the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Bacteria as paleoenvironmental proxies: the study of a cave Pleistocene profile
Cătălina Haidău
Ionuţ Cornel Mirea
Silviu Constantin
Oana Teodora Moldovan
Caves are well-known archives that preserve valuable information about the past, relevant for reconstructing past climates and environments. We sampled sediments from a 480 cm deep profile, and 16S ribosomal ribonucleic acid (rRNA) gene-based metabarcoding analyses were undertaken that complemented lithological logging, sedimentology, and optically stimulated luminescence (OSL) dating. These analyses revealed different sedimentation conditions along the profile with various water inputs. The OSL age of the sediments places the profile between 74.7 ± 12.3 to 56 ± 8 ka (base to top). However, the more recent Last Glacial Maximum (LGM) paleofloods might have occurred in the upper and lower passages of the cave. Bacterial compositions changed with depth, from soil bacteria (present in the upper part of the sediment profile) to thermophilic/sulfurous bacteria (abundant in the deeper samples of the profile). Considering the thermophilic bacteria, we could only assume their origin from a surface of hot sulfurous springs, old thermal springs, or sapropel sediments.
- Article
(4812 KB) - Full-text XML
- BibTeX
- EndNote
Caves are known archives that preserve valuable information about climate in deposits and are relevant for the reconstruction of past climate and past environments (e.g., White, 2007; Nejman et al., 2018; Constantin et al., 2021; Bernal et al., 2023; Campaña et al., 2023). Caves are also known as systems with no primary production due to the lack of plants, low nutrient input from the surface, and low in situ production (Howarth and Moldovan, 2018; Kosznik-Kwaśnicka et al., 2022). That also means that the number of biological proxies (fossil bones, fossil invertebrates, pollen) in caves to be used in paleoenvironmental and paleoclimatic studies is relatively low, and they might even be absent (Moldovan et al., 2011, 2016). Therefore, studying bacteria in cave deposits can open an avenue for paleoenvironmental research and fill some gaps related to presumed past processes in the absence of reliable proxies.
Bacteria are crucial in oligotrophic environments such as caves, as they can be primary producers and participate actively in biogeochemical cycles (Talà et al., 2021; Zada et al., 2021; Lange-Enyedi et al., 2022). Microorganisms regulate essential ecosystem processes such as the biogeochemical cycling of micro- and macronutrients (Uroz et al., 2009; Pester et al., 2012) or are implicated in the formation (Yarwood, 2018; Domeignoz-Horta et al., 2021) or decomposition of organic matter (Krishna and Mohan, 2017; Prescott and Vesterdal, 2021). They can adapt and survive depending on abiotic and biotic factors (litter inputs, moisture, temperature; Castro et al., 2010; Wani et al., 2022).
Bacterial community and structure can change over time due to modifications in the physicochemical components of an ecosystem, leading to the development of biogeographical patterns (Malard et al., 2019; Thomas et al., 2019; Bay et al., 2020; Ji et al., 2020). These patterns serve as indicators of past environmental changes. For example, the structure of bacterial communities present at deposition becomes preserved in sediment layers formed under changing environmental conditions (Frindte et al., 2020; Semenov et al., 2020; Barbato et al., 2022). Thus, such environmental conditions could be traced by reconstructing bacterial community structures from different sediment layers (Thomas et al., 2019; Frindte et al., 2020; Semenov et al., 2020; Barbato et al., 2022).
Microbial paleoenvironmental studies on soil and lake/sea sediments are more common than in caves, highlighting the importance of their diversity as an indicator of ecosystem function and environmental conditions for reconstructing the past. For instance, Xu et al. (2022) found microbial communities in lacustrine sediments that provided valuable insights into past environmental and climate changes. Influenced by abrupt environmental shifts, the distinct vertical trends in microbial community structures suggest that these communities responded dynamically to climatic events, such as aridification and cooling, around 8 million years ago. These shifts are also consistent with previous pollen evidence, indicating a transition from forest to steppe vegetation correlated with a significant uplift of the Tibetan Plateau. More et al. (2019) examined the microbial communities in the Black Sea sediments in response to substantial paleoenvironmental changes, mainly focusing on the transition around 5.2 ka. This study highlights bacterial composition changes driven by increased salinity. The research also underlines key microbial metabolic processes, shifting from methane metabolism before 5.2 ka to enhanced nitrogen and sulfur metabolisms. These changes correspond with the establishment of modern conditions in the Black Sea.
A study on paleosols (Frindte et al., 2020) that analyzed environmental DNA from different horizons within an arid paleosequence revealed key changes in microbial communities over time. The findings indicate bacterial abundance, diversity, and community composition decline with increasing soil depth and age. However, deviations from this trend were observed in paleosol horizons with higher microbial diversity and abundance, suggesting that advanced soil formation processes may have preserved more diverse microbial communities. The study also identified specific microbial taxa associated with certain soil horizons, proving that some microbial communities from ancient environments remain detectable despite burial.
Regarding the caves, most studies on paleoenvironment focused on proxies such as stable isotopes (Waltgenbach et al., 2021; Weber et al., 2021), fossil bones (Berto et al., 2021; Mirea et al., 2021; Cruz et al., 2023), fossil invertebrates (Moldovan et al., 2011, 2016; Buttler and Wilson, 2018; Romano et al., 2024; Osipova et al., 2024), or pollen (Prieto et al., 2021; Minckley et al., 2023).
Studies on cave microorganisms were performed regarding their diversity (Zhu et al., 2019; Dong et al., 2020; Dominguez-Moñino et al., 2021) and associations (Dattagupta et al., 2009; Ma et al., 2021; Zhao et al., 2024), but little attention was given to their potential as paleoclimate proxies (Epure et al., 2014, 2017; Yun et al., 2016). Furthermore, Epure et al. (2014, 2017) indicated the potential of microorganisms from old cave sediment deposits in paleoenvironment and paleoclimate reconstruction. Zepeda Mendoza et al. (2016) explored the microbial communities within a speleothem, indicating their potential as past biodiversity archives. Metagenomic analysis on a speleothem in a cave near the sea found microorganisms related to soil and marine environments. Michail et al. (2021) revealed a complex and dynamic microbial community from a stalactite core composed of bacteria from the upper-ground environment. As indicated by specific bacteria, the evidence of seasonal climate variations emphasizes environmental factors' role in shaping microbial composition over time. Overall, this research provided valuable insights into the microbial ecology of cave environments and highlights the need for further investigation into the role of microorganisms in cave deposits and paleoclimate reconstruction.
The scope of our study was to investigate the bacterial diversity from a 480 cm deep profile in Muierilor Cave, Romania, where no other biological proxy was found. This cave was studied for its evolution during the last 120 kyr. The combined optically stimulated luminescence (OSL), AMS14C, and sedimentology results, together with taphonomical analysis of the Pleistocene mammals' accumulation, indicated that most cave levels were already formed ∼ 120 ka, with the lower levels functioning periodically as vadose cave passages where sediments from the Galbenu River were deposited (Mirea et al., 2021). The bacteria identified through the 16S ribosomal ribonucleic acid (rRNA) gene-based metabarcoding were also compared to other proxies to help define past environments. Thermophiles and sulfur bacteria were amongst the high-abundance bacteria with depth, which raised questions about their occurrence, since the cave is characterized by a temperature much lower than their growth range. The possible sources of our samples are discussed, and the results strongly support the importance of investigating bacteria in old sediments, especially in the absence of other biological proxies. When cross-correlated with other proxies, our findings indicate the deposition conditions and water sources during the Pleistocene/Holocene, bringing new insights into the regional karst evolution.
2.1 Site description and sampling
Muierilor Cave (45°11′31.78′′ N, 23°45′14.07′′ E) is located at ∼ 645 m a.s.l. in Baia de Fier, southwestern Romania, being one of the most visited show caves in the country due to its archeological, paleontological, and mineralogical features (Fig. 1b). It is formed in the Polovragi–Cernădia area (Parâng Mountains complex, Southern Carpathians), where the basement is a combination of metamorphic pre-Alpine formation and granitic bodies (Hann et al., 1986), while the sedimentary deposits are represented by a mix of Upper Paleozoic and Mesozoic limestones and conglomerates along with Cenozoic deposits (gravel, sand, and clay) (Fig. 1a). The limestones in this region belong to the Oslea-Polovragi formation, made of white-gray and white limestones that can reach a thickness of 150–250 m, covering a surface of approximately 2 km2 (Bandrabur and Bandrabur, 2010; Mirea et al., 2021). Muierilor Cave is developed in Upper Jurassic–Lower Cretaceous limestone on the right side of the Galbenul Gorge. The cave system has four distinct levels, with a total length of more than 8000 m, and its cave levels are extended on an elevation range of ∼ 80 m. The significant parts of the cave include the Scientific Reserve (Level 1) and the Touristic Passage (Level 2) (Mirea et al., 2021). The fossil record in Muierilor Cave is rich, and the long history of excavations (1950–2021) of the upper and lower levels of the cave revealed numerous species. The most significant fossil accumulation is in the Urşilor Passage because of primary and secondary thanatocoenosis (Mirea et al., 2021). The highest density of the fossil remains (∼ 200 bones m2) is reported near the PMP1 excavation site and decreases to no fossils towards the PMP2 test pit (Fig. 1d.).
For this study, we sampled in PMP2 (Figs. 1d and 2), a test pit of m and with a depth of 480 cm located at the northern end of the Urşilor Passage near the restricted entrance (probably an ancient siphon) towards the Hades Passage (Mirea et al., 2021). There is no evidence of present or former percolation in this cave part: only an important accumulation of finer sediments than in PMP1 before the morphologically restricted entrance towards the Hades Passage.
Muierilor Cave is located in the Măgura Hill with forest (broad-leaved forest and mixed forest) and small pasture patches, where few agricultural activities are present (e.g., small herds of grazing cattle). Tourism activities occur mostly in the gorges and in the tourist part of the cave; there are no designed tourist paths above the cave. The few urban settlements are lower in altitude than the cave passages (developing around ∼ 400 m.a.s.l.). Therefore, land use and potential anthropogenic influences are absent or very limited.
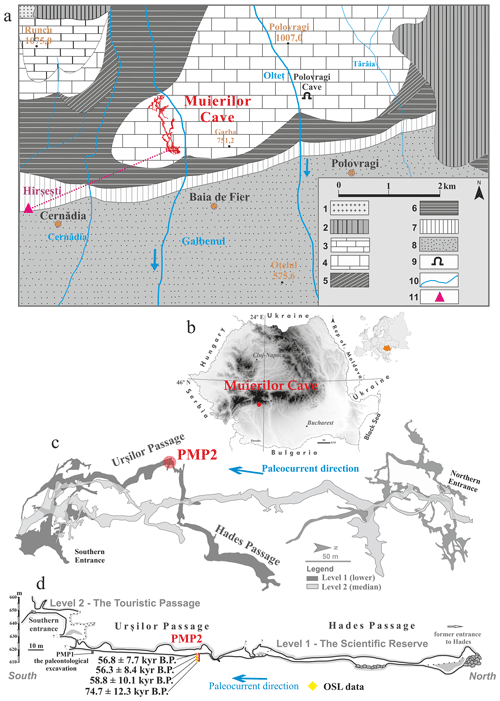
Figure 1The location of Muierilor Cave and details on the studied profile inside the cave. (a) Geological map of the Polovragi–Cernădia area (modified after Diaconu et al., 2008): 1 – Parâng Granites; 2 – metamorphic rocks; 3 – Early Jurassic (limestone); 4 – Late Jurassic (limestone); 5 – Late Cretaceous (conglomerates, sandstones, and clays); 6 – Early Miocene (marly clays); 7 – Middle Miocene (sands and clays); 8 – Late Miocene (gravels and sands); 9 – caves; 10 – rivers; 11 – location of the hot springs near Muierilor Cave (according to Ghenea et al., 1981). (b) Location of the studied cave in Romania; (c) a simplified map of the Muierilor Cave surveyed by the Emil Racovita Institute of Speleology and “Hades” Caving Club (base map courtesy of Grigore Stelian); (d) PMP2 profile with the OSL results (modified after Mirea et al., 2021).
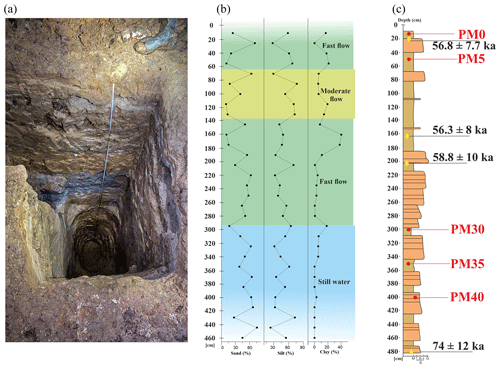
Figure 2The analyzed deposits in Muierilor Cave: (a) photo of the PMP2 section in Urşilor Passage seen from above, (b) the grain size measurements (modified after Mirea, 2020, and Mirea et al., 2021), and (c) the position of the samples (red) and the OSL ages (yellow) on the profile (modified after Mirea, 2020, and Mirea et al., 2021).
2.2 Sediment analysis and chronological framework
For the PMP2 profile, Mirea et al. (2021) conducted on-site lithological logging and grain size analyses of sediments. Optically stimulated luminescence (OSL) was used to constrain the sediment input and deposition events in the cave passages.
The PMP2 profile was excavated for sedimentology studies. It has a complex structure with sand, silts, and clay levels. It is located at the limit between the Urşilor and Hades passages (Fig. 1). Between 150 and 300 cm in depth, the sediments alternate between sand and silt (Fig. 2), while higher amounts of clay appear in the upper sector, indicating a change in the source area. In contrast, in the topmost part, alternating clay and sand, suggests the persistence of high-energy streams. The anisotropy of magnetic susceptibility (AMS) data (Fig. 2) showed that, between 420 and 300 cm in depth, the sediments were deposited from still water (Tauxe et al., 1998), most likely due to a small lateral lake formed on the main cave stream. The following 150 cm (between 300 and 150 cm) is characterized by deposition under a high-energy current flow, a moderate-flow current deposited the segment between 150 to 50 cm, and the last 50 cm shows a deposition in high, moderate currents (i.e., no particle entrainment). We therefore assume that the general flow direction was NE–SW with possible “apparent reversals”, such as those due to vortex-type flows generated by cave wall topography.
The base of the sediments in the PMP2 profile has an OSL age of 74.7 ± 12.3 ka (Fig. 1d), while the upper 2 m of the section has a significantly younger OSL age of around 58 ka.
Mirea et al. (2021) thoroughly studied the sediment deposition processes in Muierilor Cave, which highlighted some paleoflood events that had occurred during several time intervals, coinciding with warmer and wetter conditions that led to increased water input into the cave passages.
2.3 Sampling for DNA, extraction, and sequencing
For the microbiome analysis, sediment samples were taken directly into sterile Falcon tubes every 50 cm in the PMP2 profile. To avoid contamination, before sampling, the first few centimeters from the sediment surface were removed with the use of a sterilized utensil, and the sediment was taken directly with a sterile Falcon tube. Sediment samples analyzed in this study were taken from the surface of the pit (PM0) and at −50 cm (PM5), −300 cm (PM30), −350 cm (PM35), and −400 cm (PM40) deep. Not enough genetic material for metagenomics could be extracted for the samples at −100, −150, −200, and −250 cm, most likely due to the high amount of clay or other inhibitors we could not remove. Clay is known for inhibiting microorganisms (McMahon et al., 2016). The samples were transported for further laboratory analysis in an icebox and kept in the freezer at −60 °C until extraction. A quantity of 25 mg of sediment was used for DNA extraction.
We used FastPrep-24TM (MP Biomedicals) for cell disruption, and DNeasy PowerSoil (QIAGEN) was used for genomic DNA extraction, according to the manufacturer's instructions. DNA was extracted in duplicates and was quantified using SpectraMax QuickDrop (Molecular Devices). Extracted DNA was used as a template and sent for MiSeq 16S V3–V4 metagenome sequencing using a commercial company (Macrogen Europe). PCR of the V3–V4 hypervariable regions of the bacterial and archaeal SSU rRNA gene was performed using bacteria-specific primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′), according to Illumina's 16S amplicon-based metagenomic sequencing protocol.
2.4 Metabarcoding analysis and tests
Metabarcoding analysis was performed by a commercial company (Macrogen Europe) as follows: samples were analyzed using CD-HIT-OTU (Li et al., 2012) and RDnaTools (Schloss et al., 2009). Merging pairs of short reads was performed with FLASH (1.2.11) (Magoč and Salzberg, 2011). This is designed to merge pairs of reads when the original DNA fragments are shorter than twice the length of reads. CD-HIT-OTU is a multi-step pipeline to generate OTU clusters for ribosomal ribonucleic acid (rRNA) tags from 454 sequencing and Illumina platforms. CD-HIT-OTU and RDnaTools were used to filter out short reads and extra-long tails; filtered reads were clustered at 100 % identity using CD-HIT-DUP. Chimeras were identified and removed. Remaining representative reads from non-chimeric clusters are clustered into OTUs at 97 % OTU cutoff. Forward and reverse primers were removed, and, for further analysis, reads with a minimum length of 250 nt and a maximum length of 301 nt were retained. The sequencing depth varied between 79 534 and 126 869 sequences per sample, with a median of 112 595. The final dataset consisted of a total of 2692 OTU from 9 samples.
Taxonomic assignment and diversity statistics were performed by QIIME-UCLUST using NCBI targeted loci project databases 16S RefSeq version 20211127. Representative sequences from each OTU were used to assign taxonomy from phylum to species levels. We acknowledge that this approach has limitations in resolving closely related species due to the short read lengths and the conserved nature of the targeted regions. Challenges have been documented in the literature (Bailén et al., 2020; Gehrig et al., 2022; Satam et al., 2023), and we recognize that alternative sequencing approaches, such as full-length 16S rRNA gene sequencing, may provide improved taxonomic resolution.
We used the updated nomenclature for the Prokaryota taxonomic names according to Oren and Garrity (2021) and Oren et al. (2022).
The raw data were deposited in the NCBI Sequence Read Archive (SRA) under the BioProject PRJNA1161469, with BioSample accessions: SAMN43780924, SAMN43780925, SAMN43780926, SAMN43780927, SAMN43780928, SAMN43780929, SAMN43780930, SAMN43780931, and SAMN43780932.
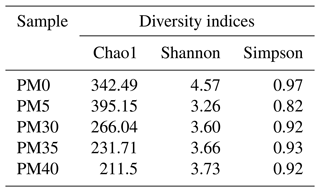
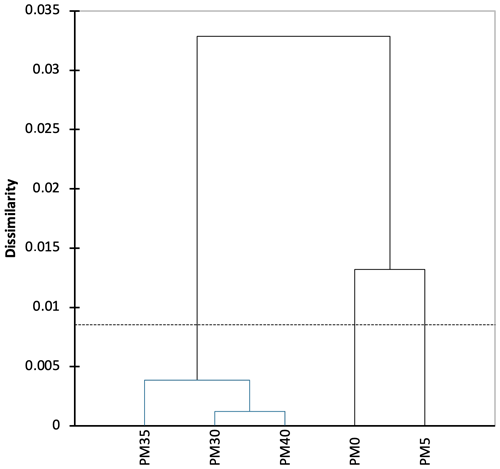
Using phyloseq (McMurdie and Holmes, 2013) in R, alpha diversity indices were calculated and differences in community composition were evaluated. The tax_glom function (taxonomic agglomeration; McMurdie and Holmes, 2013) generated counts and relative abundances at phylum, family, genus, and species levels. Alpha diversity indices, such as Shannon, Chao1, and Simpson, were calculated and used to express information about the composition of samples. Shannon considers the weight of each species in an ecosystem and gives a better description of its diversity (Konopiński, 2020). Simpson's diversity index estimates the probability that two randomly selected individuals will be identical in a sample. The less diversity, the greater the likelihood that two randomly chosen individuals will be the same species (Simpson, 1949; Zhou et al., 2020). Moreover, an abundance-based estimator of species richness, Chao1 index, was also calculated (Kim et al., 2017). To compare the bacteria diversity in the five analyzed sediment samples of the PMP2 profile, we applied agglomerative hierarchical clustering (AHC) in XLSTAT (version 2024.2.2) based on the dissimilarity matrix generated with the 256 most abundant species.
3.1 Bacteria composition in the sedimentary profile
We identified Bacteria and Archaea in the five sediment samples we analyzed from the profile at 0, −50, −300, −350, and −400 cm. This diversity was observed only at five out of the nine levels we examined; the rest of the samples did not provide enough DNA.
Only the domain Bacteria was kept for further analysis of microbial composition because the abundance of Archaea was very low (under 0.2 %), with only one species (the ammonia-oxidizing Nitrosopumilus ureiphilus) present in PM0, PM30, PM35, and PM40. Except for PM40, the other samples provided enough material for duplicates (PM0, PM5, PM30, PM35), for which the mean abundances were used for further analysis.
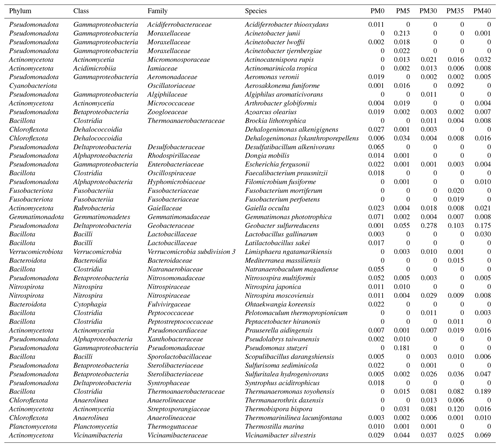
From 2692 Bacteria OTUs, those with an abundance of over 1 % were used for further analysis (see also Table A1). A total of 10 major bacterial phyla were identified in our samples (Fig. 3a), with Pseudomonadota (22 %–62 %) being the most abundant in all samples, followed by Bacillota (PM0–20 %; PM35–23 %; PM40–30 %) and Actinomycetota (PM0–10 %; PM5–13 %; PM30–20 %; PM40–17 %). The relative abundance of Pseudomonadota decreased with depth, while the relative abundances of Bacillota and Actinomycetota increased. Cyanobacteriota appeared in surprising relative abundance in PM35 (9 %).
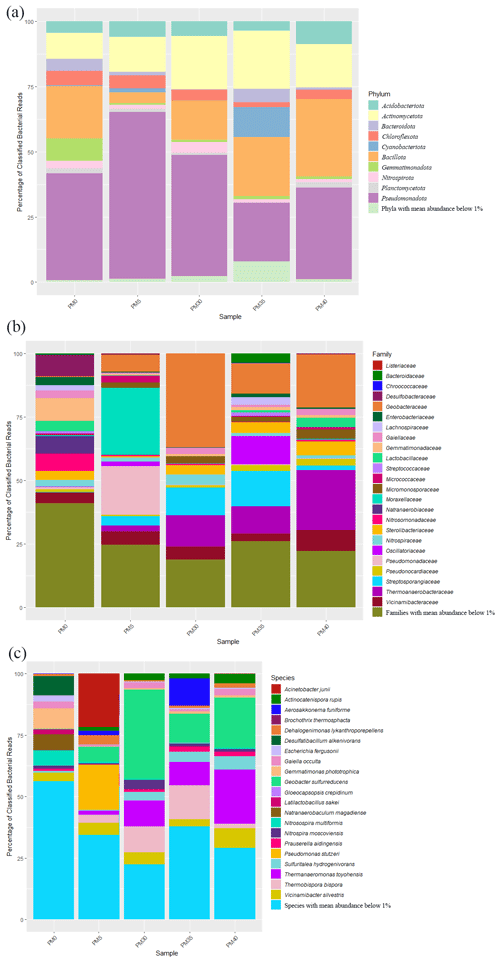
Figure 3The relative abundance of phyla (a), families (b), and species (c) in the analyzed sediment samples of Muierilor Cave (abundance >1 %).
The family composition (Fig. 3b) differed when comparing the upper with the deeper samples. In PM0, Gemmatimonadaceae and Desulfobacteraceae (8 %) and Natranaerobiaceae (6 %) were the most abundant, while, in PM5, Moraxellaceae (26 %), Pseudomonadaceae (19 %), and Geobacteraceae (6 %) were the most abundant. With deeper samples, the family relative abundances were quite similar, with Geobacteraceae (PM30−36 %; PM35−12 %; PM40−20 %), Thermoanaerobacteraceae (PM30−12 %; PM35−11 %; PM40−23 %), and Sterolibacteriaceae (PM30−3 %; PM35−5 %; PM40−5 %) amongst the highest. Streptosporangiaceae (PM30-11 %; PM35−14 %; PM40−2 %) and Vicinamibacteraceae (PM30−5 %; PM35−3 %; PM40−8 %) were present in the highest abundance in the two samples. Oscillatoriaceae was the third highest in PM35 (11 %; very low in the other samples), while Sterolibacteriaceae was the third highest in PM40 (5 %; PM30−4 %; PM35−5 %).
The most abundant species (Fig. 3c) from surface sample PM0 were Gemmatimonas phototrophica (7 %), Desulfatibacillum alkenivorans (7 %), and Natranaerobaculum magadiense (6 %), while, in PM5 (−50 cm), Acinetobacter junii (21 %), Pseudomonas stutzeri (18 %), and Geobacter sulfurreducens (6 %) were the most abundant. As in the case of family abundances, species relative abundances are similar in deeper samples, with Geobacter sulfurreducens (PM30−28 %; PM35−10 %; PM40−18 %) and Thermanaeromonas toyohensis (PM30, PM35−8 %; PM40−19 %) present in all three samples with high abundances. Thermobispora bispora (PM30−8 %; PM35−12 %) was in high abundance in the first two bottom samples (−300, −350 cm) while Sulfuritalea hydrogenivorans was in high abundance in the last two bottom samples (PM35−4 %; PM40−5 %; −350, −400 cm). Also, Nitrospira moscoviensis was found in high abundance in PM30 (3 %; PM35, PM40−0.1 %), and Actinocatenispora rupis was found in high abundance in PM40 (3 %; PM30, PM35−2 %). Vicinamibacter silvestris was one of the most abundant bacteria throughout our profile (3 %–7 %). A cyanobacterium Aerosakkonema funiforme in high abundance in PM35 (9 %; PM0−0.1 %; PM5−1 %; PM30−0.04 %) was absent in PM40.
Chao1 (Table 1) indicated that species richness was relatively higher in the surface samples (PM0 and PM5) than in deeper samples (PM30, PM35, PM40). The Shannon and Simpson diversity indices showed that surface samples (PM0) had a more diverse bacterial community than deeper samples (PM5, PM30, PM35, PM40).
3.2 The distribution of bacteria in the sedimentary profile with depth/age
During our investigation of bacterial distribution with depth in the sedimentary profile (Figs. 3–6), we found a diverse range of species associated with biogeochemical cycles, particularly sulfur (S) and iron (Fe). Additionally, we identified species specific to various environments, including soil, water, and even human-associated habitats, and those adapted to extreme conditions, such as thermophilic and halophilic environments.
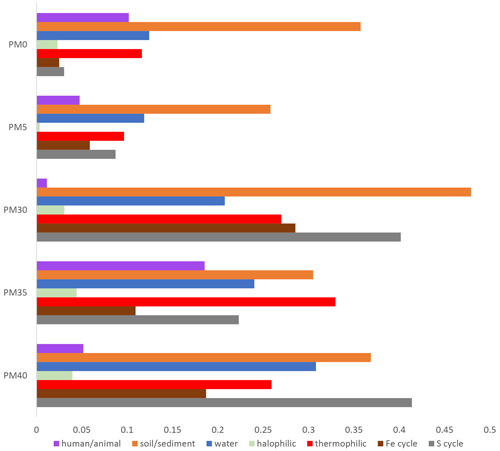
Figure 5Distribution of bacteria categories with depth in the PMP2 sediment profile of Muierilor Cave.
The AHC analysis shows a strong separation of samples PM0 and PM5 from the other samples in the profile (Fig. 4) and a significant separation of the first two samples. The three lower samples are separated from each other at lesser significance.
The family and species abundance in the profile's first two depths (PM0, PM5) comprised bacteria commonly found in surface environments. Since some of them were identified throughout the profile, the plausible explanation is the input from the surface. For example, Roseisolibacter agri, an agricultural soil bacterium (Pascual et al., 2018), was found only in PM5 and PM35. Moreover, the presence of halophilic and halotolerant bacteria (Aidingibacillus halophilus in PM30 and PM40, Algiphilus aromaticivorans in PM5 and PM30, and Halomonas lactosivorans and Saccharopolyspora deserti in all samples) could be linked to the rhizosphere because it may have plant-growth-promoting characteristics (Reang et al., 2022). Furthermore, another link to the surface environments might be the presence of animal- or human-related bacteria. Such bacteria were found in higher abundance in PM0 and were very low or absent in the other samples. For instance, Escherichia fergusonii, which causes diseases in humans and animals (Gaastra et al., 2014), was found in all samples, with the highest abundance in PM0 (2 %; and under 0.4 % in the other samples); human gut bacteria (Pianta et al., 2017; Hosomi et al., 2022), such as Blautia wexlerae and Prevotella copri, were found in low abundance in PM0 and PM35, while Megamonas funiformis, previously found in human faeces (Sakon et al., 2008), was found in PM0, PM5, and PM35.
The levels with bacteria related to slow flow or still water, while, from the fast-flow levels, no DNA could be extracted.
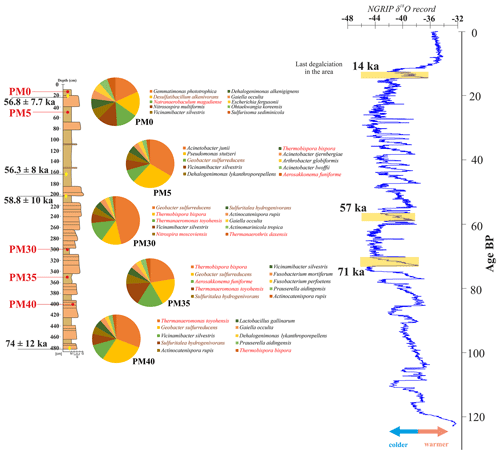
Figure 6The distribution of bacteria at different depths in the analyzed Muierilor Cave deposits (left) compared to the isotopic oxygen and temperature variations (right; modified after North Greenland Ice Core Project, 2004); only the first 10 most abundant species in each sample (red, thermophiles; brown, involved in the S cycle) are represented.
The identified bacteria species from the first two sediment samples (PM0 and PM5 at 0 and −50 cm, respectively) are common in soils, indicating a direct input from the surface during the last fast-flow episode(s). Gemmatimonadaceae, Desulfobacteraceae, and Natranaerobiaceae were the most abundant families in PM0 and were commonly found in water, marine sediments, and soil (Chee-Sanford et al., 2019). Representatives of Gemmatimonadaceae, involved in the N cycle, are soil species (Gemmatimonas aurantiaca; Chee-Sanford et al., 2019) commonly found in agricultural soils (G. kalamazoonesis; Jia et al., 2022) or are phototrophic (G. phototrophica; Koblížek et al., 2020). Desulfobacteraceae representatives were isolated from oil-polluted sediments, being involved in the S cycle (Desulfatibacillum alkenivorans, Desulfatiferula berrensis; Hakil et al., 2014; Ding et al., 2024), and Natranaerobaculum magadiense from Natranaerobiaceae was isolated from soda lake sediments, and it is obligately alkaliphilic, anaerobic, thermotolerant, and halotolerant (Zavarzina et al., 2013).
In PM5, representatives of one of the most abundant families (Moraxellaceae), such as Acinetobacter tjernbergiae, were commonly isolated from activated sludges (Yang, 2014), being involved in the P cycle, and may have potential applications in the biological removal of phosphates (Täuber et al., 2022). Psychrobacter aquimaris is a halophilic bacterium isolated from seawater (Zhang et al., 2021). Denitrifying bacteria of Pseudomonadaceae were found in our samples (Pseudomonas stutzeri; Feng et al., 2020) along with others previously isolated from marine sediments, presenting an antagonistic activity (nitrification; P. glareae; Romanenko et al., 2015). Geobacteraceae representatives were mostly isolated from sediments except for Geoalkalibacter subterraneus, which was isolated from petroleum reservoir water (Greene et al., 2009). Representatives of this family are metal reducers such as G. subterraneus, an anaerobic Fe(III)- and Mn(IV)-reducing bacterium (Greene et al., 2009); G. ferrihydriticus, an alkaliphilic, iron-reducing bacterium, isolated from lake sediments (Zavarzina et al., 2020); Geobacter argillaceus, an Fe(III)-reducing bacterium, isolated from subsurface kaolin strata (Shelobolina et al., 2007); and G. sulfurreducens, which is capable of reducing different forms of Fe(III), Mn(IV), U(VI), elemental sulfur, fumarate, and malate (Engel et al., 2020) and was isolated from surface sediment of a hydrocarbon-contaminated soil (Caccavo et al., 1994). Vicinamibacter silvestris (Vicinamibacteraceae), a soil bacterium isolated from subtropical savanna soil (Huber et al., 2016) and from agriculture and residential (park) soil (Kim et al., 2022), was found in high relative abundances in all samples.
With depth, there was also an increase in the relative abundance of Bacillota and Actinomycetota. This might be due to their resistant spores (Hashmi et al., 2020; Hazarika and Thakur, 2020), enabling the persistence unaffected by the environment for more extended periods (Hanson et al., 2012; de Rezende et al., 2013). For the deeper samples (PM30, PM35, PM40) that mark the transition from fast flow to still water, the relative abundances of families and species are quite similar amongst all three depths. Thermoanaerobacteraceae representatives are thermophilic, involved in different biogeochemical cycles, and were isolated from various substrates. Found in all three depth samples are Brockia lithotrophica, isolated from a terrestrial hot spring and involved in the S cycle (Perevalova et al., 2013); Thermanaeromonas toyohensis, isolated from a geothermal aquifer (Mori et al., 2002); and Moorella stamsii, previously isolated from a digester sludge (Alves et al., 2013). Desulfovirgula thermocuniculi (PM30) was previously isolated from a geothermal underground mine (Kaksonen et al., 2007), and Carboxydothermus hydrogenoformans (PM30, PM35), which grows with CO as its sole carbon source, was previously identified from a hot swamp (Henstra and Stams, 2004; Wu et al., 2005). The abundance of this family increases with depth.
Along the profile (see also Fig. 6), there is a transition of abundant species from soil Fe-reducing species (0 and −50 cm) to thermophilic bacteria involved in the S cycle (−300, −350, and −400 cm), with the most abundant being Geobacter sulfurreducens, Thermanaeromonas toyohensis, and Sulfuritalea hydrogenivorans. The identified thermophilic bacteria involved in the biogeochemical cycle of the S cycle are Thiobacter subterraneus (PM0, PM5; found in a hot aquifer by Hirayama et al., 2005), Thermodesulfovibrio hydrogeniphilus (all samples; found in a hot spring by Haouari et al., 2008), Thermosulfurimonas dismutans (PM5, PM30; deep-sea hydrothermal vent; Slobodkin et al., 2012), and Thioprofundum lithotrophicum (PM0, PM5, PM30, PM40; hydrothermal field; Mori et al., 2011). Acidiferrobacter thiooxydans is involved in both the Fe and S cycles (PM0; an acidophilic, thermotolerant copper mine drainage; Ma et al., 2022), and Aciditerrimonas ferrireducens is involved in the Fe cycle (PM0, PM5, PM30, PM40; acidophilic, geothermally heated field related with fumaroles emitting sulfurous gases; Itoh et al., 2011). Sulfur can naturally occur in caves, also due to the presence of fossil bones and organic decay (Onac et al., 2011; Audra et al., 2019; Misra et al., 2019; Haidău et al., 2022), with its biogeochemical cycle being driven by various microbial metabolic activities, including sulfate reduction and oxidation (Holmer and Storkholm, 2001; Takahashi et al., 2011; Fike et al., 2015; Zhu et al., 2021). With specific bacteria in our samples, we would conclude that their source must be a hot sulfurous environment around the cave.
Streptosporangiaceae, with the thermophilic representative Thermobispora bispora, was present in all samples but with higher abundance in PM30 and PM35. This bacterium was isolated from soil (Slobodkina et al., 2017). Sterolibacteriaceae, with the representative Sulfuritalea hydrogenivorans, previously isolated from freshwater lakes, increases in abundance with depth. It can oxidize thiosulfate, sulfur, or hydrogen and degrade aromatic compounds (Sperfeld et al., 2019). Nitrospira moscoviensis was previously isolated from a heating system and was reported to be moderately thermophilic (Edwards et al., 2013).
The cyanobacterium Aerosakkonema funiforme (Oscillatoriaceae), found in all samples except for PM40, was previously isolated from a mesotrophic water reservoir (Thu et al., 2012) and from a hot spring microbial mat (Moreno et al., 2023), indicating its survival at high temperatures. The increase in this cyanobacterium might be correlated with abrupt warming ∼ 70 000 years ago (Lang et al., 1999) following a cold episode that might have promoted the blooming of this taxon before it was transported underground.
Additionally, lower-abundance thermophiles were identified in the lower samples, like Thermanaerothrix daxensis and Caldilinea tarbellica found in deep hot aquifers (Grégoire et al., 2011) or Thermoanaerobaculum aquaticum (Losey et al., 2013), Thermincola carboxydiphila (Sokolova et al., 2005), and Carboxydothermus islandicus found in hot springs (Novikov et al., 2011). Bacteria tolerating high temperatures, such as Gaiella occulta (deep mineral water aquifer; Albuquerque et al., 2011), were also found.
A hydrogeological map of the area (Ghenea et al., 1981) included several mineral springs near Muierilor Cave, including thermal springs (Săcelu and Ciocadia) at less than 20 km distance. Some of them are well known in the region for having therapeutic properties (Săcelu). In contrast, others were hard to identify in the field because of their low flow rate (Hîrşeşti; see Fig. 1a). Direct proof of the existence of a thermal spring near Muierilor Cave (upstream of Galbenu River) is difficult to demonstrate due to the complex morphodynamic evolution of the river slopes. By the abundance of thermophilic bacteria, we presume that the hot spring was present in the area and was the source of old input(s) of water in the cave. Although high concentrations of S and Fe can originate from fossil bones and organic decay in caves (Audra et al., 2019; Misra et al., 2019; Haidău et al., 2022), the thermophiles point to a different possible source, a hot spring.
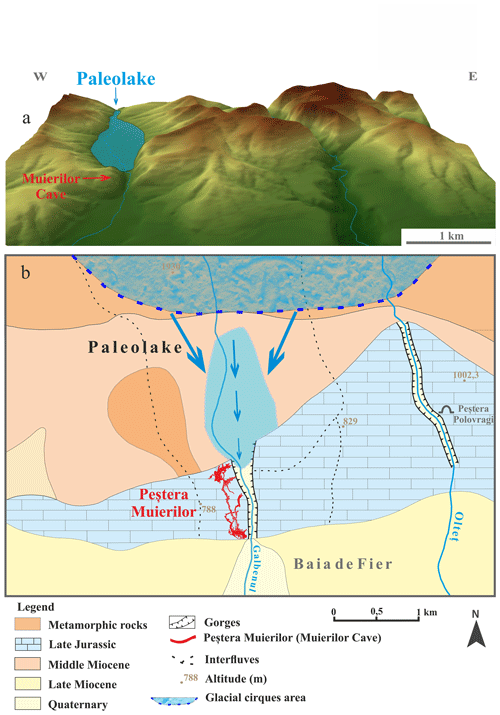
Figure 7Digital elevation model of the relief near Muierilor Cave (a) with the hypothetical position of the paleolake relative to Muierilor Cave and the flow direction during periods of high water input from the upstream mountains (b; modified after Lupu and Ilie, 1962).
Nevertheless, we do not rule out other possible sources and inputs, such as lacustrine organic sediments near the cave system. In certain conditions, sapropel sediments may form in small freshwater lakes (Leonova et al., 2019). Lupu and Ilie (1962) reported the presence of a former lake upstream of the cave system, with intermittent inflows in the cave passages related to the water availability from the snow and ice melting in the high mountains (Parâng Mountains; Fig. 7). Sapropels are characterized mainly as biogenic lake sediments, sludge sediment composed of organic matter and traces of clay, sand, or calcium carbonate (Leonova et al., 2019) with high concentrations of S, amongst others (Mg, Fe, Ca) (Taran et al., 2018; Bogush et al., 2022). Moreover, Bogush et al. (2022) found that sulfate-reducing bacteria in a sapropel core from a lake near Baikal increased with depth, probably because such bacteria are important decomposers of organic matter. Thermophilic bacteria are crucial in decomposition, especially when temperatures reach 70 °C for several weeks (Finore et al., 2023). Furthermore, S in our samples could result from the intensive decomposition, with thermophiles being active in organic matter mineralization and releasing inorganic nutrients (González et al., 2023).
The extensive clay deposits in the cave passages can also be related to the inputs from the former lake upstream of the cave system, a possible low-flow episode(s). Even though the OSL uncertainties span thousands of years, other proxies (e.g., fossil remains, speleothems) dated from the cave passages constrained sediment deposition with the flooding events from MIS 5 through the Holocene (Mirea et al., 2021). These sedimentation stages (episodes) can be associated with different climate events from MIS 5 to the Holocene (Pleistocene), with warmer periods characterized by water and sediment input in the cave.
Given the limestone's isolation within surrounding magmatic and metamorphic rocks, it is unlikely that bacteria were transported over long distances (no more than 2 km) via the epikarst network. Moreover, PMP2 is in an area of the cave with no proof of present or former percolation and no speleothems. It is near a former siphon between two different cave passages.
Microbial communities in caves are shaped by the constant input from the surface environments (Wu et al., 2015). Therefore, the possibility of such bacteria being sourced from the surface is high. Thermophiles are thought to survive only in high-temperature habitats like compost heaps (Finore et al., 2023), hot springs (Benammar et al., 2020; Kochetkova et al., 2022), or deep-sea hydrothermal vents (Miroshnichenko and Bonch-Osmolovskaya, 2006; Zeng et al., 2021). However, they were also found in cool and temperate soils (Portillo et al., 2012; González et al., 2015; Santana and González, 2015; Santana et al., 2020; Milojevic et al., 2022), strengthening the idea of microbial dispersal and the possibility of tracking their movement (Müller et al., 2014; Rime et al., 2016; Comte et al., 2017; Bell et al., 2018; Walters et al., 2022). Thermophiles can disperse on short or long distances from hot sources by water or wind (Portillo and González, 2008; Hubert et al., 2009; Perfumo and Marchant, 2010; Portillo et al., 2012; Bell et al., 2018). Soil is also a possible source. Thermophiles in temperate soils were considered vegetative viable organisms (Portillo et al., 2012; González et al., 2023), with the potential involvement in biogeochemical reactions (González et al., 2015, 2023). Recent studies on soil microbiota have included thermophiles as a permanent component despite their strict ecological requirements (Portillo et al., 2012; Santana and González, 2015; González et al., 2023). Thermophiles that inhabit the upper soil layers are believed to grow and show significant enzymatic activity during hot days (> 30 °C) to produce and stock extracellular enzymes that can help their activity under stress conditions (such as lower temperature or dryness) (Milojevic et al., 2022; Gomez et al., 2021). For example, thermophiles showed enzymatic activity for more than 100 hot days per year at around 37° N in Seville, Spain, and even only 1–2 hot days per year at 52° N in Cambridge, UK (Santana and González, 2015). There are ∼ 40 hot days in Romania per year (Micu et al., 2015), and thermophilic bacteria can survive in the soil. During extreme events, the thermophilic enzymes could decompose soil organic matter into smaller compounds (Santana et al., 2020), releasing N as ammonium (Portillo, et al., 2012) and S as sulfate (Portillo et al., 2012; Santana et al., 2021), at a higher rate than soil mesophiles (Portillo et al., 2012), indicating that the S cycle in soils is performed mainly by them (Santana et al., 2021). A possibly high abundance of thermophiles in the soil could explain their high abundance in the cave.
The presence of bacteria involved in Fe and S cycles in all our samples that date from the last interstadial could have different explanations. The deposit of fossil bones or guano (Misra et al., 2019; Haidău et al., 2022) in the cave can be an essential source of these bacteria. Detrital clay (Audra et al., 2019) can be another source of these bacteria. The depositional condition of the fossil remains from the Urşilor Passage contributed to a rapid burial (e.g., fast-flow phases), resulting in a slow diagenetic process with few mineral exchanges (mostly apatite-related minerals) on long-term sedimentation. Different minerals in the upper levels were related to phosphate-rich deposits (bone and guano degradation; Haidău et al., 2022) at the same level as the studied profile. The interconnected passages of the cave on different levels (upper and lower) by shafts can influence the concentration of various minerals by the mixed-sediment inputs.
Tracing the effects of these paleofloods on bacterial communities within the cave passages was challenging, as reconstructing the precise environmental conditions during those periods is difficult. Moreover, this study serves as supplementary information aimed at enhancing our understanding of past environments in the region. The key events contributing to sediment are the paleofloods that occurred during warmer and wetter conditions in the region. Most of our bacterial findings correlate with these events, indicating a significant influx of bacteria from outside the cave. No other major processes, such as bioturbation or collapse, have been identified in relation to the analyzed sediments.
Moreover, the bacterial abundance growth with depth could be correlated with the age of the sediments and be linked to more stable phases of the cave passage evolution when the sedimentation processes developed under a slow-energy environment (Mirea, 2020; Mirea et al., 2021). Mirea et al. (2021) showed that the top sediments within the Urşilor Passage are linked with the warm conditions of the Bølling–Allerød Interstadial, the last inflow around ∼ 14.7 ka. Therefore, this explains the different bacterial compositions correlated with sediment type and age.
The bacterial composition of a 480 cm deep profile in Muierilor Cave presented a clear difference between the upper (PM0, PM5) and bottom (PM30, PM35, PM40) samples. The composition changes with depth, from the dominance of soil-specific, Fe-reducing bacterial species to the dominance of thermophilic bacteria involved in the S cycle. The presence of bacteria involved in the Fe and S cycles can be due to the presence of an abundance of fossil bones in the cave, probably brought inside the cave together with the sediments during the episodic paleoflood events associated with the end of MIS 5a and MIS 3 (Pleistocene). Thermophiles found in higher abundance in the lower part of the profile could originate from a warm water source in the area or from the soils above the cave during a warmer period. Still, their origin is yet to be determined. The presence of lacustrine organic sediments (sapropelic sediments) near the cave system can also be considered.
This study shows that bacteria in cave deposits can be used in a multi-proxy archive to understand sediment sources and the climate during deposition, as was proposed for other cave sites and organisms (Epure et al., 2014, 2017; Moldovan et al., 2011, 2016). It shows that, for old sediments with complex depositional histories, bacteria can offer new information at the time of deposition that can support or add to the entire understanding of the paleoenvironments.
The raw data were deposited in the NCBI Sequence Read Archive (SRA) under the BioProject PRJNA1161469, with BioSample accessions SAMN43780924, SAMN43780925, SAMN43780926, SAMN43780927, SAMN43780928, SAMN43780929, SAMN43780930, SAMN43780931, and SAMN43780932. The data used in this manuscript can be accessed at https://dataview.ncbi.nlm.nih.gov/object/PRJNA1161469?reviewer=oj751r51jbshf43t890pjt85b0 (Sequence Read Archive, 2009).
OTM designed the study; CH made the extractions; CH, OTM, and ICM wrote the article; OTM, ICM, and SC made the paleoclimatic interpretation; and all authors read and approved the article.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank Alexandra Hillebrand-Voiculescu and Luchiana Faur for helping us with the sampling campaign and suggestions. We are also thankful to Stelian Grigore, Cristinel Fofirică, Arthur Dăscălescu, and Marius Iliescu (“Hades” Caving Club, Romania), the discoverers of the Hades Passage, for providing the base map of the cave.
This research was financially supported by the Ministry of Research, Innovation and Digitization grant, CNCS/CCCDI – UEFISCDI, project no. 2/2019 (DARKFOOD), within PNCDI III; the EEA Financial Mechanism (2014–2021) under project contract no. 3/2019 (KARSTHIVES 2); and grant no. PN-III-P1-1.1-PD-2021-0262 (PALEOTRACE).
This paper was edited by Petr Kuneš and reviewed by two anonymous referees.
Albuquerque, L., França, L., Rainey, F. A., Schumann, P., Nobre, M. F., and da Costa, M. S.: Gaiella occulta gen. nov., sp. nov., a novel representative of a deep branching phylogenetic lineage within the class Actinobacteria and proposal of Gaiellaceae fam. nov. and Gaiellales ord. nov., Syst. Appl. Microbiol., 34, 595–599, https://doi.org/10.1016/j.syapm.2011.07.001, 2011.
Alves, J. I., van Gelder, A. H., Alves, M. M., Sousa, D. Z., and Plugge, C. M.: Moorella stamsii sp. nov., a new anaerobic thermophilic hydrogenogenic carboxydotroph isolated from digester sludge, I. J. Syst. Evol. Microbiol., 63, 4072–4076, https://doi.org/10.1099/ijs.0.050369-0, 2013.
Audra, P., De Waele, J., Bentaleb, I., Chroòáková, A., Krištùfek, V., D'Angeli, I. M., Carbone, C., Madonia, G., Vattano, M., Scopelliti, G., Caihol, D., Vanara, N., Temovski, M., Bigot, Y.-J., Nobécourt, C.-J., Galli, E., Rull, F., and Sanz-Arranz, A.: Guano-related phosphate-rich minerals in European caves, Int. J. Speleol., 48, 75–105, https://doi.org/10.5038/1827-806X.48.1.2252, 2019.
Bailén, M., Bressa, C., Larrosa, M., and González-Soltero, R.: Bioinformatic strategies to address limitations of 16rRNA short-read amplicons from different sequencing platforms, J. Microbiol. Method., 169, 105811, https://doi.org/10.1016/j.mimet.2019.105811, 2020.
Bandrabur, G. and Bandrabur, R.: Parâng and Capaţânii Mountains, in: Karst Hydrogeology of Romania, edited by: Oraşeanu, I. and Iurkiewicz, A., Belvedere, Oradea, 69–75, ISBN 978-606-92444-0-1, 2010.
Barbato, R. A., Jones, R. M., Douglas, T. A., Esdale, J., Foley, K., Perkins, E. J., Rosten, S., and Garcia-Reyero, N.: Alaskan palaeosols in modern times: Deciphering unique microbial diversity within the late-Holocene, Holocene, 32, 909–923, https://doi.org/10.1177/09596836221101249, 2022.
Bay, S. K., McGeoch, M. A., Gillor, O., Wieler, N., Palmer, D. J., Baker, D. J., Chown, S. L., and Greening, C.: Soil bacterial communities exhibit strong biogeographic patterns at fine taxonomic resolution, mSystems, 5, 16 pp., https://doi.org/10.1128/msystems.00540-20, 2020.
Bell, E., Blake, L. I., Sherry, A., Head, I. M., and Hubert, C. R.: Distribution of thermophilic endospores in a temperate estuary indicate that dispersal history structures sediment microbial communities, Environ. Microbiol., 20, 1134–1147, https://doi.org/10.1111/1462-2920.14056, 2018.
Benammar, L., İnan Bektaş, K., Menasria, T., Beldüz, O. A., Güler, H. I., Bedaida, I. K., Gonzalez, J. M., and Ayachi, A.: Diversity and enzymatic potential of thermophilic bacteria associated with terrestrial hot springs in Algeria, Braz. J. Microbiol., 51, 1987–2007, https://doi.org/10.1007/s42770-020-00376-0, 2020.
Bernal, J. P., Revolorio, F., Cu-Xi, M., Lases-Hernández, F., Piacsek, P., Lachniet, M. S., Beddows, A. P., Lucia, G., López-Aguiar, K., Capella-Vizcaíno, S., López-Martínez, R., and Vásquez, O. J.: Variability of trace-elements and δ18O in drip water from Gruta del Rey Marcos, Guatemala; seasonal and environmental effects, and its implications for paleoclimate reconstructions, Front. Earth Sci., 11, 1112957, https://doi.org/10.3389/feart.2023.1112957, 2023.
Berto, C., Krajcarz, M. T., Moskal-del Hoyo, M., Komar, M., Sinet-Mathiot, V., Zarzecka-Szubińska, K., Krajcarz, M., Szymanek, M., Wertz, K., Marciszak, A., Mętrak, M., Suska-Malawska, M., Wilcke, A., and Kot, M.: Environment changes during Middle to Upper Palaeolithic transition in southern Poland (Central Europe), A multiproxy approach for the MIS 3 sequence of Koziarnia Cave (Kraków-Częstocgt Upland), J. Archaeol. Sci. Rep., 35, 102723, https://doi.org/10.1016/j.jasrep.2020.102723, 2021.
Bogush, A. A., Leonova, G. A., Krivonogov, S. K., Bychinsky, V. A., Bobrov, V. A., Maltsev, A. E., Tikhova, D. V., Miroshnichenko, L. V., Kondratyeva, M. L., and Kuzmina, A. E.: Biogeochemistry and element speciation in sapropel from freshwater Lake Dukhovoe (East Baikal region, Russia), Appl. Geochem., 143, 105384, https://doi.org/10.1016/j.apgeochem.2022.105384, 2022.
Buttler, C. J. and Wilson, M. A.: Paleoecology of an Upper Ordovician submarine cave-dwelling bryozoan fauna and its exposed equivalents in northern Kentucky, USA, J. Paleontol., 92, 568–576, https://doi.org/10.1017/jpa.2017.131, 2018.
Caccavo F. J., Lonergan D. J., Lovley D. R., Davis M., Stolz J. F., and McInerney M. J.: Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism, Appl. Environ. Microbiol., 60, 3752–3759, https://doi.org/10.1128/aem.60.10.3752-3759.1994, 1994.
Campaña, I., Benito-Calvo, A., Pérez-González, A., Ortega, A. I., Álvaro-Gallo, A., Miguens-Rodríguez, L., Iglesias-Cibanal, J., Bermúdez de Castro, J.M., and Carbonell, E.: Reconstructing depositional environments through cave interior facies: The case of Galería Complex (Sierra de Atapuerca, Spain), Geomorphology, 440, 108864, https://doi.org/10.1016/j.geomorph.2023.108864, 2023.
Castro, H. F., Classen, A. T., Austin, E. E., Norby, R. J., and Schadt, C. W.: Soil microbial community responses to multiple experimental climate change drivers, Appl. Environ. Microbiol., 76, 999–1007, https://doi.org/10.1128/AEM.02874-09, 2010.
Chee-Sanford, J., Tian, D., and Sanford, R.: Consumption of N2O and other N-cycle intermediates by Gemmatimonas aurantiaca strain T-27, Microbiol., 165, 1345–1354, https://doi.org/10.1099/mic.0.000847, 2019.
Comte, J., Langenheder, S., Berga, M., and Lindström, E. S.: Contribution of different dispersal sources to the metabolic response of lake bacterioplankton following a salinity change, Environ. Microbiol., 19, 251–260, https://doi.org/10.1111/1462-2920.13593, 2017.
Constantin, S., Mirea, I. C., Petculescu, A., Arghir, R. A., Măntoiu, D. Ş., Kenesz, M., Robu, M., and Moldovan, O. T: Monitoring human impact in show caves. A study of four Romanian caves, Sustainability, 13, 1619, https://doi.org/10.3390/su13041619, 2021.
Cruz, J. A., Velasco, J.A., Arroyo-Cabrales, J., and Johnson, E.: Paleoclimatic reconstruction based on the Late Pleistocene San Josecito Cave Stratum 720 Fauna Using Fossil Mammals, Reptiles, and Birds, Diversity, 15, 881, https://doi.org/10.3390/d15070881, 2023.
Dattagupta, S., Schaperdoth, I., Montanari, A., Mariani, S., Kita, N., Valley, W. J., and Macalady, L. J.: A novel symbiosis between chemoautotrophic bacteria and a freshwater cave amphipod, ISME J., 3, 935–943, https://doi.org/10.1038/ismej.2009.34, 2009.
de Rezende, J. R., Kjeldsen, K. U., Hubert, C. R. J., Finster, K., Loy, A. and Jørgensen, B. B.: Dispersal of thermophilic Desulfotomaculum endospores into Baltic Sea sediments over thousands of years, ISME J., 7, 72–84, https://doi.org/10.1038/ismej.2012.83, 2013.
Diaconu, G., Dumitraş, D., and Marincea, Ş.: Mineralogical analyses in peştera Polovragi (Olteţului gorges) and peştera Muierilor (Galbenului Gorges), Gorj County. Trav. Institut. Speol. – Emile Racovitza XLVII, Romanian Academy, 89–105, EISSN: 2067-9033, 2008.
Ding, S., Grossi, V., Hopmans, E. C., Bale, N. J., Cravo-Laureau, C., and Sinninghe Damsté, J. S.: Nitrogen and sulfur for phosphorus: Lipidome adaptation of anaerobic sulfate-reducing bacteria in phosphorus-deprived conditions, P. Natl. Acad. Sci. USA, 121, e2400711121, https://doi.org/10.1073/pnas.2400711121, 2024.
Domeignoz-Horta, L. A., Shinfuku, M., Junier, P., Poirier, S., Verrecchia, E., Sebag, D., and DeAngelis, K. M.: Direct evidence for the role of microbial community composition in the formation of soil organic matter composition and persistence, ISME Commun., 1, 64, https://doi.org/10.1038/s43705-021-00071-7, 2021.
Dominguez-Moñino, I., Jurado, V., Rogerio-Candelera, M. A., Hermosin, B., and Saiz-Jimenez, C.: Airborne bacteria in show caves from Southern Spain, Microb. Cell, 26, 247–255, https://doi.org/10.15698/mic2021.10.762, 2021.
Dong, Y., Gao, J., Wu, Q. Ai, Y., Huang, Y., Wei, W., Sun, S., and Weng, Q.: Co-occurrence pattern and function prediction of bacterial community in Karst cave, BMC Microbiol., 20, 137, https://doi.org/10.1186/s12866-020-01806-7, 2020.
Edwards, T. A., Calica, N. A., Huang, D. A., Manoharan, N., Hou, W., Huang, L., Manoharan, N., Hou, W., Huang, L., Panosyan, H., Dong, H., and Hedlund, B. P.: Cultivation and characterization of thermophilic Nitrospira species from geothermal springs in the US Great Basin, China, and Armenia, FEMS Microbiol. Ecol., 85, 283–292, https://doi.org/10.1111/1574-6941.12117, 2013.
Engel, C. E. A., Vorländer, D., Biedendieck, R., Krull, R., and Dohnt, K.: Quantification of microaerobic growth of Geobacter sulfurreducens, PLoS One, 15, e0215341, https://doi.org/10.1371/journal.pone.0215341, 2020.
Epure, L., Meleg, I. N., Munteanu, C. M., Roban, R. D., and Moldovan O. T.: Bacterial and fungal diversity of Quaternary cave sediment deposits, Geomicrobiol. J., 31, 116–127, https://doi.org/10.1080/01490451.2013.815292, 2014.
Epure, L., Muntean, V., Constantin, S., and Moldovan, O. T.: Ecophysiological groups of bacteria from cave sediments as potential indicators of paleoclimate, Quaternary Int., 432, 20–32, https://doi.org/10.1016/j.quaint.2015.04.016, 2017.
Feng, L., Yang, J., Ma, F., Pi, S., Xing, L., and Li, A.: Characterisation of Pseudomonas stutzeri T13 for aerobic denitrification: Stoichiometry and reaction kinetics, Sci. Total Environ., 717, 135181, https://doi.org/10.1016/j.scitotenv.2019.135181, 2020.
Fike, D. A., Bradley, A. S., and Rose, C. V.: Rethinking the ancient sulfur cycle, Annu. Rev. Earth Planet. Sci., 43, 593–622, https://doi.org/10.1146/annurev-earth-060313-054802, 2015.
Finore, I., Feola, A., Russo, L., Cattaneo, A., Di Donato, P., Nicolaus, B., Poli, A., and Romano, I.: Thermophilic bacteria and their thermozymes in composting processes: a review, Chem. Biol. Technol. Agric., 10, 7, https://doi.org/10.1186/s40538-023-00381-z, 2023.
Frindte, K., Lehndorff, E., Vlaminck, S. Werner, K., Kehl, M., Khormali, F., and Knief, C.: Evidence for signatures of ancient microbial life in paleosols, Sci. Rep., 10, 16830, https://doi.org/10.1038/s41598-020-73938-9, 2020.
Gaastra, W., Kusters, J. G., Van Duijkeren, E., and Lipman, L. J. A.: Escherichia fergusonii, Vet. microbiol., 172, 7–12, https://doi.org/10.1016/j.vetmic.2014.04.016, 2014.
Gehrig, J. L., Portik, D. M., Driscoll, M. D., Jackson, E., Chakraborty, S., Gratalo, D., Ashby, M., and Valladares, R.: Finding the right fit: evaluation of short-read and long-read sequencing approaches to maximize the utility of clinical microbiome data, Microb. Genom., 8, 000794, https://doi.org/10.1099/mgen.0.000794, 2022.
Ghenea, C., Bandrabur, T., and Ghenea, A.: Atlas of Romania: The underground and mineral waters map; Sheet V-2; Romanian Academy, Institute of Geography, Bucharest, Romanian Academy, Romania, EISSN: 2067-9033, 1981.
Gomez, E. J., Delgado, J. A., and González, J. M.: Influence of water availability and temperature on estimates of microbial extracellular enzyme activity, PeerJ., 9, e10994, https://doi.org/10.7717/peerj.10994, 2021.
González, J. M., Portillo, M. C., and Piñeiro-Vidal, M.: Latitude-dependent underestimation of microbial extracellular enzyme activity in soils, Int. J. Environ. Sci. Technol., 12, 2427–2434, https://doi.org/10.1007/s13762-014-0635-7, 2015.
González, J. M., Santana, M. M., Gomez, E. J., and Delgado, J. A.: Soil thermophiles and their extracellular enzymes: a set of capabilities able to provide significant services and risks, Microorganisms, 11, 1650, https://doi.org/10.3390/microorganisms11071650, 2023.
Greene, A. C., Patel, B. K., and Yacob, S.: Geoalkalibacter subterraneus sp. nov., an anaerobic Fe (III)-and Mn (IV)-reducing bacterium from a petroleum reservoir, and emended descriptions of the family Desulfuromonadaceae and the genus Geoalkalibacter, I. J. Syst. Evol. Microbiol., 59, 781–785, https://doi.org/10.1099/ijs.0.001537-0, 2009.
Grégoire, P., Bohli, M., Cayol, J. L., Joseph, M., Guasco, S., Dubourg, K., Cambar, J., Michotey, V., Bonin, P., Fardeau, M. L., and Ollivier, B.: Caldilinea tarbellica sp. nov., a filamentous, thermophilic, anaerobic bacterium isolated from a deep hot aquifer in the Aquitaine Basin, Int. J. Syst. Evol. Microbiol., 61, 1436–1441, https://doi.org/10.1099/ijs.0.025676-0, 2011.
Haidău, C., Năstase-Bucur, R., Bulzu, P., Levei, E., Cadar, O., Mirea, I. C., Faur, L., Fruth, V., Atkinson, I., Constantin, S., and Moldovan, O. T.: A 16S rRNA gene-based metabarcoding of phosphate-rich deposits in Muierilor Cave, South-Western Carpathians, Front. Microbiol., 13, 877481, https://doi.org/10.3389/fmicb.2022.877481, 2022.
Hakil, F., Amin-Ali, O., Hirschler-Rea, A., Mollex, D., Grossi, V., Duran, R., Matheron, R., and Cravo-Laureau, C.: Desulfatiferula berrensis sp. nov., an-alkene-degrading sulfate-reducing bacterium isolated from estuarine sediments, Int. J. Syst. Evol. Microbiol., 64, 540–544, https://doi.org/10.1099/ijs.0.057174-0, 2014.
Hann, H., Berza, T., Pop, G., Marinescu, F., Ricman, C., Pană, D., Săbău, G., Bindea, G., and Tatu, M.: Harta geologică a României scara 1:50 000, Foaia Polovragi, Institutul Geologic al României, Romanian Academy, Bucureşti, EISSN: 2067-9033, 1986.
Hanson, C. A., Fuhrman, J. A., Horner-Devine, M. C., and Martiny, J. B. H.: Beyond biogeographic patterns: processes shaping the microbial landscape, Nat. Rev. Microbiol., 10, 497–506, https://doi.org/10.1038/nrmicro2795, 2012.
Haouari, O., Fardeau, M. L., Cayol, J. L., Fauque, G., Casiot, C., Elbaz-Poulichet, F., Hamdi, M., and Ollivier, B.: Thermodesulfovibrio hydrogeniphilus sp. nov., a new thermophilic sulphate-reducing bacterium isolated from a Tunisian hot spring, Syst. Appl. Microbiol., 31, 38–42, https://doi.org/10.1016/j.syapm.2007.12.002, 2008.
Hashmi, I., Bindschedler, S., and Junier, P.: Firmicutes, in: Beneficial microbes in agro-ecology, edited by: Amaresan, N., Senthil Kumar, M., Annapurna, K. Kumar Krishna, and Sankaranarayanan, A., Academic Press, London, 363–396, https://doi.org/10.1016/B978-0-12-823414-3.00018-6, 2020.
Hazarika, S. N. and Thakur, D.: Actinobacteria, in Beneficial microbes in agro-ecology, edited by: Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar Krishna, and Sankaranarayanan, A., Academic Press, London, 443–47, https://doi.org/10.1016/B978-0-12-823414-3.00021-6, 2020.
Henstra, A. M. and Stams, A. J.: Novel physiological features of Carboxydothermus hydrogenoformans and Thermoterrabacterium ferrireducens, Appl. Environ. Microbiol., 70, 7236–7240, https://doi.org/10.1128/AEM.70.12.7236-7240.2004, 2004.
Hirayama, H., Takai, K., Inagaki, F., Nealson, K. H., and Horikoshi, K.: Thiobacter subterraneus gen. nov., sp. nov., an obligately chemolithoautotrophic, thermophilic, sulfur-oxidizing bacterium from a subsurface hot aquifer, I. J. Syst. Evol. Microbiol., 55, 467–472, https://doi.org/10.1099/ijs.0.63389-0, 2005.
Holmer, M. and Storkholm, P.: Sulphate reduction and sulphur cycling in lake sediments: a review, Freshw. Biol., 46, 431–451, https://doi.org/10.1046/j.1365-2427.2001.00687.x, 2001.
Hosomi, K., Saito, M., Park, J., Murakami, H., Shibata, N., Ando, M., Nagatake, T., Konishi, K., Ohno, H., Tanisawa, K., Mohsen, A., Chen, Y.-A., Kawashima, J., Natsume-Kitatani, Y., Oka, Y., Shimizu, H., Furuta, M., Tojima, Y., Sawane, K., Saija, A., Kondo, S., Yonejima, Y., Takeyama, H., Matsutani, A., Mizuguchi, K., Miyachi, M., and Kunisawa, J.: Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota, Nat. Commun., 13, 4477, https://doi.org/10.1038/s41467-022-32015-7, 2022.
Howarth, G. F. and Moldovan, T. O.: The ecological classification of cave animals and their adaptations, in: Cave Ecology, edited by: Moldovan, T. O., Kovac, L., and Halse, S., Springer, Berlin, 41–67, https://doi.org/10.1007/978-3-319-98852-8_4, 2018.
Huber, K. J., Geppert, A. M., Wanner, G., Fösel, B. U., Wüst, P. K., and Overmann, J.: The first representative of the globally widespread subdivision 6 Acidobacteria, Vicinamibacter silvestris gen. nov., sp. nov., isolated from subtropical savannah soil, I. J. Syst. Evol. Microbiol., 66, 2971–2979, https://doi.org/10.1099/ijsem.0.001131, 2016.
Hubert, C., Loy, A., Nickel, M., Arnosti, C., Baranyi, C., Brüchert, V., Ferdelman, T., Finster, K., Christensen, M. F., de Rezende, R, J., Vandieken, V., and Jørgensen, B. B.: A constant flux of diverse thermophilic bacteria into the cold Arctic seabed, J. Sci., 325, 1541–1544, https://doi.org/10.1126/science.1174012, 2009.
Itoh, T., Yamanoi, K., Kudo, T., Ohkuma, M., and Takashina, T.: Aciditerrimonas ferrireducens gen. nov., sp. nov., an iron-reducing thermoacidophilic actinobacterium isolated from a solfataric field, I. J. Syst. Evol. Microbiol., 6, 1281–1285, https://doi.org/10.1099/ijs.0.023044-0, 2011.
Ji, M., Kong, W., Stegen, J., Yue, L., Wang, F., Dong, X., Cowan, A. D., and Ferrari, B. C.: Distinct assembly mechanisms underlie similar biogeographical patterns of rare and abundant bacteria in Tibetan Plateau grassland soils, Environ. Microbiol., 22, 2261–227, https://doi.org/10.1111/1462-2920.14993, 2020.
Jia, B., Chang, X., Fu, Y., Heng, W., Ye, Z., Liu, P., Liu, L., Shoffe, A. Y., Watkins, B. C., and Zhu, L.: Metagenomic analysis of rhizosphere microbiome provides insights into occurrence of iron deficiency chlorosis in field of Asian pears, BMC Microbiol., 22, 18, https://doi.org/10.1186/s12866-021-02432-7, 2022.
Kaksonen, A. H., Spring, S., Schumann, P., Kroppenstedt, R. M., and Puhakka, J. A.: Desulfovirgula thermocuniculi gen. nov., sp. nov., a thermophilic sulfate-reducer isolated from a geothermal underground mine in Japan, I. J. Syst. Evol. Microbiol., 57, 98–102, https://doi.org/10.1099/ijs.0.64655-0, 2007.
Kim, B. R., Shin, J., Guevarra, R. B., Lee, J. H., Kim, D. W., Seol, K. H., Lee, J.-H, Kim, B. H., and Isaacson, E. R.: Deciphering diversity indices for a better understanding of microbial communities, J. Microbiol. Biotechnol., 27, 2089–2093, https://doi.org/10.4014/jmb.1709.09027, 2017.
Kim, H., Park, H.-Y., Yang, E. J., Kim, S.-H., Kim, S.-C., Oh, E.-J., Moon, J., Cho, W., Shin, W., and Yu, C.: Analysis of major bacteria and diversity of surface soil to discover biomarkers related to soil health, Toxics, 10, 117, https://doi.org/10.3390/toxics10030117, 2022.
Koblížek, M., Dachev, M., Bína, D., Piwosz, K., and Kaftan, D.: Utilization of light energy in phototrophic Gemmatimonadetes, J. Photochem. Photobiol. B., 213, 112085, https://doi.org/10.1016/j.jphotobiol.2020.112085, 2020.
Kochetkova, T. V., Podosokorskaya, O. A., Elcheninov, A. G., and Kublanov, I. V.: Diversity of thermophilic prokaryotes inhabiting Russian natural hot springs, Microbiology, 91, 1–27, https://doi.org/10.1134/S0026261722010064, 2022.
Konopiński, M. K.: Shannon diversity index: a call to replace the original Shannon's formula with unbiased estimator in the population genetics studies, PeerJ, 8, e9391, https://doi.org/10.7717/peerj.9391, 2020.
Kosznik-Kwaśnicka, K., Golec, P., Jaroszewicz, W., Lubomska, D., and Piechowicz, L.: Into the Unknown: Microbial Communities in Caves, Their Role, and Potential Use, Microorganisms, 10, 222, https://doi.org/10.3390/microorganisms10020222, 2022.
Krishna, M. P. and Mohan, M.: Litter decomposition in forest ecosystems: a review, Energy, Ecol. Environ., 2, 236–249, https://doi.org/10.1007/s40974-017-0064-9, 2017.
Lang, C., Leuenberger, M., Schwander, J., and Johnsen, S.: 16 °C Rapid Temperature Variation in Central Greenland 70,000 Years Ago, Science, 286, 934–937, http://www.jstor.org/stable/2899490 (last access: 10 November 2024), 1999.
Lange-Enyedi, N. T., Németh, P., Borsodi, A. K., Halmy, R., Czuppon, G., Kovács, I., Leél-Őssy, S., Demény, A., and Makk, J.: Calcium carbonate precipitating cultivable bacteria from different speleothems of karst caves, Geomicrobiol. J., 39, 107–122, https://doi.org/10.1080/01490451.2021.2019857, 2022.
Leonova, G. A., Maltsev, A. E., Melenevsky, V. N., Krivonogov, S. K., Kondratyeva, L. M., Bobrov, V. A., and Suslova, M. Y.: Diagenetic transformation of organic matter in sapropel sediments of small lakes (southern West Siberia and eastern Transbaikalia), Quaternary Int., 524, 40–47, https://doi.org/10.1016/j.quaint.2019.03.011, 2019.
Li, W., Fu, L., Niu, B., Wu, S., and Wooley, J.: Ultrafast clustering algorithms for metagenomic sequence analysis, Brief. Bioinform., 13, 656–668, https://doi.org/10.1093/bib/bbs035, 2012.
Losey, N. A., Stevenson, B. S., Busse, H. J., Damsté, J. S. S., Rijpstra, W. I. C., Rudd, S., and Lawson, P. A.: Thermoanaerobaculum aquaticum gen. nov., sp. nov., the first cultivated member of Acidobacteria subdivision 23, isolated from a hot spring, Int. J. Syst. Evol. Microbiol., 63, 4149–4157, https://doi.org/10.1099/ijs.0.051425-0, 2013.
Lupu, S. and Ilie, I.: Observaţii geomorfologice preliminare în bazinul rîului Galbenul, Probleme de Geografie, X, Editura Academiei Republicii Populare Romane, 1962.
Ma, L., Huang, X., Wang, H.,Yun, Y.,Cheng, X., Liu, D., Lu, X., and Qiu, X.: Microbial interactions drive distinct taxonomic and potential metabolic responses to habitats in karst cave ecosystem, Microbiol. Spectr., 9, e0115221, https://doi.org/10.1128/Spectrum.01152-21, 2021.
Ma, L., Yang, W., Huang, S., Liu, R., Li, H., Huang, X., Liu, R., Li, H., Huang, X., Xiong, J., and Liu, X.: Integrative assessments on molecular taxonomy of Acidiferrobacter thiooxydans ZJ and its environmental adaptation based on mobile genetic elements, Front. Microbiol., 13, 826829, https://doi.org/10.3389/fmicb.2022.826829, 2022.
Magoč, T. and Salzberg, S. L.: FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics, 27, 2957–2963, https://doi.org/10.1093/bioinformatics/btr507, 2011.
Malard, L. A., Anwar, M. Z., Jacobsen, C. S., and Pearce, D. A.: Biogeographical patterns in soil bacterial communities across the Arctic region, FEMS Microbiol. Ecol., 95, fiz128, https://doi.org/10.1093/femsec/fiz128, 2019.
McMahon, S., Anderson, R. P., Saupe, E. E., and Briggs, D. E. G.: Experimental evidence that clay inhibits bacterial decomposers: Implications for preservation of organic fossils, Geology, 44, 867–870, https://doi.org/10.1130/G38454.1, 2016.
McMurdie, P. J. and Holmes, S.: phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data, PLOS One, 8, e61217, https://doi.org/10.1371/journal.pone.0061217, 2013.
Michail, G., Lefkothea K., Panagiotis, M., Angeliki, R., and Ioannis, V.: Metataxonomic analysis of bacteria entrapped in a stalactite's core and their possible environmental origins, Microorganisms, 9, 2411, https://doi.org/10.3390/microorganisms9122411, 2021.
Micu, M. D., Dumitrascu, A., Cheval, S., and Birsan, V.-M.: Regional Climatic Patterns in Climate of the Romanian Carpathians, edited by: Micu, M. D., Dumitrascu, A., Cheval, S., and Birsan, V.-M., Springer Cham, Switzerland, 73–148, https://doi.org/10.1007/978-3-319-02886-6, 2015.
Milojevic, T., Cramm, M. A., Hubert, C. R. J., and Westall, F.: “Freezing” thermophiles: From one temperature extreme to another, Microorganisms, 10, 2417, https://doi.org/10.3390/microorganisms10122417, 2022.
Minckley, T. A., Clementz, M. T., and Lovelace, D.: Paleo-vegetation and environmental history of Natural Trap Cave based on pollen and carbon isotope analyses, Quaternary Int., 647, 103–111, https://doi.org/10.1016/j.quaint.2021.11.019, 2023.
Mirea, I. C.: Late Quaternary environmental changes as revealed by the sedimentary archives from Muierilor Cave, Romania, Unpublished PhD thesis, Cluj-Napoca, 138, 2020.
Mirea, I. C., Robu, M., Petculescu, A., Kenesz, M., Faur, L., Arghir, R., Tecsa, V., Timar-Gabor, A., Roban, R.-D., Panaiotu, G. C., Sharifi, A., Pourmand, A., Codrea, A. V., and Constantin, S.: Last deglaciation flooding events in the Southern Carpathians as revealed by the study of cave deposits from Muierilor Cave, Romania, Palaeogeogr. Palaeocl., 562, 110084, https://doi.org/10.1016/j.palaeo.2020.110084, 2021.
Miroshnichenko, M. L. and Bonch-Osmolovskaya, E. A.: Recent developments in the thermophilic microbiology of deep-sea hydrothermal vents, Extremophiles, 10, 85–96, https://doi.org/10.1007/s00792-005-0489-5, 2006.
Misra, P. K., Gautam, N. K., and Elangovan, V.: Bat guano: a rich source of macro and microelements essential for plant growth, Ann. Plant Soil Res., 21, 82–86, 2019.
Moldovan, O.T., Constantin, S., Panaiotu, C., Roban, R. D., Frenzel, P., and Miko, L.: Fossil invertebrates records in cave sediments and paleoenvironmental assessments: a study of four cave sites from Romanian Carpathians, Biogeosciences, 13, 483–497, https://doi.org/10.5194/bg-13-483-2016, 2016.
Moldovan, O. T., Mihevc, A., Miko, L., Constantin, S., Meleg, I. N., Petculescu, A., and Bosak, P.: Invertebrate fossils from cave sediments: a new proxy for pre-Quaternary paleoenvironments, Biogeosciences, 8, 1825–1837, https://doi.org/10.5194/bg-8-1825-2011, 2011.
More, K. D., Giosan, L., Grice, and Coolen, M. J.: Holocene paleodepositional changes reflected in the sedimentary microbiome of the Black Sea, Geobiology, 17, 436–448, https://doi.org/10.1111/gbi.12338, 2019.
Moreno, I. J., Brahamsha, B., Donia, M. S., and Palenik, B.: Diverse microbial hot spring mat communities at Black Canyon of the Colorado River, Microb. Ecol., 86, 1534–1551, https://doi.org/10.1007/s00248-023-02186-x, 2023.
Mori, K., Hanada, S., Maruyama, A., and Marumo, K.: Thermanaeromonas toyohensis gen. nov., sp. nov., a novel thermophilic anaerobe isolated from a subterranean vein in the Toyoha Mines, I. J. Syst. Evol. Microbiol., 52, 1675–1680, https://doi.org/10.1099/00207713-52-5-1675, 2002.
Mori, K., Suzuki, K. I., Urabe, T., Sugihara, M., Tanaka, K., Hamada, M., and Hanada, S.: Thioprofundum hispidum sp. nov., an obligately chemolithoautotrophic sulfur-oxidizing gammaproteobacterium isolated from the hydrothermal field on Suiyo Seamount, and proposal of Thioalkalispiraceae fam. nov. in the order Chromatiales, I. J. Syst. Evol. Microbiol., 61, 2412–2418, https://doi.org/10.1099/ijs.0.026963-0, 2011.
Müller, A. L., De Rezende, J. R., Hubert, C. R., Kjeldsen, K. U., Lagkouvardos, I., Berry, D., Jørgensen, B. B., and Loy, A.: Endospores of thermophilic bacteria as tracers of microbial dispersal by ocean currents, ISME J., 8, 1153–1165, https://doi.org/10.1038/ismej.2013.225, 2014.
Nejman, L., Lisá, L., Doláková, N., Horáček, I., Bajer, A., Novák, J., Wright, D., Sullivan, M., Wood, R., Gargett, R. H., Pacher, M., Sázelová, S., Nývltová Fišáková, M., Rohovec, J., and Králík, M.: Cave deposits as a sedimentary trap for the Marine Isotope Stage 3 environmental record: The case study of Pod Hradem, Czech Republic, Palaeogeogr. Palaeocl., 497, 201–217, https://doi.org/10.1016/j.palaeo.2018.02.020, 2018.
North Greenland Ice Core Project members: High-resolution record of Northern Hemisphere climate extending into the last interglacial period, Nature, 431, 147–151, https://doi.org/10.1038/nature02805, 2004.
Novikov, A. A., Sokolova, T. G., Lebedinsky, A. V., Kolganova, T. V., and Bonch-Osmolovskaya, E. A.: Carboxydothermus islandicus sp. nov., a thermophilic, hydrogenogenic, carboxydotrophic bacterium isolated from a hot spring, I. J. Syst. Evol. Microbiol., 61, 2532–2537, https://doi.org/10.1099/ijs.0.030288-0, 2011.
Onac, B. P., Wynn, J. G., and Sumrall, J. B.: Tracing the sources of cave sulfates: a unique case from Cerna Valley, Romania, Chem. Geol., 288, 105–114, https://doi.org/10.1016/j.chemgeo.2011.07.006, 2011.
Oren, A. and Garrity, G. M.: Valid publication of the names of forty-two phyla of prokaryotes, Int. J. Syst. Evol. Microbiol., 71, 005056, https://doi.org/10.1099/ijsem.0.005056, 2021.
Oren, A., Mareš, J., and Rippka, R.: Validation of the names Cyanobacterium and Cyanobacterium stanieri, and proposal of Cyanobacteriota phyl. nov. Int. J. Syst. Evol. Microbiol., 72, 005528, https://doi.org/10.1099/ijsem.0.005528, 2022.
Osipova, E., Danukalova, G., and Tiunov, M.: Late Pleistocene and Holocene malacological and theriological faunas from the Tetyukhinskaya Cave (South Far East, Russia) and their palaeoecological implications, Palaeoworld, 33, 241–256, https://doi.org/10.1016/j.palwor.2022.12.007, 2024.
Pascual, J., Foesel, B. U., Geppert, A., Huber, K. J., Boedeker, C., Luckner, M., Wanner, G., and Overmann, J.: Roseisolibacter agri gen. nov., sp. nov., a novel slow-growing member of the under-represented phylum Gemmatimonadetes, I. J. Syst. Evol. Microbiol., 68, 1028–1036, https://doi.org/10.1099/ijsem.0.002619, 2018.
Perevalova, A. A., Kublanov, I. V., Baslerov, R. V., Zhang, G., and Bonch-Osmolovskaya, E. A.: Brockia lithotrophica gen. nov., sp. nov., an anaerobic thermophilic bacterium from a terrestrial hot spring, Int. J. Syst. Evol. Microbiol., 63, 479–483, https://doi.org/10.1099/ijs.0.041285-0, 2013.
Perfumo, A. and Marchant, R.: Global transport of thermophilic bacteria in atmospheric dust, Environ. Microbiol. Reports, 2, 333–339, https://doi.org/10.1111/j.1758-2229.2010.00143.x, 2010.
Pester, M., Knorr, K. H., Friedrich, M. W., Wagner, M., and Loy, A.: Sulfate-reducing microorganisms in wetlands–fameless actors in carbon cycling and climate change, Front. Microbiol., 3, 72, https://doi.org/10.3389/fmicb.2012.00072, 2012.
Pianta, A., Arvikar, S., Strle, K., Drouin, E. E., Wang, Q., Costello, C. E., and Steere, A. C.: Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis, Arthritis Rheumatol., 69, 964–975, https://doi.org/10.1002/art.40003, 2017.
Portillo, M. C. and González, J. M.: Microbial communities and immigration in volcanic environments of Canary Islands (Spain), Naturwissenschaften, 95, 307–315, https://doi.org/10.1007/s00114-007-0330-3, 2008.
Portillo, M. C., Santana, M., and González, J. M.: Presence and potential role of thermophilic bacteria in temperate terrestrial environments, Naturwissenschaften, 99, 43–53, https://doi.org/10.1007/s00114-011-0867-z, 2012.
Prescott, C. E. and Vesterdal, L.: Decomposition and transformations along the continuum from litter to soil organic matter in forest soils, Forest Ecol. Manag., 498, 119522, https://doi.org/10.1016/j.foreco.2021.119522, 2021.
Prieto, A. R., Azar, P. F., and Fernández, M. M.: Holocene vegetation dynamics and human–environment interactions inferred from pollen and plant macrofossils from caves in northwestern Patagonia (Argentina), Rev. Palaeobot. Palynol., 293, 104496, https://doi.org/10.1016/j.revpalbo.2021.104496, 2021.
Reang, L., Bhatt, S., Tomar, R. S., Joshi, K., Padhiyar, S., Vyas, M. U., and Kheni, H. J.: Plant growth promoting characteristics of halophilic and halotolerant bacteria isolated from coastal regions of Saurashtra Gujarat, Sci. Rep., 12, 4699, https://doi.org/10.1038/s41598-022-08151-x, 2022.
Rime, T., Hartmann, M., and Frey, B.: Potential sources of microbial colonizers in an initial soil ecosystem after retreat of an alpine glacier, ISME J., 10, 1625–1641, https://doi.org/10.1038/ismej.2015.238, 2016.
Romanenko, L. A., Tanaka, N., Svetashev, V. I., and Mikhailov, V. V.: Pseudomonas glareae sp. nov., a marine sediment-derived bacterium with antagonistic activity, Arch. Microbiol., 197, 693–699, https://doi.org/10.1007/s00203-015-1103-6, 2015.
Romano, E., Sechi, D., Andreucci, S., Bergamin, L., D'Ambrosi, A., De Santis, C., Di Bella, L., Dinelli, E., Frezza, V., Pascucci, V., Pierfranceschi, G., and Provenzani, C.: Paleoecological reconstruction during the Holocene in the Middle Branch of Bue Marino Cave (Sardinia, Italy), Holocene, 34, 74–86, https://doi.org/10.1177/09596836231200435, 2024.
Sakon, H., Nagai, F., Morotomi, M., and Tanaka, R.: Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov., isolated from human faeces, I. J. Syst. Evol. Microbiol., 58, 970–975, https://doi.org/10.1099/ijs.0.65456-0, 2008.
Santana, M. M. and González, J. M.: High temperature microbial activity in upper soil layers, FEMS Microbiol. Lett., 362, fnv182, https://doi.org/10.1093/femsle/fnv182, 2015.
Santana, M. M., Carvalho, L., Melo, J., Araújo, M. E., and Cruz, C.: Unveiling the hidden interaction between thermophiles and plant crops: Wheat and soil thermophilic bacteria, J. Plant Interact., 15, 127–138, https://doi.org/10.1080/17429145.2020.1766585, 2020.
Santana, M. M., Dias, T., González, J. M., and Cruz, C.: Transformation of organic and inorganic sulfur-adding perspectives to new players in soil and rhizosphere, Soil Biol. Biochem., 160, 108306, https://doi.org/10.1016/j.soilbio.2021.108306, 2021.
Satam, H., Joshi, K., Mangrolia, U., Waghoo, S., Zaidi, G., Rawool, S., Thakare, R. P., Banday, S., Mishra, A. K., Das, G., and Malonia, S. K.: Next-Generation Sequencing Technology: Current Trends and Advancements, Biology, 12, 997, https://doi.org/10.3390/biology12070997, 2023.
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., Lesniewski, A. R., Oakley, B. B., Parks, H. D., Robinson, J. C., Sahl, W. J., Stres, B., Thallinger, G. G., Van Horn, J. D., and Weber, C. F.: Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microbiol., 75, 7537–7541, https://doi.org/10.1128/AEM.01541-09, 2009.
Semenov, M. V., Chernov, T. I., Zhelezova, A. D. Nikitin, D. A., Tkhakakhova, A. K., Ivanova, E. A., Xenofontova, N. A., Sycheva, S. A., Kolganova, T. V., and Kutovaya, O. V.: Microbial communities of interglacial and interstadial paleosols of the Late Pleistocene, Eurasian Soil Sc., 53, 772–779, https://doi.org/10.1134/S1064229320060101, 2020.
Sequence Read Archive (SRA): PRJNA1161469 Bacteria from cave sediments as indicator of paleoenvironments, National Library of Medicine (US), National Center for Biotechnology Information [data set], https://dataview.ncbi.nlm.nih.gov/object/PRJNA1161469?reviewer=oj751r51jbshf43t890pjt85b0 (last access: 17 February 2025), 2009.
Shelobolina, E. S., Nevin, K. P., Blakeney-Hayward, J. D., Johnsen, C. V., Plaia, T. W., Krader, P., Woodard, T., Holmes, D. E., VanPraagh, C. G., and Lovley, D. R.: Geobacter pickeringii sp. nov., Geobacter argillaceus sp. nov. and Pelosinus fermentans gen. nov., sp. nov., isolated from subsurface kaolin lenses, I. J. Syst. Evol. Microbiol., 57, 126–135, https://doi.org/10.1099/ijs.0.64221-0, 2007.
Simpson, E. H.: Measurement of diversity, Nature, 163, 688, https://doi.org/10.1038/163688a0, 1949.
Slobodkin, A. I., Reysenbach, A. L., Slobodkina, G. B., Baslerov, R. V., Kostrikina, N. A., Wagner, I. D., and Bonch-Osmolovskaya, E. A.: Thermosulfurimonas dismutans gen. nov., sp. nov., an extremely thermophilic sulfur-disproportionating bacterium from a deep-sea hydrothermal vent, I. J. Syst. Evol. Microbiol., 62, 2565–2571, https://doi.org/10.1099/ijs.0.034397-0, 2012.
Slobodkina, G. B., Baslerov, R. V., Novikov, A. A., Bonch-Osmolovskaya, E. A., and Slobodkin, A. I.: Thermodesulfitimonas autotrophica gen. nov., sp. nov., a thermophilic, obligate sulfite-reducing bacterium isolated from a terrestrial hot spring, I. J. Syst. Evol. Microbiol., 67, 301–305, https://doi.org/10.1099/ijsem.0.001619, 2017.
Sokolova, T. G., Kostrikina, N. A., Chernyh, N. A., Kolganova, T. V., Tourova, T. P., and Bonch-Osmolovskaya, E. A.: Thermincola carboxydiphila gen. nov., sp. nov., a novel anaerobic, carboxydotrophic, hydrogenogenic bacterium from a hot spring of the Lake Baikal area, I. J. Syst. Evol. Microbiol., 55, 2069–2073, https://doi.org/10.1099/ijs.0.63299-0, 2005.
Sperfeld, M., Diekert, G., and Studenik, S.: Anaerobic aromatic compound degradation in Sulfuritalea hydrogenivorans sk43H, FEMS Microbiol. Ecol., 95, fiy199, https://doi.org/10.1093/femsec/fiy199, 2019.
Takahashi, H., Kopriva, S., Giordano, M., Saito, K., and Hell, R.: Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes, Annu. Rev. Plant Biol., 62, 157–184, https://doi.org/10.1146/annurev-arplant-042110-103921, 2011.
Talà, A., Buccolieri, A., Calcagnile, M., Ciccarese, G., Onorato, M., Onorato, R., Serra, A., Spedicato, F., Tredici, M. S., Alifano, P., and Belmonte, G.: Chemotrophic profiling of prokaryotic communities thriving on organic and mineral nutrients in a submerged coastal cave, Sci. Total Environ., 755, 142514, https://doi.org/10.1016/j.scitotenv.2020.142514, 2021.
Taran, O. P., Boltenkov, V. V., Ermolaeva, N. I., Zarubina, E. Y., Delii, I. V., Romanov, R. E., and Strakhovenko, V. D.: Relations between the chemical composition of organic matter in lacustrine ecosystems and the genesis of their sapropel, Geochem. Int., 56, 256–265, https://doi.org/10.1134/S0016702918030096, 2018.
Täuber, S., Riedel, S. L., Neubauer, P., and Junne, S.: Phosphate assimilation with co-cultures of Acinetobacter tjernbergiae and Pseudomonas stutzeri, Chem.-Ing.-Tech., 94, 1300–1300, https://doi.org/10.1002/cite.202255269, 2022.
Tauxe, L., Gee, J., and Staudigel, H.: Flow directions in dikes from AMS data: The bootstrap way, J. Geophys. Res., 103, 17775–17790, 1998.
Thomas, S. P., Shanmuganathan, B., Jaiswal, M. K., Kumaresan, A., and Sadasivam, S. K.: Legacy of a Pleistocene bacterial community: Patterns in community dynamics through changing ecosystems, Microbiol. Res., 226, 65–73, https://doi.org/10.1016/j.micres.2019.06.001, 2019.
Thu, N. K., Tanabe, Y., Yoshida, M., Matsuura, H., and Watanabe, M. M.: Aerosakkonema funiforme gen. et sp. nov. (Oscillatoriales), a new gas-vacuolated oscillatorioid cyanobacterium isolated from a mesotrophic reservoir, Phycologia, 51, 672–683, https://doi.org/10.2216/11-130.1, 2012.
Uroz, S., Calvaruso, C., Turpault, M. P., and Frey-Klett, P.: Mineral weathering by bacteria: ecology, actors and mechanisms, Trends Microbiol., 17, 378–387, https://doi.org/10.1016/j.tim.2009.05.004, 2009.
Walters, K. E., Capocchi, J. K., Albright, M. B. N., Hao, Z., Brodie, L. E., and Martiny, B. J.: Routes and rates of bacterial dispersal impact surface soil microbiome composition and functioning, ISME J., 16, 2295–2304, https://doi.org/10.1038/s41396-022-01269-w, 2022.
Waltgenbach, S., Riechelmann, D. F. C., Spötl, C., Jochum, K. P., Fohlmeister, J., Schröder-Ritzrau, A., and Scholz, D.: Climate variability in central europe during the last 2500 years reconstructed from four high-resolution multi-proxy speleothem records, Geosciences, 11, 166, https://doi.org/10.3390/geosciences11040166, 2021.
Wani, A. K., Akhtar, N., Sher, F. Navarrete, A. A., and Américo-Pinheiro, J. H. P.: Microbial adaptation to different environmental conditions: molecular perspective of evolved genetic and cellular systems, Arch. Microbiol., 204, 144, https://doi.org/10.1007/s00203-022-02757-5, 2022.
Weber, M., Hinz, Y., Schöne, B. R., Jochum, K. P., Hoffmann, D., Spötl, C., Riechelmann, D. F. C., and Scholz, D.: Opposite trends in Holocene speleothem proxy records from two neighboring caves in Germany: A multi-proxy evaluation, Front. Earth Sci., 9, 642651, https://doi.org/10.3389/feart.2021.642651, 2021.
White, W. B.: Cave sediments and paleoclimate, J. Cave Karst Stud., 69, 76–93, 2007.
Wu, Y., Tan, L., Liu, W., Wang, B., Wang, J., Cai, Y., and Lin, X.: Profiling bacterial diversity in a limestone cave of the western Loess Plateau of China, Front. Microbiol., 6, 244, https://doi.org/10.3389/fmicb.2015.00244, 2015.
Xu, S., Wang, J., Zhang, X., Yang, R., Zhao, W., Huang, Z., and Wang, Y.: Lacustrine sediments bacterial community structure vertical succession of the Linxia Basin, NE Tibetan Plateau: significance for paleoenvironment reconstruction, Front. Earth Sci., 9, 714352, https://doi.org/10.3389/feart.2021.714352, 2022.
Yang, X.: Moraxellaceae, in: Encyclopedia of Food Microbiology, 2nd Edn., edited by: Batt, C. A. and Tortorello, M. L., Academic Press, London, 826–833, https://doi.org/10.1016/B978-0-12-384730-0.00441-9, 2014.
Yarwood, S. A.: The role of wetland microorganisms in plant-litter decomposition and soil organic matter formation: a critical review, FEMS Microbiol. Ecol., 94, fiy175, https://doi.org/10.1093/femsec/fiy175, 2018.
Yun, Y., Xiang, X., Wang, H., Man, B., Gong, L., Liu, Q., Dong, Q., and Wang, R.: Five-year monitoring of bacterial communities in dripping water from the Heshang Cave in Central China: implication for paleoclimate reconstruction and ecological functions, Geomicrobiol. J., 33, 1–11, https://doi.org/10.1080/01490451.2015.1062062, 2016.
Zada, S., Xie, J., Yang, M., Yang, X., Sajjad, W., Rafiq, M., Hasan, F., Zhong, H., and Wang, H.: Composition and functional profiles of microbial communities in two geochemically and mineralogically different caves, Appl. Microbiol. Biotechnol., 105, 8921–8936, https://doi.org/10.1007/s00253-021-11658-4, 2021.
Zavarzina, D. G., Zhilina, T. N., Kuznetsov, B. B., Kolganova, T. V., Osipov, G. A., Kotelev, M. S., and Zavarzin, G. A.: Natranaerobaculum magadiense gen. nov., sp. nov., an anaerobic, alkalithermophilic bacterium from soda lake sediment, I. J. Syst. Evol. Microbiol., 63, 4456–4461, https://doi.org/10.1099/ijs.0.054536-0, 2013.
Zavarzina, D. G., Gavrilov, N. S., Chistyakova, I. N., Antonova, V. A., Gracheva, A. M., Yu Merkel, A., Perevalova, A. A., Chernov, S. M., Zhilina, N. T., Yu Bychkov, A., and Bonch-Osmolovskaya, A. E.: Syntrophic growth of alkaliphilic anaerobes controlled by ferric and ferrous minerals transformation coupled to acetogenesis, ISME J., 14, 425–436, https://doi.org/10.1038/s41396-019-0527-4, 2020.
Zeng, X., Alain, K., and Shao, Z.: Microorganisms from deep-sea hydrothermal vents, Mar. Life Sci. Technol., 3, 204–230, https://doi.org/10.1007/s42995-020-00086-4, 2021.
Zepeda Mendoza, M. L., Lundberg, J., Ivarsson, M., Campos, P., Nylander, J. A., Sallstedt, T., and Dalen, L.: Metagenomic analysis from the interior of a speleothem in Tjuv-Ante's cave, northern Sweden, PLoS One, 11, e0151577, https://doi.org/10.1371/journal.pone.0151577, 2016.
Zhang, M., Han, F., Li, Y., Liu, Z., Chen, H., Li, Z., Li, Q., and Zhou, W.: Nitrogen recovery by a halophilic ammonium-assimilating microbiome: a new strategy for saline wastewater treatment, Water Res., 207, 117832, https://doi.org/10.1016/j.watres.2021.117832, 2021.
Zhao, L., Xiao, R., Zhang, S., Zhang, C., and Zhang, F.: Environmental specificity of karst cave habitats evidenced by diverse symbiotic bacteria in Opiliones, BMC Ecol. Evol., 24, 58, https://doi.org/10.1186/s12862-024-02248-9, 2024.
Zhou, J., Agichtein, E., and Kallumadi, S.: Diversifying multi-aspect search results using Simpson's diversity index, Proceedings of the 29th ACM International Conference on Information and Knowledge Management, Virtual Event Ireland, Association for Computing Machinery, New York, NY, United States, 19–23 October 2020, 2345–2348, https://doi.org/10.1145/3340531.3412163, 2020.
Zhu, H. Z., Zhang, Z. F., Zhou, N., Jiang, C. Y., Wang, B. J., Cai, L., and Liu, S. J.: Diversity, distribution and co-occurrence patterns of bacterial communities in a karst cave system, Front. microbiol., 10, 1726, https://doi.org/10.3389/fmicb.2019.01726, 2019.
Zhu, H. Z., Zhang, Z. F., Zhou, N., Jiang, C. Y., Wang, B. J., Cai, L., Wang, M.-H., and Liu, S. J.: Bacteria and metabolic potential in karst caves revealed by intensive bacterial cultivation and genome assembly, Appl. Environ. Microbiol., 8, e0244020, https://doi.org/10.1128/AEM.02440-20, 2021.