the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Mg ∕ Ca and δ18O in living planktic foraminifers from the Caribbean, Gulf of Mexico and Florida Straits
Anna Jentzen
Dirk Nürnberg
Ed C. Hathorne
Joachim Schönfeld
Past ocean temperatures and salinities can be approximated from combined stable oxygen isotopes (δ18O) and Mg ∕ Ca measurements in fossil foraminiferal tests with varying success. To further refine this approach, we collected living planktic foraminifers by net sampling and pumping of sea surface water from the Caribbean Sea, the eastern Gulf of Mexico and the Florida Straits. Analyses of δ18O and Mg ∕ Ca in eight living planktic species (Globigerinoides sacculifer, Orbulina universa, Neogloboquadrina dutertrei, Pulleniatina obliquiloculata, Globorotalia menardii, Globorotalia ungulata, Globorotalia truncatulinoides and Globorotalia tumida) were compared to measured in situ properties of the ambient seawater (temperature, salinity and δ18Oseawater) and fossil tests of underlying surface sediments. “Vital effects” such as symbiont activity and test growth cause δ18O disequilibria with respect to the ambient seawater and a large scatter in foraminiferal Mg ∕ Ca. Overall, ocean temperature is the most prominent environmental influence on δ18Ocalcite and Mg ∕ Ca. Enrichment of the heavier 18O isotope in living specimens below the mixed layer and in fossil tests is clearly related to lowered in situ temperatures and gametogenic calcification. Mg ∕ Ca-based temperature estimates of G. sacculifer indicate seasonal maximum accumulation rates on the seafloor in early spring (March) at Caribbean stations and later in the year (May) in the Florida Straits, related to the respective mixed layer temperatures of ∼26 ∘C. Notably, G. sacculifer reveals a weak positive linear relationship between foraminiferal derived δ18Oseawater estimates and both measured in situ δ18Oseawater and salinity. Our results affirm the applicability of existing δ18O and Mg ∕ Ca calibrations for the reconstruction of past ocean temperatures and δ18Oseawater reflecting salinity due to the convincing accordance of proxy data in both living and fossil foraminifers, and in situ environmental parameters. Large vital effects and seasonally varying proxy signals, however, need to be taken into account.
- Article
(9629 KB) - Full-text XML
-
Supplement
(3103 KB) - BibTeX
- EndNote
Calcite tests of planktic foraminifers are precipitated from the surrounding seawater and their stable oxygen isotope compositions (δ18Ocalcite) and Mg ∕ Ca ratios are established proxies to reconstruct past ocean conditions (e.g. Erez and Luz, 1983; Nürnberg et al., 2000). The δ18Ocalcite signature depends on the ambient seawater temperatures and oxygen isotopic compositions (δ18Oseawater) the planktic organism is thriving in. Their relationship was defined in several δ18O paleotemperature equations (e.g. Erez and Luz, 1983; Bouvier-Soumagnac and Duplessy, 1985; Bemis et al., 1998). Earlier studies showed that δ18Ocalcite reveals an offset to the equilibrium of the seawater caused by environmental factors (e.g. salinity, carbonate ion concentration [], ocean pH) and/or biological controlled processes, so-called vital effects (Weiner and Dove, 2003) (e.g. symbionts photosynthesis, respiration) as influencing factors (Spero and Lea, 1993; Spero et al., 1997; Bemis et al., 1998; Bijma et al., 1999). Symbiont activity, for example, causes a depletion of δ18Ocalcite (e.g. Spero and Lea, 1993). Different ontogenetic stages of the foraminifers result in variable stable isotopes (δ18Ocalcite and δ13Ccalcite), whereas higher δ18Ocalcite and δ13Ccalcite values are measured in tests of adult specimens (e.g. Spero and Lea, 1996). Additionally, encrustation of foraminiferal tests, at the end of the life cycle, results in higher δ18Ocalcite compared to non-encrusted specimens (e.g. Kozdon et al., 2009).
The ratios of Mg ∕ Ca in foraminiferal tests are predominantly controlled by ocean temperature. Meanwhile, robust planktic species-specific calibrations exist (e.g. Nürnberg, 1995; Nürnberg et al., 1996; Lea et al., 1999; Anand et al., 2003; Regenberg et al., 2009), which allow to reconstruct the thermal structure of the entire water column, even on timescales of millions of years. The incorporation of magnesium during calcification is largely driven by physiological processes, which may cause Mg ∕ Ca heterogeneity in single tests with high and low Mg bands in some species (Erez, 2003; Sadekov et al., 2005; Bentov and Erez, 2006; Hathorne et al., 2009; Spero et al., 2015). Further, environmental parameters (e.g. salinity, [], ocean pH) may affect foraminiferal Mg ∕ Ca (Nürnberg et al., 1996; Lea et al., 1999; Russell et al., 2004; Kisakürek et al., 2008). Most critical for proxy users are carbonate dissolution processes that considerably lower Mg ∕ Ca in foraminiferal tests (Brown and Elderfield, 1996; Rosenthal et al., 2000; Regenberg et al., 2006). Other influencing aspects, such as variable calcification depths (e.g. vertical migration through the water column during the life cycle of individuals; see Lohmann and Schweitzer, 1990; Schiebel and Hemleben, 2017) or variable seasonal abundances play a major role for the interpretation of stable isotope and Mg ∕ Ca signals of planktic foraminifers (e.g. Tedesco et al., 2007).
Whilst relatively few geochemical studies (e.g. Mg ∕ Ca) have been conducted on recent/living planktic foraminifers, either collected from the water column or cultured under controlled laboratory conditions, these studies are important for assessing different controlling factors on δ18Ocalcite and Mg ∕ Ca during biomineralisation (e.g. Kahn, 1979; Erez and Honjo, 1981; Nürnberg et al., 1996; Lea et al., 1999; Russell et al., 2004; Kisakürek et al., 2008; Spero et al., 2015).
Here, we systematically sampled the upper water column of the Caribbean, the eastern Gulf of Mexico and the Florida Straits for living tropical and subtropical planktic foraminifers using plankton nets and on-board pumping devices. δ18Ocalcite and Mg ∕ Ca analyses within bulk calcite and single chambers of living specimens collected from different depth intervals were (i) related to ocean parameters (temperature, salinity, δ18Oseawater) measured in water samples from conductivity–temperature–depth (CTD) sampling stations nearby and (ii) compared to fossil counterparts from underlying or nearby surface sediments. Our integrated approach aims to evaluate (i) vital effects under natural conditions, (ii) the ontogenetic development in particular test growth and (iii) the impact of environmental conditions on foraminiferal δ18Ocalcite and Mg ∕ Ca to further substantiate their potential as paleoceanographic proxies.
Table 1Station list of sediment, water and plankton samples obtained during cruises SO164 and M78/1 (Nürnberg et al., 2003; Schönfeld et al., 2011). MUC is the multicorer; GKG is the giant box corer; CTD is the conductivity–temperature–depth profiler; MSN is the Hydrobios Midi multiple opening–closing plankton net; PF is the plankton filter.
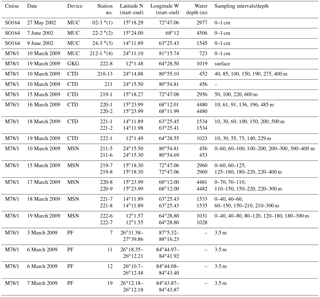
* indicates surface sediment sites close to MSN stations (1) 219, (2) 220, (3) 221 and (4) 211 (Fig. 1).
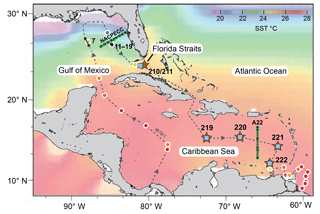
Figure 1Sea surface temperature (SST) chart of the subtropical west Atlantic (Caribbean Sea, Gulf of Mexico and Florida Straits) showing sampling locations for living planktic foraminifers (Table 1). Brown stars indicate the multiclosure net (MSN) samples and CTD stations (RV Meteor cruise M78/1). Black dots and lines indicate plankton filter samples (PF, M78/1). Blue squares indicate surface sediment samples (M78/1 and RV Sonne cruise SO164; see Regenberg et al., 2006; Steph et al., 2009). Green lines and grey dots indicate World Ocean Circulation Experiment (WOCE) transect line A22 (stations 10–15) and North American Carbon Program (NACP) line NACPECC (stations 20–28) (https://cchdo.ucsd.edu/, last access: 21 November 2018). Coloured shading indicates SST illustrated with Ocean Data View (ODV) (Schlitzer, 2009) using World Ocean Atlas 2013 (WOA13) data from January to March (Locarnini et al., 2013). Coloured dots with white outline indicate SST (3.5 m water depth) recorded during cruise M78/1 with the shipboard thermosalinograph (Schönfeld et al., 2011; Sect. S1). The grey dashed line indicates the cruise track of RV Meteor cruise M78/1 in February and March 2009. Black numbers indicate MSN and PF stations (see Table 1).
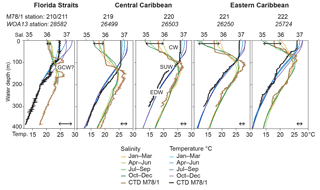
Figure 2Temperature (∘C) and salinity depth profiles in the working area. In situ CTD data measured during cruise M78/1 (March 2009, thick brown and black lines) are presented in comparison to the seasonally differentiated World Ocean Atlas 2013 (WOA13) data (Locarnini et al., 2013; Zweng et al., 2013; coloured thin lines). GCW is Gulf Common Water; CW is Caribbean water; SUW is Subtropical Underwater; EDW is 18 ∘C Sargasso Sea water. Black double arrows indicate the seasonal ranges of temperature (bottom) and salinity (top) in the uppermost water column (0–10 m water depth).
2.1 Sampling and preparation of planktic foraminifers
Analyses were performed on living foraminifers sampled from plankton nets, pumping from below the ship and fossil foraminifers from surface sediments obtained during cruises SO164 (RV Sonne) in May/June 2002 (Nürnberg et al., 2003) and M78/1 (RV Meteor) in February–March 2009 (Schönfeld et al., 2011) (Fig. 1; Table 1). To collect living planktic foraminifers, the Hydrobios Midi multiple opening–closing plankton net (MSN) with a mesh size of 100 µm was deployed at five stations in different water depth intervals (surface to max. 400 m) (Table 1). Further sampling of living specimens was accomplished by pumping seawater from 3.5 m water depth during ship's transit and subsequent filtering over a 63 µm sieve (PF samples). Immediately after sampling, the plankton samples (MSN and PF) were preserved in a mix of 50 % ethanol and seawater. The MSN samples were stained with rose Bengal (2 g L−1 ethanol). Surface sediment samples were recovered by the multicorer and United States Naval Electronic Laboratory (USNEL) giant box corer at positions close to the MSN stations (Table 1). During cruise M78/1, salinity and temperature were recorded by the RBR XR-420 CTD profiler and by the shipboard thermosalinograph (Fig. 2). For stable isotope analyses in seawater (δ18Oseawater), water samples were collected at different water depths (Table 1) with the shipboard rosette Niskin bottle system connected to the CTD profiler, filled in glass bottles (100 mL) and poisoned with 0.2 mL HgCl2 to prevent biological activity.
In the laboratory (GEOMAR, Kiel), the plankton net samples were rinsed with tap water and all foraminifers were picked wet with a glass pipette. The picked foraminifers were dried on a filter paper at room temperature, fractionated into different mesh sizes (100–125, 125–150, 150–250, 250–300, 300–400, 400–500 and >500 µm) and identified on species level after Bé (1967) and Schiebel and Hemleben (2017). For isotope and geochemical analyses, individual tests from eight different species were selected including Globigerinoides sacculifer (i.e. Trilobatus sacculifer; Spezzaferri et al., 2015) with a spherical last chamber (G. trilobus morphotype), Orbulina universa, Neogloboquadrina dutertrei, Pulleniatina obliquiloculata, Globorotalia menardii, Globorotalia ungulata, Globorotalia truncatulinoides dextral and Globorotalia tumida (Sect. S5 in the Supplement). Only test size fractions >250 µm and cytoplasm-bearing specimens with an intact calcite test were considered for analyses, indicating that the foraminifers were still alive when collected. For all species, the weighted average living depth (metres) and habitat temperature (∘C) (temperature at the weighted average living depth) were calculated based on standing stocks (individual m−3) in the water column (Table 2; see Jentzen et al., 2018).
Surface sediment samples were freeze-dried, wet sieved using tap water over a 63 µm sieve and dried at 40 ∘C. Single intact tests were picked from the 355–400 µm size fraction to be directly comparable to published data from similar Caribbean station sites (existing δ18Ocalcite data from Steph et al., 2009 and Mg ∕ Ca data from Regenberg et al., 2006).
2.2 Stable isotope analyses
Depending on the selected species and size fraction, a varying number of specimens were analysed for stable isotopes (δ18Ocalcite and δ13Ccalcite) (see Sect. S1). Prior to the measurements, the foraminiferal tests were cracked and the remaining cytoplasm was removed with a needle. The measurements were run on a Thermo Scientific MAT 253 mass spectrometer connected to an automatic carbonate preparation device Kiel CARBO IV at GEOMAR. The stable isotope results are reported relative to the Vienna Pee Dee Belemnite (V-PDB) in per mil (‰) and calibrated vs. the National Bureau of Standards (NBS) 19. The in-house standard (Solnhofen limestone) was run multiple times and after every 10 measurements with every magazine of samples and gives a long-term analytic precision of <0.06 ‰ (±1σ) for δ18Ocalcite and <0.03 ‰ (±1σ) for δ13Ccalcite, respectively.
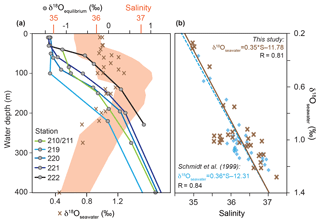
Figure 3(a) δ18Oseawater (‰ VSMOW) and colour-coded δ18Oequilibrium (‰ PDB) depth profiles at the CTD stations 210/211, 219, 220, 221 and 222 (see Fig. 1). Red shading indicates the salinity envelope of the ambient seawater from the Florida Straits and Caribbean Sea measured during cruise M78/1 matching δ18Oseawater. (b) Brown crosses indicate measured in situ salinity vs. δ18Oseawater in the Caribbean Sea and Florida Straits in the upper 600 m of the water column (see Sect. S1 for data); blue squares indicate salinity vs. δ18Oseawater from Schmidt et al. (1999; Global Seawater Oxygen-18 Database) in the upper 600 m of the water column in the Caribbean Sea.
Table 2Average weighted living depth (m), habitat temperature (∘C), symbionts information and δ18Odisequilibrium values of single species from this study and other authors.

a This study (average values). b Jentzen et al. (2018). c G. truncatulinoides dextral. d Gastrich, 1987. e Bé, 1977. f Kučera, 2007. g Facultative symbionts. h Large/thick specimens. i Seasonal variations.
Stable oxygen isotopes in seawater (δ18Oseawater) were analysed by the isotope ratio infrared spectroscopy (IRIS) analyser (Picarro model L1102-i CRDS) at the laboratory of GeoZentrum Nordbayern (Erlangen) (Van Geldern and Barth, 2012). The measurements are expressed in per mil (‰) vs. the Vienna Standard Mean Ocean Water (VSMOW). The analytical precision is better than 0.05 ‰ (±1σ).
The difference between the predicted inorganic calcite δ18O signal of the seawater (calcite formed in thermodynamic equilibrium, δ18Oequilibrium) and the δ18Ocalcite value of the foraminifer is commonly termed the vital effect (δ18Odisequilibrium) (Table 2):
To determine δ18Oequilibrium (Fig. 3a), the temperature equation of Kim and O'Neil (1997) for inorganic precipitation was applied following the relationship after Wilke et al. (2006):
with in situ temperatures (∘C) measured during cruise M78/1 by CTD and measured seawater (δ18Oseawater) values (Schönfeld et al., 2011; Sect. S1). δ18Oseawater was corrected to the V-PDB scale by subtracting 0.27 ‰ after Hut (1987).
2.3 Mg ∕ Ca analyses
The ratios of Mg ∕ Ca in foraminiferal calcite were analysed from both bulk samples comprising numerous of tests (on average ± 25 specimens) of a single species and single specimens, depending on their abundances (see Sect. S1). Prior to analyses, the samples were cleaned with a hydrogen peroxide cleaning step following Barker et al. (2003), which is suggested to be an efficient method to remove the high amount of cytoplasm in live foraminifers (Pak et al., 2004). We omitted a reductive hydrazine cleaning step, as this step is unnecessary for plankton samples. Furthermore, employing only the oxidative cleaning step allows for direct comparison to foraminiferal Mg ∕ Ca from surface sediments, which are treated similarly (Regenberg et al., 2006). For each bulk sample (plankton net and sediment), ∼400–800 µg of G. sacculifer, N. dutertrei and G. ungulata from different size fractions were used for analyses (Sect. S1). The tests were gently crushed between two glass plates, in order to open the chambers, and transferred into a vial. The samples were first rinsed with ultrapure water and ethanol, including an ultrasonic treatment. Then, 250 µL of a NaOH ∕ H2O2 solution (100 µL 30 % H2O2 and 10 mL NaOH) were added to each vial and placed for 20 min in a hot water bath (92 ∘C). For the plankton samples, these steps were repeated one to two times in order to completely remove the cytoplasm. The samples were subsequently rinsed with ultrapure water. Finally, the tests were leached with 250 µL of HNO3 (0.001 M). Prior to the element analyses, the samples were dissolved in HNO3 (0.075 M). The measurements were performed with an axial-viewing VARIAN 720 inductively coupled plasma – optical emission spectrometer (ICP-OES) at GEOMAR. The data of the measurements were normalised and trend-corrected using the ECRM 752-1 standard (3.761 mmol mol−1 Mg ∕ Ca; Greaves et al., 2008). The analytic precision is 0.1 mmol mol−1 (±2σ).
Single chambers of live collected foraminifers were analysed with an Excimer ArF 193 nm laser ablation system, coupled to an inductively coupled plasma – mass spectrometer (ICP-MS Agilent 7500cx) at GEOMAR. Single foraminifers were cleaned with a buffered hydrogen peroxide solution, in a similar way to the bulk samples. Only one specimen was put into a vial to avoid breaking the test during the cleaning process. Each test was rinsed with ultrapure water and ethanol before adding 250 µL of NaOH ∕ H2O2 solution. The samples were then placed in a hot water bath (92 ∘C) for 20 min and rinsed with ultrapure water and ethanol afterwards. Subsequently, the samples were dried at room temperature. The laser ablation technique allowed us to ablate through the test wall from the outer test surface towards the inner side. Its spot size diameter was focused to 50 or 75 µm. Ablation profiles were carried out on the last four chambers (F to F-3) (Sect. S1). The energy density of the laser was 0.9–2.6 J cm−2 and a laser repetition rate of 5 or 7 Hz was selected. The following isotopes were measured: 24Mg, 26Mg, 27Al, 43Ca, 44Ca, 55Mn, 66Zn, 88Sr, 232Th and 238U. The ablation was stopped when the test wall was penetrated. Analyses were calibrated using standard glasses 610 and 612 of the National Institute of Standards and Technology (NIST) using the values of Jochum et al. (2011). The NIST 610 and NIST 612 were ablated with an energy density of 2–3 J cm−2 after every 10 measurements of foraminiferal tests. Raw counts of elements were processed offline and 43Ca was used as internal standard to account for ablation yield. Outliers (average value ±2σ) were rejected from the results. A powder pellet of JCt-1 (giant clam shell) was used as reference and repeatedly analysed (n=15) during the ablation sessions, revealing an average Mg ∕ Ca ratio of 1.21±0.13 mmol mol−1 (standard deviation of 10.6 %, 1σ) being consistent with the consensus of solution analyses in many laboratories (Mg ∕ Ca = 1.289 mmol mol−1; Hathorne et al., 2013).
In situ temperatures (∘C) measured during cruise M78/1 (Schönfeld et al., 2011) were compared to derived Mg ∕ Ca temperature estimates. We applied different calibrations for each species to account for species-specific differences (e.g. Russell et al., 2004; Cléroux et al., 2008; Regenberg et al., 2009; see Sect. S2).
2.4 Calculation of δ18Oseawater
The combination of δ18Ocalcite and Mg ∕ Ca in foraminiferal tests allows us to estimate δ18O of the ambient seawater (Craig and Gordon, 1965; Schmidt, 1999; Fig. 3b), which is used as a proxy for surface seawater salinity. We compared our measured in situ δ18Oseawater to δ18Oseawater estimates derived from combined foraminiferal δ18Ocalcite and Mg ∕ Ca temperatures of G. sacculifer. For the calculation, we used the species-specific δ18O paleotemperature equation for G. sacculifer of Spero et al. (2003) with the species-specific Mg ∕ Ca temperature calibration for G. sacculifer of Regenberg et al. (2009).
2.5 Calcite dissolution
Calcite dissolution can affect foraminiferal Mg ∕ Ca and stable isotopes (δ18O and δ13C) as a function of the regionally different calcite saturation states in the oceans and the sensitivity of the species-specific test structure (Brown and Elderfield, 1996; Spero et al., 1997; Zeebe, 1999; Bijma et al., 1999; Regenberg et al., 2006, 2014). The calcite saturation state is defined as
and decreases from the surface (∼150–200 µmol kg−1) to ∼5000 m water depth (<0 µmol kg−1) in the eastern Caribbean Sea and Gulf of Mexico (Fig. 4). of ∼21 µmol kg−1, which is a critical threshold for the onset of selective Mg2+ ion removal from planktic foraminiferal calcite, is at ∼2500–3000 m water depth in the study area. Below this, the undersaturated water generally lowers foraminiferal Mg ∕ Ca through preferential dissolution (Regenberg et al., 2006, 2014). Furthermore, increasing concentrations and seawater pH cause decreasing δ18Ocalcite and δ13Ccalcite values of the foraminiferal tests (Spero et al., 1997; Zeebe, 1999; Bijma et al., 1999). As all plankton net samples of this study were taken from shallower than 400 m water depth, the studied living foraminifers originate from supersaturated seawater with respect to calcite ( >50 µmol kg−1) and substantial Mg2+ ion removal (loss of higher Mg ∕ Ca calcite) is not to be expected. This is not valid for fossil tests from surface sediments below 2500–3000 m water depth. At these locations (stations M78/1-220/SO164-22-2 and M78/1-219/SO164-02-3), we use the dissolution-corrected Mg ∕ Ca values from Regenberg et al. (2006, 2009) (see Sect. S1).
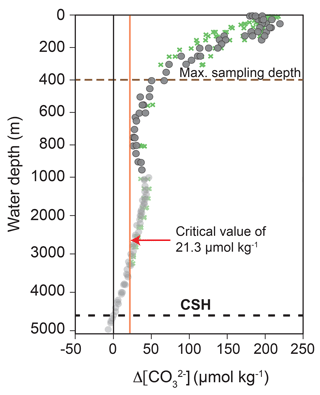
Figure 4Calcite saturation state indicated by Δ[] depth profiles of the Caribbean Sea and Gulf of Mexico. Grey dots and green crosses indicate the transects A22 (stations 10–15) and NACPECC (stations 20–28) (Fig. 1) with Δ[] being the difference between []in situ and []saturation. Alkalinity and TCO2 were taken from WOCE and NACP (https://cchdo.ucsd.edu/, last access: 21 November 2018; cruise RV Knorr in 1997, EXPOCODE: 316N151_4 and cruise RV Ronald H. Brown in 2007, EXPOCODE: 33RO20070710) to calculate []in situ using the program CO2SYS (Pierrot et al., 2006; taking the constants (K1 and K2) of Mehrbach et al., 1973 refitted by Dickson and Millero, 1987 and () from Dickson, 1990). []saturation was calculated after Jansen et al. (2002). The red vertical line indicates the critical Δ[] value of 21.3 µmol kg−1, below which selective Mg2+ ion removal starts (Regenberg et al., 2014); the black dashed line marks the calcite saturation horizon (CSH), which is defined as 0 µmol kg−1 and represents the top of the lysocline at ∼4600 m water depth; the brown dashed line indicates the maximum plankton tow sampling depth.
3.1 Hydrographical setting during sampling
The CTD and thermosalinograph data gathered during cruise M78/1 (February–March 2009) reveal low sea surface temperatures (SSTs) in the Gulf of Mexico (∼20 ∘C) and Florida Straits (∼24 ∘C) (Figs. 1, 2) comparable to the boreal winter situation (Fig. 2; Locarnini et al., 2013). Hydrographic conditions in the Caribbean vary seasonally with a large range of SSTs (range in the Florida Straits up to 5 ∘C) and salinities (SSSs; range in the Caribbean Sea up to 1) (Fig. 2), and are closely linked to the migrating Intertropical Convergence Zone (ITCZ), which is at its northernmost position (6–10∘ N) during summer (Locarnini et al., 2013; Zweng et al., 2013). The surface mixed layer extends to max. 100 m water depth in the Caribbean and is characterised by the relatively fresh Caribbean water (CW; salinity < 36). The lowest salinity is recorded in the southeastern Caribbean during summer and autumn when the Amazon and Orinoco river discharge is most intense and freshwater plumes arrive in the Caribbean Sea (Wüst, 1964; Müller-Karger et al., 1989; Chérubin and Richardson, 2007). Modified CW is transported via anticyclonic eddies (Loop Current) towards the Gulf of Mexico and Florida Straits (Vukovich, 2007). In the upper thermocline, the highly saline Subtropical Underwater (SUW; salinity > 37) prevails. This water mass originates in tropical and subtropical regions (Gallegos, 1996; Blanke et al., 2002) and resides in ∼80–160 m water depth. The 18 ∘C Sargasso Sea water (EDW) prevails in ∼200–400 m water depth entering the Caribbean Sea via the passages of the Greater Antilles (Morrison and Nowlin, 1982). The Gulf Common Water (∼23 ∘C and salinity ∼36.4; Vidal et al., 1994) possibly influences the Florida Straits' hydrography (Station 210/211) in the upper thermocline at 100–150 m, characterised by low salinity (36.5).
Seawater δ18O (δ18Oseawater) averages to ∼0.9 ‰ (VSMOW) in the uppermost 400 m water depth (Fig. 3a). Highest δ18Oseawater values (1.3 ‰) can be found in the salinity maximum at ∼60–150 m water depth, whereas the lowest value (0.3 ‰) is measured in the deepest sample at the lowest salinity. Additionally, the in situ δ18Oseawater and salinity recorded during M78/1 show a positive correlation (linear regression, r=0.81) and yield similar values as earlier datasets from the Caribbean Sea (Schmidt et al., 1999) (Fig. 3b). The δ18Oequilibrium increases with depth from ‰ to 1 ‰ depending on the decreasing ocean temperature (Figs. 2, 3a).
3.2 Vital effects on foraminiferal δ18Ocalcite
In order to address the effects of symbiont activity and life cycle on the foraminiferal oxygen isotopes, δ18Ocalcite values of living foraminifers were compared to the calculated δ18Oequilibrium of the ambient seawater and δ18Ocalcite estimates of fossil tests from underlying surface sediments.
3.2.1 Symbionts and life cycle effect on foraminiferal δ18O and δ13C
Specimens of G. sacculifer and O. universa from the mixed layer are characterised by large negative δ18Odisequilibrium values of −0.35 ‰ and −0.32 ‰, respectively (Table 2). These two species host dinoflagellates as symbionts (Gastrich, 1987), and similarly negative δ18Odisequilibrium values were reported in spinose, symbiont-bearing species caught in plankton tows from various ocean areas (Table 2 and references therein). Laboratory experiments (Spero, 1992; Spero and Lea, 1993; Bemis et al., 1998) revealed a depletion of 0.3 ‰ to 0.6 ‰ in δ18Ocalcite of O. universa and G. sacculifer under high irradiance levels related to algae photosymbiont activity. In particular, a high irradiance in the euphotic zone intensifies the photosynthetic rate in the Caribbean Sea under its prevailing oligotrophic conditions (Spero and Parker, 1985; Morel et al., 2010). Enhanced photosymbiont activity increases the O2 concentration and fosters CO2 fixation, resulting in an elevated pH within the microenvironment around the living foraminifer (Jørgensen et al., 1985; Rink et al., 1998). Both increasing pH and increasing carbonate ion concentration apparently cause a depletion of δ18Ocalcite (Spero et al., 1997; Bijma et al., 1999).
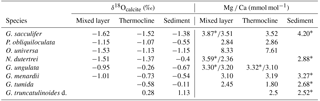
Table 3Average values of δ18Ocalcite and Mg ∕ Ca (measured on ICP-OES* and LA-ICP-MS) from the mixed layer, thermocline and surface sediment (see Sect. S1 for data). PF samples are not included in the calculations.
*Bulk samples (measured on ICP-OES).
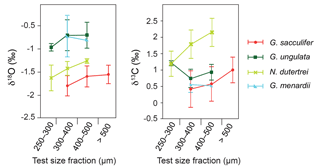
Figure 5Stable oxygen and carbon isotopes (average δ18Ocalcite and δ13Ccalcite ± standard deviations) compared to different test size fractions of living planktic foraminifers (only species with more than one analysed test size fraction are depicted).
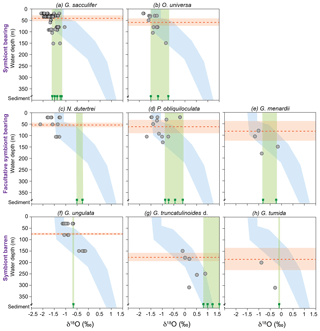
Figure 6Stable oxygen isotopes of living planktic foraminifers from the Florida Straits and the Caribbean Sea plotted vs. water depth (m) in comparison to calculated δ18Oequilibrium and surface sediment data (illustrating the vital effect). The foraminiferal dataset was differentiated into symbiont-bearing, facultative symbiont-bearing and symbiont barren species from top to bottom (Table 2; see Sect. S1 for data). Grey dots indicate foraminiferal δ18Ocalcite from MSN samples, plotted at the mean sampling depth intervals. Blue shading indicates the δ18Oequilibrium envelope of the ambient seawater from the Florida Straits and the Caribbean Sea (see Fig. 3a). Green bars indicate the range of δ18Ocalcite of fossil tests from surface sediments (green signs are the average values of single stations; see Sect. S1). Red dashed lines indicate the average weighted living depths of single species during the sampling campaign in February–March 2009 (red shaded bars are the standard deviations; Table 2). Note that all test size fractions are included.
Among all species studied, only G. sacculifer and N. dutertrei reveal a significant positive correlation (Spearman rank correlation, p<0.05) between test size and stable isotopes (δ18Ocalcite and δ13Ccalcite) (Fig. 5, Sect. S3), suggesting that ontogeny affects the isotopic fractionation processes. The species G. ungulata shows lower δ18Ocalcite values in the test size fraction <300 µm and G. menardii indicates no significant ontogenetic effect (p>0.5; Fig. 5). It should be noted that for some species we did not have enough sample material in all test size classes. However, our results are consistent with Kahn (1979), Kahn and Williams (1981), Spero and Lea (1996) and Bemis et al. (1998), who postulated that juvenile foraminifers have a larger vital effect than adult individuals, with their tests being depleted of the heavy 18O and 13C isotopes due to a higher metabolic rate (incorporation of respired CO2) and/or rapid growth rate. Rapidly growing calcitic skeletons result in a stronger kinetic isotope fractionation and cause the depletion of heavier 18O and 13C isotopes (McConnaughey, 1989).
Vertical migration of planktic species to deeper and colder water masses during their life cycle may additionally affect δ18Ocalcite, leading to commonly higher values in adult specimens (Kroon and Darling, 1995; Lončarić et al., 2006; Birch et al., 2013). Samples from the same test size fraction of all species exhibit the enrichment of heavier 18O isotopes at deeper water levels (Fig. 6; Table 3). We speculate that the increasing δ18Ocalcite at deeper water levels is a function of increasing δ18Oequilibrium of the ambient seawater, rather than ontogenetic effects itself. The surface dweller G. sacculifer reveals the largest δ18Odisequilibrium value (∼1 ‰) in the thermocline (Table 2). As a higher rate of photosynthetic processes in deeper water depths can be excluded and specimens were still alive when sampled, we suggest that G. sacculifer completed calcifying in the thermocline before reproduction. Our observation corroborates South Atlantic plankton net studies of Lončarić et al. (2006), who noted that G. sacculifer δ18Ocalcite increased with depth in the upper 60 m water depth and remained constant below the surface mixed layer, even though δ18Oequilibrium increased continuously.
3.2.2 The δ18O offset between living and fossil foraminifers
It becomes evident that almost all fossil tests from surface sediment samples, in particular N. dutertrei, P. obliquiloculata, G. truncatulinoides and G. tumida, are enriched in δ18Ocalcite (>0.5 ‰) compared to their living counterparts from the water column (Fig. 6; Table 3). δ18Ocalcite of fossil shallow dwellers G. sacculifer and O. universa are rather close to those values of specimens caught in the thermocline (average difference of 0.14 ‰ and 0.02 ‰, respectively) (Table 3). Yet, the overall discrepancy in δ18Ocalcite between fossil and living specimens may be best explained by gametogenic calcification processes or calcite crust formation, which take place during the vertical migration through the water column. At the end of the life cycle and prior to gametogenesis, various planktic foraminifer species (including G. sacculifer, O. universa, P. obliquiloculata, G. truncatulinoides, G. tumida) add a calcite crust of variable thickness on the outer surface of the test (Schiebel and Hemleben, 2017, and references therein). Based on calculations of Bouvier-Soumagnac and Duplessy (1985) and Hamilton et al. (2008), up to 25 % (∼4 µg) gametogenic calcite is added by O. universa, which is mainly secreted in colder water prior to reproduction. The tests thereby lose their glassy and transparent appearances (Bé, 1980; Deuser et al., 1981; Duplessy et al., 1981b; Hemleben et al., 1985; Schweitzer and Lohmann, 1991). Specifically, spinose species resorb their spines before releasing their gametes (Bé and Anderson, 1976; Spero, 1988). These processes result in heavier δ18Ocalcite compositions of fossil tests from surface sediments (and even individual foraminifers from sediment traps) (Duplessy et al., 1981b; Bouvier-Soumagnac and Duplessy, 1985; Bouvier-Soumagnac et al., 1986; Lin et al., 2011). Consistently, the heavy δ18Ocalcite values in adult specimens of G. truncatulinoides and G. tumida may be best explained by vertical migration into colder water masses at a late ontogenetic stage (Franco-Fraguas et al., 2011; Birch et al., 2013). Orr (1967) and Vergnaud-Grazzini (1976) recognised that living individuals of G. truncatulinoides with a thick test and pustules on the test surface are more likely to be found in deeper water masses than non-ornamented, thin-shelled specimens. As expected, such tests had δ18Ocalcite values close to those observed in surface sediments. Overall, our proxy database supports the notion that specimens of P. obliquiloculata, G. tumida and G. truncatulinoides add a thick opaque calcite layer or cortex at deeper water depths than ∼400 m. Hence, the fossil tests are enriched in δ18Ocalcite relative to the living foraminifers (up to 0.85 ‰) (Fig. 6; Table 3).
During the sampling campaign in February–March 2009, mainly juvenile specimens of N. dutertrei were found in plankton nets (mode test size fraction 150–250 µm; Jentzen et al., 2018). This finding may additionally explain the large δ18Ocalcite offset between living foraminifers and fossil tests (∼1 ‰) (Fig. 6; Table 3). Kroon and Darling (1995) recognised that small specimens of N. dutertrei have similar δ18Ocalcite values as surface dwellers and lower values than large specimens, supporting the notion on the ontogenetic-related migration to deeper water. Fairbanks et al. (1982) and Bouvier-Soumagnac and Duplessy (1985) also noted increasing δ18Ocalcite values of N. dutertrei with increasing water depth in the Panama Basin and Indian Ocean, suggesting that this species secretes substantial proportions of their tests below the mixed layer. Furthermore, living N. dutertrei from the South China Sea were depleted in δ18Ocalcite compared to individuals from sediment traps (Lin et al., 2011). Our data confirm these assumptions, as we recognised higher δ18Ocalcite values and larger individuals of N. dutertrei in surface sediments compared to the mixed layer (Fig. 6; Table 3; Jentzen et al., 2018).
The species G. menardii show increasing δ18Ocalcite values from the mixed layer to the thermocline (+0.3 ‰) and from the thermocline to the surface sediments (+0.2 ‰), pointing to decreasing ambient seawater temperatures at deeper water levels and migration within the water column (Fig. 2; Table 3). Apparently, G. ungulata is an exception to the rule, as this species does not show the enrichment of δ18Ocalcite in fossil tests compared to living specimens (Fig. 6; Table 3). Yet, the species secreted their calcite tests close to the equilibrium with the ambient seawater (0.01 ‰–0.08 ‰) throughout the water column (Table 2). The average surface sediment δ18Ocalcite value corresponds well with the depth where the highest standing stock was observed during the sampling campaign in February–March 2009 (Fig. 6; Jentzen et al., 2018).
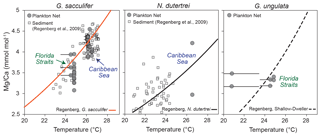
Figure 7Mg ∕ Ca values of ICP-OES bulk samples vs. temperature. Grey dots indicate the Mg ∕ Ca values of living specimens (G. sacculifer, N. dutertrei and G. ungulata), depicted at the average in situ temperature of the plankton net (MSN) intervals in the Florida Straits and Caribbean Sea recorded during cruise M78/1. Black error bars indicate the modern temperature ranges of the sampling intervals. Grey squares indicate Mg ∕ Ca ratios of fossil tests vs. δ18O calcification temperature from the Caribbean Sea and tropical Atlantic modified after Regenberg et al. (2009). Orange curve indicates the Mg ∕ Ca calibration of Regenberg et al. (2009) (surface sediments) for G. sacculifer. The black curve indicates the Mg ∕ Ca calibration of Regenberg et al. (2009) for N. dutertrei. The dashed black curve indicates the Mg ∕ Ca calibration of Regenberg et al. (2009) for shallow dwellers.
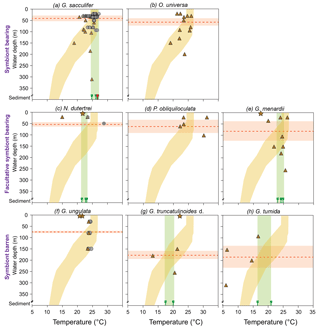
Figure 8Mg ∕ Ca derived temperature estimates of living planktic foraminifers combined from the Florida Straits, the eastern Gulf of Mexico and the Caribbean Sea in comparison to the ambient seawater temperature. The foraminiferal dataset was differentiated into symbiont-bearing, facultative symbiont-bearing and symbiont barren species from top to bottom (Table 2; see Sect. S1 for data). Grey dots indicate Mg ∕ Ca temperature estimates from bulk foraminiferal MSN samples measured by ICP-OES, depicted at the mean sampling depth intervals. Brown triangles and stars indicate Mg ∕ Ca temperature estimates derived from LA-ICP-MS measurements of single tests from MSN samples (Caribbean Sea and Florida Straits) and PF samples (Gulf of Mexico), respectively (average values; see Sect. S1). Yellow shading indicates temperature envelope (∘C) of the ambient seawater from the Florida Straits and the Caribbean Sea measured during cruise M78/1 (Fig. 2; Schönfeld et al., 2011). Note: PF samples (brown stars) were taken in 3.5 m water depth in the eastern Gulf of Mexico at SST of 20 ∘C during cruise M78/1 (Fig. 1). Green bars indicate Mg ∕ Ca derived temperature range of fossil bulk foraminiferal samples from surface sediments closest to the MSN (green sign indicates the average values of single stations in the Caribbean Sea; red sign indicates the average value of G. sacculifer in the Florida Straits; see Sect. S1). Red dashed lines indicate the average weighted living depths of single species during the sampling campaign in February–March 2009 (red bars are standard deviations; Table 2). Note that all test size fractions are included.
3.3 Mg ∕ Ca-based ocean temperature assessment from living foraminifers
In order to evaluate Mg ∕ Ca as a proxy for seawater temperature, we compared Mg ∕ Ca temperature estimates of living specimens to (i) measured in situ temperatures and (ii) Mg ∕ Ca temperature estimates of fossil tests from surface sediments. Within this study, Mg ∕ Ca analyses were performed on bulk foraminiferal samples measured by ICP-OES and single tests measured by LA-ICP-MS. ICP-OES samples of G. sacculifer, N. dutertrei and G. ungulata yield higher Mg ∕ Ca ratios on average compared to LA-ICP-MS samples from the same MSN sample (Table 3). The data indicate a difference of 0.5±0.5 mmol mol−1 for G. sacculifer (average value of eight MSN sampling intervals), 1.2 mmol mol−1 for N. dutertrei (one MSN sampling interval) and 0.17±0.05 mmol mol−1 for G. ungulata (three MSN sampling intervals). We compare the results of both methods to each other, having in mind the data discrepancy originating from the different analytical techniques. For LA-ICP-MS, only small amounts of foraminiferal calcite from single chambers are analysed and for the ICP-MS the bulk calcite from whole foraminiferal tests are measured.
Our Mg ∕ Ca ratios of eight species collected at specific ocean temperature ranges (corresponding to different water depth intervals) are in good agreement with established species-specific Mg ∕ Ca temperature calibrations (Fig. 7; see Sect. S2) and further support the foraminiferal Mg ∕ Ca dependency on ambient water temperature. Hence, we estimate Mg ∕ Ca temperatures applying the best-fitting calibration for each species (Fig. 8). Overall, all specimens collected in the surface water of the eastern Gulf of Mexico (PF samples) yield low Mg ∕ Ca temperature estimates (averaged ∼20.6 ∘C) according to the low early spring temperatures of ∼20 ∘C prevailing during cruise M78/1 (Fig. 1). Higher Mg ∕ Ca temperature estimates (∼25 ∘C) of shallow dwellers (symbiont-bearing and facultative symbiont-bearing species) in the Florida Straits and Caribbean Sea (MSN samples) point to higher temperatures in the mixed layer (>24 ∘C). Low Mg ∕ Ca ratios of deep dwellers (G. truncatulinoides and G. tumida) in the thermocline follow the decreasing ambient seawater temperatures (Fig. 8).
3.3.1 (Facultative) symbiont-bearing species
Our dataset is most complete for G. sacculifer, allowing for a detailed comparison between Mg ∕ Ca-based temperature estimates from plankton net and surface sediment samples. In the Caribbean Sea, the estimated Mg ∕ Ca temperatures for G. sacculifer (∼26 ∘C) are consistent with in situ temperatures of the mixed layer (∼26.2 ∘C), the average habitat temperature (∼26 ∘C, derived from the standing stock, Table 2) and Mg ∕ Ca temperatures derived from fossil tests (∼26 ∘C) (Fig. 8). Below 150 m water depth, the deviation between Mg ∕ Ca temperature and the ambient seawater temperature increases, which supports the former conclusion based on δ18Ocalcite that G. sacculifer completed calcifying above or within the thermocline. Lower temperature estimates of ∼24 ∘C in the Florida Straits (station 211) (Fig. 7) mirror the generally lower sea surface temperatures of ∼24.6 ∘C at this station during cruise M78/1 (Fig. 2). Here, the fossil tests from surface sediments yield higher Mg ∕ Ca ratios (+0.7 mmol mol−1) than the living specimens. The Mg ∕ Ca temperature of fossil specimens is ∼26.5 ∘C, which is rather comparable to temperatures in the Florida Straits of the mixed layer in May (Locarnini et al., 2013, Fig. 2). Foraminiferal census data from the MSN samples suppose that the highest population density of G. sacculifer, consequently also the highest flux and accumulation rate of empty tests on the seafloor, appears during early spring in the Caribbean Sea, linking this species to the warm and oligotrophic CW (∼26 ∘C) (Jentzen et al., 2018). Furthermore, high frequencies of G. sacculifer are related to the strength of the Loop Current transporting warm CW into the Gulf of Mexico (Poore et al., 2013). Therefore, we presume that a higher flux of G. sacculifer in the Florida Straits is likely to occur later in the year, presumably in May, hence after our sampling, and the fossil tests of G. sacculifer from the Caribbean Sea and Florida Straits thereby reflect different seasonal signals.
Beside the seasonal effect, millennial-scale variabilities further affect the Mg ∕ Ca signal of fossil tests from surface sediments. Regenberg et al. (2006) assumed an age range of 2–3 kyr in surface sediments (∼0–1 cm) of the Caribbean Sea. As such, the surface sediments include the record of earlier climate variations, like the Little Ice Age, when sea surface temperatures in the Caribbean were cooler by ∼2 ∘C (Watanabe et al., 2001). A large scatter of ∼0.9 mmol mol−1 Mg ∕ Ca of fossil tests from Caribbean surface sediments was therefore linked partly to past environmental variabilities (Regenberg et al., 2006). Our study, however, shows a similarly large Mg ∕ Ca scatter in living specimens collected from the same plankton nets (MSN samples, Mg ∕ Ca range up to ∼0.87 mmol mol−1; Fig. 7). Furthermore, LA-ICP-MS profiles across single chamber walls reveal a large Mg ∕ Ca variability, with decreasing Mg ∕ Ca values towards the final chamber (F) (see Sect. S4), which implies that vital effects drive Mg2+ incorporation. Earlier studies on surface sediments and culture experiments indicate an ontogenetic effect on the incorporation of Mg2+ during test growth of G. sacculifer, with lowest Mg ∕ Ca ratios in the final, newly precipitated chambers (Sadekov et al., 2005; Dueñas-Bohórquez et al., 2011). Although lower average Mg ∕ Ca ratios (∼0.3 mmol mol−1) were measured in living specimens than in fossil tests, the bulk foraminiferal samples of living G. sacculifer from the mixed layer show a significant positive correlation between Mg ∕ Ca and in situ temperatures (Pearson linear, r=0.8, p<0.05), with an overall Mg ∕ Ca scatter comparable to that of fossil specimens from surface sediments (Fig. 7).
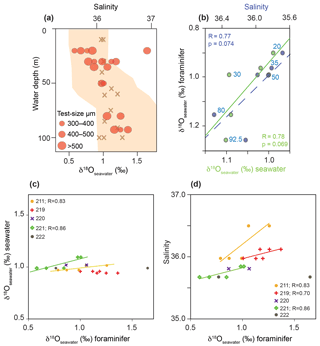
Figure 9δ18Oseawater estimates based on foraminiferal tests from living G. sacculifer compared to measured δ18Oseawater and salinity recorded during cruise M78/1 in the Caribbean Sea and Florida Straits. (a) Red dots indicate the average δ18Oseawater estimates of bulk samples from different test size fractions; brown crosses indicate the in situ δ18Oseawater (‰ VSMOW); the orange envelope indicates salinity. (b) Relationship between average δ18Oseawater estimates (foraminiferal tests), measured δ18Oseawater (seawater) and measured in situ salinity. Blue and green dots indicate average values at a specific water depth (blue numbers denote average sampling water depth in metres). (c) Relationship between δ18Oseawater estimates (foraminiferal tests) and measured δ18Oseawater (seawater) for each station. (d) Relationship between δ18Oseawater estimates (foraminiferal tests) and measured salinity for each station.
Our database for the other species is rather limited. Nonetheless, we can derive the following information. The symbiont-bearing species O. universa characteristically yields very high Mg ∕ Ca ratios in single tests (up to ∼10 mmol mol−1 on average) (see Lea et al., 1999; Russell et al., 2004). Mg ∕ Ca temperature estimates of O. universa are on average ∼1 ∘C lower than the measured in situ temperature but show decreasing values in larger depths according to lower in situ temperatures (Fig. 8; Table 3). The offset between Mg ∕ Ca temperatures of P. obliquiloculata and in situ temperatures vary from −3 to 9 ∘C. Both O. universa and P. obliquiloculata show low and high Mg2+ bands across single chambers of the tests (Sect. S4). Those bands are likely caused by physiological processes (Eggins et al., 2004; Kunioka et al., 2006; Sadekov et al., 2009; Spero et al., 2015) and reveal a large Mg ∕ Ca variability in single chambers. Single LA-ICP-MS measurements of N. dutertrei yield lower Mg ∕ Ca ratios than the ICP-OES measurements (Table 3). Here, the high Mg heterogeneity in single chambers (see Fehrenbacher et al., 2017) probably caused the large offset between the two measuring techniques (see above). However, the average derived Mg ∕ Ca temperature of plankton bulk samples (∼26.3 ∘C) at station 221 is in good agreement with the in situ temperature of the seawater at this station (∼26.5 ∘C) (Fig. 8). The difference of 0.71 mmol mol−1 Mg ∕ Ca between the living and fossil bulk samples (Table 3) supports the notion that adult specimens of N. dutertrei dwell at larger depths and continue calcifying (development of a crust; see Steinhardt et al., 2015; Fehrenbacher et al., 2017), as indicated by the lower δ18Ocalcite values and smaller specimens collected in the upper mixed layer (Jentzen et al., 2018). Living specimens of G. menardii yield a Mg ∕ Ca temperature range between ∼18 and 26.5 ∘C, which is larger but covers the temperature range of fossil tests (∼23.2–25 ∘C) and the calculated average habitat temperature (∼24.5 ∘C; Table 2) in the Florida Straits and Caribbean Sea (Fig. 8). The high Mg ∕ Ca temperature estimate in the deep sampling depth interval (Fig. 8) might indicate that G. menardii calcifies in the upper 250 m water depth.
3.3.2 Symbiont barren species
In the Florida Straits, both bulk and single Mg ∕ Ca measurements of G. ungulata yield temperature estimates of ∼24 ∘C in the mixed layer and thermocline (Fig. 8) being congruent to the average habitat temperature of 23.8 ∘C during February–March 2009 (Table 2). The average Mg ∕ Ca temperature estimates of living and fossil G. truncatulinoides (∼19 ∘C) mirror the average habitat temperature of ∼20 ∘C during February–March 2009 (Fig. 8; Table 2). The deep dweller G. tumida shows a decreasing Mg ∕ Ca temperature trend from the mixed layer to the thermocline following the decreasing in situ temperature (Fig. 8). The fossil tests of G. tumida show higher average Mg ∕ Ca ratios than the living individuals (Table 3) and yield higher temperature estimates. However, the Mg ∕ Ca temperature of fossil tests (∼19 ∘C) represents the calculated average habitat temperature (∼21.7 ∘C) far better than the living foraminifers, which show an offset to the prevailing in situ temperature of ∼7 to 17 ∘C (Fig. 8) most likely due to variable crusting of the chambers (see Sect. S4).
3.4 δ18Oseawater relationship
The combination of foraminiferal δ18Ocalcite and Mg ∕ Ca temperatures to estimate δ18Oseawater approximating paleo-salinity is a commonly accepted approach in paleoceanography (e.g. Lea et al., 2000; Schmidt et al., 2004; Nürnberg et al., 2008). Support derived from living foraminifers collected under natural conditions is still sparse. Our unique dataset on living planktic foraminifers in the mixed layer (>125 m water depth) at least allows us to test the abovementioned approach for the surface dweller G. sacculifer from the Caribbean Sea and Florida Straits (Fig. 9). As the δ18Oseawater estimates strongly depend on both the applied δ18O paleotemperature equation and empirical Mg ∕ Ca calibration, we decided to apply the δ18O paleotemperature equation of Spero et al. (2003). This equation is based on G. sacculifer cultured in laboratory settings, which takes the large disequilibrium of δ18Ocalcite in living specimens to the ambient seawater into account (Table 2). For the estimation of Mg ∕ Ca temperature, we applied the species-specific calibration of Regenberg et al. (2009) for G. sacculifer derived from fossil tests of surface sediments in the tropical Atlantic and Caribbean Sea. Our study shows that this calibration reflects our in situ temperatures very closely (Fig. 7). δ18Oseawater estimates of G. sacculifer show a weak positive linear relationship with in situ δ18Oseawater as well as with salinity for stations 211 and 221 (Fig. 9c, d). Station 219 does not show a positive linear relationship between δ18Oseawater estimates of G. sacculifer and in situ δ18Oseawater but with salinity (Fig. 9d). The average dataset of all stations shows a weak positive linear relationship (Fig. 9b); however, it is not statistically significant (p>0.05; p=0.074 for salinity and p=0.069 for δ18Oseawater, respectively). Additionally, different test size fractions influence the δ18Oseawater estimates (see Sect. 3.2.1 for δ18Ocalcite) and should be taken into account for studies reconstructing past ocean parameters (see Metcalfe et al., 2015). However, our study on living foraminifers hence provides compelling evidence that the combination of foraminiferal δ18Ocalcite and Mg ∕ Ca temperature reflecting ambient seawater properties reliably approximates the modern ocean salinity.
Our combined stable isotopes (δ18O and δ13C) and Mg ∕ Ca analyses on living planktic foraminifers, collected by MSN and PF from surface to max. 400 m water depth of the Caribbean Sea, the eastern Gulf of Mexico and the Florida Straits, allow for the following conclusions:
-
The large negative disequilibrium (between δ18Ocalcite and δ18Oequilibrium) of up to −0.35 ‰ observed for G. sacculifer and O. universa points to a strong photosynthetic activity of the host symbionts (dinoflagellates).
-
Ontogeny most likely controls δ18Ocalcite and δ13Ccalcite values. In this study, G. sacculifer and N. dutertrei show a significant increase of δ18Ocalcite and δ13Ccalcite with increasing test size.
-
Vertical migration in the water column and additional secretion of a calcite crust or gametogenic calcite (at the end of the foraminiferal life cycle) likely cause the increase of δ18Ocalcite with water depths and the enrichment of heavier 18O isotopes in fossil tests compared to living specimens.
-
The large intraspecific scatter of Mg ∕ Ca implies a strong vital effect. Nonetheless, it is evident that the ambient calcification temperature drives the Mg ∕ Ca compositions in foraminiferal tests and causes lowered Mg ∕ Ca derived temperature estimates at lowered in situ temperature.
-
The various species-specific datasets agree well with published δ18O and Mg ∕ Ca calibrations.
-
Fossil tests of G. sacculifer from surface sediments in the Caribbean Sea and Florida Straits suggest that the regional Mg ∕ Ca signatures may be seasonally biased. Mg ∕ Ca values indicate that the highest flux/accumulation rate of G. sacculifer occurs during spring (March) in the Caribbean Sea and is delayed by a few months in the Florida Straits (most likely in May) linked to prevailing seawater temperatures of ∼26 ∘C in the mixed layer.
-
Combined δ18Ocalcite and Mg ∕ Ca temperatures of G. sacculifer yield δ18Osewater estimates, which show a weak positive linear relationship with measured in situ δ18Oseawater and salinity.
The dataset of this article can be found in the Supplement and in Jentzen et al. (2018), Regenberg et al. (2006) and Steph et al. (2009).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-7077-2018-supplement.
The authors declare that they have no conflict of interest.
This study was funded by the German Research Foundation DFG (grant
SCHO605/8-1). The authors thank the captain, crew and participants of RV
Sonne cruise SO164 and RV Meteor cruise M78/1. We thank
Nadine Gehre for measuring Mg ∕ Ca on bulk samples
(ICP-OES), and Jan Fietzke and Steffanie Nordhausen for the help during laser
ablation measurements and processing the raw data. We would like to thank
Fynn Wulf and Sebastian Fessler for measuring the stable isotopes of
foraminiferal calcite, Robert van Geldern (GeoZentrum Nordbayern) for
measuring stable isotopes of seawater and Birgit Mohr (Univ. Kiel) for the
support with the preparation of scanning electron microscope photographs. We
gratefully thank Takashi Toyofuku and Brett Metcalfe for their constructive
comments, which helped to improve the manuscript.
Edited by: Lennart de Nooijer
Reviewed by:
Takashi Toyofuku and Brett Metcalfe
Anand, P., Elderfield, H., and Conte, M. H.: Calibration of Mg ∕ Ca thermometry in planktonic foraminifera from a sediment trap time series, Paleoceanography, 18, 1050, https://doi.org/10.1029/2002PA000846, 2003.
Barker, S., Greaves, M., and Elderfield, H.: A study of cleaning procedures used for foraminiferal Mg ∕ Ca paleothermometry, Geochem. Geophy. Geosy., 4, 8407, https://doi.org/10.1029/2003GC000559, 2003.
Bé, A. W. H.: Families: Globigerinidae and Globorotaliidae, Fiche no. 108, in: Fiches d`intification du zooplankton, edited by: Frasier, J. H., Conseil International pour l`Exploration de la Mer, Lamont Geological Observatory of Columbia University, Palisades, New York, 1967.
Bé, A. W. H.: An ecological, zoogeographic and taxonomic review of recent planktonic foraminifera, in: Oceanic Micropaleontology, edited by: Ramsay, A. T. S., Academic Press, London, 1977.
Bé, A. W. H.: Gametogenic calcification in a spinose planktonic foraminifer, Globigerinoides sacculifer (Brady), Mar. Micropaleontol., 5, 283–310, 1980.
Bé, A. W. H. and Anderson, O. R.: Gametogenesis in planktonic foraminifera, Science, 192, 890–892, 1976.
Bemis, B. E., Spero, H. J., Bijma, J., and Lea, D. W.: Reevaluation of the oxygen isotopic composition of planktonic foraminifera: Experimental results and revised paleotemperature equations, Paleoceanography, 13, 150–160, https://doi.org/10.1029/98PA00070, 1998.
Bentov, S. and Erez, J.: Impact of biomineralization processes on the Mg content of foraminiferal shells: A biological perspective, Geochem. Geophy. Geosy., 7, Q01P08, https://doi.org/10.1029/2005GC001015, 2006.
Bijma, J., Spero, H. J., and Lea, D. W.: Reassessing foraminiferal stable isotope geochemistry: Impact of the oceanic carbonate system (experimental results), in: Use of proxies in paleoceanography, edited by: Fischer, G. and Wefer, G., Springer Berlin, Heidelberg, 489–512, 1999.
Birch, H., Coxall, H. K., Pearson, P. N., Kroon, D., and O'Regan, M.: Planktonic foraminifera stable isotopes and water column structure: Disentangling ecological signals, Mar. Micropaleontol., 101, 127–145, 2013.
Blanke, B., Arhan, M., Lazar, A., and Prévost, G.: A Lagrangian numerical investigation of the origins and fates of the salinity maximum water in the Atlantic, J. Geophys. Res., 107, 3163, https://doi.org/10.1029/2002JC001318, 2002.
Bouvier-Soumagnac, Y. and Duplessy, J. C.: Carbon and oxygen isotopic composition of planktonic foraminifera from laboratory culture, plankton tows and Recent sediment: implications for the reconstruction of paleoclimatic conditions and of the global carbon cycle, J. Foramin. Res., 15, 302–320, 1985.
Bouvier-Soumagnac, Y., Duplessy, J. C., and Bé, A. W. H.: Isotopic composition of a laboratory cultured planktonic foraminifer O. universa, Implications for paleoclimatic reconstructions, Oceanol. Acta, 9, 519–522, 1986.
Brown, S. J. and Elderfield, H.: Variations in Mg ∕ Ca and Sr ∕ Ca ratios of planktonic foraminifera caused by postdepositional dissolution: Evidence of shallow Mg-dependent dissolution, Paleoceanography, 11, 543–551, https://doi.org/10.1029/96PA01491, 1996.
Chérubin, L. M. and Richardson, P.: Caribbean current variability and the influence of the Amazon and Orinoco fresh water plumes, Deep-Sea Res. Pt. 1, 54, 1451–1473, 2007.
Cléroux, C., Cortijo, E., Anand, P., Labeyrie, L., Bassinot, F., Caillon, N., and Duplessy, J.-C.: Mg ∕ Ca and Sr ∕ Ca ratios in planktonic foraminifera: Proxies for upper water column temperature reconstruction, Paleoceanography, 23, PA3214, https://doi.org/10.1029/2007PA001505, 2008.
Craig, H. and Gordon, L. I.: Deuterium and oxygen 18 variations in the ocean and the marine atmosphere, in: Stable Isotopes in Oceanographic Studies and Paleotemperatures, edited by: Tongiorgi, E., Spoleto, 9–130, Consiglio Nazionale Delle Ricerche Laboratorio di Geologia Nucleare, Pisa, 1965.
Deuser, W. G., Ross, E. H., Hemleben, C., and Spindler, M.: Seasonal changes in species composition, numbers, mass, size, and isotopic composition of planktonic foraminifera settling into the deep Sargasso Sea, Palaeogeogr. Palaeocl., 33, 103–127, 1981.
Dueñas-Bohórquez, A., da Rocha, R. E., Kuroyanagi, A., de Nooijer, L. J., Bijma, J., and Reichart, G.-J.: Interindividual variability and ontogenetic effects on Mg and Sr incorporation in the planktonic foraminifer Globigerinoides sacculifer, Geochim. Cosmochim. Ac., 75, 520–532, 2011.
Dickson, A. G.: Thermodynamics of the Dissociation of Boric Acid in Synthetic Seawater from 273.15 to 318.15 K, Deep-Sea Res., 37, 755–766, 1990.
Dickson, A. G. and Millero, F. J.: A Comparison of the Equilibrium Constants for the Dissociation of Carbonic Acid in Seawater Media, Deep-Sea Res., 34, 1733–1743, 1987.
Duplessy, J. C., Bé, A. W. H., and Blanc, P. L.: Oxygen and carbon isotopic composition and biogeographic distribution of planktonic foraminifera in the Indian Ocean, Palaeogeogr. Palaeocl., 33, 9–46, 1981a.
Duplessy, J. C., Blanc, P. L., and Bé, A. W. H.: Oxygen-18 enrichment of planktonic foraminifera due to gametogenic calcification below the euphotic zone, Science, 213, 1247–1250, 1981b.
Eggins, S. M., Sadekov, A., and De Deckker, P.: Modulation and daily banding of Mg ∕ Ca in Orbulina universa tests by symbiont photosynthesis and respiration: a complication for seawater thermometry?, Earth Planet. Sc. Lett., 225, 411–419, 2004.
Erez, J.: The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies, Rev. Mineral. Geochem., 54, 115–149, 2003.
Erez, J. and Honjo, S.: Comparison of isotopic composition of planktonic foraminifera in plankton tows, sediment traps and sediments, Palaeogeogr. Palaeocl., 33, 129–156, 1981.
Erez, J. and Luz, B.: Experimental paleotemperature equation for planktonic foraminifera, Geochim. Cosmochim. Ac., 47, 1025–1031, 1983.
Fairbanks, R. G., Sverdlove, M., Free, R., Wiebe, P. H., and Bé, A. W. H.: Vertical distribution and isotopic fractionation of living planktonic foraminifera from the Panama Basin, Nature, 298, 841–844, 1982.
Fehrenbacher, J. S., Russell, A. D., Davis, C. V., Gagnon, A. C., Spero, H. J., Cliff, J. B., Zhu, Z., and Martin, P.: Link between light-triggered Mg-banding and chamber formation in the planktic foraminifera Neogloboquadrina dutertrei, Nat. Commun., 8, 15441, https://doi.org/10.1038/ncomms15441, 2017.
Franco-Fraguas, P., Costa, K. B., and Toledo, F. A. D. L.: Relationship between isotopic composition (Δ18O and Δ13C) and planktonic foraminifera test size in core tops from the Brazilian Continental Margin, Braz. J. Oceanogr., 59, 327–338, 2011.
Gallegos, A.: Descriptive physical oceanography of the Caribbean Sea, in: Small Islands: Marine Science and Sustainable Development, edited by: Maul, G. A., Coast. Estuar. Stud., 51, 36–55, 1996.
Gastrich, M. D.: Ultrastructure of a new intracellular symbiotic alga found within planktonic foraminifera, J. Phycol., 23, 623–632, 1987.
Greaves, M., Caillon, N., Rebaubier, H., Bartoli, G., Bohaty, S., Cacho, I., Clarke, L., Cooper, M., Daunt, C., Delaney, M., deMenocal, P., Dutton, A., Eggins, S., Elderfield, H., Garbe-Schönberg, D., Goddard, E., Green, D., Groeneveld, J., Hastings, D., Hathorne, E., Kimoto, K., Klinkhammer, G., Labeyrie, L., Lea, D. W., Marchitto, T., Martinez-Boti, M. A., Mortyn, P. G., Ni, Y., Nürnberg, D., Paradis, G., Pena, L., Quinn, T., Rosenthal, Y., Russell, A., Sagawa, T., Sosdian, S., Stott, L., Tachikawa, K., Tappa, E., Thunell, R., and Wilson, P. A.: Interlaboratory comparison study of calibration standards for foraminiferal Mg ∕ Ca thermometry, Geochem. Geophy. Geosy., 9, Q08010, https://doi.org/10.1029/2008GC001974, 2008.
Hamilton, C. P., Spero, H. J., Bijma, J., and Lea, D. W.: Geochemical investigation of gametogenic calcite addition in the planktonic foraminifera Orbulina universa, Mar. Micropaleontol., 68, 256–267, 2008.
Hathorne, E. C., James, R. H., and Lampitt, R. S.: Environmental versus biomineralization controls on the intratest variation in the trace element composition of the planktonic foraminifera G. inflata and G. scitula, Paleoceanography, 24, PA4204, https://doi.org/10.1029/2009PA001742, 2009.
Hathorne, E. C., Gagnon, A., Felis, T., Adkins, J., Asami, R., Boer, W., Caillon, N., Case, D., Cobb, K. M., Douville, E., deMenocal, P., Eisenhauer, A., Garbe-Schoönberg, D., Geibert, W., Goldstein, S., Hughen, K., Inoue, M., Kawahata, H., Kölling, M., Cornec, F. L., Linsley, B. K., McGregor, H. V., Montagna, P., Nurhati, I. S., Quinn, T. M., Raddatz, J., Rebaubier, H., Robinson, L., Sadekov, A., Sherrell, R., Sinclair, D., Tudhope, A. W., Wei, G., Wong, H., Wu, H. C., and You, C.-F.: Interlaboratory study for coral Sr ∕ Ca and other element ∕ Ca ratio measurements, Geochem. Geophy. Geosy., 14, 3730–3750, https://doi.org/10.1002/ggge.20230, 2013.
Hemleben, C., Spindler, M., Breitinger, I., and Deuser, W. G.: Field and laboratory studies on the ontogeny and ecology of some Globorotaliid species from the Sargasso Sea off Bermuda, J. Foramin. Res., 15, 254–272, 1985.
Hut, G.: Stable isotope reference samples for geochemical and hydrological investigations, Consultants Group meeting 16–18 September 1985, International Atomic Energy Agency (I.A.E.A.), Vienna, Austria, p. 42, 1987.
Jansen, H., Zeebe, R. E., and Wolf-Gladrow, D. A.: Modeling the dissolution of settling CaCO3 in the ocean, Global Biogeochem. Cy., 16, 1027, https://doi.org/10.1029/2000GB001279, 2002.
Jentzen, A., Schönfeld, J., and Schiebel, R.: Assessment of the effect of increasing temperature on the ecology and assemblage structure of modern planktic foraminifers in the Caribbean and surrounding seas, J. Foramin. Res., 48, 251–272, 2018.
Jochum, K. P., Weis, U., Stoll, B., Kuzmin, D., Yang, Q., Raczek, I., Jacob, D. E., Stracke, A., Birbaum, K., Frick, D. A., Günther, D., and Enzweiler, J.: Determination of reference values for NIST SRM 610–617 glasses following ISO Guidelines, Geostand. Geoanal. Res., 35, 397–429, 2011.
Jørgensen, B. B., Erez, J., Revsbech, N. P., and Cohen, Y.: Symbiotic photosynthesis in a planktonic foraminiferan, Globigerinoids sacculifer (Brady), studied with microelectrodes, Limnol. Oceanogr., 30, 1253–1267, https://doi.org/10.4319/lo.1985.30.6.1253, 1985.
Kahn, M. I.: Non-equilibrium oxygen and carbon isotopic fractionation in tests of living planktonic foraminifera, Oceanol. Acta, 2, 195–208, 1979.
Kahn, M. I. and Williams, D. F.: Oxygen and carbon isotopic composition of living foraminifera from the northeast Pacific Ocean, Palaeogeogr. Palaeocl., 33, 47–69, 1981.
Kim, S.-T. and O'Neil, J. R.: Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates, Geochim. Cosmochim. Ac., 61, 3461–3475, 1997.
Kisakürek, B., Eisenhauer, A., Böhm, F., Garbe-Schönberg, D., and Erez, J.: Controls on shell Mg ∕ Ca and Sr ∕ Ca in cultured planktonic foraminiferan, Globigerinoides ruber (white), Earth Planet. Sc. Lett., 273, 260–269, 2008.
Kozdon, R., Ushikubo, T., Kita, N. T., Spicuzza, M., and Valley, J. W.: Intratest oxygen isotope variability in the planktonic foraminifer N. pachyderma: Real vs. apparent vital effects by ion microprobe, Chem. Geol., 258, 327–337, 2009.
Kroon, D. and Darling, K.: Size and upwelling control of the stable isotope composition of Neogloboquadrina dutertrei (d'Orbigny), Globigerinoides ruber (d'Orbigny) and Globigerina bulloides d'Orbigny: Examples from the Panama Basin and Arabian Sea, J. Foramin. Res., 25, 39–52, 1995.
Kučera, M.: Planktonic foraminifera as tracers of past oceanic environments, in: Developments in Marine Geology, Proxies in Late Cenozoic Paleoceanography, edited: by Hillaire-Marcel, C. and De Vernal, A., Elsevier, New York, 2007.
Kunioka, D., Shirai, K, Takahata, N., Sano, Y., Toyofuku, T., and Ujiie, Y.: Microdistribution of Mg ∕ Ca, Sr ∕ Ca, and Ba ∕ Ca ratios in Pulleniatina obliquiloculata test by using a NanoSIMS: Implication for the vital effect mechanism, Geochem. Geophy. Geosy., 7, Q12P20, https://doi.org/10.1029/2006GC001280, 2006.
Lea, D. W., Mashiotta, T. A., and Spero, H. J.: Controls on magnesium and strontium uptake in planktonic foraminifera determined by live culturing, Geochim. Cosmochim. Ac., 63, 2369–2379, 1999.
Lea, D. W., Pak, D. K., and Spero, H. J.: Climate impact of late Quaternary equatorial Pacific sea surface temperature variations, Science, 289, 1719–1724, 2000.
Lin, H.-L., Sheu, D. D.-D., Yang, Y., Chou, W.-C., and Hung, G.-W.: Stable isotopes in modern planktonic foraminifera: Sediment trap and plankton tow results from the South China Sea, Mar. Micropaleontol., 79, 15–23, 2011.
Locarnini, R. A., Mishonov, A. V., Antonov, J. I., Boyer, T. P., Garcia, H. E., Baranova, O. K., Zweng, M. M., Paver, C. R., Reagan, J. R., Johnson, D. R., Hamilton, M., and Seidov, D.: World Ocean Atlas 2013, Volume 1: Temperature, in: NOAA Atlas NESDIS 73, edited by: Levitus, S. and Mishonov, A., Silver Spring, Maryland, 2013.
Lohmann, G. P. and Schweitzer, P. N.: Globorotalia truncatulinoides Growth and chemistry as probes of the past thermocline: 1. Shell size, Paleoceanography, 5, 55–75, 1990.
Lončarić, N., Peeters, F. J. C., Kroon, D., and Brummer, G. J. A.: Oxygen isotope ecology of recent planktic foraminifera at the central Walvis Ridge (SE Atlantic), Paleoceanography, 21, PA3009, https://doi.org/10.1029/2005PA001207, 2006.
McConnaughey, T.: 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns, Geochim. Cosmochim. Ac. 53, 151–162, 1989.
Mehrbach, C., Culberson, C. H., Hawley, J. E., and Pytkowicz, R. M.: Measurement of the apparent dissociation-constants of carbonic-acid in seawater at atmospheric pressure, Limnol. Oceanogr., 18, 897–907, https://doi.org/10.4319/lo.1973.18.6.0897, 1973.
Metcalfe, B., Feldmeijer, W., de Vringer-Picon, M., Brummer, G.-J. A., Peeters, F. J. C., and Ganssen, G. M.: Late Pleistocene glacial-interglacial shell-size-isotope variability in planktonic foraminifera as a function of local hydrography, Biogeosciences, 12, 4781–4807, https://doi.org/10.5194/bg-12-4781-2015, 2015.
Morel, A., Claustre, H., and Gentili, B.: The most oligotrophic subtropical zones of the global ocean: similarities and differences in terms of chlorophyll and yellow substance, Biogeosciences, 7, 3139–3151, https://doi.org/10.5194/bg-7-3139-2010, 2010.
Morrison, J. M. and Nowlin, W. D.: General distribution of water masses within the eastern Caribbean Sea during the winter of 1972 and fall of 1973, J. Geophys. Res., 87, 4207–4229, https://doi.org/10.1029/JC087iC06p04207, 1982.
Müller-Karger, F. E., McClain, C. R., Fisher, T. R., Esaias, W. E., and Varela, R.: Pigment distribution in the Caribbean Sea: Observations from space, Prog. Oceanogr., 23, 23–64, 1989.
Nürnberg, D.: Magnesium in tests of Neogloboquadrina pachyderma sinistral from high northern and southern latitudes, J. Foramin. Res., 25, 350–368, 1995.
Nürnberg, D., Bijma, J., and Hemleben, C.: Assessing the reliability of magnesium in foraminiferal calcite as a proxy for water mass temperatures, Geochim. Cosmochim. Ac., 60, 803–814, 1996.
Nürnberg, D., Müller, A., and Schneider, R. R.: Paleo-sea surface temperature calculations in the equatorial east Atlantic from Mg ∕ Ca ratios in planktic foraminifera: A comparison to sea surface temperature estimates from U, oxygen isotopes, and foraminiferal transfer function, Paleoceanography, 15, 124–134, https://doi.org/10.1029/1999PA000370, 2000.
Nürnberg, D., Schönfeld, J., Dullo, W. C., and Rühlemann, C.: RV SONNE cruise report SO164 RASTA: Rapid climate changes in the western tropical Atlantic – Assessment of the biogenous and sedimentary record, Balboa-Balboa, 22 May–28 June 2002, GEOMAR-Report 109, 1–151, 2003.
Nürnberg, D., Ziegler, M., Karas, C., Tiedemann, R., and Schmidt, M. W.: Interacting Loop Current variability and Mississippi River discharge over the past 400 kyr, Earth Planet. Sc. Lett., 272, 278–289, 2008.
Orr, W. N.: Secondary calcification in the foraminiferal genus Globorotalia, Science, 157, 1554–1555, 1967.
Pak, D. K., Lea, D. W., and Kennett, J. P.: Seasonal and interannual variation in Santa Barbara Basin water temperatures observed in sediment trap foraminiferal Mg ∕ Ca, Geochem. Geophy. Geosy., 5, Q12008, https://doi.org/10.1029/2004GC000760, 2004.
Pierrot, D., Lewis, E., and Wallace, D. W. R.: MS Excel Program Developed for CO2 System Calculations, ORNL/CDIAC-105a, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee, 2006.
Poore, R. Z., Tedesco, K. A., and Spear, J. W.: Seasonal flux and assemblage composition of planktic foraminifers from a sediment-trap study in the northern Gulf of Mexico, J. Coastal Res., 63, 6–19, 2013.
Regenberg, M., Nürnberg, D., Steph, S., Groeneveld, J., Garbe-Schönberg, D., Tiedemann, R., and Dullo, W. C.: Assessing the effect of dissolution on planktonic foraminiferal Mg ∕ Ca ratios: Evidence from Caribbean core tops, Geochem. Geophy. Geosy. 7, Q07P15, https://doi.org/10.1029/2005GC001019, 2006.
Regenberg, M., Steph, S., Nürnberg, D., Tiedemann, R., and Garbe-Schönberg, D.: Calibrating Mg ∕ Ca ratios of multiple planktonic foraminiferal species with δ18O-calcification temperatures: Paleothermometry for the upper water column, Earth Planet. Sc. Lett., 278, 324–336, 2009.
Regenberg, M., Regenberg, A., Garbe-Schönberg, D., and Lea, D. W.: Global dissolution effects on planktonic foraminiferal Mg ∕ Ca ratios controlled by the calcite-saturation state of bottom waters, Paleoceanography, 29, 127–142, https://doi.org/10.1002/2013PA002492, 2014.
Rink, S., Kühl, M., Bijma, J., and Spero, H. J.: Microsensor studies of photosynthesis and respiration in the symbiotic foraminifer Orbulina universa, Mar. Biol., 131, 583–595, 1998.
Rosenthal, Y., Lohmann, G. P., Lohmann, K. C., and Sherrell, R. M.: Incorporation and preservation of Mg in Globigerinoides sacculifer: Implications for reconstructing the temperature and 18O ∕ 16O of seawater, Paleoceanography, 15, 135–145, https://doi.org/10.1029/1999PA000415, 2000.
Russell, A. D., Hönisch, B., Spero, H. J., and Lea, D. W.: Effects of seawater carbonate ion concentration and temperature on shell U, Mg, and Sr in cultured planktonic foraminifera, Geochim. Cosmochim. Ac., 68, 4347–4361, 2004.
Sadekov, A. Y., Eggins, S. M., and De Deckker, P.: Characterization of Mg ∕ Ca distributions in planktonic foraminifera species by electron microprobe mapping, Geochem. Geophy. Geosy., 6, Q12P06, https://doi.org/10.1029/2005GC000973, 2005.
Sadekov, A. Y., Eggins, S. M., De Deckker, P., Ninnemann, U., Kuhnt, W., and Bassinot, F.: Surface and subsurface seawater temperature reconstruction using Mg ∕ Ca microanalysis of planktonic foraminifera Globigerinoides ruber, Globigerinoides sacculifer, and Pulleniatina obliquiloculata, Paleoceanography, 24, PA3201, https://doi.org/10.1029/2008PA001664, 2009.
Schiebel, R. and Hemleben, C.: Planktic Foraminifers in the Modern Ocean, Springer-Verlag, Berlin, Heidelberg, 2017.
Schlitzer, R.: Ocean data view, available at: http://odv.awi.de (last access: 21 November 2018), 2009.
Schmidt, G. A.: Error analysis of paleosalinity calculations, Paleoceanography, 14, 422–429, https://doi.org/10.1029/1999PA900008, 1999.
Schmidt, G. A., Bigg, G. R., and Rohling, E. J.: Global Seawater Oxygen-18 Database – v1.21, available at: http://data.giss.nasa.gov/o18data (last access: 21 November 2018), 1999.
Schmidt, M. W., Spero, H. J., and Lea, D. W.: Links between salinity variation in the Caribbean and North Atlantic thermohaline circulation, Nature, 428, 160–163, https://doi.org/10.1038/nature02346, 2004.
Schönfeld, J., Bahr, A., Bannert, B., Bayer, A. S., Bayer, M., Beer, C., Blanz, T., Dullo, W. C., Flögel, S., Garlichs, T., Haley, B., Hübscher, C., Joseph, N., Kučera, M., Langenbacher, J., Nürnberg, D., Ochsenhirt, W. T., Petersen, A., Pulm, P., Titschack, J., and Troccoli, L.: Surface and Intermediate water hydrography, planktonic and benthic biota in the Caribbean Sea – Climate, Bio and Geosphere linkages (OPOKA), Cruise No. 78, Leg 1, 22 February–28 March 2009, Colón (Panama) – Port of Spain (Trinidad and Tobago), METEOR-Berichte, 1–196, 2011.
Schweitzer, P. N. and Lohmann, G. P.: Ontogeny and habitat of modern menardiiform planktonic foraminifera, J. Foramin. Res., 21, 332–346, 1991.
Shackleton, N. J., Wiseman, J. D. H., and Buckley, H. A.: Non-equilibrium isotopic fractionation between seawater and planktonic foraminiferal tests, Nature, 242, 177–179, https://doi.org/10.1038/242177a0, 1973.
Spero, H. J.: Ultrastructural examination of chamber morphogenesis and biomineralization in the planktonic foraminifer Orbulina universa, Mar. Biol., 99, 9–20, 1988.
Spero, H. J.: Do planktic foraminifera accurately record shifts in the carbon isotopic composition of seawater ∑CO2?, Mar. Micropaleontol., 19, 275–285, 1992.
Spero, H. J. and Lea, D. W.: Intraspecific stable isotope variability in the planktic foraminifera Globigerinoides sacculifer: Results from laboratory experiments, Mar. Micropaleontol., 22, 221–234, 1993.
Spero, H. J. and Lea, D. W.: Experimental determination of stable isotope variability in Globigerina bulloides: implications for paleoceanographic reconstructions, Mar. Micropaleontol., 28, 231–246, 1996.
Spero, H. J. and Parker, S. L.: Photosynthesis in the symbiotic planktonic foraminifer Orbulina universa, and its potential contribution to oceanic primary productivity, J. Foramin. Res., 15, 273–281, 1985.
Spero, H. J., Bijma, J., Lea, D. W., and Bemis, B. E.: Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes, Nature, 390, 497–500, https://doi.org/10.1038/37333, 1997.
Spero, H. J., Mielke, K. M., Kalve, E. M., Lea, D. W., and Pak, D. K.: Multispecies approach to reconstructing eastern equatorial Pacific thermocline hydrography during the past 360 kyr, Paleoceanography, 18, 1022, https://doi.org/10.1029/2002PA000814, 2003.
Spero, H. J., Eggins, S. M., Russell, A. D., Vetter, L., Kilburn, M. R., and Hönisch, B.: Timing and mechanism for intratest Mg ∕ Ca variability in a living planktic foraminifer, Earth Planet. Sc. Lett., 409, 32–42, 2015.
Spezzaferri, S., Kučera, M., Pearson, P. N., Wade, B. S., Rappo, S., Poole, C. R., Morard, R., and Stalder, C.: Fossil and Genetic Evidence for the Polyphyletic Nature of the Planktonic Foraminifera “Globigerinoides”, and Description of the New Genus Trilobatus, PLoS ONE, 10, e0128108, https://doi.org/10.1371/journal.pone.0128108, 2015.
Steinhardt, J., de Nooijer, L. L. J., Brummer, G.-J., and Reichart, G.-J.: Profiling planktonic foraminiferal crust formation, Geochem. Geophys. Geosyst., 16, 2409–2430, https://doi.org/10.1002/2015GC005752, 2015.
Steph, S., Regenberg, M., Tiedemann, R., Mulitza, S., and Nürnberg, D.: Stable isotopes of planktonic foraminifera from tropical Atlantic/Caribbean core-tops: Implications for reconstructing upper ocean stratification, Mar. Micropaleontol., 71, 1–19, 2009.
Tedesco, K., Thunell, R., Astor, Y., Muller-Karger, F.: The oxygen isotope composition of planktonic foraminifera from the Cariaco Basin, Venezuela: Seasonal and interannual variations, Mar. Micropaleontol., 62, 180–193, 2007.
Van Geldern, R. and Barth, J.: Optimization of instrument setup and post-run corrections for oxygen and hydrogen stable isotope measurements of water by isotope ratio infrared spectroscopy (IRIS), Limnol. Oceanogr.-Methods, 10, 1024–1036, 2012.
Vergnaud-Grazzini, C.: Non-equilibrium isotopic compositions of shells of planktonic foraminifera in the Mediterranean Sea, Palaeogeogr. Palaeocl., 20, 263–276, 1976.
Vidal, V. M. V., Vidal, F. V., Hernández, A. F., Meza, E., and Zambrano, L.: Winter water mass distributions in the western Gulf of Mexico affected by a colliding anticyclonic ring, J. Oceanogr., 50, 559–588, 1994.
Vukovich, F. M.: Climatology of Ocean Features in the Gulf of Mexico Using Satellite Remote Sensing Data, J. Phys. Oceanogr., 37, 689–707, https://doi.org/10.1175/JPO2989.1, 2007.
Watanabe, T., Winter, A., and Oba, T.: Seasonal changes in sea surface temperature and salinity during the Little Ice Age in the Caribbean Sea deduced from Mg ∕ Ca and 18O ∕ 16O ratios in corals, Mar. Geol., 173, 21–35, 2001.
Weiner, S. and Dove, P. M.: An overview of biomineralization processes and the problem of the vital effect, Rev. Mineral. Geochem., 54, 1–29, https://doi.org/10.2113/0540001, 2003.
Wilke, I., Bickert, T., and Peeters, F. J. C.: The influence of seawater carbonate ion concentration [] on the stable carbon isotope composition of the planktic foraminifera species Globorotalia inflata, Mar. Micropaleontol., 58, 243–258, 2006.
Wüst, G.: Stratification and circulation in the Antillean-Caribbean basins, Columbia University Press, Palisades, New York, 1964.
Zeebe, R. E.: An explanation of the effect of seawater carbonate concentration on foraminiferal oxygen isotopes, Geochim. Cosmochim. Ac., 63, 2001–2007, 1999.
Zweng, M. M, Reagan, J. R., Antonov, J. I., Locarnini, R. A., Mishonov, A. V., Boyer, T. P., Garcia, H. E., Baranova, O. K., Johnson, D. R., Seidov, D., and Biddle, M. M.: World Ocean Atlas 2013, Volume 2: Salinity, in: NOAA Atlas NESDIS 74, edited by: Levitus, S. and Mishonov, A., Silver Spring, Maryland, 2013.





