the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Improving the strength of sandy soils via ureolytic CaCO3 solidification by Sporosarcina ureae
Justin Michael Whitaker
Sai Vanapalli
Danielle Fortin
“Microbially induced carbonate precipitation” (MICP) is a biogeochemical process that can be applied to strengthen materials. The hydrolysis of urea by microbial catalysis to form carbonate is a commonly studied example of MICP. In this study, Sporosarcina ureae, a ureolytic organism, was compared to other ureolytic and non-ureolytic organisms of Bacillus and Sporosarcina genera in the assessment of its ability to produce carbonates by ureolytic MICP for ground reinforcement. It was found that S. ureae grew optimally in alkaline (pH ∼ 9.0) conditions which favoured MICP and could degrade urea (units U mL−1 represent µmol min−1 mL OD600) at levels (30.28 U mL−1) similar to S. pasteurii (32.76 U mL−1), the model ureolytic MICP organism. When cells of S. ureae were concentrated (OD600 ∼ 15–20) and mixed with cementation medium containing 0.5 M calcium chloride (CaCl2) and urea into a model sand, repeated treatments (3 × 24 h) were able to improve the confined direct shear strength of samples from 15.77 kPa to as much as 135.80 kPa. This was more than any other organism observed in the study. Imaging of the reinforced samples with scanning electron microscopy and energy-dispersive spectroscopy confirmed the successful precipitation of calcium carbonate (CaCO3) across sand particles by S. ureae. Treated samples were also tested experimentally according to model North American climatic conditions to understand the environmental durability of MICP. No statistically significant (p < 0.05, n= 3) difference in strength was observed for samples that underwent freeze–thaw cycling or flood-like simulations. However, shear strength of samples following acid rain simulations fell to 29.2 % of control MICP samples. Overall, the species S. ureae was found to be an excellent organism for MICP by ureolysis to achieve ground strengthening. However, the feasibility of MICP as a durable reinforcement technique is limited by specific climate conditions (i.e. acid rain).
- Article
(2747 KB) - Full-text XML
- BibTeX
- EndNote
Biomediated calcium carbonate (CaCO3) production is the process by which organisms induce the precipitation of calcium carbonate. With reference to bacterial CaCO3 precipitation – also known as “microbially induced carbonate precipitation”, “microbially induced calcite precipitation” (MICP) and “microbially induced calcium carbonate precipitation” (MICCP) – the phenomenon is well documented (Stocks-Fischer et al., 1999; DeJong et al., 2006; Whiffin et al., 2007; van Paassen et al., 2010). For example, cyanobacteria precipitate CaCO3 in microbial processes related to the shedding of the S layer, forming the stalagmites and stalactites in limestone caves and adding to the rocky sediments of coral reefs (Southam, 2000). Crystal aggregation of CaCO3 in the kidney, urinary tract or gallbladder have been shown to be induced by microorganisms such as Proteus mirabilis, a urease-positive organism, due to secondary infection (Worcester and Coe, 2008). Ureolytic soil organisms of the genera Sporosarcina or Bacillus can also induce CaCO3, for example, in their cycling of nitrogen with a urease enzyme (Hammes et al., 2003; Gower, 2008; Worcester and Coe, 2008). This last group of MICP producers has piqued recent engineering interests to apply them in a bioengineering and repair context.
MICP biotechnology utilizing ureolytic soil organisms, most notably Sporosarcina pasteurii, has been shown to directly reinforce or restore engineered or natural structures – such as the repair of historical monuments (Le Métayer-Levrela et al., 1999; Webster and May, 2006), marble slabs (Li and Qu, 2011) and stone heritage sites (Rodriquez-Navaro et al., 2012) – and to reduce weathering of soil embankments (Chu et al., 2012). The enzyme urease (urea amidohydrolase, EC 3.5.1.5) initiates the process, catalyzing the breakdown of urea to raise local pH and produce CaCO3 in a solution of calcium ions often supplied as calcium chloride (CaCl2), as summarized in Eqs. (1) and (2). The produced CaCO3 fills structural gaps or bridges materials (e.g. soil grains) to form a cemented product with unconfined strengths of up to 20 MPa (Whiffin et al., 2007).
Bacterial species such as Bacillus sphaericus (van Tittelboom et al., 2010) and Bacillus megaterium (Krishnapriya et al., 2015) have also been applied in material or volume strengthening. The aforementioned ureolytic soil organisms are attractive for MICP as they are “generally regarded as safe” (GRAS) bacteria with accessible substrates (i.e. urea) and an aerobic metabolism applicable to most engineering and terrestrial environments (DeJong et al., 2006).
These gram-positive organisms offer other attractive features such as spore-forming capability, allowing for long-term capsule storage in cements (Jonkers, 2011) and exopolysaccharide (EPS) secretion for improved material bonding (Bergdale, 2012).
The application of MICP in industry as a biotechnology is proposed to help reduce the need for current structure repair practices such as chemical grouting, which have been found to be environmentally detrimental in their permanence (DeJong et al., 2010), in some cases posing serious human health risks (Karol, 2003). That said, ureolytic MICP does produce excess ammonia, which can be harmful (van Paassen et al., 2010). The use of nitrifying and denitrifying bacteria could help solve this issue by oxidizing ammonia to nitrate and later nitrogen gas without affecting MICP. In fact, the work of Gat et al. (2014) has shown co-cultures of ureolytic and non-ureolytic bacteria can actually be beneficial to MICP. Alternatively, denitrifying bacteria can be used to directly induce MICP to avoid ammonia toxicity, though the level of CaCO3 is comparatively less than that of ureolytic MICP, and harmful nitrites can build up in solution (van Paassen et al., 2010). Other pathways to achieve MICP have also been explored with B. subtilis, B. megaterium and B. sphaericus (Kang et al., 2015; Li et al., 2015).
Problems in large-scale application of the MICP technology have occurred too and remain unsolved. Research by van Paassen et al. (2009) found poor sample homogeneity of MICP, as well as decreasing biomass and urease-inducing CaCO3 activity over time and increasing soil depth in a pilot 100 m3 sand study using Sporosarcina pasteurii, attributing these heterogeneities mostly to the application process. Alternative metabolisms and bacteria for large-scale applications in biomineralization of CaCO3 have also been investigated by the group (van Paassen et al., 2010). Indeed, it has been commented that the type of bacteria utilized is one of the major considerations and potential limitations in large-scale geotechnical operations (Mitchell and Santamarina, 2005).
Therefore, the search for new bacteria by which to achieve viable levels of MICP is important for optimizing the protocol best suited (in terms of performance, economics and environmental impact) for marketing in green industries (van Paassen et al., 2010; Cheng and Cord-Ruwisch, 2012; Patel, 2015). Following a literature review of the nine documented species of Sporosarcina (Claus and Fahmy, 1986), seven species were found to be urease positive and distinct from Sporosarcina pasteurii as alternative ureolytic MICP sources. While no candidate improves on some of the shortcomings of ureolytic MICP (i.e. ammonia toxicity), each candidate was found to be poorly investigated in the current MICP technology, despite fitting the ureolytic model for MICP. One candidate, Sporosarcina ureae, was selected at random for investigation as it was deemed appropriate to explore the feasibility of a single candidate species in thorough comparison to other already-published species applied in ureolytic MICP.
Thus, the primary goal of this study was to investigate the suitability of S. ureae as a MICP organism in material improvement by testing it experimentally against the previously investigated species of Sporosarcina pasteurii, Bacillus megaterium and Bacillus sphaericus. In its assessment, a parallel investigation was also performed to assess how the MICP technology, utilizing S. ureae as the candidate MICP organism, can perform under various environmental conditions including acid rain, flooding and freeze–thaw cycling concurrent with colder North American climates.
2.1 Bacteria strains, media, culture and stock conditions
Strains of Sporosarcina ureae (BGSC 70A1), Bacillus megaterium (BGSC 7A16), Lysinibacillus sphaericus (BGSC 13A4; previously known as Bacillus sphaericus; Ahmed et al., 2007) and Bacillus subtilis (BGSC 3A1T) were obtained from the Bacillus Genetic Stock Center (BGSC).
Sporosarcina pasteurii (ATCC 11859), previously known as Bacillus pasteurii (Yoon et al., 2001), was kindly donated by the group of Rodrigues et al. (University of Houston, USA). Escherichia coli DH5α™ was obtained from Thermo Fisher. S. ureae and S. pasteurii strains were grown at 30 ∘C in a modified ATCC 1832 medium as follows: 5 g L−1 yeast extract (YE) (BD Bacto™), tris base (Trizma™), 5 g L−1 ammonium sulfate (molecular biology grade, Sigma-Aldrich), 10 g L−1 urea (molecular biology grade, Sigma-Aldrich), pH 8.6. The culture broth, ATCC Medium 3 (3 g L−1 beef extract (BD Bacto™) and 5 g L−1 peptone (BD Bacto™)) was used for B. megaterium, L. sphaericus and B. subtilis. and grown at 30 ∘C, unless otherwise specified. Colonies of Bacillus and Sporosarcina were maintained on plates prepared as described supplemented with 15 g L−1 agar (BD Difco™). E. coli was grown in Luria–Bertani (LB) broth (10 g L−1 tryptone (molecular biology grade, Sigma-Aldrich), 5 g L−1 yeast extract (BD Bacto™), 10 g L−1 NaCl (molecular biology grade, Sigma-Aldrich), pH 7.5) and maintained on LB plates at 37 ∘C supplemented with 15 g L−1 agar (BD Difco™). Long-term stocks of all cultures were prepared as described (Moore and Rene, 1975) but using dry ice as the freezing agent.
2.2 Chemical and biological analysis
2.2.1 Culturing
Single colonies were lifted and grown overnight at 200 rpm in 5 mL of respective strain culture medium in a 15 mL Corning Falcon© tube. The overnight stock was combined with 200 mL of appropriate culture medium in a 500 mL Erlenmeyer flask and cultured at 175 rpm. The optical density at 600 nm (OD600) was used to track changes in turbidity of a culture volume using a BioMate UV–Vis spectrophotometer (Thermo Scientific) where 1 mL of culture volume was placed into 1.5 mL polystyrene cuvettes (Bio-Rad) of a 1 cm path length. Ultra-pure water (ddH2O) was used as a blank. At OD600 values greater than 0.4, samples of culture volumes were diluted 10–100× in tris-buffered saline (TBS; 50 mM tris base (Trizma©, Sigma-Aldrich), 150 mM NaCl (molecular biology grade, Sigma-Aldrich), pH 7.5) to maintain a linear relationship between turbidity and cell growth. When OD600 reached ∼ 0.5, the culture was twice spun at 5000 rpm for 5 min followed by a pellet re-suspension in 50 mL TBS each time. Next a fraction of volume was removed, spun at 5000 rpm for 5 min and re-suspended (OD600 ∼ 0.2) in 200 mL of a urea broth (UB) medium in a 500 mL Corning PYREX© round glass media storage bottle containing a modified Stuart's broth (Stuart et al., 1945) as follows: 20 g L−1 urea (BioReagent, Sigma-Aldrich), 5 g L−1 tris base (Trizma©, Sigma-Aldrich), 1 g L−1 glucose (reagent grade, Sigma-Aldrich), pH 8.0, with (UB-1) or without (UB-2) 10 g L−1 YE (BD Difco™). A negative control included a medium-only condition. All steps were performed aseptically with preparations incubated at 150 rpm at 30 ∘C in triplicate for each medium condition: UB-1 and UB-2. Each culture for a medium condition was staggered 10 min apart and observed for 12 h, with duplicate 2.5 mL aliquots aseptically withdrawn every 1 h, beginning at time zero (t = 0 h). The entire protocol was performed twice for a total of six data sets (n = 6), measured in duplicate, per culture in a single-medium condition.
2.2.2 Total ammonia (NH3–), pH and growth (OD600) aliquots
To evaluate different cell parameters efficiently, duplicate aliquots (2.5 mL) were taken for tracking pH, OD600 and NH3– production. In brief, first, whole aliquot volume pH was taken with a SB20 symphony pH probe (VWR). Next, 1 mL was removed for OD600 as described (Sect. 2.2.1). Finally, a 500 uL sample for NH3– analysis was retrieved and diluted in 500 uL of ddH2O and stored as described by HACH Inc. (Hach Co., 2015) with the following additional modifications made: −20 ∘C storage, one drop 5 N H2SO4.
2.2.3 Spectrophotometric analysis of NH3–
Samples were thawed and neutralized with 5 N NaOH as described by HACH Inc. (Hach Co., 2015). NH3– measurements were then performed as outlined (HACH Co., 2015) based on an adaptation of the work by Reardon et al. (1966) using a portable DR2700 HACH spectrophotometer. Samples were brought to a measureable range (0.01 to 0.50 mg L−1 NH3–N) where required. Measurements for appropriate dilutions were made by mass and corrected to volume assuming a density of 1 g L−1. Final values were reported as “U mL−1”, where U is units of µmol of NH3– produced per minute and mL is mL solution normalized to culture density (OD600) starting from t= 1 h.
2.3 Microbial cementation
2.3.1 Model sand
Industrial-quality, pure coarse silica sand (Unimin Canada Limited) was examined with the following grain distribution where D10, D50 and D60 are 10, 50 and 60 % of the cumulative mass: D10= 0.62 mm; D50= 0.88 mm; and D60= 0.96 mm. The uniformity coefficient, Cu, was 1.55, indicating a poorly graded (i.e. uniform) sand as designated by the Unified Soil Classification System (USCS) (ASTM, 2017). A poorly graded soil was used as a model due to its undesirable geotechnical characteristics in construction (i.e. settling) and tendency for instability in nature (i.e. liquefaction) (Scott, 1991; Nakata et al., 2001).
2.3.2 Cementation medium (CM) and culture
Cells of each strain were grown in 1 L of their respective medium split into two 1 L Erlenmeyer flasks containing 500 mL medium each at 175 rpm to an OD600 of ∼ 1.5–2.0 as described (Sect. 2.2.1). Cells were then harvested and successively concentrated over three runs to 50 mL. Runs involved a spin-down at 5000 rpm for 5 min followed by a pellet re-suspension in TBS. Prior to sand inoculation, 50 mL of a 2× concentrated cementation medium (2 × CM; 0.5 M CaCl2 (anhydrous granular, Sigma-Aldrich), 0.5 M urea (BioReagent, Sigma-Aldrich), 5 g L−1 YE (BD Difco™), 50 mM tris base (Trizma©, Sigma-Aldrich), pH 8) was added to the final suspension. Negative controls were 1 : 1 mixes of ddH2O and 2 × CM as well as the non-ureolytic strain B. subtilis (BGSC 3A1T) (Cruz-Ramos et al., 1997). A positive control with S. pasteurii (ATCC 11859), a ureolytic organism capable of ureolytic MICP (van Paassen et al., 2009), was also run. The procedure was repeated every 24 h to provide fresh cells for injection during cementation trials.
2.3.3 Sample preparation and cementation trial
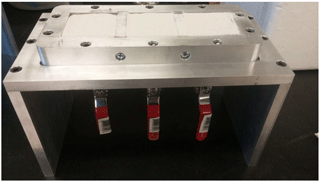
Triplicate test units were constructed from aluminum (Fig. 1), each housing a triplicate set of sample moulds measuring 60 × 60 × 15 mm. Moulds were sized according to the sample intake for the direct shear apparatus (model: ELE-26-2112/02) utilized in confined shear tests. Each mould was equipped with a drainage valve for medium replacement. Filter paper was placed over the drainage valve holes during silica sand packing to prevent material loss. Silica (autoclaved; dry cycle, 120 ∘C, 15 min) was packed to a dry density of 2.50–2.55 g cm−3 and washed three times with 25 mL of TBS. Thereafter, 25 mL of a CM suspension containing bacteria was added and incubated for 24 h. At the end of the incubation period, the CM suspension was drained and the sand washed three times with 25 mL of TBS. This was repeated twice for a total of three 24 h incubation periods. In addition, during each 24 h incubation period, 1 mL of solution was reserved for serial dilution at two times: (1) immediately after addition of CM suspension and (2) immediately before draining of CM suspension. Serial dilutions were performed using TBS onto agar plates as described (Sect. 2.1) with 0.1 mg L−1 ampicillin (Sigma-Aldrich) to measure biomass as colony-forming units (CFUs). Many species of Bacillus were found to be resistant at these Ampicillin concentrations (Environment Canada, 2015) but otherwise lethal to most contaminant bacteria. In-lab tests observed 95 % survival rates greater than 95 % for all considered Bacillus and Sporosarcina strains compared to a survival rate lower than 0.1 % among a model E. coli (DH5α™, Thermo Fisher). Ambient temperatures of treated sands were maintained at 22 ∘C, reflective of average subsurface soil temperatures of central North American climate in the summer (Mesinger et al., 2006).
2.3.4 Confined direct shear tests
Treated, drained samples were washed twice with 25 mL of ddH2O and dried in an oven for 48 h at 65 ∘C. Washing with ddH2O was done to remove salts other than CaCO3 to prevent cementation of the sand due to salt precipitation in the drying process as has been found in the literature (Jia and Jian, 2016; Zeng et al., 2018). The shear strength tests were performed in a direct shear machine (Model: ELE-26-2112/02). Unless otherwise specified, shear tests were performed on samples with an applied normal stress of 25 kPa. Shear stress was then applied to failure at a rate of 2.5 mm min−1 under dry and drained conditions. Stress–strain curves were acquired via LabVIEW data acquisition software.
2.4 Scanning electron microscopy (SEM) observation
Silica grains from the surface layer of treated, washed and dried sands were mounted on a sample holder (51 mm) using double-sided copper tape and observed to confirm the crystalline nature of the resulting precipitates using a JEOL6610LV scanning electron microscope (5 kV). Elemental composition of surface structures was analyzed, in parallel, by energy-dispersive X-ray spectroscopy (EDS).
2.5 Environmental simulation tests
2.5.1 Water flushing
The ability of cured samples to perform following a 1-month saturation period was tested. Treated silica sands were incubated with ddH2O over six periods of incubation. Each period involved injection of 25 mL of ddH2O followed by a 5-day treatment under ambient temperature of 22 ∘C. Volumes were replaced at the end of each period. No aliquots for colony counts were taken.
2.5.2 Ice water cycling
To understand the degree to which ice cycling impacted the shear strength of treated silica sand, a selected number of samples were treated over six periods of ddH2O incubation as described immediately above. However, each period began with freezing at −20 ∘C for 24 h, followed by holding for 3 days at −20 ∘C and thawing for 24 h at 22 ∘C. The selected maximum and minimum temperatures reflect those capable of being reached in Ontario winters and summer (Canada), respectively, according to Environment Canada (climatic station: Ottawa CDA) (Government of Canada, 2017).
2.5.3 Acid erosion
Formulation of an acid rain model was made according to average pH values (pH ∼ 4.4) of rainfalls reported for northeastern regions of North America (Environment Canada, 2013). The final pH was adjusted using concentrated sulfuric acid (H2SO4). One delivery volume of acid rain was equivalent to the average monthly precipitation of a North American region (April, Ottawa, Canada), calculated from records of Environment Canada (climatic station: Ottawa CDA) (Government of Canada, 2017). Rain was delivered as described for “water flushing” with ddH2O but for a single incubation period. Following incubation, the treated volumes were flushed with 25 mL of ddH2O.
2.6 Statistical processing
All statistical manipulations were performed in Excel (2007). Sample means were reported alongside the standard error of the mean (SE) or standard deviation (SD). Normality of all data sets was confirmed with the Anderson–Darling test (α= 0.05). The Student's t test (unpaired, two-tailed; α= 0.05) was utilized to compare sample means of experimental conditions for statistical significance. Prior to each t test, homogeneity of variances for data sets were determined using an F test (α= 0.05). Where variances were statistically observed as unequal, a Welch's t test was adapted to test statistical significance between two sample means.
3.1 NH3– production
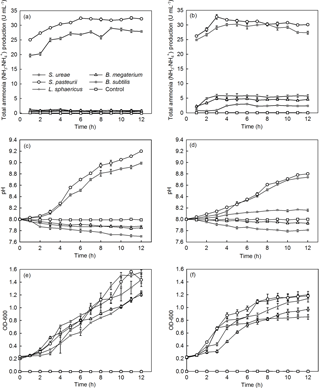
Among the different bacterial strains considered, S. pasteurii and S. ureae were capable of producing the first- and second-highest levels of NH3–, respectively, per unit of time, in both UB-1 (32.50; 29.00 U mL−1) and UB-2 (32.76; 30.28 U mL−1) media (Fig. 2a, b). Isolates of B. subtilis (2.91 U mL−1), B. megaterium (4.87 U mL−1) and L. sphaericus (5.89 U mL−1) displayed a lower peak of NH3– production in both media. When urea in medium moved from the sole source (i.e. UB-2) to one of a number of sources (i.e. UB-1) for nitrogen, NH3– production dropped to near-zero values (Fig. 2a, b) for B. subtilis (0.44 U mL−1), B. megaterium (0.56 U mL−1) and L. sphaericus (1.20 U mL−1) that were statistically significantly different (p < 0.05, n= 6) from the final UB-1 values for each species. However, isolates of S. ureae and S. pasteurii observed no statistically significant difference (p > 0.05, n= 6) between final values recorded in UB-1 and UB-2 media. Instead, a rise in production (t= 0–5 h) followed by a levelling-off in value (t= 6–12 h) was the general trend observed in UB-1 and UB-2 media (Fig. 2a, b).
3.2 Examination of bacterial abundance in culture
All strains showed a decline in growth progression when medium was restricted (i.e. UB-2) to urea as nitrogen and glucose as carbon sources (Fig. 2e, f). Growth repression was greatest in the cases of B. subtilis (−33.9 %), L. sphaericus (−26.8 %) and B. megaterium (−23.6 %) compared to S. pasteurii (−17.8 %) and S. ureae (−16.6 %).
Additionally, the final OD600 (t= 12 h) achieved for all strains in UB-2 medium was decreased compared to UB-1 medium values (t= 12 h), and the difference in value for each strain was found to be statistically significantly different (p < 0.05, n= 6). Growth cessation (i.e. stationary phase) occurred for S. ureae and S. pasteurii in both conditions but later in UB-1 (t= 11 h) compared to UB-2 (t= 9–10 h) medium (Fig. 2e, f); they grew logistically in both medium conditions. In general, growth of L. sphaericus, B. subtilis and B. megaterium in UB-2 medium followed a logistic growth curve too. However, in UB-1 medium their growth fit an exponential model, whereby an exponential growth phase was observed from t= 4 h to t= 12 h following a lag phase of growth between t= 0 h and t= 3 h.
3.3 Changes in pH
The alkalinity increased with the increase in time for the strains of S. ureae and S. pasteurii studied, in both UB-1 (8.99; 9.2) and UB-2 (8.74; 8.8) media. The lowest final pH values were observed in L. sphaericus (7.88; 8.16), B. megaterium (7.85; 7.93) and B. subtilis (7.70; 7.81) in UB-1 and UB-2 media at the end of 12 h (Fig. 2c, d).
While pH continued to rise for S. pasteurii and S. ureae in either UB-1 or UB-2 medium, it was constant for L. sphaericus, B. megaterium and B. subtilis after time in UB-1 medium as early as 6 h (L. sphaericus and B. megaterium) in UB-2 medium. While final pH values for L. sphaericus, B. megaterium and B. subtilis reached higher final (t= 12 h) values in UB-2 medium compared to UB-1, which were found to be statistically significantly different (p < 0.05, n= 6), the opposite was true for S. pasteurii and S. ureae; values in UB-2 were lower compared to UB-1, and the difference was found to be statistically significantly different for each species (p < 0.05, n= 6). In general, acidity increased with the increase in time for L. sphaericus, B. megaterium and B. subtilis in UB-1 medium. This was also true in UB-2 medium except for L. sphaericus, which showed an increase in pH over time.
3.4 Mechanical and biological behaviour in MICP reinforced sands
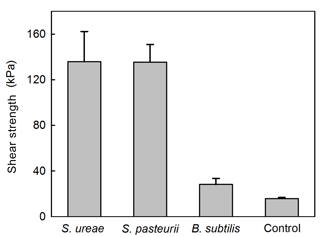
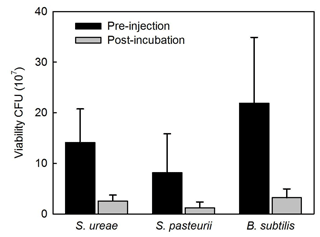
Experiments of sand consolidation with triplicate holding vessels (Fig. 1) mixed with S. ureae (135.77 kPa) or S. pasteurii (135.5 kPa) and fed MICP medium (i.e. CM-1) had improvements in their direct shear strength compared to control vessels (15.77 kPa) fed with MICP medium only. In fact, the difference in direct shear strength values for S. ureae and S. pasteurii compared to control vessels was found to be statistically significantly different (p < 0.05, n= 3). However, the difference in strength between S. ureae and S. pasteurii was not statistically significantly different (p > 0.05, n= 3). Mixtures of non-ureolytic B. subtilis (28.1 kPa) showed no statistically significant difference (p > 0.05, n= 3) in value when compared to the control (Fig. 3). While pre-injection (21.9 × 107 CFU mL−1) and post-incubation (3.2 × 107 CFU mL−1) cell abundance was highest in the case of B. subtilis (Fig. 4), all bacterial isolates showed a decrease in cell abundance when comparing pre-injection to post-incubation cell abundance with statistically significant differences (p < 0.05, n= 9). Also, the percentage loss of cell abundance, taken as the difference between post-incubation and pre-injection cell abundances divided by the initial pre-injection cell abundance (−77.7 % (S. ureae), −75.4 % (S. pasteurii), −77.7 % (B. subtilis)), was not statistically significantly different (p > 0.05, n= 9) when comparing values between species. Of note, the medium-only control had no cell growth (CFU mL−1) observed before and after incubation.
3.5 Microstructure investigation
The precipitation of calcium as CaCO3 via MICP was visualized. Sand granules from approximately the first 1 cm of sands treated with MICP solution (i.e. CM-1) combined with S. ureae are shown (Fig. 5a, b), where crystals arranged in rosette peaks (20–40 µm) can be seen across the surface of a sand grain (Fig. 5a, b). Rod-shaped structures (40–80 µm) can also be visualized, though less commonly, across grain surfaces (Fig. 5a, b). Calcium, carbon and oxygen peaks captured by EDS analysis for crystals organized in “rosette” patterns as well as in rod-shaped structures suggest CaCO3 precipitation (Fig. 5c, d).
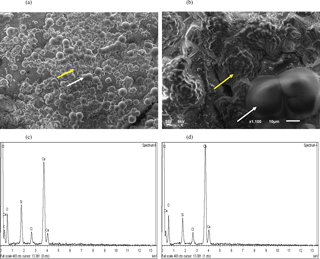
Figure 5SEM image of the (a) whole surface (bar, 100 µm) and (b) magnified (bar, 10 µm) silica granule with crystalline (yellow arrow) and amorphous (white arrow) calcium structures following bacterial treatment. EDS analysis shows the chemical composition of (c) crystalline and (d) amorphous precipitates.
3.6 Environmental durability of MICP
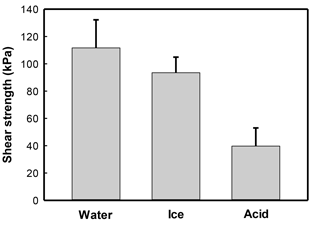
A reduction in the reinforcement of sands by CaCO3 mineralization with S. ureae inoculations was observed following exposure to acid rain as direct shear strengths reduced to 39.7 kPa (Fig. 6) or 29.2 % compared to those with no such treatment (Fig. 3). Treated sands under conditions of flooding (111.7 kPa) or freeze–thaw (93.5 kPa) rounds had better durability (i.e. strength retention) compared to acidified states, with differences in strength being statistically significantly different (p < 0.05, n= 3). In fact, no severe mechanical damage was incurred by samples treated under conditions of simulated flooding or freeze–thaw cycles (Fig. 6); when comparing the difference in their direct shear strengths to sands tested under ideal (i.e. non-environmental) conditions, these differences were found to be not statistically significantly different (p > 0.05, n= 3) (Fig. 3).
In characterizing S. ureae as a ureolytic organism in MICP, the goals of the study were to understand (1) its ability to degrade urea over time relative to other commonly applied MICP bacterial isolates and (2) its preference for urea as a nitrogen source. The strain (BGSC 70A1) was consistent in its total nitrogen (NH3–) production regardless of whether the nutrient medium included (i.e. UB-1) or did not include (i.e. UB-2) yeast extract. This can be attributed to mostly urea catabolism in UB-1 medium and entirely so in UB-2 medium as urea was the sole source of nitrogen. It is important to note that minor mineralization of the yeast extract components in UB-1 medium would likely have contributed ammonium (Gat et al., 2014) in this medium condition. This is supported by data recorded for the negative control (medium-only) in UB-1 medium with production as high as 0.12 U mL−1 (Fig. 2a, b). Also, degradation of amino acids from bacterial metabolism, such as ornithine, particularly supplied in UB-1 medium via yeast extract, could also contribute to total nitrogen in solution for this condition (Cruz-Ramos et al., 1997). For both media (UB-1 and UB-2) dissolution of ammonium as ammonia into the atmosphere would have reduced available nitrogen for measurement over time. Thus, a quantitative urea hydrolysis rate cannot be determined from the data collected, as nitrogen production over extended periods of time is a complex collection of some or all of these processes. This limits the conclusions able to be drawn as only the broad bacterial activity in medium, as regards preferences for urea as a nitrogen source, over time can be considered. For a quantitative method determining urease rates a robust protocol is presented by Lauchnor et al. (2015). Also, urea-hydrolysis-induced CaCO3 precipitation rates can be determined by measuring the decrease in dissolved Ca2+ ions over time (Harbottle et al., 2016). However, overall, the total nitrogen production over time draws support for S. ureae as a promising MICP candidate in biocement as over the time period measured it was able to produce a consistent amount of nitrogen as ammonia–ammonium in UB-1 or UB-2 medium, and ammonia production has been found to be directly proportional to CaCO3 production (Achal et al., 2009) and soil stabilization (Park et al., 2012).
As mentioned, the production of nitrogen by S. ureae in medium is due mostly, or completely, to urea catabolism, and this process is likely driven chiefly by its urease enzyme (Gruninger and Goldman, 1988; Mobley and Hausinger, 1989). Alternatively, an unknown urea-degrading enzyme other than urease could produce or contribute to the result. Notably, all Bacillus strains observed a decrease in total ammonia production when yeast extract was available (i.e. UB-1). This was not observed for S. ureae, much like S. pasteurii. Urea is a nitrogen source for bacterial growth, often catabolized by urease (Lin et al., 2012), which has been found to be controlled by nitrogen levels and pH as well as other factors which can differ between bacterial species (Mobley et al., 1995, 2001). Our observations indicate that S. ureae selects for urea in a metabolic pattern potentially similar to S. pasteurii and quite differently from the Bacillus strains investigated here, which appear to have medium-dependent metabolism of urea.
The observation that the investigated Bacillus strains have medium-dependent metabolism of urea is particularly interesting for B. subtilis as it has been applied as a non-ureolytic control organism in previous literature (Stocks-Fischer et al., 1999; Gat et al., 2014). In UB-2 medium, a non-zero total ammonia activity was measured for this strain (Fig 2a, b). This is consistent with previously published literature linking total ammonia production to urea breakdown from urease when urea is the sole source of nitrogen and urease is the assumed main catabolic enzyme – the enzyme expressed constitutively in species of Sporosarcina (Mobley et al., 1995) but in a repressible manner (i.e. activated in the absence of and other forms of nitrogen (i.e. ), with urea being the sole nitrogen source) in strains such as B. megaterium (Mobley and Hausinger, 1989) and B. subtilis (Atkinson and Fisher, 1991; Cruz-Ramos et al.,1997). This is indeed suggested by our data as it was observed for B. subtilis (and also for B. megaterium and L. sphaericus) that increased total ammonia production reached higher values in UB-2 medium compared to near-zero values in UB-1 medium with yeast extract as an alternative nitrogen source. In fact, in UB-2 medium peaks were reached within 3–6 h from near-zero values (t= 0–1 h) for all Bacillus species, further suggesting an increase in processes related to urea hydrolysis, such as urease expression, over time following a reduction in genetic repression (Fig 2a, b). This also corrolates well with growth patterns. A comparatively slow growth rate occurred (t= 8–12 h) after a comparatively fast (t= 3–7 h) rate of growth following a lag period (t= 0–2 h) for these strains, in general (Fig. 2a, b). An increase in urease, or other urea hydrolysis processes, may account for an ability to grow quickly (t= 3–7 h) despite nitrogen limitation in UB-2, as ureolysis would provide nitrogen for growth-related processes. However, growth could have been restricted, over time, due to other nutrient limitations such as glucose depletion. This would explain a continued but reduced growth rate (t= 8–12 h) (Fig. 2a, b). Alternatively, or in addition, the decreased growth could be due to decreased dissolved oxygen content in medium over time, which is required for aerobic respiration, such that each Bacillus species switched to a slower anaerobic growth pattern. An increase in harmful metabolites such as organic acids in solution over time could also have hindered growth. There is evidence that this occurred for these species in UB-1 medium as a decrease in pH over time was observed which correlates with organic acid production (Fig. 2c). Taken together, this has significance as, while B. megaterium and L. sphaericus have been investigated as candidates in ureolytic MICP, this has not been extensively the case for B. subtilis, which in this study shows ureolytic capability under specific conditions. This may guide future research on ureolytic MICP with B. subtilis, particularly where cementation media do not contain nutrient-rich additives such as yeast extract. This has been the case in some literature solutions for inducing ureolytic MICP (van Paassen et al., 2010; Cheng et al., 2013). In this study B. subtilis was included in sand solidification as a non-ureolytic strain control as the cementation medium contained yeast extract, intended for maximum biomass support and CaCO3 production rates (van Paassen et al., 2010).
It is clear that S. ureae prefers an alkaline environment, like S. pasteurii and quite different from the other isolates in trials, as in both growth conditions samples grew not only exponentially but towards an increased pH (Fig. 2c, d). Urea hydrolysis, driven potentially by urease, in this species may maintain ureolytic activity for production of the highly alkaline environment to which it is suited for growth as an alkaliphile and for its role as a nitrogen cycler (Gruninger and Goldman, 1988). These conditions are also important for CaCO3 production (Whiffin et al., 2007). It can also use the charge gradient generated from ammonium production for energy (Jahns, 1996) to support growth. A diagram of this adenosine triphosphate (ATP)-generating system coupled to ureolysis is available in the work of Jahns (1996) and Whiffin (2004). Additionally, the ammonium is an accessible nutrient (i.e. nitrogen) source (Gruninger and Goldman, 1988). This may partly account for S. ureae and S. pasteurii having the smallest change in growth between UB-1 and UB-2 medium by having the material but also energetic means to multiply. This is extremely promising as van Paassen et al. (2010) determined the CaCO3 precipitation rate is positively correlated with the number of viable microorganisms in solution. Thus, taken together, the ureolytic, pH and growth data of this study support S. ureae as superior in ureolytic action to every Bacillus strain considered except S. pasteurii. Indeed, the work of Harbottle et al. (2016) likewise found S. ureae and S. pasteurii to be about as efficient in terms of ureolytic activity. Given the current data, S. ureae and S. pasteurii are comparable as candidates for ureolytic MICP. This should prompt interest for further investigations differentiating between the two strains on such parameters as protease activity, exopolysaccharide production and biofilm levels, which are also connected to MICP capability (Achal et al., 2010), so as to identify the superior candidate. Some differential work has already been done (Sarmast et al., 2014).
To understand the macroscopic engineering aspects of S. ureae in MICP application, efforts of this study were focused on measuring and assessing its ability to strengthen model sands via urea hydrolysis to form CaCO3. In experiments with a model silica sand featuring poor geotechnical characteristics (i.e. uniform sand profile) for high susceptibility to settling and static strength decreases (Conforth, 2005), it was clearly shown that the S. ureae treatment led to consolidation of the medium in 48 h with an improvement in strength to 135.77 kPa. This was 8 times that of the control treatment (15.76 kPa) (Fig. 3). In addition, while average consolidation strengths had no statistically significant difference (p < 0.05, n= 3) between S. ureae and S. pasteurii, the peak sample strength recorded for a S. ureae mould (175.8 kPa) exceeded the maximum sample strength recorded for S. pasteurii (165.7 kPa), the typical model ureolytic organism in MICP soil strengthening. It was also well above peak average strength recorded for B. subtilis (28.1 kPa) (Fig. 3). This is as expected; B. subtilis is a non-ureolytic organism in the “good nitrogen” (Atkinson and Fisher, 1991) nutrient conditions supplied by the yeast extract of CM-1 medium. Other Bacillus species were not tested under the assumption that they too would experience repressive urea hydrolysis expression in CM-1 medium and would produce similar observations as a result. This is supported by data provided by the groups of Al Qabany et al. (2012) and van Paassen et al. (2010), which found that CaCO3 precipitation, and by inference soil strength, improved with more suitable microorganisms in MICP. Taken together, this study provides evidence that S. ureae is capable of soil improvement by ureolytic MICP similarly to S. pasteurii.
The presence of crystals as organized “rosettes” and amorphous “rods” was observed (Fig. 5a, b) along sand granules treated with S. ureae, which is evidence that it is capable of inducing prevalent formation of secondary minerals. The structures were analyzed by EDS, and the results provide support for CaCO3 formation (Fig. 5c, d). Assuming that the solution was saturated with respect to CaCO3 and that the nucleation and crystallization of the calcite polymorph was thermodynamically favoured over time, the organized deposits should represent calcite (De Yoreo and Vekilov, 2003). However, fast nucleation and crystallization can result in amorphous CaCO3 structures and could explain the rod deposits that appear amorphous in morphology under SEM (Fig. 5a, b) (Addadi et al., 2003). This observation is limited, though, as SEM cannot discriminate among CaCO3 polymorphs which can have varying morphology based on the crystallization conditions (Ni and Ratner, 2008). The exact polymorph of CaCO3 for each structure could be distinguished with techniques such as X-ray diffraction (XRD) and/or Fourier transform infrared spectroscopy (FTIR) (Anthony et al., 2003; Ni and Ratner, 2008). Assuming the rod structures are amorphous precipitates, this indicates that the treatment conditions were potentially suboptimal for the maximum precipitation of crystalline CaCO3 such as calcite over time. This could be due to high local chemical concentrations (e.g. calcium) which have been found to hinder CaCO3 crystal formation as calcite (Al Qabany et al., 2012). Investigators may be prompted to test alternative calcium concentrations from those used in this study for injections so as to increase the efficiency of crystalline CaCO3 precipitation in MICP. Finally, medium and B. subtilis treated sands gave no discernible crystal CaCO3 formation (data not shown). This provides evidence of superficial strengthening in shear tests for these treatments based on natural biofilm excretion (B. subtilis) or sporadic mineral crystallization. Thus, overall, the microscopy evidence does support that S. ureae can precipitate CaCO3 for strength improvements in soil, which was part of the goal in studying S. ureae in MICP.
When analyzing the cell viability of injections before and after incubation in treated sands, it was found that S. ureae maintained higher post-incubation (2.56 × 107 CFU) cell abundance compared to S. pasteurii (1.21 × 107 CFU) and that these differences were statistically significant (p < 0.05, n= 9) (Fig. 5). Also, both species' cell abundance was lower and found to be statistically significantly different (p < 0.05, n= 9) compared to the cell abundance for B. subtilis (3.2 × 107 CFU). This difference could be due to the solution (i.e. TBS) utilized for serial dilution of the growth medium. The TBS did not include ammonium and was not buffered at a high pH, which are two necessary conditions for the survival of alkaliphilic genera such as Sporosarcina (Morsdörf and Kaltwasser, 1989). Thus, a deflated value for S. pasteurii and S. ureae would result. Also, moulds become mostly anaerobic over time below the subsurface and within the microenvironments of sand grains as oxygen is depleted by bacterial respiration (van Paassen et al., 2010). B. subtilis cells may have survived anaerobically (Clements et al., 2002) as opposed to the obligate aerobes S. ureae and textitS. pasteurii (Claus and Fahmy, 1986), leading to higher post-incubation cell abundance for B. subtilis. However, considering the percentage loss of cell abundance calculated as described (Sect. 3.4) is comparable between all three species, this indicates that neither species outperforms the other in cell survival while in the high-salt, high-urea CM-1 medium with incubation in treated sands. That being written, the total cell abundance in S. ureae is higher compared to S. pasteurii. This is important as cells provide nucleation points for CaCO3 formation. Indeed, the literature reports designate that strength enhancement by ureolytic MICP is driven not only by urea hydrolysis activity but also by the presence of bacteria acting as nucleation sites (Stocks-Fischer et al., 1999; Gat et al., 2014). While sand surfaces can also act as nucleation points, the negatively charged bacteria cell wall attracts positively charged cations (e.g. calcium) preferentially for the controlled nucleation of CaCO3 over time. In fact, it has been shown that cell abundance in MICP treatments positively correlate with the precipitation of CaCO3 in both the rate of production and crystal size (Mitchel and Ferris, 2006). The group of Hommel et al. (2015) have even developed a model showing that calcite precipitation is proportional to cell abundance (i.e. biomass) and potentially improved soil strengths. This model assumes that the features of the cells such as biofilm production around their cell walls favour and facilitate the precipitation of CaCO3. It follows that any intact cell wall part of the biofilm can facilitate the precipitation process whether the cell itself is alive or dead. Thus, in general, more cells equates to more CaCO3 precipitation. However, in this study, S. ureae gave rise to strengths in sands that were not statistically significantly different (p > 0.05, n= 3) versus S. pasteurii treatments. This is unexpected since S. ureae had comparable ureolytic activity to S. pasteurii but higher cell abundance over time in precipitation medium. Therefore, more CaCO3 precipitation should have occurred and led to a greater strength increase in sands in S. ureae treatments. This non-linear increase in strength compared to cell abundance can be a result of a number of factors. For example, the ability of cells to precipitate CaCO3 can be hindered when an abundance of cells injected into porous material (i.e. sands) leads to pore plugging from the organic matter (i.e. cells). This has been seen to lead to a varied amount of CaCO3 precipitation throughout the volume of a mould (van Paassen et al., 2009). Where cells are distributed more evenly, they can facilitate the precipitation of CaCO3 as nucleation points (Hommel et al., 2015). This may explain why S. ureae, having a comparable NH3– activity to S. pasteurii, did not outperform it on average in undrained, direct shear strength tests despite having a higher cell abundance on average. It may also explain the broader range of strengths achieved in S. ureae (Fig. 3). For example, a suboptimal spreading mechanism could have hindered strength achievement in some moulds of S. ureae treatment where pore plugging by organic matter (i.e. cells) occurred. With this in mind, optimization of treatment protocols would help to determine whether or not S. ureae is the superior candidate compared to S. pasteurii given that it has consistently increased total cell abundance (Fig. 3) to support more nucleation of CaCO3 over time, in tandem with a NH3– production comparable to that of S. pasteurii. However, it is important to note that S. ureae cells are significantly smaller than cells of S. pasteurii (Claus and Fahmy, 1986). Therefore, the total cellular surface area available for nucleation of CaCO3 would be similar for the two species. This provides a possible explanation for why no statistically significant differences in strength were observed because if total cellular surface area was most important for precipitating CaCO3 this means there would be no difference in strengths expected for the same total cellular surface area, whether it was spread over a relatively high number of smaller cells (i.e. S. ureae) or lower number of larger cells (i.e. S. pasteurii).
It was the current authors' focus to also apply tests in conditions reflective of a Canadian environment with a relatively novel bacterial isolate (S. ureae). Sands treated with S. ureae and which underwent short-term flooding (111.67 kPa) or freeze–thaw cycling (93.47 kPa) showed no statistically significant (p > 0.05, n= 3) strength difference compared to in-lab (135.77 kPa) conditions (Fig. 6). It has been shown that MICP-treated sands maintain some porosity in materials (Cheng and Cord-Ruwisch, 2012; Chu et al., 2012) and that good strength maintenance in seasonal water saturation and freeze–thaw cycling is possible with porous materials (Litvan, 1980; Cornforth, 2005). Further studies may wish to investigate the permeability of hardened sands via S. ureae at various levels of CaCO3 precipitation to strike a balance between porosity, peak strength and endurance over time in weather simulations.
Predictably, it was seen that the acid rain model, reflective of a northern Ontario rain pH (Sect. 4.4), eroded the shear strength of sands (Fig. 6) to 35.5 % of originally observed values (Fig. 3). This is a result of the reaction of acid with CaCO3 producing units of H2O, CO2 and salt, known as weathering. A study by Cheng et al. (2013) reported similar results with a Bacillus sphaericus model. This prompts the idea that a MICP strength model, regardless of the bacteria treatment selected (S. ureae, S. pasteurii etc.) for strength enhancement, would require a time-based repair of treated volumes. This realistically limits its geotechnical and economical practicality in the industry. However, it does prompt interest to test the ability of natural buffers, such as limes and sodas, to increase the life span of MICP-induced strength enhancement by reducing acid rain degradation.
This study has worked to verify that S. ureae is a suitable organism to be applied in the soil-hardening technology currently being developed via ureolytic MICP. The authors designate it a close ureolytic MICP candidate, in performance, to the well-studied S. pasteurii and a superior one to several other Bacillus strains. As larger-scale simulations are employed, it is strongly encouraged by the authors that further optimization in the treatment procedure, regardless of the MICP organism selected, be undertaken including ideal soil buffering to reduce certain climatic effects (i.e. acid rain) and optimum volume porosity in the space to be treated to assure an economical application in industry.
The underlying research data can be accessed at Mendeley Data: http://dx.doi.org/10.17632/crnykvnt42.1 (Whitaker et al., 2018).
The specific contributions made by each co-author of the work are as follows: conceptualization, methodology, resources, investigation, project administration, formal analysis, validation, visualization, drafting, editing and final approval of the manuscript – JMW; funding acquisition, supervision, validation, project administration, editing and final approval of the manuscript – SV and DF.
The authors declare that they have no conflict of interest.
The study was funded by NSERC (Discovery Grant nos. 2016–2021 and
2015–2020) and the University of Ottawa (UROP grant 2012; USRA grant
2014).
The authors would like to acknowledge the University of Ottawa (UROP grant)
and the National Sciences and Engineering Research Council of Canada (NSERC
USRA and NSERC Discovery Grants to Danielle Fortin and Sai Vanapalli) for financial
provisioning in support of this project. Thanks are also given to Jean
Celestin, Yunlong (Harry) Lui, Penghai (Peter) Yin, Nimal De
Silva, Erika Revesz and George Mrazek, each of whom provided assistance in
shear measurements, microscopy and/or data analysis.
Edited by: Denise Akob
Reviewed by: three
anonymous referees
Achal, V., Mukherjee, A., Basu, P. C., and Reddy, M. S.: Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production, J. Ind. Microbiol. iot., 36, 981–988, https://doi.org/10.1007/s10295-009-0578-z, 2009.
Achal, V., Abhijit, M., and Reddy, M. S.: Characterization of Two Urease-Producing and Calcifying Bacillus spp. Isolated from Cement, J. Microbiol. Biotech., 20, 1571–1576, https://doi.org/10.4014/jmb.1006.06032, 2010.
Addadi, L., Raz, S., and Weiner, S.: Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization, Adv. Mater., 15, 959–970, https://doi.org/10.1002.adma.200300381, 2003.
Al Qabany, A., Soga, K., and Santamarina, C.: Factors affecting efficiency of microbially induced calcite precipitation, J. Geotech. Geoenviron., 138, 992–1001, https://doi.org/10.1061/(ASCE)GT.1943-5606.0000666, 2012.
Anthony, J. W., Bideaux, R. A., Bladh, K. W., and Nichols, M. C.: Borates, Carbonates, Sulfates, in: Handbook of Mineralogy, Chantilly, VA: Mineralogical Society of America, 5, available at: http://www.handbookofmineralogy.org (last access: May 2018), 2003.
ASTM D2487-17: Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System) available at: http://www.astm.org/cgi-bin/resolver.cgi?D2487 (last access: June 2018), 2017.
Atkinson, M. R. and Fisher, S. H. Identification of genes and gene products whose expression is activated during nitrogen-limited growth in Bacillus subtilis, J. Bacteriol., 173, 23–27, 1991.
Barabesi, C., Galizzi, A., Mastromei, G., Rossi, M., Tamburini, E., and Perito, B.: Bacillus subtilis Gene Cluster Involved in Calcium Carbonate Biomineralization, J. Bacteriol., 189, 228–235, https://doi.org/10.1128/JB.01450-06, 2007.
Bergdale, T. E., Pinkelman, R. J., Hughes, S. R., Zambelli, B., Ciurli, S., and Bang, S. S.: Engineered biosealant strains producing inorganic and organic biopolymers, J. Biotechnol., 161, 181–189, https://doi.org/10.1016/j.jbiotec.2012.07.001, 2012.
Cheng, L. and Cord-Ruwisch, R.: In situ soil cementation with ureolytic bacteria by surface percolation, J. Ecol. Eng., 42, 64–72, https://doi.org/10.1016/j.ecoleng.2012.01.013, 2012.
Cheng, L., Cord-Ruwisch, R., and Shahin, M. A.: Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation, Can. Geotech. J., 50, 81–90. https://doi.org/10.1139/cgj-2012-0023, 2013.
Chu, J., Stabnikov, V., and Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil, Geomicrobiol. J., 29, 544–549, https://doi.org/10.1080/01490451.2011.592929, 2012.
Claus, D. and Fahmy, F.: Genus Sporosarcina Kluyver and van Niel 1936, in: Bergey's Manual of Systematic Bacteriology, edited by: Mair N. S., Sneath, P. H. A., Sharpe, M. E., and Holt, J. G., The Williams & Wilkins Co., Baltimore, USA, 2, 1202–1206, 1986.
Clements, L. D., Miller, B. S., and Streips, U. N.: Comparative growth analysis of the facultative anaerobes Bacillus subtilis, Bacillus licheniformis, and Escherichia coli, Syst. Appl. Microbiol., 25, 284–286, 2002.
Cornforth, D. H.: Landslides in Practice: Investigation, Analysis, and Remedial/Preventative Options in Soils, 1st edn., Wiley, New Jersey, USA, 2005.
Cruz-Ramos, H., Glaser, P., Wray Jr., L. V., and Fisher, S. H.: The Bacillus subtilis ureABC Operon, J. Bacteriol., 179, 3371–3373, 1997.
DeJong, J. T., Fritzges, M. B., and Nüsslein, K.: Microbially Induced Cementation to Control Sand Response to Undrained Shear, J. Geotech. Geoenviron., 132, 1381–1392, https://doi.org/10.1061/(ASCE)1090-0241(2006)132:11(1381), 2006.
DeJong, J. T., Mortensen, B. M., Martinez, B. C., and Nelson, D. C.: Bio-mediated soil improvement, J. Ecol. Eng., 36, 197–210, https://doi.org/10.1016/j.ecoleng.2008.12.029, 2010.
De Yoreo, J. J. and Vekilov, P. G.: Principles of crystal nucleation and growth, Rev. Mineral. Geochem., 54, 57–93, https://doi.org/10.2113/0540057, 2003.
Environment Canada: Acid Rain FAQ, Retrieved 2016, available at: https://www.ec.gc.ca/air/default.asp?lang=En&n=7E5E9F00-1#wsDB524826 (last access: May 2018)), 2013.
Environment Canada: Final Screening Assessment for “DSL Bacillus licheniformis/subtilis group” (B. licheniformis/subtilis group, (Canada, Environment Canada, Health Canada), Environment Canada, 13–17, 2015.
Gat, D., Tsesarsky, M., Shamir, D., and Ronen, Z.: Accelerated microbial-induced CaCO3 precipitation in a defined coculture of ureolytic and non-ureolytic bacteria, Biogeosciences, 11, 2561–2569, https://doi.org/10.5194/bg-11-2561-2014, 2014.
Government of Canada: Almanac Averages and Extremes for April 21, Retrieved 2016, available at: http://climate.weather.gc.ca/climate_ data/almanac_e.html?StationID=4333&period=30&searchMethod=begins&txtStationName=Ottawa&month=4&day=2, 2017.
Gower, L.: Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization, Chem. Rev.,108, 4551–4627, https://doi.org/10.1021/cr800443h, 2008.
Gruninger, S. E. and Goldman, M.: Evidence for urea cycle activity in Sporosarcina ureae, Arch. Microbiol., 150, 394–399, https://doi.org/10.1007/BF00408313, 1988.
Hach Co.: Nitrogen Ammonia Salicylate Method [Technical Manual]: available at: https://www.hach.com/asset-get.download.jsa?id=7639983625, (last access: 7 June 2018), 2015.
Hammes, F., Boon, N., Villiers, J., Verstraete, W., and Siciliano, S. D.: Strain-Specific Ureolytic Microbial Calcium Carbonate Precipitation, Appl. Environ. Microb., 69, 4901–4909, https://doi.org/10.1128/AEM.69.8.4901-4909.2003, 2003.
Harbottle, M., Mugwar, A. J., and Botusharova, S.: Aspects of Implementation and Long Term Performance of Biologically Induced Mineralisation of Carbonates in Porous Media, 26th Goldschmidt Conference, Yokohoma, Japan, 26 June–1 July 2016, p .1051, 2016.
Hommel, J., Lauchnor, E., Phillips, A., Gerlach, R., Cunningham, A. B., Helmig, R., Ebigbo, A., and Class, H.: A revised model for microbially induced calcite precipitation: Improvements and new insights based on recent experiments, Water Resour. Res., 51, 3695–3715, https://doi.org/10.1002/2014WR016503, 2015.
Jahns, T.: Ammonium/urea-dependent generation of a proton electrochemical potential and synthesis of ATP in Bacillus pasteurii, J. Bacteriol., 178, 403–409, https://doi.org/10.1128/jb.178.2.403-409.1996, 1996.
Jia, H. and Jian, C.: Cementation of sand due to salt precipitation in drying process, Mar. Georesour. Geotec., 35, 441–445, https://doi.org/10.1080/1064119X.2016.1168498, 2016.
Jonkers, H. M.: Bacteria-based self-healing concrete, Heron, 56, 1–12, 2011.
Kang, C., Kwon, Y., and So, J.: Soil Bioconsolidation Through Microbially Induced Calcite Precipitation by Lysinibacillus sphaericus WJ-8, Geomicrobiol. J., 33, 473–478, https://doi.org/10.1080/01490451.2015.1053581, 2015.
Karol, R. H.: Chemical Grouting And Soil Stabilization, Revised And Expanded, 3rd edn., Taylor & Francis Inc., New York, NY, USA, 2003.
Krishnapriya, S., Venkatesh Babu, D. L., and Prince Arulraj, G.: Isolation and identification of bacteria to improve the strength of concrete, Microbiol. Res., 174, 48–55, https://doi.org/10.1016/j.micres.2015.03.009, 2015.
Lauchnor, E. G., Topp, D. M., Parker, A. E., and Gerlach, R.: Whole cell kinetics of ureolysis by Sporosarcina pasteurii, J. Appl. Microbiol., 118, 1321–1332, https://doi.org/10.1111/jam.12804, 2015.
Le Métayer-Levrela, G., Castaniera, S., Orialb, G., Loubièrec, J.-F, and Perthuisota, J.-P.: Applications of bacterial carbonatogenesis to the protection and regeneration of limestones in buildings and historic patrimony, Sed. Geo., 126, 25–34, https://doi.org/10.1016/S0037-0738(99)00029-9, 1999.
Li, M., Fu, Q.-L, Zhang, Q., and Achal, V.: Bio-grout based on microbially induced sand solidification by means of asparaginase activity, Sci. Rep., 5, 16128, https://doi.org/10.1038/srep16128, 2015.
Li, P. and Qu, W.: Bioremediation of historic architectural heritages by Sporosarcina pasteurii, 1st International Conference on Electric Technology and Civil Engineering, Lushan, China, 22–24 April, 1084–1087, https://doi.org/10.1109/ICETCE.2011.5775264, 2011.
Lin, W., Mathys, V., Ang, E. L., Koh, V. H., Gomez, J. M., Ang, M. L., and Alonso, S.: Urease Activity Represents an Alternative Pathway for Mycobacterium tuberculosis Nitrogen Metabolism, Infect. Immun., 80, 2771–2179, https://doi.org/10.1128/IAI.06195-11, 2012.
Litvan, G. G.: Freeze-Thaw Durability of Porous Building Materials, (Tech. No. STP691), https://doi.org/10.1520/STP36080S, 1980.
Mesinger, F., DiMego, G., Kalnay, E., and Mitchell, K.: North American Regional Reanalysis, Bulletin of the American Meteorological Society, 87, 343–360, https://doi.org/10.1175/BAMS-87-3-343, 2006.
Mitchell, A. C. and Ferris, F. G.: The influence of Bacillus pasteurii on the nucleation and growth of calcium carbonate, Geomicrobiol. J., 23, 213–226, 2006.
Mitchell, J. K. and Santamarina, J. C.: Biological considerations in geotechnical engineering, J. Geotech. Geoenviron. Eng., 131, 1222–1233, https://doi.org/10.1061/(asce)1090-0241(2005)131:10(1222), 2005.
Mobley, H. L. T. and Hausinger, R. P.: Microbial ureases: significance, regulation, and molecular characterization, Microbiol. Mol. Biol. R., 53, 85–108, 1989.
Mobley, H. L. T., Island, M. D., and Hausinger, R. P.: Molecular biology of microbial ureases, Microbiol. Rev., 59, 451–480, 1995.
Mobley, H. L., Mendz, G. L., and Hazell, S. L.: Helicobacter pylori: Physiology and Genetics, Retrieved 2016, available at: https://www.ncbi.nlm.nih.gov/books/NBK2408/ (last access: May 2018), 2001.
Moore, L. W. and Rene, V.: Liquid nitrogen storage of phytopathogenic bacteria, Phytophathology, 65, 246–250, 1975.
Mörsdorf, K. and Kaltwasser, H.: Ammonium assimilation in Proteus vulgaris, Bacillus pasteurii and Sporosarcina ureae, Arch. Microbiol., 152, 125–131, 1989.
Nakata, Y., Kato, Y., Hyodo, M., Hyde, A. F., and Murata, H.: One Dimensional Compression Behaviour of Uniformly Graded Sand Related to Particle Crushing Strength, Soils Found., 41, 39–51, https://doi.org/10.3208/sandf.41.2_39, 2001.
Ni, M. and Ratner, B. D.: Differentiation of Calcium Carbonate Polymorphs by Surface Analysis Techniques – An XPS and TOF-SIMS study, Surf. Interface Anal., 40, 1356–1361, https://doi.org/10.1002/sia.2904, 2008.
Park, J., Park, S., Kim, W., and Ghim, S.: Application of Bacillus subtilis 168 as a Multifunctional Agent for Improvement of the Durability of Cement Mortar. J. Microbiol. Biotechn., 22, 1568–1574, https://doi.org/10.4014/jmb.1202.02047, 2012.
Patel, P.: Helping Concrete Heal Itself, ACS Cent Sci., 1, 470–472, https://doi.org/10.1021/acscentsci.5b00376, 2015.
Reardon, J., Foreman, J. A., and Searcy, R. L.: New reactants for the colorimetric determination of ammonia, Clin. Chim. Acta., 14, 403–405, https://doi.org/10.1016/0009-8981(66)90120-3, 1966.
Rodriquez-Navarro, C., Jroundi, F., Schiro, M., Ruiz-Agudo, E., and González-Muñoz, M. T.: Influence of Substrate Mineralogy on Bacterial Mineralization of Calcium Carbonate: Implications for Stone Conservation, Appl. Environ. Microb., 78, 4017–4029, https://doi.org/10.1128/AEM.07044-11, 2012.
Sarmast, M., Farpoor, M. H., Sarcheshmehpoor, M., and Eghbal, M. K.: Micromorphological and Biocalcification Effects of Sporosarcina pasteurii and Sporosarcina ureae in Sandy Soil Columns, J. Agric. Sci. Technol., 16, 681–693. 2014.
Scott, J. S.: Dictionary of Civil Engineering, 4th edn., Chapman & Hall, New York, NY, USA, 1991.
Southam, G.: Bacterial Surface-Mediated Mineral Formation, in: Environmental Microbe Metal Interactions, edited by: Lovley, D., ASM Press, Washington, DC, USA, 257–276, https://doi.org/10.1128/9781555818098.ch12, 2000.
Stocks-Fischer, S., Galinat, J. K., and Bang, S. S.: Microbiological precipitation of CaCO3, Soil Biol. Biochem., 31, 1563–1571, https://doi.org/10.1016/S0038-0717(99)00082-6, 1999.
Stuart, C. A., Van Stratum, E., and Rustigian, R.: Further Studies on Urease Production by Proteus and Related Organisms, J. Bacteriol., 49, 437–444, 1945.
van Paassen, L. A., Harkers, M. P., van Zwieten, G. A., van der Zon, W. H., van der Star, W. R., and van Loosdrecht, M. C.: Scale up of BioGrout: a biological ground reinforcement method, 17th International Conference on Soil, Mechanics and Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009, 2328–2333, https://doi.org/10.3233/978-1-60750-031-5-2328, 2009.
van Paassen, L. A., Daza, C. M., Staal, M., Sorokin, D. Y., van der Zon, W., and van Loosdrecht, M. C.: Potential soil reinforcement by biological denitrification. J. Ecol. Eng., 36, 168–175, https://doi.org/10.1016/j.ecoleng.2009.03.026, 2010.
van Tittelboom, K., De Belie, N., De Muynck, W., and Verstraete, W.: Use of bacteria to repair cracks in concrete, Cem. Concr. Res., 40, 157–166, https://doi.org/10.1016/j.cemconres.2009.08.025, 2010.
Webster, A. and May, E.: Bioremediation of weathered-building stone surfaces, Trends Biotechnol., 24, 255–260, https://doi.org/10.1016/j.tibtech.2006.04.005, 2006.
Whiffin, V. S.: Microbial CaCO3 precipitation for the production of biocement, PhD Thesis, Murdoch University, Perth, Australia, 1–162, 2004.
Whiffin, V. S., van Paassen, L. A., and Harkes, M. P.: Microbial Carbonate Precipitation as a Soil Improvement Technique, Geomicrobiol. J., 24, 417–423, https://doi.org/10.1080/01490450701436505, 2007.
Whitaker, J., Fortin, D., and Vanapalli, S.: “Supplementary data – Biological, chemical and/or mechanical behaviour in liquid culture and MICP reinforced sands”, Mendeley Data, v1, available at: http://dx.doi.org/10.17632/crnykvnt42.1, last access: 18 July 2018.
Worcester, E. M. and Coe, F. L.: Nephrolithiasis, Prim. Care, 35, 369–391, https://doi.org/10.1016/j.pop.2008.01.005, 2008.
Yoon, J. H., Lee, K. C., Weiss, N., Kho, Y. H., Kang, K. H., and Park, Y. H.: Sporosarcina aquimarina sp. nov., a bacterium isolated from seawater in Korea, and transfer of Bacillus globisporus (Larkin and Stokes, 1967), Bacillus psychrophilus (Nakamura, 1984) and Bacillus pasteurii (Chester, 1898) to the genus Sporosarcina as Sporosarcina globispora comb. nov., Sporosarcina psychrophila comb. nov. and Sporosarcina pasteurii comb. nov., and emended description of the genus Sporosarcina., Int J. Syst. Evol. Micr., 51, 1079–1086, https://doi.org/10.1099/00207713-51-3-1079, 2001.
Zeng, C., Zheng, J., Cui, M., and Yao, X.: Effection of Water Content on the Strength of Bio-Cemented Sand in Various Drying Process, 2nd International Symposium on Asia Urban GeoEngineering, Changsha, China, 24–27 November 2017, 23–25, 2018.