the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Twentieth-century ecological disasters in central European monoculture pine plantations led to critical transitions in peatlands
Mariusz Lamentowicz
Piotr Kołaczek
Daria Wochal
Michał Jakubowicz
Luke Andrews
Katarzyna Marcisz
The frequency of extreme events worldwide is steadily increasing. Therefore, it is crucial to recognize the accompanying response of different ecosystems. Monoculture tree plantations with simplified ecosystem linkages in food webs are particularly vulnerable to catastrophic events like fires, wind throws, droughts, and insect outbreaks. These events threaten forests and other associated ecosystems, including peatlands, which are extremely important in regulating the global carbon cycle and, thus, mitigating the effects of a warming climate. Here, we traced a 2000-year history of the Miały peatland, which is located in one of Poland's largest pine plantation complexes, and we examined how this peatland responded to some of the largest environmental disasters observed in the 20th century across central Europe: the 1922–1924 Panolis flammea outbreak and the 1992 fire. As a disturbance proxy, we used a multi-proxy palaeoecological analysis (plant macrofossils, testate amoebae, pollen, non-pollen palynomorphs, and micro- and macrocharcoal) supported by a neodymium isotope record. We showed several critical transitions in the peatland associated with extreme events and anthropogenic impacts, which triggered significant changes in the peatland's ecological status.
- Article
(7374 KB) - Full-text XML
- BibTeX
- EndNote
In recent decades, peatlands have been subjected to intense and ever-increasing climatic and anthropogenic pressures (Zhang et al., 2022). Hydrologically unstable due to diverse anthropogenic impacts, they are becoming extremely susceptible to various types of disturbances and extreme phenomena, which are a threat to human health, cause economic losses, and contribute to the amplification of the global warming effect (Kiely et al., 2021; Page et al., 2002). Peatlands have evolved from being net CO2 sinks to CO2 emitters in every climate zone – from tropical regions (Deshmukh et al., 2021; Page et al., 2022) to the boreal realm (Ofiti et al., 2023; Turetsky et al., 2011; Wilkinson et al., 2023). This is particularly important because peatlands are valuable ecosystems accumulating a third of the world's soil carbon stocks (Parish et al., 2008), twice the entire biomass of the world's forests (Beaulne et al., 2021).
Hundreds of thousands of hectares of peatlands in Poland are located in forests, as forests cover 31 % of Poland's area, equivalent to 94 770 km2 (Statistical Office in Białystok, 2023). More than half of this forest cover comprises coniferous forests dominated by Scots pine (Pinus sylvestris L.). It is mainly the result of planned forest management in modern-day Poland in the 19th and 20th centuries (Broda, 2000). Pine monocultures were easier to manage and grew faster on poor soils, securing a continuous supply of raw material for the growing timber industry (Broda, 2000). Such an environment is particularly dangerous for Poland's peatlands, as monoculture tree plantations have simplified linkages in food webs and are therefore more sensitive to fires, strong winds, droughts, and insect outbreaks (Chapin et al., 2012), which also poses a threat to peatlands. It should be strongly emphasized here that such extreme phenomena have become more common in recent years around the world (Seidl et al., 2014; Westerling, 2016). These negative impacts have been recorded for various peatlands, including those in central and eastern Europe (Leonardos et al., 2024; Łuców et al., 2021).
It is essential to recognize how peatlands at different latitudes respond to a warming climate and how they respond to changes resulting from the management of their surroundings (land use change), including planned forests and monoculture tree plantations. Thanks to their anaerobic and acidic conditions, peatlands are excellent preservers of various types of micro- and macrofossils (Rydin and Jeglum, 2013; Tobolski, 2000). Thus, peatlands serve as valuable archives of both internal (autogenic) changes within the peatland itself and external (allogenic) changes in the surrounding environment (Marcisz et al., 2024).
Multi-proxy palaeoecological studies (including analyses of several proxies, such as testate amoebae, plant macrofossils, pollen, charcoal, and others) are an excellent tool for reconstructing the peatland development (Birks and Birks, 2006; Mitchell et al., 2000). Particularly broad insight can be provided when dendrological (Bąk et al., 2024) or geochemical methods (Fiałkiewicz-Koziełet al., 2018; Gałka et al., 2019; Marcisz et al., 2023b) are included. In recent years, the neodymium (Nd) isotope composition of the peat-hosted mineral matter has been increasingly used in palaeoecological studies. Among various applications, the method has been used to determine distant sources of atmospheric dust (Allan et al., 2013; Fagel et al., 2014; Pratte et al., 2017) and the signal associated with anthropogenic pollution (Fiałkiewicz-Koziełet al., 2016). Marcisz et al. (2023b) used this method to identify local disturbances in peat, such as fires or deforestation.
The environmental past of the largest European forest complexes, including the Noteć Forest area in Poland studied here, is insufficiently understood. These forests were affected by some of the most severe environmental disasters of the 20th century that took place in pine-dominated forests across central and eastern Europe: the 1922–1924 Panolis flammea outbreak and the 1992 fire. The only palaeoecological data documenting these events in the Noteć Forest were derived from two cores taken from the Rzecin peatland (Barabach, 2014; Lamentowicz et al., 2015; Milecka et al., 2017). However, the interpretation of these extreme events based solely on these two cores appears to leave many questions unanswered and highlights the need for further research into the impact of insect outbreaks and fires on peatland ecosystems. The changes caused by extreme events can lead a peatland to reach a critical transition, i.e. to cross a tipping point after which it does not return to its previous hydrological and trophic conditions (Dakos et al., 2019; Lenton et al., 2008, 2019). So far, peatland research has focused chiefly on the tipping points associated with changes in groundwater levels due to a warming climate, fires, pollution, carbon sequestration, or opening landscape caused by agricultural development (Fiałkiewicz-Koziełet al., 2015; Jassey et al., 2018; Lamentowicz et al., 2019a, b; Loisel and Bunsen, 2020). Except for these issues, there is a need for a broader recognition of the consequences of insect outbreaks in forest areas and the accompanying forest management.
In this article, we focus on the impact of catastrophic events on the ecosystem of the Miały peatland in the Noteć Forest (local scale) and the broad context of such disturbances for pine plantations in central and eastern Europe (regional scale). Our aims were as follows: (1) reconstruct the environmental history of the Miały peatland using multi-proxy palaeoecological analyses (including analyses of pollen, non-pollen palynomorphs, testate amoebae, plant macrofossils, and charcoal) and geochemical analyses (neodymium isotope signatures) and, through this reconstruction, identify peat layers corresponding to severe environmental catastrophic events; (2) assess the impact of such disturbances on the peatland ecosystem and understand the relation between disturbances occurring in the surrounding forest and the peatland. We hypothesized that catastrophic events in pine plantations, including insect outbreaks and fires, cause significant changes in the peatlands located in their area and even a complete change in trophic and hydrological conditions, leading to a critical transition.
2.1 Study site
The Miały peatland is located in western Poland, about 65 km northwest of Poznań (Fig. 1). It is located within the boundaries of the Noteć Forest, one of the largest forest complexes in Poland, covering an area of about 1370 km2 (Statistical Office in Białystok, 2023). The Noteć Forest is a monoculture dominated by Scots pine (Pinus sylvestris, 95 % of the tree stand) (Sukovata, 2022). A large part of the pine forest, including our research site, is located in the “Puszcza Notecka” protected landscape area. It is also a special protected area, “Puszcza Notecka” (PLB300015, since 2007), and a special area of conservation, “Dolina Miały” (PLH300042, since 2023), under Natura 2000. According to the physical geographical regionalization, the peatland is located in the Gorzów Basin mesoregion, in the Warta and Noteć inter-river sub-mesoregion. It is a high glacial–alluvial terrace covered with dunes with a relative height of 20–40 m (Kondracki, 2001). It has a temperate transitional climate. From 1981 to 2010, the average annual air temperature was 8.4 °C. The warmest month was July, with an average temperature of 18.8 °C, and the coolest month was January, with an average temperature of −1.1 °C. Average annual precipitation for 1981–2010 equalled 563 mm, with the precipitation maximum in July (69 mm) and the minimum in April (31 mm) (Institute of Meteorology and Water Management, 2025).
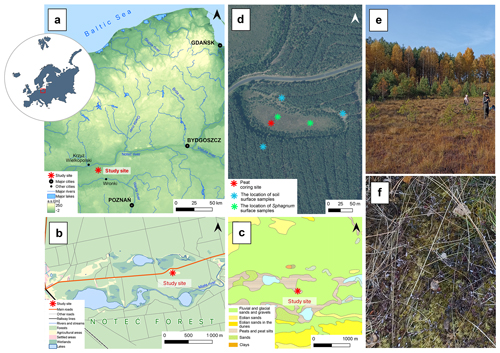
Figure 1(a–c) The location of the study site on topographic (a, b) and geological (c) maps. (d) Orthophoto of the Miały peatland with sampling points (asterisks): red – peat core sampling site; blue – soil surface sampling sites for the neodymium isotope analyses; green – Sphagnum surface sampling sites for the neodymium isotope analyses. (e) Photograph of the peatland and its forest surroundings. (f) Sphagnum mosses covered the peatland surface.
2.2 Fieldwork and sampling
The peat core was collected from the western part of the peatland in October 2021 using a Wardenaar corer (chamber dimensions: ) (Wardenaar, 1987). The entire length of the sampled peat core – a 97 cm long monolith – was analysed. The core was subsampled continuously every 1 cm, except for the first sample (0–2 cm), which contained a living layer of peat-forming vegetation. A total of 96 samples were obtained for multi-proxy analyses, including the water content in fresh material, organic matter content in dry material, ash-free bulk density, peat accumulation rate, peat carbon accumulation rate, plant macrofossils, testate amoebae, macroscopic and microscopic charcoal, pollen, and neodymium isotopes. Moreover, five surface samples of Sphagnum mosses (two samples) and soil (three samples) were taken as a reference for downcore neodymium measurements (Fig. 1), following the approach of Marcisz et al. (2023b).
2.3 Radiocarbon dating, absolute chronology, and peat accumulation rates
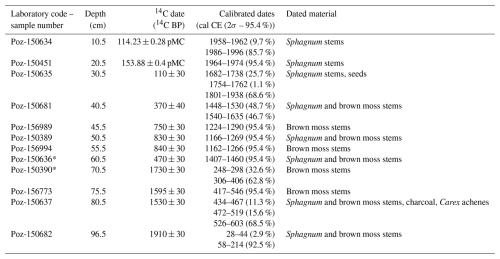
Twelve samples containing Sphagnum, brown moss stems, charcoal, and Carex achenes were used for accelerator mass spectroscopy (AMS) 14C dating of the entire length of the core, conducted at the Poznań Radiocarbon Laboratory in Poland (laboratory code marked Poz; Table 1).
The absolute chronology of the core was based on a Bayesian age–depth model using OxCal v4.4.4 (Bronk Ramsey, 2025). The P_Sequence command with a parameter k of 0.75 cm−1 calculated the model, assuming and interpolation=1 cm. The IntCal20 (Reimer et al., 2020) and Bomb21NH1 (Hua et al., 2021) atmospheric curves were used as calibration sets. The most pronounced changes in peat composition, as manifested by changes in pollen concentration, testate amoeba species composition, and species composition of plant macrofossils, which may signal changes in peat accumulation rates, were inputted using the Boundary command. In this model, the Boundary command was input at a depth of 26 cm, with a pronounced change in pollen concentration. Two dates (laboratory code Poz-150636 and Poz-150390) were rejected because they were outside the main trajectory of the model. Peat accumulation rates were retrieved from the age–depth model using the OxCal software.
Table 1The list of radiocarbon dates from Miały peatland with calibration. The outliers are marked with asterisks (*). The IntCal20 (Reimer et al., 2020) and Bomb21NH1 (Hua et al., 2021) atmospheric curves were used to calibrate the dates. pMC represents percent modern carbon. 2σ presents the confidence interval that encompasses a 95.4 % probability.
2.4 Peat properties and peat carbon accumulation rates
The water content in a wet sample (WC, %), organic matter content in a dry sample (ORG, %), ash content (ASH, g, %), ash-free bulk density (BD, g cm−3), peat accumulation rate (PAR, mm yr−1), and peat carbon accumulation rate (PCAR, ) were calculated for each of the 96 samples. For these analyses, the volume of each sample was accurately measured using calipers. Next, each sample was placed in separate crucibles, weighed, dried, and weighed again to determine the percent WC. The dried samples were burned in a muffle furnace at 550 °C for 5 h and reweighed according to the protocol of Heiri et al. (2001) to determine ASH. BD was calculated by dividing the weight of the dry sample by the volume of the fresh sample and multiplied by ORG, according to Chambers et al. (2010). PAR was calculated based on core chronology and then multiplied by the BD value obtained earlier and by 50 % to obtain PCAR, according to Loisel et al. (2014).
2.5 Plant macrofossil analysis
The plant macrofossils were analysed using the modified protocol of Mauquoy et al. (2010). Each sample of approximately 5 cm3 underwent wet sieving (mesh diameter: 200 µm). The generalized content of the sample was estimated as a percentage using a binocular microscope. Fruits, seeds, achenes, perigynia, scales, whole preserved leaves, sporangia, and opercula were counted as total numbers in each sample. The tissues of monocotyledon species and moss leaves (brown and Sphagnum mosses) were identified on slides using ×200 and ×400 magnification. The material was compared with the guides (Anderberg, 1994; Berggren, 1969; Bojňanský and Fargašová, 2007; Mauquoy and van Geel, 2007). The diagram for the analysed proxy was plotted using the “riojaPlot” package for R (Juggins, 2025).
2.6 Testate amoeba analysis
Peat samples for testate amoeba analysis were washed under 300 µm mesh following Booth et al. (2010). Testate amoebae were analysed under a light microscope with ×200 and ×400 magnification until the sum of 100 tests per sample was reached (Payne and Mitchell, 2009); however, in peat layers below 27 cm, the testate amoeba sums were lower (between 5 and 50) due to the very low concentration of tests. Several keys, including taxonomic monographs (Clarke, 2003; Mazei and Tsyganov, 2006; Meisterfeld, 2001) and online resources (Siemensma, 2025), were used to achieve the highest possible taxonomic resolution. The results of the testate amoeba analysis were used for the quantitative depth to water table (DWT) and pH reconstructions. Both reconstructions were performed in C2 software (Juggins, 2007) using the European training set (Amesbury et al., 2016). In layers with low testate amoeba sums, water table reconstruction should be viewed with caution (Payne and Mitchell, 2009).
2.7 Pollen and non-pollen palynomorph analyses
Samples for palynological analysis (volume: 3 cm3 for 0–21 cm and 1 cm3 for 21–97 cm) were prepared using standard laboratory procedures (Berglund and Ralska-Jasiewiczowa, 1986). To remove the carbonates, samples were treated with 10 % hydrochloric acid. This step was followed by digestion in hot 10 % potassium hydroxide (to remove humic compounds) and soaking in 40 % hydrofluoric acid for 24 h (to remove the mineral fraction). Next, acetolysis was carried out. Three Lycopodium tablets (Batch 280521291, containing 18 407 spores per tablet; produced by Lund University) were added to each sample during the laboratory procedures for the calculation of microfossil concentration (Stockmarr, 1971). Pollen, spores, and selected non-pollen palynomorphs (NPPs) were counted under an upright microscope (Zeiss Axio SCOPE A1) until the number of total pollen sum (TPS) grains in each sample reached at least 500, apart from 10 samples in which pollen concentrations were very low. Two of them (depths: 19–18 and 17–16 cm) were excluded due to an extremely low pollen concentration, and it was impossible to reach 100 grains included in TPS. Sporomorphs were identified with the assistance of atlases, keys (Beug, 2004; Moore et al., 1991), various publications, and the image database in the case of NPPs, for which there are no atlases (Miola, 2012; Shumilovskikh et al., 2022; Shumilovskikh and van Geel, 2020). The results of the palynological analysis were expressed as percentages, and calculations are based on the ratio of an individual taxon to the TPS, i.e. the sum of AP (arboreal pollen) and NAP (non-arboreal pollen), excluding aquatic and wetland plants (together with Cyperaceae and Ericaceae), cryptogams, and fungi. A pollen diagram was drawn using the Tilia program (Grimm, 1991).
2.8 Macro- and microcharcoal analyses
Microscopic charcoal particles (size: >10 µm) were analysed from the same slides as pollen following the standard protocol, and the number of charcoal particles and Lycopodium spores counted together exceeded 200 (Finsinger and Tinner, 2005; Tinner and Hu, 2003). Microscopic charcoal influx or accumulation rates (particles ) were calculated by multiplying the charcoal concentrations by the peat accumulation rate (PAR) (Davis and Deevey, 1964; Tinner and Hu, 2003).
A total of 96 contiguous samples (2 cm3) were prepared for macroscopic charcoal analysis. Bleaching was used to create a more visible contrast between the charcoal and the remaining organic matter, following the method described by Whitlock and Larsen (2001). The samples were sieved through a 500 µm mesh and analysed with a binocular microscope under ×60 magnification. Only charcoal fragments>600 µm were analysed to obtain the local fire signal (Adolf et al., 2018). Macroscopic charcoal influx or accumulation rates (particles ) were calculated using the charcoal concentrations and the PAR.
2.9 Neodymium isotopes
We used neodymium isotopes to assess the impact of disturbances on the Miały peatland. This method helps determine the sources of mineral matter in peat profiles, including whether it was washed into the peatland basin (i.e. the peatland had a connection to groundwater and was of minerotrophic origin) or was primarily of atmospheric origin (meaning the peatland functioned more as an ombrotrophic, rain-fed system) (Marcisz et al., 2023b). Isotopic measurements were performed from peat samples taken along the peat core as well as from reference material from the surface of the peatland and soil around it (Fig. 1). All analytical procedures and isotopic measurements were performed in the Poznań Isotope Laboratory, Poland, on a Finnigan MAT 261 multi-collector thermal ionization mass spectrometer. Details of the analytical procedures are provided by Marcisz et al. (2023b). Peat samples, as well as surface Sphagnum and soil samples from both peatlands, were dried and burned at 550 °C overnight. Prior to preparation for isotopic measurements, the ash of peat and soil samples was dissolved on a hot plate (∼100 °C for 3 d) in closed perfluoroalkoxy vials using a mixture of concentrated hydrofluoric- and nitric acids (4:1). The ash of fresh plant material was digested in 16 N HNO3. Neodymium was separated using the miniaturized chromatographic techniques described by Pin et al. (1994) and Dopieralska (2003). The analytical precision was monitored by analysing the USGS reference material BHVO-2 ( (2σ; n=2)). Neodymium (loaded as phosphate) was measured on Re in a double-filament configuration. Isotopic ratios were collected in a dynamic mode. Nd isotope ratios were normalized to . Repeated measurements of the AMES standard yielded (2σ, n=12). Nd isotope data are reported in the standard ε notation:
where CHUR denotes the present-day Chondritic Uniform Reservoir ( and ) (Jacobsen and Wasserburg, 1980).
2.10 Statistical analyses
To quantify periods of rapid vegetation change in the forest (regional scale) and on the peatland (local scale), as well as hydrological and trophic shifts on the peatland (local scale), we apply principal response curves (PrCs) to the data, as outlined by Burge et al. (2023) in their “baselines” R package. This approach allows for the identification of directional shifts and when these begin to accumulate beyond the level expected from random variation. The multivariate palynological data (pollen data and NPPs; individual taxa only) were Hellinger-transformed and reduced to a one-dimensional curve using PrCs. Thus, PrC results trace changes in the relative abundance of pollen and NPPs over time. While most rate-of-change (RoC) studies rely on a single proxy, the combination of multiple proxies, including pollen and algae, has been applied in previous research (e.g. Abrook et al., 2020).
This method is useful for detecting changes in data with a strong underlying gradient in palaeoecological studies (Van Den Brink and Ter Braak, 1999; De'ath, 1999). Generalized additive mixed models (GAMMs) were then fitted to the data, with a smoothing term accounting for temporal autocorrelation. A cubic regression spline was used as the smoothing basis, with k=20. A range of values for k was tested to ensure that the model avoids overfitting or underfitting the data. Likewise, maximum likelihood (ML) was used for consistency with Burge's framework, instead of restricted maximum likelihood (REML). However, REML was used to reanalyse the data in place of ML as a smoothing parameter, although it did not make an appreciable difference to the results.
When poor GAMM fits occurred, adaptive splines with generalized additive models (GAMs) were compared with the GAMM to assess model fits. Adaptive spline GAMs provide better fits to data exhibiting abrupt changes but cannot yet be incorporated into the GAMM framework (Simpson, 2018). Periods of significant change were identified in the GAMM models by calculating the time intervals during which the confidence intervals surrounding the first derivative did not include zero. PrCs were derived from constrained ordination of the time series palynological data, which use the “prcurve()” function (“analogue” package) in R.
The phases in the palaeoecological analyses were distinguished based on changes in plant communities obtained from palynological and plant macrofossil data.
3.1 Chronology, peat accumulation rates, and peat properties
The age–depth model shows the agreement index (Amodel) of 61 %, just above the recommended minimum of 60 % (Bronk Ramsey, 2008) (Fig. 2). The model has the highest uncertainty, with a 95.4 % confidence interval – 80 calibration years – at depths between 65.5 and 64.5 cm (ca. 840–870 cal CE; Fig. 2). The age of the oldest layer – 96.5 cm – was modelled at 130±45 (confidence interval: 1σ) cal CE (Fig. 2).
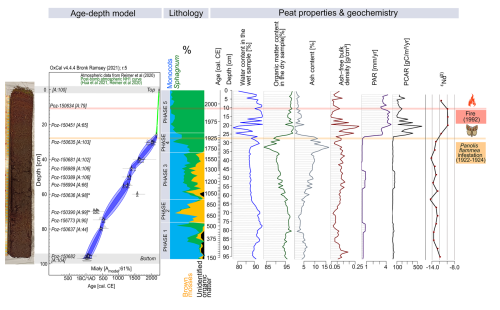
Figure 2Bayesian age–depth model (based on 14C dating) and lithology (based on plant macrofossils analysis) with palaeoecological phases of the peat profile in Miały (on the left side). Changes in the physical peat properties (water content in the wet sample, organic matter content in the dry sample, ash content, ash-free bulk density, PAR, and PCAR) and neodymium isotope signatures – εNd – are marked. The timing of the most critical catastrophic disasters in the 20th century is also marked.
The water content of the wet sample ranged from 77.0 % (22–21 cm, ca. 1965 cal CE) to 95.0 % (20–19 cm, ca. 1970 cal CE), averaging 89.4 % throughout the core (Fig. 2). Organic matter content of the dry sample ranged from 83.6 % (33–32 cm, ca. 1755–1785 cal CE) to 99.2 % (22–21 cm, ca. 1965 cal CE), with an average of 94.5 % in the entire core (Fig. 2). Bulk density ranged from 0.04 g cm−3 (15–14 cm, ca. 1980 cal CE) to 0.28 g cm−3 (21–20 cm, ca. 1965–1970 cal CE), with an average of 0.12 g cm−3 across the core (Fig. 2). Average PAR throughout the core was relatively slow at 1.3 mm yr−1, while it was fastest at 4.8 mm yr−1 (20–19 cm, ca. 1970 cal CE) and slowest at 0.2 mm yr−1 (43–42 cm, ca. 1395–1440 cal CE) (Fig. 2). The average PCAR had a value of 73.4 , with the largest value being 590.6 (21–20 cm, ca. 1965–1970 cal CE) and the smallest being 10.2 (71–70 cm, ca. 665–700 cal CE) (Fig. 2). Higher PAR and PCAR values were associated with an undecomposed acrotelm zone.
3.2 Palaeoecological analysis
3.2.1 Phase 1 (97–76 cm, ca. 130–520 cal CE): very wet peatland with a dominance of monocots surrounded by mixed forest
The local vegetation (Fig. 3) for most of this period is dominated by monocots (max 96 % of plant macrofossil content), including Carex, whose achenes are found in the peat profile. Cyperaceae pollen makes up max 6.0 % (Fig. 4). Short periods of Sphagnum dominance (max 80 %), mainly Sphagnum subgenus Cuspidata (max 40 %), occur (Fig. 3). This phase is also characterized by a high content of unidentified organic matter, reaching up to 10 % (Fig. 3).
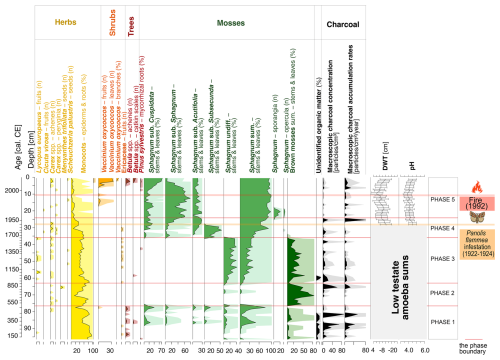
Figure 3A diagram showing macrofossil percentages and macroscopic charcoal concentrations and influx as a local fire proxy. Testate amoeba-based DWT and pH curves for the 27–0 cm layers are also presented. The timing of the most critical catastrophic disasters in the 20th century is also marked. Ten times exaggeration is presented.
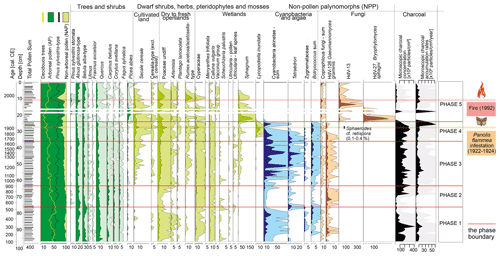
Figure 4Pollen diagram with selected taxa presented (full list of taxa is provided in the associated open dataset). Pollen percentages are shown in black, and 10 times exaggeration is marked. Microscopic charcoal concentrations and influx as an extra-local fire proxy are also presented. Two samples (depths: 19–18 and 17–16 cm) were excluded from the diagram due to an extremely low pollen concentration (no data are shown for these depths).
The low sums of testate amoebae do not allow for a statistically significant reconstruction of water and pH levels in this phase (full data in the open dataset, Bąk et al., 2025). However, among the testate amoeba taxa, Centropyxis aculeata dominates quantitatively. There is a high percentage of cyanobacteria and algae (Zygnemataceae, Botryococcus) (Fig. 4) and a maximum of the Utricularia curve in the pollen data (max 0.5 %; Fig. 4).
Pinus sylvestris-type (39.0 %–65.8 %) grains are the most frequent, but the pollen of deciduous trees is relatively common as well (Fig. 4): Betula alba type (7.4 %–26.4 %), Alnus glutinosa type (max 17.0 %), Quercus (max 15.6 %), Carpinus betulus (max 5.8 %), Corylus avellana (max 4.6 %), and Fagus sylvatica (max 3.5 %). Remains of Betula (achenes and catkin scales) are present in the plant macrofossils (Fig. 3).
The highest fire activity is recorded for ca. 310–330 cal CE, with a macroscopic charcoal concentration of ca. 70 particles cm−3 (Fig. 3) and a microscopic charcoal concentration of ca. 420 000 particles cm−3 (Fig. 4), and for ca. 430–455 cal CE, with 90 particles cm−3 of macroscopic charcoal (Fig. 3).
3.2.2 Phase 2 (76–64 cm, ca. 520–890 cal CE): moderately wet peatland and landscape closure – increase in forestation and decrease in ruderal species
The Sphagnum content decreases in favour of brown moss (max 85 %) and monocot remains (max 80 %), including Carex (achenes and perigynia of this taxon are found; Fig. 3). Cyperaceae pollen (Fig. 4) make up between 3.4 % and 8.4 %. This is the only phase in which seeds of Menyanthes trifoliata are found (Fig. 3), and the pollen curve maximum of this taxon is observed (0.3 %; Fig. 4).
Reconstructions of the DWT and trophic conditions imply a low abundance of testate amoebae, with a continuation of the quantitative dominance of C. aculeata (full data in the open dataset, Bąk et al., 2025). The share of freshwater bacteria and algae decreases significantly at this time (Fig. 4). Cyanobacteria reach a maximum of 5.9 % (Fig. 4).
This period has the highest forest cover in the peatland's surroundings. Arboreal pollen accounts for over 90 % of total pollen throughout this phase (Fig. 4). Compared to phase 1, the share of Betula alba-type pollen decreases (5.1 %–19.1 %), while the share of Pinus sylvestris-type pollen slightly increases (44.6 %–65.2 %) (Fig. 4). Admixture species – Alnus glutinosa type (max 17.9 %), Quercus (max 9.2 %), Carpinus betulus (max 6.8 %), Corylus avellana (max 5.2 %), and Fagus sylvatica (max 2.7 %) – continue to be relatively important (Fig. 4).
For the first half of phase 2, fire activity is low, but it increases in the second half. The concentration of both microscopic and macroscopic charcoal increases markedly towards the end of this phase, reaching a maximum of 61 particles cm−3 for macroscopic charcoal (Fig. 3) and ca. 293 600 particles cm−3 for microscopic charcoal (Fig. 4).
3.2.3 Phase 3 (64–36 cm, ca. 890–1660 cal CE): very wet peatland, expansion of Sphagnum mosses, development of agriculture, and gradual decrease in deciduous trees
Sphagnum mosses (max 42 %) appear again, although, due to the significant degree of the material decomposition, it was not possible to determine lower taxonomic ranks in the plant macrofossil analysis (Fig. 3). The content of the remains of monocots (max 85 %) and brown mosses (max 55 %) remains high (Fig. 3). Carex achenes are also present (Fig. 3). The percentage of Cyperaceae pollen is relatively high (2.0 %–7.0 %; Fig. 4). This is the only phase in which fruits of Lycopus europaeus are found (Fig. 3). Seeds of Scheuchzeria palustris are also present (Fig. 3).
The concentration of testate amoebae remains low; thus, the reconstruction of water levels and trophic conditions should again be treated with caution (full data in open dataset, Bąk et al., 2025). Species of the genera Centropyxis sp., Cyclopyxis sp., and Difflugia sp. dominate quantitatively. The increase in cyanobacteria (max 82.6 %) and freshwater algae, especially Tetraëdron (max 24.6 %) and Botryococcus (max 2.5 %), is significant (Fig. 4).
The share of arboreal pollen is high, ranging from 86 % to 94 %, although with a slightly decreasing trend, compounded by declines in admixture species (Fig. 4). Pinus sylvestris type represented 51 %–68 % and Betula alba type represented 6 %–15 % of total pollen. At the end of this phase, the share of Alnus glutinosa type, Quercus, Carpinus betulus, Corylus avellana, and Fagus sylvatica with respect to total pollen was 11.6 %, 5.5 %, 2.0 %, 1.1 %, and 1.6 %, respectively. The declines in the percentage of these taxa may be related to the increased contribution of cereal pollen (Fig. 4). Among cereal pollen, Secale cereale dominates, reaching a maximum of 2.2 %. The percentages of Poaceae, Artemisia, Plantago lanceolata, and Rumex acetosa/acetosella type also increased (Fig. 4).
3.2.4 Phase 4 (36–24 cm, ca. 1660–1960 cal CE): the further expansion of Sphagnum mosses and an increase in Pinus sylvestris-type pollen with an episodic extreme decrease in it
The expansion of Sphagnum is continued. The percentage of monocot remains decreases to 15 % by the end of this phase. However, the number of achenes and perigynia of Carex is higher than in any other part of the profile (Fig. 3). The percentage of Cyperaceae pollen ranges from 2.7 % to 13.0 % (Fig. 4). The initial part of the phase is dominated by the Sphagnum subgenus Subsecunda (Fig. 3). At the same time, Lycopodiella inundata appears (Fig. 4). The brown mosses completely disappear.
At the end of phase 4, the abundance of testate amoebae increases (with Galeripora discoides, Nebela tincta, and Phryganella acropodia as dominant species), which allows for statistically significant reconstructions of the water table level and pH level (Fig. 3). The abundance of cyanobacteria and algae decreases distinctly; most of them disappear entirely at the end of this phase (Fig. 4).
In the pollen dataset (Fig. 4), a further decrease in the percentage of deciduous species is observed. In the upper part of phase 4, the share of Alnus glutinosa-type, Quercus, Carpinus betulus, Corylus avellana, and Fagus sylvatica pollen with respect to total pollen is 3.4 %, 1.9 %, 1.2 %, 1.3 %, and 0.6 %, respectively. The share of Betula alba-type pollen with respect to total pollen remains at about the same level (5.9 %–12.2 %). A significant decrease in Pinus sylvestris-type pollen percentages and an increase in the percentages of Secale cereale, Poaceae, Plantago lanceolata, and Rumex acetosa/acetosella-type pollen occur in ca. 1900–1926 cal CE.
Analysis of the macroscopic charcoal data (Fig. 3) shows one local fire event (macroscopic charcoal concentration of 22 particles cm−3 and macroscopic charcoal accumulation rate of 7 particles ; 1952–1956 cal CE). The regional fire activity (Fig. 4) remained quite high (microscopic charcoal concentration of ca. 127 000–312 000 particles cm−3 and microscopic charcoal accumulation rate of ca. 3900–61 000 ).
3.2.5 Phase 5 (24–0 cm, ca. 1960–2021 cal CE): the dominance of Sphagnum mosses and the disappearance of cyanobacteria and algae, the development of microscopic fungi, and the episodic extreme collapse of the arboreal pollen curve
The uppermost part of the profile records further development of Sphagnum, initially Sphagnum subgenus Sphagnum and later Sphagnum subgenus Cuspidata. The proportion of Sphagnum subgenus Acutifolia remains stable. Sphagnum capsule remains – sporangia and opercula – appear; we link their presence with spores of the parasitic fungus Bryophytomyces sphagni (see Sect. 4). Tree remains (Betula achenes and catkin scales and Pinus sylvestris mycorrhizal roots) are abundant. Vaccinium oxycoccos leaves appear in large numbers.
At the beginning of this phase, cyanobacteria and algae disappear completely. Testate amoeba species such as G. discoides, Galeripora catinus, and N. tincta are abundant. G. discoides dominates for most of phase 5, and the abundance of N. tincta increases towards its end. The groundwater level remains constant, except for one marked fluctuation (ca. 1990–1995 cal CE), whereas the pH level increases gradually from ca. 1995 cal CE (Fig. 3). Both phenomena can be linked to the effect of the 1992 fire (see Sect. 4).
Pinus sylvestris type remains the dominant species in this phase of the profile (32.6 %–78.9 %). Compared to the previous phase, the percentage of Betula alba-type pollen increases (5.6 %–20.3 %). One significant decrease in the share of tree pollen, in particular Pinus sylvestris type, is recorded in ca. 1995 cal CE. We interpret this as decreased forest cover after the 1992 fire (see Sect. 4). At the same time, a higher share of Pinus sylvestris stomata typifies ca. 1980–2000 cal CE layers (0.2 %–3.9 %). We associate this with massive needle falls associated with the fire (see Sect. 4). Rumex acetosa/acetosella type – a taxon characteristic of open and ruderal areas (Behre, 1981) – reaches its maximum at 19.6 % (ca. 1995 cal CE), which we also interpret as an effect of the fire. The shares of other deciduous trees – Quercus (max 3.9 %), Carpinus betulus (max 1.6 %), Corylus (max 1.3 %), and Ulmus (max 0.7 %) – decrease.
3.3 Neodymium isotope analysis
The εNd values measured in the mineral matter extracted from the analysed peat samples range from −14.5 to −9.8. Most samples show a relatively low variability in the strongly negative Nd isotope ratios (), including the most negative values in layers 61–60 and 41–40 cm. Less negative εNd values (ranging from −9.9 to −9.8) are only observed in the upper part of the profile, most notably in the 21–20, 16–15, and 11–10 cm layers.
Among the reference surface samples, the mineral material from the peatland surface yielded moderately negative εNd signatures (−12.1 and −11.7), whereas the soil taken from the slopes of the peatland catchment display a strongly unradiogenic Nd isotope composition ( to −16.5; Fig. 2, Table 2). The study site is covered by young glacial material dominated by clay and sand derived from Scandinavia, transported and accumulated during the last glaciation (Marks, 2012). Previously, Nd isotope measurements in the young glacial sediments of another outwash plain covered by a pine monoculture were measured by Marcisz et al. (2023b), who reported εNd similar negative signatures ( to −16.6) to those in Miały.
3.4 Statistical analyses
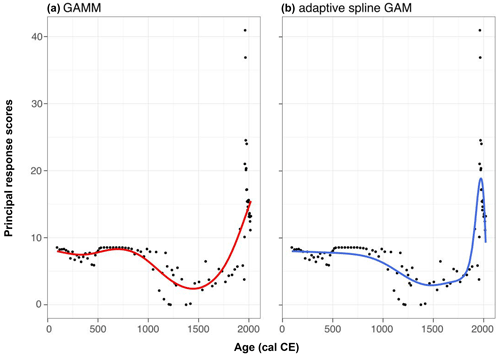
The PrC explained 73 % of the variance in the palynological data. However, the GAMM provided a relatively poor fit to the data. An adaptive spline GAM provided a better explanation of the data, with the differences between the two models primarily related to the improved fit with the more recent samples. This suggests a possible return to previous conditions, although these samples are more likely to be influenced by temporal autocorrelation. Despite this, the GAMM effectively captures the general trends in the data and provides a better fit for the earliest samples (Fig. A1). Therefore, we can proceed to use these data.
The PrC analysis revealed that changes over time occurred between the beginning of the record and 1720 cal CE. However, there is no substantial evidence of significant or rapid changes until after this time. From approximately 1000 cal CE until the 1700s, the PrC scores exhibited high variability. A significant increase in the RoC was identified for the period ca. 1725–2005, as shown in Fig. 5.
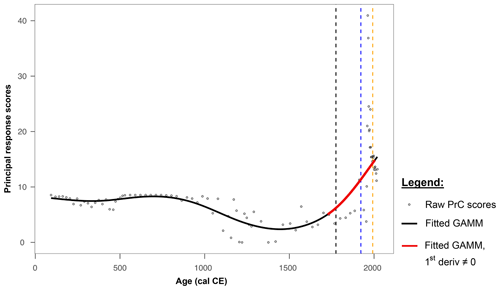
Figure 5Changes in the principal response curve derived from pollen count data (circles) fit with a GAMM fit (solid black and red lines). The red line indicates periods of rapid change. Dashed vertical lines indicate historical periods of forest management change affecting the site: the 1775 decree by Frederick the Great (black); infestation by Panolis flammea (1922–1924; blue); and the 1992 fire period (yellow).
4.1 Combining ecological, palaeoecological, geochemical, and historical data to understand long-term environmental changes
Present-day pine monoculture plantations of Poland are often perceived as typical of this region by the local population, whereas these are highly modified forests that are significantly different from the natural ones. Compared to natural potential vegetation maps, these areas should possess a large proportion of deciduous taxa, e.g. oak–hornbeam forests (Querco-Carpinetum medioeuropaeum) (Matuszkiewicz, 2008). The relatively high percentages of deciduous tree pollen compared to the percentages of Pinus sylvestris-type pollen in historical times were recorded at many sites from present-day pine monocultures in northern Poland (Bąk et al., 2024; Czerwiński et al., 2021). The development of the Polish state and agriculture in the early Middle Ages, in our data manifested by the high percentages of cereal pollen grains (including Secale cereale) and taxa characteristic for open and ruderal areas (Poaceae, Artemisia, Plantago lanceolata, and Rumex acetosa-acetosella type), caused a decline in deciduous species in the forest composition (Fig. 4). These changes in the forest structure were distinct but gradual; when planned management was introduced in 18th century, however, the contribution of admixture trees started to decrease rapidly. In 1772 CE, the area of the Noteć Forest was included in the borders of the Kingdom of Prussia as a result of the First Partition of Poland. At that time, some of the first legal regulations for planned forest management in the area appeared, including the 1775 CE decree of Frederick the Great regarding government forests in Prussia and the preference for planting pines instead of deciduous species (Bąk et al., 2024; Jaszczak, 2008). Around this time, the PrC analysis began to reveal periods of significant and rapid change in the palynological record. Since then, both the forest and, even more so, the Miały peatland within it have continued to undergo substantially rapid changes, unlike the preceding changes. The results of the PrC analysis indicated a significant RoC, confirming the occurrence of critical transitions in the peatland on a scale that was not observed in the older part of the core. The trend in the PrC aligns broadly with the patterns seen in the data, as shown by the correspondence between the PrC scores and the relative contributions of deciduous trees, arboreal pollen, Pinus sylvestris-type pollen, and NPPs.
It is commonly assumed that outwash plains or aeolian sandy dunes, remnants of the Weichselian glaciation (to 11 700 BP), which are currently covered by extensive Scots pine monoculture in northern Poland (e.g. the Noteć Forest and the Tuchola Forest) are not conducive to the growth of other species and that Pinus is a natural main forest-forming species (Magnuski, 1993; Miś, 2003). Although pollen data suggest the dominance of Pinus sylvestris type since the 2nd-century CE, a distinct admixture of Quercus, Carpinus betulus, and Corylus avellana was recognized in our study. Previous multi-proxy palaeoecological studies from the Noteć Forest exist; however, they were unable to provide information on the proportion of admixture tree species in the forest composition prior to the onset of planned forest management. The cores collected from the Rzecin peatland covered only the last 200 years and did not capture the entire background of the changes related to human activity and subsequent forest management (Barabach, 2014; Lamentowicz et al., 2015; Milecka et al., 2017; Słowiński et al., 2019). Knowledge of the historical background is essential for the interpretation of the complex response of the peatland ecosystem to a change in forest management, as it allows for the long-term tracing of reference conditions relating to both the composition of the forest and the trophic and hydrological variants of the peatland (Bąk et al., 2024). In this study, we recorded the presence of hydrophytes and later also helophytes and hygrophytes (e.g. Utricularia, Menyanthes trifoliata, Lycopus europaeus, Scheuchzeria palustris, and Cicuta virosa) in the first four phases of the peatland development (up to ca. 1960 CE; Fig. 4). Combined with the high percentages of cyanobacteria and algae (Zygnemataceae, Botryococcus, and Tetraëdron) and dominance of Centropyxis sp., Cyclopyxis sp., and Difflugia sp. among the testate amoebae, this indicates the existence of a shallow waterbody supplied not only by rainwater and runoff but also by groundwater (Figs. 3 and 4). All of these taxa disappeared in phase 5, after ca. 1960 CE.
The relative stability of the ecosystem until the 20th century appears to be in line with the moderately variable, unradiogenic neodymium isotope signatures of the mineral matter extracted from the peat samples ( to −11.6). These data are similar to the results from other peatlands in the Tuchola Forest, Poland: the Stawek peatland (−15.3 to −12.7) and Głęboczek peatland (−13.7 to −12.6) (Marcisz et al., 2023b). The notably consistent εNd values in the pre-infestation part of the studied profile point to the dominance of local sources of the mineral matter. Strongly unradiogenic εNd values are generally characteristic of the surface clastic sediments that dominate the young post-glacial landscape of northern Poland (Marcisz et al., 2023a, b).
The instability of the ecosystem witnessed after the 20th century is a consequence of the introduction of planned forest management and the planting of monoculture plantations in the late 18th century. Indeed, such forests are more sensitive to disturbance and extreme phenomena than mixed forests. The Noteć Forest fell victim to such management and faced two massive ecological disasters in the 20th century: the Panolis flammea outbreak in 1922–1924 and a fire in 1992. The consequences of the Panolis flammea outbreak were particularly severe, as they directly caused a complete change in the trophic and hydrological conditions of the peatland in the following decades, i.e. in the period around 1925–1960.
Nevertheless, all three above-mentioned disturbance factors (introduction of planned forest management, 1922–1924 outbreak, and 1992 fire) affected the condition of the peatland and were recorded as significant RoCs in the GAMM model, which can be interpreted as critical transitions (Fig. 5).
4.2 Panolis flammea outbreak (1922–1924) and its impact on peatland and pine plantations
One of the most harmful documented insect outbreaks in Poland happened in 1922–1924 CE (Broda, 2003) and covered vast areas of central and eastern Europe (today's area of Germany, Poland, Lithuania, Belarus, and part of European Russia), progressing from west to east (Ziółkowski, 1924). It was caused by Panolis flammea, one of the most dangerous primary pests of pine trees (Szmidt, 1993). As a result of the 1922–1924 Panolis flammea infestation, over 500 000 ha of forest was defoliated in Europe (Głowacka, 2009). In the Noteć Forest, the first caterpillars (found in 1921 CE) did not herald an ecological disaster (Broda, 2003). However, over the next 2 years, between 1922 and 1923, ca. 64 000 ha of the forest was destroyed (Hernik, 1979). In the Potrzebowice forest district, where our site is located, the outbreak destroyed over 90 % of the forest area (ca. 8000 ha) (Broda, 2003).
This outbreak is evidenced in our pollen record, marked by a sharp decrease in the percentage of Pinus sylvestris-type pollen (48.0 %) compared to the neighbouring layers – ca. 1875–1900 cal CE (60.6 %) and ca. 1925–1950 cal CE (62.8 %). After almost all the pine trees were destroyed and the caterpillars had nothing to eat, they attacked the deciduous trees, on which they do not usually feed (Anon, 1929). In our data, a manifestation of this shift is probably the decrease in the proportion of Betula alba-type, Alnus glutinosa-type, and Quercus pollen. This layer also shows the highest share of Poaceae (14.7 %), cereal (10.4 %), and Plantago lanceolata (2.7 %) pollen in the entire peat core. The share of Rumex acetosa/acetosella-type pollen (6.6 %) is also high. The presence of taxa characteristic of open and ruderal areas indicates that the landscape has opened up due to logging activities in the destroyed forest stands. However, in the Rzecin peatland, 8 km southeast of our site, a significant decrease in Pinus pollen has not been observed (Barabach, 2014). According to Barabach (2014), as a result of immediate human activities, heliophytes did not develop, and a natural secondary succession did not occur in the Rzecin bog's surroundings. Barabach (2014) argued that a single pine that stands alone will produce more pollen than the same pine in a compact forest stand, referring to the individual trees that survived the disaster. Later, along with wind and water, the pollen was deposited in natural depressions, including the Rzecin peatland. However, an increase in Poaceae pollen percentages has been recorded, confirming the opening of the landscape in the Rzecin bog's surroundings.
The layers corresponding to ca. 1900–1950 cal CE are the only portions of the core in which spores of Sphaerodes retispora (also known as Microthecium retisporum) were identified. This taxon occurs on other fungus (Trametes hirsuta), which inhabits dead trees and their branches, as well as recently dead and decaying wood (Bhatt et al., 2016). It mainly attacks deciduous trees, although reports of attacks on coniferous trees are known (Szwałkiewicz, 2009). Perhaps the appearance of the S. retispora spores in these layers reflects the presence of T. hirsuta on dead wood after the Panolis flammea outbreak. We also observed higher percentages of coprophilous fungi (including HdV-55 A Sordaria-type fungi) in the layer corresponding to ca. 1900–1925 cal CE (2.7 %) compared to neighbouring layers – ca. 1875–1900 cal CE (0.4 %) and ca. 1925–1950 cal CE (0.9 %). Sordaria-type coprophilous fungi can indicate the presence of open land, the presence of livestock, and wood detritus or wood burning (Lageard and Ryan, 2013; Lundqvist, 1972; Mighall et al., 2008; Wheeler et al., 2016). We point out, however, that Sordaria-type spores can also occur on the faeces of wild herbivores and are predominantly coprophilous, meaning that this taxon may include non-coprophilous species (Shumilovskikh and van Geel, 2020). Kołaczek et al. (2013), at the Jesionowa mire in southern Poland, noted the co-occurrence of a high percentage of Sordaria-type spores and a high percentage of Poaceae, cereal, Rumex acetosa/acetosella-type, and Plantago lanceolata pollen, i.e. taxa characteristic of open areas that we observed in our pollen dataset during and after the outbreak. However, in the surroundings of the Jesionowa mire, the landscape has not opened up due to deforestation, but the grazing of livestock has intensified. Synchronously, Barabach (2014) reported a massive emergence of Glomeromycota spores, which can be widely considered an indicator of soil erosion (Ejarque et al., 2010; Van Geel et al., 1989). Indeed, the deforestation associated with the outbreak resulted in increased water and wind erosion. However, Kołaczek et al. (2013) argue that Glomeromycota spores can be considered indicators of soil erosion only in lacustrine deposits. In peatlands, there is a high risk of the presence of plant species capable of forming arbuscular mycorrhizae. Glomeromycota spores then come from fungi that have colonized the roots of plants growing on the surface of the peatland.
In their study of the Rzecin peatland, Milecka et al. (2017) reported an increase in charcoal in ca. 1910–1925 cal CE. The authors linked this increase to the fires occurring in the Noteć Forest in the 1920s and 1930s. Still, it could also result from cleanup activities after the Panolis flammea outbreak, such as raking and burning litter with dead caterpillars. Barabach (2014) reported a higher content of ash and a higher charcoal concentration in the concerned interval. We did not observe increased micro- or macroscopic charcoal concentrations in the Miały peatland. It is possible that the redistribution of charcoal particles to the edges of the peatland occurred due to high water levels. A core taken closer to the edge could, therefore, give a complete answer as to the extent of burning.
Following the outbreak, an increase in the proportion of Picea abies until the early 1970s is observed in our dataset. After the outbreak, initial management plans included diversification of species composition in the newly planted forest's forest stands. Still, P. sylvestris was selected as the primary species. Other planted species included Betula (mainly along the roads), Pinus strobus, Pinus banksiana, Pinus rigida, Alnus glutinosa, Robinia pseudoacacia, and Prunus serotina (Mroczkiewicz, 1933). Considering that P. abies reaches sexual maturity after 20–30 years in open areas (Skrøppa, 2003) or even later in closed areas (ca. 40 years) (Matthias and Giesecke, 2014; Rispens, 2003), we conclude that the observed increase in P. abies pollen is an echo of the 1922–1924 outbreak.
Recognizing the ecology of past Panolis flammea outbreaks in central and eastern Europe can help model and predict its risk of occurrence in northern Europe, which is warming due to climate change. Pulgarin Díaz et al. (2022) report that, between 1970 and 2020, the range of Panolis flammea in Finland shifted nearly 5° northward, 50 years earlier than assumed. The remains of these moths could help determine the scale and ecology of historical outbreaks in central and eastern Europe and, thus, better predict their future effects in northern Europe. Unfortunately, they do not preserve well in the sediments (Bąk et al., 2024). However, we emphasize that we did not use advanced extraction methods that could potentially preserve the delicate structures of the moth wing remains (Montoro Girona et al., 2018); rather, we only undertook observation under light and stereoscopic microscopes when viewing the samples in the analyses used. We also have not encountered Panolis flammea at Miały peatland. Palaeoecological analyses such as pollen and testate amoeba analyses can support the recognition of the results of such historical outbreaks, but they cannot confirm that an outbreak occurred. There are, however, palaeoecological reconstructions of outbreaks caused by other pests whose remains are better preserved in the sediment. For example, Schafstall et al. (2022) showed the usefulness of subfossil bark beetles for reconstructing disturbances occurring in Picea abies forests in Slovakia.
4.3 Changing trophic and hydrological conditions as an effect of post-outbreak forest management
The effect of the Panolis flammea outbreak was tens of thousands of hectares of damaged forests. Damaged forests were cleaned, and the land was prepared for new planting. However, the opportunity to rebuild the forest's species structure was not seized. Easy-to-manage and fast-growing pine trees were used for forest regeneration (Ankudo-Jankowska, 2003), which caused a change in the trophic conditions of the peatland manifested by the decline in pH in our data (Fig. 3). After the infestation, in our dataset, we also notice the expansion of Sphagnum mosses, which tolerate more acidic conditions. Sphagnum content reaches 65 % for ca. 1900–1925 cal CE and 85 % for ca. 1955–1960 cal CE, further increasing in the upper part of the section (Fig. 3) and almost completely displacing monocot plants and brown mosses. We assume that more acidic conditions in the peatland after the Panolis flammea outbreak are the result of monoculture planting regimes after this devastating event, as many studies have documented the ability of various pine species to acidify the soil (Berthrong et al., 2009; Cifuentes-Croquevielle et al., 2020; Hornung, 1985; Turner and Lambert, 1988). This is confirmed by the highest percentages of Pinus sylvestris-type pollen at Miały between 1950 and 1960. This is because Pinus sylvestris in dense forest complexes begins flowering at an age of about 25–30 years (Mátyás et al., 2004).
The process of peatland acidification is a natural manifestation of peatland development over time, as long as it occurs gradually. We noted a gradual transition from a moderately rich fen to a poor fen in phase 4 (ca. 1660–1960 cal CE). However, further changes in local plant communities and hydrological and trophic conditions toward acidification occurred abruptly, characteristic of external interference. Bąk et al. (2024) pointed out that such changes are a result of forest management activities and can be caused by drainage and transformation in forest species composition. In this study, we emphasize the importance of the consequences of vulnerability and poor resilience of monoculture plantations to disturbances and extreme phenomena such as insect outbreaks.
The change in trophic conditions at this time, as well as the concomitant change in hydrological conditions, are also documented by the complete disappearance of cyanobacteria and algae (Fig. 4), indicating that the peatland was cut off from the groundwater supply. Among testate amoebae, G. discoides, N. tincta, and P. acropodia, species that tolerate unstable hydrological conditions became dominant, suggesting a lowering of the water table and substantial water table fluctuations (Lamentowicz and Mitchell, 2005; Sullivan and Booth, 2011).
This observation is supported by the concurrent change in the Nd isotopic signatures (Fig. 2). The deforestation caused by the Panolis flammea infestation is followed by an increase in the Nd isotope ratios, reaching εNd values notably higher than those observed in any of our reference samples from the peatland catchment. Therefore, the elevated εNd values, coinciding with the notably decreased ash contents, most likely reflect a decreased supply of local sediments from surface runoff and groundwater flow. This interpretation is in agreement with the acidification of the peatland; the transition in the hydrological regime likely resulted in an increased relative role of extra-local, aeolian sources of the sedimentary material (Allan et al., 2013; Fagel et al., 2014; Marcisz et al., 2023a). A specific source of such 143Nd-enriched sediments cannot, however, be identified based on the εNd record alone.
In the period of the transition of trophic and hydrological conditions in a Miały peatland (ca. 1925–1960 CE cal CE), we observed the appearance of Bryophytomyces sphagni (HdV-27). Some studies have specified that this fungus is an indicator of the change from minerotrophic to ombrotrophic conditions in a peatland, especially in association with the appearance of Sphagnum spores (van Geel et al., 2020). Although we observe numerous spores of this fungus in the narrow period of changing trophic and hydrological conditions in our dataset (ca. 1925–1960 CE cal CE), we also note that the massive number of B. sphagni spores does not necessarily indicate sudden ombrotrophication of the peatland. There are many studies in which the appearance of B. sphagni does not correlate with the ombrotrophication of the peatland (van der Linden et al., 2008; McCarroll et al., 2017; Yeloff et al., 2007). Thus, we emphasize the need for better recognition of the ecology of B. sphagni. With the appearance of B. sphagni, Gaeumannomyces caricis (HdV-126) disappears. G. caricis is a fungus associated with Carex (van Geel and Aptroot, 2006; Pals et al., 1980). In our plant macrofossil data, Sphagnum mosses, as we mentioned above, have almost completely displaced monocots, including Carex, which dominated the peatland in phases 3–5. A coincident disappearance of G. caricis, the appearance of B. sphagni, and the development of Sphagnum have been noted in the past in southwest France (Aoustin et al., 2022). These authors, among others, based on the large number of spores of B. sphagni, decided to separate the developmental phase of the object they studied, which they referred to as Sphagnum bog (Aoustin et al., 2022).
Sudden changes in trophic conditions, resulting in subsequent changes in the vegetation cover in the catchment, are one of the most common causes of critical transitions in peatlands (Lamentowicz et al., 2019b).
4.4 Fire in 1992 – the second-largest fire in the post-World War II history of Poland
Potential high and medium modern fire danger concerns 83 % of forests in Poland (65 % in Europe) (Szczygieł, 2012). This is mainly due to poor habitats and a homogeneous forest structure, with Pinus sylvestris as the dominant species. Pinus, in turn, favours the accumulation of a significant amount of dry biomass on the surface. Fire danger is also a result of the young age of the tree stands, which have not yet developed stable ecosystem links in food webs. The young stands result from planned forest management involving rapid wood harvesting and 20th-century ecological disasters (particularly insect outbreaks). Industrial pollution, increasing accessibility to the public, and climate change, resulting in prolonged droughts and water deficits, amplify the problems of forest composition and management.
The 1992 droughts were marked by fires in many regions of Poland (Polna, 2005) and other countries in central Europe (Kula and Jankovská, 2013; Somsak et al., 2009). Almost 12 000 forest fires were recorded in Poland alone, and nearly 48 000 ha of forest area burned. The largest fire in Poland's post-war history, which burned more than 9000 ha of forest (Szczygieł, 2012), occurred near the town of Kuźnia Raciborska (Silesia, southern Poland) between 26 and 30 August 1992. Two weeks prior to this event, the second-largest fire in Poland's post-war history had affected the Noteć Forest.
In the 1970s, Hernik (1979) and Ratajszczak (1979) signalled that the tree stands of the Noteć Forest were weakened by repeated insect outbreaks (Panolis flammea: 1956; Lymantria monacha: 1947 and 1964; Barbitistes constrictus: 1964; Diprion pini: 1961; Bupalus piniarius, 1966; and Dendrolimus pini: 1970). Compared to the 1922–1924 Panolis flammea outbreak, however, they were smaller, less severe, and covered different locations of the Noteć Forest, rather than a larger area. The authors stressed the need to introduce admixture species to change the age structure of the forest and reduce the fire threat. Their predictions soon turned out to be very accurate. On 2 June 1992, a fire covered about 700 ha of the Noteć Forest (Bugaj, 1992), and on 10 August of the same year, the fire consumed more than 5000 ha of forest in just 8 h (Fabijański, 1996). The total area affected was mapped in detail by the foresters (Fig. 6). Only an enclave of several hectares of deciduous old-growth forest resisted the fire.
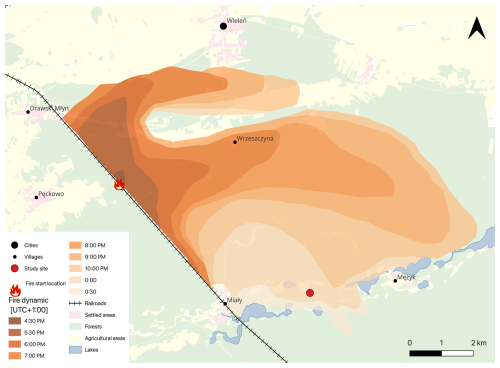
Figure 6The rate of fire spread in the Noteć Forest in 1992 (author's own work based on data provided by the State Forests, Potrzebowice forest district).
Macroscopic charcoal concentrations did not register this fire event as we expected. Although the concentrations of microscopic charcoal in ca. 1989–1991 cal CE (ca. 30 800 particles cm−3) and ca. 1991–1994 cal CE (ca. 27 500 particles cm−3) are higher than those in ca. 1986–1989 cal CE (ca. 10 000 particles cm−3) and ca. 1994–1997 cal CE (ca. 16 300 particles cm−3), these values do not reflect the actual scale of the forest destruction, especially because the fire also took place on the peatland (Fig. 6). A smaller-than-expected signal from the 1992 fire in charcoal analysis was also obtained by Barabach (2014) in the nearby Rzecin peatland. The small amount of macroscopic charcoal may be explained by the fact that more intense fires produce smaller charcoal particles (Schaefer, 1973). Additionally, before the particles are deposited, their dispersion by wind and water plays an important role (Patterson et al., 1987). Shortly after the fire reached the peatland, heavy rain had fell, reaching a value of 31.5 mm (Institute of Meteorology and Water Management, 2025). This rain stopped the fire from spreading further and significantly limited the movement of charcoal by wind.
The events are, however, well recorded by other proxies. Directly after the fire – ca. 1991–1994 cal CE and ca. 1994–1997 cal CE – a substantial decrease in the percentage of arboreal pollen, especially of Scots pine, is observed in the pollen dataset. At the same time, Pinus sylvestris stomata appear, which may indicate a fall of needles to the surface. However, we recommend a cautious approach to interpreting the presence of Pinus sylvestris stomata. While burnt Pinus sylvestris stomata would give certainty to the occurrence of fire, needle fall due to other processes should also be considered. High water levels may also have contributed to the shedding of needles by Pinus in the peatland (which we explain below). The water table rose to ground level, probably due to inundation. The rise in the groundwater level shortly after increased fire activity is a well-known phenomenon observed at other sites (Marcisz et al., 2015). The rise in water level is correlated with a high concentration (72 %) of the testate amoeba Galeripora discoides, which tolerates hydrologically unstable conditions and is abundant in disturbed ecosystems (Lamentowicz and Mitchell, 2005). Rumex acetosa/acetosella-type pollen reaches its maximum percentage, which is accompanied by an increase in the percentage of Poaceae pollen, a taxon characteristic of open areas, indicating the landscape's opening due to the forest's reduction. In their study of the Tuchola Forest peatlands, Marcisz et al. (2023b) observed pronounced decreases in the εNd values following major fire events, attesting to an increased supply of locally sourced sedimentary material favoured by forest removal. Analogously, some decrease in the εNd values following the 1992 fire is observed in the peat profile in this study. Therefore, we note that it is not always possible to unambiguously identify local fire events, even using high-resolution charcoal analysis, and that historical sources can validate the data. This is a crucial finding regarding the interpretations of palaeofire reconstructions, pointing out that even catastrophic fires can go unnoticed in the sedimentary record.
The scale and frequency of catastrophic fires, including forest and peatland fires, have been increasing for decades worldwide due to climate change (Sayedi et al., 2024). In terms of the total area burned, the year 2022 was the second-worst year ever recorded in the European Union (San-Miguel-Ayanz et al., 2023). Nearly 900 000 ha of natural area was burned, 43 % of which was located in Natura 2000 regions. In Poland, almost 7000 fires in natural areas (including more than 4800 forest fires) were recorded, resulting in approximately 2850 ha of area burned (including 2210 ha in forests). In terms of the number of fires in natural areas, more fires were recorded in France (22 800 fires; 70 300 ha), Spain (10 500; 268 000 ha), and Portugal (10 400; 110 000 ha). Therefore, forest fires in Poland were frequent but covered small areas (0.4 ha per fire on average). Most of the fires in Poland occurred in May (more than 25 %), a significant percentage of which were caused by drought. A recent study from the pine-dominated Tuchola Forest in Poland revealed a negative correlation between Scots pine growth and rainfall in May (Bąk et al., 2024), which indeed indicates a water deficit in that month. In 2022, there were 84 fire incidents in the Noteć Forest that resulted in 8.4 ha of burnt area. From 2007 to 2022, there were more than 1170 fire incidents that covered 96.7 ha. Hence, the Noteć Forest is a high-fire-risk area and, as a large monoculture forest complex, requires continuous monitoring, including within EU structures.
Understanding the functioning of peatlands that are under severe climatic pressure and have been exposed to extreme events in recent decades is crucial for their conservation and monitoring. Peatlands, as archives of environmental change, are sources of valuable information about past ecological disasters, recorded in both the palaeoecological and geochemical records. Combining these two approaches gives a complete picture of environmental changes due to fires or insect outbreaks. The conclusions of such studies can be successfully used to predict the consequences of contemporary phenomena. Particularly severe disasters can even lead to peatland ecosystems reaching critical transitions, after which there is an irreversible change in hydrological and trophic conditions, followed by a change in vegetation. We have identified many palaeo-indicators that allow a comprehensive assessment of the peatland's response to catastrophic events, both at the time of these events and on a long-term scale (Fig. 7).
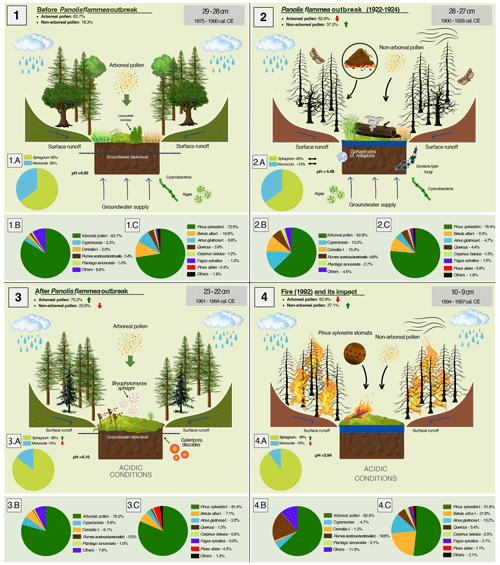
Figure 7Diagram showing environmental changes in the Miały peatland and the forest surrounding it as a result of the Panolis flammea outbreak (1922–1924; panel nos. 1 and 2), leading to a change in forest structure to a Pinus sylvestris monoculture (panel no. 3) and the consequences of poorly resilient monocultures in the form of the 1992 fire (panel no. 4). The percentages of taxa in the pie charts were taken from palynological data. Each of the four large panels corresponds to one specific layer in the peat profile – the depth of the layer and the calibrated period are marked in the upper-right corners of the panels in a grey box.
We have shown that the Miały peatland has rapidly acidified as a result of Panolis flammea infestation and forest restoration activities. We reported a significant decrease in Pinus sylvestris-type pollen during catastrophic events. Competition among plants in the peatland was won by those adapted to acidic conditions, Sphagnum mosses, which displaced monocotyledonous plants. We point out that it is difficult to identify past Panolis flammea outbreaks, as the remains of these moths do not preserve well in sediments. We emphasized a cautious approach to fungi as bioindicators of environmental change due to many ambiguous interpretations in studies. Charcoal analysis can provide information on localized fires, but it should be emphasized that not every fire is recorded in this way. For this reason, adequate validation of the data with historical sources or, if these do not exist, multi-proxy palaeoecological analyses are essential. However, we point out that other palaeo-recordings, treated cautiously, can help identify past fires, such as Pinus sylvestris stomata. To understand current or recent changes in peatlands and their surroundings, it is often not enough to analyse the recent history covering the last 100–200 years; rather, the background going back hundreds or thousands of years must be considered. Only such a combination gives a complete overview of changes due to human activity, climate change, or ecological disasters. We observed that there has been no catastrophic deforestation for more than 1800 years. Major deforestation occurred only after changes in forest management. The peatland was also hydrologically and trophically stable for most of the time analysed. Drastic changes in trophic and hydrological conditions of the Miały peatland began after the introduction of planned forest management in the late 18th century, weakening forests' resilience to environmental disasters. Particularly extreme changes occurred with the 1922–1924 Panolis flammea outbreak period and the subsequent approach to forest restoration after 30–40 years. Moreover, keeping the forest structure homogeneous led to a huge fire in 1992 (Fig. 7).
The open dataset that supports the findings of this study is available from Mendeley Data: https://doi.org/10.17632/cv5t59wf24.1 (Bąk et al., 2025).
MB: fieldwork, laboratory analyses (bulk density, carbon accumulation, plant macrofossils, and selection of plant macrofossils for AMS radiocarbon dating), age–depth modelling, data interpretation, visualization, and writing (original draft). ML: fieldwork, support with plant macrofossil analysis, data interpretation, and writing (commented on and edited the manuscript). PK: laboratory analyses (pollen and spores), age–depth modelling, data interpretation, visualization, and writing (commented on and edited the manuscript). DW: laboratory analyses (testate amoebae), testate amoeba-based reconstructions, and data interpretation. MJ: fieldwork, data interpretation, and writing (commented on and edited the manuscript). LA: statistical analyses, data interpretation, and writing (commented on and edited the manuscript). KM: funding acquisition, conceptualization, fieldwork, laboratory analyses (charcoal), data interpretation, visualization, and writing (commented on and edited the manuscript)
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank Jay Tipton for his help in the field and Małgorzata Suchorska for the laboratory preparation of pollen samples.
This research has been supported by the National Science Centre, Poland (grant no. 2020/39/D/ST10/00641).
This paper was edited by Petr Kuneš and reviewed by two anonymous referees.
Abrook, A. M., Matthews, I. P., Milner, A. M., Candy, I., Palmer, A. P., and Timms, R. G. O.: Environmental variability in response to abrupt climatic change during the Last Glacial–Interglacial Transition (16–8 cal ka BP): evidence from Mainland, Orkney, Scot. J. Geol., 56, 30–46, https://doi.org/10.1144/sjg2019-006, 2020.
Adolf, C., Wunderle, S., Colombaroli, D., Weber, H., Gobet, E., Heiri, O., van Leeuwen, J. F. N., Bigler, C., Connor, S. E., Gałka, M., La Mantia, T., Makhortykh, S., Svitavská-Svobodová, H., Vannière, B., and Tinner, W.: The sedimentary and remote-sensing reflection of biomass burning in Europe, Global Ecol. Biogeogr., 27, 199–212, https://doi.org/10.1111/geb.12682, 2018.
Allan, M., Le Roux, G., Piotrowska, N., Beghin, J., Javaux, E., Court-Picon, M., Mattielli, N., Verheyden, S., and Fagel, N.: Mid- and late Holocene dust deposition in western Europe: the Misten peat bog (Hautes Fagnes – Belgium), Clim. Past, 9, 2285–2298, https://doi.org/10.5194/cp-9-2285-2013, 2013.
Amesbury, M. J., Swindles, G. T., Bobrov, A., Charman, D. J., Lamentowicz, M., Mallon, G., Mazei, Y., Mitchell, E. A. D., Payne, R. J., Roland, T. P., Turner, E. T., and Warner, B. G.: Development of a new pan-European testate amoeba transfer function for reconstructing peatland palaeohydrology, Quaternary Sci. Rev., 152, 132–151, https://doi.org/10.1016/j.quascirev.2016.09.024, 2016.
Anderberg, A.-L.: Atlas of seeds and small fruits of Northwest-European plant species with morphological descriptions, Part 4: Resedaceae – Umbelliferae, Risbergs Tryckeri AB, Uddevalla, ISBN 91-86510-26-6, 1994.
Ankudo-Jankowska, A.: Gospodarka Lasów Państwowych na terenie województwa poznańskiego i pomorskiego w okresie międzywojennym, Studia i Materiały Ośrodka Kultury Leśnej, 5, 53–75, 2003.
Anon: Przebieg i bilans katastrofy sówkowej w Wielkopolsce, Rynek Drzewny i Budowlany, 116, 3–4, 1929.
Aoustin, D., Bertran, P., and Leroyer, C.: Late MIS 3 interstadial vegetation in coversands at Saint-Vincent-de-Paul, Southwest France, Quaternaire, 33, 193–206, https://doi.org/10.4000/quaternaire.17053, 2022.
Bąk, M., Lamentowicz, M., Kołaczek, P., Wochal, D., Matulewski, P., Kopeć, D., Wietecha, M., Jaster, D., and Marcisz, K.: Assessing the impact of forest management and climate on a peatland under Scots pine monoculture using a multidisciplinary approach, Biogeosciences, 21, 5143–5172, https://doi.org/10.5194/bg-21-5143-2024, 2024.
Bąk, M., Lamentowicz, M., Kołaczek, P., Wochal, D., Jakubowicz, M., Andrews, L., and Marcisz, K.: Dataset for paper: 20th-century ecological disasters in central European monoculture pine plantations led to critical transitions in peatlands, Mendeley Data [data set], https://doi.org/10.17632/cv5t59wf24.1, 2025. a, b, c, d
Barabach, J.: Zapis zdarzeń katastrofalnych na obszarze Puszczy Noteckiej w osadach Torfowiska Rzecin, PhD Thesis, Uniwersytet im. Adama Mickiewicza w Poznaniu, 1–129, 2014.
Beaulne, J., Garneau, M., Magnan, G., and Boucher, É.: Peat deposits store more carbon than trees in forested peatlands of the boreal biome, Sci. Rep.-UK, 11, 2657, https://doi.org/10.1038/s41598-021-82004-x, 2021.
Behre, K. E.: The interpretation of anthropogenic indicators in pollen diagrams, Pollen et Spores, 23, 225–245, 1981.
Berggren, G.: Atlas of seeds and small fruits of Northwest-European plant species (Sweden, Norway, Denmark, East Fennoscandia and Iceland) with morphological descriptions, Part 2: Cyperaceae, Berlingska Boktryckeriet, Lund, 1969.
Berglund, B. E. and Ralska-Jasiewiczowa, M.: Pollen analysis and pollen diagrams, in: Handbook of Holocene Palaeoecology and Palaeohydrology, edited by: Berglund, B. E., John Wiley & Sons, Chichester, 455–484, ISBN 0-471-90691-3, 1986.
Berthrong, S. T., Jobbágy, E. G., and Jackson, R. B.: A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation, Ecol. Appl., 19, 2228–2241, https://doi.org/10.1890/08-1730.1, 2009.
Beug, H.-J.: Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete, Verlag Dr. Friedrich Pfeil, München, ISBN 3-89937-043-0, 2004.
Bhatt, I. M., Pramod, S., Koyani, R. D., and Rajput, K. S.: Histological changes in the cell wall structure during wood decay by Trametes hirsuta and Trametes versicolor in neem (Azadirachta Indica A. Juss), J. Sustain. Forest., 35, 578–590, https://doi.org/10.1080/10549811.2016.1236277, 2016.
Birks, H. H. and Birks, H. J. B.: Multi-proxy studies in palaeolimnology, Veg. Hist. Archaeobot., 15, 235–251, https://doi.org/10.1007/s00334-006-0066-6, 2006.
Bojňanský, V. and Fargašová, A.: Atlas of seeds and fruits of central and east-european flora, The Carpathian Mountains Region, Springer, Dordrecht, ISBN 978-1-4020-5361-0, 2007.
Booth, R. K., Lamentowicz, M., and Charman, D. J.: Preparation and analysis of testate amoebae in peatland paleoenvironmental studies, Mires Peat, 7, 1–7, 2010.
Van Den Brink, P. J. and Ter Braak, C. J. F.: Principal Response Curves: analysis of time-dependent multivariate responses of biological community stress, Environ. Toxicol. Chem., 18, 138–148, 1999.
Broda, J.: Historia leśnictwa w Polsce, Wydawnictwo Akademii Rolniczej im, Augusta Cieszkowskiego w Poznaniu, Poznań, 20–70, ISBN 83-7160-216-2, 2000.
Broda, J.: Gradacje strzygoni choinówki w Lasach Państwowych Wielkopolski i Pomorza Gdańskiego w okresie międzywojennych, Studia i Materiały Ośrodka Kultury Leśnej, 5, 77–94, 2003.
Bronk Ramsey, C.: Deposition models for chronological records, Quaternary Sci. Rev., 27, 42–60, 2008.
Bronk Ramsey, C.: OxCal v4.4.4, https://c14.arch.ox.ac.uk/oxcal.html, last access: 31 July 2025.
Bugaj, P.: Płonąca puszcza, Wronieckie Sprawy, 4, 5, 1992.
Burge, O. R., Richardson, S. J., Wood, J. R., and Wilmshurst, J. M.: A guide to assess distance from ecological baselines and change over time in palaeoecological records, Holocene, 33, 905–917, https://doi.org/10.1177/09596836231169986, 2023.
Chambers, F. M., Beilman, D. W., and Yu, Z.: Methods for determining peat humification and for quantifying peat bulk density, organic matter and carbon content for palaeostudies of climate and peatland carbon dynamics, Mires Peat, 7, 1–10, 2010.
Chapin, F. S., Matson, P. A., and Vitousek, P.: Managing and Sustaining Ecosystems, in: Principles of Terrestrial Ecosystem Ecology, edited by: Chapin, F. S., Springer, 447, https://doi.org/10.1007/978-1-4419-9504-9_15, 2012.
Cifuentes-Croquevielle, C., Stanton, D. E., and Armesto, J. J.: Soil invertebrate diversity loss and functional changes in temperate forest soils replaced by exotic pine plantations, Sci. Rep.-UK, 10, 7762, https://doi.org/10.1038/s41598-020-64453-y, 2020.
Clarke, K. J.: Guide to Identification of Soil Protozoa – Testate Amoebae, edited by: Sutcliffe, D. W., Freshwater Biological Association, Ambleside, UK, 1–40, ISBN 978-0-900386-69-5, 2003.
Czerwiński, S., Guzowski, P., Lamentowicz, M., Gałka, M., Karpińska-Kołaczek, M., Poniat, R., Łokas, E., Diaconu, A.-C., Schwarzer, J., Miecznik, M., and Kołaczek, P.: Environmental implications of past socioeconomic events in Greater Poland during the last 1200 years. Synthesis of paleoecological and historical data, Quaternary Sci. Rev., 259, 106902, https://doi.org/10.1016/j.quascirev.2021.106902, 2021.
Dakos, V., Matthews, B., Hendry, A. P., Levine, J., Loeuille, N., Norberg, J., Nosil, P., Scheffer, M., and De Meester, L.: Ecosystem tipping points in an evolving world, Nat. Ecol. Evol., 3, 355–362, https://doi.org/10.1038/s41559-019-0797-2, 2019.
Davis, M. B. and Deevey, E. S.: Pollen Accumulation Rates: Estimates from Late-Glacial Sediment of Rogers Lake, Science, 145, 1293–1295, https://doi.org/10.1126/science.145.3638.1293, 1964.
De'ath, G.: Principal Curves: A New Technique for Indirect and Direct Gradient Analysis, Ecology, 80, 2237, https://doi.org/10.2307/176906, 1999.
Deshmukh, C. S., Julius, D., Desai, A. R., Asyhari, A., Page, S. E., Nardi, N., Susanto, A. P., Nurholis, N., Hendrizal, M., Kurnianto, S., Suardiwerianto, Y., Salam, Y. W., Agus, F., Astiani, D., Sabiham, S., Gauci, V., and Evans, C. D.: Conservation slows down emission increase from a tropical peatland in Indonesia, Nat. Geosci., 14, 484–490, https://doi.org/10.1038/s41561-021-00785-2, 2021.
Dopieralska, J.: Neodymium isotopic composition of conodonts as a palaeoceanographic proxy in the Variscan oceanic system, PhD Thesis, Justus-Liebig-Universität Gießen, Gießen, https://doi.org/10.22029/jlupub-9969, 2003.
Ejarque, A., Miras, Y., Riera, S., Palet, J. M., and Orengo, H. A.: Testing micro-regional variability in the Holocene shaping of high mountain cultural landscapes: a palaeoenvironmental case-study in the eastern Pyrenees, J. Archaeol. Sci., 37, 1468–1479, https://doi.org/10.1016/j.jas.2010.01.007, 2010.
Fabijański, P.: Krajobraz po pożarze, Przyroda Polska, 9, 22, 1996.
Fagel, N., Allan, M., Le Roux, G., Mattielli, N., Piotrowska, N., and Sikorski, J.: Deciphering human–climate interactions in an ombrotrophic peat record: REE, Nd and Pb isotope signatures of dust supplies over the last 2500 years (Misten bog, Belgium), Geochim. Cosmochim. Ac., 135, 288–306, https://doi.org/10.1016/j.gca.2014.03.014, 2014.
Fiałkiewicz-Kozieł, B., Śmieja-Król, B., Ostovnaya, T. M., Frontasyeva, M., Siemińska, A., and Lamentowicz, M.: Peatland microbial communities as indicators of the extreme atmospheric dust deposition, Water Air Soil Poll., 226, 97, https://doi.org/10.1007/s11270-015-2338-1, 2015.
Fiałkiewicz-Kozieł, B., Smieja-Król, B., Frontasyeva, M., Słowiński, M., Marcisz, K., Lapshina, E., Gilbert, D., Buttler, A., Jassey, V. E. J., Kaliszan, K., Laggoun-Défarge, F., Kołaczek, P., and Lamentowicz, M.: Anthropogenic- and natural sources of dust in peatland during the Anthropocene, Sci. Rep., 6, 38731, https://doi.org/10.1038/srep38731, 2016.
Fiałkiewicz-Kozieł, B., De Vleeschouwer, F., Mattielli, N., Fagel, N., Palowski, B., Pazdur, A., and Smieja-Król, B.: Record of Anthropocene pollution sources of lead in disturbed peatlands from Southern Poland, Atmos. Environ., 179, 61–68, https://doi.org/10.1016/j.atmosenv.2018.02.002, 2018.
Finsinger, W. and Tinner, W.: Minimum count sums for charcoal-concentration estimates in pollen slides: accuracy and potential errors, Holocene, 15, 293–297, 2005.
Gałka, M., Szal, M., Broder, T., Loisel, J., and Knorr, K.-H.: Peatbog resilience to pollution and climate change over the past 2700 years in the Harz Mountains, Germany, Ecol. Indic., 97, 183–193, https://doi.org/10.1016/j.ecolind.2018.10.015, 2019.
van Geel, B. and Aptroot, A.: Fossil ascomycetes in Quaternary deposits, Nova Hedwigia, 82, 313–329, https://doi.org/10.1127/0029-5035/2006/0082-0313, 2006.
Van Geel, B., Coope, G. R., and Van Der Hammen, T.: Palaeoecology and stratigraphy of the lateglacial type section at Usselo (the Netherlands), Rev. Palaeobot. Palyno., 60, 25–129, https://doi.org/10.1016/0034-6667(89)90072-9, 1989.
van Geel, B., Brinkkemper, O., van Reenen, G. B. A., van der Putten, N. N. L., Sybenga, J. E., Soonius, C., Kooijman, A. M., Hakbijl, T., and Gosling, W. D.: Multicore Study of Upper Holocene Mire Development in West-Frisia, Northern Netherlands: Ecological and Archaeological Aspects, Quaternary, 3, 12, https://doi.org/10.3390/quat3020012, 2020.
Głowacka, B.: Wstęp, in: Zabiegi agrolotnicze w ochronie lasu, edited by: Głowacka, B., Centrum Informacyjne Lasów Państwowych, Warszawa, 7–8, ISBN 978-83-89744-88-3, 2009.
Grimm, E. C.: Tilia and Tilia Graph, Illinois State Museum, 1991.
Heiri, O., Lotter, A. F., and Lemcke, G.: Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results, J. Paleolimnol., 25, 101–110, https://doi.org/10.1023/A:1008119611481, 2001.
Hernik, I.: Problematyka ochrony lasów Puszczy Noteckiej, Sylwan, 8, 49–54, 1979.
Hornung, M.: Acidification of soils by trees and forests, Soil Use Manag., 1, 24–27, https://doi.org/10.1111/j.1475-2743.1985.tb00648.x, 1985.
Hua, Q., Turnbull, J. C., Santos, G. M., Rakowski, A. Z., Ancapichún, S., De Pol-Holz, R., Hammer, S., Lehman, S. J., Levin, I., Miller, J. B., Palmer, J. G., and Turney, C. S. M.: Atmospheric radiocarbon for the period 1950–2019, Radiocarbon, 64, 1–23, https://doi.org/10.1017/RDC.2021.95, 2021.
Institute of Meteorology and Water Management: Public data, https://danepubliczne.imgw.pl/, last access: 31 July 2025.
Jacobsen, S. B. and Wasserburg, G. J.: Sm–Nd isotopic evolution of chondrites, Earth Planet. Sc. Lett., 50, 139–155, https://doi.org/10.1016/0012-821X(80)90125-9, 1980.
Jassey, V. E. J., Reczuga, M. K., Zielińska, M., Słowińska, S., Robroek, B. J. M., Mariotte, P., Seppey, C. V. W., Lara, E., Barabach, J., Słowiński, M., Bragazza, L., Chojnicki, B. H., Lamentowicz, M., Mitchell, E. A. D., and Buttler, A.: Tipping point in plant–fungal interactions under severe drought causes abrupt rise in peatland ecosystem respiration, Glob. Change Biol., 24, 972–986, https://doi.org/10.1111/gcb.13928, 2018.
Jaszczak, R.: Urządzanie lasu w Polsce do 1939 roku. Część I – początki urządzania lasu na ziemiach polskich, Sylwan, 152, 13–21, https://doi.org/10.26202/sylwan.2006126, 2008.
Juggins, S.: C2 Version 1.5 User guide. Software for ecological and palaeoecological data analysis and visualisation, Newcastle University, Newcastle upon Tyne, UK, 73, 2007.
Juggins, S.: rioja: Analysis of Quaternary Science Data, https://cran.r-project.org/web/packages/rioja/index.html, last access: 31 July 2025.
Kiely, L., Spracklen, D. V., Arnold, S. R., Papargyropoulou, E., Conibear, L., Wiedinmyer, C., Knote, C., and Adrianto, H. A.: Assessing costs of Indonesian fires and the benefits of restoring peatland, Nat. Commun., 12, 7044, https://doi.org/10.1038/s41467-021-27353-x, 2021.
Kołaczek, P., Zubek, S., Błaszkowski, J., Mleczko, P., and Margielewski, W.: Erosion or plant succession – How to interpret the presence of arbuscular mycorrhizal fungi (Glomeromycota) spores in pollen profiles collected from mires, Rev. Palaeobot. Palyno., 189, 29–37, https://doi.org/10.1016/j.revpalbo.2012.11.006, 2013.
Kondracki, J.: Geografia regionalna Polski, Wydawnictwo Naukowe PWN, Warszawa, ISBN 978-83-01-16022-7, 2001.
Kula, E. and Jankovská, Z.: Forest fires and their causes in the Czech Republic (1992–2004), J. For. Sci. (Prague), 59, 41–53, https://doi.org/10.17221/36/2012-JFS, 2013.
Lageard, J. G. and Ryan, P. A.: Microscopic fungi as subfossil woodland indicators, Holocene, 23, 990–1001, https://doi.org/10.1177/0959683612475145, 2013.
Lamentowicz, M. and Mitchell, E. A. D.: The ecology of testate amoebae (Protists) in Sphagnum in north-western Poland in relation to peatland ecology, Microb. Ecol., 50, 48–63, 2005.
Lamentowicz, M., Mueller, M., Gałka, M., Barabach, J., Milecka, K., Goslar, T., and Binkowski, M.: Reconstructing human impact on peatland development during the past 200 years in CE Europethrough biotic proxies and X-ray tomography, Quatern. Int., 357, 282–294, https://doi.org/10.1016/j.quaint.2014.07.045, 2015.
Lamentowicz, M., Kołaczek, P., Mauquoy, D., Kittel, P., Łokas, E., Słowiński, M., Jassey, V. E. J., Niedziółka, K., Kajukało-Drygalska, K., and Marcisz, K.: Always on the tipping point – A search for signals of past societies and related peatland ecosystem critical transitions during the last 6500 years in N Poland, Quaternary Sci. Rev., 225, 105954, https://doi.org/10.1016/j.quascirev.2019.105954, 2019a.
Lamentowicz, M., Gałka, M., Marcisz, K., Słowiński, M., Kajukało-Drygalska, K., Druguet Dayras, M., and Jassey, V. E. J.: Unveiling tipping points in long-term ecological records from Sphagnum-dominated peatlands, Biol. Letters, 15, 20190043, https://doi.org/10.1098/rsbl.2019.0043, 2019b.
Lenton, T. M., Held, H., Kriegler, E., Hall, J. W., Lucht, W., Rahmstorf, S., and Schellnhuber, H. J.: Tipping elements in the Earth's climate system, P. Natl. Acad. Sci. USA, 105, 1786–1793, https://doi.org/10.1073/pnas.0705414105, 2008.
Lenton, T. M., Rockström, J., Gaffney, O., Rahmstorf, S., Richardson, K., Steffen, W., and Schellnhuber, H. J.: Climate tipping points – too risky to bet against, Nature, 575, 592–595, https://doi.org/10.1038/d41586-019-03595-0, 2019.
Leonardos, L., Gnilke, A., Sanders, T. G. M., Shatto, C., Stadelmann, C., Beierkuhnlein, C., and Jentsch, A.: Synthesis and Perspectives on Disturbance Interactions, and Forest Fire Risk and Fire Severity in Central Europe, Fire, 7, 470, https://doi.org/10.3390/fire7120470, 2024.
van der Linden, M., Barke, J., Vickery, E., Charman, D. J., and van Geel, B.: Late Holocene human impact and climate change recorded in a North Swedish peat deposit, Palaeogeogr. Palaeocl., 258, 1–27, https://doi.org/10.1016/j.palaeo.2007.11.006, 2008.
Loisel, J. and Bunsen, M.: Abrupt Fen-Bog Transition Across Southern Patagonia: Timing, Causes, and Impacts on Carbon Sequestration, Front. Ecol. Evol., 8, 1–19, https://doi.org/10.3389/fevo.2020.00273, 2020.
Loisel, J., Yu, Z., Beilman, D. W., Camill, P., Alm, J., Amesbury, M. J., Anderson, D., Andersson, S., Bochicchio, C., Barber, K., Belyea, L. R., Bunbury, J., Chambers, F. M., Charman, D. J., De Vleeschouwer, F., Fiałkiewicz-Kozieł, B., Finkelstein, S. A., Gałka, M., Garneau, M., Hammarlund, D., Hinchcliffe, W., Holmquist, J., Hughes, P., Jones, M. C., Klein, E. S., Kokfelt, U., Korhola, A., Kuhry, P., Lamarre, A., Lamentowicz, M., Large, D., Lavoie, M., MacDonald, G., Magnan, G., Mäkilä, M., Mallon, G., Mathijssen, P., Mauquoy, D., McCarroll, J., Moore, T. R., Nichols, J., O'Reilly, B., Oksanen, P., Packalen, M., Peteet, D., Richard, P. J. H., Robinson, S., Ronkainen, T., Rundgren, M., Sannel, A. B. K., Tarnocai, C., Thom, T., Tuittila, E.-S., Turetsky, M., Väliranta, M., van der Linden, M., van Geel, B., van Bellen, S., Vitt, D., Zhao, Y., and Zhou, W.: A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation, Holocene, 24, 1028–1042, https://doi.org/10.1177/0959683614538073, 2014.
Łuców, D., Lamentowicz, M., Kołaczek, P., Łokas, E., Marcisz, K., Obremska, M., Theuerkauf, M., Tyszkowski, S., and Słowiński, M.: Pine Forest Management and Disturbance in Northern Poland: Combining High-Resolution 100-Year-Old Paleoecological and Remote Sensing Data, Front. Ecol. Evol., 9, 1–18, https://doi.org/10.3389/fevo.2021.747976, 2021.
Lundqvist, N.: Nordic Sordariaceae s. lat (Symbolae botanicae Upsalienses), Almqvist & Wiksells, 20, 1–374, 1972.
Magnuski, K.: Urządzeniowe aspekty postępowania gospodarczego w Puszczy Noteckiej, Sylwan, 2, 49–59, 1993.
Marcisz, K., Tinner, W., Colombaroli, D., Kołaczek, P., Słowiński, M., Fiałkiewicz-Kozieł, B., Łokas, E., and Lamentowicz, M.: Long-term hydrological dynamics and fire history over the last 2000 years in CE Europe reconstructed from a high-resolution peat archive, Quaternary Sci. Rev., 112, 138–152, https://doi.org/10.1016/j.quascirev.2015.01.019, 2015.
Marcisz, K., Belka, Z., Dopieralska, J., Jakubowicz, M., Karpińska-Kołaczek, M., Kołaczek, P., Mauquoy, D., Słowiński, M., Zieliński, M., and Lamentowicz, M.: Navigating the limitations, assumptions and conceptual pitfalls of Nd isotope research on peatlands: Reply to the comments of Le Roux et al. (2023) on “Neodymium isotopes in peat reveal past local environmental disturbances” by Marcisz et al. (2023), Sci. Total Environ., 898, 165398, https://doi.org/10.1016/j.scitotenv.2023.165398, 2023a.
Marcisz, K., Belka, Z., Dopieralska, J., Jakubowicz, M., Karpińska-Kołaczek, M., Kołaczek, P., Mauquoy, D., Słowiński, M., Zieliński, M., and Lamentowicz, M.: Neodymium isotopes in peat reveal past local environmental disturbances, Sci. Total Environ., 871, 161859, https://doi.org/10.1016/j.scitotenv.2023.161859, 2023b.
Marcisz, K., Bąk, M., Kołaczek, P., Lamentowicz, M., and Wochal, D.: Historia lasu i mokradeł zapisana w torfowiskach, in: Jak chronić torfowiska w lasach?, edited by: Lamentowicz, M. and Konczal, S., ArchaeGraph, Łódź, 29–45, ISBN 978-83-67959-36-0, 2024.
Marks, L.: Timing of the Late Vistulian (Weichselian) glacial phases in Poland, Quaternary Sci. Rev., 44, 81–88, https://doi.org/10.1016/j.quascirev.2010.08.008, 2012.
Matthias, I. and Giesecke, T.: Insights into pollen source area, transport and deposition from modern pollen accumulation rates in lake sediments, Quaternary Sci. Rev., 87, 12–23, https://doi.org/10.1016/j.quascirev.2013.12.015, 2014.
Matuszkiewicz, J.: Potential natural vegetation of Poland [Potencjalna roślinność naturalna Polski], https://www.igipz.pan.pl/Roslinnosc-potencjalna-zgik.html (last access: 31 July 2025), 2008.
Mátyás, C., Ackzell, L., and Samuel, C. J. A.: EUFORGEN Technical Guidelines for genetic conservation and use for Scots pine (Pinus sylvestris), International Plant Genetic Resources Institute, Romę, ISBN 92-9043-661-1, 2004.
Mauquoy, D. and van Geel, B.: Mire and peat macros, in: Encyclopedia of Quaternary Science, vol. 3, Elsevier, Heidelberg, 2315–2336, ISBN 978-0-444-52747-9, 2007.
Mauquoy, D., Hughes, P. D. M., and van Geel, B.: A protocol for plant macrofossil analysis of peat deposits, Mires Peat, 7, 1–5, 2010.
Mazei, Y. and Tsyganov, A. N.: Freshwater testate amoebae, KMK, Moscow, ISBN 5-87317-336-2, 2006.
McCarroll, J., Chambers, F. M., Webb, J. C., and Thom, T.: Application of palaeoecology for peatland conservation at Mossdale Moor, UK, Quatern. Int., 432, 39–47, https://doi.org/10.1016/j.quaint.2014.12.068, 2017.
Meisterfeld, R.: Testate amoebae, in: Patrimoines Naturels, vol. 50, edited by: Costello, M. J., Emblow, C. S., and White, R., Muséum National d'Histoire Naturelle – Institut d'Ecologie et de Gestion de la Biodiversité (I. E. G. B.) – Service du Patrimoine Naturel (S. P. N.), Paris, 54–57, ISBN 2-85653-538-0, 2001.
Mighall, T. M., Timpany, S., Blackford, J. J., Innes, J. B., O'Brien, C. E., O'Brien, W., and Harrison, S.: Vegetation change during the Mesolithic and Neolithic on the Mizen Peninsula, Co. Cork, south-west Ireland, Veg. Hist. Archaeobot., 17, 617–628, https://doi.org/10.1007/s00334-007-0136-4, 2008.
Milecka, K., Kowalewski, G., Fiałkiewicz-Kozieł, B., Gałka, M., Lamentowicz, M., Chojnicki, B. H., Goslar, T., and Barabach, J.: Hydrological changes in the Rzecin peatland (Puszcza Notecka, Poland) induced by anthropogenic factors: Implications for mire development and carbon sequestration, Holocene, 27, 651–664, https://doi.org/10.1177/0959683616670468, 2017.
Miola, A.: Tools for Non-Pollen Palynomorphs (NPPs) analysis: A list of Quaternary NPP types and reference literature in English language (1972–2011), Rev. Palaeobot. Palyno., 186, 142–161, https://doi.org/10.1016/j.revpalbo.2012.06.010, 2012.
Miś, R.: Charakterystyka zmian w stanie lasów Puszczy Noteckiej, Sylwan, 6, 34–46, 2003.
Mitchell, E. A. D., Buttler, A., Grosvernier, P., Rydin, H., Albinsson, C., Greenup, A. L., Heijmans, M. M. P. D., Hoosbeek, M. R., and Saarinen, T.: Relationships among testate amoebae (Protozoa), vegetation and water chemistry in five Sphagnum-dominated peatlands in Europe, New Phytol., 145, 95–106, 2000.
Montoro Girona, M., Navarro, L., and Morin, H.: A Secret Hidden in the Sediments: Lepidoptera Scales, Front. Ecol. Evol., 6, 1–5, https://doi.org/10.3389/fevo.2018.00002, 2018.
Moore, P. D., Webb, J. A., and Collinson, M. E.: Pollen Analysis, Blackwell Scientific Publications, Oxford, ISBN 0-632-02176-4, 1991.
Mroczkiewicz, L.: Zagadnienia hodowlane na terenach posówkowych, Uniwersytet Poznański, Poznań, 1933.
Ofiti, N. O. E., Schmidt, M. W. I., Abiven, S., Hanson, P. J., Iversen, C. M., Wilson, R. M., Kostka, J. E., Wiesenberg, G. L. B., and Malhotra, A.: Climate warming and elevated CO2 alter peatland soil carbon sources and stability, Nat. Commun., 14, 7533, https://doi.org/10.1038/s41467-023-43410-z, 2023.
Page, S., Mishra, S., Agus, F., Anshari, G., Dargie, G., Evers, S., Jauhiainen, J., Jaya, A., Jovani-Sancho, A. J., Laurén, A., Sjögersten, S., Suspense, I. A., Wijedasa, L. S., and Evans, C. D.: Anthropogenic impacts on lowland tropical peatland biogeochemistry, Nat. Rev. Earth Environ., 3, 426–443, https://doi.org/10.1038/s43017-022-00289-6, 2022.
Page, S. E., Siegert, F., Rieley, J. O., Boehm, H.-D. V., Jaya, A., and Limin, S.: The amount of carbon released from peat and forest fires in Indonesia during 1997, Nature, 420, 61–65, https://doi.org/10.1038/nature01131, 2002.
Pals, J. P., Van Geel, B., and Delfos, A.: Paleoecological studies in the Klokkeweel bog near hoogkarspel (prov. of Noord-Holland), Rev. Palaeobot. Palyno., 30, 371–418, https://doi.org/10.1016/0034-6667(80)90020-2, 1980.
Parish, F., Sirin, A., Charman, D. J., Joosten, H., Minayeva, T., Silvius, M., and Stringer, L.: Assessment on peatlands, biodiversity and climate change: main report, Global Environment Centre, Kuala Lumpur & Wetlands International, Wageningen, ISBN 978-983-43751-0-2, 2008.
Patterson, W. A., Edwards, K. J., and Maguire, D. J.: Microscopic Charcoal as a Fossil Indicator of Fire, Quaternary Sci. Rev., 6, 3–23, https://doi.org/10.1016/0277-3791(87)90012-6, 1987.
Payne, R. J. and Mitchell, E. A. D.: How many is enough? Determining optimal count totals for ecological and palaeoecological studies of testate amoebae, J. Paleolimnol., 42, 483–495, https://doi.org/10.1007/s10933-008-9299-y, 2009.
Pin, C., Briot, D., Bassin, C., and Poitrasson, F.: Concomitant separation of strontium and samarium-neodymium for isotopic analysis in silicate samples, based on specific extraction chromatography, Anal. Chim. Acta., 298, 209–217, https://doi.org/10.1016/0003-2670(94)00274-6, 1994.
Polna, M.: Zróżnicowanie natężenia pożarów leśnych w Polsce w latach 1990–2003, Acta Scientiarum Polonorum. Silvarum Colendarum Ratio et Industria Lignari, 4, 81–90, 2005.
Pratte, S., Garneau, M., and De Vleeschouwer, F.: Increased atmospheric dust deposition during the Neoglacial in a boreal peat bog from north-eastern Canada, Palaeogeogr. Palaeocl., 469, 34–46, https://doi.org/10.1016/j.palaeo.2016.12.036, 2017.
Pulgarin Díaz, J. A., Melin, M., and Tikkanen, O.-P.: Thermal sum drives abundance and distribution range shift of Panolis flammea in Finland, Scand. J. Forest Res., 37, 93–98, https://doi.org/10.1080/02827581.2022.2060303, 2022.
Ratajszczak, K.: Problemy gospodarki leśnej w Puszczy Noteckiej, Sylwan, 8, 29–37, 1979.
Reimer, P. J., Austin, W. E. N., Bard, E., Bayliss, A., Blackwell, P. G., Bronk Ramsey, C., Butzin, M., Cheng, H., Edwards, R. L., Friedrich, M., Grootes, P. M., Guilderson, T. P., Hajdas, I., Heaton, T. J., Hogg, A. G., Hughen, K. A., Kromer, B., Manning, S. W., Muscheler, R., Palmer, J. G., Pearson, C., van der Plicht, J., Reimer, R. W., Richards, D. A., Scott, E. M., Southon, J. R., Turney, C. S. M., Wacker, L., Adolphi, F., Büntgen, U., Capano, M., Fahrni, S. M., Fogtmann-Schulz, A., Friedrich, R., Köhler, P., Kudsk, S., Miyake, F., Olsen, J., Reinig, F., Sakamoto, M., Sookdeo, A., and Talamo, S.: The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP), Radiocarbon, 62, 725–757, https://doi.org/10.1017/RDC.2020.41, 2020.
Rispens, J. A.: Der Nadelbaumtypus. Schritte zu einem imaginativen Baumverständnis, Elemente der Naturwissenschaft, 79, 51–77, 2003.
Rydin, H. and Jeglum, J. K.: The biology of peatlands, 2nd edn., Oxford University Press, New York, ISBN 978-0-19-852871-5, 2013.
San-Miguel-Ayanz, J., Durrant, T., Boca, R., Maianti, P., Liberta`, G., Jacome Felix Oom, D., Branco, A., De Rigo, D., Suarez-Moreno, M., Ferrari, D., Roglia, E., Scionti, N., Broglia, M., Onida, M., Tistan, A., and Loffler, P.: Forest fires in Europe, Middle East and North Africa 2022, Ispra, https://doi.org/10.2760/348120, 2023.
Sayedi, S. S., Abbott, B. W., Vannière, B., Leys, B., Colombaroli, D., Romera, G. G., Słowiński, M., Aleman, J. C., Blarquez, O., Feurdean, A., Brown, K., Aakala, T., Alenius, T., Allen, K., Andric, M., Bergeron, Y., Biagioni, S., Bradshaw, R., Bremond, L., Brisset, E., Brooks, J., Brugger, S. O., Brussel, T., Cadd, H., Cagliero, E., Carcaillet, C., Carter, V., Catry, F. X., Champreux, A., Chaste, E., Chavardès, R. D., Chipman, M., Conedera, M., Connor, S., Constantine, M., Courtney Mustaphi, C., Dabengwa, A. N., Daniels, W., De Boer, E., Dietze, E., Estrany, J., Fernandes, P., Finsinger, W., Flantua, S. G. A., Fox-Hughes, P., Gaboriau, D. M., Gayo, E., Girardin, Martin, P., Glenn, J., Glückler, R., González-Arango, C., Groves, M., Hamilton, D. S., Hamilton, R. J., Hantson, S., Hapsari, K. A., Hardiman, M., Hawthorne, D., Hoffman, K., Inoue, J., Karp, A. T., Krebs, P., Kulkarni, C., Kuosmanen, N., Lacourse, T., Ledru, M.-P., Lestienne, M., Long, C., López-Sáez, J. A., Loughlin, N., Niklasson, M., Madrigal, J., Maezumi, S. Y., Marcisz, K., Mariani, M., McWethy, D., Meyer, G., Molinari, C., Montoya, E., Mooney, S., Morales-Molino, C., Morris, J., Moss, P., Oliveras, I., Pereira, J. M., Pezzatti, G. B., Pickarski, N., Pini, R., Rehn, E., Remy, C. C., Revelles, J., Rius, D., Robin, V., Ruan, Y., Rudaya, N., Russell-Smith, J., Seppä, H., Shumilovskikh, L., Sommers, W. T., Tavşanoğlu, Ç., Umbanhowar, C., Urquiaga, E., Urrego, D., Vachula, R. S., Wallenius, T., You, C., Daniau, A.-L.: Assessing changes in global fire regimes, Fire Ecol., 20, 18, https://doi.org/10.1186/s42408-023-00237-9, 2024.
Schaefer, V. J.: Some Physical Relationships of Fine Particle Smoke, in: Proceedings annual: 13 Tall Timbers Fire Ecology Conference, 22–23 March 1973, Tallahassee, Florida, 283–294, 1973.
Schafstall, N., Kuosmanen, N., Kuneš, P., Svobodová, H. S., Svitok, M., Chiverrell, R. C., Halsall, K., Fleischer, P., Knížek, M., and Clear, J. L.: Sub-fossil bark beetles as indicators of past disturbance events in temperate Picea abies mountain forests, Quaternary Sci. Rev., 275, 107289, https://doi.org/10.1016/j.quascirev.2021.107289, 2022.
Seidl, R., Schelhaas, M.-J., Rammer, W., and Verkerk, P. J.: Increasing forest disturbances in Europe and their impact on carbon storage, Nat. Clim. Chang., 4, 806, https://doi.org/10.1038/nclimate2318, 2014.
Shumilovskikh, L. S. and van Geel, B.: Non-Pollen Palynomorphs, in: Handbook for the Analysis of Micro-Particles in Archaeological Samples, edited by: Henry, E. G., Springer Cham, 65–94, https://doi.org/10.1007/978-3-030-42622-4, 2020.
Shumilovskikh, L. S., Shumilovskikh, E. S., Schlütz, F., and van Geel, B.: NPP-ID: Non-Pollen Palynomorph Image Database as a research and educational platform, Veg. Hist. Archaeobot., 31, 323–328, https://doi.org/10.1007/s00334-021-00849-8, 2022.
Siemensma, F.: Microworld, world of amoeboid organisms, https://arcella.nl/, last access: 31 July 2025.
Simpson, G. L.: Modelling Palaeoecological Time Series Using Generalised Additive Models, Front. Ecol. Evol., 6, https://doi.org/10.3389/fevo.2018.00149, 2018.
Skrøppa, T.: EUFORGEN Technical Guidelines for genetic conservation and use for Norway spruce (Picea abies), International Plant Genetic Resources Institute, Rome, ISBN 92-9043-569-0, 2003.
Słowiński, M., Lamentowicz, M., Łuców, D., Barabach, J., Brykała, D., Tyszkowski, S., Pieńczewska, A., Śnieszko, Z., Dietze, E., Jażdżewski, K., Obremska, M., Ott, F., Brauer, A., and Marcisz, K.: Paleoecological and historical data as an important tool in ecosystem management, J. Environ. Manage., 236, 755–768, https://doi.org/10.1016/j.jenvman.2019.02.002, 2019.
Somsak, L., Dlapa, P., Kollar, J., Kubicek, F., Simonovic, V., Janitor, A., Kanka, R., and Simkovic, I.: Fire impact on the secondary pine forest and soil in the Borská niziná lowland (SW Slovakia), Ekologia, 28, 52–65, https://doi.org/10.4149/ekol_2009_01_52, 2009.
Statistical Office in Białystok: Rocznik Statystyczny Leśnictwa (Statistical Yearbook of Forestry), edited by: Rozkrut, D., Główny Urząd Statystyczny, Warszawa, Białystok, 27 pp., 2023.
Stockmarr, J.: Tablets with spores used in absolute pollen analysis, Pollen et Spores, 13, 615–621, 1971.
Sukovata, L.: Gradacje foliofagów sosny w Puszczy Noteckiej – historia, prognoza i możliwości przeciwdziałania, Acta Scientiarum Polonorum Silvarum Colendarum Ratio et Industria Lignaria, 21, 93–101, https://doi.org/10.17306/J.AFW.2022.3.1, 2022.
Sullivan, M. E. and Booth, R. K.: The Potential Influence of Short-term Environmental Variability on the Composition of Testate Amoeba Communities in Sphagnum Peatlands, Microb. Ecol., 62, 80–93, https://doi.org/10.1007/s00248-011-9875-y, 2011.
Szczygieł, R.: Wielkoobszarowe pożary lasów w Polsce, Bezpieczeństwo i Technika Pożarnicza, 1, 67–78, 2012.
Szmidt, A.: Ważniejsze szkodniki, in: Biologia sosny zwyczajnej, vol. I, edited by: Białobok, S., Boratyński, A., and Bugała, W., Polska Akademia Nauk – Instytut Dendrologii w Kórniku, Poznań-Kórnik, 368–385, ISBN 83-85599-21-5, 1993.
Szwałkiewicz, J.: Uszkodzenia drzew leśnych: poradnik leśnika, Państwowe Wydawnictwo Rolne i Leśne, Warszawa, ISBN 978-09-99015-4, 2009.
Tinner, W. and Hu, F. S.: Size parameters, size-class distribution and area-number relationship of microscopic charcoal: relevance for fire reconstruction, Holocene, 13, 499–505, 2003.
Tobolski, K.: Przewodnik do oznaczania torfów i osadów jeziornych, PWN, Warszawa, ISBN 978-83-01-21683-2, 2000.
Turetsky, M. R., Donahue, W. F., and Benscoter, B. W.: Experimental drying intensifies burning and carbon losses in a northern peatland, Nat. Commun., 2, 514, https://doi.org/10.1038/ncomms1523, 2011.
Turner, J. and Lambert, M. J.: Soil properties as affected by Pinus radiata plantations, N Z J For. Sci., 18, 77–91, 1988.
Wardenaar, E. C. P.: A new hand tool for cutting peat profiles, Can. J. Bot., 65, 1772–1773, https://doi.org/10.1139/b87-243, 1987.
Westerling, A. L.: Increasing western US forest wildfire activity: sensitivity to changes in the timing of spring, Philos. T. R. Soc. B, 371, 20150178, https://doi.org/10.1098/rstb.2015.0178, 2016.
Wheeler, J., Timpany, S., Mighall, T. M., and Scott, L.: A Palaeoenvironmental Investigation of Two Prehistoric Burnt Mound Sites in Northern Ireland, Geoarchaeology, 31, 506–529, https://doi.org/10.1002/gea.21552, 2016.
Whitlock, C. and Larsen, C.: Charcoal as a fire proxy., in: Tracking environmental change using lake sediments. Terrestrial, algal, and siliceous indicators, edited by: Smol, J. P., Birks, H. J. B., and Last, W. M., vol. 3, Kluwer, Dordrecht, 75–97, ISBN 978-1-4020-0681-4, 2001.
Wilkinson, S. L., Andersen, R., Moore, P. A., Davidson, S. J., Granath, G., and Waddington, J. M.: Wildfire and degradation accelerate northern peatland carbon release, Nat. Clim. Change, 13, 456–461, https://doi.org/10.1038/s41558-023-01657-w, 2023.
Yeloff, D., Charman, D., van Geel, B., and Mauquoy, D.: Reconstruction of hydrology, vegetation and past climate change in bogs using fungal microfossils, Rev. Palaeobot. Palyno., 146, 102–145, https://doi.org/10.1016/j.revpalbo.2007.03.001, 2007.
Zhang, H., Väliranta, M., Swindles, G. T., Aquino-López, M. A., Mullan, D., Tan, N., Amesbury, M., Babeshko, K. V., Bao, K., Bobrov, A., Chernyshov, V., Davies, M. A., Diaconu, A.-C., Feurdean, A., Finkelstein, S. A., Garneau, M., Guo, Z., Jones, M. C., Kay, M., Klein, E. S., Lamentowicz, M., Magnan, G., Marcisz, K., Mazei, N., Mazei, Y., Payne, R., Pelletier, N., Piilo, S. R., Pratte, S., Roland, T., Saldaev, D., Shotyk, W., Sim, T. G., Sloan, T. J., Słowiński, M., Talbot, J., Taylor, L., Tsyganov, A. N., Wetterich, S., Xing, W., and Zhao, Y.: Recent climate change has driven divergent hydrological shifts in high-latitude peatlands, Nat. Commun., 13, 4959, https://doi.org/10.1038/s41467-022-32711-4, 2022.
Ziółkowski, J.: Szkody wyrządzone przez sówkę chojnówkę, Przegląd Leśniczy, 12, 262–268, 1924.