the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A δ11B-pH calibration for the high-latitude foraminifera species Neogloboquadrina pachyderma and Neogloboquadrina incompta
Markus Raitzsch
Gavin L. Foster
Jelle Bijma
Ulysses S. Ninnemann
Michal Kucera
Tali Lea Babila
Jessica Crumpton Banks
Mohamed M. Ezat
Audrey Morley
The boron isotopic composition of planktonic foraminifera is a powerful tool to reconstruct ocean pH and CO2 in the past. Applications to the high-latitude and polar oceans are however limited as robust calibrations between the δ11B of foraminifera and ocean pH in these regions are lacking. Here, we present a new empirical calibration for the high latitude Arctic species Neogloboquadrina pachyderma and the sub-polar to temperate species Neogloboquadrina incompta using towed specimens from the Labrador Sea, Baffin Bay, and the Nordic Seas. When paired with in situ hydrographic data, this approach allows us to avoid key assumptions used in traditional core top calibrations that are required to link shell geochemical composition to hydrographic conditions during their formation. We show that the foraminifera δ11B of the species analysed is well correlated with the δ11B of seawater borate ion. Further, the foraminiferal δ11B values are consistently lower than seawater equilibrium borate values, consistent with the interpretation of more acidic seawater in the microenvironment due to respiration. However, unlike published calibrations for non-spinose species to date the slope of the δ11B foraminifera to δ11B borate calibration is >1. We discuss several drivers of this higher sensitivity to pH and describe the possible role of vital effects in determining the boron isotopic composition of N. pachyderma and N. incompta. Finally, we apply the tow calibration to core top samples from the Nordic Seas to validate the calibration for use in the paleorecord.
- Article
(2479 KB) - Full-text XML
-
Supplement
(1048 KB) - BibTeX
- EndNote
The northern North Atlantic and the Southern Oceans are areas of strong vertical mixing and convection and as a result play a major role in air-sea gas exchange, including the release or storage of deep ocean CO2 (Takahashi et al., 2009; Ezat et al., 2017; Shuttleworth et al., 2021; Rae et al., 2018). The North Atlantic, the Nordic Seas, the Labrador Sea, and Baffin Bay in particular are areas of deep-water formation and contribute to the formation of North Atlantic deep waters (NADW), a key component of the Atlantic Meridional Overturning Circulation (AMOC). Today, NADW ventilates 26 % of the global deep ocean (Johnson, 2008) and carries dissolved CO2 to depth making the Nordic and Labrador Seas major sinks of atmospheric CO2. The extent to which CO2 is taken out of the atmosphere in these regions is dependent on several factors including temperature, the strength of the AMOC (Schmittner and Galbraith, 2008), sea-ice extent (Rysgaard et al., 2009), and biological productivity (Raven and Falkowski, 1999). Understanding the variability and magnitude of changes in surface ocean pH and CO2 fluxes in the high latitudes is critical to understand its contribution to regulate the ocean carbon cycle and global climate.
Whilst the knowledge of global atmospheric CO2 over the last 800 kilo annum (ka) is known with confidence from bubbles of ancient air trapped in Antarctic ice cores (Bereiter et al., 2015), our understanding of regional oceanic CO2 fluxes, their magnitude and temporal variability relies on marine CO2 proxy records (e.g., Martínez-Botí et al., 2015; Shuttleworth et al., 2021; Si et al., 2024). One commonly applied proxy of marine CO2 relies on the boron isotopic composition (δ11B) of foraminifera shells that tracks ocean pH (Cenozoic CO2 Proxy Integration Project Foster and Rae, 2016; Consortium et al., 2023). This proxy has been extensively applied at low and mid latitudes using species from cultures, tows, and core tops where their δ11B-pH relationship has been well calibrated (Henehan et al., 2016; Raitzsch et al., 2018; Guillermic et al., 2020), and thoroughly tested against the CO2 record from ice cores in areas where the ocean is in equilibrium with the atmosphere (Henehan et al., 2013; Chalk et al., 2017; de la Vega et al., 2023). However, reconstructing CO2 at high latitudes is particularly challenging due to the lower species diversity living in areas of the surface ocean where temperature is <5 °C and the lack of well constrained δ11B calibrations in such species (Yu et al., 2013; Ezat et al., 2017). One such species is the non-spinose planktonic foraminifera Neogloboquadrina pachyderma. It is found in abundance at latitudes above 55–60° N and exclusively in surface waters colder than 4 °C. Considering these oceanographic regions are important areas of CO2 uptake via NADW formation over the observational period, it is critical to constrain how the δ11B in this species records changes in pH and CO2.
Although the boron isotope system has a relatively well understood theoretical basis (e.g., Foster and Rae, 2016), calibrations are required to account for “vital effects” – offsets from the expected δ11B value caused by foraminiferal physiology (e.g., Henehan et al., 2016; Hönisch et al., 2003). In general, geochemical calibrations rely on at least one of these three approaches: (1) the culturing of foraminifera in a controlled laboratory environment (e.g., Gray and Evans, 2019; Lea et al., 1999), (2) the collection of foraminifera from Holocene-aged core tops or sediment traps (e.g., Anand et al., 2003; Erez and Honjo, 1981; Dekens et al., 2002) and (3) the collection of living specimen using plankton tows (e.g., Martínez-Botí et al., 2011; Mortyn and Charles, 2003; Winkelbauer et al., 2023). Whilst the culturing approach has the merit of knowing the exact seawater chemistry in which the foraminifera grew, it doesn't fully represent the environment experienced in the open ocean, such as ontogenetic vertical migration (Meilland et al., 2021), changes in biological productivity, nutrient abundance, sea ice cover, or secondary diagenetic processes such as precipitation or dissolution in the water column and sediments. Culturing foraminifera is also a time consuming and specialist activity requiring ready access to live foraminifera and careful maintenance of culture conditions, which can be challenging. The approach using core top samples accounts for all secondary alterations of the primary signal as the foraminifera is incorporated in the sedimentary archive and is thus well-suited for application in the paleoclimatic record. However, it is challenged by the difficulty to find a natural modern range of upper ocean pH (reflected by the δ11B of borate) that is sufficiently large to define a reliable calibration (e.g., Yu et al., 2013) and there remains considerable uncertainty in determining the seawater carbonate chemistry the foraminifera calcified in, especially in regions like the northern North Atlantic where there has been significant invasion of anthropogenic CO2 (Yasunaka et al., 2023). Finally, there can be uncertainty in core top sample age making the determination of seawater temperature and anthropogenic carbon contribution more uncertain.
To date, only one study has attempted to construct a δ11B calibration (Yu et al., 2013) using N. pachyderma from core top samples from the Labrador and Irminger Sea covering a relatively narrow range of pH and assuming a constant habitat depth of 50 m. Several attempts have been made to constrain the habitat depth of N. pachyderma and it is often stated that this species is a subsurface dweller (e.g., Kohfeld et al., 1996; Simstich et al., 2003) that is migrating to deeper waters prior to calcifying a thick outer crust. However, more recent direct observations of N. pachyderma habitat across the Arctic and polar oceans show that the habitat depth of N. pachyderma is variable, can be shallow (i.e. <20 m) and is primarily controlled by sea ice cover and chlorophyll intensity (Pados and Spielhagen, 2014; Greco et al., 2019) and not by diel vertical migration or the lunar phase (Greco et al., 2019). When sea-ice cover is reduced and/or when chlorophyll at the surface is low, the habitat generally deepens to 75–150 m (Greco et al., 2019). Critically, these studies have shown that heavily crusted N. pachyderma specimens can be found at any depth including the top 0–50 m (Tell et al., 2022) suggesting that there is no systematic ontogenetic vertical migration associated with reproduction or “crusting” for N. pachyderma (Manno and Pavlov, 2014; Tell et al., 2022). The considerable variability in habitat depth across the top 200 m of the surface ocean complicates the attribution of a single depth to core top calibrations and potentially results in large uncertainties when attributing modern pH and thereby δ11B of borate to foraminiferal δ11B.
To avoid the assumptions associated with traditional core top calibrations we present here a calibration using living N. pachyderma and N. incompta specimens (e.g., cytoplasm intact, no crust) collected via plankton tows from across a large range of pH in the Labrador Sea, Baffin Bay, and the Nordic Seas. This approach allows us to compare the foraminiferal δ11B with the pH of seawater and hence δ11B of seawater borate from the exact same depth at which the tows of N. pachyderma and N. incompta were collected. While this approach provides the most accurate hydrographic data for the calibration effort, the tow depth range, in some cases, integrates a large gradient in seawater carbonate chemistry and temperature, which introduces some uncertainties (reported in Table 2). We acknowledge that the depth of the plankton tows does not necessarily represent the depth of calcification. Further, the δ11B composition of non-crusted specimens analysed here may be different to crusted specimen found in marine climate archives. In order to assess the validity of the calibration we construct, we then apply the tow-based calibration to a series of high latitude core tops alongside existing data from the literature to evaluate its application to the paleorecord.
Whilst N. pachyderma and N. incompta were historically considered as two morphotypes of the same species (N. pachyderma sinistral and dextral respectively), they are now considered two separate species based on genetics, biogeography and ecological distinctions (Darling et al., 2006; Cifelli, 1961). They are however closely related; both are non-spinose species and live in polar or subpolar environments. N. pachyderma is typically found in cold open ocean waters and is the only species present in waters below 4 °C (e.g., Baffin Bay, Labrador Sea, Greenland Sea) and N. incompta dominates warmer subpolar waters of the eastern Norwegian Seas, and subpolar North Atlantic. The large geographical area of the polar North Atlantic allows us to cover a wide range of temperature, convection and CO2 flux, and as a result a large gradient of pH in this calibration.
2.1 Oceanographic setting
The dataset presented here was collected from the Nordic Seas, constituting the Greenland, Icelandic and Norwegian Seas (also sometimes called in conjunction with the Arctic Sea, the Arctic Mediterranean Sea), and the Labrador Seas. At the surface, the North Atlantic Drift enters the Nordic Seas (as “inflow”) mainly through the Iceland-Scotland ridge, and to a lesser extent the Denmark Strait (Hansen and Østerhus, 2000; Østerhus et al., 2019). North of the Iceland-Scotland ridge the North Atlantic Drift becomes the Norwegian Current, a warm and saline current that gradually cools via air-sea exchanges as it flows northward. From the Arctic Ocean surface outflow through the Fram Strait feeds the East Greenland Current, and through the Canadian Archipelago the Baffin Island Current. Once in the Nordic Seas the East Greenland Current follows the Greenland coast and becomes the West Greenland Current entering the Labrador Seas and subsequently the Baffin Bay. This current partly mixes with the Irminger current, a branch of the North Atlantic Drift, which increases its salinity. Together, the West Greenland and Irminger Currents flow northward into the Baffin Bay before recirculating southward alongside the surface Labrador Current. The Arctic waters that enter the Baffin Bay through the Canadian Archipelago travel via various sounds and straits southward and are of surface and subsurface origin (Azetsu-Scott et al., 2010). Along the way they mix with low salinity waters from precipitation, river, and sea ice meltwater before contributing to the low-density Baffin Island Current on the Western margin of the bay. The Baffin Island Current then mixes with the outflow water from the Hudson Bay and merges with the Labrador Current.
This complex system of water masses influences seawater pH in all regions. Mainly, the cold waters of Arctic origins are characterized by low pH due to the strong temperature dependent solubility of CO2 at lower temperatures. Conversely, the warmer and saline waters feeding the Norwegian margin, the Labrador Sea, and the southern part of the Baffin Bay are characterized by higher pH. These strong gradients in surface water pH also result in a large range of CO2 offsets between the surface ocean and the atmosphere (Fig. 1). As a result, this wide geographic spread and diversity in water masses allows for the large range of >0.2 seawater pH (Table 2) and diverse foraminifera habitat included in this calibration effort.
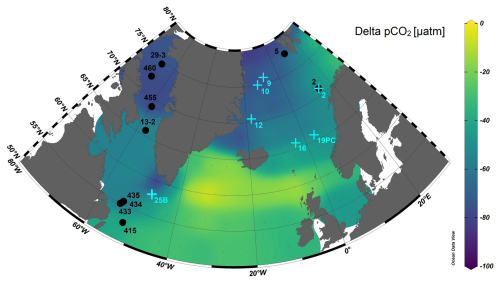
Figure 1Map of the delta partial pressure of CO2 (pCO2, in units of micro atmospheres) defined as the difference between atmospheric and seawater pCO2 (= pCO2 atmosphere – pCO2 seawater) at sea surface temperature (Takahashi et al., 2019) and location of stations where plankton tows (black circles) and core top samples (light blue crosses) were collected. Delta pCO2 for the Baffin Bay was calculated using pCO2 values reported in Nickoloff et al. (2024). Map made with Ocean Data View (Schlitzer, 2002).
2.2 Sample material
Specimens collected via plankton tows include 15 N. pachyderma samples from the Labrador Sea and Baffin Bay collected during RV Maria S. Marian cruises MSM09 in 2008, MSM44 in 2015, and MSM66 in 2017. In addition, 5 tows containing N. incompta and 5 core top samples (containing N. pachyderma) were collected from the Nordic Seas during cruise CE20009 in August 2020 (Fig. 1). Towed samples were collected from 100 µm mesh multi-nets, brought on board, and either immediately frozen at −80 °C or immediately picked into slides and frozen at −80 °C to preserve the foraminiferal cytoplasm. Frozen tows were thawed onshore and between 0.7 and 2 mg of uncrusted specimens with intact cytoplasm were hand-picked for δ11B and trace elemental to calcium analysis. The average size fraction of tow samples from the Labrador Sea ranged between 182–248 µm determined using a Keyence HX 6000 digital microscope, however all specimen sizes available were included in analysis including specimen >100 and <300 µm to ensure enough material was available. Core tops were collected from multicores recovered during cruise CE20009 (September 2020) and cruise CAGE-ARCLIM (June 2022). The multicores were immediately processed onboard and sliced at 0.5 to 1 cm resolution, and subsequently frozen at −20 °C. On land, multicore tops were thawed or freeze-dried and wet-sieved at 63 µm. 1–2 mg of N. pachyderma were picked in the 200–250 µm size fraction. When possible morphotype II was favoured when picking specimens from core tops to minimize potential depth habitat variability with morphotypes following the classification of Altuna et al. (2018, Plate 1). All specimens from core tops were encrusted and while we selected specimens from a very narrow size fraction, we did not perform a systematic analysis of the degree of encrustation of N. pachyderma in the core top samples. Therefore, we cannot exclude that post-mortem depositional processes including the precipitation of inorganic calcite, dissolution, or recrystallization impact the thickness or geochemical composition of the crust as the tests sink through the water column and settle at the seafloor. In Table S4 we list core tops that were radiocarbon dated. Accelerator mass spectrometry (AMS) radiocarbon (14C) was measured at the Keck Carbon Cycle Accelerator Mass Spectrometry facility at UC Irvine, USA. The samples for radiocarbon dating consist of the planktonic foraminifera N. pachyderma. Individuals were picked at from the 0.0–0.5 cm interval for each core top (Table S4).
2.3 Estimates of δ11B of borate
CTD (conductivity, temperature, depth) casts with Niskin bottles were deployed at each station for cruise CE20009 and water samples were collected for total alkalinity (TA) and dissolved inorganic carbon (DIC) analysis (described in Morley et al., 2024). This allows the carbonate chemistry of seawater to be fully constrained at exactly the same depth as where the tows were collected. δ11B of borate was calculated from pH (derived from TA and DIC) and derived using the following equation:
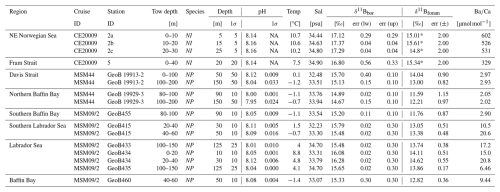
Where the total boron in seawater [B] µmol kg−1 (Lee et al., 2010), εB is the fractionation factor between the two boron species expressed as , the isotopic fractionation factor αB between B(OH)3 and B(OH) is 1.0272 as determined by Klochko et al. (2006) and the δ11B of seawater is 39.61 ‰ (Foster et al., 2010). For stations in the Labrador Sea and the Baffin Bay the water chemistry was determined using nearby cruise data collected at the same time or the same month of the year as the tow samples (Table S3). For the towed samples, the δ11B of borate was determined using the median of the hydrographic parameters within the towed sample depth interval.
For core top samples we estimate the depth of δ11B of borate using the traditional core top approach to constrain calcification depth based on paired δ18O measurements (e.g., Ravelo and Hillaire-Marcel, 2007). Briefly, we constrained calcification depth by projecting the δ18O of N. pachyderma and N. incompta onto the equilibrium δ18O of calcite in the water column (δ18O) using modern hydrographic profiles and the paleotemperature equation derived by Shackleton (1974) as follow:
Seawater temperature in degrees Celsius (T), δ18O is the equilibrium δ18O of calcite on the PDB scale and δ18Osw.SMOW is the δ18O of seawater on the SMOW scale (Table S1), either directly measured from CTD bottles or derived from salinity using the regional salinity−δ18Osw of Simstich et al. (2003). This approach yields a depth estimate for the core top foraminifera between 25 and 91 m and an average of 65 m (Morley et al., 2024, Table S4). As in Morley et al. (2024) we did not apply a systematic correction for a vital effect on δ18O values because there isn't consensus in the literature on the offset recorded in N. pachyderma collected from core top samples. Briefly, the negative δ18Oc offset from equilibrium measured in living (uncrusted) N. pachyderma collected from plankton tows is highly variable within a region, across regions, seasons, and on interannual timescales (Stangeew, 2001; Volkmann and Mensch, 2001; Pados and Spielhagen, 2014; Livsey et al., 2020). Furthermore, the addition of crust, typical for specimens recovered from core tops, increases the δ18Oc signal because crust is isotopically heavier than ontogenetic calcite (Kozdon et al., 2009; Mikis et al., 2019). Variations in the degree of encrusting can therefore either “lower”, completely “mask”, or shift the vital effect towards positive values depending on the degree of encrustation (Kozdon et al., 2009). As a result, there remains considerable uncertainty in the exact value to use when correcting for the competing signals of vital effects and crust in N. pachyderma, with studies reporting both calcification in equilibrium (Jonkers et al., 2010; Jonkers et al., 2013; Mikis et al., 2019; Jonkers et al., 2022) and out of equilibrium with seawater (Kohfeld et al., 1996; Bauch et al., 1997; Simstich et al., 2003; King and Howard, 2005). By not applying a correction for vital effects or the contribution of crust calcite we avoid biasing calcification depth estimates towards deeper depths and lower δ11Bborate values. This approach is also consistent with sediment trap studies from both the North and South Atlantic where δ18Oc values measured on crusted N. pachyderma are in equilibrium with measured water column δ18O (Jonkers et al., 2013; Mikis et al., 2019).
The pH and δ11Bborate for core tops samples was calculated by applying a correction for anthropogenic carbon on DIC (using the anthropogenic DIC of GLODAPv2, 2016) ranging ∼35 to 50 µmol kg−1 in the Nordic Sea stations in the upper 200 m (Lauvset et al., 2022). For core tops dated with AMS radiocarbon dates younger than 550 years we did not apply a correction for anthropogenic carbon (Table S4). The uncertainty for calcification depth was defined as the minimum and maximum within a ±20 m interval around the central value. We note that this approach yields an average depth of the foraminifera population signal, and that despite both species being observed to live throughout the upper 200 m of the water column (Greco et al., 2019), we assume the inferred depth is representative of where the majority of the population lives and calcifies. The δ11Bforam and corresponding δ11Bborate were fitted with a York regression (York et al., 2004) that accounts for the uncertainty in both the x and y axis.
2.4 Elemental and δ11B analysis
Analysis was conducted in two laboratories at the University of Southampton (for samples from CE20009 in the Nordics Seas) and the Alfred Wagner Institute (AWI, for tows from the Labrador Sea and Baffin Bay). Standards and reference material are presented in both laboratories to showcase reproducibility.
2.4.1 Samples from cruise CE20009
Foraminifera from tows and core tops were cleaned using a modified version of the established foraminifera method to account for higher organic content in plankton tow samples (Henehan et al., 2016; Barker et al., 2003). All foraminifera were gently crushed between two glass slides to facilitate cleaning and clay removal was carried out through sequential Milli-Q rinses and brief ultrasonication to agitate the samples (core top samples only). Both core top and tow samples were oxidatively cleaned with a solution of H2O2 (30 % by weight) buffered with 0.1M NH4OH (3.4 % of peroxide in the final oxidative mix). Samples were placed in a hot bath for 3×5 min for core tops and 3×20 min for tows to ensure the removal of additional organics, separated by brief 15 s ultrasonication. Samples were then weak acid-leached (in 0.0005 M HNO3) for 30 s and dissolved in ∼300 µL of 0.15 M HNO3. An aliquot of 20 µL (diluted in 130 µL 0.5 M HNO3) was kept for elemental analysis, and the remainder kept for boron sample preparation and analysis.
Elemental analysis was performed on an Thermo Element Inductively Coupled Plasma Mass Spectrometer (ICP-MS) at Centre for Earth Research and Analysis Southampton (CERAS) at the University of Southampton. Element-to-calcium ratios were measured against 43Ca and 48Ca, then averaged, and referenced against in house mixed element standards. Consistency standards placed at the beginning and end of each sequence were measured at the same concentration as samples to assess accuracy. For samples below one mmol mol−1, Element Ca ratios typically drift, therefore, the concentration of the samples and standards were matched to account for this effect. ratios measured included Mg and Ba. Based on the reproducibility of our in-house standards, the uncertainty for most elemental ratios is ∼5 % (at 95 % confidence; Henehan et al., 2015). In order to showcase reproducibility between coretops measured at Southampton and tows at AWI (Sect. 2.3.2), we report the long-term averages for and for NIST-C (coral reference standard) typically measured at Southampton, and the long-term average for JCP-1 typically measured at AWI and compare these to the interlaboratory assessment published in Stewart et al. (2021) and Hathorne et al. (2013), respectively (Table 1). This comparison shows that both laboratories produce consistent values without any significant interlaboratory offsets.
Samples for boron isotope analysis were first purified for boron by anion exchange chromatography in the boron-free class 100 laboratory of CERAS following the procedure of Foster (2008). A total procedure blank (TPB) was conducted for all batches of ∼10 columns and ranged 31 to 92 pg (equivalent to 1 %–5 % of the sample total boron). Samples were subsequently corrected using a long term δ11B of TPB of −7.27 ‰ from long term measurement at the University of Southampton. This gave a δ11B correction of 0.1 ‰ to 1.1 ‰. To test the effect of TPB correction on the regression slope, we present δ11B both with and without TPB correction.
The purified sample was analysed for δ11B at CERAS on a Thermo Neptune Multi Collector Inductively Coupled Plasma Mass Spectrometer (MC-ICPMS) with 1012 Ω amplifier resistors, using a standard-bracketing technique with NIST SRM 951 (Foster, 2008; Foster et al., 2013) to correct for the instrumental mass bias and drift. All other sample preparation and elemental analysis for the samples were conducted in the same CERAS laboratories at the University of Southampton.
The uncertainty on foraminifera δ11B is dependent on the boron content (Rae et al., 2011), i.e. the intensity of the 11B signal of each sample. The relationship between 11B signal and sample uncertainty was empirically determined based on the uncertainty of repeated measurements of JCp-1 Coral (Porites sp.) that has undergone the same chemical purification It is defined by the following equation (Anagnostou et al., 2019):
where [11B] is the intensity of δ11B signal in volts. Carbonate and boric acid samples were measured at CERAS and AWI and are in good agreement. The long-term δ11B value of JCp-1, AE120 and AE121 at CERAS are (at 1σ) 24.21±0.12 ‰, ‰ and 19.59±0.28 ‰ respectively, in good agreement with results of Gutjahr et al. (2021) and Stewart et al. (2021), and are comparable with values measured at AWI of 24.28±0.3 ‰ (Raitzsch et al., 2018), ‰ (n=4) and 19.5±0.79 ‰ (n=10), respectively. Samples with substantial TPB corrections have lower confidence due to higher uncertainty in the δ11B of TPB samples (given the low boron content). Applying a long term δ11B of TPB of −7.27 ‰ may not be representative of the true value. As a result, for samples with substantial TPB correction, the uncertainty of δ11BTPB was propagated into the corrected δ11B value measured on foraminifera samples (e.g., δ11Bforam).
2.4.2 Samples from the Labrador Sea and Baffin Bay
Boron (B) isotope ratios for samples from the Labrador Sea were measured following Raitzsch et al. (2018). Briefly, the cleaned samples were dissolved in 60 µL of 1 N HNO3 and loaded on a Savillex teflon lid then closed and turned upside to be micro-distilled on a hotplate to separate boron from the carbonate matrix. The micro-distillation method in Ca-rich matrix samples such as foraminifera yields a B recovery of ∼ 100 %, a low procedural blank, and accurate results, even at low B concentrations (Gaillardet et al., 2001; Wang et al., 2010; Misra et al., 2014; Raitzsch et al., 2018). The procedural blank contribution during this study was 10–50 pg B, which equates to ∼0.2 %–0.8 % of the total [B] in the micro-distillation vial. The distillate containing only boron was diluted with 2 % HNO3 and analysed for boron isotopic composition in triplicate using a Nu Plasma II multi-collector inductively coupled plasma mass spectrometry (ICPMS) at AWI (Bremerhaven, Germany) that is equipped with a customized detector array of 16 Faraday cups and 6 secondary electron multipliers (SEM), also termed ion counters (IC), where high-mass IC5 was used for 11B and IC0 for 10B.
Similar to coretop samples measured at Southampton, samples were measured for δ11B using a standard-sample-bracketing technique frequent analysis of control standard AE121 with an isotopic composition similar to that of foraminifera was monitored to ensure measurement accuracy. Each micro-distilled sample was analysed in triplicate where at least two measurements were used for averaging the δ11B value measured. Measurement uncertainties are reported as 2 standard deviations (2σ) derived from triplicate measurements or as ±0.30 ‰ determined from long-term reproducibility (2σ) of the control standard, whichever is larger. For elemental to calcium () ratios the Labrador Sea and Baffin Bay samples were analysed using a Nu AttoM high-resolution double-focusing inductively coupled plasma mass spectrometer (ICP-MS) at the AWI.
2.5 Core top samples
To evaluate the validity of the tow-based δ11B-pH calibration the core tops were considered as unknown paleo samples by applying the calibration equation derived from the plankton tows together with the revised temperature calibration recently published by Morley et al. (2024). We applied this scheme to core tops from various location in the North Atlantic, cruise CE20009 and CAGE-ARCLIM (Nordic seas, this study), RAPID-35-25B (North Atlantic, Yu et al., 2013; Moffa-Sánchez et al., 2014), and JM-FI-19PC (Norwegian sea, Ezat et al., 2017). The foraminiferal δ11B data from Ezat et al. (2017) measured with negative thermal ionization mass spectrometry (N-TIMS) was corrected for offsets in analytical techniques (using a correction of −1 ‰ defined by the foraminifera data of Farmer et al. (2016) to align with our data sets measured with MC-ICPMS. We calculate δ11B-derived pH (Eq. 1) and atmospheric CO2 and correct for CO2 differences between foraminifera habitat depth and sea surface (from CTD or GLODAP v2 pCO2 profiles) as well as local air-sea CO2 disequilibrium (Takahashi et al., 2019), and compare our result to the ice core CO2 record (Bereiter et al., 2015) over the last 500 years. Unlike comparing foraminiferal δ11B core top values to modern pH and δ11Bborate which does not account for possible sediment age variations, this approach allows for: (1) comparison of δ11B-derived CO2 with an independent record from the ice core; and (2) the knowledge of CO2 variations in the recent past to evaluate the range of CO2 (and pH) possibly recorded by the δ11B of core top foraminifera of variable ages.
For this calculation we use the constant fractionation factor determined by Klochko et al. (2006); Eq. 1). We use a δ11B of seawater of 39.61 ‰ (Foster et al., 2010), and use alkalinity as a second carbonate parameter in the CO2 calculation (Zeebe and Wolf-Gladrow, 2001). Aqueous CO2 was determined as follow:
TA is the total alkalinity measured from CTD bottle profiles. To account for uncertainty in habitat depth TA uncertainty was estimated by integrating the total range of TA within the upper 100 m of the water column. KB is the equilibrium constant of boron species in seawater (a function of temperature T, salinity S and pressure P, Dickson, 1990), BT the concentration of boron in seawater (432.6 µmol kg−1, Lee et al., 2010, [H+] the concentration of H+ determined from pH = − log[H+] (total scale), Kw the dissociation constant of water (function of T, S and P), and K1 and K2 the first and second dissociation constants of carbonic acid (function of T, S and P, Sulpis et al., 2020). The partial pressure of pCO2 (in parts per million, ppm) is determined as:
With [CO2]sw, the aqueous CO2 concentration (Eq. 4), and K0 Henry's law constant (Weiss, 1974). Following Martínez-Botí et al. (2015) and Chalk et al. (2017), the pCO2 uncertainty was determined with a Monte Carlo simulation (10 000 realisations) to account for the uncertainty of all input parameters (all with a normal distribution). An error envelope was calculated at 1 and 2σ based on the 68 % and 95 % distribution of all the realisations.
3.1 Tow samples
The δ11B of towed N. pachyderma and N. incompta samples from the Labrador and Nordic Sea (i.e. δ11Bforam) are compared to seawater δ11B borate (e.g., δ11Bborate) in Fig. 2a. The δ11Bforam of both species overlaps and both are offset to lower δ11B than ambient borate. The regression for these data, using a constant αB (Klochko et al., 2006) is best described by the following equation (with 1σ uncertainty, Fig. 2a, Table 3), with a mean square weighted deviation (mswd) of 0.58 (and p=0.28):
The slope of 1.58, being larger than unity, shows that at lower ambient δ11Bborate (lower pH), the δ11Bforam is more offset from equilibrium than at higher δ11Bborate. Furthermore, the slope lies below the 1:1 δ11Bforam : δ11Bborate line. When assessing N. pachyderma samples by themselves (i.e. tows from the Labrador Sea) the slope is steeper at 1.82. However, both slopes and intercepts are within error of each other (Table 3). We do not attempt to define a slope for N. incompta-only due to the narrow range of pH and δ11Bborate covered for these samples. The effect of TPB correction on δ11B and the regression slope (Fig. S1, Table S2) shows that the slope remains >1 when samples with high TPB are not included in the regression (Fig. S1b) or not TPB corrected (Fig. S1c). Elemental data of N. pachyderma and N. incompta show highly variable values ranging 2 to 602 µmol mol−1 (Fig. 4, Table 2).
Table 2Hydrographic dara, δ11Bforam and B/Ca measured on living N. pachyderma (NP) and N. incompta (NI) from plankton tows. See also Fig. 2. NA: not available.
* Total procedure blank corrected.
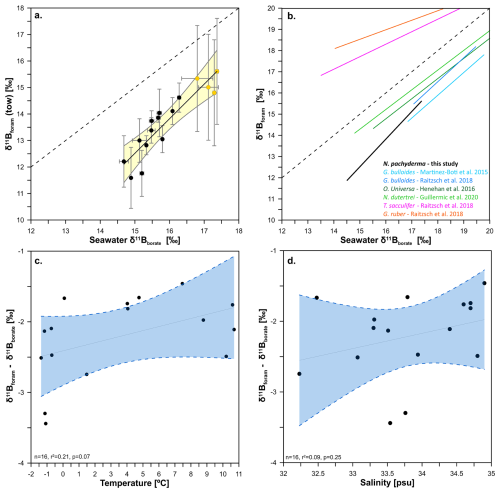
Figure 2Relationship between the δ11B of towed N. pachyderma (black circles) and N. incompta (yellow circles) with the δ11B of seawater borate. Panel (a) shows δ11B of towed specimens against δ11B of seawater borate without a temperature correction on αB on the calculation of δ11B of borate. We fitted the data with a York regression (black line) and a 95 % confidence envelope (light yellow shade), the x and y error bars represent the borate δ11B uncertainty and the foraminifera δ11B external uncertainty respectively (see methods). The 1:1 line is shown by the black dashed line. Panel (b) shows calibration equations for other planktonic foraminifera next to the tow-based calibration equation derived here for N. pachyderma. Panels c and d show the regression between δ11Bforam-δ11Bborate offset and temperature (n=16, p=0.07, r2=0.22) and salinity (n=16, p=0.25, r2=0.10) respectively. The blue envelope represents the 95 % confidence interval of the linear regression.
3.2 Relationship between δ11B, temperature and salinity
The foraminifera δ11B exhibit a significant correlation with temperature (Figs. S2, S1, r2=0.58, p≪0.05) and salinity (Fig. S2, r2=0.24, p=0.017), which is likely due to covariance between carbonate system parameters, temperature, and salinity. To evaluate the influence of temperature and salinity on the proxy system we plot them against the offset between δ11Bforam and predicted δ11Bborate (Fig. 2c and d) and in both cases the correlation is not significant (i.e. p<0.05).
3.3 Applying the calibration to core top samples.
Whilst tows provide information about the original signal imprinted in well constrained calcifying environments, the application in the paleorecord relies on sediment samples with foraminifera that incorporate the calcification depth during ontogeny and post depositional environment processes in terms of sedimentation rate, corrosiveness of porewaters etc. The tow-based foraminifera δ11B calibration equation based on temperatures calculated from the -δ18O correction scheme (Morley et al., 2024) yields reconstructed atmospheric pCO2 concentrations that span the pre-industrial (280 ppm) to modern range (420 ppm).
N. pachyderma and N. incompta are both non-spinose asymbiotic genetically sister species inhabiting the high latitude oceans (Darling et al., 2006). While their geographic distribution in the North Atlantic overlaps slightly at temperatures between 6–10 °C (Darling et al., 2006) each species inhabits distinct environmental conditions: warm Atlantic waters at subpolar latitudes of high pH for N. incompta, and cold polar waters of lower pH for N. pachyderma. Combining them in our study therefore allows us to cover a wider range of pH, and hence δ11Bborate, improving the overall calibration. The minor difference in the calibration slopes when considering N. pachyderma alone or if both species are combined (Table 3) further support this approach.
4.1 Potential drivers of δ11B sensitivity and alteration of microenvironment
Our new calibration shows that the δ11Bforam recorded by N. pachyderma and N. incompta is lower than the δ11B of seawater borate (1:1 line, Fig. 2). This result is in agreement with other published calibrations of the non-spinose species, Globoconella inflata, the symbiont-bearing spinose species O. universa (Henehan et al., 2016), and the symbiont-barren spinose species G. bulloides (Martínez-Botí et al., 2015). Further we observe that the slope of the regression is >1 (Fig. 4e, Table 3). This observation contrasts with previously published δ11B calibrations that show either a slope close to unity in line with the expected pH sensitivity from δ11B of borate in seawater such as that found for epifaunal benthic foraminifera (Rae et al., 2011), G. bulloides (Martínez-Botí et al., 2015), O. universa (Henehan et al., 2016) and corals Desmophyllum dianthus (Anagnostou et al., 2012; McCulloch et al., 2012) and Balanophyllia elegans (Gagnon et al., 2021), or a slope <1 like the spinose symbiont-bearing species G. ruber (Henehan et al., 2013) and T. sacculifer (Sanyal et al., 2001; Martínez-Botí et al., 2015).
Interpretations of a weaker sensitivity (i.e. slope <1) to pH for G. ruber and T. sacculifer invoke photosymbiont activity, calcification and/or foraminifera metabolism, altering the pH of the diffusion-controlled microenvironment immediately surrounding the living foraminifera (e.g. Henehan et al., 2016; Hönisch et al., 2003; Zeebe et al., 2003). In this scenario, lower pH in the microenvironment relative to ambient seawater immediately surrounding N. pachyderma and N. incompta specimens could arise from the CO2 flux from foraminiferal respiration and calcification, which is offset/compensated for by CO2 drawdown by symbionts in the photosymbiont bearing spinose foraminifera (Zeebe et al., 1999; Rink et al., 1998). Alternative explanations for the patterns seen in photosymbiont bearing foraminifera invoke the incorporation of the isotopically heavy B(OH)3 at slower calcification rates at lower pH, suggested to increase δ11Bforam resulting in a shallow δ11Bforam –δ11Bborate slope (Uchikawa et al., 2015; Farmer et al., 2019). Whether or not calcification rates impact δ11Bforam in N. pachyderma in this way remains to be determined because little is known about the exact foraminiferal calcification rates in their natural environment. Inferred calcification rates based on laboratory culturing experiments and temperature dependent modelling suggest that Neogloboquadrinoids have lower calcification rates overall than other planktonic foraminifera species (Lombard et al., 2011). Furthermore, recent advances in three-dimensional imaging techniques (microCT scans) propose that growth patterns of Neogloboquadrinoids through ontogeny follow a distinct growth trajectory that unlike other spinose species slows at maturity for the final whorl of chambers (Burke et al., 2020), which constitute 70 % or more of the total pre-gametogenic CaCO3. Whole-shell measurements of non-crusted tows, such as those here, are likely dominated by the isotopic composition recorded in these terminal chambers. In this scenario, if boric acid incorporation at low pH is responsible for <1 slopes in some species of foraminifera, it is unlikely to play a role in the Neogloboquadrinoids data presented here as low growth rates are associated with elevated δ11B above borate rather than below borate (Fig. 2b; Farmer et al., 2019; Uchikawa et al., 2015).
In the following sections we explore additional mechanisms that could explain the lower-than-expected δ11Bforam values and high sensitivity of N. pachyderma to low seawater borate (slope >1).
4.1.1 Foraminiferal microenvironment and calcification
Non-spinose foraminifera are generally devoid of photosynthetic symbionts; the pH of the micro-environment around such foraminifera is expected to more acidic than the ambient seawater. The respiration and calcification of foraminifera both release CO2 into the microenvironment during these processes, which have been suggested as the main drivers explaining the lower-than-expected δ11Bforam values in symbiont-barren species (Foster, 2008; Hönisch et al., 2003; Martínez-Botí et al., 2015; Yu et al., 2013).
To assess the calcification environment for N. pachyderma we estimated microenvironmental pH and the calcite saturation state (Ωcalcite) for our plankton tow dataset. Briefly, we derived Ωcalcite at calcification depth using hydrographic profiles (TA and DIC) which yields on average Ωcalcite=2.2. One way to approximate the conditions within the micro-environment of a living foraminiferal, without turning to complex diffusion-reaction modelling (e.g., Zeebe et al., 2003), is to simply model the effect of removing TA and DIC in a 2:1 ratio to simulate the influence of calcification (cf. Hönisch et al., 2019). Following Hönisch et al. (2019) we modelled the progressive removal of up to 200 and 400 µmol kg−1 from the ambient DIC and TA, respectively (as in Hönisch et al., 2019; Fig. 3). We note that this decrease in DIC and TA may be counteracted by diffusion. Taking the δ11B values of the N. pachyderma and N. incompta at face value, this treatment implies an average pH of 7.4 in the microenvironment with Ωcalcite=0.4. This suggests that N. pachyderma calcifies in waters undersaturated with respect to calcite.
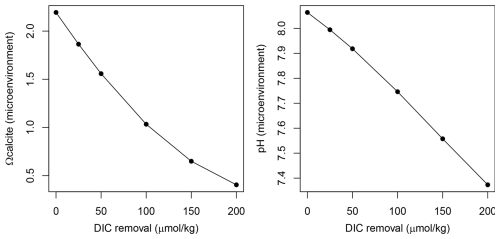
Figure 3Theoretical effect of calcification (inducing a decrease of TA : DIC in a 2:1 ratio) on the Omega calcite and pH of seawater in the foraminifera microenvironment.
Calcification in foraminifera, like in many marine carbonates (Gilbert et al., 2022), takes place in an internal space within a fluid, the calcifying fluid, that is ultimately modified from ambient seawater carbonate chemistry (de Nooijer et al., 2014, and references therein). Benthic foraminifera have been observed to upregulate the pH in their calcifying fluid as high as 9 (de Nooijer et al., 2009) by the removal of protons via a Ca-ATPase enzymatic pump (Toyofuku et al., 2017) and/or alkalinity elevation of seawater vacuoles to facilitate calcification (Bentov et al., 2009). It is likely that DIC is also elevated in the calcifying fluid by passive CO2 diffusion and active HCO pumping (Ujiié et al., 2023). Indeed, genes relating to these processes were recently identified in foraminifera by Ujiié et al. (2023).
Whether or not N. pachyderma upregulates its internal pH remains to be determined, however, active proton pumping as a mechanism for N. pachyderma calcification aligns well with the recent biomineralization model proposed for Mg incorporation in N. pachyderma outlined in Morley et al. (2024). Briefly, they demonstrated that the preferential exclusion of Mg2+ into N. pachyderma is compromised in unfavourable calcification environments (e.g., at low seawater [CO] and SST >5 °C) in order to maintain energy for proton removal (see also Evans et al., 2018; Zeebe and Sanyal, 2002). This observation invokes two important processes: (1) the generation and transport of bicarbonate and protons across the calcifying membrane and (2) that proton pumping may be less efficient for N. pachyderma at temperatures below 5 °C and low sea water [CO]. The latter process may provide an explanation for the steep δ11Bforam : δ11Bborate slope observed here in Eq. (1), considering that the samples collected from the coldest and most acidic environments lead to the largest offset from a slope = 1 (Fig. 2c).
Assuming N. pachyderma upregulate their internal pH, the lower-than-expected δ11Bforam values, suggesting a calcification fluid that is undersaturated with respect to calcite, remain puzzling. Following Gagnon et al. (2021) these observations could be reconciled if we consider boric acid diffusion into the calcifying fluid as part of the biomineralization process. If the diffusion of boric acid between the calcifying fluid and the microenvironment around the foraminifera is fast, while the exchange of seawater between the two is slow, it is possible for the concentration of boric acid internally to be the same as that in the micro-environment. Since the δ11B of each aqueous boron species is set by their relative proportions, this sets the δ11B of borate in the calcifying fluid to be equal to the δ11B of borate externally, even if the pH internally is significantly greater than the micro-environment. While this model reconciles the upregulation of pH in the calcifying fluid enabling calcification with δ11Bforam values representative of the (undersaturated) microenvironment surrounding the foraminifera, it awaits direct determination of the calcifying fluid pH in N. pachyderma to validate it.
4.1.2 The effect and impact of temperature on the boron isotope fractionation factor αB
The influence of temperature on the aqueous boron isotope fractionation factor αB has been the subject of debate (Hönisch et al., 2019) even though there is a theoretical thermodynamic basis for αB to be influenced by temperature (Zeebe, 2005), its magnitude is uncertain and it has not been conclusively demonstrated experimentally. So far, the fractionation αB has been determined at 25 and 40 °C at a salinity of 35 (Klochko et al., 2006). However, large uncertainties prevent a conclusive quantification of the temperature dependence of αB, which has been used to argue against a significant temperature sensitivity of αB over the temperature range used in most δ11B-pH calibrations and paleo-pH studies (Foster and Rae, 2016). In Fig. 2, we show the calibration equation with constant αB as defined in Klochko et al. (2006) and in Fig. S2c with a temperature dependent αB as defined in Hönisch et al. (2019).
Due to the large temperature gradient in our dataset, αB corrected for temperature has a significant effect on the slope and intercept (Fig. S2b and c). Like in other symbiont-barren planktonic foraminifera the temperature correction, when applied to N. pachyderma, reduces the large negative offset between δ11Bforam and predicted δ11Bborate however the microenvironmental pH remains slightly below the predicted δ11Bforam: δ11Bborate line. Therefore, even with a temperature dependent αB N. pachyderma would still require manipulation of microenvironmental pH to reconcile the disequilibrium isotopic offset from seawater values. Furthermore, there is no significant correlation between the offsets of N. pachyderma δ11B from δ11Bborate, and temperature (Fig. 2c), arguing against a strong influence of a temperature dependent αB. Thus, although a temperature dependence on the fractionation factor is likely to exist, our data suggests that it is not significant over the temperature range relevant for N. pachyderma.
4.2 The marine aggregate habitat hypothesis
It has been recently suggested that some non-spinose foraminifera live in marine aggregate (also referred to as marine snow or particulate organic matter). This has been indirectly observed in laboratory culture for N. pachyderma (Davis et al., 2020), G. truncatulinoides (Richey et al., 2022) and from plankton net for G. menardii and N. dutertrei (Fehrenbacher et al., 2018). Geochemical analysis of the shells of these species tend to show elevated (Fehrenbacher et al., 2018; Richey et al., 2022; Fritz-Endres et al., 2022; Hupp and Fehrenbacher, 2023) compared to species that have not been observed in marine aggregates, and well above values expected if precipitating in seawater given coefficient partition defined from laboratory experiment (e.g., Hönisch et al., 2011). Barium sulphate BaSO4 (barite) in pelagic environment is thought to precipitate via microbial activity (e.g., González-Munoz et al., 2003; Martinez-Ruiz et al., 2020) or through supersaturation of barium and sulphur in microenvironment that promotes precipitation abiotically (Dehairs et al., 1980; Bishop, 1988; Horner and Crockford, 2021, and references therein). Hence foraminifera calcifying in such a microenvironment would have their shell enriched in barium compared to the bulk seawater. Such a microenvironment could be a concern for paleo-pH reconstructions as we expect variable pH alteration in marine aggregates. Marine aggregates dominated by algae can result in a pH increase if the signal is dominated by photosynthesis (e.g., Ploug et al., 1999) whilst aggregates dominated by organic matter degradation will release CO2 and decrease pH by 0.22–0.91 (Alldredge and Cohen, 1987). Due to the technical challenges to collect intact marine aggregates from the water column and to measure pH with microelectrodes on sometimes floating aggregates, studies on pH alterations in marine aggregates are rare (Alldredge and Cohen, 1987) and further constraints are needed to quantify pH changes on multiple aggregates where N. pachyderma and N. incompta are observed to live.
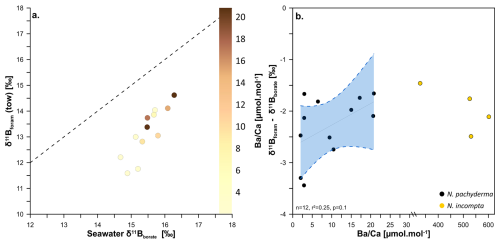
Figure 4Relationship between the δ11B of towed N. pachyderma (coloured circles with the δ11B of seawater borate in relation to calcite ratio. Panel (a) shows δ11B of towed specimens against δ11B of seawater borate while the colour gradient is showing the respective values. The 1:1 line is shown by the black dashed line. Panel (b) is showing the regression between δ11Bforam-δ11Bborate offset of towed N. pachyderma specimens and values (n=12, p=0.1, r2=0.25). The data are fitted with a linear regression (black line) and a 95 % confidence envelope (blue shade). Also shown in panel (b) are the high values for N. incompta in yellow circles; please note the axis break.
If barium concentration is an accurate metric to trace this behaviour in N. pachyderma and N. incompta we would expect samples with high to record a distinct pH and δ11B from samples with low- samples. Figure 4a shows some samples have higher barium than what is predicted by seawater following the partition coefficient DBa=0.11 of other non-spinose foraminifera (Fehrenbacher et al., 2018), yet do not show any anomalous δ11B compared to low- samples. The elevated results (>30 µmol mol−1) need to be caveated by the fact that samples were not treated for barite removal during the cleaning process (as in Fehrenbacher et al., 2018), as this would lead to unacceptable sample loss for δ11B measurement. The ratio in N. incompta is distinct from N. pachyderma, with a range of 328–779 µmol mol−1 (vs. 1–21 µmol mol−1 for N. pachyderma). The species difference and trend of values is observed in both tows and core tops. This striking contrast between the two species points towards a distinct behavioural difference between the two species, potentially suggesting N. incompta is more prone to live in marine aggregates or has a specific feeding behaviour that would favour Ba enrichment during calcification. The lack of any deviation in δ11B space for these high-Ba samples (Fig. 4b) suggest that the Ba enrichment observed did not result in a pH-altered microenvironment in marine aggregates influencing the δ11Bforam and instead that Ba increase may be caused by other processes (e.g. feeding behaviour). In the absence of direct evidence of aggregate environment associated with high for these two species, we conclude that the observed increase in , either does not result in significant pH change or if it does, it does not compromise the δ11B proxy in the species examined here. The hypothesis that these species live in marine aggregate, and the link with Ba-enrichment requires further investigation through direct observation of intact marine aggregates in the water column (e.g. collected with a marine snow catcher) or newly formed in cultures, as well as direct measurement of pH change in the interstitial fluid of the aggregates and in foraminifera shells.
4.3 δ11B-derived CO2 for core top values
A comparison of available δ11Bforam values measured on N. pachyderma from previously published cores tops (Ezat et al., 2017; Henehan et al., 2016; Yu et al., 2013) with our tow-based calibration dataset shows that there is generally good agreement between N. pachyderma collected from tows and core tops (Fig. 5). We note here that we recalculated seawater borate for the Yu et al. (2013) dataset using GLODAP v2 at 50 m instead of CARINA that was used in the original study. We used an anthropogenic DIC correction (CANT) on δ11Bborate values for all core tops pre-dating the onset of anthropogenic CO2 emissions (see Tables S4 and S6), The core tops analysed in this study fall within the calibration uncertainties of the tow-based calibration equation except for Station 16. Further, the majority of the Yu et al. (2013) data falls below the calibration equation derived using the plankton tows.
There could be several reasons for the offsets between calibration studies. First, the estimated calcification depth of 50 m for these samples may be too shallow. A shift to a deeper depth habitat (e.g. 75–150 m) would reduce δ11Bborate and thereby result in a better overall fit with the tow dataset (Fig. S3). However, in the absence of stable isotopes we cannot constrain habitat depth with better accuracy. Further, the modern water column data including the correction factor for anthropogenic carbon to estimate seawater borate may not represent the actual calcification environment of N. pachyderma preserved in core tops. For example, when the correction factor for anthropogenic carbon is removed for the Yu et al. (2013) dataset, the offset to the tow based calibration dataset is resolved. However, the pre-industrial ages for all but one core top in this dataset would strongly argue for the use of the correction factor for anthropogenic carbon. For station 16, it is possible that the sample from the multicore top 0–0.5 cm is modern or partially modern and should not be corrected for anthropogenic carbon. This is supported by the age model for gravity core M23411 (Baumann, 2007) collected at the same location as Station 16 providing Holocene sedimentation rates of 143 years per 0.5 cm. Therefore, the top 0–0.5 cm of sediments at this station could include a significant contribution of specimens grown after the onset of fossil fuel burning. However, in the absence of a date from the multicore top we cannot confirm it.
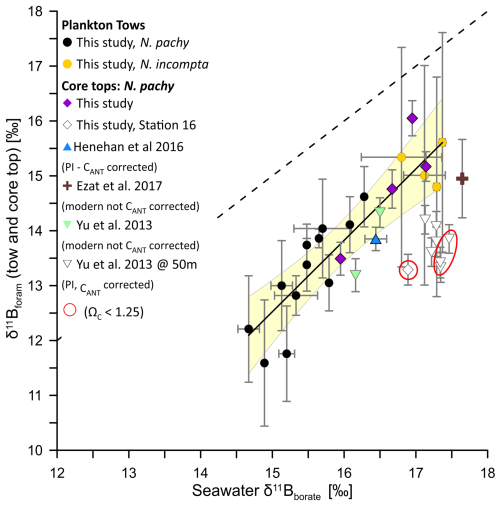
Figure 5Compilation of plankton tows and core top δ11Bforam versus δ11Bborate. Here we plot our tow and core top results together with all available δ11Bforam values measured on N. pachyderma (Henehan et al., 2016; Ezat et al., 2017; Yu et al., 2013). PI stands for preindustrial and CANT for anthropogenic carbon correction. The 1:1 line is shown by the black dashed line, and the solid black line shows the York regression of the tow dataset as shown in Fig. 2 with the 95 % confidence envelope (light yellow shade). The Yu et al. (2013) data is plotted with δ11Bborate values estimated at 50 m. Please view Fig. S3 for δ11Bborate values estimated at 150 m.
Alternatively, the lower than expected δ11Bforam values from the Yu et al. (2013) dataset may result from different size fractions used in the analysis. Here, core tops were sieved at a narrow size fraction of 200–250 µm, while Yu et al. (2013) used a larger range of 150–250 µm. Indeed, the offsets of N. pachyderma δ11Bforam from δ11Bborate are much larger for the Yu et al. (2013) dataset that includes smaller specimens. However, we note that our core tops are not offset from our plankton tow dataset which includes all size fractions. This would argue against a size effect. However, the apparent size effect could be reconciled if it is placed in the context of dissolution. For example, it has been observed that small foraminifera are more affected by dissolution than larger individuals as a result of their higher surface area to volume ratio (Berger and Piper, 1972; Berger et al., 1982). The cumulative effect of smaller size fractions and partial dissolution may therefore explain the apparent offset in the Yu et al. (2013) dataset (Ni et al., 2007).
Finally, δ11B values recorded in crust calcite may be lower than in ontogenetic calcite. This has previously been suggested for T. sacculifer, where crust calcite is lower in δ11B relative to ontogenetic calcite because symbionts are expelled or digested before gametogenic calcification begins (Ni et al., 2007). This loss of photosymbionts decreases the local pH due to the lack of photosynthetic activity and the δ11Bborate of the microenvironment would be lower than ambient seawater values. Consequently, the crust calcite will be isotopically lighter than the ontogenetic calcite (Ni et al., 2007). However, N. pachyderma is asymbiotic and modern ecological observations would argue against a habitat migration to deeper depth during gametogenesis (Tell et al., 2022; Manno and Pavlov, 2014; Greco et al., 2019) and therefore it is unlikely that a difference in hydrographic conditions would be responsible for a geochemical signature difference between ontogenetic and crust calcite. These ecological observations are supported by geochemical analysis of living (no crust) and dead (crust) N. pachyderma specimens collected from the same plankton nets (Hupp and Fehrenbacher, 2023), N. incompta and N. dutertrei specimen grown and crusted in culture under constant temperatures (Davis et al., 2017; WestgÅrd et al., 2023; Fehrenbacher et al., 2017) and N. pachyderma specimen from sediment traps (Jonkers et al., 2016).
The mechanisms responsible for the geochemical offsets observed in ontogenetic and crust calcite for N. pachyderma (e.g., % and δ18Oc up to 2 ‰), remain unresolved, but are more likely linked to changes in the biomineralization process than hydrography. For example, faster calcification rates during the formation of crust calcite relative to the last whirl of chambers could result in variable kinetic fractionation during ontogeny and therefore variation in the degree of isotopically heavy B(OH)3 incorporated into the foraminiferal calcite in crusted specimen (Uchikawa et al., 2015; Farmer et al., 2019). If so, the degree of crusting would be an additional factor to consider when preparing specimens for analysis. Furthermore, the more soluble ontogenetic calcite (Wycech et al., 2018), could be prone to preferential dissolution (water column and sediments) relative to crust calcite, leaving core top N. pachyderma from sites close to undersaturation depleted in δ11B values (Hönisch and Hemming, 2004; Ni et al., 2007; Seki et al., 2010). Indeed, we note that the low δ11B values from Station 16 and from some stations of the Yu et al. (2013) dataset have low bottom water ΩC <1.25 values (Fig. 4a, Table S5). Unfortunately, calcification rates are difficult to determine in culture for N. pachyderma as temperature control, especially at low temperatures, prevents prolonged periods of observations. Furthermore, there is no systematic δ11B offset between crusted and uncrusted specimens preventing us at this stage from estimating a geochemical offset value between ontogenetic and crust calcite (e.g., Kozdon et al., 2009). Ongoing developments in δ11B single shell analysis vs. MC-ICPMS laser ablation (Standish et al., 2019; Mayk et al., 2020; Raitzsch et al., 2020) N. pachyderma may provide enhanced spatial resolution to address the foraminifera test isotopic heterogeneity.
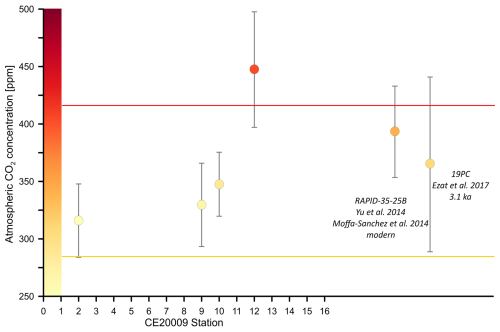
Figure 6Atmospheric CO2 concentration [ppm] derived using boron isotopes, and δ18Oc from CE20009 core tops collected from the Nordic Seas (except station 16). Also shown are two core tops that have paired and d18Oc values for N. pachyderma available. One from the Yu et al. (2013) dataset that we paired with and δ18Oc from Moffa-Sánchez et al. (2014) and the other is from Ezat et al. (2017). The CO2 uncertainty was calculated with a Monte Carlo simulation (see methods) See also Table S6.
To assess whether the tow-based calibration can be used to estimate past pH and atmospheric CO2 concentrations beyond these caveats we now treat the core tops as if they were an unknown paleo-sample. δ11B derived CO2 estimates from core tops in this study (except for station 16) provide CO2 values that are consistent with atmospheric CO2 observations made post 1900 (Fig. 6). These results suggest that despite the low sedimentation rates; a proportion of modern foraminifera shells capture the anthropogenic CO2 signal within the top 0–0.5 cm of surface sediments in the Nordic Seas. We also calculated atmospheric CO2 using published core tops from Ezat et al. (2017) (which has larger uncertainties due to the additional uncertainties linked to differing analytical methods) and one core top from Yu et al. (2013) that we were able to pair with values from the same multicore top published in Moffa-Sánchez et al. (2014). The highest reconstructed CO2 values consistent with modern atmospheric CO2 were measured in core tops that were confirmed modern by AMS 14C dating (e.g., Station 12 and RAPID-35-25B). Applying the new calibration with a slope >1 to the downcore data of Yu et al. (2013), will elevate reconstructed surface seawater CO2. As a result, regions off Iceland in the polar North Atlantic may not have served as a strong sink of CO2 to the atmosphere during the glacial and deglacial period as hypothesized in Yu et al. (2013). A detailed analysis of existing and new palaeoceanographic datasets will be the object of a forthcoming manuscript.
Here we present the first tow-based open ocean δ11B calibration of the non-spinose foraminifera N. pachyderma and N. incompta in the North Atlantic, complemented by core tops from similar locations. The use of tows allows us to precisely constrain the water chemistry in which foraminifera grew, bypassing the assumptions of foraminifera habitat depth and water chemistry typically encountered in core-top-based calibrations. We show that the signal recorded by these species is consistently below what is predicted by aqueous δ11Bborate, in line with other non-spinose foraminifera and that there is little evidence to support a strong influence of a temperature dependent αB.
We propose instead that the lower-than-expected δ11Bforam values are a result of active proton pumping during calcification raising the pH in the calcification fluid while the microenvironment surrounding the cell is undersaturated with respect to calcite as a result of respiration and calcification. The seemingly opposing signals can be reconciled when considering rapid boric acid diffusion between the calcifying fluid and the microenvironment around the foraminifera (Gagnon et al., 2021).
The addition of core top samples in the calibration does not significantly alter the slope of the calibration, giving confidence in the application of this tow-based calibration equation in downcore sediments. This is further supported when combining the δ11B calibration with the -δ18O correction scheme to reconstruct past atmospheric CO2 concentrations. Given the rapid rise in recent atmospheric CO2 concentrations, highly resolved marine archives when independently dated (e.g. 210Pb), may be used to reconstruct ocean surface CO2 and air-sea CO2 fluxes over the past 200 years/the last glacial cycles and thereby provide crucial insight into ocean-atmosphere carbon fluxes since the onset of anthropogenic carbon emissions over deepwater formation regions. The application of the δ11B calibration to recent marine archives may therefore allow us to answer critical open questions about the role of the Nordic Seas as a carbon sink when deepwater formation was reduced (stronger) and sea ice extent was greater (reduced).
All data needed to evaluate the conclusions in the paper are presented in the paper and/or the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-6765-2025-supplement.
EdlV carried out palaeoceanographic sample preparation and geochemical analysis of plankton tows and core tops from expedition CE20009, compiled and analysed all datasets, and wrote the original draft of the manuscript. AM conceptualized the project, acquired funding, carried out palaeoceanographic sample preparation of plankton tows from the Labrador Sea, coordinated the input of all co-authors and finalized the manuscript for publication. MR performed trace element and boron isotope analysis on N. pachyderma plankton tow samples from the Labrador Sea. JB supervised AM at the Alfred Wegner Institute and oversaw trace element and boron isotope analysis. UN oversaw all stable isotope analyses of water and foraminifera samples from CE20009 and contributed to the final version of this manuscript. GF oversaw trace element and boron isotope analysis on N. pachyderma plankton tow samples from CE20009 and contributed to the final version of this manuscript. MK advised AM during project conceptualization, supervised her during her MSCA-IF at MARUM, University of Bremen, and contributed to the final version of this manuscript. TB provided support to EdlV during boron isotope analysis and contributed to the final version of this manuscript. JCB carried out palaeoceanographic sample preparation of plankton tows from expedition CE20009. ME provided sample material and contributed to the final version of this manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We gratefully acknowledge the support of the crew on the RV Celtic Explorer sailing under Master Anthony Hobin.
This research was funded by the EU H2020 Marie Skłodowska-Curie Actions Project ARCTICO (grant no. 838529) and the Marine Institute of Ireland Research Programme 2014-2020 (grant no. PDOC/19/05/02) awarded to Audrey Morley. In addition, Audrey Morley received financial support from Research Ireland and the Geological Survey of Ireland under the SFI Frontiers for the Future Programme 21/FFP-P/10261 and Grant in Aid funding from the Marine Institute for research expedition CE20009 on the RV Celtic Explorer. Markus Raitzsch acknowledges DFG (German Research Foundation) for funding through research grant no. RA 2068/4-1.
This paper was edited by Chiara Borrelli and reviewed by two anonymous referees.
Alldredge, A. L. and Cohen, Y.: Can microscale chemical patches persist in the sea? Microelectrode study of marine snow, fecal pellets, Science, 235, 689–691, 1987.
Altuna, N. E. B., Pieńkowski, A. J., Eynaud, F., and Thiessen, R.: The morphotypes of Neogloboquadrina pachyderma: Isotopic signature and distribution patterns in the Canadian Arctic Archipelago and adjacent regions, Marine Micropaleontology, 142, 13–24, 2018.
Anagnostou, E., Huang, K.-F., You, C.-F., Sikes, E., and Sherrell, R.: Evaluation of boron isotope ratio as a pH proxy in the deep sea coral Desmophyllum dianthus: Evidence of physiological pH adjustment, Earth and Planetary Science Letters, 349, 251–260, 2012.
Anagnostou, E., Williams, B., Westfield, I., Foster, G., and Ries, J.: Calibration of the pH-δ11B and temperature-Mg/Li proxies in the long-lived high-latitude crustose coralline red alga Clathromorphum compactum via controlled laboratory experiments, Geochimica et Cosmochimica Acta, 254, 142–155, 2019.
Anand, P., Elderfield, H., and Conte, M. H.: Calibration of thermometry in planktonic foraminifera from a sediment trap time series, Paleoceanography, 18, 1050, https://doi.org/10.1029/2002pa000846, 2003.
Azetsu-Scott, K., Clarke, A., Falkner, K., Hamilton, J., Jones, E. P., Lee, C., Petrie, B., Prinsenberg, S., Starr, M., and Yeats, P.: Calcium carbonate saturation states in the waters of the Canadian Arctic Archipelago and the Labrador Sea, Journal of Geophysical Research: Oceans, 115, C11021, https://doi.org/10.1029/2009JC005917, 2010.
Barker, S., Greaves, M., and Elderfield, H.: A study of cleaning procedures used for foraminiferal paleothermometry, Geochemistry Geophysics Geosystems, 4, 8407, https://doi.org/10.1029/2003GC000559, 2003.
Bauch, D., Carstens, J., and Wefer, G.: Oxygen isotope composition of living Neogloboquadrina pachyderma (sin.) in the Arctic Ocean, Earth and Planetary Science Letters, 146, 47–58, 1997.
Baumann, A.: Dinoflagellaten-Zysten als Paläoumweltindikatoren im Spätquartär des Europäischen Nordmeeres, PhD thesis, Universität Bremen, https://doi.org/10.23689/fidgeo-314, 2007.
Bentov, S., Brownlee, C., and Erez, J.: The role of seawater endocytosis in the biomineralization process in calcareous foraminifera, Proceedings of the National Academy of Sciences, 106, 21500–21504, 2009.
Bereiter, B., Eggleston, S., Schmitt, J., Nehrbass-Ahles, C., Stocker, T. F., Fischer, H., Kipfstuhl, S., and Chappellaz, J.: Revision of the EPICA Dome C CO2 record from 800 to 600 kyr before present, Geophysical Research Letters, 42, 542–549, 2015.
Berger, W. H. and Piper, D. J. W.: Planktonic foraminifera: differential settling, dissolution, and redeposition, Limnology Oceanography, 17, 275–287, 1972.
Berger, W. H., Bonneau, M.-C., and Parker, F. L.: Foraminifera on the deep-sea floor: lysocline and dissolution rate, Oceanologica Acta, 5, 249–258, 1982.
Bishop, J. K.: The barite-opal-organic carbon association in oceanic particulate matter, Nature, 6162, 341–343, 1988.
Burke, J. E., Renema, W., Schiebel, R., and Hull, P. M.: Three-dimensional analysis of inter-and intraspecific variation in ontogenetic growth trajectories of planktonic foraminifera, Marine Micropaleontology, 155, 101794, https://doi.org/10.1016/j.marmicro.2019.101794, 2020.
Chalk, T. B., Hain, M. P., Foster, G. L., Rohling, E. J., Sexton, P. F., Badger, M. P., Cherry, S. G., Hasenfratz, A. P., Haug, G. H., and Jaccard, S. L.: Causes of ice age intensification across the Mid-Pleistocene Transition, Proceedings of the National Academy of Sciences, 114, 13114–13119, 2017.
Cifelli, R.: Globigerina incompta, a new species of pelagic foraminifera from the North Atlantic, Contributions from the Cushman Foundation for Foraminiferal Research, 12, 83–86, 1961.
Consortium, C. C. P. I. P., Hönisch, B., Royer, D. L., Breecker, D. O., Polissar, P. J., Bowen, G. J., Henehan, M. J., Cui, Y., Steinthorsdottir, M., and McElwain, J. C.: Toward a Cenozoic history of atmospheric CO2, Science, 382, eadi5177, https://doi.org/10.1126/science.adi5177, 2023.
Darling, K. F., Kucera, M., Kroon, D., and Wade, C. M.: A resolution for the coiling direction paradox in Neogloboquadrina pachyderma, Paleoceanography, 21, PA2011, https://doi.org/10.1029/2005PA001189, 2006.
Davis, C. V., Fehrenbacher, J. S., Hill, T. M., Russell, A. D., and Spero, H. J.: Relationships Between Temperature, pH, and Crusting on Ratios in Laboratory-Grown Neogloboquadrina Foraminifera, Paleoceanography, 32, 1137–1152, 2017.
Davis, C. V., Livsey, C. M., Palmer, H. M., Hull, P. M., Thomas, E., Hill, T. M., and Benitez-Nelson, C. R.: Extensive morphological variability in asexually produced planktic foraminifera, Science Advances, 6, eabb8930, https://doi.org/10.1126/sciadv.abb8930, 2020.
Dehairs, F., Chesselet, R., and Jedwab, J.: Discrete suspended particles of barite and the barium cycle in the open ocean, Earth and Planetary Science Letters, 49, 528–550, https://doi.org/10.1016/0012-821X(80)90094-1, 1980.
Dekens, P. S., Lea, D. W., Pak, D. K., and Spero, H. J.: Core top calibration of in tropical foraminifera: Refining paleotemperature estimation, Geochemistry Geophysics Geosystems, 3, 1022, https://doi.org/10.1029/2001gc000200, 2002.
de la Vega, E., Chalk, T. B., Hain, M. P., Wilding, M. R., Casey, D., Gledhill, R., Luo, C., Wilson, P. A., and Foster, G. L.: Orbital CO2 reconstruction using boron isotopes during the late Pleistocene, an assessment of accuracy, Clim. Past, 19, 2493–2510, https://doi.org/10.5194/cp-19-2493-2023, 2023.
de Nooijer, L. J., Toyofuku, T., and Kitazato, H.: Foraminifera promote calcification by elevating their intracellular pH, Proceedings of the National Academy of Sciences, 106, 15374–15378, 2009.
de Nooijer, L. D., Spero, H., Erez, J., Bijma, J., and Reichart, G.-J.: Biomineralization in perforate foraminifera, Earth-Science Reviews, 135, 48–58, 2014.
Dickson, A.: Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K, Deep Sea Research Part A, 37, 755–766, 1990.
Erez, J. and Honjo, S.: Comparison of isotopic composition of planktonic foraminifera in plankton tows, sediment traps and sediments, Palaeogeogr. Palaeoclimatol. Palaeocol., 33, 129–156, 1981.
Evans, D., Müller, W., and Erez, J.: Assessing foraminifera biomineralisation models through trace element data of cultures under variable seawater chemistry, Geochimica et Cosmochimica Acta, 236, 198–217, 2018.
Ezat, M. M., Rasmussen, T. L., Hönisch, B., Groeneveld, J., and Demenocal, P.: Episodic release of CO2 from the high-latitude North Atlantic Ocean during the last 135 kyr, Nature Communications, 8, 14498, https://doi.org/10.1038/ncomms14498, 2017.
Farmer, J. R., Hönisch, B., and Uchikawa, J.: Single laboratory comparison of MC-ICP-MS and N-TIMS boron isotope analyses in marine carbonates, Chemical Geology, 447, 173–182, 2016.
Farmer, J. R., Branson, O., Uchikawa, J., Penman, D. E., Hönisch, B., and Zeebe, R. E.: Boric acid and borate incorporation in inorganic calcite inferred from B/Ca, boron isotopes and surface kinetic modeling, Geochimica et Cosmochimica Acta, 244, 229–247, 2019.
Fehrenbacher, J. S., Russell, A. D., Davis, C. V., Gagnon, A. C., Spero, H. J., Cliff, J. B., Zhu, Z., and Martin, P.: Link between light-triggered Mg-banding and chamber formation in the planktic foraminifera Neogloboquadrina dutertrei, Nature Communications, 8, 15441, https://doi.org/10.1038/ncomms15441, 2017.
Fehrenbacher, J. S., Russell, A. D., Davis, C. V., Spero, H. J., Chu, E., and Hönisch, B.: ratios in the non-spinose planktic foraminifer Neogloboquadrina dutertrei: Evidence for an organic aggregate microhabitat, Geochimica et Cosmochimica Acta, 236, 361–372, 2018.
Foster, G.: Seawater pH, pCO2 and [CO2-3] variations in the Caribbean Sea over the last 130 kyr: a boron isotope and B/Ca study of planktic foraminifera, Earth and Planetary Science Letters, 271, 254–266, 2008.
Foster, G. L. and Rae, J. W.: Reconstructing ocean pH with boron isotopes in foraminifera, Annual Review of Earth and Planetary Sciences, 44, 207–237, 2016.
Foster, G., Pogge von Strandmann, P. A., and Rae, J.: Boron and magnesium isotopic composition of seawater, Geochemistry, Geophysics, Geosystems, 11, Q08015, https://doi.org/10.1029/2010GC003201, 2010.
Foster, G. L., Hönisch, B., Paris, G., Dwyer, G. S., Rae, J. W., Elliott, T., Gaillardet, J., Hemming, N. G., Louvat, P., and Vengosh, A.: Interlaboratory comparison of boron isotope analyses of boric acid, seawater and marine CaCO3 by MC-ICPMS and NTIMS, Chemical Geology, 358, 1–14, 2013.
Fritz-Endres, T., Fehrenbacher, J. S., Russell, A. D., and Cynar, H.: Increased productivity in the equatorial pacific during the deglaciation inferred from the ratios of non-spinose planktic foraminifera, Paleoceanography and Paleoclimatology, 37, e2022PA004506, https://doi.org/10.1029/2022PA004506, 2022.
Gagnon, A. C., Gothmann, A. M., Branson, O., Rae, J. W., and Stewart, J. A.: Controls on boron isotopes in a cold-water coral and the cost of resilience to ocean acidification, Earth and Planetary Science Letters, 554, 116662, https://doi.org/10.1016/j.epsl.2020.116662, 2021.
Gaillardet, J., Lemarchand, D., Göpel, C., and Manhès, G.: Evaporation and sublimation of boric acid: application for boron purification from organic rich solutions, Geostandards Newsletter, 25, 67–75, 2001.
Gilbert, P. U., Bergmann, K. D., Boekelheide, N., Tambutté, S., Mass, T., Marin, F., Adkins, J. F., Erez, J., Gilbert, B., and Knutson, V.: Biomineralization: Integrating mechanism and evolutionary history, Science advances, 8, eabl9653, https://doi.org/10.1126/sciadv.abl9653, 2022.
González-Munoz, M. T., Fernández-Luque, B., Martínez-Ruiz, F., Ben Chekroun, K., Arias, J. M., Rodríguez-Gallego, M., Martínez-Canamero, M., de Linares, C., and Paytan, A.: Precipitation of barite by Myxococcus xanthus: possible implications for the biogeochemical cycle of barium, Applied and Environmental Microbiology, 69.9, https://doi.org/10.1128/AEM.69.9.5722-5725.2003, 2003.
Gray, W. R. and Evans, D.: Nonthermal influences on in planktonic foraminifera: A review of culture studies and application to the last glacial maximum, Paleoceanography and Paleoclimatology, 34, 306–315, 2019.
Greco, M., Jonkers, L., Kretschmer, K., Bijma, J., and Kucera, M.: Depth habitat of the planktonic foraminifera Neogloboquadrina pachyderma in the northern high latitudes explained by sea-ice and chlorophyll concentrations, Biogeosciences, 16, 3425–3437, https://doi.org/10.5194/bg-16-3425-2019, 2019.
Guillermic, M., Misra, S., Eagle, R., Villa, A., Chang, F., and Tripati, A.: Seawater pH reconstruction using boron isotopes in multiple planktonic foraminifera species with different depth habitats and their potential to constrain pH and pCO2 gradients, Biogeosciences, 17, 3487–3510, https://doi.org/10.5194/bg-17-3487-2020, 2020.
Gutjahr, M., Bordier, L., Douville, E., Farmer, J., Foster, G. L., Hathorne, E. C., Hönisch, B., Lemarchand, D., Louvat, P., and McCulloch, M.: Sub-permil interlaboratory consistency for solution-based boron isotope analyses on marine carbonates, Geostandards and Geoanalytical Research, 45, 59–75, 2021.
Hansen, B. and Østerhus, S.: North atlantic–nordic seas exchanges, Progress in oceanography, 45, 109–208, 2000.
Hathorne, E. C., Gagnon, A., Felis, T., Adkins, J., Asami, R., Boer, W., Caillon, N., Case, D., Cobb, K. M., and Douville, E.: Interlaboratory study for coral Sr/Ca and other element Ca ratio measurements, Geochemistry, Geophysics, Geosystems, 14, 3730–3750, 2013.
Henehan, M. J., Rae, J. W., Foster, G. L., Erez, J., Prentice, K. C., Kucera, M., Bostock, H. C., Martínez-Botí, M. A., Milton, J. A., and Wilson, P. A.: Calibration of the boron isotope proxy in the planktonic foraminifera Globigerinoides ruber for use in palaeo-CO2 reconstruction, Earth and Planetary Science Letters, 364, 111–122, 2013.
Henehan, M. J., Foster, G. L., Rae, J. W., Prentice, K. C., Erez, J., Bostock, H. C., Marshall, B. J., and Wilson, P. A.: Evaluating the utility of B/Ca ratios in planktic foraminifera as a proxy for the carbonate system: A case study of Globigerinoides ruber, Geochemistry, Geophysics, Geosystems, 16, 1052–1069, 2015.
Henehan, M. J., Foster, G. L., Bostock, H. C., Greenop, R., Marshall, B. J., and Wilson, P. A.: A new boron isotope-pH calibration for Orbulina universa, with implications for understanding and accounting for `vital effects', Earth and Planetary Science Letters, 454, 282–292, 2016.
Hönisch, B. and Hemming, N. G.: Ground-truthing the boron isotope-paleo-pH proxy in planktonic foraminifera shells: Partial dissolution and shell size effects, Paleoceanography, 19, PA4010, https://doi.org/10.1029/2004PA001026, 2004.
Hönisch, B., Bijma, J., Russell, A. D., Spero, H. J., Palmer, M. R., Zeebe, R. E., and Eisenhauer, A.: The influence of symbiont photosynthesis on the boron isotopic composition of foraminifera shells, Marine Micropaleontology, 49, 87–96, 2003.
Hönisch, B., Allen, K. A., Russell, A. D., Eggins, S. M., Bijma, J., Spero, H. J., Lea, D. W., and Yu, J.: Planktic foraminifers as recorders of seawater , Marine Micropaleontology, 79, 52–57, https://doi.org/10.1016/j.marmicro.2011.01.003, 2011.
Hönisch, B., Eggins, S. M., Haynes, L. L., Allen, K. A., Holland, K. D., and Lorbacher, K.: Boron proxies in paleoceanography and paleoclimatology, Wiley, ISBN 9781119010678, 2019.
Horner, T. J. and Crockford, P. W.: Barium isotopes: Drivers, dependencies, and distributions through space and time, Cambridge University Press, Cambridge University Press, ISBN 9781108865845, 2021.
Hupp, B. N. and Fehrenbacher, J. S.: Geochemical differences between alive, uncrusted and dead, crusted shells of Neogloboquadrina pachyderma: Implications for paleoreconstruction, Paleoceanography and Paleoclimatology, 38, e2023PA004638, https://doi.org/10.1029/2023PA004638, 2023.
Johnson, G. C.: Quantifying Antarctic bottom water and North Atlantic deep water volumes, Journal of Geophysical Research: Oceans, 113, C05027, https://doi.org/10.1029/2007JC004477, 2008.
Jonkers, L., Brummer, G. J. A., Peeters, F. J., van Aken, H. M., and De Jong, M. F.: Seasonal stratification, shell flux, and oxygen isotope dynamics of left-coiling N. pachyderma and T. quinqueloba in the western subpolar North Atlantic, Paleoceanography, 25, PA2204, https://doi.org/10.1029/2009PA001849, 2010.
Jonkers, L., Heuven, S., Zahn, R., and Peeters, F. J.: Seasonal patterns of shell flux, δ18O and δ13C of small and large N. pachyderma (s) and G. bulloides in the subpolar North Atlantic, Paleoceanography, 28, 164–174, 2013.
Jonkers, L., Buse, B., Brummer, G.-J. A., and Hall, I. R.: Chamber formation leads to banding in the planktonic foraminifer Neogloboquadrina pachyderma, Earth and Planetary Science Letters, 451, 177–184, 2016.
Jonkers, L., Brummer, G.-J. A., Meilland, J., Groeneveld, J., and Kucera, M.: Variability in Neogloboquadrina pachyderma stable isotope ratios from isothermal conditions: implications for individual foraminifera analysis, Clim. Past, 18, 89–101, https://doi.org/10.5194/cp-18-89-2022, 2022.
King, A. L. and Howard, W. R.: δ18O seasonality of planktonic foraminifera from Southern Ocean sediment traps: Latitudinal gradients and implications for paleoclimate reconstructions, Marine Micropaleontology, 56, 1–24, 2005.
Klochko, K., Kaufman, A. J., Yao, W., Byrne, R. H., and Tossell, J. A.: Experimental measurement of boron isotope fractionation in seawater, Earth and Planetary Science Letters, 248, 276–285, 2006.
Kohfeld, K. E., Fairbanks, R. G., Smith, S. L., and Walsh, I. D.: Neogloboquadrina pachyderma (sinistral coiling) as paleoceanographic tracers in polar oceans: Evidence from Northeast Water Polynya plankton tows, sediment traps, and surface sediments, Paleoceanography, 11, 679–699, 1996.
Kozdon, R., Ushikubo, T., Kita, N., Spicuzza, M., and Valley, J.: Intratest oxygen isotope variability in the planktonic foraminifer N. pachyderma: Real vs. apparent vital effects by ion microprobe, Chemical Geology, 258, 327–337, 2009.
Lauvset, S. K., Lange, N., Tanhua, T., Bittig, H. C., Olsen, A., Kozyr, A., Alin, S., Álvarez, M., Azetsu-Scott, K., Barbero, L., Becker, S., Brown, P. J., Carter, B. R., da Cunha, L. C., Feely, R. A., Hoppema, M., Humphreys, M. P., Ishii, M., Jeansson, E., Jiang, L.-Q., Jones, S. D., Lo Monaco, C., Murata, A., Müller, J. D., Pérez, F. F., Pfeil, B., Schirnick, C., Steinfeldt, R., Suzuki, T., Tilbrook, B., Ulfsbo, A., Velo, A., Woosley, R. J., and Key, R. M.: GLODAPv2.2022: the latest version of the global interior ocean biogeochemical data product, Earth Syst. Sci. Data, 14, 5543–5572, https://doi.org/10.5194/essd-14-5543-2022, 2022.
Lea, D., Mashiotta, T. A., and Spero, H. J.: Controls on magnesium and strontium uptake in planktonic foraminifera determined by live culturing, Geochimica et Cosmochimica Acta, 63, 2369–2379, 1999.
Lee, K., Kim, T.-W., Byrne, R. H., Millero, F. J., Feely, R. A., and Liu, Y.-M.: The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans, Geochimica et Cosmochimica Acta, 74, 1801–1811, 2010.
Livsey, C. M., Kozdon, R., Bauch, D., Brummer, G. J. A., Jonkers, L., Orland, I., Hill, T. M., and Spero, H. J.: High-resolution and δ18O patterns in modern Neogloboquadrina pachyderma from the Fram Strait and Irminger Sea, Paleoceanography and Paleoclimatology, 35, e2020PA003969, https://doi.org/10.1029/2020PA003969, 2020.
Lombard, F., Labeyrie, L., Michel, E., Bopp, L., Cortijo, E., Retailleau, S., Howa, H., and Jorissen, F.: Modelling planktic foraminifer growth and distribution using an ecophysiological multi-species approach, Biogeosciences, 8, 853–873, https://doi.org/10.5194/bg-8-853-2011, 2011.
Manno, C. and Pavlov, A.: Living planktonic foraminifera in the Fram Strait (Arctic): absence of diel vertical migration during the midnight sun, Hydrobiologia, 721, 285–295, 2014.
Martínez-Botí, M., Mortyn, P., Schmidt, D., Vance, D., and Field, D.: in foraminifera from plankton tows: Evaluation of proxy controls and comparison with core tops, Earth and Planetary Science Letters, 307, 113–125, 2011.
Martínez-Botí, M. A., Marino, G., Foster, G. L., Ziveri, P., Henehan, M. J., Rae, J. W., Mortyn, P. G., and Vance, D.: Boron isotope evidence for oceanic carbon dioxide leakage during the last deglaciation, Nature, 518, 219, https://doi.org/10.1038/nature14155, 2015.
Martinez-Ruiz, F., Paytan, A., Gonzalez-Munoz, M., Jroundi, F., Abad, M. d. M., Lam, P. J., Horner, T. J., and Kastner, M.: Barite precipitation on suspended organic matter in the mesopelagic zone, Frontiers in Earth Science, 8, 567714, https://doi.org/10.3389/feart.2020.567714, 2020.
Mayk, D., Fietzke, J., Anagnostou, E., and Paytan, A.: LA-MC-ICP-MS study of boron isotopes in individual planktonic foraminifera: A novel approach to obtain seasonal variability patterns, Chemical Geology, 531, 119351, https://doi.org/10.1016/j.chemgeo.2019.119351, 2020.
McCulloch, M., Trotter, J., Montagna, P., Falter, J., Dunbar, R., Freiwald, A., Försterra, G., Correa, M. L., Maier, C., and Rüggeberg, A.: Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation, Geochimica et Cosmochimica Acta, 87, 21–34, 2012.
Meilland, J., Siccha, M., Kaffenberger, M., Bijma, J., and Kucera, M.: Population dynamics and reproduction strategies of planktonic foraminifera in the open ocean, Biogeosciences, 18, 5789–5809, https://doi.org/10.5194/bg-18-5789-2021, 2021.
Mikis, A., Hendry, K. R., Pike, J., Schmidt, D. N., Edgar, K. M., Peck, V., Peeters, F. J. C., Leng, M. J., Meredith, M. P., Jones, C. L. C., Stammerjohn, S., and Ducklow, H.: Temporal variability in foraminiferal morphology and geochemistry at the West Antarctic Peninsula: a sediment trap study, Biogeosciences, 16, 3267–3282, https://doi.org/10.5194/bg-16-3267-2019, 2019.
Misra, S., Owen, R., Kerr, J., Greaves, M., and Elderfield, H.: Determination of δ11B by HR-ICP-MS from mass limited samples: Application to natural carbonates and water samples, Geochimica et Cosmochimica Acta, 140, 531–552, 2014.
Moffa-Sánchez, P., Hall, I. R., Barker, S., Thornalley, D. J., and Yashayaev, I.: Surface changes in the eastern Labrador Sea around the onset of the Little Ice Age, Paleoceanography, 29, 160–175, 2014.
Morley, A., de la Vega, E., Raitzsch, M., Bijma, J., Ninnemann, U., Foster, G., Chalk, T. B., Meilland, J., Cave, R., and Büscher, J.: A solution for constraining past marine Polar Amplification, Nature Communications, 15, 9002, https://doi.org/10.1038/s41467-024-53424-w, 2024.
Mortyn, P. G. and Charles, C. D.: Planktonic foraminiferal depth habitat and δ18O calibrations: Plankton tow results from the Atlantic sector of the Southern Ocean, Paleoceanography, 18, 1037, https://doi.org/10.1029/2001PA000637, 2003.
Ni, Y., Foster, G. L., Bailey, T., Elliott, T., Schmidt, D. N., Pearson, P., Haley, B., and Coath, C.: A core top assessment of proxies for the ocean carbonate system in surface-dwelling foraminifers, Paleoceanography, 22, PA3212, https://doi.org/10.1029/2006PA001337, 2007.
Nickoloff, G., Else, B., Ahmed, M., Burgers, T., Miller, L., and Papakyriakou, T.: Strong pCO2 undersaturation in an Arctic sea: A decade of spatial and temporal variability in Baffin Bay, Journal of Geophysical Research: Oceans, 129, e2023JC020595, https://doi.org/10.1029/2023JC020595, 2024.
Østerhus, S., Woodgate, R., Valdimarsson, H., Turrell, B., de Steur, L., Quadfasel, D., Olsen, S. M., Moritz, M., Lee, C. M., Larsen, K. M. H., Jónsson, S., Johnson, C., Jochumsen, K., Hansen, B., Curry, B., Cunningham, S., and Berx, B.: Arctic Mediterranean exchanges: a consistent volume budget and trends in transports from two decades of observations, Ocean Sci., 15, 379–399, https://doi.org/10.5194/os-15-379-2019, 2019.
Pados, T. and Spielhagen, R. F.: Species distribution and depth habitat of recent planktic foraminifera in Fram Strait, Arctic Ocean, Polar Research, 33, 22483, https://doi.org/10.3402/polar.v33.22483, 2014.
Ploug, H., Grossart, H.-P., Azam, F., and Jorgensen, B. B.: Photosynthesis, respiration, and carbon turnover in sinking marine snow from surface waters of Southern California Bight: implications for the carbon cycle, Marine Ecology Progress Series, 179, 1–11, 1999.
Rae, J. W., Foster, G. L., Schmidt, D. N., and Elliott, T.: Boron isotopes and B/Ca in benthic foraminifera: Proxies for the deep ocean carbonate system, Earth and Planetary Science Letters, 302, 403–413, 2011.
Rae, J. W., Burke, A., Robinson, L., Adkins, J. F., Chen, T., Cole, C., Greenop, R., Li, T., Littley, E., and Nita, D.: CO2 storage and release in the deep Southern Ocean on millennial to centennial timescales, Nature, 562, 569–573, 2018.
Raitzsch, M., Bijma, J., Benthien, A., Richter, K.-U., Steinhoefel, G., and Kučera, M.: Boron isotope-based seasonal paleo-pH reconstruction for the Southeast Atlantic–A multispecies approach using habitat preference of planktonic foraminifera, Earth and Planetary Science Letters, 487, 138–150, 2018.
Raitzsch, M., Rollion-Bard, C., Horn, I., Steinhoefel, G., Benthien, A., Richter, K.-U., Buisson, M., Louvat, P., and Bijma, J.: Technical note: Single-shell δ11B analysis of Cibicidoides wuellerstorfi using femtosecond laser ablation MC-ICPMS and secondary ion mass spectrometry, Biogeosciences, 17, 5365–5375, https://doi.org/10.5194/bg-17-5365-2020, 2020.
Ravelo, A. C. and Hillaire-Marcel, C.: Chapter eighteen the use of oxygen and carbon isotopes of foraminifera in paleoceanography, Developments in marine geology, 1, 735–764, 2007.
Raven, J. A. and Falkowski, P. G.: Oceanic sinks for atmospheric CO2, Plant, Cell & Environment, 22, 741–755, 1999.
Richey, J. N., Fehrenbacher, J. S., Reynolds, C. E., Davis, C. V., and Spero, H. J.: Barium enrichment in the non-spinose planktic foraminifer, Globorotalia truncatulinoides, Geochimica et Cosmochimica Acta, 333, 184–199, 2022.
Rink, S., Kühl, M., Bijma, J., and Spero, H.: Microsensor studies of photosynthesis and respiration in the symbiotic foraminifer Orbulina universa, Marine Biology, 131, 583–595, 1998.
Rysgaard, S., Bendtsen, J., Pedersen, L. T., Ramløv, H., and Glud, R. N.: Increased CO2 uptake due to sea ice growth and decay in the Nordic Seas, Journal of Geophysical Research: Oceans, 114, C09011, https://doi.org/10.1029/2008JC005088, 2009.
Sanyal, A., Bijma, J., Spero, H., and Lea, D. W.: Empirical relationship between pH and the boron isotopic composition of Globigerinoides sacculifer: Implications for the boron isotope paleo-pH proxy, Paleoceanography, 16, 515–519, 2001.
Schlitzer, R.: Interactive analysis and visualization of geoscience data with Ocean Data View, Computers & Geosciences, 28, 1211–1218, https://doi.org/10.1016/S0098-3004(02)00040-7, 2002.
Schmittner, A. and Galbraith, E. D.: Glacial greenhouse-gas fluctuations controlled by ocean circulation changes, Nature, 456, 373–376, 2008.
Seki, O., Foster, G. L., Schmidt, D. N., Mackensen, A., Kawamura, K., and Pancost, R. D.: Alkenone and boron-based Pliocene pCO2 records, Earth and Planetary Science Letters, 292, 201–211, 2010.
Shackleton, N.: Attainment of isotopic equilibrium between ocean water and the benthonic foraminifera genus Uvigerina: Isotopic changes in the ocean during the last glacial, Colloq. Int. C.N.R.S., 219, 203–209, 1974.
Shuttleworth, R., Bostock, H., Chalk, T. B., Calvo, E., Jaccard, S., Pelejero, C., Martínez-García, A., and Foster, G.: Early deglacial CO2 release from the Sub-Antarctic Atlantic and Pacific oceans, Earth and Planetary Science Letters, 554, 116649, https://doi.org/10.1016/j.epsl.2020.116649, 2021.
Si, W., Novak, J. B., Richter, N., Polissar, P., Ma, R., Santos, E., Nirenberg, J., Herbert, T. D., and Aubry, M.-P.: Alkenone-derived estimates of Cretaceous p CO2, Geology, 52, 555–559, 2024.
Simstich, J., Sarnthein, M., and Erlenkeuser, H.: Paired δ 18 O signals of Neogloboquadrina pachyderma (s) and Turborotalita quinqueloba show thermal stratification structure in Nordic Seas, Marine Micropaleontology, 48, 107–125, 2003.
Standish, C. D., Chalk, T. B., Babila, T. L., Milton, J. A., Palmer, M. R., and Foster, G. L.: The effect of matrix interferences on in situ boron isotope analysis by laser ablation multi-collector inductively coupled plasma mass spectrometry, Rapid Communications in Mass Spectrometry, 33, 959–968, 2019.
Stangeew, E.: Distribution and Isotopic Composition of Living Planktonic Foraminifera N. pachyderma (sinistral) and T. quinqueloba in the High Latitude North Atlantic, PhD thesis, Christian-Albrechts Universität Kiel, https://nbn-resolving.org/urn:nbn:de:gbv:8-diss-4645 (last access: 13 November 2025), 2001.
Stewart, J. A., Anagnostou, E., and Foster, G. L.: An improved boron isotope pH proxy calibration for the deep-sea coral Desmophyllum dianthus through sub-sampling of fibrous aragonite, Chemical Geology, 447, 148–160, 2016.
Stewart, J. A., Christopher, S. J., Kucklick, J. R., Bordier, L., Chalk, T. B., Dapoigny, A., Douville, E., Foster, G. L., Gray, W. R., and Greenop, R.: NIST RM 8301 boron isotopes in marine carbonate (simulated coral and foraminifera solutions): Inter-laboratory δ11B and trace element ratio value assignment, Geostandards and Geoanalytical Research, 45, 77–96, 2021.
Sulpis, O., Lauvset, S. K., and Hagens, M.: Current estimates of K1* and K2* appear inconsistent with measured CO2 system parameters in cold oceanic regions, Ocean Sci., 16, 847–862, https://doi.org/10.5194/os-16-847-2020, 2020.
Takahashi, T., Sutherland, S. C., Wanninkhof, R., Sweeney, C., Feely, R. A., Chipman, D. W., Hales, B., Friederich, G., Chavez, F., and Sabine, C.: Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans, Deep Sea Research Part II, 56, 554–577, 2009.
Takahashi, T., Sutherland, S. C., and Kozyr, A.: Global ocean surface water partial pressure of CO2 database: Measurements performed during 1957–2018 (version 2018), NOAA/NCEI/OCADS NDP-088 (V2018) Rep., 25 pp., https://www.ncei.noaa.gov (last access: 27 February 2025), 2019.
Tell, F., Jonkers, L., Meilland, J., and Kucera, M.: Upper-ocean flux of biogenic calcite produced by the Arctic planktonic foraminifera Neogloboquadrina pachyderma, Biogeosciences, 19, 4903–4927, https://doi.org/10.5194/bg-19-4903-2022, 2022.
Toyofuku, T., Matsuo, M. Y., de Nooijer, L. J., Nagai, Y., Kawada, S., Fujita, K., Reichart, G.-J., Nomaki, H., Tsuchiya, M., and Sakaguchi, H.: Proton pumping accompanies calcification in foraminifera, Nature Communications, 8, 14145, https://doi.org/10.1038/ncomms14145, 2017.
Uchikawa, J., Penman, D. E., Zachos, J. C., and Zeebe, R. E.: Experimental evidence for kinetic effects on B/Ca in synthetic calcite: implications for potential B (OH) 4- and B (OH) 3 incorporation, Geochimica et Cosmochimica Acta, 150, 171–191, 2015.
Ujiié, Y., Ishitani, Y., Nagai, Y., Takaki, Y., Toyofuku, T., and Ishii, S. i.: Unique evolution of foraminiferal calcification to survive global changes, Science Advances, 9, eadd3584, https://doi.org/10.1126/sciadv.add3584, 2023.
Volkmann, R. and Mensch, M.: Stable isotope composition (δ18O, δ13C) of living planktic foraminifers in the outer Laptev Sea and the Fram Strait, Marine Micropaleontology, 42, 163–188, 2001.
Wang, B.-S., You, C.-F., Huang, K.-F., Wu, S.-F., Aggarwal, S. K., Chung, C.-H., and Lin, P.-Y.: Direct separation of boron from Na-and Ca-rich matrices by sublimation for stable isotope measurement by MC-ICP-MS, Talanta, 82, 1378–1384, 2010.
Weiss, R. F.: Carbon dioxide in water and seawater: The solubility of a non-ideal gas, Marine Chemistry, 2, 203–215, 1974.
WestgÅrd, A., Ezat, M. M., Chalk, T. B., Chierici, M., Foster, G. L., and Meilland, J.: Large-scale culturing of Neogloboquadrina pachyderma, its growth in, and tolerance of, variable environmental conditions, Journal of Plankton Research, 45, 732–745, 2023.
Winkelbauer, H. A., Hoogakker, B. A., Chance, R. J., Davis, C. V., Anthony, C. J., Bischoff, J., Carpenter, L. J., Chenery, S. R., Hamilton, E. M., and Holdship, P.: Planktic foraminifera iodine/calcium ratios from plankton tows, Frontiers in Marine Science, 10, 1095570, https://doi.org/10.3389/fmars.2023.1095570, 2023.
Wycech, J. B., Kelly, D. C., Kitajima, K., Kozdon, R., Orland, I. J., and Valley, J. W.: Combined effects of gametogenic calcification and dissolution on δ18O measurements of the planktic foraminifer Trilobatus sacculifer, Geochemistry, Geophysics, Geosystems, 19, 4487–4501, 2018.
Yasunaka, S., Manizza, M., Terhaar, J., Olsen, A., Yamaguchi, R., Landschützer, P., Watanabe, E., Carroll, D., Adiwira, H., and Müller, J. D.: An assessment of CO2 uptake in the Arctic Ocean from 1985 to 2018, Global Biogeochemical Cycles, 37, e2023GB007806, https://doi.org/10.1029/2023GB007806, 2023.
York, D., Evensen, N. M., Martínez, M. L., and De Basabe Delgado, J.: Unified equations for the slope, intercept, and standard errors of the best straight line, American journal of physics, 72, 367–375, 2004.
Yu, J., Thornalley, D. J., Rae, J. W., and McCave, N. I.: Calibration and application of B/Ca, Cd/Ca, and δ11B in Neogloboquadrina pachyderma (sinistral) to constrain CO2 uptake in the subpolar North Atlantic during the last deglaciation, Paleoceanography, 28, 237–252, 2013.
Zeebe, R. E.: Stable boron isotope fractionation between dissolved B(OH)3 and B(OH)4-, Geochimica et Cosmochimica Acta, 69, 2753–2766, https://doi.org/10.1016/j.gca.2004.12.011, 2005.
Zeebe, R. E. and Sanyal, A.: Comparison of two potential strategies of planktonic foraminifera for house building: Mg2+ or H+ removal?, Geochimica et Cosmochimica Acta, 66, 1159–1169, 2002.
Zeebe, R. E. and Wolf-Gladrow, D.: CO2 in seawater: equilibrium, kinetics, isotopes, 65, Gulf Professional Publishing, ISBN 9780444509468, 2001.
Zeebe, R. E., Bijma, J., and Wolf-Gladrow, D. A.: A diffusion-reaction model of carbon isotope fractionation in foraminifera, Marine Chemistry, 64, 199–127, 1999.
Zeebe, R. E., Wolf-Gladrow, D. A., Bijma, J., and Hönisch, B.: Vital effects in foraminifera do not compromise the use of δ11B as a paleo-pH indicator: Evidence from modeling, Paleoceanography, 18, 1043, https://doi.org/10.1029/2003PA000881, 2003.






