the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Methanogenesis by CO2 reduction dominates lake sediments with different organic matter compositions
Guangyi Su
Julie Tolu
Clemens Glombitza
Jakob Zopfi
Moritz F. Lehmann
Mark A. Lever
Carsten J. Schubert
Microbial methane production is a key reaction involved in the terminal step of anaerobic degradation of organic matter. The energy substrates of methane-producing microorganisms are largely generated during the breakdown of larger organic molecules by fermentative microorganisms, wherein the products of fermentation may vary with the chemical compositions of these larger molecules. Due to differences in energy substrates among methane-producing microorganisms, it is thus possible that organic matter compositional variations select for different communities of methane producers. Here, we investigate the sources and compositions of OC in sediments of Lake Geneva and how both are potentially linked to methane production. Differences in dominant long-chain fatty acid abundances and carbon isotopic compositions suggest the predominance of diagenetically altered phytoplankton-derived OC at a profundal site (PS) and temporally highly variable sources of both aquatic and terrestrial OC in a deltaic location. Despite these differences, radiotracer-based methanogenesis rate measurements and stable isotopic signatures of methane indicate significant methane production that is dominated by CO2 reduction (>95 % of total methanogenesis) in both locations. Matching this interpretation, members of well-known CO2-reducing Methanoregula sp. dominate both sites. Similarly, no clear effect of OC source on methane production rates was evident. Our data demonstrate that OC of diverse sources and diagenetic states supports microbial methane production, but the data do not indicate a clear impact of the OC source on the dominant methanogenic pathway or the community structure of methanogenic microorganisms in lacustrine sediments.
- Article
(2827 KB) - Full-text XML
-
Supplement
(784 KB) - BibTeX
- EndNote
Lakes represent an important source of methane (CH4) emissions to the atmosphere (Bastviken et al., 2011). Methane is a potent greenhouse gas with a global warming potential more than 27 times that of carbon dioxide on a 100-year basis (GWP-100) (IPCC, 2021). Although recent evidence suggests that various living organisms can produce methane under oxic conditions (Ernst et al., 2022), it is still believed that most methane in lake sediments is produced via the anaerobic decomposition of organic carbon (OC) through a process known as canonical methanogenesis. Canonical methanogenesis (from here on referred to as methanogenesis) is the final step in the degradation of organic matter and is catalysed by anaerobic archaea, capable of using several substrates including H2CO2, acetate, and methylated compounds (Lyu et al., 2018). It is still poorly understood how methane production in lake sediments is regulated and to what extent the methanogenic potential is related to OC quality. In this regard, microbial organic matter degradation reactions (e.g. hydrolysis, fermentation, and anaerobic oxidation) play a critical role by sequentially breaking down organic macromolecules, such as proteins, carbohydrates, and lipids, to acetate and H2CO2, which are then used as substrates by methanogenic archaea (Demirel and Scherer, 2008).
Freshwater lakes cover only a small portion of the Earth's surface (<3 %) (Downing et al., 2006) compared to oceans (71 %), yet, the annually accumulated OC in lakes is comparable to what is stored in the oceans (Mendonça et al., 2017). In many lakes, most of the organic matter in sediments is derived from autochthonous aquatic organisms like phytoplankton and aquatic macrophytes (Dean and Gorham, 1998). On the other hand, allochthonous OC such as detritus of terrestrial vegetation can also account for a significant fraction of sedimentary organic matter (Larsen et al., 2011), for example, within river deltas (Randlett et al., 2015). In small or oligotrophic lakes and/or high-latitude or high-altitude lakes, OC sedimentation may in fact be dominated by land-derived organic matter, and it has previously been observed that high carbon burial efficiency in sediments is linked to a high proportion of allochthonous OC (Sobek et al., 2009). In general, OC from aquatic biomass such as phytoplankton mainly comprises relatively labile compounds (Parsons et al., 1961). In contrast, allochthonous OC (e.g. terrestrial plants) contains more complex structural and biochemically recalcitrant compounds, such as cellulose and lignin (Opsahl and Benner, 1995), which are more effectively preserved over time (Han et al., 2020, 2022). The different biochemical compositions and characteristics of OC in lake sediments and the associated differential susceptibility to hydrolytic attack and microbial breakdown into smaller carbon compounds (Lehmann et al., 2002, 2020) might substantially affect methane production and emission, as has been suggested in previous studies (Grasset et al., 2018; West et al., 2012). However, laboratory experiments performed on short timescales (i.e. within weeks or months) involving the spiking of sediments with fresh algal and/or plant organic materials do not accurately reflect the effects of enhanced OC contribution to natural sediments on methane production. This is because the compositions and lability of original organic materials may change greatly during sedimentation due to oxidative destruction and microbial alteration during sinking in the water column (Kawamura et al., 1987). Moreover, allochthonous OC found in lake sediments is often the less reactive (i.e. less bio-degradable) remnant of land-derived debris, where the more reactive fractions have already been removed on land or during fluvial transport (Raymond and Bauer, 2001). Lastly, while it is known that organic matter bioavailability decreases over time as labile components are selectively remineralized (Grasset et al., 2018), the effects of organic matter quality and OC degradation state, as well as of their interactions, on methane production remain uncertain.
Lipid biomarkers and their stable carbon isotopic compositions can be used to infer sources and diagenetic state of lacustrine organic matter (Dai et al., 2005; Meyers and Ishiwatari, 1993). Different chain lengths of fatty acids and n-alkanes can be used for the distinction between aquatic phytoplankton and terrestrial plants (Cranwell, 1976; Eglinton and Hamilton, 1967). In addition, primary producers growing in different habitats (e.g. terrestrial plants and freshwater phytoplankton) have distinct isotopic compositions due to the differences in C sources and biochemical pathways through which inorganic carbon is assimilated and incorporated into biomass (Cloern et al., 2002). For example, terrestrial C3 plants incorporate carbon dioxide from the atmosphere using the C3 Calvin–Benson pathway and, consequently, have an average bulk isotopic value of approximately −28 ‰, while the isotopic values of various aquatic plants are often significantly less negative (e.g. an average of -20 ‰ for benthic diatoms) (Cloern et al., 2002; O'Leary, 1981). Moreover, stable carbon isotope measurements can be used to trace the carbon flow from organic matter degradation to methane in lake sediments. The isotopic signatures of methane and methane precursors (e.g. dissolved inorganic carbon, bulk OC) can be used to assess the relative contribution of the major pathways (i.e. CO2 reduction and acetoclastic methanogenesis) to total methane production (Conrad, 2005; Whiticar, 1999). To date, it remains unclear which organic compounds represent the main precursors of molecules that are ultimately converted into methane in older sediments, in which more labile OC fractions, such as OC from microalgal cells, have largely been remineralized already. Previous research in Lake Geneva revealed higher benthic methane fluxes but lower total mineralization rates of organic matter in deltaic sediments compared to at profundal sites (PSs) with reduced riverine impact (Randlett et al., 2015). The observed differences imply that, despite less efficient total remineralization that leads to elevated OC burial rates in deltaic sediments, high methane production is sustained by the high input of allochthonous OC (Sollberger et al., 2014).
Here, we investigated relationships between methanogenesis rates and pathways and the sources and degradation states of sedimentary organic matter in profundal and deltaic sediments of Lake Geneva. We combined methanogenic rate measurements by radiotracer incubations with 14C-labelled bicarbonate and acetate with compositional (lipids, pyrolysis–GC-MS (gas chromatography–mass spectrometry)) and stable carbon isotopic analyses of OC and methane, as well as sedimentation rate measurements (based on Pb-210, Cs-137). Additional quantitative analyses of a methanogenic marker gene (mcrA) and analyses of methanogenic community structure (16S rRNA gene sequences) provided insights into the abundances and identities of in situ methanogenic populations. Based on this multi-disciplinary data set, we identified potential relationships between sediment OC sources and degradation status, rates, and pathways and the organisms involved in the microbial production of methane.
2.1 Study sites
Lake Geneva is the largest western European lake. The Rhone River, the main tributary to the lake in the northeastern part, has a catchment area of 5220 km2 and accounts for about 68 % of the total water discharge and large amounts of suspended sediment and fine-particle loading to the lake (Burrus et al., 1989). The Rhone River inflow brings in large amounts of terrestrial OC, which is mostly deposited near the river mouth as an important contribution to deltaic sediments. On the other hand, sedimentation in the deeper part of the lake is usually dominated by phytoplankton-derived OC (Gallina et al., 2017).
2.2 Sample collection and processing
Sediment cores were collected using a larger gravity corer (14 cm inner diameter) at a profundal site (46°25′54′′ N, 6°47′33′′ E; water depth: 240 m) and a smaller gravity corer (6.5 cm inner diameter) at a deltaic site (DS; 46°24′58′′ N, 6°51′34′′ E; water depth: 128 m) in August and December 2019, respectively. Profundal samples for different analyses were obtained from a single sediment core at 2 cm vertical resolution. Samples for the analysis of dissolved methane concentrations were collected on site with cut-off syringes through pre-drilled holes in the core liner covered with adhesive tape. Sediment samples of 2 cm3 were fixed with 5 mL 10 % NaOH in 120 mL serum bottles, which were capped immediately with thick butyl rubber stoppers and an aluminium crimp cap. Additionally, two replicate samples were taken from the same depth for methanogenesis rate measurements (described below). Sediment porewater was extracted with Rhizon samplers (Rhizosphere Research Products, Wageningen, Netherlands) connected to 20 mL syringes through pre-drilled small holes, with 2 cm distance between them. Porewater samples for dissolved inorganic carbon (DIC) concentration measurements and stable carbon isotope analyses were stored in 4 mL glass vials without headspace at 4 °C. Separate porewater sample aliquots for the quantification of acetate and other volatile fatty acids were stored in combusted (450 °C for 5 h) glass vials at −20 °C until further analysis. The sediment core was then extruded in the lab and sectioned into 2 cm segments. Samples were taken from each segment and stored frozen (−20 °C) until further analysis for bulk parameters and lipid biomarkers. Using the gravity corer equipped with the smaller core liner, four sediment cores were obtained from the deltaic site. One core was used for methane concentration measurements, and a second one was used for rate measurements (both at 2 cm resolution; sample collection is as described above). The third core was used for porewater extraction in the lab using Rhizon samplers, and the last one was split open for the determination of porosity and the analysis of bulk parameters and lipid biomarkers. An additional small-diameter sediment core was taken at the profundal site during the second sampling campaign for porosity analysis.
2.3 Rate measurements of methanogenesis using radio-labelled substrates
Methanogenesis rates (MGRs) were determined using a radioisotope-based approach. We determined the modes of methane production and activity rates in incubation experiments with radio-labelled acetate and bicarbonate. At each depth, samples (2.5 cm3) were collected through pre-drilled holes using 3 mL cut-off plastic syringes, which were closed with rubber stoppers and stored at 4 °C. Upon arrival of the samples in the laboratory, 10 µL of anoxic 14C-labelled bicarbonate (∼16 kBq, Perkin–Elmer) or 2-14C-labelled (i.e. 14C label in the methyl group) acetate solution (∼18 kBq, Perkin–Elmer) was injected for the MGR measurements to determine CO2 reduction (MGRDIC) or acetoclastic (MGRAc) methanogenesis rates, respectively. Immediately after the tracer injection through the stoppers, all samples including killed controls (i.e. samples that were transferred to 10 mL 5 % NaOH solution immediately after tracer injection) were incubated under an N2 atmosphere at in situ temperature (4 °C) in the dark for 48 h.
To stop microbial activity in the incubations with 14C-labelled substrates, samples were transferred into 120 mL serum bottles containing 10 mL aqueous NaOH (5 % wt/wt), immediately crimp-sealed with butyl rubber stoppers, and vigorously shaken. The 14C activity in the different carbon pools was determined as previously described (Su et al., 2019). Briefly, the headspace of a fixed sample is purged with air (30 mL min−1 for 30 min) through a heated (850 °C) quartz tube filled with copper oxide, where the 14CH4 (product of methanogenesis during incubation) is combusted to 14CO2. The 14CO2 is then captured in two sequential traps of scintillation vials (20 mL) containing 8 mL of 1:7 phenylethylamine and methoxyethanol. The cumulative radioactivity of both traps is then determined by liquid scintillation counting (2200CA Tri-Carb Liquid Scintillation Analyzer) after adding 8 mL of a scintillation cocktail (Ultima Gold, PerkinElmer) to each vial and thorough mixing using a vortex mixer. For incubations with bicarbonate, the residual 14C-bicarbonate was measured as 14CO2, released from the alkaline liquid phase after adding 2.5 mL of 32 % HCl. The remaining radioactivity (possibly explained by inorganic carbon assimilation into biomass) was determined in a 1 mL aliquot of the acidified mixture (amended with 4 mL Ultima Gold) by liquid scintillation counting. Incubation bottles with 14C-acetate were also acidified with 2.5 mL of 32 % HCl after extraction of 14CH4, and 14CO2 from microbial acetate oxidation was subsequently purged and trapped as described above. To determine the residual 14C-acetate in the incubation vial, 1 mL of the acidified mixture was mixed with 4 mL Ultima Gold for scintillation counting (Beulig et al., 2018). The control samples were processed in the same way as the incubated samples after the termination of incubation. The methanogenesis rates with DIC () and acetate () as substrates were calculated using Eqs. (1) and (2), respectively, modified from a previous study (Beulig et al., 2018).
[DIC] and [Ac] are concentrations of DIC and acetate in the sediment porewater, respectively. and ADIC represent the activities of produced methane and DIC (i.e. residual DIC in bicarbonate-amended incubations and product DIC in acetate-amended incubations) at the end of the incubation (in counts per minute, CPM). AR and AAc represent the remaining radioactivity of DIC-incubated samples (i.e. biomass and metabolic intermediates) and the residual 14C-acetate radioactivity (possibly also including a small fraction of 14C in metabolic intermediates and incorporated into biomass), respectively. φ is the porosity of the sediment samples. The factor 1.08 accounts for the isotopic fractionation of 14C (Hansen et al., 2001). t represents the incubation time in days. Measured 14CH4 activities in all incubation samples were blank-corrected by subtracting the 14CH4 activity measured in the killed control (typically close to background radioactivity) incubated with the same amounts of 14C-labelled substrates. The activities in the 14CH4 pool were considered to be zero if the blank-corrected value was negative.
2.4 Porewater methane and nutrient analyses
Methane concentrations were measured in the headspace of NaOH-preserved samples using a gas chromatograph (GC, Agilent 6890N) with a flame ionization detector and helium as a carrier gas. Methane concentrations in the wet sediments were then calculated based on the ratio of headspace to sample volume. The C isotopic composition (13C12C) of methane was determined in the same samples using a pre-concentration unit (TraceGas, Micromass, UK) connected to an isotope ratio mass spectrometer (IRMS; GV Instruments, Isoprime). Stable C-isotope values are reported in the conventional δ notation (in ‰) relative to the Vienna Pee Dee Belemnite standard (VPDB), with a reproducibility of 0.5 ‰ based on replicate measurements of methane standards. A carbon analyser (TOC-L, Shimadzu, Kyoto, Japan), equipped with a non-dispersive infrared detector (NDIR), was used to quantify dissolved inorganic carbon (DIC) concentrations in sediment porewaters. To determine the carbon isotopic composition of DIC, a porewater aliquot of 1–2 mL was introduced into a He-purged exetainer (Labco Ltd) and acidified with ∼200 µL 85 % H3PO4. After 2 h of equilibration at 37 °C, the CO2 released from the aqueous phase was analysed using a preparation system (MultiFlow, Isoprime) coupled to an IRMS (Micromass, Isoprime). The standard deviation for replicate measurements of samples and standards was <0.2 ‰. The apparent carbon isotope fractionation factor (αc) during methanogenesis was estimated using δ13C values of methane and DIC (Eq. 3).
Values of αc greater than 1.065 were interpreted to indicate a predominance of CO2 reduction over acetoclastic methanogenesis (Conrad, 2005). Porewater samples for acetate and other volatile fatty acids were analysed with two-dimensional ion chromatography (2D IC), as described previously (Glombitza et al., 2014), with modifications reported in Schaedler et al. (2018). Samples were filtered through pre-washed (10 mL Ultrapure Type-I water) disposable syringe filters (Acrodisc® IC grade, 0.2 µm pore size, 13 mm diameter). The first 0.5 mL sample after filtration was discarded before collecting the samples for 2D IC analysis. We used a dual Dionex ICS6000 instrument (Thermo Scientific) equipped with a Dionex AS24 column (2 mm diameter) for the first dimension and a Dionex AS11HC column (2 mm diameter) for the second dimension. Quantification was done from the conductivity detector signal with a series of five external standards between 0.5 and 100 µM.
2.5 Bulk sediment analyses
The total organic carbon (TOC) contents of sediment samples were determined by the difference between total carbon and total inorganic carbon. Samples for total carbon were measured with an Elementar Vario Pyro Cube CN elemental analyser (Elementar, Germany), and samples for total inorganic carbon were analysed by coulometry using a UIC CM5015 coulometer (Joliet, IL, USA). Sediment cores were dated by gamma spectrometry using 137Cs on freeze-dried and ground sediment for the determination of sedimentation rates, as described previously (Randlett et al., 2015). The δ13C-TOC was determined on decalcified samples (Schubert and Nielsen, 2000). Briefly, freeze-dried and homogenized samples were treated with 5 mL 10 % HCl in 15 mL Falcon tubes overnight. After centrifugation, the supernatant was discarded, and the solid phase was washed and centrifuged three times with 5 mL Milli-Q water. Samples were dried at 50 °C for 48 h prior to analysis. The δ13C-TOC was then assessed by elemental analysis–isotope ratio mass spectrometry (EA, Pyro Cube, Elementar and IRMS, Isoprime, UK). The reproducibility based on replicate measurements of standards and samples was better than 0.2 ‰.
2.6 Pyrolysis gas chromatography–mass spectrometry
The sedimentary organic matter composition was characterized at the molecular level using a pyrolyser equipped with an autosampler (EGA/PY-3030D and AS-1020E, FrontierLabs, Japan) connected to a gas chromatograph (Trace 1310, Thermo Scientific) and a mass spectrometer (ISQ 7000, Thermo Scientific), following the optimized method (Tolu et al., 2015). Depending on the sample, an aliquot of 2–3 mg of dry sediment was pyrolysed at 450 °C. A data-processing pipeline, including chromatogram smoothing, alignment background correction, and multivariate curve resolution by alternate regression, was used to automatically detect and integrate the peaks and to extract their mass spectra in the R computational environment (R Core Team, 2024). To optimize the number of detected peaks, data processing was performed independently for the sediments from the deltaic site (DS) and the profundal site (PS). Individual peaks were identified using “NIST MS Search 2” software, which includes the library “NIST/EPA/NIH 2011”, complemented by spectra from published studies. The relative abundances of these identified pyrolytic organic compounds (Table S2 in the Supplement) were calculated for each sample by normalization to the sum of their peak areas, set at 100 %.
2.7 Lipid extraction, separation, and quantification
At both sites, sediment samples from five depths (1, 5, 11, 19, and 29 cm) were selected based on redox conditions (1 cm: oxic and suboxic; all others: anoxic), OC content, and methanogenesis rates (the latter two variables had peak value in the deeper layers) and then were lyophilized and homogenized for subsequent lipid extraction. Prior to extraction, an internal standard mix (5α-androstane, 3-eicosanone, n-C 19:0 fatty acid and n-C19 alkanol) was added to each sample for the quantification of single biomarkers. Lipids were extracted in 20 mL of 7:3 dichloromethanemethanol (DCM/MeOH) in a microwave reaction system (SolvPro, Anton Paar, Graz, Austria), as described previously (Ladd et al., 2018). Total lipid extracts (TLEs) were obtained by successively rinsing the samples with DCM after centrifugation and then were concentrated using a Multivapor P-6 (Büchi Labortechnik AG, Switzerland). TLEs were further evaporated to dryness and saponified in 3 mL methanolic KOH solution (∼1 N) at 80 °C for 3 h. Neutral compounds were extracted by liquid–liquid extraction using hexane, and fatty acids (FAs) were then extracted from the remaining aqueous phase with hexane after acidification (pH<2). The fatty acid fraction was treated using 1 mL of BF3 in methanol (14 % , Sigma Aldrich) at 80 °C for 2 h and was converted to fatty acid methyl esters (FAMEs). Neutral compounds were further separated into four different fractions using 500 mg6 mL pre-packed Si gel columns (filling quantity/volume, Biotage, Uppsala, Sweden). Briefly, the neutral fraction was dissolved in 4 mL hexane and transferred onto the column, followed by 4 mL hexane/DCM () and then 4 mL DCM/MeOH () and, finally, 4 mL MeOH with the elution of hydrocarbon, ketone, alcohol, and remaining polar compounds, respectively. The alcohol fraction was acetylated in 25 µL acetic anhydride and 200 µL pyridine at 70 °C for 30 min.
All fractions were quantified using a gas chromatograph equipped with a flame ionization detector (GC-FID) (GC-2010 Plus, Shimadzu, Japan). Samples were injected onto an InertCap 5MS/NP column (, GL Sciences, Japan) using an AOC-20i autosampler (Shimadzu) through a split–splitless injector operated in splitless mode at 280 °C. The column was heated from 70 to 130 °C at 20 °C min−1 and then to 320 °C at 4 °C min−1 and was held at 320 °C for 20 min. FAMEs were identified by comparing their retention times to those of laboratory standards (i.e. a fatty acid methyl ester mix and bacterial acid methyl ester from Supelco, reference nos. 47885-U and 47080-U, respectively) and were quantified by normalization to the internal n-C 19:0 fatty acid standard. Identification of hydrocarbons was performed by comparing their retention times to those of an external standard containing C14 to C40 n-alkanes (Sigma Aldrich). The alcohols were characterized using a gas chromatography–mass spectrometer (GC-MS, QP2020, Shimadzu, Japan) under identical chromatographic conditions. Acquired mass spectra were identified through comparison with published data.
2.8 Compound-specific stable carbon isotope analysis
The stable carbon isotope composition of FAMEs, n-alkanes, and alcohols was determined by gas chromatography–isotope ratio mass spectrometry (GC-IRMS) using a Delta V Advantage IRMS (Thermo Scientific) with a ConFlow IV (Thermo Scientific). Samples were injected with a TriPlus RSH autosampler into a programmable temperature vaporizer (PTV) inlet operated in splitless mode at 280 °C on a GC-1310 gas chromatograph (Thermo Scientific, Bremen, Germany). The GC was equipped with a 30 m DB-5MS fused silica capillary column (0.25 mm i.d., 0.25 µm film thickness). The GC oven was heated from 80 to 215 °C at 15 °C min−1 and then to 320 °C at 5 °C min−1 and was held at 320 °C for 10 min. Column effluent was combusted at 1020 °C. Compound-specific δ13C values were reported relative to the VPDB scale and were calibrated externally using known δ13C values of an alkane mixture (n-C, Arndt Schimmelmann, Indiana University, USA), which were run at the beginning and the end of each sequence, as well as after every sixth sample injection. The standard deviation for replicate measurements for these standards averaged 0.4 ‰, with the average offset from their known values being less than 0.5 ‰. The isotopic values of FAMEs and acetylated alcohols were additionally corrected for the introduction of carbon atoms during the derivatization step.
2.9 DNA extraction, PCR amplification, Illumina sequencing, and data analysis
DNA was extracted from selected sediment samples of Lake Geneva, where both high and low methanogenic activities were detected (DS: 11, 19, 21, and 27 cm; PS: 5, 11, 19, and 29 cm), using FastDNA SPIN Kit for Soils (MP Biomedicals) following the manufacturer's instructions. A two-step polymerase chain reaction (PCR) approach was applied to prepare the library for Illumina sequencing at the Genomics Facility Basel, as described in detail previously (Su et al., 2020, 2023). The PCR was performed using the universal primers 515F-Y and 926R targeting the V4 and V5 regions of the 16S rRNA gene. These primers cover the majority (∼84.7 %) of the 16S rRNA gene sequences of methanogenic archaea and are well-suited for assessing community structures of methanogens in environmental samples (Table S4 in the Supplement). Data were then analysed with Phyloseq (McMurdie and Holmes, 2013) in the R environment (R Core Team, 2024). Raw sequence data were deposited at NCBI Short Read Archive under the BioProject ID no. PRJNA736863, with accession nos. from SAMN19667760 to SAMN19667767.
2.10 Quantitative PCR (qPCR)
The abundance of methanogens in Geneva sediments was determined by quantitative PCR (qPCR) with the primer set mcrIRD and 2 µL DNA as the template (Lever and Teske, 2015). qPCR reactions of all DNA samples were performed using the SensiFAST SYBR No-ROX Kit (Bioline) on a Mic (Magnetic Induction Cycler) real-time PCR machine (Bio Molecular Systems, Inc). An initial denaturing step of 95 °C for 3 min was followed by 40 cycles of 5 s at 95 °C, 10 s at 56 °C, and 22 s at 72 °C. The specificity of the amplification was assessed by examining the melting curves from 72 °C to 95 °C. The calibration curve was generated using a serial of 10-fold dilutions of pGEM-T Easy plasmid DNA (Promega, USA) carrying a single copy of the target gene (mcrIRD-F/mcrIRD-R). The number of gene copies in plasmid DNA was calculated using the equation reported previously (Ritalahti et al., 2006). We further compared the absolute gene copy numbers of mcrA between the two sites using the Wilcoxon signed-rank test (“wilcox.test”), implemented in the R package “stats” (R Core Team, 2024).
3.1 Sediment geochemistry at the two study sites
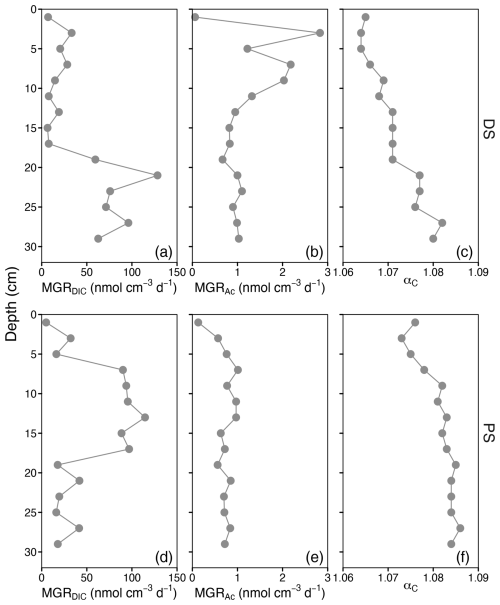
The two sites displayed different hydrochemical and geochemical characteristics (Fig. 1, Table S1 and Fig. S1 in the Supplement). Dating of the sediments revealed that the deltaic site (DS) had a slightly higher sedimentation rate (0.58 cmyr−1) than the profundal site (PS) (0.43 cmyr−1). At the deltaic site, two distinct peaks in methane concentration (PS, ∼7 mM) were observed at 9 and 23 cm, respectively (Fig. 1a). At the profundal site, concentrations of methane increased with depth and remained relatively constant at ∼4 mM below 15 cm (Fig. 1e). At both sites, the porewater concentrations of DIC increased with depth, and considerable amounts accumulated in the sediment porewater. The depth-integrated DIC concentrations in the top 30 cm were 1964 mmol m−2 at the PS and 3490 mmol m−2 at the DS; however, based on the sedimentation over the last 50 years, the depth-integrated DIC concentrations were 1302 mmol m−2 at the PS (21 cm) and 3490 mmol m−2 at the DS (29 cm). Acetate, as a potential methanogenic substrate, showed concentration maxima of 25.9 µM (DS) and 3.8 µM (PS) close to the sediment surface. The methane δ13C values decreased with depth in both deltaic sediments (from −64.9 ‰ to −72.2 ‰, Fig. 1b) and profundal sediments (from −72.7 ‰ to −74.6 ‰, Fig. 1f). Overall, the observed methane carbon isotope values at both sites fall within a range that is typical for biogenic production (Whiticar, 1999). Vertical profiles of δ13C-DIC show a similar pattern at the two sites, with the lowest values of −5.5 ‰ at 5 cm at the DS (Fig. 1b) and −5.2 ‰ at 3 cm at the PS (Fig. 1f). At the PS, TOC concentrations increase slightly with depth, with the mean content almost doubling compared to the DS (Fig. 1c and g, Table S1). With respect to its stable carbon isotope composition, δ13C-TOC increased slightly with depth at the PS, from −28 ‰ in surficial sediments to −26 ‰ below 7 cm, whereas δ13C-TOC values remained relatively constant at the DS throughout the sampled sediment column (). The elemental C N ratios in the sediments of DS show a very high variability with depth and range from 6.7 to 16.8, with a mean value of 10.7 (Fig. 1d and Table S1), while the values at the PS are lower and less variant along the sediment core (7.9±0.3, Fig. 1 h).
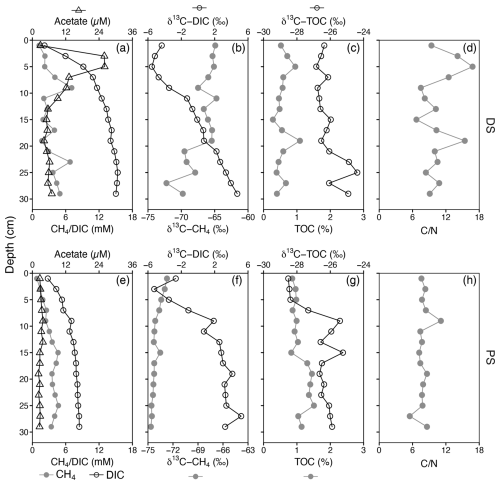
Figure 1Sediment geochemistry as a function of depth at a deltaic site (DS, a–d) and a profundal site (PS, e–h) in Lake Geneva. (a, e) Profiles of dissolved methane, dissolved inorganic carbon (DIC), and acetate concentrations. (b, f) Stable carbon isotopic signatures (δ13C) of CH4 and DIC (in ‰ vs. VPDB). (c, g) Concentrations of total organic carbon (TOC, % of dry weight) and its carbon isotopic composition (in ‰ vs. VPDB). (d, h) Molar ratios of total organic carbon to total nitrogen (C N).
3.2 Composition of sedimentary organic matter
To investigate the molecular composition of sedimentary organic matter at the two different sites, sediment samples were further analysed using pyrolysis–GC-MS. We identified a total of 65 individual organic compounds (Table S2), which can be classified into the following compound groups: carbohydrates, N compounds, n-alkenes, n-alkanes, phenols, lignin oligomers, and (poly)aromatics. Both carbohydrates and N compounds were the most abundant organic-compound groups among the pyrolysis products, with no statistical difference between the DS and the PS (Fig. 2a and b). At both sites, the carbohydrates consisted mainly of compounds such as butenal, methylfuraldehyde, and furanone, and N compounds were dominated by pyrrole, pyridine, methyl-pyrrole, and methyl-pyridine (Table S2), which are indicative of degraded products of carbohydrates and proteins (Schellekens et al., 2009; Tolu et al., 2017). Most strikingly, sediments at the DS displayed significantly higher relative abundances of lignin (p<0.05) and phenols (p<0.01) compared to profundal sediments (Fig. 3d and e). In addition, we observed significantly higher relative abundances of n-alkenes at the PS (Fig. 3c). This group contains monounsaturated short-chain n-C15 to n-C18 (Table S2), which were the most abundant organic compounds among the n-alkenes.
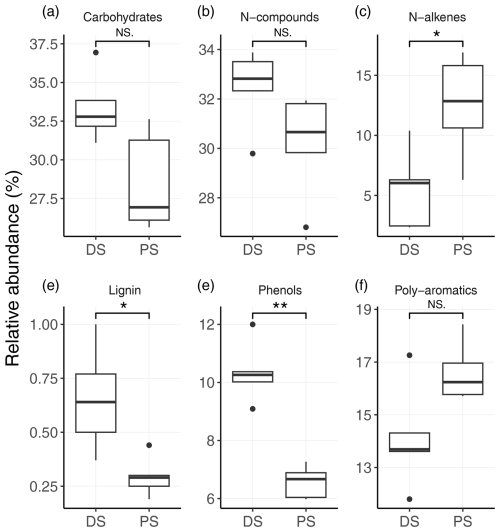
Figure 2Relative abundances (in %) of different biochemical classes of organic compounds in sediments at the deltaic (DS) and profundal site (PS) of Lake Geneva. (a) Carbohydrates, (b) N compounds, (c) n-alkenes, (d) lignin, (e) phenols, and (f) poly-aromatics. Statistical differences of compound abundances between the two sites were determined with the Wilcoxon signed-rank test. Significance levels are as follows: ** p<0.01, * p<0.05, and NS shows no significant differences between the two sites (p>0.05).
3.3 Lipid concentration, distribution, and stable carbon isotopic signature
Typical lipid biomarkers derived from aquatic phytoplankton (e.g. short-chain fatty acids) and terrestrial plants (e.g. long-chain n-alkanes) were present in both profundal and deltaic sediments, yet concentrations of some of these biomarkers were strikingly different between the two sites, as well as between different depths within the same site (Fig. 3 and Table S3). Among the fatty acids, n-C16:0 was by far the most abundant in all measured samples, with a slight increase in δ13C values with depth at both sites. At the DS, the unsaturated fatty acid n-C16:1ω7 was the most abundant monounsaturated fatty acid, with the highest concentration observed at 11 cm. At the PS, n-C16:1 (ω7 and ω5) and n-C18:1 (ω9 and ω7) decreased in concentration with depth and were 2 to 4 times more abundant in the upper sediment layers (0–2, 4–6, and 10–12 cm) than in the lower parts (18–20 and 28–30 cm). The short-chain fatty acids were generally depleted in 13C, with stronger 13C depletions observed in surface sediments, particularly for the unsaturated fatty acid n-C16:1ω5 at the PS.
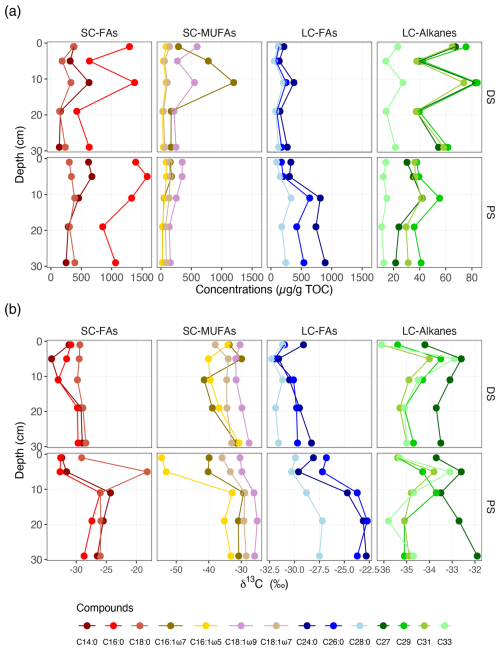
Figure 3Vertical distribution of specific fatty acids and n-alkanes in deltaic (DS) and profundal (PS) sediments of Lake Geneva. (a) Concentrations (expressed as µg lipid g−1 TOC) and (b) compound-specific δ13C values (in ‰ vs. VPDB). SC-FAs denotes short-chain fatty acids (C14:0, C16:0, and C18:0), SC-MUFAs denotes short-chain monounsaturated fatty acids (C16:1 and C18:1), LC-FAs denotes long-chain fatty acids (C24:0, C26:0, and C28:0), and LC-alkanes denotes long-chain n-alkanes (C27, C29, C31, and C33).
In contrast to short-chain fatty acids, C24:0 was most abundant among the long-chain fatty acids (i.e. C24:0, C26:0, and C28:0), with relatively higher concentrations found in profundal sediments. Most strikingly, an apparent increase in the concentrations of these long-chain fatty acids was observed in the sediments of the PS, with consistently high concentrations in the lower parts (10–30 cm). Meanwhile, the δ13C values of these compounds decreased dramatically with depth. By comparison, concentrations of long-chain fatty acids at the DS were variable at different sediment depths, but their δ13C values remained relatively constant (e.g. C28:0, Fig. 3b) and showed less variability with depth compared to the PS (e.g. C24:0 and C26:0). Although the concentrations of long-chain n-alkanes at the DS were twice as high as those at the PS, their δ13C values (particularly for C29, C31, and C33) were very similar at the two sites (Fig. 3b).
3.4 Relative importance of methanogenic pathways
Methane production through CO2 reduction was observed at both sites and at all depths. At the deltaic site, the highest activity was found i the lower part of the sediment core (19–29 cm), while, in the profundal sediment, maximum rates of CO2 reduction via this pathway were detected within the upper sediments at depths between 7 and 17 cm (Fig. 4b, e and Table S1). In contrast, the rates of methane production via acetate fermentation (i.e. acetoclastic methanogenesis) were low, with maxima of 2.8 and 1.0 observed at 3 cm (PS) and 7 cm (DS), respectively. The areal methanogenesis rates by CO2 reduction within the top 30 cm at both sites were 2 orders of magnitude higher than those of acetoclastic methanogenesis (Table S1). Acetate oxidation activity was very low at both the PS and the DS, with depth-integrated (0–30 cm) rates of 0.1 and 0.2 , respectively (Table S1). At both sites, the fractionation factor αc, which is approximated based on the difference between the measured δ13C-DIC and δ13C-CH4 values, increased with depth (Fig. 4f), ranging from 1.064 to 1.071 (DS) and from 1.073 to 1.084 (PS).
3.5 Abundance and diversity of methanogenic archaea
On average, gene copy numbers of the methyl coenzyme M reductase gene (mcrA) in the deltaic sediments of Lake Geneva were significantly higher than those in the profundal sediments (p<0.05; Fig. 5a). At both sites, Methanoregula and Methanothrix dominated the methanogenic guild, followed by Methanosarcina and Methanobacterium (Fig. 5b). Within the methanogenic community, the mean relative abundances of Methanothrix and Methanosarcina were both higher at the DS than those at the PS. By comparison, Methanoregula was the most abundant genus in the methanogenic community at the PS.
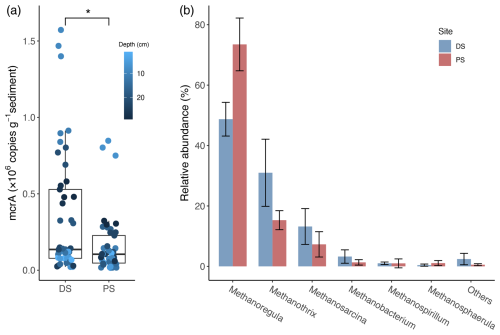
Figure 5Abundance and diversity of methanogens at the deltaic site (DS) and the profundal site (PS) in Lake Geneva. (a) Absolute abundances of the mcrA gene encoding the α subunit of the methyl coenzyme M reductase. The statistical difference between the two sites was determined with the Wilcoxon signed-rank test, and the asterisk denotes the significance level (* p<0.05). (b) Mean relative abundances of different methanogenic groups (% of the total methanogens). Data are based on read abundances of 16S rRNA gene sequences.
3.6 Correlation analysis between geochemical parameters, methane production rates, and microbial communities
The alpha diversity measures indicate that the community structure of microorganisms in the sediments at the DS were more diverse than at the PS (Fig. S2). Principal coordinate analysis (PCoA) revealed significantly different microbial community compositions at the two investigated sites, as indicated by the clear separation of the data by the first principal coordinate, explaining 50.8 % and 44.5 % of the observed variance for archaea and bacteria, respectively (Fig. S3 in the Supplement). For both archaeal and bacterial communities, PCoA plots show a very close aggregation of the deep sediment samples at the PS (17, 23, and 29 cm), indicating that their community structures are highly similar. Conversely, there is quite some variance among the samples at the more dynamic deltaic site, with its variable deposition history. The measured rates of both CO2 reduction and acetoclastic methanogenesis tend to be higher in sediment samples harbouring a more diverse microbial community (Fig. S4), but the relation is weak and, particularly for acetoclastic methanogenesis, not significant. Pearson correlation analysis between methane production rates and environmental parameters shows that methane production rates from both MGRDIC and MGRAc were positively but not significantly correlated with the concentration of short-chain n-alkanes (correlation coefficients of 0.46 and 0.51 for MGRDIC and MGRAc, respectively; Fig. S5 in the Supplement). δ13C–CH4 values showed a positive correlation with the abundance of carbohydrates, lignin, and long-chain n-alkanes and significant negative correlation with the abundance of long-chain fatty acids and total organic carbon. C N ratios were negatively correlated with the concentrations of both short-chain and long-chain fatty acids (Fig. S5).
Our results indicate a dominance of methane production by CO2 reduction in both profundal and deltaic sediments of Lake Geneva. This inference is supported by radiotracer measurements, measured carbon isotopic compositions, and methanogenic community analyses, which revealed members of CO2-reducing Methanoregula to be the dominant group of methanogens. Thus, CO2 reduction was observed as the primary MGR process in both sediments of the PS, which predominantly contained diagenetically altered phytoplankton-derived OC, and sediments of the DS, characterized by variable sources of aquatic and terrestrial OC. We conclude, therefore, that the dominant pathway of methanogenesis does not primarily depend on the chemical composition of sedimentary organic matter. Other factors that affect production rates of different electron donors (e.g. H2, acetate) could play a more important role, and this will be discussed below.
4.1 Difference in terms of organic carbon sources and diagenetic alteration
The deltaic sediments in this study displayed a strikingly large range of C N ratios (6.7–16.8), whereas C N ratios of profundal samples remained relatively constant throughout the sediment core (7.9±0.3). Fresh organic matter from lacustrine phytoplankton, which is protein-rich (Parsons et al., 1961), typically has low C N ratios of 6–9 (Meyers and Ishiwatari, 1993). In contrast, bulk sediment containing large portions of terrestrial vascular plants often displays much higher C N ratios, sometimes at least 3 times greater, due to its high content of high-carbon structural components and refractory organics such as lignin (Hedges et al., 1986). A comparison of carbon isotope and bulk C N values to data from previous studies (Lamb et al., 2006) confirmed the presence of both aquatic and terrestrial sources in the deltaic sediments and the dominance of organic matter of autochthonous origin (e.g. algal biomass) at the PS (Fig. 6). At the DS, variable microbial community structures and significant changes in C N ratios with depth reflect a highly variable depositional history, indicating differing origins of sedimentary organic matter. Specifically, peak C N ratios of 16.8 at 5 cm and 15.4 at 19 cm were likely derived from terrestrial organic matter in these intercalated layers, while relatively lower ratios (∼10) at other depths suggest a mixture of aquatic and terrestrial sources. Even lower values (∼7) in some deltaic sediment layers may indicate predominantly autochthonous deposition of phytoplankton particles.
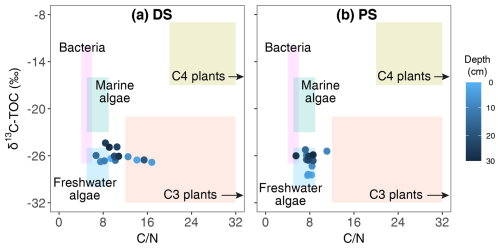
Figure 6Comparison of δ13C and bulk C N values of Lake Geneva sediments from the deltaic site (DS, a) and the profundal site (PS, b) to elemental and isotopic indicators of bulk organic matter produced by bacteria and marine and freshwater algae, as well as terrestrial C3 and C4 plants (Lamb et al., 2006).
Indeed, at both sites, sediments contained OC from aquatic biomass, as verified by the short-chain fatty acids, indicative of freshwater algae (Cranwell, 1976) and/or in situ production by bacteria (Volkman et al., 1998). However, contrary to the findings from the combined carbon isotope and bulk C N ratios, both lignin and phenols (Fig. 2) and long-chain n-alkanes (Fig. 3a) derived from terrestrial plants were detected in profundal sediments, although the concentrations of these compounds were lower at the PS than at the DS. Long-chain fatty acids (i.e. C24:0 and C26:0), which are typically assumed to originate from terrestrial plant waxes (Cranwell, 1974), make up a proportionally more important fraction of lipids in deeper sediment layers, particularly below 5 cm depth. However, the distinct carbon isotope compositions of these long-chain fatty acids between the two sites (Fig. 3b and Table S3; δ13C is, on average, ∼7 ‰ lower at the DS than at the PS) suggest different OC sources.
Indeed, Chikaraishi et al. (2004) reported that long-chain fatty acids (i.e. C24:0 and C26:0) have δ13C values of for a variety of terrestrial vascular plants, whereas the δ13C for aquatic plants is significantly less negative (). At the PS, δ13C values for C24:0 and C26:0 fatty acids (below 5 cm depth) were close to those indicative of freshwater aquatic plants (Chikaraishi et al., 2004). Previous studies have shown that both aquatic macrophytes and microalgae may represent potential sources of long-chain fatty acids (Ficken et al., 2000; Volkman et al., 1998). However, it seems somewhat contradictory to conclude that long-chain fatty acids were primarily derived from aquatic vascular plants simply because the observed C N ratios at the PS were too low. Indeed, microalgae such as diatoms were estimated to contribute from 30 % to 80 % of the fatty acid pool of C24:0 to C28:0 in intertidal sandy sediment (Volkman et al., 1980). Thus, the long-chain fatty acids observed in the profundal sediments may also have been derived from microalgae (Volkman et al., 1998). This, in turn, could explain why TOC was more depleted in terms of 13C in the surface sediments at the PS, which does not necessarily indicate the origins of terrestrial plants (Cloern et al., 2002).
The carbon isotopic composition of long-chain n-alkanes (i.e. C27−31), characteristic for land-derived organic matter (Chikaraishi et al., 2004), was almost identical between the PS and DS (Fig. 3b, ranging from −35 ‰ to −33 ‰), suggesting a similar terrestrial source. At the DS, if long-chain n-alkanes and long-chain fatty acids were derived from the same terrestrial source, they should have similar C-isotopic values. However, the slightly lower δ13C values of long-chain fatty acids at the DS (on average, −30 ‰) imply a mixture of both aquatic and terrestrial sources, which is consistent with the C N ratios observed at this site.
Turning to other organic matter (OM) biomarkers, phytol concentrations were considerably higher at the PS (with no clear depth trend) compared to at the DS, whereas no clear difference in terms of cholesterol concentrations was observed between the sites (with depth-dependent concentration decreases at both sites). For brassicasterol, there seems to be a clear depth-related decrease at both sites, with slightly higher concentrations in surface sediments (0–2 and 4–6 cm) at the PS compared to at the DS. Phytol, a side chain of chlorophyll, can originate from both phytoplankton and terrestrial plants (Shi et al., 2001). Due to its rapid degradation under intense-light and oxic conditions in terrestrial environments, terrestrially derived phytol (or chlorophyll) is generally of minor importance in aquatic sediments (Meyers and Takeuchi, 1981), and so it can be assumed that most of the sedimentary phytol is derived from aquatic sources (Ladd et al., 2018). As for cholestanol and brassicasterol (Table S3), the former can originate from both zooplankton and phytoplankton (Bechtel and Schubert, 2009), while the latter is a lipid biomarker mainly derived from phytoplankton in aquatic sediments (Volkman, 1986). Hence, based on at least two of the biomarkers presented here, we have putative evidence for a shift towards more autochthonous organic matter at the profundal site, as could be expected.
4.2 Methanogenesis is mainly driven by CO2 reduction
Our rate measurements using trace 14C-labelled substrates clearly showed that CO2 reduction played a dominant role in methane formation in both the PS and DS sediments compared to acetoclastic methanogenesis. As we did not measure rates of methylotrophic methanogenesis, we are unable to determine the relative importance of this pathway. However, since we detected neither common methylated compounds such as methanol in the sediment porewater nor methanogens that exclusively perform methylotrophic methanogenesis at any of the studied sites, we argue that this pathway is likely to be of minor importance with regard to its the contribution to the overall methane production at these sites. Indeed, H2CO2 and acetate are usually the main substrates in freshwater environments (Lyu et al., 2018), and, to date, no direct evidence for the occurrence of methylotrophic methanogenesis has been documented for lake sediments. Methylotrophic methanogenesis has been shown to be an important pathway mainly in sulfate reduction zones of marine sediments, where methanogens can utilize methylated compounds (e.g. methanol and trimethylamine) as non-competitive substrates for methane production (Xiao et al., 2018; Xu et al., 2021; Zhuang et al., 2018).
The finding that CO2 reduction pathways dominated methane production throughout the sediment cores at both sites was further supported by the observed apparent fractionation factor αc (DS value of 1.071±0.001 and PS value of 1.081±0.001; Fig. 4c, f and Table S1). Rates for CO2 reduction in Lake Geneva were similar at the two studied sites but were much higher than those previously reported in other lakes at similar depths (Kuivila et al., 1989). The observed low rates for acetoclastic methanogenesis, on the other hand, were comparable to those in other lake sediments (Kuivila et al., 1989; Schulz and Conrad, 1996). Methane production via acetate fermentation was traditionally believed to play a more important role than that via CO2 reduction in lake sediments (Whiticar, 1999; Whiticar et al., 1986). However, more recent evidence seems to contradict this paradigm, demonstrating that CO2 reduction can indeed be a much more significant methane-producing pathway than acetoclastic methanogenesis (Blair et al., 2018; Conrad et al., 2011; Meier et al., 2024). While the dominance of CO2 reduction in lake sediments has been reported previously, our results reinforce this conclusion with consistent geochemical and isotopic evidence across contrasting sedimentary regimes in Lake Geneva.
At both sites, Methanoregula was the most abundant methanogenic genus, particularly in deep sediments where the highest CO2 reduction rates were observed. This genus, commonly found in freshwater lakes (Bartosiewicz et al., 2024; Berberich et al., 2020; Meier et al., 2024), is known to perform hydrogenotrophic methanogenesis (i.e. CO2 reduction with hydrogen as the electron donor). Methanothrix, the second most abundant methanogen, primarily performs acetoclastic methanogenesis. At the PS, Methanoregula dominated at all investigated depths, in accordance with the high rates by CO2 reduction. While acetoclastic methanogenesis played only a minor role in total methane production, higher rates were observed for deltaic sediments, where they coincided with a lower apparent fractionation factor. This observation is consistent with higher concentrations of acetate and a higher relative abundance of acetate-consuming Methanothrix, though the underlying drivers are unclear and do not appear to be linked to OC sources or chemical compositions. While Methanothrix has long been considered to exclusively use acetate as substrate for methanogenesis (Jetten et al., 1992; Smith and Ingram-Smith, 2007), there is now evidence that some species can also perform CO2 reduction via direct interspecies electron transfer (Rotaru et al., 2014; Zhou et al., 2023).
Although overall methane production rates were similar at both sites, the qPCR data at hand indicated that bulk methane production was not primarily controlled by (i.e. proportional to) the cell abundance of methanogens. Instead, it was likely to be regulated by other environmental factors, such as limited substrate supply (i.e. H2 and acetate), stemming from rates of hydrolysis (Kristensen et al., 1995) and/or fermentation rates (Valentine et al., 1994) within the sediments. Since methanogens rely on syntrophic and other heterotrophic bacteria to generate these key substrates, the activity and composition of the broader microbial community may play a significant role in shaping methane production rates (Beulig et al., 2018; Liu and Whitman, 2008; Meier et al., 2024). In Lake Geneva sediments, rates of both CO2 reduction and acetoclastic methanogenesis increased with microbial Shannon diversity. More diverse microbial communities tend to possess more diverse organic matter degradation capacities, resulting in a higher production of acetate and H2CO2, which, in turn, promotes overall methane production (Conrad, 2020). In addition, our microbial abundance and diversity data were insufficient to fully characterize the carbon metabolism of microorganisms involved in H2 and acetate production, which could directly impact CO2 reduction and acetoclastic methanogenesis and potentially explain the observed methane production differences. The acetate concentrations were below 26 µM at both sites but were well within the range of what has been measured in profundal sediment of Lake Constance, where acetoclastic methanogenesis was dominant (Schulz and Conrad, 1996). Hence, the consistently low rates of acetoclastic methanogenesis throughout the sediment cores at both sites likely resulted from low turnover rates of acetate during organic matter degradation rather than being driven by low acetate concentrations.
4.3 Effect of organic carbon characteristics on methanogenesis
Despite the differences in OC sources and quality between the two sites, the overall methane production rates were comparable (Fig. 4b, e and Table S1). However, the sedimentary zones of maximum methane production and the depth-dependent rates of both metabolic modes of methanogenesis varied. This likely reflects differences in OC quality and degradation pathways, which influence the balance between remineralization to H2CO2 versus acetate (Conrad, 2020), resulting in spatial variability in sedimentary methane production.
Potential methane production rates have been shown to correlate positively with the quantity of sediment organic matter (Berberich et al., 2020). However, at both the DS and PS in Lake Geneva, the sediment layers of maximum rates of CO2 reduction did not coincide with higher TOC contents. Instead, combined rate and isotopic measurements revealed that methanogenesis rates via CO2 reduction corresponded to sediment samples with 13C-enriched bulk organic carbon (Figs. 1 and 4). This suggests that the source and microbial decomposition of organic matter, rather than its quantity, primarily influenced the methanogenic activity via the CO2 reduction pathway.
Methane production rates in lake sediments can be quite low across various latitudes but can also increase significantly with the addition of fresh OC (Bartosiewicz et al., 2024; Schwarz et al., 2008; West et al., 2012). Indeed, high lipid contents in phytoplankton biomass have been shown to enhance methane production in both engineered systems and lake sediments (West et al., 2015; Zhao et al., 2014). Additionally, existing evidence suggests that terrestrial OC can stimulate methane ebullition in reservoirs (DelSontro et al., 2011; Sobek et al., 2012). It has also been suggested that the availability of easily degradable organic matter controls both methanogenic pathways and methanogenic archaeal communities (Liu et al., 2017). However, the influence of organic matter source or composition on the relative importance of methane production pathways and their corresponding rates remains poorly constrained and has rarely been explored. At the PS, within the zone of high methanogenic activity via CO2 reduction, TOC became less depleted in 13C, a trend also observed, though less pronounced, in the deltaic sediments. The δ13C shift of TOC within the most active zone at the PS likely reflects either the selective loss of isotopically light organic matter fractions or changes in organic matter sources, as indicated by depth-related variations in both short- and long-chain fatty acid concentrations.
Contrary to previous studies demonstrating that algal deposition stimulated the activity of acetoclastic methanogens (Schulz and Conrad, 1995; Schwarz et al., 2008), our results revealed higher acetate concentrations in tandem with higher acetoclastic methanogenesis occurring in surface sediments of the deltaic site, where high C N ratios indicated terrestrial inputs. This suggests that allochthonous OC may play a role in acetate production and acetoclastic methanogenesis. At the PS, autochthonous OM was the dominant OC source to the sediments, and the CH4 produced likely resulted from the decomposition of buried algal detritus (Fig. 6). This interpretation is also supported by the 2-fold higher brassicasterol, the 3-fold higher phytol, and the 3–4-fold higher long-chain fatty acid concentrations (with elevated δ13C values) at the PS compared to at the DS. These biomarkers, in combination with lower C N ratios, are indicative of algal material as the dominant organic contributor to early diagenetic methane production.
While the dominant sedimentary organic matter source (i.e. the partitioning between autochthonous versus allochthonous OC inputs into the sediments) varied between the two studied sites, the similar overall methane production rates imply that methanogenesis occurred ubiquitously in these lacustrine sediments, independently of the OC sources, as well as showing their apparent susceptibility to organic matter degradation and/or their overall diagenetic state. This finding aligns with a recent study across the river and/or deltaic–pelagic continuum in a reservoir system in southwestern Ohio (Berberich et al., 2020) and has important implications for understanding the lacustrine methane cycle. On the one hand, eutrophication in lakes and other aquatic systems often increases the supply of autochthonous organic carbon, thereby enhancing methanogenesis and leading to increased methane emissions (Davidson et al., 2018). On the other hand, the potential of terrestrial organic matter to contribute to lacustrine methane production can be significantly impacted by anthropogenic activities, such as dam construction (Li et al., 2023), or a result of deforestation and soil erosion, which alter sediment inputs into the lake (Bélanger et al., 2017). Our study demonstrates that diverse OC sources and varying diagenetic states in both profundal and deltaic sediments support substantial rates of methane formation through CO2 reduction. However, identifying the exact fractions of organic matter that were degraded and that fuelled methane production remains challenging. Understanding these mechanisms is essential for predicting how changes in OC inputs and sediment dynamics may influence methane production and emissions from lacustrine environments.
Our study demonstrates that both profundal and deltaic sediments of Lake Geneva are significant sources of methane despite clear differences in the origins and compositions of organic matter. The profundal site is dominated by aquatic OC, while the deltaic site features a more variable mix of OC sources, including substantial terrestrial contributions at certain depths. Methane production at both sites is driven by CO2 reduction, which accounted for over 95 % of total methane production and was most probably mediated by Methanoregula. While phylogenetic data suggest a link between methanogen communities and methanogenetic pathways, the broader microbial community dynamics, particularly those involved in H2 and acetate production, remain insufficiently understood.
At the profundal site, methane production is mainly associated with the decomposition of aquatic organic matter as terrestrial OC is less abundant. At the deltaic site, acetoclastic methanogenesis appears to be linked to the higher terrestrial organic matter inputs. Nevertheless, the overall dominance of methanogenesis via CO2 reduction at both sites suggests that the depositional regime and the OC source and composition are not the primary determinants of the prevailing methane-producing pathway. Instead, other environmental factors such as substrate availability or microbial community dynamics may influence methane production, although the specific mechanisms remain uncertain. Future research should focus on unravelling these interactions to better predict if and how changes in OC inputs and sediment dynamics may impact methane emissions from lacustrine environments.
Raw reads of the 16S rRNA sequencing data are available on the NCBI GenBank under BioProject ID no. PRJNA736863 and under accession nos. SRR14794332–SRR14794339.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-4449-2025-supplement.
CJS, GS, and MAL conceived the research. CJS acquired the funding for this study. GS performed the field work, lab experiments, and data analyses. JT assisted with pyrolysis–GC data analysis. CG and MAL measured porewater volatile fatty acids and analysed the data. JZ and MFL supported the rate measurements and molecular analyses. GS wrote the original draft of the paper. All of the authors helped to revise and approved the submitted version of the paper.
At least one of the (co-)authors is a member of the editorial board of Biogeosciences. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This research was supported by the EAWAG internal funds. We thank Alois Zwyssig, Sandra Schmid, and Cameron Callbeck for the assistance with the field sample collection. We also thank Serge Robert for the laboratory assistance, including the lipid sample preparation and laboratory analyses, and for his help in the elemental analysis of the samples. Patrick Kathriner is acknowledged for the laboratory support. We are also grateful to Thomas Kuhn at the University of Basel for the laboratory support with the radioisotope measurements.
This research has been supported by the Eidgenössische Anstalt für Wasserversorgung Abwasserreinigung und Gewässerschutz.
This paper was edited by Susanne Liebner and reviewed by Christian Knoblauch and one anonymous referee.
Bartosiewicz, M., Przytulska, A., Birkholz, A., Zopfi, J., and Lehmann, M. F.: Controls and significance of priming effects in lake sediments, Glob. Chang. Biol., 30, 1–13, https://doi.org/10.1111/gcb.17076, 2024.
Bastviken, D., Tranvik, L. J., Downing, J. A., Crill, P. M., and Enrich-Prast, A.: Freshwater methane emissions offset the continental carbon sink, Science, 331, 50–50, https://doi.org/10.1126/science.1196808, 2011.
Bechtel, A. and Schubert, C. J.: A biogeochemical study of sediments from the eutrophic Lake Lugano and the oligotrophic Lake Brienz, Switzerland, Org. Geochem., 40, 1100–1114, https://doi.org/10.1016/j.orggeochem.2009.06.009, 2009.
Bélanger, É., Lucotte, M., Moingt, M., Paquet, S., Oestreicher, J., and Rozon, C.: Altered nature of terrestrial organic matter transferred to aquatic systems following deforestation in the Amazon, Appl. Geochem., 87, 136–145, https://doi.org/10.1016/j.apgeochem.2017.10.016, 2017.
Berberich, M. E., Beaulieu, J. J., Hamilton, T. L., Waldo, S., and Buffam, I.: Spatial variability of sediment methane production and methanogen communities within a eutrophic reservoir: Importance of organic matter source and quantity, Limnol. Oceanogr., 65, 1336–1358, https://doi.org/10.1002/lno.11392, 2020.
Beulig, F., Røy, H., Glombitza, C., and Jørgensen, B. B.: Control on rate and pathway of anaerobic organic carbon degradation in the seabed, P. Natl. Acad. Sci. USA, 115, 367–372, https://doi.org/10.1073/pnas.1715789115, 2018.
Blair, N. E., Leithold, E. L., Thanos Papanicolaou, A. N., Wilson, C. G., Keefer, L., Kirton, E., Vinson, D., Schnoebelen, D., Rhoads, B., Yu, M., and Lewis, Q.: The C-biogeochemistry of a Midwestern USA agricultural impoundment in context: Lake Decatur in the intensively managed landscape critical zone observatory, Biogeochemistry, 138, 171–195, https://doi.org/10.1007/s10533-018-0439-9, 2018.
Burrus, D., Thomas, R. L., Dominik, J., and Vernet, J.-P.: Recovery and concentration of suspended solids in the upper Rhone River by continuous flow centrifugation, Hydrol. Process., 3, 65–74, https://doi.org/10.1002/hyp.3360030107, 1989.
Chikaraishi, Y., Naraoka, H., and Poulson, S. R.: Hydrogen and carbon isotopic fractionations of lipid biosynthesis among terrestrial (C3, C4 and CAM) and aquatic plants, Phytochemistry, 65, 1369–1381, https://doi.org/10.1016/j.phytochem.2004.03.036, 2004.
Cloern, J. E., Canuel, E. A., and Harris, D.: Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system, Limnol. Oceanogr., 47, 713–729, https://doi.org/10.4319/lo.2002.47.3.0713, 2002.
Conrad, R.: Quantification of methanogenic pathways using stable carbon isotopic signatures: a review and a proposal, Org. Geochem., 36, 739–752, https://doi.org/10.1016/j.orggeochem.2004.09.006, 2005.
Conrad, R.: Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: A mini review, Pedosphere, 30, 25–39, https://doi.org/10.1016/S1002-0160(18)60052-9, 2020.
Conrad, R., Noll, M., Claus, P., Klose, M., Bastos, W. R., and Enrich-Prast, A.: Stable carbon isotope discrimination and microbiology of methane formation in tropical anoxic lake sediments, Biogeosciences, 8, 795–814, https://doi.org/10.5194/bg-8-795-2011, 2011.
Conrad, R., Klose, M., and Enrich-Prast, A.: Acetate turnover and methanogenic pathways in Amazonian lake sediments, Biogeosciences, 17, 1063–1069, https://doi.org/10.5194/bg-17-1063-2020, 2020.
Cranwell, P. A.: Monocarboxylic acids in lake sediments: Indicators, derived from terrestrial and aquatic biota, of paleoenvironmental trophic levels, Chem. Geol., 14, 1–14, https://doi.org/10.1016/0009-2541(74)90092-8, 1974.
Cranwell, P. A.: Decomposition of aquatic biota and sediment formation: organic compounds in detritus resulting from microbial attack on the alga Ceratium hirundinella, Freshwater Biol., 6, 41–48, https://doi.org/10.1111/j.1365-2427.1976.tb01589.x, 1976.
Dai, J., Sun, M.-Y., Culp, R. A., and Noakes, J. E.: Changes in chemical and isotopic signatures of plant materials during degradation: Implication for assessing various organic inputs in estuarine systems, Geophys. Res. Lett., 32, L13608, https://doi.org/10.1029/2005GL023133, 2005.
Davidson, T. A., Audet, J., Jeppesen, E., Landkildehus, F., Lauridsen, T. L., Søndergaard, M., and Syväranta, J.: Synergy between nutrients and warming enhances methane ebullition from experimental lakes, Nat. Clim. Change, 8, 156–160, https://doi.org/10.1038/s41558-017-0063-z, 2018.
Dean, W. E. and Gorham, E.: Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands, Geology, 26, 535, https://doi.org/10.3133/fs05899, 1998.
DelSontro, T., Kunz, M. J., Kempter, T., Wüest, A., Wehrli, B., and Senn, D. B.: Spatial heterogeneity of methane ebullition in a large tropical reservoir, Environ. Sci. Technol., 45, 9866–9873, https://doi.org/10.1021/es2005545, 2011.
Demirel, B. and Scherer, P.: The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review, Rev. Environ. Sci. Biotechnol., 7, 173–190, https://doi.org/10.1007/s11157-008-9131-1, 2008.
Downing, J. A., Prairie, Y. T., Cole, J. J., Duarte, C. M., Tranvik, L. J., Striegl, R. G., McDowell, W. H., Kortelainen, P., Caraco, N. F., Melack, J. M., and Middelburg, J. J.: The global abundance and size distribution of lakes, ponds, and impoundments, Limnol. Oceanogr., 51, 2388–2397, https://doi.org/10.4319/lo.2006.51.5.2388, 2006.
Eglinton, G. and Hamilton, R. J.: Leaf epicuticular waxes, Science, 156, 1322–1335, https://doi.org/10.1126/science.156.3780.1322, 1967.
Ernst, L., Steinfeld, B., Barayeu, U., Klintzsch, T., Kurth, M., Grimm, D., Dick, T. P., Rebelein, J. G., Bischofs, I. B., and Keppler, F.: Methane formation driven by reactive oxygen species across all living organisms, Nature, 603, 482–487, https://doi.org/10.1038/s41586-022-04511-9, 2022.
Ficken, K. J., Li, B., Swain, D. L., and Eglinton, G.: An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes, Org. Geochem., 31, 745–749, https://doi.org/10.1016/S0146-6380(00)00081-4, 2000.
Gallina, N., Beniston, M., and Jacquet, S.: Estimating future cyanobacterial occurrence and importance in lakes: a case study with Planktothrix rubescens in Lake Geneva, Aquat. Sci., 79, 249–263, https://doi.org/10.1007/s00027-016-0494-z, 2017.
Glombitza, C., Pedersen, J., Røy, H., and Jørgensen, B. B.: Direct analysis of volatile fatty acids in marine sediment porewater by two-dimensional ion chromatography-mass spectrometry, Limnol. Oceanogr.-Meth., 12, 455–468, https://doi.org/10.4319/lom.2014.12.455, 2014.
Grasset, C., Mendonça, R., Villamor Saucedo, G., Bastviken, D., Roland, F., and Sobek, S.: Large but variable methane production in anoxic freshwater sediment upon addition of allochthonous and autochthonous organic matter, Limnol. Oceanogr., 63, 1488–1501, https://doi.org/10.1002/lno.10786, 2018.
Han, X., Schubert, C. J., Fiskal, A., Dubois, N., and Lever, M. A.: Eutrophication as a driver of microbial community structure in lake sediments, Environ. Microbiol., 22, 3446–3462, https://doi.org/10.1111/1462-2920.15115, 2020.
Han, X., Tolu, J., Deng, L., Fiskal, A., Schubert, C. J., Winkel, L. H. E., and Lever, M. A.: Long-term preservation of biomolecules in lake sediments: potential importance of physical shielding by recalcitrant cell walls, PNAS Nexus, 1, pgac076, https://doi.org/10.1093/pnasnexus/pgac076, 2022.
Hansen, L. K., Jakobsen, R., and Postma, D.: Methanogenesis in a shallow sandy aquifer, Rømø, Denmark, Geochim. Cosmochim. Ac., 65, 2925–2935, https://doi.org/10.1016/S0016-7037(01)00653-6, 2001.
Hedges, J. I., Clark, W. A., Quay, P. D., Richey, J. E., Devol, A. H., and Santos, M.: Compositions and fluxes of particulate organic material in the Amazon River, Limnol. Oceanogr., 31, 717–738, https://doi.org/10.4319/lo.1986.31.4.0717, 1986.
IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S. L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M. I., Huang, M., Leitzell, K., Lonnoy, E., Matthews, J. B. R., Maycock, T. K., Waterfield, T., Yelekçi, O., Yu, R., and Zhou, B., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, https://doi.org/10.1017/9781009157896, 2021.
Jetten, M. S. M., Stams, A. J. M., and Zehnder, A. J. B.: Methanogenesis from acetate: a comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp., FEMS Microbiol. Lett., 88, 181–198, https://doi.org/10.1111/j.1574-6968.1992.tb04987.x, 1992.
Kawamura, K., Ishiwatari, R., and Ogura, K.: Early diagenesis of organic matter in the water column and sediments: Microbial degradation and resynthesis of lipids in Lake Haruna, Org. Geochem., 11, 251–264, https://doi.org/10.1016/0146-6380(87)90036-2, 1987.
Kristensen, E., Ahmed, S. I., and Devol, A. H.: Aerobic and anaerobic decomposition of organic matter in marine sediment: Which is fastest?, Limnol. Oceanogr., 40, 1430–1437, https://doi.org/10.4319/lo.1995.40.8.1430, 1995.
Kuivila, K. M., Murray, J. W., Devol, A. H., and Novelli, P. C.: Methane production, sulfate reduction and competition for substrates in the sediments of Lake Washington, Geochim. Cosmochim. Ac., 53, 409–416, https://doi.org/10.1016/0016-7037(89)90392-X, 1989.
Ladd, S. N., Nelson, D. B., Schubert, C. J., and Dubois, N.: Lipid compound classes display diverging hydrogen isotope responses in lakes along a nutrient gradient, Geochim. Cosmochim. Ac., 237, 103–119, https://doi.org/10.1016/j.gca.2018.06.005, 2018.
Lamb, A. L., Wilson, G. P., and Leng, M. J.: A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and CN ratios in organic material, Earth Sci. Rev., 75, 29–57, https://doi.org/10.1016/j.earscirev.2005.10.003, 2006.
Larsen, S., Andersen, T., and Hessen, D. O.: Climate change predicted to cause severe increase of organic carbon in lakes, Glob. Change Biol., 17, 1186–1192, https://doi.org/10.1111/j.1365-2486.2010.02257.x, 2011.
Lehmann, M. F., Bernasconi, S. M., Barbieri, A., and McKenzie, J. A.: Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis, Geochim. Cosmochim. Ac., 66, 3573–3584, https://doi.org/10.1016/S0016-7037(02)00968-7, 2002.
Lehmann, M. F., Carstens, D., Deek, A., McCarthy, M., Schubert, C. J., and Zopfi, J.: Amino acid and amino sugar compositional changes during in vitro degradation of algal organic matter indicate rapid bacterial re-synthesis, Geochim. Cosmochim. Ac., 283, 67–84, https://doi.org/10.1016/j.gca.2020.05.025, 2020.
Lever, M. A. and Teske, A. P.: Diversity of methane-cycling archaea in hydrothermal sediment investigated by general and group-specific PCR primers, Appl. Environ. Microb., 81, 1426–1441, https://doi.org/10.1128/AEM.03588-14, 2015.
Li, B., Wang, H., Lai, A., Xue, J., Wu, Q., Yu, C., Xie, K., Mao, Z., Li, H., Xing, P., and Wu, Q. L.: Hydrogenotrophic pathway dominates methanogenesis along the river-estuary continuum of the Yangtze River, Water Res., 240, 120096, https://doi.org/10.1016/j.watres.2023.120096, 2023.
Liu, Y. and Whitman, W. B.: Metabolic, Phylogenetic, and Ecological Diversity of the Methanogenic Archaea, Ann. NY Acad. Sci., 1125, 171–189, https://doi.org/10.1196/annals.1419.019, 2008.
Liu, Y., Conrad, R., Yao, T., Gleixner, G., and Claus, P.: Change of methane production pathway with sediment depth in a lake on the Tibetan plateau, Palaeogeogr. Palaeocl., 474, 279–286, https://doi.org/10.1016/j.palaeo.2016.06.021, 2017.
Lyu, Z., Shao, N., Akinyemi, T., and Whitman, W. B.: Methanogenesis, Curr, Biol., 28, R727–R732, https://doi.org/10.1016/j.cub.2018.05.021, 2018.
McMurdie, P. J. and Holmes, S.: Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data, PLoS One, 8, 1–11, https://doi.org/10.1371/journal.pone.0061217, 2013.
Meier, D., van Grinsven, S., Michel, A., Eickenbusch, P., Glombitza, C., Han, X., Fiskal, A., Bernasconi, S., Schubert, C. J., and Lever, M. A.: Hydrogen–independent CO2 reduction dominates methanogenesis in five temperate lakes that differ in trophic states, ISME Communications, 4, ycae089, https://doi.org/10.1093/ismeco/ycae089, 2024.
Mendonça, R., Müller, R. A., Clow, D., Verpoorter, C., Raymond, P., Tranvik, L. J., and Sobek, S.: Organic carbon burial in global lakes and reservoirs, Nat. Commun., 8, 1–6, https://doi.org/10.1038/s41467-017-01789-6, 2017.
Meyers, P. A. and Ishiwatari, R.: Lacustrine organic geochemistry-an overview of indicators of organic matter sources and diagenesis in lake sediments, Org. Geochem., 20, 867–900, https://doi.org/10.1016/0146-6380(93)90100-P, 1993.
Meyers, P. A. and Takeuchi, N.: Environmental changes in Saginaw Bay, Lake Huron recorded by geolipid contents of sediments deposited since 1800, Environ. Geol., 3, 257–266, https://doi.org/10.1007/BF02473517, 1981.
O'Leary, M. H.: Carbon isotope fractionation in plants, Phytochemistry, 20, 553–567, https://doi.org/10.1016/0031-9422(81)85134-5, 1981.
Opsahl, S. and Benner, R.: Early diagenesis of vascular plant tissues: Lignin and cutin decomposition and biogeochemical implications, Geochim. Cosmochim. Ac., 59, 4889–4904, https://doi.org/10.1016/0016-7037(95)00348-7, 1995.
Parsons, T. R., Stephens, K., and Strickland, J. D. H.: On the Chemical Composition of Eleven Species of Marine Phytoplankters, J. Fish. Res. Board Can., 18, 1001–1016, https://doi.org/10.1139/f61-063, 1961.
R Core Team: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing [online], https://www.r-project.org/ (last access: 20 January 2025), 2024.
Randlett, M.-E., Sollberger, S., Del Sontro, T., Müller, B., Corella, J. P., Wehrli, B., and Schubert, C. J.: Mineralization pathways of organic matter deposited in a river–lake transition of the Rhone River Delta, Lake Geneva, Environ. Sci. Process Impacts, 17, 370–380, https://doi.org/10.1039/C4EM00470A, 2015.
Raymond, P. A. and Bauer, J. E.: Riverine export of aged terrestrial organic matter to the North Atlantic Ocean, Nature, 409, 497–500, https://doi.org/10.1038/35054034, 2001.
Ritalahti, K. M., Amos, B. K., Sung, Y., Wu, Q., Koenigsberg, S. S., and Löffler, F. E.: Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains, Appl. Environ. Microb., 72, 2765–2774, https://doi.org/10.1128/AEM.72.4.2765-2774.2006, 2006.
Rotaru, A.-E., Shrestha, P. M., Liu, F., Shrestha, M., Shrestha, D., Embree, M., Zengler, K., Wardman, C., Nevin, K. P., and Lovley, D. R.: A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane, Energy Environ. Sci., 7, 408–415, https://doi.org/10.1039/C3EE42189A, 2014.
Schaedler, F., Lockwood, C., Lueder, U., Glombitza, C., Kappler, A., and Schmidt, C.: Microbially mediated coupling of Fe and N cycles by nitrate-reducing Fe (II)-oxidizing bacteria in littoral freshwater sediments, Appl. Environ. Microb., 84, 1–14, https://doi.org/10.1128/AEM.02013-17, 2018.
Schellekens, J., Buurman, P., and Pontevedra-Pombal, X.: Selecting parameters for the environmental interpretation of peat molecular chemistry – A pyrolysis-GC/MS study, Org. Geochem., 40, 678–691, https://doi.org/10.1016/j.orggeochem.2009.03.006, 2009.
Schubert, C. J. and Nielsen, B.: Effects of decarbonation treatments on δ13C values in marine sediments, Mar. Chem., 72, 55–59, https://doi.org/10.1016/S0304-4203(00)00066-9, 2000.
Schulz, S. and Conrad, R.: Effect of algal deposition on acetate and methane concentrations in the profundal sediment of a deep lake (Lake Constance), FEMS Microbiol. Ecol., 16, 251–259, https://doi.org/10.1016/0168-6496(94)00088-E, 1995.
Schulz, S. and Conrad, R.: Influence of temperature on pathways to methane production in the permanently cold profundal sediment of Lake Constance, FEMS Microbiol. Ecol., 20, 1–14, https://doi.org/10.1016/0168-6496(96)00009-8, 1996.
Schwarz, J. I. K. K., Eckert, W., and Conrad, R.: Response of the methanogenic microbial community of a profundal lake sediment (Lake Kinneret, Israel) to algal deposition, Limnol. Oceanogr., 53, 113–121, https://doi.org/10.4319/lo.2008.53.1.0113, 2008.
Shi, W., Sun, M. Y., Molina, M., and Hodson, R. E.: Variability in the distribution of lipid biomarkers and their molecular isotopic composition in Altamaha estuarine sediments: Implications for the relative contribution of organic matter from various sources, Org. Geochem., 32, 453–467, https://doi.org/10.1016/S0146-6380(00)00189-3, 2001.
Smith, K. S. and Ingram-Smith, C.: Methanosaeta, the forgotten methanogen?, Trends Microbiol., 15, 150–155, https://doi.org/10.1016/j.tim.2007.02.002, 2007.
Sobek, S., Durisch-Kaiser, E., Zurbrügg, R., Wongfun, N., Wessels, M., Pasche, N., and Wehrli, B.: Organic carbon burial efficiency in lake sediments controlled by oxygen exposure time and sediment source, Limnol. Oceanogr., 54, 2243–2254, https://doi.org/10.4319/lo.2009.54.6.2243, 2009.
Sobek, S., DelSontro, T., Wongfun, N., and Wehrli, B.: Extreme organic carbon burial fuels intense methane bubbling in a temperate reservoir, Geophys. Res. Lett., 39, 2–5, https://doi.org/10.1029/2011GL050144, 2012.
Sollberger, S., Corella, J. P., Girardclos, S., Randlett, M. E., Schubert, C. J., Senn, D. B., Wehrli, B., and DelSontro, T.: Spatial heterogeneity of benthic methane dynamics in the subaquatic canyons of the Rhone River Delta (Lake Geneva), Aquat. Sci., 76, 89–101, https://doi.org/10.1007/s00027-013-0319-2, 2014.
Su, G., Niemann, H., Steinle, L., Zopfi, J., and Lehmann, M. F.: Evaluating radioisotope-based approaches to measure anaerobic methane oxidation rates in lacustrine sediments, Limnol. Oceanogr.-Meth., 17, 429–438, https://doi.org/10.1002/lom3.10323, 2019.
Su, G., Zopfi, J., Yao, H., Steinle, L., Niemann, H., and Lehmann, M. F.: Manganese/iron-supported sulfate-dependent anaerobic oxidation of methane by archaea in lake sediments, Limnol. Oceanogr., 65, 863–875, https://doi.org/10.1002/lno.11354, 2020.
Su, G., Lehmann, M. F., Tischer, J., Weber, Y., Lepori, F., Walser, J.-C., Niemann, H., and Zopfi, J.: Water column dynamics control nitrite-dependent anaerobic methane oxidation by Candidatus “Methylomirabilis” in stratified lake basins, ISME J., 17, 693–702, https://doi.org/10.1038/s41396-023-01382-4, 2023.
Tolu, J., Gerber, L., Boily, J.-F., and Bindler, R.: High-throughput characterization of sediment organic matter by pyrolysis–gas chromatography/mass spectrometry and multivariate curve resolution: A promising analytical tool in (paleo)limnology, Anal. Chim. Acta., 880, 93–102, https://doi.org/10.1016/j.aca.2015.03.043, 2015.
Tolu, J., Rydberg, J., Meyer-Jacob, C., Gerber, L., and Bindler, R.: Spatial variability of organic matter molecular composition and elemental geochemistry in surface sediments of a small boreal Swedish lake, Biogeosciences, 14, 1773–1792, https://doi.org/10.5194/bg-14-1773-2017, 2017.
Valentine, D. W., Holland, E. A., and Schimel, D. S.: Ecosystem and physiological controls over methane production in northern wetlands, J. Geophys. Res., 99, 1563, https://doi.org/10.1029/93JD00391, 1994.
Volkman, J. K.: A review of sterol markers for marine and terrigenous organic matter, Org. Geochem., 9, 83–99, https://doi.org/10.1016/0146-6380(86)90089-6, 1986.
Volkman, J. K., Johns, R. B., Gillan, F. T., Perry, G. J., and Bavor, H. J.: Microbial lipids of an intertidal sediment – I. Fatty acids and hydrocarbons, Geochim. Cosmochim. Ac., 44, 1133–1143, https://doi.org/10.1016/0016-7037(80)90067-8, 1980.
Volkman, J. K., Barrett, S. M., Blackburn, S. I., Mansour, M. P., Sikes, E. L., and Gelin, F.: Microalgal biomarkers: A review of recent research developments, Org. Geochem., 29, 1163–1179, https://doi.org/10.1016/S0146-6380(98)00062-X, 1998.
West, W. E., Coloso, J. J., and Jones, S. E.: Effects of algal and terrestrial carbon on methane production rates and methanogen community structure in a temperate lake sediment, Freshwater Biol., 57, 949–955, https://doi.org/10.1111/j.1365-2427.2012.02755.x, 2012.
West, W. E., McCarthy, S. M., and Jones, S. E.: Phytoplankton lipid content influences freshwater lake methanogenesis, Freshwater Biol., 2261–2269, https://doi.org/10.1111/fwb.12652, 2015.
Whiticar, M. J.: Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane, Chem. Geol., 161, 291–314, https://doi.org/10.1016/S0009-2541(99)00092-3, 1999.
Whiticar, M. J. J., Faber, E., and Schoell, M.: Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation – Isotope evidence, Geochim. Cosmochim. Ac., 50, 693–709, https://doi.org/10.1016/0016-7037(86)90346-7, 1986.
Xiao, K.-Q., Beulig, F., Røy, H., Jørgensen, B. B., and Risgaard-Petersen, N.: Methylotrophic methanogenesis fuels cryptic methane cycling in marine surface sediment, Limnol. Oceanogr., 63, 1519–1527, https://doi.org/10.1002/lno.10788, 2018.
Xu, L., Zhuang, G. C., Montgomery, A., Liang, Q., Joye, S. B., and Wang, F.: Methyl-compounds driven benthic carbon cycling in the sulfate-reducing sediments of South China Sea, Environ. Microbiol., 23, 641–651, https://doi.org/10.1111/1462-2920.15110, 2021.
Zhao, B., Ma, J., Zhao, Q., Laurens, L., Jarvis, E., Chen, S., and Frear, C.: Efficient anaerobic digestion of whole microalgae and lipid-extracted microalgae residues for methane energy production, Bioresource Technol., 161, 423–430, https://doi.org/10.1016/j.biortech.2014.03.079, 2014.
Zhou, J., Smith, J. A., Li, M., and Holmes, D. E.: Methane production by Methanothrix thermoacetophila via direct interspecies electron transfer with Geobacter metallireducens, mBio, 2–3, https://doi.org/10.1128/mbio.00360-23, 2023.
Zhuang, G.-C., Heuer, V. B., Lazar, C. S., Goldhammer, T., Wendt, J., Samarkin, V. A., Elvert, M., Teske, A. P., Joye, S. B., and Hinrichs, K.-U.: Relative importance of methylotrophic methanogenesis in sediments of the Western Mediterranean Sea, Geochim. Cosmochim. Ac., 224, 171–186, https://doi.org/10.1016/j.gca.2017.12.024, 2018.