the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Reviews and syntheses: Present, past, and future of the oxygen minimum zone in the northern Indian Ocean
Greg Cowie
Birgit Gaye
Joaquim Goes
Helga do Rosário Gomes
Raleigh R. Hood
Zouhair Lachkar
Henrike Schmidt
Joachim Segschneider
Arvind Singh
Decreasing concentrations of dissolved oxygen in the ocean are considered one of the main threats to marine ecosystems as they jeopardize the growth of higher organisms. They also alter the marine nitrogen cycle, which is strongly bound to the carbon cycle and climate. While higher organisms in general start to suffer from oxygen concentrations < ∼ 63 µM (hypoxia), the marine nitrogen cycle responds to oxygen concentration below a threshold of about 20 µM (microbial hypoxia), whereas anoxic processes dominate the nitrogen cycle at oxygen concentrations of < ∼ 0.05 µM (functional anoxia). The Arabian Sea and the Bay of Bengal are home to approximately 21 % of the total volume of ocean waters revealing microbial hypoxia. While in the Arabian Sea this oxygen minimum zone (OMZ) is also functionally anoxic, the Bay of Bengal OMZ seems to be on the verge of becoming so. Even though there are a few isolated reports on the occurrence of anoxia prior to 1960, anoxic events have so far not been reported from the open northern Indian Ocean (i.e., other than on shelves) during the last 60 years. Maintenance of functional anoxia in the Arabian Sea OMZ with oxygen concentrations ranging between > 0 and ∼ 0.05 µM is highly extraordinary considering that the monsoon reverses the surface ocean circulation twice a year and turns vast areas of the Arabian Sea from an oligotrophic oceanic desert into one of the most productive regions of the oceans within a few weeks. Thus, the comparably low variability of oxygen concentration in the OMZ implies stable balances between the physical oxygen supply and the biological oxygen consumption, which includes negative feedback mechanisms such as reducing oxygen consumption at decreasing oxygen concentrations (e.g., reduced respiration). Lower biological oxygen consumption is also assumed to be responsible for a less intense OMZ in the Bay of Bengal. According to numerical model results, a decreasing physical oxygen supply via the inflow of water masses from the south intensified the Arabian Sea OMZ during the last 6000 years, whereas a reduced oxygen supply via the inflow of Persian Gulf Water from the north intensifies the OMZ today in response to global warming. The first is supported by data derived from the sedimentary records, and the latter concurs with observations of decreasing oxygen concentrations and a spreading of functional anoxia during the last decades in the Arabian Sea. In the Arabian Sea decreasing oxygen concentrations seem to have initiated a regime shift within the pelagic ecosystem structure, and this trend is also seen in benthic ecosystems. Consequences for biogeochemical cycles are as yet unknown, which, in addition to the poor representation of mesoscale features in global Earth system models, reduces the reliability of estimates of the future OMZ development in the northern Indian Ocean.
- Article
(9720 KB) - Full-text XML
- BibTeX
- EndNote
The rise of atmospheric oxygen concentrations to nearly present-day levels was a precondition for the evolution of complex life forms and accompanied the appearance of algae and planktonic cyanobacteria at about 800 to 500 million years ago (Brocks et al., 2017; Canfield, 2014; Lenton and Watson, 2011; Lyons et al., 2014; Sánchez-Baracaldo, 2015). Numerically, planktonic cyanobacteria are still the most abundant plankton clade in the ocean and exert a strong control on the energy transfer into the marine biosphere by transforming nitrogen gas (N2) into ammonium (NH) (nitrogen fixation, Fig. 1, Falkowski et al., 2004). Since algae, which in addition to cyanobacteria comprise marine primary producers, cannot fix nitrogen gas, they rely on the supply of fixed nitrogen (NH, NO, NO) for the production of organic matter. Energy yielded by the respiration of organic matter produced by cyanobacteria and algae sustains heterotrophic life in the ocean, while chemoautotrophic organisms oxidize degradation products such as methane and ammonium to gain energy for running their metabolisms (Dalsgaard et al., 2003; Kuypers et al., 2001; Middelburg, 2011). In the absence of elementary oxygen, oxygen bound to sulfur (e.g., sulfate) or nitrogen (nitrate and nitrite) can also be utilized to oxidize organic matter and its degradation products. Since nitrate and nitrite are (like ammonium) accessible to algae, their use as oxidizing agents reduces the availability of fixed nitrogen in the ocean. Accordingly, decreasing oxygen concentrations could exert a negative feedback on marine primary production by lowering the availability of fixed nitrogen for algal production (Canfield et al., 2019; McElroy, 1983).
Anoxic conditions emerge only rarely in the ocean but appear to be common in microenvironments within particles via which organic matter, which is produced in the sunlit surface ocean, is exported into the deep sea (e.g., Bianchi et al., 2018; Naqvi et al., 2000; Weeks et al., 2002). However, already at low levels of dissolved oxygen, aerobic and anaerobic processes occur simultaneously and compete against each other (Bristow et al., 2017; Gaye et al., 2013). Chemoautotrophic microbes use the available elementary oxygen to oxidize ammonium to nitrite and further to nitrate (nitrification: NH → NO → NO), while microbes carrying out anaerobic processes transform ammonium as well as nitrate and nitrite into N2 (Fig. 1). Among these anaerobic processes heterotrophic denitrification and the chemoautotrophic anaerobic oxidation of ammonium (anammox) are the most relevant. Denitrification reduces nitrate in a sequence of several steps via nitrite to N2 (NO → NO → N2), whereas anammox bacteria utilize nitrite to oxidize ammonium (NO NH → N2). Thus, nitrite plays an important role in the competition between these anaerobic and aerobic processes because, independent of its formation via nitrification and denitrification, it can either be oxidized to nitrate or reduced to N2. Concentrations of dissolved oxygen strongly influence the fate of nitrite and thereby exert a control on the availability of fixed nitrogen in the ocean (Bristow et al., 2016; Gaye et al., 2013).
According to experiments and in situ observations, anammox sets in when oxygen concentrations drop below ∼ 20 µM, while denitrification occurs at oxygen concentrations of approximately < 6 µM (Fig. 2, Bristow et al., 2016; Dalsgaard et al., 2014; Kalvelage et al., 2011). Consequently, anammox competes with nitrification for nitrite at an oxygen level between 6 and 10 µM and additionally with denitrification at an oxygen level < 6 µM. Decreasing oxygen concentrations favor anammox and denitrification, while, in addition to the influence of oxygen, the quality of the supplied organic matter appears to also control the relative importance of denitrification vs. anammox for the reduction of nitrite to N2 (Babbin et al., 2014; Bristow et al., 2016; Ward et al., 2009). However, since denitrification and anammox ultimately produce N2 at the expense of fixed nitrogen, the term denitrification is used as a synonym for both processes in the following discussion if anammox is not specifically mentioned.
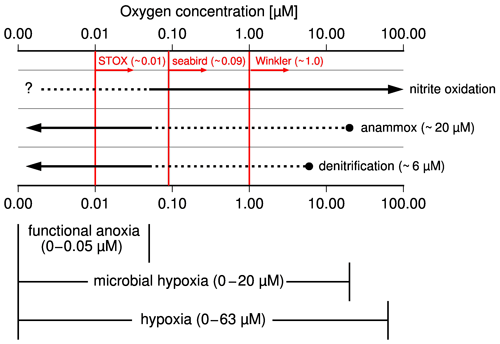
Figure 2Schematic illustration showing the occurrence of microbial processes at varying oxygen levels, the subdivision of hypoxia into microbial hypoxia, and functional anoxia as well as detection limits of methods used to measure concentration of dissolved oxygen in seawater in red. Broken lines indicate processes which occur but do not control the fate of nitrite (reduction to N2 versus oxidation to nitrate).
Hypoxia, which means low oxygen concentrations, describes oxygen concentrations below which higher organisms start to suffer from the lack of oxygen (Ekau et al., 2010; Vaquer-Sunyer and Duarte, 2008). Accordingly, an oxygen concentration of 60–63 µM is commonly applied as an upper limit of hypoxia in fisheries and ecology. Since such high threshold values do not reflect oxygen-dependent changes in the nitrogen cycle, it is suggested to subdivide hypoxia into microbial hypoxia and functional anoxia (Fig. 2). Functional anoxia was defined already in other works (Canfield et al., 2019; Thamdrup et al., 2012) and covers oxygen levels below which denitrification dominates the nitrogen cycle. Microbial hypoxia is suggested here as the range at which decreasing oxygen levels progressively offset the oxygen inhibition of denitrification. Since this starts with the occurrence of anammox, we consider 20 µM as the upper threshold of microbial hypoxia, whereas anoxia (zero oxygen) terminates hypoxia and therewith also functional anoxia and microbial hypoxia. Because oxygen detection limits of classical Winkler titration (∼ 1 µM), seabird sensors (∼ 0.09 µM), and the newly developed switchable trace oxygen sensors (STOX, ∼ 0.01 µM) are too high to prove anoxia (Thamdrup et al., 2012; Ulloa et al., 2012), the appearance of hydrogen sulfide is generally considered an indicator of anoxia.
Based on data obtained from the World Ocean Atlas, the total volume of waters characterized by oxygen concentrations < 20 µM in the global ocean is approximately 15 × 1015 m3, of which 21 % (3.13 × 1015 m3) is located in the northern Indian Ocean (Fig. 3c, Acharya and Panigrahi, 2016; Garcia et al., 2010). The largest proportion of this oxygen-poor water body is in the Arabian Sea (2.5 × 1015 m3) and only a small fraction in the Bay of Bengal (0.6 × 1015 m3). In comparison to the Bay of Bengal oxygen minimum zone (OMZ), with a mean concentration of dissolved oxygen of 14.51 ± 4 µM, the Arabian Sea OMZ is more intense, as indicated by mean concentration of dissolved oxygen of 10.45 ± 3 µM (Acharya and Panigrahi, 2016). Notably, in regions where these OMZs impinge on continental margins, sediments and benthic communities are exposed to semi-permanent bottom-water hypoxia. The Arabian Sea and the Bay of Bengal together are currently home to ∼ 59 % of the Earth's marine sediments exposed to hypoxia (Helly and Levin, 2004).
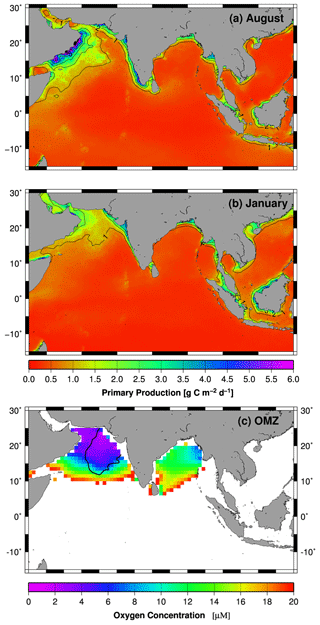
Figure 3(a, b) Monthly mean primary production rates (Behrenfeld and Falkowski, 1997) covering the periods between 2002 and 2014. (c) Minimum oxygen concentration in the water column of the Indian Ocean. Oxygen concentrations > 20 µM are indicated by the white color. The data were obtained from the World Ocean Atlas 2013 (Boyer et al., 2013). The black line indicates the extent of the secondary nitrate maximum (SNM) in 1997 (Rixen et al., 2014). The maps were produced with Generic Mapping Tools.
Denitrification, within sediments (benthic denitrification) and under hypoxic conditions in the water column, is by far the largest sink of nitrate in the ocean (Gruber, 2004). Estimates of benthic and water-column denitrification rates are still fraught with large uncertainties on global as well as on regional scales. Global-scale estimates of benthic and water-column denitrification range between 65 and 300 Tg N yr−1 and 39 and 270 Tg N yr−1, respectively (Eugster and Gruber, 2012; Gruber, 2004; Somes et al., 2013). Although denitrification in the Arabian Sea has been much more intensively studied than in the Bay of Bengal, estimates of benthic (1 to 6.8 Tg N yr−1) and water-column (1 to 33 Tg N yr−1) denitrification in the Arabian still reveal a wide range (Bange et al., 2000; Bristow et al., 2017; Deuser et al., 1978; Gaye et al., 2013; Howell et al., 1997; Naqvi et al., 1982; Somasundar et al., 1990). However, these estimates imply that on average the Arabian Sea contributes approximately 2 % and 11 % to the global mean benthic and water-column denitrification, respectively, although published data indicate that the estimated benthic denitrification rates might be too low. According to these more recent data, the benthic denitrification at the Pakistan continental margin amounts to up to 10.5 Tg N yr−1 (Schwartz et al., 2009; Somes et al., 2013), which exceeds the former budget (6.8 Tg N yr−1) of the entire Arabian Sea sediments (Bange et al., 2000). In line with a severe depletion of nitrate in bottom waters on the Indian shelf (Naqvi et al., 2010) this implies that benthic denitrification rates contribute > 2 % to the global mean benthic denitrification rate. This further emphasizes the role of the northern Indian Ocean OMZ for the marine nitrogen cycle, which was considered one of the least understood OMZs in the world's ocean (Schmidt et al., 2020; Segschneider et al., 2018). The aim of this paper is to provide a short background on the development of OMZs and recent trends in the OMZs of the Indian Ocean as well as to discuss biological and physical drivers, the past and future development, and ecosystem responses to changes in the intensity of OMZs in the northern Indian Ocean.
2.1 Oxygen minimum zones
The first large ocean-going oceanographic expeditions discovered OMZs in the Pacific, Atlantic, and Indian Ocean between the end of the 19th and the first third of the 20 century (Sewell and Fage, 1948, and references therein). Their occurrence was explained by the consumption of oxygen during the respiration of organic matter exported from the sunlit surface ocean and a sluggish horizontal renewal of water within the OMZ (Dietrich, 1936; Seiwell, 1937). Due to the main respiration depth of exported organic matter, OMZs mostly develop at water depths between approximately 100 and 1000 m (Suess, 1980), and oxygen concentrations within OMZs generally decrease with an increasing age of the water mass within the OMZ (Karstensen et al., 2008).
Sverdrup (1938) presented the first OMZ model showing that oxygen concentrations within the OMZ represent the balance between biological oxygen consumption and oxygen supply. Primary production and fluxes of oxygen across the air–sea interface are the sources of dissolved oxygen in surface waters. Vertical mixing and subduction of oxygen-enriched surface waters during the deep and mode water formation at high latitudes are in turn the main processes ventilating the interior of the ocean (McCartney, 1977; Sverdrup, 1938). Accordingly, Broecker and Peng (1982) introduced a model in which upwelling of oxygen-enriched deep water at the lower boundary and vertical mixing at the upper boundary serve to ventilate the OMZ, whereas the respiration of exported organic matter decreases oxygen concentrations.
More recent studies emphasize the influence of mesoscale eddies on the development of the OMZ (e.g., Chelton et al., 2011; Fassbender et al., 2018; Lachkar et al., 2016; Oschlies and Garcon, 1998; Resplandy et al., 2019). Mesoscale eddies emerge from baroclinic and barotropic instabilities related to the shear of horizontal currents and affect the vertical and lateral transport of water. This results in a patchiness of environmental conditions with complex and non-linear impacts on the OMZ (Fassbender et al., 2018; McGillicuddy, 2016). For instance, upward movements of water can increase the biological oxygen consumption by increasing nutrient inputs into the surface waters and thereby the biological production. Conversely, downward water movements could lower the biological production (Gruber et al., 2011) and increase oxygen concentrations in the OMZ additionally by increasing the supply of oxygen-enriched surface waters into the OMZ. In particular, stirring of oxygen by eddies along isopycnal surfaces has been suggested to modulate the intensity and distribution of low-oxygen waters in the ocean (Gnanadesikan et al., 2012, 2013). In the eastern tropical Atlantic and Pacific Ocean, recent work has highlighted the role of eddies in enhancing ocean mixing in regions of sluggish large-scale circulation, thus contributing to the ventilation of OMZs located there (Bettencourt et al., 2015; Brandt et al., 2015; Gnanadesikan et al., 2013). In this context, long-term changes in oxygen concentrations have been linked to changes in the intensity of eddy activity. For instance, Brandt et al. (2010) have shown that a reduction in filamentation and the strength of alternating zonal jets associated with mesoscale eddies between the periods 1972–1985 and 1999–2008 has contributed to a reduction in the ventilation of the OMZ located in the tropical north Atlantic. Eddy trapping in turn maintains properties of the trapped fluid over relatively long time periods. This favors the development of localized OMZs, which propagate laterally along with eddies as seen, e.g., in the open North Atlantic Ocean and off Peru in the Pacific Ocean (Bourbonnais et al., 2015; d'Ovidio et al., 2013; Fiedler et al., 2016; Karstensen et al., 2017; Schütte et al., 2016).
2.2 Spatial and temporal variability of the Arabian Sea OMZ
In the Atlantic Ocean and Pacific Ocean, hypoxic OMZs are associated with highly productive major eastern boundary current upwelling systems. In the Indian Ocean, the geographic setting prevents the development of such an upwelling system. However, a major monsoon-driven upwelling system emerges in the western Arabian Sea off the Arabian Peninsula, and a smaller one develops along the Indian southwest coast during the northern hemispheric summer (Fig. 3a). Initially described by Schott (1935), the upwelling system in the western Arabian Sea was subject to intense studies including the International Indian Ocean Expedition (IIOE) between 1959 and 1965 and the Joint Global Ocean Flux Study (JGOFS) with its field phase between 1994 and 1997 (e.g., Bauer et al., 1991; Brock et al., 1991; Bruce, 1974; Currie et al., 1973; Sastry and D'Souza, 1972; Wyrtki, 1973). However, contrary to expectations, the OMZ is most intense in the central and eastern Arabian Sea and not in the western Arabian Sea where the productivity is highest (Fig. 3c, Antoine et al., 1996; Naqvi, 1991). The offshore advection of upwelling-driven blooms, which increases the organic carbon export into the central Arabian Sea (Rixen et al., 2006), contributes to this eastward displacement of the OMZ, but monsoon-driven and seasonally varying physical oxygen supply mechanisms are assumed to be the main processes causing it.
Numerical model studies have shown that, on an annual timescale, mesoscale eddies and filaments dominate the vertical supply of oxygen to the OMZ and the lateral transport of ventilated waters into the central and northern Arabian Sea (Resplandy et al., 2011, 2012). In line with these numerical model studies and previous field work (Rixen and Ittekkot, 2005; Sen Gupta and Naqvi, 1984; Swallow, 1984), McCreary et al (2013) also highlighted the important role of vertical eddy mixing in the ventilation of the western Arabian Sea in addition to the inflow of oxygen-enriched Indian Ocean Central Water (ICW).
The ICW originates from convective mixing as Subantarctic Mode Water (SAMW) in the southern Indian Ocean (McCartney, 1977; Sverdrup et al., 1942). It enters the western Arabian Sea along with Timor Sea Water and the Subtropical Subsurface Water via the Somali Current (Schott and McCreary, 2001; Stramma et al., 1996; You, 1997). To a lesser extent, it is also carried into the eastern Arabian Sea along with an undercurrent, which compensates for the poleward-flowing West Indian Coastal Current (WICC, Fig. 4a, Schmidt et al., 2020; Shenoy et al., 2020; Shetye et al., 1990). Due to the much stronger inflow of ICW in the western Arabian Sea, the OMZ retreats eastwards in summer (Rixen et al., 2014). In winter the OMZ expands westwards due to weaker inflow of ICW and the seasonal reversal of the surface ocean circulation – counterclockwise during the winter monsoon and clockwise during the summer monsoon.
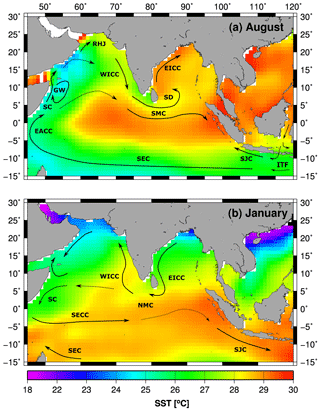
Figure 4(a, b) Monthly mean sea surface temperature in the Indian Ocean (Smith et al., 2008) and the surface ocean circulation simplified and redrawn from Schott and McCreary (2001). The arrows indicate the South Equatorial Current (SEC), South Monsoon Current (SMC), Sri Lanka Dome (SD), East Indian Coastal Current (EICC), South Java Current (SJC), Indonesian Throughflow (ITF), Somali Current (SC), Great Whirl (GW), Ras al Hadd Jet (RHJ), West Indian Coastal Current (WICC), and North Monsoon Current (NMC). The maps were produced with Generic Mapping Tools.
Data compiled by Acharya and Panigrahi (2016) indicate the response of the OMZ to the monsoon-driven seasonality in the Arabian Sea in more detail. These authors analyzed the Word Ocean Atlas 2013 (WOA13) and data obtained from the Global Ocean Data Analysis Project. Thereby, they took methodical biases into account, corrected the WOA13 data, and applied a 20 µM threshold to define the OMZ. Based on the corrected data set, these authors determined the areal extension and maximum thickness of the OMZ and calculated the mean oxygen concentration within the OMZ. According to their data, the OMZ has its lowest areal extension in summer (Fig. 5a) due to the inflow of ICW. However, since the shrinking horizontal extension of the OMZ is associated with a thickening, the OMZ also has its largest volume in summer, which in turn is accompanied by the lowest concentration of oxygen within the OMZ (Fig. 5b). The summer thickening of the OMZ is favored by the negative wind stress curl and the associated downwelling in the central Arabian Sea (Brock et al., 1991; Rao et al., 1989) and is coincident with the confluence of ICW that enters the Arabian Sea in the west and east as mentioned before. The low oxygen concentrations reflect in turn impacts of the enhanced upwelling-driven productivity and the associated increased oxygen consumption in the OMZ, which apparently exceeds the increased physical oxygen supply via eddy stirring and the inflow of ICW.
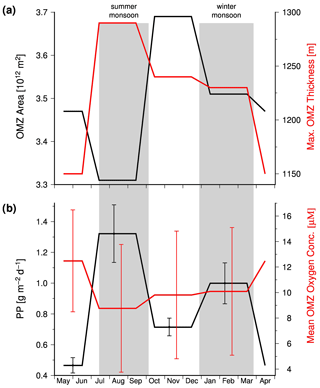
Figure 5(a) The mean seasonal areal extension and maximum thickness of the Arabian Sea OMZ. (b) Seasonal mean satellite-derived primary production which was obtained from the ocean primary production website (http://www.science.oregonstate.edu/ocean.productivity/, last access: 15 August 2020) and the seasonal mean oxygen concentration within the Arabian Sea OMZ. The satellite data covered the period between 2002 and 2019 and were averaged seasonally for the Arabian Sea north of 10∘. The OMZ data are obtained from Table 5 in Acharya and Panigrahi (2016).
In order to test whether the monsoon controls the intensity of the OMZ via its impact on the biological production, satellite-derived primary production values were obtained from the ocean primary production website (http://www.science.oregonstate.edu/ocean.productivity/, last access: 15 August 2020). The data cover the period between 2002 and 2019 and were averaged seasonally for the Arabian Sea north of 10∘. In general, the obtained mean primary production rates were inversely linked to the OMZ oxygen concentrations (Fig. 6a), which supports the hypothesis that monsoon-driven changes in the biological carbon production and export exert a strong control on the intensity of the OMZ on a seasonal timescale. However, the autumn season appears to be an exception. During this season the OMZ oxygen concentrations were lower than expected from the low primary production rate, indicating an even lower physical oxygen supply. One explanation could be the strongly reduced inflow of ICW in combination with a low air–sea oxygen supply in response to the warming and the resulting enhanced stratification of surface waters after the upwelling season.
2.3 The Bay of Bengal OMZ
Similar to the Arabian Sea, upwelling-favorable winds also occur in the Bay of Bengal during the summer monsoon (Hood et al., 2017; Shetye and Shenoi, 1988). However, the productivity in the Bay of Bengal is lower than in the Arabian Sea and shows only a weakly pronounced seasonality (Fig. 3a, b). Nevertheless, sediment trap studies have shown that, despite a lower productivity, organic carbon fluxes into the deep Bay of Bengal are almost as high as those in the central and eastern Arabian Sea, due to a ballast effect associated with high loadings of lithogenic mineral material (Rao et al., 1994; Rixen et al., 2019b). Ballast minerals, supplied from land via rivers or as dust, protect organic matter against bacterial attacks by adsorbing organic molecules to atomic lattices (Armstrong et al., 2002) and accelerate the sinking speed of particles (Haake and Ittekkot, 1990; Hamm, 2002; Ramaswamy et al., 1991). Enhanced sinking speeds reduce respiration in shallower waters and thereby increase the flux of organic matter to deeper waters (Banse, 1990; Ittekkot, 1993). A stronger ballast effect, in addition to the lower primary production, was assumed to lower the oxygen consumption in the Bay of Bengal in comparison to that in the Arabian Sea OMZ (Al Azhar et al., 2017; Rao et al., 1994). High freshwater fluxes largely cause the lower oxygen consumption in the Bay of Bengal and also reduce the physical oxygen supply.
In addition to their role as supplier of ballast minerals, high freshwater fluxes from river runoff, but also from precipitation, form a buoyant low-salinity surface layer that isolates nutrient-enriched subsurface water and increases stratification (Kumar et al., 1996). The increased stratification weakens vertical mixing as well as upwelling and thereby the biological productivity and the physical oxygen supply. Furthermore, in addition to the reduced vertical oxygen supply, the inflow of oxygen-poor Arabian Sea Water also lowers the lateral oxygen supply into the Bay of Bengal OMZ. The inflow of Arabian Sea Water into the Bay of Bengal is marked by a broad salinity maximum that occurs below the low-salinity surface layer to a water depth of approximately 1000 m (Rao et al., 1994, and references therein).
The influence of eddies on the OMZ has been studied (Kumar et al., 2007; Prasanna Kumar et al., 2004; Sarma and Bhaskar, 2018; Sarma et al., 2018; Singh et al., 2015), but due to the pronounced interannual variability (Chen et al., 2012; Johnson et al., 2019) and the eddies' non-linear behavior, impacts of eddies on the Bay of Bengal OMZ are difficult to quantify. For instance, Sarma and Baskhar (2018) focused on anticyclonic eddies sampled by bio-Argo floats between 2012 and 2016 in the Bay of Bengal and showed that anticyclonic eddies are formed on the eastern side of the basin and propagate westward. They ventilate the layer between 150 and 300 m and weaken the OMZ. Such episodic injection of oxygen could be a mechanism that enhances the oxygen supply by reducing impacts of the strong stratification on vertical mixing. On the other hand, Sarma et al. (2018) showed that while anticyclonic eddies supply oxygen to the subsurface layer and hence weaken the OMZ, cyclonic eddies inject nutrients into the euphotic zone and thus enhance productivity and oxygen consumption at depth.
2.4 Recent trends in the Bay of Bengal and the Arabian Sea
Today, the balance between physical oxygen supply and biological oxygen consumption is disturbed as indicated by the expansion of hypoxia, which is an increasingly common feature in coastal waters (Altieri et al., 2017; Diaz and Rosenberg, 2008). It is called the “spreading of dead zones” because their occurrence is often associated with mass mortality of fish and invertebrates (e.g., Weeks et al., 2002). Eutrophication and global warming mainly cause the spreading of dead zones by enhancing the production of organic matter and decreasing the oxygen supply due to a reduced solubility of oxygen in warmer surface waters. Since decreasing concentrations of dissolved oxygen have also been widely observed in the open ocean (i.e., beyond coastal systems) during the last 50 years, deoxygenation of the ocean is considered one of the main threats to pelagic ecosystems (Breitburg et al., 2018; Keeling et al., 2009; Schmidtko et al., 2017; Stramma et al., 2010a, b, 2008).
However, there are indications that the Arabian Sea and apparently also the Bay of Bengal OMZ was more intense in the recent past, i.e., prior to the Indian Ocean Expedition (IIOE, 1959–1965), than thereafter. For instance, Carruthers et al. (1959) described mass mortality of fish along the Arabian and Indian coast as well as in the central Arabian Sea at around 62.5∘ E and 9∘ N and identified oxygen depletion as the most likely trigger (Carruthers et al., 1959). This view was supported by a report on the occurrence of hydrogen sulfide from the northeastern Arabian Sea and off Oman at Ras al Hadd (Ivanenkov and Rozanov, 1961). Furthermore, hydrogen sulfide was also detected in the northwestern Bay of Bengal (Ivanenkov and Rozanov, 1961). These were the only reports on the occurrence of hydrogen sulfide in the northern Indian Ocean until Naqvi et al. (2000) discovered an anoxic event that developed along the western Indian coast off Mumbai in the late summer of 1999. Such strong events do not evolve every year (Gupta et al., 2016; Sudheesh et al., 2016), but their appearance shows that the spreading of dead zones in coastal regions does not spare the Indian shelf (Altieri et al., 2017; Diaz and Rosenberg, 2008; Diaz et al., 2019).
In contrast to these shelf processes, global, observation-based syntheses of OMZs (beyond continental shelves) reveal only a weak decrease in dissolved oxygen concentrations in the OMZs of the Arabian Sea and the Bay of Bengal in comparison to OMZs of the South Atlantic Ocean and the Pacific Ocean (Ito et al., 2017; Naqvi, 2019; Schmidtko et al., 2017; Stramma et al., 2008). The detailed analysis of all oxygen data available from the central Arabian Sea by Banse et al. (2014) ascribes this to opposing regional trends within the Arabian Sea. The authors analyzed oxygen data, which were measured between 1959 and 2004 in the Arabian Sea and in the depth range between 100 and 500 m. Biases caused by different analytical procedures were taken into account, and oxygen data were compiled for sub-regions within the Arabian Sea. The results showed that oxygen concentrations increased in the southern part of the Arabian Sea and declined in the central Arabian Sea. Follow-up studies also reported decreasing oxygen concentrations in the western and northern Arabian Sea (Piontkovski and Al-Oufi, 2015; Queste et al., 2018). In the northern Arabian Sea, dissolved oxygen concentrations in the surface mixed layer largely reflect the trend seen in the OMZ, as indicated by a compilation of dissolved oxygen data covering the period from the 1960s to 2010 (Gomes et al., 2014).
Since STOX data for the Arabian Sea were unavailable prior to 2007, the data compiled by Banse et al. (2014) do not resolve any changes in the oxygen concentrations below 0.09 µM (Fig. 2). Such low oxygen concentrations have recently been measured in the Arabian Sea and in the Bay of Bengal as well as in the OMZ of the eastern Pacific Ocean (Bristow et al., 2017; Jensen et al., 2011; Thamdrup et al., 2012). In the latter study, it was shown that nitrite accumulates in the water column at oxygen concentrations of < 0.05 µM. In the Arabian Sea OMZ, the accumulation of nitrite was first described during the John Murray expedition of 1933–1934 (Gilson, 1937). It is called the secondary nitrite maximum (SNM) and assumed to indicate active denitrification (Naqvi, 1991). The role of the SNM as an indicator of active denitrification is further supported by profiles of stable isotope ratios of nitrogen in nitrate (δ15N) and nitrite (NO) concentration profiles (Fig. 7, Gaye et al., 2013; Rixen et al., 2014). Since denitrification increases δ15N in the water column due to the preferential uptake of the lighter 14NO (Cline and Kaplan, 1975; Mariotti et al., 1981), low nitrate concentrations correspond to high δ15N within the SNM. Assuming a similar response of the nitrogen cycle to low oxygen concentration in the Arabian Sea as in the eastern Pacific Ocean suggests that the Arabian Sea SNM is characterized by oxygen concentrations of about ∼ 0.05 µM and in turn that denitrification dominates the nitrogen cycle at such low oxygen concentrations. Nevertheless, an isotope tracer study indicated that the re-oxidation of nitrite to nitrate reduced the formation of N2 by 50 % to 60 % (Gaye et al., 2013), which implies an active competition between aerobic and anaerobic processes even at such low oxygen concentrations. However, since anaerobic denitrification dominates the competition, as indicated by the accumulation of nitrite, the depletion of nitrate, and the δ15N maxima, an oxygen concentration of ∼ 0.05 µM is seen as a threshold below which functional anoxia occurs (Fig. 2).
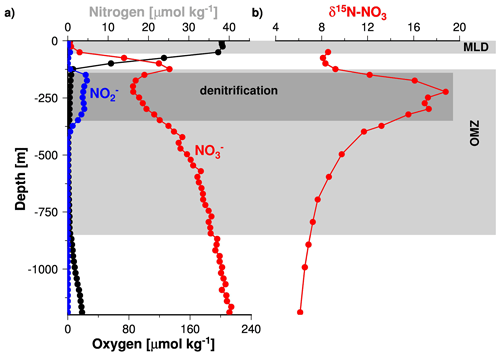
Figure 7Vertical profiles of nitrite, nitrate, and dissolved oxygen (a) as well as δ15N of nitrate (b) measured during the RV Meteor cruise M74/1b in 2007. The figure was obtained from Rixen et al. (2014).
Naqvi (1991) was the first to use the SNM to map the spatial extent of functional anoxia in the Arabian Sea. His analysis was based on data obtained during the International Indian Ocean Expedition between 1959 and 1965 and cruises thereafter but did not include data from Ivanenkov and Rozanov (1961) and the JGOFS. The data of Ivanenkov and Rozanov (1961) indicate a more intense OMZ, including the occurrence of hydrogen sulfide as mentioned earlier and a larger extent of the SNM than calculated by Naqvi (1991), although there are doubts regarding the reliability of the older data (Sen Gupta and Naqvi, 1984). Comparison of JGOFS data, collected in 1994/1995, with those compiled by Naqvi (1991) shows that the SNM has expanded south- and westwards (Rixen et al., 2014). This implies, in accordance with decreasing oxygen concentrations, an expansion of the OMZ in the Arabian Sea, which might have started in the early 1990s. However, the reliability of this trend has been questioned by Naqvi (2019). While it is acknowledged that the data are too sparse to have unquestionable confidence in this trend, it is difficult to assess the doubts raised by Naqvi (2019), as these are based on data derived from sediment cores that do not cover the most recent past.
In the Bay of Bengal, a pronounced SNM has not yet been detected, although a recent study presented data from seven stations in the northern Bay of Bengal and revealed oxygen concentrations which partly drop below ∼ 0.05 µM at four sites (Bristow et al., 2017). Incubation experiments were carried out, but with one exception these failed to prove denitrification. At the one exceptional station, a denitrification rate of 0.9 nmol L−1 d−1 was measured, which falls much below denitrification rates of > 20 nmol L−1 d−1 as measured by Ward et al. (2009) in the Arabian Sea. However, these results indicate that the Bay of Bengal OMZ is on the verge of functional anoxia, with re-oxidation of nitrite to nitrate as yet preventing significant denitrification (Bristow et al., 2017). However, outbreaks of hydrogen sulfide as seen in the upwelling systems off Peru (Schunck et al., 2013) and Namibia (Weeks et al., 2002) have so far not been reported in the northern Indian Ocean during the last 50 years, other than in bottom waters on the Indian shelf as mentioned earlier. This implies that the physical oxygen supply and the biological oxygen consumption maintained hypoxic conditions and prevented persistent anoxia in the Arabian Sea and functional anoxia in the Bay of Bengal OMZ.
Maintenance of functional anoxia in the Arabian Sea OMZ with oxygen concentrations ranging between > 0 and ∼ 0.05 µM is highly extraordinary considering that the monsoon-driven seasonality reverses the surface ocean currents and turns the Arabian Sea from an oligotrophic oceanic desert into one of the most productive regions in the world's ocean within weeks. This, on the one hand, suggests that there are feedback mechanisms counteracting impacts of the monsoon on the intensity of the OMZ. On the other hand, the recent expansion of the OMZ in the Arabian Sea and the first indication of denitrification in the Bay of Bengal OMZ indicate that there are also processes overriding effects of these feedback mechanisms.
3.1 Biological feedback mechanism
Based on the growing evidence that low concentrations of dissolved oxygen slow down the respiration of organic matter in the water column and thereby the biological oxygen consumption (Aumont et al., 2015; Cavan et al., 2017; Laufkötter et al., 2017; Thamdrup et al., 2012; Van Mooy et al., 2002), it has been hypothesized that an oxygen–related feedback mechanism stabilizes the Arabian Sea OMZ (Rixen et al., 2019a). This mechanism operates in the upper part of the OMZ, which hosts the seasonal thermocline but also affects the base of the OMZ and thereby its thickness as discussed before (Fig. 8).
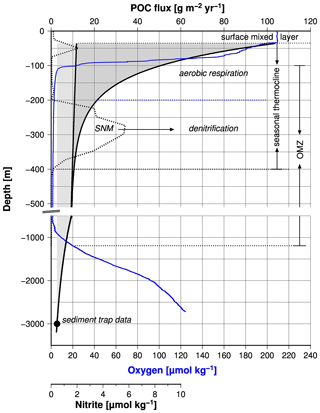
Figure 8Fluxes of protected and free particulate organic carbon versus water depth (black line). The fluxes were calculated according to the equation introduced by Armstrong et al. (2002) and data measured by a sediment trap in the central Arabian Sea. The black circle shows the long-term mean organic carbon fluxes measured by sediment traps in the central Arabian Sea. The blue and broken black lines indicate concentrations of dissolved oxygen and nitrite measured during the cruise M74 in 2007 in the central Arabian Sea (Station 450). Rixen et al. (2014) provide further information about the sediment trap study and the RV Meteor cruise M74. The broken horizontal lines mark the depth of the OMZ, the surface mixed layer, and the seasonal thermocline. The latter is subdivided into an aerobic upper and a lower anaerobic part. SNM means secondary nitrite maximum.
The seasonal thermocline is the subsurface layer from which water is introduced into the euphotic zone via physical processes such as upwelling, vertical mixing, and eddy-driven transports on a seasonal timescale. Nutrients supplied by these mechanisms largely sustain the productivity of pelagic ecosystems and the associated export production (Eppley and Peterson, 1979). Hence, the seasonal thermocline is the main nutrient reservoir of pelagic ecosystems, and to fulfill this role the vast majority of the exported organic matter must be respired within the seasonal thermocline. Accordingly, the seasonal thermocline represents the main zone of respiration and, similar to soils on land, accommodates the nutrient recycling machinery of the pelagic ecosystem. Nutrient losses from the seasonal thermocline, via particle fluxes into the deep sea, denitrification, and lateral advection, must be compensated for by nutrient inputs in order to maintain the productivity (Rixen et al., 2019a). Nitrogen fixation, river discharges, and atmospheric deposition can be important nutrient sources, but in the Arabian Sea lateral inflow of water masses from the south via the cross-equatorial cell are the main source balancing nutrient losses from the seasonal thermocline (Bange et al., 2000; Gaye et al., 2013). Accordingly, a significant negative impact of denitrification on primary and export production and the associated oxygen consumption appear to be unlikely on seasonal to centennial timescales in the Arabian Sea.
The SNM, which occurs at water depths between 200 and 400 m in the central Arabian Sea and as deep as 500 m in the eastern Arabian Sea, divides the seasonal thermocline into an aerobic part at water depths between ∼ 40 and 200 m and an anaerobic part down to the base of the SNM (Fig. 8). The depth of the seasonal thermocline of approximately 300–400 m corresponds to the depth range of vertically migrating zooplankton as observed during the large summer bloom in the Arabian Sea (Smith, 2001) and roughly matches the water depth range from where subsurface water is introduced via upwelling into the euphotic zone in the western Arabian Sea (Brock et al., 1992; Rixen et al., 2000). Furthermore, nitrate concentrations, which decrease within the SNM, remain above 10 µM (Fig. 7), suggesting that supply of decomposable organic matter (rather than nitrate availability) limits denitrification, as also suggested by other studies (Bristow et al., 2016; Ward et al., 2009). A substrate limitation at a water depth of 400 to 500 m and the arrival of organic matter at sediment traps deployed in the deep sea at a water depth of 3000 m support the concept of export production that is divided between free (reactive) and protected (low reactivity) organic matter (Armstrong et al., 2002). This partition is based on the assumption that ballast-associated, protected organic matter is preferentially exported to deeper waters as fast-sinking particles, whereas the slow-sinking free organic matter is preferentially respired within the seasonal thermocline. If decreasing oxygen concentrations reduce the respiration of free organic matter, this prevents a further depletion of oxygen and the development of anoxia by reducing the oxygen consumption within the seasonal thermocline. The consequence is an increasing export of free organic matter out of the seasonal thermocline, which could enhance the oxygen consumption at the base of the OMZ. This would imply that low oxygen concentrations within the OMZ are accompanied by a thickening of the OMZ, which can be seen on a seasonal timescale by the negative correlation between mean OMZ oxygen concentrations and the maximum thickness of the OMZ (Fig. 6b).
3.2 Biological oxygen consumption
The role of an oxygen-dependent, biologically driven feedback mechanism, counteracting impacts of the monsoon on the intensity of the OMZ, depends on the local oxygen consumption. If this is too low, remote processes instead of the local monsoon-driven carbon export rates need to be considered as forces controlling the intensity of the OMZ. The apparent oxygen utilization (AOU), in addition to mixing analyses, provides information that helps to estimate the role of the local oxygen consumption for maintaining the intensity of the OMZ in the Arabian Sea. The AOU represents the oxygen deficit caused by biological oxygen consumption and is calculated by subtracting the measured oxygen concentration from the temperature- and salinity-dependent oxygen saturation concentration. This approach is based on the assumption that the water mass of interest was saturated with respect to oxygen during its formation at the surface and, since then, the respiration of exported organic matter consumed oxygen within the water mass.
A mixing analysis based on data measured during the JGOFS in 1994/1995 reveals that oxygen deficits inherited from ICW contribute approximately 25 % to the AOU determined in the Arabian Sea OMZ (Rixen and Ittekkot, 2005). Accordingly, the respiration of organic matter produced in the Arabian Sea largely causes the low oxygen concentration in the Arabian Sea OMZ, which emphasizes the role of the monsoon-driven productivity in the Arabian Sea and the oxygen-dependent biologically driven feedback mechanism as local drivers for the OMZ development.
However, it should be noted that in the Arabian Sea, mean satellite-derived export production rates were too low to explain a high biological oxygen consumption considering a residence time of water within the OMZ of 10 years (Rixen and Ittekkot, 2005). The mismatch reflects uncertainties caused by the poorly constrained residence time of water within the OMZ and export production rates. Even though residence times of water within the Arabian Sea and Bay of Bengal OMZ of 10 and 12 years seem to be well accepted (Bristow et al., 2017; Olson et al., 1993), there are also estimates ranging from 1 to 51 years for the Arabian Sea OMZ (Naqvi and Shailaja, 1993; Sen Gupta and Naqvi, 1984). In the Arabian Sea, satellite-derived export production rates vary by a factor of 10 (Rixen et al., 2019b), and an even larger variability can be seen on a global scale. Global export production rates derived from satellite-data, numerical models extrapolated from sediment trap data and based on estimates of the biological oxygen demand vary approximately between 1.8 and 27.5 Pg C yr−1. In general estimates based on the biological oxygen demand, in line with inverse modeling studies, call for higher export production rates (Burd et al., 2010; del Giorgio and Duarte, 2002; Schlitzer, 2000; Schlitzer, 2002), whereas satellite and sediment trap data, as well as numerical model studies, suggest lower export production rates (Emerson, 2014). Nevertheless, considering estimates within the upper range (higher carbon export and longer residence times of water in the OMZ), the AOU determined in the Arabian Sea could be explained (Rixen and Ittekkot, 2005).
3.3 Physical oxygen supply mechanisms as driver of OMZ changes
The OMZs in the northern Indian Ocean are melting pots collecting the influence of a variety of water masses with different origins and histories (e.g., Morcos et al., 2012; Schott and McCreary, 2001; You, 1997). Mixing analyses indicate that the Arabian Sea OMZ contains in addition to ICW also Arabian Sea Water (ASW) as well as Persian Gulf and Red Sea Water (RSW; Acharya and Panigrahi, 2016; Hupe and Karstensen, 2000; Rixen and Ittekkot, 2005). The Persian Gulf Water (PGW) is a dense and oxygen-rich surface water that subducts in the northern Arabian Sea and strongly contributes to the ventilation of the upper OMZ (Lachkar et al., 2019; Schmidt et al., 2020). Regional model simulations have shown that eddies control the transport and the spreading of the PGW into the Gulf of Oman (Lachkar et al., 2019; Queste et al., 2018; Vic et al., 2015). Projected future warming of the Persian Gulf can, in turn, lower the oxygen concentrations of the PGW and its sinking in the Gulf of Oman. The consequence is a drop of oxygen concentrations at depths between 200 and 300 m in the northern Arabian Sea (Lachkar et al., 2019). Such a reduced physical oxygen supply could explain the observed intensification of the Arabian Sea OMZ and the expansion of the SNM (see above). Furthermore, it implies that global warming impacts on the physical oxygen supply override effects of the biologically driven feedback mechanism.
3.4 Implications
Understanding the processes controlling oxygen consumption within OMZs, such as export production and the residence time of water, is still fraught with large uncertainties. Nevertheless, it seems that there are oxygen-dependent, biologically driven feedback mechanisms counteracting impacts of the monsoon on the intensity of the OMZ. This could explain the absence of persistent anoxia and functional anoxia in the Arabian Sea and Bay of Bengal OMZs. However, the recent expansion of the OMZ in the Arabian Sea and the first indication of denitrification in the Bay of Bengal OMZ indicate that there are other processes overriding effects of these feedback mechanisms. The postulated decrease in the physical oxygen supply caused by the inflow of warmer and hence more oxygen-depleted PGW agrees with the observed decreasing oxygen concentrations and expansion of the SNM in the Arabian Sea. In the Bay of Bengal, a response to global warming is more difficult to establish due to strong interannual variations in the intensity of the eddy activity. The fact that eddies affect both the supply of oxygen (through ventilation) and its consumption (through biological productivity) in a non-trivial manner increases the difficulties to adequately parameterize the effects of eddies on dissolved oxygen in coarse-resolution models.
4.1 Sediment δ15N records of OMZ strength
On millennial and even longer timescales, sedimentary records have been used to trace changes in OMZ intensities. The δ15N values of particulate nitrogen in sediments are often used as tracers of OMZ intensity because they reflect major shifts in the pool of fixed nitrogen due to denitrification, as discussed before (Altabet et al., 1995, 1999; Brandes et al., 1998; Ganeshram et al., 1995). Locally, eolian and riverine nitrogen supply affects δ15N values (Kendall et al., 2007; Voss et al., 2006), but in the Indian Ocean sedimentary records of δ15N reflect the balance between denitrification and nitrogen fixation. Deep water nitrate has an average δ15N value of ∼ 5 ‰ (Sigman et al., 2005), but, due to denitrification, the δ15N of nitrate in the Arabian Sea increases to values > 17 ‰ (Fig. 7). Convective mixing, eddy pumping, and especially upwelling move nitrate-deficient water masses from the OMZ to the surface, so nitrate with high δ15N values is transported into the euphotic zone. After assimilation into biomass by phytoplankton, 15N-enriched particulate matter sinks through the water column to the seafloor where the signal of denitrification, and hence OMZ intensity, is preserved in sediments (Altabet et al., 1995; Gaye-Haake et al., 2005; Naqvi et al., 1998; Suthhof et al., 2001). Early diagenesis may raise sedimentary δ15N values by 2 ‰–5 ‰, and the diagenetic effect increases with water depth (Altabet, 2006; Tesdal et al., 2013). Nevertheless, the relative changes in δ15N in deep-sea sediments record variations in the OMZ intensity, while records from the continental slopes are subjected to negligible diagenetic enrichments so that they retain the signal of the nitrogen source (Altabet et al., 1999; Gaye et al., 2018).
4.2 OMZ fluctuations during the Holocene
A sediment core from the northern Bay of Bengal (Contreras-Rosales et al., 2016) indicates that the highest δ15N values (and thus the lowest OMZ oxygen concentrations) recorded in the core prevailed during the Holocene and the Last Glacial Maximum (LGM), with a δ15N range between 4.4 ‰ and 5.0 ‰ (i.e., in the range of the average value of deep ocean waters; see above). Therefore, denitrification in the past 21 000 years can be ruled out in the Bay of Bengal from a paleoceanographic perspective (Contreras-Rosales et al., 2016). The δ15N values at the core top (4.6 ‰) were similar to values in sediment trap materials (3.7 ‰–4.5 ‰) and were explained by a mixture of nutrients or suspended matter from the Ganges–Brahmaputra–Meghna river system with nitrate from subsurface water (Contreras-Rosales et al., 2016; Gaye-Haake et al., 2005; Unger et al., 2006). Enhanced δ15N values in the early Holocene to 6000 BP (BP means before present, where present means 1950) coincide with a stronger monsoon and were attributed to enhanced supply of nitrate from the subsurface, which has elevated δ15N compared to the depleted values of the riverine end-member (Sarkar et al., 2009). However, to our knowledge there is only one published sediment record from the Bay of Bengal spanning the entire Holocene (Contreras-Rosales et al., 2016), so we know nothing about the spatial variability within the basin.
In contrast to the Bay of Bengal, denitrification in the Arabian Sea has prevailed during the warm interstadials of the Pleistocene and during the entire Holocene, as can be discerned from sedimentary δ15N values > 6 ‰, with maxima of > 11 ‰ (Agnihotri et al., 2003; Higginson et al., 2004; Kessarkar et al., 2018; Möbius et al., 2011; Pichevin et al., 2007). Productivity increased with the onset of the Holocene as the summer monsoon strengthened and monsoonal upwelling off Somalia and Oman commenced and became a permanent feature of the Holocene Arabian Sea (Böning and Bard, 2009; Gaye et al., 2018). A rise of δ15N by at least 2 ‰ shows that an onset of upwelling immediately strengthened the OMZ and led to denitrification across the entire basin in the beginning of the Holocene (Böll et al., 2015; Gaye et al., 2018).
Furthermore, southward retreat of Antarctic Sea Ice is assumed to have reduced ventilation of the Arabian Sea OMZ through its influences on the formation of the oxygen-enriched ICW and associated meridional overturning circulation of the upper Indian Ocean (Böning and Bard, 2009; Naidu and Govil, 2010). A decline in δ15N by about 1 ‰ is found in the early Holocene until 6000 BP in high-resolution sediment cores from the western, northern, and eastern Arabian Sea and indicates that the OMZ weakened and became less persistent during this period (Fig. 9a, b). More vigorous upwelling, discernible from benthic foraminifera, may have led to a better ventilation of the basin as it is associated with an increased inflow of ICW from the south (Das et al., 2017). As discussed before, the inflow of ICW increases the physical oxygen supply and, furthermore, reduces the residence time of OMZ waters (Böning and Bard, 2009; Pichevin et al., 2007).

Figure 9(a) Locations of high-resolution cores (circles) and areas of model simulations (boxes). (b) Increasing δ15N values in high-resolution cores from the Arabian Sea (note inverted scale) show increasing denitrification since about 6000–8000 BP; data from the northern (yellow; light brown), eastern (red), western (blue), and southwestern (black) Arabian Sea. Sediment cores: SO90-63KA (Burdanowitz et al., 2019), RC27-23 (Altabet et al., 2002), NIOP-905P (Ivanochko et al., 2005), SK148-55 (Kessarkar et al., 2018), and MD04-2876 (Pichevin et al., 2007) parallel with (c) sinking oxygen concentrations in biogeochemical model simulations driven by the Kiel Climate Model/PISCES model in the northern (yellow), eastern (red), western (blue), and southern Arabian Sea (dark grey). See text for definition of regions. Model results are 20-year running means.
After 6000 BP, increasing δ15N values indicate a strengthening of the OMZ across the entire basin (Fig. 9a, b). It is hypothesized that a weaker ventilation is responsible for decreasing oxygen concentrations and that this could be due to reduced inflow of ICW, as it was blocked by the enhanced inflow of PGW and RSW since the sea level high stand at 6000 BP (Pichevin et al., 2007). Furthermore, a southward shift of the West Indian Coastal Current and the associated poleward undercurrent lowered the inflow of ICW and thereby the ventilation of the OMZ in the eastern Arabian Sea (Mahesh and Banakar, 2014).
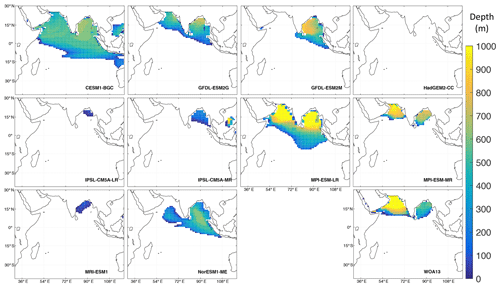
Figure 10Thickness of the OMZ (oxygen concentration < 20 µM) in 10 ESMs from the fifth Coupled Model Intercomparison Project (CMIP5; Taylor et al., 2012) and in observations from oxygen climatologies of the World Ocean Atlas 2013 (Garcia et al., 2013; bottom right). The model data cover the period from 1900–1999 and are taken from the “historical” experiment. For more information on the models see Cabré et al. (2015) (Table A1). The maps were produced with MATLAB.
4.3 Holocene model simulations
In order to give an additional, model-based estimate of the OMZ evolution in the Indian Ocean, transient model simulations over the Holocene were performed with the global atmosphere–ocean Kiel Climate Model (KCM, Park et al., 2009) and the marine biogeochemistry model PISCES (Aumont et al., 2003).
In a first step, KCM was forced with transient orbital parameters and greenhouse gas concentrations from 9500 BP to present. In a second step, the PISCES model was forced with the ocean physical fields from the above KCM experiment in so-called offline mode. This model setup comprised a ventilation age tracer of the water masses (see Segschneider et al., 2018, for a more detailed description of the model components and experiment setup). While the oceanic grid in this setup was refined to a meridional resolution of 0.5∘ near the Equator to allow a better representation of equatorial waves, the long integrations (9500 model years) required a coarse model resolution that is far from eddy resolving. The ballast scheme for the export of particulate organic carbon (POC) also neglected the lithogenic ballast effect, which is important in the Arabian Sea and Bay of Bengal as discussed before.
From these model experiments, temperature and oxygen fields have been analyzed and compared to sedimentary records mainly in the Arabian Sea. Here the model results were subdivided into areas corresponding to the binned sediment core regions specified in Gaye et al. (2018) (Fig. 9a; north: 62–68∘ E, 20–25∘ N; east: 68–75∘ E, 13–20∘ N; west: 54–60∘ E, 15–20∘ N; south: 48–55∘ E, 7–12∘ N). The simulated oxygen concentrations in the Arabian Sea are generally somewhat too high at the surface due to a cold bias of the KCM, but the observed near-surface gradients of oxygen concentrations are very well matched. However, in the deeper layers the model overestimates oxygen concentrations (not shown; see Supplement Fig. A.1c in Segschneider et al., 2018). As a result, simulated oxygen concentrations in the model Arabian Sea are nowhere low enough for denitrification to occur (denitrification sets in at 6 µM in the PISCES model, with a transition phase to full denitrification at lower oxygen concentrations). Moreover, no nitrogen isotopes are simulated in the current model version. Comparison to the δ15N data from the sediment cores is, therefore, restricted to a qualitative assessment.
The simulated oxygen concentrations (averaged between 200 and 800 m depth) show the lowest concentrations in the northern Arabian Sea (initially around 80 µM in the early Holocene, yellow curve in Fig. 9c). The concentrations are 10 µM higher in the western Arabian Sea (blue line) and a further 5 µM higher in the eastern Arabian Sea (red line), while they are much higher in the southern Arabian Sea (starting at 155 µM, grey line). Oxygen concentrations are fairly constant over the first 2500 years and then gradually decrease until the late Holocene. This decrease is strongest in the northern Arabian Sea (−20 µM) and quite similar in the western and eastern Arabian Sea (−10 µM). This is in qualitative agreement with the Holocene trends of δ15N data (Fig. 9b) that show the highest δ15N values (indicating strong denitrification and thus low oxygen) for the shallow northern core and lower δ15N for the western and eastern cores.
Simulated export production and water mass age in the Arabian Sea have been discussed for an earlier model experiment with the same model setup (but accelerated forcing) by Gaye et al. (2018) and in more detail for the global OMZs including the Indian Ocean for the model experiment analyzed here by Segschneider et al. (2018). While simulated export production in the Arabian Sea is fairly constant throughout the Holocene (Fig. 7 in Gaye et al., 2018), ventilation age is increasing throughout the Holocene concurrent with decreasing oxygen concentrations (Fig. 15 in Segschneider et al., 2018). This implies that changes in the ocean circulation and the associated inflow of oxygen-enriched ICW largely influenced the OMZ during the Holocene after the onset of upwelling at the beginning of the Holocene.
4.4 Implications
The δ15N sedimentary records reveal the difference in the late Pleistocene and Holocene history of denitrification in the Arabian Sea and Bay of Bengal. Oxygen concentrations in the Bay of Bengal never declined below the threshold of denitrification, whereas denitrification prevailed in the Arabian Sea during the warm interstadials and the entire Holocene. A data–model comparison shows that the age of the OMZ water mass increased after 6000 BP in both basins (not shown for the Bay of Bengal), coinciding with a strengthening of the OMZ and denitrification in the Arabian Sea. Based on the model results of constant export production and increasing water mass age, it is concluded that a reduced ventilation is responsible for decreasing oxygen concentration. The similar temporal evolution of observed OMZ intensity and modeled oxygen concentration in the Arabian Sea under orbital and greenhouse gas forcing thus indicates that the mid- to late Holocene OMZ intensification may be related to oceanic circulation rather than to local processes in the northern Indian Ocean. The progressive oxygen loss over the Holocene may thus be the result of orbital and greenhouse gas forcing in a qualitatively similar way to the much stronger variations simulated for LGM to mid-Holocene changes (Bopp et al., 2017).
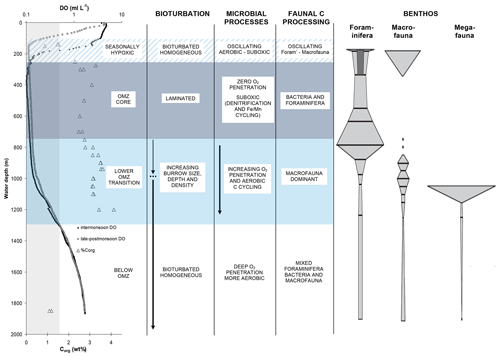
Figure 11A summary of water-column conditions, sediment properties, benthic communities, and processes influencing carbon cycling across the OMZ on the Indus margin of the Arabian Sea (modified from Cowie and Levin, 2009, and reprinted with the permission of Elsevier). Water-column dissolved oxygen (DO) concentration profiles are shown for intermonsoon (April–May) and late-to-postmonsoon (September–October) periods. Organic carbon (Corg) concentrations (weight percent) are for surficial (0–2 cm) sediments. The vertical shaded zone indicates OMZ boundaries as defined by DO ≤ 0.5 mL L−1. Shaded depth ranges denote the OMZ core (∼ 250–750 m, near-uniform DO of ≤ 0.1 mL L−1), a lower OMZ transition zone (∼ 750–1300 m) in which DO and the numbers of and activity of macrofauna increase with station depth, and a seasonally hypoxic zone (∼ 100–250 m) in which the upper OMZ boundary shoals during the summer monsoon season. Faunal classes are as defined by Gooday et al. (2009).
5.1 Global models
For future climate projections we rely on Earth system models (ESM). Although these models reproduce large-scale features and global OMZ trends, they suffer from considerable mismatches between measured and model oxygen concentrations in the ocean (Bopp et al., 2013; Cabré et al., 2015; Oschlies et al., 2008, 2018). In comparison to observational data, they underestimate oxygen losses significantly (e.g., Oschlies et al., 2018, and references therein), and simulated volumes of OMZs differ considerably. Unresolved physical oxygen supply mechanisms, poorly constrained biological oxygen consumption rates, and their hardly known responses to global change cause these uncertainties (e.g., Oschlies et al., 2018; Segschneider and Bendtsen, 2013). Furthermore, feedbacks caused by the strong coupling of the marine oxygen and nitrogen cycles complicate long-term predictions (Fu et al., 2018; Oschlies et al., 2019).
Especially in the Indian Ocean, global coupled biogeochemical ESMs struggle to represent the OMZs (Fig. 10, Oschlies et al., 2008). In most ESMs the east–west contrast between the Arabian Sea and Bay of Bengal is opposite to what observations show, with most global models producing lower oxygen concentrations in the Bay of Bengal than in the Arabian Sea. Furthermore, half of the models cannot simulate hypoxic conditions in the Arabian Sea at all. A comparison of the thickness of the hypoxic layer in the northern Indian Ocean shows a disagreement among all models (Fig. 10). The maximum simulated volume (8.2 × 1015 m3, CESM1-BGC) is more than twice the hypoxic volume found from observations (3.1 × 1015 m3, WOA13). Moreover, this volume extends too far horizontally and does not cover the thickness of the observed OMZ in the Arabian Sea (Fig. 10).
To some degree, this problem may be attributed to the fact that ESMs are not tuned for the northern Indian Ocean. In addition, global models generally have coarser resolution to reduce computational costs and thus are far from eddy resolving, as for the KCM (results discussed in the previous section). Eddy transport is parameterized in the ESMs, but these still fail to represent the OMZs in the northern Indian Ocean. Even though the next generation of ESMs already targeted this problem, by providing high-resolution options including mesoscale processes in models used in the Coupled Model Intercomparison Project Phase 6 (CMIP6), there are only moderate improvements in subsurface oxygen representation (Kwiatkowski et al., 2020). The CMIP6 models still tend to overestimate oxygen concentrations in the Arabian Sea (Séférian et al., 2020).
5.2 Future projections
The poor representation of the OMZs of the northern Indian Ocean in ESMs reduces the reliability of future projections of potential changes in the OMZs related to natural and anthropogenic forcing and thus their ecological impacts and possible feedbacks to climate change. Global models suggest a general decline of oxygen for the entire ocean, but there is no clear trend visible in the Indian Ocean (Oschlies et al., 2017). However, an older set of ESMs analyzed in Cocco et al. (2013) suggests a future decrease in oxygen in the subtropical Indian Ocean in the upper mixed layer and a small increase in the western tropical Indian Ocean. This increasing oxygen concentration is also seen in response to climate change in the RCP8.5 and RCP2.6 scenarios of the Coupled Model Intercomparison Project Phase 5 (CMIP5, Bopp et al., 2013). Specifically, Bopp et al. (2013) showed that a decrease in productivity is consistently simulated across all CMIP5 models and scenarios in the tropical Indian Ocean and that, by 2100, all models project an increase in the volume of waters with an oxygen concentration below 80 µM, relative to 1990–1999. This response is more consistent than that of the previous generation of ESMs, i.e., changes varying from −26 % to +16 % over 1870–2099 under the SRES-A2 scenario (Cocco et al., 2013).
However, for lower oxygen levels, there is less agreement among the CMIP5 models and also compared to observations regarding the volume of the OMZ (Bopp et al., 2013). Specifically, for the volume of waters < 50 µM, four models project an expansion of 2 % to 16 % (both GFDL-ESMs, HadGEM2-ES and CESM1-BGC), whereas two other models project a slight contraction of 2 % (NorESM1-ME and MPI-ESM-MR). For the volume of waters with an oxygen concentration < 5 µM, only one model (IPSL-CM5A-MR) is close to the volume estimated from observations and projects a large expansion of this volume (+30 % in the 2090s). These results for low-oxygen waters (oxygen concentrations of 5–50 µM) agree with those of Cocco et al. (2013), with large model–data and model–model discrepancies, and simulated responses varying in sign for the evolution of these volumes under climate change (Bopp et al., 2013).
Globally, the models agree on a negative oxygen trend over the 21st century driven by declining solubility of oxygen in surface waters through global warming (Resplandy, 2018; Schmidtko et al., 2017) and a reduced ventilation by changes in the ocean's circulation (Bopp et al., 2017). This holds true even though these models take into consideration the negative feedback caused by reduced tropical export production due to increased stratification of the upper water column (Fu et al., 2018; Palter and Trossman, 2018). Uncertainties and disagreements among the models arise from subtle differences in timing and magnitude of these opposing trends (Bopp et al., 2017). Waters with low oxygen saturation are particularly sensitive to impacts of climate warming (Fu et al., 2018) as well as vertical diffusivity that is parameterized by the mixing coefficient in the models (Duteil et al., 2012) and also mesoscale eddy transport and the lateral mixing coefficient (Bahl et al., 2019; Lachkar et al., 2016). Globally, reduced mixing across the mixed layer explains 75 % of the reduced subduction, but regionally changes in wind patterns that cause modulations in Ekman pumping and subduction are more important (Couespel et al., 2019). Thus, future trends in the northern Indian Ocean OMZs derived from the ESMs are highly uncertain, with projected potential increases or decreases in the volume of low-oxygen waters, depending on the model and the oxygen levels under consideration (Bopp et al., 2013; Cocco et al., 2013).
5.3 Implications
The OMZ in the Indian Ocean is the one we know least about, but it may also be the OMZ with the most complex dynamics in terms of forcing and variability. As discussed before, regional eddy-resolving modeling studies have been able to reproduce the OMZs, and thus they have helped us to better understand the interplay between physical and biogeochemical drivers (Lachkar et al., 2019; McCreary et al., 2013; Resplandy et al., 2012; Resplandy et al., 2011). Global models still struggle to reproduce the Indian Ocean OMZ. One explanation for this is the coarse resolution of these models; i.e., they cannot resolve the mesoscale and submesoscale processes that ventilate the subsurface waters, and they underestimate coastal upwelling during the monsoon seasons and, therefore, also primary production and biological oxygen demand. As a result, the oxygen trend in the tropical Indian Ocean remains unclear. However, in addition to poor representation of mesoscale and submesoscale features in global models, large uncertainties stem also from largely unknown biogeochemical and ecosystem responses to global physical changes.
6.1 Benthic ecosystems
6.1.1 Benthic communities
Hypoxia has major consequences at the sea floor for benthic communities and for the biogeochemical processes they drive. Benthic communities and processes in the Bay of Bengal have thus far received less study than those of the Arabian Sea. It is however clear that oxygen exerts an important control on benthic communities across the margins of both basins (e.g, Ingole et al., 2010; Raman et al., 2015). There are grain-size related contrasts in communities across the shelves but also clear oxygen-related patterns across the upper slope depth ranges where mid-water oxygen minima impinge on the sea floor (Fig. 11). In the Arabian Sea, the degree to which this oxygen effect is expressed varies between margins due to differing degrees of bottom-water ventilation. On the Pakistan margin, where ventilation and bottom-water oxygen levels are lowest, hypoxia-resistant foraminifera are the only fauna to persist at the core of the OMZ, and macro- and megafauna are totally absent (Gooday et al., 2009). By contrast, on the Indian margin, and even off Oman, where upwelling-driven productivity and delivery of organic matter to sediments are particularly high, macrofauna generally persist across the entire margin, albeit in reduced numbers and diversity at the OMZ core (e.g., Ingole et al., 2010; Levin et al., 2000).
Further, across the OMZ boundaries, clear “edge effects” have been observed: sharp changes in community composition and faunal abundance linked to different oxygen thresholds (e.g., Levin et al., 2009b). These have also been observed on other hypoxia-impacted margins in the eastern Pacific and off SW Africa (e.g., Levin et al., 1991), as well as in hypoxic basins such as the Baltic Sea, and at sites impacted by excess organic matter input (e.g., Rosenberg, 2001). While there are some common patterns, specific oxygen thresholds are difficult to constrain because of inter-margin and inter-basin differences in faunal assemblages, which are also affected by local differences in factors such as food availability and predator avoidance, as well as inter-study differences in the availability and quality of bottom-water oxygen data.
6.1.2 Benthic ecosystem function
The strong but variable cross-OMZ gradients in bottom-water oxygen and benthic communities translate to contrasts in benthic ecosystem function, which also varies between margins. For example, the numbers, size, and depth of faunal burrows, and the extent of bioturbation and bio-irrigation, change across the OMZ boundaries (e.g., Cowie and Levin, 2009; Smith et al., 2000). In the extreme case, this leads to total absence of bioturbation and bio-irrigation at the core of the OMZ off Pakistan, as well as the resulting presence of annually laminated (varved) sediments, which are not observed on the better-ventilated margins of the Arabian Sea or in the Bay of Bengal. In the Arabian Sea, there are also clear oxygen-dependent differences in benthic community organic matter processing, as have been revealed by tracer incubation experiments. For example, a threshold oxygen concentration occurs, above which macrofauna dominate short-term organic matter (OM) processing, and below which meiofauna and bacteria dominate. This was illustrated on the Pakistan margin both at sites that spanned the lower OMZ boundary and at a shelf-edge site that underwent strong seasonal change in bottom-water oxygen levels, from fully oxygenated (intermonsoon) to hypoxic (summer monsoon) (e.g., Andersson et al., 2008; Pozzato et al., 2013; Woulds et al., 2007, 2009).
Further, the edge effect seen in benthic community composition also has been observed in faunal OM processing. At sites in the lower OMZ transition zone, the polychaete Linopherus sp. showed clear morphological adaptation to low oxygen levels and overwhelmingly dominated both the benthic community and also the uptake and processing of organic matter (Jeffreys et al., 2012). These results, and those of other experiments (e.g., Hunter et al., 2012; White et al., 2019), illustrate that faunal assemblage composition may represent an important factor determining the pattern of seafloor processing but also the composition, bioavailability, and fate of residual organic matter. It is certainly clear that faunal digestive processes are recorded in the composition of organic matter deposited across the margins (e.g., Jeffreys et al., 2009; Smallwood et al., 1999). In summary, oxygen-dependent cross-margin variability in benthic communities and ecosystem function (feeding, bioturbation, and bio-irrigation) may be important contributors to the role that oxygen exposure plays in controlling organic carbon distribution and burial across Arabian Sea margins, although other factors, most notably hydrodynamic processes, are also important (e.g., Cowie, 2005; Cowie et al., 2009; Koho et al., 2013; Kurian et al., 2018).
6.1.3 Sediment redox conditions and microbial processes
Alongside the contrasts in faunal communities, bioturbation, and irrigation, there are cross-OMZ differences in sediment redox conditions and microbial processes. Again, these are expressed to varying degrees on the different margins of the Arabian Sea (Cowie, 2005) and will be less apparent in the Bay of Bengal due to the less intense oxygen depletion at the OMZ core. In the Arabian Sea, sulfate reduction has generally been shown to be surprisingly limited in near-surface sediments (top ∼ 50 cm) (e.g., Cowie, 2005; Law et al., 2009), and redox conditions overall have been shown to be only moderately reducing (e.g., Crusius et al., 1996) relative to rates and conditions observed on upwelling/OMZ margins in other basins. Nonetheless, Pakistan margin sediments, and possibly those on other Arabian Sea margins, are home to significant rates of denitrification and anammox (e.g., Schwartz et al., 2009; Sokoll et al., 2012) and authigenic phosphorous (P) burial (e.g., Filippelli and Cowie, 2017; Kraal et al., 2012). These phenomena represent important sink terms in the N and P biogeochemical cycles, and, along with sediment–water nutrient fluxes that vary in direction, magnitude, and N : P stoichiometry across the OMZ, serve as potential controls on pelagic nutrient inventories.
Finally, there is evidence that Pakistan margin sediments (and possibly OMZ sediments on other margins) sequester important amounts of “dark” (non-photosynthetic) carbon arising from anammox and possibly other chemoautotrophic processes occurring in overlying waters or within the sediments (e.g., Cowie et al., 1999, 2009; Lengger et al., 2019). It is a term that is currently underestimated or ignored in carbon budgets and biogeochemical models. On the Pakistan margin, there are also chemosynthetic bacterial mats associated with methane seeps (Himmler et al., 2018)
6.1.4 Implications
As mentioned above, the coastal hypoxia on the western Indian shelf can reach anoxic conditions in nearshore bottom waters (e.g., Naqvi et al., 2000). Apart from mortality of benthic (as well as pelagic) fauna under extreme conditions, details of the effects of seasonal hypoxia on benthic communities in the shelf and coastal waters of Arabian Sea and Bay of Bengal are not well documented. Thus, while seasonal contrasts in benthic community organic matter processing were reported on the Pakistan shelf (see above), it is not otherwise clear if or how benthic communities have adapted to the recurring, possibly intensifying, hypoxia. What is clear is that wholesale seasonal changes occur in benthic microbial processes and in the magnitudes and directions of sediment–water nutrient fluxes (e.g., Pratihary et al., 2014).
Potential benthic ecosystem and biogeochemical consequences of projected intensification and expansion of hypoxia have been the subject of multiple reviews (e.g., Levin et al., 2009a; Middelburg and Levin, 2009; Stramma et al., 2008). Intensification of hypoxia within the Arabian Sea and Bay of Bengal OMZs would predictably drive distributions in benthic communities, sediment characteristics, and biogeochemical processes towards those currently observed off Pakistan. This would result in potentially expanded depth ranges devoid of macro- and megafauna (and thus bioturbation and irrigation) but also shifts in the locations and composition of edge populations associated with oxygen gradients at OMZ boundaries. Other hypoxia-related phenomena might also impact on benthic ecosystems. These include the increasing prevalence of Noctiluca and jellyfish and their potential impacts on food webs and organic matter export to depth. Mass deposition of jelly fish on the seafloor off Oman (Billett et al., 2006) has major impacts on seafloor communities and processes (Sweetman et al., 2016).
It is not yet clear what the net effect of such changes would be on carbon burial, but changes in faunal populations and transition from hypoxic to fully anoxic conditions could have major impacts on benthic N and P cycling and sediment–water nutrient fluxes (and N : P ratios), as has been observed with expanding hypoxia in the Baltic Sea (Jilbert et al., 2011; Karlson et al., 2007). Intensification of existing seasonal coastal hypoxic zones, or shoaling of upper OMZ boundaries (currently close to shelf-edge depth) into shelf waters, could have particularly pronounced impacts on benthic (and pelagic) fauna – with direct implications in terms of food security for large human populations – and on biogeochemical processes.
Intensification or increased duration of coastal hypoxia could lead to increasing occurrence of mass mortality or to reduced ability of faunal populations to recover between hypoxic events. It would also result in expanded areas of reducing sediments and potential changes to carbon sequestration, N and P cycling, and N2O emissions (Middelburg and Levin, 2009). Further, the magnitudes and the dramatic seasonal changes in benthic processes and nutrient fluxes seen at sites on the western Indian shelf (Pratihary et al., 2014) imply that expanded or intensified hypoxia could, through benthic–pelagic coupling, have major influences on nutrient inventories and processes occurring in shallow overlying waters.
6.2 Pelagic ecosystems
There is a large body of evidence on the effects of hypoxia on macro-organisms. This includes reduced diel migration depths, vertical habitat compression, and shoaling distributions of fishery species and their prey (Breitburg et al., 2018). However, few reports exist on the effects of hypoxia on phytoplankton, the primary producers of the marine ecosystem. This is because it is generally understood that biological consequences of reduced oxygen concentrations are likely to be most notable for the 200–300 m layer, as these waters impinge on the euphotic zone (Stramma et al., 2010b). There have been several reports of coastal upwelling bringing up nutrient-enriched and hypoxic waters onto continental shelves stimulating production and increasing local biological oxygen demand (Stramma et al., 2010b).
In the Arabian Sea, the increasing expansion of hypoxia discussed in Sect. 2.4 both on the shelf and offshore is the predominant driver for a shift in the base of the pelagic ecosystem from mainly autotrophy before 2000 (Garrison et al., 1998, 2000; Smith et al., 1998a) to greater dependence on mixotrophy more recently (Gomes et al., 2014). A large-scale, ongoing study conducted by the National Institute of Oceanography, India, from 2003 onward, in support of India's ocean color program, first documented the appearance of extensive blooms of the green mixotrophic dinoflagellate, Noctiluca scintillans (Noctiluca).
6.2.1 Noctiluca blooms
Noctiluca is a large (∼ 1 mm in diameter) dinoflagellate, capable of sustaining itself via photosynthesis from its green free-swimming endosymbionts, Pedinomonas noctilucae (Wang et al., 2016), and/or by ingestion of exogenous prey (Goes and Gomes, 2016; Gomes et al., 2009, 2014; Prakash et al., 2008, 2017). Within a decade and a half, Noctiluca, has taken over the once diatom-dominated food chain of the Arabian Sea and the Sea of Oman, forming large green mats that can be observed from space with regular predictability (Fig. 12).
Figure 12(a) NOAA Suomi-VIIRS-derived Chl a concentrations on 6 February 2018 showing Noctiluca blooms in the Sea of Oman in association with a cyclonic eddy. For projecting the Chl a concentrations the Google Earth low-resolution land elevation map was used (© Google Earth). (b) Noctiluca blooms along the coast of Muscat on 6 February 2018.
In their quest to find the ecological drivers of Noctiluca, Gomes et al. (2014) conducted on-deck dissolved oxygen amendment experiments in the central and western Arabian Sea during the winter monsoons of 2009, 2010, and 2011 to provide the first conclusive evidence that the growth of green Noctiluca blooms was being facilitated by hypoxia. Results from this study showed that green Noctiluca is predisposed to hypoxic waters and is able to fix CO2 more efficiently under hypoxic conditions than at higher concentrations of dissolved oxygen. In contrast, diatoms and other phytoplankton showed a > 50 % decrease in CO2 fixation rates under lower oxygen concentrations. While it appears that green Noctiluca thrives in hypoxic waters, it is unclear whether Noctiluca is capable of modulating its intracellular environment in order to maximize photosynthetic rates by its endosymbionts. However, the regular occurrence of Noctiluca seems to be in line with the decreasing oxygen concentrations in the Arabian Sea as discussed in the earlier sections.
Continuous long-term sampling at a coastal station (Bay of Bandar Khayran), from 2001, and at another location offshore from 2005, both in the Sea of Oman, supplemented by field observations, provide a mesocosm-like situation to study the ecophysiological characteristics that underpin Noctiluca's recent success. The glider-based study of Piontkovski et al. (2017) and an earlier observational study (Goes and Gomes, 2016) showed that prior to appearing as surface blooms in late winter, Noctiluca are seen at deeper depths close to the oxycline, often as large, actively photosynthesizing subsurface blooms advantaged by the intrusion of hypoxic waters into the euphotic column and the higher concentrations of nutrients beneficial for endosymbiont photosynthesis. In this region, Noctiluca blooms (Al-Azri et al., 2015; Al-Hashmi et al., 2015) are found in association with a large cyclonic eddy that facilitates the up-shoaling of low-oxygen, high-nutrient waters to the surface (Gomes et al., 2009; Harrison et al., 2017). As the water column warms and stabilizes, a requirement for dinoflagellates to proliferate, Noctiluca blooms, as mixotrophs with phagotrophic abilities, proliferate, now advantaged by the plentiful food of phytoplankton, associated bacteria, and detritus. Altimetry data show furthermore that this semi-permanent cyclonic and mesoscale eddy is responsible for sustaining this bloom for a prolonged period along the coasts of Oman and Iran even until February (Gomes et al., 2009). Both cyclonic and anticyclonic eddies disperse Noctiluca eastwards into the central and eastern Arabian Sea, ultimately engulfing the entire northern Arabian Sea (Gomes et al., 2009).
A more recent study (Lotliker et al., 2018) refutes the connection between Noctiluca's blooms and the spread of hypoxia. However, their conclusions were not backed by any experimental data and their O2 data were from Bio-ARGO floats that were located south of where Noctiluca blooms occur. Sensor calibration was also a contentious issue, and Lotliker et al. (2018) provided only six calibration points for a data set that spanned from February 2013 to April 2016. However, we are still uncertain if these large blooms will further intensify the Arabian Sea OMZ. Nonetheless, we are aware that Noctiluca is not a preferred food for most zooplankton but is a cnidarian such as a jellyfish voraciously grazed upon by gelatinous tunicates such as salps. During our field campaigns, we have seen large swarms of salps, known to be efficient filter feeders, devouring Noctiluca (Gomes et al., 2014) and depositing large pellets. Salp pellets are known to be fast sinking (up to 2700 m d−1) and carbon-rich (up to 37 % dry weight), contributing disproportionately to carbon flux compared with other zooplankton (Henschke et al., 2016; Martin et al., 2017).
6.2.2 Zooplankton migration
Vertical oxygen gradients of the OMZ set the limits to the horizontal and vertical distribution of zooplankton, affecting their distribution, diel vertical migration, and ecological functions strongly (Saltzman and Wishner, 1997; Wishner et al., 2008). Recent modeling studies (Aumont et al., 2018) estimate that about one-third of the epipelagic biomass performs diurnal vertical migrations.
In general, most zooplankton taxa show minimum abundances in the core of the OMZ and higher abundances in well-oxygenated waters above or beneath the OMZ (Böttger-Schnack, 1996; Saltzman and Wishner, 1997; Wishner et al., 1995). Certain zooplankton, however, have developed vertical migration strategies that enable them to pass through or even live within the OMZ (Gonzalez and Quiñones, 2002; Herring et al., 1998; Longhurst, 1967). The ability to do so has been linked to the presence of lactic dehydrogenase (LDH), an enzyme associated with anaerobic metabolism (Escribano, 2006; Gonzalez and Quiñones, 2002).
In the Arabian Sea, almost 85 % of the epipelagic mesozooplankton biomass is found within the upper aerobic part of the seasonal thermocline or approximately in the upper 100 m. Below this region, in the anaerobic part of the seasonal thermocline, zooplankton concentrations decline sharply (Banse, 1994; Böttger-Schnack, 1996; Smith and Madhupratap, 2005; Wishner et al., 1998). The most comprehensive study of this region has been the JGOFS (Smith et al., 1998b; Smith and Madhupratap, 2005), which concluded the following vis-à-vis zooplankton distributions and the OMZ: (1) exclusion from the suboxic core of the OMZ of most zooplankton biomass; (2) the occurrence of extremely high abundances of a few species of diel vertical migrators at depth during the daytime, well within the suboxic zone; (3) organism-specific (and probably species-specific) distribution boundaries at the upper and lower edges of the OMZ; (4) very high biomass of diel vertical migrators that moved between the surface waters at night and the suboxic waters during the day, with many of these animals spending the day at depths where the oxygen was less than 4.5 µM; and (5) aggregation of mesozooplankton communities to surface layers in locations where the OMZ was forced upwards due to physical processes but where they are susceptible to predators.
A comparison between a eutrophic, more oxygenated onshore station and an offshore station with a strong OMZ elucidated the influence of depth and oxygen concentrations, as well as other factors on the copepod distribution in the Arabian Sea (Wishner et al., 2008). The extent and intensity of the oxycline at the lower boundary of the OMZ, and its spatial and temporal variability over the year of sampling, was an important factor affecting distributional patterns. Calanoid copepod species showed vertical zonation through the lower OMZ, but no apparent diel vertical migration for either calanoid or non-calanoid copepods was observed at these midwater depths. Subzones of the OMZ, termed the OMZ core, the lower oxycline, and the sub-oxycline, had different copepod communities and ecological interactions. The calanoid copepod community was most diverse in the most oxygenated environments (oxygen > 6.25 µM), but the rank order of abundance of species was similar in the lower oxycline and sub-oxycline. Some species were absent or much scarcer in the OMZ core. It thus appears that the vertical zonation of copepod species through the lower OMZ oxycline is probably a complex interplay between physiological limitation by low oxygen, potential predator control, and potential food resources.
Only one species in the Arabian Sea, Pleuromamma indica, has displayed the ability to survive and thrive in hypoxic waters. This species is not only observed in large numbers in hypoxic waters (Goswami et al., 1992; Haq et al., 1973; Saraswathy and Iyer, 1986; Vinogradov and Voronina, 1962), but is also capable of migrating daily through the well-oxygenated surface layer (Saraswathy and Iyer, 1986). There are also indications that the increased abundance of P. indica in recent years is tied to the geographically more widespread oxygen depletion.
While a considerable body of information is available on the OMZ as a determinant of zooplankton distribution, less is known on the extent of the effects of diel migration on oxygen depletion in OMZs of the world. Using measurements from shipboard acoustic Doppler current profilers (ADCPs) and a global biogeochemical model, Bianchi et al. (2013) found that by clustering in the upper margins of OMZs, vertical migrators accentuate organic matter breakdown in these waters, exacerbating the oxygen deficit. Aumont et al. (2018) used a fully coupled model to simulate the net impact of diurnal vertical migration on dissolved oxygen of the entire pelagic ecosystem on a global scale. Respiration and egestion by migratory organisms induce a modest decrease in oxygen between 150 and 500 m, which reaches about 5 µM averaged globally at 500 m, although less so in the Arabian Sea and the Bay of Bengal. Three distinct vertical layers could be distinguished over the global ocean: (1) vertical migration generates a positive dissolved oxygen anomaly in the subsurface above 200 m that can exceed 10 µM, which is explained by less intense respiration in the seasonal thermocline; (2) further below, down to about 1000 m diel vertical migration produces a depletion in oxygen from respiration by migrators with greatest depletion at middle and high latitudes; and (3) finally, in the bathypelagic domain (below 1000 m), oxygen levels are increased by almost 2 µM as a result of a slightly lower oxygen consumption.
6.2.3 Implications
We are still uncertain if the recent emergence and persistence of Noctiluca blooms will further intensify the Arabian Sea's OMZ. Satellite-derived Chlorophyll a trends (1980–2019) reveal an almost 3-fold increase in phytoplankton biomass, with increases particularly in the northwestern and central Arabian Sea (Goes et al., 2020). With respect to repercussions for the food chain, we are aware that Noctiluca is not a preferred food for most zooplankton but is voraciously grazed upon by gelatinous animals such as salps. High gelatinous zooplankton biomass is often observed in regions of persistent low oxygen concentrations (Lucas et al., 2014), suggesting that the recent appearance of extensive blooms of Noctiluca reflect the intensification of the Arabian Sea OMZ. The earlier, comprehensive JGOFS studies of the 1990s, which investigated the vertical migration and distributions patterns of the Arabian Sea's OMZ, have not been repeated even on a moderate scale as acute piracy and political instability have hindered campaigns to the region. Thus, while modeling (Lachkar et al., 2018, 2019) and data compilation studies (Banse et al., 2014; Rixen et al., 2014) suggest the expansion of the OMZ in the Arabian Sea, as discussed earlier, little is known of its effect on zooplankton distribution and vertical migration, and this also holds true for the Bay of Bengal OMZ.
The Arabian Sea and the Bay of Bengal are home to ∼ 59 % of the Earth's marine sediments exposed to severe oxygen depletion and approximately 21 % of the total volume of oxygen-depleted waters. The Arabian Sea OMZ is larger, more intense, and reveals functional anoxia in its upper part, whereas the smaller and less intense Bay of Bengal OMZ only seems to be on the verge of becoming functionally anoxic. Since oxygen concentrations within this range can presently only be measured by STOX sensors and are below detecting limits of standard methods, our understanding of the response of the nitrogen cycle to such low oxygen concentrations is based on only a few measurements and suffers from the lack of data.
Although there are a few reports on the occurrence of anoxia prior to the first large international Indian Ocean Expedition (IIOE), anoxic events have so far not been reported from the open northern Indian Ocean (i.e., beyond coastal waters) during the last 60 years. Maintenance of functional anoxia in the Arabian Sea OMZ is highly extraordinary considering the impact of the monsoon-driven seasonality on the surface ocean circulation and the productivity in the Arabian Sea. Stable balances between physical oxygen supply and biological oxygen consumption, including feedback mechanisms caused by the negative influence of decreasing oxygen concentrations on the biological oxygen consumption, seem to have prevented the occurrence of persistent anoxic conditions in the Arabian Sea OMZ and functional anoxia in the Bay of Bengal OMZ. A reduced biological oxygen consumption due to a lower productivity and a stronger ballast effect is in line with a less intense Bay of Bengal OMZ. The lower oxygen consumption in the Bay of Bengal is largely driven by river discharges, which supply huge amounts of ballast minerals and lower the nutrient supply from subsurface waters into the sunlit surface ocean by enhancing the stratification in the surface ocean. However, there is still very little known about the interannual variability of the Indian Ocean OMZs, as there are limited long-term observational data and the influence of the remote forcing processes that drive this variability (e.g., Indian Ocean Dipole, IOD; and El Niño–Southern Oscillation, ENSO) is not fully understood.
Results obtained from the global atmosphere–ocean Kiel Climate Model and eddy-resolving regional models indicate that a decreasing inflow of oxygen-enriched water masses from the south (ICW) intensified the Arabian Sea OMZ during the last 6000 years, whereas a decreasing oxygen concentration within inflowing Persian Gulf Water intensifies the OMZ in response to global warming. These trends significantly affect benthic and pelagic ecosystems. The regular occurrence of Noctiluca is an example of a new phenomenon that is assumed to herald a regime shift within the pelagic ecosystem of the Arabian Sea in response to declining concentrations of dissolved oxygen. Comprehensive studies investigating possible repercussions on the OMZ through, e.g., impacts on the export production and vertical migration and distributions of zooplankton are missing. Accordingly, these recent changes augment the problems that arise when trying to represent the Indian Ocean OMZ in models and thus in projecting the impact of the changing monsoon system on productivity and OMZ development under global change scenarios. This holds true for the CMIP5 models and is hardly improved in the new CMIP6 models.
The paper was written jointly by all co-authors. TR coordinated the writing processes, and co-authors focused on specific sections as listed in the following: TR and ZL focused on Sects. 1–3; BG and JS focused on Sect. 4; HS and RRH focused on Sect. 5; GC focused on Sect. 6.2; and JG, HdRG, and AS focused on Sect. 6.2.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Understanding the Indian Ocean system: past, present and future (BG/ACP/OS/SE inter-journal SI)”. It is not associated with a conference.
This work was initiated in December 2018 during a core group meeting of the Second International Indian Ocean Expedition (IIOE-2), in Kiel, Germany, for which we thank the organizers and the entire core group. We would also like to thank the many scientists, technicians, officers and their crews of the numerous research vessels as well as all the colleagues and the various national funding agencies that made this work possible. In particular, we would like to thank the three anonymous reviewers, Annie Bourbonnais, and Viviane Menezes for their constructive and valuable comments, which helped a lot to improve the manuscript. Tim Rixen, Birgit Gaye, and Joachim Segschneider are grateful for the financial support of the research projects CARIMA (Natural versus anthropogenic controls of past monsoon variability in Central Asia recorded in marine archives), CAHOL (Central Asian HOLocene Climate), and MASCARA (Saya de Malha Bank Carbonate Geochemistry) by the German Federal Ministry of Education and Research (BMBF) with the grant numbers 03G0806 and 03G0806B (CARIMA), 03G0864A (CAHOL), and 03G0270B (MASCARA). Joaquim Goes and Helga do Rosário Gomes are supported by grants from the National Aeronautics and Space Administration (NASA; NNX17AG66G-ECO4CAST), the National Science Foundation (NSF; 2019983), and the Gordon and Betty Moore Foundation. Paul Wessels and Walther H. F. Smith are acknowledged for providing the generic mapping tools (GMT).
This paper was edited by Viviane Menezes and reviewed by Annie Bourbonnais and three anonymous referees.
Acharya, S. S. and Panigrahi, M. K.: Eastward shift and maintenance of Arabian Sea oxygen minimum zone: Understanding the paradox, Deep-Sea Res. Pt. I, 115, 240–252, 2016.
Agnihotri, R., Bhattacharya, S. K., Sarin, M. M., and Somayajulu, B. L. K.: Changes in surface productivity and subsurface denitrification during the Holocene: a multiproxy study from the eastern Arabian Sea, The Holocene, 13, 701–713, 2003.
Al Azhar, M., Lachkar, Z., Lévy, M., and Smith, S.: Oxygen Minimum Zone Contrasts Between the Arabian Sea and the Bay of Bengal Implied by Differences in Remineralization Depth, Geophys. Res. Lett., 44, 11106–111114, 2017.
Al-Azri, A. R., Al-Hashmi, K. A., Al-Habsi, H., Al-Azri, N., and Al-Khusaibi, S.: Abundance of harmful algal blooms in the coastal waters of Oman: 2006–2011, Aquat. Ecosyst. Health, 18, 269–281, 2015.
Al-Hashmi, K. A., Smith, S. L., Claereboudt, M., Piontkovski, S. A., and Al-Azri, A.: Dynamics of potentially harmful phytoplankton in a semi-enclosed bay in the Sea of Oman, B. Mar. Sci.e, 91, 141–166, 2015.
Altabet, M. A.: Isotopic Tracers of the Marine Nitrogen Cycle: Present and Past, in: Marine Organic Matter: Biomarkers, Isotopes and DNA, The Handbook of Environmental Chemistry, edited by: Volkman, J. K., Springer, Berlin, Heidelberg, 2006.
Altabet, M. A., Francois, R., Murray, D. W., and Prell, W. L.: Climate-related variations in denitrification in the Arabian Sea from sediment 15N ∕ 14N ratios, Nature, 373, 506–509, 1995.
Altabet, M. A., Murray, D. W., and Prell, W. L.: Climatically linked oscillations in Arabian Sea denitrification over the past 1 m.y.: Implications for the marine N cycle, Paleoceanography, 14, 732–743, 1999.
Altabet, M. A., Higginson, M. J., and Murray, D. W.: The effect of millennial-scale changes in Arabian Sea denitrification on atmospheric CO2, Nature, 415, 159–162, 2002.
Altieri, A. H., Harrison, S. B., Seemann, J., Collin, R., Diaz, R. J., and Knowlton, N.: Tropical dead zones and mass mortalities on coral reefs, P. Natl. Acad. Sci., 114, 3660, https://doi.org/10.1073/pnas.1621517114, 2017.
Andersson, J. H., Woulds, C., Schwartz, M., Cowie, G. L., Levin, L. A., Soetaert, K., and Middelburg, J. J.: Short-term fate of phytodetritus in sediments across the Arabian Sea Oxygen Minimum Zone, Biogeosciences, 5, 43–53, https://doi.org/10.5194/bg-5-43-2008, 2008.
Antoine, D., André, J.-M., and Morel, A.: Oceanic primary production – 2. Estimation at global scale from satellite (coastal zone color scanner) chlorophyll, Global Biogeochem. Cy., 10, 57–69, 1996.
Armstrong, R. A., Lee, C., Hedges, J. I., Honjo, S., and Wakeham, S.: A new, mechanistic model for organic carbon fluxes in the ocean: based on the quantitative association of POC with ballast minerals, Deep-Sea Res., 49, 219–236, 2002.
Aumont, O., Maier-Reimer, E., Blain, S., and Monfray, P.: An ecosystem model of the global ocean including Fe, Si, P colimitations, Global Biogeochem. Cy., 17, 1060, https://doi.org/10.1029/2001GB001745, 2003.
Aumont, O., Ethé, C., Tagliabue, A., Bopp, L., and Gehlen, M.: PISCES-v2: an ocean biogeochemical model for carbon and ecosystem studies, Geosci. Model Dev., 8, 2465–2513, https://doi.org/10.5194/gmd-8-2465-2015, 2015.
Aumont, O., Maury, O., Lefort, S., and Bopp, L.: Evaluating the Potential Impacts of the Diurnal Vertical Migration by Marine Organisms on Marine Biogeochemistry, Global Biogeochem. Cy., 32, 1622–1643, 2018.
Babbin, A. R., Keil, R. G., Devol, A. H., and Ward, B. B.: Organic Matter Stoichiometry, Flux, and Oxygen Control Nitrogen Loss in the Ocean, Science, 344, 406–408, 2014.
Bahl, A., Gnanadesikan, A., and Pradal, M. A.: Variations in Ocean Deoxygenation Across Earth System Models: Isolating the Role of Parameterized Lateral Mixing, Global Biogeochem. Cy., 33, 703–724, 2019.
Bange, H. W., Rixen, T., Johansen, A. M., Siefert, R. L., Ramesh, R., Ittekkot, V., Hoffmann, M. R., and Andreae, M. O.: A revised nitrogen budget for the Arabian Sea, Global Biogeochem. Cy., 14, 1283–1297, 2000.
Banse, K.: New views on the degradation and disposition of organic particles as collected by sediment traps in the open sea, Deep-Sea Res., 37, 1177–1195, 1990.
Banse, K.: Grazing and Zooplankton Production as Key Controls of Phytoplankton Production in the Open Ocean, Oceanography, 7, 13–20, 1994.
Banse, K., Naqvi, S. W. A., Narvekar, P. V., Postel, J. R., and Jayakumar, D. A.: Oxygen minimum zone of the open Arabian Sea: variability of oxygen and nitrite from daily to decadal timescales, Biogeosciences, 11, 2237–2261, https://doi.org/10.5194/bg-11-2237-2014, 2014.
Bauer, S., Hitchcock, G. L., and Olson, D. B.: Influence of monsoonally-forced Ekman dynamics upon surface layer depth and plankton biomass distribution in the Arabian Sea, Deep-Sea Res., 38, 531–553, 1991.
Behrenfeld, M. J. and Falkowski, P. G.: Photosynthetic rates derived from satellite-based chlorophyll concentration, Limnol. Oceanogr., 42, 1–20, 1997.
Bettencourt, J. H., López, C., Hernández-García, E., Montes, I., Sudre, J., Dewitte, B., Paulmier, A., and Garçon, V.: Boundaries of the Peruvian oxygen minimum zone shaped by coherent mesoscale dynamics, Nat. Geosci., 8, 937–940, 2015.
Bianchi, D., Galbraith, E. D., Carozza, D. A., Mislan, K. A. S., and Stock, C. A.: Intensification of open-ocean oxygen depletion by vertically migrating animals, Nat. Geosci., 6, 545–548, 2013.
Bianchi, D., Weber, T. S., Kiko, R., and Deutsch, C.: Global niche of marine anaerobic metabolisms expanded by particle microenvironments, Nat. Geosci., 11, 263–268, 2018.
Billett, D. S. M., Bett, B. J., Jacobs, C. L., Rouse, I. P., and Wigham, B. D.: Mass deposition of jellyfish in the deep Arabian Sea, Limnol.d Oceanogr., 51, 2077–2083, 2006.
Böll, A., Schulz, H., Munz, P., Rixen, T., Gaye, B., and Emeis, K.-C.: Contrasting sea surface temperature of summer and winter monsoon variability in the northern Arabian Sea over the last 25 ka, Palaeogeogr. Palaeocl., 426, 10–21, 2015.
Böning, P. and Bard, E.: Millenial/centennial-scale thermocline ventilation changes in the Indian Ocean as reflected by aragonite preservation and geochemical variations in the Arabian Sea sediments, Geochim. Cosmochim. Ac., 73, 6771–6788, 2009.
Bopp, L., Resplandy, L., Orr, J. C., Doney, S. C., Dunne, J. P., Gehlen, M., Halloran, P., Heinze, C., Ilyina, T., Séférian, R., Tjiputra, J., and Vichi, M.: Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models, Biogeosciences, 10, 6225–6245, https://doi.org/10.5194/bg-10-6225-2013, 2013.
Bopp, L., Resplandy, L., Untersee, A., Le Mezo, P., and Kageyama, M.: Ocean (de)oxygenation from the Last Glacial Maximum to the twenty-first century: insights from Earth System models, Philos. Trans. Roy. Soc. A, 375, 20160323, https://doi.org/10.5194/bg-10-6225-2013, 2017.
Böttger-Schnack, R.: Vertical structure of small metazoan plankton, especially noncalanoid copepods, I. Deep Arabian Sea, J. Plank. Res., 18, 1073–1101, 1996.
Bourbonnais, A., Altabet, M. A., Charoenpong, C. N., Larkum, J., Hu, H., Bange, H. W., and Stramma, L.: N-loss isotope effects in the Peru oxygen minimum zone studied using a mesoscale eddy as a natural tracer experiment, Global Biogeochem. Cy., 29, 793–811, 2015.
Boyer, T. P., Antonov, J. I., Baranova, O. K., Coleman, C., Garcia, H. E., Grodsky, A., Johnson, D. R., Locarnini, R. A., Mishonov, A. V., O'Brien, T. D., Paver, C. R., Reagan, J. R., Seidov, D., Smolyar, I. V., and Zweng, M. M.: World Ocean Database 2013, Silver Spring, MD, NOAA Printing Officce, (NOAA Atlas NESDIS, 72), 208 pp., available at: http://hdl.handle.net/11329/357 (last access: 21 January 2018), 2013.
Brandes, J. A., Devol, A. H., Yoshinari, T., Jayakumar, A., and Naqvi, S. W. A.: Isotopic composition of nitrate in the central Arabian Sea and eastern tropical North Pacific: A tracer for mixing and nitrogen cycles, Limnol. Oceanogr., 43, 1680–1689, 1998.
Brandt, P., Hormann, V., Körtzinger, A., Visbeck, M., Krahmann, G., Stramma, L., Lumpkin, R., and Schmid, C.: Changes in the ventilation of the oxygen minimum zone of the tropical North Atlantic, J. Phys. Oceanogr., 40, 1784–1801, 2010.
Brandt, P., Bange, H. W., Banyte, D., Dengler, M., Didwischus, S.-H., Fischer, T., Greatbatch, R. J., Hahn, J., Kanzow, T., Karstensen, J., Körtzinger, A., Krahmann, G., Schmidtko, S., Stramma, L., Tanhua, T., and Visbeck, M.: On the role of circulation and mixing in the ventilation of oxygen minimum zones with a focus on the eastern tropical North Atlantic, Biogeosciences, 12, 489–512, https://doi.org/10.5194/bg-12-489-2015, 2015.
Breitburg, D., Levin, L. A., Oschlies, A., Grégoire, M., Chavez, F. P., Conley, D. J., Garçon, V., Gilbert, D., Gutiérrez, D., Isensee, K., Jacinto, G. S., Limburg, K. E., Montes, I., Naqvi, S. W. A., Pitcher, G. C., Rabalais, N. N., Roman, M. R., Rose, K. A., Seibel, B. A., Telszewski, M., Yasuhara, M., and Zhang, J.: Declining oxygen in the global ocean and coastal waters, Science, 359, eaam7240, https://doi.org/10.1126/science.aam7240, 2018.
Bristow, L. A., Dalsgaard, T., Tiano, L., Mills, D. B., Bertagnolli, A. D., Wright, J. J., Hallam, S. J., Ulloa, O., Canfield, D. E., Revsbech, N. P., and Thamdrup, B.: Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters, P. Natl. Acad. Sci., 113, 10601, https://doi.org/10.1073/pnas.1600359113, 2016.
Bristow, L. A., Callbeck, C. M., Larsen, M., Altabet, M. A., Dekaezemacker, J., Forth, M., Gauns, M., Glud, R. N., Kuypers, M. M. M., Lavik, G., Milucka, J., Naqvi, S. W. A., Pratihary, A., Revsbech, N. P., Thamdrup, B., Treusch, A. H., and Canfield, D. E.: N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone, Nat. Geosci., 10, 24–29, 2017.
Brock, J. C., McClain, C. R., Luther, M. E., and Hay, W. W.: The Phytplankton Bloom in the Northwestern Arabian Sea During the Southwest Monsoon of 1979, J. Geophys. Res., 96, 20623–20642, 1991.
Brock, J. C., McClain, C. R., and Hay, W. W.: A Southwest Monsoon Hydrographic Climatology for the Northwestern Arabian Sea, Journal of Geophysical Research, 97, 9455-9465, 1992.
Brocks, J. J., Jarrett, A. J. M., Sirantoine, E., Hallmann, C., Hoshino, Y., and Liyanage, T.: The rise of algae in Cryogenian oceans and the emergence of animals, Nature, 548, 578–581, https://doi.org/10.1038/nature23457, 2017.
Broecker, W. S. and Peng, T.-H.: Tracers in the sea, Lamont-Doherty Geological Observatory, Columbia University, Palisades, New York, 690 pp., 1982.
Bruce, J. G.: Some details of upwelling off the Somali and Arabian Coasts, J. Mar. Res., 32, 419–423, 1974.
Burd, A. B., Hansell, D. A., Steinberg, D. K., Anderson, T. R., Arístegui, J., Baltar, F., Beaupré, S. R., Buesseler, K. O., DeHairs, F., Jackson, G. A., Kadko, D. C., Koppelmann, R., Lampitt, R. S., Nagata, T., Reinthaler, T., Robinson, C., Robison, B. H., Tamburini, C., and Tanaka, T.: Assessing the apparent imbalance between geochemical and biochemical indicators of meso- and bathypelagic biological activity: What the @$,ôØ! is wrong with present calculations of carbon budgets?, Deep-Sea Res. Pt. II, 57, 1557–1571, 2010.
Burdanowitz, N., Gaye, B., Hilbig, L., Lahajnar, N., Lückge, A., Rixen, T., and Emeis, K.-C.: Holocene monsoon and sea level-related changes of sedimentation in the northeastern Arabian Sea, Deep-Sea Res. Pt. II, 166, 6–18, https://doi.org/10.1016/j.dsr2.2019.03.003, 2019.
Cabré, A., Marinov, I., Bernardello, R., and Bianchi, D.: Oxygen minimum zones in the tropical Pacific across CMIP5 models: mean state differences and climate change trends, Biogeosciences, 12, 5429–5454, https://doi.org/10.5194/bg-12-5429-2015, 2015.
Canfield, D.: Oxygen, a four billion year history, Princeton University Press, Prniceton, New Jersey, USA, 2014.
Canfield, D. E., Kraft, B., Löscher, C. R., Boyle, R. A., Thamdrup, B., and Stewart, F. J.: The regulation of oxygen to low concentrations in marine oxygen-minimum zones, J. Mar. Res., 77, 297–324, 2019.
Carruthers, J. N., Gogate, S. S., Naidu, J. R., and Laevastu, T.: Shorewards Upslope of the Layer of Minimum Oxygen Off Bombay: Its Influence on Marine Biology, Especially Fisheries, Nature, 183, 1084–1087, 1959.
Cavan, E. L., Trimmer, M., Shelley, F., and Sanders, R.: Remineralization of particulate organic carbon in an ocean oxygen minimum zone, Nat. Commun., 8, 14847, https://doi.org/10.1038/ncomms14847, 2017.
Chelton, D. B., Gaube, P., Schlax, M. G., Early, J. J., and Samelson, R. M.: The Influence of Nonlinear Mesoscale Eddies on Near-Surface Oceanic Chlorophyll, Science, 334, 328, https://doi.org/10.1126/science.1208897, 2011.
Chen, G., Wang, D., and Hou, Y.: The features and interannual variability mechanism of mesoscale eddies in the Bay of Bengal, Cont. Shelf Res., 47, 178–185, 2012.
Cline, J. D. and Kaplan, I. R.: Isotopic fractionation of dissolved nitrate during denitrification in the eastern tropical north pacific ocean, Mar.e Chem., 3, 271–299, 1975.
Cocco, V., Joos, F., Steinacher, M., Frölicher, T. L., Bopp, L., Dunne, J., Gehlen, M., Heinze, C., Orr, J., Oschlies, A., Schneider, B., Segschneider, J., and Tjiputra, J.: Oxygen and indicators of stress for marine life in multi-model global warming projections, Biogeosciences, 10, 1849–1868, https://doi.org/10.5194/bg-10-1849-2013, 2013.
Contreras-Rosales, L. A., Schefuß, E., Meyer, V., Palamenghi, L., Lückge, A., and Jennerjahn, T. C.: Origin and fate of sedimentary organic matter in the northern Bay of Bengal during the last 18 ka, Glob. Planet. Change, 146, 53–66, 2016.
Couespel, D., Lévy, M., and Bopp, L.: Major Contribution of Reduced Upper Ocean Oxygen Mixing to Global Ocean Deoxygenation in an Earth System Model, Geophys. Res. Lett., 46, 12239–12249, 2019.
Cowie, G.: The biogeochemistry of Arabian Sea surficial sediments: A review of recent studies, Prog. Oceanogr., 65, 260–289, 2005.
Cowie, G. L., Calvert, S. E., Pedersen, T. F., Schulz, H., and von Rad, U.: Organic content and preservational controls in surficial shelf and slope sediments from the Arabian Sea (Pakistan margin), Mar. Geol., 161, 23–38, 1999.
Cowie, G. L. and Levin, L. A.: Benthic biological and biogeochemical patterns and processes across an oxygen minimum zone (Pakistan margin, NE Arabian Sea), Deep-Sea Res. Pt. II, 56, 261–270, 2009.
Cowie, G. L., Mowbray, S., Lewis, M., Matheson, H., and McKenzie, R.: Carbon and nitrogen elemental and stable isotopic compositions of surficial sediments from the Pakistan margin of the Arabian Sea, Deep-Sea Res. Pt. II, 56, 271–282, 2009.
Crusius, J., Calvert, S., Pedersen, T., and Sage, D.: Rhenium and molybdenum enrichments in sediments as indicators of oxic, suboxic and sulfidic conditions of deposition, Earth Planet. Sc. Lett., 145, 65–78, 1996.
Currie, R. I., Fisher, A. E., and Hargreaves, P. M.: Arabian Sea Upwelling. in: Biology of the Indian Ocean, edited by: Zeitschel, B., Ecological Studies 3, Springer Verlag, Berlin, 1973.
d'Ovidio, F., De Monte, S., Penna, A. D., Cotté, C., and Guinet, C.: Ecological implications of eddy retention in the open ocean: a Lagrangian approach, J. Phys. A, 46, 254023, https://doi.org/10.1088/1751-8113/46/25/254023, 2013.
Dalsgaard, T., Canfield, D. E., Petersen, J., Thamdrup, B., and Acuna-Gonzalez, J.: N2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica, Nature, 422, 606–608, 2003.
Dalsgaard, T., Stewart, F. J., Thamdrup, B., De Brabandere, L., Revsbech, N. P., Ulloa, O., Canfield, D. E., and DeLong, E. F.: Oxygen at Nanomolar Levels Reversibly Suppresses Process Rates and Gene Expression in Anammox and Denitrification in the Oxygen Minimum Zone off Northern Chile, mBio, 5, e01966-01914, https://doi.org/10.1128/mBio.01966-14, 2014.
Das, M., Singh, R. K., Gupta, A. K., and Bhaumik, A. K.: Holocene strengthening of the Oxygen Minimum Zone in the northwestern Arabian Sea linked to changes in intermediate water circulation or Indian monsoon intensity?, Paleogeogr. Paleoclimatol., 483, 125–135, 2017.
del Giorgio, P. A. and Duarte, C. M.: Respiration in the open ocean, Nature, 420, 379–384, 2002.
Deuser, W. G., Ross, E. H., and Mlodzinska, Z. J.: Evidence for and rate of denitrification in the Arabian Sea, Deep-Sea Res., 25, 431–445, 1978.
Diaz, R. J. and Rosenberg, R.: Spreading Dead Zones and Consequences for Marine Ecosystems, Science, 321, 926–929, 2008.
Diaz, R. J., Rosenberg, R., and Sturdivant, K.: Hypoxia in estuaries and semi-enclosed seas, in: Ocean deoxygenation: Everyone's problem, edited by: Laffoley, D. and Baxter, J. M., IUCN, Gland, Switzerland 2019.
Dietrich, G.: Aufbau und Bewegung von Golfstrom und Agulhasstrom, Naturwissenschaften, 24, 225–230, 1936.
Duteil, O., Koeve, W., Oschlies, A., Aumont, O., Bianchi, D., Bopp, L., Galbraith, E., Matear, R., Moore, J. K., Sarmiento, J. L., and Segschneider, J.: Preformed and regenerated phosphate in ocean general circulation models: can right total concentrations be wrong?, Biogeosciences, 9, 1797–1807, https://doi.org/10.5194/bg-9-1797-2012, 2012.
Ekau, W., Auel, H., Pörtner, H.-O., and Gilbert, D.: Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish), Biogeosciences, 7, 1669–1699, https://doi.org/10.5194/bg-7-1669-2010, 2010.
Emerson, S.: Annual net community production and the biological carbon flux in the ocean, Global Biogeochem. Cy., 28, 14–28, 2014.
Eppley, R. W. and Peterson, B. J.: Particulate organic matter flux and planktonic new production in the deep ocean, Nature, 282, 677–680, 1979.
Escribano, R.: Zooplankton interactions with the oxygen minimum zone in the eastern south pacific, Gayana (Concepción), 70, 19–21, 2006.
Eugster, O. and Gruber, N.: A probabilistic estimate of global marine N-fixation and denitrification, Global Biogeochem. Cy., 26, GB4013, https://doi.org/10.1029/2012GB004300, 2012.
Falkowski, P. G., Katz, M. E., Knoll, A. H., Quigg, A., Raven, J. A., Schofield, O., and Taylor, F. J. R.: The Evolution of Modern Eukaryotic Phytoplankton, Science, 305, 354–360, 2004.
Fassbender, A. J., Bourbonnais, A., Clayton, S., Gaube, P., Omand, M., Franks, P. J. S., Altabet, M. A., and McGillicuddy Jr., D. J.: Interpreting mosaics of ocean biogeochemistry, EOS, 99, https://doi.org/10.1029/2018EO109707, 2018.
Fiedler, B., Grundle, D. S., Schütte, F., Karstensen, J., Löscher, C. R., Hauss, H., Wagner, H., Loginova, A., Kiko, R., Silva, P., Tanhua, T., and Körtzinger, A.: Oxygen utilization and downward carbon flux in an oxygen-depleted eddy in the eastern tropical North Atlantic, Biogeosciences, 13, 5633–5647, https://doi.org/10.5194/bg-13-5633-2016, 2016.
Filippelli, G. and Cowie, G.: Carbon and Phosphorus Cycling in Arabian Sea Sediments across the Oxygen Minimum Zone, J. Oceanogr. Mar.e Res., 5, 171, https://doi.org/10.4172/2572-3103.1000171, 2017.
Fu, W., Primeau, F., Keith Moore, J., Lindsay, K., and Randerson, J. T.: Reversal of Increasing Tropical Ocean Hypoxia Trends With Sustained Climate Warming, Global Biogeochem. Cy., 32, 551–564, 2018.
Ganeshram, R. S., Pedersen, T. F., Calvert, S. E., and Murray, J. W.: Large changes in oceanic nutrient inventories from glacial to interglacial periods, Nature, 376, 755–758, 1995.
Garcia, H. E., Locarnini, R. A., Boyer, T. P., Antonov, J. I., Zweng, M. M., Baranova, O. K., and Johnson, D. R.: World Ocean Atlas 2009, in: NOAA Atlas NESDIS 71, edited by: Levitus, S., U.S. Government Printing Office, Washington, D.C., 2010.
Garcia, H. E., Locarnini, R. A., Boyer, T. P., Antonov, J. I., Baranova, O. K., Zweng, M. M., Reagan, J. R., and Johnson, D. R.: World Ocean Atlas 2013, Vol. 3, Dissolved Oxygen, Apparent Oxygen Utilization, and Oxygen Saturation, edited by: S. Levitus, A. Mishonov Technical Ed., NOAA Atlas NESDIS 75, 27 pp., 2014.
Garrison, D. L., Gowing, M. M., and Hughes, M. P.: Nano- and microplankton in the northern Arabian Sea during the Southwest Monsoon, August-September 1995 A US-JGOFS study, Deep-Sea Res. Pt. II, 45, 2269–2299, 1998.
Garrison, D. L., Gowing, M. M., Hughes, M. P., Campbell, L., Caron, D. A., Dennett, M. R., Shalapyonok, A., Olson, R. J., Landry, M. R., and Brown, S. L.: Microbial food web structure in the Arabian Sea: a US JGOFS study, Deep-Sea Res. Pt. II, 47, 1387–1422, 2000.
Gaye, B., Nagel, B., Dähnke, K., Rixen, T., and Emeis, K.-C.: Evidence of parallel denitrification and nitrite oxidation in the ODZ of the Arabian Sea from paired stable isotopes of nitrate and nitrite, Global Biogeochem. Cy., 27, GB004115, https://doi.org/10.1002/2011gb004115, 2013.
Gaye, B., Böll, A., Segschneider, J., Burdanowitz, N., Emeis, K.-C., Ramaswamy, V., Lahajnar, N., Lückge, A., and Rixen, T.: Glacial–interglacial changes and Holocene variations in Arabian Sea denitrification, Biogeosciences, 15, 507–527, https://doi.org/10.5194/bg-15-507-2018, 2018.
Gaye-Haake, B., Lahajnar, N., Emeis, K.-C., Unger, D., Rixen, T., Suthhof, A., Ramaswamy, V., Schulz, H., Paropkari, A. L., Guptha, M. V. S., and Ittekkot, V.: Stable nitrogen isotopic ratios of sinking particles and sediments from the northern Indian Ocean, Mar. Chem., 96, 243–255, 2005.
Gilson, H. C.: The nitrogen cycle, Scientific Reports John Murray Expedition 1933–1934, Vol. 2, 21–81, 1937.
Gnanadesikan, A., Dunne, J. P., and John, J.: Understanding why the volume of suboxic waters does not increase over centuries of global warming in an Earth System Model, Biogeosciences, 9, 1159–1172, https://doi.org/10.5194/bg-9-1159-2012, 2012.
Gnanadesikan, A., Bianchi, D., and Pradal, M.-A.: Critical role for mesoscale eddy diffusion in supplying oxygen to hypoxic ocean waters, Geophys. Res. Lett., 40, 5194–5198, 2013.
Goes, J. I. and Gomes, H.: An ecosystem in transition: the emergence of mixotrophy in the Arabian Sea, in: Aquatic Microbial Ecology and Biogeochemistry: A Dual Perspective, edited by: Glibert, P. and Kana, T., Springer International Publishing, p. 245, 2016.
Goes, J. I., Tian, H., Gomes, H. d. R., Anderson, O. R., Al-Hashmi, K., deRada, S., Luo, H., Al-Kharusi, L., Al-Azri, A., and Martinson, D. G.: Ecosystem state change in the Arabian Sea fuelled by the recent loss of snow over the Himalayan-Tibetan Plateau region, Sci. Rep., 10, 7422, https://doi.org/10.1038/s41598-020-64360-2, 2020.
Gomes, d. R. H., Goes, J. I., Matondkar, S. G. P., Buskey, E. J., Basu, S., Parab, S., and Thoppil, P.: Massive outbreaks of Noctiluca scintillans blooms in the Arabian Sea due to spread of hypoxia, Nat. Commun., 5, 4862, https://doi.org/10.1038/ncomms5862, 2014.
Gomes, H., Goes, J. I., Matondkar, S. G. P., Parab, S. G., Al-Azri, A., and Thoppil, P. G.: Unusual Blooms of the Green Noctiluca Miliaris (Dinophyceae) in the Arabian Sea during the Winter Monsoon, in: Indian Ocean: Biogeochemical Processes and Ecological Variability, edited by: Wiggert, J. D., Hood, R. R., Naqvi, S. W. A., Smith, S. L., and Brink, K. H., AGU Book Series, American Geophysical Union, 2009.
Gonzalez, R. R. and Quiñones, R. A.: Ldh activity in Euphausia mucronata and Calanus chilensis: implications for vertical migration behaviour, J. Plank. Res., 24, 1349–1356, 2002.
Gooday, A. J., Levin, L. A., Aranda da Silva, A., Bett, B. J., Cowie, G. L., Dissard, D., Gage, J. D., Hughes, D. J., Jeffreys, R., Lamont, P. A., Larkin, K. E., Murty, S. J., Schumacher, S., Whitcraft, C., and Woulds, C.: Faunal responses to oxygen gradients on the Pakistan margin: A comparison of foraminiferans, macrofauna and megafauna, Deep-Sea Res. Pt. II, 56, 488–502, 2009.
Goswami, S. C., Saparia, J. S., and Bhargava, R. M. S.(Eds.): Zooplankton standing stock assessment and fishery resources in the Indian seas, Oxford & IBH Publishing Co., New Delhi, 217–225, 1992.
Gruber, N.: The dynamics of the marine nitrogen cycle and its influence on atmospheric CO2, in: Carbon-Climate Interactions, edited by: Follows, M. and Oguz, T., NATO ASI Series, New York, 2004.
Gruber, N., Lachkar, Z., Frenzel, H., Marchesiello, P., Munnich, M., McWilliams, J. C., Nagai, T., and Plattner, G.-K.: Eddy-induced reduction of biological production in eastern boundary upwelling systems, Nat. Geosci., 4, 787–792, 2011.
Gupta, G. V. M., Sudheesh, V., Sudharma, K. V., Saravanane, N., Dhanya, V., Dhanya, K. R., Lakshmi, G., Sudhakar, M., and Naqvi, S. W. A.: Evolution to decay of upwelling and associated biogeochemistry over the southeastern Arabian Sea shelf, J. Geophys. Res.-Biogeo., 121, 159–175, 2016.
Haake, B. and Ittekkot, V.: Die Wind-getriebene “biologische Pumpe” und der Kohlenstoffentzug im Ozean, Naturwissenschaften, 77, 75–79, 1990.
Hamm, C. E.: Interactive aggregation and sedimentation of diatoms and clay-sized lithogenic material, Limnol. Oceanogr., 47, 1790–1795, 2002.
Haq, S. M., Khan, J. A., and Chugtai, S.: The Distribution and Abundance of Zooplankton along the Coast of Pakistan during Postmonsoon and Premonsoon Periods, in: The Biology of the Indian Ocean, edited by: Zeitzschel, B. and Gerlach, S. A., Springer Berlin Heidelberg, Berlin, Heidelberg, 1973.
Harrison, P. J., Piontkovski, S., and Al-Hashmi, K.: Understanding how physical-biological coupling influences harmful algal blooms, low oxygen and fish kills in the Sea of Oman and the Western Arabian Sea, Mar. Pollut. Bull., , 114, 25–34, https://doi.org/10.1016/j.marpolbul.2016.11.008, 2017.
Helly, J. J. and Levin, L. A.: Global distribution of naturally occurring marine hypoxia on continental margins, Deep-Sea Res. Pt. I, 51, 1159–1168, 2004.
Henschke, N., Everett, J. D., Richardson, A. J., and Suthers, I. M.: Rethinking the Role of Salps in the Ocean, Trends Ecol. Evol., 31, 720–733, 2016.
Herring, P. J., Fasham, M. J. R., Weeks, A. R., Hemmings, J. C. P., Roe, H. S. J., Pugh, P. R., Holley, S., Crisp, N. A., and Angel, M. V.: Across-slope relations between the biological populations, the euphotic zone and the oxygen minimum layer off the coast of Oman during the southwest monsoon (August, 1994), Prog. Oceanogr., 41, 69–109, 1998.
Higginson, M. J., Altabet, M. A., Murray, D. W., Murray, R. W., and Herbert, T. D.: Geochemical evidence for abrupt changes in relative strength of the Arabian monsoons during a stadial/interstadial climate transition, Geochim. Cosmochim. Ac., 68, 3807–3826, 2004.
Himmler, T., Smrzka, D., Zwicker, J., Kasten, S., Shapiro, R. S., Bohrmann, G., and Peckmann, J.: Stromatolites below the photic zone in the northern Arabian Sea formed by calcifying chemotrophic microbial mats, Geology, 46, 339–342, 2018.
Hood, R. R., Beckley, L. E., and Wiggert, J. D.: Biogeochemical and ecological impacts of boundary currents in the Indian Ocean, Prog. Oceanogr., 156, 290–325, 2017.
Howell, E. A., Doney, S. C., Fine, R. A., and Olson, D. B.: Geochemical estimates of denitrification in the Arabian Sea and the Bay of Bengal during WOCE, Geophys. Res. Lett., 24, 2549–2552, 1997.
Hunter, W. R., Levin, L. A., Kitazato, H., and Witte, U.: Macrobenthic assemblage structure and organismal stoichiometry control faunal processing of particulate organic carbon and nitrogen in oxygen minimum zone sediments, Biogeosciences, 9, 993–1006, https://doi.org/10.5194/bg-9-993-2012, 2012.
Hupe, A. and Karstensen, J.: Redfield stoichiometry in Arabian Sea subsurface waters, Global Biogeochem. Cy., 14, 357–372, 2000.
Ingole, B. S., Sautya, S., Sivadas, S., Singh, R., and Nanajkar, M.: Macrofaunal community structure in the western Indian continental margin including the oxygen minimum zone, Mar. Ecol., 31, 148–166, 2010.
Ito, T., Minobe, S., Long, M. C., and Deutsch, C.: Upper ocean O2 trends: 1958–2015, Geophys. Res. Lett., 44, 4214–4223, 2017.
Ittekkot, V.: The abiotically driven biological pump in the ocean and short-term fluctuations in atmospheric CO2 contents, Glob. Planet. Change, 8, 17–25, 1993.
Ivanenkov, V. N. and Rozanov, A. G.: Hydrogen sulphide contamination of the intermediate water layers of the Arabian Sea and the Bay of Bengal, Okeanologiya, 1, 443–449, 1961.
Ivanochko, T. S., Ganeshram, R. S., Brummer, G.-J. A., Ganssen, G., Jung, S. J. A., Moreton, S. G., and Kroon, D.: Variations in tropical convection as an amplifier of global climate change at the millennial scale, Earth Planet. Sc. Lett., 235, 302–314, 2005.
Jeffreys, R. M., Wolff, G. A., and Cowie, G. L.: Influence of oxygen on heterotrophic reworking of sedimentary lipids at the Pakistan margin, Deep-Sea Res. Pt. II, 56, 358–375, 2009.
Jeffreys, R. M., Levin, L. A., Lamont, P. A., Woulds, C., Whitcraft, C. R., Mendoza, G. F., Wolff, G. A., and Cowie, G. L.: Living on the edge: single-species dominance at the Pakistan oxygen minimum zone boundary, Mar. Ecol. Prog. Ser., 470, 79–99, 2012.
Jensen, M. M., Lam, P., Revsbech, N. P., Nagel, B., Gaye, B., Jetten, M. S. M., and Kuypers, M. M. M.: Intensive nitrogen loss over the Omani Shelf due to anammox coupled with dissimilatory nitrite reduction to ammonium, ISME J., 5, 1660–1670, 2011.
Jilbert, T., Slomp, C. P., Gustafsson, B. G., and Boer, W.: Beyond the Fe-P-redox connection: preferential regeneration of phosphorus from organic matter as a key control on Baltic Sea nutrient cycles, Biogeosciences, 8, 1699–1720, https://doi.org/10.5194/bg-8-1699-2011, 2011.
Johnson, K. S., Riser, S. C., and Ravichandran, M.: Oxygen variability controls denitrification in the bay of Bengal oxygen minimum zone, Geophys. Res. Lett., 46, 804–811, 2019.
Kalvelage, T., Jensen, M. M., Contreras, S., Revsbech, N. P., Lam, P., Günter, M., LaRoche, J., Lavik, G., and Kuypers, M. M. M.: Oxygen Sensitivity of Anammox and Coupled N-Cycle Processes in Oxygen Minimum Zones, PLOS ONE, 6, e29299, https://doi.org/10.1371/journal.pone.0029299, 2011.
Karlson, K., Bonsdorff, E., and Rosenberg, R.: The Impact of Benthic Macrofauna for Nutrient Fluxes from Baltic Sea Sediments, AMBIO, 36, 161–167, 167, 2007.
Karstensen, J., Stramma, L., and Visbeck, M.: Oxygen minimum zones in the eastern tropical Atlantic and Pacific oceans, Prog. Oceanogr., 77, 331–350, 2008.
Karstensen, J., Schütte, F., Pietri, A., Krahmann, G., Fiedler, B., Grundle, D., Hauss, H., Körtzinger, A., Löscher, C. R., Testor, P., Vieira, N., and Visbeck, M.: Upwelling and isolation in oxygen-depleted anticyclonic modewater eddies and implications for nitrate cycling, Biogeosciences, 14, 2167–2181, https://doi.org/10.5194/bg-14-2167-2017, 2017.
Keeling, R. F., Körtzinger, A., and Gruber, N.: Ocean Deoxygenation in a Warming World, Annu. Rev. Mar. Sci., 2, 199–229, 2009.
Kendall, C., Elliott, E. M., and Wankel, S. D.: Tracing anthropogenic inputs of nitrogen to ecosystems, in: Stable Isotopes in Ecology and Environmental Science, edited by: Michener, R. H. and Lajtha, K., Blackwell Publshing, 2007.
Kessarkar, P. M., Naqvi, S. W. A., Thamban, M., Fernandes, L. L., Siebert, C., Rao, V. P., Kawahata, H., Ittekkot, V., and Frank, M.: Variations in Denitrification and Ventilation Within the Arabian Sea Oxygen Minimum Zone During the Holocene, Geochem. Geophy. Geosy., 19, 2179–2193, 2018.
Koho, K. A., Nierop, K. G. J., Moodley, L., Middelburg, J. J., Pozzato, L., Soetaert, K., van der Plicht, J., and Reichart, G.-J.: Microbial bioavailability regulates organic matter preservation in marine sediments, Biogeosciences, 10, 1131–1141, https://doi.org/10.5194/bg-10-1131-2013, 2013.
Kraal, P., Slomp, C. P., Reed, D. C., Reichart, G.-J., and Poulton, S. W.: Sedimentary phosphorus and iron cycling in and below the oxygen minimum zone of the northern Arabian Sea, Biogeosciences, 9, 2603–2624, https://doi.org/10.5194/bg-9-2603-2012, 2012.
Kumar, D., M., Naqvi, S. W. A., George, M. D., and Jayakumar, A.: A sink for atmospheric carbon dioxide in the northeastern Indian Ocean, J. Geophys. Res., 101, 18121–18125, 1996.
Kumar, S. P., Nuncio, M., Ramaiah, N., Sardesai, S., Narvekar, J., Fernandes, V., and Paul, J. T.: Eddy-mediated biological productivity in the Bay of Bengal during fall and spring intermonsoons, Deep-Sea Res. Pt. I, 54, 1619–1640, 2007.
Kurian, S., Kessarkar, P. M., Purnachandra Rao, V., Reshma, K., Sarkar, A., Pattan, J. N., and Naqvi, S. W. A.: Controls on organic matter distribution in oxygen minimum zone sediments from the continental slope off western India, J. Mar. Syst., 207, 103–118, https://doi.org/10.1016/j.jmarsys.2018.09.003, 2018. 103118, 2018.
Kuypers, M. M. M., Sleikers, A. O., Lavik, G., Schmid, M., Jorgensen, B. B., Kuenen, J. G., Damsté, J. S. S., Strous, M., and Jetten, M. S. M.: Anaerobic ammonium oxidation by anammox bacteria in the Black Sea, Nature, 422, 608–611, 2001.
Kwiatkowski, L., Torres, O., Bopp, L., Aumont, O., Chamberlain, M., Christian, J. R., Dunne, J. P., Gehlen, M., Ilyina, T., John, J. G., Lenton, A., Li, H., Lovenduski, N. S., Orr, J. C., Palmieri, J., Santana-Falcón, Y., Schwinger, J., Séférian, R., Stock, C. A., Tagliabue, A., Takano, Y., Tjiputra, J., Toyama, K., Tsujino, H., Watanabe, M., Yamamoto, A., Yool, A., and Ziehn, T.: Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production decline from CMIP6 model projections, Biogeosciences, 17, 3439–3470, https://doi.org/10.5194/bg-17-3439-2020, 2020.
Lachkar, Z., Smith, S., Lévy, M., and Pauluis, O.: Eddies reduce denitrification and compress habitats in the Arabian Sea, Geophys. Res. Lett., 43, 9148–9156, 2016.
Lachkar, Z., Lévy, M., and Smith, S.: Intensification and deepening of the Arabian Sea oxygen minimum zone in response to increase in Indian monsoon wind intensity, Biogeosciences, 15, 159–186, https://doi.org/10.5194/bg-15-159-2018, 2018.
Lachkar, Z., Lévy, M., and Smith, K. S.: Strong Intensification of the Arabian Sea Oxygen Minimum Zone in Response to Arabian Gulf Warming, Geophys. Res. Lett., 46, 5420–5429, 2019.
Laufkötter, C., John, J. G., Stock, C. A., and Dunne, J. P.: Temperature and oxygen dependence of the remineralization of organic matter, Global Biogeochem. Cy., 31, 1038–1050, 2017.
Law, G. T. W., Cowie, G. L., Breuer, E. R., Schwartz, M. C., Martyn Harvey, S., Woulds, C., Shimmield, T. M., Shimmield, G. B., and Doig, K. A.: Rates and Regulation of Microbially Mediated Aerobic and Anaerobic Carbon Oxidation Reactions in Continental Margin Sediments from the Northeastern Arabian Sea (Pakistan Margin), Indian Ocean Biogeochemical Processes and Ecological Variability, in: Geophysical Monograph Series, 299–319, https://doi.org/10.1029/2008GM000765, 2009.
Lengger, S. K., Rush, D., Mayser, J. P., Blewett, J., Schwartz-Narbonne, R., Talbot, H. M., Middelburg, J. J., Jetten, M. S. M., Schouten, S., Sinninghe Damsté, J. S., and Pancost, R. D.: Dark carbon fixation in the Arabian Sea oxygen minimum zone contributes to sedimentary organic carbon (SOM), Global Biogeochem. Cy., 33, 1715–1732, https://doi.org/10.1029/2019GB006282, 2019.
Lengger, S., Rush, D., Mayser, J. P., Blewett, J., Schwartz-Narbonne, R., Talbot, H., Middelburg, J. J., Jetten, M. S. M., Schouten, S., Sinninghe Damsté, J. S., and Pancost, R. D.: Dark carbon fixation contributes to sedimentary organic carbon in the Arabian Sea oxygen minimum zone, Global Biogeochem. Cy., 33, 1715–1732, https://doi.org/10.1029/2019GB006282, 2020.
Lenton, T. M. and Watson, A. J.: Revolutions that made the Earth, Oxford University Press, Oxford, 196 pp., 2011.
Levin, L. A., Huggett, C. L., and Wishner, K. F.: Control of deep-sea benthic community structure by oxygen and organic-matter gradients in the eastern Pacific Ocean, J. Mar. Res., 49, 763–800, 1991.
Levin, L. A., Ekau, W., Gooday, A. J., Jorissen, F., Middelburg, J. J., Naqvi, S. W. A., Neira, C., Rabalais, N. N., and Zhang, J.: Effects of natural and human-induced hypoxia on coastal benthos, Biogeosciences, 6, 2063–2098, https://doi.org/10.5194/bg-6-2063-2009, 2009a.
Levin, L. A., Whitcraft, C. R., Mendoza, G. F., Gonzalez, J. P., and Cowie, G.: Oxygen and organic matter thresholds for benthic faunal activity on the Pakistan margin oxygen minimum zone (700–1100 m), Deep-Sea Res. Pt. II, 56, 449–471, 2009b.
Levin, L. A., Gage, J. D., Martin, C., and Lamont, P. A.: Macrobenthic community structure within and beneath the oxygen minimum zone, NW Arabian Sea, Deep-Sea Res. Pt. II, 47, 189–226, 2000.
Longhurst, A. R.: Vertical distribution of zooplankton in relation to the eastern Pacific oxygen minimum, Deep-Sea Res. Oceanogr.c Abstract., 14, 51–63, 1967.
Lotliker, A. A., Baliarsingh, S. K., Trainer, V. L., Wells, M. L., Wilson, C., Udaya Bhaskar, T. V. S., Samanta, A., and Shahimol, S. R.: Characterization of oceanic Noctiluca blooms not associated with hypoxia in the Northeastern Arabian Sea, Harmful Algae, 74, 46–57, 2018.
Lucas, C. H., Jones, D. O. B., Hollyhead, C. J., Condon, R. H., Duarte, C. M., Graham, W. M., Robinson, K. L., Pitt, K. A., Schildhauer, M., and Regetz, J.: Gelatinous zooplankton biomass in the global oceans: geographic variation and environmental drivers, Glob. Ecol. Biogeogr., 23, 701–714, 2014.
Lyons, T. W., Reinhard, C. T., and Planavsky, N. J.: The rise of oxygen in Earth/'s early ocean and atmosphere, Nature, 506, 307–315, 2014.
Mahesh, B. S. and Banakar, V. K.: Change in the intensity of low-salinity water inflow from the Bay of Bengal into the Eastern Arabian Sea from the Last Glacial Maximum to the Holocene: Implications for monsoon variations, Paleogeogr. Paleocl., 397, 31–37, 2014.
Mariotti, A., Germon, J. C., Hubert, P., Kaiser, P., Letolle, R., Tardieux, A., and Tardieux, P.: Experimental determination of nitrogen kinetic isotope fractionation: Some principles; illustration for the denitrification and nitrification processes, Plant Soil, 62, 413–430, 1981.
Martin, B., Koppelmann, R., and Kassatov, P.: Ecological relevance of salps and doliolids in the northern Benguela Upwelling System, J. Plank. Res., 39, 290–304, 2017.
McCartney, M. S.: Subantarctic Mode Water, in: A Voyage of Discovery: George Deacon 70th Anniversary Volume, edited by: Angel, M. V., Supplement to Deep-Sea Research, Pergamon Press, Oxford, UK, 1977.
McCreary Jr., J. P., Yu, Z., Hood, R. R., Vinaychandran, P. N., Furue, R., Ishida, A., and Richards, K. J.: Dynamics of the Indian-Ocean oxygen minimum zones, Prog. Oceanogr., 112/113, 15–37, 2013.
McElroy, M. B.: Marine biological controls on atmospheric CO2 and climate, Nature, 302, 328–329, 1983.
McGillicuddy, D. J.: Mechanisms of Physical-Biological-Biogeochemical Interaction at the Oceanic Mesoscale, Annu. Rev. Mar. Sci.e, 8, 125–159, 2016.
Middelburg, J. J.: Chemoautotrophy in the ocean, Geophys. Res.h Lett., 38, L24604, https://doi.org/10.1029/2011gl049725, 2011.
Middelburg, J. J. and Levin, L. A.: Coastal hypoxia and sediment biogeochemistry, Biogeosciences, 6, 1273–1293, https://doi.org/10.5194/bg-6-1273-2009, 2009.
Möbius, J., Gaye, B., Lahajnar, N., Bahlmann, E., and Emeis, K.-C.: Influence of diagenesis on sedimentary 15∘ N in the Arabian Sea over the last 130 kyr, Mar. Geol., 284, 127–138, 2011.
Morcos, S. A. and AbdAllah, A. M.: Oceanography of the Gulf of Aden: John Murray–Mabahiss Expedition 1933–1934 Revisited, Egypt. J. Aquat. Res., 38, 77–91, https://doi.org/10.1016/j.ejar.2012.12.001, 2012.
Naidu, P. D. and Govil, P.: New evidence on the sequence of deglacial warming in the tropical Indian Ocean, J. Quaternary Sci., 25, 1138–1143, 2010.
Naqvi, S. A. S.: Evidence for ocean deoxygenation and its patterns: Indian Ocean, in: Ocean deoxygenation: Everyone's problem, edited by: Laffoley, D. and Baxter, J. M., IUCN, Gland, Switzerland, 2019.
Naqvi, S. W. A. and Shailaja, M. S.: Activity of the respiratory electron transport system and respiration rates within the oxygen minimum layer of the Arabian Sea, Deep-Sea Res. Pt. II, 40, 687–695, 1993.
Naqvi, S. W. A., Noronha, R. J., and Reddy, C. V. G.: Denitrification in the Arabian Sea, Deep-Sea Res., 29, 459–469, 1982.
Naqvi, S. W. A., Yoshinari, T., Jayakumar, A., Altabet, M. A., Narvekar, P. V., Devol, A. H., Brandes, J. A., and Codispoti, L. A.: Budgetary and biogeochemical implications of N2O isotope signatures in the Arabian Sea, Nature, 394, 462–464, 1998.
Naqvi, S. W. A., Jayakumar, D. A., Narvekar, P. V., Naik, H., Sarma, V. V. S. S., D'Souza, W., Joseph, S., and George, M. D.: Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf, Nature, 408, 346–349, 2000.
Naqvi, S. W. A., Moffett, J. W., Gauns, M. U., Narvekar, P. V., Pratihary, A. K., Naik, H., Shenoy, D. M., Jayakumar, D. A., Goepfert, T. J., Patra, P. K., Al-Azri, A., and Ahmed, S. I.: The Arabian Sea as a high-nutrient, low-chlorophyll region during the late Southwest Monsoon, Biogeosciences, 7, 2091–2100, https://doi.org/10.5194/bg-7-2091-2010, 2010.
Naqvi, W. A.: Geographical extent of denitrification in the Arabian Sea in relation to some physical processes, Oceanol. Ac., 14, 281–290, 1991.
Olson, D. B., Hitchcock, G. L., Fine, R. A., and Warren, B. A.: Maintenance of the low-oxygen layer in the central Arabian Sea, Deep-Sea Res. Pt. II, 40, 673–685, 1993.
Oschlies, A., Duteil, O., Getzlaff, J., Koeve, W., Landolfi, A., and Schmidtko, S.: Patterns of deoxygenation: sensitivity to natural and anthropogenic drivers, Philosophical Transactions of the Royal Society A: Mathematical, Phys. Eng. Sci., 375, 20160325, https://doi.org/10.1098/rsta.2016.0325, 2017.
Oschlies, A., Brandt, P., Stramma, L., and Schmidtko, S.: Drivers and mechanisms of ocean deoxygenation, Nat. Geosci., 11, 467–473, 2018.
Oschlies, A. and Garcon, V.: Eddy-induced enhancement of primary production in a model of the North Atlantic Ocean, Nature, 394, 266–269, https://doi.org/10.1038/28373 1998.
Oschlies, A., Schulz, K. G., Riebesell, U., and Schmittner, A.: Simulated 21st century's increase in oceanic suboxia by CO2-enhanced biotic carbon export, Global Biogeochem. Cy., 22, GB4008, https://doi.org/10.1029/2007gb003147, 2008.
Oschlies, A., Koeve, W., Landolfi, A., and Kähler, P.: Loss of fixed nitrogen causes net oxygen gain in a warmer future ocean, Nat. Commun., 10, 2805, https://doi.org/10.1038/s41467-019-10813-w, 2019.
Palter, J. B. and Trossman, D. S.: The Sensitivity of Future Ocean Oxygen to Changes in Ocean Circulation, Global Biogeochem. Cy., 32, 738–751, 2018.
Park, W., Keenlyside, N., Latif, M., Stroh, A., Redler, R., Roeckner, E., and Madec, G.: Tropical Pacific Climate and Its Response to Global Warming in the Kiel Climate Model, J. Climate, 22, 71–92, 2009.
Pichevin, L., Bard, E., Martinez, P., and Billy, I.: Evidence of ventilation changes in the Arabian Sea during the late Quaternary: Implication for denitrification and nitrous oxide emission, Global Biogeochem. Cy., 21, GB4008, https://doi.org/10.1029/2006gb002852, 2007.
Piontkovski, S. A. and Al-Oufi, H. S.: The Omani shelf hypoxia and the warming Arabian Sea, Int. J. Environ. Stud., 72, 256–264, 2015.
Piontkovski, S. A., Queste, B. Y., Al-Hashmi, K. A., Al-Shaaibi, A., Bryantseva, Y. V., and Popova, E. A.: Subsurface algal blooms of the northwestern Arabian Sea, Mar. Ecol. Prog. Ser.s, 566, 67–78, 2017.
Pozzato, L., van Oevelen, D., Moodley, L., Soetaert, K., and Middelburg, J. J.: Carbon processing at the deep-sea floor of the Arabian Sea oxygen minimum zone: A tracer approach, J. Sea Res., 78, 45–58, 2013.
Prakash, S., Ramesh, R., Sheshshayee, M. S., Dwivedi, R. M., and Raman, M.: Quantification of new production during a winter Noctiluca scintillans bloom in the Arabian Sea, Geophys. Res. Lett., 35, L08604, https://doi.org/10.1029/2008gl033819, 2008.
Prakash, S., Roy, R., and Lotliker, A.: Revisiting the Noctiluca scintillans paradox in northern Arabian Sea, Current Sci., 113, 1429–1434, 2017.
Prasanna Kumar, S., Nuncio, M., Narvekar, J., Kumar, A., Sardesai, d. S., De Souza, S., Gauns, M., Ramaiah, N., and Madhupratap, M.: Are eddies nature's trigger to enhance biological productivity in the Bay of Bengal?, Geophys. Res. Lett., 31, L07309, https://doi.org/10.1029/2003GL019274, 2004.
Pratihary, A. K., Naqvi, S. W. A., Narvenkar, G., Kurian, S., Naik, H., Naik, R., and Manjunatha, B. R.: Benthic mineralization and nutrient exchange over the inner continental shelf of western India, Biogeosciences, 11, 2771–2791, https://doi.org/10.5194/bg-11-2771-2014, 2014.
Queste, B. Y., Vic, C., Heywood, K. J., and Piontkovski, S. A.: Physical Controls on Oxygen Distribution and Denitrification Potential in the North West Arabian Sea, Geophys. Res. Lett., 45, 4143–4152, 2018.
Raman, A. V., Damodaran, R., Levin, L. A., Ganesh, T., Rao, Y. K. V., Nanduri, S., and Madhusoodhanan, R.: Macrobenthos relative to the oxygen minimum zone on the East Indian margin, Bay of Bengal, Mar. Ecol., 36, 679–700, 2015.
Ramaswamy, V., Nair, R. R., Manganini, S., Haake, B., and Ittekkot, V.: Lithogenic fluxes to the deep Arabian Sea measured by sediment traps, Deep-Sea Res., 38, 169–184, 1991.
Rao, C. K., Naqvi, S. W. A., Kumar, M. D., Varaprasad, S. J. D., Jayakumar, D. A., George, M. D., and Singbal, S. Y. S.: Hydrochemistry of the Bay of Bengal: possible reasons for a different water-column cycling of carbon and nitrogen from the Arabian Sea, Mar. Chem., 47, 279–290, 1994.
Rao, R. R., Molinari, R. L., and Festa, J. F.: Evolution of the Climatological Near-Surface Thermal Structure of the Tropical Indian Ocean – 1. Description of Mean Monthly Mixed Layer Depth, and Sea Surface Temperature, Surface Current, and Surface Meteorological Fields, J. Geophys. Res., 94, 10801–10815, 1989.
Resplandy, L.: Will ocean zones with low oxygen levels expand or shrink?, Nature, 557, 314–315, 2018.
Resplandy, L., Lévy, M., Madec, G., Pous, S., Aumont, O., and Kumar, D.: Contribution of mesoscale processes to nutrient budgets in the Arabian Sea, J. Geophys. Res.-Ocean., 116, C11007, https://doi.org/10.1029/2011JC007006, 2011.
Resplandy, L., Lévy, M., Bopp, L., Echevin, V., Pous, S., Sarma, V. V. S. S., and Kumar, D.: Controlling factors of the oxygen balance in the Arabian Sea's OMZ, Biogeosciences, 9, 5095–5109, https://doi.org/10.5194/bg-9-5095-2012, 2012.
Resplandy, L., Lévy, M., and McGillicuddy Jr., D. J.: Effects of Eddy-Driven Subduction on Ocean Biological Carbon Pump, Global Biogeochem. Cy., 33, 1071–1084, 2019.
Rixen, T. and Ittekkot, V.: Nitrogen deficits in the Arabian Sea, implications from a three component mixing analysis, Deep-Sea Res. Pt. II, 1879–1891, 2005.
Rixen, T., Haake, B., and Ittekkot, V.: Sedimentation in the western Arabian Sea: the role of coastal and open-ocean upwelling, Deep-Sea Res. Pt. II, 47, 2155–2178, 2000.
Rixen, T., Goyet, C., and Ittekkot, V.: Diatoms and their influence on the biologically mediated uptake of atmospheric CO2 in the Arabian Sea upwelling system, Biogeosciences, 3, 1–13, https://doi.org/10.5194/bg-3-1-2006, 2006.
Rixen, T., Gaye, B., and Emeis, K.-C.: The monsoon, carbon fluxes, and the organic carbon pump in the northern Indian Ocean, Prog. Oceanogr., 175, 24–39, 2019a.
Rixen, T., Gaye, B., Emeis, K.-C., and Ramaswamy, V.: The ballast effect of lithogenic matter and its influences on the carbon fluxes in the Indian Ocean, Biogeosciences, 16, 485–503, https://doi.org/10.5194/bg-16-485-2019, 2019b.
Rixen, T., Baum, A., Gaye, B., and Nagel, B.: Seasonal and interannual variations in the nitrogen cycle in the Arabian Sea, Biogeosciences, 11, 5733–5747, https://doi.org/10.5194/bg-11-5733-2014, 2014.
Rosenberg, R.: Marine benthic faunal successional stages and related sediment activity, Sci. Mar., 66, 107–119, 2001.
Saltzman, J. and Wishner, K. F.: Zooplankton ecology in the eastern tropical Pacific oxygen minimum zone above a seamount: 2. Vertical distribution of copepods, Deep-Sea Res. Pt. I, 44, 931–954, 1997.
Sánchez-Baracaldo, P.: Origin of marine planktonic cyanobacteria, Sci. Rep., 5, 17418, https://doi.org/10.1038/srep17418, 2015.
Saraswathy, M. and Iyer, H. K.: Ecology of Pleuromamma indica Wolfenden (Copepoda – Calanoida) in the Indian Ocean, Indian J. Mar. Sci., 15, 219–222, 1986.
Sarkar, A., Sengupta, S., McArthur, J. M., Bera, M. K., Bushan, R., Samanta, A., and Agrawal, S.: Evolution of Ganges-Brahmaputra western delta plain: clues from sedimentology and carbon isotopes, Quaternary Sci. Rev., 28, 2564–2581, 2009.
Sarma, V. and Udaya Bhaskar, T.: Ventilation of oxygen to oxygen minimum zone due to anticyclonic eddies in the Bay of Bengal, J. Geophys. Res.-Biogeo., 123, 2145–2153, 2018.
Sarma, V., Jagadeesan, L., Dalabehera, H., Rao, D., Kumar, G., Durgadevi, D., Yadav, K., Behera, S., and Priya, M.: Role of eddies on intensity of oxygen minimum zone in the Bay of Bengal, Cont. Shelf Res., 168, 48–53, 2018.
Sastry, J. S. and D'Souza, R. S.: Upwelling & Upward Mixing in the Arabian Sea, Indian J. Mar. Sci., 1, 17–27, 1972.
Schlitzer, R.: Applying the adjoint method for biogeochemical modeling, Export of particulate organic matter in the World Ocean, in: Inverse Methods in Biogeochemical Cycles, edited by: Kasibhata, P., AGU Monograph, AGU, 2000.
Schlitzer, R.: Carbon export fluxes in the Southern Ocean: results from inverse modeling and comparison with satellite-based estimates, Deep-Sea Res. Pt. II, 49, 1623–1644, 2002.
Schmidt, H., Czeschel, R., and Visbeck, M.: Seasonal variability of the circulation in the Arabian Sea at intermediate depth and its link to the Oxygen Minimum Zone, Ocean Sci. Discuss., https://doi.org/10.5194/os-2020-9, in review, 2020.
Schmidtko, S., Stramma, L., and Visbeck, M.: Decline in global oceanic oxygen content during the past five decades, Nature, 542, 335–339, https://doi.org/10.1038/nature21399, 2017.
Schott, F. and McCreary Jr., J. P.: The monsoon circulation of the Indian Ocean, Prog. Oceanogr., 51, 1–123, 2001.
Schott, G.: Geographie des Indischen und Stillen Ozeans, Boysen, Hamburg, Germany, 1935.
Schunck, H., Lavik, G., Desai, D. K., Großkopf, T., Kalvelage, T., Löscher, C. R., Paulmier, A., Contreras, S., Siegel, H., Holtappels, M., Rosenstiel, P., Schilhabel, M. B., Graco, M., Schmitz, R. A., Kuypers, M. M. M., and LaRoche, J.: Giant Hydrogen Sulfide Plume in the Oxygen Minimum Zone off Peru Supports Chemolithoautotrophy, PLOS ONE, 8, e68661, https://doi.org/10.1371/journal.pone.0068661, 2013.
Schütte, F., Karstensen, J., Krahmann, G., Hauss, H., Fiedler, B., Brandt, P., Visbeck, M., and Körtzinger, A.: Characterization of “dead-zone” eddies in the eastern tropical North Atlantic, Biogeosciences, 13, 5865–5881, https://doi.org/10.5194/bg-13-5865-2016, 2016.
Schwartz, M. C., Woulds, C., and Cowie, G. L.: Sedimentary denitrification rates across the Arabian Sea oxygen minimum zone, Deep-Sea Res. Pt. II, 56, 324–332, 2009.
Séférian, R., Berthet, S., Yool, A., Palmiéri, J., Bopp, L., Tagliabue, A., Kwiatkowski, L., Aumont, O., Christian, J., Dunne, J., Gehlen, M., Ilyina, T., John, J. G., Li, H., Long, M. C., Luo, J. Y., Nakano, H., Romanou, A., Schwinger, J., Stock, C., Santana-Falcón, Y., Takano, Y., Tjiputra, J., Tsujino, H., Watanabe, M., Wu, T., Wu, F., and Yamamoto, A.: Tracking Improvement in Simulated Marine Biogeochemistry Between CMIP5 and CMIP6, Curr. Clim. Change Rep., 6, 95–119, 2020.
Segschneider, J. and Bendtsen, J.: Temperature-dependent remineralization in a warming ocean increases surface pCO2 through changes in marine ecosystem composition, Global Biogeochem. Cy., 27, 1214–1225, 2013.
Segschneider, J., Schneider, B., and Khon, V.: Climate and marine biogeochemistry during the Holocene from transient model simulations, Biogeosciences, 15, 3243–3266, https://doi.org/10.5194/bg-15-3243-2018, 2018.
Seiwell, H. R.: The minimum oxygen concentration in the western basin of the North Atlantic, Papers Phys. Oceanogr. Meteorol., 5, 3–18, 1937.
Sen Gupta, R. and Naqvi, S. W. A.: Chemical Oceanography of the Indian Ocean, North of the Equator, Deep-Sea Res., 31, 671–706, 1984.
Sewell, R. B. S. and Fage, L.: Minimum Oxygen Layer in the Ocean, Nature, 162, 949–951, 1948.
Shenoy, D. M., Suresh, I., Uskaikar, H., Kurian, S., Vidya, P. J., Shirodkar, G., Gauns, M. U., and Naqvi, S. W. A.: Variability of dissolved oxygen in the Arabian Sea Oxygen Minimum Zone and its driving mechanisms, J. Mar. Syst., 204, 103310, https://doi.org/10.1016/j.jmarsys.2020.103310, 2020.
Shetye, S. R. and Shenoi, S. S. C.: Seasonal cycle of surface circulation in the coastal North Indian Ocean, P. Indian A. S.-Earth, 97, 53–62, 1988.
Shetye, S. R., Gouveia, A. D., Shenoi, S. S. C., Sundar, D., Michael, G. S., Almeida, A. M., and Santanam, K.: Hydrography and circulation off the west coast of India during the Southwest Monsoon 1987, J. Mar. Res., 48, 359–378, 1990.
Sigman, D. M., Granger, J., DiFiore, P. J., Lehmann, M. F., Ho, R., Cane, G., and van Geen, A.: Coupled nitrogen and oxygen isotope measurements of nitrate along the eastern North Pacific margin, Global Biogeochem. Cy., 19, GB4022, https://doi.org/10.1029/2005GB002458, 2005.
Singh, A., Gandhi, N., Ramesh, R., and Prakash, S.: Role of cyclonic eddy in enhancing primary and new production in the Bay of Bengal, J. Sea Res., 97, 5–13, 2015.
Smallwood, B. J., Wolff, G. A., Bett, B. J., Smith, C. R., Hoover, D., Gage, J. D., and Patience, A.: Megafauna Can Control the Quality of Organic Matter in Marine Sediments, Naturwissenschaften, 86, 320–324, 1999.
Smith, C. R., A. Levin, L., Hoover, D. J., McMurtry, G., and Gage, J. D.: Variations in bioturbation across the oxygen minimum zone in the northwest Arabian Sea, Deep-Sea Res. Pt. II, 47, 227–257, 2000.
Smith, S. L.: Understanding the Arabian Sea: Reflections on the 1994–1996 Arabian Sea Expedition, Deep-Sea Res. Pt. II, 48, 1385–1402, 2001.
Smith, S. L. and Madhupratap, M.: Mesozooplankton of the Arabian Sea: Patterns influenced by seasons, upwelling, and oxygen concentrations, Prog. Oceanogr., 65, 214–239, 2005.
Smith, S. L., Roman, M., Prusova, I., Wishner, K., Gowing, M., Codispoti, L. A., Barber, R., Marra, J., and Flagg, C.: Seasonal response of zooplankton to monsoonal reversals in the Arabian Sea, Deep-Sea Res. Pt. II, 45, 2369–2403, 1998a.
Smith, S. L., Codispoti, L. A., Morrison, J. M., and Barber, R. T.: The 1994–1996 Arabian Sea Expedition: An integrated, interdisciplinary investigation of the response of the northwestern Indian Ocean to monsoonal forcing, Deep-Sea Res. Pt. II, 45, 1905–1915, 1998b.
Smith, T. M., Reynolds, R. W., Peterson, T. C., and Lawrimore, J.: Improvements to NOAA's historival merged land-ocean surface temperature analysis (1880–2006), J. Climate, 21, 2283–2296, 2008.
Sokoll, S., Holtappels, M., Lam, P., Collins, G., Schlüter, M., Lavik, G., and Kuypers, M.: Benthic Nitrogen Loss in the Arabian Sea Off Pakistan, Front. Microbiol., 3, 1–17, 2012.
Somasundar, K., Rajendran, A., Dileep Kumar, M., and Sen Gupta, R.: Carbon and nitrogen budgets of the Arabian Sea, Mar. Chem., 30, 363–377, 1990.
Somes, C. J., Oschlies, A., and Schmittner, A.: Isotopic constraints on the pre-industrial oceanic nitrogen budget, Biogeosciences, 10, 5889–5910, https://doi.org/10.5194/bg-10-5889-2013, 2013.
Stramma, L., Fischer, J., and Schott, F.: The flow field off southwest India at 8∘ N during the southwest monsoon of August 1993, J. Mar. Res., 54, 55-72, 1996.
Stramma, L., Johnson, G. C., Sprintall, J., and Mohrholz, V.: Expanding oxygen-minimum zones in the tropical oceans, Science, 320, 655–658, 2008.
Stramma, L., Johnson, G. C., Firing, E., and Schmidtko, S.: Eastern Pacific oxygen minimum zones: Supply paths and multidecadal changes, J. Geophys. Res., 115, C09011, https://doi.org/10.1029/2009jc005976, 2010a.
Stramma, L., Schmidtko, S., Levin, L. A., and Johnson, G. C.: Ocean oxygen minima expansions and their biological impacts, Deep-Sea Res. Pt. I, 57, 587–595, 2010b.
Sudheesh, V., Gupta, G. V. M., Sudharma, K. V., Naik, H., Shenoy, D. M., Sudhakar, M., and Naqvi, S. W. A.: Upwelling intensity modulates N2O concentrations over the western Indian shelf, J. Geophys. Res.-Ocean., 121, 8551–8565, 2016.
Suess, E.: Particulate organic carbon flux in the oceans – surface productivity and oxygen utilization, Nature, 288, 260–263, 1980.
Suthhof, A., Ittekkot, V., and Gaye-Haake, B.: Millenial-scale oscillation of denitrification intensitiy in the Arabian Sea during the late Quaternary and its potential influence on atmospheric N2O and global climate, Global Biogeochem. Cy., 15, 637–650, 2001.
Sverdrup, H. U.: On the Explanation of the Oxygen Minima and Maxima in the Oceans1), ICES J. Mar. Sci., 13, 163–172, 1938.
Sverdrup, H. U., Johnson, M. W., and Flemming, R. H.: The Oceans, their physics chemistry and general biology, Prentice-Hall, Englewood Cliffs, N.J., 1942.
Swallow, J. C.: Some aspects of the physical oceanography of the Indian Ocean, Deep-Sea Res. Pt. A, 31, 639–650, 1984.
Sweetman, A. K., Chelsky, A., Pitt, K. A., Andrade, H., van Oevelen, D., and Renaud, P. E.: Jellyfish decomposition at the seafloor rapidly alters biogeochemical cycling and carbon flow through benthic food-webs, Limnol. Oceanogr., 61, 1449–1461, 2016.
Taylor, K. E., Stouffer, R. J., and Meehl, G. A.: An Overview of CMIP5 and the Experiment Design, Bull. Am. Meteorol. Soc., 93, 485–498 https://doi.org/10.1175/BAMS-D-11-00094.1, 2012.
Tesdal, J.-E., Galbraith, E. D., and Kienast, M.: Nitrogen isotopes in bulk marine sediment: linking seafloor observations with subseafloor records, Biogeosciences, 10, 101–118, https://doi.org/10.5194/bg-10-101-2013, 2013.
Thamdrup, B., Dalsgaard, T., and Revsbech, N. P.: Widespread functional anoxia in the oxygen minimum zone of the Eastern South Pacific, Deep-Sea Res. Pt. I, 65, 36–45, 2012.
Ulloa, O., Canfield, D. E., DeLong, E. F., Letelier, R. M., and Stewart, F. J.: Microbial oceanography of anoxic oxygen minimum zones, P. Natl. Acad. Sci., 109, 15996, https://doi.org/10.1073/pnas.1205009109, 2012.
Unger, D., Schaefer, P., Ittekkot, V., and Gaye, B.: Nitrogen isotopic composition of sinking particles from the southern Bay of Bengal: Evidence for variable nitrogen sources, Deep-Sea Res., 1, 53, 1658–1676, 2006.
Van Mooy, B. A. S., Keil, R. G., and Devol, A. H.: Impact of suboxia on sinking particulate organic carbon: Enhanced carbon flux and preferential degradation of amino acids via denitrification, Geochim. Cosmochim. Ac., 66, 457–465, 2002.
Vaquer-Sunyer, R. and Duarte, C. M.: Thresholds of hypoxia for marine biodiversity, P. Natl. Acad. Sci., 105, 15452, https://doi.org/10.1073/pnas.0803833105, 2008.
Vic, C., Roullet, G., Capet, X., Carton, X., Molemaker, M. J., and Gula, J.: Eddy topography interactions and the fate of the Persian Gulf Outflow, J. Geophys. Res.-Ocean., 120, 6700–6717, 2015.
Vinogradov, M. E. and Voronina, N. M.: Influence of the oxygen deficit on the distribution of plankton in the Arabian Sea, Deep-Sea Res. Oceanogr. Abstracts, 9, 523–530, 1962.
Voss, M., Deutsch, B., Elmgren, R., Humborg, C., Kuuppo, P., Pastuszak, M., Rolff, C., and Schulte, U.: Source identification of nitrate by means of isotopic tracers in the Baltic Sea catchments, Biogeosciences, 3, 663–676, https://doi.org/10.5194/bg-3-663-2006, 2006.
Wang, L., Lin, X., Goes, J. I., and Lin, S.: Phylogenetic Analyses of Three Genes of Pedinomonas noctilucae, the Green Endosymbiont of the Marine Dinoflagellate Noctiluca scintillans, Reveal its Affiliation to the Order Marsupiomonadales (Chlorophyta, Pedinophyceae) under the Reinstated Name Protoeuglena noctilucae, Protist, 167, 205–216, 2016.
Ward, B. B., Devol, A. H., Rich, J. J., Chang, B. X., Bulow, S. E., Naik, H., Pratihary, A., and Jayakumar, A.: Denitrification as the dominant nitrogen loss process in the Arabian Sea, Nature, 461, 78–81, 2009.
Weeks, S. J., Currie, B., and Bakun, A.: Massive emissions of toxic gas in the Atlantic, Nature, 415, 493–494, 2002.
White, C. M., Woulds, C., Cowie, G. L., Stott, A., and Kitazato, H.: Resilience of benthic ecosystem C-cycling to future changes in dissolved oxygen availability, Deep-Sea Res. Pt. II, 161, 29–37, 2019.
Wishner, K. F., Ashjian, C. J., Gelfman, C., Gowing, M. M., Kann, L., Levin, L. A., Mullineaux, L. S., and Saltzman, J.: Pelagic and benthic ecology of the lower interface of the Eastern Tropical Pacific oxygen minimum zone, Deep-Sea Res. Pt. I, 42, 93–115, 1995.
Wishner, K. F., Gowing, M. M., and Gelfman, C.: Mesozooplankton biomass in the upper 1000 m in the Arabian Sea: overall seasonal and geographic patterns, and relationship to oxygen gradients, Deep-Sea Res. Pt. II, 45, 2405–2432, 1998.
Wishner, K. F., Gelfman, C., Gowing, M. M., Outram, D. M., Rapien, M., and Williams, R. L.: Vertical zonation and distributions of calanoid copepods through the lower oxycline of the Arabian Sea oxygen minimum zone, Prog. Oceanogr., 78, 163–191, 2008.
Woulds, C., Cowie, G. L., Levin, L. A., Andersson, J. H., Middelburg, J. J., Vandewiele, S., Lamont, P. A., Larkin, K. E., Gooday, A. J., Schumacher, S., Whitcraft, C., Jeffreys, R. M., and Schwartz, M.: Oxygen as a control on sea floor biological communities and their roles in sedimentary carbon cycling, Limnol. Oceanogr., 52, 1698–1709, 2007.
Woulds, C., Andersson, J. H., Cowie, G. L., Middelburg, J. J., and Levin, L. A.: The short-term fate of organic carbon in marine sediments: Comparing the Pakistan margin to other regions, Deep-Sea Res. Pt. II, 56, 393–402, 2009.
Wyrtki, K.: Physical Oceanography of the Indian Ocean, in: The Biology of the Indian Ocean, edited by: Zeitschel, B., Springer Verlag, Berlin, Heidelberg, New York, 1973.
You, Y.: Seasonal variations of thermocline circulation and ventilation in the Indian Ocean, J. Geophys. Res., 102, 10391–10422, 1997.