the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Soil signals of key mechanisms driving greater protection of organic carbon under aspen compared to spruce forests in a North American montane ecosystem
Lena Wang
Sharon A. Billings
Daniel R. Hirmas
Keira Johnson
Devon Kerins
Julio Pachon
Curtis Beutler
Karla M. Jarecke
Vaishnavi Varikuti
Micah Unruh
Hoori Ajami
Holly Barnard
Alejandro N. Flores
Kenneth Williams
Pamela L. Sullivan
Soil organic carbon (SOC) is often retained more effectively in aspen-dominated forests compared to coniferous forests in North America, yet the reasons why are unclear. A potential driver could be differences in SOC protection mechanisms. Over decades to centuries, chemical (e.g., mineral association) and physical (e.g., aggregation) processes can work to preserve SOC stocks, which can vary across cover types. To investigate this hypothesis, we evaluate controls on SOC concentrations in the Coal Creek watershed (CO, USA), a montane ecosystem dominated by quaking aspen and Engelmann spruce and underlain by granite and sandstone. We examined a combination of biological, chemical, physical, and environmental conditions to evaluate potential abiotic and biotic mechanisms of SOC preservation at multiple depths. As expected, we observed greater SOC concentrations under aspen compared to spruce. Growing-season soil moisture, temperature, and CO2 and O2 varied with slope position and aspect, and thus forest cover type. Dissolved organic carbon (DOC) was lower under aspen compared to spruce. Exo-enzyme data indicate that aspen soil microbes likely access more organically bound resources; consistent with this, soil organic N exhibited higher δ15N values, hinting at a greater degree of organic matter processing. Finally, aspen soils exhibited greater root abundance, and aspen mineral soils revealed smaller mean aggregate diameters compared to conifer sites. Our data suggest enhanced biotic activities in aspen-dominated forest soils that promote both chemical and physical protection of SOC in aspen- relative to spruce-dominated forests, which may have implications for DOC export.
- Article
(3603 KB) - Full-text XML
-
Supplement
(1818 KB) - BibTeX
- EndNote
The distribution and composition of temperate montane forests are changing (Alexander, 1987; Anderegg et al., 2013), driven by increasing air temperature, earlier snowmelt, earlier onset and extent of the growing season (Godsey et al., 2014; Mote et al., 2018; Rhoades et al., 2018), and increasing frequency and intensity of disturbance (e.g., drought, fire, logging, and insect infestation; Canelles et al., 2021). For example, aspen stands have lost substantial live density and basal area to Engelmann spruce, sub-alpine fir, and Douglas Fir since 1964, with an increasing rate of decline since 1994 (Alexander, 1987; Coop et al., 2014). Changes in montane forest cover can directly impact soil organic carbon (SOC) stability. Given that SOC influences the availability of nutrients, soil stability, ecosystem water fluxes, and biosphere–atmosphere exchange of greenhouse gases (Jackson et al., 2017), and that global SOC reservoirs represent far more C than plant biomass and the atmosphere (Scharlemann et al., 2014), unraveling drivers of SOC stability remains an important research goal (Billings et al., 2021). Between paired aspen and conifer stands at numerous sites throughout North America, SOC pools differ substantially (review in Laganière et al., 2017). Studies consistently show that C under conifers is more readily destabilized than under aspen (Woldeselassie et al., 2012; Laganiere et al., 2013; Boča et al., 2020; Román Dobarco et al., 2021). Further, SOC pools in aspen-dominated environments tend to be composed of larger stocks of mineral-associated organic C (MAOC), which is a relatively stable SOC fraction, than those under conifers (66 % compared to 48 % MAOC to SOC, respectively; Román Dobarco and Van Miegroet, 2014; Román Dobarco et al., 2021). Yet, the mechanisms driving such differences in SOC stability under aspen and conifer remain elusive.
Examining soil physical attributes and how they can differ with plant cover type may help us understand differences in MAOC fate in aspen vs. conifer forests. For example, soil aggregation is a key process promoting SOC protection in many soil types (Blanco-Canqui and Lal, 2004). Soil aggregation refers to the clustering or binding of soil particles into larger units. This process is promoted by interactions among colloidal material and binding compounds (microaggregates; Six et al., 2004; Blanco-Canqui and Lal, 2004; Weil and Brady, 2017; Araya and Ghezzehei, 2019) and among particulate organic C (POC; Cotrufo et al., 2019) and clay minerals or clay-sized particles (Six et al, 2000). The collapse and formation of aggregates influence the protection of SOC. For example, the breakdown of macroaggregates into microaggregates often leads to the release of dissolved organic C (DOC; Cincotta et al., 2019), some of which can undergo microbial uptake and mineralization to CO2. In contrast, aggregate formation can limit soil microbial access to SOC on aggregates' interiors, helping to shield it from exo-enzymatic attack (Jastrow, 1996; Six et al., 2000; Woolf and Lehmann, 2019).
Multiple mechanisms may drive differences in soil aggregation across aspen and conifer soils. First, soils beneath conifers are often more acidic (Popenoe et al., 1992; Buck and St. Clair, 2012) and thus may promote a greater abundance of relatively small aggregates, given that increases in soil solution [H+] can weaken soil aggregation processes (Stătescu et al., 2013). Second, differences in rooting abundance among aspen and conifers may drive differences in aggregate formation across these cover types. Aspen tends to produce shallow roots that generally extend to ∼ 30 cm deep (Shepperd et al., 2006), while conifers tend to develop both lateral and tap roots, the latter of which can extend relatively deep into the soil and bedrock (Mauer and Palátová, 2012). Spruce tends to exhibit lower fine-root biomass compared to aspen (Mekontchou et al., 2020). Fine roots may promote aggregate formation through enmeshment processes, while coarse roots may promote aggregate collapse because of roots perforating aggregates (Bronick and Lal, 2005). Differences in soil moisture between aspen and conifers driven by differences in aspect, foliar cover, and transpiration rates (Buck and St. Clair, 2012) may also influence aggregate stability, as rapid changes in soil moisture can cause aggregates to burst while a gradual increase in moisture can stabilize aggregates (Amézketa, 1999). Combined, these concurrent and competing processes may drive differences in soil aggregation between aspen- and conifer-dominated soils in ways that are difficult to predict due to complex and non-linear interactions, and require the synthesis of findings across biological and pedological disciplines to understand.
Soil moisture and temperature not only influence physical aggregation processes, and thus the protection and preservation of SOC, but also the degree to which microbes transform SOC into CO2 or alter the transport of organic C pools to depth. Where soil moisture is higher, greater transport of organic C pools into the subsurface may be feasible, potentially increasing the amount of organic matter sorbed to minerals at greater depths (Mikutta et al., 2019). Conversely, DOC leaching may increase, and subsequent DOC export could reduce SOC concentrations (Roulet and Moore, 2006; Monteith et al., 2007). Soil temperature also may drive differences in SOC transformations across aspen and conifer sites, given that aspect exerts strong control on aspen distribution. Soil temperatures tend to be warmer under the sunnier, aspen-dominated stands compared to conifer stands (Buck and St. Clair, 2012). In a temperature-limited montane system, warmer temperatures under aspen stands may increase microbial metabolic activity and turnover, and thus accelerate microbial necromass formation, a process linked to greater stocks of relatively persistent SOC (Liang et al., 2019), perhaps due to necromass-promoting aggregate formation and stabilization (Sae-Tun et al., 2022). Thus, understanding soil water movement and solute transport – traditionally studied by hydrologists and soil biogeochemists – along with biological and soil formation processes is key to explaining patterns of SOC transformation.
Finally, differences in the chemical composition of aspen and conifer biomass and their root exudates may explain differences in MAOC stocks between the two stand types (Boča et al., 2020). For example, aspen litter tends to exhibit lower lignin concentrations than coniferous litterfall (Moore et al., 2006). The Microbial Efficiency – Matrix Stabilization (MEMS) framework (Cotrufo et al., 2013) would suggest this more labile plant material may be easier for soil microbes to assimilate and transform into microbial necromass, which can become more physically or chemically protected through aggregation and chemical bonding (Kleber et al., 2007; von Lützow et al., 2008; Cotrufo et al., 2013) and lead to relatively more persistent stocks of SOC (Liang et al., 2019; Buckeridge et al., 2022). Differences in microbial activities between aspen and conifer may further be exacerbated by differences in root exudation between these species. For example, Norway spruce can exhibit lower exudation rates than silver birch (Sadnes et al., 2005), and deciduous trees appear to experience greater exudation rates than pines (Wang et al., 2021). Though many studies explore the biotic, chemical, physical, and hydrologic processes that can influence SOC transformations and preservation, these processes are rarely examined at the same time. Thus, it remains unclear why conifer-dominated forests consistently harbor smaller amounts of SOC, and why aspen-dominated forests exhibit greater SOC stabilization.
Here, we use a holistic, critical-zone approach – integrating physical, chemical, and biological processes from the vegetation canopy to bedrock (Chorover et al., 2007) – drawing on data from biology, hydrology, pedology, and other disciplines to understand SOC dynamics and drivers. We explore a suite of abiotic and biotic factors as they relate to SOC pool sizes across two forest cover types at Coal Creek, a watershed in central Colorado, USA, dominated by Engelmann spruce (Picea engelmannii) on the north-facing hillslopes, and aspen (Populus tremuloides) on the south-facing hillslopes. Coal Creek has experienced relatively high variability in stream water DOC concentrations in recent years (2005–2019; Leonard et al., 2022). The mysterious, almost tripling of stream DOC concentrations in some years (2018–2019) may indicate recent shifts in upslope biogeochemical processes such as greater forest stress associated with climate change (Leonard et al., 2022) and subsequent changes in hydrologic flow paths (Zhi et al., 2020; Kerins and Li, 2023) that influence C transport from soil profiles to stream water. We test the hypothesis that higher soil organic carbon (SOC) stocks commonly observed under aspen stands – relative to conifer-dominated soils – are driven by enhanced microbial activity in aspen soils. We further hypothesize that aspen-dominated soils contain more stable microaggregates than spruce soils, driven by higher microbial activity and associated increases in necromass production. Differences in rooting strategies and ecohydrologic factors (e.g., evapotranspiration, soil moisture) between aspen and spruce likely exert secondary controls on C stability. Finally, we hypothesize that the proliferation of fine roots in aspen soils is associated with smaller water-stable aggregates, whereas the deeper, coarser roots in spruce soils promote vertical movement of water and dissolved C down through the soil profile, potentially leading to greater DOC export to streams compared to aspen systems.
To test these hypotheses, we quantified multiple metrics describing basic abiotic conditions, SOC pools, soil microbial activities, soil aggregate-size distributions, and rooting distributions on five hillslopes dominated by either spruce or aspen, underlain by two contrasting lithologies and located at two hillslope positions (i.e., backslope and footslope). We aim to clarify some of the mechanisms governing aspen- and conifer-dominated forest soil microbial activity, soil aggregation, and soil moisture dynamics and their impact on SOC protection and potential DOC transport into surface water, illuminating the possible trajectories of SOC and DOC in rapidly changing, montane forest watersheds.
Coal Creek (53 km2) is a high-elevation (2715 m), headwater tributary of the Upper Colorado River Basin located in the Colorado Rocky Mountains near the town of Crested Butte (Fig. 1). Coal Creek is a sub-catchment of the larger East River watershed (300 km2) and falls within the research domains of the U.S. Department of Energy-funded Watershed Function Science Focus Area and Rocky Mountain Biological Laboratory (RMBL). The watershed is seasonally snow-covered from November through June. The area has a continental, subarctic climate with long, cold winters and short, cool summers. The mean annual temperature is 0.9 °C and the mean annual precipitation is 670 mm (Carroll et al., 2018), with approximately 60 % falling as snow between October and May. This area has been warming since the 1980s and the fraction of snow has been decreasing at roughly 1 % yr−1 (Zhi et al., 2020). Due to these warming temperatures, the growing season in Crested Butte appears to be extending (Wadgymar et al., 2018).
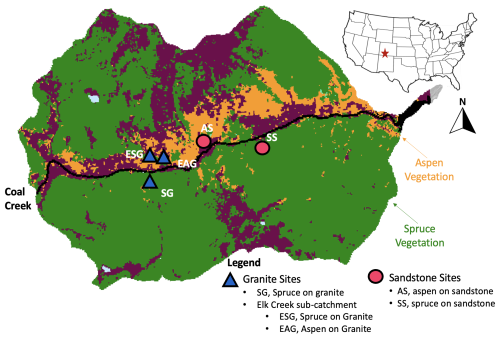
Figure 1A map of the Coal Creek catchment. Colors represent land cover types, where aspen (orange) are dominantly at lower south-facing slopes while conifer (green) are on both north- and south-facing slopes. Shapes represent lithology type, where granite sites (blue triangles) are in the western part of the catchment and sandstone sites (pink circles) are in the eastern part of the catchment. AS is aspen sandstone, and SS is spruce sandstone. ESG and EAG are spruce granite and aspen granite, respectively. They are in Elk Creek, a sub-catchment of Coal Creek. While ESG is on a dominantly south-facing slope, it is north facing within the Elk Creek catchment. SG is also a spruce granite site. Note that all sites reside at contrasting hillslope positions: backslope = AS and SG, and footslopes = SS, ESG, and EAG.
The geology of Coal Creek is underlain by sandstone, siltstone, shale, and coal units from the Mesa Verde Formation, variegated claystone and shale from the Wasatch Formation, and some intrusive granite diorite, granite, quartz, and monzonite that are Middle Tertiary aged (Gaskill et al., 1991). Soils are predominantly mapped as carbonate-free Alfisols, Mollisols, and Inceptisols (Soil Survey Staff, 2023a).
Spruce, aspen, and alpine meadows can be found in the Coal Creek watershed. North-facing slopes are dominated by Engelmann spruce, while aspen and Engelmann spruce can be found on south-facing slopes. We focused on five sites during this study. Three of our sites lie within the main drainage of Coal Creek, including two spruce sites (spruce sandstone, SS; spruce granite, SG) and one aspen (aspen sandstone (AS). The last two sites are located in Elk Creek, a sub-catchment of Coal Creek, which includes one spruce site and one aspen site, both underlain by granite (Elk spruce granite, ESG, and Elk aspen granite, EAG). While ESG is on a dominantly south-facing slope, it is north facing within the Elk Creek catchment.
To quantify the impact of aspen vs. conifer land cover on soil organic C dynamics at Coal Creek, we dug two pits roughly 1 m deep at all five sites. The first series of pits were dug in the summer 2020 and 2021 (Table 1). The second series of pits were dug in the summer of 2022. Aspen was dominant at two sites (AS and EAG), while spruce was dominant at three sites (SS, ESG, and SG). Because aspen- and conifer-dominated forests in this region tend to occur on hillslopes of contrasting aspects, it was not possible to isolate land cover from aspect effects (e.g., temperature, radiation). While the sites were selected based on their land cover, other key ecosystem features, underlying lithology (either granite or sandstone), and hillslope position (either backslopes or footslopes) also differed across the sites (Fig. 1). We address these site features as potential sources of variation in our response variables in the discussion.
Soil pits were described following Schoeneberger et al. (2012), then each pit face was photographed with a high-resolution, digital single-lens reflex camera (D5600, Nikon, Minato City, Tokyo, Japan) to quantify rooting depth distributions following Billings et al. (2018). Bulk soil samples were collected by depth every 10 cm for the first set of pits (2020–2021), and by horizon for the second set of pits (2022). Samples were then immediately stored in a refrigerator or freezer (DOC, microbial biomass C, exo-enzyme assays, nitrate) until they could be ground, sieved to 2 mm, and analyzed. Twice in the summer of 2022 (late June and mid-August), soil was collected from three auger-sampling locations within ∼ 100 m of each pit to characterize soil chemistry (i.e., SOC, DOC, pH). Soils were augured at 10 cm intervals to 110 cm (or deepest possible depth), and samples were stored in coolers with ice packs in the field, transported back to the lab, and stored at 4 °C (most analyses) or frozen (SOC, DOC).
3.1 Measuring soil organic C and nitrogen dynamics
We assessed SOC and soil organic nitrogen (SON) concentrations and stocks, and the likelihood of SOC and SON degradation by microbes, by analyzing bulk soil samples at 10 cm intervals. We determined SOC and SON on subsamples (∼ 75 mg) via an elemental analyzer (vario MACRO cube, Elementar, Ronkonkoma, NY). We used SOC and SON concentration measurements to calculate each subsample's C:N ratio. To determine stocks of SOC in each horizon, we multiplied SOC concentrations by soil bulk density obtained in each horizon. Bulk density was measured using a three-dimensional laser scanner (3D Scanner Ultra HD, NextEngine, Inc., Santa Monica, CA) following Rossi et al. (2008).
We measured extractable DOC to estimate organic C that can be relatively easily mobilized and transported out of the soil profiles; note that this differs from DOC measured in soil porewater using lysimeters, and instead represents a salt-extractable pool. We analyzed soil samples at 10 cm intervals to auger refusal collected at each site during the growing season. Soil samples were extracted within 3 months of collection date. A total of 7.5 g of soil at field moisture was extracted with 30 mL of simulated rainwater (Laegdsmand et al., 1999). The extracted soil solutions were comprised of 47.9 µM NaNO3, 4.69 µM KCl, 23.81 µM CaCl2 × 2 H2O, 12.09 µM MgSO4 × 7 H2O, and 18.24 µM (NH4)2SO4, and adjusted to a pH of 4.2 ± 0.5 using HCl. Samples were placed on a shaker table for 30 min and centrifuged at 80 Hz (s−1) for 15 min. Samples were filtered through 0.45 µm nylon syringe filters and 50 mL acid-washed syringes. Filtered samples were stored in 10 mL centrifuge tubes, frozen and shipped overnight in a cooler with dry ice to the University of Kansas. DOC was analyzed from the thawed samples using a violet-pink Mn (III) pyrophosphate solution and a microplate reader (Biotek, UT).
To better understand the potential for microbial activity in these soils, we quantified microbial biomass C by horizon from pits dug in the summer of 2022 (Brookes et al., 1985). We exposed 5 g of each soil sample to chloroform for 24 h. To these fumigated sub-samples and to 5 g of unfumigated sub-samples we added 20 mL of 0.5 M K2SO4 and shook for 30–40 min at 220 rpm. These samples were filtered through a 0.45 µm polyethersulfone filter and their DOC concentration was determined via colorimetry (Bartlett and Ross, 1988) on a Synergy HT microplate reader (Agilent, USA). To assess the degree to which soil microbial communities were generating exo-enzymes that catalyze soil organic matter decay and thus can provide assimilable C- and N-rich compounds, we quantified potential activity rates of two such enzymes. We measured activity of β-glucosidase and N-acetyl-β-D-glucosaminidase, herein referred to as BGase and NAGase, which are linked to microbial C (BGase), and N and C (NAGase) acquisition (Sinsabaugh and Moorhead, 1994; Allison, 2014; Stone et al., 2014), using 4-methylumbelliferyl β-D-glucopyranoside (for BGase) and 4-methylumbelliferyl N-acetyl-β-D-glucosaminide (for NAGase) fluorescent tags. These tags were added to slurries made from approximately 1 g of soil and pH-adjusted 50 mM sodium acetate. We pipetted the blended sample into the desired substrate and incubated all plates at 25 °C for 18 h. Fluorescence from a Synergy HT plate reader (Agilent, USA) was used as a proxy for each enzyme's capacity to cleave monomers from the respective molecules undergoing decay (DeForest, 2009; German et al., 2011).
We quantified salt-extractable because of its importance as a biotically available form of N, and also because of its status as a readily leachable ion. As such, it can serve as an indicator of each soil's capacity to undergo elemental loss in surface soil with hydrologic fluxes, and provides a valuable point of comparison to DOC values. We extracted ∼ 10 g (fresh weight) of each soil sample with 0.5 M K2SO4 and repeated the shaking and filtering steps described above for microbial biomass C. Extracts were analyzed for (Synergy HT, Agilent, USA) using Shand et al. (2008), a microplate-based approach that relies on hydrazine sulfate and sulfanilamide to generate a color intensity directly related to concentration.
We also quantified soil organic matter δ15N, given these signatures' value as an indicator of the degree to which soil microbes have processed soil organic matter (Nadelhoffer and Fry 1988; Billings and Richter 2006). Sub-samples of each soil were dried, ground to fine powder, and weighed into a tin capsule for analysis. Values of δ15N were obtained at the Kansas State University Stable Isotope Lab, where an Elementar EA vario PYRO cube linked to an Elementar GeovisION isotope ratio mass spectrometer determine N concentration and δ15N, respectively.
3.2 Measuring soil chemical and physical properties
To better assess possible differences in the chemical and physical controls on SOC stability, we also measured pH, effective cation exchange capacity (ECEC), soil texture, and wet aggregate size distribution (ASD). We focused on pH as it is known to strongly control microbial communities and MAOC (Kleber et al., 2015). The soil pH was determined in a 1:1 H2O soil slurry (Soil Survey Staff, 2023b). We focused on ECEC because ECEC has a high positive correlation with SOC, clay content, and aluminum and iron oxides (Solly et al., 2020), which are highly correlated with the formation of MAOC (Kleber et al., 2015). ECEC was determined by summing Ca, Mg, and K extracted using a Mehlich-3 solution (Culman, 2019). Mehlich-3 extraction was used instead of an ammonium acetate extraction because the soil samples had a pH of < 7.5 and there is very little to no calcium carbonate. In these conditions Mehlich-3 and ammonium acetate extractions yield similar ECEC values (Rutter et al., 2022).
We examined soil texture at each pit for several reasons. First, the total amount of clay is important to MAOC, and second, texture is known to impact the distribution and connectivity of pores. This connectivity influences how easily oxygen can diffuse into a soil profile and thus processes such as microbial respiration (Schjønning et al., 1999; Moldrup et al., 2013), and further regulates water and solute transport down-profile. Soil texture was analyzed on pit samples collected from 2020–2021 using a laser diffraction unit (Bettersizer S3, Bettersize Instruments, Dandong, Liaoning, China). Five grams of soil was sieved to 2 mm, and organic matter was removed by treating samples with 30 % hydrogen peroxide. A total of 10 mL of 10 % sodium hexametaphosphate (HMP) was added to the solution to prevent flocculation. The soil solution was pipetted into the Bettersizer until obscuration levels were between 14–20. We set clay–silt and silt–sand boundaries to be 6.6 and 60.33 µm, respectively (Makó et al., 2017).
We quantified aggregate size distributions as one key metric of soil structure. Aggregate-size distributions were measured on each soil horizon following Nimmo and Perkins (2002). Briefly, around 25 g of the largest air-dried aggregates were fully saturated with a Dickson apparatus (Dickson et al., 1991), and placed on a Yoder device where sieves (#4, 10, 17, 70) and soil samples were raised and lowered in the water 2.8 cm per stroke at a rate of 36 strokes per minute for 10 min. Following this agitation in water, the sieves with their respective aggregates were placed in a drying oven at 105 °C for 12 h. The soil material remaining on each sieve was dispersed with 200 mL of 2 g L−1 HMP, mixed for 10 min, passed through the sieve again, and oven dried at 105 °C for 2 h. Weights were recorded and mass fractions of water-stable aggregates were then calculated. Sieves divided aggregates into five classes: aggregates > 4.76 mm, aggregates between 2 and 4.76 mm, aggregates between 0.21 and 1 mm, and aggregates less than 0.21 mm. To simplify our analysis, we agglomerated these into three classes following Souza et al. (2023): fine aggregates (< 0.21 mm), intermediate aggregates (0.21–4.76 mm), and coarse aggregates (> 4.76 mm). A weighted geometric mean aggregate diameter (GMD) was calculated for each triplicate using the mass fractions of each aggregate-size class; the mean and standard deviation were calculated from these triplicate values to represent the aggregate diameter of each sample. The GMD values were divided by SOC content and the resulting values were used to characterize the propensity of C to form aggregates.
3.3 Measuring rooting distributions
To determine the relationship between roots and C stability and transport, we measured the fraction of soil volume containing fine and coarse roots throughout the soil profiles using images collected from all 10 pits (e.g., 2020/2021 and 2022). We used ImageJ (Schneider et al., 2012) to overlay each image with a 1 cm × 1 cm grid. We then manually checked each 1 cm × 1 cm grid cell for the presence of a fine root (diameter < 1 mm) or coarse root (diameter > 1 mm) and noted these presence/absence scores for each grid cell. Our focus is the soil volume containing roots and thus directly influenced by roots. As such, only presence/absence and not count data were recorded, and in any cell containing both fine and coarse roots the presence of only the coarse root(s) was recorded given their greater volume (Billings et al., 2018). These measures are thus a conservative measure of direct root influence on soil volumes, derived at the centimeter scale for soil pedons. Centimeter-scale cell presence/absence data were transformed into the fraction of each 1 cm thick layer containing roots.
3.4 Sensor data
Soil sensor arrays were installed in the first set of pits (2020/2021) at the completion of sampling. Sensors were installed at depths of 15, 45, and 110 cm (or deepest depth; Table 1) to monitor soil temperature (°C) and volumetric water content (VWC; EC-5, Meter Group, Pullman, WA), matric potential (kPa; Teros 21, Meter Group, Pullman, WA), O2 concentration (%; IB201806, Apogee Instruments, Logan, UT), and CO2 concentration (ppm; F0275476, Eosense, Dartmouth, Canada). Data were collected every 30 min for moisture, matric potential, and temperature, and hourly for O2 and CO2 given the power requirements. We focus on CO2 and O2 as they are indicators of soil microbial and root biotic activities, including heterotrophic respiration. Microbial activity directly and indirectly affects the formation of MAOC, SOC stabilization, and microaggregation (Dohnalkova et al., 2022). We used sensor data to investigate additional environmental controls on C dynamics. We converted O2 from millivolt readings to % by adding calibrated values to the millivolt value of O2. Each calibrated value was specific to the sensor installed and determined using atmospheric concentrations prior to installation. To focus on the growing season, we selected data from 15 June–29 August, which was 14 d before the first sample was collected (29 June) and ending 14 d after the last sample was collected (15 August). AS reflects 2021 data, SS reflects average daily 2021 and 2022, and ESG, EAG, and SG reflect 2022 data. These differences were because pits were installed with sensors in different years and some of the instrumentation had power outages and other unforeseen issues. We averaged daily temperature and VWC by week, and examined average and standard deviation of the O2 and CO2 over the growing season.
3.5 Data analysis
Spatial replicates controlling for all ecosystem-scale factors were not feasible in this study. Instead, we advance our understanding of SOC stability by examining a more diverse suite of biotic and abiotic ecosystem characteristics than is often the case in SOC-focused work. Our work begins to unravel the complex interactions among cover-type characteristics, soil properties, and hydrologic settings in SOC dynamics. We used Wilcoxon rank sum tests to determine if differences between aspen and spruce concentrations of SOC, DOC, total soil nitrogen and nitrate, and ratios of DOC to SOC were significant. We used linear mixed effects (LME) methods via the R package lme4 (Bates et al., 2015) to assess the influence of vegetation type, depth, and their interaction (N=5) on soil abiotic conditions, various forms of soil nitrogen and C and δ15N, ASD, and root abundances. We tested whether variables were normally distributed using the Shapiro–Wilk test and transformed the data to achieve a normal distribution if they were non-normal. The soil chemical properties of SOC, DOC, DOC:SOC, and ECEC were log transformed, while C:N data were transformed with the function . Root fractions and soil solution pH did not require transformation to meet model assumptions. We assessed whether vegetation type exerted a meaningful influence on the previously mentioned variables by constructing four models. The two simplest models included only vegetation type or depth, both as fixed effects. A third model included those fixed effects additively (e.g., vegetation + depth), and a fourth model included their interaction. We resolved the lack of independence of soil depth within each pedon by incorporating site identifiers as a random effect term in the model. We then tested the normality of the model residuals using the Shapiro–Wilk test. For all models that passed this test, we compared the model fits using analysis of variance and visually examined model residual errors for homogeneity of variance; the best model fit was selected based on the lowest Akaike information criterion following Hauser et al. (2020). We interpret the results of these LME models conservatively, given the low number of replicate sites for each land cover type. We could not perform an LME model on microbial biomass and enzyme data due to the relatively limited number of samples. This limited our ability to include vegetation*depth interactions in those models.
4.1 Soil properties and development
Clay, silt, and sand content at the aspen sites (AS and EAG; Fig. S1 in the Supplement) and one of the conifer sites (ESG) exhibited little variation with depth (average 33.1 % clay and 18.8 % sand), while the two other conifer sites had a greater sand and lower silt and clay content, particularly at depths greater than 25 cm (SS and SG; Fig. S1). Cation exchange capacity was similar among the aspen and conifer sites, with averages of 7.2 ± 4.9 and 8.2 ± 6.8 (meq per 100 g soil), respectively, with elevated values at the surface that declined with depth (Fig. S2).
We were able to access and describe soil profiles to approximately 100 cm (Fig. 2, Table S1 in the Supplement). All sites had weak to moderately strong subangular blocky structure throughout the soil profile, and most sites had weak to moderately strong granular structure in A and upper B horizons. Dendritic tubular pores, interpreted to be abandoned root channels, were present throughout the soil profile of aspen sites, while they were less common in the conifer soil profiles. Aspen sites exhibited faint organic stains and organoargillans (i.e., dark, organic stained clay films) throughout the soil profile, while conifer sites had clay bridges and krotovina throughout the soil profile. The krotovina suggest greater bioturbation under conifer than aspen. Both vegetation types exhibited ferriargillans (i.e., clay coats that include Fe oxides), clay films, and charcoal, although ferriargillans and clay films were more prominent under conifer. Clay bridges, organoargillans, and ferriargillans indicate illuviation. Lithologic discontinuities were identified in SS, ESG, and EAG, indicating colluvial inputs into these footslope pedons.
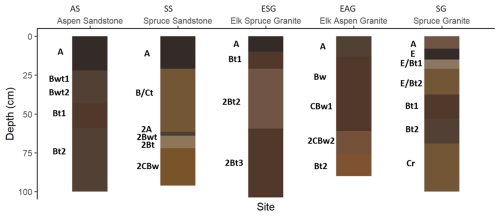
Figure 2Soil profiles described at each site. Horizon colors represent the moist color of the soils as matched to the soil-color or Munsell chart.
Soils at both aspen sites (AS and EAG) are Ustic Haplocryolls with thick, SOC-enriched surface horizons (mollic epipedons) and showing evidence of incipient subsoil development in the form of moderately thick cambic horizons. Soils under conifer sites are Typic Haplocryepts (SS and SG) and Eutric Haplocryalfs (ESG). Although surface horizons under conifer were not as well developed (ochric epipedons), the subsurface showed similar incipient pedogenesis in the form of cambic horizons for SS and SG and greater development in the case of ESG, where an argillic horizon was identified between 19 and 90 cm below the mineral surface.
4.2 Soil abiotic conditions
To understand how soil abiotic conditions are linked to SOC forms and processing pathways, we focused our analysis of soil temperature and moisture during the growing season (June–August; Fig. 3). As expected, soil temperature increased at all sites as the growing season progressed, peaking in mid to late July, with the warmest temperatures observed near the surface and lower variability observed at depth. We also observed that the aspen sites (AS and EAG), which are on south-facing slopes, are warmer than conifer sites with an average surface soil (15 cm deep) temperature of 14.3 ± 1.2 and 10.4 ± 1.0 °C, respectively, during the growing season. Aspen sites were generally drier than conifer. The average volumetric water content in the surface soils (15 cm deep) at aspen sites was 0.15 ± 0.05 and the average volumetric water content at spruce sites was 0.24 ± 0.05 cm3 cm−3.
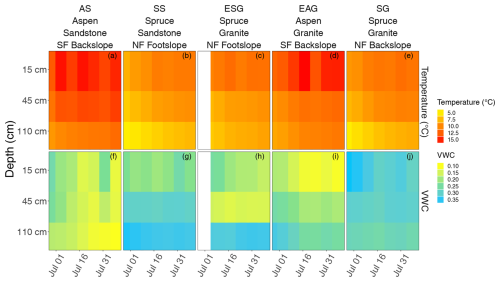
Figure 3Temperature (a–e; °C) and volumetric water content (VWC (cm3 cm−3); f–j) data for aspen sandstone (AS; a, f), spruce sandstone (SS; b, g), spruce granite (ESG; c and h), aspen granite (EAG, d, i), and spruce granite (SG; e, j). AS reflects 2021 data; SS reflects averaged 2021 and 2022; ESG, EAG, and SG reflect 2022 data.
We also examined soil pH. Across the entire soil profile, pH was similar at the aspen and spruce sites (5.6 ± 0.3 and 5.3 ± 0.4, respectively) but their depth trends differed with spruce soils having slightly more acidic pH near the surface compared to the aspen (Fig. S2). This trend reversed at approximately 60 cm, where the aspen soils became slightly more acidic compared to the conifer soils.
4.3 Soil organic C and nitrogen
Across all sites, SOC concentrations ranged from 46.0–62.6 mg g−1 near the surface (5 cm deep) to 4.8 to 29.0 mg g−1 at depth (Fig. 4a). SOC concentrations were generally higher under aspen compared to spruce sites (p < 0.0001; Fig. 4a), but LME models also suggest that the best-fit model included a significant interaction between vegetation and depth (p < 0.001), suggesting that SOC declines with depth for both vegetation types but to a greater extent under spruce compared to aspen. Stocks of SOC for all depth intervals ranged between 0.01 and 1.31 kg m−2 (Fig. 4b); these values did not exhibit consistent declines with depth or clear differences across cover type.
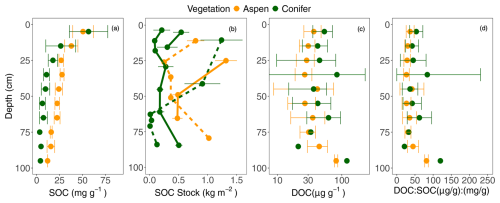
Figure 4(a) Soil organic carbon (SOC) concentrations, (b) SOC stock [by horizon per pit; solid lines indicate sites underlain by granite and dotted lines aspen], (c) dissolved organic carbon (DOC), and (d) the ratio of dissolved organic carbon to soil organic carbon (DOC:SOC) with depth under two different vegetation types, aspen (orange) and spruce (green) at the Coal Creek catchment, Colorado, USA. Values represent mean ± standard deviation.
In contrast to SOC concentrations, DOC was generally higher under the spruce stands compared to aspen. Similar to SOC, a model including a significant interaction between vegetation and depth was the best predictor of DOC values (p < 0.001), likely reflecting variable DOC values at different depths in both vegetation types (Fig. 4c). The DOC:SOC ratio also exhibited a significant interaction between vegetation type and depth (Fig. 4d; p = 0.0007). As with DOC, this significant interaction likely reflects variable ratio values for each cover type across depths.
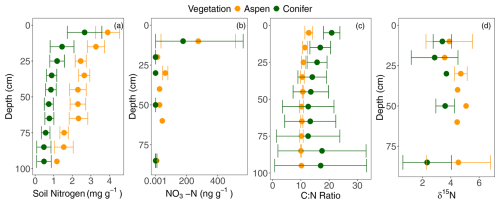
Figure 5(a) Soil nitrogen, (b) soil nitrate, (c) carbon to nitrogen ratio (C:N), and (d) δ15N with depth under two different vegetation types, aspen (orange) and spruce (green), at the Coal Creek catchment, Colorado, USA. Values represent mean ± standard deviation. For each mean and standard deviation, where error bars are not visible the deviation is smaller than the point.
Total soil nitrogen ranged from 0.2 mg g−1 at depth to 4.63 mg g−1 near the surface. A model including an interaction between vegetation type and depth was the best fit (p = 0.003; Fig. 5a). Aspen values were greater than those in spruce-dominated soils at all depths; the significant interaction implies that the decline with depth was greater in spruce soils. Nitrate concentrations averaged 214 ± 323 ng g−1 near the surface and 2.3 ± 3.8 ng g−1 at depth. Nitrate was elevated under the aspen compared to the spruce sites (Fig. 5b), and values under both vegetation types varied with depth. A model that included both depth and vegetation type with no interaction was a meaningfully better predictor of nitrate concentrations than either depth or vegetation alone (p = 0.035), and including a depth–vegetation interaction did not improve model fit. Aspen soil C:N averaged 10.9 ± 1.1 and remained fairly constant with depth (Fig. 5c). The spruce sites showed greater variation with depth with a similar mean value of 19.3 in the top 20 cm but widely variable values at the deepest points, ranging from 4.6 to 28.7 (Fig. 5c). Including the interaction between vegetation type and soil depth improved model fit (p = 0.0008), indicating that C:N varied more with depth in spruce soils than in aspen soils, where values stayed fairly constant. The lowest C:N value, found at depth in one of the spruce forest, suggests that the soil organic matter there has been heavily processed by microbes (Ziegler et al., 2017).
δ15N signatures showed less distinct depth trends compared to the total nitrogen and nitrate, mirroring the relative lack of clear depth trends in C:N. Though variation across sites limited our ability to find statistically significant differences across vegetation types or a significant influence of depth, δ15N of soil organic matter in spruce plots tended to be lower than that of aspen (Fig. 5d), hinting that soil N has undergone more microbial processing (Nadelhoffer and Fry 1988; Billings and Richter 2006) under aspens compared to under conifers. This interpretation is consistent with the mean spruce C:N values being greater than those in aspen forests (Fig. 5c).
4.4 Biotic activity
4.4.1 Roots
The LME models indicate that models including vegetation–depth interactions were the most effective at describing total and coarse root fractions (p < 0.001), with generally greater root abundances in the aspen compared to the spruce (Fig. 6a and c). In contrast, vegetation type offered no additional explanatory power to the depth-dependent fine root abundance (p > 0.05; Fig. 6b), suggesting that the greater total root abundance under aspen was driven more by the coarse root fraction. The difference between aspen and spruce root abundances were continuous with depth for the total root fraction but more punctuated with coarse root fraction. For example, higher coarse root fractions were observed from 30–60 cm and greater than 90 cm for the aspen as compared to the spruce. Interestingly, overall spruce root fractions decreased faster with depth than aspen root fractions. When we standardized DOC with rooting abundance, we found generally greater concentrations of DOC per unit root abundance under spruce soils, particularly with respect to total and fine roots (Fig. 7).
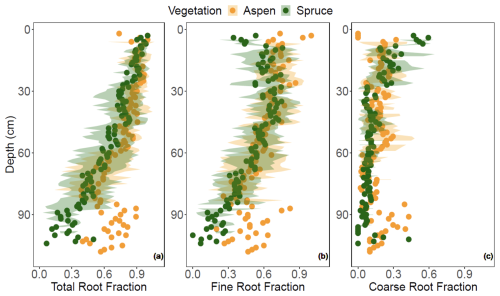
Figure 6(a) Total, (b) fine, and (c) coarse root fractions quantified at 1 cm depth interval under two different vegetation types, aspen (orange) and spruce (green), at the Coal Creek catchment, Colorado, USA. Values represent mean (points) ± standard deviation (shading).
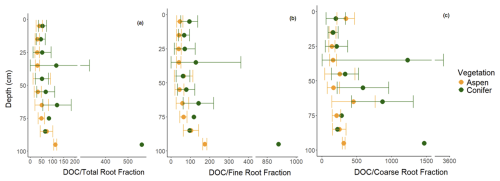
Figure 7DOC divided by mean (a) total, (b) fine, and (c) coarse root fractions every 10 cm under two different vegetation types, aspen (orange) and spruce (green), at the Coal Creek catchment, Colorado, USA. Values represent mean (points) ± standard deviation (bars). Root fractions represent the count of fine (< 1 mm) or coarse (≥ 1 mm) roots in 10 cm depth increments.
4.4.2 Enzyme activity and microbial biomass
Exo-enzyme activity, their ratios, and microbial biomass C decreased from the surface with depth (Fig. 8a–d). Exo-enzyme activity standardized by microbial biomass lacked distinct depth trends (data not shown). Beta values of exponential decay curves fit to these data, merged for each cover type, were larger (more negative) for the spruce sites compared to aspen, indicating steeper declines in exo-enzymatic activities, microbial biomass C, and BGase activity relative to NAGase activity in spruce-dominated forest soils.
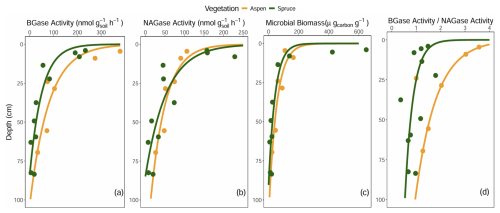
Figure 8Soil (a) β-glucosidase (BGase), (b) β-N-acetyl glucosaminidase (NAGase), (c) microbial biomass, and (d) the ratio of BGase to NAGase at the Coal Creek catchment, Colorado, USA. Each point represents one site and one depth, and curves represent exponential fit of the data, with each curve defined by multiple spruce and aspen sites.
4.4.3 Soil O2 and CO2
We examined soil O2 and CO2 concentrations during the growing season to better understand patterns of respiration (Fig. 9). Soil CO2 concentrations increased with depth across all sites, while O2 concentrations were more variable. Soil O2 concentrations remained relatively stable at aspen sites and at spruce granite sites (AS, EAG, and SG). However, O2 concentrations decreased with depth at the remaining two spruce sites – one sandstone and one granite (SS and ESG).
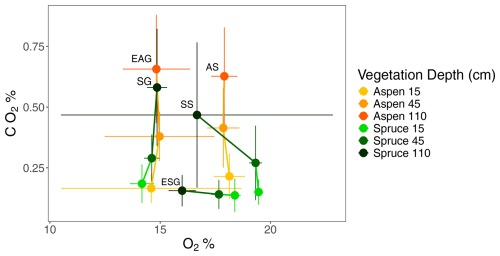
Figure 9Soil gas concentrations of O2 (%) and CO2 (%) at aspen (orange) and spruce (green) sites during the growing season at depths 15 cm (light), 45 cm (medium), and 110 cm (dark) at the Coal Creek catchment, Colorado. Values represent mean (points) ± standard deviation (bars) with lines connecting depths within each profile. The shallowest depth of each site is labeled: AS, aspen sandstone; EAG, Elk aspen granite; ESG, Elk spruce granite; SG, spruce granite; SS, spruce sandstone.
4.5 Soil aggregates
The mean geometric diameter of soil aggregates was generally smaller under aspen compared to spruce (Fig. 10a). Aspen aggregates tended to be finer, with fewer intermediate and coarse aggregates compared to the spruce soil (Fig. 10b–d). LME models indicated that vegetation–depth interactions were the most meaningful in driving all three aggregate size classes (p < 0.001). To explore the propensity of SOC to promote and stabilize aggregation in these soils, we standardized aggregates by SOC concentrations (Fig. 11; Souza et al., 2023). LME models indicate that an interaction between vegetation type and depth contributes to variation in the fine aggregate:SOC data (Fig. 11a; p < 0.0001), suggesting that in the bottom third of the profile, SOC in spruce soils also contributes to fine aggregate formation. Adding an interaction between vegetation and depth to the model improved model fit for coarse aggregate:SOC values (p < 0.0001), highlighting a higher coarse aggregate:SOC ratio in the spruce sites that varied little with depth compared to aspen soils (Fig. 11b) and suggesting that SOC in spruce-dominated soils exhibits a higher propensity to form coarse aggregates throughout the soil profile.
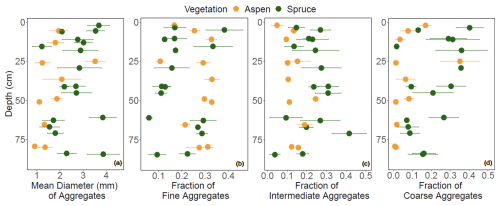
Figure 10(a) Diameter of soil aggregates, and fraction of (b) fine aggregates (< 0.21 mm), (c) intermediate aggregates (0.21–4.76 mm), and (d) coarse aggregates (> 4.76 mm) that contribute to the overall soil aggregate diameter at the Coal Creek catchment, Colorado, USA. Values represent (a) geometric mean or (b–d) mean (points) ± standard deviation (bars). Colors are associated with vegetation: aspen (orange) and spruce (green).
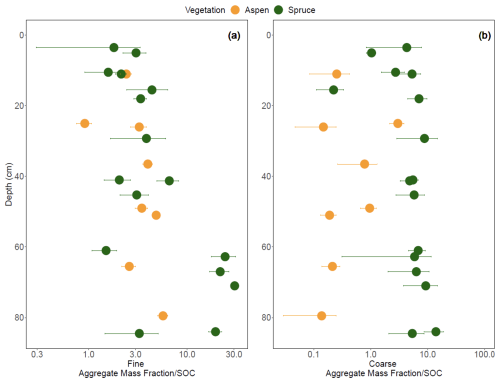
Figure 11The fraction of (a) fine aggregates (< 0.21 mm) and (b) coarse aggregates (> 4.76 mm) divided by the fraction of SOC at the Coal Creek catchment, Colorado, USA. Values represent mean (points) ± standard deviation (bars). Colors are associated with vegetation: aspen (orange) and spruce (green). Please note that each aggregate size class is divided by the total SOC, not the C associated with each size class.
By integrating knowledge from biology, pedology, hydrology, and soil chemistry, we were better able to understand how multiple factors interact to drive observed SOC patterns in aspen and conifer montane forests. Our data indicate that differences in SOC protection contribute to the commonly observed patterns of elevated SOC storage in soils beneath aspen compared to conifer stands (Woldeselassie et al., 2012, Laganiere et al., 2013, Boča et al., 2020, Román Dobarco et al., 2021). Furthermore, our study suggests that aspen-dominated soils may experience enhanced degrees of microbial transformation of SOC, with the products of those transformations exhibiting a greater tendency to reside in relatively small aggregates and thus protect C to a greater degree (Fig. 12). Consistent with this idea, we also observed less DOC loss in aspen soils compared to soil under spruce stands and slightly higher concentrations of DOC per unit root abundance under the spruce stands. These differences suggest greater infiltration of DOC to deeper horizons in spruce soils compared to those in aspen stands. It is important to highlight that spatial replicates controlling for all factors of interest at an ecosystem scale were not feasible, but that our work moves beyond considerations of vegetation biomass characteristics that often dominate investigations of contrasting SOC dynamics. Instead we begin to unravel the complex interactions among cover type characteristics, soil properties, and hydrologic settings (e.g., hillslope position). Below, we discuss the drivers of SOC form and fate in greater detail, and interpret these findings in light of recent increases in stream water DOC concentration in this spruce-dominated watershed.
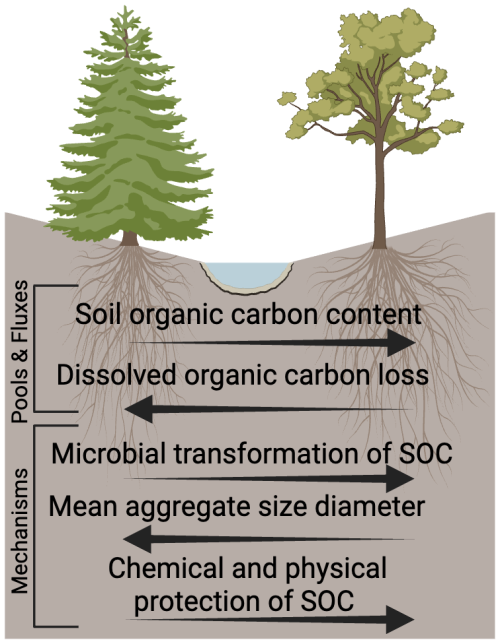
Figure 12Summary of observations across aspen and spruce sites at Coal Creek, CO (USA) that are interpreted to indicate a greater amount of chemical and physical protection of SOC under aspen sites.
5.1 Microbial data are consistent with the MEMS framework
Our data provide multiple lines of evidence that SOC protection, and thus C fate, in these montane forests is largely controlled by biotic processes linked to soil mineral material. Here, greater values of total N, nitrate, BGase, and δ15N, and lower C:N under aspen compared to spruce (Figs. 5 and 8), suggest a greater degree of microbially processed organic matter under the aspen stands, where greater SOC contents were measured (Fig. 4). These data hint that the microbial community under aspen stands functions in a manner consistent with the MEMS framework (Cotrufo et al., 2013), transforming relatively labile leaf litter (e.g., under aspen) into byproducts more readily stabilized within soil profiles to a greater extent than appears to occur with slower-turnover litterfall (e.g., spruce). Differences in litterfall composition and thus decay rates across aspen and conifer species have been widely reported, with generally lower lignin and higher nitrogen content in aspen litter (Moore et al., 2006). Our inference about litterfall differences promoting microbial byproduct stabilization is consistent with findings from across western Canada, where investigators observe relatively more active microbial communities under aspen compared to paired spruce stands throughout a growing season (Norris et al., 2016). Specifically, one interpretation of these C:N, δ15N, exo-enzyme, SOC, and DOC data at our sites is that tree species-specific composition of litterfall appears to have prompted greater microbial activity (Fig. 8), likely promoting greater contributions of microbial necromass to the SOC pool. This, in turn, may promote greater SOC retention in aspen-dominated soils; though investigation of specific necromass-derived compounds in these soils (e.g., Liang et al., 2019) is beyond the scope of this work, it represents a valuable way forward to test this inference.
5.2 SOC transformations likely influence aggregate sizes and the probability of destabilization
The smaller aggregate sizes in aspen-dominated soils further support the notion that SOC stability is enhanced by higher microbial activity and increased necromass production rates. SOC is better protected and has generally longer mean residence times in smaller aggregates than larger aggregates (Six and Jastrow, 2002; Six et al., 2004). Literature hints that the larger-size aggregates (Fig. 10c) and greater propensity for C to form large aggregates (Fig. 11b) observed in the spruce-dominated soils at our sites may be due to a greater abundance of particulate organic matter in spruce compared to aspen forest soils (Cotrufo et al., 2015, 2019); this may be the case since spruce litterfall is more difficult to decompose. Taken together, these lines of evidence are consistent with aspen-dominated forests harboring SOC pools that tend to promote relatively small aggregate formation that can preserve SOC to a greater extent – especially at depth, where MAOC tends to dominate SOC pools (Jackson et al., 2017).
Our soil data also indicate that SOC pools in aspen soils are more strongly dominated by MAOC compared to those in spruce soils. The greater abundances of smaller aggregates and total soil nitrogen and nitrate concentrations, and lower C:N ratios (Fig. 5a–c), in aspen compared to conifer soils are consistent with relatively greater MAOC than POC concentrations (Kögel-Knabner et al., 2008; Ye et al., 2018; Sokol et al., 2022). Combined with the lower DOC:SOC ratio in aspen-dominated soils, these data suggest that a greater fraction of SOC in aspen-dominated soils is mineral-bound and relatively difficult to transform into microbially available pools of DOC. We interpret these data to suggest that microbially mediated transformations of SOC promote differences in the abundance of MAOC and the physical structure of soil aggregates that lead to differences in the SOC protection.
5.3 Roots may indirectly regulate depth profiles of DOC losses
Roots can influence SOC stability through their promotion of both physical and chemical protection (Sullivan et al., 2022b). Specifically, roots can play an important role in the formation and breakdown of soil aggregates (Oades, 1984; Singer et al., 1992; Le Bissonnais, 1996; Attou et al., 1998), they can create biopores that can support the transport of DOC in deeper soil layers (Angers and Caron, 1998; Zhang et al., 2015; Lucas et al., 2019), and root exudates can prime microbial activity, enhance decomposition, and support the formation of MAOC (Jilling et al., 2021; Fossum et al., 2022). Our data revealed little direct correspondence of root abundance with SOC. However, per unit root abundance, spruce soils appear to harbor more DOC compared to aspen (Fig. 7). This pattern – especially evident in total and fine root abundance – suggests that DOC moves more readily through spruce soil profiles, potentially leading to greater DOC losses to stream water compared to aspen-dominated soils. A complementary explanation would be that there are differences in the amount of DOC exudation by roots between the two species, and indeed such differences in exudation rates have been hypothesized in the literature (Buck and St. Clair, 2012; Boča et al., 2020). We might expect that greater exudation would lead to a greater increase in the MAOC pool and enhanced C stability (Even and Cotrufo, 2024), which could explain the lower values of DOC relative to SOC observed under aspen.
5.4 Aspect exerts some control on Coal Creek SOC dynamics
South-facing slopes tend to be warmer and drier than north-facing slopes in the Northern Hemisphere (Burnett et al., 2008), and thus they can prompt more microbial decomposition of SOC. In our study aspen cover occurs where soil temperatures are warmer (Fig. 2). As such, it is possible that the exo-enzymatic signals of generally greater microbial activity in aspen-dominated soils compared to spruce-dominated soils (Fig. 8) are prompted more by enhanced soil temperatures than by differences in aspen and spruce organic matter characteristics, and that enhanced soil temperatures also contribute to smaller soil aggregates, perhaps also due to greater microbial activity. We note that the volumetric fraction of soil moisture was also lower in the aspen, particularly at the shallowest soils, but that aspen soils appear to stay sufficiently moist (0.10–0.20 under aspen vs. 0.20–0.30 under the spruce) to support microbial activity responses to the higher temperatures. Consistent with this idea, soil CO2 and O2 concentrations generally suggest that microbial activities in the warmer, aspen-dominated soils are greater than in the cooler, spruce-dominated soils. Cooler, wetter conditions of the spruce-dominated soils, particularly following snow melt, may prompt a deeper infiltration of moisture and DOC down the soil profile, leading to the elevated DOC/root biomass observed under the spruce stands. While disentangling the impact of elevated soil temperatures from that of the chemical composition of organic inputs from aspen trees within the soil profile is difficult, soil nitrogen and δ15N data are consistent with the idea that litterfall chemistry, and not just temperatures, promoted greater microbial activities in the aspen-dominated soils. We suggest that investigating the comprehensive, integrated effects of warmer, aspen-dominated sites on SOC dynamics compared to cooler, spruce-dominated sites offers a straightforward approach to assessing landscape-scale transitions in watershed C dynamics.
Widespread increases in stream water DOC concentrations have been reported around the world in recent decades (Evans et al., 2005; Alvarez-Cobelas et al., 2012; Stanley et al., 2012; Pagano et al., 2014). Increases in stream water DOC concentration can harm global water quality by altering light and thermal regimes, nutrient cycling (e.g., Morris et al., 1995; Cory et al., 2015), the transport and bioavailability of heavy metals (e.g., Dupré et al., 1999), and creating harmful disinfection byproducts (Leonard et al., 2022). Consistent with these global trends, recent findings at Coal Creek also report increasing DOC concentrations (Leonard et al., 2022; Kerins et al., 2024). As such, our research may help to shed light on drivers of stream water DOC, and thus has implications for changing drinking water quality in the region. Specifically, our work hints that differences in aggregate-size distributions may play an underappreciated role in influencing stream water C chemistry. Aggregate size can be modulated by vegetation type (i.e., smaller aggregates associated with aspen; Fig. 11; Neris et al., 2012; Zhao et al., 2017), and aggregation and disaggregation both represent mechanisms that can influence the transport of DOC to streams (Fan et al., 2022). Larger aggregates appear more prone to induce DOC transport into streams due to their relatively greater propensity to undergo fragmentation and associated loss of DOC (Cincotta et al., 2019; Fan et al., 2022).
Understanding how these different types of vegetation affect the chemical and physical properties of soil, and how this influences C release, is further complicated by climate change. Increasing temperature, a phenomenon evident in many Rocky Mountain environments including Coal Creek (Zhi et al., 2020), can cause aggregates to become less stable (Lavee et al., 1996; Wang et al., 2016), soil microbes to increase their C demand (Belay-Tedla et al., 2009; Hu et al., 2017), and recalcitrant C to undergo decay more rapidly (Luo et al., 2009). Dry soil conditions, which are often prompted by warming (Lakshmi et al., 2003), can induce a decrease in microbial biomass, which is often incorporated into stable aggregates (Gillabel et al., 2007). In addition to warming-induced changes to subsurface properties and function, changing stand composition prompted by warming and drying can alter C dynamics. Some research indicates a high mortality rate among aspen stands and the expansion of conifer stands associated with increases in drought (Anderegg et al., 2013, Brewen et al., 2021), while others indicate the expansion of bark beetles and wildfires may promote the encroachment of aspen into conifer stands (Andrus et al., 2021). Our work suggests that the distribution of spruce and aspen in a watershed may influence soil release of DOC and its subsequent transport into streams, given that spruce vegetation appears to be associated with larger aggregates (Fig. 10), a potential for greater DOC loss per unit SOC (Fig. 4c), greater sand content (depth > 25 cm; Fig. S1) and thus likely greater values of hydraulic conductivity, and generally higher soil moisture content (Fig. 3) compared to aspen-dominated soils. Thus, shifts in stand composition associated with perturbations linked to large-scale global changes have the potential to influence DOC transport from the hillslope to the stream.
Our work explores the interplay of different forest cover types and abiotic conditions in governing soil microbial activities, which then influence the propensity of SOC pools to form and stabilize soil aggregates of different sizes. In turn, these processes appear to promote varying capacities of a soil to protect SOC from destabilization. Our work contributes to the ongoing efforts to investigate suites of biotic and abiotic features across whole ecosystems – the critical zone (Richter and Billings, 2015) – to better understand SOC dynamics (e.g., Keller, 2019; Mainka et al., 2022; Wasner et al., 2024). It also provides a foundation for future studies that could incorporate more spatially replicated sites across key environmental gradients. Specifically, our data suggest that organic matter from aspen supports higher microbial transformation rates and greater stabilization of SOC, reducing the likelihood that labile SOC is transported down the soil profile. Consequently, aspen-dominated stands may be less likely to promote the movement of DOC across the landscape and into streams. This phenomenon may be driven by greater rates of microbial necromass formation and generation of relatively smaller aggregates, and highlights how models like MEMS (Cotrufo et al., 2013) can be important for projecting not just CO2 release to the atmosphere and SOC stabilization, but down-profile and downstream C transport as well. Though soil temperature differences likely played a role in the greater soil microbial activity in aspen, the generally higher nitrogen in aspen soils lends credence to the idea that litterfall chemistry itself played a key role in the higher rates of soil microbial activity. As such, the patterns that emerge in our data suggest that processes that control land cover ultimately also control SOC dynamics and soil structure in ways that may directly impact the delivery of organic C pools deep within soil profiles and stream water quality, and be sensitive to changing climatic conditions. Here, we demonstrate how the critical zone paradigm offers a valuable approach for examining, interrogating, and understanding watersheds, linking vegetation dynamics to subsurface processes and ultimately to the flux of water and C from hillslopes to streams.
Soil sensor and soil properties data can be obtained at HydroShare, http://www.hydroshare.org/resource/9948ad04a9a74246ad9bd5f8decb40b9 (Sullivan and Li, 2022a).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-6097-2025-supplement.
LW: conceptualization, data curation, formal analysis, investigation, methodology, visualization writing – original draft, writing – review and editing. SAB: conceptualization, funding acquisition, investigation, methodology, writing – original draft, writing – review and editing. LL: conceptualization, investigation, funding acquisition, writing – review and editing. DRH: data curation, funding acquisition, methodology, investigation, writing – review and editing. KJ: data curation, investigation, writing – review and editing. DK: investigation, writing – review and editing. JP: data curation, investigation, writing – review and editing. CB: investigation, writing – review and editing. KMJ: data curation, investigation, writing – review and editing. VV: data curation, investigation, writing – review and editing. mu: writing – review and editing. HA: data curation, investigation, funding acquisition, methodology, writing – review and editing. HRB: investigation, funding acquisition, writing – review and editing. ANF: funding acquisition, writing – review and editing. KHW: funding acquisition, investigation, writing – review and editing. PLS: conceptualization, funding acquisition, methodology, project administration, supervision, visualization, writing – original draft, writing – review and editing.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We would like to thank Reece Gregory, Nicole Hornslein, Ariel Mollhagen, and Michael Mackenzie.
This material is based upon work supported by the National Science Foundation, under grant nos. NSF 2121694 (Pamela L. Sullivan), NSF 2012796 (Pamela L. Sullivan), and NSF 2012669 (Holly Barnard), and the Department of Energy, under grant nos. DE-SC0020146 (Li Li and Pamela L. Sullivan), NSF 2121639 (Sharon A. Billings), and NSF 2121760 (Hoori Ajami and Daniel R. Hirmas). This material is partially based upon work supported as part of the Watershed Function Scientific Focus Area funded by the US Department of Energy, Office of Science, Office of Biological and Environmental Research under contract no. DE-AC02-05CH11231. Finally, our understanding of these sites benefited from data provided for project https://doi.org/10.46936/mone.proj.2023.60933/60008945 awarded to Sharon A. Billings and Pamela L. Sullivan by the Molecular Observation Network (MONet) at the Environmental Molecular Sciences Laboratory (https://ror.org/04rc0xn13, last access: 15 January 2025), a DOE Office of Science user facility sponsored by the Biological and Environmental Research program under contract no. DE-AC05-76RL01830.
This paper was edited by Erika Buscardo and reviewed by three anonymous referees.
Alexander, R. R.: Ecology, silviculture, and management of the Engelmann spruce–subalpine fir type in the central and southern Rocky Mountains (No. 659), U. S. Dep. Agric. For. Serv., 1987.
Allison, S. D.: Modeling adaptation of carbon use efficiency in microbial communities, Front. Microbiol., 5, 571, https://doi.org/10.3389/fmicb.2014.00571, 2014.
Alvarez-Cobelas, M., Angeler, D. G., Sánchez-Carrillo, S., and Almendros, G.: A worldwide view of organic carbon export from catchments, Biogeochemistry, 107, 275–293, https://doi.org/10.1007/s10533-010-9553-z, 2012.
Amézketa, E.: Soil aggregate stability: a review, J. Sustain. Agr., 14, 83–151, https://doi.org/10.1300/J064v14n02_08, 1999.
Anderegg, L. D., Anderegg, W. R., Abatzoglou, J., Hausladen, A. M., and Berry, J. A.: Drought characteristics' role in widespread aspen forest mortality across Colorado, USA, Glob. Change Biol., 19, 1526–1537, https://doi.org/10.1111/gcb.12146, 2013.
Andrus, R. A., Hart, S. J., Tutland, N., and Veblen, T. T.: Future dominance by quaking aspen expected following short-interval, compounded disturbance interaction, Ecosphere, 12, e03345, https://doi.org/10.1002/ecs2.3345, 2021.
Angers, D. A. and Caron, J.: Plant-induced changes in soil structure: processes and feedbacks, Biogeochemistry, 42, 55–72, https://doi.org/10.1023/A:1005944025343, 1998.
Araya, S. N. and Ghezzehei, T. A.: Using machine learning for prediction of saturated hydraulic conductivity and its sensitivity to soil structural perturbations, Water Resour. Res., 55, 5715–5737, https://doi.org/10.1029/2018WR024357, 2019.
Attou, F., Bruand, A., and Le Bissonnais, Y.: Effect of clay content and silt–clay fabric on stability of artificial aggregates, Eur. J. Soil Sci., 49, 569–577, https://doi.org/10.1046/j.1365-2389.1998.4940569.x, 1998.
Bartlett, R. J., and Ross, D. S.: Colorimetric determination of oxidizable carbon in acid soil solutions, Soil Science Society of America Journal, 52, 1191–1192, https://doi.org/10.2136/sssaj1988.03615995005200040055x, 1988.
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., Dai, B., Grothendieck, G., Green, P., and Bolker, M. B.: Package `lme4’. convergence, 12, 2, https://cran.r-project.org/web/packages/lme4/lme4.pdf (last access: 20 June 2025), 2015.
Belay-Tedla, A., Zhou, X., Su, B., Wan, S.,and Luo, Y.: Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping, Soil Biol. Biochem., 41, 110–116, https://doi.org/10.1016/j.soilbio.2008.10.003, 2009.
Billings, S. A. and Richter, D. D.: Changes in stable isotopic signatures of soil nitrogen and carbon during forty years of forest development, Oecologia, 148, 325–333, https://doi.org/10.1007/s00442-006-0366-7, 2006.
Billings, S. A., Hirmas, D., Sullivan, P. L., Lehmeier, C. A., Bagchi, S., Min, K., Brecheisen, Z., Hauser, E., Stair, R., Flournoy, R., and deB Richter, D.: Loss of deep roots limits biogenic agents of soil development that are only partially restored by decades of forest regeneration, Elem. Sci. Anth., 6, 34, https://doi.org/10.1525/elementa.287, 2018.
Billings, S. A., Lajtha, K., Malhotra, A., Berhe, A. A., de Graaff, M.-A., Earl, S., Fraterrigo, J., Georgiou, K., Grandy, S., Hobbie, S. E., Moore, J. A. M., Nadelhoffer, K., Pierson, D., Rasmussen, C., Silver, W. L., Sulman, B. N., Weintraub, S., and Wieder, W.: Soil organic carbon is not just for soil scientists: Measurement recommendations for diverse practitioners, Ecol. Appl., 31, https://doi.org/10.1002/eap.2290, 2021.
Blanco-Canqui, H., and Lal, R.: Mechanisms of carbon sequestration in soil aggregates. Critical reviews in plant sciences, 23, 481–504, https://doi.org/10.1080/07352680490886842, 2004.
Boča, A., Jacobson, A. R., and Van Miegroet, H.: Aspen soils retain more dissolved organic carbon than conifer soils in a sorption experiment, Front. For. Glob. Change, 3, 594473, https://doi.org/10.3389/ffgc.2020.594473, 2020.
Brewen, C. J., Berrill, J. P., Ritchie, M. W., Boston, K., Dagley, C. M., Jones, B., Coppoletta, M., and Burnett, C. L.: 76-year decline and recovery of aspen mediated by contrasting fire regimes: Long-unburned, infrequent and frequent mixed-severity wildfire, PLOS ONE, 16, e0232995, https://doi.org/10.1371/journal.pone.0232995, 2021.
Bronick, C. J. and Lal, R.: Soil structure and management: a review, Geoderma, 124, 3–22, https://doi.org/10.1016/j.geoderma.2004.03.005, 2005.
Brookes, P. C., Landman, A., Pruden, G., and Jenkinson, D. S.: Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil, Soil Biol. Biochem, 17, 837–842, 1985.
Buck, J. R. and St. Clair, S. B.: Aspen increase soil moisture, nutrients, organic matter and respiration in Rocky Mountain forest communities, PLoS One, 7, e52369, https://doi.org/10.1371/journal.pone.0052369, 2012.
Buckeridge, K. M., Creamer, C., and Whitaker, J.: Deconstructing the microbial necromass continuum to inform soil carbon sequestration, Funct. Ecol., 36, 1396–1410, https://doi.org/10.1111/1365-2435.14014, 2022.
Burnett, B. N., Meyer, G. A., and McFadden, L. D.: Aspect-related microclimatic influences on slope forms and processes, northeastern Arizona, J. Geophys. Res., 113, F03002, https://doi.org/10.1029/2007JF000789, 2008.
Canelles, Q., Aquilué, N., James, P. M. A., Lawler, J., and Brotons, L.: Global review on interactions between insect pests and other forest disturbances, Landsc. Ecol., 36, 945–972, https://doi.org/10.1007/s10980-021-01209-7, 2021.
Carroll, R. W., Bearup, L. A., Brown, W., Dong, W., Bill, M., and Willlams, K. H.: Factors controlling seasonal groundwater and solute flux from snow‐dominated basins, Hydrological Processes, 32, 21870–2202, https://doi.org/10.1002/hyp.13151, 2008.
Chorover, J., Kretzschmar, R., Garcia-Pichel, F., and Sparks, D. L.: Soil biogeochemical processes within the critical zone, Elements, 3, 321–326, https://doi.org/10.2113/gselements.3.5.321, 2007.
Cincotta, M. M., Perdrial, J. N., Shavitz, A., Libenson, A., Landsman-Gerjoi, M., Perdrial, N., Armfield, J., Adler, T., and Shanley, J. B.: Soil aggregates as a source of dissolved organic carbon to streams: an experimental study on the effect of solution chemistry on water extractable carbon, Front. Environ. Sci., 172, https://doi.org/10.3389/fenvs.2019.00172, 2019.
Coop, J. D., Barker, K. J., Knight, A. D., and Pecharich, J. S.: Aspen (Populus tremuloides) stand dynamics and understory plant community changes over 46 years near Crested Butte, Colorado, USA, Forest Ecol. Manag., 318, 1 12, https://doi.org/10.1016/j.foreco.2014.01.019, 2014.
Cory, R. M., Harrold, K. H., Neilson, B. T., and Kling, G. W.: Controls on dissolved organic matter (DOM) degradation in a headwater stream: the influence of photochemical and hydrological conditions in determining light-limitation or substrate-limitation of photo-degradation, Biogeosciences, 12, 6669–6685, https://doi.org/10.5194/bg-12-6669-2015, 2015.
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K., and Paul, E.: The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter?, Glob. Change Biol., 19, 988–995, https://doi.org/10.1111/gcb.12113, 2013.
Cotrufo, M. F., Soong, J. L., Horton, A. J., Campbell, E. E., Haddix, M. L., Wall, D. H., and Parton, W. J.: Formation of soil organic matter via biochemical and physical pathways of litter mass loss, Nat. Geosci., 8, 776–779, https://doi.org/10.1038/ngeo2520, 2015.
Cotrufo, M. F., Ranalli, M. G., Haddix, M. L., Six, J., and Lugato, E.: Soil carbon storage informed by particulate and mineral-associated organic matter, Nat. Geosci., 12, 989–994, https://doi.org/10.1038/s41561-019-0484-6, 2019.
Culman, S.: Calculating Cation Exchange Capacity, Base Saturation, and Calcium Saturation, Ohioline, https://ohioline.osu.edu/factsheet/anr-81 (last access: 20 June 2025), 2019.
DeForest, J. L.: The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA, Soil Biol. Biochem., 41, 1180–1186, https://doi.org/10.1016/j.soilbio.2009.02.029, 2009.
Dickson, E. L., Rasiah, V., and Groenevelt, P. H.: Comparison of four prewetting techniques in wet aggregate stability determination, Can. J. Soil Sci., 71, 67–72, https://doi.org/10.4141/cjss91-006, 1991.
Dohnalkova, A. C., Tfaily, M. M., Chu, R. K., Smith, A. P., Brislawn, C. J., Varga, T., Crump, A. R., Kovarik, L., Thomashow, L. S., Harsh, J. B., and Keller, C. K.: Effects of Microbial-Mineral Interactions on Organic Carbon Stabilization in a Ponderosa Pine Root Zone: A Micro-Scale Approach, Front. Earth Sci., 10, 799694, https://doi.org/10.3389/feart.2022.799694, 2022.
Dupré, B., Viers, J., Dandurand, J. L., Polve, M., Bénézeth, P., Vervier, P., and Braun, J. J.: Major and trace elements associated with colloids in organic-rich river waters: ultrafiltration of natural and spiked solutions, Chem. Geol., 160, 63–80, https://doi.org/10.1016/S0009-2541(99)00060-1, 1999.
Evans, C. D., Monteith, D. T., and Cooper, D. M.: Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts, Environ. Pollut., 137, 55–71, https://doi.org/10.1016/j.envpol.2004.12.031, 2005.
Even, R. J. and Cotrufo, M. F.: The ability of soils to aggregate, more than the state of aggregation, promotes protected soil organic matter formation, Geoderma, 442, 116760, https://doi.org/10.1016/j.geoderma.2023.116760, 2024.
Fan, L., Lehmann, P., Zheng, C., and Or, D.: Vegetation‐promoted soil structure inhibits hydrologic landslide triggering and alters carbon fluxes, Geophysical Research Letters, 49, e2022GL100389, https://doi.org/10.1029/2022GL100389, 2022.
Fossum, C., Estera-Molina, K. Y., Yuan, M., Herman, D. J., Chu-Jacoby, I., Nico, P. S., Morrison, K. D., Pett-Ridge, J., and Firestone, M. K.: Belowground allocation and dynamics of recently fixed plant carbon in a California annual grassland, Soil Biol. Biochem., 165, 108519, https://doi.org/10.1016/j.soilbio.2021.108519, 2022.
Gaskill, D. L., Mutschler, F. E., Kramer, J. H., Thomas, J. A., and Zahony, S. G.: Geologic map of the gothic quadrangle, Gunnison County, Colorado, The Survey, https://doi.org/10.3133/gq1689, 1991.
German, D. P., Weintraub, M. N., Grandy, A. S., Lauber, C. L., Rinkes, Z. L., and Allison, S. D.: Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies, Soil Biol. Biochem., 43, 1387–1397, https://doi.org/10.1016/j.soilbio.2011.03.017, 2011.
Gillabel, J., Denef, K., Brenner, J., Merckx, R., and Paustian, K.: Carbon sequestration and soil aggregation in center-pivot irrigated and dryland cultivated farming systems, Soil Sci. Soc. Am. J., 71, 1020–1028, https://doi.org/10.2136/sssaj2006.0215, 2007.
Godsey, S. E., Kirchner, J. W., and Tague, C. L.: Effects of changes in winter snowpacks on summer low flows: case studies in the Sierra Nevada, California, USA, Hydrol. Process., 28, 5048–5064, https://doi.org/10.1038/s41612-018-0012-1, 2014.
Hauser, E., Richter, D. D., Markewitz, D., Brecheisen, Z., and Billings, S. A.: Persistent anthropogenic legacies structure depth dependence of regenerating rooting systems and their functions, Biogeochemistry, 147, 259–275, 2020.
Hu, Y., Wang, Z., Wang, Q., Wang, S., Zhang, Z., Zhang, Z., and Zhao, Y.: Climate change affects soil labile organic carbon fractions in a Tibetan alpine meadow, J. Soils Sediments, 17, 326–339, https://doi.org/10.1007/s11368-016-1565-4, 2017.
Jackson, R. B., Lajtha, K., Crow, S. E., Hugelius, G., Kramer, M. G., and Pineiro, G.: The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls, Annu. Rev. Ecol. Evol. S., 48, 419–445, https://doi.org/10.1146/annurev-ecolsys-112414-054234, 2017.
Jastrow, J. D.: Soil aggregate formation and the accrual of particulate and mineral-associated organic matter, Soil Biol. Biochem., 28, 665–676, https://doi.org/10.1016/0038-0717(95)00159-X, 1996.
Jastrow, J. D., Miller, R. M., and Boutton, T. W.: Carbon dynamics of aggregate-associated organic matter estimated by carbon-13 natural abundance, Soil Sci. Soc. Am. J., 60, 801–807, https://doi.org/10.2136/sssaj1996.03615995006000030017x, 1996.
Jilling, A., Keiluweit, M., Gutknecht, J. L., and Grandy, A. S.: Priming mechanisms providing plants and microbes access to mineral-associated organic matter, Soil Biol. Biochem., 158, 108265, https://doi.org/10.1016/j.soilbio.2021.108265, 2021.
Keller, C. K.: Carbon exports from terrestrial ecosystems: A Critical-Zone framework, Ecosystems, 22, 1691–1705, https://doi.org/10.1007/s10021-019-00375-9, 2019.
Kerins, D. and Li, L.: High dissolved carbon concentration in arid rocky mountain streams, Environmental Science & Technology, 57, 4656–4667, 2023.
Kerins, D., Sadayappan, K., Zhi, W., Sullivan, P. L., Williams, K. H., Carroll, R. W., Barnard, H., Sprenger, M., Wenming, D., Williams, K., Pedrial, J., and Li, L.: Hydrology outweighs temperature in driving production and export of dissolved carbon in a snowy mountain catchment, Water Resour. Res., 60, e2023WR036077, https://doi.org/10.1029/2023WR036077, 2024.
Kleber, M., Sollins, P., and Sutton, R.: A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces, Biogeochemistry, 85, 9–24, https://doi.org/10.1007/s10533-007-9103-5, 2007.
Kleber, M., Eusterhues, K., Keiluweit, M., Mikutta, C., Mikutta, R., and Nico, P. S.: Mineral–organic associations: formation, properties, and relevance in soil environments, Adv. Agron., 130, 1–140, https://doi.org/10.1016/bs.agron.2014.10.005, 2015.
Kögel-Knabner, I., Guggenberger, G., Kleber, M., Kandeler, E., Kalbitz, K., Scheu, S., Eusterhues, K., and Leinweber, P.: Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry, J. Plant Nutr. Soil Sc., 171, 61–82, https://doi.org/10.1002/jpln.200700048, 2008.
Laegdsmand, M., Villholth, K. G., Ullum, M., and Jensen, K. H.: Processes of colloid mobilization and transport in macroporous soil monoliths, Geoderma, 93, 33–59, https://doi.org/10.1016/S0016-7061(99)00041-5, 1999.
Laganiere, J., Paré, D., Bergeron, Y., Chen, H. Y., Brassard, B. W., and Cavard, X.: Stability of soil carbon stocks varies with forest composition in the Canadian boreal biome, Ecosystems, 16, 852–865, https://doi.org/10.1007/s10021-013-9658-z, 2013.
Laganière, J., Boča, A., Van Miegroet, H., and Paré, D.: A tree species effect on soil that is consistent across the species' range: the case of aspen and soil carbon in North America, Forests, 8, 113, https://doi.org/10.3390/f8040113, 2017.
Lakshmi, V., Jackson, T. J., and Zehrfuhs, D.: Soil moisture–temperature relationships: results from two field experiments, Hydrol. Process., 17, 3041–3057, https://doi.org/10.1002/hyp.1275, 2003.
Lavee, H., Sarah, P., and Imeson, A.: Aggregate stability dynamics as affected by soil temperature and moisture regimes, Geografiska Annaler: Series A, Physical Geography, 78, 73–82, https://doi.org/10.1080/04353676.1996.11880453, 1996.
Le Bissonnais, Y.: Aggregate stability and assessment of soil crustability and erodibility: I, Theory and methodology, Eur. J. Soil Sci., 47, 425–437, https://doi.org/10.1111/j.1365-2389.1996.tb01843.x, 1996.
Leonard, L. T., Vanzin, G. F., Garayburu-Caruso, V. A., Lau, S. S., Beutler, C. A., Newman, A. W., Mitch, W. A., Stegen, J. C., Williams, K. H., and Sharp, J. O.: Disinfection byproducts formed during drinking water treatment reveal an export control point for dissolved organic matter in a subalpine headwater stream, Water Research X, 15, 100144, https://doi.org/10.1016/j.wroa.2022.100144, 2022.
Liang, C., Amelung, W., Lehmann, J., and Kästner, M.: Quantitative assessment of microbial necromass contribution to soil organic matter, Glob. Change Biol., 25, 3578–3590, https://doi.org/10.1111/gcb.14781, 2019.
Lucas, M., Schlüter, S., Vogel, H. J., and Vetterlein, D.: Roots compact the surrounding soil depending on the structures they encounter, Sci. Rep., 9, 16236, https://doi.org/10.1038/s41598-019-52665-w, 2019.
Luo, C., Xu, G., Wang, Y., Wang, S., Lin, X., Hu, Y., Zhang, Z., Chang, X., Duan, J., Su, A., and Zhao, X.: Effects of grazing and experimental warming on DOC concentrations in the soil solution on the Qinghai-Tibet plateau, Soil Biol. Biochem., 41, 2493–2500, https://doi.org/10.1016/j.soilbio.2009.09.006, 2009.
Mainka, M., Summerauer, L., Wasner, D., Garland, G., Griepentrog, M., Berhe, A. A., and Doetterl, S.: Soil geochemistry as a driver of soil organic matter composition: insights from a soil chronosequence, Biogeosciences, 19, 1675–1689, https://doi.org/10.5194/bg-19-1675-2022, 2022.
Makó, A., Tóth, G., Weynants, M., Rajkai, K., Hermann, T., and Tóth, B.: Pedotransfer functions for converting laser diffraction particle‐size data to conventional values, European Journal of Soil Science, 68, 769–782, https://doi.org/10.1111/ejss.12456, 2017.
Mauer, O. and Palátová, E.: Root system development in Douglas fir (Pseudotsuga menziesii [Mirb.] Franco) on fertile sites, Journal of Forest Science, 58, 400–409, https://doi.org/10.17221/94/2011-JFS, 2012.
Mekontchou, C. G., Houle, D., Bergeron, Y., and Drobyshev, I.: Contrasting root system structure and belowground interactions between black spruce (Picea mariana (Mill.) B. S.P) and trembling aspen (Populus tremuloides Michx) in boreal mixed woods of eastern Canada, Forests, 11, 127, https://doi.org/10.3390/f11020127, 2020.
Mikutta, R., Turner, S., Schippers, A., Gentsch, N., Meyer-Stüve, S., Condron, L. M., Peltzer, D. A., Richardson, S. J., Eger, A., Hempel, G., and Kaiser, K.: Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient, Sci. Rep., 9, 10294, https://doi.org/10.1038/s41598-019-46501-4, 2019.
Moldrup, P., Deepagoda, T. C., Hamamoto, S., Komatsu, T., Kawamoto, K., Rolston, D. E., and de Jonge, L. W.: Structure-dependent water-induced linear reduction model for predicting gas diffusivity and tortuosity in repacked and intact soil, Vadose Zone J., 12, https://doi.org/10.2136/vzj2013.01.0026, 2013.
Monteith, D. T., Stoddard, J. L., Evans, C. D., De Wit, H. A., Forsius, M., Høgåsen, T., Wilander, A., Skjelkvåle, B. L., Jeffries, D. S., Vuorenmaa, J., and Keller, B.: Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry, Nature, 450, 537–540, https://doi.org/10.1038/nature06316, 2007.
Moore, T. R., Trofymow, J. A., Prescott, C. E., Fyles, J., and Titus, B. D.: Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests, Ecosystems, 9, 46–62, https://doi.org/10.1007/s10021-004-0026-x, 2006.
Morris, D. P., Zagarese, H., Williamson, C. E., Balseiro, E. G., Hargreaves, B. R., Modenutti, B., and Queimalinos, C.: The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon, Limnol. Oceanogr., 40, 1381–1391, 1995.
Mote, P. W., Li, S., Lettenmaier, D. P., Xiao, M., and Engel, R.: Dramatic declines in snowpack in the western US, Npj Climate and Atmospheric Science, 1, 2, https://doi.org/10.1038/s41612-018-0012-1, 2018.
Nadelhoffer, K. J. and Fry, B.: Nitrogen-15 and carbon-13 abundances in forest soil organic matter, Soil Sci. Soc. Am. J., 52,1633-1640, 1988.
Neris, J., Jiménez, C., Fuentes, J., Morillas, G., and Tejedor, M.: Vegetation and land-use effects on soil properties and water infiltration of Andisols in Tenerife (Canary Islands, Spain), Catena, 98, 55–62, https://doi.org/10.1016/j.catena.2012.06.006, 2012.
Nimmo, J. R. and Perkins, K. S.: 2.6 Aggregate stability and size distribution. Methods of soil analysis: part 4 physical methods, 5, 317–328, https://doi.org/10.2136/sssabookser5.4.c14, 2002.
Norris, C. E., Quideau, S. A., and Oh, S. W.: Microbial utilization of double-labeled aspen litter in boreal aspen and spruce soils, Soil Biol. Biochem., 100, 9–20, https://doi.org/10.1016/j.soilbio.2016.05.013, 2016.
Oades, J. M.: Soil organic matter and structural stability: mechanisms and implications for management, Plant Soil, 76, 319–337, https://doi.org/10.1007/BF02205590, 1984.
Pagano, T., Bida, M., and Kenny, J. E.: Trends in levels of allochthonous dissolved organic carbon in natural water: a review of potential mechanisms under a changing climate, Water, 6, 2862–2897, https://doi.org/10.3390/w6102862, 2014.
Popenoe, J. H., Bevis, K. A., Gordon, B. R., Sturhan, N. K., and Hauxwell, D. L.: Soil-vegetation relationships in Franciscan terrain of northwestern California, Soil Sci. Soc. Am. J., 56, 1951–1959, https://doi.org/10.2136/sssaj1992.03615995005600060050x, 1992.
Rhoades, A. M., Ullrich, P. A., and Zarzycki, C. M.: Projecting 21st century snowpack trends in western USA mountains using variable-resolution CESM, Clim. Dynam., 50, 261–288, https://doi.org/10.1007/s00382-017-3606-0, 2018.
Richter, D. D., and Billings, S. A.: One physical system: Tansley's ecosystem as Earth's critical zone, New Phytologist, 206, 900–912, 2015.
Román Dobarco, M. and Van Miegroet, H.: Soil Organic Carbon Storage and Stability in the Aspen-Conifer Ecotone in Montane Forests in Utah, USA, Forests, 5, 666–688, https://doi.org/10.3390/f5040666, 2014.
Román Dobarco, M., Jacobson, A. R., and Van Miegroet, H.: Chemical composition of soil organic carbon from mixed aspen-conifer forests characterized with Fourier transform infrared spectroscopy, Eur. J. Soil Sci., 72, 1410–1430, https://doi.org/10.1111/ejss.13065, 2021.
Roulet, N. and Moore, T. R.: Browning the waters, Nature, 444, 283–284, https://doi.org/10.1038/444283a, 2006.
Rossi, A. M., Hirmas, D. R., Graham, R. C., and Sternberg, P. D.: Bulk density determination by automated three-dimensional laser scanning, Soil Sci. Soc. Am. J., 72, 1591–1593, https://doi.org/10.2136/sssaj2008.0072N, 2008.
Rutter, E. B., Ruiz Diaz, D., and Hargrave, L. M.: Evaluation of Mehlich‐3 for determination of cation exchange capacity in Kansas soils, Soil Science Society of America Journal, 86, 146–156, https://doi.org/10.1002/saj2.20354, 2022.
Sadnes, A., Eldhuset, T. D., and Wollebaek, G.: Organic acids in root exudates and soil solution of Norway spruce and silver birch, Soil Biol. Biochem., 37, 259–269, https://doi.org/10.1016/j.soilbio.2004.07.036, 2005.
Sae-Tun, O., Bodner, G., Rosinger, C., Zechmeister-Boltenstern, S., Mentler, A., and Keiblinger, K.: Fungal biomass and microbial necromass facilitate soil carbon sequestration and aggregate stability under different soil tillage intensities, Appl. Soil Ecol., 179, 104599, https://doi.org/10.1016/j.apsoil.2022.104599, 2022.
Scharlemann, J. P., Tanner, E. V., Hiederer, R., and Kapos, V.: Global soil carbon: understanding and managing the largest terrestrial carbon pool, Carbon. Manag., 5, 81–91, https://doi.org/10.4155/cmt.13.77, 2014.
Schjønning, P., Thomsen, I. K., Møberg, J. P., de Jonge, H., Kristensen, K., and Christensen, B. T.: Turnover of organic matter in differently textured soils: I, Physical characteristics of structurally disturbed and intact soils, Geoderma, 89, 177–198, https://doi.org/10.1016/S0016-7061(98)00083-4, 1999.
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W.: NIH Image to ImageJ: 25 years of image analysis, Nature Methods, 9, 671–675, https://doi.org/10.1038/nmeth.2089, 2012.
Schoeneberger, P. J., Wysocki, D. A., Benham E. C., and Soil Survey Staff: Field book for describing and sampling soils, Version 3.0, Natural Resources Conservation Service, National Soil Survey Center, Lincoln, NE, https://www.govinfo.gov/content/pkg/GOVPUB-A57-PURL-gpo174414/pdf/GOVPUB-A57-PURL-gpo174414.pdf (last access: 20 June 2025), 2012.
Shand, C. A., Williams, B. L., and Coutts, G.: Determination of N-species in soil extracts using microplate techniques, Talanta, 74, 648–654, https://doi.org/10.1016/j.talanta.2007.06.039, 2008.
Shepperd, W., Rogers, P. C., Burton, D. and Bartos, D. L.: Ecology, management, and restoration of aspen in the Sierra Nevada, Gen. Tech. Rep, RMRS-GTR-178, Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station 122 p., 178, https://doi.org/10.2737/RMRS-GTR-178, 2006.
Singer, M. J., Southard, R. J., Warrington, D. N., and Janitzky, P.: Stability of synthetic sand-clay aggregates after wetting and drying cycles, Soil Sci. Soc. Am. J., 56, 1843–1848. 1992.
Sinsabaugh, R. L. and Moorhead, D. L.: Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition, Soil Biol. Biochem., 26, 1305–1311, https://doi.org/10.1016/0038-0717(94)90211-9, 1994.
Six, J. and Jastrow, J. D.: Organic matter turnover, in: Encyclopedia of Soil Science, Marcel Dekker, New York., 10, 2002.
Six, J., Paustian, K., Elliott, E. T., and Combrink, C.: Soil structure and organic matter I. Distribution of aggregate‐size classes and aggregate‐associated carbon, Soil Science Society of America Journal, 64, 681–689, https://doi.org/10.2136/sssaj2000.642681x, 2000.
Six, J., Bossuyt, H., Degryze, S., and Denef, K.: A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics, Soil Till. Res., 79, 7–31, https://doi.org/10.1016/j.still.2004.03.008, 2004.
Soil Survey Staff: Natural Resources Conservation Service, United States Department of Agriculture, Web Soil Survey, http://websoilsurvey.sc.egov.usda.gov/, last access: 21 May 2023a.
Soil Survey Staff: Kellogg Soil Survey Laboratory methods manual. Soil Survey Investigations Report, 42, Version 6.0. United States Department of Agriculture (USDA), Natural Resources Conservation Service (NRCS), https://www.nrcs.usda.gov/sites/default/files/2023-01/SSIR42.pdf (last access: 20 June 2025), 2023b.
Sokol, N. W., Whalen, E. D., Jilling, A., Kallenbach, C., Pett-Ridge, J., and Georgiou, K.: Global distribution, and formation and fate of mineral-associated soil organic matter under a changing climate: A trait-based perspective, Funct. Ecol., 36, 1411–1429, https://doi.org/10.1111/1365-2435.14040, 2022.
Solly, E. F., Weber, V., Zimmermann, S., Walthert, L., Hagedorn, F., and Schmidt, M. W.: A critical evaluation of the relationship between the effective cation exchange capacity and soil organic carbon content in Swiss forest soils, Front. For. Glob. Change, 3, 98, https://doi.org/10.3389/ffgc.2020.00098, 2020.
Souza, L. F., Hirmas, D. R., Sullivan, P. L., Reuman, D. C., Kirk, M. F., Li, L., Ajami, H., Wen, H., Sarto, M. V., Loecke, T. D., Rudick, A. K., and Billings, S. A.: Root distributions, precipitation, and soil structure converge to govern soil organic carbon depth distributions, Geoderma, 437, 116569, https://doi.org/10.1016/j.geoderma.2023.116569, 2023.
Stanley, E. H., Powers, S. M., Lottig, N. R., Buffam, I., and Crawford, J. T.: and Contemporary changes in dissolved organic carbon (DOC) in human-dominated rivers: is there a role for DOC management?, Freshwater Biol., 57, 26–42, 2012.
Stătescu, F., Zaucă, D. C., and Pavel, L. V.: Soil structure and water-stable aggregates, Environ. Eng. Manag. J., 12, https://doi.org/10.30638/eemj.2013.091, 2013.
Stone, M. M., DeForest, J. L., and Plante, A. F.: Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory, Soil Biol. Biochem., 75, 237–247, https://doi.org/10.1016/j.soilbio.2014.04.017, 2014.
Sullivan, P. and Li, L.: DWCZ CO – Coal Creek (CC), HydroShare, [data set], http://www.hydroshare.org/resource/9948ad04a9a74246ad9bd5f8decb40b9 (last access: 20 June 2025), 2022a.
Sullivan, P. L., Billings, S. A., Hirmas, D., Li, L., Zhang, X., Ziegler, S., Murenbeeld, K., Ajami, H., Guthrie, A., Singha, K., and Giménez, D.: Embracing the dynamic nature of soil structure: A paradigm illuminating the role of life in critical zones of the Anthropocene, Earth-Sci. Rev., 225, 103873, https://doi.org/10.1016/j.earscirev.2021.103873, 2022b.
von Lützow, M., Kögel-Knabner, I., Ludwig, B., Matzner, E., Flessa, H., Ekschmitt, K., Guggenberger, G., Marschner, B., and Kalbitz, K.: Stabilization mechanisms of organic matter in four temperate soils: Development and application of a conceptual model, J. Plant Nutr. Soil Sc., 171, 111–124, https://doi.org/10.1002/jpln.200700047, 2008.
Wadgymar, S. M., Ogilvie, J. E., Inouye, D. W., Weis, A. E., and Anderson, J. T.: Phenological responses to multiple environmental drivers under climate change: insights from a long-term observational study and a manipulative field experiment, New Phytol., 218, 517–529, https://doi.org/10.1111/nph.15029, 2018.
Wang, Q., Xiao, J., Ding, J., and Zou, T.: Differences in root exudate inputs and rhizosphere effects on soil N transformation between deciduous and evergreen trees, Plant Soil, 458, https://doi.org/10.1007/s11104-019-04156-0, 2021.
Wang, Y., Gao, S., Li, C., Zhang, J., and Wang, L.: Effects of temperature on soil organic carbon fractions contents, aggregate stability and structural characteristics of humic substances in a Mollisol, J. Soils Sediments, 16, 1849–1857, https://doi.org/10.1007/s11368-016-1379-4, 2016.
Wasner, D., Abramoff, R., Griepentrog, M., Venegas, E. Z., Boeckx, P., and Doetterl, S.: The role of climate, mineralogy and stable aggregates for soil organic carbon dynamics along a geoclimatic gradient, Glob. Biogeochem. Cy., 38, e2023GB007934, https://doi.org/10.1029/2023GB007934, 2024.
Weil, R. R. and Brady, N. C.: The nature and properties of soils, 15 edn., Pearson, ISBN 9780133254488, 2017.
Woldeselassie, M., Van Miegroet, H., Gruselle, M. C., and Hambly, N.: Storage and stability of soil organic carbon in aspen and conifer forest soils of northern Utah, Soil Sci. Soc. Am. J., 76, 2230–2240, https://doi.org/10.2136/sssaj2011.0364, 2012.
Woolf, D. and Lehmann, J.: Microbial models with minimal mineral protection can explain long-term soil organic carbon persistence, Sci. Rep., 9, https://doi.org/10.1038/s41598-019-43026-8, 2019.
Ye, C., Chen, D., Hall, S. J., Pan, S., Yan, X., Bai, T., Guo, H., Zhang, Y., Bai, Y., and Hu, S.: Reconciling multiple impacts of nitrogen enrichment on soil carbon: plant, microbial and geochemical controls, Ecol. Lett., 21, 1162–1173, https://doi.org/10.1111/ele.13083, 2018.
Zhang, Y., Niu, J., Yu, X., Zhu, W., and Du, X.: Effects of fine root length density and root biomass on soil preferential flow in forest ecosystems, For. Syst., 24, 12, https://doi.org/10.5424/fs/2015241-06048, 2015.
Zhao, D., Xu, M., Liu, G., Ma, L., Zhang, S., Xiao, T., and Peng, G.: Effect of vegetation type on microstructure of soil aggregates on the Loess Plateau, China, Agr. Ecosyst. Environ., 242, 1–8, https://doi.org/10.1016/j.agee.2017.03.014, 2017.
Zhi, W., Williams, K. H., Carroll, R. W., Brown, W., Dong, W., Kerins, D., and Li, L.: Significant stream chemistry response to temperature variations in a high-elevation mountain watershed, Commun. Earth Environ., 1, 43, https://doi.org/10.1038/s43247-020-00039-w, 2020.
Ziegler, S. E., Benner, R., Billings, S. A., Edwards, K. A., Philben, M., Zhu, X., and Laganière, J.: Climate warming can accelerate carbon fluxes without changing soil carbon stocks, Front. Earth Sci., https://doi.org/10.3389/feart.2017.00002, 2017.
- Abstract
- Introduction
- Study area
- Methods
- Results
- Discussion
- Changes in SOC destabilization and release have implications for stream water quality
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Study area
- Methods
- Results
- Discussion
- Changes in SOC destabilization and release have implications for stream water quality
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement