the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Sensitivity of tropical woodland savannas to El Niño droughts
Simone Matias Reis
Yadvinder Malhi
Ben Hur Marimon Junior
Beatriz Schwantes Marimon
Huanyuan Zhang-Zheng
Igor Araújo
Renata Freitag
Edmar Almeida de Oliveira
Karine da Silva Peixoto
Luciana Januário de Souza
Ediméia Laura Souza da Silva
Eduarda Bernardes Santos
Kamila Parreira da Silva
Maélly Dállet Alves Gonçalves
Cécile Girardin
Cecilia Dahlsjö
Oliver L. Phillips
Imma Oliveras Menor
The 2015–2016 El Niño event led to one of the hottest and most intense droughts for many tropical forests, profoundly impacting forest productivity. However, we know little about how this event affected the Cerrado, the largest savanna in South America. Here, we report on 5 years of productivity of the dominant vegetation types in the Cerrado, namely savanna (cerrado) and transitional forest–savanna (cerradão), continuously tracked before, during, and after the El Niño. Between 2014 and 2019, we carried out intensive monitoring of the productivity of key vegetation components (stems, leaves, roots). Cerradão productivity declined strongly by 29 % during the El Niño event. The most impacted component was stem productivity, which was reduced by 58 %. By contrast, cerrado productivity varied little over the years, and while the most affected component was fine roots, declining by 38 % during the event, fine-root productivity recovered soon after the El Niño. The two vegetation types also showed contrasting patterns in terms of the allocation of productivity to canopy, wood, and fine-root production. Our findings demonstrate that the cerradão can show low resistance and resilience to climatic disturbances due to the slow recovery of productivity. This suggests that the transitional Amazon–Cerrado ecosystems between South America's largest biomes may be particularly vulnerable to drought, enhanced by climate change.
- Article
(1225 KB) - Full-text XML
-
Supplement
(422 KB) - BibTeX
- EndNote
The 2015–2016 El Niño event led to some of the most intense tropical droughts in 100 years, as well as record maximum temperatures, occurring on top of decades of long-term warming (Jiménez-Muñoz et al., 2016; Liu et al., 2017). While the 2015–2016 climate anomaly affected most of the tropics, it was especially strong in South and Central America (Gloor et al., 2018; Powers et al., 2020). Intense droughts can increase tree mortality and affect the carbon sequestration capacity of forests, as shown by long-term ground-based monitoring (e.g., Phillips et al., 2009; Feldpausch et al., 2016; Rifai et al., 2018; Bennett et al., 2023). Satellite-based analyses also reveal the impacts of climate anomalies on carbon dynamics (Palmer, 2018; Fan et al., 2019), providing a synoptic view of ecosystem productivity. However, we still lack ground-based, tree-level measurements of net primary productivity (NPP) through extreme tropical-climate events, hindering our understanding of key aspects of the vegetation carbon cycle response, such as recovery following drought events and NPP allocation. Measuring these ecosystem responses directly is helped by tracking long-term forest dynamics in permanent plots but, in particular, requires high-fidelity process-based measurements that are sustained over time. These are exceptionally challenging to conduct and require long-term dedication to measurements before, during, and after major climate events like the 2015–2016 El Niño.
We know especially little about how El Niño events affect the productivity of savanna ecosystems in the extensive Amazonia–Cerrado transition in South America. This contains a mixture of Amazon and Cerrado species, making the species composition of this region unique and diverse (Ratter et al., 1973; Marimon et al., 2006; Morandi et al., 2016). Despite its ecological importance, the region has been greatly impacted by deforestation (∼ 41 % between 1984 and 2014) so that, today, only fragments of native vegetation remain (e.g., Marques et al., 2020). In recent decades, the remaining vegetation has been affected by increasing temperatures, frequent wildfires, extreme drought events, and a long-term trend toward longer dry seasons (e.g., Reis et al., 2018; Silvério et al., 2019; Nogueira et al., 2019; Matricardi et al., 2020; Araújo et al., 2021a). Deforestation, together with increases in temperature and reductions in precipitation during El Niño events, increases wildfire occurrence and carbon emissions, reducing the capacity of the vegetation to act as a carbon sink (Covey et al., 2021; Gatti et al., 2021). As the Amazonia–Cerrado transition is the driest, warmest, and most fragmented region in the Amazon basin (e.g., Matricardi et al., 2020; Marques et al., 2020; Covey et al., 2021; Reis et al., 2022), it is especially vital to understand better how climate change and extreme climate events impact productivity dynamics here.
The transition is composed naturally of a mosaic of vegetation, with the typical cerrado (referred to as cerrado hereafter) and woodland savanna (i.e., cerradão) being the most common in the regions (Ratter et al., 1973; Marimon et al., 2006; Oliveras and Malhi, 2016). Despite co-existing in the same space, the cerrado and cerradão vegetation formations show contrasting characteristics (Marimon Junior and Haridasan, 2005; Marimon et al., 2006). The cerradão is a transitional forest–savanna characterized by a closed canopy; an understory formed by small shrubs and herbs, with few grasses; and an average height of the tree stratum that varies from 8 to 15 m, with tree cover of 50 % to 90 % (Ribeiro and Walter, 2008; Oliveras and Malhi 2016), while the cerrado is a savanna vegetation type with a discontinuous canopy; trees and shrubs with a grass understory; and a low average height of just 3 to 6 m, with tree cover of 20 % to 50 % (Marimon Junior and Haridasan, 2005; Ribeiro and Walter, 2008).
In the cerrado, most species are deciduous, shedding their leaves during the dry season, whereas, in the cerradão, brevi-deciduous and/or evergreen species predominate (Ribeiro and Walter, 2008). This phenological difference has direct implications for the tolerance to water and thermal stress. The cerrado species exhibit conservative water use strategies, characterized by smaller stomata and higher trichome density, which reduce water loss and protect the leaves from overheating (Araújo et al., 2021b, 2023). In contrast, trees in the cerradão group have larger stomata and a lower density of trichomes, which may result in higher stomatal conductance and, consequently, greater water demand (Araújo et al., 2021b).
Among species that co-occur in both vegetation types, individuals in the cerrado shed their leaves earlier in the dry season than those in the cerradão, a strategy that prevents damage to photosynthetic apparatus during the driest and hottest period of the year (Araújo et al., 2021a). In the cerradão, later leaf senescence prolongs tree activity under water deficits, making them more vulnerable to rising temperatures, both under current conditions and in future projections (Araújo et al., 2021a). Trees in the cerradão are also taller than those in the cerrado, a trait that may increase their sensitivity to drought. Taller trees tend to have wider xylem vessels, making them more susceptible to embolism risk under severe water stress (Olson et al., 2018; Araújo et al., 2024). These contrasting strategies suggest that the responses of these two vegetation types to climatic disturbances such as El Niño events may differ substantially and, in particular, that the physiological and anatomical characteristics of cerradão vegetation may make it more susceptible to marked temperature increases and prolonged water deficits.
Here, by setting up and sustaining intensive, long-term monitoring plots that experience a similar climate for cerradão and cerrado, we aimed to quantify and compare the effect of the 2015–2016 El Niño on the carbon cycle (productivity and allocation) of the two vegetation types. Our guiding questions and hypotheses are outlined below.
-
Did the 2015–2016 El Niño affect total productivity and the productivity and partitioning of different compartments (canopy, stem, and fine root) in the cerradão and cerrado?
We hypothesize the following:-
H1. Cerrado and cerradão NPPs respond differently to El Niño events due to their distinct structural, anatomical, and eco-physiological strategies. We predict that the 2015–2016 El Niño reduced total productivity in both environments but that this was more severe in the cerradão, where traits such as taller trees, larger stomata, greater maximum stomatal pore opening, and reduced water loss control increase vulnerability to drought (e.g., Araújo et al., 2021a, b, 2023, 2024; Jancoski et al., 2022).
-
H2. The productivity decline should be more pronounced in the canopy and stem of the cerradão, whereas, in the cerrado, the reduction may have been less significant due to its higher water use resilience (Ball, 2010). During drought, cerrado plants are expected to reallocate resources from the aboveground compartments (canopy and stem) to fine roots, enhancing deep-water access, whereas cerradão trees, with greater investment in vertical growth, experience increased water stress and reduced productivity (Comas et al., 2013; Pérez-Ramos et al., 2013; Scalon et al., 2022).
-
-
Did the cerradão and cerrado regain productivity after the El Niño?
-
H3. The cerrado is expected to recover its productivity more quickly than the cerradão. In the cerradão, recovery may be slower due to greater structural damage and impairment of the trees' hydraulic systems, such as xylem vessel embolism (Jancoski, 2019). The cerrado is expected to exhibit greater resilience due to its conservative water use strategy and capacity for re-sprouting after extreme drought periods (Jancoski et al., 2022). In the cerradão, prolonged stress may have reduced the recovery rate, especially in trees that suffered embolism or partial canopy mortality (Reis et al., 2022; Araújo et al., 2024).
-
2.1 Study sites
We conducted this study in two long-term plots: one in the cerradão (a transitional forest–savanna) and another in the cerrado (typical cerrado; savanna), both located in Bacaba Municipal Park, Nova Xavantina, Mato Grosso State, central Brazil. The park covers approximately 500 ha in the transition zone between the Cerrado (Brazilian savanna) and the Amazonia. Since the two plots are only ∼ 300 m apart, they experience similar climatic conditions, classified as Aw (tropical with dry winters) in Köppen's system (Alvares et al., 2013). The region has two well-defined seasons: a cooler dry season (April to September) and a hot rainy season (October to March). According to the Brazilian National Institute of Meteorology (INMET) (station no. 83319), the mean monthly temperature is 24.8 °C, and the total annual precipitation is 1440 mm (Peixoto et al., 2017). The park's average altitude is ∼ 250 m. There is no evidence of a shallow water table which might buffer the impact of climate extremes on vegetation (Marimon Junior and Haridasan, 2005).
Each plot covers 1 ha and was established in 2002 (Marimon Junior and Haridasan, 2005), with multiple re-censuses having been conducted since then. Since 2010, these plots have been part of the PELD project (Cerrado-Amazonia Forest Transition: ecological and socio-environmental bases for conservation), the RAINFOR network (Amazonia Forest Inventory Network; ForestPlots.net et al., 2021), and the ForestPlots.net database. Since 2014, they have also been integrated into the GEM network (Global Ecosystems Monitoring network; Malhi et al., 2021). These plots have supported numerous studies on topics including soil properties, species composition and diversity, biomass, nutrient allocation, and tree dynamics (e.g., Marimon Junior and Haridasan, 2005; Marimon et al., 2014; Scalon et al., 2022). Partial carbon cycles for the cerradão plot, including litterfall, soil CO2 efflux and carbon stocks in fine roots, litter layer, and stems, have been published previously (Peixoto et al., 2017; Peixoto et al., 2018). Here, we provide the first comprehensive description of net primary productivity in both plots, along with an extended time series that sheds light on the aftermath of the 2015–2016 El Niño event.
The plots have remained fire-free since 2008. The cerradão plot is a transitional forest–savanna ecosystem with overlapping savanna and forest species, a closed canopy, and dominant species such as Hirtella glandulosa Spreng. and Tachigali vulgaris L.G. Silva and H.C. Lima. Ratter et al. (1973) classified this vegetation type as Hirtella glandulosa cerradão. In contrast, the cerrado plot is characterized by an open canopy with trees and shrubs, a grass understory, and two dominant tree species: Qualea parviflora Mart. and Davilla elliptica A.St.-Hil. (Marimon Junior and Haridasan, 2005; Marimon et al., 2014). However, the cerrado vegetation has been densifying, with reduced grass cover, possibly due to fire exclusion (Morandi et al., 2015).
Soil properties are similar across the plots, consisting of sandy loams classified as yellow latosol, which are acidic (pH < 5.0) and dystrophic (Ca2+ ∼ 0.4 cmolc kg−1), with high levels of exchangeable aluminum (Al3+ > 1.3 cmolc kg−1). However, the cerradão soil has a higher clay content and greater water-holding capacity than the cerrado soil, potentially explaining the contrasting vegetation types at these adjacent sites (Marimon Junior and Haridasan, 2005). In the cerrado plot, the average tree height is 3.7 m, with a basal area of ∼ 14.9 m2 ha−1, while, in the cerradão, trees are taller on average (6.4 m), with a higher basal area (∼ 21.4 m2 ha−1) (Marimon Junior and Haridasan, 2005). Both plots contain 77 tree species and similar tree densities (1890 trees in the cerrado and 1884 trees in the cerradão) (Marimon Junior and Haridasan, 2005).
2.2 Site climate and the El Niño 2015–2016 event
We used climate variables – air temperature, relative air humidity, and precipitation – from a time series recorded at a meteorological station (World Weather Station 83319) located approximately 800 m from the plots. We calculated the maximum climatological water deficit (MCWD), a key measure of tropical-forest water stress (see Aragão et al., 2007). For this calculation, we assumed a standardized evapotranspiration (ET) rate of 100 mm per month for wet-season tropical forests (Aragão et al., 2007).
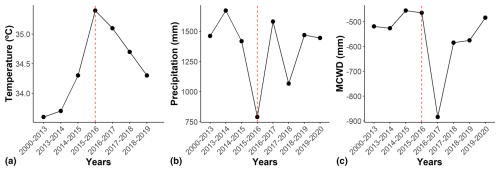
Figure 1Climate variables between 2000 and 2020 for the cerrado and cerradão. We show (a) temperature (°C), (b) precipitation (mm yr−1), and (c) maximum climatological water deficit (MCWD, mm in a rolling year), with the first month of the dry season (May) representing the beginning of each year's climatic calendar. The temperature indicates the average maximum monthly temperatures. The dashed red line indicates the El Niño periods. Climatic data are from meteorological station no. 83319 of the Brazilian National Institute of Meteorology (INMET). See Table S1 in the Supplement for data.
We used the hydrological year to define the period from May 2015 to April 2016 as representative of the climate conditions during the 2015–2016 El Niño–Southern Oscillation event based on Aragão et al. (2007) and Liu et al. (2017). During the event, the site experienced record-high mean annual and mean monthly maximum temperatures (26.0 and 35.4 °C, respectively) and record-low total annual precipitation (790.2 mm). Additionally, in September 2016, the annual MCWD reached a record low of −883.7 mm (Fig. 1; Table S1 in the Supplement).
2.3 Field methods and measurement uncertainties
We followed the GEM protocol manual (Marthews et al., 2014; Malhi et al., 2021) to collect data for this study. We measured the main components of NPP, including canopy (leaves, twigs, reproductive parts, and others), wood (stems and branches), and fine roots. Additionally, we estimated other NPP components, such as canopy (leaf herbivory) and wood (coarse root). The field method measurements and uncertainties are described below.
-
Litterfall net primary productivity (NPPlitterfall). We collected litterfall – dead organic material production (< 2 cm diameter) – every 14 d from January 2014 to December 2019. We used 0.2827 m2 circular collectors placed 1 m above the ground at the center of each of the 25 subplots in each plot (cerradão and cerrado). We separated litter into leaves, twigs, reproductive parts (flowers, fruits, and seeds), and unidentifiable material. We calculated NPPlitterfall as follows: NPPlitterfall = NPPcanopy − loss to leaf herbivory. We oven-dried at 65 °C to a constant mass; weighed it; and then separated it into leaves, twigs, reproductive parts, and others. We estimated litter to contain 49.2 % carbon based on mean values from Amazonia (Patiño et al., 2012). We calculated errors as the standard error associated with variation among the litter traps (collectors).
-
Loss to leaf herbivory (NPPherbivory). We estimated leaf herbivory loss based on Neyret et al. (2016), who observed that herbivory loss was 3.11 % in NXV-01 and 4.43 % in NXV-02. Data collection was conducted between March and May 2014. Each leaf's fractional herbivory (H) was calculated as H = (Anh − Ah) Anh, where Ah is the area of each leaf, including the damage caused by herbivory, and Anh is the leaf area prior to herbivory (Neyret et al., 2016). We derived the average H value for all leaves collected per litterfall trap and then calculated plot-level means. A systematic uncertainty of +50 % was assigned to the values for error propagation.
-
Aboveground coarse-wood net primary productivity (NPPstem). To estimate stem NPP, we used the data measured every 2–3 years, collected between 2013 and 2020, in the cerradão and cerrado plots. All trees ≥ 5 cm in diameter were surveyed to determine the growth rate of surviving trees and the rate of recruitment of new trees. The default measurement point was set at 30 cm (DAS30cm) above the soil surface instead of a typical forest diameter at breast height of 1.3 m. The biomass of each stem was calculated using the specific allometric equation of Rezende et al. (2006) for the Cerrado: , where C is the aboveground carbon stocks (kg), D is the diameter (30 cm above the soil), and H is the height (m). We measured the total height using a Leica DISTO laser measurement device. The authors assumed that dry stem biomass consists of 50 % carbon. A systematic uncertainty of +25 % was assigned to recognize systematic error in the use of allometry.
-
Branch turnover net primary productivity (NPPbranch turnover). Every 3 months, between 2014 and 2019, we collected branchfall > 2 cm diameter (excluding that associated with dead trees) within four 1 m × 100 m transects in each plot (cerrado and cerradão). Small branches were cut to include only the transect-crossing component and then were removed and weighed. Larger branches had their dimensions taken (diameter at three points) and were assigned a wood density value according to decomposition class (Harmon et al., 1995). See the RAINFOR-GEM manual (Marthews et al., 2014, p. 74) for a description of the decomposition status and surface area formulas. Errors were calculated as the standard error associated with the variation among transects.
-
Coarse-root net primary productivity (NPPcoarse root). Root biomass was estimated based on Miranda et al. (2014), specific to the vegetation types of the Cerrado. Based on this study, the ratio of the root (belowground) to shoot (aboveground) biomass is 1.37 for the cerrado and 0.22 for the cerradão. A recent study using 144 plots found a similar relationship, with a ratio of the root (belowground) to shoot (aboveground) biomass of 1.58 in Brazilian savannas (Terra et al., 2023). A systematic uncertainty of +20 % was assigned to values for error propagation. Although we did not measure this component, we find it useful to include this information given the scarcity of such estimates for savannas.
-
Fine-root net primary productivity (NPPfine root). Every 3 months, from September 2014 to February 2020, we collected fine roots in each plot (cerradão and cerrado) using 16 ingrowth cores (mesh cages: 12 cm diameter, 30 cm depth). Fine roots were manually removed from soil samples in four 10 min time steps, following a method that corrects for the underestimation of hard-to-extract root biomass (Metcalfe et al., 2007). This method was used to predict root extraction beyond 40 min (up to 120 min); typically, an additional 33 % correction factor was applied for fine roots not collected within 40 min. A correction for fine-root productivity below 30 cm depth (Galbraith et al., 2013) increased the value by 39 %. Errors were calculated as the standard error associated with the variation among sampling points. Root-free soil was then reinserted into the ingrowth core. Collected roots were thoroughly rinsed, oven-dried at 65 °C to a constant mass, and weighed. This process was repeated for each subsequent measurement.
For total NPP (calculated as the sum of several components; see Eq. (1) below), the uncertainty value is calculated by combining the uncertainty of each component by error propagation (Hughes and Hase, 2010; Malhi et al., 2015). The uncertainty of each component is explained above.
2.4 NPP calculation
We measured the NPP in the two plots between 2014 and 2020, as described above. We calculated all major components of NPP using the following equations:
The calculations above neglect several small NPP components, such as NPP lost through volatile organic compound emissions (NPPVOC), unmeasured litter trapped in the canopy, or litter dropped from understory flora below the litter traps (1 m). However, in central Amazonia, Malhi et al. (2009) found that NPPVOC represents a relatively minor fraction of total NPP (0.13 + 0.06 Mg C ha−1 yr−1). For belowground NPP, we do not include root exudates and mycorrhizae, which contribute less than 2 Mg C ha−1 yr−1 and represent a modest portion of carbon fluxes (Malhi et al., 2017). Therefore, we focus on canopy, wood, and fine-root productivity, which, together, account for over 85 % of NPP (see Riutta et al., 2018, and their references).
We calculated the relative allocation to the main NPP components (woody, canopy, and fine-root NPP) for leaves, fine roots, and stems using the following equation:
2.5 Data analyses
Our analyses focused on comparing NPP across years (2014 to 2019), comprising the periods before, during, and after the El Niño 2015–2016 event, in both cerrado and cerradão. To compare total canopy NPP across years in each vegetation type (cerradão and cerrado), we performed a repeated-measure ANOVA. The statistical model considered the year to be a fixed factor, while litter traps were included as a random effect to account for the hierarchical structure of the data over time. When significant differences were detected, we used Tukey's post hoc test to compare total canopy NPP between years. We applied the same analysis to compare stem and fine-root NPP across different years in each plot. For stem NPP, we used subplots as random effects, and for fine-root NPP, we used ingrowth cores as random effects. In cases where residuals violated ANOVA assumptions, we applied Friedman's non-parametric test. We performed all analyses in the R environment, with a significance level of 0.05.
3.1 Total NPP and its allocation
During the El Niño event, total NPP in the cerradão decreased by 29 % (6.6 ± 0.6 Mg C ha−1 yr−1) and reached a level similar to that of the cerrado (6.6 ± 1.3 Mg C ha−1 yr−1; Fig. 2, Table S2). By 2018, it remained 13 % lower than pre-El Niño conditions (Fig. 2). In contrast, total NPP in the cerrado showed little variation before, during, and after the El Niño.
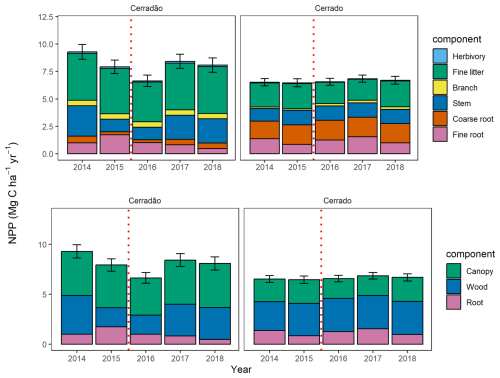
Figure 2Mean total annual net primary productivity (NPP) between 2014 and 2018, split into its components (above) and annual NPP allocation into the canopy, wood, and root components (below) for the cerradão and cerrado. The branch data from the cerradão were collected in 2014 and repeated in other years. The error bars represent the standard error for total NPP. The dashed red line indicates the El Niño periods.
Throughout the study period, NPP allocation in the cerrado exhibited little interannual variation and showed no clear drought signal. The primary axis of interannual variation was between canopy investment and root allocation, while woody allocation remained constant (Figs. 2 and 3). However, in the cerradão, a clear drought signal was observed, with increased investment in fine roots during the drought and reduced investment in woody growth. Canopy allocation remained relatively constant.
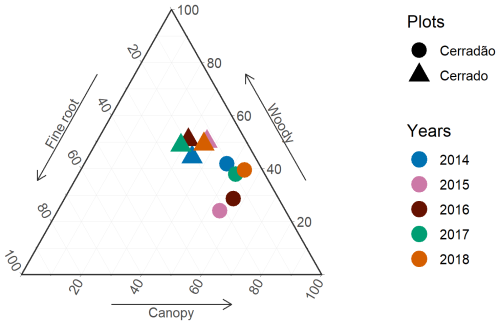
Figure 3Relative allocation (% of total) of net primary productivity (NPP) to canopy, woody, and fine-root NPP in the cerrado and cerradão. Woody components include stems, coarse roots, and branch turnover. Fine root includes fine-root NPP only (no root exudates). Canopy includes litterfall and herbivory.
3.2 Canopy NPP
Canopy productivity was affected after the El Niño event in both the cerradão (F = 2.8, p = 0.01, −16.7 %) and the cerrado (F = 6.7, p < 0.001, −16.2 %) (Fig. 4). However, the NPP of this component had fully recovered within 2 years after the event. When analyzing leaf NPP, the primary component of NPP litterfall, the cerrado exhibited a pattern similar to total NPP litterfall, with a 13.2 % decline in 2016, followed by recovery. In contrast, the cerradão showed a 12 % increase in the year the El Niño began, followed by a 28 % decline in 2016 and subsequent fluctuations in the following years. Notably, in the cerradão, branch (twig) production increased following the event, and, by 2018, its production had doubled compared to previous years.
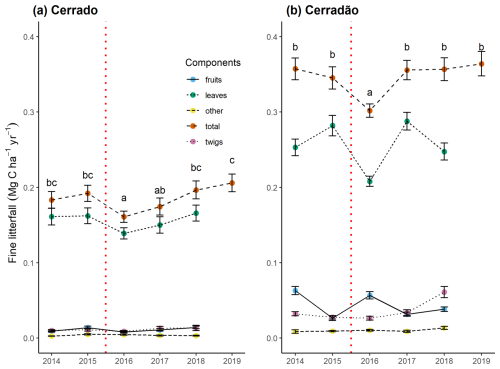
Figure 4Mean monthly productivity in canopy litterfall and its components for the cerrado (a) and cerradão (b) between 2014 and 2019: fruits – flower, fruit, and seed fall; leaves – leaf fall; other – not identified; total – total canopy fine litterfall (as measured in litter traps); and twigs – twig fall (< 2 cm). The error bars represent the standard error. The dashed red line indicates the El Niño periods. Different letters denote significant differences between years in total canopy fine litterfall (Tukey's post hoc test).
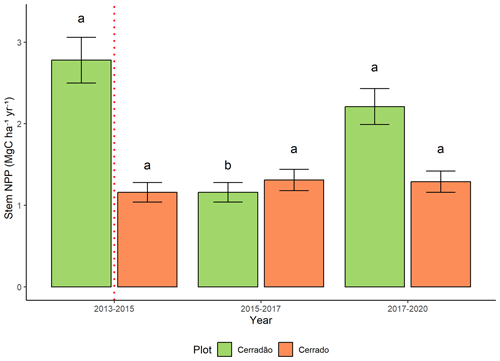
3.3 Stem NPP
In the cerradão, the most affected component was stem net primary productivity (NPP), which declined by 58 % during and after the El Niño (F = 15.6, p < 0.001; Fig. 5). By 2019, it remained 21 % lower than pre-El Niño conditions. This decline was primarily driven by two key species in this transitional forest, Hirtella glandulosa Spreng. and Tachigali vulgaris L.G. Silva and H.C. Lima, which contributed 22 % and 17 % to NPP after the El Niño, respectively. Before the event, T. vulgaris was the dominant contributor to NPP (26 %). In the cerrado, stem productivity was unaffected by the El Niño event (Fig. 5).
3.4 Fine-root NPP
In the cerradão, fine-root net primary productivity (NPPfr) increased significantly (+42 %) during the El Niño event (F = 17.3, p < 0.001) but declined in the following years (Fig. 6). In contrast, the cerrado exhibited the opposite pattern. NPPfr decreased by 38 % during the event (F = 5.6, p = 0.001; Figs. 2 and 6). However, this component re-established itself shortly after the El Niño but experienced another decline of approximately 38 % in 2018.
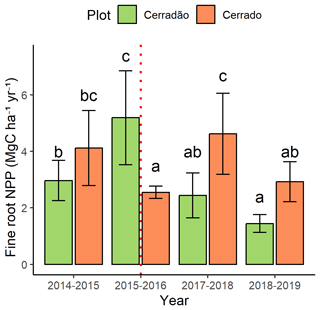
Figure 6Fine-root net primary productivity (NPPfr) for the cerrado (light green) and cerradão (orange) between September 2014 and August 2019. The error bars represent the standard error. The dashed red line indicates the El Niño periods. Different letters denote significant differences between years in each plot (Tukey's post hoc test).
The cerradão and cerrado showed contrasting responses to the 2015–2016 El Niño-associated drought event. The cerrado appears to be more resistant as total NPP and stem NPP were not impacted by the El Niño event, and the components that experienced a reduction (e.g., production of fine roots and canopy productivity) soon re-established themselves. In contrast, the cerradão exhibited lower resistance as all NPP components were affected during the El Niño event, including total NPP and stem NPP. Furthermore, although most components recovered, fine-root production remained significantly lower than pre-event levels (−51 %), and stem production, while not statistically significant, was still 20 % lower. Our findings demonstrate the high sensitivity of the cerradão to extreme drought events.
4.1 Total NPP and its allocation
The decline in total NPP during the El Niño in the cerradão was primarily driven by reduced stem growth (−58 %), followed by a decrease in fine-litter production (−16 %). Each of these parameters will be discussed in detail later. The total productivity of the cerradão was more affected (−29 %) than that of the Amazonian rainforest (−7.6 % to −8.5 %) during the El Niño drought of 2015–2016 (Machado-Silva et al., 2021). Moreover, the reduction in stem productivity was much larger (−58 %; −1.62 Mg C ha−1) than that estimated for tropical forests as a whole (−8.3 % in 1997–1998 and −9 % in 2015–2016, Rifai et al., 2018; −0.40 Mg C ha−1, Bennett et al., 2023). This demonstrates the high sensitivity of this vegetation to climate anomalies.
NPP partitioning between canopy, wood, and fine roots varies substantially within tropical ecosystems (Zhang-Zheng et al., 2024). Reports on NPP partitioning changes under drought were very scarce. Doughty et al. (2014) found that NPP portioning to roots decreases while partitioning to leaves increases during drought. On the other hand, we see such a pattern only very slightly in the cerrado in 2015. Our cerradão site, however, shows a marked decrease in NPP partitioning to wood, which was not observed in Amazonia forests. One possibility is that these shifting strategies reflect points on an aridity continuum from sub-humid Amazonian forest through transitional or seasonally dry forests through to savanna. Alternatively, the differences in soil fertility may play a role, changing the costs and advantages of investment in fine-root production.
4.2 Canopy NPP
The cerradão adopted the strategy of shedding more leaves at the onset of the El Niño. However, both the cerradão and cerrado showed a significant reduction in leaf litter production toward the end of the event (Fig. 4). The observed patterns in leaf litter production suggest that both cerradão and cerrado responded to the extreme drought conditions induced by the 2015–2016 El Niño but with distinct temporal dynamics. The early onset of leaf shedding in the cerradão indicates a shift in its typical phenological strategy, likely as an adaptive response to water stress since full or partial deciduousness, along with strong stomatal regulation, appears to be a common water regulation strategy during the dry season for cerrado species but not for cerradão species (Araújo et al., 2021a; Jancoski et al., 2022). The accelerated leaf abscission at the beginning of the El Niño may have functioned as a short-term mechanism to reduce transpiration and prevent excessive hydraulic stress.
Despite these initial adjustments, both vegetation types exhibited a marked decline in leaf litter production toward the end of the event, suggesting that prolonged drought imposed significant physiological constraints on canopy maintenance. This reduced litterfall could be attributed to a combination of factors, including lower overall canopy productivity, leaf damage resulting from extended drought stress, or a decrease in new-leaf formation. During periods of soil water stress, it is well known that plants often shed their leaves as a strategy to minimize water loss and avoid potential mortality (e.g., Brando et al., 2008). This leaf loss likely contributed to the observed decline in litterfall as the plants prioritize conserving water over maintaining canopy coverage. Interestingly, this leaf loss strategy can also have benefits for nutrient cycling; when leaves drop, the nutrients they contain are released into the litter layer and soil, where they can be reabsorbed by the plants as they re-establish leaf growth after the high-stress period (e.g., Oliveira et al., 2017). Thus, while the reduction in litterfall may initially appear to be detrimental, it can also facilitate nutrient availability for future growth, highlighting the complex interactions between water stress, leaf dynamics, and ecosystem productivity. This response aligns with findings from other tropical and subtropical ecosystems, where extreme drought events disrupt typical phenological cycles and lead to declines in aboveground productivity (Sippel et al., 2018; Duan et al., 2018). The convergence in response at the end of the El Niño highlights the widespread impact of severe climatic anomalies on carbon allocation strategies in the Cerrado biome. While cerradão species initially adjusted by shedding more leaves, the prolonged water deficit ultimately constrained their ability to maintain productivity, leading to reductions in leaf turnover similar to those observed in the cerrado. This suggests that, despite differences in initial strategies, extreme drought events may override ecosystem-specific adaptations, emphasizing the vulnerability of both vegetation types to future increases in climate variability.
4.3 Stem NPP
The results indicating a significant decline in stem net primary productivity (NPP) in the cerradão during and after the El Niño event highlight the vulnerability of this ecosystem to extreme climatic conditions. The 58 % reduction in NPP, along with the continued 21 % decrease by 2019, compared to pre-El Niño conditions suggests that the structural integrity and growth potential of the dominant species in this transitional forest were notably compromised. This decline can be attributed primarily to two dominant species, Hirtella glandulosa and Tachigali vulgaris, which play crucial roles in the ecological dynamics of the cerradão (Reis et al., 2015, 2017). The significant contribution of H. glandulosa to NPP after the El Niño event (22 %) indicates that, while this species was able to maintain some level of productivity, it still suffered under the adverse conditions imposed by the drought. Meanwhile, T. vulgaris, which was the dominant contributor to NPP prior to the event (26 %), experienced a decline in its growth or survivorship, reflecting its sensitivity to prolonged drought stress (Prestes et al., 2024). This shift in species dominance and productivity highlights the intricate interdependencies among species within the cerradão, emphasizing the importance of specific species in maintaining overall forest productivity.
In contrast, the cerrado exhibited a resilience in stem productivity during the same climatic event, with no significant changes noted (F = 1.3, p = 0.28). This resilience may be attributed to the inherent differences in water use strategies between the two ecosystems. The cerrado, characterized by its conservative water use strategies, may have been better adapted to cope with the drought conditions brought about by the El Niño, allowing for sustained stem productivity (Jancoski et al., 2022; Araújo et al., 2023). The contrasting responses of stem NPP between these ecosystems underscore the potential for differential impacts of climate extremes, driven by the distinct ecological strategies employed by their resident species. The decline in stem NPP in the cerradão has implications for carbon storage and overall ecosystem health. As stem productivity is closely linked to biomass accumulation, the reduced NPP could lead to long-term alterations in carbon dynamics within this forest type. Additionally, decreased stem growth may affect the structural complexity of the forest, with potential consequences for habitat provision and biodiversity. The persistent reduction in stem productivity even after the El Niño event suggests a lagged response in the ecosystem's recovery, possibly due to lingering effects of drought stress or nutrient limitations. This highlights the need for further monitoring of these ecosystems to understand recovery trajectories and to inform conservation strategies.
4.4 Fine-root NPP
The observed changes in fine-root net primary productivity (NPPfr) during the El Niño event reveal significant differences in how the cerradão and cerrado ecosystems respond to extreme climatic conditions. In the cerradão, there was a notable increase in fine-root NPPfr of 42 % during the El Niño event (F = 17.3, p < 0.001). This increase suggests that the cerradão, characterized by taller trees and greater leaf area (Araújo et al., 2023), may have adapted to drought conditions by investing more resources into fine-root growth. This response could be a strategy to enhance water absorption capabilities during a period of soil elevated atmospheric demand and potential soil moisture deficits (Metcalfe et al., 2008). However, this strategy does not ameliorate drought risk as tree mortality was high (Prestes et al., 2024) despite a high investment in fine roots. Yet, following the El Niño event, NPPfr in the cerradão declined in subsequent years. This decline may indicate that the initial increase in root production could not be sustained in the long term due to prolonged drought stress or nutrient limitations, leading to a reduction in overall root biomass and productivity.
The cerrado, on the other hand, exhibited an opposite pattern, with a marked reduction in NPPfr during the El Niño. This reduction in fine-root productivity suggests that the cerrado, which typically employs a more conservative water use strategy (Araújo et al., 2021b, 2023), experienced greater stress during the drought. The decrease in fine-root NPPfr may reflect the challenges these species faced in maintaining root function under extreme conditions, resulting in a lower investment in root growth. The strategy observed in the cerrado was similar to that of tropical dry forests, reflecting root phenological patterns linked to water availability (Kummerow et al., 1990; Kavanagh and Kellman, 1992). Interestingly, after the El Niño event, fine-root productivity in the cerrado re-established itself, indicating some level of resilience and recovery. However, this recovery was short-lived as NPPfr experienced another decline of approximately 38 % in 2018. This subsequent decline may be attributed to the residual effects of the El Niño event, including persistent water deficits or nutrient availability issues, which may have hindered the full recovery of fine-root productivity.
Cerradão is an important transitional vegetation type within the Amazon–Cerrado ecotone, connecting two of Brazil's major biomes: the Cerrado and the Amazon. However, this vegetation type is highly vulnerable to climatic events (as shown in the present study), wildfires (Reis et al., 2015; 2017), and windstorms (Reis et al., 2022). One of its most dominant trees, T. vulgaris, which plays a key role in carbon uptake, showed strong sensitivity to El Niño events. Thus, if these extreme drought events continue to become more frequent and intense, the cerradão may release more carbon than it absorbs, consistently with a regional-scale atmospheric result for southeastern Amazonia (Gatti et al., 2021). Moreover, as a transitional zone between the Cerrado and the Amazon, the cerradão plays an important role in maintaining the ecological balance along this interface. Our results suggest that the increasing frequency of El Niño events could disrupt this transition, creating conditions for the progressive degradation of forests along the edges of the Amazon. This highlights the urgent need for actions to mitigate the impacts of climate change in this sensitive region.
The data used to produce the figures are available as a data package on ForestPlots.net: https://doi.org/10.5521/2025_4 (Reis et al., 2025) and in the Supplement. The data used to do the analyses are available on request from ForestPlot.net: https://www.forestplots.net/en/join-forestplots/working-with-data (ForestPlots.net, 2025).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-3949-2025-supplement.
SMR wrote the paper with input from all of the authors (YM, BHM Jr., RF, BSM, HZ, IA, CAJG, EAO, KSP, LJS, ELS, EBS, KPS, MDAG, CALD, OLP, and IOM). YM, BHM Jr., and IOM were involved in planning and supervised the work. SMR, RF, EAO, KSP, LJS, ELS, EBS, KPS, and MDAG performed the field measurements. SMR and HZ performed the analyses and made the figures. IOM and YM provided the funding. All of the authors discussed the results and contributed to the final paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank the team of the Laboratório de Ecologia Vegetal – Plant Ecology Laboratory at the UNEMAT (Universidade do Estado de Mato Grosso) campus in Nova Xavantina, especially Carla Heloísa Luz de Oliveira, Camila Borges, Erica Prestes Ferreira, Luiz Macedo Schuwaab Júnior, Erika Camila Oliveira, Izabel Amorim, Eder Carvalho das Neves, Kelen Alves Cavalheiro, and Poliana Alves Cavalheiro for the help in collecting the field data. We also thank the National Council for Scientific and Technological Development (CNPq) for the financial support of the projects PELD (“Cerrado-Amazonia Transition: ecological and socio-environmental bases for Conservation”, stages II, III, and IV), PPBIO (“Phytogeography of the Amazon-Cerrado Transition Zone”), and FAPEMAT. We also thank the CNPq for the research productivity grant PQ1 to Beatriz Schwantes Marimon and Ben Hur Marimon Junior. Simone Matias Reis was funded by a postdoctoral fellowship from NERC and FAPESP.
This research has been supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant nos. 403725/2012-7, 441244/2016-5, 441572/2020-0, and 457602/2012-0), the Fundação de Amparo à Pesquisa do Estado de Mato Grosso (grant nos. 164131/2013 and 0589267/2016), the Fundação de Amparo à Pesquisa do Estado de São Paulo (grant no. BIO-RED 2015/50517-5), the Natural Environment Research Council (grant no. BIO-RED 2015/50517-5), and UK Research and Innovation (UKRI).
This paper was edited by David Medvigy and reviewed by two anonymous referees.
Alvares, C. A., Stape, J. L., Sentelhas, P. C., Gonçalves, J. D. M., and Sparovek, G.: Köppen's climate classification map for Brazil, Meteorol. Z., 22, 711–728, https://doi.org/10.1127/0941-2948/2013/0507, 2013.
Aragão, L. E. O., Malhi, Y., Roman-Cuesta, R. M., Saatchi, S., Anderson, L. O., and Shimabukuro, Y. E.: Spatial patterns and fire response of recent Amazonian droughts, Geophys. Res. Lett., 34, L07701, https://doi.org/10.1029/2006GL028946, 2007.
Araújo, I., Marimon, B. S., Scalon, M. C., Fauset, S., Junior, B. H. M., Tiwari, R., Galbraith, D. R., and Gloor, M. U.: Trees at the Amazonia-Cerrado transition are approaching high temperature thresholds, Environ. Res. Lett., 16, 034047, https://doi.org/10.1088/1748-9326/abe3b9, 2021a.
Araújo, I., Marimon, B. S., Scalon, M. C., Cruz, W. J., Fauset, S., Vieira, T. C., Galbraith, D. R., and Gloor, M. U.: Intraspecific variation in leaf traits facilitates the occurrence of trees at the Amazonia–Cerrado transition, Flora, 279, 151829, https://doi.org/10.1016/j.flora.2021.151829, 2021b.
Araújo, I., Scalon, M. C., Amorim, I., Menor, I. O., Cruz, W. J., Reis, S. M., Simione, P. F., and Marimon, B. S.: Morpho-anatomical traits and leaf nutrient concentrations vary between plant communities in the Cerrado–Amazonia transition?, Flora, 306, 152366, https://doi.org/10.1016/j.flora.2023.152366, 2023.
Araújo, I., Marimon, B. S., Junior, B. H. M., Oliveira, C. H., Silva, J. W., Beú, R. G., da Silva, I. V., Simioni, P. F., Tavares, J. V., Phillips, O. L., Gloor, M. U., and Galbraith, D. R.: Taller trees exhibit greater hydraulic vulnerability in southern Amazonian forests, Environ. Exp. Bot., 226, 105905, https://doi.org/10.1016/j.envexpbot.2024.105905, 2024.
Ball, R. A.: Ecophysiological leaf traits of Cerrado woody plants, PhD thesis, University of Alberta, 105 pp., https://doi.org/10.7939/R35M2C, 2010.
Bennett, A. C., Sousa, T. R, Monteagudo-Mendoza, A., Esquivel-Muelbert, A., Morandi, P. S., Souza, F. C., Castro, W., Duque, L. F., Llampazo, G. F., dos Santos, R. M., Ramos, E., Torre, E. V., Alvarez-Davila, E., Baker, T. R., Costa, F. R. C., Lewis, S. L., Marimon, B. S., Schietti, J., Burban, B., Berenguer, E., Araujo-Murakami, A., Correa, Z. R., Lopez, W., Santana, F. D., Viscarra, L. J., Elias, F., Vasquez Martinez, R., Marimon-Junior, B. H., Galbraith, D., Sullivan, M. J. P., Emilio, T., Prestes, N. C. C. S., Barlow, J., Fagundes, N. C. A., Oliveira, E. A., Alvarez Loayza, P., Alves, L. F., Vieira, S. A., Maia, V. A., Aragão, L. E. O. C., Arets, E. J. M. M., Arroyo, L., Bánki, O., Baraloto, C., Camargo, P. B., Barroso, J., Bento da Silva, W., Bonal, D., Santos, A. B. M., Brienen, R. J. W., Brown, F., Castilho, C. V., Ribeiro, S. C., Moscoso, V. C., Chavez, E., Comiskey, J. A., Cornejo Valverde, F., Dávila Cardozo, N., de Aguiar-Campos, N., Melo, L. O., del Aguila Pasquel, J., Derroire, G., Disney, M., do Socorro, M. A., Dourdain, A., Feldpausch, T. R., Ferreira, J., Forni Martins, V., Gardner, T., Gloor, E., Sibauty, G. G., Guillen, R., Hase, E., Hérault, B., Honorio Coronado, E. N., Huaraca Huasco, W., Janovec, J. P., Jimenez-Rojas, E., Joly, C., Kalamandeen, M., Killeen, T. J., Farrapo, C. L., Levesley, A., Lizon Romano, L., Lopez Gonzalez, G., Maës dos Santos, F. A., Magnusson, W. E., Malhi, Y., Reis, S. M. A., Melgaço, K., Melo Cruz, O. A., Mendoza Polo, I., Montañez, T., Morel, J. D., Núñez Vargas, M. P., Oliveira de Araújo, R., Pallqui Camacho, N. C., Parada Gutierrez, A., Pennington, T., Pickavance, G. C., Pipoly, J., Pitman, N. C. A., Quesada, C., Ramirez Arevalo, F., Ramírez-Angulo, H., Ramos, R. F., Richardson, J. E., de Souza, C. R., Roopsind, A., Schwartz, G., Silva, R. C., Silva Espejo, J., Silveira, M., Singh, J., Soto Shareva, Y., Steininger, M., Stropp, J., Talbot, J., ter Steege, H., Terborgh, J., Thomas, R., Valenzuela Gamarra, L., van der Heijden, G., van der Hout, P., Zagt, R., and Phillips, O. L.: Sensitivity of South American tropical forests to an extreme climate anomaly, Nat. Clim. Change, 13, 967–974, https://doi.org/10.1038/s41558-023-01776-4, 2023.
Brando, P. M., Nepstad, D. C., Davidson, E. A., Trumbore, S. E., Ray, D., and Camargo, P.: Drought effects on litterfall, wood production and belowground carbon cycling in an Amazon forest: results of a throughfall reduction experiment, Philos. T. Roy. Soc. B, 363, 1839–1848, https://doi.org/10.1098/rstb.2007.0031, 2008.
Comas, L. H., Becker, S. R., Cruz, V. M. V., Byrne, P. F., and Dierig, D. A.: Root traits contributing to plant productivity under drought, Front. Plan. Sci., 4, 442, https://doi.org/10.3389/fpls.2013.00442, 2013.
Covey, K., Soper, F., Pangala, S., Bernardino, A., Pagliaro, Z., Basso, L., Cassol, H., Fearnside, P., Navarrete, D., Novoa, S., Sawakuchi, H., Lovejoy, T., Marengo, J., Peres, C. A., Baillie, J., Bernasconi, P., Camargo, J., Freitas, C., Hoffman, B., Nardoto, G. B., Nobre, I., Mayorga, J., Mesquita, R., Pavan, S., Pinto, F., Rocha, F., de Assis Mello, R., Thuault, A., Bahl, A. A., and Elmore, A.: Carbon and beyond: The biogeochemistry of climate in a rapidly changing Amazon, Front. For. Glob. Change, 4, 618401, https://doi.org/10.3389/ffgc.2021.618401, 2021.
Doughty, C. E., Malhi, Y., Araujo-Murakami, A., Metcalfe, D. B., Silva-Espejo, J. E., Arroyo, L., Heredia, J. P., Pardo-Toledo, E., Mendizabal, L. M., Rojas-Landivar, V. D., Vega-Martinez, M., Flores-Valencia, M., Sibler-Rivero, R., Moreno-Vare, L., Viscarra, L. J., Chuviru-Castro, T., Osinaga-Becerra, M., and Ledezma, R.: Allocation trade-offs dominate the response of tropical forest growth to seasonal and interannual drought, Ecology, 95, 2192–2201, https://doi.org/10.1890/13-1507.1, 2014.
Duan, S., He, H. S., and Spetich, M.: Effects of growing-season drought on phenology and productivity in the west region of central hardwood forests, USA, Forests, 9, 377, https://doi.org/10.3390/f9070377, 2018.
Fan, L., Wigneron, J.-P., Ciais, P., Chave, J., Brandt, M., Fensholt, R., Saatchi, S. S., Bastos, A., Al-Yaari, A., Hufkens, K., Qin, Y., Xiao, X., Chen, C., Myneni, R. B., Fernandez-Moran, R., Mialon, A., Rodriguez-Fernandez, N. J., Kerr, Y., Tian, F., and Peñuelas, J.: Satellite-observed pantropical carbon dynamics, Nat. Plants, 5, 944–951, https://doi.org/10.1038/s41477-019-0478-9, 2019.
Feldpausch, T. R., Phillips, O. L., Brienen, R. J. W., Gloor, E., Lloyd, J., Lopez-Gonzalez, G., Monteagudo-Mendoza, A., Malhi, Y., Alarcón, A., Álvarez Dávila, E., Alvarez-Loayza, P., Andrade, A., Aragao, L. E. O. C., Arroyo, L., Aymard, G. A. C., Baker, T. R., Baraloto, C., Barroso, J., Bonal, D., Castro, W., Chama, V., Chave, J., Domingues, T. F., Fauset, S., Groot, N., Honorio Coronado, E., Laurance, S., Laurance, W. F., Lewis, S. L., Licona, J. C., Marimon, B. S., Marimon-Junior, B. H., Mendoza Bautista, C., Neill, D. A., Oliveira, E. A., Oliveira dos Santos, C., Pallqui Camacho, N. C., Pardo-Molina, G., Prieto, A., Quesada, C. A., Ramírez, F., Ramírez-Angulo, H., Réjou-Méchain, M., Rudas, A., Saiz, G., Salomão, R. P., Silva-Espejo, J. E., Silveira, M., ter Steege, H., Stropp, J., Terborgh, J., Thomas-Caesar, R., van der Heijden, G. M. F., Vásquez Martinez, R., Vilanova, E., and Vos, V. A.: Amazon forest response to repeated droughts, Global Biogeochem. Cy., 30, 964–982, https://doi.org/10.1002/2015GB005133, 2016.
ForestPlots.net, Blundo, C., Carilla, J., Grau, R., Malizia, A., Malizia, L., Osinaga-Acosta, O., Bird, M., Bradford, M., Catchpole, D., Ford, A., Graham, A., Hilbert, D., Kemp, J., Laurance, S., Laurance, W., Ishida, F. Y., Marshall, A., Waite, C., Woell, H., Bastin, J.-F., Bauters, M., Beeckman, H., Boeckx, P., Bogaert, J., De Canniere, C., de Haulleville, T., Doucet, J.-L., Hardy, O., Hubau, W., Kearsley, E., Verbeeck, H., Vleminckx, J., Brewer, S. W., Alarcón, A., Araujo-Murakami, A., Arets, E., Arroyo, L., Chavez, E., Fredericksen, T., Guillén Villaroel, R., Gutierrez Sibauty, G., Killeen, T., Licona, J. C., Lleigue, J., Mendoza, C., Murakami, S., Parada Gutierrez, A., Pardo, G., Peña-Claros, M., Poorter, L., Toledo, M., Villalobos Cayo, J., Viscarra, L. J., Vos, V., Ahumada, J., Almeida, E., Almeida, J., Almeida de Oliveira, E., Alves da Cruz, W., Alves de Oliveira, A., Alvim Carvalho, F., Amorim Obermuller, F., Andrade, A., Antunes Carvalho, F., Vieira, S. A., Aquino, A. C., Aragão, L., Araújo, A. C., Assis, M. A., Aboin Gomes, J. A. M., Baccaro, F., Barbosa de Camargo, P., Barni, P., Barroso, J., Bernacci, L. C., Bordin, K., Brilhante de Medeiros, M., Broggio, I., Camargo, J. L., Cardoso, D., Carniello, M. A., Casarin Rochelle, A. L., Castilho, C., Castro, A. A. J. F., Castro, W., Cerruto Ribeiro, S., Costa, F., Costa de Oliveira, R., Coutinho, I., Cunha, J., da Costa, L., da Costa Ferreira, L., da Costa Silva, R., da Graça Zacarias Simbine, M., de Andrade Kamimura, V., de Lima, H. C., de Oliveira Melo, L., de Queiroz, L., de Sousa Lima, J. R., do Espírito Santo, M., Domingues, T., dos Santos Prestes, N. C., Silva Carneiro, S. E., Elias, F., Eliseu, G., Emilio, T., Farrapo, C. L., Fernandes, L., Ferreira, G., Ferreira, J., Ferreira, L., Ferreira, S., Fragomeni Simon, M., Freitas, M. A., García, Q. S., Manzatto, A. G., Graça, P., Guilherme, F., Hase, E., Higuchi, N., Iguatemy, M., Imbrozio Barbosa, R., Jaramillo, M., Joly, C., Klipel, J., do Amaral, I. L., Levis, C., Lima, A. S., Lima Dan, M., Lopes, A., Madeiros, H., Magnusson, W. E., Manoel dos Santos, R., Marimon, B., Marimon Junior, B. H., Marotti Martelletti Grillo, R., Martinelli, L., Matias Reis, S., Medeiros, S., Meira-Junior, M., Metzker, T., Morandi, P., Moreira do Nascimento, N., Moura, M., Müller, S. C., Nagy, L., Nascimento, H., Nascimento, M., Nogueira Lima, A., Oliveira de Araújo, R., Oliveira Silva, J., Pansonato, M., Pavan Sabino, G., Pedra de Abreu, K. M., Pena Rodrigues, P. J. F., Piedade, M., Rodrigues, D., Rodrigues Pinto, J. R., Quesada, C., Ramos, E., Ramos, R., Rodrigues, P., Rodrigues de Sousa, T., Salomão, R., Santana, F., Scaranello, M., Scarton Bergamin, R., Schietti, J., Schöngart, J., Schwartz, G., Silva, N., Silveira, M., Simão Seixas, C., Simbine, M., Souza, A. C., Souza, P., Souza, R., Sposito, T., Stefani Junior, E., do Vale, J. D., Vieira, I. C. G., Villela, D., Vital, M., Xaud, H., Zanini, K., Zartman, C. E., Ideris, N. K. H., Metali, F. H., Abu Salim, K., Saparudin, M. S., Mat Serudin, R., Sukmaria Sukri, R., Begne, S., Chuyong, G., Djuikouo, M. N., Gonmadje, C., Simo-Droissart, M., Sonké, B., Taedoumg, H., Zemagho, L., Thomas, S., Baya, F., Saiz, G., Silva Espejo, J., Chen, D., Hamilton, A., Li, Y., Luo, T., Niu, S., Xu, H., Zhou, Z., Álvarez-Dávila, E., Andrés Escobar, J. C., Arellano-Peña, H., Cabezas Duarte, J., Calderón, J., Corrales Bravo, L. M., Cuadrado, B., Cuadros, H., Duque, A., Duque, L. F., Espinosa, S. M., Franke-Ante, R., García, H., Gómez, A., González-M., R., Idárraga-Piedrahíta, Á., Jimenez, E., Jurado, R., López Oviedo, W., López-Camacho, R., Melo Cruz, O. A., Mendoza Polo, I., Paky, E., Pérez, K., Pijachi, A., Pizano, C., Prieto, A., Ramos, L., Restrepo Correa, Z., Richardson, J., Rodríguez, E., Rodriguez M., G. M., Rudas, A., Stevenson, P., Chudomelová, M., Dancak, M., Hédl, R., Lhota, S., Svatek, M., Mukinzi, J., Ewango, C., Hart, T., Kasongo Yakusu, E., Lisingo, J., Makana, J.-R., Mbayu, F., Toirambe, B., Tshibamba Mukendi, J., Kvist, L., Nebel, G., Báez, S., Céron, C., Griffith, D. M., Guevara Andino, J. E., Neill, D., Palacios, W., Peñuela-Mora, M. C., Rivas-Torres, G., Villa, G., Demissie, S., Gole, T., Gonfa, T., Ruokolainen, K., Baisie, M., Bénédet, F., Betian, W., Bezard, V., Bonal, D., Chave, J., Droissart, V., Gourlet-Fleury, S., Hladik, A., Labrière, N., Naisso, P., Réjou-Méchain, M., Sist, P., Blanc, L., Burban, B., Derroire, G., Dourdain, A., Stahl, C., Nssi Bengone, N., Chezeaux, E., Evouna Ondo, F., Medjibe, V., Mihindou, V., White, L., Culmsee, H., Durán Rangel, C., Horna, V., Wittmann, F., Adu-Bredu, S., Affum-Baffoe, K., Foli, E., Balinga, M., Roopsind, A., Singh, J., Thomas, R., Zagt, R., Murthy, I. K., Kartawinata, K., Mirmanto, E., Priyadi, H., Samsoedin, I., Sunderland, T., Yassir, I., Rovero, F., Vinceti, B., Hérault, B., Aiba, S.-I., Kitayama, K., Daniels, A., Tuagben, D., Woods, J. T., Fitriadi, M., Karolus, A., Khoon, K. L., Majalap, N., Maycock, C., Nilus, R., Tan, S., Sitoe, A., Coronado G., I., Ojo, L., de Assis, R., Dalberg Poulsen, A., Sheil, D., Arévalo Pezo, K., Buttgenbach Verde, H., Chama Moscoso, V., Cordova Oroche, J. C., Cornejo Valverde, F., Corrales Medina, M., Davila Cardozo, N., de Rutte Corzo, J., del Aguila Pasquel, J., Flores Llampazo, G., Freitas, L., Galiano Cabrera, D., García Villacorta, R., Garcia Cabrera, K., García Soria, D., Gatica Saboya, L., Grandez Rios, J. M., Hidalgo Pizango, G., Honorio Coronado, E., Huamantupa-Chuquimaco, I., Huaraca Huasco, W., Huillca Aedo, Y. T., Marcelo Peña, J. L., Monteagudo Mendoza, A., Moreano Rodriguez, V., Núñez Vargas, P., Palacios Ramos, S. C., Pallqui Camacho, N., Peña Cruz, A., Ramirez Arevalo, F., Reyna Huaymacari, J., Reynel Rodriguez, C., Paredes, M. A. R., Bayona, L. R., Gonzales, R. d. P. R., Peña, M. E. R., Revilla, N. S., Shareva, Y. C. S., Trujillo, R. T., Gamarra, L. V., Martinez, R. V., Arenas, J. V., Amani, C., Ifo, S. A., Bocko, Y., Boundja, P., Ekoungoulou, R., Hockemba, M., Nzala, D., Fofanah, A., Taylor, D., Dios, G. B., Cayuela, L., Cerda, Í. G. l., Macía, M., Stropp, J., Playfair, M., Wortel, V., Gardner, T., Muscarella, R., Priyadi, H., Rutishauser, E., Chao, K., Munishi, P., Bánki, O., Bongers, F., Boot, R., Fredriksson, G., Reitsma, J., Steege, H. t., Andel, T. v., Meer, P. v. d., Hout, P. v. d., Nieuwstadt, M. v., Ulft, B. v., Veenendaal, E., Vernimmen, R., Zuidema, P., Zwerts, J., Akite, P., Bitariho, R., Chapman, C., Gerald, E., Leal, M., Mucunguzi, P., Abernethy, K., Alexiades, M., Baker, T. R., Banda, K., Banin, L., Barlow, J., Bennett, A., Berenguer, E., Berry, N., Bird, N. M., Blackburn, G. A., Brearley, F., Brienen, R., Burslem, D., Carvalho, L., Cho, P., Coelho, F., Collins, M., Coomes, D., Cuni-Sanchez, A., Dargie, G., Dexter, K., Disney, M., Draper, F., Duan, M., Esquivel-Muelbert, A., Ewers, R., Fadrique, B., Fauset, S., Feldpausch, T. R., França, F., Galbraith, D., Gilpin, M., Gloor, E., Grace, J., Hamer, K., Harris, D., Jeffery, K., Jucker, T., Kalamandeen, M., Klitgaard, B., Levesley, A., Lewis, S. L., Lindsell, J., Lopez-Gonzalez, G., Lovett, J., Malhi, Y., Marthews, T., McIntosh, E., Melgaço, K., Milliken, W., Mitchard, E., Moonlight, P., Moore, S., Morel, A., Peacock, J., Peh, K. S., Pendry, C., Pennington, R. T., Pereira, L. d. O., Peres, C., Phillips, O. L., Pickavance, G., Pugh, T., Qie, L., Riutta, T., Roucoux, K., Ryan, C., Sarkinen, T., Valeria, C. S., Spracklen, D., Stas, S., Sullivan, M., Swaine, M., Talbot, J., Taplin, J., Heijden, G. v. d., Vedovato, L., Willcock, S., Williams, M., Alves, L., Loayza, P. A., Arellano, G., Asa, C., Ashton, P., Asner, G., Brncic, T., Brown, F., Burnham, R., Clark, C., Comiskey, J., Damasco, G., Davies, S., Fiore, T. D., Erwin, T., Farfan-Rios, W., Hall, J., Kenfack, D., Lovejoy, T., Martin, R., Montiel, O. M., Pipoly, J., Pitman, N., Poulsen, J., Primack, R., Silman, M., Steininger, M., Swamy, V., Terborgh, J., Thomas, D., Umunay, P., Uriarte, M., Torre, E. V., Wang, O., Young, K., Aymard, G. A., Hernández, L., Fernández, R. H., Ramírez-Angulo, H., Salcedo, P., Sanoja, E., Serrano, J., Torres-Lezama, A., Le, T. C., Le, T. T., and Tran, H. D.: Taking the pulse of Earth's tropical forests using networks of highly distributed plots, Biol. Conserv., 260, 108849, https://doi.org/10.1016/j.biocon.2020.108849, 2021.
ForestPlots.net: WORKING WITH DATA, ForestPlot.net [data set], https://www.forestplots.net/en/join-forestplots/working-with-data, last access: 9 August 2025.
Galbraith, D., Malhi, Y., Affum-Baffoe, K., Castanho, A. D., Doughty, C. E., Fisher, R. A., Lewis, S. L., Peh, K. S.-H., Phillips, O. L., Quesada, C. A., Sonké, B., and Lloyd, J.: Residence times of woody biomass in tropical forests, Plant Ecol. Divers., 6, 139–157, https://doi.org/10.1080/17550874.2013.770578, 2013.
Gatti, L. V., Basso, L. S., Miller, J. B., Gloor, M., Domingues, L. G., Cassol, H. L. G., Tejada, G., Aragão, L. E. O. C., Nobre, C., Peters, W., Marani, L., Arai, E., Sanches, A. H., Corrêa, S. M., Anderson, L., Von Randow, C., Correia, C. S. C., Crispim, S. P., and Neves, R. A.: Amazonia as a carbon source linked to deforestation and climate change, Nature, 595, 388–393, https://doi.org/10.1038/s41586-021-03629-6, 2021.
Gloor, E., Wilson, C., Chipperfield, M. P., Chevallier, F., Buermann, W., Boesch, H., Parker, R., Somkuti, P., Gatti, L. V., Correia, C., Domingues, L. G., Peters, W., Miller, J., Deeter, M. N., and Sullivan, M. J.: Tropical land carbon cycle responses to 2015/16 El Niño as recorded by atmospheric greenhouse gas and remote sensing data, Philos. T. Roy. Soc. B, 373, 20170302, https://doi.org/10.1098/rstb.2017.0302, 2018.
Harmon, M. E., Whigham, D. F., Sexton, J., and Olmsted, I.: Decomposition and mass of woody detritus in the dry tropical forests of the northeastern Yucatan Peninsula, Mexico, Biotropica, 27, 305–316, 1995.
Hughes, I. G. and Hase, T. P. A. (Eds.): Measurements and their uncertainties: A practical guide to modern error analysis, Oxford University Press, New York, 153 pp., ISBN 9780199566327, 2010.
Jancoski, H. S.: Características morfofuncionais de árvores em resposta à sazonalidade climática e herbivoria na transição Cerrado-Amazônia, PhD thesis, Universidade do Estado de Mato Grosso, 89 pp., https://portal.unemat.br/media/files/halina-soares.pdf (last access: 9 August 2025), 2019.
Jancoski, H. S., Schwantes Marimon, B., Scalon, M. C., de V. Barros, F., Marimon-Junior, B. H., Carvalho, E., Oliveira, R. S., and Oliveras Menor, I.: Distinct leaf water potential regulation of tree species and vegetation types across the Cerrado–Amazonia transition, Biotropica, 54, 431–443, https://doi.org/10.1111/btp.13064, 2022.
Jiménez-Muñoz, J. C., Mattar, C., Barichivich, J., Santamaría-Artigas, A., Takahashi, K., Malhi, Y., Sobrino, J. A., and Van Der Schrier, G.: Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016, Sci. Rep., 6, 1–7, https://doi.org/10.1038/srep33130, 2016.
Kavanagh, T., and Kellman M.: Seasonal Pattern of Fine Root Proliferation in a Tropical Dry Forest, Biotropica, 24, 157–165, https://doi.org/10.2307/2388669, 1992.
Kummerow, J., Castillanos, J., Maas, M., and Larigauderie, A.: Production of fine roots and the seasonality of their growth in a Mexican deciduous dry forest, Vegetatio, 90, 73–80, https://doi.org/10.1007/BF00045590, 1990.
Liu, J., Bowman, K. W., Schimel, D. S., Parazoo, N. C., Jiang, Z., Lee, M., Bloom, A. A., Wunch, D., Frankenberg, C., Sun, Y., O'Dell, C. W., Gurney, K. R., Menemenlis, D., Gierach, M., Crisp, D., and Eldering, A.: Contrasting carbon cycle responses of the tropical continents to the 2015–2016 El Niño, Science, 358, eaam5690, https://doi.org/10.1126/science.aam5690, 2017.
Machado-Silva, F., Peres, L. F., Gouveia, C. M., Enrich-Prast, A., Peixoto, R. B., Pereira, J. M., Marotta, H., Fernandes, P. J. F., and Libonati, R.: Drought resilience debt drives NPP decline in the Amazon Forest, Global Biogeochem. Cy., 35, e2021GB007004, https://doi.org/10.1029/2021GB007004, 2021.
Malhi, Y., Aragao, L. E. O., Metcalfe, D. B., Paiva, R., Quesada, C. A., Almeida, S., Anderson, L., Brando, P., Chambers, J. Q., da Costa, A. C. L., Hutyra, L. R., Oliveira, P., Patiño, S., Pyle, E. H., Robertson, A. L., and Teixeira, L. M.: Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests, Glob. Change Biol., 15, 1255–1274, https://doi.org/10.1111/j.1365-2486.2008.01780.x, 2009.
Malhi, Y., Doughty, C. E., Goldsmith, G. R., Metcalfe, D. B., Girardin, C. A., Marthews, T. R., del Aguila-Pasquel, J., Aragão, L. E. O. C., Araujo-Murakami, A., Brando, P., da Costa, A. C. L., Silva-Espejo, J. E., Farfán Amézquita, F., Galbraith, D. R., Quesada, C. A., Rocha, W., Salinas-Revilla, N., Silvério, D., Meir, P., and Phillips, O. L.: The linkages between photosynthesis, productivity, growth and biomass in lowland Amazonian forests, Glob. Change Biol., 21, 2283–2295, https://doi.org/10.1111/gcb.12859, 2015.
Malhi, Y., Girardin, C. A., Goldsmith, G. R., Doughty, C. E., Salinas, N., Metcalfe, D. B., Huaraca Huasco, W., Silva-Espejo, J. E., del Aguilla-Pasquell, J., Farfán Amézquita, F., Aragão, L. E. O. C., Guerrieri, R., Ishida, F. Y., Bahar, N. H. A., Farfan-Rios, W., Phillips, O. L., Meir, P., and Silman, M.: The variation of productivity and its allocation along a tropical elevation gradient: a whole carbon budget perspective, New Phytol., 214, 1019–1032, https://doi.org/10.1111/nph.14189, 2017.
Malhi, Y., Girardin, C., Metcalfe, D. B., Doughty, C. E., Aragão, L. E., Rifai, S. W., Oliveras, I., Shenkin, A., Aguirre-Gutiérrez, J., Dahlsjö, C. A. L., Riutta, T., Berenguer, E., Moore, S., Huaraca Huasco, W., Salinas, N., Da Costa, A. C. L., Bentley, L. P., Adu-Bredu, S., Marthews, T. R., Meir, P., and Phillips, O. L.: The Global Ecosystems Monitoring network: Monitoring ecosystem productivity and carbon cycling across the tropics, Biol. Conserv., 253, 108889, https://doi.org/10.1016/j.biocon.2020.108889, 2021.
Marimon, B. S., Lima, E. S., Duarte, T. G., Chieregatto, L. C., and Ratter, J. A.: Observations on the vegetation of Northeastern Mato Grosso, Brazil. IV. An analysis of the Cerrado-Amazonian Forest ecotone, Edinb. J. Bot., 63, 323–341, https://doi.org/10.1017/S0960428606000576, 2006.
Marimon, B. S., Marimon-Junior, B. H., Feldpausch, T. R., Oliveira-Santos, C., Mews, H. A., Lopez-Gonzalez, G., Lloyd, J., Franczak, D. D., de Oliveira, E. A., Maracahipes, L., Miguel, A., Lenza, E., and Phillips, O. L.: Disequilibrium and hyperdynamic tree turnover at the forest–cerrado transition zone in southern Amazonia, Plant Ecol. Divers., 7, 281–292, https://doi.org/10.1080/17550874.2013.818072, 2014.
Marimon Junior, B. H. and Haridasan, M.: Comparação da vegetação arbórea e características edáficas de um cerradão e um cerrado sensu stricto em áreas adjacentes sobre solo distrófico no leste de Mato Grosso, Brasil, Acta Bot. Bras., 19, 913–926, https://doi.org/10.1590/S0102-33062005000400026, 2005.
Marques, E. Q., Marimon-Junior, B. H., Marimon, B. S., Matricardi, E. A., Mews, H. A., and Colli, G. R.: Redefining the Cerrado–Amazonia transition: implications for conservation, Biodivers. Conserv., 29, 1501–1517, https://doi.org/10.1007/s10531-019-01720-z, 2020.
Marthews, T. R., Riutta, T., Oliveras-Menor, I., Urrutia, R., Moore, S., Metcalfe, D., Malhi, Y., Phillips, O., Huaraca Huasco, W., Ruiz Jaén, M., Girardin, C., Butt, N., and Cain, R.: Measuring Tropical Forest Carbon Allocation and Cycling: A RAINFOR-GEM Field Manual for Intensive Census Plots (v3.0), Global Ecosystems Monitoring Network, Oxford, 121 pp., https://www.researchgate.net/publication/273448136_Measuring_Tropical_Forest_Carbon_Allocation_and_Cycling_v30 (last access: 9 August 2025), 2014.
Matricardi, E. A. T., Skole, D. L., Costa, O. B., Pedlowski, M. A., Samek, J. H., and Miguel, E. P.: Long-term forest degradation surpasses deforestation in the Brazilian Amazon, Science, 369, 1378–1382, https://doi.org/10.1126/science.abb3021, 2020.
Metcalfe, D. B., Meir, P., Aragao, L. E. O. C., Malhi, Y., Da Costa, A. C. L., Braga, A., Gonçalves, P. H. L., de Athaydes, J., de Almeida, S. S., and Williams, M.: Factors controlling spatio-temporal variation in carbon dioxide efflux from surface litter, roots, and soil organic matter at four rain forest sites in the eastern Amazon, J. Geophys. Res.-Biogeo., 112, G04001, https://doi.org/10.1029/2007JG000443, 2007.
Metcalfe, D. B., Meir, P., Aragão, L. E. O., da Costa, A. C., Braga, A. P., Gonçalves, P. H. L., Silva Junior, J. A., de Almeida, S. S., Dawson, L. A., Malhi, Y., and Williams, M.: The effects of water availability on root growth and morphology in an Amazon rainforest, Plant Soil, 311, 189–199, https://doi.org/10.1007/s11104-008-9670-9, 2008.
Miranda, S. D. C., Bustamante, M., Palace, M., Hagen, S., Keller, M., and Ferreira, L. G.: Regional variations in biomass distribution in Brazilian savanna woodland, Biotropica, 46, 125–138, https://doi.org/10.1111/btp.12095, 2014.
Morandi, P. S., Marimon-Junior, B. H., De Oliveira, E. A., Reis, S. M., Valadão, M. X., Forsthofer, M., Passos, F. B., and Marimon, B. S.: Vegetation succession in the Cerrado–Amazonian forest transition zone of Mato Grosso state, Brazil, Edinb. J. Bot., 73, 83–93, https://doi.org/10.1017/S096042861500027X, 2015.
Morandi, P. S., Marimon, B. S., Eisenlohr, P. V., Marimon-Junior, B. H., Oliveira-Santos, C., Feldpausch, T. R., de Oliveira, E. A., Reis, S. M., Lloyd, J., and Phillips, O. L.: Patterns of tree species composition at watershed-scale in the Amazon “arc of deforestation”: implications for conservation, Environ. Conserv., 43, 317–326, https://doi.org/10.1017/S0376892916000278, 2016.
Neyret, M., Bentley, L. P., Oliveras, I., Marimon, B. S., Marimon-Junior, B. H., de Oliveira, E. A., Passos, F. B., Ccoscco, R. C., dos Santos, J., Reis, S. M., Morandi, P. S., Paucar, G. R., Robles Cáceres, A., Valdez Tejeira, Y., Yllanes Choque, Y., Salinas, N., Shenkin, A., Asner, G. P., Díaz, S., Enquist, B. J., and Malhi, Y.: Examining variation in the leaf mass per area of dominant species across two contrasting tropical gradients in light of community assembly, Ecol. Evol., 6, 5674–5689, https://doi.org/10.1002/ece3.2281, 2016.
Nogueira, D. S., Marimon, B. S., Marimon-Junior, B. H., Oliveira, E. A., Morandi, P., Reis, S. M., Elias, F., Neves, E. C., Feldpausch, T. R., and Phillips, O. L.: Impacts of Fire on Forest Biomass Dynamics at the Southern Amazon Edge, Environ. Conserv. 46, 285–292, https://doi.org/10.1017/S0376892919000110, 2019.
Oliveira, B., Marimon Junior, B. H., Mews, H. A., Valadão, M. B. X., and Marimon, B. S.: Unraveling the ecosystem functions in the Amazonia–Cerrado transition: evidence of hyperdynamic nutrient cycling, Plant Ecol., 218, 225–239, https://doi.org/10.1007/s11258-016-0681-y, 2017.
Oliveras, I. and Malhi, Y.: Many shades of green: the dynamic tropical forest–savannah transition zones, Philos. T. Roy. Soc. B, 371, 20150308, https://doi.org/10.1098/rstb.2015.0308, 2016.
Olson, M. E., Soriano, D., Rosell, J. A., Anfodillo, T., Donoghue, M. J., Edwards, E. J., León-Gómez, C., Dawson, T., Camarero Martínez, J. J., Castorena, M., Echeverría, A., Espinosa, C. I., Fajardo, A., Gazol, A., Isnard, S., Lima, R. S., Marcati, C. R., and Méndez-Alonzo, R.: Plant height and hydraulic vulnerability to drought and cold, P. Natl. Acad. Sci. USA, 115, 7551–7556, https://doi.org/10.1073/pnas.1721728115, 2018.
Palmer, P. I.: The role of satellite observations in understanding the impact of El Nino on the carbon cycle: current capabilities and future opportunities, Philos. T. Roy. Soc. B, 373, 20170407, https://doi.org/10.1098/rstb.2017.0407, 2018.
Patiño, S., Fyllas, N. M., Baker, T. R., Paiva, R., Quesada, C. A., Santos, A. J. B., Schwarz, M., ter Steege, H., Phillips, O. L., and Lloyd, J.: Coordination of physiological and structural traits in Amazon forest trees, Biogeosciences, 9, 775–801, https://doi.org/10.5194/bg-9-775-2012, 2012.
Peixoto, K. S., Marimon-Junior, B. H., Marimon, B. S., Elias, F., de Farias, J., Freitag, R., Mews, E. A., Neves, E. C., Prestes, N. C. C. S., and Malhi, Y.: Unravelling ecosystem functions at the Amazonia-Cerrado transition: II. Carbon stocks and CO2 soil efflux in cerradao forest undergoing ecological succession, Acta Oecol., 82, 23–31, https://doi.org/10.1016/j.actao.2017.05.005, 2017.
Peixoto, K. D. S., Marimon-Junior, B. H., Cavalheiro, K. A., Silva, N. A., das Neves, E. C., Freitag, R., Mews, E. A., Valadao, M. B. X., and Marimon, B. S.: Assessing the effects of rainfall reduction on litterfall and the litter layer in phytophysiognomies of the Amazonia–Cerrado transition, Braz. J. Bot., 41, 589–600, https://doi.org/10.1007/s40415-018-0443-2, 2018.
Pérez-Ramos, I. M., Volaire, F., Fattet, M., Blanchard, A., and Roumet, C.: Tradeoffs between functional strategies for resource-use and drought-survival in Mediterranean rangeland species, Environ. Exp. Bot., 87, 126–136, https://doi.org/10.1016/j.envexpbot.2012.09.004, 2013.
Phillips, O. L., Aragão, L. E. O. C., Lewis, S. L., Fisher, J. B., Lloyd, J., López-González, G., Malhi, Y., Monteagudo, A., Peacock, J., Quesada, C. A., van der Heijden, G., Almeida, S., Amaral, I., Arroyo, L., Aymard, G., Baker, T. R., Bánki, O., Blanc, L., Bonal, D., Brando, P., Chave, J., de Oliveira, Á. C. A., Dávila Cardozo, N., Czimczik, C. I., Feldpausch, T. R., Freitas, M. A., Gloor, E., Higuchi, N., Jiménez, E., Lloyd, G., Meir, P., Mendoza, C., Morel, A., Neill, D. A., Nepstad, D., Patiño, S., Peñuela, M. C., Prieto, A., Ramírez, F., Schwarz, M., Silva, J., Silveira, M., Thomas, A. S., ter Steege, H., Stropp, J., Vásquez, R., Zelazowski, P., Álvarez Dávila, E., Andelman, S., Andrade, A., Chao, K.-J., Erwin, T., Di Fiore, A., Honorio C., E., Keeling, H., Killeen, T. J., Laurance, W. F., Peña Cruz, A., Pitman, N. C. A., Núñez Vargas, P., Ramírez-Angulo, H., Rudas, A., Salomão, R., Silva, N., Terborgh, J., and Torres-Lezama, A.: Drought sensitivity of the Amazon rainforest, Science, 323, 1344–1347, https://doi.org/10.1126/science.1164033, 2009.
Powers, J. S., Vargas G. G., Brodribb, T. J., Schwartz, N. B., Pérez-Aviles, D., Smith-Martin, C. M., Becknell, J. M., Aureli, F., Blanco, R., Calderón-Morales, E., Calvo-Alvarado, J. C., Calvo-Obando, A. J., Chavarría, M. M., Carvajal-Vanegas, D., Jiménez-Rodríguez, C. D., Chacon, E. M., Schaffner, C. M., Werden, L. K., Xu, X., and Medvigy, D.: A catastrophic tropical drought kills hydraulically vulnerable tree species, Glob. Change Biol., 26, 3122–3133, https://doi.org/10.1111/gcb.15037, 2020.
Prestes, N. C., Marimon, B. S., Morandi, P. S., Reis, S. M., Marimon Junior, B. H., Cruz, W. J., Oliveira, E. A., Lucas H. Mariano, L. H., Elias, F., Santos, D. M., Esquivel-Muelbert, A., and Phillips, O. L.: Impact of the extreme 2015–16 El Niño climate event on forest and savanna tree species of the Amazonia-Cerrado transition, Flora, 319, 152597, https://doi.org/10.1016/j.flora.2024.152597, 2024.
Ratter, J. A., Richards, P. W., Argent, G., and Gifford, D. R.: Observations on the vegetation of northeastern Mato Grosso: I. The woody vegetation types of the Xavantina-Cachimbo Expedition area, Philos. T. Roy. Soc. B, 266, 449–492, https://doi.org/10.1098/rstb.1973.0053, 1973.
Reis, S. M., Lenza, E., Marimon, B. S., Gomes, L., Forsthofer, M., Morandi, P. S., Marimon Junior, B. H., Feldpausch, T. R., and Elias, F.: Post-fire dynamics of the woody vegetation of a savanna forest (Cerradão) in the Cerrado-Amazon transition zone, Acta Bot. Bras., 29, 408–416, https://doi.org/10.1590/0102-33062015abb0009, 2015.
Reis, S. M., de Oliveira, E. A., Elias, F., Gomes, L., Morandi, P. S., Marimon, B. S., Marimon Junior, B. H., Neves, E. C., Oliveira, O., and Lenza, E.: Resistance to fire and the resilience of the woody vegetation of the “Cerradão” in the “Cerrado”–Amazon transition zone, Braz. J. Bot., 40, 193–201, https://doi.org/10.1007/s40415-016-0336-1, 2017.
Reis, S. M., Marimon, B. S., Marimon Junior, B. H., Morandi, P. S., Oliveira, E. A. D., Elias, F., and Phillips, O. L.: Climate and fragmentation affect forest structure at the southern border of Amazonia, Plant Ecol. Divers., 11, 13–25, 2018.
Reis, S. M., Marimon, B. S., Esquivel-Muelbert, A., Marimon Junior, B. H., Morandi, P. S., Elias, F., de Oliveira, E. A., Galbraith, D., Feldpausch, T. R., Menor, I. O., Malhi, Y., and Phillips, O. L.: Climate and crown damage drive tree mortality in southern Amazonian edge forests, J. Ecol., 110, 876–888, https://doi.org/10.1111/1365-2745.13849, 2022.
Reis, S. M., Malhi, Y., Marimon Junior, B. H., Marimon, B. S., Zhang-Zheng, H., Araújo, I., Freitag, R., de Oliveira, E. A., Peixoto, K. S., Souza, L. J., Souza da Silva, E. L., Bernardes Santos, E., Silva, K. P., Gonçalves, M. D. A., Girardin, C., Dahlsjö, C., Phillips, O., and Oliveras Menor, I.: Datapackage from: Sensitivity of tropical woodland savannas to El Niño droughts, ForestPlots.net [data set], https://doi.org/10.5521/2025_4, 2025.
Rezende, A. V., Vale, A. D., Sanquetta, C. R., Figueiredo Filho, A., and Felfili, J. M.: Comparação de modelos matemáticos para estimativa do volume, biomassa e estoque de carbono da vegetação lenhosa de um cerrado sensu stricto em Brasília, DF, Sci. For., 71, 65–73, https://doi.org/10.5902/1980509827065, 2006.
Ribeiro, J. F. and Walter, B. M. T.: As principais fitofisionomias do bioma Cerrado, in: Cerrado Ecologia e Fauna, edited by: Sano, S. M., Almeida, S. P., and Ribeiro, J. F., Embrapa Informação Tecnológico, Brasília, 153–221, ISBN 9788573833973, 2008.
Rifai, S. W., Girardin, C. A. J., Berenguer, E., del Aguila-Pasquel, J., Dahlsjö, C. A. L., Doughty, C. E., Jeffery, K. J., Moore, S., Oliveras, I., Riutta, T., Rowland, L. M., Araujo Murakami, A., Addo-Danso, S. D., Brando, P., Burton, C., Evouna Ondo, F., Duah-Gyamfi, A., Farfán Amézquita, F., Freitag, R., Hancco Pacha, F., Huaraca Huasco, W., Ibrahim, F., Mbou, A. T., Mihindou Mihindou, V., Peixoto, K. S., Rocha, W., Rossi, L. C., Seixas, M., Silva-Espejo, J. E., Abernethy, K. A., Adu-Bredu, S., Barlow, J., da Costa, A. C. L., Marimon, B. S., Marimon-Junior, B. H., Meir, P., Metcalfe, D. B., Phillips, O. L., White, L. J. T., and Malhi, Y.: ENSO Drives interannual variation of forest woody growth across the tropics, Philos. T. R. Soc. B, 373, 20170410, https://doi.org/10.1098/rstb.2017.0410, 2018.
Riutta, T., Malhi, Y., Kho, L. K., Marthews, T. R., Huaraca Huasco, W., Khoo, M., Tan, S., Turner, E., Reynolds, G., Both, S., Burslem, D. F. R. P., Teh, Y. A., Vairappan, C. S., Majalap, N., and Ewers, R. M.: Logging disturbance shifts net primary productivity and its allocation in Bornean tropical forests, Glob. Change Biol., 24, 2913–2928, https://doi.org/10.1111/gcb.14068, 2018.
Scalon, M. C., Oliveras Menor, I., Freitag, R., Peixoto, K. S., Rifai, S. W., Marimon, B. S., Marimon Junior, B. H., and Malhi, Y.: Contrasting strategies of nutrient demand and use between savanna and forest ecosystems in a neotropical transition zone, Biogeosciences, 19, 3649–3661, https://doi.org/10.5194/bg-19-3649-2022, 2022.
Silvério, D. V., Brando, P. M., Bustamante, M. M., Putz, F. E., Marra, D. M., Levick, S. R., and Trumbore, S. E.: Fire, fragmentation, and windstorms: A recipe for tropical forest degradation, J. Ecol., 107, 656–667, https://doi.org/10.1111/1365-2745.13076, 2019.
Sippel, S., Reichstein, M., Ma, X., Mahecha, M. D., Lange, H., Flach, M., and Frank, D.: Drought, heat, and the carbon cycle: a review, Curr. Clim. Change Rep., 4, 266–286, https://doi.org/10.1007/s40641-018-0103-4, 2018.
Terra, M. C., Nunes, M. H., Souza, C. R., Ferreira, G. W., do Prado-Junior, J. A., Rezende, V. L., Maciel, R., Mantovani, V., Rodrigues, A., Augusto Morais, V. A., Scolforo, J. R. S., and de Mello, J. M.: The inverted forest: Aboveground and notably large belowground carbon stocks and their drivers in Brazilian savanas, Sci. Total Environ., 867, 161320, https://doi.org/10.1016/j.scitotenv.2022.161320, 2023.
Zhang-Zheng, H., Adu-Bredu, S., Duah-Gyamfi, A., Moore, S., Addo-Danso, S. D., Amissah, L., Valentini, R., Djagbletey, G., Anim-Adjei, K., Quansah, J., Sarpong, B., Owusu-Afriyie, K., Gvozdevaite, A., Tang, M., Ruiz-Jaen, M. C., Ibrahim, F., Girardin, C. A. J., Rifai, S., Dahlsjö, C. A. L., Riutta, T., Deng, X., Sun, Y., Prentice, I. C., Oliveras Menor, I., and Malhi, Y.: Contrasting carbon cycle along tropical forest aridity gradients in West Africa and Amazonia, Nat. Commun., 15, 3158, https://doi.org/10.1038/s41467-024-47202-x, 2024.