the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Biological response of eelgrass epifauna, Taylor's Sea hare (Phyllaplysia taylori) and eelgrass isopod (Idotea resecata), to elevated ocean alkalinity
Nicholas D. Ward
Peter J. Regier
Mallory C. Ringham
Matthew D. Eisaman
Marine carbon dioxide removal (mCDR) approaches are under development to mitigate the effects of climate change by sequestering carbon in stable reservoirs, with the potential co-benefit of local reductions in coastal acidification impacts. One such method is ocean alkalinity enhancement (OAE). A specific OAE method is the generation of aqueous alkalinity via electrochemistry to enhance the alkalinity of the receiving water by the extraction of acid from seawater, thereby avoiding the issues of solid dissolution kinetics and the release of impurities into the ocean from alkaline minerals. While electrochemical acid extraction is a promising method for increasing the carbon dioxide sequestration potential of the ocean, the biological effects of increasing seawater alkalinity and pH within an OAE project site are relatively unknown. This study aims to address this knowledge gap by testing the effects of increased pH and alkalinity, delivered in the form of aqueous NaOH, on two eelgrass epifauna in the US Pacific Northwest, Taylor's sea hare (Phyllaplysia taylori) and eelgrass isopod (Idotea resecata), chosen for their ecological importance as salmon prey and for their role in eelgrass ecosystems. Four-day experiments were conducted in closed bottles to allow measurements of the evolution of carbonate species throughout the experiment, with water refreshed twice daily to maintain elevated pH, across pHNBS (NBS standard scale) treatments ranging from 7.8 to 9.3. Sea hares experienced mortality in all pH treatments, ranging from 37 % mortality at pHNBS 7.8 to 100 % mortality at pHNBS 9.3. Isopods experienced lower mortality rates in all treatment groups, ranging from 13 % at pHNBS 7.8 to 21 % at pHNBS 9.3, which did not significantly increase with higher pH treatments. These experiments represent an extreme of constant exposure to elevated pH and alkalinity, which should be considered in the context of both the natural variation and the dilution of alkalinity experienced by marine communities across an OAE project site. Different invertebrate species will likely have different responses to increased pH and alkalinity, depending on their physiological vulnerabilities. Investigation of the potential vulnerabilities of local marine species will help inform the decision-making process regarding mCDR planning and permitting.
- Article
(698 KB) - Full-text XML
-
Supplement
(1047 KB) - BibTeX
- EndNote
Among the many other impacts of climate change, increased levels of atmospheric CO2 drive global decreases in ocean pH and calcium carbonate (CaCO3) saturation states (Doney et al., 2009; Doney et al., 2020). This process, known as ocean acidification (OA), poses a significant threat to marine organisms and ecosystems (Doney et al., 2020). OA can lead to various detrimental effects on marine life, including decreases in survival, growth, calcification, development, and abundance, particularly for slow-moving or sessile animals (Kroeker et al., 2013). Marine invertebrates can experience physiological effects such as oxidative stress, decreased immunity, decreased growth and development, and lower reproductive success (Shi and Li, 2023). OA can be particularly harmful to organisms in early life stages, affecting fertilization, larval development, dispersal, and settlement (Ross et al., 2011). Moreover, OA can negatively impact food web dynamics and ecosystem processes (Fabry et al., 2008).
Active removal of 5–15 Gt of atmospheric CO2 per year, in addition to drastic decreases in CO2 emissions, is necessary to avoid the worst effects of climate change (IPCC, 2022; Rogelj et al., 2018). An increasing focus on methods of enhancing the ocean's natural carbon sink through marine carbon dioxide removal (mCDR) is driven by the ocean's natural capacity to store CO2 (National Academies of Sciences, Engineering, and Medicine, 2022). One method of mCDR, ocean alkalinity enhancement (OAE), aims to store atmospheric CO2 in seawater in the dissolved bicarbonate phase in response to a disequilibrium in pCO2 (partial pressure of carbon dioxide) across the air–sea boundary of the surface ocean, induced by a change in seawater alkalinity (Cross et al., 2023; Oschlies et al., 2023). Because OAE deployments can result in local increases in pH and alkalinity, they could help to locally mitigate some of the effects of acidification on marine ecosystems – including those arising from both ocean acidification and other sources of coastal acidification, such as nutrient pollution. Some OAE approaches aim to add natural or industrial alkaline materials to ocean or coastal environments (Feng et al., 2017; Köhler et al., 2010; Montserrat et al., 2017; Rigopoulos et al., 2018; Harvey, 2008; Ilyina et al., 2013; Kheshgi, 1995; La Plante, 2023; Moras et al., 2022; Nduagu, 2012; Rau, 2008; Renforth and Henderson, 2017; Shaw et al., 2022). Electrochemical OAE approaches process salt (e.g., sodium chloride – NaCl) to generate aqueous acid (hydrochloric acid – HCl), which is removed from the system, and base (sodium hydroxide – NaOH), which is mixed with the seawater stream and returned to the ocean, thereby enhancing the alkalinity of the surrounding water (de Lannoy et al., 2018; Eisaman et al., 2018; Eisaman et al., 2023; Lu et al., 2022; Ringham et al., 2024; Tyka et al., 2022; Wang et al., 2023). The increased surface alkalinity drives additional ocean uptake of atmospheric CO2, which is ultimately stored in seawater as dissolved bicarbonate (Cross et al., 2023; Eisaman et al., 2023; Ringham et al., 2024). While OAE could be a promising avenue for reducing atmospheric CO2, the biological response of marine organisms and the impact of locally increasing pH and alkalinity on ecosystems remain largely unknown.
Changes in ocean pH can have implications for marine life and the health of marine ecosystems. pH shifts can affect the physiology of aquatic organisms by disrupting acid–base regulation, which is essential for cellular function; inhibiting fixation and respiration of CO2; and reducing nutrient uptake (Tresguerres et al., 2020; Tresguerres et al., 2023). Multicellular marine organisms rely on intracellular and extracellular pH gradients and modulation for metabolic processes. This is regulated through an acid–base balance, which can be disrupted if environmental conditions, such as pH or CO2, are significantly altered (National Research Council, 2010). Many organisms have the ability to control their internal pH to an extent, but some may be able to acclimate to local environmental changes better than others at the cost of high metabolic demand (Portner et al., 2000). Previous studies have explored the impact of pH changes, particularly in the context of OA, on a variety of marine organisms, but only a few works have studied the impacts of increasing pH and/or alkalinity relevant to the context of OAE. One particularly relevant study, Albright et al. (2016), quantified the net calcification of a coral reef flat to alkalinity enrichment by the addition of NaOH into seawater in situ, indicating that this method could mitigate acidification impacts at local scales. Another study investigated the effects of increased ocean alkalinity on red calcifying algae. The algae experienced a 60 % increase in carbonate production when alkalinity was increased from 2694 to 3454 µEq L−1 (microequivalents per liter; resulting in a pH increase from 7.97 to 8.2), but these alkalinity and pH increases had no significant negative impacts on primary productivity, respiration, or photophysiology (Gore et al., 2019). A study of the exposure of European green crabs to calcium hydroxide at two concentrations (0.28 and 0.54 mmol L−1), to raise the pHNBS (8.0–8.7), showed a physiological disruption of acid–base regulation, respiratory alkalosis, and hyperkalemia (Cripps et al., 2013). Female crabs were more susceptible to increases in pH (from pHNBS 8.03 to 8.77), which affected their physiology, although no mortality in any control or treatment group was observed. Another study on the aquaculture application of calcium hydroxide to reduce biofouling found that short-term exposure of bivalves (blue mussel, eastern oyster, and bay scallop) to a 12.7 pH solution (resulting in a seawater pH increase from ambient to pH 9.3–11.7) resulted in short-term behavioral stress, but no mortality was found, likely due to the quick dilution of the alkaline solution. The bivalves were then exposed to a moderately elevated pH (9.2) consistently for 3 d, during which time they experienced prolonged closure of their valves, indicating an avoidance behavior. However, responses were short-lived, the behavior ceased when treatment was completed, and no mortality was observed (Comeau et al., 2017). Recent focus has been given to the environmental impacts of OAE (Cyronak et al., 2024), but more research is needed to move the mCDR field forward, following best practices for collaborative OAE research (Oschlies et al., 2023). It is important to note that air-equilibrated OAE approaches will cause less drastic pH changes than a non-equilibrated approach (Hartmann et al., 2023).
Determination of the potential impacts on local species and those of cultural, economic, or ecological importance is of critical importance in characterizing OAE deployments. In the Pacific Northwest region of the USA and Canada, eelgrass (Zostera marina) is critical to many nearshore ecosystems, playing key roles such as providing habitat for other species and acting as a food source, directly and indirectly supporting food webs (Thayer and Phillips, 1977). Eelgrass ecosystems provide a variety of supporting services, such as refuge, nursery habitat, foraging areas, and habitat areas for reproduction, as well as regulating services, such as shoreline protection, sediment stability, water quality improvement, and climate change regulation (Sherman and DeBruyckere, 2018). Pacific salmon, both a culturally and commercially important species, rely on valuable eelgrass habitat. This ecosystem provides foraging opportunities for juvenile salmon that promote growth and survival during their critical early life stage (Kennedy et al., 2018). Pacific Northwest eelgrass ecosystems are at risk from a variety of threats, including invasive species, anthropogenic contaminants, and global shifts in temperature and sea level rise (Sherman and DeBruyckere, 2018). Although manipulation experiments have shown that acidic conditions may alleviate stress and promote eelgrass productivity (Zayas-Santiago et al., 2020; Zimmerman et al., 2017), the potential impacts on eelgrass epifauna under either acidic or alkaline treatments remains unknown.
Eelgrass isopods (Idotea resecata) typically range from Alaska, USA, to California, USA, and are found in eelgrass ecosystems. Isopods crawl on eelgrass blades and feed on epiphytic diatoms, playing a significant role in food webs as a prey source for many fish species, including Pacific salmon (Bridges, 1973; Ricketts and Calvin, 1952; Welton and Miller, 1980). Taylor's sea hares (Phyllaplysia taylori), a gastropod mollusk and species of sea slug, typically range from British Columbia, Canada, to California, USA, and spend their lives on the blades of eelgrass, feeding on epiphytic diatoms (Beeman, 1963). As grazers, Taylor's sea hares are main contributors to reducing the epiphyte load on eelgrass blades (Lewis and Boyer, 2014). Sea hares are herbivores and use their green coloration and vertical stripes as camouflage from predation among the eelgrass blades (Bridges, 1973). In the eelgrass ecosystem, both isopods and sea hares graze on the epiphytic algae, which block the eelgrass from the sun and limit photosynthesis. This grazing reduces the epiphyte load on the eelgrass blades, allowing for continued photosynthesis (Lewis and Boyer, 2014). Research on OA effects on these two species of invertebrates is limited; however, one study (Hughes et al., 2017) investigated OA effects on P. taylori and I. resecata and found a negative quadratic relationship between change in pH and body mass, indicating an optimum pH of between 7.4 and 7.5, with mass decreasing as the pH nears 7.0 and 7.8. This study also observed low sea hare mortality and high isopod mortality in response to OA (Hughes et al., 2017). Studies investigating OA effects on other species of sea hares and isopods can provide helpful context. When exposed to OA conditions, the California sea hare, Aplysia californica, experienced altered behavior and acid–base regulation (Zlatkin and Heuer, 2019; Zlatkin et al., 2020). When exposed to OA conditions, the behavior of the sea hare Stylocheilus striatus was altered, showing reduced speed and foraging success as well as increased metabolic demand (Horwitz et al., 2020). When exposed to OA conditions, another species of isopod, Idotea balthica, exhibited 100 % mortality under high-pCO2 conditions (Wood et al., 2014). Eelgrass mesograzer species' sensitivity to shifts in environmental changes, such as pH, salinity, and temperature, are likely to affect their feeding behavior on epiphytes, which can lead to indirect effects on the growth and productivity of the eelgrass ecosystem (Hughes et al., 2017; Tanner et al., 2019). The effects of OA and OAE on other macroinvertebrates are limited, whereas the effects of OAE on the two species examined are unknown.
Marine epifauna local to the Pacific Northwest experience substantial natural variability in pH over daily to seasonal timescales. Over the course of the year, pHNBS in Puget Sound surface layer waters can vary by more than one pH unit, with even greater variability at the numerous river outlets (e.g., pHNBS 6.6–8.6) around the region (Bianucci et al., 2018; Fassbender et al., 2018). Based on Cotter et al. (2022), pHNBS within an eelgrass meadow at the entrance of Sequim Bay varied between 8.02 and 8.22 between low and high tide and was consistently higher than outside of the meadow. Unpublished data show tidal variation in the pHNBS in the tidal channel of Sequim Bay that ranges from 7.6 to 8.2. This variability is driven primarily by tides, diel productivity patterns, river discharge, and seasonal weather variability. While eelgrass epifauna may be used to natural daily and seasonal variations in pH, constant exposure to higher pH due to OAE deployments may have significant impacts on marine communities. Local hydrodynamics at individual OAE deployments will determine the dilution of an alkalinity release from a point source. The mixing zone, or the regulated local area around the point source directly impacted by the OAE deployment, may experience consistently elevated pH conditions relative to baseline conditions, returning to levels indistinguishable from natural variability by the edge of the mixing zone. Point-source OAE deployments in the USA may be regulated under the 1972 Clean Water Act through the National Pollutant Discharge Elimination System (NPDES), which requires a biological assessment of the project site. Dilution models paired with habitat assessments can help to identify potential exposures of marine communities within range of an OAE project, such as if eelgrass meadows could be consistently exposed to elevated pH.
The objective of this study was to assess the biological responses of eelgrass epifauna (Taylor's sea hares and isopods) to increased pH and alkalinity levels to inform future mCDR field trials and identify knowledge gaps pertaining to laboratory and field trials in the context of OAE. The study helps to estimate the level of mortality and potential behavioral trends of eelgrass invertebrates if exposed to these high pH and alkalinity conditions continuously outside the bounds of typical coastal variability. This work is a step towards informing safe bounds of operation for OAE interventions that may impact specific species in eelgrass meadows in the Pacific Northwest and identifying knowledge gaps and future laboratory experiments representing the conditions relevant to point-source OAE deployments.
2.1 Laboratory experiments
The experiments were conducted at the Pacific Northwest National Laboratory (PNNL) marine research laboratory in Sequim, WA, USA. Eelgrass has been cultivated in outdoor mesocosm tanks on the dock at this facility for over 20 years, supplied with unfiltered seawater from the entrance of Sequim Bay. The PNNL wet laboratory facility has abundant access to raw seawater, which comes on demand directly from the bay via the facility's seawater intake, located near the seafloor at about 10 m depth by the mouth of Sequim Bay. pHNBS measured close to the facility's seawater intake displays large variability throughout the day and year, ranging from 7.6 to 8.3 in general (https://mcrldata.pnnl.gov, last access: 27 August 2024).
Separate experiments were conducted for adult sea hares and eelgrass isopods, with collections from the eelgrass ecosystem tanks occurring from July to September 2023. This collection included three respective batches of 120 sea hares (late July to early August 2023) and isopods (late August to late September 2023). Sea hares and isopods were gently collected by hand or with nets from the outdoor eelgrass tanks and transferred to three acclimation tanks in the on-site wet laboratory. Acclimation tanks were filled with about 2.5 cm of sediment collected from one of the outdoor eelgrass tanks and had continuously flowing raw seawater from Sequim Bay to provide water flow to the organisms. The flow rate was not measured but allowed for the water to rapidly refresh and maintain a temperature as close to the natural environment as possible. Animals were provided daily with eelgrass blades and diatom masses from the outdoor tanks as habitat and food sources in the acclimation tanks. The animals acclimated for 1 week, during which time they were checked daily for mortality, and any deceased animals were removed from the tanks. At the end of acclimation, 100 animals were randomly collected from the surviving individuals to enter the experiments. After acclimation, animals were not fed for the remainder of the experiment, based on the standards for acute toxicity tests with macroinvertebrates (ASTM International, 2000). This means that malnourishment may have led to some mortality, in both treatment groups and the non-pH-adjusted controls.
Preparation of pH treatments was always completed in a separate container from the organisms to avoid exposing animals to incorrect pH levels. To start the experiment and for each water change, 7 L of unfiltered seawater was gently poured into an 8 L bucket, and the pH, salinity, temperature, and dissolved oxygen (DO) of the seawater were measured using a YSI ProDSS probe. The accuracy of the probe is ± 0.2 pH units. The probe was calibrated daily using an NBS (standard scale) buffer, ensuring it was within ± 0.05 pHNBS before use. The control group pHNBS was the pH of Sequim Bay water at the time of water change (generally around 7.8 ± 0.3 due to natural variability). Temperature, salinity, initial pH readings, and water volume were used to calculate the amount of NaOH needed to reach the desired pHNBS for each treatment group, using a CO2SYS Excel macro (Pierrot et al., 2021). Three pHNBS treatments were targeted for these experiments: 8.3 (low treatment), 8.8 (medium treatment), and 9.3 (high treatment). If the initial pHNBS was higher (closer to the target pHNBS), less NaOH was added to reach the target pHNBS. Commercial aqueous NaOH (0.5 M, Honeywell Advanced Materials 352576X1L) was used to replicate the 0.5 M concentration of NaOH derived from an electrodialysis method that creates acid and base from seawater (Eisaman et al., 2023; Ringham et al., 2024). The PNNL facility is now capable of generating NaOH via electrodialysis, but this was not available at the time of the experiments. The pHNBS of the seawater was measured as aqueous NaOH was added to the bucket and adjusted on a drop-by-drop basis until it was within 0.1 of the target pHNBS.
The characterization of each seawater treatment for measured pHNBS, salinity, temperature, DO, and alkalinity addition and the calculated dissolved inorganic carbon, total alkalinity (TA), and aragonite saturation state are included in Table 1 (see Sects. 2.2 and 3.1). After a brief pause to allow for the homogeneous mixing of NaOH into the seawater, a gas sample (see Sect. 2.2) was collected for pCO2 analysis, and the seawater was then carefully transferred to fill (to the top) six 1 L glass jars without creating bubbles. For each of the sea hare and isopod experiments, 5 animals were randomly picked from the acclimation tanks and placed into five of the six jars per pH treatment (resulting in a total of 25 animals per treatment). Lids were closed tight on the jars, and the jars were then placed randomly on a laboratory water table (Fig. 1) to account for potential variances in environmental parameters (e.g., light and air temperature). Lids were not perforated to limit air exchange and abiotic alteration of pH (i.e., atmospheric equilibration, which would decrease the intended pH treatment and increase pCO2 as CO2 diffused into the seawater in response to the NaOH addition). For each pH treatment, a sixth jar was filled with control or treated seawater, but no organisms were added; this jar was used as a chemical control. Each three rounds of the experiments for both sea hares and isopods comprised a total of 24 jars: 5 jars at control pH (no NaOH added) with 5 animals each for a total of 25 animals, 1 chemical control (without animals) at control pH, 5 jars at each of the three pH treatments (NaOH added) with 5 animals each for a total of 25 animals per treatment, and 1 chemical control (without animals) for each of the three pH treatments (NaOH added).
Table 1Summary of the overall range (mean ± 1 SD) in measured initial and final (with and without organisms) temperature, salinity, dissolved oxygen (DO), and pH of seawater prior to each refresh. The initial time point represents the water that was then poured into all jars prior to either adding organisms or sealing for a no-organism control. The pHNBS and pCO2 values measured at the initial time point for the ambient pH treatment (i.e., pHNBS=7.8 without NaOH addition) were used to calculate the initial TA. The measured pH and calculated alkalinity after NaOH addition (initial TA + alkalinity added) were then used to calculate pCO2, dissolved inorganic carbon (DIC), and aragonite saturation going forward (see Sect. 2.2). Diel and tidal variability in water quality over the time that the water was drawn to refresh the jars each day resulted in variability between each round of the experiment.
* Measured pHNBS values reported here include the +0.17 unit offset described in Sect. 2.2.
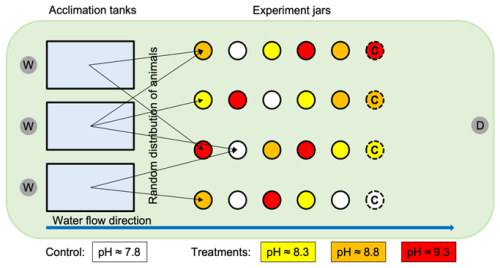
Figure 1Experimental setup of the laboratory water table, including the 1 L glass jars (red, orange, and yellow circles) used in the experiment and the acclimation tanks (blue rectangles). The white circles indicate control jars with pHNBS≈7.8 (no NaOH added), yellow circles indicate the low treatment with pHNBS≈8.3, orange circles indicate the medium treatment with pHNBS≈8.8, and red circles indicate the high treatment with pHNBS≈9.3. The dashed circles with “C” in them indicate the chemical control jars in which only treated (or control) seawater was added without the presence of sea hares or isopods. Animals were distributed randomly from the acclimation tanks to the experiment jars, as indicated by the black arrows. The circles with “W” in them indicate the water input, the circle with “D” in it indicates the drain on the water table, and the blue arrow indicates the water flow along the table.
The water change process was repeated twice a day to ensure proper oxygenation and pH treatment in the jars and to remove any excrement or deceased organisms. For each water change, used water from all of the jars within a treatment group was carefully pooled into a single bucket, and pHNBS, salinity, temperature, and DO within this bucket were measured. Water quality measurements were also taken from the chemical control from each treatment group. Water was refilled as described above, with water quality and gas analyses repeated with each water refill. Organisms were checked for mortality; any casualties were removed from the jars; and any noticeable behavior, such as reproduction or cannibalism among the animals, was also noted. Between water changes, a standpipe was inserted into the drain of the water table to create a water bath of about 10 cm to keep the jars at a cooler temperature akin to the natural seawater in Sequim Bay. The temperature of the water from Sequim Bay ranged from 10.9 to 15.3°C depending on the time of the day and month of the year. The experiment was conducted over 4 d and was repeated three times for both sea hares and isopods, with a new batch of animals each time.
2.2 pCO2 sampling and carbonate speciation calculations
To collect gas samples from the initial seawater prior to base addition, a water sample was gently poured into a 300 mL bottle, which was allowed to overflow to eliminate headspace and avoid extraneous bubbles. A total of 60 mL of nitrogen (N2) was injected with a syringe into the bottle; simultaneously, 60 mL of water was removed from the bottle with another syringe. This created a headspace of N2 at the top of the bottle. After vigorously shaking the bottle for 1 min to distribute the N2 throughout the water sample, the gas sample was extracted using a unique plastic syringe for each sample. pCO2 of the gas sample was then measured on a Picarro G2508 cavity ring-down spectrometer with a flow limiter installed on the inlet to reduce gas flow rates (e.g., Regier et al., 2023). The above methodology was only applied to samples collected prior to adding NaOH, which had pCO2 values above atmospheric saturation. Adding NaOH to seawater results in pCO2 values well below atmospheric saturation, and the headspace equilibration method is only reliable when pCO2 is at or above atmospheric saturation (i.e., > 420 ppm) (Koschorreck et al., 2021).
We employed a series of calculations using the CO2SYS Excel macro (Pierrot et al., 2021) to evaluate carbonate speciation (e.g., total alkalinity – TA; dissolved inorganic carbon – DIC; and aragonite saturation – Ω). Directly measuring DIC and TA was not practical given the numbers of replications, treatments, and water refreshes that were performed. Therefore, we focused on measurements of pCO2 and pHNBS and only derived DIC and TA to provide context for these experiments. We note that there are significant uncertainties in each of the pCO2 and pHNBS measurements that propagate to the DIC and TA calculations. Because the pHNBS probe had an accuracy of ± 0.2 units, we first compared data from the initial, untreated seawater used for these experiments with similar untreated samples collected from the same seawater intake (n=10) that were robustly analyzed for TA using an Apollo AS-ALK3 titrator as part of a different research effort (Table S1). While these data are not a one-to-one match with the initial seawater used in our experiments, they provide a realistic range of carbonate chemistry conditions for untreated seawater across the same range of salinity. The average TA of the 10 samples directly analyzed for TA was 2168 µmol kg−1. We then calculated TA for the initial, untreated water samples used for this study based on pH and pCO2 and found that these calculations significantly underestimated TA. Therefore, we adjusted the measured pHNBS values from our probe by a Δ pH of +0.17 units, which is within the uncertainty of the probe. This pH correction resulted in an average calculated TA of 2171 µmol kg−1 for the initial, untreated water used for the experiment, which is only 0.1 % higher than the known, directly measured TA of our facility's seawater intake. The pHNBS values reported throughout the paper represent our corrected values.
With the TA of the initial, untreated seawater calculated, we then calculated the alkalinity that was added to adjust the seawater pHNBS to the target 8.3, 8.8, and 9.3 treatments based on the known concentration and volume of NaOH added to each treatment. Alkalinity of the treated seawater was calculated as the sum of the initial pre-base-addition seawater alkalinity and the amount of alkalinity added as NaOH. With pHNBS directly measured and TA estimated for the NaOH-treated samples, we were then able to calculate the remaining unmeasured carbonate species (i.e., DIC, pCO2, and aragonite saturation) while avoiding the issue of unreliable pCO2 measurements below atmospheric saturation, albeit with much higher uncertainty than if DIC and TA were directly measured. The goal of these calculations was not to precisely quantify TA and DIC dynamics but, rather, to provide context for the range of alkalinity additions used in this study and the biological responses that are the focal point of this study.
2.3 Data analyses
All data analyses other than CO2SYS calculations were conducted in version 4.3.1 of the R statistical software (R Core Team, 2023). Data exploration, visualization, and analyses were completed using the following libraries: “ggplot2”, “tidyverse”, “dplyr”, “ggpubr”, “readxl”, “rstatix, and “car” (Fox and Weisberg, 2019; Kassambara, 2022; Kassambara, 2023; Pinheiro et al., 2023; Wickham, 2016; Wickham et al., 2019; Wickham et al., 2023; Wickham and Bryan, 2023; Zeileis and Hothorn, 2002). One-way analysis of variance (ANOVA) tests were applied to mortality data from the four different treatment groups to determine whether differences between groups were statistically significant. When p values indicated a significant (p<0.05) difference between treatments based on ANOVA tests, Tukey's honest significant difference (HSD) tests were used to identify significant differences between individual levels of pH treatment. Tukey's HSD tests were selected because they enable one to specify which groups to compare, rather than looking for the significance between all treatments via ANOVA. Next, t tests were conducted to compare average mortality from each pHNBS treatment (8.3, 8.8, and 9.3) to the control pHNBS (7.8). A simple linear regression was fitted to the mortality data for both sea hares and isopods using the linear model function in R to determine if pHNBS explained the variation in mortality data for each species.
Chemical toxicity tests use a lethal concentration 50 (LC50) value to measure the toxicity of a substance and its concentration that results in 50 % mortality of a test subject (Government of Canada, 2024). While LC50 tests generally measure the concentration of a particular substance, we were more interested in looking at the overall effect that the pHNBS values had on the organisms' mortality, as opposed to the actual amount of NaOH added to achieve said pHNBS. Therefore, for each round of sea hare and isopod experiments, the pH treatment at which 50 % mortality occurred was used as an LC50 value. The lowest-observed-effect concentration (LOEC) and no-observed-effect concentration (NOEC) are typically defined as the lowest tested concentration that is significantly different from the control and the tested concentration immediately below the LOEC that is not statistically significant, respectively, when compared to the control (Quantics Biostatistics, 2016). We used the LOEC and NOEC as the lowest tested pH that was statistically different from the control and the tested pHNBS immediately below the LOEC that was not statistically different from the control, respectively. Carbonate chemistry data are shown as the average of all replicates ± 1 standard deviation. Note that standard deviation for pHNBS values was well below the manufacturer's stated uncertainty; therefore, for this parameter, we display the known uncertainty of ± 0.2 units.
3.1 Experimental conditions
Carbonate speciation parameters were evaluated for geochemical context for the biological experiments. The initial pHNBS prior to base addition averaged 8.0 ± 0.2 and 7.9 ± 0.1 for the sea hare and isopod experiments, respectively (Table 1). The initial measured pCO2 prior to base addition was above atmospheric saturation for both the sea hare and isopod experiments (647 ± 159 and 753 ± 134 ppm, respectively). The initial seawater TA prior to base addition or incubation as calculated from the pHNBS and pCO2 was 2173 ± 522 and 2170 ± 359 µmol kg−1 for the sea hare and isopod experiments, respectively. Immediately after base addition and prior to closing off the jars, the pCO2 calculated from the measured pHNBS and enhanced alkalinity (i.e., initial calculated TA plus the measured addition of NaOH) dropped to below atmospheric saturation for all treatments and was near zero for the pHNBS 9.3 treatment (Table 1). On average, 212, 612, and 1142 µmol kg−1 of alkalinity was added to the pHNBS 8.3, 8.8, and 9.3 sea hare experiments and 233, 637, and 1,246 µmol kg−1 of alkalinity was added to the pHNBS 8.3, 8.8, and 9.3 isopod experiments, respectively. The maximum aragonite saturation state calculated increased from 1.4 ± 0.7 to 16.2 ± 0.2 in the sea hare experiments and from 1.3 ± 0.6 prior to base addition to 16.7 ± 1.2 in the pHNBS 9.3 treatment for the isopod experiments (Table 1).
The initial DIC calculated from measured pHNBS and pCO2 was 2062 ± 476 and 2078 ± 315 µmol kg−1 for sea hares and isopods, respectively. We assume that there was no significant change in DIC due to atmospheric uptake of CO2 following the alkalinity addition because of the short timeline of the experiments and the disruption in air–sea gas exchange caused by the closed-lid experimental setup. DIC increase throughout the incubations in both the jars with and without organisms can be attributed to either microbial or animal respiration. However, given the large uncertainty in our carbonate measurement methods and calculations, observed changes in DIC,pCO2, and alkalinity were negligible and not a focus of this study.
Table 2Summary table of ecotoxicological results from each week of the experiment for sea hares and isopods. NOEC represents the no-observed-effect concentration, LOEC represents the lowest-observed-effect concentration, and LC50 represents the lethal concentration 50. pH is reported using the NBS scale.
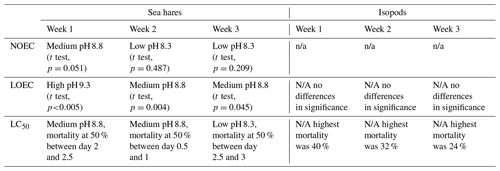
n/a: not applicable.
Other water quality parameters changed slightly in the final (i.e., incubated) versus initial time points (Table 1). Dissolved O2 (DO) declined as a result of animal and microbial respiration but remained oxic between each round of seawater refreshes. Likewise, pHNBS decreased slightly, but the measured pHNBS values of each treatment were still distinct from one another (Table 1).
3.2 Animal mortality
Sea hare mortality was observed in the control and all treatment groups, ranging from the lowest total mortality (37 %) in the control group at pHNBS 7.8 to 100 % mortality (all three rounds resulted in 100 % mortality) at pHNBS 9.3 when averaged over all three rounds of the experiment (Fig. 2a). Results from each individual round of the experiment are provided in the Supplement. At this highest pHNBS target (9.3), 100 % mortality was observed after the eighth water refresh (day 4) in the first round, after the fourth water refresh (day 2) in the second round, and after the fifth water refresh (day 3) in the third and final round. The other treatment groups never saw 100 % mortality, with the low-pHNBS treatment (8.3) resulting in 57 % mortality averaged over the three rounds and the medium-pHNBS treatment (8.8) resulting in 80 % mortality averaged over the three rounds. Overall, sea hares experienced 50 % mortality at pHNBS 8.3 after the sixth water refresh (day 3), whereas 50 % mortality was reached after the fifth water refresh (day 3) at pHNBS 8.8 (although as early as second water refresh in one of the three rounds) and after the second water refresh (day 1) at pHNBS 9.3 (see the Supplement for the LC50 plots). A 50 % mortality rate did not occur in the control group (pHNBS 7.8). The lowest-observed-effect concentration (LOEC) that caused significantly more mortality than the control group for sea hares was a target pHNBS of 9.3 in week 1 (t test, p<0.005) and a target pHNBS of 8.8 in weeks 2 and 3 (t test, p=0.004, p=0.045), corresponding to an alkalinity addition of ∼ 1247 and ∼ 638 µmol kg−1, respectively (Table 2). The no-observed-effect concentration (NOEC) for sea hares was a target pHNBS of 8.3, corresponding to an alkalinity addition of ∼ 234 µmol kg−1. For isopods, the LOEC and NOEC could not be determined, as no mortality in any treatment group was statistically higher than mortality in the control group.
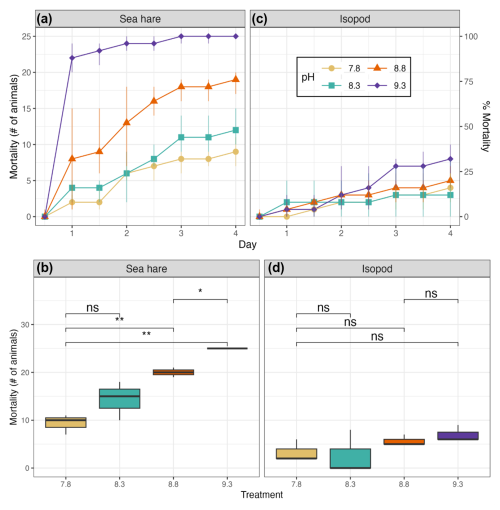
Figure 2Average mortality of sea hares (a) and isopods (c) is shown over the 4 d period over the three rounds of the experiment and for each treatment group. Yellow circles indicate the chemical control at pHNBS≈7.8 with no addition of alkalinity, blue squares indicate pHNBS≈8.3, orange triangles indicate pHNBS≈8.8, and purple diamonds indicate the highest alkalinity treatment at pHNBS≈9.3. Mortality of sea hares (b) and isopods (d) in each treatment group were averaged over the three rounds of the experiment. The t test p values are represented as ns (non-significant) and (significant, p value <0.05). The y axes on the left represent the number of animals, whereas the y axes on the right represent percentages.
The ANOVA test showed significant differences in sea hare mortality between the four treatment groups (ANOVA, p<0.005). Further analysis with a Tukey test (Table 4) showed that the mortality in the high-pH treatment group was significantly higher than mortality in the control and low-pH treatment groups (Tukey HSD, p<0.001 and p=0.002, respectively) and mortality in the medium-pH treatment group was significantly higher than in the control group (Tukey HSD, p=0.002).
Averaged over the course of all three rounds, a clear trend in increasing mortality correlating with increasing pH can be observed for sea hares (Fig. 2b). The low treatment group displayed the most variation in mortality over the three rounds, whereas the high treatment group showed the least variation in mortality (due to each round resulting in 100 % mortality). The average mortality was not significantly higher in the low treatment group compared to the control group, but it was significantly higher for both the medium and high treatment groups (Fig. 2b).
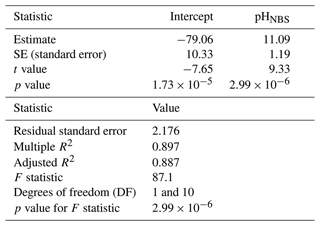
A simple linear regression model was fitted to sea hare mortality to test if pH explained the variation in the data. Results indicate that pH is a significant predictor of sea hare mortality (lm, ) and that pH explains a large percentage of the variation in sea hare mortality (lm, R2 = 0.897) (Table 3).
Table 4The ANOVA and Tukey HSD p values for sea hare and isopod mortality comparisons between treatments. Asterisks (*) indicate a significant p value of p<0.05.
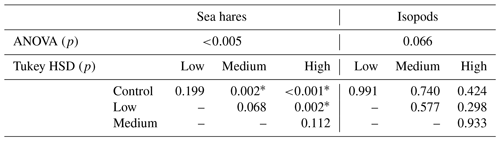
Isopod average mortality appears to show a slight increasing trend with each treatment group; however, the overlap between treatment groups is considerable and shows high levels of variation in results between the three rounds of experiments (Fig. 2d). Isopod mortality never reached 50 % for any treatment group in any round of the experiment (Fig. 2d). Averaged over the three experimental rounds, the control group (pHNBS 7.8) experienced 13 % mortality, whereas the high treatment group (pHNBS 9.3) experienced 21 % mortality. There were no significant differences in mortality between any of the treatment groups (ANOVA, p=0.066; Table 4). In addition to the low mortality observed in every treatment group, isopod molting and reproduction was also observed throughout each round of the experiment.
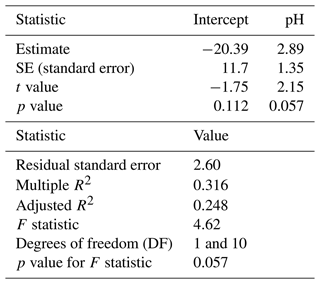
A simple linear regression model was fitted to isopod mortality to test if pH explained the variation in the data. Results indicate that pH is not a significant predictor of isopod mortality (lm, p=0.057) and that a small percentage of variability in mortality can be explained by pH (lm, R2 = 0.316) (Table 5).
Few previous studies have investigated the effect of increased pH and alkalinity on various marine organisms, as briefly described in Sect. 1. Results from these previous studies and the present experiments indicate that different species will likely have different responses to increased pH, depending on their physiological vulnerabilities (Albright et al., 2016; Comeau et al., 2017; Cripps et al., 2013; Gore et al., 2019). Investigation of the potential vulnerabilities of local marine species can help inform decision-making in the mCDR planning process regarding the placement of highly alkaline seawater outflow pipes in nearshore environments. This will also allow for targeted monitoring of specific species that might be more sensitive within the mixing zone of mCDR projects.
4.1 Chemical response
In the experimental setup, the glass jars were closed tightly and contained active microbial communities from the raw seawater. The PNNL wet laboratory has typical indoor laboratory lighting without windows for natural light. We did not subject the jars to grow lights or nighttime lighting; therefore, algal production inside the jars likely slowed or stopped while microbial respiration continued. This respiration would have resulted in CO2 production and O2 consumption, leading to high pCO2 measurements in the chemical control jars. In the jars with organisms present, CO2 production was a result of microbial and animal respiration, which could lead to increasing pCO2 and declining pH. To maintain our target pHNBS treatment, water was refreshed twice daily with seawater treated with the appropriate amount of NaOH to reach the desired pH treatment. When sealing water in a closed system without natural light, the outcome is generally net heterotrophy and CO2 production. It is essentially impossible to culture organisms with no changes in CO2 in a closed system, without using a chemostat system that constantly refreshes the water. We note that the large uncertainties in our seawater carbonate measurements and calculations limit the further description of CO2 dynamics in this study, which primarily required an understanding of enhanced alkalinity and increased pH at each treatment level.
It is worth noting that precipitation was visually observed but rapidly dissipated upon mixing during the initial treatment of seawater with NaOH, indicating that this was likely Mg(OH)2 (Hartmann et al., 2023; Ringham et al., 2024; Suitner et al., 2024). No additional precipitation was observed after incubation or before refreshing water throughout the experiments at any treatment level, suggesting that these experiments did not surpass thresholds for runaway CaCO3 precipitation, a condition in which more alkalinity is removed by precipitation than was initially added by the alkalinity treatment (Moras et al., 2022; Hartmann et al., 2023; Suitner et al., 2024). Determination of precipitation thresholds is a major area of research for OAE, as CaCO3 precipitation can reverse the intended effect of OAE by removing alkalinity from the surface ocean and releasing CO2 to the water column. In addition, the increased turbidity resulting from precipitation may impact photosynthesis and predator–prey interactions in the natural environment. Understanding connections between changes in carbonate chemistry and biological activity is crucial for characterizing potential interactions between OAE and the biota in the receiving environment.
4.2 Biological responses
The two eelgrass epifauna invertebrates investigated in this study, sea hares and isopods, responded differently to seawater treated with NaOH. In increasingly alkaline environments with increasing pH, sea hares exhibited higher mortality in a shorter amount of time than isopods. A previous study investigating resilience to ocean acidification in sea hares and isopods found opposing results, in that sea hare mortality was low while isopod mortality was high; nonetheless, the authors suspected that isopod mortality was likely not due to pH (Hughes et al., 2017).
In addition to showing higher survivorship, isopods exhibited growth and reproductive behaviors during the experiments. The process of molting for eelgrass isopods promotes growth and also provides the animals with an opportunity to reproduce (Kuris et al., 2007; Sadro, 2001). The isopods in the experiment exhibited molting in the control group and all treatment groups in all three rounds of the experiment, indicating that growth and reproduction still occurred in highly alkaline waters. The variation in mortality between the control and treatment groups was small and statistically not significant, demonstrating that the mortality observed was likely not due to variations in pH. During the experiment, isopods were observed cannibalizing each other in the control and all treatment groups. It is possible that the mortality we observed during the experiment could have been due to lack of food rather than changes in pH. A previous study showed that Idotea resecata individuals were the fastest daily consumers of eelgrass among six common mesograzer species (Best and Stachowicz, 2012), suggesting they may not handle 4 d of fasting well. However, there is limited information on eelgrass isopod biology, and this warrants further studies. Besides the high mortality, the sea hares did not exhibit any observable behaviorial changes due to the pH treatment.
Nearshore ecosystems are likely targets for OAE projects in the near term for many reasons, including (1) reduced challenging logistics of permitting and developing offshore facilities and the need for power and material movement from an OAE project site and (2) the potential co-benefits of locally increasing pH to counter to acidification trends. The experiments presented here establish the responses and limits of sea hares and eelgrass isopods if continuously exposed to high pH levels. In an actual OAE deployment (field trial or commercial scale), such outcomes would only be observed within the acute mixing zone if eelgrass meadows and their associated epifauna were present; if the animals were unable to move away; and if alkalinity releases were continuous, with the trajectory of the point-source alkalinity plume traveling across the eelgrass habitat in such a way as to expose the animals to high pH and alkalinity levels without acclimatization and with constant exposure. While NPDES regulations may require OAE outfalls and mixing zones to be located away from eelgrass populations, nearby meadows may grow toward a mixing zone over time if the conditions are suitable. Thus, it is necessary to better understand what to potentially expect for these meadows and their epifaunal communities in order to provide sound scientific information to regulatory agencies and OAE developers. The PNNL-Sequim campus, located near an eelgrass bed, currently hosts a demonstration electrodialysis OAE system that generates alkalinity from seawater in a laboratory setting, and it will likely perform field trials in the future. The data produced by the present experiments are essential for assessing the potential impact of such trials and deployments, and they will also will help to pave the way for responsible permitting and regulation of OAE deployments.
Species-specific research is critical to understanding physiological and behavioral changes in organisms exposed to mCDR-generated conditions. In future work, extrapolation to ecosystem-level impacts is of major interest in this field, as mCDR projects are evaluated on the “additionality” of climate mitigation strategies; potential environmental impacts; and, critically, comparison to the business-as-usual counterfactual, in the absence of mCDR interventions, which includes the progression of OA. This study is a step between standard chemical toxicity studies and incubations targeted at representing constant, elevated pH and alkalinity within the acute mixing zone of an OAE intervention. However, modifications to the experimental design could result in studies that more realistically represent the plume and mixing zone of an OAE deployment.
4.3 Limitations and future recommendations
The species in this study were chosen for several reasons: they are ecologically important as a feedstock for culturally and economically valuable salmon and serve an important niche in grazing on eelgrass, they are locally available in PNNL mesocosm tanks, and they represent a higher trophic level (i.e., macroinvertebrates) compared with the species typically studied for biological responses to OAE (i.e., primary producers, especially phytoplankton). However, we note that little information is available on the impact of pH (high or low) on these organisms under natural or OA conditions, complicating the OAE research on these species. In addition, alternate methods for the characterization of seawater carbonate chemistry would allow for improved resolution of the existing study. DIC and TA titration were not available on-site at the beginning of this experiment, which resulted in the use of less-precise pH sensing (YSI) and in pCO2 gas measurements that are less precise at undersaturation. Improved sampling protocols would allow for higher confidence that animals were exposed to consistent pH and alkalinity treatments across water changes. To avoid air exchange causing the pH to drop below the target level, we secured the experiment jars with airtight lids. This also prevented any animals from escaping the experiment. While this kept the pHNBS within 0.1 of our target pHNBS, it did cause a decrease in dissolved oxygen levels between water changes (on average, −2.67 mg L−1 for sea hares and −0.57 mg L−1 for isopods). This was mitigated by refreshing the water twice daily; however, in an ideal experiment, we would have a flow-through system with a continuous flow of treated water so the organisms would have ample oxygen and the water would not need to be manually refreshed, reducing the need for physically handling the organisms, which would, in turn, reduce unnecessary stress on sensitive species. Additionally, a flow-through system of unfiltered, untreated water as a chemical control would allow for a comparison between pH shifts due to natural variability and consistently elevated pH. Due to funding and technical considerations, such as wastewater safety, a flow-through system was not feasible at the time of this study. We note that pH scaling is an important factor in interpreting results: pH in the NBS and total scales may differ by more than 0.1 unit. Comparison of biological responses to elevated alkalinity and pH between studies or between laboratory manipulation and natural conditions should include careful consideration of this factor.
Following ASTM 2000 standards for acute toxicity tests (ASTM International, 2000), the experiments were conducted under starvation conditions. Thus, the observed responses in the survivability of the sea hares and isopods might have been intensified by this starvation, leading to an overstretched result from an OAE perspective. The pH and alkalinity sensitivity of healthy (not malnourished) invertebrates in the mixing zone of an OAE outfall may differ from what was observed in the experiments, potentially with greater survivability at higher pH. In addition to the pH treatments conducted in this study, alkalinity additions that reflect more complex scenarios could be added, including the following: acclimation of organisms to increasing levels of alkalinity to represent initial test phases of OAE deployments; experiments conducted under varying temperature, salinity, and initial carbonate chemistry conditions to represent the range of natural seasonal variation; and varied durations of high alkalinity and pH exposure to represent realistic OAE deployments and the potential variation in dilution and advection of an alkalinity plume in time and space. Additionally, the dependence of mortality on the duration of exposure is critical to the practical application of these results. In practice, the maximum pH resulting from an OAE intervention will be observed at the outfall or point of dispersal. The pH will decrease rapidly with distance through the mixing zone until the alkaline plume has diluted enough to where it will be indistinguishable from natural variation in the open environment (Ho et al., 2023; Wang et al., 2023). If the species are mobile over an area larger than the mixing zone radius, they may only experience the mixing zone maximum pH for some period of time. However, immobile species or species with small mobility ranges (such as sea hares and isopods) located in the mixing zone will continuously experience altered water quality. Understanding the potential exposure of OAE projects on marine organisms will likely involve a combination of near-field dilution modeling of the release of alkalinity into seawater and in situ sensing for pH changes within the mixing zone. Studies investigating the impact of increasing alkalinity and pH on specific species should take into account the natural chemical variations experienced before an OAE perturbation, the range of chemical changes during an OAE perturbation based on the expected dilution of alkalinity and pH in time and space at varying distances from an outfall, and the potential for acclimatization to increased alkalinity and pH as OAE projects scale up from pilot experiments.
Experiments like these will provide important baseline information for optimizing, permitting, and deploying OAE systems, particularly in coastal waters where shallow well-mixed waters interact strongly with benthic biological communities that host organisms (such as sea hares and isopods) that are critical to marine food webs. Future experimental research should focus on additional commercially, culturally, or ecologically important species that are relevant to OAE deployment sites. This work should be scaled up to understand the effects of alkalinity enhancement at an ecosystem level, using both in situ and ex situ mesocosms, allowing for more realistic representations of natural ecosystems with both benthic and pelagic species, while confining the alkalinity treatment (Riebesell et al., 2023). In parallel, numerical modeling studies that most accurately depict the realistic impacts and extent of an OAE project on seawater carbonate and other chemistries in time and space will allow for a more accurate prediction of the habitats and species that will be impacted by the release of alkalinity, the extent to which they are exposed to conditions of elevated pH and alkalinity, and the potential indirect impacts on supported food webs. This will, in turn, inform future experimental design and monitoring needs for OAE deployment.
Raw data are available from https://doi.org/10.25921/53s1-h309 (Jones et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-1615-2025-supplement.
KJ: investigation, formal analysis, visualization, writing – original draft preparation, and writing – review and editing. LGH: conceptualization, methodology, supervision, writing – original draft preparation, and writing – review and editing. NDW: funding acquisition, methodology, project administration, writing – original draft preparation, and writing – review and editing. PJR: formal analysis, visualization, writing – original draft preparation, and writing – review and editing. MCR and MDE: writing – original draft preparation and writing – review and editing.
During the period of this work, Matthew D. Eisaman was a co-founder and chief scientific advisor at Ebb Carbon, Inc.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Environmental impacts of ocean alkalinity enhancement”. It is not associated with a conference.
This study was led by Pacific Northwest National Laboratory, which is operated for the US Department of Energy by Battelle Memorial Institute under contract DE-AC05-76RL01830. Financial support was provided by the US Department of Energy's Water Power Technologies Office Laboratory Research Program. We would like to thank Brendan Carter, who led the Electrochemical Acid Sequestration to Ease Ocean Acidification project, for his assistance throughout and Chinmayee Subban for her leadership on this project. We thank the Ebb Carbon team members, especially Tyson Minck, for helping us understand the electrochemical OAE process and for assisting with the experimental setup. The team also acknowledges Kira Burch and Grace Weber (former PNNL interns) and Tristen Myers (PNNL researcher) for their help running the experiments, Jakob Bueche (PNNL staff member) for assisting with the experimental setup, and Ioana Bociu and Corey O'Donnell for assisting with permitting and compliance.
The authors wish to express that Matthew Eisaman was a dedicated and inspiring scientist and innovator. We are deeply saddened by his passing and aspire to honor his legacy in the pursuit of sustainable scientific solutions.
This research has been supported by the Water Power Technologies Office (grant no. 80654).
This paper was edited by Kai G. Schulz and reviewed by two anonymous referees.
Albright, R., Caldeira, L., Hosfelt, J., Kwiatkowski, L., Maclaren, J. K., Mason, B. M., Nebuchina, Y., Ninokawa, A., Pongratz, J., Ricke, K. L., Rivlin, T., Schneider, K., Sesboüé, M., Shamberger, K., Silverman, J., Wolfe, K.., Zhu, K., and Caldeira, K.: Reversal of ocean acidification enhances net coral reef calcification, Nature, 531, 362–365, 2016.
ASTM International: Standard Guide for Conducting Acute Toxicity Tests on Test Materials with Fishes, Macroinvertebrates, and Amphibians, in: Annual Book of Standards, Vol. 11.06, ASTM International, Pennsylvania, USA, 23, https://doi.org/10.1520/E0729-23, 2000.
Beeman, R.: Notes on the California Species of Aplysia (Gastropoda, Opisthobranchia), Veliger, 5, 145–147, 1963.
Best, R. J. and Stachowicz, J. J.: Trophic cascades in seagrass meadows depend on mesograzer variation in feeding rates, predation susceptibility, and abundance, Mar. Ecol. Prog. Ser., 456, 29–42, https://doi.org/10.3354/meps09678, 2012.
Bianucci, L., Long, W., Khangaonkar, T., Pelletier, G., Ahmed, A., Mohamedali, T., Roberts, M., and Figueroa-Kaminsky, C.: Sensitivity of the regional ocean acidification and carbonate system in Puget Sound to ocean and freshwater inputs, Elementa, 6, 22, https://doi.org/10.1525/elementa.151, 2018.
Bridges, C.: Larval development and life history of Phyllaplysia taylori dall (Opistobranchiata: Anaspidea), M.S. Thesis, University of the Pacific, 115 pp., https://scholarlycommons.pacific.edu/uop_etds/1798 (last access: 27 August 2024), 1973.
Comeau, L. A., Sonier, R., Guyondet, T., Landry, T., Ramsay, A., and Davidson, J.: Behavioural response of bivalve molluscs to calcium hydroxide, Aquaculture, 466, 78–85, https://doi.org/10.1016/j.aquaculture.2016.09.045, 2017.
Cotter, E., Regier, P., Walters, E., Ward, N., and Cavagnaro, R. J.: Autonomous measurements of carbon dioxide uptake in a blue carbon habitat, Pacific Northwest National Laboratory, 19 pp., https://doi.org/10.2172/1984287, 2022.
Cripps, G., Widdicombe, S., Spicer, J. I., and Findlay, H. S.: Biological impacts of enhanced alkalinity in Carcinus maenas, Mar. Pollut. Bull., 71, 190–198, https://doi.org/10.1016/j.marpolbul.2013.03.015, 2013.
Cross, J., Sweeney, C., Jewett, E., Feely, R., McElhany, P., Carter, B., Stein, T., Kitch, G., and Gledhill, D.: Strategy for NOAA Carbon Dioxide Removal Research, NOAA, 81 pp., https://doi.org/10.25923/gzke-8730, 2023.
Cyronak, T., Kapsenberg, L., Palter, J., Schulz, K. K. G., and Grasse, P.: Environmental impacts of ocean alkalinity enhancement [Special Issue]. Biogeosciences, 2024.
de Lannoy, C.-F., Eisaman, M.D., Jose, A., Karnitz, S. D., DeVaul, R. W., Hannun, K., and Rivest, J. L. B.: Indirect ocean capture of atmospheric CO2: Part I. Prototype of a negative emissions technology, Int. J. Greenh. Gas Con., 70, 243–253, 2018.
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A.: Ocean Acidification: The Other CO2 Problem, Annu. Rev. Mar. Sci., 1, 169–192, https://doi.org/10.1146/annurev.marine.010908.163834, 2009.
Doney, S. C., Busch, D. S., Cooley, S. R., and Kroeker, K. J.: The Impacts of Ocean Acidification on Marine Ecosystems and Reliant Human Communities, Annu. Rev. Env. Resour., 45, 83–112, https://doi.org/10.1146/annurev-environ-012320-083019, 2020.
Eisaman, M. D., Rivest, J. L. B., Karnitz, S. D., De Lannoy, C.-F., Jose, A., DeVaul, R. W., and Hannun, K.: Indirect Ocean Capture of Atmospheric CO2: Part II. Understanding the Cost of Negative Emissions, Int. J. Greenh. Gas Con., 70, 254–261, https://doi.org/10.1016/j.ijggc.2018.02.020, 2018.
Eisaman, M. D., Geilert, S., Renforth, P., Bastianini, L., Campbell, J., Dale, A. W., Foteinis, S., Grasse, P., Hawrot, O., Löscher, C. R., Rau, G. H., and Rønning, J.: Assessing the technical aspects of ocean-alkalinity-enhancement approaches, in: Guide to Best Practices in Ocean Alkalinity Enhancement Research, edited by: Oschlies, A., Stevenson, A., Bach, L. T., Fennel, K., Rickaby, R. E. M., Satterfield, T., Webb, R., and Gattuso, J.-P., Copernicus Publications, State Planet, 2-oae2023, 3, https://doi.org/10.5194/sp-2-oae2023-3-2023, 2023.
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C.: Impacts of ocean acidification on marine fauna and ecosystem processes, ICES J. Mar. Sci., 65, 414–432, https://doi.org/10.1093/icesjms/fsn048, 2008.
Fassbender, A. J., Alin, S. R., Feely, R. A., Sutton, A. J., Newton, J. A., Krembs, C., Bos, J., Keyzers, M., Devol, A., Ruef, W., and Pelletier, G.: Seasonal carbonate chemistry variability in marine surface waters of the US Pacific Northwest, Earth Syst. Sci. Data, 10, 1367–1401, https://doi.org/10.5194/essd-10-1367-2018, 2018.
Feng, E. Y., Koeve, W., Keller, D. P., and Oschlies, A.: Model-Based Assessment of the CO2 Sequestration Potential of Coastal Ocean Alkalinization, Earth's Future, 5, 1252–1266, 2017.
Fox, J. and Weisberg, S.: An R Companion to Applied Regression, 3rd Edn., Sage, Thousand Oaks CA, R [code], https://www.john-fox.ca/Companion/ (last access: 27 August 2024), 2019.
Gore, S., Renforth, P., and Perkins, R.: The potential environmental response to increasing ocean alkalinity for negative emissions, Mitig. Adapt. Strateg. Glob. Change, 24, 1191–1211, https://doi.org/10.1007/s11027-018-9830-z, 2019.
Government of Canada: What is a LD50 and LC50?: https://www.ccohs.ca/oshanswers/chemicals/ld50.html, last access: 29 January 2024.
Hartmann, J., Suitner, N., Lim, C., Schneider, J., Marín-Samper, L., Arístegui, J., Renforth, P., Taucher, J., and Riebesell, U.: Stability of alkalinity in ocean alkalinity enhancement (OAE) approaches–consequences for durability of CO2 storage, Biogeosciences, 20, 781–802, https://doi.org/10.5194/bg-20-781-2023, 2023.
Harvey, L.: Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions, J. Geophys. Res.-Ocean., 113, C04028, https://doi.org/10.1029/2007JC004373, 2008.
Ho, D. T., Bopp, L., Palter, J. B., Long, M. C., Boyd, P. W., Neukermans, G., and Bach, L. T.: Monitoring, reporting, and verification for ocean alkalinity enhancement, in: Guide to Best Practices in Ocean Alkalinity Enhancement Research, edited by: Oschlies, A., Stevenson, A., Bach, L. T., Fennel, K., Rickaby, R. E. M., Satterfield, T., Webb, R., and Gattuso, J.-P., Copernicus Publications, State Planet, 2-oae2023, 12, https://doi.org/10.5194/sp-2-oae2023-12-2023, 2023.
Horwitz, R., Norin, T., Watson, S., Pistevos, J., Beldade, R., Hacquart, S., Gattuso, J-P., Rodolfo-Metalpa, R., Vidal-Dupiol, J., Killen, S., and Mills, S.: Near-future ocean warming and acidification alter foraging behaviour, locomotion, and metabolic rate in a keystone marine mollusc, Sci. Rep., 10, 5461, https://doi.org/10.1038/s41598-020-62304-4, 2020.
Hughes, B. B., Lummis, S. C., Anderson, S. C., and Kroeker, K. J.: Unexpected resilience of a seagrass system exposed to global stressors, Glob. Change Biol., 24, 224–234, https://doi.org/10.1111/gcb.13854, 2017.
Ilyina, T., Wolf-Gladrow, D., Munhoen, G., and Heinze, C.: Assessing the potential of calcium-based artificial ocean akalinization to mitigate rising atmospheric CO2 and ocean acidification, Geophys. Res. Lett., 40, 5909–591, https://doi.org/10.1002/2013GL057981, 2013.
IPCC: Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Masson Delmotte, V., Zhai, P., Pirani, A., Connors, S. L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M. I., Huang, M., Leitzell, K., Lonnoy, E., Matthews, J. B. R., Maycock, T. K., Waterfield, T., Yelekçi, O., Yu, R., and Zhou, B.: Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 3–32, https://doi.org/10.1017/9781009157896.001, 2022.
Jones, K., Hemery, L. G., Ward, N. D., Regier, P. J., Ringham, M. C., and Eisaman, M. D.: Biological response of eelgrass epifauna, Taylor’s Sea hare (Phyllaplysia taylori) and eelgrass isopod (Idotea resecata), to elevated ocean alkalinity from 2023-07-24 to 2023-09-29, National Centers for Environmental Information [data set], https://doi.org/10.25921/53s1-h309, 2025.
Kassambara, A.: Ggpubr: “Ggplot2” Based Publication Ready Plots, R [code], https://rpkgs.datanovia.com/ggpubr/ (last access: 27 August 2024), 2022.
Kassambara, A.: rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R package version 0.7.2, R [code], https://CRAN.R-project.org/package=rstatix (last access: 27 August 2024), 2023.
Kennedy, L. A., Juanes, F., and El-Sabaawi, R.: Eelgrass as Valuable Nearshore Foraging Habitat for Juvenile Pacific Salmon in the Early Marine Period, Mar. Coast Fish., 10, 190–203, https://doi.org/10.1002/mcf2.10018, 2018.
Kheshgi, H. S.: Sequestering atmospheric carbon dioxide by increasing ocean alkalinity, Energy, 20, 915–922, 1995.
Köhler, P., Hartmann, J., and Wolf-Gladrow, D. A.: Geoengineering potential of artificially enhanced silicate weathering of olivine, P. Natl. Acad. Sci. USA, 107, 20228–20233, 2010.
Koschorreck, M., Prairie, Y. T., Kim, J., and Marcé, R.: Technical note: CO2 is not like CH4 – limits of and corrections to the headspace method to analyse pCO2 in fresh water, Biogeosciences, 18, 1619–1627, https://doi.org/10.5194/bg-18-1619-2021 2021.
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., Duarte, C. M., and Gattuso, J.-P.: Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming, Glob. Change Biol., 19, 1884–1896, https://doi.org/10.1111/gcb.12179, 2013.
Kuris, A., Sadeghian, P., Carlton, J., and Campos, E.: Decapoda, in: The Light and Smith Manual: Intertidal invertebrates from central California to Oregon, University of California Press, 632–656, https://doi.org/10.2307/jj.5973107, 2007.
La Plante, E., Chen, X., Bustillos, S., Bouissonnie, A., Traynor, T., Jassby, D., Corsini, L., Simonetti, D., and Sant, G.: Electrolytic seawater mineralization and the mass balances that demonstrate carbon dioxide removal, ACS EST Engg, https://doi.org/10.1021/acsestengg.3c00004, 2023.
Lewis, J. T. and Boyer, K. E.: Grazer Functional Roles, Induced Defenses, and Indirect Interactions: Implications for Eelgrass Restoration in San Francisco Bay, Diversity, 6, 751–770, https://doi.org/10.3390/d6040751, 2014.
Lu, X., Ringham, M., Hirtle, N., Hillis, K., Shaw, C., Herndon, J., Carter, B. R., and Eisaman, M. D.: Characterization of an Electrochemical Approach to Ocean Alkalinity Enhancement, AGU Fall Meeting Abstracts, 2022AGUFMGC31C..01L, 2022.
Montserrat, F., Renforth, P., Hartmann, J., Leermakers, M., Knops, P., and Meysman, F. J. R.: Olivine dissolution in seawater: implications for CO2 sequestration through enhanced weathering in coastal environments, Environ. Sci. Technol., 51, 3960–3972, 2017.
Moras, C. A., Bach, L. T., Cyronak, T., Joannes-Boyau, R., and Schulz, K. G.: Ocean alkalinity enhancement – avoiding runaway CaCO3 precipitation during quick and hydrated lime dissolution, Biogeosciences, 19, 3537–3557, https://doi.org/10.5194/bg-19-3537-2022, 2022.
National Academies of Sciences, Engineering, and Medicine: A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration, National Academies Press, 308 pp., https://doi.org/10.17226/26278, 2022.
National Research Council: Effects of Ocean Acidification on the Physiology of Marine Organisms, in: Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean, National Academies Press, 45–58, https://doi.org/10.17226/12904, 2010.
Nduagu, E.: Production of Mg(OH)2 from Mg-silicate rock for CO2 mineral sequestration, Dissertation for Abo Akademi University, ISBN: 978-952-12-2821-6, 2012.
Oschlies, A., Stevenson, A., Bach, L. T., Fennel, K., Rickaby, R. E. M., Satterfield, T., Webb, R., and Gattuso, J.-P. (Eds.): Guide to Best Practices in Ocean Alkalinity Enhancement Research, Copernicus Publications, State Planet, 2-oae2023, https://doi.org/10.5194/sp-2-oae2023, 2023.
Pierrot, D., Epitalon, J.-M., Orr, J. C., Lewis, E., and Wallace, D. W. R.: MS Excel program developed for CO2 system calculations – version 3.0, GitHub, https://github.com/dpierrot/co2sys_xl (last access: 27 January 2025), 2021.
Pinheiro, J., Bates, D., and R Core Team: nlme: Linear and Nonlinear Mixed Effects Models, R package version 3.1-164, R [code], https://CRAN.R-project.org/package=nlme (last access: 27 August 2024), 2023.
Portner, H. O., Bock, C., and Reipschläger, A.: Modulation of the Cost of pHi Regulation During Metabolic Depression: A 31P-NMR Study in Invertebrate (Sipunculus Nudus) Isolated Muscle, J. Exp.Biol., 203, 2417–2428, https://doi.org/10.1242/jeb.203.16.2417, 2000.
Quantics Biostatistics: Ecotoxicology – NOEC and LOEC, https://www.quantics.co.uk/blog/ecotoxicology-noec-and-loec/ (last access: 22 August 2024), 2016.
R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, R [code], https://www.R-project.org/ (last access: 27 August 2024), 2023.
Rau, G. H.: Electrochemical splitting of calcium carbonate to increase solution alkalinity: Implications for mitigation of carbon dioxide and ocean acidity, Environ. Sci. Technol., 42, 8935–8940, 2008.
Regier, P., Ward, N.D., Izquierdo, A., Baldwin, A.H., Day, D., McElhinny, J., Patel, K., Vargas, R., Zheng, J., Exchange Consortium, and Myers-Pigg, A.: Coastal inundation regime moderates the short-term effects of sediment and soil additions on seawater oxygen and greenhouse gas dynamics: a microcosm experiment, Front. Mar. Sci., 10, 1308590, https://doi.org/10.3389/fmars.2023.1308590, 2023.
Renforth, P. and Henderson, G.: Assessing ocean alkalinity for carbon sequestration, Rev. Geophys., 55, 636–674, 2017.
Ricketts, E. F. and Calvin, J.: Between Pacific tides: Iodetea resecata, in: Oregon Estuarine Invertebrates: Rudys' Illustrated Guide to Common Species, 3rd Edn., edited by: Hiebert, T. C., Butler, B. A., and Shanks, A. L.,University of Oregon Libraries and Oregon Institute of Marine Biology, Charleston, OR, account of the habits and habitats of some five hundred of the common, conspicuous seashore invertebrates of the Pacific Coast between Sitka, Alaska, and Northern Mexico, Stanford, Stanford University Press, Stanford, 1952.
Riebesell, U., Basso, D., Geilert, S., Dale, A. W., and Kreuzburg, M.: Mesocosm experiments in ocean alkalinity enhancement research, in: Guide to Best Practices in Ocean Alkalinity Enhancement Research, edited by: Oschlies, A., Stevenson, A., Bach, L. T., Fennel, K., Rickaby, R. E. M., Satterfield, T., Webb, R., and Gattuso, J.-P., Copernicus Publications, State Planet, 2-oae2023, 6, https://doi.org/10.5194/sp-2-oae2023-6-2023, 2023.
Rigopoulos, I., Harrison, A. L., Delimitis, A., Ioannou, I., Efstathiou, A. M., Kyratsi, T., and Oelkers, E. H.: Carbon sequestration via enhanced weathering of peridotites and basalts in seawater, Appl. Geochem., 91, 197–207, 2018.
Ringham, M. C., Hirtle, N., Shaw, C., Lu, X., Herndon, J., Carter, B. R., and Eisaman, M. D.: An assessment of ocean alkalinity enhancement using aqueous hydroxides: kinetics, efficiency, and precipitation thresholds, Biogeosciences, 21, 3551–3570, 2024, https://doi.org/10.5194/bg-21-3551-2024
Rogelj, J., Popp, A., Calvin, K. V., Luderer, G., Emmerling, J., Gernaat, D., Fujimori, S., Strefler, J., Hasegawa, T., Marangoni, G., Krey, V., Kriegler, E., Riahi, K., van Vuuren, D. P., Doelman, J., Drouet, L., Edmonds, J., Fricko, O., Harmsen, M., Havlík, P., Humpenöder, F., Stehfest, E., and Tavoni, M.: Scenarios towards limiting global mean temperature increase below 1.5 °C, Nat. Clim. Change, 8, 325–332, https://doi.org/10.1038/s41558-018-0091-3, 2018.
Ross, P. M., Parker, L., O'Connor, W. A., and Bailey, E. A.: The Impact of Ocean Acidification on Reproduction, Early Development and Settlement of Marine Organisms, Water, 3, 1005–1030, https://doi.org/10.3390/w3041005, 2011.
Sadro, S.: Arthropoda: Decapoda, in: Identification guide to larval marine invertebrates of the Pacific Northwest, Oregon State University Press, ISBN 9780870715310, 176–178, 2001.
Shaw, C., Ringham, M. C., Lu, X., Carter, B. R., Eisaman, M. D., and Tyka, M.: Understanding the Kinetics of Electrochemically derived Magnesium Hydroxide for Ocean Alkalinity Enhancement, in: AGU Fall Meeting Abstracts, Vol. 2022, Chicago, IL, 12–16 December 2022, GC32I-0713, 2022.
Sherman, K. and DeBruyckere, L.: Eelgrass Habitats on the U.S. West Coast: State of the Knowledge of Eelgrass Ecosystem Services and Eelgrass Extent, Pacific Marine and Estuarine Fish Habitat Partnership for The Nature Conservancy, 67 pp., https://www.pacificfishhabitat.org/wp-content/uploads/2017/09/EelGrass_Report_Final_ForPrint_web.pdf (last access: 30 March 2024), 2018.
Shi, Y. and Li, Y.: Impacts of ocean acidification on physiology and ecology of marine invertebrates: A comprehensive review, Aquat. Ecol., 58, 207–226, https://doi.org/10.1007/s10452-023-10058-2, 2023.
Suitner, N., Faucher, G., Lim, C., Schneider, J., Moras, C. A., Riebesell, U., and Hartmann, J.: Ocean alkalinity enhancement approaches and the predictability of runaway precipitation processes: results of an experimental study to determine critical alkalinity ranges for safe and sustainable application scenarios, Biogeosciences, 21, 4587–4604, https://doi.org/10.5194/bg-21-4587-2024, 2024.
Tanner, R. L., Faye, L. E., and Stillman, J. H.: Temperature and salinity sensitivity of respiration, grazing, and defecation rates in the estuarine eelgrass sea hare, Phyllaplysia taylori, Mar. Biol., 166, 109, https://doi.org/10.1007/s00227-019-3559-4, 2019.
Thayer, G. and Phillips, R.: Importance of Eelgrass Beds in Puget Sound, Mar. Fish. Rev., 39, 18–22, 1977.
Tresguerres, M., Clifford, A. M., Harter, T. S., Roa, J. N., Thies, A. B., Yee, D. P., and Brauner, C. J.: Evolutionary links between intra- and extracellular acid–base regulation in fish and other aquatic animals, J. Exp. Zool., 333, 449–465, https://doi.org/10.1002/jez.2367, 2020.
Tresguerres, M., Kwan, G. T., and Weinrauch, A.: Evolving views of ionic, osmotic and acid–base regulation in aquatic animals, J.Exp. Biol., 226, jeb245747, https://doi.org/10.1242/jeb.245747, 2023.
Tyka, M. D., Arsdale, C. V., and Platt, J. C.: CO2 capture by pumping surface acidity to the deep ocean, Energy Environ. Sci., 15, 786–798, https://doi.org/10.1039/D1EE01532J, 2022.
Wang, H., Pilcher, D. J., Kearney, K. A., Cross, J. N., Shugart, O. M., Eisaman, M. D., and Carter, B. R.: Simulated Impact of Ocean Alkalinity Enhancement on Atmospheric CO2 Removal in the Bering Sea, Earth's Future, 11, e2022EF002816, https://doi.org/10.1029/2022EF002816, 2023.
Welton, L. L. and Miller, M. A.: Isopoda and Tanaidacea: the isopods and allies, in: Intertidal invertebrates of California, edited by: Morris, R. H., Abbott, D. P., and Haderlie, E. C., Stanford University Press, California, 536–558, 1980.
Wickham, H.: ggplot2: Elegant Graphics for Data Analysis, Springer-Verlag New York, R [code], ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org (last access: 27 August 2024), 2016.
Wickham, H. and Bryan, J.: readxl: Read Excel Files, R [code], https://readxl.tidyverse.org, https://github.com/tidyverse/readxl (last access: 27 August 2024), 2023.
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J., Kuhn, M., Pedersen, T. L., Miller, E., Bache, S. M., Müller, K., Ooms, J., Robinson, D., Seidel, D. P., Spinu, V., Takahashi, K., Vaughan, D., Wilke, C., Woo, K., and Yutani, H.: Welcome to the tidyverse, Journal of Open Source Software, 4, 1686, https://doi.org/10.21105/joss.01686, 2019.
Wickham, H., François, R., Henry, L., Müller, K., and Vaughan, D.: dplyr: A Grammar of Data Manipulation, R package version 1.1.4, R [code], https://github.com/tidyverse/dplyr, https://dplyr.tidyverse.org (last access: 27 August 2024), 2023.
Wood, H., Sköld, H., and Eriksson, S.: Health and population-dependent effects of ocean acidification on the marine isopod Idotea balthica, Mar. Biol., 161, 2423–2431, https://doi.org/10.1007/s00227-014-2518-3, 2014.
Zayas-Santiago, C. C., Rivas-Ubach, A., Kuo, L.-J., Ward, N. D., and Zimmerman, R. C.: Metabolic Profiling Reveals Biochemical Pathways Responsible for Eelgrass Response to Elevated CO2 and Temperature, Sci. Rep., 10, 4693, https://doi.org/10.1038/s41598-020-61684-x, 2020.
Zeileis, A. and Hothorn, T.: Diagnostic Checking in Regression Relationships, R [code], R News, 2, 7–10, https://CRAN.R-project.org/doc/Rnews/ (last access: 27 August 2024), 2002.
Zimmerman, R. C., Hill, V. J., Jinuntuya, M., Celebi, B., Ruble, D., Smith, M., Cedeno, T., and Swingle, W. M.: Experimental impacts of climate warming and ocean carbonation on eelgrass Zostera marina, Mar. Ecol. Prog. Ser., 566, 1–15, https://doi.org/10.3354/meps12051, 2017.
Zlatkin, R. and Heuer, R.: Ocean acidification affects acid-base physiology and behaviour in a model invertebrate, the California sea hare (Aplysia californica), Roy. Soc. Open Sci., 6, 191041, https://doi.org/10.1098/rsos.191041, 2019.
Zlatkin, R., Grosell, M., and Heuer, R.: The effects of Ocean Acidification in the California sea hare (Aplysia californica), Federation of American Societies for Experimental Biology, 34, p. 1, https://doi.org/10.1096/fasebj.2020.34.s1.07006, 2020.