the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Cyanobacteria net community production in the Baltic Sea as inferred from profiling pCO2 measurements
Jens Daniel Müller
Bernd Schneider
Ulf Gräwe
Peer Fietzek
Marcus Bo Wallin
Anna Rutgersson
Norbert Wasmund
Siegfried Krüger
Gregor Rehder
Organic matter production by cyanobacteria blooms is a major environmental concern for the Baltic Sea, as it promotes the spread of anoxic zones. Partial pressure of carbon dioxide (pCO2) measurements carried out on Ships of Opportunity (SOOP) since 2003 have proven to be a powerful tool to resolve the carbon dynamics of the blooms in space and time. However, SOOP measurements lack the possibility to directly constrain depth-integrated net community production (NCP) in moles of carbon per surface area due to their restriction to the sea surface. This study tackles the knowledge gap through (1) providing an NCP best guess for an individual cyanobacteria bloom based on repeated profiling measurements of pCO2 and (2) establishing an algorithm to accurately reconstruct depth-integrated NCP from surface pCO2 observations in combination with modelled temperature profiles.
Goal (1) was achieved by deploying state-of-the-art sensor technology from a small-scale sailing vessel. The low-cost and flexible platform enabled observations covering an entire bloom event that occurred in July–August 2018 in the Eastern Gotland Sea. For the biogeochemical interpretation, recorded pCO2 profiles were converted to , which is the dissolved inorganic carbon concentration normalised to alkalinity. We found that the investigated bloom event was dominated by Nodularia and had many biogeochemical characteristics in common with blooms in previous years. In particular, it lasted for about 3 weeks, caused a drawdown of 90 µmol kg−1, and was accompanied by a sea surface temperature increase of 10 ∘C. The novel finding of this study is the vertical extension of the drawdown up to the compensation depth located at around 12 m. Integration of the drawdown across this depth and correction for vertical fluxes leads to an NCP best guess of ∼1.2 mol m−2 over the productive period.
Addressing goal (2), we combined modelled hydrographical profiles with surface pCO2 observations recorded by SOOP Finnmaid within the study area. Introducing the temperature penetration depth (TPD) as a new parameter to integrate SOOP observations across depth, we achieve an NCP reconstruction that agrees to the best guess within 10 %, which is considerably better than the reconstruction based on a classical mixed-layer depth constraint.
Applying the TPD approach to almost 2 decades of surface pCO2 observations available for the Baltic Sea bears the potential to provide new insights into the control and long-term trends of cyanobacteria NCP. This understanding is key for an effective design and monitoring of conservation measures aiming at a Good Environmental Status of the Baltic Sea.
- Article
(4769 KB) - Full-text XML
- BibTeX
- EndNote
1.1 Net community production (NCP) in marine ecosystems
Net community production (NCP) of organic matter triggers many biogeochemical processes that control the functioning and state of marine ecosystems. Globally relevant examples are the biological carbon pump (Henson et al., 2011; Sanders et al., 2014) and the establishment of oxygen minimum zones (Gilly et al., 2013; Oschlies et al., 2018). In this biogeochemical context, we define NCP as the net amount of carbon fixed in organic matter (gross production minus respiration) that is produced in a defined water volume over a defined period. This definition implies that the choice of an integration depth is a critical component of any NCP estimate. Traditionally, NCP is constrained to the depth of the euphotic zone, the compensation depth at which gross production equals respiration, or the mixed-layer depth (Sarmiento and Gruber, 2006). Of those approaches, only the integration to the compensation depth is directly linked to the vertical distribution of carbon fixation and remineralisation and therefore quantifies the amount of formed organic matter that can potentially be exported. The reliable quantification of this potential export is a prerequisite to understand subsequent biogeochemical transformation of the organic matter and its imprint on environmental conditions in any aquatic system.
1.2 Baltic Sea
On a regional scale, NCP quantification is of particular importance to study deoxygenation of stratified water bodies caused by the remineralisation of organic matter that was exported across a permanent pycnocline. This hydrographical situation is typically encountered in semi-enclosed, silled estuaries such as the Baltic Sea. The deep basins of the Baltic Sea receive substantial amounts of oxygenated, salty water from the North Sea only during occasional major inflow events. Between inflow events, those water masses can stagnate for more than a decade below the permanent halocline (Mohrholz et al., 2015), which is located at around 60 m water depth in the Central Baltic Sea. The export of organic matter into the deep waters is considered the ultimate cause for the expansion of anoxic areas in the Baltic Sea, which are today among the largest anthropogenically induced anoxic areas in the world (Carstensen et al., 2014). Although the actual oxygenation state of the deep basins of the Baltic Sea is modulated by the frequency and strength of inflow events (Mohrholz et al., 2015; Neumann et al., 2017) and the biogeochemical properties of the inflowing waters (Meier et al., 2018), the long-term expansion of the anoxic water body was primarily attributed to increased nutrient inputs from land (Jokinen et al., 2018; Meier et al., 2019; Carstensen et al., 2014; Mohrholz, 2018) that fuelled the organic matter production in surface waters. Therefore, a quantitative and mechanistic understanding of organic matter production is key to understand, predict, and eventually counteract the expansion of the anoxic areas. Such measures to reduce eutrophication and deep water anoxia actually represent a core component of the EU Marine Strategy Framework Directive (MSFD), which is implemented as the HELCOM Baltic Sea Action Plan (BSAP) and aims at a Good Environmental Status (GES) of the Baltic Sea.
1.3 Cyanobacteria blooms
The annual cycle of organic matter production in the Central Baltic Sea can be broadly divided into two phases (Schneider and Müller, 2018). The first production phase is the spring bloom, which is controlled by the availability of nitrate and shifted from being dominated by diatoms to dinoflagellates in the late 1980s (Wasmund et al., 2017; Spilling et al., 2018). After a so-called blue water period with close-to-zero NCP rates, the second production phase consists of midsummer blooms dominated by nitrogen-fixing cyanobacteria that develop in most years depending on meteorological conditions. Although cyanobacteria NCP is yet poorly constrained, its relative contribution to the annual NCP in the Eastern Gotland Sea in 2009 was estimated on the order of 40 % (Schneider and Müller, 2018; Schneider et al., 2014), though the uncertainty of this estimate is high. This preliminary estimate further needs to be interpreted with care as cyanobacteria NCP varies significantly between years and regions. The blooming of cyanobacteria is limited to the months of June to August (Kownacka et al., 2020) and represents a common feature of the Baltic Sea ecosystem at least since the 1960s (Finni et al., 2001). The blooms are a major public concern, because they produce toxins and form thick surface scums, lowering the recreational value of the Baltic Sea. From a biogeochemical perspective, the ability to fix nitrogen makes cyanobacteria independent from nitrate and aggravates the eutrophication state of the Baltic Sea. Whether their growth is limited by the availability of phosphate remains an ongoing debate (Nausch et al., 2012), although the highly variable C:P ratio of their biomass (Nausch et al., 2009) indicates phenotypic plasticity. Other ongoing debates in the field of cyanobacteria research address the fate of the produced organic matter and its transfer into the food web (Karlson et al., 2015), the intensification of cyanobacteria blooms through positive feedback loops between organic matter production, deep water anoxia, and the release of phosphate from anoxic sediments (Vahtera et al., 2007), as well as their response to ongoing changes in salinity, temperature, and the partial pressure of carbon dioxide, pCO2 (Olofsson et al., 2019, 2020). The limited understanding of the factors that control the blooms hinders the reliable prediction of the future state of the Baltic Sea and therefore the prioritisation of conservation measures (Elmgren, 2001). In particular, it remains challenging to disentangle how expected trends – including warming, reduced nutrient loads, and increasing pCO2 – might impact cyanobacteria growth (Meier et al., 2019; Saraiva et al., 2019). A long-term hindcast of cyanobacteria NCP and the attribution of its strength to prevailing environmental conditions in particular years could improve our understanding of controlling factors and facilitate more reliable predictions of the blooms. However, such a hindcast of cyanobacteria NCP was so far impossible due to missing vertically resolved observations that would allow the constraint of their organic matter production.
1.4 Quantification of NCP
Striving for a better understanding of the ecosystem impact of cyanobacteria blooms, the accurate quantification of produced organic matter is key. In this regard, NCP could in principle be quantified directly as an increase in particulate organic carbon (POC). However, POC measurements would not detect the amount of organic matter that was exported between observations (Wasmund et al., 2005) and also fail to achieve the required spatio-temporal resolution due to a low degree of automation. As an alternative, it is possible to quantify NCP through the drawdown of dissolved inorganic carbon (CT) from the water column (Schneider et al., 2003). From a biogeochemical perspective, the determination of NCP in terms of carbon is ideal, because carbon is the major component of organic matter and directly related to the amount of oxygen (O2) that is consumed during remineralisation. In principle, NCP could also be estimated from O2 time series. However, the equilibrium reactions of carbon dioxide (CO2) in seawater result in slower re-equilibration of CO2 with the atmosphere compared to O2 (Wanninkhof, 2014). This results in substantially longer preservation of the CT signal and thus a lower uncertainty contribution of required air–sea CO2 flux corrections, making CT the preferred tracer for NCP. During the Baltic Sea spring bloom, the tracing of nutrient drawdown is a meaningful alternative to quantify NCP and convincingly leads to comparable results to the CT approach (Wasmund et al., 2005). However, time series of nutrient drawdown do not allow for determining NCP of algae blooms dominated by nitrogen-fixing organisms and those with highly variable C:P ratios. As both characteristics are typical for Baltic Sea cyanobacteria blooms (Nausch et al., 2009), the well-established CT approach remains the favourable method to determine midsummer NCP in this region. However, it should be noted that NCP estimates derived from this approach include the formation of POC and dissolved organic carbon (DOC). The produced DOC contributes ∼20 % to NCP (Hansell and Carlson, 1998; Schneider and Kuss, 2004) and is not likely to be vertically exported.
1.5 Previous studies
Among previous attempts to trace and quantify the organic matter production of cyanobacteria blooms, automated pCO2 measurements on the Ship of Opportunity (SOOP) Finnmaid played a pivotal role. Those measurements were started in 2003, and it was demonstrated that highly accurate time series of changes (not absolute values) in CT can be derived from pCO2 observations (Schneider et al., 2006). The conversion from pCO2 to CT relies on a fixed alkalinity (AT) estimate and is applicable under the condition that internal sinks and sources of AT can be excluded, which is the case in the Baltic Sea due to the absence of calcifying plankton (Tyrrell et al., 2008). The derived parameter is comparable to directly measured CT normalised to AT and in the following referred to as . For several years of SOOP observations, it was shown that the drawdown during midsummer cyanobacteria blooms occurs in pulses of days to weeks, primarily during calm, sunny days. Further, it was found that the drawdown correlates well with the co-occurring increase in sea surface temperature (SST), rather than with absolute SST. This relationship was attributed to a common driver, which is the light dose received by the water mass under consideration (Schneider and Müller, 2018).
Despite the successful investigation of cyanobacteria blooms through SOOP pCO2 observations, providing a depth-integrated estimate of NCP in units of moles carbon fixed per surface area remains challenging due to the restriction of SOOP observations to surface waters. Previous studies aiming at a depth-integrated NCP estimate either simply assumed that the drawdown reached as far down as the water inlet of the measurement system (Schneider and Müller, 2018) or relied on a modelled mixed-layer depth for the vertical integration of surface observations (Schneider et al., 2014). However, in the absence of any vertically resolved measurements, neither approach could be validated. Likewise, remote sensing approaches were capable to resolve the spatial coverage of the blooms (Hansson and Hakansson, 2007; Kahru and Elmgren, 2014) but failed to detect their vertical extent (Kutser et al., 2008) and quantify NCP. Finally, regular research vessel cruises allowed for the determination of a full suite of biogeochemical parameters from discrete water samples and even the experimental determination of carbon fixation rates through 14C incubations (Wasmund et al., 2001, 2005). Such incubation experiments can provide valuable information about instantaneous rates of NCP, but – in contrast to time series observations such as obtained by SOOP measurements – do not allow the integration of observed changes over time and constrain budgets of biogeochemical transformations. This integration over time requires several weeks of repeated observations to resolve the progression of entire bloom events, ideally covering a station network to average bloom patchiness.
1.6 This study
This study builds upon the previous success to determine NCP based on pCO2 time series but extends the approach to vertically resolved observations for the first time. The primary goals of this study are to
-
provide a best guess for the depth-integrated NCP of an individual cyanobacteria bloom based on the full suite of depth-resolved in situ measurements and
-
establish an algorithm to reconstruct depth-integrated NCP based on surface pCO2 observations and modelled hydrographical profiles.
Achieving goal (2) and applying the algorithm to almost 2 decades of SOOP pCO2 observations in the Baltic Sea would not only allow the determination of long-term trends of cyanobacteria NCP, but also enable disentangling its drivers through a comparison of NCP estimates from different years characterised by particular environmental conditions such as SST, pCO2, and nutrient availability.
2.1 Overview
Profiling in situ sensor measurements and water sampling were performed on board the 27 ft sailing vessel SV Tina V in the framework of the field sampling campaign “BloomSail”. The study area was located in the Central Baltic Sea and extended about 25 nautical miles from the coast of Gotland into the Eastern Gotland Basin (Fig. 1). Measurements were performed during eight cruises covering the period 6 July to 16 August 2018 (Fig. 2).
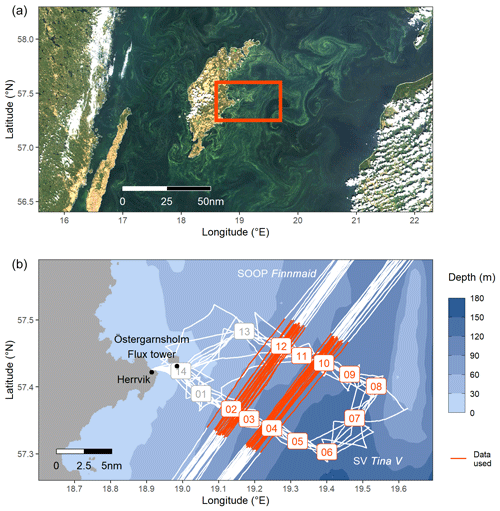
Figure 1(a) Extent of the cyanobacteria bloom on 26 July, detectable as greenish patterns in a true-colour satellite image (MODIS Aqua–Terra, NASA Worldview) showing the Central Baltic Sea around the island of Gotland. The box indicates the study area as shown in (b), a bathymetric map with the cruise tracks of SV Tina V (BloomSail campaign) and SOOP Finnmaid. BloomSail stations and the SOOP sub-transects used in this study are highlighted in red. The ICOS flux tower for atmospheric measurements is located on the island of Östergarnsholm.
Figure 2Overview on profiling sensor measurements performed at stations 02–12 (Fig. 1). Individual sampling events are displayed as points, whereas vertical lines indicate the mean date of each cruise event.
A custom-made sensor package configured at IOW's Innovative Instrumentation department was deployed to perform pCO2 and conductivity, temperature, and depth (CTD) measurements. The sensor package was either towed near the sea surface while cruising or lowered to at least 25 m water depth at designated profiling stations. This study focuses exclusively on the vertical profiles recorded at stations 02–12 (Fig. 1b), whereas profiles at stations with water depths less than 60 m were not taken into account to avoid the impact of coastal processes. In addition to the sensor measurements, discrete samples for dissolved inorganic carbon (CT), total alkalinity (AT), and phytoplankton counts were collected. Track coordinates were continuously recorded with a tablet computer (Galaxy Tab Active, Samsung Electronics, Suwon, South Korea).
In addition to the field sampling campaign, atmospheric measurements of wind speed and pCO2,atm were provided by an ICOS (Integrated Carbon Observation System) station permanently operated on the island Östergarnsholm (Fig. 1b). Furthermore, sea surface pCO2 and temperature (SST) were also determined on the SOOP Finnmaid, regularly crossing the field study area (Fig. 1b). High-resolution hydrographical model data were obtained from the Generalized Estuarine Turbulence Model (GETM) along a vertical section following the Finnmaid track.
2.2 Field sampling campaign
2.2.1 CTD measurements
CTD measurements were performed with a SBE 16 SeaCAT instrument (serial number 2557; Sea-Bird Electronics, Bellevue, USA). Pre- and post-deployment calibrations of the instrument were carried out in the accredited calibration laboratory of the IOW in the time span of a few months around the deployments and confirmed that the temperature and conductivity sensors achieved the typical accuracy of better than ±0.01 ∘C and ±0.01 S m−1, respectively. The manual operation of the sensor package was guided by real-time display of data submitted through a strain-relieved cable. Data stored on an internal memory were used for analysis. The CTD logging frequency was 15 s, and observations were linearly interpolated to match the higher measurement frequency of the pCO2 sensor (for additional details see Appendix A2). The CTD instrument supplied auxiliary sensors with power and served as a central unit to record and transmit analogue output signals.
2.2.2 pCO2 sensor measurements
The submersible CO2 sensor used in this study, a CONTROS HydroC® CO2 (formerly Kongsberg Maritime Contros, Kiel, Germany; now -4H-JENA engineering, Jena, Germany), uses membrane equilibration of a headspace and subsequent optical non-dispersive infrared (NDIR) absorption to determine the pCO2 in water (Fietzek et al., 2014).
A pre- and post-deployment calibration of the sensor was performed by the manufacturer. pCO2 data were post-processed taking into account the pre- and post-deployment calibration polynomials, as well as zeroing signals regularly recorded during each deployment. Given the statistics of the pre- and post-deployment calibration, the small drift encountered throughout the deployment and the otherwise smooth performance and regular cleaning of the sensor during the deployment, the accuracy of the drift-corrected pCO2 data is considered to be within 1 % of reading as also found by Fietzek et al. (2014). For details concerning sensor calibration, configuration, and signal post-processing, see Appendices A1–A3.
Although the pCO2 sensor achieves low and reproducible response times through active pumping of water onto the membrane, a correction of the response time (τ) was applied following previously developed procedures (Miloshevich et al., 2004; Fiedler et al., 2013; Atamanchuk et al., 2015). After the response time correction, the mean absolute pCO2 difference between the up- and downcast profile was <2.5 µatm in the upper 5 m of the water column and <7.5 µatm across the upper 20 m (Fig. A2). For details concerning the response time correction, see Appendix A4.
The biogeochemical interpretation of the pCO2 data was based on downcast profiles only. Since downcasts were started after complete equilibration of the pCO2 sensor in near-surface waters, the applied response time correction has only a minor impact on the derived NCP estimate.
2.2.3 Discrete CT, AT, and phytoplankton sampling
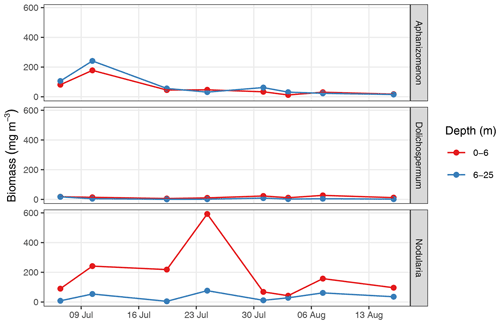
Discrete water samples were collected with a manually released Niskin bottle. The sampling was restricted to stations 07 and 10 (Fig. 1b) due to logistic constraints. The sampling depth was estimated based on the length of the released line. CT and AT samples were filled into 250 mL SCHOTT-DURAN bottles and poisoned with 200 µL saturated HgCl2 solution within 24 h after sampling. Samples were stored dark and cool, transported to IOW, and analysed in the laboratory within no more than 21 d after sampling. CT was determined with an automated infrared inorganic carbon analyser (AIRICA, Marianda, Kiel, Germany), and AT was analysed by open cell titration (Dickson et al., 2007). CT and AT measurements were referenced to certified reference materials from batch 173 (Dickson et al., 2003). The mean observed AT was used for the calculation of from pCO2 (see Sect. 2.5.2), while measured CT was only used for comparison to calculated values and not directly included in the NCP calculation. Phytoplankton samples were fixed with Lugol solution, and cyanobacteria community composition and biomass were determined by microscopic counts of the genera Aphanizomenon, Dolichospermum, and Nodularia according to the Utermöhl method (HELCOM, 2017). For details on the analysis of discrete samples, see Appendix B.
2.3 Atmospheric measurements
Meteorological observations were provided by the ICOS flux tower (Fig. 1b) located on the southernmost tip of the Island of Östergarnsholm (57.43010∘ N, 18.98415∘ E; Rutgersson et al., 2020). Atmospheric pCO2,atm was recorded with an atmospheric profile system (AP200, Campbell Scientific, Logan, USA) mounted with a CO2–H2O gas analyser (LI-840A, LI-COR Biosciences, Lincoln, USA). Wind speed was measured with a wind monitor (Young, Michigan, USA) at 12 m above mean sea level. Wind speed and pCO2 data were averaged over 30 min intervals for further analysis. Measured wind speed was converted to U10, the wind speed at 10 (Winslow et al., 2016), to be consistent with the gas exchange parameterisation (see Sect. 2.5.2).
2.4 calculation
The dissolved inorganic carbon concentration () was calculated from the measured profiles of temperature and response-time-corrected pCO2 (Schneider et al., 2014), as well as the mean AT (1720 µmol kg−1) and mean salinity (6.9) determined from discrete samples collected across the upper 20 m of the water column and over the entire observation period (Fig. B1). Calculations were performed with the R package seacarb (Gattuso et al., 2020), using the CO2 dissociation constants for estuarine waters from Millero (2010).
The calculated represents an alkalinity- and salinity-normalised estimate of the dissolved inorganic carbon concentration. is suitable to accurately determine changes rather than absolute values of the dissolved inorganic carbon concentration and therefore the preferred variable to quantify NCP. The uncertainty in the determination of changes of is below 2 µmol kg−1 when the mean AT is constrained within the observed standard deviation of ±27 µmol kg−1 (see Appendix C1 for a detailed assessment).
2.5 NCP best guess
The determination of our NCP best guess relies on the interpretation of observed temporal changes in (Δ) across the water column. Conceptually, our calculations follow the idea of a one-dimensional box model approach, which does not resolve regional variability within the research area; i.e. it neglects lateral water mass transport. The calculation of the underlying Δ profiles requires a vertical gridding of measured profiles into discrete depth intervals δz and their regional averaging across all stations (for details see Sect. 2.5.1). According to Eq. (1), we derive the column inventory of incremental changes of Δ (iΔ) between two cruise events through vertical integration of Δ from the sea surface to the compensation depth (CD), i.e. the depth (z) at which no net drawdown of CO2 was observed:
Correcting iΔ for the cumulative CO2 fluxes between two cruise events caused by air–sea gas exchange (Fair-sea; see Sect. 2.5.2) and vertical mixing (Fmix, see Sect. 2.5.3) leads to incremental NCP estimates according to
Incremental NCP estimates between cruise events are further added up to derive cumulative NCP over the study period. We refer to the derived NCP estimate as our best guess, as it is well-constrained by high-quality measurements and therefore as close to the truth as currently possible.
2.5.1 Vertical gridding and regional averaging
The vertical gridding of individual profiles was achieved by calculating mean values within depth intervals (δz) of 1 m. Downcast profiles with missing observations from two or more depth intervals caused by zeroing measurements of the pCO2 sensor were discarded, which affected 8 out of 86 recorded profiles. For each of eight cruise events (Fig. 2), regionally averaged profiles were further calculated as mean values within each depth interval across all stations. Based on those mean, vertically gridded cruise profiles, incremental and cumulative changes over time were calculated for each depth interval. Throughout the paper, observations averaged across the upper 0–6 m of the water column are referred to as surface observations.
2.5.2 Air–sea CO2 flux
The air–sea gas exchange of CO2 (Fair-sea) was calculated from sea surface pCO2, salinity, and temperature, in combination with pCO2,atm and U10 according to Wanninkhof (2014). For the calculation, sea surface observations were linearly interpolated to match the temporal resolution of atmospheric measurements. A negative sign of Fair-sea indicates uptake of CO2 from the atmosphere.
2.5.3 Vertical entrainment flux of CO2 through mixing
Between 6 June and 7 August, vertical mixing of into the surface layer (Fmix) was neglected, because a stable thermocline coincided with the integration depth for the NCP calculation (i.e. the compensation depth). However, clear signals for significant vertical entrainment of across this layer were observed between 7 and 16 August (Fig. 3). This entrainment was quantified assuming an instantaneous complete vertical mixing to 17 m water depth after 7 August. For this simplified scenario, Fmix was estimated based on a mass balance of , which behaves conservatively with respect to mixing (see Appendix C2 for details). A negative sign of Fmix indicates entrainment of CO2 into the surface layer.
2.6 NCP reconstruction from surface pCO2 observations and hydrographical profiles
Our calculation of depth-integrated NCP from a time series of surface pCO2 observations, such as provided by SOOP, also relies on the conversion of pCO2 to . Incremental changes of in the surface water further need to be multiplied with an estimate of the integration depth (ID) to derive an inventory change. Taking the air–sea flux of CO2 (Sect. 2.5.2) into account, the NCP reconstruction can be determined as
In the lack of vertical observations, we tested two alternative approximations of ID, which are
- •
-
mixed-layer depth (MLD) and
- •
-
temperature penetration depth (TPD).
MLD and TPD are described in detail in Sect. 2.6.3. The two parameterisations were further applied to the following two test data sets, both of which contain the required surface pCO2 and vertically resolved temperature and salinity data:
- •
-
in situ data from the BloomSail campaign without pCO2 data at depth (SV Tina V (surface only))
- •
-
combined SOOP surface pCO2 observations and modelled salinity and temperature profiles (SOOP Finnmaid + GETM model).
For both data sets, time series were calculated based on the same observed mean AT as used to derive the NCP best guess (Sect. 2.4). Please note that neither the MLD nor the TPD approach allows us to resolve vertical entrainment fluxes, because profiles of are not reconstructed (compare Sect. 2.5.3). Based on all possible combinations of two time series and two integration depth constraints, four reconstructed NCP time series were derived and compared to the best guess (i.e. the estimate based on the vertically resolved pCO2 observations from this study).
2.6.1 SOOP Finnmaid surface pCO2
SOOP Finnmaid regularly commutes between Helsinki in Finland and Travemünde in Germany, thereby crossing the entire Central Baltic Sea and our study area on the east coast of Gotland every 1–2 d. On board SOOP Finnmaid, pCO2 is measured with a bubble-type equilibrator system supplied with water from an inlet at around 3 m water depth. Details of the measurement set-up are described in Schneider et al. (2014), and data are submitted on a regular basis to the Surface Ocean CO2 Atlas SOCAT (Bakker et al., 2016). The primary measurement system used to determine pCO2 in this study is a NDIR sensor (LI-6262, LI-COR Biosciences, Lincoln, USA). The ferrybox unit is also equipped with an additional methane–carbon dioxide analyser (greenhouse gas analyser DLT 100, type 908-0011, Los Gatos Research, San Jose, USA), providing independent pCO2 observations (Gülzow et al., 2011). Intercomparison of both systems is routinely used to ensure the correct functioning of the instrumentation. In this study, a data gap caused by malfunctioning of the primary LI-COR system was filled by including data recorded with the Los Gatos system on six cruises between 8 and 16 July (see Appendix D for details). The mean regional pCO2, sea surface temperature (SST), and salinity (SSS) were calculated for each crossing of the study area (Fig. 1b). Based on the mean pCO2 and SST values, was calculated following the procedure outlined in Sect. 2.4. A remaining gap in the SOOP time series was filled with two in situ observations from the BloomSail campaign (19 and 24 July).
2.6.2 GETM model temperature and salinity
Surface SOOP measurements were complemented with vertically resolved salinity and temperature data from the output of a numerical ocean model of the Baltic Sea. The deployed General Estuarine Turbulence Model (GETM) has a horizontal resolution of 1 nautical mile and 50 vertical terrain-following levels. The uppermost level has a thickness of maximum 50 cm to properly represent SST and ocean–atmosphere fluxes. The computation of the atmospheric fluxes is based on the parameterisation of Kara et al. (2005). The model covers the entire Baltic Sea and the period 1961–2019. A detailed analysis of the model performance is given in Placke et al. (2018) and Gräwe et al. (2019). For the present study, we used a model run restarted in 2003 with the atmospheric forcing from the operational reanalysis data set of the German weather service (Zängl et al., 2015). Additionally, we implemented the Langmuir-circulation parameterisation of Axell (2002), to account for wind-wave-induced variation in the mixed-layer depth. Model results were averaged over 24 h and interpolated to a standardised section with 2 km horizontal and 1 m vertical resolution, which follows the mean Finnmaid cruise track. Based on this standard section, daily mean profiles within the study area (characterised by little regional variability) were computed and linearly interpolated to match the exact times of Finnmaid crossings.
2.6.3 Parameterisation of the integration depth (ID)
In this study, two parameters were used to integrate surface observations across depth, namely the classical mixed-layer depth (MLD) and the newly introduced temperature penetration depth (TPD).
MLD was defined as the shallowest depth at which seawater density exceeds the density at the surface by more than 0.1 kg m−3 (Roquet et al., 2015). According to this definition, MLD characterises the thermohaline structure of the water column and often (but not necessarily) approximates the depth to which surface water masses are actively mixed. The definition through a fixed density threshold further implies that gradual changes of temperature with depth are not reflected by this parameter.
TPD characterises the mean penetration depth of surface warming that occurred between two sampling events. TPD was defined as the integrated warming signal across the water column, i.e. the sum of all positive temperature changes within 1 m depth intervals, divided by the SST increase (for a graphical illustration see Fig. C4a). According to this definition and in contrast to MLD, TPD takes gradual changes of temperature across depth into account and does not require a fixed threshold value. TPD is only applicable when SST increases and has units of metres. To illustrate the TPD concept, it should be noted that a homogeneous warming signal that ceases abruptly at 10 m water depth would result in the same TPD as a warming signal that decreases linearly from the surface to 20 m water depth (TPD is 10 m in both cases). The TPD approach is motivated by the assumption that primary production and temperature increase are both primarily controlled by the light dose that a water parcel received (Schneider et al., 2014) and therefore show similar vertical patterns.
In analogy to TPD, the penetration depth of drawdown (CPD) was defined as the integrated loss of across the water column divided by the decrease in at the surface (Fig. C4b).
3.1 Dynamics of temperature, pCO2, , and phytoplankton biomass
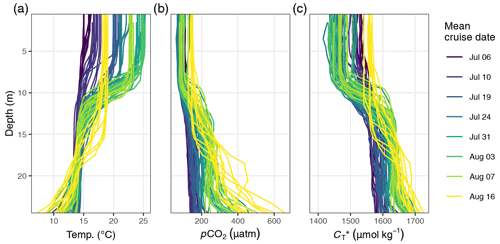
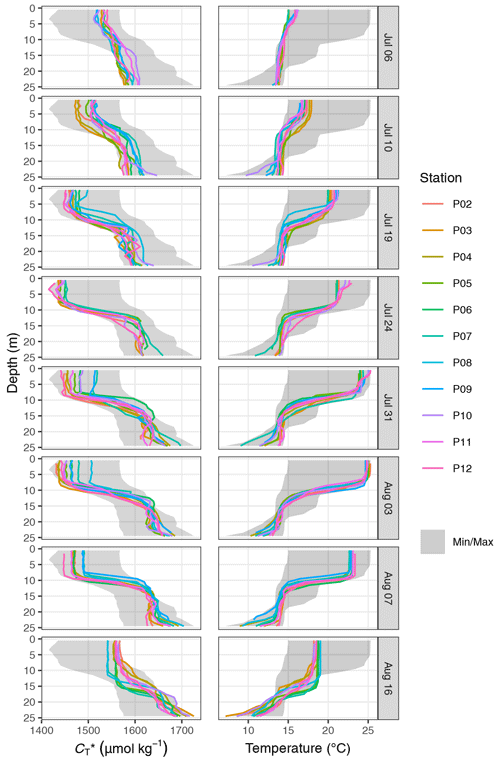
Between 6 July and 16 August, a total number of 78 complete vertical CTD and pCO2 downcast profiles were recorded (Figs. 2 and 3). was calculated and profiles were regionally averaged for each of the eight cruise events (Fig. 4). Since the first cruise of the BloomSail expedition on 6 July, sea surface temperature (SST) increased steadily from ∼ 15 ∘C to peak values of ∼25 ∘C (Figs. 4 and 5) observed on 3 August. Sea surface pCO2 was already as low as ∼ 100 µatm at the beginning of July (Fig. 5a) and decreased further to the lowest values of ∼70 µatm on 24 July. The drop in pCO2 and the simultaneous increase in SST correspond to a decrease in of almost 90 µmol kg−1 (Fig. 4). During this period of intense primary production, the regional variability of SST, pCO2, and across stations was low compared to their temporal change (Figs. 5a and b; and C3). The regional variability is slightly higher when including the coastal stations 01, 13, and 14 (results not shown) but is generally lower than one could expect from the bloom patchiness typically observed through remote sensing (Fig. 1a). With respect to pCO2 dynamics, it should be noted that (i) the observed temperature increase and drawdown have opposing effects on pCO2, and (ii) the change of pCO2 per change in is generally low at low absolute pCO2. The observed dynamics in surface waters are clearly attributable to the primary production activity of phytoplankton and go along with an observed increase in the biomass of Nodularia sp. (Fig. B2), which also peaked on 24 July. Furthermore, we found that calculated from pCO2 agreed with derived from discrete samples within the uncertainty range attributed to regional variability (Fig. 5c).
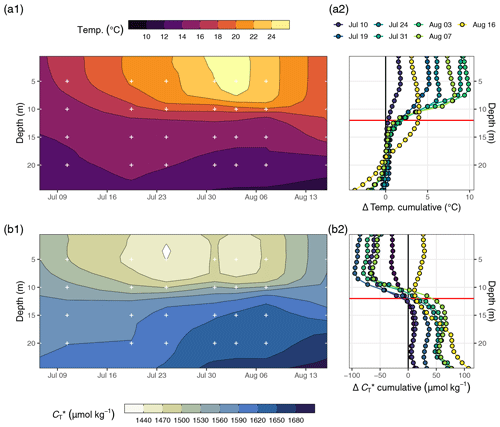
Figure 4(a) Temperature and (b) between 6 July and 16 August displayed as (1) Hovmöller plots and (2) profiles of cumulative changes since the first cruise on 6 July. Mean cruise dates are indicated by the horizontal position of white symbols in (a1) and (b1), and the compensation depth of 12 m is indicated as a red horizontal line in (a2) and (b2).
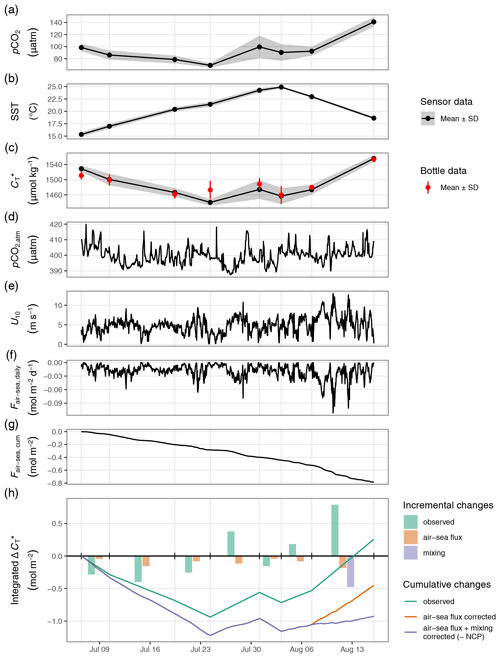
Figure 5Time series displaying from top to bottom sea surface observation of (a) pCO2, (b) temperature, and (c) with grey ribbons indicating the standard deviation across stations and red symbols representing discrete sample data; atmospheric observations of (d) pCO2,atm, (e) wind speed at 10 m, (f) daily and (g) cumulative air–sea fluxes of CO2; and (h) the derived water column inventory changes of . In (h), bars represent incremental changes between cruise events (vertical grid lines), whereas lines represent cumulative changes since the first cruise. Colours distinguish observed changes from values referring to the applied air–sea CO2 flux and mixing correction. Net community production (NCP) is equal to the negative value of flux and mixing-corrected cumulative changes of (purple line) and peaks on 24 July.
Between the extremes of pCO2 and (minimum on 24 July) and SST (maximum on 3 August), a noticeable increase in surface was observed on 31 July, which was accompanied by a higher regional variability across the station network (Fig. 5a and c). The temporary increase was limited to the north-eastern stations 07–10 (Fig. C3) and paralleled by a drop in salinity and elevated AT at the same stations (Fig. B1). It is therefore attributable to the lateral exchange of water masses. All signals of this lateral intrusion vanished within a week. At the other stations (02–06 and 11–12), no noticeable signs of water mass exchange or changes were observed between 24 July and 3 August, indicating that the production and respiration of organic matter were balanced during this period. During the first 2 weeks of August the study area was affected by increased wind speeds, causing a decrease in SST back to ∼18 ∘C. The simultaneous return of surface pCO2 to ∼150 µatm corresponded to a increase of ∼100 µmol kg−1.
The observed surface warming and drawdown extended vertically to a water depth of ∼10 m (Fig. 4). On the first cruise day (6 July), the vertical distribution of and temperature was still relatively homogeneous. at 25 m water depth was ∼70 µmol kg−1 higher than at the surface. Likewise, the temperature gradient covered only ∼3 ∘C from 16 ∘C at the very surface to 13 ∘C at depth. The warming of surface waters caused an increasingly stable thermocline to be established at around 10 m water depth, which reached a temperature gradient of ∼10 ∘C across 5 m on 3 August. Likewise, continuous and uniform drawdown of within the surface layer enhanced the vertical gradient to >150 µmol kg−1 between the surface and 25 m water depth. The drawdown was observed to a maximum depth of 12 m.
Between 7 and 16 August the SST drop of ∼6∘C was accompanied by a temperature increase in deeper water layers (11–17 m) of up to 5 ∘C. This vertical redistribution of heat indicates vertical mixing of water masses, which was also reflected in a steep increase in in the surface water and a loss of between 11–17 m (Figs. 3 and 4).
3.2 NCP best guess based on profiling measurements
Net community production (NCP) was determined through vertical integration of the observed drawdown of from the surface to the compensation depth located at 12 m. The determined compensation depth reflects the maximum penetration depth of the incremental (i.e. between cruise days), as well as the cumulative (i.e. from 6–24 July), drawdown (Fig. 4). Likewise, about 95 % of the cumulative warming signal, which refers to positive temperature changes integrated over depth, occurred above 12 m.
Until 24 July, the depth-integrated drawdown amounted to ∼0.9 mol m−2 (Fig. 5h). This observed drawdown was corrected for air–sea fluxes of CO2. Between 6 July and 7 August, the cumulative air–sea flux (Fair-sea, cum) amounted to around −0.5 mol m−2 (Fig. 5g), with a negative sign representing CO2 uptake from the atmosphere. In the absence of noticeable vertical mixing, this flux was entirely added to the observed drawdown. Only between 7 and 16 August, when mixing to about 17 m water depth was observed, was a significant fraction of the CO2 taken up from the atmosphere transported below 12 m water depth. To account for the partial loss of airborne CO2 to deeper waters during this 9 d period, only of Fair-sea, cum during this time (−0.2 mol m−2), which is the fraction that would remain in the upper water column, was added to the observed drawdown. In addition, a significant amount of entrainment (−0.5 mol m−2) into the surface layer was caused by the vertical mixing between 7 and 16 August (Figs. 5h and C2).
After correction for air–sea fluxes and vertical entrainment of CO2, the cumulative changes of depth-integrated represent the NCP between the sea surface and the compensation depth at 12 m (Fig. 5h). The peak NCP value of ∼1.2 mol m−2 was observed on 24 July and is of primary interest because it reflects the amount of organic matter that was produced and is potentially available to be either exported or remineralised. The temporary drop in the NCP best guess on 31 July is due to the lateral exchange of water masses as described in Sect. 3.1. Deriving the NCP time series without the stations affected by later exchange of water masses (07–10) results in an almost identical NCP estimate on 24 July but a reduced drop on 31 July (data not shown). In both cases, no signs of continued NCP were observed after 24 July. Accordingly, our interpretation of the reconstructed NCP based on surface pCO2 observations will focus on the comparison to the peak value of our NCP best guess on 24 July.
3.3 NCP reconstruction based on surface pCO2 and hydrographical profiles
The reconstruction of depth-integrated NCP was tested for two data sets containing the same type of information, namely the observed changes in surface pCO2 and vertical profiles of seawater salinity and temperature. The first data set “SV Tina V (surface only)” contains the surface pCO2 data recorded during the BloomSail expedition, as well as the complete CTD profiles. The second data set (“SOOP Finnmaid + GETM model”) combines surface pCO2 observations from SOOP Finnmaid with seawater salinity and temperature as estimated with the GETM model.
An almost identical decrease in surface of ∼50 µmol kg−1 was determined between 6 and 16 July (Fig. 6a), based on the completely independent pCO2 data recorded on SOOP Finnmaid and SV Tina V. Likewise, a very similar increase in between 6 and 15 August was determined from both independent observational data sets. The good agreement between the independent observations justifies that a data gap due to failure of instrumentation on the SOOP was filled with two observations from SV Tina V on 19 and 24 July (open circles in Fig. 6a).
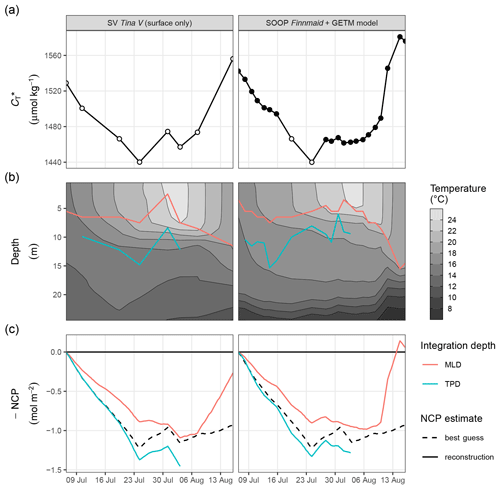
Figure 6Time series illustrating the reconstruction of depth-integrated NCP from surface and vertically resolved hydrographical parameters. Displayed are results based on two test data sets, namely observations from SV Tina V without data at depth (left panels) and a combination of SOOP and modelled hydrographical data (right panels). From top to bottom, panels represent (a) surface , (b) the vertical distribution of temperature together with the mixed-layer depth (MLD) and temperature penetration depth (TPD) for each cruise day, and (c) depth-integrated NCP comparing the reconstructions (solid lines) with the best guess (dashed black line) according to Fig. 5. Please note that a data gap in the SOOP record was filled with two observations from SV Tina V (open circles in a).
Good agreement was also found for the spatio-temporal dynamics of observed and modelled seawater temperature (Fig. 6b). Observed and modelled SST agreed within 1 ∘C over the entire observation period, despite an absolute change spanning almost 10 ∘C. Slightly higher deviations between observed and modelled temperature were found around the thermocline, where the observational record revealed a stronger temperature gradient. This difference is likely due to an imperfect representation of Langmuir circulation in the model (Axell, 2002), whereas the absence of increased light attenuation caused by phytoplankton particles was previously found to have only minor impacts on modelled SST dynamics (Löptien and Meier, 2011). Most importantly, the mean temperature penetration depths (TPDs) derived from the observational and model data differ by less than 1 m, indicating that surface warming and the integrated heat uptake are accurately represented by the model. The TPD (mean ± SD) over the observed productive period between 6 and 24 July was determined as 12.3±2.5 and 11.4±2.3 m for the observational and model data, respectively (Fig. 6b). The TPD estimates are considerably higher than the respective mixed-layer depth (MLD) estimates (6.0±1.9 and 5.5±1.2 m) and agree better with the observed penetration depth of drawdown, indicating that TPD is the favourable parameterisation of the integration depth.
Based on SOOP observations before 6 July, first signs of the onset of the investigated bloom event were detected already on 3 July. Between 3 and 6 July, an SST increase of ∼1 ∘C was accompanied by a drawdown of ∼10 µmol kg−1 (data not shown). Still, in the absence of any vertically resolved observation for this time period, the following comparison of the reconstructions to the best guess needs to be restricted to the period 6–24 July, during which the bulk of NCP occurred.
The NCP reconstruction based on TPD is generally higher than the MLD-based estimate (Fig. 6c). Comparing peak cumulative NCP estimates for 24 July, the TPD approach results in a ∼10 % overestimation compared to the best guess, i.e. the value derived from vertically resolved measurements. In contrast, the MLD-based NCP estimate is ∼30 % lower than the best guess. The reconstructed NCP estimates are very similar for both test data sets, as the good agreement between the underlying , MLD, and TPD time series suggests.
Comparing the deviation between the best guess and reconstructed NCP estimates in light of the lateral variability observed within the study area, it must be emphasised that between 6 and 24 July, the mean standard deviation of pCO2 and across stations amounted to ±6 µatm and ±11 µmol kg−1, respectively. This is higher than the likely uncertainty associated with the pCO2 measurements (see Methods), as well as its response time correction (see Methods and Appendix A4) or conversion to (see Appendix C1). Therefore, the lateral variability of seawater chemistry and the production signal are generally considered the highest source of uncertainty to our NCP estimates. Still, this lateral variability is small compared to the signal to be resolved (i.e. the drawdown of ∼90 µmol kg−1). However, on a relative scale the lateral variability is about as large as the difference between the best guess and the TPD-based NCP reconstruction (∼10 %), suggesting that the bias of the reconstruction falls within the uncertainty range of the best guess. In contrast, the lateral variability is smaller than the deviation between the best guess and the MLD-based NCP reconstruction.
All reconstructed NCP estimates include the correction of air–sea fluxes of CO2, but it is impossible to quantify and correct vertical entrainment fluxes due to mixing, because the vertical distribution of across the water column can not be resolved. The strong deviation between the best guess NCP and the MLD-based reconstruction on 16 August is due to this missing correction of vertical mixing. This deviation highlights that the reconstruction approach is only applicable to production periods with a stable or shoaling thermocline. The TPD-based approach does not allow for any estimate during the last 2 weeks of the observation period, as the TPD is per definition only applicable to periods of warming surface waters.
4.1 Comparison to previous studies
Having in mind the application of our NCP reconstruction approach to surface pCO2 observation collected since 2003, it is important to examine whether the biogeochemical dynamics of the examined cyanobacteria bloom in 2018 is representative for those in other years. Unfortunately, only a few previous studies aimed at the quantification of cyanobacteria growth as a component of the Baltic Sea carbon budget. One exception is the interpretation of SOOP Finnmaid data by Schneider et al. (2014). Focusing on the period from June to August and taking into consideration individual production pulses observed in the years 2005, 2008, 2009, and 2011, the authors found average daily rates of drawdown ranging from 3 to 8 , which comprises the mean rate of 5 determined in this study (i.e. the average drawdown of ∼90 µmol kg−1 over 18 d, Fig. 4). The individual production events identified by Schneider et al. (2014) lasted 1 to 5 weeks, similar to the bloom duration described in this study. Finally, Schneider et al. (2014) also provided a depth-integrated NCP estimate based on daily modelled mixing depths, which ranged from 3–20 m and were derived from the vertical distribution of a tracer 1 d after its injection into the surface. Although this approach is primarily useful to estimate the vertical distribution of air–sea CO2 fluxes and does not necessarily reflect the vertical extent of organic matter production, their determined midsummer NCP estimates (1–2.1 mol m−2) are on the same order of magnitude as the best guess derived in this study. It should be noted that the NCP estimates by Schneider et al. (2014) refer to the cumulative NCP of one to three production pulses per year, whereas our estimate of ∼1.2 mol m−2 refers to a single bloom event.
Wasmund et al. (2001) conducted 14C incubation experiments at different water depths to determine instantaneous rates of daytime primary production during a cyanobacteria bloom. Their reported carbon fixation rates in surface waters (0.4–0.8 ) are on the same order of magnitude as the mean rate found in this study (5 , equivalent to 0.2 ), despite representing daytime production rates and diurnal averages. More important than the agreement between the fixation rates at the sea surface, is the fact that Wasmund et al. (2001) also found significantly lower carbon fixation rates below 10 m water depth (<0.2 ), which agrees well with the depth distribution of NCP observed in this study.
Furthermore, the succession of different cyanobacteria genera observed in 2018, with the Nodularia-dominated bloom following an earlier presence of Aphanizomenon (Fig. B2), was previously described as a typical pattern (Wasmund, 2017), as well as the fact that increased wind speed and turbulence can inhibit N fixation of cyanobacteria and cause the termination of the bloom (Wasmund, 1997).
In conclusion, the bloom event duration, drawdown, and NCP, as well as the vertical extent of carbon fixation and the succession of the bloom observed in this study, agree well with observations in previous years, and distinct differences cannot be found. We therefore conclude that the findings of this study are representative for Baltic Sea cyanobacteria blooms in general, although the SST and pCO2 levels in 2018 were at the upper and lower ends, respectively, of the conditions observed in previous years (Schneider and Müller, 2018).
4.2 Biogeochemical relevance and interpretation
Our best guess of cumulative NCP on 24 July (∼ 1.2 mol m−2) represents the net amount of organic matter that was produced throughout the bloom event in the surface waters above the compensation depth at 12 m. After subtracting ∼20 % dissolved organic carbon (DOC) production (Hansell and Carlson, 1998; Schneider and Kuss, 2004), our NCP estimate equals the produced particulate organic carbon (POC) that is potentially available for export. In contrast, NCP estimates derived from other traditional methods for the integration across depth (such as the lower bound of the euphotic zone or the mixed-layer depth) would not directly relate to the POC export potential.
However, the potential POC export constraint by our NCP estimate is not equivalent to the supply of organic matter to the deep waters of the Gotland Basin, because POC might be (partly) remineralised before sinking beneath the permanent halocline. Remineralisation of POC that occurs during the bloom event above the compensation depth is – according to our definition of NCP – already included in our estimate. In contrast, any additional remineralisation of POC that occurs between the compensation depth and the halocline, or above the compensation depth after the end of the bloom event, reduces the organic matter supply to the deep waters and thereby mitigates deoxygenation. Indeed, our profiling measurements indicate a steady accumulation of beneath the compensation depth (Fig. 4), likely fuelled by the remineralisation of organic matter. However, our measurements do not allow the constraint of the budget of this accumulation, and we could not attribute the source of organic matter.
In contrast to shallow remineralisation processes, the deepening of the mixed layer that marked the end of the studied bloom event may facilitate the efficient transport of POC from the surface layer to depth. Focusing on the accumulation of remineralisation products beneath 150 m in the Gotland Basin, a previous study revealed that – in accordance with the main input of POC during the productive period – remineralisation rates exhibit a pronounced seasonality (Schneider et al., 2010). This seasonality was found to be most pronounced in the water layers closest to the sediment surface, suggesting that beneath 150 m the remineralisation takes place mainly at the sediment surface and is of minor importance during particle sinking through the deep water column. The pronounced seasonality further confirms that surface organic matter production and deep water oxygen consumption are indeed tightly coupled, despite a potential degradation of POC before export across the permanent halocline.
We conclude that NCP estimates determined with the methods developed in this study are of direct relevance to quantify the drivers for deep water deoxygenation. However, a better understanding of the organic matter remineralisation processes would be required to close the budget of biogeochemical transformations. New observational platforms, such as recently deployed biogeochemical ARGO floats (Haavisto et al., 2018), will complement the existing SOOP infrastructure and help to provide the required observational constraints throughout the water column.
4.3 Recommendations and caveats for NCP reconstruction from SOOP and model data
The good agreement between our best guess and the TPD-based NCP reconstruction on 24 July (Fig. 6c) indicates that it is possible to determine NCP from surface pCO2 observations and vertically resolved seawater temperature with little uncertainty. For the NCP calculation based on surface pCO2 observations from SOOP and modelled temperature profiles, we recommend
-
converting surface pCO2 to based on a mean AT estimate for the region under consideration,
-
identifying production pulses dominated by cyanobacteria as periods characterised by a decrease in that occur between June and August,
-
integrating observed surface changes to the temperature penetration depth (TPD) estimated from modelled temperature profiles, rather than using a mixed-layer depth (MLD) estimate, and
-
performing the integration individually for each production pulse and limiting NCP reconstruction to periods characterised by a stable or shoaling thermocline.
It should be emphasised that lateral variability and water mass transport are critical for observation-based NCP estimates and constitute the largest source of uncertainty in our estimates. However, SOOP observations allow averaging of observations across large regions, which reduces the impact of lateral water mass transport (Schneider and Müller, 2018). The region for spatial averaging should be chosen large enough to avoid as much as possible the influence of lateral perturbations which depend on the surface dynamics and the biogeochemical gradients in the surrounding area. Yet, the region for spatial averaging should be chosen small enough to ensure that variations in pCO2 within the region are small compared to the temporal changes of interest. Another critical aspect of the recommended NCP reconstruction approach is the restriction to periods of a stable or shoaling thermocline. While in principle it is possible that net organic matter production could also occur during periods of a deepening thermocline, this process was neither observed in this study nor in previous years (Schneider and Müller, 2018) and is in line with findings from the long-term cyanobacteria monitoring programme unravelling that increased wind speed causes the termination of the bloom (Wasmund, 1997). We thus conclude that reconstructed NCP estimates are not affected by a systematic underestimation due to this temporal restriction. Likewise, the required mean AT estimate should not restrict the applicability of our approach even if AT is not directly measured. For the Baltic Sea, it was demonstrated (Schneider et al., 2003) that AT estimated from the known AT–S relationship (Müller et al., 2016) is sufficiently accurate to convert pCO2 to (see also Appendix C1).
The NCP reconstruction approach presented in this study was derived from observations covering a single bloom event within the Central Baltic Sea. In the lack of comparable comprehensive observational data that underlie our best guess, the applicability of this approach could not be tested for other regions or bloom events. However, the dynamics and intensity of the bloom event described here are comparable to previous independent descriptions of cyanobacteria blooms. Therefore, it is assumed that underlying biogeochemical mechanisms are representative and that the NCP reconstruction approach can be applied to other cyanobacteria bloom events. Specifically, we assume that the findings represented here can be applied to evaluate past and future pCO2 observations made on Finnmaid and other SOOPs in the Central Baltic Sea without compromise. However, larger uncertainties should be expected when applying our NCP reconstruction approach to other regions outside the Central Baltic Sea.
In this study, the depth-integrated quantification of NCP that occurred during a cyanobacteria bloom in the Baltic Sea in 2018 is achieved through the interpretation of profiling measurements of pCO2 that covered the entire bloom event. Furthermore, it is demonstrated that this best guess can be reconstructed with small bias from SOOP pCO2 observations and modelled temperature profiles. Recommendations to apply our reconstruction approach to the comprehensive long-term record of surface pCO2 data available for the Baltic Sea are given. The application of this approach will allow for the detection and attribution of trends in cyanobacteria NCP over decades. In particular the comparison of NCP estimates of bloom events that occurred under different environmental conditions will provide a better understanding of the controlling factors. Factors to be tested include the environmental parameters used to constrain NCP (pCO2, SST, and TPD), but also additional observations of nutrients and phytoplankton composition routinely determined on the SOOP Finnmaid and in the framework of the Baltic Sea monitoring programme. The recently started initiative to deploy biogeochemical ARGO floats in the Baltic Sea will further aid in linking surface NCP estimates and deep water deoxygenation and thereby constrain biogeochemical budgets in the Baltic Sea. Ultimately, this knowledge will inform the design and monitoring of conservation measures aiming at a Good Environmental Status of the Baltic Sea and potentially other regions.
A1 Sensor calibration
The CONTROS HydroC® CO2 sensor used in this study (serial number CO2-0618-001) was calibrated in water by the manufacturer at 15 ∘C before (June 2018) and after (October 2018) the deployment for a measuring range of 100 to 500 µatm. The pre- and post-deployment calibration polynomials met the six steps per calibration with an R2 of 0.999999 (pre) and 0.999993 (post) at an RMSE of 0.13 µatm (pre) and 0.43 µatm (post). The time between the calibrations was about 107 d, and the sensor runtime during this interval was about 506 h or little more than 21 d. The zero drift observed between the two calibrations was only 0.89 µatm.
A2 Sensor configuration and operation
The instrument periodically records zeroing values, during which the CO2 within the gas stream is scrubbed by a soda lime cartridge. Zeroings of 2 min duration were recorded every 5 h during the field deployment. A period of 600 s after the zeroing was flagged as a flush period, during which the sensor signal recovers to environmental conditions. Recordings during the flush and zeroing periods were removed before further biogeochemical interpretation.
For the majority of the measurements, the sensor was operated with a 8 W pump (SBE-5T; Sea-Bird Electronics, Bellevue, USA), and the logging interval was set to 1 s. Only for the first two cruise days on 6 and 10 July was a 1 W pump (SBE-5M, Sea-Bird Electronics) used and the logging interval set to 10 s.
The downcast profiles were always recorded continuously and with a steady profiling speed of ∼2 m min−1. The upcast profiles were performed either continuously as well or with a stop to record an equilibrated reference pCO2 value at a desired depth. Only continuous downcast profiles were used for biogeochemical interpretation.
Zeroing signals were recorded by the CTD unit from the analogue sensor output, as well as in the internal sensor memory. Both records were used to ensure exact temporal match of the CTD and pCO2 time series. Only pCO2 data stored with higher temporal resolution in the internal memory were used during further analysis.
A3 Data post-processing
A drift correction as discussed in Fietzek et al. (2014) was applied to the field data to improve the data quality. This post-processing considers information from the pre- and post-deployment calibrations (i.e. concentration dependent or span drift) and the regular in situ zeroings (i.e. zero drift).
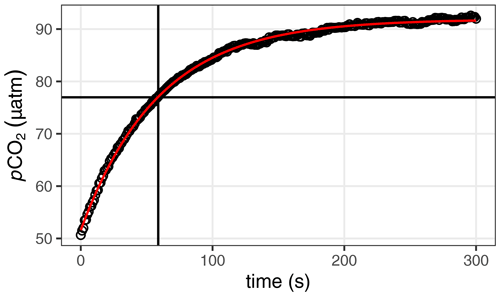
Figure A1Exemplary determination of the response time τ through fitting an exponential function (red curve) to the pCO2 signal recovery following a zeroing measurement. The determined response time τ and pCO2(t=τ) are indicated by a vertical and horizontal line, respectively.
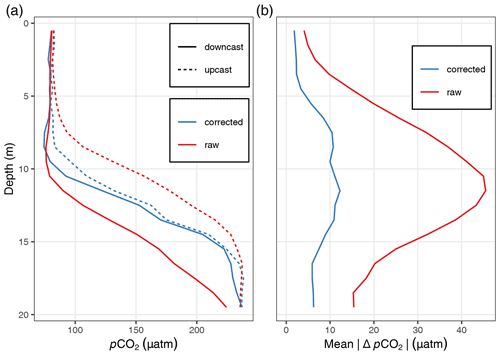
Figure A2Comparison of pCO2 profiles before (raw) and after (corrected) response time correction: (a) exemplary up- and downcast pCO2 profiles at one station and (b) mean absolute pCO2 difference between up- and downcast profiles across all profiles included in the quality assessment.
The first 60 s within every zeroing interval was discarded to only consider smooth zero-gas measurements that are not affected by the signal drop from ambient pCO2 to the zero value. Zero signals for every point of the deployment were obtained by linear interpolation of the zero measurements. In case of data gaps larger than 2 h within the deployment data, the course of the two zero signals before or after the gap was linearly extrapolated forward or backward, respectively, instead of an interpolation over the time of the measuring gap. A concentration-dependent drift of the sensor was considered by transforming the pre- into the post-deployment calibration polynomial according to the actual sensor runtime (and not according to the course of the zero measurements as applied within Fietzek et al., 2014).
Approximately 100 unrealistic outliers were found within the sensor temperature record (Tsensor parameter) of the HydroC®. These were identified to be electronic artefacts and the values replaced by the constant temperatures recorded before and after these events that only lasted a few seconds at most.
A4 pCO2 response time correction
The pCO2 response time correction applies a common “growth-law equation” and follows a two-step procedure (Miloshevich et al., 2004) that was previously successfully applied to pCO2 data recorded with the same type of instrument as used in this study (Fiedler et al., 2013; Fietzek et al., 2014; Atamanchuk et al., 2015). In a first step, the actual in situ response times (τ) of the sensor were determined by fitting an exponential function to the signal recovery following a zeroing (Sect. A4.1). In a second step, the determined τ values were used to correct the signal delay (Sect. A4.2). A quality assessment of the pCO2 response time correction is given in Sect. A4.3.
A4.1 Response time determination
In situ response times (τ) were determined from pCO2 data recorded during the flush period after each zeroing. Data recorded during the initial 20 s of each flush period were removed as those are affected by the mixing of residual gas volumes inside the sensor. Individual τ values were determined by fitting the non-linear model
where pCO2(t) is the recorded pCO2 at time t, pCO2(t0) and pCO2(tend) are the fitted pCO2 values at the beginning and the end of the equilibration process, and dt is the time since the beginning of the equilibration process. In situ τ was determined for a fit interval length of 300 s. Flush periods were discarded when the mean of absolute residuals from the fit exceeded 1 % of the final pCO2, a condition which indicated unstable environmental pCO2 (e.g. due to unintended heaving of the sensor package).
Similar to previous studies, a decrease in τ with increasing in situ temperature was found. The dependence of τ on temperature was fitted with linear regression models, separately for the deployments with the 1 W and 8 W pumps. The sensor was carefully cleaned after each cruise, and no signs of a changing sensor response time over time as an indicator of fouling on the sensor's membrane were detected.
A4.2 Correction procedure
For each recorded pCO2 value, the corresponding τ was calculated from measured in situ temperature. The response time correction was then applied based on a rearranged version of Eq. (A1):
where pCO2,insitu is the true in situ pCO2 time series, pCO2,obs the pCO2 time series as recorded by the sensor, and τ the response time for the interval between ti and ti+1. A rolling mean with a window width of 30 s was applied to the response-time-corrected pCO2,insitu time series to remove short-term noise. Please note that throughout the rest of the paper pCO2,insitu is referred to as pCO2.
A4.3 Quality assessment
The improvements by the response time correction were investigated based on the difference between up- and downcast pCO2 profiles vertically gridded into 1 m depth intervals. To focus this quality assessment on the conditions in near-surface waters which are the subject of this study, profiles were discarded which exceeded a maximum depth of 30 m and/or a maximum pCO2 of 300 µatm. Those profiles were excluded only for the quality assessment (not for the biogeochemical interpretation) to avoid a bias through exposure to very high pCO2 at greater depth. Furthermore, profiles were removed with a maximum number of missing observations from two or more depth intervals, which occasionally occurred when a sensor zeroing started while profiling. Based on this subset of response-time-corrected pCO2 profiles, it was found that the mean absolute pCO2 difference between the up- and downcast profile was <2.5 µatm averaged across the upper 5 m of the water column and <7.5 µatm across the upper 20 m. The highest offset was found at around 10 m water depth and results from the steep environmental pCO2 gradient around the thermocline.
B1 CT and AT
Dissolved inorganic carbon (CT) was determined from discrete bottle samples with an automated infrared inorganic carbon analyser (AIRICA, Marianda, Kiel, Germany). The analysis relies on the stripping of CO2 through acidification. The released CO2 is transported with a nitrogen carrier gas stream to an infrared detection unit (LI-7000, LI-COR Biosciences, Lincoln, USA), where the peak area is determined. Comparison to measurements performed on certified reference materials (CRM Batch 173; Dickson et al., 2003) allows for the calculation of CT. Triplicated measurements were performed on each sample, and a precision of 2 µmol kg−1 was achieved.
Total alkalinity (AT) was analysed by open cell titration of 125–140 g of sample. The method involves a two-stage titration. After a first single addition of hydrochloric acid to achieve a pH 4–3.5, AT is determined during a continued, stepwise titration to pH 3, during which pH is recorded potentiometrically (Dickson et al., 2007). Measurements were referenced to CRM batch 173 (Dickson et al., 2003).
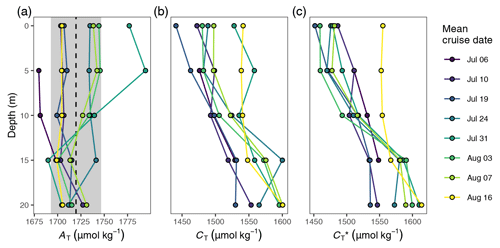
Figure B1Vertical profiles of (a) AT, (b) CT, and (c) CT normalised to the mean alkalinity (). Shown are cruise mean values for discrete samples taken at stations 07 and 10. The dashed line and grey area in (a) indicate the mean ±1 standard deviation of AT across the upper 20 m of the water column.
calculated for discrete samples refers to a classical alkalinity-normalised CT and was defined as . derived from discrete samples, or pCO2 sensor data are directly comparable (Fig. 5c) because they are referenced to the same mean AT of the discrete samples (1720 µmol kg−1).
B2 Phytoplankton
Phytoplankton samples were fixed with Lugol solution within no more than 24 h after sampling. Samples were stored dark, before being transported to IOW and analysed in the laboratory within no more than 3 months after sampling. Phytoplankton community composition and biomass were determined by the Utermöhl method (HELCOM, 2017), which relies on microscope counts and the conversion of cell shape and size to biomass units.
C1 Conversion from pCO2 to
The approach to estimate temporal changes (rather than absolute values) in the dissolved inorganic carbon concentration (CT) from a pCO2 time series was previously established and theoretically examined (Schneider et al., 2014, and references therein). It relies on a fixed estimate of alkalinity (AT) and is only applicable when noticeable internal changes in AT can be excluded, as is the case in the Baltic Sea due to the absence of calcifying plankton (Tyrrell et al., 2008). To avoid confusion with measured or absolute CT values and for consistency with previous studies, the calculated variable is referred to as .
To evaluate the applicability of this approach under the specific pCO2 and temperature conditions observed in summer 2018, we calculated changes between 6 and 24 July for a range of AT values covering 3 times the standard deviation of AT observations (Fig. B1). For assumed AT values of 1747 and 1693 µmol kg−1, which is 1 standard deviation of the observations (27 µmol kg−1) higher and lower than the mean AT (1720 µmol kg−1), the bias of the derived change in amounts to ±1.6 µmol kg−1. This bias is <2 % compared to the signal of interest, i.e. the absolute drawdown of (89 µmol kg−1).
It should be noted that the bias assessment presented here reflects two types of errors, namely (i) the assignment of an erroneous mean AT value for the calculation and (ii) the lateral exchange of water masses with different AT but identical initial pCO2 during the observation period. The robustness of this approach to the latter aspect is the reason why pCO2 observations are more suitable to determine NCP than direct CT measurements, when those are not normalised to corresponding AT measurements.
C2 Calculation of the vertical entrainment flux of
The vertical entrainment flux of that occurred across the 12 m integration depth layer between 7 and 16 August was estimated assuming an instantaneous complete vertical mixing to 17 m water depth after 7 August. For this scenario, the hypothetical homogeneous concentration after the mixing event (mix) equals the mean volume-weighted concentration between 0–17 m (Fig. C2). Furthermore, the entrainment flux (flux) into the surface water column (0–12 m) is equal to the concentration difference between observed on 7 August and mix, integrated from 12 to 17 m.
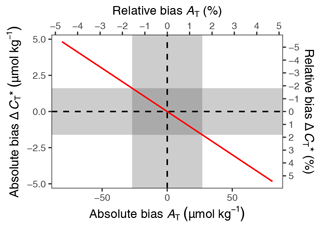
Figure C1Bias of changes in as a function of the bias in mean AT used for calculation (see Fig. B1). Results correspond to the pCO2 and temperature conditions observed in this study and are expressed in absolute and relative units. Grey areas highlight ±1 standard deviation around the mean AT.
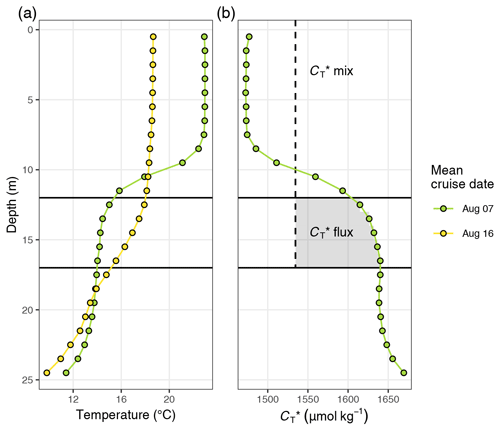
Figure C2Illustration of the approximation of the entrainment flux of due to vertical mixing. (a) The estimated deepening of the mixed layer from 12 to 17 m water depth between 7 and 16 August is based on the observed changes in the temperature profiles. (b) Assuming a complete, instantaneous mixing of the water column after 7 August, the hypothetical homogeneous concentration of (mix) can be used to approximate the entrainment flux of (grey area).
C3 Individual stations profiles
C4 Temperature penetration depth (TPD) concept
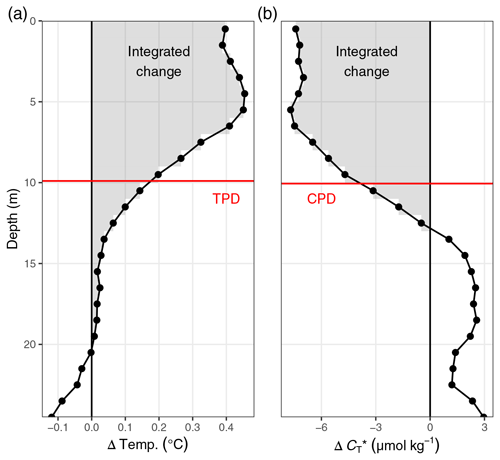
Figure C4Illustration of the temperature and penetration depth concept, short TPD, and CPD. Shown are exemplary profiles of incremental changes of (a) temperature and (b) observed between the cruises on 6 and 10 July. TPD and CPD (red horizontal lines) are defined as the depth-integrated positive (for temperature) and negative (for ) changes (grey areas) divided by the change at the surface. TPD and CPD are expressed in units of metres.
For SOOP Finnmaid transects recorded between 7 and 16 July, pCO2 data were not available from the LI-COR system because of technical failure. Therefore, data generated by the Los Gatos (LGR) system were used to fill the gap. Unfortunately, the comparison of LI-COR and LGR measurements before 7 July indicated a small leakage in the LGR system, which was later also physically detected and fixed. The resulting difference between the two systems was clearly correlated with absolute pCO2, as expected from contamination with ambient air. For data from the transect on 5 July, the linear regression model pCO2,true=pCOCO2,LGR−24.2 was fitted, assuming that the LI-COR system had delivered the “true” pCO2,true before its failure. Assuming further that the effect of the contamination remained constant, this relationship was then applied to reconstruct pCO2,true from pCO2,LGR for the period without LI-COR data. To validate this adjustment, pCO2,true was also reconstructed from pCO2,LGR on 4 July and compared to pCO2 directly measured with the LI-COR system. The mean difference was below 2 µatm for the entire transect as well as for a data subset within the study region, giving confidence to the high accuracy of the adjusted pCO2,true. It should be noted that the adjusted SOOP pCO2 data recorded between 7 and 16 July agree well with the in situ pCO2 recorded by the sailing campaign; i.e. the standard deviations of all surface measurements in the study region overlap.
Code and data underlying this study are archived at https://doi.org/10.5281/zenodo.4553314 (Müller, 2021).
JDM was the lead author of this study and involved in all parts of it, in particular the conceptualisation, funding acquisition, project administration, field sampling, data curation, visualisation and analysis, and manuscript writing. BS was involved in the conceptualisation, formal analysis, and original draft preparation. UG generated and processed the GETM model data. PF supported the HydroC® sensor configuration and performed the data post processing. MBW and AR provided the atmospheric data and supported the field campaign logistically. NW was responsibly for the collection, analysis, and interpretation of phytoplankton samples. SK designed and assembled the sensor package. GR was involved in the conceptualisation, funding acquisition, and project administration and supervision. All authors contributed to the internal review and editing of the manuscript.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The BloomSail expedition would not have been possible without the tremendous support of a fantastic group of people. In particular, 13 sailors supported the activities at sea. In the order of presence on board, those are Friederike Saathoff, Yannic Wocken, Andreas Kubatzki, Amanda Nylund, Rainer Kroiß, Ben Ole Grabler, Anna von Brandis, Fynn Pasewald, Ronja Topp, Matthias Kreuzburg, Katharina Klehmet, Mareike Körner, and Bastian Wulf. Monika Gerth supported the expedition by providing regular remote sensing products. Stefan Otto, Jenny Jeschek, and Paul Cwierz supported the analysis of discrete samples. Furthermore, the authors are thankful for logistic and administrative support received from IOW (Beatrix Blabusch, Barbara Hentzsch, Kristin Beck) as well as in the harbour of Herrvik (Mats Hanson, Kenneth Broscheit). Technical equipment of the sailing vessel was supported among others by Segelwerkstatt Warnemünde, Lopolight, Delius Klasing, BMS, and Kadematic. We acknowledge the use of imagery from the NASA Worldview application (https://worldview.earthdata.nasa.gov/, last access: 11 April 2020), part of the NASA Earth Observing System Data and Information System (EOSDIS). We acknowledge detailed and very constructive suggestions from the two anonymous reviewers.
This research has been supported by the National Geographic Society (grant no. EC-178R-18), in-house funding received from the IOW, and through BONUS, the joint Baltic Sea research and development programme (Art 185) funded by the European Union’s Seventh Programme for research, technological development and demonstration and the German Federal Ministry of Education and Research (grant no. 03F0773A; project BONUS INTEGRAL). Measurements on the SOOP Finnmaid are a German contribution to the Integrated Carbon Observation System (ICOS) and received funding through the German Federal Ministry of Education and Research and the German Federal Ministry of Transport and Digital Infrastructure. The ICOS station Östergarnsholm is funded by the Swedish Research Council and Uppsala University.
This paper was edited by Emilio Marañón and reviewed by two anonymous referees.
Atamanchuk, D., Tengberg, A., Aleynik, D., Fietzek, P., Shitashima, K., Lichtschlag, A., Hall, P. O. J., and Stahl, H.: Detection of CO2 leakage from a simulated sub-seabed storage site using three different types of pCO2 sensors, Int. J. Greenh. Gas Con., 38, 121–134, https://doi.org/10.1016/j.ijggc.2014.10.021, 2015. a, b
Axell, L. B.: Wind-driven internal waves and Langmuir circulations in a numerical ocean model of the southern Baltic Sea, J. Geophys. Res., 107, 3204, https://doi.org/10.1029/2001JC000922, 2002. a, b
Bakker, D. C. E., Pfeil, B., Landa, C. S., Metzl, N., O'Brien, K. M., Olsen, A., Smith, K., Cosca, C., Harasawa, S., Jones, S. D., Nakaoka, S., Nojiri, Y., Schuster, U., Steinhoff, T., Sweeney, C., Takahashi, T., Tilbrook, B., Wada, C., Wanninkhof, R., Alin, S. R., Balestrini, C. F., Barbero, L., Bates, N. R., Bianchi, A. A., Bonou, F., Boutin, J., Bozec, Y., Burger, E. F., Cai, W.-J., Castle, R. D., Chen, L., Chierici, M., Currie, K., Evans, W., Featherstone, C., Feely, R. A., Fransson, A., Goyet, C., Greenwood, N., Gregor, L., Hankin, S., Hardman-Mountford, N. J., Harlay, J., Hauck, J., Hoppema, M., Humphreys, M. P., Hunt, C. W., Huss, B., Ibánhez, J. S. P., Johannessen, T., Keeling, R., Kitidis, V., Körtzinger, A., Kozyr, A., Krasakopoulou, E., Kuwata, A., Landschützer, P., Lauvset, S. K., Lefèvre, N., Lo Monaco, C., Manke, A., Mathis, J. T., Merlivat, L., Millero, F. J., Monteiro, P. M. S., Munro, D. R., Murata, A., Newberger, T., Omar, A. M., Ono, T., Paterson, K., Pearce, D., Pierrot, D., Robbins, L. L., Saito, S., Salisbury, J., Schlitzer, R., Schneider, B., Schweitzer, R., Sieger, R., Skjelvan, I., Sullivan, K. F., Sutherland, S. C., Sutton, A. J., Tadokoro, K., Telszewski, M., Tuma, M., van Heuven, S. M. A. C., Vandemark, D., Ward, B., Watson, A. J., and Xu, S.: A multi-decade record of high-quality fCO2 data in version 3 of the Surface Ocean CO2 Atlas (SOCAT), Earth Syst. Sci. Data, 8, 383–413, https://doi.org/10.5194/essd-8-383-2016, 2016. a
Blischak, J. D., Carbonetto, P., and Stephens, M.: Creating and sharing reproducible research code the workflowr way, F1000Res., 8, 1749, https://doi.org/10.12688/f1000research.20843.1, 2019.
Carstensen, J., Andersen, J. H., Gustafsson, B. G., and Conley, D. J.: Deoxygenation of the Baltic Sea during the last century, P. Natl. Acad. Sci. USA, 111, 5628–5633, https://doi.org/10.1073/pnas.1323156111, 2014. a, b
Dickson, A. G., Afghan, J. D., and Anderson, G. C.: Reference materials for oceanic CO2 analysis: a method for the certification of total alkalinity, Mar. Chem., 80, 185–197, https://doi.org/10.1016/S0304-4203(02)00133-0, 2003. a, b, c
Dickson, A. G., Sabine, C. L. and Christian, J. R. (Eds.): Guide to Best Practices for Ocean CO2 Measurements, PICES Special Publication 3, 191 pp., 2007. a, b
Fiedler, B., Fietzek, P., Vieira, N., Silva, P., Bittig, H. C., and Körtzinger, A.: In Situ CO2 and O2 Measurements on a Profiling Float, J. Atmos. Ocean. Tech., 30, 112–126, https://doi.org/10.1175/JTECH-D-12-00043.1, publisher: American Meteorological Society, 2013. a, b
Fietzek, P., Fiedler, B., Steinhoff, T., and Körtzinger, A.: In situ Quality Assessment of a Novel Underwater pCO2 Sensor Based on Membrane Equilibration and NDIR Spectrometry, J. Atmos. Ocean. Tech., 31, 181–196, https://doi.org/10.1175/JTECH-D-13-00083.1, publisher: American Meteorological Society, 2014. a, b, c, d, e
Finni, T., Kononen, K., Olsonen, R., and Wallström, K.: The History of Cyanobacterial Blooms in the Baltic Sea, Ambio, 30, 172–178, https://doi.org/10.1579/0044-7447-30.4.172, publisher: Royal Swedish Academy of Sciences, 2001. a
Gattuso, J.-P., Epitalon, J.-M., Lavigne, H., and Orr, J.: seacarb: Seawater Carbonate Chemistry, version 3.2.14, available at: https://CRAN.R-project.org/package=seacarb (last access: 1 April 2021), 2020. a
Gilly, W. F., Beman, J. M., Litvin, S. Y., and Robison, B. H.: Oceanographic and Biological Effects of Shoaling of the Oxygen Minimum Zone, Annu. Rev. Mar. Sci., 5, 393–420, https://doi.org/10.1146/annurev-marine-120710-100849, _eprint: https://doi.org/10.1146/annurev-marine-120710-100849, 2013. a
Gräwe, U., Klingbeil, K., Kelln, J., and Dangendorf, S.: Decomposing Mean Sea Level Rise in a Semi-Enclosed Basin, the Baltic Sea, J. Climate, 32, 3089–3108, https://doi.org/10.1175/JCLI-D-18-0174.1, 2019. a
Gülzow, W., Rehder, G., Schneider, B., Deimling, J. S. v., and Sadkowiak, B.: A new method for continuous measurement of methane and carbon dioxide in surface waters using off-axis integrated cavity output spectroscopy (ICOS): An example from the Baltic Sea, Limnol. Oceanogr.-Meth., 9, 176–184, https://doi.org/10.4319/lom.2011.9.176, 2011. a
Haavisto, N., Tuomi, L., Roiha, P., Siiriä, S.-M., Alenius, P., and Purokoski, T.: Argo Floats as a Novel Part of the Monitoring the Hydrography of the Bothnian Sea, Front. Mar. Sci., 5, 324, https://doi.org/10.3389/fmars.2018.00324, 2018. a
Hansell, D. A. and Carlson, C. A.: Net community production of dissolved organic carbon, Global Biogeochem. Cy., 12, 443–453, https://doi.org/10.1029/98GB01928, 1998. a, b
Hansson, M. and Hakansson, B.: The Baltic Algae Watch System – a remote sensing application for monitoring cyanobacterial blooms in the Baltic Sea, J. Appl. Remote Sens., 1, 011507, https://doi.org/10.1117/1.2834769, 2007. a
HELCOM: Guidelines for monitoring phytoplankton species composition abundance and biomass, available at: https://www.helcom.fi/wp-content/uploads/2019/08/Guidelines-for-monitoring-phytoplankton-species-composition-abundance-and-biomass.pdf (last access: 6 November 2020), 2017. a, b
Henson, S. A., Sanders, R., Madsen, E., Morris, P. J., Moigne, F. L., and Quartly, G. D.: A reduced estimate of the strength of the ocean's biological carbon pump, Geophys. Res. Lett., 38, L04606, https://doi.org/10.1029/2011GL046735, 2011. a
Jokinen, S. A., Virtasalo, J. J., Jilbert, T., Kaiser, J., Dellwig, O., Arz, H. W., Hänninen, J., Arppe, L., Collander, M., and Saarinen, T.: A 1500-year multiproxy record of coastal hypoxia from the northern Baltic Sea indicates unprecedented deoxygenation over the 20th century, Biogeosciences, 15, 3975–4001, https://doi.org/10.5194/bg-15-3975-2018, 2018. a
Kahru, M. and Elmgren, R.: Multidecadal time series of satellite-detected accumulations of cyanobacteria in the Baltic Sea, Biogeosciences, 11, 3619–3633, https://doi.org/10.5194/bg-11-3619-2014, 2014. a
Kara, A. B., Hurlburt, H. E., and Wallcraft, A. J.: Stability-Dependent Exchange Coefficients for Air–Sea Fluxes, J. Atmos. Ocean. Tech., 22, 1080–1094, https://doi.org/10.1175/JTECH1747.1, 2005. a
Karlson, A. M. L., Duberg, J., Motwani, N. H., Hogfors, H., Klawonn, I., Ploug, H., Barthel Svedén, J., Garbaras, A., Sundelin, B., Hajdu, S., Larsson, U., Elmgren, R., and Gorokhova, E.: Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs, Ambio, 44, 413–426, https://doi.org/10.1007/s13280-015-0660-x, 2015. a
Kownacka, J., Busch, S., Göbel, J., Gromisz, S., Hällfors, H., Huseby, S., Jaanus, A., Jakobsen, H. H., Johansen, M., Johansson, M., Liebeke, N., Kraśniewski, W., Kremp, A., Lehtinen, S., Olenina, I., and Wasmund, N.: Cyanobacteria biomass, 1990–2019, available at: https://helcom.fi/wp-content/uploads/2020/09/BSEFS-Cyanobacteria-biomass-1990-2019-1.pdf, last access: 16 November 2020. a
Kutser, T., Metsamaa, L., and Dekker, A. G.: Influence of the vertical distribution of cyanobacteria in the water column on the remote sensing signal, Estuar. Coast. Shelf Sci., 78, 649–654, https://doi.org/10.1016/j.ecss.2008.02.024, 2008. a
Löptien, U. and Meier, H. E. M.: The influence of increasing water turbidity on the sea surface temperature in the Baltic Sea: A model sensitivity study, J. Marine Syst., 88, 323–331, https://doi.org/10.1016/j.jmarsys.2011.06.001, 2011. a
Meier, H. E. M., Väli, G., Naumann, M., Eilola, K., and Frauen, C.: Recently Accelerated Oxygen Consumption Rates Amplify Deoxygenation in the Baltic Sea, J. Geophys. Res.-Oceans, 123, 3227–3240, https://doi.org/10.1029/2017JC013686, _eprint: https://doi.org/10.1029/2017JC013686, 2018. a
Meier, H. E. M., Eilola, K., Almroth-Rosell, E., Schimanke, S., Kniebusch, M., Höglund, A., Pemberton, P., Liu, Y., Väli, G., and Saraiva, S.: Disentangling the impact of nutrient load and climate changes on Baltic Sea hypoxia and eutrophication since 1850, Clim. Dynam., 53, 1145–1166, https://doi.org/10.1007/s00382-018-4296-y, 2019. a, b
Millero, F. J.: Carbonate constants for estuarine waters, Mar. Freshwater Res., 61, 139, https://doi.org/10.1071/MF09254, 2010. a
Miloshevich, L. M., Paukkunen, A., Mel, H. V., and Oltmans, S. J.: Development and Validation of a Time-Lag Correction for Vaisala Radiosonde Humidity Measurements, J. Atmos. Ocean. Tech., 21, 1305–1327, https://doi.org/10.1175/1520-0426(2004)021<1305:DAVOAT>2.0.CO;2, 2004. a, b
Mohrholz, V.: Major Baltic Inflow Statistics – Revised, Front. Mar. Sci., 5, 152–166, https://doi.org/10.3389/fmars.2018.00384, 2018. a
Mohrholz, V., Naumann, M., Nausch, G., Krüger, S., and Gräwe, U.: Fresh oxygen for the Baltic Sea – An exceptional saline inflow after a decade of stagnation, J. Marine Syst., 148, 152–166, https://doi.org/10.1016/j.jmarsys.2015.03.005, 2015. a, b
Müller, J. D., Schneider, B., and Rehder, G.: Long-term alkalinity trends in the Baltic Sea and their implications for CO2-induced acidification, Limnol. Oceanogr., 61, 1984–2002, https://doi.org/10.1002/lno.10349, 2016. a
Müller, J. D.: jens-daniel-mueller/BloomSail: bg-2021-40, Zenodo [data set], https://doi.org/10.5281/zenodo.4553314, 2021. a
Nausch, M., Nausch, G., Lass, H. U., Mohrholz, V., Nagel, K., Siegel, H., and Wasmund, N.: Phosphorus input by upwelling in the eastern Gotland Basin (Baltic Sea) in summer and its effects on filamentous cyanobacteria, Estuar. Coast. Shelf Sci., 83, 434–442, https://doi.org/10.1016/j.ecss.2009.04.031, 2009. a, b
Nausch, M., Nausch, G., Mohrholz, V., Siegel, H., and Wasmund, N.: Is growth of filamentous cyanobacteria supported by phosphate uptake below the thermocline?, Estuar. Coast. Shelf Sci., 99, 50–60, https://doi.org/10.1016/j.ecss.2011.12.011, 2012. a
Neumann, T., Radtke, H., and Seifert, T.: On the importance of Major Baltic Inflows for oxygenation of the central Baltic Sea, J. Geophys. Res.-Oceans, 122, 1090–1101, https://doi.org/10.1002/2016JC012525, 2017. a
Olofsson, M., Torstensson, A., Karlberg, M., Steinhoff, F. S., Dinasquet, J., Riemann, L., Chierici, M., and Wulff, A.: Limited response of a spring bloom community inoculated with filamentous cyanobacteria to elevated temperature and pCO2, Bot. Mar., 62, 3–16, https://doi.org/10.1515/bot-2018-0005, 2019. a
Olofsson, M., Suikkanen, S., Kobos, J., Wasmund, N., and Karlson, B.: Basin-specific changes in filamentous cyanobacteria community composition across four decades in the Baltic Sea, Harmful Algae, 91, 101685, https://doi.org/10.1016/j.hal.2019.101685, 2020. a
Oschlies, A., Brandt, P., Stramma, L., and Schmidtko, S.: Drivers and mechanisms of ocean deoxygenation, Nat. Geosci., 11, 467–473, https://doi.org/10.1038/s41561-018-0152-2, 2018. a
Placke, M., Meier, H. E. M., Gräwe, U., Neumann, T., Frauen, C., and Liu, Y.: Long-Term Mean Circulation of the Baltic Sea as Represented by Various Ocean Circulation Models, Front. Mar. Sci., 5, 287, https://doi.org/10.3389/fmars.2018.00287, 2018. a
Roquet, F., Madec, G., McDougall, T. J., and Barker, P. M.: Accurate polynomial expressions for the density and specific volume of seawater using the TEOS-10 standard, Ocean Model., 90, 29–43, https://doi.org/10.1016/j.ocemod.2015.04.002, 2015. a
Rutgersson, A., Pettersson, H., Nilsson, E., Bergström, H., Wallin, M. B., Nilsson, E. D., Sahlée, E., Wu, L., and Mårtensson, E. M.: Using land-based stations for air–sea interaction studies, Tellus A, 72, 1–23, https://doi.org/10.1080/16000870.2019.1697601, 2020. a
Sanders, R., Henson, S. A., Koski, M., De La Rocha, C. L., Painter, S. C., Poulton, A. J., Riley, J., Salihoglu, B., Visser, A., Yool, A., Bellerby, R., and Martin, A. P.: The Biological Carbon Pump in the North Atlantic, Prog. Oceanogr., 129, 200–218, https://doi.org/10.1016/j.pocean.2014.05.005, 2014. a
Saraiva, S., Markus Meier, H. E., Andersson, H., Höglund, A., Dieterich, C., Gröger, M., Hordoir, R., and Eilola, K.: Baltic Sea ecosystem response to various nutrient load scenarios in present and future climates, Clim. Dynam., 52, 3369–3387, https://doi.org/10.1007/s00382-018-4330-0, 2019. a
Sarmiento, J. L. and Gruber, N.: Ocean Biogeochemical Dynamics, Princeton University Press, Princeton, New Jersey, US, 2006. a
Schneider, B. and Kuss, J.: Past and present productivity of the Baltic Sea as inferred from pCO2 data, Cont. Shelf Res., 24, 1611–1622, https://doi.org/10.1016/j.csr.2004.06.023, 2004. a, b
Schneider, B. and Müller, J. D.: Biogeochemical Transformations in the Baltic Sea, Springer Oceanography, Springer International Publishing, Cham, https://doi.org/10.1007/978-3-319-61699-5, 2018. a, b, c, d, e, f, g
Schneider, B., Nausch, G., Nagel, K., and Wasmund, N.: The surface water CO2 budget for the Baltic Proper: a new way to determine nitrogen fixation, J. Marine Syst., 42, 53–64, https://doi.org/10.1016/S0924-7963(03)00064-2, 2003. a, b
Schneider, B., Kaitala, S., and Maunula, P.: Identification and quantification of plankton bloom events in the Baltic Sea by continuous pCO2 and chlorophyll a measurements on a cargo ship, J. Marine Syst., 59, 238–248, https://doi.org/10.1016/j.jmarsys.2005.11.003, 2006. a
Schneider, B., Nausch, G., and Pohl, C.: Mineralization of organic matter and nitrogen transformations in the Gotland Sea deep water, Mar. Chem., 119, 153–161, https://doi.org/10.1016/j.marchem.2010.02.004, 2010. a
Schneider, B., Gustafsson, E., and Sadkowiak, B.: Control of the mid-summer net community production and nitrogen fixation in the central Baltic Sea: An approach based on pCO2 measurements on a cargo ship, J. Marine Syst., 136, 1–9, https://doi.org/10.1016/j.jmarsys.2014.03.007, 2014. a, b, c, d, e, f, g, h, i
Spilling, K., Olli, K., Lehtoranta, J., Kremp, A., Tedesco, L., Tamelander, T., Klais, R., Peltonen, H., and Tamminen, T.: Shifting Diatom–Dinoflagellate Dominance During Spring Bloom in the Baltic Sea and its Potential Effects on Biogeochemical Cycling, Front. Mar. Sci., 5, 327, https://doi.org/10.3389/fmars.2018.00327, 2018. a
Tyrrell, T., Schneider, B., Charalampopoulou, A., and Riebesell, U.: Coccolithophores and calcite saturation state in the Baltic and Black Seas, Biogeosciences, 5, 485–494, https://doi.org/10.5194/bg-5-485-2008, 2008. a, b
Vahtera, E., Conley, D. J., Gustafsson, B. G., Kuosa, H., Pitkänen, H., Savchuk, O. P., Tamminen, T., Viitasalo, M., Voss, M., Wasmund, N., and Wulff, F.: Internal Ecosystem Feedbacks Enhance Nitrogen-fixing Cyanobacteria Blooms and Complicate Management in the Baltic Sea, Ambi0, 36, 186–194, https://doi.org/10.1579/0044-7447(2007)36[186:IEFENC]2.0.CO;2, 2007. a
Wanninkhof, R.: Relationship between wind speed and gas exchange over the ocean revisited: Gas exchange and wind speed over the ocean, Limnol. Oceanogr.-Meth., 12, 351–362, https://doi.org/10.4319/lom.2014.12.351, 2014. a, b
Wasmund, N.: Occurrence of cyanobacterial blooms in the baltic sea in relation to environmental conditions, Int. Rev. Ges. Hydrobio., 82, 169–184, https://doi.org/10.1002/iroh.19970820205, _eprint: https://doi.org/10.1002/iroh.19970820205, 1997. a, b
Wasmund, N.: Recruitment of bloom-forming cyanobacteria from winter/spring populations in the Baltic Sea verified by a mesocosm approach, Boreal Environ. Res., 22, 445–455, 2017. a
Wasmund, N., Voss, M., and Lochte, K.: Evidence of nitrogen fixation by non-heterocystous cyanobacteria in the Baltic Sea and re-calculation of a budget of nitrogen fixation, Mar. Ecol. Prog. Ser., 214, 1–14, https://doi.org/10.3354/meps214001, 2001. a, b, c
Wasmund, N., Nausch, G., Schneider, B., Nagel, K., and Voss, M.: Comparison of nitrogen fixation rates determined with different methods: a study in the Baltic Proper, Mar. Ecol. Prog. Ser., 297, 23–31, https://doi.org/10.3354/meps297023, 2005. a, b, c
Wasmund, N., Kownacka, J., Göbel, J., Jaanus, A., Johansen, M., Jurgensone, I., Lehtinen, S., and Powilleit, M.: The Diatom/Dinoflagellate Index as an Indicator of Ecosystem Changes in the Baltic Sea 1. Principle and Handling Instruction, Front. Mar. Sci., 4, 22, https://doi.org/10.3389/fmars.2017.00022, 2017. a
Winslow, L. A., Zwart, J. A., Batt, R. D., Dugan, H. A., Woolway, R. I., Corman, J. R., and Read, J. S.: LakeMetabolizer: An R package for estimating lake metabolism from free-water oxygen using diverse statistical models, available at: https://cran.r-project.org/web/packages/LakeMetabolizer/index.html (last access: 1 October 2020), 2016. a
Zängl, G., Reinert, D., Rípodas, P., and Baldauf, M.: The ICON (ICOsahedral Non-hydrostatic) modelling framework of DWD and MPI-M: Description of the non-hydrostatic dynamical core, Q. J. Roy. Meteor. Soc., 141, 563–579, https://doi.org/10.1002/qj.2378, 2015. a
- Abstract
- Introduction
- Methods
- Results
- Discussion
- Conclusions
- Appendix A: pCO2 sensor measurements
- Appendix B: Discrete samples
- Appendix C: Net community production estimation
- Appendix D: SOOP Finnmaid pCO2
- Code and data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Abstract
- Introduction
- Methods
- Results
- Discussion
- Conclusions
- Appendix A: pCO2 sensor measurements
- Appendix B: Discrete samples
- Appendix C: Net community production estimation
- Appendix D: SOOP Finnmaid pCO2
- Code and data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References