestel et al., 2018). As alternatives, molecular-level techniques can detect how temperature affects plant and soil organic matter, microbial growth, and their community composition under climate warming (Feng et al., 2008; Xue et al., 2016; Pold et al., 2017).
Since the molecular structure of organic material has long been thought to determine long-term decomposition rates in soil humic substances, solid-state cross-polarization magic-angle spinning carbon-13 nuclear magnetic resonance (CPMAS 13C NMR) and diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy have been successfully applied in studies on changes in SOC chemical structure during organic matter decomposition without any physical or chemical destruction (Schmidt et al., 2011). The structure of SOC could be very complex, but by combining both techniques (solid-state 13C NMR and DRIFT), complementary information could be obtained on aromatic and aliphatic components (Ferrari et al., 2011).
Despite the importance of the response of SOC stocks to warming and N enrichment in the intact ecosystem, results about the chemical stabilization mechanisms (i.e., molecular structure of SOC) in alpine meadows remained controversial. This knowledge gap is significant because the Tibetan Plateau (TP) stores a large C pool, with 36.6 Pg C stored in the top 3 m of the soil, accounting for 23.5 % of China's total organic soil-stored C and 2.5 % of the global pool of soil C, which is of great importance in regulating future global climate change and C emission (Genxu et al., 2002; J. Ding et al., 2019). At the same time, the TP has experienced climate warming at a rate that is 2 times faster than that in other regions worldwide and is predicted to lead to great soil C losses via microbial respiration in the future (Biskaborn et al., 2019). In addition, during recent decades, the TP has been subject to high levels of N enrichment driven by agricultural activities (up to ∼8.0 ) (Gao et al., 2007; Li et al., 2012; Zhang and Fu, 2020) and atmospheric N deposition (1 ) (Lü and Tian, 2007; Yu et al., 2019), with an annual rate of increase in deposition (0.053 ) (Liu et al., 2013; J. Wang et al., 2019), and this kind of enrichment has been shown to induce soil C loss and affect SOC stabilization in this typical N-constrained ecosystem (Xiao et al., 2021).
Since temperature is one of the main drivers of the vegetation growth and decomposition of organic matter, ongoing climate change may alter biophysical processes, with consequences for ecosystem functioning, especially in highly sensitive cold regions such as the alpine meadow on the TP (Piao et al., 2006; Yang et al., 2008). However, how and to what extent chemical stabilization of SOC shifts may occur, and consequently SOC storage and C-climate feedback would respond to warming and N enrichment in an alpine meadow ecosystem, remain largely unknown. Here, we used soils from a 9-year experiment with a two-way factorial design involving soil warming (daytime: 1.80 ∘C; nighttime: 0.77 ∘C) and control plots and N enrichments (0, 5, 10, and 15 , marked as N0, N1, N2, and N3, respectively) (Liu et al., 2016) on the TP to examine the changes in the stock and molecular structure of SOC.
We hypothesized that 9-year N enrichment and warming would affect SOC stocks and the chemical structure of the SOC. N enrichment below a certain threshold may favor C sequestration in the alpine grassland ecosystem, but warming may result in C loss. Added N would stimulate hydrolytic enzyme activity, while warming would repress enzyme activity. Finally, we hypothesized that variation in the enzyme response to N and temperature would emerge as an important explanation for variability in the effect of added N and warming on SOC stock.
2.1 Site description
Plot sampling was conducted in a grassland ecosystem located on the eastern edge of the Tibetan Plateau, Maqu County, Gansu Province, China ( N, E; 3500 m above sea level; Fig. 1; NOAA, 2015), in August 2019. The grassland ecosystem of the TP covers an area of about 1.53×106 km2, accounting for nearly 60 % of the total area of the TP (Liu et al., 2016). Alpine meadow is the main vegetation type in this area, and the plant community is dominated by perennial herbaceous species of Poaceae, Ranunculaceae, and Asteraceae. The area of alpine meadow accounts for more than 44 % of the area of alpine grasslands, and its SOC storage accounts for 56 % of the SOC storage of alpine grasslands on the whole TP (Yang et al., 2008). The soil in the alpine meadow is classified as Mat-Cryic Cambisol (Hou et al., 2019). This region has a typical plateau continental climate. The mean annual precipitation is 620 mm, and most falls in the growing season (summer). The mean annual temperature is 1.2 ∘C, with the lowest monthly mean temperature occurring in January (−10.7 ∘C) and the highest monthly mean temperature occurring in July (11.7 ∘C). During the past several decades, the mean annual temperatures in the region have risen at a rate of 0.58 ∘C per decade (Liu et al., 2016).
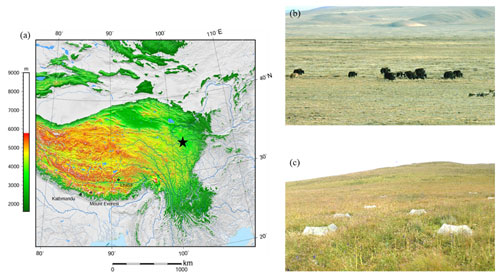
Figure 1Elevation map of the studied sites (a; the pentagram refers to the sampling point, NOAA, 2015), photo of the alpine meadow (b), and the diagram of the warming treatment (open-top chamber) (c). Note: the map (a) was cited from the Wikimedia Commons website (Tibet and surrounding areas above 1600 m, created using Generic Mapping Tools).
2.2 Experimental design and soil sampling
A field-based warming experiment was established in June 2011 with a split-plot block design in which both temperature (open-top chamber, +1.80 ∘C in the daytime and +0.77 ∘C in the nighttime at the soil surface) and nitrogen (0, 5, 10, and 15 , corresponding to N0, N1, N2, and N3, respectively) were manipulated, with six replicates per treatment (Liu et al., 2016). The 48 plots (8 treatments (N0, N1, N2, N3, WN0, WN1, WN2, WN3) with 6 replicates each treatment) with roughly the same species diversity and community structure were 5 m×5 m and were separated by 1 m from adjacent edges. Additional details can be found in our previous studies (Sun et al., 2023). Surface layer (0–10 cm) soils were collected from these 48 plots using an auger 4 cm in diameter in August 2019. Then, the fresh soil samples were transported to the laboratory on ice.
2.3 Soil analysis
Soil microbial biomass carbon (MBC) was measured according to the chloroform fumigation extraction method using a total organic carbon (TOC) analyzer (Multi N/C 3100, Analytik Jena GmbH, Germany) (Vance et al., 1987). The soil pH was determined in a 1:5 soil : water suspension with a pH meter (PHS-3D, Rex, Shanghai, China). Bulk density samples were dried at 105 ∘C for 48 h and calculated by dividing the oven-dried soil mass by the steel cylinder volume (100 cm3) because coarse fragments (stones or large roots) were not obtained in ring samples. For SOC analysis, air-dried soil was ground and HCl-fumigated (Komada et al., 2008), and then the SOC concentration was determined with an elemental analyzer (FlashSmart, Thermo Fisher Scientific, USA). The SOC stocks (0–10 cm) were calculated by multiplying the SOC concentration by the bulk density (Walter et al., 2016). At these 48 sites, all plants in three plots (50 cm×50 cm) were harvested and dried to determine the aboveground biomass (AGB).
We measured the activity of four extracellular enzymes in the soil at an in situ pH (Nie et al., 2013). The absorbance of the C degradation enzymes β-D-cellubiosidase (CB), α-glucosidase (AG), β-glucosidase (BG), and β-xylosidase (XYL) was measured using a Tecan infinite M200 microplate fluorometer (Grodig, Austria) with 365 nm excitation and 460 nm emission filters. The activities were expressed as dry soil (). We combined CB, AG, BG, and XYL into an EnC.
2.4 SOC molecular structure examination using NMR spectroscopy
The soil samples used for NMR spectroscopy analysis were pre-treated using hydrofluoric (HF) acid (2 %) to eliminate paramagnetic materials, e.g., ferric ions and manganese ions, that may affect the NMR signals (Skjemstad et al., 1994; Schmidt et al., 1997; Mathers et al., 2002). The solid-state NMR spectra (13C-CPMAS) were recorded on a Bruker AVANCE III 600 MHz instrument (Bruker Instrument Inc., Billerica, MA, USA). The acquisition conditions were set at a frequency of 75.5 MHz, with 20 kHz spectral width, 5 kHz spinning speed, 2 ms contact time, and 2.5 s recycle time. The regions of 0–210 ppm spectra were plotted.
We examined seven chemical shift regions to represent the main C functional groups (Golchin et al., 1997; Sun et al., 2019). We report proportions of each chemical shift area and calculated four ratios indicative of the characteristics of soil organic matter. The alkyl C, the most persistent fraction of SOC, comes from original plant biopolymers (such as cutin, suberin, and waxes) or from metabolic products of soil microorganisms (Ussiri and Johnson, 2003). As these materials decompose, the relative abundance of O-alkyl C in the litter materials decreases, and there is a progressive increase in alkyl C (Bonanomi et al., 2013). Therefore, the ratio of alkyl C to O-alkyl C (AO-A = ) is an index representing the extent of SOC decomposition. The higher this ratio, the higher the decomposition degree of SOC (Wang et al., 2015). Aromaticity () was used to indicate the complexity of a molecular structure (Dai et al., 2001). The ratio of aliphatic Caromatic C (aliparom), , also indicates the molecular structure of soil C, with a higher aliparom meaning less aromatic nuclear structure in humus. The hydrophobic Chydrophilic C (HBHI) ratio, , was used to reflect the stability of soil aggregation (Spaccini et al., 2006; Wang et al., 2010). The higher values of the HBHI ratio indicated that SOC was more hydrophobic (Cao et al., 2016), which in turn implied that SOC was more stable (Spaccini et al., 2006; Wu et al., 2014).
2.5 Bulk soil organic matter composition using DRIFT spectroscopy
Because of the large number of variables affecting a spectrum, it is extremely difficult to obtain a complete and fine molecular structure from a single spectrum without additional knowledge obtained by other spectroscopic techniques (Ferrari et al., 2011). So, we employed another complementary molecular-level analysis, DRIFT spectroscopy, which is a useful method for the characterization of organic matter (Olk et al., 2000) and humic substances (Mao et al., 2008; Francioso et al., 2009), to explore potential shifts in SOC composition in response to warming and N enrichment. To characterize warming and N-induced changes in SOC composition, 6 mg of a ground soil sample was examined by DRIFT spectroscopy. Mid-infrared spectra were recorded using a Bruker TENSOR 27 spectrometer (Billerica, MA, USA) from 4000 to 400 cm−1 (average of 16 scans per sample at 4 cm−1 resolution). Infrared absorption bands were represented by functional groups. Infrared absorption bands were represented by functional groups as follows: aliphatic C−H (2900 cm−1), aromatic esters, carbonylcarboxyl C=O (1735–1720 cm−1), aromatic C=C (1660–1600 cm−1, 1430–1380 cm−1), lignin-like residues (1515–1500 cm−1), phenoliccellulose (1260–1210 cm−1), and aromatic C−H (880, 805, and 745 cm−1) carbon (Niemeyer et al., 1992; Leifeld, 2006; Chatterjee et al., 2012). A summary of the absorption bands associated with different compound classes can be found in Fig. S2 in the Supplement. Additional details can be found in our previous study (Ofiti et al., 2021).
2.6 Statistical analysis
All the data are presented as the mean values of six field replicates. Any significant differences in soil physicochemical properties among the different N enrichment levels and warming treatments were identified by using two-way ANOVA followed by Tukey's heavily significant difference (HSD) post hoc test, with differences considered to be statistically significant at P<0.05. The statistical analysis was conducted using SPSS 13.0 and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria, 2013). To access the direct and indirect effects of external factors on SOC stock, structural equation modeling (SEM) was performed using the R packages plspm and piecewiseSEM (Li et al., 2020). For this purpose, firstly, all data were tested for normality using the Kolmogorov–Smirnov test, and the non-normal variables were log-transformed. Secondly, we established a prior model based on prior knowledge of effects and relationships among the driving factors. Finally, we selected the best model based on overall goodness of fits, including the chi-squared (χ2) statistic, degrees of freedom (dfs), whole-model P value, goodness-of-fit index, and root-mean-square error of approximation (Schermelleh-Engel et al., 2003).
3.1 Bulk soil properties
N enrichment and warming showed a significant interaction effect for pH, AGB, EnC, , and SOC stock (P<0.05, Fig. 2). AGB, EnC, and SOC stocks increased significantly under N enrichment, but the increment decreased with rising N addition concentration (P<0.05, Fig. 2b, c, and f). Warming exacerbates soil acidification and decreased the AGB, EnC, MBC, and SOC stock significantly. Both N enrichment and warming significantly decreased the ratio (P<0.05, Fig. 2d). Except for MBC, the N and warming interactions significantly altered soil physicochemical properties (Fig. 2, Table S1 in the Supplement).
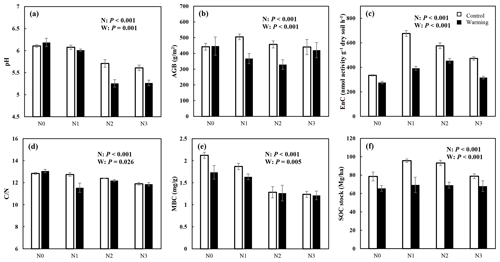
Figure 2N and warming-induced changes in the soil properties (mean ± SE, n=6). Control (white bar) and warmed plots (black bar) at four different levels of simulated N deposition. N0, N1, N2, and N3 indicate N enrichments of 0, 5, 10, and 15 , respectively. The parameters are soil pH (a); AGB, aboveground biomass (b); EnC, C-degrading enzymes (c); , the ratio of soil C concentration to N concentration (d); MBC, microbial biomass carbon (e); and SOC, soil organic carbon stock (f).
3.2 SOC speciation as seen by DRIFT and NMR spectroscopy
Changes in SOC molecular composition became apparent in DRIFT and NMR spectra (Figs. 3 and 4 and Figs. S2 and S3 in the Supplement). In all N-enrichment and warming treatments, there was a statistically nonsignificant change in the SOC composition and molecular structure observed by both DRIFT and NMR spectra. The relative abundance of carbonylcarboxyl C=O, C=C aromatic compounds, and lignin-like residues decreased slightly after N enrichment, though not significantly. The relative abundance of the phenoliccellulose groups remained stable in all the individual and interaction treatments (Figs. 3 and S2).
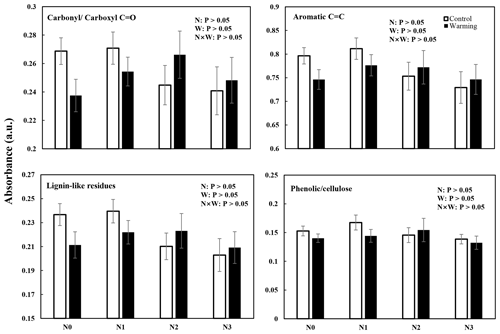
Figure 3N and warming-induced changes in the relative abundance of different functional groups identifiable by diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy in warmed and control plots (mean ± SE, n=6). The spectral regions were assigned to aromatic carbonylcarboxyl C=O groups, aromatic C=C groups, lignin-like residues, and cellulosephenolic groups.
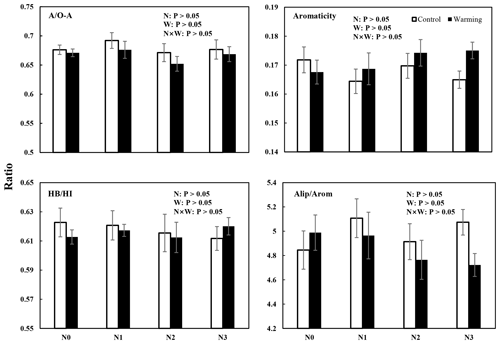
Figure 4Four different SOC chemical structural complexity indexes (mean ± SE, n=6) from solid-state 13C CPMAS NMR spectra of soil samples from different treatments. AO-A = alkyl CO-alkyl C; HBHI = hydrophobic Chydrophilic C; aliparom = aliphatic Caromatic C.
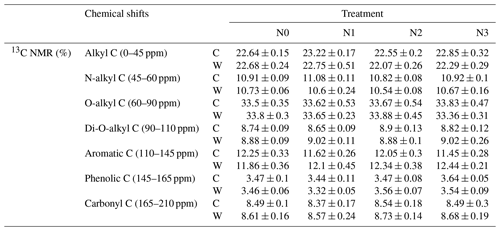
The results of 13C NMR spectroscopy indicated the relative abundance of different C components (Table 1, Figs. 4 and S3), showing that the proportion of the seven C functional groups did not change in soils under N-enrichment and warming treatments. The relative proportions of the seven C functional groups were stable in the eight treatments in the following abundance order: O-alkyl C (mean 33 %), followed by alkyl C (mean 22 %), aromatic C (mean 12 %), N-alkyl C (11 %), carbonyl C and di-O-alkyl C (mean 8 %), and finally phenolic C (mean 3 %) (Table 1, Fig. S3). The four indexes which can represent the extent of SOC decomposition observed by NMR spectra also showed no significant difference under all the N-enrichment and warming treatments (Fig. 4), suggesting that SOC showed a similar degradation state at all N-level enrichments and warming treatments as well as the interaction effects.
3.3 Factors driving the SOC stocks
We then developed SEM to assess the direct and indirect effects of soil variables on the SOC stocks (Fig. 5). The SEM results revealed strong connections among global change, biotic, and edaphic factors (Fig. 5), demonstrating a need to consider their interactions when predicting SOC stock and its response to individual and interactive effects of N enrichment and warming. Overall, the SEM explained 44 %, 55 %, and 21 % of the variance in SOC stock driven by N enrichment, warming treatment, and interaction effects, respectively. In both N and warming patterns, C-degrading enzymes showed an important indirect factor in regulating SOC stock. N enrichment had a positive effect on SOC stock by enhancing enzyme activities. In contrast, warming had a negative effect on SOC stock by inhibiting microbial enzymes. In addition, warming had a strong negative direct effect on SOC stock (Fig. 5b). However, no significant direct or indirect pathways for the interaction effects of N and warming on SOC stocks were observed (Fig. 5c).
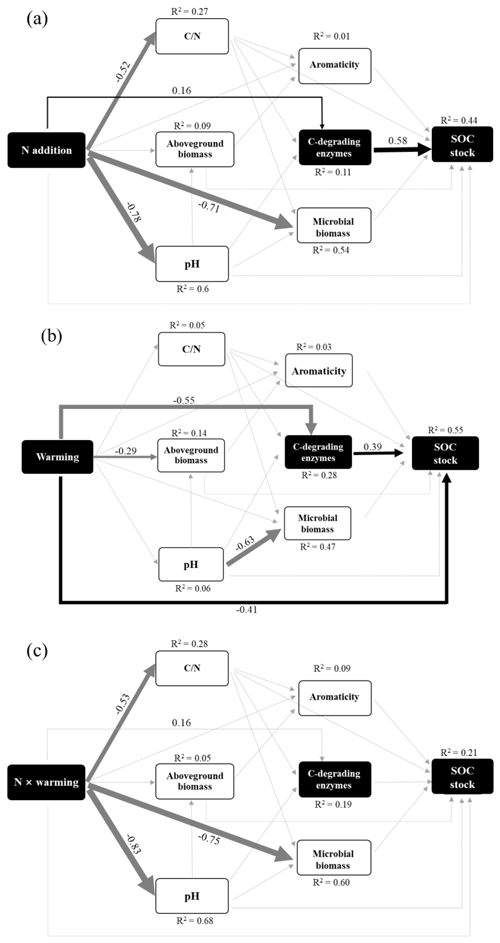
Figure 5The factors regulating the SOC stock under (a) N enrichment, (b) warming treatment, and (c) interactive effects of N and warming. In the SEM analysis, black arrows represent significant positive pathways, gray arrows represent significant negative pathways, and gray dashed arrows indicate nonsignificant pathways. Values next to the arrows represent standardized effect sizes with statistical significance (* P<0.05; ** P<0.01; *** P<0.001). The thickness of the arrow represents the standardized effect sizes. C-degrading enzyme indicates the sum of β-D-cellubiosidase (CB), α-glucosidase (AG), β-glucosidase (BG), and β-xylosidase (XYL). Goodness-of-fit statistics for the model are shown as follows: (a) χ2=4.53, P=0.53, GFI=0.99, RMSEA<0.001; (b) χ2=4.47, P=0.486, GFI=0.99, RMSEA<0.001.
4.1 Effects of warming and N enrichment on soil C pool size
It is suggested that small N inputs can decrease CO2 emissions by changing the interaction between plants and soil microbes in N-limited ecosystems, e.g., by increasing plant productivity and root biomass and then organic C inputs to the soil by promoting N availability and thus retarding litter and SOC decomposition (Franklin et al., 2003; Mo et al., 2008; Zhou et al., 2014). However, in an alpine grassland, Jiang et al. (2012) found that both plant growth and microbial activity were generally N-limited, but the ability of plants to capture soil inorganic N was much stronger than that of soil microorganisms. When N was added, increased N availability resulted in increased plant growth, microbial activity, and plant biomass (Micks et al., 2004). Therefore, the decomposition of litter and SOM is enhanced by increasing the quantity of litter input or by elevating microbial activity, and consequently soil functions would shift from C sequestration to C loss. The increased N has consequently reduced the soil pH by 0.26 globally in only 1 decade, which may significantly influence the microbial community composition and activity and then the SOC sequestration capacity (Geisseler and Scow, 2014; Tian and Niu, 2015; Raza et al., 2021). This speculation is consistent with our results that N input below a threshold level (e.g., 10 g m−2 in this study) may be beneficial for C sequestration in alpine meadows of the TP and can partly explain the patterns of SOC pool sizes under various N-enrichment levels in this study. Specifically, the SOC stock increased following N enrichment, but as the N addition concentration increased, this growth progressively diminished, eventually even disappearing. Our results revealed that alpine grassland ecosystems on the TP may become a potential C source in future scenarios of increasing N enrichment.
In our study presented here, the 9 years of warming resulted in a very significant SOC loss of 14 % to 28 % (Fig. 2 and Fig. S1 in the Supplement). The TP stored large amounts of SOC because of the permafrost soil, where limited C decomposition has led to the accumulation of large SOC stocks (Hengl et al., 2014; Schuur et al., 2015). Previous studies showed that the vulnerability of soils with large C stocks derives from the high temperature sensitivity of C decomposition and biogeochemical restrictions to the processes driving soil C inputs (Davidson and Janssens, 2006; García-Palacios et al., 2021). In contrast to this, in soils with low initial C stocks, small losses coming from accelerated decomposition induced by rising temperature may be offset by concurrent increases in plant growth and soil C stabilization (Day et al., 2008; Macias-Fauria et al., 2012; Crowther et al., 2015). However, in areas with larger SOC stocks, accelerated decomposition exceeds the potential C accumulation of plant growth, contributing to a significant C loss to the atmosphere (Luo et al., 2019).
4.2 Effects of N enrichment and warming on SOC chemical compositions
SOC chemical composition is controlled not only by the chemistry of the plant material input into the soil, but also by the microbial processing and degradation of SOC (Baldock et al., 1992). Although N addition can stimulate plant growth and increase litter fall, it can also accelerate or slow down microbial processing of plant residues, thus altering the chemical composition of SOC (J.-J. Wang et al., 2019). Surprisingly, we observed that the SOC molecular structure remained unchanged in all N-enrichment and warmed plots (Figs. 3 and 4).
As the predominant chemical component of SOC across all treatments in our study, O-alkyl C is mainly composed of carbohydrates, peptides, and other labile organic components derived from the fresh material, which could be preferentially degraded compared with more resistant components such as alkyl C (Simpson and Simpson, 2012; He et al., 2018). So, we hypothesized that this result could indicate that N and warming may have the same impact on the input of fresh plants on the TP. Unlike O-alkyl C, lipids represent the main source of alkyl C (aliphatic chains), which is derived from the original plant biopolymers. Lignin and tannin represent the main source of aromatic C, together with phenolic C, mainly originating from lignin and amino acids of peptides (Baldock et al., 1992). All these components are more resistant to microbial decomposition than labile O-alkyl C (Simpson and Simpson, 2012). Our results suggested that the proportions of the stable SOC chemical structures remained the same between the different N-enrichment concentrations and warming treatments, indicating the synchronous degradation of SOC. The alkylO-alkyl ratio and aromaticity, normally regarded as the indicator of the relative stage of SOC degradation and widely used as an indicator to reflect the complexity of SOC chemical structures (Baldock et al., 1992), exhibited no significant difference among N-enrichment or warming treatments, suggesting that all N levels and warming treatments exerted similar effects on the degradation of SOC and aromatic and complex molecular structures (Zhang et al., 2013).
Infrared spectroscopy of SOC showed a statistically nonsignificant change in the treated plots and the control plots, consistent with a previous study which showed that the 4.5 years of +4 ∘C whole-soil warming did not change the relative abundance of carbonylcarboxyl C=O and C=C aromatic compounds in the surface soils (above 20 cm) from a forest (Ofiti et al., 2021). Collectively, the above results suggested that the molecular structure of surface SOC may not be as sensitive to long-term warming as we thought before (Atanassova and Doerr, 2011; H. Chen et al., 2018). Surface SOC is dominated by recent (less transformed) plant-litter inputs, which is less degraded and transformed than subsoil SOC (Ofiti et al., 2021). The lack of change in plant- and microorganism-derived organic matter in the surface soil may be due to slight drying and warming near the surface (Soong et al., 2021), which could have inhibited or resulted in relatively fewer surface inputs. We noticed that warming significantly reduced AGB under N1 and N2 enrichments in this study (Fig. 2). Compared with labile SOC, stable SOC can be more vulnerable to priming once microbes are provided with exogenous C substrates. This high vulnerability of stable SOC to priming warrants more attention in future studies on SOC cycling and global change (Zhang et al., 2022). Overall, the stabilized functional SOC molecular structure suggests that soil warming and N enrichment had similarly affected the labile and stabilized SOC of this C-rich grassland soil at the level of chemical stability of organic C molecules, along with the C loss.
4.3 Regulating factors of SOC stock
Our interpretation that prolonged warming could reduce SOC storage is further supported by the simultaneous reduction in different C pool sizes characterized by various chemical structural complexities with long-term warming. Many previous studies have shown that microorganisms preferentially use the labile C pool for community utilization and turnover after short-term warming (Melillo et al., 2002; Kirschbaum, 2004). However, after the initial microbial assimilation of readily accessible SOC with warming, soil microorganisms can acclimate to C starvation through utilization of chemically less available C with continued warming (Chen et al., 2020). This transformation in microbial preferences of C substrates can be facilitated by changes in C-degrading enzyme activities (Crowther and Bradford, 2013).
Our results indicated that C-degrading enzymes could play a key role in regulating soil C storage (Fig. 5a and b), which is in line with previous explanations for continued soil C loss with long-term warming, such as shifts in microbial community and physiology (Melillo et al., 2017; Metcalfe 2017), changes in microbial carbon use efficiency (Tucker et al., 2013), and increased microbial accessibility to litter and SOC (Doetterl et al., 2015; Bailey et al., 2019), which are all closely related to changes in microbial C-degrading enzyme activities. For example, warming decreased the abundance of lignin-derived compounds but increased ligninase activity in a mixed temperate forest (Feng et al., 2008). Although only cellulase activity was measured in our study, a previous meta-analysis study has shown significantly increasing ligninase activity after warming, enhancing the evidence of microbial response to recalcitrant C pools and the evidence of simultaneous loss of different C fractions after long-term warming (J. Chen et al., 2018). Microbial utilization of recalcitrant C pools could substantially accelerate overall soil C loss. This is because depolymerization of these recalcitrant macromolecules increases microbial accessibility to litter and SOC that was protected by recalcitrant C pools before (Schmidt et al., 2011; Lehmann and Kleber, 2015; Paustian et al., 2016).
While N fertilization exerts both direct and indirect impacts on SOC, its influence on carbonates is direct, leading to continuous losses. This not only serves as a source of atmospheric CO2 (Kim et al., 2020; Raza et al., 2020; Zamanian et al., 2018) but also degrades soil structure and affects physical, chemical, and biological properties (Meng and Li, 2019). Under acidic conditions, this process induces fundamental changes in microbial community composition and enzyme activity critical for SOC stability (Rowley et al., 2020). In ecosystems characterized by N limitation, such as alpine grasslands, N enrichment enhances N availability, accelerating the decomposition of labile organic C, especially in these otherwise C-rich ecosystems. This in turn results in decreased soil C availability (Craine et al., 2007; Janssens et al., 2010; Song et al., 2017). A previous study at our research site revealed a significant reduction in the soil labile C pool within the particulate organic C fraction with increasing N enrichment, signifying a decline in soil C availability (Chen et al., 2019). Our findings demonstrate that N enrichment significantly stimulates EnC activities and enhances microbial demand for C (Fig. 2), aligning with prior research indicating that added N stimulates the activity of soil cellulose-degrading enzymes (e.g., CB and BG) (Carreiro et al., 2000; Saiya-Cork et al., 2002; Chen et al., 2017). This stimulation may be attributed to the increase in C-acquiring enzymes resulting from heightened microbial demand for C, especially in N-limited ecosystems (Keeler et al., 2009). Previous studies suggest that N enrichment could induce C limitation by reducing plant allocation to fine root production, leading to lower C input into the soil (Treseder, 2008). Thus, we propose that factors beyond the thermal environment, such as N enrichment, can modulate soil enzymes and alter substrate availability. Moreover, these processes can mediate the strength of the soil C–climate feedback. Although N enrichment may increase soil C sinks, this increase may be counteracted by warming. In summary, our results suggest that warming and N enrichment have antagonistic interaction effects on SOC stock, with differential effects on the contribution of alpine meadows to the soil C pool, which may explain the result that the N and warming interaction did not show significant direct or indirect effects on SOC storage in the SEM results. All these results underscore the importance of considering soil C availability and enzymatic activity responses, which collectively determine the response of the C balance to multiple environmental changes, for a more comprehensive understanding of C storage dynamics.
Based on a 9-year warming (+1.80 ∘C in the daytime and +0.77 ∘C in the nighttime at the soil surface) and different levels of N-enrichment experiments (0, 5, 10, and 15 ), we examined the responses of SOC stocks and their molecular components in a Tibetan alpine meadow ecosystem. In summary, our results show little effects of soil warming and N enrichment on the chemical composition of bulk soil despite ongoing C loss in the warmed plots of the study site (Fig. 2). The SOC molecular structure suggested that the easily decomposable and stabilized SOC is synchronously affected after 9-year warming and N treatments despite the large changes in SOC stocks. Given the long residence time of some SOC (Schmidt et al., 2011), the similar loss of all measurable chemical forms of SOC under global change treatments could have important climate consequences. While we found little effects of soil warming on SOC chemistry and the molecular structure of bulk soil, consistent and long-lasting changes could appear with prolonged soil warming and decreasing SOC stocks in the following years. In this process, the importance of enzyme activity must be emphasized, which has been found to be an important indirect factor in regulating changes in SOC stocks.
The data that support the findings of this study and those not presented within the article and its Supplement are available from https://doi.org/10.5281/zenodo.8289311 (Sun et al., 2023b).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-21-575-2024-supplement.
MN developed the original ideas presented in the paper. HS performed the overall analysis with assistance from JintL, JinqL, and NOEO. XL and SZ organized the field experiment. HS, MWIS, and MN wrote the first draft, and all the authors jointly revised the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This work was supported by the Shuguang Program supported by the Shanghai Education Development Foundation and Shanghai Municipal Education Commission (grant no. 21SG02), the Shanghai Pilot Program for Basic Research – Fudan University 21TQ1400100 (grant no. 21TQ004), the Science and Technology Department of Shanghai (grant no. 21DZ1201902), the Shanghai Pujiang Program (grant no. 2020PJD003).
This research has been supported by the Program of Shanghai Academic Research Leader (grant no. 21XD1420700), the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant no. 172744), and the National Natural Science Foundation of China (grant nos. 91951112 and 32101377).
This paper was edited by Sara Vicca and reviewed by Qiufang Zhang and one anonymous referee.
Allison, S. D., Wallenstein, M. D., and Bradford, M. A.: Soil-carbon response to warming dependent on microbial physiology, Nat. Geosci., 3, 336–340, https://doi.org/10.1038/ngeo846, 2010.
Atanassova, I. and Doerr, S.: Changes in soil organic compound composition associated with heat-induced increases in soil water repellency, Eur. J. Soil Sci., 62, 516–532, https://doi.org/10.1111/j.1365-2389.2011.01350.x, 2011.
Bailey, V. L., Pries, C. H., and Lajtha, K.: What do we know about soil carbon destabilization?, Environ. Res. Lett., 14, 083004, https://doi.org/10.1088/1748-9326/ab2c11, 2019.
Baldock, J., Oades, J., Waters, A., Peng, X., Vassallo, A., and Wilson, M.: Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy, Biogeochemistry, 16, 1–42, https://doi.org/10.1007/BF00024251, 1992.
Biskaborn, B. K., Smith, S. L., Noetzli, J., Matthes, H., Vieira, G., Streletskiy, D. A., Schoeneich, P., Romanovsky, V. E., Lewkowicz, A. G., Abramov, A., Allard, M., Boike, J., Cable, W. L., Christiansen, H. H., Delaloye, R., Diekmann, B., Drozdov, D., Etzelmüller, B., Grosse, G., Guglielmin, M., Ingeman-Nielsen, T., Isaksen, K., Ishikawa, M., Johansson, M., Johannsson, H., Joo, A., Kaverin, D., Kholodov, A., Konstantinov, P., Kröger, T., Lambiel, C., Lanckman, J.-P., Luo, D., Malkova, G., Meiklejohn, I., Moskalenko, N., Oliva, M., Phillips, M., Ramos, M., Sannel, A. B. K., Sergeev, D., Seybold, C., Skryabin, P., Vasiliev, A., Wu, Q., Yoshikawa, K., Zheleznyak, M., and Lantuit, H.: Permafrost is warming at a global scale, Nat. Commun., 10, 264, https://doi.org/10.1038/s41467-018-08240-4, 2019.
Bonanomi, G., Incerti, G., Giannino, F., Mingo, A., Lanzotti, V., and Mazzoleni, S.: Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than and LigninN ratios, Soil Biol. Biochem., 56, 40–48, https://doi.org/10.1016/j.soilbio.2012.03.003, 2013.
Bradford, M. A., Wieder, W. R., Bonan, G. B., Fierer, N., Raymond, P. A., and Crowther, T. W.: Managing uncertainty in soil carbon feedbacks to climate change, Nat. Clim. Change, 6, 751–758, https://doi.org/10.1038/nclimate3071, 2016.
Cao, Z. Y., Wang, Y., Li, J., Zhang, J. J., and He, N. P.: Soil organic carbon contents, aggregate stability, and humic acid composition in different alpine grasslands in Qinghai-Tibet Plateau, J. Mt. Sci., 13, 2015–2027, https://doi.org/10.1007/s11629-015-3744-y, 2016.
Carreiro, M., Sinsabaugh, R., Repert, D., and Parkhurst, D.: Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition, Ecology, 81, 2359–2365, https://doi.org/10.1890/0012-9658(2000)081[2359:MESELD]2.0.CO;2, 2000.
Chatterjee, S., Santos, F., Abiven, S., Itin, B., Stark, R. E., and Bird, J. A.: Elucidating the chemical structure of pyrogenic organic matter by combining magnetic resonance, mid-infrared spectroscopy and mass spectrometry, Org. Geochem., 51, 35–44, https://doi.org/10.1016/j.orggeochem.2012.07.006, 2012.
Chen, H., Yang, Z., Chu, R. K., Tolic, N., Liang, L., Graham, D. E., Wullschleger, S. D., and Gu, B.: Molecular insights into Arctic soil organic matter degradation under warming, Environ. Sci. Technol., 52, 4555–4564, https://doi.org/10.1021/acs.est.7b05469, 2018.
Chen, J., Luo, Y., Li, J., Zhou, X., Cao, J., Wang, R.-W., Wang, Y., Shelton, S., Jin, Z., Walker, L. M., Feng, Z., Niu, S., Feng, W., Jian, S., and Zhou, L.: Costimulation of soil glycosidase activity and soil respiration by nitrogen addition, Global Change Biol., 23, 1328–1337, https://doi.org/10.1111/gcb.13402, 2017.
Chen, J., Luo, Y., García-Palacios, P., Cao, J., Dacal, M., Zhou, X., Li, J., Xia, J., Niu, S., Yang, H., Shelton, S., Guo, W., and van Groenigen, K. J.: Differential responses of carbon-degrading enzyme activities to warming: Implications for soil respiration, Global Change Biol., 24, 4816–4826, https://doi.org/10.1111/gcb.14394, 2018.
Chen, J., Elsgaard, L., van Groenigen, K. J., Olesen, J. E., Liang, Z., Jiang, Y., Laerke, P. E., Zhang, Y., Luo, Y., Hungate, B. A., Sinsabaugh, R. L., and Jorgensen, U.: Soil carbon loss with warming: New evidence from carbon-degrading enzymes, Global Change Biol., 26, 1944–1952, https://doi.org/10.1111/gcb.14986, 2020.
Chen, Y., Liu, X., Hou, Y., Zhou, S., and Zhu, B.: Particulate organic carbon is more vulnerable to nitrogen addition than mineral-associated organic carbon in soil of an alpine meadow, Plant Soil, 458, 1–11, https://doi.org/10.1007/s11104-019-04279-4, 2019.
Contosta, A. R., Frey, S. D., and Cooper, A. B.: Soil microbial communities vary as much over time as with chronic warming and nitrogen additions, Soil Biol. Biochem., 88, 19–24, https://doi.org/10.1016/j.soilbio.2015.04.013, 2015.
Craine, J., Morrow, C., and Fierer, N.: Microbial nitrogen limitation increase decomposition, Ecology, 88, 2105–2113, https://doi.org/10.1890/06-1847.1, 2007.
Crowther, T. W. and Bradford, M. A.: Thermal acclimation in widespread heterotrophic soil microbes, Ecol. Lett., 16, 469–477, https://doi.org/10.1111/ele.12069, 2013.
Crowther, T. W., Thomas, S. M., Maynard, D. S., Baldrian, P., Covey, K., Frey, S. D., van Diepen, L. T., and Bradford, M. A.: Biotic interactions mediate soil microbial feedbacks to climate change, P. Natl. Acad. Sci. USA, 112, 7033–7038, https://doi.org/10.1073/pnas.1502956112, 2015.
Dai, K. O. H., Johnson, C. E., and Driscoll, C. T.: Organic matter chemistry and dynamics in clear-cut and unmanaged hardwood forest ecosystems, Biogeochemistry, 54, 51–83, https://doi.org/10.1023/A:1010697518227, 2001.
Davidson, E. A. and Janssens, I. A.: Temperature sensitivity of soil carbon decomposition and feedbacks to climate change, Nature, 440, 165–173, https://doi.org/10.1038/nature04514, 2006.
Day, T. A., Ruhland, C. T., and Xiong, F. S.: Warming increases aboveground plant biomass and C stocks in vascular-plant-dominated Antarctic tundra, Global Change Biol., 14, 1827–1843, https://doi.org/10.1111/j.1365-2486.2008.01623.x, 2008.
Devaraju, N., Bala, G., Caldeira, K., and Nemani, R.: A model based investigation of the relative importance of CO2-fertilization, climate warming, nitrogen deposition and land use change on the global terrestrial carbon uptake in the historical period, Clim. Dynam., 47, 173–190, https://doi.org/10.1007/s00382-015-2830-8, 2015.
Ding, J., Wang, T., Piao, S., Smith, P., Zhang, G., Yan, Z., Ren, S., Liu, D., Wang, S., Chen, S., Dai, F., He, J., Li, Y., Liu, Y., Mao, J., Arain, A., Tian, H., Shi, X., Yang, Y., Zeng, N., and Zhao, L.: The paleoclimatic footprint in the soil carbon stock of the Tibetan permafrost region, Nat. Commun., 10, 4195, https://doi.org/10.1038/s41467-019-12214-5, 2019.
Ding, X., Chen, S., Zhang, B., Liang, C., He, H., and Horwath, W. R.: Warming increases microbial residue contribution to soil organic carbon in an alpine meadow, Soil Biol. Biochem., 135, 13–19, https://doi.org/10.1016/j.soilbio.2019.04.004, 2019.
Dlamini, P., Chivenge, P., and Chaplot, V.: Overgrazing decreases soil organic carbon stocks the most under dry climates and low soil pH: A meta-analysis shows, Agr. Ecosyst. Environ., 221, 258–269, https://doi.org/10.1016/j.agee.2016.01.026, 2016.
Doetterl, S., Stevens, A., Six, J., Merckx, R., Van Oost, K., Casanova Pinto, M., Casanova-Katny, A., Muñoz, C., Boudin, M., and Zagal Venegas, E.: Soil carbon storage controlled by interactions between geochemistry and climate, Nat. Geosci., 8, 780–783, https://doi.org/10.1038/ngeo2516, 2015.
Feng, X., Simpson, A. J., Wilson, K. P., Dudley Williams, D., and Simpson, M. J.: Increased cuticular carbon sequestration and lignin oxidation in response to soil warming, Nat. Geosci., 1, 836–839, https://doi.org/10.1038/ngeo361, 2008.
Ferrari, E., Francioso, O., Nardi, S., Saladini, M., Ferro, N. D., and Morari, F.: DRIFT and HR MAS NMR characterization of humic substances from a soil treated with different organic and mineral fertilizers, J. Mol. Struct., 998, 216–224, https://doi.org/10.1016/j.molstruc.2011.05.035, 2011.
Francioso, O., Montecchio, D., Gioacchini, P., Cavani, L., Ciavatta, C., Trubetskoj, O., and Trubetskaya, O.: Structural differences of Chernozem soil humic acids SEC–PAGE fractions revealed by thermal (TG–DTA) and spectroscopic (DRIFT) analyses, Geoderma, 152, 264–268, https://doi.org/10.1016/j.geoderma.2009.06.011, 2009.
Franklin, O., Högberg, P., Ekblad, A., and Ågren, G. I.: Pine forest floor carbon accumulation in response to N and PK additions: bomb 14C modelling and respiration studies, Ecosystems, 6, 644–658, https://doi.org/10.1007/s10021-002-0149-x, 2003.
Gao, Y., Luo, P., Wu, N., Yi, S., and Chen, H.: Biomass and nitrogen responses to grazing intensity in an alpine meadow on the eastern Tibetan Plateau, Pol. J. Ecol., 55, 469–479, https://doi.org/10.1007/BF02557215, 2007.
García-Palacios, P., Crowther, T. W., Dacal, M., Hartley, I. P., Reinsch, S., Rinnan, R., Rousk, J., van den Hoogen, J., Ye, J.-S., and Bradford, M. A.: Evidence for large microbial-mediated losses of soil carbon under anthropogenic warming, Nat. Rev. Earth Environ., 2, 507–517, https://doi.org/10.1038/s43017-021-00178-4, 2021.
Geisseler, D. and Scow, K.: Long-term effects of mineral fertilizers on soil microorganisms – A review, Soil Biol. Biochem., 75, 54–63, https://doi.org/10.1016/j.soilbio.2014.03.023, 2014.
Genxu, W., Ju, Q., Guodong, C., and Yuanmin, L.: Soil organic carbon pool of grassland soils on the Qinghai-Tibetan Plateau and its global implication, Sci. Total Environ., 291, 207–217, https://doi.org/10.1016/S0048-9697(01)01100-7, 2002.
Golchin, A., Clarke, P., Baldock, J. A., Higashi, T., Skjemstad, J. O., and Oades, J. M.: The effects of vegetation and burning on the chemical composition of soil organic matter in a volcanic ash soil as shown by 13C NMR spectroscopy. I. Whole soil and humic acid fraction, Geoderma, 76, 155–174, https://doi.org/10.1016/S0016-7061(96)00104-8, 1997.
Guan, S., An, N., Zong, N., He, Y., Shi, P., Zhang, J., and He, N.: Climate warming impacts on soil organic carbon fractions and aggregate stability in a Tibetan alpine meadow, Soil Biol. Biochem., 116, 224–236, https://doi.org/10.1016/j.soilbio.2017.10.011, 2018.
He, Y., He, X., Xu, M., Zhang, W., Yang, X., and Huang, S.: Long-term fertilization increases soil organic carbon and alters its chemical composition in three wheat-maize cropping sites across central and south China, Soil Till. Res., 177, 79–87, https://doi.org/10.1016/j.still.2017.11.018, 2018.
Hengl, T., de Jesus, J. M., MacMillan, R. A., Batjes, N. H., Heuvelink, G. B., Ribeiro, E., Samuel-Rosa, A., Kempen, B., Leenaars, J. G., and Walsh, M. G.: SoilGrids1 km – global soil information based on automated mapping, PLoS ONE, 9, e105992, https://doi.org/10.1371/journal.pone.0105992, 2014.
Hicks Pries, C. E., Castanha, C., Porras, R. C., and Torn, M. S.: The whole-soil carbon flux in response to warming, Science, 355, 1420, https://doi.org/10.1126/science.aal1319, 2017.
Hobbie, S. E., Miley, T. A., and Weiss, M. S.: Carbon and nitrogen cycling in soils from acidic and nonacidic tundra with different glacial histories in Northern Alaska, Ecosystems, 5, 0761–0774, https://doi.org/10.1007/s10021-002-0185-6, 2002.
Hou, Y., Chen, Y., Chen, X., He, K., and Zhu, B.: Changes in soil organic matter stability with depth in two alpine ecosystems on the Tibetan Plateau, Geoderma, 351, 153–162, https://doi.org/10.1016/j.geoderma.2019.05.034, 2019.
Janssens, I. A., Dieleman, W., Luyssaert, S., Subke, J. A., Reichstein, M., Ceulemans, R., Ciais, P., Dolman, A. J., Grace, J., Matteucci, G., Papale, D., Piao, S. L., Schulze, E. D., Tang, J., and Law, B. E.: Reduction of forest soil respiration in response to nitrogen deposition, Nat. Geosci., 3, 315–322, https://doi.org/10.1038/ngeo844, 2010.
Jiang, C., Yu, G., Li, Y., Cao, G., Yang, Z., Sheng, W., and Yu, W.: Nutrient resorption of coexistence species in alpine meadow of the Qinghai-Tibetan Plateau explains plant adaptation to nutrient-poor environment, Ecol. Eng., 44, 1–9, https://doi.org/10.1016/j.ecoleng.2012.04.006, 2012.
Keeler, B. L., Hobbie, S. E., and Kellogg, L. E. J. E.: Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition, Ecosystems, 12, 1–15, https://doi.org/10.1007/s10021-008-9199-z, 2009.
Kim, J. H., Jobbágy, E. G., Richter, D. D., Trumbore, S. E., and Jackson, R. B.: Agricultural acceleration of soil carbonate weathering, Global Change Biol., 26, 5988–6002, https://doi.org/10.1111/gcb.15207, 2020.
Kirschbaum, M. U.: Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss?, Global Change Biol., 10, 1870–1877, https://doi.org/10.1111/j.1365-2486.2004.00852.x, 2004.
Komada, T., Anderson, M. R., and Dorfmeier, C. L.: Carbonate removal from coastal sediments for the determination of organic carbon and its isotopic signatures, δ13C and Δ14C: comparison of fumigation and direct acidification by hydrochloric acid, Limnol. Oceanogr.-Meth., 6, 254–262, https://doi.org/10.4319/lom.2008.6.254, 2008.
Lehmann, J. and Kleber, M.: The contentious nature of soil organic matter, Nature, 528, 60–68, https://doi.org/10.1038/nature16069, 2015.
Leifeld, J.: Application of diffuse reflectance FT-IR spectroscopy and partial leastsquares regression to predict NMR properties of soil organic matter, Eur. J. Soil Sci., 57, 846–857, https://doi.org/10.1111/j.1365-2389.2005.00776.x, 2006.
Li, B., He, Y., Wang, Z., Luo, P., and Hong, Q.: Decomposition process and scientific management of yak dung on alpine meadow in the eastern Qinghai-Tibetan Plateau, Pratacultural Sci., 29, 1302–1306, 2012 (in Chinese abstract).
Li, J., Nie, M., Pendall, E., Reich, P. B., Pei, J., Noh, N. J., Zhu, T., Li, B., and Fang, C.: Biogeographic variation in temperature sensitivity of decomposition in forest soils, Global Change Biol., 26, 1873–1885, https://doi.org/10.1111/gcb.14838, 2020.
Li, Q., Tian, Y., Zhang, X., Xu, X., Wang, H., and Kuzyakov, Y.: Labile carbon and nitrogen additions affect soil organic matter decomposition more strongly than temperature, Appl. Soil Ecol., 114, 152–160, https://doi.org/10.1016/j.apsoil.2017.01.009, 2017.
Liang, C. and Balser, T. C.: Warming and nitrogen deposition lessen microbial residue contribution to soil carbon pool, Nat. Commun., 3, 1222, https://doi.org/10.1016/j.apsoil.2017.01.009, 2012.
Liu, X., Zhang, Y., Han, W., Tang, A., Shen, J., Cui, Z., Vitousek, P., Erisman, J. W., Goulding, K., Christie, P., Fangmeier, A., and Zhang, F.: Enhanced nitrogen deposition over China, Nature, 494, 459–462, https://doi.org/10.1038/nature11917, 2013.
Liu, X., Lyu, S., Zhou, S., and Bradshaw, C. J.: Warming and fertilization alter the dilution effect of host diversity on disease severity, Ecology, 97, 1680–1689, https://doi.org/10.1890/15-1784.1, 2016.
Lü, C. and Tian, H.: Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data, J. Geophys. Res., 112, D22S05, https://doi.org/10.1029/2006JD007990, 2007.
Lu, M., Zhou, X., Yang, Q., Li, H., Luo, Y., Fang, C., Chen, J., Yang, X., and Li, B.: Responses of ecosystem carbon cycle to experimental warming: a meta-analysis, Ecology, 94, 726–738, https://doi.org/10.1890/12-0279.1, 2013.
Luo, R., Fan, J., Wang, W., Luo, J., Kuzyakov, Y., He, J.-S., Chu, H., and Ding, W.: Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan Plateau, Sci. Total Environ., 650, 303–312, https://doi.org/10.1016/j.scitotenv.2018.09.038, 2019.
Macias-Fauria, M., Forbes, B. C., Zetterberg, P., and Kumpula, T.: Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems, Nat. Clim. Change, 2, 613–618, https://doi.org/10.1038/nclimate1558, 2012.
Mack, M., Schuur, E., Bret-Harte, M., Shaver, G., and Chapin, F. J. N.: Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization, Nature, 431, 440–443, https://doi.org/10.1038/nature02887, 2004.
Mao, J., Yao, J., Wang, L., and Liu, W.: Easily prepared high-quantum-yield CdS quantum dots in water using hyperbranched polyethylenimine as modifier, J. Colloid Interf. Sci., 319, 353–356, https://doi.org/10.1016/j.jcis.2007.10.027, 2008.
Mathers, N. J., Xu, Z., Berners-Price, S. J., Perera, M. S., and Saffigna, P. G.: Hydrofluoric acid pre-treatment for improving 13C CPMAS NMR spectral quality of forest soils in south-east Queensland, Australia, Soil Res., 40, 665–674, https://doi.org/10.1071/SR01073, 2002.
Melillo, J. M., Steudler, P., Aber, J. D., Newkirk, K., Lux, H., Bowles, F., Catricala, C., Magill, A., Ahrens, T., and Morrisseau, S.: Soil warming and carbon-cycle feedbacks to the climate system, Science, 298, 2173–2176, https://doi.org/10.1126/science.1074153, 2002.
Melillo, J. M., Frey, S. D., Deangelis, K. M., Werner, W. J., Bernard, M. J., Bowles, F. P., Pold, G., Knorr, M. A., and Grandy, A. S.: Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world, Science, 358, 101–105, https://doi.org/10.1126/science.aan2874, 2017.
Meng, J. and Li, X.-A.: Effects of carbonate on the structure and properties of loess and the corresponding mechanism: an experimental study of the Malan loess, Xi'an area, China, B. Eng. Geol. Environ., 78, 4965–4976, https://doi.org/10.1007/s10064-018-01457-z, 2019.
Metcalfe, D. B.: Microbial change in warming soils, Science, 358, 41–42, https://doi.org/10.1126/science.aap7325, 2017.
Micks, P., Aber, J. D., Boone, R. D., and Davidson, E. A.: Short-term soil respiration and nitrogen immobilization response to nitrogen applications in control and nitrogen-enriched temperate forests, Forest Ecol. Manag., 196, 57–70, https://doi.org/10.1016/j.foreco.2004.03.012, 2004.
Mo, J., Zhang, W., Zhu, W., Gundersen, P., Fang, Y., Li, D., and Wang, H.: Nitrogen addition reduces soil respiration in a mature tropical forest in southern China, Global Change Biol., 14, 403–412, https://doi.org/10.1111/j.1365-2486.2007.01503.x, 2008.
Moscatelli, M. C., Lagomarsino, A., De Angelis, P., and Grego, S.: Short-and medium-term contrasting effects of nitrogen fertilization on C and N cycling in a poplar plantation soil, Forest Ecol. Manag., 255, 447–454, https://doi.org/10.1016/j.foreco.2007.09.012, 2008.
Nie, M., Pendall, E., Bell, C., Gasch, C. K., Raut, S., Tamang, S., and Wallenstein, M. D.: Positive climate feedbacks of soil microbial communities in a semi-arid grassland, Ecol. Lett., 16, 234–241, https://doi.org/10.1111/ele.12034, 2013.
Niemeyer, J., Chen, Y., and Bollag, J.-M.: Characterization of humic acids, composts, and peat by diffuse reflectance Fourier-transform infrared spectroscopy, Soil Sci. Soc. Am. J., 56, 135–140, https://doi.org/10.2136/sssaj1992.03615995005600010021x, 1992.
Ofiti, N. O. E., Zosso, C. U., Soong, J. L., Solly, E. F., Torn, M. S., Wiesenberg, G. L. B., and Schmidt, M. W. I.: Warming promotes loss of subsoil carbon through accelerated degradation of plant-derived organic matter, Soil Biol. Biochem., 156, 108185, https://doi.org/10.1016/j.soilbio.2021.108185, 2021.
Olk, D. C., Brunetti, G., and Senesi, N.: Decrease in Humification of Organic Matter with Intensified Lowland Rice Cropping A Wet Chemical and Spectroscopic Investigation, Soil Sci. Soc. Am. J., 64, 1337–1347, https://doi.org/10.2136/sssaj2000.6441337x, 2000.
Paustian, K., Lehmann, J., Ogle, S., Reay, D., Robertson, G. P., and Smith, P.: Climate-smart soils, Nature, 532, 49–57, https://doi.org/10.1038/nature17174, 2016.
Pendall, E., Bridgham, S., Hanson, P. J., Hungate, B., Kicklighter, D. W., Johnson, D. W., Law, B. E., Luo, Y. Q., Megonigal, J. P., Olsrud, M., Ryan, M. G., and Wan, S. Q.: Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models, New Phytol., 162, 311–322, https://doi.org/10.1111/j.1469-8137.2004.01053.x, 2004.
Piao, S., Fang, J., and He, J.: Variations in vegetation net primary production in the Qinghai-Xizang Plateau, China, from 1982 to 1999, Climatic Change, 74, 253–267, https://doi.org/10.1007/s10584-005-6339-8, 2006.
Poeplau, C., Kätterer, T., Leblans, N. I. W., and Sigurdsson, B. D.: Sensitivity of soil carbon fractions and their specific stabilization mechanisms to extreme soil warming in a subarctic grassland, Global Change Biol., 23, 1316–1327, https://doi.org/10.1111/gcb.13491, 2017.
Pold, G., Grandy, A. S., Melillo, J. M., and DeAngelis, K. M.: Changes in substrate availability drive carbon cycle response to chronic warming, Soil Biol. Biochem., 110, 68–78, https://doi.org/10.1016/j.soilbio.2017.03.002, 2017.
Raza, S., Miao, N. A., Wang, P., Ju, X., Chen, Z., Zhou, J., and Kuzyakov, Y.: Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands, Global Change Biol., 26, 3738–3751, https://doi.org/10.1111/gcb.15101, 2020.
Raza, S., Zamanian, K., Ullah, S., Kuzyakov, Y., Virto, I., and Zhou, J.: Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation, J. Clean. Prod., 315, 128036, https://doi.org/10.1016/j.jclepro.2021.128036, 2021.
Riggs, C. E. and Hobbie, S. E.: Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils, Soil Biol. Biochem., 99, 54–65, https://doi.org/10.1016/j.soilbio.2016.04.023, 2016.
Rinnan, R., Michelsen, A., Bååth, E., and Jonasson, S.: Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem, Global Change Biol., 13, 28–39, https://doi.org/10.1111/j.1365-2486.2006.01263.x, 2007.
Romero-Olivares, A. L., Allison, S. D., and Treseder, K. K.: Soil microbes and their response to experimental warming over time: A meta-analysis of field studies, Soil Biol. Biochem., 107, 32–40, https://doi.org/10.1016/j.soilbio.2016.12.026, 2017.
Rowley, M. C., Grand, S., Adatte, T., and Verrecchia, E. P.: A cascading influence of calcium carbonate on the biogeochemistry and pedogenic trajectories of subalpine soils, Switzerland, Geoderma, 361, 114065, https://doi.org/10.1016/j.geoderma.2019.114065, 2020.
Saiya-Cork, K., Sinsabaugh, R., and Zak, D.: The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil, Soil Biol. Biochem., 34, 1309–1315, https://doi.org/10.1016/S0038-0717(02)00074-3, 2002.
Schermelleh-Engel, K., Moosbrugger, H., and Müller, H.: Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures, Methods of Psychological Research Online, 8, 23–74, 2003.
Schmidt, M., Knicker, H., Hatcher, P. G., and Kogel-Knabner, I.: Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10 % hydrofluoric acid, Eur. J. Soil Sci., 48, 319–328, https://doi.org/10.1111/j.1365-2389.1997.tb00552.x, 1997.
Schmidt, M. W., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I. A., Kleber, M., Kögel-Knabner, I., Lehmann, J., and Manning, D. A.: Persistence of soil organic matter as an ecosystem property, Nature, 478, 49–56, https://doi.org/10.1038/nature10386, 2011.
Schuur, E. A., McGuire, A. D., Schädel, C., Grosse, G., Harden, J. W., Hayes, D. J., Hugelius, G., Koven, C. D., Kuhry, P., and Lawrence, D. M.: Climate change and the permafrost carbon feedback, Nature, 520, 171–179, https://doi.org/10.1038/nature14338, 2015.
Simpson, M. J. and Simpson, A. J.: The chemical ecology of soil organic matter molecular constituents, J. Chem. Ecol., 38, 768–784, https://doi.org/10.1007/s10886-012-0122-x, 2012.
Sistla, S. A., Moore, J. C., Simpson, R. T., Gough, L., Shaver, G. R., and Schimel, J. P.: Long-term warming restructures Arctic tundra without changing net soil carbon storage, Nature, 497, 615–618, https://doi.org/10.1038/nature12129, 2013.
Skjemstad, J., Clarke, P., Taylor, J., Oades, J., and Newman, R.: The removal of magnetic materials from surface soils-a solid state 13C CP/MAS NMR study, Soil Res., 32, 1215–1229, https://doi.org/10.1071/SR9941215, 1994.
Song, Y., Song, C., Meng, H., Swarzenski, C. M., Wang, X., and Tan, W.: Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China, Ecol. Eng., 100, 175–185, https://doi.org/10.1016/j.ecoleng.2016.12.025, 2017.
Soong, J. L., Castanha, C., Hicks Pries, C. E., Ofiti, N., Porras, R. C., Riley, W. J., Schmidt, M. W., and Torn, M. S.: Five years of whole-soil warming led to loss of subsoil carbon stocks and increased CO2 efflux, Sci. Adv., 7, eabd1343, https://doi.org/10.1126/sciadv.abd1343, 2021.
Spaccini, R., Mbagwu, J., Conte, P., and Piccolo, A.: Changes of humic substances characteristics from forested to cultivated soils in Ethiopia, Geoderma, 132, 9–19, https://doi.org/10.1016/j.geoderma.2005.04.015, 2006.
Sun, H., Jiang, J., Cui, L., Feng, W., Wang, Y., and Zhang, J.: Soil organic carbon stabilization mechanisms in a subtropical mangrove and salt marsh ecosystems, Sci. Total Environ., 673, 502–510, https://doi.org/10.1016/j.scitotenv.2019.04.122, 2019.
Sun, H., Chen, H., Li, J., Zhang, Y., Liu, X., Li, J., and Nie, M.: Nitrogen enrichment enhances thermal acclimation of soil microbial respiration, Biogeochemistry, 162, 343–357, https://doi.org/10.1007/s10533-023-01014-1, 2023a.
Sun, H., Schmidt, M. W. I., Li, J., Li, J., Liu, X., Ofiti, N. O. E., Zhou, S., and Nie, M.: Nine years of warming and nitrogen addition in the Tibetan grassland promoted loss of soil organic carbon but did not alter the bulk change of chemical structure, Zenodo [data set], https://doi.org/10.5281/zenodo.8289311, 2023.
Tian, D. and Niu, S.: A global analysis of soil acidification caused by nitrogen addition, Environ. Res. Lett., 10, 024019, https://doi.org/10.1088/1748-9326/10/2/024019, 2015.
Treseder, K. K.: Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies, Ecol. Lett., 11, 1111–1120, https://doi.org/10.1111/j.1461-0248.2008.01230.x, 2008.
Tucker, C. L., Bell, J., Pendall, E., and Ogle, K.: Does declining carbon-use efficiency explain thermal acclimation of soil respiration with warming?, Global Change Biol., 19, 252–263, https://doi.org/10.1111/gcb.12036, 2013.
Ussiri, D. A. and Johnson, C. E.: Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods, Geoderma, 111, 123–149, https://doi.org/10.1016/S0016-7061(02)00257-4, 2003.
Van Gestel, N., Shi, Z., Van Groenigen, K. J., Osenberg, C. W., Andresen, L. C., Dukes, J. S., Hovenden, M. J., Luo, Y., Michelsen, A., and Pendall, E.: Predicting soil carbon loss with warming, Nature, 554, E4–E5, https://doi.org/10.1038/nature20150, 2018.
Vance, E. D., Brookes, P. C., and Jenkinson, D. S.: An extraction method for measuring soil microbial biomass C, Soil Biol. Biochem., 19, 703–707, https://doi.org/10.1016/0038-0717(87)90052-6, 1987.
Vaughn, L. J. and Torn, M. S.: 14C evidence that millennial and fast-cycling soil carbon are equally sensitive to warming, Nat. Clim. Change, 9, 467–471, https://doi.org/10.1038/s41558-019-0468-y, 2019.
von Lützow, M. and Kögel-Knabner, I.: Temperature sensitivity of soil organic matter decomposition – what do we know?, Biol. Fert. Soils, 46, 1–15, https://doi.org/10.1007/s00374-009-0413-8, 2009.
Walter, K., Don, A., Tiemeyer, B., and Freibauer, A.: Determining Soil Bulk Density for Carbon Stock Calculations: A Systematic Method Comparison, Soil Sci. Soc. Am. J., 80, 579–591, https://doi.org/10.2136/sssaj2015.11.0407, 2016.
Wang, C., He, N., Zhang, J., Lv, Y., and Wang, L.: Long-term grazing exclusion improves the composition and stability of soil organic matter in Inner Mongolian grasslands, PLoS ONE, 10, e0128837, https://doi.org/10.1371/journal.pone.0128837, 2015.
Wang, H., Liu, S.-R., Mo, J.-M., Wang, J.-X., Makeschin, F., and Wolff, M.: Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China, Ecol. Res., 25, 1071–1079, https://doi.org/10.1007/s11284-010-0730-2, 2010.
Wang, J., Song, B., Ma, F., Tian, D., Li, Y., Yan, T., Quan, Q., Zhang, F., Li, Z., Wang, B., Gao, Q., Chen, W., and Niu, S.: Nitrogen addition reduces soil respiration but increases the relative contribution of heterotrophic component in an alpine meadow, Funct. Ecol., 33, 2239–2253, https://doi.org/10.1111/1365-2435.13433, 2019.
Wang, J.-J., Bowden, R. D., Lajtha, K., Washko, S. E., Wurzbacher, S. J., and Simpson, M. J.: Long-term nitrogen addition suppresses microbial degradation, enhances soil carbon storage, and alters the molecular composition of soil organic matter, Biogeochemistry, 142, 299–313, https://doi.org/10.1007/s10533-018-00535-4, 2019.
Wu, J., Lin, H., Meng, C., Jiang, P., and Fu, W.: Effects of intercropping grasses on soil organic carbon and microbial community functional diversity under Chinese hickory (Carya cathayensis Sarg.) stands, Soil Res., 52, 575–583, https://doi.org/10.1071/SR14021, 2014.
Xiao, J., Dong, S., Zhao, Z., Han, Y., Li, S., Shen, H., and Ding, C.: Stabilization of soil organic carbon in the alpine meadow is dependent on the nitrogen deposition level on the Qinghai-Tibetan Plateau, Ecol. Eng., 170, 106348, https://doi.org/10.1016/j.ecoleng.2021.106348, 2021.
Xue, K., Yuan, M. M., Shi, Z. J., Qin, Y., Deng, Y., Cheng, L., Wu, L., He, Z., Van Nostrand, J. D., and Bracho, R.: Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming, Nat. Clim. Change, 6, 595–600, https://doi.org/10.1038/nclimate2940, 2016.
Yang, Y., Fang, J., Tang, Y., Ji, C., Zheng, C., He, J., and Zhu, B.: Storage, patterns and controls of soil organic carbon in the Tibetan grasslands, Global Change Biol., 14, 1592–1599, https://doi.org/10.1111/j.1365-2486.2008.01591.x, 2008.
Yu, G., Jia, Y., He, N., Zhu, J., Chen, Z., Wang, Q., Piao, S., Liu, X., He, H., Guo, X., Wen, Z., Li, P., Ding, G., and Goulding, K.: Stabilization of atmospheric nitrogen deposition in China over the past decade, Nat. Geosci., 12, 424–429, https://doi.org/10.1038/s41561-019-0352-4, 2019.
Zamanian, K., Zarebanadkouki, M., and Kuzyakov, Y.: Nitrogen fertilization raises CO2 efflux from inorganic carbon: a global assessment, Global Change Biol., 24, 2810–2817, https://doi.org/10.1111/gcb.14148, 2018.
Zhang, H. R. and Fu, G.: Responses of plant, soil bacterial and fungal communities to grazing vary with pasture seasons and grassland types, northern Tibet, Land Degrad. Dev., 32, 1821–1832, https://doi.org/10.1002/ldr.3835, 2020.
Zhang, Q., Feng, J., Li, J., Huang, C.-Y., Shen, Y., Cheng, W., and Zhu, B.: A distinct sensitivity to priming effect between labile and stable soil organic carbon, New Phytol., 237, 88–99, https://doi.org/10.1111/nph.18458, 2022.
Zhang, T., Li, Y., Chang, S. X., Jiang, P., Zhou, G., Liu, J., and Lin, L.: Converting paddy fields to Lei bamboo (Phyllostachys praecox) stands affected soil nutrient concentrations, labile organic carbon pools, and organic carbon chemical compositions, Plant Soil, 367, 249–261, https://doi.org/10.1007/s11104-012-1551-6, 2013.
Zhou, L., Zhou, X., Zhang, B., Lu, M., Luo, Y., Liu, L., and Li, B.: Different responses of soil respiration and its components to nitrogen addition among biomes: a meta-analysis, Global Change Biol., 20, 2332–2343, https://doi.org/10.1111/gcb.12490, 2014.