the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Microbial sulfur cycling across a 13 500-year-old lake sediment record
Paula C. Rodriguez
Cara Magnabosco
Longhui Deng
Stefano M. Bernasconi
Hendrik Vogel
Marina Morlock
Mark A. Lever
The sulfur cycle is very important in lake sediments, despite the much lower sulfate concentrations in freshwater than seawater. To date, little is known about the formation and preservation of organic and inorganic sulfur compounds in such sediments, especially in the sulfate-depleted subsurface. Here, we investigated the fate of buried S-compounds down to 10 m sediment depth, which represents the entire ∼ 13.5 kyr sedimentary history of the sulfate-rich alpine Lake Cadagno. Chemical profiles of sulfate and reduced sulfur reveal that sulfate from lake water is depleted at the sediment surface with the concomitant formation of iron sulfide minerals. An underlying aquifer provides a second source of sulfate and other oxidants to the deepest and oldest sediment layers, generating an inverse redox gradient with ongoing sulfate consumption. The isotopic offsets between pools of humic acid sulfur, acid-volatile sulfur (AVS), and chromium-reducible sulfur (CRS) in both surface and deep sediments suggest differential timing of formation, with sulfide oxidation to sulfur/polysulfides playing an integral role in organic matter sulfurization. Although sulfate is depleted in the central part of the sediment column, dsrB gene libraries reveal a potential for microbial sulfur reduction throughout the sediment column, with sequences in sulfate-depleted layers being dominated by Chloroflexota. Collectively, our data indicate an active sulfur cycle that is driven by uncultivated microorganisms in deep sulfate-depleted sediments of Lake Cadagno.
- Article
(3207 KB) - Full-text XML
-
Supplement
(426 KB) - BibTeX
- EndNote
The biological sulfur cycle exerts an important control on organic matter burial and thus plays a major role in the global cycling of carbon, oxygen, nitrogen, and iron. In anoxic marine sediments, microbial reduction of sulfate (SO) to hydrogen sulfide (ΣH2S) is quantitatively the most important respiration reaction, remineralizing upwards of 30 % of the total organic carbon flux to the seafloor (Baloza et al., 2022; Bowles et al., 2014; Jørgensen, 1982). Even in freshwater systems, where sulfate concentrations are typically 100–1000 times lower than in seawater, high rates of microbial sulfate reduction can be sustained by rapid reoxidation of ΣH2S by FeIII, MnIV, and possibly by redox-active organic substances, e.g., certain humic acids (Hansel et al., 2015; Pester et al., 2012).
Sulfur isotopic fractionation provides important insights into microbial sulfur cycling in the past and present by recording signatures of these processes within different sulfur pools, including sulfide minerals. The preferential reduction of 32S- over 34S-sulfate generates isotopic fractionations of 3 ‰ to 75 ‰ between sulfate and hydrogen sulfide in microbial cultures (e.g., Bradley et al., 2016; Brunner and Bernasconi, 2005; Detmers et al., 2001; Habicht and Canfield, 1997; Kaplan and Rittenberg, 1964; Rudnicki et al., 2001; Sim et al., 2011; Wortmann et al., 2001). Nonetheless, the dynamics and controls on the magnitude of sulfur isotope fractionation by sulfate-reducing microorganisms have proven to be complex to understand in the environment. While pyrite δ34S values supposedly record the S isotopic composition of sulfide in porewater fluids, major isotopic differences (between 10 ‰ and 40 ‰) have been observed between coexisting sedimentary pyrite and dissolved H2S (Chanton et al., 1987; Canfield et al., 1992; Brüchert and Pratt, 1996; Raven et al., 2016). These discrepancies have been explained by processes such as sediment remobilization, bioturbation, or post-depositional sediment–fluid interactions (Fike et al., 2015; Jørgensen et al., 2004). While the δ34S composition of pyrite has been widely used to interpret global changes in the Earth's sulfur cycle or microbial metabolic pathways, an earlier body of work (e.g., Schwarcz and Burnie, 1973; Goldhaber and Kaplan, 1975, 1980; Maynard, 1980) showed that δ34S isotopic variations in marine sediments are largely controlled by local physical factors, such as sedimentation rate, along with the supply of Fe and OM. In open systems, the sulfate pool is constantly replenished with light 32S-sulfate, whereas under conditions of rapid sedimentation, sulfate in sediment pore spaces is sealed off from overlying waters and the sulfate pool undergoes Rayleigh distillation (Hartmann and Nielsen, 2012). Closed versus open system sulfate reduction can thus explain the large variability in observed fractionations between sulfide and sulfate, which is recorded in sediments by authigenic pyrite (e.g., Och and Shields-Zhou, 2012; Bryant et al., 2023).
While pyrite is the dominant S pool in most marine sediments, the biggest S pool in many freshwater sediments is organic S (Losher, 1989; Mitchell et al., 1981; Nriagu and Soon, 1985; Urban et al., 1999). This organic S originates from both the settling of seston material and the microbial reduction of water column-derived sulfate to hydrogen sulfide, which then reacts with sedimentary organic matter (Damsté et al., 1998; David and Mitchell, 1985; Losher, 1989; Putschew et al., 1996; Rudd et al., 1986). The sulfurization of organic matter tends to promote organic matter resistance to microbial degradation and is thus believed to contribute substantially to long-term preservation of organic carbon in sediments (Damsté and De Leeuw, 1990; Hebting et al., 2006) and to petroleum formation (Orr and Damsté, 1990). Though it is likely that some microorganisms are capable of degrading fractions of this organic S pool, their activity and identity are unknown.
Recently, the metabolic capacity for sulfur cycling has been expanded to new phylogenetic groups based on the detection of specific marker genes for sulfur cycling within these taxa (e.g., Anantharaman et al., 2018). Although the presence of such genes must be interpreted with caution, their distribution across environments can help illuminate the distributions of putative sulfur-reducing and sulfur-oxidizing microbial communities. Thiosulfohydrolase of the sulfur oxidation (Sox) enzyme system (soxB) is one such marker gene and has been widely employed to characterize the diversity of sulfur-oxidizing bacteria (SOB) (Meyer et al., 2007). Another example is dissimilatory sulfite reductase (dsrAB), an enzyme that catalyzes the reduction of sulfite to sulfide and is used by all known sulfate reducers (Klein et al., 2001).
Because low rates of microbial sulfur cycling continue in sulfate-depleted marine sediments (Brunner et al., 2016; Holmkvist et al., 2011; Pellerin et al., 2018; Treude et al., 2014), such processes may likewise occur in sulfate-depleted sediments of lakes and leave a lasting imprint on the lake sulfur geochemical record. Here, we investigate the potential for microbial sulfur cycling in Lake Cadagno, which is an intermediate system between freshwater and seawater, due to its elevated sulfate concentrations (1–2 mM). We combine chemical and isotopic analyses of major S and C phases with quantification and sequencing of S-cycling genes (dsrB, soxB) to investigate S cycling across the complete ∼ 13.5 kyr sedimentary history of Lake Cadagno.
2.1 Geological setting and sampling
The meromictic Lake Cadagno, located in the Swiss Alps, contains 1–2 mM dissolved sulfate, which originates from the dissolution of sulfate-bearing dolomite bedrock via subaquatic springs. Since its formation ∼ 13.5 kyr, Lake Cadagno has undergone a complex redox history, transitioning from seasonal stratification around 12.5 kyr to complete euxinia about 10.9 kyr (Berg et al., 2022; Wirth et al., 2013). Preliminary analyses of sulfur phases in surface and deep sediments (Berg et al., 2022) reveal two sulfate depletion zones (SDZ). For high-resolution analyses, short cores were retrieved from the deepest part of the lake (46.55060° N and 8.71201° E) using a UWITEC gravity corer fitted with plastic liners that are 1 m in length and 9 cm in inner diameter. Deep cores of 3 m long and 6 cm in diameter core sections were recovered using a percussion piston-coring system (Uwitec, AT). More details are provided in Berg et al. (2022). Cores from three parallel boreholes were reserved for nondestructive imaging, porewater extraction, and solid-phase analyses, respectively.
In brief, porewater was extracted using syringes connected to Rhizons (0.2 µm pore size, Rhizosphere) pre-flushed with 2–3 mL of porewater to remove contaminant air. Porewater was then distributed into separate vials with appropriate fixatives for downstream analyses described below. For solid-phase samples, windows were cut into the core liners using a hand-held vibrating saw and potentially contaminated sediment in contact with the liner was scraped away. Samples were then taken using sterile, cut-off syringes and frozen (−20 °C) in separate aliquots for DNA and solid-phase extractions.
Additional samples for sulfate isotope analyses were obtained in June 2020 from one surface spring (at SwissGrid coordinates 2′697′763, 1′155′959) and one subaquatic spring at approximately 5 m depth (2′697′521, 1′156′044) located on the south side of the lake.
Porewater samples in 1991 were also collected from the deepest point in the lake by diving to the bottom and inserting a dialysis porewater sampler directly into the sediment, which was recovered 2 weeks later. Porewater was extracted using a dialysis porewater sampler (Brandl and Hanselmann, 1991) consisting of 80 × 20 × 1.5 cm Plexiglas sheets containing 41 rows of cylindrical dialysis chambers, each 1.5 cm in diameter and 1.5 cm deep. The chambers were covered on both sides with membranes held in place by 3 mm thick Plexiglas overlays. The membranes sealed the single chambers and prevented exchanges between them. Up to five individual samples of 2.6 mL were collected from each row. Divers positioned the sampler in the sediment, leaving 10 cm extending above the sediment–water interface to collect bottom water samples, and it was allowed to incubate for 2 weeks. After recovery, the water of the individual chambers was immediately extracted from the chambers with a syringe and injected into a vial containing a silver nitrate solution to precipitate silver sulfide.
2.2 Porewater and solid-phase analyses
Samples for analysis of dissolved metals (Fe, Mn) were acidified with 5 µL of 30 % HCl per 2 mL to prevent precipitation and measured by inductively coupled plasma optical emission spectroscopy (ICP-OES, Agilent Technologies 5100). Samples for dissolved sulfate were immediately frozen at −20 °C until analysis by ion chromatography (DX-ICS-1000, DIONEX). A part of this sample was reserved for precipitation as BaSO and S-isotopic analysis as described below.
The silver sulfide precipitate from 1991 porewater samples was recovered by centrifugation, dried, and preserved for later sulfur isotope analysis. The remaining solution was acidified to pH < 2, and sulfate was precipitated with BaCl2, recovered by filtration, and dried for analysis.
Total carbon (TC) and total sulfur (TS) were determined from oven-dried sediment (70 °C) by EA-IRMS (elemental analyzer isotope ratio mass spectrometry) as described below. Total organic carbon (TOC) was determined after acid extraction of inorganic carbon with concentrated 6 N HCl. Total inorganic carbon (TIC) was calculated as the difference between TC and TOC. Solid, reactive iron was determined on anoxically treated, freeze-dried samples by extraction with 0.25 mM HCl and subsequent photometric determination of Fe(II) Fe(III) in the supernatant (Stookey, 1970).
2.3 Solid-phase sulfur extractions
Sequential sulfur extractions were performed on freeze-dried samples based on the protocol of Ferdelman et al. (1991). First, elemental sulfur was extracted under N2 atmosphere three times with degassed 100 % methanol. During each step, the methanol-sample mixture was sonicated for 10 min in an ice bath, centrifuged, and then the methanol was pipetted into a clean vial. Methanol extracts were analyzed by ultrahigh pressure liquid chromatography (UPLC) using a Waters Acquity H-class instrument with an Acquity UPLC BEH C18, 1.7 µm, 2.1 × 50 mm column (Waters, Japan) and a PDA (photodiode array) detector (absorbance wavelength set to 265 nm). The injection volume was 10 µL, with methanol as eluent, flowing at 0.2 mL min−1. Elemental sulfur eluted at 4.14 min.
Next, humic acids were extracted three times, or until the supernatant was clear, with degassed 0.1 M NaOH and collected in 50 mL Falcon tubes. Silicates were precipitated from the base extracts by the addition of saturated NaCl solution (5 mL per 45 mL extract) and removed by centrifugation and decanting. The basic extract was acidified to pH 1.5 with concentrated HCl, allowed to stand at 4 °C overnight, and centrifuged to precipitate humic acids. These were washed three times with distilled water to remove salts prior to drying and C, N, and S analysis.
Finally, acid-volatile sulfur (AVS) and chromium-reducible sulfur (CRS) were extracted from the remaining sediment using the two-step acid Cr-II method (Fossing and Jørgensen, 1989; Kallmeyer et al., 2004). For the AVS fraction, 6 N HCl was added to the sediment in a reaction flask under an N2 atmosphere, and H2S was trapped by bubbling through a 5 % Zn-acetate solution for 2 h. The CRS fraction was subsequently obtained by adding 20 mL of the organic solvent dimethyl sulfoxide and 16 mL of CrCl2 solution and reacting again for 2 h. AVS and CRS fractions, collected as ZnS, were quantified photometrically as above, pelleted by centrifugation, rinsed with MilliQ, and dried at 50 °C prior to δ34S analyses as described below.
2.4 Isotopic analyses
Isotopic compositions of sulfur in the sedimentary AVS, CRS, and humic acid sulfur (HAS) fractions and of dissolved sulfate from sediment porewater, a subaquatic spring, and two surface springs were determined using a Flash-EA 1112 (Thermo Fisher) coupled to an isotope ratio mass spectrometer (IRMS, Delta V, Thermo Fisher) by addition of vanadium pentoxide as a catalyst. Isotope ratios are reported in the conventional δ-notation with respect to the Vienna-Cañon Diabolo Troilite (VCDT) standard for sulfur. The system was calibrated for sulfur using the international standards for sulfide and sulfate: IAEA-S1 (δ34S = −0.3 ‰), IAEA-S2 (δ34S = +22.67 ‰), IAEA-S3 (δ34S = −32.55 ‰) and IAEA-SO5 (δ34S = +0.49 ‰), IAEA-SO6 (δ34S = −34.05 ‰), NBS-127 (δ34S = +21.1 ‰). Reproducibility of the measurements was better than 0.2 ‰. This method also produced the weight % sulfur in the humic acid extracts.
The 1991 samples were measured at ETH Zürich by combusting 1–2 mg of silver sulfide sealed in quartz glass tubes with copper oxide turnings at 950 °C. The SO2 was separated from other combustion products using a pentane–liquid nitrogen slush and was measured on a VG 903 mass spectrometer. The system was calibrated with IAEA-S1, IAEA-S3, and NBS 28. Reproducibility of the standard δ34S value was better than ±0.3 ‰.
2.5 DNA extraction and sulfur-cycling gene analyses
DNA was extracted from frozen sediment according to lysis protocol II of Lever et al. (2015) as outlined in Berg et al. (2022). The dsrB gene was PCR-amplified using the dsrB F1a–h/4RSI1a–f primer mixtures from Lever et al. (2013). soxB genes were amplified using the recently designed soxB-837F1a–l/soxB_1170R1a–g primer mixtures (Deng et al., 2022). Quantitative PCRs (qPCR) were performed on a LightCycler 480 II system using the reagent mixtures outlined in Jochum et al. (2017). The thermal cycler settings were (1) enzyme activation and initial denaturation at 95 °C for 5 min; (2) 60 cycles of (a) denaturation at 95 °C for 30 s, (b) annealing at 56 °C (dsrB) or 60 °C (soxB) for 30 s, (c) elongation at 72 °C for 25 s, and (d) fluorescence acquisition at 82 °C (dsrB) or 86 °C (soxB) for 5 s; and (3) a stepwise melting curve from 60 to 95 °C to check for primer specificity. Plasmids containing full-length dsrAB and soxB genes of Desulfotomaculum carboxydivorans and Thiobacillus denitrificans, respectively, were applied as qPCR standards.
dsrB gene sequences were phylogenetically annotated using the ARB software (Ludwig et al., 2004) based on an updated version of the dsrAB database published in Müller et al. (2015). This database was expanded by adding dsrAB gene sequences from subsequently published metagenomes, as well as closest BLAST hits to dsrB gene sequences detected in Lake Cadagno. The phylogenetic annotation was based on a dsrAB gene bootstrap tree that was built by ARB Neighbor-Joining with Jukes–Cantor correction using diverse dsrAB reads that covered the entire dsrB gene amplicon sequence and were at least 750 bp in length. The shorter amplicon sequences from Lake Cadagno, as well as the closest BLAST hits that were < 750 bp long, were added using the ARB Parsimony option combined with a newly designed amplicon-specific dsrB filter that removed hypervariable regions.
3.1 Sulfur geochemistry in Lake Cadagno sediments
The complete sedimentary sequence from Lake Cadagno is approximately 950 cm long, covering a period of ∼ 13.5 kyr (Berg et al., 2022). Sediments are characterized by relatively fine-grained pelagic lacustrine sediments intercalated with frequent coarser-grained flood- and mass movement-derived deposits containing remobilized littoral lake and terrestrial sediment in the upper 790 cm, underlain by light-colored fine-grained deposits of late glacial origin (Fig. 1). The sediment can thus be divided into three distinct lithostratigraphic units representing an early oxic lake (950–790 cm; 13.5 to 12.5 kyr), a redox transition interval (790–760 cm; 12.5 to 10.9 kyr), and the euxinic period (above 760 cm; 10.9 kyr to present). High-resolution mapping of element geochemistry on split core surfaces (Fig. 1) reveals that the accumulation of sulfur is restricted to sediments deposited after the onset of periodic anoxia (transition interval) to permanently reducing conditions (euxinic interval). Fe and S were normalized against Ti, which represents the lithogenic fraction unaltered by redox processes in the aquatic environment. The correlation between S Ti and Fe Ti suggests the presence of authigenic iron sulfide phases. The largest S excursions are located at 300, 560, and 637, which correspond to lacustrine deposits according to the lithological sequence.
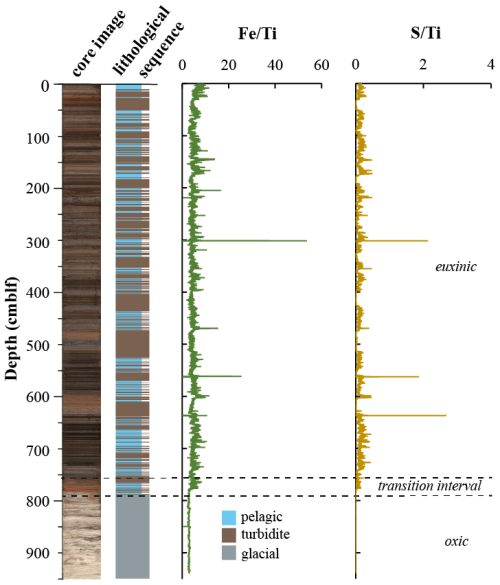
Figure 1Lithological profile determined from a composite core image of the sedimentary sequence retrieved from Lake Cadagno. XRF profiles of S Ti and Fe Ti are from Berg et al. (2022). Changes in lake redox chemistry are denoted by dashed lines.
To obtain further insights into sulfur redox cycling in these sediments, major solid sulfur phases were quantified (Fig. 2a). Elemental sulfur (S0) is the most abundant solid sulfur phase in surface sediments at 300 µmol g−1 dry sediment. The parallel decrease in S0 and FeIII (Fig. 2b) with depth indicates increasingly reducing conditions. Both AVS (mostly amorphous FeS and mackinawite) and HAS exhibit a peak at 10 cm depth, which coincides with a peak in dissolved Fe2+ and the steepest decrease in sulfate concentrations (Fig. 2c). Below this depth, AVS, HAS, and S0 decrease in parallel with sulfate at the expense of CRS formation. Most carbon is in organic form in the Lake Cadagno sediments, with measurable contributions (< 2 wt %) of total inorganic carbon (TIC) present only in the top few centimeters and again at 14 cm depth (Fig. 2b).
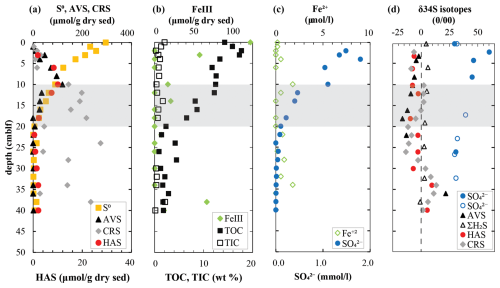
Figure 2Geochemistry of (a) major solid-phase sulfur pools along with (b) FeIII and total organic and inorganic carbon and (c) dissolved species involved in sulfur cycling in the surface sediments of Lake Cadagno. (d) Isotope ratios of major sulfur pools with data from 1991 represented as open symbols overlain on profiles from 2019. The shaded gray region indicates the sulfate depletion zone.
Notably, δ34S sulfate profiles measured more than 30 years apart coincide surprisingly well (Fig. 2d). Sulfate in the upper sediments is highly enriched in 34S, increasing from +24 ‰ in the bottom waters to +60 ‰ within the upper 2 cm. This zone coincides with strong decreases in TOC and isotopically light 13C-DIC values where high rates of organic matter mineralization have been inferred (Berg et al., 2022; Gajendra et al., 2023). There is a small but consistent offset between δ34S in reduced sulfur pools of H2S, AVS, HAS, and CRS but no real trends. The widest fluctuations of δ34S values were measured in the AVS with −16 ‰ at +16 cm and 22 ‰ at 34 cm depth.
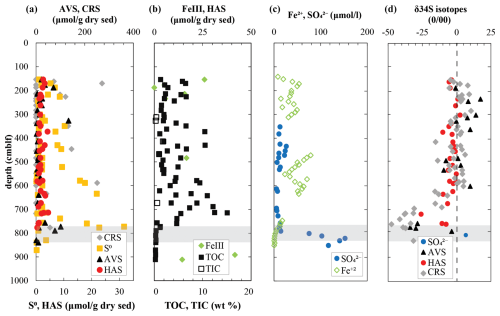
Figure 3Geochemistry of (a) major solid-phase sulfur pools along with (b) FeIII and total organic and inorganic carbon and (c) dissolved species involved in sulfur cycling in the deep sediments of Lake Cadagno. (d) Isotope ratios of major sulfur pools. Note that sufficient porewater was obtained to measure sulfate isotopes by pooling five deep sediment samples, which are thus an average value. The shaded gray region indicates the sulfate depletion zone.
In mid-column sediments between 150 and 760 cm, S0 and AVS are barely or not detectable, whereas HAS concentrations are relatively constant (0.42–4.40 µmol g−1 dry sed) and CRS levels fluctuate widely (0–270 µmol g−1 dry sed). The highest concentrations of CRS and HAS are associated with lacustrine deposits (Fig. 3a; see also Tables S1 and S2 in the Supplement). In the deep euxinic sediments, exceptionally high CRS contents were detected in a handful of samples that are typically lacustrine deposits and follow broadly the same trend as solid FeIII and dissolved Fe2+ concentrations (Fig. 3b, c).
At 760 cm, a second SDZ has been described based on an upward-diffusing gradient of sulfate thought to originate from a subterranean aquifer (Berg et al., 2022). The change in redox conditions at 775 cm depth is marked by a small peak in AVS (9 µmol g−1 dry sed) and S0 (23 µmol g−1 dry sed). In concurrence with extant oxidizing conditions, late glacial sediments below 790 cm are poor in reduced sulfur and organic matter but contain measurable iron oxides and up to 0.2 mmol L−1 sulfate in porewaters (Fig. 3b, c). These sulfate concentrations are much lower than concentrations of sulfate in lake bottom water (1.8 mmol L−1) but are in the same general range as a subaquatic spring (0.27 mmol L−1) and a surface spring (0.17 mmol L−1).
Sufficient sulfate for isotopic analyses could only be obtained by pooling porewater from the entire late glacial sediment sequence (790–910 cm). This averaged sulfate isotopic signature is relatively light (+7 ‰; Fig. 3d), which is more similar to values measured in subaquatic (+12 ‰) and surface (+15 ‰) springs. Isotopic values of reduced sulfur pools fluctuate between positive and negative values with no discernible trend in the mid-column sediment from 150 to 600 cm depth. In the deep SDZ, δ34S-AVS becomes strongly negative, exhibiting values as low as −34 ‰, coinciding with lighter porewater sulfate (+6.8 ‰). CRS is more depleted in 34S than AVS in this zone, with extremely light values of −47.5 ‰ at 760 cm, which is equivalent to a fractionation of 54 ‰ compared to deep porewater sulfate. δ34S-HAS is consistently less negative than AVS and CRS, varying between 0 ‰ and −26 ‰ down to 750 cm depth in respective sediment layers. No significant difference in HAS isotopic composition was found between sediment layer types.
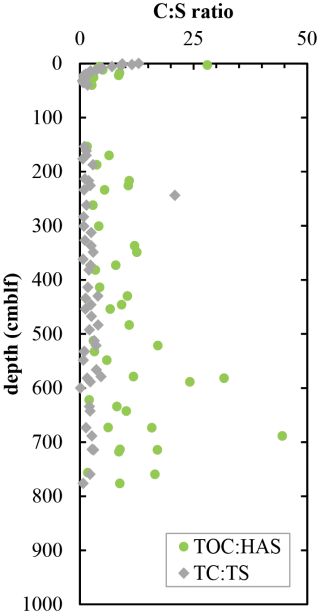
C to S ratios of organic matter are expected to decrease when sulfide reacts with organic matter to form organic S or when microorganisms preferentially degrade organic carbon and leave behind organic S. TC : TS decreases from 12 at the surface to about 1.3 at 20 cm depth and remains relatively constant throughout the deeper sediments (Fig. 4). Most of this carbon is in organic form, with measurable contributions of total inorganic carbon (TIC) present only in surface sediment and again at 400 cm depth (< 2 wt %; Fig. 2b). The apparent outlier at 243 cm is due to very low sulfur concentrations measured in this sample. A part of the total organic sulfur could be measured as HAS, and the ratios of TOC : HAS exhibit a decrease from the surface to 25 cm depth (Fig. 4). Below 150 cm depth, ratios of TOC : HAS fluctuate widely, following the same trend as TOC (r2 = 0.31) with an even stronger correlation (r2 = 0.81) among terrestrial sediments only. In fact, there is a significant difference (p < 0.0001) in TOC : HAS ratios between sediment layers of lacustrine and terrestrial origin (Student's t-test).
3.2 Genetic potential for microbial sulfur cycling
Abundances of sulfur-cycling microorganisms in the Lake Cadagno sediment column were assessed by qPCR of functional genes for sulfate reduction (dsrB) and sulfur oxidation (soxB) (Fig. 5). Copy numbers of dsrB gradually decrease from surface sediments (4.23 × 108 copies g−1 wet sediment) to the upper SDZ (7.17 × 106 copies g−1, 44 cm depth). Throughout the SDZ (35 cm and below), gene copy numbers remain relatively stable between 1.58 × 106 and 2.9 × 107 copies g−1 wet sediment. Within the lower sulfate–methane depletion zone at around 810 cm depth, dsrB copy numbers drop off greatly, to values of 101 and 3.10 × 103 copies g−1 before increasing again to 2.77 × 104 copies g−1 in parallel with increasing sulfate concentrations in the underlying oxic, glacial interval (Fig. 5; also see Fig. 2c).
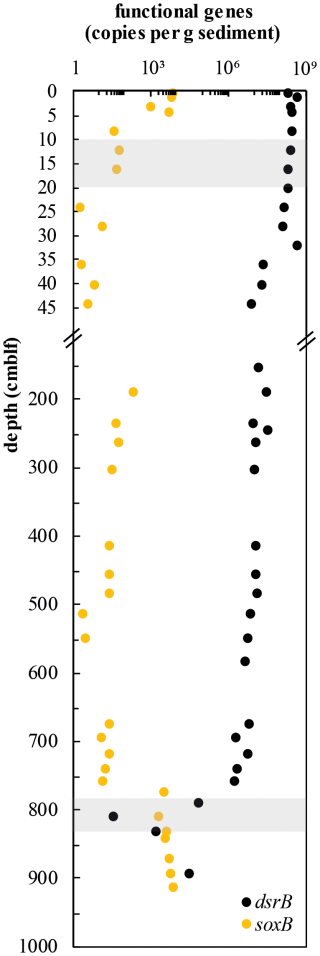
Figure 5Depth profiles of dsrB and soxB gene copy numbers. Copies of both genes were detectable by qPCR in all samples targeted. Shaded gray regions indicate sulfate–methane depletion zones.
Surprisingly, soxB was detectable throughout the entire sediment column. The highest values (up to 6.45 × 103 copies g−1 sediment) were found in the sulfate-rich surface sediments down to the SDZ. In mid-column sediments, sulfur oxidation gene copies were much lower (1.84 × 100–1.93 × 102 copies g−1) before increasing again in the lower SDZ and reaching a second peak in the glacial sediment layer (6.59 × 103 copies g−1). This increase in sulfur oxidation potential matches the oxidizing, and most likely oxic, conditions in this deep glacial sediment layer that are indicated by the presence of Fe-oxides, elemental sulfur, and sulfate (Fig. 3a–c). Sulfur-oxidizing bacteria appear to make up a large part of the total microbial population in this layer, with an average ratio of soxB to 16S DNA copies g−1 sediment of 1.17 ± 1.34. At the same time, 16S qPCR data indicate a drop in microbial population size from 108 copies g−1 sediment in the lower SDZ to 103 to 105 copies g−1 in the deep glacial layer (Berg et al., 2022).
3.3 Diversity of sulfate-reducing microorganisms
Sequencing of the sulfate reduction gene (dsrB) revealed a diverse assembly of potential sulfate reducers in Lake Cadagno sediments (Fig. 6). The majority of sequences could not be classified beyond the supergroup level, indicating that they belong to novel lineages. Overall, the sulfate reducers identified in our gene amplicon libraries were consistent with those identified in 16S rRNA gene libraries, with high relative abundances of Deltaproteobacteria, Nitrospirae, and Chloroflexota (Berg et al., 2022). The community profile shows a clear differentiation between surface sediment and deeper sulfate-depleted layers, and there is a net decrease in taxonomic diversity with depth and sediment age.
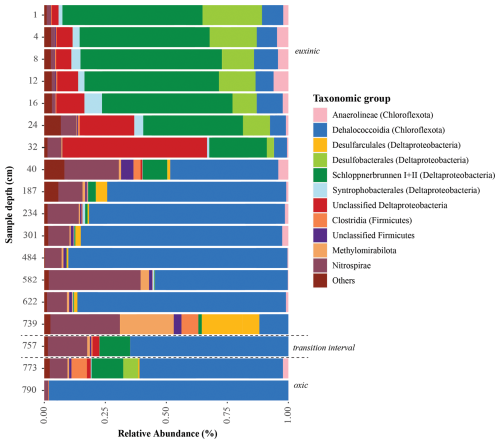
Figure 6Taxonomic classification of functional genes dsrB recovered from the Lake Cadagno sediment depths. Sediment geological transitions are indicated with dashed lines.
Similar to other sulfate-rich sedimentary environments, Lake Cadagno surface sediments harbor highly abundant (> 80 % relative abundance) Desulfobacterota (formerly Deltaproteobacteria). Most of these belong to uncultured members of the order Desulfobacterales, clade Schlöppnerbrunnen I + II (originally identified in peatland soils), and other unclassified Deltaproteobacteria. In addition, reads belonging to the genus Desulfomonile, of which members are known to also disproportionate sulfur intermediates (Slobodkin and Slobodkina, 2019), are well represented in sediments between 4 and 28 cm below the sediment surface (2 %–8 % of the total community).
Below the SDZ at 40 cm, there is a shift in the sulfate-reducing microbial assemblage toward the dominance of uncultivated Chloroflexota. A second compositional shift occurs in deeper layers around the redox transition interval at 739 cm depth. Genes for sulfate reduction in these layers affiliate with Clostridiales, Dehalococcoidia, Methylomirabilales, and the phylum Nitrospirae. Sulfate reducers belonging to Desulfobacterota also reappear close to the redox transition but are distinct from those in surface sediments, affiliating mostly with the species Desulfoarculus baarsii (classified within the order Desulfarculales). In the deep glacial sediment (> 790 cm), the diversity of microbial sulfate reducers is reduced (98 % of dsrB sequence reads) to Chloroflexota from the classes Anaerolineae and Dehalococcoidia.
4.1 Cycles of sulfate reduction and sulfide oxidation drive isotope fractionation in both surface and deep sediments
The relatively heavy isotopic signature of sulfate in Lake Cadagno bottom waters (δ34S = +24 ‰) compared to the δ34S of source waters observed in subaquatic +12 ‰ and surface springs +15 ‰ indicates active sulfate reduction in the anoxic lower part of the water column. These values are consistent with previously measured values of surface (+12 ‰) and bottom water (+30 ‰) sulfate (Canfield et al., 2010). The δ34S values of sulfate in the Lake Cadagno springs are the same as those of other springs in the Valle Leventina (Steingruber et al., 2020), indicating dissolution of gypsum dolomite in the marine evaporites from the Middle Triassic (δ34S circa +15 ‰) as the main source (Bernasconi et al., 2017).
In Lake Cadagno surface sediments (above the first mass movement deposit at 30 cm), the relatively large differences (44 ‰ to 66 ‰) in δ34S between sulfate and CRS are in the same range as those measured in sediment incubations (Canfield et al., 2010). Interestingly, δ34S sulfate profiles measured more than 30 years apart coincide surprisingly well, with sulfate becoming progressively heavier with depth (Fig. 2d), as is typical of closed system sulfate reduction.
Microbial sulfur cycling in the sulfate-rich uppermost sediment layer (0–24 cm) appears to be primarily driven by Desulfobacterota. Below this depth, mostly uncultured groups of unclassified Deltaproteobacteria and Dehalococcoidia possess an unexplored genetic potential for sulfate reduction. The high input of labile, microbial organic carbon from the overlying water column supports very high rates of anaerobic organic carbon mineralization within the upper 20 cm (Gajendra et al., 2023). As a result, TOC drops from 15 wt %–20 wt % at the lake floor to values of ≤ 5 wt % below 20 cm. Microbial sulfate reduction appears to be primarily linked to the degradation of this organic matter (Berg et al., 2022), though sulfate-driven AOM may additionally occur within the top 2–3 cm (Schubert et al., 2011). Vertical shifts in dominant S-cycling microorganisms from surface sediments (Schloppnerbrunnen I + II, Desulfobacterales (all Desulfobacterota) to unclassified Desulfobacterota in deeper layers suggest a key influence of sulfate concentrations and organic matter quality on sulfate-reducing microbial community structure. Herein, Desulfobacterales include several known sulfate-reducing and sulfur-disproportionating genera, such as Desulfobulbus, Desulfovibrio, and Desulfomonile (Cypionka et al., 1998; Hashimoto et al., 2022; Wasmund et al., 2017). Similar shifts in dominant sulfate-reducing communities from significant abundances of known groups with cultured representatives in surface layers to dominance of physiologically unclassified taxa in deeper layers have been reported from marine sediments (Jochum et al., 2017). We also detected genes for sulfur oxidation despite the anoxic nature of these sediments. These genes possibly belong to the facultatively anaerobic bacterial genus Sulfuricurvum, which was previously identified in surface sediments (Berg et al., 2022). Sufficient iron oxides (and likely manganese oxides) are also available to drive reoxidation of sulfide to elemental sulfur (Fig. 2a, b).
As sulfate concentrations drop below detection below 30 cm depth, there is a clear shift in dominance from Desulfobacterota to Chloroflexota (Fig. 6). Members of this phylum have so far not been demonstrated to perform dissimilatory sulfur cycling, but Chloroflexota dsrB sequences have been found in deep sedimentary marine environments (Vuillemin et al., 2020). In the mid-column sediments, dsrB gene abundances remain high, suggesting that sulfate/sulfur reduction likely continues in sulfate-depleted sediments parallel to fermentation and methanogenic metabolisms. It is possible that sulfate or other oxidized sulfur species, which are regenerated by sulfur oxidation reactions with metal oxides, support these communities of S-cycling microorganisms. Alternatively, the high abundances of Chloroflexota from the class Dehalococcoidia could suggest alternative sulfur-based metabolic activities. Several studies have proposed Chloroflexota to be involved in the metabolism of organic sulfur compounds (Mehrshad et al., 2018; Wasmund et al., 2014), with genomic analyses from deep sea sediments indicating genetic potential for dimethylsulfide, methane sulfonate, and alkane sulfonate metabolisms (Liu et al., 2022). Overall, our findings are consistent with studies of marine sediments demonstrating active sulfate reduction below the zone of sulfate depletion (Brunner et al., 2016; Holmkvist et al., 2011; Pellerin et al., 2018; Treude et al., 2014) and suggest that deep sulfate reduction can also occur in lake sediments.
An oxidizing front and a constant groundwater supply of sulfate at the transition between euxinic and deep glacial deposits appear to sustain continuous microbial sulfur cycling at 760–800 cm depth. Pools of reduced sulfur are, on average, more depleted in δ34S in the deep glacial sediments than in surface sediments, but the differences between porewater sulfate and HAS (εsulfate-HAS = 14 ‰–32 ‰), AVS (εsulfate-AVS = 9 ‰–45 ‰), and CRS (εsulfate-CRS = 36 ‰–53 ‰) in these deep layers are actually smaller. Nonetheless, the values should be taken with caution as only one value of δ34S-sulfate (+7 ‰), representing a pooled average of samples from 790 to 850 cm sediment depth, could be measured below 760 cm due to low porewater yields. This relatively light pool of sulfate could represent a mixture of groundwater sulfate (+12 ‰) and sulfate produced through oxidation of sedimentary AVS and CRS by groundwater-derived oxidants. The potential for sulfur oxidation is supported by a strong increase in soxB gene copies (Fig. 5), along with the presence of abundant elemental S0 (Fig. 3a) at the deep redox transition. Light sulfate is likely the main determinant of the highly negative δ34S values of reduced sulfur.
4.2 Rapid degradation and sulfurization are controlled by organic matter quality
Major changes in solid-phase sulfur pools occur within the upper 20 cm of sediment, corresponding to the very high rates of organic matter degradation and microbial sulfate reduction (Berg et al., 2022). These changes include a shift from elemental S and AVS as the dominant S pools at 0–10 cm to CRS (which contains pyrite) as the main S pool between 10 and 20 cm. This CRS likely derives from chemical reactions of AVS (containing FeS) and elemental sulfur (including polysulfides) (Luther, 1991). Below 20 cm, CRS remains the dominant S pool and, moreover, shows a highly significant (p < 0.001) enrichment in lacustrine layers versus mass movement deposits (Student's t-test). The elevated CRS in lacustrine layers confirms the notion that most iron sulfides are of authigenic (rather than terrestrial) origin and result from anaerobic breakdown of lacustrine organic matter driving sulfate reduction.
In addition to CRS formation, we observe the sulfurization of organic matter in these (< 100-year-old) surface sediments, as indicated by the strong increase in HAS (up to 10 µmol g−1 sed) in the top 0–10 cm, along with a drop in TOC : HAS ratios (Fig. 4). This observation is consistent with previous studies on Lake Cadagno and other lakes in Switzerland, which reported that most organic matter sulfurization occurs within the initial decades after sediment deposition (Hebting et al., 2006; Urban et al., 1999). Although sulfurized humic acids persist in the older sediment layers, a substantial fraction of the HAS appears to be lost at 10-15 cm depth. The resulting release of sulfide from HAS could explain the more positive δ34S-CRS values despite the concurrent depletion in 34S-sulfate at 10–15 cm sediment depth. This would match previous observations on estuarine sediments, where sulfide released from fulvic acids was shown to contribute to 34S-enriched CRS (Brüchert, 1998).
It is also possible that variations in HAS concentrations are simply due to sediment origin, as shown by the strong correlation between TOC : HAS and sediment layer type (p < 0.0001). Lacustrine sediment enriched in HAS is interspersed with HAS-poor mass movement deposits containing predominantly terrestrial material beginning at 18–22 cm depth and also forming thicker deposits, such as from 31 to 55 cm depth (Fig. 1). This suggests that fresh lacustrine organic matter from the lake water column is more easily sulfurized than refractory terrestrial organic matter from the lake watershed.
While humic acids comprise only a small part of total organic sulfur in some sediments, they have been shown to be a highly persistent and major component of buried organic carbon (Huc and Durand, 1977). In Lake Cadagno sediments, we estimate that an average of 2.6 ± 3.2 % of total organic carbon is extracted as HA and is expected to contain reduced sulfur in the form of organic sulfides or thiols (Brüchert, 1998; Ferdelman et al., 1991; Urban et al., 1999). While sulfurized carotenoids were detected by Hebting et al. (2006), the chemical composition of most of this sulfurized organic matter remains unclear. It is worth noting that while most lipid-rich algal and microbial materials are rapidly degraded in surface sediments, carbohydrates appear to be selectively preserved, thus increasing their contribution to total organic matter in deeper layers (Gajendra et al., 2023). This effective preservation of carbohydrates could be related to macromolecular matrices that are rich in degradation-resistant structural polymers (e.g., hemicelluloses and pectin in microalgal and terrestrial plant biomass; Gajendra et al., 2023). In addition, the high chemical reactivity of carbohydrates with hydrogen sulfide could play a role. Past research has shown that carbonyl functional groups are more reactive with inorganic sulfur species than hydroxyl groups, explaining why carbohydrates with a carbonyl group in the C1 position constitute a major part of sulfurized organic matter in marine sediments (Damsté et al., 1998) and in laboratory experiments (Kok et al., 2000). The same could be the case in the deeper sediment layers of Lake Cadagno. Herein, sulfurization could even contribute to the effective preservation of carbohydrates in these layers. Alternatively, it is possible that sulfur incorporated into carbohydrates is selectively preserved as organic S because the surrounding carbohydrate matrices are degradation resistant. Nonetheless, further research is needed to ascertain the extent to which diagenesis has influenced the total organic sulfur pool (e.g., kerogen sulfur and polar bitumen sulfur) and thus carbon preservation over time.
Interestingly, organic sulfur in the deepest part of the Lake Cadagno sediment column (> 600 cm sediment depth) is more enriched in δ34S than co-occurring pyrite. This is consistent with measurements from marine systems (Anderson and Pratt, 1995; Brüchert, 1998; Goldhaber and Kaplan, 1980; Raven et al., 2016, 2023; Werne et al., 2003). Because the fractionation factor of organic matter sulfurization is almost negligible (Amrani and Aizenshtat, 2004), it is likely that differences in the timing of formation explain why the δ34S-HAS in deeper layers is, on average, higher than the δ34S-CRS. HAS may originate in shallow sediment layers, e.g., from 34S-enriched polysulfides produced by partial sulfide oxidation (Brüchert, 1998; Putschew et al., 1996; Werne et al., 2008), and preserves its chemical signature during burial due to high chemical stability. By contrast, groundwater-derived oxidants may cause part of the CRS pool to be re-oxidized and undergo additional fractionation cycles in this deepest sediment layer that directly overlies the aquifer.
Here, we report the first biogeochemical and isotopic data on sulfur cycling in ancient (up to 13.5 kyr) sediments of Lake Cadagno. With its moderate water column sulfate concentrations (2mM), this lake represents an intermediate between typical high-sulfate marine and low-sulfate lake sedimentary environments. In addition to confirming the rapid sulfurization of organic matter, we document two separate zones of reduced sulfur formation within the same sediment column: one close to the surface and a second in deeply buried sediments at the base of the sediment column. This deep sulfate-reduction zone is fed by sulfate-rich, oxidizing groundwater, which both drives the reoxidation of sulfides and leaves an isotopic imprint on newly formed reduced sulfur compounds. The deep sulfate reduction zone in Lake Cadagno has parallels to terrestrial aquifers (Porowski et al., 2019), submarine groundwater discharge sites (McAllister et al., 2015), and even deep crustal aquifers (Engelen et al., 2008), within which reworking and overprinting of buried δ34S by new δ34S signatures is likely to occur. Deep groundwater-driven sulfur cycling may be more widespread than presently recognized, as submarine groundwater seepage is a common phenomenon and the basaltic ocean crust is the largest aquifer system on Earth.
Deep sulfur cycling in Lake Cadagno appears to be driven by a diverse and uncultivated biosphere. Most dsrB lineages recovered here belong to novel taxa whose role in sulfur and carbon cycling has yet to be revealed. These microorganisms, such as Chloroflexota, are not classical sulfate-reducing bacteria that have been physiologically characterized in the laboratory, and much is yet to be understood about their metabolisms. Nonetheless, the recovery of sulfur oxidation genes (soxB) from presumably anoxic surface sediments and sulfate reduction genes (dsrB) from sulfate-depleted deep sediments opens new questions about deep sources of oxidants, which could drive continued sulfur cycling in such reducing environments.
dsrB gene sequences have been deposited in the NCBI database under Bioproject number PRJNA991470. All data are provided in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-5483-2025-supplement.
JSB performed sediment sampling and chemical analyses, synthesized the data, and wrote the manuscript. PCR and LD performed microbial community analyses and interpretations under the supervision of CM. SB provided S-isotope data from 1991 to 2019. HV and MM performed sediment sampling and sedimentological characterizations and dating. MAL supervised this project. All co-authors contributed to the revision and editing of this manuscript.
At least one of the (co-)authors is a member of the editorial board of Biogeosciences. The peer-review process was guided by an independent editor, and the authors have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank the entire 2019 Cadagno sampling crew for assistance in the field and especially the Alpine Biology Center Foundation (Switzerland) for the use of its research facilities. We also acknowledge Iso Christl, Rachele Ossola, and Jorge Spangenberg for their support with chemical analyses. We would like to thank Kurt Hanselmann for the dialysis porewater samplers and for the support in the field sampling in 1991.
This research has been supported by the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant no. 182096). Longhui Deng was sponsored by the Science and Technology Commission of Shanghai Municipality, Shanghai Pujiang Program (22PJ1404800) (grant no. 23R1428500).
This paper was edited by Tina Treude and reviewed by Morgan Raven and one anonymous referee.
Amrani, A. and Aizenshtat, Z.: Mechanisms of sulfur introduction chemically controlled: δ34S imprint, Org. Geochem., 35, 1319–1336, https://doi.org/10.1016/j.orggeochem.2004.06.019, 2004.
Anantharaman, K., Hausmann, B., Jungbluth, S. P., Kantor, R. S., Lavy, A., Warren, L. A., Rappé, M. S., Pester, M., Loy, A., Thomas, B. C., and Banfield, J. F.: Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle, ISME J., 12, 1715–1728, https://doi.org/10.1038/s41396-018-0078-0, 2018.
Anderson, T. F. and Pratt, L. M.: Isotopic Evidence for the Origin of Organic Sulfur and Elemental Sulfur in Marine Sediments, in: Geochemical Transformations of Sedimentary Sulfur, American Chemical Society, vol. 612, 378–396, https://doi.org/10.1021/bk-1995-0612.ch021, 1995.
Baloza, M., Henkel, S., Geibert, W., Kasten, S., and Holtappels, M.: Benthic Carbon Remineralization and Iron Cycling in Relation to Sea Ice Cover Along the Eastern Continental Shelf of the Antarctic Peninsula, J. Geophys. Res.-Oceans, 127, e2021JC018401, https://doi.org/10.1029/2021JC018401, 2022.
Berg, J. S., Lepine, M., Laymand, E., Han, X., Vogel, H., Morlock, M. A., Gajendra, N., Gilli, A., Bernasconi, S. M., Schubert, C. J., Su, G., and Lever, M. A.: Ancient and Modern Geochemical Signatures in the 13,500-Year Sedimentary Record of Lake Cadagno, Front. Earth Sci., 9, https://doi.org/10.3389/feart.2021.754888, 2022.
Bernasconi, S. M., Meier, I., Wohlwend, S., Brack, P., Hochuli, P. A., Bläsi, H., Wortmann, U. G., and Ramseyer, K.: An evaporite-based high-resolution sulfur isotope record of Late Permian and Triassic seawater sulfate, Geochim. Cosmochim. Acta, 204, 331–349, https://doi.org/10.1016/j.gca.2017.01.047, 2017.
Bowles, M. W., Mogollón, J. M., Kasten, S., Zabel, M., and Hinrichs, K.-U.: Global rates of marine sulfate reduction and implications for sub-sea-floor metabolic activities, Science, 344, 889–891, https://doi.org/10.1126/science.1249213, 2014.
Bradley, A. S., Leavitt, W. D., Schmidt, M., Knoll, A. H., Girguis, P. R., and Johnston, D. T.: Patterns of sulfur isotope fractionation during microbial sulfate reduction, Geobiology, 14, 91–101, https://doi.org/10.1111/gbi.12149, 2016.
Brandl, H., and Hanselmann, K. W.: Evaluation and application of dialysis porewater samplers for microbiological studies at sediment-water interfaces, Aquatic Sciences, 53, 55–73, 1991.
Brüchert, V., and Pratt, L. M.: Contemporaneous early diagenetic formation of organic and inorganic sulfur in estuarine sediments from St. Andrew Bay, Florida, USA, Geochimica et Cosmochimica Acta, 60, 2325–2332, 1996.
Brüchert, V.: Early diagenesis of sulfur in estuarine sediments: the role of sedimentary humic and fulvic acids, Geochim. Cosmochim. Acta, 62, 1567–1586, https://doi.org/10.1016/S0016-7037(98)00089-1, 1998.
Brunner, B. and Bernasconi, S. M.: A revised isotope fractionation model for dissimilatory sulfate reduction in sulfate reducing bacteria, Geochim. Cosmochim. Acta, 69, 4759–4771, https://doi.org/10.1016/j.gca.2005.04.015, 2005.
Brunner, B., Arnold, G. L., Røy, H., Müller, I. A., and Jørgensen, B. B.: Off Limits: Sulfate below the Sulfate-Methane Transition, Front. Earth Sci., 4, https://doi.org/10.3389/feart.2016.00075, 2016.
Bryant, R. N., Houghton, J. L., Jones, C., Pasquier, V., Halevy, I., and Fike, D. A.: Deconvolving microbial and environmental controls on marine sedimentary pyrite sulfur isotope ratios, Science, 382, 912–915, https://doi.org/10.1126/science.adg6103, 2023.
Canfield, D. E., Boudreau, B. P., Mucci, A., and Gundersen, J. K.: The early diagenetic formation of organic sulfur in the sediments of Mangrove Lake, Bermuda, Geochimica et Cosmochimica Acta, 62, 767–781, 1998.
Canfield, D. E., Farquhar, J., and Zerkle, A. L.: High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog, Geology, 38, 415–418, https://doi.org/10.1130/G30723.1, 2010.
Chanton, J. P., Martens, C. S., and Goldhaber, M. B.: Biogeochemical cycling in an organic-rich coastal marine basin. 7. Sulfur mass balance, oxygen uptake and sulfide retention, Geochimica et Cosmochimica Acta, 51, 1187–1199, 1987.
Cypionka, H., Smock, A. M., and Böttcher, M. E.: A combined pathway of sulfur compound disproportionation in Desulfovibrio desulfuricans, FEMS Microbiol. Lett., 166, 181–186, https://doi.org/10.1111/j.1574-6968.1998.tb13888.x, 1998.
Damsté, J. S. and De Leeuw, J. W.: Analysis, structure and geochemical significance of organically-bound sulphur in the geosphere: State of the art and future research, Org. Geochem., 16, 1077–1101, https://doi.org/10.1016/0146-6380(90)90145-P, 1990.
Damsté, J. S., Kok, M. D., Köster, J., and Schouten, S.: Sulfurized carbohydrates: an important sedimentary sink for organic carbon?, Earth Planet. Sci. Lett., 164, 7–13, https://doi.org/10.1016/S0012-821X(98)00234-9, 1998.
David, M. B. and Mitchell, M. J.: Sulfur constituents and cycling in waters, seston, and sediments of an oligotrophic lake, Limnol. Oceanogr., 30, 1196–1207, https://doi.org/10.4319/lo.1985.30.6.1196, 1985.
Deng, L., Meile, C., Fiskal, A., Bölsterli, D., Han, X., Gajendra, N., Dubois, N., Bernasconi, S. M., and Lever, M. A.: Deposit-feeding worms control subsurface ecosystem functioning in intertidal sediment with strong physical forcing, PNAS Nexus, 1, pgac146, https://doi.org/10.1093/pnasnexus/pgac146, 2022.
Detmers, J., Brüchert, V., Habicht, K. S., and Kuever, J.: Diversity of Sulfur Isotope Fractionations by Sulfate-Reducing Prokaryotes, Appl. Environ. Microbiol., 67, 888–894, https://doi.org/10.1128/AEM.67.2.888-894.2001, 2001.
Engelen, B., Ziegelmüller, K., Wolf, L., Köpke, B., Gittel, A., Cypionka, H., Treude, T., Nakagawa, S., Inagaki, F., Lever, M. A., and Steinsbu, B. O.: Fluids from the Oceanic Crust Support Microbial Activities within the Deep Biosphere, Geomicrobiol. J., 25, 56–66, https://doi.org/10.1080/01490450701829006, 2008.
Ferdelman, T. G., Church, T. M., and Luther, G. W.: Sulfur enrichment of humic substances in a Delaware salt marsh sediment core, Geochim. Cosmochim. Acta, 55, 979–988, https://doi.org/10.1016/0016-7037(91)90156-Y, 1991.
Fike, D. A., Bradley, A. S., and Rose, C. V.: Rethinking the Ancient Sulfur Cycle, Annu. Rev. Earth Planet. Sci., 43, 593–622, https://doi.org/10.1146/annurev-earth-060313-054802, 2015.
Fossing, H. and Jørgensen, B. B.: Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method, Biogeochemistry, 8, 205–222, https://doi.org/10.1007/BF00002889, 1989.
Gajendra, N., Berg, J. S., Vogel, H., Deng, L., Wolf, S. M., Bernasconi, S. M., Dubois, N., Schubert, C. J., and Lever, M. A.: Carbohydrate compositional trends throughout Holocene sediments of an alpine lake (Lake Cadagno), Front. Earth Sci., 11, 1047224, https://doi.org/10.3389/feart.2023.1047224, 2023.
Goldhaber, M. B. and Kaplan, I. R.: Controls and consequences of sulfate reduction rates in recent marine sediments, Soil Sci., 119, 42, https://doi.org/10.2136/sssaspecpub10.c2, 1975.
Goldhaber, M. B. and Kaplan, I. R.: Mechanisms of sulfur incorporation and isotope fractionation during early diagenesis in sediments of the gulf of California, Mar. Chem., 9, 95–143, https://doi.org/10.1016/0304-4203(80)90063-8, 1980.
Habicht, K. S. and Canfield, D. E.: Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments, Geochim. Cosmochim. Acta, 61, 5351–5361, https://doi.org/10.1016/S0016-7037(97)00311-6, 1997.
Hansel, C. M., Lentini, C. J., Tang, Y., Johnston, D. T., Wankel, S. D., and Jardine, P. M.: Dominance of sulfur-fueled iron oxide reduction in low-sulfate freshwater sediments, ISME J., 9, 2400–2412, https://doi.org/10.1038/ismej.2015.50, 2015.
Hartmann, M. and Nielsen, H.: δ34S values in recent sea sediments and their significance using several sediment profiles from the western Baltic Sea, Isotopes Environ. Health Stud., 48, 7–32, https://doi.org/10.1080/10256016.2012.660528, 2012.
Hashimoto, Y., Shimamura, S., Tame, A., Sawayama, S., Miyazaki, J., Takai, K., and Nakagawa, S.: Physiological and comparative proteomic characterization of Desulfolithobacter dissulfuricans gen. nov., sp. nov., a novel mesophilic, sulfur-disproportionating chemolithoautotroph from a deep-sea hydrothermal vent, Front. Microbiol., 13, https://doi.org/10.3389/fmicb.2022.1042116, 2022.
Hebting, Y., Schaeffer, P., Behrens, A., Adam, P., Schmitt, G., Schneckenburger, P., Bernasconi, S. M., and Albrecht, P.: Biomarker Evidence for a Major Preservation Pathway of Sedimentary Organic Carbon, Science, 312, 1627–1631, https://doi.org/10.1126/science.1126372, 2006.
Holmkvist, L., Kamyshny, A., Vogt, C., Vamvakopoulos, K., Ferdelman, T. G., and Jørgensen, B. B.: Sulfate reduction below the sulfate–methane transition in Black Sea sediments, Deep-Sea Res. Part Oceanogr. Res. Pap., 58, 493–504, https://doi.org/10.1016/j.dsr.2011.02.009, 2011.
Huc, A. Y. and Durand, B. M.: Occurrence and significance of humic acids in ancient sediments, Fuel, 56, 73–80, https://doi.org/10.1016/0016-2361(77)90046-1, 1977.
Jochum, L. M., Chen, X., Lever, M. A., Loy, A., Jørgensen, B. B., Schramm, A., and Kjeldsen, K. U.: Depth Distribution and Assembly of Sulfate-Reducing Microbial Communities in Marine Sediments of Aarhus Bay, Appl. Environ. Microbiol., 83, https://doi.org/10.1128/AEM.01547-17, 2017.
Jørgensen, B. B.: Mineralization of organic matter in the sea bed – the role of sulphate reduction, Nature, 296, 643–645, https://doi.org/10.1038/296643a0, 1982.
Jørgensen, B. B., Böttcher, M. E., Lüschen, H., Neretin, L. N., and Volkov, I. I.: Anaerobic methane oxidation and a deep H2S sink generate isotopically heavy sulfides in Black Sea sediments 1, Geochim. Cosmochim. Acta, 68, 2095–2118, https://doi.org/10.1016/j.gca.2003.07.017, 2004.
Kallmeyer, J., Ferdelman, T. G., Weber, A., Fossing, H., and Jørgensen, B. B.: A cold chromium distillation procedure for radiolabeled sulfide applied to sulfate reduction measurements, Limnol. Oceanogr. Methods, 2, 171–180, https://doi.org/10.4319/lom.2004.2.171, 2004.
Kaplan, I. R. and Rittenberg, S. C. Y. 1964: Microbiological Fractionation of Sulphur Isotopes, Microbiology, 34, 195–212, https://doi.org/10.1099/00221287-34-2-195, 1964.
Klein, M., Friedrich, M., Roger, A. J., Hugenholtz, P., Fishbain, S., Abicht, H., Blackall, L. L., Stahl, D. A., and Wagner, M.: Multiple Lateral Transfers of Dissimilatory Sulfite Reductase Genes between Major Lineages of Sulfate-Reducing Prokaryotes, J. Bacteriol., 183, 6028–6035, https://doi.org/10.1128/JB.183.20.6028-6035.2001, 2001.
Kok, M. D., Schouten, S., and Sinninghe Damsté, J. S.: Formation of insoluble, nonhydrolyzable, sulfur-rich macromolecules via incorporation of inorganic sulfur species into algal carbohydrates, Geochim. Cosmochim. Acta, 64, 2689–2699, https://doi.org/10.1016/S0016-7037(00)00382-3, 2000.
Lever, M. A., Rouxel, O., Alt, J. C., Shimizu, N., Ono, S., Coggon, R. M., Shanks, W. C., Lapham, L., Elvert, M., Prieto-Mollar, X., Hinrichs, K.-U., Inagaki, F., and Teske, A.: Evidence for Microbial Carbon and Sulfur Cycling in Deeply Buried Ridge Flank Basalt, Science, 339, 1305–1308, https://doi.org/10.1126/science.1229240, 2013.
Lever, M. A., Torti, A., Eickenbusch, P., Michaud, A. B., Šantl-Temkiv, T., and Jørgensen, B. B.: A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types, Front. Microbiol., 6, https://doi.org/10.3389/fmicb.2015.00476, 2015.
Liu, R., Wei, X., Song, W., Wang, L., Cao, J., Wu, J., Thomas, T., Jin, T., Wang, Z., Wei, W., Wei, Y., Zhai, H., Yao, C., Shen, Z., Du, J., and Fang, J.: Novel Chloroflexi genomes from the deepest ocean reveal metabolic strategies for the adaptation to deep-sea habitats, Microbiome, 10, 75, https://doi.org/10.1186/s40168-022-01263-6, 2022.
Losher, A.: The sulfur cycle in freshwater lake sediments and implications for the use of C/S ratios as indicators of past environmental changes, Dr. diss., ETH Zurich, https://doi.org/10.3929/ethz-a-000569398, 1989.
Ludwig, W., Strunk, O., Westram, R., Richter, L., Meier, H., Yadhukumar, Buchner, A., Lai, T., Steppi, S., Jobb, G., Förster, W., Brettske, I., Gerber, S., Ginhart, A. W., Gross, O., Grumann, S., Hermann, S., Jost, R., König, A., Liss, T., Lüßmann, R., May, M., Nonhoff, B., Reichel, B., Strehlow, R., Stamatakis, A., Stuckmann, N., Vilbig, A., Lenke, M., Ludwig, T., Bode, A., and Schleifer, K.-H.: ARB: a software environment for sequence data, Nucleic Acids Research, 32, 1363–1371, https://doi.org/10.1093/nar/gkh293, 2004.
Luther, G. W.: Pyrite synthesis via polysulfide compounds, Geochim. Cosmochim. Acta, 55, 2839–2849, https://doi.org/10.1016/0016-7037(91)90449-F, 1991.
Maynard, J. B.: Sulfur isotopes of iron sulfides in Devonian-Mississippian shales of the Appalachian basin: control by rate of sedimentation, Am. J. Sci. US, 280, https://doi.org/10.2475/ajs.280.8.772, 1980.
McAllister, S. M., Barnett, J. M., Heiss, J. W., Findlay, A. J., MacDonald, D. J., Dow, C. L., Luther III, G. W., Michael, H. A., and Chan, C. S.: Dynamic hydrologic and biogeochemical processes drive microbially enhanced iron and sulfur cycling within the intertidal mixing zone of a beach aquifer, Limnol. Oceanogr., 60, 329–345, https://doi.org/10.1002/lno.10029, 2015.
Mehrshad, M., Rodriguez-Valera, F., Amoozegar, M. A., López-García, P., and Ghai, R.: The enigmatic SAR202 cluster up close: shedding light on a globally distributed dark ocean lineage involved in sulfur cycling, ISME J., 12, 655–668, https://doi.org/10.1038/s41396-017-0009-5, 2018.
Meyer, B., Imhoff, J. F., and Kuever, J.: Molecular analysis of the distribution and phylogeny of the soxB gene among sulfur-oxidizing bacteria – evolution of the Sox sulfur oxidation enzyme system, Environ. Microbiol., 9, 2957–2977, https://doi.org/10.1111/j.1462-2920.2007.01407.x, 2007.
Mitchell, M. J., Landers, D. H., and Brodowski, D. F.: Sulfur constituents of sediments and their relationship to lake acidification, Water. Air. Soil Pollut., 16, 351–359, https://doi.org/10.1007/BF01046915, 1981.
Müller, A. L., Kjeldsen, K. U., Rattei, T., Pester, M., and Loy, A.: Phylogenetic and environmental diversity of DsrAB-type dissimilatory (bi)sulfite reductases, ISME J., 9, 1152–1165, https://doi.org/10.1038/ismej.2014.208, 2015.
Nriagu, J. O. and Soon, Y. K.: Distribution and isotopic composition of sulfur in lake sediments of northern Ontario, Geochim. Cosmochim. Acta, 49, 823–834, https://doi.org/10.1016/0016-7037(85)90175-9, 1985.
Och, L. M. and Shields-Zhou, G. A.: The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling, Earth-Sci. Rev., 110, 26–57, https://doi.org/10.1016/j.earscirev.2011.09.004, 2012.
Orr, W. L. and Damsté, J. S.: Geochemistry of Sulfur in Petroleum Systems, in: Geochemistry of Sulfur in Fossil Fuels, American Chemical Society, vol. 429, 2–29, https://doi.org/10.1021/bk-1990-0429.ch001, 1990.
Pellerin, A., Antler, G., Røy, H., Findlay, A., Beulig, F., Scholze, C., Turchyn, A. V., and Jørgensen, B. B.: The sulfur cycle below the sulfate-methane transition of marine sediments, Geochim. Cosmochim. Acta, 239, 74–89, https://doi.org/10.1016/j.gca.2018.07.027, 2018.
Pester, M., Knorr, K.-H., Friedrich, M. W., Wagner, M., and Loy, A.: Sulfate-reducing microorganisms in wetlands – fameless actors in carbon cycling and climate change, Front. Microbiol., 3, https://doi.org/10.3389/fmicb.2012.00072, 2012.
Porowski, A., Porowska, D., and Halas, S.: Identification of Sulfate Sources and Biogeochemical Processes in an Aquifer Affected by Peatland: Insights from Monitoring the Isotopic Composition of Groundwater Sulfate in Kampinos National Park, Poland, Water, 11, 1388, https://doi.org/10.3390/w11071388, 2019.
Putschew, A., Scholz-Böttcher, B. M., and Rullkötter, J.: Early diagenesis of organic matter and related sulphur incorporation in surface sediments of meromictic Lake Cadagno in the Swiss Alps, Org. Geochem., 25, 379–390, https://doi.org/10.1016/S0146-6380(96)00143-X, 1996.
Raven, M. R., Sessions, A. L., Fischer, W. W., and Adkins, J. F.: Sedimentary pyrite δ34S differs from porewater sulfide in Santa Barbara Basin: Proposed role of organic sulfur, Geochim. Cosmochim. Acta, 186, 120–134, https://doi.org/10.1016/j.gca.2016.04.037, 2016.
Raven, M. R., Crockford, P. W., Hodgskiss, M. S. W., Lyons, T. W., Tino, C. J., and Webb, S. M.: Organic matter sulfurization and organic carbon burial in the Mesoproterozoic, Geochim. Cosmochim. Acta, 347, 102–115, https://doi.org/10.1016/j.gca.2023.02.020, 2023.
Rudd, J. W. M., Kelly, C. A., and Furutani, A.: The role of sulfate reduction in long term accumulation of organic and inorganic sulfur in lake sediments1, Limnol. Oceanogr., 31, 1281–1291, https://doi.org/10.4319/lo.1986.31.6.1281, 1986.
Rudnicki, M. D., Elderfield, H., and Spiro, B.: Fractionation of sulfur isotopes during bacterial sulfate reduction in deep ocean sediments at elevated temperatures, Geochim. Cosmochim. Acta, 65, 777–789, https://doi.org/10.1016/S0016-7037(00)00579-2, 2001.
Schubert, C. J., Vazquez, F., Lösekann-Behrens, T., Knittel, K., Tonolla, M., and Boetius, A.: Evidence for anaerobic oxidation of methane in sediments of a freshwater system (Lago di Cadagno), FEMS Microbiol. Ecol., 76, 26–38, https://doi.org/10.1111/j.1574-6941.2010.01036.x, 2011.
Schwarcz, H. P. and Burnie, S. W.: Influence of sedimentary environments on sulfur isotope ratios in clastic rocks: a review, Miner. Deposita, 8, 264–277, https://doi.org/10.1007/BF00203208, 1973.
Sim, M. S., Bosak, T., and Ono, S.: Large Sulfur Isotope Fractionation Does Not Require Disproportionation, Science, 333, 74–77, https://doi.org/10.1126/science.1205103, 2011.
Slobodkin, A. I. and Slobodkina, G. B.: Diversity of Sulfur-Disproportionating Microorganisms, Microbiology, 88, 509–522, https://doi.org/10.1134/S0026261719050138, 2019.
Steingruber, S. M., Bernasconi, S. M., and Valenti, G.: Climate Change-Induced Changes in the Chemistry of a High-Altitude Mountain Lake in the Central Alps, Aquat. Geochem., https://doi.org/10.1007/s10498-020-09388-6, 2020.
Stookey, L. L.: Ferrozine – a new spectrophotometric reagent for iron, ACS Publ., https://doi.org/10.1021/ac60289a016, 1970.
Treude, T., Krause, S., Maltby, J., Dale, A. W., Coffin, R., and Hamdan, L. J.: Sulfate reduction and methane oxidation activity below the sulfate-methane transition zone in Alaskan Beaufort Sea continental margin sediments: Implications for deep sulfur cycling, Geochim. Cosmochim. Acta, 144, 217–237, https://doi.org/10.1016/j.gca.2014.08.018, 2014.
Urban, N. R., Ernst, K., and Bernasconi, S.: Addition of sulfur to organic matter during early diagenesis of lake sediments, Geochim. Cosmochim. Acta, 63, 837–853, https://doi.org/10.1016/S0016-7037(98)00306-8, 1999.
Vuillemin, A., Kerrigan, Z., D'Hondt, S., and Orsi, W. D.: Exploring the abundance, metabolic potential and gene expression of subseafloor Chloroflexi in million-year-old oxic and anoxic abyssal clay, FEMS Microbiol. Ecol., 96, fiaa223, https://doi.org/10.1093/femsec/fiaa223, 2020.
Wasmund, K., Schreiber, L., Lloyd, K. G., Petersen, D. G., Schramm, A., Stepanauskas, R., Jørgensen, B. B., and Adrian, L.: Genome sequencing of a single cell of the widely distributed marine subsurface Dehalococcoidia, phylum Chloroflexi, ISME J., 8, 383–397, https://doi.org/10.1038/ismej.2013.143, 2014.
Wasmund, K., Mußmann, M., and Loy, A.: The life sulfuric: microbial ecology of sulfur cycling in marine sediments, Environ. Microbiol. Rep., 9, 323–344, https://doi.org/10.1111/1758-2229.12538, 2017.
Werne, J. P., Lyons, T. W., Hollander, D. J., Formolo, M. J., and Sinninghe Damsté, J. S.: Reduced sulfur in euxinic sediments of the Cariaco Basin: sulfur isotope constraints on organic sulfur formation, Chem. Geol., 195, 159–179, https://doi.org/10.1016/S0009-2541(02)00393-5, 2003.
Werne, J. P., Lyons, T. W., Hollander, D. J., Schouten, S., Hopmans, E. C., and Sinninghe Damsté, J. S.: Investigating pathways of diagenetic organic matter sulfurization using compound-specific sulfur isotope analysis, Geochim. Cosmochim. Acta, 72, 3489–3502, https://doi.org/10.1016/j.gca.2008.04.033, 2008.
Wirth, S. B., Gilli, A., Niemann, H., Dahl, T. W., Ravasi, D., Sax, N., Hamann, Y., Peduzzi, R., Peduzzi, S., Tonolla, M., Lehmann, M. F., and Anselmetti, F. S.: Combining sedimentological, trace metal (Mn, Mo) and molecular evidence for reconstructing past water-column redox conditions: The example of meromictic Lake Cadagno (Swiss Alps), Geochim. Cosmochim. Acta, 120, 220–238, https://doi.org/10.1016/j.gca.2013.06.017, 2013.
Wortmann, U. G., Bernasconi, S. M., and Böttcher, M. E.: Hypersulfidic deep biosphere indicates extreme sulfur isotope fractionation during single-step microbial sulfate reduction, Geology, 29, 647–650, https://doi.org/10.1130/0091-7613(2001)029<0647:HDBIES>2.0.CO;2, 2001.