the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Temperature and nutrient availability influence radial growth of Picea abies at opposite slopes in a treeline ecotone
Hana Kuželová
Tomáš Chuman
Jelena Lange
Jan Tumajer
Václav Treml
Treeline ecotones in complex mountain landscapes are exposed to pronounced differences in irradiation and soil nutrient availability. Different amounts of nutrients and direct solar energy can influence tree stem growth, especially in lower parts of a treeline ecotone, where trees are still temperature limited, though located below the upper margin of tree life. We hypothesized that, at two sites located on north- and south-facing slopes, differences in nutrient availability outperform temperature differences in modulating stem growth rates, while growth phenology is driven by temperature seasonality. To test this hypothesis, we compared the growth phenology and kinetics of Picea abies in the lower part of a treeline ecotone between a north-facing slope with relatively nutrient-rich soils and a south-facing slope with nutrient-poor soils. We analysed intra-annual wood formation, soil and air microclimate, and soil and needle nutrient contents. Our results showed that thermal differences between south- and north-facing slopes are small but nontrivial, involving higher daytime temperature at the south-facing slope and longer irradiation at the north-facing slope during the middle part of the growing season. The timings of growth onset and maximum growth rate were almost synchronized between both slopes. Accordingly, annual stem growth at both sites was most sensitive to the meteorological conditions at the start of the growing season and around the summer solstice. However, the absolute growth rate was higher on the north-facing slope, consistent with a higher availability and content of base cations in the soil and needles. Our results suggest that temperature governs growth phenology at the lower part of the treeline ecotone, but nutrient availability modulates the growth rate in the peak season when temperature no longer limits cambial activity. We demonstrated that the effect of nutrient availability can be superior to the effect of slope aspect for stem growth rates of Picea abies located in the lower part of a treeline ecotone in a temperate mountain range.
- Article
(2223 KB) - Full-text XML
-
Supplement
(644 KB) - BibTeX
- EndNote
In cold environments, tree stem growth is tightly linked to temperature oscillations during the growing season (Rossi et al., 2016; Körner, 2021), which is reflected in the seasonal phenology of wood formation, that is, cambial division and xylem differentiation (Cuny et al., 2015). There are minimum temperature thresholds for both photosynthesis and the processes linked to the investment of non-structural carbohydrates into developing xylem, the latter being lower and thus representing the ultimate limitation of stem growth (Fatichi et al., 2019). This is apparent along elevation transects where both non-structural carbohydrates and nutrient contents in twigs, leaves and sapwood increase towards the treeline as a result of increasing sink limitation of the growth (Hoch and Körner, 2012; Fajardo and Piper, 2017; Doležal et al., 2019). While no exceptions from the pure sink limitation of tree growth have been found for broadleaved treelines in the Southern Hemisphere (Fajardo and Piper, 2017), some studies examining conifers in the permafrost zone have highlighted the important role of nutrient availability in co-limiting stem growth (Sullivan et al., 2015; Dial et al., 2022). Although this is probably not generally valid for conifer treelines outside the permafrost zone (Hagedorn et al. 2020; Körner, 2021), the nutrient and moisture co-limitation of tree growth can play a role at local upper tree limits (boundaries of realized niche) or in the lower part of a treeline ecotone, i.e. several tens to a hundred metres below the treeline (Möhl et al., 2018; Körner and Hoch, 2023). Such ecotones are common as current treelines often lag behind the pace of warming (Lu et al., 2021; Shi et al., 2022).
The low-temperature limit of tree growth at its cold range boundary is evidenced by growth resumption after exceeding a certain temperature threshold, as shown both by warming/cooling experiments (Gričar et al., 2006; Lenz et al.; 2013) and by observations in natural treeline settings (Körner and Hoch, 2006; Rossi et al., 2007). Indirectly, the prevailing low-temperature limitation of tree growth at cold sites is supported by similar thermal limits of global treelines (Körner and Paulsen, 2004). Furthermore, tree-ring chronologies from treelines are significantly correlated with growing season temperature (Chagnon et al., 2023), and calibrated thermal limits of wood formation models agree with those based on direct or experimental observations (Tumajer et al., 2021).
Recently, tree growth in cold biomes tends to accelerate in some areas, which has often been attributed to warming and an extension of the growing season (Shi et al., 2020; Li et al., 2023). However, in forest stands near the treeline, observed growth enhancement has been connected to increased nitrogen supply in some regions (Kolář et al., 2015; Möhl et al., 2018; Etzold et al., 2020). Sullivan et al. (2015) showed better performance in shoot, stem and root growth at microsites relatively richer in nitrogen at the Arctic treeline due to warmer soils and a higher snowpack accelerating nutrient cycles (Dawes et al., 2017). Not only nitrogen (N) but also phosphorus (P, namely the stoichiometry of N and P) was suggested as a limiting factor of tree occurrence at some treeline ecotone sites in the Himalayas (Müller et al., 2017). Apart from N and P, the role of other nutrients, especially base cations, has been largely neglected at tree stands near their cold distribution margins. Recently, there is a growing body of literature showing that base cations can play a vital role under certain conditions in limiting tree growth in the treeline ecotone (Drollinger et al., 2017) or in montane forests (Körner, 2022; Oulehle et al., 2023).
Local evidence of growth enhancements at sites relatively enriched by N and P is not necessarily in conflict with the ultimate role of the low-temperature limitation of tree growth at the treeline (Körner, 2012) or with observations of increasing nutrient concentrations in leaves with elevation near cold margins of the tree distribution (Fajardo and Piper, 2017). This evidence rather suggests that nutrient availability together with low temperature might co-determine growth dynamics at upper tree limits whose position is not in equilibrium with the current climate (Lu et al., 2021; Körner and Hiltbrunner, 2024).
In a complex mountain relief, a high variation in topoclimatic and soil conditions can create a heterogenous mosaic of sites differing in local surface temperature (Jochner et al., 2017; Kuželová and Treml, 2020) as well as nutrient content (Liptzin et al., 2013; Mayor et al., 2017). Probably the best-known topoclimatic effect is the so-called slope exposure phenomenon, which suggests that south-facing slopes in the Northern Hemisphere outside the tropics are warmer than north-facing slopes, and vice versa in the Southern Hemisphere (Körner, 2012). This phenomenon is less pronounced on forested slopes (Paulsen and Körner, 2001). Sites on opposite slopes might differ not only in insolation and surface temperature but also in nutrient availability, as surface temperature might influence litter decomposition through surface moisture and snowmelt patterns (Dawes et al., 2017; Ellison et al., 2019; Stark et al., 2023).
In this study, we use an opposite slope aspect design in a complex research setting, which allows us to cover multiple site properties that potentially influence tree growth performance in the lower part of the treeline ecotone. This is a typical situation for the majority of current treelines which are located below the physiological limit of tree existence as the pace of warming is much faster than the upward shift of treelines (Lu et al., 2021). We carried out detailed observations of Picea abies trees in terms of intra-annual wood formation and inter-annual climate–growth responses together with analyses of site thermal and moisture properties and soil and foliar nutrient contents. We tested the general assumption that at the lower part of a treeline ecotone the crucial phases of tree growth, such as growth resumption and timing of the peak growth rate, are driven by thermal and solar constraints (Rossi et al., 2006b, 2007). However, we hypothesize that the growth rate is influenced by nutrient availability, given the positive effect of nutrient availability on absolute tree growth that has been shown at some cold-limited sites (Möhl et al., 2018; Sullivan et al., 2015).
2.1 Study area
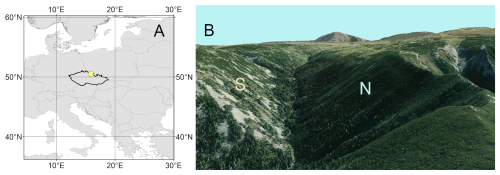
Our study focuses on a dominant tree species of a treeline ecotone, Picea abies [L.] Karst., growing on N- and S-facing slopes in the Krkonoše, Czech Republic (50°43′ N, 15°40′ E; site elevation 1250 , Fig. 1). The Krkonoše, with the highest peak Sněžka (1602 ), are characterized by high annual precipitation sums (on average 1400–1600 mm) and a mean annual temperature of 0.5 °C in the uppermost locations (Metelka et al., 2007). Snow cover in the treeline ecotone lasts from November until May, with a maximum snow depth of about 2 m (Metelka et al., 2007). The treeline ecotone is situated at elevations ranging from 1250 to 1450 m.
Two sites on opposite slopes (N-facing slope, S-facing slope) were established in the lower part of the treeline ecotone in the Bílé Labe valley (Fig. 1). The canopy cover at both sites was 20 %, and the tree height of adult individuals ranged between 8 and 13 m. Picea abies stands are gradually replaced by shrubland dominated by Pinus mugo in upslope direction. Both sites are located on steep slopes (inclination between 20 and 30°) with frequent patches of screes. The N-facing site is located on the transition between gneiss as well as mica-schist (upper part) and granodiorites (lower part). The S-facing site is underlaid by granites. Skeletic Leptosols and Skeletic Podzols are the dominant soil types on both slopes.
2.2 Microclimatic monitoring
We measured air and soil temperatures and soil water potential to characterize site microclimatic conditions from 2012 to 2015 (to 2014 for soil water potential). Air temperature was recorded using sensors in radiation shields hanging in the tree crown approximately 7 m above the ground (one sensor at each site). Three sensors recorded the soil temperature and soil water potential of the root zone (mineral soil, −10 cm depth) at each site at places fully shaded by tree crowns. We used gypsum block soil water potential sensors to measure available soil moisture (measuring range 0 to −2 MPa, Delmhorst, EMS Brno). Air and soil temperatures and soil water potential were measured and stored at 1 h intervals. Both air and soil temperature sensors have an accuracy of ± 0.2 °C (http://www.emsbrno.cz, last access: 10 January 2024).
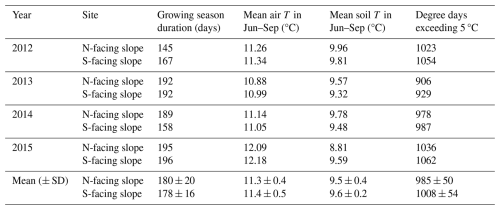
For each year and site, we calculated various variables that characterize the intra-annual meteorological patterns that are important for woody plants. Following Körner and Hiltbrunner (2018), we used soil temperature to define the duration of the meteorological growing season, i.e. the period when meteorological conditions potentially permit wood formation. We used the continuous period where soil temperature values were above 3.2 °C, roughly corresponding to a mean weekly air temperature of 0 °C (Körner and Paulsen, 2004). For both sites and for all years, we identified dates of the start, the end and the duration of the meteorological growing season. Additionally, we computed mean air and soil temperatures for the period June to September for each site and year. Lastly, we calculated degree days by integrating all mean daily air temperatures exceeding 5 °C for each site and year.
Site insolation was estimated using the ArcGIS solar radiation tool (ESRI, 2020). Based on a digital elevation model with 5 m resolution, we modelled the duration of direct insolation in hours and the solar irradiance (W m−2) for both sites and each day of the year. For each site, the insolation depended on the sun's position, slope aspect and inclination, and the location of surrounding ridges and peaks that shaded the sites for certain parts of the day and year.
2.3 Soil and foliar nutrient analyses
Soil samples were collected in two campaigns in October 2013 and October 2023. In the first campaign we took two soil samples, and in the second campaign we took three soil samples from the topmost 10 cm of the mineral soil at each site. Each sample from each campaign was pooled from five subsamples distributed randomly over each study site and properly mixed. Air-dried soils were sieved to remove the size fraction > 2 mm. Samples were analysed for exchangeable pH (CaCl2), cation exchange capacity (CEC); total soil N; soil organic carbon (Cox); and plant-available concentrations of Ca, Mg, K, and P with the Mehlich III extraction solution (Mehlich, 1984). Soil samples were analysed in the accredited laboratory of the Research Institute for Soil and Water Conservation, Prague. Differences between sites in measured soil variables were tested using the Kruskal–Wallis test implemented in R (R Development Core Team, 2023). For this purpose, we merged samples from both campaigns.
To compare the nutrient concentrations in needles between sites and to validate whether they reflect nutrient availability in soils, we collected current-year and previous-year needles from six trees at each site in October 2023. Sampled trees included those used for xylogenesis research. The long lag between the sampling of wood formation (2012–2014) and the sampling of foliar macroelements (2023) might influence absolute values of the determined elements because of their inter-annual variability, but not the difference between sites, which remains constant (Novotný et al., 2018). Branches from the upper part of the crown were cut using a telescopic long-reach pruner. Current-year and previous-year needles were sampled from all cut branches and pooled per site. Pooled samples of 1000 current-year and 1000 previous-year needles were dried and then analysed for the content of main macroelements and microelements. The analyses of the main macroelements and microelements followed a standard ICP Forests protocol (Rautio et al., 2020). The foliage K, Ca, Mg and P were determined using ICP-OES (inductively coupled plasma optical emission spectroscopy) after needle decomposition in a microwave oven. The total S and N contents were analysed using a Leco CNS elemental analyser (Elementar Analysensysteme GmbH, Germany).
2.4 Wood formation
Six (2012) to eight trees (2013–2014) at each site were monitored in terms of wood formation (xylogenesis) over the three growing seasons (Table S1 in the Supplement), which overlapped with the period of microclimatic measurements. A new set of healthy and dominant/co-dominant trees was selected for sampling each season to avoid possible impacts of previous year's sampling on ongoing cambial activity. Wood microcores were sampled using a Trephor puncher (Rossi et al., 2006a) at a stem height of 1 ± 0.2 m. Each sample contained the xylem of the current year, the cambial zone, the phloem, and one or more previous complete annual rings. The distance between adjacent sampling points on a stem was always greater than 3 cm to avoid effects of sampling on wood formation. Sampling intervals ranged from 7 to 10 d during the period from April to October, which significantly exceeds the typical duration of a growing season in a treeline environment (Treml et al., 2015). Once sampled, the microcores were immersed in a formaldehyde–ethanol–acetic acid fixative. The laboratory procedures followed Gričar et al. (2006). The microcores were dehydrated using a successive series of ethanol and xylol-substitute and were then embedded in paraffin. 12 µm thick cross sections were cut using a rotary microtome. The paraffin was removed, and samples were dehydrated using a successive series of xylol-substitute and ethanol solutions with descending/ascending ethanol concentrations. The cross sections were then stained with safranine and astra blue and mounted on permanent slides using Canada balsam.
The cells in the following wood phenological phases were counted for each cross section under 400–500× magnification using an optical microscope following Rossi et al. (2003): cells in the cambial zone, enlarging cells, wall-thickening cells and mature cells. The number of cells in each developmental stage was counted in three radial files and subsequently averaged. The number of cells in the preceding tree ring was counted for three radial files and averaged. For each tree, the start and end dates of each developmental phase (onset of cambial activity, enlarging phase, cell wall thickening phase, mature phase) and the overall duration of cambial activity were determined according to Rossi et al. (2007).
The counts of cells developed over the course of the growing season were fitted by a Gompertz function using the R package CAVIAR (Rathgeber et al., 2018). Next, the following parameters were determined from the Gompertz equation for each tree: the maximum daily cell production rate, the day of maximum cell production rate (both called critical dates; Rathgeber et al., 2018), and the mean daily production rate in the period when 90 % of cells were formed. Between-site differences of critical dates and production rates were tested using the Kruskal–Wallis test implemented in R (R Development Core Team, 2023).
Logistic regressions were calculated to identify temperature thresholds at which the wood formation resumes (Rossi et al., 2008), with active/inactive wood formation as the explained binary variable and with the 7 d backward mean soil and air temperature as explanatory variables. Only observations before the summer solstice were considered (Treml et al., 2019). The start of the active wood formation was alternatively defined by the occurrence of the first new cells in the cambial zone or the first enlarging cells. All calculations were performed in R (R Development Core Team, 2023).
2.5 Climate–growth relationships of tree-ring chronologies
Wooden cores were extracted at 1 m stem height from 45–50 randomly selected dominant and co-dominant individuals of Picea abies at each site in October 2013 using an increment borer (5 mm in diameter) (Table S3 in the Supplement). Following standard laboratory procedures (fixation of cores to wooden supports, air-drying, sanding), tree-ring widths were measured using the WinDendro system (scanner and software with semi-automatic ring detection) (Regent Instruments, 2021). The resulting tree-ring series were visually and statistically cross-dated using PAST 5 software (Knibbe, 2013; Speer, 2010). We focused on high-frequency (inter-annual) growth variability preserved in tree-ring data. Therefore, tree-ring series were standardized with a cubic smoothing spline with a 40 year window length at a 50 % frequency cutoff, and the autocorrelation was removed using autoregressive modelling (Cook and Peters, 1981). The residual tree-ring chronology for each site was built by averaging tree-ring series of individual trees. We calculated Pearson correlations between tree-ring chronologies and climatic time series with daily resolution and ran the correlation analysis for time spans from 10 to 30 consecutive days (Jevšenak, 2019). Daily climatic data (mean daily temperature, daily precipitation totals) from the nearest meteorological station Labská/Vrbatova bouda (1320 , 8 km westwards from our sites) were available and used from 1961 after filling data gaps using the neighbouring station. Before calculating correlations, temperature data were standardized in the same way as tree-ring data (Ols et al., 2023).
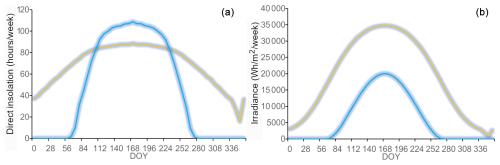
Figure 2Modelled duration of direct insolation (a) and irradiance (b) plotted against the day of year (DOY) on the north-facing (blue) and south-facing (orange) sites.
Table 1Thermal characteristics of the growing season on the north-facing and south-facing sites calculated based on on-site measurements.
In order to characterize long-term growth rate, we converted tree-ring widths to basal area increments and calculated mean basal area increment for each tree (Speer, 2010). Mean basal area increments and their confidence intervals were then compared between sites considering tree age. Tree-ring and temperature data standardization and climate–growth correlations were performed using the dplR (Bunn et al., 2023) and dendroTools (Jevšenak and Levanič, 2018) R packages (R Development Core Team, 2023).
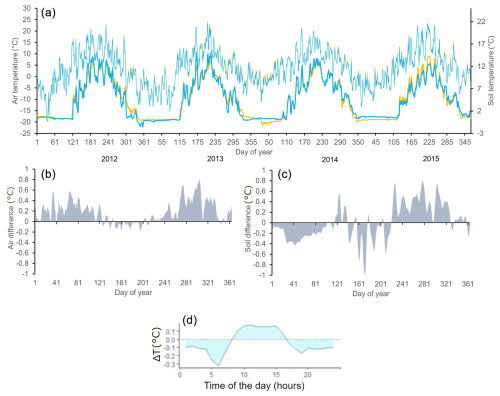
Figure 3(a) Daily means of soil (bold lines, bottom) and air temperatures (thin lines, top) for the north-facing (blue) and south-facing (orange) slopes for the period 2012–2015. (b) Differences between south- and north-facing slopes (temperature south minus temperature north) are smoothed by a 5 d moving average (2012–2015) for mean daily air temperature and (c) the same for mean daily soil temperature. (d) Differences in the course of daily air temperature (temperature south minus temperature north; hourly interval) for June–August (2012–2015).
3.1 Solar radiation and temperature differences
Irradiance was considerably higher on the S-facing slope than on the N-facing slope over the entire year (Fig. 2b). The N-facing slope was characterized by more than 5 months in winter without direct insolation (Fig. 2a). The duration of direct insolation was accordingly longer on the S-facing slope, except for the period between the end of April and mid-August when the weekly duration of insolation was longer on the N-facing slope, reflecting the effect of the complex topographical setting (Fig. 1b). Mean air temperature during the main growing season (June–September) was about 0.1 °C higher at the S-facing slope in most years, but this difference was smaller than the measurement error (Table 1, Fig. 3). Similarly, degree days were slightly higher at the S-facing slope (Table 1). Interestingly, differences in air temperature during the main part of the growing season showed a pronounced daily pattern with a warmer S-facing slope during the day and a warmer N-facing slope at night (Fig. 3d).
At both sites, soil temperature oscillated under the snowpack close to 0 °C usually until the day of year (DOY) 110–120 (ca. end of April) and then abruptly increased (Fig. 3a). Soils tended to be cooler on the S-facing slope during the winter, possibly due to deeper freezing under a thinner snowpack. Soil temperature was higher on the S-facing slope at the very beginning and towards the end of the growing season, while soils on the N-facing slope tended to be warmer in the peak growing season (Fig. 3c), which roughly corresponds to the period when daily direct insolation is longer on the N-facing slope (Fig. 2a). As a result, mean soil temperature was slightly warmer (0.2–0.3 °C) at the N-facing slope over the June–September period in most years, with differences again being close to the measurement error, except in 2015 when the S-facing slope was substantially warmer (Table 1). There was no systematic pattern in the duration of the meteorologically defined growing season (Table 1).
Both sites exhibited several periods with significant negative soil water potentials (Fig. S1 in the Supplement), occurring mostly in the summers of 2013 (both N- and S-facing slopes) and 2014 (N-facing slope) but also in the winters of 2012 (N-facing slope) and 2014 (S-facing slope).
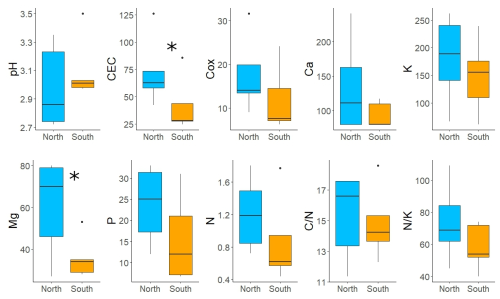
Figure 4Differences in soil characteristics between south-facing (orange) and north-facing (blue) slopes. Variables: exchangeable pH; cation exchange capacity (CEC) (meq kg−1); soil organic carbon (Cox) (%); plant-available concentrations of Ca, Mg, K, and P with the Mehlich III extraction solution (mg kg−1); total soil N (%); and ratios of and . The analytical results for Ca that were below the detection limit were replaced by of the detection limit. Asterisks denote statistically significant differences.
3.2 Nutrient content in soil and foliage
Soils at both sites were strongly acidic with low CEC, P content and plant-available base cations, except for K (Fig. 4, Table S2 in the Supplement). The concentrations of base cations were systematically higher on the N-facing slope than on the S-facing slope. Statistically significant differences were detected for CEC and Mg (p < 0.05, Fig. 4). The concentration of Ca was below the detection limit for half of the samples. The content of Cox and N was high, with a favourable ratio at both sites.
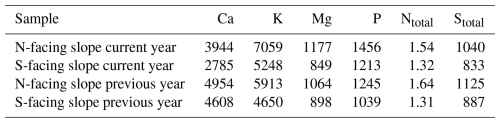
In line with soil nutrient analysis, foliar macroelements were higher on the N-facing slope than on the S-facing slope both in current-year and previous-year needles (Table 2). For the current-year needles, the concentrations of base cations (Ca, K, Mg) were about 25 %–29 % higher on the N-facing slope. The content of P and N was also substantially higher (16 %–21 %) on the N-facing slope (Table 2).
3.3 Wood formation
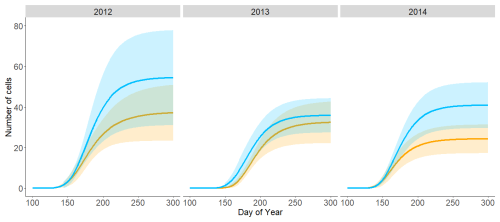
The N-facing slope exhibited a higher number of newly formed cells each year, with the greatest difference compared to the S-facing slope found in 2014 (Fig. 5, Fig. S2 in the Supplement). Although the difference was systematic across years and all parts of the growing season, it was statistically non-significant due to the limited number of sampled trees and natural between-tree variability in xylogenesis. The higher number of cells on the N-facing slope resulted from consistently higher mean and maximum cell formation rates, i.e. faster stem growth; the difference was statistically significant in 2014 (p < 0.05) (Fig. 5, Fig. S3 in the Supplement). Note that while the tree age distribution was comparable between sites in 2012 and 2014, trees on the N-facing slope were about 50 years older in 2013 (Table S1).
Critical dates of wood formation, such as dates of the beginning, peak and end of wood formation, did not show any consistent pattern, and no difference was statistically significant (Fig. S3). The variability in critical dates among trees on the S-facing slope was usually higher than on the N-facing slope. Similarly to critical dates derived from the Gompertz equation, there were no consistent differences in dates of cell phenological phases between sites based on raw cell development data (Fig. S4 in the Supplement). The duration of cell wall thickening was significantly longer on the N-facing slope in 2013 and 2014.
Logistic regressions with growth resumption indicated by the first enlarging cells (binary response variable) and soil temperature (predictor) better fitted the data than regressions with cambial division (active/inactive – binary response variable) and air temperature (predictor) (Fig. S5 in the Supplement). Growth onset represented by the occurrence of the first cambial cells occurred at 3.3 and 3.9 °C soil temperatures and 3.6 and 4.5 °C air temperatures on the S-facing slope and N-facing slope, respectively. In contrast, thresholds for the first enlarging cells were very similar at both sites and occurred at 4.7 °C of soil temperature at both sites and 6.4 and 6.3 °C of air temperatures on the S-facing slope and N-facing slope, respectively.
3.4 Tree-ring width chronologies and their climate response
Tree-ring chronologies from both sites share their high-frequency variability (Fig. S6 in the Supplement). Tree-ring series from the south-facing slope are generally younger; the chronology characteristics such as mean sensitivity and expressed population signal are similar (Table S3). Mean basal area increments are significantly larger at the N-facing slope than at the S-facing slope (Fig. 6), consistent with the higher cell formation rates at the N-facing slope (Fig. 5). Both sites showed two prominent periods with significant temperature–growth correlations, indicating crucial parts of the season for annual ring width formation – the beginning of the growing season centred around DOY 125 (first week of May) and the peak growing season centred around DOY 180 (end of June, Fig. 7). Positive temperature–growth correlations were slightly stronger on the N-facing slope, with the maximum correlation coefficient exceeding 0.6 for a 25 d period centred around DOY 179. There was a significant negative correlation of growth at both sites with precipitation centred around DOY 115–125, which overlaps the positive effect of temperature at the beginning of the growing season (Fig. 7). The remaining significant climate–growth correlations are restricted to very short periods and might be stochastic.
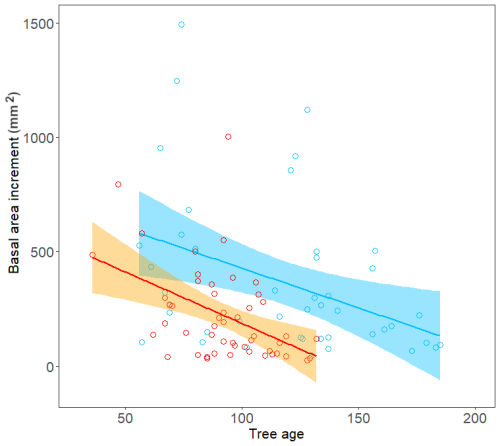
Figure 6Relationship between mean tree basal area increment (mm2) and tree age for trees growing on the north-facing site (blue) and the south-facing site (orange).
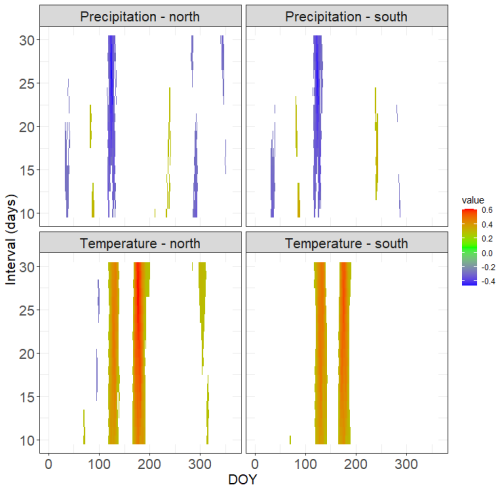
Figure 7Climate–growth correlations for tree-ring width chronologies of the north- and south-facing sites calculated over the period 1961–2013. Only statistically significant correlations are shown. The time window is centred over the respective day of the year (DOY). The y axis shows the length of the window of the number of consecutive days for which the correlations were computed. Tree-ring chronologies are shown in Fig. S6.
We present a study that combines observations of radial stem growth at weekly temporal resolution with the analysis of sub-daily local microclimate and site nutrient availability at cold sites located at the lower part of the treeline ecotone about 150 m below the local tree maxima. We found that phenological dates, particularly the onset of wood formation, as well as radial growth in relative terms, were strongly temperature-limited at both sites as expected, but the absolute growth rate was higher on the N-facing slope than on the S-facing slope. This is in line with our finding that soils were considerably richer in nutrients on the N-facing slope, which was also warmer than the S-facing slope during the nighttime.
4.1 Thermal and nutrient limitation of growth
We expected to find either the same thermal conditions within tree patches on both slopes or a slightly warmer S-facing slope (Paulsen and Körner, 2001), leading to similar growth onset, duration and growth rate. However, while we did find that both sites showed similar thermal conditions and thus similar constraints and timing of beginning and peak growth phases, the N-facing slope exhibited a systematically higher seasonal growth rate, albeit significant only in 2014. Consistently, significantly higher basal area increments were observed for trees growing on the N-facing slope than on the S-facing slope.
Tree growth at cold sites has been considered predominantly limited by low temperature (Babst et al., 2019). Accordingly, we found clear support for the thermal limitation hypothesis as our temperature thresholds for growth resumption were very similar at both sites (between 3.3 and 6.4 °C depending on the temperature variable) and similar to the values published elsewhere (Rossi et al., 2007; Körner, 2021). Furthermore, radial stem growth at both sites, expressed as annual tree-ring width, showed very high sensitivity to variations in temperature during the identical periods of the year. Ring-width chronologies correlated with temperature mainly during the beginning of the growing season in early May, which is indicative for the onset of growth in the early growing season (Castagneri et al., 2017; Carrer et al., 2017; Li et al., 2023). In the same part of the season, tree-ring chronologies displayed negative correlations with precipitation. Precipitation usually falls in the form of snow at that time and delays the onset of the growing season. The second period with a significantly positive response of radial growth to temperature is the peak growing season at the end of June, when the rates of cell division and enlargement culminate (Castagneri et al., 2017; Rossi et al., 2006b). Our data thus show that the timing of growth resumption and the peak rate of cell production are strongly constrained by temperature.
Notably, irrespective of the similar thermal constraints of stem growth on both slopes, the absolute production rate of new tracheids was always higher on the N-facing slope. Differences between sites were not significant at the 5 % probability level except 2014, probably due to the limited number of sampled trees, though similar or higher in our study than what is a common standard in wood formation studies (Cuny et al., 2015; Huang et al., 2020). Cell counts were higher each year on the N-facing slope, consistent with significantly higher stem growth over the entire lifespan of trees on the N-facing slope compared to the S-facing slope (Fig. 6). Factors responsible for the observed differences in growth rate could generally include differences in microclimate, tree age (Rathgeber et al., 2011; Zeng et al., 2018) or accessibility of nutrients (Drollinger et al., 2017). Tree age did not differ between sites in the years with the greatest differences in cell counts (2012, 2014). In 2013 trees were significantly older on the N-facing slope than on the S-facing slope. However, we still observed a greater number of newly formed cells on the N-facing slope, contrary to theoretical expectation as older trees usually form fewer cells than younger trees (Lundqvist et al., 2018).
It is unclear to what extent microclimatic differences were decisive in our study since we found between-site thermal differences within or close to the measurement error of the thermistors. The S-facing slope tended to be slightly more favourable with respect to air temperature and degree day sums. However, the N-facing slope was warmer during the night when stem water potentials are highest with intense cell expansion and division (Zweifel et al., 2021), which may thus benefit stem growth there. Differences in soil temperature were ambiguous: the S-facing slope tended to be warmer than the N-facing slope at the beginning of the growing season, but the N-facing slope was warmer during the peak growing season, leading to slightly warmer soils on N-facing slope for the June–September period. The S-facing slope was substantially warmer only in the warmest year of the measurement period (2015).
Overall, our temperature measurements showed that a part of the growing season was characterized by air and soil temperatures well above the growth-limiting threshold of > 5 °C (Körner, 2021), potentially allowing for the influence of other growth-limiting factors. One plausible explanation for the observed growth differences could thus be nutrient availability. Specifically, we suggest the better growth performance of trees at the N-facing slope may be due to a higher availability of base cations and perhaps also P, as can be seen from nutrient concentrations measured both in soils and in needles. Higher concentrations of leaf macronutrients at the N-facing slope could also potentially indicate a greater sink limit of growth (Hoch and Körner, 2012; Fajardo and Piper, 2017). However, in light of higher growth rates and concentrations of macronutrients in soils of the N-facing slope, we interpret this pattern as a consequence of higher uptake of nutrients reflected in the source-driven higher growth rate (Ellison et al., 2019).
Not surprisingly, in an ecosystem saturated with N (Novotný et al., 2018), the main between-site differences cannot be attributed to N availability but to other nutrients such as Ca, Mg or P. This applies especially to very acidic soils with a pH of around 3, as in our case, where leaching of base cations is likely (Lucas et al., 2011). In environments rich in C and N, stoichiometric requirements for building new biomass mean that other nutrients are limiting, especially base cations and P (Mellert and Ewald, 2014; Norby et al., 2022; Körner, 2022). So far, most studies accentuated N availability as an important constraint of the growth performance of trees at cold sites (Möhl et al., 2018; Gustafson et al., 2021), but these studies focused on ecosystems not saturated with N. The important role of P for tree growth has also been shown in forest ecosystems near their cold margin (Hagedorn et al., 2020; Ellison et al., 2019). Consistent with the traditional Liebig law of the minimum (Liebig, 1840), our study highlights the importance of general stoichiometric principles of nutrient requirements for the production of new biomass, which may also play a crucial role in the growth rate of trees at cold sites.
The source of the higher nutrient content in soils of our N-facing slope is not entirely clear. Metamorphic rocks prevalent on the N-facing slope are generally richer in Mg, Ca and K than granites prevalent on the S-facing slope, while the content of P is comparable between metamorphites and granites (Czech Geological Survey, 2024). Additionally to weathering, higher nighttime temperatures and slightly higher soil temperatures in the peak growing season on the N-facing slope may have enhanced decomposition rates and thus, indirectly, growth performance. Similar relationships between higher soil temperatures, higher soil nutrient content and enhanced tree growth have been observed at subarctic treeline ecotones (Sullivan et al., 2015; Dial et al., 2024).
4.2 Implications for tree growth in topographically complex cold landscapes
Our data generally support the idea that the thermal differences between high-elevation slopes under forest cover are relatively subtle in the temperate zone (Paulsen and Körner, 2001; Treml and Banaš, 2008; Rita et al., 2021). Additionally, we would like to highlight two striking patterns related to our N-facing and S-facing sites that may be generalizable. First, the duration of direct sunlight during the growing season was longer on the N-facing slope, probably due to incoming morning and evening sunlight from the northeast and northwest, respectively. This phenomenon might increase with increasing latitudes and be stronger at less steep slopes. An extreme example is the midnight irradiation of north-facing slopes beyond the polar circle (Kirchhefer, 2000). Second, probably as a consequence, nighttime air temperature was higher on the N-facing slope compared to the S-facing slope, leading to equal mean daily air temperature on both sites (the S-facing slope was warmer during the warmest part of the day). However, since the differences in night temperature were rather high, topographical effects on local air mass ventilation resulting in relatively lower radiative cooling on the N-facing slope than on the S-facing slope should also be considered in our case (Barry, 2008). Higher nighttime air temperature on the N-facing slope may also be the cause of warmer soils in some years but with differences close to the measurement error. We conclude that the thermal differences between tree stands growing on supposedly warmer more growth-favourable south-facing slopes and cooler north-facing slopes can be so subtle that they may be overridden by local topography with related relief shading and local circulation.
Faster growth of trees on soils richer in nutrients also implies that the advance of current upper tree limits might be faster on fertile soils because seedlings could potentially reach a mature and reproductive age earlier (Dial et al., 2022). This remains to be rigorously tested, although some studies have already suggested a greater potential for treeline advancement on fertile soils (Rousi et al., 2018; Gustafson et al., 2021).
We demonstrated that in the lower part of a treeline ecotone in temperate mountains, thermal differences between south-facing and north-facing slopes can be subtle and may be overridden by relief shading and local air circulation. Crucial phases of stem growth, particularly the onset of wood formation and timing of peak growth rate, were constrained by temperature and day length. Consequently, phenology of wood formation was similar between slopes. However, the absolute growth rate was systematically higher on the north-facing slope with soils considerably enriched by nutrients. Our results suggest a joint effect of nutrient-driven absolute growth rate together with the thermally constrained growth phenology at sites close to the cold range limit of trees. These findings are essential for understanding stem growth trends at treelines whose current position often lags behind the pace of warming.
The data used for the analyses together with R scripts are available here: https://doi.org/10.5281/zenodo.14619874 (jantumajer, 2025).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-3807-2025-supplement.
HK and VT conceptualized the study. HK, and VT performed data processing. TC and JT contributed to data analyses. HK and VT led the paper writing. JL, TC and JT contributed to paper writing (comments and revisions).
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Treeline ecotones under global change: linking spatial patterns to ecological processes”. It is not associated with a conference.
We appreciate the authority of the Krkonoše National Park for the permission to conduct research and for the logistical support. We further thank Jakub Kašpar and Šárka Zákravská for their help with fieldwork. We are grateful to two anonymous reviewers for their constructive comments to the earlier version of the manuscript.
This research has been supported by the Grantová Agentura České Republiky (grant no. 22-26519S) and the Ministerstvo Školství, Mládeže a Tělovýchovy (Johannes Amos Comenius Programme, project No. CZ.02.01.01/00/22_008/0004605, Natural and Anthropogenic Georisks).
This paper was edited by Matteo Garbarino and reviewed by two anonymous referees.
Babst, F., Bouriaud, O., Poulter, B., Trouet, V., Girardin, M. P., and Frank, D. C.: Twentieth century redistribution in climatic drivers of Global Tree Growth, Science Advances, 5, eaat4313, https://doi.org/10.1126/sciadv.aat4313, 2019.
Barry, R. G.: Mountain Weather and climate, Cambridge University Press, Cambridge, United Kingdom, https://doi.org/10.1017/CBO9780511754753, 2008.
Bunn, A., Korpela, M., Biondi, F., Campelo, F., Mérian, P., Qeadan, F., and Zang, C.: dplR: Dendrochronology Program Library in R, R package version 1.7.4, The Comprehensive R Archive Network, https://CRAN.R-project.org/package=dplR (last access: 10 January 2024), 2023.
Carrer, M., Castagneri, D., Prendin, A. L., Petit, G., and von Arx, G.: Retrospective analysis of wood anatomical traits reveals a recent extension in tree cambial activity in two high-elevation conifers, Front. Plant Sci., 8, 737, https://doi.org/10.3389/fpls.2017.00737, 2017.
Castagneri, D., Fonti, P., von Arx, G., and Carrer, M.: How does climate influence xylem morphogenesis over the growing season? insights from long-term intra-ring anatomy in Picea abies, Ann. Bot., 119, 1011–1020, https://doi.org/10.1093/aob/mcw274, 2017.
Chagnon, C., Moreau, G., D'Orangeville, L., Caspersen, J., Labrecque-Foy, J.-P., and Achim, A.: Strong latitudinal gradient in temperature-growth coupling near the treeline of the Canadian Subarctic Forest, Frontiers in Forests and Global Change, 6, 1–12, https://doi.org/10.3389/ffgc.2023.1181653, 2023.
Cook, E. R. and Peters, K.: The smoothing spline a new approach to standardizing forest interior tree-ring width series for dendroclimatic studies, Tree Ring Bull., 41, 45–53, 1981.
Cuny, H. E., Rathgeber, C. B., Frank, D., Fonti, P., Mäkinen, H., Prislan, P., Rossi, S., del Castillo, E. M., Campelo, F., Vavrčík, H., Camarero, J. J., Bryukhanova, M. V., Jyske, T., Gričar, J., Gryc, V., De Luis, M., Vieira, J., Čufar, K., Kirdyanov, A. V., Oberhuber, W., Treml, V., Huang, J.-G., Li, X., Swidrak, I., Deslauriers, A., Liang, E., Nöjd, P., Gruber, A., Nabais, C., Morin, H., Krause, C., King, G., and Fournier, M.: Woody biomass production lags stem-girth increase by over one month in coniferous forests, Nat. Plants, 1, 15160, https://doi.org/10.1038/nplants.2015.160, 2015.
Czech Geological Survey: Lithogeochemical database of the Czech Geological Survey, Czech Geological Survey, http://www.geology.cz/litogeochemie (last access: 10 January 2024), 2024.
Dawes, M. A., Schleppi, P., Hättenschwiler, S., Rixen, C., and Hagedorn, F.: Soil warming opens the nitrogen cycle at the Alpine Treeline, Glob. Change Biol., 23, 421–434, https://doi.org/10.1111/gcb.13365, 2017.
Dial, R. J., Maher, C. T., Hewitt, R. E., and Sullivan, P. F.: Sufficient conditions for rapid range expansion of a boreal conifer, Nature, 608, 546–551, https://doi.org/10.1038/s41586-022-05093-2, 2022.
Dial, R. J., Maher, C. T., Hewitt, R. E., Wockenfuss, A. M., Wong, R. E., Crawford, D. J., Zietlow, M. G., and Sullivan, P. F.: Arctic sea ice retreat fuels Boreal Forest Advance, Science, 383, 877–884, https://doi.org/10.1126/science.adh2339, 2024.
Dolezal, J., Kopecky, M., Dvorsky, M., Macek, M., Rehakova, K., Capkova, K., Borovec, J., Schweingruber, F., Liancourt, P., and Altman, J.: Sink limitation of plant growth determines tree line in the arid Himalayas, Funct. Ecol., 33, 553–565, https://doi.org/10.1111/1365-2435.13284, 2019.
Drollinger, S., Müller, M., Kobl, T., Schwab, N., Böhner, J., Schickhoff, U., and Scholten, T.: Decreasing nutrient concentrations in soils and trees with increasing elevation across a treeline ecotone in Rolwaling Himal, Nepal, J. Mt. Sci., 14, 843–858, https://doi.org/10.1007/s11629-016-4228-4, 2017.
Ellison, S. B., Sullivan, P. F., Cahoon, S. M., and Hewitt, R. E.: Poor nutrition as a potential cause of divergent tree growth near the Arctic treeline in Northern Alaska, Ecology, 100, e02878, https://doi.org/10.1002/ecy.2878, 2019.
ESRI: ArcGIS Desktop: Release 10.7.1, Environmental Systems Research Institute, Redlands, CA, 2020.
Etzold, S., Ferretti, M., Reinds, G. J., Solberg, S., Gessler, A., Waldner, P., Schaub, M., Simpson, D., Benham, S., Hansen, K., Ingerslev, M., Jonard, M., Karlsson, P. E., Lindroos, A.-J., Marchetto, A., Manninger, M., Meesenburg, H., Merilä, P., Nöjd, P., Rautio, P., Sanders, T. G. M., Seidling, W., Skudnik, M., Thimonier, A., Verstraeten, A., Vesterdal, L., Vejpustkova, M., and de Vries, W.: Nitrogen deposition is the most important environmental driver of growth of pure, even-aged and managed European forests, Forest Ecol. Manag., 458, 117762, https://doi.org/10.1016/j.foreco.2019.117762, 2020.
Fajardo, A. and Piper, F. I.: An assessment of carbon and nutrient limitations in the formation of the southern Andes Tree Line, J. Ecol., 105, 517–527, https://doi.org/10.1111/1365-2745.12697, 2017.
Fatichi, S., Pappas, C., Zscheischler, J., and Leuzinger, S.: Modelling carbon sources and sinks in terrestrial vegetation, New Phytol., 221, 652–668, https://doi.org/10.1111/nph.15451, 2019.
Gričar, J., Zupančič, M., Čufar, K., Koch, G., Schmitt, U., and Oven, P.: Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies), Ann. Bot., 97, 943–951, https://doi.org/10.1093/aob/mcl050, 2006.
Gustafson, A., Miller, P. A., Björk, R. G., Olin, S., and Smith, B.: Nitrogen restricts future sub-arctic treeline advance in an individual-based dynamic vegetation model, Biogeosciences, 18, 6329–6347, https://doi.org/10.5194/bg-18-6329-2021, 2021.
Hagedorn, F., Dawes, M. A., Bubnov, M. O., Devi, N. M., Grigoriev, A. A., Mazepa, V. S., Nagimov, Z. Y., Shiyatov, S. G., and Moiseev, P. A.: Latitudinal decline in stand biomass and productivity at the elevational treeline in the Ural Mountains despite a common thermal growth limit, J. Biogeogr., 47, 1827–1842, https://doi.org/10.1111/jbi.13867, 2020.
Hoch, G. and Körner, C.: Global patterns of mobile carbon stores in trees at the high-Elevation Tree Line, Global Ecol. Biogeogr., 21, 861–871, https://doi.org/10.1111/j.1466-8238.2011.00731.x, 2012.
Huang, J.-G., Ma, Q., Rossi, S., Biondi, F., Deslauriers, A., Fonti, P., Liang, E., Mäkinen, H., Oberhuber, W., Rathgeber, C. B., Tognetti, R., Treml, V., Yang, B., Zhang, J.-L., Antonucci, S., Bergeron, Y., Camarero, J. J., Campelo, F., Čufar, K., Cuny, H. E., De Luis, M., Giovannelli, A., Gričar, J., Gruber, A., Gryc, V., Güney, A., Guo, X., Huang, W., Jyske, T., Kašpar, J., King, G., Krause, C., Lemay, A., Liu, F., Lombardi, F., Martinez del Castillo, E., Morin, H., Nabais, C., Nöjd, P., Peters, R. L., Prislan, P., Saracino, A., Swidrak, I., Vavrčík, H., Vieira, J., Yu, B., Zhang, S., Zeng, Q., Zhang, Y., and Ziaco, E.: Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers, P. Natl. Acad. Sci. USA, 117, 20645–20652, https://doi.org/10.1073/pnas.2007058117, 2020.
jantumajer: jantumajer/TreelineAspect: v3 (Version v3), Zenodo [code and data set], https://doi.org/10.5281/zenodo.14619874, 2025.
Jevšenak, J.: Daily Climate Data reveal stronger climate-growth relationships for an extended European tree-ring network, Quaternary Sci. Rev., 221, 105868, https://doi.org/10.1016/j.quascirev.2019.105868, 2019.
Jevšenak, J. and Levanič, T.: DendroTools: R package for studying linear and nonlinear responses between tree-rings and daily environmental data, Dendrochronologia, 48, 32–39, https://doi.org/10.1016/j.dendro.2018.01.005, 2018.
Jochner, M., Bugmann, H., Nötzli, M., and Bigler, C.: Tree growth responses to changing temperatures across space and time: A fine-scale analysis at the treeline in the Swiss alps, Trees, 32, 645–660, https://doi.org/10.1007/s00468-017-1648-x, 2017.
Kirchhefer, A. J.: The influence of slope aspect on tree-ring growth of Pinus sylvestris L. in northern Norway and its implications for climate reconstruction, Dendrochronologia, 18, 27–40, 2000.
Knibbe, B.: Personal Analysis System for Tree-ring Research 5, Instruction Manual, SCIEM, Vienna, Austria, 2013.
Körner, C.: Alpine treelines functional ecology of the global high elevation tree limits, Springer, Basel, Switzerland, https://doi.org/10.1007/978-3-0348-0396-0, 2012.
Körner, C.: The cold range limit of trees, Trend. Ecol. Evol., 36, 979–989, https://doi.org/10.1016/j.tree.2021.06.011, 2021.
Körner, C.: The forest's nutrient cycle drives its carbon cycle, Tree Physiology, 42, 425–427, https://doi.org/10.1093/treephys/tpab170, 2022.
Körner, C. and Hiltbrunner, E.: The 90 ways to describe plant temperature, Perspect. Plant Ecol., 30, 16–21, https://doi.org/10.1016/j.ppees.2017.04.004, 2018.
Körner, C. and Hiltbrunner, E.: Rapid advance of climatic tree limits in the eastern alps explained by on-site temperatures, Reg. Environ. Change, 24, 98, https://doi.org/10.1007/s10113-024-02259-8, 2024.
Körner, C. and Hoch, G.: A test of treeline theory on a montane Permafrost Island, Arct. Antarct. Alp. Res., 38, 113–119, https://doi.org/10.1657/1523-0430(2006)038[0113:atotto]2.0.co;2, 2006.
Körner, C. and Hoch, G.: Not every high-latitude or high-elevation forest edge is a treeline, J. Biogeogr., 50, 838–845, https://doi.org/10.1111/jbi.14593, 2023.
Körner, C. and Paulsen, J.: A world-wide study of high altitude treeline temperatures, J. Biogeogr., 31, 713–732, https://doi.org/10.1111/j.1365-2699.2003.01043.x, 2004.
Kolář, T., Čermák, P., Oulehle, F., Trnka, M., Štěpánek, P., Cudlín, P., Hruška, J., Büntgen, U., and Rybníček, M.: Pollution Control enhanced spruce growth in the “Black Triangle” near the Czech–Polish border, Sci. Total Environ., 538, 703–711, https://doi.org/10.1016/j.scitotenv.2015.08.105, 2015.
Kuželová, H. and Treml, V.: Landscape-scale variability of air and soil temperature related to tree growth in the treeline ecotone, Alpine Bot., 130, 75–87, https://doi.org/10.1007/s00035-020-00233-8, 2020.
Lenz, A., Hoch, G., and Körner, C.: Early season temperature controls cambial activity and total tree ring width at the Alpine treeline, Plant Ecol. Divers., 6, 365–375, https://doi.org/10.1080/17550874.2012.711864, 2013.
Li, X., Liang, E., Camarero, J. J., Rossi, S., Zhang, J., Zhu, H., Fu, Y.,Sun, J., Wang, T., Piao, S., and Peñuelas J.: Warming-induced phenological mismatch between trees and shrubs explains high-elevation forest expansion, Natl. Sci. Rev., 10, nwad182, https://doi.org/10.1093/nsr/nwad182, 2023.
Liebig, J.: Die organische chemie in ihrer Anwendung auf Agricultur und Physiologie, Vieweg, Braunschweig, Germany, https://doi.org/10.5962/bhl.title.42117, 1840.
Liptzin, D., Sanford, R. L., and Seastedt, T. R.: Spatial patterns of total and available N and P at Alpine Treeline, Plant Soil, 365, 127–140, https://doi.org/10.1007/s11104-012-1379-0, 2013.
Lu, X., Liang, E., Wang,Y., Babst, F., and Camarero, J. J.: Mountain treelines climb slowly despite rapid climate warming, Global Ecol. Biogeogr., 30, 305–315, https://doi.org/10.1111/geb.13214, 2021.
Lucas, R. W., Klaminder, J., Futter, M. N., Bishop, K. H., Egnell, G., Laudon, H., and Högberg, P.: A meta-analysis of the effects of nitrogen additions on base cations: Implications for plants, soils, and streams, Forest Ecol. Manag., 262, 95–104, https://doi.org/10.1016/j.foreco.2011.03.018, 2011.
Lundqvist, S.-O., Seifert, S., Grahn, T., Olsson, L., García-Gil, M. R., Karlsson, B., and Seifert, T.: Age and weather effects on between and within ring variations of number, width and coarseness of tracheids and radial growth of young Norway spruce, Eur. J. For. Res., 137, 719–743, https://doi.org/10.1007/s10342-018-1136-x, 2018.
Mayor, J. R., Sanders, N. J., Classen, A. T., Bardgett, R. D., Clément, J. C., Fajardo, A., Lavorel, S., Sundqvist, M. K., Bahn, M., Chisholm, C., Cieraad, E., Gedalof, Z., Grigulis, K., Kudo, G., Oberski, D. L., and Wardle, D. A.: Elevation alters ecosystem properties across temperate treelines globally, Nature, 542, 91–95, https://doi.org/10.1038/nature21027, 2017.
Mehlich, A.: Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant, Commun. Soil Sci. Plan., 15, 1409–1416, https://doi.org/10.1080/00103628409367568, 1984.
Mellert, K. H. and Ewald, J.: Nutrient limitation and site-related growth potential of Norway spruce (Picea abies [L.] karst) in the Bavarian alps, Eur. J. For. Res., 133, 433–451, https://doi.org/10.1007/s10342-013-0775-1, 2014.
Metelka, L., Mrkvica, Z., and Halásová, O.: Climate, in Krkonoše – nature, history, life, Baset, Prague, pp. 147–155, ISBN: 978-80-7340-104-7, 2007.
Möhl, P., Mörsdorf, M. A., Dawes, M. A., Hagedorn, F., Bebi, P., Viglietti, D., Freppaz, M., Wipf, S., Körner, C., Thomas, F. M., and Rixen, C.: Twelve years of low nutrient input stimulates growth of trees and dwarf shrubs in the treeline ecotone, J. Ecol., 107, 768–780, https://doi.org/10.1111/1365-2745.13073, 2018.
Müller, M., Oelmann, Y., Schickhoff, U., Böhner, J., and Scholten, T.: Himalayan treeline soil and foliar stoichiometry indicate nutrient shortage with elevation, Geoderma, 291, 21–32, https://doi.org/10.1016/j.geoderma.2016.12.015, 2017.
Norby, R. J., Warren, J. M., Iversen, C. M., Childs, J., Jawdy, S. S., and Walker, A. P.: Forest stand and canopy development unaltered by 12 years of CO2 Enrichment, Tree Physiol., 42, 428–440, https://doi.org/10.1093/treephys/tpab107, 2022.
Novotný, R., Lomský, B., and Šrámek, V.: Changes in the phosphorus and nitrogen status and supply in the young spruce stands in the Lužické, the jizerské and the Orlické Mts. in the Czech Republic during the 2004–2014 period, Eur. J. For. Res., 137, 879–894, https://doi.org/10.1007/s10342-018-1146-8, 2018.
Ols, C., Klesse, S., Girardin, M. P., Evans, M. E. K., DeRose, R. J., and Trouet, V.: Detrending climate data prior to climate–growth analyses in dendroecology: A common best practice?, Dendrochronologia, 79, 126094, https://doi.org/10.1016/j.dendro.2023.126094, 2023.
Oulehle, F., Urban, O., Tahovská, K., Kolář, T., Rybníček, M., Büntgen, U., Hruška, J., Čáslavský, J., and Trnka, M.: Calcium availability affects the intrinsic water-use efficiency of temperate forest trees, Commun. Earth Environ., 4, 199, https://doi.org/10.1038/s43247-023-00822-5, 2023.
Paulsen, J. and Körner, C.: GIS-analysis of tree-line elevation in the Swiss alps suggests no exposure effect, J. Veg. Sci., 12, 817–824, https://doi.org/10.2307/3236869, 2001.
R Development Core Team: R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. 2023.
Rautio, P., Fürst, A., Stefan, K., Raitio, H., Bartels, U.: Part XII: Sampling and Analysis of Needles and Leaves, Version 2020-3, in: Manual on methods and criteria for harmonized sampling, assessment, monitoring and analysis of the effects of air pollution on forests, edited by: UNECE ICP Forests Programme Co-ordinating Centre, Thünen Institute of Forest Ecosystems, Eberswalde, Germany, 2020.
Rathgeber, C. B., Rossi, S., and Bontemps, J.-D.: Cambial activity related to tree size in a mature silver-fir plantation, Ann. Bot., 108, 429–438, https://doi.org/10.1093/aob/mcr168, 2011.
Rathgeber, C. B., Santenoise, P., and Cuny, H. E.: Caviar: An R package for checking, displaying and processing wood-formation-monitoring data, Tree Physiol., 38, 1246–1260, https://doi.org/10.1093/treephys/tpy054, 2018.
Regent Instruments: WinDendro (Version 2021), Quebec, Canada, 2021.
Rita, A., Bonanomi, G., Allevato, E., Brghetti, M., Cesarano, G., Mogaveno, V., Rossi, S., Saulino, L., Zotti, M., and Saracino, A.: Topography modulates near-ground microclimate in the Mediterranean Fagus sylvatica treeline, Sci. Rep., 11, 8122, https://doi.org/10.1038/s41598-021-87661-6, 2021.
Rossi, S., Deslauriers, A., and Morin, H.: Application of the gompertz equation for the study of xylem cell development, Dendrochronologia, 21, 33–39, https://doi.org/10.1078/1125-7865-00034, 2003.
Rossi, S., Deslauriers, A., and Anfodillo, T.: Assessment of cambial activity and xylogenesis by microsampling tree species: An example at the alpine timberline, IAWA J., 27, 383–394, https://doi.org/10.1163/22941932-90000161, 2006a.
Rossi, S., Deslauriers, A., Anfodillo, T., Morin, H., Saracino, A., Motta, R., and Borghetti, M.: Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length, New Phytol., 170, 301–310, https://doi.org/10.1111/j.1469-8137.2006.01660.x, 2006b.
Rossi, S., Deslauriers, A., Anfodillo, T., and Carraro, V.: Evidence of threshold temperatures for xylogenesis in conifers at high altitudes, Oecologia, 152, 1–12, https://doi.org/10.1007/s00442-006-0625-7, 2007.
Rossi, S., Deslauriers, A., Griçar, J., Seo, J., Rathgeber, C. B., Anfodillo, T., Morin, H., Levanic, T., Oven, P., and Jalkanen, R.: Critical temperatures for xylogenesis in conifers of cold climates, Global Ecol. Biogeogr., 17, 696–707, https://doi.org/10.1111/j.1466-8238.2008.00417.x, 2008.
Rossi, S., Anfodillo, T., Čufar, K., Cuny, H. E., Deslauriers, A., Fonti, P., Frank, D., Gričar, J., Gruber, A., Huang, J., Jyske, T., Kašpar, J., King, G., Krause, C., Liang, E., Mäkinen, H., Morin, H., Nöjd, P., Oberhuber, W., Prislan, P., Rathgeber, C. B. K., Saracino, A., Swidrak, I., and Treml, V.: Pattern of xylem phenology in conifers of cold ecosystems at the Northern Hemisphere, Glob. Change Biol., 22, 3804–3813, https://doi.org/10.1111/gcb.13317, 2016.
Rousi, M., Possen, B. J., Ruotsalainen, S., Silfver, T., and Mikola, J.: Temperature and soil fertility as regulators of tree line Scots pine growth and survival—implications for the acclimation capacity of northern populations, Glob. Change Biol., 24, 545–559, https://doi.org/10.1111/gcb.13956, 2018.
Shi, C., Schneider, L., Hu, Y., Shen, M., Sun, C., Xia, J., Forbes, B. C., Shi, P., Zhang, Y., and Ciais, P.: Warming-induced unprecedented high-elevation forest growth over the monsoonal Tibetan Plateau, Environ. Res. Lett., 15, 054011, https://doi.org/10.1088/1748-9326/ab7b9b, 2020.
Shi, H., Zhou, Q., He, R., Zhang, Q., and Dang, H.: Climate warming will widen the lagging gap of global treeline shift relative to densification, Agr. Forest Meteorol., 318, 108917, https://doi.org/10.1016/j.agrformet.2022.108917, 2022.
Speer, J. H.: Fundamentals of Tree Ring Research, University of Arizona Press, Tucson, ISBN: 9780816526857, 2010.
Stark, S., Kumar, M., Myrsky, E., Vuorinen, J., Kantola, A. M., Telkki, V.-V., Sjögersten, S., Olofsson, J., and Männistö, M. K.: Decreased soil microbial nitrogen under vegetation `shrubification' in the subarctic forest–Tundra Ecotone: The potential role of increasing nutrient competition between plants and soil microorganisms, Ecosystems, 26, 1504–1523, https://doi.org/10.1007/s10021-023-00847-z, 2023.
Sullivan, P. F., Ellison, S. B., McNown, R. W., Brownlee, A. H., and Sveinbjörnsson, B.: Evidence of soil nutrient availability as the proximate constraint on growth of treeline trees in northwest Alaska, Ecology, 96, 716–727, https://doi.org/10.1890/14-0626.1, 2015.
Treml, V. and Banaš, M.: The effect of exposure on Alpine treeline position: A case study from the high sudetes, Czech Republic, Arct. Antarct. Alp. Res., 40, 751–760, https://doi.org/10.1657/1523-0430(07-060)[treml]2.0.co;2, 2008.
Treml, V., Kašpar, J., Kuželová, H., and Gryc, V.: Differences in intra-annual wood formation in Picea abies across the treeline ecotone, Giant Mountains, czech republic, Trees, 29, 515–526, https://doi.org/10.1007/s00468-014-1129-4, 2015.
Treml, V., Hejda, T., and Kašpar, J.: Differences in growth between shrubs and trees: How does the stature of woody plants influence their ability to thrive in cold regions?, Agr. Forest Meteorol., 271, 54–63, https://doi.org/10.1016/j.agrformet.2019.02.036, 2019.
Tumajer, J., Kašpar, J., Kuželová, H., Shishov, V. V., Tychkov, I. I., Popkova, M. I., Vaganov, E. A., and Treml, V.: Forward modeling reveals multidecadal trends in cambial kinetics and phenology at treeline, Front. Plant Sci., 12, 613643, https://doi.org/10.3389/fpls.2021.613643, 2021.
Zeng, Q., Rossi, S., and Yang, B.: Effects of age and size on xylem phenology in two conifers of northwestern China, Front. Plant Sci., 8, 2264, https://doi.org/10.3389/fpls.2017.02264, 2018.
Zweifel, R., Sterck, F., Braun, S., Buchmann, N., Eugster, W., Gessler, A., Häni, M., Peters, R. L., Walthert, L., Wilhelm, M., Ziemińska, K., and Etzold, S.: Why trees grow at night, New Phytol., 231, 2174–2185, https://doi.org/10.1111/nph.17552, 2021.