the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Algal richness in BSCs in forests under different management intensity with some implications for P cycling
Karen Baumann
Peter Leinweber
Tatiana Mikhailyuk
Ulf Karsten
Biological soil crusts (BSCs) are highly important communities in drylands and disturbed areas worldwide, where the higher vegetation is sparse, with a diverse microalgal community as the key component. They perform important ecological functions, such as stabilization of soil and nutrient enrichment. In temperate regions BSCs are also common, but generally less studied. Changes in land use and land use intensity strongly influence biodiversity per se and ecosystem processes, as can be seen particularly in densely populated regions like Europe. However, systematic studies on the effect of land use gradients, i.e., forest management intensity, on BSCs have been missing up to now. To close this knowledge gap and enhance the understanding of management effects on BSCs from pine and beech forests under different management regimes, key primary producers of these communities (eukaryotic microalgae and cyanobacteria) were studied. Phototrophic microorganisms were identified morphologically and categorized as either coccal taxa, which typically occur in high diversity, or filamentous taxa, which have the potential to initiate BSC formation. In total, 51 algal species were recorded, most of them from the phylum Chlorophyta, followed by Streptophyta and Stramenopiles, and only 1 cyanobacterial taxon. The most abundant crust-initiating filamentous algae were three species of Klebsormidium (Streptophyta), a ubiquitous genus regularly occurring in BSCs because of its broad ecophysiological tolerance. Increasing management intensity in the forests resulted in a higher number of algal species; especially the number of coccal taxa increased. Furthermore, the proportion of inorganic phosphorus showed tendencies towards a negative correlation with the number of algal species. Thus, management of forests has an impact on the diversity of phototrophic organisms in BSCs, which might in turn affect their biogeochemical P cycling.
- Article
(2214 KB) - Full-text XML
-
Supplement
(55 KB) - BibTeX
- EndNote
Biological soil crusts (BSCs) occur as important vegetation on all continents on Earth, predominantly in arid and semi-arid habitats, but also in temperate regions (e.g., Belnap et al., 2001; Weber et al., 2016). In semiarid and arid environments, BSCs were studied, for example, in deserts of Israel and the USA but also in polar regions (Borchhardt et al., 2017; Flechtner et al., 1998; Kidron et al., 2010). In temperate regions, dunes with sparse vascular plant vegetation or disturbed areas in open sites (e.g., former mining sites) typically promote the development of BSCs (T. Fischer et al., 2010; Langhans et al., 2009; Lukešová, 2001; Schulz et al., 2016; Szyja et al., 2018).
Even though there is a rising interest in BCSs as global players in terrestrial nitrogen fixation (Elbert et al., 2012), reports on BSCs from forests are very rare (Seitz et al., 2017). Under mesic conditions, BSCs have to compete with highly competitive vascular plants, which strongly limit their development. In forests, light limitation and the occurrence of litter additionally restrict the development of BSCs on the forest ground. Therefore, any disturbance of the higher vegetation changes the competitive situation, allowing the development of BSCs. Disturbances occur frequently in temperate forests. They include litter-free spots at hillslopes, tree falls, pits of wild boars, and molehill-like humps, as well as human-induced disturbances such as skid trails and clear-cut areas. An increase in tree falls after storm events is a growing problem in Europe, especially with a rise in the number and strength of storms potentially caused by the global climate change (Schwierz et al., 2010). In places where a substantial disturbance of intact forest ecosystems had occurred BSCs typically represent pioneer vegetation for the colonialization of bare soil. BSC organisms initiate the biological introduction of carbon and nutrients into soil, promoting the regrowth of vascular plants (Seitz et al., 2017) and erosion protection after heavy disturbance and destruction of intact forest ecosystems.
Destruction of BSC cover caused by land use has numerous negative effects such as an increase in soil erosion, changes in water regime, and C and N losses from the topsoil (Barger et al., 2006; Belnap, 2003). Studies dealing with the effect of land use on BSCs were mainly conducted in arid and semiarid regions. These studies showed strong negative effects of intensive livestock grazing on BSC cover due to trampling and reported a subsequent BSC recovery period of up to 27 years (Concostrina-Zubiri et al., 2014; Gomez et al., 2004; Williams et al., 2008). Also, ploughing in Australian sand plains reduced the BSC cover dramatically (Daryanto et al., 2013). In contrast to reports from arid areas there are no studies on the effect of land use in temperate regions, nor on the effect of land use activities other than grazing or human activities on BSCs. Further, reports on how disturbances in continuous vegetation might promote the development of BSCs are missing.
BSCs can be characterized as “ecosystem engineers” since they form water-stable aggregates, which have an important ecological role in primary production, nitrogen cycling, mineralization, water retention, and stabilization of soils (Castillo-Monroy et al., 2010; Evans and Johansen, 1999; Lewis, 2007). While the role of BSC in the C- and N-cycle is well documented, little is known about their role in P cycling. Recent studies indicated that the number of microalgal species in BSCs can be related to the soil P content (Baumann et al., 2017; Schulz et al., 2016). Nevertheless, the effect of environmental factors that shape BSC communities and in turn affect soil characteristics is still unstudied.
Together with the macroscopic lichens and bryophytes, cyanobacteria and eukaryotic microalgae represent the most important phototrophic components of BSCs (Belnap et al., 2001). Eukaryotic microalgae, essential components of biocrust communities as major contributors to C fixation (Büdel et al., 2016; Szyja et al., 2018), are still the least studied phototrophs in BSCs. BSC microalgae can be divided into two functional groups: (i) filamentous and (ii) single-celled, i.e., coccoid. Filamentous green algae are major BSC-forming taxa that stabilize soil particles by gluing them together due to the excretion of sticky mucilage. They usually occur in high biomass but low diversity. Coccoid algae are attached to the soil particles or other algae and typically occur in high diversity but low biomass (Büdel et al., 2016).
Filamentous cyanobacteria, especially representatives from the genus Microcoleus, are often dominant phototrophic organisms in BSCs from drylands and dunes of temperate regions (Garcia-Pichel et al., 2001; Schulz et al., 2016). They are described as important members of BSC communities due to their ability to produce sticky mucilage sheaths and extracellular polymeric substances, thus forming a network between soil particles (Gundlapally and Garcia-Pichel, 2006). In temperate regions, this key function is often carried out by the filamentous eukaryotic algae, such as Klebsormidium, Xanthonema, or Zygogonium (Fischer and Subbotina, 2014; Lukešová, 2001; Pluis, 1994).
In a previous study, we indicated that the BSC's algal richness is related to P cycling (Baumann et al., 2017). The data implied that BSCs were involved in the transformation of inorganic P to organic P compounds, thus playing a key role in the biological P cycling in temperate soils. However, BSC algal species richness was only considered as a sum parameter; detailed information on species occurrence is still missing. Therefore, in the present study we focused on the identification of algal species and the effect of silvicultural management intensity on algal species richness in BSCs collected from the same plots as Baumann et al. (2017) and additional sampling sites. The correlation of BSC algal richness with C, N, and P content, and in particular different P fractions, was investigated in order to uncover the link between biogeochemical cycles and BSC alga species. The aim of the present study was to characterize for the first time algal community in the BSCs from disturbed sites in temperate forests of different silvicultural management intensities.
2.1 Study site
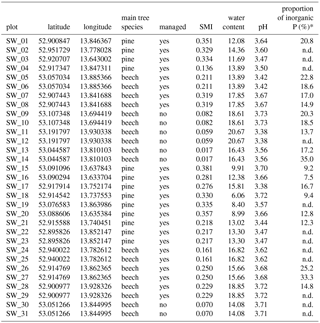
BSC samples were collected in June 2014 and 2015 from the plots of the “German Biodiversity Exploratories” project with natural protected forests and managed forest (age-class forest) (M. Fischer et al., 2010). Forest plots were located in the Schorfheide-Chorin Biosphere Reserve in northeastern Germany; the plots differed in the dominant tree species: Scots pine (Pinus sylvestris L.) or European beech (Fagus sylvatica L.). Samples were taken from the disturbed areas where BSCs developed on the litter-free bare soil (for illustration, see Fig. 1). The top millimeters of soil, where BSC had been visually detected as a green cover, were collected on a spatula. After transportation to the lab the upper 2 mm of BSC were separated from the adhering soil underneath with a razor blade before being stored dry in paper bags. In total, 31 BSCs were collected from 13 pine and 18 beech plots, of which 23 were managed and 8 were natural forest plots (Table 1).
Table 1General information on study sites: sample location, main tree species, management status, silvicultural management index (SMI), water content and pH from bulk soil analyses, and proportion of inorganic P as % of total P. n.d.: not determined; * Taken from Baumann et al. (2017).
2.2 Culturing, identification, and richness of algae
Solid 3N-Bolds Basal Medium (1.5 % agar) with vitamins (Starr and Zeikus, 1993) was used for the establishment of enrichment cultures. Several 7–10 mm2 BSC pieces were cleaned with forceps to remove all roots and leaves, in order to avoid the growth of fungi and bacteria, and were placed on the surface of an agar plate under sterile conditions. Plates were incubated at 20 ∘C, 30–35 µmol photons m−2 s−1 (Osram Lumilux Cool White lamps L36W/840) under a light/dark cycle of 16:8 h L:D. The plates were regularly inspected and colonies were identified after 4 to 6 weeks' incubation, using a light microscope (BX51, Olympus) with Nomarski differential interference optics and 1000 × magnification. Photomicrographs were taken with an Olympus UC30 camera attached to the microscope and processed with the cellSens Entry software (Olympus). For direct observation of BSC samples, pieces of BSC were rewetted with tap water, put on a glass slide, and analyzed with the above-mentioned microscope at 400 × magnification. Mucilage of algae was stained with an aqueous solution of methylene blue.
Morphological identification of algae and cyanobacteria was based on the standard syllabus (Ettl and Gärtner, 1995) and more recent taxonomic publications on certain algal groups (Darienko et al., 2010; Kostikov et al., 2002; Mikhailyuk et al., 2015). Phototrophic microorganisms were identified as Cyanobacteria, Chlorophyta, Streptophyta, and some Stramenopiles (Eustigmatophyceae). Diatoms were regularly found in direct observations but were excluded from the analyses as the mentioned enrichment cultivation was not suitable for this group of microalgae (e.g., Schulz et al., 2016).
Since the enrichment cultivation did not provide clear information on the abundance of each identified taxon, we used the total number of algae and cyanobacteria species per sample, also known as species richness, as the measure of alpha diversity. As a measure of beta diversity, the similarity between the plots was shown by presence/absence of individual species, combining the total number and the identity of all algal taxa observed. Furthermore, the identified algae and cyanobacteria were categorized based on their life form (filamentous or coccal), since different life forms differ in their ecological function. The proportion of filamentous algae in the total number of algae was used for statistical analyses.
2.3 Environmental variables
The natural and managed forest plots were characterized by different silvicultural management intensity. In natural forests, no management was conducted, meaning that fallen trees were left in place and no trees were cut. In managed age-class forests, the forest stands were regularly disturbed by tree cuts, removal of dead trees, and usage of skid trails. To evaluate the effect of management, the silvicultural management index (SMI) was used. This index takes into account the tree species, forest stand density and age, as well as the aboveground living and dead wood biomass (Schall and Ammer, 2013). High stand density is reflected by a high SMI; therefore, natural forests have a lower SMI than managed forests, and a pine stand has a higher SMI than a beech stand (Schall and Ammer, 2013).
To assess potential links between BSC organisms and environmental parameters, the species' richness, presence/absence of individual algal species, and proportion of filamentous algae were related to the following environmental parameters: dominant tree species (pine or beech), silvicultural management intensity (SMI), pH, and water content of the bulk soil (Table 1, for all 31 samples). Additionally, for a subset of 19 BSC samples, data on total C, N and P content and organic and inorganic P compounds, for labile, moderately labile and stable P, were included. Element data were presented in detail by Baumann et al. (2017), and are thus not presented in this paper.
2.4 Statistical analyses
All statistical analyses were done using the R version 3.3.0 (R Development Core Team, 2009) statistical software. Analysis of variance (ANOVA) was conducted to reveal the effect of environmental parameters on algal and cyanobacteria richness, and proportion of filamentous species; the best predictors for their variance were selected by backward elimination stepwise regression analysis based on the BIC (Bayesian information criterion) using the “step” command in R. The correlation between environmental parameters was determined by Pearson correlation (“cor” and “cor.test” commands in R).
To reveal correlations of single environmental parameters with the presence or absence of individual algal species, PerMANOVA (with the “adonis” function in R, Anderson, 2001) was applied using the Bray–Curtis dissimilarity index (Bray and Curtis, 1957), including a permutation test with 1000 permutations. The adonis function allows application of non-Euclidean distance metrics and handles both categorical and continuous predictors. For analysis of co-correlation of environmental factors, Pearson correlation was used. To test significant differences of environmental factors between tree species, an unpaired two-tailed t-test was performed. Differences with a p-value below or equal to 0.05 were taken as significant.
3.1 Algae identification
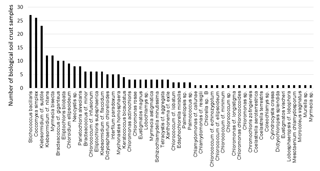
In total 51 different algae species and one cyanobacterium were detected in enrichment cultures of all 31 BSC samples. Stichococcus bacillaris was the most ubiquitous taxon, observed in 27 out of 31 samples, followed by Coccomyxa simplex and Klebsormidium cf. subtile in 26 and 23 out of 31 samples, respectively. All other algal species were detected in less than 50 % of the BSC samples; 22 algal species were observed exclusively in one sample (Fig. 2). The richness of algae (total species number) at each plot ranged from 3 to 14 species with a mean of 8 and a standard deviation of 2.6 (a complete species list is provided in Supplement Table S1).
The phylum Chlorophyta made up 81 % of all detected algal species, followed by Streptophyta (11 %) and Stramenopiles (6 %). Cyanobacteria were rare in these BSCs: only one species, Microcoleus vaginatus, was observed in only one sample.
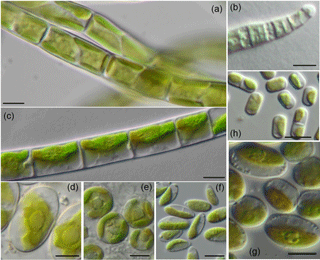
Figure 3Filamentous and examples of coccal algae from forest BSCs: algae with strong filaments: A-Xanthonema cf. exile, B-Microcoleus vaginatus, C-Klebsormidium cf. flaccidum; coccal algae: D-Chloroidium ellipsoideum, E-Eustigmatos magnus, F-Coccomyxa simplex; algae with short or easily disintegrated filaments: G-Stichococcus bacillaris, H-Interfilum paradoxum; scale bar = 5 µm.
The identified algal species were differentiated according to their life form (Fig. 3). Five species with strong filaments (Klebsormidium cf. flaccidum, K. cf. subtile, K. cf. nitens, Xanthonema cf. exile, Microcoleus vaginatus) and two species with short or easily disintegrating filaments (Interfilum paradoxum, Stichococcus bacillaris) were found. In each BSC at least two different filamentous taxa were detected, indicating their importance for the BSC formation. Genus Klebsormidium seemed to be highly important for BSCs in forest since it was registered in every BSC sample (Table S1).
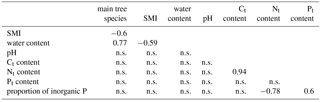
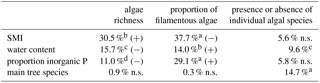
Table 2Significant Pearson correlation coefficients to reveal correlations between environmental factors, which might affect or be affected by the richness of algae. This co-correlation analysis should support the correct interpretation of potential important factors for the alga community. SMI – silvicultural management index; n.s. – not significant.
3.2 Correlation of algae richness with plot characteristics and nutrient content
The gravimetric water content of the bulk soil was negatively correlated with the SMI; the pH was neither correlated with the water content, nor with the SMI nor with the dominant tree species (Table 2). The N content was positively correlated with the C content, and N as well as C content were independent of the SMI and pH. Total P and the proportion of inorganic P were independent of the C and N content, as well as of pH and SMI (Table 2).
The richness of algal species and the proportion of filamentous algae in BSCs only correlated with SMI, water content and proportion of inorganic P (Table 3). The remaining tested parameters (C and N content, total P, proportion of organic P, pH, dominant tree species, and soil horizon) were excluded by stepwise model simplification based on the BIC. This means that these factors had no measurable effect on the algal species richness or on the proportion of filamentous algae. The SMI was positively correlated with the species richness, meaning that a higher SMI resulted in a higher species richness (Fig. 4); especially the proportion of coccal algae was increased. BSCs with higher algal richness tended to have lower proportions of inorganic P.
The presence/absence of individual algal species in BSCs significantly correlated with the dominant tree species (15 % explained variance) and with the soil water content (10 % explained variance). The SMI and proportion of inorganic P explained each 5 % of the variance, but this was not significant (Table 3). Therefore, we concluded that the dominant tree species and the soil water content affect the composition of algal species in BSCs.
4.1 Species composition and abundance
In total, 51 microalgal species and one cyanobacterium were identified in all sampled BSCs (Fig. 2), which is a similar or slightly lower species richness compared to the other reports on BSCs from temperate regions at open sites (Langhans et al., 2009; Schulz et al., 2016), but similar or higher compared to the previous reports on algae from forest bulk soil (Khaybullina et al., 2010; Novakovskaya and Patova, 2008; Starks et al., 1981). Nevertheless, the given number most probably underestimates the real algal richness, since our results are based on the enrichment cultivation followed by morphological identification. Enrichment cultivation promotes the growth of only culturable algae, which represent only a small part of all phototrophic microorganisms in BSCs (Langhans et al., 2009). A recent paper, comparing metagenomic data of a polar BSC with data based on enrichment cultivation and morphological identification of the algae, showed that only about 10 % of the metagenomic data could be confirmed by morphological identification (Rippin et al., 2018). Furthermore, it is not always possible to distinguish dormant from currently active microalgae. However, direct observation of a BSC sample under the microscope gives at least a first hint of the dominant active organisms. With this approach we could confirm that all filamentous algae were abundant and vital in the BSC samples. The morphological identification of algae has known challenges: for example, sibling species have similar characteristics but are genetically distant (Potter et al., 1997). To overcome these limitations, researchers proposed combining molecular and morphological methods of identification, since molecular techniques alone can also fail to detect some taxa, as a result of unsuccessful DNA extraction, inappropriate primers, etc. (Büdel et al., 2009; Garcia-Pichel et al., 2001).
All observed algal species are known to be terrestrial taxa; most of them were already reported from other BSCs (Büdel et al., 2016, and references therein; Ettl and Gärtner, 1995). Chlorophyceae were the most abundant phylum, which is typical for temperate regions (Büdel et al., 2016). Especially most of the unicellular taxa belong to the Chlorophyta (genera such as Chlamydomonas, Chloromonas, Chlorococcum, and Tetracystis). A high richness of Chlorophyta is characteristic of humid habitats and typical for forest soils (Hoffmann, 1989).
Cyanobacteria were represented by only one species. While they are often reported as predominant species in BSCs of arid regions such as Israel and drylands of the USA (Garcia-Pichel et al., 2001; Kidron et al., 2010), cyanobacteria are less abundant in temperate regions (Gypser et al., 2016; Langhans et al., 2009; Pluis, 1994) and even rare in acidic soils, which corresponds to the forest plots of our Schorfheide-Chorin study site (Hoffmann et al., 2007; Lukešová, 2001; Lukešová and Hoffmann, 1996). It seems that cyanobacteria play only a minor role in forest ecosystems, with consequences for the taxa's ecological traits. For example, the ability for nitrogen fixation in phototrophic organisms was only reported for cyanobacteria and never observed in eukaryotic algae. In forest ecosystems, litter and other decomposable biomass might have provided sufficient mineral nitrogen compounds, which could have led to the absence of nitrogen-fixing organisms in these systems in contrast to nitrogen-poor habitats such as dunes or deserts where cyanobacteria are dominant (Langhans et al., 2009; Schulz et al., 2016).
The filamentous alga Klebsormidium was found in nearly all BSCs of our study, whereas species with similar strong filaments (Microcoleus and Xanthonema) were only found occasionally. Filamentous algae can be regarded as key players in BSC communities, because of their BSC-initiating potential by building tight networks among soil particles (Büdel et al., 2016). In some forest BSCs, moss protonema can exert a similar function, due to their filamentous nature (Weber et al., 2016). However, in the forest ecosystems of Schorfheide-Chorin the green algae Klebsormidium seems to be the most important BSC-initiating alga. This genus can tolerate a wide range of environmental factors and has a cosmopolitan distribution in numerous terrestrial habitats (Karsten et al., 2016; Rindi et al., 2011, and references therein). Its presence in other terrestrial habitats, such as natural rocks in lowlands and mountainous areas (Mikhailyuk et al., 2008), caves (Vinogradova and Mikhailyuk, 2009), sand dunes (Schulz et al., 2016), tree barks (Freystein et al., 2008), acidic post-mining sites (Lukešová, 2001), urban walls (Rindi and Guiry, 2004), and building facades (Barberousse et al., 2006), is well documented. As many other terrestrial algae, Klebsormidium is tolerant to light exposure during dehydration (Gray et al., 2007). This is a typical situation, which BSC algae have to cope with, since the increase in light intensity in the morning is often associated with dehydration (Raanan et al., 2016). A recent study in central Europe, however, observed that Klebsormidium is sensitive to increasing light during cellular water loss (Pierangelini et al., 2017). The distribution of Klebsormidium in nearly all BSC samples from Schorfheide-Chorin forest may be explained by a lower solar radiation and lower evaporation rates in forest ecosystems compared with the open habitats (e.g., inland dunes) where besides Klebsormidium other filamentous algae are dominant (Langhans et al., 2009; Pluis, 1994). Also, the forest soil is rather acidic (pH min: 3.23, pH max: 3.86; Table 1), which supports a dominance of Klebsormidium (Škaloud et al., 2014). Thus, the low light availability, low water evaporation, and acidic soil conditions plausibly explain the presence and the dominance of Klebsormidium as a potential BSC-initiating algal taxon in nearly all BSCs from Schorfheide-Chorin forest plots.
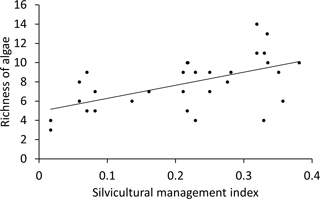
Figure 4Plot of algae richness in BSCs from forests over the silvicultural management index (SMI). Natural forest has a low SMI, managed forests a high SMI; the line indicates the best linear fit (slope: 13.6, p<0.001 (ANOVA)).
Table 3Effect of environmental factors on algae richness, filamentous algae proportion (both estimated by ANOVA) and presence or absence of individual algal species (estimated by PerMANOVA) quantified by the percentage of explained variance. The significance level is indicated by a – p<0.001, b – p<0.01, c – p<0.05, and d – . ns – not significant; (+) indicates positive correlation, (−) negative correltaion.
Three morphospecies of the genus Klebsormidium were identified in the investigated samples (Fig. 2). All three morphospecies were reported from other aeroterrestrial habitats in central Europe (Glaser et al., 2017; Mikhailyuk et al., 2015). Klebsormidium exhibits morphological features, which can be easily recognized. However, the identification down to species level is difficult due to the high morphological plasticity (Lokhorst, 1996). And still, in times of molecular identification, the debate on species definition in the genus Klebsormidium is ongoing (Mikhailyuk et al., 2015; Rindi et al., 2017). Therefore, the definition of clades within Klebsormidium was and still is a helpful tool to differentiate between morpho- or geno-types (Rindi et al., 2011). Studies comparing these Klebsormidium clades from different localities observed global ubiquity on the one hand, and local endemism on the other (Ryšánek et al., 2014). Clade composition seems to differ depending on the habitat: Klebsormidium cf. flaccidum (B∕C clade) was abundant in both closed and open habitats, whereas K. cf. nitens and K. cf. subtile (E clade) were predominantly distributed in forest BSCs (Glaser et al., 2017; Mikhailyuk et al., 2015). In our study, however, BSCs from forests contained more often Klebsormidium cf. subtile and K. cf. nitens than K. cf. flaccidum. In desiccation experiments the recovery rates of these clades were similar (Donner et al., 2017a, b). It is still open which of the environmental factors cause the observed habitat preferences of the different clades. Additional ecophysiological experiments including potential environmental factors, such as light regimes, desiccation frequency, and duration, as well as soil parameters such as pH, in combination with transcriptomic approaches might explain these conspicuous habitat preferences of Klebsormidium clades.
4.2 Correlation with SMI
The silvicultural management index (SMI) was used to estimate the forest management intensity. It takes into account the tree species, forest stand age and density. However, intensively managed forest did not necessarily inherit more disturbed sites suitable for the BSC development. In contrast, BSC development is limited in forests with high density (typical for intensively managed forest stands). However, managed forests have a higher risk for complete stand loss, because of either regular clear-cut or strong storms; it is more likely to lose a large part of pine stands with high density compared to natural beech forest.
The richness of algal species as well as the proportion of coccal algae were positively correlated with the silvicultural management index (SMI; Fig. 4). This means that more algal species were discovered in BSCs from managed than from natural forest ecosystems. This finding agrees with conclusions of high algal richness on disturbed or cultivated soils (Gollerbakh and Shtina, 1969; Hoffmann, 1989). The SMI reflects the effect of management practice on the dominant tree species and the stand density. Most biodiversity exploratory studies on forest-soil microorganisms observed a stronger effect of the dominant tree species than of the SMI on the microbial community (Goldmann et al., 2015; Kaiser et al., 2016; Purahong et al., 2014); only one study on litter decaying fungi and bacteria indicated a significant difference between natural and managed beech forests (Purahong et al., 2015). Kaiser et al. (2016) discussed that the different tree species influence soil bacteria by shifting the pH in soil; hence, tree species was designated as the main predictor for bacterial community composition. However, the bulk soil pH did not differ significantly between beech and pine forest in Schorfheide-Chorin (Table 1); hence, the algae in BSCs were not affected by this abiotic parameter. Therefore, we rejected an effect of the SMI via the pH on the BSC algal species richness in Schorfheide-Chorin.
However, the SMI combines other potential factors, which could explain its positive correlation with the richness of algal species as well as the proportion of coccal algae. Water and light availability might have affected BSC microalgae due to forest stand density and tree species. Forest plots in Schorfheide-Chorin were dominated by either beech or pine trees, which affect the light regime differently: in beech forests the canopy shade changes over the year, with usually higher solar radiation on the ground in winter and spring than in summer, while in pine forests no such light fluctuations occur. Also, the stand density, another parameter of the SMI, could affect the light regime on the ground: higher density would result in less photosynthetic active radiation for photosynthetically active soil microorganisms. The radiation is often coupled with evaporation of soil moisture (Raanan et al., 2016); hence, the stand density could have an indirect effect on the BSC organisms via an altered water regime. Thus, the SMI was expected to affect the algal richness in BSCs via lower light availability and lower evaporation rates. This assumption is well supported by the two-way analysis of water content and SMI. Nevertheless, it should be noted that the water content was measured in the bulk soil, which might differ from the one of BSCs. For future studies on microalgae in BSCs it would be important to examine also the incident light on the ground as well as the BSC water content.
Although the SMI positively affected the algal richness, the presence or absence of individual algal taxa was not correlated with the SMI, but with the main tree species. Broadleaf litter has a higher quality in terms of a more favorable C:N and C:P ratio compared to coniferous litter (Cleveland and Liptzin, 2007; McGroddy et al., 2004). It might have been that the community in the pine forest promoted algal species which could cope with a suboptimal ratio. But as mentioned above, both light regime and water availability differ between the two forest types and could also have contributed to the observed differences in the occurrence of algal species.
4.3 Correlation with C, N, and P
BSCs have different important ecological functions, such as the enhancement of the nutrient content in the top soil layer (Baumann et al., 2017; Evans and Johansen, 1999). To assess the relationship between BSC community and biogeochemical cycling in BSCs, the content of total C, N, and P and additionally the different P fractions (organic, inorganic, labile, and stable fractions) were correlated with algal richness. Although a correlation between the richness of algae and the total C, N, and P content was not observed, the presence of BSCs clearly led to an increased content of total C, N, and P and in particular a higher proportion of organic P (Baumann et al., 2017). These results indicate that algal species are functionally redundant, and that a BSC community with low species richness still has a functional role in increasing C, N, and P content. A more detailed analysis of the P fractions gave a slightly different picture: the proportion of inorganic P was positively correlated with the proportion of filamentous algae and showed a tendency to a negative correlation with the richness of BSC algae. Soluble inorganic phosphate can be assimilated by organisms, and it originates either from the weathering of P-containing minerals, desorption of mineral-bound phosphates, or the mineralization of organic matter (Mackey and Paytan, 2009). Thus, a low amount of inorganic P could indicate a high uptake rate of BSC organisms, and thus a more closed P cycle due to the higher algal richness (Baumann et al., 2017).
BSCs are able to coexist with continuous forests, because natural and human-induced disturbances regularly provide free space (e.g., tree fall, skid trails) for BSCs to develop. For the first time, algal richness in BSCs from such disturbed sites in temperate forests under different management intensities were described. The rather acidic forest soil supported a clear dominance of streptophycean Klebsormidium morphotypes as the main BSC-initiating filamentous algae, while cyanobacteria played a negligible role. Higher forest management intensity resulted in a higher richness of algae, especially in a higher proportion of coccal taxa. It is reasonable to assume that the silvicultural management intensity in forests affects the algal richness due to the higher forest stand density in managed forests, which changes the light and water regime. Increasing algal richness in BSCs was supposed to enhance biogeochemical cycling of nutrients, but this hypothesis could not be proven. Nevertheless, the fraction of inorganic P showed tendencies towards a negative correlation with BSC algae, especially with filamentous species. Consequently, the present study gives the first hint of a relation between the biogeochemical cycles in BSCs and algal species. This relation should be studied in more detail, e.g., by gene expression analyses to understand whether and how algae in BSCs influence the cycling of P. Also, forthcoming studies should include other BSC-associated organisms, such as fungi and bacteria, to identify key players and the ecological role of BSCs in the P cycle.
Data are publicly available and stored in BExIS (available at https://www.bexis.uni-jena.de/PublicData/PublicData.aspx?DatasetId=20686, last access: 6 July 2018; Koenig-Ries et al., 2011).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-4181-2018-supplement.
KG, KB, PL, and UK designed the experiments and collected soil samples. KG, KB, and TM carried out the lab work. KG prepared the manuscript with contributions from all co-authors.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Biological soil crusts and their role in biogeochemical processes and cycling”. It is a result of the BIOCRUST3 conference, Moab, USA, 26 to 30 September 2016.
The authors would like to thank Nadine Borchhardt for her help during BSC sampling. Water content and pH data were provided by Ingo Schöning, Theresa Klötzing, and Marion Schrumpf (Max Planck Institute for Biogeochemistry, Jena, Germany). Special thanks go to Elena Samolov for her contribution to English corrections.
We thank the managers of the three Exploratories, Martin Gorke, and all former
managers for their work in maintaining the plot and project infrastructure,
Christiane Fischer for giving support through the central office, Michael
Owonibi for managing the central database, and Markus Fischer, Eduard
Linsenmair, Dominik Hessenmöller, Daniel Prati, Ingo Schöning,
François Buscot, Ernst-Detlef Schulze, Wolfgang W. Weisser, and the late
Elisabeth Kalko for their role in setting up the Biodiversity
Exploratories project. The work has been funded by DFG Priority Program 1374
“Infrastructure-Biodiversity-Exploratories” (subproject Crustfunction –
KA899/28-1 and LE903/12-1). Fieldwork permits were issued by the responsible
state environmental offices of Baden-Württemberg, Thuringia, and
Brandenburg (according to §72 BbgNatSchG). Tatiana Mikhailyuk thanks the
Alexander von Humboldt Foundation for financial support.
Edited by: Bettina Weber
Reviewed by: two
anonymous referees
Anderson, M. J.: A new method for non-parametric multivariate analysis of variance, Austral. Ecol., 26, 32–46, https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x, 2001.
Barberousse, H., Tell, G., Yéprémian, C., and Couté, A.: Diversity of algae and cyanobacteria growing on building facades in France, Algol. Stud., 120, 81–105, 2006.
Barger, N. N., Herrick, J. E., Van Zee, J., and Belnap, J.: Impacts of Biological Soil Crust Disturbance and Composition on C and N Loss from Water Erosion, Biogeochem., 77, 247–263, https://doi.org/10.1007/s10533-005-1424-7, 2006.
Baumann, K., Glaser, K., Mutz, J.-E., Karsten, U., MacLennan, A., Hu, Y., Michalik, D., Kruse, J., Eckhardt, K.-U., Schall, P., and Leinweber, P.: Biological soil crusts of temperate forests: Their role in P cycling, Soil Biol. Biochem., 109, 156–166, https://doi.org/10.1016/j.soilbio.2017.02.011, 2017.
Belnap, J.: The world at your feet: desert biological soil crusts, Front. Ecol. Environ., 1, 181–189, 2003.
Belnap, J., Büdel, B., and Lange, O. L.: Biological soil crusts: characteristics and distribution, in: Biological soil crusts: structure, function, and management, Vol. 1, edited by: Belnap, J. and Lange, O. L., 3–30, Springer-Verlag, Berlin, Heidelberg, 2001.
Borchhardt, N., Schiefelbein, U., Abarca, N., Boy, J., Mikhailyuk, T., Sipman, H. J. M., and Karsten, U.: Diversity of algae and lichens in biological soil crusts of Ardley and King George islands, Antarctica, Antarct. Sci., 29, 1–9, https://doi.org/10.1017/S0954102016000638, 2017.
Bray, J. R. and Curtis, J. T.: An ordination of the upland forest communities of southern Wisconsin, Ecol. Monogr., 27, 325–349, https://doi.org/10.2307/1942268, 1957.
Büdel, B., Darienko, T., Deutschewitz, K., Dojani, S., Friedl, T., Mohr, K. I., Salisch, M., Reisser, W., and Weber, B.: Southern african biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency, Microb. Ecol., 57, 229–247, https://doi.org/10.1007/s00248-008-9449-9, 2009.
Büdel, B., Dulić, T., Darienko, T., Rybalka, N., and Friedl, T.: Cyanobacteria and Algae of Biological Soil Crusts, in: Biological Soil Crusts: An Organizing Principle in Drylands, edited by: Weber, B., Büdel, B., and Belnap, J., 55–80, Springer International Publishing, Cham., 2016.
Castillo-Monroy, A., Maestre, F., Delgado-Baquerizo, M., and Gallardo, A.: Biological soil crusts modulate nitrogen availability in semi-arid ecosystems: insights from a Mediterranean grassland, Plant Soil, 333, 21–34, https://doi.org/10.1007/s11104-009-0276-7, 2010.
Cleveland, C. C. and Liptzin, D.: C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass?, Biogeochemistry, 85, 235–252, https://doi.org/10.1007/s10533-007-9132-0, 2007.
Concostrina-Zubiri, L., Huber-Sannwald, E., Martínez, I., Flores, J. L., Reyes-Agüero, J. A., Escudero, A., and Belnap, J.: Biological soil crusts across disturbance–recovery scenarios: effect of grazing regime on community dynamics, Ecol. Appl., 24, 1863–1877, 2014.
Darienko, T., Gustavs, L., Mudimu, O., Menendez, C. R., Schumann, R., Karsten, U., Friedl, T., and Pröschold, T.: Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta), Eur. J. Phycol., 45, 79–95, https://doi.org/10.1080/09670260903362820, 2010.
Daryanto, S., Eldridge, D. J., and Wang, L.: Ploughing and grazing alter the spatial patterning of surface soils in a shrub-encroached woodland, Geoderma, 200–201, 67–76, https://doi.org/10.1016/j.geoderma.2013.02.006, 2013.
Donner, A., Glaser, K., Borchhardt, N., and Karsten, U.: Ecophysiological Response on Dehydration and Temperature in Terrestrial Klebsormidium (Streptophyta) Isolated from Biological Soil Crusts in Central European Grasslands and Forests, Microb. Ecol., 73, 850–864, https://doi.org/10.1007/s00248-016-0917-3, 2017a.
Donner, A., Ryšánek, D., Mikhailyuk, T., and Karsten, U.: Ecophysiological traits of various genotypes of a green key alga in biological soil crusts from the semi-arid Colorado Plateau, USA, J. Appl. Phycol., 29, 2911–2923, https://doi.org/10.1007/s10811-017-1158-7, 2017b.
Elbert, W., Weber, B., Burrows, S., Steinkamp, J., Büdel, B., Andreae, M. O., and Pöschl, U.: Contribution of cryptogamic covers to the global cycles of carbon and nitrogen, Nat. Geosci., 5, 459–462, https://doi.org/10.1038/ngeo1486, 2012.
Ettl, H. and Gärtner, G.: Syllabus der Boden-, Luft- und Flechtenalgen, Spektrum Akademischer Verlag, 1995.
Evans, R. D. and Johansen, J. R.: Microbiotic crusts and ecosystem processes, Crit. Rev. Plant Sci., 18, 183–225, https://doi.org/10.1080/07352689991309199, 1999.
Fischer, M., Bossdorf, O., Gockel, S., Hänsel, F., Hemp, A., Hessenmöller, D., Korte, G., Nieschulze, J., Pfeiffer, S., Prati, D., Renner, S., Schöning, I., Schumacher, U., Wells, K., Buscot, F., Kalko, E. K. V., Linsenmair, K. E., Schulze, E.-D., and Weisser, W. W.: Implementing large-scale and long-term functional biodiversity research: The Biodiversity Exploratories, Basic Appl. Ecol., 11, 473–485, https://doi.org/10.1016/j.baae.2010.07.009, 2010.
Fischer, T. and Subbotina, M.: Climatic and soil texture threshold values for cryptogamic cover development: a meta analysis, Biologia (Bratisl.), 69, 1520–1530, https://doi.org/10.2478/s11756-014-0464-7, 2014.
Fischer, T., Veste, M., Wiehe, W., and Lange, P.: Water repellency and pore clogging at early successional stages of microbiotic crusts on inland dunes, Brandenburg, NE Germany, CATENA, 80, 47–52, https://doi.org/10.1016/j.catena.2009.08.009, 2010.
Flechtner, V. R., Johansen, J. R., and William, H. C.: Algal composition of microbiotic crusts from the central desert of Baja California, Mexico, Gt. Basin Nat., 58, 295–311, 1998.
Freystein, K., Salisch, M., and Reisser, W.: Algal biofilms on tree bark to monitor airborne pollutants, Biologia (Bratisl.), 63, 866–872, https://doi.org/10.2478/s11756-008-0114-z, 2008.
Garcia-Pichel, F., Lopez-Cortes, A., and Nubel, U.: Phylogenetic and Morphological Diversity of Cyanobacteria in Soil Desert Crusts from the Colorado Plateau, Appl. Environ. Microbiol., 67, 1902–1910, https://doi.org/10.1128/AEM.67.4.1902-1910.2001, 2001.
Glaser, K., Donner, A., Albrecht, M., Mikhailyuk, T., and Karsten, U.: Habitat-specific composition of morphotypes with low genetic diversity in the green algal genus Klebsormidium (Streptophyta) isolated from biological soil crusts in Central European grasslands and forests, Eur. J. Phycol., 52, 188–199, https://doi.org/10.1080/09670262.2016.1235730, 2017.
Goldmann, K., Schöning, I., Buscot, F., and Wubet, T.: Forest Management Type Influences Diversity and Community Composition of Soil Fungi across Temperate Forest Ecosystems, Front. Microbiol., 6, 1300, https://doi.org/10.3389/fmicb.2015.01300, 2015.
Gollerbakh, M. M. and Shtina, E. A.: Soil Algae, Nauka, Leningrad, 1969.
Gomez, E. D., Garland, J. L., and Roberts, M. S.: Microbial structural diversity estimated by dilution-extinction of phenotypic traits and T-RFLP analysis along a land-use intensification gradient, Fems Microbiol. Ecol., 49, 253–259, 2004.
Gray, D. W., Lewis, L. A., and Cardon, Z. G.: Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives, Plant Cell Environ., 30, 1240–1255, https://doi.org/10.1111/j.1365-3040.2007.01704.x, 2007.
Gundlapally, S. R. and Garcia-Pichel, F.: The Community and Phylogenetic Diversity of Biological Soil Crusts in the Colorado Plateau Studied by Molecular Fingerprinting and Intensive Cultivation, Microb. Ecol., 52, 345–357, https://doi.org/10.1007/s00248-006-9011-6, 2006.
Gypser, S., Herppich, W. B., Fischer, T., Lange, P., and Veste, M.: Photosynthetic characteristics and their spatial variance on biological soil crusts covering initial soils of post-mining sites in Lower Lusatia, NE Germany, Flora – Morphol. Distrib. Funct. Ecol. Plants, 220, 103–116, https://doi.org/10.1016/j.flora.2016.02.012, 2016.
Hoffmann, L.: Algae of terrestrial habitats, Bot. Rev., 55, 77–105, 1989.
Hoffmann, L., Ector, L., and Kostikov, I.: Algal Flora from Limed and Unlimed Forest Soils in the Ardenne (Belgium), Syst. Geogr. Plants, 77, 15–90, 2007.
Kaiser, K., Wemheuer, B., Korolkow, V., Wemheuer, F., Nacke, H., Schöning, I., Schrumpf, M., and Daniel, R.: Driving forces of soil bacterial community structure, diversity, and function in temperate grasslands and forests, Sci. Rep., 6, 33696, https://doi.org/10.1038/srep33696, 2016.
Karsten, U., Herburger, K., and Holzinger, A.: Living in biological soil crust communities of African deserts—Physiological traits of green algal Klebsormidium species (Streptophyta) to cope with desiccation, light and temperature gradients, J. Plant Physiol., 194, 2–12, https://doi.org/10.1016/j.jplph.2015.09.002, 2016.
Khaybullina, L. S., Gaysina, L. A., Johansen, J. R., and Krautová, M.: Examination of the terrestrial algae of the Great Smoky Moutains National Park, USA, Fottea, 10, 201–215, 2010.
Kidron, G. J., Vonshak, A., Dor, I., Barinova, S., and Abeliovich, A.: Properties and spatial distribution of microbiotic crusts in the Negev Desert, Israel, CATENA, 82, 92–101, https://doi.org/10.1016/j.catena.2010.05.006, 2010.
Koenig-Ries, B., Ostrowski, A., Petzold, E., and Nieschulze, J.: BExIS – Biodiversity Exploratories Information System, TDWG 2011 Annual Conference, available at: https://www.bexis.uni-jena.de/PublicData/PublicData.aspx?DatasetId=20686 (last access: 6 July 2018), 2011.
Kostikov, I., Darienko, T., Lukešova, A., and Hoffmann, L.: Revision of the classification system of Radiococcaceae Fott ex Komárek (except the Subfamily Dictyochlorelloideae) (Chlorophyta)., Algol. Stud., 104, 23–58, 2002.
Langhans, T. M., Storm, C., and Schwabe, A.: Community Assembly of Biological Soil Crusts of Different Successional Stages in a Temperate Sand Ecosystem, as Assessed by Direct Determination and Enrichment Techniques, Microb. Ecol., 58, 394–407, https://doi.org/10.1007/s00248-009-9532-x, 2009.
Lewis, L. A.: Chlorophyta on land: independent lineages of green eukaryotes from arid lands, in: Algae and Cyanobacteria in Extreme Environments, edited by: Seckbach, J., Springer Netherlands, Dordrecht., 2007.
Lokhorst, G. M.: Comparative Taxonomic Studies on the Genus Klebsormidium (Charophyceae) in Europe, Gustav Fischer, Stuttgart., 1996.
Lukešová, A.: Soil algae in brown coal and lignite post-mining areas in Central Europe (Czech Republic and Germany), Restor. Ecol., 9, 341–350, https://doi.org/10.1046/j.1526-100X.2001.94002.x, 2001.
Lukešová, A. and Hoffmann, L.: Soil algae from acid rain impacted forest areas of the Krušné hory Mts. 1. Algal communities, Vegetatio, 125, 123–136, 1996.
Mackey, K. R. M. and Paytan, A.: Phosphorus cycle, in: Encyclopedia of Microbiology, Vol. 3, edited by: Schaechter, M., 322–334., 2009.
McGroddy, M. E., Daufresne, T., and Hedin, L. O.: Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios, Ecology, 85, 2390–2401, 2004.
Mikhailyuk, T., Sluiman, H. J., Massalski, A., Mudimu, O., Demchenko, E. M., Kondratyuk, S. Y., and Friedl, T.: New streptophyte green algae from terrestrial habitats and an assessment of the genus Interfilum (Klebsormidiophyceae, Streptophyta), J. Phycol., 44, 1586–1603, https://doi.org/10.1111/j.1529-8817.2008.00606.x, 2008.
Mikhailyuk, T., Glaser, K., Holzinger, A., and Karsten, U.: Biodiversity of Klebsormidium (Streptophyta) from alpine biological soil crusts (Alps, Tyrol, Austria, and Italy), edited by: Gabrielson, P., J. Phycol., 51, 750–767, https://doi.org/10.1111/jpy.12316, 2015.
Novakovskaya, I. and Patova, E.: Green algae in spruce forests in the north-east of European Russia, Biologia (Bratisl.), 63, 836–842, https://doi.org/10.2478/s11756-008-0109-9, 2008.
Pierangelini, M., Ryšánek, D., Lang, I., Adlassnig, W., and Holzinger, A.: Terrestrial adaptation of green algae Klebsormidium and Zygnema (Charophyta) involves diversity in photosynthetic traits but not in CO2 acquisition, Planta, 256, 971–986, https://doi.org/10.1007/s00425-017-2741-5, 2017.
Pluis, J. L. A.: Algal crust formation in the inland dune area, Laarder Wasmeer, the Netherlands, Plant Ecol., 113, 41–51, 1994.
Potter, D., Lajeunesse, T. C., Saunders, G. W., and Anderson, R. A.: Convergent evolution masks extensive biodiversity among marine coccoid picoplankton, Biodivers. Conserv., 6, 99–107, 1997.
Purahong, W., Hoppe, B., Kahl, T., Schloter, M., Schulze, E.-D., Bauhus, J., Buscot, F., and Krüger, D.: Changes within a single land-use category alter microbial diversity and community structure: molecular evidence from wood-inhabiting fungi in forest ecosystems, J. Environ. Manage., 139, 109–119, 2014.
Purahong, W., Kapturska, D., Pecyna, M. J., Jariyavidyanont, K., Kaunzner, J., Juncheed, K., Uengwetwanit, T., Rudloff, R., Schulz, E., Hofrichter, M., Schloter, M., Krüger, D., and Buscot, F.: Effects of Forest Management Practices in Temperate Beech Forests on Bacterial and Fungal Communities Involved in Leaf Litter Degradation, Microb. Ecol., 69, 905–913, https://doi.org/10.1007/s00248-015-0585-8, 2015.
Raanan, H., Oren, N., Treves, H., Berkowicz, S. M., Hagemann, M., Pade, N., Keren, N., and Kaplan, A.: Simulated soil crust conditions in a chamber system provide new insights on cyanobacterial acclimation to desiccation: Simulation of BSC conditions and acclimation, Environ. Microbiol., 18, 414–426, https://doi.org/10.1111/1462-2920.12998, 2016.
R Development Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Austria [online], 2009.
Rindi, F. and Guiry, M. D.: Composition and spatial variability of terrestrial algal assemblages occurring at the bases of urban walls in Europe, Phycologia, 43, 225–235, 2004.
Rindi, F., Mikhailyuk, T. I., Sluiman, H. J., Friedl, T., and López-Bautista, J. M.: Phylogenetic relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta), Mol. Phylogenet. Evol., 58, 218–231, https://doi.org/10.1016/j.ympev.2010.11.030, 2011.
Rindi, F., Rysanek, D., and Skaloud, P.: Problems of epitypification in morphologically simple green microalgae: a case study of two widespread species of Klebsormidium (Klebsormidiophyceae, Streptophyta), Fottea, 17, 78–88, https://doi.org/10.5507/fot.2016.017, 2017.
Rippin, M., Borchhardt, N., Williams, L., Colesie, C., Jung, P., Büdel, B., Karsten, U., and Becker, B.: Genus richness of microalgae and Cyanobacteria in biological soil crusts from Svalbard and Livingston Island: morphological versus molecular approaches, Polar Biol., 41, 909–923, https://doi.org/10.1007/s00300-018-2252-2, 2018.
Ryšánek, D., Hrčková, K., and Škaloud, P.: Global ubiquity and local endemism of free-living terrestrial protists: phylogeographic assessment of the streptophyte alga Klebsormidium: Global biogeography of a terrestrial protist, Environ. Microbiol., 17, 689–698, https://doi.org/10.1111/1462-2920.12501, 2014.
Schall, P. and Ammer, C.: How to quantify forest management intensity in Central European forests, Eur. J. For. Res., 132, 379–396, https://doi.org/10.1007/s10342-013-0681-6, 2013.
Schulz, K., Mikhailyuk, T., Dreßler, M., Leinweber, P., and Karsten, U.: Biological Soil Crusts from coastal dunes at the Baltic Sea: cyanobacterial and algal biodiversity and related soil properties, Microb. Ecol., 71, 178–193, https://doi.org/10.1007/s00248-015-0691-7, 2016.
Schwierz, C., Köllner-Heck, P., Zenklusen Mutter, E., Bresch, D. N., Vidale, P.-L., Wild, M., and Schär, C.: Modelling European winter wind storm losses in current and future climate, Clim. Change, 101, 485–514, https://doi.org/10.1007/s10584-009-9712-1, 2010.
Seitz, S., Nebel, M., Goebes, P., Käppeler, K., Schmidt, K., Shi, X., Song, Z., Webber, C. L., Weber, B., and Scholten, T.: Bryophyte-dominated biological soil crusts mitigate soil erosion in an early successional Chinese subtropical forest, Biogeosciences, 14, 5775–5788, https://doi.org/10.5194/bg-14-5775-2017, 2017.
Škaloud, P., Lukešova, A., Malavasi, V., Ryšánek, D., Hrčková, K., and Rindi, F.: Molecular evidence for the polyphyletic origin of low pH adaptation in the genus Klebsormidium (Klebsormidiophyceae, Streptophyta), Plant Ecol. Evol., 147, 333–345, https://doi.org/10.5091/plecevo.2014.989, 2014.
Starks, T. L., Shubert, L. E., and Trainor, F. R.: Ecology of soil algae: a review, Phycologia, 20, 65–80, 1981.
Starr, R. C. and Zeikus, J. A.: UTEX the culture collection of algae at the University of Texas at Austin, J. Phycol., 29 (Suppl.), 1–106, 1993.
Szyja, M., Büdel, B., and Colesie, C.: Ecophysiological characterization of early successional biological soil crusts in heavily human-impacted areas, Biogeosciences, 15, 1919–1931, https://doi.org/10.5194/bg-15-1919-2018, 2018.
Vinogradova, O. N. and Mikhailyuk, T. I.: Algal flora of the caves and grottoes of the National Nature Park “Podilsky Tovtry” (Ukraine), Int. J. Algae, 11, 289–304, https://doi.org/10.1615/InterJAlgae.v11.i3.80, 2009.
Weber, B., Büdel, B., and Belnap, J.: Biological soil crusts: an organizing principle in drylands, Springer, Nature, Berlin, 2016.
Williams, W. J., Eldridge, D. J., and Alchin, B. M.: Grazing and drought reduce cyanobacterial soil crusts in an Australian Acacia woodland, J. Arid Environ., 72, 1064–1075, https://doi.org/10.1016/j.jaridenv.2007.11.017, 2008.