the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Estimation of metabolic dynamics of restored seagrass meadows in a Southeast Asia islet: insights from ex situ benthic incubation
Mariche Bandibas-Natividad
Jian-Jhih Chen
Hsin-Yu Chou
Lan-Feng Fan
Yi-Le Shen
Seagrass meadows are vital carbon sinks, but their function is threatened by rapid decline, driving restoration efforts to enhance coastal recovery and carbon removal. The role of restored seagrass meadows as carbon sources or sinks depends largely on organic carbon metabolism and carbonate dynamics. Here, we employed ex situ core incubation to quantify gross primary productivity (GPP), community respiration (R), net ecosystem metabolism (NEM), and net ecosystem calcification (NEC) in restored seagrass (SG) and adjacent bare sediment (BS). SG exhibited significantly higher GPP ( vs. , p<0.01) and NEM ( vs. , p<0.01) than BS, indicating enhanced autotrophy and carbon sink potential. SG also exhibited net calcification (), while BS showed net dissolution (); however, high NEC variability resulted in no statistically significant difference (p>0.05). These findings suggest that restored seagrass enhances organic carbon sequestration despite variability in carbonate fluxes. Ex situ incubations enable simultaneous measurements of organic and inorganic carbon metabolism, although complementary in situ validation remains essential. Overall, this study highlights the potential of seagrass restoration to strengthen coastal carbon sinks and contribute to climate change mitigation.
- Article
(4466 KB) - Full-text XML
- BibTeX
- EndNote
Seagrass meadows are marine angiosperms comprising approximately 72 species globally (Short et al., 2011). Although they occupy just 0.1 % of the ocean's surface and have limited taxonomic diversity, they are highly productive and ecologically significant ecosystems in the marine environments (Fourqurean et al., 2012; Short et al., 2011). These meadows play essential roles in nutrient and carbon cycling and serve as key habitats for many marine species (Duarte et al., 2010; Fourqurean et al., 2012). Due to their relatively complex structure, seagrass meadows capture and retain organic carbon (Corg) in the sediment, making them one of the major carbon reservoirs globally (Duarte et al., 2005; Mcleod et al., 2011). Previous estimates suggest that seagrasses account for approximately 15 % of the total global carbon sequestered in benthic sediments (Duarte et al., 2013), with burial rates 35 times those of tropical rainforests (Mcleod et al., 2011). Nevertheless, carbon storage capacity can vary depending on species-specific traits, geographical location, and environmental conditions (Duarte et al., 2010; Fourqurean et al., 2012).
In spite of their ecological significance, seagrass meadows have experienced a global decline, driven primarily by human-induced activities such as coastal development, eutrophication, and deteriorating water quality (Orth et al., 2006; Waycott et al., 2009). Since 1980, the global coverage of seagrass has decreased by 110 km2 annually, with the rate of decline increasing (Waycott et al., 2009). The loss is frequently associated with increased water column turbidity and epiphytic shading, which reduce the light for seagrass photosynthesis, leading to meadow degradation (Campbell et al., 2003; Orth et al., 2006). Degradation also diminishes their capacity to modify local pH and influence the dynamics of dissolved oxygen (DO) and dissolved inorganic carbon (DIC) (Hendriks et al., 2014). Moreover, the continued loss of seagrass ecosystems raises concerns that vast amounts of previously sequestered carbon could be released back in the atmosphere, converting seagrasses from carbon sinks to carbon sources and intensifying global climate change (Macreadie et al., 2013). The ongoing decline could potentially release up to 299 Tg of carbon annually, contributing roughly 10 % of CO2 emissions associated with anthropogenic land-use changes (Fourqurean et al., 2012).
In response to these challenges, seagrass restoration has emerged as a critical strategy to mitigate environmental degradation, enhance coastal resilience, and address global climate change (Juska and Berg et al., 2022). Protecting and restoring seagrass meadows aligns with international goals like the Paris Agreement, as these ecosystems offer significant potential for long-term carbon storage and climate regulation (Fourqurean et al., 2012). However, despite growing restoration efforts, there remains limited understanding of their success, particularly regarding benthic metabolism and carbon dynamics (Kindeberg et al., 2024). While studies from temperate regions, such as the Zostera marina restoration in the Virginia Coast (Rheuban et al., 2014), have provided valuable insights, data from tropical regions – including Southeast Asia, a global hotspot for seagrass diversity – remain scarce (Duarte et al., 2010; Ward et al., 2022; Chou et al., 2023). It represents a critical gap in our knowledge of the impact of restoration efforts on carbon removal and ocean acidification mitigation.
Although there is increasing consensus on the potential of “Blue Carbon” storage in seagrass meadows as a climate change mitigation strategy, the biogeochemical cycling within these ecosystems is complex. Several processes, including ecosystem calcification, anaerobic metabolism, and bioturbation, can counteract net organic carbon (OC) sequestration (Van Dam et al., 2021). These processes regulate local DIC and total alkalinity (AT) budgets, adding complexity to accurately quantifying carbon sequestration (Kindeberg et al., 2024). Overlooking these processes can result in significant overestimates of local carbon sequestration rates and misinterpretations of the role seagrass meadows play in mitigating climate change, potentially leading to inaccurate assessments of their carbon sink capacity (Johannessen, 2023; Chen et al., 2024; Fan et al., 2024).
Several methodologies were developed to quantify benthic metabolism, which is a crucial component of biogeochemical cycling, including the photosynthesis–irradiance curve (Kraemer and Alberte, 1993), the open-water O2 mass balance approach (Odum, 1956; Chou et al., 2023), and aquatic eddy covariance (Berg et al., 2022; Juska and Berg, 2022). While these methods provide important data, they might overlook the complexities of bioturbation, remineralization, and carbonate dynamics (Olivé et al., 2016; Ward et al., 2022; Juska and Berg, 2022). In this study, we aim to address these knowledge gaps by quantifying organic carbon metabolism (net ecosystem metabolism, NEM) and carbonate dynamics (i.e., net ecosystem calcification, NEC) in restored seagrass (SG) meadows and adjacent bare sediment (BS) habitats on a Southeast Asia islet, using an innovative ex situ benthic incubation.
2.1 Study site
The Penghu Islands, located in the southern part of Taiwan Strait (Fig. 1), host a range of seagrass species. Notably, four species have been reported: Halophila ovalis, Halodule pinifolia, Halodule uninervis, and Zostera japonica (Yang et al., 2002). The sampling location (23°38′18.38′′ N and 119°33′46.48′′ E) is a restoration meadow dominated by H. uninervis and H. ovalis. This restoration site encompasses approximately 3 ha (Allen Coral Atlas, 2020), with seagrass percent cover varying from 20 %–90 %. These seagrasses are subtidal, with water depths ranging from 1.7–4.4 m. The substrate in this area is composed of carbonate sand. The area supports a diverse community of bivalves (e.g., Pinna sp.), gastropods, echinoderms, and various fish species, all of which were observed during the sampling.
2.2 Ex situ core incubation system
The ex situ benthic core methodology used in this study was adapted from Chen et al. (2019) (Fig. 2). This approach has been widely employed in various studies to assess nutrient concentrations and benthic metabolism in coastal ecosystems and estuaries (Eyre and Ferguson, 2005; Maher and Eyre, 2011). Typically, the ex situ core incubation involves 150 L treatment tanks containing aerated water. Each tank can accommodate 10 polycarbonate plexiglass cores, 10 cm in diameter and 50 cm in height. The tanks were equipped with magnetic stir bars driven by a centrally located rotating motor fitted with a magnet. Each core was sealed with a plexiglass lid containing two ports, one of which is for probe insertion (Eyre and Ferguson, 2005). This method was used to quantify seagrass metabolism, particularly in subtidal systems where in situ measurements are often logistically challenging. While ex situ conditions may differ from natural underwater environments, we carefully designed our setup to closely replicate field conditions, including natural light exposure and ambient temperature, to ensure ecological relevance.
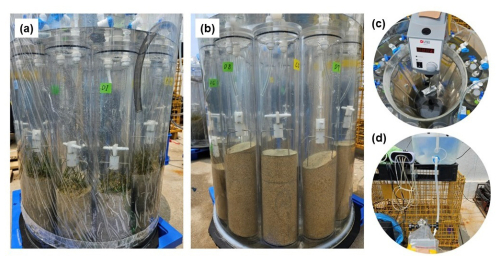
Figure 2Ex situ benthic chamber setup for measuring metabolic rates and carbonate dynamics in seagrass meadows and bare sediment. The chambers contain seagrass samples (a), while the chambers contain bare sediment (b). Insets show close-ups of the central rotating motor with a magnet setup for water circulation (c) and the setup for continuous seawater supply (d).
2.3 Sediment core collection and pre-incubation
The incubation was conducted on 12–13 April 2024. Twenty intact sediment cores, comprising both seagrass and bare sediment, were collected on-site using the plexiglass tubes. The cores were inserted about 20 cm into the sediment, keeping approximately 1.9 L of water. Each core was sealed with a gas-tight plexiglass plate at the bottom. The samples were brought back to the incubation site within 2 h of collection and allowed to settle for 24 h. Additionally, 150 L of water was collected on-site for continuous supply during the experiment.
At the incubation site, the cores were uncovered and placed in 150 L tanks filled with aerated seawater. They were kept at in situ temperature, exposed to natural sunlight, and continuously recirculated. The stirring rate was controlled to prevent sediment resuspension (Ferguson et al., 2004). The cores underwent a 24 h pre-incubation period to promote stable sediment profiles. The seagrass composition within the collected cores for ex situ core incubation was dominated by H. uninervis and H. ovalis. The shoot count of H. uninervis ranged from 20–40 shoots per 0.008 m2, while H. ovalis ranged from 2–20 shoots per 0.008 m2.
2.4 Sample collection and analysis
Following pre-incubation, the cores were tightly closed using a plexiglass lid. Temperature, salinity, and pH were determined using a YSI ProDSS Multiparameter water quality sonde, while DO (mg L−1) was measured with a Thermo Scientific DO probe. Both probes were calibrated with calibration standards. Measurements were taken at midnight (24:00 LT) with 2 h intervals and ended at noon. Photosynthetically active radiation (PAR) levels were measured using a SQ-420X Smart Quantum Sensor positioned atop the incubation tank.
After measurements, three 150 mL seawater samples were collected separately from the SG and BS cores using a syringe for DIC and pH analysis. The water samples were processed with 60 µL HgCl2 solution to stop any biological activity. DIC analysis was performed using a non-dispersive infrared method with a DIC analyzer (AS-C3, Apollo SciTech Inc.), following the approach of Dickson et al. (2007) and our past studies (Chou et al., 2018; 2021; Fan et al., 2024). For each DIC run, we used certified reference material (Batch no. 206) sourced from A. G. Dickson at Scripps Institution of Oceanography to check for drift and systematic bias. pH values were measured spectrophotometrically in total scale at 25 °C following Clayton and Byrne (1993). Data from DIC and pH, along with actual temperature and salinity, were used to calculate the AT, partial pressure of CO2 (pCO2), and aragonite saturation state (ΩAr) using the Excel macro CO2SYS version 2.1 (Pelletier et al., 2011). The dissociation constants for carbonic acid applied in these calculations were obtained from Mehrbach et al. (1973) and subsequently refined by Dickson and Millero (1987).
2.5 Benthic flux rate calculations
Areal rates of R, GPP, net primary productivity (NPP), and NEM were calculated based on changes in DO concentrations, following Eq. (1) (Eyre et al., 2011). Respiration rates were determined from concentration data collected during the initial dark period (midnight to dawn) (Eq. 2). NPP was calculated based on light O2 flux measurements from dawn to noon (Eq. 3). We implemented a 6 h dark incubation period to ensure oxygen concentrations remained above 80 % (Eyre et al., 2002) and a 6 h light incubation period to prevent oxygen from reaching supersaturated levels (Olivé et al., 2016). Hourly GPP rates were computed as the difference between R and NPP rates (Eq. 4). NEM was calculated using Eq. (5). Positive values indicate autotrophy, while negative values represent heterotrophy.
where F=flux rate (); Ct0 and Ct1=concentration in the overlying water at the start and end of the time period (µmol L−1), respectively; V=volume of overlying water in the core (L); A=surface area in the sediment core (m2); and T=incubation period (h).
NEC rates () were estimated from the change of total alkalinity, assuming these changes are only due to CaCO3 precipitation and dissolution (Eq. 6) (Roth et al., 2019; Van Dam et al., 2019):
Here, ΔnAT=change in nAT () over the Δt (time), h=volume/area, and p=water density. The −0.5 scalar factor was applied to account for the stoichiometric relationship, where 1 mol of CaCO3 consumes 2 mol of AT. Day and night incubations (lasting 12 h) were conducted simultaneously with organic carbon metabolism to obtain daily NEC fluxes. The dark period (midnight to dawn) was used to measure nighttime dissolution, while the light period (dawn to noon) was used for daytime calcification. NEC is positive with AT consumption, indicating CaCO3 precipitation, and negative with AT production, indicating CaCO3 dissolution.
In this study, both hourly and daily rates were reported. Hourly rates allow us to examine diel variations in metabolic processes, while daily rates provide an integrated view of overall carbon dynamics, facilitating comparison with existing literature.
2.6 Statistical analysis
Independent sample T tests were applied to compare metabolic rates (R, NPP, GPP, NEM, NEC) between SG and BS using SPSS v. 17. Data were subjected to a normality test before performing the analysis. Least-squares linear regression was employed to assess the correlation between changes in DO in the SG and BS. The Mann–Whitney U test was applied for carbonate chemistry analysis due to the non-normal distribution of data.
3.1 Water quality and carbonate chemistry
Diurnal patterns of water quality and carbonate parameters for SG and BS during the 2 d ex situ core incubation are illustrated in Figs. 3 and 4, respectively. The temperature in both treatments ranged from 22–29 °C, while salinity levels spanned 35–36. These values were similar to in situ measurements obtained from the seagrass beds using a CTD profiler. During the daytime (6:00 AM to 12:30 PM LT), PAR levels ranged from 26 to a peak of 1662 , with the highest intensities observed at midday. The average PAR measured 953 on the first day of incubation, increasing slightly to 1026 on the second day. DO saturation levels were more variable in SG than BS, with values ranging from 54 %–224 % and 92 %–123 %, respectively. DO saturation levels in both treatments followed a diel pattern, with lower nighttime and higher daytime values.
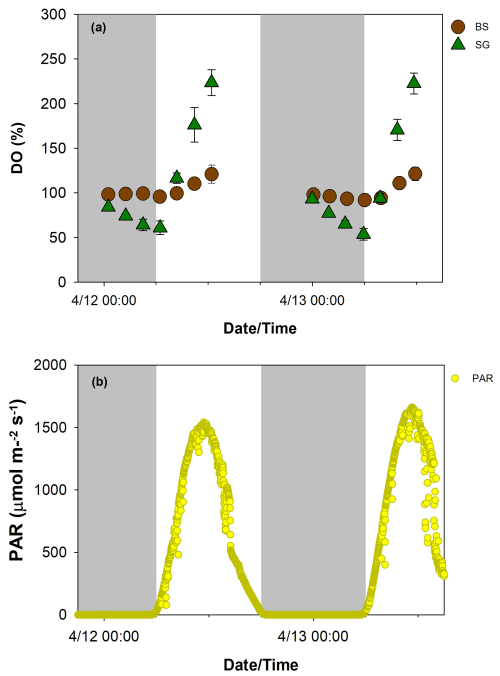
Figure 3Diurnal pattern of dissolved oxygen (DO, a) in replanted seagrass (SG, green triangle) and bare sediment (BS, brown circle) (n=9, mean ± SD) and photosynthetically active radiation (PAR, b) during the 2 d (12–13 April 2024) incubation.
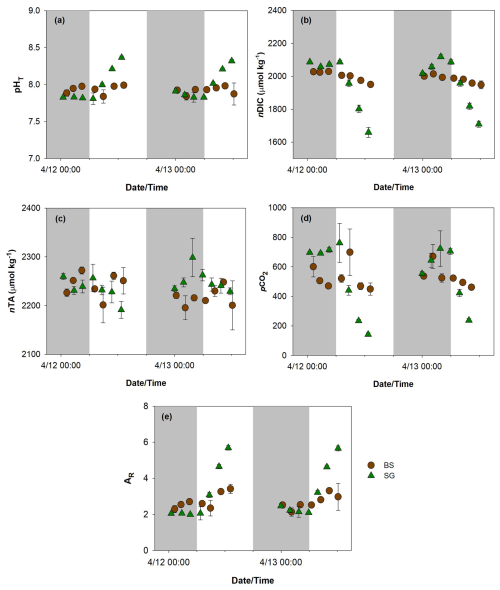
Figure 4Total scale pH (pHT, a), normalized dissolved inorganic carbon (nDIC, b), normalized total alkalinity (nAT, c), partial pressure of carbon dioxide (pCO2, d), and aragonite saturation state (ΩAR, e) in replanted seagrass (SG, green triangle) and bare sediment (BS, brown circle) during the 2 d (12–13 April 2024) incubation. n=3, mean ± SD.
Both nDIC () and pHT displayed greater diurnal fluctuations at SG compared to the BS. At SG, nDIC ranged from 1660–2118 µmol kg−1 (mean ± SD: ) and followed a diel pattern. pHT ranged from 7.81–8.37 at SG (mean ± SD: 7.99±0.2), following the opposite trend to nDIC, with values decreasing at night and increasing during the day. At the BS site, these parameters were less variable, with nDIC values ranging from 1948–2029 µmol kg−1 and pHT from 7.84–7.99, with mean values of and 7.93±0.1, respectively. Similarly, the calculated nAT was also more fluctuating in SG than BS, with mean values of 2243±24 and , respectively. The calculated pCO2 displayed a broader range at SG (142–762 µatm; mean ± SD: 510±231) compared to BS (450–699 µatm; mean ± SD: 524±82), suggesting a more dynamic carbon cycling potentially driven by seagrass metabolic activity. The mean ΩAr was higher in SG (3.14±1) compared to BS (2.72±0.4), indicating more favorable conditions for calcification at the seagrass site. The Mann–Whitney test on carbonate chemistry revealed no significant distinction between SG and BS (pHT p=0.713; nDIC, p=0.419; nAT, p=0.679; ΩAr, p=0.511).
3.2 Respiration, gross primary production, and net ecosystem metabolism
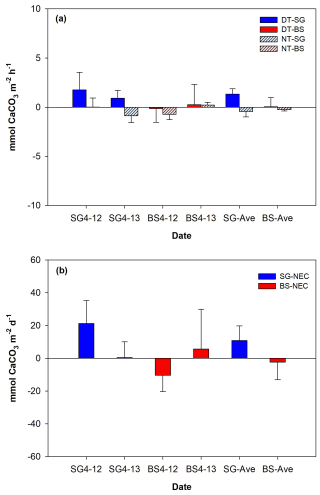
Figure 5 illustrates the comparison of metabolic rates (mean ± SD) between SG and BS. The mean respiration rates in SG () were significantly higher than in BS (), by approximately an 8-fold difference (p<0.01). The mean GPP in SG was , which is 35-fold higher than in BS () (p<0.01). GPP was always higher than R in both systems, with mean ratios of 3.4 and 1.9 in SG and BS, respectively. For NEM, both systems displayed positive values, indicating net autotrophy, with SG being 10-fold higher () compared to BS () (p<0.01). Both R and GPP in SG and BS increased on the second day of incubation (SG (R: −3.1 vs. ; GPP: 23.3 vs. 24.7 ); BS (R: −0.4 vs. ; GPP: 2.7 vs. 3.1 )), while NEM in SG (218.04 vs. 198.4 ) and BS (22.3 vs. 17.8 ) showed a slight decrease. However, these changes were not statistically significant.
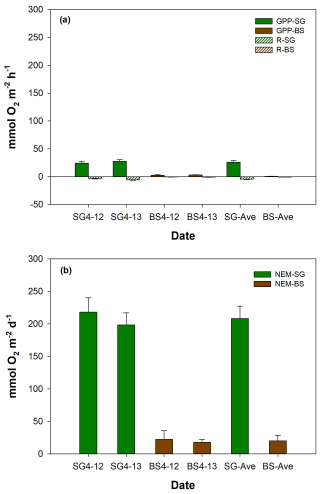
Figure 5Mean (± SD, standard deviation) values of (a) metabolic rates such as respiration (R), gross primary productivity (GPP), and (b) net ecosystem metabolism (NEM) of restored seagrass (SG, green bars) and bare sediment (BS, brown bars) in Penghu during the 2 d (12–13 April 2024) incubation (n=9).
3.3 Calcium carbonate precipitation, dissolution, and net ecosystem calcification
The NEC values (mean ± SD) over a diel cycle for SG and BS demonstrated differences in their overall carbonate dynamics (Fig. 6). Over the 2 d incubation period, SG exhibited a net-calcifying system with positive daily NEC means (10.9±15.7 ), driven by daytime calcification () despite nighttime dissolution (). In contrast, BS supported a net-dissolving system with mean daily NEC (). Mean daytime calcification and nighttime dissolution were 0.1±1.6 and , respectively. Both systems followed a general diurnal pattern, with positive NEC during the day (calcifying) and negative at night (dissolving).
Seagrass meadows are widely recognized as an important blue carbon ecosystem with substantial potential to mitigate anthropogenic CO2 emissions. Although research on seagrass ecosystems has grown in recent years, significant gaps remain in understanding their carbon dynamics. In particular, the balance of organic and inorganic carbon processes within these systems is not fully understood. Meanwhile, global seagrass coverage continues to decline, which has increased the urgency of restoration efforts (Waycott et al., 2009). Restoring seagrass meadows to enhance carbon sequestration has become increasingly important. Currently, most studies on restored seagrass meadows focus primarily on the burial of particulate organic carbon (Greiner et al., 2013), with far fewer exploring both organic metabolism and carbonate cycling in restored seagrass meadows. Here, we present the first dataset on carbon uptake through metabolic rates and calcification measurements in restored seagrass meadows within tropical regions.
4.1 Restoration of seagrass enhances metabolic rates
The metabolic rates estimated in present study were comparable to those recorded in other seagrass meadows (Table 1). Our GPP in SG was 24 % and 37 % higher than the tropical and global averages, respectively, but 38 % lower than Dongsha Island, Taiwan (Chou et al., 2023). It is also comparable to measurements reported for H. uninervis in Tropical Australia (Table 1). Conversely, the R values estimated in this study were roughly half of the tropical and global averages (Duarte et al., 2010). Our NEM (214 ) is within the range of previous estimates for tropical seagrass meadows (−477.28–484.20 ) and global estimates (−477.28–531.63 ). In addition to these global comparisons, our study reveals a clear distinction in metabolic rates (e.g., GPP, R, NEM) between SG and BS. The GPP and R in restored seagrass meadows were 35 and 7 times greater than in BS. The relatively higher metabolic rates in seagrass meadows compared to bare sediments have also been observed in other studies (Table 1). For instance, a 2-year-old restored Halodule wrightii meadow demonstrated a 13-fold increase in NEM relative to bare sediment (Egea et al., 2023). Similarly, Posidonia oceanica exhibited a notable 70-fold increase in metabolic rates compared to bare sediment (Barron et al., 2006). Furthermore, Zostera marina exhibits net autotrophy, while bare sediments are net heterotrophy (Attard et al., 2019; Chen et al., 2019). Such patterns highlight the fundamental ecological functions restored seagrass meadows play relative to unvegetated/bare sediments. The increase in GPP reflects the enhanced carbon fixation capacity of seagrass meadows, while the elevated R indicates active organic matter decomposition and microbial respiration (Duarte and Krause-Jensen, 2017). According to Duarte et al. (2010), seagrass meadows generally act as autotrophic (NEM>0) CO2 sinks when GPP exceeds 186 and shift to heterotrophy (NEM<0) when GPP falls below this threshold. Based on this threshold, our mean GPP for restored seagrass exceeded the value for autotrophy, resulting in a positive NEM, which is consistent with their global assessment. The NEM observed in SG was 10 times higher than in BS, suggesting that SG sequesters significantly more carbon than BS. These findings highlight the finding that seagrass restoration significantly boosts metabolic rates and enhances carbon cycling. Given the increasing loss of global seagrass cover, restoration not only boosts ecosystem productivity but also strengthens the ability of coastal systems to remove carbon, thereby contributing to climate change mitigation efforts.
Table 1Comparison of metabolic rates from global estimates. GPP and R values are expressed in units and NEM in .
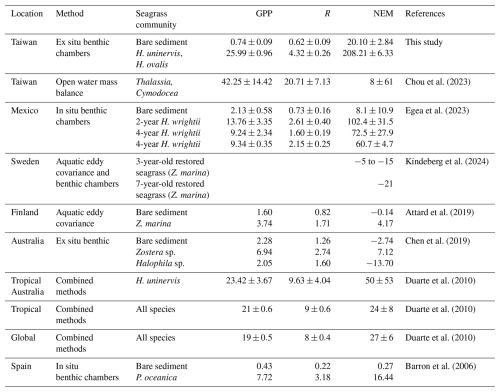
The daily values of R and GPP reported in the literature were divided by 24 and 12, respectively, to calculate the hourly values.
Key drivers of elevated metabolic rates in tropical meadows include greater PAR availability, aboveground biomass, and higher temperatures (Ganguly et al., 2017; Ward et al., 2022). Many tropical species grow near their optimal photosynthetic and physiological conditions (Lee et al., 2007; Koch et al., 2012), efficiently capturing light in shallow, clear waters, which contributes to higher NEM (Ralph et al., 2007). In our study, DO variation corresponds to light intensity (Figs. 3 and 7), suggesting that the elevated GPP observed in seagrass meadows could be driven by higher light intensity. This is likely due to the relatively lower canopy cover of H. uninervis and density in SG, which reduces shading within the seagrass. As a result, more light penetrates to the leaves, increasing their photosynthetic surface area and contributing to NEM (Ralph et al., 2007). In contrast, lower respiration rates in the SG area were likely due to the sediment characteristics and organic matter quality in this habitat. The seagrass beds are situated in carbonate-rich sediments, which typically contain less organic matter than siliciclastic or muddy sediments (Belshe et al., 2018; Kindeberg et al., 2018). This limits the availability of substrates for microbial decomposition. Moreover, the organic matter derived from seagrass detritus is generally more refractory and less labile, further reducing its accessibility for microbial breakdown and thereby suppressing heterotrophic respiration (Ren et al., 2024). Although seagrasses are capable of transporting oxygen to their belowground tissues via internal aerenchyma (Borum et al., 2006), which can support aerobic respiration, the combined effect of low organic content and poor substrate lability limits microbial activity and oxygen consumption.
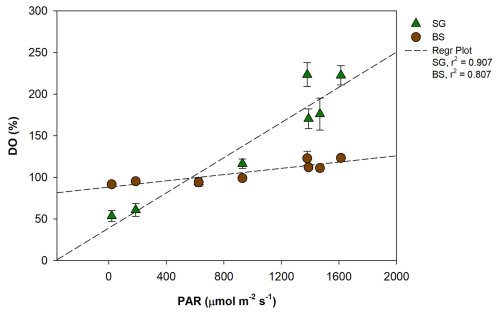
Figure 7Regression plot between photosynthetically active radiation (PAR, ) and dissolved oxygen (DO, %) in restored seagrass (SG, green triangle) and bare sediment (BS, brown circle). Error bars represent standard deviation (SD).
Several studies indicate that restored seagrass can achieve primary productivity and carbon sequestration levels comparable to natural meadows, although recovery depends on the extent of degradation, restoration success, and site-specific habitat conditions (Oreska et al., 2017; Marbà et al., 2015). For example, long-term research in Florida Bay demonstrated that sediment carbon sequestration rates and plant biomass took nearly a decade to match those of natural meadows (Greiner et al., 2013). The ability of restored meadows to maintain net autotrophy is crucial for their role as carbon sinks (Kennedy et al., 2010). This is particularly relevant for climate change mitigation strategies, where the conservation and rehabilitation of this ecosystem are recognized as natural climate solutions (Griscom et al., 2017). Nonetheless, a recent investigation on restored seagrass exhibits net heterotrophy, as observed by Kindeberg et al. (2024) in both 3- and 7-year-old meadows in Sweden. A similar pattern also reported in some natural seagrass meadows in Australia (Chen et al., 2019) (Table 1). This discrepancy underscores the variability in seagrass productivity and metabolic processes based on geographical location and environmental conditions, highlighting the need for region-specific assessments to fully understand seagrass ecosystem dynamics. Long-term studies should also consider temporal and annual variations.
4.2 Calcification dynamics in restored seagrass
Our results show that restored seagrass meadows exhibit significantly higher CaCO3 cycling – both formation and dissolution – compared to bare sediments. This corroborates with prior studies, which documented enhanced carbonate dynamics in vegetated habitats relative to unvegetated sediments. For instance, P. oceanica and Thalassia testudinum meadows have been shown to promote both CaCO3 production and dissolution (Burdige and Zimmerman, 2002; Barrón et al., 2006), with tropical seagrass ecosystems displaying similar patterns (Chou et al., 2021; Fan et al., 2024). Further, our data revealed a typical diurnal pattern, with positive values during daytime (net calcifying) and negative values during nighttime (net dissolving). These findings align with previous estimates, such as those in Florida Bay, which reported similar diurnal calcification dynamics (Yates and Halley, 2006).
The variations of CaCO3 production and dissolution in surface waters and sediment are related to the carbon cycle through photosynthesis and respiration (Yates and Halley, 2006). During daylight hours, photosynthesis raises pH and reduces CO2 levels in the water, creating favorable conditions for calcium carbonate precipitation – a process referred to as light-enhanced calcification (Schneider et al., 2009). We found a significant positive correlation between PAR and nAT changes (r2=0.52, p<0.05), suggesting that increased light availability may enhance calcification by photoautotrophs in restored seagrass areas during the day (Fig. 8). Additionally, our data showed a significant negative correlation between nAT flux and NEM (r2=0.54, p<0.01), indicating that higher photosynthetic activity (positive NEM) promotes calcification by consuming AT, while lower NEM or net heterotrophy contributes to AT production, likely through carbonate dissolution or anaerobic decomposition (Fig. 9). Similar relationships between photosynthesis and calcification have been reported in marine calcifiers (Mallon et al., 2022), and the influence of epiphytic organisms in promoting calcification during active photosynthesis has been highlighted in seagrass meadows such as P. oceanica (Barrón et al., 2006). At night, carbonate dissolution predominates as aerobic respiration produces CO2 and carbonic acid in sediment porewater (Eyre et al., 2014), lowering carbonate saturation and driving mineral dissolution (Burdige and Zimmerman, 2002; Burdige et al., 2008; Chou et al., 2021; Fan et al., 2024). The degree of dissolution is directly linked to the rate of organic matter decomposition, which depends on the quantity of organic matter, its reactivity, and oxygen availability (Andersson et al., 2005; Morse et al., 2006). High shoot density and root biomass in restored seagrass meadows enhance organic matter supply and decomposition in sediment, further driving nighttime dissolution (Kindeberg et al., 2020).
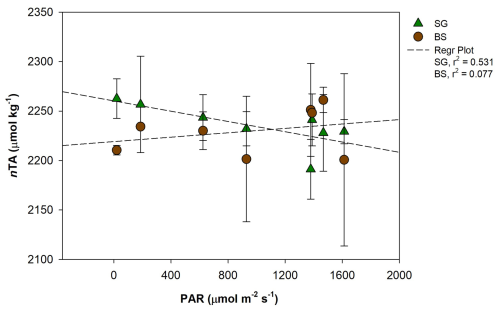
Figure 8Regression plot between photosynthetically active radiation (PAR, ) and normalized total alkalinity (nAT, µmol kg−1) in restored seagrass (SG, green triangle) and bare sediment (BS, brown circle). Error bars represent standard deviation (SD).
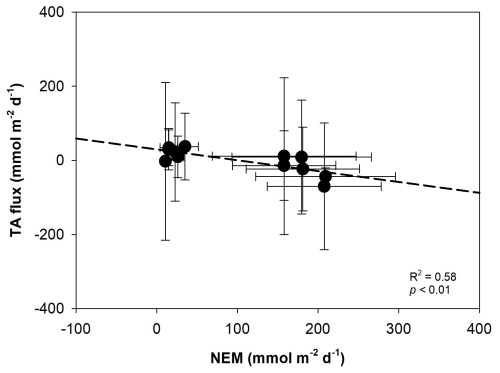
Figure 9Linear regression showing the relationship between total alkalinity (AT, ) flux and net ecosystem metabolism (NEM, ) in restored seagrass meadows and bare sediment. Error bars represent standard deviation (SD).
Over cumulative days, our NEC measurements indicate that restored seagrass meadows support overall net calcification, whereas BS supports net dissolution. Our estimates are similar to those from Australia (Walker and Woelkerling, 1988) and 7 times higher than Mediterranean seagrass net calcification rates (Barrón et al., 2006), which are 295 (8.8 ) and 51 (1.40 ), respectively. In contrast, our findings are lower than those reported in the Caribbean region of Mexico, where ex situ estimates ranged from 14–153 (Enríquez and Schubert, 2014). This highlights the enhanced carbonate production potential in tropical seagrass meadows. A positive net calcification system occurs when CaCO3 precipitation exceeds dissolution within the system (Kleypas et al., 2001; Eyre et al., 2014). Restoration of seagrass meadows provides a substrate for diverse calcifying organisms, including crustose coralline algae, bryozoans, foraminifera, and serpulids, which enhance carbonate production (Beavington-Penney et al., 2005). Epiphytes on seagrass leaves significantly contribute to CaCO3 production, with tropical seagrass meadows typically supporting higher carbonate loads than temperate ones. Reported production rates span 180 in Jamaica (Land, 1970) to 2800 in Barbados (Patriquin, 1972), underscoring regional variability in seagrass-associated calcification. Moreover, fluctuations in concentrations are crucial in regulating the capacity of calcifying organisms to form CaCO3. Our data reveal a higher mean ΩAr in SG (3.14±1) compared to BS (2.72±0.4). Seagrass photosynthesis raises pH and ΩAr, enhancing the calcification of surrounding calcifying organisms (De Beer and Larkum, 2001). However, the consumption of AT by calcifiers during the calcification process releases CO2, potentially counteracting pH increases and partially offsetting the net carbon uptake potential of seagrass ecosystems (Alongi et al., 2008; Mazarrasa et al., 2015; Saderne et al., 2019). This highlights the dual role of seagrass restoration in supporting biodiversity and CO2 uptake while influencing carbonate and carbon flux dynamics. Although the restored seagrass meadow in our study functions as a net-calcifying system, AT fluxes between SG and BS showed no significant difference.
4.3 Net carbon uptake of seagrass restoration
In order to estimate the net carbon uptake potential of seagrass restoration, we applied the photosynthesis-quotient (PQ) of 1 to calculate CO2 uptake from organic carbon metabolism (Gattuso et al., 1998; Ward et al., 2022). In terms of carbonate dynamics, we applied Φ, as described by Humphreys et al. (2018), to calculate the size of CO2 source or sink for each system. In the SG system, which is net calcifying, Φ indicates a CO2 source, with 0.61 mol of CO2 released into the seawater for each mole of CaCO3 precipitated. In contrast, the BS system, which is net dissolving, Φ represents a CO2 sink, with 0.65 mol of CO2 absorbed for each mole of CaCO3 dissolved. These values are comparable to previous findings, which reported a CO2 flux-to-CaCO3 precipitation ratio of 0.63 (Frankignoulle et al., 1994; Smith, 2013; Mazarrasa et al., 2015). The calculated results show that total carbon uptake from NEM was 208 mmol CO2 m2 d−1 in SG and 20 mmol CO2 m2 d−1 BS. For NEC, the carbon release in SG was 6.6 CO2 m2 d−1, while for BS, an additional CO2 uptake was . Consequently, the net carbon uptake is 202 and 22 mmol CO2 m2 d−1 for SG and BS, respectively. Our results demonstrate that the primary productivity of restored seagrass through photosynthesis exceeds the rates of calcification by 31-fold, suggesting that restored seagrass can act as a net carbon sink. However, further assessments are necessary to capture temporal variations, as our current measurements are based on daily observations and one season only.
4.4 Limitations of ex situ benthic incubation and future research
We tested the ex situ benthic core incubation approach for restored seagrass meadows, drawing from the existing utilities in some coastal areas and freshwater ecosystems for sulfate and nutrient fluxes (Eyre and Ferguson, 2005, Chen et al., 2019). Overall, the ex situ benthic incubation method provides a significant advantage by measuring both organic and inorganic carbon dynamics simultaneously, addressing a critical gap in previous methods that often overlook carbonate dynamics (Johannessen, 2023). This approach is also useful for assessing seagrass metabolism in subtidal meadows, where collecting data is challenging due to high labor costs and weather conditions. Moreover, some in situ autonomous methods are often expensive and constrained operational periods of only a few weeks due to challenges like sensor error and biofouling (Yates and Halley, 2003; Takeshita et al., 2016). While this approach provides several advantages, one notable limitation is its applicability. Currently, the design is primarily suited for small seagrass, like H. ovalis, H. uninervis, and Z. japonica. It may not be adequate for larger species, like Enhalus acoroides and large Thalassia hemprichii, due to differences in size and growth characteristics. Moreover, we suggest validating the ex situ results with in situ data to ensure comparability with natural conditions, particularly the effects of light attenuation. Our measurements were obtained under ex-situ conditions in a shallow water column, which likely exposed the cores to higher irradiance than would be encountered in situ at different seagrass depths (2–4 m). While previous research has shown that ex situ and in situ incubations can yield comparable metabolic estimates, supporting the validity of our approach (Maher and Eyre, 2011), we acknowledge the need for future in situ incubations to more accurately capture the natural light environment experienced by seagrass leaves. Future research should integrate ex situ results with in situ data with different geographic and environmental settings to enhance the generalizability of the findings. This will provide a more accurate assessment of seagrass ecosystems' role in global carbon cycling and inform more effective coastal management and conservation practices.
This study investigates the organic carbon metabolism and carbonate dynamics of replanted SG compared to BS using the ex situ core incubation method. The results show that SG has higher GPP and NEM, while exhibiting similar NEC, making it a stronger carbon sink than BS. The findings highlight the role of seagrass restoration in enhancing carbon removal and contribute to a growing body of literature that highlights the ecological value of restored seagrass meadows. This study represents the first simultaneous quantitative estimate of the effect of both organic carbon metabolism and carbonate dynamics on carbon sequestration of restored seagrass in Southeast Asia, providing valuable insights into the region's carbon dynamics. We emphasize the need for long-term research on metabolic rates and carbonate dynamics to account for temporal variations and to fully understand the implications of these processes in carbon sequestration. This will also help optimize restoration strategies aimed at maximizing carbon sink potential and mitigating ocean acidification. Furthermore, ex situ benthic incubation proves to be a valuable tool for assessing carbon fluxes in seagrass meadows, particularly those dominated by pioneering species, although further in situ assessments are necessary for comprehensive validation.
The data supporting the findings of this study are available in the DRYAD repository at https://doi.org/10.5061/dryad.d7wm37qd0 (Natividad et al., 2025).
WCC and JJC conceptualized the research and spearheaded the implementation. JJC, MBN, HYC, and YLS facilitated sample collection and analysis. MBN and JJC performed the data analysis and drafted and revised the manuscript. WCC and LFF reviewed and revised the manuscript. All authors were involved in the finalization of the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We are grateful to Hsin-Chiao Chang, Yuhann Yokie-Tai, Ping-Chun Chen, and Xin-Yi Wang for the field sampling and laboratory assistance and to Adrian Lansigan and Wencelito Hintural for generating the map.
This research has been supported by the National Science and Technology Council (grant-nos. NSTC 113-2119-M-019-008 and NSTC 113-2611-M-019-011).
This paper was edited by Paul Stoy and reviewed by two anonymous referees.
Allen Coral Atlas: Imagery, maps and monitoring of the world's tropical coral reefs, Zendodo [data set], https://doi.org/10.5281/zenodo.3833242, 2020.
Alongi, D. M., Trott, L. A., Undu, M. C., and Tirendi, F.: Benthic microbial metabolism in seagrass meadows along a carbonate gradient in Sulawesi, Indonesia, Aquat. Microb. Ecol., 51, 141–152, https://doi.org/10.3354/ame01191, 2008.
Andersson, A. J., Mackenzie, F. T., and Lerman, A.: Coastal ocean and carbonate systems in the high CO2 world of the Anthropocene, Am. J. Sci., 305, 875–918, https://doi.org/10.2475/ajs.305.9.875, 2005.
Attard, K. M., Rodil, I. F., Glud, R. N., Berg, P., Norkko, J., and Norkko, A.: Seasonal ecosystem metabolism across shallow benthic habitats measured by aquatic eddy covariance, Limnol. Oceanogr. Lett., 4, 79–86, https://doi.org/10.1002/lol2.10107, 2019.
Barrón, C., Duarte, C. M., Frankignoulle, M., and Borges, A. V.: Organic carbon metabolism and carbonate dynamics in a Mediterranean seagrass (Posidonia oceanica), meadow, Estuar. Coasts., 29, 417–426, https://doi.org/10.1007/bf02784990, 2006.
Beavington-penney, S. J., Wright, V. P., and Racey, A.: Sediment production and dispersal on foraminifera-dominated early Tertiary ramps: The Eocene El Garia Formation, Tunisia, Sedimentology, 52, 537–569, https://doi.org/10.1111/j.1365-3091.2005.00709.x, 2005.
Belshe, E. F., Hoeijmakers, D., Herran, N., Mtolera, M., and Teichberg, M.: Seagrass community-level controls over organic carbon storage are constrained by geophysical attributes within meadows of Zanzibar, Tanzania, Biogeosciences, 15, 4609–4626, https://doi.org/10.5194/bg-15-4609-2018, 2018.
Berg, P., Huettel, M., Glud, R. N., Reimers, C. E., and Attard, K. M. Aquatic Eddy Covariance: The method and its contributions to defining oxygen and carbon fluxes in marine environments, Ann. Rev. Mar. Sci., 14, 431–455, https://doi.org/10.1146/annurev-marine-042121-012329, 2022.
Borum, J., Sand-Jensen, K., Binzer, T., Pedersen, O., and Greve, T. M.: Oxygen movement in seagrasses, in: Seagrasses: Biology, Ecology and Conservation, edited by: Larkum, A. W. D., Orth, R. J., and Duarte, C. M., Springer, Dordrecht, 255–270, https://doi.org/10.1007/978-1-4020-2983-7_10, 2006.
Burdige, D. J. and Zimmerman, R. C.: Impact of sea grass density on carbonate dissolution in Bahamian sediments, Limnol. Oceanogr., 47, 1751–1763, https://doi.org/10.4319/lo.2002.47.6.1751, 2002.
Burdige, D. J., Zimmerman, R. C., and Hu, X.: Rates of carbonate dissolution in permeable sediments estimated from porewater profiles: the role of sea grasses, Limnol. Oceanogr., 53, 549–565, https://doi.org/10.4319/lo.2008.53.2.0549, 2008.
Campbell, S., Miller, C., Steven, A., and Stephens, A.: Photosynthetic responses of two temperate seagrasses across a water quality gradient using chlorophyll fluorescence, J. Exp. Mar. Biol. Ecol., 291, 57–78, https://doi.org/10.1016/s0022-0981(03)00090-x, 2003.
Chen, J., Wells, N., Erler, D., and Eyre, B.: Importance of habitat diversity to changes in benthic metabolism over land-use gradients: evidence from three subtropical estuaries, Mar. Ecol. Prog. Ser., 631, 31–47, https://doi.org/10.3354/meps13147, 2019.
Chen, T.-Y., Chen, J.-J., and Chou, W.-C.: Rethinking blue carbon: unlocking invisible carbon sinks, Environ. Res. Lett., 19, 101001, https://doi.org/10.1088/1748-9326/ad7044, 2024.
Chou, W., Fan, L., Yang, C., Chen, Y., Hung, C., Huang, W., Shih, Y., Soong, K., Tseng, H., Gong, G., Chen, H., and Su, C.: A unique DIEL pattern in carbonate chemistry in the seagrass meadows of Dongsha Island: the enhancement of metabolic carbonate dissolution in a semienclosed lagoon, Front. Mar. Sci., 8, https://doi.org/10.3389/fmars.2021.717685, 2021.
Chou, W., Fan, L., Hung, C., Shih, Y., Huang, W., Lui, H., and Chen, T.: Dynamics of O2 and pCO2 in a Southeast Asia seagrass meadow: Metabolic rates and carbon sink capacity, Front. Mar. Sci., 10, https://doi.org/10.3389/fmars.2023.1076991, 2023.
Chou, W.-C., Chu, H.-C., Chen, Y.-H., Syu, R.-W., Hung, C.-C., and Soong, K.: Short-term variability of carbon chemistry in two contrasting seagrass meadows at Dongsha island: implications for pH buffering and CO2 sequestration, Estuar. Coast. Shelf Sci., 210, 36–44, https://doi.org/10.1016/j.ecss.2018.06.006, 2018.
Clayton, T. D. and Byrne, R. H.: Spectrophotometric seawater pH measurements: total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results, Deep-Sea Res. I: Oceanogr. Res. Pap., 40, 2115–2129, https://doi.org/10.1016/0967-0637(93)90048-8, 1993.
De Beer, D. and Larkum, A. W. D.: Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors, Plant Cell Environ., 24, 1209– 1217, https://doi.org/10.1046/j.1365-3040.2001.00772.x, 2001.
Dickson, A. G. and Millero, F. J.: A Comparison of the Equilibrium Constants for the Dissociation of Carbonic Acid in Seawater Media, Deep-Sea Res. I: Oceanogr. Res. Pap., 34, 1733–1743, https://doi.org/10.1016/0198-0149(87)90021-5, 1987.
Dickson, A. G., Sabine, C. L., and Christian, J. R. (Eds.): Guide to best practices for ocean CO2 measurements, PICES Special Publication 3, 191 pp., ISBN 1-897176-07-4, 2007.
Duarte, C. M. and Krause-Jensen, D.: Export from Seagrass Meadows Contributes to Marine Carbon Sequestration, Front. Mar. Sci., 4, 13, https://doi.org/10.3389/fmars.2017.00013, 2017.
Duarte, C. M., Middelburg, J. J., and Caraco, N.: Major role of marine vegetation on the oceanic carbon cycle, Biogeosciences, 2, 1–8, https://doi.org/10.5194/bg-2-1-2005, 2005.
Duarte, C. M., Marbà, N., Gacia, E., Fourqurean, J. W., Beggins, J., Barrón, C., and Apostolaki, E. T.: Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows, Global Biogeochem. Cy., 24, https://doi.org/10.1029/2010gb003793, 2010.
Duarte, C. M., Sintes, T., and Marbà, N.: Assessing the CO2 capture potential of seagrass restoration projects. J. Appl. Ecol., 50, 1341–1349, https://doi.org/10.1111/1365-2664.12155, 2013.
Egea, L., Infantes, E., and Jiménez-Ramos, R.: Loss of POC and DOC on seagrass sediments by hydrodynamics, Sci. Total Environ., 901, 165976, https://doi.org/10.1016/j.scitotenv.2023.165976, 2023.
Enríquez, S. and Schubert, N.: Direct contribution of the seagrass Thalassia testudinum to lime mud production, Nat. Commun., 5, 3835, https://doi.org/10.1038/ncomms4835, 2014.
Eyre, B. D. and Ferguson, A. J. P.: Benthic metabolism and nitrogen cycling in a subtropical east Australian estuary (Brunswick): Temporal variability and controlling factors, Limnol. Oceanogr., 50, 81–96, https://doi.org/10.4319/lo.2005.50.1.0081, 2005.
Eyre, B. D., Rysgaard, S., Dalsgaard, T., Christensen, P. B.: Comparison of isotope pairing and N2: Ar methods for measuring sediment denitrification – assumptions, modifications, and implications, Estuaries, 25, 1077–1087, https://doi.org/10.4319/lo.2002.47.4.1043, 2002.
Eyre, B. D. and Ferguson, A. J. P.: Benthic metabolism and nitrogen cycling in a subtropical east Australian estuary (Brunswick): temporal variability and controlling factors, Limnol. Oceanogr., 50, 81–96, https://doi.org/10.4319/lo.2005.50.1.0081, 2005.
Eyre, B. D., Ferguson, A. J. P., Webb, A., Maher, D., and Oakes, J. M.: Denitrification, N-fixation and nitrogen and phosphorus fluxes in different benthic habitats and their contribution to the nitrogen and phosphorus budgets of a shallow oligotrophic sub-tropical coastal system (southern Moreton Bay, Australia), Biogeochemistry, 102, 111–133, https://doi.org/10.1007/s10533-010-9425-6, 2011.
Eyre, B. D., Andersson, A. J., and Cyronak, T.: Benthic coral reef calcium carbonate dissolution in an acidifying ocean, Nat. Clim. Change, 4, 969–976, https://doi.org/10.1038/nclimate2380, 2014.
Fan, L.-F., Kang, E.-C., Natividad, M. B., Hung, C.-C., Shih, Y.-Y., Huang, W.-J., and Chou, W.-C.: The role of benthic TA and DIC fluxes on carbon sequestration in seagrass meadows of Dongsha Island, J. Mar. Sci. Eng., 12, 2061, https://doi.org/10.3390/jmse12112061, 2024.
Ferguson, A., Eyre, B., and Gay, J.: Benthic nutrient fluxes in euphotic sediments along shallow sub-tropical estuaries, northern New South Wales, Australia, Aquat. Microb. Ecol., 37, 219–235, https://doi.org/10.3354/ame037219, 2004.
Frankignoulle, M., Canon, C., and Gattuso, J.-P.: Marine calcification as a source of carbon dioxide: Positive feedback of increasing atmospheric CO2, Limnol. Oceanogr., 39, 458–462, 1994.
Fourqurean, J. W., Duarte, C. M., Kennedy, H., Marbà, N., Holmer, M., Mateo, M. A., Apostolaki, E. T., Kendrick, G. A., Krause-Jensen, D., McGlathery, K. J., and Serrano, O.: Seagrass ecosystems as a globally significant carbon stock, Nat. Geosci., 5, 505–509, https://doi.org/10.1038/ngeo1477, 2012.
Ganguly, D., Singh, G., Ramachandran, P., Selvam, A. P., Banerjee, K., and Ramachandran, R.: Seagrass metabolism and carbon dynamics in a tropical coastal embayment, Ambio. Oct., 46, 667–679, https://doi.org/10.1007/s13280-017-0916-8, 2017.
Gattuso, J.-P., Frankignoulle, M., and Wollast, R.: Carbon and carbonate metabolism in coastal aquatic ecosystems, Annu. Rev. Ecol. Syst., 29, 405–434, https://doi.org/10.1146/annurev.ecolsys.29.1.405, 1998.
Greiner, J. T., McGlathery, K. J., Gunnell, J., and McKee, B. A.: Seagrass restoration enhances “Blue carbon” sequestration in coastal waters, PLoS One, 8, e72469, https://doi.org/10.1371/journal.pone.0072469, 2013.
Griscom, B. W., Adams, J., Ellis, P. W., Houghton, R. A., Lomax, G., Miteva, D. A., Schlesinger, W. H., Shoch, D., Siikamäki, J. V., Smith, P., Woodbury, P., Zganjar, C., Blackman, A., Campari, J., Conant, R. T., Delgado, C., Elias, P., Gopalakrishna, T., Hamsik, M. R., Herrero, M., Kiesecker, J., Landis, E., Laestadius, L., Leavitt, S. M., Minnemeyer, S., Polasky, S., Potapov, P., Putz, F. E., Sanderman, J., Silvius, M., Wollenberg, E., and Fargione, J: Natural climate solutions, P. Natl. A Sci., 114, 11645–11650, https://doi.org/10.1073/pnas.1710465114, 2017.
Hendriks, I. E., Olsen, Y. S., Ramajo, L., Basso, L., Steckbauer, A., Moore, T. S., Howard, J., and Duarte, C. M.: Photosynthetic activity buffers ocean acidification in seagrass meadows, Biogeosciences, 11, 333–346, https://doi.org/10.5194/bg-11-333-2014, 2014.
Humphreys, M. P., Daniels, C. J., Wolf-Gladrow, D. A., Tyrrell, T., and Achterberg, E. P.: On the influence of marine biogeochemical processes over CO2 exchange between the atmosphere and ocean, Mar. Chem., 199, 1–11, https://doi.org/10.1016/j.marchem.2017.12.006, 2018.
Johannessen, S. C.: How to quantify blue carbon sequestration rates in seagrass meadow sediment: geochemical method and troubleshooting, Carbon Footprints, 2, https://doi.org/10.20517/cf.2023.37, 2023.
Juska, I. and Berg, P.: Variation in seagrass meadow respiration measured by aquatic eddy covariance Limnol. Oceanogr. Lett. 7, 410–418, https://doi.org/10.1002/lol2.10276, 2022.
Kennedy, H., Beggins, J., Duarte, C. M., Fourqurean, J. W., Holmer, M., Marbà, N., and Middelburg, J. J.: Seagrass sediments as a global carbon sink: Isotopic constraints, Global Biogeochem. Cy., 24, https://doi.org/10.1029/2010gb003848, 2010.
Kindeberg, T., Ørberg, S. B., Röhr, M. E., Holmer, M., and Krause-Jensen, D.: Sediment stocks of carbon, nitrogen, and phosphorus in Danish eelgrass meadows, Front. Mar. Sci., 5, 474, https://doi.org/10.3389/fmars.2018.00474, 2018.
Kindeberg, T., Bates, N. R., Courtney, T. A., Cyronak, T., Griffin, A., Mackenzie, F. T., Paulsen, M. L., and Andersson, A. J.: Porewater carbonate chemistry dynamics in a temperate and a subtropical seagrass system, Aquat. Geochem., 26, 375–399, https://doi.org/10.1007/s10498-020-09378-8, 2020.
Kindeberg, T., Attard, K. M., Hüller, J., Müller, J., Quintana, C. O., and Infantes, E.: Structural complexity and benthic metabolism: resolving the links between carbon cycling and biodiversity in restored seagrass meadows, Biogeosciences, 21, 1685–1705, https://doi.org/10.5194/bg-21-1685-2024, 2024.
Kleypas, J. A., Buddemeier, R. W., and Gattuso, J. P.: The future of coral reefs in an age of global change, Int. J. Earth Sci., 90, 426–437, https://doi.org/10.1007/s005310000125, 2001.
Koch, M., Bowes, G., Ross, C., and Zhang, X.: Climate change and ocean acidification effects on seagrasses and marine macroalgae, Glob. Change Biol., 19, 103–132, https://doi.org/10.1111/j.1365-2486.2012.02791.x, 2012.
Kraemer, G. P. and Alberte, R. S.: Age-related patterns of metabolism and biomass in subterranean tissues of Zostera marina L. (eelgrass), Mar. Ecol. Prog. Ser., 95, 193–203, 1993.
Land, L. S. Carbonate mud; production by epibiont growth on Thalassia testudinum, J. Sediment. Res., 40, 1361–1363, https://doi.org/10.1306/74D721B7-2B21-11D7-8648000102C1865D, 1970.
Lee, K., Park, S. R., and Kim, Y. K.: Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. J. Exp. Mar. Biol. Ecol., 350, 144–175, https://doi.org/10.1016/j.jembe.2007.06.016, 2007.
Macreadie, P. I., Baird, M. E., Trevathan-Tackett, S. M., Larkum, A. W. D., and Ralph, P. J.: Quantifying and modelling the carbon sequestration capacity of seagrass meadows – A critical assessment, Mar. Pollut. Bull., 83, 430–439, https://doi.org/10.1016/j.marpolbul.2013.07.038, 2013.
Maher, D. and Eyre, B.: Benthic carbon metabolism in southeast Australian estuaries: habitat importance, driving forces, and application of artificial neural network models, Mar. Ecol. Prog. Ser., 439, 97–115, https://doi.org/10.3354/meps09336, 2011.
Mallon, J., Cyronak, T., Hall, E. R., Banaszak, A. T., Exton, D. A., and Bass, A. M.: Light-driven dynamics between calcification and production in functionally diverse coral reef calcifiers, Limnol. Oceanogr., 67, 434–449, https://doi.org/10.1002/lno.12002, 2022.
Marbà, N., Arias-Ortiz, A., Masqué, P., Kendrick, G. A., Mazarrasa, I., Bastyan, G. R., Garcia-Orellana, J., and Duarte, C. M.: Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks, J. Ecol., 103, 296–302, https://doi.org/10.1111/1365-2745.12370, 2015.
Mazarrasa, I., Marbà, N., Lovelock, C. E., Serrano, O., Lavery, P. S., Fourqurean, J. W., Kennedy, H., Mateo, M. A., Krause-Jensen, D., Steven, A. D. L., and Duarte, C. M.: Seagrass meadows as a globally significant carbonate reservoir, Biogeosciences, 12, 4993–5003, https://doi.org/10.5194/bg-12-4993-2015, 2015.
Mcleod, E., Chmura, G. L., Bouillon, S., Salm, R., Björk, M., Duarte, C. M., Lovelock, C. E., Schlesinger, W. H., and Silliman, B. R.: A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2, Front. Ecol. Environ., 9, 552–560, https://doi.org/10.1890/110004, 2011.
Mehrbach, C., Culberson, C. H., Hawley, J. E., and Pytkowicx, R. M.: Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure, Limnol. Oceanogr., 18, 897–907, https://doi.org/10.4319/lo.1973.18.6.0897, 1973.
Morse, J. W., Andersson, A. J., and Mackenzie, F. T.: Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and “ocean acidification”: Role of high-Mg calcites, Geochim. Cosmochim. Ac., 70, 5814–5830, https://doi.org/10.1016/j.gca.2006.08.017, 2006.
Natividad, M. B., Chen, J.-J., Chou, H.-Y., Fan, L.-F., Shen, Y. L., and Chou, W.-C.: Estimation of metabolic dynamics of restored seagrass meadows in a Southeast Asia Islet: Insights from ex situ benthic incubation, Dryad [data set], https://doi.org/https://doi.org/10.5061/dryad.d7wm37qd0, 2025.
Odum, H. T.: Primary production in flowing waters, Limnol. Oceanogr., 1, 102–117, 1956.
Olivé, I., Silva, J., Costa, M. M., and Santos, R.: Estimating seagrass community metabolism using benthic chambers: The effect of incubation time, Estuar. Coasts, 39, 138–144, https://doi.org/10.1007/s12237-015-9973-z, 2016.
Oreska, M. P. J., Wilkinson, G. M., McGlathery, K. J., Bost, M., and McKee, B. A.: Non-seagrass carbon contributions to seagrass sediment blue carbon, Limnol. Oceanogr., 63, https://doi.org/10.1002/lno.10718, 2017.
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Olyarnik, S., Short, F. T., Waycott, M., and Williams, S. L.: A global crisis for seagrass ecosystems, OUP Academic., https://doi.org/10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2, 2006.
Patriquin, D.G: The origin of nitrogen and phosphorus for growth of the marine angiosperm Thalassia testudinum, Mar. Biol. 15, 35–46, https://doi.org/10.1007/BF00347435, 1972.
Pelletier, G., Lewis, E., and Wallace, D. W. R.: CO2SYS. XLS: A Calculator for the CO2 System in Seawater for Microsoft Excel/VBA, Version 16, Washington State Department of Ecology, Washington DC, https://www.ecy.wa.gov/programs/eap/models.html (last access: 22 September 2025), 2011.
Ralph, P., Durako, M., Enríquez, S., Collier, C., and Doblin, M.: Impact of light limitation on seagrasses, J. Exp. Mar. Biol. Ecol., 350, 176–193, https://doi.org/10.1016/j.jembe.2007.06.017, 2007.
Ren, Y., Liu, S., Luo, H., Jiang, Z., Liang, J., Wu, Y., Huang, X., and Macreadie, P. I.: Seagrass decline weakens sediment organic carbon stability, Sci. Total Environ., 937, 173523, https://doi.org/10.1016/j.scitotenv.2024.173523, 2024.
Rheuban, J. E., Berg, P., and McGlathery, K. J.: Ecosystem metabolism along a colonization gradient of eelgrass (Zostera marina) measured by eddy correlation, Limnol. Oceanogr., 59, 1376–1387, 2014.
Roth, F., Wild, C., Carvalho, S., Rädecker, N., Voolstra, C. R., Kürten, B., Anlauf, H., El-Khaled, Y. C., Carolan, R., and Jones, B. H.: An in situ approach for measuring biogeochemical fluxes in structurally complex benthic communities, Methods Ecol. Evol., 10, 712–725, https://doi.org/10.1111/2041-210x.13151, 2019.
Saderne, V., Geraldi, N. R., Macreadie, P. I., Maher, D. T., Middelburg, J. J., Serrano, O., Almahasheer, H., Arias-Ortiz, A., Cusack, M., Eyre, B. D., Fourqurean, J. W., Kennedy, H., Krause-Jensen, D., Kuwae, T., Lavery, P. S., Lovelock, C. E., Marbà, N., Masqué, P., Mateo, M. A., Mazarrasa, I., McGlathery, K. J., Oreska, M. P. J., Sanders, C. J., Santos, I. R., Smoak, J. M., Tanaya, T., Watanabe, K., and Duarte, C. M.: Role of carbonate burial in Blue Carbon budgets, Nat. Commun., 10, 1106, https://doi.org/10.1038/s41467-019-08842-6, 2019.
Schneider, K., Levy, O., Dubinsky, Z., and Erez, J.: In situ diel cycles of photosynthesis and calcification in hermatypic corals, Limnol. Oceanogr., 54, 1995–2002, https://doi.org/10.4319/lo.2009.54.6.1995, 2009.
Short, F. T., Polidoro, B., Livingstone, S. R., Carpenter, K. E., Bandeira, S., Bujang, J. S., Calumpong, H. P., Carruthers, T. J. B., Coles, R. G., Dennison, W. C., Erftemeijer, P. L. A., Fortes, M. D., Freeman, A. S., Jagtap, T. G., Kamal, A. H. M., Kendrick, G. A., Kenworthy, W. J., La Nafie, Y. A., Nasution, I. M., Orth, R. J., Prathep, A., Sanciangco, J. C., van Tussenbroek, B., Vergara, S. G., Waycott, M., and Zieman, J. C.: Extinction risk assessment of the world’s seagrass species, Biol. Conserv., 144, 1961–1971, https://doi.org/10.1016/j.biocon.2011.04.010, 2011.
Smith, S. V.: Parsing the oceanic calcium carbonate cycle: a net atmospheric carbon dioxide source, or a sink?, L&O e-Books, Association for the Sciences of Limnology and Oceanography (ASLO), Waco, TX, ISBN 978-0-9845591-2-1, https://doi.org/10.4319/svsmith.2013.978-0-9845591-2-1, 2013.
Takeshita, Y., McGillis, W., Briggs, E. M., Carter, A. L., Donham, E. M., Martz, T. R., Price, N. N., and Smith, J. E.: Assessment of net community production and calcification of a coral reef using a boundary layer approach, J. Geophys. Res.-Oceans, 121, 5655–5671, 2016.
Van Dam, B. R., Lopes, C., Osburn, C. L., and Fourqurean, J. W.: Net heterotrophy and carbonate dissolution in two subtropical seagrass meadows, Biogeosciences, 16, 4411–4428, https://doi.org/10.5194/bg-16-4411-2019, 2019.
Van Dam, B. R., Zeller, M. A., Lopes, C., Smyth, A. R., Böttcher, M. E., Osburn, C. L., Zimmerman, T., Pröfrock, D., Fourqurean, J. W., and Thomas, H.: Calcification-driven CO2 emissions exceed “Blue Carbon” sequestration in a carbonate seagrass meadow, Sci. Adv., 7, https://doi.org/10.1126/sciadv.abj1372, 2021.
Walker, D. and Woelkerling, W.: Quantitative study of sediment contribution by epiphytic coralline red algae in seagrass meadows in Shark Bay, Western Australia, Mar. Ecol. Prog. Ser., 43, 71–77, 1988.
Ward, M., Kindinger, T. L., Hirsh, H. K., Hill, T. M., Jellison, B. M., Lummis, S., Rivest, E. B., Waldbusser, G. G., Gaylord, B., and Kroeker, K. J.: Reviews and syntheses: Spatial and temporal patterns in seagrass metabolic fluxes, Biogeosciences, 19, 689–699, https://doi.org/10.5194/bg-19-689-2022, 2022.
Waycott, M., Duarte, C. M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., Olyarnik, S., Calladine, A., Fourqurean, J. W., Heck, K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Short, F. T., and Williams, S. L.: Accelerating loss of seagrasses across the globe threatens coastal ecosystems, P. Natl. A. Sci., 106, 12377–12381, https://doi.org/10.1073/pnas.0905620106, 2009.
Yang, Y.-P., Fong, S.-C., and Liu, H.-Y.: Taxonomy and distribution of seagrasses in Taiwan, Taiwania, 47, 54–61, 2002.
Yates, K. K. and Halley, R. B.: Measuring coral reef community metabolism using new benthic chamber technology, Coral Reefs, 22, 247–255, https://doi.org/10.1007/s00338-003-0314-5, 2003.
Yates, K. K. and Halley, R. B.: Diurnal variation in rates of calcification and carbonates sediment dissolution in Florida Bay, Estuar. Coasts, 29, 24–39, 2006.