the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Magnesium (Mg∕Ca, δ26Mg), boron (B∕Ca, δ11B), and calcium (Ca2+) geochemistry of Arctica islandica and Crassostrea virginica extrapallial fluid and shell under ocean acidification
Blanca Alvarez Caraveo
Maxence Guillermic
Alan Downey-Wall
Louise P. Cameron
Jill N. Sutton
John A. Higgins
Justin B. Ries
Katie Lotterhos
The geochemistry of biogenic carbonates has long been used as proxies to record changing seawater parameters. However, the effect of ocean acidification (OA) on seawater chemistry and organism physiology could impact isotopic signatures and how elements are incorporated into the shell. In this study, we investigated the geochemistry of three reservoirs important for biomineralization – seawater, the extrapallial fluid (EPF), and the shell – in two bivalve species: Crassostrea virginica and Arctica islandica. Additionally, we examined the effects of three ocean acidification conditions (ambient: 500 ppm CO2, moderate: 900 ppm CO2, and high: 2800 ppm CO2) on the geochemistry of the same three reservoirs for C. virginica. We present data on calcification rates, EPF pH, measured elemental ratios (, ), and isotopic signatures (δ26Mg, δ11B). In both species, comparisons of seawater and EPF and , Ca2+, and δ26Mg indicate that the EPF has a distinct composition that differs from seawater. Shell δ11B did not faithfully record seawater pH, and δ11B-calculated pH values were consistently higher than pH measurements of the EPF with microelectrodes, indicating that the shell δ11B may reflect a localized environment within the entire EPF reservoir. In C. virginica, EPF and , as well as absolute concentrations of Mg2+, B, and Ca2+, were all significantly affected by ocean acidification, indicating that OA affects the physiological pathways regulating or storing these ions, an observation that complicates their use as proxies. Reduction in EPF Ca2+ may represent an additional mechanism underlying reduction in calcification in C. virginica in response to seawater acidification. The complexity of dynamics of EPF chemistry suggests boron proxies in these two mollusk species are not straightforwardly related to seawater pH, but ocean acidification does lead to both a decrease in microelectrode pH and boron-isotope-based pH, potentially showing applicability of boron isotopes in recording physiological changes. Collectively, our findings show that bivalves have high physiological control over the internal calcifying fluid, which presents a challenge in using boron isotopes for reconstructing seawater pH.
- Article
(3369 KB) - Full-text XML
-
Supplement
(405 KB) - BibTeX
- EndNote
The elemental geochemistry of marine biogenic carbonate shells is widely used to track and reconstruct environmental change (e.g., Broecker and Peng, 1982; Elderfield et al., 2006). The incorporation of elements within the skeleton of marine calcifiers has been shown to be correlated with different environmental parameters, such as temperature (Dunbar et al., 1994; Alibert and McCulloch 1997) and pH (e.g., Hemming and Hanson, 1992; Hönisch et al., 2004; McCulloch et al., 2018). However, elemental and isotopic signatures of biogenic carbonate deviate from inorganic carbonate grown under the same conditions, complicating the use and interpretation of these theoretical models for paleo-reconstructions (e.g., Urey et al., 1951; Craig, 1953; Weiner and Dove, 2003). “Vital effects” are the physiological processes that alter the geochemistry of biominerals and consequently offset the environmental signal incorporated in biogenic carbonates, which includes the different biomineralization strategies that can modify the chemistry of the calcification fluid (Urey et al., 1951; Weiner and Dove, 2003). For organisms to calcify, a semi-isolated calcification space will be, to varying degrees, separated from seawater for supersaturation to be achieved in support of calcification (Weiner and Dove, 2003). The geochemistry of the calcification fluid can be altered due to isolation from the parent fluid as well as the modulation of the calcification fluid chemistry via methods of passive or active ion transport to the site of calcification (Weiner and Dove 2003; McCulloch et al., 2017; Sutton et al., 2018; Liu et al., 2020). A mechanistic understanding of such vital effects is desirable for the accurate interpretation of geochemical proxies preserved in the shells of these organisms.
Bivalve extrapallial fluid (EPF) is an internal fluid reservoir physically semi-separated from seawater that circulates in the pallial cavity, between the mantle organ and shell (Wilbur and Saleuddin, 1983). Seawater enters the pallial cavity when valves are open, and then the internal hemolymph fluid circulates within the organs of the mollusk and finally can also be transported across the mantle to the EPF (Zhao et al., 2018). Bivalve mollusk shell calcification is thought to occur at the interface of the EPF and growing shell where the ions for calcification interact with organic matrices, such as polypeptide molecules and proteins within the EPF that act as a scaffolding template for nucleation and are important in the calcification process (e.g., Crenshaw, 1972; Wilbur and Bernhardt, 1984; Addadi et al., 2006; Checa 2018). Unlike the calcifying fluid reservoirs in most organisms, bivalve EPF has a large enough volume that it can be directly sampled, allowing for direct measurements of the reservoir to compare with seawater geochemistry and elucidate in situ changes in EPF chemistry. A foundational study by Crenshaw (1972) found that, in three mollusk species, the EPF calcification fluid had a different chemical composition and pH from seawater and hemolymph fluid (Crenshaw et al., 1972). A previous study on the king scallop, Pecten maximus, by Cameron et al. (2019) showed that EPF pH was lower than seawater and also depended on seawater pCO2 and temperature. Additionally, Ramesh et al. (2017) used a microelectrode approach to show that pH and [] were elevated proximal to the growing shell in larval Mytilus edulis shells. This result using microelectrodes suggests a potential difference in pH between the bulk EPF and the pH close to the site of calcification. In the quahog Arctica islandica, Stemmer et al. (2019) reported synchronous short-term fluctuations in EPF Ca2+ and the pH at the outer mantle epithelium surface, providing further support that the extrapallial fluid of mollusks is a discrete fluid under biological control. Understanding the elemental composition and isotope signatures of mollusk internal fluid reservoirs, mechanisms of calcification, and ion transport to the site of calcification is critical to understanding these vital effects. It may also give insight into the sensitivity of bivalves to pCO2-induced ocean acidification, a major environmental challenge for bivalves, which are typically amongst the more sensitive group of marine calcifier species to acidification (Ries et al., 2009; Kroeker et al., 2011; Gazeau et al., 2013; Stewart-Sinclair et al., 2020).
Mollusks have long been recognized as valuable archives for climate reconstructions, given their annual resolution growth bands, long lifespans, and wide geographic distributions (Gibson et al., 2001; Peharda et al., 2021). The geochemistry of bivalve shells acts as an archive that provides information on the internal calcification fluid they precipitate their shells from. For example, shell has been shown to be correlated to EPF pH in bivalves such as M. edulis (Heinemann et al., 2012) and Mercenaria mercenaria (Ulrich et al., 2021) and can potentially be useful in understanding the internal carbonate chemistry within the calcification fluid. Shell δ11B is used as a proxy for seawater pH in foraminifera (Foster and Rae, 2016) and calcification fluid pH in corals (McCulloch et al., 2017; Eagle et al., 2022), but it seems to be offset from theoretical pH calculations in bivalves like M. edulis (e.g., Heinemann et al., 2012; Liu et al., 2020), M. mercenaria (Liu et al., 2020), and Crassostrea virginica (Liu et al., 2020). Shell is used as a temperature proxy in bivalves (Wanamaker et al., 2008; Schöne et al., 2011); however, mollusks can regulate and actively exclude Mg2+ from their shells (e.g., Lorens and Bender, 1977; Planchon et al., 2013), showing that biological regulation of the internal fluids for shell formation can have a strong influence on Mg-based geochemical proxies. Furthermore, Mg isotope analyses can potentially inform the Mg2+ transport process in mollusks. Although few Mg isotopic studies on mollusks have been done, a study by Planchon et al. (2013) investigated the δ26Mg of Ruditapes philippinarum tissues, shell, and the EPF and found that seawater and EPF Mg isotopic signatures were similar, suggesting that seawater is the source of Mg2+ ions within the EPF. Additionally, they found that Mg isotopic signatures of some specimens deviated from inorganically precipitated aragonite, suggesting an ability to physiologically alter or regulate Mg2+ within the EPF (Planchon et al., 2013).
Marine calcifiers are thought to be particularly sensitive to ocean acidification because of lowered saturation state of calcite (Ωcalcite) and availability of carbonate ions they need to precipitate their shells (Orr et al., 2005). However, marine calcifiers can exhibit extremely different calcification responses to ocean acidification (e.g., Ries, 2011; Kroeker et al., 2013). Bivalves show similar variable responses to ocean acidification (Gazeau et al., 2013). A study conducted by Waldbusser et al. (2015) found that juvenile Crassostrea gigas and Mytilus galloprovincialis had developmental and growth sensitivities to decreasing seawater Ωcalcite. Furthermore, a study by Fitzer et al. (2016) found that M. edulis shell crystallography was affected by ocean acidification, compromising the organism's shell. Additionally, studies have found that exposure to ocean acidification conditions could affect trace element uptake in bivalve shells (Norrie et al., 2018; Zhao et al., 2020). The effect of ocean acidification on bivalve shell geochemistry is of particular consequence for paleoclimate reconstructions due to primary or secondary effects such as calcification or physiological impairment (e.g., Michaelidis et al., 2005; Waldbusser et al., 2015; Norrie et al., 2018; Zhao et al., 2020).
Few taxa have been studied using combined geochemical tracer work to determine the chemistry of calcification fluid pools and sources of ions to the calcification front. To date, one study has investigated the and δ11B of shell and the EPF of the bivalve M. edulis (Heinemann et al., 2012). Mollusk extrapallial fluid is an attractive target to investigate geochemical vital effects because not only can it be probed with electrodes, but it can also be extracted and analyzed. In this study, we investigate the δ11B, , δ26Mg, and of the extracted EPF and aragonite shell of the quahog, A. islandica, and the calcite shell of the eastern oyster, C. virginica. This allows for the investigation of the tripartite fractionation between seawater, the EPF, and shell. Individuals were kept in controlled laboratory experiments, with EPF pH determined by microelectrodes and other physiological parameters, such as calcification rate, determined by conventional methods (Downey-Wall et al., 2020). Additionally, in order to examine if elemental ratios and isotopic signatures can be impacted under ocean acidification, specimens of C. virginica were also cultured in three different treatments of pCO2: ambient, moderate, and high ocean acidification conditions. Geochemical analyses of the seawater, shell, and the EPF thereby allow novel insights into the transport of ions from seawater to the EPF and the fractionation of isotopes and elements between the EPF and shell for both species. Additionally, it gave insight into how the same analyses can change for C. virginica under acidified conditions.
2.1 Experimental conditions
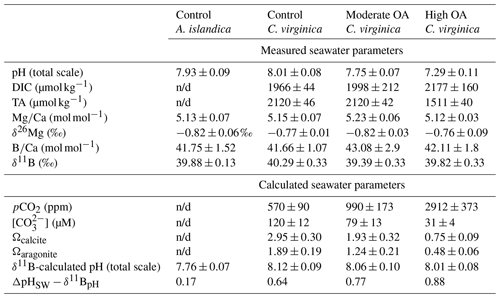
Adult A. islandica specimens were collected from Beals Island, Maine, USA ( N, W), in March 2018, transferred to Northeastern University's Marine Science Center, and maintained in the lab until March 2019. The average A. islandica shell length was 5.8±0.5 (n=6). For A. islandica, seawater was maintained at a pH of 7.93±0.09, temperature of 9±1 °C, and salinity of 35 in the control conditions (Cameron 2020).
A detailed explanation of the collection and culturing of C. virginica is outlined in Downey-Wall et al. (2020). Adult C. virginica specimens were collected from three intertidal sites on Plum Island Sound, Massachusetts, USA (Site 1 at N, W; Site 2 at N, W; Site 3 at N, W) in April 2017 and transferred to Northeastern University's Marine Science Center. The average C. virginica shell length was 9.5±2.4 cm (n=107). Specimens were acclimated to laboratory conditions for 33 d and then transferred to experimental tanks. Seawater salinity and temperature were monitored and maintained throughout the experiment. Crassostrea virginica seawater was maintained at a temperature of 17.2±1 °C and salinity of 31. C. virginica specimens were exposed to control (mean ; ), moderate ocean acidification (OA) (990±29 ppm, ), or high OA (2912±59 ppm, ) treatments. Target pCO2 treatment was achieved by mixing compressed pCO2 and compressed ambient air using solenoid-valve-controlled mass flow controllers at flow rates that target pCO2 conditions. The treated seawater was introduced to the flow-through aquaria at a rate of 150 mL min−1. For the acidification experiment, tank salinity, temperature, and dissolved inorganic carbon (DIC) and TA were measured for the duration of the experiment and used to calculate pH (total scale), Ωcalcite, [], [], [CO2], and pCO2 of each tank using CO2SYS version 2.1 (Pierrot et al., 2011; Downey-Wall et al., 2020). Measured and calculated seawater parameters are reported in Table 1. Oysters were fed 1 % Shellfish Diet 1800®twice daily following best practices outlined in Helm et al. (2004).
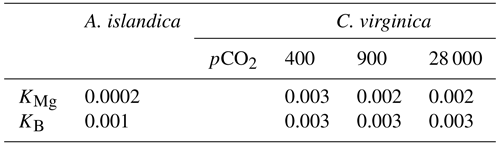
Table 1Seawater carbonate chemistry parameters (pH, DIC, TA, Ω, δ11B-calculated EPF pH, and pH) and seawater geochemical parameters (, δ26Mg, , δ11B) for both C. virginica and A. islandica under control conditions and C. virginica for OA conditions. Parameters that were unable to be measured due to insufficient sample size or unable to be calculated are marked with “n/d”.
2.2 Calcification rate measurements
Net calcification rate for C. virginica specimens (n=35) was calculated in Downey-Wall et al. (2020) using the buoyant weighing technique. Buoyant weight was measured by submerging oysters in a 27.65 L tank (48 cm long, 24 cm wide, and 24 cm deep) filled with seawater. Specimens were placed on a bottom-loading scale (Cole-Parmer Symmetry S-PT 413E, precision=0.001 g) and weighed three times. At the end of the experiment, an empirical linear relationship was created between buoyant weight and dry-shell weight of shucked oysters following the same methodology in Ries et al. (2009). The root mean square error (RMSE) for the dry weight and buoyant weight model was 1.939 mg. Calcification was calculated as the difference in calculated dry weight at the start and end of the experiment over the number of days. This number was then divided by the initial weight and multiplied by 100 to get the percent change in calcification.
2.3 Extrapallial fluid sampling
Sampling of the extrapallial fluid (EPF) for both species was previously described in Downey-Wall et al. (2020). Briefly, a hole was drilled into the shell to expose the EPF cavity, and a port was inserted and sealed with epoxy to directly sample the EPF with a syringe and prevent seawater intrusion (Fig. 1). Oysters recovered for 4 d before being transferred to experimental tanks for acclimation before the experiment. To sample the EPF, oysters were removed from the tanks, and the EPF was extracted by inserting a sterile 5 mL syringe with a flexible 18-gauge polypropylene tip through the port. EPF samples were stored in 2 mL microcentrifuge tubes and refrigerated at 6 °C for further analysis. EPF pH (Total scale) was measured directly after extraction using a micro-pH probe. EPF measurements were collected at the end of the experiment, on day 71 for C. virginica and day 14 for A. islandica. EPF pH diel variability was also explored by measuring EPF pH at six time points to produce time series for both species in a 24 h period.
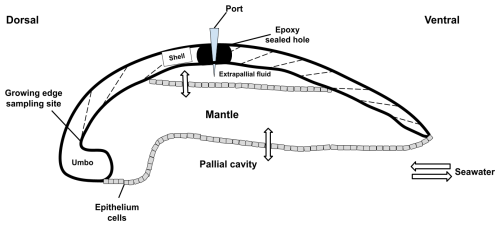
Figure 1Schematic of a bivalve cross section from the dorsal to the ventral sides. Seawater enters the pallial cavity, and ions can diffuse or be transported across the mantle organ to the extrapallial fluid space. A hole was drilled through the top of the shell into the extrapallial fluid space and sealed with epoxy following port insertion. The shell was drilled on the inner side of the growing edge of the shell to sample new growth.
2.4 Shell sampling
Following EPF extraction, bivalves were shucked and cleaned in 90 % ethanol. The cleaned shells were dried at room temperature for 48 h and sealed in plastic bags for analysis. Whole shells were cut into cross sections from the hinge to the margin using a circular saw while being rinsed with ethanol during sectioning to prevent mineralogical changes from heat exposure. For skeletal geochemical and elemental ratio analysis, the inner (lamellar) layer of the oyster shell was gently shaved with a diamond-tipped Dremel tool. Care was taken to ensure sampling the most recently deposited material right near the growing edge of the bivalve, located below the umbo of the oyster shell (Fig. 1). Cross sections showed growth bands and aided in sampling the newest growth under experimental conditions. For A. islandica, we wanted to compare ambient conditions, so new growth was not necessary to sample. About 5 mg of ground powder was stored in sealed microcentrifuge tubes.
2.5 Elemental ratio analysis
For the shells, about 2.5 mg of powder was subsampled from each specimen shell and oxidatively cleaned with 0.3 % hydrogen peroxide in a 0.1 N sodium hydroxide solution to remove organic matter as described in Barker et al. (2003). Carbonate samples were dissolved in 1 N double-distilled HCl. Elemental ratios were measured on a Thermo Fisher Scientific Element XR HR-ICP-MS at the Pôle Spectrométrie Océan (PSO; Plouzané, France) after Ca analyses on an Agilent ICP-AES Varian 710 at the University of California, Los Angeles (UCLA, Los Angeles, USA). Data quality and external reproducibility were maintained and quantified via repeated measurements of the international standard JCP-1 during a particular session (Gutjahr et al., 2021). Typical measured concentrations of procedural blanks for the trace element analyses for sessions in which samples are diluted to 30 ppm Ca are 7Li<3 %, 11B<4 %, 25Mg<0.1 %, 87Sr<0.1 %, and 43Ca<0.1 %. Typical analytical uncertainties on the elemental ratios are 0.3 µmol mol−1 for , 21 µmol mol−1 for , 0.09 mmol mol−1 for , and 0.01 mmol mol−1 for (2 SD, n=28).
For EPF and seawater samples, 10 µL of sample was added to 490 µL of a solution of . Mono-elemental solution of indium was added to reach a concentration of 1 ppb to monitor any matrix effect or drift of the instrument during a particular session. Standards were prepared by diluting an in-house seawater standard spiked with indium. International standard NRC-NASS-6 was used to ensure quality of the data.
2.6 Boron isotope analyses
Boron purification for the different samples was achieved via microdistillation following the method described in Guillermic et al. (2021) and originally developed by Gaillardet et al. (2001) and modified for the Ca-rich matrix by Wang et al. (2010). Approximately 2.5–3.0 mg of oxidatively cleaned shell powders was dissolved in 1 N HCl. For the EPF, 25 µL of the EPF was added to 40 µL of 1 N HCl. For the seawater, 50 µL of concentrated HCl was added to 450 µL of seawater; 60 µL of each of the solutions was loaded for microdistillation. Boron isotopes were analyzed at the PSO, Plouzané, on a Thermo Neptune inductively coupled plasma mass spectrometry (MC-ICP-MS) equipped with a 1011 Ω Faraday cup.
The certified boron isotope liquid standard ERM©AE120 (, Vogl et al., 2011) was used to monitor reproducibility and drift during each session. Samples measured for boron isotopes in carbonates were typically run at 80 ppb B (∼30 ng B per 0.5 m), whereas samples of the EPF and seawater were typically run at 150–200 ppb B (∼150 ng B per mL). Sensitivity of 11B was 10 mV ppb−1 B (e.g., 10 mV for 1 ppb B) in wet plasma at a 50 µL min−1 sample aspiration rate. Procedural boron blanks ranged from 0.3–0.4 ng B, and the acid blank during analyses was measured at 3 mV on the 11B, indicating a total blank contribution of <2 % of the sample signal with no memory effect within and across sessions. External reproducibility was ensured by the measurements of a carbonate standard microdistilled at the same time as the samples. The result for the isotopic composition of the JCP-1 is (2 SE, n=41), within the error of published values (24.36±0.45 ‰, 2 SD; Gutjahr et al., 2021).
2.7 Magnesium isotope analyses
Carbonate samples were dissolved in a 0.1 N buffered acetic acid ammonium hydroxide solution over 4 h in a sonicator. Samples were then centrifuged, and aliquots of the supernatant were transferred into cleaned 15 mL centrifuge tubes. Aliquots of the bulk supernatants were then diluted ∼30-fold, and calcium and magnesium were separated and purified in different runs via a Thermo Scientific Dionex ICS-5000+ ion chromatograph equipped with a fraction collector according to established methods outlined by Husson et al. (2015). EPF samples contained organics that obscured elution profiles, thus limiting the elemental yield and purification. Therefore, samples were digested on a hot plate in hydrogen peroxide and nitric acid to remove organics prior to purification. Seawater and EPF samples were purified through the Thermo Scientific Dionex ICS-5000+ ion chromatograph using another elution method than for carbonate samples. Seawater and carbonate standards were also purified at the same time to ensure quality of the method.
Samples were then dried and then rehydrated in a solution of 2 % nitric acid. Magnesium isotopic ratios were measured at Princeton University using a Thermo Neptune+ (MC-ICP-MS) spectrometer according to methods outlined in Higgins et al. (2018) and Ahm et al. (2021). Samples were introduced via an ESI Apex-IR sample introduction system. Magnesium isotope ratios () were measured in low-resolution mode, with every sample bracketed by the analysis of standards. Results are reported relative to the Dead Sea Magnesium-3 standard (DSM-3). Long-term external precision on magnesium isotope results at the Higgins Lab (Princeton) was determined through repeated measurements of the Cambridge-1 standard (, 2 SD, n=19) and modern seawater (, 2 SD, n=21) and is reported in Ahm et al. (2021). Measured standards during the analytical session are given for the Cambridge-1 standard (, 2 SD, n=2) and for modern seawater (, 2 SD, n=2).
2.8 Calculation of boron proxies and EPF carbonate chemistry
The use of boron proxies to reconstruct pH and [] of the precipitating solution (i.e., the organism's calcifying fluid) is based upon boron speciation and fractionation in seawater (Hemming and Hanson, 1992; Hönisch et al., 2004). In seawater-type solutions, the speciation of boric acid [B(OH)3] and borate ion [] varies as a function of pH (Hemming and Hanson 1992). In addition to the pH dependence of their relative abundances, the boron proxy also relies upon the large isotopic fractionation between the two boron species (Klochko et al., 2006; Nir et al., 2015). A key assumption of the proxy is that boron, in the form of borate ion, is the predominant form incorporated into the crystal lattice of calcite via carbonate ion substitution during the precipitation of calcium carbonate (Hemming and Hanson, 1992). The δ11B of the carbonate () should then, in theory, reflect the boron isotopic composition of the borate ion () in the bivalve calcifying fluid (extrapallial fluid), which in turn reflects pH of the calcifying (extrapallial) fluid.
The boron isotopic signature of the shell (δ11Bcarb) was used to calculate pH of the calcifying fluid (pHCF) using the following equation (Hemming and Hanson, 1992; Zeebe and Wolf-Gladrow, 2001):
In Eq. (1), pKB is the dissociation constant, δ11Bsw represents the measured boron isotopic composition of seawater, δ11Bcarb represents the boron isotopic composition of the shell, and represents the boron isotopic fractionation factorfractionation between boric acid and borate ion (Klochko et al., 2006).
The saturation states of calcite (Ωcalcite) and aragonite (Ωaragonite) of the EPF for each species were calculated using temperature, salinity, pressure, measured EPF Ca2+, measured EPF Mg2+, pH either from microelectrode pH or δ11B-calculated pH, and literature values of DIC (3000 for A. islandica from Stemmer et al., 2019, and 4200 for C. virginica from McNally et al., 2022). The saturation states were calculated using Seacarbx with a maximum input of Mg2+ allowed by the code for samples presenting higher EPF Mg2+ than the limit allowed by the code (Raitzsch et al., 2021). Those saturation state values are limited by the fact that no direct measurements of EPF DIC were performed during this study, and a range of Ca2+ and Mg2+ values were measured in the EPF, resulting in a range of calculated saturation states. The apparent partition coefficient was calculated as the ratio of ECa for the mineral over the ECa for seawater.
2.9 Statistical analysis
All statistical tests were performed and data graphed using GraphPad Prism software version 9 (GraphPad Software Inc.; San Diego, CA, USA). Prior to statistical analyses, a Shapiro–Wilks test was run to determine normality, and a Brown–Forsythe test was used to determine heterogeneity of variance of residuals. Only two comparative t-test data did not meet requirements, so a nonparametric Mann–Whitney u test was run in place of a t test. A t test and Mann–Whitney u tests were performed in order to test whether there was a difference between seawater and EPF geochemical parameters and between the EPF of both species under ambient conditions. A one-way ANOVA with pH as a three-level factor was used to test whether pH had a significant effect on our geochemical data. ANOVA and t test significance was achieved if the p value was less than 0.05. Regression analysis was performed on a GraphPad Prism, and significance was denoted if the slope of the regression was statistically non-zero.
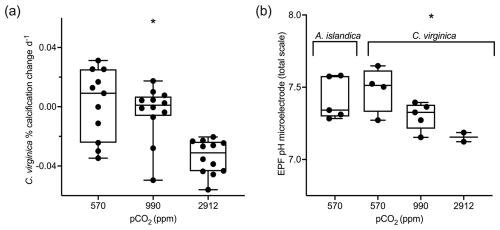
Figure 2(a) Box plots showing percent calcification change over the experiment for C. virginica for each treatment. (b) Averaged microelectrode EPF pH for A. islandica under control conditions and C. virginica for OA treatments. Stars denote a statistically significant ANOVA (at significance p<0.05).
3.1 Culturing experiment, calcification rates, and seawater and EPF chemistry
Crassostrea virginica specimens were previously cultured in experimental tanks with seawater that was continuously bubbled with gas mixtures comprising three pCO2 levels: 400, 900, and 2800 ppm (Downey-Wall et al., 2020). The tank seawater saturation state of calcite (Ωcalcite) was calculated for C. virginica under the ocean acidification experiment and not A. islandica. As seawater pCO2 increased, seawater Ωcalcite decreased. Only the highest pCO2 treatment produced calcite saturation states Ωcalcite<1, which does not favor calcification (Table 1). Similarly to Ωcalcite, calcification rates were also only measured for the C. virginica OA experiment. pCO2 treatment had a significant effect on C. virginica calcification, with the percent change in calcification per day decreasing with increasing pCO2. There was also variability in calcification between specimens within each treatment (Fig. 2a).
In this study, we present unpublished EPF pH microelectrode data for A. islandica cultured under a single control condition (400 ppm pCO2), and we present published EPF microelectrode data for the C. virginica acidification experiment of Downey-Wall et al. (2020). Under control seawater conditions the EPF pH of A. islandica was 7.41, compared to 7.48 for C. virginica. The EPF pH values of both species were not statistically different (t test p>0.05), and the average EPF pH of both species was well under seawater pH (Fig. 2b). Additionally pCO2 treatment also had a significant effect on C. virginica EPF pH (ANOVA p<0.05), with microelectrode-measured EPF pH decreasing as pCO2 increased (Fig. 2b). Downey-Wall et al. (2020) also report that pCO2 treatment had a significant effect on EPF pH (linear model, p<0.05) and that at the highest pCO2 treatment, EPF pH was significantly lower than seawater pH (post hoc p<0.05; see Downey-Wall et al., 2020). We calculated the change in pH (ΔpH) as the EPF pH subtracted from seawater pH to show the magnitude EPF pH is downregulated relative to external seawater. The ΔpH for A. islandica was 0.52 and was similar to the control condition ΔpH for C. virginica, which was 0.53 (Table 2). Under OA treatments, ΔpH for C. virginica decreased with decreasing seawater pH. The ΔpH for the control treatment was 0.53, the moderate OA treatment was 0.46, and the high OA treatment was 0.08.
Table 2Measured extrapallial fluid (EPF) carbonate chemistry parameters (pH, DIC, TA, Ω, δ11B-calculated EPF pH, and pH) and shell geochemical parameters (, δ26Mg, , and δ11B) for both C. virginica and A. islandica under control conditions and C. virginica for OA conditions. Parameters that were unable to be measured due to insufficient sample size or unable to be calculated are marked with “n/d”.
3.2 Comparison of A. islandica and C. virginica geochemistry of seawater, the EPF, and bivalve shell
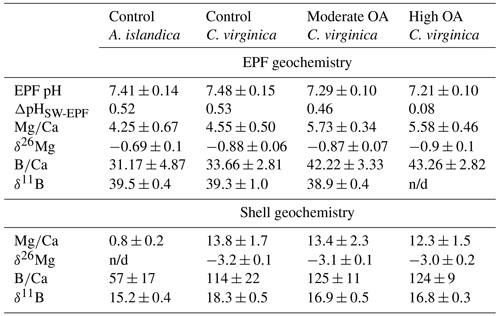
There was a significant decrease in EPF compared to seawater for both A. islandica and C. virginica (t test, n=2, p<0.05; Fig. 3a and b). The of C. virginica EPF was and significantly higher than A. islandica EPF, which was (Fig. 3d; Table 2). For both species, the low EPF vs. seawater was driven by higher Ca2+ concentrations in the EPF relative to seawater (Fig. 3h and i). Considering the elemental concentrations alone, instead of as a ratio, there was no significant difference in EPF Mg2+ or Ca2+ concentrations between species (Fig. 3g and j). Shell for the calcitic C. virginica was and significantly higher than the aragonitic A. islandica shell, which was , in line with shell polymorph mineralogy. The apparent partition coefficient (KMg) between the seawater and the shell was 0.003 in C. virginica and 0.002 in A. islandica (Table 3). KMg between the EPF and shell was 0.003 in C. virginica and 0.002 in A. islandica. KMg between seawater and the EPF is 0.9 for C. virginica and 0.8 for A. islandica (Table 3). C. virginica seawater and EPF δ26Mg were and , respectively, and displayed a significant decrease in EPF δ26Mg compared to seawater for C. virginica (t test, n1=3, n2=5, p<0.05; Fig. 3k and l). For A. islandica, seawater and EPF δ26Mg were and , respectively, but no statistical analysis could be done between the two reservoirs owing to the small sample size (Tables 1 and 2). The average shell δ26Mg for C. virginica was , but A. islandica shell δ26Mg could not be analyzed because of low shell [Mg2+] content and limited sample material.
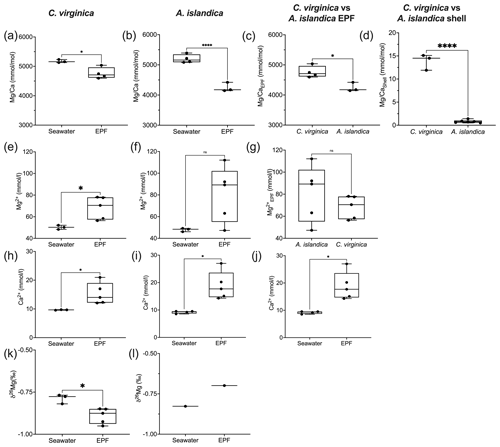
Figure 3Box plots of comparing seawater and extrapallial fluid for (a) C. virginica and (b) A. islandica, (c) comparing EPF between species, and (d) shell between species. Box plots of [Mg2+] comparing seawater and extrapallial fluid for (e) C. virginica and (f) A. islandica, (g) comparing EPF [Mg2+] between species. Box plots of [Ca] comparing seawater and extrapallial fluid for (h) C. virginica and (i) A. islandica, (j) comparing EPF [Ca] between species. Box plots of δ26Mg comparing seawater and extrapallial fluid for (k) C. virginica and (l) A. islandica. Stars denote statistically different means, and “ns” signifies nonsignificant mean differences in a pairwise t test or Mann–Whitney u test (at significance p<0.05). No comparison was tested on (l) due to limited sample size.
Arctica islandica EPF was and was significantly lower than seawater , which was (t test, n1=7, n2=5, p<0.05; Fig. 4a). C. virginica EPF was and was significantly lower than seawater , which was (t test, n1=6, n2=5, p<0.05; Fig. 4b) The boron concentration was not significantly different between seawater and the EPF for both C. virginica and A. islandica (Fig. 4e and f). There was no significant difference in shell or EPF between C. virginica and A. islandica (Fig. 4c and d). The apparent partition coefficient (KB) between the seawater and the shell was 0.003 in C. virginica and 0.001 in A. islandica. KB between the EPF and shell was 0.003 in C. virginica and 0.002 in A. islandica. KB between seawater and the EPF is 0.8 in C. virginica and 0.7 for A. islandica (Table 3). There was no significant difference in δ11B between seawater and the EPF for both species in the control condition (Fig. 4h–l). There was also no significant difference in EPF δ11B between species (Fig. 4j); however, there was a significant difference in shell δ11B between C. virginica and A. islandica (t test, n1=10, n2=3, p<0.05; Fig. 4k). Under control conditions, shell δ11B was measured to be 15.26±0.41 ‰ (2 SD, n=3) for C. virginica and 18.34±0.59 ‰ (2 SD, n=3) for A. islandica.
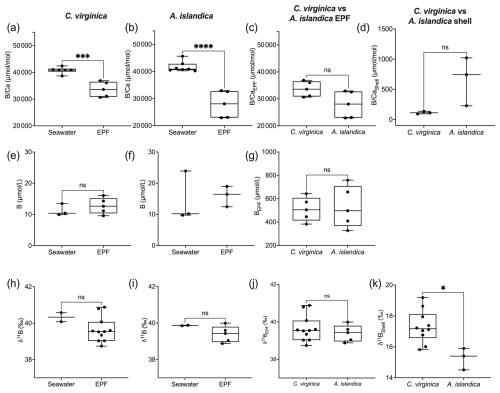
Figure 4Box plots of comparing seawater and extrapallial fluid for (a) C. virginica and (b) A. islandica, (c) comparing EPF between species, and (d) shell between species. Box plots of [B] comparing seawater and extrapallial fluid for (e) C. virginica and (f) A. islandica, (g) comparing EPF [B] between species. Box plots of δ11B comparing seawater and extrapallial fluid for (h) C. virginica and (i) A. islandica, (j) comparing EPF δ11B between species, and (k) shell δ11B between species. Stars denote statistically different means, and “ns” signifies nonsignificant mean differences in a pairwise t test or Mann–Whitney u test (at significance p<0.05).
The control condition δ11B-calculated EPF pH for C. virginica was 8.12±0.08 ‰ (2 SD, n=3) and for A. islandica 7.93±0.09 ‰ (2 SD, n=3), which yielded a statistically significant difference between the two species (t test, n1=3, n2=3, p<0.05; Fig. 5a). For C. virginica, the δ11B-calculated EPF was 0.1 pH units higher than the seawater pH and 0.6 lower than measured EPF pH. Conversely, the A. islandica δ11B-calculated EPF was 0.1 pH units lower than the seawater pH and 0.3 higher than the measured EPF pH (Fig. 5).
Figure 5(a) Box plot of δ11B-calculated pH for C. virginica and A. islandica. (b) Box plot of measured microelectrode pH for C. virginica and A. islandica. The gray line shows seawater pH for C. virginica and A. islandica. Stars denote statistically different means, and “ns” signifies nonsignificant mean differences in a pairwise t test (at significance p<0.05).
In Table 4, the EPF aragonite saturation state (Ωaragonite) for A. islandica and EPF calcite saturation state (Ωcalcite) for C. virginica were calculated using the averaged measured EPF pH and averaged δ11B-calculated EPF pH, averaged measured Mg2+ and Ca2+, and literature values of DIC (3000 µmol L−1 for A. islandica taken from Stemmer et al. (2019) and 4200 µmol L−1 for C. virginica from McNally et al. (2022). Under control conditions, the A. islandica Ωaragonite and C. virginica Ωcalcite was calculated using δ11B-calculated EPF pH and measured EPF pH (Table 4). Under the ocean acidification experiment, EPF Ωcalcite decreased with decreasing seawater pH when using either EPF pH or δ11B-calculated EPF pH to calculate EPF Ωcalcite. There were large differences in A. islandica Ωaragonite and C. virginica Ωcalcite when using either EPF pH (Ωaragonite=1.7 and Ωcalcite=3.7) or the δ11B-calculated pH (Ωaragonite=3.8 and Ωcalcite=15.4).
3.3 C. virginica ocean acidification experiment geochemistry
In the C. virginica acidification experiment, EPF but not shell was found to increase as EPF pH decreased (regression, n=10, p<0.05; Fig. 6a and b). OA treatment had a significant effect on shell (ANOVA, n=10, p<0.05; Fig. 6a and b). The concentration of both Ca2+ and Mg2+ in the EPF decreased with decreasing EPF pH (regression, n=10, p<0.05; Fig. 6c and d). However, when binning by seawater pH treatments, only the Ca2+ and Mg2+ of the ambient condition were significantly elevated compared to the moderate and high ocean acidification treatments (Tukey HSD, n1=4, n2=3, p<0.05; Fig. 6c and d). The EPF and shell δ26Mg did not change as a function of the EPF or seawater pH (Figs. 6e and f and 5e and f).
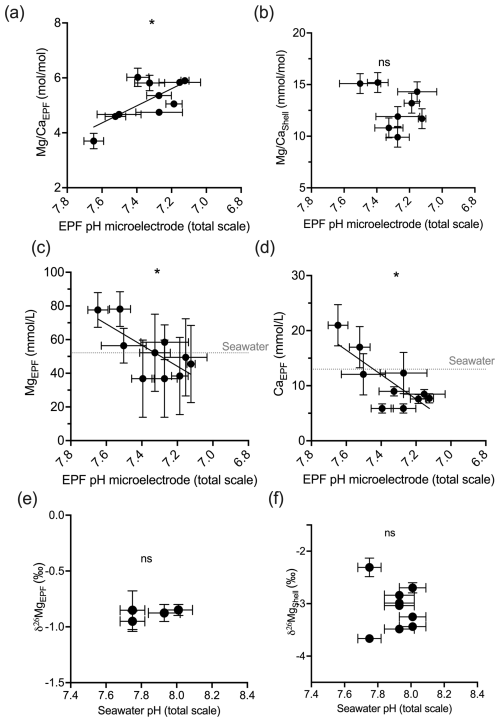
Figure 6Scatterplots showing C. virginica individual specimen (a) EPF and (b) shell across corresponding microelectrode pH. Additionally, scatterplots (c) EPF Mg2+, (d) EPF Ca2+, (e) EPF δ26Mg, and (f) shell δ26Mg across microelectrode EPF pH. Dotted gray lines on (c) and (d) show the average Mg2+ and Ca2+ seawater concentration, respectively. Stars denote statistically significantly nonzero regression slopes, and “ns” signifies nonsignificant regressions (at significance p<0.05).
Under OA conditions, EPF but not shell was found to increase as seawater pH decreased (ANOVA p<0.05; compare Fig. 7a and b). The EPF but not shell was found to increase as EPF pH decreased (regression p<0.05; Fig. 7a and b). The boron concentration of the EPF, but not the shell, significantly decreased with decreasing EPF pH (regression p<0.05; Fig. 7c). The EPF B concentration increased with increasing seawater pH (ANOVA p<0.05; Fig. 7c); however, shell boron concentrations did not significantly change with seawater pH. Due to small EPF sample volume, the EPF for the oysters in the lowest seawater pH treatment was not measured for δ11B. There was a significant difference in mean EPF δ11B between the control pH treatment, which was 39.39 ‰, and moderate pH treatment, which was 38.92 ‰ (t test, n1=11, n2=7, p<0.05; Fig. 7e and f). The difference between seawater δ11B and EPF δ11B was 0.91 ‰ for the control treatment and decreased to 0.47 ‰ for the moderate pH treatment. Shell δ11B, but not EPF δ11B, significantly decreased with decreasing EPF pH (regression p<0.05; Fig. 7e and f).
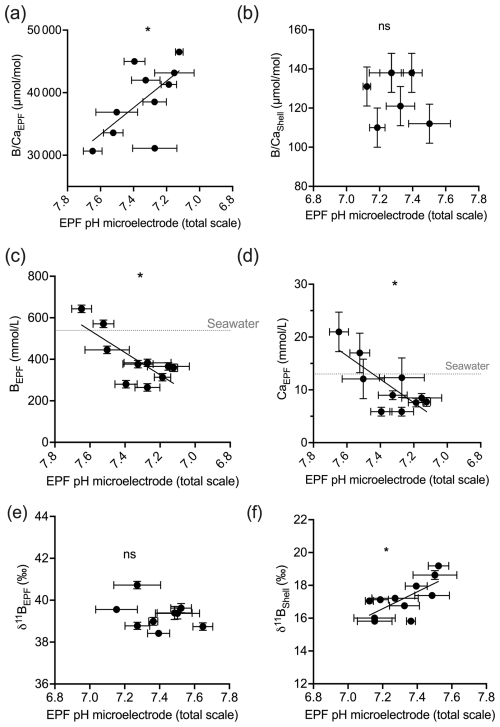
Figure 7Scatterplots showing C. virginica individual specimen (a) EPF and (b) shell across corresponding microelectrode EPF pH. Additionally, scatterplots of (c) EPF B, (d) EPF Ca2+, (e) EPF δ11B, and (f) shell δ11B across microelectrode EPF pH. Dotted gray lines in (c) and (d) show the average B and Ca2+ seawater concentration, respectively. Stars denote statistically significantly nonzero regression slopes, and “ns” signifies nonsignificant regressions (at significance p<0.05).
Figure 8a shows the measured EPF pH, the δ11B-calculated EPF, and seawater to EPF 1:1 pH line graphed across the C. virginica acidification experiment. The slope of the measured microelectrode EPF pH vs. seawater pH linear regression was 0.36. It lies below the seawater to EPF 1:1 pH line but intersects the seawater to EPF 1:1 pH line at lowest pH or highest pCO2 culture conditions (Fig. 8). Conversely, the slope of the δ11B-calculated EPF pH vs. seawater pH linear regression was 0.14. It lies above the seawater to EPF 1:1 pH line but intersects the seawater to EPF 1:1 pH line at higher culture pH conditions (Fig. 8).
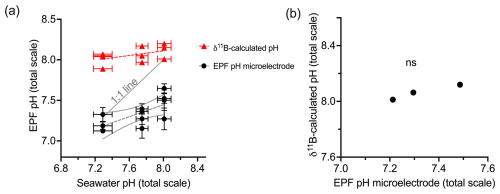
Figure 8(a) Scatterplot of δ11B-calculated pH and microelectrode EPF pH across seawater pH treatments. The gray line shows the 1:1 seawater to EPF pH line. The δ11B-calculated pH regression line had a slope of 0.14 seawater pH/EPF pH. The microelectrode EPF pH line had a slope of 0.36. Panel (b) shows the averaged δ11B-calculated pH vs. microelectrode EPF pH. The “ns” signifies a nonsignificant regression (at significance p<0.05).
For the C. virginica acidification experiment, Downey-Wall et al. (2020) measured the EPF pH of individual specimens in each acidification treatment over a 24 h period (ntotal=108 and n=6 per time point per treatment). Figure 9 shows how the EPF pH for each individual fluctuated over 24 h. Ambient treatment EPF pH ranged from 6.63–7.94, moderate OA treatment ranged from 6.68–7.88, and high OA treatment ranged from 6.78–7.47. The control treatment EPF pH of individuals did intersect the averaged seawater pH for the treatment tanks; however, the EPF pH in the moderate and high pH treatments fell below the corresponding average treatment seawater pH lines. For all treatments, the time series EPF pH lines fell below the corresponding average treatment δ11B-calculated EPF pH line.
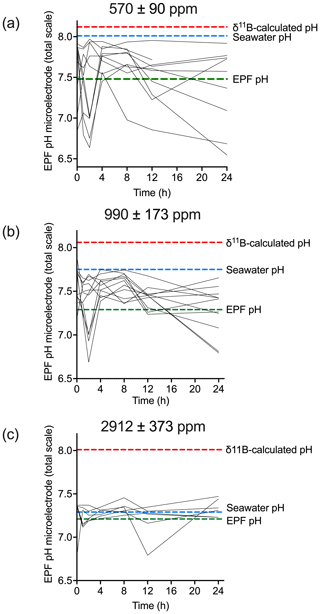
Figure 9Time series (in hours) of microelectrode EPF pH over a 24 h period for (a) control (n=10), (b) moderate (n=11), and (c) high pCO2 treatments (n=6). Each line represents the microelectrode EPF pH for each individual specimen measured in that treatment. The dotted red line shows the corresponding average δ11B-calculated pH for the treatment, the dotted blue line shows the average seawater pH for the treatment, and the dotted green line shows average EPF pH.
4.1 Comparison of A. islandica and C. virginica Mg2+ and Ca2+ geochemistry of seawater, the EPF, and bivalve shell
This study examined tripartite element and isotope fractionation between different reservoirs involved in the biomineralization of two bivalves species: aragonitic A. islandica and calcitic C. virginica. Marine bivalves source ions for internal fluids from seawater, and previous studies by Crenshaw (1972) have highlighted that the extrapallial fluid is chemically different from seawater. The ions sourced from seawater are modulated either passively or actively across the outer mantle epithelium (OME) cells into the extrapallial cavity, where biomineralization occurs (Zhao et al., 2018). The exact mechanisms behind bivalve biomineralization are still a topic of active research, and evidence has been put forth for several distinct pathways, primarily regulation of calcification constituents across the OME or transport of a precursor phase of CaCO3 to promote calcification (Addadi et al., 2003; Checa 2018).
In this study we found that the extrapallial fluid is chemically distinct from seawater. Here we show that, under ambient conditions, both the EPF and of both C. virginica and A. islandica were lower than those of seawater, indicating that the EPF has a distinct geochemical make-up from seawater (Fig. 3). This is consistent with the anatomical understanding in bivalves that the extrapallial fluid is semi-isolated from seawater, and its geochemistry can be influenced by ion fluxes across the OME as well as other ion pathways (Crenshaw 1972; Sillanpää et al., 2018; Stemmer et al., 2019). However, we also find that, for both and , this result is driven by an increase in absolute Ca2+ in the EPF, so we do not find evidence for dilution or concentration of the absolute Mg2+ or B in the EPF (Fig. 3). Previous work on bivalves has shown that magnesium can inhibit calcite crystal nucleation, and there is evidence for exclusion of Mg2+ from the EPF (Lorens and Bender, 1977). In line with other studies, we show that C. virginica and A. islandica have lower in the EPF than seawater (Lorens and Bender, 1977; Planchon et al., 2013); however, we note that the EPF trend is driven by changes in EPF Ca2+. C. virginica and A. islandica EPF were significantly different, with lower EPF for A. islandica, possibly due to different controls over EPF Ca2+ between both species. The partition coefficient between EPF and the shell was calculated to be 0.003 for C. virginica and 0.0002 for A. islandica, which is consistent with previous studies on bivalves and with the mineralogical difference between the calcite produced by C. virginica and the aragonite produced by A. islandica (Ulrich et al., 2021).
We found that the EPF δ26Mg of C. virginica was depleted compared to seawater δ26Mg (Fig. 3). Our δ26Mg values for the EPF and shell were in line with previous work on bivalves (Planchon et al., 2013). Planchon et al. (2013) found a (2 SD, n=5) difference between EPF and seawater in the aragonitic Manila clam, Ruditapes philippinarum. Similarly, in the present study, a difference of was observed for the calcitic C. virginica, but no δ26Mg data were collected for A. islandica due to sample limitation. Both Planchon et al. (2013) and the present study show depleted EPF δ26Mg relative to seawater δ26Mg, indicating a potential biological modulation of EPF Mg2+, which has been previously attributed to heavier isotopes being incorporated into soft tissues or magnesium fixation within organic molecules (Planchon et al., 2013). However, it is important to note that the difference between EPF and seawater δ26Mg is low, and the δ26Mg fractionation between the shell and seawater (2.43 ‰ ) was slightly larger than but still in line with inorganic calcite precipitation studies (Mavromatis et al., 2013; Saulnier et al., 2012).
4.2 Comparison of A. islandica and C. virginica EPF pH and boron geochemistry of seawater, EPF, and bivalve shell
The boron isotopes and proxies have been used as paleo-pH and proxies, recording changes in seawater carbonate chemistry in the shells of foraminifera (e.g., Hemming and Hanson 1992; Sanyal et al., 2001; Foster and Rae, 2016). In different taxa, however, there is evidence that these proxies monitor changes in the carbonate chemistry of the internal calcifying fluid, which may be different from seawater geochemistry (e.g., Allison and Finch 2010; Cornwall et al., 2017; Sutton et al., 2018; Guillermic et al., 2021). In the present study, we constrained the and δ11B of the main reservoirs involved in the biomineralization (seawater, extrapallial fluid, and shell) of C. virginica and A. islandica.
We found an incongruence between seawater pH, measured EPF pH, and δ11B-calculated pH. For both C. virginica and A. islandica, microelectrode EPF pH was lower than seawater pH. These findings are similar to previous work on bivalves, which also show that the EPF pH is lower than seawater pH (Crenshaw 1972; Heinemann et al., 2012; Stemmer et al., 2019; Cameron et al., 2019). Microelectrode EPF pH between species was found to not be significantly different, indicating a similar downregulation in pH compared to seawater. However, our δ11B-based EPF pH was different between species (Fig. 5). Using boron isotope systematics, this translated to a δ11B-calculated EPF pH of 7.76±0.07 for A. islandica and 8.12±0.09 for C. virginica. Although boron isotopes have been shown to probe the internal calcification fluid of certain taxa, like corals (e.g., Allison and Finch 2010), our results show an incongruence between measured EPF pH and δ11B-calculated pH.
4.3 C. virginica ocean acidification effects on Mg2+ and Ca2+ geochemistry of seawater, EPF, and bivalve shell
In the complementary study by Downey-Wall et al. (2020), it was found that the C. virginica calcification rates decreased with seawater pH (Downey-Wall et al., 2020; Fig. 2). The reduction of calcification under ocean acidification conditions is well documented in other seawater pH experiments on different bivalve species (e.g., Ries et al., 2009; Beniash et al., 2010; Waldbusser et al., 2011; Downey-Wall et al., 2020). This result is consequential as the shell is important in protecting the animal from predation, desiccation, and the effects of transient changes in seawater chemistry (Gosling, 2008). Under ocean acidification treatments, the average microelectrode EPF pH of C. virginica was lower than seawater pH. This is in line with other simulated ocean acidification studies that also found a decrease in EPF pH (Michaelidis et al., 2005; Thomsen et al., 2013; Zittier et al.; 2015, Cameron et al., 2019; Downey-Wall et al., 2020). However, the change in pH between EPF and seawater pH (pH) decreased with decreasing pH, resulting in an EPF pH that was closer to seawater pH under acidified conditions (Figs. 8a and 9c).
Only C. virginica was cultured under ocean acidification (OA) treatments representing control, moderate, and high OA treatments. As mentioned above, the control experiment showed elevation of EPF Ca2+ and EPF Mg2+ relative to seawater. However, as EPF pH decreased, the EPF Ca2+ and Mg2+ significantly decreased as well (Fig. 6). Ion transporters such as voltage-gated Ca2+-channels tend to also affect chemically similar ions like Mg2+, and a reduction of such a transporter could possibly explain the similar trends in Ca2+ and Mg2+ concentrations under OA (Hess et al., 1986). Under OA conditions, EPF Ca2+ decreased to concentrations that were similar to or below seawater Ca2+, indicating a reduced ability of the organism to upregulate these ions under OA conditions. Previous studies have found a similar tight coupling between pH and Ca2+. For example, Stemmer et al. (2019) found synchronous patterns between pH and Ca2+ dynamics in A. islandica that they explained to be the result of calcium-transporting ATPase, which exchanges protons and calcium ions across the mantle and has proven to be important for acid–base regulation and calcium transport in bivalves (Stemmer et al., 2019; Sillanpää et al., 2018; Sillanpää et al., 2020). Although calcium-transporting ATPase could explain this increase in Ca2+ under ambient conditions, this transport mechanism may be reduced under acidified conditions, thereby impairing the bivalve's ability to regulate protons and calcium ions in the extrapallial fluid, rendering EPF Ca2+ and pH more similar to that of seawater.
Alternatively, the simultaneous reduction in Ca2+ and Mg2+ under OA conditions could point to an ion storage mechanism. The reduction of both calcium and magnesium within the EPF under moderate and high OA treatments could possibly be linked to changes of storage and budgets of ions under stressful conditions (Mount et al., 2004; Johnstone et al., 2015; Wang et al., 2017). Further, several studies have highlighted significant changes in bivalve Ca2+ ion transport and storage in different extracellular and subcellular compartments associated with shell damage and repair under acidified conditions (Sillanpää et al., 2016; Mount et al., 2004; Fitzer et al., 2016). Lastly, the EPF Ca2+ could simply reflect the balance between calcification and dissolution of the shell, as exemplified in a study on C. virginica conducted by Ries et al. (2016), which found that under similarly low saturation states, localized shell calcification was maintained despite net dissolution of the shell. Regardless of the exact mechanism, the reduction in extrapallial fluid Ca2+ under ocean acidification is a significant result that could impact the ability of bivalves to calcify by decreasing the CaCO3 saturation state of the EPF.
4.4 C. virginica ocean acidification effects on boron geochemistry
Similarly to ambient conditions, the calculated δ11B-based pH for C. virginica is systematically higher than microelectrode EPF pH (Fig. 8). Both δ11B-based pH and measured EPF pH record a decrease in pH under acidified conditions (regression p<0.05 for microelectrode pH). However, the offset between microelectrode EPF pH and the δ11B-calculated pH was 0.3 pH units and increased to 0.6 and 0.8 pH units for the moderate and high OA treatments, respectively (Fig. 8). This demonstrates that, under OA conditions, the incongruence between δ11B-based pH and measured EPF pH increases and potentially renders the seawater pH proxy impractical, even after species-specific empirical calibration. Under OA conditions, shell δ11B was not correlated with changes in seawater pH but was significantly correlated to microelectrode pH (Fig. 7f). These data indicate that microelectrode EPF pH does not fully resolve δ11B vital effects or discrepancies.
However, it is important to note the differences in timescales associated with δ11B-calculated EPF pH and microelectrode pH. Our microelectrode pH measurements, although averaged across several time points, show snapshots in time and are variable due different behavioral scenarios such as open (feeding, high pH) and closed (respiring into a closed system, low pH) cycles. Conversely, the δ11B represents an integrated average of EPF pH over the interval that the sampled shell was formed, which could range from days to weeks. Furthermore, the δ11B method will only record EPF pH at the site of calcification when the shell is forming, which can skew the archiving of the δ11B pH signal in the shell to higher values because the crystal only forms when saturation states and calcification rates are higher. This potential bias is also consistent with our δ11B-calculated EPF pH data being higher than the microelectrode pH data and similar to trends seen in corals (Cameron et al., 2022).
A possible explanation for the incongruence between δ11B-based pH and measured EPF pH arises from boron isotope systematics. The boron isotope proxy assumes that only the charged borate ion is incorporated as BO4 into the mineral, but it has been shown that boric acid can also be incorporated as BO3, and NMR studies have shown the presence of BO3 in the shells of different marine organisms (Rollion Bard et al., 2011; Cusack et al., 2015). However, the presence of BO3 does not obviously translate to a strong bias in the δ11B signature of the mineral due to the potential re-coordination of BO4 to BO3 within the crystal lattice (Klochko et al., 2009). A simple calculation shows that 14 %–17 % boric acid incorporation could explain the observed difference between EPF pH and δ11B-calculated pH for C. virginica, which could very well explain the discrepancy. Alternatively, shell δ11B could also be affected by seawater or extrapallial fluid DIC, which bivalves are known to modulate under ambient and OA conditions (Crenshaw 1972, Stemmer et al., 2019). Gagnon et al. (2021) found that the shell δ11B of deep-water coral is independently sensitive to changes in seawater DIC as a result of diffusion of boric acid (Gagnon et al., 2021). Though no similar studies have looked at the same effect in bivalves, this mechanism is still possible. Taken together, these findings could explain the offset between δ11B-based pH and seawater or EPF pH. Nevertheless, this remains speculative as there is no further evidence of boric acid incorporation in these species.
The difference between microelectrode EPF pH and δ11B-based EPF pH implies that pH measured with boron isotopes probes a localized site of calcification rather than the entire EPF pool measured with microelectrode. A spatial and temporal study conducted by Stemmer et al. (2019) measured the EPF of Arctica islandica and showed highly dynamic changes in pH, Ca2+, and DIC from the surface of the shell to the outer mantle epithelium (OME), with localized environment at the OME reaching pH values up to 9.5. Due to this high variability, it is possible that the EPF microelectrode measurements in this study did not capture the full variability of the EPF. Stemmer et al. (2019) presented EPF pH values measured at the shell surface ranging [7.1–7.6] for A. islandica, comparable to the values measured from microelectrode in this study (Fig. 9). Additionally, Stemmer et al. (2019) found large influxes of DIC which could not have been explained just from metabolic activity but instead indicated intense DIC pumping and bursts of calcification. These findings are in line with the holistic view of biomineralization outlined in Checa (2018) and Johnstone et al. (2015), who argue that crystal deposition is a series of periodic events under biological regulation. In our study, a time series of microelectrode EPF pH shows that at no point, during ventilation and closed cycles, does the EPF pH reach the δ11B-calculated pH (Fig. 9). The fact that microelectrode EPF pH is systematically lower than seawater pH for both of our bivalve species may reflect localized differences in pH associated with zones of calcification. The two environments (site of calcification and bulk EPF) can act distinctly, with low pH and high DIC EPF being a source of carbon for the site of calcification in the bulk EPF and elevated pH of the site of calcification supporting the conversion of the DIC species to [] in support of mineral precipitation. Further work would be needed to assess this highly dynamic and localized environment; however, our study shows that boron isotopes may reflect the pH of the microenvironment where calcification occurs within the EPF, which has previously been inferred by prior studies using non-geochemical approaches (Ramesh et al., 2017, 2018; Stemmer et al., 2019).
In this study, we used numerous approaches constraining the geochemical composition of and partitioning between the tripartite reservoirs of the bivalve mineralization system: seawater, EPF, and shell. Comparisons of seawater and extrapallial fluid and , Ca2+, and δ26Mg indicate that the EPF has a distinct composition that differs from seawater. Additionally, our OA experiments show that the EPF and , as well as absolute Mg2+, B, and Ca2+, all were significantly affected by CO2-induced ocean acidification, demonstrating that the biological pathways regulating or storing these ions involved in calcification are impacted by ocean acidification. Decreased calcium ion concentration within the extrapallial fluid due to OA could impair calcification by lowering the saturation state of the EPF with respect to CaCO3. Additionally, our results show that shell δ11B does not faithfully record seawater pH. However, shell δ11B is correlated with EPF pH, despite an offset from in situ microelectrode pH measurements. Both microelectrode pH and δ11B-calculated pH decreased with decreasing pH. However, the δ11B-calculated pH values were consistently higher than microelectrode pH measurements, indicating that the shell δ11B may reflect pH at a more localized site of calcification, rather than pH of the bulk EPF. Furthermore, the offset between the δ11B-calculated pH and microelectrode pH increased with decreasing pH under ocean acidification, indicating OA has a larger effect on bulk pH of the EPF measured via microelectrode than on site of calcification pH – the latter of which the bivalve may have more physiological control over to ensure continued calcification even under chemically unfavorable conditions. These complex dynamics of EPF chemistry suggest that boron proxies in these two bivalve species are not straightforwardly related to seawater pH, precluding utilization of those species for reconstructing the carbonate chemistry of seawater. Moreover, the δ11B proxy may not be suitable for reconstructing seawater pH for bivalves with high physiological control over their internal calcifying fluid and is further complicated under conditions of moderate and extreme ocean acidification, where δ11B EPF pH deviates further from bulk microelectrode pH, possibly due to the effect of DIC on shell δ11B or the tendency for shell δ11B to reflect EPF pH at the more localized site of calcification, rather than pH of the bulk EPF.
Code used in this paper can be found in the Supplement of Raitzsch et al. (2021).
All data are published in this paper or in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-2831-2025-supplement.
LPC, ADW, JBR, and KL designed the experiments and carried them out. BAC, MG, and RAE developed the geochemical study. BAC and MG performed geochemical analysis with the help of JNS and JAH. BAC, MG, and RAE prepared the manuscript with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors would like to thank Celine Liorzou, Yoan Germain, and Anne Trinquier for their technical support at the PSO. Additionally, the authors would like to thank Stefania Gili for her technical support at Princeton University.
Blanca Alvarez Caraveo was supported by the National Science Foundation Graduate Research Fellowship Program under grant no. DGE-2034835 and the UC Eugene Cota-Robles Fellowship. Blanca Alvarez Caraveo, Maxence Guillermic, and Robert A. Eagle are supported by the ocean science work of Center for Diverse Leadership in Science, which is funded by a grant from the David and Lucile Packard Foundation (grant no. 85180), the National Science Foundation (grant no. NSF-RISE-2024426), and by gifts from Oceankind and Dalio Philanthropies. The Center for Diverse Leadership in Science is also supported by grant no. NSF-RISE-2228198, the Waverley Street Foundation, the Silicon Valley Community Foundation, and the Sloan Foundation. Katie Lotterhos and Justin B. Ries were supported by the National Science Foundation (grant no. BIO-OCE 1635423).
This paper was edited by Niels de Winter and reviewed by two anonymous referees.
Addadi, L., Raz, S., and Weiner, S.: Taking Advantage of Disorder: Amorphous calcium carbonate and its roles in biomineralization, Advanced Materials, 15, 959–970, https://doi.org/10.1002/adma.200300381, 2003.
Addadi, L., Joester, D., Nudelman, F., and Weiner, S.: Mollusc Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes, Chem.-Eur. J., 12, 980–987, https://doi.org/10.1002/chem.200500980, 2006.
Ahm, A.-S. C., Bjerrum, C. J., Hoffman, P. F., Macdonald, F. A., Maloof, A. C., Rose, C. V., Strauss, J. V., and Higgins, J. A.: The Ca and Mg isotope record of the Cryogenian Trezona carbon isotope excursion, Earth Planet. Sc. Lett., 568, 117002, https://doi.org/10.1016/j.epsl.2021.117002, 2021.
Alibert, C. and McCulloch, M. T.: Strontium/calcium ratios in modern porites corals From the Great Barrier Reef as a proxy for sea surface temperature: Calibration of the thermometer and monitoring of ENSO, Paleoceanography, 12, 345–363, https://doi.org/10.1029/97PA00318, 1997.
Allison, N. and Finch, A. A.: δ11B, Sr, Mg and B in a modern Porites coral: the relationship between calcification site pH and skeletal chemistry, Geochim. Cosmochim. Ac., 74, 1790–1800, https://doi.org/10.1016/j.gca.2009.12.030, 2010.
Barker, S., Greaves, M., and Elderfield, H. A study of cleaning procedures used for foraminiferal paleothermometry, Geochem. Geophy. Geosy., 4, 8407, https://doi.org/10.1029/2003GC000559, 2003.
Beniash, E., Ivanina, A., Lieb, N. S., Kurochkin, I., and Sokolova, I. M.: Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica, Mar. Ecol. Prog. Ser., 419, 95–108, https://doi.org/10.3354/meps08841, 2010.
Broecker, W. S. and Peng, T.-H.: Tracers in the Sea, Lamont-Doherty Geological Observatory, Columbia University Palisades, New York, b1–b2, https://doi.org/10.1017/S0033822200005221, New York, 1982.
Cameron, L. P.: Understanding Patterns of Bivalve Vulnerability and Resilience to Ocean Acidification: Insights from Field Studies, Tank Experiments and Novel Physiological Studies, Dissertation Northeastern University, ISBN 9798641900292, 2020.
Cameron, L. P., Reymond, C. E., Bijma, J., Büscher, J. V., de Beer, D., Guillermic, M., Eagle, R. A., Gunnell, J., Müller-Lundin, F., Schmidt-Grieb, G. M., Westfield, I., Westphal, H., Ries, J. B.: Impacts of warming and acidification on coral calcification linked to photosymbiont loss and deregulation of calcifying fluid pH, Journal of Marine Science and Engineering, 10, 1106, https://doi.org/10.3390/jmse10081106, 2022.
Cameron, L. P., Reymond, C. E., Müller-Lundin, F., Westfield, I., Grabowski, J. H., Westphal, H., and Ries, J. B.: Effects of temperature and ocean acidification on the extrapallial fluid pH, calcification rate, and condition factor of the king scallop Pecten maximus, J. Shellfish Res., 38, 763–777, https://doi.org/10.2983/035.038.0327, 2019.
Checa, A. G.: Physical and Biological Determinants of the fabrication of molluscan shell microstructures, Frontiers in Marine Science, 5, 353, https://doi.org/10.3389/fmars.2018.00353, 2018.
Cornwall, C. E., Comeau, S., and McCulloch, M. T.: Coralline algae elevate pH at the site of calcification under ocean acidification, Glob. Change Biol., 23, 4245–4256, https://doi.org/10.1111/gcb.13673, 2017.
Craig, H.: The geochemistry of the stable carbon isotopes, Geochim. Cosmochim. Ac., 3, 53–92, https://doi.org/10.1016/0016-7037(53)90001-5, 1953.
Crenshaw, M. A.: The inorganic composition of molluscan extrapallial fluid, Biol. Bull., 143, 506–512, 1972.
Cusack, M., Kamenos, N. A., Rollion-Bard, C., and Tricot, G.: Red coralline algae assessed as marine pH proxies using 11B MAS NMR, Sci. Rep.-UK, 5, 8175, https://doi.org/10.1038/srep08175, 2015.
Downey-Wall, A. M., Cameron, L. P., Ford, B. M., McNally, E. M., Venkataraman, Y. R., Roberts, S. B., Ries, J. B., and Lotterhos, K. E.: Ocean acidification induces subtle shifts in gene expression and DNA methylation in mantle tissue of the Eastern oyster (Crassostrea virginica), Frontiers in Marine Science, 7, 566419, https://doi.org/10.3389/fmars.2020.566419, 2020.
Dunbar, R. B., Wellington, G. M., Colgan, M. W., and Glynn, P. W.: Eastern Pacific sea surface temperature since 1600 A. D.: The δ18O record of climate variability in Galápagos Corals, Paleoceanography, 9, 291–315, https://doi.org/10.1029/93PA03501, 1994.
Eagle, R. A., Guillermic, M., De Corte, I., Alvarez Caraveo, B., Bove, C. B., Misra, S., Cameron, L. P., Castillo, K. D., and Ries, J. B.: Physicochemical Control of Caribbean Coral Calcification Linked to Host and Symbiont Responses to Varying pCO2 and Temperature, Journal of Marine Science and Engineering, 10, 1075, https://doi.org/10.3390/jmse10081075, 2022.
Elderfield, H., Yu, J., Anand, P., Kiefer, T., and Nyland, B.: Calibrations for benthic foraminiferal paleothermometry and the carbonate ion hypothesis, Earth Planet. Sc. Lett., 250, 633–649, https://doi.org/10.1016/j.epsl.2006.07.041, 2006.
Fitzer, S. C., Chung, P., Maccherozzi, F., Dhesi, S. S., Kamenos, N. A., Phoenix, V. R., and Cusack, M.: Biomineral shell formation under ocean acidification: a shift from order to chaos, Sci. Rep.-UK, 6, 21076, https://doi.org/10.1038/srep21076, 2016.
Foster, G. L. and Rae, J. W. B.: Reconstructing Ocean pH with Boron Isotopes in Foraminifera, Annu. Rev. Earth Pl. Sc., 44, 207–237, https://doi.org/10.1146/annurev-earth-060115-012226, 2016.
Gagnon, A. C., Gothmann, A. M., Branson, O., Rae, J. W. B., and Stewart, J. A.: Controls on boron isotopes in a cold-water coral and the cost of resilience to ocean acidification, Earth Planet. Sc. Lett., 554, 116662, https://doi.org/10.1016/j.epsl.2020.116662, 2021.
Gaillardet, J., Lemarchand, D., Göpel, C., and Manhès, G.: Evaporation and Sublimation of Boric Acid: Application for Boron Purification from Organic Rich Solutions, Geostandard. Newslett., 25, 67–75, https://doi.org/10.1111/j.1751-908X.2001.tb00788.x, 2001.
Gazeau, F., Parker, L. M., Comeau, S., Gattuso, J.-P., O'Connor, W. A., Martin, S., Pörtner, H.-O., and Ross, P. M.: Impacts of ocean acidification on marine shelled molluscs, Mar. Biol., 160, 2207–2245, https://doi.org/10.1007/s00227-013-2219-3, 2013.
Gibson, R., Barnes, M., and Atkinson, R.: Molluscs as archives of environmental change, Oceanogr. Mar. Biol., 39, 103–164, 2001.
Gosling, E.: Bivalve molluscs: biology, ecology and culture, John Wiley & Sons, 2008.
Guillermic, M., Cameron, L. P., De Corte, I., Misra, S., Bijma, J., De Beer, D., Reymond, C. E., Westphal, H., Ries, J. B., and Eagle, R. A.: Thermal stress reduces pocilloporid coral resilience to ocean acidification by impairing control over calcifying fluid chemistry, Science Advances, 7, eaba9958, https://doi.org/10.1126/sciadv.aba9958, 2021.
Gutjahr, M., Bordier, L., Douville, E., Farmer, J., Foster, G. L., Hathorne, E. C., Hönisch, B., Lemarchand, D., Louvat, P., McCulloch, M., Noireaux, J., Pallavicini, N., Rae, J. W. B., Rodushkin, I., Roux, P., Stewart, J. A., Thil, F., and You, C.: Sub-Permil Interlaboratory Consistency for Solution-Based Boron Isotope Analyses on Marine Carbonates, Geostand. Geoanal. Res., 45, 59–75, https://doi.org/10.1111/ggr.12364, 2021.
Heinemann, A., Fietzke, J., Melzner, F., Böhm, F., Thomsen, J., Garbe-Schönberg, D., and Eisenhauer, A.: Conditions of Mytilus edulis extracellular body fluids and shell composition in a pH-treatment experiment: Acid-base status, trace elements and δ11B, Geochem. Geophy. Geosy., 13, 2011GC003790, https://doi.org/10.1029/2011GC003790, 2012.
Helm, M. M., Bourne, N., and Lovatelli, A.: The hatchery culture of bivalves: a practical manual, FAO, Rome, ISBN 92-5-105224-7, XXI, 177 pp., 2004.
Hemming, N. G. and Hanson, G. N.: Boron isotopic composition and concentration in modern marine carbonates, Geochim. Cosmochim. Ac., 56, 537–543, https://doi.org/10.1016/0016-7037(92)90151-8, 1992.
Hess, P., Lansman, J. B., and Tsien, R. W.: Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells, J. Gen. Physiol., 88, 293–319, https://doi.org/10.1085/jgp.88.3.293, 1986.
Higgins, J. A., Blättler, C. L., Lundstrom, E. A., Santiago-Ramos, D. P., Akhtar, A. A., Crüger Ahm, A.-S., Bialik, O., Holmden, C., Bradbury, H., Murray, S. T., and Swart, P. K.: Mineralogy, early marine diagenesis, and the chemistry of shallow-water carbonate sediments, Geochim. Cosmochim. Ac., 220, 512–534, https://doi.org/10.1016/j.gca.2017.09.046, 2018.
Hönisch, B., Hemming, N., Grottoli, A. G., Amat, A., Hanson, G. N., and Bijma, J.: Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects, Geochim. Cosmochim. Ac., 68, 3675–3685, https://doi.org/10.1016/j.gca.2004.03.002, 2004.
Husson, J. M., Higgins, J. A., Maloof, A. C., and Schoene, B.: Ca and Mg isotope constraints on the origin of Earth's deepest δ13C excursion, Geochim. Cosmochim. Ac., 160, 243–266, https://doi.org/10.1016/j.gca.2015.03.012, 2015.
Johnstone, M. B., Gohad, N. V., Falwell, E. P., Hansen, D. C., Hansen, K. M., and Mount, A. S.: Cellular orchestrated biomineralization of crystalline composites on implant surfaces by the eastern oyster, Crassostrea virginica (Gmelin, 1791), J. Exp. Mar. Biol. Ecol., 463, 8–16, https://doi.org/10.1016/j.jembe.2014.10.014, 2015.
Klochko, K., Kaufman, A. J., Yao, W., Byrne, R. H., and Tossell, J. A.: Experimental measurement of boron isotope fractionation in seawater, Earth Planet. Sc. Lett., 248, 276–285, https://doi.org/10.1016/j.epsl.2006.05.034, 2006.
Klochko, K., Cody, G. D., Tossell, J. A., Dera, P., and Kaufman, A. J.: Re-evaluating boron speciation in biogenic calcite and aragonite using 11B MAS NMR, Geochim. Cosmochim. Ac., 73, 1890–1900, https://doi.org/10.1016/j.gca.2009.01.002, 2009.
Kroeker, K. J., Micheli, F., Gambi, M. C., and Martz, T. R.: Divergent ecosystem responses within a benthic marine community to ocean acidification, P. Natl. Acad. Sci. USA, 108, 14515–14520, https://doi.org/10.1073/pnas.1107789108, 2011.
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., and Gattuso, J. P.: Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming, Glob. Change Biol., 19, 1884–1896, https://doi.org/10.1111/gcb.12179, 2013.
Liu, Y.-W., Sutton, J. N., Ries, J. B., and Eagle, R. A.: Regulation of calcification site pH is a polyphyletic but not always governing response to ocean acidification, Science Advances, 6, eaax1314, https://doi.org/10.1126/sciadv.aax1314, 2020.
Lorens, R. B. and Bender, M. L.: Physiological exclusion of magnesium from Mytilus edulis calcite, Nature, 269, 793–794, https://doi.org/10.1038/269793a0, 1977.
Mavromatis, V., Gautier, Q., Bosc, O., and Schott, J.: Kinetics of Mg partition and Mg stable isotope fractionation during its incorporation in calcite, Geochim. Cosmochim. Ac., 114, 188–203, https://doi.org/10.1016/j.gca.2013.03.024, 2013.
McCulloch, M. T., D'Olivo, J. P., Falter, J., Holcomb, M., and Trotter, J. A.: Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation, Nat. Commun., 8, 15686, https://doi.org/10.1038/ncomms15686, 2017.
McCulloch, M. T., D’Olivo, J. P., Falter, J., Georgiou, L., Holcomb, M., Montagna, P., and Trotter, J. A.: Boron isotopic systematics in scleractinian corals and the role of pH up-regulation. Boron isotopes: The fifth element, edited by: Marschall, H. and Foster, G., 145–162, Advances in Isotope Geochemistry, Springer, Cham, https://doi.org/10.1007/978-3-319-64666-4_6, 2018.
McNally, E. M., Downey-Wall, A. M., Titmuss, F. D., Cortina, C., Lotterhos, K., and Ries, J. B.: Parental exposure of Eastern oysters (Crassostrea virginica) to elevated pCO2 mitigates its negative effects on early larval shell growth and morphology, Limnol. Oceanogr., 67, 1732–1745, https://doi.org/10.1002/lno.12162, 2022.
Michaelidis, B., Ouzounis, C., Paleras, A., and Pörtner, H. O.: Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis, Mar. Ecol. Prog. Ser., 293, 109–118, https://doi.org/10.3354/meps293109, 2005.
Mount, A. S., Wheeler, A. P., Paradkar, R. P., and Snider, D.: Hemocyte-Mediated Shell Mineralization in the Eastern Oyster, Science, 304, 297–300, https://doi.org/10.1126/science.1090506, 2004.
Nir, O., Vengosh, A., Harkness, J. S., Dwyer, G. S., and Lahav, O.: Direct measurement of the boron isotope fractionation factor: Reducing the uncertainty in reconstructing ocean paleo-pH, Earth Planet. Sc. Lett., 414, 1–5, https://doi.org/10.1016/j.epsl.2015.01.006, 2015.
Norrie, C. R., Dunphy, B. J., Ragg, N. L. C., and Lundquist, C. J.: Ocean acidification can interact with ontogeny to determine the trace element composition of bivalve shell, Limnol. Oceanogr. Lett., 3, 393–400, https://doi.org/10.1002/lol2.10090, 2018.
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R. M., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R. G., Plattner, G., Rodgers, K. B., Sabine, C. L., Sarmiento, J. L., Schlitzer, R., Slater, R. D., Totterdell, I. J., Weirig, M., Yamanaka, Y., and Yool, A.: Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms, Nature, 437, 681–686, https://doi.org/10.1038/nature04095, 2005.
Peharda, M., Schöne, B. R., Black, B. A., and Correge, T.: Advances of sclerochronology research in the last decade, Palaeogeogr. Palaeocl., 570, 110371, https://doi.org/10.1016/j.palaeo.2021.110371, 2021.
Pierrot, D. E., Wallace, D. W. R., and Lewis, E.: MS Excel program developed for CO2 system calculations, Carbon dioxide information analysis center, https://doi.org/10.3334/cdiac/otg.co2sys_xls_cdiac105a, 2011.
Planchon, F., Poulain, C., Langlet, D., Paulet, Y.-M., and André, L.: Mg-isotopic fractionation in the manila clam (Ruditapes philippinarum): New insights into Mg incorporation pathway and calcification process of bivalves, Geochim. Cosmochim. Ac., 121, 374–397, https://doi.org/10.1016/j.gca.2013.07.002, 2013.
Raitzsch, M., Bijma, J., Bickert, T., Schulz, M., Holbourn, A., and Kučera, M.: Atmospheric carbon dioxide variations across the middle Miocene climate transition, Clim. Past, 17, 703–719, https://doi.org/10.5194/cp-17-703-2021, 2021.
Ramesh, K., Hu, M. Y., Thomsen, J., Bleich, M., and Melzner, F.: Mussel larvae modify calcifying fluid carbonate chemistry to promote calcification, Nat. Commun., 8, 1709, https://doi.org/10.1038/s41467-017-01806-8, 2017.
Ramesh, K., Melzner, F., Griffith, A. W., Gobler, C. J., Rouger, C., Tasdemir, D., and Nehrke, G.: In vivo characterization of bivalve larval shells: a confocal Raman microscopy study, J. R. Soc. Interface, 15, 20170723, https://doi.org/10.1098/rsif.2017.0723, 2018.
Ries, J. B.: A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification, Geochim. Cosmochim. Ac., 75, 4053–4064, https://doi.org/10.1016/j.gca.2011.04.025, 2011.
Ries, J. B., Cohen, A. L., and McCorkle, D. C.: Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification, Geology, 37, 1131–1134, https://doi.org/10.1130/G30210A.1, 2009.
Ries, J. B., Ghazaleh, M. N., Connolly, B., Westfield, I., and Castillo, K. D.: Impacts of seawater saturation state () and temperature (10, 25 °C) on the dissolution kinetics of whole-shell biogenic carbonates, Geochim. Cosmochim. Ac., 192, 318–337, https://doi.org/10.1016/j.gca.2016.07.001, 2016.
Rollion-Bard, C., Blamart, D., Trebosc, J., Tricot, G., Mussi, A., and Cuif, J.-P.: Boron isotopes as pH proxy: A new look at boron speciation in deep-sea corals using 11B MAS NMR and EELS, Geochim. Cosmochim. Ac., 75, 1003–1012, https://doi.org/10.1016/j.gca.2010.11.023, 2011.
Sanyal, A., Bijma, J., Spero, H., and Lea, D. W.: Empirical relationship between pH and the boron isotopic composition of Globigerinoides sacculifer: Implications for the boron isotope paleo-pH proxy, Paleoceanography, 16, 515–519, https://doi.org/10.1029/2000PA000547, 2001.
Saulnier, S., Rollion-Bard, C., Vigier, N., and Chaussidon, M.: Mg isotope fractionation during calcite precipitation: An experimental study, Geochim. Cosmochim. Ac., 91, 75–91, https://doi.org/10.1016/j.gca.2012.05.024, 2012.
Schöne, B. R., Zhang, Z., Radermacher, P., Thébault, J., Jacob, D. E., Nunn, E. V., and Maurer, A. F.: and ratios of ontogenetically old, long-lived bivalve shells (Arctica islandica) and their function as paleotemperature proxies, Palaeogeogr. Palaeocl., 302, 52–64, https://doi.org/10.1016/j.palaeo.2010.03.016, 2011.
Sillanpää, J. K., Ramesh, K., Melzner, F., Sundh, H., and Sundell, K.: Calcium mobilisation following shell damage in the Pacific oyster, Crassostrea gigas, Mar. Genom., 27, 75–83, https://doi.org/10.1016/j.margen.2016.03.001, 2016.
Sillanpää, J. K., Sundh, H., and Sundell, K. S.: Calcium transfer across the outer mantle epithelium in the Pacific oyster, Crassostrea gigas, P. R. Soc. B, 285, 20181676, https://doi.org/10.1098/rspb.2018.1676, 2018.
Sillanpää, J. K., Cardoso, J. C. dos R., Félix, R. C., Anjos, L., Power, D. M., and Sundell, K.: Dilution of seawater affects the Ca2+ transport in the outer mantle epithelium of Crassostrea gigas, Front. Physiol., 11, 496427, https://doi.org/10.3389/fphys.2020.00001, 2020.
Stemmer, K., Brey, T., Gutbrod, M. S., Beutler, M., Schalkhausser, B., and De Beer, D.: In situ measurements of pH, Ca2+, and DIC dynamics within the extrapallial fluid of the ocean quahog Arctica islandica, J. Shellfish Res., 38, 71–78, https://doi.org/10.2983/035.038.0107, 2019.
Stewart-Sinclair, P. J., Last, K. S., Payne, B. L., and Wilding, T. A.: A global assessment of the vulnerability of shellfish aquaculture to climate change and ocean acidification, Ecol. Evol., 10, 3518–3534, https://doi.org/10.1002/ece3.6149, 2020.
Sutton, J. N., Liu, Y.-W., Ries, J. B., Guillermic, M., Ponzevera, E., and Eagle, R. A.: δ11B as monitor of calcification site pH in divergent marine calcifying organisms, Biogeosciences, 15, 1447–1467, https://doi.org/10.5194/bg-15-1447-2018, 2018.
Thomsen, J., Casties, I., Pansch, C., Körtzinger, A., and Melzner, F.: Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments, Glob. Change Biol., 19, 1017–1027, https://doi.org/10.1111/gcb.12109, 2013.
Ulrich, R. N., Guillermic, M., Campbell, J., Hakim, A., Han, R., Singh, S., Stewart, J. D., Román-Palacios, C., Carroll, H. M., and De Corte, I.: Patterns of element incorporation in calcium carbonate biominerals recapitulate phylogeny for a diverse range of marine calcifiers, Front. Earth Sci., 9, 641760, https://doi.org/10.3389/feart.2021.641760, 2021.
Urey, H. C., Lowenstam, H. A., Epstein, S., and McKinney, C. R.: Measurement of paleotemperatures and temperatures of the Upper Cretaceous of England, Denmark, and the southeastern United States, Geol. Soc. Am. Bull., 62, 399–416, https://doi.org/10.1130/0016-7606(1951)62[399:MOPATO]2.0.CO;2, 1951.
Vogl, J., Rosner, M., and Pritzkow, W.: Development and validation of a single collector SF-ICPMS procedure for the determination of boron isotope ratios in water and food samples, J. Anal. Atom. Spectrom., 26, 861–869, https://doi.org/10.1039/C0JA00220H, 2011.
Waldbusser, G. G., Voigt, E. P., Bergschneider, H., Green, M. A., and Newell, R. I. E.: Biocalcification in the Eastern Oyster (Crassostrea virginica) in Relation to Long-term Trends in Chesapeake Bay pH, Estuar. Coast., 34, 221–231, https://doi.org/10.1007/s12237-010-9307-0, 2011.
Waldbusser, G. G., Hales, B., Langdon, C. J., Haley, B. A., Schrader, P., Brunner, E. L., Gray, M. W., Miller, C. A., and Gimenez, I.: Saturation-state sensitivity of marine bivalve larvae to ocean acidification, Nat. Clim. Change, 5, 273–280, https://doi.org/10.1038/nclimate2479, 2015.
Wanamaker Jr., A. D., Kreutz, K. J., Wilson, T., Borns Jr., H. W., Introne, D. S., and Feindel, S.: Experimentally determined and ratios in juvenile bivalve calcite for Mytilus edulis: implications for paleotemperature reconstructions, Geo-Mar. Lett., 28, 359–368, https://doi.org/10.1007/s00367-008-0112-8, 2008.
Wang, B.-S., You, C.-F., Huang, K.-F., Wu, S.-F., Aggarwal, S. K., Chung, C.-H., and Lin, P.-Y.: Direct separation of boron from Na-and Ca-rich matrices by sublimation for stable isotope measurement by MC-ICP-MS, Talanta, 82, 1378–1384, https://doi.org/10.1016/j.talanta.2010.07.010, 2010.
Wang, X., Wang, M., Jia, Z., Qiu, L., Wang, L., Zhang, A., and Song, L.: A Carbonic Anhydrase Serves as an Important Acid-Base Regulator in Pacific Oyster Crassostrea gigas Exposed to Elevated CO2: Implication for Physiological Responses of mollusc to Ocean Acidification, Mar. Biotechnol., 19, 22–35, https://doi.org/10.1007/s10126-017-9734-z, 2017.
Weiner, S. and Dove, P. M.: An overview of biomineralization processes and the problem of the vital effect, Rev. Mineral. Geochem., 54, 1–29, https://doi.org/10.1007/s10126-017-9734-z, 2003.
Wilbur, K. M. and Saleuddin, A. S. M.: Shell formation. In the mollusca, Academic Press, 235–287, edited by: Saleuddin, A. S. M. and Wilbur, K. M., https://doi.org/10.1016/B978-0-12-751404-8.50014-1, 1983.
Wilbur, K. M. and Bernhardt, A. M.: Effects of amino acids, magnesium, and molluscan extrapallial fluid on crystallization of calcium carbonate: In vitro experiments, Biol. Bull., 166, 251–259, https://doi.org/10.2307/1541446, 1984.
Zeebe, R. E. and Wolf-Gladrow, D.: CO2 in Seawater: Equilibrium, Kinetics, Isotopes, edited by: Zeebe, R. E. and Wolf-Gladrow, D., Gulf Professional Publishing, 65, ISBN 0444509461, 2001.
Zhao, L., Milano, S., Walliser, E. O., and Schöne, B. R.: Bivalve shell formation in a naturally CO2-enriched habitat: Unraveling the resilience mechanisms from elemental signatures, Chemosphere, 203, 132–138, https://doi.org/10.1016/j.chemosphere.2018.03.180, 2018.
Zhao, L., Milano, S., Tanaka, K., Liang, J., Deng, Y., Yang, F., Walliser, E. O., and Schöne, B. R.: Trace elemental alterations of bivalve shells following transgenerational exposure to ocean acidification: Implications for geographical traceability and environmental reconstruction, Sci. Total Environ., 705, 135501, https://doi.org/10.1016/j.scitotenv.2019.135501, 2020.
Zittier, Z. M., Bock, C., Lannig, G., and Pörtner, H. O.: Impact of ocean acidification on thermal tolerance and acid–base regulation of Mytilus edulis ( L.) from the North Sea, J. Exp. Mar. Biol. Ecol., 473, 16–25, https://doi.org/10.1016/j.jembe.2015.08.001, 2015.