the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Aphotic N2 fixation along an oligotrophic to ultraoligotrophic transect in the western tropical South Pacific Ocean
Katyanne M. Shoemaker
Pia H. Moisander
Jutta Niggemann
Thorsten Dittmar
Solange Duhamel
Olivier Grosso
Mireille Pujo-Pay
Sandra Hélias-Nunige
Alain Fumenia
Sophie Bonnet
The western tropical South Pacific (WTSP) Ocean has been recognized as a global hot spot of dinitrogen (N2) fixation. Here, as in other marine environments across the oceans, N2 fixation studies have focused on the sunlit layer. However, studies have confirmed the importance of aphotic N2 fixation activity, although until now only one had been performed in the WTSP. In order to increase our knowledge of aphotic N2 fixation in the WTSP, we measured N2 fixation rates and identified diazotrophic phylotypes in the mesopelagic layer along a transect spanning from New Caledonia to French Polynesia. Because non-cyanobacterial diazotrophs presumably need external dissolved organic matter (DOM) sources for their nutrition, we also identified DOM compounds using Fourier transform ion cyclotron resonance mass spectrometry (FTICRMS) with the aim of searching for relationships between the composition of DOM and non-cyanobacterial N2 fixation in the aphotic ocean. N2 fixation rates were low (average 0.63 ± 0.07 nmol N L−1 d−1) but consistently detected across all depths and stations, representing ∼ 6–88 % of photic N2 fixation. N2 fixation rates were not significantly correlated with DOM compounds. The analysis of nifH gene amplicons revealed a wide diversity of non-cyanobacterial diazotrophs, mostly matching clusters 1 and 3. Interestingly, a distinct phylotype from the major nifH subcluster 1G dominated at 650 dbar, coinciding with the oxygenated Subantarctic Mode Water (SAMW). This consistent pattern suggests that the distribution of aphotic diazotroph communities is to some extent controlled by water mass structure. While the data available are still too scarce to elucidate the distribution and controls of mesopelagic non-cyanobacterial diazotrophs in the WTSP, their prevalence in the mesopelagic layer and the consistent detection of active N2 fixation activity at all depths sampled during our study suggest that aphotic N2 fixation may contribute significantly to fixed nitrogen inputs in this area and/or areas downstream of water mass circulation.
- Article
(792 KB) - Full-text XML
-
Supplement
(1634 KB) - BibTeX
- EndNote
Pelagic N2 fixation is considered the greatest input of fixed nitrogen to the oceans, adding up to ∼ 100–107 Tg N per year (Galloway et al., 2004; Codispoti, 2007; Gruber and Galloway, 2008; Jickells et al., 2017). In the sunlit layer of the warm oligotrophic tropical and subtropical oceans, cyanobacterial diazotrophs such as Trichodesmium, UCYN-B and diatom–diazotroph associations (DDAs) dominate fixed nitrogen inputs via N2 fixation (Zehr, 2011). In colder and less oligotrophic waters at higher latitudes, other diazotrophs including UCYN-A and non-cyanobacterial groups may be more competitive (Moisander et al., 2010, 2014; Bonnet et al., 2015; Langlois et al., 2015), considerably expanding the latitudinal range over which N2 fixation is considered significant in predictive biogeochemical models. In the past decade, several studies have retrieved nifH sequences from the dark ocean, some also accompanied by low N2 fixation rates (Hamersley et al., 2011; Bonnet et al., 2013; Rahav et al., 2013). Due to the immense volume of the dark ocean, aphotic N2 fixation could influence the global nitrogen budget substantially. However, the number of published aphotic N2 fixation rates is scant and our understanding of the metabolism and ecology of aphotic diazotrophs is still limited, hindering our ability to evaluate their impact on global fixed nitrogen inputs (Moisander et al., 2017).
Non-cyanobacterial nifH sequences fall into four established nifH gene clusters (Chien and Zinder, 1996), are the most numerous in nifH gene databases (Riemann et al., 2010) and are spread throughout the global ocean (Farnelid et al., 2011; Langlois et al., 2015). As discussed in Bombar et al. (2016), the growth and activity of non-cyanobacterial diazotrophs may be controlled by (i) the presence of oxygen because oxygen destroys the nitrogenase enzyme, (ii) the availability of fixed nitrogen because N2 fixation becomes too energetically expensive when reduced nitrogen forms are readily available in the environment and (iii) the availability of energy because non-cyanobacterial diazotrophs may not be able to photosynthesize and thus rely on external fixed carbon sources. However, aphotic diazotrophic activity has been found both in oxygen-deficient regions such as the oxygen minimum zone of the eastern tropical South Pacific (Bonnet et al., 2013; Loescher et al., 2014) and the fully oxygenated mesopelagic waters in the Mediterranean Sea (Rahav et al., 2013; Benavides et al., 2016). Moreover, while fixed nitrogen availability should theoretically shut down N2 fixation, significant N2 fixation rates have been measured in the nitrate-rich mesopelagic waters of the western tropical South Pacific (WTSP) (Benavides et al., 2015). Finally, energy is likely made available to heterotrophic non-cyanobacterial diazotrophs through labile dissolved organic matter (DOM). Aphotic N2 fixation rates have been related to the presence of relatively labile DOM compounds such as transparent exopolymeric particles (TEP) in the WTSP (Benavides et al., 2015) or peptides and unsaturated aliphatics in the Mediterranean Sea (Benavides et al., 2016). The addition of small labile DOM molecules such as carbohydrates or amino acids has been shown to enhance aphotic N2 fixation in various environments (Bonnet et al., 2013; Rahav et al., 2013; Loescher et al., 2014; Benavides et al., 2015). However, some photic non-cyanobacterial diazotrophs also bear genes for the degradation of refractory DOM compounds (e.g., aromatic hydrocarbons; Bentzon-Tilia et al., 2015). It is thus reasonable to expect that aphotic non-cyanobacterial diazotrophs may be able to exploit diverse DOM sources. Unfortunately, the current lack of genome information from non-cyanobacterial aphotic diazotrophs does not allow us to assess how they are affected by DOM composition and lability.
The WTSP has been recently recognized as a global hot spot of photic N2 fixation, harboring among the highest N2 fixation rates ever recorded (∼ 600 µmol N m−2 d−1; Bonnet et al., 2017), mostly attributed to Trichodesmium and UCYN-B (Bonnet et al., 2009, 2015; Berthelot et al., 2017; Stenegren et al., 2018). To the eastern border of this region, the ultraoligotrophic South Pacific Gyre (GY) has low photic N2 fixation rates (Raimbault and Garcia, 2008), which have been mainly attributed to small unicellular diazotrophs such as UCYN-A and Gammaproteobacteria (Bonnet et al., 2009; Halm et al., 2011; Stenegren et al., 2018). Despite its potentially immense implications in global fixed nitrogen inputs, the aphotic N2 fixation potential of the WTSP remains mostly unexplored (Benavides et al., 2015). Here we quantify N2 fixation and describe the communities based on the nifH gene in the mesopelagic layer along a ∼ 5000 km transect in the WTSP spanning from oligotrophic to ultraoligotrophic conditions (Moutin et al., 2017).
2.1 Hydrography, nutrients, chlorophyll a and dissolved organic carbon
The OUTPACE cruise (Oligotrophy to Ultraoligotrophy South Pacific Experiment; https://doi.org/10.17600/15000900) took place onboard the R/V L'Atalante from 20 February to 2 April 2015 (i.e., during austral summer), sailing westwards from New Caledonia to French Polynesia (see Fig. 2 in Moutin et al., 2017). Temperature, salinity, chlorophyll fluorescence and oxygen data were obtained using an SBE 9 plus CTD mounted on a General Oceanics rosette frame fitted with 24–12 L Niskin bottles.
Seawater samples were collected with Niskin bottles mounted on a rosette frame at 15 short-duration (SD, 8 h) and 3 long-duration (LD, 7 days) stations (Moutin et al., 2017). Samples for the determination of the inorganic nutrients nitrate (NO, nitrite (NO and phosphate (PO were collected in 20 mL acid-washed polyethylene flasks, poisoned with 1 % mercury chloride and analyzed onshore using an AA3 Bran + Luebbe autoanalyzer. The detection limit for both NO and PO was 0.05 µM. Samples for the determination of dissolved organic carbon (DOC) were collected in combusted glass bottles and immediately filtered through two mounted precombusted (4 h, 450 ∘C) 25 mm GF/F filters (0.7 µm, Whatman) using a custom-made all-glass–Teflon filtration syringe system. Filtered seawater was directly collected in precombusted glass ampoules and acidified to pH 2 with orthophosphoric acid. Ampoules were immediately sealed and stored cold (4 ∘C) and in the dark until analyses by high-temperature catalytic oxidation on a Shimadzu TOC-L analyzer according to Sohrin and Sempéré (2005). Typical analytical precision is ±0.1–0.5 (SD) or 0.2–0.5 % (coefficient of variation, CV). Consensus reference materials were injected every 12 to 17 samples to control for stable operating conditions. Chlorophyll a (Chl a) concentrations were determined from 500 mL samples filtered through GF/F filters. Chl a was extracted in methanol and measured by fluorometry (Herbland et al., 1985).
2.2 DOM analysis
Samples for ultra-high-resolution mass spectrometry analyses were collected in acid-cleaned 2 L transparent polycarbonate bottles and extracted (solid-phase) via Agilent PPL cartridges as described in Dittmar et al. (2008). After extraction, the cartridges were rinsed with acidified ultrapure water and frozen at −20 ∘C. Subsequently, the samples were dried by flushing with high-purity N2 and eluted with 6 mL of methanol. The efficiency of the extraction was 47.3 ± 3.9 % on a carbon basis. Methanol extracts were molecularly characterized on a 15 Tesla Fourier transform ion cyclotron resonance mass spectrometer (Solarix FTICRMS) using an electrospray ionization source in negative mode (Bruker Apollo II). Molecular formulae were ascribed to the detected masses as outlined in Seidel et al. (2014). The aromaticity and unsaturation degree of each compound were evaluated according to its molecular formula and were presented as the modified aromaticity index (AI-mod) and double bond equivalents (DBE), respectively (Koch and Dittmar, 2006). In addition, we ascribed the identified molecular formulae to compound groups according to established molar ratios, AI-mod, DBE and heteroatom contents (Seidel et al., 2014). To reveal compositional differences among samples, we performed a principal coordinate analysis (PCoA) on Bray–Curtis distance matrices, including all detected molecular formulae and their respective relative FTICRMS signal intensities. The PCoA scores were correlated against all hydrographic and biological variables measured in our study.
2.3 N2 fixation rates
Seawater was sampled at each SD station from 200, 500, 650 and 800 dbar in quadruplicate 4.3 L transparent polycarbonate bottles covered with black cloth. Each bottle was spiked with 6 mL of 15N2 gas (98.9 % Euriso-top), inverted 20–30 times and incubated in the dark at 8 ∘C in temperature-regulated incubators onboard. After 24 h of incubation, each pair of bottles was filtered onto two separate precombusted GF/F filters (two bottles concentrated per filter) and stored at −20 ∘C until analyzed with an Integra2 analyzer calibrated every 10 samples using reference material (IAEA-N1). To obtain accurate N2 fixation rates we (1) measured the δ15N of background N2 in the incubation on each incubation bottle by membrane inlet mass spectrometry analyses (MIMS; Kana et al., 1994) with obtained values of 7.548 ± 0.557 at % (Bonnet et al., 2018), (2) collected time zero samples in duplicate at each depth and station to determine the natural δ15N of ambient particulate nitrogen (PN) and (3) subtracted blank GF/F PN values from our results. N2 fixation rates were calculated with the equations of Montoya et al. (1996). Considering the PN linearity limit of the mass spectrometer (2.32 µg N), 3 times the standard deviation of time zero values (natural δ15N of PN), and our usual filtration volume (8.6 L) and incubation time (24 h), our volumetric N2 fixation rate detection limit was 0.027 nmol N L−1 d−1. The minimum quantifiable rate calculated using standard propagation of errors via the observed variability between replicate samples was 0.006 nmol N L−1 d−1.
2.4 Flow cytometry
Samples for cell enumeration were collected at the same stations and depths as samples for the quantification of N2 fixation rates. Samples of 1.8 mL were fixed (0.25 % electron microscopy grade paraformaldehyde, w∕v) for 10–15 min at room temperature in the dark, flash-frozen in liquid nitrogen and stored at −80 ∘C for later analysis using a BD Influx flow cytometer (BD Biosciences, San Jose, CA, USA). Samples were thawed at room temperature in the dark, and reference beads (Fluoresbrite, YG, 1 µm) were added to each sample. The non-pigmented bacterioplankton (hereafter bacteria) were discriminated in a sample aliquot stained with SYBR Green I DNA dye (1 : 10 000 final). Because the Prochlorococcus population cannot be uniquely distinguished in the SYBR stained samples in the upper water column, bacteria were determined as the difference between the total cell numbers of the SYBR stained sample and Prochlorococcus enumerated in unstained samples. Particles were excited at 488 nm (plus 457 nm for unstained samples), and forward scatter (FSC; < 15∘) , green fluorescence (530 ∕ 40 nm), orange fluorescence (580 ∕ 30 nm) and red fluorescence (> 650 nm) emissions were measured. Bacteria were discriminated based on their green fluorescence and FSC characteristics. Cytograms were analyzed using FCS Express 6 Flow Cytometry Software (De Novo Software, CA, US).
2.5 DNA extraction, sequencing and sequence analysis
Samples for DNA extraction were collected in duplicate in 4.3 L polycarbonate bottles and the total volume was filtered immediately through 0.2 µm Supor filters (Pall Gelman). The filters were stored in bead beater tubes and kept at −80 ∘C until analysis. Samples were collected from four depths (200, 500, 650 and 850 dbar) at all SD stations, with the exception of station SD5 where seawater for DNA analyses was only collected at 500 dbar and station SD14 where only 200 and 800 dbar were analyzed. DNA was extracted using the Qiagen Plant kit, with additional freeze–thaw, bead beating and Proteinase K steps for sample preparation before the kit purification, and elution to 100 µL as previously described (Moisander et al., 2008). PCR was conducted using degenerate, nested nifH primers (Zehr et al., 2001) and the second-round primers modified for Illumina library preparation using a Bio-Rad C1000 thermocycler. The PCR mix was composed of 2.5 µL of 10X Platinum Taq PCR buffer (Thermo Fisher), MgCl (2.5 mM final), dNTPs (0.2 mM final), primers (1 µM final), 0.11 µL Platinum Taq and 4 µL of DNA extract (1 µL on second round) adjusted to 25 µL total volume with nuclease free water. To alleviate PCR biases, PCR was conducted in triplicate in each round of reactions for which first-round triplicate reaction products were pooled, then 1 µL was used as a template in triplicate reactions on the second round. A negative PCR control with water as a template was included and treated in parallel with samples through all the subsequent steps. Subsamples of the amplification products were checked via 1.2 % TAE gel electrophoresis. The second-round products showing a band of the expected size were pooled and purified using a magnetic bead protocol (Ampure, Beckman Coulter). The purified products were barcoded for Illumina (San Diego, CA, USA) MiSeq sequencing with Nextera indexes using the manufacturer protocol. The indexed products were purified again with magnetic beads, then quantified with a plate reader (Molecular Devices, Sunnyvale, CA, USA) using PicoGreen (Thermo Fisher). The indexed samples were adjusted to equal concentrations and pooled for multiplexing during sequencing. The pooled sample was shipped to the Tufts University sequencing center (Boston, MA, USA) for paired end sequencing (2 × 300 bp). The quality of the pooled sample and select individual samples was checked with a bioanalyzer before the run. The resulting sequences were paired within mothur (Schloss et al., 2009), and reads containing ambiguities or more than eight homopolymers were discarded. The sequences were assigned to OTUs (at 97 % cutoff) using the UCLUST denovo picking method (Edgar, 2010) implemented in MacQIIME v1.9.1 (Caporaso et al., 2010). Low-abundance OTUs consisting of fewer than 15 sequences across all samples were discarded from further processing. A representative sequence of each OTU was extracted from the data and quality processed in ARB (Ludwig et al., 2004), removing sequences that did not conceptually translate or were otherwise of poor quality. These OTUs were removed from further analysis. Remaining sequences were aligned based on protein alignment of the nifH fragments in a public nifH database (https://www.jzehrlab.com/nifh, last access: 15 September 2017). Aligned protein sequences were assigned to nifH clusters using a decision tree statistical model, CART (Frank et al., 2016). The sequence data were normalized to the proportion of total reads in each sample. Total relative abundances of sequences that fell in major nifH clusters were used to create a heatmap within R Studio using the “vegan” statistical package (Oksanen et al., 2015). Sequences were further classified through a locally run blastp using the nifH database as a reference (April 2015 database update). A neighbor-joining tree was built in ARB with the 100 most abundant OTUs across all samples.
3.1 Hydrography, nutrients, DOC and bacterial abundance
Sections of hydrographic variables (temperature, salinity) are shown in Fig. 3a–b in Moutin et al. (2017), and nutrient concentrations (NOx – i.e., NONO – and PO are shown in Fig. 5a–b in Fumenia et al. (2018). DOC and bacterial abundance are shown in Fig. S1 in the Supplement. All variables show a clear divide between the Melanesian Archipelago waters (MA; stations SD1 to SD12) and the South Pacific Gyre (GY; eastwards of SD12; Moutin et al., 2017). Lower temperatures and salinity values (< 8 ∘C and ∼ 35, respectively) were measured below 450 to 600 dbar in the MA, while they were detected at shallower depths eastwards in the GY (Fig. 3a–b in Moutin et al., 2017). Nutrient concentrations were high throughout the mesopelagic zone west of New Caledonia (> 30 µM NOx and > 2 µM PO; Fig. 3a–b in Fumenia et al., 2018). Sailing eastwards, NOx in the MA region was < 5 µM between 150 and 250 dbar, with high concentrations > 30 µM being detected at ∼ 680 dbar. Such high NOx concentrations were detected at shallower depths (500 to 600 dbar) in the GY. PO followed a similar pattern, with the highest concentrations detected at depths > 500 dbar reaching 2.5 µM (Fig. 3a–b in Fumenia et al., 2018). DOC concentrations presented a pattern opposite to that of inorganic nutrients, with < 300 dbar presenting concentrations of 50–60 µM, lowering to < 40 µM below 600 dbar (Fig. S1a). Bacterial abundance was > 1 × 105 cells mL−1 down to 300 dbar in the MA waters, while its numbers decreased abruptly in the GY, especially east of SD12 (Fig. S1b)
3.2 High-resolution analysis of DOM (FTICRMS)
FTICRMS analysis of DOM yielded ∼ 13 500 molecular formulae in each sample, covering a mass range between 150 and 1000 Da (measured in daltons). Each molecular formula was assigned to a given compound group as described in Seidel et al. (2014). According to this grouping, 4–31 % of all compounds detected were oxygen-poor (O ∕ C < 0.5) unsaturated aliphatics, 16 % were oxygen-rich (O ∕ C > 0.5) unsaturated aliphatics and 13 % were polyphenols, while saturated fatty acids, sugars and peptides represented < 3 %. Compounds usually regarded as labile DOM (peptides, sugars and saturated fatty acids) were relatively more abundant in the MA (data not shown).
3.3 Aphotic N2 fixation rates
Aphotic N2 fixation rates were measurable at all stations and depths and ranged between 0.05 and 0.68 nmol N L−1 d−1 (Fig. 1). These rates did not seem to follow any longitudinal or vertical pattern. However, the rates observed at station SD13 (where a massive surface concentration of chlorophyll was observed; de Verneil et al., 2017) were on average ∼ 5-fold higher than at the other stations (average 0.63 ± 0.07 nmol N L−1 d−1).
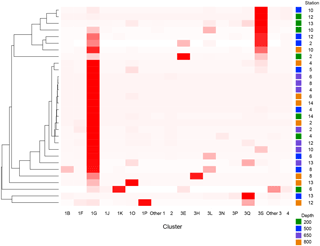
Figure 2Heatmap of nifH clusters and subclusters across the stations. The Bray–Curtis distances were used to build the dendrogram on the left. Distances were calculated with relative abundances of sequences by subcluster. Subclusters within clusters 1 and 3 that had low relative abundances throughout all samples have been grouped as “other”.
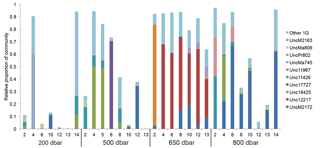
Figure 3Relative abundance of subcluster 1G over pressure levels (depths) and stations. Each bar represents the relative abundance of subcluster 1G in the total community for each sample. Samples are arranged by station within each of the pressure levels sampled. Blastp was used to assign OTUs to a top hit in the nifH reference database. The major sequence types in the nifH database found in subcluster 1G are shown with different colors.
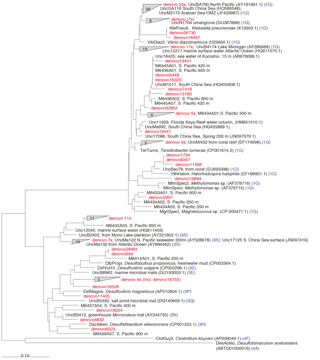
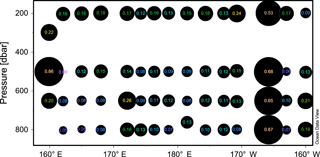
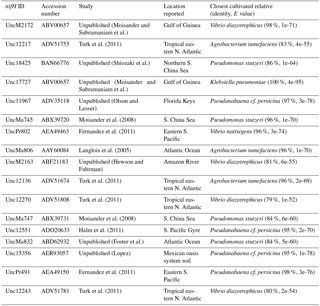
Figure 4The nifH amino acid neighbor-joining tree with the 100 most abundant OTUs from this study. Sequences beginning with “denovo”, shown in red, are randomly chosen representative sequences from these OTUs (OTUs binned at 97 % identity). The clusters in the tree are grouped at an approximately > 95 % sequence identity. The tree includes reference sequences (if uncultivated, the names of these sequences start with “Unc”). The reference sequences are shown with accession numbers from the nifH database and with the cluster identifier shown in blue if indicated in the nifH database. Additional sequences are included from a previous study in the South Pacific mesopelagic layers (Benavides et al., 2015); these clones are labeled with the original clone names M64XXAXX and depths.
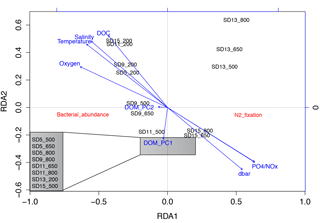
Figure 5Redundancy analysis (RDA) ordination biplot showing the relationship between N2 fixation rates, bacterial abundance, depth, environmental variables (temperature, salinity and oxygen), dissolved organic matter (DOM) principal coordinates, dissolved organic carbon (DOC) and inorganic nutrient concentrations. The samples within the grey box were so similar to each other that their labels overlapped when plotted, so in the figure they are listed in a zoom-out box on the left bottom corner of the panel.
3.4 Diazotroph community composition
There were 3317–146 864 (mean = 88 867, SD = 42 574) sequences per sample after pairing and the QA ∕ QC steps. The negative control resulted in only eight reads belonging to six OTUs. These sequences are likely a result of misbinning at the time of sequencing, and therefore the negative control was removed from downstream analysis. Shannon diversity at the 97 % OTU level was not significantly affected by either depth or station (one-way ANOVA p > 0.05). The majority of nifH sequences fell to clusters 1 and 3, although clusters 2 and 4 were represented in the transect at low relative abundances (Fig. 2). Within cluster 1, subcluster 1G, which contains Gammaproteobacteria, accounted for over half of the total sequences (56 %, SD = 38 %). With a few exceptions, in samples with lower proportions of 1G, subcluster 3S was the most abundant group. Cluster 3S had high relative abundances at station SD10 and in the 200 and 500 dbar depths of stations SD2 and SD12, as well as at 200 dbar at station SD13. The cyanobacterial subcluster 1B was observed at a very low relative abundance throughout all stations and depths (average 0.5 % of total community) and included Trichodesmium, UCYN-A and Richelia. Consistent variations in community composition among depths and stations were not detected via cluster-based analysis; however, patterns emerged when observing data at the OTU level in abundant clusters (Fig. 3). In subcluster 1G, reads most closely matching an unclassified bacterium from the tropical North Atlantic (Unc12217, E value = 2.82 × 10−70; Table 1, Fig. 4) in the nifH database dominated the communities in the 650 dbar depth, and this phylotype was found only at minor proportions in other depths. This phylotype had an approximately 83 % amino acid identity with Agrobacterium tumefaciens (Table 1, Fig. 4). This phylotype was present at high proportions across all other 650 dbar samples except at station SD2, the westernmost station. Although identified as “Other 1G” in Fig. 3, this trend was also true for several other groups present only at the 650 dbar depth (best matches with database sequences Unc12243, Unc12270 and UncPr491; Table 1). An additional group was found only at 650 dbar in the easternmost stations (SD10–SD13), closely matching a sequence from the Amazon River (UncM2163, E value = 4.22 × 10−74; Table 1). Within the nifH cluster 3, subcluster 3S was the most widespread and abundant, with representation from three groups in the reference database: Unc12045, UncB2403 and UncMa132. Sequences with the best matches with Unc12045 (Genbank ID: ADV51583; Turk et al., 2011) and UncB2403 (AAP48957; Steward et al., 2004) were present at stations SD2, SD4, SD10 and SD12, but had the highest relative abundance at station SD10, 500 dbar at station SD2, and the 200 and 500 dbar depths of station SD12. UncMa132 (AAS98182; unpublished) related groups were recovered from all stations at a low relative abundance, with the highest relative abundance in the 200 dbar samples from stations SD2, SD10, SD12 and SD13 as well as the 800 dbar samples from stations SD4 and SD10. These 3S groups have no closely related cultivated isolates, with the closest similarity with Spirochaeta aurantia (74–78 % similarity, AF325792). All of the top 100 most abundant OTUs fell in clusters 1 and 3 (Fig. 4). The majority fell in the cluster 1G, with several additional phylotypes present in addition to the major ones discussed above. Several OTUs were closely related to previously described sequences from the South Pacific Ocean mesopelagic layers (Benavides et al., 2015). Magnetococcus sp., Methylomonas sp. and Teredinibacter turnerae were among the closest cultivated representatives to the cluster 1G OTUs recovered (Fig. 4). Among the OTUs that fell into cluster 3, Desulfovibrio spp. were the closest cultivated representatives in the NCBI database.
3.5 N2 fixation and diazotrophs related to in situ environmental parameters
Bonferroni-corrected Spearman rank correlations showed that N2 fixation rates were only significantly correlated with temperature (ρ= 0.263, p = 0.045), salinity (ρ= 0.284, p = 0.029) and DOC concentrations (ρ= 0.269, p = 0.042; note that these hydrographic variables and DOC were intercorrelated; data not shown; Table S1 in the Supplement). A redundancy analysis (RDA; Fig. 5) including hydrographic variables, inorganic nutrients, DOC, bacterial abundance, N2 fixation rates and DOM PCoA scores indicated that N2 fixation rates were not related to DOM compositional variability (Table S1). N2 fixation rates from shallower depths such as those from 200 dbar were significantly related to temperature, salinity, oxygen and DOC concentrations, while the first principal coordinate of DOM related to the majority of the samples. Stations SD13 and SD15 (easternmost part of the transect, within the GY) were partly related to depth and NOx concentrations (samples from 650 and 800 dbar), while the rest of the samples of the profile appeared very distant in the RDA plot (Fig. 5).
The aphotic N2 fixation activity measured in the WTSP was low (average 0.18 ± 0.07 nmol N L−1 d−1) but consistently detected across all depths and stations (Fig. 1), representing on average 13 and 51 % of photic N2 fixation in the MA and GY waters, respectively (Bonnet et al., 2018), or 6–88 % of overall photic N2 fixation across the whole OUTPACE transect. It is pertinent to note that aphotic N2 fixation rates may be underestimated if a significant percentage of the non-cyanobacterial diazotroph population is smaller than 0.7 µm (the nominal pore size of GF/F filters), as measured N2 fixation rates in non-cyanobacteria-dominated environments have been reported to be significantly higher when smaller pore size filters are used (Bombar et al., 2018). Aphotic N2 fixation may contribute significantly to global fixed nitrogen inputs if widespread throughout the ocean's mesopelagic zone (or deeper). Unfortunately, our ability to assess this contribution remains hindered by the lack of specific N2 fixation methods and our poor understanding of the ecophysiology of non-cyanobacterial diazotrophs (Bombar et al., 2016; Moisander et al., 2017).
N2 fixation rates in aphotic environments correlate with different DOM compound groups in different regions (Benavides et al., 2015, 2016), and nifH gene expression varies among non-cyanobacterial diazotroph phylotypes when exposed to conditions presumed to enhance their N2 fixation activity (Severin et al., 2015). N2 fixation was detected as low rates across an oligotrophic-to-ultraoligotrophic transect in the WTSP. These rates were not significantly correlated with DOM compounds as identified by FTICRMS, although they were positively correlated with DOC concentrations. Non-cyanobacterial diazotroph communities are usually highly diverse in aphotic marine waters (Hewson et al., 2007). If such phylogenetic diversity also entails a broad metabolic diversity and different affinities for DOM compounds, correlations between DOM and non-cyanobacterial diazotroph abundance, identity and/or N2 fixation activity will likely be blurred. Such ecophysiological heterogeneity may also be reflected by the lack of longitudinal and vertical patterns in the N2 fixation rates observed along the transect (Fig. 1).
Some depth- and longitude-related patterns were observed within the potential diazotroph community, one of the major patterns being a distinct A. tumefaciens-related phylotype from the major nifH subcluster 1G dominating at the 650 dbar depth. These sequences were unique from the 1G sequences found at other depths and, with the exception of station SD2, were found uniformly across stations. A potential driver for the depth variation seen at 650 dbar is the presence of the oxygenated Subantarctic Mode Water (SAMW) at this depth (Fumenia et al., 2018). The high concentration of oxygen in this water mass (190–220 µmol kg−1) could potentially be linked to this change in diazotroph community to members that can withstand higher levels of oxygen. To our knowledge, this is the first study identifying a relationship between nifH community composition and large-scale oceanographic circulation patterns in mesopelagic depths. Unfortunately, the coverage of our samples throughout the mesopelagic zone was not enough to represent all the different water masses present and to identify patterns in N2 fixation activity or diversity of diazotrophs according to water mass distribution (see T∕S diagram in Fig. S2).
Members of the 1G subcluster include a variety of Gammaproteobacteria, and this group has previously been reported at high numbers in tropical surface waters including in the WTSP (Messer et al., 2017). In mesopelagic waters, transcripts from the 1G subcluster have been reported in an oxygen-deficient zone in the Arabian Sea at a depth of 175 m (Jayakumar et al., 2012), and genes have been reported from the WTSP from depths of 350–600 m (Benavides et al., 2015). Closely related cultivated isolates to the sequences found in this study include members of the Gammaproteobacterial genera Vibrio, Pseudomonas, Klebsiella and Agrobacterium. The cyanobacterium Pseudanabaena also had a relatively close relationship to the 650 dbar subcluster 1G sequences. When the proportion of sequences from subcluster 1G was low, members of cluster 3, primarily subcluster 3S, tended to have higher relative abundances (mostly east of the Tonga Trench, located between stations SD9 and SD10; Fig. S1). The three major matches of sequences in this study for subcluster 3S were sequences reported from the surface waters in the North Pacific Subtropical Gyre, the eastern North Atlantic and a hypersaline lake. The phylotype most commonly observed at 200 dbar was most similar to sequences reported in the tropical North Pacific (AAS98182). Cluster 3 is typically considered to contain obligate and facultative anaerobes including Spirochaeta and Desulfovibrio. Cluster 3 diazotrophs were present in low gene copy numbers in North Atlantic surface waters, even when NO concentrations were high (Langlois et al., 2008). Members of cluster 3 have also been recovered in the mesopelagic WTSP (Benavides et al., 2015), although the present study reports longitudinal variation as a primary driver of cluster 3 phylotype diversity and relative abundance.
The cyanobacterial subcluster 1B including Trichodesmium, UCYN-A and Richelia was observed at a very low relative abundance throughout all stations and depths (average 0.5 % of total community), in agreement with the findings of Caffin et al. (2017), who detected those phylotypes in sediment traps deployed during the OUTPACE cruise at 150 and 325 m. Dead Trichodesmium colonies are thought to be mainly degraded in the photic zone (Letelier and Karl, 1998), although the detection of Trichodesmium in sediment traps and seawater samples obtained from the mesopelagic zone (Agustí et al., 2015; Chen et al., 2003; Pabortsava et al., 2017) suggests that decayed dense blooms likely sink fast down the water column. The detection of cyanobacterial diazotroph nifH sequences in the mesopelagic zone calls into question whether the N2 fixation rates measured are solely attributable to non-cyanobacterial diazotrophs (Moisander et al., 2017). Cyanobacterial photosynthetic diazotrophs reach the mesopelagic zone through sinking and sedimentation and thus are unlikely diazotrophically active when devoid of light. However, recent studies have detected photosynthetically active diatoms at depths overpassing the mesopelagic zone (Agustí et al., 2015), indicating that dead cell packages can be exported vertically at high speed. Whether cyanobacterial diazotrophs remain active when they reach the aphotic layer or whether they die or shut down N2 fixation on the way remains an open question.
The data presented here are a significant contribution to the scarce overall availability of aphotic N2 fixation rates, specifically the few available rates from the WTSP. Despite our limited knowledge of the ecophysiology of aphotic non-cyanobacterial diazotrophs (Bombar et al., 2016), their ubiquity in the mesopelagic layer and the consistent detection of N2 fixation activity at all depths sampled during our study suggest that aphotic N2 fixation may contribute to fixed nitrogen input in this area.
All data and metadata are available at the French INSU/CNRS LEFE CYBER database (scientific coordinator: Hervé Claustre; data manager and webmaster: Catherine Schmechtig) at the following web address: http://www.obs-vlfr.fr/proof/php/outpace/outpace.php (INSU/CNRS LEFE CYBER, 2017).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-3107-2018-supplement.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Interactions between planktonic organisms and biogeochemical cycles across trophic and N2 fixation gradients in the western tropical South Pacific Ocean: a multidisciplinary approach (OUTPACE experiment)”. It is not associated with a conference.
This is a contribution of the OUTPACE (Oligotrophy from Ultra-oligoTrophy
PACific Experiment) project (https://outpace.mio.univ-amu.fr/, last access: May 2018) funded by the
French research national agency (ANR-14-CE01-0007-01), the LEFE-CYBER
program (CNRS-INSU), the GOPS program (IRD) and the CNES (BC T23, ZBC
4500048836). The OUTPACE cruise (https://doi.org/10.17600/15000900) was
managed by MIO (OSU Institut Pythéas, AMU) from Marseilles (France). The
authors thank the crew of the R/V L'Atalante for outstanding on-ship operations.
Mar Benavides
was funded by the People Programme (Marie Skłodowska-Curie Actions) of the
European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant
agreement number 625185. The NSF OCE-1733610 award to Pia H. Moisander supported Pia H. Moisander and
Katyanne M. Shoemaker. Solange Duhamel was funded by National Science Foundation award OCE-1434916 and by
support from the Vetlesen Foundation.
Edited by: Douglas G. Capone
Reviewed by: Luisa I. Falcon and two anonymous referees
Agustí, S., González-Gordillo, J. I., Vaqué, D., Estrada, M., Cerezo, M. I., Salazar, G., Gasol, J. M., and Duarte, C. M.: Ubiquitous healthy diatoms in the deep sea confirm deep carbon injection by the biological pump, Nat. Commun., 6, 1–8, https://doi.org/10.1038/ncomms8608, 2015.
Benavides, M., H. Moisander, P., Berthelot, H., Dittmar, T., Grosso, O., and Bonnet, S.: Mesopelagic N2 fixation related to organic matter composition in the Solomon and Bismarck Seas (Southwest Pacific), PLOS ONE, 10, 1–19, https://doi.org/10.1371/journal.pone.0143775, 2015.
Benavides, M., Bonnet, S., Hernández, N., Martínez-Pérez, A. M., Nieto-Cid, M., Álvarez-Salgado, X. A., Baños, I., Montero, M. F., Mazuecos, I. P., Gasol, J. M., Osterholz, H., Dittmar, T., Berman-Frank, I., and Arístegui, J.: Basin-wide N2 fixation in the deep waters of the Mediterranean Sea, Global Biogeochem. Cy., 30, 1–19, https://doi.org/10.1002/2015GB005326, 2016.
Bentzon-Tilia, M., Severin, I., Hansen, L. H., and Riemann, L.: Genomics and Ecophysiology of Heterotrophic Nitrogen-Fixing Bacterial Isolated from Estuarine Surface Water, Am. Soc. Microbiol., 6, 1–11, https://doi.org/10.1128/mBio.00929-15, 2015.
Berthelot, H., Benavides, M., Moisander, P. H., Grosso, O., and Bonnet, S.: High-nitrogen fixation rates in the particulate and dissolved pools in the Western Tropical Pacific (Solomon and Bismarck Seas), Geophys. Res. Lett., 44, 8414–8423, https://doi.org/10.1002/2017GL073856, 2017.
Bombar, D., Paerl, R. W., and Riemann, L.: Marine Non-Cyanobacterial Diazotrophs: Moving beyond Molecular Detection, Trends Microbiol., 24, 916–927, https://doi.org/10.1016/j.tim.2016.07.002, 2016.
Bombar, D., Paerl, R. W., Anderson, R., and Riemann, L.: Filtration via Conventional Glass Fiber Filters in 15N2 Tracer Assays Fails to Capture All Nitrogen-Fixing Prokaryotes, Front. Mar. Sci., 5, https://doi.org/10.3389/fmars.2018.00006, 2018.
Bonnet, S., Biegala, I. C., Dutrieux, P., Slemons, L. O., and Capone, D. G.: Nitrogen fixation in the western equatorial Pacific: Rates, diazotrophic cyanobacterial size class distribution, and biogeochemical significance, Global Biogeochem. Cy., 23, 1–13, https://doi.org/10.1029/2008GB003439, 2009.
Bonnet, S., Dekaezemacker, J., Turk-Kubo, K. A., Moutin, T., Hamersley, R. M., Grosso, O., Zehr, J. P., and Capone, D. G.: Aphotic N2 fixation in the eastern tropical South Pacific Ocean, PLOS ONE, 8, 1–14, https://doi.org/10.1371/journal.pone.0081265, 2013.
Bonnet, S., Rodier, M., Turk-Kubo, K. A., Germineaud, C., Menkes, C., Ganachaud, A., Cravatte, S., Raimbault, P., Campbell, E., Quéroué, F., Sarthou, G., Desnues, A., Maes, C., and Eldin, G.: Contrasted geographical distribution of N2 fixation rates and nifH phylotypes in the Coral and Solomon Seas (southwestern Pacific) during austral winter conditions, Global Biogeochem. Cy., 29, 1874–1892, https://doi.org/10.1002/2015GB005117, 2015.
Bonnet, S., Caffin, M., Berthelot, H., and Moutin, T.: Hot spot of N2 fixation in the western tropical South Pacific pleads for a spatial decoupling between N2 fixation and denitrification, P. Natl. Acad. Sci. USA, 114(14), E2800–E2801, https://doi.org/10.1073/pnas.1619514114, 2017.
Bonnet, S., Caffin, M., Berthelot, H., Grosso, O., Benavides, M., Helias-Nunige, S., Guieu, C., Stenegren, M., and Foster, R. A.: In depth characterization of diazotroph activity across the Western Tropical South Pacific hot spot of N2 fixation, Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-567, in review, 2018.
Caffin, M., Moutin, T., Foster, R. A., Bouruet-Aubertot, P., Doglioli, A. M., Berthelot, H., Grosso, O., Helias-Nunige, S., Leblond, N., Gimenez, A., Petrenko, A. A., de Verneil, A., and Bonnet, S.: Nitrogen budgets following a Lagrangian strategy in the Western Tropical South Pacific Ocean: the prominent role of N2 fixation (OUTPACE cruise), Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-468, in review, 2017.
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., Fierer, N., Peña, A. G., Goodrich, J. K., Gordon, J. I., Huttley, G. a, Kelley, S. T., Knights, D., Koenig, J. E., Ley, R. E., Lozupone, C. a, Mcdonald, D., Muegge, B. D., Pirrung, M., Reeder, J., Sevinsky, J. R., Turnbaugh, P. J., Walters, W. a, Widmann, J., Yatsunenko, T., Zaneveld, J., and Knight, R.: correspondence QIIME allows analysis of high- throughput community sequencing data Intensity normalization improves color calling in SOLiD sequencing, Nat. Publ. Gr., 7, 335–336, https://doi.org/10.1038/nmeth.f.303, 2010.
Chen, Y. L. L., Chen, H. Y., and Lin, Y. H.: Distribution and downward flux of Trichodesmium in the South China Sea as influenced by the transport from the Kuroshio Current, Mar. Ecol. Prog. Ser., 259, 47–57, https://doi.org/10.3354/meps259047, 2003.
Chien, Y.-T. and Zinder, S. H.: Cloning, functional organization, transcript studies, and phylogenetic analysis of the complete nitrogenase structural genes (nifHDK2) and associated genes in the archaeon Methanosarcina barkeri 227, J. Bacteriol., 178, 143–148, 1996.
Codispoti, L. A.: An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory, Biogeosciences, 4, 233–253, https://doi.org/10.5194/bg-4-233-2007, 2007.
de Verneil, A., Rousselet, L., Doglioli, A. M., Petrenko, A. A., and Moutin, T.: The fate of a southwest Pacific bloom: gauging the impact of submesoscale vs. mesoscale circulation on biological gradients in the subtropics, Biogeosciences, 14, 3471–3486, https://doi.org/10.5194/bg-14-3471-2017, 2017.
Dittmar, T., Koch, B., Hertkorn, N., and Kattner, G.: A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater, Limnol. Oceanogr.-Meth., 6, 230–235, https://doi.org/10.4319/lom.2008.6.230, 2008.
Edgar, R. C.: Search and clustering orders of magnitude faster than BLAST, Bioinformatics, 26, 2460–2461, https://doi.org/10.1093/bioinformatics/btq461, 2010.
Farnelid, H., Andersson, A. F., Bertilsson, S., Al-Soud, W. A., Hansen, L. H., Sørensen, S., Steward, G. F., Hagström, Å., and Riemann, L.: Nitrogenase gene amplicons from global marine surface waters are dominated by genes of non-cyanobacteria, PLOS ONE, 6, e19223, https://doi.org/10.1371/journal.pone.0019223, 2011.
Fernandez, C., Farías, L., and Ulloa, O.: Nitrogen fixation in denitrified marine waters, PLoS One, 6, e19223, https://doi.org/10.1371/journal.pone.0020539, 2011.
Frank, I. E., Turk-Kubo, K. A., and Zehr, J. P.: Rapid annotation of nifH gene sequences using classification and regression trees facilitates environmental functional gene analysis, Environ. Microbiol. Rep., 8, 905–916, https://doi.org/10.1111/1758-2229.12455, 2016.
Fumenia, A., Moutin, T., Bonnet, S., Benavides, M., Petrenko, A., Helias Nunige, S., and Maes, C.: Excess nitrogen as a marker of intense dinitrogen fixation in the Western Tropical South Pacific Ocean: impact on the thermocline waters of the South Pacific, Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-557, in review, 2018.
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., Asner, G. P., Cleveland, C. C., Green, P. A., Holland, E. A., Karl, D. M., Michaels, A. F., Porter, J. H., Townsend, A. R., and Vo, C. J.: Nitrogen cycles: past, present, and future, Biogeochemistry, 70, 53–226, 2004.
Gruber, N. and Galloway, J. N.: An Earth-system perspective of the global nitrogen cycle, Nature, 451, 293–296, https://doi.org/10.1038/nature06592, 2008.
Halm, H., Lam, P., Ferdelman, T. G., Lavik, G., Dittmar, T., Laroche, J., D'Hondt, S., and Kuypers, M. M. M.: Heterotrophic organisms dominate nitrogen fixation in the south pacific gyre, ISME J., 6, 1238–1249, https://doi.org/10.1038/ismej.2011.182, 2011.
Hamersley, M. R., Turk, K. A., Leinweber, A., Gruber, N., Zehr, J. P., Gunderson, T., and Capone, D. G.: Nitrogen fixation within the water column associated with two hypoxic basins in the Southern California Bight, Aquat. Microb. Ecol., 63, 193–205, https://doi.org/10.3354/ame01494, 2011.
Herbland, A., Le Bouteiller, A., and Raimbault, P.: Size structure of phytoplankton biomass in the equatorial Atlantic Ocean, Deep-Sea Res., 32, 819–836, https://doi.org/10.1016/j.dsr2.2013.07.011, 1985.
Hewson, I., Moisander, P. H., Achilles, K. M., Carlson, C. A., Jenkins, B. D., Mondragon, E. A., Morrison, A. E., and Zehr, J. P.: Characteristics of diazotrophs in surface to abyssopelagic waters of the Sargasso Sea, Aquat. Microb. Ecol., 46, 15–30, https://doi.org/10.3354/ame046015, 2007.
INSU/CNRS LEFE CYBER: OUTPACE (Oligotrophy to UlTra-oligotrophy PACific Experiment), available at: http://www.obs-vlfr.fr/proof/php/outpace/outpace.php (last access: 8 May 2018), 2017.
Jayakumar, A., Al-Rshaidat, M. M. D., Ward, B. B., and Mulholland, M. R.: Diversity, distribution, and expression of diazotroph nifH genes in oxygen-deficient waters of the Arabian Sea, FEMS Microbiol. Ecol., 82, 597–606, https://doi.org/10.1111/j.1574-6941.2012.01430.x, 2012.
Jickells, T. D., Buitenhuis, E., Altieri, K., Baker, A. R., Capone, D., Duce, R. A., Dentener, F., Fennel, K., Kanakidou, M., LaRoche, J., Lee, K., Liss, P., Middelburg, J. J., Moore, J. K., Okin, G., Oschlies, A., Sarin, M., Seitzinger, S., Sharples, J., Singh, A., Suntharalingam, P., Uematsu, M., and Zamora, L. M.: A reevaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean, Global Biogeochem. Cy., 31, 289–305, https://doi.org/10.1002/2016GB005586, 2017.
Kana, T. M., Darkangelo, C., Hunt, M. D., Oldham, J. B., Bennett, G. E., and Cornwell, J. C.: Membrane lnlet Mass Spectrometer for Rapid Environmental Water Samples, Anal. Chem., 66, 4166–4170, 1994.
Koch, B. P. and Dittmar, T.: From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter, Rapid Commun. Mass Sp., 20, 926–932, https://doi.org/10.1002/rcm.2386, 2006.
Langlois, R., Großkopf, T., Mills, M., Takeda, S. and LaRoche, J.: Widespread distribution and expression of Gamma A (UMB), an uncultured, diazotrophic, γ-proteobacterial nifH phylotype, PLOS ONE, 10, 1–17, https://doi.org/10.1371/journal.pone.0128912, 2015.
Langlois, R. J., Hümmer, D., and LaRoche, J.: Abundances and distributions of the dominant nifH phylotypes in the Northern Atlantic Ocean, Appl. Environ. Microb., 74, 1922–1931, https://doi.org/10.1128/AEM.01720-07, 2008.
Letelier, R. M. and Karl, D. M.: Trichodesmium spp. physiology and nutrient fluxes in the North Pacific subtronical gyre, Aquat. Microb. Ecol., 15, 265–276, https://doi.org/10.3354/ame015265, 1998.
Loescher, C. R., Großkopf, T., Desai, F. D., Gill, D., Schunck, H., Croot, P. L., Schlosser, C., Neulinger, S. C., Pinnow, N., Lavik, G., Kuypers, M. M. M., Laroche, J., and Schmitz, R. A.: Facets of diazotrophy in the oxygen minimum zone waters off Peru, ISME J., 8, 2180–2192, https://doi.org/10.1038/ismej.2014.71, 2014.
Ludwig, W., Strunk, O., Westram, R., Richter, L., Meier, H., Yadhukumar, A., Buchner, A., Lai, T., Steppi, S., Jacob, G., Förster, W., Brettske, I., Gerber, S., Ginhart, A. W., Gross, O., Grumann, S., Hermann, S., Jost, R., König, A., Liss, T., Lüßbmann, R., May, M., Nonhoff, B., Reichel, B., Strehlow, R., Stamatakis, A., Stuckmann, N., Vilbig, A., Lenke, M., Ludwig, T., Bode, A., and Schleifer, K. H.: ARB: A software environment for sequence data, Nucleic Acids Res., 32, 1363–1371, https://doi.org/10.1093/nar/gkh293, 2004.
Messer, L. F., Brown, M. V., Furnas, M. J., Carney, R. L., McKinnon, A. D., and Seymour, J. R.: Diversity and activity of diazotrophs in great barrier reef surface waters, Front. Microbiol., 8, 1–16, https://doi.org/10.3389/fmicb.2017.00967, 2017.
Moisander, P. H., Beinart, R. A., Voss, M., and Zehr, J. P.: Diversity and abundance of diazotrophic microorganisms in the South China Sea during intermonsoon, ISME J., 2, 954–967, https://doi.org/10.1038/ismej.2008.51, 2008.
Moisander, P. H., Beinart, R. A., Hewson, I., White, A. E., Johnson, K. S., Carlson, C. A., Montoya, J. P., and Zehr, J. P.: Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain, Science, 327, 1512–1514, https://doi.org/10.1126/science.1185468, 2010.
Moisander, P. H., Serros, T., Paerl, R. W., Beinart, R. A., and Zehr, J. P.: Gammaproteobacterial diazotrophs and nifH gene expression in surface waters of the South Pacific Ocean, ISME J., 8, 1962–1973, https://doi.org/10.1038/ismej.2014.49, 2014.
Moisander, P. H., Benavides, M., Bonnet, S., Berman-Frank, I., White, A. E., and Riemann, L.: Chasing after non-cyanobacterial nitrogen fixation in marine pelagic environments, Front. Microbiol., 8, 1736, https://doi.org/10.3389/fmicb.2017.01736, 2017.
Montoya, J. P., Voss, M., Kahler, P. and Capone, D. G.: A Simple, High-Precision, High-Sensitivity Tracer Assay for N2 Fixation, Appl. Environ. Microb., 62, 986–993, 1996.
Moutin, T., Doglioli, A. M., de Verneil, A., and Bonnet, S.: Preface: The Oligotrophy to the UlTra-oligotrophy PACific Experiment (OUTPACE cruise, 18 February to 3 April 2015), Biogeosciences, 14, 3207–3220, https://doi.org/10.5194/bg-14-3207-2017, 2017.
Oksanen, J., Blanchet, G., Klindt, R., Legendre, P., Minchin, P. R., O'Hara, R. B., Simpson, G. L., Solymos, P., Stevens, M. H. H., and Wagner, H.: Vegan: Community ecology package, R package version 2.3-4, available at: http://CRAN.R-project.org/package=vegan (last access: 15 September 2017), 2015.
Pabortsava, K., Lampitt, R. S., Benson, J., Crowe, C., McLachlan, R., Le Moigne, F. A. C., Mark Moore, C., Pebody, C., Provost, P., Rees, A. P., Tilstone, G. H. and Woodward, E. M. S.: Carbon sequestration in the deep Atlantic enhanced by Saharan dust, Nat. Geosci., 10, 189–194, https://doi.org/10.1038/ngeo2899, 2017.
Rahav, E., Bar-Zeev, E., Ohayon, S., Elifantz, H., Belkin, N., Herut, B., Mulholland, M. R., and Berman-Frank, I.: Dinitrogen fixation in aphotic oxygenated marine environments, Front. Microbiol., 4, 1–11, https://doi.org/10.3389/fmicb.2013.00227, 2013.
Raimbault, P. and Garcia, N.: Evidence for efficient regenerated production and dinitrogen fixation in nitrogen-deficient waters of the South Pacific Ocean: impact on new and export production estimates, Biogeosciences, 5, 323–338, https://doi.org/10.5194/bg-5-323-2008, 2008.
Riemann, L., Farnelid, H., and Steward, G. F.: Nitrogenase genes in non-cyanobacterial plankton: Prevalence, diversity and regulation in marine waters, Aquat. Microb. Ecol., 61, 235–247, https://doi.org/10.3354/ame01431, 2010.
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., Lesniewski, R. A., Oakley, B. B., Parks, D. H., Robinson, C. J., Sahl, J. W., Stres, B., Thallinger, G. G., Van Horn, D. J. and Weber, C. F.: Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microb., 75, 7537–7541, https://doi.org/10.1128/AEM.01541-09, 2009.
Seidel, M., Beck, M., Riedel, T., Waska, H., Suryaputra, I. G. N. A., Schnetger, B., Niggemann, J., Simon, M., and Dittmar, T.: Biogeochemistry of dissolved organic matter in an anoxic intertidal creek bank, Geochim. Cosmochim. Ac., 140, 418–434, https://doi.org/10.1016/j.gca.2014.05.038, 2014.
Severin, I., Bentzon-tilia, M., Moisander, P. H., and Riemann, L.: Nitrogenase expression in estuarine bacterioplankton influenced by organic carbon and availability of oxygen, FEMS Microbiol. Lett., 362, 1–26, 2015.
Sohrin, R. and Sempéré, R.: Seasonal variation in total organic carbon in the northeast Atlantic in 2000–2001, J. Geophys. Res., 110, C10S90, https://doi.org/10.1029/2004JC002731, 2005.
Stenegren, M., Caputo, A., Berg, C., Bonnet, S., and Foster, R. A.: Distribution and drivers of symbiotic and free-living diazotrophic cyanobacteria in the western tropical South Pacific, Biogeosciences, 15, 1559–1578, https://doi.org/10.5194/bg-15-1559-2018, 2018.
Steward, G. F., Jenkins, B. D., Ward, B. B., and Zehr, J. P.: Development and testing of a DNA macroarray to assess nitrogenase (nifH) gene diversity, Appl. Environ. Microb., 70, 1455–1465, 2004.
Turk, K. A., Rees, A. P., Zehr, J. P., Pereira, N., Swift, P., Shelley, R., Lohan, M., Woodward, E. M. S., and Gilbert, J.: Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic, ISME J., 5, 1201–1212, https://doi.org/10.1038/ismej.2010.205, 2011.
Zehr, J., Waterbury, J., and Turner, P.: Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean, Nature, 715, 25–28, https://doi.org/10.1038/news010809-11, 2001.
Zehr, J. P.: Nitrogen fixation by marine cyanobacteria, Trends Microbiol., 19, 162–173, https://doi.org/10.1016/j.tim.2010.12.004, 2011.