the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Reviews and syntheses: Contribution of sulfate to aerobic methane oxidation in upland soils – a mini-review
Rui Su
Kexin Li
Nannan Wang
Fenghui Yuan
Ying Zhao
Yunjiang Zuo
Ying Sun
Liyuan He
Lixin Yang
Lihua Zhang
Methane (CH4) is a potent greenhouse gas, and its global warming potential is 28 times higher than carbon dioxide (CO2). Various environmental factors influence aerobic CH4 oxidation in soil. The sulfate (SO) ion is the main component of atmospheric deposition and has been increasing in recent years. It promotes CH4 production and anaerobic CH4 oxidation; however, the impact of SO on aerobic CH4 oxidation in soils has not yet been comprehensively summarized. We synthesize current research on the effects of SO on aerobic CH4 oxidation, examining both its macroscopic manifestations and microscale pathways. Through a literature review, we found that SO enhances aerobic CH4 oxidation by 0 %–42 %; moreover, it has been found that various physicochemical properties and processes in the soil are influenced by the addition of SO, which in turn affects aerobic CH4 oxidation. This review enhances our understanding of the role of SO in promoting aerobic CH4 oxidation. It lays the foundation for future research with two primary goals: (1) validating these findings by quantifying CH4 flux and aerobic oxidation rates and (2) elucidating the underlying microbial processes through experimental research. Concurrently, the review provides directions for further investigation into the impact of SO on aerobic CH4 oxidation.
- Article
(3294 KB) - Full-text XML
- BibTeX
- EndNote
Methane (CH4) is an important greenhouse gas, and its atmospheric concentration has increased since preindustrial times (Place, 2024; Praeg et al., 2016). Its global warming potential is 28 times higher than carbon dioxide (CO2), owing to its superior heat absorption efficiency (IPCC, 2013). Methanotrophs (aerobic methanotrophs) consume CH4 under certain conditions (Le Mer and Roger, 2001), reducing the atmospheric CH4 concentration (Singh et al., 2010). Consequently, methanotrophs are crucial microbes that play an indispensable role in regulating and mitigating the CH4-related greenhouse effect on Earth. Soil aerobic CH4 oxidation is the sole known biological sink for atmospheric CH4 (Ho et al., 2019; Murguia-Flores et al., 2018), contributing to 5 %–7 % of the global annual atmospheric CH4 uptake (Saunois et al., 2020). Upland soils are the primary biological CH4 sink (Bodelier, 2011; Guo et al., 2023), owing to methanotroph-mediated CH4 consumption (Song et al., 2024). This represents the second-largest atmospheric CH4 consumption sink, surpassed only by hydroxyl radical depletion (Deng et al., 2019). Aerobic CH4 oxidation in soils is influenced by many factors, such as the soil water content, soil texture, soil type, temperature, soil pH, soil inorganic nitrogen content, and metal availability, and many of these factors have been extensively reviewed (Shukla et al., 2013; Mishra et al., 2018). However, the effect of SO, a significant ionic component of acid deposition, on aerobic CH4 oxidation has not yet been reviewed.
Acid rain, involving the deposition of SO and other acidic compounds, remains a globally significant environmental issue (Chen et al., 2020; Qi et al., 2022). The three largest affected regions are Europe, North America, and China (Li et al., 2021). SO is the major ion in acid rain (Wright and Henriksen, 1978) and has profound impacts on substances and biochemical processes in soils. As a crucial component of terrestrial ecosystems, soils serve as the ultimate receptor of acid deposition. SO deposition induces soil acidification (Huang et al., 2019), alters soil plant diversity (Li et al., 2022), affects microbial properties (Wang et al., 2018), and limits grass yield potential (Klessa et al., 1989), as well as reducing the activities of soil enzymes such as cellulase, invertase, and polyphenol oxidase (Tie et al., 2020). SO can inhibit CH4 production (methanogenesis) and promote anaerobic CH4 oxidation, playing a crucial role in anaerobic CH4 biogeochemical processes. SO suppresses methanogenesis, primarily due to its thermodynamic and kinetic preference as an electron acceptor (Granberg et al., 2001; Schimel, 2004), leading to decreased CH4 emissions (Gauci et al., 2004). SO has also been shown to facilitate anaerobic CH4 oxidation by anaerobic methanotrophic archaea in diverse ecosystems, such as oceans (Boetius et al., 2000), wetlands (La et al., 2022), and paddy fields (Fan et al., 2021), acting as a crucial electron acceptor. Despite these well-documented effects on anaerobic CH4 biogeochemical processes, the influence of SO on aerobic CH4 oxidation, particularly in upland soils, remains underexplored. Given the increasing global deposition of SO due to industrial activities, understanding its impact on aerobic CH4 oxidation is essential for predicting future CH4 dynamics and developing effective climate mitigation strategies.
In this review, we have analyzed the literature on the effects of SO on aerobic CH4 oxidation. Our analysis not only reveals evidence suggesting that SO promotes aerobic CH4 oxidation but also identifies supporting evidence from related studies. In this work, we reviewed references about the influence of SO on soil properties, substances, or biochemical processes, aiming to elucidate any microscale pathways on aerobic CH4 oxidation through variations in soil substances or processes. Our analysis reveals that SO may affect aerobic CH4 oxidation. Based on the available literature, three out of five studies that investigated the influence of SO on aerobic CH4 oxidation were able to demonstrate a positive effect on aerobic CH4 oxidation thus, we infer that SO favors aerobic CH4 oxidation. This review summarizes the microscale pathways by which SO influences aerobic CH4 oxidation and highlights the importance of future research in this area. By providing a comprehensive synthesis of existing knowledge, this work serves as a valuable reference for future experimental studies. Furthermore, the findings of this review will contribute to a deeper understanding of global CH4 cycling, particularly in the context of increasing SO deposition. Moving forward, we aim to experimentally validate the impact of aerobic CH4 oxidation following SO addition and elucidate the underlying microbial mechanisms involved.
2.1 Aerobic CH4 oxidation processes
Aerobic CH4 oxidation is mediated by methanotrophs, a group of specialized microorganisms (Chistoserdova et al., 2005). In soils, aerobic CH4 oxidation can be classified into two distinct forms based on the concentration of CH4 (Walsh et al., 2009). The first form, known as high-affinity oxidation, occurs at CH4 concentrations close to atmospheric levels (< 2 ppm) and is carried out by high-affinity methanotrophs (Chowdhury and Dick, 2013). This process is commonly observed in upland soils, particularly in environments with high NH concentrations (Ho et al., 2019; Le Mer and Roger, 2001). The second form, referred to as low-affinity oxidation, occurs at CH4 concentrations exceeding 40 ppm and is mediated by low-affinity methanotrophs (Chowdhury and Dick, 2013). This form is typically found in wetland environments, where CH4 concentrations are significantly higher than atmospheric levels (Bechtold et al., 2025). Aerobic CH4 oxidation converts CH4 to CO2 in four steps: ① methane monooxygenase (MMO) oxidizes CH4 to methanol (CH3OH), ② methanol dehydrogenase (MDH) oxidizes CH3OH to formaldehyde (HCHO), ③ formaldehyde dehydrogenase (FADH) oxidizes HCHO to formate (HCOOH), and ④ formate dehydrogenase (FDH) oxidizes HCOOH to CO2 (Fig. 1, paths ①–④) (Mancinelli, 1995).
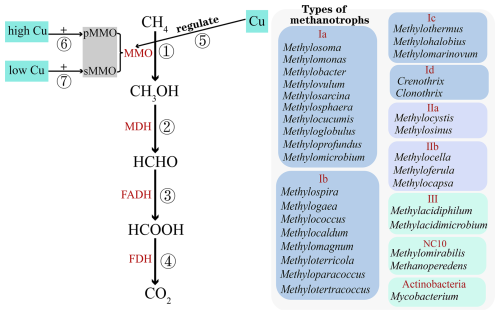
Figure 1Diagram of the aerobic methane oxidation process and the classification of methanotrophs: ① CH4 is oxidized to methanol (CH3OH) by methane monooxygenase (MMO); ② CH3OH is oxidized to formaldehyde (HCHO) by methanol dehydrogenase (MDH); ③ HCHO is oxidized to formate (HCOOH) by formaldehyde dehydrogenase (FADH); ④ HCOOH is oxidized to CO2 by formate dehydrogenase (FDH); ⑤ Cu controls two MMOs' expression; ⑥ high Cu concentration regulates pMMO expression in soil; and ⑦ low Cu concentration regulates sMMO expression in soil.
2.2 Methanotrophs
Methanotrophs constitute a distinct subset of methylotrophs, primarily dependent on the one-carbon compound CH4 as their sole source of carbon and energy (Hanson and Hanson, 1996). In the traditional classification system, proteobacterial methanotrophs were categorized into type I (Methylococcaceae and Crenotrichaceae), type II (Methylocystaceae and Beijerinckiaceae), and type X (Methylococcaceae) (Li et al., 2020) based on their cell membrane arrangement, chemotaxonomic properties, physiological characteristics, and phylogenetic locations. However, due to the discovery of non-canonical methanotrophs, the traditional classification system has become outdated. Consequently, methanotrophs are now classified into seven categories based on phylogenetic analysis: types I-A (Methylomonadaceae), I-B (Methylococcaceae), I-C (Methylothermaceae), I-D (Crenotrichaceae), II-A (Methylocystaceae), II-B (Beijerinckiaceae), III (Methylacidiphilaceae), and NC10 (Fenibo et al., 2023). Methylomonadaceae, Methylococcaceae, Methylothermaceae, and Crenotrichaceae belong to the class Gammaproteobacteria, while Methylocystaceae and Beijerinckiaceae are classified under Alphaproteobacteria. Methylacidiphilaceae belongs to the phylum Verrucomicrobia. The composition of different types of methanotrophs is shown in Fig. 1 (Fenibo et al., 2023). Notably, only four genera – Methylocella, Methylacidimicrobium, Methylacidiphilum, and Methylomirabilis – are capable of carbon fixation via the Calvin–Benson–Bassham (CBB) cycle (Fenibo et al., 2023; Op den Camp et al., 2009). Among actinobacterial methanotrophs, Candidatus Mycobacterium methanotrophicum is classified with the Mycobacterium genus (van Spanning et al., 2022). Methanotrophs utilize two forms of methane monooxygenase (MMO): soluble cytoplasmic monooxygenase (sMMO) and particulate membrane–bound monooxygenase (pMMO). Except for Methylocella silvestris and Methyloferula stellata, all methanotrophs possess pMMO. sMMO has only been detected in a few specific genera, namely Methylomonas sp., Methylomicrobium sp., Methylosinus sp., and Methylococcus capsulatus (DiSpirito et al., 2016). Copper (Cu) concentration differentially regulates MMO expression (Fig. 1 ⑤): high Cu concentrations induces pMMO (Fig. 1 ⑥), whereas low Cu concentrations triggers sMMO (Fig. 1 ⑦) (Hakemian and Rosenzweig, 2007).
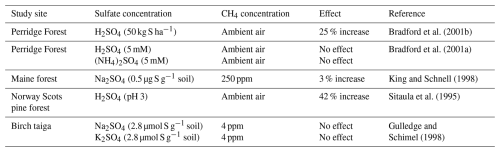
Sulfates, including SO and sulfuric acid (H2SO4), enhance aerobic CH4 oxidation within a range of 0 %–42 % (Table 1); therefore, we hypothesize that SO may stimulate aerobic CH4 oxidation. For example, in a temperate mixed deciduous woodland, the cumulative uptake of aerobic CH4 oxidation was 25 % higher in the experimental group with H2SO4 addition compared to the control group during the final quarter of the study period (Bradford et al., 2001b). Similar results were reported by Sitaula et al. (1995). In another study, King and Schnell (1998) found that adding SO (Na2SO4) increased aerobic CH4 oxidation by 3 % at a CH4 concentration of 250 ppm compared to the control group, although this result was not statistically significant. The lack of significance may be attributed to the insufficient concentration gradient of Na2SO4 in the experimental setup, which limited the ability to fully assess the effects of SO on aerobic CH4 oxidation. Therefore, we propose that the observed enhancement of aerobic CH4 oxidation following H2SO4 addition is primarily due to the increase in SO concentration.
The promotional effect of SO on aerobic CH4 oxidation is further supported by comparisons with other anions under similar cationic conditions. Benstead and King (2001) observed that HNO3 exerted a stronger inhibitory effect on aerobic CH4 oxidation under equivalent soil acidic conditions than H2SO4. This finding is consistent with the results of Bradford et al. (2001a), who experimentally confirmed the inhibitory effect of nitrate (NO) on aerobic CH4 oxidation (Dunfield and Knowles, 1995; Wang and Ineson, 2003). When H2SO4 and HNO3 were added to the soil to achieve H+ concentrations of 10 and 1 µmol H+ per gram of fresh weight (gfw), respectively, both acids inhibited aerobic CH4 oxidation to a similar extent. However, H2SO4 exhibited a lesser inhibitory effect than HNO3. We hypothesize that SO may promote aerobic CH4 oxidation, as evidenced by the findings of Benstead and King (2001) and Bradford et al. (2001a).
However, not all studies support the hypothesis that SO promotes aerobic CH4 oxidation. For instance, Bradford et al. (2001a) observed no significant difference in aerobic CH4 oxidation between low (564 µM) and high (1408 µM) concentrations of H2SO4 compared to the control group. This discrepancy may be due to differences in H2SO4 concentration across studies. Similarly, Hu et al. (2018) reported no significant effect of SO on aerobic CH4 oxidation. Based on the available evidence, SO promotes aerobic CH4 oxidation within a range of 0 %–42 %. Although the mechanisms by which SO influences aerobic CH4 oxidation are not yet fully understood, we have identified potential microscopic pathways through which SO may affect this aerobic process by reviewing the relevant literature.
The impact of SO on aerobic CH4 oxidation – particularly its enhancement mechanisms – remains poorly understood. Our literature review identifies two promotive pathways: (i) SO direct effects on methanotroph activity and community structure (Fig. 2, path d) (Bradford et al., 2001b; Sitaula et al., 1995), thereby modulating CH4 oxidation; and (ii) SO indirect effects through alterations in soil physicochemical properties (Fan et al., 2017), substrate availability (Bjorneras et al., 2019; Palmer et al., 2013; Xu et al., 2017), and nutrient dynamics (Islam, 2012) (Fig. 2), which subsequently influence methanotrophic activity and ultimately affect CH4 oxidation.
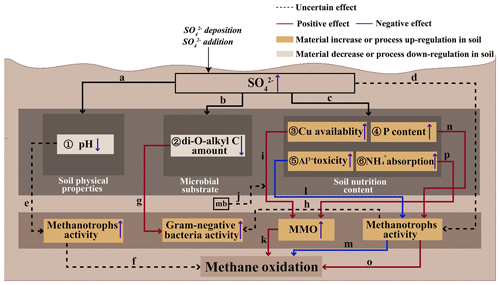
Figure 2Conceptual diagram of the potential microscopic mechanisms by which sulfate influences aerobic methane oxidation in upland soil: ① SO decreases soil pH (Fasth et al., 1991; Tie et al., 2020); ② SO decreases the soil di-O-alkyl C amount (Xu et al., 2017); ③ SO increases soil Cu availability (Islam, 2012); ④ SO increases the soil P content by increasing soil acid phosphatase activity (Lv et al., 2014; Veraart et al., 2015); ⑤ SO increases soil Al3+ toxicity (Hu et al., 2013; Sogn and Abrahamsen, 1998); ⑥ SO increases NH absorption (Bradford et al., 2001b; Gulledge and Schimel, 1998; King and Schnell, 1998); (a) changes in soil physical properties due to increased soil SO content; (b) changes in soil microbial substrate due to increased soil SO content; (c) SO may promote CH4 oxidation; (d) SO affects the activity or community size of methanotrophs in soils (Bradford et al., 2001b; Sitaula et al., 1995); (e) decreased pH may inhibit or stimulate soil CH4 oxidation (Sitaula et al., 1995); (f) methanotroph activity affects CH4 oxidation; (g) decreased di-O-alkyl C amount increases soil Gram-negative bacteria activity (Xu et al., 2017); (h) the increased activity of Gram-negative bacteria may stem from the enhanced activity of methanotrophs; (i) elevated Cu availability stimulates soil aerobic CH4 oxidation (Ho et al., 2013); (j) mb (methanobactin) is expected to accelerate Cu uptake (Knapp et al., 2007); (k) enhanced MMO activity facilitates aerobic CH4 oxidation. (l) elevated Al3+ toxicity inhibits soil methanotroph activity (Nanba and King, 2000; Shukla et al., 2013); (m) decreased methanotroph activity inhibits soil CH4 oxidation; (n) elevated P content increases soil methanotroph activity (Zhang et al., 2011); (o) elevated methanotroph activity stimulates soil CH4 oxidation (Bradford et al., 2001b; Sitaula et al., 1995); and (p) the increased adsorption of NH enhances the availability of MMO to soil methanotrophs.
First, the addition of SO alters soil physicochemical properties (Fig. 2, path a), i.e., particularly by reducing soil pH (Fig. 2 ①). Soil acidification increases due to enhanced base cation leaching associated with SO addition (Hu et al., 2013), leading to a decrease in the pH of forest soils (Fasth et al., 1991; Tie et al., 2020). The addition of H2SO4 has been shown to promote aerobic CH4 oxidation by altering the activity or community structure of methanotrophs (Bradford et al., 2001b; Sitaula et al., 1995). However, in experiments involving H2SO4 addition, it remains unclear whether the observed enhancement in aerobic CH4 oxidation is primarily due to the decreased pH (Fig. 2, path e) or the increase in SO concentration (Fig. 2, path d). Generally, CH4 consumption is greater under higher-pH conditions in forest soils (Brumme and Borken, 1999; Silver et al., 1999); therefore, the reduction in soil pH may lead to a decrease in aerobic CH4 oxidation. However, in acidic soils, a decrease in pH has been shown to increase aerobic CH4 oxidation (Sitaula et al., 1995). Consequently, when evaluating the impact of SO addition on aerobic CH4 oxidation, it is essential to consider the initial soil pH (Fig. 2, path e), as methanotrophs exhibit different pH preferences in acidic and alkaline environments (Shukla et al., 2013).
Second, SO addition can alter the soil microbial substrate (Fig. 2, path b), particularly by decreasing the soil di-O-alkyl C content (Fig. 2 ②) (Xu et al., 2017). In a subtropical forest, SO addition has been shown to increase the activity of Gram-negative bacteria in soil by reducing the litter di-O-alkyl carbon (di-O-alkyl C) (Fig. 2 ② and path g) (Xu et al., 2017). Di-O-alkyl C is a component of soil organic carbon (SOC). SOC degradation is accelerated when the percentage of di-O-alkyl C is high (Huang et al., 2021); conversely, when the content of di-O-alkyl C is low, SOC degradation slows down, leading to a greater availability of substrates for microorganisms, including methanotrophs. Methanotrophs, which are Gram-negative bacteria (Schimel and Gulledge, 1998), may exhibit increased activity in response to SO addition. This enhancement of methanotroph activity (Fig. 2, path h) can ultimately promote aerobic CH4 oxidation (Fig. 2, path o).
Third, SO can alter the soil nutrition content (Fig. 2, path c), specifically increasing the soil Cu availability (Fig. 2 ③) (Islam, 2012), the phosphorus (P) content (Fig. 2 ④) by enhancing acid phosphatase activity (Lv et al., 2014; Veraart et al., 2015), the Al3+ (aluminum ion) toxicity (Fig. 2 ⑤) (Hu et al., 2013; Sogn and Abrahamsen, 1998), and the NH absorption (Bradford et al., 2001b; Gulledge and Schimel, 1998; King and Schnell, 1998) (Fig. 2 ⑥). Cu is a crucial component in aerobic CH4 oxidation processes, with its critical role stemming from its high abundance in catalytically active pMMO complexes – where it directly participates in CH4 oxidation and facilitates electron transfer from endogenous reductants to molecular oxygen (Balasubramanian and Rosenzweig, 2007; Semrau et al., 2010). This process drives the conversion of CH4 to methanol (Dassama et al., 2016). It was anticipated that methanobactin secreted by methanotrophs during aerobic CH4 oxidation would facilitate Cu uptake (Knapp et al., 2007); however, the specific mechanism by which methanobactin affects Cu uptake remains unclear (Fig. 2, path j). For methanotrophs capable of expressing both sMMO and pMMO, the expression of these enzymes is regulated by the availability of Cu, a phenomenon known as the classic “copper switch” (Stanley et al., 1983). Under Cu-deficient conditions, these methanotrophs express sMMO. However, as the ratio of Cu to biomass increases, the expression of sMMO significantly decreases, while the expression of pMMO increases (Semrau et al., 2018). Notably, nearly all methanotrophs possess pMMO (Koo and Rosenzweig, 2021); therefore, increased Cu availability can enhance the expression of pMMO. Research indicates that Cu can serve as a promoter of aerobic CH4 oxidation (Ho et al., 2013). Therefore, SO addition may promote aerobic CH4 oxidation by increasing the availability of soil Cu, thereby enhancing the expression of pMMO (Fig. 2, path i and k).
A positive correlation has been found between P and aerobic CH4 oxidation in soils (Veraart et al., 2015; Zhang et al., 2020). P can potentially enhance the activity of soil methanotrophs (Fig. 2, path n) (Zhang et al., 2011), with an increase in soil P content achieved through the hydrolysis of organic compounds, including nucleic acids, phospholipids, and phosphate esters, by acid and alkaline phosphatases (Veraart et al., 2015). The addition of SO accelerated acid phosphatase activity, thereby increasing the soil P content (Lv et al., 2014). Therefore, we hypothesize that SO may indirectly enhance aerobic CH4 oxidation through the augmentation of soil P content, subsequently promoting the activity of methanotrophs in the soil (Fig. 2, path n and o). It is well established that Al3+ inhibits aerobic CH4 oxidation (Tamai et al., 2007; Tamai et al., 2003). Additionally, soil acidification resulting from SO addition has been shown to intensify the toxicity of Al3+ in forest soils (Fig. 2 ⑤) (Hu et al., 2013; Sogn and Abrahamsen, 1998). The increase in Al3+ can inhibit the activity of methanotrophs (Nanba and King, 2000; Shukla et al., 2013) (Fig. 2, path l), thereby inhibiting aerobic CH4 oxidation (Fig. 2, path m). Therefore, SO addition may directly affect methanotrophs by enhancing the toxicity of Al3+ in the soil, thereby inhibiting aerobic CH4 oxidation (Fig. 2, path o). When NH4Cl and (NH4)2SO4 were added to the soil at the same molar concentration of NH, the inhibitory effect of (NH4)2SO4 on aerobic CH4 oxidation was weaker than that of NH4Cl (Adamsen and King, 1993; Bradford et al., 2001a; King and Schnell, 1998). NH has been found to inhibit aerobic CH4 oxidation (Bronson and Mosier, 1994; Dunfield and Knowles, 1995), and the key mechanism is the competition between CH4 and NH for the same MMO enzyme (Gulledge et al., 2004). Due to the similar molecular structures of CH4 and NH, MMO can oxidize both CH4 (to CH3OH) and NH (to NO). The inhibitory effect of NH4Cl is greater than that of (NH4)2SO4, as SO may enhance the adsorption of NH onto cation exchange sites in the soil (Bradford et al., 2001b; Gulledge and Schimel, 1998; King and Schnell, 1998) (Fig. 2 ⑥). This reduced availability of NH limits its ability to compete with methanotrophs for MMO enzymes, thereby increasing the availability of MMO (Fig. 2, path p), promoting aerobic CH4 oxidation (Fig. 2, path k), and further intensifying the inhibitory effect of NH4Cl compared to (NH4)2SO4. In conclusion, SO served as a facilitator of aerobic CH4 oxidation, mitigating the inhibitory effects of NH on this process.
This review synthesizes the double-scale mechanisms by which SO influences aerobic CH4 oxidation. Macroscopically, SO enhances aerobic CH4 oxidation rates by 0 %–42 %. Mechanistic studies demonstrate that this regulation occurs through SO-driven alteration of environmental factors (e.g., pH, availability, Al3+ toxicity, and NH absorption), which subsequently modulate methanotroph physiology and MMO activity. Based on synthesized evidence, we hypothesize a net stimulatory effect of SO on aerobic CH4 oxidation. Validating this hypothesis requires deeper mechanistic insights; therefore, future research should prioritize quantifying aerobic CH4 oxidation responses to SO exposure while elucidating underlying microbial mechanisms. This integrated approach is projected to advance CH4 mitigation strategies amid rising global SO deposition.
All raw data can be provided by the corresponding authors upon request.
RS finished writing the manuscript; KL, NW, FY, YZ, YS, YZ, LY, and LH gave constructive comments and revised the structure and content of the article; XX and LZ reviewed and edited the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors would like to express their sincere gratitude to the anonymous reviewers for their valuable comments and suggestions, which provided critical guidance for the revision and improvement of the manuscript.
This study was supported by the National Natural Science Foundation of China (grant nos. 32471777 and 32271681), the Joint Funds of China's National Natural Science Foundation (grant no. U2006215); the Academic Team Leadership Program (grant no. 2024XSYL01), Minzu University of China; the National Science Foundation (grant no. 2145130) and the SPRUCE and NGEE Arctic projects, supported by the Office of Biological and Environmental Research, Office of Science, US Department of Energy; the Major Program of the National Natural Science Foundation of China (grant no. 42494823); the National Key Research and Development Program of China (grant no. 2024YFF0808703); and the Young Scientists Innovation Funds of State Key Laboratory of Black Soils Conservation and Utilization (grant no. 2023HTDGZQN-03).
This paper was edited by Frank Hagedorn and reviewed by two anonymous referees.
Adamsen, A. P. S. and King, G. M.: Methane consumption in temperate and subarctic forest soils rates, vertical zonation, and responses to water and nitrogen, Appl. Environ. Microbiol., 59, 485–490, https://doi.org/10.1128/aem.59.2.485-490.1993, 1993.
Balasubramanian, R. and Rosenzweig, A. C.: Structural and mechanistic insights into methane oxidation by particulate methane monooxygenase, Accounts Chem. Res., 40, 573–580, https://doi.org/10.1021/ar700004s, 2007.
Bechtold, E. K., Ellenbogen, J. B., Villa, J. A., Ferreira, D. K. d. M., Oliverio, A. M., Kostka, J. E., Rich, V. I., Varner, R. K., Bansal, S., Ward, E. J., Bohrer, G., Borton, M. A., Wrighton, K. C., and Wilkins, M. J.: Metabolic interactions underpinning high methane fluxes across terrestrial freshwater wetlands, Nat. Commun., 16, https://doi.org/10.1038/s41467-025-56133-0, 2025.
Benstead, J. and King, G. M.: The effect of soil acidification on atmospheric methane uptake by a Maine forest soil, FEMS Microbiol. Ecol., 34, 207–212, https://doi.org/10.1111/j.1574-6941.2001.tb00771.x, 2001.
Bjorneras, C., Skerlep, M., Floudas, D., Persson, P., and Kritzberg, E. S.: High sulfate concentration enhances iron mobilization from organic soil to water, Biogeochemistry, 144, 245–259, https://doi.org/10.1007/s10533-019-00581-6, 2019.
Bodelier, P. L. E.: Interactions between nitrogenous fertilizers and methane cycling in wetland and upland soils, Curr. Opin. Environ. Sustain., 3, 379–388, https://doi.org/10.1016/j.cosust.2011.06.002, 2011.
Boetius, A., Ravenschlag, K., Schubert, C., Rickert, D., Widdel, F., Gieseke, A., Amann, R., Jorgensen, B., Witte, U., and Pfannkuche, O.: A marine microbial consortium apparently mediating anaerobic oxidation of methane, Nature, 407, 623–626, https://doi.org/10.1038/35036572, 2000.
Bradford, M. A., Ineson, P., Wookey, P. A., and Lappin-Scott, H. M.: The effects of acid nitrogen and acid sulphur deposition on CH4 oxidation in a forest soil: a laboratory study, Soil Biol. Biochem., 33, 1695–1702, https://doi.org/10.1016/s0038-0717(01)00091-8, 2001a.
Bradford, M. A., Wookey, P. A., Ineson, P., and Lappin-Scott, H. M.: Controlling factors and effects of chronic nitrogen and sulphur deposition on methane oxidation in a temperate forest soil, Soil Biol. Biochem., 33, 93–102, https://doi.org/10.1016/S0038-0717(00)00118-8, 2001b.
Bronson, K. F. and Mosier, A. R.: Suppression of methane oxidation in aerobic soil by nitrogen fertilizers, nitrification inhibitors, and urease inhibitors, Biol. Fert. Soils, 17, 263–268, https://doi.org/10.1007/bf00383979, 1994.
Brumme, R. and Borken, W.: Site variation in methane oxidation as affected by atmospheric deposition and type of temperate forest ecosystem, Global Biogeochem. Cy., 13, 493–501, https://doi.org/10.1029/1998gb900017, 1999.
Chen, X., Zhang, J. E., and Wei, H.: Physiological responses of earthworm under acid rain stress, Int. J. Env. Res. Public Health., 17, 7246, https://doi.org/10.3390/ijerph17197246, 2020.
Chistoserdova, L., Vorholt, J. A., and Lidstrom, M. E.: A genomic view of methane oxidation by aerobic bacteria and anaerobic archaea, Genome Biol., 6, 1–6, https://doi.org/10.1186/gb-2005-6-2-208, 2005.
Chowdhury, T. R. and Dick, R. P.: Ecology of aerobic methanotrophs in controlling methane fluxes from wetlands, Appl. Soil Ecol., 65, 8–22, https://doi.org/10.1016/j.apsoil.2012.12.014, 2013.
Dassama, L. M., Kenney, G. E., Ro, S. Y., Zielazinski, E. L., and Rosenzweig, A. C.: Methanobactin transport machinery, P. Natl. Acad. Sci. USA, 113, 13027–13032, https://doi.org/10.1073/pnas.1603578113, 2016.
Deng, Y., Che, R., Wang, F., Conrad, R., Dumont, M., Yun, J., Wu, Y., Hu, A., Fang, J., Xu, Z., Cui, X., and Wang, Y.: Upland Soil Cluster Gamma dominates methanotrophic communities in upland grassland soils, Sci. Total Environ., 670, 826–836, https://doi.org/10.1016/j.scitotenv.2019.03.299, 2019.
DiSpirito, A. A., Semrau, J. D., Murrell, J. C., Gallagher, W. H., Dennison, C., and Vuilleumier, S.: Methanobactin and the link between copper and bacterial methane oxidation, Microbiol. Mol. Biol. R., 80, 387–409, https://doi.org/10.1128/mmbr.00058-15, 2016.
Dunfield, P. and Knowles, R.: Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol, Appl. Environ. Microbiol., 61, 3129–3135, https://doi.org/10.1128/aem.61.8.3129-3135.1995, 1995.
Fan, J. L., Xu, Y. H., Chen, Z. M., Xiao, J., Liu, D. Y., Luo, J. F., Bolan, N., and Ding, W. X.: Sulfur deposition suppressed nitrogen-induced soil N2O emission from a subtropical forestland in southeastern China, Agr. Forest Meteorol., 233, 163–170, https://doi.org/10.1016/j.agrformet.2016.11.017, 2017.
Fan, L. C., Schneider, D., Dippold, M. A., Poehlein, A., Wu, W. C., Gui, H., Ge, T. D., Wu, J. S., Thiel, V., Kuzyakov, Y., and Dorodnikov, M.: Active metabolic pathways of anaerobic methane oxidation in paddy soils, Soil Biol. Biochem., 156, 108215, https://doi.org/10.1016/j.soilbio.2021.108215, 2021.
Fasth, W. J., David, M. B., and Vance, G. F.: Sulfate retention and cation leaching of forest soils in response to acid additions, Can. J. Forest Res., 21, 32–41, https://doi.org/10.1139/x91-005, 1991.
Fenibo, E. O., Selvarajan, R., Wang, H. Q., Wang, Y., and Abia, A. L. K.: Untapped talents: insight into the ecological significance of methanotrophs and its prospects, Sci. Total Environ., 903, 166145, https://doi.org/10.1016/j.scitotenv.2023.166145, 2023.
Gauci, V., Matthews, E., Dise, N., Walter, B., Koch, D., Granberg, G., and Vile, M.: Sulfur pollution suppression of the wetland methane source in the 20th and 21st centuries, P. Natl. Acad. Sci. USA, 101, 12583–12587, https://doi.org/10.1073/pnas.0404412101, 2004.
Granberg, G., Sund, I., Svensson, B. H., and Nilsson, M.: Effect of temperature, and nitrogen and sulfur deposition, on methane emission from a boreal mire, Ecology, 82, 1982–1998, 2001.
Gulledge, J. and Schimel, J.: Low-concentration kinetics of atmospheric CH4 oxidation in soil and mechanism of NH inhibition, Appl. Environ. Microbiol., 64, 4291–4298, 1998.
Gulledge, J., Hrywna, Y., Cavanaugh, C., and Steudler, P. A.: Effects of long-term nitrogen fertilization on the uptake kinetics of atmospheric methane in temperate forest soils, FEMS Microbiol. Ecol., 49, 389–400, https://doi.org/10.1016/j.femsec.2004.04.013, 2004.
Guo, J. H., Feng, H. L., Peng, C. H., Chen, H., Xu, X., Ma, X. H., Li, L., Kneeshaw, D., Ruan, H. H., Yang, H. Q., and Wang, W. F.: Global climate change increases terrestrial soil CH4 emissions, Global Biogeochem. Cy., 37, e2021GB007255, https://doi.org/10.1029/2021gb007255, 2023.
Hakemian, A. S. and Rosenzweig, A. C.: The biochemistry of methane oxidation, Annu. Rev. Biochem., 76, 223–241, https://doi.org/10.1146/annurev.biochem.76.061505.175355, 2007.
Hanson, R. S. and Hanson, T. E.: Methanotrophic bacteria, Microbiol. Rev., 60, 439–471, https://doi.org/10.1128/mmbr.60.2.439-471.1996, 1996.
Ho, A., Lüke, C., Reim, A., and Frenzel, P.: Selective stimulation in a natural community of methane oxidizing bacteria: effects of copper on pmoA transcription and activity, Soil Biol. Biochem., 65, 211–216, https://doi.org/10.1016/j.soilbio.2013.05.027, 2013.
Ho, A., Lee, H. J., Reumer, M., Meima-Franke, M., Raaijmakers, C., Zweers, H., de Boer, W., Van der Putten, W. H., and Bodelier, P. L. E.: Unexpected role of canonical aerobic methanotrophs in upland agricultural soils, Soil Biol. Biochem., 131, 1–8, https://doi.org/10.1016/j.soilbio.2018.12.020, 2019.
Hu, M. J., Wilson, B. J., Sun, Z. G., Huang, J. F., and Tong, C.: Effects of nitrogen and sulphate addition on methane oxidation in the marsh soil of a typical subtropical estuary (Min River) in China, Chem. Ecol., 34, 610–623, https://doi.org/10.1080/02757540.2018.1464153, 2018.
Hu, Y. L., Jung, K., Zeng, D. H., and Chang, S. X.: Nitrogen- and sulfur-deposition-altered soil microbial community functions and enzyme activities in a boreal mixedwood forest in western Canada, Can. J. Forest Res., 43, 777–784, https://doi.org/10.1139/cjfr-2013-0049, 2013.
Huang, J., Zhou, K. J., Zhang, W., Liu, J. X., Ding, X., Cai, X. A., and Mo, J. M.: Sulfur deposition still contributes to forest soil acidification in the Pearl River Delta, South China, despite the control of sulfur dioxide emission since 2001, Environ. Sci. Pollut. Res., 26, 12928–12939, https://doi.org/10.1007/s11356-019-04831-w, 2019.
Huang, R. L., Crowther, T. W., Sui, Y. Y., Sun, B., and Liang, Y. T.: High stability and metabolic capacity of bacterial community promote the rapid reduction of easily decomposing carbon in soil, Commun. Biol., 4, 1376, https://doi.org/10.1038/s42003-021-02907-3, 2021.
IPCC: Climate change 2013: the physical science basis, in: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia, Y., Bex, V., and Midgley, P. M., Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535 pp., https://doi.org/10.1017/CBO9781107415324, 2013.
Islam, M.: The effect of different rates and forms of sulfur on seed yield and micronutrient uptake by chickpea, Plant Soil Environ., 58, 399–404, https://doi.org/10.17221/145/2012-pse, 2012.
King, G. M. and Schnell, S.: Effects of ammonium and non-ammonium salt additions on methane oxidation by Methylosinus trichosporium OB3b and Maine forest soils, Appl. Environ. Microbiol., 64, 253–257, 1998.
Klessa, D. A., Frame, J., Golightly, R. D., and Harkess, R. D.: The effect of fertilizer sulfur on grass production for silage, Grass Forage Sci., 44, 277–281, https://doi.org/10.1111/j.1365-2494.1989.tb02165.x, 1989.
Knapp, C. W., Fowle, D. A., Kulczycki, E., Roberts, J. A., and Graham, D. W.: Methane monooxygenase gene expression mediated by methanobactin in the presence of mineral copper sources, P. Natl. Acad. Sci. USA, 104, 12040–12045, https://doi.org/10.1073/pnas.0702879104, 2007.
Koo, C. W. and Rosenzweig, A. C.: Biochemistry of aerobic biological methane oxidation, Chem. Soc. Rev., 50, 3424–3436, https://doi.org/10.1039/d0cs01291b, 2021.
La, W., Han, X., Liu, C. Q., Ding, H., Liu, M., Sun, F., Li, S., and Lang, Y.: Sulfate concentrations affect sulfate reduction pathways and methane consumption in coastal wetlands, Water Res., 217, 118441, https://doi.org/10.1016/j.watres.2022.118441, 2022.
Le Mer, J. and Roger, P.: Production, oxidation, emission and consumption of methane by soil: a review, Eur. J. Soil Biol., 37, 25–50, https://doi.org/10.1016/S1164-5563(01)01067-6, 2001.
Li, C., Liu, X., Meng, M. J., Zhai, L., Zhang, B., Jia, Z. H., Gu, Z. Y., Liu, Q. Q., Zhang, Y. L., and Zhang, J. C.: The use of Biolog Eco microplates to compare the effects of sulfuric and nitric acid rain on the metabolic functions of soil microbial communities in a subtropical plantation within the Yangtze River Delta region, Catena, 198, 105039, https://doi.org/10.1016/j.catena.2020.105039, 2021.
Li, C. H., Wang, B., Fang, Z., Yu, H. L., and Huang, J. Y.: Plant species diversity is driven by soil base cations under acid deposition in desert coal-mining region in northwestern China, Ecol. Indic., 145, 109682, https://doi.org/10.1016/j.ecolind.2022.109682, 2022.
Li, Y., Liu, Y. W., Pan, H., Hernández, M., Guan, X. M., Wang, W., Zhang, Q. C., Luo, Y., Di, H. J., and Xu, J. M.: Impact of grazing on shaping abundance and composition of active methanotrophs and methane oxidation activity in a grassland soil, Biol. Fert. Soil., 56, 799–810, https://doi.org/10.1007/s00374-020-01461-0, 2020.
Lv, Y., Wang, C. Y., Jia, Y. Y., Wang, W. W., Ma, X., Du, J. J., Pu, G. Z., and Tian, X. J.: Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China, Appl. Soil Ecol., 79, 1–9, https://doi.org/10.1016/j.apsoil.2013.12.002, 2014.
Mancinelli, R.: The regulation of methane oxidation in soil, Annu. Rev. Microbiol., 49, 581–605, 1995.
Mishra, V. K., Shukla, R., and Shukla, P. N.: Inhibition of Soil Methane Oxidation by Fertilizer Application: an Intriguing but Persistent Paradigm, Environ. Pollut. Protect., 3, 57–69, https://doi.org/10.22606/epp.2018.32001, 2018.
Murguia-Flores, F., Arndt, S., Ganesan, A. L., Murray-Tortarolo, G., and Hornibrook, E. R. C.: Soil Methanotrophy Model (MeMo v1.0): a process-based model to quantify global uptake of atmospheric methane by soil, Geosci. Model Dev., 11, 2009–2032, https://doi.org/10.5194/gmd-11-2009-2018, 2018.
Nanba, K. and King, G.: Response of atmospheric methane consumption by maine forest soils to exogenous aluminum salts, Appl. Environ. Microbiol., 66, 3674–3679, https://doi.org/10.1128/AEM.66.9.3674-3679.2000, 2000.
Op den Camp, H. J., Islam, T., Stott, M. B., Harhangi, H. R., Hynes, A., Schouten, S., Jetten, M. S., Birkeland, N. K., Pol, A., and Dunfield, P. F.: Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia, Environ. Microbiol. Rep., 1, 293–306, https://doi.org/10.1111/j.1758-2229.2009.00022.x, 2009.
Palmer, S. M., Clark, J. M., Chapman, P. J., van der Heijden, G. M. F., and Bottrell, S. H.: Effects of acid sulphate on DOC release in mineral soils: the influence of SO retention and Al release, Eur. J. Soil Sci., 64, 537–544, https://doi.org/10.1111/ejss.12048, 2013.
Place, S. E.: Examining the role of ruminants in sustainable food systems, Grass Forage Sci., 79, 135–143, https://doi.org/10.1111/gfs.12673, 2024.
Praeg, N., Wagner, A. O., and Illmer, P.: Plant species, temperature, and bedrock affect net methane flux out of grassland and forest soils, Plant Soil, 410, 193–206, https://doi.org/10.1007/s11104-016-2993-z, 2016.
Qi, S. Q., Li, X. X., Luo, J., Han, R. F., Chen, Q. Q., Shen, D. S., and Shentu, J.: Soil heterogeneity influence on the distribution of heavy metals in soil during acid rain infiltration: experimental and numerical modeling, J. Environ. Manage., 322, 116144, https://doi.org/10.1016/j.jenvman.2022.116144, 2022.
Saunois, M., Stavert, A. R., Poulter, B., Bousquet, P., Canadell, J. G., Jackson, R. B., Raymond, P. A., Dlugokencky, E. J., Houweling, S., Patra, P. K., Ciais, P., Arora, V. K., Bastviken, D., Bergamaschi, P., Blake, D. R., Brailsford, G., Bruhwiler, L., Carlson, K. M., Carrol, M., Castaldi, S., Chandra, N., Crevoisier, C., Crill, P. M., Covey, K., Curry, C. L., Etiope, G., Frankenberg, C., Gedney, N., Hegglin, M. I., Höglund-Isaksson, L., Hugelius, G., Ishizawa, M., Ito, A., Janssens-Maenhout, G., Jensen, K. M., Joos, F., Kleinen, T., Krummel, P. B., Langenfelds, R. L., Laruelle, G. G., Liu, L., Machida, T., Maksyutov, S., McDonald, K. C., McNorton, J., Miller, P. A., Melton, J. R., Morino, I., Müller, J., Murguia-Flores, F., Naik, V., Niwa, Y., Noce, S., O'Doherty, S., Parker, R. J., Peng, C., Peng, S., Peters, G. P., Prigent, C., Prinn, R., Ramonet, M., Regnier, P., Riley, W. J., Rosentreter, J. A., Segers, A., Simpson, I. J., Shi, H., Smith, S. J., Steele, L. P., Thornton, B. F., Tian, H., Tohjima, Y., Tubiello, F. N., Tsuruta, A., Viovy, N., Voulgarakis, A., Weber, T. S., van Weele, M., van der Werf, G. R., Weiss, R. F., Worthy, D., Wunch, D., Yin, Y., Yoshida, Y., Zhang, W., Zhang, Z., Zhao, Y., Zheng, B., Zhu, Q., Zhu, Q., and Zhuang, Q.: The Global Methane Budget 2000–2017, Earth Syst. Sci. Data, 12, 1561–1623, https://doi.org/10.5194/essd-12-1561-2020, 2020.
Schimel, J.: Playing scales in the methane cycle: from microbial ecology to the globe, P. Natl. Acad. Sci. USA, 101, 12400–12401, https://doi.org/10.1073/pnas.0405075101, 2004.
Schimel, J. and Gulledge, J.: Microbial community structure and global trace gases, Glob. Change Biol., 4, 745–758, https://doi.org/10.1046/j.1365-2486.1998.00195.x, 1998.
Semrau, J. D., DiSpirito, A. A., and Yoon, S.: Methanotrophs and copper, FEMS Microbiol. Rev., 34, 496–531, https://doi.org/10.1111/j.1574-6976.2010.00212.x, 2010.
Semrau, J. D., DiSpirito, A. A., Gu, W., and Yoon, S.: Metals and Methanotrophy, Appl. Environ. Microbiol., 84, https://doi.org/10.1128/aem.02289-17, 2018.
Shukla, P. N., Pandey, K. D., and Mishra, V. K.: Environmental determinants of soil methane oxidation and methanotrophs, Crit. Rev. Env. Sci. Tec., 43, 1945–2011, https://doi.org/10.1080/10643389.2012.672053, 2013.
Silver, W. L., Lugo, A. E., and Keller, M.: Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils, Biogeochemistry, 44, 301–328, https://doi.org/10.1007/bf00996995, 1999.
Singh, B. K., Bardgett, R. D., Smith, P., and Reay, D. S.: Microorganisms and climate change: terrestrial feedbacks and mitigation options, Nat. Rev. Microbiol., 8, 779–790, https://doi.org/10.1038/nrmicro2439, 2010.
Sitaula, B. K., Bakken, L. R., and Abrahamsen, G.: CH4 uptake by temperate forest soil: effect of N input and soil acidification, Soil Biol. Biochem., 27, 870–880, https://doi.org/10.1016/0038-0717(95)00017-9, 1995.
Sogn, T. and Abrahamsen, G.: Effects of N and S deposition on leaching from an acid forest soil and growth of Scots pine (Pinus sylvestris L.) after 5 years of treatment, Forest Ecol. Manage., 103, 177–190, https://doi.org/10.1016/S0378-1127(97)00188-6, 1998.
Song, H., Peng, C., Zhu, Q., Chen, Z., Blanchet, J.-P., Liu, Q., Li, T., Li, P., and Liu, Z.: Quantification and uncertainty of global upland soil methane sinks: Processes, controls, model limitations, and improvements, Earth-Sci. Rev., 252, https://doi.org/10.1016/j.earscirev.2024.104758, 2024.
Stanley, S. H., Prior, S. D., Leak, D. J., and Dalton, H.: Copper stress underlies the fundamental change in intracellular location of methane mono-oxygenase in methane-oxidizing organisms – studies in batch and continuous cultures, Biotechnol. Lett., 5, 487–492, https://doi.org/10.1007/bf00132233, 1983.
Tamai, N., Takenaka, C., Ishizuka, S., Water-soluble Al inhibits methane oxidation at atmospheric concentration levels in Japanese forest soil, Soil Biol. Biochem., 39, 1730–1736, https://doi.org/10.1016/j.soilbio.2007.01.029, 2007.
Tamai, N., Takenaka, C., Ishizuka, S., and Tezuka, T.: Methane flux and regulatory variables in soils of three equal-aged Japanese cypress (Chamaecyparis obtusa) forests in central Japan, Soil Biol. Biochem., 35, 633–641, https://doi.org/10.1016/s0038-0717(03)00010-5, 2003.
Tie, L. H., Fu, R., Penuelas, J., Sardans, J., Zhang, S. B., Zhou, S. X., Hu, J. X., and Huang, C. D.: The additions of nitrogen and sulfur synergistically decrease the release of carbon and nitrogen from litter in a subtropical forest, Forests, 11, 1280, https://doi.org/10.3390/f11121280, 2020.
van Spanning, R. J. M., Guan, Q., Melkonian, C., Gallant, J., Polerecky, L., Flot, J. F., Brandt, B. W., Braster, M., Iturbe Espinoza, P., Aerts, J. W., Meima-Franke, M. M., Piersma, S. R., Bunduc, C. M., Ummels, R., Pain, A., Fleming, E. J., van der Wel, N. N., Gherman, V. D., Sarbu, S. M., Bodelier, P. L. E., and Bitter, W.: Methanotrophy by a Mycobacterium species that dominates a cave microbial ecosystem, Nat. Microbiol., 7, 2089–2100, https://doi.org/10.1038/s41564-022-01252-3, 2022.
Veraart, A. J., Steenbergh, A. K., Ho, A., Kim, S. Y., and Bodelier, P. L. E.: Beyond nitrogen: the importance of phosphorus for CH4 oxidation in soils and sediments, Geoderma, 259–260, 337–346, https://doi.org/10.1016/j.geoderma.2015.03.025, 2015.
Walsh, C., O'Regan, B., and Moles, R.: Incorporating methane into ecological footprint analysis: A case study of Ireland, Ecol. Econ., 68, 1952–1962, https://doi.org/10.1016/j.ecolecon.2008.07.008, 2009.
Wang, Q., Kwak, J.-H., Choi, W.-J., and Chang, S. X.: Decomposition of trembling aspen leaf litter under long-term nitrogen and sulfur deposition: effects of litter chemistry and forest floor microbial properties, Forest Ecol. Manage., 412, 53–61, https://doi.org/10.1016/j.foreco.2018.01.042, 2018.
Wang, Z. P. and Ineson, P.: Methane oxidation in a temperate coniferous forest soil: effects of inorganic N, Soil Biol. Biochem., 35, 427–433, https://doi.org/10.1016/s0038-0717(02)00294-8, 2003.
Wright, R. F. and Henriksen, A.: Chemistry of small Norwegian lakes, with special reference to acid precipitation, Limnol. Oceanogr., 23, 487–498, https://doi.org/10.4319/lo.1978.23.3.0487, 1978.
Xu, Y. H., Fan, J. L., Ding, W. X., Gunina, A., Chen, Z. M., Bol, R., Luo, J. F., and Bolan, N.: Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additions: links to microbial community composition and activity, Geoderma, 286, 116–124, https://doi.org/10.1016/j.geoderma.2016.10.032, 2017.
Zhang, L. H., Yuan, F. H., Bai, J. H., Duan, H. T., Gu, X. Y., Hou, L. Y., Huang, Y., Yang, M. G., He, J. S., Zhang, Z. H., Yu, L. J., Song, C. C., Lipson, D. A., Zona, D., Oechel, W., Janssens, I. A., and Xu, X. F.: Phosphorus alleviation of nitrogen-suppressed methane sink in global grasslands, Ecol. Lett., 23, 821–830, https://doi.org/10.1111/ele.13480, 2020.
Zhang, T., Zhu, W., Mo, J., Liu, L., and Dong, S.: Increased phosphorus availability mitigates the inhibition of nitrogen deposition on CH4 uptake in an old-growth tropical forest, southern China, Biogeosciences, 8, 2805–2813, https://doi.org/10.5194/bg-8-2805-2011, 2011.
- Abstract
- Introduction
- The microbial aerobic CH4 oxidation processes
- Soil CH4 oxidation in response to SO addition
- Microscale pathways by which SO addition influences aerobic CH4 oxidation
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Abstract
- Introduction
- The microbial aerobic CH4 oxidation processes
- Soil CH4 oxidation in response to SO addition
- Microscale pathways by which SO addition influences aerobic CH4 oxidation
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References