the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Tracing terrestrial versus marine sources of dissolved organic carbon in a coastal bay using stable carbon isotopes
Shin-Ah Lee
Tae-Hoon Kim
The sources of dissolved organic matter (DOM) in coastal waters are diverse, and they play different roles in the biogeochemistry and ecosystems of the ocean. In this study, we measured dissolved organic carbon (DOC) and nitrogen (DON), the stable carbon isotopic composition of dissolved organic carbon (δ13C-DOC), and fluorescent dissolved organic matter (FDOM) in coastal bay waters surrounded by large cities (Masan Bay, Republic of Korea) to determine the different DOM sources in this region. The surface seawater samples were collected in two sampling campaigns (August 2011 and August 2016). The salinities were in the range of 10–21 in 2011 and 25–32 in 2016. In 2011, excess DOC was observed in high-salinity (16–21) waters; the excess DOC source was found to be mainly from marine autochthonous production according to the δ13C-DOC values (−23.7 ‰ to −20.6 ‰), the higher concentrations of protein-like FDOM, and the lower DOC∕DON (C∕N) ratios (8–15). In contrast, excess DOC observed in high-salinity waters in 2016 was characterized by low FDOM, more depleted δ13C values (−28.8 ‰ to −21.1 ‰), and high C∕N ratios (13–45), suggesting that the source of excess DOC is terrestrial C3 plants by direct land–seawater interactions. Our results show that multiple DOM tracers such as δ13C-DOC, FDOM, and C∕N ratios are powerful for determining different sources of DOM occurring in coastal waters.
- Article
(3132 KB) - Full-text XML
-
Supplement
(181 KB) - BibTeX
- EndNote
Dissolved organic matter (DOM) plays an important role in biogeochemical cycles (e.g., de-oxygenation, acidification, and photochemistry) and ecosystems of the ocean (Hansell and Carlson, 2002). DOM composition depends on its parent organic matter and subsequent biogeochemical processes. DOM in coastal waters originates from various sources including (1) in situ production by primary production, exudation of aquatic plants, and aquatic plant degradation (Markager et al., 2011; Carlson and Hansell, 2015); (2) terrestrial sources by the degradation of soil and terrestrial plant matter (Opsahl and Benner, 1997; Bauer and Bianchi, 2011); and (3) anthropogenic sources such as industrial, agricultural, and domestic sewage (Griffith and Raymond, 2011).
Depending on the origin and composition of DOM, its behavior and cycling are different; the labile fraction of DOM decomposes rapidly through microbially or photochemically mediated processes, whereas refractory DOM is resistant to degradation and can persist in the ocean for millennia. In the coastal ocean, organic matter from terrestrial plant litter or soils appears to be more refractory (Cauwet, 2002) and thus often behaves conservatively. In addition, refractory DOM is produced in the ocean by the bacterial transformation of labile DOM, which reshapes its composition (Tremblay and Benner, 2006; Jiao et al., 2010). However, it is still very difficult to determine the sources and characteristics of DOM in coastal waters.
There are many approaches to distinguish the source of DOM in coastal areas using various tracers (Faganeli et al., 1988; Benner and Opsahl, 2001; Chen et al., 2004; Baker and Spencer, 2004; Cawley et al., 2012; and Lee and Kim, 2018). The stable carbon isotopic composition of dissolved organic carbon (δ13C-DOC) has been used to distinguish different sources. In general, δ13C values derived from C3 and C4 land plants are in the range of −23 ‰ to −34 ‰ and −9 ‰ to −17 ‰ (Deines, 1980), respectively, while those derived from marine phytoplankton range from −18 ‰ to −22 ‰ (Kelley et al., 1998; Coffin and Cifuentes, 1999). In addition, the optically active fraction of DOM known as fluorescent DOM (FDOM) has been successfully used for characterizing DOM (Coble et al., 1990; Coble, 1996). The fluorescence excitation–emission matrices and parallel factor analysis (EEMs–PARAFAC) technique has been applied to trace the source of humic-like versus protein-like DOM in coastal waters and estuaries (Chen et al., 2004; Jaffé et al., 2004; and Murphy et al., 2008). Dissolved organic carbon (DOC) and nitrogen (DON) ratios, DOC∕DON, are often used to differentiate allochthonous versus autochthonous sources. The C∕N ratios of terrestrial organic carbon are usually higher than 12, while those of marine organic carbon from phytoplankton are almost constant, ranging from 6 to 8 (Milliman et al., 1984; Lobbes et al., 2000). However, the interpretation of the isotopic ratio from a bulk sample in complex coastal environments is somewhat complicated by the overlap of isotopic ranges. Thus, several studies have used δ13C-DOC combined with FDOM (Osburn and Stedmon, 2011; Osburn et al., 2011; Ya et al., 2015; and Lu et al., 2015) or carbon isotope ratios combined with the C∕N ratio (Thornton and McManus, 1994; Andrews et al., 1998; Wang et al., 2004; McCallister et al., 2006; and Pradhan et al., 2014) to discriminate different sources of DOM in estuarine and coastal waters. As far as we know, these three tracers have not yet been used together to determine DOM sources in coastal waters.
Our study aimed to discriminate between DOM sources in coastal waters, where various sources are present, using δ13C-DOC, FDOM, and DOC∕DON ratios together. Masan Bay is surrounded by cities with thousands of industrial plants and a population of 1.1 million people. In association with large anthropogenic nutrient loading, this area has been recognized as a highly eutrophic embayment (Lee and Min, 1990; Yoo, 1991; and Hong et al., 2010). Red tides and a hypoxic water mass in the bottom layer of the bay have occurred annually in spring and summer (Lee et al., 2009). In addition, there are potential point sources from sewage treatment plants (STPs) which manage domestic and industrial wastewater from the cities of Masan and Changwon. Lee et al. (2011) revealed the origins of sewage and organic matter using dissolved sterols in Masan Bay. They reported that the water samples from creeks, the inner bay, and a nearby STP were affected by sewage sources. Oh et al. (2017) showed that the excess DOC in bay water is produced by phytoplankton production. Therefore, Masan Bay is a suitable place to test the applicability of these multiple tracers in order to determine different DOM sources in other coastal regions around the world.
2.1 Study site
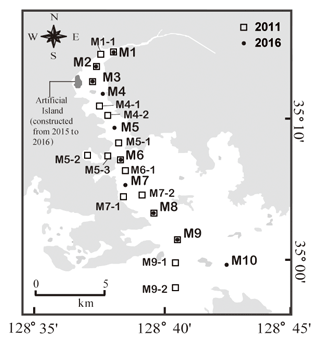
Masan Bay is located on the southeast coast of the Republic of Korea with an area of approximately 80 km2 (Fig. 1). The annual precipitation is approximately 1500 mm, and most of the precipitation occurs in the summer monsoon season. The amount of freshwater discharge into this bay is approximately 2.5×108 m3 yr−1, with significant seasonal variation. The tide is semidiurnal, showing a maximum tidal amplitude of ∼1.9 m (average amplitude = 1.3 m) during the sampling period. Due to topographic conditions, the current is very weak (2–3 cm s−1), and the residence times of water in the inner bay and in the entire bay are approximately 54 and 23 d, respectively (Lee et al., 2009). In the middle of the bay, an artificial island was constructed in 2015–2016 (Fig. 1) with an area of 0.64 km2. The artificial island may have resulted in changes in water currents, residence times, and biogeochemical conditions.
2.2 Sampling
Sampling was conducted in August 2011 and August 2016 in Masan Bay. Water samples were collected from the surface at 17 sites in 2011 and 10 sites in 2016. The bay receives a large amount of freshwater discharge from the northernmost part of the region. The average surface water temperatures were 30.4±2.3 ∘C in 2011 and 26.5±0.7 ∘C in 2016. All water samples were filtered through pre-combusted glass fiber filters (GF/F, Whatman). Samples for FDOM analysis were stored at 4 ∘C in pre-combusted amber vials. Samples of DOC, total dissolved nitrogen (TDN), and δ13C-DOC analysis were stored in pre-combusted glass ampoules after acidifying to a pH of ∼2 with 6 M HCl. Samples analyzed for dissolved inorganic nitrogen (DIN) were stored frozen in a HDPE bottle (Nalgene) prior to analyses.
2.3 Analytical methods
The concentrations of DOC and TDN were determined using a high-temperature catalytic oxidation (HTCO) analyzer (TOC-VCPH, Shimadzu, Japan). The standardization for DOC analysis was performed using a calibration curve of acetanilide (C∕N ratio = 8) in ultra-pure water. The acidified samples were purged with pure air carrier gas for 2 min to remove dissolved inorganic carbon. Samples were carried into a combustion tube heated to 720 ∘C where the DOC was converted quantitatively to CO2. CO2 gas was detected by a nondispersive infrared detector (NDIR). Our DOC and TDN methods were verified using deep seawater reference samples, 44–46 µmol L−1 for DOC and 32–34 µmol L−1 for TDN, which were produced by the University of Miami (Hansell Organic Biogeochemistry Lab, USA). Inorganic nutrients were measured using nutrient auto-analyzers (FUTURA+, Alliance Instruments, for 2011 samples; QuAAtro39, SEAL Analytical Ltd., for 2016 samples). Reference seawater materials (KANSO Technos, Japan) were used for the verification of analytical accuracy. DON concentrations were calculated based on the difference between the TDN and DIN concentrations.
The values of δ13C-DOC were determined using a TOC–IRMS instrument (IR-MS from Isoprime, UK coupled with a Vario TOC cube from Elementar, Germany). The analytical method is the same as that used by Kim et al. (2015) and Lee and Kim (2018). Low carbon water (<2 µM; University of Miami, Hansell Organic Biogeochemistry Lab) was measured for blank corrections and used for preparing all standard samples. The blank correction procedure is the same as that reported previously (Panetta et al., 2008; De Troyer et al., 2010). Certified IAEA-CH-6 sucrose (International Atomic Energy Agency; ‰) was used for standardization. The standard solution was measured every 10 samples to monitor the drifting effect. Our measured values of δ13C-DOC in the deep seawater reference (University of Miami) samples were ±0.3 ‰ relative to the values provided by Panetta et al. (2008) and Lang et al. (2007).
FDOM was determined using a spectrofluorometer (FluoroMate FS-2, SCINCO) within 2 d of the sampling time. EEMs were collected for the emission (Em) wavelength range of 240–600 nm with 2 nm intervals and an excitation (Ex) wavelength range of 240–500 nm with 5 nm intervals. Each sample value was subtracted from the signal of Milli-Q water produced daily to remove Raman scattering peaks. All data were converted to quinine sulfate units (QSU) using a quinine sulfate standard solution dissolved in 0.1 N sulfuric acid at Ex/Em of 350/450 nm. We did not correct EEM data for inner filter effects before measurements, because the inner filter effects were found to be negligible for coastal water samples using this instrument (Lee and Kim, 2018). EEMs–PARAFAC was performed in MATLAB (R2013a) using a DOMFluor toolbox, and the three components (C1–C3) were validated by split-half analysis (Figs. S1 and S2 in the Supplement).
3.1 Horizontal distributions of DOM
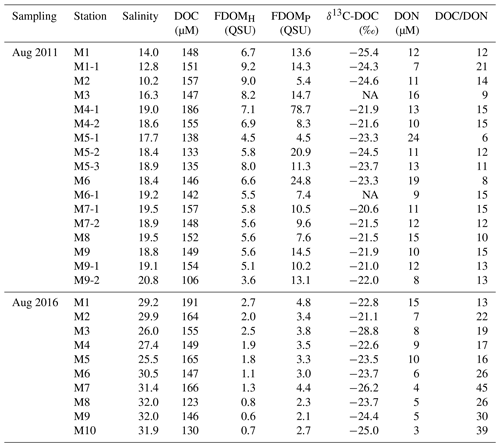
The salinity of surface seawater in August 2011 ranged from 10 to 21, while the salinity in August 2016 ranged from 25 to 32 (Table 1 and Fig. 2). The concentrations of DOC in both sampling periods ranged from 100 to 200 µM (Fig. 2), which fall within the DOC ranges commonly observed in coastal waters (Gao et al., 2010; Osburn and Stedmon, 2011; and Kim et al., 2012). The highest concentration of DOC in 2011 (186 µM) was observed at station M4-1 in the middle of the bay, whereas the highest concentration of DOC in 2016 (191 µM) was observed at station M1, which is the innermost station in the bay. DOC concentrations were lowest at the outermost stations in both sampling periods. Concentrations of DON were in the range of 7–24 µM in 2011 and 3–15 µM in 2016, with the highest value at station M5-1 in 2011 and at M1 in 2016 (Fig. 2).
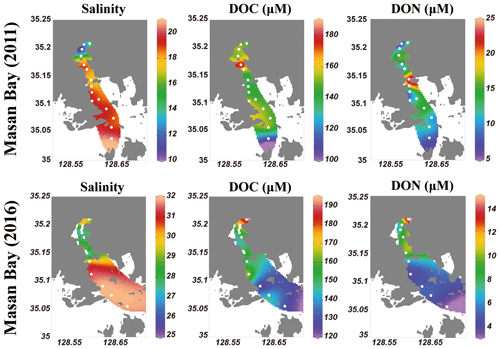
Figure 2Surface distributions of salinity, DOC, and DON in Masan Bay, Republic of Korea, in 2011 and 2016.
Table 1Salinity, DOC, FDOMH, FDOMP, δ13C-DOC, DON, and the DOC∕DON ratio in surface water of Masan Bay in August 2011 and August 2016.
NA: not available.
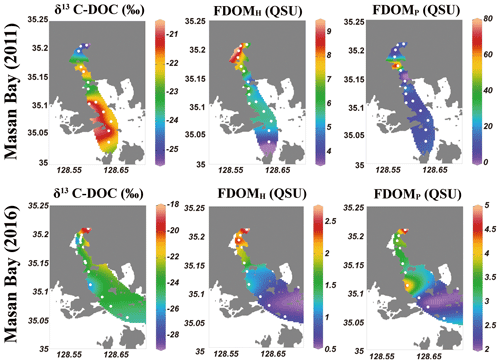
EEMs–PARAFAC dataset analyses identified three components in the surface water samples. EEMs contour plots and split-half validation results for three components are shown in the Supplement (Figs. S1 and S2). Based on the comparison with data in the OpenFluor (Murphy et al., 2014), Component 1 (FDOMH; Ex/Em = 322/405 nm) is associated with a terrestrial humic-like component (Liu et al., 2019; Dalmagro et al., 2019; and Chen et al., 2016). Component 2 (FDOMM; Ex/Em = 386/450 nm) is also associated with an allochthonous humic-like component (Wünsch et al., 2017). Component 3 (FDOMP; Ex/Em = 280/330 nm) is associated with a protein-like component, which is a product of microbial processes (Liu et al., 2019; Murphy et al., 2011; and Osburn et al., 2011). We use Component 1 as a representative of terrestrial humic-like FDOM (FDOMH) in this study because there was a significant correlation (r2=0.95) between Component 1 and Component 2.
FDOMH is known to indicate humic substances from terrestrial, anthropogenic, or agricultural sources (Coble, 2007), whereas FDOMP is likely related to autochthonous or anthropogenic sources (Coble, 1996; Hudson et al., 2007). The intensities of FDOMH and FDOMP in 2011 were in the range of 3.6–9.2 and 4–79 QSU, respectively (Fig. 3). The intensities of FDOMH and FDOMP in 2016 were in the range of 2.7–0.6 and 4.8–2.1 QSU, respectively (Fig. 3). An exceptionally high concentration of FDOMP was observed at station M4-1 (78 QSU) relative to that of other stations (2–25 QSU) in 2011 (Fig. 4d).
3.2 Origin of excess DOM
The plot of DOC versus salinity in 2011 (Fig. 4a) shows two different mixing trends. The first slope shows a slight increase in DOC with decreasing salinity toward the innermost stations, including M1, M1-1, and M2 (Fig. 4a, Group 1). The second trend is a sharp rise in DOC (excess DOC in 2011) to the maximum value at stations with salinities between 18 and 22 (Fig. 4a, Group 2), indicating that there are other DOC sources at the high-salinity stations, besides the two end-member source mixing. The plot of DOC versus salinity shows that DOC in 2016 was in a range similar to that of 2011, although there was much less influence from freshwater (Fig. 4a, Group 3). This plot shows that there was additional DOC (excess DOC) in 2016 in the high-salinity water in the bay. The potential sources of excess DOC occurring in this bay water may include terrestrial freshwater from creeks, STP water, direct land–seawater interaction, and in situ biological production. The creek water may also include various anthropogenic sources (i.e., industrial, agricultural, and domestic sewage) as well as natural land sources. There are no salt-marsh or wetland habitats in Masan Bay. To determine the main sources of the excess DOC using δ13C-DOC, FDOM, and DOC∕DON ratios, the stations with excess DOC are separated into three groups (Group 1, Group 2 in 2011, and Group 3 in 2016) (Fig. 4a).
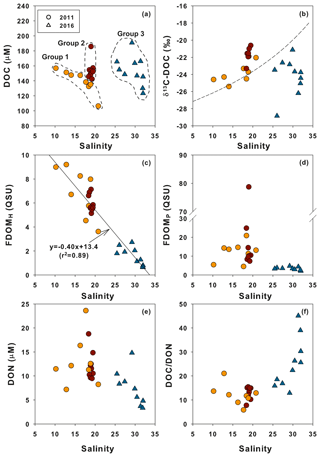
Figure 4Relationships between salinity and (a) DOC, (b) δ13C-DOC, (c) FDOMH, (d) FDOMP, (e) DON, and (f) DOC∕DON ratio values. The DOC concentrations are divided into three groups based on probable different sources (in the dashed circles). The dashed line (b) represents the binary conservative mixing line for δ13C-DOC between the terrestrial end-member and the marine end-member. The solid line (c) represents a linear regression fit of the data.
Group 1 includes low-salinity water stations (M1, M1-1, M2, M3, M5-1, M5-2, and M5-3) observed in 2011 (Fig. 1). δ13C-DOC values from Group 1 ranged from −25.4 ‰ to −23.3 ‰. We plotted a conservative mixing curve of δ13C-DOC for two end-member source mixing (Spiker, 1980; Raymond and Bauer, 2001). The assumed end-member values of DOC and δ13C-DOC were 185 µM and −28 ‰ (Raymond and Bauer, 2001), respectively, for the terrestrial end-member (S=0) and 100 µM and −18 ‰ (Kelley et al., 1998), respectively, for the marine end-member (S=34). The δ13C values from Group 1 fall on the mixing line or are slightly heavier than the mixing line, within 1.5 ‰, indicating conservative mixing between the terrestrial C3 land plants (−23 ‰ to −32 ‰; Deines, 1980) in freshwater and open-ocean seawater. The slightly heavier values could be produced by in situ biological production during the mixing processes. As such, the plot of δ13C-DOC values versus C∕N ratios also indicates that the excess DOC in Group 1 is from freshwater DOC (Fig. 5a).
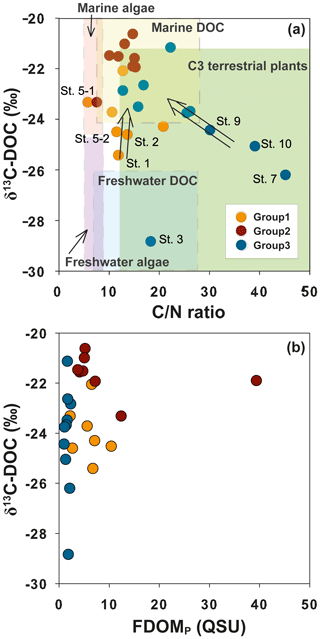
Figure 5Relationships between δ13C-DOC values and (a) the DOC∕DON (C∕N) ratio and (b) FDOMP in Masan Bay, Republic of Korea. The ranges of the DOC∕DON ratio and δ13C-DOC values for each group are based on the values reported by Lamb et al. (2006) and Beaupré (2015).
Group 2 includes high-salinity water stations (M4-1, M4-2, M6, M6-1, M7-1, M7-2, M8, M9, and M9-1) observed in 2011 (Fig. 1). The δ13C-DOC values from Group 2 were in the range of −23.3 ‰ to −20.6 ‰ and were more enriched than the conservative mixing curve. These values are close to the marine δ13C-DOC values (−22 ‰ to −18 ‰) (Fry et al., 1998), except for one station (M6), in this group (−23.3 ‰). The δ13C-DOC values from Group 2 suggest that excess DOM was added in situ by biological production in seawater. As such, the plot of δ13C-DOC values versus C∕N ratios also indicates that the excess DOC of Group 2 is produced by marine phytoplankton (Fig. 5a).
Group 3 includes high-salinity water stations (M1, M2, M3, M4, M5, M6, and M7) observed in 2016 (Fig. 1). Although all data were collected in the same wet season (August), the salinity ranges from both campaigns were different from those in 2011, with a narrow high-salinity range in 2016. The δ13C-DOC values from Group 3 also show significantly different values relative to those sampled in 2011 (Group 1 and Group 2). The δ13C-DOC values from Group 3 were depleted (−28.8 ‰ and −21.1 ‰) relative to the conservative mixing curve (Fig. 4b). The plot of δ13C-DOC values versus C∕N ratios indicates that the excess DOC in Group 3 is from C3 terrestrial plants through direct land–seawater interactions (including the possible sources from a newly built artificial island), based on the fact that the excess DOC occurred in high-salinity (26–32) waters (Fig. 5a).
FDOMH had a significant negative correlation with salinity (r2=0.89). The concentrations were highest for Group 1 and lowest for Group 3. This result indicates that humic DOM in this region was mainly from a terrestrial source and behaved conservatively in the freshwater and seawater mixing zone. This trend is commonly observed in coastal waters worldwide (Coble et al., 1998; Mayer et al., 1999). However, the concentration of FDOMP had no correlation with salinity. In general, FDOMP shows nonconservative behavior in many estuaries owing to the extra source of DOC produced by in situ biological production (Benner and Opsahl, 2001). In the study region, a remarkably high FDOMP concentration was observed at station M4-1 in 2011, where the DOC concentration was highest. This trend also supports the argument that, based on the δ13C-DOC results, the main source of DOC at this station is from in situ biological production. We observed the decoupling of DOC and FDOMH because FDOMH is not the major portion of DOC in this bay.
Masan Bay has many potential land sources of DOM from different creeks. In addition, the treated sewage outflow from a STP is located near station M7-1 (Fig. 1). Many studies have been conducted to identify organic pollutants from STPs (Kannan et al., 2010; Lee et al., 2011). In our study, however, station M7-1 samples did not have different DOM characteristics but rather had the following features:
-
The concentrations of DOC, FDOMH, and FDOMP compared with salinity did not show anomalously higher or lower trends, relative to the other stations nearby.
-
The δ13C-DOC values at M7-1 (−20.6 ‰) were close to the marine values (Fry et al., 1998), similar to those from other stations nearby, although these values are known to be lighter in some US wastewater treatment plants (−26 ‰) (Griffith et al., 2009).
-
A fulvic-like peak was not observed, although a significantly higher fulvic-like peak (Ex/Em 320–340 nm/410–430 nm) was observed in treated sewage (Baker, 2001).
-
The increase in FDOMP intensities at stations M7-1 and M7-2 were insignificant relative to those at stations M6-1 and M8, although FDOMP is often used as a tracer of anthropogenic material including treated effluents (Hudson et al., 2007).
Thus, we conclude that the concentration of DOC at station M7-1 was not influenced by the STP. This STP appears to reduce TOC concentrations to a level that cannot influence DOC concentrations resulting from the other mixing sources, as shown in several other estuaries (Abril et al., 2002).
In general, anomalously high FDOMP was observed in anthropogenic sources (Coble, 1996; Baker and Inverarity, 2004). The δ13C values for sewage effluents generally ranged from −22 ‰ to −28.5 ‰ (Andrews et al., 1998; Barros et al., 2010), and those for STP effluents ranged from −24 ‰ to −28 ‰ (Griffith et al., 2009). The δ13C vs. FDOMP plot (Fig. 5b) shows that there was no increase in FDOMP concentrations in samples which had depleted δ13C values. Thus, we conclude that there was no significant DOC input from untreated sewage or STP sources in this bay.
We determined the sources of DOM in 2011 and 2016 using the δ13C-DOC, FDOM, and DOC∕DON ratios. The main sources were separated into three groups based on DOC concentrations versus salinity plots. The DOM concentrations in the first group in 2011, which included the lowest salinity waters, were found to be mixtures of terrestrial DOM and open-ocean DOM sources based on the δ13C values of −25.4 ‰ to −23.3 ‰ and a good correlation between FDOMH and salinity. The excess DOC concentrations in the second group in high-salinity waters in 2011 were found to be produced by in situ biological production based on more enriched δ13C-DOC values (−22.0 ‰ to −20.6 ‰), high FDOMP concentrations, and low C∕N ratios. The excess DOC concentrations in the third group in high-salinity waters in 2016 seemed to be produced by a direct interaction between land and seawater based on more depleted δ13C-DOC values (−28.8 ‰ and −21.1 ‰), low FDOM concentrations, and high C∕N ratios. Our results show that using a combination of multiple DOM tracers including δ13C-DOC, FDOM, and C∕N ratios is a powerful method for determining different sources of DOM occurring in coastal waters
All data used in this paper can be accessed by email to the corresponding author upon request.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-17-135-2020-supplement.
GK conceptualized the study. SAL and THK collected the samples. SAL performed the analyses. SAL and GK wrote the paper, and all authors contributed to the interpretation and discussion of the results.
The authors declare that they have no conflict of interest.
We thank members of the Environmental and Marine Biogeochemistry Laboratory (EMBL) for their assistance with sampling and laboratory analyses. We would like to thank two anonymous reviewers for constructive discussion and valuable comments.
This research has been supported by the National Research Foundation of Korea (NRF) (grant nos. NRF-2015R1A2A1A10054309 and NRF-2018R1A2B3001147) funded by the South Korean government (MEST).
This paper was edited by Clare Woulds and reviewed by two anonymous referees.
Abril, G., Nogueira, M., Etcheber, H., Cabeçadas, G., Lemaire, E., and Brogueira, M.: Behaviour of organic carbon in nine contrasting European estuaries, Estuar. Coast. Shelf S., 54, 241–262, 2002.
Andrews, J., Greenaway, A., and Dennis, P.: Combined carbon isotope and C∕N ratios as indicators of source and fate of organic matter in a poorly flushed, tropical estuary: Hunts Bay, Kingston Harbour, Jamaica, Estuar. Coast. Shelf S., 46, 743–756, 1998.
Baker, A.: Fluorescence excitation-emission matrix characterization of some sewage-impacted rivers, Environ. Sci. Technol., 35, 948–953, 2001.
Baker, A. and Inverarity, R.: Protein-like fluorescence intensity as a possible tool for determining river water quality, Hydrol. Process., 18, 2927–2945, 2004.
Baker, A. and Spencer, R. G.: Characterization of dissolved organic matter from source to sea using fluorescence and absorbance spectroscopy, Sci. Total Environ., 333, 217–232, 2004.
Barros, G. V., Martinelli, L. A., Novais, T. M. O., Ometto, J. P. H., and Zuppi, G. M.: Stable isotopes of bulk organic matter to trace carbon and nitrogen dynamics in an estuarine ecosystem in Babitonga Bay (Santa Catarina, Brazil), Sci. Total Environ., 408, 2226–2232, 2010.
Bauer, J. E. and Bianchi, T. S.: Dissolved organic carbon cycling and transformation, in: Treatise on estuarine and coastal science, edited by: Wolanski, E. and Mcluski, D. S., 5, 7–67, Academic Press, Waltham, 2011.
Beaupré, S. R.: Chapter 6 – The Carbon Isotopic Composition of Marine DOC, in: Biogeochemistry of Marine Dissolved Organic Matter (Second Edition), edited by: Hansell, D. A. and Carlson, C. A., Academic Press, Boston, 335–368, 2015.
Benner, R. and Opsahl, S.: Molecular indicators of the sources and transformations of dissolved organic matter in the Mississippi river plume, Org. Geochem., 32, 597–611, 2001.
Carlson, C. A. and Hansell, D. A.: Chapter 3 – DOM Sources, Sinks, Reactivity, and Budgets, in: Biogeochemistry of Marine Dissolved Organic Matter (Second Edition), edited by: Hansell, D. A. and Carlson, C. A., Academic Press, Boston, 65–126, 2015.
Cauwet, G.: Chapter 12 – DOM in the Coastal Zone, in: Biogeochemistry of Marine Dissolved Organic Matter, edited by: Hansell, D. A. and Carlson, C. A., Academic Press, San Diego, 579–609, 2002.
Cawley, K. M., Ding, Y., Fourqurean, J., and Jaffé, R.: Characterising the sources and fate of dissolved organic matter in Shark Bay, Australia: a preliminary study using optical properties and stable carbon isotopes, Mar. Freshwater Res., 63, 1098–1107, https://doi.org/10.1071/MF12028, 2012.
Chen, R. F., Bissett, P., Coble, P., Conmy, R., Gardner, G. B., Moran, M. A., Wang, X., Wells, M. L., Whelan, P., and Zepp, R. G.: Chromophoric dissolved organic matter (CDOM) source characterization in the Louisiana Bight, Mar. Chem., 89, 257–272, 2004.
Chen, M., Kim, J.-H., Nam, S.-I., Niessen, F., Hong, W.-L., Kang, M.-H., and Hur, J. J.: Production of fluorescent dissolved organic matter in Arctic Ocean sediments, Sci. Rep.-UK, 6, 39213, https://doi.org/10.1038/srep39213, 2016.
Coble, P. G.: Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy, Mar. Chem., 51, 325–346, 1996.
Coble, P. G.: Marine optical biogeochemistry: the chemistry of ocean color, Chem. Rev., 107, 402–418, 2007.
Coble, P. G., Green, S. A., Blough, N. V., and Gagosian, R. B.: Characterization of dissolved organic matter in the Black Sea by fluorescence spectroscopy, Nature, 348, 432–435, https://doi.org/10.1038/348432a0, 1990.
Coble, P. G., Del Castillo, C. E., and Avril, B.: Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon, Deep-Sea Res. Pt. II, 45, 2195–2223, 1998.
Coffin, R. B. and Cifuentes, L. A.: Stable isotope analysis of carbon cycling in the Perdido Estuary, Florida, Estuaries, 22, 917–926, 1999.
Dalmagro, H. J., Lathuillière, M. J., Sallo, F. d. S., Guerreiro, M. F., Pinto, O. B., de Arruda, P. H., Couto, E. G., and Johnson, M. S.: Streams with Riparian Forest Buffers versus Impoundments Differ in Discharge and DOM Characteristics for Pasture Catchments in Southern Amazonia, Water, 11, 390, https://doi.org/10.3390/w11020390, 2019.
Deines, P.: Chapter 9 – The isotopic composition of reduced organic carbon, in Handbook of environmental isotope geochemistry, edited by: Fritz, P. and Fontes, J. C., Elsevier Science, Amsterdam, 329–406, 1980.
De Troyer, I., Bouillon, S., Barker, S., Perry, C., Coorevits, K., and Merckx, R.: Stable isotope analysis of dissolved organic carbon in soil solutions using a catalytic combustion total organic carbon analyzer-isotope ratio mass spectrometer with a cryofocusing interface, Rapid Commun. Mass Sp., 24, 365–374, 2010.
Faganeli, J., Malej, A., Pezdic, J., and Malacic, V.: C : N : P ratios and stable c-isotopic ratios as indicators of sources of organic-matter in the gulf of trieste (northern adriatic), Oceanol. Acta, 11, 377–382, 1988.
Fry, B., Hopkinson, C. S., Nolin, A., and Wainright, S. C.: 13C∕12C composition of marine dissolved organic carbon, Chem. Geol., 152, 113–118, 1998.
Gao, L., Fan, D., Li, D., and Cai, J.: Fluorescence characteristics of chromophoric dissolved organic matter in shallow water along the Zhejiang coasts, southeast China, Mar. Environ. Res., 69, 187–197, 2010.
Griffith, D. R. and Raymond, P. A.: Multiple-source heterotrophy fueled by aged organic carbon in an urbanized estuary, Mar. Chem., 124, 14–22, 2011.
Griffith, D. R., Barnes, R. T., and Raymond, P. A.: Inputs of fossil carbon from wastewater treatment plants to US rivers and oceans, Environ. Sci. Technol., 43, 5647–5651, 2009.
Hansell, D. A. and Carlson, C. A.: Biogeochemistry of Marine Dissolved Organic Matter, Academic Press, San Diego, 774 pp. 2002.
Hong, S. H., Kannan, N., Jin, Y., Won, J. H., Han, G. M., and Shim, W. J.: Temporal trend, spatial distribution, and terrestrial sources of PBDEs and PCBs in Masan Bay, Korea, Mar. Pollut. Bull., 60, 1836–1841, 2010.
Hudson, N., Baker, A., and Reynolds, D.: Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters – a review, River Res. Appl., 23, 631–649, 2007.
Jaffé, R., Boyer, J., Lu, X., Maie, N., Yang, C., Scully, N., and Mock, S.: Source characterization of dissolved organic matter in a subtropical mangrove-dominated estuary by fluorescence analysis, Mar. Chem., 84, 195–210, 2004.
Jiao, N., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., Kirchman, D. L., Weinbauer, M. G., Luo, T., and Chen, F.: Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean, Nat. Rev. Microbiol., 8, 593–599, 2010.
Kannan, N., Hong, S. H., Yim, U. H., Kim, N. S., Ha, S. Y., Li, D., and Shim, W. J.: Dispersion of organic contaminants from wastewater treatment outfall in Masan Bay, Korea, J. Toxicol. Env. Health Sci., 2, 200–206, 2010.
Kelley, C. A., Coffin, R. B., and Cifuentes, L. A.: Stable isotope evidence for alternative bacterial carbon sources in the Gulf of Mexico, Limnol. Oceanogr., 43, 1962–1969, 1998.
Kim, T.-H., Waska, H., Kwon, E., Suryaputra, I. G. N., and Kim, G.: Production, degradation, and flux of dissolved organic matter in the subterranean estuary of a large tidal flat, Mar. Chem., 142, 1–10, 2012.
Kim, T.-H., Kim, G., Lee, S. A., and Dittmar, T.: Extraordinary slow degradation of Dissolved Organic Carbon (DOC) in a cold marginal sea, Sci. Rep.-UK, 5, 13808, https://doi.org/10.1038/srep13808, 2015.
Lamb, A. L., Wilson, G. P., and Leng, M. J.: A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C∕N ratios in organic material, Earth-Sci. Rev., 75, 29–57, 2006.
Lang, S. Q., Lilley, M. D., and Hedges, J. I.: A method to measure the isotopic (13C) composition of dissolved organic carbon using a high temperature combustion instrument, Mar. Chem., 103, 318–326, 2007.
Lee, C.-W. and Min, B.-Y.: Pollution in Masan Bay, a matter of concern in South Korea, Mar. Pollut. Bull., 21, 226–229, 1990.
Lee, H. J., Hong, S. H., Kim, M., Ha, S. Y., An, S. M., and Shim, W. J.: Tracing origins of sewage and organic matter using dissolved sterols in Masan and Haengam Bay, Korea, Ocean Sci. J., 46, 95–103, 2011.
Lee, S.-A. and Kim, G.: Sources, fluxes, and behaviors of fluorescent dissolved organic matter (FDOM) in the Nakdong River Estuary, Korea, Biogeosciences, 15, 1115–1122, https://doi.org/10.5194/bg-15-1115-2018, 2018.
Lee, Y.-W., Hwang, D.-W., Kim, G., Lee, W.-C., and Oh, H.-T.: Nutrient inputs from submarine groundwater discharge (SGD) in Masan Bay, an embayment surrounded by heavily industrialized cities, Korea, Sci. Total Environ., 407, 3181–3188, 2009.
Liu, C., Du, Y., Yin, H., Fan, C., Chen, K., Zhong, J., and Gu, X.: Exchanges of nitrogen and phosphorus across the sediment-water interface influenced by the external suspended particulate matter and the residual matter after dredging, Environ. Pollut., 246, 207–216, 2019.
Lobbes, J. M., Fitznar, H. P., and Kattner, G.: Biogeochemical characteristics of dissolved and particulate organic matter in Russian rivers entering the Arctic Ocean, Geochim. Cosmochim. Ac., 64, 2973–2983, 2000.
Lu, Y., Edmonds, J. W., Yamashita, Y., Zhou, B., Jaegge, A., and Baxley, M.: Spatial variation in the origin and reactivity of dissolved organic matter in Oregon-Washington coastal waters, Ocean Dynam., 65, 17–32, 2015.
Markager, S., Stedmon, C. A., and Søndergaard, M.: Seasonal dynamics and conservative mixing of dissolved organic matter in the temperate eutrophic estuary Horsens Fjord, Estuar. Coast. Shelf S., 92, 376–388, 2011.
Mayer, L. M., Schick, L. L., and Loder, T. C.: Dissolved protein fluorescence in two Maine estuaries, Mar. Chem., 64, 171–179, 1999.
McCallister, S. L., Bauer, J. E., Ducklow, H. W., and Canuel, E. A.: Sources of estuarine dissolved and particulate organic matter: a multi-tracer approach, Org. Geochem., 37, 454–468, 2006.
Milliman, J. D., Qinchun, X., and Zuosheng, Y.: Transfer of particulate organic carbon and nitrogen from the Yangtze River to the ocean, Am. J. Sci., 284, 824–834, 1984.
Murphy, K. R., Stedmon, C. A., Waite, T. D., and Ruiz, G. M.: Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy, Mar. Chem., 108, 40–58, 2008.
Murphy, K. R., Hambly, A., Singh, S., Henderson, R. K., Baker, A., Stuetz, R., and Khan, S. J.: Organic matter fluorescence in municipal water recycling schemes: toward a unified PARAFAC model, Environ. Sci. Technol., 45, 2909–2916, 2011.
Murphy, K. R., Stedmon, C. A., Wenig, P., and Bro, R.: OpenFluor – an online spectral library of auto-fluorescence by organic compounds in the environment, Anal. Methods-UK, 6, 658–661, 2014.
Oh, Y. H., Lee, Y.-W., Park, S. R., and Kim, T.-H.: Importance of dissolved organic carbon flux through submarine groundwater discharge to the coastal ocean: Results from Masan Bay, the southern coast of Korea, J. Marine Syst., 173, 43–48, 2017.
Opsahl, S. and Benner, R.: Distribution and cycling of terrigenous dissolved organic matter in the ocean, Nature, 386, 480–482, 1997.
Osburn, C. L. and Stedmon, C. A.: Linking the chemical and optical properties of dissolved organic matter in the Baltic-North Sea transition zone to differentiate three allochthonous inputs, Mar. Chem., 126, 281–294, 2011.
Osburn, C. L., Wigdahl, C. R., Fritz, S. C., and Saros, J. E.: Dissolved organic matter composition and photoreactivity in prairie lakes of the US Great Plains, Limnol. Oceanogr., 56, 2371–2390, 2011.
Panetta, R. J., Ibrahim, M., and Gélinas, Y.: Coupling a High-Temperature Catalytic Oxidation Total Organic Carbon Analyzer to an Isotope Ratio Mass Spectrometer To Measure Natural-Abundance δ13C-Dissolved Organic Carbon in Marine and Freshwater Samples, Anal. Chem., 80, 5232–5239, 2008.
Pradhan, U., Wu, Y., Shirodkar, P., Zhang, J., and Zhang, G.: Sources and distribution of organic matter in thirty five tropical estuaries along the west coast of India-a preliminary assessment, Estuar. Coast. Shelf S., 151, 21–33, 2014.
Raymond, P. A. and Bauer, J. E.: DOC cycling in a temperate estuary: a mass balance approach using natural 14C and 13C isotopes, Limnol. Oceanogr., 46, 655–667, 2001.
Spiker, E.: The Behavior of C-14 and C-13 in Estuarine Water-Effects of Insitu CO2 Production and Atmospheric Exchange, Radiocarbon, 22, 647–654, 1980.
Thornton, S. and McManus, J.: Application of organic carbon and nitrogen stable isotope and C∕N ratios as source indicators of organic matter provenance in estuarine systems: evidence from the Tay Estuary, Scotland, Estuar. Coast. Shelf S., 38, 219–233, 1994.
Tremblay, L. and Benner, R.: Microbial contributions to N-immobilization and organic matter preservation in decaying plant detritus, Geochim. Cosmochim. Ac., 70, 133–146, 2006.
Wang, X.-C., Chen, R. F., and Gardner, G. B.: Sources and transport of dissolved and particulate organic carbon in the Mississippi River estuary and adjacent coastal waters of the northern Gulf of Mexico, Mar. Chem., 89, 241–256, 2004.
Wünsch, U. J., Murphy, K. R., and Stedmon, C. A.: The one-sample PARAFAC approach reveals molecular size distributions of fluorescent components in dissolved organic matter, Environ. Sci. Technol., 51, 11900–11908, 2017.
Ya, C., Anderson, W., and Jaffé, R.: Assessing dissolved organic matter dynamics and source strengths in a subtropical estuary: Application of stable carbon isotopes and optical properties, Cont. Shelf Res., 92, 98–107, 2015.
Yoo, K.: Population dynamics of dinoflagellate community in Masan Bay with a note on the impact of environmental parameters, Mar. Pollut. Bull., 23, 185–188, 1991.