the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Carbon and nitrogen dynamics in subsoils after 20 years of added precipitation in a Mediterranean grassland
Sora L. Kim
Asmeret Asefaw Berhe
Precipitation is a major driver of ecosystem change and the physiochemical characteristics of soil. Under different climate change scenarios, increased drought frequency and changing precipitation are predicted to impact Mediterranean ecosystems, including those in Northern California. Subsoils are large carbon (C) reservoirs; however, most studies that have investigated precipitation effects on soil organic matter (SOM) have primarily focused on near-surface soils. Recent studies have indicated different responses to environmental perturbation in surface (<30 cm) versus deep (>30 cm) soils due to important differences in physiochemical characteristics. Here, we present soil data at depth (∼300 cm) from a 20-year precipitation manipulation experiment. We determined changes in total elemental concentration and stable isotope composition of soil C, N, δ13C, and δ15N for an ambient control versus additional precipitation in the winter and spring months. The addition of winter precipitation resulted in the largest cumulative C stock (0–300 cm); however, there were no statistically significant changes in C stock throughout the depth profile. Nevertheless, there was evidence of the vertical translocation of C to deep soil layers, specifically of plant-derived C, with both winter and spring precipitation additions. The precipitation addition in winter also resulted in the highest subsoil C stock compared to the control (ambient) and spring treatments. Overall, added winter precipitation led to the best conditions for C accumulation, as the added precipitation coincided with lower temperatures and improved growing conditions at our field site. This study highlights the importance of the timing of precipitation events, especially with regard to deep C stocks (>1 m).
- Article
(4101 KB) - Full-text XML
-
Supplement
(557 KB) - BibTeX
- EndNote
Deep soil, ranging from 1 to 3 m, can account for 30 % to 50 % of the total soil profile carbon (C) stock (Jobbágy and Jackson, 2000), but most published studies have only sampled to 50 cm or shallower (Yost and Hartemink, 2020). As a result, considerable uncertainty is associated with estimations of deep global soil organic carbon (SOC) stocks. Current estimates of global SOC stocks to 1 m have converged to 1100–1500 Pg, but estimates to 3 m depths are 2800 ± 700 Pg (Jackson et al., 2017). There are many reasons for the lack of data on deep soils, including logistical and cost issues associated with sampling to such great depth. However, deep soils are an active C pool and have not been fully accounted for as a potential sink or source under current and future climate change. The limited number of studies on deep soils have shown that subsoil C storage is affected by climate change, land use, and management change. For example, the introduction of switchgrass significantly increased subsoil C stocks (Slessarev et al., 2020), whereas intensive agricultural management of grasslands decreased C stocks at 100 cm depth in Great Britain (Ward et al., 2016). Land use change and its effects on subsoil C are also moderated by climate (Guo and Gifford, 2002). The transition from forest to pasture increased soil C in ecosystems with a mean annual precipitation (MAP) of between 2000 and 3000 mm, but it decreased soil C in ecosystems with MAP amounts of less than 1000 mm and greater than 3000 mm (Guo and Gifford, 2002). However, most deep SOC responses to global change can only be hypothesized due to the few manipulative experiments on deep soils (Hicks Pries et al., 2023). Given that subsoils can be affected by climate and land use change and act as a significant reservoir of stable C, more experimental studies are needed.
Deep soils have key physical, chemical, and biological features that make them significant reservoirs of C as well as a target for long-term C sequestration. Physical characteristics of deep soils include a greater bulk density and finer texture that result in a greater physical protection of C (Button et al., 2022; Plante et al., 2006) and lead to a lower accessibility of soil C compounds for microbial communities at depth (Wilpiszeski et al., 2019). In terms of biological properties, there is also less microbial biomass in subsoils compared to the surface, although there is evidence of microbial biomass that can be reactivated with the presence of deep roots and/or mechanical disruption (Jilling et al., 2018; Min et al., 2021). Deep soils often have a greater surface area with respect to mineral surfaces, such as oxyhydroxides (Mikutta et al., 2006; Rumpel and Kögel-Knabner, 2011). These mineral surfaces are important for C stabilization and allow for protective and stabilizing associations for C compounds that would otherwise be quickly decomposed (Kleber et al., 2005; Porras et al., 2017; Schmidt et al., 2011). When this deep C stabilization takes place, older radiocarbon ages of subsoil C are often observed and are interpreted as a greater residence time (McFarlane et al., 2013; Rumpel, 2004; Rumpel and Kögel-Knabner, 2011; Sollins et al., 2009). This combination of physical, chemical, and biological features demonstrates the potential of deep soils to hold significant amounts of stabilized C.
Subsoils in grassland ecosystems are particularly important to consider due to their large global land area and their ability to store large amounts of C belowground (Bai and Cotrufo, 2022; Berhe et al., 2012; Chou et al., 2008). This large source of belowground biomass is due to deeply rooting grasses, and it is estimated that 60 % of the grassland net primary productivity (NPP) is stored belowground and is more likely to be incorporated in soil organic matter (SOM) (Jackson et al., 2017). This belowground NPP results in a large soil C stock, and a meta-analysis found that grasslands have approximately 43 % their C stock stored from 1 to 3 m (Jobbágy and Jackson, 2000). It has been estimated that 44 % of the variability in SOC stock uncertainty is associated with the spatial scale and soil profile depth, especially due to the lack of data at greater depths, and this results in significant uncertainty when estimating grassland C stocks globally (Maillard et al., 2017). Sampling deeper soils to understand the stabilization mechanisms and destabilization processes of C at depths greater than 1 m will be key for deep grassland soils to serve as a C source or sink under climate change conditions with changing moisture and temperature regimes.
Currently, we lack measurements of not only the physiochemical characteristics of deep soils but also the effects of environmental perturbation on these soils. However, several recent studies have indicated different responses to environmental perturbation in surface (<30 cm) versus deep (>30 cm) soils (Berhe et al., 2008; Hicks Pries et al., 2017; Min et al., 2020, 2021). Nevertheless, key questions remain regarding (1) whether deep soil C is vulnerable to climate change and (2) the dynamics of other key nutrients at depth, like nitrogen and phosphorus. These dynamics are important because decoupling of these nutrients from each other could impact and alter C cycling. For example, increasing aridity was found to decouple key nutrients like phosphorus (Delgado-Baquerizo et al., 2013). Long-term field experiments are needed to test hypotheses (at the field scale) about the impact of environmental perturbation on deep soils. There are still limited data on SOC concentrations with increased precipitation in grasslands (Bai and Cotrufo, 2022). A study that looked at 30 years of precipitation augmentation in a grassland ecosystem found minimal changes in bulk C or N, but it did observe greater mineral-associated organic matter (MAOM) in the top 30 cm (Rocci et al., 2023). Overall, there are few long-term environmental manipulations, and even fewer studies have specifically examined the impacts of long-term manipulation on deep soils. This makes the Angelo manipulation experiment, which has been ongoing for 20 years, a particularly good site to explore questions regarding changes in soil biogeochemistry with decadal-scale precipitation shifts.
Previous work at the Angelo experiment has suggested important biotic feedbacks, both plant and microbial, with changing precipitation amount and seasonality. The long-term Angelo experiment is testing the impacts of increased precipitation combined with changing seasonality. More specifically, it is testing the impact of shifting seasonality of precipitation to the spring months (March–June) in a Mediterranean climate, where most of the precipitation for the water year typically takes place in the winter months (November–February). This site had multiple studies occur at the 6- and 10-year mark of the experiment (Berhe et al., 2012; Cruz-Martínez et al., 2012; Hawkes et al., 2011; Suttle et al., 2007), but this work represents one of the first long-term follow-up studies on the experiment to great depth. Previous work at the Angelo experiment has suggested that plant and fungal communities are especially sensitive to changes in precipitation amount and precipitation seasonality. More specifically, it was found that added spring precipitation caused reductions in plant diversity (Suttle et al., 2007), while fungal communities were less diverse under both winter and spring precipitation additions (Hawkes et al., 2011). Microbial communities, on the other hand, were relatively more robust and resilient to changing water conditions (Cruz-Martínez et al., 2009); however, a later study suggested that longer-term studies might be missing important short-term variation driven in part by rainfall fluctuation (Cruz-Martínez et al., 2012). More recent work on microbial communities at the Angelo experiment has suggested that extended rainfall decreased the depth-based differentiation in microbial community composition (Diamond et al., 2018). Seven years of changing precipitation seasonality and precipitation amount has also been found to affect soil biogeochemical processes, with increased winter precipitation diminishing the role of oxyhydroxides in C stabilization (Berhe et al., 2012). While significant work on biotic responses to changing precipitation regimes has been done at Angelo, less work has been done on soil biogeochemistry, especially at depth (>50 cm).
We wanted to examine the intersection of a long-term precipitation manipulation experiment and its potential impacts on deep soils in a Northern California grassland to understand whether subsoil C stocks might be affected by climate change. The objectives of this study were to determine how changes in the amount and timing of rainfall in a California grassland ecosystem affect (a) the distribution of C stocks to 3 m, (b) the chemical composition of organic matter entering the soil and its distribution throughout the soil profile, and (c) the associations between inputs and C stocks. Our hypotheses were largely based on a previous study at the precipitation experiment that focused on belowground processes (Berhe et al., 2012). This study found a lower C concentration with added winter precipitation and a higher C concentration with added spring precipitation. Authors also found an accumulation of easily assimilated and less decomposed SOM and reduced rates of decomposition with spring precipitation addition. Based on this, we hypothesized that (a) there would be a reduction in C stocks with winter precipitation addition. We also hypothesized that there would be (b) an accumulation of aliphatic functional groups with spring precipitation addition and (c) a stronger association between inputs and C stocks with spring precipitation addition.
2.1 Site description
The Angelo Precipitation Experiment was established in a meadow at Angelo Coast Range Reserve in Mendocino County, California ( N, W). The dominant vegetation at Angelo is a mix of Aira spp., Bromus spp, and Briza spp. (Foley et al., 2023). The site is at an elevation of 1350 m a.s.l. and experiences a Mediterranean climate with wet, cool winters and warm, dry summers. At Angelo, soils are part of the Holohan–Hollowtree–Casabonne Complex and are classified as Ultic Haploxeralfs. The parent material is largely greywacke and mudstone, derived from Cretaceous marine greywacke sandstones and mudstones of the Franciscan Complex, and the site overlays a bedrock terrace of the South Fork Eel River (Berhe et al., 2012).
2.2 The rainfall addition
The Angelo Coast Range Reserve rainfall manipulation experiment was established in 2000 and set up to reflect changes in rainfall patterns predicted by the Hadley Centre for Climate Prediction and Research (HadCM2) and the Canadian Centre for Climate Modeling and Analysis (CCM1) for Northern California over the next 50–100 years. For this experiment, 36 large circular plots (70 m2) were regularly spaced across 2.7 ha meadows. Plots were set up in a randomized block design for three separate treatments: (1) an ambient rainfall control, (2) a winter addition of precipitation, and (3) a spring addition of precipitation. Water addition treatments were administered by adding 14–16 mm of water every third day over 3 months (Fig. 1). For the winter treatment, this water addition was administered from January to March, whereas it was administered from April to June for the spring treatment (Fig. 1). This water addition resulted in a 20 % increase over the mean annual precipitation (Suttle et al., 2007). The supplemental water for the water addition experiment was collected from a spring above the meadow and distributed evenly over the surface of each plot using a sprinkler system (Rain Bird® Rain Curtain™).
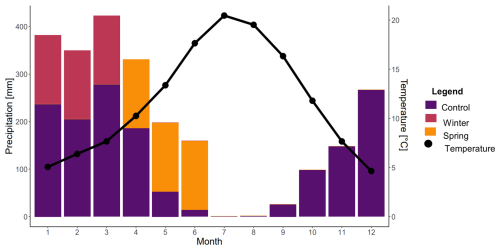
Figure 1Precipitation and temperature over a year at Angelo Coast Range Reserve. The control indicates ambient precipitation, whereas the added precipitation for the winter and spring treatments is shown in the months that it is added. Data were sourced from Dendra (a cyber-infrastructure project for real-time data storage) for Angelo from 2012 to 2022. Months are numbered.
Plots were assigned treatments in a randomized complete block design to take spatial biases into account (Suttle et al., 2007). Within each experimental block, treatment assignment was randomized among the plots and was then re-randomized for the next block (and so on). This resulted in each block containing a single replicate of each experimental treatment. This design maximized the likelihood of capturing any pre-existing differences that might exist in terms of physical or biological conditions across the grassland.
2.3 Soil sampling
In October 2020, samples were collected using a Geoprobe system to the depth of resistance (approximately 3 m), with four replicates per treatment (ambient, winter, and spring). For all cores, samples were collected at consistent 10 cm intervals (0–10, 10–20, and so on). This resulted in a total of 345 collected soil samples across all treatments and depths.
After samples were collected, they were transported in coolers with ice packs and stored in a 4 °C cold room for approximately 4 months until they could be subsampled and analyzed. Long storage times occurred due to a lack of access to laboratory facilities during the COVID-19 pandemic and subsequent shutdown procedures. When samples could be processed, a subsample was removed from each sample, and air-dried for 7 d at room temperature. Soil samples were tested for carbonates by observing the presence and degree of effervescence with a few drops of 1 M hydrochloric acid. Following air-drying, the sample was then sieved to 2 mm. A further subsample from the processed air-dried sample was taken for ball-milling (using a SamplePrep 8000M ball mill) to a homogenous particle size.
We measured a suite of physical and chemical properties of these samples, specifically bulk density, soil pH, and gravimetric water content. We collected bulk density at Angelo using Geoprobe cores and calculated C stocks with these bulk density estimates. We subsampled each depth increment to estimate water content and then calculated the dry mass of soil in a 10 cm increment. Bulk density was calculated as the mass of the dry <2 mm fraction to correct for the impact of rock and root volume on soil C and N stocks (Throop et al., 2012). Soil pH was measured in 1:1 soil : water and soil : CaCl2 slurries.
2.4 Elemental and isotopic analyses
For elemental and isotopic analysis of C and N, soil samples were air-dried, sieved to 2 mm, and ground (using both a mortar and pestle and SamplePrep 8000M ball mill). The δ13C and δ15N values and elemental C and N contents of all samples were measured at the Stable Isotope Ecosystem Laboratory (SIELO) of the University of California, Merced (UC Merced). Briefly, samples were weighed into tin capsules and combusted in a Costech 4010 elemental analyzer coupled with a Delta V Plus continuous-flow isotope ratio mass spectrometer. C and N isotope compositions were corrected for instrumental drift, mass linearity, and standardized to the international Vienna Peedee Belemnite (VPDB; δ13C) and atmospheric air (AIR; δ15N) scales using the USGS 41A and USGS 40 standard reference materials. Mean δ13C values for USGS 40 and 41a were (mean ± standard deviation with n indicated) −26.4 ‰ ± 0.1 ‰ (n=118) and 36.5 ‰ ± 0.2 ‰ (n=59), respectively, and mean δ15N values were −4.5 ‰ ± 0.1 ‰ (n=118) and 47.5 ‰ ± 0.1 ‰ (n=59), respectively. Elemental C and N content were determined via linear regression of CO2 and N2 sample gas peak areas against the known C and N contents of USGS 40, USGS 41a, and Costech acetanilide. All isotope compositions are expressed using standard delta notation.
Stable isotopes are a staple tool in the realm of soil science due to their role as ecological integrators, and they can be a useful tool in understanding environmental perturbations in deep soils. In particular, C3 plants have a well-documented physiological response to increasing aridity that leads to high δ13C values (Farquhar et al., 1989; Kohn, 2010). δ13C has been shown to vary with MAP due to discrimination against 13C in drier areas because stomata have to remain more closed to minimize water loss (Krüger et al., 2023). This interaction between precipitation and the stable isotope values of plant matter means that the inputs for formed C will be affected by climate. These altered stable isotope values could act as a potential tracer for plant inputs into soil. There is a well-documented pattern of increasing δ13C values with depth; a disruption of this pattern by 13C-depleted plant matter inputs could be a good indicator of formed C being distributed throughout the profile. Furthermore, diffuse reflectance mid-infrared Fourier transform spectroscopy (DRIFTS) is a complementary analysis that can characterize the chemical composition of soil C. DRIFTS can measure the vibrational frequency of ecologically relevant functional groups, like aliphatic, aromatic, and amide functional groups (Mainka et al., 2022; Margenot et al., 2015; Parikh et al., 2014). Together, stable isotopes and DRIFTS can provide important information about incoming plant matter as well as microbial activity in soils.
2.5 Diffuse reflectance mid-infrared Fourier transform spectroscopy (DRIFTS)
To measure the presence of functional groups that are important for organic matter and mineral surfaces related to soil C across our study systems, we used diffuse reflectance mid-infrared Fourier transform spectroscopy (DRIFTS). DRIFTS measures the vibrational frequencies of functional groups in a sample and is well suited for analyzing soils due to the minimal sample preparation needed for this technique. We performed analyses on bulk soil samples that were ball-milled to a homogenous consistency to avoid interferences that could affect baselines or peak widths. We used a Bruker IFS 66V/S spectrophotometer (Ettlingen, Germany) with a Praying Mantis apparatus (Harrick Scientific, Ossining, NY) at the Nuclear Magnetic Resonance Facility at UC Merced. It is important to note that potassium bromide (KBr) was used as a background reference, but samples were not diluted with KBr. Samples were initially dried in a desiccator following homogenization to remove interference from water. Absorption was measured between 4000 and 400 cm−1 averaged over 300 scans with an aperture of 4 mm. Functional groups for simple plant C (aliphatic C−H; λ: 2976–2898 cm−1), complex plant C (aromatic C=C; λ: 1550–1500 cm−1), and microbially derived C (amide/quinone/ketone, CO; aromatic, CC, carboxylate COO; λ: 1660–1580 cm−1) were assigned following Mainka et al. (2022), also shown in Table 1 (Mainka et al., 2022; Parikh et al., 2014; Vranova et al., 2013). We excluded wavenumbers that overlapped with signals from mineral compounds, specifically from 1400 to 400 cm−1, from the analysis (Margenot et al., 2015; Parikh et al., 2014). We integrated the area under the curve in R (v4.2.1; R Core Team, 2022) for our functional groups of interest (aliphatic, aromatic, and amide). Bounds for integration are reported in Table 1 as “range.” We then normalized the area under the curve for our functional groups of interest to 100 %. We also calculated ratios of (1) simple plant C to microbial C and (2) complex plant C to microbial C. A low ratio of simple plant C to microbial C indicated microbial oxidation of plant-derived C, whereas a high ratio of simple plant C to microbial C indicated a high supply of aliphatic plant C to soil. Additionally, a low ratio of complex plant C to microbial C indicated more microbial oxidation of plant C, whereas a high ratio of complex plant C to microbial C indicated a high supply of aromatic plant compounds to the soil.
2.6 Statistical methods and model fitting
All statistical analyses were performed using R statistical software (v4.2.1; R Core Team, 2022). Differences between treatments for C stocks were evaluated using a Kruskal–Wallis test within each 10 cm (Fig. 3b) or 50 cm (Fig. 3a), depth interval depending on the analysis. We used a Kruskal–Wallis test combined with a pairwise Wilcoxon test due to it being a nonparametric statistical test. We determined that our data were nonparametric using a Shapiro–Wilk test of normality. Statistical significance was evaluated using α=0.05.
To better understand relationships between the C stock and inputs, we performed a linear regression on C stock and C:N ratio values. While C:N ratio values integrate many processes, there is evidence to suggest that a higher C:N ratio (>20) indicates plant inputs, whereas low C:N ratio values (<8) indicate microbial inputs (Bell et al., 2014; Knicker, 2011; Nierop et al., 2001). We interpreted a high C:N ratio as being indicative of more plant inputs, whereas a low C:N ratio would be indicative of more microbial inputs and decomposition.
To test the relative importance of biotic and abiotic factors on δ15N and C within treatments and to account for possible nonlinear relationships, we used a hierarchical generalized additive mixed model (GAMM) (Pedersen et al., 2019). To fit the GAMM, we used the “mgcv” package (Wood, 2017). GAMMs are a type of generalized linear model where the predictor is defined by a number of smooth functions of covariates. Models avoid overfitting by penalizing each smooth function, i.e., by penalizing the “wiggliness” of the fit. We also checked concurvity (the extension of collinearity to a GAMM) using the mgcv package. We used smooth functions based on thin plate regression splines (which are the default), and residuals approximated a “scat” or scaled t distribution, which was assessed from residuals using the DHARMa package (Hartig et al., 2024). In order to compare the relative importance of abiotic (depth) and biotic (DRIFTS ratios) variables, we constructed different models: one model had all terms included, one only included depth, and one only included our DRIFTS ratios of interest that indicate microbial oxidation and plant inputs. All models were compared using the Akaike information criterion (AIC), and the full model with all predictors and our depth model had the lowest AIC; therefore, for parsimony, not all of the models run were included.
3.1 Variation in physical, chemical, and isotopic parameters across treatments and depths
Physical parameters, such as bulk density, were relatively similar across treatments (Fig. 2a and b), although there were important differences in the surface material. The control treatment had the highest bulk density at 10 cm (1.7 ± 0.1 g cm−3), whereas the spring and winter treatments (1.4 ± 0.4 g cm−3) had lower bulk density (Fig. 2a). Bulk density increased across the depth profile and eventually converged to similar values across all treatments (∼1.8–2 g cm−3) (Fig. 2a). C (%) was greatest at the surface (0–30 cm) in the control (2.91 %–0.71 %) and winter (3.26 %–0.99 %) treatments, but it quickly dropped off to similar values at approximately 50 cm (∼0.3 %) (Fig. 2b). N (%) was present in low concentrations (<0.2 %) across the entire profile and across treatments (Fig. 2c). However, the C:N ratio varied in key ways throughout the depth profile and between treatments (Fig. 2d). While the C:N ratio was the greatest at 10 cm in the control (18.7 ± 4.09) compared to the winter (13.9 ± 1.78) and spring treatments (10.9 ± 0.96), the winter and spring treatments had an elevated C:N ratio from 30 to 100 cm (Fig. 2d). Changes in physical and elemental parameters were largely limited to the surface, and they converged past 1 m in most cases.
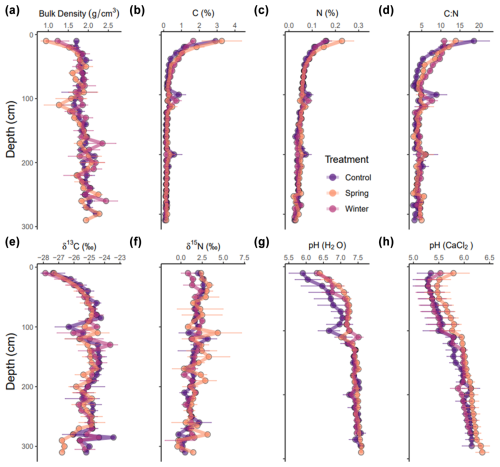
Figure 2(a–h) Physical and chemical parameters for all treatments across the depth profile. All data are shown as means with their standard error (n=3 for each treatment). Panel (a) shows bulk density (in g cm−3) for all treatments. Panels (b) and (c) show C (%) and N (%), respectively. Panel (d) shows C:N ratios. Panels (e) and (f) show δ13C and δ15N stable isotope values, respectively. Panels (g) and (h) show the pH in H2O and CaCl2, respectively.
Stable isotope values to 3 m were highly variable across the depth profile and across treatments, especially around 100 cm depth. We did not observe treatment effects from added precipitation on δ13C and δ15N values (Fig. 2e and f, respectively). We expected the well-documented pattern of increasing δ13C values with depth in soils (Natelhoffer and Fry, 1988) to be moderated by the decreased δ13C of formed C due to the added precipitation from the manipulation experiment in both the winter and spring treatments. We observed slight differences in the overall distribution of δ13C values in the winter and spring treatment throughout the depth profile; however, except for the low δ13C values that we recorded around 300 cm depth in the spring treatment plots, we did not observe any statistically significant differences in δ13C values throughout the profile.
In terms of chemical parameters, differences in pH between treatments were also limited to the top 1 m and converged to similar values from 1 to 3 m (Fig. 2g and h). However, it is important to note that the spring and winter treatments had higher pH (H2O) compared to the control treatment to 1 m. Only the spring treatment had an elevated pH (CaCl2), while the control and winter treatments were similar throughout the entire depth profile. The pH values in water and CaCl2 indicate slightly acidic to neutral soil pH across the soil profile for all treatments.
3.2 C stocks and elemental relationships across depth increments
We found no statistically significant differences in the C stock across the 3 m depth profile or at the surface (top 50 cm) based on our Kruskal–Wallis test. All discussion of these results in this paragraph refers to trends between calculated cumulative stocks and across depth profiles due to the lack of statistical significance determined by the Kruskal–Wallis tests. We determined changes in the overall C stock across the entire depth profile of the treatment plots and observed important changes in seasonality. We found that the winter treatment had the greatest C stock value (200.5 ± 34.5 g cm−2; Table 2), followed by the control treatment (191.2 ± 36.7 g cm−2), whereas the spring treatment had the smallest cumulative C stocks (171.4 ± 13.7 g cm−2) over the entire profile (0–300 cm) (Table 1). The soil C stocks sharply dropped below 50 cm in all of the treatment plots. Proportionally, soils from 1 to 3 m held 35 % of the C stock in the control treatment, 33 % in the winter treatment, and 37 % in the spring treatment (Fig. 3). In addition, a more detailed investigation of the surface revealed greater C stocks from 0 to 50 cm in both the winter and control treatments (Fig. 3). There was also a relatively greater C amount in the winter and spring treatments at 150 cm. Interestingly, the winter C stock at 300 cm was decreased compared to both the control and spring treatments. Overall, however, the winter treatment had the greatest overall C stock, whereas the spring treatment had the lowest value.
Table 2Calculated cumulative carbon stocks for each treatment to examine how total carbon stocks might be changing with precipitation addition. The winter and control treatments had the greatest cumulative carbon stocks based on three cores of 0–300 cm per treatment (n=9); standard error is shown in parentheses.
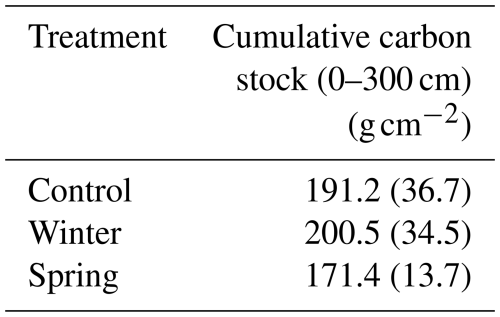
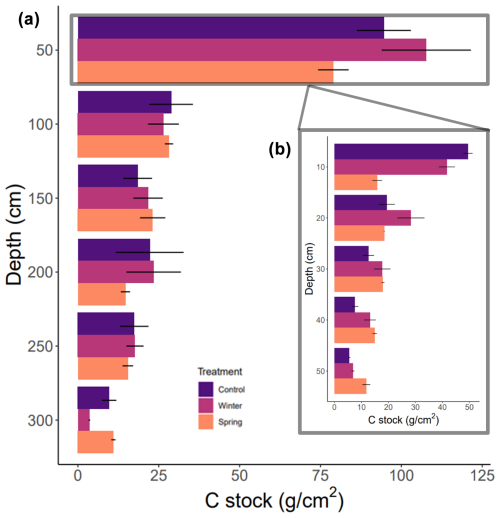
Figure 3Carbon stocks throughout 0–300 cm cores across the control treatment as well as winter and spring precipitation additions. (a) The average calculated carbon stocks in 50 cm depth increments with standard error. (b) The inset includes higher-resolution information for the top 50 cm in 10 cm increments.
To further examine the relationships between soil C processes and inputs at the precipitation manipulation experiment, we looked at relationships between the C stock and C:N ratio across depth increments (Fig. 4). There is evidence to support that high C:N ratio values are indicative of plant tissues (Bell et al., 2014; Nierop et al., 2001), whereas low C:N ratio values are indicative of microbial byproducts such as amino acids and amino sugars (Knicker, 2011). Overall, C:N ratio values integrate multiple processes that can be difficult to disentangle, such the balance between inputs and decomposition (Conen et al., 2008). We expected to see significant positive relationships, indicating a tight relationship between C stock and plant inputs. We did indeed see positive and significant relationships across all treatments. We found a slightly more positive relationship in the winter treatment than the control. In the spring treatment, we saw a much narrower range in the C:N ratio values compared to the winter and control treatments. This analysis highlighted the unique distribution of both the C:N ratio and C stock in the spring treatment.
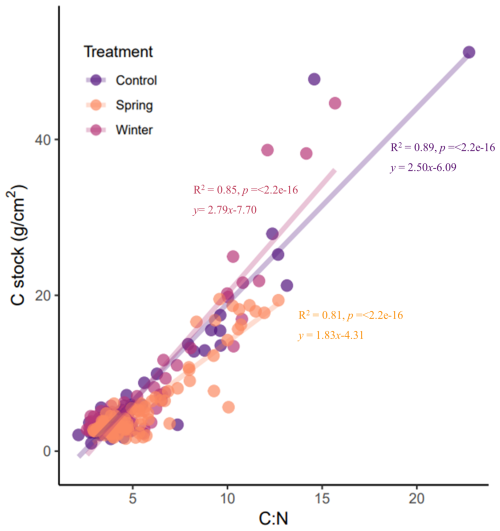
Figure 4Linear regressions of the C:N ratio and C stock for all depths and treatments reveal differences in nutrient dynamics between the spring treatments. All linear regressions are significant, but the slope for the winter treatment is more gentle compared with the control and spring treatments, which have similar slopes and a higher C:N ratio at the surface.
3.3 Variation in functional group chemistry across treatments
The DRIFTS spectra show differences across treatments and depths, especially in areas of interest for biological OM inputs (Fig. 5). We observed shifts in the functional group chemistry of SOM across treatments, both proportionally (Fig. 6) and in relationship to elemental and isotopic data (Table 3). We calculated proportional relationships by normalizing the area under the curve for our functional groups of interest to 100 %. An important observation from the DRIFTS spectra is the proportional contribution of microbially associated functional groups across all three treatments (>50 %; Fig. 6a–c). Simple plant-derived organic matter (aliphatics) was approximately 30 % of the total, with some slight variation, while complex plant matter (aromatic) functional groups comprised the smallest fraction, representing about 20 % of the total (Fig. 6). Furthermore, differences in simple and plant-derived OM diverged between treatments from 200 to 250 cm, where the control treatment seemed to have the greatest aromatic or complex plant-derived OM proportionally (Fig. 6a), and also based on the complex plant : microbial ratio (Fig. 6e). Our DRIFTS data overall suggested that the dominant functional groups were similar across depth and treatment and that the dominant functional group was largely microbially associated organic matter.
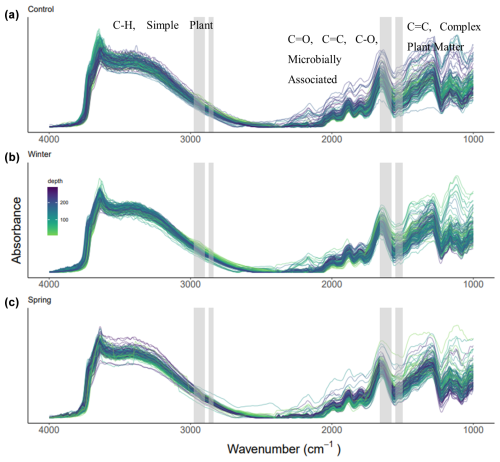
Figure 5(a–c) DRIFTS spectra across treatments and depths, labeled with the wavenumbers of interest: aliphatic compounds and simple plant matter (2976–2998 and 2870–2839 cm−1); aromatic compounds and complex plant matter (1550–1500 cm−1); amide, quinone, ketone stretch, aromatic and/or carboxylate stretch, and microbially associated OM (1660–1580 cm−1). The control, winter, and spring treatments are shown in panels (a), (b), and (c), respectively, and colors represent the depth gradient.
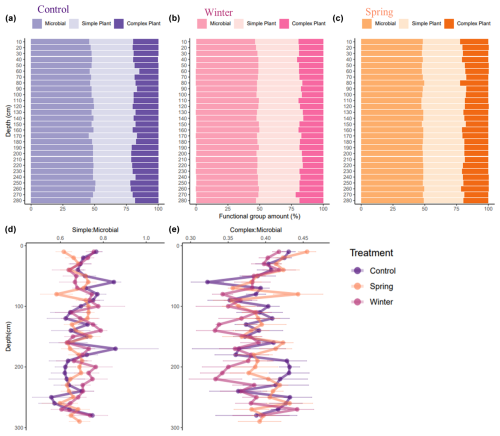
Figure 6(a–e) Proportions of integrated area for areas of interest in DRIFTS data, which indicate the dominance of microbially associated OM across treatments and depths. Areas of interest in DRIFTS spectra include simple plant-derived functional groups (2976–2998 and 2870–2839 cm−1), complex plant-derived functional groups (1550–1500 cm−1), and microbially associated OM (1660–1580 cm−1). The proportional area of interest for each 10 cm depth interval for the (a) control treatment, (b) winter treatment, and (c) spring treatments is also shown. The ratios of (d) simple plant matter to microbial plant matter and (e) complex plant matter to microbial plant matter by depth are also shown, along with their averages and standard error, for each 10 cm depth interval.
We further analyzed the relationship of the shifts in SOM functional group chemistry with elemental and isotopic data to better understand abiotic versus biotic controls on SOM storage and processing across treatments. We chose to more closely examine δ15N, due to the fact that it is coupled to biotic processes (Dijkstra et al., 2008; Hobbie and Ouimette, 2009), and C (%) to potentially understand relationships of biotic and abiotic factors with C storage. We fit hierarchical GAMMs to better predict both δ15N (Table 3a) and C concentrations (Table 3b). GAMMs were fit to try and predict δ15N and C (%) to better understand if δ15N is actually related to our DRIFTS ratios of interest (which we interpreted as biotic factors) and C storage. By visualizing our GAMM, we saw significant negative relationships in the winter and spring treatments regarding the relationship between the simple plant : microbial ratio and δ15N values (Fig. S2a in the Supplement). We also observed a similar pattern for relationships between the complex plant : microbial ratio and δ15N values (Fig. S2b). Overall, we were able to account for greater variation in both δ15N and C (%) using GAMMs. The model including all factors for C (%) performed better () than the model for δ15N values (). For both the C concentration and δ15N, depth was a key factor across all treatments. Importantly, however, the simple plant : microbial DRIFTS ratio was a significant predictor of δ15N for the winter treatment, and the complex plant : microbial DRIFTS ratio was a significant predictor for both the winter and spring (Table 3a).
C translocation was a key mechanism in this experiment, and there was a clear impact on the distribution of C stocks and functional group chemistry between the topsoil and subsoil from adding more water to the soil profile. We saw the greatest cumulative C stocks in the winter treatment, especially compared to the spring precipitation addition. Furthermore, plant phenology in an annual grassland varies greatly from the winter months to the spring. Spring temperatures are increasing, evaporative stress is greater, and annual plants are usually reaching the end of their life span in this season. We think that greater C is accumulating in the winter treatment due to precipitation addition coinciding with lower temperatures and lesser microbial activity. In sum, what we are likely seeing is increased plant inputs in the winter treatment and greater transport to deeper layers, whereas in the spring, due to higher temperatures and greater evaporative stress, there is more gaseous loss of C, less water, and less C moved to deeper soil layers.
4.1 C translocation and accrual as a result of added precipitation
We observed clear signals of greater C translocation throughout the soil profile as a result of added precipitation, but where this C accumulated was based on seasonality. Added precipitation seemed to lead to greater transport of C throughout the profile in the winter and spring treatment especially. However, we did not see any statistically significant differences in C stocks across the subsoil or surface C stock measurements (Fig. 3a and b). This could be due to significant variability introduced from fixed-depth bulk density measurements due to changing soil volume over time (von Haden et al., 2020). We observed significant evidence for slightly higher C in the winter treatment being plant-derived based on both the C:N ratio and DRIFTS data. We also saw evidence of greater biotic processing in the spring treatment when comparing δ15N to the simple plant matter : microbially associated DRIFTS ratio (Table 3) and the overall lower and more constrained C:N ratio values (Fig. 4). We also saw positive and significant trends when we related C concentrations to the ratio of complex plant matter to microbially associated OM (Fig. S2b), highlighting a unique relationship between complex plant inputs and C, especially in the winter and spring, that may be related to biotic inputs. It is important to note that evidence of subsoil C accumulation in the winter treatment was only made possible by incorporating measurements from deeper than 1 m and that depth was a significant predictor in our GAMMs across all treatments (Table 3). Previous work has suggested that the main sources of organic matter into subsoils are plant-derived compounds (roots and root exudates), dissolved organic matter (DOM), and bioturbation (Rumpel and Kögel-Knabner, 2011). Work at the Angelo experiment has shown evidence of changing rooting patterns and greater overall biomass with increased precipitation at this site (Suttle et al., 2007). Rooting depth and root exudation rates are affected by the availability of soil water, and the direction of the response is strongly dependent on the species (Li et al., 2021; Souza et al., 2023; Staszel et al., 2022). However, it has been found, through 13CO2 pulse labeling studies, that drought reduces the transfer of newly fixed C to microbes (Fuchslueger et al., 2014; Ruehr et al., 2009), while manipulative experiments with rainfall addition increase root C exudation rates (Li et al., 2021). A recent meta-analysis noted that decreased precipitation also slows the belowground C cycle, while precipitation increases promote nearly every aspect, such as C stock, substrate supply, microbial activity, and respiration (Abbasi et al., 2020). This is due to interactions between precipitation and biological entities, namely, plants and microbes. Increased precipitation, root respiration, and belowground NPP are positively correlated with soil water availability, and they enhance plant growth and photosynthetic rates (Heisler-White et al., 2008; Maire et al., 2015). Wetting of dry soil also has a dramatic impact on soil microbes due to increased substrate availability and the reactivation of dormant microbes, yielding respiration pulses known as the Birch effect (Salazar et al., 2018; Schimel et al., 2007; Skopp et al., 1990). Overall, greater precipitation in the winter could contribute to greater root exudation in surface soils that then gets quickly fixed by soil microbes. Our results show that, in the winter treatment plots, the additional precipitation in the already wet winter season likely increases root exudation, where this increased C input coincides with lower temperatures and lesser biological activity in soil. In contrast, in the spring treatment, soil C is exposed to greater microbial processing. Thus, there is greater movement of this plant-derived input moving down the profile in both the spring and winter treatments, although it moves to greater depths in the spring treatment, likely as DOM. Recent plot-scale studies have proposed that OM formation in subsoils is linked to a complex cascade model, in which OM is sorbed, microbially processed, and remobilized in cycles as it migrates down the profile (Liebmann et al., 2020).
4.2 Biotic shifts as a result of changing precipitation amount and seasonality
Changing plant phenology throughout the growing season and changes in plant community composition could be contributing to the differences that we see in the C stock accrual in our treatment plots. Increased precipitation has been shown to increase NPP in grasslands, but it also alters the plant community composition and reduces diversity (Song et al., 2019; Suttle et al., 2007). Furthermore, a higher plant species richness has been associated with increased soil organic C (Prommer et al., 2020). At the Angelo Precipitation Experiment, it has been shown that the plant community composition responses to changing precipitation are based on seasonality, with spring precipitation addition resulting in reduced plant diversity, whereas the winter treatment maintained diversity close to the control (ambient) plots (Suttle et al., 2007). More specifically, Suttle et al. (2007) found that annual grass biomass was greater and plant richness was lower with spring precipitation addition. However, the spring treatment in our experiment still accumulated C in surface soils. This could be because, according to mesocosm experiments, invasive annual plants, such as cheatgrass, have been shown to accumulate both C and N due to higher rates of root exudation (Morris et al., 2016). A recent meta-analysis further examined the feedback between annuals and litter and rhizosphere inputs, finding that invasive plants may support more decomposers that stimulate more nutrient release from litter (Zhang et al., 2019). The simplification of the plant community in the spring treatment plots as well as the potential for greater root exudates and greater stimulation of decomposition by annual grasses at the surface could be leading to the surface C stock accumulation that we observed from 0 to 50 cm in the winter and spring plots. Other studies have suggested that a longer and later wet season would result in significant losses of C due to increased soil respiration (Chou et al., 2008). Increases in NPP accompanied by increased gaseous C flux are consistent with what is found in larger meta-analyses, where increased precipitation stimulates plant growth and ecosystem C fluxes (Wu et al., 2011). With respect to the Angelo Precipitation Experiment, there is still an open question regarding how these changes in plant community will interact with the potential stimulation of gaseous C flux.
Isotope values can also be affected by interactions between plants and climatic conditions, specifically due to the physiological responses of plants to soil water conditions. The δ13C value of C3 plants is tied to the climatic regime (Krüger et al., 2023); furthermore, the plants in this ecosystem are largely C3, meaning that differences in plant isotope values and formed C are likely driven by physiological responses to changing climatic regime and soil water. More specifically, there is less discrimination against the heavy isotope when plant stomata have to close more often, which is the case in arid environments (Casson and Gray, 2008; Driesen et al., 2020; Farquhar et al., 1989; Kohn, 2010; Krüger et al., 2023; Madhavan et al., 1991). Overall, this means that more precipitation would drive δ13C values down (more negative). There is also evidence that plant stomata opening is driven by soil water potential (Carminati and Javaux, 2020). Specifically, we think that formed C in plant inputs is lower with respect to the δ13C value and could be driving down δ13C values in the soil profile. However, we lack isotopic measurements of plant tissue; therefore, we present this as a valuable avenue for future work. Soil δ13C values in the spring and winter treatments were not significantly different from the control, and the high variability in these measurements makes it hard to detect if we are seeing this pattern in our data; nevertheless, there is some evidence of slightly lower δ13C values in the spring and winter treatments from 0 to 100 cm and again from 120 to 200 cm (Fig. 2e).
Although plant communities are sensitive to changing precipitation regimes, microbes are resilient to precipitation shifts. Specifically, there is evidence that the microbial community structure is resilient to long-term shifts in precipitation seasonality, even if there are shifts in the plant community structure (Cruz-Martínez et al., 2009). This capacity is likely because the climatic history of Mediterranean ecosystems would select for microbial populations resilient to soil moisture fluctuations (Cruz-Martínez et al., 2009). While microbial communities are resilient to long-term changes in moisture regime, there is evidence that they can respond rapidly to immediate changes in environmental conditions, which may be missed in long-term studies (Cruz-Martínez et al., 2012). For example, subsoil microbial communities quickly respond to added C (Min et al., 2021), and old C can be quickly mineralized (Fontaine et al., 2007). These studies suggest that increases in C translocation to subsoils could stimulate the loss of ancient buried C through a potential priming effect, where additions of fresh C can enhance the decomposition of harder-to-decompose or mineral-associated C (Keiluweit et al., 2015; Kuzyakov et al., 2000). While this study lacked explicit measurement of microbial communities with precipitation addition, we did observe increased translocation of C throughout the depth profile with precipitation addition. This ability of subsoil microbial communities to quickly take advantage of fresh C inputs could affect the long-term sequestration potential of subsoils affected by increased precipitation.
4.3 Implications for the C sequestration potential of deep soils in grasslands
While our results suggest possible C accrual in subsoils with the winter addition of precipitation, it is important to consider the mechanisms for the destabilization of subsoil C. The addition of fresh C to subsoils is identified as a potential destabilization mechanism due to priming effects (Rumpel and Kögel-Knabner, 2011). The addition of C (both microbial and plant derived) in subsoils in the winter treatment could fundamentally alter C cycling in subsoils. Further work is still needed regarding what proportion of added C can become associated with minerals in subsoils or be quickly mineralized by soil microbes. Current evidence suggests that fresh C is quickly mineralized at depth (Fontaine et al., 2007), but few studies have looked at fresh C partitioning to the mineral-associated fraction in subsoils. This added C could also be affecting the microbial community structure as well as increasing the formation of necromass at depth. There is also evidence that changing porosity and soil structure impacts the structure of microbial communities in soils (Wilpiszeski et al., 2019). Overall, interactions between changing soil water conditions and C addition to subsoils is dependent on the seasonality of this added precipitation in a Mediterranean grassland, and this could affect the sequestration potential of subsoils in grasslands under climate change.
This study leveraged a long-term (20-year) precipitation manipulation experiment to investigate how changing precipitation amount and seasonality would affect soil C, N, and functional group chemistry in deep soils of a California grassland. We measured a suite of soil chemical characteristics, stable isotopes, and C stocks and performed diffuse reflectance mid-infrared Fourier transform spectroscopy (DRIFTS) on all samples at 10 cm increments for the depth interval from 0 to 3 m, and we found greater cumulative C stocks in the winter treatment. Across all treatments, we found that soils from 1 to 3 m held nearly one-third of the overall C stock. These results suggest that added precipitation over the winter in Mediterranean grasslands can alter plant inputs and enhance C stocks in deep soils, highlighting the importance of measuring soil C and functional group chemistry to greater depths. This study found that increased precipitation has the potential to increase C translocation to deeper layers and that added winter precipitation causes a shift towards a more abiotic control on soil C cycling. Thus, changing precipitation amount and precipitation seasonality need to be taken into account when considering the C sequestration potential of Mediterranean grasslands.
The code can be made available from the corresponding author upon reasonable request. The code for this project is largely for visualization of the data.
The data are available at https://doi.org/10.5061/dryad.2280gb64q (Wahab et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-3915-2025-supplement.
Conceptualization of the project was led by AAB. Fieldwork and lab work was carried out by LMW. Formal analysis and investigation was done by LMW with supervision and contributions from all authors. LMW prepared the manuscript with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank Debbo Elias, Charlotte Calvario, Todd Longbottom, Ronnie Hall, Xin Gao, and Jing Yan for assistance with field sampling and lab analyses. In addition, this study would not have been possible without field support from Peter Steel from Angelo Coast Range Reserve, especially in August 2020 during the COVID-19 pandemic. Finally, we would like to thank Moritz Mainka and the anonymous reviewer for improving the manuscript.
This research was supported by the Department of Energy, the Sigma Xi Grants in Aid of Research, the UC Natural Reserve System Mathias Graduate Student Research Grant Program, and a UC Merced Committee on Research Senate Award. Moreover, this work was supported by the Department of Energy Office of Science Graduate Student Research (SCGSR) award. Work at LLNL was conducted under the auspices of DOE Contract DE-AC52-07NA27344 and supported by the US Department of Energy Biological and Environmental Research award SCW1632.
This paper was edited by Anja Rammig and reviewed by Moritz Mainka and one anonymous referee.
Abbasi, A. O., Salazar, A., Oh, Y., Reinsch, S., del Rosario Uribe, M., Li, J., Rashid, I., and Dukes, J. S.: Reviews and syntheses: Soil responses to manipulated precipitation changes – an assessment of meta-analyses, Biogeosciences, 17, 3859–3873, https://doi.org/10.5194/bg-17-3859-2020, 2020.
Bai, Y. and Cotrufo, M. F.: Grassland soil carbon sequestration: Current understanding, challenges, and solutions, Science, 377, 603–608, https://doi.org/10.1126/science.abo2380, 2022.
Bell, C., Carrillo, Y., Boot, C. M., Rocca, J. D., Pendall, E., and Wallenstein, M. D.: Rhizosphere stoichiometry: are ratios of plants, soils, and enzymes conserved at the plant species-level?, New Phytol., 201, 505–517, https://doi.org/10.1111/nph.12531, 2014.
Berhe, A., Suttle, K. B., Burton, S. D., and Banfield, J. F.: Contingency in the direction and mechanics of soil organic matter responses to increased rainfall, Plant Soil, 358, 371–383, https://doi.org/10.1007/s11104-012-1156-0, 2012.
Berhe, A. A., Harden, J. W., Torn, M. S., and Harte, J.: Linking soil organic matter dynamics and erosion-induced terrestrial carbon sequestration at different landform positions, J. Geophys. Res., 113, G04039, https://doi.org/10.1029/2008JG000751, 2008.
Button, E. S., Pett-Ridge, J., Murphy, D. V., Kuzyakov, Y., Chadwick, D. R., and Jones, D. L.: Deep-C storage: Biological, chemical and physical strategies to enhance carbon stocks in agricultural subsoils, Soil Biol. Biochem., 170, 108697, https://doi.org/10.1016/j.soilbio.2022.108697, 2022.
Carminati, A. and Javaux, M.: Soil Rather Than Xylem Vulnerability Controls Stomatal Response to Drought, Trends Plant Sci., 25, 868–880, https://doi.org/10.1016/j.tplants.2020.04.003, 2020.
Casson, S. and Gray, J. E.: Influence of environmental factors on stomatal development, New Phytol., 178, 9–23, https://doi.org/10.1111/j.1469-8137.2007.02351.x, 2008.
Chou, W. W., Silver, W. L., Jackson, R. D., Thompson, A. W., and Allen-Diaz, B.: The sensitivity of annual grassland carbon cycling to the quantity and timing of rainfall, Glob. Change Biol., 14, 1382–1394, https://doi.org/10.1111/j.1365-2486.2008.01572.x, 2008.
Conen, F., Zimmermann, M., Leifeld, J., Seth, B., and Alewell, C.: Relative stability of soil carbon revealed by shifts in δ15N and C:N ratio, Biogeosciences, 5, 123–128, https://doi.org/10.5194/bg-5-123-2008, 2008.
Cruz-Martínez, K., Suttle, K. B., Brodie, E. L., Power, M. E., Andersen, G. L., and Banfield, J. F.: Despite strong seasonal responses, soil microbial consortia are more resilient to long-term changes in rainfall than overlying grassland, ISME J., 3, 738–744, https://doi.org/10.1038/ismej.2009.16, 2009.
Cruz-Martínez, K., Rosling, A., Zhang, Y., Song, M., Andersen, G. L., and Banfield, J. F.: Effect of Rainfall-Induced Soil Geochemistry Dynamics on Grassland Soil Microbial Communities, Appl. Environ. Microb., 78, 7587–7595, https://doi.org/10.1128/AEM.00203-12, 2012.
Delgado-Baquerizo, M., Maestre, F. T., Gallardo, A., Bowker, M. A., Wallenstein, M. D., Quero, J. L., Ochoa, V., Gozalo, B., García-Gómez, M., Soliveres, S., García-Palacios, P., Berdugo, M., Valencia, E., Escolar, C., Arredondo, T., Barraza-Zepeda, C., Bran, D., Carreira, J. A., Chaieb, M., Conceição, A. A., Derak, M., Eldridge, D. J., Escudero, A., Espinosa, C. I., Gaitán, J., Gatica, M. G., Gómez-González, S., Guzman, E., Gutiérrez, J. R., Florentino, A., Hepper, E., Hernández, R. M., Huber-Sannwald, E., Jankju, M., Liu, J., Mau, R. L., Miriti, M., Monerris, J., Naseri, K., Noumi, Z., Polo, V., Prina, A., Pucheta, E., Ramírez, E., Ramírez-Collantes, D. A., Romão, R., Tighe, M., Torres, D., Torres-Díaz, C., Ungar, E. D., Val, J., Wamiti, W., Wang, D., and Zaady, E.: Decoupling of soil nutrient cycles as a function of aridity in global drylands, Nature, 502, 672–676, https://doi.org/10.1038/nature12670, 2013.
Diamond, S., Andeer, P., Li, Z., Crits-Christoph, A., Burstein, D., Anantharaman, K., Lane, K. R., Thomas, B. C., Pan, C., Northen, T., and Banfield, J. F.: Processing of grassland soil C−N compounds into soluble and volatile molecules is depth stratified and mediated by genomically novel bacteria and archaea, bioRxiv [preprint], https://doi.org/10.1101/445817, 2018.
Dijkstra, P., LaViolette, C. M., Coyle, J. S., Doucett, R. R., Schwartz, E., Hart, S. C., and Hungate, B. A.: 15N enrichment as an integrator of the effects of C and N on microbial metabolism and ecosystem function, Ecol. Lett., 11, 389–397, https://doi.org/10.1111/j.1461-0248.2008.01154.x, 2008.
Driesen, E., Van Den Ende, W., De Proft, M., and Saeys, W.: Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review, Agronomy, 10, 1975, https://doi.org/10.3390/agronomy10121975, 2020.
Farquhar, G. D., Ehleringer, J. R., and Hubick, K. T.: Carbon Isotope Discrimination and Photosynthesis, Annu. Rev. Plant Physio., 40, 503–537, 1989.
Foley, M. M., Blazewicz, S. J., McFarlane, K. J., Greenlon, A., Hayer, M., Kimbrel, J. A., Koch, B. J., Monsaint-Queeney, V. L., Morrison, K., Morrissey, E., Hungate, B. A., and Pett-Ridge, J.: Active populations and growth of soil microorganisms are framed by mean annual precipitation in three California annual grasslands, Soil Biol. Biochem., 177, 108886, https://doi.org/10.1016/j.soilbio.2022.108886, 2023.
Fontaine, S., Barot, S., Barré, P., Bdioui, N., Mary, B., and Rumpel, C.: Stability of organic carbon in deep soil layers controlled by fresh carbon supply, Nature, 450, 277–280, https://doi.org/10.1038/nature06275, 2007.
Fuchslueger, L., Bahn, M., Fritz, K., Hasibeder, R., and Richter, A.: Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow, New Phytol., 201, 916–927, https://doi.org/10.1111/nph.12569, 2014.
Guo, L. B. and Gifford, R. M.: Soil carbon stocks and land use change: a meta analysis, Global Change Biol., 8, 345–360, https://doi.org/10.1046/j.1354-1013.2002.00486.x, 2002.
Hartig, F.: DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R package version 0.4.7, https://CRAN.R-project.org/package=DHARMa (last access: 25 February 2025), 2024.
Hawkes, C. V., Kivlin, S. N., Rocca, J. D., Huguet, V., Thomsen, M. A., and Suttle, K. B.: Fungal Community Responses to Precipitation, Global Change Biol., 17, 1637–1645, https://doi.org/10.1111/j.1365-2486.2010.02327.x, 2011.
Heisler-White, J. L., Knapp, A. K., and Kelly, E. F.: Increasing precipitation event size increases aboveground net primary productivity in a semi-arid grassland, Oecologia, 158, 129–140, https://doi.org/10.1007/s00442-008-1116-9, 2008.
Hicks Pries, C. E., Castanha, C., Porras, R. C., and Torn, M. S.: The whole-soil carbon flux in response to warming, Science, 355, 1420–1423, https://doi.org/10.1126/science.aal1319, 2017.
Hicks Pries, C. E., Ryals, R., Zhu, B., Min, K., Cooper, A., Goldsmith, S., Pett-Ridge, J., Torn, M., and Berhe, A. A.: The Deep Soil Organic Carbon Response to Global Change, Annu. Rev. Ecol. Evol. S., 54, 375–401, https://doi.org/10.1146/annurev-ecolsys-102320-085332, 2023.
Hobbie, E. A. and Ouimette, A. P.: Controls of nitrogen isotope patterns in soil profiles, Biogeochemistry, 95, 355–371, https://doi.org/10.1007/s10533-009-9328-6, 2009.
Jackson, R. B., Lajtha, K., Crow, S. E., Hugelius, G., Kramer, M. G., and Piñeiro, G.: The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls, Annu. Rev. Ecol. Evol. S., 48, 419–445, https://doi.org/10.1146/annurev-ecolsys-112414-054234, 2017.
Jilling, A., Keiluweit, M., Contosta, A. R., Frey, S., Schimel, J., Schnecker, J., Smith, R. G., Tiemann, L., and Grandy, A. S.: Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes, Biogeochemistry, 139, 103–122, https://doi.org/10.1007/s10533-018-0459-5, 2018.
Jobbágy, E. G. and Jackson, R. B.: The vertical distribution of soil organic carbon and its relation to climate and vegetation, Ecol. Appl., 10, 423–436, https://doi.org/10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2, 2000.
Keiluweit, M., Bougoure, J. J., Nico, P. S., Pett-Ridge, J., Weber, P. K., and Kleber, M.: Mineral protection of soil carbon counteracted by root exudates, Nat. Clim. Change, 5, 588–595, https://doi.org/10.1038/nclimate2580, 2015.
Kleber, M., Mikutta, R., Torn, M. S., and Jahn, R.: Poorly crystalline mineral phases protect organic matter in acid subsoil horizons, Eur. J. Soil Sci., 56, 050912034650054, https://doi.org/10.1111/j.1365-2389.2005.00706.x, 2005.
Knicker, H.: Soil organic N – An under-rated player for C sequestration in soils?, Soil Biol. Biochem., 43, 1118–1129, https://doi.org/10.1016/j.soilbio.2011.02.020, 2011.
Kohn, M. J.: Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate, P. Natl. Acad. Sci. USA, 107, 19691–19695, https://doi.org/10.1073/pnas.1004933107, 2010.
Krüger, N., Finn, D. R., and Don, A.: Soil depth gradients of organic carbon-13 – A review on drivers and processes, Plant Soil, 495, 113–136, https://doi.org/10.1007/s11104-023-06328-5, 2023.
Kuzyakov, Y., Friedel, J. K., and Stahr, K.: Review of mechanisms and quantification of priming effects, Soil Biol., 32, 1485–1498, https://doi.org/10.1016/S0038-0717(00)00084-5, 2000.
Li, C., Liu, L., Zheng, L., Yu, Y., Mushinski, R. M., Zhou, Y., and Xiao, C.: Greater soil water and nitrogen availability increase C:N ratios of root exudates in a temperate steppe, Soil Biol. Biochem., 161, 108384, https://doi.org/10.1016/j.soilbio.2021.108384, 2021.
Liebmann, P., Wordell-Dietrich, P., Kalbitz, K., Mikutta, R., Kalks, F., Don, A., Woche, S. K., Dsilva, L. R., and Guggenberger, G.: Relevance of aboveground litter for soil organic matter formation – a soil profile perspective, Biogeosciences, 17, 3099–3113, https://doi.org/10.5194/bg-17-3099-2020, 2020.
Madhavan, S., Treichel, I., and O'Leary, M. H.: Effects of Relative Humidity on Carbon Isotope Fractionation in Plants, Bot. Acta, 104, 292–294, https://doi.org/10.1111/j.1438-8677.1991.tb00232.x, 1991.
Maillard, É., McConkey, B. G., and Angers, D. A.: Increased uncertainty in soil carbon stock measurement with spatial scale and sampling profile depth in world grasslands: A systematic analysis, Agr. Ecosyst. Environ., 236, 268–276, https://doi.org/10.1016/j.agee.2016.11.024, 2017.
Mainka, M., Summerauer, L., Wasner, D., Garland, G., Griepentrog, M., Berhe, A. A., and Doetterl, S.: Soil geochemistry as a driver of soil organic matter composition: insights from a soil chronosequence, Biogeosciences, 19, 1675–1689, https://doi.org/10.5194/bg-19-1675-2022, 2022.
Maire, V., Wright, I. J., Prentice, I. C., Batjes, N. H., Bhaskar, R., Van Bodegom, P. M., Cornwell, W. K., Ellsworth, D., Niinemets, Ü., Ordonez, A., Reich, P. B., and Santiago, L. S.: Global effects of soil and climate on leaf photosynthetic traits and rates, Global Ecol. Biogeogr., 24, 706–717, https://doi.org/10.1111/geb.12296, 2015.
Margenot, A., Calderón, F., and Parikh, S. J.: Limitations and Potential of Spectral Subtractions in Fourier-Transform Infrared Spectroscopy of Soil Samples, Soil Sci. Soc. Am. J., 80, 10–26, https://doi.org/10.2136/sssaj2015.06.0228, 2015.
McFarlane, K. J., Torn, M. S., Hanson, P. J., Porras, R. C., Swanston, C. W., Callaham, M. A., and Guilderson, T. P.: Comparison of soil organic matter dynamics at five temperate deciduous forests with physical fractionation and radiocarbon measurements, Biogeochemistry, 112, 457–476, https://doi.org/10.1007/s10533-012-9740-1, 2013.
Mikutta, R., Kleber, M., Torn, M. S., and Jahn, R.: Stabilization of Soil Organic Matter: Association with Minerals or Chemical Recalcitrance?, Biogeochemistry, 77, 25–56, https://doi.org/10.1007/s10533-005-0712-6, 2006.
Min, K., Berhe, A. A., Khoi, C. M., Van Asperen, H., Gillabel, J., and Six, J.: Differential effects of wetting and drying on soil CO2 concentration and flux in near-surface vs. deep soil layers, Biogeochemistry, 148, 255–269, https://doi.org/10.1007/s10533-020-00658-7, 2020.
Min, K., Slessarev, E., Kan, M., McFarlane, K., Oerter, E., Pett-Ridge, J., Nuccio, E., and Berhe, A. A.: Active microbial biomass decreases, but microbial growth potential remains similar across soil depth profiles under deeply-vs. shallow-rooted plants, Soil Biol. Biochem., 162, 108401, https://doi.org/10.1016/j.soilbio.2021.108401, 2021.
Morris, K. A., Stark, J. M., Bugbee, B., and Norton, J. M.: The invasive annual cheatgrass releases more nitrogen than crested wheatgrass through root exudation and senescence, Oecologia, 181, 971–983, https://doi.org/10.1007/s00442-015-3544-7, 2016.
Natelhoffer, K. J. and Fry, B.: Controls on Natural Nitrogen-15 and Carbon-13 Abundances in Forest Soil Organic Matter, Soil Sci. Soc. Am. J., 52, 1633–1640, https://doi.org/10.2136/sssaj1988.03615995005200060024x, 1988.
Nierop, K. G. J., Van Lagen, B., and Buurman, P.: Composition of plant tissues and soil organic matter in the first stages of a vegetation succession, Geoderma, 100, 1–24, https://doi.org/10.1016/S0016-7061(00)00078-1, 2001.
Parikh, S. J., Goyne, K. W., Margenot, A. J., Mukome, F. N. D., and Calderón, F. J.: Soil Chemical Insights Provided through Vibrational Spectroscopy, in: Advances in Agronomy, vol. 126, Elsevier, 1–148, https://doi.org/10.1016/B978-0-12-800132-5.00001-8, 2014.
Pedersen, E. J., Miller, D. L., Simpson, G. L., and Ross, N.: Hierarchical generalized additive models in ecology: an introduction with mgcv, PeerJ, 7, e6876, https://doi.org/10.7717/peerj.6876, 2019.
Plante, A. F., Conant, R. T., Stewart, C. E., Paustian, K., and Six, J.: Impact of Soil Texture on the Distribution of Soil Organic Matter in Physical and Chemical Fractions, Soil Sci. Soc. Am. J., 70, 287–296, https://doi.org/10.2136/sssaj2004.0363, 2006.
Porras, R. C., Hicks Pries, C. E., McFarlane, K. J., Hanson, P. J., and Torn, M. S.: Association with pedogenic iron and aluminum: effects on soil organic carbon storage and stability in four temperate forest soils, Biogeochemistry, 133, 333–345, https://doi.org/10.1007/s10533-017-0337-6, 2017.
Prommer, J., Walker, T. W. N., Wanek, W., Braun, J., Zezula, D., Hu, Y., Hofhansl, F., and Richter, A.: Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity, Global Change Biol., 26, 669–681, https://doi.org/10.1111/gcb.14777, 2020.
R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (last access: 21 July 2025), 2022.
Rocci, K. S., Bird, M., Blair, J. M., Knapp, A. K., Liang, C., and Cotrufo, M. F.: Thirty years of increased precipitation modifies soil organic matter fractions but not bulk soil carbon and nitrogen in a mesic grassland, Soil Biol. Biochem., 185, 109145, https://doi.org/10.1016/j.soilbio.2023.109145, 2023.
Ruehr, N. K., Offermann, C. A., Gessler, A., Winkler, J. B., Ferrio, J. P., Buchmann, N., and Barnard, R. L.: Drought effects on allocation of recent carbon: from beech leaves to soil CO2 efflux, New Phytol., 184, 950–961, https://doi.org/10.1111/j.1469-8137.2009.03044.x, 2009.
Rumpel, C.: Location and chemical composition of stabilized organic carbon in topsoil and subsoil horizons of two acid forest soils, Soil Biol. Biochem., 36, 177–190, https://doi.org/10.1016/j.soilbio.2003.09.005, 2004.
Rumpel, C. and Kögel-Knabner, I.: Deep soil organic matter – a key but poorly understood component of terrestrial C cycle, Plant Soil, 338, 143–158, https://doi.org/10.1007/s11104-010-0391-5, 2011.
Salazar, A., Sulman, B. N., and Dukes, J. S.: Microbial dormancy promotes microbial biomass and respiration across pulses of drying-wetting stress, Soil Biol. Biochem., 116, 237–244, https://doi.org/10.1016/j.soilbio.2017.10.017, 2018.
Schimel, J., Balser, T. C., and Wallenstein, M.: Microbial Stress-Response Physiology and its implcations for ecosystem function, Ecology, 88, 1386–1394, https://doi.org/10.1890/06-0219, 2007.
Schmidt, M. W. I., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I. A., Kleber, M., Kögel-Knabner, I., Lehmann, J., Manning, D. A. C., Nannipieri, P., Rasse, D. P., Weiner, S., and Trumbore, S. E.: Persistence of soil organic matter as an ecosystem property, Nature, 478, 49–56, https://doi.org/10.1038/nature10386, 2011.
Skopp, J., Jawson, M. D., and Doran, J. W.: Steady-State Aerobic Microbial Activity as a Function of Soil Water Content, Soil Sci. Soc. Am. J., 54, 1619, https://doi.org/10.2136/sssaj1990.03615995005400060018x, 1990.
Slessarev, E. W., Nuccio, E. E., McFarlane, K. J., Ramon, C. E., Saha, M., Firestone, M. K., and Pett-Ridge, J.: Quantifying the effects of switchgrass (Panicum virgatum) on deep organic C stocks using natural abundance 14C in three marginal soils, GCB Bioenergy, 12, 834–847, https://doi.org/10.1111/gcbb.12729, 2020.
Sollins, P., Kramer, M. G., Swanston, C., Lajtha, K., Filley, T., Aufdenkampe, A. K., Wagai, R., and Bowden, R. D.: Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization, Biogeochemistry, 96, 209–231, https://doi.org/10.1007/s10533-009-9359-z, 2009.
Song, J., Wan, S., Piao, S., Knapp, A. K., Classen, A. T., Vicca, S., Ciais, P., Hovenden, M. J., Leuzinger, S., Beier, C., Kardol, P., Xia, J., Liu, Q., Ru, J., Zhou, Z., Luo, Y., Guo, D., Adam Langley, J., Zscheischler, J., Dukes, J. S., Tang, J., Chen, J., Hofmockel, K. S., Kueppers, L. M., Rustad, L., Liu, L., Smith, M. D., Templer, P. H., Quinn Thomas, R., Norby, R. J., Phillips, R. P., Niu, S., Fatichi, S., Wang, Y., Shao, P., Han, H., Wang, D., Lei, L., Wang, J., Li, X., Zhang, Q., Li, X., Su, F., Liu, B., Yang, F., Ma, G., Li, G., Liu, Y., Liu, Y., Yang, Z., Zhang, K., Miao, Y., Hu, M., Yan, C., Zhang, A., Zhong, M., Hui, Y., Li, Y., and Zheng, M.: A meta-analysis of 1119 manipulative experiments on terrestrial carbon-cycling responses to global change, Nat. Ecol. Evol., 3, 1309–1320, https://doi.org/10.1038/s41559-019-0958-3, 2019.
Souza, L. F. T., Hirmas, D. R., Sullivan, P. L., Reuman, D. C., Kirk, M. F., Li, L., Ajami, H., Wen, H., Sarto, M. V. M., Loecke, T. D., Rudick, A. K., Rice, C. W., and Billings, S. A.: Root distributions, precipitation, and soil structure converge to govern soil organic carbon depth distributions, Geoderma, 437, 116569, https://doi.org/10.1016/j.geoderma.2023.116569, 2023.
Staszel, K., Lasota, J., and Błońska, E.: Effect of drought on root exudates from Quercus petraea and enzymatic activity of soil, Sci. Rep., 12, 7635, https://doi.org/10.1038/s41598-022-11754-z, 2022.
Suttle, K. B., Thomsen, M. A., and Power, M. E.: Species Interactions Reverse Grassland Responses to Changing Climate, Science, 315, 640–642, https://doi.org/10.1126/science.1136401, 2007.
Throop, H. L., Archer, S. R., Monger, H. C., and Waltman, S.: When bulk density methods matter: Implications for estimating soil organic carbon pools in rocky soils, J. Arid Environ., 77, 66–71, https://doi.org/10.1016/j.jaridenv.2011.08.020, 2012.
von Haden, A. C., Yang, W. H., and DeLucia, E. H.: Soils' dirty little secret: Depth-based comparisons can be inadequate for quantifying changes in soil organic carbon and other mineral soil properties, Global Change Biol., 26, 3759–3770, https://doi.org/10.1111/gcb.15124, 2020.
Vranova, V., Rejsek, K., and Formanek, P.: Aliphatic, Cyclic, and Aromatic Organic Acids, Vitamins, and Carbohydrates in Soil: A Review, Sci. World J., 2013, 1–15, https://doi.org/10.1155/2013/524239, 2013.
Ward, S. E., Smart, S. M., Quirk, H., Tallowin, J. R. B., Mortimer, S. R., Shiel, R. S., Wilby, A., and Bardgett, R. D.: Legacy effects of grassland management on soil carbon to depth, Global Change Biol., 22, 2929–2938, https://doi.org/10.1111/gcb.13246, 2016.
Wahab, L. M., Kim, S., and Berhe, A. A.: Carbon and nitrogen dynamics in subsoils after 20 years of added precipitation in a Mediterranean grassland, Dryad [data set], https://doi.org/10.5061/dryad.2280gb64q, 2025.
Wilpiszeski, R. L., Aufrecht, J. A., Retterer, S. T., Sullivan, M. B., Graham, D. E., Pierce, E. M., Zablocki, O. D., Palumbo, A. V., and Elias, D. A.: Soil Aggregate Microbial Communities: Towards Understanding Microbiome Interactions at Biologically Relevant Scales, Appl. Environ. Microb., 85, e00324-19, https://doi.org/10.1128/AEM.00324-19, 2019.
Wood, S. N.: Generalized additive models: an introduction with R, Chapman and Hall/CRC, https://doi.org/10.1201/9781315370279, 2017.
Wu, Z., Dijkstra, P., Koch, G. W., Peñuelas, J., and Hungate, B. A.: Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation, Global Change Biol., 17, 927–942, https://doi.org/10.1111/j.1365-2486.2010.02302.x, 2011.
Yost, J. L. and Hartemink, A. E.: How deep is the soil studied – an analysis of four soil science journals, Plant Soil, 452, 5–18, https://doi.org/10.1007/s11104-020-04550-z, 2020.
Zhang, P., Li, B., Wu, J., and Hu, S.: Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis, Ecol. Lett., 22, 200–210, https://doi.org/10.1111/ele.13181, 2019.