the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Reviews and syntheses: Iron – a driver of nitrogen bioavailability in soils?
Xia Zhu-Barker
Patricia Lazicki
William Horwath
An adequate supply of bioavailable nitrogen (N) is critical to soil microbial communities and plants. Over the last decades, research efforts have rarely considered the importance of reactive iron (Fe) minerals in the processes that produce or consume bioavailable N in soils compared to other factors such as soil texture, pH, and organic matter (OM). However, Fe is involved in both enzymatic and non-enzymatic reactions that influence the N cycle. More broadly, reactive Fe minerals restrict soil organic matter (SOM) cycling through sorption processes but also promote SOM decomposition and denitrification in anoxic conditions. By synthesizing available research, we show that Fe plays diverse roles in N bioavailability. Fe affects N bioavailability directly by acting as a sorbent, catalyst, and electron transfer agent or indirectly by promoting certain soil features, such as aggregate formation and stability, which affect N turnover processes. These roles can lead to different outcomes in terms of N bioavailability, depending on environmental conditions such as soil redox shifts during wet–dry cycles. We provide examples of Fe–N interactions and discuss the possible underlying mechanisms, which can be abiotic or microbially meditated. We also discuss how Fe participates in three complex phenomena that influence N bioavailability: priming, the Birch effect, and freeze–thaw cycles. Furthermore, we highlight how Fe–N bioavailability interactions are influenced by global change and identify methodological constraints that hinder the development of a mechanistic understanding of Fe in terms of controlling N bioavailability and highlight the areas of needed research.
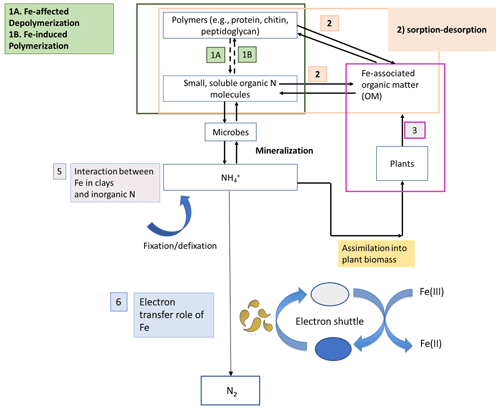
Nitrogen (N) bioavailability is a critical limiting factor for terrestrial ecosystem productivity (Vitousek and Howarth, 1991). The largest pool of N in these ecosystems is found in soils which contain 133–140 Pg of total N globally within the first top 100 cm of soil (Batjes, 1996). A clear description of the factors controlling N bioavailability in soils is needed to design agricultural practices that meet crop demand and mitigate N loss to the environment. A large range of literature exists regarding the effects of soil texture, organic matter (OM), mineral N inputs, pH, moisture, and microbial communities on N mineralization. However, emerging theories on soil organic matter (SOM) dynamics are increasingly emphasizing the role of soil mineralogy (Cotrufo et al., 2013; Lehmann and Kleber, 2015; Blankinship et al., 2018; Daly et al., 2021; Whalen et al., 2022). While these reviews have largely focused on carbon (C) cycling, the role of minerals is rarely considered in N cycling (Jilling et al., 2018). Since C and N cycles are interconnected in soils (Gärdenäs et al., 2011; Feng et al., 2019), they should be regulated by the same factors, including mineralogy type (Wade et al., 2018). Increasing evidence shows that Fe specifically represents a major control over N biological transformations, including mineralization (Wade et al., 2018), nitrification (X. Huang et al., 2016; Han et al., 2018), and denitrification (Wang et al., 2016; Zhu et al., 2014), as well as their abiotic analogous reactions, such as chemo-denitrification (Burger and Venterea, 2011) and Fe-mediated hydroxylamine (NH2OH) oxidation to nitrous oxide (N2O) (Bremner et al., 1980). These reactions and others (Fig. 1) are likely to operate ubiquitously in soils due to the close proximity between Fe minerals and SOM since most of the latter is contained in association with the former (Wagai and Mayer, 2007; Lalonde et al., 2012).
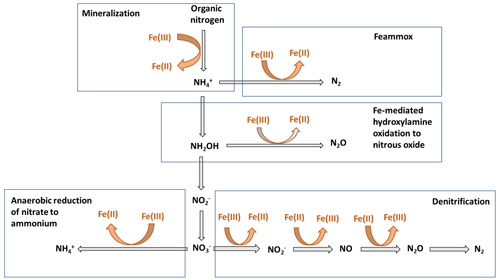
Figure 1Schematic representation depicting the different stages of the N cycle in which Fe plays a role. Adapted from Zhu-Barker et al. (2015).
The characteristic properties of individual Fe minerals and N compounds and how these properties are influenced by the soil environment likely drive the aforementioned reactions as well. First, Fe exists in a variety of polymorphs (Navrotsky et al., 2008) and is a redox-sensitive element that cycles between Fe(II) and Fe(III) states, as controlled by soil Eh and pH. While Fe(III) promotes N stabilization within mineral associations, Fe(III) mobilization, when it is reduced to Fe(II), can release N into solution. Fe reactivity is also driven by the amount and sign of surface charge, surface topography, particle size, crystallinity (Petridis et al., 2014; Li et al., 2015a), and the presence and type of organic matter (OM) coverage (Kaiser and Zech, 2000a; Kleber et al., 2007; Boland et al., 2014; Henneberry et al., 2016; Daugherty et al., 2017; Gao et al., 2018; Poggenburg et al., 2018). Second to this, soil N exists predominantly in organic forms (ON), mostly as proteins and peptides and, to a lesser extent, as amino-sugars and nucleic acids (Schulten and Schnitzer, 1997; Kögel-Knabner, 2006; Knicker, 2011). Proteins are intrinsically reactive towards soil minerals due to a number of properties, including hydrophobicity, surface charge distribution, surface area, number and type of functional groups, conformation, and size (Lützow et al., 2006). N from these compounds is generally not directly bioavailable due to molecular size constraints on microbial cell uptake (Schimel and Bennett, 2004). Depolymerization reactions, carried out by the activity of extracellular enzymes, such as peptidases, transform these polymers into soluble, low-molecular-weight organic monomers (e.g., short oligopeptides, amino acids). Recent research shows that the size of amino acids available for mineralization is controlled by peptidase activity but more so by protein availability, both of which are affected by the interactions with Fe minerals. Therefore, Fe may drive gross amino acid production in soils (Noll et al., 2019).
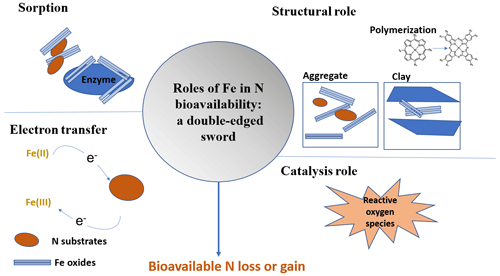
Therefore, the impact of Fe on N cycling can be significant; thus, our aim here is to review the role of Fe in controlling N bioavailability. To do so, we categorize the processes by which Fe affects OM dynamics into four different categories or roles (Fig. 2). In the sorbent role, OM interacts with Fe(III) through adsorption, co-precipitation, or surface coatings (Eusterhues et al., 2005; Wagai and Mayer, 2007; Lalonde et al., 2012). These associations increase OM storage by decreasing its availability to extracellular enzymes and decomposition processes (Lalonde et al., 2012). In fact, the content of Fe minerals is a major predictor of soil sorptive capacity (Mayes et al., 2012). In the structural role, Fe minerals participate in the formation of soil aggregates (X. W. Zhang et al., 2016) and increase soil structural stability (Barral et al., 1998; Xue et al., 2019). Aggregates can increase OM stability and retention in soils by protecting it from the decomposer community and their enzymes (Van Veen and Kuikman, 1990; Kleber et al., 2021). Moreover, Fe(III) can facilitate the formation of large polymers of OM that promote its stability. Thirdly, Fe's electron transfer role depends on its oxidation state. Fe(III) serves as a sink of electrons, while Fe(II) functions as a source of electrons. During anoxic periods, dissimilatory Fe(III) reduction can be coupled with the oxidation of OM, which accounts for a significant amount of C loss under anoxic conditions (Roden and Wetzel, 1996; Dubinsky et al., 2010). This process can release previously adsorbed or coprecipitated C, thereby increasing its susceptibility to degradation. Finally, Fe has a catalysis role, whereby Fe acts as a catalyst for the production of reactive oxygen species (ROSs) that are potent oxidants of OM. This happens through Fenton reactions that are prevalent in various soils such as cultivated soils (Hall and Silver, 2013; Chen et al., 2020), arctic soils (Trusiak et al., 2018) and desert soils (Hall et al., 2012; Georgiou et al., 2015). These reactions are an overlooked but potentially important pathway for OM transformation in soils and sediments and N bioavailability (Lipson et al., 2010; Wang et al., 2017; Trusiak et al., 2018; Merino et al., 2020; Kleber et al., 2021).
While these roles of Fe in controlling C cycling have been studied extensively, their effects on N bioavailability are not well explored. This review seeks to underpin these suggested relationships and provide mechanistic descriptions of how Fe controls N bioavailability in soils. Moreover, we detail how Fe participates in three complex phenomena that influence N bioavailability: priming, the Birch effect, and freeze–thaw cycles. Finally, we explore how Fe–N bioavailability interactions are influenced by global change. This information is needed to construct reliable models with improved predictive power of N cycling in terrestrial ecosystems (Wade et al., 2018) and will offer new possibilities for land management.
2.1 Sorbent role
This section aims to elucidate how Fe as a sorbent interacts with both inorganic N (ammonium – , nitrate – ) and ON and how these interactions modulate N bioavailability. The opposite process of sorption, known as desorption, can affect N bioavailability since sorbed N can be released back into the soil solution. By comprehending the dynamics of both the sorption and desorption of N onto and from Fe minerals, we can gain more insights into mechanisms affecting its bioavailability in soils.
2.1.1 ON bioavailability
Soil microbes rely heavily on their ability to access ON and the effectiveness of their extracellular enzymes in breaking it down into assimilable forms. Fe minerals can modulate N bioavailability by controlling both ON accessibility and extracellular-enzyme activity through sorption. Nevertheless, the outcomes in terms of N bioavailability are variable and depend on several factors, including soil conditions; the interplay between ON, extracellular enzymes, and Fe mineral surfaces; and the potential for desorption to occur. Here, we discuss these intricate interactions which can result in distinct patterns of N bioavailability across soils.
Does extracellular-enzyme sorption to Fe oxides affect their participation in N mineralization?
Soil microbes produce various types of extracellular enzymes, including substrate-specific enzymes (e.g., proteases and aminopeptidases) and non-specific oxidative enzymes (e.g., laccase and peroxidase), to acquire N (Caldwell, 2005; Sinsabaugh et al., 2009; Hassan et al., 2013). While the latter enzymes are typically associated with C cycling, their importance for N mineralization has also been demonstrated (Zhu et al., 2014; Kieloaho et al., 2016). Many of these enzymes become adsorbed to Fe minerals when released in soil. The effect of such immobilization on enzyme activities and consequent N bioavailability remains uncertain due to conflicting reports in the literature. Numerous studies have documented a decrease in enzyme activity combined with increased persistence in soil and resistance to proteolysis (Sarkar and Burns, 1984; Gianfreda et al., 1995; Bayan and Eivazi, 1999; Rani et al., 2000; Tietjen and Wetzel, 2003; Kelleher et al., 2004; Yan et al., 2010; Schimel et al., 2017; Li et al., 2020), while others have reported opposing effects (Quiquampoix and Ratcliffe, 1992; Quiquampoix et al., 1995; Servagent-Noinville et al., 2000). For instance, Fe adsorption reduced the activity of urease (Gianfreda et al., 1995; Bayan and Eivazi, 1999; Li et al., 2020) but increased the activity of N-acetyl-glucosaminidase (NAG) (Allison, 2006; Olagoke et al., 2020). These contradicting effects can have multiple explanations. First, enzyme-active sites can become occluded upon adsorption, limiting the diffusion of ON towards the binding sites and lower ON decomposition. Site occlusion can be caused by conformational changes in the enzyme structure (Datta et al., 2017), Fe-induced aggregation (Olagoke et al., 2020), or unfavorable attachment orientation on mineral surfaces (Baron et al., 1999; Yang et al., 2019). Second, Fe oxides can inhibit the activity of extracellular enzymes by constraining ON availability. Along a chronosequence of 120 kyr, Turner et al. (2014) found that Fe oxides inhibited the activities of urease and proteases more strongly than aminopeptidases, possibly due to the preferential adsorption of urea and proteins over peptides (Turner et al., 2014). Third, enzyme activity may be influenced by soil mineral content, as observed by (Olagoke et al., 2020). In mineral-poor soils, enzymes may have higher and more persistent activity due to limited adsorption sites, potentially leading to improved microbial C and N use efficiencies by allowing microbes to invest in biomass production instead of enzyme production. Finally, a new mechanism was proposed by Chacon et al. (2019) where Fe minerals, specifically goethite, can induce abiotic protein fragmentation with subsequent loss of activity (Chacon et al., 2019), but further investigation is needed to determine its occurrence in soil and its implications for enzyme activity and N bioavailability. Beyond sorption, the reduced metals that are prevalent in waterlogged soil have been found to exert an inhibitory or stimulating effect on enzyme activity. Specifically, Fe(II) stimulated the activity of oxidative enzymes (Van Bodegom et al., 2005; Sinsabaugh, 2010) but strongly inhibited that of urease (Gotoh and Patrick, 1974; Tabatabai, 1977; Pulford and Tabatabai, 1988; Gu et al., 2019). Amidase activity, on the other hand, appeared to be unaffected by waterlogging (Pulford and Tabatabai, 1988). In light of the current state of knowledge, there is a need for more research to comprehend how the intricate relationships between Fe minerals and enzymes regulate N bioavailability in soils.
Does the sorption of ON to Fe oxides affect their bioavailability?
Many studies have demonstrated that poorly crystalline Fe minerals, such as ferrihydrite, control the sorption of ON in soils (Kaiser and Zech, 2000b; Dümig et al., 2012; Keiluweit et al., 2012; Dippold et al., 2014). Indeed, Fe minerals interact with a wide range of N-containing moieties via adsorption or co-precipitation processes. The latter process is particularly important in organic N stabilization as it incorporates N into the pool of mineral-associated OM (MAOM) (Leinweber and Schulten, 2000; Keiluweit et al., 2012; Swenson et al., 2015; Heckman et al., 2018; Zhao et al., 2020). During these processes, Fe can form strong chemical bonds with N-containing moieties; for instance, goethite forms a stronger bond with the SOM functional group ammonia () than with carboxylate, phosphate, or methyl groups (Newcomb et al., 2017). The bond strength between N and mineral surfaces varies considerably across different environments due to differences in binding mechanisms, mineral and N properties, soil properties such as pH and ion strength, and the presence of antecedent SOM on mineral surfaces (Lützow et al., 2006). However, protein may adsorb irreversibly to mineral surfaces over a wide range of solution pH levels and resist desorption (Hlady and Buijs, 1996; Yu et al., 2013); desorption is perceived to be a necessary step for extracellular enzymes to proceed with N mineralization. Similarly, nucleic acid molecules persist for a long time on clay minerals (Yu et al., 2013) and are shielded from degradation.
Advances in spectroscopic techniques have generated new conceptual models of organo-mineral associations, such as the zonal structure model of organo-mineral associations, which postulates that organic compounds self-organize on mineral particle surfaces (Kleber et al., 2007). In this model, amphiphilic SOM compounds with N-bearing and oxidized functional groups directly interact with mineral surfaces to form the contact zone, whereas hydrophobic groups face outwards, creating a region of high hydrophobicity, the hydrophobic zone. Additional organic molecules attach to this zone, forming an outer layer termed the kinetic zone. Multiple recent observations support this model, including (1) the preferential enrichment of N-containing moieties on Fe mineral surfaces (Kopittke et al., 2018; Possinger et al., 2020); (2) the preferential adsorption of N compounds over other organic compound classes on Fe mineral surfaces (Gao et al., 2017); and (3) the partial sorption of some organic compounds, including amino acids, to Fe minerals (Amelung et al., 2002; Dippold et al., 2014). This model has implications for N bioavailability because, in contrast to the contact zone, the weakly sorbed N in the kinetic zone likely exchanges with soil solution and is more available. Recent research on the chemical composition of C and N at the organo-organic and organo-mineral interfaces of the model found that alkyl C and less N occurred at the former, whereas oxidized C and more N occurred at the latter (Possinger et al., 2020). The authors of this study hypothesized that the processes stabilizing C and N at these interfaces are different, considering that the association between SOM rich in -alkyl C and Fe oxides explained the stabilization of -alkyl C in soils (Schöning et al., 2005). In addition to protecting a fraction of bioavailable N, Vogel et al. (2014, 2015) found that sorption can retard the movement of N in soils, thereby increasing N retention by decreasing its accessibility to degradation mechanisms. More insight is needed to advance the understanding of ON bioavailability from organo-mineral associations.
Desorption of ON from Fe minerals
The desorption of ON from Fe-organic associations occurs due to several destabilization mechanisms, including surface displacement by competitive sorption, oxidative and reductive dissolution of Fe minerals (Kleber et al., 2015), and local disequilibrium in soil chemistry. Once released, ON may become accessible to microbial degradation or diffuse into microbial cells. The following is a discussion of the different destabilization mechanisms of Fe-organic associations in soils and factors influencing them:
- a.
ON desorption by oxidation and reductive dissolution of Fe minerals
The oxidation and the reduction of Fe minerals in response to variations in soil pH and redox conditions can significantly compromise the stability of Fe–organo associations and release Fe and OM into the soil solution as a consequence. In fact, Fe reduction can decrease mineral sorption capacity and release Fe ions, which can lower soil pH, and promote the solubilization of Fe–mineral associations. However, the extent of OM mobilized remains unpredictable due to knowledge gaps related to mechanisms of mineral resistance to reduction and their controlling factors in soils. For instance, short-range-order (SRO) Fe oxides can resist both chemical and microbial reduction due to co-precipitation of Fe with SRO aluminosilicates or physical protection within microaggregates (Henneberry et al., 2012; Shimizu et al., 2013; Eusterhues et al., 2014; Filimonova et al., 2016; Suda and Makino, 2016; Coward et al., 2018; Tamrat et al., 2019). Conversely, Fe oxidation can also solubilize Fe–organo associations by decreasing pH or generating hydroxyl radicals through Fenton chemistry, which oxidize OM abiotically. In addition to these effects, redox alterations to mineral properties can also affect OM cycling. For instance, the transformation of amorphous Fe minerals to more crystalline forms can promote long-term OM stabilization and decrease its turnover rates (Hall et al., 2018) since crystalline forms are more resistant to reduction. Chen et al. (2020) also found that crystalline forms were not associated with C release from Fe associations (Chen et al., 2020).
- b.
N desorption by local disequilibrium in soil chemistry
OM in soils can be desorbed from mineral surfaces due to the establishment of local disequilibrium conditions. Such conditions result from depletion of dissolved organic matter (DOM) in the soil solution due to microbial uptake, for example, promoting the release of OM from MAOM until DOM concentrations in the soil solution are in equilibrium with sorbed OM. This process is likely affected by the strength of bonds between N and Fe minerals; in fact, interaction forces vary considerably: strong interactions are favored by polyvalent cation bridges and ligand exchange, whereas weak interactions occur by hydrogen bonds or van der Waals forces (Kleber et al., 2015). While the relationship between particular binding mechanisms and N desorption from minerals has not yet been established in real soil conditions, multiple studies in model systems demonstrated that OM bound by ligand exchange was more resistant to desorption than other mechanisms (Wang and Lee, 1993; Gu et al., 1994, 1995; Mikutta et al., 2007). Therefore, it is likely to be less affected by the dynamic-equilibria principle, and less N will be made available (Kleber et al., 2015).
- c.
N desorption by surface displacement via competitive sorption
ON associated with Fe can be displaced by the input of highly sorptive organic compounds. For instance, Scott and Rothstein (2014) observed that weakly bound, N-rich hydrophilic compounds were easily displaced by stronger binding compounds (e.g., hydrophobic compounds), leading to the downward migration of N to subsurface and mineral horizons.
- d.
Is desorption of N from organo-mineral associations a prerequisite to N mineralization?
As mentioned earlier, desorption of protein from mineral surfaces is often perceived to be the primary pathway by which ON becomes accessible to microbial degradation (Schimel and Bennett, 2004). However, protein adsorption to Fe minerals is an irreversible process (Rabe et al., 2011), which restricts proteolytic activity. Recently, the direct proteolysis of protein at the mineral surface was investigated, as ferrihydrite- and goethite-adsorbed protein was found to be degraded without prior desorption (Tian et al., 2020). Substrate–enzyme complexes were formed directly at the surface of minerals. Together with the zonal structure of organo-mineral associations, this finding challenges the long-standing assumption that Fe minerals impair protein bioavailability through acting as a sorbent. The reader is referred to Keiluweit and Kuyper (2020) for a more expanded discussion of this mechanism (Keiluweit and Kuyper, 2020).
2.1.2 Inorganic N bioavailability
Fe plays a crucial role in regulating inorganic N bioavailability in soils, with Fe present in clay minerals being a prominent example of this interconnection. In fact, Fe present in clays accounts for 30 %–50 % of total Fe in soils and sediments and can be located in both the octahedral and tetrahedral sheets of 1:1 and 2:1 clay minerals or exist as a coating on their surfaces (Favre et al., 2006; Stucki, 2013). On the one hand, the reduction of this Fe has been demonstrated to enable the abiotic fixation of (Zhang and Scherer, 2000; Deroo et al., 2021). This occurs through the increasing negative charge and cation exchange capacity of clays (Pentráková et al., 2013). In addition, the reductive dissolution of coated Fe on clay minerals promotes diffusion into or out of clay interlayers (Zhang and Scherer, 2000). After de-fixation, the fixed pool can serve as a source of bioavailable N (Deroo et al., 2021). On the other hand, the oxidation of clay's Fe(II) can be involved in the processes that cause the loss of bioavailable N. For instance, Zhao et al. (2013) found that the oxidation of Fe(II) present in nontronite causes the loss of as dinitrogen (N2) (Zhao et al., 2013). The potential importance of such processes in N bioavailability should be considered, especially in highly weathered soils with high clay content.
2.2 Structural role of Fe in controlling N bioavailability
This section explores the structural role of Fe in regulating the availability of N. Two aspects are examined, namely the influence of structural Fe in aggregates on N bioavailability and the polymerization of ON induced by Fe.
2.2.1 Fe, soil aggregates, and N bioavailability
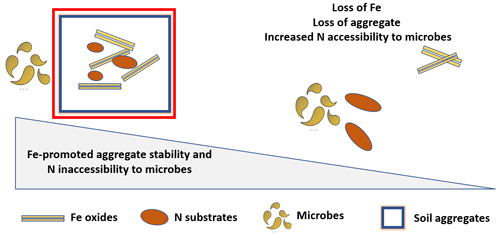
Fe oxides play a critical role in the formation and the stability of microaggregate in soils, ultimately affecting N bioavailability. These oxides serve as nuclei for microaggregate formation and as a binding agent, forming bridges between them (Barral et al., 1998; Pronk et al., 2012; Peng et al., 2015; Wei et al., 2016). In fact, it was shown that the partial or complete removal of mineral-forming components, for example due to Fe reduction, can initiate aggregate turnover and destabilization (Michalet, 1993; Cornell and Schwertmann, 2003). The relative importance of Fe in aggregate stability depends on several properties that are expected to affect N bioavailability, such as Fe mineral and SOM content, mineral identity and degree of crystallinity, and soil redox conditions. In particular, Fe promotes the formation and stability of aggregates in soils with low OM and high Fe content (Barral et al., 1998; Wu et al., 2016). Duiker et al. (2003) showed that poorly crystalline Fe minerals are more important than crystalline minerals for aggregate stabilization (Duiker et al., 2003).
Many studies suggest that Fe-mediated micro-aggregation may slow down or suppress N mineralization and stabilize OM. For example, Silva et al. (2015) reported that applying Fe-rich biosolids in a tropical-soil chronosequence induced rapid formation of microaggregates and significantly increased SOC (Silva et al., 2015). Similarly, Bugeja and Castellano (2018) observed a positive correlation between ammonium oxalate-extractable Fe (AmOx-F) and C and N in microaggregates, indicating that Fe and microaggregate stabilization are interconnected (Bugeja and Castellano, 2018). Mendes et al. (1999) showed that readily mineralizable N levels correlate positively with increased aggregate size in soils (Mendes et al., 1999). In addition, numerous studies reported the accumulation of N in microaggregates (Golchin et al., 1994; Rodionov et al., 2001). For instance, Wagai et al. (2020) observed joint accumulation of OM with a low C:N ratio and pedogenic Fe and Al oxides in the meso-density fractions (1.8–2.4 g cm−3) of five soil orders collected from different climate zones. These observations are explained by the fact that microaggregate N is relatively more persistent than macroaggregate N because microaggregate turnover is relatively slow and not available to microbial degradation, which provides longer-term stabilization of OM (Cambardella and Elliott, 1993; Six et al., 2002). We also hypothesize that there is another pathway by which Fe-promoted aggregation may decrease N mineralization based on the information that aggregates of different sizes influence microbial community composition, and therefore the activities of N mineralization enzymes, differently (Muruganandam et al., 2009). Therefore, it will be useful to examine the distribution and the activities of these enzymes among soil aggregate size classes along a gradient of increasing Fe mineral content in soils.
2.2.2 Does Fe-induced ON polymerization increase the recalcitrance of N?
Little is known about Fe (mineral)-induced OM polymerization in soils. Some evidence exists that Fe oxides induce both C and N polymerization of SOM (Piccolo et al., 2011; Li et al., 2012; Johnson et al., 2015; Zou et al., 2020). In a long-term organic-fertilization experiment, Yu et al. (2020) proposed that the Fe-catalyzed formation of reactive oxygen species (ROSs) allows C monomers to recombine into large, recalcitrant C biopolymers through the formation of intramolecular bonds. A similar process was observed by Piccolo et al. (2011). Similarly, hydrohematite, maghemite, lepidocrocite, and hematite can induce the oxidative polymerization of hydroquinone, with rates depending on the type of minerals (Huang, 1990). Synthetic ferrihydrite and goethite were demonstrated to induce peptide bond formation between aspartate chains (Matrajt and Blanot, 2004), as well as the abiotic formation of amino acids from simple organics such as pyruvate and glyoxylate (Barge et al., 2019). The environmental conditions in these experiments were similar to those occurring in natural systems, such as in Fe-containing sediments (Barge et al., 2019). More studies of abiotic polymerization by minerals must be envisaged to understand its relevance to N bioavailability in soils.
2.3 Catalytic role of Fe in controlling N bioavailability
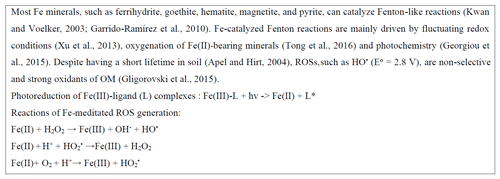
Emerging research has revealed that ROSs derived from Fe-catalyzed Fenton reactions (Box 1) are implicated in N mineralization. These reactions may involve abiotic or coupled biotic–abiotic processes causing N to mineralize, as explained below. In desert soils, the reaction of light with hematite generates ROSs, which can oxidize amino acids to nitrous oxide (N2O) (Georgiou et al., 2015) and N oxide gases (Hall et al., 2012). Compared to soils containing water, desert soils accumulate photo-generated superoxides and peroxidases via complexation of with surface transition metal oxides. When these soils are wetted, the accumulated ROSs are subjected to dismutation and hydrolysis, leading to the generation of HO• and subsequent OM oxidation. While this mechanism is strictly abiotic, soil microorganisms in diverse ecosystems were found to use Fe-generated HO• to acquire organic C and N (Diaz et al., 2013; Shah et al., 2016; J. Zhang et al., 2016; Op De Beeck et al., 2018). For instance, a boreal forest fungus (Paxillus involutus) may use radical oxidation to stimulate N mineralization in various ways (Op De Beeck et al., 2018): (1) to liberate from amine groups of proteins, peptides, and amino acids according to mechanisms reviewed in Stadtman and Levine (2003), (2) to facilitate the accessibility of protein N in SOM complexes to proteolytic degradation, and (3) to enhance protein vulnerability to proteolysis and increase the activity of proteolytic enzymes (J. Zhang et al., 2016).
Despite their involvement in N liberation, ROSs may promote the formation of stable and protective Fe-associated OM complexes. In a long-term fertilization experiment conducted by Yu et al. (2020), Fe mobilized by Fenton reactions formed new short-range-order (SRO) Fe minerals, which promoted C and N storage. Moreover, ROSs generated from catalytic reactions involving Fe can also cause enzyme oxidation and subsequent loss of activity (Huang et al., 2013).
2.4 Electron transfer role of Fe in N bioavailability
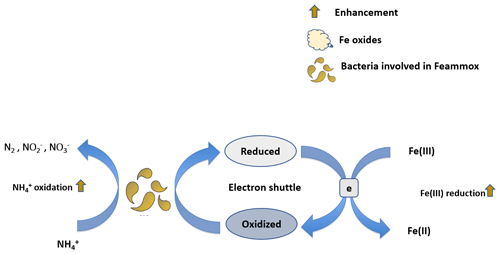
Electron transfer to Fe(III) oxides, both biotically or abiotically, is a critical step in many processes favoring the gain or the loss of N from soils and sediments (Sahrawat, 2004; Ding et al., 2014). The ability of Fe(III) minerals to accept electrons, or their reducibility, varies greatly with crystallinity, particle size, solution pH, ambient Fe(II) concentration, the presence of adsorbates, and aggregation level (Roden, 2004, 2006). Here, we explore relationships between mineral reducibility and anaerobic oxidation associated with Fe reduction (Feammox) and anaerobic OM oxidation to illustrate two examples of N processes that are involved in bioavailable N production and loss. Starting with Feammox, this process occurs mostly in acidic soils and has been estimated to metabolize 7.8–61 in paddy soils, accounting for about 3.9 %–31 % of N fertilizer loss (Ding et al., 2014). The terminal products of this process are either N2, , or , with N2 as the dominant product (Yang et al., 2012). Feammox rates are strongly positively correlated with the concentrations of microbially reducible Fe(III) (Ding et al., 2014; Li et al., 2015b; Ding et al., 2019, 2020). Moreover, Fe(III) enhances the activity, distribution, and diversity of microbial communities involved in Feammox (S. Huang et al., 2016; Ding et al., 2017). A series of incubation studies investigated the effects of different Fe sources on Feammox, and the results demonstrated that only ferrihydrite and goethite, not ferric chloride, lepidocrocite, hematite, or magnetite, served as electron acceptors for Feammox (Huang and Jaffé, 2015, 2018). These observations can be explained by a possible accumulation of free Fe(II), which halted Feammox, or due to the limited ability of Fe reducers in reducing certain minerals (Huang et al., 2014). It is notable that chelates (Park et al., 2009) and electron shuttles (Zhou et al., 2016) can facilitate electron transfer to Fe(III) minerals (Fig. 3), which enhances their reduction rates and related N processes. For instance, the addition of electron shuttles increased potential N loss by Feammox by 17 %–340 % compared to no addition (Zhou et al., 2016). Similarly to Feammox, production rates in submerged soils and sediments were found to be strongly correlated with reducible Fe(II) production rates (Sahrawat and Narteh, 2001; Sahrawat, 2004).
The electron-donating capacity of Fe minerals is also involved in N bioavailability. In fact, many Fe(II) species, including soluble Fe(II)- and Fe(III)-bearing minerals such as siderite and magnetite, can act as electron donors (Benz et al., 1998; Chaudhuri et al., 2001) for reduction coupled with Fe oxidation, which promotes the loss of as gases. For denitrification, it was found that N2O emissions from flooded soils with contrasting Fe(II) levels were regulated by Fe(II) electron-donating capacity: the electrons donated reached 16.2 % and 32.9 % in soils with low and high Fe(II) content, respectively. Soil with high Fe(II) content emitted less N2O and more N2, suggesting an improved denitrification efficiency due to an electron flow which exceeded the demand for N2O production (Wang et al., 2016).
This section is concerned with the role of Fe in the three phenomena that affect N bioavailability in soils: priming, The Birch effect, and freeze–thaw cycles. Priming occurs when new input of labile C influences (positive or negative) the decomposition of native SOM (Kuzyakov et al., 2000). The Birch effect is a short-term pulse in C and N mineralization caused by soil drying and rewetting. The freeze–thaw cycles refer to the alternation between freezing and thawing temperatures in a soil which can liberate decomposable OM and accelerate soil respiration. We offer this perspective to facilitate understanding of the mechanisms by which Fe affects N bioavailability through these three phenomena.
3.1 Priming
3.1.1 Fe-mediated priming in soils under oxidizing conditions
Investigations of the patterns and drivers of priming across both local and broad geographical scales indicate that SOM stabilization mechanisms, including associations with Fe oxides, regulate priming and explain most of its variation (Chen et al., 2019; Jeewani et al., 2021). Positive priming, where new inputs increase SOM mineralization, is negatively related to MAOM concentration due to Fe constraining microbial access of sorbed organics to microbial degradation (Bruun et al., 2010; Porras et al., 2018). In the rhizosphere, plant and microbial exudates can disrupt Fe-organic associations by means of both chemical and biological mechanisms. Chemical mechanisms include stripping Fe from associations through surface complexation, facilitating Fe reduction (Zinder et al., 1986; Keiluweit et al., 2015; Ding et al., 2021) and displacing sorbed organics into the soil solution (Zinder et al., 1986; Keiluweit et al., 2015; Ding et al., 2021). Biological mechanisms stimulate the production of N-acquiring enzymes as microbes are supplied with carbon and energy (Jilling et al., 2018; Yuan et al., 2018; Jiang et al., 2021; Jilling et al., 2021). Such mechanisms enhance positive priming by increasing the accessibility of C and N to microbes and facilitating N mining. However, the susceptibility of Fe-organic associations to these mechanisms varies; for example, ferrihydrite associations are affected by both chemical and biological processes, while goethite associations are more susceptible to chemical processes (Li et al., 2021). Therefore, the ability of microbes and plant communities to trigger specific destabilization mechanisms of the dominant mineral in their environment affects how much N can be made available from mineral associations (Jilling et al., 2018; Li et al., 2021).
Beyond SOM stabilization, Fe oxides may regulate priming by altering microbial community composition and soil C and N content (Heckman et al., 2009; Heckman et al., 2018), potentially by restricting nutrient availability and changing the structural properties of dissolved organic matter (DOM). For instance, the application of goethite to soil limits P and N bioavailability while increasing the aromatic content of water-extractable organic matter (WEOM), which may lower the ratio of fungi to bacteria (Heckman et al., 2012) and alter C and N cycling as a consequence (Silva-Sánchez et al., 2019; Wardle et al., 2004).
3.1.2 Fe-mediated priming in soils under reducing conditions
Recently, Fe-mediated priming in soils under reducing conditions has received growing interest. Dunham-Cheatham et al. (2020) found that glucose application to a soil under anoxic–oxic transition induced a novel type of priming by facilitating the reductive dissolution of Fe(III)–C associations under anoxic conditions followed by a dramatic increase of OC mineralization when oxic conditions were restored (Dunham-Cheatham et al., 2020). Li et al. (2021) found that the roles of Fe in anaerobic OM mineralization can be shifted by microbial biomass C (MBC). In soil with low MBC, both ferrihydrite and goethite protected the added acetate from decomposition through sorption processes. In soil with high MBC, however, goethite acted as an electron acceptor and increased acetate decomposition, whereas ferrihydrite predominantly adsorbed the added substrate. Priming decreased in both low- and high-MBC soils but more so in low-MBC soil (Li et al., 2021). Lecomte et al. (2018) demonstrated that Fe(III)-reducing microorganisms have a competitive advantage of colonizing plant roots in the rhizosphere due to their capacity for providing Fe(II) for plant nutrition in exchange for C-rich exudates and for performing denitrification (Lecomte et al., 2018). These exudates are probably used as a C source in the denitrification process or to destabilize Fe-organic associations and to release sorbed C and N (Dunham-Cheatham et al., 2020). More research into Fe-mediated priming in strictly anoxic soils, or at the oxic–anoxic transition, is needed.
3.2 Birch effect
Although many studies have been done on N mineralization and nitrification (Birch, 1958, 1959, 1960, 1964; Wilson and Baldwin, 2008), the studies on the Birch effect have mainly focused on C. The Birch effect for N was described as a rapid increase in N mineralization rates as an air-dried soil is rewetted. Soil moisture is accompanied by increased production. This pattern is likely explained by multiple interacting mechanisms, including the dissolution of organo-mineral bonds, which increases the accessibility of substrates to microbial degradation. In addition, the highly dynamic nature of wet–dry cycles can trigger rapid electron transfer from and to Fe oxides, known as the cryptic Fe cycle, which can affect N bioavailability. During the wet period, Fe(III) oxides can be used as an electron acceptor and can be reduced to Fe(II), which can abiotically react with to form or with nitrite () to form N2O. This Fe(II) can be converted back to Fe(III) oxides during the dry period, which may sorb OM and protect it against further degradation or generate oxidative radicals through Fenton reactions that break down organics, including N compounds. The cryptic Fe cycle can also induce rapid redox-induced mineral transformations, such as the transformation of amorphous Fe oxides into more crystalline forms, which decreases soil sorption capacity and nutrient retention (Attygalla et al., 2016; Wilmoth et al., 2018; Chen et al., 2020). Therefore, this cryptic Fe cycle will have a varied effect on the role of Fe in controlling N bioavailability over short spatiotemporal scales, which may either increase or decrease bioavailable N. Further research is needed to detangle these interactions.
3.3 Fe in the context of freeze–thaw cycles: the case of permafrost-affected soils
Permafrost-affected soils store large amounts of OC and ON as a result of SOM stabilization due to freezing of SOM and cryoturbation. Along a permafrost soil chronosequence, Joss et al. (2022) found a higher percentage of FeOM in cryoturbated horizons compared to in organic or mineral horizons in soil. Cryoturbation also favors the accumulation of SOM with a high C:N ratio at deeper soil depths (Treat et al., 2016a), which may also be present as associations with Fe minerals or in particulate organic matter. Upon thawing, this tremendous amount of SOC and total N (TN) facilitates high gross N turnover rates by heterotrophic processes. For instance, Treat et al. (2016b) observed increased N availability during long thaw seasons in tundra soils, whereas other authors reported higher N2O emissions from increased denitrification (Cui et al., 2016; Yang et al., 2016, 2018). This is partly because SOC and SOM, previously trapped in FeOM associations, are released and exposed to microbial degradation (Harden et al., 2012; Gentsch et al., 2015; Mueller et al., 2015; Patzner et al., 2020). In fact, Patzner et al. (2020) found that, along a thaw gradient, the amount of dissolved organic carbon (DOC) increased, as did the abundance of Fe(III)-reducing bacteria which use Fe(III) as a terminal electron acceptor and oxidize OM. The importance of these mechanisms in N destabilization likely depends on the extent to which Fe dissolution contributes to soil OM persistence in redox-dynamic permafrost (Patzner et al., 2020). More investigations of Fe control over N bioavailability in permafrost-affected soils are needed, especially with the recent development pointing out that mineral N cycling is as important as ON cycling in the active layers of these soils (Ramm et al., 2022).
Anticipated future climate scenarios indicate substantial fluctuations in precipitation and temperature patterns, accompanied by increasing levels of atmospheric CO2. These changes, along with alterations in land use, have the potential to significantly impact Fe–N bioavailability interactions in various ways, as detailed below.
4.1 Impact of variability in precipitation and temperature
The extreme variation in precipitation can lead to an increase in the occurrence of the Birch effect and a potential disruption of the dynamic of N availability in soils. Fe plays multiple roles in this process; in drier soils, Fe can protect ON from decomposition through the formation of stable Fe–organic associations. However, the reaction of Fe with light can induce Fenton reactions, leading to ON decomposition (Georgiou et al., 2015). In wetter soil, the destabilization of ON can increase as a result of fluctuations in redox conditions, the potential occurrence of cryptic Fe cycling, and modifications of mineral properties. In addition to precipitation, climate change is causing temperature to rise sharply, leading to extensive thawing of permafrost soils. As a consequence, redox-meditated heterotrophic N turnover processes and the destabilization of Fe–organic associations are expected to increase ON loss from these soils. Furthermore, rising temperatures will increase microbial metabolism, which will subsequently destabilize SOM.
4.2 Impact of elevated atmospheric CO2
The effects of rising atmospheric CO2 concentration (eCO2) levels on Fe–N bioavailability interactions are not well understood. Recent research showed that eCO2 stimulates root and microbial respiration, which can decrease soil redox potential, causing Fe reduction to proceed (Cheng et al., 2010). The production of Fe(II), which increased by 64 % under eCO2 treatment, caused substantial losses of via Feammox in a 15-year free-air CO2 enrichment (FACE) study in rice paddy systems. Feammox was meditated by autotrophic anaerobes that may use soil CO2 as a C source to couple anaerobic ammonium oxidation and Fe reduction (C. Xu et al., 2020). eCO2 can also increase the destabilization of mineral-associated organic nitrogen (MAON) via priming as eCO2 increases root biomass and associated exudate production at deeper soil depths, enabling the liberation of a large amount of deep soil N from these associations (Iversen, 2010). This increased turnover of N from MAOM would probably be substantial under future eCO2.
4.3 Impact of land use change
Land use change involving the conversion to agriculture can decrease soil organic N (SON) (García-Oliva et al., 2006). We hypothesize that this decline in SON is influenced by the effects of land use change on Fe cycling. For example, it was observed that the crystallinity of Fe oxides increased when forests were converted to agricultural fields in the southern Piedmont, USA (Li and Richter, 2012). Additionally, Tan et al. (2019) showed that land use change from fallow to paddy soils promoted Fe reduction by decreasing soil pH and increasing the electron-shuttling capacity of SOM (Tan et al., 2019), which may accelerate N turnover through processes such as Feammox. To conclude, global change affects the roles of Fe in N bioavailability, which may in turn affect the balance between Fe-meditated SON destabilization and protection
Attempts at understanding controls and drivers of N bioavailability, a fundamental soil ecosystem property, often omit the role of Fe minerals. However, the tendency of proteins to associate strongly with minerals and the involvement of the latter in both enzymatic and non-enzymatic reactions that influence the N cycle have motivated this review, which specifically focuses on Fe–N bioavailability interactions (Fig. 4). Including Fe in current models of SOM is challenging because the mechanisms by which Fe controls N storage, stabilization, bioavailability, and loss are complex and remain incompletely understood. This is because the present knowledge is, on the one hand, based on OM–mineral correlations, which is a simplistic approach since correlations tend to be specific for certain soil conditions and types (Wagai et al., 2020; Kleber et al., 2021), and on the other hand, knowledge is impeded by limitations in the analytical framework used to explore these interactions. In this section, we highlight the challenges and opportunities for future research.
5.1 Sorbent role of Fe in controlling N bioavailability
5.1.1 Organic nitrogen
The sorbent role of Fe in controlling N bioavailability is multifaceted. Sorption can protect ON from decomposition by reducing the activity of enzymes and limiting the accessibility of ON to degradation mechanisms. However, a fraction of sorbed ON is bioavailable (Bird et al., 2002; Kleber et al., 2007) or can be made available by processes such as priming or displacement by competitive organics. Thus, the concept of sorptive stabilization of N substrates does not stand as a conclusive explanation for ON persistence in soils and should rather be revisited. In this context, sorption to Fe minerals may impose spatial constraints on the accessibility of ON substances to microbes as sorption can locate ON in physically isolated spaces such as micropores, microaggregates, or microdomains of densely arranged clays, which slows down its decomposition and decreases its bioavailability (Kleber et al., 2021).
Research on Fe-meditated ON depolymerization has mostly focused on proteins (Wanek et al., 2010; Noll et al., 2019; Reuter et al., 2020) since proteins alone constitute 60 % or more of the N in plant and microbial cells (Fuchs, 1999) and are strongly sorbed to Fe surfaces. However, not all soil- and mineral-associated N is protein. Rather, N exists in a variety of chemical forms, including microbial cell wall compounds. Using Fourier transform infrared spectroscopy (FTIR) and isotope pool dilution (IPD), multiple studies have shown the importance of microbial cell wall depolymerization in the delivery of soil N (Hu et al., 2017, 2018, 2020). In addition, depolymerization of membrane lipids and nucleic acids is not yet characterized despite the detection of their degradation products in soils (Warren, 2021). This leads to the following question: how important is the chemical form of Fe-associated N in determining soil N bioavailability? This is relevant since the molecular characteristic of different N forms influences the type and strength of bonding with minerals, which may affect N bioavailability. For instance, Fe oxyhydroxides bind amino sugars more strongly than proteins in boreal forests (Keiluweit and Kuyper, 2020), likely allowing less mineralization from the former compared to the latter compounds.
5.1.2 Inorganic nitrogen
Despite a small number of studies relating structural Fe in clays and aggregates to N bioavailability, the dynamics of these interactions and the relevant mechanisms remain elusive. Several questions remain to be resolved, including the following: are the original structure and physicochemical characteristics of clay minerals restored upon reoxidation of its structural Fe? If so, what are the implications for release and fixation and other processes that influence the loss and gain of bioavailable N?
5.2 Structural role of Fe in controlling N bioavailability
Research is needed to comprehend the implications of Fe's structural role in controlling N bioavailability and its potential influence on soil dynamics and nutrient-cycling processes. Specifically, it remains uncertain how significant the loss of Fe through solubilization and reduction is to microaggregate instability and N bioavailability in soils. Furthermore, the relevance and occurrence of Fe-induced carbon ON polymerization in soils still require confirmation as observations of this phenomenon have thus far been limited to laboratory settings.
5.3 The role of Fe as a catalyst in controlling N bioavailability
Assessing the importance of Fe-meditated ROS generation in N bioavailability is a formidable challenge. In fact, despite being common in soils, ROSs have extremely short lifetimes and are highly reactive towards other soil constituents such as carbonates and bromide (Kleber et al., 2021), which complicates their detection in soils. They are produced by both abiotic and biotic pathways, and the contribution of each pathway to N bioavailability remains elusive. Additionally, rates and mechanisms of ROS production from these two pathways are still not known. Such information is particularly important to understand N dynamics in environments conducive to ROS formation, such as oxic or anoxic zones, environments with intense solar radiation, or in boreal forests where fungi use ROS-based mechanisms to access Fe-sorbed N. In contrast to their decomposition role, Yu et al. (2020) found an important role of Fe-meditated ROS production in OM polymerization, which increases the recalcitrance of OM and its resistance to degradation mechanisms (Yu et al., 2020). This finding sheds light on other controls and pathways relevant to N bioavailability. For example, under what conditions can the role of Fe-mediated ROS generation in N bioavailability be shifted from decomposition to protection? And how will this evolve in a changing world where solar radiation is becoming more intense and where the frequencies of extreme events (e.g., droughts, rain) is increasing?
5.4 Electron transfer role of Fe in controlling N bioavailability
The capacity of Fe to act as an electron acceptor and donor can affect bioavailable N loss from soils by processes such as Feammox and denitrification. To further understand these processes, more research is needed on cryptic Fe cycling and on the controls over the oxidation–reduction dynamics of Fe in soil since preservation of oxidized Fe promotes N stabilization within mineral associations. For instance, the effects of added electron shuttles on the extent and the rate of Fe(III) reduction and the associated loss of N via Feammox have been investigated; however, the capacity of SOM and organo-Fe associations to transfer electrons has received less attention (Sposito, 2011; Z. Xu et al., 2020). The characterization and mapping of spatiotemporal redox heterogeneity also deserves attention (Wilmoth, 2021).
5.5 A varied analytical approach is needed to characterize Fe–N interactions
To understand the roles of Fe in controlling N bioavailability, a varied analytical approach must be adopted to enable a more holistic and multidimensional view of these interactions considering all the possible outcomes of Fe reactions on N as driven by the physicochemical and biological characteristics of soil and management. This approach is essential to provide realistic turnover rates of N and to decipher the underlying mechanisms of Fe–N reactions in soil in contrast to the findings of controlled laboratory experiments, which do not represent soil in its complexity and heterogeneity. This approach should also capture variations in the processes of interest at multiple scales and within multiple time dimensions. Here, we the present most common and powerful techniques that can be combined in the framework of this varied approach to understand Fe–N interactions. Note that an extensive list of techniques is out of the scope of this review.
5.5.1 Imaging techniques
Techniques such as synchrotron X-ray absorption spectroscopy (XAS) and synchrotron X-rays allow the identification and the characterization of structural and chemical properties of minerals, as well as their oxidation states. They can also be used to determine the speciation of SON and dissolved organic N (DON), as well as the structural characteristics of soil, such as pore size and pore connectivity. These information could help, for example, to characterize the fine-scale redox heterogeneity (Wilmoth, 2021) that affects Fe cycling and its interconnection with N bioavailability. In addition, these techniques are used to observe and investigate the 3D structure of organo-Fe minerals in soils. Kleber et al. (2021) called for using them in studies of enzyme activity because they allow the investigation of the natural structure of organo-mineral associations without alteration (Kleber et al., 2021). However, while using advanced imaging techniques reveals information at fine scales, upscaling such data is challenging (Wagai et al., 2020).
5.5.2 Microbial techniques
Microbial techniques provide information on the identity of microbial taxa regulating the soil biogeochemical processes in question. These include techniques such as metatranscriptomics, which can be used to distinguish the biological from the abiotic pathways used to direct redox reactions (Wilmoth, 2021), and metagenomics, which were used recently to explore coupled nutrient interactions, including coupled Fe–N reactions (Ma et al., 2021).
5.5.3 Isotope techniques
Isotopes can be used to determine gross rates and the investigation of the pathways and mechanisms of the processes in question. They can also be used to determine OM pools with varying turnover rates. Stable isotope probing, which is a high-resolution technique, can also be used to trace the microbial uptake of N as affected by Fe minerals, as well as its fate in soil environments.
5.5.4 molecular-characterization techniques
Molecular-characterization techniques, which include FTIR, allow the identification of different soil organic molecules and the analysis of their bonding mode and strength with minerals.
5.6 Concluding comment
As a final commentary on Fe–N bioavailability interactions, we propose the following questions: how much N can be mobilized by Fe-related mechanisms? What are the controls on these interactions? And how important are certain mechanisms relative to others in securing N bioavailability in the context of global change? Do reactions observed in laboratory settings occur naturally in soils? We also urge the field to develop new methods and techniques, such as those capable of detecting low concentrations of ROSs and their fate in soil environments or the products of mineral-induced OM polymerization.
No data sets were used in this article.
IS conducted the literature review and wrote the paper. XZB, PL, and WH proofread, edited, reviewed, and provided guidance and advice on the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We acknowledge funding from Mohammed VI Polytechnic University and the J. G. Boswell Endowed Chair in Soil Science. We would also like to express our gratitude to the anonymous reviewers whose invaluable insights and constructive feedback greatly contributed to improving the quality and impact of our paper.
This paper was edited by Serita Frey and reviewed by two anonymous referees.
Allison, S. D.: Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes, Biogeochemistry, 81, 361–373, https://doi.org/10.1007/s10533-006-9046-2, 2006.
Amelung, W., Lobe, I., and Du Preez, C. C.: Fate of microbial residues in sandy soils of the South African Highveld as influenced by prolonged arable cropping, Eur. J. Soil Sci., 53, 29–35, https://doi.org/10.1046/j.1365-2389.2002.00428.x, 2002.
Apel, K. and Hirt, H.: Reactive oxygen species: metabolism, oxidative stress, and signal transduction, Annu. Rev. Plant. Biol., 55, 373–399, https://doi.org/10.1146/annurev.arplant.55.031903.141701, 2004.
Attygalla, N. W., Baldwin, D. S., Silvester, E., Kappen, P., and Whitworth, K. L.: The severity of sediment desiccation affects the adsorption characteristics and speciation of phosphorus, Environ. Sci.-Proc. Imp., 18, 64–71, https://doi.org/10.1039/c5em00523j, 2016.
Barge, L. M., Flores, E., Baum, M. M., VanderVelde, D. G., and Russell, M. J.: Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems, P. Natl. Acad. Sci. USA, 116, 4828–4833, https://doi.org/10.1073/pnas.1812098116, 2019.
Baron, M. H., Revault, M., Servagent-Noinville, S., Abadie, J., and Quiquampoix, H.: Chymotrypsin adsorption on montmorillonite: enzymatic activity and kinetic FTIR structural analysis, J. Colloid. Interf. Sci., 214, 319–332, https://doi.org/10.1006/jcis.1999.6189, 1999.
Barral, M. T., Arias, M., and Guérif, J.: Effects of iron and organic matter on the porosity and structural stability of soil aggregates, Soil Till. Res., 46, 261–272, https://doi.org/10.1016/s0167-1987(98)00092-0, 1998.
Batjes N. H.: Total carbon and nitrogen in the soils of the world, Eur. J. Soil Sci., 47, 151–163, https://doi.org/10.1111/j.1365-2389.1996.tb01386.x, 1996.
Bayan, M. R. and Eivazi, F.: Selected enzyme activities as affected by free iron oxides and clay particle size, Commun. Soil Sci. Plan., 30, 1561–1571, https://doi.org/10.1080/00103629909370308, 1999.
Benz, M., Brune, A., and Schink, B.: Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria, Arch. Microbiol., 169, 159–165, https://doi.org/10.1007/s002030050555, 1998.
Birch, H. F.: The effect of soil drying on humus decomposition and nitrogen availability, Plant Soil, 10, 9–31, https://doi.org/10.1007/bf01343734, 1958.
Birch, H. F.: Further observations on humus decomposition and nitrification, Plant Soil, 11, 262–286, https://doi.org/10.1007/bf01435157, 1959.
Birch, H. F.: Nitrification in soils after different periods of dryness, Plant Soil, 12, 81–96, https://doi.org/10.1007/bf01377763, 1960.
Birch, H. F.: Mineralisation of plant nitrogen following alternate wet and dry conditions. Plant Soil, 20, 43–49, https://doi.org/10.1007/bf01378096, 1964.
Bird, J. A., van Kessel, C., and Horwath W. R.: Nitrogen dynamics in humic fractions under alternative straw management in temperate rice, Soil Sci. Soc. Am. J., 66, 478–488, https://doi.org/10.2136/sssaj2002.4780, 2002.
Blankinship, J. C., Berhe, A. A., Crow, S. E., Druhan, J. L., Heckman, K. A., Keiluweit, M., Lawrence, C. R., Marín-Spiotta, E., Plante, A. F., Rasmussen, C., Schädel, C., Schimel, J. P., Sierra, C. A., Thompson, A., Wagai, R., and Wieder W. R. Improving understanding of soil organic matter dynamics by triangulating theories, measurements, and models, Biogeochemistry, 140, 1–13, https://doi.org/10.1007/s10533-018-0478-2, 2018.
Boland, D. D., Collins, R. N., Miller, C. J., Glover, C. J., and Waite, T. D.: Effect of solution and solid-phase conditions on the Fe(II)-accelerated transformation of ferrihydrite to lepidocrocite and goethite, Environ. Sci. Technol., 48, 5477–5485, https://doi.org/10.1021/es4043275, 2014.
Bremner J. M., Blackmer A. M., and Waring S. A.: Formation of nitrous oxide and dinitrogen by chemical decomposition of hydroxylamine in soils, Soil Biol. Biochem., 12, 263–269, https://doi.org/10.1016/0038-0717(80)90072-3, 1980.
Bruun, T. B., Elberling, B., and Christensen, B. T.: Lability of soil organic carbon in tropical soils with different clay minerals, Soil Biol. Biochem., 42, 888–895, https://doi.org/10.1016/j.soilbio.2010.01.009, 2010.
Bugeja, S. and Castellano, M.: Physicochemical organic matter stabilization across a restored grassland chronosequence, Soil Sci. Soc. Am. J., 82, 1559–1567, https://doi.org/10.2136/sssaj2018.07.0259, 2018.
Burger, M. and Venterea, R. T.: Effects of nitrogen fertilizer types on nitrous oxide emissions, in: Understanding greenhouse gas emissions from agricultural management, edited by: Guo, L., Gunasekara, A. S., and McConnell, L. L., ACS Sym. Ser., 1072, 179–202, https://doi.org/10.1021/bk-2011-1072, 2011.
Caldwell, B. A.: Enzyme activities as a component of soil biodiversity: A review, Pedobiologia, 49, 637–644, https://doi.org/10.1016/j.pedobi.2005.06.003, 2005.
Cambardella, C. A. and Elliott, E. T.: Carbon and nitrogen distribution in aggregates from cultivated and native grassland soils, Soil Sci. Soc. Am. J., 57, 1071–1076, https://doi.org/10.2136/sssaj1993.03615995005700040032x, 1993.
Chacon, S. S., Reardon, P. N., Burgess, C. J., Purvine, S., Chu, R. K., Clauss, T. R., Walter, E., Myrold, D. D., Washton, N., and Kleber, M.: Mineral surfaces as agents of environmental proteolysis: mechanisms and controls, Environ. Sci. Technol., 53, 3018–3026, https://doi.org/10.1021/acs.est.8b05583, 2019.
Chaudhuri, S. K., Lack, J. G., and Coates, J. D.: Biogenic magnetite formation through anaerobic biooxidation of Fe(II), Appl. Environ. Microb., 67, 2844–2848, https://doi.org/10.1128/aem.67.6.2844-2848.2001, 2001.
Chen, C., Hall, S. J., Coward, E., and Thompson, A.: Iron-mediated organic matter decomposition in humid soils can counteract protection, Nat. Commun., 11, 1–13, https://doi.org/10.1038/s41467-020-16071-5, 2020.
Chen, L., Liu, L., Qin, S., Yang, G., Fang, K., Zhu, B., Kuzyakov, Y., Chen, P., Xu, Y., and Yang, Y.: Regulation of priming effect by soil organic matter stability over a broad geographic scale, Nat. Commun., 10, 5112, https://doi.org/10.1038/s41467-019-13119-z, 2019.
Cheng, L., Zhu, J., Chen, G., Zheng, X., Oh, N. H., Rufty, T. W., Richter, D., and Hu, S.: Atmospheric CO2 enrichment facilitates cation release from soil, Ecol. Lett., 13, 284–291, https://doi.org/10.1111/j.1461-0248.2009.01421.x, 2010.
Cornell, R. M. and Schwertmann, U.: The iron oxides: structure, properties, reactions, occurrences, and uses, Wiley-VCH, Weinheim, 664, https://doi.org/10.1002/3527602097, 2003.
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K., and Paul, E.: The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter?, Glob. Change Biol., 19, 988–995, https://doi.org/10.1111/gcb.12113, 2013.
Coward, E. K., Thompson, A., and Plante, A. F.: Contrasting Fe speciation in two humid forest soils: Insight into organomineral associations in redox-active environments, Geochim. Cosmochim. Ac., 238, 68–84, https://doi.org/10.1016/j.gca.2018.07.007, 2018.
Cui, Q., Song, C., Wang, X., Shi, F., Wang, L., and Guo, Y.: Rapid N2O fluxes at high level of nitrate nitrogen addition during freeze-thaw events in boreal peatlands of northeast China, Atmos. Environ., 135, 1–8, https://doi.org/10.1016/j.atmosenv.2016.03.053, 2016.
Daly, A. B., Jilling, A., Bowles, T. M., Buchkowski, R. W., Frey, S. D., Kallenbach, C. M., Keiluweit, M., Mooshammer, M., Schimel J. P., and Grandy A. S.: A holistic framework integrating plant-microbe-mineral regulation of soil bioavailable nitrogen, Biogeochemistry, 154, 211–229, https://doi.org/10.1007/s10533-021-00793-9, 2021.
Datta, R., Anand, S., Moulick, A., Baraniya, D., Imran Pathan, S., Rejsek, K., Vranová, V., Sharma, M., Sharma, D., Kelkar, A., and Formánek, P.: How enzymes are adsorbed on soil solid phase and factors limiting its activity: A Review, Int. Agrophys., 31, 287–302, https://doi.org/10.1515/intag-2016-0049, 2017.
Daugherty, E. E., Gilbert, B., Nico, P. S., and Borch, T.: Complexation and Redox Buffering of Iron(II) by Dissolved Organic Matter, Environ. Sci. Technol., 51, 11096–11104, https://doi.org/10.1021/acs.est.7b03152, 2017.
Deroo, H., Akter, M., Mendoza, O., Boeckx, P., and Sleutel, S.: Control of paddy soil redox condition on gross and net ammonium fixation and defixation, Geoderma, 400, 115151, https://doi.org/10.1016/j.geoderma.2021.115151, 2021.
Diaz, J. M., Hansel, C. M., Voelker, B. M., Mendes, C. M., Andeer, P. F., and Zhang, T.: Widespread production of extracellular superoxide by heterotrophic bacteria, Science, 340, 1223–1226, https://doi.org/10.1126/science.1237331, 2013.
Ding, B., Li, Z., and Qin, Y.: Nitrogen loss from anaerobic ammonium oxidation coupled to Iron(III) reduction in a riparian zone, Environ. Pollut., 231, 379–386, https://doi.org/10.1016/j.envpol.2017.08.027, 2017.
Ding, B., Chen, Z., Li, Z., Qin, Y., and Chen, S.: Nitrogen loss through anaerobic ammonium oxidation coupled to Iron reduction from ecosystem habitats in the Taihu estuary region, Sci. Total Environ., 662, 600–606, https://doi.org/10.1016/j.scitotenv.2019.01.231, 2019.
Ding, B., Qin, Y., Luo, W., and Li, Z.: Spatial and seasonal distributions of Feammox from ecosystem habitats in the Wanshan region of the Taihu watershed, China, Chemosphere, 239, 124742, https://doi.org/10.1016/j.chemosphere.2019.124742, 2020.
Ding, L. J., An, X. L., Li, S., Zhang, G. L., and Zhu, Y. G.: Nitrogen Loss through Anaerobic Ammonium Oxidation Coupled to Iron Reduction from Paddy Soils in a Chronosequence, Environ. Sci. Technol., 48, 10641–10647, https://doi.org/10.1021/es503113s, 2014.
Ding, Y., Ye, Q., Liu, M., Shi, Z., and Liang, Y.: Reductive release of Fe mineral-associated organic matter accelerated by oxalic acid, Sci. Total Environ., 763, 142937, https://doi.org/10.1016/j.scitotenv.2020.142937, 2021.
Dippold, M., Biryukov, M., and Kuzyakov, Y.: Sorption affects amino acid pathways in soil: Implications from position-specific labeling of alanine, Soil Biol. Biochem., 72, 180–192, https://doi.org/10.1016/j.soilbio.2014.01.015, 2014.
Dubinsky, E. A., Silver, W. L., and Firestone, M. K.: Tropical forest soil microbial communities couple iron and carbon biogeochemistry, Ecology, 91, 2604–2612, https://doi.org/10.1890/09-1365.1, 2010.
Duiker, S. W., Rhoton, F. E., Torrent, J., Smeck, N. E., and Lal, R.: Iron (hydr)oxide crystallinity effects on soil aggregation, Soil. Sci. Soc. Am. J., 67, 606–611, https://doi.org/10.2136/sssaj2003.6060, 2003.
Dümig, A., Häusler, W., Steffens, M., and Kögel-Knabner, I.: Clay fractions from a soil chronosequence after glacier retreat reveal the initial evolution of organo–mineral associations, Geochim. Cosmochim. Ac., 85, 1–18, https://doi.org/10.1016/j.gca.2012.01.046, 2012.
Dunham-Cheatham, S. M., Zhao, Q., Obrist, D., and Yang, Y.: Unexpected mechanism for glucose-primed soil organic carbon mineralization under an anaerobic–aerobic transition, Geoderma, 376, 114535, https://doi.org/10.1016/j.geoderma.2020.114535, 2020.
Eusterhues, K., Rumpel, C., and Kögel-Knabner, I.: Organo-mineral associations in sandy acid forest soils: importance of specific surface area, iron oxides and micropores, Eur. J. Soil Sci., 56, 753–763, https://doi.org/10.1111/j.1365-2389.2005.00710.x, 2005.
Eusterhues, K., Hädrich, A., Neidhardt, J., Küsel, K., Keller, T. F., Jandt, K. D., and Totsche, K. U.: Reduction of ferrihydrite with adsorbed and coprecipitated organic matter: microbial reduction by Geobacter bremensis vs. abiotic reduction by Na-dithionite, Biogeosciences, 11, 4953–4966, https://doi.org/10.5194/bg-11-4953-2014, 2014.
Favre, F., Stucki, J. W., and Boivin, P.: Redox properties of structural Fe in ferruginous smectite. A discussion of the standard potential and its environmental implications, Clay. Clay Miner., 54, 466–472, https://doi.org/10.1346/ccmn.2006.0540407, 2006.
Feng, J., Wei, K., Chen, Z., Lü, X., Tian, J., Wang, C., and Chen, L.: Coupling and decoupling of soil carbon and nutrient cycles across an aridity gradient in the drylands of northern China: evidence from ecoenzymatic stoichiometry, Global Biogeochem. Cy., 33, 559–569, https://doi.org/10.1029/2018gb006112, 2019.
Filimonova, S., Kaufhold, S., Wagner, F., Häusler, W., and Kögel-Knabner, I.: The role of allophane nano-structure and Fe oxide speciation for hosting soil organic matter in an allophanic Andosol, Geochim. Cosmochim. Ac., 180, 284–302, https://doi.org/10.1016/j.gca.2016.02.033, 2016.
Fuchs, H.: Das lernende Unternehmen, in: Die Kunst, (k)eine perfekte Führungskraft zu sein, Gabler Verlag, Wiesbaden, 110–110, https://doi.org/10.1007/978-3-322-82766-1_40, 1999.
Gao, J., Jansen, B., Cerli, C., Helmus, R., Mikutta, R., Dultz, S., Guggenberger, G., and Kalbitz, K.: Competition and surface conditioning alter the adsorption of phenolic and amino acids on soil minerals, Eur. J. Soil Sci., 68, 667–677, https://doi.org/10.1111/ejss.12459, 2017.
Gao, J., Jansen, B., Cerli, C., Helmus, R., Mikutta, R., Dultz, S., Guggenberger, G., Vogel, C., and Kalbitz, K.: Organic matter coatings of soil minerals affect adsorptive interactions with phenolic and amino acids, Eur. J. Soil Sci., 69, 613–624, https://doi.org/10.1111/ejss.12562, 2018.
García-Oliva, F., Lancho, J. F. G., Montaño, N. M., and Islas, P.: Soil carbon and nitrogen dynamics followed by a forest-to-pasture conversion in Western Mexico, Agroforest. Syst., 66, 93–100, https://doi.org/10.1007/s10457-005-2917-z, 2006.
Gärdenäs, A. I., Ågren, G. I., Bird, J. A., Clarholm, M., Hallin, S., Ineson, P., Kätterer, T., Knicker, H., Nilsson, S. I., Näsholm, T., Ogle, S., Paustian, K., Persson, T., and Stendahl, J.: Knowledge gaps in soil carbon and nitrogen interactions – From molecular to global scale, Soil Biol. Biochem., 43, 702–717, https://doi.org/10.1016/j.soilbio.2010.04.006, 2011.
Garrido-Ramírez, E. G., Theng, B. K., and Mora, M. L.: Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—a review, Appl. Clay Sci., 47, 182–192, https://doi.org/10.1016/j.clay.2009.11.044, 2010.
Gentsch, N., Mikutta, R., Shibistova, O., Wild, B., Schnecker, J., Richter, A., Urich, T., Gittel, A., Šantrůčková, H., Bárta, J., Lashchinskiy, N., Mueller, C. W., Fuß, R., and Guggenberger, G.: Properties and bioavailability of particulate and mineral-associated organic matter in Arctic permafrost soils, Lower Kolyma Region, Russia, Eur. J. Soil Sci., 66, 722–734, https://doi.org/10.1111/ejss.12269, 2015.
Georgiou, C. D., Sun, H. J., McKay, C. P., Grintzalis, K., Papapostolou, I., Zisimopoulos, D., Panagiotidis, K., Zhang, G., Koutsopoulou, E., Christidis, G. E., and Margiolaki, I.: Evidence for photochemical production of reactive oxygen species in desert soils, Nat. Commun., 6, 1–11, https://doi.org/10.1038/ncomms8100, 2015.
Gianfreda, L., Rao, M. A., and Violante, A.: Formation and activity of urease-tannate complexes affected by aluminum, iron, and manganese, Soil Sci. Soc. Am. J., 59, 805–810, https://doi.org/10.2136/sssaj1995.03615995005900030024x, 1995.
Gligorovski, S., Strekowski, R., Barbati, S., and Vione, D.: Environmental implications of hydroxyl radicals (OH), Chem. Rev., 115, 13051–13092, https://doi.org/10.1021/cr500310b, 2015.
Golchin, A., Oades, J., Skjemstad, J., and Clarke, P.: Soil structure and carbon cycling, Soil Res., 32, 1043–1068, https://doi.org/10.1071/sr9941043, 1994.
Gotoh, S. and Patrick Jr., W. H.: Transformation of iron in a waterlogged soil as influenced by redox potential and pH, Soil Sci. Soc. Am. J., 38, 66–71, https://doi.org/10.2136/sssaj1974.03615995003800010024x, 1974.
Gu, B., Schmitt, J., Chen, Z., Liang, L., and McCarthy, J. F.: Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models, Environ. Sci. Technol., 28, 38–46, https://doi.org/10.1021/es00050a007, 1994.
Gu, B., Schmitt, J., Chen, Z., Liang, L., and McCarthy, J. F.: Adsorption and desorption of different organic matter fractions on iron oxide, Geochim. Cosmochim. Ac., 59, 219–229, https://doi.org/10.1016/0016-7037(94)00282-q, 1995.
Gu, C., Zhang, S., Han, P., Hu, X., Xie, L., Li, Y., Brooks, M., Liao, X., and Qin, L.: Soil enzyme activity in soils subjected to flooding and the effect on nitrogen and phosphorus uptake by oilseed rape, Front. Plant Sci., 10, 386, https://doi.org/10.3389/fpls.2019.00368, 2019.
Hall, S. J., and Silver, W. L.: Iron oxidation stimulates organic matter decomposition in humid tropical forest soils, Glob. Change Biol., 19, 2804–2813, https://doi.org/10.1111/gcb.12229, 2013.
Hall, S. J., Silver, W. L., and Amundson, R.: Greenhouse gas fluxes from Atacama Desert soils: a test of biogeochemical potential at the Earth's arid extreme, Biogeochemistry, 111, 303–315, https://doi.org/10.1007/s10533-011-9650-7, 2012.
Hall, S. J., Berhe, A. A., and Thompson, A.: Order from disorder: do soil organic matter composition and turnover co-vary with iron phase crystallinity?, Biogeochemistry, 140, 93–110, https://doi.org/10.1007/s10533-018-0476-4, 2018.
Han, J., Shi, L., Yakun, W., Chen, Z., and Wu, L.: The regulatory role of endogenous iron on greenhouse gas emissions under intensive nitrogen fertilization in subtropical soils of China, Environ. Sci. Pollut. R., 25, 14511–14520, https://doi.org/10.1007/s11356-018-1666-2, 2018.
Harden, J. W., Koven, C. D., Ping, C. L., Hugelius, G., McGuire, A. D., Camill, P., Jorgenson, T., Kuhry, P., Michaelson, G. J., and O'Donnell, J. A.: Field information links permafrost carbon to physical vulnerabilities of thawing, Geophys. Res. Lett., 39, L15704, https://doi.org/10.1029/2012gl051958, 2012.
Hassan, W., Chen, W., Cai, P., and Huang, Q.: Oxidative enzymes, the ultimate regulator: implications for factors affecting their efficiency, J. Environ. Qual., 42, 1779–90, https://doi.org/10.2134/jeq2013.05.0204, 2013.
Heckman, K., Welty-Bernard, A., Rasmussen, C., and Schwartz, E.: Geologic controls of soil carbon cycling and microbial dynamics in temperate conifer forests, Chem. Geol., 267, 12–23, https://doi.org/10.1016/j.chemgeo.2009.01.004, 2009.
Heckman, K. A., Welty-Bernard, A., Vázquez-Ortega, A., Schwartz, E., Chorover, J., and Rasmussen, C.: The influence of goethite and gibbsite on soluble nutrient dynamics and microbial community composition, Biogeochemistry, 112, 179–195, https://doi.org/10.1007/s10533-012-9715-2, 2012.
Heckman, K., Throckmorton, H., Horwath, W. R., Swanston, C. W., and Rasmussen, C.: Variation in the molecular structure and radiocarbon abundance of mineral-associated organic matter across a lithosequence of forest soils, Soil Syst., 2, 36, https://doi.org/10.3390/soilsystems2020036, 2018.
Henneberry, Y. K., Kraus, T. E. C., Nico, P. S., and Horwath, W. R.: Structural stability of coprecipitated natural organic matter and ferric iron under reducing conditions, Org. Geochem., 48, 81–89, https://doi.org/10.1016/j.orggeochem.2012.04.005, 2012.
Henneberry, Y., Kraus, T. E. C., Krabbenhoft, D. P., and Horwath, W. R.: Investigating the temporal effects of metal-based coagulants to remove mercury from solution in the presence of dissolved organic matter, Environ. Manage., 57, 220–228, https://doi.org/10.1007/s00267-015-0601-2, 2016.
Hlady, V. V. and Buijs, J.: Protein adsorption on solid surfaces, Curr. Opin. Biotech., 7, 72–77, https://doi.org/10.1016/s0958-1669(96)80098-x, 1996.
Hu, Y., Zheng, Q., and Wanek, W.: Flux analysis of free amino sugars and amino acids in soils by isotope tracing with a novel liquid chromatography/high resolution mass spectrometry platform, Anal. Chem., 89, 9192–9200, https://doi.org/10.1021/acs.analchem.7b01938, 2017.
Hu, Y., Zheng, Q., Zhang, S., Noll, L., and Wanek, W.: Significant release and microbial utilization of amino sugars and d-amino acid enantiomers from microbial cell wall decomposition in soils, Soil Biol. Biochem., 123, 115–125, https://doi.org/10.1016/j.soilbio.2018.04.024, 2018.
Hu, Y., Zheng, Q., Noll, L., Zhang, S., and Wanek, W.: Direct measurement of the in situ decomposition of microbial-derived soil organic matter, Soil Biol. Biochem., 141, 107660, https://doi.org/10.1016/j.soilbio.2019.107660, 2020.
Huang, P. M.: Role of soil minerals in transformations of natural organics and xenobiotics in soil, in: Soil Biochemistry, edited by: Bollag, J. M. and Stotzki, G., Routledge, New York, 29–116, https://doi.org/10.1201/9780203739389-2, 1990.
Huang, S. and Jaffé, P. R.: Characterization of incubation experiments and development of an enrichment culture capable of ammonium oxidation under iron-reducing conditions, Biogeosciences, 12, 769–779, https://doi.org/10.5194/bg-12-769-2015, 2015.
Huang, S. and Jaffé, P. R.: Isolation and characterization of an ammonium-oxidizing iron reducer: Acidimicrobiaceae sp. A6, PLOS ONE, 13, e0194007, https://doi.org/10.1371/journal.pone.0194007, 2018.
Huang, S., Chen, C., Peng, X., and Jaffé, P. R.: Environmental factors affecting the presence of Acidimicrobiaceae and ammonium removal under iron-reducing conditions in soil environments, Soil Biol. Biochem., 98, 148–158, https://doi.org/10.1016/j.soilbio.2016.04.012, 2016.
Huang, X., Kanerva, P., Salovaara, H., Loponen, J., and Sontag-Strohm, T.: Oxidative modification of a proline-rich gliadin peptide, Food Chem., 141, 2011–2016, https://doi.org/10.1016/j.foodchem.2013.05.066, 2013.
Huang, X., Gao, D., Peng, S., and Tao, Y.: Effects of ferrous and manganese ions on anammox process in sequencing batch biofilm reactors, J. Environ. Sci., 26, 1034–1039, https://doi.org/10.1016/s1001-0742(13)60531-8, 2014.
Huang, X., Zhu-Barker, X., Horwath, W. R., Faeflen, S. J., Luo, H., Xin, X., and Jiang, X.: Effect of iron oxide on nitrification in two agricultural soils with different pH, Biogeosciences, 13, 5609–5617, https://doi.org/10.5194/bg-13-5609-2016, 2016.
Iversen, C. M.: Digging deeper: fine-root responses to rising atmospheric CO concentration in forested ecosystems, New Phytol., 186, 346–357, https://doi.org/10.1111/j.1469-8137.2009.03122.x, 2010.
Jeewani, P. H., Van Zwieten, L., Zhu, Z., Ge, T., Guggenberger, G., Luo, Y., and Xu, J.: Abiotic and biotic regulation on carbon mineralization and stabilization in paddy soils along iron oxide gradients, Soil Biol. Biochem., 160, 108312, https://doi.org/10.1016/j.soilbio.2021.108312, 2021.
Jiang, Z., Liu, Y., Yang, J., Brookes, P. C., and Gunina, A.: Rhizosphere priming regulates soil organic carbon and nitrogen mineralization: The significance of abiotic mechanisms, Geoderma, 385, 114877, https://doi.org/10.1016/j.geoderma.2020.114877, 2021.
Jilling, A., Keiluweit, M., Contosta, A. R., Frey, S., Schimel, J., Schnecker, J., Smith, R. G., Tiemann, L., and Grandy, A. S.: Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes, Biogeochemistry, 139, 103–122, https://doi.org/10.1007/s10533-018-0459-5, 2018.
Jilling, A., Keiluweit, M., Gutknecht, J. L. M., and Grandy, A. S.: Priming mechanisms providing plants and microbes access to mineral-associated organic matter, Soil Biol. Biochem., 158, 108265, https://doi.org/10.1016/j.soilbio.2021.108265, 2021.
Johnson, K., Purvis, G., Lopez-Capel, E., Peacock, C., Gray, N., Wagner, T., März, C., Bowen, L., Ojeda, J., Finlay, N., Robertson, S., Worrall, F., and Greenwell, C.: Towards a mechanistic understanding of carbon stabilization in manganese oxides, Nat. Commun., 6, 7628, https://doi.org/10.1038/ncomms8628, 2015.
Joss, H., Patzner, M. S., Maisch, M., Mueller, C. W., Kappler, A., and Bryce, C.: Cryoturbation impacts iron-organic carbon associations along a permafrost soil chronosequence in northern Alaska, Geoderma, 413, 115738, https://doi.org/10.1016/j.geoderma.2022.115738, 2022.
Kaiser, K. and Zech, W.: Dissolved organic matter sorption by mineral constituents of subsoil clay fractions, J. Plant Nutr. Soil Sc., 163, 531–535, https://doi.org/10.1002/1522-2624(200010)163:5<531::aid-jpln531>3.0.co;2-n, 2000a.
Kaiser, K. and Zech, W.: Sorption of dissolved organic nitrogen by acid subsoil horizons and individual mineral phases, Eur. J. Soil Sci., 51, 403–411, https://doi.org/10.1046/j.1365-2389.2000.00320.x, 2000b.
Keiluweit, M. and Kuyper, T. W.: Proteins unbound – how ectomycorrhizal fungi can tap a vast reservoir of mineral-associated organic nitrogen, New Phytol., 228, 406–408, https://doi.org/10.1111/nph.16796, 2020.
Keiluweit, M., Bougoure, J. J., Zeglin, L. H., Myrold, D. D., Weber, P. K., Pett-Ridge, J., Kleber, M., and Nico, P. S.: Nano-scale investigation of the association of microbial nitrogen residues with iron (hydr)oxides in a forest soil O-horizon, Geochim. Cosmochim. Ac., 95, 213–226, https://doi.org/10.1016/j.gca.2012.07.001, 2012.
Keiluweit, M., Bougoure, J. J., Nico, P. S., Pett-Ridge, J., Weber, P. K., and Kleber, M.: Mineral protection of soil carbon counteracted by root exudates, Nat. Clim. Change, 5, 588–595, https://doi.org/10.1038/nclimate2580, 2015.
Kelleher, B. P., Willeford, K. O., Simpson, A. J., Simpson, M. J., Stout, R., Rafferty, A., and Kingery, W. L.: Acid phosphatase interactions with organo-mineral complexes: influence on catalytic activity, Biogeochemistry, 71, 285–297, https://doi.org/10.1023/b:biog.0000049348.53070.6f, 2004.
Kieloaho, A. J., Pihlatie, M., Dominguez Carrasco, M., Kanerva, S., Parshintsev, J., Riekkola, M. L., Pumpanen, J., and Heinonsalo, J.: Stimulation of soil organic nitrogen pool: The effect of plant and soil organic matter degrading enzymes, Soil Biol. Biochem., 96, 97–106, https://doi.org/10.1016/j.soilbio.2016.01.013, 2016.
Kleber, M., Sollins, P., and Sutton, R.: A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces, Biogeochemistry, 85, 9–24, https://doi.org/10.1007/s10533-007-9103-5, 2007.
Kleber, M., Eusterhues, K., Keiluweit, M., Mikutta, C., Mikutta, R., and Nico, P. S.: Mineral–organic associations: formation, properties, and relevance in soil environments, in: Advances in Agronomy, edited by: Sparks, D. L., Academic Press, Elsevier, 1–140, https://doi.org/10.1016/bs.agron.2014.10.005, 2015.
Kleber, M., Bourg, I. C., Coward, E. K., Hansel, C. M., Myneni, S. C. B., and Nunan, N.: Dynamic interactions at the mineral–organic matter interface, Nat. Rev. Earth. Environ., 2, 402–421, https://doi.org/10.1038/s43017-021-00162-y, 2021.
Knicker, H.: Soil organic N - An under-rated player for C sequestration in soils?, Soil Biol. Biochem., 43, 1118–1129, https://doi.org/10.1016/j.soilbio.2011.02.020, 2011.
Kögel-Knabner, I.: Chemical structure of organic N and organic P in soil, in: nucleic acids and proteins in soil, edited by: Nannipieri, P. and Smalla, K., Springer, Berlin, Heidelberg, 23–48, https://doi.org/10.1007/3-540-29449-x_2, 2006.
Kopittke, P. M., Hernandez-Soriano, M. C., Dalal, R. C., Finn, D., Menzies, N. W., Hoeschen, C., and Mueller, C. W.: Nitrogen-rich microbial products provide new organo-mineral associations for the stabilization of soil organic matter, Glob. Change Biol., 24, 1762–1770, https://doi.org/10.1111/gcb.14009, 2018.
Kuzyakov, Y., Friedel, J. K., and Stahr, K.: Review of mechanisms and quantification of priming effects, Soil Biol. Biochem., 32, 1485–1498, https://doi.org/10.1016/s0038-0717(00)00084-5, 2000.
Kwan, W. P. and Voelker, B. M.: Rates of hydroxyl radical generation and organic compound oxidation in mineral-catalyzed Fenton-like systems, Environ. Sci. Technol., 37, 1150–1158, https://doi.org/10.1021/es020874g, 2003.
Lalonde, K., Mucci, A., Ouellet, A., and Gélinas, Y.: Preservation of organic matter in sediments promoted by iron, Nature, 483, 198–200, https://doi.org/10.1038/nature10855, 2012.
Lecomte, S. M., Achouak, W., Abrouk, D., Heulin, T., Nesme, X., and Haichar, F. E. Z.: Diversifying anaerobic respiration strategies to compete in the rhizosphere, Front. Environ. Sci., 6, 139, https://doi.org/10.3389/fenvs.2018.00139, 2018.
Lehmann, J., and Kleber, M.: The contentious nature of soil organic matter, Nature, 528, 60–66, https://doi.org/10.1038/nature16069, 2015.
Leinweber, P. and Schulten, H. R.: Nonhydrolyzable forms of soil organic nitrogen: Extractability and composition, J. Plant. Nutr. Soil Sci., 163, 433–439, https://doi.org/10.1002/1522-2624(200008)163:4<433::aid-jpln433>3.0.co;2-f, 2000.
Li, C., Zhang, B., Ertunc, T., Schaeffer, A., and Ji, R.: Birnessite-induced binding of phenolic monomers to soil humic substances and nature of the bound residues, Environ. Sci. Technol., 46, 8843–8850, https://doi.org/10.1021/es3018732, 2012.
Li, H., Bölscher, T., Winnick, M., Tfaily, M. M., Cardon, Z. G., and Keiluweit, M.: Simple plant and microbial exudates destabilize mineral-associated organic matter via multiple pathways, Environ. Sci. Technol., 55, 3389–3398, https://doi.org/10.1021/acs.est.0c04592, 2021.
Li, J. and Richter, D. D.: Effects of two-century land use changes on soil iron crystallinity and accumulation in Southeastern Piedmont region, USA, Geoderma, 173–174, 184–191, https://doi.org/10.1016/j.geoderma.2011.12.021, 2012.
Li, X., Li, H., and Yang, G.: Promoting the adsorption of metal ions on kaolinite by defect sites: a molecular dynamics study, Sci. Rep.-UK., 5, 14377, https://doi.org/10.1038/srep14377, 2015a.
Li, X., Hou, L., Liu, M., Zheng, Y., Yin, G., Lin, X., Cheng, L., Li, Y., and Hu, X.: Evidence of nitrogen loss from anaerobic ammonium oxidation coupled with ferric iron reduction in an intertidal wetland, Environ. Sci. Technol., 49, 11560–11568, https://doi.org/10.1021/acs.est.5b03419, 2015b.
Li, Y., Wang, M., Zhang, Y., Koopal, L. K., and Tan, W.: Goethite effects on transport and activity of lysozyme with humic acid in quartz sand, Colloid. Surface. A., 604, 125319, https://doi.org/10.1016/j.colsurfa.2020.125319, 2020.
Lipson, D. A., Jha, M., Raab, T. K., and Oechel, W. C.: Reduction of iron (III) and humic substances plays a major role in anaerobic respiration in an Arctic peat soil, J. Geophys. Res.-Biogeo., 115, G00I06, https://doi.org/10.1029/2009jg001147, 2010.
Lützow, M. V., Kögel-Knabner, I., Ekschmitt, K., Matzner, E., Guggenberger, G., Marschner, B., and Flessa, H.: Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions – a review, Eur. J. Soil Sci., 57, 426–445, https://doi.org/10.1111/j.1365-2389.2006.00809.x, 2006.
Ma, B., Stirling, E., Liu, Y., Zhao, K., Zhou, J., Singh, B. K., Tang, C., Dahlgren, R. A., and Xu, J.: Soil biogeochemical cycle couplings inferred from a function-taxon network, Research, 2021, 7102769, https://doi.org/10.34133/2021/7102769, 2021.
Matrajt, G. and Blanot, D.: Properties of synthetic ferrihydrite as an amino acid adsorbent and a promoter of peptide bond formation, Amino Acids, 26, 153–158, https://doi.org/10.1007/s00726-003-0047-3, 2004.
Mayes, M. A., Heal, K. R., Brandt, C. C., Phillips, J. R., and Jardine, P. M.: Relation between soil order and sorption of dissolved organic carbon in temperate subsoils, Soil Sci. Soc. Am. J., 76, 1027–1037, https://doi.org/10.2136/sssaj2011.0340, 2012.
Mendes, I. C., Bandick, A. K., Dick, R. P., and Bottomley, P. J.: Microbial biomass and activities in soil aggregates affected by winter cover crops, Soil Sci. Soc. Am. J., 63, 873–881, https://doi.org/10.2136/sssaj1999.634873x, 1999.
Merino, C., Kuzyakov, Y., Godoy, K., Cornejo, P., and Matus, F.: Synergy effect of peroxidase enzymes and Fenton reactions greatly increase the anaerobic oxidation of soil organic matter, Sci. Rep.-UK, 10, 1–12, https://doi.org/10.1038/s41598-020-67953-z, 2020.
Michalet, R.: Hematite identification in pseudo-particles of Moroccan rubified soils, Clay Miner., 28, 233–242, https://doi.org/10.1180/claymin.1993.028.2.05, 1993.
Mikutta, R., Mikutta, C., Kalbitz, K., Scheel, T., Kaiser, K., and Jahn, R.: Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms, Geochim. Cosmochim. Ac., 71, 2569–2590, https://doi.org/10.1016/j.gca.2007.03.002, 2007.
Mueller, C. W., Rethemeyer, J., Kao-Kniffin, J., Löppmann, S., Hinkel, K. M., and G. Bockheim, J.: Large amounts of labile organic carbon in permafrost soils of northern Alaska, Glob. Change Biol., 21, 2804–2817, https://doi.org/10.1111/gcb.12876, 2015.
Muruganandam, S., Israel, D. W., and Robarge, W. P.: Activities of nitrogen-mineralization enzymes associated with soil aggregate size fractions of three tillage systems, Soil Sci. Soc. Am. J., 73, 751–759, https://doi.org/10.2136/sssaj2008.0231, 2009.
Navrotsky, A., Mazeina, L., and Majzlan, J.: Size-driven structural and thermodynamic complexity in iron oxides, Science, 319, 1635, https://doi.org/10.1126/science.1148614, 2008.
Newcomb, C. J., Qafoku, N. P., Grate, J. W., Bailey, V. L., and De Yoreo, J. J.: Developing a molecular picture of soil organic matter–mineral interactions by quantifying organo–mineral binding, Nat. Commun., 8, 1–8, https://doi.org/10.1038/s41467-017-00407-9, 2017.
Noll, L., Zhang, S., Zheng, Q., Hu, Y., and Wanek, W.: Wide-spread limitation of soil organic nitrogen transformations by substrate availability and not by extracellular enzyme content, Soil Biol. Biochem., 133, 37–49, https://doi.org/10.1016/j.soilbio.2019.02.016, 2019.
Olagoke, F. K., Kaiser, K., Mikutta, R., Kalbitz, K., and Vogel, C.: Persistent activities of extracellular enzymes adsorbed to soil minerals, Microorganisms, 8, 1796, https://doi.org/10.3390/microorganisms8111796, 2020.
Op De Beeck, M., Troein, C., Peterson, C., Persson, P., and Tunlid, A.: Fenton reaction facilitates organic nitrogen acquisition by an ectomycorrhizal fungus, New Phytol., 218, 335–343, https://doi.org/10.1111/nph.14971, 2018.
Park, W., Nam, Y. K., Lee, M. J., and Kim, T. H.: Anaerobic ammonia-oxidation coupled with Fe3+ reduction by an anaerobic culture from a piggery wastewater acclimated to /Fe3+ medium, Biotechnol. Bioproc. E., 14, 680–685, https://doi.org/10.1007/s12257-009-0026-y, 2009.
Patzner, M. S., Mueller, C. W., Malusova, M., Baur, M., Nikeleit, V., Scholten, T., Hoeschen, C., Byrne, J. M., Borch, T., Kappler, A., and Bryce, C.: Iron mineral dissolution releases iron and associated organic carbon during permafrost thaw, Nat. Commun., 11, 1–11, https://doi.org/10.1038/s41467-020-20102-6, 2020.
Peng, X., Yan, X., Zhou, H., Zhang, Y., and Sun, H.: Assessing the contributions of sesquioxides and soil organic matter to aggregation in an Ultisol under long-term fertilization, Soil Till. Res., 46, 89–98, https://doi.org/10.1016/j.still.2014.04.003, 2015.
Pentráková, L., Su, K., Pentrák, M., and Stucki, J. W.: A review of microbial redox interactions with structural Fe in clay minerals, Clay Miner., 48, 543–560, https://doi.org/10.1180/claymin.2013.048.3.10, 2013.
Petridis, L., Ambaye, H., Jagadamma, S., Kilbey, S. M., Lokitz, B. S., Lauter, V., and Mayes, M. A.: Spatial arrangement of organic compounds on a model mineral surface: implications for soil organic matter stabilization, Environ. Sci. Technol., 48, 79–84, https://doi.org/10.1021/es403430k, 2014.
Piccolo, A., Spaccini, R., Nebbioso, A., and Mazzei, P.: Carbon sequestration in soil by in situ catalyzed photo-oxidative polymerization of soil organic matter, Environ. Sci. Technol., 45, 6697–6702, https://doi.org/10.1021/es201572f, 2011.
Poggenburg, C., Mikutta, R., Schippers, A., Dohrmann, R., and Guggenberger, G.: Impact of natural organic matter coatings on the microbial reduction of iron oxides, Geochim. Cosmochim. Ac., 224, 223–248, https://doi.org/10.1016/j.gca.2018.01.004, 2018.
Porras, R. C., Hicks Pries, C. E., Torn, M. S., and Nico, P. S.: Synthetic iron (hydr)oxide-glucose associations in subsurface soil: Effects on decomposability of mineral associated carbon, Sci. Total Environ., 613–614, 342–351, https://doi.org/10.1016/j.scitotenv.2017.08.290, 2018.
Possinger, A. R., Zachman, M. J., Enders, A., Levin, B. D. A., Muller, D. A., Kourkoutis, L. F., and Lehmann, J.: Organo–organic and organo–mineral interfaces in soil at the nanometer scale, Nat. Commun., 11, 6103, https://doi.org/10.1038/s41467-020-19792-9, 2020.
Pronk, G., Heister, K., Ding, G. C., Smalla, K., and Kögel-Knabner, I., Development of biogeochemical interfaces in an artificial soil incubation experiment; aggregation and formation of organo-mineral associations, Geoderma, 189–190, 585–594, https://doi.org/10.1016/j.geoderma.2012.05.020, 2012.
Pulford, I. D. and Tabatabai, M. A.: Effect of waterlogging on enzyme activities in soils, Soil Biol. Biochem., 20, 215–219, https://doi.org/10.1016/0038-0717(88)90039-9, 1988.
Quiquampoix, H. and Ratcliffe, R. G.: A 31P NMR study of the adsorption of bovine serum albumin on montmorillonite using phosphate and the paramagnetic cation Mn2+: modification of conformation with pH, J. Colloid. Interf. Sci., 148, 343–352, https://doi.org/10.1016/0021-9797(92)90173-j, 1992.
Quiquampoix, H., Abadie, J., Baron, M., Leprince, F., Matumoto-Pintro, P., Ratcliffe, R. G., and Staunton, S.: Mechanisms and consequences of protein adsorption on soil mineral surfaces, ACS Sym. Ser., 602, 321–333, https://doi.org/10.1021/bk-1995-0602.ch023, 1995.
Ramm, E., Liu, C. Ambus, P., Butterbach-Bahl, K., Hu, B., Martikainen, P. J., Marushchak, M. E., Mueller, C. W., Rennenberg, H., Schloter, M., Siljanen, H. M. P., Voigt, C., Werner, C., Biasi, C., and Dannenmann, M., A review of the importance of mineral nitrogen cycling in the plant-soil-microbe system of permafrost-affected soils – changing the paradigm, Environ. Res. Lett., 17, 013004, https://doi.org/10.1088/1748-9326/ac417e, 2022.
Rabe, M., Verdes, D., and Seeger, S.: Understanding protein adsorption phenomena at solid surfaces, Adv. Colloid Interfac., 162, 87–106, https://doi.org/10.1016/j.cis.2010.12.007, 2011.
Rani, A. S., Das, M. L. M., and Satyanarayana, S.: Preparation and characterization of amyloglucosidase adsorbed on activated charcoal, J. Mol. Catal. B-Enzym., 10, 471–476, https://doi.org/10.1016/s1381-1177(99)00116-2, 2000.
Reuter, H., Gensel, J., Elvert, M., and Zak, D.: Evidence for preferential protein depolymerization in wetland soils in response to external nitrogen availability provided by a novel FTIR routine, Biogeosciences, 17, 499–514, https://doi.org/10.5194/bg-17-499-2020, 2020.
Roden, E. E.: Analysis of long-term bacterial vs. chemical Fe(III) oxide reduction kinetics, Geochim. Cosmochim. Ac., 68, 3205–3216, https://doi.org/10.1016/j.gca.2004.03.028, 2004.
Roden, E. E.: Geochemical and microbiological controls on dissimilatory iron reduction, C. R. Geosci., 338, 456–467, https://doi.org/10.1016/j.crte.2006.04.009, 2006.
Roden, E. E. and Wetzel, R. G.: Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments, Limnol. Oceanogr., 41, 1733–1748, https://doi.org/10.4319/lo.1996.41.8.1733, 1996.
Rodionov, A., Amelung, W., Urusevskaja, I., and Zech, W.: Origin of the enriched labile fraction (ELF) in Russian Chernozems with different site history, Geoderma, 102, 299–315, https://doi.org/10.1016/s0016-7061(01)00038-6, 2001.
Sahrawat, K. L.: Ammonium production in submerged soils and sediments: the role of reducible iron, Commun. Soil Sci. Plan., 35, 399–411, https://doi.org/10.1081/css-120029721, 2004.
Sahrawat, K. L. and Narteh, L. T.: Organic matter and reducible iron control of ammonium production in submerged soils, Commun. Soil Sci. Plan., 32, 1543–1550, https://doi.org/10.1081/css-100104211, 2001.
Sarkar, J. M. and Burns, R. G.: Synthesis and properties of β-d-glucosidasephenolic copolymers as analogues of soil humic-enzyme complexes, Soil Biol. Biochem., 16, 619–625, https://doi.org/10.1016/0038-0717(84)90082-8, 1984.
Schimel, J., Becerra, C. A., and Blankinship, J.: Estimating decay dynamics for enzyme activities in soils from different ecosystems, Soil Biol. Biochem., 114, 5–11, https://doi.org/10.1016/j.soilbio.2017.06.023, 2017.
Schimel, J. P. and Bennett, J.: Nitrogen mineralization: challenges of a changing paradigm, Ecology, 85, 591–602, https://doi.org/10.1890/03-8002, 2004.
Schöning, I., Knicker, H., and Kögel-Knabner, I.: Intimate association between -alkyl carbon and iron oxides in clay fractions of forest soils, Org. Geochem., 36, 1378–1390, https://doi.org/10.1016/j.orggeochem.2005.06.005, 2005.
Schulten, H. R. and Schnitzer, M.: The chemistry of soil organic nitrogen: a review, Biol. Fert. Soils, 26, 1–15, https://doi.org/10.1007/s003740050335, 1997.
Scott, E. E. and Rothstein, D. E.: The dynamic exchange of dissolved organic matter percolating through six diverse soils, Soil Biol. Biochem., 69, 83–92, https://doi.org/10.1016/j.soilbio.2013.10.052, 2014.
Servagent-Noinville, S., Revault, M., Quiquampoix, H., and Baron, M.: Conformational changes of bovine serum albumin induced by adsorption on different clay surfaces: FTIR analysis. J. Colloid. Interf. Sci., 221, 273–283, https://doi.org/10.1006/jcis.1999.6576, 2000.
Shah, F., Nicolás, C., Bentzer, J., Ellström, M., Smits, M., Rineau, F., Canbäck, B., Floudas, D., Carleer, R., Lackner, G., Braesel, J., Hoffmeister, D., Henrissat, B., Ahrén, D., Johansson, T., Hibbett, D. S., Martin, F., Persson, P., and Tunlid, A.: Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors, New Phytol., 209, 1705–1719, https://doi.org/10.1111/nph.13722, 2016.
Shimizu, M., Zhou, J., Schröder, C., Obst, M., Kappler, A., and Borch, T.: Dissimilatory reduction and transformation of ferrihydrite-humic acid coprecipitate, Environ. Sci. Technol., 47, 13375–13384, https://doi.org/10.1021/es402812j, 2013.
Silva, L. C., Doane, T. A., Corrêa, R. S., Valverde, V., Pereira, E. I., and Horwath, W. R.: Iron-mediated stabilization of soil carbon amplifies the benefits of ecological restoration in degraded lands, Ecol. Appl., 25, 1226–1234, https://doi.org/10.1890/14-2151.1, 2015.
Silva-Sánchez, A., Soares, M., and Rousk, J.: Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality, Soil Biol. Biochem., 134, 25–35, https://doi.org/10.1016/j.soilbio.2019.03.008, 2019.
Sinsabaugh, R. L.: Phenol oxidase, peroxidase and organic matter dynamics of soil, Soil Biol. Biochem., 42, 391–404, https://doi.org/10.1016/j.soilbio.2009.10.014, 2010.
Sinsabaugh, R. L., Hill, B. H., and Follstad Shah, J. J.: Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment, Nature, 462, 795–798, https://doi.org/10.1038/nature08632, 2009.
Six, J., Feller, C., Denef, K., Ogle, S. M., de Moraes Sa, J. C. , and Albrecht, A.: Soil organic matter, biota and aggregation in temperate and tropical soils - Effects of no-tillage, Agronomie, 22, 755–775, https://doi.org/10.1051/agro:2002043, 2002.
Sposito, G.: Electron shuttling by natural organic matter: twenty years after, in: Aquatic redox chemistry, edited by: Tratnyek, P. G., Grundl, T. J., and Haderlein, S. B., ACS Symp. Ser., 113–127, https://doi.org/10.1021/bk-2011-1071.ch006, 2011.
Stadtman, E. R. and Levine, R. L.: Free radical-mediated oxidation of free amino acids and amino acid residues in proteins, Amino Acids, 25, 207–218, https://doi.org/10.1007/s00726-003-0011-2, 2003.
Stucki, J. W.: Properties and behaviour of iron in clay minerals, in: Developments in clay science, edited by: Bergaya F. and Lagaly, G., Elsevier, 559–611, https://doi.org/10.1016/b978-0-08-098258-8.00018-3, 2013.
Suda, A. and Makino, T.: Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review, Geoderma, 270, 68–75, https://doi.org/10.1016/j.geoderma.2015.12.017, 2016.
Swenson, T. L., Bowen, B. P., Nico, P. S., and Northen, T. R.: Competitive sorption of microbial metabolites on an iron oxide mineral, Soil Biol. Biochem., 90, 34–41, https://doi.org/10.1016/j.soilbio.2015.07.022, 2015.
Tabatabai, M.: Effects of trace elements on urease activity in soils, Soil Biol. Biochem., 9, 9–13, https://doi.org/10.1016/0038-0717(77)90054-2, 1977.
Tamrat, W. Z., Rose, J., Grauby, O., Doelsch, E., Levard, C., Chaurand, P., and Basile-Doelsch, I.: Soil organo-mineral associations formed by co-precipitation of Fe, Si and Al in presence of organic ligands, Geochim. Cosmochim. Ac., 260, 15–28, https://doi.org/10.1016/j.gca.2019.05.043, 2019.
Tan, W., Yuan, Y., Zhao, X., Dang, Q., Yuan, Y., Li, R., Cui, D., and Xi, B.: Soil solid-phase organic matter-mediated microbial reduction of iron minerals increases with land use change sequence from fallow to paddy fields, Sci. Total Environ., 676, 378–386, https://doi.org/10.1016/j.scitotenv.2019.04.288, 2019.
Tian, Z., Wang, T., Tunlid, A., and Persson, P.: proteolysis of iron oxide-associated bovine serum albumin, Environ. Sci. Technol., 54, 5121–5130, https://doi.org/10.1021/acs.est.0c00860, 2020.
Tietjen, T. and Wetzel, R. G.: Extracellular enzyme-clay mineral complexes: Enzyme adsorption, alteration of enzyme activity, and protection from photodegradation, Aquat. Ecol., 37, 331–339, https://doi.org/10.1023/b:aeco.0000007044.52801.6b, 2003.
Tong, M., Yuan, S., Ma, S., Jin, M., Liu, D., Cheng, D., Liu, X., Gan, Y., and Wang, Y.: Production of abundant hydroxyl radicals from oxygenation of subsurface sediments, Environ. Sci. Technol., 50, 214–221, https://doi.org/10.1021/acs.est.5b04323, 2016.
Treat, C. C., Jones, M. C., Camill, P., Gallego-Sala, A., Garneau, M., Harden, J. W., Hugelius, G., Klein, E. S., Kokfelt, U., Kuhry, P., Loisel, J., Mathijssen, P. J. H., O'Donnell, J. A., Oksanen, P. O., Ronkainen, T. M., Sannel, A. B. K., Talbot, J., Tarnocai, C., and Väliranta, M.: Effects of permafrost aggradation on peat properties as determined from a pan-Arctic synthesis of plant macrofossils, J. Geophys. Res.-Biogeo., 121, 78–94, https://doi.org/10.1002/2015jg003061, 2016a.
Treat, C. C., Wollheim, W. M., Varner, R. K., and Bowden, W. B.: Longer thaw seasons increase nitrogen availability for leaching during fall in tundra soils, Environ. Res. Lett., 11, 064013, https://doi.org/10.1088/1748-9326/11/6/064013, 2016b.
Trusiak, A., Treibergs, L. A., Kling, G. W., and Cory, R. M.: The role of iron and reactive oxygen species in the production of CO2 in arctic soil waters, Geochim. Cosmochim. Ac., 224, 80–95, https://doi.org/10.1016/j.gca.2017.12.022, 2018.
Turner, S., Schippers, A., Meyer-Stüve, S., Guggenberger, G., Gentsch, N., Dohrmann, R., Condron, L. M., Eger, A., Almond, P. C., Peltzer, D. A., Richardson, S. J., and Mikutta, R.: Mineralogical impact on long-term patterns of soil nitrogen and phosphorus enzyme activities, Soil Biol. Biochem., 68, 31–43, https://doi.org/10.1016/j.soilbio.2013.09.016, 2014.
Van Bodegom, P. M., Broekman, R., Van Dijk, J., Bakker, C., and Aerts, R.: Ferrous iron stimulates phenol oxidase activity and organic matter decomposition in waterlogged wetlands, Biogeochemistry, 76, 69–83, https://doi.org/10.1007/s10533-005-2053-x, 2005.
Van Veen, J. A., and Kuikman, P. J.: Soil structural aspects of decomposition of organic matter by micro-organisms, Biogeochemistry, 11, 213–233, https://doi.org/10.1007/bf00004497, 1990.
Vitousek, P. M. and Howarth, R. W.: Nitrogen limitation on land and in the sea: How can it occur?, Biogeochemistry, 13, 87–115, https://doi.org/10.1007/bf00002772, 1991.
Vogel, C., Mueller, C. W., Höschen, C., Buegger, F., Heister, K., Schulz, S., Schloter, M., and Kögel-Knabner, I.: Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils, Nat. Commun., 5, 1–7, https://doi.org/10.1038/ncomms3947, 2014.
Vogel, C., Heister, K., Buegger, F., Tanuwidjaja, I., Haug, S., Schloter, M., and Kögel-Knabner, I.: Clay mineral composition modifies decomposition and sequestration of organic carbon and nitrogen in fine soil fractions, Biol. Fert. Soils, 51, 427–442, https://doi.org/10.1007/s00374-014-0987-7, 2015.
Wade, J., Waterhouse, H., Roche, L. M., and Horwath, W. R.: Structural equation modeling reveals iron (hydr)oxides as a strong mediator of N mineralization in California agricultural soils, Geoderma, 315, 120–129, https://doi.org/10.1016/j.geoderma.2017.11.039, 2018.
Wagai, R. and Mayer, L. M.: Sorptive stabilization of organic matter in soils by hydrous iron oxides, Geochim. Cosmochim. Ac., 71, 25–35, https://doi.org/10.1016/j.gca.2006.08.047, 2007.
Wagai, R., Kajiura, M., and Asano, M.: Iron and aluminum association with microbially processed organic matter via meso-density aggregate formation across soils: organo-metallic glue hypothesis, SOIL, 6, 597–627, https://doi.org/10.5194/soil-6-597-2020, 2020.
Wanek, W., Mooshammer, M., Blöchl, A., Hanreich, A., and Richter, A.: Determination of gross rates of amino acid production and immobilization in decomposing leaf litter by a novel 15N isotope pool dilution technique, Soil Biol. Biochem., 42, 1293–1302, https://doi.org/10.1016/j.soilbio.2010.04.001, 2010.
Wang, B., Lerdau, M., and He, Y.: Widespread production of nonmicrobial greenhouse gases in soils, Glob. Change Biol., 23, 4472–4482, https://doi.org/10.1111/gcb.13753, 2017.
Wang, M., Hu, R., Zhao, J., Kuzyakov, Y., and Liu, S.: Iron oxidation affects nitrous oxide emissions via donating electrons to denitrification in paddy soils, Geoderma, 271, 173–180, https://doi.org/10.1016/j.geoderma.2016.02.022, 2016.
Wang, X.-C. and Lee, C.: Adsorption and desorption of aliphatic amines, amino acids and acetate by clay minerals and marine sediments, Mar. Chem., 44, 1–23, https://doi.org/10.1016/0304-4203(93)90002-6, 1993.
Wardle, D. A., Bardgett, R. D., Klironomos, J. N., Setälä, H., van der Putten, W. H., and Wall, D. H.: Ecological linkages between aboveground and belowground biota, Science, 304, 1629–1633, https://doi.org/10.1126/science.1094875, 2004.
Warren, C.: What are the products of enzymatic cleavage of organic N?, Soil Biol. Biochem., 154, 108152, https://doi.org/10.1016/j.soilbio.2021.108152, 2021.
Wei, Y., Wu, X., Xia, J., Shen, X., and Cai, C.: Variation of Soil Aggregation along the Weathering Gradient: Comparison of Grain Size Distribution under Different Disruptive Forces, PLOS ONE, 11, e0160960, https://doi.org/10.1371/journal.pone.0160960, 2016.
Whalen, E. D., Grandy, A. S., Sokol, N. W., Keiluweit, M., Ernakovich, J., Smith, R. G., and Frey, S. D.: Clarifying the evidence for microbial- and plant-derived soil organic matter, and the path toward a more quantitative understanding, Glob. Change Biol., 28, 7167–7185, https://doi.org/10.1111/gcb.16413, 2022.
Wilmoth, J. L.: Redox heterogeneity entangles soil and climate interactions, Sustainability, 13, 10084, https://doi.org/10.3390/su131810084, 2021.
Wilmoth, J. L., Moran, M. A., and Thompson, A.: Transient O2 pulses direct Fe crystallinity and Fe(III)-reducer gene expression within a soil microbiome, Microbiome, 6, 1–14, https://doi.org/10.1186/s40168-018-0574-5, 2018.
Wilson, J. S. and Baldwin, D. S.: Exploring the “Birch effect” in reservoir sediments: influence of inundation history on aerobic nutrient release, Chem. Ecol., 4, 379–386, https://doi.org/10.1080/02757540802497582, 2008.
Wu, X., Cai, C., Wang, J., Wei, Y., and Wang, S.: Spatial variations of aggregate stability in relation to sesquioxides for zonal soils, South-central China, Soil Till. Res., 157, 11–22, https://doi.org/10.1016/j.still.2015.11.005, 2016.
Xu, C., Zhang, K., Zhu, W., Xiao, J., Zhu, C., Zhang, N., Yu, F., Li, S., Zhu, C., Tu, Q., Chen, X., Zhu, J., Hu, S., Koide, R. T., Firestone, M. K., and Cheng, L.: Large losses of ammonium-nitrogen from a rice ecosystem under elevated CO2, Sci. Adv., 6, 1–13, https://doi.org/10.1126/sciadv.abb7433, 2020.
Xu, J., Sahai, N., Eggleston, C. M., and Schoonen, M. A. A.: Reactive oxygen species at the oxide/water interface: Formation mechanisms and implications for prebiotic chemistry and the origin of life, Earth. Planet. Sc. Lett., 363, 156–167, https://doi.org/10.1016/j.epsl.2012.12.008, 2013.
Xu, Z., Yang, Z., Wang, H., and Jiang, J.: Assessing redox properties of natural organic matters with regard to electron exchange capacity and redox-active functional groups, J. Chem., 2020, 1–8, https://doi.org/10.1155/2020/2698213, 2020.
Xue, B., Huang, L., Huang, Y., Zhou, F., Li, F., Kubar, K. A., Li, X., Lu, J., and Zhu, J.: Roles of soil organic carbon and iron oxides on aggregate formation and stability in two paddy soils, Soil Till. Res., 187, 161–171, https://doi.org/10.1016/j.still.2018.12.010, 2019.
Yan, J., Pan, G., Li, L., Quan, G., Ding, C., and Luo, A.: Adsorption, immobilization, and activity of β-glucosidase on different soil colloids, J. Colloid. Interf. Sci., 348, 565–570, https://doi.org/10.1016/j.jcis.2010.04.044, 2010.
Yang, G., Peng, Y., Marushchak, M. E., Chen, Y., Wang, G., Li, F., Zhang, D., Wang, J., Yu, J., Liu, L., Qin, S., Kou, D., and Yang, Y.: Magnitude and pathways of increased nitrous oxide emissions from uplands following permafrost thaw, Environ. Sci. Technol., 52, 9162–9169, https://doi.org/10.1021/acs.est.8b02271, 2018.
Yang, W. H., Weber, K. A., and Silver, W. L.: Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction, Nat. Geosci.,5, 538–541, https://doi.org/10.1038/ngeo1530, 2012.
Yang, Z., Gao, J., Yang, M., and Sun, Z.: Effects of freezing intensity on soil solution nitrogen and microbial biomass nitrogen in an alpine grassland ecosystem on the Tibetan Plateau, China, J. Arid Land., 8, 749–759, https://doi.org/10.1007/s40333-016-0012-0, 2016.
Yang, Z., Liao, Y., Fu, X., Zaporski, J., Peters, S., Jamison, M., Liu, Y., Wullschleger, S. D., Graham, D. E., and Gu, B.: Temperature sensitivity of mineral-enzyme interactions on the hydrolysis of cellobiose and indican by β-glucosidase, Sci. Total Environ., 686, 1194–1201, https://doi.org/10.1016/j.scitotenv.2019.05.479, 2019.
Yu, G. H., Sun, F. S., Yang, L., He, X. H., and Polizzotto, M. L.: Influence of biodiversity and iron availability on soil peroxide: Implications for soil carbon stabilization and storage, Land Degrad. Dev., 31, 463–472, https://doi.org/10.1002/ldr.3463, 2020.
Yu, W. H., Li, N., Tong, D. S., Zhou, C. H., Lin, C. X., and Xu, C. Y.: Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review, Appl. Clay Sci., 80–81, 443–452, https://doi.org/10.1016/j.clay.2013.06.003, 2013.
Yuan, Y., Zhao, W., Zhang, Z., Xiao, J., Li, D., Liu, Q., Yin, H., and Yin, H.: Impacts of oxalic acid and glucose additions on N transformation in microcosms via artificial roots, Soil Biol. Biochem., 121, 16–23, https://doi.org/10.1016/j.soilbio.2018.03.002, 2018.
Zhang, J., Presley, G. N., Hammel, K. E., Ryu, J. S., Menke, J. R., Figueroa, M., Hu, D., Orr, G., and Schilling, J. S.: Localizing gene regulation reveals a staggered wood decay mechanism for the brown rot fungus Postia placenta, P. Natl. Acad. Sci. USA, 113, 10968–10973, https://doi.org/10.1073/pnas.1608454113, 2016.
Zhang, X. W., Kong, L. W., Cui, X. L., and Yin, S.: Occurrence characteristics of free iron oxides in soil microstructure: evidence from XRD, SEM and EDS, B. Eng. Geol. Environ., 75, 1493–1503, https://doi.org/10.1007/s10064-015-0781-2, 2016.
Zhang, Y. and Scherer, H.: Mechanisms of fixation and release of ammonium in paddy soils after floodingII. Effect of transformation of nitrogen forms on ammonium fixation, Biol. Fert. Soils, 31, 517–521, https://doi.org/10.1007/s003740000202, 2000.
Zhao, L., Dong, H., Kukkadapu, R., Agrawal, A., Liu, D., Zhang, J., and Edelmann, R. E.: Biological oxidation of Fe(II) in reduced nontronite coupled with nitrate reduction by Pseudogulbenkiania sp. Strain 2002, Geochim. Cosmochim. Ac., 119, 231–247, https://doi.org/10.1016/j.gca.2013.05.033, 2013.
Zhao, Q., Callister, S. J., Thompson, A. M., Kukkadapu, R. K., Tfaily, M. M., Bramer, L. M., Qafoku, N. P., Bell, S. L., Hobbie, S. E., Seabloom, E. W., Borer, E. T., and Hofmockel, K. S.: Strong mineralogic control of soil organic matter composition in response to nutrient addition across diverse grassland sites, Sci. Total Environ., 736, 137839, https://doi.org/10.1016/j.scitotenv.2020.137839, 2020.
Zhou, G. W., Yang, X. R., Li, H., Marshall, C. W., Zheng, B. X., Yan, Y., Su, J. Q., and Zhu, Y. G.: Electron shuttles enhance anaerobic ammonium oxidation coupled to Iron(III) reduction, Environ. Sci. Technol., 50, 9298–9307, https://doi.org/10.1021/acs.est.6b02077, 2016.
Zhu, B., Gutknecht, J. L. M., Herman, D. J., Keck, D. C., Firestone, M. K., and Cheng, W.: Rhizosphere priming effects on soil carbon and nitrogen mineralization, Soil Biol. Biochem., 76, 183–192, https://doi.org/10.1016/j.soilbio.2014.04.033, 2014.
Zhu-Barker, X., Cavazos, A. R., Ostrom, N. E., Horwath, W. R., and Glass, J. B.: The importance of abiotic reactions for nitrous oxide production, Biogeochemistry, 126, 251–267, https://doi.org/10.1007/s10533-015-0166-4, 2015.
Zinder, B., Furrer, G., and Stumm, W.: The coordination chemistry of weathering: II. Dissolution of Fe(III) oxides, Geochim. Cosmochim. Ac., 50, 1861–1869, https://doi.org/10.1016/0016-7037(86)90244-9, 1986.
Zou, J., Huang, J., Zhang, H., and Yue, D.: Evolution of humic substances in polymerization of polyphenol and amino acid based on non-destructive characterization, Front. Environ. Sci. En., 15, 5, https://doi.org/10.1007/s11783-020-1297-y, 2020.
- Abstract
- Introduction
- Roles of Fe in controlling N bioavailability
- Involvement of Fe in soil phenomena that affect N bioavailability
- Impact of global change on Fe–N bioavailability interactions
- Synthesis and outlook
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Review statement
- References
- Abstract
- Introduction
- Roles of Fe in controlling N bioavailability
- Involvement of Fe in soil phenomena that affect N bioavailability
- Impact of global change on Fe–N bioavailability interactions
- Synthesis and outlook
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Review statement
- References