the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Temporal and spatial influences of environmental factors on the distribution of mesopelagic organisms in the North Atlantic Ocean
Jian Hui Li
Jie Yang
Ge Chen
Mesopelagic organisms play a critical role in marine ecosystems and the global carbon cycle, acting as key intermediaries between trophic levels through diel vertical migration (DVM) and seasonal vertical migration (SVM). However, the seasonal vertical migration patterns of these organisms, and the environmental drivers influencing them, remain insufficiently understood. Here, we analyzed 83 603 backscattering coefficient (bbp) profiles obtained from 720 BGC-Argo floats deployed in the North Atlantic Ocean from 2010 to 2021. This extensive dataset enabled the identification of bbp spikes, allowing us to investigate the diurnal and seasonal vertical distributions of mesopelagic organisms, as indicated by these bbp spikes. Additionally, we examined the horizontal heterogeneity in these distributions and their correlations with key environmental variables. Our findings reveal distinct diurnal migrations, characterized by multilayered aggregations predominantly in the mid-ocean during daylight hours, with prominent signals at depths around 150, 330, 650, and 780 m. At night, a strong scattering layer forms in the upper ocean, with signals concentrated at depths shallower than 350 m, particularly in the top 100 m. Seasonal analyses shows that, in spring and winter, the average bbp spike intensity is lower in the upper ocean than in the mid-ocean, although the frequency of bbp spikes is higher in the upper ocean. In contrast, summer and autumn – especially summer – exhibit both higher mean bbp spike intensity and higher frequency near the surface. Spatially, mesopelagic organisms migrate deeper in the northeast and remain shallower in the southwest, correlating with higher temperatures and shallower distributions of mesopelagic organisms. Random forest analysis revealed that the vertical temperature gradient was the most influential environmental factor affecting the distribution of mesopelagic organisms year-round, with a relative importance of 26.03 %. Other critical factors include latitude, dissolved oxygen, photosynthetically active radiation (PAR), salinity, mixed-layer depth (MLD), and surface chlorophyll concentration, with relative importance values of 13.92 %, 13.71 %, 8.66 %, 8.29 %, 8.23 %, and 8.09 %, respectively. This study enhances our understanding of the mechanisms driving carbon transfer to the deep ocean and the energy and material cycles within marine ecosystems, providing a basis for future fisheries management in mesopelagic environments.
- Article
(6236 KB) - Full-text XML
- BibTeX
- EndNote
The mesopelagic organisms, comprising species such as zooplankton, shrimp, squid, fish, and jellyfish, are estimated to harbor around 1 billion tonnes of biomass, representing a significant fraction of global fish biomass (Irigoien et al., 2014). This biome is a crucial component of marine ecosystems, serving as a vital link between primary producers and higher trophic levels and playing a fundamental role in the ocean’s energy transfer and nutrient cycling (Klevjer et al., 2016; Kruse et al., 2010). A prominent behavioral adaptation in the mesopelagic zone is the diel vertical migration (DVM), wherein organisms undertake extensive vertical movements to optimize survival and foraging efficiency. During daylight hours, they inhabit depths of several hundred meters in the mesopelagic zone to minimize predation risk, while, at night, they migrate to the epipelagic zone to exploit food resources. This migration is recognized as one of the largest-scale migrations on Earth (Kapelonis et al., 2023; Hays, 2003; Petrusevich et al., 2020). Additionally, mesopelagic organisms undergo seasonal vertical migration (SVM), adjusting their vertical distribution in response to environmental fluctuations (Robinson et al., 2010). Both DVM and SVM drive the active export of organic and inorganic materials – through excretion, defecation, respiration, and mortality – into deeper ocean layers (Lourenço and Jany, 2021). These processes are not only ecologically important but also play a significant role in biogeochemical cycling, carbon sequestration, and mitigating global climate change (Govindarajan et al., 2023; Hazen, 2022; Gjoesaeter et al., 1980; Hays, 2003; Ramirez-Llodra et al., 2010; Robinson et al., 2010; Bailey, 2021).
Traditional methods for detecting and sampling mesopelagic organisms, including trawl sampling and acoustic surveys, have been widely used in previous studies. Although trawl sampling is more frequently used, it suffers from limitations in spatial and temporal resolution and from biases related to evasion and selectivity, which impede accurate estimates of migration timing, rate, and extent (Sutton, 2013; Underwood et al., 2020; Luo et al., 2000). Acoustic sampling offers greater precision but is restricted by the spatial and temporal coverage due to platform constraints, such as vessels. Additionally, the resolution of acoustic sensors often fails to detect small, dispersed, and weakly scattering species at depth, and the high costs associated with traditional active acoustic methods, including ADCP and scientific echosounders, further limit extensive in situ observations (Haëntjens et al., 2020; Chai et al., 2020; Underwood et al., 2020; Nakao et al., 2021). Recently, the increased deployment of BGC-Argo floats has enhanced accuracy and broadened the scope of applications in various studies, including inter-annual analyses of phytoplankton communities, quantification of carbon export from the ocean interior, and observations of mesocosm flux decay due to fragmentation processes (Rembauville et al., 2017; Xing et al., 2020; Wang and Fennel, 2022; Galí et al., 2022; Briggs et al., 2020; Boyd et al., 2019). Bio-optical sensors mounted on these floats have proven effective in detecting a range of bio-optical properties, rendering them powerful tools for large-scale spatial detection of mesopelagic organisms (Claustre et al., 2019; Haëntjens et al., 2020).
In recent years, significant progress has been made in utilizing backscattering coefficient (bbp) spike signals from BGC-Argo floats to study marine biological processes. These signals have shown a strong correlation with mesopelagic biological information, as evidenced by their high concordance with acoustic trawl observations (Haëntjens et al., 2020). Specifically, the bbp spike signals are mainly produced by larger particles that are closely related to biological aggregations (Briggs et al., 2011). For instance, the extensive diatom blooms in the North Atlantic each spring lead to a substantial increase in particulate matter, consisting of fresh phytoplankton aggregates that rapidly sink to the seafloor (Lampitt, 1985; Honjo and Manganini, 1993). Occasionally, large spikes in optical profiles are also interpreted as aggregates or zooplankton (Bishop et al., 1999; Gardner et al., 2000; Bishop and Wood, 2008). The bbp signal captures the entire particle assemblage, including zooplankton, detritus, bacteria, and mineral particles. Notably, significant increases in bbp are observed when small zooplankton dominate the mixed-layer community (Rembauville et al., 2017; Petit, 2023). Furthermore, satellite-based lidar inversion of bbp signals has revealed that zooplankton activity can cause pronounced bbp spikes, particularly at night, with these spikes being most evident in the surface ocean layers, offering insights into zooplankton diel vertical migration (DVM) globally (Behrenfeld et al., 2019). Collectively, these studies indicate that bbp spike signals not only reflect the presence of large particulate matter and tiny zooplankton but also capture the diel vertical migration of zooplankton, providing a powerful tool for understanding marine biological dynamics.
Despite significant advancements in understanding mesopelagic ecosystems, large-scale detection of mesopelagic organisms remains challenging, leading to considerable uncertainties in biomass estimates that range from billions to hundreds of tonnes (Gjoesaeter et al., 1980; Sarant, 2014). The patterns of diel vertical migration (DVM) and seasonal vertical migration (SVM), their adaptive mechanisms, and the multifactorial influences on these behaviors are still poorly understood (Bandara et al., 2021). However, recent studies have shown that the aggregation and vertical migration of mesopelagic organisms are regulated by a complex interplay of multidimensional environmental variables. Long-term factors, such as food availability, light, oceanic physicochemical properties, and dissolved oxygen levels, form the fundamental drivers (Fennell and Rose, 2015; Della Penna and Gaube, 2020; Devine et al., 2021). Short-term factors, including cloud cover, ocean currents, and lunar phases, also dynamically influence these behaviors (Lampert et al., 1989; Parra et al., 2019; Klevjer et al., 2020; Hauss et al., 2016). Collectively, these findings indicate that the spatiotemporal distribution of mesopelagic organisms results from the interaction of macro-scale oceanic physical environments, micro-scale nutrient cycling, and periodic fluctuations. To address these challenges, we leveraged backscattering bbp and spike signals from BGC-Argo floats in the mid- and high-latitude regions of the North Atlantic. By examining diurnal and seasonal vertical migrations, analyzing horizontal distribution patterns, and identifying key environmental drivers using random forest modeling, we aimed to elucidate the mechanisms shaping mesopelagic ecosystems across diverse spatiotemporal scales.
2.1 Study area
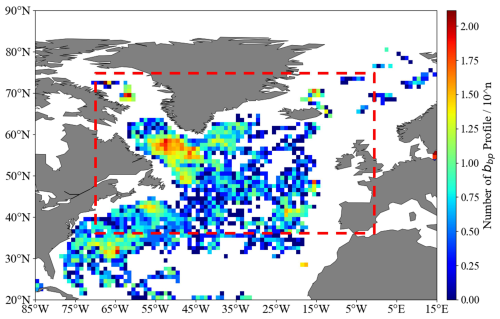
The North Atlantic (35–75° N, 0–70° W) is a critical region for global carbon cycling and marine ecosystems. Mesopelagic fish, through large-scale diel vertical migrations, efficiently transfer carbon and nutrients between the euphotic zone and deep sea, playing a key role in material cycling and energy flow (Lusher et al., 2016). Annually, demersal and pelagic fish on the UK–Ireland continental slope capture and store over 1 million tonnes of carbon dioxide equivalent (Trueman et al., 2014). Combined with planktonic regulation of carbon export via the biological carbon pump (Brun et al., 2019), these processes form the biological basis of the regional carbon cycle. The North Atlantic basin, spanning a large latitudinal gradient, experiences significant seasonal variations in solar radiation and primary production, which strongly influence mesozooplankton community dynamics. High-latitude overturning circulation and subduction processes of the subtropical circulation drive deep-sea dissolved organic carbon (DOC) transport, forming a key mechanism of the biological pump and contributing significantly to the global carbon cycle (Hansell et al., 2002; Falk-Petersen et al., 2009). Thus, the North Atlantic is central to addressing global climate change, preserving biodiversity, and guiding sustainable marine resource use. Figure 1 is based on backscattering coefficient (bbp) profiles collected by BGC-Argo floats across the study area, using 1° × 1° grid statistics.
2.2 Data
The dataset used in this study includes BGC-Argo profiles and remote sensing data collected from 2010 to 2021. The BGC-Argo dataset consists of profiles from 720 floats, capturing key parameters such as chlorophyll a (Chl), dissolved oxygen, salinity, temperature, and particle backscattering at 700 nm (bbp 700). A total of 83 603 valid profiles were selected based on the detection of bbp 700 spikes. Remote sensing data comprise sea surface temperature (SST) and chlorophyll a (Chl). The SST data, obtained from the National Oceanic and Atmospheric Administration (NOAA), consist of optimally interpolated fields with a spatial resolution of 0.25°, derived from a fusion of Advanced Very High Resolution Radiometer (AVHRR) observations from multiple platforms, providing high accuracy and broad spatial coverage. The Chl data, sourced from GlobColour, are a Level 3 ocean color product with a 0.25° resolution, combining outputs from multiple chlorophyll sensors to ensure data continuity, enhance spatial and temporal coverage, and reduce noise. Satellite-derived parameters such as SST and Chl provide context for tracking surface ocean dynamics influencing mesopelagic distributions and examining large-scale seasonal and annual trends. They also establish temporal baselines and environmental context for BGC-Argo data, particularly in regions with limited in situ measurements or where large-scale trends are assessed. In addition to these key parameters, we incorporated two additional variables to enhance our analysis: photosynthetically active radiation (PAR) and mixed-layer depth (MLD). These variables provide important insights into light conditions and the vertical structure of the ocean, both of which are critical for understanding the dynamics of mesopelagic organisms. For PAR, we utilized a high-resolution, long-term global gridded PAR product (2010–2018) provided by Tang et al. (2021), which has a temporal resolution of 3 h. Unlike solar altitude, which is based on latitude and time and may not fully capture the temporal and spatial variability in PAR, this dataset offers a more accurate and detailed representation of light availability. For MLD, we used data from the hybrid algorithm and threshold method (Holte et al., 2017). Among them, the hybrid algorithm was preferred for its accuracy, especially in regions such as the Labrador Sea (LS) and Irminger Sea, where the threshold method overestimates MLD by 10 % in winter.
2.3 Methods
2.3.1 BGC-Argo spike layer observations method
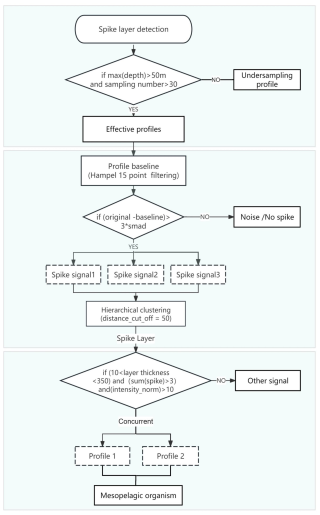
To investigate the aggregation patterns of mesopelagic organisms, we utilized a previously established spike layer detection algorithm (Haëntjens et al., 2020) to extract bbp spike signals. The extraction process involved several key steps. Initially, we filtered bbp profiles, selecting those with more than 30 sampling points and a maximum depth greater than 50 m. Subsequently, the raw bbp signal of each profile was smoothed using a 15-point Hampel filter, establishing a baseline signal. Next, we computed the difference between the original bbp signal and the baseline. Signals exhibiting differences exceeding twice the smad were identified as spike signals (see Eq. 1 for smad calculation, where smad represents the minimum threshold of the profile and bbp(n) represents all bbp signals in each profile). These detected spike signals were subsequently clustered hierarchically using a depth parameter of 50 m, and the results were categorized based on distinct features. Spike signals with identical features that occur simultaneously in two or more profiles are aggregated into a spike layer. To avoid the misalignment of spike layer positions across different profiles during statistical analysis, we have made certain improvements to the reference method by extracting the internal spike point information of the aggregated layers. For each layer, we quantified the intensity, depth, and spike count of each spike point, which were then recorded for further analysis. Furthermore, the bbp spike signals we analyzed include not only zooplankton but also spikes from sinking material aggregates with high precision (>90 %) (Haëntjens et al., 2020). The spike layer extraction workflow is illustrated in Fig. 2.
where smad represents the minimum threshold of the profile, defined as the standardized median absolute deviation of the signal distribution. bbp(i) represents each backscattering coefficient bbp value in the profile, while bbp(n) refers to the set of all bbp values in the profile. The calculation of the median is performed on the deviations of all spike values from the median of spikes in the profile. The term, the inverse complementary error function evaluated at , serves as a scaling factor for standardizing smad.
We firstly removed outliers from the environmental profiles and interpolated missing data points. Subsequently, a 25-point median filter followed by a mean filter was applied to the environmental data to minimize the influence of outliers and missing values on the analysis accuracy. After preprocessing, we calculated the temperature gradients for each profile, along with the mean dissolved oxygen and salinity values over depth intervals of 0–200, 200–500, and 500–800 m. Given the higher variability in chlorophyll and temperature in the upper and middle layers, we averaged these parameters over depth ranges of 0–50, 50–200, and 200–500 m. These averaged values served as environmental inputs for the random forest model. Additionally, sea surface chlorophyll and sea surface temperature data, with a spatial resolution of 0.25° and a temporal resolution of 1 d, were integrated with the BGC-Argo profile data to enhance the analysis.
2.3.2 Statistical method
To clarify the daily vertical migration of mesopelagic organisms, the water column was partitioned into 10 m depth intervals, and profiles were categorized into daytime and nighttime based on the local solar time. In each interval, the spiking signals were normalized by calculating the proportion of spiking points relative to the total number of detected points, and the environmental factors were averaged.
For the seasonal analysis, we categorized profiles containing spiking layers and selected those with comprehensive environmental data, resulting in 1045 profiles for spring, 1722 for summer, 1739 for autumn, and 801 for winter. Referring to established literature and conventional definitions of depth ranges for the upper (0–200 m) and middle (200–800 m) oceanic zones, we quantified the intensity and frequency of spiking signals for each season. To account for seasonal variations in the number of profiles, we normalized the frequency distributions. Frequency, defined as the number of spikes per unit depth, represents the likelihood of aggregation for specific taxa. Intensity, measured as the median number of bbp signals within each pinnacle layer, serves as a proxy for species composition (size) or abundance within the mesopelagic zone. To account for seasonal variations in both the number of profiles and the overall signal strength, both frequency and intensity distributions were normalized. Specifically, in each 10 m depth bin, the frequency and intensity were normalized by dividing the total number of spike points and the signal strength, respectively, by the total number of detected spike points across the total number of profiles for each season. This normalization procedure minimized potential biases arising from seasonal differences in sampling effort or signal intensity, enabling meaningful comparisons of vertical distribution patterns across seasons.
To capture the complex, nonlinear relationships influencing the distribution of mesopelagic organisms, we employed a random forest model, building upon methodologies from previous studies (De Forest and Drazen, 2009; Scales et al., 2016; Cuttitta et al., 2018; Villafaña and Rivadeneira, 2018; Song et al., 2022; Alexander et al., 2023). We firstly conducted Spearman correlation analysis to explore the associations between the depth and intensity of spiking signals within the pinnacle layer and environmental variables. Subsequently, the random forest model was applied to elucidate the regression relationships between mesopelagic organism densities and environmental factors, offering a more detailed understanding of their interactions. The random forest model was parameterized with 500 trees (ntree), balancing performance and computational efficiency. We used the default number of variables per split (mtry), where the value of mtry was set to the square root of the total number of input features. This configuration allowed the model to capture intricate, nonlinear patterns in the data. The model exhibited robustness in handling high-dimensional data, achieving an R2 value of 0.64, indicating moderate explanatory power without signs of overfitting.
3.1 Diurnal vertical migration
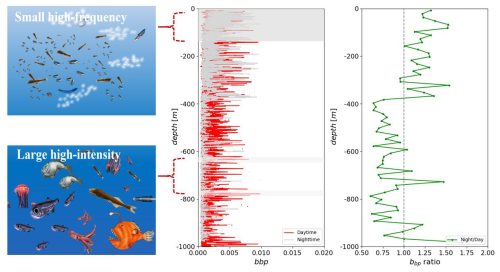
Our findings reveal a distinct diurnal migration pattern with a multilayered structure in mesopelagic organisms. Normalized data indicate prominent intensity bands within the bbp spike layer at depths of approximately 150, 330, 650, and 780 m during daylight hours. In contrast, nighttime observations show a strong scattering layer at a shallower depth of around 350 m. Notably, the mean intensity of the bbp spike layer at depths shallower than 380 m is 1.24 times higher at night compared to daytime, increasing to 1.28 times at depths shallower than 100 m. Conversely, at a depth of approximately 380 m, the average daytime intensity is 1.17 times higher than nighttime values (Fig. 3a, b).
These patterns align with observations by Klevjer et al. (2016) in the southern North Atlantic, where mesopelagic organisms exhibited significant aggregation between 400–600 m after dawn, followed by a substantial migration to the upper layers (0–200 m) after dusk. In addition, Grimaldo et al. (2020) reported three distinct sound scattering layers (SSLs) between 46–50° N and 21–26° W, with layers observed at 100–250, 300–360, and 420–700 m during daylight hours. These findings correspond with our observations, where mesopelagic organisms’ backscatter during the day is predominantly concentrated in the mesopelagic layers. This is further supported by Fennell and Rose (2015), who found higher deep scattering layer (DSL) densities in years with increased sea temperatures at the depths of major DSL concentration (400–600 m) in the western North Atlantic. Furthermore, Klevjer et al. (2020), in their study of the Irminger Sea, located northeast of our study area, observed a weak, non-migrating layer at approximately 700 m. This depth coincides with the lower edge of the scattering layer observed in the northeastern region of our study area, providing additional context for the consistency of our results across neighboring regions in the North Atlantic.
3.2 Seasonal vertical migration
Seasonal analysis reveals notable variability in the intensity of bbp spike layer signals across different ocean layers. The average intensity between the upper and middle layers exhibits minor differences in spring and autumn (below 10 %), whereas more substantial disparities are observed in summer and winter (over 50 %). In spring and winter, bbp spike intensity in the upper ocean is generally lower than in the middle layer, with the opposite pattern in summer and autumn. In the upper-ocean layer, the spike intensity peaks at 0.002290 m−1 during summer and reaches its minimum at 0.001635 m−1 in winter. Within the middle layer, the highest spike intensity is observed in winter at 0.005748 m−1, while the lowest intensity of 0.001502 m−1 occurs in autumn (Table 1).
Table 1Seasonal average intensity of mesopelagic organism aggregation in the upper and middle layers of the ocean; the spike intensity denotes the highest intensity recorded for a particular layer, with the depth indicating the precise location.
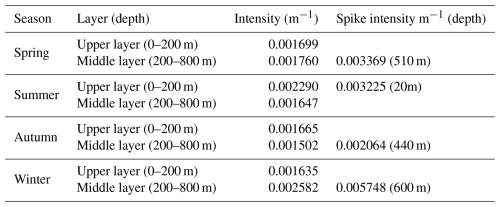
The distribution of extreme values in the bbp spike layer intensity exhibits clear seasonal patterns. In spring, extreme signals appear around 350, 510, and 700 m, with a spike intensity at 510 m (0.003369 m−1). During summer, the highest intensity shifts to the near-surface layer, around 20 m (0.003225 m−1). In autumn, intensity is primarily concentrated between 300 and 600 m, with a spike around 440 m (0.002064 m−1). Winter signals concentrate between 200 and 700 m, with maximum intensity at 600 m (0.005748 m−1) (Fig. 4). These findings suggest that the maximum intensity of the bbp spike layer predominantly occurs in the middle-ocean layer during spring and winter, reflecting a multilayer aggregation pattern, while, in summer, the highest intensity is near the surface. In autumn, the difference between the upper and middle layers is less pronounced, consistent with the findings of Loisel et al. (2002) for the same region. Additionally, the depth of the strongest bbp spike signal demonstrates a distinct seasonal dynamic: it is deepest in winter (around 600 m), ascends in spring (approximately 510 m), rises further to near-surface levels in summer (around 100 m), and descends in autumn (about 440 m).
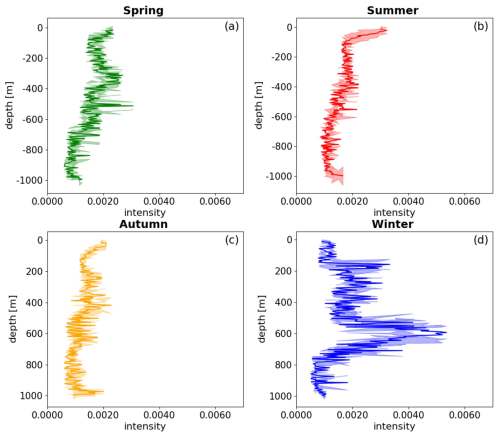
Figure 4The diagram depicts the density distribution of mesopelagic organisms in different seasons. The four seasons are represented by panels (a), (b), (c), and (d), corresponding to spring, summer, autumn, and winter, respectively. The shaded areas correspond to the spike layer, while the error bars indicate the standard deviation of the depth range.
The normalized frequency distribution of the bbp spike layer across different seasons (Fig. 5) reveals a consistent trend of relatively high-frequency aggregation of mesopelagic organisms at depths shallower than approximately 350 m. In spring and winter, the average frequency of bbp spike signals within the upper 350 m is elevated by factors of 1.28 and 1.33, respectively, compared to deeper waters. Additionally, there is a significant increase in the proportion of organisms migrating to the upper 200 m during spring, rising from 1.17 in winter to 1.58, indicating a notable shift toward shallower depths. In summer and autumn, the mean frequency of bbp spike signals at depths shallower than 350 m is 1.85 and 4.15 times higher, respectively, than at greater depths. Notably, there is a pronounced aggregation of high-frequency signals in the near-surface layer, shallower than 50 m.
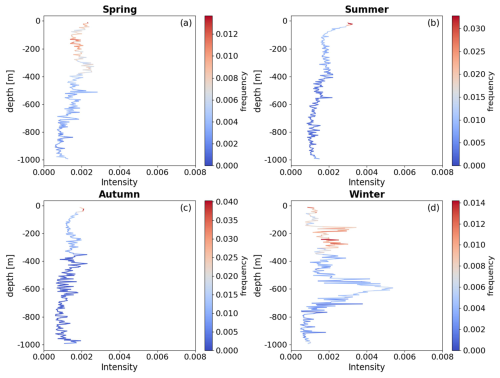
Figure 5This figure illustrates the intensity and density of the vertical distribution of mesopelagic organisms, with red areas indicating multiple occurrences of these organisms throughout the mesopelagic zone. The color bar represents the proportion of occurrences of mesopelagic organisms within each 5 m bin relative to the total seasonal frequency.
3.3 Horizontal spatial distribution
The horizontal spatial distribution of mesopelagic organisms was analyzed by calculating the mean depths of all bbp spike layers within a 1° × 1° grid across mid- and high-latitude regions of the North Atlantic (Fig. 6). The results indicate a predominantly shallow distribution in the northwestern North Atlantic, with mean depths around 200 m. In contrast, the Labrador Sea shows a deeper average bbp spike layer depth of approximately 400 m, while the Irminger Sea averages around 300 m. In the eastern Iceland Sea, organisms are found at substantially greater depths, averaging around 800 m. Prominent frontal zones, including the Greenland–Icelandic–Norwegian, East Greenland, Labrador Front (II), and North Atlantic Drift fronts, show bbp spike layers at depths ranging from several tens of meters to a few hundred meters.
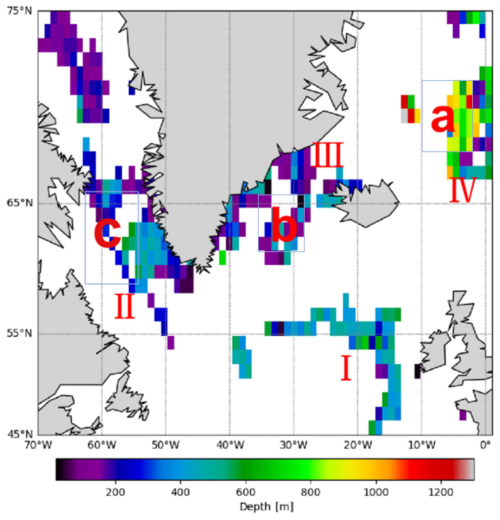
Figure 6The figure shows the spatial differences in the depth distribution of mesopelagic organisms, with a spatial resolution of 1° × 1°. The map shows the Iceland Sea (a), Irminger Sea (b), and Labrador Sea (c). The North Atlantic Drift Front (I), Labrador Front (II), East Greenland Front (III), and Greenland–Iceland–Norway Front (IV) are indicated.
3.4 Environmental driving factors
Random forest variable importance analysis revealed that the vertical temperature gradient made the greatest contribution to the model, accounting for 26.03 % of the variance. Following this, latitude (13.92 %), dissolved oxygen at 500 m (13.71 %), photosynthetically active radiation (PAR; 8.66 %), salinity at 500 m (8.29 %), mixed-layer depth (MLD; 8.23 %), chlorophyll concentration (8.09 %), temperature (7.10 %), and solar altitude (6.68 %) were also found to be significant. Among these, the vertical temperature gradient was the most influential in determining the seasonal and spatial distribution of mesopelagic organisms. Latitude, as a key geographical factor, also exerted a considerable influence on the spatial distribution patterns. Excluding the northeastern regions, mesopelagic organisms were generally found at shallower depths in higher latitudes. The model's response curves further elucidated the relationships between environmental factors and the aggregation depth of mesopelagic organisms in the open ocean. Within certain ranges, increasing latitude, higher dissolved oxygen levels, greater mixing, reduced light penetration, and decreasing temperatures all corresponded to shallower aggregation depths for midwater organisms. Across all regions, the distributions in summer and autumn tended to be shallower, whereas spring and winter distributions were generally deeper. These observations partially explain the consistency between the spatial distribution of midwater organisms and the heterogeneity of the physiological environment. In contrast, when considering the intensity of biological aggregation as a response variable, stronger signals from mesopelagic organisms typically originated from shallower depths. It is important to note that, while random forest analysis can capture broad trends within specific ranges of environmental variability, the detailed seasonal differences across individual subregions require further multifactorial analysis for a more comprehensive understanding.
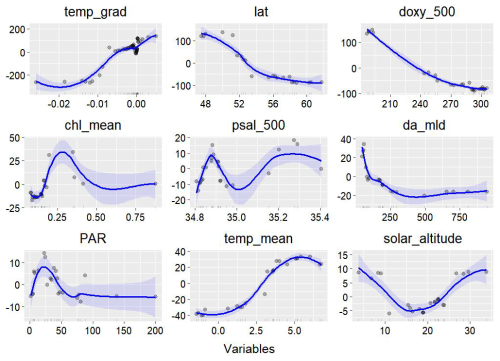
Figure 7Response curves from the random forest model, with the blue line indicating the influence of various environmental factors. The small black ticks along the horizontal axis represent the distribution density of the data, while the gray points represent individual data points. The x axis displays the range of feature values. The y axis shows the accumulated local effect (ALE) of each feature on the response variable (p), which reflects the anomaly in depth change of the primary spike layer. Positive values indicate a deepening of the spike, while negative values indicate a shoaling.
During the daytime, mesopelagic organisms predominantly reside within the mesopelagic zone (150–800 m), forming distinct signal bands. Conversely, nocturnal migrations lead these organisms to occupy shallower pelagic strata, particularly those below 380 m. Despite these observations, the environmental dynamics within these shallower strata remain insufficiently defined. Comparative analyses indicate that elevated chlorophyll concentrations, more favorable thermal conditions, and reduced nocturnal illumination in the upper pelagic layers collectively reduce predation risk and avoid hypoxic conditions. These factors create a more favorable environment for mesopelagic organisms, thereby enhancing their nocturnal migrations and intensifying the bbp signal in the upper pelagic layers, consistent with satellite-based lidar observations (Behrenfeld et al., 2019). Additionally, the interaction between diurnal bbp spiking layer characteristics and environmental factors such as chlorophyll and temperature emphasizes the importance of thermal and salinity gradients. Enhanced spiking signals are observed above these gradients, driven by increased food availability and physiological predispositions favoring aggregation in regions of chlorophyll maxima and thermal gradients (Sameoto, 1986). These findings align with random forest model results, which demonstrate that pronounced temperature gradients correlate with shallower mesopelagic distributions.
Despite the limited availability of seasonal data, our observations across all regions reveal a consistent pattern: the vertical distribution of mesopelagic organisms is shallower during summer and autumn and deeper during spring and winter. This trend is largely attributable to the light-driven seasonal patterns that govern mesopelagic organism distribution. The seasonal variations in the backscattering coefficient (bbp) spike layer intensity are influenced by a suite of environmental factors, including water temperature, ocean currents, dissolved oxygen levels, light availability, and food sources (Bianchi et al., 2013; Klevjer et al., 2016). The impact of these factors varies significantly across different regions and seasons (Klevjer et al., 2020), leading to fluctuations in the mean intensity, intensity maxima distribution, and frequency of bbp spike layer signals within the mesopelagic layer. During spring and winter, the mean intensity of the bbp spike layer in the upper mesopelagic zone decreases, while its frequency increases relative to the middle mesopelagic layer. This shift is likely driven by the organisms' preference for specific depths influenced by lower temperatures, deeper mixed layers, limited light availability, and reduced phytoplankton concentrations in the upper layers during these seasons.
As spring progresses and temperatures and light levels rise, the mixed layer becomes shallower and phytoplankton blooms increase. In response, mesopelagic organisms migrate to the upper layers to exploit improved foraging opportunities, resulting in higher-frequency aggregations and a relative decrease in the mean intensity of the mesopelagic spike signal (Allan et al., 2021; Henson et al., 2012; Lutz et al., 2007; Woodd-Walker et al., 2002; Briggs et al., 2011; Vedenin et al., 2022). In the cooler months of spring and winter, strong downwelling increases surface water density, while salinity differences and stratification in high latitudes and the Atlantic Ocean facilitate the transfer of dissolved oxygen to deeper waters. Consequently, mesopelagic organisms migrate to greater depths in search of suitable habitats and food resources, thereby avoiding elevated predation pressure in surface waters (Freeman, 2006; Garcia-Soto et al., 2021; Yin et al., 2024). This migration results in a higher concentration of organisms in the middle layer and leads to a multilayer aggregation phenomenon. The correlation between dissolved oxygen in the 200–500 m layer and the negative correlation in the 500–800 m zone indicate a distinct oxygen minimum zone around 500–600 m, delineating the emergence of a prominent mesopelagic signal layer at approximately 600 m depth. During summer and autumn, the mean frequency of bbp spike signals at depths shallower than 350 m is 1.85 and 4.15 times higher, respectively, than at greater depths. Notably, there is a pronounced aggregation of high-frequency signals in the near-surface layer, shallower than 50 m. In summer, a stable shallow mixed layer isolates the surface from deeper waters, concentrating mesopelagic organisms in the upper middle layer. High-intensity and high-frequency signal layers emerge at the ocean’s surface during summer and autumn. In autumn, these strong signals are frequently associated with chlorophyll maxima around 200 m depth. Increased solar radiation enhances phytoplankton photosynthesis, significantly boosting primary productivity and providing abundant food resources for larger marine organisms (Flombaum et al., 2013). Warmer sea surface temperatures also create favorable conditions for species thriving in warmer waters, promoting the survival, reproduction, and growth of larger marine organisms (Chen et al., 2019; Bova et al., 2021). Additionally, ocean circulation and upwelling transport nutrient-rich deep waters to the surface, attracting larger marine species to feed during the day.
Our analysis of bbp spike signal frequency and intensity reveals significant seasonal differences between the upper and middle layers of the ocean. In spring and winter, although the average bbp spike intensity in the upper ocean is lower than in the middle layer (where spike values are primarily distributed), mesopelagic organisms still aggregate at specific depths in the middle layer and migrate to the upper ocean for foraging. In contrast, in summer and autumn, especially summer, both the average intensity and frequency of bbp spikes are significantly higher in the upper layer than in the middle layer, with a marked concentration in the near-surface zone. This shift indicates a seasonal change in mesopelagic behavior, with a heightened preference for upper-layer habitats and foraging during warmer months. A similar pattern in the mesopelagic scatterers of intermediate to deep layers was noted by Powell and Ohman (2015), who investigated the scattering characteristics of migratory and non-migratory zooplankton in frontal regions. Their study found that shallower migratory layers, which consist of smaller but more abundant scatterers, are more homogeneously distributed at finer scales. In contrast, deeper non-migratory layers likely consist of fewer but larger scatterers, and these are associated with a lower abundance of organisms, which are likely non-migratory in nature. The 400–500 m depth range of the mesopelagic layer, typically inhabited by non-swimming species or crustaceans, is shaped by vertical fluxes of organic carbon and particulate matter (Marohn et al., 2021; Liu, 2011; Sikder et al., 2019; Henson et al., 2012; Lutz et al., 2007). Based on our findings, lower-intensity but higher-frequency signals may correspond to smaller-sized plankton or particle-based signals, while higher intensity and lower frequency signals are likely associated with larger, but fewer, organisms. This distribution pattern may be driven by multiple mechanisms: firstly, larger mesopelagic organisms, with stronger swimming abilities, tend to migrate to deeper waters to avoid currents, while smaller organisms remain in the upper layers (Lin and Costello, 2023; Sorochan et al., 2023). Secondly, during spring and winter, the deeper mixed layer and unstable water column in the North Atlantic, along with transient stratification events often disrupted by storms, favor the accumulation of organic matter in the deeper mixed layer, resulting in increased biotic aggregation frequencies in the mid-ocean (Dall'Olmo et al., 2016). These mechanisms collectively shape the vertical distribution and seasonal dynamics of mesopelagic organisms, providing new insights into the structure and function of marine ecosystems.
Spatially, our findings on the spatial distribution of mesopelagic organisms align well with Klevjer's study of four North Atlantic basins, with the shallowest distributions around 200 m in the Labrador Sea (LS) and the deepest at 500–600 m in the Icelandic Sea (ICS) (Klevjer et al., 2020). Our study area is situated in a high-latitude region, and, with the exception of the unique Norwegian Sea area, the distribution of mesopelagic organisms follows a different mechanism across other regions. As latitude increases, dissolved oxygen levels rise, light penetration diminishes, and temperatures decline. In this context, mesopelagic organisms tend to aggregate at shallower depths. This behavior indicates that, despite the higher predation risk associated with the shallower distribution of the deep scattering layer (DSL) in the North Atlantic's high-latitude regions, these organisms still prefer areas with richer dissolved oxygen. The general depth distribution in the northeastern part of our study area is much deeper, whereas the distribution of mesopelagic organisms along the left coastline of Greenland at the same latitude is much shallower. Even at the same latitude, there is considerable variability in the depth distribution; therefore, it is misleading to directly infer that mesopelagic organisms become shallower with increasing latitude. Considering the complexity of the North Atlantic, factors such as sea ice coverage, the North Atlantic Oscillation, and various current systems could influence the distribution of midwater organisms (Gu et al., 2024; Puerta et al., 2020; Lynch-Stieglitz et al., 2024), highlighting the need to address different regions separately.
Currently, the two primary mechanisms driving mesopelagic aggregation are temperature-dependent physiology and light-dependent foraging. Diel vertical migration (DVM) of midwater fish is highly correlated with latitude. Our study area’s northeastern section, including the Greenland Sea, Iceland Sea, and Norwegian Sea, is the only deep-sea basin above the Arctic Circle that remains largely ice-free throughout the year (Klevjer et al., 2015). For the distribution of mesopelagic organisms, a hypothesis suggests that, due to the extreme light climate in high-latitude areas, the foraging conditions are poor, limiting the success of mesopelagic fish in these environments. The persistent daylight in summer limits safe foraging in the upper layers during “nighttime”, while continuous darkness in winter may restrict visual foraging at any time of day (Kaartvedt, 2008). Therefore, we hypothesize that seasonal differences in our results are primarily driven by light conditions, but latitude-driven distribution differences cannot be fully explained by light alone. While it is theoretically expected that the light comfort zone remains consistent across oceans with varying levels of light penetration, Aksnes et al. (2009) highlight that oxygen-poor waters, in contrast to oxygen-rich waters, exhibit reduced light penetration. The mechanism linking light attenuation to dissolved oxygen may involve microbial heterotrophic degradation of particulate organic matter, leading to the release of CDOM, which exacerbates light attenuation in oxygen-deprived waters (Aksnes et al., 2009; Nelson and Siegel, 2013; Catalá et al., 2015). From a biological distribution perspective, our results challenge the general assumption that mesopelagic organisms tend to inhabit deeper layers in clearer waters and shallower layers in waters with higher light attenuation coefficients (Braun et al., 2023). In high-latitude regions, we observe that mesopelagic organisms tend towards shallower distributions, which contradicts the expected pattern where light attenuation should correlate with deeper distributions. This discrepancy may be linked to CDOM spikes associated with zooplankton foraging and excretion behavior, producing fluorescent proteins or amino-acid-like fluorescence. This is fundamentally different from the mesopelagic bbp spike signals we detected, which reflect aggregates of zooplankton or sinking materials. Therefore, in high-latitude regions, the latitude-driven distribution of zooplankton or sinking material aggregates is not solely influenced by light conditions. Environmental differences also suggest that the western coast, influenced by the Greenland cold water current, has lower temperatures and reduced nutrient availability. The colder sea temperatures may reduce the activity of large predators, providing relatively safe habitats and suitable nutrient conditions for mesopelagic organisms (Chawarski et al., 2022). Previous studies have suggested that the light climate in high latitudes limits the northward extension of larger mesopelagic fish populations, as both summer light nights and winter darkness limit food availability; in the ICS, migration into the epipelagic zone is restricted by nocturnal light levels (Kaartvedt, 2008; Norheim et al., 2016). Langbehn et al. (2022) found that, in high latitudes, light conditions primarily regulate the distribution and population dynamics of mesopelagic fish, with temperature playing a secondary role. In winter, as daylight diminishes, prey disperses, and most organisms remain dormant in deeper waters. Cold temperatures and low metabolic demands enable mesopelagic fish to conserve energy despite limited food availability. In summer, warmer temperatures and longer daylight hours force mesopelagic fish to forage near the surface, but increased predation risk drives them to venture outside the optimal light zone in search of food (Langbehn et al., 2022). Our results also indicate a clear trend of deeper biological distributions in spring and winter, which is similar to the long overwintering phase of squid species that feed and reproduce in deeper waters (Berge et al., 2012).
In polar regions, ocean ecosystems are considerably shaped by seasonal changes in light and sea surface temperature. While light plays a crucial role in the vertical migration of zooplankton and fish, affecting their predation and survival (Kaartvedt, 2008; Ljungström et al., 2021), temperature directly affects physiological rates (Gillooly et al., 2001). Our study region is also influenced by polar water masses and by acoustic and oceanographic measurements, and several studies have demonstrated that latitude-driven variations in upper-layer communities align with the polar boundary defined by deep-sea temperature gradients (Saupe et al., 2019; Sallée et al., 2021). As mesopelagic organisms transition into polar water masses, the acoustic backscattering of these organisms suddenly weakens, and vertical scattering increases, altering the structure of the mesopelagic zone (Ingvaldsen et al., 2023). In conclusion, our findings demonstrate that light is the primary driver of the seasonal distribution of mesopelagic organisms in the study area, particularly in high-latitude regions, whereas vertical temperature gradients govern their vertical distribution.
Mesopelagic organisms also exhibit significant aggregation behaviors in frontal zones, where alternating downwelling and upwelling currents induce vertical displacements with substantial ecological impacts. In addition to these vertical mechanisms, mesoscale fronts also separate water masses through horizontal mixing, creating potential habitats for zooplankton (Martin, 2003). These horizontal processes, combined with light availability and nutrient dynamics, shape the spatial distribution of mesopelagic organisms and their aggregation behaviors in frontal zones (Powell and Ohman, 2015). Together, these mechanisms highlight the ecological complexity and importance of frontal regions. Frontal regions are characterized by steep environmental gradients, such as variations in sea surface temperature, chlorophyll concentration, sea surface height, and dissolved oxygen. These factors significantly influence fish distribution (Owen, 1981; Woodd-Walker et al., 2002). Frontal zones, marked by distinct thermal gradients resulting from the convergence of different water masses, also serve as biodiversity and productivity hotspots (Longhurst, 2007). In the study area, an average of three to four water masses interact, with polar fronts demarcating the boundary between Atlantic and polar water masses. The interaction between colder northern waters and terrestrial runoff creates gradients of declining temperature and salinity, forming distinct physiographic environments that influence mesopelagic distribution (Sutton et al., 2017; Astthorsson et al., 2007). Notably, the Greenland–Iceland–Norway Front, characterized by a significant temperature-salinity gradient, corresponds to deeper mesopelagic aggregations, driven by the separation of colder Arctic and warmer Atlantic waters and the resulting temperature–salinity gradient, which critically impacts marine productivity and spatial distribution. Moreover, vertical mixing within frontal zones enhances nutrient upwelling, supporting higher primary productivity and providing abundant food resources for mesopelagic organisms (Ljungström et al., 2021). Previous studies have similarly highlighted the role of water masses and frontal zones in influencing mesopelagic distributions (Yin et al., 2024).
Comparative analysis using acoustic trawl and satellite lidar detection confirms that bbp from BGC-Argo effectively capture the biological signal of mesopelagic organisms. During the day, mesopelagic organisms predominantly inhabit the middle layers, exhibiting multilayered aggregation patterns. At night, reduced light levels lower predation risks, driving a general upward migration into the upper layers, where pronounced diel vertical migration (DVM) is observed.
Seasonally, the mean intensity of bbp spikes in the upper layers remains lower than in the middle layers during spring and winter, although the frequency of these spikes in the upper layers is higher. In contrast, summer and autumn show an increase in both the intensity and the frequency of bbp signals in the upper layers, particularly near the surface. This seasonal shift reflects a change in habitat utilization, with mesopelagic organisms becoming more active in the upper layers for foraging. The depth of the strongest bbp signal exhibits a periodic pattern, becoming more shallow from winter through spring and summer and deepening in autumn, which corresponds to seasonal fluctuations in mixed-layer concentration.
Horizontally, the study area reveals deeper distributions in the northeast and shallower distributions in the southwest. In the northwestern North Atlantic, mesopelagic organisms typically reside at an average depth of 200 m, while, in the eastern Iceland Sea, they are found at greater depths, around 800 m. The Labrador Sea features an average signal layer depth of 400 m, whereas the Irminger Sea has it at approximately 300 m. Oceanic fronts, such as the Greenland–Iceland–Norway, East Greenland, Labrador, and North Atlantic Drift fronts, present pronounced temperature gradients, favorable light conditions, and nutrient-rich waters, attracting significant concentrations of mesopelagic organisms and leading to substantial biological aggregations.
Spatiotemporal distribution patterns highlight that mesopelagic depth distribution is influenced by multiple environmental factors. Correlation and random forest analyses underscore temperature as a primary determinant year-round, with temperature gradients emerging as the most significant factor affecting mesopelagic distribution in spike layers. Seawater salinity, dissolved oxygen, sea surface chlorophyll concentration, and latitude also play important roles.
BGC-Argo data provide valuable insights into the spatial distribution and seasonal variability of mesopelagic organisms, promoting our understanding of organic carbon transfer to the deep sea, ecosystem energy and material cycling, and fisheries management. Future research should incorporate additional environmental factors, such as eddies, currents, and oceanic fronts, to further elucidate the complex dynamics influencing mesopelagic organisms. Despite the extensive vertical profile data provided by BGC-Argo, clustering effects and limited sampling of certain environmental parameters suggest that advancements in lidar technology could substantially improve mesopelagic organism detection capabilities.
BGC-Argo data are accessible via the Biogeochemical-Argo portal at https://biogeochemical-argo.org/data-access.php (last access: 26 July 2025). The GlobColour sea surface chlorophyll (Chl) dataset can be found at http://hermes.acri.fr/index.php (last access: 26 July 2025). Sea surface temperature data are available at https://doi.org/10.1175/JCLI-D-20-0166.1 (Huang et al., 2020). The direct URL to access the dataset is: https://data.tpdc.ac.cn/zh-hans/data/16795f34-cd08-48b0-a1d6-4f85d195ad9e/ (last access: 10 October 2025). The new PAR product is downloadable at https://doi.org/10.11888/RemoteSen.tpdc.271909 (Tang, 2021). MLD data were sourced from http://mixedlayer.ucsd.edu (last access: 26 July 2025).
JY was responsible for data collection, funding acquisition, equipment provision, and refining the article's logical structure. JHL conducted the experiments, obtained the experimental results, completed the experimental methods, and drafted the article. GC provided critical intellectual input and constructive suggestions during the manuscript revision phase.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
The authors thank Nils Haëntjens for providing the code for the spike identification algorithm and appreciate the contributions of previous researchers to biogeochemical identification in the ocean. Data from the Biogeochemical-Argo portal, GlobColour sea surface chlorophyll, and sea surface temperature datasets were invaluable for this work. We gratefully acknowledge the financial support provided by the National Natural Science Foundation of China under grant no. 42530404.
This paper was edited by Andrew Thurber and reviewed by two anonymous referees.
Aksnes, D. L., Dupont, N., Staby, A., Fiksen, Ø., Kaartvedt, S., and Aure, J.: Coastal water darkening and implications for mesopelagic regime shifts in Norwegian fjords, Marine Ecology Progress Series, 387, 39–49, https://doi.org/10.3354/meps08120, 2009. a, b
Allan, E. A., DiBenedetto, M. H., Lavery, A. C., Govindarajan, A. F., and Zhang, W. G.: Modeling characterization of the vertical and temporal variability of environmental DNA in the mesopelagic ocean, Sci. Rep., 11, 21273, https://doi.org/10.1038/s41598-021-00288-5, 2021. a
Astthorsson, O. S., Gislason, A., and Jonsson, S.: Climate variability and the Icelandic marine ecosystem, Deep-Sea Res. Pt. II, 54, 2456–2477, https://doi.org/10.1016/j.dsr2.2007.07.030, 2007. a
Bailey, R.: Life in the Mesopelagic Zone of the Ocean, https://www.thoughtco.com/mesopelagic-zone-4685646 (last access: 18 June 2025), 2021. a
Bandara, K., Varpe, Ø., Wijewardene, L., Tverberg, V., and Eiane, K.: Two hundred years of zooplankton vertical migration research, Biological Reviews, 96, 1547–1589, https://doi.org/10.1111/brv.12715, 2021. a
Behrenfeld, M. J., Gaube, P., Della Penna, A., O’malley, R. T., Burt, W. J., Hu, Y., Bontempi, P. S., Steinberg, D. K., Boss, E. S., Siegel, D. A., Hostetler, C. A., Tortell, P. D., and Doney, S. C.: Global satellite-observed daily vertical migrations of ocean animals, Nature, 576, 257–261, https://doi.org/10.1038/s41586-019-1796-9, 2019. a, b
Berge, J., Varpe, Ø., Moline, M. A., Wold, A., Renaud, P., Daase, M., and Falk-Petersen, S.: Retention of ice-associated amphipods: possible consequences for an ice-free Arctic Ocean, Biol. Lett., 8, 1012–1015, https://doi.org/10.1098/rsbl.2012.0517, 2012. a
Bianchi, D., Galbraith, E. D., Carozza, D. A., Mislan, K., and Stock, C. A.: Intensification of open-ocean oxygen depletion by vertically migrating animals, Nat. Geosci., 6, 545–548, https://doi.org/10.1038/NGEO1837, 2013. a
Bishop, J. K. and Wood, T.: Particulate matter chemistry and dynamics in the twilight zone at VERTIGO ALOHA and K2 sites, Deep-Sea Res. Pt. I, 55, 1684–1706, https://doi.org/10.1016/j.dsr.2008.07.012, 2008. a
Bishop, J. K., Calvert, S. E., and Soon, M. Y.: Spatial and temporal variability of POC in the northeast Subarctic Pacific, Deep-Sea Res. Pt. II, 46, 2699–2733, https://doi.org/10.1016/S0967-0645(99)00081-8, 1999. a
Bova, S., Rosenthal, Y., Liu, Z., Godad, S. P., and Yan, M.: Seasonal origin of the thermal maxima at the Holocene and the last interglacial, Nature, 589, 548–553, https://doi.org/10.1038/s41586-020-03155-x, 2021. a
Boyd, P. W., Claustre, H., Levy, M., Siegel, D. A., and Weber, T.: Multi-faceted particle pumps drive carbon sequestration in the ocean, Nature, 568, 327–335, https://doi.org/10.1038/s41586-019-1098-2, 2019. a
Braun, C. D., Della Penna, A., Arostegui, M. C., Afonso, P., Berumen, M. L., Block, B. A., Brown, C. A., Fontes, J., Furtado, M., Gallagher, A. J., Golet, W. J., Kneebone, J., Macena, B. C. L., Mucientes, G., Orbesen, E. S., Queiroz, N., Shea, B. D., Schratwieser, J., Sims, D. W., Skomal, G. B., Snodgrass, D., and Thorrold, S. R.: Linking vertical movements of large pelagic predators with distribution patterns of biomass in the open ocean, P. Natl. Acad. Sci. USA, 120, e2306357120, https://doi.org/10.1073/pnas.2306357120, 2023. a
Briggs, N., Perry, M. J., Cetinić, I., Lee, C., D'Asaro, E., Gray, A. M., and Rehm, E.: High-resolution observations of aggregate flux during a sub-polar North Atlantic spring bloom, Deep-Sea Res. Pt. I, 58, 1031–1039, https://doi.org/10.1016/J.DSR.2011.07.007, 2011. a, b
Briggs, N., Dall’Olmo, G., and Claustre, H.: Major role of particle fragmentation in regulating biological sequestration of CO2 by the oceans, Science, 367, 791–793, https://doi.org/10.1126/science.aay1790, 2020. a
Brun, P., Stamieszkin, K., Visser, A. W., Licandro, P., Payne, M. R., and Kiørboe, T.: Climate change has altered zooplankton-fuelled carbon export in the North Atlantic, Nat. Ecol. Evol., 3, 416–423, https://doi.org/10.1038/s41559-018-0780-3, 2019. a
Catalá, T. S., Reche, I., Álvarez, M., Khatiwala, S., Guallart, E., Benítez-Barrios, V. M., Fuentes-Lema, A., Romera-Castillo, C., Nieto-Cid, M., Pelejero, C., Fraile-Nuez, E., Ortega-Retuerta, E., Marrasé, C., and Álvarez-Salgado, X. A.: Water mass age and aging driving chromophoric dissolved organic matter in the dark global ocean, Global Biogeochem. Cycles, 29, 917–934, https://doi.org/10.1002/2014GB005048, 2015. a
Chai, F., Johnson, K. S., Claustre, H., Xing, X., Wang, Y., Boss, E., Riser, S., Fennel, K., Schofield, O., and Sutton, A.: Monitoring ocean biogeochemistry with autonomous platforms, Nat. Rev. Earth Environ., 1, 315–326, https://doi.org/10.1038/s43017-020-0053-y, 2020. a
Chawarski, J., Klevjer, T. A., Cote, D., and Geoffroy, M.: Evidence of temperature control on mesopelagic fish and zooplankton communities at high latitudes, Front. Marine Sci., 9, 917985, https://doi.org/10.3389/fmars.2022.917985, 2022. a
Chen, B., Smith, S. L., and Wirtz, K. W.: Effect of phytoplankton size diversity on primary productivity in the North Pacific: trait distributions under environmental variability, Ecol. Lett., 22, 56–66, https://doi.org/10.1111/ele.13167, 2019. a
Claustre, H., Johnson, K. S., and Takeshita, Y.: Observing the global ocean with biogeochemical-Argo, Annu. Rev. Marine Sci., 12, 23–48, https://doi.org/10.1146/annurev-marine-010419-010956, 2019. a
Dall'Olmo, G., Dingle, J., Polimene, L., Brewin, R. J., and Claustre, H.: Substantial energy input to the mesopelagic ecosystem from the seasonal mixed-layer pump, Nat. Geosci., 9, 820–823, https://doi.org/10.1038/ngeo2818, 2016. a
Della Penna, A. and Gaube, P.: Mesoscale eddies structure mesopelagic communities, Front. Marine Sci., 7, 454, https://doi.org/10.3389/fmars.2020.00454, 2020. a
Devine, B., Fennell, S., Themelis, D., and Fisher, J. A.: Influence of anticyclonic, warm-core eddies on mesopelagic fish assemblages in the Northwest Atlantic Ocean, Deep-Sea Res. Pt. I, 173, 103555, https://doi.org/10.1016/j.dsr.2021.103555, 2021. a
Falk-Petersen, S., Mayzaud, P., Kattner, G., and Sargent, J. R.: Lipids and life strategy of Arctic Calanus, Marine Biol. Res., 5, 18–39, https://doi.org/10.1080/17451000802512267, 2009. a
Fennell, S. and Rose, G.: Oceanographic influences on deep scattering layers across the North Atlantic, Deep-Sea Res. Pt. I, 105, 132–141, https://doi.org/10.1016/J.DSR.2015.09.002, 2015. a, b
Flombaum, P., Gallegos, J. L., Gordillo, R. A., Rincón, J., Zabala, L. L., Jiao, N., Karl, D. M., Li, W. K., Lomas, M. W., Veneziano, D., Vera, C. S., Vrugt, J. A., and Martiny, A. C.: Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus, P. Natl. Acad. Sci. USA, 110, 9824–9829, https://doi.org/10.1073/pnas.1307701110, 2013. a
Freeman, A.: Size-dependent trait-mediated indirect interactions among sea urchin herbivores, Behavioral Ecology, 17, 182–187, https://doi.org/10.1093/beheco/arj014, 2006. a
Galí, M., Falls, M., Claustre, H., Aumont, O., and Bernardello, R.: Bridging the gaps between particulate backscattering measurements and modeled particulate organic carbon in the ocean, Biogeosciences, 19, 1245–1275, https://doi.org/10.5194/bg-19-1245-2022, 2022. a
Garcia-Soto, C., Cheng, L., Caesar, L., Schmidtko, S., Jewett, E. B., Cheripka, A., Rigor, I., Caballero, A., Chiba, S., Báez, J. C., Zielinski, T., and Abraham, J. P.: An overview of ocean climate change indicators: Sea surface temperature, ocean heat content, ocean pH, dissolved oxygen concentration, arctic sea ice extent, thickness and volume, sea level and strength of the AMOC (Atlantic Meridional Overturning Circulation), Front. Marine Sci., 8, 642372, https://doi.org/10.3389/fmars.2021.642372, 2021. a
Gardner, W. D., Richardson, M. J., and Smith Jr., W. O.: Seasonal patterns of water column particulate organic carbon and fluxes in the Ross Sea, Antarctica, Deep-Sea Res. Pt. II, 47, 3423–3449, https://doi.org/10.1016/S0967-0645(00)00074-6, 2000. a
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M., and Charnov, E. L.: Effects of size and temperature on metabolic rate, Science, 293, 2248–2251, https://doi.org/10.1126/science.1061967, 2001. a
Gjoesaeter, J., Kawaguchi, K., Fao, R. E. D., and FIR: A review of the world resources of mesopelagic fish, Fao Fish Tech. Rap., 93, Food and Agriculture Organization of the United Nations (FAO), Rome, ISBN 92-5-100924-4, 1980. a, b
Govindarajan, A. F., Llopiz, J. K., Caiger, P. E., Jech, J. M., Lavery, A. C., McMonagle, H., Wiebe, P. H., and Zhang, W.: Assessing mesopelagic fish diversity and diel vertical migration with environmental DNA, Front. Marine Sci., 10, 1219993, https://doi.org/10.3389/fmars.2023.1219993, 2023. a
Grimaldo, E., Grimsmo, L., Alvarez, P., Herrmann, B., Møen Tveit, G., Tiller, R., Slizyte, R., Aldanondo, N., Guldberg, T., Toldnes, B., Carvajal, A., Schei, M., and Selnes, M.: Investigating the potential for a commercial fishery in the Northeast Atlantic utilizing mesopelagic species, ICES Journal of Marine Science, 77, 2541–2556, https://doi.org/10.1093/icesjms/fsaa114, 2020. a
Gu, Q., Gervais, M., Danabasoglu, G., Kim, W. M., Castruccio, F., Maroon, E., and Xie, S.-P.: Wide range of possible trajectories of North Atlantic climate in a warming world, Nat. Commun., 15, 4221, https://doi.org/10.1038/s41467-024-48401-2, 2024. a
Haëntjens, N., Della Penna, A., Briggs, N., Karp-Boss, L., Gaube, P., Claustre, H., and Boss, E.: Detecting mesopelagic organisms using biogeochemical-Argo floats, Geophys. Res. Lett., 47, e2019GL086088, https://doi.org/10.1029/2019GL086088, 2020. a, b, c, d, e
Hansell, D. A., Carlson, C. A., and Suzuki, Y.: Dissolved organic carbon export with North Pacific Intermediate Water formation, Global Biogeochem. Cycles, 16, 7–1, https://doi.org/10.1029/2000GB001361, 2002. a
Hauss, H., Christiansen, S., Schütte, F., Kiko, R., Edvam Lima, M., Rodrigues, E., Karstensen, J., Löscher, C. R., Körtzinger, A., and Fiedler, B.: Dead zone or oasis in the open ocean? Zooplankton distribution and migration in low-oxygen modewater eddies, Biogeosciences, 13, 1977–1989, https://doi.org/10.5194/bg-13-1977-2016, 2016. a
Hays, G. C.: A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations, Hydrobiologia, 503, 163–170, https://doi.org/10.1023/B:HYDR.0000008476.23617.b0, 2003. a, b
Hazen, E. L.: Climate change “heard” in the ocean depths, Nat. Clim. Change, 12, 891–892, https://doi.org/10.1038/s41558-022-01484-5, 2022. a
Henson, S. A., Sanders, R., and Madsen, E.: Global patterns in efficiency of particulate organic carbon export and transfer to the deep ocean, Global Biogeochem. Cycles, 26, https://doi.org/10.1029/2011GB004099, 2012. a, b
Holte, J., Talley, L. D., Gilson, J., and Roemmich, D.: An Argo mixed layer climatology and database, Geophys. Res. Lett., 44, 5618–5626, https://doi.org/10.1002/2017GL073426, 2017. a
Honjo, S. and Manganini, S. J.: Annual biogenic particle fluxes to the interior of the North Atlantic Ocean; studied at 34 N 21 W and 48 N 21 W, Deep-Sea Res. Pt. II, 40, 587–607, https://doi.org/10.1016/0967-0645(93)90034-K, 1993. a
Huang, B., Liu, C., Banzon, V., Freeman, E., Graham, G., Hankins, B., Smith, T., and Zhang, H.-M.: Improvements of the Daily Optimum Interpolation Sea Surface Temperature (DOISST) Version 2.1, Journal of Climate, 34, 2923–2939, https://doi.org/10.1175/JCLI-D-20-0166.1, 2020. a
Ingvaldsen, R. B., Eriksen, E., Gjøsæter, H., Engås, A., Schuppe, B. K., Assmann, K. M., Cannaby, H., Dalpadado, P., and Bluhm, B. A.: Under-ice observations by trawls and multi-frequency acoustics in the Central Arctic Ocean reveals abundance and composition of pelagic fauna, Sci. Rep., 13, 1000, https://doi.org/10.1038/s41598-023-27957-x, 2023. a
Irigoien, X., Klevjer, T. A., Røstad, A., Martinez, U., Boyra, G., Acuña, J. L., Bode, A., Echevarria, F., Gonzalez-Gordillo, J. I., Hernandez-Leon, S., Agusti, S., Aksnes, D. L., Duarte, C. M., and Kaartvedt, S.: Large mesopelagic fishes biomass and trophic efficiency in the open ocean, Nat. Commun., 5, 3271, https://doi.org/10.1038/ncomms4271, 2014. a
Kaartvedt, S.: Photoperiod may constrain the effect of global warming in arctic marine systems, J. Plankton Res., 30, 1203–1206, https://doi.org/10.1093/plankt/fbn075, 2008. a, b, c
Kapelonis, Z., Siapatis, A., Machias, A., Somarakis, S., Markakis, K., Giannoulaki, M., Badouvas, N., and Tsagarakis, K.: Seasonal patterns in the mesopelagic fish community and associated deep scattering layers of an enclosed deep basin, Sci. Rep., 13, 17890, https://doi.org/10.21203/rs.3.rs-2947537/v1, 2023. a
Klevjer, T., Norheim, E., Aksnes, D., Strand, E., Knutsen, T., Melle, W., and Wiebe, P.: Zooplankton and micronekton vertical distribution and diel vertical migration behaviour in the Northern North Atlantic Ocean, International Council for the Exploration of the Sea (ICES), ICES CM 2015/S:11, 2015. a
Klevjer, T. A., Irigoien, X., Røstad, A., Fraile-Nuez, E., Benítez-Barrios, V. M., and Kaartvedt, S.: Large scale patterns in vertical distribution and behaviour of mesopelagic scattering layers, Sci. Rep., 6, 19873, https://doi.org/10.1038/srep19873, 2016. a, b, c
Klevjer, T. A., Melle, W., Knutsen, T., and Aksnes, D. L.: Vertical distribution and migration of mesopelagic scatterers in four north Atlantic basins, Deep-Sea Res. Pt. II, 180, 104811, https://doi.org/10.1016/j.dsr2.2020.104811, 2020. a, b, c, d
Kruse, S., Brey, T., and Bathmann, U.: Role of midwater chaetognaths in Southern Ocean pelagic energy flow, Marine Ecology Progress Series, 416, 105–113, https://doi.org/10.3354/meps08773, 2010. a
Lampert, W., Fleckner, W., Pott, E., Schober, U., and Strkel, K. U.: Herbicide effects on planktonic systems of different complexity, Hydrobiologia, 188–189, 415–424, 1989. a
Lampitt, R.: Evidence for the seasonal deposition of detritus to the deep-sea floor and its subsequent resuspension, Deep-Sea Res. Pt. A, 32, 885–897, https://doi.org/10.1016/0198-0149(85)90034-2, 1985. a
Langbehn, T. J., Aksnes, D. L., Kaartvedt, S., Fiksen, Ø., Ljungström, G., and Jørgensen, C.: Poleward distribution of mesopelagic fishes is constrained by seasonality in light, Global Ecology and Biogeography, 31, 546–561, https://doi.org/10.1111/geb.13446, 2022. a, b
Lin, H.-Y. and Costello, M. J.: Body size and trophic level increase with latitude, and decrease in the deep-sea and Antarctica, for marine fish species, PeerJ, 11, e15880, https://doi.org/10.7717/peerj.15880, 2023. a
Liu, R.: Progress of marine biodiversity studies in China seas, Biodiversity Science, 19, 614, https://doi.org/10.17520/biods.2022526, 2011. a
Ljungström, G., Langbehn, T. J., and Jørgensen, C.: Light and energetics at seasonal extremes limit poleward range shifts, Nat. Clim. Change, 11, 530–536, 2021. a, b
Loisel, H., Nicolas, J.-M., Deschamps, P.-Y., and Frouin, R.: Seasonal and inter-annual variability of particulate organic matter in the global ocean, Geophys. Res. Lett., 29, 49–1, https://doi.org/10.1029/2002GL015948, 2002. a
Longhurst, A.: Ecological Geography of the Sea, Academic Press, https://doi.org/10.1016/B978-0-12-455521-1.X5000-1, 2007. a
Lourenço, S. and Jany, M.: Zooplankton Diel Vertical Migration impact on particulate matter flux in the Atlantic, Ph.D. thesis, WASCAL, 2021. a
Luo, J., Ortner, P. B., Forcucci, D., and Cummings, S. R.: Diel vertical migration of zooplankton and mesopelagic fish in the Arabian Sea, Deep-Sea Res. Pt. II, 47, 1451–1473, https://doi.org/10.1016/S0967-0645(99)00150-2, 2000. a
Lusher, A. L., O'Donnell, C., Officer, R., and O'Connor, I.: Microplastic interactions with North Atlantic mesopelagic fish, ICES Journal of marine science, 73, 1214–1225, https://doi.org/10.1093/ICESJMS/FSV241, 2016. a
Lutz, M. J., Caldeira, K., Dunbar, R. B., and Behrenfeld, M. J.: Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean, J. Geophys. Res.-Oceans, 112, https://doi.org/10.1029/2006JC003706, 2007. a, b
Lynch-Stieglitz, J., Vollmer, T. D., Valley, S. G., Blackmon, E., Gu, S., and Marchitto, T. M.: A diminished North Atlantic nutrient stream during Younger Dryas climate reversal, Science, 384, 693–696, https://doi.org/10.1126/science.adi5543, 2024. a
Marohn, L., Schaber, M., Freese, M., Pohlmann, J.-D., Wysujack, K., Czudaj, S., Blancke, T., and Hanel, R.: Distribution and diel vertical migration of mesopelagic fishes in the Southern Sargasso Sea – observations through hydroacoustics and stratified catches, Marine Biodiversity, 51, 1–24, https://doi.org/10.1007/s12526-021-01216-6, 2021. a
Martin, A.: Phytoplankton patchiness: the role of lateral stirring and mixing, Prog. Oceanogr., 57, 125–174, https://doi.org/10.1016/S0079-6611(03)00085-5, 2003. a
Nakao, L. T. H., Krueger, C. P., and Bleninger, T.: Benchmarking for using an acoustic Doppler current profiler for bathymetric survey, Environmental Monitoring and Assessment, 193, 356, https://doi.org/10.1007/s10661-021-09073-3, 2021. a
Nelson, N. B. and Siegel, D. A.: The global distribution and dynamics of chromophoric dissolved organic matter, Annual review of marine science, 5, 447–476, https://doi.org/10.1146/annurev-marine-120710-100751, 2013. a
Norheim, E., Klevjer, T. A., and Aksnes, D. L.: Evidence for light-controlled migration amplitude of a sound scattering layer in the Norwegian Sea, Marine Ecology Progress Series, 551, 45–52, https://doi.org/10.3354/meps11731, 2016. a
Owen, R. W.: Fronts and eddies in the sea: mechanisms, interactions and biological effects, Analysis of marine ecosystems, 98, 3–5, 1981. a
Parra, D., Valverde, L., Pino, F. J., and Patel, M. K.: A review on the role, cost and value of hydrogen energy systems for deep decarbonisation, Renewable and Sustainable Energy Reviews, 101, 279–294, https://doi.org/10.1016/j.rser.2018.11.010, 2019. a
Petit, F.: Developement and exploitation of new approaches for observation of phytoplankton community composition from BGC-Argo floats in open ocean, Ph.D. thesis, Sorbonne Université, 2023. a
Petrusevich, V. Y., Dmitrenko, I. A., Niemi, A., Kirillov, S. A., Kamula, C. M., Kuzyk, Z. Z. A., Barber, D. G., and Ehn, J. K.: Impact of tidal dynamics on diel vertical migration of zooplankton in Hudson Bay, Ocean Sci., 16, 337–353, https://doi.org/10.5194/os-16-337-2020, 2020. a
Powell, J. R. and Ohman, M. D.: Changes in zooplankton habitat, behavior, and acoustic scattering characteristics across glider-resolved fronts in the Southern California Current System, Prog. Oceanogr., 134, 77–92, https://doi.org/10.1016/j.pocean.2014.12.011, 2015. a, b
Puerta, P., Johnson, C., Carreiro-Silva, M., Henry, L.-A., Kenchington, E., Morato, T., Kazanidis, G., Rueda, J. L., Urra, J., Ross, S., Wei, C.-L., González-Irusta, J. M., Arnaud-Haond, S., and Orejas, C.: Influence of water masses on the biodiversity and biogeography of deep-sea benthic ecosystems in the North Atlantic, Front. Marine Sci., 7, 239, https://doi.org/10.3389/fmars.2020.00239, 2020. a
Ramirez-Llodra, E., Brandt, A., Danovaro, R., De Mol, B., Escobar, E., German, C. R., Levin, L. A., Martinez Arbizu, P., Menot, L., Buhl-Mortensen, P., Narayanaswamy, B. E., Smith, C. R., Tittensor, D. P., Tyler, P. A., Vanreusel, A., and Vecchione, M.: Deep, diverse and definitely different: unique attributes of the world's largest ecosystem, Biogeosciences, 7, 2851–2899, https://doi.org/10.5194/bg-7-2851-2010, 2010. a
Rembauville, M., Briggs, N., Ardyna, M., Uitz, J., Catala, P., Penkerc'h, C., Poteau, A., Claustre, H., and Blain, S.: Plankton assemblage estimated with BGC-Argo floats in the Southern Ocean: Implications for seasonal successions and particle export, J. Geophys. Res.-Oceans, 122, 8278–8292, https://doi.org/10.1002/2017jc013067, 2017. a, b
Robinson, C., Steinberg, D. K., Anderson, T. R., Arístegui, J., Carlson, C. A., Frost, J. R., Ghiglione, J.-F., Hernández-León, S., Jackson, G. A., Koppelmann, R., Quéguiner, B., Ragueneau, O., Rassoulzadegan, F., Robison, B. H., Tamburini, C., Tanaka, T., Wishner, K. F., and Zhang, J.: Mesopelagic zone ecology and biogeochemistry–a synthesis, Deep-Sea Res. Pt. II, 57, 1504–1518, https://doi.org/10.1016/j.dsr2.2010.02.018, 2010. a, b
Sallée, J.-B., Pellichero, V., Akhoudas, C., Pauthenet, E., Vignes, L., Schmidtko, S., Garabato, A. N., Sutherland, P., and Kuusela, M.: Summertime increases in upper-ocean stratification and mixed-layer depth, Nature, 591, 592–598, https://doi.org/10.1038/s41586-021-03303-x, 2021. a
Sameoto, D.: Influence of the biological and physical environment on the vertical distribution of mesozooplankton and micronekton in the eastern tropical Pacific, Marine Biology, 93, 263–279, https://doi.org/10.1016/j.dsr2.2010.02.018, 1986. a
Sarant, L.: Little mesopelagic fish have big carbon capture impact, Nature Middle East, https://api.semanticscholar.org/CorpusID:87615417 (last access: 19 June 2025), 2014. a
Saupe, E. E., Myers, C. E., Townsend Peterson, A., Soberón, J., Singarayer, J., Valdes, P., and Qiao, H.: Spatio-temporal climate change contributes to latitudinal diversity gradients, Nat. Ecol. Evol., 3, 1419–1429, https://doi.org/10.1038/s41559-019-0962-7, 2019. a
Sikder, M. N. A., Abdullah Al, M., Xu, G., Hu, G., and Xu, H.: Spatial variations in trophic-functional patterns of periphytic ciliates and indications to water quality in coastal waters of the Yellow Sea, Environmental Science and Pollution Research, 26, 2592–2602, https://doi.org/10.1007/s11356-018-3744-x, 2019. a
Sorochan, K., Plourde, S., and Johnson, C.: Near-bottom aggregations of Calanus spp. copepods in the southern Gulf of St. Lawrence in summer: significance for North Atlantic right whale foraging, ICES Journal of Marine Science, 80, 787–802, https://doi.org/10.1093/icesjms/fsad003, 2023. a
Sutton, T.: Vertical ecology of the pelagic ocean: classical patterns and new perspectives, Journal of fish biology, 83, 1508–1527, https://doi.org/10.1111/jfb.12263, 2013. a
Sutton, T. T., Clark, M. R., Dunn, D. C., Halpin, P. N., Rogers, A. D., Guinotte, J., Bograd, S. J., Angel, M. V., Perez, J. A. A., Wishner, K., Haedrich, R. L., Lindsay, D. J., Drazen, J. C., Vereshchaka, A., Piatkowski, U., Morato, T., Błachowiak-Samołyk, K., Robison, B. H., Gjerde, K. M., Pierrot-Bults, A., and Heino, M.: A global biogeographic classification of the mesopelagic zone, Deep-Sea Res. Pt. I, 126, 85–102, https://doi.org/10.1016/j.dsr.2017.05.006, 2017. a
Tang, W.: A long-term and high-resolution global gridded photosynthetically active radiation product (1984–2018), National Tibetan Plateau/Third Pole Environment Data Center [data set], https://doi.org/10.11888/RemoteSen.tpdc.271909, 2021. a
Tang, W., Qin, J., Yang, K., Jiang, Y., and Pan, W.: Mapping long-term and high-resolution global gridded photosynthetically active radiation using the ISCCP H-series cloud product and reanalysis data, Earth Syst. Sci. Data, 14, 2007–2019, https://doi.org/10.5194/essd-14-2007-2022, 2022. a
Trueman, C., Johnston, G., O'hea, B., and MacKenzie, K.: Trophic interactions of fish communities at midwater depths enhance long-term carbon storage and benthic production on continental slopes, P. Roy. Soc. B, 281, 20140669, https://doi.org/10.1098/rspb.2014.0669, 2014. a
Underwood, M. J., García-Seoane, E., Klevjer, T. A., Macaulay, G. J., and Melle, W.: An acoustic method to observe the distribution and behaviour of mesopelagic organisms in front of a trawl, Deep-Sea Res. Pt. II, 180, 104873, https://doi.org/10.1016/j.dsr2.2020.104873, 2020. a, b
Vedenin, A., Musaeva, E., and Vereshchaka, A.: Three-dimensional distribution of mesoplankton assemblages in the Central Atlantic, Global Ecology and Biogeography, 31, 1345–1365, https://doi.org/10.1111/geb.13509, 2022. a
Wang, B. and Fennel, K.: Biogeochemical-Argo data suggest significant contributions of small particles to the vertical carbon flux in the subpolar North Atlantic, Limnol. Oceanogr., 67, 2405–2417, https://doi.org/10.1002/lno.12209, 2022. a
Woodd-Walker, R. S., Ward, P., and Clarke, A.: Large-scale patterns in diversity and community structure of surface water copepods from the Atlantic Ocean, Marine Ecology Progress Series, 236, 189–203, https://doi.org/10.3354/meps236189, 2002. a, b
Xing, X., Wells, M. L., Chen, S., Lin, S., and Chai, F.: Enhanced winter carbon export observed by BGC-Argo in the Northwest Pacific Ocean, Geophys. Res. Lett., 47, e2020GL089847, https://doi.org/10.1029/2020GL089847, 2020. a
Yin, Z., Zhang, X., Wang, S., and Xu, Y.: Spatiotemporal variation of dissolved oxygen concentration in the northern Gulf of Mexico during the period of 1992–2017, Marine Pollution Bulletin, 198, 115771, https://doi.org/10.1016/j.marpolbul.2023.115771, 2024. a, b