the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Fire in lichen-rich subarctic tundra changes carbon and nitrogen cycling between ecosystem compartments but has minor effects on stocks
Ramona J. Heim
Andrey Yurtaev
Anna Bucharova
Wieland Heim
Valeriya Kutskir
Klaus-Holger Knorr
Christian Lampei
Alexandr Pechkin
Dora Schilling
Farid Sulkarnaev
Norbert Hölzel
Fires are predicted to increase in Arctic regions due to ongoing climate change. Tundra fires can alter carbon and nutrient cycling and release a substantial quantity of greenhouse gases with global consequences. Yet, the long-term effects of tundra fires on carbon (C) and nitrogen (N) stocks and cycling are still unclear. Here we used a space-for-time approach to investigate the long-term fire effects on C and N stocks and cycling in soil and aboveground living biomass. We collected data from three large fire scars (>44, 28, and 12 years old) and corresponding control areas and used linear mixed-effect models in a Bayesian framework to analyse long-term development of C and N stocks and cycling after fire.
We found that tundra fires had no long-term effect on total C and N stocks because a major part of the stocks was located belowground in soils which were largely unaltered by fire. However, fire had a strong long-term effect on stocks in the aboveground vegetation, mainly due to the reduction in the lichen layer. Fire reduced N concentrations in graminoids and herbs on the younger fire scars, which affected respective ratios and may indicate an increased post-fire competition between vascular plants. Aboveground plant biomass was depleted in 13C in all three fire scars. In soil, the relative abundance of 13C changed with time after fire.
Our results indicate that in lichen-rich subarctic tundra ecosystems, the contribution of fires to the release of additional carbon to the atmosphere might be relatively small as soil stocks appear to be resilient within the observed time frame.
- Article
(2500 KB) - Full-text XML
-
Supplement
(1094 KB) - BibTeX
- EndNote
Arctic regions are warming faster than the worldwide average, with strong impacts on the global carbon cycle (Post et al., 2019; Schuur et al., 2015). Warming is expected to expose ecosystems at high latitudes to increased fire frequency and extent (Chen et al., 2021; Hu et al., 2015; Moritz et al., 2012; Young et al., 2016). Wildfires in the Arctic can feed back on climate change because they release carbon stored in the tundra ecosystems (Lasslop et al., 2020; Veraverbeke et al., 2021). This effect may be substantial because Arctic and subarctic regions store huge amounts of the global carbon (C) (ca. 1700×109 t in terrestrial soils; Schuur et al., 2015).
Wildfires enhance the release of stored carbon from active ecosystems in multiple ways. Combustion of living and dead biomass rapidly transfers large amounts of stored C to the atmosphere (Mack et al., 2011). Wildfires also burn the insulating and reflecting layers of soil organic material and vegetation, which increases soil temperatures through irradiation (Chambers et al., 2005; Jiang et al., 2015). Warmer soil temperatures raise microbial activity, which in turn promotes faster decomposition processes (Jansson and Hofmockel, 2020). Soil warming induces permafrost thaw and active-layer deepening, which exposes organic matter in deeper layers to decomposition, resulting in a massive release of carbon to the atmosphere (Estop-Aragonés et al., 2020; Schuur et al., 2009). In summary, the rate of C loss through fires is substantial and faster than other climate-driven processes influencing C cycling in northern latitudes (Mack et al., 2011).
Fires also affect the stored N, which has consequences for vegetation and ecosystem functioning because N limits productivity in tundra ecosystems (Oulehle et al., 2016). Fires release substantial parts of the accumulated nitrogen (N) pool to the atmosphere. However, soil warming and related deeper thaw may increase plant-available inorganic N through higher mineralization rates (Aerts, 2006; Salmon et al., 2016). This increases the productivity of shrubs and graminoids because they can reach the nutrients mineralized in deeper soil layers than cryptogams (Dormann and Woodin, 2002; Oulehle et al., 2016; Salmon et al., 2016). As a consequence, the cover of vascular plants increases after fires at the expense of previously dominating cryptogams, which cannot profit from the enhanced nitrogen availability as they do not reach deep soil layers or cannot take up nitrogen from the soil (Bret-Harte et al., 2013; Jandt et al., 2008; Narita et al., 2015; Turetsky et al., 2012). Such profound changes in vegetation cover, in turn, influence ecosystem functioning and processes, such as carbon and nitrogen cycling and C and N stocks (Longton, 1997; Sancho et al., 2016; Turetsky, 2003).
Studies including nutrient cycles in soil and vegetation related to long-term effects of tundra fires are rare, and therefore the effect of fires on the carbon cycle of tundra ecosystems is relatively unknown (Mack et al., 2011). This is unfortunate because the reactions of the tundra ecosystem to altered temperature have a time lag and are thus relatively slow (Rinnan et al., 2007). The effects of fire on the functioning of the tundra ecosystem may thus be visible only after more than a decade (Blok et al., 2018). Therefore, long-term studies are essential (Chapin et al., 2000).
One widely used tool to unravel C and nutrient cycling processes is the use of stable isotopes (Peterson and Fry, 1987). The relative abundance of the heavy carbon isotope (13C) in the living aboveground biomass and in the soil can provide insight into post-fire environmental site conditions. In soil, increased activity of microbes after fire is reflected in the residual enrichment of 13C in the remaining soil organic matter as decomposers prefer isotopically light material (12C) (Ehleringer et al., 2000). In the aboveground biomass, the isotope ratio is related to the isotopic composition of the source CO2, and thus the isotope ratio in leaves may reflect the lower levels of 13C in CO2 which is available for photosynthesis through respiration from soils during decomposition (Dawson et al., 2002; Farquhar et al., 1989).
The isotope ratios of N can be used for a better understanding of post-fire N cycling processes by providing information about the association with mycorrhizal fungi. During N transfer from mycorrhizal fungi to the plant, the heavy 15N gets enriched in the mycorrhizae and depleted in the plants. During succession, the associated mycorrhizal fungi have to colonize the plants first, and therefore 15N would be expected to be enriched in early successional stages and depleted in later successional stages (Hobbie et al., 2005).
Here, we studied the long-term effect of fire on tundra nutrient stocks and cycling using a space-for-time approach. We focused on three large fire scars (>44, 28, and 12 years old) that represent a gradient of post-fire succession. We analysed C and N stocks, concentrations, and isotope ratios in vegetation and soil. We hypothesized that
- 1.
fire leads to decreased ecosystem C and N stocks;
- 2.
fire affects long-term C and N cycling, evident through
- a.
an increased N concentration and a decreased ratio in vascular plants after fire
- b.
a depletion of 13C in aboveground biomass and a relative enrichment of 13C in soil after fire
- c.
increased relative abundance of 15N in leaves of vascular plants on the younger fire scars.
- a.
2.1 Study area
The study area was located in western Siberia within the Yamalo-Nenets Autonomous Okrug (region) between the rivers Pur and Taz, north of the Arctic Circle (centre at N, E; total study area size ca. 70 km2) (Heim et al., 2021a). The region has a subarctic climate with a growing season from mid-June to early September and with a mean annual temperature of −8.1 ∘C, mean January temperature of −26.2 ∘C, mean July temperature of 14.4 ∘C, and annual precipitation of 482 mm (Kazakov, 2019). The vegetation in the region represents the transition zone between the forest–tundra to the south and the shrub–tundra to the north (Yurkovskaya, 2011). Compared to other previously investigated tundra areas, e.g. moist acidic tundra in the Anaktuvuk River region in Alaska (Mack et al., 2011), the region is a relatively dry and well-drained upland area. The vegetation is dominated by reindeer lichens (mostly Cladonia spp., around 70 % cover), with abundant shrubs and dwarf shrubs, such as Betula nana (around 25 % cover) and Vaccinium uliginosum (around 10 % cover) and occasional graminoids and bryophytes with low cover. The landscape is sparsely dotted with larch trees (Larix sibirica).
Soils are Cryosols (IUSS Working Group WRB, 2015), which developed in silty, loess-like parent material and soil organic layer thickness was generally low at all sites. Bulk density ranged from 0.6 to 1.6 g cm−3 and pH from 3.8 to 6.3 (Fig. S1 in the Supplement). The mineral soil was covered by living lichen and moss at unburned sites, lying directly on the mineral layer. The 0–5 cm layer in our study is the upper layer of the mineral soil, which was at unburned sites found directly under the lichen layer. Soil temperature in 12 cm depth was higher on burnt sites but regenerated to control levels after >44 years (predicted mean difference to control plots after 12, 28, and <44 years: 6.29, 6.29, and 0.24 ∘C) (Heim et al., 2021a). This was also the case in active-layer depth, which was lower on burnt sites but regenerated to control levels after >44 years (predicted mean difference to control plots after 12, 28, and <44 years: −33.02, −32.20, and 6.62 cm) (Heim et al., 2021a).
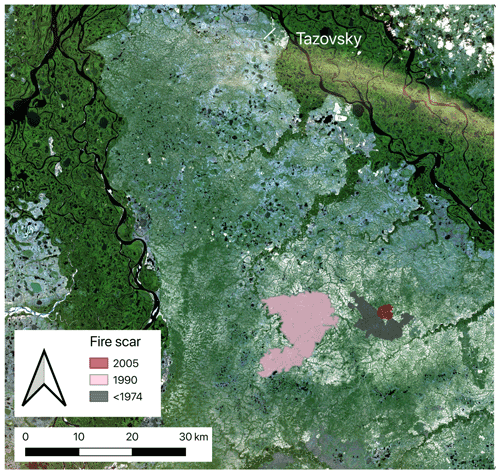
Figure 1Study area with fire scars in the north of western Siberia. Edited figure from Heim et al. (2021a). Satellite image source: Copernicus Sentinel-2 data (2019).
The main cause of fires in the study region is lightning (Kornienko, 2018), but the number of human-induced fires increases because of expanding transport and settlement infrastructure due to oil and gas exploitation (Mollicone et al., 2006; Vilchek and Bykova, 1992; Yu et al., 2015).
2.2 Sampling design and data collection
To examine the long-term impact of fire on C and N cycling in soil and aboveground biomass, we studied three large fire scars (aged 12, 28, and >44 years) and adjacent unburnt control sites (Fig. 1). All three fire scars were located close to each other (<10 km distance between scars) and therefore had similar environmental conditions. We detected the three fire scars visually with the help of satellite images. We used annual Landsat images back to the year 1985 in Google Earth Timelapse (Gorelick et al., 2017) and older Landsat images back to 1973, which we downloaded via the USGS earth explorer (U.S. Geological Survey, 2018) (for details see Heim et al., 2021a). The oldest fire scar (3500 ha) was already visible in the first satellite image from 1973 (Landsat 1). The medium-aged scar (ca. 12 500 ha) burnt in 1990, and the youngest scar (542 ha) burnt in 2005.
We collected samples in July 2017 at fire scars burned before 1973 and in 2005 (that is >44 and 12 years after fire, respectively) and in July 2018 in the area burnt in 1990 (28 years after fire). Environmental conditions of the tundra ecosystem are stable with low inter-annual variability (Dahl, 1975), and climatic conditions in the 2 sampling years were similar (Kazakov, 2019). It is thus unlikely that collecting in 2 subsequent years biased the results.
We placed 10 sampling locations along the fire border of each fire scar (at least 300 m apart from each other). At each location, we placed one sampling plot in the burnt and one sampling plot at the unburnt site, resulting in a total of 60 plots (30 pairs). Each site included a 10 m × 10 m plot and a soil profile, located 2–5 m next to the plot. The plots at each location were placed as close to each other as possible, but at least 100 m apart (50 m minimum plot distance from the fire border to avoid edge effects).
We sampled aboveground biomass in five subplots of the 10 m × 10 m plot. The subplots were situated in the four corners and in the centre of the plot. While the subplots for shrubs, herbs, and graminoids were squares of 0.3 m×0.3 m, the subplots for lichens and bryophytes were 0.1 m×0.1 m and were randomly placed within the 0.3 m×0.3 m squares. Biomass of each type (shrubs, graminoids, herbs, bryophytes, lichens) was separately sampled, and all subplots were pooled in one bag per type.
Soil was sampled in layers ranging from 0–5, 5–30, and 30–60 cm depth. In each layer, we placed three cylinders (200 cm3) horizontally and distributed them over the whole range and sampled the soil of one layer in one plastic bag.
2.3 Laboratory analyses and stock calculations
In the field, aboveground biomass was stored well ventilated, while soil was stored in airtight plastic bags. We dried aboveground biomass and soil in the laboratory at 60 ∘C to a constant weight. For determination of C and N concentrations and stable-isotopic composition, samples were ground in a ball mill (tungsten carbide cups, MM400, Retsch, Haan, Germany) and weighed into tin capsules for analyses on an elemental analyser (EA 3000, EuroVector, Padua, Italy) coupled to an isotope ratio mass spectrometer (EA–IRMS; Nu Horizon, Nu Instruments, Wrexham, UK). Calibrations were done using certified working standards (IVA Analysentechnik, Meerbusch, Germany) and reference materials (IAEA 600; Coplen et al., 2006; USGS 35, Böhlke et al., 2003). We could not analyse nitrogen isotopic composition in the soil samples as the N concentrations were generally too low.
We express the stable-isotope composition using the common delta notation (in per mille, ‰) as a ratio relative to an internationally accepted reference standard: , where E is the element, xx is the mass of the heavier isotope, and R is the abundance ratio of the isotopes (e.g. 15N : 14N) (Dawson et al., 2002). Higher δ values indicate a higher abundance of the heavier isotope (Dawson et al., 2002). We furthermore analysed bulk density. We dried a subsample of soil samples at 105 ∘C and calculated bulk density using the following formula: bulk density = dry soil weightsoil volume. Based on the bulk density, we calculated soil stocks for each range with the following formula: stocksoil = bulk density ⋅ concentration100 ⋅ range size. C and N stocks for vegetation were calculated as follows: stockvegetation = dry mass ⋅ sampling area ⋅ concentration100. We use the term “total stocks” for combined soil and vegetation stocks.
2.4 Statistical analysis
We carried out all statistics in R Version 4.0.3. (R Core Team, 2020) and fitted all models in a Bayesian framework using the function brm() from the package brms (Bürkner, 2017, 2018). For testing hypothesis 1 (fire leads to decreased ecosystem C and N stocks), we summarized aboveground biomass and soil stocks per sampling site. We fitted two linear models with log-transformed stocks (C stocks, N stocks) as dependent variables. Fire scar (12, 28, >44 years), burn status (burnt, unburnt), and their interaction were included as independent variables and location (paired plots) as a random factor. To obtain the posterior distribution, we used improper priors and ran 6000 iterations (warm-up=3000) with 4 chains. The posterior distribution is a probability distribution that summarizes updated beliefs about the parameter after observing the data and is thus a result of the prior distribution and the likelihood function (Korner-Nievergelt et al., 2015).
We present mean values together with the 95 % credible interval (CrI) of the simulated posterior distribution. The 95 % CrI is the range in which the true value is expected with a probability of 0.95. We calculated the posterior probabilities by using the proportion of simulated values of the posterior distribution of C and N stocks on burnt sites smaller than the proportion of simulated values of the posterior distribution of stocks on unburnt control sites. Therefore, a posterior probability of 1 would indicate that the stocks on the burnt sites were significantly lower than on unburnt control sites. A posterior probability of 0 would mean the opposite, and a posterior probability of 0.5 indicates that stocks at burnt and unburnt sites did not differ.
To analyse the impacts of fire on aboveground biomass and soil stocks separately, we fitted four linear models with log-transformed stocks (aboveground biomass C stocks, aboveground biomass N stocks, soil C stocks, soil N stocks) as dependent variables. Fire scar (12, 28, >44 years), burn status (burnt, unburnt), and vegetation type for biomass (shrub leaves, herbs, graminoids, bryophytes, lichens) or depth for soil (0–5, 5–30, 30–60 cm) were included as independent variables with all possible interactions. Sites were nested in location as a random factor. To obtain the posterior distribution, we used improper priors and ran 4000 iterations (warmup=2000) with 4 chains. For the models of ratio in biomass and C concentration in soil, we used adapt_delta=0.95 and ran 6000 iterations (warmup=3000) for better convergence. Bayesian R2 was calculated with the function bayes_R2 from the package brms (Bürkner, 2017, 2018).
For testing the hypothesis 2 (fire affected long-term C and N cycling), we first calculated the differences between burnt and unburnt control plots (burnt − unburnt) for concentrations, ratios, and isotope ratios as this enabled a more straightforward and clearer interpretation (see raw data in Figs. S2 and S3 in the Supplement). A negative difference in the C concentration, therefore, indicates that fire decreased the C concentration. A negative difference in the ratio indicates that fire decreased the ratio – through either decreased C concentrations or increased N concentrations. A negative difference in δ13C (Δ13C) indicates that fire decreased δ13C (and thus caused a depletion of 13C).
We fitted nine linear models with concentrations, -ratio, and isotope ratios (C in biomass, C in soil, N in biomass, N in soil, in biomass, in soil, δ13C in biomass, δ13C in soil, δ15N in biomass) as dependent variables. Fire scar age (12, 28, >44 years), burn status (burnt, unburnt), and vegetation type for biomass (shrub leaves, herbs, graminoids, bryophytes, lichens) or depth for soil (0–5, 5–30, 30–60 cm) were included as independent variables with all possible interactions. Sites were nested in location as a random factor. We controlled for non-constant variances in type and depth, adapting sigma (e.g. sigma 0 + type). To obtain the posterior distribution, we used improper priors and ran 4000 iterations (warmup=2000) with 4 chains.
3.1 Fire influence on C and N stocks in aboveground biomass and soil
Fire had no long-term effects on combined soil and vegetation stocks (hereafter total stocks) (C: Pburnt_12<control_12=0.68, Pburnt_28<control_28=0.94, Pburnt_44<control_44=0.16; N: Pburnt_12<control_12=0.40, Pburnt_28<control_28=0.81, Pburnt_44<control_44=0.18) (Fig. 2 and Tables S6 and S7 in the Supplement). The net losses and gains for the youngest fire scar were −2.17 kg C m−2 and 0.09 kg N m−2, for the intermediate −9.85 kg C m−2 and −0.33 kg N m−2, and for the oldest 5.41 kg C m−2 and 0.30 kg N m−2.
Soils at burnt plots had less C at the intermediate fire scar in 5–30 cm depth. In the oldest fire scar, soils stored more N and C in the upper soil layer in comparison to the unburnt control (Table 1 and Tables S7 and S8 in the Supplement), pointing towards a replenishment from the belowground organs of vascular plants in the course of succession.
In contrast to soil, C and N stocks in vegetation were strongly reduced through fire (Fig. 2). In the two younger stages vegetation C stocks had only about 15 % of the unburnt reference, and even after more than 44 years they reached on average only 39 % of the unburnt reference. Vegetation N stocks recovered significantly fast and in the oldest stage already reached 74 % of the unburned reference (Fig. 2). This was mainly because fire substantially reduced lichens and consequently the C and N stored in them. The depletion of C and N in lichens lasted for more than 4 decades (Tables 1, S7, and S8). In contrast, fire increased C and N stocks in herbs in the intermediate fire scar and in bryophytes in the oldest one (Table 1). In the oldest fire scars the proportion of C and N stored in bryophytes clearly dominated the stocks of C and N in aboveground living vegetation. For both elements, absolute values were ca. 3 times higher than in unburned controls (Fig. 2). Thus, after 44 years the dominant role for storing C and N in aboveground vegetation shifted from lichens to bryophytes, and lichens were still far from gaining back their former absolutely dominant importance as C and N sink.
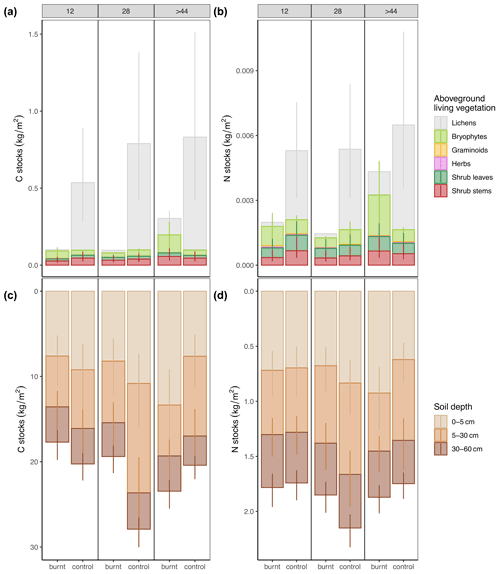
Figure 2Predicted mean values for C and N stocks in aboveground biomass and soil in regard to time after fire on burnt and control plots. Vertical lines are 95 % credible intervals (CrIs). Model structure and parameters as well as corresponding CrIs and R2 are shown in Tables S6–S8. C and N stocks for shrub stems are calculated as described in Fig. S4 in the Supplement. Compare (a) and (b) with aboveground dry weight in Fig. S5 in the Supplement.
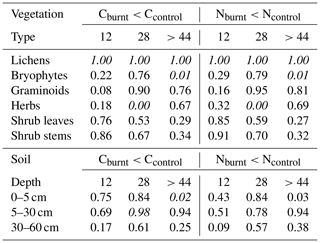
Table 1Probabilities of stocks on burnt plots to be lower than stocks on unburnt control plots. If probabilities are higher than 0.975 (stocks on burnt plots are lower compared to unburnt plots) or smaller than 0.025 (stocks on burnt plots are higher), they are written in italics. If probabilities are 0.5 there is no difference between stocks on burnt plots and stocks on control plots.
3.2 Fire influence on C and N cycling
Fire affected C and N concentrations and isotope ratios in aboveground biomass and soil (Fig. 3a–h, Tables S9 and S10 in the Supplement). While fire had generally strong long-term effects on C and N concentrations as well as on isotope ratios in vegetation, only the upper soil layer showed differences in concentrations and isotope ratios. Overall, long-term fire effects on C and N concentrations and isotope ratios were most pronounced in lichens.
Fire had a positive effect on N concentrations in lichens and a negative effect on N concentrations in graminoids and herbs (Fig. 3c). Those effects disappeared in all groups after >44 years post-fire. The N concentrations were reflected in the ratios, which increased in graminoids and herbs but decreased in lichens (Fig. 3e). While fire significantly increased C and N concentrations in the upper soil layer on the oldest fire scar, we could not detect a fire effect on the deeper soil layers (Fig. 3d and f).
Lichens on all three fire scars were relatively depleted in 13C in comparison to unburnt plots (Fig. 3g). This was also the case in graminoids on the two younger fire scars (Fig. 3g).
In soil, the abundance of 13C changed with time after fire. The upper soil layer of the youngest fire scar was enriched in 13C in comparison to unburnt plots (Fig. 3h). This relationship turned with time since fire, and we observed a relative depletion of 13C in the soils of the oldest fire scar in comparison to unburnt plots.
Fire increased the abundance of 15N in lichens (Fig. 3i). This effect was still visible 4 decades after fire. We found no significant long-term fire effects on δ15N in vascular plants.
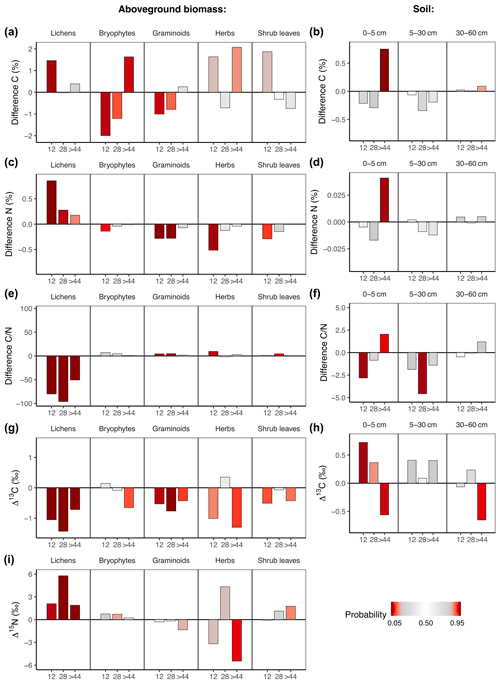
Figure 3Difference (burnt−unburnt) between posterior means of C and N concentrations and isotope ratios in regard to time after fire in aboveground biomass and soil. Colour intensity indicates the probability P of the value from an unburnt control plot to be higher than the value from the burnt plot. If P is very high, there is a high probability of a negative fire impact. If P is very low, there is a high probability of a positive fire impact.
Fire had no long-term effects on total C and N stocks in subarctic dry tundra. This is probably because most of it was stored in soils (97.4 % of C and 99.7 % of N) which were largely unaffected by fire. In contrast, stocks in aboveground vegetation were strongly reduced through fire, and the effect persisted even after more than 44 years. Striking results reporting high losses of carbon through tundra fires (Mack et al., 2011) received a lot of attention and were covered by media outlets (e.g. Rosen, 2017). While Mack et al. (2011) mainly investigated the combustion loss, we investigated the long-term fire consequences on stocks. In our study, recovery of vegetation took place, and hence there was a regeneration of the carbon stocks. The effect of fire on tundra carbon and nitrogen stocks is variable and dependent on many factors. First, losses are strongly mediated by fire severity through the consumption of organic matter (Schuur et al., 2003). Fire severity was relatively high for the fire investigated in Alaska (Mack et al., 2011) but intermediate in our study (Heim et al., 2021a), which may partly explain the lack of a strong effect in our study (Maslov et al., 2020).
Our results support recent findings of He et al. (2021), who claim that soil organic carbon loss through tundra fires may be overestimated because the soil organic layer in many tundra areas is much lower (see also our findings) than in the area of the extreme Anaktuvuk River fire with predominantly wet organic soils (Histosols).
In addition to the thick organic layer, which usually is combusted by fire (He et al., 2021), moist tundra ecosystems typically store much higher amounts of carbon per unit area because of a higher soil organic matter content in comparison to dry tundra ecosystems (Marion et al., 1997). Through increased temperature-sensitive decomposition after fire (De Baets et al., 2016), burning can thus indirectly lead to higher decomposition rates and ecosystem carbon loss in wet tundra ecosystems. Depending on tundra type, fire impacts can thus have varying severity of fire impact on biogeochemical cycling. Second, the thickness of the soil organic matter plays an important role. Because the decomposition of plant material is limited in tundra ecosystems by low temperatures and the often wet and anaerobic conditions, organic matter accumulates (Jonasson et al., 2001). A reduction in this soil organic layer leads to decreases in the amount of stored C (Mack et al., 2004). Thus, exceptionally large losses of C and N stocks were reported from wildfires in rather wet tundra ecosystems with a thick soil organic layer or from peatlands (Mack et al., 2011; Turetsky et al., 2015). Our study was located in an upland area with well-drained mineral soils and a thin organic layer, and in such conditions, fires seem to cause only minor losses (Loranty et al., 2014). Thick organic soil layers, as reported, for example, for a site in Alaska (21.5 cm; Mack et al., 2011), may store particularly large amounts of C and N that are potentially lost if burnt. However, much shallower organic layers have been reported for north-eastern Siberia (6.3 cm; Loranty et al., 2014) and were also typical of our study area (average thickness of 4.3 cm). Therefore, potential losses upon burning can be expected to be much lower, and, moreover, the importance of the more stable mineral soil carbon stock less susceptible to losses from burning relatively increases. However, a direct comparison of these studies is not possible as we investigated the system during regeneration and not directly after combustion. Further studies are needed to draw more general conclusions about the role of vegetation type and environmental settings (for example well-drained upland soils) for wildfire-induced losses of C and N in tundra ecosystems. In the long term, fire even increased C and N stocks in the upper soil layer, which was probably due to plant community turnover. The vegetation on burnt plots did not return to control levels within >44 years and was dominated by bryophytes; herbaceous plants; and shrubs, especially Betula nana (Heim et al., 2021a). Vascular plants, and especially woody species, accumulate more soil C in roots and litter (Cahoon et al., 2016), and due to root exudates and their ability of symbiotic N fixation burnt plots can also contain more N than unburnt lichen-dominated plots (Maslov et al., 2018).
In contrast to soil, fire substantially reduced C and N stocks in vegetation. Nevertheless, as vegetation stored much less C and N in comparison to soil, those effects were negligible for the estimation of total ecosystem stocks. In unburnt tundra, a large proportion of the aboveground C and N stocks was stored in reindeer lichens, which dominated the vegetation before fire (Heim et al., 2021a). As reindeer lichens recover very slowly, they were rare even several decades after fire (Frost et al., 2020; Heim et al., 2021a) and did not reach the C and N stocks of unburnt sites. Instead, C and N were stored in shrubs and bryophytes, a pattern that reflects the vegetation recovery (Frost et al., 2020). However, shrubs and bryophytes did not compensate for C and N loss in the burnt lichen biomass.
4.1 Fire affected long-term C and N cycling
Fire had a long-term effect on C and N concentrations and stable-isotope ratios in aboveground vegetation, especially lichens, which suggests a strong, long-lasting effect of fire on C and N cycling in the ecosystem. The long-term fire effects on nutrient cycling in soil were minor and most apparent in the topsoil.
Fire did not increase N concentrations in vascular plants. Originally, we expected that fire enhances mineralization rates, which releases previously unavailable N that can be taken up by vascular plants (Aerts, 2006; Salmon et al., 2016). However, our results do not support this hypothesis. On the contrary, we even found signs of nitrogen limitation in herbs and graminoids at the younger fire scars, as indicated by lower N concentration and higher ratios in the biomass. A possible explanation for this pattern may be linked to enhanced competition for nitrogen among vascular plants, which increased during post-fire succession (Bret-Harte et al., 2013; Heim et al., 2021a). Unburnt plots were dominated by lichens, which obtain large parts of their nutrients from the atmosphere (Asplund and Wardle, 2017) and thus did not compete for available soil N. Therefore, vascular plants at unburnt plots may have relatively more available soil N. Lichens reflected long-term impacts of fire on N cycling. We found high N concentrations on burnt plots of the youngest fire scar. The disappearance of this effect with time since fire might be related to the fact that younger lichens generally contain more N (Kytöviita and Crittenden, 2007).
Soil N concentrations were only increased in the upper soil layer of the oldest fire scar. This pattern is less likely linked to the temperature-mediated increased microbial activity as the soil temperature in the oldest fire scar recovered to control levels (Heim et al., 2021a). Rather, this might be explained by the increased cover of vascular plants, which produce more root exudates, have symbiotic nitrogen fixation, and lead to easily decomposable falloff litter (Maslov et al., 2018; McLaren et al., 2017).
The lower soil δ13C on burnt plots of the oldest fire scar (where soil temperatures returned to control levels) is probably linked to the altered vegetation composition with more shrubs and compound-specific variations in δ13C in litter. In plants, easily decomposing substances are comparatively enriched in 13C, while more refractory substances, such as lignin, are relatively depleted in 13C (Ågren et al., 1996). Increased shrub litter and a higher proportion of lignin at the oldest fire scar may thus result in a depletion of heavy 13C in the remaining organic matter of the soil (Ågren et al., 1996).
Why δ13C in vascular and lower plants on fire scars is decreased in our study remains relatively unclear as variations in δ13C are usually complex and not straightforward to interpret (Dawson et al., 2002). Therefore, our findings could not be related with certainty to a process described in the literature. One reason for the decreased δ13C might be the lower 13C content of the CO2 in the ambient air of fire scars. A lower 13C content of the CO2 can be explained by increased decomposition rates (Dawson et al., 2002; Lakatos et al., 2007). However, we could not detect a decrease in C stocks in our data that would allow the assumption of increased mineralization.
In our case, water availability may be a better explanation for the observed shifts towards lower 13C values in vegetation as increased water availability is common in post-fire permafrost landscapes (Holloway et al., 2020). This is in line with the frequently observed negative correlations of indicators for humidity and tree ring 13C (e.g. Holzkämper et al., 2012) as 13C reflects stomatal conductance as affected by moisture availability or drought stress. Dawson et al. (2002) state that “but unlike in vascular plants, delta tends to increase with water limitation in nonvascular plant taxa” (Williams and Flanagan, 1996, 1998). Our soil moisture data, however, do not support this, which might be due to the timing of sampling (soil moisture is also probably the most variable parameter in space and time). We did not find an effect of fire on the abundance of 15N in the biomass of vascular plants. This was surprising because we expected that 15N in leaves of vascular plants on younger fire scars would be increased, while it would decrease in later successional stages, through the progressing colonization with mycorrhizae (Hobbie et al., 2005).
The lack of an effect of past fires on δ15N may indicate that the belowground mycorrhizae remained relatively unaffected by fire, and the resprouting strategy of tundra shrubs facilitates the resilience of dominant fungi against tundra fires (Hewitt et al., 2013). However, on closer inspection of the shrub species Betula nana on the intermediate fire scar, we detected an enrichment of 15N in leaves of the burnt plots in comparison to plants on unburnt plots (Fig. S11 in the Supplement). This indicates that the shrubs on the fire scar may not have been re-colonized again by mycorrhizae (Hobbie et al., 2005). We probably did not find an overall effect of fire on shrubs because we included too many species in the analysis that interact with different mycorrhizae or no mycorrhiza at all, which makes a pattern unrecognizable. While fire had no significant effect on δ15N in vascular plants, the positive effect of fire on δ15N in lichens probably reflects the age of the thallus. Ellis et al. (2003) showed that there is a consistent pattern of δ15N distribution in the lichen thallus, with the highest δ15N content in the apices and a minimum located 2–4 cm below the apex. The high δ15N can thus be related to the fact that lichen thalli in burnt areas are younger and thus smaller in comparison to unburnt stands (Heim et al., 2021a).
While our results demonstrate that tundra fires do not generally reduce C and N stocks, we show that fire disturbance is an important long-term driver of C and N cycling in the subarctic tundra ecosystem of northern Siberia. We found that lichens play an important role in the storage of C and N in the aboveground biomass.
We did not find recovery to pre-fire conditions in terms of C and N cycling, even after more than 44 years.
Overall, our results strongly suggest that in lichen-dominated subarctic tundra ecosystems, the contribution of wildfires to the release of additional carbon to the atmosphere might be relatively small as soil stocks appear to be resilient.
The data and R-scripts that support the findings of this study are openly available in “Zenodo” at https://doi.org/10.5281/zenodo.5582683 (Heim et al., 2021b) and https://doi.org/10.5281/zenodo.5583511 (Heim et al., 2021c).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-19-2729-2022-supplement.
RJH was involved in conceptualisation, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualisation, and writing of the original draft; AY was involved in data curation, investigation, methodology, project administration, resources, validation, and review and editing; AB was involved in supervision, validation, and review and editing. WH was involved in investigation, validation, and review and editing. VK was involved in investigation. KHK was involved in methodology, resources, validation, and review and editing. CL was involved in formal analysis, investigation, validation, and review and editing. AP was involved in investigation. DS was involved in investigation. FS was involved in investigation. NH was involved in conceptualisation, funding acquisition, methodology, project administration, resources, supervision, validation, and review and editing.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We would like to thank Julia Aminova, Denis Bazyk, Leya Brodt, Marvin Diek, Bettina Haas, Liv Jessen, Olga Konkova, Ksenia Maryasicheva, Michael Moskowchenko, and Daniel Rieker for help with data collection. We are grateful to the Department for Science and Innovation of the Yamalo-Nenets Autonomous Okrug (Alexei Titovsky), the Interregional Expedition Center “Arctic” (Elena Nyukina), and the Arctic Research Center of the Yamalo-Nenets Autonomous Okrug (Andrey Lobanov) for the great financial and logistic support and fruitful cooperation. For the trustful collaboration, we also thank Andrei Tolstikov (University of Tyumen). The C and N analyses of this study were carried out in the laboratory of the Institute of Landscape Ecology of the University of Münster. The assistance of Ulrike Berning-Mader is greatly acknowledged.
This research has been supported by the Studienstiftung des Deutschen Volkes (scholarship grant) and the Westfälische Wilhelms-Universität Münster (Open Access Publication Fund of the University of Münster grant).
This paper was edited by Akihiko Ito and reviewed by two anonymous referees.
Aerts, R.: The freezer defrosting: global warming and litter decomposition rates in cold biomes, J. Ecol., 94, 713–724, 2006. a, b
Ågren, G. I., Bosatta, E., and Balesdent, J.: Isotope discrimination during decomposition of organic matter: a theoretical analysis, Soil Sci. Soc. Am. J., 60, 1121–1126, 1996. a, b
Asplund, J. and Wardle, D. A.: How lichens impact on terrestrial community and ecosystem properties, Biol. Rev., 92, 1720–1738, https://doi.org/10.1111/brv.12305, 2017. a
Blok, D., Faucherre, S., Banyasz, I., Rinnan, R., Michelsen, A., and Elberling, B.: Contrasting above- and belowground organic matter decomposition and carbon and nitrogen dynamics in response to warming in High Arctic tundra, Glob. Change Biol., 24, 2660–2672, 2018. a
Böhlke, J. K., Mroczkowski, S. J., and Coplen, T. B.: Oxygen isotopes in nitrate: New reference materials for 18O : 17O : 16O measurements and observations on nitrate-water equilibration, Rapid Commun. Mass Sp., 17, 1835–1846, 2003. a
Bret-Harte, M. S., Mack, M. C., Shaver, G. R., Huebner, D. C., Johnston, M., Mojica, C. A., Pizano, C., and Reiskind, J. A.: The response of Arctic vegetation and soils following an unusually severe tundra fire, Philos. T. Roy. Soc. B, 368, 20120490, https://doi.org/10.1098/rstb.2012.0490, 2013. a, b
Bürkner, P.-C.: brms: An R Package for Bayesian Multilevel Models Using Stan, J. Stat. Softw., 80, 1–28, https://doi.org/10.18637/jss.v080.i01, 2017. a, b
Bürkner, P.-C.: Advanced Bayesian Multilevel Modeling with the R Package brms, R J., 10, 395–411, https://doi.org/10.32614/RJ-2018-017, 2018. a, b
Cahoon, S. M. P., Sullivan, P. F., and Post, E.: Greater abundance of Betula nana and early onset of the growing season increase ecosystem CO2 uptake in West Greenland, Ecosystems, 19, 1149–1163, 2016. a
Chambers, S. D., Beringer, J., Randerson, J. T., and Chapin, I. S.: Fire effects on net radiation and energy partitioning: Contrasting responses of tundra and boreal forest ecosystems, J. Geophys. Res.-Atmos., 110, 1–9, https://doi.org/10.1029/2004JD005299, 2005. a
Chapin III, F. S., McGuire, A. D., Randerson, J., Pielke, R., Baldocchi, D., Hobbie, S. E., Roulet, N., Eugster, W., Kasischke, E., and Rastetter, E. B.: Arctic and boreal ecosystems of western North America as components of the climate system, Glob. Change Biol., 6, 211–223, 2000. a
Chen, Y., Hu, F. S., and Lara, M. J.: Divergent shrub-cover responses driven by climate, wildfire, and permafrost interactions in Arctic tundra ecosystems, Glob. Change Biol., 27, 652–663, 2021. a
Coplen, T. B., Brand, W. A., Gehre, M., Gröning, M., Meijer, H. A. J., Toman, B., and Verkouteren, R. M.: New guidelines for δ13C measurements, Anal. Chem., 78, 2439–2441, 2006. a
Dahl, E.: Stability of tundra ecosystems in Fennoscandia, in: Fennoscandian tundra ecosystems, Springer, 231–236, https://doi.org/10.1007/978-3-642-66276-8_29, 1975. a
Dawson, T. E., Mambelli, S., Plamboeck, A. H., Templer, P. H., and Tu, K. P.: Stable isotopes in plant ecology, Annu. Rev. Ecol. Syst., 33, 507–559, 2002. a, b, c, d, e, f
De Baets, S., Van de Weg, M. J., Lewis, R., Steinberg, N., Meersmans, J., Quine, T. A., Shaver, G. R., and Hartley, I. P.: Investigating the controls on soil organic matter decomposition in tussock tundra soil and permafrost after fire, Soil Biol. Biochem., 99, 108–116, 2016. a
Dormann, C. F. and Woodin, S. J.: Climate change in the Arctic: using plant functional types in a meta-analysis of field experiments, Funct. Ecol., 16, 4–17, 2002. a
Ehleringer, J. R., Buchmann, N., and Flanagan, L. B.: Carbon isotope ratios in belowground carbon cycle processes, Ecol. Appl., 10, 412–422, 2000. a
Ellis, C. J., Crittenden, P. D., Scrimgeour, C. M., and Ashcroft, C.: The natural abundance of 15N in mat-forming lichens, Oecologia, 136, 115–123, 2003. a
Estop-Aragonés, C., Olefeldt, D., Abbott, B. W., Chanton, J. P., Czimczik, C. I., Dean, J. F., Egan, J. E., Gandois, L., Garnett, M. H., and Hartley, I. P.: Assessing the Potential for Mobilization of Old Soil Carbon After Permafrost Thaw: A Synthesis of 14C Measurements From the Northern Permafrost Region, Global Biogeochem. Cy., 34, e2020GB006672, https://doi.org/10.1029/2020GB006672, 2020. a
Farquhar, G. D., Ehleringer, J. R., and Hubick, K. T.: Carbon isotope discrimination and photosynthesis, Annu. Rev. Plant Biol., 40, 503–537, 1989. a
Frost, G. V., Loehman, R. A., Saperstein, L. B., Macander, M. J., Nelson, P. R., Paradis, D. P., and Natali, S. M.: Multi-decadal patterns of vegetation succession after tundra fire on the Yukon-Kuskokwim Delta, Alaska, Environ. Res. Lett., 15, 25003, https://doi.org/10.1088/1748-9326/ab5f49, 2020. a, b
Gorelick, N., Hancher, M., Dixon, M., Ilyushchenko, S., Thau, D., and Moore, R.: Google Earth Engine: Planetary-scale geospatial analysis for everyone, Remote Sens. Environ., 202, 18–27, 2017. a
He, J., Chen, D., Jenkins, L., and Loboda, T. V.: Impacts of wildfire and landscape factors on organic soil properties in Arctic tussock tundra, Environ. Res. Lett., 16, 085004, https://doi.org/10.1088/1748-9326/ac1192, 2021. a, b
Heim, R. J., Bucharova, A., Brodt, L., Kamp, J., Rieker, D., Soromotin, A. V., Yurtaev, A., and Hoelzel, N.: Post-fire vegetation succession in the Siberian subarctic tundra over 45 years, Sci. Total Environ., 760, 143425, https://doi.org/10.1016/j.scitotenv.2020.143425, 2021a. a, b, c, d, e, f, g, h, i, j, k, l
Heim, R. J., Yurtaev, A., Bucharova, A., Heim, W., Kutskir, V., Knorr, K.-H., Lampei, C., Pechkin, A., Schilling, D., Sulkarnaev, F., and Hoelzel, N.: Dataset for “Fire in lichen-rich subarctic tundra changes carbon and nitrogen cycling between ecosystem compartments but has minor effects on stocks”, Zenodo [data set], https://doi.org/10.5281/zenodo.5582683, 2021b. a
Heim, R. J., Yurtaev, A., Bucharova, A., Heim, W., Kutskir, V., Knorr, K.-H., Lampei, C., Pechkin, A., Schilling, D., Sulkarnaev, F., and Hoelzel, N.: R-Code for “Fire in lichen-rich subarctic tundra changes carbon and nitrogen cycling between ecosystem compartments but has minor effects on stocks”, Zenodo [code], https://doi.org/10.5281/zenodo.5583511, 2021c. a
Hewitt, R. E., Bent, E., Hollingsworth, T. N., Chapin III, F. S., and Taylor, D. L.: Resilience of Arctic mycorrhizal fungal communities after wildfire facilitated by resprouting shrubs, Ecoscience, 20, 296–310, 2013. a
Hobbie, E. A., Jumpponen, A., and Trappe, J.: Foliar and fungal 15N : 14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: testing analytical models, Oecologia, 146, 258–268, 2005. a, b, c
Holloway, J. E., Lewkowicz, A. G., Douglas, T. A., Li, X., Turetsky, M. R., Baltzer, J. L., and Jin, H.: Impact of wildfire on permafrost landscapes: A review of recent advances and future prospects, Permafrost Periglac., 31, 371–382, 2020. a
Holzkämper, S., Tillman, P. K., Kuhry, P., and Esper, J.: Comparison of stable carbon and oxygen isotopes in Picea glauca tree rings and Sphagnum fuscum moss remains from subarctic Canada, Quaternary Res., 78, 295–302, 2012. a
Hu, F. S., Higuera, P. E., Duffy, P., Chipman, M. L., Rocha, A. V., Young, A. M., Kelly, R., and Dietze, M. C.: Arctic tundra fires: Natural variability and responses to climate change, Front. Ecol. Environ., 13, 369–377, https://doi.org/10.1890/150063, 2015. a
IUSS Working Group WRB: World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps, World Soil Resources Reports, No. 106, FAO, Rome, ISBN 978-92-5-108369-7, 2015. a
Jandt, R., Joly, K., Meyers, C. R., and Racine, C.: Slow Recovery of Lichen on Burned Caribou Winter Range in Alaska Tundra: Potential Influences of Climate Warming and Other Disturbance Factors, Arct. Antarct. Alp. Res., 40, 89–95, https://doi.org/10.1657/1523-0430(06-122)[JANDT]2.0.CO;2, 2008. a
Jansson, J. K. and Hofmockel, K. S.: Soil microbiomes and climate change, Nat. Rev. Microbiol., 18, 35–46, https://doi.org/10.1038/s41579-019-0265-7, 2020. a
Jiang, Y., Rocha, A. V., O'Donnell, J. A., Drysdale, J. A., Rastetter, E. B., Shaver, G. R., and Zhuang, Q.: Contrasting soil thermal responses to fire in Alaskan tundra and boreal forest, J. Geophys. Res.-Earth, 120, 363–378, https://doi.org/10.1002/2014jf003180, 2015. a
Jonasson, S., Chapin III, F. S., and Shaver, G. R.: Biogeochemistry in the Arctic: patterns, processes, and controls, in: Global biogeochemical cycles in the climate system, Elsevier, 139–150, https://doi.org/10.1016/B978-012631260-7/50012-1, 2001. a
Kazakov, K.: Spravochno-informatsionnyy portal “Pogoda i klimat” [Reference information portal “Weather and Climate”], http://www.pogodaiklimat.ru/msummary.php?m=all&y=all&id=23256, last access: 2 May 2019. a, b
Korner-Nievergelt, F., Roth, T., Felten, S. V., Guelat, J., Almasi, B., and Korner-Nievergelt, P.: Bayesian Data Analysis in Ecology using Linear Models with R, BUGS and Stan, Elsevier, London, ISBN 978-0-12-801370-0, 2015. a
Kornienko, S. G.: Cartography of pyrogenic violations of the vegetation cover on the Tazovsky Peninsula with satellite data, Actual Problems of Oil and Gas, 1, 1–11, 2018. a
Kytöviita, M.-M. and Crittenden, P. D.: Growth and nitrogen relations in the mat-forming lichens Stereocaulon paschale and Cladonia stellaris, Ann. Bot.-London, 100, 1537–1545, 2007. a
Lakatos, M., Hartard, B., and Máguas, C.: The stable isotopes δ13C and δ18O of lichens can be used as tracers of microenvironmental carbon and water sources, Terrestrial Ecology, 1, 77–92, 2007. a
Lasslop, G., Hantson, S., Harrison, S. P., Bachelet, D., Burton, C., Forkel, M., Forrest, M., Li, F., Melton, J. R., and Yue, C.: Global ecosystems and fire: multi-model assessment of fire-induced tree cover and carbon storage reduction, Glob. Change Biol., 26, 5027–5041, https://doi.org/10.1111/gcb.15160, 2020. a
Longton, R. E.: The role of bryophytes and lichens in polar ecosystems, Special Publication-British Ecological Society, 13, 69–96, 1997. a
Loranty, M. M., Natali, S. M., Berner, L. T., Goetz, S. J., Holmes, R. M., Davydov, S. P., Zimov, N. S., and Zimov, S. A.: Siberian tundra ecosystem vegetation and carbon stocks four decades after wildfire, J. Geophys. Res.-Biogeo., 119, 2144–2154, https://doi.org/10.1002/2014jg002730, 2014. a, b
Mack, M. C., Schuur, E. A. G., Bret-Harte, M. S., Shaver, G. R., and Chapin, F. S.: Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization, Nature, 431, 440–443, 2004. a
Mack, M. C., Bret-Harte, M. S., Hollingsworth, T. N., Jandt, R., Schuur, E. A., Shaver, G. R., and Verbyla, D. L.: Carbon loss from an unprecedented Arctic tundra wildfire, Nature, 475, 489–492, https://doi.org/10.1038/nature10283, 2011. a, b, c, d, e, f, g, h, i
Marion, G. M., Bockheim, J. G., and Brown, J.: Arctic soils and the ITEX experiment, Glob. Change Biol., 3, 33–43, 1997. a
Maslov, M. N., Maslova, O. A., Pozdnyakov, L. A., and Kopeina, E. I.: Biological activity of soils in mountain tundra ecosystems under postpyrogenic restoration, Eurasian Soil Sci.+, 51, 692–700, 2018. a, b
Maslov, M. N., Maslova, O. A., and Kopeina, E. I.: Changes in the pools of total and labile soil organic carbon during post-fire succession in the Khibiny Mountain tundra ecosystems, Eurasian Soil Sci.+, 53, 330–338, 2020. a
McLaren, J. R., Buckeridge, K. M., van de Weg, M. J., Shaver, G. R., Schimel, J. P., and Gough, L.: Shrub encroachment in Arctic tundra: Betula nana effects on above-and belowground litter decomposition, Ecology, 98, 1361–1376, 2017. a
Mollicone, D., Eva, H. D., and Achard, F.: Ecology: Human role in Russian wild fires, Nature, 440, 436, 2006. a
Moritz, M. A., Parisien, M.-A., Batllori, E., Krawchuk, M. A., Van Dorn, J., Ganz, D. J., and Hayhoe, K.: Climate change and disruptions to global fire activity, Ecosphere, 3, 1–22, 2012. a
Narita, K., Harada, K., Saito, K., Sawada, Y., Fukuda, M., and Tsuyuzaki, S.: Vegetation and Permafrost Thaw Depth 10 Years after a Tundra Fire in 2002, Seward Peninsula, Alaska, Arct. Antarct. Alp. Res., 47, 547–559, https://doi.org/10.1657/AAAR0013-031, 2015. a
Oulehle, F., Rowe, E. C., Myška, O., Chuman, T., and Evans, C. D.: Plant functional type affects nitrogen use efficiency in high-Arctic tundra, Soil Biol. Biochem., 94, 19–28, 2016. a, b
Peterson, B. J. and Fry, B.: Stable isotopes in ecosystem studies, Annu. Rev. Ecol. Syst., 18, 293–320, 1987. a
Post, E., Alley, R. B., Christensen, T. R., Macias-Fauria, M., Forbes, B. C., Gooseff, M. N., Iler, A., Kerby, J. T., Laidre, K. L., and Mann, M. E.: The polar regions in a 2 ∘C warmer world, Science Advances, 5, eaaw9883, https://doi.org/10.1126/sciadv.aaw9883, 2019. a
R Core Team: R: A Language and Environment for Statistical Computing, Vienna, Austria, http://www.r-project.org/index.html (last access: 24 May 22), 2020. a
Rinnan, R., Michelsen, A., Bååth, E., and Jonasson, S.: Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem, Glob. Change Biol., 13, 28–39, 2007. a
Rosen, Y.: Warming, fires, warming, fires: How tundra wildfires could create an unstoppable cycle, https://www.adn.com/alaska- news/science/2016/05/30/warming-fires-warming-fires-how- tundra-wildfires-could-create-an-unstoppable-cycle/ (last access: 24 May 2022), 2017. a
Salmon, V. G., Soucy, P., Mauritz, M., Celis, G., Natali, S. M., Mack, M. C., and Schuur, E. A. G.: Nitrogen availability increases in a tundra ecosystem during five years of experimental permafrost thaw, Glob. Change Biol., 22, 1927–1941, 2016. a, b, c
Sancho, L. G., Belnap, J., Colesie, C., Raggio, J., and Weber, B.: Carbon budgets of biological soil crusts at micro-, meso-, and global scales, in: Biological soil crusts: an organizing principle in drylands, Springer, 287–304, https://doi.org/10.1007/978-3-319-30214-0_15, 2016. a
Schuur, E. A. G., Trumbore, S. E., Mack, M. C., and Harden, J. W.: Isotopic composition of carbon dioxide from a boreal forest fire: Inferring carbon loss from measurements and modeling, Global Biogeochem. Cy., 17, 1, 2003. a
Schuur, E. A. G., Vogel, J. G., Crummer, K. G., Lee, H., Sickman, J. O., and Osterkamp, T. E.: The effect of permafrost thaw on old carbon release and net carbon exchange from tundra, Nature, 459, 556–559, 2009. a
Schuur, E. A. G., McGuire, A. D., Schädel, C., Grosse, G., Harden, J. W., Hayes, D. J., Hugelius, G., Koven, C. D., Kuhry, P., and Lawrence, D. M.: Climate change and the permafrost carbon feedback, Nature, 520, 171–179, 2015. a, b
Turetsky, M. R.: The role of bryophytes in carbon and nitrogen cycling, Bryologist, 106, 395–409, 2003. a
Turetsky, M. R., Bond-Lamberty, B., Euskirchen, E., Talbot, J., Frolking, S., McGuire, A. D., and Tuittila, E.: The resilience and functional role of moss in boreal and arctic ecosystems, New Phytol., 196, 49–67, 2012. a
Turetsky, M. R., Benscoter, B., Page, S., Rein, G., Van Der Werf, G. R., and Watts, A.: Global vulnerability of peatlands to fire and carbon loss, Nat. Geosci., 8, 11–14, https://doi.org/10.1038/ngeo2325, 2015. a
U.S. Geological Survey: Earth Explorer, https://earthexplorer.usgs.gov/ (last access: 24 May 2022), 2018. a
Veraverbeke, S., Delcourt, C. J. F., Kukavskaya, E., Mack, M., Walker, X., Hessilt, T., Rogers, B., and Scholten, R. C.: Direct and longer-term carbon emissions from arctic-boreal fires: a short review of recent advances, Current Opinion in Environmental Science and Health, 23, 100277, https://doi.org/10.1016/j.coesh.2021.100277, 2021. a
Vilchek, G. E. and Bykova, O. Y.: The origin of regional ecological problems within the northern Tyumen Oblast, Russia, Arctic Alpine Res., 24, 99–107, 1992. a
Williams, T. G. and Flanagan, L. B.: Effect of changes in water content on photosynthesis, transpiration and discrimination against 13CO2 and C18O16O in Pleurozium and Sphagnum, Oecologia, 108, 38–46, 1996. a
Williams, T. G. and Flanagan, L. B.: Measuring and modelling environmental influences on photosynthetic gas exchange in Sphagnum and Pleurozium, Plant Cell Environ., 21, 555–564, 1998. a
Young, A. M., Higuera, P. E., Duffy, P. A., and Hu, F. S.: Climatic thresholds shape northern high-latitude fire regimes and imply vulnerability to future climate change, Ecography, 39, 1–12, 2016. a
Yu, Q., Epstein, H. E., Engstrom, R., Shiklomanov, N., and Strelestskiy, D.: Land cover and land use changes in the oil and gas regions of Northwestern Siberia under changing climatic conditions, Environ. Res. Lett., 10, 124020, https://doi.org/10.1088/1748-9326/10/12/124020, 2015. a
Yurkovskaya, T. K.: Vegetation map [Karta rastitel'nosti], in: National Atlas of Soils of the Russian Federation [Natsional'nyy atlas pochv Rossiyskoy Federatsii], edited by: Shoba, S. A., Alyabina, I. O., Urusevskaya, I. S., and Chernova, O. V., Moscow State University, Moscow, 46–48, https://doi.org/10.31111/geobotmap/1984.3, 2011. a





